
LBA45: Phase 1b/2, Open Label, Multicenter, Study of the Combination of SD-101 and Pembrolizumab in Patients with Advanced Melanoma Who Are Naïve to Anti-PD-1/L1 Therapy (SYNERGY-001/KEYNOTE-184, NCT02521870) G. Long1, M. Milhem2, A. Amin3, C. J. Hoimes4, T. Medina5, R. Conry6, C. Lao7, G. Daniels8, S. Reddy9, I. Mehmi10, R. Andtbacka11, M. Barve12, M. Shaheen13, T. Tueting14, M. Chisamore15, B. Xing16, A. Candia16, E. Gamelin16, R. Janssen16, A. Ribas17 1Melanoma Institute Australia, The University of Sydney and Royal North Shore and Mater Hospitals, Sydney, Australia; 2University of Iowa, Iowa City, USA; 3Levine Cancer Institute, Charlotte, USA; 4Case Western Reserve University, Cleveland, USA; 5University of Colorado Cancer Center, Aurora, USA; 6University of Alabama School of Medicine, Birmingham, USA; 7University of Michigan Health System, Ann Arbor, USA; 8University of California, San Diego, USA; 9Stanford University, Stanford, USA; 10Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, USA; 11University of Utah, Salt Lake City, USA; 12Mary Crowley Cancer Research, Dallas, USA; 13University of Arizona Cancer Center North, Tucson, USA; 14University Hospital Magdeburg, Magdeburg, Germany; 15Merck & Co., Inc., Kenilworth, NJ, USA; 16Dynavax Technologies Corporation, Berkeley, USA; 17Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, USA BACKGROUND PD-1 blockade has significantly improved outcomes in advanced melanoma, yet durable responses are elicited in less than half of the patients, therefore this remains an area of unmet need.1 KEYTRUDA® (pembrolizumab) is an anti-PD-1 monoclonal antibody (mAb) that is approved by the FDA to treat patients with unresectable or metastatic melanoma.1 SD-101 is a synthetic class-C CpG-oligodeoxynucleotide, agonist of toll-like receptor 9 (TLR9). SD-101 stimulates human plasmacytoid dendritic cells to release interferon-alpha and mature into efficient antigen-presenting cells, enhancing both innate and adaptive immune responses.2 Preclinical studies in multiple mouse tumor models demonstrated that intratumoral injection of SD-101, in combination with PD-1 blockade, suppressed the growth of tumors not only at the injected site, but also at distant non-injected sites.3 In a previous phase 1b/2 study of patients with indolent non-Hodgkin’s lymphoma, treatment of a single lesion with low dose radiation and intratumoral SD-101 induced abscopal tumor shrinkage in 83% of patients.4 Here, we report the latest results from the phase 1b dose escalation and phase 2 expansion cohort of patients with advanced melanoma naïve to anti-PD-1/L1 therapy who were treated with the combination of SD-101 and pembrolizumab. (Updates data presented at ASCO 2018 (Abstract 9513)5. Results of the phase 1b portion of this study were published in Ribas, A., et al., Cancer Discovery (2018).6 Characteristics 2 mg/lesion n = 47 8 mg/lesion n = 40 Median age, years (Min, Max) 66 (36, 85) 66 (33, 89) > 65 years, n (%) 30 (64) 23 (58) Male, n (%) 33 (70) 26 (65) ECOG PS 0, n (%) 30 (64) 30 (75) Baseline LDH, median (Q1, Q3) > ULN, n (%) 193 (162, 234) 8 (17) 195 (177, 238) 10 (25) Stage at Screening, n (%) IIIC 10 (21) 8 (20) IV 37 (79) 32 (80) M1a 16 (34) 11 (28) M1b 9 (19) 9 (23) M1c 12 (26) 12 (30) PD-L1 Expression, n (%)* Positive (≥1%) 19 (40) 13 (33) Negative (<1%) 15 (32) 15 (38) Pending 13 (28) 12 (30) Prior lines of therapy, 0 / 1 / 2 / ≥3. n (%) 34 / 11 / 2 / 0 (72 / 23 / 4 / 0) 28 / 11 / 0 / 1 (70 / 28 / 0 / 3) Table 1. Demographics and Baseline Characteristics (Phase 1b/2) ECOG PS = Eastern Cooperative Oncology Group performance status; LDH = lactate dehydrogenase; Q1 = first quartile; Q3 = third quartile; ULN = upper limit of normal; * Expression determined by tumor proportion score using Dako 22C3 antibody Safety Table 2. Safety Summary Event, N (%) 2 mg/lesion n = 47 8 mg/lesion n = 40 Total N = 87 Any Treatment-related AE 43 (91) 37 (93) 80 (92) Grade 3-4 12 (26) 16 (40) 28 (32) Chills 4 (9) 1 (3) 5 (6) Myalgia 5 (11) 0 5 (6) Fatigue 2 (4) 3 (8) 5 (6) Headache 4 (9) 2 (5) 6 (7) Malaise 3 (6) 2 (5) 5 (6) Any irAEs 10 (21) 6 (15) 16 (18) Grade 3-4 3 (6) 1 (3) 4 (5) AEs leading to d/c of either or both drug 7 (15) 13 (33) 20 (23) SAEs 14 (30) 15 (38) 29 (33) Death 0 1 (3) 1 (1) irAE = immune-related adverse event; d/c = discontinuation; SAE = serious adverse event. Note: death was considered not related to drug Table 3. Immune-Related Adverse Events 2 mg/lesion n = 47 8 mg/lesion n = 40 Objective response rate, n (%) [95% CI] 33 (70) [56, 81] 19 (48) [33, 63] Complete response 5 (11) 2 (5) Partial response 28 (60) 17 (43) Time to response, median (months) 2.1 2.3 Duration of response, median (months) (95% CI) Not reached (9.0, NE) Not reached (8.2, NE) Stable disease, n (%) 4 (9) 8 (20) Disease Control Rate, n (%) 37 (79) 27 (68) Progressive disease, n (%) 6 (13) 9 (23) Non-evaluable*, n (%) 4 (9) 4 (10) CONCLUSIONS ACKNOWLEDGEMENTS The addition of 2 mg/lesion of SD-101 to pembrolizumab appears to increase immune activity in the tumor microenvironment and efficacy compared with 8 mg/lesion in similar patient populations The ORR in the 2 mg/lesion SD-101 group (70%) was higher than in the 8 mg/lesion SD-101 group (48%) The median PFS in the 2 mg/lesion group (15.2+ months) was significantly longer than in the 8 mg/lesion group (10.4 months) The median DOR in both groups has not been reached Responses occurred in patients with PD-L1 negative tumors and PD-L1 positive tumors Tumor shrinkage occurred in injected lesions, and non-injected visceral lesions including in the liver and lung The combination of SD-101 and pembrolizumab was well tolerated, consistent with previous reports AEs associated with SD-101 were transient, mild to moderate flu-like symptoms that were manageable with over-the-counter medications No increase in immune-related AEs over pembrolizumab monotherapy was observed7,8 Clinical responses were supported by immunologic data consistent with the mechanism of SD-101 Increases in CD8+ cells, NK cells, cytotoxic cells and Th1 cells in the tumor microenvironment were observed in both SD-101 dose groups but were higher in the 2 mg group and appeared to correlate with enhanced clinical efficacy SD-101 is also being investigated in patients with anti-PD-1/L1 resistant/refractory advanced melanoma The addition of SD-101 (8 mg/lesion) to pembrolizumab appears to restore tumor sensitivity to a PD-1 inhibitor in a significant percentage of these patients with an ORR of 21.4% (see ESMO Abstract 3781) Keytruda (pembrolizumab) package insert USA. Merck Sharp & Dohme Corp, Whitehouse Station, NJ. 2014 Guiducci C, et al. J Exp Med 2006;203:1999-2008. Wang S, et al., Proc Natl Acad Sci U S A, 2016. 