Table of Contents
| Filed by the Registrant o | ||||||||
| Filed by a Party other than the Registrant þ | ||||||||
| Check the appropriate box: | ||||||||
| þ | Preliminary Proxy Statement | o | Confidential, for Use of the Commission Only | |||||
| o | Definitive Proxy Statement | (as permitted by Rule 14a-6(e)(2)) | ||||||
| o | Definitive Additional Materials | |||||||
| o | Soliciting Material Pursuant to Rule 14a-12 | |||||||
| Payment of Filing Fee (Check the appropriate box): | ||||
| þ | No fee required. | |||
| o | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. | |||
| (1) | Title of each class of securities to which transaction applies: | |||
| (2) | Aggregate number of securities to which transaction applies: | |||
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined): | |||
| (4) | Proposed maximum aggregate value of transaction: | |||
| (5) | Total fee paid: | |||
| o | Fee paid previously with preliminary materials: | |||
| o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the form or schedule and the date of its filing. | |||
| (1) | Amount Previously Paid: | |||
| (2) | Form, Schedule or Registration Statement No.: | |||
| (3) | Filing Party: | |||
| (4) | Date Filed: | |||
| 2 | ||||
| 3 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| 9 | ||||
| 9 | ||||
| 12 | ||||
| 12 | ||||
| 12 | ||||
| 13 | ||||
| 13 | ||||
| 13 | ||||
| Annex A | A- | 1 | ||
| Annex B | B- | 1 | ||
| Annex C | C- | 1 | ||
| Proxy Card |
i
Table of Contents
LLOYD I. MILLER
| § | AGAINST the issuance of stock in the proposed merger: To BLOCK THIS MERGER, Pharmos shareholders should vote AGAINST the issuance of up to 19,500,000 shares of the Company’s common stock in connection with the proposed acquisition by the Company of Vela Pharmaceuticals, Inc. (the “Merger”) |
VOTE AGAINST THE ISSUANCE OF ADDITIONAL STOCK.
enclosed GOLD proxy, voting AGAINST the issuance of additional shares.
| § | All valid proxies received before the Special Meeting will be voted, and shareholders have the power to revoke their proxies at any time before they are exercised. | ||
| § | Please do not return any proxy sent to you by the Company. |
Table of Contents
2
Table of Contents
| § | Conserving cash by increasing Board oversight of product development expenses. | ||
| § | Partnering with other companies to develop Pharmos products. | ||
| § | Re-prioritizing and re-focusing current product development projects to allocate cash more efficiently. | ||
| § | Reducing administrative and overhead costs by increasing Board scrutiny of expenditures. | ||
| § | Avoiding acquisitions and business combinations on terms that reduce pro forma cash balances. |
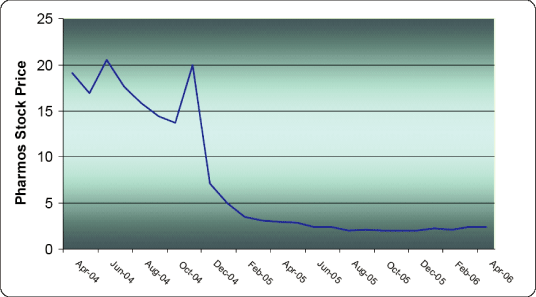
| Over the past two years, the price of Pharmos common stock steadily declined from $19.10 a share on April 30, 2004 to $2.47 a share by May 1, 2006. The steep decline of the Company’s stock price is more clearly illustrated by the graph provided on page 7 of the preliminary proxy statement for the Special Meeting of Pharmos Stockholders. Mr. Miller believes that during the past two years, the Pharmos Board of Directors, including the Company’s current nominees |
3
Table of Contents
| for re-election to the Board, Haim Aviv, Mony Ben Dor and Abraham Sartani, had immediate oversight responsibility for both the Company and the investment of its shareholders. However, during the two consecutive years of falling Pharmos stock prices, these directors failed to take effective action to protect shareholder interests. | |||
| In light of the LOW STOCK PRICE at which the Company’s common stock is currently valued, Mr. Miller opposes the issuance of Pharmos stock in the acquisition of Vela Pharmaceuticals, Inc., a company currently suffering from a cash shortfall. |
| As stated in the Company’s Form 10-K filed with the SEC on May 12, 2006, as of that date, there were 19,065,783 shares of Pharmos common stock outstanding. Under the terms of the Agreement and Plan of Merger, filed as an attachment to the Company’s Form 8-K filed on March 15, 2006, if the issuance of common stock in connection with the Merger is approved, the Company will issue up to an additional 19,500,000 shares of common stock to the owners of Vela. | |||
