Exhibit 99.1

Developing Bacteriophage Therapies for Patients with Antibiotic - Resistant Infections June 6, 2017 NYSE MKT: APHB

2 This presentation contains “forward - looking” statements that involve risks, uncertainties and assumptions. If the risks or uncer tainties materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such for war d - looking statements. All statements other than statements of historical fact could be deemed forward - looking, including, but not limited to: the potential future of antibiotic resistance; the expected timing of additional clinical trials, including Phase I or II clinica l t rials; the drug product candidates to be supplied by AmpliPhi for clinical trials; planned business strategies, including our personalized pr eci sion phage (P 3 ) therapy strategy and the expected benefits thereof; bacteriophage technology being uniquely positioned to address the globa l t hreat of antibiotic resistance; our expectations with respect to the $1.8 million Australian tax rebate; the protection of intellec tua l property; the activities to be performed by specific parties in connection with clinical trials; the potential use of bacteriophages to tre at bacterial infections; timing for manufacturing scale - up and the purification and formulation of bacteriophages; research and development p lans; the development of bacteriophage - based therapies; the ability to select combinations of phages to formulate product candidates; the ability to manufacture product candidates; the safety and efficacy of product candidates; potential and expected financing ar ran gements; collaborations with third parties and the potential markets for product candidates; potential market growth; the development of personalized precision phage therapies and planned approach for administering such therapies; the potential benefits of perso nal ized precision phage therapies; and any statements of assumptions underlying any of the items mentioned. These statements are base d o n estimates and information available to us at the time of this presentation and are not guarantees of future performance. Actu al results could differ materially from our current expectations. You should not rely upon forward - looking statements as predictions of fut ure events. Although we believe that the expectations reflected in the forward - looking statements are reasonable, we cannot guarante e that the future results, levels of activity, performance or events and circumstances reflected in the forward - looking statements will be achieved or occur. Moreover, we undertake no obligation to update publicly any forward - looking statements for any reason to conform these statements to actual results or to changes in our expectations except as required by law. We refer you to the documents that we file from time to time with the Securities and Exchange Commission (the “SEC”), specifi cal ly our Annual Report on Form 10 - K and our Quarterly Report on Form 10 - Q filed with the SEC. These documents, including the sections the rein entitled “Risk Factors,” identify important factors that could cause the actual results to differ materially from those conta ine d in forward - looking statements. Safe Harbor Statement
3 Antibiotic resistance is a major global health threat AmpliPhi is developing therapies to treat multidrug - resistant (MDR) infections • Bacteriophages can precisely target bacteria, have a differentiated mechanism of action and are potentially synergistic with antibiotics • 10+ years of bacteriophage R&D, with $60M+ invested • Wholly owned cGMP manufacturing facility dedicated to phage production AB - SA01 targeting S. aureus positioned for Phase 2 in chronic rhinosinusitis • Two Phase 1 studies completed (chronic rhinosinusitis and topical) • Positive feedback from Type B meeting with FDA in Q1 2017 AB - PA01 targeting P. aeruginosa in preclinical for cystic fibrosis (CF) • Positive feedback received from UK Medicines and Healthcare products Regulatory Agency (MHRA) New strategy expected to provide clinical evidence by treating patients with serious or life - threatening infections under compassionate use in 2017 AmpliPhi Biosciences Overview

4 “ The world is headed for a post - antibiotic era, in which common infections and minor injuries which have been treatable for decades can once again kill .” Dr. Keiji Fukuda, WHO’s Assistant Director - General for Health Security, 2014