113(46): p. E7240-e7249 Frank, M.J., et al. Cancer Discovery 2018. DOI: 10.1158/2159-8290. Ribas A. et a; J Clin Oncol 36, 2018 (suppl; abstr 9513) Ribas A, et al. Cancer Discovery 2018; DOI: 10.1158/2159-8290.CD-18-0280. Ribas A, et al. JAMA. 2016;315(15):1600-1609. Specenier P. Expert Opin Biol Ther. 2017;17(6):765-780) This study was sponsored by Dynavax Technologies Corporation in collaboration with Merck & Co., Inc., Kenilworth, NJ USA. We thank the patients and their families and caregivers for participating in the study; the participating study teams; and Tripta Dahiya for contributions to the analysis of the data (Dynavax). Copies of this poster obtained through QR (Quick Response) and/or text key codes are for personal use only and may not be reproduced without written permission of the authors. Corresponding Author: Georgina Long (georgina.long@sydney.edu.au) METHODS Figure 1. Study Design Primary Endpoint: Objective response rate assessed by RECIST v1.1 Secondary Endpoints: Safety and tolerability, progression-free survival, duration of response, and immunophenotype of the tumor environment RESULTS Table 4. Best Overall Response by RECIST v1.1 (ITT Population) SD-101 2 mg i.t. + Pembrolizumab 200 mg i.v. SD-101 4 mg i.t. + Pembrolizumab 200 mg i.v. SD-101 8 mg i.t. + Pembrolizumab 200 mg i.v. SD-101 1 mg i.t.† + Pembrolizumab 200 mg i.v. SD-101 2 mg i.t. in up to 4 lesions + Pembrolizumab 200 mg i.v. OR SD-101 8 mg i.t. in one lesion + Pembrolizumab 200 mg i.v. Phase 1b Dose Escalation* Phase 2 Dose Expansion *DLT period 29 days; †3 patients received 1 mg/lesion; i.t.= intratumoral; i.v. = intravenous. Data Cutoff: September 21, 2018 *Patients discontinued prior to first scan: 2 mg—clinical progression (n=2), irAE (n=1), withdrew consent (n=1); 8 mg—clinical progression (n=1), unrelated AE/death (n=1); irAE (n=1), withdrew consent (n=1). CI = confidence interval; ITT= Intention to treat; NE=not estimable NOTE: Two patients in the 2 mg group with recently reported PRs are not reflected in the figures 2 mg/lesion 8 mg/lesion PFS (Kaplan-Meier method) 6-month rate (95% CI) 85% (70, 93) 60% (42, 73) Median (months) (95% CI) not reached (15.2, NE) 10.4 (4.2, NE) Follow-up, median (months) 5.9 6.9 ORR= objective response rate; PD-L1 expression based on tumor proportion score (Dako 22C3 antibody) Figure 5. Progression-free Survival (ITT Population) Figure 7. Percent Change From Baseline Over Time in Non-injected Target Lesions by Organ System (2 mg SD-101 Per Lesion) PD-L1 Expression 2 mg/lesion 8 mg/lesion N ORR (%) N ORR (%) ≥1% 19 79 13 62 <1 % 15 80 15 33 Pending/missing 13 46 12 50 Phase 1b/2 Trial (SYNERGY-001/KEYNOTE-184) No prior anti-PD-1/L1 therapy At least one injectable lesion CT, computed tomography scan SCREENING TREATMENT TREATMENT 0 1 2 3 4 6 9 12 15 18 21 24 0 1 2 3 4 6 9 12 15 18 21 24.... CT CT CT Biopsy Biopsy Biopsy Biopsy Pembrolizumab Weeks SD-101 Weeks 51 Figure 8. Changes in Immune Activity in the Tumor Microenvironment -100 -80 -60 -40 -20 0 20 40 60 80 100 120 Percent Change from Baseline, % Patients 2 mg/lesion 8 mg/lesion 0.0 0.2 0.4 0.6 0.8 1.0 0 5 10 15 20 25 Months Survival Probability Log-rank test p = 0.02 2 mg/lesion 8 mg/lesion + Censored 2 mg 47 24 9 3 2 0 8 mg 40 23 16 2 1 0 Values above the graphs represent the means and 95% confidence intervals. Methods: biopsies of the injected tumor were collected at screening (prior to dosing) and post-dose. Biopsies were analyzed by the nCounter® PanCancer Immune Profiling Panel (NanoString Technologies, Inc., Seattle WA) to evaluate the immunophenotype of the tumor environment. Nanostring data were analyzed using the nSolver™ Analysis Software Natural Killer Cells 2 mg/lesion 8 mg/lesion Pre: 46.2 ± 18.4 Post: 92.3 ± 31.3 Ave Fold change = 7.11 p < 0.0006 N=23 Pre: 61.4 ± 22.5 Post: 104.7 ± 28.9 Ave fold change = 2.36 