| The shares that may be issued to Vela’s owners consist of 11,500,000 shares that will be issued at closing, and an additional 8,000,000 shares that may be issued after closing, contingent on two milestones. The two milestones are successful completion of a Phase IIb clinical trial for one of Vela’s products and the filing of a new drug application with the U.S. FDA and do not require Vela’s products to produce any revenues. Therefore, if the issuance of common stock in connection with the Merger is approved and the milestones are met, Pharmos might MORE THAN DOUBLE its currently existing number of shares of outstanding common stock. | |||
| Even if Vela is unable to achieve the requisite milestones that would trigger the issuance of the additional 8,000,000 shares of common stock in connection with the Merger, the number of outstanding shares of Pharmos common stock will still increase by 11,500,000 shares, so that Vela’s owners will own more than one-third of the combined company. The milestones could trigger the issuance of additional shares so that Vela’s owners may own MORE THAN HALF of the combined company, even if Vela’s products NEVER PRODUCE ANY REVENUES. | |||
| Ultimately, if the issuance of shares in connection with the Merger is approved, THE POWER OF YOUR VOTE WILL BE SIGNIFICANTLY DILUTED. For the reasons stated, Mr. Miller believes that the consideration proposed to be paid by Pharmos to the owners of Vela would result in excessive dilution of the equity interests of Pharmos shareholders. |
| As stated in Note 13 to the Pharmos Financial Statements in its Form 10-K filed with the SEC on March 21, 2006, as of December 31, 2005, the Company had NET OPERATING LOSSES OF APPROXIMATELY $120,000,000 in the United States alone. This loss reflects the Company’s continued inability, since its inception, to convert research and development expenses into sales revenue – or in other words, to realize profits from its cash burn. | |||
| In these preliminary proxy materials, when discussing the Company’s prolonged period of cash burn, Mr. Miller is referring to the current Board’s failure to achieve profits, in light of continuing cash expenditures evidenced by current net operating losses totaling $120,000,000. |
| As stated in Note 1 to Vela’s Financial Statements for the year ended December 31, 2005, included in the Pharmos preliminary proxy statement filed with the SEC on May 3, 2006, as of year end, Vela had “CUMULATIVE LOSSES OF APPROXIMATELY $62,174,000, negative working capital and negative cash flows from operations.” Furthermore, because of Vela’s failure to take a single product beyond its research and development phase since the company’s inception in 1998, the Financial Statements also clearly state that “If a merger or sale [of Vela] does not occur, the Company’s ability to continue as a going concern [will be] dependent upon the continued sale of its securities for funds to meet its cash requirements.” | |||
| VELA IS A CASH-POOR COMPANY. If the Merger is effectuated, Pharmos will be called upon to provide the necessary cash infusion to develop Vela’s products. However, as noted above, Pharmos itself is currently reporting net operating losses of $120,000,000. Therefore, if |
4
Table of Contents
| shareholders approve the issuance of common stock in connection with the Merger, Pharmos, as the surviving company, faces significant increases in its cash requirements. According to the Pharmos Form 8-K filed on June 6, 2006, Pharmos expects a cash burn of $19 to $20 million in 2006 - nearly half of its estimated cash balance of $40 million. | |||
| § | Statements in this proxy statement that Pharmos should avoid diverting funds to Vela: | ||
| The Pharmos Board emphasizes its desire to incorporate Vela’s lead drug, dextofisopam, into the Pharmos product pipeline. However, dextofisopam will require SIGNIFICANT ADDITIONAL CASH EXPENDITURES from Pharmos before the drug can advance into the next stage of clinical trials. Because Vela has no cash balances to fund its products, Pharmos cash balances will be diverted to develop Vela’s products, further delaying development of existing Pharmos products. | |||
| In light of already significant net operating losses at Pharmos, Mr. Miller believes that Pharmos should instead seek to RE-FOCUS and RE-PRIORITIZE its own internal product development projects, so as to allocate current cash expenditures more effectively. Then, Pharmos will be better suited to attract partnering opportunities with other pharmaceutical companies, which will allow the Company to share development costs and reduce cash burn. |
5
Table of Contents
The members of the Committee, like you, are Pharmos shareholders, and like you, are committed to the goal of maximizing shareholder value.
