Exhibit 99.1

1 Barrington Research Healthcare Conference NASDAQ: NEOL Laurence P. Birch President and Chief Executive Officer

2 Safe Harbor Statement Various remarks that we may make about future expectations, plans and prospects for the Company constitute forward-looking statements for purposes of the Safe-Harbor provisions under The Private Securities Litigation Reform Act of 1995. Such forward-looking statements are necessarily current estimates reflecting the judgment of the Company’s management and involve risks and uncertainties that could cause actual results to differ materially from those indicated by these forward-looking statements as a result of various important factors, including those discussed in the Risk Factors section of our most recent Annual Report on Form 10-K and in our most recently filed quarterly reports on Form 10-Q which are on file with the Securities and Exchange Commission.

3 Dedicated to Discovery & Development Engaged in the research, development and commercialization of new and innovative drugs for various cancers and other therapeutic applications Drug development program includes two proprietary platforms: Tumor-targeting toxin platform, which includes development for a new indication of Pulmonary Fibrosis NeoLipid® drug delivery system Multiple drug product candidates advanced to various stages of clinical development

4 Investment Highlights Multiple drug candidates in various points of clinical development Cintredekin Besudotox, Indication GBM: Confirmatory Phase III LEP-ETU: Phase II LE-DT: Phase I Robust Pipeline Additional indications for Cintredekin Besudotox, IL-13 Product initiatives funded and progressing Trials to capitalize on broader indications of cancer Optimal Cost Structure Significantly reduced cash consumption rate Lean organization focused primarily on the development of drug candidates Continue to identify new out-license and partnership opportunities Resources available to fund current candidates in development well into 2009

5 Completed Restructuring Initiative Reorganization strategy, originally announced in April 2007 Key Initiatives Completed Reduced annualized cash consumption levels to approximately $5 million Realigned cost structure to optimize resources to progress several drug candidates to next strategic event Streamlined organization to include a core team of technical employees to support development activities and ensure regulatory compliance for all drug candidates Formed a new management team with relevant experience and expertise, including Dr. Aquilur Rahman, NeoPharm’s co-founder and Chief Scientific Advisor

6 Near-Term Strategy Focus on optimizing NeoPharm’s drug development activities As of March 31, 2008, cash on hand of $18.2 million is sufficient to fund currently anticipated development activities well into 2009 Ensure regulatory compliance with all drug candidates Progress several liposomal drug product candidates through Phase II completion Commence confirmatory Phase III for IL-13 for the treatment of GBM File IND for Cintredekin Besudotox for the treatment of IPF Protect and enhance the value of NeoPharm’s intellectual property