p = 0.0076 N=16 Cytotoxic Cells 2 mg/lesion 8 mg/lesion Pre: 37.1 ± 20.8 Post: 142.0 ± 49.2 Ave fold change = 9.86 p < 0.0001 N=23 Pre: 57.0 ± 34.5 Post: 129.4 ± 39.5 Ave fold change = 6.40 p = 0.0052 N=16 Th1 Helper T Cells 2 mg/lesion 8 mg/lesion Pre: 20.6 ± 10.4 Post: 75.1 ± 19.4 Ave fold change = 9.54 p < 0.0001 N=23 Pre: 31.5 ± 15.4 Post: 62.2 ± 13.1 Ave fold change = 3.53 p = 0.0021 N=16 CD8 T Cells 2 mg/lesion 8 mg/lesion Pre: 35.1 ± 18.7 Post: 136.7 ± 42.3 Ave fold change = 11.14 p < 0.0001 N=23 Pre: 46.7 ± 25.8 Post: 91.3 ± 31.7 Ave fold change = 4.61 p = 0.0214 N=16 Figure 2. Best Percent Change From Baseline in All Target Lesions Table 6. Progression-free Survival (ITT Population) Table 5. Responses in Both PD-L1 Negative and Positive Tumors Figure 3. Duration of Follow Up and Patient Status Figure 4. Responses in Two Non-injected Liver Lesions in a Patient with PD-L1 Expression of 1% who Received 2 mg of SD-101 in a Single Lesion Baseline 190 Days Baseline 190 Days Figure 6. Percent Change From Baseline Over Time for Target Lesions in Patients Who Received 2 mg or 8 mg SD-101 Per Lesion All Injected Target Lesions All Non-injected Target Lesions Efficacy Event 2 mg/lesion n = 47 8 mg/lesion n = 40 Total N = 87 irAEs all grades, n (%) Hypothyroidism 7 (15) 3 (8) 10 (12) Pneumonitis 2 (4) 1 (3) 3 (3) Myositis 1 (2) 1 (3) 2 (2) Autoimmune retinopathy 0 1 (3) 1 (1) Autoimmune hepatitis 0 1 (3) 1 (1) Myasthenia gravis 0 1 (3) 1 (1) Colitis 1 (2) 0 1 (1) Autoimmune colitis 1 (2) 0 1 (1) Hypophysitis 2 (4) 0 2 (2) Hyperthyroidism 1 (2) 0 1 (1) Autoimmune myocarditis 0 1 (3) 1 (1) Optic neuritis 0 1 (3) 1 (1) REFERENCES 2 mg/lesion Patients 0 100 200 300 400 500 600 700 800 900 1000 Partial Response Complete Response Progressive Disease Stable Disease Ongoing Days 8 mg/lesion 0 100 200 300 400 500 600 700 800 900 1000 Patients Partial Response Complete Response Progressive Disease Stable Disease Ongoing Days Immune-Related Biomarkers Patients: Unresectable Stage IIIC, Stage IV Metastatic Melanoma ECOG performance status of 0 or 1 . CI = confidence interval; ITT = intention to treat; NE=not estimable; PFS = progression-free survival Exhibit 99.2

Abstract 3781: Phase 1b/2, Open-Label, Multicenter Study of Intratumoral SD-101 in Combination With Pembrolizumab in Patients With Advanced Melanoma Resistant/Refractory to Anti-PD-1/PD-L1 Therapy (SYNERGY-001/KEYNOTE-184, NCT02521870) A. Ribas1, I. Mehmi2, T. Medina3, C. Lao4, S. Kummar5, A. Amin6, S. Deva7, A. K. Salama8, T. Tueting9, M. Milhem10, C. J. Hoimes11, G. Daniels12, M. Shaheen13, S. Jang14, M. Barve15, A. Powell16, S. Chandra17, E. V. Schmidt18, R. Janssen19, G. V. Long20 1Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, USA; 2Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, USA; 3University of Colorado Cancer Center, Aurora, USA; 4University of Michigan Health System, Ann Arbor, USA; 5Stanford University School of Medicine, Stanford, USA; 6Levine Cancer Institute, Charlotte, USA; 7Auckland City Hospital, Auckland, NZ; 8Duke University, Durham, USA; 9University Hospital Madgeburg, Madgeburg, DE; 10University of Iowa, Iowa City, USA; 11Case Western Reserve University, Cleveland, USA; 12University of California, San Diego, USA; 13University of Arizona Cancer Center North, Tucson, USA; 14Melanoma and Skin Cancer Center, Inova Char Cancer Institute, Fairfax, USA; 15Mary Crowley Cancer Research, Dallas, USA; 16Affinity Research, Nedlands, AU; 17Northwestern University, Chicago, USA; 18Merck & Co., Inc., Kenilworth, NJ, USA; 