LLOYD I. MILLER, III (age 52) is a registered investment advisor and has been a member of the Chicago Board of Trade since 1978 and a member of the Chicago Stock Exchange since 1996. Mr. Miller graduated from Brown University in 1977 with a Bachelor’s Degree. Mr. Miller is currently a director of Stamps.com, American BankNote Corporation and Aldila, Inc. Mr. Miller previously served on the board of directors of several other companies, including Anacomp, Denny’s Corporation, Vulcan International, Celeritek, Inc., Dynabazaar, Inc. (formerly FairMarket, Inc.) and American Controlled Industries. Mr. Miller’s principal occupation is investing assets held by Mr. Miller on his own behalf and on behalf of his family.
RAYMOND E. McKEE, PH.D. (age 59) served as Vice President, Investor Relations and Corporate Development at Pharmos from 2004 to March 2006, Pharmos previously employed Dr. McKee to serve as Vice President, Business Development from 2000-2002. He served as Vice President, Business Development at Collgard Biopharmaceuticals from 2002-2004. Previously, Dr. McKee was a Senior Consultant at ISO Health Care from 2000-2004, Vice President at Innovir Labratories, Inc. from 1998-1999, Biotechnology Editor/Senior Analyst at the Genesis Group from 1994-1998, a partner in Technology Partners from 1990-1994, Director of Technology Transfer and Development at Lifecodes Corporation from 1987-1990, Director of Life Sciences at Allied Instrumentation Laboratories from 1984-1986, Director of Research and Development at Ventrex Laboratories from 1981-1984 and Project Manager at Abbott Laboratories from 1977-1981. Dr. McKee completed his Post Doctoral Research in Bioanalytical Chemistry at University of Texas Medical School in 1977, received his Ph.D. in Biochemistry from Purdue University in 1975 and received his Bachelors Degree from Susquehanna University in 1969.
GERARD SOULA (age 61) is the founder of Flamel Technologies SA and from 1990 to 2006 served in various executive positions as President-Directeur General (Chairman of the Board of Directors, President and Chief Executive Officer), Director of Research and Development and as a member of Flamel’s Board of Directors. Dr. Soula earned a degree from the Institut d’Administration des Entreprises in 1971. After receiving his Doctorat-es Sciences in organic chemistry from Marseille University in 1973, he joined Rhone-Poulenc’s Research Center in Lyon, France. In 1984, he received the Rhone-Poulenc Innovation Award for his discovery of TDA, a phase transfer catalyst. From 1981 to 1990, he served as both the Head of Research of the Silicon Group and the Director of Research of the Polymer Materials Department of Rhone-Poulenc.
