Exhibit 99.1

Key Opinion Leader Event Phage - Based Therapeutics for the Treatment of Antimicrobial - Resistant Infections June 26, 2019 New York, NY

2 I This presentation contains “forward - looking” statements that involve risks, uncertainties and assumptions . If the risks or uncertainties materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward - looking statements . All statements other than statements of historical fact could be deemed forward - looking, including, but not limited to : the potential future of antibiotic resistance ; the ability for bacteriophage therapies to disrupt and destroy biofilms and restore sensitivity to antibiotics ; the expected benefits of the merger between AmpliPhi Biosciences Corporation and C 3 J Therapeutics, Inc . , and acquisition of a synthetic phage platform from Synthetic Genomics, Inc .; the planned development strategy, presenting data to regulatory agencies and defining planned clinical studies ; the expected timing of additional clinical trials, including Phase 1 b/Phase 2 or registrational clinical trials ; the drug product candidates to be supplied by Armata for clinical trials ; bacteriophage technology being uniquely positioned to address the global threat of antibiotic resistance ; the protection of intellectual property, including pending and issued patents, in applicable jurisdictions ; the activities to be performed by specific parties in connection with clinical trials or expanded access cases ; the potential use of bacteriophages to treat bacterial infections ; research and development plans ; the development of bacteriophage - based therapies ; the ability to select combinations of phages to formulate product candidates ; the ability to manufacture product candidates ; pursuit of additional indications ; the safety and efficacy of product candidates ; collaborations with third parties and the potential markets and market opportunities for product candidates ; potential market growth ; our partnership with Merck, known as MSD outside of the United States and Canada ; our ability to achieve our company’s vision, including improvements through engineering and success of clinical trials ; our ability to obtain financing on terms and in amounts that are acceptable to us ; our ability to meet anticipated milestones for 2019 and 2020 ; and any statements of assumptions underlying any of the items mentioned . These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future performance . Actual results could differ materially from our current expectations . You should not rely upon forward - looking statements as predictions of future events . Although we believe that the expectations reflected in the forward - looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward - looking statements will be achieved or occur . Moreover, we undertake no obligation to update publicly any forward - looking statements for any reason to conform these statements to actual results or to changes in our expectations except as required by law . We refer you to the documents that we file from time to time with the Securities and Exchange Commission (the “SEC”), including our Annual Report on Form 10 - K, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K . These documents, including the sections therein entitled “Risk Factors,” identify important factors that could cause the actual results to differ materially from those contained in forward - looking statements . Forward Looking Statement

