UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
(Rule 14a-101)
Filed by the Registrant ¨ Filed by a Party other than the Registrant x
Check the appropriate box:
| ¨ | Preliminary Proxy Statement | |
| ¨ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | |
| ¨ | Definitive Proxy Statement | |
| ¨ | Definitive Additional Materials | |
| x | Soliciting Material Pursuant to §240.14a-12 | |
SIGMA-ALDRICH CORPORATION
(Name of Registrant as Specified In Its Charter)
MERCK KGaA
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| x | No fee required. | |||
| ¨ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. | |||
| (1) | Title of each class of securities to which transaction applies:
| |||
| (2) | Aggregate number of securities to which transaction applies:
| |||
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined):
| |||
| (4) | Proposed maximum aggregate value of transaction:
| |||
| (5) | Total fee paid:
| |||
| ¨ | Fee paid previously with preliminary materials. | |||
| ¨ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. | |||
| (1) | Amount Previously Paid:
| |||
| (2) | Form, Schedule or Registration Statement No.:
| |||
| (3) | Filing Party:
| |||
| (4) | Date Filed:
| |||
Filed by: Merck KGaA
Pursuant to Rule 14a-12
under the Securities Exchange Act of 1934
Subject Company: Sigma-Aldrich Corporation
Commission File No. 000-08135
The following materials are available to shareholders of Sigma-Aldrich Corporation in connection with the previously announced transaction between Sigma-Aldrich Corporation and Merck KGaA on the transaction website hosted by Merck KGaA located at http://www.emdgroup.com/emd/media/topics/001.html:
1) Press Release
2) Investor Presentation
3) Ad Hoc Release
4) Factsheet
5) MerckNet Report
6) Script of CEO/CFO Video
7) Townhall Presentation

News Release | Your Contact | |||||
Nicole Mommsen |
+49 151 1454 2005 | |||||
| Markus Talanow | +49 6151 72-7144 | |||||
| Investor Relations | +49 6151 72-3321 |
September 22, 2014
Merck KGaA to Acquire Sigma-Aldrich to Enhance Position in Attractive Life Science Industry
| • | Merck KGaA to acquire Sigma-Aldrich for $140 per share in cash, valuing company at approx. $17 billion (€13.1 billion) |
| • | Acquisition expands EMD Millipore’s global reach, increasing the company’s presence in North America and adding exposure to fast-growing Asian markets |
| • | Customers benefit from broader offering of complementary products and capabilities and leading e-commerce platform in the industry |
| • | Merck KGaA plans to maintain significant presence in St. Louis, MO, and Billerica, MA |
| • | Life Science contribution to Merck KGaA earnings more than doubles |
| • | Transaction expected to be immediately accretive to EPS pre and EBITDA margin |
| • | Merck KGaA to host media conference call today at 8:00 AM EDT / 2:00 PM CET |
Darmstadt, Germany and St. Louis, MO, September 22, 2014 – Merck KGaA, a leading company for innovative and top-quality high-tech products in the pharmaceutical, chemical and life science sectors, and Sigma-Aldrich today announced that they have entered into a definitive agreement under which Merck KGaA, Darmstadt, Germany, will acquire Sigma-Aldrich for $17.0 billion (€13.1 billion), establishing one of the leading players in the $130 billion global life science industry.
Merck KGaA, Darmstadt, Germany, will acquire all of the outstanding shares of Sigma-Aldrich for $140 per share in cash. The agreed price represents a 37% premium to the latest closing price of $102.37 on September 19, 2014, and a 36% premium to the one-month average closing price. The transaction is expected to be immediately accretive to Merck KGaA, Darmstadt,
| Merck KGaA | ||||
Frankfurter Strasse 250 64293 Darmstadt Hotline +49 6151 72-5000 www.emdgroup.com | Head Media Relations -62445 Spokesperson: -9591 / -7144 / -6328 Fax +49 6151 72-3138 media.relations@merckgroup.com | |||
Page 1 of 7

News Release
Germany’s EPS pre and EBITDA margin. Merck KGaA, Darmstadt, Germany, expects to achieve annual synergies of approximately €260 million (approximately $340 million), which should be fully realized within three years after closing.
“This transaction marks a milestone on our transformation journey aimed at turning our three businesses into sustainable growth platforms”, said Karl-Ludwig Kley, Chairman of Merck KGaA, Darmstadt, Germany’s Executive Board. “For our life science business it’s even more than that: it’s a quantum leap. In one of the world’s key industries two companies that fit perfectly together have found each other to present a much broader product offering to our global customers in research, pharma and biopharma manufacturing and diagnostic and testing labs. As such, the combination of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich will secure stable growth and profitability in an industry that is driven by trends such as the globalization of research and manufacturing. What’s more, the combination gives us the possibility to invest even more in innovation going forward. We are delighted to make this compelling proposition to Sigma-Aldrich’s shareholders, who will obtain full and certain cash value for their shares.”
Rakesh Sachdev, President and Chief Executive Officer of Sigma-Aldrich, said, “We are excited to join forces with Merck KGaA, Darmstadt, Germany, a distinguished industry leader. The combined company will be well-positioned to deliver significant customer benefits, including a broader, complementary range of products and capabilities, greater investment in breakthrough innovations, enhanced customer service, and a leading e-commerce and distribution platform in the industry. This transaction is a clear validation of our success in transforming Sigma-Aldrich into a customer-focused and solutions-oriented global organization. This is a testament to the strength of the Sigma-Aldrich brand and the accomplishments of our 9,000 employees worldwide. We believe this is a very positive outcome for our shareholders, who will receive a significant premium, and our employees, who will benefit from enhanced opportunities as part of a larger, more global organization.”
The combined company will be able to serve life science customers around the world with a highly attractive set of established brands and an efficient supply chain that can support the delivery of more than 300,000 products. In the Laboratory & Academia business, together EMD
Page 2 of 7

News Release
Millipore and Sigma-Aldrich will offer their customers a complementary range of products across laboratory chemicals, biologics and reagents. In pharma and biopharma production, Sigma-Aldrich will complement EMD Millipore’s existing products and capabilities with additions along the entire value chain of drug production and validation.
Shaped over almost 350 years by a family of owners, Merck today is known as a successful and values-driven business and a well-respected employer in the life science industry. With a common focus on employees, customers and communities, the combination represents a strong operational and cultural fit. Merck KGaA, Darmstadt, Germany, has great respect for Sigma-Aldrich’s rich history, customer-centric culture and commitment to corporate social responsibility and believes that the combination will afford new opportunities to employees at both companies. Merck KGaA, Darmstadt, Germany, plans to maintain a significant presence in St. Louis, and in Billerica, following completion of the transaction, as well as in important EMD Millipore sites such as Darmstadt and Molsheim, France.
Merck KGaA, Darmstadt, Germany, has successfully integrated a number of life science businesses in recent years, evaluating each company and combining the strongest operations, most efficient processes and most innovative programs that best support the future growth of the combined company. Merck KGaA, Darmstadt, Germany, intends to apply the same principles to the acquisition of Sigma-Aldrich in order to ensure a seamless integration. An integration team, which will include representatives from both companies, will be established to oversee and facilitate the integration process.
The combined life science business will have solid growth potential, strong and sustainable cash flow, and meaningful efficiency potential on an operational level. Based on fiscal year 2013 financials, the business would have had combined sales of €4.7 billion ($6.1 billion), an increase of 79% and combined EBITDA pre (earnings before interest, taxes, depreciation and amortization before one-time items) of €1.5 billion ($2.0 billion), which is an increase of 139%. Merck KGaA, Darmstadt, Germany, Group’s sales would have increased by approximately 19%. For the same period, the acquisition would have increased Merck KGaA, Darmstadt, Germany, Group’s EBITDA pre by approximately 24% and improved Group EBITDA pre margin from approximately 30% to approximately 33% including expected synergies.
Page 3 of 7

News Release
The transaction has been unanimously approved by Sigma-Aldrich’s Board of Directors. A merger agreement will be presented to Sigma-Aldrich shareholders for approval at a special meeting of shareholders.
The transaction has the full support of Merck KGaA, Darmstadt, Germany’s Executive Board and E. Merck KG including its Board of Partners, and a Merck KGaA, Darmstadt, Germany, shareholder vote will not be required. Bridge financing has been secured for the all-cash transaction, and Merck KGaA, Darmstadt, Germany, expects the final financing structure will comprise a combination of cash on Merck KGaA, Darmstadt, Germany’s balance sheet, bank loans and bonds. Closing is expected in mid-year 2015, subject to regulatory approvals and other customary closing conditions.
Guggenheim Securities and J.P. Morgan are acting as financial advisers to Merck KGaA, Darmstadt, Germany. Skadden, Arps, Slate, Meagher & Flom LLP is acting as legal adviser to Merck KGaA, Darmstadt, Germany. Morgan Stanley & Co. LLC is acting as financial adviser to Sigma-Aldrich and Sidley Austin LLP is acting as legal adviser.
Page 4 of 7

News Release
Notes to editors
| • | Documents regarding the transaction are accessiblehere. |
| • | For the media, aconference call will be held with the Merck KGaA, Darmstadt, Germany management |
at 8:00 AM EDT / 2:00 PM CET
| +1 (888)424-8151 | Audience US Toll Free | |
| +1 (847)585-4422 | Audience US Toll | |
| 0800 222 2013 | Audience Germany Freephone | |
| 069 222 215 20 | Audience Germany-Frankfurt Local | |
| Audience Passcode | 9203 983 |
| • | For analysts and investors, aconference call with the Merck KGaA, Darmstadt, Germany management will be held 9:00 AM EDT / 3:00 PM CET |
| +1 (888) 424-8151 | Audience US Toll Free | |
| +1 (847)585-4422 | Audience US Toll | |
| 0800 222 2013 | Audience Germany Freephone | |
| 069 222 215 20 | Audience Germany-Frankfurt Local | |
| Audience Passcode | 6411 816 |
| • | Merck onFacebook,Twitter,Linkedin |
| • | Merck KGaA, Darmstadt, Germany photos and video footage can be foundhere |
| • | Merck KGaA, Darmstadt, Germany stock symbols |
Reuters: MRCG,Bloomberg: MRK GY,Dow Jones: MRK.DE
Frankfurt Stock Exchange:ISIN: DE 000 659 9905 – WKN: 659 990
| • | Sigma-Aldrich stock symbols |
Reuters: SIAL.O, Bloomberg: SIAL:US
Contacts Sigma-Aldrich
Karen Miller
Senior Vice President, Corporate Development & Corporate Communications
Karen.Miller@sial.com
+1-314-286-7996
Quintin Lai
Vice President - Investor Relations and Strategy
investorrelations@sial.com
+1-314-898-4643
Page 5 of 7

News Release
All Merck KGaA, Darmstadt, Germany, press releases are distributed by e-mail at the same time they become available on the EMD Group Website. In case you are a resident of the USA or Canada please go towww.emdgroup.com/subscribe to register again for your online subscription of this service as our newly introduced geo-targeting requires new links in the email. You may later change your selection or discontinue this service.
About Merck KGaA, Darmstadt, Germany
Merck KGaA of Darmstadt, Germany, is a leading company for innovative and top-quality high-tech products in the pharmaceutical and chemical sectors. Its subsidiaries in Canada and the United States operate under the umbrella brand EMD. Around 39,000 employees work in 66 countries to improve the quality of life for patients, to further the success of customers and to help meet global challenges. The company generated total revenues of € 11.1 billion in 2013 with its four divisions: Biopharmaceuticals, Consumer Health, Performance Materials and Life Science Tools. Merck KGaA of Darmstadt, Germany is the world’s oldest pharmaceutical and chemical company – since 1668, the name has stood for innovation, business success and responsible entrepreneurship. Holding an approximately 70 percent interest, the founding family remains the majority owner of the company to this day.
About Sigma-Aldrich
Sigma-Aldrich, a leading Life Science and Technology company focused on enhancing human health and safety, manufactures and distributes more than 230,000 chemicals, biochemicals and other essential products to more than 1.4 million customers globally in research and applied labs as well as in industrial and commercial markets. With three distinct business units - Research, Applied and SAFC Commercial - Sigma-Aldrich is committed to enabling science to improve the quality of life. The Company operates in 37 countries, has approximately 9,000 employees worldwide and had sales of $2.7 billion in 2013. For more information about Sigma-Aldrich, please visit its website at www.Sigma-Aldrich.com.
Cautionary Note Regarding Forward-Looking Statements
This communication may include “forward-looking statements.” Statements that include words such as “anticipate,” “expect,” “should,” “would,” “intend,” “plan,” “project,” “seek,” “believe,” “will,” and other words of similar meaning in connection with future events or future operating or financial performance are often used to identify forward-looking statements. All statements in this communication, other than those relating to historical information or current conditions, are forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond control of Merck KGaA, Darmstadt, Germany, which could cause actual results to differ materially from such statements.
Risks and uncertainties relating to the proposed transaction with Sigma-Aldrich Corporation (“Sigma-Aldrich”) include, but are not limited to: the risk Sigma-Aldrich’s shareholders do not approve the transaction; uncertainties as to the timing of the transaction; the risk that regulatory or other approvals required for the transaction are not obtained or are obtained subject to conditions that are not anticipated; competitive responses to the transaction; litigation relating to the transaction; uncertainty of the expected financial performance of the combined company following completion of the proposed transaction; the ability of Merck KGaA, Darmstadt, Germany, to achieve the cost-savings and synergies contemplated by the proposed transaction within the expected time frame; the ability of Merck KGaA, Darmstadt, Germany, to promptly and effectively integrate the businesses of Sigma-Aldrich and Merck KGaA, Darmstadt, Germany; the effects of the business combination of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich, including the combined company’s future financial condition, operating results, strategy and plans; the implications of the proposed transaction on certain employee benefit plans of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich; and disruption from the proposed transaction making it more difficult to maintain relationships with customers, employees or suppliers.
Additional risks and uncertainties include, but are not limited to: the risks of more restrictive regulatory requirements regarding drug pricing, reimbursement and approval; the risk of stricter regulations for the manufacture, testing and
Page 6 of 7

News Release
marketing of products; the risk of destabilization of political systems and the establishment of trade barriers; the risk of a changing marketing environment for multiple sclerosis products in the European Union; the risk of greater competitive pressure due to biosimilars; the risks of research and development; the risks of discontinuing development projects and regulatory approval of developed medicines; the risk of a temporary ban on products/production facilities or of non-registration of products due to non-compliance with quality standards; the risk of an import ban on products to the United States due to an FDA warning letter; the risks of dependency on suppliers; risks due to product-related crime and espionage; risks in relation to the use of financial instruments; liquidity risks; counterparty risks; market risks; risks of impairment on balance sheet items; risks from pension obligations; risks from product-related and patent law disputes; risks from antitrust law proceedings; risks from drug pricing by the divested Generics Group; risks in human resources; risks from e-crime and cyber attacks; risks due to failure of business-critical information technology applications or to failure of data center capacity; environmental and safety risks; unanticipated contract or regulatory issues; a potential downgrade in the rating of the indebtedness of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich; downward pressure on the common stock price of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich and its impact on goodwill impairment evaluations; the impact of future regulatory or legislative actions; and the risks and uncertainties detailed by Sigma-Aldrich with respect to its business as described in its reports and documents filed with the U.S. Securities and Exchange Commission (the “SEC”).
The foregoing review of important factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere, including the Report on Risks and Opportunities Section of the most recent annual report and quarterly report of Merck KGaA, Darmstadt, Germany, and the Risk Factors section of Sigma-Aldrich’s most recent reports on Form 10-K and Form 10-Q. Any forward-looking statements made in this communication are qualified in their entirety by these cautionary statements, and there can be no assurance that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, us or our business or operations. Except to the extent required by applicable law, we undertake no obligation to update publicly or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Important Additional Information
This communication is being made in respect of the proposed merger transaction involving Sigma-Aldrich and Merck KGaA, Darmstadt, Germany. The proposed merger will be submitted to the stockholders of Sigma-Aldrich for their consideration. In connection therewith, Sigma-Aldrich intends to file relevant materials with the SEC, including a preliminary proxy statement and a definitive proxy statement. The definitive proxy statement will be mailed to the stockholders of Sigma-Aldrich. BEFORE MAKING ANY VOTING OR ANY INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION AND ANY OTHER RELEVANT DOCUMENTS FILED OR TO BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Investors and security holders may obtain free copies of the proxy statement, any amendments or supplements thereto and other documents containing important information about Sigma-Aldrich, once such documents are filed with the SEC, through the website maintained by the SEC at www.sec.gov. Copies of the documents filed with the SEC by Sigma-Aldrich will be available free of charge on Sigma-Aldrich’s website at www.sigmaaldrich.com under the heading “SEC Documents” within the “Investor Info” section in the “Investors” portion of Sigma-Aldrich’s website. Shareholders of Sigma-Aldrich may also obtain a free copy of the definitive proxy statement by contacting Sigma-Aldrich’s Investor Relations Department at (314) 898-4643.
Sigma-Aldrich and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Sigma-Aldrich is set forth in its proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 21, 2014, its annual report on Form 10-K for the fiscal year ended December 31, 2013, which was filed with the SEC on February 6, 2014, and in subsequent documents filed with the SEC, each of which can be obtained free of charge from the sources indicated above. Other information regarding the participants in the proxy solicitation of the stockholders of Sigma-Aldrich and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the preliminary and definitive proxy statements and other relevant materials to be filed with the SEC when they become available.
Page 7 of 7

Acquisition of Sigma-Aldrich
Taking Merck’s life science business to the next level
Karl-Ludwig Kley, CEO
Marcus Kuhnert, CFO
M
September 22, 2014

M
Disclaimer Publication of Merck KGaA, Darmstadt, Germany. In the United States and Canada the subsidiaries of Merck KGaA, Darmstadt, Germany operate under the umbrella brand EMD.

