SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
FOR ANNUAL AND TRANSITION REPORTS PURSUANT TO SECTIONS 13
OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
(MARK ONE)
| | | |
| [X] | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2003
OR
| | | |
| [ ] | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO .
COMMISSION FILE NUMBER: 0-28440
ENDOLOGIX, INC.
(EXACT NAME OF REGISTRANT AS SPECIFIED IN ITS CHARTER)
| | | |
DELAWARE
(STATE OF INCORPORATION) | | 68-0328265
(I.R.S. EMPLOYER IDENTIFICATION NO.) |
13900 ALTON PARKWAY, SUITE 122, IRVINE, CALIFORNIA 92618
(ADDRESS OF PRINCIPAL EXECUTIVE OFFICES, INCLUDING ZIP CODE)
REGISTRANT’S TELEPHONE NUMBER, INCLUDING AREA CODE: (949) 595-7200
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT: NONE
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT: COMMON
STOCK, $.001 PAR VALUE.
Indicate by check mark whether the registrant:(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
Indicate by a check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]
Indicate by a check mark whether the registrant is an accelerated filer (as defined in Rule 12b-2 of the Act). Yes [ ] No [X]
1
TABLE OF CONTENTS
As of June 30, 2003, the aggregate market value of the voting stock held by non-affiliates of the Registrant was approximately $63,324,000 (based upon the closing price for shares of the Registrant’s Common Stock as reported by the NASDAQ National Market for June 30, 2003, the last trading date of our second fiscal quarter). Shares of Common Stock held by each officer, director and holder of 5% or more of the outstanding Common Stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
On March 11, 2004, approximately 31,676,945 shares of the Registrant’s Common Stock, $.001 par value, were outstanding.
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act. We have based these forward-looking statements largely on our current expectations and projections about future events and trends affecting the financial condition of our business. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions including, among other things:
| • | | research and development of our products; |
| |
| • | | development and management of our business and anticipated trends on our business; |
| |
| • | | our ability to attract, retain and motivate qualified personnel; |
| |
| • | | our ability to attract and retain customers; |
| |
| • | | the market opportunity for our products and technology; |
| |
| • | | the nature of regulatory requirements that apply to us, our suppliers and competitors and our ability to obtain and maintain any required regulatory approvals; |
| |
| • | | our future capital expenditures and needs; |
| |
| • | | our ability to obtain financing on commercially reasonable terms; |
| |
| • | | our ability to compete; |
| |
| • | | general economic and business conditions; and |
| |
| • | | other risks set forth under “Risk Factors” in this Annual Report on Form 10-K. |
You can identify forward-looking statements generally by the use of forward-looking terminology such as “believes,” “expects,” “may,” “will,” “intends,” “plans,” “should,” “could,” “seeks,” “pro forma,” “anticipates,” “estimates,” “continues,” or other variations thereof, including their use in the negative, or by discussions of strategies, opportunities, plans or intentions.
Unless otherwise required by law, we undertake no obligation to publicly update or revise any forward-looking statements, either as a result of new information, future events or otherwise after the date of this Annual Report on Form 10-K. The forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results to differ in significant ways from any future results expressed or implied by the forward-looking statements.
2
PART I
Item 1. Business
Introduction
We develop, manufacture, sell and market minimally invasive therapies for the treatment of cardiovascular disease. Our products, the PowerLink System and PowerWeb System, are catheter-based alternative treatments for abdominal aortic aneurysm, or AAA. AAA is a weakening of the wall of the aorta, the largest artery of the body. Once AAA develops, it continues to enlarge and if left untreated becomes increasingly susceptible to rupture. The overall patient mortality rate for ruptured AAAs is approximately 75%, making it the 13th leading cause of death in the United States today.
The PowerLink System, and its predecessor the PowerWeb System, is a catheter and endoluminal graft, or ELG, system. The self-expanding stainless steel stent cage is covered by ePTFE, a common surgical graft material. The PowerLink ELG is implanted in the abdominal aorta, which is accessed through the femoral artery. Once deployed into its proper position, the blood flow is shunted away from the weakened or “aneurysmal” section of the aorta, reducing pressure and the potential for the aorta to rupture. We believe that implantation of our products will reduce the mortality and morbidity rates associated with conventional AAA surgery, as well as provide a clinical alternative to many patients that could not undergo conventional surgery.
Prior to developing the PowerLink System, we developed various catheter-based systems to treat cardiovascular disease. We licensed our proprietary Focus balloon technology to Guidant Corporation for use in Guidant’s coronary stent delivery systems. Sales of our PowerLink System in Europe and royalties from the Guidant license are the primary source of our current revenues.
We were incorporated in California in March 1992 under the name Cardiovascular Dynamics, Inc. and reincorporated in Delaware in June 1993. In January 1999, we merged with privately held Radiance Medical Systems, Inc. and changed our name to Radiance Medical Systems, Inc. and in May 2002, we merged with privately held Endologix, Inc., and changed our name to Endologix, Inc.
We file periodic electronic reports with the Securities Exchange Commission. You may read and copy any materials the Company files with the SEC at the SEC’s Public Reference Room at 450 Fifth Street, N.W., Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an internet site (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. We also maintain an internet site (www.endologix.com) at which we make electronic copies of our most recently filed reports available as soon as reasonably practicable after filing such material with the SEC or you may request copies by writing to us at: Endologix, Inc., attention: Investor Relations, 13900 Alton Parkway, Suite 122, Irvine, CA 92618.
Industry Background
Atherosclerosis is a type of arteriosclerosis. Atherosclerosis is the thickening and hardening of arteries. Some hardening of arteries occurs naturally as people grow older. Atherosclerosis involves deposits of fatty substances, cholesterol, cellular waste products, calcium and other substances on the inner lining of an artery. Atherosclerosis is a slow, complex disease that starts in childhood and often progresses with age.
Atherosclerosis also can reduce the integrity and strength of the vessel wall, causing the vessel wall to expand or balloon out. This is an aneurysm. Aneurysms are commonly diagnosed in the aorta, which is the body’s largest artery. The highest incidence of aortic aneurysms occurs in the segment below the opening of the arteries that feed the kidneys, the renal arteries, to where the aorta divides into the two iliac arteries that travel down the legs. Once diagnosed, patients with AAA require either a combination of medical therapy and non-invasive monitoring, or they must undergo a major surgery procedure to repair the aneurysm.
3
For years, physicians have been interested in less invasive methods to treat AAA disease as an alternative to the current standard of surgical repair. The high morbidity and mortality rates of surgery is well-documented, yet medical management for this condition carries the catastrophic risk of aneurysm rupture. Physicians and commercial interests alike began investigating catheter-based alternatives to repair an aneurysm from within, utilizing surgical grafts in combination with expandable wire cages or scaffolds to exclude blood flow and pressure from the weakened segment of the aorta.
We believe the appeal of the PowerLink System for patients, physicians, and health-care payors is compelling. The current standard of treatment is a highly invasive, open surgical procedure requiring a large incision in the patient’s abdomen, withdrawal of the patient’s intestines to provide access to the aneurysm, and the cross clamping of the aorta to stop blood flow. This procedure typically lasts two to four hours and is performed under general anesthesia. This surgery has an operative mortality rate estimated to range from 4% to 10%. In addition, complication rates vary depending upon patient risk classification, ranging from 15% for low-risk patients to 40% for high-risk patients. The average cost of conventional AAA surgery is approximately $28,000, excluding physicians’ fees. The typical recovery period for conventional AAA surgery includes a hospital stay of 10 to 15 days and post-hospital convalescence of 8 to 12 weeks. Our minimally invasive treatment of AAA requires only a small incision in the femoral artery of the leg, minimizing both hospital lengths of stay and the amount of time required for convalescence. Many patients can be treated utilizing only local anesthesia. These benefits led many physicians and commercial concerns to invest time, money and energy to develop these technologies.
Market Opportunity
In the United States alone, an estimated 1.7 million people have an AAA, yet there are only about 220,000 diagnosed each year. Although AAA is one of the most serious cardiovascular diseases, most AAAs are never detected. Approximately 70% to 80% of AAA patients do not have symptoms at the time of initial diagnosis, and AAAs generally are discovered inadvertently during procedures to diagnose unrelated medical conditions. Once an AAA develops, it continues to enlarge and if left untreated, becomes increasingly susceptible to rupture. The overall patient mortality rate for ruptured aneurysms is approximately 75%. We estimate that each year, of those patients diagnosed with AAA, approximately 60,000 undergo conventional surgery, 15,000 to 20,000 are treated with a commercially available ELG and the remainder are put under “watchful waiting”. AAAs generally are more prevalent in people over the age of 60 and are more common in men than in women. The market opportunity outside of the U.S. for these technologies is estimated to be equal in size to that in the U.S.
Patients diagnosed with an AAA larger than five centimeters can be classified into one of three categories: those patients opting for elective surgery, patients who refuse surgery due to the clinical risks of an open procedure, and those who are considered at high risk for an open procedure. These high-risk patients and those refusing surgery will populate the initial patient pool for less invasive techniques. In addition, we believe that ELGs could be applied to as much as 60% of the approximately 60,000 surgeries performed in the United States each year.
| | | In addition to the current pool of potential patients, we expect that the number of persons seeking treatment for their condition will increase based on the following factors: |
| • | | Elderly Population Growth Rate. In 2000, the age 65 and over population in the United States numbered approximately 34 million, or 12.4% of the total population, while growing at a higher rate than the overall U.S. population. In the United States, the vast majority of AAA procedures are performed in patients age 65 and over. |
| |
| • | | Increasing Expectations of Maintaining Active Lifestyles. Baby boomers, on average, exercise more frequently and live more active lifestyles than the average American. As baby boomers age, their more active lifestyle, combined with their strong desire to maintain the quality of life to which they are accustomed, make them increasingly likely to seek minimally invasive alternatives and forego the long convalescence period required by conventional surgical alternatives. |
4
| • | | Increased Screening Will Increase the Patient Pool. Medical journals report that AAA screening at age 65 reduces mortality from AAA disease. A recently published article in the Lancet, a British medical journal, demonstrated that population screening at age 65 can reduce the mortality associated with AAA and that the screening is cost effective. We believe that like colonoscopy or mammography, growth of the use of non-invasive, inexpensive testing and minimally invasive alternatives for treatment of AAA will increase the number of patients seeking screening for this serious medical condition. |
| |
| • | | Improved Endoluminal Devices. We believe improved clinical results of endoluminal repair devices should convert many “watchful waiting” and surgical candidates to ELG procedures. Next generation endovascular AAA repair systems address shortfalls of first and second generation stent grafts, and longer follow-up should enhance acceptance of ELGs as viable therapy. |
Endologix’s Products
PowerLink System
Our PowerLink System consists of a self-expanding stainless steel stent cage covered with ePTFE, a common surgical graft material. The PowerLink ELG is implanted in the abdominal aorta, gaining access by a small incision through the femoral artery. Once deployed into its proper position, the blood flow is shunted away from the weakened, or aneurysmal, section of the aorta, reducing pressure and the potential for the aorta to rupture.
We believe the PowerLink System is a superior design that overcomes the inherent limitations of early generation devices and offers the following advantages:
| • | | One-Piece, Bifurcated ELG. This eliminates many of the problems associated with early generation multi-piece systems. Our products eliminate much of the guidewire manipulation required during the procedure to assemble the component parts of a modular system, thereby simplifying the procedure. In addition, in the follow-up period, there can be no limb detachment with a one-piece system. We believe this should result in continued long- term exclusion of the aneurysm, and improved clinical results. |
| |
| • | | Fully Supported. The main body and limbs of the PowerLink System are fully supported by a stainless steel cage. The stainless steel cage greatly reduces or eliminates the risk of kinking in even tortuous anatomy, eliminating the need for additional procedures or costly peripheral stents. Kinking results in reduced blood flow and limb thrombosis. |
| |
| • | | Unique, Minimally Invasive Delivery Mechanism. The PowerLink System requires only a small surgical incision in one leg. The other leg needs only placement of a non-surgical introducer sheath, three millimeters in diameter. Other ELGs typically need surgical exposure of the femoral artery in both legs to introduce the multiple components. Our unique delivery mechanism and downsizing of the catheter permits our technology to be used in patients having small or very tortuous access vessels. We believe the ease of use of the PowerLink System will improve clinical results, simplify the procedure, and lead to product adoption. |
| |
| • | | Self-Expanding. The stent is formed from a stainless steel variant in a proprietary configuration that is protected by our patent portfolio. This proprietary design expands to the proper size of the target aorta and eliminates the need for hooks or barbs for attachment. Based on our results to date, the PowerLink System has an excellent record for successful deployments. |
| |
| • | | Single Wire and Long Main Body Design. The long main body of the stent cage is made of a single length of wire, shaped into its appropriate configuration. There can be no individual stent migration since the main body is made of a single stent. In addition the long main body places the PowerLink near or at the aortic bifurcation, which minimizes the risk of device migration during the follow up period. |
5
Limitations of Earlier Technology
Our technology is dramatically different than devices currently available commercially. Despite enthusiasm by physicians and patients alike for minimally invasive technology, we believe early generation devices have achieved a limited market penetration due to design limitations and related complications. The published clinical literature details many of the deficiencies of these approaches. In our opinion, early generation devices have the following limitations:
| • | | Assembly Required. Multi-piece, or modular, systems require assembly within the aneurysm sac by mating the various device components. These systems can be more difficult to implant and lead to long operative times. In addition, there are a number of reports of component detachment during the follow-up period. Component detachment can lead to a leak and a re-pressurization of the sac. We believe this results in increased risk of AAA rupture, often requiring a highly invasive, open surgical procedure to repair the detachment. |
| |
| • | | Lack of Support. ELGs with non-supported systems do not have integrated stent cages to support the ELG’s main body or limbs. Due to the tortuous anatomy, non-supported systems have demonstrated a high propensity to kink, particularly in the limbs, leading to thrombosis, which is a blockage of blood flow through the ELG. This requires a second procedure using balloon angioplasty and/or stent placement to correct the condition. This also adds additional device costs and may require a second hospitalization. |
| |
| • | | Use of Individual Stents. Early generation ELGs utilized individual stents sutured together to create an endoskeleton or cage, as opposed to the PowerLink System that is made of a single stent body construction. Over the past two years, reports of suture breakage in other competitor’s devices, resulting in individual stent separation and migration, have been prevalent. This resulted in unusual wear of the polyester graft material leading to perforations of the graft. These patients required surgery to remove the ELG followed by a conventional open surgery procedure. We believe this was the primary cause for one manufacturer to recall its product and to temporarily suspend its U.S. human clinical trial. |
PowerLink Products
Variations in patient anatomies require an adaptive technology. We designed our PowerLink System, with multiple aortic cuffs, limb extensions, bifurcated main body lengths and diameters to simplify procedures, improve clinical results, and drive product adoption by offering physicians a full line of products that are adaptable for treatment of the majority of patients with AAA disease.
PowerLink Infrarenal Bifurcated Systems. The PowerLink Infrarenal Bifurcated System is available in multiple diameters and lengths and can treat patients that have an aortic neck up to 26 millimeters in diameter. The infrarenal device is made of a stainless steel cage covered by thin-walled ePTFE and attaches below the renal arteries.We use thin-walled ePTFE to permit the graft to be used in a wide range of neck diameters, which allows us to treat a wide variety of anatomies with a standard device making it easier for hospital purchasing patterns. During 2003 approximately 55% of our AAA product sales were from infrarenal product sales. Based upon management’s views regarding physician preferences for infrarenal and suprarenal devices and sales trends for our legacy products, we expect infrarenal product sales to account for approximately 50% of our sales when selling both the infrarenal and suprarenal devices in a market. We have obtained the CE Mark for this product in Europe, and are in the follow-up portion of an arm of a Phase II pivotal trial in the United States. We anticipate obtaining United States Food and Drug Administration, or FDA marketing approval for this system in the second half of 2004.
PowerLink Suprarenal Bifurcated System. The PowerLink Suprarenal Bifurcated System is available in multiple diameters and lengths and can treat patients that have an aortic neck up to 26 millimeters in diameter. The suprarenal model has a segment of uncovered stent at the proximal end that permits the operator to place the device more proximally, over the opening of the renal arteries in patients with short or angulated aortic necks. The uncovered stent permits continuous blood flow to the renal arteries, thereby mitigating the risk of kidney complications. We expect this product to account for approximately 40% of our sales. We have obtained the CE Mark for this product in Europe, and are
6
currently enrolling patients in an arm of a Phase II pivotal trial in the U.S. Assuming that our Phase II data for our infrarenal device will support the filing of a supplemental PMA application for the suprarenal device, we believe that we will complete enrollment in the suprarenal arm of the study in the second half of 2004 and we would be approved for marketing in the second half of 2005.
PowerLink Aorto-Uni-iliac Systems. The PowerLink Aorto-Uni-iliac System is available for patients with AAA and either bilateral common iliac artery aneurysms or iliac access conditions that make the placement of any bifurcated graft problematic. As in the PowerLink Bifurcated System, the Aorto-Uni-iliac Systems are available in an infrarenal or suprarenal configuration. We have obtained the CE Mark for these products in Europe.
PowerLink Aortic Cuffs and Limb Extensions. The PowerLink Aortic Cuffs and Limb Extensions permit the physician to treat a greater number of patients. Aortic cuffs are available in 25 to 28 millimeters in diameter and multiple lengths. They also are available in the infrarenal or suprarenal configurations. Limb extensions are 20 millimeters and 16 millimeters in diameter with various lengths, allowing the physician to customize the technology to a given individual. We have obtained the CE Mark for this product in Europe, and these devices are included in the follow-up portion of an arm of a Phase II pivotal trial in the United States. We anticipate obtaining FDA marketing approval for these products in the second half of 2004.
XL Bifurcated System. The XL Bifurcated System is a stent graft that can treat large aortic diameters less than or equal to 32 millimeters in diameter in AAA patients with large aortic necks. We have obtained the CE Mark for this product in Europe.
Clinical Trials
PowerLink and PowerWeb Systems
The PowerLink System and the PowerWeb System have been implanted in clinical trials and post regulatory approval in more than 1,400 patients worldwide. Clinical investigators so far are reporting successful short to long-term results. Trial results from key studies are summarized below.
Pivotal U.S. Phase II Clinical Trial. We believe that the requisite patient enrollment has been achieved in the infrarenal arm of our two arm U.S. pivotal Phase II trial which is studying the PowerLink System for elective endovascular aneurysm repair. As of March 2003, 193 patients had been treated with the infrarenal PowerLink System . On January 8, 2004, Endologix submitted its completed pre-market approval, or PMA, application to the FDA, reporting results on 184 patients. The completed PMA application includes the pivotal clinical trial results as the final step in the modular PMA process. Two of the four previously submitted modules have already been reviewed and accepted. On February 13, 2004, the FDA accepted the PMA application as fileable. Acceptance of the filing means that the FDA has made a threshold determination that the PMA application is sufficiently complete to move forward in the FDA review and approval process. Queries regarding the remaining two modules in addition to the clinical results will be addressed during the final PMA review period. The fileable date marks the continuance of a 180-day review period, which began January 8, 2004. We expect to meet with the FDA in April to discuss any major issues from the agency’s review. At that time, the FDA will also determine the need for an FDA panel meeting before moving forward with its decision regarding U.S. marketing approval.
In December 2002, preliminary data on the pivotal clinical trial was reported in the Journal of Vascular Surgery by study investigator Dr. Jeffrey Carpenter. We believe that the data submitted in the PMA application is not materially different than the preliminary data previously reported. The pivotal clinical study cohort presented in the PMA application consists in part of the following fundamental data:
Enrollment of 259 patients: 193 PowerLink test patients and 66 surgical controls:
| | • | | Deployment Success – 97.8% |
| |
| | • | | Mean Length of Hospital Stay – 3.4 days |
| |
| | • | | 30 day Mortality (2) – 1.09% (not device related) |
7
One year clinical follow up was submitted on 184 patients:
| | • | | AAA Rupture – 0 |
| |
| | • | | Secondary Procedures: (10) – 5.4% to treat endoleaks |
(4) – 2.2% to treat device related obstructions
CT scans were analyzed by an Independent Core Lab:
| | • | | 13.2% endoleak rate at one year, 5.6% endoleak at two years |
| |
| | • | | > 5mm Device Migrations (5) – 3.8% |
| |
| | • | | Wire Fractures – 0 |
| |
| | • | | Material Failure – 0 |
As of March 2004, of the 193 patient enrollees required for the second arm of U.S. Pivotal Phase II clinical trial, 66 patients had been treated with the suprarenal PowerLink System and 46 have completed the required 12 month follow-up period. The infrarenal and suprarenal devices are similar, except that the wire stent in the suprarenal device is extended above the graft material to allow the physician to anchor the top of the device above the renal arteries without obstructing them.
Europe. In September 2002, we completed clinical trials in France. Fourteen centers used the PowerLink System for elective endovascular aneurysm repair in 64 patients recruited during a 13-month interval. Seven patients had intra-operative complications and all were treated successfully. Within one month of follow-up, two adverse events required reintervention. One surgical conversion and one endovascular procedure of proximal cuff placement were performed. The stent graft demonstrated a low endoleak rate and there were no aneurysm ruptures, device migration or materials failure. Survival rate free from severe complication was reported to be 97%.
Japan.Shonin Clinical Trial on the PowerWeb System. In November 2001, we completed the first AAA clinical trial in Japan, including the required 6 month follow up. Six centers used our earlier generation device, the PowerWeb System, for elective endovascular aneurysm repair in 79 patients. The patient age range was 40 to 89 years, with a mean age range of 70 to 79 years. The effectiveness of the PowerWeb System was measured based on whether there was a persistent endoleak, device migration, device damage, or change in aneurysm sac shape over a 6 month follow period. Only 2.9% of all patients and 1.7% of patients implanted with bifurcated devices experienced these problems. Safety of the PowerWeb System was based on adverse events, which occurred in 22 patients after treatment, of which five were device related. The total safety evaluation ratings demonstrated that 68 patients (98.5%) were treated safely. In conclusion, trial results showed a combined rating of effectiveness and safety for 66 patients (95.6%) and the clinicians recommended approval of the PowerWeb System as a low invasive medical device for aneurysms.
Along with Cosmotec Co., Ltd., our Japanese distributor, we contracted with Medical Industries Corp., or MIC, a prestigious in-country caretaker consulting firm to conduct the study. Tokyo Medical University was the Principal Investigative Site with Professor Shin Ishimaru, M.D. as the Principal Investigator. Professor Ishimaru has published extensively and participates as a faculty member for many surgical congresses.
In July 2002, we submitted our PowerWeb System for approval by the Japanese Ministry of Health, or MOH . We were the first company to submit for the Shonin utilizing a complete Japanese patient cohort, and we anticipate that approval will be received in the second half of 2004. We will then file the necessary Partial Change to update the ELG to a current configuration, as well as submit the dossier necessary to be eligible to obtain hospital reimbursement. We expect the device will be eligible for insurance reimbursement sometime in the second half of 2005.
The PowerWeb System is the predecessor to the PowerLink System. The difference between the PowerLink and PowerWeb Systems designs is mainly that wire segments are linked together by shaping the wire in the PowerLink design to form the device, whereas the wire segments are sutured together in the PowerWeb design.
8
RDX System
Prior to our merger with the private company (former) Endologix, Inc., we developed proprietary devices to deliver radiation to prevent the recurrence of blockages in arteries following balloon angioplasty, vascular stenting and other interventional treatments of blockages in coronary and peripheral arteries. We incorporated our proprietary RDX technology into catheter-based systems that deliver beta radiation to the site of a treated blockage in an artery in order to decrease the likelihood of restenosis.
We have completed a U.S. pivotal trial for the RDX System and anticipate submitting safety data only for submission to the FDA in April 2004. Following our 2001 restructuring, we decided not to pursue approval to market the RDX System from the FDA (see Note 14 to consolidated financial statements). As part of the restructuring, we discontinued our pursuit of Japanese clinical trials and stopped sales and marketing of the device in Europe and elsewhere.
We have also completed a feasibility trial for sapheneous vein grafts and peripheral vascular use of the RDX System, and the final report was submitted to the FDA in February 2004. We do not plan to file for a Phase II trial for SVG, peripheral or any other application of the device.
Our Strategy
Our objective is to become a premier supplier of endovascular surgery products that repair diseased or damaged vascular structures as an alternative to open surgery. As part of our core strategy, we intend to:
| • | | Demonstrate a Significant Technology Advantage. Our strategy has been to develop technology that addresses the limitations of the early generation devices, and execute clinical studies to substantiate the superiority of its technology. Being “first to market” has not been an advantage in the AAA market thus far, as other devices approved for marketing in the United States have undergone post-approval recalls and/or temporary sales suspensions. Upon receipt of an FDA marketing approval we will conduct a limited product launch to demonstrate the effectiveness of our sales model and product acceptance. |
| |
| • | | Establish the PowerLink System as the Standard of Care for AAA Repair. We intend to establish our products as the standard of care for elective treatment of AAAs. We plan to coordinate each market rollout by selectively targeting top tier medical institutions and training their staff at our various clinical investigational sites. |
| |
| • | | Execute a Global Marketing Strategy and Address Key Markets. We have obtained the right to affix the CE Mark, and are evaluating our distribution options in Europe. Because of limitations on device reimbursement in Europe, we have sought to limit our capital commitments by establishing sales through a distributor or to sell direct, on a limited basis. We intend to establish a direct sales organization in the United States upon receipt of FDA approval. |
| |
| • | | Increase Public Awareness. When we receive regulatory approval for our technology, we intend to promote our endovascular procedure for patients by trying to increase public awareness of AAA disease and by supporting the merits of early detection and endovascular treatment. Recently published articles report that baseline testing for AAA can reduce the incidence of rupture. |
| |
| • | | Continue to Develop Core Competencies. We believe we have demonstrated core competencies in developing catheter-based solutions that address a large unmet clinical need that we identified after close consultation with key physicians. Our focus at this time is the aortic aneurysm. In the future, we intend to develop additional devices to expand the application of our core competencies. |
| |
| • | | Evaluate Opportunities Based on non-AAA Intellectual Property. We have an extensive patent portfolio that includes not only patents involved with brachytherapy but many patents pertaining to the delivery of substances into the bloodstream of the artery, or direct delivery of |
9
| | | substances into the wall of the artery. We plan on evaluating product opportunities that may be available as a result of these patents. |
Marketing and Sales
PowerLink System
United States.We anticipate a U.S. product launch for the infrarenal PowerLink in the second half of 2004. The primary customer and decision maker for these devices in the U.S. is the vascular surgeon. The market is fairly concentrated with estimates of 1,000 to 1,500 potential general and vascular surgeons, and a limited number of interventional cardiologists and radiologists, in approximately 1,000 hospitals. Upon receipt of an FDA marketing approval we will conduct a limited product launch to demonstrate the effectiveness of our sales model and product acceptance.
Europe. The market for ELGs in Europe is influenced by vascular surgeons, interventional radiologists and, to a lesser extent, interventional cardiologists who perform catheter directed treatment of AAA. The European market is less concentrated than the domestic market. We have obtained the right to affix the CE Mark to our family of PowerLink products. Europe represents a smaller market opportunity due to capitated hospital budgets and a selling price that is typically less than in the U.S. We currently sell our devices through independent distributors or sell direct on a limited basis. We will participate in and share the costs of attending key cardiovascular conferences in Europe. We expect to continue to interface with key opinion leaders in Europe.
Rest of World, excluding Japan. We have obtained marketing approval in a number of countries, including China, Australia, Argentina, Brazil and South Africa and have initial clinical experience in each of these locales.
PowerWeb System
Japan. We believe we will be the first company to enter the Japanese market for ELGs with a commercial device in the second half of 2005, depending upon the Ministry of Health’s approval our device and ELG reimbursement. Cosmotec will market our technology with a combination of clinical specialists and a vascular sales force. Cosmotec has seven sales offices throughout the country and a sales force of over 70 persons.
Legacy Products
In late 2001, three companies published the first clinical study data for drug-coated stents, a competing technology to our radiation catheter system. While our RDX system used beta radiation to treat restenosis resulting from angioplasty procedures, drug coated stents have drugs that inhibit cell proliferation to limit restenosis. Though drug coated stent feasibility trials were on a relatively small cohort of patients, all three companies reported restenosis rates near or at zero percent. Considering the efficacy, ease of use and probable cost effectiveness of drug-coated stents compared to our radiation catheter system, we determined that the market for the radiation based system likely will be limited. The other products we sold at the end of 2001, our Focus technology products, were nearing the end of their marketable lives due to competing products.
As a result, in order to conserve cash and to position ourselves to take advantage of strategic alternatives, we decided in September 2001 to restructure our operations. In December 2001, we discontinued all sales and marketing activities for our Focus technology coronary stents, coronary stent delivery systems, balloon dilatation catheters and RDX radiation therapy catheter systems.
In June 1998, we entered into a technology license agreement with Guidant, an international interventional cardiology products company, granting them a 10 year license to manufacture and distribute stent delivery products using our Focus technology. The original territory for the license was the United States and Canada, but has expanded with the expiration of distribution relations in other countries. Under the agreement, technology developed by either party was to be owned by that party while technology developed jointly was to be owned jointly and included in the license at no additional cost to Guidant. If for any calendar year, after timely written notice by us to Guidant of a shortfall in royalty payments below the
10
annual minimum royalty required, they elect not to pay us at least the minimum royalty, we can cancel the agreement. Also, as Guidant has paid to date the aggregate payment amount required under the contract, they can at any time, with or without cause, terminate the agreement upon thirty days notice. We are entitled to receive royalties on Guidant’s sales. In the year ended December 31, 2003, we recorded $2.6 million in royalties. We anticipate that royalties from Guidant will continue to decline substantially in 2004 and thereafter as competition from drug-coated stents, which began in the second quarter of 2003, increases and as Guidant introduces more non-licensed products.
Manufacturing
We manufacture our endovascular products at our facilities in Irvine, California. Based upon our forecasted production requirements, we believe that our current manufacturing facilities will be sufficient for our needs through 2005.
Our current manufacturing process is labor intensive and involves shaping and forming a stainless steel wire cage, sewing graft material together to form the outside skin of the device and suturing the graft material on to the cage. While we plan to make process improvements in 2004 to reduce the labor component of the production, the majority of the direct cost comes from the ePTFE graft material, which has pricing set by our agreement with Impra, Inc.
