SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K/A
FOR ANNUAL AND TRANSITION REPORTS PURSUANT TO SECTIONS 13
OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
(MARK ONE)
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2002
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
FOR THE TRANSITION PERIOD FROM __________ TO __________ .
COMMISSION FILE NUMBER: 0-28440
ENDOLOGIX, INC.
(EXACT NAME OF REGISTRANT AS SPECIFIED IN ITS CHARTER)
| | | |
| DELAWARE | | 68-0328265 |
| (STATE OF INCORPORATION) | | (I.R.S. EMPLOYER IDENTIFICATION NO.) |
13900 ALTON PARKWAY, SUITE 122, IRVINE, CALIFORNIA 92618
(ADDRESS OF PRINCIPAL EXECUTIVE OFFICES, INCLUDING ZIP CODE)
REGISTRANT’S TELEPHONE NUMBER, INCLUDING AREA CODE: (949) 457-9546
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
| | | |
| TITLE OF EACH CLASS | | NAME OF EACH EXCHANGE ON WHICH REGISTERED |
| |
|
| NONE | | NONE |
SECURITIES TO BE REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT: COMMON
STOCK, $.001 PAR VALUE.
Indicate by check mark whether the registrant:(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesx Noo
Indicate by a check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.x
Indicate by a check mark whether the registrant is an accelerated filer (as defined in Rule 12b-2 of the Act). Yeso Nox
TABLE OF CONTENTS
PART III
Item 10.Directors and Executive Officers of the Registrant
The following table sets forth information as of March 14, 2003 with respect to our directors and executive officers:
| | | | | | | |
| NAME | | AGE | | POSITION |
| |
| |
|
| Franklin D. Brown | | | 59 | | | Executive Chairman |
| Paul McCormick | | | 49 | | | President and Chief Executive Officer |
| David M. Richards | | | 43 | | | Chief Financial Officer and Corporate Secretary |
| Joseph A. Bishop | | | 38 | | | Vice President, Research and Development |
| Karen Uyesugi | | | 47 | | | Vice President, Clinical and Regulatory Affairs |
| Jeffrey H. Thiel | | | 47 | | | Director |
| Jeffrey F. O’Donnell | | | 43 | | | Director |
| Maurice Buchbinder, M.D. | | | 49 | | | Director |
| Michael R. Henson | | | 57 | | | Director |
| Edward M. Diethrich, M.D. | | | 67 | | | Director |
Franklin D. Brown.Mr. Brown serves as our Executive Chairman and has been a director since 1997. Following the merger with the former Endologix in May 2002, Mr. Brown was our Chief Executive Officer and Chairman until January 2003, when he was elected Executive Chairman. Mr. Brown previously served as the Chairman and Chief Executive Officer of the former Endologix, Inc. since joining that company in 1998. From October 1994 until the sale of the company in September 1997, Mr. Brown served as Chairman, President and Chief Executive Officer at Imagyn Medical, Inc. From 1986 until the sale of the company in 1994, Mr. Brown served as President and Chief Executive Officer of Pharmacia Deltec, Inc., an ambulatory drug delivery company. Mr. Brown also serves on the boards of directors of Triage Medical, Inc., Qualigen, Inc. and ATI Medical, Inc., all private companies.
Paul McCormick. Mr. McCormick is our President and Chief Executive Officer and has been a director since May 2002. Mr. McCormick has more than 24 years in the medical device industry. The majority of his career has been in emerging medical technologies. Mr. McCormick joined the former Endologix in January 1998 as Vice President of Sales and Marketing, and served as President and Chief Operating Officer from January 2001 until the merger in May 2002. He then served in the same position with us until January 2003 when he became President and Chief Executive Officer. Previously, he held various sales and marketing positions at Progressive Angioplasty Systems, a company that was purchased by United States Surgical Corporation, Heart Technology, purchased by Boston Scientific, Trimedyne Inc., and United States Surgical Corporation.
David M. Richards. Mr. Richards joined us in September 1996 and serves as our Chief Financial Officer and Corporate Secretary. From September 1996 to October 2001, Mr. Richards served as our Controller.
Joseph A. Bishop. Mr. Bishop joined us in August 1996 and serves as our Vice President of Research and Development. As part of Radiance Medical Systems Inc., Mr. Bishop served as the Vice President of Operations from August 2000 to May 2002, Director of Manufacturing from May 1998 to August 2000, and from August 1996 to May 1998 held several management and engineering positions. Prior to joining us, Mr. Bishop held several manufacturing supervision positions with Guidant Corporation from June 1986 to August 1996.
Karen Uyesugi. Ms. Uyesugi has 23 years of both domestic and international regulatory experience in the medical device and pharmaceutical industry. The majority of her career has been involved with a wide variety of Class III and Class II medical devices ranging from implantable cardiovascular devices, neurosurgery, and general surgery products. Ms. Uyesugi has served as our Vice President, Clinical and Regulatory Affairs since the merger with the former Endologix in May 2002. Prior to joining the former Endologix in July 1998, Ms. Uyesugi held various positions in regulatory, clinical, and quality assurance at Neuro Navigational Corporation, Trimedyne, Inc., Baxter Healthcare, Shiley Inc., and Allergan Pharmaceuticals.
Jeffrey H. Thiel.Mr. Thiel has served as a Director since 2001. Mr. Thiel joined the Company in October 1996, and had previously served as Chief Executive Officer of the Company from January 1, 2001 until May 29, 2002. He served as President and Chief Operating Officer from September 1999 to December 2000. From February 1999 to September 1999, Mr. Thiel served as Executive Vice President and from October 1996 to February 1999 as Vice President, Operations. From May 1995 to October 1996, Mr. Thiel served as Director of Operations of BEI Medical Systems. Mr. Thiel also serves on the board of directors of Micrus Corporation, a private company.