M
Merck KGaA
Darmstadt · Germany
Disclaimer
Cautionary Note Regarding Forward-Looking Statements
This communication may include “forward-looking statements.” Statements that include words such as “anticipate,” “expect,” “should,” “would,” “intend,” “plan,” “project,” “seek,” “believe,” “will,” and other words of similar meaning in connection with future events or future operating or financial performance are often used to identify forward-looking statements. All statements in this communication, other than those relating to historical information or current conditions, are forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond control of Merck KGaA, Darmstadt, Germany, which could cause actual results to differ materially from such statements.
Risks and uncertainties relating to the proposed transaction with Sigma-Aldrich Corporation (“Sigma-Aldrich”) include, but are not limited to: the risk Sigma-Aldrich’s shareholders do not approve the transaction; uncertainties as to the timing of the transaction; the risk that regulatory or other approvals required for the transaction are not obtained or are obtained subject to conditions that are not anticipated; competitive responses to the transaction; litigation relating to the transaction; uncertainty of the expected financial performance of the combined company following completion of the proposed transaction; the ability of Merck KGaA, Darmstadt, Germany, to achieve the cost-savings and synergies contemplated by the proposed transaction within the expected time frame; the ability of Merck KGaA, Darmstadt, Germany, to promptly and effectively integrate the businesses of Sigma-Aldrich and Merck KGaA, Darmstadt, Germany; the effects of the business combination of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich, including the combined company’s future financial condition, operating results, strategy and plans; the implications of the proposed transaction on certain employee benefit plans of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich; and disruption from the proposed transaction making it more difficult to maintain relationships with customers, employees or suppliers.
Additional risks and uncertainties include, but are not limited to: the risks of more restrictive regulatory requirements regarding drug pricing, reimbursement and approval; the risk of stricter regulations for the manufacture, testing and marketing of products; the risk of destabilization of political systems and the establishment of trade barriers; the risk of a changing marketing environment for multiple sclerosis products in the European Union; the risk of greater competitive pressure due to biosimilars; the risks of research and development; the risks of discontinuing development projects and regulatory approval of developed medicines; the risk of a temporary ban on products/production facilities or of non-registration of products due to non-compliance with quality standards; the risk of an import ban on products to the United States due to an FDA warning letter; the risks of dependency on suppliers; risks due to product-related crime and espionage; risks in relation to the use of financial instruments; liquidity risks; counterparty risks; market risks; risks of impairment on balance sheet items; risks from pension obligations; risks from product-related and patent law disputes; risks from antitrust law proceedings; risks from drug pricing by the divested Generics Group; risks in human resources; risks from e-crime and cyber attacks; risks due to failure of business-critical information technology applications or to failure of data center capacity; environmental and safety risks; unanticipated contract or regulatory issues; a potential downgrade in the rating of the indebtedness of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich; downward pressure on the common stock price of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich and its impact on goodwill impairment evaluations; the impact of future regulatory or legislative actions; and the risks and uncertainties detailed by Sigma-Aldrich with respect to its business as described in its reports and documents filed with the U.S. Securities and Exchange Commission (the “SEC”).
The foregoing review of important factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere, including the Report on Risks and Opportunities Section of the most recent annual report and quarterly report of Merck KGaA, Darmstadt, Germany, and the Risk Factors section of Sigma-Aldrich’s most recent reports on Form 10-K and Form 10-Q. Any forward-looking statements made in this communication are qualified in their entirety by these cautionary statements, and there can be no assurance that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, us or our business or operations. Except to the extent required by applicable law, we undertake no obligation to update publicly or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Important Additional Information
This communication is being made in respect of the proposed merger transaction involving Sigma-Aldrich and Merck KGaA, Darmstadt, Germany. The proposed merger will be submitted to the stockholders of Sigma-Aldrich for their consideration. In connection therewith, Sigma-Aldrich intends to file relevant materials with the SEC, including a preliminary proxy statement and a definitive proxy statement. The definitive proxy statement will be mailed to the stockholders of Sigma-Aldrich. BEFORE MAKING ANY VOTING OR ANY INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION AND ANY OTHER RELEVANT DOCUMENTS FILED OR TO BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Investors and security holders may obtain free copies of the proxy statement, any amendments or supplements thereto and other documents containing important information about Sigma-Aldrich, once such documents are filed with the SEC, through the website maintained by the SEC at www.sec.gov. Copies of the documents filed with the SEC by Sigma-Aldrich will be available free of charge on Sigma-Aldrich’s website at www.sigmaaldrich.com under the heading “SEC Documents” within the “Investor Info” section in the “Investors” portion of Sigma-Aldrich’s website. Shareholders of Sigma-Aldrich may also obtain a free copy of the definitive proxy statement by contacting Sigma-Aldrich’s Investor Relations Department at +1 (314) 898 4643.
Sigma-Aldrich and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Sigma-Aldrich is set forth in its proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 21, 2014, its annual report on Form 10-K for the fiscal year ended December 31, 2013, which was filed with the SEC on February 6, 2014, and in subsequent documents filed with the SEC, each of which can be obtained free of charge from the sources indicated above. Other information regarding the participants in the proxy solicitation of the stockholders of Sigma-Aldrich and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the preliminary and definitive proxy statements and other relevant materials to be filed with the SEC when they become available.
3

M
Merck KGaA
Darmstadt · Germany
Agenda
Strategic rationale and transaction overview
Introduction of Sigma-Aldrich and the combined business
Financial impacts
Summary
M
Merck KGaA
Darmstadt · Germany
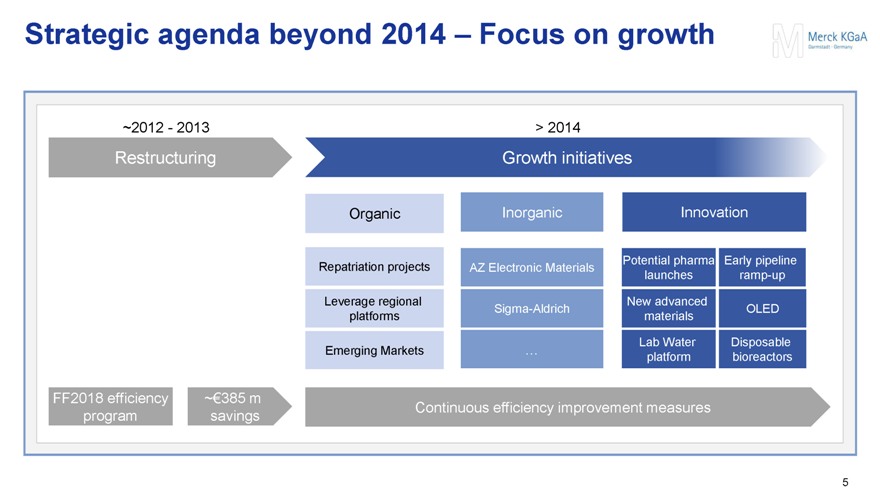
Strategic agenda beyond 2014 – Focus on growth
~2012 - 2013 > 2014
Restructuring Growth initiatives
Organic Inorganic Innovation
Repatriation projects AZ Electronic Materials Potential pharma launches Early pipeline ramp-up
Leverage regional platforms Sigma-Aldrich New advanced materials OLED
Emerging Markets … Lab Water platform Disposable bioreactors
FF2018 efficiency program ~€385 m savings Continuous efficiency improvement measures
5

M
Merck KGaA
Darmstadt · Germany
Sigma-Aldrich – Next step to enhance
Merck Millipore, a leading life sciences company
Attractive life science industry
Attractive industry driven by sustainable underlying market trends
Stable growth pattern, offering additional growth opportunities
Strong companies with healthy margins
Taking Merck’s life science business to the next level
Strong track record of delivering profitable growth
Adding scale with step change acquisition
6

M
Merck KGaA
Darmstadt · Germany
A compelling transaction rationale
Increasing scale – expanding position in attractive life science industry
Enhancing value for our customers
Strategic and operational fit
Broadens product range and ease of doing business for Laboratories & Academia
Complements Process Solutions product offering
Closing the gap in U.S. – adequate presence in all geographies
Leveraging existing platforms for global innovation rollout
Financial fit
Further diversification of revenue stream
Substantial synergy potential
Immediately accretive to EPS pre* and EBITDA margin
Solid investment grade rating will be maintained
*EPS pre one-time items and amortization, especially from purchase price allocation (PPA)
7

M
Merck KGaA
Darmstadt · Germany
Transaction overview
PRICE
US$ 140 per share, all-cash offer
PREMIUM
Premium of 37% to unaffected share price; 36% to one-month volume-weighted average price (VWAP)
CERTAINTY
Recommended by Sigma-Aldrich Board of Directors; No Merck shareholder vote required
FINANCING
Bridge financing secured; Final structure: cash, bank loans and bonds; Strong combined cash flows for rapid deleveraging; solid investment grade rating will be maintained
TIMING
Subject to regulatory approvals; expected closing mid-year 2015
*Closing share price as of September 19, 2014
8
M
Merck KGaA
Darmstadt · Germany
Agenda
Strategic rationale and transaction overview
Introduction of Sigma-Aldrich and the combined business
Financial impacts
Summary
M
Merck KGaA
Darmstadt · Germany
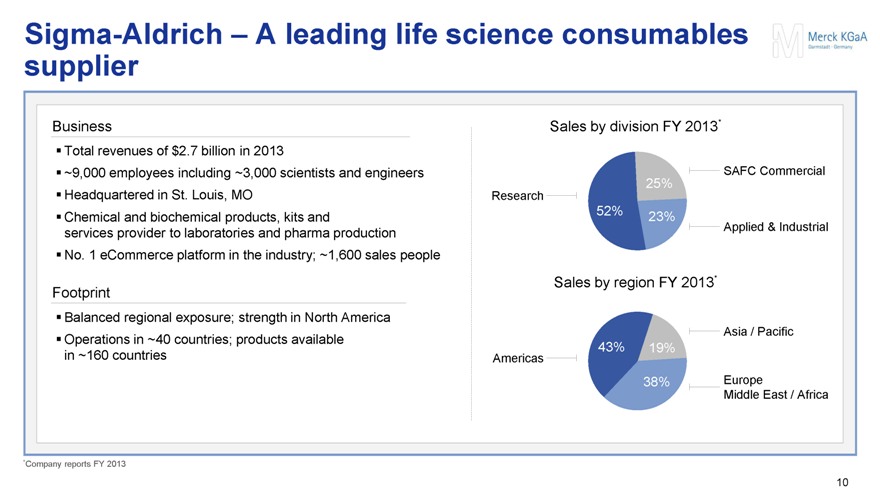
Sigma-Aldrich – A leading life science consumables supplier
Business
Total revenues of $2.7 billion in 2013
~9,000 employees including ~3,000 scientists and engineers
Headquartered in St. Louis, MO
Chemical and biochemical products, kits and services provider to laboratories and pharma production
No. 1 eCommerce platform in the industry; ~1,600 sales people
Footprint
Balanced regional exposure; strength in North America
Operations in ~40 countries; products available in ~160 countries
Sales by division FY 2013*
SAFC Commercial
25%
Research
52% 23%
Applied & Industrial
Sales by region FY 2013*
Asia / Pacific
Americas 43% 19%
38% Europe
Middle East / Africa
*Company reports FY 2013
10
M
Merck KGaA
Darmstadt · Germany
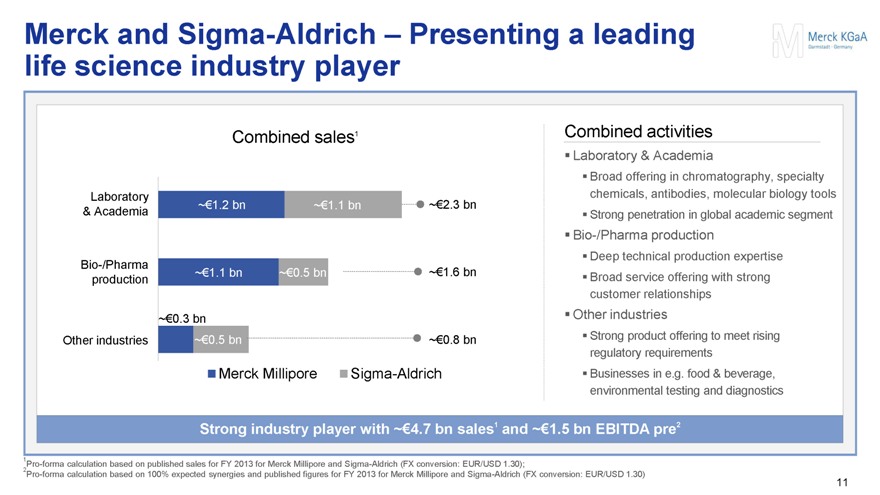
Merck and Sigma-Aldrich – Presenting a leading life science industry player
Combined sales1
Laboratory & Academia ~€1.2 bn ~€1.1 bn ~€2.3 bn
Bio-/Pharma production ~€1.1 bn ~€0.5 bn ~€1.6 bn
~€0.3 bn Other industries ~€0.5 bn ~€0.8 bn
Merck Millipore Sigma-Aldrich
Combined activities
Laboratory & Academia
Broad offering in chromatography, specialty chemicals, antibodies, molecular biology tools
Strong penetration in global academic segment
Bio-/Pharma production
Deep technical production expertise
Broad service offering with strong customer relationships
Other industries
Strong product offering to meet rising regulatory requirements
Businesses in e.g. food & beverage, environmental testing and diagnostics
Strong industry player with ~€4.7 bn sales1 and ~€1.5 bn EBITDA pre2
1Pro-forma calculation based on published sales for FY 2013 for Merck Millipore and Sigma-Aldrich (FX conversion: EUR/USD 1.30);
2Pro-forma calculation based on 100% expected synergies and published figures for FY 2013 for Merck Millipore and Sigma-Aldrich (FX conversion: EUR/USD 1.30)
11
M
Merck KGaA
Darmstadt · Germany
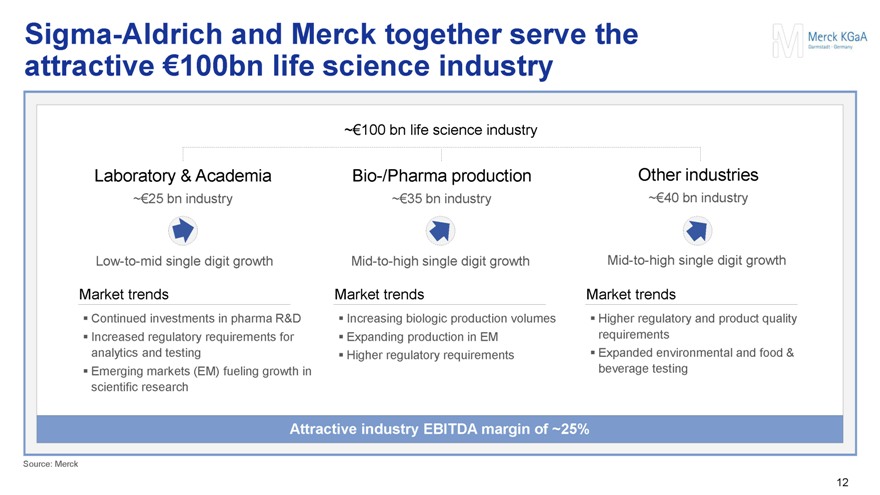
Sigma-Aldrich and Merck together serve the attractive €100bn life science industry
Laboratory & Academia
~€25 bn industry
Low-to-mid single digit growth
Market trends
Continued investments in pharma R&D
Increased regulatory requirements for analytics and testing
Emerging markets (EM) fueling growth in scientific research
~€100 bn life science industry
Bio-/Pharma production
~€35 bn industry
Mid-to-high single digit growth
Market trends
Increasing biologic production volumes
Expanding production in EM
Higher regulatory requirements
Other industries
~€40 bn industry
Mid-to-high single digit growth
Market trends
Higher regulatory and product quality requirements
Expanded environmental and food & beverage testing
Attractive industry EBITDA margin of ~25%
Source: Merck
12
M
Merck KGaA
Darmstadt · Germany
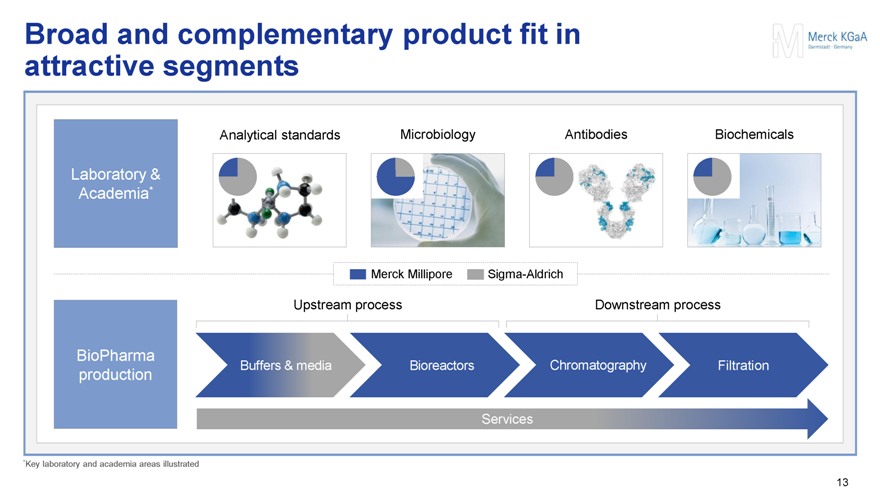
Broad and complementary product fit in attractive segments
Laboratory & Academia*
Analytical standards Microbiology Antibodies Biochemicals
Merck Millipore Sigma-Aldrich
BioPharma production
Upstream process Downstream process
Buffers & media Bioreactors Chromatography Filtration
Services
*Key laboratory and academia areas illustrated
13
M
Merck KGaA
Darmstadt · Germany
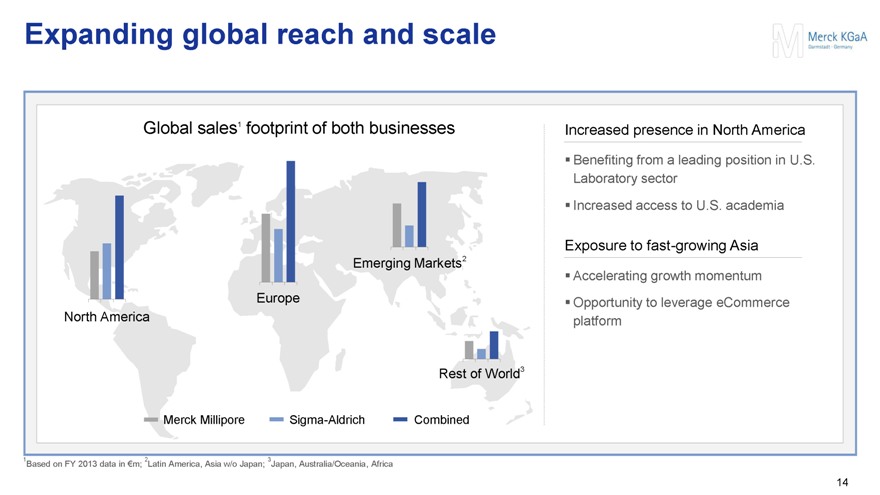
Expanding global reach and scale
Global sales1 footprint of both businesses
Emerging Markets2
Europe
North America
Rest of World3
Merck Millipore
Sigma-Aldrich
Combined
Increased presence in North America
Benefiting from a leading position in U.S. Laboratory sector
Increased access to U.S. academia
Exposure to fast-growing Asia
Accelerating growth momentum
Opportunity to leverage eCommerce platform
1Based on FY 2013 data in €m; 2Latin America, Asia w/o Japan; 3Japan, Australia/Oceania, Africa
14