Impra, Inc.In February 1999, we entered into a supply agreement with Impra, Inc., a subsidiary of C.R. Bard, Inc for the supply of ePTFE. The supply agreement expires in December 2007 and is automatically renewable on a year-by-year basis, for additional one-year periods, unless either party gives the other party notice of its intention not to renew within 30 days from the expiration date of the applicable renewal period. Under the terms of the agreement, we have agreed to purchase certain quantities of ePTFE for our endovascular products, with built in annual quantity increases. In January 2002, the agreement was amended, increasing the minimum purchase requirements for 2002 and thereafter, and increasing the prices each year after 2002 according to the general increase in the Consumer Price Index, with an additional increase if we receive FDA approval to commercially distribute our devices in the U.S.
Legacy Products. We stopped production of our non-PowerLink products, in Irvine, California in December 2001. We also terminated our manufacturing agreement with Bebig GmbH for the production of RDX catheters in Europe due to our decision to restructure operations in late 2001.
Patents and Proprietary Information
We have an aggressive program to develop intellectual property in the United States, Europe and Asia. We are building a portfolio of apparatus and method patents covering various aspects of our current and future technology. In the AAA area, we have 14 U.S. patents issued, covering 296 claims, and 9 pending U.S. patent applications. Our current AAA area patents begin expiring in 2017 and the last patent expires in 2019. We intend to continue to file for patent protection to strengthen our intellectual property position as we continue to develop our technology.
In addition to our AAA intellectual property, we own or have the rights to 37 issued U.S. patents, one issued European patent and one Japanese patent relating to intravascular radiation, stents, and various catheter technologies. The non AAA patents begin expiring in 2012 and the last patent expires in 2018. Our technology license to Guidant is supported by seven U.S. patents and one Japanese patent. These patents begin expiring in 2014 and the last patent expires in 2016.
Our policy is to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications to protect technology, inventions and improvements that are important to the development of our business. We require our employees, consultants and advisors to execute confidentiality agreements in connection with their employment, consulting or advisory relationships. We also require employees, consultants and advisors who may work on our products to agree to disclose and assign to us all inventions conceived during the work day, using our property or which relate to our business. We cannot assure you that any issued patents will provide competitive advantages for our products or that they will not be challenged or circumvented by our competitors.
11
Competition
We believe that the primary competitive factors in the market for AAA devices are:
| • | | clinical effectiveness, as defined by product safety, ease of use, reliability and durability; |
| |
| • | | price; |
| |
| • | | availability of third-party reimbursement; |
| |
| • | | distribution capability; |
| |
| • | | time necessary to develop products successfully; and |
| |
| • | | ability to receive regulatory approval. |
We expect that significant competition in the endovascular grafting market will develop over time. Three manufacturers, Medtronic, W.L. Gore, and Cook have obtained FDA marketing approval for their ELGs. However, we believe that our technology offers significant clinical advantages over currently available technologies. The cardiovascular device industry is marked by rapid technological improvements and, as a result, physicians are quick to seize upon improved designs. Significant market share and revenue can be captured by designs demonstrating superior clinical outcomes. We believe deliverability of the device, dependability of the clinical results and the durability of the product design are the most important product characteristics. The PowerLink System is the only available one-piece bifurcated, fully supported ELG, and we believe that the PowerLink System will offer improved deliverability, dependability, and durability.
Companies that are “first to market” in the United States with a new technique must underwrite the significant and expensive challenge of physician training and proctoring. In addition, the first generation companies have borne these costs as well as costs of addressing reimbursement issues. We believe that our PowerLink System represents next generation technology that is poised to take advantage of a well-prepared market. The chart below compares the PowerLink System with competing AAA systems.
Below is a chart that details the stent graft characteristics of the minimally-invasive AAA stent grafts being sold in Europe and/or the United States. We believe that earlier generation technology devices experienced material failures and complications due to their reliance on multi-piece designs, designs that did not include a stent cage to support the entire graft, or designs with hooks or barbs to hold their devices in place (See the section above entitled “Limitations of Earlier Technology” for a discussion of these factors). Because our PowerLink and PowerWeb stent grafts are single piece, fully supported designs that use radial force and column strength to maintain fixation, we believe that our grafts may offer us a competitive advantage. Because material failures that have been experienced typically occur over the first few years of the implant, and we have a limited amount of long-term clinical data, it is difficult to determine if our design will continue to show a low incidence of material failure.
12
| | | | | | | | | |
| | | Stent Graft Characteristics
| | |
Mfg.
| | Single Piece?
| | Fully Supported?
| | Fixation
| | FDA Status
|
Endologix
PowerLink | | Yes | | Yes | | Radial Force &
Column Strength | | In Trial |
| |
Medtronic
AneuRx, Talent | | No | | Yes | | Radial Force | | Approved -AneuRx
In Trial - Talent |
| |
Cook
Zenith | | No | | Yes | | Radial Force & Barbs | | Approved |
| |
WL Gore
Excluder | | No | | Yes | | Radial Force & Barbs | | Approved |
| |
Johnson and Johnson
Fortron | | No | | Yes | | Radial Force & Barbs | | In Trial |
In addition to the competitors mentioned above, the following devices are known to have development programs for new devices: Terumo-Vascutek and Boston Scientific.
Most of our competitors have substantially greater capital resources than we do and also have greater resources and expertise in the areas of research and development, obtaining regulatory approvals, manufacturing and marketing. We cannot assure you that competitors and potential competitors will not succeed in developing, marketing and distributing technologies and products that are more effective than those we will develop and market or that would render our technology and products obsolete or noncompetitive. We may be unable to compete effectively against such competitors and other potential competitors based upon their manufacturing, marketing and sales resources.
Any product we develop that gains regulatory clearance or approval will have to compete for market acceptance and market share. An important factor in such competition may be the timing of market introduction of competitive products. Accordingly, we expect the relative speed with which we can develop products, gain regulatory approval and reimbursement acceptance and supply commercial quantities of the product to the market to be an important competitive factor. In addition, we believe that the primary competitive factors for products addressing AAA include deliverability, safety, efficacy, ease of use, reliability, service and price. We also believe that physician relationships, especially relationships with leaders in the interventional cardiology community, also are important competitive factors.
Third-Party Reimbursement
In the United States, medical institutions are the primary purchasers of our products. Medical institutions then bill various third-party payors, such as Medicare, Medicaid, and other government programs and private insurance plans, for the healthcare services and products provided to patients. Government agencies, private insurers and other payors determine whether to provide coverage for a particular procedure and reimburse hospitals for medical treatment at a fixed rate based on the diagnosis-related group established by the U.S. Centers for Medicare and Medicaid Services, or CMS. The fixed rate of reimbursement is based on the procedure performed, and is unrelated to the specific devices used in that procedure.
Reimbursement of interventional procedures utilizing our products currently is covered under a diagnosis-related group. Some payors may deny reimbursement if they determine that the device used in a treatment was unnecessary, inappropriate or not cost-effective, experimental or used for a non-approved indication. Therefore, we cannot assure you that reimbursement for any new procedure we develop will be available to hospitals and other users of our products, or that future reimbursement policies of payors will not hamper our ability to sell new products on a profitable basis.
In October 2000, the CMS issued a guideline regarding the proper coding of our procedures for billing purposes. CMS instructed that code 39.71, for endovascular graft repair of aneurysm, be utilized. For purposes of hospital reimbursement, the majority of patients using the PowerLink System device will be classified under DRG 110, Major Cardiovascular Procedures with Complication/Comorbidity. In the
13
latest data published by CMS, the national average reimbursement for DRG 110 exceeded $21,000. In Europe, reimbursement for the procedure, including the device, typically comes from the hospital’s general fund and is usually from about half to three-quarters of the reimbursement available in the U.S.
Outside the United States, market acceptance of products depends partly upon the availability of reimbursement within the prevailing healthcare payment systems. Reimbursement systems vary significantly by country, and by region within some countries, and reimbursement approvals must be obtained on a country-by-country basis. Reimbursement is obtained from a variety of sources, including government sponsored healthcare and private health insurance plans.
Some countries have centrally organized healthcare systems, but in most cases there is a degree of regional autonomy either in deciding whether to pay for a particular procedure or in setting the reimbursement level. The manner in which new devices enter the healthcare system depends on the system. There may be a national appraisal process leading to a new procedure or product coding, or it may be a local decision made by the relevant hospital department. The latter is particularly the case where a global payment is made that does not detail specific technologies used in the treatment of a patient. Most foreign countries also have private insurance plans that may reimburse patients for alternative therapies. Although not as prevalent as in the United States, managed care is gaining prevalence in certain European countries.
Upon obtaining the Shonin in Japan, equivalent to FDA approval of a PMA application in the U.S., our next step will be to establish the level of reimbursement, which will drive hospital pricing. We believe that the level of reimbursement in Japan will approximate that of the United States.
We believe that reimbursement in the future will be subject to increased restrictions such as those described above, both in the United States and in other countries. The general escalation in medical costs has led to and probably will continue to create increased pressures on the health care providers to reduce the cost of products and services, including any products we develop. If third party reimbursements are inadequate to provide us with a profit on any products we develop, our efforts to develop and market products in the future may fail.
Government Regulation
The manufacturing and marketing of our products are subject to extensive and rigorous government regulation in the United States and in other countries. Prior to commercialization, new products must meet rigorous governmental agency requirements for pre-clinical and clinical testing and patient follow-up. Federal regulations control the ongoing safety, efficacy, manufacture, storage, labeling, record-keeping, and marketing of all medical devices. We cannot sell or market our products without U.S. or foreign government regulatory approvals.
If a medical device manufacturer establishes that a newly developed device is “substantially equivalent” to a legally marketed Class I or Class II device, or to a Class III device that the FDA has not called for a PMA, the manufacturer may seek clearance from the FDA to market the device by filing a premarket notification with the FDA under Section 510(k) of the Federal Food, Drug, and Cosmetic Act. All of the 510(k) clearances received for our catheters were based on substantial equivalence to legally marketed devices. We cannot assure you that the FDA will grant us timely 510(k) clearance for any of our future products or significant modifications of our existing products. In addition, if the FDA has concerns about the safety or effectiveness of any of our products, it could act to withdraw approval or clearances of those products or request that we present additional data.
If substantial equivalence cannot be established, or if the FDA determines the device or the particular application for the device requires a more rigorous review to assure safety and effectiveness, the FDA will require the manufacturer to submit a PMA which must be reviewed and approved by the FDA prior to sales and marketing of the device in the United States. The PMA process is significantly more complex, expensive and time consuming than the 510(k) clearance process and typically requires the submission of clinical data. The PowerLink System is subject to this PMA process.
14
FDA regulations require us to register as a medical device manufacturer with the FDA. Additionally, the California Department of Health Services, or CDHS, requires us to register as a medical device manufacturer within the state. Because of this, the FDA and the CDHS inspect us on a routine basis for compliance with QSR regulations. These regulations require that we manufacture our products and maintain related documentation in a prescribed manner with respect to manufacturing, testing and control activities. We have undergone and expect to continue to undergo regular QSR inspections in connection with the manufacture of our products at our facilities. Further, the FDA requires us to comply with various FDA regulations regarding labeling. The Medical Device Reporting laws and regulations require us to provide information to the FDA on deaths or serious injuries alleged to have been associated with the use of our devices, as well as product malfunctions that likely would cause or contribute to death or serious injury if the malfunction were to recur. In addition, the FDA prohibits an approved device from being marketed for unapproved applications.
Failure to comply with applicable regulatory requirements can, among other consequences, result in fines, injunctions, civil penalties, suspensions or loss of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. In addition, government regulations may be established in the future that could prevent or delay regulatory clearance or approval of our products. Delays in receipt of clearances or approvals, failure to receive clearances or approvals or the loss of previously received clearances or approvals would have a material adverse effect on our business, financial condition and results of operations.
We are subject to other federal, state and local laws, regulations and recommendations relating to safe working conditions, laboratory and manufacturing practices. We cannot accurately predict the extent of government regulation that might result from any future legislation or administrative action. Failure to comply with regulatory requirements could have a material adverse effect on our business, financial condition and results of operations.
Our international sales are subject to regulatory requirements in the countries in which our products are sold. The regulatory review process varies from country to country and may in some cases require the submission of clinical data. We most likely would rely on distributors in such foreign countries to obtain the requisite regulatory approvals. We cannot assure you, however, that we would obtain such approvals on a timely basis or at all. In addition, the FDA must approve the export to certain countries of devices which require a PMA but are not yet approved domestically.
In Europe, we need to comply with the requirements of the Medical Devices Directive, or MDD, and affix the CE Mark on our products to attest to such compliance. To achieve compliance, our products must meet the “Essential Requirements” of the MDD relating to safety and performance and we must successfully undergo verification of our regulatory compliance, or conformity assessment, by a Notified Body selected by us. The level of scrutiny of such assessment depends on the regulatory class of the product.
In December 1996, we received ISO 9001/EN46001 certification from our Notified Body with respect to the manufacturing of all of our products in our Irvine facilities. In February 2003, we received ISO 9001:1994 and ISO 13485:1996 certification. These certifications demonstrate that we manufacture our products in accordance with certain international quality requirements. A manufacturer must receive ISO 9001/EN46001 certification prior to applying for the CE Mark of specific products. We are subject to continued supervision by our Notified Body and will be required to report any serious adverse incidents to the appropriate authorities. We also must comply with additional requirements of individual nations. Failure to maintain compliance required for the CE Mark could have a material adverse effect upon our business, financial condition and results of operations. We cannot assure you that we will be able to achieve or maintain such compliance on all or any product or that we will be able to produce products timely and profitably while complying with the MDD and other regulatory requirements.
Product Liability
The manufacture and marketing of medical devices carries the risk of financial exposure to product liability claims. Our products are used in situations in which there is a high risk of serious injury or death. Such risks will exist even with respect to those products that have received, or in the future may receive, regulatory approval for commercial sale. We are currently covered under a product liability insurance policy with coverage limits of $3.0 million per occurrence and $3.0 million per year in the aggregate. We cannot assure you that our product liability insurance is adequate or that such insurance
15
coverage will remain available at acceptable costs. We also cannot assure you that we will not incur significant product liability claims in the future. A successful claim brought against us in excess of its insurance coverage could have a material adverse effect on our business, financial condition and results of operations. Additionally, adverse product liability actions could negatively affect the reputation and sales of our products and our ability to obtain and maintain regulatory approval for our products, as well as substantially divert the time and effort of management away from our operations.
Employees
As of December 31, 2003, we had 41 employees, including sixteen in manufacturing, nine in research and development, seven in clinical affairs, two in sales and marketing and seven in administration. We believe that the success of our business will depend, in part, on our ability to attract and retain qualified personnel. Our employees are not subject to a collective bargaining agreement, and we believe we have good relations with our employees.
Research and Development
We spent $6.7 million in 2003, $6.2 million in 2002, and $14.6 million in 2001 on research and development, including clinical studies. During 2003, we spent $6.1 million on the development of PowerLink AAA products and $561,000 on the development of the RDX.
Our focus is to continually develop innovative and cost effective medical device technology for the treatment of aortic aneurysms, specifically abdominal aortic aneurysms. To achieve the dynamics required to rapidly implement these projects, our research and development is structured into three main development areas: New Product Development, Current Product Enhancements and Process Improvements. The objective is to bring a specific focus to each critical area of development and to facilitate multiple projects on parallel paths.
Current Projects
| • | | PowerLink XL. It is estimated that 10% of the potential AAA patients require a larger endoluminal graft device than our current 28 mm device. The PowerLink XL is a 34mm bifurcated device, designed to compete in this market. The scope of this project consists of two design variations, the PowerLink Bifurcated Assembly, and the PowerLink Bifurcated Suprarenal Assembly. |
| |
| • | | TDC Delivery Catheter. The goal of this project is to improve the performance of the delivery catheter for simpler and quicker deployment of the endoluminal graft, while remaining compatible with all of our current stent-graft designs. |
Item 2. Properties
Currently, we lease facilities aggregating approximately 20,000 square feet in Irvine, California under various lease agreements that expire in March 2005. We believe that our facilities are adequate to meet our operational requirements through the term of our lease.
Item 3. Legal Proceedings
On September 15, 1999, EndoSonics Corporation, which was a wholly-owned subsidiary of Jomed N.V. until July 2003, filed a complaint for declaratory relief in the Superior Court in Orange County, California, claiming that under a May 1997 agreement between the parties, EndoSonics had rights to combine our Focus balloon technology with EndoSonics’ ultrasound imaging transducer on the same catheter with a coronary vascular stent. In February 2001 the court ruled in our favor, ruling that Jomed-EndoSonics had no such rights to include a stent with the Focus balloon and ultrasound imaging transducer. Under the judgment, we were entitled to recover approximately $468,000 of our legal fees and costs we had previously expensed, plus interest. In May 2001, Jomed-EndoSonics appealed the judgment, and in January 2003 the appeals court upheld the judgment in our favor. In February 2003, we agreed to accept
16
payment of the judgment for legal fees and costs of $468,000, which was recorded as a reduction to general and administrative expenses, and interest due of $94,000, all of which was collected by March 31, 2003.
In July 2002, we terminated our contracts with two of our European distributors of PowerLink products for non-performance. In October 2002, we commenced an arbitration proceeding against the distributors to recover delinquent receivables of $376,000. In response, the distributors filed counterclaims for breach of contract, intentional and negligent misrepresentation and concealment of material facts in which they claim damages of $1.0 million. In February 2003, the parties agreed to a mutual release of claims made in the arbitration action and signed a new distribution agreement. The European distributors paid $320,000 to us in full settlement of delinquent receivables, net of product returns for $47,000 and expense reimbursement of $17,000. We also accepted a one-time exchange of products valued at $80,000.
We are a party to ordinary disputes arising in the normal course of business. Management is of the opinion that the outcome of these matters will not have a material adverse effect on our consolidated financial position, results of operations or cash flows.
Item 4. Submission of Matters to a Vote of Security Holders
The annual meeting of stockholders was conducted on October 28, 2003. The following actions were taken at this meeting:
a. In the election of directors, the following is a tabulation of the votes:
| | | | | | | | | | | | | |
| | | Number of Shares
|
Name
| | For
| | Withheld
| | Broker Non-Votes
|
| Franklin D. Brown | | | 22,154,754 | | | | 1,703,706 | | | | — | |
| Edward M. Diethrich | | | 22,174,188 | | | | 1,684,272 | | | | — | |
The following directors continued their terms on the Board following the annual meeting: Maurice Buchbinder, M.D., Paul McCormick, Jeffrey O’Donnell and Michael Henson (Filling a vacancy, Gregory D. Waller was appointed to the Board on November 11, 2003. Michael Henson resigned from the Board and was replaced by Roderick de Greef on November 26, 2003.).
| | b. | | Approval of amendment to Certification of Incorporation to increase the number of authorized shares of common stock from 30,000,000 to 50,000,000 shares. The following is a tabulation of the votes: |
| | | | | | | | | | | | | |
| | | Number of Shares
|
For
| | Against
| | Withheld
| | Broker Non-Votes
|
| 22,274,149 | | | 574,940 | | | | 1,009,371 | | | | — | |
| | c. | | Approval of amendment to Employee Stock Purchase Plan to increase the number of authorized shares of common stock purchasable thereunder from 200,000 to 400,000 shares. The following is a tabulation of the votes: |
| | | | | | | | | | | | | |
| | | Number of Shares
|
For
| | Against
| | Withheld
| | Broker Non-Votes
|
| 14,992,601 | | | 787,538 | | | | 1,058,499 | | | | 7,019,822 | |
17
| | d. | | Ratification of PricewaterhouseCoopers LLP as independent auditors of the Company for the fiscal year ending December 31, 2003. The following is a tabulation of the votes: |
| | | | | | | | | | | | | |
| | | Number of Shares
|
For
| | Against
| | Withheld
| | Broker Non-Votes
|
| 22,790,471 | | | 56,610 | | | | 1,011,375 | | | | — | |
The total outstanding shares available for voting at the meeting was 27,976,540.
PART II
ITEM 5. Market for Registrant’s Common Equity and Related Stockholder Matters
Our common stock commenced trading on the NASDAQ National Market on June 20, 1996 and is traded under the symbol “ELGX.” The following table sets forth the high and low sale prices for our common stock as reported on the NASDAQ National Market for the periods indicated.
| | | | | | | | | |
| | | HIGH
| | LOW
|
| Year Ended December 31, 2002 | | | | | | | | |
| First Quarter | | $ | 2.10 | | | $ | 1.25 | |
| Second Quarter | | | 1.43 | | | | .87 | |
| Third Quarter | | | 1.20 | | | | .72 | |
| Fourth Quarter | | | 1.35 | | | | .69 | |
| Year Ended December 31, 2003 | | | | | | | | |
| First Quarter | | $ | 1.91 | | | $ | .91 | |
| Second Quarter | | | 3.44 | | | | 1.50 | |
| Third Quarter | | | 4.15 | | | | 2.77 | |
| Fourth Quarter | | | 4.04 | | | | 3.43 | |
| Year Ending December 31, 2004 | | | | | | | | |
| First Quarter (through March 11, 2004) | | $ | 7.26 | | | $ | 3.73 | |
On March 11, 2004 the closing sale price of our common stock on the NASDAQ National Market was $5.50 per share and there were 317 record holders of our common stock.
Dividend Policy
We have never paid any dividends. We currently intend to retain all earnings, if any, for use in the expansion of our business and therefore do not anticipate paying any dividends in the foreseeable future.
18
ITEM 6.Selected Financial Data
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31,
|
| | | 1999
| | 2000
| | 2001
| | 2002
| | 2003
|
| | | | | | | (In thousands, except per share data) | | | | |
Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
| Revenue: | | | | | | | | | | | | | | | | | | | | |
| Product | | $ | 3,856 | | | $ | 2,139 | | | $ | 1,111 | | | $ | 834 | | | $ | 1,395 | |
| License | | | 2,855 | | | | 6,800 | | | | 6,528 | | | | 6,565 | | | | 2,595 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total revenue | | | 6,711 | | | | 8,939 | | | | 7,639 | | | | 7,399 | | | | 3,990 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Cost of sales: | | | | | | | | | | | | | | | | | | | | |
| Cost of product sales | | | 2,823 | | | | 1,465 | | | | 1,149 | | | | 460 | | | | 625 | |
| Cost of sales from restructuring (2) | | | — | | | | — | | | | 601 | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total cost of sales | | | 2,823 | | | | 1,465 | | | | 1,750 | | | | 460 | | | | 625 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Gross profit | | | 3,888 | | | | 7,474 | | | | 5,889 | | | | 6,939 | | | | 3,365 | |
| Operating costs and expenses: | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 8,610 | | | | 11,508 | | | | 14,605 | | | | 6,155 | | | | 6,711 | |
| Marketing and sales | | | 1,989 | | | | 842 | | | | 1,305 | | | | 982 | | | | 787 | |
| General and administrative | | | 2,468 | | | | 3,097 | | | | 2,582 | | | | 2,435 | | | | 2,083 | |
| Charge for acquired in-process research and development (1) | | | 4,194 | | | | — | | | | — | | | | 4,501 | | | | — | |
| Restructuring charges (2) | | | — | | | | — | | | | 4,617 | | | | 168 | | | | — | |
| Minority interest | | | (6 | ) | | | (26 | ) | | | (65 | ) | | | (27 | ) | | | (16 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Total operating costs and expenses | | | 17,255 | | | | 15,421 | | | | 23,044 | | | | 14,214 | | | | 9,565 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Loss from operations | | | (13,367 | ) | | | (7,947 | ) | | | (17,155 | ) | | | (7,275 | ) | | | (6,200 | ) |
| Other income | | | 2,587 | | | | 2,484 | | | | 1,514 | | | | 708 | | | | 285 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Net loss | | $ | (10,780 | ) | | $ | (5,463 | ) | | $ | (15,641 | ) | | $ | (6,567 | ) | | $ | (5,915 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Basic and diluted net loss per share | | $ | (0.98 | ) | | $ | (0.46 | ) | | $ | (1.20 | ) | | $ | (0.33 | ) | | $ | (0.23 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Shares used in computing basic and diluted net loss per share | | | 10,951 | | | | 11,749 | | | | 13,086 | | | | 19,718 | | | | 25,845 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | |
| | | December 31,
|
| | | 1999
| | 2000
| | 2001
| | 2002
| | 2003
|
| | | (In thousands) |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 2,051 | | | $ | 6,311 | | | $ | 3,327 | | | $ | 2,606 | | | $ | 4,402 | |
| Marketable securities available-for-sale | | | 20,004 | | | | 24,046 | | | | 16,983 | | | | 7,104 | | | | 8,377 | |
| Working capital | | | 9,793 | | | | 23,202 | | | | 15,111 | | | | 9,411 | | | | 15,020 | |
| Total assets | | | 29,873 | | | | 38,454 | | | | 23,330 | | | | 33,907 | | | | 35,343 | |
| Accumulated deficit | | | (40,333 | ) | | | (45,796 | ) | | | (61,437 | ) | | | (68,004 | ) | | | (73,919 | ) |
| Total stockholders’ equity | | | 25,111 | | | | 35,240 | | | | 19,758 | | | | 31,476 | | | | 33,875 | |
| (1) | | The charges for acquired in-process research and development for the year ended December 31, 1999 relates to our acquisition of the former Radiance Medical Systems, Inc. The charge for acquired in-process research and development for the year ended December 31, 2002 relates to our merger with the former Endologix, Inc. These charges represent the portion of the purchase price allocated to the acquired research and development projects, which, at the date of the acquisition, were in process, had not reached technological feasibility and had no alternative future use (Note 2 to the Consolidated Financial Statements). |
| (2) | | Due to the competitive market, in order to conserve cash prior to filing a Pre-Market Approval Application with the FDA for our radiation catheter, or RDX system, and to take advantage of strategic alternatives, we decided in September 2001 to restructure our operations. The restructuring plan included the discontinuance of product manufacturing and marketing, Japanese clinical trials for the RDX system, and new research and development projects, and the involuntary termination of 55 employees. As a result of the restructuring plan, we recorded a $344,000 charge, comprised of |
19
| | | manufacturing facility set up and sub-license fees and non-cancelable commitments under the agreements with our third party manufacturer in Europe, Bebig GmbH, $20,000 in other non- cancelable commitments, $601,000 for the write-off of inventory that would not be used to fulfill outstanding catheter and stent technology product orders, $1.1 million for employee termination benefits, and $42,000 for other exit costs (Note 14 to the Consolidated Financial Statements). |
| |
| | | In addition, we concluded that certain RDX technology equipment and intangible assets, previously acquired in fiscal 1999 related to the RDX technology, were impaired resulting in a charge of $390,000 and $2.1 million. We concluded the assets would not generate future cash flows. Because we also decided to cease manufacturing of our other product lines, subject to fulfillment of outstanding orders, we recorded a charge of $40,000 for equipment used in the production of other catheter and stent technology products. We also wrote off $269,000 for the carrying value of furniture, computers, software and leasehold improvements that were no longer being used. During the fourth quarter of 2001, the Company completed its evaluation of its facility needs and recorded a $309,000 restructuring charge for non-cancelable lease commitments, net of estimated sublease income of $256,000. |
| |
| | | During the fourth quarter of 2002, we reassessed our restructuring accrual for non-cancelable lease commitments in light of diminished opportunity for sublease arrangements prior to the lease term expirations in October 2003, and recorded an additional $168,000 restructuring charge. |
Summarized Quarterly Data (unaudited)
| | | | | | | | | | | | | | | | | |
| | | March 31
| | June 30
| | September 30
| | December 31
|
| | | (In thousands, except per share amounts) |
2003: | | | | | | | | | | | | | | | | |
| Product sales | | $ | 490 | | | $ | 295 | | | $ | 285 | | | $ | 325 | |
| Total revenues | | | 1,162 | | | | 989 | | | | 919 | | | | 920 | |
| Gross profit | | | 905 | | | | 881 | | | | 786 | | | | 793 | |
| Net loss | | | (1,190 | ) | | | (1,653 | ) | | | (1,492 | ) | | | (1,580 | ) |
| Basic and diluted net loss per share | | | (0.05 | ) | | | (0.07 | ) | | | (0.05 | ) | | | (0.06 | ) |
2002(1): | | | | | | | | | | | | | | | | |
| Product sales | | $ | — | | | $ | 140 | | | $ | 387 | | | $ | 307 | |
| Total revenues | | | 1,768 | | | | 1,940 | | | | 2,133 | | | | 1,558 | |
| Gross profit | | | 1,699 | | | | 1,857 | | | | 1,963 | | | | 1,420 | |
| Net income (loss) | | | 548 | | | | (4,862 | ) | | | (1,178 | ) | | | (1,075 | ) |
| Basic net income (loss) per share | | | 0.04 | | | | (0.28 | ) | | | (0.05 | ) | | | (0.04 | ) |
| Diluted net income (loss) per share | | | 0.04 | | | | (0.28 | ) | | | (0.05 | ) | | | (0.04 | ) |
(1) In the quarters ended June 30, and September 30, 2002, we recorded a charge of $4.4 million and $63,000, respectively, for acquired in-process research and development relating to our merger with the former Endologix. These charges represent the portion of the purchase price allocated to the acquired research and development projects, which, at the date of the acquisition, were in process, had not reached technological feasibility and had no alternative future use (Note 2 to the Consolidated Financial Statements).
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read in conjunction with “Selected Consolidated Financial Data” and our Consolidated Financial Statements and the related notes included in this Annual Report on Form 10-K. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of various factors including the risks we discuss in “Risk Factors” and elsewhere in this Annual Report on Form 10-K.
20
Overview
Our Business
We are engaged in the development, manufacture, sales and marketing of minimally invasive therapies for the treatment of vascular disease. Our primary focus is the development of the PowerLink System, a catheter-based alternative treatment to surgery for AAA. AAA is a weakening of the wall of the aorta, the largest artery of the body. Once AAA develops, it continues to enlarge and if left untreated becomes increasingly susceptible to rupture.
The PowerLink System is a catheter and endoluminal graft, or ELG system. The self-expanding stainless steel cage is covered by ePTFE, a common surgical graft material. The PowerLink ELG is implanted in the abdominal aorta, gaining access through the femoral artery. Once deployed into its proper position, the blood flow is shunted away from the weakened or “aneurismal” section of the aorta, reducing pressure and the potential for the aorta to rupture. We believe that implantation of the PowerLink System will reduce the mortality and morbidity rates associated with conventional AAA surgery.
We completed Japanese clinical trials for our AAA technology in November 2001 and have submitted for Japanese MOH approval to commercialize the product. As the clinical trial in Japan was completed in 2001, only minor expenses relating to the clinical trial are included in our results for 2003. We believe that Japanese MOH review should be completed in the second half of 2004. We will then file the necessary partial change to update the ELG to a current configuration, as well as submit the dossier necessary to be eligible to obtain hospital reimbursement. We expect the device will be eligible for insurance reimbursement sometime in the second half of 2005.