1
Jeffrey O’Donnell.Mr. O’Donnell has served on the board since June 1998. Mr. O’Donnell served as the Company’s President from January 1998 until March 1999, and Chief Executive Officer from June 1998 until March 1999. From November 1995 to January 1998, Mr. O’Donnell served as the Company’s Vice President, Sales and Marketing. Mr. O’Donnell has served as President and Chief Executive Officer of PhotoMedex since November 1999. From March 1999 to November 1999, Mr. O’Donnell served as the President and Chief Executive Officer of X-Site Medical. From January 1994 to May 1995, Mr. O’Donnell served as the President and Chief Executive Officer of Kensey Nash Corporation, a diversified medical device company. Mr. O’Donnell is a member of the board of directors of Escalon Medical Corporation, a manufacturer and distributor of cardiovascular and ophthalmology devices.
Maurice Buchbinder, M.D.Dr. Buchbinder has served on the board since January 1999. Dr. Buchbinder was a co-founder and member of the board of directors of the (former) Radiance from August 1997 to January 1999. Since 1995, Dr. Buchbinder has served as the Director of Interventional Cardiology at Sharp Memorial Hospital, San Diego, California and as the Director of Interventional Cardiology at the Foundation for Cardiovascular Research, Scripps Memorial Hospital, La Jolla, California. From 1985 to 1995, Dr. Buchbinder served at various intervals as the Professor of Medicine and the Associate Professor of Medicine, Cardiology Division, UCSD Medical Center, San Diego, California. Dr. Buchbinder is Board certified, Diplomat, from the American Board of Cardiovascular Diseases and the American Board of Internal Medicine.
Michael R. Henson.Mr. Henson is a founder of the MedFocus Fund, LLC, a venture capital fund, and has been a general partner since it was formed in November 2000. Mr. Henson joined the Company in February 1992 as President, Chief Executive Officer and Chairman of the Board of Directors, and currently serves as a Director. From June 1997 until March 1999, Mr. Henson served as Chairman of the Board, Chief Executive Officer and President of the Company. Prior to joining the Company, Mr. Henson served as the Chief Executive officer of Endosonics Corporation from 1988 to February 1995, and as Chairman of the Board from February 1993 to November 1996. From April 1983 to February 1988, Mr. Henson served as President and Chief Executive Officer of Trimedyne, Inc., a manufacturer of medical lasers and catheters. Mr. Henson also serves on the board of directors of several private medical device companies including Devax, Inc., Triage Medical, Inc., Endonetics, Inc., and Micrus Corporation.
Edward M. Diethrich, M.D.Dr. Diethrich has served as a Director for the Company since May 2002. Dr. Diethrich was a Director for the former Endologix, Inc. from 1997 until its merger with the Company on May 29, 2002. Dr. Diethrich has been the Medical Director and Chief of Cardiovascular Surgery of the Arizona Heart Hospital since 1997, and has been the Director and Chief of Cardiovascular Surgery at the Arizona Heart Institute from 1971 to the present.
2
Item 11.Executive Compensation
The following table sets forth the salary and bonus earned for the three fiscal years ended December 31, 2002, by our Chief Executive Officers and executive officers for the 2002 fiscal year. All the individuals named in the table are referred to as the “Named Executive Officers.”
Endologix, Inc.
Summary Compensation Table
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Long Term |
| | | | | | | | | | | | | | | Compensation |
| | | | | | | | | | | | | | | Shares |
| | | | | | | Annual Compensation | | Underlying |
| | | | | | | Salary | | Bonus | | Options |
| | | | | | |
| |
| |
|
| Franklin D. Brown (a) | | | 2002 | | | | 196,285 | | | | 47,250 | | | | 20,000 | (h) |
| Chairman and Chief Executive Officer | | | 2001 | | | | — | | | | — | | | | — | |
| | | | 2000 | | | | — | | | | — | | | | — | |
| Paul McCormick (b) | | | 2002 | | | | 119,583 | | | | 29,025 | | | | — | |
| President | | | 2001 | | | | — | | | | — | | | | — | |
| | | | 2000 | | | | — | | | | — | | | | — | |
| Jeffrey H. Thiel (c) | | | 2002 | | | | 125,591 | | | | — | | | | 40,000 | |
| Chief Executive Officer and President | | | 2001 | | | | 218,462 | | | | — | | | | 25,000 | |
| | | | 2000 | | | | 178,286 | | | | 58,500 | | | | 75,000 | |
| Joseph A. Bishop (d) | | | 2002 | | | | 138,715 | | | | 27,298 | | | | 50,000 | |
| Vice President, Research | | | 2001 | | | | 125,000 | | | | — | | | | 15,000 | |
| and Operations | | | 2000 | | | | 106,665 | | | | 26,800 | | | | 30,000 | |
| Paul A. Molloy (e) | | | 2002 | | | | 187,615 | | | | 12,353 | | | | 50,000 | |
| Senior Vice President, Sales | | | 2001 | | | | 166,154 | | | | — | | | | 50,000 | |
| & Marketing | | | 2000 | | | | — | | | | — | | | | — | |
| David M. Richards (f) | | | 2002 | | | | 119,231 | | | | 24,713 | | | | 50,000 | |
| Chief Financial Officer and | | | 2001 | | | | 99,789 | | | | — | | | | 5,000 | |
| Secretary | | | 2000 | | | | 94,343 | | | | 10,700 | | | | 4,000 | |
| Karen Uyesugi (g) | | | 2002 | | | | 93,333 | | | | 26,780 | | | | 70,000 | |
| Vice President Clinical and | | | 2001 | | | | — | | | | — | | | | — | |
| Regulatory Affairs | | | 2000 | | | | — | | | | — | | | | — | |
| (a) | | Mr. Brown currently serves as our Executive Chairman. He served as our Chief Executive Officer and Chairman following the merger with (former) Endologix on May 29, 2002 until January 1, 2003. |
| |
| (b) | | Mr. McCormick currently serves as our Chief Executive Officer and President. He served as our President and Chief Operating Officer following the merger on May 29, 2002 with (former) Endologix until January 1, 2003. |
| |
| (c) | | Mr. Thiel served as the President and Chief Executive Officer until the merger with the (former) Endologix on May 29, 2002. Mr. Thiel continues to serve on our Board. |
| |
| (d) | | Mr. Bishop was elected Vice President of Operations on August 21, 2000 and has served as Director of Manufacturing since May 25, 1998. |
| |
| (e) | | Mr. Molloy served as our Senior Vice President, Sales & Marketing from January 15, 2001 until November 22, 2002. |
| |
