Table of Contents
SCHEDULE 14A INFORMATION
EXCHANGE ACT OF 1934 (AMENDMENT NO. )
o Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
þ Definitive Proxy Statement
o Definitive Additional Materials
o Soliciting Material Pursuant to § 240.14a-12
| þ | No fee required. | |
| o | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| (1) | Title of each class of securities to which transaction applies: | ||
| (2) | Aggregate number of securities to which transaction applies: | ||
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): | ||
| (4) | Proposed maximum aggregate value of transaction: | ||
| (5) | Total fee paid: |
| o | Fee paid previously with preliminary materials. | |
| o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| (1) | Amount previously paid: | ||
| (2) | Form, Schedule or Registration Statement No.: | ||
| (3) | Filing Party: | ||
| (4) | Date Filed: |
Table of Contents

| 1. | To approve the proposed issuance of our common stock and warrants to purchase our common stock to certain investors pursuant to the Securities Purchase Agreement, dated as of October 6, 2005. | |
| 2. | To approve the proposed amendment of our certificate of incorporation to increase the authorized number of shares of our common stock by 50,000,000. | |
| 3. | To approve the proposed amendment of the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (i) to increase the number of shares of our common stock that may be issued under the plan by 16,000,000, (ii) to increase the number of shares of our common stock that may be issued under the plan to any eligible person in any calendar year by 6,000,000 and (iii) to eliminate the current minimum restriction period requirements with respect to restricted stock granted under the plan. | |
| 4. | To approve the proposed decrease in the number of issued and outstanding shares of our common stock by means of a one-for-five reverse stock split. | |
| 5. | To transact such other business that may properly come before the meeting or any adjournment thereof. |
| By order of the board of directors, | |
 | |
| Steven B. Engle | |
| Chairman and Chief Executive Officer |
Table of Contents
| Proposal 1 | The proposed issuance of shares of our common stock and warrants to purchase shares of our common stock to certain investors pursuant to the Securities Purchase Agreement. | |
| Proposal 2 | The proposed amendment of our certificate of incorporation to increase the authorized number of shares of our common stock by 50,000,000. | |
| Proposal 3 | The proposed amendment of the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (i) to increase the number of shares of our common stock that may be issued under the plan by 16,000,000, (ii) to increase the number of shares of our common stock that may be |
Table of Contents
| issued under the plan to any eligible person in any calendar year by 6,000,000 and (iii) to eliminate the current minimum restriction period requirements with respect to restricted stock granted under the plan. | ||
| Proposal 4 | The proposed decrease in the number of issued and outstanding shares of our common stock by means of a one-for-five reverse stock split. |
2
Table of Contents
3
Table of Contents
4
Table of Contents
5
Table of Contents
6
Table of Contents
7
Table of Contents
| • | we will issue an aggregate of 88,000,000 shares of our common stock to the Investors; | |
| • | if the authorized share increase is approved at the special meeting pursuant to Proposal 2, we will issue the closing warrants to the Investors to purchase up to an additional 22,000,000 shares of our common stock at an exercise price of $1.00 per share; | |
| • | if the authorized share increase is not approved at the special meeting pursuant to Proposal 2, we will issue (i) the first closing warrants to the Investors to purchase the remaining number of authorized shares under our certificate of incorporation following the closing (expected to be approximately 1,000,000) at an exercise price of $1.00 per share and (ii) the second closing warrants to the Investors to purchase the number of shares equal to 22,000,000 less the number of shares covered by the first closing warrants at an exercise price of $1.00 per share; and | |
| • | the Investors will collectively deliver $66 million to us, representing the purchase price for the common stock and the closing warrants (or the first closing warrants and the second closing warrants, as applicable). |
| Affirmative Covenants. We agreed to, prior to the closing under the purchase agreement or a contingent warrant trigger: (i) pay and discharge all lawful taxes, assessments and governmental charges, (ii) maintain valid workers’ compensation insurance policies, (iii) preserve and maintain our corporate existence, rights, franchises and privileges, (iv) keep adequate books and records, (v) comply with the requirements of all applicable laws, (vi) take all actions necessary to ensure that all material patents are kept in force and (vii) notify the Investors in writing of any event that would cause any of our representations to be materially inaccurate or that would cause us to not materially comply with the agreements and conditions in the purchase agreement. |
8
Table of Contents
| SEC Filings, Budget and Other Information. Subject to the closing, until December 31, 2008, we agreed to deliver copies of the following to each Investor that owns at least 75% of the shares of our common stock it originally purchased at the closing: (i) all filings we make with the Securities and Exchange Commission, promptly after they become public information; and (ii) our annual budget, at least 30 days prior to the commencement of the applicable fiscal year. We have also agreed to prepare and submit to, and obtain the approval from our board of directors for, our annual budget for the succeeding fiscal year at least thirty days prior to the commencement of each fiscal year. Each Investor has agreed, if it elects to receive any material nonpublic information in the annual budget provided to it under this covenant, to keep such nonpublic information confidential to the extent necessary to comply with applicable securities laws. | |
| Negative Covenants. We agreed, among other matters, to the following negative covenants: (i) until December 31, 2008, and so long as Essex Woodlands continues to own at least 75% of the shares of our common stock it purchased under the purchase agreement, we will not, without Essex Woodlands’ prior written consent, enter into any material transaction that could result in the sale, transfer or assignment of, or grant of any exclusive or non-exclusive license to, any patent or patent application for Riquent® in the United States; and (ii) prior to the closing under the purchase agreement, we will not solicit, initiate or encourage submission of any financing proposal by any person or entity to acquire any equity interest in us or to finance our business or operations, or enter into any such transaction (except for transactions on terms superior to those contained in the purchase agreement). | |
| Indemnification. We agreed to defend, protect, indemnify and hold harmless each Investor and each holder of the shares purchased under the purchase agreement, the warrants or the shares issuable upon exercise of the warrants, and their affiliates and other agents or representatives, from and against any and all losses and claims incurred as a result of any breach of our representations, warranties and covenants under the purchase agreement. | |
| Obligations. We and each of the Investors agreed to use commercially reasonable efforts to timely satisfy each of the conditions to closing under the purchase agreement. | |
| Certain Filings. We agreed to timely make, or obtain exemptions from, the filings required under applicable federal and state securities laws with respect to the issuance of the shares purchased under the purchase agreement, the warrants and the shares issuable upon exercise of the warrants. | |
| Reservation of Shares. We agreed to take all actions necessary, subject to approval by our stockholders of the required increase in our authorized number of shares of common stock, to at all times have authorized, and reserved for issuance, no less than 100% of the shares of our common stock issuable upon exercise of the outstanding warrants. | |
| Waivers to Existing Registration Rights. With respect to any existing agreements or arrangements under which we are obligated to register the sale of any of our securities under the Securities Act of 1933, as amended (the “Securities Act”), we agreed to use commercially reasonable efforts to obtain waivers so that such obligations, if any, would be satisfied by our actions to file registration statements under the registration rights agreement. | |
| Board Nominees; Director Protections. We agreed to ensure that (i) our board of directors will consist of no more than nine authorized directors, (ii) two individuals designated by Essex Woodlands, one individual designated by Frazier and one individual jointly designated by Essex Woodlands and Frazier are properly nominated for election as directors on our board of directors and (iii) each of such designees is at all times covered and insured by our directors and officers liability insurance. We have also agreed to reimburse each designee for all reasonable expenses he incurs in attending any board of directors meetings, to enter into indemnification agreements with each such designee and to pay each designee the same compensation that is paid to other non-employee directors for serving as directors. | |
| Preemptive Rights. Subject to the closing, we have agreed that, until December 31, 2008 and so long as any Investor continues to own at least 75% of the shares of common stock it purchased under the purchase agreement, such Investor will have a right of first refusal to purchase up to its pro rata share |
9
Table of Contents
| (based on the percentage of our outstanding common stock held by it) of any equity securities or debt securities convertible into equity securities on the same price and terms and conditions as we offer such securities to other potential investors. This right of first purchase will not apply to any issuance of: |
| • | shares of our common stock (or options therefor) to our officers, directors, employees or consultants pursuant to stock purchase or option plans on terms approved by our board of directors; | |
| • | shares of our common stock, options or warrants to purchase shares of our common stock to financial institutions or lessors in connections with commercial credit agreements, equipment financings or similar transactions, provided such issuances are primarily for other than equity financing purposes; | |
| • | shares of our common stock, options or warrants to purchase shares of our common stock pursuant to (i) joint ventures, technology licensing or research and development activities, (ii) distribution or manufacture of our products or services, (iii) the purchase of advertising placement or (iv) any other transaction involving corporate partners, in each case primarily for other than equity financing purposes; and | |
| • | shares of our common stock, options or warrants to purchase shares of our common stock in connection with bona fide acquisitions, mergers or similar transactions. |
| Listing. We have agreed to comply with all requirements of the Nasdaq National Market or the Nasdaq Capital Market, as applicable, and to use our best efforts to list the shares issued under the purchase agreement and upon exercise of the warrants on the Nasdaq National Market until all such shares are sold or, if such listing is suspended, obtain listing on the Nasdaq Capital Market until the Nasdaq National Market listing is restored. | |
| Stockholder Approvals. We have agreed to use our best efforts to obtain the approval of our stockholders with respect to the transactions described in Proposals 1 through 4 of this proxy statement. We have also agreed, in the event that any of Proposals 2 through 4 is not approved at the special meeting, to call a second meeting of stockholders within 45 days following the closing under the purchase agreement for the purpose of seeking approval for each proposal that was not approved. |
| • | our representations and warranties in the purchase agreement are true, correct and complete in all material respects on and as of the closing date; | |
| • | we have performed and complied in all material respects with all of our agreements and conditions contained in the purchase agreement required to be performed or complied with prior to or at the closing; | |
| • | we have obtained the approval of our stockholders to complete the transactions contemplated by the purchase agreement; | |
| • | the receipt by each Investor of legal opinions in form and substance acceptable to Investors who have committed to purchase a majority of the shares to be sold under the purchase agreement; | |
| • | the number of authorized directors on our board of directors has been expanded to nine, and the two individuals designated by Essex Woodlands, the individual designated by Frazier and the individual jointly designated by Essex Woodlands and Frazier have been elected or appointed as directors on our board of directors; | |
| • | one of Essex Woodlands’ designees and Frazier’s designee to our board have been elected or appointed to the compensation committee of our board of directors; |
10
Table of Contents
| • | we have delivered to the Investors our certificate of incorporation, certificates of the Delaware and California Secretaries of State certifying as to our corporate good standing and certificates of our secretary and president certifying as to certain of our corporate documentation and fulfillment of closing conditions; | |
| • | we have paid or made adequate arrangements for payment, in accordance with our agreement, of the fees and expenses of the parties in connection with the transactions under the purchase agreement; | |
| • | all governmental or regulatory authorizations, approvals, filings or permits, if any, that are required in connection with the closing have been obtained; | |
| • | our common stock has been designated for quotation on the Nasdaq National Market or the Nasdaq Capital Market and not suspended from trading; | |
| • | we have delivered irrevocable instructions to our transfer agent to issue certificates registered in the name of each Investor for the shares of our common stock to be issued pursuant to the purchase agreement; | |
| • | no material adverse effect has occurred or become known with respect to our business, assets, liabilities (contingent or otherwise), operations, condition (financial or otherwise), or results of operations; | |
| • | we have taken all actions necessary under our stockholder rights plan to ensure that it will not be triggered by the transactions contemplated by the purchase agreement; | |
| • | we have taken all actions necessary to ensure that no material change in control provision or restriction is triggered by the purchase agreement and related agreements; and | |
| • | the Investors have committed to purchase at least $66 million of shares of our common stock in the aggregate at the closing under the purchase agreement. |
| • | the representations and warranties made by such Investor are true, correct and complete in all material respects on and as of the closing date; | |
| • | such Investor has performed and complied in all material respects with all of its agreements and conditions contained in the purchase agreement required to be performed or complied with prior to or at the closing; and | |
| • | we have obtained the approval of our stockholders with respect to the transactions contemplated by the purchase agreement. |
11
Table of Contents
| The Closing Warrants. |
| • | if after October 6, 2005, we pay a dividend in or make a distribution of shares of our common stock to all holders of our outstanding common stock, the exercise price will be reduced by a fraction reflecting the ratio between the number of shares of our common stock outstanding as of the record date fixed with respect to such dividend or distribution and the total number of outstanding shares of our common stock immediately after such dividend or distribution; and | |
| • | if the outstanding shares of our common stock are subdivided into a greater number of shares or combined into a smaller number of shares after October 6, 2005, the exercise price will be proportionately reduced or increased, respectively. |
12
Table of Contents
| • | the date we consummate an equity financing transaction not permitted under the non-solicitation provisions of the purchase agreement, including the consummation of any financing under any proposal with terms superior to those contained in the purchase agreement; | |
| • | the first business day after the date on which our stockholders fail to approve Proposal 1; | |
| • | December 30, 2005; and | |
| • | the date after the holder receives notice of any of certain extraordinary transactions, including certain mergers, exchanges with respect to our common stock and the sale or transfer of all or substantially all of our assets. |
13
Table of Contents
| • | if after October 6, 2005, we pay a dividend in or make a distribution of shares of our common stock to all holders of our outstanding common stock, the exercise price will be reduced by a fraction reflecting the ratio between the number of outstanding shares of our common stock as of the record date fixed with respect to such dividend or distribution and the total number of shares of our common stock outstanding immediately after such dividend or distribution; and | |
| • | if the outstanding shares of our common stock are subdivided into a greater number of shares or combined into a smaller number of shares after October 6, 2005, the exercise price will be proportionately reduced or increased, respectively. |
| • | the shares of our common stock that are sold to the Investors pursuant to the purchase agreement; |
14
Table of Contents