We believe that the requisite patient enrollment has been achieved in the infrarenal arm of our two arm U.S. pivotal Phase II trial which is studying the PowerLink System for elective endovascular aneurysm repair. On January 8, 2004, we submitted our completed PMA application to the FDA. The completed PMA application includes the pivotal clinical trial results as the final step in the modular PMA process. Two of the four previously submitted modules have already been reviewed and accepted. On February 13, 2004, the FDA accepted the PMA application as fileable. Acceptance of the filing means that the FDA has made a threshold determination that the PMA application is sufficiently complete to move forward in the FDA review and approval process. Queries regarding the remaining two modules in addition to the clinical results will be addressed during the final PMA review period. The fileable date marks the continuance of a 180-day review period, which began January 8, 2004. During this review period, Endologix expects to meet with the FDA in April to discuss any major issues from the agency’s review. At that time, the FDA will also determine the need for an FDA panel meeting before moving forward with its decision regarding U.S. marketing approval, which we anticipate in the second half of 2004.
We are continuing to enroll patients in the other arm of our Phase II U.S. clinical trial, for a suprarenal version of the PowerLink System, to support a PMA application with the FDA in order to market it in the United States. We have to follow all of the patients for 12 months, following their enrollment in the study, before final data collection and submission for PMA to the FDA. The infrarenal and suprarenal devices are similar, except that the wire stent in the suprarenal device is extended above the graft material to allow the physician to anchor the top of the device above the renal arteries without obstructing them.
Prior to the acquisition of the former Endologix and the restructuring that occurred during the third and fourth quarters of 2001 (see below under the captions “Merger with Former Endologix, Inc.” and “Company Restructuring” and Notes 2 and 14 to the Consolidated Financial Statements), we were researching, developing and marketing a radiation therapy catheter for the treatment of blockages in arteries after angioplasty, or restenosis. Prior to that we developed, manufactured and marketed other catheter and stent products for treatment of cardiovascular disease.
Over the past few years, our source of revenues has shifted gradually from direct sales of catheter and stent products to royalties from licenses of our stent delivery technology. In June 1998, we licensed Guidant Corporation rights to manufacture and distribute products using our Focus technology for the delivery of stents. In exchange, we received milestone payments based upon the transfer of know-how to Guidant, and continue to receive royalty payments based upon the sale of products by Guidant using the Focus technology. The payments under the Guidant license are the primary source of our existing revenues. See Note 5 to the Consolidated Financial Statements for more information on the Guidant agreement.
21
We have experienced an operating loss for each of the last five years. Our results of operations have varied significantly from quarter to quarter, and we expect that our results of operations will continue to vary significantly in the future. Our operating results depend upon several factors, including:
| • | | the timing and amount of expenses associated with clinical testing and development of the PowerLink system and remaining clinical testing of the radiation catheter system; |
| |
| • | | the progress and success of clinical trials and regulatory approvals; |
| • | | varying product sales by Guidant Corporation, our licensee; |
| |
| • | | our ability to penetrate markets following regulatory approval; and, |
| |
| • | | outcomes from future partnering or technology acquisition agreements, if any. |
During 2004, we will continue enrolling patients in a pivotal clinical trial in the U.S., begin one or two other trials in the U.S. and prepare for and begin actively marketing our AAA products in the U.S. As a result, we anticipate that our expenses will be substantially higher and result in operating losses through at least 2004.
Company Restructuring
In late 2001, three companies published the first clinical study data for drug-coated stents, a competing technology to our radiation catheter system. While our RDX system uses beta radiation to treat restenosis resulting from angioplasty procedures, drug coated stents have drugs that inhibit cell proliferation to limit restenosis. Though drug coated stent feasibility trials were on a relatively small cohort of patients, all three companies reported restenosis rates near or at zero percent. Considering the efficacy, ease of use and probable cost effectiveness of drug-coated stents compared to our radiation catheter system, we determined that the market for the radiation based system likely will be limited.
As a result, in order to conserve cash and to position ourselves to take advantage of strategic alternatives, we restructured our business and later decided not to file a PMA for the radiation catheter system but to still complete the clinical studies. We submitted the final reports for the coronary and saphenous vein graft feasibility trials to the FDA in the first quarter of 2003 and expect to submit the final reports for the remaining studies, the pivotal coronary and peripheral trials, in the first quarter of 2004.
Merger with Former Endologix, Inc.
Reasons for the Merger
In September 2001, as part of a restructuring plan driven by the success of drug-coated stents, we began investigating other medical device technologies for commercialization. In the fourth quarter of 2001, we began discussions with Endologix, Inc. (“former Endologix”), a privately held developer and manufacturer of the PowerLink System, an endoluminal stent graft for minimally invasive treatment of AAAs. Based on our investigation of the PowerLink System, we believed that it was a novel device for treatment of abdominal aortic aneurysms, and that clinical results to date indicated that the PowerLink System had several features and benefits that may provide a better clinical outcome in comparison to devices that were currently on the market. We believed that the acquisition of former Endologix’s technology would provide us with a new and unique medical device technology for a promising and potentially lucrative market.
Merger Transaction
In May 2002, we acquired all of the capital stock of former Endologix. We paid stockholders of former Endologix $0.75 cash for each share of former Endologix common stock, for an aggregate of $8.4 million, and issued one share of our common stock for each share of former Endologix common stock, for an aggregate of 11,140,541 shares.
In addition, we agreed to pay contingent consideration in the amount of $5.6 million in the event a PMA approval is received in the U.S. for the PowerLink System on or before March 31, 2004, or $2.8 million if a PMA approval is received by June 30, 2004. We may choose to pay the contingent consideration, if payable, in cash or common stock at our sole discretion. As of December 31, 2003, a
22
PMA approval has not yet been obtained and such contingent consideration has not been recorded in the consolidated financial statements.
In the course of negotiations of the merger, we agreed to forgive a loan of $100,000 and accrued interest of $37,000 owed by our former chief executive officer, as an incentive for him to assist with the post-closing transition and integration issues given that he would no longer have an ongoing executive management position with us. As a result of this arrangement, we expensed $137,000 to administrative expenses.
We accounted for the acquisition for as a purchase under SFAS No. 141, “Business Combinations.” In accordance with SFAS No. 141, we allocated the purchase price based on the fair value of the assets acquired and liabilities assumed. In the merger, we acquired, in addition to the net tangible assets of the business, intangible assets such as the PowerLink and PowerWeb (an earlier version of the PowerLink) technologies, both developed and in-process, the Endologix trade name and PowerLink and PowerWeb trademarks, and goodwill. We employed valuation techniques reflecting recent guidelines from the AICPA on approaches and procedures for identifying and allocating the purchase price to assets to be used in research and development activities, including acquired in-process research and development, or IPR&D. To value IPR&D and developed technology, we estimated their future net cash flows and discounted them to their present value. To value trademarks and tradenames, we estimated the royalties that would have been paid for their use and discounted them to their net present value.
To determine the proper allocation of purchase price to technology assets, we first determined whether technological feasibility had been reached for a particular technology based upon whether it had been approved for sale by the appropriate regulatory body, or, in the absence of regulatory approval, whether there existed any material costs yet to be incurred, material changes to the technology to be completed or material risks of approval for sale. Then, we considered whether the technology had any alternative future uses.
If technological feasibility of projects had not been reached and the technology had no alternative future uses, we considered the technology to be IPR&D. The IPR&D is comprised of technological development efforts aimed at the discovery of new, technologically advanced knowledge, the conceptual formulation and design of possible alternatives, as well as the testing of process and product cost improvements. Specifically, these technologies included, but were not limited to, research and development efforts towards U.S. commercialization and expansion of the PowerLink product line to include a larger size of the device.
We then estimated that we would spend $6,700 to complete the regulatory process for U.S. commercialization of the PowerLink System by mid-2004. We also estimated that we would spend $6,600 to complete the research and development and regulatory approval process for a larger size PowerLink System for commercialization in Europe by late 2002, and in the U.S. by mid-2007.
We then determined the weighted average stage of completion for IPR&D projects was approximately 60% for U.S. commercialization of the PowerLink System and 33% for the development and commercialization of the larger size of the PowerLink System as of merger date. The cash flows from revenues forecasted in each period are reduced by related expenses, capital expenditures, the cost of working capital, and an assigned contribution to the core technologies serving as a foundation for the research and development. The discount rates applied to the individual technology’s net cash flows were 40%, based upon the level of risk associated with a particular technology and the current return on investment requirements of the market.
The amount of merger consideration allocated to IPR&D was then determined by estimating the stage of completion of each IPR&D project at the date of the merger, estimating the cash flows for the future research and development, clinical trials and release of products employing these technologies, all as described above, and discounting the net cash flows to their present values. As a result of the foregoing determinations, we expensed the portion of the purchase price allocated to IPR&D of $4.5 million during the year ended December 31, 2002.
We also determined the fair value of developed technology at the merger date to be $14.1 million, which represents the acquired, aggregate fair value of individually identified technologies that were fully
23
developed at the time of the merger. As with the IPR&D, the developed technology was valued using the income approach and a discount rate of 30%, in context of the business enterprise value of the former Endologix. We determined a value of $2.7 million for trademarks and tradenames based upon the estimated royalty that would have to be paid for the right to use these assets if they had not been acquired by us, and a discount rate of 35%. The residual amount of $3.6 million was allocated to goodwill. The developed technology that we acquired consisted of a large diameter (34 mm) cuff, which would be used to adapt a PowerLink System to patients with larger diameter aortas, and the PowerLink System. Although we do not believe that there has been a material change to the development timeline or costs for the technologies our development of the European market for these technologies has taken longer than we expected. As a result, we have not realized the sales revenue and operating results originally projected for 2003 and do not expect to meet the projected results for 2004 due to a later than expected U.S. regulatory approval date for the PowerLink System. We originally projected U.S. regulatory approval for the infrarenal PowerLink System in the first half of 2004 but it appears that approval will not occur, if it is granted, until the second half of 2004. As a result, we believe that product sales and operating results for 2004 will be adversely affected. We do not, however, believe that the projected results over the lives of the products will be adversely affected. Failure to achieve projected results, which could for example result from delays in the development of the technology, or our inability to gain regulatory approval in key markets will adversely affect our future operating results and financial condition and may result in a write-down or write-off of intangible assets acquired in the merger. The trademarks and trade names have an indefinite life and the developed technology is being amortized over ten years. See Note 2 to the consolidated financial statements for further description of the accounting for the merger.
Significant Accounting Policies and Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to collectibility of customer accounts, whether the cost of inventories can be recovered, the value assigned to and estimated useful life of intangible assets, the realization of tax assets and estimates of tax liabilities, contingent liabilities and the potential outcome of litigation. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of its consolidated financial statements:
| • | | allowances for accounts receivable and inventory; |
| |
| • | | long-lived assets, including intangible assets; |
| |
| • | | indefinite lived assets; and, |
| |
| • | | income taxes. |
We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. These estimates are based on our review of the aging of customer balances, correspondence with the customer, and the customer’s payment history. If additional information becomes available to us indicating the financial condition of the customer is deteriorating, additional allowances may be required. We write down our inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand, as driven by economic and market conditions, and the product’s shelf life. If actual demand, or economic or market conditions are less favorable than those projected by management, additional inventory write-downs may be required. We revised our estimate of demand for our Focus technology products in 2001, which resulted in the write-down of inventory. We record an impairment charge, or expense, for long-lived assets whenever events or changes in circumstances indicate that the value recorded for the asset may not be recoverable. Future changes in operations, such as our decision to discontinue new sales of our Focus product, adverse market conditions or the introduction of competing technologies, such as drug-coated stents, among other things, could cause us to write down the asset (i.e., record an expense) to better reflect our current estimate of its value. Our goodwill will be tested for impairment annually, or more frequently if events or changes in circumstances indicate that the goodwill is impaired. Factors that may impact whether there is a potential impairment include a significant decrease in our stock price and our evaluation of a control premium that may be used when estimating our total fair value. Our stock price may continue to decline, or other factors may arise, which could result in goodwill impairment in future periods.
We reduce our deferred tax assets to zero due to uncertainties concerning the future realization of the related tax benefits, primarily due to our history of losses. In the event we were to determine that we would be able to realize some or all of the tax benefit of the deferred tax assets, the valuation allowance would be reduced, resulting in increased income in the period such determination was made.
24
Results of Operations
Comparison of Years Ended December 31, 2002 and 2003
Product Sales. Sales increased 67% to $1.4 million in the year ended December 31, 2003 from $834,000 in the year ended December 31, 2002. While adversely impacted by lower U.S. clinical trial sales, product revenue for 2003 significantly increased, primarily due to the impact in 2003 of a full year of PowerLink product sales, compared with seven months of PowerLink product sales in 2002, following the merger with former Endologix. Our U.S. clinical trial sales for 2003 decreased, compared with 2002 as we completed enrollment in the infrarenal arm of our pivotal U.S. clinical trial in the first quarter of 2003, and we stopped enrollment in the suprarenal arm of the trial for six months while we awaited review of the interim results by our data safety monitoring board, which occurred in November 2003. We anticipate that product sales for 2004 will be substantially higher than for 2003, assuming that we receive FDA approval to market our PowerLink infrarenal products in the second half of 2004.
License Revenue.License revenue decreased 60% to $2.6 million in the year ended December 31, 2003 from $6.6 million in the year ended December 31, 2002. Our technology license agreement with Guidant resulted in $2.3 million and $6.0 million in royalties in 2003 and 2002, respectively. We recognized $196,000 in minimum royalties in 2002 and $261,000 in minimum royalties in 2003, under our agreement with Escalon Medical Corporation (“Escalon”). We currently do not expect to receive more than the minimum royalties due under the Escalon agreement, which we will recognize as revenue when cash is collected due to the uncertainty of collection. We recognized $360,000 in deferred distributor fees in 2002 and none in 2003 under a distribution agreement with Cosmotec Ltd. of Japan (“Cosmotec”), regarding the distribution of our radiation therapy products in Japan through our joint venture. In December 2002 we agreed with Cosmotec not to distribute the products and to dissolve the joint venture, at which time we recognized the remaining deferred distributor fee of $299,000 as revenue.
Changes in Guidant’s sales of licensed products will significantly impact future revenues. Although the license agreement with Guidant expires in June 2005, if at any time Guidant discontinued selling licensed products, we would not receive royalties from them in excess of the minimum annual amount of $250,000. In September 2002, we believe that Guidant replaced certain licensed products with unlicensed products in the U.S. market and, as a result, royalties on licensed products sales decreased materially from quarter to quarter, thereafter. We believe that the introduction of drug-coated stents in the first half of 2003 has also had a material negative effect on Guidant’s sales of licensed products, and we anticipate a continuing reduction in royalties from Guidant in 2004.
Cost of Product Revenue. The cost of product revenue increased 36% to $625,000 in the year ended December 31, 2003 from $460,000 in the year ended December 31, 2002. This increase was attributable primarily to having a full year of AAA product sales in 2003 and only seven months of AAA product sales in 2002. Secondarily, the cost of product revenue for 2002 includes a $64,000 write-off of RDX product inventory. We do not expect our inventory reserves to increase significantly in the future based upon our projected demand for the infrarenal PowerLink System after we obtain FDA approval, which is expected in the second half of 2004.
Gross Profit.Gross profit decreased 52% to $3.4 million in the year ended December 31, 2003 from $6.9 million in the year ended December 31, 2002. The decrease in gross profit was due primarily a decrease in royalties received from Guidant, which do not have an associated cost of product revenue.
Gross profit on product sales increased 106% to $770,000 in the year ended December 31, 2003 from $374,000 in the year ended December 31, 2002, attributable primarily to having a full year of AAA product sales in 2003 and only seven months of AAA product sales in 2002. 2002 results include an $80,000 charge for product exchanges as part of the settlement and release agreement with two European distributors (See Note 15 to consolidated financial statements regarding the settlement and release agreement).
Research and Development. Research and development expenses increased 9% to $6.7 million in the year ended December 31, 2003 from $6.2 million in the year ended December 31, 2002. The increase resulted primarily from an increase in PowerLink technology development expenses of $3.2 million, partially offset by a decrease of $2.7 million in spending on radiation technology development as we are nearing the completion of the related clinical studies. Though the costs for the infrarenal PowerLink
25
System clinical study will decrease substantially for 2004, because we anticipate continuing the suprarenal PowerLink System clinical study through 2004 and we are planning to begin one or two more U.S. clinical trials in 2004, the first of which should begin in the second quarter, we anticipate that research and development expenses for 2004 will be comparable or higher than in 2003, depending upon the number of clinical studies we begin in 2004 .
Marketing and Sales. Marketing and sales expenses decreased 20% to $787,000 in the year ended December 31, 2003 from $982,000 in the year ended December 31, 2002. This decrease was primarily the result of a reduction in sales and marketing administrative staffing. We anticipate that 2004 marketing and sales expenses will be materially higher compared to the total expenses for 2003 as we will be staffing for a limited U.S. sales launch for the PowerLink infrarenal products in the second half of 2004.
General and Administrative. General and administrative expenses decreased 14% to $2.1 million in the year ended December 31, 2003 from $2.4 million in the year ended December 31, 2002. The decrease resulted primarily from a reimbursement in 2003 of legal costs and expenses of $468,000, which were previously expensed as general and administrative expenses, as part of a legal settlement with Jomed-Endosonics, now part of Volcano Therapeutics. A further reduction in general and administrative expenses resulting from bad debt recoveries of $136,000 and lower legal costs of $122,000, which was offset by increases in outside service costs of $131,000, including strategic advisor fees, insurance costs of $114,000 and audit costs of $50,000. We anticipate that general and administrative expenses will be materially higher in 2004, compared with 2003, not only because of the 2003 expense reduction from a $468,000 legal costs and expenses reimbursement, but also because of increases in expenses to help support the U.S. product launch.
Charge for Acquired In-Process Research and Development.We recognized a charge of $4.5 million in the year ended December 31, 2002 as a result of the merger with the former Endologix (See Note 2 to consolidated financial statements).
Restructuring Charges. We recognized restructuring charges totaling $168,000 in the year ended December 31, 2002 based upon our reassessment and elimination of estimated sub-lease income we anticipated receiving to offset rent expenditures for non-cancelable lease commitments.
Other Income (Expense).Other income decreased 60% to $285,000 for the year ended December 31, 2003 from $708,000 in the year ended December 31, 2002. The decrease in other income was due primarily to the decrease in interest income of $306,000, resulting from a 39% lower average cash balance and a lower average interest rate on invested cash. Secondarily, the decrease was due to a $103,000 decrease in net gains on the sale of assets, $85,000 of which resulted from changes in realized net gains on the sale of marketable securities.
Comparison of Years Ended December 31, 2001 and 2002
Product Sales. Sales decreased 25% to $834,000 in the year ended December 31, 2002 from $1.1 million in the year ended December 31, 2001, as a result of the discontinuance of manufacturing and marketing of our Focus technology and RDX products as part of our 2001 restructuring plan. The discontinuance of those product lines was partially offset by product sales for seven months of 2002 of our AAA products, following the merger with the former Endologix.
License Revenue.License revenue increased 1% to $6.6 million in the year ended December 31, 2002 from $6.5 million in the year ended December 31, 2001. Our technology license agreement with Guidant resulted in $6.4 million and $6.0 million in royalties in 2001 and 2002, respectively. We recognized $17,000 in minimum royalties in 2001 and $196,000 in minimum royalties in 2002, under our agreement with Escalon. We currently do not expect to receive more than the minimum royalties due under the Escalon agreement, which we will recognize as revenue when cash is collected due to the uncertainty of collection. We recognized $81,000 in deferred distributor fees in 2001 and $360,000 in 2002 under a distribution agreement with Cosmotec regarding the distribution of our radiation therapy products in Japan through our joint venture. In December 2002, we agreed with Cosmotec not to distribute the products and to dissolve the joint venture, at which time we recognized the remaining deferred distributor fee of $299,000 as revenue.
26
Cost of Product Sales. The cost of product sales decreased 60% to $460,000 in the year ended December 31, 2002 from $1.1 million in the year ended December 31, 2001. This decrease was attributable primarily to a lower average cost of sales for AAA products, compared with that for our former Focus technology and RDX products sold in 2001, partially offset by a one-time $80,000 charge for product exchanges as part of the settlement and release agreement with European distributors (See Note 15 to consolidated financial statements regarding the settlement and release agreement), and a 25% decrease in product sales to $834,000 in 2002 from $1.1 million in 2001.
Cost of Sales from Restructuring.Due to our restructuring and discontinuance of the marketing of existing products (i.e., Focus technology and RDX products) that we announced in the third quarter of 2001, we wrote-off $601,000 of inventory that would not be used to fulfill existing customer orders. We did not have any corresponding write-offs of inventory in 2002 due to the restructuring.
Gross Profit.Gross profit increased 18% to $6.9 million in the year ended December 31, 2002 from $5.9 million in the year ended December 31, 2001. The increase in gross profit was due primarily an inventory write off of $601,000 in 2001 as a result of the restructuring and to sales of higher margin AAA products in 2002.
Gross profit on product sales increased to $374,000 in the year ended December 31, 2002 from $(38) in the year ended December 31, 2001, due primarily to sales of higher margin AAA products in 2002, partially offset by an $80,000 charge for product exchanges as part of the settlement and release agreement with two European distributors (See Note 15 to consolidated financial statements regarding the settlement and release agreement), and an inventory write off of $601,000 in 2001 as a result of the restructuring.
Research and Development. Research and development expenses decreased 58% to $6.2 million in the year ended December 31, 2002 from $14.6 million in the year ended December 31, 2001. The decrease was primarily due to discontinued research and development projects as part of our September 2001 restructuring plan. Corresponding expenses on AAA research and development did not commence until the merger with the former Endologix.
Marketing and Sales. Marketing and sales expenses decreased 25% to $982,000 in the year ended December 31, 2002 from $1.3 million in the year ended December 31, 2001. This decrease was primarily the result of our discontinuance of marketing and sales of our then existing products as part of our September 2001 restructuring plan.
General and Administrative. General and administrative expenses decreased 6% to $2.4 million in the year ended December 31, 2002 from $2.6 million in the year ended December 31, 2001. The decrease was due primarily to lower legal expenses relating mainly to the EndoSonics Corporation lawsuit (See Note 15 to the consolidated financial statements), and lower bad debt expense in 2002, as bad debt expense of $131,000, net of recoveries, in 2001 was due primarily to the restructuring.
Charge for Acquired In-Process Research and Development.We recognized a charge of $4.5 million in the year ended December 31, 2002 as a result of the merger with the former Endologix (See Note 2 to consolidated financial statements).
Restructuring Charges. As a result of the restructuring we began in September 2001, we recognized restructuring charges totaling $4.6 million in the year ended December 31, 2001. The charges consisted of a $2.1 million impairment charge for previously acquired RDX developed technology, $1.1 million of involuntary employee termination costs, a $699,000 impairment charge for manufacturing and other operating assets, a $344,000 charge, comprised of manufacturing facility setup and sub-license fees and non-cancelable commitments under agreements with Bebig, $20,000 in other non-cancelable commitments, $309,000 of non-cancelable lease commitments, net of estimated sublease income of $256,000, and $42,000 of other non-cancelable commitments and exit costs. We recognized restructuring charges totaling $168,000 in the year ended December 31, 2002 based upon our reassessment and elimination of estimated sub-lease income we anticipated receiving to offset rent expenditures for non-cancelable lease commitments.
27
Other Income (Expense).Other income decreased 53% to $708,000 for the year ended December 31, 2002 from $1.5 million in the year ended December 31, 2001. The decrease in other income was due primarily to the decrease in interest income of $818,000, resulting from the use of $8.4 million in cash as merger consideration to the shareholders of the former Endologix in 2002, coupled with continuing losses from operations.
Liquidity and Capital Resources
Since inception, we have financed our operations primarily by:
| • | | selling our equity securities; |
| |
| • | | licensing our technologies; |
| |
| • | | commercial sales, and |
| |
| • | | entering into international product distribution agreements. |
Prior to our initial public offering in 1996, we raised an aggregate of approximately $11.4 million from the private sales of preferred and common stock. In 1996, we closed our initial public offering of common stock, with net proceeds of approximately $42.8 million after deducting underwriting discounts and commissions and other expenses of the offering.
In 1997 we raised an aggregate of $577,000 through private sales of common stock to strategic partners.
In 1999, we granted Cosmotec the exclusive distribution rights to market our vascular radiation therapy products in Japan. We received $1.0 million from Cosmotec as an upfront cash payment. As part of the transaction with Cosmotec, in August 1999 we acquired a 51% interest, for $233,000, in a joint venture with an affiliate of Cosmotec. The joint venture was formed to gain regulatory approval of and provide distribution for the radiation catheter system in Japan. However, under the 2001 restructuring plan, we discontinued preparations for Japanese clinical trials. In December 2002, we agreed with Cosmotec and its affiliate to dissolve the joint venture.
In June 2000, we borrowed $1.0 million from Cosmotec and recorded $1.4 million in debt to reflect the fair value of the 5%, $1.0 million face amount convertible debenture. In September 2000, Cosmotec converted the debenture into 142,857 shares of our common stock at $7.00 per share.
In October 2000, we sold in a secondary offering 686,000 shares of our common stock held in treasury and 814,000 shares of our newly issued common stock. We received $13.0 million in net proceeds, after deducting underwriting discounts, commissions and other expenses.
As a result of our company restructuring beginning in September 2001, we expended the following amounts during the years ended December 31, 2001 through 2003 (SeeCompany Restructuring, in the Overview section, above, for additional information):
| | | | | | | | | | | | | | | | | |
| | | 2001
| | 2002
| | 2003
| | Totals
|
| Employee termination benefits | | $ | 474,000 | | | $ | 619,000 | | | $ | — | | | $ | 1,093,000 | |
| Non-cancelable commitments | | | 371,000 | | | | 221,000 | | | | 248,000 | | | | 840,000 | |
| | | |
| | | |
| | | |
| | | |
| |
| Totals | | $ | 845,000 | | | $ | 840,000 | | | $ | 248,000 | | | $ | 1,933,000 | |
| | | |
| | | |
| | | |
| | | |
| |
In July 2002, the board of directors authorized a program for repurchases of our outstanding common stock of up to $l.5 million under certain parameters. As of December 31, 2003, we have repurchased an aggregate of 495,000 shares for $661,000.
In October 2002, we repaid a 10%, $1.0 million convertible debenture and accrued interest due to Cosmotec that was assumed in the merger with the former Endologix.
28
In July 2003, we closed a private placement of 4,000,000 shares of our common stock at $2.25 per share. The proceeds of the private placement, net of issuance costs, amounted to $8.4 million.
In March 2004, we closed a private placement of 3,200,000 shares of our common stock at $5.10 per share, which resulted in aggregate net proceeds of $15.3 million, after deduction of transaction expenses.
Net cash used by operating activities was $5.0 million for the year ended December 31, 2003, compared with $2.2 million for the same period of 2002. The increase for 2003 reflects a full year of operations supporting the development of the PowerLink System products, following the merger with the former private company Endologix at the end of May 2002.
At December 31, 2003, we had cash, cash equivalents and marketable securities available for sale of $12.8 million. We expect to continue to incur substantial costs and cash outlays in 2004 to support our research and development efforts.
For the years ended December 31, 2003 and 2002, we have incurred net losses of $5.9 million and $6.6 million, respectively. As of December 31, 2003, we had an accumulated deficit of approximately $73.9 million. We believe that current cash and cash equivalents, marketable securities and cash generated by operations are sufficient to meet anticipated cash needs for operating and capital expenditures through at least June 30, 2005. Unanticipated reductions in royalty revenue, failure of the market to accept our products, or failure to reduce certain discretionary expenditures, if necessary, could have a material adverse effect on the our ability to achieve our intended business objectives.
Our future capital requirements will depend on many factors, including:
| • | | our research and development programs |
| |
| • | | the scope and results of clinical trials; |
| |
| • | | the regulatory approval process; |
| |
| • | | the costs involved in intellectual property rights enforcement or litigation; |
| |
| • | | competitive products; |
| |
| • | | the establishment of manufacturing capacity; |
| |
| • | | the emphasis on sales and marketing capabilities; |
| |
| • | | the establishment of collaborative relationships with other parties; and, |
| |
| • | | the ability to develop technology and to commercialize products. |
We believe that the funds received as part of our private placement completed in March 2004 and the funds we had at the time will be sufficient to support operations, including a limited U.S. market launch of the PowerLink System, through at least June 30, 2005. Thereafter, we may need to raise additional funds to support operations through additional financings, including debt, private or public equity offerings and collaborative arrangements with existing or new corporate partners. We cannot assure you that we will be able to raise funds on favorable terms, or at all. Equity financings may dilute the interests of the existing shareholders. If we obtain funds through arrangements with collaborative partners or others, we may be required to grant rights to certain technologies or products that we would not otherwise grant.
Accounts Receivable. Trade accounts receivable, net, decreased 62% to $239,000 at December 31, 2003 from $622,000 at December 31, 2002. The decrease is due primarily to the settlement of a legal action with our European distributor and their payment of cash of $320,000, returns allowed of $47,000 and a credit for expenses incurred of $17,000, reducing their outstanding receivables that had been outstanding at December 31, 2002.
Other Receivables. Other receivables decreased 35% to $656,000 at December 31, 2003 from $1.0 million at December 31, 2002 due primarily to a decrease of the royalty receivable from Guidant of $358,000. SeeComparisons of Years Ended December 31, 2002 and 2003in subsectionsLicense Revenue, regarding Guidant royalty revenues, above.
Inventories. Inventories increased 36% to $2.8 million at December 31, 2003 from $2.0 million at December 31, 2002. The increase was due primarily to Impra component inventory purchases, based upon contractual minimum quantity purchase requirements, in excess of total material usage in products sold.
29
We anticipate inventory levels will continue to grow during 2004, as contractual minimum quantity purchase requirements will exceed the amount of materials utilized in products sold until 2005. As our component cost will materially increase if the PowerLink receives FDA approval, and we believe that we will be able to utilize the inventory on hand at December 31, 2003, as well as our minimum purchase total for 2004, we will consider component purchases in excess of the minimum requirement prior to approval, which we anticipate in the second half of 2004. See discussion of minimum purchase requirements regarding Impra contract, above, within theLiquidity and Capital Resource section.
Intangibles. Intangibles, net decreased to $14.5 million at December 31, 2003 from $15.9 million at December 31, 2002. The decrease in intangibles is due to amortization of $1.4 million.