M
Merck KGaA
Darmstadt · Germany
Leveraging operational excellence to deliver superior value to customers
Delivering innovative workflow solutions to increase customers’ efficiency
Product innovation
Broad technology and platforms
Recurring winners of renowned innovation awards
Mobius FlexReady
Duolink
Amnis
Efficient supply chain for >300,000 products
Process innovation
Best in class customer experience; e.g. 24 hour delivery in major markets
Top-notch customer interface supported by eCommerce platform
eCommerce platform
Supply chain
Efficient work flow solutions and unique customer experience
15

M
Merck KGaA
Darmstadt · Germany
Agenda
Strategic rationale and transaction overview
Introduction of Sigma-Aldrich and the combined business
Financial impacts
Summary
M
Merck KGaA
Darmstadt · Germany
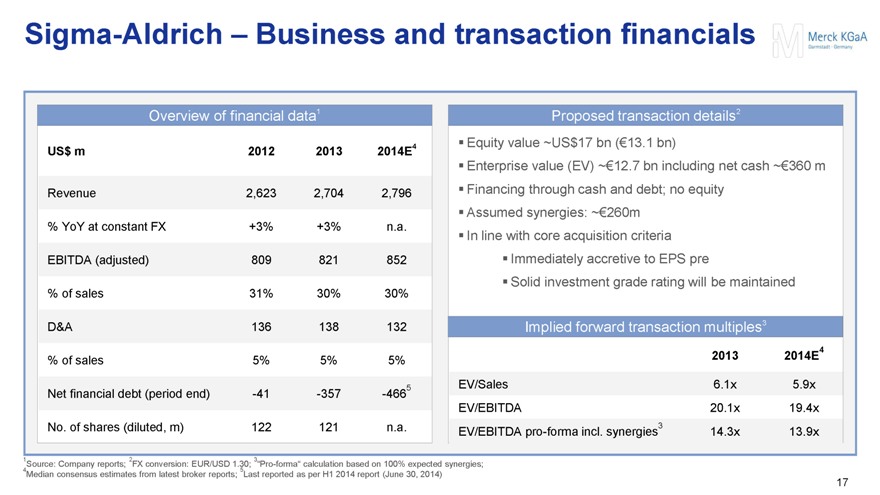
Sigma-Aldrich – Business and transaction financials
Overview of financial data1
US$ m 2012 2013 2014E4
Revenue 2,623 2,704 2,796
% YoY at constant FX +3% +3% n.a.
EBITDA (adjusted) 809 821 852
% of sales 31% 30% 30%
D&A 136 138 132
% of sales 5% 5% 5%
Net financial debt (period end) -41 -357 -4665
No. of shares (diluted, m) 122 121 n.a.
Proposed transaction details2
Equity value ~US$17 bn (€13.1 bn)
Enterprise value (EV) ~€12.7 bn including net cash ~€360 m
Financing through cash and debt; no equity
Assumed synergies: ~€260m
In line with core acquisition criteria
Immediately accretive to EPS pre
Solid investment grade rating will be maintained
Implied forward transaction multiples3
2013 2014E4
EV/Sales 6.1x 5.9x
EV/EBITDA 20.1x 19.4x
EV/EBITDA pro-forma incl. synergies3 14.3x 13.9x
1Source: Company reports; 2FX conversion: EUR/USD 1.30; 3“Pro-forma” calculation based on 100% expected synergies;
4Median consensus estimates from latest broker reports; 5Last reported as per H1 2014 report (June 30, 2014)
17
M
Merck KGaA
Darmstadt · Germany
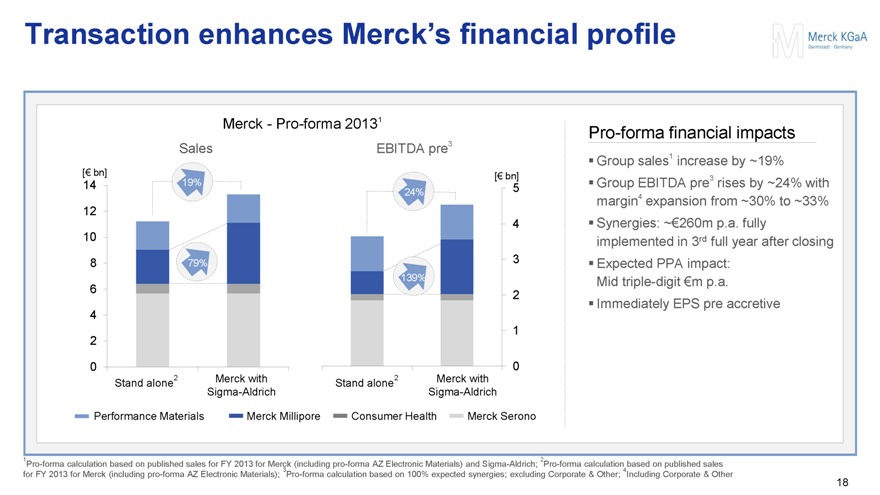
Transaction enhances Merck’s financial profile
Merck - Pro-forma 20131
Sales EBITDA pre3
[€ bn] [€ bn]
14 19%
24% 5 12 4 10 8 79% 3 139% 6 2 4 1 2 0 0
Stand alone2
Merck with Sigma-Aldrich
Stand alone2
Merck with Sigma-Aldrich
Performance Materials
Merck Millipore
Consumer Health
Merck Serono
Pro-forma financial impacts
Group sales1 increase by ~19%
Group EBITDA pre3 rises by ~24% with margin4 expansion from ~30% to ~33%
Synergies: ~€260m p.a. fully implemented in 3rd full year after closing
Expected PPA impact: Mid triple-digit €m p.a.
Immediately EPS pre accretive
1Pro-forma calculation based on published sales for FY 2013 for Merck (including pro-forma AZ Electronic Materials) and Sigma-Aldrich; 2Pro-forma calculation based on published sales for FY 2013 for Merck (including pro-forma AZ Electronic Materials); 3Pro-forma calculation based on 100% expected synergies; excluding Corporate & Other; 4Including Corporate & Other
18
M
Merck KGaA
Darmstadt · Germany
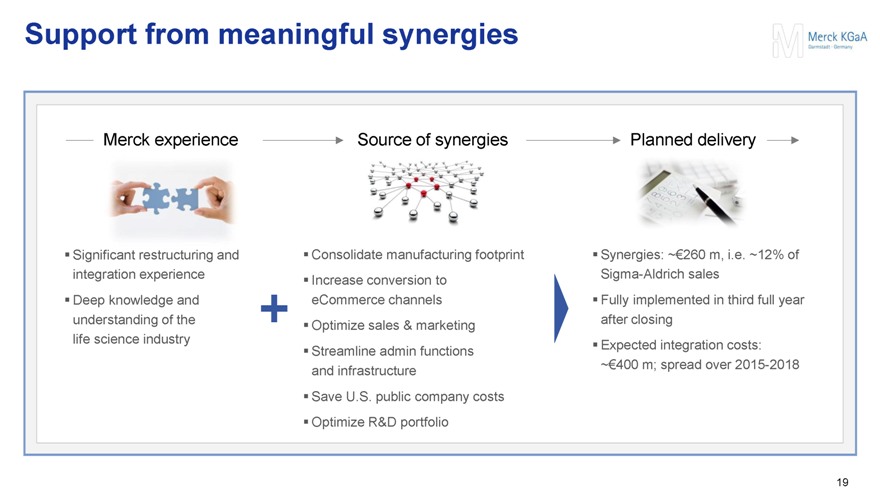
Support from meaningful synergies
Merck experience
Significant restructuring and integration experience
Deep knowledge and understanding of the life science industry
Source of synergies
Consolidate manufacturing footprint
Increase conversion to eCommerce channels
Optimize sales & marketing
Streamline admin functions and infrastructure
Save U.S. public company costs
Optimize R&D portfolio
Planned delivery
Synergies: ~€260 m, i.e. ~12% of Sigma-Aldrich sales
Fully implemented in third full year after closing
Expected integration costs:
~€400 m; spread over 2015-2018
19
M
Merck KGaA
Darmstadt · Germany
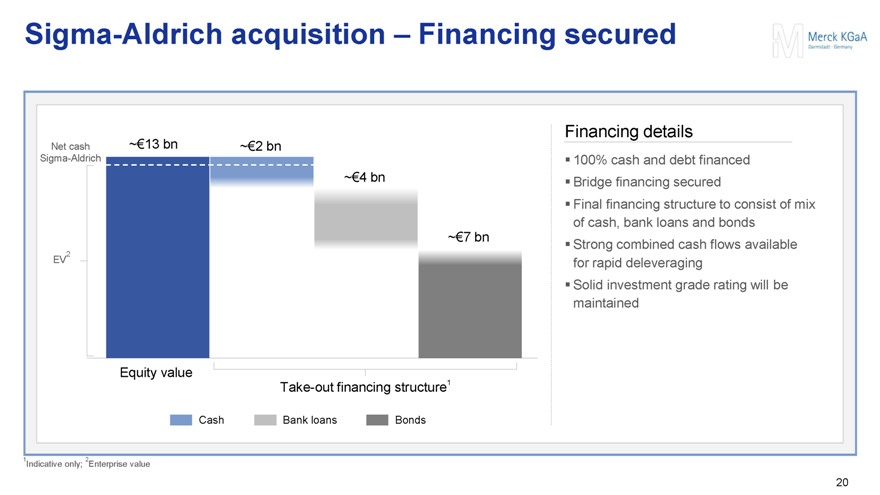
Sigma-Aldrich acquisition – Financing secured
Net cash ~€13 bn ~€2 bn
Sigma-Aldrich
~€4 bn
~€7 bn
EV2
Equity value
Take-out financing structure1
Cash
Bank loans
Bonds
Financing details
100% cash and debt financed
Bridge financing secured
Final financing structure to consist of mix of cash, bank loans and bonds
Strong combined cash flows available for rapid deleveraging
Solid investment grade rating will be maintained
1Indicative only; 2Enterprise value
20

M
Merck KGaA
Darmstadt · Germany
Agenda
Strategic rationale and transaction overview
Introduction of Sigma-Aldrich and the combined business
Financial impacts
Summary

M
Merck KGaA
Darmstadt · Germany
Sigma-Aldrich acquisition – Taking Merck Millipore to the next level
Expanding position in the attractive life science industry, poised for sustainable growth
Enhancing value for customers via strengthened offering, reach, and operational excellence
Sigma-Aldrich and Merck Millipore will be core earnings contributor to Merck and generate sustainable and growing cash flow
Consistent with Merck’s core acquisition criteria and corporate transformation journey
22

M
Merck KGaA
Darmstadt · Germany
M
Merck KGaA
Darmstadt · Germany
Appendix
M
Merck KGaA
Darmstadt · Germany
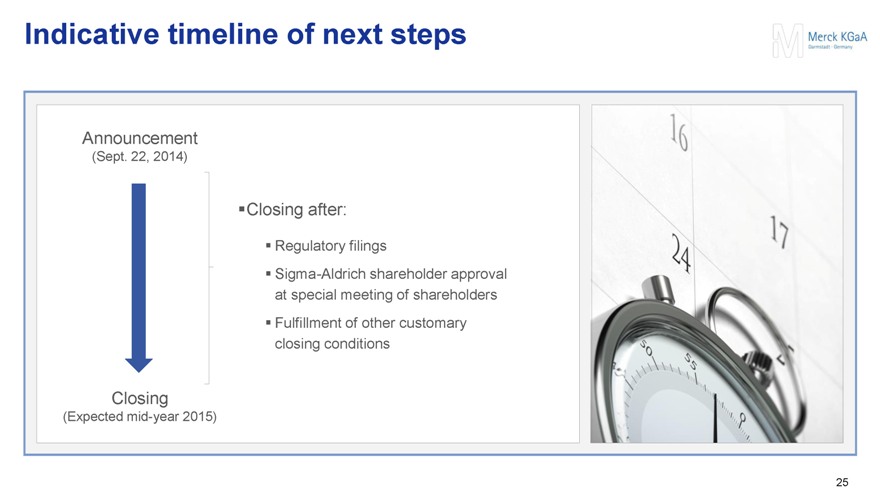
Indicative timeline of next steps
Announcement
(Sept. 22, 2014)
Closing after:
Regulatory filings
Sigma-Aldrich shareholder approval at special meeting of shareholders
Fulfillment of other customary closing conditions
Closing
(Expected mid-year 2015)
25
M
Merck KGaA
Darmstadt · Germany
Investor Relations contact details
Constantin Fest
Head of Investor Relations
+49 6151 72-5271
constantin.fest@merckgroup.com
Alessandra Heinz
Assistant Investor Relations
+49 6151 72-3321
alessandra.heinz@merckgroup.com
Svenja Bundschuh
Assistant Investor Relations
+49 6151 72-3744
svenja.bundschuh@merckgroup.com
Eva Sterzel
AGM, Capital Market Events, IR-Media
+49 6151 72-5355
eva.sterzel@merckgroup.com
Annett Weber
Institutional Investors / Analysts
+49 6151 72-63723
annett.weber@merckgroup.com
Julia Schwientek
Institutional Investors / Analysts
+49 6151 72-7434
julia.schwientek@merckgroup.com
Olliver Lettau
Analysts, Fixed Income, Private Investors
+49 6151 72-34409 olliver.lettau@merckgroup.com
Email: investor.relations@merckgroup.com Web: www.investors.merck.de Fax: +49 6151 72-913321
26