IMPACT CURRENT PHARMOS SHAREHOLDERS
6
Table of Contents
It is time for a change
7
Table of Contents
| § | Issuance of stock at closing. Vela shareholders will receive 11,500,000��million shares of Pharmos common stock immediately upon the closing of the Merger in exchange for all outstanding Vela securities, after conversion of any outstanding Vela bridge notes. | ||
| § | Payment of $5,000,000 in cash. In addition, at the closing, the Company will pay $5,000,000 in cash to or on behalf of Vela to repay all accrued interest and a portion of the principal amount of Vela’s outstanding bridge note indebtedness and to satisfy some of Vela’s liabilities. | ||
| § | Contingent issuance of additional stock if milestones are met. Vela’s shareholders will also receive up to an additional 8,000,000 Pharmos shares upon the achievement of milestones related to Vela’s Phase II product candidate, dextofisopam. These milestones do not require Vela’s products to produce any revenues. The number of shares to be issued in connection with the Merger and achievement of the milestones may also be adjusted in the future to account for any stock splits, stock dividends or other similar recapitalizations. |
8
Table of Contents
9
Table of Contents
| § | delivering a later dated proxy to Mr. Miller’s proxy solicitor, using the enclosed postage paid envelope; or | ||
| § | delivering a later dated proxy to the secretary of Pharmos; or | ||
| § | delivering a written revocation to either Mr. Miller’s proxy solicitor or the Secretary of Pharmos; or | ||
| § | voting in person at the Special Meeting. |
| § | submitting a new proxy card or voting instruction form to your broker or nominee; or | ||
| § | attending the Special Meeting and voting in person, provided you have obtained a signed legal proxy from the record holder giving you the right to vote your shares. |
10
Table of Contents
11
Table of Contents
12
Table of Contents
13
Table of Contents
Let your voice be heard.
Please mark, sign and date the enclosed GOLD proxy card and return it promptly in the enclosed
postage-prepaid envelope.
1200 Wall Street West, 3rd Floor
Lyndhurst, New Jersey 07071
(800) 581-4729
14
Table of Contents
WHO MAY BE DEEMED TO BE PARTICIPANTS IN THE SOLICITATION OF PROXIES
A-1
Table of Contents
MR. MILLER, DR. McKEE, DR. SOULA AND THEIR ASSOCIATES
SEE LEGEND BELOW FOR EXPLANATION OF ENTITIES
| ENTITY | DATE | BUY/SELL | ||||||
| A-4 | 6/14/2005 | 977 | ||||||
| A-4 | 6/15/2005 | 3,618 | ||||||
| A-4 | 6/16/2005 | 3,996 | ||||||
| A-4 | 6/21/2005 | 3,467 | ||||||
| A-4 | 6/27/2005 | 20,000 | ||||||
| A-4 | 6/28/2005 | 18,000 | ||||||
| A-4 | 6/29/2005 | 2,078 | ||||||
| A-4 | 7/5/2005 | 45,952 | ||||||
| A-4 | 7/6/2005 | 11,600 | ||||||
| A-4 | 7/7/2005 | 105,190 | ||||||
| A-4 | 7/8/2005 | 15,200 | ||||||
| A-4 | 7/11/2005 | 12,460 | ||||||
| A-4 | 7/12/2005 | 17,640 | ||||||
| A-4 | 7/13/2005 | 14,733 | ||||||
| A-4 | 7/14/2005 | 24,024 | ||||||
| A-4 | 7/18/2005 | 8,317 | ||||||
| A-4 | 7/19/2005 | 44,926 | ||||||
| M-2 | 8/5/2005 | 5,000 | ||||||
| M-2 | 8/8/2005 | 46,587 | ||||||