7 NeoPharm Product Portfolio Cintredekin Besudotox (Two Indications) Recurrent Glioblastoma Multiforme (GBM) Confirmatory Phase III trial expected to begin late 2008 Idiopatic Pulmonary Fibrosis Anticipated IND filing end of 2008 NeoLipid® Platform LEP-ETU (liposomal paclitaxel) An anti-cancer agent that is designed to prevent cell division by promoting the assembly and stabilization of microtubules Currently in Phase II clinical development LE-DT (liposomal docetaxel) Potential alternative therapy for patients allergic or less responsive to Taxotere® Currently in Phase I clinical development LE-rafAON Designed and developed to inhibit activated c-raf-1 gene, which has been associated with radiation and chemotherapy resistance. c-raf gene-specific antisense Anticipated IND filing 2Q’08
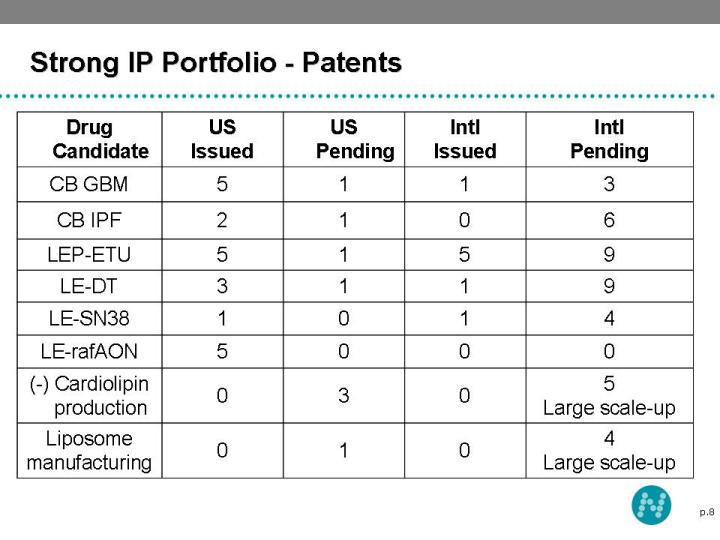
8 Strong IP Portfolio - Patents
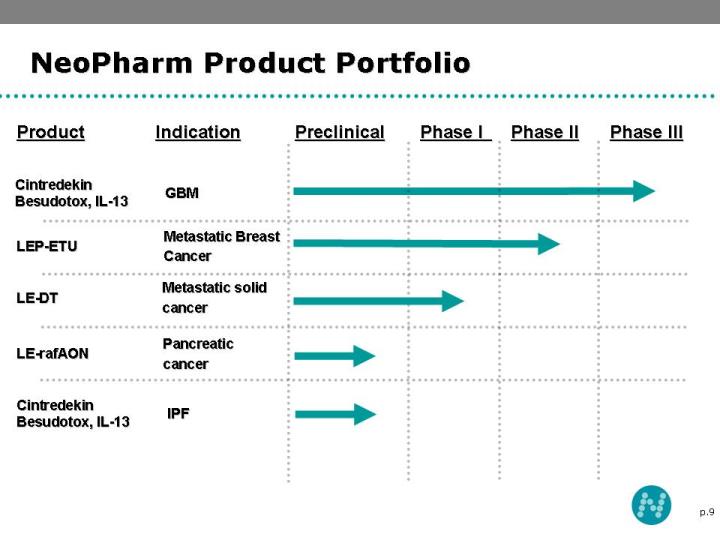
9 NeoPharm Product Portfolio LE-DT LEP-ETU IPF Cintredekin Besudotox, IL-13 GBM Cintredekin Besudotox, IL-13 LE-rafAON Metastatic solid cancer Metastatic Breast Cancer Phase III Phase II Phase I Indication Product Preclinical Pancreatic cancer
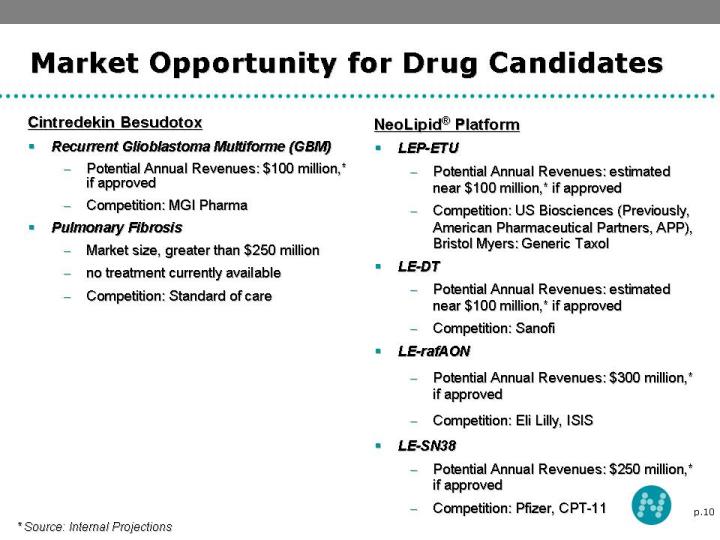
10 Market Opportunity for Drug Candidates Cintredekin Besudotox Recurrent Glioblastoma Multiforme (GBM) Potential Annual Revenues: $100 million,* if approved Competition: MGI Pharma Pulmonary Fibrosis Market size, greater than $250 million no treatment currently available Competition: Standard of care NeoLipid® Platform LEP-ETU Potential Annual Revenues: estimated near $100 million,* if approved Competition: US Biosciences (Previously, American Pharmaceutical Partners, APP), Bristol Myers: Generic Taxol LE-DT Potential Annual Revenues: estimated near $100 million,* if approved Competition: Sanofi LE-rafAON Potential Annual Revenues: $300 million,* if approved Competition: Eli Lilly, ISIS LE-SN38 Potential Annual Revenues: $250 million,* if approved Competition: Pfizer, CPT-11 * Source: Internal Projections