M
Merck KGaA
Darmstadt · Germany
Media Relations contact details
Dr. Walter Huber
Head of Group Communications
+49 6151 72-2287
walter.huber@merckgroup.com
Nicole Mommsen
Head of Media Relations
+49 6151 72-62445
nicole.mommsen@merckgroup.com
Silke Klotz
Assistant Media Relations
+49 6151 72-4342
silke.klotz@merckgroup.com
Markus Talanow
Financial Communications,
Chemicals
+49 6151 72-7144
markus.talanow@merckgroup.com
Dr. Gangolf Schrimpf
Pharma
+49 6151 72-9591
gangolf.schrimpf@merckgroup.com
Gerhard Lerch
HR, IT, Corporate Responsibility
+49 6151 72-6328
gerhard.lerch@merckgroup.com
Email: media.relations@merckgroup.com Web: www.media.merck.de Fax: +49 6151 72-5000
27
Merck KGaA: Merck to Acquire Sigma-Aldrich to Enhance Position in Attractive Life Science Industry
Merck KGaA / Key word(s): Offer
22.09.2014 11:56
Dissemination of an Ad hoc announcement according to § 15 WpHG, transmitted by DGAP - a service of EQS Group AG.
The issuer is solely responsible for the content of this announcement.
Merck, a leading company for innovative and top-quality high-tech products in the pharmaceutical, chemical and life science sectors, and Sigma-Aldrich Corporation (“Sigma-Aldrich”), today have entered into a definitive agreement under which Merck will acquire Sigma-Aldrich for $17.0 billion (EUR13.1 billion), establishing one of the leading players in the $130 billion global life science industry.
Merck will acquire all of the outstanding shares of Sigma-Aldrich for $140 per share in cash. The agreed price represents a 37% premium to the latest closing price of $102.37 on September 19, 2014, and a 36% premium to the one-month average closing price. The transaction is expected to be immediately accretive to Merck’s EPS pre and EBITDA margin. Merck expects to achieve annual synergies of approximately EUR260 million (approximately $340 million), which should be fully realized within three years after closing.
Bridge financing has been secured for the all-cash transaction, and Merck expects the final financing structure will comprise a combination of cash on Merck’s balance sheet, bank loans and bonds. Closing is expected mid-year 2015, subject to regulatory approvals, approval by a special meeting of the shareholders of Sigma-Aldrich and other customary closing conditions.
Information and Explanation of the Issuer to this News:
Cautionary Note Regarding Forward-Looking Statements
This communication may include “forward-looking statements.” Statements that include words such as “anticipate,” “expect,” “should,” “would,” “intend,” “plan,” “project,” “seek,” “believe,” “will,” and other words of similar meaning in connection with future events or future operating or financial performance are often used to identify forward-looking statements. All statements in this communication, other than those relating to historical information or current conditions, are forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond control of Merck KGaA, Darmstadt, Germany, which could cause actual results to differ materially from such statements.
Risks and uncertainties relating to the proposed transaction with Sigma-Aldrich Corporation (“Sigma-Aldrich”) include, but are not limited to: the risk Sigma-Aldrich’s shareholders do not approve the transaction; uncertainties as to the timing of the transaction; the risk that regulatory or other approvals required for the transaction are not obtained or are obtained subject to conditions that are not anticipated; competitive responses to the transaction; litigation relating to the transaction; uncertainty of the expected financial performance of the combined company following completion of the proposed transaction; the ability of Merck KGaA, Darmstadt, Germany, to achieve the cost-savings and synergies contemplated by the proposed transaction within the expected time frame; the ability of Merck KGaA, Darmstadt, Germany, to promptly and effectively integrate the businesses of Sigma-Aldrich and Merck KGaA, Darmstadt, Germany; the effects of the business combination of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich, including the combined company’s future financial condition, operating results, strategy and plans; the implications of the proposed transaction on certain employee benefit plans of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich; and disruption from the proposed transaction making it more difficult to maintain relationships with customers, employees or suppliers.
Additional risks and uncertainties include, but are not limited to: the risks of more restrictive regulatory requirements regarding drug pricing, reimbursement and approval; the risk of stricter regulations for the manufacture, testing and marketing of products; the risk of destabilization of political systems and the establishment of trade barriers; the risk of a changing marketing environment for multiple sclerosis products in the European Union; the risk of greater competitive pressure due to biosimilars; the risks of research and development; the risks of discontinuing development projects and regulatory approval of developed medicines; the risk of a temporary ban on products/production facilities or of non-registration of products due to non-compliance with quality standards; the risk of an import ban on products to the United States due to an FDA warning letter; the risks of dependency on suppliers; risks due to product-related crime and espionage; risks in relation to the use of financial instruments; liquidity risks; counterparty risks; market risks; risks of impairment on balance sheet items; risks from pension obligations; risks from product-related and patent law disputes; risks from antitrust law proceedings; risks from drug pricing by the divested Generics Group; risks in human resources; risks from e-crime and cyber attacks; risks due to failure of business-critical information technology applications or to failure of data center capacity; environmental and safety risks; unanticipated contract or regulatory issues; a potential downgrade in the rating of the indebtedness of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich; downward pressure on the common stock price of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich and its impact on goodwill impairment evaluations; the impact of future regulatory or legislative actions; and the risks and uncertainties detailed by Sigma-Aldrich with respect to its business as described in its reports and documents filed with the U.S. Securities and Exchange Commission (the “SEC”).
The foregoing review of important factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere, including the Report on Risks and Opportunities Section of the most recent annual report and quarterly report of Merck KGaA, Darmstadt, Germany, and the Risk Factors section of Sigma-Aldrich’s most recent reports on Form 10-K and Form 10-Q. Any forward-looking statements made in this communication are qualified in their entirety by these cautionary statements, and there can be no assurance that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, us or our business or operations. Except to the extent required by applicable law, we undertake no obligation to update publicly or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Important Additional Information
This communication is being made in respect of the proposed merger transaction involving Sigma-Aldrich and Merck KGaA, Darmstadt, Germany. The proposed merger will be submitted to the stockholders of Sigma-Aldrich for their consideration. In connection therewith, Sigma-Aldrich intends to file relevant materials with the SEC, including a preliminary proxy statement and a definitive proxy statement. The definitive proxy statement will be mailed to the stockholders of Sigma-Aldrich. BEFORE MAKING ANY VOTING OR ANY INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION AND ANY OTHER RELEVANT DOCUMENTS FILED OR TO BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Investors and security holders may obtain free copies of the proxy statement,
2
any amendments or supplements thereto and other documents containing important information about Sigma-Aldrich, once such documents are filed with the SEC, through the website maintained by the SEC at www.sec.gov. Copies of the documents filed with the SEC by Sigma-Aldrich will be available free of charge on Sigma-Aldrich’s website at www.sigmaaldrich.com under the heading “SEC Documents” within the “Investor Info” section in the “Investors” portion of Sigma-Aldrich’s website. Shareholders of Sigma-Aldrich may also obtain a free copy of the definitive proxy statement by contacting Sigma-Aldrich’s Investor Relations Department at (314) 898-4643.
Sigma-Aldrich and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Sigma-Aldrich is set forth in its proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 21, 2014, its annual report on Form 10-K for the fiscal year ended December 31, 2013, which was filed with the SEC on February 6, 2014, and in subsequent documents filed with the SEC, each of which can be obtained free of charge from the sources indicated above. Other information regarding the participants in the proxy solicitation of the stockholders of Sigma-Aldrich and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the preliminary and definitive proxy statements and other relevant materials to be filed with the SEC when they become available.
22.09.2014 The DGAP Distribution Services include Regulatory Announcements, Financial/Corporate News and Press Releases.
Media archive at www.dgap-medientreff.de and www.dgap.de
| Language: | English | |
| Company: | Merck KGaA | |
| Frankfurter Str. 250 | ||
| 64293 Darmstadt | ||
| Germany | ||
| Phone: | +49 (0) 6151 72 - 3321 | |
| Fax: | +49 (0) 6151 72 - 913321 | |
| E-mail: | investor.relations@merckgroup.com | |
| Internet: | www.merck.de | |
| ISIN: | DE0006599905 | |
| WKN: | 659990 | |
| Indices: | DAX | |
| Listed: | Regulierter Markt in Frankfurt (Prime Standard); Freiverkehr in Berlin, Düsseldorf, Hamburg, Hannover, München, Stuttgart; Terminbörse EUREX; London, SIX |
| End of Announcement | DGAP News-Service |
3
Factsheet
| Company |  |  | ||||
| Ticker | FWB: MRK | NASDAQ: SIAL | ||||
| Description | Merck KGaA of Darmstadt, Germany is a leading company for innovative and top-quality high-tech products in the pharmaceutical and chemical sectors. In Canada and the United States, Merck operates under the name EMD.
With its four divisions – Biopharmaceuticals, Consumer Health, Performance Materials, Life Science Tools – EMD generated total revenues of € 11.1 billion in 2013.
Around 39,000 EMD employees work in 66 countries to improve the quality of life for patients, to further the success of customers and to help meet global challenges.
EMD is the world’s oldest pharmaceutical and chemical company – since 1668, the company has stood for innovation, business success and responsible entrepreneurship. Holding an approximately 70 percent interest, the founding family remains the majority owner of the company to this day.
www.emdgroup.com | Sigma-Aldrich, a leading Life Science and Technology company focused on enhancing human health and safety, manufactures and distributes more than 230,000 chemicals, biochemicals and other essential products to more than 1.4 million customers globally in research and applied labs as well as in industrial and commercial markets.
With three distinct business units – Research, SAFC Commercial, Applied – Sigma-Aldrich is committed to enabling science to improve the quality of life.
The Company operates in 37 countries, has approximately 9,000 employees worldwide and had sales of $2.7 billion in 2013.
www.sigma-aldrich.com | ||||
Key Facts & Figures FY 2013 |
EMD Group |
• US$ 2,704 million revenue | ||||
• € 11,095 million revenue |
• US$ 821 million EBITDA (adjusted) | |||||
• € 3,253 million EBITDA-pre |
• ~230,000 products and solutions | |||||
• 39,000 employees | • One of the leading e-commerce and supply chain | |||||
platforms | ||||||
• ~ 9,000 employees (~500 Ph. Ds) | ||||||
| EMD Millipore | ||||||
• € 2,645 million revenue | ||||||
• € 643 million EBITDA pre | ||||||
• ~ 60,000 products and solutions | ||||||
• ~ 10,000 employees (600 R&D) | ||||||
Combined Business |
• ~€ 4.7 billion1 pro forma 2013 revenue | |||||
• ~€ 1.5 billion2 pro forma 2013 EBITDA pre | ||||||
• 2 million total customers | ||||||
• More than 300,000 total products | ||||||
| 1) | Pro forma calculation based on published sales for FY 2013 for EMD Millipore and Sigma-Aldrich (FX conversion: EUR/USD 1.30); 2) Pro forma calculation based on 100% expected synergies and published figures for FY 2013 for EMD Millipore and Sigma-Aldrich (FX conversion: EUR/USD 1.30) | |||||
Products |
|
| ||||
1
Factsheet
Strategic Rationale | • | Increasing scale – expanding position in attractive life science industry | ||
• |
Enhancing value for our customers | |||
| • | Broadens product range and ease of doing business for Laboratories & Academia | |||
| • | Complements Process Solutions product offering | |||
| • | Closing the gap in U.S. – adequate presence in all geographies | |||
| • | Leveraging existing platforms for global innovation rollout | |||
| Financial Fit | • | Further diversification of revenue stream | ||
| • | Substantial synergy potential | |||
| • | Immediately accretive to EPS pre* and EBITDA margin | |||
| • | Solid investment grade rating will be maintained | |||
Proposed acquisition |
• |
US$ 140 per share all-cash offer | ||
• |
Price represents 37% premium to the latest closing price of US$ 102.37 on September 19, 2014, and a 36% premium to the one-month volume-weighted average price (VWAP) | |||
Transaction details |
• |
Fully financed with cash, bank loans and bonds | ||
• |
Strong combined cash flows for rapid deleveraging; solid investment grade rating will be maintained | |||
| • | Expected synergies of € 260 million fully achieved in the third year after closing | |||
| • | Subject to regulatory approvals; expected closing mid-year 2015 | |||
| • | Recommended by Sigma-Aldrich Board of Directors; No EMD shareholder vote required | |||
| Commitment to the U.S. | • | EMD plans to maintain a significant presence in St. Louis, MO as well as in Billerica, MA following the transaction | ||
Cautionary Note Regarding Forward-Looking Statements
This communication may include “forward-looking statements.” Statements that include words such as “anticipate,” “expect,” “should,” “would,” “intend,” “plan,” “project,” “seek,” “believe,” “will,” and other words of similar meaning in connection with future events or future operating or financial performance are often used to identify forward-looking statements. All statements in this communication, other than those relating to historical information or current conditions, are forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond control of Merck KGaA, Darmstadt, Germany, which could cause actual results to differ materially from such statements.
Risks and uncertainties relating to the proposed transaction with Sigma-Aldrich Corporation (“Sigma-Aldrich”) include, but are not limited to: the risk Sigma-Aldrich’s shareholders do not approve the transaction; uncertainties as to the timing of the transaction; the risk that regulatory or other approvals required for the transaction are not obtained or are obtained subject to conditions that are not anticipated; competitive responses to the transaction; litigation relating to the transaction; uncertainty of the expected financial performance of the combined company following completion of the proposed transaction; the ability of Merck KGaA, Darmstadt, Germany, to achieve the cost-savings and synergies contemplated by the proposed transaction within the expected time frame; the ability of Merck KGaA, Darmstadt, Germany, to promptly and effectively integrate the businesses of Sigma-Aldrich and Merck KGaA, Darmstadt, Germany; the effects of the business combination of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich, including the combined company’s future financial condition, operating results, strategy and plans; the implications of the proposed transaction on certain employee benefit plans of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich; and disruption from the proposed transaction making it more difficult to maintain relationships with customers, employees or suppliers.
Additional risks and uncertainties include, but are not limited to: the risks of more restrictive regulatory requirements regarding drug pricing, reimbursement and approval; the risk of stricter regulations for the manufacture, testing and marketing of products; the risk of destabilization of political systems and the establishment of trade barriers; the risk of a changing marketing environment for multiple sclerosis products in the European Union; the risk of greater competitive pressure due to biosimilars; the risks of research and development; the risks of discontinuing development projects and regulatory approval of developed medicines; the risk of a temporary ban on products/production facilities or of non-registration of products due to non-compliance with quality standards; the risk of an import ban on products to the United States due to an FDA warning letter; the risks of dependency on suppliers; risks due to product-related crime and espionage; risks in relation to the use of financial instruments; liquidity risks; counterparty risks; market risks; risks of impairment on balance sheet items; risks from pension obligations; risks from product-related and patent law disputes; risks from
2
Factsheet
antitrust law proceedings; risks from drug pricing by the divested Generics Group; risks in human resources; risks from e-crime and cyber attacks; risks due to failure of business-critical information technology applications or to failure of data center capacity; environmental and safety risks; unanticipated contract or regulatory issues; a potential downgrade in the rating of the indebtedness of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich; downward pressure on the common stock price of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich and its impact on goodwill impairment evaluations; the impact of future regulatory or legislative actions; and the risks and uncertainties detailed by Sigma-Aldrich with respect to its business as described in its reports and documents filed with the U.S. Securities and Exchange Commission (the “SEC”).
The foregoing review of important factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere, including the Report on Risks and Opportunities Section of the most recent annual report and quarterly report of Merck KGaA, Darmstadt, Germany, and the Risk Factors section of Sigma-Aldrich’s most recent reports on Form 10-K and Form 10-Q. Any forward-looking statements made in this communication are qualified in their entirety by these cautionary statements, and there can be no assurance that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, us or our business or operations. Except to the extent required by applicable law, we undertake no obligation to update publicly or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Important Additional Information
This communication is being made in respect of the proposed merger transaction involving Sigma-Aldrich and Merck KGaA, Darmstadt, Germany. The proposed merger will be submitted to the stockholders of Sigma-Aldrich for their consideration. In connection therewith, Sigma-Aldrich intends to file relevant materials with the SEC, including a preliminary proxy statement and a definitive proxy statement. The definitive proxy statement will be mailed to the stockholders of Sigma-Aldrich. BEFORE MAKING ANY VOTING OR ANY INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION AND ANY OTHER RELEVANT DOCUMENTS FILED OR TO BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Investors and security holders may obtain free copies of the proxy statement, any amendments or supplements thereto and other documents containing important information about Sigma-Aldrich, once such documents are filed with the SEC, through the website maintained by the SEC at www.sec.gov. Copies of the documents filed with the SEC by Sigma-Aldrich will be available free of charge on Sigma-Aldrich’s website at www.sigmaaldrich.com under the heading “SEC Documents” within the “Investor Info” section in the “Investors” portion of Sigma-Aldrich’s website. Shareholders of Sigma-Aldrich may also obtain a free copy of the definitive proxy statement by contacting Sigma-Aldrich’s Investor Relations Department at (314) 898-4643.
Sigma-Aldrich and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Sigma-Aldrich is set forth in its proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 21, 2014, its annual report on Form 10-K for the fiscal year ended December 31, 2013, which was filed with the SEC on February 6, 2014, and in subsequent documents filed with the SEC, each of which can be obtained free of charge from the sources indicated above. Other information regarding the participants in the proxy solicitation of the stockholders of Sigma-Aldrich and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the preliminary and definitive proxy statements and other relevant materials to be filed with the SEC when they become available.
3
EN - MerckNet Report
Homepage teaser:Merck to Acquire Sigma-Aldrich to Enhance Position in Attractive Life Science Industry –Merck and Sigma-Aldrich, one of the leading US-based life science and technology companies, today announced that they have entered into a definitive agreement under which Merck will acquire Sigma-Aldrich for $17.0 billion (€13.1 billion), establishing one of the leading players in the $130 billion global life science industry.Read more
Text: Merck announced today that it has entered into a definitive agreement under which Merck will acquire all of the outstanding shares of Sigma-Aldrich for $140 per share in cash. The agreement values Sigma-Aldrich at $17.0 billion (€13.1 billion). The transaction is expected to close by mid-year 2015 subject to regulatory approvals and customary closing conditions. Merck’s Chairman of the Executive Board stated in today’spress release [link to release], that the combination of Merck and Sigma-Aldrich marks a quantum leap for Merck’s life science business.
With a common focus on employees, customers and communities, the combination represents a strong operational and cultural fit. Together, the two companies will be able to offer a much broader product offering to our global customers in research, pharma and biopharma manufacturing and diagnostic and testing labs. The combined company will invest more in innovation going forward, enhancing the customer benefits, and increasing the company’s presence in North America and adding exposure to fast-growing Asian markets.
In the Laboratory & Academia business, the combined company will offer their customers a complementary range of products across laboratory chemicals, biologics and reagents. In pharma and biopharma production, Sigma-Aldrich will complement Merck Millipore’s existing products and capabilities with additions along the entire value chain of drug production and validation.
Merck has great respect for Sigma-Aldrich’s rich history, customer-centric culture and commitment to corporate social responsibility and believes that the combination will afford new opportunities to employees at both companies. An integration team, which will include representatives from both companies, will be established to oversee and facilitate the integration process.
Learn more: Video interview with Karl-Ludwig Kley, the Chairman of the Executive Board, Marcus Kuhnert, Member of the Executive Board and Chief Financiel Officer and Rakesh Sachdev, Sigma-Aldrich’s CEO about why the transaction represents a compelling strategic and cultural fit.Link to video interview
Cautionary Note Regarding Forward-Looking Statements
This communication may include “forward-looking statements.” Statements that include words such as “anticipate,” “expect,” “should,” “would,” “intend,” “plan,” “project,” “seek,” “believe,” “will,” and other words of similar meaning in connection with future events or future operating or financial performance are often used to identify forward-looking statements. All statements in this communication, other than those relating to historical information or current conditions, are forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond control of Merck KGaA, Darmstadt, Germany, which could cause actual results to differ materially from such statements.
Risks and uncertainties relating to the proposed transaction with Sigma-Aldrich Corporation (“Sigma-Aldrich”) include, but are not limited to: the risk Sigma-Aldrich’s shareholders do not approve the transaction; uncertainties as to the timing of the transaction; the risk that regulatory or other approvals required for the transaction are not obtained or are obtained subject to conditions that are not anticipated; competitive responses to the transaction; litigation relating to the transaction; uncertainty of the expected financial performance of the combined company following completion of the proposed transaction; the ability of Merck KGaA, Darmstadt, Germany, to achieve the cost-savings and synergies contemplated by the proposed transaction within the expected time frame; the ability of Merck KGaA, Darmstadt, Germany, to promptly and effectively integrate the businesses of Sigma-Aldrich and Merck KGaA, Darmstadt, Germany; the effects of the business combination of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich, including the combined company’s future financial condition, operating results, strategy and plans; the implications of the proposed transaction on certain employee benefit plans of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich; and disruption from the proposed transaction making it more difficult to maintain relationships with customers, employees or suppliers.
Additional risks and uncertainties include, but are not limited to: the risks of more restrictive regulatory requirements regarding drug pricing, reimbursement and approval; the risk of stricter regulations for the manufacture, testing and marketing of products; the risk of destabilization of political systems and the establishment of trade barriers; the risk of a changing marketing environment for multiple sclerosis products in the European Union; the risk of greater competitive pressure due to biosimilars; the risks of research and development; the risks of discontinuing development projects and regulatory approval of developed medicines; the risk of a temporary ban on products/production facilities or of non-registration of products due to non-compliance with quality standards; the risk of an import ban on products to the United States due to an FDA warning letter; the risks of dependency on suppliers; risks due to product-related crime and espionage; risks in relation to the use of financial instruments; liquidity risks; counterparty risks; market risks; risks of impairment on balance sheet items; risks from pension obligations; risks from product-related and patent law disputes; risks from antitrust law proceedings; risks from drug pricing by the divested Generics Group; risks in human resources; risks from e-crime and cyber attacks; risks due to failure of business-critical information technology applications or to failure of data center capacity; environmental and safety risks; unanticipated contract or regulatory issues; a potential downgrade in the rating of the indebtedness of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich; downward pressure on the common stock price of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich and its impact on goodwill impairment evaluations; the impact of future regulatory or legislative actions; and the risks and uncertainties detailed by Sigma-Aldrich with respect to its business as described in its reports and documents filed with the U.S. Securities and Exchange Commission (the “SEC”).
2
The foregoing review of important factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere, including the Report on Risks and Opportunities Section of the most recent annual report and quarterly report of Merck KGaA, Darmstadt, Germany, and the Risk Factors section of Sigma-Aldrich’s most recent reports on Form 10-K and Form 10-Q. Any forward-looking statements made in this communication are qualified in their entirety by these cautionary statements, and there can be no assurance that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, us or our business or operations. Except to the extent required by applicable law, we undertake no obligation to update publicly or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Important Additional Information
