- EXEL Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
DEF 14A Filing
Exelixis (EXEL) DEF 14ADefinitive proxy
Filed: 12 Apr 18, 12:00am
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the Securities
Exchange Act of 1934 (Amendment No. )
Filed by the Registrant☒
Filed by a Party other than the Registrant☐
Check the appropriate box:
☐ | Preliminary Proxy Statement | |
☐ | Confidential, for Use of the Commission Only (as permitted by Rule14a-6(e)(2)) | |
☒ | Definitive Proxy Statement | |
☐ | Definitive Additional Materials | |
☐ | Soliciting Material Pursuant to§240.14a-12 |

EXELIXIS, INC.
(Exact name of registrant as specified in its charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
☒ | No fee required. | |||||
☐ | Fee computed on table below per Exchange Act Rules14a-6(i)(1) and0-11. | |||||
| (1 | ) | Title of each class of securities to which transaction applies: | ||||
| (2 | ) | Aggregate number of securities to which transaction applies: | ||||
| (3 | ) | Per unit price or other underlying value of transaction computed pursuant to Exchange ActRule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): | ||||
| (4 | ) | Proposed maximum aggregate value of transaction: | ||||
| (5 | ) | Total fee paid: | ||||
☐ | Fee paid previously with preliminary materials. | |||||
☐ | | Check box if any part of the fee is offset as provided by Exchange Act Rule0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. | ||||
| (1 | ) | Amount Previously Paid: | ||||
| (2 | ) | Form, Schedule or Registration Statement No.: | ||||
| (3 | ) | Filing Party: | ||||
| (4 | ) | Date Filed: | ||||

210 East Grand Ave.
South San Francisco, CA 94080
NOTICEOF ANNUAL MEETINGOF STOCKHOLDERS
To be held on MAY 23, 2018
To the Stockholders of Exelixis, Inc.:
NOTICE IS HEREBY GIVEN that the Annual Meeting of Stockholders of Exelixis, Inc., a Delaware corporation (“Exelixis”), will be held on Wednesday, May 23, 2018, at 8:00 a.m., local time, at Exelixis’ offices located at 210 East Grand Avenue, South San Francisco, California 94080 for the following purposes:
| 1. | To elect the three Class I nominees for director named in the Proxy Statement accompanying this Notice to hold office until the 2021 Annual Meeting of Stockholders. |
| 2. | To ratify the selection by the Audit Committee of the Board of Directors of Ernst & Young LLP as Exelixis’ independent registered public accounting firm for the fiscal year ending December 28, 2018. |
| 3. | To approve, on an advisory basis, the compensation of Exelixis’ named executive officers, as disclosed in the Proxy Statement accompanying this Notice. |
| 4. | To conduct any other business properly brought before the meeting. |
These items of business are more fully described in the Proxy Statement accompanying this Notice.
We are mailing to most of our stockholders a Notice of Internet Availability of Proxy Materials (the “Notice”) instead of a paper copy of this Proxy Statement and our 2017 Annual Report. The Notice contains instructions on how to access those documents over the Internet. The Notice also contains instructions on how to request a paper copy of our proxy materials, including this Proxy Statement, our 2017 Annual Report and a form of proxy card or voting instruction card. All stockholders who do not receive a Notice will receive a paper copy of the proxy materials by mail. We believe that this process will allow us to provide our stockholders with the information they need in a more timely manner, while reducing the environmental impact and lowering the costs of printing and distributing our proxy materials.
The record date for the Annual Meeting is March 29, 2018. Only stockholders of record at the close of business on that date may vote at the meeting or any postponement or adjournment thereof.
Important notice regarding the availability of proxy materials for the Annual Meeting of Stockholders to be held on May 23, 2018, at 8:00 a.m., local time, at Exelixis’ offices located at 210 East Grand Avenue, South San Francisco, CA 94080.
The Proxy Statement and Annual Report to stockholders are available at www.exel-annualstockholdermeeting.com.
The Board of Directors recommends that you vote “FOR” Proposal Nos.1-3 identified above.
|
By Order of the Board of Directors

JEFFREY J. HESSEKIEL
Executive Vice President and General Counsel
South San Francisco, California
April 12, 2018
YOUR VOTE IS IMPORTANT
WHETHER OR NOT YOU PLAN TO ATTEND THE 2018 ANNUAL MEETING OF STOCKHOLDERS, TO ENSURE THAT YOU ARE REPRESENTED AT THE MEETING AND TO ENSURE THAT A QUORUM IS PRESENT, WE URGE YOU TO VOTE YOUR PROXY ONLINE, BY TELEPHONE OR BY RETURNING A PROXY CARD BY MAIL AS INSTRUCTED IN THE NOTICE OF AVAILABILITY OF PROXY MATERIALS. EVEN IF YOU HAVE VOTED BY PROXY, YOU MAY STILL VOTE IN PERSON IF YOU ATTEND THE ANNUAL MEETING. PLEASE NOTE, HOWEVER, THAT IF YOU HOLD YOUR SHARES THROUGH A BROKER, BANK OR OTHER NOMINEE, THEN THAT ENTITY IS THE STOCKHOLDER OF RECORD, AND YOU WILL NEED TO FOLLOW THE INSTRUCTIONS ON THE INSTRUCTION FORM THEY SEND TO YOU AND THEY WILL VOTE YOUR SHARES AS YOU DIRECT, OR YOU MUST OBTAIN A PROXY ISSUED IN YOUR NAME FROM THAT ENTITY TO VOTE YOUR SHARES.
Questions and Answers | Proxy Materials and Voting

210 East Grand Ave.
South San Francisco, CA 94080
PROXY STATEMENT
FORTHE 2018 ANNUAL MEETINGOF STOCKHOLDERS
MAY 23, 2018
QUESTIONS AND ANSWERS ABOUT THESE PROXY MATERIALS AND VOTING
Why am I receiving these materials?
We have made these materials available to you on the Internet or, upon your request, have delivered printed versions of these materials to you by mail because the Board of Directors, or the Board, of Exelixis, Inc. (sometimes referred to as “we,” “us” or “Exelixis”) is soliciting your proxy to vote at the 2018 Annual Meeting of Stockholders, or the Annual Meeting, including at any adjournments or postponements of the meeting. The Annual Meeting will be held on Wednesday, May 23, 2018, at 8:00 a.m., local time, at our offices at the address set forth above. We invite you to attend the Annual Meeting to vote on the proposals described in this proxy statement. However, you do not need to attend the Annual Meeting to vote your shares. Instead, you may simply complete, sign and return a proxy card, or follow the instructions below or in the Notice of Internet Availability of Proxy Materials described below to submit your proxy over the telephone or on the Internet.
We intend to send or make available these materials to stockholders on April 12, 2018.
What is included in these proxy materials?
These proxy materials include:
| ›› | The Notice of the 2018 Annual Meeting of Stockholders; |
| ›› | The Proxy Statement for the Annual Meeting; and |
| ›› | Our Annual Report on Form10-K for the fiscal year ended December 29, 2017, as filed with the Securities and Exchange Commission, or SEC, on February 26, 2018, or the Annual Report. |
If you requested printed versions by mail, these proxy materials also include the proxy card or voting instruction form for the Annual Meeting.
Why did I receive aone-page notice in the mail regarding Internet availability of proxy materials instead of a full set of printed proxy materials?
Pursuant to rules adopted by the SEC, we have elected to use the Internet as the primary means of furnishing proxy materials to our stockholders this year. This method allows us to deliver the proxy materials to you more quickly, lowers our costs significantly and helps to conserve natural resources. Accordingly, we are sending a Notice of Internet Availability of Proxy Materials, or Notice of Availability, to our stockholders who have not asked us to provide proxy materials in printed form. All stockholders receiving a Notice of Availability can request a printed set of proxy materials. Moreover, all stockholders can access the proxy materials atwww.exel-annualstockholdermeeting.com, irrespective of whether they receive a Notice of Availability or a printed copy of the proxy materials. Instructions on how to access the proxy materials on the Internet or how to request a printed copy may be found in the Notice of Availability and in this Proxy Statement.
In addition, a stockholder may ask to receive proxy materials in printed form by mail or electronically by email on an ongoing basis. We encourage stockholders to take advantage of the option to receive proxy materials electronically by email to help reduce the environmental impact of our annual meeting and to reduce costs associated with the physical printing and mailing of materials. If you choose to receive future proxy materials by email, you will receive an email message next year with instructions containing a link to those materials and a link to the proxy voting website. Your election to receive proxy materials by email will remain in effect until you terminate it.
2018 Proxy Statement 1
Who may vote at the Annual Meeting?
Only stockholders of record at the close of business on March 29, 2018, the Record Date, will be entitled to vote at the Annual Meeting. On the Record Date, there were 296,659,164 shares of common stock outstanding and entitled to vote.
Stockholder of Record: Shares Registered in Your Name
If on March 29, 2018, your shares were registered directly in your name with our transfer agent, Computershare Trust Company, N.A., then you are a stockholder of record. As a stockholder of record, you may vote in person at the meeting or vote by proxy. Whether or not you plan to attend the Annual Meeting, we urge you to vote by proxy over the telephone or on the Internet as instructed below, or complete and mail the proxy card if you received printed materials.
Beneficial Owner: Shares Registered in the Name of a Broker, Bank or Other Stockholder of Record
If on March 29, 2018, your shares were held in an account at a brokerage firm, bank, dealer, or other similar organization, then you are the beneficial owner of shares held in “street name,” and these proxy materials are being forwarded to you by that organization. The organization holding your shares is considered to be the stockholder of record for purposes of voting at the Annual Meeting. As a beneficial owner, you have the right to direct your broker, bank or other stockholder of record regarding how to vote the shares in your account, and you will receive instructions from your broker, bank or other stockholder of record that must be followed in order for your broker, bank or other stockholder of record to vote your shares per your instructions. You are also invited to attend the Annual Meeting. However, since you are not the stockholder of record, you may not vote your shares in person at the meeting unless you request and obtain a valid proxy from your broker, bank or other stockholder of record giving you the right to vote such shares in person at the Annual Meeting.
What am I voting on?
There are three matters scheduled for a vote at the 2018 Annual Meeting. They are as follows:
| ›› | Election of the three Class I nominees for director named herein to hold office until the 2021 Annual Meeting of Stockholders; |
| ›› | Ratification of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 28, 2018; and |
| ›› | Advisory approval of the compensation of our named executive officers, as disclosed in this Proxy Statement. |
How do I vote?
Whether or not you plan to attend the meeting, we urge you to vote by proxy to ensure your vote is counted. You may still attend the meeting and vote in person even if you have already voted by proxy.
Stockholder of Record: Shares Registered in Your Name
If you are a stockholder of record, you have four ways to vote.
 In Person | ›› | To vote in person, come to the Annual Meeting and we will give you a ballot when you arrive. You must bring valid photo identification such as a driver’s license or passport and may be asked to provide proof of stock ownership, such as your account statement, as of the Record Date, March 29, 2018. | ||
 Via Internet | ›› | To vote on the Internet, go to www.investorvote.com/EXEL and follow the instructions provided in the Notice of Availability. Your vote must be received by 11:59 p.m., Eastern Time, on May 22, 2018, to be counted. | ||
 By Telephone | ›› | To vote by telephone, request a paper or email copy of the proxy materials by following the instructions provided in the Notice of Availability and call the number provided with the proxy materials to transmit your voting instructions. Your vote must be received by 11:59 p.m. Eastern Time, on May 22, 2018, to be counted. | ||
 By Mail | ›› | To vote by mail, request a paper copy of the proxy materials by following the instructions provided in the Notice of Availability and complete, sign and date the proxy card enclosed with the paper copy of the proxy materials and return it promptly in the envelope that will be provided. If you return your signed proxy card to us before the Annual Meeting, we will vote your shares as you direct. | ||
2 Exelixis, Inc.
Questions and Answers | Proxy Materials and Voting
Beneficial Owner: Shares Registered in the Name of Broker, Bank or Other Stockholder of Record (i.e., “Street Name”)
If you are a beneficial owner of shares registered in the name of your broker, bank, or other stockholder of record, you should have received the Notice of Availability containing voting instructions from that organization rather than from us. You must follow these instructions for your bank, broker or other stockholder of record to vote your shares per your instructions. Alternatively, many brokers and banks provide the means to grant proxies to vote shares by telephone and via the Internet. If your shares are held in an account with a broker, bank or other stockholder of record providing such a service, you may grant a proxy to vote those shares by telephone or over the Internet as instructed by your broker, bank or other stockholder of record. To vote in person at the Annual Meeting, you must obtain a valid proxy from your broker, bank or other stockholder of record. Follow the instructions from your broker, bank or other stockholder of record included with these proxy materials, or contact your broker, bank or other stockholder of record to request a proxy form.
We provide Internet proxy voting to allow you to vote your shares online, with procedures designed to ensure the authenticity and correctness of your proxy vote instructions. However, please be aware that you must bear any costs associated with your Internet access, such as usage charges from Internet access providers and telephone companies. |
How many votes do I have?
On each matter to be voted upon, you have one vote for each share of common stock you own as of the Record Date, March 29, 2018.
How are proxies voted?
All shares represented by valid proxies received prior to the taking of the vote at the Annual Meeting will be voted and, where a stockholder specifies by means of a proxy a choice with respect to any matter to be acted upon, the shares will be voted in accordance with the stockholder’s instructions.
What if I return a proxy card but do not make specific choices?
If you are a stockholder of record and you return a signed and dated proxy card without marking any voting selections, your shares will be voted on the proposals as follows:
| ›› | “For” the election of Drs. Cohen and Poste and Mr. Wyszomierski as described in Proposal 1; |
| ›› | “For” the ratification of our selection of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 28, 2018, as described in Proposal 2; and |
| ›› | “For” the advisory approval of the compensation of our named executive officers as described in Proposal 3. |
If any other matter is properly presented at the Annual Meeting, your proxyholder (one of the individuals named on your proxy card) will vote your shares using his best judgment.
If you are a beneficial owner of shares registered in the name of your broker, bank or other stockholder of record and you do not provide the broker, bank or other stockholder of record holding your shares with voting instructions, your broker, bank or other stockholder of record will determine if it has the discretionary authority to vote on the particular matter. If you are a beneficial owner whose shares are held of record by a broker and you do not provide voting instructions, your shares will not be voted on any proposal on which the broker does not have discretionary authority to vote. This is called a “brokernon-vote.” In these cases, the broker can register your shares as being present at the Annual Meeting for purposes of determining the presence of a quorum, but will not be able to vote on those matters for which specific authorization is required under the rules of the New York Stock Exchange.
If you are a beneficial owner whose shares are held of record by a broker, your broker has discretionary voting authority under the rules of the New York Stock Exchange to vote your shares on Proposal No. 2 (the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 28, 2018), even if your broker does not receive voting instructions from you. However, your broker does not have discretionary authority to vote on Proposal No. 1 or Proposal No. 3 without voting instructions from you, in which case a brokernon-vote will occur and your shares will not be voted on Proposal No. 1 or Proposal No. 3.
Who is paying for this proxy solicitation?
We are soliciting proxies and will bear the entire cost of soliciting proxies, including the preparation, printing and mailing of the Notice of Availability, the Notice of Annual Meeting, the Proxy Statement, the proxy card and any additional information
2018 Proxy Statement 3
furnished to stockholders. Copies of solicitation materials will be furnished to banks, brokerage houses, fiduciaries and custodians holding in their names shares of our common stock beneficially owned by others to forward to such beneficial owners. We may reimburse persons representing beneficial owners of our common stock for their costs of forwarding solicitation materials to such beneficial owners. Original solicitation of proxies by mail may be supplemented by telephone, telegram or personal solicitation by our directors, officers or other regular employees. No additional compensation will be paid to directors, officers or other regular employees for such services.
What does it mean if I receive more than one Notice of Availability or proxy card?
If you receive more than one Notice of Availability or proxy card, your shares are registered in more than one name or are registered in different accounts. Please follow the instructions on each Notice of Availability or proxy card to ensure that all of your shares are voted.
Can I change my vote after submitting my proxy?
Yes. You can revoke your proxy at any time before the final vote at the Annual Meeting. You may revoke your proxy in the following ways:
Stockholder of Record: Shares Registered in Your Name
| ›› | Your proxy may be revoked by filing with the Secretary of Exelixis at our principal executive office, Exelixis, Inc., 210 East Grand Avenue, South San Francisco, California 94080, either (1) a written notice of revocation or (2) a duly executed proxy card bearing a later date. |
| ›› | Your proxy may also be revoked by granting a subsequent proxy by telephone or on the Internet (your latest telephone or Internet proxy is the one that is counted). |
| ›› | Your proxy may also be revoked by attending the Annual Meeting and voting in person. Attendance at the Annual Meeting will not, by itself, revoke your proxy. |
Beneficial Owner: Shares Registered in the Name of Broker, Bank or Other Stockholder of Record
| ›› | If your shares are held by your broker or bank as nominee or agent, you should follow the instructions provided by your broker or bank to revoke any prior voting instructions. |
What is the quorum requirement for the Annual Meeting?
A majority of the outstanding shares entitled to vote at the Annual Meeting must be present at the Annual Meeting, either in person or by proxy, to hold a valid meeting. This is called a “quorum.”
If you are a stockholder of record, your shares will be counted towards the quorum only if you vote in person at the meeting or have properly voted by proxy on the Internet, by telephone or by submitting a proxy card by mail or at the Annual Meeting. You may vote “For,” “Against” or “Abstain” with respect to Proposals 1, 2 and 3. Abstentions will be counted towards the number of shares considered to be present at the meeting for purposes of determining whether a quorum is present.
If you are a beneficial owner holding your shares in “street name” then only the broker, bank or other stockholder of record can vote your shares unless you obtain a valid proxy from the broker, bank or other stockholder of record. See “What if I return a proxy card but do not make specific choices?” above. Shares represented by “brokernon-votes” will be counted in determining whether there is a quorum present. Votes will be counted by the inspector of election appointed for the Annual Meeting. If there is no quorum, either the chairman of the Annual Meeting or the holders of a majority of shares present at the Annual Meeting in person or represented by proxy may adjourn the Annual Meeting to another date.
How many votes are needed to approve each proposal, how are votes counted, and how are abstentions and brokernon-votes treated?
| ›› | Proposal1-Election of Directors: Directors in an uncontested election, such as this one, are elected by a majority of the votes cast. Each of the three Class I nominees must receive “For” votes from the holders of a majority of shares cast with respect to such director (i.e., the number of shares voted “For” a director must exceed the number of shares voted “Against” that director). Abstentions and brokernon-votes, if any, are not counted for purposes of electing directors and will have no effect on the results of this vote. |
4 Exelixis, Inc.
Questions and Answers | Proxy Materials and Voting
| ›› | Proposal2-Ratification of Ernst & Young LLP:The affirmative vote of a majority of shares present in person or represented by proxy at the Annual Meeting and entitled to vote on the proposal is required to ratify the selection of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 28, 2018. Abstentions will have the effect of votes against this proposal. Brokers generally have discretionary authority to vote on the ratification of our independent accounting firm; thus we do not expect any brokernon-votes on this proposal. To the extent there are any brokernon-votes, they will have no effect on the results of this proposal. |
| ›› | Proposal3-Advisory Vote on Executive Compensation: The affirmative vote of a majority of shares present in person or by represented proxy at the Annual Meeting and entitled to vote on the proposal is required to approve thenon-binding, advisory vote on executive compensation. Abstentions will be counted toward the tabulation of votes cast on the proposal and will have the same effect as votes against this proposal. Brokernon-votes will have no effect and will not be counted towards the vote total. Since the vote is advisory, it is not binding on the Board or on us. Nevertheless, the views expressed by our stockholders, whether through this vote or otherwise, are important to the Compensation Committee and the Board and, accordingly, the Compensation Committee and Board intend to consider the results of this vote in making determinations in the future regarding executive compensation arrangements. Your vote will serve as an additional tool to guide the Compensation Committee and Board in continuing to improve the alignment of our executive compensation programs with business objectives and performance and with the interests of our stockholders. |
Do I have dissenters’ rights?
No. We are organized as a corporation under Delaware law. Under the Delaware General Corporation Law, our stockholders are not entitled to dissenters’ rights with respect to any of the proposals set forth in this Proxy Statement and we will not independently provide the stockholders with any such rights.
How can I find out the results of the voting at the Annual Meeting?
Preliminary voting results will be announced at the Annual Meeting. In addition, final voting results will be published in a current report on Form8-K that we expect to file within four business days after the Annual Meeting. If final voting results are not available to us in time to file a Form8-K within four business days after the Annual Meeting, we intend to file a Form8-K to publish preliminary results and, within four business days after the final results are known to us, file an additional Form8-K to publish the final results.
Will other matters be voted on at the Annual Meeting?
We are not aware of any matters to be presented at the Annual Meeting other than those described in this Proxy Statement. If any other matters not described in the Proxy Statement are properly presented at the meeting, proxies will be voted in accordance with the best judgment of the proxyholders.
What proxy materials are available on the Internet?
This Proxy Statement and our 2017 Annual Report are available atwww.exel-annualstockholdermeeting.com.
What is the deadline for submitting stockholder proposals for the 2019 Annual Meeting?
To be considered for inclusion in the 2019 proxy materials, your proposal must be submitted in writing by December 13, 2018, to Exelixis’ Secretary at Exelixis, Inc., 210 East Grand Avenue, South San Francisco, California 94080, and you must comply with all applicable requirements of Rule14a-8 promulgated under the Securities Exchange Act of 1934, as amended. However, if our 2019 Annual Meeting of Stockholders is held before April 23, 2019, or after June 22, 2019, then the deadline will be a reasonable time prior to the time that we make our proxy materials available to our stockholders, either online or in printed form.
If you wish to submit a proposal or nominate a director at the 2019 Annual Meeting of Stockholders, but you are not requesting that your proposal or nomination be included in next year’s proxy materials, you must submit your proposal in writing, in the manner set forth in our Bylaws, to Exelixis’ Secretary at Exelixis, Inc., 210 East Grand Avenue, South San Francisco, California 94080, to be received no earlier than the close of business on February 22, 2019, and no later than the close of business on March 24, 2019. However, if our 2019 Annual Meeting of Stockholders is held before April 23, 2019, or after June 22, 2019, then you must notify Exelixis’ Secretary, in writing, not earlier than the close of business on the 90th day prior to the date of the 2019 Annual Meeting of Stockholders and not later than the close of business on the later of (i) the 60th day prior to the date of the 2019 Annual Meeting of Stockholders or (ii) if we publicly announce the date of the
2018 Proxy Statement 5
2019 Annual Meeting of Stockholders fewer than 70 days prior to the date of the 2019 Annual Meeting of Stockholders, the 10th day following the day that we first make such public announcement of the date of the 2019 Annual Meeting of Stockholders. We also advise you to review our Bylaws, which contain additional requirements about advance notice of stockholder proposals and director nominations. The chairperson of the 2019 Annual Meeting of Stockholders may determine, if the facts warrant, that a matter has not been properly brought before the meeting and, therefore, may not be considered at the meeting. In addition, unless prohibited by Rule14a-4(c) promulgated under the Securities Exchange Act of 1934, as amended, our management will have discretionary authority to vote all shares for which it has proxies for any such stockholder proposal or director nomination, including in opposition to such stockholder proposal or director nomination.
How may I obtain a printed copy of the Proxy Materials?
Instructions on how to obtain a printed copy of the proxy materials are set forth in the Notice of Availability.
Directions to the Annual Meeting
210 East Grand Avenue
South San Francisco, CA 94080
From San Francisco International Airport/San Jose | From the City of San Francisco | |||||
| Via Highway 101 North | Via Highway 101 South | |||||
• Highway 101 North
• Take exit 425A towards Grand Avenue
• Turn right onto Grand Avenue
• Turn right at first light onto East Grand Avenue
• 210 East Grand Avenue will be the first building on your right after crossing light at Harbor Way. | • Highway 101 South
• Take exit 425A towards Grand Avenue
• Turn left at first light onto Airport Boulevard
• Turn left at first light onto Grand Avenue
• Grand Avenue becomes East Grand Avenue
• 210 East Grand Avenue will be the first building on your right after crossing light at Harbor Way | |||||
Directions to our Annual Meeting may also be found on our website at:www.exelixis.com/about/locations-and-directions.
6 Exelixis, Inc.
Proposal 1 | Election of Class I Directors
PROPOSAL 1
ELECTIONOF CLASS I DIRECTORS
Our Certificate of Incorporation and Bylaws provide that the Board is divided into three classes, with each class having a three-year term. As of the date of this Proxy Statement, the Board has twelve members — three Class I directors, five Class II directors and four Class III directors. On April 5, 2018, the Board elected Maria C. Freire, Ph.D. to serve as a member of the Board. The Board elected Dr. Freire to the Board as a Class II director (so that her term will expire at next year’s annual meeting) after weighing various factors, including the timing of Dr. Freire’s election to our Board and the proximity of that timing to our deadline for finalizing our proxy materials and the Board’s desire that our stockholders have the opportunity to vote on her election to the Board in the near term. The term of office for each of the three directors in Class I will expire at the Annual Meeting. Each of the director nominees set forth in this Proxy Statement is currently a director of Exelixis who was previously elected by the stockholders. If elected at the Annual Meeting, each of these nominees would serve until the 2021 Annual Meeting and until his successor is elected and has qualified, or, if sooner, until the director’s death, resignation or removal.
As this is an uncontested election, each director will be elected by a majority of the votes cast at the meeting with respect to the election of that director. A majority of the votes cast means that the number of shares voted “for” a director must exceed the number of votes cast “against” that director. If any nominee becomes unavailable for election as a result of an unexpected occurrence, your shares will be voted for the election of such substitute nominee as the Board, after receiving the recommendation of the Nominating and Corporate Governance Committee of the Board, may propose. Each person nominated for election has agreed to serve if elected, and we have no reason to believe that any nominee will be unable to serve.