11 Benchmarks for Success Second Quarter 2008 Executed Letter of Intent with SIRO for Confirmatory Phase III – Cintredekin Besudotox, IL-13 Commencement of Phase I – LE-DT IND Submission – LE-rafAON Third Quarter 2008 Commencement of Phase III Confirmatory Trial – Cintredekin Besudotox, IL-13 Commencement of Phase I – LE-rafAON Fourth Quarter 2008 Completion of Phase II – LEP-ETU IND Submission - IL-13 for the treatment of Idiopathic Pulmonary Fibrosis

12 Product Portfolio Technologies

13 Cintredekin Besudotox For the Treatment of Recurrent Glioblastoma

14 Cintredekin Besudotox - Tumor Targeting Platform Cintredekin Besudotox (IL13-PE38QQR) is a novel recombinant tumor targeting protein, consisting of IL-13 and truncated Pseudomonas exotoxin A Malignant Glioma cells express IL-13 receptors at high density which permit binding of IL13-PE38QQR, cytotoxin internalization and cell death
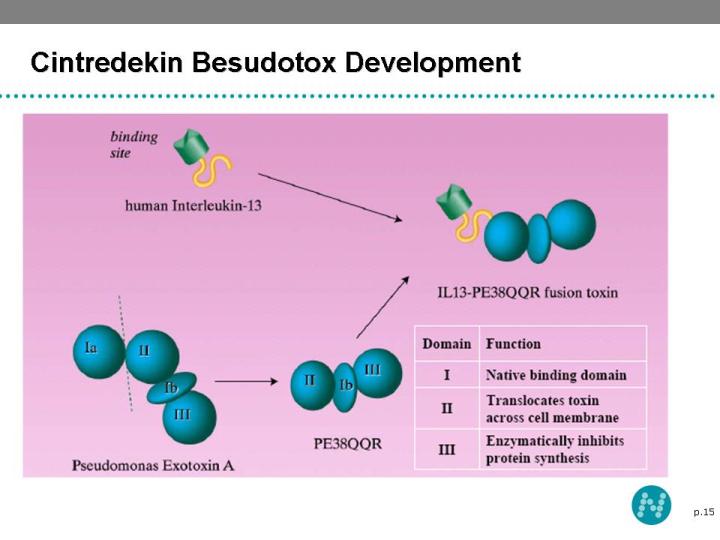
15 Cintredekin Besudotox Development
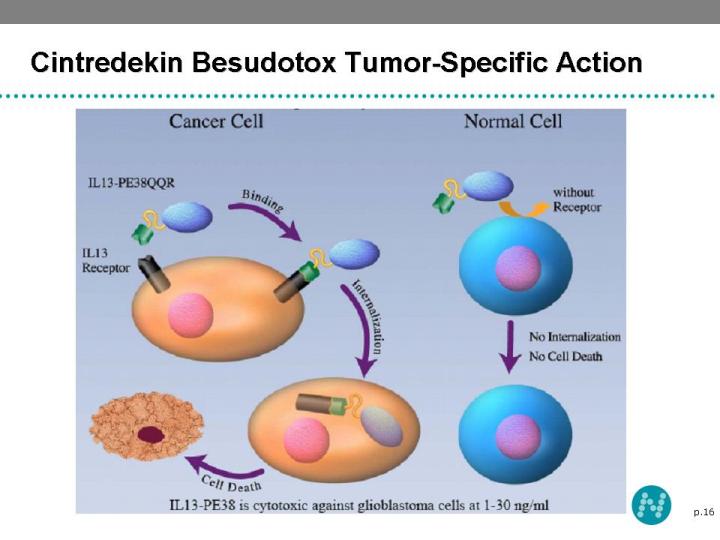
16 Cintredekin Besudotox Tumor-Specific Action
17 Convection Enhanced Delivery (CED) Cintredekin Besudotox (IL13-PE38QQR) is delivered to patients by a convection enhanced delivery (CED) system CED utilizes a pressure gradient to distribute macro-molecules to clinically significant volumes of tissue by bulk flow CED delivery avoids plasma dilution and minimizes systemic exposure Bypasses Blood-Brain Barrier Achieves larger distribution volumes than obtained by diffusion (cm vs. mm)
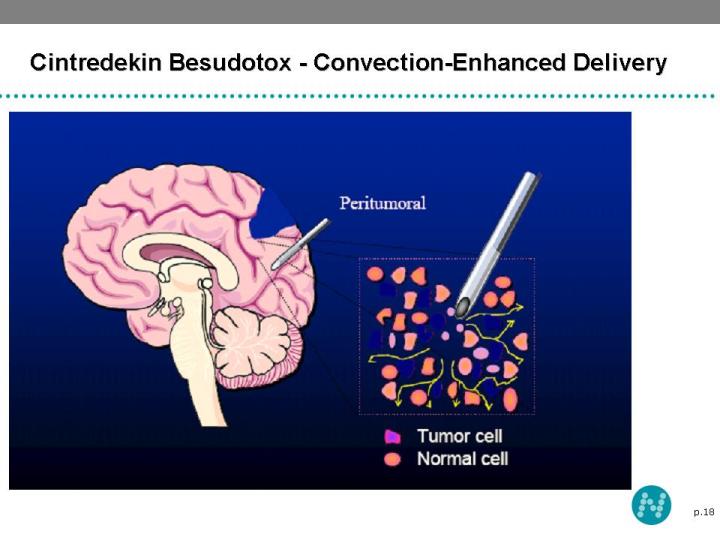
18 Cintredekin Besudotox - Convection-Enhanced Delivery