This communication is being made in respect of the proposed merger transaction involving Sigma-Aldrich and Merck KGaA, Darmstadt, Germany. The proposed merger will be submitted to the stockholders of Sigma-Aldrich for their consideration. In connection therewith, Sigma-Aldrich intends to file relevant materials with the SEC, including a preliminary proxy statement and a definitive proxy statement. The definitive proxy statement will be mailed to the stockholders of Sigma-Aldrich. BEFORE MAKING ANY VOTING OR ANY INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION AND ANY OTHER RELEVANT DOCUMENTS FILED OR TO BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Investors and security holders may obtain free copies of the proxy statement, any amendments or supplements thereto and other documents containing important information about Sigma-Aldrich, once such documents are filed with the SEC, through the website maintained by the SEC at www.sec.gov. Copies of the documents filed with the SEC by Sigma-Aldrich will be available free of charge on Sigma-Aldrich’s website at www.sigmaaldrich.com under the heading “SEC Documents” within the “Investor Info” section in the “Investors” portion of Sigma-Aldrich’s website. Shareholders of Sigma-Aldrich may also obtain a free copy of the definitive proxy statement by contacting Sigma-Aldrich’s Investor Relations Department at (314) 898-4643.
Sigma-Aldrich and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Sigma-Aldrich is set forth in its proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 21, 2014, its annual report on Form 10-K for the fiscal year ended December 31, 2013, which was filed with the SEC on February 6, 2014, and in subsequent documents filed with the SEC, each of which can be obtained free of charge from the sources indicated above. Other information regarding the participants in the proxy solicitation of the stockholders of Sigma-Aldrich and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the preliminary and definitive proxy statements and other relevant materials to be filed with the SEC when they become available.
3
Interview with Karl-Ludwig Kley, Chairman of the Executive Board, Merck KGaA, Darmstadt, Germany and Rakesh Sachdev, President and CEO, Sigma-Aldrich
| Q: | Why are you acquiring Sigma-Aldrich? |
| A: | Karl-Ludwig Kley (K-L K): Well, before turning to answer your question let me say this. We are currently in the US, so whenever I refer to Merck, which is my company, I mean Merck KGaA, Darmstadt, Germany. The original Merck. |
Well, why are we acquiring Sigma-Aldrich? It’s simply a compelling value proposition. They are two great companies joining each other in a sector which is growing. Going forward, in a sector which is full of challenges, full of customer needs, together we will find answers for our customers. Both companies are built on innovation, on science, and we can deliver what the customer wants.
| Q: | Why does the deal make sense from your perspective? From the Sigma-Aldrich perspective? |
| A: | Rakesh Sachdev (RS): First of all, let me say that this combination is a clear testament to the success of the performance of Sigma-Aldrich, our 9,000 employees and what we have created. And we have created a very customer-intimate, and customer-focused company. |
Clearly the combination and this proposal offers a significant benefit to our shareholders because it offers a premium – a significant premium – and it’s immediate in cash. It clearly offers a lot of benefits to our customers because the value proposition is significantly enhanced through the combination of the strength of Merck as well as that of Sigma-Aldrich. We will bring more innovation to the customers. We will bring more complementary products and solutions.
And, finally for our employees, this is also very compelling because as a part of a much larger, more global organisation, as we develop more opportunities with our customers, it will translate into more opportunities for our employees on both sides.
| Q: | Why is now the right time to be making such a sizeable acquisition in life sciences? |
| A: | K-L K: Well, we see a lot of things going on in the sector. We see a lot of technical developments… of technological developments. We see our customers demanding more and more; asking for more global solutions, asking for more service in the sector itself. Now it’s time to give more answers to customers’ needs. |
The second aspect comes from the internal side. We have finished our efficiency programme, rolled out the efficiency measures and we are ready to take the next step. And the next step is strengthening our life science business with this deal. It’s not just a milestone, it’s a quantum leap in our business.
| Q: | When you look at the two businesses individually, why do you think they will be better as a combination? |
| A: | RS: First of all we have a shared perspective on the importance of the whole life sciences industry and clearly as a combination with the strengths that we bring together. We have a complementary geographic presence. We have complementary products. When the customers benefits, the business benefits, and the employees benefit. And that’s the real thesis behind this combination. |
| Q: | So, tell me a little bit more about the benefits to your business from your perspective. How is this really going to propel you to the next level? |
| A: | K-L K: Firstly geographically. Currently we are under-represented in the US and with the strong presence of Sigma-Aldrich in the US market, we’ll have a size which really allows us to take products and service to the customer throughout this great country. |
Together we can team up in Asian countries and be very helpful to our customers there. Our business to the academia and lab market is doubling with this acquisition. Regarding our sales to the biopharmaceutical industry, we have very complementary products. And then – and I have to make huge complements to Rakesh and his great company – we can build everything on the best e-commerce platform in this sector. This is something which will really drive this deal and is helpful for everybody involved.
| Q: | Do you want to add something about the e-commerce platform that comes with this deal? |
| A: | RS: We believe Sigma-Aldrich has a leading e-commerce platform today in the life sciences industry. We typically have about 70 million visitors on our website every year and we conduct transactions there. But it’s not just for conducting transactions. There is a lot of scientific content that we deliver to our customers who rely on our e-commerce platform, which in turn also gives us a lot of information about our customers that we use to develop new products, drive new innovation. So it’s really a two-way street. And I think it’s going to be incredibly helpful as we put these two businesses – Merck and Sigma-Aldrich – together, to be able to capitalise on that strength. |
| Q: | Now you both mentioned your customers, but won’t this deal ultimately lead to a period of disruption for them and then loss of choice? |
| A: | K-L K: I strongly believe customers are becoming more and more demanding. They are asking for global solutions. They are asking for extended |
2
services. They are asking for a broad range of possibilities and this is something we can offer. I believe customer service will improve and the opportunities for customers to develop their products further will be better than it is today.
SR: Just to pick up on what Karl said. Two years ago we realigned Sigma-Aldrich really to deliver solutions to our customers and we have moved the company from being a products-focused company to a solutions-focused company. The value proposition that we can provide together now with complementary products, with complementary science is going to take us even further down the solutions path for these customers. So I think our customers will have a lot more choice, but they will also have much better solutions from the combined company.
| Q: | Just stepping back for a moment, why are you making an acquisition in life sciences and not strengthening your pharma business? Is it an indication that the oldest pharma business in the world is stepping back from pharma and research? |
| A: | K-L K: No, no. We won’t let anyone become older than us! So, we will stay in pharma. We remain committed to pharma. We have just informed the public and the investors and the analysts last week actually about the status of our pharma business. We are delivering very stable sales on our existing portfolio. We had some, we believe, exciting news to share about the pharma pipeline and we will also commit the necessary resources to develop our pipeline products further; be it in partnership or be it alone. So, our pharma business will go on and remain an integral part of our company. |
| Q: | Tell me a little bit about why this deal is an attractive value proposition for your shareholders? |
| A: | RS: Well, for our shareholders, as I have said, this offers a significant premium from Merck. It’s immediate. It’s in cash. It’s certain. So, I think our shareholders should be pleased with what they are receiving from Merck. |
| Q: | But, from your perspective, you’re paying a premium to the all-time high share price of Sigma-Aldrich. So, is that a real good value proposition for your shareholders or are you overpaying? |
| A: | K-L K: If you want to buy a Rolls Royce you don’t get it a bargain price. If you invest to buy the best e-commerce platform in the industry, if you invest to combine these science driven businesses into one greater good you have to pay the price, the value which is represented in this company. |
On financial terms, for our shareholders, it’s attractive. It’s immediately EPS accretive. It meets all our financial criteria. We have a history of fast deleveraging. So, we believe that we can also offer the rating agencies a value proposition which will keep our rating on investment grade. So, for all financial stakeholders it’s an attractive value proposition.
3
| Q: | Tell me a little bit more about the synergies and how many jobs might possibly be at stake? |
| A: | K-L K: Well, a deal of that size of course means a lot of synergies, but it would be premature to talk about synergies today. Once we have the regulatory approvals, once the shareholders have approved the deal then we would have to sit together and find out what this means. |
But let me say two things. One, of course, is a very clear commitment to St. Louis. The city has been the home of Sigma-Aldrich for decades and we not only respect this, but we treasure the values which are embedded in this operation. And certainly St. Louis will play a major role in taking the combined company forward.
The other message I want to make here very clearly is that – and this is something we have in common – we have a culture which is based on values; on respecting people, on integrity. When it comes to defining synergies and executing them, we will do it with the highest respect to those people who have made the companies what they are today.
| Q: | When would you expect this transaction to close? What are the next steps that need to be taken? |
| A: | RS: I think we are expecting the transaction to close by mid-year 2015. We have to go through the normal regulatory approvals and also getting the shareholder vote from the Sigma-Aldrich shareholders. So, we will be working through that in the course of the coming weeks and months. |
| Q: | And finally, what would be your message to your staff as they go through this integration process? |
| A: | RS: When I sit back and think about Sigma-Aldrich, we have been in business for 80 years and we are a science-based company. We have had a very constant mission for 80 years and that is to enable science to improve the quality of life. In fact, our people get excited coming to work every morning because they know in some form or fashion they’re going to be improving the quality of life. And what’s great is that Merck has the same mission, is to improve the quality of life. I think it’s going to be very interesting for the employees of Sigma-Aldrich and Merck to come together because they speak the same language. The culture and the values, as Karl said, are very, very similar and there is going to be some great opportunities for the employees of the combined company as we take this combined business forward to the next level. |
[End]
Cautionary Note Regarding Forward-Looking Statements
This communication may include “forward-looking statements.” Statements that include words such as “anticipate,” “expect,” “should,” “would,” “intend,” “plan,” “project,” “seek,” “believe,” “will,” and
4
other words of similar meaning in connection with future events or future operating or financial performance are often used to identify forward-looking statements. All statements in this communication, other than those relating to historical information or current conditions, are forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond control of Merck KGaA, Darmstadt, Germany, which could cause actual results to differ materially from such statements.
Risks and uncertainties relating to the proposed transaction with Sigma-Aldrich Corporation (“Sigma-Aldrich”) include, but are not limited to: the risk Sigma-Aldrich’s shareholders do not approve the transaction; uncertainties as to the timing of the transaction; the risk that regulatory or other approvals required for the transaction are not obtained or are obtained subject to conditions that are not anticipated; competitive responses to the transaction; litigation relating to the transaction; uncertainty of the expected financial performance of the combined company following completion of the proposed transaction; the ability of Merck KGaA, Darmstadt, Germany, to achieve the cost-savings and synergies contemplated by the proposed transaction within the expected time frame; the ability of Merck KGaA, Darmstadt, Germany, to promptly and effectively integrate the businesses of Sigma-Aldrich and Merck KGaA, Darmstadt, Germany; the effects of the business combination of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich, including the combined company’s future financial condition, operating results, strategy and plans; the implications of the proposed transaction on certain employee benefit plans of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich; and disruption from the proposed transaction making it more difficult to maintain relationships with customers, employees or suppliers.
Additional risks and uncertainties include, but are not limited to: the risks of more restrictive regulatory requirements regarding drug pricing, reimbursement and approval; the risk of stricter regulations for the manufacture, testing and marketing of products; the risk of destabilization of political systems and the establishment of trade barriers; the risk of a changing marketing environment for multiple sclerosis products in the European Union; the risk of greater competitive pressure due to biosimilars; the risks of research and development; the risks of discontinuing development projects and regulatory approval of developed medicines; the risk of a temporary ban on products/production facilities or of non-registration of products due to non-compliance with quality standards; the risk of an import ban on products to the United States due to an FDA warning letter; the risks of dependency on suppliers; risks due to product-related crime and espionage; risks in relation to the use of financial instruments; liquidity risks; counterparty risks; market risks; risks of impairment on balance sheet items; risks from pension obligations; risks from product-related and patent law disputes; risks from antitrust law proceedings; risks from drug pricing by the divested Generics Group; risks in human resources; risks from e-crime and cyber attacks; risks due to failure of business-critical information technology applications or to failure of data center capacity; environmental and safety risks; unanticipated contract or regulatory issues; a potential downgrade in the rating of the indebtedness of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich; downward pressure on the common stock price of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich and its impact on goodwill impairment evaluations; the impact of future regulatory or legislative actions; and the risks and uncertainties detailed by Sigma-Aldrich with respect to its business as described in its reports and documents filed with the U.S. Securities and Exchange Commission (the “SEC”).
The foregoing review of important factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere, including the Report on Risks and Opportunities Section of the most recent annual report and quarterly report of Merck KGaA, Darmstadt, Germany, and the Risk Factors section of Sigma-Aldrich’s most recent reports on Form 10-K and Form 10-Q. Any forward-looking statements made in this communication are qualified in their entirety by these cautionary statements, and there can be no assurance that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, us or our business or operations. Except to the extent required by applicable law, we undertake no obligation to update publicly or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
5
Important Additional Information
This communication is being made in respect of the proposed merger transaction involving Sigma-Aldrich and Merck KGaA, Darmstadt, Germany. The proposed merger will be submitted to the stockholders of Sigma-Aldrich for their consideration. In connection therewith, Sigma-Aldrich intends to file relevant materials with the SEC, including a preliminary proxy statement and a definitive proxy statement. The definitive proxy statement will be mailed to the stockholders of Sigma-Aldrich. BEFORE MAKING ANY VOTING OR ANY INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION AND ANY OTHER RELEVANT DOCUMENTS FILED OR TO BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Investors and security holders may obtain free copies of the proxy statement, any amendments or supplements thereto and other documents containing important information about Sigma-Aldrich, once such documents are filed with the SEC, through the website maintained by the SEC at www.sec.gov. Copies of the documents filed with the SEC by Sigma-Aldrich will be available free of charge on Sigma-Aldrich’s website at www.sigmaaldrich.com under the heading “SEC Documents” within the “Investor Info” section in the “Investors” portion of Sigma-Aldrich’s website. Shareholders of Sigma-Aldrich may also obtain a free copy of the definitive proxy statement by contacting Sigma-Aldrich’s Investor Relations Department at (314) 898-4643.
Sigma-Aldrich and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Sigma-Aldrich is set forth in its proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 21, 2014, its annual report on Form 10-K for the fiscal year ended December 31, 2013, which was filed with the SEC on February 6, 2014, and in subsequent documents filed with the SEC, each of which can be obtained free of charge from the sources indicated above. Other information regarding the participants in the proxy solicitation of the stockholders of Sigma-Aldrich and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the preliminary and definitive proxy statements and other relevant materials to be filed with the SEC when they become available.
6
Interview with Marcus Kuhnert, CFO, Merck KGaA, Darmstadt Germany
| Q: | You’re paying a considerable premium even to the all-time high share price of Sigma-Aldrich. So does this transaction really represent a good deal for your shareholders? |
| A: | Yes it does. The deal has a compelling strategic rationale and will for sure create value for our shareholders. Sigma-Aldrich has a very attractive growth potential and its profitability is well above the average of its industry. |
When we looked at the company we also identified significant synergy potential which will make the deal financially attractive and, of course, we’re sticking to our core acquisition criteria, namely EPS pre will be accretive right from the beginning. The EBITDA margin will be accretive for the Group, as well as for the division. And we will of course earn back our cost of capital. Following our conservative financial policy we will maintain investment grade credit rating.
| Q: | So, do you think this price will be attractive to Sigma-Aldrich shareholders? Have you offered them enough to get this deal across the line? |
| A: | Yes. I think we have made a very attractive offer and offered a very attractive premium to Sigma-Aldrich shareholders. The Board of Directors has accepted our offer after extensive discussions and we, as well as Sigma-Aldrich’s management, are very confident that Sigma-Aldrich shareholders will accept the offer in a special meeting to be held within the next months. |
| Q: | So, are you able to provide some certainty that the transaction will go ahead? |
| A: | As I already said, Sigma-Aldrich’s Board of Directors has recommended the transaction and we are now waiting for the approval of the shareholders holding the majority of Sigma-Aldrich shares which we expect to get in their special meeting which will be held in the next months. |
The transaction has already been approved by our major shareholder. The bridge financing is in place and the transaction is, of course, subject to the regulatory approval to be granted by the relevant authorities.
| Q: | What certainty can you give to shareholders that you can achieve the stated synergies? |
| A: | We have a good understanding regarding the source and the level of the synergies coming from our own analysis, from talks with Sigma-Aldrich’s management and, of course, also from the due diligence that we have just conducted. |
On top of that, we have gained extensive experience over the last couple of years in the areas of integration, restructuring and execution in the life science segment and we are very committed to generate and to realise the full amount of synergies. We believe that we will be able to realise the full amount of synergies in the third year after closing. This is industry standard and realistic given the strong operational and cultural fit between the two companies.
| Q: | What level of financing capacity is now available for other acquisitions and when might you be ready to look at making a larger acquisition? |
| A: | Now, following the transaction, we will clearly use the strong and growing cash flow of the combined businesses in order to rapidly deleverage. While we will be continuing to screen the market for attractive opportunities, I can clearly say that for the time beginning the clear focus will be on rapid deleveraging. |
[End]
Cautionary Note Regarding Forward-Looking Statements
This communication may include “forward-looking statements.” Statements that include words such as “anticipate,” “expect,” “should,” “would,” “intend,” “plan,” “project,” “seek,” “believe,” “will,” and other words of similar meaning in connection with future events or future operating or financial performance are often used to identify forward-looking statements. All statements in this communication, other than those relating to historical information or current conditions, are forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond control of Merck KGaA, Darmstadt, Germany, which could cause actual results to differ materially from such statements.
Risks and uncertainties relating to the proposed transaction with Sigma-Aldrich Corporation (“Sigma-Aldrich”) include, but are not limited to: the risk Sigma-Aldrich’s shareholders do not approve the transaction; uncertainties as to the timing of the transaction; the risk that regulatory or other approvals required for the transaction are not obtained or are obtained subject to conditions that are not anticipated; competitive responses to the transaction; litigation relating to the transaction; uncertainty of the expected financial performance of the combined company following completion of the proposed transaction; the ability of Merck KGaA, Darmstadt, Germany, to achieve the cost-savings and synergies contemplated by the proposed transaction within the expected time frame; the ability of Merck KGaA, Darmstadt, Germany, to promptly and effectively integrate the businesses of Sigma-Aldrich and Merck KGaA, Darmstadt, Germany; the effects of the business combination of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich, including the combined company’s future financial condition, operating results, strategy and plans; the implications of the proposed transaction on certain employee benefit plans of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich; and disruption from the proposed transaction making it more difficult to maintain relationships with customers, employees or suppliers.
Additional risks and uncertainties include, but are not limited to: the risks of more restrictive regulatory requirements regarding drug pricing, reimbursement and approval; the risk of stricter regulations for the manufacture, testing and marketing of products; the risk of destabilization of political systems and the establishment of trade barriers; the risk of a changing marketing environment for multiple sclerosis products in the European Union; the risk of greater competitive pressure due to biosimilars; the risks of research and development; the risks of discontinuing development projects and regulatory approval of developed medicines; the risk of a temporary ban on products/production facilities or of non-registration
2
of products due to non-compliance with quality standards; the risk of an import ban on products to the United States due to an FDA warning letter; the risks of dependency on suppliers; risks due to product-related crime and espionage; risks in relation to the use of financial instruments; liquidity risks; counterparty risks; market risks; risks of impairment on balance sheet items; risks from pension obligations; risks from product-related and patent law disputes; risks from antitrust law proceedings; risks from drug pricing by the divested Generics Group; risks in human resources; risks from e-crime and cyber attacks; risks due to failure of business-critical information technology applications or to failure of data center capacity; environmental and safety risks; unanticipated contract or regulatory issues; a potential downgrade in the rating of the indebtedness of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich; downward pressure on the common stock price of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich and its impact on goodwill impairment evaluations; the impact of future regulatory or legislative actions; and the risks and uncertainties detailed by Sigma-Aldrich with respect to its business as described in its reports and documents filed with the U.S. Securities and Exchange Commission (the “SEC”).
The foregoing review of important factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere, including the Report on Risks and Opportunities Section of the most recent annual report and quarterly report of Merck KGaA, Darmstadt, Germany, and the Risk Factors section of Sigma-Aldrich’s most recent reports on Form 10-K and Form 10-Q. Any forward-looking statements made in this communication are qualified in their entirety by these cautionary statements, and there can be no assurance that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, us or our business or operations. Except to the extent required by applicable law, we undertake no obligation to update publicly or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Important Additional Information
This communication is being made in respect of the proposed merger transaction involving Sigma-Aldrich and Merck KGaA, Darmstadt, Germany. The proposed merger will be submitted to the stockholders of Sigma-Aldrich for their consideration. In connection therewith, Sigma-Aldrich intends to file relevant materials with the SEC, including a preliminary proxy statement and a definitive proxy statement. The definitive proxy statement will be mailed to the stockholders of Sigma-Aldrich. BEFORE MAKING ANY VOTING OR ANY INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION AND ANY OTHER RELEVANT DOCUMENTS FILED OR TO BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Investors and security holders may obtain free copies of the proxy statement, any amendments or supplements thereto and other documents containing important information about Sigma-Aldrich, once such documents are filed with the SEC, through the website maintained by the SEC at www.sec.gov. Copies of the documents filed with the SEC by Sigma-Aldrich will be available free of charge on Sigma-Aldrich’s website at www.sigmaaldrich.com under the heading “SEC Documents” within the “Investor Info” section in the “Investors” portion of Sigma-Aldrich’s website. Shareholders of Sigma-Aldrich may also obtain a free copy of the definitive proxy statement by contacting Sigma-Aldrich’s Investor Relations Department at (314) 898-4643.
Sigma-Aldrich and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Sigma-Aldrich is set forth in its proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 21, 2014, its annual report on Form 10-K for the fiscal year ended December 31, 2013, which was filed with the SEC on February 6, 2014, and in subsequent documents filed with the SEC, each of which can be obtained free of charge from the sources indicated above. Other information regarding the participants in the proxy solicitation of the stockholders of Sigma-Aldrich and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the preliminary and definitive proxy statements and other relevant materials to be filed with the SEC when they become available.
3