| (f) | | Mr. Richards was appointed Chief Financial Officer and Secretary on February 19, 2002 and was acting Chief Financial Officer beginning November 11, 2001. From September 16, 1996 to October 31, 2001, Mr. Richards was Controller. |
| |
| (g) | | Ms. Uyesugi became our Vice President of Clinical and Regulatory Affairs following the Merger with (former) Endologix on May 29, 2002. |
| |
| (h) | | Options were granted before employment as our Chief Executive Officer based upon Board participation. |
3
OPTION GRANTS IN LAST FISCAL YEAR
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | Potential Realizable Value |
| | | | | | | | | | | | | | | | | | | of Options At Assumed |
| | | | | | | | | | | | | | | | | | | Annual Rates of Stock |
| | | | | | | | | | | | | | | | | | | Price Appreciation for |
| | | Individual Grants | | Option Term (4) |
| | |
| |
|
| | | Number of | | % of Total Options | | | | | | | | | | | | | | | | |
| | | Securities | | Granted To | | Exercise of Base | | | | | | | | | | | | |
| | | Underlying Options | | Employees in Fiscal | | Price ($/Share) | | | | | | | | | | | | |
| Name | | Granted (1) | | Year (2) | | (3) | | Expiration Date | | 5%($)(4) | | 10%($)(4) |
| |
| |
| |
| |
| |
| |
|
| Franklin D. Brown | | | 20,000 | | | | — | %(5) | | $ | 1.56 | | | | 2/19/12 | | | $ | 19,621 | | | $ | 49,725 | |
| Paul McCormick | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Jeffrey H. Thiel | | | 25,000 | | | | 7 | | | | 1.55 | | | | 2/7/12 | | | | 24,370 | | | | 61,758 | |
| | | | 15,000 | | | | — | | | | 0.84 | | | | 10/15/12 | | | | 7,924 | | | | 20,081 | |
| | | | 40,000 | | | | 7 | | | | | | | | | | | | 32,294 | | | | 81,739 | |
| Joseph A. Bishop | | | 50,000 | | | | 14 | | | | 1.55 | | | | 2/7/12 | | | | 48,739 | | | | 123,515 | |
| Paul A. Molloy | | | 50,000 | | | | 14 | | | | 1.55 | | | | 2/7/12 | | | | 48,739 | | | | 123,515 | |
| David M. Richards | | | 50,000 | | | | 14 | | | | 1.07 | | | | 7/17/12 | | | | 33,646 | | | | 85,265 | |
| Karen Uyesugi | | | 70,000 | | | | 20 | | | | 0.85 | | | | 8/6/12 | | | | 37,419 | | | | 94,828 | |
| (1) | | The options listed in the table were granted under our 1996 Stock Options/Stock Issuance Plan. The options have a maximum term of ten years measured from the date of grant. Twenty-five percent (25%) of the options are exercisable upon the optionee’s completion of one year of service measured from the date of grant, and the balance are exercisable in a series of successive equal monthly installments upon the optionee’s completion of each additional month of service over the next 36 months thereafter. |
| |
| (2) | | Based upon options granted for an aggregate of 353,000 shares to employees in 2002, including the Named Executive Officers. |
| |
| (3) | | The exercise price may be paid in cash, in shares of our common stock valued at fair market value on the exercise date or through a cashless exercise procedure involving a same-day sale of the purchased shares. |
| |
| (4) | | The 5% and 10% assumed annual rates of compounded stock price appreciation are mandated by rules of the Securities and Exchange Commission. There can be no assurance provided to any executive officer or any other holder of Radiance’s securities that the actual stock price appreciation over the option term will be at the assumed 5% and 10% levels or at any other defined level. Unless the market price of the Common Stock appreciates over the option term, no value will be realized from the option grants made to the executive officers. |
| |
| (5) | | Options granted for service on our Board of Directors. |
4
AGGREGATED OPTION EXERCISES IN LAST FISCAL YEAR
AND FISCAL YEAR-END OPTION VALUES
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Number of Securities | | Value of Unexercised |
| | | | | | | | | | | Underlying Unexercised | | In-the-Money Options at |
| | | Shares Acquired on | | Aggregate Value | | Options at FY-End | | FY-End (2) |
| Name | | Exercise | | Realised $ (1) | | Exercisable | | Unexercisable | | Exercisable | | Unexercisable |
| |
| |
| |
| |
| |
| |
|
| Franklin D. Brown | | | — | | | $ | — | | | | 42,500 | | | | 20,000 | | | $ | — | | | $ | — | |
| Paul McCormick | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Jeffrey H. Thiel | | | — | | | | — | | | | 217,687 | | | | 95,313 | | | | — | | | | 150 | |
| Joseph A. Bishop | | | — | | | | — | | | | 76,512 | | | | 73,488 | | | | — | | | | — | |
| Paul A. McCormick | | | — | | | | — | | | | 76,512 | | | | 73,488 | | | | — | | | | — | |
| David M. Richards | | | — | | | | — | | | | 49,340 | | | | 56,833 | | | | — | | | | — | |
| Karen Uyesugi | | | — | | | | — | | | | — | | | | 70,000 | | | | — | | | | — | |
| (1) | | Based on the fair market value on the date of exercise less the exercise price payable for such shares. |
| |
| (2) | | Based on the fair market value of our common stock at year-end, $0.85 per share, less the exercise price payable for such shares. All of the options for the officers listed had exercise prices in excess of $0.85, with the exception of 15,000 for Mr. Thiel. |
Compensation of Directors
Non-employee directors each receive a fee of $1,000 per quarter, $1,000 for each Board meeting attended and reimbursement for certain travel expenses and other out-of-pocket costs. Members of Committees of the Board each receive an additional fee of $500 for each Committee meeting attended. Non-employee Board members are eligible to receive periodic option grants under the Automatic Option Grant Program in effect under our 1996 Stock Option/Stock Issuance Plan. Each individual who first becomes a non-employee Board member, whether elected by the stockholders or appointed by the Board, automatically will be granted, at the time of such initial election or appointment, an option to purchase 15,000 shares of Common Stock at the fair market value per share of Common Stock on the grant date. Each option has a maximum term of ten years. On the date of each Annual Meeting of Stockholders, each individual who is to continue to serve as a non-employee Board member after the Annual meeting will receive an additional option grant to purchase 15,000 shares of Common Stock, provided such individual has been a member of the Board for at least six months.