19Dynavax Technologies Corporation, Berkeley, USA; 20Melanoma Institute Australia, University of Sydney, AU BACKGROUND PD-1 blockade has significantly improved outcomes in advanced melanoma, yet durable responses are elicited in less than half of the patients, therefore this remains an area of unmet need.1 KEYTRUDA® (pembrolizumab) is an anti-PD-1 monoclonal antibody (mAb) that is approved by the FDA to treat patients with unresectable or metastatic melanoma.1 SD-101 is a synthetic class-C, CpG-oligodeoxynucleotide, toll-like receptor nine (TLR9) agonist, which stimulates human plasmacytoid dendritic cells (PDCs) to release interferon-alpha and mature into efficient antigen-presenting cells, enhancing both innate and adaptive immune responses (Figure 1).2 Preclinical studies of anti-PD-1 non-responder mouse tumor models demonstrated that intratumoral injection of SD-101, in combination with PD-1 blockade, suppressed the growth of tumors not only at the injected site, but also at distant un-injected sites.3 In the phase 1b portion of this study, intratumoral injections of SD-101 in combination with pembrolizumab demonstrated clinical responses in both injected and distant lesions of patients with metastatic melanoma.4 Here, we report the results from a phase 2 expansion cohort of patients with advanced melanoma resistant/refractory (R/R) to anti-PD-1/PD-L1 therapy who were treated with the combination of SD-101 and pembrolizumab. Preliminary results from the phase 1b portion of this study were presented at AACR 2018 (Abstract: CT139) and published in Cancer Discovery (2018): Ribas, A., et al.5,6 Figure 1. Both Innate and Adaptive Immune Responses Are Increased by Intratumoral Injection of SD-101 Characteristics N = 30 Median age, years (Min, Max) 64 (53, 76) Male, n (%) 23 (76.7) ECOG PS 0/1, n (%) 30 (100) Baseline LDH, mean (SD) ≤ULN, n (%) >1 to ≤2 ULN, n (%) >2 ULN, n (%) 223 (3.0, 1886.0) 17 (55%) 9 (29%) 4 (13%) Stage at Screening, n (%) IIIC 10 (33.3) IV 19 (63.3) Missing 1 (3.3) Metastases involving: Skin/Subcutaneous tissue 20 (66.7) Lymph Nodes 15 (50) Liver 9 (30) Lung 6 (20) Bones 3 (10) Other 13 (43.3) PD-L1 expression, n (%)* Positive (≥1%) 4 (13.3) Negative (<1%) 13 (43.3) Pending 13 (43.3) Table 1. Demographics and Baseline Characteristics ECOG PS = Eastern Cooperative Oncology Group performance status; LDH = lactate dehydrogenase; SD = standard deviation; ULN = upper limit of normal; * Expression determined using Dako 22C3 antibody Table 2. Baseline Disease Characteristics Characteristics, n (%) N = 30 Prior radiotherapy 6 (20) Prior surgery 28 (93.3) Prior lines of therapy, 1/2/≥3 11/8/11 PD1/PD-L1 therapy Resistant* 18 (60) Refractory** 12 (40) Prior CTLA-4 therapy 12 (40) Event, n (%) N = 30 irAEs all grades 4 (13.3) Hypothyroidism 1 (3.3) Pneumonitis 1 (3.3) Autoimmune hepatitis 1 (3.3) Pancreatitis 1 (3.3) Table 5. Safety Summary Event, N (%) N = 30 Any Treatment-related AE 24 (80) Grade 3-4 12 (40) Chills 1 (3.3) Myalgia 2 (6.6) Injection-site pain 1 (3.3) Fatigue 2 (6.6) Headache 0 Malaise 2 (6.6) Vomiting 3 (10) Any irAEs 4 (13.3) Grade 3-4 2 (6.6) AEs leading to d/c of either or both drug 4 (13.3) SAEs 3 (10) Death** 1 (3.3) Table 6. Immune-Related Adverse Events irAE = immune-related adverse event; d/c = discontinuation; SAE = serious adverse event **Death not related to study treatment Efficacy Table 3. Best Overall Response in mITT by RECIST Best ORR, n (%) N = 29 (mITT*) Objective response rate 6 (21.4) Complete response 1 (3.4) Partial