Exhibit 99.1

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above

Link to searchable text of slide shown above
Link to searchable text of slide shown above
Link to searchable text of slide shown above

Link to searchable text of slide shown above
Searchable text section of graphics shown above
[LOGO] | | Creating Nitric Oxide Enhancing Medicines |
[GRAPHIC]
JP Morgan Healthcare | | |
Conference | | January 12, 2004 |
| | |
Michael D. Loberg, Ph.D. | | |
President and CEO | | |
Forward Looking Statement | | [GRAPHIC] |
Any remarks that we may make about NitroMed’s future expectations, progress, plans, prospects, and target dates for research, development and clinical trial milestones, regulatory submissions, marketing approvals and product launches constitute forward looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including those discussed in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2003, which is on file with the SEC. The forward looking information provided by NitroMed represents our estimates as of today. Subsequent events may cause our estimates to change. We may elect to update our estimates at some point in the future, but specifically disclaim any obligation to do so.
[LOGO]
Leader in Nitric Oxide-based drug development
• Near-term commercial opportunity - BiDil®
• Active strategic collaborations – [LOGO] [LOGO]
• Transitioning to integrated pharmaceutical company
• Merck and NitroMed advanced lead NO-based COX-2 Inhibitor into Phase I testing;
$5MM milestone payment
• Boston Scientific and NitroMed extended NO drug coated stent agreement for 2 more years;
$3MM R&D payment
• BiDil® confirmatory clinical trial recruitment on schedule;
expected to conclude in Q3 2004.
[GRAPHIC]
BiDil®
(Isosorbide Dinitrate and Hydralazine)
NO-Enhancing Medicine
To Treat African Americans with Heart Failure
To reduce mortality and hospitalizations and improve quality-of-life in African Americans with heart failure
Attractive Commercial Potential
• Large, unmet medical need
• First-in-class Nitric Oxide Enhancing therapy
• Pricing flexibility
• Reached by focused sales force
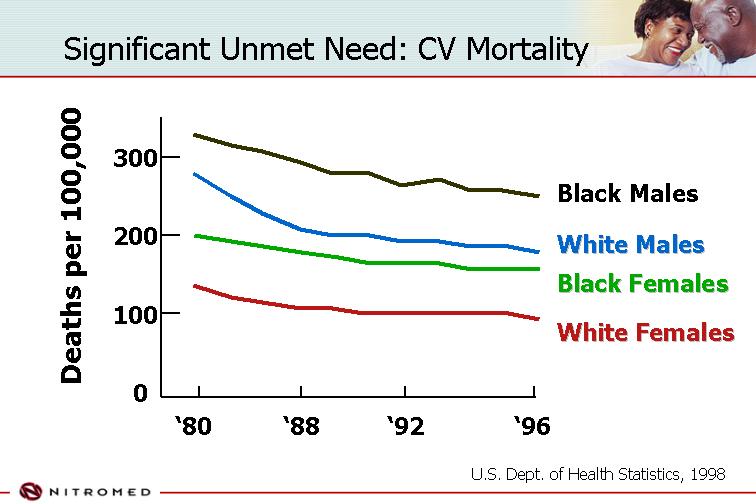
Significant Unmet Need: CV Mortality | | |
[CHART]
U.S. Dept. of Health Statistics, 1998
Heart Failure In African Americans | | |
• African Americans suffer disproportionately
• 18% of the disease; 11% of the population
• African Americans experience unique etiology
• younger age
• lower incidence of coronary artery disease
• higher incidence of hypertension
• Altered response to approved therapies
Yancy CW. J Card Fail. 2000;6:183-186.