M
Merck KGaA
Darmstadt · Germany
Merck and Merck Millipore Townhall
Announced Sigma-Aldrich acquisition
Taking our life science business to the next level
September 22, 2014
Bernd Reckmann, Member of the Merck Executive Board, Head of Chemicals Udit Batra, President & CEO, Merck Millipore
1

M
Merck KGaA
Darmstadt · Germany
Disclaimer
Cautionary Note Regarding Forward-Looking Statements
This communication may include “forward-looking statements.” Statements that include words such as “anticipate,” “expect,” “should,” “would,” “intend,” “plan,” “project,” “seek,” “believe,” “will,” and other words of similar meaning in connection with future events or future operating or financial performance are often used to identify forward-looking statements. All statements in this communication, other than those relating to historical information or current conditions, are forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements in the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond control of Merck KGaA, Darmstadt, Germany, which could cause actual results to differ materially from such statements.
Risks and uncertainties relating to the proposed transaction with Sigma-Aldrich Corporation (“Sigma-Aldrich”) include, but are not limited to: the risk Sigma-Aldrich’s shareholders do not approve the transaction; uncertainties as to the timing of the transaction; the risk that regulatory or other approvals required for the transaction are not obtained or are obtained subject to conditions that are not anticipated; competitive responses to the transaction; litigation relating to the transaction; uncertainty of the expected financial performance of the combined company following completion of the proposed transaction; the ability of Merck KGaA, Darmstadt, Germany, to achieve the cost-savings and synergies contemplated by the proposed transaction within the expected time frame; the ability of Merck KGaA, Darmstadt, Germany, to promptly and effectively integrate the businesses of Sigma-Aldrich and Merck KGaA, Darmstadt, Germany; the effects of the business combination of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich, including the combined company’s future financial condition, operating results, strategy and plans; the implications of the proposed transaction on certain employee benefit plans of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich; and disruption from the proposed transaction making it more difficult to maintain relationships with customers, employees or suppliers.
Additional risks and uncertainties include, but are not limited to: the risks of more restrictive regulatory requirements regarding drug pricing, reimbursement and approval; the risk of stricter regulations for the manufacture, testing and marketing of products; the risk of destabilization of political systems and the establishment of trade barriers; the risk of a changing marketing environment for multiple sclerosis products in the European Union; the risk of greater competitive pressure due to biosimilars; the risks of research and development; the risks of discontinuing development projects and regulatory approval of developed medicines; the risk of a temporary ban on products/production facilities or of non-registration of products due to non-compliance with quality standards; the risk of an import ban on products to the United States due to an FDA warning letter; the risks of dependency on suppliers; risks due to product-related crime and espionage; risks in relation to the use of financial instruments; liquidity risks; counterparty risks; market risks; risks of impairment on balance sheet items; risks from pension obligations; risks from product-related and patent law disputes; risks from antitrust law proceedings; risks from drug pricing by the divested Generics Group; risks in human resources; risks from e-crime and cyber attacks; risks due to failure of business-critical information technology applications or to failure of data center capacity; environmental and safety risks; unanticipated contract or regulatory issues; a potential downgrade in the rating of the indebtedness of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich; downward pressure on the common stock price of Merck KGaA, Darmstadt, Germany, or Sigma-Aldrich and its impact on goodwill impairment evaluations; the impact of future regulatory or legislative actions; and the risks and uncertainties detailed by Sigma-Aldrich with respect to its business as described in its reports and documents filed with the U.S. Securities and Exchange Commission (the “SEC”).
The foregoing review of important factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere, including the Report on Risks and Opportunities Section of the most recent annual report and quarterly report of Merck KGaA, Darmstadt, Germany, and the Risk Factors section of Sigma-Aldrich’s most recent reports on Form 10-K and Form 10-Q. Any forward-looking statements made in this communication are qualified in their entirety by these cautionary statements, and there can be no assurance that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, us or our business or operations. Except to the extent required by applicable law, we undertake no obligation to update publicly or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Important Additional Information
This communication is being made in respect of the proposed merger transaction involving Sigma-Aldrich and Merck KGaA, Darmstadt, Germany. The proposed merger will be submitted to the stockholders of Sigma-Aldrich for their consideration. In connection therewith, Sigma-Aldrich intends to file relevant materials with the SEC, including a preliminary proxy statement and a definitive proxy statement. The definitive proxy statement will be mailed to the stockholders of Sigma-Aldrich. BEFORE MAKING ANY VOTING OR ANY INVESTMENT DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION AND ANY OTHER RELEVANT DOCUMENTS FILED OR TO BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION. Investors and security holders may obtain free copies of the proxy statement, any amendments or supplements thereto and other documents containing important information about Sigma-Aldrich, once such documents are filed with the SEC, through the website maintained by the SEC at www.sec.gov. Copies of the documents filed with the SEC by Sigma-Aldrich will be available free of charge on Sigma-Aldrich’s website at www.sigmaaldrich.com under the heading “SEC Documents” within the “Investor Info” section in the “Investors” portion of Sigma-Aldrich’s website. Shareholders of Sigma-Aldrich may also obtain a free copy of the definitive proxy statement by contacting Sigma-Aldrich’s Investor Relations Department at +1 (314) 898 4643.
Sigma-Aldrich and certain of its directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Sigma-Aldrich is set forth in its proxy statement for its 2014 annual meeting of stockholders, which was filed with the SEC on March 21, 2014, its annual report on Form 10-K for the fiscal year ended December 31, 2013, which was filed with the SEC on February 6, 2014, and in subsequent documents filed with the SEC, each of which can be obtained free of charge from the sources indicated above. Other information regarding the participants in the proxy solicitation of the stockholders of Sigma-Aldrich and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the preliminary and definitive proxy statements and other relevant materials to be filed with the SEC when they become available.
2

M
Merck KGaA
Darmstadt · Germany
Content for today
Today’s News Highlights
The Combination: Fits Our Strategy
What is Our Responsibility Now?
3

