Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
AND RISK FACTORS SUMMARY
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the consolidated financial statements and the related notes included elsewhere in this Form 10-Q and in our Annual Report on Form 10-K for the year ended December 31, 2020. Some of the information contained in this discussion and analysis, particularly with respect to our plans and strategy for our business and related financing, includes forward-looking statements within the meanings of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, including statements regarding expectations, beliefs, intentions or strategies for the future. When used in this report, the terms “anticipate,” “believe,” “estimate,” “expect,” “can,” “continue,” “could,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and words or phrases of similar import, as they relate to our company or our management, are intended to identify forward-looking statements. We intend that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are only predictions and reflect our views as of the date they are made with respect to future events and financial performance, and we undertake no obligation to update or revise, nor do we have a policy of updating or revising, any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events, except as may be required under applicable law. Forward-looking statements are subject to many risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward-looking statements as a result of several factors including those set forth under “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2021.
Examples of the risks and uncertainties include, but are not limited to, the following:
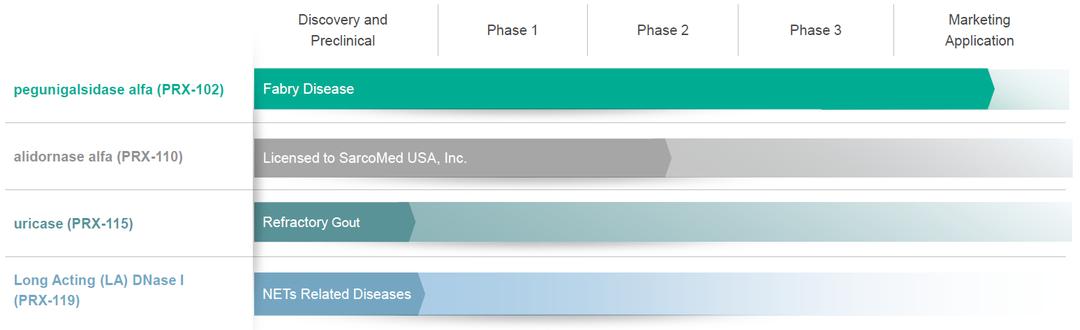
●risks related to the timing and progress of the preparation of an updated BLA addressing the CRL for PRX-102;
●risks related to the timing, progress and likelihood of final approval by the FDA of a resubmitted BLA for PRX-102 and, if approved, whether PRX-102 will be commercially successful;
●the risk that the FDA, the European Medicines Agency, or EMA, or other foreign regulatory authorities may not accept or approve a marketing application we file for PRX-102 or any of our other product candidates;
●failure or delay in the commencement or completion of our preclinical studies and clinical trials, which may be caused by several factors, including: slower than expected rates of patient recruitment; unforeseen safety issues; determination of dosing issues; lack of effectiveness during clinical trials; inability or unwillingness of medical investigators and institutional review boards to follow our clinical protocols; inability to monitor patients adequately during or after treatment; and or lack of sufficient funding to finance our clinical trials;
●the risk that the results of our clinical trials will not support the applicable claims of safety or efficacy and that our product candidates will not have the desired effects or will have undesirable side effects or other unexpected characteristics;
●risks relating to our ability to make required payments under our outstanding convertible notes, including the 2024 Notes, or any other indebtedness as they come due and our ability to obtain additional financing and raise capital as necessary should the regulatory approval process become more extended;
●risks associated with the COVID-19 outbreak and variants, which may adversely impact our business, preclinical studies and clinical trials;
●risks relating to our evaluation and pursuit of strategic alternatives;
●risks relating to our ability to manage our relationship with our collaborators, distributors or partners;
●risks relating to changes to interim, topline or preliminary data from clinical trials that we announce or publish;
●risks related to any transactions we may effect in the public or private equity markets to raise capital to finance future research and development activities, general and administrative expenses and working capital;
●risk of significant lawsuits, including stockholder litigation, which is common in the life sciences sector;
●our dependence on performance by third-party providers of services and supplies;
●the impact of development of competing therapies and/or technologies by other companies;