All director nominees set forth in this Proxy Statement have tendered an irrevocable resignation as a director conditioned upon (i) such director failing to receive a majority of the votes cast at the Annual Meeting, and (ii) acceptance by the Board of such resignation. If a director nominee who is serving as a director at the time of the election does not receive a majority of the votes cast at the Annual Meeting, the Nominating and Corporate Governance Committee will act to determine whether to accept the director’s conditional resignation and will submit such recommendation for prompt consideration by the Board. The Board will act on the Nominating and Corporate Governance Committee’s recommendation within ninety days following certification of the stockholder vote. In making their decision, the Nominating and Corporate Governance Committee will evaluate the best interests of Exelixis and its stockholders and shall consider all factors and information deemed relevant. The director who tenders his conditional resignation shall not participate in the Nominating and Corporate Governance Committee’s recommendation or Board action regarding whether to accept the conditional resignation of such director. If the Board determines not to accept the conditional resignation of a director, the Board will promptly disclose its decision-making process and decision to reject the conditional resignation in a Form8-K furnished to the Securities and Exchange Commission, or the SEC.
Set forth below is biographical information for each person nominated and each person whose term of office as a director will continue after the Annual Meeting. Incorporated within each biography is a description of the specific experience, qualifications, attributes and skills of each director or director nominee that led our Board to conclude that the individual should serve as a director as of the date of this Proxy Statement.
2018 Proxy Statement 7
Class I Director Nominees
for Election for a Three-Year Term Expiring at the 2021 Annual Meeting
Charles Cohen, Ph.D. Chief Executive Officer, Perform Biologics, Inc. | Director since 1995
Age 67
Key Qualifications and Expertise: Our Board has concluded that Dr. Cohen should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his training as a scientist, his knowledge and experience with respect to the biotechnology, pharmaceutical and healthcare industries, his broad leadership experience resulting from service on various boards and as a chief executive officer, and his knowledge and experience with respect to finance matters. | |
Charles Cohen, Ph.D., has been a director since November 1995. Dr. Cohen is an independent investor and since March 2015, has served as Chief Executive Officer and as a member of the board of directors of Perform Biologics, Inc., a private biotechnologystart-up company. Previously, Dr. Cohen served as Chief Executive Officer and as a member of the board of directors of On Target Therapeutics, LLC, a private biotechnology start-up company, from June 2015 to June 2017. From May 2007 to December 2015, Dr. Cohen was a managing director of Synthesis Capital (formerly Advent Healthcare Ventures), a venture capital firm. From 2003 to 2007, Dr. Cohen was Vice President of Advent International, a global private equity firm. From 2000 to 2002, Dr. Cohen was the Chief Executive Officer of Cellzome AG, a post-genomics biotechnology company. Prior to that time, Dr. Cohenco-founded Creative BioMolecules, Inc., a biotechnology company, in 1982 and was one of its directors and its Chief Executive Officer from 1985 to 1995. Dr. Cohen served as a member of the board of directors of the following publicly-held companies: Anadys Pharmaceuticals, Inc., a biopharmaceutical company dedicated to improving patient care by developing novel medicines for the treatment of hepatitis C, from 2000 to 2005; and Anesiva, Inc., a biopharmaceutical company focused on the development and commercialization of novel pharmaceutical products for pain management from 2005 to 2007. Dr. Cohen also served as the Chairman of the Supervisory Board of Cellzome AG, prior to its acquisition by GlaxoSmithKline in May 2012, and as the Chief Executive Officer of several other companies. Dr. Cohen received his Ph.D. from New York University School of Medicine. |
George Poste, DVM, Ph.D., FRS Chief Scientist, Complex Adaptive Systems Initiative | Director since 2004
Age 73
Key Qualifications and Expertise: Our Board has concluded that Dr. Poste should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his training as a scientist, his knowledge and experience with respect to the life sciences, healthcare and pharmaceutical industries, his broad leadership experience resulting from service on various boards, and his knowledge and experience with policymaking, regulatory issues and other governmental matters. | |
George Poste, DVM, Ph.D., FRS, has been a director since August 2004. Since February 2009, Dr. Poste has been the Chief Scientist at Complex Adaptive Systems Initiative and Regents’ Professor and Del E. Webb Professor of Health Innovation at Arizona State University. From May 2003 to February 2009, Dr. Poste served as the director of the Biodesign Institute at Arizona State University. Dr. Poste has served as the Chief Executive Officer of Health Technology Networks, a consulting company that specializes in the application of genomic technologies and computing in healthcare, since 2000. From 1992 to 1999, he was the Chief Science and Technology Officer and President, R&D, of SmithKline Beecham Corporation, a pharmaceutical company. Dr. Poste served on the Defense Science Board of the U.S. Department of Defense from 2001 to 2010 and is a member of other organizations dedicated to advancing defenses against bioweapons and biowarfare. Dr. Poste has served as a member of the board of directors of Monsanto Company, a publicly-held provider of agricultural products and solutions, since 2003, and as a member of the board of directors of Caris Life Sciences, a privately-held medical diagnostics company, since 2009. From April 2000 until August 2009, Dr. Poste served as theNon-Executive Chairman of Orchid Cellmark, Inc., a publicly-held DNA forensics company. Dr. Poste is a Fellow of the Royal Society, the UK Academy of Medical Sciences, Hoover Institution, Stanford University, and various other prestigious organizations and has been awarded honorary doctorates from several universities. Dr. Poste holds a D.V.M. in veterinary medicine and a Ph.D. in Virology from the University of Bristol, England. |
8 Exelixis, Inc.
Proposal 1 | Class I Director Nominees
Jack L. Wyszomierski Former Executive Vice President and Chief Financial Officer, VWR International, LLC | Director since 2004
Age 62
Key Qualifications and Expertise: Our Board has concluded that Mr. Wyszomierski should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his extensive financial reporting, accounting and finance experience, as well as his experience in the healthcare and life sciences industries. These qualities have also formed the basis for the Board’s decision to appoint Mr. Wyszomierski as a member and chairman of the Audit Committee. | |
Jack L. Wyszomierski, has been a director since February 2004. From June 2004 to June 2009, Mr. Wyszomierski served as the Executive Vice President and Chief Financial Officer of VWR International, LLC, a supplier of laboratory supplies, equipment and supply chain solutions to the global research laboratory industry. From 1982 to 2003, Mr. Wyszomierski held positions of increasing responsibility within the finance group at Schering-Plough Corporation, a health care company, culminating with his appointment as Executive Vice President and Chief Financial Officer in 1996. Prior to joining Schering-Plough, he was responsible for capitalization planning at Joy Manufacturing Company, a producer of mining equipment, and was a management consultant at Data Resources, Inc. Mr. Wyszomierski has served: as a member of the board of directors of XOMA Corporation, a publicly-held company that discovers, develops novel antibody therapeutics, since August 2010; as a member of the board of directors of Athersys, Inc., a publicly-held company engaged in the discovery and development of therapeutic product candidates, since June 2010; as a member of the board of directors of Solenis LLC, a privately-held manufacturer of chemical products, since August 2014; and as a member of the board of directors of SiteOne Landscape Supply, Inc., a publicly-held company that distributes landscape supply products, since April 2016. Mr. Wyszomierski previously served as a member of the board of directors of: Unigene Laboratories, Inc., a publicly-held biopharmaceutical company, from April 2010 to July 2013; and AssuraMed Holding, Inc., a privately-held distributor of home healthcare products, from January 2011 until its acquisition by Cardinal Health Inc. in March 2013. Mr. Wyszomierski holds a M.S. in Industrial Administration and a B.S. in Administration, Management Science and Economics from Carnegie Mellon University. |
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” EACH NAMED NOMINEE.
Class II Directors
Continuing in Office Until the 2019 Annual Meeting
Carl B. Feldbaum, Esq. President Emeritus, Biotechnology Industry Organization | Director since 2007
Age 74
Key Qualifications and Expertise: Our Board has concluded that Mr. Feldbaum should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his training as an attorney, his knowledge and experience with respect to the biotechnology, pharmaceutical and healthcare industries, his broad leadership experience resulting from service on various boards and as an executive officer and his knowledge and experience with policymaking, regulatory issues and other governmental matters. | |
Carl B. Feldbaum, Esq., has been a director since February 2007. Mr. Feldbaum serves as a member emeritus of the board of directors of BIO Ventures for Global Health, anon-profit organization, and is president emeritus of the Biotechnology Industry Organization (BIO), which represents more than 1,000 biotechnology companies, academic institutions and state biotechnology centers internationally. Mr. Feldbaum served as president of BIO from 1993 until his retirement in 2005. Prior to joining BIO, Mr. Feldbaum was chief of staff to Senator Arlen Specter of Pennsylvania. He also was president and founder of Palomar Corporation, a national security “think tank” in Washington, D.C. Before founding Palomar Corporation, Mr. Feldbaum was Assistant to the Secretary of Energy and served as the Inspector General for defense intelligence in the U.S. Department of Defense. Mr. Feldbaum served as a member of the board of directors of the following publicly-held companies: Actelion, Ltd, a biopharmaceutical company focused on the discovery, development and commercialization of innovative drugs for diseases with significant unmet medical needs (acquired by Johnson & Johnson in 2017), from 2005 to 2015; Trovagene, Inc., a precision medicine biotechnology company focused on the development of oncology therapeutics for improved cancer care, from 2014 to 2015; and Connetics Corporation, a specialty pharmaceutical company focused on the development and commercialization of innovative therapeutics for the dermatology market, from 2005 until its acquisition by Stiefel Laboratories, Inc. in 2006. Mr. Feldbaum holds an A.B. in Biology from Princeton University and a J.D. from the University of Pennsylvania Law School. |
2018 Proxy Statement 9
Maria C. Freire, Ph.D. President and Executive Director, Foundation for the National Institutes of Health | Director since 2018
Age 64
Key Qualifications and Expertise: Our Board has concluded that Dr. Freire should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to her training as a scientist, her knowledge and experience with respect to medical research, the pharmaceutical industry and government healthcare policymaking, as well as her leadership experience in the public sector. | |
Maria C. Freire, Ph.D., has been a director since April 2018. Since November 2012, Dr. Freire has served as President and Executive Director and as a member of the board of directors of the Foundation for the National Institutes of Health. From March 2008 to November 2012, she served as President and as a member of the board of directors of the Albert and Mary Lasker Foundation. Prior to joining the Lasker Foundation, Dr. Freire served as President and Chief Executive Officer of the Global Alliance for TB Drug Development from 2001 to 2008 and Director of the Office of Technology Transfer at the National Institutes of Health from 1995 to 2001. Dr. Freire has served on the board of directors of Alexandria Real Estate Equities, Inc. a publicly-held urban office real estate investment trust uniquely focused on collaborative life science and technology campuses, since April 2012, and has served on the boards of numerous national and international organizations, including the Science Board of the U.S. Food and Drug Administration, the World Health Organization Commission on Intellectual Property Rights, Innovation and Public Health and the United Nations Secretary General’s High Level Panel on Access to Medicines. Dr. Freire is also a member of the National Academy of Medicine and the Council on Foreign Relations, and she is the recipient of numerous awards, including a 2017 Gold Stevie Award for “Woman of the Year,” the U.S. Department of Health and Human Services Secretary’s Award for Distinguished Service, the Arthur S. Fleming Award and the Bayh-Dole Award. Dr. Freire holds a Ph.D. in Biophysics from the University of Virginia and a B.S. from the Universidad Peruana Cayetano Heredia in Lima, Peru. |
Alan M. Garber, M.D., Ph.D. Provost of Harvard University | Director since 2005
Age 62
Key Qualifications and Expertise: Our Board has concluded that Dr. Garber should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his training as a physician and economist, his knowledge and experience with respect to the life sciences, healthcare and pharmaceutical industries, and his knowledge and experience with policymaking, regulatory issues and other governmental matters. | |
Alan M. Garber, M.D., Ph.D., has been a director since January 2005. He became Provost of Harvard University, Mallinckrodt Professor of Health Care Policy at Harvard Medical School, and a Professor in the Harvard Kennedy School of Government and in the Department of Economics at Harvard in September 2011. Before moving to Harvard, from 1998 until August 2011, he was the Henry J. Kaiser Jr. Professor, a Professor of Medicine, and a Professor (by courtesy) of Economics, Health Research and Policy, and of Economics in the Graduate School of Business at Stanford University. Dr. Garber also served as the Director of the Center for Primary Care and Outcomes Research and the Center for Health Policy at Stanford. During his tenure at Stanford University, Dr. Garber also served as a Senior Fellow at the Freeman Spogli Institute for International Studies and as a staff physician at the VA Palo Alto Health Care System. Dr. Garber has served as a member of the board of directors of Vertex Pharmaceuticals Incorporated, a publicly-held biotechnology company focused on developing and commercializing therapies for the treatment of cystic fibrosis, since June 2017. Dr. Garber is a member of the National Academy of Medicine, the American Society of Clinical Investigation, the Association of American Physicians, the American Academy of Arts and Sciences and the Board on Science, Technology, and Economic Policy at the National Academies. He is a Fellow of the American College of Physicians, the Royal College of Physicians and the American Association for the Advancement of Science. Dr. Garber is also a Research Associate with the National Bureau of Economic Research and served as founding Director of its Health Care Program for nineteen years. He has also served as a member of the National Advisory Council on Aging at the National Institutes of Health, as a member of the Board of Health Advisers of the Congressional Budget Office and as Chair of the Medicare Evidence Development and Coverage Advisory Committee at the Centers for Medicare and Medicaid Services. Dr. Garber previously served on the editorial board of acclaimed scientific journals and has received numerous awards and honors. Dr. Garber holds an A.B. summa cum laude, an A.M. and a Ph.D., all in Economics, from Harvard University, and an M.D. with research honors from Stanford University. |
10 Exelixis, Inc.
Proposal 1 | Class II Directors
Vincent T. Marchesi, M.D., Ph.D. Professor of Pathology and Cell Biology, Yale University | Director since 2005
Age 82
Key Qualifications and Expertise: Our Board has concluded that Dr. Marchesi should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his training as a physician and scientist and his research and experience in the fields of healthcare and life sciences, with a particular focus on biotechnology. | |
Vincent T. Marchesi, M.D., Ph.D., has been a director since May 2001. Since 1973, Dr. Marchesi has been a Professor of Pathology and Cell Biology at Yale University and, since 1991, the Director of the Boyer Center for Molecular Medicine at Yale University. In 1982, Dr. Marchesico-founded Molecular Diagnostics, Inc., a diagnostic development company. Dr. Marchesi was formerly Chair of Pathology at theYale-New Haven Hospital. Dr. Marchesi holds an M.D. from Yale University and a Ph.D. from Oxford University, and is a member of the National Academy of Sciences and the Institute of Medicine.
|
Julie Anne Smith President and Chief Executive Officer, Nuredis, Inc. | Director since 2016
Age 47
Key Qualifications and Expertise: Our Board has concluded that Ms. Smith should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to her knowledge and experience with respect to biotechnology, healthcare and pharmaceutical industries and her broad leadership experience resulting from service as an executive in the pharmaceutical industry. | |
Julie Anne Smith, has been a director since September 2016. Since July 2017, Ms. Smith has served as President and Chief Executive Officer and sits on the board of directors of Nuredis, Inc., a privately-held biotechnology company developing novel therapies to treat inherited neurodegenerative diseases caused by nucleotide repeat expansions. Previously, Ms. Smith served as President and Chief Executive Officer of Raptor Pharmaceutical Corp. from January 2015 until the company’s acquisition by Horizon Pharma plc in October 2016, where she also served as Executive Vice President and Chief Operating Officer from 2012 to 2014. From 2008 to 2012, Ms. Smith served as Chief Commercial Officer of Enobia Pharmaceuticals prior to the company’s acquisition by Alexion Pharmaceuticals, Inc. Previously, Ms. Smith served as Vice President of Commercial at Jazz Pharmaceuticals from 2006 to 2008, as Vice President, Global Marketing at Genzyme General from 2001 to 2005, and helped to establish the commercial operations division for the biotechstart-up, Novazyme Pharmaceuticals, from 2000 to 2001. Ms. Smith began her industry career at Bristol-Myers Squibb Company, joining the company in 1996 as a Product Manager and rising to become a Director of channel strategy and design. Ms. Smith has served on the board of directors of Audentes Therapeutics, Inc. a publicly-held clinical stage biotechnology company focused on developing and commercializing gene therapy products for patients suffering from serious, life-threatening rare diseases caused by single gene defects, since December 2016 and previously, Ms. Smith was a Director on the Health and Emerging Companies Sections of the Biotechnology Industry Organization (BIO) board. Ms. Smith holds a B.S. in biological and nutritional sciences from Cornell University. |
Class III Directors
Continuing in Office Until the 2020 Annual Meeting
Michael M. Morrissey, Ph.D. President and Chief Executive Officer, Exelixis, Inc. | Director since 2010
Age 57
Key Qualifications and Expertise: Our Board has concluded that Dr. Morrissey should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his leadership role as the President and Chief Executive Officer of Exelixis. Beyond his role as Exelixis’ principal executive officer, the Board also considered Dr. Morrissey’s extensive qualifications, including his training as a scientist, his significant knowledge and experience with respect to the biotechnology, healthcare and pharmaceutical industries, comprehensive leadership background resulting from service as an executive in the biotechnology industry, and his ability to bring historic knowledge and continuity to the Board. | |
Michael M. Morrissey, Ph.D., has served as a director and as Exelixis’ President and Chief Executive Officer since July 2010. Dr. Morrissey has held positions of increasing responsibility at Exelixis since he joined the company in February 2000, including serving as President of Research and Development from January 2007 until July 2010. From 1991 to 2000, Dr. Morrissey held several positions at Berlex Biosciences, last holding the position of Vice President, Discovery Research. Earlier in his career, Dr. Morrissey served as a Senior Scientist and Project Team Leader in Medicinal Chemistry at CIBA-Geigy Corporation. He is the author of numerous scientific publications in medicinal chemistry and drug discovery and an inventor on 70 issued U.S. patents and 25 additional published U.S. patent applications. Dr. Morrissey holds a B.S. (Honors) in Chemistry from the University of Wisconsin and a Ph.D. in Chemistry from Harvard University.
|
2018 Proxy Statement 11
Stelios Papadopoulos, Ph.D. Co-Founder and Chairman of the Board, Exelixis, Inc. | Director since 1994
Age 69
Key Qualifications and Expertise: Our Board has concluded that Dr. Papadopoulos should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his training as a scientist, his knowledge and experience with respect to the biotechnology, healthcare and pharmaceutical industries, his broad leadership experience resulting from extensive service on various boards, his knowledge and experience with respect to finance matters, and his ability to bring historic knowledge and continuity to the Board. | |
Stelios Papadopoulos, Ph.D., aco-founder of Exelixis, has been a director since December 1994 and the Chairman of the Board since January 1998. Dr. Papadopoulos retired as Vice Chairman of Cowen & Co., LLC in August 2006 after six years as an investment banker with the firm, where he focused on the biotechnology and pharmaceutical sectors. Prior to joining Cowen & Co., he spent 13 years as an investment banker at PaineWebber, Incorporated, where he was most recently Chairman of PaineWebber Development Corp., a PaineWebber subsidiary focusing on biotechnology. He joined PaineWebber in April 1987 from Drexel Burnham Lambert, where he was a Vice President in the Equity Research Department covering the biotechnology industry. Prior to Drexel, he was a biotechnology analyst at Donaldson, Lufkin & Jenrette. Before coming to Wall Street in 1985, Dr. Papadopoulos was on the faculty of the Department of Cell Biology at New York University Medical Center. He continues his affiliation with New York University Medical Center as an Adjunct Associate Professor of Cell Biology. Dr. Papadopoulos is aco-founder of Anadys Pharmaceuticals, Inc., a publicly-held biopharmaceutical company dedicated to improving patient care by developing novel medicines for the treatment of hepatitis C, acquired byHoffmann-La Roche Inc. in November 2011. Dr. Papadopoulos served as a member of the board of directors of Anadys Pharmaceuticals from 2000 to 2011 and as its chairman in 2011, prior to its acquisition. Dr. Papadopoulos has also served as a member of the board of directors of three other publicly-held companies: BG Medicine, Inc., a diagnostics company focused on the development and commercialization of cardiovascular diagnostic tests, since 2003; Biogen, Inc., a biopharmaceutical company focused on the treatment of serious diseases, since 2008 and as its chairman since 2014; and Regulus Therapeutics Inc., a biopharmaceutical company focused on the development of medicines targeting microRNAs, since 2008, and as its chairman since 2013. Dr. Papadopoulos was alsoco-founder and member of the board of directors of Cellzome Inc., a privately-held drug discovery company acquired by GlaxoSmithKline in May 2012. In thenot-for-profit sector, Dr. Papadopoulos is aco-founder and Chairman of Fondation Santé, a member of the board of visitors of Duke Health, and a member of the Global Advisory Board of the Duke Institute for Health Innovation. Dr. Papadopoulos holds a Ph.D. in Biophysics and an M.B.A. in Finance, both from New York University. |
George A. Scangos, Ph.D. Chief Executive Officer, Vir Biotechnology, Inc. | Director since 1996
Age 69
Key Qualifications and Expertise: Our Board has concluded that Dr. Scangos should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his prior leadership role as our President and Chief Executive Officer. Beyond his prior role as our principal executive officer, the Board also considered Dr. Scangos’ extensive qualifications, including his training as a scientist, his significant knowledge and experience with respect to the biotechnology, healthcare and pharmaceutical industries, his comprehensive leadership background resulting from service on various boards and as an executive in the biotechnology industry, and his ability to bring historic knowledge and continuity to the Board. | |
George A. Scangos, Ph.D., has been a director since October 1996. Since January 2017, Dr. Scangos has served as Chief Executive Officer and as a member of the board of directors of Vir Biotechnology, Inc., a privately held biotechnology company focused on fighting infectious diseases. From July 2010 to December 2016, Dr. Scangos served as Chief Executive Officer and as a member of the board of directors of Biogen Inc., a publicly-held biopharmaceutical company focused on the treatment of serious diseases. Prior to joining the Biogen organization, from October 1996 to July 2010, Dr. Scangos served as our President and Chief Executive Officer. From September 1993 to October 1996, Dr. Scangos served as President of Biotechnology at Bayer Corporation, a pharmaceutical company, and was responsible for research, business and process development, manufacturing, engineering, and quality assurance. Dr. Scangos has served as a member of the board of directors of various publicly-held companies, including: Agilent Technologies, Inc., a global leader in life sciences, diagnostics and applied chemical markets, since 2014; and Anadys Pharmaceuticals, Inc., a biopharmaceutical company dedicated to improving patient care by developing novel medicines for the treatment of hepatitis C, from 2003 to 2010. Dr. Scangos has also served as a member of the board of directors of our former subsidiary, TaconicArtemis GmbH (previously known as Artemis Pharmaceuticals GmbH), until 2010 and Entelos, Inc. from 1997 to 2010, and he currently serves as a director of Decibel Therapeutics, Inc. and Fondation Santé. Dr. Scangos previously served as the Chair of the California Healthcare Institute (CHI), as a member of the Board of the Global Alliance for TB Drug Development, and as a member of the board of directors of BayBio. Dr. Scangos is also a member of the Board of Advisors of the University of California, San Francisco School of Pharmacy, and the National Board of Advisors of the University of California, Davis School of Medicine. Dr. Scangos was a Jane Coffin Childs Post-Doctoral Fellow at Yale University and a faculty member at Johns Hopkins University. Dr. Scangos currently holds an appointment as Adjunct Professor of Biology at Johns Hopkins University. Dr. Scangos holds a B.A. in Biology from Cornell University and a Ph.D. in Microbiology from the University of Massachusetts. |
12 Exelixis, Inc.
Proposal 1 | Class III Directors
Lance Willsey, M.D. Founding Partner, DCF Capital | Director since 1997
Age 56
Key Qualifications and Expertise: Our Board has concluded that Dr. Willsey should continue to serve as a director of Exelixis as of the date of this Proxy Statement due to his skill as a physician, his knowledge and experience with respect to the life sciences and healthcare industries, and his knowledge and experience with respect to finance matters. | |
Lance Willsey, M.D., has been a director since April 1997. Dr. Willsey was a founding partner of DCF Capital, a hedge fund focused on investing in the life sciences, from July 1998 to March 2002, and currently is a consultant to institutional investors in the field of oncology. Since 2000, Dr. Willsey has served on the Visiting Committee of the Department of Genitourinary Oncology at the Dana-Farber Cancer Institute at Harvard Medical School. From July 1997 to July 1998, Dr. Willsey served on the Staff Department of Urologic Oncology at the Dana-Farber Cancer Institute. From July 1996 to July 1997, Dr. Willsey served on the Staff Department of Urology at Massachusetts General Hospital at Harvard University School of Medicine, where he was a urology resident from July 1992 to July 1996. From 2000 to 2010, Dr. Willsey served a member of the board of directors of Exact Sciences Corporation, a publicly-held molecular diagnostics company focused on the early detection and prevention of the deadliest forms of cancer. Dr. Willsey holds a B.S. in Physiology from Michigan State University and an M.S. in Biology and an M.D., both from Wayne State University. |
Corporate Governance
Corporate Governance Guidelines
We have adopted written corporate governance guidelines, which may be viewed atwww.exelixis.com under the caption “Investors & Media-Corporate Governance.” This document includes guidelines for determining director independence and qualifications for directors. Our Board regularly reviews, and modifies from time to time, our corporate governance guidelines, Board committee charters and Board practices. Please note that information found on, or accessible through, our website is not a part of, and is not incorporated into, this Proxy Statement.
Code of Conduct and Ethics
We have adopted a Code of Conduct and Ethics that applies to all directors, officers and employees, including the principal executive officer, principal financial officer and principal accounting officer. The Code of Conduct and Ethics is posted on our website atwww.exelixis.com under the caption “Investors & Media-Corporate Governance.” We intend to satisfy the disclosure requirement under Item 5.05 of Form8-K regarding an amendment to, or waiver from, a provision of this Code of Conduct and Ethics by posting such information on our website, at the address and location specified above and, to the extent required by the listing standards of the Nasdaq Stock Market, by filing a Current Report on Form8-K with the SEC, disclosing such information.