Accounts Payable and Accrued Expenses. Accounts payable and accrued expenses decreased 37% to $1.5 million at December 31, 2003 from $2.3 million at December 31, 2002. This decrease was attributable to lower accruals for restructuring charges of $672,000, including $619,000 for involuntary employee terminations and $53,000 for non-cancelable commitments, and clinical expenses of $624,000, partially offset by an increase in accounts payable and other expense accruals of $532,000.
Minority Interest. Minority interest decreased to zero at December 31, 2003, from $83,000 at December 31, 2002. In December 2002, we agreed with Cosmotec, and its affiliate, our joint venture partner, to release each other from the distribution and joint venture agreements, respectively, as we had no plans to pursue regulatory approval of the RDX product, and to dissolve the joint venture, Radiatec. The dissolution of the joint venture was completed in May 2003 (see Note 3 to the Consolidated Financial Statements).
Commitments
In February 1999, the former Endologix agreed to purchase a key component for its PowerLink product from Impra, Inc., a subsidiary of C.R. Bard, Inc. and then a related party, under a supplier agreement that expires in December 2007, and then automatically renews, on a year by year basis, for additional one year periods without notice, unless a party provides notice not to renew within thirty days from the expiration of the renewal period. Under the terms of the agreement, we have agreed to purchase certain unit quantities of the component, with built in annual quantity increases, or the agreement may be canceled. In January 2002, the agreement was amended to increase the minimum quantity purchase requirements for 2002 and thereafter and increase the prices each year after 2002 according to the general increase in the Consumer Price Index. For the year ended December 31, 2003, we have purchased or committed to purchase $1.0 million under the supplier agreement. In 2004, we anticipate purchasing a total of approximately $1.4 million in materials. However, if we receive FDA approval to commercially distribute devices using the component and we have not received a commitment from the supplier to provide the component at the current price, the total price that we believe we will pay Impra for the component will materially increase and could cost as much as approximately $2.0 million. We believe that U.S. commercialization will occur during 2004. We are economically dependent on this vendor as it is the sole source for the key component.
As of December 31, 2003, expected future cash payments related to contractual obligations and commercial commitments were as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | Total
| | 2004
| | 2005
| | 2006
| | 2007
|
| Contractual Obligations (a): | | | | | | | | | | | | | | | | | | | | |
| Operating lease obligations. | | $ | 370 | | | $ | 283 | | | $ | 83 | | | $ | 4 | | | $ | — | |
| Purchase obligation (b) | | | 10,294 | | | | 1,357 | | | | 2,574 | | | | 2,960 | | | | 3,403 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | $ | 10,664 | | | $ | 1,640 | | | $ | 2,657 | | | $ | 2,964 | | | $ | 3,403 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| (a) | | There are no contractual obligations that extend past 2007. |
| |
| (b) | | Represents estimates of obligations under Impra component purchase contract. The actual amounts paid may be materially different primarily due to the fact that the agreement provides that the |
30
| | | component price to be paid increases materially following FDA approval of the PowerLink System, and we do not currently know when or if we will receive such FDA approval. For the purpose of this forecast, we have assumed that we will purchase all of the materials to meet the minimum purchase requirement for 2004 before the PowerLink System is approved. Additionally, the total cost of the components is determined by the mix of sizes of graft material that we purchase, as well as the number of components purchased. Under the agreement, each year we must buy 115% of the minimum or actual number of units purchased, whichever is higher, in the prior year. The cost of the component is determined by the size of the graft piece purchased, and we do not currently know what sizes we will be purchasing after 2004. For 2005, we estimated the sizes to be purchased and for years thereafter until the contract terminates at the end of 2007, we assumed that the minimum amount purchased increased 15% each year. Please see the paragraph, above, for more information on the Impra agreement. |
Risk Factors
Certain factors may affect our business and future results. Some of the information included herein contains forward-looking statements. These statements can be identified by the use of forward-looking terms such as “may,” “will,” “expect,” “anticipates,” “estimate,” “continue,” or other similar words. These statements discuss future expectations, projections or results of operations or of financial condition or state other “forward-looking” information. When considering these forward-looking statements, you should keep in mind the risk factors and other cautionary statements we make. These risk factors could cause our actual results to differ materially from those contained in any forward-looking statement. If any of the following risks actually occur, our business could be harmed and the trading price of our common stock could decline.
Risks Related To Our Business
We expect to incur losses for the foreseeable future and may never achieve profitability.
From our formation in 1992 to December 31, 2003, we have incurred a cumulative net loss of approximately $73.9 million. We incurred a net loss of $5.9 million for the year ended December 31, 2003 and incurred a net loss of $6.6 million for the year ended December 31, 2002. As we do not anticipate receiving FDA approval until the second half of 2004, we do not expect to be profitable in 2004, and it is possible that we may never achieve profitability.
We cannot assure you that we will be able to obtain regulatory approvals for the PowerLink AAA system.
We need to complete a U.S. pivotal human clinical trial for the PowerLink system. The PowerLink system is the only product we have under development and it has not been approved for marketing by the FDA. Prior to granting approval, the FDA may require more information or clarification of information provided in our regulatory submissions, or more clinical studies, which could require significant additional expenditures. If granted, the FDA may impose limitations on the uses for which or how we may market the PowerLink system. Should we experience delays or be unable to obtain regulatory approvals, we may never generate significant revenues, and our business prospects will be substantially impaired.
In Japan, we have completed our clinical trials for the PowerWeb System and are working with the Ministry of Health for regulatory approval. While we believe that we will receive regulatory approval in Japan in the second half of 2004, because this is the first AAA device submitted for approval, it is difficult for us to determine when or whether the device will be approved and if approved, when and whether the technology will obtain hospital reimbursement from the Japanese Medical authorities and permit commercialization.
In addition, any design, vendor or material change to the PowerWeb or PowerLink System may require regulatory approval. If we do not receive regulatory approval, we may not be able to commercialize the product.
If we receive regulatory approval for our products and decide to market them, we will need to grow rapidly. Rapid growth may strain the capabilities of our managers, operations and facilities and, consequently, could harm our business.
If we obtain the required U. S. regulatory approval for the PowerLink system, commercial-scale production will require us to expand our operations. Rapid growth may strain our managerial and other
31
organizational resources. Our ability to manage our growth will depend on the ability of our officers and key employees to:
| | • | | manage the simultaneous manufacture of different products efficiently and integrate the manufacture of new products with existing product lines; |
| |
| | • | | address difficulties in scaling up production of new products, including problems involving production yields, quality control and assurance, component supply and shortages of qualified personnel; and, |
| |
| | • | | implement and improve our operational, management information and financial control systems. |
We rely on a single vendor to supply our graft material for the PowerLink system, and any disruption in our supply could delay or prevent us from completing our clinical trials or from producing the product for sale.
Currently, we rely on Impra, a subsidiary of C.R. Bard, to supply us with graft , which is a primary component for the PowerLink system. Our reliance on a sole source supplier exposes our operations to disruptions in supply caused by:
| | • | | failure of our supplier to comply with regulatory requirements; |
| |
| | • | | any strike or work stoppage; |
| |
| | • | | disruptions in shipping; |
| |
| | • | | a natural disaster caused by fire, floods or earthquakes; |
| |
| | • | | a supply shortage experienced by our sole source supplier; and |
| |
| | • | | the fiscal health and manufacturing strength of our sole source supplier. |
Although we retain a significant stock of the graft material, the occurrence of any of the above disruptions in supply or other unforeseen events that could cause a disruption in supply from our sole source graft supplier may cause us to halt or delay our clinical trials. Because we do not have alternative suppliers, our sales and profitability would be harmed in the event of a disruption.
We are currently only developing a single technology, the PowerLink system.
Because of limited resources, we are currently only developing a single technology, the PowerLink system. If we are unable to commercialize the PowerLink system and reach positive cash flow from operations, we may not be able to fund development and commercialization of an alternative technology.
Our operations are capital intensive, and we may need to raise additional funds in the future to fund our operations.
Our activities are capital intensive. Although we believe that our existing cash resources and anticipated cash generated from operations will be sufficient to meet our planned capital requirements through at least June 30, 2005, we will require additional capital to fund on-going operations, including our anticipated full market product launch in the U.S. in 2005. Our cash requirements in the future may be significantly different from our current estimates and depend on many factors, including:
| | • | | the scope and results of our clinical trials; |
| |
| | • | | the time and costs involved in obtaining regulatory approvals; |
32
| | • | | the costs involved in obtaining and enforcing patents or any litigation by third parties regarding intellectual property; the establishment of high volume manufacturing and sales and marketing capabilities; and, |
| |
| | • | | our success in entering into collaborative relationships with other parties. |
To finance these activities, we may seek funds through additional rounds of financing, including private or public equity or debt offerings and collaborative arrangements with corporate partners. We may be unable to raise funds on favorable terms, or not at all. The sale of additional equity or convertible debt securities could result in additional dilution to our stockholders. If we issue debt securities, these securities could have rights superior to holders of our common stock, and could contain covenants that will restrict our operations. We might have to obtain funds through arrangements with collaborative partners or others that may require us to relinquish rights to our technologies, product candidates or products that we otherwise would not relinquish. If adequate funds are not available, we might have to delay, scale back or eliminate one or more of our development programs, which would impair our future prospects.
Our primary source of revenues is our Focus technology license agreement with Guidant.
Our current and future revenues depend on the number of stent delivery systems that incorporate our Focus technology that are sold by Guidant Corporation. Under our license agreement with Guidant, we receive royalty payments only from Guidant’s sale of products using the Focus technology. Approximately 58% of our total revenues in the year ended December 31, 2003 were from Guidant. Our license revenues declined substantially following the release of unlicensed products by Guidant and introduction of drug-coated stents and may continue to decline precipitously. In any event, we expect that our revenues from Guidant will decline over the next few years as technological changes in the stent market make our Focus stent technology obsolete.
We will need to devote significant resources to market our products and technology to physicians in order to achieve market acceptance. If we fail to achieve market acceptance, our business will suffer.
Because the FDA and other regulatory agencies have approved other minimally-invasive AAA graft systems, we believe that unless we can demonstrate clinically superior results and are able to convince physicians of the superiority of the device, we may not be able to successfully market the products. Other companies may have superior resources to market similar products or technologies or have superior technologies and products to market. Therefore, even if our products gain regulatory approval, we will need to spend significant resources prior to achieving market acceptance. Any failure of our products to achieve commercial acceptance, or any inability on our part to devote the requisite resources necessary to market our products, will harm our business.
We may rely on third-party distributors to sell and market any product we develop. They may do so ineffectively.
We may depend on medical device distributors and strategic relationships, some of which may be with our competitors, to distribute the PowerLink system or any other product we develop. Significant consolidation among medical device suppliers has made it increasingly difficult for smaller suppliers like us to distribute products effectively without a relationship with one or more of the major suppliers. Consequently, we may enter into agreements with third parties to distribute any product we develop. If we enter into such relationships, we will depend directly on their efforts to market our product, yet we will be unable to control their efforts completely. If our distributors fail to market and sell our products effectively, our operating results and business may suffer substantially, or we may have to make significant additional expenditures to market our products.
33
The market for our products is highly competitive, and competing medical device technologies may prove more effective in treating these conditions than our product candidates.
Competition in the market for devices used in the treatment of vascular disease is intense, and we expect it to increase. The PowerLink system and other potential products will compete with treatment methods that are well established in the medical community, as well as treatments based on new technologies. We face competition from manufacturers of other catheter-based AAA graft devices and pharmaceutical products intended to treat vascular disease.
The most significant devices that pose a competitive challenge to us include:
| | • | | Medtronic’s AneuRx ,W. L. Gore’s Excluder, and the Cook Zenith AAA system which are available in the U.S. and Europe; |
| |
| | • | | Other AAA graft systems by Medtronic, Johnson and Johnson, currently with more limited availability, and |
| |
| | • | | other technologies in various phases of development, including pharmaceutical solutions. |
Any of these treatments could prove to be more effective or may achieve greater market acceptance than the PowerLink system. Even if these treatments are not as effective as the PowerLink system, many of the companies pursuing these treatments and technologies have:
| | • | | significantly greater financial, management and other resources; |
| |
| | • | | more extensive research and development capability; |
| |
| | • | | established market positions; and, |
| |
| | • | | larger sales and marketing organizations. |
In addition, we believe that many of the purchasers and potential purchasers of our competitors’ products prefer to purchase medical devices from a single source. Accordingly, many of our competitors will have an advantage over us because of their size and range of product offerings.
Our future operating results are difficult to predict and may vary significantly from quarter to quarter. This fluctuation may negatively impact our stock price in the future.
Because the PowerLink system is still in the research and development phase, we cannot predict when, if ever, we will have revenues based on the U.S. sales of the PowerLink system. Also, our current revenues are attributable primarily to a license agreement with Guidant, which limits our ability to predict future revenues. Moreover, we expect revenues pursuant to the license agreement with Guidant to diminish in the future as technology changes. In addition to the foregoing factors, our quarterly revenues and results of operations have fluctuated in the past and may fluctuate in the future due to:
| | • | | the conduct of clinical trials; |
| |
| | • | | the timing of regulatory approvals; |
| |
| | • | | fluctuations in our expenses associated with expanding our operations; |
| |
| | • | | new product introductions both in the United States and internationally; |
| |
| | • | | variations in foreign exchange rates; and, |
| |
| | • | | changes in third-party payors’ reimbursement policies. |
34
| Therefore, we believe that period to period comparison of our operating results may not necessarily be reliable indicators of our future performance. It is likely that in some future period our operating results will not meet your expectations or those of public market analysts. |
Any unanticipated change in revenues or operating results is likely to cause our stock price to fluctuate since such changes reflect new information available to investors and analysts. New information may cause investors and analysts to revalue our stock, which could cause a decline in value.
Risks Related To Our Industry
Our products and manufacturing activities are subject to extensive governmental regulation that could make it more expensive and time consuming for us to introduce new and improved products.
Our products must comply with regulatory requirements imposed by the FDA and similar agencies in foreign countries. These requirements involve lengthy and detailed laboratory and clinical testing procedures, sampling activities, an extensive FDA review process and other costly and time-consuming procedures. It often takes companies several years to satisfy these requirements, depending on the complexity and novelty of the product. We also are subject to numerous additional licensing and regulatory requirements relating to safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances. Some of the most important requirements we face include:
| | • | | FDA pre-market approval process; |
| |
| | • | | California Department of Health Services requirements; |
| |
| | • | | ISO 9001/EN46001 certification; and, |
| |
| | • | | European Union CE Mark requirements. |
Government regulation may impede our ability to conduct clinical trials and to manufacture the PowerLink system and other prospective products. Government regulation also could delay our marketing of new products for a considerable period of time and impose costly procedures on our activities. The FDA and other regulatory agencies may not approve any of our products on a timely basis, if at all. Any delay in obtaining, or failure to obtain, such approvals could impede our marketing of any proposed products and reduce our product revenues.
In addition, even after receipt of approval and market launch, our products remain subject to strict regulatory controls on manufacture, marketing and use. We may be forced to modify or recall our product after release. Any such action could have a material affect on the reputation of our products and on our business and financial position.
Further, regulations may change, and any additional regulation could limit or restrict our ability to use any of our technologies, which could harm our business. We could also be subject to new federal, state or local regulations that could affect our research and development programs and harm our business in unforeseen ways. If this happens, we may have to incur significant costs to comply with such laws and regulations.
We cannot predict the extent to which third-party payors may provide reimbursement for the use of our products.
Our success in marketing products based on novel or innovative technology depends in large part on whether domestic and international government health administrative authorities, private health insurers and other organizations will reimburse customers for the cost of our product. Reimbursement systems in international markets vary significantly by country and by region within some countries, and reimbursement approvals must be obtained on a country-by-country basis. Further, many international
35
markets have government managed healthcare systems that control reimbursement for new devices and procedures. In most markets there are private insurance systems as well as government-managed systems. We cannot assure you that sufficient reimbursement will be available for any product that we may develop, in either the United States or internationally, to establish and maintain price levels sufficient to realize an appropriate return on the development of our new products.
If government and third party payors do not provide adequate coverage and reimbursement for our new products, it will be very difficult for us to market our products to doctors and hospitals, and we may not achieve commercial success.
We may be unable to protect our intellectual property from infringement. A failure to protect our technology may affect our business negatively.
The market for medical devices is subject to frequent litigation regarding patent and other intellectual property rights. It is possible that our patents or licenses may not withstand challenges made by others or protect our rights adequately.
Our success depends in large part on our ability to secure effective patent protection for our products and processes in the United States and internationally. We have filed and intend to continue to file patent applications for various aspects of our technology. However, we face the risks that:
| | • | | we may fail to secure necessary patents prior to or after obtaining regulatory clearances, thereby permitting competitors to market competing products; and |
| |
| | • | | our already-granted patents may be re-examined, re-issued or invalidated. |
We also own trade secrets and confidential information that we try to protect by entering into confidentiality agreements with other parties. We cannot be certain that any of the confidentiality agreements will be honored or, if breached, that we would have sufficient remedies to protect our confidential information. Further, our competitors may independently learn our trade secrets or develop similar or superior technologies. To the extent that our consultants, key employees or others apply technological information to our projects that they develop independently or others develop, disputes may arise regarding the ownership of proprietary rights to such information such disputes may not be resolved in our favor. If we are unable to protect our intellectual property adequately, our business and commercial prospects likely will suffer.
If our current products or licensed products infringe upon the intellectual property of our competitors, the sale of these products may be challenged and we may have to defend costly and time-consuming infringement claims.
We may need to engage in expensive and prolonged litigation to assert any of our rights or to determine the scope and validity of rights claimed by other parties. With no certainty as to the outcome, litigation could be too expensive for us to pursue. Our failure to pursue litigation could result in the loss of our rights that could hurt our business substantially. In addition, the laws of some foreign countries do not protect our intellectual property rights to the same extent as the laws of the United States, if at all.
Our failure to obtain rights to intellectual property of third parties or the potential for intellectual property litigation could force us to do one or more of the following:
| | • | | stop selling, making or using our products that use the disputed intellectual property; |
| |
| | • | | obtain a license from the intellectual property owner to continue selling, making, licensing or using our products, which license may not be available on reasonable terms, or at all; |
| |
| | • | | redesign our products or services; and |
| |
| | • | | subject us to significant liabilities to third parties. |
36
| If any of the foregoing occurs, we may be unable to manufacture and sell our products or license our technology and may suffer severe financial harm. Whether or not an intellectual property claim is valid, the cost of responding to it, in terms of legal fees and expenses and the diversion of management resources, could harm our business. |
We may face product liability claims that could result in costly litigation and significant liabilities.
Clinical testing, manufacturing and marketing of our products may expose us to product liability claims. Although we have, and intend to maintain insurance, the coverage limits of our insurance policies may not be adequate and one or more successful claims brought against us may have a material adverse effect on our business, financial condition and results of operations. Additionally, adverse product liability actions could negatively affect the reputation and sales of our products and our ability to obtain and maintain regulatory approval for our products.
Other Risks
The price of our stock may fluctuate unpredictably in response to factors unrelated to our operating performance.
The stock market periodically experiences significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These broad market fluctuations may cause the market price of our common stock to drop. In particular, the market price of securities of small medical device companies, like ours, has been very unpredictable and may vary in response to:
| • | | announcements by us or our competitors concerning technological innovations; |
| |
| • | | introductions of new products; |
| |
| • | | FDA and foreign regulatory actions; |
| |
| • | | developments or disputes relating to patents or proprietary rights; |
| |
| • | | failure of our results of operations to meet the expectations of stock market analysts and investors; |
| |
| • | | changes in stock market analyst recommendations regarding our common stock; |
| |
| • | | changes in healthcare policy in the United States or other countries; and |
| |
| • | | general stock market conditions. |
Some provisions of our charter documents may make takeover attempts difficult, which could depress the price of our stock and inhibit your ability to receive a premium price for your shares.
Provisions of our amended and restated certificate of incorporation could make it more difficult for a third party to acquire control of our business, even if such change in control would be beneficial to our stockholders. Our amended and restated certificate of incorporation allows our board of directors to issue up to five million shares of preferred stock and to fix the rights and preferences of such shares without stockholder approval. Any such issuance could make it more difficult for a third party to acquire our business and may adversely affect the rights of our stockholders. In addition, our board of directors is divided into three classes for staggered terms of three years. These provisions may delay, deter or prevent a change in control of us, adversely affecting the market price of our common stock.
37
Substantial future sales of our common stock in the public market may depress our stock price and make it difficult for you to recover the full value of your investment in our shares.
We have approximately 31,677,000 shares of common stock outstanding, net of treasury stock, most of which are freely tradable. The market price of our common stock could drop due to sales of a large number of shares or the perception that such sales could occur. These factors also could make it more difficult to raise funds through future offerings of common stock.
Recent Accounting Pronouncements
In May 2003, the FASB issued SFAS 150, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity. SFAS 150 establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity. It requires that an issuer classify a financial instrument that is within its scope as a liability (or an asset in some circumstances). Many of those instruments were previously classified as equity. This Statement is effective for financial instruments entered into or modified after May 31, 2003 (except for mandatorily redeemable noncontrolling interests). For all instruments that existed prior to May 31, 2003, SFAS 150 is effective at the beginning of the first interim period beginning after June 15, 2003 (except for mandatorily redeemable noncontrolling interests). For mandatorily redeemable noncontrolling interests, the FASB has deferred certain provisions of SFAS 150. The adoption of SFAS 150 did not have a material effect on our consolidated financial position, results of operations or cash flows.
In December 2003 the SEC issued Staff Accounting Bulletin (SAB) No. 104, Revenue Recognition. SAB No. 104 revises and rescinds certain sections of SAB No. 101 in order to make this interpretive guidance consistent with current authoritative accounting and auditing guidance and SEC rules and regulations. Accordingly there is no impact to our results of operations, financial position or cash flows as a result of the issuance of SAB No. 104.
In December 2003, the FASB issued Interpretation No. 46R, Consolidation of Variable Interest Entities (FIN 46R). FIN 46R requires the application of either FIN 46 or FIN 46R by Public Entities to all Special Purpose Entities (SPE) created prior to February 1, 2003 as of December 31, 2003 for calendar year-end companies. FIN 46R is applicable to all non-SPEs created prior to February 1, 2003 at the end of the first interim or annual period ending after March 15, 2004. For all entities created subsequent to January 31, 2003, Public Entities were required to apply the provisions of FIN 46. The adoption of FIN 46 did not have a material impact on our consolidated financial position, results of operations or cash flows. The adoption of FIN 46R for SPEs did not have an impact to our consolidated financial position, results of operations or cash flows, and we do not believe the adoption of FIN 46R for non-SPEs will have a material impact to our consolidated financial position, results of operations or cash flows.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We do not believe that we currently have material exposure to interest rate, foreign currency exchange rate or other relevant market risks.
Interest Rate and Market Risk. Our exposure to market risk for changes in interest rates relates primarily to our investment profile. We do not use derivative financial instruments in our investment portfolio. We place our investments with high credit quality issuers and, by policy, limit the amount of credit exposure to any one issuer. We are averse to principal loss and try to ensure the safety and preservation of our invested funds by limiting default risk, market risk, and reinvestment risk. We attempt to mitigate default risk by investing in only the safest and highest credit quality securities and by constantly positioning our portfolio to respond appropriately to a significant reduction in a credit rating of any investment issuer or guarantor. At December 31, 2003, our investment portfolio included only high-grade corporate bonds and commercial paper and government bonds all with remaining maturities of less than two years and denominated in U.S. dollars.
38
The table below provides information about our available-for-sale investment portfolio. For investment securities, the table presents principal cash flows and related weighted average fixed interest rates by expected maturity dates.
Principal amounts by expected maturity in the subsequent twelve-month periods ending December 31:
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Fair Value at |
| | | | | | | | | | | | | | | December 31, |
| | | 2004
| | 2005
| | Total
| | 2003
|
| | | (in thousands, except interest rates) |
| Cash and cash equivalents | | $ | 4,311 | | | | — | | | $ | 4,311 | | | $ | 4,306 | |
| Weighted average interest rate | | | 0.25 | % | | | — | | | | 0.25 | % | | | | |
| Investments | | $ | 8,127 | | | $ | 200 | | | $ | 8,327 | | | $ | 8,377 | |
| Weighted average interest rate | | | 1.94 | % | | | 6.21 | % | | | 2.04 | % | | | | |
| Total portfolio | | $ | 12,438 | | | $ | 200 | | | $ | 12,638 | | | $ | 12,683 | |
| Weighted average interest rate | | | 1.36 | % | | | 6.21 | % | | | 1.43 | % | | | | |
Foreign Currency Exchange Risk. We do not currently have material foreign currency exposure as the majority of our assets are denominated in U.S. currency and our foreign-currency based transactions are not material. Accordingly, we do not have a significant currency exposure at December 31, 2003.
Item 8. Financial Statements
| | | | | |
| | | PAGE
|
| Report of Independent Auditors | | | F-1 | |
| Financial Statements: | | | | |
| Consolidated Balance Sheets | | | F-2 | |
| Consolidated Statements of Operations | | | F-3 | |
| Consolidated Statements of Stockholders’ Equity | | | F-4 | |
| Consolidated Statements of Cash Flows | | | F-5 | |
| Notes to Consolidated Financial Statements | | | F-6 | |
The financial statement schedule listed under Part IV, Item 15, is filed as part of this Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our “disclosure controls and procedures” as of the end of the period covered by this report, pursuant to Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures, as of the end of the period covered by this report, were effective in timely alerting them to material information relating to us required to be included in our periodic SEC filings. There has been no change in our internal control over financial reporting during the fourth fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
39
PART III
Item 10. Directors and Officers of the Registrant
The following table sets forth information as of March 11, 2004 with respect to our directors and executive officers:
| | | | | | | |
NAME
| | AGE
| | POSITION
|
| Franklin D. Brown | | | 60 | | | Executive Chairman and Director |
| Paul McCormick | | | 50 | | | President and Chief Executive Officer and Director |
| David M. Richards | | | 44 | | | Chief Financial Officer and Corporate Secretary |
| Stefan Schreck, Ph.D. | | | 44 | | | Vice President, Research and Development |
| Karen Uyesugi | | | 48 | | | Vice President, Clinical and Regulatory Affairs |
| Maurice Buchbinder, M.D. | | | 50 | | | Director |
| Roderick de Greef | | | 43 | | | Director |
| Edward M. Diethrich, M.D. | | | 68 | | | Director |
| Jeffrey F. O’Donnell | | | 44 | | | Director |
| Gregory D. Waller | | | 54 | | | Director |
Franklin D. Brown. Mr. Brown serves as our Executive Chairman and has been a director since 1997. Following the merger with the former Endologix in May 2002, Mr. Brown was our Chief Executive Officer and Chairman until January 2003, when he was elected Executive Chairman. Mr. Brown previously served as the Chairman and Chief Executive Officer of the former Endologix, Inc. since joining that company in 1998. From October 1994 until the sale of the company in September 1997, Mr. Brown served as Chairman, President and Chief Executive Officer at Imagyn Medical, Inc. From 1986 until the sale of the company in 1994, Mr. Brown served as President and Chief Executive Officer of Pharmacia Deltec, Inc., an ambulatory drug delivery company. Mr. Brown also serves on the boards of directors of Triage Medical, Inc. and ATI Medical, Inc., both private companies.
Paul McCormick. Mr. McCormick is our President and Chief Executive Officer and has been a director since May 2002. Mr. McCormick has more than 24 years in the medical device industry. The majority of his career has been in emerging medical technologies. Mr. McCormick joined the former Endologix in January 1998 as Vice President of Sales and Marketing, and served as President and Chief Operating Officer from January 2001 until the merger in May 2002. He then served in the same position with us until January 2003 when he became President and Chief Executive Officer. Previously, he held various sales and marketing positions at Progressive Angioplasty Systems, Heart Technology, Trimedyne Inc., and United States Surgical Corporation.
David M. Richards. Mr. Richards joined us in September 1996 and serves as our Chief Financial Officer and Corporate Secretary. From September 1996 to October 2001, Mr. Richards served as our Controller.
Stefan G Schreck, Ph.D.Dr. Schreck joined us in February 2004 and serves as our Vice President of Research and Development. Dr. Schreck has more than 20 years of experience in research and development of medical products. Prior to joining Endologix, Dr. Schreck held increasingly more responsible R&D management positions in the medical device industry. From May 1995 to April 2000, Dr. Schreck served as Director of Research in Baxter Healthcare’s Heart Valve Division. From April 2000 to August 2002, Dr. Schreck served as Senior Director R&D at Edwards Lifesciences and was responsible for the development of all surgical heart valve repair and replacement products. From August 2002 to February 2004, Dr. Schreck served as President & CEO of MediMorph Solutions Inc., an engineering and management consulting firm for the medical device industry that he founded.
Karen Uyesugi. Ms. Uyesugi has 23 years of both domestic and international regulatory experience in the medical device and pharmaceutical industry. The majority of her career has been involved with a wide variety of Class III and Class II medical devices ranging from implantable cardiovascular devices, neurosurgery, and general surgery products. Ms. Uyesugi has served as our Vice President, Clinical and Regulatory Affairs since the merger with the former Endologix in May 2002. Prior to joining
40
the former Endologix in July 1998, Ms. Uyesugi held various positions in regulatory, clinical, and quality assurance at Neuro Navigational Corporation, Trimedyne, Inc., Baxter Healthcare, Shiley Inc., and Allergan Pharmaceuticals.
Maurice Buchbinder, M.D.Dr. Buchbinder has served on our Board of Directors since January 1999. Dr. Buchbinder was a co-founder and member of the board of directors of the (former) Radiance from August 1997 to January 1999. Since 1995, Dr. Buchbinder has served as the Director of Interventional Cardiology at Sharp Memorial Hospital, San Diego, California and as the Director of Interventional Cardiology at the Foundation for Cardiovascular Research, Scripps Memorial Hospital, La Jolla, California. From 1985 to 1995, Dr. Buchbinder served at various intervals as the Professor of Medicine and the Associate Professor of Medicine, Cardiology Division, UCSD Medical Center, San Diego, California. Dr. Buchbinder is Board certified, Diplomat, from the American Board of Cardiovascular Diseases and the American Board of Internal Medicine.