19 Cintredekin Besudotox For the Treatment of Idiopathic Pulmonary Fibrosis

20 Cintredekin Besudotox - Idiopathic Pulmonary Fibrosis New Indication Idiopathic Pulmonary Fibrosis (IPF) may result from a malfunctioning immune response Repeated immune-related damage and scarring of lungs destroys the elasticity, which affects the patients’ ability to breath Cell initiating the immune response to lung show over-expressed IL-13 receptors Selective destruction of cells presenting IL-13 receptors reduce the further damage and improve lung function in animal disease model of pulmonary fibrosis
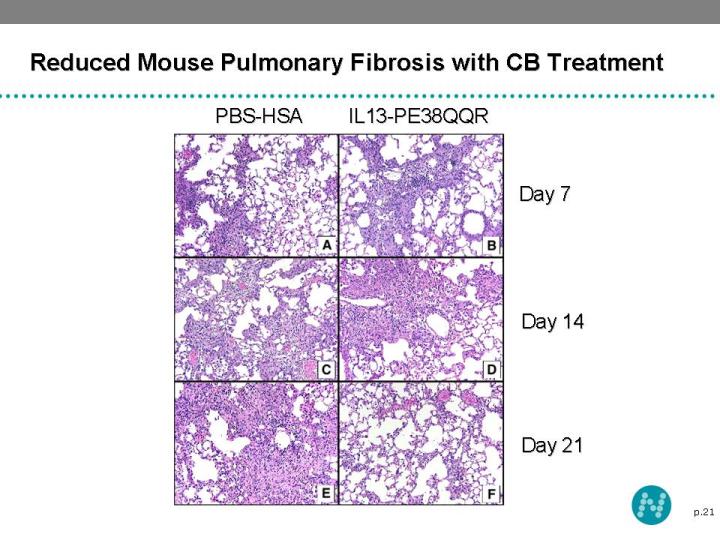
21 Reduced Mouse Pulmonary Fibrosis with CB Treatment
22 NeoLipid® Drug Delivery Platform