| M-2 | 8/9/2005 | 30,680 | ||||||
| M-2 | 8/11/2005 | 45,702 | ||||||
| M-2 | 8/12/2005 | 4,310 | ||||||
| M-2 | 8/15/2005 | 8,468 | ||||||
| M-2 | 8/16/2005 | 17,332 | ||||||
| M-2 | 8/17/2005 | 1,100 | ||||||
| M-2 | 8/18/2005 | 17,667 | ||||||
| M-2 | 8/19/2005 | 26,192 | ||||||
| M-2 | 8/22/2005 | 78,876 | ||||||
| M-2 | 8/23/2005 | 100,000 | ||||||
| M-2 | 8/24/2005 | 54,316 | ||||||
| M-2 | 8/25/2005 | 4,100 | ||||||
| M-2 | 8/25/2005 | 4,900 | ||||||
| M-2 | 8/26/2005 | 41,584 | ||||||
| M-2 | 8/29/2005 | 13,435 | ||||||
| M-2 | 8/30/2005 | 39,834 | ||||||
| M-2 | 8/31/2005 | 34,840 | ||||||
| M-2 | 9/1/2005 | 3,660 | ||||||
| M-2 | 10/12/2005 | 8,231 | ||||||
| M-2 | 10/13/2005 | 600 | ||||||
B-1
Table of Contents
| ENTITY | DATE | BUY/SELL | ||||||
| M-2 | 10/14/2005 | 81,600 | ||||||
| M-2 | 10/17/2005 | 62,765 | ||||||
| M-2 | 10/18/2005 | 77,213 | ||||||
| M-2 | 10/19/2005 | 2,822 | ||||||
| M-2 | 10/20/2005 | 154 | ||||||
| M-2 | 11/1/2005 | 13,665 | ||||||
| M-2 | 11/2/2005 | 19,100 | ||||||
| M-2 | 11/3/2005 | 6,278 | ||||||
| M-2 | 11/28/2005 | 6,820 | ||||||
| M-2 | 11/28/2005 | 3,526 | ||||||
| M-2 | 11/29/2005 | 11,353 | ||||||
| M-2 | 11/30/2005 | 9,522 | ||||||
| M-2 | 12/2/2005 | 8,697 | ||||||
| M-2 | 12/5/2005 | 27,743 | ||||||
| M-2 | 12/6/2005 | 5,003 | ||||||
| M-2 | 12/7/2005 | 595 | ||||||
| M-2 | 12/8/2005 | 17,650 | ||||||
| M-2 | 12/9/2005 | 19,009 | ||||||
| M-2 | 12/12/2005 | 20,500 | ||||||
| M-2 | 12/13/2005 | 7,125 | ||||||
| M-2 | 12/14/2005 | 4,806 | ||||||
| M-2 | 12/15/2005 | 1,010 | ||||||
| M-2 | 12/16/2005 | 60 | ||||||
| M-2 | 12/19/2005 | 1,000 | ||||||
| M-2 | 12/20/2005 | 1,520 | ||||||
| M-2 | 12/21/2005 | 66 | ||||||
| M-2 | 12/22/2005 | 5,443 | ||||||
| M-2 | 12/23/2005 | 4,620 | ||||||
| M-2 | 12/27/2005 | 25,817 | ||||||
| M-2 | 12/28/2005 | 1,226 | ||||||
| M-2 | 12/29/2005 | 25,000 | ||||||
| M-2 | 12/30/2005 | 97,051 | ||||||
| M-2 | 1/3/2006 | 8,300 | ||||||
| M-2 | 1/4/2006 | 9,262 | ||||||
| Gerard Soula | 1/4/2006 | 25,000 | ||||||
| M-2 | 1/5/2006 | 500 | ||||||
| M-2 | 1/9/2006 | 1,590 | ||||||
| M-2 | 1/9/2006 | 426 | ||||||
| M-2 | 1/10/2006 | 10,548 | ||||||
| M-2 | 1/11/2006 | 1,845 | ||||||
| M-2 | 2/9/2006 | 20,000 | ||||||
| M-2 | 2/10/2006 | 520 | ||||||
| M-2 | 2/13/2006 | 37,635 | ||||||
| Gerard Soula | 2/14/2006 | 25,000 | ||||||
| M-2 | 2/17/2006 | 500 | ||||||
| M-2 | 2/21/2006 | 500 | ||||||
| M-2 | 5/1/2006 | 325 | ||||||
| LIM | 5/10/2006 | 1,506 | ||||||
| M-2 | 5/10/2006 | 20,000 | ||||||
| LIM | 5/11/2006 | 8,494 | ||||||
| M-2 | 5/12/2006 | 3,497 | ||||||
B-2
Table of Contents
| ENTITY | DATE | BUY/SELL | ||||||
| M-2 | 5/15/2006 | 49,113 | ||||||
| M-2 | 5/23/2006 | 4,496 | ||||||
| M-2 | 5/24/2006 | 9,108 | ||||||
| M-2 | 5/30/2006 | 3,648 | ||||||
| M-2 | 5/31/2006 | 1,900 | ||||||
| M-2 | 6/6/2006 | 9,814 | ||||||
| M-2 | 6/7/2006 | 1,034 | ||||||
| M-2 | 6/8/2006 | 6,966 | ||||||
| M-2 | 6/13/2006 | 19,000 | ||||||
| M-2 | 6/14/2006 | 3,200 | ||||||
| Gerard Soula | 6/15/2006 | 10,000 | ||||||
| M-2 | 6/15/2006 | 13,263 | ||||||
| M-2 | 6/16/2006 | 3,580 | ||||||