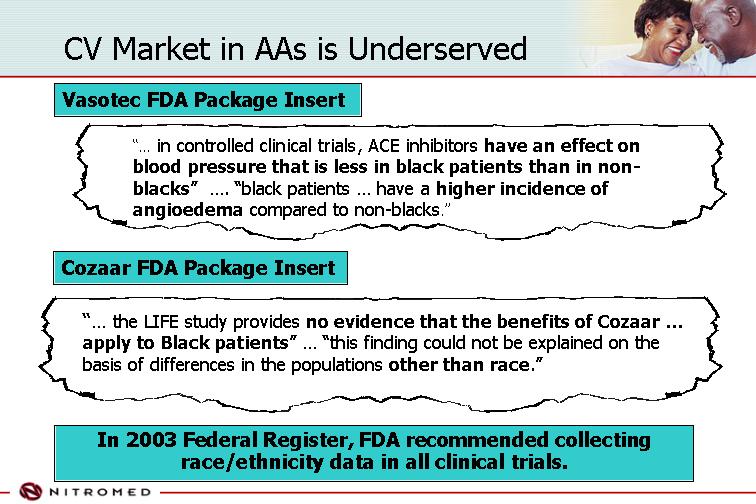
CV Market in AAs is Underserved | | |
Vasotec FDA Package Insert
“… in controlled clinical trials, ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks” …. “black patients … have a higher incidence of angioedema compared to non-blacks.”
Cozaar FDA Package Insert
“… the LIFE study provides no evidence that the benefits of Cozaar … apply to Black patients” … “this finding could not be explained on the basis of differences in the populations other than race.”
In 2003 Federal Register, FDA recommended collecting race/ethnicity data in all clinical trials.
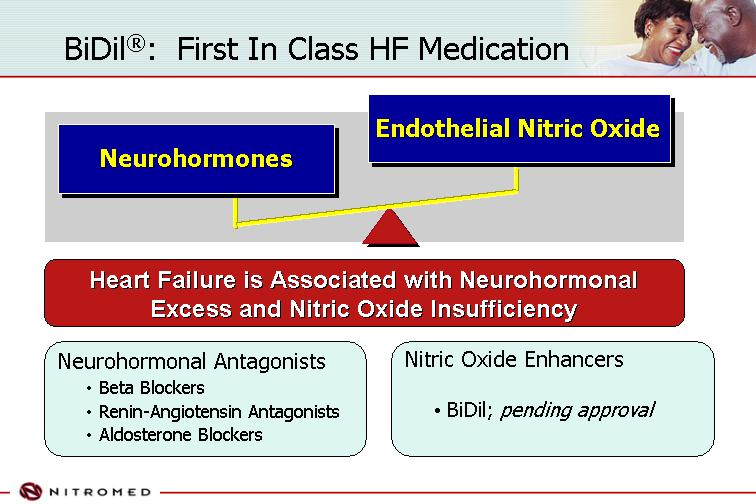
BiDil®: First In Class HF Medication
Neurohormones | Endothelial Nitric Oxide |
[GRAPHIC] |
Heart Failure is Associated with Neurohormonal Excess and Nitric Oxide Insufficiency
Neurohormonal Antagonists | Nitric Oxide Enhancers |
| • | | Beta Blockers | • | BiDil; pending approval |
| • | | Renin-Angiotensin Antagonists | | |
| • | | Aldosterone Blockers | | |
| | | | | | |
Attractive Commercial Potential
• U.S. heart failure market ~$50 billion annually
• BiDil market opportunity
~ 0.9 MM diagnosed AA heart failure patients at current Coreg pricing ($3.40/day)
~ $1.1 billion market opportunity
• Relatively easy to penetrate
• add-on therapy
• expected to be only drug indicated to improve outcomes in African Americans
• non-price sensitive
• reachable by <200 person sales force
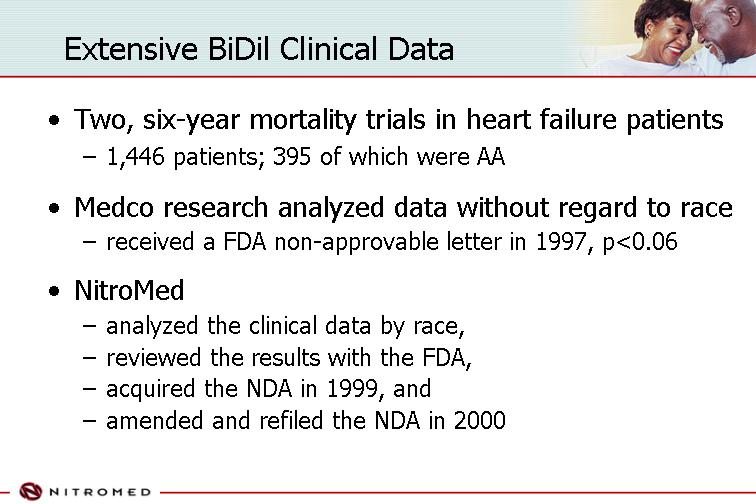
Extensive BiDil Clinical Data
• Two, six-year mortality trials in heart failure patients
• 1,446 patients; 395 of which were AA