Each initial option grant vests four over years, and each annual option grant vests upon the completion of one year of Board service. The option grants also vests immediately upon the optionee’s death or permanent disability or an acquisition of the Company by merger or asset sale or a hostile change in control of the Company.
Officers are appointed to serve at the discretion of the Board of Directors, until their successors are appointed. There are no family relationships among executive officers or directors of Endologix. There are no arrangements or understandings involving any director or any nominee regarding such person’ status as a director or nominee.
Some of our directors received additional compensation as a result of our merger with the (former) Endologix. In recognition of their service to the Company, the compensation committee awarded each of William G. Davis, Edward M. Leonard and Gerard von Hoffman, directors of the Company until May 2002, an option to acquire 25,000 shares of our common stock, which became fully vested on completion of the merger in May 2002. In addition, the compensation committee amended an existing option to acquire 6,000 shares of our common stock held by Mr. Davis to have an exercise period of five years from the date we signed the merger agreement. Pursuant to Mr. Thiel’s employment agreement with the Company, upon his termination as a result of the merger, Mr. Thiel is entitled to thirteen months’ severance pay and continued benefits for one year. Additionally, Mr. Thiel’s stock options that would have vested over the following year vested immediately upon his termination. Finally, the Company forgave a $100,000 loan, together with accrued interest thereon, that the Company made to Mr. Thiel. See “Item 13 – Certain Relationships and Related Transactions: for a more complete description of the loan.
5
Management Contracts and Termination of Employment and Change in Control Agreements
We entered into an employment agreement with Mr. Brown, our Executive Chairman, effective October 18, 2002. The agreement has a three-year term and it automatically renews for successive one-year terms thereafter. The agreement provides for an annual increases in base salary as may be determined by the Compensation Committee of our board of directors. In 2002, as Chairman and CEO, Mr. Brown’s base salary was $300,000 and he was eligible to receive an annual cash bonus of up to 35% of his base salary as well as incentive-based stock options. Commencing January 1, 2003, as Executive Chairman, Mr. Brown’s base salary is $200,000, and he is eligible to receive incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Mr. Brown’s employment without cause, he is entitled to his base salary and continued benefits for six months. Additionally, Mr. Brown’s stock options that would have vested over the following six months will vest immediately upon his termination. Lastly, Mr. Brown would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination. In the event of a change in control, Mr. Brown is entitled to his base salary and continued benefits for twelve months and all of Mr. Brown’s stock options will accelerate and vest and all of our rights to repurchase his restricted stock will terminate. Finally, Mr. Brown would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination.
We entered into an employment agreement with Mr. McCormick, our Chief Executive Officer and President, effective October 18, 2002. The agreement has a three-year term and it has automatically renewed for successive one-year terms thereafter. The agreement has an automatically renewing one-year term and is subject to annual increases in base salary as may be determined by the Compensation Committee of our board of directors. In 2002, as President and Chief Operating Officer, Mr. McCormick’s base salary was $210,000 and he was eligible to receive an annual cash bonus of up to 35% of his base salary as well as incentive-based stock options. Mr. McCormick’s current base salary is $240,000, and he is eligible to receive an annual cash bonus of up to 35% of his base salary as well as incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Mr. McCormick’s employment without cause, he is entitled to his base salary and continued benefits for six months. Additionally, Mr. McCormick’s stock options that would have vested over the following six months will vest immediately upon his termination. Lastly, Mr. McCormick would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination. In the event of a change in control, Mr. McCormick is entitled to his base salary and continued benefits for twelve months and all of Mr. McCormick’s stock options will accelerate and vest and all of our rights to repurchase his restricted stock will terminate. Finally, Mr. McCormick would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination.
We entered into an employment agreement with Mr. Thiel, our Chief Executive Officer, and effective February 1, 1999, as amended December 10, 1999, December 22, 2000, and February 7, 2002. The original agreement had a two-year term and it has automatically renewed for successive one-year terms thereafter. Mr. Thiel’s base salary for fiscal 2002 was $220,000 and he was eligible to receive an annual cash bonus and incentive-based stock options. The agreement included executive fringe benefits as is customary for our other executives. Mr. Thiel’s employment agreement terminated as a result of the merger with (former )Endologix in May 2002. Pursuant to his employment agreement, Mr. Thiel is entitled to thirteen month’ severance pay and continued benefits for one year. Additionally, we granted Mr. Thiel an incentive stock option to acquire 25,000 shares of our common stock at fair market value on the date of grant, and Mr. Thiel’s stock options that would have vested over the following year vested immediately upon his termination. Finally, the Company also forgave a $100,000 loan, together with accrued interest thereon.