response 5 (17.2) Duration of response (months), median (min, max) 2.1 (1.0, 6.1) Stable disease 5 (17.2) Disease Control Rate 11 (38.0) Progressive disease 11 (37.9) Tumor IFN DC T cell SD-101 induces IFN and DC maturation Dendritic cells take up antigens from dying tumor cells and migrate to the lymph nodes Chemokines IFN IFN SD-101 (CXCL9, 10) Tumor antigens Lymph node DC Dying tumor T cell PD-L1 PD1 Anti-PD1 Dying tumor NK cell IFNs stimulate tumor killing by NK cells Tumor Cell Tumor-specific T cells activated by DC and IFN in lymph node Uninjected tumors are destroyed by CTL generated in the SD-101 injected sites Blood vessel CTL CTL CTL Dying tumor CTL Dying tumor CTL CTL = cytotoxic (CD8+) T cell; DC = dendritic cells; IFN = interferon; NK = natural killer CONCLUSIONS REFERENCES Patient Best Response TPS 1 PR 0 2 SD 0 3 SD 0 4 PD 0 5 PD 0 6 PD 0 7 PR <1 8 SD <1 9 PD <1 10 SD 1 11 SD 1 12 PD 1 13 PD 1 14 CR 3 15 PR 10 16 PR 10 17 PD 90 Table 4. PD-L1 Expression Levels and Efficacy Figure 5. Patient Response History and Current Status The combination of SD-101 and pembrolizumab was well tolerated, consistent with previous reports: AEs associated with SD-101 were transient, mild to moderate injection-site reactions and flu-like symptoms that were manageable with over-the-counter medications Low incidence of immune-related AEs Preliminary data showed encouraging efficacy, with an ORR of 21.4% Responses were observed in both SD-101 injected and non-injected lesions; however, it is too early to determine the durability of responses Responses and disease control were observed in PD-L1 positive and negative tumors The addition of SD-101 to pembrolizumab appears to restore tumor sensitivity to PD-1 inhibitor in patients who are R/R to anti-PD1/PD-L1 therapy Based on results from the phase 2 study in PD-1/PD-L1 naive patients demonstrating higher efficacy of multiple 2mg injections (up to 4 injections) compared to 1 injection of 8 mg, a phase 2 study with the new dosing of 2 mg per injection is ongoing Keytruda (pembrolizumab) for injection, for intravenous use [package insert]. Merck Sharp & Dohme Corp, Whitehouse Station, NJ USA. 2014. Guiducci C, et al. J Exp Med 2006;203:1999-2008. Wang S, et al. Proc Natl Acad Sci U S A, 2016. 113(46):E7240-e7249. Leung ACF, et al. Journal of Clinical Oncology 2017;35:15 (suppl):9550. Ribas A, et al. Cancer Res 2018;78 (13 Supplement):CT139. Ribas A, et al. Cancer Discovery 2018; DOI: 10.1158/2159-8290.CD-18-0280. This study was sponsored by Dynavax Technologies Corporation in collaboration with Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. We thank the patients and their families and caregivers for participating in the study; the participating study teams and Albert Candia, Kavya Kazipeta, and Tripta Dahiya for contributions to the analysis of the data (Dynavax Technologies Corporation). Copies of this poster obtained through QR (Quick Response) and/or text key codes are for personal use only and may not be reproduced without written permission of the authors. Corresponding Author: Antoni Ribas (aribas@mednet.ucla.edu) CT, computed tomography scan SCREENING TREATMENT TREATMENT 0 1 2 3 4 6 9 12 15 18 21 24 0 1 2 3 4 6 9 12 15 18 21 24 CT CT CT Pembrolizumab Weeks SD-101 Weeks TPS: tumor proportion score; PD-L1 expression data are pending for 13 patients PD-L1 negative PD-L1 positive METHODS Ongoing Phase 1b/2, Open-label, Multicenter, Expansion Study of Intratumoral SD-101 in Combination With Pembrolizumab (SYNERGY-001, MEL-01, NCT02521870) Patients: Stage IIIC, Stage IV Metastatic Melanoma Resistant or refractory to prior anti-PD-1/PD-L1 therapy ECOG performance status of 0 or 1 At least 2 lesions that qualify as a target lesion per RECIST v1.1, and 1 of the qualifying lesions must be easily accessible and amenable to multiple intratumoral injections. The target lesion should be of sufficient size such that the required tumor biopsies do not significantly affect tumor assessment per RECIST v1.1. Study Treatment: SD-101 was administered intratumorally in one target lesion, 8 mg Weekly x 4 doses then Q3W x 7 doses* Pembrolizumab was administered IV (200 mg Q3W) Figure 2. Study Design Primary Endpoint: Overall response rate assessed by RECIST v1.1 Secondary Endpoints: Safety and tolerability, duration of response, and immunophenotype of the tumor environment RESULTS SD-101 induces PDCs to secrete high levels of interferon-alpha, a potent immunomodulatory cytokine that is able to boost NK cell cytotoxic activity and induce recruitment of T cells. In addition, SD-101 induces DC maturation cross-presentation of tumor associated antigens, inducing CD8+ T cell responses. STUDY OBJECTIVE To confirm the safety profile and assess the efficacy of SD-101 and pembrolizumab combination therapy in patients with advanced melanoma who were R/R to anti-PD-1 therapy. Figure 3. Best Percent Change from Baseline in Target Lesions Figure 4. Percent Change from Baseline Over Time Days Complete Response Partial Response Stable Disease Progressive Disease Ongoing Patients 0 50 100 150 200 250 300 350 400 100 80 60 40 20 0 -20 -40 -60 -80 -100 Percent change from baseline, % Non-Injected Lesions Patients 100 80 60 40 20 0 -20 -40 -60 -80 -100 Percent change from baseline, % All Target Lesions Patients 100 80 60 40 20 0 -20 -40 -60 -80 -100 Percent change from baseline, % Injected Lesions Patients Note: Patients receiving 1 and 2 mg dosing were excluded (1 of 4 patients who received 1 or 2 mg dosing experienced a partial response) *mITT: excluding patients on treatment but did not yet have their first CT scan and tumor assessment *Out of 38 patients, 4 patients treated with 4 mg per injection and 4 patients treated with up to 2 mg per injection in other cohorts were excluded; 30 patients have been treated with 8 mg per injection. SAFETY *Resistant: progressive disease after at least 3 months on treatment and initial objective response or stable disease. A disease with a relapse while on adjuvant treatment or within 6 months after the end of an adjuvant treatment is also considered resistant. **Refractory: tumor initially resistant, best confirmed response is progressive disease while on treatment.
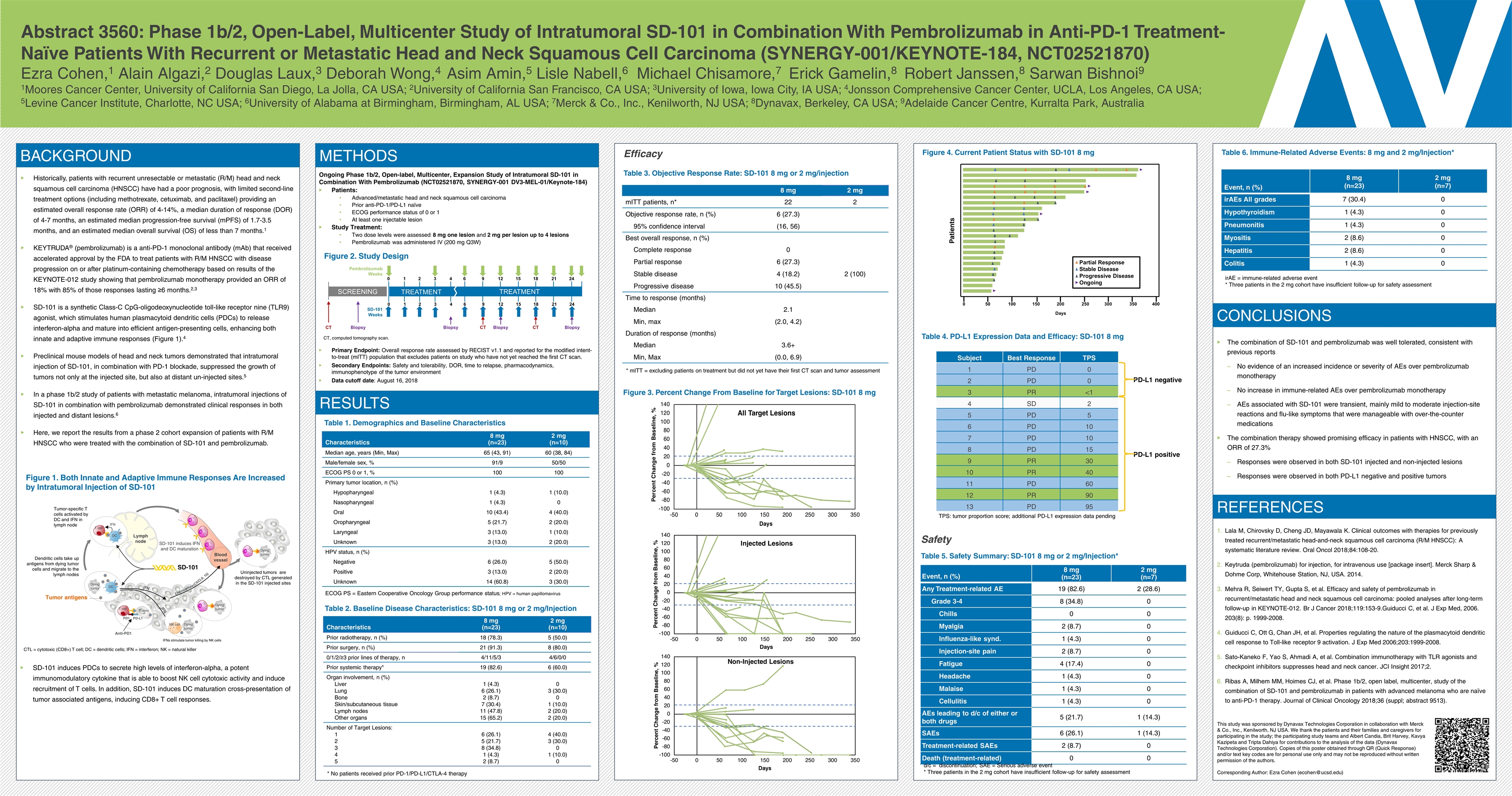
Abstract 3560: Phase 1b/2, Open-Label, Multicenter Study of Intratumoral SD-101 in Combination With Pembrolizumab in Anti-PD-1 Treatment-Naïve Patients With Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (SYNERGY-001/KEYNOTE-184, NCT02521870) Ezra Cohen,1 Alain Algazi,2 Douglas Laux,3 Deborah Wong,4 Asim Amin,5 Lisle Nabell,6 Michael Chisamore,7 Erick Gamelin,8 Robert Janssen,8 Sarwan Bishnoi9 1Moores Cancer Center, University of California San Diego, La Jolla, CA USA; 2University of California San Francisco, CA USA; 3University of Iowa, Iowa City, IA USA; 4Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA USA; 5Levine Cancer Institute, Charlotte, NC USA; 6University of Alabama at Birmingham, Birmingham, AL USA; 7Merck & Co., Inc., Kenilworth, NJ USA; 8Dynavax, Berkeley, CA USA; 