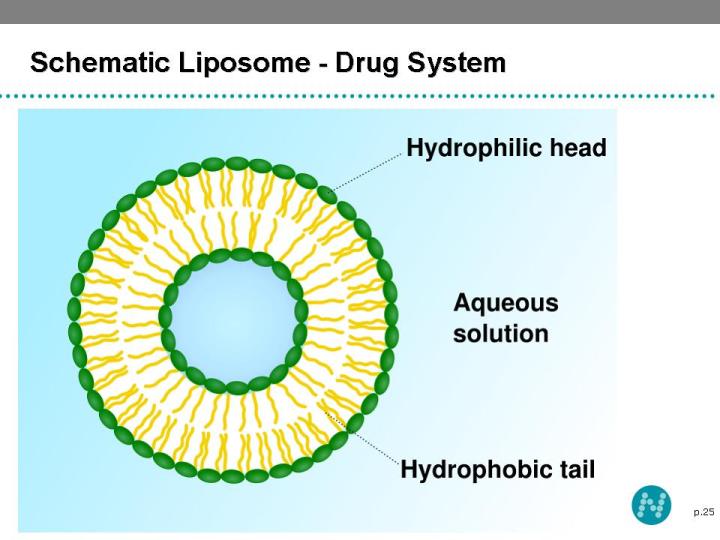
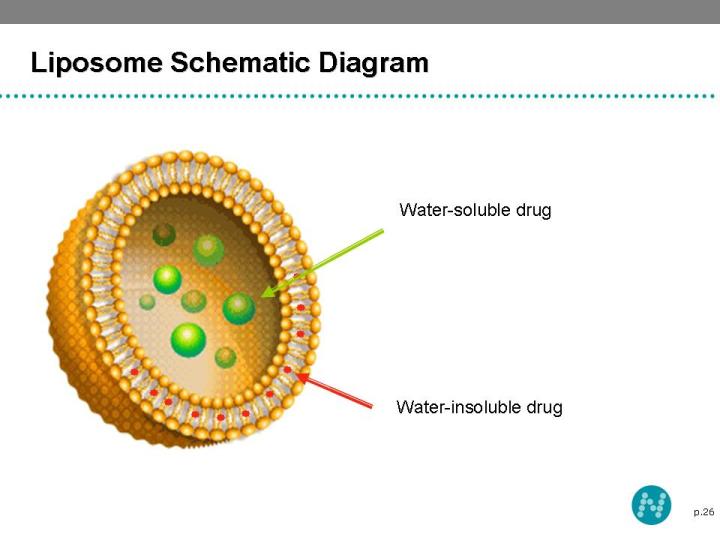
23 NeoLipid® Drug Delivery Platform A liposome is a spherical vesicle with a bilayer membrane, composed of phospholipid and cholesterol Liposomes has central hydrophilic and peripheral hydrophobic regions Liposomes are used to deliver a wide range of drugs The hydrophilic region contains the hydrophilic (water soluble) and hydrophobic region carries the hydrophobic (lipid soluble) drugs

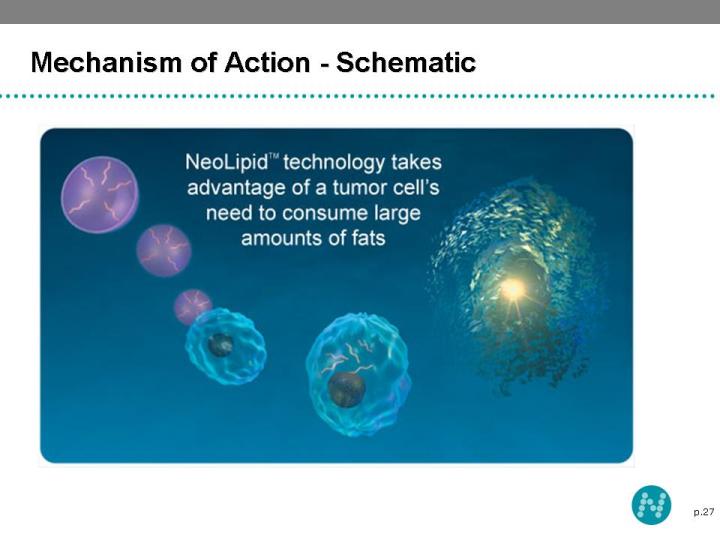
24 Mechanism of Action Liposomes are made of lipids (fats) Cancer cells need to consume large amount of fats to sustain their extremely rapid growth They recognize the liposomal drugs as a potential source of nutrition Cancer cells absorb the liposomes loaded with anti-cancer drugs as a source of fat Once the anti-cancer drugs are released from the liposome in the cells, the cancer cells are killed
25 Schematic Liposome - Drug System
26 Liposome Schematic Diagram
27 Mechanism of Action - Schematic

28 Advantages of NeoLipid® Drug Delivery Technology NeoPharm’s NeoLipid® Technology offers the following potential advantages: Reduced drug toxicity and multi drug resistance by using unique lipid, cardiolipin Improved pharmacokinetics and drug release profile Enhanced intracellular penetration Higher therapeutic efficacy (anticancer activity) Increased stability for our lyophilized easy to use products which is especially important during storage, reconstitution and administration to the patients