| • | the shares of our common stock issuable upon exercise of the warrants; | |
| • | any shares of our capital stock issued or issuable with respect to the shares of our common stock referred to above as a result of any stock split, stock dividend, recapitalization, exchange or similar event or otherwise, without regard to any limitations on the issuance of such shares of our common stock; and | |
| • | any of our securities issued upon the reclassification of any of the securities referred to above. |
| • | at any time prior to the expiration of the registration period, the number of shares of our common stock available for sale under a registration statement is insufficient to cover all of the registrable securities and we propose to file a registration statement relating to an offering for our own account or the account of others; | |
| • | if our offering is being underwritten, each holder requesting registration of its shares must accept the terms of the underwriting as agreed between us and our underwriter or underwriters; | |
| • | if the underwriters determine, in their reasonable good faith opinion, that marketing or other factors dictate that a limitation on the number of shares of our common stock which may be included in the offering is necessary to facilitate and not adversely affect the offering, we will include in such registration (i) first, all securities we propose to sell for our own account and (ii) second, up to the full number of securities proposed to be registered for the account of each of the holders, pro rata on the basis of the number of registrable securities held by such holder; and | |
| • | we are not obligated to register securities under this section of the registration rights agreement if the registration statement that we intend to file relates to (i) securities to be offered by us solely in connection with any acquisition of any entity or business or (ii) equity securities issuable under stock option or other employee benefit plans. |
15
Table of Contents
| • | if any registration statement required to be filed by us under the registration rights agreement is not filed or declared effective within the applicable filing deadline or effectiveness deadline, respectively; | |
| • | if, on any day during the registration period (other than during certain periods which we have been allowed under the agreement to delay disclosure of material non-public information concerning us), any registrable security required to be registered cannot be sold as a matter of law or because we have failed to timely perform our obligations under the agreement; or | |
| • | if any period during which we delay disclosure of material non-public information concerning us exceeds the length allowed under the agreement. |
| • | promptly prepare and file a registration statement and use our best efforts to cause such registration statement to become effective under the time frames described above, and keep the registration statement effective until the expiration of the registration period; | |
| • | prepare and file any amendments and supplements to the registration statement or prospectus as required by law, and comply with all legal requirements with respect to the disposition of registrable securities; | |
| • | permit holders’ legal counsel to review and comment on the registration statement and any amendments and supplements to such registration statement, and not file any such document with the SEC to which such legal counsel reasonably objects; | |
| • | furnish each holder whose registrable securities are included in any registration statement, without charge, copies of such registration statements and such other documents as reasonably requested by such holder; | |
| • | use our best efforts to register and qualify the securities, in any jurisdiction in the U.S. that may be required, under all state and local securities laws of such jurisdiction, and maintain such registration and qualification during the registration period; | |
| • | notify the Investors’ designated legal counsel and each Investor in writing, among other matters, (i) of the occurrence of any event that causes any information contained in any registration statement to become inaccurate, (ii) when a prospectus amendment or supplement has been filed, (iii) of any request by the SEC for amendments or supplements to a registration statement and (iv) of our reasonable determination that an amendment to any registration statement would be appropriate; | |
| • | make available for inspection by (i) any holder, (ii) their legal counsel and (iii) the accounting firm retained by the holders, all of our financial and other records and corporate documents and properties that are pertinent, which are requested for any purpose related to the holders’ rights or our obligations under the registration rights agreement, provided that each such inspector agrees to hold such records in strict confidence (unless certain exceptions are applicable); |
16
Table of Contents
| • | hold in confidence and not disclose any information concerning an Investor provided to us (unless certain exceptions are applicable); | |
| • | use our best efforts to (i) cause all registrable securities covered by a registration statement to be listed on each securities exchange on which our other securities of the same class are then listed and (ii) secure designation and quotation of all of the registrable securities covered by such registration statement on the Nasdaq stock market; and we are required to pay all fees and expenses in connection with satisfying these obligations; | |
| • | provide a transfer agent and registrar for all registrable securities registered under any registration statement not later than the effective date of such registration statement; | |
| • | if requested by a holder, incorporate in a prospectus supplement or amendment, as necessary, such information as such holder requests to be included relating to such holder and the sale and distribution of registrable securities; and | |
| • | promptly notify the holders in writing of the existence of any material non-public information with respect to which we have delayed disclosure to the public, provided that such periods of delay in disclosure must not exceed (i) 30 consecutive days and (ii) an aggregate of 60 days during any consecutive 365-day period. |
| • | such holder agrees in writing with the transferee to assign such rights, and a copy of such agreement is furnished to us within a reasonable time after assignment; | |
| • | within a reasonable time after such transfer or assignment, we are furnished with written notice of the name of the transferee or assignee and the securities with respect to which such registration rights are being transferred or assigned; | |
| • | the transferee or assignee agrees in writing with us to be bound by all of the provisions contained in the registration rights agreement; and | |
| • | the transfer was made in accordance with the requirements of the purchase agreement and the warrants. |
17
Table of Contents
18
Table of Contents
| • | there were 74,152,686 shares of common stock issued and outstanding; | |
| • | there were options to purchase 10,815,992 shares of common stock outstanding, and the same number of shares of common stock reserved for issuance upon exercise of such options; | |
| • | there were 485,515 shares of common stock reserved for issuance upon the exercise of awards not yet granted under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan; | |
| • | there were 530,283 shares of common stock reserved for issuance under the La Jolla Pharmaceutical Company 1995 Employee Stock Purchase Plan; and | |
| • | there were warrants outstanding and warrants issuable in connection with the purchase agreement to purchase up to 3,707,634 shares of common stock, and the same number of shares of common stock reserved for issuance upon the exercise of such warrants. |
19
Table of Contents
20
Table of Contents
21
Table of Contents
22
Table of Contents
23
Table of Contents
| Award Types |
24
Table of Contents
25
Table of Contents
| • | if the recipient’s sales price exceeds the purchase price paid for the shares upon exercise of the Incentive Stock Option, the recipient will recognize capital gain equal to the excess, if any, of the sales price over the fair market value of the shares on the date of exercise, and will recognize ordinary income equal to the excess, if any, of the lesser of the sales price or the fair market value of the shares on the date of exercise over the purchase price paid for the shares upon exercise of the Incentive Stock Option; or | |
| • | if the recipient’s sales price is less than the purchase price paid for the shares upon exercise of the Incentive Stock Option, the recipient will recognize a capital loss equal to the excess of the purchase price paid for the shares upon exercise of the Incentive Stock Option over the sales price of the shares. |
26
Table of Contents
27
Table of Contents
28
Table of Contents
29
Table of Contents
30
Table of Contents
31
Table of Contents
32
Table of Contents
| (a) | (b) | (c) | ||||||||||
| Number of | ||||||||||||
| Securities | ||||||||||||
| Remaining | ||||||||||||
| Number of | Weighted- | Available for | ||||||||||
| Securities to Be | Average Exercise | Future Issuance | ||||||||||
| Issued upon | Price of | Under Equity | ||||||||||
| Exercise of | Outstanding | Compensation | ||||||||||
| Outstanding | Options, | Plans (Excluding | ||||||||||
| Options, Warrants | Warrants and | Securities Reflected | ||||||||||
| Plan Category | and Rights | Rights | in Column (a)) | |||||||||
| Equity compensation plans approved by security holders | 8,978,464 | (1)(2) | $ | 4.44 | (3) | 864,958 | (4)(5)(6) | |||||
| Equity compensation plans not approved by security holders | — | — | — | |||||||||
| (1) | Outstanding options to purchase shares of our common stock under the La Jolla Pharmaceutical Company 1994 Stock Incentive Plan (the “1994 Plan”) and the 2004 Plan as of December 31, 2004. |
| (2) | As of October 14, 2005, there were outstanding options to purchase 10,815,992 shares of our common stock under the 1994 Plan and the 2004 Plan. |
| (3) | As of October 14, 2005, the weighted average exercise price of outstanding options under the 1994 Plan and the 2004 Plan was $3.29. |
| (4) | Includes 655,367 shares subject to the 2004 Plan and 209,591 shares subject to the La Jolla Pharmaceutical Company 1995 Employee Stock Purchase Plan (the “1995 ESPP”) as of December 31, 2004. |
| (5) | As of October 14, 2005, there were 485,515 shares available for issuance under the 2004 Plan and 530,283 shares available for purchase under the 1995 ESPP. |
| (6) | If our stockholders approve both Proposal 2 and Proposal 3, the number of shares available under the 2004 Plan will be increased by 16,000,000. |
33
Table of Contents
| Long-Term | |||||||||||||||||||||
| Compensation- | |||||||||||||||||||||
| Awards- | |||||||||||||||||||||
| Annual Compensation | Securities | All Other | |||||||||||||||||||
| Underlying | Compensation | ||||||||||||||||||||
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Options (#) | ($) | ||||||||||||||||
| Steven B. Engle | 2004 | $ | 418,855 | $ | 144,474 | 300,000 | $ | — | |||||||||||||
| Chief Executive Officer and | 2003 | 392,798 | 140,664 | 300,000 | — | ||||||||||||||||
| Chairman of the Board | 2002 | 374,946 | 77,406 | 515,000 | — | ||||||||||||||||
| Matthew D. Linnik, Ph.D. | 2004 | 290,577 | 87,998 | 122,000 | — | ||||||||||||||||
| Chief Scientific Officer and | 2003 | 275,445 | 77,035 | 150,000 | — | ||||||||||||||||
| Executive Vice President of | 2002 | 251,158 | 40,661 | 150,000 | — | ||||||||||||||||
| Research | |||||||||||||||||||||
| Bruce K. Bennett, Jr.(1) | 2004 | 201,065 | 52,302 | 70,000 | — | ||||||||||||||||
| Vice President of Manufacturing | 2003 | 188,269 | 52,567 | 90,000 | — | ||||||||||||||||
| 2002 | 170,731 | 458 | 130,000 | — | |||||||||||||||||
| Kenneth R. Heilbrunn, M.D.(2) | 2004 | 289,401 | 75,190 | 80,000 | — | ||||||||||||||||
| Vice President of | 2003 | 275,050 | 54,195 | 90,000 | — | ||||||||||||||||
| Clinical Development | 2002 | 158,823 | 458 | 145,000 | 25,000 | (3) | |||||||||||||||
| William J. Welch(4) | 2004 | 233,184 | 60,743 | 90,000 | — | ||||||||||||||||
| Vice President of Sales and | 2003 | 212,827 | 57,189 | 82,500 | — | ||||||||||||||||
| Marketing | 2002 | 193,091 | 31,181 | 75,000 | — | ||||||||||||||||
| (1) | Mr. Bennett joined us in January 2002. As a result, the amounts paid to him in 2002 reflect only a partial year’s compensation. |
| (2) | Dr. Heilbrunn joined us in June 2002. As a result, the amounts paid to him in 2002 reflect only a partial year’s compensation. Dr. Heilbrunn’s employment was terminated effective as of April 1, 2005. |
| (3) | The amount consisted of relocation expense reimbursement. |
| (4) | Mr. Welch resigned effective as of July 29, 2005. |
34
Table of Contents
| Individual Grants | ||||||||||||||||||||||||
| Potential Realizable Value | ||||||||||||||||||||||||
| Percent of | at Assumed Annual Rates | |||||||||||||||||||||||
| Number of | Total Options | of Stock Price | ||||||||||||||||||||||
| Securities | Granted to | Appreciation for Option | ||||||||||||||||||||||
| Underlying | Employees in | Exercise | Term(4) | |||||||||||||||||||||
| Options | Fiscal Year | Price | Expiration | |||||||||||||||||||||
| Name | Granted (#)(1) | (%) | ($/share)(2) | Date(3) | 5% ($) | 10% ($) | ||||||||||||||||||
Steven B. Engle(5) | 300,000 | 17.04 | $ | 2.96 | 5/21/14 | $ | 558,458 | $ | 1,415,243 | |||||||||||||||
Matthew D. Linnik(5) | 122,000 | 6.93 | 2.96 | 5/21/14 | 227,106 | 575,532 | ||||||||||||||||||
Bruce K. Bennett, Jr.(5) | 70,000 | 3.98 | 2.96 | 5/21/14 | 130,307 | 330,223 | ||||||||||||||||||
| Kenneth R. Heilbrunn* | 80,000 | 4.54 | 2.96 | 5/21/14 | 148,922 | 377,398 | ||||||||||||||||||
| William J. Welch* | 90,000 | 5.11 | 2.96 | 5/21/14 | 167,538 | 424,573 | ||||||||||||||||||
| * | Indicates that the person is no longer employed by us. |
| (1) | A portion of the options were granted under the 1994 Plan and a portion of the options were granted under the 2004 Plan. The 1994 and 2004 Plans are administered by the compensation committee of the board of directors which has broad discretion and authority to construe and interpret the 1994 and 2004 Plans and to modify outstanding options. All granted options vest and become exercisable pursuant to the 1994 and 2004 Plans between the date of the grant and May 21, 2007. |
| (2) | The exercise price and tax withholding obligations related to the exercise may be paid by delivery of cash or already owned shares or offset by the underlying shares, subject to certain conditions. The exercise price for each grant is the market price of our common stock on the date of grant. |
| (3) | All of the options are exercisable for a term of 10 years, subject to earlier termination upon certain events related to termination of employment or if we experience a change in control. |
| (4) | The potential realizable values listed are based upon an assumption that the market price of our common stock appreciates at the stated rate, compounded annually, from the date of grant to the expiration date. The 5% and 10% assumed rates of appreciation are determined by the rules of the SEC and do not represent our estimate of the future market value of the common stock. Actual gains, if any, are dependent upon the future market price of our common stock. |
| (5) | In our current fiscal year ending December 31, 2005, as of October 14, 2005, we have granted to Steven B. Engle, Matthew D. Linnik and Bruce Bennett, Jr. options to purchase 700,000, 253,000 and 184,000 shares of our common stock, respectively. |
35
Table of Contents
| Number of Securities | ||||||||||||||||||||||||
| Underlying Unexercised | Value of Unexercised In-the- | |||||||||||||||||||||||
| Options at Fiscal Year End | Money Options at Fiscal | |||||||||||||||||||||||
| Shares | Value | (#) | Year End ($)(2) | |||||||||||||||||||||
| Acquired on | Realized | |||||||||||||||||||||||
| Name | Exercise (#) | ($)(1) | Exercisable | Unexercisable | Exercisable | Unexercisable | ||||||||||||||||||
| Steven B. Engle | — | — | 250,000 | — | $ | 305,786 | — | |||||||||||||||||
| Matthew D. Linnik | — | — | 82,792 | — | 98,605 | — | ||||||||||||||||||
| Bruce K. Bennett, Jr. | — | — | — | — | — | — | ||||||||||||||||||
| Kenneth R. Heilbrunn* | — | — | — | — | — | — | ||||||||||||||||||
| William J. Welch* | — | — | — | — | — | — | ||||||||||||||||||
| * | Indicates that the person is no longer employed by us. |
| (1) | This amount represents the difference between the exercise price of the options and the market price of our common stock on the date of exercise. |
| (2) | These amounts represent the difference between the exercise price of the in-the-money options and the market price of our common stock on December 31, 2004, the last trading day of 2004. The closing price of our common stock on that day on the Nasdaq National Market was $1.67. Options are in-the-money if the market value of the shares covered thereby is greater than the option exercise price. |
36
Table of Contents
37
Table of Contents
38
Table of Contents
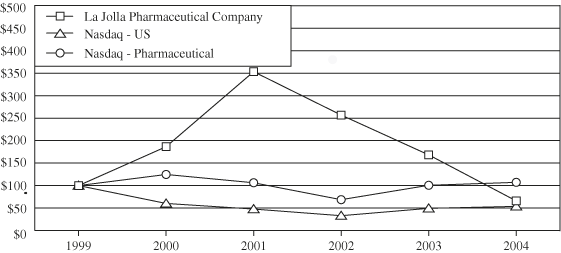
| 12/31/1999 | 12/31/2000 | 12/31/2001 | 12/31/2002 | 12/31/2003 | 12/31/2004 | ||||||||||||||||||||||||||
| La Jolla Pharmaceutical Company | 100 | 186.45 | 353.22 | 256.82 | 168.31 | 65.98 | |||||||||||||||||||||||||
| Nasdaq — US | 100 | 60.31 | 47.84 | 33.07 | 49.45 | 53.81 | |||||||||||||||||||||||||
| Nasdaq — Pharmaceuticals | 100 | 124.73 | 106.31 | 68.69 | 100.69 | 107.22 | |||||||||||||||||||||||||
39
Table of Contents
| • | each person who is known by us to be the beneficial owner of more than 5% of our common stock; | |
| • | each of our directors and nominees; | |
| • | each of our current named executive officers and the two former named executive officers who ceased to be employed during the current fiscal year; and | |
| • | all of our directors and executive officers as a group. |