Introduction Todd R. Patrick Chief Executive Officer

Bacteriophages – A Primer Magda Barbu, Ph.D. Senior Director, Synthetic Biology

5 I The most ubiquitous organisms on Earth Natural predators of bacteria – Bacteria - eater (“ phagein ” – to devour) Highly targeted Prior history as therapeutic agent – Antibiotics displaced phage use, drug - resistant threat revitalized phage use Bacteriophages Phage Micrograph Phage Plaques Source: M. Wurtzbiozentrum , University of Basel
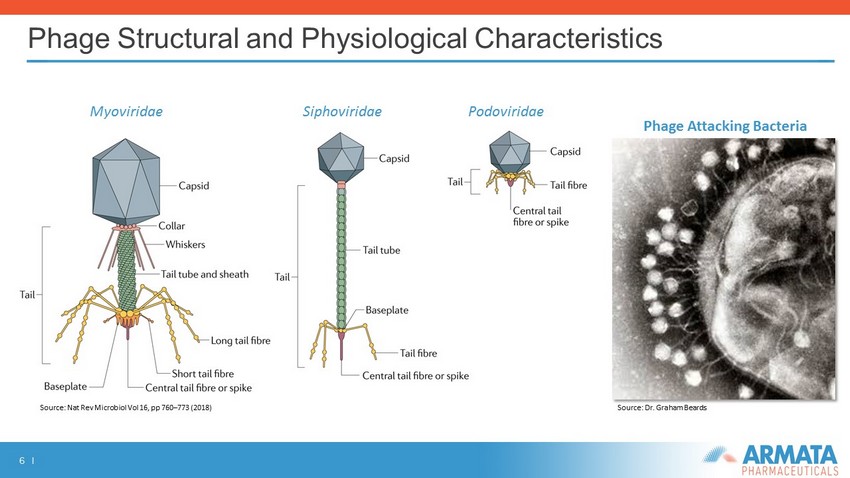
6 I Phage Structural and Physiological Characteristics Phage Attacking B acteria Myoviridae Siphoviridae Podoviridae Source: Nat Rev Microbiol Vol 16, pp 760 – 773 (2018 ) Source: Dr. Graham Beards
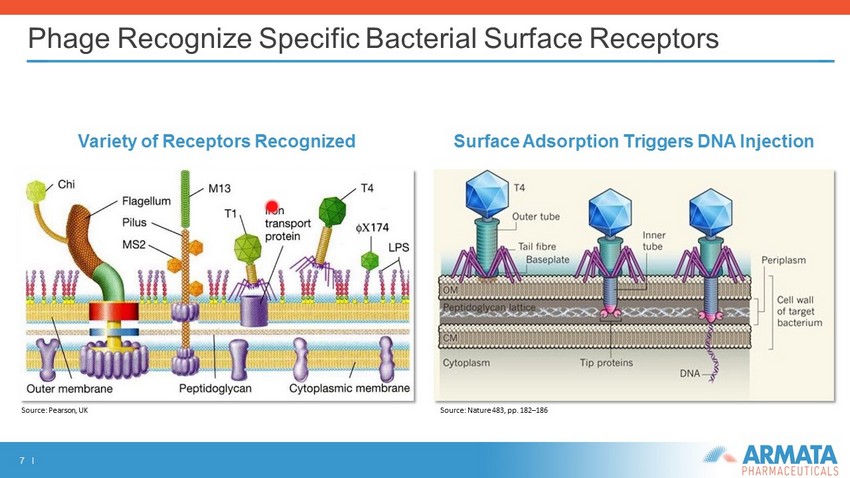
7 I Phage Recognize Specific Bacterial Surface Receptors Variety of Receptors Recognized Surface Adsorption T riggers DNA Injection Source: Pearson, UK Source: Nature 483, pp. 182 – 186
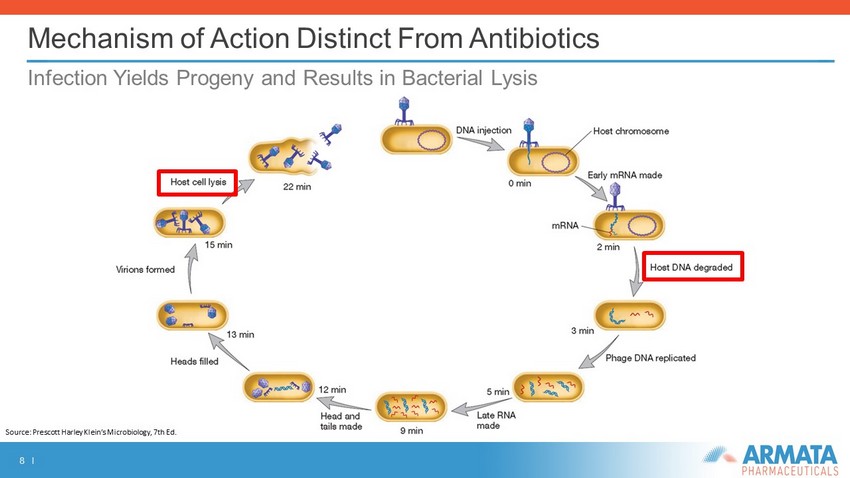
8 I Mechanism of Action Distinct From Antibiotics Infection Yields Progeny and Results in Bacterial Lysis Source: Prescott Harley Klein’s Microbiology, 7th Ed.
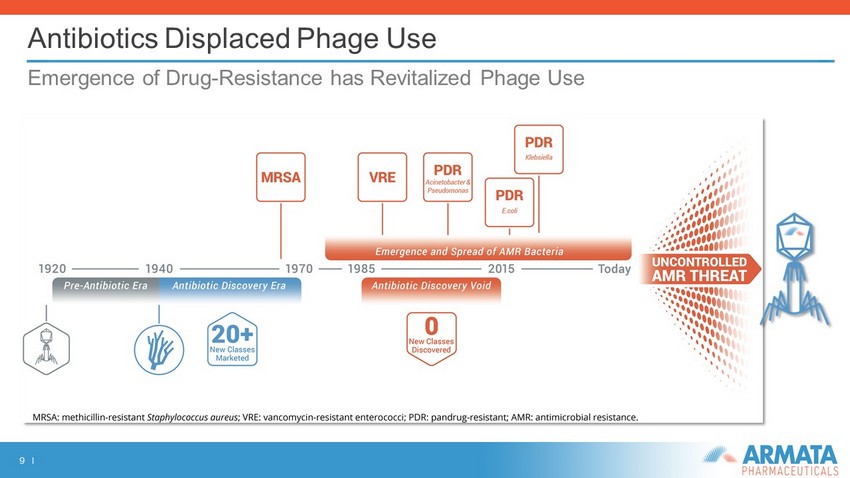
9 I Antibiotics Displaced Phage Use Emergence of Drug - Resistance has Revitalized Phage Use

10 I Deadly Infections Successfully Treated With Phage
11 I First Phage Therapy Center in US Signals Growing Acceptance

12 I Phage Therapy Now And In the Future Compelling Attributes of Phage • Life - changing outcomes in EIND cases – Sufficient proof to advance to formal clinical trials • Highly specific bactericidal agents – Minimal disruption of microbiome • Mechanism of action distinct from antibiotics • Replication at site of infection • Formulation versatility, treatment flexibility Improvements Through Engineering • Expanded host range • Improved biofilm disruption • Preventing emergence of resistance • Phage Dx to identify treatable patients • IP for best - in - class therapeutics Moving Phage Into Formal Trials and Towards Commercialization
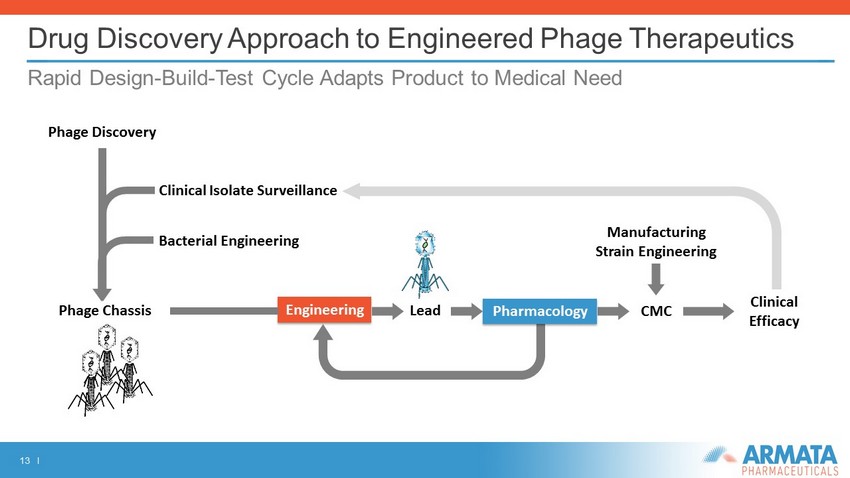
13 I Drug Discovery Approach to Engineered Phage Therapeutics Rapid Design - Build - Test Cycle Adapts Product to Medical Need Engineering Phage Chassis CMC Clinical Efficacy Phage Discovery Clinical Isolate Surveillance Pharmacology Lead Manufacturing Strain Engineering Bacterial Engineering