Year 2050 Looming Era of Drug - Resistant Superbugs 5 Est. $100 trillion worldwide cumulative cost Imagine the world without effective antibiotics - if we fail to act, this could become our reality Est. 10,000,000 annual deaths worldwide Source: 2016 O’Neill Report to UK Government
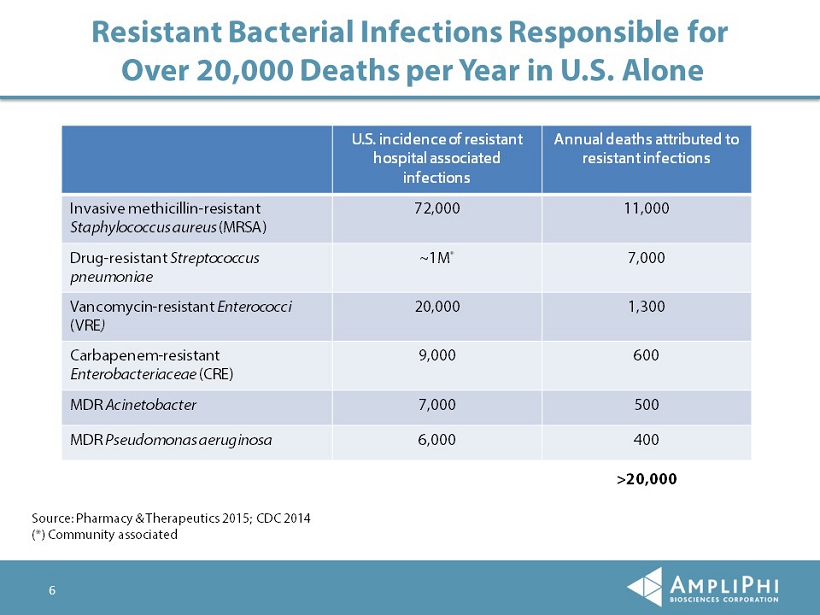
6 Resistant Bacterial Infections Responsible for Over 20,000 Deaths per Year in U.S. Alone Source: Pharmacy & Therapeutics 2015; CDC 2014 (*) Community associated U.S. incidence of resistant hospital associated infections Annual deaths attributed to resistant infections Invasive methicillin - resistant Staphylococcus aureus (MRSA) 72,000 11,000 Drug - resistant Streptococcus pneumoniae ~1M * 7,000 Vancomycin - resistant Enterococci (VRE ) 20,000 1,300 Carbapenem - resistant Enterobacteriaceae (CRE) 9,000 600 MDR Acinetobacter 7,000 500 MDR Pseudomonas aeruginosa 6,000 400 >20,000
The Story of Mr. P: Patient with Deadly Infection • 68 - year - old male • Contracted abdominal MDR Acinetobacter baumannii infection while traveling • Received multiple courses of antibiotics over 4 - month period • Vancomycin, meropenem , colistin , tigecycline , azithromycin, and rifampin • Critically ill; in a coma for several weeks 7

The Story of Mr. P: Preparing Personalized Phage Therapy • Multi - disciplinary team including UCSD, Texas A&M, SDSU, U.S. Navy, and AmpliPhi • Bacterial isolates screened against phage library • Personalized phage formulations provided within 10 days • Phage administered IP and IV, under Emergency IND allowed by FDA 8

The Story of Mr. P: Successful Outcome • Patient emerged from coma 4 days after initial phage administration • 5 days after initial phage dose, bacteria became sensitive to antibiotics • Continued phage administration (for selective pressure) and antibiotics • Patient experienced durable microbiological cure • Released from the hospital and doing well 9

Presentation at Scientific Conference “Intravenous applications of phage therapy to treat a terminally ill patient who was infected with a multidrug - resistant A. baumannii ” by Dr. Biswajit Biswas of the U.S. Navy’s Medical Research Center - Fredrick at Centennial Celebration of Bacteriophage Research Institut Pasteur, Paris April 26, 2017 10