| Amount and Nature of | Percent of | ||||||||
| Name and Address of Beneficial Owner(1) | Beneficial Ownership(2) | Class (%)(3) | |||||||
| Alejandro Gonzalez Cimadevilla | |||||||||
| Ruben Dario #223 5-A Chapultepec Morales Mexico, D.F. 05 11570 | 7,157,951 | (4) | 9.7 | ||||||
| Columbia Wanger Asset Management, LP | |||||||||
| 227 West Monroe Street, Suite 3000 Chicago, IL 60606 | 5,625,000 | (5) | 7.6 | ||||||
| Thomas H. Adams, Ph.D. | 172,767 | (6) | * | ||||||
| Josefina T. Elchico | 62,737 | (7) | * | ||||||
| Steven B. Engle | 2,296,351 | (8) | 3.1 | ||||||
| Robert A. Fildes, Ph.D. | 203,768 | (9) | * | ||||||
| Paul C. Jenn, Ph.D. | 338,979 | (10) | * | ||||||
| Stephen M. Martin | 100,172 | (11) | * | ||||||
| Craig R. Smith, M.D. | 32,056 | (12) | * | ||||||
| Bruce K. Bennett, Jr. | 284,405 | (13) | * | ||||||
| Kenneth R. Heilbrunn, M.D. | 102,155 | (14) | * | ||||||
| Matthew D. Linnik, Ph.D. | 668,715 | (15) | * | ||||||
| William J. Welch | 158,648 | (16) | * | ||||||
| All directors and executive officers as a group (12 persons) | 4,977,397 | (17) | 6.7 | ||||||
| * | Less than 1% |
| (1) | Unless otherwise indicated, the address for each beneficial owner is care of La Jolla Pharmaceutical Company, 6455 Nancy Ridge Drive, San Diego, California 92121. | |
| (2) | The table below includes the number of shares underlying options that are exercisable within 60 days from October 14, 2005. All information with respect to beneficial ownership is based upon filings made by the respective beneficial owners with the SEC or information provided to the Company by such beneficial owners. Except as indicated in the footnotes to this table, the persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to community property laws. The table does not include shares of restricted stock that may be issued to our key employees pursuant to the retention agreements we entered into in connection with the purchase agreement. | |
| (3) | On October 14, 2005, there were 74,152,686 shares of common stock outstanding. Shares not outstanding that are subject to options exercisable by the holder thereof within 60 days of October 14, 2005 are deemed outstanding for the purposes of calculating the number and percentage owned by such stockholder, but not deemed outstanding for the purpose of calculating the percentage owned by each other stockholder listed. The percentage owned by each of the stockholders listed does not include |
40
Table of Contents
| shares of restricted stock that may be issued to our key employees pursuant to the retention agreements we entered into in connection with the purchase agreement. | ||
| (4) | Based on information provided to us by the stockholder as of October 6, 2005. | |
| (5) | Based on the Form 13-F Holdings Report filed on August 16, 2005. | |
| (6) | Includes 165,767 shares subject to options that are exercisable within 60 days. | |
| (7) | Includes 37,953 shares subject to options that are exercisable within 60 days. | |
| (8) | Includes 2,294,867 shares subject to options that are exercisable within 60 days. | |
| (9) | Includes 120,704 shares subject to options that are exercisable within 60 days. |
| (10) | Includes 333,305 shares subject to options that are exercisable within 60 days. |
| (11) | Includes 99,972 shares subject to options that are exercisable within 60 days. |
| (12) | All shares are subject to options that are exercisable within 60 days. |
| (13) | Includes 221,831 shares subject to options that are exercisable within 60 days. |
| (14) | Based upon the Form 4 filed by the reporting person on April 5, 2005, and includes 90,000 shares subject to options that are exercisable within 60 days. Dr. Heilbrunn’s employment was terminated effective as of April 1, 2005. |
| (15) | Includes 662,218 shares subject to options that are exercisable within 60 days. |
| (16) | Based upon the Form 4 filed by the reporting person on May 20, 2005, and includes 156,739 shares subject to options that are exercisable within 60 days. Mr. Welch resigned effective as of July 29, 2005. |
| (17) | Includes 4,718,964 shares subject to options that are exercisable within 60 days. |
41
Table of Contents
| By order of the board of directors, | |
 | |
| Steven B. Engle | |
| Chairman of the Board and Chief Executive Officer |
42
Table of Contents
A-1
Table of Contents
A-2
Table of Contents
A-3
Table of Contents
A-4
Table of Contents
A-5
Table of Contents
A-6
Table of Contents
| (1) the name of the Company and its Subsidiaries, all assumed fictional business names, trademarks, service marks, trade dress, logos and trade names, whether or not registered, including all common law rights, and registrations and applications for registrations thereof (collectively,“Marks”); | |
| (2) all patents and patent applications (including divisions, continuations, continuations-in-part, reissues, reexaminations and extensions thereof) either granted or pending with a domestic, international or foreign patent office or other governing body (collectively,“Patents”); | |
| (3) all registered and unregistered copyrights in both published works and unpublished works including the content of proprietary computer software and internet web sites in which the Company or any Subsidiary has rights (collectively,“Copyrights”); and | |
| (4) all know-how, trade secrets, confidential or proprietary information, customer lists, software, technical information, data, compounds, compositions of matter, formulas, process technology, plans, drawings and blue prints (collectively,“Trade Secrets”), which are not the subject of Marks, Patents or Copyrights. |
| (1) All of the Patents that the Company or any Subsidiary owns and, to the knowledge of the Company, all of the Patents that either has rights to pursuant to license agreements with third parties, are |
A-7
Table of Contents
| currently in compliance with applicable formal legal requirements (including payment of required filing, examination and maintenance fees and filing of required proofs of working or use), and are valid and enforceable. To the knowledge of the Company, there is no fact that would form a reasonable basis for belief that the patent applications within the Patents would be unenforceable or invalid if issued as patents. All of the Patents that Company or any Subsidiary owns and, to the knowledge of the Company, all of the Patents that either has rights to pursuant to license agreements with third parties, were prosecuted or are being prosecuted in full compliance with the Duty of Candor required by the United States Patent & Trademark Office and any similar requirement of any corresponding foreign agencies, specifically, all material information known to the Company, its subsidiary or its licensors, as appropriate, during the prosecution of such Patents was disclosed to the United States Patent & Trademark Office or corresponding foreign agency during prosecution, and in connection with such prosecution, the Company, its Subsidiary or its licensors, as appropriate, did not make any material misstatements, material omissions or material misleading statements. | |
| (2) Except as otherwise indicated inSection 3.12(E)of the Disclosure Schedule, no Patent within the Intellectual Property Assets has been or is now involved in any interference proceeding, reissue proceeding, reexamination proceeding, or opposition proceeding. To the knowledge of the Company, there is no potentially interfering patent or patent application of any third party with respect to the Patents. | |
| (3) Except as otherwise indicated inSection 3.12(E)of the Disclosure Schedule, (a) to the Company’s knowledge, no Patent within the Intellectual Property Assets is infringed or has been challenged or threatened in any way, and (b) none of the products manufactured or sold or currently contemplated to be manufactured or sold, nor any process or know-how used or currently contemplated to be used, by the Company or any Subsidiary infringes or is alleged in writing to infringe any patent or other proprietary right of any other person or entity. Neither the Company or any Subsidiary nor, to the Company’s knowledge, its licensors has received any notices or threats of infringement or conflict with the intellectual property right of any third party, and there is no pending Proceeding (as defined below) by others including any claim or allegation that the Company or any Subsidiary is infringing any intellectual property right of any third party. As used in thisSection 3.12,“Proceeding” means any action, arbitration, audit, examination, investigation, hearing, litigation, or suit (whether civil, criminal, administrative, judicial or investigative, whether formal or informal and whether public or private) commenced, brought, conducted or heard by or before, or otherwise involving, any Governmental Entity or arbitrator. | |
| (4) All products made, used or sold by or on behalf of the Company or any Subsidiary under the Patents within the Intellectual Property Assets have been or will be marked with the proper patent notice to the extent feasible. | |
| (5) The Company or, to the Company’s knowledge, its licensors, as applicable, own all right, title and interest in the Patents within the Intellectual Property Assets and are identified in the records of the United States Patent and Trademark Office and corresponding foreign agencies as holders of record of such Patents. All right, title and interest in the issued patents and pending patent applications with the Patents have been assigned to the Company or, to the Company’s knowledge, its licensors, as applicable. Except as otherwise set forth in the Disclosure Schedule, there is no other entity or individual that has any right, title or interest in any of the issued patents and pending patent applications within the Patents other than the Company and, to the Company’s knowledge, its licensors, as applicable. |
| (1) Except as set forth inSection 3.12(F)of the Disclosure Schedule, all Marks have been registered with the United States Patent and Trademark Office, are currently in compliance with all applicable formal legal requirements (including the timely post-registration filing of affidavits of use and incontestability and renewal applications), are valid and enforceable. |
A-8
Table of Contents
| (2) No Mark within the Intellectual Property Assets has been or is now involved in any opposition proceeding, invalidation proceeding, or cancellation proceeding and, to the knowledge of the Company, no such action is threatened with respect to any of the Marks. | |
| (3) To the knowledge of the Company, there is no potentially interfering trademark or trademark application of any other person or entity. | |
| (4) No Mark within the Intellectual Property Assets has been challenged or threatened in any way and none of the Marks within the Intellectual Property Assets used by the Company or any Subsidiary infringes or is alleged to infringe any trade name, trademark or service mark of any other person or entity and, to the knowledge of the Company, no Mark is infringed by any other person or entity. | |
| (5) All products and materials containing a Mark bear the proper federal registration notice where permitted by law. |
| (1) The Company and its Subsidiaries have taken all reasonable precautions to protect the secrecy, confidentiality and value of its Trade Secrets (including the enforcement by the Company or the Subsidiary of a policy requiring each employee or contractor to execute proprietary information and confidentiality agreements substantially in its standard form, and all current and former employees and contractors of the Company and its Subsidiaries who have had access to Trade Secrets or contributed to or participated in the conception or development of Trade Secrets have executed such an agreement). | |
| (2) The Company has good title to and an absolute right (but not necessarily exclusive) to use the Trade Secrets. The Trade Secrets are not part of the public knowledge or literature and, to the knowledge of the Company, have not been used, divulged or appropriated either for the benefit of any person or entity or to the detriment of the Company or any Subsidiary. No Trade Secret is subject to any adverse claim or has been challenged or threatened in writing in any way or infringes any intellectual property right of any other person or entity. |
A-9
Table of Contents
A-10
Table of Contents
A-11
Table of Contents
A-12
Table of Contents
A-13
Table of Contents
| “THESE SECURITIES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED. THEY MAY NOT BE SOLD, OFFERED FOR SALE, TRANSFERRED, PLEDGED OR HYPOTHECATED IN THE ABSENCE OF A REGISTRATION STATEMENT IN EFFECT WITH RESPECT TO SUCH SECURITIES UNDER SUCH ACT OR AN OPINION OF COUNSEL OR OTHER EVIDENCE REASONABLY SATISFACTORY TO THE COMPANY THAT SUCH REGISTRATION IS NOT REQUIRED OR UNLESS SOLD PURSUANT TO RULE 144 UNDER SUCH ACT.” |
A-14
Table of Contents
| (A) The Company and each Subsidiary will pay and discharge all lawful taxes, assessments and governmental charges or levies imposed upon it or upon its income or property before the date on which penalties attach thereto, and all lawful claims which, if unpaid, would become a material lien or charge on any properties of the Company or any Subsidiary; provided, however, that neither the Company nor any Subsidiary will be required to pay any tax, assessment, charge, levy or claim which is being contested in good faith and by appropriate proceedings if the Company or the Subsidiary will have set aside on its books reserves, if any, to the extent required by GAAP with respect thereto. | |
| (B) The Company shall maintain, or shall cause to be maintained valid policies of workers’ compensation insurance and insurance with responsible and reputable insurance companies or associations in such amounts, types and covering such risks as are acceptable to the Board of Directors of the Company and are customarily carried by similarly situated companies engaged in similar businesses and owning similar properties in the same general areas in which the Company or such Subsidiary operates, including, without limitation, insurance against loss, damage, fire, theft, public liability, products liability, clinical trial liability and other risks. |
A-15
Table of Contents
| (C) The Company and each Subsidiary shall preserve and maintain its corporate existence, rights, franchises and privileges in the jurisdiction of its incorporation, and shall qualify and remain qualified, and cause each Subsidiary to qualify and remain qualified, as a foreign corporation in each jurisdiction in which such qualification is necessary or desirable in view of its business and operations or the ownership or lease of its properties and the failure to so qualify, individually or in the aggregate, would have a Company Material Adverse Effect. The Company shall, and shall cause each Subsidiary to, secure, preserve and maintain all Intellectual Property Assets owned or possessed by it, except where the failure to so secure, preserve and maintain such Intellectual Property Assets would not have a Company Material Adverse Effect. | |
| (D) The Company and each Subsidiary shall keep adequate records and books of account in which complete entries will be made in accordance with GAAP, reflecting all financial transactions of the Company and any Subsidiary, and in which, for each fiscal year, all proper reserves for depreciation, depletion, returns of merchandise, obsolescence, amortization, taxes, bad debts and other purposes in connection with its business shall be made. | |
| (E) The Company and each Subsidiary will comply with the requirements of all applicable laws, rules, regulations and orders of any governmental authority, where noncompliance could have a Company Material Adverse Effect. | |
| (F) The Company will take such actions as are necessary to ensure that all material Patents are kept in force and maintained in compliance with formal legal requirements (including payment of required filing, examination and maintenance fees and filing of required proofs of working or use), unless otherwise determined by the Board of Directors in advance of the failure to take any such actions with respect to any particular Patent. | |
| (G) The Company will notify the Purchasers in writing of any events, circumstances, facts or occurrences of which the Company becomes aware that would result (i) in any representation or warranty of the Company in this Agreement being untrue or incorrect in any material respect (or, to the extent such representation or warranty is already qualified as to materiality in this Agreement, being untrue or incorrect in any respect) or (ii) in the Company not complying in all material respects with the agreements and conditions contained in this Agreement. |
| (A) Promptly after they become public information, copies of all filings (including exhibits thereto) made by the Company with the Commission. | |
| (B) Subject to the last sentence of this paragraph, a copy of the Annual Budget (as defined below) at least 30 days prior to the commencement of the applicable fiscal year. At least 30 days prior to the commencement of each fiscal year, the Company will prepare and submit to, and obtain in respect thereof the approval from the Board of Directors, the operating budgets, operating expenses, profit and loss projections, cash flow projections and a capital expenditure budget (the“Annual Budget”) for the succeeding fiscal year. If the Annual Budget contains any material nonpublic information regarding the Company or its securities (“Nonpublic Information”), the Company shall, prior to providing the Annual Budget to a Purchaser, advise such Purchaser of such fact (but not the Nonpublic Information itself) in writing and, if the Purchaser thereafter elects in writing to receive the Annual Budget, the Company shall provide the Annual Budget to such Purchaser. Each Purchaser that receives Nonpublic Information pursuant to thisSection 5.2(B)shall keep such information confidential to the extent necessary to comply with applicable securities laws (including, without limitation, Regulation FD). |