M
Merck KGaA
Darmstadt · Germany
Content for today
Today’s News Highlights
The Combination: Fits Our Strategy
What is Our Responsibility Now?
4
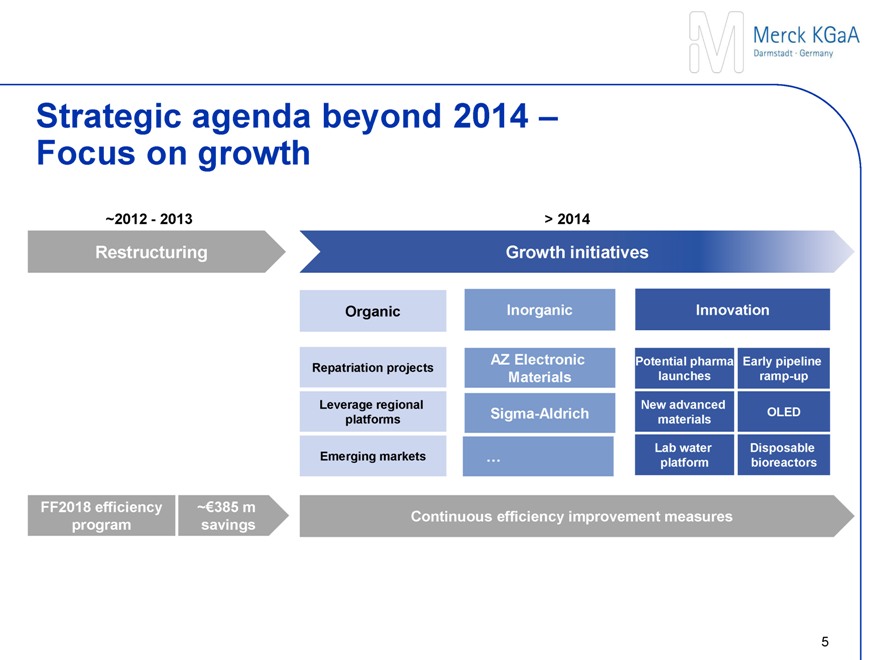
M
Merck KGaA
Darmstadt · Germany
Strategic agenda beyond 2014 – Focus on growth
~2012 - 2013
> 2014
Restructuring
Growth initiatives
Organic
Inorganic
Innovation
Repatriation projects
AZ Electronic
Materials
Potential pharma launches
Early pipeline ramp-up
Leverage regional
New advanced
platforms
Sigma-Aldrich
materials
OLED
Lab water
Disposable
Emerging markets
platform
bioreactors
FF2018 efficiency
~€385 m
program
savings
Continuous efficiency improvement measures
5

M
Merck KGaA
Darmstadt · Germany
Sigma-Aldrich – Next step to enhance Merck’s position in attractive life science industry
Attractive life science industry
Attractive industry driven by
sustainable underlying market trends
Stable growth pattern, offering
additional growth opportunities
Strong companies with healthy
margins
Taking Merck’s life science business to the next level
Strong track record to deliver profitable growth
Adding scale with step change acquisition
6
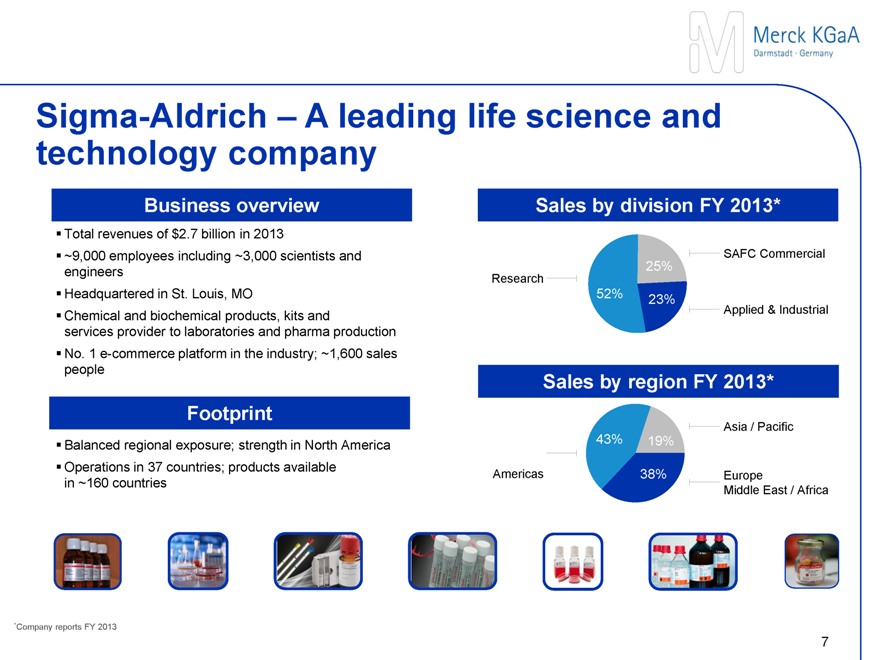
M
Merck KGaA
Darmstadt · Germany
Sigma-Aldrich – A leading life science and technology company
Business overview
Total revenues of $2.7 billion in 2013
~9,000 employees including ~3,000 scientists and engineers
Headquartered in St. Louis, MO
Chemical and biochemical products, kits and services provider to laboratories and pharma production No. 1 e-commerce platform in the industry; ~1,600 sales people
Footprint
Balanced regional exposure; strength in North America
Operations in 37 countries; products available in ~160 countries
Sales by division
FY 2013*
SAFC Commercial
25%
Research
52%
23%
Applied & Industrial
Sales by region FY
2013*
Asia / Pacific
43%
19%
Americas
38%
Europe
Middle East / Africa
*Company reports FY 2013
7
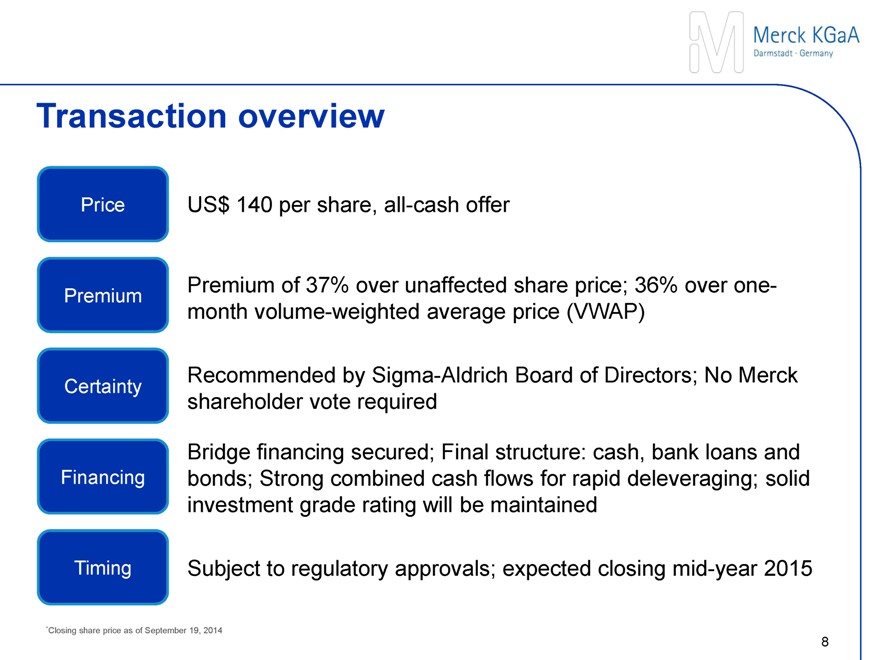
M
Merck KGaA
Darmstadt · Germany
Transaction overview
Price
US$ 140 per share, all-cash offer
Premium
Premium of 37% over unaffected share price; 36% over one-
month volume-weighted average price (VWAP)
Certainty
Recommended by Sigma-Aldrich Board of Directors; No Merck
shareholder vote required
Bridge financing secured; Final structure: cash, bank loans and
Financing
bonds; Strong combined cash flows for rapid deleveraging; solid
investment grade rating will be maintained
Timing
Subject to regulatory approvals; expected closing mid-year 2015
*Closing share price as of September 19, 2014
8
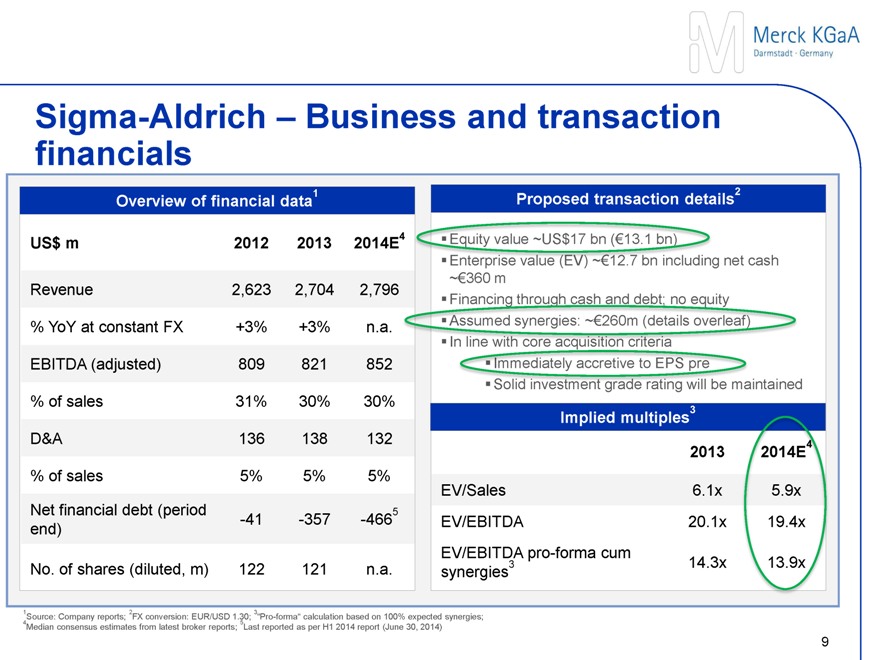
M
Merck KGaA
Darmstadt · Germany
Sigma-Aldrich – Business and transaction financials
Overview of financial data1
US$ m 2012 2013 2014E4
Revenue 2,623 2,704 2,796
% YoY at constant FX +3% +3% n.a.
EBITDA (adjusted) 809 821 852
% of sales 31% 30% 30%
D&A 136 138 132
% of sales 5% 5% 5%
Net financial debt (period end) 5 -41 -357 -466
No. of shares (diluted, m) 122 121 n.a.
Proposed transaction details2
Equity value ~US$17 bn (€13.1 bn)
Enterprise value (EV) ~€12.7 bn including net cash ~€360 m
Financing through cash and debt; no equity
Assumed synergies: ~€260m (details overleaf)
In line with core acquisition criteria
Immediately accretive to EPS pre
Solid investment grade rating will be maintained
Implied multiples3
2013 2014E4
EV/Sales 6.1x 5.9x
EV/EBITDA 20.1x 19.4x
EV/EBITDA pro-forma cum 14.3x 13.9x
synergies3
1Source: Company reports; 2FX conversion: EUR/USD 1.30; 3“Pro-forma” calculation based on 100% expected synergies; 4Median consensus estimates from latest broker reports; 5Last reported as per H1 2014 report (June 30, 2014)
9
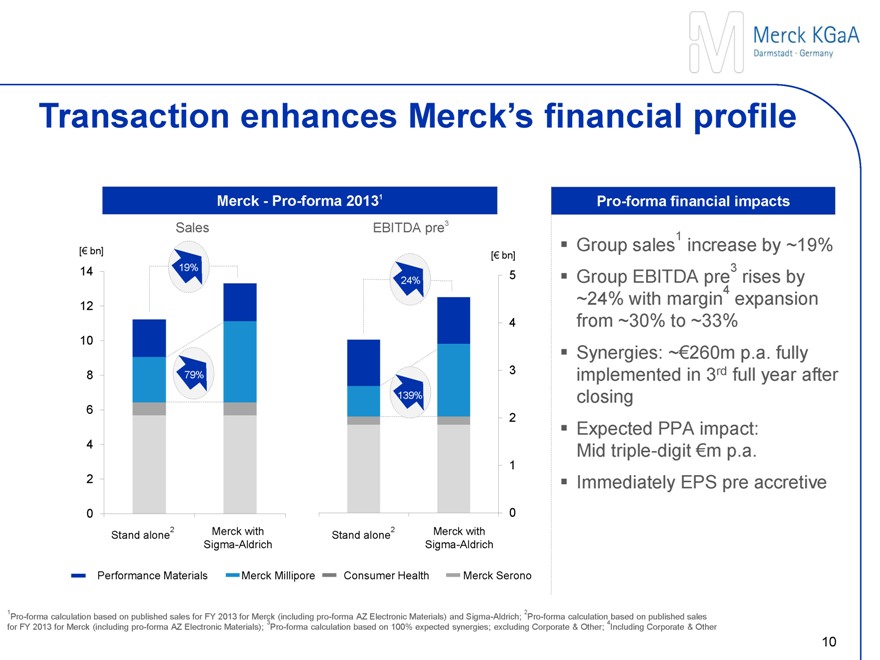
M
Merck KGaA
Darmstadt · Germany
Transaction enhances Merck’s financial profile
Merck - Pro-forma 20131
Sales EBITDA pre3
[€ bn] [€ bn]
14 19% 5
24%
12
4
10
8 79% 3
139%
6 2
4
1
2
0 0
Stand alone2 Merck with Stand alone2 Merck with
Sigma-Aldrich Sigma-Aldrich
Performance Materials Merck Millipore Consumer Health Merck Serono
Pro-forma financial impacts
Group sales1 increase by ~19%
Group EBITDA pre3 rises by ~24% with margin4 expansion from ~30% to ~33%
Synergies: ~€260m p.a. fully implemented in 3rd full year after closing
Expected PPA impact: Mid triple-digit €m p.a.
Immediately EPS pre accretive
1Pro-forma calculation based on published sales for FY 2013 for Merck (including pro-forma AZ Electronic Materials) and Sigma-Aldrich; 2Pro-forma calculation based on published sales for FY 2013 for Merck (including pro-forma AZ Electronic Materials); 3Pro-forma calculation based on 100% expected synergies; excluding Corporate & Other; 4Including Corporate & Other
10
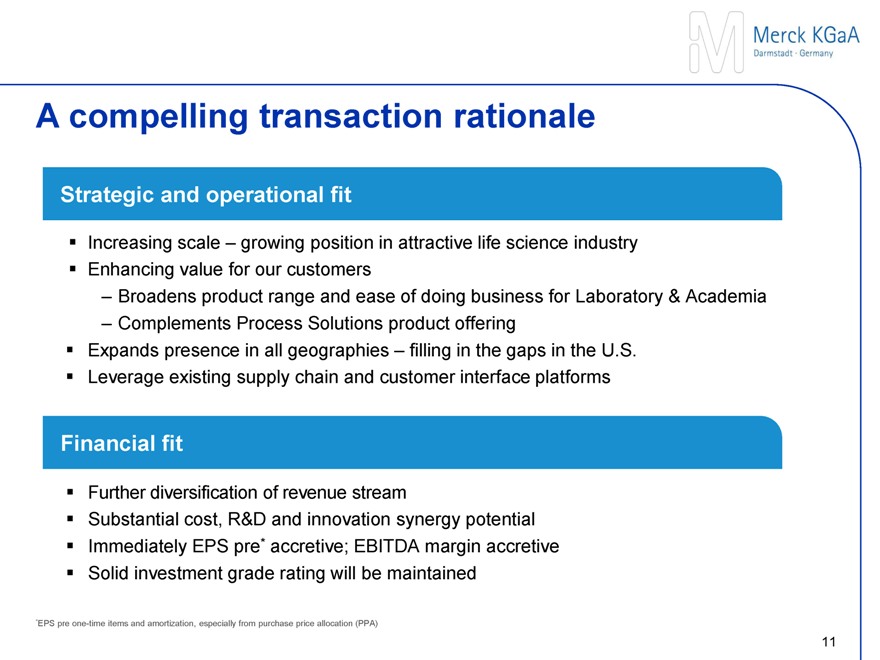
M
Merck KGaA
Darmstadt · Germany
A compelling transaction rationale
Strategic and operational fit
Increasing scale – growing position in attractive life science industry
Enhancing value for our customers
– Broadens product range and ease of doing business for Laboratory & Academia
– Complements Process Solutions product offering
Expands presence in all geographies – filling in the gaps in the U.S.
Leverage existing supply chain and customer interface platforms
Financial fit
Further diversification of revenue stream
Substantial cost, R&D and innovation synergy potential
Immediately EPS pre* accretive; EBITDA margin accretive
Solid investment grade rating will be maintained
*EPS pre one-time items and amortization, especially from purchase price allocation (PPA)
11