Roderick de Greef.Mr. de Greef has served on our Board of Directors since November 2003. Mr. de Greef has served as the Executive Vice President, Chief Financial Officer and Secretary of Cardiac Science, Inc. since March 2001. From 1995 to 2001, Mr. de Greef provided corporate finance advisory services to a number of early stage companies including Cardiac Science, where he was instrumental in securing equity capital beginning in 1997, and advising on merger and acquisition activity. From 1989 to 1995, Mr. de Greef was Vice President and Chief Financial Officer of BioAnalogics, Inc. and International BioAnalogics, Inc., both publicly held, development stage medical technology companies located in Portland, Oregon. From 1986 to 1989, Mr. de Greef was Controller and then Chief Financial Officer of publicly held Brentwood Instruments, Inc. Mr. de Greef has a B.A. in Economics and International Relations from California State University at San Francisco and an M.B.A. from the University of Oregon. Mr. de Greef also serves on the boards of BioLife Solutions, Inc. a public biotechnology company located in Binghamton, New York and DentalView, Inc. an Irvine-based privately held medical device company.
Edward M. Diethrich, M.D.Dr. Diethrich has served on our Board of Directors since May 2002. Dr. Diethrich was a Director for the former Endologix, Inc. from 1997 until its merger with us on May 29, 2002. Dr. Diethrich has been the Medical Director and Chief of Cardiovascular Surgery of the Arizona Heart Hospital since 1997, and has been the Director and Chief of Cardiovascular Surgery at the Arizona Heart Institute from 1971 to the present.
Jeffrey O’Donnell. Mr. O’Donnell has served on our Board of Directors since June 1998. Mr. O’Donnell served as our President from January 1998 until March 1999, and Chief Executive Officer from June 1998 until March 1999. From November 1995 to January 1998, Mr. O’Donnell served as our Vice President, Sales and Marketing. Mr. O’Donnell has served as President and Chief Executive Officer of PhotoMedex since November 1999. From March 1999 to November 1999, Mr. O’Donnell served as the President and Chief Executive Officer of X-Site Medical. From January 1994 to May 1995, Mr. O’Donnell served as the President and Chief Executive Officer of Kensey Nash Corporation, a diversified medical device company. Mr. O’Donnell is a member of the board of directors of Escalon Medical Corporation, a publicly held manufacturer and distributor of cardiovascular and ophthalmology devices, Cardiac Science, Inc., a publicly held medical device manufacturer for external cardiac defibrillators and RMI, a private medical device manufacturer.
Gregory D. Waller.Mr. Waller has served on our Board of Directors since November 2003. Mr. Waller has served as Vice President-Finance, Chief Financial Officer and Treasurer of Sybron Dental Specialties, Inc., a manufacturer and marketer of consumable dental products, since August 1993 and was formerly the Vice President and Treasurer of Kerr, Ormco and Metrex. Mr. Waller joined Ormco in December 1980 as Vice President and Controller and served as Vice President of Kerr European Operations from July 1989 to August 1993. Mr. Waller has an MBA with a concentration in accounting from California State University, Fullertion
Audit Committee and Audit Committee Financial Expert
The members of our audit committee are Gregory D. Waller, Roderick de Greef and Jeffrey F. O’Donnell, all of whom satisfy the independence and financial literary standards established by the Securities and Exchange Commission and the NASDAQ National Market. Our Board of Directors has
41
determined that Messrs. Waller, de Greef and O’Donnell all qualify as an “audit committee financial expert” as that term is defined by the rules and regulations of the Securities and Exchange Commission.
Section 16(a) Beneficial Ownership Reporting Compliance
The members of our Board of Directors, our executive officers and persons who hold more than 10% of our outstanding common stock are subject to the reporting requirements of Section 16(a) of the Securities Exchange Act of 1934 which requires them to file reports with respect to their ownership of the common stock and their transactions in such common stock. Based upon (i) the copies of Section 16(a) reports that Endologix received from such persons for their 2003 fiscal year transactions in the common stock and their common stock holdings and/or (ii) the written representations received from one or more of such persons that no annual Form 5 reports were required to be filed by them for the 2003 fiscal year, we believe that all reporting requirements under Section 16(a) for such fiscal year were met in a timely manner by our executive officers, Board members and greater than ten-percent stockholders, with the following exceptions:
Sales of common stock on September 23 and 24 , 2003 by Michael Henson, a former director, were reported on a Form 4 filed on October 7, 2003;
Sales of common stock from September 29 through October 14 , 2003 by Jeffrey O’Donnell were reported on a Form 4 filed on October 20, 2003 and a gift to an irrevocable trust not under investment control by Jeffrey O’Donnell on September 5, 2003 was reported on a Form 5 filed on March 24, 2004;
Automatic annual option grants to Jeffrey O’Donnell, Edward Diethrich, M.D. and Maurice Buchbinder for their service on the Board of Directors, which were granted on October 28, 2003 was reported on a Form 4 filed on November 12, 2003, in the case of Messrs. O’Donnell and Diethrich and November 13, 2003, in the case of Mr. Buchbinder;
A sale of common stock on November 17 , 2003 by Franklin Brown was reported on a Form 4 filed on November 20, 2003.
Code of Ethics
We have adopted a code of ethics that applies to our principal executive officer, principal financial officer and principal accounting officer. A copy of our code of ethics is attached as Exhibit 14 to this Annual Report Form 10-K. We intend to satisfy the disclosure requirement under Item 10 of Form 8-K regarding an amendment to, or waiver from, a provision of this code of ethics by posting such information on our website, www.endologix.com.
Item 11. Executive Compensation
SUMMARY COMPENSATION TABLE
The following table sets forth the salary and bonus earned for the three fiscal years ended December 31, 2003, by our Chief Executive Officer and our executive officers whose salary and bonus exceeded $100,000 for the 2003 fiscal year. All of the individuals named in the table are referred to as the “Named Executive Officers.”
42
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Long Term |
| | | | | | | | | | | | | | | Compensation |
| | | | | | | Annual Compensation
| |
Shares |
| | | | | | | | | | | | | | | Underlying |
| | | | | | | Salary
| | Bonus
| | Options
|
| Franklin D. Brown (a) | | | 2003 | | | $ | 200,000 | | | $ | — | | | | — | |
| Chairman of the Board | | | 2002 | | | | 196,285 | | | | 47,250 | | | | 20,000 | (b) |
| of Directors | | | 2001 | | | | — | | | | — | | | | — | |
| Paul McCormick | | | 2003 | | | $ | 240,000 | | | $ | 50,400 | | | | 100,000 | |
| President and Chief Executive | | | 2002 | | | | 119,583 | | | | 29,025 | | | | — | |
| Officer | | | 2001 | | | | — | | | | — | | | | — | |
| Joseph A. Bishop (c) | | | 2003 | | | $ | 141,000 | | | $ | 35,151 | | | | — | |
| Vice President, Research | | | 2002 | | | | 138,715 | | | | 27,298 | | | | 50,000 | |
| and Operations | | | 2001 | | | | 125,000 | | | | — | | | | 15,000 | |
| David M. Richards | | | 2003 | | | $ | 136,000 | | | $ | 32,477 | | | | 30,000 | |
| Chief Financial Officer and | | | 2002 | | | | 119,231 | | | | 24,713 | | | | 50,000 | |
| Secretary | | | 2001 | | | | 99,789 | | | | — | | | | 5,000 | |
| Karen Uyesugi (d) | | | 2003 | | | $ | 170,000 | | | $ | 38,250 | | | | 30,000 | |
| Vice President Clinical and | | | 2002 | | | | 93,333 | | | | 26,780 | | | | 70,000 | |
| Regulatory Affairs | | | 2001 | | | | — | | | | — | | | | — | |
| (a) | | Mr. Brown currently serves as our Executive Chairman. He served as our Chief Executive Officer and Chairman following the merger with (former) Endologix on May 29, 2002 until January 1, 2003. |
| |
| (b) | | Options were granted before employment as our Chief Executive Officer based upon Board participation. |
| |
| (c) | | Mr. Bishop resigned his position with us on January 9, 2004. |
| |
| (d) | | Ms. Uyesugi became our Vice President of Clinical and Regulatory Affairs following the Merger with (former) Endologix on May 29, 2002. |
43
OPTION GRANTS IN LAST FISCAL YEAR
The following table sets forth the number and potential realizable value of options granted to each of the Named Executive Officers during the year ended December 31, 2003.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | Potential Realizable Value |
| | | | | | | | | | | | | | | | | | | of Options At Assumed |
| | | | | | | | | | | | | | | | | | | Annual Rates of Stock |
| | | | | | | | | | | | | | | | | | | Price Appreciation for |
| | | | | | | Individual Grants
| | | | | | Option Term (4)
|
| | | | | | | % of Total | | | | | | | | |
| | | Number of | | Options | | | | | | | | |
| | | Securities | | Granted To | | | | | | | | |
| | | Underlying | | Employees | | Exercise of | | | | | | |
| | | Options | | in Fiscal | | Base Price | | Expiration | | | | |
Name
| | Granted (1)
| | Year (2)
| | ($/Share) (3)
| | Date
| | 5% ($) (4)
| | 10% ($) (4)
|
| Franklin D. Brown | | | — | | | | — | | | | — | | | | — | | | $ | — | | | $ | — | |
| Paul McCormick | | | 100,000 | | | | 18.1 | % | | $ | 3.92 | | | | 12/11/2013 | | | | 246,527 | | | | 624,747 | |
| Joseph A. Bishop | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| David M. Richards | | | 30,000 | | | | 5.5 | % | | $ | 3.92 | | | | 12/11/2013 | | | | 73,958 | | | | 187,424 | |
| Karen Uyesugi | | | 30,000 | | | | 5.5 | % | | $ | 3.92 | | | | 12/11/2013 | | | | 73,958 | | | | 187,424 | |
| (1) | | The options listed in the table were granted under our 1996 Stock Options/Stock Issuance Plan. The options have a maximum term of ten years measured from the date of grant. Twenty-five percent (25%) of the options are exercisable upon the optionee’s completion of one year of service measured from the date of grant, and the balance are exercisable in a series of successive equal monthly installments upon the optionee’s completion of each additional month of service over the next 36 months thereafter. |
| |
| (2) | | Based upon options granted for an aggregate of 550,000 shares to employees in 2003, including the Named Executive Officers. |
| |
| (3) | | The exercise price may be paid in cash or in shares of our common stock valued at fair market value on the exercise date. |
| |
| (4) | | The 5% and 10% assumed annual rates of compounded stock price appreciation are mandated by rules of the Securities and Exchange Commission. There can be no guarantee that the actual stock price appreciation over the option term will be at the assumed 5% and 10% levels or at any other defined level. Unless the market price of the Common Stock appreciates over the option term, no value will be realized from the option grants made to the executive officers. |
AGGREGATED OPTION EXERCISES IN LAST FISCAL YEAR
AND FISCAL YEAR-END OPTION VALUES
The following table sets forth information with respect to the exercise of options held by the Named Executive Officers during the year ended December 31, 2003.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Number of Securities | | Value of Unexercised In-the- |
| | | Shares | | Aggregate | | Underlying Unexercised | | Money Options at |
| | | Acquired | | Value | | Options at FY-End | | FY-End (2) |
| | | on | | Realized $ | |
| |
|
Name
| | Exercise
| | (1)
| | Exercisable
| | Unexercisable
| | Exercisable
| | Unexercisable
|
| Franklin D. Brown | | | — | | | $ | — | | | | 51,667 | | | | 10,833 | | | $ | 30,657 | | | $ | 25,891 | |
| Paul McCormick | | | — | | | | — | | | | — | | | | 100,000 | | | | — | | | | 3,000 | |
| Joseph A. Bishop | | | — | | | | — | | | | 165,939 | | | | 46,561 | | | | 69,623 | | | | 64,999 | |
| David M. Richards | | | 12,500 | | | | 25,375-- | | | | 59,527 | | | | 64,146 | | | | 30,443 | | | | 93901 | |
| Karen Uyesugi | | | 20,416 | | | | 50,863 | | | | 2,917 | | | | 76,667 | | | | 9,043 | | | | 145,568 | |
| (1) | | Based on the fair market value on the date of exercise less the exercise price payable for such shares. |
| |
| (2) | | Based on the fair market value of our common stock at year-end, $3.95 per share, less the exercise price payable for such shares. |
Compensation of Directors
Non-employee directors each receive a fee of $1,000 per quarter, $1,000 for each Board meeting attended and reimbursement for certain travel expenses and other out-of-pocket costs. Members of Committees of the Board each receive an additional fee of $500 for each Committee meeting attended. Non-employee Board members are eligible to receive periodic option grants under the Automatic Option Grant Program in effect under our 1996 Stock Option/Stock Issuance Plan. Each individual who first becomes a non-employee Board member, whether elected by the stockholders or appointed by the Board, automatically will be granted, at the time of such initial election or appointment, an option to purchase 25,000 shares of Common Stock at the fair market value per share of Common Stock on the grant date. Each option has a maximum term of ten years. On the date of each Annual Meeting of Stockholders, each individual who is to continue to serve as a non-employee Board member after the Annual meeting will receive an additional option grant to purchase 15,000 shares of Common Stock, provided such individual has been a member of the Board for at least six months.
44
Each initial option grant vests over four years, with 25% of the options vesting after one year and the remaining options vesting on a pro rata basis over the next 36 months thereafter, and each annual option grant vests upon the completion of one year of Board service. The option grants also vests immediately upon the optionee’s death or permanent disability or an acquisition of Endologix by merger or asset sale or a hostile change in control of Endologix.
There are no arrangements or understandings involving any director or any nominee regarding such person’ status as a director or nominee.
Management Contracts and Termination of Employment and Change in Control Agreements
We entered into an employment agreement with Mr. Brown, our Executive Chairman, effective October 18, 2002. The agreement has a three-year term and it automatically renews for successive one-year terms thereafter. The agreement provides for annual increases in base salary as may be determined by the Compensation Committee of our Board of Directors. Mr. Brown’s base salary is $200,000, and he is eligible to receive incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Mr. Brown’s employment without cause, he is entitled to his base salary and continued benefits for six months. Additionally, Mr. Brown’s stock options that would have vested over the following six months will vest immediately upon his termination. Lastly, Mr. Brown would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination. In the event Mr. Brown’s employment is terminated in connection with a change in control, he is entitled to his base salary and continued benefits for twelve months and all of his stock options will accelerate and vest and all of our rights to repurchase his restricted stock will terminate. Finally, Mr. Brown would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination.
We entered into an employment agreement with Mr. McCormick, our Chief Executive Officer and President, effective October 18, 2002. The agreement has a three-year term and it automatically renews for successive one-year terms thereafter. The agreement provides for annual increases in base salary as may be determined by the Compensation Committee of our Board of Directors. Mr. McCormick’s base salary is $260,000 and he is eligible to receive an annual cash bonus of up to 35% of his base salary as well as incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Mr. McCormick’s employment without cause, he is entitled to his base salary and continued benefits for six months. Additionally, Mr. McCormick’s stock options that would have vested over the following six months will vest immediately upon his termination. Lastly, Mr. McCormick would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination. In the event Mr. McCormick’s employment is terminated in connection with a change in control, he is entitled to his base salary and continued benefits for twelve months and all of his stock options will accelerate and vest and all of our rights to repurchase his restricted stock will terminate. Finally, Mr. McCormick would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination.
We entered into an employment agreement with Mr. Richards, our Chief Financial Officer and Secretary, effective October 18, 2002. The agreement has a three-year term and it automatically renews for successive one-year terms thereafter. The agreement provides for annual increases in base salary as may be determined by the Compensation Committee of our Board of Directors. Mr. Richards’ base salary is $141,440, and he is eligible to receive an annual cash bonus of up to 30% of his base salary as well as incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Mr. Richards’ employment without cause, he is entitled to his base salary and continued benefits for six months and all stock options that would have vested over the following six months will vest immediately upon his termination. Mr. Richards would also be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination. In the event Mr. Richard’s employment is terminated in connection with a change in control, he is entitled to his base salary and continued benefits for twelve months and all of his stock options will accelerate and vest and all of our rights to repurchase his restricted stock will terminate. Finally, Mr. Richards’ would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination.
45
We entered into an employment agreement with Ms. Uyesugi, Vice President of Clinical and Regulatory Affairs, effective October 18, 2002. The agreement has a three-year term and it automatically renews for successive one-year terms thereafter. The agreement provides for annual increases in base salary as may be determined by the Compensation Committee of our Board of Directors. Ms. Uyesugi’s base salary is $176,800, and she is eligible to receive an annual cash bonus of up to 30% of her base salary as well as incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Ms. Uyesugi’s employment without cause, she is entitled to her base salary and continued benefits for six months and all stock options that would have vested over the following six months will vest immediately upon her termination. Lastly, Ms. Uyesugi would be entitled to a prorated payment equal to the target bonus amount for which she would have been eligible for the year of termination. In the event Ms. Uyesugi is terminated in connection with a change in control, she is entitled to her base salary and continued benefits for twelve months and all stock options will accelerate and vest and all of our rights to repurchase her restricted stock will terminate. Finally, Ms. Uyesugi would be entitled to a prorated payment equal to the target bonus amount for which she would have been eligible for the year of termination.
We entered into an employment agreement with Mr. Schreck, Vice President of Research and Development, effective February 23, 2004. The agreement expires on October 18, 2005, unless sooner terminated pursuant to the terms and provisions of the agreement. Unless thirty days notice is provided by either party before the expiration date, the agreement automatically renews for successive one-year terms thereafter. The agreement provides for annual increases in base salary as may be determined by the Compensation Committee of our Board of Directors. Mr. Schreck’s base salary is $170,000, and he is eligible to receive an annual cash bonus of up to 30% of his base salary as well as incentive-based stock options. The agreement includes executive fringe benefits as is customary for our other executives. If we terminate Mr. Schreck’s employment without cause, he is entitled to his base salary and continued benefits for six months and all stock options that would have vested over the following six months will vest immediately upon her termination. Lastly, Mr. Schreck would be entitled to a prorated payment equal to the target bonus amount for which she would have been eligible for the year of termination. In the event Mr. Schreck is terminated in connection with a change in control, he is entitled to his base salary and continued benefits for twelve months and all stock options will accelerate and vest and all of our rights to repurchase his restricted stock will terminate. Finally, Mr Schreck would be entitled to a prorated payment equal to the target bonus amount for which he would have been eligible for the year of termination.
Compensation Committee Members
Franklin D. Brown and Edward M. Diethrich, M.D. are the compensation committee members. Mr. Brown currently serves as our Executive Chairman and up until December 31, 2002 served as our Chief Executive Officer, both of which are officer positions.
Compensation Committee Interlocks and Insider Participation
None of our executive officers served on the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our Board of Directors or Compensation Committee.
Board Compensation Committee Report on Executive Compensation
The Compensation Committee of the Board of Directors makes recommendations to the full Board with respect to the base salary and bonuses to be paid to our executive officers each fiscal year. In addition, the Compensation Committee has the authority to administer the Endologix 1996 Stock Option/Stock Issuance Plan with respect to option grants and stock issuances made thereunder to officers and other key employees. The following is a summary of the policies of the Compensation Committee that affect the compensation paid to executive officers, as reflected in the tables and text set forth elsewhere in this Annual Report on Form 10-K.
General Compensation Policy. Our compensation policy is designed to attract and retain qualified key executives critical to our success and to provide such executives with performance-based incentives tied to the achievement of certain milestones. One of the Compensation Committee’s primary objectives is to have a substantial portion of each officer’s total compensation contingent upon our performance as well
46
as upon the individual’s contribution to our success as measured by his personal performance. Accordingly, each executive officer’s compensation package is comprised primarily of three elements.
| • | | base salary which reflects individual performance and expertise and is designed to be competitive with salary levels in the industry; |
| |
| • | | variable performance awards payable in cash and tied to our achievement of certain goals; and |
| |
| • | | long-term stock-based incentive awards that strengthen the mutuality of interests between the executive officers and our stockholders. |
The following are the principal factors that the Compensation Committee considered in establishing the components of each executive officer’s compensation package for the 2003 fiscal year. However, the Compensation Committee may in its discretion apply different factors, particularly different measures of financial performance, in setting executive compensation for future fiscal years.
Base Salary. The base salary levels for the executive officers were established by the Board for the 2003 fiscal year on the basis of the following factors: personal performance, the estimated salary levels in effect for similar positions at a select group of companies with which we compete for executive talent, and internal comparability considerations. Although the Compensation Committee reviewed various compensation surveys, the Board did not rely upon any specific survey for comparative compensation purposes. Instead, the Board made its decisions as to the appropriate market level of base salary for each executive officer on the basis of its understanding of the salary levels in effect for similar positions at those companies with which we compete for executive talent. The Compensation Committee on an annual basis will review base salaries, and adjustments will be made in accordance with the factors indicated above.
Annual Incentive Compensation. The Endologix Employee Bonus Plan provides the Board of Directors with discretionary authority to award cash bonuses to executive officers and employees in accordance with recommendations made by the Compensation Committee. The Compensation Committee’s recommendations are based upon the extent to which financial and performance targets (established semi-annually by the Compensation Committee) are met and the contribution of each such officer and employee to the attainment of such targets. For fiscal year 2003, the performance targets for each of the executive officers included gross sales, cash flow, engineering product goals and regulatory goals. The weight given to each factor varied from individual to individual.
Long-Term Incentive Compensation. The 1996 Stock Option/Stock Issuance Plan also provides the Board with the ability to align the interests of the executive officer with those of the stockholders and provide each individual with a significant incentive to manage Endologix from the perspective of an owner with an equity stake in the business. The number of shares subject to each option grant is based upon the officer’s tenure, level of responsibility and relative position in Endologix. We have established general guidelines for making options grants to the executive officers in an attempt to target a fixed number of unvested option shares based upon the individual’s position with Endologix and their existing holdings of unvested options. However, we do not adhere strictly to these guidelines and will vary the size of the option grant made to each executive officer as it feels the circumstances warrant. Each grant allows the officer to acquire shares of our common stock at a fixed price per share (the market price on the grant date) over a specified period of time (up to 10 years from the date of grant). The option normally vests in periodic installments over a four-year period, contingent upon the executive officer’s continued employment with us. Accordingly, the option will provide a return to the executive officer only if he or she remains in our employ and the market price of our common stock appreciates over the option term.
CEO Compensation. The Compensation Committee set the base salary for Paul McCormick, our Chief Executive Officer at a level which is designed to provide a salary competitive with salaries paid to chief executive officers of similarly-sized companies in the industry and commensurate with each such individual’s experience. Mr. McCormick’s experience at the (former) Endologix, given the central role of the CEO in commercializing the technology acquired in the merger, was an important determinant in setting his compensation. As the Company’s activities for 2003 were mainly for research and development, the Compensation Committee did not intend to have the base salary component of compensation affected to
47
any significant degree by our financial performance, but by the Company’s achievement of clinical and regulatory milestones.
Compensation Committee
Franklin D. Brown
Edward M. Diethrich, M.D.
48
Stock Performance Graph
The graph depicted below shows Endologix’s stock price as an index assuming $100 invested on December 31, 1998, along with the composite prices of companies listed on the CRSP Total Return Index for National Association of Securities Dealers Automated Quotation (“NASDAQ”) Stock Market, and the NASDAQ Medical Device Manufacturers’ Index.
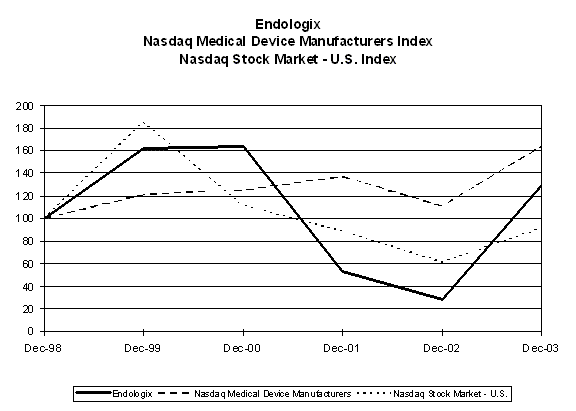
2004 PROXY PERFORMANCE GRAPH DATA
ANNUAL DATA SERIES
SCALED PRICES: Stock and index prices scaled to 100 at 12/31/98
| | | | | | | | | | | | | |
| | | | | | | Nasdaq Medical | | |
| | | | | | | Device | | Nasdaq Stock Market |
DATES
| | Endologix
| | Manufacturers
| | - U.S.
|
| Dec-98 | | | 100.00 | | | | 100.00 | | | | 100.00 | |
| Dec-99 | | | 161.44 | | | | 121.11 | | | | 185.43 | |
| Dec-00 | | | 163.40 | | | | 124.94 | | | | 111.83 | |
| Dec-01 | | | 52.94 | | | | 137.25 | | | | 88.76 | |
| Dec-02 | | | 27.78 | | | | 111.09 | | | | 61.37 | |
| Dec-03 | | | 129.08 | | | | 164.29 | | | | 91.75 | |
49
Item 12. Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information known to us regarding the ownership of our common stock as of March 11, 2004 by: (i) each stockholder known to us to be a beneficial owner of more than five percent (5%) of our common stock; (ii) each director; (iii) each Named Executive Officer; and (iv) all of our current directors and officers as a group.
| | | | | | | | | |
| | | Number of Shares | | Percentage of |
Name and Address (1)
| | Beneficially Owned (2)
| | Outstanding Shares (3)
|
Federated Investors, Inc. a portfolio of Federated Equity Funds 140 East 45th Street, 43rd Floor New York, New York 10017 | | | 4,155,556 | | | | 13.1 | % |
| Franklin D. Brown (4) | | | 468,033 | | | | 1.5 | % |
| Paul McCormick | | | 468,304 | | | | 1.5 | % |
| David M. Richards (5) | | | 93,506 | | | | * | |
| Karen Uyesugi (6) | | | 43,140 | | | | * | |
| Joseph A. Bishop (7) | | | 25,625 | | | | * | |
| Maurice Buchbinder, M.D. (8) | | | 970,640 | | | | 3.0 | % |
| Roderick de Greef | | | | | | | * | |
| Edward B. Diethrich, M.D. (9) | | | 1,008,000 | | | | 3.2 | % |
| Jeffrey F. O’Donnell (10) | | | 213,533 | | | | * | |
| Gregory D. Waller | | | | | | | * | |
| All directors and officers as a group (9 persons) (11) | | | 3,290,781 | | | | 10.2 | % |
| * | | Represents beneficial ownership of less than 1% |
| |
| (1) | | Unless otherwise indicated, the business address of each holder is: c/o Endologix, Inc., 13900 Alton Parkway, Suite 122, Irvine, CA 92618. |
| |
| (2) | | The number of shares of common stock beneficially owned includes any shares issuable pursuant to stock options that may be exercised within 60 days after March 11, 2004. Shares issuable pursuant to such options are deemed outstanding for computing the ownership percentage of the person holding such options but are not deemed to be outstanding for computing the ownership percentage of any other person. |
| |
| (3) | | Applicable percentages are based on 31,676,945 shares outstanding on March 11, 2004, plus the number of shares such stockholder can acquire within 60 days after March 11, 2004. |
| |
| (4) | | Includes 53,333 shares subject to options exercisable within 60 days after March 11, 2004, and 359,700 shares held in a family trust. |
| |
| (5) | | Includes 64,528 shares subject to options exercisable within 60 days after March 11, 2004. |
| |
| (6) | | Includes 10,209 shares subject to options exercisable within 60 days after March 11, 2004. |
| |
| (7) | | Includes 25,625 shares subject to options exercisable within 90 days after Mr. Bishop’s employment departure date of January 9, 2004. |
| |
| (8) | | Includes 249,440 shares subject to options exercisable within 60 days after March 11, 2004, and 18,200 shares held in a family trust. |
50
| (9) | | Includes 1,002,375 held by T&L Investments L.P. Dr. and Mrs. Edward B. Diethrich hold a total of 98% of the voting and dispositive power over the shares through a 98% ownership of the capital stock of EBDFam, Inc., the general partner in T&L Investments LP. Includes 5,625 shares subject to options exercisable within 60 days after March 11, 2004. |
| |
| (10) | | Includes 211,250 shares subject to options exercisable within 60 days after March 11, 2004. |
| |
| (11) | | Includes 620,010 shares subject to options exercisable within 60 days after March 11, 2004. |
Securities Authorized for Issuance under Equity Compensation Plans as of December 31, 2003
Equity Compensation Plan Information
| | | | | | | | | | | | | |
| | | Number of securities to be | | Weighted average | | Number of securities |
| | | issued upon exercise of | | exercise price of | | remaining available for |
Plan category
| | outstanding options
| | outstanding options
| | future issuance
|
| | | (a) | | (b) | | (c) |
| Equity compensation plans approved by security holders: | | | | | | | | | | | | |
| 1996 Stock Option/Stock Issuance Plan | | | 2,049,801 | | | $ | 3.46 | | | | 225,975 | |
| Employee Stock Purchase Plan | | | — | | | | — | | | | 205,768 | |
| Equity compensation plans not approved by security holders: | | | | | | | | | | | | |
| 1997 Supplemental Stock Option Plan | | | 88,500 | | | $ | 4.24 | | | | 1,500 | |
| | | |
| | | |
| | | |
| |
| Total | | | 2,138,301 | | | $ | 3.50 | | | | 227,475 | |
| | | |
| | | |
| | | |
| |
1997 Supplemental Stock Option Plan.
This stock option plan is used to provide compensation to non-employees, typically as part of a consulting services arrangement. The plan authorizes the issuance of non-qualified stock options only. The Company accounts for non-employee stock-based awards, in which goods or services are the consideration received for the stock options issued, in accordance with the provisions of SFAS No.123 and related interpretations (See Note 1 and 11 to the consolidated financial statements for additional information on recognition of expense associated with non-employee option grants under the 1997 Supplemental Stock Option Plan).
Item 13. Certain Relationships and Related Transactions
In May 2002, we merged with (former) Endologix. Under the terms of the merger agreement, we paid $0.75 for each share of Endologix common stock, for an aggregate amount of $8.4 million, and issued one share of our common stock for each share of Endologix common stock, not to exceed an aggregate issuance of 11,140,541 shares. In addition, we are obligated to pay contingent consideration in the amount of $2.8 million in the event pre-market approval, or PMA, is received for our PowerLink System on or before June 30, 2004. We may choose to pay the contingent consideration, if payable, in cash or common stock at our sole discretion.