A-16
Table of Contents
| (A) At any time prior to December 31, 2008 when Essex or its successor or affiliates continue to own at least 75% of the Shares originally purchased by Essex at the Closing, the Company will not enter into any material transaction that could result in sale, transfer, assignment of, or grant of any exclusive or non-exclusive license in or to, any Patent related to Riquent® in the United States, in each case without the prior written consent of Essex Woodlands Health Ventures Fund VI, LP (“Essex”). | |
| (B) Prior to the Closing, the Company shall not, and it shall not authorize or permit any officer, director or employee of or any investment banker, broker, attorney, accountant, or other representative retained by the Company to, solicit, initiate or encourage (including by way of furnishing information) submission of any proposal or offer from any person or entity which constitutes, or may reasonably be expected to lead to, a Financing Proposal. As used herein, a“Financing Proposal” shall mean any proposal or offer to acquire in any manner a direct or indirect equity interest in the Company or its assets, or to finance the business or operations of the Company (other than standard senior indebtedness for borrowed money from a bank or similar financial institution, trade debt in the ordinary course of business consistent with past practice, and issuances under the Company Stock Plans in the ordinary course of business). If the Company receives a Financing Proposal, the Company shall notify the Purchasers immediately and shall provide to the Purchasers a copy of any written documentation in connection therewith. Prior to the Closing, the Company shall not enter into any transaction pursuant to which any person or entity would acquire in any manner a direct or indirect equity interest in the Company or its assets, or would finance the business or operations of the Company (other than standard senior indebtedness for borrowed money from a bank or similar financial institution, trade debt in the ordinary course of business consistent with past practice, and issuances under the Company Stock Plans in the ordinary course of business). | |
| (C) The Company will not make any commitment or binding obligation to take any action that would be prohibited under thisSection 5.3. | |
| (D) The Company will not allow any Subsidiary to take any action (or make a commitment or binding obligation to take any action) that would be prohibited under thisSection 5.3if taken by the Company. | |
| (E) Nothing contained in thisSection 5.3shall prevent the Company’s Board of Directors (the“Board”) from (i) making any disclosure to its stockholders, if, in the good faith judgment of the Board, failure to so disclose would be inconsistent with its obligations under applicable law; (ii) providing (or authorizing the provision of) information to, or engaging in (or authorizing) such discussions or negotiations with, any person who has made a bona fide, detailed and non-contingent written Financing Proposal received after the date hereof which did not result from a breach of thisSection 5.3; or (iii) recommending or consummating such a Financing Proposal which did not result from such a breach to its stockholders if and only to the extent that, in the case of actions referred to in clause (ii) or (iii), (x) such Financing Proposal is a Superior Proposal (as defined below), and (y) the Board, after having consulted with and considered the advice of outside counsel to the Board, determines in good faith that providing such information or engaging in such negotiations or discussions or making such recommendation is required in order to discharge the directors’ fiduciary duties to the Company and its stockholders in accordance with the Delaware General Corporation Law. For purposes of this Agreement, a“Superior Proposal” means a bona fide, detailed and non-contingent written Financing Proposal by a third party on terms that the Board determines in its good faith judgment, after receiving the advice of its financial advisors (and its legal advisors regarding the prospects for regulatory approval), to be more favorable from a financial point of view to the Company and its stockholders than the transactions contemplated hereby, after taking into account the likelihood of consummation of such transaction on the terms set forth therein, taking into account all legal, financial (including the financing terms of any such proposal), regulatory and other aspects of such proposal and any other relevant factors permitted under applicable law (including any costs, delays and uncertainty regarding consummation of such Financing Proposal), after giving the Purchasers at least ten (10) Business Days to respond to such third-party Financing Proposal once the Board has notified the Purchasers that in the absence of any further action by the Purchasers it would consider such Financing Proposal to be a Superior Proposal. |
A-17
Table of Contents
| (F) Each Purchaser that receives Nonpublic Information pursuant to thisSection 5.3shall keep such information confidential to the extent necessary to comply with applicable securities laws (including, without limitation, Regulation FD). |
A-18
Table of Contents
A-19
Table of Contents
A-20
Table of Contents
| (a) The Company shall, as promptly as practicable following the execution and delivery of this Agreement, establish a record date, duly call, give notice of, convene and hold a meeting of its stockholders (the“Stockholders Meeting”) (it being agreed and understood that the Company shall use its reasonable best efforts to duly call and give notice of such meeting by no later than October 28, 2005, which meeting shall occur not later than 30 Business Days following the date on which the Proxy Statement (as defined below) is first mailed to the stockholders of the Company) for the purpose of seeking stockholder approval of (i) the sale and issuance of the Shares, the Closing Warrants and the |
A-21
Table of Contents
| Warrant Shares and any other matters contemplated by this Agreement or the Ancillary Agreements that are required to be approved by the stockholders under applicable law or the rules of the Commission or the Principal Market or Capital Market, as applicable (the“Transaction Approval”), (ii) an amendment to the Certificate to authorize an increase in the number of shares of Common Stock authorized by the Certificate by 50,000,000, which shares are to be reserved for exercise in full of the Warrants, for issuance in connection with the Company’s existing equity incentive plans and for issuance in connection with other corporate purposes (the“Share Authorization Approval”), (iii) an amendment to the Certificate, to become effective following the Closing, to authorize the combination of the Company’s outstanding shares of Common Stock into a lesser number of shares of Common Stock, using a ratio approved by the Board and agreed upon prior to the filing of the Proxy Statement by Purchasers who have agreed to purchase at least 50% of the Shares to be sold at Closing (which ratio shall result in no more than 5 shares combining into a single share, and no fewer than 2 shares combining into a single share) (the“Reverse Split Approval”), and (iv) an amendment to the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan to (A) increase the number of shares available thereunder by 16 million shares; (B) amend the vesting requirements with respect to restricted stock to accommodate the terms of the retention agreements entered into in connection with the execution of this Agreement; and (C) increase the maximum number of shares that may be granted to any person in any calendar year from 1 million shares to 7 million shares. (the“Option Approval”) (collectively, the“Proposals”). In connection therewith, the Company will, as promptly as practicable following the date hereof, prepare and file with the Commission proxy materials (including a proxy statement and form of proxy) for use at the Stockholders Meeting. The Company will use its reasonable best efforts to have the Proxy Statement “cleared” by the Commission’s staff as promptly as practicable and, as promptly as practicable thereafter, cause the Proxy Statement, in definitive form, to be mailed to the stockholders of the Company. Each Purchaser shall promptly furnish in writing to the Company such information relating to such Investor and its investment in the Company as the Company may reasonably request for inclusion in the Proxy Statement. The Company will comply in all material respects with Section 14(a) of the Exchange Act and the rules promulgated thereunder in relation to any proxy statement (as amended or supplemented, the“Proxy Statement”) and any form of proxy to be sent to the stockholders of the Company in connection with the Stockholders Meeting, and the Proxy Statement shall not, on the date that the Proxy Statement (or any amendment thereof or supplement thereto) is first mailed to stockholders or at the time of the Stockholders Meeting, contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein not false or misleading, or omit to state any material fact necessary to correct any statement in any earlier communication with respect to the solicitation of proxies or the Stockholders Meeting which has become false or misleading. No filing of, or amendment or supplement to, the Proxy Statement will be made by the Company without providing the Purchasers a reasonable opportunity to review and comment thereon. If the Company should discover at any time prior to the Stockholders Meeting, any event relating to the Company or any of its Subsidiaries or any of their respective affiliates, officers or directors that is required to be set forth in a supplement or amendment to the Proxy Statement, in addition to the Company’s obligations under the Exchange Act, the Company will promptly inform the Purchasers thereof. | |
| (b) Following the Closing, if the Company shall not have obtained the Share Authorization Approval,the Reverse Split Approval or the Option Approval, the Company shall, as promptly as practicable following the Closing Date, establish a record date, duly call, give notice of, convene and hold a meeting of its stockholders (the“Second Stockholders Meeting”) which shall occur not later than the 45th day following the date of the Closing, subject to any delays that may result from the Commission’s review, if any, of the proxy statement with respect to such Second Stockholders Meeting (the“Second Stockholders Meeting Deadline”) for the purpose of seeking each of such approvals that was not obtained at the Stockholders Meeting. In connection therewith, the Company will, as promptly as practicable following the Closing Date, prepare and file with the Commission proxy materials (including a proxy statement and form of proxy) for use at the Second Stockholders Meeting. The Company will use its reasonable best efforts to have the such proxy statement “cleared” by the Commission’s staff as promptly as practicable and, as promptly as practicable thereafter, cause such proxy statement, in |
A-22
Table of Contents
| definitive form, to be mailed to the stockholders of the Company. Each Purchaser shall promptly furnish in writing to the Company such information relating to such Investor and its investment in the Company as the Company may reasonably request for inclusion in the Second Proxy Statement (as defined below). The Company will comply in all material respects with Section 14(a) of the Exchange Act and the rules promulgated thereunder in relation to any proxy statement (as amended or supplemented, the“Second Proxy Statement”) and any form of proxy to be sent to the stockholders of the Company in connection with the Second Stockholders Meeting, and the Second Proxy Statement shall not, on the date that the Second Proxy Statement (or any amendment thereof or supplement thereto) is first mailed to stockholders or at the time of the Second Stockholders Meeting, contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein not false or misleading, or omit to state any material fact necessary to correct any statement in any earlier communication with respect to the solicitation of proxies or the Second Stockholders Meeting which has become false or misleading. No filing of, or amendment or supplement to, the Second Proxy Statement will be made by the Company without providing the Purchasers a reasonable opportunity to review and comment thereon. If the Company should discover at any time prior to the Second Stockholders Meeting, any event relating to the Company or any of its Subsidiaries or any of their respective affiliates, officers or directors that is required to be set forth in a supplement or amendment to the Second Proxy Statement, in addition to the Company’s obligations under the Exchange Act, the Company will promptly inform the Purchasers thereof. | |
| (c) The Company acknowledges that, prior to the date hereof, the Board of Directors of the Company has approved the calling of the Stockholders Meeting and, if necessary, the Second Stockholders Meeting. Subject to their fiduciary obligations under applicable law (as determined in good faith by the Company’s Board of Directors after consultation with the Company’s outside counsel), the Company’s Board of Directors shall recommend to the Company’s stockholders that the stockholders vote in favor of the Proposals (the“Company Board Recommendation”) at both the Stockholders Meeting and the Second Stockholders Meeting (if any) and take all commercially reasonable action (including, without limitation, hiring McKenzie Partners or another proxy solicitation firm of nationally recognized standing) to solicit the approval of the stockholders for the Proposals at both the Stockholders Meeting and the Second Stockholders Meeting (if any) unless the Board of Directors shall have modified, amended or withdrawn the Company Board Recommendation pursuant to the provisions of the immediately succeeding sentence. The Company covenants that the Board of Directors of the Company shall not modify, amend or withdraw the Company Board Recommendation at either the Stockholders Meeting or the Second Stockholders Meeting (if any) unless the Board of Directors (after consultation with the Company’s outside counsel) shall determine in the good faith exercise of its business judgment that maintaining the Company Board Recommendation would violate its fiduciary duty to the Company’s stockholders. Whether or not the Company’s Board of Directors modifies, amends or withdraws the Company Board Recommendation pursuant to the immediately preceding sentence, the Company shall in accordance with Section 146 of the Delaware General Corporation Law and the provisions of the Certificate and Bylaws, (i) take all action necessary to convene the Stockholders Meeting (and, if necessary, the Second Stockholders Meeting) to consider and vote upon the approval of the Proposals in accordance with the requirements of Section 5.14(a) and 5.14(b) and (ii) submit the Proposals at the Stockholders Meeting (and, as necessary, at the Second Stockholders Meeting) to the stockholders of the Company for their approval in accordance with the requirements of Section 5.14(a) and 5.14(b). |
| (A) The representations and warranties contained inSection 3shall be true, correct and complete in all material respects (except to the extent that any of such representations and warranties is already qualified as to materiality inSection 3, in which case such representations and warranties shall be true, |
A-23
Table of Contents
| correct and complete without further qualification) on and as of the Closing Date (except for representations and warranties that speak only as of a specific date (which shall be true, correct and complete as of such date)), with the same effect as though such representation and warranty had been made on and as of that date. | |
| (B) The Company shall have performed and complied in all material respects with all agreements and conditions contained in this Agreement required to be performed or complied with by the Company prior to or at the Closing. | |
| (C) The Company shall have obtained the Transaction Approval from its stockholders, and shall have complied in all material respects with the terms ofSection 5.14with respect to the Share Authorization Approval. | |
| (D) Each Purchaser shall have received (i) an opinion from Gibson, Dunn & Crutcher LLP, counsel for the Company, dated the Closing Date and addressed to the Purchasers, in form and substance reasonably acceptable to Purchasers who have committed to purchase a majority of the Shares, and (ii) a “freedom to operate” opinion from Morrison & Foerster, counsel for the Company, dated the Closing Date and addressed to the Purchasers, in form and substance reasonably acceptable to Purchasers who have committed to purchase a majority of the Shares. | |
| (E) The Company, each of the Purchasers and the other parties identified therein shall have entered into the Registration Rights Agreement in the form attached hereto asExhibit D(the“Rights Agreement”). | |
| (F) The Company shall have expanded the number of authorized directors of its Board of Directors to nine and, conditioned only upon the Closing, (i) Martin P. Sutter and James Topper shall each have been elected or appointed as a “Class 3 Director” (as defined in the Certificate), with Mr. Sutter being the initial Essex Designee and Mr. Topper being the initial Frazier Designee, (ii) Frank Young shall have been elected or appointed as a “Class 2 Director” (as defined in the Certificate), as an initial Essex Designee, and (iii) Nader Naini shall have been elected or appointed as a “Class 1 Director” (as defined in the Certificate), as the initial Joint Designee. | |