14 I Natural Synthetic 0 20 40 60 80 100 E r a d i c a t i o n ( % ) Synthetic Phage Product Candidate Improving Natural Phage Through Engineering Wider Host Range and Improved Growth Wide host range Synthetic Phage 7 68 Phage C Phage A Phage B 4 38 Phage Progenies Natural 70 Synthetic 122 Improved Biofilm Eradication Benefits Observed in an Animal Model 0 12 24 36 48 60 72 0 20 40 60 80 100 Hours M o u s e s u r v i v a l ( % ) Vehicle WT Phage B WHR Phage B Synthetic Natural
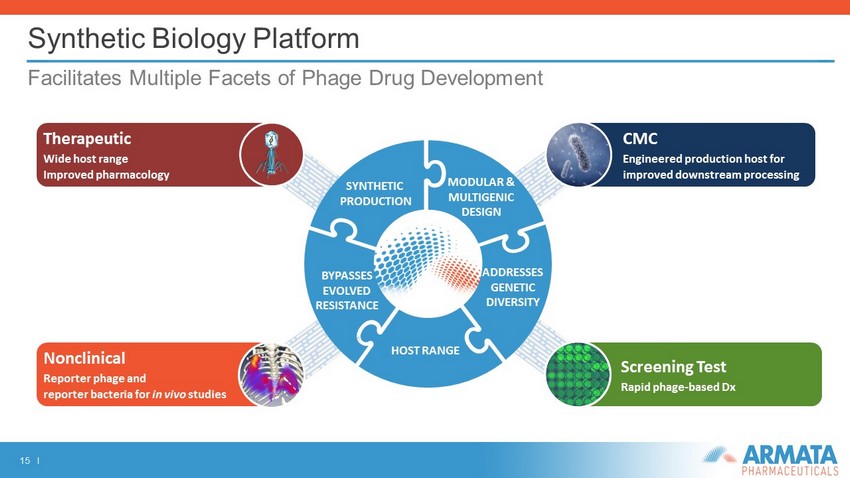
15 I Synthetic Biology Platform Facilitates Multiple Facets of Phage Drug Development Screening Test Rapid phage - based Dx Therapeutic Wide host range Improved pharmacology MODULAR & MULTIGENIC DESIGN BYPASSES EVOLVED RESISTANCE ADDRESSES GENETIC DIVERSITY HOST RANGE SYNTHETIC PRODUCTION CMC Engineered production host for improved downstream processing Nonclinical Reporter phage and reporter bacteria for in vivo studies

Bacteriophage Therapy for Serious Bacterial Infections: Why Now? Robert T. Schooley, MD Professor of Medicine University of California San Diego

17

Paper presented at French Academy of Sciences, Sept. 3, 1917 (translated in Res. Micro. 158 :2007) Courtesy of Ryland Young, Texas A&M University 18

Arrowsmith: Sinclair Lewis 19

advertisement for the Bacteriophage Laboratory, Paris, 1936 Courtesy of Ryland Young, Texas A&M University 20

The 1930s: A Fork in the Road Western Medicine Russia and Former Soviet Republics 21