51
As set forth below, some of our officers and directors may be entitled to receive milestone payments due to their ownership of common stock of the (former) Endologix at the time of the merger. The payment would be due if we receive FDA approval of our PMA application for the PowerLink System on or before June 30, 2004.
| | | | | |
Director or Officer
| | Amount Payable
|
| Franklin D. Brown | | $ | 240,000 | |
| Paul McCormick | | | 108,750 | |
| Karen Uyesugi | | | 37,500 | |
| Edward B. Diethrich, M. D. | | | 312,500 | |
| Jeffrey F. O’Donnell | | | 15,000 | |
Item 14. Principal Accountant Fees and Services
The audit committee reviews and pre-approves all non-audit services to be performed by our independent auditors, PricewaterhouseCoopers LLP. Such pre-approval is on a project by project basis and therefore the our Audit Committee has not adopted a pre-approval policy with respect to such non-audit services.
The following table sets forth the aggregate fees billed to us by PricewaterhouseCoopers LLP for the fiscal years ended December 31, 2002 and December 31, 2003:
| | | | | | | | | |
| | | December 31, | | December 31, |
| | | 2002
| | 2003
|
| Audit Fees (1) | | $ | 113,900 | | | $ | 150,360 | |
| Audit-Related Fees (2) | | | 115,425 | | | | — | |
| Tax Fees | | | — | | | | — | |
| All Other Fees | | | — | | | | — | |
| | | |
| | | |
| |
| Total | | $ | 229,325 | | | $ | 150,360 | |
| | | |
| | | |
| |
(1) Includes fees for professional services rendered for the audit of our annual financial statements, reviews of the financial statements included in quarterly reports on Form 10-Q, and issuance of consents.
(2) Includes fees for review of 2002 merger transaction.
PART IV
Item 15. Exhibits, Financial Statement Schedules, And Reports on Form 8-K
(a) The following documents are filed as a part of this Annual Report on Form 10-K:
1. Financial Statements.
| |
| Report of Independent Auditors |
| Consolidated Balance Sheets — December 31, 2002 and 2003 |
| Consolidated Statements of Operations for the years ended December 31, 2001, 2002 and 2003 |
| Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2001, 2002 and 2003 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2001, 2002 and 2003 |
| Notes to Consolidated Financial Statements for the years ended December 31, 2001, 2002 and 2003 |
52
2. Financial Statement Schedule.
II — Valuation and Qualifying Accounts
Schedules not listed above have been omitted because they are not applicable or are not required to be set forth herein as such information is included in the Consolidated Financial Statements or the notes thereto.
3. Exhibits.
Reference is made to Item 15(c) of this Annual Report on Form 10-K.
(b) REPORTS ON FORM 8-K.
We filed a Report on Form 8-K as of November 7, 2003 announcing the financial results for the 3rd quarter of 2003.
(c) EXHIBITS.
| | | |
| EXHIBIT | | |
NUMBER
| | DESCRIPTION
|
| 2.4(1) | | Agreement and Plan of Merger dated November 3, 1998 by and between CardioVascular Dynamics, Inc. and Radiance Medical Systems, Inc. |
| | | |
| 2.5(2) | | Assets Sale and Purchase Agreement dated January 21, 1999 by and between the Company and Escalon Medical Corp. |
| | | |
| 2.5.1(3) | | Amendment and Supplement to Assets Sale and Purchase Agreement and Release dated February 28, 2001 by and between the Company and Escalon Medical Corp. |
| | | |
| 2.5.2(3) | | Short-Term Note, Exhibit 1, to Amendment and Supplement to Assents Sale and Purchase Agreement and Release dated February 28, 2001 by and between the Company and Escalon Medical Corp. |
| | | |
| 2.5.3(3) | | Long-Term Note, Exhibit 2, to Amendment and Supplement to Assents Sale and Purchase Agreement and Release dated February 28, 2001 by and between the Company and Escalon Medical Corp. |
| | | |
| 2.6(4) | | Agreement and Plan of Merger, dated as of February 8, 2002, by and among the Company, RMS Acquisition Corp. and Endologix, Inc. |
| | | |
| 3.1 | | Restated Certificate of Incorporation as currently in effect. |
| | | |
| 3.2(5) | | Amended and Restated Bylaws |
| | | |
| 4.1(6) | | Specimen Certificate of Common Stock |
| | | |
| 10.1(7) | | Form of Indemnification Agreement entered into between the Registrant and its directors and officers |
| | | |
| 10.3(7) | | The Registrant’s Employee Stock Purchase Plan and forms of agreement thereunder |
| | | |
| 10.15(7) | | Industrial Lease dated February 23, 1995 by and between the Irvine Company and the Company |
| | | |
| 10.22(8) | | Supplemental Stock Option Plan |
53
| | | |
| EXHIBIT | | |
NUMBER
| | DESCRIPTION
|
| 10.24(9)* | | License Agreement by and between the Company and Guidant dated June 19, 1998 |
| | | |
| 10.25(10) | | 1996 Stock Option/Stock Issuance Plan (as Amended and Restated as of April 8, 1997, March 12, 1998 and November 3, 1998) |
| | | |
| 10.26(11) | | 1997 Stock Option Plan (As Amended as of June 15, 1998) assumed by Registrant pursuant to its acquisition of Radiance Medical Systems, Inc. on January 14, 1999 |
| | | |
| 10.36(12) | | Form of Employment Agreement dated August 21, 2000 by and between the Company and Joseph A. Bishop |
| | | |
| 10.40*(13) | | Supply Agreement dated as of February 12, 1999, and as amended August 4, 1999, November 16, 1999, March 10, 2000, and January 31, 2001 by and between the Company and Impra, Inc. |
| | | |
| 10.40.1* (13) | | Amendment to Supply Agreement dated January 17, 2002 by and between the Company and Impra, Inc. |
| | | |
| 10.41(14) | | Form of Indemnification Agreement dated October 1, 2002 by and between the Company and its officers and directors. |
| | | |
| 10.42 (15) | | Form of Employment Agreement dated October 18, 2002 by and between the Company and its officers, excluding Joseph A. Bishop. |
| | | |
| 10.42.2 (15) | | Schedule of Parties to the Employment Agreement. |
| | | |
| 10.43 | | Amendment to Employment Agreement dated October 18, 2002 by and between the Company and Franklin D. Brown, dated December 17, 2003, which is attached as Exhibit 10.43. |
| | | |
| 10.44 | | Amendment to Employment Agreement dated October 18, 2002 by and between the Company and Paul McCormick, dated December 17, 2003, which is attached as Exhibit 10.44. |
| | | |
| 10.45 | | Form of Employment Agreement dated February 26, 2004 by and between the Company and Stefan G. Schreck, which is attached as Exhibit 10.45. |
| | | |
| 14 | | Form of the Code of Ethics for Our Chief Executive Officer and Principal Financial Officers, which is attached as Exhibit 14. |
54
| | | |
| EXHIBIT | | |
NUMBER
| | DESCRIPTION
|
| 21.1 | | List of Subsidiaries |
| | | |
| 23.1 | | Consent of PricewaterhouseCoopers LLP |
| | | |
| 24.1 | | Power of Attorney (included on signature page hereto) |
| | | |
| 31.1 | | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a)/15d-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 31.2 | | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a)/15d-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.1 | | Certification of Chief Executive Officer, Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.2 | | Certification of Chief Financial Officer, Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
* Portions of this exhibit are omitted and were filed separately with the Securities and Exchange Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934.
(1) Previously filed as Exhibit 2.4 to the Company’s Report on Form 8-K filed with the Securities and Exchange Commission as of November 12, 1998.
(2) Previously filed as Exhibit 2 to the Company’s Report on Form 8-K filed with the Securities and Exchange Commission as of February 5, 1999.
(3) Previously filed as an exhibit to the Company’s Report on Form 10-K filed with the Securities and Exchange Commission on March 29, 2001.
(4) Previously filed as Annex I to the Company’s Proxy Statement on Schedule 14A filed with the Securities Exchange Commission on April 26, 2002.
(5) Previously filed as Exhibit 3.4 to the Company’s Report on Form 10-Q filed with the Securities and Exchange Commission on November 16, 1998.
(6) Previously filed as an exhibit to Amendment No. 2 to the Company’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on June 10, 1996.
(7) Previously filed as an exhibit to the Company’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on May 3, 1996.
(8) Previously filed as an exhibit to the Company’s Registration Statement on Form S-8 filed with the Securities and Exchange Commission on December 12, 1997.
(9) Previously filed as Exhibit 10.24 to the Company’s Report on Form 10-Q filed with the Securities and Exchange Commission as of August 11, 1998.
(10) Previously filed as Annex III to the Company’s Proxy Statement on Schedule 14A filed with the Securities and Exchange Commission on December 18, 1998.
(11) Previously filed as Exhibit 99.2 to the Company’s Registration Statement on Form S-8 filed with the Securities and Exchange Commission on February 17, 1999.
55
(12) Previously filed as Exhibit 10.36 to Amendment No. 1 to the Company’s Registration Statement on Form S-2 filed with the Securities and Exchange Commission on September 11, 2000.
(13) Previously filed as an exhibit to the Company’s Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2002.
(14) Previously filed as an exhibit to the Company’s Report on Form 10-Q filed with the Securities and Exchange Commission on November 13, 2002.
(15) Previously filed as an exhibit to the Company’s Report on Form 10-K filed with the Securities and Exchange Commission on March 27, 2003.
56
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this amendment to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| | | ENDOLOGIX, INC. |
| | | | | |
| Date: March 25, 2004 | | By: | | /s/ PAUL MCCORMICK |
| | | |
|
| | | | Paul McCormick |
| | | | Chief Executive Officer and Director |
| | | | (Principal Executive Officer) |
POWER OF ATTORNEY
We, the undersigned directors and officers of Endologix, Inc., do hereby constitute and appoint Paul McCormick and David M. Richards, and each of them, as our true and lawful attorney-in-fact and agents with power of substitution, to do any and all acts and things in our name and behalf in our capacities as directors and officers and to execute any and all instruments for us and in our names in the capacities indicated below, which said attorney-in-fact and agent may deem necessary or advisable to enable said corporation to comply with the Securities and Exchange Act of 1934, as amended, and any rules, regulations and requirements of the Securities and Exchange Commission, in connection with this Annual Report on Form 10-K, including specifically but without limitation, power and authority to sign for us or any of us in our names in the capacities indicated below, any and all amendments (including post-effective amendments) hereto; and we do hereby ratify and confirm all that said attorney-in-fact and agent, shall do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, the following persons on behalf of the registrant and in the capacities and on the dates indicated have signed this report below.
| | | | | |
SIGNATURE
| | TITLE
| | DATE
|
/s/ PAUL MCCORMICK
(Paul McCormick) | | Chief Executive Officer
and Director
(Principal Executive Officer) | | March 25, 2004 |
| | | | | |
/s/ DAVID M. RICHARDS
(David M. Richards) | | Chief Financial Officer, and
Secretary (Principal Financial
and Accounting Officer) | | March 25, 2004 |
| | | | | |
/s/ FRANKLIN D. BROWN
(Franklin D. Brown) | | Executive Chairman and Director | | March 25, 2004 |
| | | | | |
/s/ MAURICE BUCHBINDER, M.D.
(Maurice Buchbinder, M.D.) | | Director | | March 25, 2004 |
| | | | | |
/s/ RODERICK DE GREEF.
(Roderick de Greef) | | Director | | March 25, 2004 |
| | | | | |
/s/ EDWARD DIETHRICH, M.D.
(Edward Diethrich, M.D.) | | Director | | March 25, 2004 |
| | | | | |
/s/ JEFFREY F. O’DONNELL
(Jeffrey F. O’Donnell) | | Director | | March 25, 2004 |
| | | | | |
/s/ GREGORY D. WALLER
(Gregory D. Waller) | | Director | | March 25, 2004 |
57
Report of Independent Auditors
To the Board of Directors and Stockholders
of Endologix, Inc.
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of Endologix, Inc. and its subsidiaries at December 31, 2003 and 2002, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2003 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. These financial statements and financial statement schedule are the responsibility of the Company’s management; our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits. We conducted our audits of these statements in accordance with auditing standards generally accepted in the United States of America, which require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
Orange County, California
March 10, 2004
F-1
ENDOLOGIX, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| | | | | | | | | |
| | | December 31,
|
| | | 2002
| | 2003
|
| ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 2,606 | | | $ | 4,402 | |
| Marketable securities available-for-sale, including unrealized gains of $23 and $6 | | | 5,053 | | | | 8,166 | |
| Accounts receivable, net of allowance for doubtful accounts of $165 and $16 | | | 622 | | | | 239 | |
| Other receivables | | | 1,004 | | | | 656 | |
| Inventories | | | 2,043 | | | | 2,780 | |
| Other current assets | | | 431 | | | | 245 | |
| | | |
| | | |
| |
| Total current assets | | | 11,759 | | | | 16,488 | |
| | | |
| | | |
| |
| Property and equipment: | | | | | | | | |
| Furniture and equipment | | | 195 | | | | 209 | |
| Leasehold improvements | | | 30 | | | | 54 | |
| | | |
| | | |
| |
| | | | 225 | | | | 263 | |
| Less accumulated depreciation and amortization | | | (40 | ) | | | (122 | ) |
| | | |
| | | |
| |
| Net property and equipment | | | 185 | | | | 141 | |
| Marketable securities available-for-sale, including unrealized gains of $30 and $1 | | | 2,051 | | | | 211 | |
| Goodwill | | | 3,602 | | | | 3,602 | |
| Intangibles, net | | | 15,939 | | | | 14,534 | |
| Other assets | | | 371 | | | | 367 | |
| | | |
| | | |
| |
| Total assets | | $ | 33,907 | | | $ | 35,343 | |
| | | |
| | | |
| |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 2,348 | | | $ | 1,468 | |
| | | |
| | | |
| |
| Total current liabilities | | | 2,348 | | | | 1,468 | |
| Minority interest | | | 83 | | | | — | |
| | | |
| | | |
| |
| Total liabilities | | | 2,431 | | | | 1,468 | |
| | | |
| | | |
| |
| Commitments and contingencies (Notes 10 and 15) | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Preferred stock, $0.001 par value; 5,000,000 shares authorized, no shares issued and outstanding | | | | | | | | |
| Common stock, $0.001 par value; 50,000,000 shares authorized, 24,314,000 and 28,576,000 shares issued and outstanding | | | 24 | | | | 28 | |
| Additional paid-in capital | | | 99,495 | | | | 108,279 | |
| Accumulated deficit | | | (68,004 | ) | | | (73,919 | ) |
| Treasury stock, at cost, 227,000 and 495,000 shares | | | (205 | ) | | | (661 | ) |
| Accumulated other comprehensive income | | | 166 | | | | 148 | |
| | | |
| | | |
| |
| Total stockholders’ equity | | | 31,476 | | | | 33,875 | |
| | | |
| | | |
| |
| Total liabilities and stockholders’ equity | | $ | 33,907 | | | $ | 35,343 | |
| | | |
| | | |
| |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-2
ENDOLOGIX, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| | | | | | | | | | | | | |
| | | Year Ended December 31,
|
| | | 2001
| | 2002
| | 2003
|
| Revenue: | | | | | | | | | | | | |
| Product | | $ | 1,111 | | | $ | 834 | | | $ | 1,395 | |
| License | | | 6,528 | | | | 6,565 | | | | 2,595 | |
| | | |
| | | |
| | | |
| |
| Total revenue | | | 7,639 | | | | 7,399 | | | | 3,990 | |
| | | |
| | | |
| | | |
| |
| Cost of sales: | | | | | | | | | | | | |
| Cost of product sales | | | 1,149 | | | | 460 | | | | 625 | |
| Cost of sales from restructuring | | | 601 | | | | — | | | | — | |
| | | |
| | | |
| | | |
| |
| Total cost of sales | | | 1,750 | | | | 460 | | | | 625 | |
| | | |
| | | |
| | | |
| |
| Gross profit | | | 5,889 | | | | 6,939 | | | | 3,365 | |
| | | |
| | | |
| | | |
| |
| Operating costs and expenses: | | | | | | | | | | | | |
| Research and development | | | 14,605 | | | | 6,155 | | | | 6,711 | |
| Marketing and sales | | | 1,305 | | | | 982 | | | | 787 | |
| General and administrative | | | 2,582 | | | | 2,435 | | | | 2,083 | |
| Charge for acquired in-process research and development | | | — | | | | 4,501 | | | | — | |
| Restructuring charges | | | 4,617 | | | | 168 | | | | — | |
| Minority interest in losses of subsidiary | | | (65 | ) | | | (27 | ) | | | (16 | ) |
| | | |
| | | |
| | | |
| |
| Total operating costs and expenses | | | 23,044 | | | | 14,214 | | | | 9,565 | |
| | | |
| | | |
| | | |
| |
| Loss from operations | | | (17,155 | ) | | | (7,275 | ) | | | (6,200 | ) |
| | | |
| | | |
| | | |
| |
| Other income (expense): | | | | | | | | | | | | |
| Interest income | | | 1,426 | | | | 608 | | | | 302 | |
| Gain on sale of assets | | | 89 | | | | 111 | | | | 8 | |
| Other income (expense), net | | | (1 | ) | | | (11 | ) | | | (25 | ) |
| | | |
| | | |
| | | |
| |
| Total other income | | | 1,514 | | | | 708 | | | | 285 | |
| | | |
| | | |
| | | |
| |
| Net loss | | $ | (15,641 | ) | | $ | (6,567 | ) | | $ | (5,915 | ) |
| | | |
| | | |
| | | |
| |
| Basic and diluted net loss per share | | $ | (1.20 | ) | | $ | (0.33 | ) | | $ | (0.23 | ) |
| | | |
| | | |
| | | |
| |
| Shares used in computing basic and diluted net loss per share | | | 13,086 | | | | 19,718 | | | | 25,845 | |
| | | |
| | | |
| | | |
| |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-3
ENDOLOGIX, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional | | | | | | Treasury |
| | |
| | Paid-In | | Accumulated | |
|
| | | Shares
| | Amount
| | Capital
| | Deficit
| | Shares
| | Amount
|
Balance at December 31, 2000 | | | 13,049,000 | | | $ | 13 | | | $ | 80,678 | | | $ | (45,796 | ) | | | — | | | $ | — | |
| Exercise of common stock options | | | 21,000 | | | | — | | | | 38 | | | | — | | | | — | | | | — | |
| Employee stock purchase plan | | | 52,000 | | | | — | | | | 216 | | | | — | | | | — | | | | — | |
| Amortization of deferred compensation | | | — | | | | — | | | | (97 | ) | | | — | | | | — | | | | — | |
| Net loss | | | — | | | | — | | | | — | | | | (15,641 | ) | | | — | | | | — | |
| Unrealized gain on investments, net | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Unrealized exchange rate loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance at December 31, 2001 | | | 13,122,000 | | | | 13 | | | | 80,835 | | | | (61,437 | ) | | | — | | | | — | |
| Exercise of common stock options | | | 39,000 | | | | — | | | | 40 | | | | — | | | | — | | | | — | |
| Employee stock purchase plan | | | 12,000 | | | | — | | | | 16 | | | | — | | | | — | | | | — | |
| Common stock issued in conjunction with a business combination | | | 11,141,000 | | | | 11 | | | | 18,626 | | | | — | | | | — | | | | — | |
| Common stock repurchased | | | — | | | | — | | | | — | | | | — | | | | (227,000 | ) | | | (205 | ) |
| Amortization of deferred compensation | | | — | | | | — | | | | (22 | ) | | | — | | | | — | | | | — | |
| Net loss | | | — | | | | — | | | | — | | | | (6,567 | ) | | | — | | | | — | |
| Unrealized holding loss arising during the period, net | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Reclassification adjustment for realized gains included in loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Unrealized exchange rate gain | | | — | | | | — | | | | — | | | | — | | | | | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance at December 31, 2002 | | | 24,314,000 | | | | 24 | | | | 99,495 | | | | (68,004 | ) | | | (227,000 | ) | | | (205 | ) |
| Exercise of common stock options | | | 139,000 | | | | — | | | | 184 | | | | — | | | | — | | | | — | |
| Employee stock purchase plan | | | 123,000 | | | | — | | | | 95 | | | | — | | | | — | | | | — | |
| Sale of Common stock | | | 4,000,000 | | | | 4 | | | | 8,353 | | | | — | | | | — | | | | — | |
| Common stock repurchased | | | — | | | | — | | | | — | | | | — | | | | (268,000 | ) | | | (456 | ) |
| Amortization of deferred compensation | | | — | | | | — | | | | 75 | | | | — | | | | — | | | | — | |
| Compensation from modification of Director’s stock options | | | — | | | | — | | | | 77 | | | | — | | | | — | | | | — | |
| Net loss | | | — | | | | — | | | | — | | | | (5,915 | ) | | | — | | | | — | |
| Unrealized holding loss arising during the period, net | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Unrealized exchange rate gain | | | — | | | | — | | | | — | | | | — | | | | | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance at December 31, 2003 | | | 28,576,000 | | | $ | 28 | | | $ | 108,279 | | | $ | (73,919 | ) | | | (495,000 | ) | | $ | (661 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
[Additional columns below]
[Continued from above table, first column(s) repeated]
| | | | | | | | | | | | | |
| | | Accumulated | | | | |
| | | Other | | | | |
| | | Comprehensive | | Stockholders’ | | Comprehensive |
| | | Income
| | Equity
| | Loss
|
Balance at December 31, 2000 | | $ | 345 | | | $ | 35,240 | | | $ | (5,266 | ) |
| | | | | | | | | | | |
| |
| Exercise of common stock options | | | — | | | | 38 | | | | | |
| Employee stock purchase plan | | | — | | | | 216 | | | | | |
| Amortization of deferred compensation | | | — | | | | (97 | ) | | | | |
| Net loss | | | — | | | | (15,641 | ) | | $ | (15,641 | ) |
| Unrealized gain on investments, net | | | 115 | | | | 115 | | | | 115 | |
| Unrealized exchange rate loss | | | (113 | ) | | | (113 | ) | | | (113 | ) |
| | | |
| | | |
| | | |
| |
Balance at December 31, 2001 | | | 347 | | | | 19,758 | | | $ | (15,639 | ) |
| | | | | | | | | | | |
| |
| Exercise of common stock options | | | — | | | | 40 | | | | | |
| Employee stock purchase plan | | | — | | | | 16 | | | | | |
| Common stock issued in conjunction with a business combination | | | — | | | | 18,637 | | | | | |
| Common stock repurchased | | | — | | | | (205 | ) | | | — | |
| Amortization of deferred compensation | | | — | | | | (22 | ) | | | — | |
| Net loss | | | — | | | | (6,567 | ) | | $ | (6,567 | ) |
| Unrealized holding loss arising during the period, net | | | (141 | ) | | | (141 | ) | | | (141 | ) |
| Reclassification adjustment for realized gains included in loss | | | (62 | ) | | | (62 | ) | | | (62 | ) |
| Unrealized exchange rate gain | | | 22 | | | | 22 | | | | 22 | |
| | | |
| | | |
| | | |
| |
Balance at December 31, 2002 | | | 166 | | | | 31,476 | | | $ | (6,748 | ) |
| | | | | | | | | | | |
| |
| Exercise of common stock options | | | — | | | | 184 | | | | | |
| Employee stock purchase plan | | | — | | | | 95 | | | | | |
| Sale of Common stock | | | — | | | | 8,357 | | | | | |
| Common stock repurchased | | | — | | | | (456 | ) | | | | |
| Amortization of deferred compensation | | | — | | | | 75 | | | | | |
| Compensation from modification of Director’s stock options | | | — | | | | 77 | | | | | |
| Net loss | | | — | | | | (5,915 | ) | | $ | (5,915 | ) |
| Unrealized holding loss arising during the period, net | | | (46 | ) | | | (46 | ) | | | (46 | ) |
| Unrealized exchange rate gain | | | 28 | | | | 28 | | | | 28 | |
| | | |
| | | |
| | | |
| |
Balance at December 31, 2003 | | $ | 148 | | | $ | 33,875 | | | $ | (5,933 | ) |
| | | |
| | | |
| | | |
| |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-4
ENDOLOGIX, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | | |
| | | Year Ended December 31,
|
| | | 2001
| | 2002
| | 2003
|
| Operating activities: | | | | | | | | | | | | |
| Net loss | | $ | (15,641 | ) | | $ | (6,567 | ) | | $ | (5,915 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
| Acquired in-process research and development charge | | | — | | | | 4,501 | | | | — | |
| Restructuring charges | | | 3,453 | | | | 168 | | | | — | |
| Depreciation and amortization | | | 1,057 | | | | 867 | | | | 1,494 | |
| Amortization of deferred compensation | | | (97 | ) | | | (22 | ) | | | 75 | |
| Stock-based compensation | | | — | | | | — | | | | 77 | |
| Bad debt expense (recovery) | | | 131 | | | | (3 | ) | | | (139 | ) |
| Minority interest in losses of subsidiary | | | (65 | ) | | | (27 | ) | | | (16 | ) |
| Loss (gain) on disposal of assets | | | (19 | ) | | | (69 | ) | | | 17 | |
| Forgiveness of officer loan | | | — | | | | 137 | | | | — | |
| Changes (net of effects of acquisition): | | | | | | | | | | | | |
| Accounts receivable | | | 295 | | | | 215 | | | | 522 | |
| Inventories | | | 411 | | | | (575 | ) | | | (737 | ) |
| Other receivables and current assets | | | 79 | | | | 1,837 | | | | 534 | |
| Accounts payable and accrued expenses | | | 523 | | | | (2,331 | ) | | | (880 | ) |
| Deferred revenue | | | (81 | ) | | | (360 | ) | | | — | |
| | | |
| | | |
| | | |
| |
| Net cash used in operating activities | | | (9,954 | ) | | | (2,229 | ) | | | (4,968 | ) |
| | | |
| | | |
| | | |
| |
| Investing activities: | | | | | | | | | | | | |
| Purchases of available-for-sale securities | | | (17,157 | ) | | | (9,510 | ) | | | (9,175 | ) |
| Maturities of available-for-sale securities | | | 24,335 | | | | 19,254 | | | | 7,856 | |
| Capital expenditures for property and equipment | | | (329 | ) | | | (87 | ) | | | (42 | ) |
| Final distribution to subsidiary minority interest shareholder | | | — | | | | — | | | | (67 | ) |
| Purchase of (former) Endologix, net of cash acquired of $2,097 | | | — | | | | (7,033 | ) | | | — | |
| | | |
| | | |
| | | |
| |
| Net cash provided by (used in) investing activities | | | 6,849 | | | | 2,624 | | | | (1,428 | ) |
| | | |
| | | |
| | | |
| |
| Financing activities: | | | | | | | | | | | | |
| Proceeds from sale of common stock, net of expenses | | | — | | | | — | | | | 8,357 | |
| Proceeds from sale of common stock under employee stock purchase plan | | | 216 | | | | 16 | | | | 95 | |
| Proceeds from exercise of stock options | | | 38 | | | | 40 | | | | 184 | |
| Repayment of note payable | | | — | | | | (1,000 | ) | | | — | |
| Purchases of treasury stock | | | — | | | | (205 | ) | | | (456 | ) |
| | | |
| | | |
| | | |
| |
| Net cash provided by (used in) financing activities | | | 254 | | | | (1,149 | ) | | | 8,180 | |
| | | |
| | | |
| | | |
| |
| Effect of exchange rate changes on cash and cash equivalents | | | (133 | ) | | | 33 | | | | 12 | |
| | | |
| | | |
| | | |
| |
| Net (decrease) increase in cash and cash equivalents | | | (2,984 | ) | | | (721 | ) | | | 1,796 | |
| Cash and cash equivalents, beginning of year | | | 6,311 | | | | 3,327 | | | | 2,606 | |
| | | |
| | | |
| | | |
| |
| Cash and cash equivalents, end of year | | $ | 3,327 | | | $ | 2,606 | | | $ | 4,402 | |
| | | |
| | | |
| | | |
| |
| Supplemental disclosure of non-cash financing activities: | | | | | | | | | | | | |
| In May 2002, the Company acquired all of the stock of (former) Endologix. The following is a summary of the transaction: | | | | | | | | | | | | |
| Fair value of assets acquired, including intangible assets | | | | | | $ | 25,664 | | | | | |
| Acquired in-process research and development | | | | | | | 4,501 | | | | | |
| Cash paid | | | | | | | (9,129 | ) | | | | |
| Merger consideration due | | | | | | | (12 | ) | | | | |
| Common stock issued | | | | | | | (18,637 | ) | | | | |
| | | | | | | |
| | | | | |
| Liabilities assumed | | | | | | $ | 2,387 | | | | | |
| | | | | | | |
| | | | | |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-5
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In Thousands, Except Share and Per Share Amounts)
1. Business, Basis of Presentation and Summary of Significant Accounting Policies
Business and Basis of Presentation
Endologix, Inc. (formerly named Radiance Medical Systems, Inc. and Cardiovascular Dynamics, Inc. and referred to as “Endologix” or the “Company”) was incorporated in California in March 1992 and reincorporated in Delaware in June 1993. In January 1999, the Company merged with privately held Radiance Medical Systems, Inc. (“former Radiance”), and changed its name to Radiance Medical Systems, Inc. In May 2002, the Company merged with privately held Endologix, Inc., and changed its name to Endologix, Inc. (Note 2).
Since the merger in May 2002, the Company has been engaged in the development, manufacture, sales and marketing of minimally invasive therapies for the treatment of vascular disease. The Company’s primary focus is the development of the PowerLink System, a catheter-based alternative treatment for abdominal aortic aneurysms, or AAA. AAA is a weakening of the wall of the aorta, the largest artery of the body.
Prior to restructuring in September 2001 (Note 14) and the merger in May 2002 (Note 2) the Company was developing proprietary devices to deliver radiation to prevent the recurrence of blockages in arteries following balloon angioplasty, vascular stenting, arterial bypass surgery and other interventional treatments of blockages in coronary and peripheral arteries. The Company also manufactured, licensed and sold angioplasty catheters and stent products primarily through medical device distributors.
The consolidated financial statements include the accounts of the Company and its wholly and majority-owned subsidiaries. Intercompany transactions have been eliminated in consolidation. The Company operates in a single business segment.
For the years ended December 31, 2003 and 2002, the Company has incurred net losses of $5.9 million and $6.6 million, respectively. As of December 31, 2003, the Company had an accumulated deficit of approximately $73.9 million. In March 2004, the Company closed a private placement of 3,200,000 shares of common stock at $5.10 per share, which resulted in aggregate net proceeds of $15.3 million, after deduction of transaction expenses (Note 17). Management believes that current cash and cash equivalents, and marketable securities are sufficient to meet anticipated cash needs for operating and capital expenditures through at least December 31, 2004. Unanticipated reductions in royalty revenue, failure of the market to accept the Company’s products, failure to raise capital to fund operations beyond 2004, or failure to reduce certain discretionary expenditures, if necessary, could have a material adverse effect on the Company’s ability to achieve its intended business objectives.