| (G) Subject to each being deemed eligible to serve on the compensation committee of the Board of Directors pursuant to the terms of the charter of the compensation committee and applicable listing standards, conditioned only upon the Closing, Martin P. Sutter and James Topper shall each have been elected or appointed to the compensation committee of the Board of Directors of the Company. | |
| (H) The Company shall have delivered to the Purchasers: (i) the Certificate, as in effect as of the Closing Date, which shall be in the form of the Certificate on the date of this Agreement (or as amended by an amendment made in compliance withSection 5.14), certified by the Secretary of State of the State of Delaware; (ii) certificates, as of a recent practicable date, as to the corporate good standing of the Company issued by the Secretaries of State of the States of Delaware and California and the Secretary of each other State in which the Company is qualified to do business; (iii) a certificate of the Secretary or Assistant Secretary of the Company, dated as of the Closing Date, certifying as to (a) the By-laws of the Company (which shall be in the same form as the By-laws on the date of this Agreement), (b) the signatures and titles of the officers of the Company executing this Agreement or any of the Ancillary Agreements, and (c) resolutions of the Board of Directors and stockholders of the Company, authorizing and approving all matters in connection with this Agreement, the Ancillary Agreements and the transactions contemplated hereby and thereby, in each case that is respectively required to be approved by them; and (iv) a certificate, executed by the President of the Company, dated the Closing Date, certifying to the fulfillment of the conditions specified inSection 6.1. | |
| (I) The Company will have paid or made adequate arrangements for payment in accordance with the provisions ofSection 7.3, the fees and disbursements of the Purchasers and their counsel at the Closing. |
A-24
Table of Contents
| (J) All authorizations, approvals, filings or permits, if any, of or with any governmental authority or regulatory body of the United States or of any state or any Self-Regulatory Organizations (including NASDAQ) that are required in connection with the lawful issuance and sale of the Shares, Warrants and Warrant Shares shall be duly made, obtained and effective as of the Closing Date. | |
| (K) The Common Stock shall be designated for quotation on the NASDAQ National Market (the“Principal Market”) or the NASDAQ Capital Market (the“Capital Market”), and shall not have been suspended by the Commission or the Principal Market from trading on the Principal Market (unless, in connection with a suspension by the Principal Market, the Company shall have been designated for quotation on the Capital Market and not later been suspended therefrom). | |
| (L) No stop order or suspension of trading shall have been imposed by the applicable Principal Market or Capital Market on which the Common Stock is listed for trading or approved for quotation, the Commission or any other governmental or regulatory body with respect to public trading in the Common Stock. | |
| (M) The Company shall have delivered the Irrevocable Transfer Agent Instructions to the Company’s transfer agent. | |
| (N) From the date of this Agreement through the Closing Date, no Company Material Adverse Effect shall have occurred or become known (whether or not arising in the ordinary course of business). | |
| (O) The Company shall have taken all corporate and other action and proceedings in connection with the Rights Agreement between the Company and American Stock Transfer & Trust Company, as amended (the“Rights Plan”) and the “Rights” (as defined in the Rights Plan) or otherwise, as is necessary to ensure that the Company’s stockholders do not receive or become entitled to receive any Series A Junior Participating Preferred Stock (or other securities or rights) as a result of the transactions contemplated by this Agreement and the Ancillary Agreements, and all such actions and proceedings, and all documentation incident thereto, shall be reasonably satisfactory in substance and form to Essex and its counsel. | |
| (P) The Company shall have taken all corporate and other action and proceedings necessary to ensure that no material change in control provision or restriction is triggered (and not waived) as a result of the transactions contemplated by this Agreement and the Ancillary Agreements (excluding the payment of the incentive bonuses pursuant to the terms of the retention agreements entered into on the date hereof in connection with the execution of this Agreement), and all such actions and proceedings, and all documentation incident thereto, shall be reasonably satisfactory in substance and form to Essex and its counsel. | |
| (Q) Such Purchaser, together with all other Purchasers that have fulfilled the conditions ofSection 6.2, shall have committed to purchase at least $66,000,000 of Shares in the aggregate at the Closing. |
| (A) The representations and warranties of such Purchaser contained inSection 4shall be true, correct and complete in all material respects (except to the extent that any of such representations and warranties is already qualified as to materiality inSection 4, in which case such representations and warranties shall be true, correct and complete without further qualification) on and as of the Closing Date, with the same effect as though such representations and warranties had been made on and as of that date. | |
| (B) Such Purchaser shall have performed and complied in all material respects with all agreements and conditions contained in this Agreement required to be performed or complied with by such Purchaser prior to or at the Closing. | |
| (C) The Company shall have obtained the Transaction Approval from its stockholders. |
A-25
Table of Contents
A-26
Table of Contents
| La Jolla Pharmaceutical Company 6455 Nancy Ridge Drive San Diego, CA 92121 Attention: Chief Executive Officer Fax: (858) 626-2851 |
| Gibson, Dunn & Crutcher LLP 4 Park Plaza Irvine, CA 92614 Attention: Mark W. Shurtleff, Esq. Fax: (949) 451-4220 |
A-27
Table of Contents
A-28
Table of Contents
A-29
Table of Contents
| The Company: | La Jolla Pharmaceutical Company a Delaware corporation By: /s/Steven B. Engle Title: Chairman and CEO | |
| Purchasers: | Essex Woodlands Health Ventures Fund VI, LP By: Essex Woodlands Health Ventures VI, L.P., its General Partner By: Essex Woodlands Health Ventures VI, L.L.C., its General Partner By: /s/Martin P. Sutter | |
| Frazier Healthcare V, LP By: FHMV, LP, its General Partner By: FHMV, LLC, its General Partner By: /s/James N. Topper | ||
A-30
Table of Contents
| Domain Public Equity Partners, L.P. |
| By: | Domain Public Equity Associates, L.L.C., |
| its General Partner |
| By: | /s/Lisa A. Kraeutler |
| Lisa A. Kraeutler, Attorney-in-Fact | |
| /s/Alejandro Gonzalez Cimadevilla | |
| Alejandro Gonzalez Cimadevilla | |
| Special Situations Fund III, L.P. |
| By: | /s/Austin W. Marxe |
| Austin W. Marxe, its General Partner | |
| Special Situations Cayman Fund, L.P. |
| By: | /s/Austin W. Marxe |
| Austin W. Marxe, its General Partner | |
| Special Situations Private Equity Fund, L.P. |
| By: | /s/Austin W. Marxe |
| Austin W. Marxe, its General Partner | |
| Special Situations Life Sciences Fund, L.P. |
| By: | /s/Austin W. Marxe |
| Austin W. Marxe, its General Partner |
A-31
Table of Contents
| Sutter Hill Ventures, | |
| A California Limited Partnership |
| By: | /s/William H. Younger, Jr. |
| Name: William H. Younger, Jr. | |
| ANVEST, L.P. |
| By: | /s/David Anderson |
| David Anderson, General Partner | |
| G. Leonard Baker, Jr. and Mary Anne Baker, Co-trustees of the Baker Revocable Trust U/A/D 2/3/03 |
| By: | /s/G. Leonard Baker, Jr. |
| G. Leonard Baker, Jr., Trustee | |
| William H. Younger, Jr. and Lauren L. Younger, Co-trustees of the Younger Living Trust U/A/D 1/20/95 |
| By: | /s/William H. Younger, Jr. |
| William H. Younger, Jr., Trustee |
A-32
Table of Contents
| Tench Coxe and Simone Otus Coxe, | |
| Co-trustees of the Coxe Revocable Trust U/A/D 4/23/98 |
| By: | /s/Tench Coxe |
| Tench Coxe, Trustee | |
| /s/James C. Gaither | |
| James C. Gaither | |
| Jeffrey W. Bird and Christina R. Bird as Trustees of Jeffrey W. and Christina R. Bird Trust Agreement Dated 10/31/00 |
| By: | /s/Jeffrey W. Bird |
| Jeffrey W. Bird, Trustee | |
| Saunders Holdings, L.P. |
| By: | /s/G. Leonard Baker, Jr. |
| G. Leonard Baker, Jr., General Partner | |
| Robert Yin and Lily Yin as Trustees of Yin Family Trust Dated March 1, 1997 |
| By: | /s/Robert Yin |
| Robert Yin, Trustee |
A-33
Table of Contents
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan | |
| FBO Sherryl W. Hossack | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan | |
| FBO David L. Anderson | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan | |
| FBO William H. Younger | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan | |
| FBO Tenche Coxe | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan | |
| FBO David E. Sweet | |
| /s/Vicki M. Bandel | |
A-34
Table of Contents
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan | |
| FBO David E. Sweet (Rollover) | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan | |
| FBO Lynne M. Brown | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan | |
| FBO Patricia Tom (Post) | |
| /s/Vicki M. Bandel | |
A-35
Table of Contents
| Shares under | Shares under | |||||||||||||||||
| Shares | Closing Warrant | Closing Warrant | ||||||||||||||||
| Number of | under | (if Share Authorization | (if Share Authorization | |||||||||||||||
| Shares | Contingent | Approval received | Approval not received | Aggregate | ||||||||||||||
| Purchaser Name and Address | Purchased | Warrant | prior to Closing) | prior to Closing) | Purchase Price | |||||||||||||
| Essex Woodlands Health Ventures Fund VI, L.P. 10001 Woodloch Forest Drive Waterway Plaza Two, Suite 175 The Woodlands, TX 77380 Attn: Martin P. Sutter Fax: (281) 364-9755 | 33,333,334 | 1,404,407 | 8,333,334 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 25,000,000 | ||||||||||||
| with a copy to (which shall not constitute notice): Baker & McKenzie LLP 130 E. Randolph Drive Chicago, IL 60601 Attn: Bruce Zivian, Esq. Fax: (312) 698-2469 | ||||||||||||||||||
| Frazier Healthcare V, LP 601 Union Street, Suite 3200 Seattle, WA 98101 Attn: James Topper Fax: (206) 621-1848 | 20,000,000 | 842,644 | 5,000,000 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 15,000,000 | ||||||||||||
| Alejandro Gonzalez Cimadevilla Ruben Dario #223 5-A Chapultepec Morales Mexico D.F. ZIP 11570 Fax: (5255) 52818008 | 14,666,666 | 617,939 | 3,666,666 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 11,000,000 | ||||||||||||
| Domain Public Equity Partners, L.P. One Palmer Square, Suite 515 Princeton, NJ 08542 Attn: Nicole Vitullo Fax: (609) 683-4581 | 6,000,000 | 252,793 | 1,500,000 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 4,500,000 | ||||||||||||
| Special Situations Fund III, L.P. 153 E. 53rd Street, 55th Floor New York, NY 10022 Attn: Marianne Hicks Fax: 212-207-6515 | 4,766,667 | 200,830 | 1,191,667 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 3,575,000 | ||||||||||||
| with a copy to (which shall not constitute notice): Lowenstein Sandler PC 65 Livingston Avenue Roseland, NJ 07068 Attn: John D. Hogoboom, Esq. Fax: (973) 597-2383 | ||||||||||||||||||
A-36
Table of Contents
| Shares under | Shares under | |||||||||||||||||
| Shares | Closing Warrant | Closing Warrant | ||||||||||||||||
| Number of | under | (if Share Authorization | (if Share Authorization | |||||||||||||||
| Shares | Contingent | Approval received | Approval not received | Aggregate | ||||||||||||||
| Purchaser Name and Address | Purchased | Warrant | prior to Closing) | prior to Closing) | Purchase Price | |||||||||||||
| Special Situations Cayman Fund, L.P. 153 E. 53rd Street, 55th Floor New York, NY 10022 Attn: Marianne Hicks Fax: 212-207-6515 | 1,283,333 | 54,070 | 320,833 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 962,500 | ||||||||||||
| with a copy to (which shall not constitute notice): Lowenstein Sandler PC 65 Livingston Avenue Roseland, NJ 07068 Attn: John D. Hogoboom, Esq. Fax: (973) 597-2383 | ||||||||||||||||||
| Special Situations Private Equity Fund, L.P. 153 E. 53rd Street, 55th Floor New York, NY 10022 Attn: Marianne Hicks Fax: 212-207-6515 | 1,283,333 | 54,070 | 320,833 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 962,500 | ||||||||||||
| with a copy to (which shall not constitute notice): Lowenstein Sandler PC 65 Livingston Avenue Roseland, NJ 07068 Attn: John D. Hogoboom, Esq. Fax: (973) 597-2383 | ||||||||||||||||||
| Special Situations Life Sciences Fund, L.P. 153 E. 53rd Street, 55th Floor New York, NY 10022 Attn: Marianne Hicks Fax: 212-207-6515 | 666,667 | 28,088 | 166,667 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 500,000 | ||||||||||||
| with a copy to (which shall not constitute notice): Lowenstein Sandler PC 65 Livingston Avenue Roseland, NJ 07068 Attn: John D. Hogoboom, Esq. Fax: (973) 597-2383 | ||||||||||||||||||
| Sutter Hill Ventures, a California Limited Partnership 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | 4,474,935 | 188,539 | 1,118,734 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 3,356,201.25 | ||||||||||||
| Anvest, L.P. c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | 33,334 | 1,404 | 8,334 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 25,000.50 | ||||||||||||
A-37
Table of Contents
| Shares under | Shares under | |||||||||||||||||
| Shares | Closing Warrant | Closing Warrant | ||||||||||||||||
| Number of | under | (if Share Authorization | (if Share Authorization | |||||||||||||||
| Shares | Contingent | Approval received | Approval not received | Aggregate | ||||||||||||||
| Purchaser Name and Address | Purchased | Warrant | prior to Closing) | prior to Closing) | Purchase Price | |||||||||||||
| G. Leonard Baker, Jr. and Mary Anne Baker, Co-Trustees of The Baker Revocable Trust U/A/D 2/3/03 c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | 136,038 | 5,732 | 34,010 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 102,028.50 | ||||||||||||
| Saunders Holdings, L.P. c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | 136,038 | 5,732 | 34,010 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 102,028.50 | ||||||||||||
| William H. Younger, Jr. and Lauren L. Younger, Co-Trustees of The Younger Living Trust U/A/D 1/20/95 c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | 156,026 | 6,574 | 39,007 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 117,019.50 | ||||||||||||
| Tench Coxe and Simone Otus Coxe, Co-Trustees of The Coxe Revocable Trust U/A/D 4/23/98 c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | 241,089 | 10,156 | 60,267 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 180,816.75 | ||||||||||||
| James C. Gaither c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | 63,246 | 2,665 | 15,812 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 47,434.50 | ||||||||||||
A-38
Table of Contents
| Shares under | Shares under | |||||||||||||||||
| Shares | Closing Warrant | Closing Warrant | ||||||||||||||||
| Number of | under | (if Share Authorization | (if Share Authorization | |||||||||||||||
| Shares | Contingent | Approval received | Approval not received | Aggregate | ||||||||||||||
| Purchaser Name and Address | Purchased | Warrant | prior to Closing) | prior to Closing) | Purchase Price | |||||||||||||
| Jeffrey W. Bird and Christina R. Bird as Trustees of Jeffrey W. and Christina R. Bird Trust Agreement Dated 10/31/00 c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | 104,415 | 4,399 | 26,104 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 78,311.25 | ||||||||||||
| Robert Yin and Lily Yin as Trustees of Yin Family Trust Dated March 1, 1997 c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | 5,640 | 238 | 1,410 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 4,230.00 | ||||||||||||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO Sherryl W. Hossack Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 | 7,500 | 316 | 1,875 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 5,625.00 | ||||||||||||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO David L. Anderson Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 | 166,547 | 7,017 | 41,637 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 124,910.25 | ||||||||||||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO William H. Younger, Jr. Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 | 156,026 | 6,574 | 39,007 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 117,019.50 | ||||||||||||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO Tench Coxe Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 | 266,666 | 11,235 | 66,667 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 199,999.50 | ||||||||||||
A-39
Table of Contents
| Shares under | Shares under | ||||||||||||||||||
| Shares | Closing Warrant | Closing Warrant | |||||||||||||||||
| Number of | under | (if Share Authorization | (if Share Authorization | ||||||||||||||||
| Shares | Contingent | Approval received | Approval not received | Aggregate | |||||||||||||||
| Purchaser Name and Address | Purchased | Warrant | prior to Closing) | prior to Closing) | Purchase Price | ||||||||||||||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO David E. Sweet Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 | 6,666 | 281 | 1,667 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 4,999.50 | |||||||||||||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO David E. Sweet (Rollover) Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 | 25,554 | 1,077 | 6,389 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 19,165.50 | |||||||||||||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO Lynne M. Brown Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 | 9,000 | 379 | 2,250 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 6,750.00 | |||||||||||||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO Patricia Tom (Post) Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 | 11,280 | 475 | 2,820 | Warrant A: See Ft. 1 Warrant B: See Ft. 2 | $ | 8,460.00 | |||||||||||||