Director Independence
We have adopted standards for director independence pursuant to Nasdaq listing standards, which require that a majority of the members of a listed company’s board of directors qualify as “independent,” as affirmatively determined by the board of directors. An “independent director” means a person other than an officer or employee of Exelixis or one of our subsidiaries, or another individual having a relationship that, in the opinion of the Board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Consistent with these considerations, after review of all relevant transactions or relationships between each director, or any of his or her family members, and Exelixis, its senior management and its independent registered public accounting firm, the Board has affirmatively determined that Drs. Cohen, Freire, Garber, Marchesi, Papadopoulos, Poste, Scangos and Willsey, Messrs. Wyszomierski and Feldbaum and Ms. Smith, who are eleven of the twelve members of the Board, represent a majority of the Board and are independent. Dr. Morrissey, our President and Chief Executive Officer, is not independent by virtue of his employment with Exelixis. In addition, the Board has also determined that: (i) all directors who serve on the Audit, Compensation and Nominating and Corporate Governance Committees are independent under applicable Nasdaq listing standards; and (ii) all members of the Audit Committee meet the independence requirements under the Securities Exchange Act of 1934, as amended.
Board Leadership Structure
The Board does not have a formal policy on whether the role of chairman and chief executive officer should be separate or combined. Our corporate governance guidelines provide that the Board will select its chairman and the chief executive officer in the manner it considers to be in the best interests of our company. Currently, we have an independent chairman of the board separate from the chief executive officer. The Board believes this bifurcated structure provides for sufficient
2018 Proxy Statement 13
independent oversight of management and strong Board leadership, while allowing for the effective management of company affairs. The Board believes that if the positions of chairman and chief executive officer are combined, the appointment of a lead independent director would be necessary for effective governance. Accordingly, our corporate governance guidelines provide that if the roles are combined, the independent directors of the Board must appoint a lead independent director. Our corporate governance guidelines further provide that the lead independent director would: (i) preside at all meetings of the Board at which the chairman is not present, including executive sessions of the independent directors; (ii) have the authority to call meetings of the independent directors; (iii) serve as the principal liaison on Board-wide issues between the independent directors and the chairman; and (iv) have such other authority and duties as the Board may from time to time determine. The Board believes that this flexible approach provides it with the ability to establish a leadership structure, based upon its judgment that is in the best interests of our company and those of our stockholders at any given time.
Role of the Board in Risk Oversight
Management is responsible for identifying the various risks facing our company, including, without limitation, strategic, operational, financial, regulatory and cyber-security risks that may exist from time to time. Management is also charged with the responsibility of implementing appropriate risk management policies and procedures and managing our risk exposure on aday-to-day basis. While we do not have a formal risk oversight policy, the Board, as a whole and through its various committees, conducts the risk oversight function for our company. In its risk oversight role, the Board evaluates whether management has reasonable controls in place to address material risks currently facing our company and those we may face in the future. The Board and its committees meet at regularly scheduled and special meetings throughout the year at which they are presented with information regarding risks facing the company. The Board delegates certain of its risk oversight responsibilities to its various committees as follows:
| ›› | Our Audit Committee oversees the management of risks related to financial reporting, trading in our securities, fraud, information technology and cyber-security, including a regular review of the existence or status of any such risks and evaluating the steps management has taken or proposes to take to mitigate them. Our Audit Committee also reviews any proposed related party transactions to ensure we do not engage in transactions that would create a conflict of interest or result in harm to us. |
| ›› | Our Compensation Committee oversees the management of risks related to our compensation policies and practices, including structuring and reviewing our executive compensation programs, considering whether such programs are in line with our strategic objectives and incentivizing appropriate risk-taking. |
| ›› | Our Nominating and Corporate Governance Committee oversees the management of risks related to our compliance programs, including healthcare compliance, and corporate governance matters, including director independence and Board composition and succession. |
Also, the Board is presented with frequent business updates during monthly teleconferences among our Board and senior management. Following consideration of the information provided by management, the Board provides feedback, makes recommendations and, as needed, issues directives to management to address our risk exposure.
Prohibitions on Derivative, Hedging, Monetization and Other Transactions
We maintain an insider trading policy that applies to directors and employees, including our executive officers, which prohibits certain transactions in our stock, including short sales, puts, calls or other transactions involving derivative securities, hedging or monetization transactions, purchases of Exelixis securities on margin or borrowing against an account in which Exelixis securities are held, or pledging Exelixis stock as collateral for a loan.
Stockholder Communications with the Board
Security holders may send communications to the Board by mail at 210 East Grand Avenue, South San Francisco, California 94080, by facsimile at(650) 837-7951 or bye-mail at info@exelixis.com, each of the foregoing sent “Attn: Board of Directors.”
Stock Ownership Guidelines
In February 2018, the Board adopted Stock Ownership Guidelines applicable to our directors and Named Executive Officers (as defined below) to further align their financial interests with those of our stockholders, as well as to promote sound
14 Exelixis, Inc.
Proposal 1 | Corporate Governance
corporate governance. For ournon-employee directors, our Stock Ownership Guidelines provide an ownership target equal to the lesser of 3,000 shares or a value equivalent to three times the annual cash Board retainer. Under the guidelines, we expectnon-employee directors to achieve their stock ownership targets within five years of becoming subject to these guidelines. The policy includes procedures for granting exemptions in the case of severe financial hardship. Ownership targets for our Named Executive Officers (including those serving on our Board) are described below under Compensation Discussion and Analysis—Other Compensation Information—Stock Ownership Guidelines.
In determining ownership levels for eachnon-employee director under our Stock Ownership Guidelines, credit is provided for shares held outright (including shares owned through trusts, the Amended and Restated Exelixis, Inc. 401(k) Plan, or the 401(k) Plan, or by a spouse), as well as 50% of the number of vested, but unexercised, stock options. No credit is provided for restricted stock units until they vest. The values for all shares determined to be held by ournon-employee directors and Named Executive Officers are based on the200-day average stock price as of the measurement date. As of February 15, 2018, the date the Board adopted the Stock Ownership Guidelines, all of ournon-employee directors serving on the Board as of such date had met the required ownership targets.
Board Committees and Meetings
The Board held five meetings during 2017 and all of our directors attended at least 75% of the total meetings of the Board and of the committees on which they served. The independent directors met four times in regularly scheduled executive sessions.
During 2017, the Board had four standing committees: an Audit Committee, Compensation Committee, Nominating and Corporate Governance Committee and Research & Development Committee. Committee membership in 2017 was as follows:
Board Member | Audit Committee | Compensation Committee | Nominating and Corporate Governance Committee | Research & Development | ||||
Charles Cohen, Ph.D. | Member | Chair | Member | |||||
Carl B. Feldbaum, Esq. | Member | Member | ||||||
Maria C. Freire, Ph.D.* | ||||||||
Alan M. Garber, M.D., Ph.D. | Chair | Member | ||||||
Vincent T. Marchesi, M.D., Ph.D. | Member | Member | ||||||
Stelios Papadopoulos, Ph.D. | Member | Member | ||||||
George Poste, D.V.M., Ph.D., FRS | Member | Chair | ||||||
George A. Scangos, Ph.D. | Member** | Member | ||||||
Julie A. Smith | Member | Member | ||||||
Lance Willsey, M.D. | Member | Member | ||||||
Jack L. Wyszomierski | Chair*** | Member | ||||||
Number of Meetings Held in Fiscal 2017 | 4 | 12 | 3 | 2 |
| * | Dr. Freire became a director on April 5, 2018 and did not serve on any committee of the Board during 2017. Dr. Freire was also appointed to the Nominating and Corporate Governance Committee and the Research & Development Committee on April 5, 2018. |
| ** | On May 9, 2017, Dr. Scangos resigned from the Audit Committee. |
| *** | Designated by the Board as an “audit committee financial expert.” |
Audit Committee
The Audit Committee of the Board oversees our corporate accounting and financial reporting process, ensures the integrity of our financial statements and has been designated as the Qualified Legal Compliance Committee within the meaning of Rule 205.2(k) of Title 17, Chapter II of the Code of Federal Regulations. The Audit Committee performs several functions, such as evaluating the performance of, and assessing the qualifications of, the independent registered public accounting firm; determining whether to retain or terminate the existing independent registered public accounting firm or to appoint and
2018 Proxy Statement 15
engage a new independent registered public accounting firm; reviewing and approving the engagement of the independent registered public accounting firm to perform any proposed permissible services and appropriate compensation thereof; reviewing, providing oversight of, and approving related party transactions; establishing procedures, as required under applicable law, for the receipt, retention and treatment of complaints received by Exelixis regarding accounting, internal accounting controls or auditing matters and the confidential and anonymous submission by employees of concerns regarding questionable accounting or auditing matters; overseeing our risk assessment and risk management processes with respect to financial reporting fraud, including receiving reports on a quarterly basis concerning the existence or status of any significant financial reporting or fraud risks and discussing and evaluating the steps management has taken or proposes to take to mitigate such risks; reviewing our tax strategy, the status of any material tax audits and proceedings and any other material tax matters; reviewing the financial statements to be included in our Annual Report on Form10-K; discussing with management and the independent registered public accounting firm the results of the annual audit and the results of our quarterly financial statement reviews; and resolving any disagreements between the independent registered public accounting firm and management. The Audit Committee also has the specific responsibilities and authority necessary to comply with the listing standards of the Nasdaq Stock Market applicable to audit committees.
The Board has determined that Mr. Wyszomierski is an “audit committee financial expert” as defined in applicable SEC rules.
The Audit Committee’s report is set forth in “Report of the Audit Committee” below. The Audit Committee has a written charter, which is available on our website atwww.exelixis.com under the caption “Investors & Media-Corporate Governance.”
Compensation Committee
The purpose of the Compensation Committee is to: oversee our compensation policies, plans and programs; review and determine the compensation to be paid to officers and directors; work with management to address any conflict of interest with any compensation adviser engaged by management or the Compensation Committee; review with management our Compensation Discussion and Analysis, and to consider whether to recommend that it be included in our proxy statements and other filings; and prepare and review the Compensation Committee’s report included in our annual proxy statement or Annual Report on Form10-K, as applicable, in accordance with applicable rules and regulations of the SEC. The Compensation Committee also establishes and reviews general policies relating to compensation and benefits of employees, including executive officers, and performs such other functions regarding compensation as the Board may delegate. The Compensation Committee also administers the issuance of stock options and other awards under our stock plans.
The Compensation Committee’s report is set forth in “Compensation Committee Report” below. Information on the Compensation Committee’s processes and procedures for consideration of executive compensation are addressed in “Compensation Discussion and Analysis” below. For information regarding our processes and procedures for the consideration and determination of director compensation, please see “Compensation of Directors” below. The Compensation Committee has a written charter, which is available on our website atwww.exelixis.com under the caption “Investors & Media-Corporate Governance.”
Compensation Consultants.The Compensation Committee retained Compensia, Inc., or Compensia, a national compensation consulting firm, as its external compensation consultant to assist the Compensation Committee in its duties related to executive andnon-employee director compensation. Compensia did not perform any work for us other than the executive compensation andnon-employee director compensation consulting services provided to the Compensation Committee and reported directly to the Compensation Committee or through its chairperson. Fees paid to Compensia in 2017 did not exceed $120,000. At the direction of the Compensation Committee, management retained Radford, a compensation consulting firm serving technology and life sciences companies, principally to provide benchmark and industry compensation data for executive and broad-based compensation analyses. In consideration for compensation related services provided during 2017 we paid Radford an aggregate of $49,587. Radford also provided our management with access to the Radford Global Life Sciences Survey, Radford Global Sales Survey and Radford U.S. Benefits Survey, for which we paid Radford an aggregate of $13,123 in 2017. Radford is an Aon Hewitt company and an affiliate of Aon Risk Services which provided insurance brokerage services to us during 2017 at a total cost of $239,417.
Nominating and Corporate Governance Committee
The purpose of the Nominating and Corporate Governance Committee is to: oversee all aspects of our corporate governance functions on behalf of the Board; make recommendations to the Board regarding corporate governance issues; identify,
16 Exelixis, Inc.
Proposal 1 | Board Committees and Meetings
review and evaluate candidates to serve as directors; serve as a focal point for communication between such candidates,non-committee directors and management; recommend such candidates to the Board and make such other recommendations to the Board regarding affairs relating to the directors; and develop a set of corporate governance principles for Exelixis. The Nominating and Corporate Governance Committee also oversees our compliance programs, including our healthcare compliance program created to prevent fraud and abuse in federal healthcare programs andnon-compliance with applicable U.S. Food and Drug Administration and other healthcare laws. The Nominating and Corporate Governance Committee has a written charter, which is available on our website atwww.exelixis.com under the caption “Investors & Media-Corporate Governance.”
Director Qualifications; Diversity.The Nominating and Corporate Governance Committee does not have a fixed set of minimum qualifications for candidates for membership on the Board. Instead, in considering candidates for directorship, the Nominating and Corporate Governance Committee will generally consider all relevant factors, including the candidate’s applicable expertise and demonstrated excellence in his or her field, the usefulness of such expertise to us, the availability of the candidate to devote sufficient time and attention to the affairs of Exelixis, the existence of any relationship that would interfere with the exercise of the candidate’s independent judgment, and the candidate’s demonstrated character and judgment. In the review process, the Nominating and Corporate Governance Committee evaluates prospective candidates for directorship in the context of the existing membership of the Board (including the qualities and skills of the existing directors), our operating requirements and the long-term interests of our stockholders. While the Board does not have a formal policy with regard to the consideration of diversity in identifying director nominees, the Nominating and Corporate Governance Committee believes that the factors considered above enable it to identify director candidates that possess key industry knowledge as well as a variety of different professional and personal backgrounds, attributes (including, but not limited to, gender and national origin), experiences, skills and expertise such that the Board, as a group, can best fulfill its responsibilities to our stockholders. The Nominating and Corporate Governance Committee is authorized to access external resources as it deems necessary or appropriate to fulfill its defined responsibilities, including engagement of executive search firms to help identify director candidates.
Director Nominations.The Nominating and Corporate Governance Committee considers and assesses all candidates recommended by our directors, officers and stockholders. Evaluations of candidates generally involve a review of background materials, internal discussions and interviews with selected candidates as appropriate. If, after its review, the Nominating and Corporate Governance Committee supports a candidate, it would recommend the candidate for consideration by the full Board. The Nominating and Corporate Governance Committee considers stockholder recommendations for directors using the same criteria as potential nominees recommended by the members of the Nominating and Corporate Governance Committee or others. The Nominating and Corporate Governance Committee has not received any recommended nominations from any stockholder holding 5% or more of our common stock in connection with the 2018 Annual Meeting.
Stockholders who wish to recommend individuals for consideration by the Nominating and Corporate Governance Committee to become nominees for election to the Board may do so by delivering a written recommendation to the Nominating and Corporate Governance Committee within the timeframe specified in our Bylaws that is applicable to matters to be brought before an Annual Meeting of Stockholders as set forth under “Questions and Answers About These Proxy Materials and Voting” above. Such communications should be sent to the following address: Exelixis, Inc., 210 East Grand Ave., South San Francisco, California 94080, Attn: Nominating and Corporate Governance Committee of the Board. Submissions must include (a) the name, age, business address and residence address of such person, (b) the principal occupation or employment of such person, (c) the class and number of shares of the corporation which are beneficially owned by such person, (d) a description of all arrangements or understandings between the stockholder and each nominee and any other person or persons (naming such person or persons) pursuant to which the nominations are to be made by the stockholder, (e) a statement whether such person, if elected, intends to comply with the corporation’s Corporate Governance Guidelines, including with respect to matters relating to the election of directors, and (f) any other information relating to such person that is required to be disclosed in solicitations of proxies for election of directors, or is otherwise required, in each case pursuant to Regulation 14A under the 1934 Act (including without limitation such person’s written consent to being named in the proxy statement, if any, as a nominee and to serving as a director if elected).
Research & Development Committee
The Research & Development Committee, which was established in January 2006, is responsible for advising Exelixis and the Board on matters of scientific importance as the Board, in consultation with management, may designate from time to time.
2018 Proxy Statement 17
Annual Meeting; Attendance
The Board does not have a formal policy with respect to the attendance of its members at Annual Meetings of Stockholders. Dr. Morrissey was the only member of the Board in attendance at the 2017 Annual Meeting of Stockholders.
18 Exelixis, Inc.
Compensation of Directors
COMPENSATION OF DIRECTORS
The compensation program for ournon-employee directors is intended to be competitive and fair so that we can attract optimal talent to our Board and recognize the time and effort required of a director given the size and complexity of our operations. In accordance with its charter, our Compensation Committee is responsible for recommending to the Board for approval the annual compensation for ournon-employee directors and acts on behalf of the Board in discharging the Board’s responsibilities with respect to overseeing our compensation policies fornon-employee directors. In February 2017, the Board approved changes to thenon-employee director compensation program following consideration of market data prepared by the Compensation Committee’s independent compensation adviser, Compensia, and the recommendation of the Compensation Committee. All changes were effective beginning with the first quarter of fiscal 2017.
Cash Compensation Arrangements
Eachnon-employee director receives an annual cash retainer for his or her service on the Board, as well as an annual cash retainer if he or she serves as the Chairman of the Board, on a committee or as the chair of a committee. In addition, ournon-employee directors receive fees for attendance at Board meetings and at their respective committee meetings.
The table below provides information regarding the cash compensation arrangements for ournon-employee directors for 2017. Dr. Morrissey receives no compensation in his capacity as a member of the Board.
Service | Fee Type | Cash Compensation ($) | |||||
Board | Retainer Fee | 25,000 | |||||
| Additional Chair Retainer Fee | 30,000 | ||||||
| Regular Meeting Fee | 2,500 | ||||||
| Special Meeting Fee (1) | 1,000 | ||||||
Audit Committee | Retainer Fee | 6,000 | |||||
| Additional Chair Retainer Fee | 15,000 | ||||||
| Meeting Fee (2) | 1,000 | ||||||
Compensation Committee | Retainer Fee | 5,000 | |||||
| Additional Chair Retainer Fee | 10,000 | ||||||
| Meeting Fee (2) | 1,000 | ||||||
Nominating & Corporate Governance Committee | Retainer Fee | 5,000 | |||||
| Additional Chair Retainer Fee | 10,000 | ||||||
| Meeting Fee (2) | 1,000 | ||||||
Research & Development Committee | Retainer Fee | 10,000 | |||||
| Additional Chair Retainer Fee | 10,000 | ||||||
| Meeting Fee (2) | 5,000 | ||||||
| (1) | Meeting at which minutes are generated. |
| (2) | In-person meeting or teleconference at which minutes are generated. |
Equity Compensation Arrangements
Ournon-employee directors are also eligible to receive equity as part of their Board service, including an initial award upon joining the Board and an annual award on the day following each Annual Meeting of Stockholders. Grants to ournon-employee directors are made under our 2017 Equity Incentive Plan, or 2017 Plan, pursuant to theNon-Employee Director Equity Compensation Policy, or 2017 Directors’ Policy, as adopted by the Board. Historically, ournon-employee directors would receive initial awards and annual awards in the form of an option to purchase a specific andpre-determined number of shares of our common stock. However, to address continued changes in the trading price of our common stock, the 2017 Directors’ Policy moved to a value-based approach for determining the number of shares subject to these equity awards. During 2017, under the terms of the 2017 Directors’ Policy,non-employee directors were eligible to receive aone-time initial award having a value equal to approximately $400,000 when they first join the Board and an annual award having a value equal to approximately $250,000 on the day following each Annual Meeting of Stockholders. Each initial
2018 Proxy Statement 19
award and annual award will be divided between approximately 50% in the form of a nonstatutory stock option and approximately 50% in the form of an RSU award, based on a valuation methodology established by the Board; provided, however, that with respect to annual awards, eachnon-employee director may instead elect to receive 100% of such equity grants in the form of a nonstatutory stock option. The total number of options and RSUs granted as part of the initial award and each annual award is determined using a formula based upon the Black-Scholes Merton option pricing model and the average of the daily closing sale prices of our common stock for all of the trading days during the30-day calendar period ending on (and including) the last calendar day immediately prior to the grant date of such initial award or annual award. The fair value of such initial award or annual award, as determined in accordance with the 2017 Directors’ Policy, may be greater or lesser than the grant date fair value reflected in the “Director Compensation Table” below. This is a result of the different calculation employed to determine the grant date fair value of each initial award or annual award, which is determined using a formula based upon the Black-Scholes Merton option pricing model and the closing sale price of our common stock on the grant date.
Options granted under the 2017 Plan in accordance with the 2017 Directors’ Policy are not incentive stock options under the Internal Revenue Code of 1986, as amended, or the Code. The exercise price of automatic grants of stock options under the 2017 Plan is equal to 100% of the fair market value of a share of common stock on the grant date. Under the terms of the 2017 Directors’ Policy, theone-time initial options are immediately exercisable but are subject to a repurchase right and will vest at the rate of 25% of the underlying shares on the first anniversary of the grant date and monthly thereafter over the next three years. The annual options are exercisable immediately but are subject to a repurchase right and will vest monthly in equal parts over aone-year period. As long as thenon-employee director continues to serve with us or with an affiliate of us, the option will continue to vest and be exercisable during its term. When the option holder’s service terminates, we will have the right to repurchase any unvested shares acquired upon exercise of the option at the original exercise price without interest. The post-termination exercise period for the vested portion of the options granted to ournon-employee directors is generally set to terminate the earlier of three years after anon-employee director’s service terminates or the remainder of the term of the option, as described in the form of option agreement fornon-employee directors under the 2017 Plan. The options granted pursuant to the 2017 Directors’ Policy have a term that does not exceed seven years. Under the terms of the 2017 Directors’ Policy, theone-time initial RSU awards will vest at the rate of 25% of the underlying shares on each of the first four anniversaries of the grant date, so long as thenon-employee director continues to serve with us or with an affiliate with us. The annual RSU awards will vest at the rate of 100% of the shares on the first anniversary of the grant date, so long as thenon-employee director continues to serve with us or with an affiliate with us. In the event of a change in control, 100% of thenon-employee director’s outstanding and unvested equity awards will immediately vest, and any applicable repurchase rights of the Company will terminate. In the event of a change in control, the vesting of each award granted under the 2017 Directors’ Plan (or under our priorNon-Employee Director Equity Compensation Policy, or the Prior Directors’ Policy, under the 2014 Equity Incentive Plan, or the 2014 Plan) will be accelerated in full to a date prior to the effective time of such change in control (contingent upon the effectiveness of such change in control) as the Board will determine (or, if the Board does not determine such a date, to the date that is five days prior to the effective time of such change in control).
On September 22, 2016, Ms. Smith Joined the Board and became eligible to receive an equity award pursuant to the terms of the Prior Directors’ Policy. At the time Ms. Smith joined the Board, the Compensation Policy determined that it would be appropriate to grant Ms. Smith an initial option award having a value equal to approximately $200,000, which reflected the value of an initial option award at the time of the most recent Board compensation analysis prior to Ms. Smith joining the Board. Accordingly, the Board granted Ms. Smith an option to purchase 26,000 shares of our common stock as herone-time initial option award. However, on February 23, 2017, in connection with the Board’s adoption of the 2017 Directors’ Policy and the increase in the target value of theone-time initial awards to $400,000, the Board granted Ms. Smith a supplemental award in the form of an option to purchase an additional 16,620 shares of our common stock, such that the total value of both her initial award and supplemental award was equal to approximately $400,000.
Reimbursement of Expenses
The members of the Board are eligible for reimbursement of certain expenses incurred in connection with their attendance of Board meetings and their service on the Board in accordance with our policy.
20 Exelixis, Inc.
Compensation of Directors
Director Compensation Table
The following table shows compensation information for ournon-employee directors for the fiscal year ended December 29, 2017. Discussion of Dr. Freire’s compensation is not included, as she did not become a director until April 5, 2018.