Significant Accounting Policies and Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, the Company evaluates its estimates, including those related to collectibility of customer accounts, whether the cost of inventories can be recovered, the value assigned to and estimated useful life of intangible assets, the realization of tax assets and estimates of tax liabilities, contingent liabilities and the potential outcome of litigation. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions.
Cash and Cash Equivalents
Cash and cash equivalents includes cash on hand, demand deposits, money market funds and debt securities with original maturities of three months or less from the date of purchase.
F-6
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
Marketable Securities Available-For-Sale
The Company accounts for its investments pursuant to Statement of Financial Accounting Standards (“SFAS”) No. 115, Accounting for Certain Investments in Debt and Equity Securities.
The Company has classified its entire investment portfolio as available-for-sale. Available-for-sale securities are stated at fair value with unrealized gains and losses included in accumulated other comprehensive income, net of realized gains and losses. Management evaluates the classification of its securities based on the Company’s short-term cash needs. The amortized cost of debt securities is adjusted for amortization of premiums and accretions of discounts to maturity. Such amortization is included in interest income. Realized gains of $70, $69 and $-0- for the years ended December 31, 2001, 2002 and 2003, respectively, are included in other income. The cost of securities sold is based on the specific identification method.
Inventories
Inventories are stated at the lower of cost, determined on an average cost basis, or market value.
Property and Equipment
Property and equipment are stated at cost and depreciated on a straight-line basis over the estimated useful lives of the assets. Leasehold improvements are amortized over the term of the lease or the estimated useful life of the asset, whichever is shorter. Maintenance and repairs are expensed as incurred while renewals or betterments are capitalized. Upon sale or disposition of property and equipment, any gain or loss is included in the statement of operations. The estimated useful lives for furniture and equipment range from three to seven years and the estimated useful life for leasehold improvements is seven years. Following the restructuring in September 2001 (Note 14), the estimated useful lives of production-related equipment were reduced to three months to reflect the estimated time necessary to manufacture products to complete existing Focus technology product orders and RDX systems for the remaining clinical trials.
Intangible Assets
In accordance with SFAS No. 142, “Goodwill and Other Intangible Assets,” goodwill and other intangible assets with indeterminate lives are not subject to amortization but are tested for impairment annually or whenever events or changes in circumstances indicate that the asset might be impaired. The Company performed their annual impairment analysis as of June 30, 2003 and will continue to test for impairment annually as of June 30. No impairment was indicated. Other intangible assets with finite lives are subject to amortization, and impairment reviews are performed in accordance with SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets.” Intangible assets, totaling $20,360, were acquired in the acquisition of the (former) Endologix. Specifically, $3,602, $2,708 and $14,050 was recorded as goodwill, trademarks and tradenames and developed technology, respectively, and $4,501 was expensed as acquired in-process research and development. The developed technology is being amortized over its estimated useful life of 10 years. During the years ended December 31, 2002 and 2003, the Company recorded $819 and $1,405 in amortization expense for the developed technology.
Prior to SFAS No. 142, intangible assets acquired in connection with business combinations were amortized on the straight-line method over their estimated useful lives. Intangible assets, totaling $4,567, from the 1998 purchase of a controlling interest in and 1999 acquisition of privately held Radiance Medical Systems, Inc. (the “former Radiance”) were being amortized over three to seven years, respectively. Based upon a valuation of intangible assets acquired in the purchase of a controlling interest in and acquisition of the former Radiance, $3,266 and $1,301 were recorded as developed technology and covenants not to compete, respectively. During the years ended December 31, 2001 and 2002, the Company recorded $675
F-7
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
and $-0- of amortization expense, respectively, for developed technology and covenants not to compete (see Note 14 for discussion of restructuring activities and impairment of these assets).
Long-Lived Assets
In accordance with SFAS No. 144, long-lived assets and intangible assets with determinate lives are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company evaluates potential impairment by comparing the carrying amount of the asset with the estimated undiscounted future cash flows associated with the use of the asset and its eventual disposition. Should the review indicate that the asset is not recoverable, the Company’s carrying value of the asset would be reduced to its estimated fair value, which is measured by future discounted cash flows.
In September 2001, the Company decided to restructure its operations and discontinue marketing and manufacturing of the RDX system. As a result, the Company recorded impairment charges for its intangible assets and property and equipment totaling $2,111 and $699, respectively (Note 14).
Fair Value of Financial Instruments
The carrying amount of all financial instruments approximates fair value because of the short maturities of the instruments.
Concentrations of Credit Risk and Significant Customers
The Company maintains its cash and cash equivalents in deposit accounts and in pooled investment accounts administered by a major financial institution.
The Company sells its products primarily to medical institutions and distributors worldwide. The Company performs on going credit evaluations of its customers’ financial condition and generally does not require collateral from customers. Management believes that an adequate allowance for doubtful accounts has been provided.
In June 1998, the Company signed a technology license agreement with Guidant Corporation (“Guidant”), an international interventional cardiology products company, granting Guidant the right to manufacture and distribute products using the Company’s Focus technology for stent deployment. During 2001, 2002 and 2003, the Company recognized royalty revenue from Guidant of $6,429, $6,010 and $2,334, respectively, which represented 84%, 81% and 58% of total revenues, respectively (Note 5). There are no other customers or licensees who represented greater than 10% of the Company’s total revenues. As of December 31, 2002 and 2003, receivables from Guidant amounted to $887 and $530, respectively.
As of December 31, 2002 and 2003, accounts receivable from Bolton Medical Distribution S.A. amounted to $329 and $87, respectively, and accounts receivable from Klinikum Nurnberg Nord amounted to $11 and $28, respectively. No other single customer accounted for more than 10% of the Company’s accounts receivable balance at December 31, 2002 or 2003.
F-8
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
Product Sales by Geographic Region
The Company had product sales by region as follows:
| | | | | | | | | | | | | |
| | | Year Ended December 31,
|
| | | 2001
| | 2002
| | 2003
|
| Europe | | $ | 416 | | | $ | 315 | | | $ | 940 | |
| United States | | | 40 | | | | 376 | | | | 189 | |
| Asia | | | 138 | | | | — | | | | 25 | |
| Middle East | | | 174 | | | | — | | | | — | |
| Latin America | | | 165 | | | | 51 | | | | 96 | |
| Other | | | 178 | | | | 92 | | | | 145 | |
| | | |
| | | |
| | | |
| |
| | | $ | 1,111 | | | $ | 834 | | | $ | 1,395 | |
| | | |
| | | |
| | | |
| |
U.S. product sales for the year ended December 31, 2002 and 2003 were for sales of the PowerLink System product to hospitals that are conducting clinical trials. During 2003, approximately 55% and 45% of our AAA products sales were from infrarenal and suprarenal products, respectively. Product sales in the year ended December 31, 2001 were for products which have been discontinued.
Revenue Recognition
The Company sells directly to hospitals and to distributors. The Company recognizes revenue when there is persuasive evidence of an arrangement with the customer that states a fixed and determinable price and terms, delivery of the product has occurred in accordance with the shipment terms, and collectibility of the receivable is reasonably assured. The Company has no post delivery obligations to its customers other than a limited warranty, which is discussed under Product Warranty below.
The Company earns royalty revenue, which is included in license revenue in the consolidated statement of operations, as a result of the sale of product rights and technologies to third parties. Royalties are recognized upon the sale of products, subject to the royalty, by the third party. License revenues are recognized ratably over the estimated life of the agreement (Note 5).
Shipping Costs
Shipping costs billed to customers are included in revenue with the related costs in costs of goods sold.
Foreign Currency Translation
The local currency is the functional currency for the Company’s foreign subsidiaries. Accordingly, the assets and liabilities of foreign subsidiaries are translated at the rates of exchange at the balance sheet date. The income and expense items of these subsidiaries are translated at average monthly rates of exchange. The resulting translation gains and losses are included as a component of accumulated other comprehensive income on the consolidated balance sheet. Gains and losses resulting from foreign currency transactions, which are denominated in a currency other than the respective entity's functional currency are included in the consolidated statement of operations.
Stock-Based Compensation
The Company has elected to follow Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB 25”), and related interpretations in accounting for its employee stock options because the alternative fair value accounting provided for under SFAS No. 123 (SFAS No. 123), “Accounting for Stock-Based Compensation,” and amended by SFAS No. 148, “Accounting for Stock-Based Compensation-Transition and Disclosure,” requires use of option valuation models that were not developed for use in valuing employee stock options. Under the provisions of APB 25, the Company recognizes compensation expense only to the extent that the exercise price of the Company’s employee stock options is less than the market price of the underlying stock on the date of grant. Pro forma
F-9
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
information regarding net loss and loss per share is required by SFAS No. 123, which also requires that the information be determined as if the Company has accounted for its employee stock options granted under the fair value method. The fair value for these options was estimated at the date of grant using the Black-Scholes option pricing model. The Black-Scholes model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including the expected stock price volatility.
Because the Company’s employee stock options have characteristics significantly different from those of traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing models do not necessarily provide a reliable single measure of the fair value of its employee stock options.
In calculating pro forma information regarding net loss and net loss per share, the fair value was estimated at the date of grant using a Black-Scholes option pricing model with the following weighted-average assumptions: risk-free interest rate of 4.3%, 2.7% and 2.8%; a dividend yield of 0%, 0% and 0%; volatility of the expected market price of the Company’s common stock of 70.0%, 80.0% and 79.0%; and a weighted-average expected life of the options of 5.0, 5.0 and 5.0 years for 2001, 2002 and 2003, respectively.
For purposes of pro forma disclosures, the estimated fair value of the options is amortized to expense over the options’ vesting period. The Company’s pro forma information for the years ended December 31, 2001, 2002 and 2003 follows:
| | | | | | | | | | | | | |
| | | 2001
| | 2002
| | 2003
|
| Net loss, as reported | | $ | (15,641 | ) | | $ | (6,567 | ) | | $ | (5,915 | ) |
| Add: Stock-based employee compensation expense included in reported net loss, net of related tax effects | | | — | | | | — | | | | 77 | |
| Deduct: Total stock-based employee compensation expense determined under fair value based method for all awards, net of related tax effects | | | (1,183 | ) | | | (361 | ) | | | (134 | ) |
| | | |
| | | |
| | | |
| |
| Pro forma net loss | | $ | (16,824 | ) | | $ | (6,928 | ) | | $ | (5,972 | ) |
| Basic and diluted net loss per share, as reported | | $ | (1.20 | ) | | $ | (0.33 | ) | | $ | (0.23 | ) |
| Basic and diluted net loss per share, pro forma | | $ | (1.29 | ) | | $ | (0.35 | ) | | $ | (0.23 | ) |
The Company accounts for non-employee stock-based awards, in which goods or services are the consideration received for the stock options issued, in accordance with the provisions of SFAS No.123 and related interpretations. Compensation expense for non-employee stock-based awards is recognized in accordance with FASB Interpretation 28, “Accounting for Stock Appreciation Rights and Other Variable Stock Options or Award Plans, an Interpretation of APB Opinions No. 15. and 25” (FIN 28). Under SFAS No. 123 and FIN 28, the Company records compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation recorded during the service period is adjusted in subsequent periods for changes in the stock options’ fair value.
Income Taxes
The Company follows SFAS No. 109, “Accounting for Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in different periods for financial statement purposes versus tax return purposes. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each year-end based on enacted tax laws and statutory rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets when it is more likely than not that a portion of such assets will not be recoverable through future taxable income.
F-10
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
Net Loss Per Share
Net loss per common share is computed using the weighted average number of common shares outstanding during the periods presented. Because of the net losses during the years ended December 31, 2001, 2002 and 2003, options to purchase the common stock of the Company were excluded from the computation of net loss per share because the effect would have been antidilutive. If they were included, the number of shares used to compute net loss per share would have been increased by approximately 261,000 shares, 122,000 shares and 366,000 shares for the years ended December 31, 2001, 2002 and 2003, respectively. However, options to purchase approximately 1,337,000 shares at a weighted average exercise price of $5.73, 1,521,000 shares at a weighted average exercise price of $4.06, and 1,496,000 shares at a weighted average exercise price of $4.50 that were outstanding during 2001, 2002 and 2003, respectively, would have still been excluded from the computation of diluted loss per share because the options’ exercise price was greater than the average market price of the common shares.
Research and Development Costs
Research and development costs are expensed as incurred.
Comprehensive Income (Loss)
The Company accounts for elements of comprehensive income (loss) pursuant to SFAS No. 130, “Reporting Comprehensive Income.” Comprehensive income (loss) includes unrealized holding gains and losses and other items that have been previously excluded from net income (loss) and reflected instead in stockholders’ equity. Comprehensive income (loss) includes net loss, the effect of foreign currency translation adjustments, and unrealized holding gains (losses) on marketable securities classified as available-for-sale.
Product Warranty
Customers may request, within six months of shipment to end users, replacement products for products they receive that do not meet the manufacturer’s 60 days of shipment to distributors and within product specifications. No other warranties are offered and the Company disclaims responsibility for any consequential or incidental damages associated with the use of the products. Historically, the Company has not experienced a material amount of returns as a result of its product warranty. The Company records an accrual for an estimate of returns at the time revenue is recognized. To date, such returns have been insignificant.
Recent Accounting Pronouncements
In May 2003, the FASB issued SFAS 150, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity. SFAS 150 establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity. It requires that an issuer classify a financial instrument that is within its scope as a liability (or an asset in some circumstances). Many of those instruments were previously classified as equity. This Statement is effective for financial instruments entered into or modified after May 31, 2003 (except for mandatorily redeemable noncontrolling interests). For all instruments that existed prior to May 31, 2003, SFAS 150 is effective at the beginning of the first interim period beginning after June 15, 2003 (except for mandatorily redeemable noncontrolling interests). For mandatorily redeemable noncontrolling interests, the FASB has deferred certain provisions of SFAS 150. The adoption of SFAS 150 did not have a material effect on the Company’s consolidated financial position, results of operations or cash flows.
In December 2003 the SEC issued Staff Accounting Bulletin (SAB) No. 104, Revenue Recognition. SAB No. 104 revises and rescinds certain sections of SAB No. 101 in order to make this interpretive guidance consistent with current authoritative accounting and auditing guidance and SEC rules and regulations. Accordingly there is no impact to the Company’s results of operations, financial position or cash flows as a result of the issuance of SAB No. 104.
F-11
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
In December 2003, the FASB issued Interpretation No. 46R, Consolidation of Variable Interest Entities (FIN 46R). FIN 46R requires the application of either FIN 46 or FIN 46R by Public Entities to all Special Purpose Entities (SPE) created prior to February 1, 2003 as of December 31, 2003 for calendar year-end companies. FIN 46R is applicable to all non-SPEs created prior to February 1, 2003 at the end of the first interim or annual period ending after March 15, 2004. For all entities created subsequent to January 31, 2003, Public Entities were required to apply the provisions of FIN 46. The adoption of FIN 46 did not have a material impact on the Company’s consolidated financial position, results of operations or cash flows. The adoption of FIN 46R for SPEs did not have an impact to the Company’s consolidated financial position, results of operations or cash flows, and the Company does not believe the adoption of FIN 46R for non-SPEs will have a material impact to the Company’s consolidated financial position, results of operations or cash flows.
2. Merger and Sale of Assets
Endologix, Inc.
Reasons for the Merger
In September 2001, the Company decided to search for additional commercial opportunities by evaluating technologies outside of vascular radiation therapy, then the primary operational focus. Positive data had been presented, and was continuing to be presented, from several major medical device companies, on the effectiveness of drug-coated stents to prevent restenosis, or re-blockage of arteries. As a result, the Company believed the market for its radiation catheter would be limited.
In the fourth quarter of 2001, the Company began discussions with Endologix, Inc. (“former Endologix”), a privately held developer and manufacturer of the PowerLink System, an endoluminal stent graft for minimally invasive treatment of abdominal aortic aneurysms. Based on its investigation of the PowerLink System, the Company believed that it was a novel device and that clinical results to date indicated that the PowerLink System had several features and benefits that may provide a better clinical outcome in comparison to devices currently on the market.
The Company believed that the acquisition of former Endologix’s technology would provide the Company with a new and different medical device technology for a promising and potentially lucrative market.
Merger Transaction
In May 2002, the Company acquired all of the capital stock of former Endologix. The Company paid stockholders of former Endologix $0.75 cash for each share of former Endologix common stock, for an aggregate of $8,355, and issued one share of Radiance common stock for each share of former Endologix common stock, for an aggregate of 11,141,000 shares. The results of former Endologix have been included in the consolidated financial statements since May 2002.
In addition, the Company agreed to pay contingent consideration in the amount of $5,579 in the event pre-market approval, or PMA, is received in the U.S. for the PowerLink System on or before March 31, 2004, or $2,790 if PMA approval is received by June 30, 2004. The Company may choose to pay the contingent consideration, if payable, in cash or common stock at its sole discretion. As of December 31, 2003, PMA approval has not yet been obtained and such contingent consideration has not been recorded in the consolidated financial statements. Any contingent payment made will be capitalized as an addition to the purchase price.
The acquisition was accounted for as a purchase under SFAS No. 141, “Business Combinations.” In accordance with SFAS No. 141, the Company allocated the purchase price based on the fair value of the assets acquired and liabilities assumed. In the merger, the Company acquired, in addition to the net
F-12
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
tangible assets of the business, intangible assets such as the PowerLink and PowerWeb (an earlier version of the PowerLink) technologies, both developed and in-process, the Endologix trade name and PowerLink and PowerWeb trademarks, and goodwill. The Company employed valuation techniques reflecting recent guidelines from the AICPA on approaches and procedures for identifying and allocating the purchase price to assets to be used in research and development activities, including acquired in-process research and development, or IPR&D. To value IPR&D and developed technology, the Company estimated their future net cash flows and discounted them to their present value. To value trademarks and tradenames, the Company estimated the royalties that would have been paid for their use and discounted them to their net present value.
To determine the proper allocation of purchase price to technology assets, the Company first determined whether technological feasibility had been reached for a particular technology based upon whether it had been approved for sale by the appropriate regulatory body, or, in the absence of regulatory approval, whether there existed any material costs yet to be incurred, material changes to the technology to be completed or material risks of approval for sale. Then, the Company considered whether the technology had any alternative future uses.
If technological feasibility of projects had not been reached and the technology had no alternative future uses, the Company considered the technology to be IPR&D. The IPR&D is comprised of technological development efforts aimed at the discovery of new, technologically advanced knowledge, the conceptual formulation and design of possible alternatives, as well as the testing of process and product cost improvements. Specifically, these technologies included, but were not limited to, research and development efforts towards U.S. commercialization and expansion of the PowerLink product line to include a larger size of the device.
The Company then estimated that it would spend $6,700 to complete the regulatory process for U.S. commercialization of the PowerLink System by mid-2004. The Company also estimated that it would spend $6,600 to complete the research and development and regulatory approval process for a larger size PowerLink System for commercialization in Europe by late 2002, and in the U.S. by mid-2007.
The Company then determined the weighted average stage of completion for IPR&D projects was approximately 60% for U.S. commercialization of the PowerLink System and 33% for the development and commercialization of the larger size of the PowerLink System as of merger date. The cash flows from revenues forecasted in each period are reduced by related expenses, capital expenditures, the cost of working capital, and an assigned contribution to the core technologies serving as a foundation for the research and development. The discount rates applied to the individual technology’s net cash flows were 40%, based upon the level of risk associated with a particular technology and the current return on investment requirements of the market.
The amount of merger consideration allocated to IPR&D was then determined by estimating the stage of completion of each IPR&D project at the date of the merger, estimating the cash flows for the future research and development, clinical trials and release of products employing these technologies, all as described above, and discounting the net cash flows to their present values. As a result of the foregoing determinations, the Company expensed the portion of the purchase price allocated to IPR&D of $4,501 during the year ended December 31, 2002.
The Company also determined the fair value of developed technology at the merger date to be $14,050, which represents the acquired, aggregate fair value of individually identified technologies that were fully developed at the time of the merger. As with the IPR&D, the developed technology was valued using the income approach and a discount rate of 30%, in context of the business enterprise value of the former Endologix. The Company determined a value of $2,708 for trademarks and tradenames based upon the estimated royalty that would have to be paid for the right to use these assets if they had not been acquired by the Company, and a discount rate of 35%. The residual amount of $3,602 was allocated to goodwill. The trademarks and tradenames have an indefinite life and the developed technology is being amortized over ten years. The Company recognized amortization expense on intangible assets of $819 and
F-13
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
$1,405 during the years ended December 31, 2002 and 2003, respectively. The amortization expense on intangible assets for the next five years will be $1,405 per year.
The components of the purchase price and allocation are as follows:
| | | | | |
| Purchase Price: | | | | |
| Stock consideration (11,140,541 shares at $1.67/share*) | | $ | 18,637 | |
| Cash | | | 8,355 | |
| Acquisition costs | | | 786 | |
| | | |
| |
| Total | | $ | 27,778 | |
| | | |
| |
| Allocation of Purchase Price: | | | | |
| Current assets | | $ | 4,961 | |
| Property and equipment | | | 135 | |
| Other long-term assets | | | 34 | |
| Current liabilities | | | (2,387 | ) |
| Note receivable from shareholder | | | 174 | |
| IPR&D | | | 4,501 | |
| Developed technology | | | 14,050 | |
| Trademarks and tradenames | | | 2,708 | |
| Goodwill | | | 3,602 | |
| | | |
| |
| Total | | $ | 27,778 | |
| | | |
| |
*- Determined as the Nasdaq average closing price for the three business days before, the day of the merger announcement, and the three business days thereafter.
The following pro forma data summarizes the results of operations for the periods indicated as if the Endologix merger had been completed as of the beginning of the periods presented. The pro forma data gives effect to actual operating results prior to the merger, adjusted to include the pro forma effect of amortization of identified intangible assets.
| | | | | | | | | |
| | | Year Ended
|
| | | December 31, 2001
| | December 31, 2002
|
| Proforma: | | | | | | | | |
| Revenue | | $ | 10,674 | | | $ | 8,688 | |
| Net loss | | $ | (21,518 | ) | | $ | (5,378 | ) |
| Net loss per share basic and diluted | | $ | (0.89 | ) | | $ | (0.22 | ) |
| Weighted-average shares outstanding | | | 24,226,000 | | | | 24,266,000 | |
The above pro forma calculations do not include the charge of $4,501 for acquired IPR&D.
Sale of Vascular Access Assets
In January 1999, the Company sold substantially all of the properties and assets used exclusively in its Vascular Access product line to Escalon under an Assets Sale and Purchase Agreement (“Agreement”). Under the terms of the Agreement, the Company was entitled to receive royalty payments upon the sale of products for a five-year period beginning in 1999. In 2000, the Company recognized as revenue the pro-rata minimum royalty due of $300. In February 2001, the Company amended the Agreement with Escalon regarding the payment of royalties. As payment for $182 in royalties due the Company in the first quarter of 2001, Escalon issued 50,000 shares of Escalon common stock to the Company with a fair value of $100, which approximated the market price as quoted on NASDAQ, a prime plus one percent interest bearing note receivable due in January 2002 for $65, and cash of $17. The Company recognized a loss of $20 upon the sale of the shares of Escalon common stock in the fourth quarter of 2001. The note receivable was paid in January 2002.
F-14
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
Additionally, the Company received a prime (4.0% at December 31, 2003) plus one percent interest bearing note receivable for $718, payable in eleven equal quarterly installments from April 2002 to October 2004, representing the remaining minimum royalties, on a discounted basis, due for 2001 to 2003 under the Agreement. Additional royalties above the minimums will only be paid under the amended agreement if related product sales exceed $3,000 annually. The Company is recognizing interest income and royalty revenue under the $718 note receivable on a cash basis, as collection of this note receivable was not reasonably assured. Accordingly, the note receivable and deferred revenue are not recorded on the consolidated balance sheet. Interest income of $47, $34 and $22 was recognized in 2001, 2002 and 2003, respectively. The Company did not recognize royalty revenue from the agreement in 2001, but did recognize royalty revenue of $196 in 2002 and $261 in 2003.
3. Deferred Revenue
Deferred Distributor Fees
In June 1999, the Company granted Cosmotec distribution rights to market its vascular radiation therapy products in Japan. The Company received $1,000 as cash payment in exchange for the distribution rights and was recognizing the payment as revenue ratably over the estimated seven-year term of the distribution agreement. The cash received in excess of revenue recognized had been recorded as deferred revenue. In conjunction with the granting of distribution rights, the Company issued a $1,000 convertible debenture to Cosmotec. The convertible debenture was issued at below its estimated fair value resulting in a $377 reduction in the deferred revenue recorded by the Company. In December 2002, the Company and Cosmotec agreed to mutually release their obligations under the distribution agreement due to discontinuance of plans to distribute the Company’s vascular radiation therapy products in Japan. As a result, the then remaining deferred revenue of $299 was recorded as revenue. The Company recognized $81 and $360 of revenue during the years ended December 31, 2001 and 2002, respectively.
In December 2002, the Company and its 51% owned joint venture partner agreed to commence the dissolution of the joint venture, which was completed in May 2003. Included in the Company’s balance sheet for this joint venture at December 31, 2002 is $173 in cash and $83 in minority interest.
4. Note Payable
In September 2001, the former Endologix issued a $1,000 unsecured subordinated convertible note to Cosmotec. The note was assumed by the Company in its merger with former Endologix. The note bore interest at 10% and the total in principal and interest of $1,106 was paid in full in October 2002.
5. License Agreements
EndoSonics Corporation
In 1995 and 1997, the Company entered into license agreements with EndoSonics pursuant to which the Company granted EndoSonics the non-exclusive, royalty-free right to certain technology for use in the development and sale of certain products. In exchange, the Company received the non-exclusive, royalty-free right to utilize certain of EndoSonics’ product regulatory filings to obtain regulatory approval of the Company’s products (Note 15).
Guidant Corporation
In June 1998, the Company signed a technology license agreement with Guidant granting Guidant the right to manufacture and distribute stent delivery products using the Company’s Focus technology. Under the agreement, the Company was entitled to receive certain milestone payments based upon the transfer of the technology to Guidant, and royalty payments based upon the sale of products using the Focus technology. For the years ended December 31, 2001, 2002 and 2003, the Company recorded $6,429, $6,010, and $2,334, respectively, in royalties under the agreement. At December 31, 2002 and 2003, $887
F-15
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
and $530, respectively, due under this agreement are included in other receivables on the consolidated balance sheet.
6. Marketable Securities Available-for-Sale
The Company’s investments in debt securities are diversified among high credit quality securities in accordance with the Company’s investment policy. A major financial institution manages the Company’s investment portfolio. As of December 31, 2003, $8,166 and $211 of the Company’s debt securities had contractual maturities more than 90 days and less than one year and between one to two years, respectively.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2002
| | December 31, 2003
|
| | | | | | | Gross | | | | | | | | | | Gross | | |
| | | | | | | Unrealized | | | | | | | | | | Unrealized | | |
| | | | | | | Holding | | Fair | | | | | | Holding | | Fair |
| | | Cost
| | Gains
| | Value
| | Cost
| | Gains
| | Value
|
| U.S. Treasury and other agencies debt securities | | $ | 4,023 | | | $ | 38 | | | $ | 4,061 | | | $ | 7,059 | | | $ | 4 | | | $ | 7,063 | |
| Corporate debt securities | | | 3,028 | | | | 15 | | | | 3,043 | | | | 1,311 | | | | 3 | | | | 1,314 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | $ | 7,051 | | | $ | 53 | | | $ | 7,104 | | | $ | 8,370 | | | $ | 7 | | | $ | 8,377 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
7. Inventories
Inventories consisted of the following:
| | | | | | | | | |
| | | December 31,
|
| | | 2002
| | 2003
|
| Raw materials | | $ | 1,069 | | | $ | 1,647 | |
| Work in process | | | 174 | | | | 496 | |
| Finished goods | | | 800 | | | | 637 | |
| | | |
| | | |
| |
| | | $ | 2,043 | | | $ | 2,780 | |
| | | |
| | | |
| |
8. Intangibles
Intangibles consisted of the following:
| | | | | | | | | |
| | | December 31,
|
| | | 2002
| | 2003
|
| Developed technology | | $ | 14,050 | | | $ | 14,050 | |
| Accumulated amortization | | | (819 | ) | | | (2,224 | ) |
| | | |
| | | |
| |
| | | | 13,231 | | | | 11,826 | |
| Trademarks and tradenames | | | 2,708 | | | | 2,708 | |
| | | |
| | | |
| |
| Intangible assets, net | | $ | 15,939 | | | $ | 14,534 | |
| | | |
| | | |
| |
The intangibles were acquired in the merger with the former Endologix (Note 2).
F-16
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
9. Accounts Payable and Accrued Expenses
Accounts payable and accrued expenses consisted of the following:
| | | | | | | | | |
| | | December 31,
|
| | | 2002
| | 2003
|
| Accounts payable | | $ | 740 | | | $ | 662 | |
| Accrued payroll and related expenses | | | 871 | | | | 511 | |
| Accrued restructuring charges | | | 248 | | | | — | |
| Accrued clinical expenses | | | 405 | | | | 225 | |
| Other accrued expenses | | | 84 | | | | 70 | |
| | | |
| | | |
| |
| | | $ | 2,348 | | | $ | 1,468 | |
| | | |
| | | |
| |
At December 31, 2002, accrued restructuring charges are comprised of $248 in non-cancelable commitments (Note 14).
10. Commitments and Contingencies
Sole-Source, Related-Party Supplier Agreement
In February 1999, the former Endologix agreed to purchase a key component for its PowerLink product from Impra, Inc., a subsidiary of C.R. Bard, Inc., which at the time was a significant shareholder and thus a related party, under a supplier agreement that expires in December 2007, and which then automatically renews, on a year by year basis, for additional one year periods without notice, unless a party provides notice not to renew within thirty days from the expiration of the renewal period. Under the terms of the agreement, the Company has agreed to purchase certain unit quantities of the component, with built in annual quantity increases. In January 2002, the agreement was amended, increasing the minimum quantity purchase requirements for 2002 and thereafter and increasing the prices each year after 2002 according to the general increase in the Consumer Price Index. For the year ended December 31, 2003, the Company purchased $906 and is committed to purchase an additional $140 of the key component under the supplier agreement. During 2004, the Company is required under the supplier agreement to purchase a minimum number of units, which depending on the units purchased, could range in cost from $1,357 to $2,001. If the Company receives FDA approval to commercially distribute devices using the component, the price that the Company will pay Impra for the component will materially increase. The Company believes that U.S. commercialization could occur during 2004.
