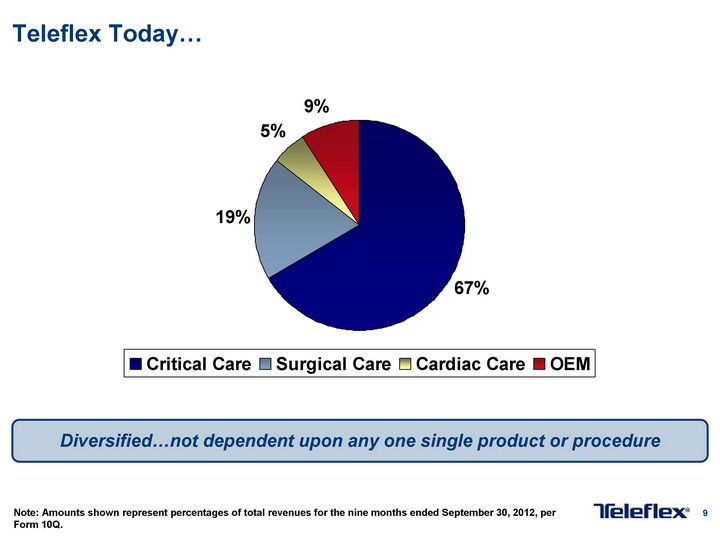
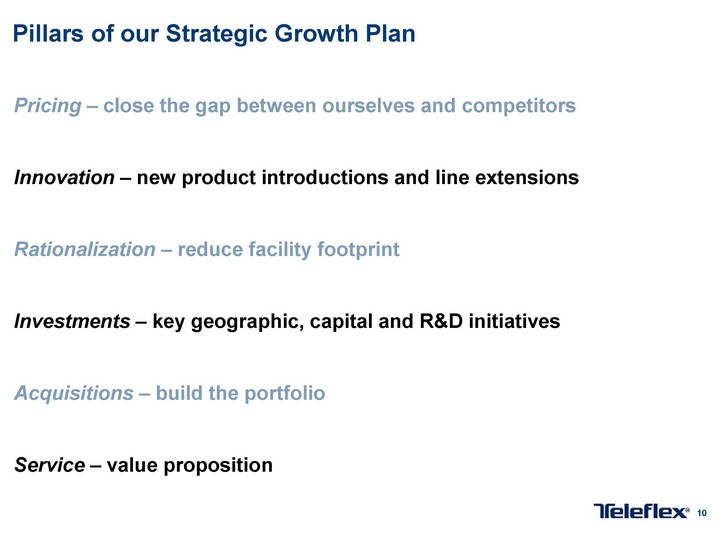
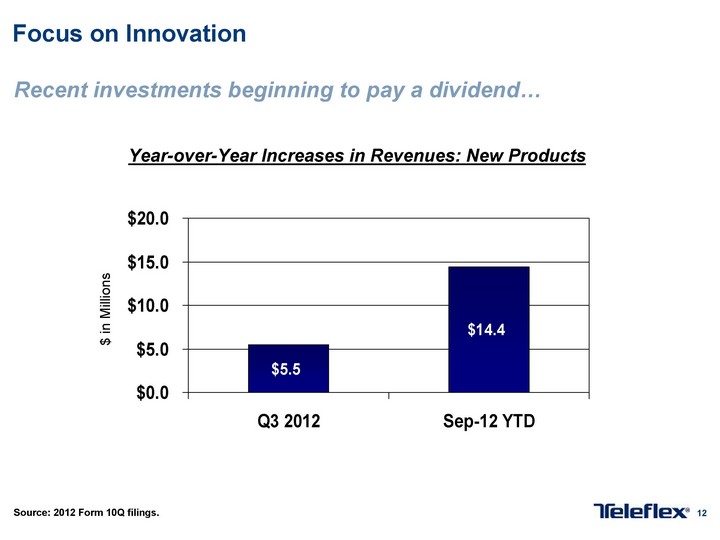
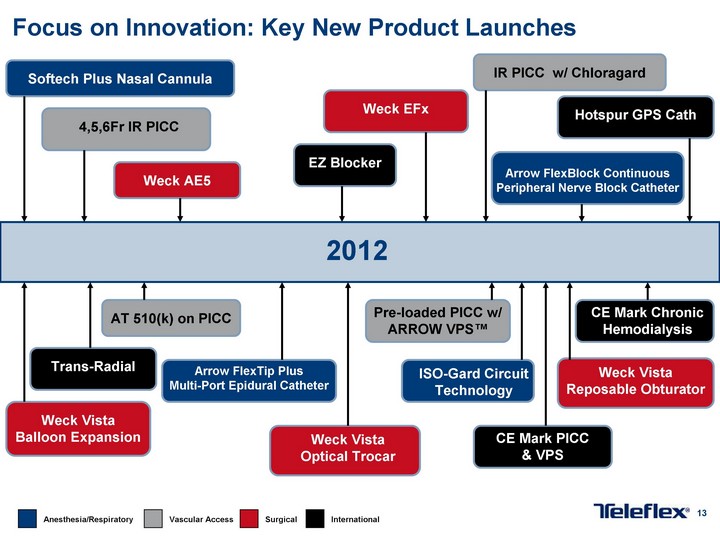
Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
Filing exhibits
TFX similar filings
- 26 Feb 13 Departure of Directors or Certain Officers
- 21 Feb 13 Regulation FD Disclosure
- 21 Feb 13 Teleflex Reports Fourth Quarter and Full Year 2012 Results
- 13 Dec 12 Regulation FD Disclosure
- 31 Oct 12 Regulation FD Disclosure
- 31 Oct 12 Teleflex Reports Third Quarter 2012 Results
- 14 Aug 12 Teleflex to Acquire Global Laryngeal Mask Leader
Filing view
External links