The Power of Bacteriophages as Targeted Antibacterials Harnessing ancient predator - prey relationship to kill bacteria • Naturally occurring viruses • infect and kill only specific bacterial strains • penetrate biofilms Phages are the most abundant and diverse organisms on Earth • Humans evolved with phage in and on us • There are a variety of phage types capable of infecting and killing most, if not all, strains of bacteria Discovered in 1915 and used broadly in U.S. and Europe prior to development of antibiotics • Efficacy of approach has been broadly, yet anecdotally, demonstrated • Modern technology provides solutions to the early challenges of phage purification and characterization 11
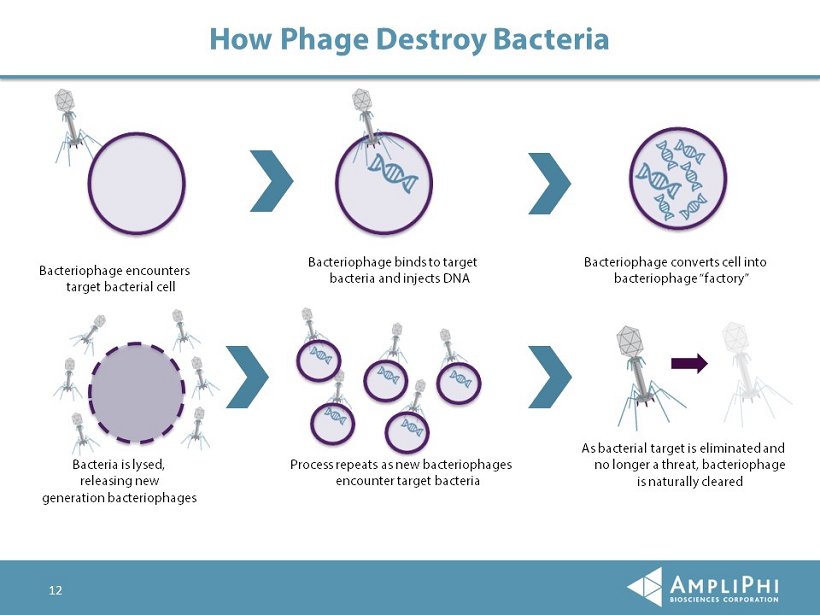
How Phage Destroy Bacteria Bacteriophage encounters target bacterial cell Bacteriophage binds to target bacteria and injects DNA Bacteriophage converts cell into bacteriophage “factory” Bacteria is lysed, releasing new generation bacteriophages Process repeats as new bacteriophages encounter target bacteria As bacterial target is eliminated and no longer a threat, bacteriophage is naturally cleared 12

13 Patent protection ▪ Protection for sequential administration of phage and antibiotics ▪ Prosecuting both biofilm - dependent and independent induced sensitivity ▪ Broad species coverage in EU, Australia, Japan, Canada; U.S. protection for Pseudomonas Phages can exert selection pressure on MDR bacteria to induce sensitivity to antibiotics ▪ Bacteria have limited degrees of freedom to mutate around therapies ▪ Phage attack causes evolutionary trade - off in bacteria, restoring antibiotic sensitivity ▪ E.g., efflux pump* ▪ Recent publications demonstrate POC Phage Therapy Has Potential to Reinvigorate Antibiotic Efficacy (*) Chan et al. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa . Scientific Reports 2016

Development Pipeline Indication Collaborator Discovery Preclinical Phase 1 Phase 2 AB - SA01 (3 - Phage cocktail targeting Staphylococcus aureus ) Chronic Rhinosinusitis (CRS) Seek partner for Phase 2 Topical indications / wound care AB - PA01 (4 - Phage cocktail targeting Pseudomonas aeruginosa ) Cystic Fibrosis (CF) Seek partner or foundation funding CRS Otitis Seek partner 14
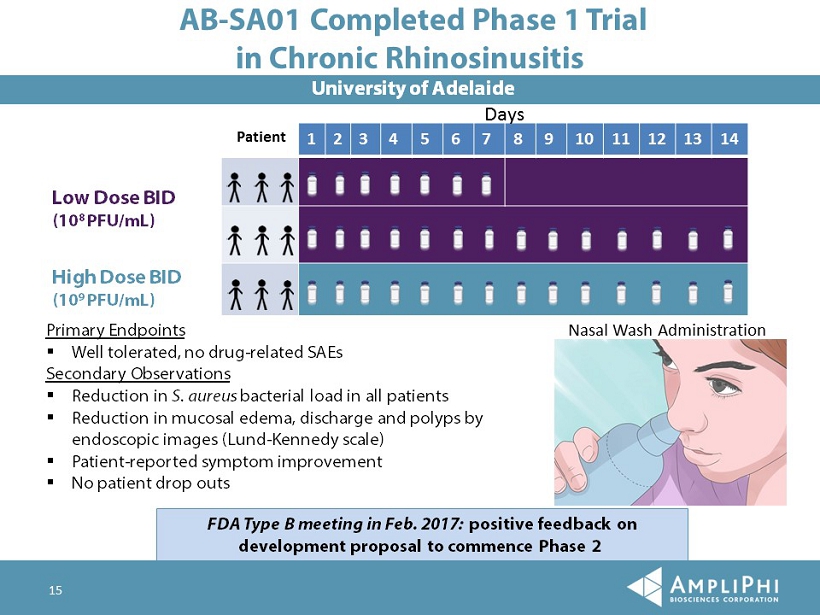
15 University of Adelaide Patient 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Days AB - SA01 Completed Phase 1 Trial in Chronic Rhinosinusitis Low Dose BID (10 8 PFU/mL) High Dose BID (10 9 PFU/mL) Primary Endpoints ▪ Well tolerated, no drug - related SAEs Secondary Observations ▪ Reduction in S. aureus bacterial load in all patients ▪ Reduction in mucosal edema, discharge and polyps by endoscopic images (Lund - Kennedy scale) ▪ Patient - reported symptom improvement ▪ No patient drop outs Nasal Wash Administration FDA Type B meeting in Feb. 2017: positive feedback on development proposal to commence Phase 2