| M-2 | 6/19/2006 | 14,068 | ||||||
| M-2 | 6/20/2006 | 9,152 | ||||||
B-3
Table of Contents
Trust A-4 (“A4”) | Lloyd I. Miller, III (“Miller”) is the advisor to Trust A-4 (the “Trust”). | |
| Alan Goldman, VP PNC Bank, N.A. 500 PNC Center 201 East Fifth Street Cincinnati, OH 45202 | Trust A-4 was created pursuant to a Declaratory Judgment, signed by the Honorable Wayne F. Wilke for the Court of Common Pleas, Probate Division, Hamilton County, Ohio, on October 27, 1992, pursuant to which Trust A was split into four separate trusts. The Trusts were created pursuant to an Amended and Restated Trust Agreement (the “Trust Agreement”), dated September 20, 1983. | |
| Mr. Miller was named as advisor to PNC Bank, Ohio, N.A. (formerly The Central Trust Company, N.A., Cincinnati Ohio), the trustee named in the Trust Agreement. Such appointment became effective on April 22, 1990, the date of death of Lloyd I. Miller, the grantor of the Trusts. | ||
| All of the shares Mr. Miller may be deemed to beneficially own as advisor to the Trust were purchased by funds generated and held by the Trust. | ||
Milfam II, L.P. (“M-2”) | Mr. Miller is the manager of Milfam LLC, an Ohio limited liability company established pursuant to the Operating Agreement of Milfam LLC, dated as of December 10, 1996. | |
| Steve Hendrickson Northern Trust Company 50 South Lasalle Street Chicago, IL 60675 | Milfam LLC is the managing general partner of Milfam II, L.P. a Georgia limited partnership established, pursuant to the Partnership Agreement for Milfam II, L.P., dated December 11, 1996. | |
| All of the shares Mr. Miller may be deemed to beneficially own as the manager of the managing general partner of Milfam II, L.P. were purchased with money contributed to Milfam II, L.P. by its partners, or money generated and held by Milfam II, L.P. | ||
Lloyd I. Miller, III (“LIM”) | Mr. Miller is the record owner of 1,506 of the LIM shares and the beneficial holder of all 10,000 LIM shares. | |
| Lloyd I. Miller, III | ||
| 4550 Gordon Drive | ||
| Naples, Florida 34102 |
580 Walnut Street
Cincinnati, OH 45202
B-4
Table of Contents
MR. LLOYD I. MILLER, III
| (3) Amount and | ||||||||||||
| (1) Title of | (2) Name and address | nature of beneficial | ||||||||||
| class | of beneficial owner | ownership | (4) Percent of class | |||||||||
| Common Stock | Lloyd I. Miller, III | |||||||||||
| 4550 Gordon Drive | 1,795,035 | 9.4 | % | |||||||||
| Naples, Florida 34102 | ||||||||||||
B-5
Table of Contents
BENEFICIAL OWNERS AND MANAGEMENT
| § | each person who was known by the Company to own beneficially more than 5% of any class of the Company’s Common Stock, | ||
| § | each of the Company’s Directors, and | ||
| § | all current Directors and executive officers of the Company as a group. |
| Amount of | ||||||||||
| Beneficial | Percentage of | |||||||||
| Name and Address of Beneficial Ownership | Ownership | Total (1) | ||||||||
| Haim Aviv, Ph.D. (2) c/o Pharmos Ltd, Kiryat Weitzman Rehovot 76326, Israel | 437,024 | 2.3 | % | |||||||