We entered into an employment agreement with Mr. Bishop, our Vice President, Research and Development, effective August 21, 2000. The agreement has an automatically renewing one-year term and is subject to annual increases in base salary as may be determined by the Compensation Committee of our board of directors. Mr. Bishop’s base salary is $141,000, and he is eligible to receive an annual cash bonus of up to 30% of his base salary as well as incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Mr. Bishop’s employment without cause, he is entitled to his base salary and continued benefits for the remainder of the current term and all stock options that would have vested over the following year will vest immediately upon his termination. In the event of a change in control, all of Mr. Bishop’s stock options will accelerate and vest and all of our rights to repurchase his restricted stock will terminate.
We entered into an employment agreement with Mr. Molloy, our former Senior Vice President, Sales and Marketing, effective January 15, 2001. The agreement had an automatically renewing one-year term and was subject to annual increases in base salary as may be determined by the Compensation Committee of our board of directors. Mr. Molloy’s base salary under the agreement was $180,000 and he was eligible to receive annual cash bonus of up to 30% of his base salary, as well as incentive-based stock options. The agreement included executive fringe benefits as was customary for our other executives. Following the termination of his employment with the Company on November 22,
6
2002, Mr. Molloy is entitled to receive one year of severance pay and continued benefits. He also received a pro rata portion of the target bonus he was entitled to for 2002.
We entered into an employment agreement with Mr. Richards, our Chief Financial Officer and Secretary, effective October 18, 2002. The agreement has a three-year term and it has automatically renewed for successive one-year terms thereafter. The agreement has an automatically renewing one-year term and is subject to annual increases in base salary as may be determined by the Compensation Committee of our board of directors. Mr. Richards’ base salary is $136,000, and he is eligible to receive an annual cash bonus of up to 30% of his base salary as well as incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Mr. Richards’ employment without cause, he is entitled to his base salary and continued benefits for six months and all stock options that would have vested over the following six months will vest immediately upon his termination. Mr. Richards would also be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination. In the event of a change in control, Mr. Richards is entitled to his base salary and continued benefits for twelve months and all of Mr. Richards’ stock options will accelerate and vest and all of our rights to repurchase his restricted stock will terminate. Finally, Mr. Richards’ would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination.
We entered into an employment agreement with Ms. Uyesugi, Vice President of Clinical and Regulatory Affairs, effective October 18, 2002. The agreement has a three-year term and it has automatically renewed for successive one-year terms thereafter. The agreement has an automatically renewing one-year term and is subject to annual increases in base salary as may be determined by the Compensation Committee of our board of directors. Ms. Uyesugi’s base salary is $170,000, and she is eligible to receive an annual cash bonus of up to 30% of her base salary as well as incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Ms. Uyesugi’s employment without cause, she is entitled to her base salary and continued benefits for six months and all stock options that would have vested over the following six months will vest immediately upon her termination. Lastly, Ms. Uyesugi would be entitled to a prorated payment equal to the target bonus amount for which she would have been eligible for the year of termination. In the event of a change in control, Ms. Uyesugi is entitled to her base salary and continued benefits for twelve months and all stock options will accelerate and vest and all of our rights to repurchase her restricted stock will terminate. Finally, Ms. Uyesugi would be entitled to a prorated payment equal to the target bonus amount for which she would have been eligible for the year of termination
Compensation Committee Interlocks and Insider Participation
From January 2002 until the merger in May 2002, the members of the Compensation Committee were Maurice Buchbinder, M.D., Franklin D. Brown and Jeffrey F. O’Donnell. Jeffrey F. O’Donnell served as our President and Chief Executive Officer until March 1999. Dr. Buchbinder served as our Medical Director on a consulting basis. Following the merger, Dr. Buchbinder and Mr. Brown left the committee and Mr. Henson joined it. Mr. Henson served as our CEO from inception in 1992 until June 1998 and from March 1999 until January 2001. Mr. Henson also served as our President from inception until January 1998 and from March 1999 until September 1999. No other member of the Compensation Committee was at any time during the 2002 fiscal year or at any other time an officer or employee of the Company.
None or our executive officers served on the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our Board of Directors or Compensation Committee.
Board Compensation Committee Report on Executive Compensation
The Compensation Committee of the Board of Directors makes recommendations to the full Board with respect to the base salary and bonuses to be paid to our executive officers each fiscal year. In addition, the Compensation Committee has the authority to administer the Endologix 1996 Stock Option/Stock Issuance Plan with respect to option grants and stock issuances made thereunder to officers and other key employees. The following is a summary of the policies of the Compensation Committee that affect the compensation paid to executive officers, as reflected in the tables and text set forth elsewhere in this Annual Report on Form 10-K/A.
General Compensation Policy.Our compensation policy is designed to attract and retain qualified key executives critical to our success and to provide such executives with performance-based incentives tied to the achievement of certain milestones. One of the Compensation Committee’s primary objectives is to have a substantial portion of each officer’s total compensation contingent upon our performance as well as upon the individual’s contribution to our success as measured by his personal performance. Accordingly, each executive officer’s compensation package is comprised primarily of three elements.
| | • | | base salary which reflects individual performance and expertise and is designed to be competitive with salary levels in the industry; |
| |
| | • | | Variable performance awards payable in cash and tied to the Company’s achievement of certain goals; and |
7
| | • | | long-term stock-based incentive awards that strengthen the mutuality of interests between the executive officers and our stockholders. |
The following are the principal factors that the Compensation Committee considered in establishing the components of each executive officer’s compensation package for the 2002 fiscal year. However, the Committee may in its discretion apply different factors, particularly different measures of financial performance, in setting executive compensation for future fiscal years.
Base Salary.The base salary levels for the executive officers were established by the Board for the 2002 fiscal year on the basis of the following factors: personal performance, the estimated salary levels in effect for similar positions at a select group of companies with which we compete for executive talent, and internal comparability considerations. Although the Compensation Committee reviewed various compensation surveys, the Board did not rely upon any specific survey for comparative compensation purposes. Instead, the Board made its decisions as to the appropriate market level of base salary for each executive officer on the basis of its understanding of the salary levels in effect for similar positions at those companies with which we compete for executive talent. The Compensation Committee on an annual basis will review base salaries, and adjustments will be made in accordance with the factors indicated above.