1941: Second AMA report further discredits the “ d’Herelle ” phage theory 1934 Commissioned JAMA Report Courtesy of Ryland Young, Texas A&M University 22
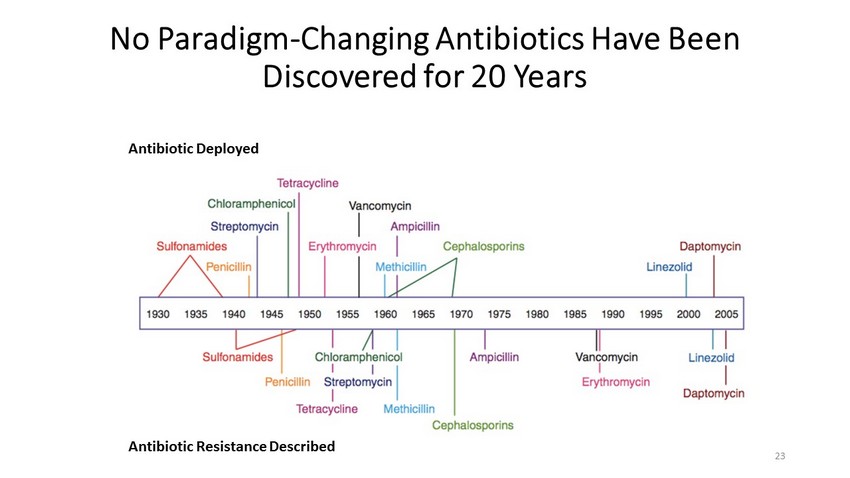
No Paradigm - Changing Antibiotics Have Been Discovered for 20 Years Antibiotic Resistance Described Antibiotic Deployed 23
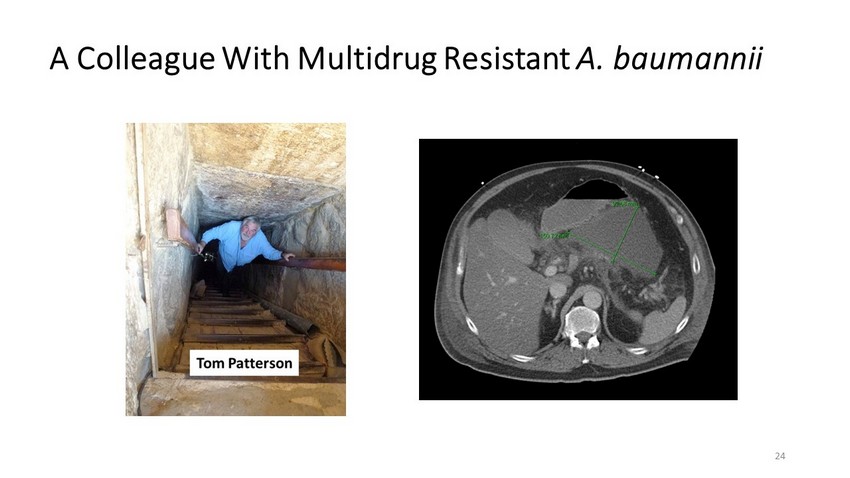
A Colleague With Multidrug Resistant A. baumannii 24

95 96 97 98 99 100 101 102 103 104 0 5000 10000 15000 20000 25000 30000 15-Jan 22-Jan 29-Jan 5-Feb 12-Feb 19-Feb 26-Feb 4-Mar 11-Mar T max (degrees F) White Blood Cell Count (per mm3) Date WBC Tmax B. thetaiotaomicron / Acinetobacter C. glabrata B. thetaiotaomicron Blood Peritoneal fluid 25

26
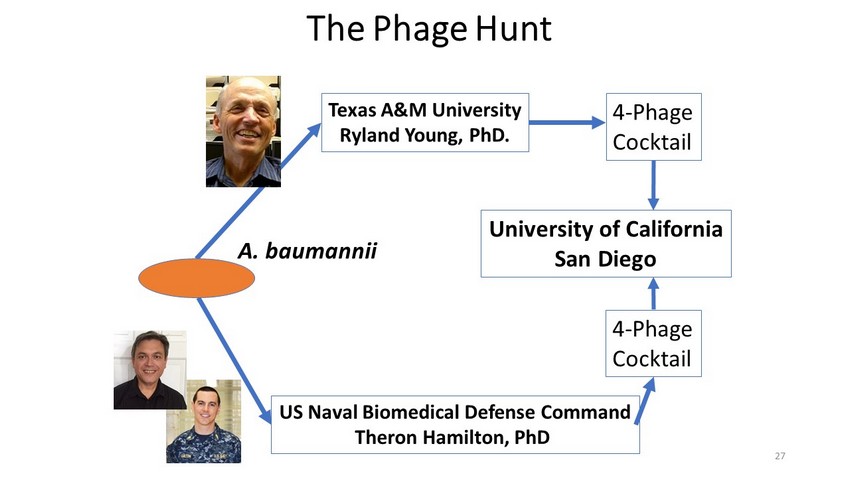
The Phage Hunt Texas A&M University Ryland Young, PhD. US Naval Biomedical Defense Command Theron Hamilton, PhD 4 - Phage Cocktail 4 - Phage Cocktail University of California San Diego A. baumannii 27
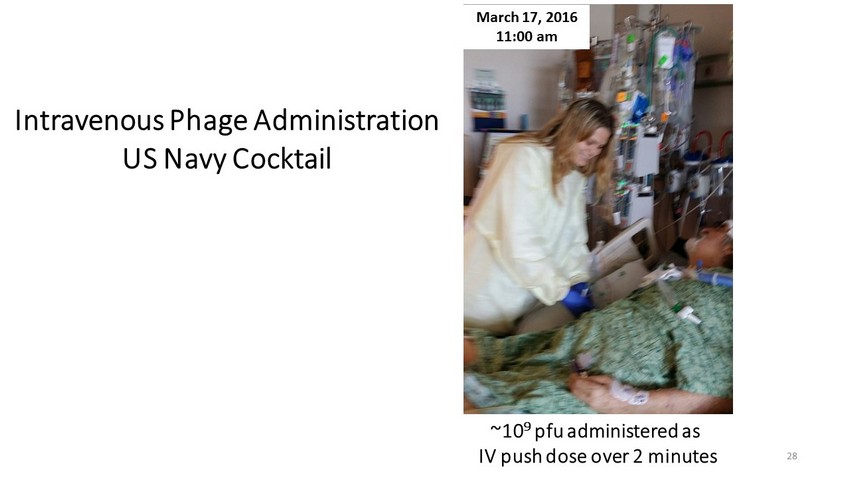
Intravenous Phage Administration US Navy Cocktail March 17, 2016 11:00 am ~10 9 pfu administered as IV push dose over 2 minutes 28

