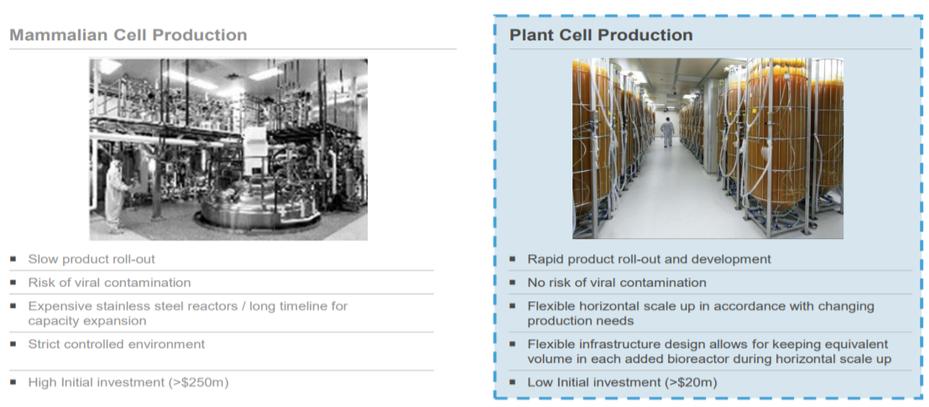
Research and Development Expenses, Net
Research and development expenses were $27.2 million for the nine months ended September 30, 2020, a decrease of $7.8 million, or 22%, compared to $35.0 million of research and development expenses for the nine months ended September 30, 2019. The decrease is primarily due to the completion of two out of the three phase III clinical trials of PRX-102 and reduced costs related to the BALANCE Study as well as a decrease in costs related to manufacturing of our drug in development as some of the manufactured drug product and related costs have been recorded as inventory.
We expect research and development expenses to continue to be our primary expense as we enter into a more advanced stage of preclinical and clinical trials for certain of our product candidates.
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $8.2 million for the nine months ended September 30, 2020, an increase of $1.3 million, or 19%, compared to $6.9 million for the nine months ended September 30, 2019. The increase resulted primarily from a $1.3 million increase in share-based compensation costs.
Financial Expenses, Net
Financial expenses net were $6.8 million for the nine months ended September 30, 2020, an increase of $1.1 million, or 20%, compared to financial expenses net of $5.7 million for the nine months ended September 30, 2019. The increase resulted primarily from expenses related to our outstanding convertible notes equal to $1.3 million.
Liquidity and Capital Resources
Our sources of liquidity include our cash balances and bank deposits. At September 30, 2020, we had $13.5 million in cash and cash equivalents and $27.8 million in bank deposits. We have primarily financed our operations through equity and debt financings, business collaborations, and grants funding.
During the nine months ended September 30, 2020, we completed a private placement of common stock and warrants for net proceeds of approximately $41.3 million. In connection with the offering, we issued 17,604,423 unregistered shares of our common stock at a purchase price per share of $2.485 and warrants to purchase an additional 17,604,423 shares of common stock at an exercise price of $2.36 per share. In September 2020, we received cash proceeds of $472,000 from the exercise of 200,000 warrants.
We believe that our cash and cash equivalents and bank deposits as of September 30, 2020 are sufficient to satisfy our capital needs for at least 12 months from the date that these financial statements are issued. In addition, as of September 30, 2020, we had outstanding 2021 Notes amounting to $57.9 million which are due on November 15, 2021, unless the notes are refinanced, restructured or converted before that date.
We expect that by the maturity date of the 2021 Notes, our cash and cash equivalents and short term deposits, combined with the cash we anticipate to generate from the satisfaction of milestones under the Chiesi US Agreement and from our revenues, will be sufficient to satisfy the 2021 Notes payment. If, prior to the maturity date of the 2021 Notes, we will not have sufficient cash available to satisfy the full principal amount of the outstanding 2021 Notes, and to otherwise finance our future cash needs, our management may pursue corporate collaborations, licensing or similar arrangements, public or private equity offerings, and/or debt financings or restructuring, sell shares under our ATM Sales Agreement, and if necessary, may need to scale back operations, curtail or cease operations. Other than the ATM Sales Agreement, we do not have any commitments for future external funding, except with respect to the development-related payments and milestone payments that may become payable under the Chiesi Agreements. There is no assurance that we will be successful in obtaining the level of financing needed for our operations.
Cash Flows
Net cash used in operations was 18.6 million for the nine months ended September 30, 2020. In response to the COVID-19 pandemic, a higher number of subjects in our ongoing clinical trials opted for home care treatments over in-site treatments which resulted in an immaterial amount of additional expenses. The net loss for the nine months ended September 30, 2020 of $6.9 million was increased by a $15.1 million decrease in contracts liability and a $5.1 million increase in inventories, partially offset by an increase of $3.3 million in accounts payable and accruals, $2.5 million amortization of debt issuance costs and debt discount and a $2.1 million in share-based compensation. Net cash used in investing activities for the nine months ended September 30, 2020 was $27.5 million and