Director Compensation for Fiscal 2017
| Fees Earned or Paid in Cash ($) | Stock Awards (Restricted Stock Units) ($)(1) | Option Awards ($)(2) | Total ($) | |||||||||||||
Charles Cohen, Ph.D. | 94,500 | — | 233,501 | 328,001 | ||||||||||||
Carl B. Feldbaum, Esq. | 61,500 | — | 233,501 | 295,001 | ||||||||||||
Alan M. Garber, M.D., Ph.D. | 74,500 | — | 233,501 | 308,001 | ||||||||||||
Vincent T. Marchesi, M.D., Ph.D. | 74,500 | 114,348 | 116,159 | 305,007 | ||||||||||||
Stelios Papadopoulos, Ph.D. | 97,500 | 114,348 | 116,159 | 328,007 | ||||||||||||
George Poste, D.V.M., Ph.D., FRS | 75,500 | 114,348 | 116,159 | 306,007 | ||||||||||||
George A. Scangos, Ph.D. | 62,500 | — | 233,501 | 296,001 | ||||||||||||
Julie A. Smith | 61,500 | — | 426,844 | (3) | 488,344 | |||||||||||
Lance Willsey, M.D. | 74,500 | — | 233,501 | 308,001 | ||||||||||||
Jack L. Wyszomierski | 70,500 | 114,348 | 116,159 | 301,007 | ||||||||||||
| (1) | On May 25, 2017, each of Drs. Marchesi, Papadopoulos and Poste and Mr. Wyszomierski were granted an RSU award pursuant to the 2017 Directors’ Policy, which accounted for approximately 50% of the total value of their annual award. Amounts shown in this column reflect the grant date fair value of the RSU award as computed in accordance with Financial Accounting Standards Board, or FASB, Accounting Standards Codification Topic 718, or ASC 718. The assumptions used to calculate the value of RSUs are set forth in Note 8 of the Notes to Consolidated Financial Statements included in our Annual Report on Form10-K for the fiscal year ended December 27, 2017, filed with the SEC on February 26, 2018. See “Equity Compensation Arrangements” above for a description of the RSU awards made tonon-employee directors on May 25, 2017. |
| Only one RSU award was granted to each of Drs. Marchesi, Papadopoulos and Poste and Mr. Wyszomierski during fiscal 2017 and, accordingly, the grant date fair value of that RSU award is reflected in the table. The aggregate number of shares subject to all RSUs held by each of thesenon-employee directors as of December 29, 2017, is as follows: Dr. Marchesi—5,843; Dr. Papadopoulos—5,843; Dr. Poste—5,843; and Mr. Wyszomierski—5,843. None of the othernon-employee directors received any RSU awards during fiscal 2017, and none of the othernon-employee directors held any outstanding RSU awards as of December 29, 2017. |
| (2) | On May 25, 2017, eachnon-employee director was granted an option to purchase our common stock pursuant to the 2017 Directors’ Policy. Each of Drs. Cohen, Garber, Scangos and Willsey, Mr. Feldbaum and Ms. Smith elected to receive 100% of their annual award in the form of options to purchase our common stock, while each of Drs. Marchesi, Papadopoulos and Poste and Mr. Wyszomierski elected to receive approximately 50% of their annual award in the form of options to purchase our common stock and approximately 50% of their annual award in the form of an RSU award. Amounts shown in this column reflect the aggregate grant date fair value for the option awards granted in fiscal 2017 as computed in accordance with FASB ASC 718. The assumptions used to calculate the value of option awards are set forth in Note 8 of the Notes to Consolidated Financial Statements included in our Annual Report on Form10-K for the fiscal year ended December 29, 2017, filed with the SEC on February 26, 2018. See “Equity Compensation Arrangements” above for a description of the option grants made tonon-employee directors on May 25, 2017. There can be no assurance that the options will ever be exercised (in which case no value will actually be realized by the director) or that the value on exercise of stock options will be equal to the grant date fair value shown in this column. |
| With the exception of Ms. Smith, who was also granted a supplemental option award on February 23, 2017, only one stock option award was granted to eachnon-employee director during fiscal 2017 and, accordingly, the grant date fair value of that stock option is reflected in the table. The aggregate number of shares subject to all stock options held by each of our currentnon-employee directors as of December 29, 2017, is as follows: Dr. Cohen—360,137; Mr. Feldbaum—269,287; Dr. Garber—301,697; Dr. Marchesi—272,940; Dr. Papadopoulos—353,727; Dr. Poste—226,348; Dr. Scangos—333,510; Ms. Smith—66,111; Dr. Willsey—350,883; and Mr. Wyszomierski—295,339. |
2018 Proxy Statement 21
| (3) | On February 23, 2017, Ms. Smith was granted a supplemental award in the form of an option to purchase 16,620 shares or our common stock in connection with the Board’s adoption of the 2017 Directors’ Policy and the increase in the target value of theone-time initial awards to $400,000. The grant date fair value of her supplemental award was $193,343 and the grant date fair value of her annual award was $233,501, in each case as computed in accordance with FASB ASC 718. The assumptions used to calculate the value of this option award is set forth in Note 8 of the Notes to Consolidated Financial Statements included in our Annual Report on Form10-K for the fiscal year ended December 29, 2017, filed with the SEC on February 26, 2018. See “Equity Compensation Arrangements” above for a description of the supplemental option grant made to Ms. Smith on February 23, 2018. There can be no assurance that the option will ever be exercised (in which case no value will actually be realized by the director) or that the value on exercise of the stock option will be equal to the grant date fair value described above. |
22 Exelixis, Inc.
Proposal 2 | Ratification of Selection of Independent Registered Public Accounting Firm
PROPOSAL 2
RATIFICATIONOF SELECTIONOF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Audit Committee of the Board has selected Ernst & Young LLP as Exelixis’ independent registered public accounting firm for the fiscal year ending December 28, 2018. The Board, on behalf of the Audit Committee, has further directed that management submit the selection of the independent registered public accounting firm for ratification by the stockholders at the Annual Meeting. Ernst & Young LLP has audited our financial statements for each of the seventeen fiscal years in the period ended December 29, 2017. Representatives of Ernst & Young LLP are expected to be present at the Annual Meeting. They will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Neither our Bylaws nor other governing documents or law require stockholder ratification of the selection of Ernst & Young LLP as Exelixis’ independent registered public accounting firm. However, the Board is submitting the selection of Ernst & Young LLP to the stockholders for ratification as a matter of good corporate governance. If the stockholders fail to ratify the selection, the Audit Committee of the Board will reconsider whether or not to retain that firm. Even if the selection is ratified, the Audit Committee of the Board in its discretion may direct the appointment of a different independent registered public accounting firm at any time during the year if they determine that such a change would be in the best interests of Exelixis and its stockholders.
The affirmative vote of the holders of a majority of the shares present in person or represented by proxy and entitled to vote at the Annual Meeting is required to ratify the selection of Ernst & Young LLP. Abstentions will be counted toward the tabulation of votes cast on proposals presented to the stockholders and will have the same effect as a vote against this proposal. Brokernon-votes, if any, will have no effect on the results of this vote.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” PROPOSAL 2.
Principal Accountant Fees and Services
The aggregate fees billed by Ernst & Young LLP for the last two fiscal years for the services described below are as follows:
| Fiscal Year Ended | ||||||||
December 29, 2017 | December 30, 2016 | |||||||
Audit Fees (1) | $ | 1,658,395 | $ | 1,703,009 | ||||
Audit-Related Fees (2) | 220,000 | — | ||||||
Tax Fees(3) | 208,152 | — | ||||||
All Other Fees(4) | 1,955 | 1,995 | ||||||
Total Fees | $ | 2,088,502 | $ | 1,705,004 | ||||
| (1) | “Audit fees” consist of fees billed for professional services rendered for the audit of our consolidated financial statements and review of the interim consolidated financial statements included in quarterly reports and services that are normally provided by Ernst & Young LLP in connection with statutory and regulatory filings and other engagements such as comfort letters, consents, and review of documents filed with the SEC. |
| (2) | “Audit-related fees” consist of fees billed for assurance and related services that are reasonably related to the performance of the audit or review of our consolidated financial statements and are not reported under “Audit fees.” During fiscal 2017 these services included consultations relating to various transactions. We did not incur any “Audit-related” fees during fiscal 2016. |
| (3) | “Tax fees” include fees for tax compliance, tax advice and tax planning. No tax fees were billed during fiscal 2016. |
2018 Proxy Statement 23
| (4) | “All other fees” consist of fees for products and services other than the services described above. During fiscal 2017 and 2016, these fees related to anon-line subscription to an Ernst & Young, LLP database. |
All fees described above werepre-approved by the Audit Committee. The Audit Committee has determined that the rendering of the services other than audit services by Ernst & Young LLP is compatible with maintaining the independence of the independent registered public accounting firm.
Pre-Approval of Services
During 2017 and 2016, the Audit Committee of the Boardpre-approved the audit andnon-audit services to be performed by Exelixis’ independent registered public accounting firm, Ernst & Young LLP.Non-audit services are defined as services other than those provided in connection with an audit or a review of our financial statements. The Audit Committeepre-approves all audit andnon-audit services rendered by Ernst & Young LLP. The Audit Committee generallypre-approves specified services in the defined categories of audit services, audit-related services, tax services and all other services up to specified amounts.Pre-approval may also be given as part of the Audit Committee’s approval of the scope of the engagement of the independent registered public accounting firm or on an individual explicitcase-by-case basis before the independent auditor is engaged to provide each service. The Audit Committee or its chairman, whom the Audit Committee has designated as aone-person subcommittee withpre-approval authority,pre-approved all audit fees, audit-related fees, tax fees and other fees in 2017 and 2016. Anypre-approvals by the subcommittee must be and were presented to the Audit Committee at its next scheduled meeting.
24 Exelixis, Inc.
Report of the Audit Committee
REPORTOFTHE AUDIT COMMITTEE
The material in this report is not “soliciting material,” is not deemed “filed” with the Securities and Exchange Commission and is not deemed to be incorporated by reference in any filing of Exelixis under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
In connection with the audited consolidated financial statements for the fiscal year ended December 29, 2017, of Exelixis, Inc. (“Exelixis”), the Audit Committee of the Board of Directors of Exelixis has:
(1) reviewed and discussed the audited financial statements for the fiscal year ended December 29, 2017, with management of Exelixis;
(2) discussed with Ernst & Young LLP, Exelixis’ independent registered public accounting firm (“Ernst & Young”), the matters required to be discussed by Auditing Standard No. 1301,Communications with Audit Committees, as adopted by the Public Company Accounting Oversight Board (“PCAOB”); and
(3) received the written disclosures and the letter from Ernst & Young required by applicable requirements of the PCAOB regarding Ernst & Young’s communications with the Audit Committee concerning independence, and has discussed with Ernst & Young that accounting firm’s independence.
Based on the foregoing, the Audit Committee has recommended to the Board of Directors that the audited financial statements be included in Exelixis’ Annual Report on Form10-K for the fiscal year ended December 29, 2017.
Audit Committee:
Jack L. Wyszomierski, Chairman
Charles Cohen
Stelios Papadopoulos
2018 Proxy Statement 25
PROPOSAL 3
ADVISORY VOTEONTHE COMPENSATIONOFTHE NAMED EXECUTIVE OFFICERS
Our stockholders are entitled to vote to approve, on an advisory basis, the compensation, as disclosed in this Proxy Statement, of our Chief Executive Officer, Chief Financial Officer and the other executive officers appearing in the table entitled “Summary Compensation Table” later in this Proxy Statement (collectively, the Named Executive Officers). This vote is not intended to address any specific item of compensation, but rather the overall compensation of our Named Executive Officers and the philosophy, policies and practices described in this Proxy Statement.
The compensation of our Named Executive Officers subject to the vote is disclosed in “Compensation Discussion and Analysis,” and the compensation tables and the related narrative disclosure contained in this Proxy Statement. As discussed in the “Compensation Discussion and Analysis” section of this Proxy Statement, the success of biopharmaceutical companies is significantly influenced by their work forces. We believe it is critical to our business that we retain our core team of highly qualified employees, including our executive officers. Large pharmaceutical and biopharmaceutical companies and strong local competitors have aggressively recruited our executives and other skilled employees, with the most critical positions at our company among those that are the most in demand. In light of these circumstances, we have designed our executive compensation program to help attract and retain highly qualified individuals with relevant experience in the biopharmaceutical industry to manage the varied aspects of our evolving business. The primary objective of our executive compensation program is to retain and motivate our core team of highly qualified employees, including our Named Executive Officers, and align their compensation with our critical business objectives and performance, as well as with the interests of our stockholders.
The Board encourages our stockholders to read the disclosures set forth in the “Compensation Discussion and Analysis” section of this Proxy Statement to review the correlation between compensation and performance, as well as compensation actions taken in 2017. The Board believes that our executive compensation program effectively aligns executive pay with our performance and results in the attraction and retention of highly talented executives.
Accordingly, the Board recommends that our stockholders vote FOR the following resolution:
“RESOLVED, that the compensation paid to the our Named Executive Officers, as disclosed pursuant to Item 402 of RegulationS-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion, is hereby APPROVED.”
Required Vote and Board of Directors Recommendation
Advisory approval of Proposal 3 requires the affirmative vote of a majority of the shares present or represented by proxy and entitled to vote at the Annual Meeting. Abstentions will be counted toward the tabulation of votes cast on the proposal and will have the same effect as votes against this proposal. Brokernon-votes will have no effect.
Our stockholders have expressed a preference, and our Board has determined, to hold an advisory vote on executive compensation annually. We are presenting this Proposal 3 as required by Section 14A of the Securities Exchange Act of 1934, as amended. Our Board believes that approval of Proposal 3 is in our best interests and the best interests of our stockholders for the reasons stated above. Because the vote is advisory, it is not binding on the Board or on us. Nevertheless, the views expressed by our stockholders, whether through this vote or otherwise, are important to management and the Board and, accordingly, the Compensation Committee and Board intend to consider the results of this vote in making determinations in the future regarding executive compensation arrangements. Your vote will serve as an additional tool to guide the Compensation Committee and Board in continuing to improve the alignment of our executive compensation programs with business objectives and performance and with the interests of our stockholders.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” PROPOSAL 3.
26 Exelixis, Inc.
Security Ownership of Certain Beneficial Owners and Management
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding the ownership of our common stock as of March 2, 2018, (except as noted) by: (i) each director and nominee for director; (ii) each of the executive officers named in the Summary Compensation Table; (iii) all current executive officers and directors of Exelixis as a group; and (iv) all those known by us to be beneficial owners of more than five percent of our common stock.
| Beneficially Owned(1) | ||||||||
Name of Beneficial Owner | Number of Shares of Common Stock | Percentage of Total | ||||||
Executive Officers and Directors | ||||||||
Michael M. Morrissey, Ph.D. (2) | 3,731,695 | 1.2 | % | |||||
Gisela M. Schwab, M.D. (3) | 1,954,029 | * | ||||||
Christopher J. Senner (4) | 420,608 | * | ||||||
Jeffrey J. Hessekiel, J.D. (5) | 860,729 | * | ||||||
Peter Lamb, Ph.D. (6) | 1,124,691 | * | ||||||
Charles Cohen, Ph.D. (7) | 608,317 | * | ||||||
Carl B. Feldbaum, Esq. (8) | 304,020 | * | ||||||
Maria C. Freire, Ph.D. (9) | — | * | ||||||
Alan M. Garber, M.D., Ph.D. (10) | 348,026 | * | ||||||
Vincent T. Marchesi, M.D., Ph.D. (11) | 393,010 | * | ||||||
Stelios Papadopoulos, Ph.D. (12) | 1,367,198 | * | ||||||
George Poste, D.V.M., Ph.D., FRS (13) | 288,903 | * | ||||||
George A. Scangos, Ph.D. (14) | 1,820,753 | * | ||||||
Julie A. Smith (15) | 66,874 | * | ||||||
Lance Willsey, M.D. (16) | 789,156 | * | ||||||
Jack L. Wyszomierski (17) | 368,299 | * | ||||||
All current directors, executive officers as a group (17 persons) (18) | 14,807,376 | 4.8 | % | |||||
5% Stockholders | ||||||||
FMR LLC (19) 245 Summer Street Boston, Massachusetts 02210 | 36,439,460 | 12.3 | % | |||||
The Vanguard Group (20) 100 Vanguard Blvd. Malvern, Pennsylvania 19355 | 26,351,871 | 8.9 | % | |||||
Meditor Group Ltd. (21) Wessex House, 3rd Floor 45 Reid Street Hamilton HM12, Bermuda | 16,484,638 | 5.6 | % | |||||
BlackRock, Inc. (22) 55 East 52nd Street New York, New York 10055 | 16,321,767 | 5.5 | % | |||||
| * | Less than one percent. |
| (1) | This table is based upon information supplied by executive officers and directors and upon information gathered by us about principal stockholders known to us. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, we believe that each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. Applicable percentages are based on |
2018 Proxy Statement 27
| 296,528,278 shares outstanding on March 2, 2018, adjusted as required by rules promulgated by the SEC. The percentage of beneficial ownership as to any person as of a particular date is calculated by dividing the number of shares beneficially owned by such person, which includes the number of shares as to which such person has the right to acquire voting or investment power within 60 days of March 2, 2018, by the sum of the number of shares outstanding as of such date plus the number of shares as to which such person has the right to acquire voting or investment power within 60 days of March 2, 2018. Consequently, the denominator for calculating beneficial ownership percentages may be different for each beneficial owner. |
| (2) | Includes 169,698 shares held by Michael M. Morrissey and Meghan D. Morrissey, Trustees of the Morrissey Family Living Trust dated July 21, 1994, as amended. Also includes 3,538,217 shares Dr. Morrissey has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018. Also includes 17,127 shares held by Mr. Morrissey under our 401(k) Plan, determined based upon information provided in plan statements. |
| (3) | Includes 1,767,053 shares Dr. Schwab has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018. Also includes 14,287 shares held by Dr. Schwab under our 401(k) Plan, determined based upon information provided in plan statements. |
| (4) | Includes 333,437 shares Mr. Senner has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018. Also includes 2,174 shares held by Mr. Senner under our 401(k) Plan, determined based upon information provided in plan statements. |
| (5) | Includes 608,999 shares Mr. Hessekiel has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018. Also includes 475 shares held by Mr. Hessekiel under our 401(k) Plan, determined based upon information provided in plan statements. |
| (6) | Includes 1,048,967 shares Dr. Lamb has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018. Also includes 16,515 shares held by Dr. Lamb under our 401(k) Plan, determined based upon information provided in plan statements. |
| (7) | Includes 360,137 shares Dr. Cohen has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 5,873 of which would be subject to repurchase by us, if so exercised. |
| (8) | Includes 269,287 shares Mr. Feldbaum has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 5,873 of which would be subject to repurchase by us, if so exercised. |
| (9) | Dr. Freire became a director on April 5, 2018 and concurrently was granted a one-time initial award pursuant to the 2017 Directors’ Policy, consisting of (i) an option to purchase 28,240 shares of our common stock and (ii) an RSU award representing 14,120 shares of our common stock, each of which will vest in accordance with the terms of the 2017 Directors’ Policy, described under “Compensation of Directors—Equity Compensation Arrangements” above. However, as the one-time initial award was granted subsequent to March 2, 2018, the effective date of this table, she did not have beneficial ownership of any of these shares as of March 2, 2018. |
| (10) | Includes 286,697 shares Dr. Garber has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 5,873 of which would be subject to repurchase by us, if so exercised. |
| (11) | Includes 272,940 shares Dr. Marchesi has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 2,922 of which would be subject to repurchase by us, if so exercised. |
| (12) | Includes 338,727 shares Dr. Papadopoulos has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 2,922 of which would be subject to repurchase by us, if so exercised. |
| (13) | Includes 211,348 shares Dr. Poste has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 2,922 of which would be subject to repurchase by us, if so exercised. |
| (14) | Includes 8,963 shares held by Dr. Scangos and Leslie S. Wilson, as Trustees of The Jennifer Wilson Scangos Trust, and 8,963 shares held by Dr. Scangos and Leslie S. Wilson, as Trustees of The Katherine Wilson Scangos Trust. Also includes 333,510 shares Dr. Scangos has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 5,873 of which would be subject to repurchase by us, if so exercised. Also includes 5,669 shares held by Dr. Scangos under our 401(k) Plan, determined based upon information provided in plan statements. |
| (15) | Includes 66,111 shares Ms. Smith has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 35,130 of which would be subject to repurchase by us, if so exercised. |
| (16) | Includes 320,883 shares Dr. Willsey has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 5,873 of which would be subject to repurchase by us, if so exercised. |
| (17) | Includes 295,339 shares Mr. Wyszomierski has the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 2,922 of which would be subject to repurchase by us, if so exercised. |
28 Exelixis, Inc.
Security Ownership of Certain Beneficial Owners and Management
| (18) | Total number of shares includes 4,407,836 shares of common stock held by our current directors and executive officers as of March 2, 2018, and entities affiliated with such directors and executive officers. Also includes 10,333,192 shares our directors and executive officers have the right to acquire pursuant to options exercisable within 60 days of March 2, 2018, 76,183 of which would be subject to repurchase by us, if so exercised. Also includes 66,348 shares held by our current directors and executive officers under our 401(k) Plan, determined based upon information provided in plan statements. |
| (19) | FMR LLC reported that (a) it has sole voting power with respect to 9,813,751 of these shares, (b) FMR LLC and Abigail P. Johnson each have sole dispositive power of all of these shares and (c) Fidelity Growth Company Fund has sole voting power with respect to 14,889,267 of these shares. Ms. Johnson is a Director, the Vice Chairman and the Chief Executive Officer of FMR LLC. FMR LLC also reports that FMR Co., Inc. beneficially owns 5% or greater of our common stock. The foregoing information is based solely on a Schedule 13G/A filed with the SEC on February 13, 2018, which provides information only as of December 29, 2017, and, consequently, the beneficial ownership of these reporting persons may have changed between December 29, 2017 and March 2, 2017. |
| (20) | The Vanguard Group reported that it has sole voting power over 159,258 of such shares, shared voting power over 39,980 of these shares, sole dispositive power over 26,175,453 of such shares and shared dispositive power over 176,418 of such shares. The information is based solely on a Schedule 13G/A, filed with the SEC on February 9, 2018, which provides information only as of December 31, 2017, and, consequently, the beneficial ownership of Vanguard may have changed between December 31, 2017 and March 2, 2018. |
| (21) | These shares are beneficially owned by Meditor Group Ltd., or Meditor, and Meditor European Master Fund Ltd., or MEMF, an investment management client and subsidiary of Meditor. Meditor reported that it and MEMF each have shared voting and dispositive power over the shares. The foregoing information is based solely on a Schedule 13G/A filed with the SEC on January 4, 2018, which provides information only as of December 31, 2017, and, consequently, the beneficial ownership of Meditor and MEMF may have changed between December 31, 2017 and March 2, 2018. |
| (22) | BlackRock, Inc. reported that it has sole voting power over 15,258,340 of such shares, and that it has sole dispositive power over all of the shares. The information is based solely on a Schedule 13G/A, filed with the SEC on January 29, 2018, which provides information only as of December 31, 2017, and, consequently, the beneficial ownership of BlackRock may have changed between December 31, 2017 and March 2, 2018. |
2018 Proxy Statement 29
EXECUTIVE OFFICERS
The following chart sets forth certain information regarding our executive officers as of March 29, 2018:
Name | Age | Position | ||
Michael M. Morrissey, Ph.D. (1) | 57 | President and Chief Executive Officer | ||
Gisela M. Schwab, M.D. | 61 | President, Product Development and Medical Affairs and Chief Medical Officer | ||
Christopher J. Senner | 50 | Executive Vice President and Chief Financial Officer | ||
Patrick J. Haley | 42 | Senior Vice President, Commercial | ||
Jeffrey J. Hessekiel, J.D. | 49 | Executive Vice President and General Counsel | ||
Peter Lamb, Ph.D. | 57 | Executive Vice President, Scientific Strategy and Chief Scientific Officer |
| (1) | Please see “Class III Directors Continuing in Office until the 2020 Annual Meeting” in this Proxy Statement for Dr. Morrissey’s biography. |
Gisela M. Schwab, M.D. President, Product Development and Medical Affairs and Chief Medical Officer |
Gisela M. Schwab, M.D., has served as President, Product Development and Medical Affairs and Chief Medical Officer since February 2016. Previously she served as Executive Vice President and Chief Medical Officer from January 2008 to February 2016 and as Senior Vice President and Chief Medical Officer from September 2006 to January 2008. From 2002 to 2006, she held the position of Senior Vice President and Chief Medical Officer at Abgenix, Inc., a human antibody-based drug development company. She also served as Vice President, Clinical Development, at Abgenix from 1999 to 2001. Prior to working at Abgenix, from 1992 to 1999, she held positions of increasing responsibility at Amgen Inc., most recently as Director of Clinical Research and Hematology/Oncology Therapeutic Area Team Leader. From August 2011 through July 2014, Dr. Schwab served as a member of the board of directors of Topotarget A/S, a publicly-held biopharmaceutical company. Since June 2012 she has served as a member of the board of directors of Cellerant Therapeutics, Inc. a privately-held biopharmaceutical company and since March 2015, she has served as a member of the board of directors of Nordic Nanovector A.S., a Norwegian biotechnology company. She received her Doctor of Medicine degree from the University of Heidelberg, trained at the University of Erlangen-Nuremberg and the National Cancer Institute and is board certified in internal medicine and hematology and oncology. |
Christopher J. Senner Executive Vice President and Chief Financial Officer |
Christopher J. Senner, has served as Executive Vice President and Chief Financial Officer (and in such capacity, as our principal financial officer and principal accounting officer, as defined under applicable securities laws) since July 2015. Prior to joining Exelixis, Mr. Senner served as Vice President, Corporate Finance for Gilead Sciences, Inc., a biopharmaceutical company, from March 2010 to July 2015, where he was accountable for controllership, tax, treasury and corporate and operational financial planning. Mr. Senner previously spent eighteen years at Wyeth, a pharmaceutical company acquired by Pfizer Inc. in 2009, in a variety of financial roles with increasing responsibility, most notably as Chief Financial Officer of Wyeth’s U.S. pharmaceuticals business and the BioPharma Business Unit. Mr. Senner holds a B.S. in Finance from Bentley College. |
P.J. Haley Senior Vice President, Commercial |
P.J. Haley, has served as the company’s Senior Vice President, Commercial since December 2016 and has held positions of progressive Commercial leadership since September 2010, serving as Vice President, Commercial from November 2014 to November 2016, Executive Director, Sales & Marketing from September 2013 to October 2014, Senior Director, Marketing from March 2012 to August 2013, and as Director, Marketing from September 2010 to February 2012. Prior to joining Exelixis, from 2007 to 2010, he held positions of increasing responsibility at Genentech, Inc., on the Avastin marketing team, most recently Group Product Manager. Between 2003 and 2007, Mr. Haley served in various sales and marketing roles at Amgen, Inc. He served as an analyst at PWC Securities, Lehman Brothers and Accenture from 1998 to 2001. Mr. Haley holds a Masters of Business Administration from University of Michigan, Ross School of Business, and a Bachelor of Arts in Art History and Medieval and Renaissance Studies from Duke University. |
30 Exelixis, Inc.
Executive Officers
Jeffrey J. Hessekiel, J.D. Executive Vice President and General Counsel |
Jeffrey J. Hessekiel, J.D., has served as Executive Vice President and General Counsel since February 2014 and as its Secretary from October 2014 to September 2017. From 2012 to 2014, he held the position of Senior Counsel at Arnold & Porter Kaye Scholer LLP, where he advised emerging growth and public companies, primarily in the life sciences sector, on complex legal issues connected with strategic transactions, healthcare compliance programs and investigations, and regulatory matters. Prior to working with Arnold & Porter, from 2002 to 2012, he held positions of increasing responsibility at Gilead Sciences, Inc., most recently as Chief Compliance & Quality Officer where he was responsible for the creation and management of Gilead’s Corporate Compliance & Quality department. From 1998 to 2002, Mr. Hessekiel held the position of Associate, working on both litigation and corporate matters for Wilson Sonsini Goodrich and Rosati PC. Mr. Hessekiel also worked as an Associate focusing on litigation matters for Heller Ehrman LLP from 1996 to 1998. Prior to joining Heller Ehrman LLP, Mr. Hessekiel also worked for several internationalnon-governmental organizations. Mr. Hessekiel received his J.D. from The George Washington University Law School and is admitted to practice in California. Mr. Hessekiel received a B.A. in Political Science from Duke University. |
Peter Lamb, Ph.D. Executive Vice President, Scientific Strategy and Chief Scientific Officer |
Peter Lamb, Ph.D., has served as Executive Vice President, Scientific Strategy and Chief Scientific Officer since February 2016. Previously, he served as Executive Vice President, Discovery Research and Chief Scientific Officer from September 2009 to February 2016, as Senior Vice President, Discovery Research and Chief Scientific Officer from January 2007 until September 2009, as Vice President, Discovery Pharmacology from December 2003 until January 2007 and as Senior Director, Molecular Pharmacology and Structural Biology from October 2000 until December 2003. Prior to joining Exelixis, from June 1992 until September 2000, Dr. Lamb held positions of increasing responsibility at Ligand Pharmaceuticals, a pharmaceutical company, most recently serving as Director of Transcription Research. Dr. Lamb has held post-doctoral research fellowships at the Carnegie Institution, Department of Embryology, with Dr. S.L. McKnight and the University of Oxford with Dr. N.J. Proudfoot, working in the field of gene regulation. He has authored numerous articles in the fields of gene expression, signal transduction and oncology, and is an author on multiple issued and pending U.S. patents. He has a Ph.D. in Molecular Biology from the ICRF/University of London and a B.A. in Biochemistry from the University of Cambridge. |
2018 Proxy Statement 31
COMPENSATION OF EXECUTIVE OFFICERS
Compensation Discussion and Analysis
This Compensation Discussion and Analysis explains the strategy, design, and decision-making related to our compensation programs and practices for our principal executive officer, our principal financial officer and our three other most highly compensated executive officers, who we refer to collectively as our Named Executive Officers. This Compensation Discussion and Analysis is intended to provide perspective on the information contained in the tables that follow this discussion. For fiscal 2017, our Named Executive Officers were:
Named Executive Officers | Title | |
Michael M. Morrissey, Ph.D. | President and Chief Executive Officer | |
Gisela M. Schwab, M.D. | President, Product Development and Medical Affairs and Chief Medical Officer | |
Christopher J. Senner | Executive Vice President and Chief Financial Officer | |
Jeffrey J. Hessekiel, J.D. | Executive Vice President and General Counsel | |
Peter Lamb, Ph.D. | Executive Vice President, Scientific Strategy and Chief Scientific Officer |
While the principal purpose of this Compensation Discussion and Analysis is to discuss the compensation of our Named Executive Officers, many of the programs discussed apply to other members of senior management who, together with the Named Executive Officers, are collectively referred to as our executive officers.