| David Schlachet (3) Syneron Medical Ltd. Industrial Zone, Tavor Building P.O.B. 550 Yokneam Illit, 20692 Israel | 25,187 | * | * | |||||||
| Mony Ben Dor (4) 40 Hakukia St Rishon Le Zion 75548, Israel | 17,187 | * | * | |||||||
| Georges Anthony Marcel M.D., Ph.D.(3) 9 ue de Magdebourg 75116 Paris, France | 14,000 | * | * | |||||||
| Elkan R. Gamzu, Ph.D. (5) enERGetics, 199 Wells Avenue, Suite 302 Newton, MA 02459 | 20,750 | * | * | |||||||
| Lawrence F. Marshall, M.D. (3) University of California, San Diego Regents Court Bldg., Suite 200 4130 LaJolla Village Drive LaJolla, CA 92037-1480 | 12,875 | * | * | |||||||
| Abraham Sartani. M.D. c/o Recordati SpA, Via Civitali, 1 20148 Milano, Italy | — | — | ||||||||
C-1
Table of Contents
| Amount of | ||||||||||
| Beneficial | Percentage of | |||||||||
| Name and Address of Beneficial Ownership | Ownership | Total (1) | ||||||||
| Alan L. Rubino (3) c/o Pharmos Corporation 99 Wood Avenue South, Suite 311 Iselin, NJ 08830 | 40,000 | * | ||||||||
| James A. Meer (6) c/o Pharmos Corporation 99 Wood Avenue South, Suite 311 Iselin, NJ 08830 | 47,750 | * | ||||||||
| All Directors and Executive Officers as a group (9 persons)(8) | 614,773 | 3.2 | % | |||||||
| Lloyd Miller, III (9) 4550 Gordon Drive Naples, FL 34102 | 1,508,351 | 7.9 | % | |||||||
| * | Indicates ownership of less than 1%. | |
| (1) | Based on 19,065,784 shares of Common Stock outstanding, plus each individual’s currently exercisable warrants or options. Assumes that no other individual will exercise any warrants and/or options. | |
| (2) | Consists of 170,423 outstanding shares and 228,626 shares issuable upon exercise of currently exercisable warrants and/or options. | |
| (3) | Consists entirely of shares issuable upon exercise of currently exercisable warrants and/or options. | |
| (4) | Consists of 1,000 outstanding shares and 17,625 shares issuable upon exercise of currently exercisable warrants and/or options. | |
| (5) | Consists of 2,000 outstanding shares and 18,750 shares issuable upon exercise of currently exercisable warrants and/or options. | |
| (6) | Consists of 12,000 outstanding shares and 35,750 shares issuable upon exercise of currently exercisable warrants and/or options | |
| (7) | Consists of 223,398 outstanding shares and 391,375 shares issuable upon exercise of currently exercisable warrants and/or options. | |
| (8) | This information is as of December 31, 2004 based on a Form 13G filed by Lloyd Miller on February 9, 2006. |
C-2
Table of Contents
NOT BY THE BOARD OF DIRECTORS OF PHARMOS CORPORATION
If you return this proxy, properly executed, without specifying a choice,
your shares will be voted AGAINST the Proposal
acquisition by the Company of Vela Pharmaceuticals, Inc.
| LLOYD I. MILLER, III RECOMMENDS A VOTE AGAINST THE PROPOSAL | ||||
| o FOR | o AGAINST | o ABSTAIN | ||
Table of Contents
as may properly come before the meeting, or any adjournment or postponements thereof,
as set forth in the proxy statement provided herewith.
| Dated: | ||||||
| Signature | ||||||
| Signature, if held jointly | ||||||
| Title: | ||||||
The Altman Group, Inc., toll free at (800) 581-4729.