• Medco research analyzed data without regard to race
• received a FDA non-approvable letter in 1997, p<0.06
• NitroMed
• analyzed the clinical data by race,
• reviewed the results with the FDA,
• acquired the NDA in 1999, and
• amended and refiled the NDA in 2000
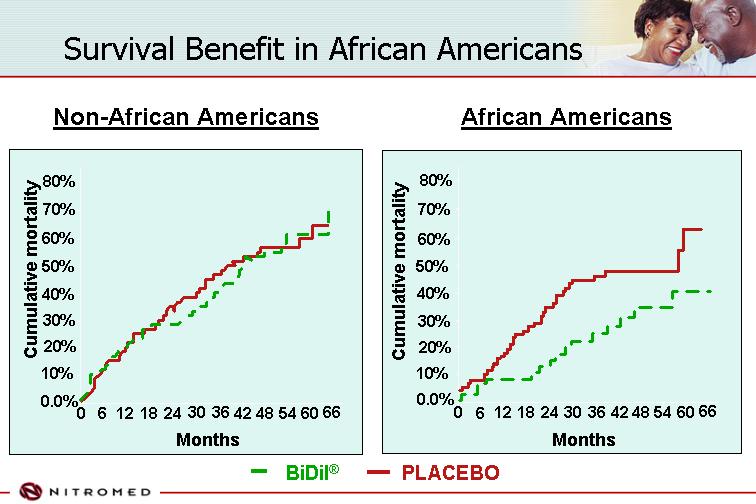
Survival Benefit in African Americans
Non-African Americans
[CHART]
African Americans
[CHART]
FDA Response
“… a single, clearly positive study in a black CHF population would be a basis for approval of BiDil® for the treatment of heart failure in blacks.”
“… if the results of the study are convincing this would provide sufficient clinical evidence to support approval of BiDil®.”
Robert Temple, M.D., Director Drug Evaluation I
A-HeFT
African-American Heart Failure Trial
A PLACEBO-CONTROLLED TRIAL OF BIDIL® ADDED TO STANDARD THERAPY IN AFRICAN-AMERICAN PATIENTS WITH HEART FAILURE
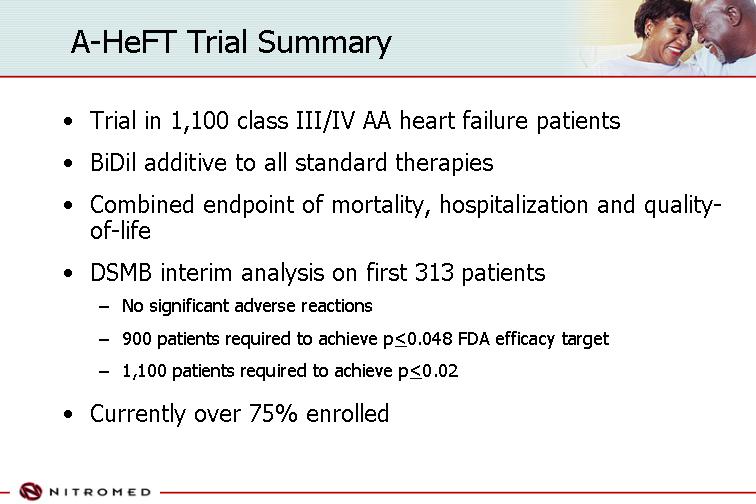
A-HeFT Trial Summary
• Trial in 1,100 class III/IV AA heart failure patients
• BiDil additive to all standard therapies
• Combined endpoint of mortality, hospitalization and quality-of-life
• DSMB interim analysis on first 313 patients
• No significant adverse reactions
• 900 patients required to achieve p<0.048 FDA efficacy target
• 1,100 patients required to achieve p<0.02
• Currently over 75% enrolled
BiDil® Coalition
[LOGO]
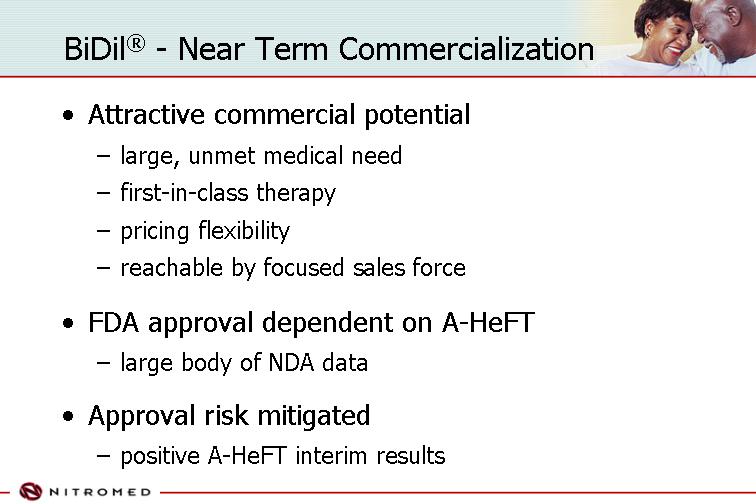
BiDil® - Near Term Commercialization
• Attractive commercial potential
• large, unmet medical need
• first-in-class therapy
• pricing flexibility
• reachable by focused sales force