Annual Incentive Compensation.The Endologix Employee Bonus Plan provides the Board of Directors with discretionary authority to award cash bonuses to executive officers and employees in accordance with recommendations made by the Compensation Committee. The Compensation Committee’s recommendations are based upon the extent to which financial and performance targets (established semi-annually by the Compensation Committee) are met and the contribution of each such officer and employee to the attainment of such targets. For fiscal year 2002, the performance targets for each of the Named Executive Officers included gross sales, cash flow, engineering product goals and regulatory goals. The weight given to each factor varied from individual to individual.
Long-Term Incentive Compensation.The 1996 Stock Option/Stock Issuance Plan also provides the Board with the ability to align the interests of the executive officer with those of the stockholders and provide each individual with a significant incentive to manage Endologix from the perspective of an owner with an equity stake in the business. The number of shares subject to each option grant is based upon the officer’s tenure, level of responsibility and relative position in the Company. We have established general guidelines for making options grants to the executive officers in an attempt to target a fixed number of unvested option shares based upon the individual’s position with Endologix and their existing holdings of unvested options. However, we do not adhere strictly to these guidelines and will vary the size of the option grant made to each executive officer as it feels the circumstances warrant. Each grant allows the officer to acquire shares of our common stock at a fixed price per share (the market price on the grant date) over a specified period of time (up to 10 years from the date of grant). The option normally vests in periodic installments over a four-year period, contingent upon the executive officer’s continued employment with the Company. Accordingly, the option will provide a return to the executive officer only if he or she remains in our employ and the market price of our common stock appreciates over the option term.
CEO Compensation.The Compensation Committee set the base salary for Jeffrey H. Thiel, our Chief Executive Officer from January 1, 2001 until the merger on May 29, 2002, Franklin D. Brown, our Chief Executive Officer from May 29, 2002 until December 31, 2002, and Paul McCormick, our current Chief Executive Officer at a level which is designed to provide a salary competitive with salaries paid to chief executive officers of similarly-sized companies in the industry and commensurate with each such individual’s experience. Mr. Thiel’s experience at the Company and his work helping to create and execute our restructuring plan, searching for and investigating strategic alternatives, and completing a corporate transaction during the fiscal year, were important factors in setting his total compensation. Mr. Brown’s and Mr. McCormick’s experience at the (former) Endologix, given the central role of the CEO in commercializing the technology acquired in the merger, was an important determinant in setting their compensation. The Compensation Committee did not intend to have the base salary component of compensation affected to any significant degree by our performance. As an incentive to participate in the success and increased value of Endologix and to align Mr. Thiel’s interests with the long-term interest of our stockholders, granted Mr. Thiel options prior to the merger with (former) Endologix.
| | | |
| | | Compensation Committee |
| | | |
| | | Michael R. Henson
Jeffrey F. O’Donnell |
8
Audit Committee Report
From January 2002 until the merger with former Endologix in May 2002, the members of the Audit Committee were William Davis, Edward Leonard and Gerard Von Hoffmann. Following the merger, the members of the audit committee are Maurice Buchbinder, M.D., Edward Diethrich, M.D., and Jeffrey F. O’Donnell. The Audit Committee of the Board of Directors (the “Audit Committee”) is composed of independent directors as required and in compliance with the listing standards of the NASDAQ National Market. The Audit Committee operates pursuant to a written charter adopted by the Board of Directors.
Stock Performance Graph
The graph depicted below shows Endologix’s stock price as an index assuming $100 invested on December 31, 1997, along with the composite prices of companies listed on the CRSP Total Return Index for National Association of Securities Dealers Automated Quotation (“NASDAQ”) Stock Market, the J.P. Morgan H&Q Total Return Index for Healthcare Technology Companies (Excluding Biotechnology) (for the period ending December 31, 2001) and the NASDAQ Medical Device Manufacturers’ Index. J.P. Morgan discontinued the J.P. Morgan Index H&Q Index at the end of 2001.
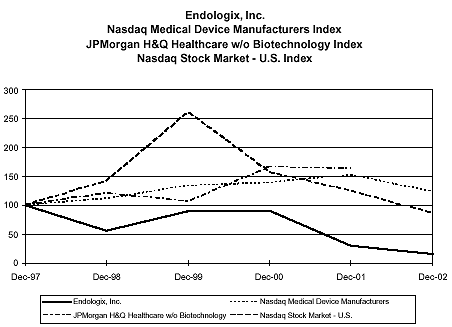
2003 PROXY PERFORMANCE GRAPH DATA
ANNUAL DATA SERIES
SCALED PRICES: Stock and index prices scaled to 100 at 12/31/97
| | | | | | | | | | | | | | | | | |
| | | | | | | JPMorgan H&Q | | | | | | NASDAQ Medical |
| | | | | | | Healthcare w/o | | NASDAQ Stock Market | | Device |
| DATES | | Endologix, Inc. | | Biotechnology | | - U.S. | | Manufacturers |
| |
| |
| |
| |
|
| Dec-97 | | | 100.00 | | | | 100.00 | | | | 100.00 | | | | 100.00 | |
| Dec-98 | | | 55.64 | | | | 121.51 | | | | 140.99 | | | | 111.33 | |
| Dec-99 | | | 89.82 | | | | 106.16 | | | | 261.48 | | | | 134.83 | |
| Dec-00 | | | 90.91 | | | | 166.07 | | | | 157.42 | | | | 139.10 | |
| Dec-01 | | | 29.45 | | | | 163.82 | | | | 124.89 | | | | 152.93 | |
| Dec-02 | | | 15.45 | | | | #N/A | | | | 86.33 | | | | 123.78 | |
9
Note: Assumes $100 invested on 12/31/97 in Endologix, the NASDAQ Stock Market, the NASDAQ Medical Device Manufacturers’ Index and the JP Morgan H&Q Healthcare – Excluding Biotech Index. Assumes reinvestment of dividends.