Thomas Patterson and Theron Hamilton, April 2018 29

30
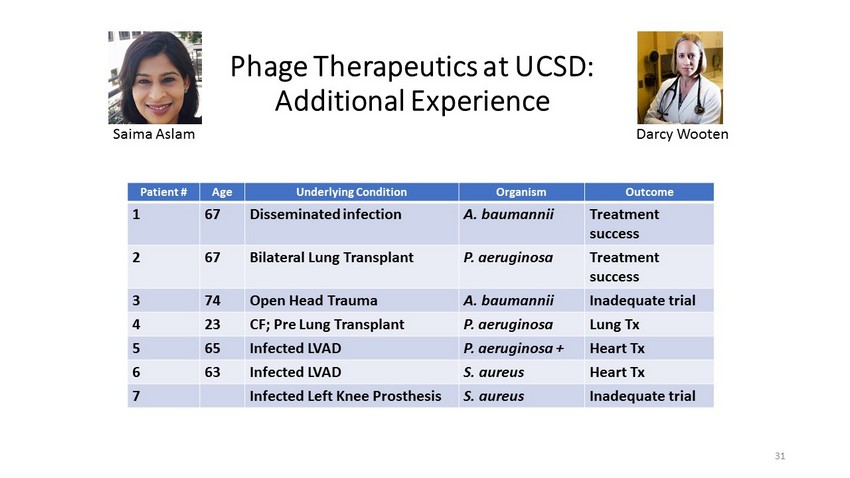
Phage Therapeutics at UCSD: Additional Experience Patient # Age Underlying Condition Organism Outcome 1 67 Disseminated infection A. baumannii Treatment success 2 67 Bilateral Lung Transplant P. aeruginosa Treatment success 3 74 Open Head Trauma A. baumannii Inadequate trial 4 23 CF; Pre Lung Transplant P. aeruginosa Lung Tx 5 65 Infected LVAD P. aeruginosa + Heart Tx 6 63 Infected LVAD S. aureus Heart Tx 7 Infected Left Knee Prosthesis S. aureus Inadequate trial Darcy Wooten Saima Aslam 31
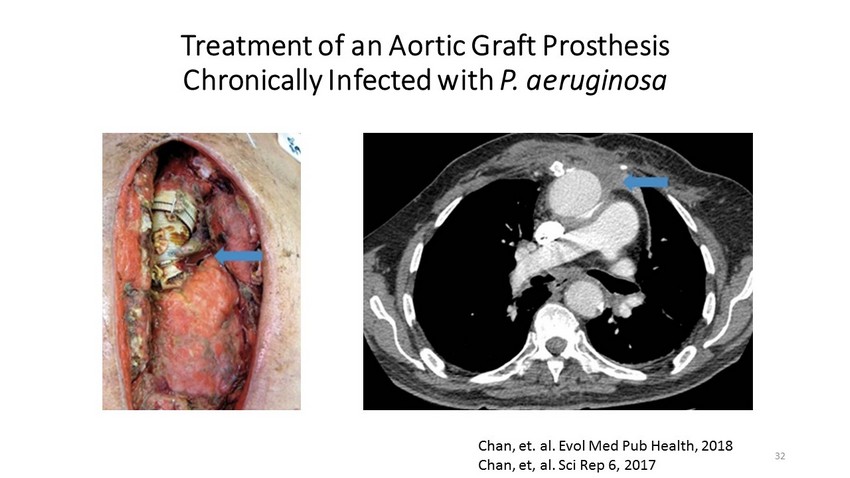
Treatment of an Aortic Graft Prosthesis Chronically Infected with P. aeruginosa Chan, et. al. Evol Med Pub Health, 2018 Chan, et, al. Sci Rep 6, 2017 32

ID Week Abstract 1642 33

Phage Therapy - 28 Day Outcomes Age Gender WMP0XX Indication(s) Duration of phage therapy Doses IV Clinical outcome at EoT Apache II 28 days mortality 65 M 001 PVE 14 days BID IV 28 success 18 No 47 M 003 PVE 3 days BID IV 6 failure 25 Yes, uncontrolled septic shock 43 F 004 IE (Right) 14 days BID IV 28 success 6 No 39 M 005 OM 14 days BID IV 28 success 3 No 87 M 006 IE 2 days BID IV 4 improvement 23 Yes, elected not to dialyse 89 M 007 OM 13 days BID IV 25 success 21 Yes, palliative care mode 36 F 008 IE (Right) 14 days BID IV 28 success 7 No 69 F 009 PVE 14 days BID IV 28 success 25 No 21 M 010 PVE 28 days BID IV (14 days pre - + post - surgery) 56 success 17 No 64 M 011 IE 14 days BID IV 4 Not susceptible, No assessment 25 Yes, complications of sepsis 71 M 012 OM 14 days BID IV 28 success 12 No 81 M 013 PVE 14 days BID IV 28 success 17 Yes, palliative care mode 70 M 014 IE 14 days BID IV 28 success TBC Well at d26 - All Staph patients met SIRS/SOFA sepsis criteria; all bacteraemic >2 consecutive days - 3 patients had bacteriophage x 3 days or less – 8 of remaining 10 did well - Left IE n=8 3/5 prosthetic IE survived to day 90; 2 without surgery 34