16 U.S. Army under CRADA Primary Endpoints (all subjects) • AB - SA01 well tolerated throughout the trial ▪ No treatment - emergent AEs considered definitely or probably related to AB - SA01 ▪ No severe or high - grade treatment - emergent AEs ▪ No discontinuation of treatment due to treatment - emergent AEs ▪ All laboratory values and vital signs within normal ranges Subject 1 2 3 Days Topically administered AB - SA01 and placebo to opposite forearms, covered with occlusive dressings Treatment for 3 consecutive days and monitored for 2 weeks following treatment ▪ Low Dose QD ( 10 8 PFU/mL) ▪ High Dose QD ( 10 9 PFU/mL) AB - SA01 Completed Topical Phase 1 Trial

Positive FDA Feedback at Type B Meeting in February 2017 FDA expressed support for precision phage therapy for patients with serious or life - threatening infections • “CBER acknowledged that phage therapy is an exciting approach to treatment of multidrug - resistant organisms and expressed a commitment to addressing the unique regulatory challenges that might arise during product development.” Data from compassionate - use cases could inform approval pathway • “CBER stated that the clinical safety and effectiveness data collected during development, including from emergency case studies, could inform future discussions for clinical development and ultimately, the regulatory pathway to approval.” FDA is open for continued discussion • FDA suggested various potential next steps, e.g., Master File, Type C Meeting, etc. 17
• Provide personalized precisely targeted phage therapies for patients with antibiotic - resistant infections under compassionate use • Serve patients who have few or no other therapeutic options • Demonstrate real - world clinical evidence • Optimize treatment regimens and expand therapeutic phage libraries • Support path to commercial approval based on real - world evidence 18 Personalized Precision Therapies for MDR Infections
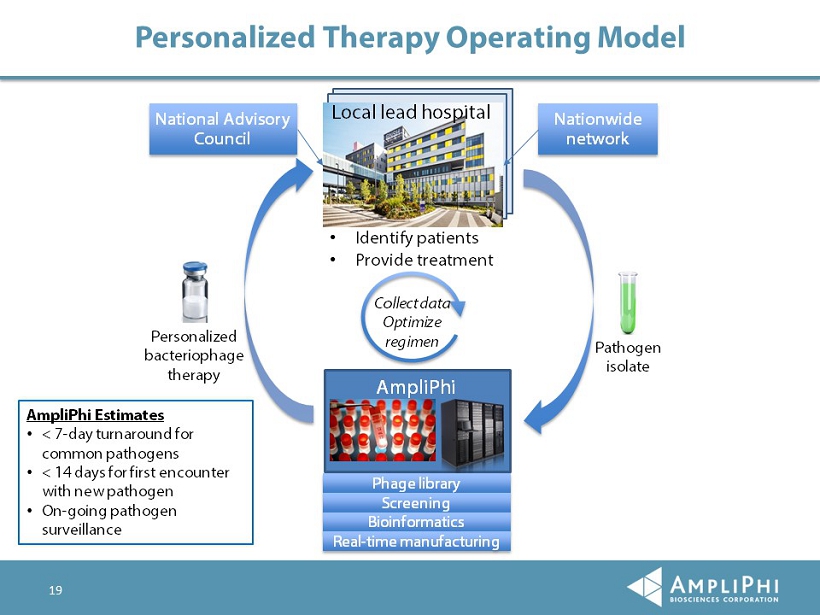
19 Personalized Therapy Operating Model Nationwide network • Identify patients • Provide treatment Collect data Optimize regimen AmpliPhi Estimates • < 7 - day turnaround for common pathogens • < 14 days for first encounter with new pathogen • On - going pathogen surveillance National Advisory Council Pathogen isolate Personalized bacteriophage therapy AmpliPhi Local lead hospital Phage library Screening Bioinformatics Real - time manufacturing