Section 16(a) Beneficial Ownership Reporting Compliance
The members of the Board of Directors, the executive officers of the Company and persons who hold more than 10% of our outstanding common stock are subject to the reporting requirements of Section 16(a) of the Securities Exchange Act of 1934 which requires them to file reports with respect to their ownership of the common stocks and their transactions in such Common Stocks. Based upon (i) the copies of Section 16(a) reports that Endologix received from such persons for their 2002 fiscal year transactions in the common stock and their common stock holdings and/or (ii) the written representations received from one or more of such persons that no annual Form 5 reports were required to be filed by them for the 2002 fiscal year, we believe that all reporting requirements under Section 16(a) for such fiscal year were met in a timely manner by its executive officers, Board members and greater than ten-percent stockholders.
10
Item 12.Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information known to the Company regarding the ownership of Endologix’s common stock as of April 8, 2003 by: (i) each stockholder known to Endologix to be a beneficial owner of more than five percent (5%) of the Company’s common stock; (ii) each director; (iii) each Named Executive Officer; and (iv) all current directors and officers of Endologix as a group.
| | | | | | | | | |
| | | Number of Shares | | | | |
| | | Beneficially Owned | | Percentage of |
| Name and Address | | (1) | | Outstanding Shares (2) |
| |
| |
|
Bartholomew Family Trust (3)
Myles & Amy Douglas, Trustees
605 Skyhawk Ranch Road
Gardnerville, Nevada 89410 | | | 1,937,000 | | | | 8.1 | % |
C.R. Bard (4)
730 Central Avenue
Murray Hill, N.J. 07974 | | | 1,428,571 | | | | 6.0 | % |
T&L Investments LP(5)
2632 N. 20th Street
Phoenix, AZ 85006 | | | 1,252,375 | | | | 5.2 | % |
| Franklin D. Brown (6) | | | 1,013,750 | | | | 4.2 | % |
| Paul McCormick | | | 452,338 | | | | 1.9 | % |
| Jeffrey H. Thiel (7) | | | 321,450 | | | | 1.3 | % |
| Joseph Bishop (8) | | | 106,467 | | | | * | |
| Paul A. Molloy | | | — | | | | — | |
| David M. Richards (9) | | | 54,376 | | | | * | |
| Karen Uyesugi | | | 150,000 | | | | * | |
| Maurice Buchbinder, MD (10) | | | 939,702 | | | | 3.9 | % |
| Edward Diethrich, M.D. (5) | | | 1,252,375 | | | | 5.2 | % |
Michael R. Henson (11)
13700 Alton Parkway, Suite 158
Irvine, CA 92618 | | | 1,914,681 | | | | 7.9 | % |
| Jeffrey F. O’Donnell (12) | | | 308,976 | | | | 1.3 | % |
| All directors and officers as a group (10 persons) (13) | | | 5,261,740 | | | | 21.0 | % |
| Total Principal Stockholders | | | 9,879,686 | | | | 39.4 | % |
| * | | Represents beneficial ownership of less than 1% |
| |
| (1) | | The number of shares of Common Stocks beneficially owned includes any shares issuable pursuant to stock options that may be exercised within 60 days after April 8, 2003. Shares issuable pursuant to such options are deemed outstanding for computing percentage of the person holder such options but are not deemed to be outstanding for computing the percentage of any other person. |
| |
| (2) | | Applicable percentages are based on shares outstanding on April 8, 2003, plus the number of shares such stockholder can acquire within 60 days of April 8, 2003. |
| |
| (3) | | The Bartholomew Family Trust had shares voting and sole dispositive power of 1,937,000 shares. |
11
| (4) | | Pursuant to a Schedule 13/GA filed with the Commission on June 28, 2002, C. R. Bard reported that it had shares voting and sole dispositive power over 1,428,571 shares. |
| |
| (5) | | T&L Investments LP held 1,252,375. Dr. and Mrs. Edward B. Diethrich hold a total of 98% of the voting and dispositive power over the shares through a 98% ownership of the capital stock of EBDFam, Inc., the general partner in T&L Investments LP. |
| |
| (6) | | Includes 48,750 shares subject to options exercisable within 60 days after April 8, 2003. |
| |
| (7) | | Includes 257,479 shares subject to options exercisable within 60 days after April 8, 2003. Mr. Thiel shares voting and investment power with his spouse as co-trustee with respect to 36,471 shares that are held in a revocable trust. |
| |
| (8) | | Includes 100,052 shares subject to options exercisable within 60 days after April 8, 2003. |
| |
| (9) | | Includes 51,819 shares subject to options exercisable within 60 days after April 8, 2003. |
| |
| (10) | | Includes 218,502 shares subject to options exercisable within 60 days after April 8, 2003, and 18,200 shares held in a family trust. |
| |
| (11) | | Includes 141,146 shares subject to options exercisable with 60 days after April 8, 2003, 488,295 shares held in a family trust, 51,000 shares held in Mr. Henson’s daughter’s custodial account, and 10,500 shares held in Mr. Henson’s spouse’s IRA account. Mr. Henson’s spouse is the beneficial owner of 25,000 shares of the Company’s common stock, to which Mr. Henson disclaims beneficial ownership. |
| |
| (12) | | Includes 304,165 shares subject to options exercisable within 60 days after April 8, 2003. |
| |
| (13) | | Includes 1,121,913 shares subject to options exercisable within 60 days after April 8, 2003. |
Item 13.Certain Relationships and Related Transactions
In May 2002, the Company merged with (former) Endologix. Under the terms of the merger agreement, we issued $0.75 for each share of Endologix common stocks, for an aggregate amount of $8.4 million, and one share of our common stock for each share of Endologix common stock, not to exceed an aggregate issuance of 11,140,541 shares. In addition, we may pay contingent consideration in the amount of $5.6 million in the event pre-market approval, or PMA, is received for Endologix’s PowerLink System on or before March 31, 2004, or $2.8 million if PMA approval is received by June 30, 2004. We may choose to pay the contingent consideration, if payable, in cash or common stock at our sole discretion.