Executive Summary
Our Business
We are a biotechnology company committed to the discovery, development and commercialization of new medicines to improve care and outcomes for people with cancer. Since our founding in 1994, three products discovered at Exelixis have progressed through clinical development, received regulatory approval, and entered the marketplace. Two are derived from cabozantinib, an inhibitor of multiple tyrosine kinases including MET, AXL and VEGF receptors: CABOMETYX® (cabozantinib) approved for advanced renal cell carcinoma, or RCC, and COMETRIQ® (cobimetinib) approved for progressive, metastatic medullary thyroid cancer, or MTC. The third product, COTELLIC® (cobimetinib), is a formulation of cobimetinib, an inhibitor of MEK, and is marketed under a collaboration with Genentech, Inc. (a member of the Roche Group), or Genentech; it is approved as part of a combination regimen to treat advanced melanoma. Both cabozantinib and cobimetinib have shown potential in a variety of forms of cancer and are the subject of broad clinical development programs for multiple potential oncology indications.
2017 Financial Performance Highlights
We delivered strong financial results in fiscal 2017, increasing total revenue by 136% year over year and ending fiscal 2017 with a healthy cash and investments balance of $457.2 million, of which $452.0 million was available for operations and that we plan to reinvest in our business in order to drive the anticipated expansion and depth of our product offerings. Other key financial highlights from fiscal 2017 include:
| ›› | Cabozantinib franchise net product revenue of $349.0 million, resulting in our second consecutive year of record net product revenue; |
| ›› | Total revenue of $452.5 million, reflecting both strong product demand and significant financial contributions generated from our collaborations; |
| ›› | Total operating expenses of $286.6 million, up 31% from the prior fiscal year, demonstrating disciplined expense management by operating within the parameters of our issued guidance; |
| ›› | Our first full year of operating profit, resulting in diluted earnings per share of $0.49, up from diluted net loss per share of $(0.28) from the prior fiscal year; and |
| ›› | Retired all of our outstanding debt, including our $80.0 million term loan with Silicon Valley Bank and $113.9 million of Secured Convertible Notes due 2018 held by entities associated with Deerfield Management Company, L.P., or the Deerfield Notes. |
32 Exelixis, Inc.
Compensation of Executive Officers | Compensation Discussion and Analysis
Stock Price Performance
Our strong financial and corporate performance in fiscal 2017 also resulted in meaningful value creation for our stockholders. As illustrated below, in fiscal 2017 our stock price continued to significantly outperform both our 2017 peer group (described below) as a whole, as well as the Russell 3000 Index.
FY2015-FY2017 Total Shareholder Return vs 2017 Peer Group
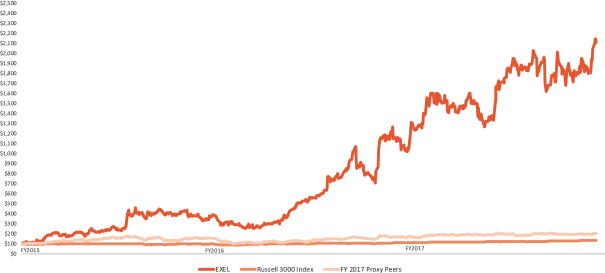
The foregoing graph compares, for the three-year period ended December 31, 2017, the cumulative total stockholder return for our common stock, the Russell 3000 Index and an average of the companies that form our 2017 peer group. The graph assumes that $100 was invested on December 31, 2014 in each of our common stock, the Russell 3000 Index and in each company in our 2017 peer group, and assumes reinvestment of any dividends. It places our corporate performance in context, highlighting the strength of our results versus our 2017 peers as a whole and the market. When considering corporate performance factors, the Compensation Committee’s decision-making in 2017 was rooted in the understanding that our Named Executive Officers led our company to achieve this exceptional success on a relative basis. The stock price performance on the graph is not necessarily indicative of future stock price performance.
2017 Corporate Performance Highlights
In fiscal 2017, we continued to drive growth by executing on our strategy. Key highlights of our corporate performance included:
Successful Commercialization of CABOMETYX for Previously Treated Advanced RCC
| ›› | In the U.S., we expanded our distribution channel in order to improve patient access to CABOMETYX and continued to focus on educating physicians on CABOMETYX’s unique product profile, which translated into strong product demand in 2017. |
| ›› | We worked closely with our partner Ipsen Pharma SAS, or Ipsen, in support of its regulatory strategy and commercialization efforts for CABOMETYX outside the U.S. and Japan, which culminated in regulatory approvals in Australia, Switzerland and South Korea, as well as launches and completed and/or ongoing pricing and reimbursement negotiations in a majority of European Union, or EU, member states. |
Expansion of CABOMETYX Label as a Treatment for Previously Untreated Advanced RCC
| ›› | On December 19, 2017, approximately two months ahead of the assigned Prescription Drug User Fee Act, or PDUFA, action date, the U.S. Food and Drug Administration, or FDA, approved CABOMETYX for an expanded indication to include previously untreated patients with advanced RCC, the most common form of advanced kidney cancer in adults. |
2018 Proxy Statement 33
| ›› | Ipsen submitted to the European Medicines Agency, or EMA, the regulatory dossier for CABOMETYX as a treatment for previously untreated, advanced or metastatic RCC in the EU on August 28, 2017; the filing was subsequently validated on September 8, 2017. |
Positive Results from the CELESTIAL Trial in Previously Treated Advanced HCC following Second Interim Analysis
| ›› | In October 2017, we announced that CELESTIAL, our company-sponsored, global phase 3 trial comparing cabozantinib to placebo in patients with advanced hepatocellular carcinoma, or HCC, who had previously progressed on or were intolerant to sorafenib and up to one additional therapy, met its primary endpoint, with cabozantinib providing a statistically significant and clinically meaningful improvement in overall survival compared to placebo. |
| ›› | Based on the results of CELESTIAL, on March 15, 2018, we submitted a supplemental New Drug Application, or sNDA, to the FDA, for CABOMETYX as a treatment for patients with previously treated advanced HCC. |
Evaluation of Cabozantinib in Combination with Multiple Immune Checkpoint Inhibitors
| ›› | We initiated a phase 3 pivotal trial in previously untreated, advanced or metastatic RCC in collaboration with Bristol-Myers Squibb Company, or BMS, which, pursuant to its amended protocol, is evaluating the combination of cabozantinib with BMS’s immune checkpoint inhibitor, nivolumab. |
| ›› | We initiated a phase 1/2 trial in collaboration with BMS in patients with both previously treated and previously untreated advanced HCC, evaluating cabozantinib in combination with nivolumab and in combination with both nivolumab and ipilimumab. |
| ›› | We initiated a phase 1b trial with various expansion cohorts evaluating cabozantinib and atezolizumab, F.Hoffmann-La Roche Ltd’s, or Roche’s,PD-L1 checkpoint inhibitor, in patients with advanced genitourinary malignancies, which, pursuant to its amended protocol, includes expansion cohorts fornon-small cell lung cancer, or NSCLC, and castration-resistant prostate cancer, or CRPC, in addition to the previously included RCC and urothelial carcinoma. |
Expansion of Global Partnership for Cabozantinib to Japan
| ›› | On January 30, 2017, we entered into a collaboration and license agreement with Takeda Pharmaceutical Company Limited, or Takeda, for the commercialization and further clinical development of cabozantinib in Japan. |
| ›› | In December 2017, Takeda confirmed that the first patient was enrolled in its bridging study evaluating cabozantinib in second-line advanced RCC in Japan. |
Continued Development of COTELLIC
| ›› | Genentech advanced the development program for cobimetinib, with three phase 3 pivotal trials exploring the combination of cobimetinib with atezolizumab or atezolizumab alone in colorectal carcinoma (IMblaze370) and BRAF wild-type melanoma (IMspire170), and the combination of cobimetinib with atezolizumab and vemurafenib in BRAF V600 mutant melanoma (IMspire150). |
| ›› | Enrollment for IMblaze370 was completed in the first quarter of 2017, and Genentech has announced thattop-line results for the trial are expected during the first half of 2018. |
34 Exelixis, Inc.
Compensation of Executive Officers | Compensation Discussion and Analysis
2017 Compensation Program Highlights
In 2017, we achieved significant corporate and financial milestones that helped position us to be able to deliver upon our goal of driving the expansion and depth of our product offerings. The Compensation Committee believes that the 2017 compensation of our employees, including our Named Executive Officers, reflects not only those achievements, but will also encourage appropriate efforts towards the achievement of our commercial objectives and both short-term and long-term research and development goals.
In light of the above, the Board and Compensation Committee took the following key actions with respect to 2017 compensation for our Named Executive Officers:
Key Compensation Actions
|
Description
| |
Modest Salary Increases for Named Executive Officers | In February 2017, the Compensation Committee increased base salaries of our Named Executive Officers by between 3.5% and 6% over salaries for 2016. | |
Granted Substantial Portion of Long-Term Incentive Compensation in the Form of Stock Options | In October 2017, as part of our ongoing equity incentive compensation program, the Compensation Committee granted stock options and restricted stock units, or RSUs, to focus our Named Executive Officers on the company’s long-term performance. For Dr. Morrissey, the mix was 75% stock options and 25% RSUs, and for the other Named Executive Officers, the mix was 50% stock options and 50% RSUs. Stock options are a key aspect of aligning our Named Executive Officers’ compensation with the interests of our stockholders because they provide a return to our Named Executive Officers only if the market price of our common stock appreciates over the stock option term. | |
Approved Annual Cash Bonuses Aligned with Strong Company Performance | In February 2018, the Compensation Committee approved the payment of cash bonuses in the amount of 100% of each Named Executive Officer’s 2017 target cash bonuses resulting from the Compensation Committee’s assessment of the overall achievement of our corporate goals during 2017, and the contribution of each Named Executive Officer toward such achievements. |
Compensation Practices and Governance Highlights
Pay for Performance | Link the compensation of our Named Executive Officers to the success of our corporate goals | |
Stockholder Alignment | Align the interests of our Named Executive Officers with those of our stockholders through the use of long-term equity incentives | |
Equity Plan Features | Apply a maximum7-year term for stock options No repricing of underwater stock options without prior stockholder approval 2017 Plan includes minimum vesting requirements of no less than one year for all types of awards, subject to limited exceptions | |
Stock Ownership Guidelines | Apply stock ownership guidelines to directors and executive officers to further align their interests with those of our stockholders | |
Change in Control Provisions | No excessive change in control or severance payments Provide “double-trigger” change in control benefits No taxgross-ups on severance or change in control benefits | |
Perquisites, Retirement and | Named Executive Officers do not receive excessive perquisites or post-termination retirement or pension benefits that are not available to all employees generally | |
Prohibition on Hedging and | Prohibit hedging and purchases on margin by executive officers and directors. | |
Meaningful Limits on Pledging | Limit pledging of our common stock by executive officers and directors to circumstances where the individual can clearly demonstrate the financial capacity to repay the loan without resort to the pledged securities. No executive officers or directors pledged our common stock during 2017. |
2018 Proxy Statement 35
Objectives of the Compensation Program
The primary goals of our executive compensation program are to:
| ›› | Provide market-competitive compensation that attracts, retains and motivates our executive officers to achieve our short- and long-term business objectives; |
| ›› | Align our executive officers’ compensation with the interests of our stockholders; and |
| ›› | Reward our executive officers for our success in achieving our corporate goals. |
The success of any biopharmaceutical company is significantly influenced by the quality of its work force. We believe it is critical to our business that we retain our core team of highly qualified employees, including our Named Executive Officers. As a testament to their high value in the marketplace, large pharmaceutical and biopharmaceutical companies and strong local competitors have aggressively recruited our executives and other skilled employees, with the most critical positions at our company among those that are the most in demand. In light of these circumstances, each year we design our executive compensation program to help attract and retain highly qualified individuals with relevant experience in the biopharmaceutical industry to manage the varied aspects of our growing business operations. In fact, our Named Executive Officers have an average tenure of over ten years with Exelixis, which we believe is evidence of the effectiveness of our compensation program with respect to retention.
How We Determine Executive Compensation
Role of the Compensation Committee, Compensation Consultants and Executive Officers in Compensation Decisions
The Compensation Committee is responsible for determining and approving the compensation packages offered to our Named Executive Officers. When appropriate, the Compensation Committee will solicit the input of, or present its recommendations on compensation matters for consideration and approval to, the full Board. The Compensation Committee acts on behalf of the Board in discharging the Board’s responsibilities with respect to overseeing our compensation policies, plans, and programs, and establishing and reviewing general policies relating to compensation and benefits of our employees. The Compensation Committee also administers our equity and other incentive plans. The Compensation Committee does not delegate any of its functions to others in determining executive compensation.
Under its charter, the Compensation Committee has the authority to obtain the advice and assistance from external advisors to assist it in the performance of its duties. In accordance with this authority, the Compensation Committee engages external advisors to advise on executive officer compensation, including base salaries, bonus targets and equity compensation, as well as director compensation. These advisors also prepare industry compensation data, assist with the development of our peer group, and advise the Compensation Committee on best industry practices and relevant changes to regulations that may impact the Compensation Committee’s decision-making process with regard to executive officer and director compensation determinations. In November 2016, the Compensation Committee retained the consulting firm Compensia, to compile biopharmaceutical industry compensation data for the purpose of developing a peer group for fiscal 2017 and to help us evaluate the compensation of our executive officers and directors against that peer group. Compensia also prepared a market analysis of long-term incentive compensation of our executive officers compared to our peer group. This market analysis was reviewed with the Compensation Committee and used to guide the Compensation Committee’s decisions regarding annual long-term equity compensation determinations in October 2017.
Dr. Morrissey, our President and Chief Executive Officer, also participates in the Compensation Committee’s deliberations with respect to Named Executive Officer compensation other than his own, and is not present during deliberation and voting on his compensation. Each year, Dr. Morrissey and other senior management develop annual corporate business goals for the company, which are reviewed and, subject to their input, approved by the Compensation Committee and the Board. In determining Named Executive Officer compensation recommendations for 2017, our Chief Executive Officer solicited the input of and received documentary support from our senior human resources personnel. The Compensation Committee also received documentary support, including peer group and industry data, from third-party sources with respect to base salaries, target annual cash bonuses, and equity compensation. In particular, in January 2017, at the direction of the Compensation Committee, our senior human resources personnel retained Radford to provide a market analysis of executive compensation of our executive officers compared to our peer group that was previously approved by the Compensation Committee based on advice from Compensia. This market analysis was reviewed with the Compensation Committee and used to guide the Compensation Committee’s decisions regarding 2017 base salaries and 2017 bonus targets in February 2017.
36 Exelixis, Inc.
Compensation of Executive Officers | Compensation Discussion and Analysis
The Compensation Committee did not engage any other consultants in 2017 with respect to executive and/or director compensation matters, other than Compensia and Radford. The Compensation Committee assessed the independence of both Compensia and Radford pursuant to SEC rules and concluded that the work performed by both compensation consultants for the Compensation Committee had not raised any conflict of interest.
Compensation Committee Process
In setting the level of salary, cash bonus and equity compensation for our Named Executive Officers, the Compensation Committee typically considers various factors, including:
| ›› | the performance of the company during the prior year; |
| ›› | the performance of each Named Executive Officer during the prior year; |
| ›› | the criticality of each Named Executive Officer’s skill set and expected future contributions to our business; |
| ›› | the growing complexity of our business resulting in increased workloads and responsibilities of our Named Executive Officers; |
| ›› | the appropriate mix of compensation for each Named Executive Officer; |
| ›› | the percentage of vested versus unvested equity awards held by each Named Executive Officer; and |
| ›› | market data for our industry and specific peer group. |
The Compensation Committee balances each of these factors against our cash resources and the critical need to prioritize clinical development investments over other expenditures, as well as our aggregate equity burn rate. When establishing each element of a Named Executive Officer’s compensation, the Compensation Committee also typically takes into consideration the executive officer’s historical salary, cash bonus and equity compensation, as well as his or her total current and potential compensation. Using this process, our Compensation Committee strives to ensure that our executive compensation program as a whole is competitive. In determining the amount and mix of compensation elements and whether each element provides the correct incentives and rewards for performance consistent with our short and long-term goals and objectives, the Compensation Committee relies on its subjective judgment about each individual rather than adopting a formulaic approach to compensatory decisions.
The Compensation Committee believes that it is important when making its compensation decisions to be informed as to the current practices of comparable publicly-held companies. To this end, the Compensation Committee reviews market data, which include competitive information relating to compensation levels for comparable positions in the biotechnology and life sciences sector.
Stockholder Advisory Vote on Executive Compensation
We provide our stockholders with the opportunity to cast an annual advisory vote on our executive compensation program, which the Compensation Committee takes into account when determining the compensation of the company’s Named Executive Officers. At our annual meeting of stockholders held in May 2017, approximately 97% of the votes present and entitled to vote on thesay-on-pay proposal voted in favor of the proposal. Our Compensation Committee considered the outcome of the vote on thesay-on-pay proposal to be an endorsement of the Compensation Committee’s policies and practices and has continued to conduct its review of executive compensation generally consistent with past practice.
Competitive Assessment—Peer Group and Market Data
The Compensation Committee reviews and makes adjustments to the composition of the peer group as often as deemed necessary, to account for changes in both our business and the businesses of the companies in the peer group. As discussed above, the Compensation Committee, in consultation with Compensia, developed a peer group to be used as a reference point for setting base salaries, bonus targets and annual equity incentive compensation for 2017. The Compensation Committee does not have a specific target compensation level for the Named Executive Officers and does not otherwise use a formulaic approach to setting pay at a particular positioning within the market data.
In developing the 2017 peer group, the Compensation Committee considered the significant evolution of our business and meaningful developments in comparison to the 2016 peer group companies. It also considered what other companies might make suitable additions to the 2017 list. The key qualitative and quantitative considerations that influenced the development
2018 Proxy Statement 37
of the 2017 peer group were: (1) industry—including, biotechnology and pharmaceutical companies; (2) therapeutic area, including companies with an oncology product focus, while avoiding the inclusion of companies marketing solely orphan and rare disease drugs; (3) stage of business, as reflected by the existence of a marketed product; (4) location of headquarters and whether the company is traded on a major U.S. exchange; (5) market capitalization between 0.25x—4.0x of our then-current market capitalization (approximately $750 million—approximately $12.0 billion) and (6) revenue between 0.5x—5.0x of our then revenue (approximately $60 million—approximately $600 million). Due to the limited number of companies that had comparable revenue growth expectations after taking into consideration the possibility that our revenue could grow appreciably due to increased CABOMETYX sales during 2017, the Compensation Committee expanded the top end of the revenue range from 2.0x of current revenue to 5.0x.
Following this analysis, the Compensation Committee identified the following 19 publicly-traded, U.S—based biotechnology/pharmaceutical companies as our peer group to be used in reviewing compensation for 2017:
Fiscal 2017 Peer Group | ||||
ACADIA Pharmaceuticals Inc. | Intercept Pharmaceuticals, Inc. | Neurocrine Biosciences, Inc. | ||
Acorda Therapeutics, Inc. | Ionis Pharmaceuticals, Inc. | Seattle Genetics, Inc. | ||
Alkermes Public Limited Company | Ironwood Pharmaceuticals, Inc. | Supernus Pharmaceuticals, Inc. | ||
ARIAD Pharmaceuticals, Inc. | Lexicon Pharmaceuticals, Inc. | TESARO, Inc. | ||
Corcept Therapeutics Incorporated | Momenta Pharmaceuticals, Inc. | The Medicines Company | ||
Eagle Pharmaceuticals, Inc. | Nektar Therapeutics | Vanda Pharmaceuticals, Inc. | ||
Halozyme Therapeutics, Inc. | ||||
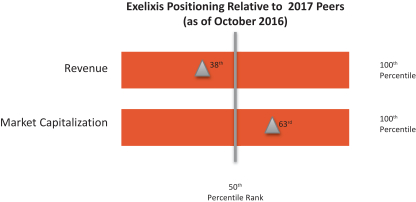
Of these companies, 13 companies (ARIAD Pharmaceuticals, Inc., Corcept Therapeutics Incorporated, Eagle Pharmaceuticals, Inc., Halozyme Therapeutics, Inc., Intercept Pharmaceuticals, Inc., Ironwood Pharmaceuticals, Inc., Ionis Pharmaceuticals, Inc., Momenta Pharmaceuticals, Inc., Nektar Therapeutics, Neurocrine Biosciences, Inc., Seattle Genetics, Inc., Supernus Pharmaceuticals, Inc. and Vanda Pharmaceuticals Inc.) were in our 2016 peer group. The Compensation Committee did not include in our 2017 peer group six companies (Alnylam Pharmaceuticals, Inc., Array BioPharma, Inc., FibroGen, Inc., Ophthotech Corporation, Portola Pharmaceuticals, Inc. and PTC Therapeutics, Inc.) that were in our 2016 peer group, because they were either a clinical-stage company or had a market capitalization that fell far below the selection criteria.
In addition to the analysis of the compensation data from the peer group, due to our anticipated growth during 2017, the Compensation Committee also reviewed the compensation levels and disclosed program design for executive officers of Endo International plc, United Therapeutics Corporation, Jazz Pharmaceuticals Public Limited Company, Medivation, Inc., BioMarin Pharmaceutical Inc., Incyte Corporation, Vertex Pharmaceuticals Incorporated and Alexion Pharmaceuticals, Inc. to provide perspective on what executive officer pay would look like at the next stage of growth. We refer to this group of companies as our reference peer group.
38 Exelixis, Inc.
Compensation of Executive Officers | Compensation Discussion and Analysis
Elements of Compensation
Our executive compensation program consists of three principal components: base salary, annual discretionary cash bonus, and equity incentive compensation. Our Named Executive Officers are also eligible to participate, on the same basis as other employees, in our 401(k) Plan, our employee stock purchase plan and other benefit programs generally available to all employees. The Compensation Committee has not established any formal policies or guidelines for allocating compensation between current and long-term incentive compensation, or between cash andnon-cash compensation.
Base Salary. We pay a base salary to each of our Named Executive Officers to provide an appropriate and competitive base level of current cash income. The Compensation Committee reviews annually each Named Executive Officer’s base salary and sets salary generally based on the criticality of such officer’s skill set and expected future contributions to our business, the performance of the Named Executive Officer during the prior year, the growing complexity of our business and market data for our industry and specific peer group, with each of these factors balanced against the critical need to effectively manage our cash resources to prioritize clinical development investments over other expenditures, as well as other elements of the executive officer’s compensation.
Annual Cash Bonus. We pay annual cash bonuses to our Named Executive Officers to align the Named Executive Officers’ compensation with our business objectives, and to enable us to retain and reward executive officers who fulfill or exceed performance expectations. Our Compensation Committee determines bonus targets (expressed as a percentage of base salary) based on the seniority of the applicable position. The bonus targets are reviewed annually by the Compensation Committee, taking into consideration market data for our industry and specific peer group. Actual cash bonuses have been discretionary but subject to the Compensation Committee’s assessment as to the achievement of our corporate goals on an overall basis and the Compensation Committee’s subjective determination of each Named Executive Officer’s contribution toward the achievement of such goals. Whether or not a bonus is paid is solely within the discretion of our Compensation Committee, which will weigh the relative importance of achievement or failure to achieve certain of our corporate goals against the achievement or failure to achieve others, all in the context of our overall annual performance. The actual bonus awarded in any year, if any, may be more or less than the target, depending on individual performance and the achievement of our corporate goals, as well as other factors, including the critical need to effectively manage our cash resources to prioritize clinical development investments over other expenditures.