35

Phage Hunt Science Education Alliance - Phages Hunters Advancing Genomics and Evolutionary Science 36
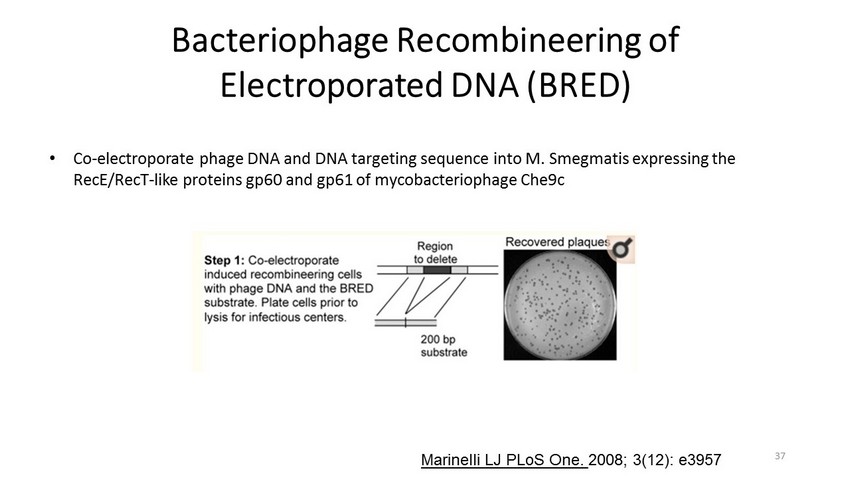
Bacteriophage Recombineering of Electroporated DNA (BRED) • Co - electroporate phage DNA and DNA targeting sequence into M. Smegmatis expressing the RecE / RecT - like proteins gp60 and gp61 of mycobacteriophage Che9c Marinelli LJ PLoS One . 2008; 3(12): e3957 37
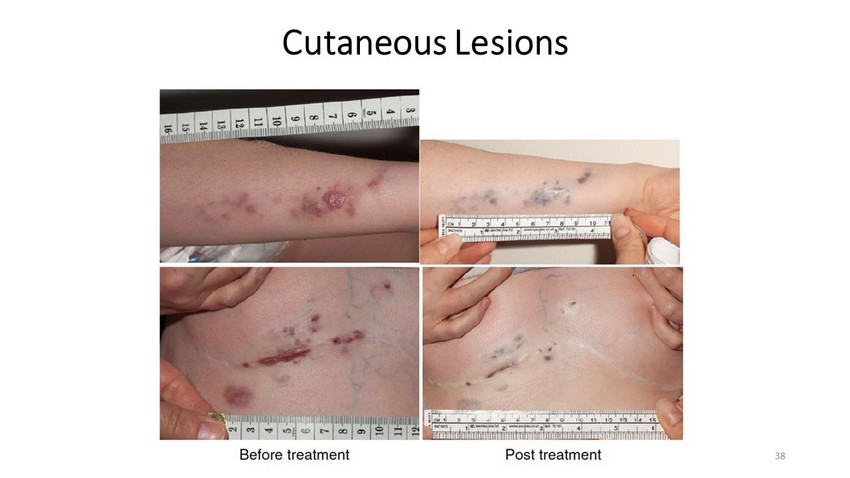
Cutaneous Lesions 38

Contemporary Phage Therapy: Lessons Learned from EIND Experiences • Safety – With current technologies there are no significant concerns regarding the safety of parenteral administration – Regulatory agencies are quite comfortable with safety profiles including the administration of genetically altered phages • Efficacy – Clinical outcomes have generally been favorable – Antimicrobial activity has been demonstrated by in vivo resistance selection – Phage resistant mutants are often substantially less fit – Additive or synergistic activity with antibiotics demonstrated • Pharmacology – Systemic distribution is well documented with intravenous administration 39

Where Do We Go From Here? Execute a series of rigorously designed prospective clinical trials using traditional approaches that would be used to evaluate any new antimicrobial agent 40
Bacteriophage Therapy in 2019 What has Changed Since 1917? • Much better understanding of issues related to host range • Major improvements in phage characterization and selection technologies (currently phenotypic - > potentially genotypic later) • Much better technology for expanding and preparing phage at GMP manufacturing levels of production • Better understanding of phage PK/PD with parenteral therapy • Real potential for exploiting phage - antibiotic synergy • Major possibilities with engineered phage in the future 41
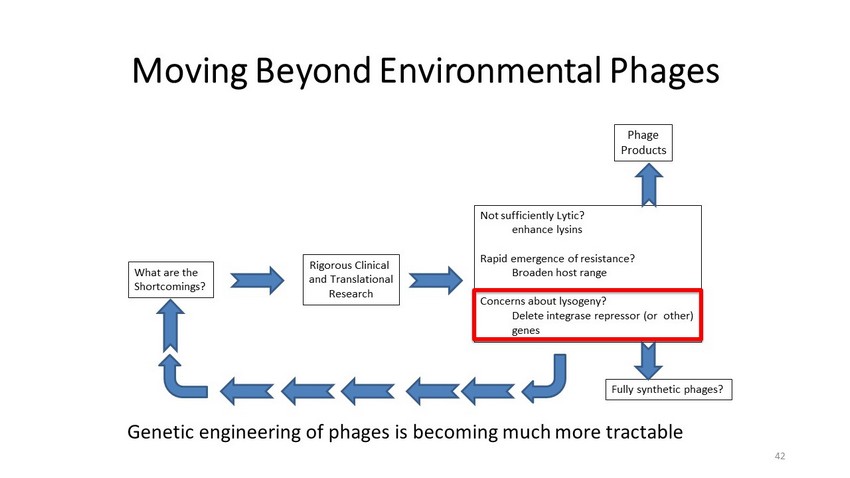
Moving Beyond Environmental Phages Fully synthetic phages? Rigorous Clinical and Translational Research What are the Shortcomings? Not sufficiently Lytic? enhance lysins Rapid emergence of resistance? Broaden host range Concerns about lysogeny? Delete integrase repressor (or other) genes Genetic engineering of phages is becoming much more tractable Phage Products 42