| Total: | 88,000,000 | 3,707,634 | 22,000,000 | $ | 66,000,000 | ||||||||||||||
| Ft. 1 | Warrant A equals (Aggregate Purchase Price)/$66,000,000 * Number of Warrant Shares Authorized to Be Issued on Closing Date. |
| Ft. 2 | Warrant B equals Number of Shares under Closing Warrant (if Share Authorization Approval received prior to Closing) minus Number of Shares under Warrant A. |
A-40
Table of Contents
A-41
Table of Contents
A-42
Table of Contents
A-43
Table of Contents
A-44
Table of Contents
A-45
Table of Contents
B-1
Table of Contents
B-2
Table of Contents
B-3
Table of Contents
| Net Number = | (A × B) - (A × C) | |||
| B | ||||
| A = the total number of shares with respect to which this Warrant is then being exercised. |
| B = | the Closing Price of the Common Stock on the Trading Day immediately preceding the date of the Exercise Notice. |
| C = the Exercise Price then in effect for the applicable Warrant Shares at the time of such exercise. |
| 3. | Date; Duration; Mandatory Exchange; Redemption and Cancellation. |
B-4
Table of Contents
| 4. | Covenants as to Common Stock. |
| 6. | Warrant Holder not Deemed a Stockholder. |
B-5
Table of Contents
| 7. | Compliance With Securities Laws. |
| 8. | Ownership and Transfer. |
B-6
Table of Contents
| 9. | Adjustment of Exercise Price and Number of Shares Issuable. |
B-7
Table of Contents
| 10. | Extraordinary Transaction Defined. |
B-8
Table of Contents
| 11. | Lost, Stolen, Mutilated or Destroyed Warrants. |
| La Jolla Pharmaceutical Company | |
| 6455 Nancy Ridge Drive | |
| San Diego, CA 92121 | |
| Attention: Chief Executive Officer | |
| Fax: (858) 626-2851 |
| Gibson, Dunn & Crutcher LLP | |
| 4 Park Plaza | |
| Irvine, CA 92614 | |
| Attention: Mark W. Shurtleff, Esq. | |
| Fax: (949) 451-4220 |
| American Stock Transfer & Trust Company | |
| 59 Maiden Lane | |
| Plaza Level | |
| New York, NY 10038 | |
| Fax: (718) 921-8124 |
B-9
Table of Contents
| 14. | Governing Law; Jurisdiction; Venue; Waiver of Jury Trial. |
| 15. | Descriptive Headings. |
B-10
Table of Contents
| La Jolla Pharmaceutical Company |
| By: |
| Name: |
| Title: |
B-11
Table of Contents
B-12
Table of Contents
| La Jolla Pharmaceutical Company |
| By: |
| Name: |
| Title: |
B-13
Table of Contents
| Name and Address of Assignee | Federal Tax Identification Number | Number of Warrant Shares | ||
| o | Inside the United States to a Qualified Institutional Purchaser pursuant to and in compliance with the Securities Act of 1933, as amended; or |
| o | Inside the United States to an Accredited Investor pursuant to and in compliance with the Securities Act of 1933, as amended; or |
| o | Pursuant to and in compliance with Rule 144 under the Securities Act of 1933, as amended; |
| Name of Holder (print): |
| (Signature): |
| (By:) |
| (Title:) |
B-14
Table of Contents
| 1. | Definitions. |
C-1
Table of Contents
| 2. | Exercise of Warrant. |
C-2
Table of Contents
C-3
Table of Contents
| Net Number = | (A × B) - (A × C) | |||
| B | ||||
| A = | the total number of shares with respect to which this Warrant is then being exercised. |
| B = | the Closing Price of the Common Stock on the Trading Day immediately preceding the date of the Exercise Notice. |
| C = | the Exercise Price then in effect for the applicable Warrant Shares at the time of such exercise. |
| 3. | Date; Duration; Mandatory Exchange; Redemption and Cancellation. |
C-4
Table of Contents
| 4. | Covenants as to Common Stock. |
| 5. | Taxes. |
| 6. | Warrant Holder Not Deemed a Stockholder. |
C-5
Table of Contents
| 7. | Compliance with Securities Laws. |
| 8. | Ownership and Transfer. |
C-6
Table of Contents
| 9. | Adjustment of Exercise Price and Number of Shares Issuable. |
C-7
Table of Contents
| 10. | Extraordinary Transaction Defined. |
C-8
Table of Contents
| 11. | Lost, Stolen, Mutilated or Destroyed Warrants. |
| 12. | Notice. |
| La Jolla Pharmaceutical Company | |
| 6455 Nancy Ridge Drive | |
| San Diego, CA 92121 | |
| Attention: Chief Executive Officer | |
| Fax: (858) 626-2851 |
| Gibson, Dunn & Crutcher LLP | |
| 4 Park Plaza | |
| Irvine, CA 92614 | |
| Attention: Mark W. Shurtleff, Esq. | |
| Fax: (949) 451-4220 |
| American Stock Transfer & Trust Company | |
| 59 Maiden Lane | |
| Plaza Level | |
| New York, NY 10038 | |
| Fax: (718) 921-8124 |
C-9
Table of Contents
| 13. | Amendments. |
| 14. | Governing Law; Jurisdiction; Venue; Waiver of Jury Trial. |
| 15. | Descriptive Headings. |
C-10
Table of Contents
| La Jolla Pharmaceutical Company |
| By: |
| Name: |
| Title: |
C-11
Table of Contents
C-12
Table of Contents
| Name of Holder (print): | ||
| (Signature): | ||
| (By:) | ||
| (Title:) | ||
| Tax ID No. | ||
| Dated: , |
C-13
Table of Contents
| La Jolla Pharmaceutical Company |
| By: | ||
| Name: | ||
| Title: |
C-14
Table of Contents
| Name and Address of Assignee | Federal Tax Identification Number | Number of Warrant Shares | ||
| o | Inside the United States to a Qualified Institutional Purchaser pursuant to and in compliance with the Securities Act of 1933, as amended; or | |
| o | Inside the United States to an Accredited Investor pursuant to and in compliance with the Securities Act of 1933, as amended; or | |
| o | Pursuant to and in compliance with Rule 144 under the Securities Act of 1933, as amended; |
| Name of Holder (print): | ||
| (Signature): | ||
| (By:) | ||
| (Title:) | ||
| Dated: , |
C-15
Table of Contents
| 1. | Definitions. |
D-1
Table of Contents
| 2. | Exercise of Warrant. |
D-2
Table of Contents
D-3
Table of Contents
| Net Number = | (A × B) - (A × C) | |
| B |
D-4
Table of Contents
| A = | the total number of shares with respect to which this Warrant is then being exercised. | |
| B = | the Closing Price of the Common Stock on the Trading Day immediately preceding the date of the Exercise Notice. | |
| C = | the Exercise Price then in effect for the applicable Warrant Shares at the time of such exercise. |
| 3. | Date; Duration; Mandatory Exchange; Redemption and Cancellation. |
| 4. | Covenants as to Common Stock. |
D-5
Table of Contents
| 5. | Taxes. |
| 6. | Warrant Holder not Deemed a Stockholder. |
| 7. | Compliance with Securities Laws. |
D-6
Table of Contents
| 8. | Ownership and Transfer. |
| 9. | Adjustment of Exercise Price and Number of Shares Issuable. |
D-7
Table of Contents
D-8
Table of Contents
| 10. | Extraordinary Transaction Defined. |
| 11. | Lost, Stolen, Mutilated or Destroyed Warrants. |
| 12. | Notice. |
| La Jolla Pharmaceutical Company | |
| 6455 Nancy Ridge Drive | |
| San Diego, CA 92121 | |
| Attention: Chief Executive Officer | |
| Fax: (858) 626-2851 |
| Gibson, Dunn & Crutcher LLP | |
| 4 Park Plaza | |
| Irvine, CA 92614 | |
| Attention: Mark W. Shurtleff, Esq. | |
| Fax: (949) 451-4220 |
D-9
Table of Contents
| American Stock Transfer & Trust Company | |
| 59 Maiden Lane | |
| Plaza Level | |
| New York, NY 10038 | |
| Fax: (718) 921-8124 |
| 13. | Amendments. |
| 14. | Governing Law; Jurisdiction; Venue; Waiver of Jury Trial. |
D-10
Table of Contents
| 15. | Descriptive Headings. |
| La Jolla Pharmaceutical Company |
| By: |
| Name: |
| Title: |
D-11
Table of Contents
| o | “Cash Exercise” with respect to [ ] Warrant Shares; and/or | |
| o | “Cashless Exercise” with respect to [ ] Warrant Shares (to the extent permitted by the terms of the Warrant). |
D-12
Table of Contents
| Name of Holder (print): | ||
| (Signature): | ||
| (By:) | ||
| (Title:) | ||
| Tax ID No. | ||
| Dated: |
D-13
Table of Contents
| La Jolla Pharmaceutical Company |
| By: |
| Name: |
| Title: |
D-14
Table of Contents
| Name and Address of Assignee | Federal Tax Identification Number | Number of Warrant Shares | ||
| o | Inside the United States to a Qualified Institutional Purchaser pursuant to and in compliance with the Securities Act of 1933, as amended; or | |
| o | Inside the United States to an Accredited Investor pursuant to and in compliance with the Securities Act of 1933, as amended; or | |
| o | Pursuant to and in compliance with Rule 144 under the Securities Act of 1933, as amended; |
| Name of Holder (print): | ||
| (Signature): | ||
| (By:) | ||
| (Title:) | ||
| Dated: | ||
D-15
Table of Contents
| 1. | Definitions. |
E-1
Table of Contents
| 2. | Registration. |
E-2
Table of Contents
E-3
Table of Contents
| 3. | Related Obligations. |
E-4
Table of Contents
E-5
Table of Contents
E-6
Table of Contents
E-7
Table of Contents
| 4. | Obligations of the Investors. |
E-8
Table of Contents
| 5. | Expenses of Registration. |
| 6. | Indemnification. |
E-9
Table of Contents
E-10
Table of Contents
E-11
Table of Contents
| 7. | Contribution. |
| 8. | Reports Under the Exchange Act. |
| 9. | Assignment of Registration Rights. |
E-12
Table of Contents
| 10. | Amendment of Registration Rights. |
| 11. | Miscellaneous. |
| If to the Company: | |
| La Jolla Pharmaceutical Company | |
| 6455 Nancy Ridge Drive | |
| San Diego, CA 92121 | |
| Attention: Chief Executive Officer | |
| Fax: (858) 626-2851 |
| Gibson, Dunn & Crutcher LLP | |
| 4 Park Plaza | |
| Irvine, CA 92614 | |
| Attention: Mark W. Shurtleff | |
| Fax: (949) 451-4220 |
| If to Legal Counsel: | |
| Baker & McKenzie LLP | |
| 130 East Randolph Drive | |
| Chicago, Illinois 60601 | |
| Attention: Bruce Zivian, Esq. | |
| Fax: (312) 698-2469 |
E-13
Table of Contents
E-14
Table of Contents
| The Company: | La Jolla Pharmaceutical Company | |
| a Delaware corporation | ||
| By: /s/Steven B. Engle | ||
| Name: Steven B. Engle | ||
| Title: Chairman and CEO | ||
| Purchasers: | Essex Woodlands Health Ventures Fund VI, LP | |
| By: Essex Woodlands Health Ventures VI, L.P. | ||
| Its: General Partner | ||
| By: Essex Woodlands Health Ventures VI, L.L.C. | ||
| Its: General Partner | ||
| By: /s/Martin P. Sutter | ||
E-15
Table of Contents
| Domain Public Equity Partners, L.P. |
| By: | Domain Public Equity Associates, L.L.C., |
| its General Partner |
| By: | /s/Lisa A. Kraeutler |
| Lisa A. Kraeutler, Attorney-in-Fact | |
| /s/Alejandro Gonzalez Cimadevilla | |
| Alejandro Gonzalez Cimadevilla | |
| Special Situations Fund III, L.P. |
| By: | /s/Austin W. Marxe |
| Austin W. Marxe, its General Partner | |
| Special Situations Cayman Fund, L.P. |
| By: | /s/Austin W. Marxe |
| Austin W. Marxe, its General Partner | |
| Special Situations Private Equity Fund, L.P. |
| By: | /s/Austin W. Marxe |
| Austin W. Marxe, its General Partner | |
| Special Situations Life Sciences Fund, L.P. |
| By: | /s/Austin W. Marxe |
| Austin W. Marxe, its General Partner | |
| Sutter Hill Ventures, | |
| a California limited partnership |
| By: | /s/William H. Younger, Jr. |
| William H. Younger Jr. | |
| Managing Director of the General Partner |
E-16
Table of Contents
| ANVEST, L.P. |
| By: | /s/David Anderson |
| David Anderson, General Partner | |
| G. Leonard Baker, Jr. and Mary Anne Baker, Co-Trustees of the Baker Revocable Trust U/ A/ D 2/3/03 |
| By: | /s/G. Leonard Baker, Jr. By David E. Sweet |
| Under Power of Attorney | |
| G. Leonard Baker, Jr., Trustee | |
| William H. Younger, Jr. and Lauren L. Younger, Co-Trustees of the Younger Living Trust U/ A/ D 1/20/95 |
| By: | /s/William H. Younger, Jr. |
| William H. Younger, Jr., Trustee | |
| Tench Coxe and Simone Otus Coxe, Co-Trustees of the Coxe Revocable Trust U/ A/ D 4/23/98 |
| By: | /s/Tench Coxe By David E. Sweet |
| Under Power of Attorney | |
| Tench Coxe, Trustee | |
| /s/James C. Gaither By David E. Sweet | |
| Under Power of Attorney | |
| James C. Gaither | |
| Jeffrey W. Bird and Christina R. Bird as Trustees of Jeffrey W. and Christina R. Bird Trust Agreement Dated 10/31/00 |
| By: | /s/Jeffrey W. Bird |
| Jeffrey W. Bird, Trustee | |
| Saunders Holdings, L.P. |
| By: | /s/G. Leonard Baker, Jr. |
| By David E. Sweet Under Power of Attorney | |
| G. Leonard Baker, Jr., General Partner |
E-17
Table of Contents
| Robert Yin and Lily Yin as Trustees of Yin Family Trust Dated March 1, 1997 |
| By: | /s/Robert Yin |
| Robert Yin, Trustee | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan FBO Sherryl W. Hossack | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan FBO David L. Anderson | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan FBO William H. Younger | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan FBO Tenche Coxe | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan FBO David E. Sweet | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan FBO David E. Sweet (Rollover) | |
| /s/Vicki M. Bandel | |
E-18
Table of Contents
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan FBO Lynne M. Brown | |
| /s/Vicki M. Bandel | |
| Wells Fargo Bank, N.A. FBO | |
| SHV Profit Sharing Plan FBO Patricia Tom (Post) | |
| /s/Vicki M. Bandel | |
E-19
Table of Contents
| Investor Address | Investor’s Representatives’ Address | |||
| Investor Name | and Facsimile Number | and Facsimile Number | ||
| Essex Woodlands Health Ventures Fund VI, L.P. | 10001 Woodloch Forest Drive Waterway Plaza Two, Suite 175 The Woodlands, TX 77380 Attn: Martin P. Sutter Fax: (281) 364-9755 | Baker & McKenzie LLP 130 E. Randolph Drive Chicago, IL 60601 Attn: Bruce Zivian, Esq. Fax: (312) 698-2469 | ||
| Frazier Healthcare V, LP | 601 Union Street, Suite 3200 Seattle, WA 98101 Attn: James Topper Fax: (206) 621-1848 | |||
| Alejandro Gonzalez Cimadevilla | Ruben Dario #223 5-A Chapultepec Morales Mexico D.F. ZIP 11570 Fax: (5255) 52818008 | |||
| Domain Public Equity Partners, L.P. | One Palmer Square, Suite 515 Princeton, NJ 08542 Attn: Nicole Vitullo Fax: (609) 683-4581 | |||
| Special Situations Fund III, L.P. | 153 E. 53rd Street, 55th Floor New York, NY 10022 Attn: Marianne Hicks Fax: 212-207-6515 | Lowenstein Sandler PC 65 Livingston Avenue Roseland, NJ 07068 Attn: John D. Hogoboom, Esq. Fax: (973) 597-2383 | ||
| Special Situations Cayman Fund, L.P. | 153 E. 53rd Street, 55th Floor New York, NY 10022 Attn: Marianne Hicks Fax: 212-207-6515 | Lowenstein Sandler PC 65 Livingston Avenue Roseland, NJ 07068 Attn: John D. Hogoboom, Esq. Fax: (973) 597-2383 | ||
| Special Situations Private Equity Fund, L.P. | 153 E. 53rd Street, 55th Floor New York, NY 10022 Attn: Marianne Hicks Fax: 212-207-6515 | Lowenstein Sandler PC 65 Livingston Avenue Roseland, NJ 07068 Attn: John D. Hogoboom, Esq. Fax: (973) 597-2383 | ||
| Special Situations Life Sciences Fund, L.P. | 153 E. 53rd Street, 55th Floor New York, NY 10022 Attn: Marianne Hicks Fax: 212-207-6515 | Lowenstein Sandler PC 65 Livingston Avenue Roseland, NJ 07068 Attn: John D. Hogoboom, Esq. Fax: (973) 597-2383 | ||
| Sutter Hill Ventures | 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax: 650-493-5600 | |||
| Anvest, L.P. | c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 |
E-20
Table of Contents
| Investor Address | Investor’s Representatives’ Address | |||
| Investor Name | and Facsimile Number | and Facsimile Number | ||
| G. Leonard Baker, Jr. and Mary Anne Baker, Co-Trustees of The Baker Revocable Trust U/A/D 2/3/03 | c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | |||
| Saunders Holdings, L.P. | c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | |||
| William H. Younger, Jr. and Lauren L. Younger, Co-Trustees of The Younger Living Trust U/ A/ D 1/20/95 | c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | |||
| Tench Coxe and Simone Otus Coxe, Co-Trustees of The Coxe Revocable Trust U/ A/ D 4/23/98 | c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | |||
| James C. Gaither | c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | |||
| Jeffrey W. Bird and Christina R. Bird as Trustees of Jeffrey W. and Christina R. Bird Trust Agreement Dated 10/31/00 | c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | |||
| Robert Yin and Lily Yin as Trustees of Yin Family Trust Dated March 1, 1997 | c/o Sutter Hill Ventures 755 Page Mill Road Suite A-200 Palo Alto, CA 94304 Attn: Robert Yin Fax (650) 493-5600 | |||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO Sherryl W. Hossack | Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 Fax: 415-956-9362 | |||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO David L. Anderson | Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 Fax: 415-956-9362 |
E-21
Table of Contents
| Investor Address | Investor’s Representatives’ Address | |||
| Investor Name | and Facsimile Number | and Facsimile Number | ||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO William H. Younger, Jr. | Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 Fax: 415-956-9362 | |||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO Tench Coxe | Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 Fax: 415-956-9362 | |||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO David E. Sweet | Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 Fax: 415-956-9362 | |||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO David E. Sweet (Rollover) | Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 Fax: 415-956-9362 | |||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO Lynne M. Brown | Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 Fax: 415-956-9362 | |||
| Wells Fargo Bank, N.A. FBO SHV Profit Sharing Plan FBO Patricia Tom (Post) | Attention: Vicki Bandel 420 Montgomery Street, 2nd Fl. San Francisco, CA 94101 Fax: 415-956-9362 |
E-22
Table of Contents
| (a) Incentive Election #1. If you elect Incentive Election #1, you will receive $52,462.00 (the “Closing Cash Amount”) in cash within two days after the Closing. On the 180th day after the Closing, subject to Section 7, you will receive an additional incentive payment in cash equal to the Closing Cash Amount (the “Second Cash Payment”). | |
| (b) Incentive Election #2. If you elect Incentive Election #2, you will receive an amount equal to the Closing Cash Amount within two business days after the Closing. In addition, you will receive 69,949 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (the “Plan”). The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. | |