Equity Incentive Compensation. We have granted, and intend to continue to grant, stock options and RSUs, and potentially other types of equity incentive awards, to align our Named Executive Officers’ compensation with achievement of the company’s key business objectives and its long-term performance, thereby linking their compensation to the interests of our stockholders. The Compensation Committee believes that stock options are an effective equity-based tool to motivate our Named Executive Officers to pursue critical company interests aggressively, because options only have value if the value of our company as reflected by our stock price increases over time. The Compensation Committee believes that RSUs are another effective instrument for employee retention, and that they also have incentive value since the value of RSUs increases as our stock price increases over time. RSUs are also generally less dilutive to our stockholders than stock options. Further, RSUs continue to have incentive value even in the event of a severe stock price decline, unlike stock options that can lose their incentive value in such an event. Because of the overall importance to our success of aggressively pursuing our strategic goals, as well as the critical need to effectively manage our cash resources to prioritize clinical development investments over other expenditures, a significant portion of the Named Executive Officers’ total compensation typically has consisted of, and is expected to continue to consist of, equity-based awards in the form of stock options and RSUs.
Change in Control and Severance Benefit Plan. We have adopted a Change in Control and Severance Benefit Plan, in which all of our Named Executive Officers participate, to maintain the competitiveness of our executive compensation program and to remove or at least reduce an executive’s potential personal bias against a takeover attempt. It also provides incentives to our executive officers to remain with our company, and objectively evaluate and facilitate an acquisition of our company should consideration of such a transaction be determined appropriate by the Board and in the best interests of our stockholders. A description of this plan is set forth below under the heading “Potential Payments Upon Termination or Change in Control.” This plan is a double-trigger plan, in which each participant receives benefits under the plan only if the participant is terminated without cause or is constructively terminated in connection with a change in control, rather than a single-trigger plan, in which each participant would receive benefits under the plan if a change in control occurs or the participant resigns for any reason after a change in control. We adopted a double-trigger plan because it protects the participants from post-change in control events that are not related to the participants’ performance, encourages them to stay throughout a transition period in the event of a change in control, and does not provide for benefits for a participant who remains with the surviving company in a comparable position. The Compensation Committee believes that these benefits are an important element of the Named Executive Officers’ retention and motivation and are consistent with
2018 Proxy Statement 39
compensation arrangements provided in a competitive market for executive talent, and that the benefits of such severance rights agreements, including generally requiring a release of claims against us as a condition to receiving any severance benefits are in our best interests.
Other Benefits. We have a 401(k) Plan in which substantially all of our employees, including our Named Executive Officers, are entitled to participate. Employees contribute their own funds, as salary deductions, on apre-tax basis. Contributions may be made up to plan limits, subject to government limitations. We match employee contributionsdollar-for-dollar up to $8,500 in each calendar year, in the form of Exelixis common stock. Our employee stock purchase plan allows for eligible employees to purchase shares of our common stock at a price equal to the lower of 85% of the closing price on the first day of thesix-month offering period or 85% of the closing price on the final day of such offering period, subject to specified limits. We provide health care, dental and vision benefits to all full-time employees, including our Named Executive Officers. These and other standard health and welfare benefits are available to all eligible employees, subject to applicable laws. We provide these benefits because we believe they are consistent and competitive with the market practices of our peers.
2017 Compensation Decisions
Through our compensation program, we seek to align pay and performance. To that end, a significant portion of our Named Executive Officers’ compensation is “at risk” because it is variable, performance-based and in large part dependent on the success of our company.At-risk compensation includes long-term equity based awards, the value of which depends on increases in the price of our common stock, and annual incentive cash bonuses, which relate to the overall level of achievement of our company performance goals and, other than for Dr. Morrissey, each Named Executive Officer’s individual contributions toward the achievement of such performance goals. The following charts highlight the pay mix for our Chief Executive Officer and all of our other Named Executive Officers as a group.
Chief Executive Officer Pay Mix1 91% of CEO 2017 Compensation is Considered At-Risk | All Other Named Executive Officers (as a group) Pay Mix1 85% of All Other Named Executive Officers (as a group) 2017 Compensation is Considered At-Risk
| |
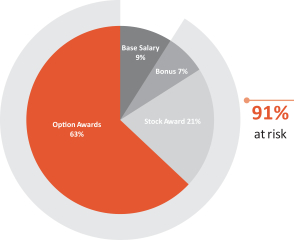 | 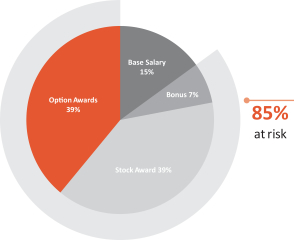 |
| 1 | Percentages calculated from values reported in the 2017 Summary Compensation Table |
2017 Base Salaries
In considering the appropriate level of base salaries for our Named Executive Officers, the Compensation Committee did not apply a formula, but rather employed a holistic analysis emphasizing: (1) the individual contribution of the Named Executive Officer to our exceptional 2016 commercial, regulatory and financial achievements; (2) the criticality of each Named Executive Officer’s skill set and relative expected future contributions to our business; (3) the growing complexity of our business resulting in increased workloads and responsibilities of each of our Named Executive Officers; and (4) other competing business priorities for our company that require substantial investment. The Compensation Committee also considered the base salaries of similarly situated executives at our peer group companies, and in contemplation of the potential continuation of our rapid growth, of similarly situated executives at our reference peer group companies, for an understanding of whether our compensation program is competitively positioned to retain our highly qualified Named Executive Officers.
40 Exelixis, Inc.
Compensation of Executive Officers | Compensation Discussion and Analysis
Following such analysis, in February 2017, the Compensation Committee determined that each Named Executive Officer should receive a modest increase in his or her base salary for 2017 as follows:
Name | 2016 Base Salary | 2017 Base Salary | Percentage Increase | |||||||||
Michael M. Morrissey, Ph.D. | $ | 850,000 | $ | 901,000 | 6.00 | % | ||||||
Gisela M. Schwab, M.D. | $ | 600,000 | $ | 636,000 | 6.00 | % | ||||||
Christopher J. Senner | $ | 540,000 | $ | 567,000 | 5.00 | % | ||||||
Jeffrey J. Hessekiel, J.D. | $ | 489,038 | $ | 511,045 | 4.50 | % | ||||||
Peter Lamb, Ph.D. | $ | 460,000 | $ | 476,100 | 3.50 | % | ||||||
2017 Annual Cash Bonuses
In February 2017, the Compensation Committee determined 2017 target annual cash bonuses (expressed as a percentage of base salary) should remain at the same levels as 2016 for all Named Executive Officers other than Dr. Morrissey (i.e., 50% for Dr. Schwab and 45% for each of Mr. Senner, Mr. Hessekiel and Dr. Lamb). The Compensation Committee’s decision regarding 2017 target bonuses was based on its subjective assessment that the percentages of base salaries previously established were appropriate and continued to align us competitively with our 2017 peer group. Following a review of a market analysis prepared by Radford, in order to better align Dr. Morrissey’s total cash compensation with those of chief executive officers of the companies included in our 2017 peer group and in recognition of his greater role in determining the course of, and ability to influence the future of the company, as well as the importance of his leadership to the company’s achievement of its 2017 business and financial objectives, the Compensation Committee approved an increase to Dr. Morrissey’s 2017 target annual bonus to 75% (expressed as a percentage of base salary). The target bonus amounts for the company’s cash bonuses are intended to serve as general guidelines for the determination of actual bonuses and are not designed to set formulaic payout levels. The Compensation Committee determined at the time that it would not be appropriate to apply an objective formulaic approach during a period of such rapid change and evolution for the company.
In connection with establishing the bonus program for 2017, the Compensation Committee reviewed management’s proposed commercial, product development, pipeline development, finance and legal goals and recommended them to the Board for approval. In February 2017, the Board then reviewed and approved the proposed company goals for 2017, as identified in the table below. In selecting these goals, the Compensation Committee and the Board believed that they were appropriate drivers for our business, as they provided a balance between the efforts necessary to continue to execute on a successful commercial launch for CABOMETYX and generate further growth in our business, all while maintaining a solid financial position, which together, would enhance stockholder value. At the time the 2017 corporate performance goals were set, the Compensation Committee and management believed that such goals were challenging and achieving them would require not only continued strong commercial performance, favorable clinical trial results, business development opportunities, and prudent fiscal and legal management, but also a high level of effort and execution on the part of our Named Executive Officers.
During 2017, management reported regularly to the Compensation Committee and the Board on the status of the company’s performance against these goals, including in formal meetings in February, May, September and December. In February 2018, the Compensation Committee and the Board evaluated the company’s performance in relation to the 2017 goals, as well as, other than for Dr. Morrissey, each Named Executive Officer’s individual contribution to the achievement of those goals. As further described below, the Compensation Committee concluded that 2017 was a year of meaningful
2018 Proxy Statement 41
accomplishments during which the Companypartially-met, met or exceeded each of the goals, as highlighted by the corporate achievements identified in the table below.
| CORPORATE GOALS | ACHIEVEMENTS | EVALUATION | ||||
| Product Development | File a sNDA for CABOMETYX as a treatment for patients with previously untreated advanced RCC based on CABOSUN results | ›› Based on the results from CABOSUN, filed a sNDA with the FDA for CABOMETYX as a treatment for patients with previously untreated advanced RCC. The FDA granted Priority Review of the filing and assigned a PDUFA action date of February 15, 2018.
›› Received FDA approval for CABOMETYX as a treatment for patients with previously untreated advanced RCC on December 19, 2017, two months ahead of the assigned PDUFA date. | Exceeded | |||
| Conduct a second interim analysis, or IA, for CELESTIAL, and if positive, file a sNDA for CABOMETYX as a treatment for patients with previously treated advanced HCC | ›› Completed a second IA for CELESTIAL, which met its primary endpoint, with cabozantinib providing a statistically significant and clinically meaningful improvement in overall survival compared to placebo.
›› Following internal preparations and discussions with the FDA during 2017, filed a sNDA on March 15, 2018 for CABOMETYX as a treatment for patients with previously treated advanced HCC. | Partially Met | ||||
| Initiate late stage development of cabozantinib in combination with an immunotherapy in patients with previously untreated advanced RCC and in bladder cancer | ›› Initiated CheckMate 9ER, a phase 3 pivotal trial evaluating the combination of cabozantinib with nivolumab in previously untreated, advanced or metastatic RCC and CheckMate 040 evaluating the same combination and also cabozantinib with both nivolumab and ipilimumab in a phase 1/2 trial in both previously treated and previously untreated advanced HCC.
›› Initiated a phase 1b trial evaluating cabozantinib and atezolizumab in patients with advanced genitorurinary malignancies, which, pursuant to its amended protocol includes expansion cohorts for NSCLC and CRPC, in addition to the previously included RCC and UC. | Partially Met | ||||
| Continue the broad evaluation of cabozantinib in other tumor types through externally-sponsored studies | ›› Managed 31 ongoing and 29 planned externally sponsored trials evaluating the clinical and therapeutic potential of cabozantinib, including those administered through our Cooperative Research and Development Agreement with National Cancer Institute’s Cancer Therapy Evaluation Program and our investigator sponsored trial program. | Met | ||||
| Support cabozantinib worldwide regulatory filings by cabozantinib partners | ›› Supported EMA validation of the regulatory dossier submitted by Ipsen, for CABOMETYX as a treatment for patients with previously untreated advanced RCC in the EU.
›› Supported Ipsen’s receipt of regulatory approvals in Australia, Switzerland and South Korea for CABOMETYX as a treatment for advanced RCC in adults following prior VEGF targeted therapy, launches in France, Germany, Italy, Spain and the United Kingdom and associated pricing and reimbursement negotiations in the EU member states. | Exceeded |
42 Exelixis, Inc.
Compensation of Executive Officers | Compensation Discussion and Analysis
| CORPORATE GOALS | ACHIEVEMENTS | EVALUATION | ||||
| Commercial | Continue to execute successful commercial launch for CABOMETYX in the U.S. and exceed the net product revenue target established by the Board | ›› Generated cabozantinib franchise net product revenue of $349.0 million during 2017. | Exceeded | |||
| Develop and implement launch strategy to ensure organizational readiness should the company receive FDA approval for CABOMETYX as a treatment for patients with previously untreated advanced RCC and/or patients with previously treated advanced HCC | ›› The organization was launch-ready immediately upon the FDA’s approval for CABOMETYX as a treatment for patients with previously untreated advanced RCC on December 19, 2017.
›› Continued launch preparedness efforts in line with regulatory timelines for a potential approval of CABOMETYX as a treatment for patients with previously treated advanced HCC. | Met | ||||
| Pipeline Development | Support licensing efforts for a late preclinical/clinical oncology asset and rapidly advance development of the asset oncein-house | ›› Conducted a worldwide landscape review of oncology opportunities, followed by due diligence efforts, site visits and early stage partnering discussions for multiplepre-clinical and clinical stage oncology assets, resulting in the execution of an exclusive license and collaboration agreement with StemSynergy Therapeutics, Inc., or StemSynergy, on January 8 2018. | Partially-met | |||
| Complete the initial build out of an internal discovery team and advance initial potential programs to a preclinicalgo/no-go decision (i.e., proof of concept to initiate full discovery program) | ›› Hired the necessary full-time employees to enable restart of internal discovery work, conducted multipleproof-of-concept experiments and advanced one program to preclinical development. | Met | ||||
| Finance and Legal | Manage cash balance to a target established by the Board, and manage operating expenses within the budget approved by the Board | ›› Managed operating expenses to $286.6 million and ended fiscal 2017 with a cash balance of $457.2 million. | Met | |||
| Execute an agreement with a partner for the development and commercialization of cabozantinib in Japan | ›› Entered into an exclusive licensing agreement with Takeda to commercialize current and potential future cabozantinib indications in Japan. | Met | ||||
| Effectively manage the JAMS arbitration hearing in the dispute with Genentech/Roche regarding cobimetinib | ›› Amended our collaboration agreement with Genentech in connection with the resolution of our arbitration, which provides for a favorably- revised revenue and cost-sharing arrangement, that became effective as of July 1, 2017, and that is applicable to current and all potential future commercial uses of COTELLIC. | Met | ||||
| Repay outstanding debt with Silicon Valley Bank and the Deerfield Notes | ›› Retired all outstanding debt, including our $80.0 million term loan with Silicon Valley Bank and our Deerfield Notes in the amount of $123.8 million. | Met |
2018 Proxy Statement 43
The Compensation Committee did not assign any weighting to specific company goals in making its assessment of overall performance, but rather made its assessment of our overall performance against the goals from a holistic perspective. The Compensation Committee also recognized the individual contributions, other than for Dr. Morrissey, toward achievement of the corporate goals and the combined contribution of our Named Executive Officers and cohesive management effort that led to these 2017 corporate achievements. Specifically, the Compensation Committee considered the following contributions from each Named Executive Officer, other than Dr. Morrissey:
| ›› | Dr. Schwab’s overall leadership of our product development and medical affairs organizations and her performance with respect to the execution of our product development goals in 2017, particularly with respect to the FDA’s approval of CABOMETYX as a treatment for patients with previously untreated advanced RCC, positivetop-line results from CELESTIAL, support of cabozantinib worldwide regulatory filings by collaboration partners, and the initiation of multiple clinical trials of cabozantinib in combination with immunotherapy agents; |
| ›› | Mr. Senner’s overall leadership of our finance organization and his performance with respect to the execution of our financial goals in 2017, particularly with respect to the repayment of all of our outstanding debt; |
| ›› | Mr. Hessekiel’s overall leadership of our legal and compliance organizations and his performance with respect to our business and legal goals in 2017, particularly with respect to the resolution of our arbitration with Genentech; and |
| ›› | Dr. Lamb’s overall leadership of our research and discovery organization and his performance with respect to our pipeline development goals, particularly with respect to the progress on the license and collaboration agreement with StemSynergy and the rebuilding of our discovery organization. |
Although the Compensation Committee determined that, overall, our corporate and financial performance in 2017 exceeded expectations and that this exceptional performance could reasonably have justified above-target bonus payments for Named Executive Officers, the Compensation Committee was conscious of the importance of financial discipline at this particular juncture in our company’s history and the critical need to effectively manage our cash resources to prioritize clinical development investments over other expenditures. Therefore, in February 2018, the Compensation Committee approved annual cash bonus payments at 100% of each Named Executive Officer’s target amount, as reflected in the table below.
Name | 2017 Target (% of 2017 | 2017 Bonus Payout (% of 2017 Target | 2017 Bonus Payout | |||||||||
Michael M. Morrissey, Ph.D. | 75 | % | 100 | % | $ | 675,750 | ||||||
Gisela M. Schwab, M.D. | 50 | % | 100 | % | $ | 318,000 | ||||||
Christopher J. Senner | 45 | % | 100 | % | $ | 255,150 | ||||||
Jeffrey J. Hessekiel, J.D. | 45 | % | 100 | % | $ | 229,970 | ||||||
Peter Lamb, Ph.D. | 45 | % | 100 | % | $ | 214,245 | ||||||
2017 Equity Incentive Awards
In October 2017, the Compensation Committee reviewed the annual equity compensation awards for our Named Executive Officers for 2017. In determining the form of equity incentive grants for the Named Executive Officers, the Compensation Committee:
| ›› | evaluated the status of equity incentive awards held by the Named Executive Officers to assess the retention and incentive values of those awards; |
| ›› | determined the appropriate size and value of new equity incentive awards for our Named Executive Officers, taking into consideration the need to be competitive in the market to retain the talent required to continue to execute on the successful commercial launch of CABOMETYX and further advance the cabozantinib development program; and |
| ›› | determined the grant of a mix of 75% stock options and 25% RSUs for Dr. Morrissey, and 50% stock options and 50% RSUs for all other Named Executive Officers, to align our Named Executive Officer’s interests with the interests of our stockholders, while allowing us to minimize dilution and deliver an appropriate level of compensation. |
When determining the appropriate size and value of equity incentive awards, the Compensation Committee asked Compensia to provide guidance with respect to implementing a program that would incentivize our Named Executive
44 Exelixis, Inc.
Compensation of Executive Officers | Compensation Discussion and Analysis
Officers to drive toward the achievement of key company priorities and increase stockholder value over the long-term, while maintaining competitive market practices and being mindful of the company’s equity burn rate. Compensia prepared a market analysis of long-term incentive compensation of our executive officers compared to our 2017 peer group. However, due to the significant appreciation of our stock price over the course of 2017, at the time the 2017 equity awards were granted, our market capitalization was positioned at the 85th percentile of our 2017 peer group. While we have historically focused on grant date dollar value when determining the size of ongoing equity awards, the Compensation Committee determined that as a result of this increase in market capitalization, it would be more prudent to evaluate equity grants for the Named Executive Officers as a percentage of market capitalization of a selectsub-set of our 2017 peer group, including Seattle Genetics, Inc., Alkermes Public Limited Company and TESARO, Inc. and one member of our reference peer group Jazz Pharmaceuticals Public Limited Company because the Compensation Committee believed that each of these companies were a good comparator based on its current financial and business characteristics and trajectory. Reviewing equity grant values as a percent of market capitalization can provide an understanding of how much of a company’s market capitalization is used for executive equity awards and can help to normalize for varying market capitalizations. At the direction of the Compensation Committee, Compensia prepared an analysis taking the last annual grant reported in each of those companies proxy statements divided by the market capitalization of each company at the time of grant to determine the percent of market capitalization granted to each Named Executive Officer of those peer companies. The Compensation Committee reviewed the materials provided by Compensia and determined that the equity awards for each of our Named Executive Officers should be sized substantially below the 50th percentile of the equity grants as a percent of market capitalization of this select peer group.
As part of the decision making process, the Compensation Committee considered the challenges of managing a rapidly evolving organization from one rooted in drug discovery and development to one focused on product commercialization, as well as Exelixis’ need to retain a cohesive management team to facilitate the continued commercial success of CABOMETYX, and the achievement of development and regulatory objectives that would position the company for future success. The Compensation Committee also believed it was important that the value of the equity awards continue to reflect the individual performance of each Named Executive Officer during the 2017 fiscal year to date and criticality of each Named Executive Officer’s skill set and expected future contributions to our business, and in October 2017, approved the following grants to the Named Executive Officers:
Name | Number of Shares Subject to Time- Based Stock Options | Number of Shares Subject to RSUs | ||||||
Michael M. Morrissey, Ph.D. | 480,000 | 80,000 | ||||||
Gisela M. Schwab, M.D. | 150,000 | 75,000 | ||||||
Christopher J. Senner | 125,000 | 62,500 | ||||||
Jeffrey J. Hessekiel, J.D. | 100,000 | 50,000 | ||||||
Peter Lamb, Ph.D. | 110,000 | 55,000 | ||||||
The stock options for each of the Named Executive Officers have an exercise price of $24.41 per share, the fair market value of our common stock on the date of grant (October 3, 2017), and expire seven years from the date of grant or earlier upon termination of service with us. All of the stock options vest over a four year period following the grant date and the RSUs vest annually over approximately a four year period following the first quarterly vesting date (i.e., February 15th, May 15th, August 15th and November 15th) following the date of grant. Vesting of these stock options and RSUs will cease upon termination of service as an employee for any reason. The Named Executive Officers are party to our Change in Control and Severance Benefit Plan, which provides for acceleration of vesting of the award in the event of certain specified change in control events involving us, for the reasons discussed above.
Other Compensation Information
Timing of Equity Awards
Annual grants of equity awards to our executive officers, including our Named Executive Officers, are generally determined and approved atpre-scheduled Compensation Committee meetings, with such meeting date typically serving as the grant date. However, the Compensation Committee may sometimes approve the grant of equity awards to executive officers and other employees in advance of its next scheduled meeting, either at a special meeting or by unanimous written consent, in
2018 Proxy Statement 45
connection with certain new hires, promotions and other circumstances where the Compensation Committee deems it appropriate to make such grants. The grant dates for these equity awards are typically the same date that a newly hired officer begins employment or the effective date of an officer’s promotion, as applicable. Grants made to new hires below the level of Vice President, are approved on a monthly basis by our Equity Award Committee, comprised solely of Dr. Morrissey, acting in his capacity as a director pursuant to authority delegated to him by the Compensation Committee. All stock options are granted with an exercise price that is not less than the closing price of our common stock on The Nasdaq Global Select Market on the grant date. We have no plan or practice to time option grants in coordination with the release ofnon-public information, and we do not time the release ofnon-public information to affect the value of executive compensation.
Stock Ownership Guidelines
In February 2018, the Board adopted Stock Ownership Guidelines for our Named Executive Officers in order to further align their financial interests with those of our stockholders, as well as to promote sound corporate governance. The Stock Ownership Guidelines for our Named Executive Officers provide for ownership targets as follows:
Position | Ownership Level | |
Chief Executive Officer | Lesser of 160,000 shares or a value equivalent to 5 times annual base salary | |
Other Named Executive Officers | Lesser of 25,000 shares or a value equivalent to 1.5 times annual base salary |
In considering appropriate guidelines for our executive officers and directors, the Compensation Committee reviewed a market analysis of stock ownership guidelines of our peer group established by the Compensation Committee for 2018 executive and director compensation determinations. The approved guidelines are representative of the median of our 2018 peer group and provide for a “lesser of” approach to address stock price volatility often experienced by biotechnology companies.
All Named Executive Officers are expected to achieve their stock ownership targets within five years of becoming subject to these guidelines. The policy includes procedures for granting exemptions in the case of severe financial hardship. In determining ownership levels for each Named Executive Officer under our Stock Ownership Guidelines, credit is provided for shares held outright (including shares owned through trusts, our 401(k) Plan, or by a spouse), as well as 50% of the number of vested, but unexercised, stock options. No credit is provided for restricted stock units until they vest. The values for all shares determined to be held by Named Executive Officers are based on the200-day average stock price as of the measurement date. As of February 15, 2018, the date the stock ownership guidelines were adopted by the Board, all of our Named Executive Officers had met the required ownership targets.
“Clawback” Policy
The Compensation Committee has not yet established a policy to recover bonuses from our executive officers if the performance objectives that led to a bonus determination were to be restated, or found not to have been met to the extent originally believed by the Compensation Committee. As a public company, if we are required to restate our financial results due to our material noncompliance with any financial reporting requirements under the federal securities laws as a result of misconduct, our Chief Executive Officer and Chief Financial Officer may be legally required to reimburse us for any bonus or other incentive-based or equity-based compensation they receive, in accordance with the provisions of Section 304 of the Sarbanes-Oxley Act of 2002. Further, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or Dodd-Frank Act, requires that the SEC promulgate rules which would require that, in the event we are required to restate our financial statements, we “claw back” any bonuses paid based on financial performance that would not have been paid if based on the restated financial performance. The SEC has not yet finalized its “claw back” rules. We expect that the Compensation Committee and Board willre-evaluate the potential adoption of a “claw back” policy after any such rules are promulgated.
Accounting and Tax Considerations
Under FASB ASC 718, we are required to estimate and record an expense for each award of equity compensation (including stock options and RSUs) over the vesting period of the award. As long as stock options and RSUs remain the sole components of our long-term compensation program, we expect to record stock-based compensation expense on an ongoing basis according to ASC 718. Compensation expense relating to awards subject to performance conditions is recognized if it is probable that the performance goals will be achieved. The probability of achievement of such goals is assessed on a quarterly basis. The Compensation Committee has considered, and may in the future consider, the grant of restricted stock to our executive officers in lieu of stock option grants and/or RSU awards.