29 Development Activities

30 Cintredekin Besudotox - GBM For the treatment of Recurrent Glioblastoma Multiforme (GBM) Based on the outcome of the PRECISE Trial, on March 28, 2007 the FDA concluded that an additional Phase III trial would be required The PRECISE Trial did not meet the primary endpoint of a statistically significant difference, or separation, in the overall survival curves versus the Gliadel Wafer®, or Gliadel As recently announced NeoPharm has signed a letter of intent to commence a confirmatory Phase III trial The primary objective would be overall survival against standard of care as suggested by the FDA Secondary objective will focus on Progression Free Survival, or PFS, for patients with GBM

31 Cintredekin Besudotox –IPF For the treatment of Idiopathic Pulmonary Fibrosis Licensing agreement initiated with NIH in June 2007 Approximately 40,000 Americans die each year from pulmonary fibrosis Few treatments exist for this disease and no known cure Compiling all pre-clinical data available, which has shown positive preliminary evidence IND submission currently expected in late 2008
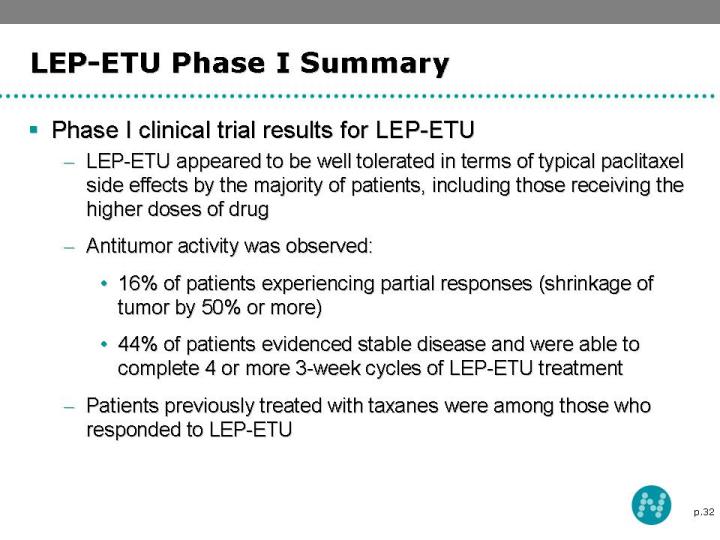
32 LEP-ETU Phase I Summary Phase I clinical trial results for LEP-ETU LEP-ETU appeared to be well tolerated in terms of typical paclitaxel side effects by the majority of patients, including those receiving the higher doses of drug Antitumor activity was observed: 16% of patients experiencing partial responses (shrinkage of tumor by 50% or more) 44% of patients evidenced stable disease and were able to complete 4 or more 3-week cycles of LEP-ETU treatment Patients previously treated with taxanes were among those who responded to LEP-ETU

33 LEP-ETU Phase II Trial First Patients enrolled March 31, 2008 Anticipate enrollment completion by end of third quarter 2008 Target enrollment of 35 patients in five centers Trial Design: Evaluate the anti-tumor effect and safety/tolerability of LEP-ETU in metastatic breast cancer The primary objective of this Phase II trial will be to assess the Overall Response Rate of patients with metastatic breast cancer after treatment with a dose of 275 mg/m2 LEP-ETU administered over 90 minutes, with Secondary objectives will be to evaluate the Progression-Free Survival (PFS) of patients treated with LEP-ETU and the safety of LEP-ETU, in particular peripheral neuropathy
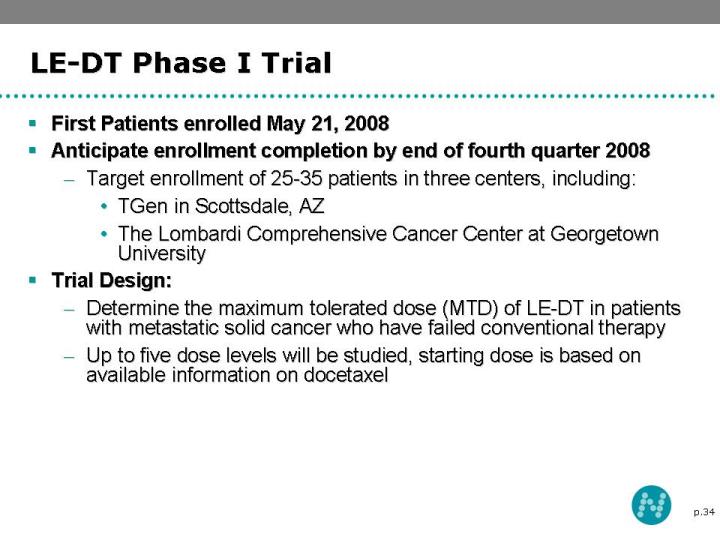
34 LE-DT Phase I Trial First Patients enrolled May 21, 2008 Anticipate enrollment completion by end of fourth quarter 2008 Target enrollment of 25-35 patients in three centers, including: TGen in Scottsdale, AZ The Lombardi Comprehensive Cancer Center at Georgetown University Trial Design: Determine the maximum tolerated dose (MTD) of LE-DT in patients with metastatic solid cancer who have failed conventional therapy Up to five dose levels will be studied, starting dose is based on available information on docetaxel