20 • Seek partners and grant funding for AB - PA01 CF and AB - SA01 CRS • Focus on compassionate - use strategy to demonstrate clinical efficacy • Treat at least 10 patients in 2017, suffering from high unmet need, serious or life - threatening MDR infections (e.g., endocarditis, PJI, CF, cUTI ) • Start with AB - SA01 and AB - PA01 • Based on compassionate - use data, define clinical indications for pivotal development of AB - SA01 and AB - PA01 • Present data to FDA and define studies required for registration • Initiate Phase 2/registrational studies potentially as early as mid - 2018 Regulatory Strategy: Leverage Compassionate - use Clinical Data to Inform Path to Approval

21 • In May 2017, the Company completed an underwritten offering of common stock and warrants for approximately $9.1M of net proceeds • After considering the recent fundraise, the Company expects existing cash resources to be sufficient to fund operations through mid - 2018 • In addition, the Company filed for $1.8M in tax rebates from the Australian Government; expect receipt in mid - 2017 subject to Australian tax authorities review • 8.7M common shares outstanding and 17.3M fully diluted as of June 1, 2017 * • Historically, partnerships have helped support development Funding and Capitalization *Share amounts outstanding include common stock only. Fully diluted includes outstanding warrants and stock options. Fully di lut ed amount does not include shares issuable under the 2016 Equity Incentive Plan or the ESPP Plan or the shares that are potentia lly issuable pursuant to the Common Stock Issuance Agreement. See the most recent Quarterly Report on Form 10 - Q as filed with the SEC.
Management Paul Grint, M.D. Chief Executive Officer Igor Bilinsky, Ph.D. Chief Operating Officer Steve Martin Chief Financial Officer Alex Gaidamaka, Ph.D., D.V.M. VP CMC Sandra Morales, Ph.D. VP Research Carrie Langlais Furr , Ph.D. VP Regulatory & Project Management 22 Board of Directors Jeremy Curnock Cook, Chair Louis Drapeau Wendy Johnson Mike Perry, D.V.M., Ph.D . Vijay Samant Paul Grint, M.D. ~25 in R&D and Manufacturing Team

23 Antibiotic resistance is a major global health threat AmpliPhi is developing therapies to treat MDR infections • Bacteriophages can precisely target bacteria, have a differentiated mechanism of action and are potentially synergistic with antibiotics • 10+ years of bacteriophage R&D, with $60M+ invested • Wholly owned cGMP manufacturing facility dedicated to phage production AB - SA01 targeting S. aureus positioned for Phase 2 in chronic rhinosinusitis • Two Phase 1 studies completed (chronic rhinosinusitis and topical) • Positive feedback from Type B meeting with FDA in Q1 2017 AB - PA01 targeting P. aeruginosa in preclinical for cystic fibrosis (CF) • Positive feedback received from UK Medicines and Healthcare products Regulatory Agency (MHRA) New capital - efficient strategy expected to demonstrate clinical evidence by treating patients with serious or life - threatening infections under compassionate use in 2017 Summary






