46 Exelixis, Inc.
Compensation of Executive Officers | Compensation Discussion and Analysis
Section 162(m) of the Code, as amended by the Tax Cuts and Jobs Act, limits the deductibility for federal income tax purposes to not more than $1 million of compensation paid to “covered employees” in a calendar year. Prior to the recent enactment of the Tax Cuts and Jobs Act, compensation that qualified as “performance-based compensation” under Section 162(m) of the Code was not subject to this deduction limitation. Pursuant to the Tax Cuts and Jobs Act, this exception for “performance-based compensation” under Section 162(m) of the Code was repealed, with respect to taxable years beginning after December 31, 2017, and subject to a transition rule for written binding contracts which were in effect on November 2, 2017, and which were not modified in any material respect on or after such date. As a result, compensation paid to any of our “covered employees” in excess of $1 million per taxable year generally will not be deductible unless among other requirements, it is intended to qualify, and is eligible to qualify, as “performance-based compensation” under Section 162(m) of the Code pursuant to the transition relief provided by the Tax Cuts and Jobs Act. Certain equity awards issued prior to the transition date were designed to permit us to qualify for the performance-based exception, but because of certain ambiguities and uncertainties as to the application and interpretation of Section 162(m) of the Code and the regulations issued thereunder, including the uncertain scope of the transition relief provided by the Tax Cuts and Jobs Act, no assurance can be given that any compensation paid by the Company will be eligible for such transition relief and, therefore, eligible for the “performance-based compensation” exception under Section 162(m) of the Code. To maintain flexibility in compensating our executive officers in a manner designed to promote our objectives, the Compensation Committee has not adopted a policy that requires all compensation to be deductible. The Compensation Committee is continuing to assess the impact of Section 162(m) of the Code, as amended, on its compensation programs, and intends to provide future compensation in a manner consistent with our best interests and those of our stockholders.
Compensation Policies and Practices as They Relate to Risk Management
In 2017, the Compensation Committee reviewed our compensation policies and practices and concluded that the mix and design of these policies and practices are not reasonably likely to encourage our employees to take excessive risks. In connection with its evaluation, the Compensation Committee considered, among other things, the structure, philosophy and design characteristics of our primary incentive compensation plans and programs in light of our risk management and governance procedures, as well as other factors that may calibrate or balance potential risk-taking incentives. Based on this assessment, the Compensation Committee concluded that risks arising from our compensation policies and practices for all employees, including executive officers, are not reasonably likely to have a material adverse effect on us.
Forward-Looking Statements
This Compensation Discussion and Analysis contains forward-looking statements, including, without limitation, statements related to: our plans to drive the expansion and depth of our product offerings; Genentech’s expectation thattop-line results for the IMblaze370 trial are expected during the first half of 2018; potential approval of CABOMETYX as a treatment for patients with previously treated advanced HCC; and other statements that are not historical fact. Words such as “focused,” “plan,” “intends,” “expected,” “could, “will” or other similar expressions identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward-looking. In addition, any statements that refer to expectations, projections or other characterizations of future events or circumstances are forward-looking statements. These forward-looking statements are based upon our current plans, assumptions, beliefs, expectations, estimates and projections. Forward-looking statements involve risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in the forward-looking statements as a result of these risks and uncertainties, which include, without limitation: the degree of market acceptance of CABOMETYX, COMETRIQ and COTELLIC and the availability of coverage and reimbursement for these products; risks and uncertainties related to regulatory review and approval processes and our and our collaborators’ compliance with applicable legal and regulatory requirements; risks related to the potential failure of cabozantinib and cobimetinib, both alone and in combination with other therapies, to demonstrate safety and efficacy in clinical testing; the availability of data at the referenced times; our dependence on our relationship with our collaboration partners, including the level of their investment in the resources necessary to successfully commercialize cabozantinib and cobimetinib in the territories where they are approved; our ability and the ability of our collaborators to conduct clinical trials of cabozantinib and cobimetinib, both alone and in combination with other therapies, sufficient to achieve a positive completion; the level of costs associated with our commercialization, research and development,in-licensing or acquisition of product candidates, and other activities; our dependence on third-party vendors; our ability to protect our intellectual property rights; market competition, including the potential for competitors to obtain approval for generic versions of our marketed products; changes in economic and business conditions, and other factors discussed under the caption “Risk Factors” in our annual report on Form10-K filed with the Securities and Exchange Commission on February 26, 2018, and in our future filings with the SEC. The forward-looking statements made in this
2018 Proxy Statement 47
Compensation Discussion and Analysis speak only as of the date of this proxy statement. We expressly disclaim any duty, obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based.
48 Exelixis, Inc.
Compensation of Executive Officers | Summary of Compensation
Summary of Compensation
The following table shows for the fiscal years ended December 29, 2017, December 30, 2016 and January 1, 2016 (referred to as fiscal years 2017, 2016 and 2015, respectively), compensation awarded to, paid to or earned by our Chief Executive Officer, our Chief Financial Officer, and our other three most highly compensated executive officers in 2017, which we refer to as our “Named Executive Officers.”
Summary Compensation Table
Name and Principal Position | Year (1) | Salary ($)(2) | Bonus ($)(3) | Stock Awards ($)(4) | Option Awards ($)(5) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||
Michael M. Morrissey, Ph.D. | 2017 | 891,781 | 675,750 | 1,952,800 | 5,911,152 | 8,100 | 9,439,583 | |||||||||||||||||||||
President and Chief | 2016 | 841,154 | 800,000 | 918,600 | 3,700,434 | 7,950 | 6,268,138 | |||||||||||||||||||||
Executive Officer | 2015 | 824,423 | 480,001 | (6) | — | 2,693,750 | 9,326 | 4,007,500 | ||||||||||||||||||||
Gisela M. Schwab, M.D. | 2017 | 629,493 | 318,000 | 1,830,750 | 1,847,235 | 8,100 | 4,633,578 | |||||||||||||||||||||
President, Product Development | 2016 | 591,186 | 375,000 | 294,800 | 1,257,579 | 8,511 | 2,527,076 | |||||||||||||||||||||
and Medical Affairs and Chief | 2015 | 565,800 | 275,092 | (6) | — | 1,358,996 | 7,950 | 2,207,838 | ||||||||||||||||||||
Medical Officer | ||||||||||||||||||||||||||||
Christopher J. Senner* | 2017 | 562,120 | 255,150 | 1,525,625 | 1,539,363 | 8,100 | 3,890,358 | |||||||||||||||||||||
Executive Vice President and | 2016 | 532,923 | 303,750 | 294,800 | 1,062,084 | 7,950 | 2,201,507 | |||||||||||||||||||||
Chief Financial Officer | 2015 | 226,923 | (7) | 225,002 | (6) | 366,000 | 1,809,593 | — | 2,627,518 | |||||||||||||||||||
Jeffrey J. Hessekiel, J.D. | 2017 | 507,067 | 229,970 | 1,220,500 | 1,231,490 | 7,076 | 3,196,103 | |||||||||||||||||||||
Executive Vice President and | 2016 | 484,918 | 275,084 | 239,525 | 862,943 | — | 1,862,470 | |||||||||||||||||||||
General Counsel | 2015 | 480,332 | 209,588 | (6) | — | 1,062,021 | — | 1,751,941 | ||||||||||||||||||||
Peter Lamb, Ph.D. | 2017 | 473,190 | 214,245 | 1,342,550 | 1,354,639 | 8,100 | 3,392,724 | |||||||||||||||||||||
Executive Vice President, | 2016 | 453,546 | 258,750 | 239,525 | 967,207 | 7,950 | 1,926,978 | |||||||||||||||||||||
Scientific Strategy and Chief | 2015 | 437,199 | 190,588 | (6) | — | 1,028,921 | 9,464 | 1,666,172 | ||||||||||||||||||||
Scientific Officer | ||||||||||||||||||||||||||||
| * | Mr. Senner joined Exelixis in July 2015. |
| (1) | The compensation reflected in the Summary Compensation Table reflects a52-week period for each of fiscal 2017. 2016 and 2015. |
| (2) | The amount in this column represents the amount actually paid to each Named Executive Officer for fiscal 2017. For information regarding 2017 base salaries, please see “Compensation Discussion and Analysis—2017 Compensation Decisions—2017 Base Salaries.” |
| (3) | The amount in this column represents discretionary cash bonuses awarded for services rendered during the indicated fiscal years by the Named Executive Officers (equity was issued in lieu of cash for bonuses for 2015). For a description of the company’s discretionary cash bonus program for 2017, see “Compensation Arrangements—Annual Cash Bonuses” following the Grants of Plan Based Awards table. |
| (4) | Amounts shown in this column reflect the aggregate grant date fair value in the indicated fiscal year for the RSU awards as computed in accordance with FASB ASC 718. The assumptions used to calculate the value of RSU awards are set forth in Note 8 of the Notes to Consolidated Financial Statements included in our Annual Report on Form10-K for the fiscal year ended December 29, 2017, filed with the SEC on February 26, 2018. |
| (5) | Amounts shown in this column do not reflect compensation actually received or amounts that may be realized in the future by the Named Executive Officers. The amounts shown reflect the aggregate grant date fair value in the indicated fiscal years for option awards as computed in accordance with FASB ASC 718. The assumptions used to calculate the value of option awards are set forth in Note 8 of the Notes to Consolidated Financial Statements included in our Annual Report on Form10-K for the fiscal year ended December 29, 2017, filed with the SEC on February 26, 2018. The grant date fair values presented in the table for the performance-based option awards assume achievement of the highest level of performance conditions, and excludes estimates of forfeiture. There can be no assurance that the stock option awards will ever be exercised (in which case no value will actually be realized by the executive) or that the value on exercise will be equal to the FASB ASC 718 value shown in this column. |
2018 Proxy Statement 49
| (6) | Represents the aggregate grant date fair value for the RSU award issued to the Named Executive Officer in lieu of a cash bonus for 2015 as computed in accordance with FASB ASC 718. The assumptions used to calculate the value of the RSU award is set forth in Note 8 of the Notes to Consolidated Financial Statements included in our Annual Report on Form10-K for the fiscal year ended December 29, 2017, filed with the SEC on February 26, 2018. |
| (7) | Mr. Senner’s base salary for 2015 was $500,000 per year. The amount shown represents the amount of salary he was actually paid in fiscal 2015, taking into account his start date in July 2015. |
Grants of Plan-Based Awards
The following table shows for the fiscal year ended December 29, 2017, certain information regarding grants of plan-based awards to the Named Executive Officers:
Grants of Plan-Based Awards in Fiscal 2017
| Grant Date | All other stock (#)(1) | All other option (#)(2) | Exercise or Awards ($/Sh) | Grant Date Option Awards ($)(3) | ||||||||||||||||
Michael M. Morrissey, Ph.D. | 10/3/2017 | 480,000 | 24.41 | 5,911,152 | ||||||||||||||||
| 10/3/2017 | 80,000 | 1,952,800 | ||||||||||||||||||
Gisela M. Schwab, M.D. | 10/3/2017 | 150,000 | 24.41 | 1,847,235 | ||||||||||||||||
| 10/3/2017 | 75,000 | 1,830,750 | ||||||||||||||||||
Christopher J. Senner | 10/3/2017 | 125,000 | 24.41 | 1,539,363 | ||||||||||||||||
| 10/3/2017 | 62,500 | 1,525,625 | ||||||||||||||||||
Jeffrey J. Hessekiel, J.D. | 10/3/2017 | 100,000 | 24.41 | 1,231,490 | ||||||||||||||||
| 10/3/2017 | 50,000 | 1,220,500 | ||||||||||||||||||
Peter Lamb, Ph.D. | 10/3/2017 | 110,000 | 24.41 | 1,354,639 | ||||||||||||||||
| 10/3/2017 | 55,000 | 1,342,550 | ||||||||||||||||||
| (1) | The RSU award was granted pursuant to our 2017 Plan. The RSU award will vest as to 1/4th of the shares subject to the RSU award on November 15, 2018 and thereafter as to 1/4th of the original number of shares subject to the RSU award on each succeeding November 15th thereafter until fully vested. Vested shares will be delivered to the Named Executive Officer on the vesting date, provided that delivery may be delayed pursuant to the terms of the award agreement. Vesting is subject to acceleration as described under the caption “—Potential Payments Upon Termination or Change in Control” below. |
| (2) | The option award was granted pursuant to our 2017 Plan and expires seven years from the date of grant or earlier upon termination of service. The option will vest as to 1/4th of the original number of shares subject to the option on theone-year anniversary of the grant date and will continue to vest thereafter as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the grant date. Vesting is subject to acceleration as described under the caption “—Potential Payments Upon Termination or Change in Control” below. |
| (3) | Amounts shown in this column do not reflect compensation actually received or amounts that may be realized in the future by the Named Executive Officers. The amounts shown in this column reflect the aggregate grant date fair value in fiscal year 2017 for the option award or the RSU award as computed in accordance with FASB ASC 718. The assumptions used to calculate the value of the option awards and the RSU awards are set forth in Note 8 of the Notes to Consolidated Financial Statements included in our Annual Report on Form10-K for the fiscal year ended December 29, 2017, filed with the SEC on February 26, 2018. There can be no assurance that the stock option award will ever be exercised (in which case no value will actually be realized by the executive) or that the value on exercise will be equal to the FASB ASC 718 value shown in this column. |
50 Exelixis, Inc.
Compensation of Executive Officers | Compensation Arrangements
Compensation Arrangements
Base Salaries. For a description of actions taken by the Compensation Committee with respect to base salaries for our Named Executive Officers for fiscal 2017, please see “Compensation Discussion and Analysis—2017 Compensation Decisions—2017 Base Salaries” above.
Annual Cash Bonuses. Each year the Compensation Committee considers the payment of annual cash bonuses to the Named Executive Officers for services rendered in the past year. Historically, the payment of bonuses has been entirely within the discretion of the Compensation Committee. While the Compensation Committee established general guidelines related to bonus target amounts, and the payment of actual cash bonuses was generally aligned with the achievement of our corporate goals and the Compensation Committee’s subjective determination of each Named Executive Officer’s contribution toward the achievement of such goals, the Compensation Committee exercised broad discretion in determining the amount of cash bonuses and did not attempt to quantify the level of achievement of corporate goals or the extent to which each Named Executive Officer contributed to Exelixis’ overall success. Accordingly, we do not consider the bonuses reported in the Summary Compensation Table to be“Non-Equity Incentive Plan Compensation” within the meaning of applicable SEC rules.
For additional information with respect to annual bonuses for our Named Executive Officers for fiscal 2017, please see “Compensation Discussion and Analysis—2017 Compensation Decisions—2017 Annual Cash Bonuses” above.
Stock Awards and Option Awards. Our 2017 Plan provides for the grant of RSUs and compensatory stock options to our Named Executive Officers and other employees. In October 2017, we granted time-based stock options and time-based RSU awards to all of our Named Executive Officers to further our longer-term business strategy.
For information regarding stock option grants to the Named Executive Officers in fiscal 2017, including the number of options granted, the exercise price and vesting conditions related thereto, please see “Compensation Discussion and Analysis—2017 Compensation Decisions—2017 Equity Incentive Awards” above. As a general matter, the vested portion of options granted to our Named Executive Officers will expire three months after each Named Executive Officer’s termination of continuous service, subject to extension in certain termination situations or events that can accelerate the vesting, as described under “Potential Payments Upon Termination or Change in Control” below.
Employment Agreements.We have no employment agreements with our Named Executive Officers.
Change in Control and Severance Benefit Plan. Each of our Named Executive Officers participates in our Change in Control and Severance Benefit Plan, a description of which is included below under the heading “Potential Payments Upon Termination or Change in Control.”
Other Compensatory Arrangements. Please see “Compensation Discussion and Analysis—Elements of Compensation—Other Benefits” above for a description of other executive compensatory arrangements, including our 401(k) Plan and other benefits.
2018 Proxy Statement 51
Outstanding Equity Awards at Fiscal Year–End
The following table shows certain information regarding outstanding equity awards at December 29, 2017, for the Named Executive Officers.
Outstanding Equity Awards at December 29, 2017
| Option Awards | Stock Awards | |||||||||||||||||||||||||||
| Grant | Number of Securities Underlying Unexercised Options (#)(1) | Number of Securities Underlying Unexercised Options (#)(1) | Option Exercise Price | Option Expiration | Number of Shares or Units of Stock That Have Not Vested | Market Value of Shares or Units of Stock That Have Not Vested | ||||||||||||||||||||||
Name | Date | Exercisable | Unexercisable | ($) | Date | (#)(2) | ($)(3) | |||||||||||||||||||||
Michael M. Morrissey, Ph.D. | 12/16/2008 | 50,000 | 5.04 | 12/15/2018 | ||||||||||||||||||||||||
| 2/26/2009 | 25,000 | 4.42 | 2/25/2019 | |||||||||||||||||||||||||
| 12/9/2009 | 300,000 | 7.18 | 12/8/2019 | |||||||||||||||||||||||||
| 9/28/2011 | 450,000 | 5.50 | 9/27/2018 | |||||||||||||||||||||||||
| 9/21/2012 | 402,000 | 5.555 | 9/20/2019 | |||||||||||||||||||||||||
| 9/18/2013 | 960,000 | 5.51 | 9/17/2020 | |||||||||||||||||||||||||
| 9/19/2014 | 804,551 | 1.70 | 9/18/2021 | |||||||||||||||||||||||||
| 9/16/2015 | 281,250 | 218,750 | (4) | 6.21 | 9/15/2022 | |||||||||||||||||||||||
| 2/11/2016 | 68,750 | 81,250 | (5) | 4.20 | 2/10/2023 | |||||||||||||||||||||||
| 9/26/2016 | 112,500 | 247,500 | (10) | 15.31 | 9/25/2023 | |||||||||||||||||||||||
| 9/26/2016 | 45,000 | (7) | 1,368,000 | |||||||||||||||||||||||||
| 10/3/2017 | 480,000 | (11) | 24.41 | 10/2/2024 | ||||||||||||||||||||||||
| 10/3/2017 | 80,000 | (12) | 2,432,000 | |||||||||||||||||||||||||
Gisela M. Schwab, M.D. | 12/16/2008 | 37,499 | 5.04 | 12/15/2018 | ||||||||||||||||||||||||
| 2/26/2009 | 7,293 | 4.42 | 2/25/2019 | |||||||||||||||||||||||||
| 12/9/2009 | 177,700 | 7.18 | 12/8/2019 | |||||||||||||||||||||||||
| 9/28/2011 | 112,500 | 5.50 | 9/27/2018 | |||||||||||||||||||||||||
| 9/21/2012 | 123,000 | 5.555 | 9/20/2019 | |||||||||||||||||||||||||
| 9/18/2013 | 320,000 | 5.51 | 9/17/2020 | |||||||||||||||||||||||||
| 9/19/2014 | 500,000 | 1.70 | 9/18/2021 | |||||||||||||||||||||||||
| 2/5/2015 | 250,000 | 1.90 | 2/4/2022 | |||||||||||||||||||||||||
| 9/16/2015 | 137,812 | 107,188 | (4) | 6.21 | 9/15/2022 | |||||||||||||||||||||||
| 2/11/2016 | 34,375 | 40,625 | (5) | 4.20 | 2/10/2023 | |||||||||||||||||||||||
| 9/22/2016 | 37,500 | 82,500 | (6) | 14.74 | 9/21/2023 | |||||||||||||||||||||||
| 9/22/2016 | 15,000 | (7) | 456,000 | |||||||||||||||||||||||||
| 10/3/2017 | 150,000 | (11) | 24.41 | 10/2/2024 | ||||||||||||||||||||||||
| 10/3/2017 | 75,000 | (12) | 2,280,000 | |||||||||||||||||||||||||
Christopher J. Senner | 7/15/2015 | 111,458 | 138,542 | (8) | 3.66 | 7/14/2022 | ||||||||||||||||||||||
| 9/16/2015 | 126,562 | 98,438 | (4) | 6.21 | 9/15/2022 | |||||||||||||||||||||||
| 9/22/2016 | 37,500 | 82,500 | (6) | 14.74 | 9/21/2023 | |||||||||||||||||||||||
| 9/22/2016 | 15,000 | (7) | 456,000 | |||||||||||||||||||||||||
| 10/3/2017 | 125,000 | (11) | 24.41 | 10/2/2024 | ||||||||||||||||||||||||
| 10/3/2017 | 62,500 | (12) | 1,900,000 | |||||||||||||||||||||||||
52 Exelixis, Inc.
Compensation of Executive Officers | Outstanding Equity Awards at Fiscal Year–End
| Option Awards | Stock Awards | |||||||||||||||||||||||||||
| Grant | Number of Securities Underlying Unexercised Options (#)(1) | Number of Securities Underlying Unexercised Options (#)(1) | Option Exercise Price | Option Expiration | Number of Shares or Units of Stock That Have Not Vested | Market Value of Shares or Units of Stock That Have Not Vested | ||||||||||||||||||||||
Name | Date | Exercisable | Unexercisable | ($) | Date | (#)(2) | ($)(3) | |||||||||||||||||||||
Jeffrey J. Hessekiel, J.D. | 2/10/2014 | 120,416 | 9,584 | (9) | 7.27 | 2/9/2021 | ||||||||||||||||||||||
| 9/19/2014 | 145,000 | 1.70 | 9/18/2021 | |||||||||||||||||||||||||
| 2/5/2015 | 172,698 | 1.90 | 2/4/2022 | |||||||||||||||||||||||||
| 9/16/2015 | 106,875 | 83,125 | (4) | 6.21 | 9/15/2022 | |||||||||||||||||||||||
| 9/22/2016 | 30,468 | 67,032 | (6) | 14.74 | 9/21/2023 | |||||||||||||||||||||||
| 9/22/2016 | 12,187 | (7) | 370,485 | |||||||||||||||||||||||||
| 10/3/2017 | 100,000 | (11) | 24.41 | 10/2/2024 | ||||||||||||||||||||||||
| 10/3/2017 | 50,000 | (12) | 1,520,000 | |||||||||||||||||||||||||
Peter Lamb, Ph.D. | 9/21/2012 | 123,000 | 5.555 | 9/20/2019 | ||||||||||||||||||||||||
| 9/18/2013 | 168,000 | 5.51 | 9/17/2020 | |||||||||||||||||||||||||
| 9/19/2014 | 400,000 | 1.70 | 9/18/2021 | |||||||||||||||||||||||||
| 2/5/2015 | 175,000 | 1.90 | 2/4/2022 | |||||||||||||||||||||||||
| 9/16/2015 | 106,875 | 83,125 | (4) | 6.21 | 9/15/2022 | |||||||||||||||||||||||
| 2/11/2016 | 18,333 | 21,667 | (5) | 4.20 | 2/10/2023 | |||||||||||||||||||||||
| 9/22/2016 | 30,468 | 67,032 | (6) | 14.74 | 9/21/2023 | |||||||||||||||||||||||
| 9/22/2016 | 12,187 | (7) | 370,485 | |||||||||||||||||||||||||
| 10/3/2017 | 110,000 | (11) | 24.41 | 10/2/2014 | ||||||||||||||||||||||||
| 10/3/2017 | 55,000 | (12) | 1,672,000 | |||||||||||||||||||||||||
| (1) | Option awards granted prior to January 26, 2010 were issued under our 2000 Equity Incentive Plan, or 2000 Plan and have vested in full and expire ten years from the date of grant or earlier upon termination of continuous service. There were no option awards granted to Named Executive Officers on or after January 26, 2010 and prior to May 18, 2011. Option awards granted on or after May 18, 2011, and prior to May 28, 2014, were issued under our 2011 Equity Incentive Plan, or 2011 Plan, and are either subject to time-based vesting or were subject to performance-based vesting and expire seven years from the date of grant or earlier upon termination of continuous service. Vesting of awards granted under the 2011 Plan still subject to vesting is set forth in the applicable footnote accompanying the entry. All option awards granted pursuant to our 2011 Plan subject to time-based vesting have vested in part, and the remaining unvested portion will vest as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the vesting commencement date. All option awards granted under our 2011 Plan subject to performance-based vesting have either been cancelled or are vested in full. Option awards granted on or after May 28, 2014, and prior to May 24, 2017, were issued under our 2014 Plan and are either subject to time-based vesting or were subject to performance-based vesting and expire seven years from the date of grant or earlier upon termination of continuous service. Vesting of awards granted under the 2014 Plan is set forth in the applicable footnote accompanying the entry. Option awards granted pursuant to our 2014 Plan subject to time-based vesting vest as to 1/4th of the original number of shares subject to the option on theone-year anniversary of the vesting commencement date and thereafter as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the vesting commencement date. Option awards granted pursuant to our 2014 Plan subject to performance-based vesting are vested in full. Option awards granted on or after May 24, 2017 were issued under our 2017 Plan and are subject to time-based vesting and expire seven years from the date of grant or earlier upon termination of continuous service. Vesting of awards granted under the 2017 Plan is set forth in the applicable footnote accompanying the entry. Option awards granted pursuant to our 2017 Plan subject to time-based vesting vest as to 1/4th of the original number of shares subject to the option on theone-year anniversary of the vesting commencement date and thereafter as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the vesting commencement date. The 2017 Plan also provides for the grant of option awards subject to performance-based vesting, but as of December 29, 2017, no such awards have been granted. Vesting of all options issued to our Named Executive Officers are subject to acceleration as described under the caption “Potential Payments Upon Termination or Change in Control” below. |
| (2) | RSU awards granted prior to May 28, 2014 were issued under our 2011 Plan, RSU awards granted on or after May 28, 2014 and prior to May 24, 2017 were issued under our 2014 Plan, and RSU awards granted on or after May 24, 2017 were issued under our 2017 Plan. RSU awards generally vest as to 1/4th of the original number of shares subject to the RSU award on the first established RSU vesting date following the one year anniversary of the grant date and thereafter as to 1/4th of the original number of shares subject to the RSU award on each anniversary of the first established RSU vesting date following the one year anniversary of the grant date, until fully-vested. We have established February 15th, May 15th, August 15th and November 15th as RSU vesting dates. Vested shares will be delivered to the Named Executive Officer on the applicable vesting date, provided that delivery may be delayed pursuant to the terms of the award agreement. Vesting of all RSU awards issued to our Named Executive Officers is subject to acceleration as described under the caption “Potential Payments Upon Termination or Change in Control” below. |
2018 Proxy Statement 53
| (3) | For purposes of determining market value, we assumed a stock price of $30.40, the closing sale price per share of our common stock on December 29, 2017, the last business day of our last fiscal year. |
| (4) | Option vests as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the grant date with a final vesting date of September 16, 2019 (assuming that such options are not accelerated). |
| (5) | Option vests as to 1/4th of the original number of shares subject to the option on theone-year anniversary of the grant date and thereafter as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the grant date with a final vesting date of February 11, 2020 (assuming that such options are not accelerated). |
| (6) | Option vests as to 1/4th of the original number of shares subject to the option on theone-year anniversary of the grant date and thereafter as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the grant date with a final vesting date of September 22, 2020 (assuming that such options are not accelerated). |
| (7) | RSUs vest as to 1/4th of the original number of shares subject to the RSU award on each November 15th with a final vesting date of November 15, 2020 (assuming that such RSUs are not accelerated). |
| (8) | Option vests as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the grant date with a final vesting date of July 15, 2019 (assuming that such options are not accelerated). |
| (9) | Option vests as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the grant date with a final vesting date of February 10, 2018 (assuming that such options are not accelerated). |
| (10) | Option vests as to 1/4th of the original number of shares subject to the option on theone-year anniversary of the grant date and thereafter as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the grant date with a final vesting date of September 26, 2020 (assuming that such options are not accelerated). |
| (11) | Option vests as to 1/4th of the original number of shares subject to the option on theone-year anniversary of the grant date and thereafter as to 1/48th of the original number of shares subject to the option on each monthly anniversary of the grant date with a final vesting date of October 3, 2021 (assuming that such options are not accelerated). |
| (12) | RSUs vest as to 1/4th of the original number of shares subject to the RSU award on each November 15th with a final vesting date of November 15, 2021 (assuming that such RSUs are not accelerated). |
Option Exercises and Stock Vested
The following table includes certain information with respect to stock options exercised and stock awards that vested during the fiscal year ended December 29, 2017.