• FDA approval dependent on A-HeFT
• large body of NDA data
• Approval risk mitigated
• positive A-HeFT interim results
[LOGO]
Our Pipeline
and
Strategic Collaborations
Making the best medicines better.
[LOGO] | | NO-based COX-2 Inhibitors |
• Exclusive WW research and licensing collaboration
• NO-Based COX-2 inhibitors
• improved GI and renal profiles
• extensive NitroMed IP
• $6 Billion market
• Lead candidate in Phase I clinical testing
• Two milestones and $23 million income in 2003
• Potential of up to $350 million in fees and milestones
• Traditional pharmaceutical royalties
• Exclusive research & development license agreement
• extended for additional 2 years in January 2004
• 3 products
• Nitric Oxide properties used to reduce restenosis
• antiplatelet, antiproliferative and promotes vascular re-endothelializaton
• $6.8 billion market in 2006
• Extensive NitroMed IP
• To date, $5.5 million in fees, R&D funding, and equity
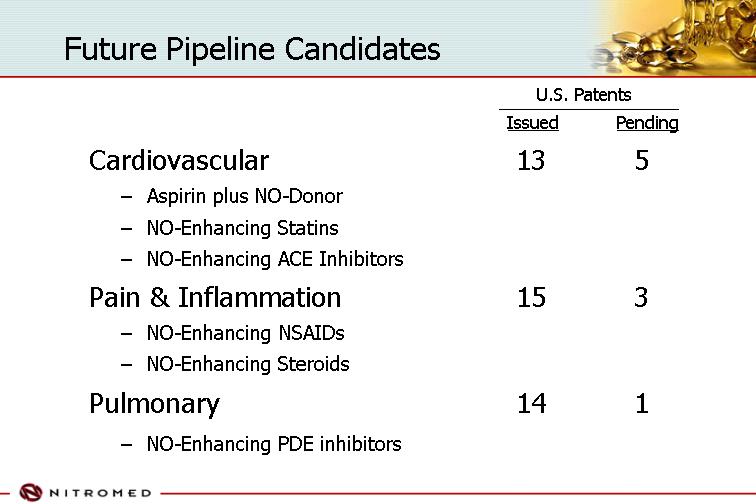
Future Pipeline Candidates
| | | | U.S. Patents | |
| | | | Issued | | Pending | |
Cardiovascular | | | 13 | | 5 | |
| • | Aspirin plus NO-Donor | | | | | |
| • | NO-Enhancing Statins | | | | | |
| • | NO-Enhancing ACE Inhibitors | | | | | |
| | | | | | | | |
Pain & Inflammation | | | 15 | | 3 | |
| • | NO-Enhancing NSAIDs | | | | | |
| • | NO-Enhancing Steroids | | | | | |
| | | | | | | | |
Pulmonary | | | 14 | | 1 | |
| • | NO-Enhancing PDE inhibitors | | | | | |
NitroMed Corporate Details
FINANCIAL GUIDANCE
• 2003 Year-End Financial Guidance
• total R&D Revenue of $12 to $13 MM
• total Operating Expenses of $21 to $24 MM