43

R&D Update Brian Varnum, Ph.D. President and Chief Development Officer

45 I Pipeline Multiple Opportunities for Value Creation Pathogen Indications Discovery Preclinical Phase 1 Phase 2 Staphylococcus aureus Bacteremia Pseudomonas aeruginosa Respiratory Undisclosed Undisclosed Partnered AP - SA01 Phage libraries to address market expansion and new indications
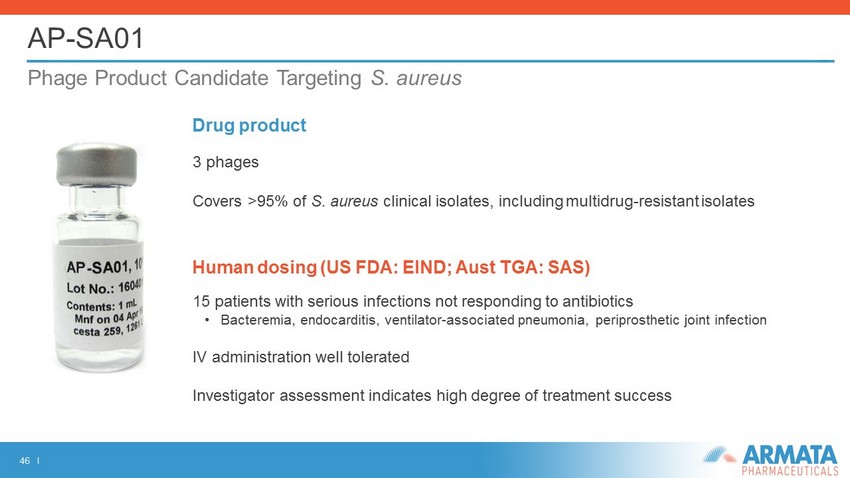
46 I AP - SA01 Phage Product Candidate Targeting S. aureus Drug product 3 phages Covers >95% of S. aureus clinical isolates, including multidrug - resistant isolates Human dosing (US FDA: EIND; Aust TGA: SAS) 15 patients with serious infections not responding to antibiotics • Bacteremia, endocarditis, ventilator - associated pneumonia, periprosthetic joint infection IV administration well tolerated Investigator assessment indicates high degree of treatment success
47 I Advanced Through Pre - IND Meeting • Highlighted prior human exposure under expanded access programs in U.S. and Australia • General agreement on proposed trial designs (Phase 1/2) • Nonclinical data not required; in line with FDA’s flexible approach to non - traditional antimicrobials Phase 1/2 Bacteremia Study • KOLs engaged; study protocol being finalized • CRO bidding initiated • Non - dilutive financing opportunity advancing AP - SA01: Regulatory and Clinical Status Source: Twitter Sept. 16, 2018
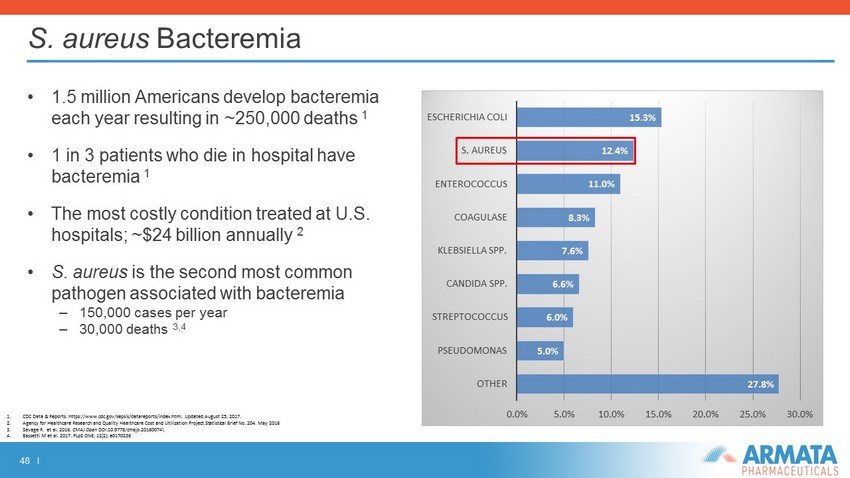
48 I S. aureus Bacteremia • 1.5 million Americans develop bacteremia each year resulting in ~250,000 deaths 1 • 1 in 3 patients who die in hospital have bacteremia 1 • The most costly condition treated at U.S. hospitals; ~$24 billion annually 2 • S. aureus is the second most common pathogen associated with bacteremia – 150,000 cases per year – 30,000 deaths 3,4 1. CDC Data & Reports. https://www.cdc.gov/sepsis/datareports/index.html. Updated August 25, 2017. 2. Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project Statistical Brief No. 204. May 2016 3. Savage R. et al. 2016. CMAJ Open DOI:10.9778/cmajo.20160074 \ 4. Bassetti M et al. 2017. PLoS ONE; 12(2): e0170236 27.8% 5.0% 6.0% 6.6% 7.6% 8.3% 11.0% 12.4% 15.3% 0.0% 5.0% 10.0% 15.0% 20.0% 25.0% 30.0% OTHER PSEUDOMONAS STREPTOCOCCUS CANDIDA SPP. KLEBSIELLA SPP. COAGULASE ENTEROCOCCUS S. AUREUS ESCHERICHIA COLI