The Company is economically dependent on this vendor as it is the sole source for the key component.
Operating Leases
The Company leases its administrative, research and manufacturing facilities and certain equipment under long-term, non-cancelable lease agreements that have been accounted for as operating leases. Certain of these leases include renewal options and require the Company to pay operating costs, including property taxes, insurance and maintenance as prescribed by the agreements.
Future minimum payments by year under non-cancellable operating leases with initial terms in excess of one year were as follows as of December 31, 2003:
| | | | | |
| Year Ending December 31, | | | | |
| 2004 | | $ | 283 | |
| 2005 | | | 83 | |
| 2006 | | | 4 | |
| | | |
| |
| | | $ | 370 | |
| | | |
| |
During the fourth quarter of 2001, the Company completed its evaluation of facility needs and recorded a $309 restructuring charge for non-cancelable lease commitments, net of estimated sublease income of $256. During the fourth quarter of 2002, the Company reassessed its restructuring accrual for non-cancelable lease commitments in light of diminished opportunity for sublease arrangements prior to the lease term expirations in October 2003, and recorded an additional charge of $168 (Note 14).
F-17
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
Rental expense charged to operations for all operating leases during the years ended December 31, 2001, 2002 and 2003, was approximately $487, $295 and $301, respectively, exclusive of restructuring costs.
The Company subleased some of its facilities through November 2003. Rental income recorded for all subleased facilities during the years ended December 31, 2001, 2002 and 2003, was approximately $100, $207 and $209, respectively.
Employment Agreements and Retention Plan
The Company has entered into employment agreements with its officers and one manager (“key employees”) under which severance payments and benefits would become payable in the event of termination by the Company for any reason other than cause or upon a change in control or corporate transaction, or by the key employee for good reason, as such terms are defined in the agreement. If due, the severance payment will generally be equal to six months of the key employee’s then current salary for termination by the Company without cause or by the key employee for good reason, and generally be equal to twelve months of salary upon a change in control or corporate transaction.
Additionally, in December 2002, the Board of Directors approved an employee retention plan. In the event of a sale of the Company, employees other than those with employment agreements would receive a severance payment equal to two to three months of their then current salary.
Contract Manufacturing Agreement with Bebig GmbH
In July 1999, the Company entered into a two-year contract manufacturing agreement with Bebig GmbH (“Bebig”) to activate the radioactive sources and complete final assembly of the RDX system in Europe. The agreement was amended in July 2000, February 2001 and May 2001. Under the agreement as amended, the Company paid an aggregate of $1,620 in 2001, including $194 related to the cancellation of the arrangement in connection with the Company’s restructuring in 2001(Note 14).
In conjunction with the contract manufacturing agreement, the Company entered into a three year sub-license agreement with Bebig for certain radiation technology that it believed might be useful in the development of its radiation therapy products. During 2001, the Company recorded $125 in license fee expense and paid an additional $150 for license rights through November 2002. All license fees due under the license agreement for prior periods were offset by payments under the manufacturing agreement.
The Company has expensed, as research and development, all costs associated with the contract manufacturing and license agreements with Bebig, except those expensed as restructuring costs.
In September 2001, the Company implemented a restructuring plan that included the discontinuance of European manufacturing (Note 14). As a result, the remaining non-cancelable contractual commitments due under the agreements with Bebig (including the remaining minimum sub-license fee), totaling $344, was included as a component of the restructuring charge that was paid in the fourth quarter of 2001. No amounts are owed to Bebig at December 31, 2003.
11. Stockholders’ Equity
Authorized Shares of Common Stock
In October 2003, shareholders approved an increase in the number of authorized shares of common stock from 30,000,000 to 50,000,000 shares.
Sale of Common Stock
In July 2003, the Company announced the completion of its private placement of 4,000,000 shares of its common stock at a purchase price of $2.25 per share. The Company received aggregate gross proceeds
F-18
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
of $9,000 for the newly issued shares of common stock. The proceeds of the private placement, net of issuance costs, amounted to $8,357.
Treasury Stock
In July 2002, the board of directors authorized a program for repurchases of the Company’s outstanding common stock of up to $1,500 under certain parameters. During the year ended December 31, 2002, the Company utilized $205 to repurchase 227,000 shares of its common stock at a weighted average share price of $.90 per share. During the year ended December 31, 2003, the Company utilized $456 to repurchase 268,000 shares of its common stock at a weighted average share price of $1.71 per share.
Stock Option Plan
In May 1996, the Company adopted the 1996 Stock Option/Stock Issuance Plan (the “1996 Plan”) that is the successor to the Company’s 1995 Stock Option Plan. In September 1997, the Company adopted the 1997 Supplemental Stock Option Plan (the “1997 Plan”). Under the terms of the 1996 and 1997 Plans, eligible key employees, directors, and consultants can receive options to purchase shares of the Company’s common stock at a price not less than 100% for incentive stock options and 85% for nonqualified stock options of the market value of the Company’s common stock on the date of grant. At December 31, 2003, the Company had authorized 3,450,000 and 90,000 shares of common stock for issuance under the 1996 and 1997 Plan, respectively. At December 31, 2003, the Company had 225,975 shares and 1,500 shares of common stock available for grant under the 1996 and 1997 Plan, respectively. The options granted under the Plans are exercisable over a maximum term of ten years from the date of grant and generally vest over a four-year period. The activity under both plans is summarized below:
| | | | | | | | | | | | | | | | | | | | | |
| | | Option Price | | Number | | Options |
| | | Per Share
| | of Shares
| | Exercisable
|
| Balance at December 31, 2000 | | $ | 0.11 | | | to | | $ | 13.19 | | | | 2,131,228 | | | | 1,129,863 | |
| | | | | | | | | | | | | | | | | | | |
| |
| Granted | | $ | 1.55 | | | to | | $ | 6.88 | | | | 333,700 | | | | | |
| Exercised | | $ | 1.00 | | | to | | $ | 3.50 | | | | (21,417 | ) | | | | |
| Forfeited | | $ | 1.55 | | | to | | $ | 13.19 | | | | (271,250 | ) | | | | |
| | | |
| | | |
| | | |
| | | |
| | | | | |
| Balance at December 31, 2001 | | $ | 0.11 | | | to | | $ | 13.19 | | | | 2,172,261 | | | | 1,525,275 | |
| | | | | | | | | | | | | | | | | | | |
| |
| Granted | | $ | 0.77 | | | to | | $ | 1.73 | | | | 603,000 | | | | | |
| Exercised | | $ | 0.11 | | | to | | $ | 1.50 | | | | (39,020 | ) | | | | |
| Forfeited | | $ | 1.00 | | | to | | $ | 13.19 | | | | (790,359 | ) | | | | |
| | | |
| | | |
| | | |
| | | |
| | | | | |
| Balance at December 31, 2002 | | $ | 0.11 | | | to | | $ | 13.19 | | | | 1,945,882 | | | | 1,254,930 | |
| | | | | | | | | | | | | | | | | | | |
| |
| Granted | | $ | 2.81 | | | to | | $ | 3.92 | | | | 550,000 | | | | | |
| Exercised | | $ | 0.11 | | | to | | $ | 1.50 | | | | (138,831 | ) | | | | |
| Forfeited | | $ | 1.07 | | | to | | $ | 7.00 | | | | (218,750 | ) | | | | |
| | | |
| | | |
| | | |
| | | |
| | | | | |
| Balance at December 31, 2003 | | $ | 0.11 | | | to | | $ | 13.19 | | | | 2,138,301 | | | | 1,340,333 | |
| | | |
| | | | | | | |
| | | |
| | | |
| |
The following table summarizes information regarding stock options outstanding at December 31, 2003:
| | | | | | | | | | | | | | | | | | | | | |
Options Outstanding
| | Options Exercisable
|
| | | | | | | Weighted- | | | | | | | | |
| | | | | | | Average | | Weighted | | | | | | |
| | | | | | | Remaining | | Average | | | | | | Weighted-Average |
| Range of | | Options | | Contractual | | Exercise | | Options | | Exercise |
Exercise Prices
| | Outstanding
| | Life (Years)
| | Price
| | Exercisable
| | Price
|
| $ 0.11 - - 0.85 | | | 245,878 | | | | 6.4 | | | $ | 0.56 | | | | 174,585 | | | $ | 0.44 | |
| 1.00 - 2.50 | | | 367,750 | | | | 6.4 | | | | 1.45 | | | | 219,999 | | | | 1.53 | |
| 2.69 - 3.92 | | | 853,173 | | | | 7.1 | | | | 3.69 | | | | 303,173 | | | | 3.46 | |
| 4.47 - 5.63 | | | 550,400 | | | | 5.0 | | | | 4.87 | | | | 532,683 | | | | 4.88 | |
| 6.59 - 8.75 | | | 101,100 | | | | 6.0 | | | | 7.01 | | | | 93,226 | | | | 7.02 | |
| 13.19 | | | 20,000 | | | | 6.6 | | | | 13.19 | | | | 16,667 | | | | 13.19 | |
| | | |
| | | | | | | | | | | |
| | | | | |
| 0.11 - 13.19 | | | 2,138,301 | | | | 6.7 | | | $ | 3.50 | | | | 1,340,333 | | | $ | 3.68 | |
| | | |
| | | | | | | | | | | |
| | | | | |
F-19
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
The weighted-average grant-date fair value of options granted during 2001, 2002 and 2003 where the exercise price on the date of grant was equal to the stock price on that date, was $4.64, $1.26, and $3.82, respectively.
Deferred compensation is being amortized over the vesting period of the related non-employee options, which is generally four years. During the years ended December 31, 2001, 2002 and 2003, $(97), $(22) and $75, respectively, was recorded as compensation expense for the change in the fair value of unvested non-employee option grants. During the years ended December 31, 2001, 2002 and 2003, 30,000, 20,000, and 20,000 options, respectively, were granted to non-employees. As of December 31, 2001, 2002 and 2003, a total of 244,932, 182,600 and 283,600 non-employee stock options, respectively, were outstanding.
No compensation expense was recorded in the financial statements for stock options issued to employees for 2001, 2002 and 2003 because the options were granted with an exercise price equal to the market price of the Company’s common stock on the date of grant. On October 1, 2003, based upon an agreement with a departing Board member, all of the member’s existing options with an exercise price of $5.00 and below were cancelled and re-granted with a five-year life at the original grant price (218,000 options at an average exercise price of $3.87) and the existing options with an exercise price above $5.00 (95,000 options at an average exercise price of $6.43) were cancelled. As a result of the regrant of options, the Company recorded $77 in compensation expense in 2003, which represented the difference between the original exercise price and fair value of the Company’s common stock on the date of the modification.
Stock Purchase Plan
Under the terms of the Company’s 1996 Employee Stock Purchase Plan (the “Purchase Plan”), eligible employees can purchase common stock through payroll deductions at a price equal to the lower of 85% of the fair market value of the Company’s common stock at the beginning or end of the applicable offering period. In October 2003, an additional 200,000 shares of common stock were approved for issuance under the Purchase Plan. During 2001, 2002 and 2003, a total of approximately 52,000, 12,000, and 123,000 shares of common stock, respectively, were purchased at an average price of $4.17, $1.35, and $0.77 per share, respectively.
12. Income Taxes
Significant components of the Company’s deferred tax assets and (liabilities) are as follows at December 31:
| | | | | | | | | |
| | | 2002
| | 2003
|
| Net operating loss carryforwards | | $ | 17,775 | | | $ | 20,396 | |
| Accrued expenses | | | 157 | | | | 43 | |
| Tax credits | | | 3,773 | | | | 4,485 | |
| Bad debt reserve | | | 128 | | | | 7 | |
| Depreciation | | | 263 | | | | 121 | |
| Amortization | | | 449 | | | | 111 | |
| Inventory write-downs | | | 501 | | | | 33 | |
| Capitalized research and development | | | 2,430 | | | | 1,372 | |
| Developed technology | | | (6,675 | ) | | | (5,789 | ) |
| Deferred compensation amortization | | | 558 | | | | 649 | |
| Other | | | 188 | | | | 82 | |
| | | |
| | | |
| |
| Deferred tax assets | | | 19,547 | | | | 21,510 | |
| Valuation allowance | | | (19,547 | ) | | | (21,510 | ) |
| | | |
| | | |
| |
| Net deferred tax assets | | $ | — | | | $ | — | |
| | | |
| | | |
| |
Based upon the Company’s history of continuing operating losses, realization of its deferred tax assets does not meet the “more likely than not” criteria under SFAS No. 109 and, accordingly, a valuation allowance for the entire deferred tax asset amount has been recorded.
The valuation allowance increased by $5,889 in 2001, decreased by $446 in 2002 and increased by $1,963 in 2003.
F-20
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
In connection with the acquisition of the former Endologix in May 2002, the Company acquired deferred tax assets of $6,233 and deferred tax liabilities of $6,675.
The Company’s effective tax rate differs from the statutory rate of 35% due primarily to research and development and other tax credits offset by federal and state losses that were recorded without tax benefit.
At December 31, 2003, the Company has net operating loss carryforwards for federal and state income tax purposes of approximately $56,821 and $18,457, respectively, which begin to expire in 2009 and 2004, respectively. In addition, the Company has research and development and other tax credits for federal and state income tax purposes of approximately $2,418, and $1,957, respectively, which begin to expire in 2005. The state research and development credits do not expire for California purposes. In addition, the Company has approximately $110 of California Manufacturers’ Investment Credits, which begin to expire in 2005.
As of December 31, 2003, a portion of the state valuation allowance related to the tax benefits of stock option deductions are included in the Company’s net operating loss carryforwards. At such time as the valuation allowance is reduced (if at all, subject to the “change in ownership” limitations described below), the benefit will be first credited to income tax expense. Thereafter, the benefit will be credited to additional paid-in capital.
Because of the “change of ownership” provision of the Tax Reform Act of 1986, utilization of the Company’s net operating loss and research credit carryforwards may be subject to an annual limitation against taxable income in future periods. As a result of the annual limitation, a portion of these carryforwards may expire before ultimately becoming available to reduce future income tax liabilities.
The results of operations for the years ended December 31, 2001, 2002 and 2003 include the net losses of the Company’s wholly-owned German and majority-owned Japanese subsidiaries of $93, $33, and $28, respectively.
13. Employee Benefit Plan
The Company provides a 401(k) Plan for all employees 21 years of age or older with over 3 months of service. Under the 401(k) Plan, eligible employees voluntarily contribute to the Plan up to 15% of their salary through payroll deductions. Employer contributions may be made by the Company at its discretion based upon matching employee contributions, within limits, and profit sharing provided for in the Plan. No employer contributions were made in 2001, 2002 or 2003.
14. Restructuring Charges
In September 2001, two companies published clinical study data for drug-coated stents, a competing technology to the Company’s radiation catheter system. That data demonstrated the high level of efficacy of drug-coated stents in preventing restenosis. Considering that efficacy, and the ease of use and probable cost effectiveness of drug-coated stents compared to the Company’s radiation catheter system, the Company determined that the market for the radiation-based system likely would be limited.
As a result, in order to conserve cash prior to assessing the outcome of its clinical study on its radiation catheter and deciding whether to make a PMA filing, and to be in position to take advantage of strategic alternatives, the Company decided in September 2001 to restructure its operations. The restructuring plan was comprised of the following: a) discontinue marketing and manufacturing of the radiation catheter in Europe and other international markets in the third quarter of 2001, b) discontinue marketing and manufacturing of products using the Company’s other stent and catheter technology, subject to the fulfillment of outstanding orders, which was completed in the fourth quarter of 2001, c) cease preparations for clinical trials for the radiation catheter in Japan, d) terminate 55 employees on an involuntary basis, which was completed in the first quarter of 2002, and e) search for additional commercial opportunities by evaluating technologies outside of radiation therapy. The involuntarily
F-21
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
terminated employees consisted of 28 employees in manufacturing, 19 employees in research and development, 3 employees in sales and marketing and 5 employees in administration.
As a result of the restructuring plan, the Company recorded a $344 charge, comprised of manufacturing facility set up and sub-license fees and non-cancelable commitments under the agreements with Bebig, the Company’s former third-party European manufacturer for its radiation catheter products, $20 in other non-cancelable commitments, $601 for the write-off of inventory that will not be used to fulfill the outstanding product orders, $1,093 for employee termination benefits and $42 for other exit costs.
The Company concluded that no future cash flows were expected to be generated from the radiation catheter technology. As a result, the net carrying value of the Company’s equipment related to the technology and its intangible assets, consisting of acquired technology and employment contracts were written down to zero, resulting in a charge of $390 and $2,111, respectively, during 2001.
The Company also evaluated the estimated cash flows expected to be generated from equipment used in the production of its other discontinued products, including any possible cash flows associated with the equipment’s eventual disposition and recorded a charge of $40 based on estimated discounted cash flows, and revised the estimated useful life of the equipment.
The Company also wrote off $269 for the carrying value of furniture, computers, software and leasehold improvements that were no longer being used.
During the fourth quarter of 2001, the Company completed its evaluation of its facility needs and recorded a $309 restructuring charge for non-cancelable lease commitments, net of estimated sublease income of $256. During the fourth quarter of 2002, the Company reassessed its restructuring accrual for non-cancelable lease commitments in light of diminished opportunity for sublease arrangements prior to the lease term expirations in October 2003, and recorded an additional $168 restructuring charge.
The following is a summary of the restructuring-related liability payments and adjustments during the years ended December 31, 2001, 2002 and 2003:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Employee | | | | | | | | | | |
| | | Termination | | Property and | | Intangible | | Non-Cancelable | | Other | | |
| | | Benefits
| | Equipment
| | Assets
| | Commitments
| | Charges
| | Totals
|
| Charges | | $ | 1,093 | | | $ | 699 | | | $ | 2,111 | | | $ | 672 | | | $ | 42 | | | $ | 4,617 | |
| Cash payments | | | (474 | ) | | | — | | | | — | | | | (371 | ) | | | — | | | | (845 | ) |
| Non-cash charges | | | — | | | | (699 | ) | | | (2,111 | ) | | | — | | | | (42 | ) | | | (2,852 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Liability balance December 31, 2001 | | | 619 | | | | — | | | | — | | | | 301 | | | | — | | | | 920 | |
| Cash payments | | | (619 | ) | | | — | | | | — | | | | (221 | ) | | | — | | | | (840 | ) |
| Adjustments | | | — | | | | — | | | | — | | | | 168 | | | | — | | | | 168 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Liability balance December 31, 2002 | | | — | | | | — | | | | — | | | | 248 | | | | — | | | | 248 | |
| Cash payments | | | — | | | | — | | | | — | | | | (248 | ) | | | — | | | | (248 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| Liability balance December 31, 2003 | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
F-22
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
15. Legal Matters
On September 15, 1999, EndoSonics Corporation, which was a wholly-owned subsidiary of Jomed N.V. until July 2003, filed a complaint for declaratory relief in the Superior Court in Orange County, California, claiming that under a May 1997 agreement between the parties, EndoSonics had rights to combine the Company’s Focus balloon technology with an EndoSonics’ ultrasound imaging transducer on the same catheter with a coronary vascular stent. In February 2001 the court ruled in the Company’s favor, ruling that Jomed-EndoSonics had no such rights to include a stent with the Focus balloon and ultrasound imaging transducer. Under the judgment, the Company was entitled to recover approximately $468 of its legal fees and costs it had previously expensed, plus interest. In May 2001, Jomed-EndoSonics appealed the judgment, and in January 2003 the appeals court upheld the judgment in the favor of the Company. In February 2003, the Company agreed to accept payment of the judgment for legal fees and costs of $468, which was recorded as a reduction to general and administrative expenses, and interest due of $94, all of which was collected by March 31, 2003.
In July 2002, the Company terminated its contracts with two of its European distributors of PowerLink products for non-performance. In October 2002, the Company commenced an arbitration proceeding against the distributors to recover delinquent receivables of $376. In response, the distributors filed counterclaims for breach of contract, intentional and negligent misrepresentation and concealment of material facts in which they claim damages of $1,000. In February 2003, the parties agreed to a mutual release of claims made in the arbitration action and signed a new distribution agreement. The European distributors paid $320 to the Company in full settlement of delinquent receivables, net of product returns for $47 and expense reimbursement of $17. The Company also accepted a one-time exchange of products valued at $80.
The Company is a party to ordinary disputes arising in the normal course of business. Management is of the opinion that the outcome of these matters will not have a material adverse effect on the Company’s consolidated financial position, results of operations or cash flows.
16. Related Party Transactions
Notes Receivable from Officers
In September 1998, an officer of the former Endologix purchased common stock and the former Endologix accepted as payment a $174 five-year, 6% note receivable, which was acquired by the Company in the merger with former Endologix. The note receivable was paid in July 2002.
In January 1997, the Company loaned $100 to its then president. The note was collateralized by a second trust deed on the executive’s home and had a five-year term with interest compounding semi-annually at 6%. The principal and interest was originally due January 2002 and had been extended to January 2004. As part of the separation agreement with the officer following the merger with the former Endologix in May 2002, the debt, including interest, totaling $137 was forgiven and expensed to general and administrative expenses.
F-23
ENDOLOGIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(In Thousands, Except Share and Per Share Amounts)
In October 1997, former Radiance loaned $10 to its then director of research and development. When former Radiance was acquired, the loan was continued and former Radiance’s director of research and development became the Company’s vice president of clinical affairs. The note was not collateralized and had a six month term with interest compounding semi-annually at 6%. The principal and interest was originally due March 1998 and had been extended to March 2003. In April 2002, the loan and interest due of $13, was paid in full.
17. Subsequent Event
On March 10, 2004, the Company announced the completion of its private placement of 3,200,000 shares of its common stock at a price of $5.10 per share. The Company received aggregate gross proceeds of $16,320 for the newly issued shares of common stock. The proceeds of the private placement, net of commissions and other expenses, amounted to approximately $15,300.
F-24
(b) FINANCIAL STATEMENT SCHEDULE
ENDOLOGIX, INC.
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
YEARS ENDED DECEMBER 31, 2003, 2002, AND 2001
(In Thousands)
| | | | | | | | | | | | | | | | | | | | | |
Column A
| | Column B
| | Column C
| | Column D
| | Column E
|
| | | | | | | Additions (Reductions)
| | | | | | |
| | | Balance at | | Charges to | | Charged | | | | | | Balance at |
| | | Beginning | | Costs and | | to Other | | | | | | End of |
Description
| | of Period
| | Expenses
| | Accounts
| | Deductions(a)
| | Period
|
Year ended December 31, 2003 | | | | | | | | | | | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 165 | | | $ | (139 | ) | | $ | — | | | $ | (10 | ) | | $ | 16 | |
| Reserve for excess and obsolete inventories | | $ | 1,158 | | | $ | (93 | ) | | $ | — | | | $ | (983 | ) | | $ | 82 | |
| Income tax valuation allowance | | $ | 19,547 | | | $ | 1,963 | | | $ | — | | | $ | — | | | $ | 21,510 | |
Year ended December 31, 2002 | | | | | | | | | | | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 244 | | | $ | (3 | ) | | $ | — | | | $ | (76 | ) | | $ | 165 | |
| Reserve for excess and obsolete inventories | | $ | 985 | | | $ | 238 | | | $ | — | | | $ | (65 | ) | | $ | 1,158 | |
| Income tax valuation allowance | | $ | 19,993 | | | $ | (446 | ) | | $ | — | | | $ | — | | | $ | 19,547 | |
Year ended December 31, 2001 | | | | | | | | | | | | | | | | | | | | |
| Allowance for doubtful accounts | | $ | 113 | | | $ | 131 | | | $ | — | | | $ | — | | | $ | 244 | |
| Reserve for excess and obsolete inventories | | $ | 343 | | | $ | 817 | | | $ | — | | | $ | (175 | ) | | $ | 985 | |
| Income tax valuation allowance | | $ | 14,104 | | | $ | 5,889 | | | $ | — | | | $ | — | | | $ | 19,993 | |
| (a) | | Deductions represent the actual write-off of accounts receivable balances or the disposal of inventory. |
F-25
EXHIBIT INDEX
| | | |
| EXHIBIT | | |
NUMBER
| | DESCRIPTION
|
| 2.4(1) | | Agreement and Plan of Merger dated November 3, 1998 by and between CardioVascular Dynamics, Inc. and Radiance Medical Systems, Inc. |
| | | |
| 2.5(2) | | Assets Sale and Purchase Agreement dated January 21, 1999 by and between the Company and Escalon Medical Corp. |
| | | |
| 2.5.1(3) | | Amendment and Supplement to Assets Sale and Purchase Agreement and Release dated February 28, 2001 by and between the Company and Escalon Medical Corp. |
| | | |
| 2.5.2(3) | | Short-Term Note, Exhibit 1, to Amendment and Supplement to Assents Sale and Purchase Agreement and Release dated February 28, 2001 by and between the Company and Escalon Medical Corp. |
| | | |
| 2.5.3(3) | | Long-Term Note, Exhibit 2, to Amendment and Supplement to Assents Sale and Purchase Agreement and Release dated February 28, 2001 by and between the Company and Escalon Medical Corp. |
| | | |
| 2.6(4) | | Agreement and Plan of Merger, dated as of February 8, 2002, by and among the Company, RMS Acquisition Corp. and Endologix, Inc. |
| | | |
| 3.1 | | Restated Certificate of Incorporation as currently in effect. |
| | | |
| 3.2(5) | | Amended and Restated Bylaws |
| | | |
| 4.1(6) | | Specimen Certificate of Common Stock |
| | | |
| 10.1(7) | | Form of Indemnification Agreement entered into between the Registrant and its directors and officers |
| | | |
| 10.3(7) | | The Registrant’s Employee Stock Purchase Plan and forms of agreement thereunder |
| | | |
| 10.15(7) | | Industrial Lease dated February 23, 1995 by and between the Irvine Company and the Company |
| | | |
| 10.22(8) | | Supplemental Stock Option Plan |
| | | |
| 10.24(9)* | | License Agreement by and between the Company and Guidant dated June 19, 1998 |
| | | |
| 10.25(10) | | 1996 Stock Option/Stock Issuance Plan (as Amended and Restated as of April 8, 1997, March 12, 1998 and November 3, 1998) |
| | | |
| 10.26(11) | | 1997 Stock Option Plan (As Amended as of June 15, 1998) assumed by Registrant pursuant to its acquisition of Radiance Medical Systems, Inc. on January 14, 1999 |
| | | |
| 10.36(12) | | Form of Employment Agreement dated August 21, 2000 by and between the Company and Joseph A. Bishop |
| | | |
| 10.40*(13) | | Supply Agreement dated as of February 12, 1999, and as amended August 4, 1999, November 16, 1999, March 10, 2000, and January 31, 2001 by and between the Company and Impra, Inc. |
| | | |
| 10.40.1*(13) | | Amendment to Supply Agreement dated January 17, 2002 by and between the Company and Impra, Inc. |
II-1
| | | |
| EXHIBIT | | |
NUMBER
| | DESCRIPTION
|
| 10.41(14) | | Form of Indemnification Agreement dated October 1, 2002 by and between the Company and its officers and directors. |
| | | |
| 10.42 (15) | | Form of Employment Agreement dated October 18, 2002 by and between the Company and its officers, excluding Joseph A. Bishop. |
| | | |
| 10.42.2 (15) | | Schedule of Parties to the Employment Agreement. |
| | | |
| 10.43 | | Amendment to Employment Agreement dated October 18, 2002 by and between the Company and Franklin D. Brown, dated December 17, 2003, which is attached as Exhibit 10.43. |
| | | |
| 10.44 | | Amendment to Employment Agreement dated October 18, 2002 by and between the Company and Paul McCormick, dated December 17, 2003, which is attached as Exhibit 10.44. |
| | | |
| 10.45 | | Form of Employment Agreement dated February 26, 2004 by and between the Company and Stefan G. Schreck, which is attached as Exhibit 10.45. |
| | | |
| 14 | | Form of the Code of Ethics for Our Chief Executive Officer and Principal Financial Officers, which is attached as Exhibit 14. |
II-2
| | | |
| EXHIBIT | | |
NUMBER
| | DESCRIPTION
|
| 21.1 | | List of Subsidiaries |
| | | |
| 23.1 | | Consent of PricewaterhouseCoopers LLP |
| | | |
| 24.1 | | Power of Attorney (included on signature page hereto) |
| | | |
| 31.1 | | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a)/15d-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 31.2 | | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a)/15d-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.1 | | Certification of Chief Executive Officer, Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.2 | | Certification of Chief Financial Officer, Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
* Portions of this exhibit are omitted and were filed separately with the Securities and Exchange Commission pursuant to the Company’s application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934.
(1) Previously filed as Exhibit 2.4 to the Company’s Report on Form 8-K filed with the Securities and Exchange Commission as of November 12, 1998.
(2) Previously filed as Exhibit 2 to the Company’s Report on Form 8-K filed with the Securities and Exchange Commission as of February 5, 1999.
(3) Previously filed as an exhibit to the Company’s Report on Form 10-K filed with the Securities and Exchange Commission on March 29, 2001.
(4) Previously filed as Annex I to the Company’s Proxy Statement on Schedule 14A filed with the Securities Exchange Commission on April 26, 2002.
(5) Previously filed as Exhibit 3.4 to the Company’s Report on Form 10-Q filed with the Securities and Exchange Commission on November 16, 1998.
(6) Previously filed as an exhibit to Amendment No. 2 to the Company’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on June 10, 1996.
(7) Previously filed as an exhibit to the Company’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on May 3, 1996.
(8) Previously filed as an exhibit to the Company’s Registration Statement on Form S-8 filed with the Securities and Exchange Commission on December 12, 1997.
(9) Previously filed as Exhibit 10.24 to the Company’s Report on Form 10-Q filed with the Securities and Exchange Commission as of August 11, 1998.
(10) Previously filed as Annex III to the Company’s Proxy Statement on Schedule 14A filed with the Securities and Exchange Commission on December 18, 1998.
(11) Previously filed as Exhibit 99.2 to the Company’s Registration Statement on Form S-8 filed with the Securities and Exchange Commission on February 17, 1999.
II-3
(12) Previously filed as Exhibit 10.36 to Amendment No. 1 to the Company’s Registration Statement on Form S-2 filed with the Securities and Exchange Commission on September 11, 2000.
(13) Previously filed as an exhibit to the Company’s Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2002.
(14) Previously filed as an exhibit to the Company’s Report on Form 10-Q filed with the Securities and Exchange Commission on November 13, 2002.
(15) Previously filed as an exhibit to the Company’s Report on Form 10-K filed with the Securities and Exchange Commission on March 27, 2003.
II-4