• Cash & Marketable Securities of $96 to $98 MM
• 2004 Year-End Financial Guidance
• total R&D Revenue of $8 to $10 MM
• total Operating Expenses of $28 to $33 MM
• Cash & Marketable Securities of $66 to $76 MM
Anticipated Upcoming Milestones
BiDil®
Interim Safety Assessment | | Q1 2004 | |
| | | |
Close of Recruitment | | Q3 2004 | |
| | | |
Clinical Results | | Q2 2005 | |
| | | |
NDA Supplement Filing | | Q3 2005 | |
| | | |
BiDil Launch | | Q1 2006 | |
*Assuming FDA approval
[LOGO]
Phase I Trial Initiated | | Q4 2003 | |
Product Development
Stent Trials | | ND | |
| | | |
New Pharma Deals | | ND | |
Company Highlights
• Late stage BiDil opportunity
• large, unsatisfied market
• reduced development risk
• all commercial rights retained
• Strong strategic collaborations [LOGO] [LOGO]
• large markets
• market leaders
• Inherently lower-risk business model
• leading patent position
• multiple pre-clinical programs
• Experienced management team
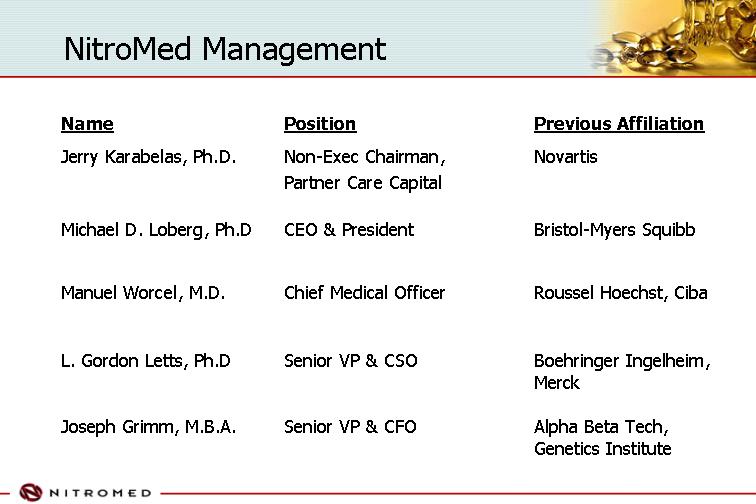
NitroMed Management
Name | | Position | | Previous Affiliation |
| | | | |
Jerry Karabelas, Ph.D. | | Non-Exec Chairman, Partner Care Capital | | Novartis |
| | | | |
Michael D. Loberg, Ph.D | | CEO & President | | Bristol-Myers Squibb |
| | | | |
Manuel Worcel, M.D. | | Chief Medical Officer | | Roussel Hoechst, Ciba |
| | | | |
L. Gordon Letts, Ph.D | | Senior VP & CSO | | Boehringer Ingelheim, Merck |
| | | | |
Joseph Grimm, M.B.A. | | Senior VP & CFO | | Alpha Beta Tech, Genetics Institute |
[LOGO]
Creating Nitric Oxide-Enhancing Medicines



