M
Merck KGaA
Darmstadt · Germany
Content for today
Today’s News Highlights
The Combination: Fits Our Strategy
What is Our Responsibility Now?
12
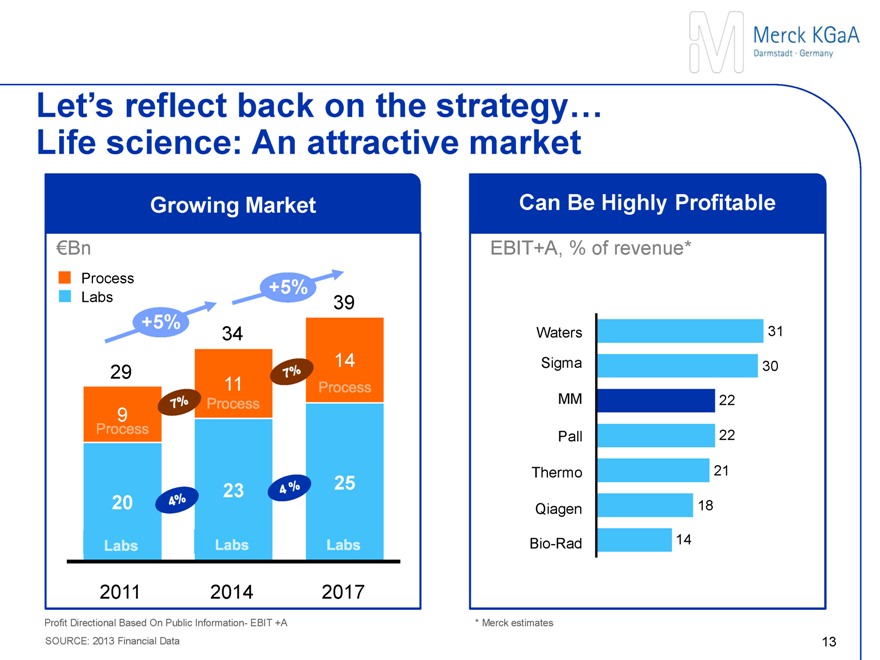
M
Merck KGaA
Darmstadt · Germany
Let’s reflect back on the strategy…
Life science: An attractive market
Growing Market
€Bn
Process
Labs
+5% +5%
29 34 39
9 7% 11 7% 14
Process Process Process
20 4% 23 4% 25
Labs Labs Labs
2011 2014 2017
Can Be Highly Profitable
EBIT+A, % of revenue*
Waters 31
Sigma 30
MM 22
Pall 22
Thermo 21
Qiagen 18
Bio-Rad 14
Profit Directional Based On Public Information- EBIT +A *Merck estimates
SOURCE: 2013 Financial Data
13
M
Merck KGaA
Darmstadt · Germany
We also understood what was needed to succeed in the marketplace
Labs
1. Broad and attractive product offering
2. Simple customer interface
3. Organization able to deal with complexity
Process
1. Deeply technical field force
2. Product depth in developed markets
3. Broad product range in emerging markets
14
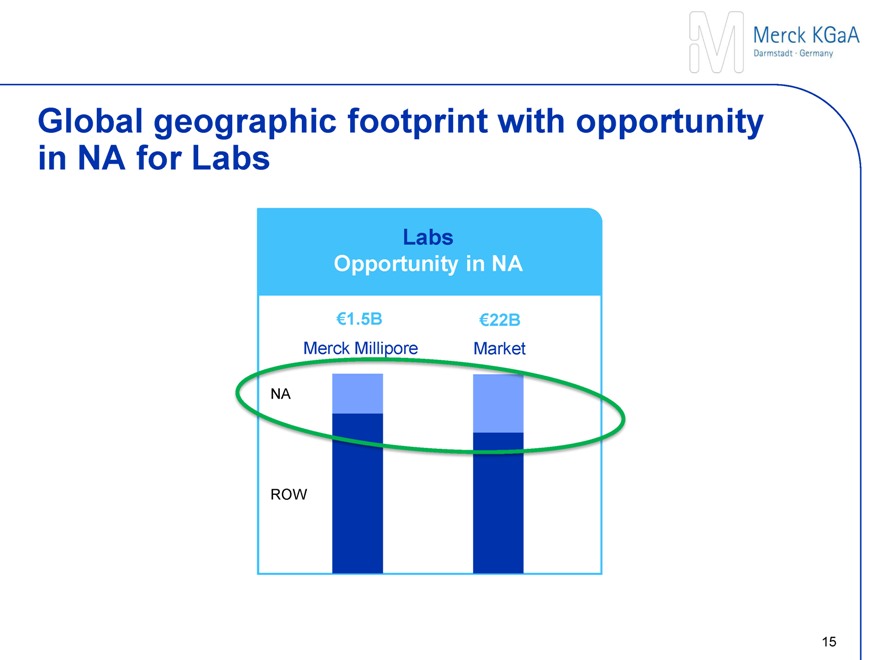
M Merck KGaA
Darmstadt · Germany
Global geographic footprint with opportunity in NA for Labs
Labs
Opportunity in NA
€1.5B
€22B
Merck Millipore
Market
NA
ROW
15

M Merck KGaA
Darmstadt · Germany
Broad and complementary product fit in attractive segments
Laboratory & Academia* Analytical standards Microbiology Antibodies Biochemicals
Merck Millipore Sigma-Aldrich
Bio-/Pharma production
Upstream process Downstream process
Buffers & media
Bioreactors
Chromatography
Filtration
Services
*Key laboratory and academia areas illustrated
16

M Merck KGaA
Darmstadt · Germany
Leverage operational excellence to deliver superior value to customers
Product innovation
Delivering innovative workflow solutions to increase customers’ efficiency
Broad technology and platforms
Recurring winners of renowned innovation awards
Mobius FlexReady CellASIC Amnis
Process innovation
Efficient supply chain for >300,000 products
Best in class customer experience; e.g. 24 hour delivery in major markets
Top-notch customer interface supported by e-commerce platform
e-commerce platform Supply chain
Efficient work flow solutions and unique customer experience
17
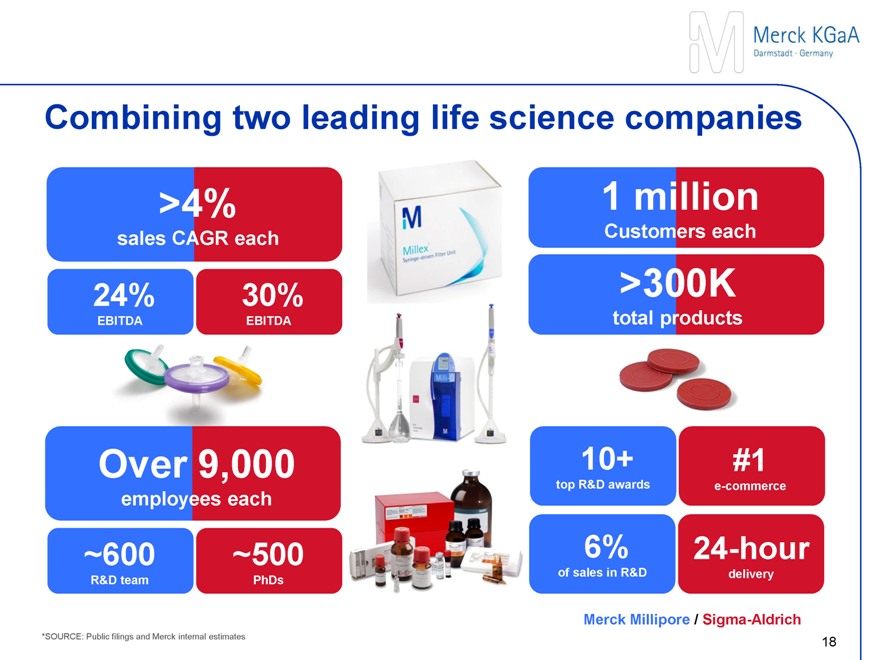
M Merck KGaA
Darmstadt · Germany
Combining two leading life science companies
>4%
sales CAGR each
24% EBITDA
30% EBITDA
1 million
Customers each
>300K
total products
Over 9,000
employees each
~600
R&D team
~500
PhDs
10+
top R&D awards
6%
of sales in R&D
#1
e-commerce
24-hour
delivery
Merck Millipore / Sigma-Aldrich
*SOURCE: Public filings and Merck internal estimates
18

M Merck KGaA
Darmstadt · Germany
Commitment to our local communities
Merck/Merck Millipore Sigma-Aldrich
Habitat for Humanity
Greater Boston
World Health
Organization
GPHF
GLOBAL PHARMA HEALTH FUND E.V.
RESPONSIBLE CARE®
OUR COMMITMENT TO SUSTAINABILITY
Midleton College
Spartam Nactus Es Hanc Exorna
ISO
ISO 50001
ENERGY MANAGEMENT
Charity:water
A Leader in Life Science and Corporate Citizenship
Newsweek
GREEN RANKINGS 2014
ISO
AUTISM SPEAKS
It’s time to listen
STEMpact
GLOBAL100
CDP
CLIMATE PERFORMANCE LEADER 2013
SIGMA-ALDRICH
SCIENCE PARTNERS
346 Year Heritage
80 Year Heritage
19
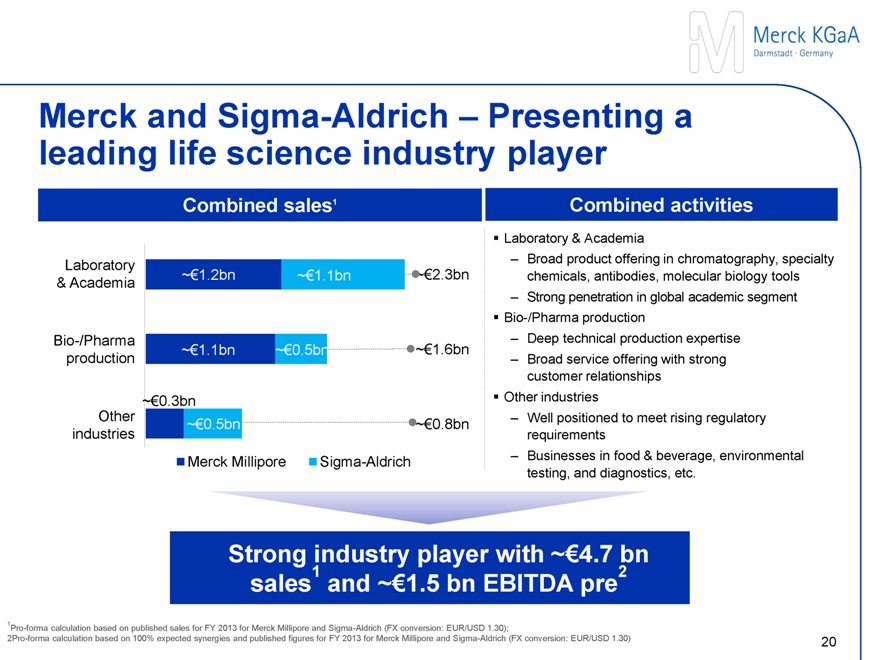
M
Merck KGaA
Darmstadt · Germany
Merck and Sigma-Aldrich – Presenting a leading life science industry player
Combined sales1
Laboratory
& Academia
~€1.2bn
~€1.1bn
~€ 2.3bn
Bio-/Pharma production
~€1.1bn
~€0.5bn
~€ 1.6bn
~€0.3bn
Other industries
~€0.5bn
~€ 0.8bn
Merck Millipore
Sigma-Aldrich
Combined activities
Laboratory & Academia
– Broad product offering in chromatography, specialty chemicals, antibodies, molecular biology tools
– Strong penetration in global academic segment
Bio-/Pharma production
– Deep technical production expertise
– Broad service offering with strong customer relationships
Other industries
– Well positioned to meet rising regulatory requirements
– Businesses in food & beverage, environmental testing, and diagnostics, etc.
Strong industry player with ~€4.7 bn
sales1 and ~€1.5 bn EBITDA pre2
1Pro-forma calculation based on published sales for FY 2013 for Merck Millipore and Sigma-Aldrich (FX conversion: EUR/USD 1.30);
2Pro-forma calculation based on 100% expected synergies and published figures for FY 2013 for Merck Millipore and Sigma-Aldrich (FX conversion: EUR/USD 1.30)
20
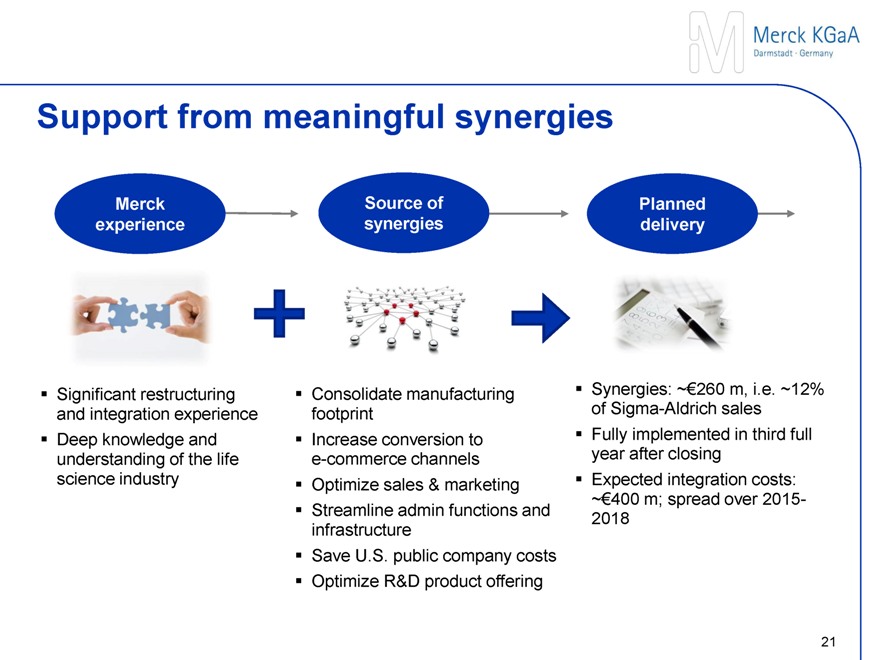
M
Merck KGaA
Darmstadt · Germany
Support from meaningful synergies
Merck experience
Significant restructuring and integration experience
Deep knowledge and understanding of the life science industry
Source of synergies
Consolidate manufacturing footprint
Increase conversion to e-commerce channels
Optimize sales & marketing
Streamline admin functions and infrastructure
Save U.S. public company costs
Optimize R&D product offering
Planned delivery
Synergies: ~€260 m, i.e. ~12% of Sigma-Aldrich sales
Fully implemented in third full year after closing
Expected integration costs: ~€400 m; spread over 2015- 2018
21

M
Merck KGaA
Darmstadt · Germany
Sigma-Aldrich acquisition takes Merck Millipore to the next level
Expanding position in attractive life science industry, poised for sustainable growth
Enhancing value for customers via strengthened offering, reach and operational excellence
Sigma-Aldrich and Merck Millipore will be core earnings contributor to Merck and generate sustainable and growing cash flow
Consistent with Merck’s core acquisition criteria and corporate transformation journey
22

M
Merck KGaA
Darmstadt · Germany
Content for today
Today’s News Highlights
The Combination: Fits Our Strategy
What is Our Responsibility Now?
23
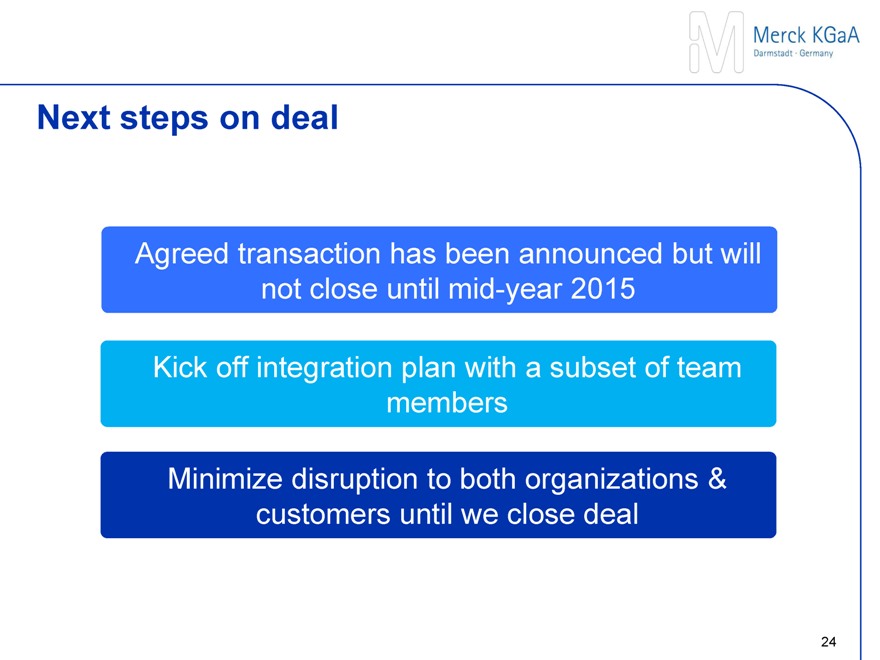
M
Merck KGaA
Darmstadt · Germany
Next steps on deal
Agreed transaction has been announced but will not close until mid-year 2015
Kick off integration plan with a subset of team members
Minimize disruption to both organizations & customers until we close deal
24

M
Merck KGaA
Darmstadt · Germany
Our Merck Millipore responsibility now
Customers, Customers, Customers
Deliver Performance: Close Q3 & FY2014
Avoid Assumptions
25

M
Merck KGaA
Darmstadt · Germany
Our Merck responsibility now
Business as usual
Close the transaction in mid-year 2015
Focus on growth opportunities across all businesses
26

M
Merck KGaA
Darmstadt · Germany
Taking Merck to the next level
In Line with the Group Strategy
Beneficial Diversification of Revenues and Sustainable Profitability
More Choice for More Customers
Product and Process innovation – operational excellence & e-commerce)
27

CONFIDENTIAL – FOR INTERNAL USE ONLY
Questions & Answers