| (c) Incentive Election #3. If you elect Incentive Election #3, you will receive 139,898 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan. The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. |
F-1
Table of Contents
F-2
Table of Contents
F-3
Table of Contents
F-4
Table of Contents
| Very truly yours, | |
| La Jolla Pharmaceutical Company |
| By: | /s/Steven B. Engle |
| Steven B. Engle | |
| Chief Executive Officer |
Signature
Print Name
F-5
Table of Contents
| | |
| | |
| |
| Signature | |
| Print Name | |
| Date |
F-6
Table of Contents
| (a) Incentive Election #1. If you elect Incentive Election #1, you will receive $50,500.00 (the “Closing Cash Amount”) in cash within two days after the Closing. On the 180th day after the Closing, subject to Section 7, you will receive an additional incentive payment in cash equal to the Closing Cash Amount (the “Second Cash Payment”). | |
| (b) Incentive Election #2. If you elect Incentive Election #2, you will receive an amount equal to the Closing Cash Amount within two business days after the Closing. In addition, you will receive 67,333 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (the “Plan”). The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. | |
| (c) Incentive Election #3. If you elect Incentive Election #3, you will receive 134,667 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan. The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. |
F-7
Table of Contents
F-8
Table of Contents
F-9
Table of Contents
F-10
Table of Contents
| Very truly yours, | |
| La Jolla Pharmaceutical Company |
| By: | /s/Steven B. Engle |
| Steven B. Engle | |
| Chief Executive Officer |
| /s/Josefina T. Elchico | |
| Signature | |
| Josefina T. Elchico | |
| Print Name |
F-11
Table of Contents
| | |
| | |
| |
| Signature | |
| Print Name | |
| Date |
F-12
Table of Contents
| (a) Incentive Election #1. If you elect Incentive Election #1, you will receive $109,200.00 (the “Closing Cash Amount”) in cash within two days after the Closing. On the 180th day after the Closing, subject to Section 7, you will receive an additional incentive payment in cash equal to the Closing Cash Amount (the “Second Cash Payment”). | |
| (b) Incentive Election #2. If you elect Incentive Election #2, you will receive an amount equal to the Closing Cash Amount within two business days after the Closing. In addition, you will receive 145,600 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (the “Plan”). The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. | |
| (c) Incentive Election #3. If you elect Incentive Election #3, you will receive 291,200 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan. The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. | |
| (d) Incentive Election #4. If you elect Incentive Election #4, you will receive $54,600.00 in cash within two business days after the Closing. In addition, you will receive 72,800 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (the “Plan”). The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. On the 180th day after the Closing, subject to Section 7, you will receive an additional incentive payment in cash equal to $109,200.00 (the “Second Cash Payment”). |
F-13
Table of Contents
F-14
Table of Contents
F-15
Table of Contents
F-16
Table of Contents
| Very truly yours, | |
| La Jolla Pharmaceutical Company |
| By: | /s/Steven M. Martin |
| Steven M. Martin | |
| Director |
F-17
Table of Contents
| | |
| | |
| | |
| |
| Signature | |
| Print Name | |
| Date |
F-18
Table of Contents
| (a) Incentive Election #1. If you elect Incentive Election #1, you will receive $49,241.00 (the “Closing Cash Amount”) in cash within two days after the Closing. On the 180th day after the Closing, subject to Section 7, you will receive an additional incentive payment in cash equal to the Closing Cash Amount (the “Second Cash Payment”). | |
| (b) Incentive Election #2. If you elect Incentive Election #2, you will receive an amount equal to the Closing Cash Amount within two business days after the Closing. In addition, you will receive 65,654 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (the “Plan”). The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. | |
| (c) Incentive Election #3. If you elect Incentive Election #3, you will receive 131,308 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan. The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. |
F-19
Table of Contents
F-20
Table of Contents
F-21
Table of Contents
F-22
Table of Contents
| Very truly yours, | |
| La Jolla Pharmaceutical Company |
| By: | /s/Steven B. Engle |
| Steven B. Engle | |
| Chief Executive Officer |
Signature
Print Name
F-23
Table of Contents
| | |
| | |
| |
| Signature | |
| Print Name | |
| Date |
F-24
Table of Contents
| (a) Incentive Election #1. If you elect Incentive Election #1, you will receive $76,371.00 (the “Closing Cash Amount”) in cash within two days after the Closing. On the 180th day after the Closing, subject to Section 7, you will receive an additional incentive payment in cash equal to the Closing Cash Amount (the “Second Cash Payment”). | |
| (b) Incentive Election #2. If you elect Incentive Election #2, you will receive an amount equal to the Closing Cash Amount within two business days after the Closing. In addition, you will receive 101,827 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (the “Plan”). The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. | |
| (c) Incentive Election #3. If you elect Incentive Election #3, you will receive 203,655 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan. The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. |
F-25
Table of Contents
F-26
Table of Contents
F-27
Table of Contents
F-28
Table of Contents
| Very truly yours, | |
| La Jolla Pharmaceutical Company |
| By: | /s/Steven B. Engle |
| Steven B. Engle | |
| Chief Executive Officer |
| /s/Matthew D. Linnik | |
| Signature | |
| Matthew D. Linnik | |
| Print Name |
F-29
Table of Contents
| | |
| | |
| |
| Signature | |
| Print Name | |
| Date |
F-30
Table of Contents
| (a) Incentive Election #1. If you elect Incentive Election #1, you will receive $46,469.00 (the “Closing Cash Amount”) in cash within two days after the Closing. On the 180th day after the Closing, subject to Section 7, you will receive an additional incentive payment in cash equal to the Closing Cash Amount (the “Second Cash Payment”). | |
| (b) Incentive Election #2. If you elect Incentive Election #2, you will receive an amount equal to the Closing Cash Amount within two business days after the Closing. In addition, you will receive 61,958 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (the “Plan”). The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. | |
| (c) Incentive Election #3. If you elect Incentive Election #3, you will receive 123,916 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan. The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. |
F-31
Table of Contents
F-32
Table of Contents
F-33
Table of Contents
F-34
Table of Contents
| Very truly yours, | |
| La Jolla Pharmaceutical Company |
| By: | /s/Steven B. Engle |
| Steven B. Engle | |
| Chief Executive Officer |
| /s/Theodora Reilly | |
| Signature | |
| Theodora Reilly | |
| Print Name |
F-35
Table of Contents
| | |
| | |
| |
| Signature | |
| Print Name | |
| Date |
F-36
Table of Contents
| (a) Incentive Election #1. If you elect Incentive Election #1, you will receive $44,256.00 (the “Closing Cash Amount”) in cash within two days after the Closing. On the 180th day after the Closing, subject to Section 7, you will receive an additional incentive payment in cash equal to the Closing Cash Amount (the “Second Cash Payment”). | |
| (b) Incentive Election #2. If you elect Incentive Election #2, you will receive an amount equal to the Closing Cash Amount within two business days after the Closing. In addition, you will receive 59,008 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (the “Plan”). The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. | |
| (c) Incentive Election #3. If you elect Incentive Election #3, you will receive 118,015 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan. The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. |
F-37
Table of Contents
F-38
Table of Contents
F-39
Table of Contents
F-40
Table of Contents
| Very truly yours, | |
| La Jolla Pharmaceutical Company |
| By: | /s/Steven B. Engle |
| Steven B. Engle | |
| Chief Executive Officer |
| /s/Gail A. Sloan | |
| Signature | |
| Gail A. Sloan | |
| Print Name |
F-41
Table of Contents
| | |
| | |
| |
| Signature | |
| Print Name | |
| Date |
F-42
Table of Contents
| (a) Incentive Election #1. If you elect Incentive Election #1, you will receive $43,388.00 (the “Closing Cash Amount”) in cash within two days after the Closing. On the 180th day after the Closing, subject to Section 7, you will receive an additional incentive payment in cash equal to the Closing Cash Amount (the “Second Cash Payment”). | |
| (b) Incentive Election #2. If you elect Incentive Election #2, you will receive an amount equal to the Closing Cash Amount within two business days after the Closing. In addition, you will receive 57,851 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (the “Plan”). The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. | |
| (c) Incentive Election #3. If you elect Incentive Election #3, you will receive 115,701 shares of restricted stock under the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan. The shares will be issued as soon as practicable after the Closing and, subject to Section 8, the Company’s repurchase right with respect to such shares will lapse with respect to all of the shares on the one year anniversary of the Closing. |
F-43
Table of Contents
F-44
Table of Contents
F-45
Table of Contents
F-46
Table of Contents
| Very truly yours, | |
| La Jolla Pharmaceutical Company |
| By: | /s/Steven B. Engle |
| Steven B. Engle | |
| Chief Executive Officer |
| /s/Andrew Wiseman | |
| Signature | |
| Andrew Wiseman | |
| Print Name |
F-47
Table of Contents
| | |
| | |
| |
| Signature | |
| Print Name | |
| Date |
F-48
Table of Contents
| 1. The name of the Corporation is: La Jolla Pharmaceutical Company. | |
| 2. The Certificate of Incorporation of the Corporation is hereby amended by striking out Article IV thereof and by substituting in lieu of said Article the following new Article: |
| The Corporation is authorized to issue two classes of stock designated “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock that this Corporation is authorized to issue is Two Hundred Thirty Three Million (233,000,000), consisting of Two Hundred Twenty Five Million (225,000,000) shares of Common Stock, par value $0.01 per share, and Eight Million (8,000,000) shares of Preferred Stock, par value $0.01 per share. | |
| The Board is hereby authorized to issue the shares of Preferred Stock in one or more series, to fix the number of shares of any such series of Preferred Stock, to determine the designation of any such series, and to fix the rights, preferences, and privileges and the qualifications, limitations or restrictions of the series of Preferred Stock to the full extent permitted under the Delaware General Corporation Law. The authority of the Board with respect to any series of Preferred Stock shall include, without limitation, the power to fix or alter the dividend rights, dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions, if any), the redemption price or prices, and the liquidation preferences and the number of shares constituting any such additional series and the designation thereof, or any of them; and to increase or decrease the number of authorized shares of any series subsequent to the issue of that series, but not below the number of shares of such series then outstanding. In case the authorized number of shares of any series shall be so decreased, the shares constituting such decrease shall resume the status which they had prior to the adoption of the resolution originally fixing the number of shares of such series. |
| 3. The amendment of the Certificate of Incorporation herein certified has been duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware. |
| Name: | |
| Title: |
G-1
Table of Contents
| (a) “Administrator” means the Board or a Committee that has been delegated the authority to administer the Plan. | |
| (b) “Award” means an Incentive Award or a Nonemployee Director’s Option. | |
| (c) “Award Document” means an award agreement duly executed on behalf of the Company and by the Recipient or, in the Administrator’s discretion, a confirming memorandum issued by the Company to the Recipient. | |
| (d) “Board” means the Board of Directors of the Company. | |
| (e) “Change in Control” means the following and shall be deemed to occur if any of the following events occur: |
| (i) Except as provided by subsection (iii) hereof, the acquisition (other than from the Company) by any person, entity or “group,” within the meaning of Section 13(d)(3) or 14(d)(2) of the Exchange Act (excluding, for this purpose, the Company or its subsidiaries, or any employee benefit plan of the Company or its subsidiaries which acquires beneficial ownership of voting securities of the Company), of beneficial ownership (within the meaning of Rule 13d-3 promulgated under the Exchange Act) of forty percent (40%) or more of either the then outstanding shares of Common Stock or the combined voting power of the Company’s then outstanding voting securities entitled to vote generally in the election of directors; or | |
| (ii) Individuals who, as of the effective date of the Plan, constitute the Board (the “Incumbent Board”) cease for any reason to constitute at least a majority of the Board, provided that any person becoming a director subsequent to the date hereof whose election, or nomination for election by the Company’s stockholders, is or was approved by a vote of at least a majority of the directors then comprising the Incumbent Board (other than an election or nomination of an individual whose initial assumption of office is in connection with an actual or threatened election contest relating to the election of the directors of the Company, as such terms are used in Rule 14a-11 of Regulation 14A promulgated under the Exchange Act) shall be, for purposes of the Plan, considered as though such person were a member of the Incumbent Board; or | |
| (iii) Approval by the stockholders of the Company of a reorganization, merger or consolidation with any other person, entity or corporation, other than: |
| (A) a merger or consolidation which would result in the persons holding the voting securities of the Company outstanding immediately prior thereto continuing to hold more than fifty percent (50%) of the combined voting power of the voting securities of the Company or its successor which are outstanding immediately after such merger or consolidation, or | |
| (B) a merger or consolidation effected to implement a recapitalization of the Company (or similar transaction) in which no person acquires forty percent (40%) or more of the combined voting power of the Company’s then outstanding voting securities; or |
H-1
Table of Contents
| (iv) Approval by the stockholders of the Company of a plan of complete liquidation of the Company or an agreement for the sale or other disposition by the Company of all or substantially all of the Company’s assets. |
| Notwithstanding the foregoing, a Change in Control shall not be deemed to have occurred (1) if the “person” is an underwriter or underwriting syndicate that has acquired the ownership of 50% or more of the combined voting power of the Company’s then outstanding voting securities solely in connection with a public offering of the Company’s securities, or (2) if the “person” is an employee stock ownership plan or other employee benefit plan maintained by the Company that is qualified under the provisions of the Employee Retirement Income Security Act of 1974, as amended. | |
| (f) “Code” means the Internal Revenue Code of 1986, as amended. Where the context so requires, a reference to a particular Code section shall also refer to any successor provision of the Code to such section. | |
| (g) “Committee” means the committee appointed by the Board to administer the Plan. | |
| (h) “Common Stock” means the common stock of the Company, $0.01 par value. | |
| (i) “Company” means La Jolla Pharmaceutical Company. | |
| (j) “Dividend Equivalent” means a right granted by the Company under Section 2.07 to a holder of an Option, Stock Appreciation Right, or other Incentive Award denominated in shares of Common Stock to receive from the Company during the Applicable Dividend Period (as defined in Section 2.07) payments equivalent to the amount of dividends payable to holders of the number of shares of Common Stock underlying such Option, Stock Appreciation Right, or other Incentive Award. | |
| (k) “Eligible Person” means any director, Employee or consultant of the Company or any Related Corporation. | |
| (l) “Employee” means an individual who is in the employ of the Company (or any Parent or Subsidiary) subject to the control and direction of the employer entity as to both the work to be performed and the manner and method of performance. | |
| (m) “Exchange Act” means the Securities Exchange Act of 1934, as amended. Where the context so requires, a reference to a particular section of the Exchange Act or rule thereunder shall also refer to any successor provision to such section or rule. | |
| (n) “Exercise Price” means the price at which the Holder may purchase shares of Common Stock underlying an Option. | |