35 LE-rafAON Pre-Clinical Designed and developed to inhibit activated c-raf-1 gene, which has been associated with radiation and chemotherapy resistance. c-raf gene-specific antisense
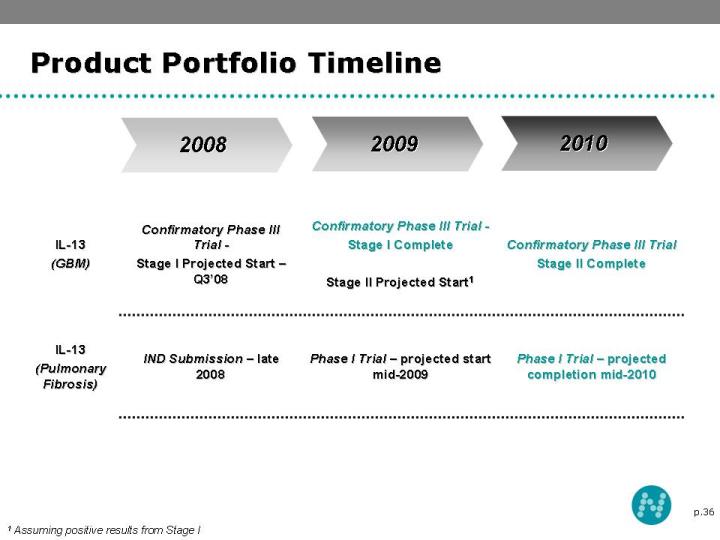
36 Product Portfolio Timeline 2008 2010 2009 1 Assuming positive results from Stage I
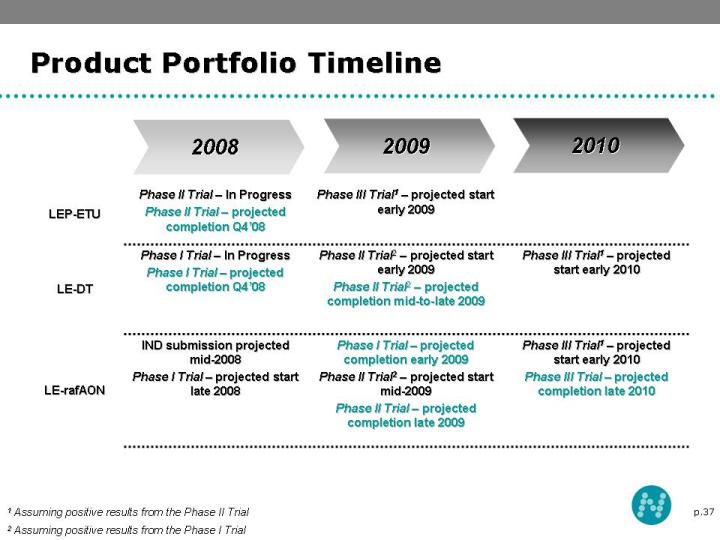
37 Product Portfolio Timeline 2008 2010 2009 1 Assuming positive results from the Phase II Trial 2 Assuming positive results from the Phase I Trial

38 Conclusion Multiple drug candidates in various points of clinical development Cintredekin Besudotox, Indication GBM: Confirmatory Phase III LEP-ETU: Phase II LE-DT: Phase I Robust Pipeline Additional indications for Cintredekin Besudotox, IL-13 Product initiatives funded and progressing Trials to capitalize on broader indications of cancer Optimal Cost Structure Significantly reduced cash consumption rate Lean organization focused primarily on the development of drug candidates Continue to identify new out-license and partnership opportunities Resources available to fund current candidates in development well into 2009





