Options Exercised and Stock Vested in Fiscal 2017
| Option Awards | Stock Awards | |||||||||||||||
Name | Number of Shares Acquired on Exercise(#) | Value Realized on | Number of Shares Acquired on Vesting(#) | Value Realized on Vesting($)(2) | ||||||||||||
Michael M. Morrissey, Ph.D. | 970,449 | 19,617,032 | 15,000 | 371,250 | ||||||||||||
Gisela M. Schwab, M.D. | 262,508 | 3,704,392 | 5,000 | 123,750 | ||||||||||||
Christopher J. Senner | 100,000 | 2,313,000 | 5,000 | 123,750 | ||||||||||||
Jeffrey J. Hessekiel | 182,302 | 3,222,445 | 4,063 | 100,559 | ||||||||||||
Peter Lamb, Ph.D. | 185,000 | 2,110,367 | 4,063 | 100,559 | ||||||||||||
| (1) | “Value Realized on Exercise” reflects the price at which the shares acquired upon exercise (the closing market price of our common stock on the exercise date) of the stock options were sold, net of the exercise price for acquiring the shares. |
| (2) | “Value Realized on Vesting” reflects the product of the fair market value of our common stock on the applicable vesting date multiplied by the number of units vested and does not necessarily reflect proceeds actually received by the Named Executive Officers. |
54 Exelixis, Inc.
Compensation of Executive Officers | Potential Payments Upon Termination or Change in Control
Potential Payments Upon Termination or Change in Control
Change in Control and Severance Benefit Plan
In December 2005, the Board, upon recommendation of the Compensation Committee, adopted a Change in Control and Severance Benefit Plan that provides for certain severance benefits to our officers in connection with specified termination events. Eligible plan participants may include any employee having a rank of vice president or above, which includes the Named Executive Officers. We amended our Change in Control and Severance Benefit Plan in December 2008 and again in December 2010 to bring the plan into compliance with Section 409A of the Code and other rules governing such plans. We further amended our Change in Control and Severance Benefit Plan in September 2017 to, among other compliance-related changes, increase the cash payments and COBRA benefits for plan participants, including our Named Executive Officers, following termination events not in connection with a change in control.
If a Named Executive Officer’s employment terminates due to an involuntary termination without cause or a constructive termination, or Covered Termination, during a period starting one month prior to and ending 13 months following a change in control, or a Change in Control Termination, then the Named Executive Officer would be entitled to the following benefits under the plan:
| ›› | a cash payment paid in installments pursuant to our regularly scheduled payroll periods equal to the sum of the Named Executive Officer’s base salary and target bonus for (i) 18 months for Named Executive Officers (other than the Chief Executive Officer) and (ii) 24 months for the Chief Executive Officer; |
| ›› | the vesting of up to all of the Named Executive Officer’s options and RSUs will accelerate in full, and the exercise period of such options will be extended to the later of: (i) 12 months after the later of (x) the participant’s termination date, or (y) the change in control; and (ii) the post-termination exercise period provided for in the applicable option agreement; the plan also provides that any reacquisition or repurchase rights held by us in respect of common stock issued or issuable pursuant to any stock awards granted under any of our equity incentive plans shall lapse; |
| ›› | payment of COBRA premiums, or the cash equivalent thereof, for any health, dental or vision plan sponsored by Exelixis for a period of up to (i) 18 months for Named Executive Officers (other than the Chief Executive Officer) and (ii) 24 months for the Chief Executive Officer; and |
| ›› | payment of outplacement services for (i) 18 months for Named Executive Officers (other than the Chief Executive Officer), subject to a $30,000 limit and (ii) 24 months for the Chief Executive Officer, subject to a $50,000 limit. |
In the event of a Covered Termination of a Named Executive Officer that is not also a Change in Control Termination, such Named Executive Officer would be entitled to receive a cash severance benefit under the plan equal to 12 months of base salary paid in installments pursuant to our regularly scheduled payroll periods. In such circumstances, we would also pay for a period of up to 12 months such Named Executive Officer’s COBRA premiums, or the cash equivalent thereof, for any health, dental or vision plan that we sponsored and that the Named Executive Officer is enrolled in. However, such Named Executive Officer would not be entitled to any vesting acceleration benefits by virtue of such termination.
The payments and benefits described above are subject to certain reductions and offsets if, for example, the Named Executive Officer received other severance benefits from us pursuant to a written employment agreement. In addition, if any of the severance benefits payable under the plan would constitute a “parachute payment” subject to the excise tax imposed by Section 4999 of the Code, a Named Executive Officer may receive a reduced amount of the affected severance benefits, The plan does not provide for the gross up of any excise taxes imposed by Section 4999 of the Code. No Named Executive Officer would receive benefits under the plan if (i) the Named Executive Officer has entered into an individually negotiated employment agreement that provides for severance or change in control benefits, (ii) the Named Executive Officer voluntarily terminates employment with us to accept employment with another entity that is controlled by us or is otherwise affiliated with us or (iii) the Named Executive Officer does not confirm in writing that he or she is subject to agreements with us relating to proprietary and confidential information. In addition, as a general matter, to be eligible to receive benefits under the plan and if requested by Exelixis, a Named Executive Officer must execute a general waiver and release of claims, and such release must become effective in accordance with its terms.
Treatment of Equity Awards
Pursuant to our 2000 Plan, 2011 Plan, 2014 Plan and 2017 Plan, in the event of an asset sale, merger or consolidation in which we are not the surviving corporation, or a reverse merger in which we are the surviving corporation but our common stock is converted by virtue of the merger into other property, then any surviving or acquiring corporation may assume
2018 Proxy Statement 55
outstanding stock awards or substitute similar stock awards for those under the plan. If any surviving or acquiring corporation refuses to assume such outstanding stock awards or substitute similar stock awards, stock awards held by participants whose service has not terminated will be accelerated in full. In addition, if any person, entity or group acquires beneficial ownership of more than 50% of our combined voting power, then stock awards held by participants whose service has not terminated will be accelerated in full.
The following table sets forth the potential severance payments and benefits under our Change in Control and Severance Benefit Plan to which a Named Executive Officer would be entitled in connection with specified termination events, as if such Named Executive Officers’ employment terminated as of December 29, 2017, the last day of our last fiscal year. In addition, the table sets forth the amounts to which such Named Executive Officers would be entitled under our equity plans either (i) in connection with a change in control transaction in which the successor corporation did not assume or substitute outstanding stock awards, or (ii) an entity or group acquired more than 50% of our combined voting power, in each case, as of December 29, 2017. There are no other agreements, arrangements or plans that entitle any of the above-mentioned Named Executive Officers to severance, perquisites or other enhanced benefits upon termination of employment, other than certain extensions of the termination date to avoid violation of registration requirements under the Securities Act of 1933, as amended, or for such Named Executive Officer’s death or disability.
Potential Payments Table
The following table shows the potential payments upon termination of employment or a change in control for the Named Executive Officers. The table assumes that the triggering event took place on December 29, 2017, the last day of our 2017 fiscal year.
Potential Payments Upon Termination or Change in Control Table
Name | Benefit | Change in Control and Severance Benefit Plan | Equity Plans | |||||||||||
Involuntary Termination Without Cause or Constructive Termination in Connection with a Change of Control ($)(1) | Involuntary Termination Without Cause or Constructive Termination Not in Connection with a Change in Control ($)(2) | Certain Change of Control Transactions without Termination ($)(3) | ||||||||||||
Michael M. Morrissey, Ph.D. | Base Salary | 1,802,000 | 901,000 | — | ||||||||||
| Bonus | 1,351,500 | — | — | |||||||||||
| Vesting Acceleration(4) | 17,830,288 | — | 17,830,288 | |||||||||||
| COBRA Payments | 60,672 | 30,336 | — | |||||||||||
| Outplacement Services | 50,000 | — | — | |||||||||||
Benefit Total | 21,094,460 | 931,336 | 17,830,288 | |||||||||||
Gisela M. Schwab, M.D. | Base Salary | 954,000 | 636,000 | — | ||||||||||
| Bonus | 477,000 | — | — | |||||||||||
| Vesting Acceleration(4) | 8,583,703 | — | 8,583,703 | |||||||||||
| COBRA Payments | 51,019 | 34,013 | — | |||||||||||
| Outplacement Services | 30,000 | — | — | |||||||||||
Benefit Total | 10,095,722 | 670,013 | 8,583,703 | |||||||||||
Christopher J. Senner | Base Salary | 850,500 | 567,000 | — | ||||||||||
| Bonus | 382,725 | — | — | |||||||||||
| Vesting Acceleration(4) | 10,482,528 | — | 10,482,528 | |||||||||||
| COBRA Payments | 45,504 | 30,336 | — | |||||||||||
| Outplacement Services | 30,000 | — | — | |||||||||||
Benefit Total | 11,791,257 | 597,336 | 10,482,528 | |||||||||||
56 Exelixis, Inc.
Compensation of Executive Officers | Potential Payments Upon Termination or Change in Control
Name | Benefit | Change in Control and Severance Benefit Plan | Equity Plans | |||||||||||
Involuntary Termination Without Cause or Constructive Termination in Connection with a Change of Control ($)(1) | Involuntary Termination Without Cause or Constructive Termination Not in Connection with a Change in Control ($)(2) | Certain Change of Control Transactions without Termination ($)(3) | ||||||||||||
Jeffrey J. Hessekiel, J.D. | Base Salary | 766,568 | 511,045 | — | ||||||||||
| Bonus | 344,955 | — | — | |||||||||||
| Vesting Acceleration(4) | 5,771,678 | — | 5,771,678 | |||||||||||
| COBRA Payments | 28,647 | 19,098 | — | |||||||||||
| Outplacement Services | 30,000 | — | — | |||||||||||
Benefit Total | 6,941,848 | 530,143 | 5,771,678 | |||||||||||
Peter Lamb, Ph.D. | Base Salary | 714,150 | 476,100 | — | ||||||||||
| Bonus | 321,368 | — | — | |||||||||||
| Vesting Acceleration(4) | 6,329,575 | — | 6,329,575 | |||||||||||
| COBRA Payments | 17,729 | 11,819 | — | |||||||||||
| Outplacement Services | 30,000 | — | — | |||||||||||
Benefit Total | 7,412,822 | 487,919 | 6,329,575 | |||||||||||
| (1) | These benefits would be payable under the Change in Control and Severance Benefit Plan if the involuntary termination without cause or constructive termination occurred during a period starting one month prior to and ending 13 months following the change in control. |
| (2) | These benefits would be payable under the Change in Control and Severance Benefit Plan if the involuntary termination without cause occurred more than one month before the change in control or if the involuntary termination without cause or a constructive termination occurred more than 13 months following the change in control. |
| (3) | These benefits would be payable under the 2000 Plan and/or the 2011 Plan and/or the 2014 Plan and/or the 2017 Plan if either (i) a successor corporation does not assume outstanding stock awards in a change of control transaction or (ii) a person, entity or group acquires beneficial ownership of more than 50% of our combined voting power, and, in each case, the Named Executive Officers do not terminate employment in connection with such a transaction or event. |
| (4) | Assumes that the triggering event occurred on December 29, 2017, the last day of our last fiscal year, when the closing sale price per share of our common stock was $30.40. The amount of the vesting acceleration is determined by: (i) aggregating for all accelerated options, the amount equal to (A) the excess, if any, of $30.40 over the relevant exercise price of the option, multiplied by (B) the number of shares underlying unvested options at such exercise price as of December 29, 2017; and (ii) aggregating for all accelerated RSUs, the amount equal to (X) $30.40 multiplied by (Y) the number of shares underlying the unvested RSUs. There can be no assurance that a similar triggering event would produce the same or similar results as those estimated if such event occurs on any other date or at a time when our closing sale price is different. |
CEO Pay Ratio
We believe that we provide fair and equitable compensation to our employees through a combination of competitive base pay, incentives, retirement plans and other benefits. In accordance with Item 402(u) of RegulationS-K, promulgated by the Dodd Frank Act, we determined the ratio of: (a) the annual total compensation of our Chief Executive Officer; to (b) the median of the annual total compensation of all of our employees, except for our Chief Executive Officer, both calculated in accordance with the requirements of Item 402(c)(2)(x) of RegulationS-K.
To determine the median of the annual total compensation of all of our employees, except for our Chief Executive Officer, we used the following methodology:
| ›› | To identify our employee population, we used tax and payroll records to determine all full-time, part-time, temporary and seasonal employees, excluding our Chief Executive Officer, who were employed as of October 31, 2017. |
2018 Proxy Statement 57
| ›› | To identify the median of the annual total compensation of all of our employees from our employee population, we calculated each employee’s “target total direct compensation,” which consists of: (i) fiscal 2017 base salary (using a reasonable estimate of the hours worked and overtime actually paid during fiscal 2017 for hourly employees); (ii) target cash bonus; and (iii) the grant date fair value of any equity awards granted during fiscal 2017 (using the same methodology that we use for estimating the value of the equity awards granted to our Named Executive Officers and reported in our Summary Compensation Table). |
| ›› | In making this determination, we annualized the base salary and target cash bonus for employees who were employed by us for less than the entirety of fiscal 2017. |
Because the median employee we initially identified had anomalous compensation characteristics, we substituted another employee with substantially similar compensation to the originally identified median employee during fiscal 2017.
Once our representative median employee was identified in the manner described above, we calculated the annual total compensation of the representative median employee using the same methodology that we used to determine the annual total compensation of our Chief Executive Officer, as reported in the Summary Compensation Table included in this proxy statement. For fiscal 2017, the median of the annual total compensation of our employees (other than our Chief Executive Officer) was $275,254 and the annual total compensation of our Chief Executive Officer, as reported in the Summary Compensation Table included in this Proxy Statement, was $9,439,583. Based on this information, the ratio of the annual total compensation of our Chief Executive Officer to the median of the annual total compensation of all employees was 34 to 1.
This pay ratio represents our reasonable estimate calculated in a manner consistent with Item 402(u) of RegulationS-K and applicable guidance, which provide significant flexibility in how companies identify the median employee. Each company may use a different methodology and make different assumptions particular to that company. As a result, and as explained by the SEC when it adopted these rules, in considering the pay ratio disclosure, stockholders should keep in mind that the rule was not designed to facilitate comparisons of pay ratios among different companies, even companies within the same industry, but rather to allow stockholders to better understand and assess each particular company’s compensation practices and pay ratio disclosures. Neither the Compensation Committee nor our management used our fiscal 2017 Chief Executive Officer to median employee pay ratio in making compensation decisions.
58 Exelixis, Inc.
Compensation of Executive Officers | Compensation Committee Report
COMPENSATION COMMITTEE REPORT
The material in this report is not “soliciting material,” is not deemed “filed” with the Securities and Exchange Commission and is not deemed to be incorporated by reference in any filing of Exelixis under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
The Compensation Committee of the Board of Directors of Exelixis, Inc., consisting solely of independent directors, has reviewed and discussed with management the Compensation Discussion and Analysis contained in this Proxy Statement and, based on this review and discussion, the Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this Proxy Statement and incorporated into our Annual Report on Form10-K for the year ended December 29, 2017.
Compensation Committee:
Charles Cohen, Chairman
Carl B. Feldbaum
Vincent T. Marchesi
Julie A. Smith
Lance Willsey
2018 Proxy Statement 59
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
During 2017, the Compensation Committee comprised Drs. Cohen, Marchesi and Willsey, Mr. Feldbaum and Ms. Smith. None of the members of the Compensation Committee during 2017 has at any time been an officer or employee of Exelixis, except that Dr. Cohen served as our acting Chief Scientific Officer from December 1995 to April 1997, and was named an officer of one of our former subsidiaries from 2001 through March 2005, for which he did not receive any compensation. No interlocking relationship exists between the Board or Compensation Committee and the board of directors or compensation committee of any other company, nor has any interlocking relationship existed in the past.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The Board recognizes that related party transactions can present a heightened risk of potential or actual conflicts of interests. The Board adopted a written Statement of Policy with respect to transactions entered into with related parties. Under this policy, the Audit Committee has been tasked with responsibility to review and approve related party transactions. The policy provides that management shall present related party transactions to the Audit Committee for approval. The policy does not prevent management from entering into any related party transaction without prior approval of the Audit Committee, so long as such related party transaction is thereafter presented to the Audit Committee for ratification. If ratification is not forthcoming, then management shall make all reasonable efforts to cancel or annul such transaction.
Under the policy, a “related party” includes: any senior officer (including each executive officer or officer subject to Section 16 of the Securities Exchange Act of 1934, as amended) or director of Exelixis; a person who is an immediate family member of a senior officer, director or director nominee; a security holder who is known to own of record or beneficially more than 5% percent of any class of our securities; a person who is an immediate family member of such security holder; or an entity which is owned or controlled by one of the aforementioned persons, or an entity in which one of the aforementioned persons has a substantial ownership interest in or control over such entity.
All related party transactions shall be disclosed in our applicable filings with the SEC as required under SEC rules.
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our directors and executive officers and persons who own more than ten percent of a registered class of our equity securities to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock and other equity securities of Exelixis. Officers, directors and greater than ten percent stockholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us and written representations that no other reports were required, during the fiscal year ended December 29, 2017, all Section 16(a) filing requirements applicable to our officers, directors and greater than ten percent beneficial owners were complied with, except as follows: certain shares of our common stock held by Mr. Haley were inadvertently omitted from Mr. Haley’s Form 3, originally filed on December 21, 2016, and the number of shares beneficially owned by Mr. Haley was corrected in a subsequent Form 5 filing for the fiscal year ended December 29, 2017.
60 Exelixis, Inc.
HOUSEHOLDING OF PROXY MATERIALS
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers, banks and other fiduciaries) to satisfy the delivery requirements for Notices of Internet Availability of Proxy Materials or other Annual Meeting materials with respect to two or more stockholders sharing the same address by delivering a single Notice or other Annual Meeting materials addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
This year, a number of brokers with account holders who are our stockholders will be householding proxy materials. A single Notice will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that they will be householding communications to your address, householding will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in householding and would prefer to receive a separate Notice, please notify your broker or direct your written request to Investor Relations, Exelixis, Inc., 210 East Grand Avenue, South San Francisco, California 94080 or contact Exelixis, Inc., Investor Relations at(650) 837-7000. Stockholders who currently receive multiple copies of the Notice at their address and would like to request householding of their communications should contact their broker.
ANNUAL REPORT ON FORM10-K
A copy of our Annual Report on Form10-K for the fiscal year ended December 29, 2017, including the consolidated financial statements, schedules and list of exhibits, and any particular exhibit specifically requested, is available without charge upon written request to: Investor Relations, Exelixis, Inc., 210 East Grand Avenue, South San Francisco, California 94080.
OTHER MATTERS
The Board knows of no other matters that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before the meeting, it is the intention of the persons named in the accompanying proxy to vote on such matters in accordance with their best judgment.
By Order of the Board of Directors

JEFFREY J. HESSEKIEL
Executive Vice President and General Counsel
South San Francisco, California
April 12, 2018
2018 Proxy Statement 61
|  | |||||||||||
Electronic Voting Instructions | ||||||||||||
| Available 24 hours a day, 7 days a week! | ||||||||||||
Instead of mailing your proxy, you may choose one of the voting methods outlined below to vote your proxy. | ||||||||||||
VALIDATION DETAILS ARE LOCATED BELOW IN THE TITLE BAR. | ||||||||||||
Proxies submitted by the Internet or telephone must be received by 11:59 p.m., Eastern Time, on May 22, 2018. | ||||||||||||
|
Vote by Internet
• Go towww.investorvote.com/EXEL
• Or scan the QR code with your smartphone
• Follow the steps outlined on the secure website | |||||||||||
Vote by telephone
• Call toll free 1-800-652-VOTE (8683) within the USA, US territories & Canada on a touch tone telephone
• Follow the instructions provided by the recorded message | ||||||||||||
Using ablack ink pen, mark your votes with anX as shown in this example. Please do not write outside the designated areas. | ☒ | |||||||||||

q IF YOU HAVE NOT VOTED VIA THE INTERNETOR TELEPHONE, FOLD ALONG THE PERFORATION, DETACH AND RETURN THE BOTTOM PORTION IN THE ENCLOSED ENVELOPE. q
|
A | Proposals — The Board of Directors recommends a voteFOR all the nominees listed in Proposal 1 andFOR Proposals 2 and 3.
| |||
| 1. To elect the three Class I nominees for director named in the accompanying Proxy Statement to hold office until the 2021 Annual Meeting of Stockholders | + | |||
| For | Against | Abstain | For | Against | Abstain | For | Against | Abstain | ||||||||||||||||
01 - Charles Cohen, Ph.D. |
☐ |
☐ |
☐ |
02 - George Poste, DVM, Ph.D., FRS |
☐ |
☐ |
☐ |
03 - Jack L. Wyszomierski |
☐ |
☐ |
☐ |
| For | Against | Abstain | For | Against | Abstain | |||||||||||
2. To ratify the selection by the Audit Committee of the Board of Directors of Ernst & Young LLP as Exelixis’ independent registered public accounting firm for the fiscal year ending December 28, 2018. | ☐ | ☐ | ☐ | 3. To approve, on an advisory basis, the compensation of Exelixis’ named executive officers, as disclosed in the accompanying Proxy Statement. | ☐ | ☐ | ☐ | |||||||||
| B | Non-Voting Items |
| Change of Address — Please print your new address below. | Comments — Please print your comments below. | Meeting Attendance | ||||||||
| Mark the box to the right if you plan to attend the Annual Meeting. | ☐ |
| C | Authorized Signatures — This section must be completed for your vote to be counted. — Date and Sign Below | |||
NOTE: Please sign as name appears hereon. Joint owners should each sign. When signing as attorney, executor, administrator, trustee or guardian, please give full title as such. | ||||
| Date (mm/dd/yyyy) — Please print date below. | Signature 1 — Please keep signature within the box. | Signature 2 — Please keep signature within the box. | ||||||
| / / | ||||||||
| ⬛ |  | + | ||||||
02TAYA
Important notice regarding the Internet availability of proxy materials for the 2018 Annual Meeting of Stockholders.
The Proxy Statement and the 2017 Annual Report to Stockholders are available at:
http://exel-annualstockholdermeeting.com
q IF YOU HAVE NOT VOTED VIA THE INTERNETOR TELEPHONE, FOLD ALONG THE PERFORATION, DETACH AND RETURN THE BOTTOM PORTION IN THE ENCLOSED ENVELOPE.q

Proxy — EXELIXIS, INC.
|
PROXY SOLICITED BY THE BOARD OF DIRECTORS
FOR THE ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON MAY 23, 2018
The undersigned hereby appoints Michael M. Morrissey, Christopher J. Senner and Jeffrey J. Hessekiel, and each of them, as attorneys and proxies of the undersigned, with full power of substitution, to vote all of the shares of stock of Exelixis, Inc. that the undersigned may be entitled to vote at the Annual Meeting of Stockholders of Exelixis, Inc. to be held at the offices of Exelixis, Inc. at 210 East Grand Avenue, South San Francisco, CA 94080 on Wednesday, May 23, 2018, at 8:00 a.m. (local time), and at any and all postponements, continuations and adjournments thereof, with all powers that the undersigned would possess if personally present, upon and in respect of the following matters and in accordance with the following instructions, with discretionary authority as to any and all other matters that may properly come before the meeting.
UNLESS A CONTRARY DIRECTION IS INDICATED, THIS PROXY WILL BE VOTED “FOR” ALL NOMINEES LISTED IN PROPOSAL 1 AND “FOR” PROPOSALS 2 AND 3, AS MORE SPECIFICALLY DESCRIBED IN THE PROXY STATEMENT. IF SPECIFIC INSTRUCTIONS ARE INDICATED, THIS PROXY WILL BE VOTED IN ACCORDANCE THEREWITH.
(Continued and to be marked, dated and signed, on the other side)