As set forth below, some of the officers and directors of Endologix may be entitled to receive milestone payments due to their ownership of common stock of the (former) Endologix at the time of the merger. The payment would be due if the Company receives FDA Approval of its PMA Application for the PowerLink System on or before March 31, 2004 or June 30, 2004.
| | | | | | | | | |
| | | Amount Payable if PMA | | Amount Payable if PMA |
| | | received on or before | | received on or before |
| Director or Officer | | March 31, 2004 | | June 30, 2004 |
| |
| |
|
| Franklin D. Brown | | $ | 480,000 | | | $ | 240,000 | |
| Paul McCormick | | | 217,500 | | | | 108,750 | |
| Jeffrey H. Thiel | | | 12,000 | | | | 6,000 | |
| Karen Uyesugi | | | 75,000 | | | | 37,500 | |
| Edward B. Diethrich, M.D. | | | 625,000 | | | | 312,500 | |
| Michael R. Henson | | | 635,500 | | | | 317,750 | |
| Jeffrey F. O’Donnell | | | 30,000 | | | | 15,000 | |
12
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this amendment to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| | | ENDOLOGIX, INC. |
| | | | | |
| Date: April 28, 2003 | | By: | | /s/ PAUL McCORMICK |
| | | | |
|
| | | | | Paul McCormick
Chief Executive Officer and Director
(Principal Executive Officer) |
| | | | | |
| Date: April 28, 2003 | | By: | | /s/ DAVID M. RICHARDS |
| | | | |
|
| | | | | David M. Richards
Chief Financial Officer and Secretary
(Principal Financial and Accounting Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, the following persons on behalf of the registrant and in the capacities and on the dates indicated have signed this report below.
| | | | | |
| SIGNATURE | | TITLE | | DATE |
| |
| |
|
/s/ PAUL McCORMICK
(Paul McCormick) | | Chief Executive Officer
and Director
(Principal Executive Officer) | | April 28, 2003 |
| | | | | |
/s/ DAVID M. RICHARDS
(David M. Richards) | | Chief Financial Officer, and
Secretary (Principal
Financial and Accounting
Officer) | | April 28, 2003 |
| | | | | |
/s/ FRANKLIN D. BROWN
(Franklin D. Brown) | | Executive Chairman/Director | | April 28, 2003 |
| | | | | |
/s/ MICHAEL R. HENSON
(Michael R. Henson) | | Director | | April 28, 2003 |
| | | | | |
/s/ JEFFREY H. THIEL
(Jeffrey H. Thiel) | | Director | | April 28, 2003 |
| | | | | |
/s/ JEFFREY F. O’DONNELL
(Jeffrey F. O’Donnell) | | Director | | April 28, 2003 |
| | | | | |
/s/ EDWARD DIETHRICH, M.D.
(Edward Diethrich, M.D.) | | Director | | April 28, 2003 |
| | | | | |
(Maurice Buchbinder, M.D.) | | Director | | April 28, 2003 |
13
CERTIFICATIONS
I, Paul McCormick, certify that:
| 1. | | I have reviewed this annual report on Form 10-K/A of Endologix, Inc. |
| |
| 2. | | Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report; |
| |
| 3. | | Based on my knowledge, the financial statements, and other financial information included in this annual report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this annual report; |
| |
| 4. | | The registrant’s other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and have: |
| | a) | | designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared; |
| |
| | b) | | evaluated the effectiveness of the registrant’s disclosure controls and procedures as of a date within 90 days prior to the filing date of this annual report (the “Evaluation Date”); and |
| |
| | c) | | presented in this annual report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date; |
| 5. | | The registrant’s other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant’s auditors and the audit committee of registrant’s board of directors (or persons performing the equivalent functions): |
| | a) | | all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant’s ability to record, process, summarize and report financial data and have identified for the registrant’s auditors any material weaknesses in internal controls; and |
| |
| | b) | | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal controls; and |
| 6. | | The registrant’s other certifying officers and I have indicated in this annual report whether there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses. |
| | | |
| Date: April 28, 2003 | | /s/ PAUL MCCORMICK |
| | |
|
| | | Paul McCormick
Chief Executive Officer |
14
CERTIFICATIONS
I, David M. Richards, certify that:
| 1. | | I have reviewed this annual report on Form 10-K/A of Endologix, Inc. |
| |
| 2. | | Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report; |
| |
| 3. | | Based on my knowledge, the financial statements, and other financial information included in this annual report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this annual report; |
| |
| 4. | | The registrant’s other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and have: |
| | a) | | designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared; |
| |
| | b) | | evaluated the effectiveness of the registrant’s disclosure controls and procedures as of a date within 90 days prior to the filing date of this annual report (the “Evaluation Date”); and |
| |
| | c) | | presented in this annual report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date; |
| 5. | | The registrant’s other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant’s auditors and the audit committee of registrant’s board of directors (or persons performing the equivalent functions): |
| | a) | | all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant’s ability to record, process, summarize and report financial data and have identified for the registrant’s auditors any material weaknesses in internal controls; and |
| |
| | b) | | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal controls; and |
| 6. | | The registrant’s other certifying officers and I have indicated in this annual report whether there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses. |
| | | |
| Date: April 28, 2003 | | /s/ DAVID M. RICHARDS |
| | |
|
| | | David M. Richards
Chief Financial Officer |
15
EXHIBIT INDEX
The following exhibits are filed herewith:
| | | |
| Exhibit 99.1 | | Certification of Chief Executive Officer, Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| Exhibit 99.2 | | Certification of Chief Financial Officer, Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