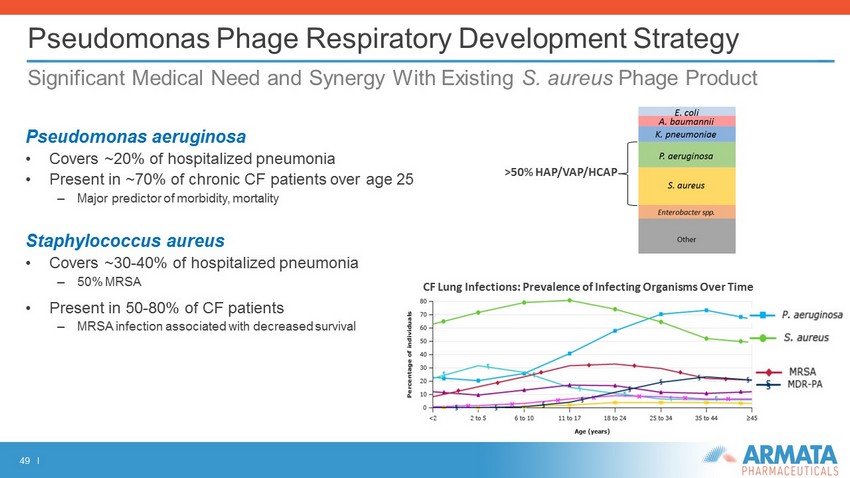
49 I Pseudomonas Phage Respiratory Development Strategy Significant Medical Need and Synergy With Existing S. aureus Phage Product Pseudomonas aeruginosa • Covers ~20% of hospitalized pneumonia • Present in ~70% of chronic CF patients over age 25 – Major predictor of morbidity, mortality Staphylococcus aureus • Covers ~30 - 40% of hospitalized pneumonia – 50% MRSA • Present in 50 - 80% of CF patients – MRSA infection associated with decreased survival >50% HAP/VAP/HCAP CF Lung Infections: Prevalence of Infecting Organisms Over Time
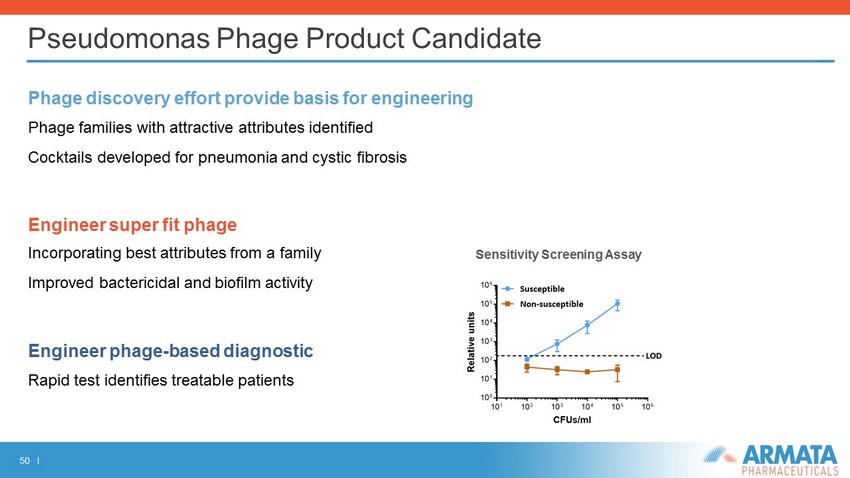
50 I Pseudomonas Phage Product Candidate Phage discovery effort provide basis for engineering Phage families with attractive attributes identified Cocktails developed for pneumonia and cystic fibrosis Engineer super fit phage Incorporating best attributes from a family Improved bactericidal and biofilm activity Engineer phage - based diagnostic Rapid test identifies treatable patients Sensitivity Screening Assay

51 I • Liquid formulation for first - in - human trial – Suitable for IV and inhalation • Successful manufacturing of first 2 Pseudomonas phage – GMP lots at intended Phase 1 scale Pseudomonas Phage Production and Formulation Utilizing Armata’s Proprietary Phage - Specific GMP Test Specification Phage 1 Phage 2 Potency (PFU/mL) ≥1 x 10 9 1.76 x 10 9 1.79 x 10 9 Endotoxin (EU/mL) ≤100 0.25 2.5 Sterility No growth No growth No growth

52 I AP - SA01 • Initiate Phase 1/2 clinical study in bacteremia Pseudomonas phage product candidate • Conduct Pre - IND meeting and file IND • Initiate first - in - human clinical study Anticipated Milestones 2019/2020

53 I Investment Highlights A World - Leader in Phage Therapeutics Lead candidate, AP - SA01, positioned to enter clinical development Natural phage discovery and synthetic biology yield robust pipeline Merck partnership to develop proprietary synthetic phage to target an undisclosed infectious disease agent Phage - specific GMP drug manufacturing facilities Strong Board and Executive leadership team Sound capital structure with $16 million cash at May 9 th merger Closing

Thank You!





















