| (o) “Fair Market Value” of capital stock of the Company shall be determined with reference to the closing price of such stock on the day in question (or, if such day is not a trading day in the U.S. securities markets, on the nearest preceding trading day), as reported with respect to the principal market or trading system on which such stock is then traded; or, if no such closing prices are reported, the mean between the high bid and low ask prices that day on the principal market or national quotation system on which such shares are then quoted; provided, however, that when appropriate, the Administrator in determining Fair Market Value of capital stock of the Company may take into account such other factors as may be deemed appropriate under the circumstances. Notwithstanding the foregoing, the Fair Market Value of capital stock for purposes of grants of Incentive Stock Options shall be determined in compliance with applicable provisions of the Code. The Fair Market Value of rights or property other than capital stock of the Company means the fair market value thereof as determined by the Administrator on the basis of such factors as it may deem appropriate. | |
| (p) “Holder” means the Recipient of an Award or any permitted assignee holding the Award. | |
| (q) “Incentive Award” means any Option (other than a Nonemployee Director’s Option), Restricted Stock, Stock Appreciation Right, Stock Payment, Performance Award or Dividend Equivalent granted or sold to an Eligible Person under this Plan. |
H-2
Table of Contents
| (r) “Incentive Stock Option” means an Option that qualifies as an incentive stock option under Section 422 (or any successor section) of the Code and the regulations thereunder. | |
| (s) “Just Cause Dismissal” shall mean a termination of a Recipient’s Service for any of the following reasons: (i) the Recipient violates any reasonable rule or regulation of the Company or the Recipient’s superiors or the Chief Executive Officer or President of the Company that (A) results in damage to the Company or (B) after written notice to do so, the Recipient fails to correct within a reasonable time; (ii) any willful misconduct or gross negligence by the Recipient in the responsibilities assigned to him or her; (iii) any willful failure to perform his or her job; (iv) any wrongful conduct of a Recipient which has an adverse impact on the Company or which constitutes fraud, embezzlement or dishonesty; (v) the Recipient’s performing services for any other person or entity which competes with the Company while he or she is providing Service, without the written approval of the Chief Executive Officer or President of the Company; or (vi) any other conduct that the Administrator determines constitutes Just Cause for Dismissal; provided, however, that if the term of concept has been defined in an employment agreement between the Company and the Recipient, then Just Cause Dismissal shall have the definition set forth in such employment agreement. The foregoing definition shall not in any way preclude or restrict the right of the Company or any Related Corporation to discharge or dismiss any Recipient or other person in the Service of the Company or any Related Corporation for any other acts or omissions but such other acts or omission shall not be deemed, for purposes of the Plan, to constitute grounds for Just Cause Dismissal. | |
| (t) “Nonemployee Director” means a director of the Company who is not an Employee of the Company or any of its Related Corporations. | |
| (u) “Nonemployee Director’s Option” means a Nonqualified Stock Option granted to a Nonemployee Director pursuant to Article III of the Plan. | |
| (v) “Nonqualified Stock Option” means an Option that does not qualify as an Incentive Stock Option. | |
| (w) “Option” means a right to purchase stock of the Company granted under this Plan, and can be an Incentive Stock Option or a Nonqualified Stock Option. | |
| (x) “Parent” means any corporation (other than the Company) in an unbroken chain of corporations ending with the Company, provided each corporation in the unbroken chain (other than the Company) owns, at the time of the determination, stock possessing 50% or more of the total combined voting power of all classes of stock in one of the other corporations in such chain. | |
| (y) “Performance Award” means an award, payable in cash, Common Stock or a combination thereof, which vests and becomes payable over a period of time upon attainment of performance criteria established in connection with the grant of the award. | |
| (z) “Performance-Based Compensation” means performance-based compensation as described in Section 162(m) of the Code and the regulations thereunder. If the amount of compensation an Eligible Person will receive under any Incentive Award is not based solely on an increase in the value of Common Stock after the date of grant or award, the Administrator, in order to qualify an Incentive Award as performance-based compensation under Section 162(m) of the Code and the regulations thereunder, can condition the grant, award, vesting, or exercisability of such an award on the attainment of a preestablished, objective performance goal. For this purpose, a preestablished, objective performance goal may include one or more of the following performance criteria: (i) cash flow, (ii) earnings per share (including earnings before interest, taxes, and amortization), (iii) return on equity, (iv) total stockholder return, (v) return on capital, (vi) return on assets or net assets, (vii) income or net income, (viii) operating margin, (ix) return on operating revenue, (x) attainment of stated goals related to the Company’s research and development or clinical trials programs, (xi) attainment of stated goals related to the Company’s capitalization, costs, financial condition, or results of operations, and (xii) any other similar performance criteria. |
H-3
Table of Contents
| (aa) “Permanent Disability” shall mean the inability of the Recipient to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment which can be expected to result in death or has lasted or can be expected to last for a continuous period of twelve months or more. | |
| (bb) “Plan” means the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan as set forth in this document. | |
| (cc) “Purchase Price” means the purchase price (if any) to be paid by a Recipient for Restricted Stock as determined by the Administrator (which price shall be at least equal to the minimum price required under applicable laws and regulations for the issuance of Common Stock). | |
| (dd) “Recipient” means an Eligible Person who has received an Award hereunder. | |
| (ee) “Related Corporation” means either a Parent or Subsidiary. | |
| (ff) “Restricted Stock” means Common Stock that is the subject of an award made under Section 2.04 and which is nontransferable and subject to a substantial risk of forfeiture until specific conditions are met as set forth in this Plan and in any Award Document. | |
| (gg) “Securities Act” means the Securities Act of 1933, as amended. | |
| (hh) “Service” means the performance of services for the Company or its Related Corporations by a person in the capacity of an Employee, a director or a consultant, except to the extent otherwise specifically provided in the Award Document. | |
| (ii) “Stock Appreciation Right” means a right granted under Section 2.05 to receive a payment that is measured with reference to the amount by which the Fair Market Value of a specified number of shares of Common Stock appreciates from a specified date, such as the date of grant of the Stock Appreciation Right, to the date of exercise. | |
| (jj) “Stock Payment” means a payment in shares of Common Stock to replace all or any portion of the compensation (other than base salary) that would otherwise become payable to a Recipient. | |
| (kk) “Subsidiary” means any corporation (other than the Company) in an unbroken chain of corporations beginning with the Company, provided each corporation in the unbroken chain (other than the last corporation) owns, at the time of the determination, stock possessing 50% or more of the total combined voting power of all classes of stock in one of the other corporations in such chain. |
H-4
Table of Contents
H-5
Table of Contents
H-6
Table of Contents
H-7
Table of Contents
| (i) Termination for Cause. Except as otherwise provided by the Administrator, in the event of a Just Cause Dismissal of a Recipient, all of the outstanding Options granted to such Recipient shall expire and become unexercisable as of the date of such Just Cause Dismissal. | |
| (ii) Termination Other Than for Cause. Subject to subsection (i) above and except as otherwise provided by the Administrator, in the event of a Recipient’s termination of Service from the Company or its Related Corporations due to: |
| (A) any reason other than Just Cause Dismissal, death, or Permanent Disability, or normal retirement, the outstanding Options granted to such Recipient, whether or not vested, shall expire and become unexercisable as of the earlier of (1) the date such Options would expire in accordance |
H-8
Table of Contents
| with their terms if the Recipient had remained in Service or (2) three calendar months after the date the Recipient’s Service terminated in the case of Incentive Stock Options, or six months after the Recipient’s Service terminated, in the case of Nonqualified Stock Options. | |
| (B) death or Permanent Disability, the outstanding Options granted to such Recipient, whether or not vested, shall expire and become unexercisable as of the earlier of (1) the date such Options would expire in accordance with their terms if the Recipient had remained in Service or (2) twelve months after the date of termination. | |
| (C) normal retirement, the outstanding Options granted to such Recipient, whether or not vested, shall expire and become unexercisable as of the earlier of (A) the date such Options expire in accordance with their terms or (B) twenty-four months after the date of retirement. |
| (iii) Termination of Director Service. In the event that a Director shall cease to be a Nonemployee Director, all outstanding Options (other than a Nonemployee Director’s Option) granted to such Recipient shall be exercisable, to the extent already vested and exercisable on the date such Recipient ceases to be a Nonemployee Director and regardless of the reason the Recipient ceases to be a Nonemployee Director until the fifth anniversary of the date such Director ceases to be a Nonemployee Director; provided that the Administrator may extend such post-termination period to up to the expiration date of the Option. |
| (i) No Transfer. The shares of Restricted Stock may not be sold, assigned, transferred, pledged, hypothecated or otherwise disposed of, alienated or encumbered until the restrictions are removed or expire; |
H-9
Table of Contents
| (ii) Certificates. The Administrator may require that the certificates representing shares of Restricted Stock granted or sold to a Holder pursuant to the Plan remain in the physical custody of an escrow holder or the Company until all restrictions are removed or expire; | |
| (iii) Restrictive Legends. Each certificate representing shares of Restricted Stock granted or sold to a Holder pursuant to the Plan will bear such legend or legends making reference to the restrictions imposed upon such Restricted Stock as the Administrator in its discretion deems necessary or appropriate to enforce such restrictions; and | |
| (iv) Other Restrictions. The Administrator may impose such other conditions on Restricted Stock as the Administrator may deem advisable including, without limitation, restrictions under the Securities Act, under the Exchange Act, under the requirements of any stock exchange or upon which such Restricted Stock or shares of the same class are then listed and under any blue sky or other securities laws applicable to such shares. |
| (i) A Stock Appreciation Right granted in connection with an Option granted under this Plan will entitle the holder of the related Option, upon exercise of the Stock Appreciation Right, to surrender such Option, or any portion thereof to the extent unexercised, with respect to the number of shares as to which such Stock Appreciation Right is exercised, and to receive payment of an amount computed pursuant to Section 2.05(b)(iii). Such Option will, to the extent surrendered, then cease to be exercisable. | |
| (ii) A Stock Appreciation Right granted in connection with an Option hereunder will be exercisable at such time or times, and only to the extent that, the related Option is exercisable, and will not be transferable except to the extent that such related Option may be transferable. | |
| (iii) Upon the exercise of a Stock Appreciation Right related to an Option, the Holder will be entitled to receive payment of an amount determined by multiplying: (i) the difference obtained by subtracting the Exercise Price of a share of Common Stock specified in the related Option from the Fair Market Value of a share of Common Stock on the date of exercise of such Stock Appreciation Right (or as of such other date or as of the occurrence of such event as may have been specified in the instrument evidencing the grant of the Stock Appreciation Right), by (ii) the number of shares as to which such Stock Appreciation Right is exercised. |
H-10
Table of Contents
H-11
Table of Contents
H-12
Table of Contents
H-13
Table of Contents
| Notice of Grant of Stock Options and Option Agreement | La Jolla Pharmaceutical Co. ID: 33-0361285 6455 Nancy Ridge Drive San Diego, CA 92121 (858) 452-6600 | |||
Name: | Option Number: | |||
| Plan: | 2004 | |||
Address: | ID: | |||
| Shares | Vest Type | Full Vest | Expiration | |||||||||||
| ------------------------------------ Date | ||
| Name | Date | |
H-14
Table of Contents
| 1. The name of the Corporation is: La Jolla Pharmaceutical Company. | |
| 2. The Certificate of Incorporation of the Corporation is hereby amended by striking out Article IV thereof and by substituting in lieu of said Article the following new Article: |
| The Corporation is authorized to issue two classes of stock designated “Common Stock” and “Preferred Stock.” The total number of shares of all classes of stock that this Corporation is authorized to issue is Two Hundred Thirty Three Million (233,000,000), consisting of Two Hundred Twenty Five Million (225,000,000) shares of Common Stock, par value $0.01 per share, and Eight Million (8,000,000) shares of Preferred Stock, par value $0.01 per share. | |
| The Board is hereby authorized to issue the shares of Preferred Stock in one or more series, to fix the number of shares of any such series of Preferred Stock, to determine the designation of any such series, and to fix the rights, preferences, and privileges and the qualifications, limitations or restrictions of the series of Preferred Stock to the full extent permitted under the Delaware General Corporation Law. The authority of the Board with respect to any series of Preferred Stock shall include, without limitation, the power to fix or alter the dividend rights, dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions, if any), the redemption price or prices, and the liquidation preferences and the number of shares constituting any such additional series and the designation thereof, or any of them; and to increase or decrease the number of authorized shares of any series subsequent to the issue of that series, but not below the number of shares of such series then outstanding. In case the authorized number of shares of any series shall be so decreased, the shares constituting such decrease shall resume the status which they had prior to the adoption of the resolution originally fixing the number of shares of such series. | |
| Effective at 5:00 p.m. on , 2005 (the “Effective Time”), the issued and outstanding Common Stock of the Corporation will be reverse split on a one-for-five basis so that each five shares of Common Stock issued and outstanding immediately prior to the Effective Time shall automatically be converted into and reconstituted as one share of Common Stock (the “Reverse Split”). No fractional shares will be issued by the Corporation as a result of the Reverse Split, and, as of the Effective Time, stockholders otherwise entitled to receive fractions of shares shall have no further interest as a stockholder in respect of such fractions of shares. In lieu of such fractions of shares, the Corporation will pay the holders thereof cash in an amount equal to (i) the value of such fractional shares based on the closing price per share of the Common Stock as reported on the Nasdaq National Market or the Nasdaq Capital Market, as applicable, on the day preceding the Effective Time or (ii) if the Common Stock is not then listed on the Nasdaq National Market or the Nasdaq Capital Market, the fair market value of the Common Stock as determined by the Corporation’s board of directors. |
I-1
Table of Contents
| 3. The amendment of the Certificate of Incorporation herein certified has been duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware. |
| Name: | |
| Title: |
I-2
Table of Contents
PROXY CARD
| 1. | Approval of the proposed issuance of common stock and warrants to purchase common stock to certain investors pursuant to the Securities Purchase Agreement, dated as of October 6, 2005. | ||
| FOR o AGAINST o ABSTAIN o | |||
| 2. | Approval of the proposed amendment of our certificate of incorporation to increase the authorized number of shares of our common stock by 50,000,000. | ||
| FOR o AGAINST o ABSTAIN o | |||
| 3. | Approval of the proposed amendment to the La Jolla Pharmaceutical Company 2004 Equity Incentive Plan (i) to increase the number of shares of common stock that may be issued under the plan by 16,000,000, (ii) to increase the number of shares of common stock that may be issued under the plan to any eligible person in any calendar year by 6,000,000 and (iii) to eliminate the minimum restriction period requirement with respect to restricted stock granted under the plan. | ||
| FOR o AGAINST o ABSTAIN o | |||
| 4. | Approval of the proposed decrease in the number of issued and outstanding shares of common stock by means of a one-for-five reverse stock split. | ||
| FOR o AGAINST o ABSTAIN o | |||
| 5. | In their discretion, the proxies are authorized to consider and vote upon such other business as may properly come before the special meeting or any adjournment or postponement thereof. |
Table of Contents
| Dated , 2005 | ||||
| Signatures(s) of stockholder |