9Adelaide Cancer Centre, Kurralta Park, Australia BACKGROUND Historically, patients with recurrent unresectable or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) have had a poor prognosis, with limited second-line treatment options (including methotrexate, cetuximab, and paclitaxel) providing an estimated overall response rate (ORR) of 4-14%, a median duration of response (DOR) of 4-7 months, an estimated median progression-free survival (mPFS) of 1.7-3.5 months, and an estimated median overall survival (OS) of less than 7 months.1 KEYTRUDA® (pembrolizumab) is a anti-PD-1 monoclonal antibody (mAb) that received accelerated approval by the FDA to treat patients with R/M HNSCC with disease progression on or after platinum-containing chemotherapy based on results of the KEYNOTE-012 study showing that pembrolizumab monotherapy provided an ORR of 18% with 85% of those responses lasting ≥6 months.2,3 SD-101 is a synthetic Class-C CpG-oligodeoxynucleotide toll-like receptor nine (TLR9) agonist, which stimulates human plasmacytoid dendritic cells (PDCs) to release interferon-alpha and mature into efficient antigen-presenting cells, enhancing both innate and adaptive immune responses (Figure 1).4 Preclinical mouse models of head and neck tumors demonstrated that intratumoral injection of SD-101, in combination with PD-1 blockade, suppressed the growth of tumors not only at the injected site, but also at distant un-injected sites.5 In a phase 1b/2 study of patients with metastatic melanoma, intratumoral injections of SD-101 in combination with pembrolizumab demonstrated clinical responses in both injected and distant lesions.6 Here, we report the results from a phase 2 cohort expansion of patients with R/M HNSCC who were treated with the combination of SD-101 and pembrolizumab. Figure 1. Both Innate and Adaptive Immune Responses Are Increased by Intratumoral Injection of SD-101 Characteristics 8 mg (n=23) 2 mg (n=10) Median age, years (Min, Max) 65 (43, 91) 60 (38, 84) Male/female sex, % 91/9 50/50 ECOG PS 0 or 1, % 100 100 Primary tumor location, n (%) Hypopharyngeal 1 (4.3) 1 (10.0) Nasopharyngeal 1 (4.3) 0 Oral 10 (43.4) 4 (40.0) Oropharyngeal 5 (21.7) 2 (20.0) Laryngeal 3 (13.0) 1 (10.0) Unknown 3 (13.0) 2 (20.0) HPV status, n (%) Negative 6 (26.0) 5 (50.0) Positive 3 (13.0) 2 (20.0) Unknown 14 (60.8) 3 (30.0) Table 1. Demographics and Baseline Characteristics ECOG PS = Eastern Cooperative Oncology Group performance status; HPV = human papillomavirus Table 2. Baseline Disease Characteristics: SD-101 8 mg or 2 mg/Injection Characteristics 8 mg (n=23) 2 mg (n=10) Prior radiotherapy, n (%) 18 (78.3) 5 (50.0) Prior surgery, n (%) 21 (91.3) 8 (80.0) 0/1/2/≥3 prior lines of therapy, n 4/11/5/3 4/6/0/0 Prior systemic therapy* 19 (82.6) 6 (60.0) Organ involvement, n (%) Liver Lung Bone Skin/subcutaneous tissue Lymph nodes Other organs 1 (4.3) 6 (26.1) 2 (8.7) 7 (30.4) 11 (47.8) 15 (65.2) 0 3 (30.0) 0 1 (10.0) 2 (20.0) 2 (20.0) Number of Target Lesions: 1 2 3 4 5 6 (26.1) 5 (21.7) 8 (34.8) 1 (4.3) 2 (8.7) 4 (40.0) 3 (30.0) 0 1 (10.0) 0 Safety Event, n (%) 8 mg (n=23) 2 mg (n=7) irAEs All grades 7 (30.4) 0 Hypothyroidism 1 (4.3) 0 Pneumonitis 1 (4.3) 0 Myositis 2 (8.6) 0 Hepatitis 2 (8.6) 0 Colitis 1 (4.3) 0 Table 5. Safety Summary: SD-101 8 mg or 2 mg/Injection* Event, n (%) 8 mg (n=23) 2 mg (n=7) Any Treatment-related AE 19 (82.6) 2 (28.6) Grade 3-4 8 (34.8) 0 Chills 0 0 Myalgia 2 (8.7) 0 Influenza-like synd. 1 (4.3) 0 Injection-site pain 2 (8.7) 0 Fatigue 4 (17.4) 0 Headache 1 (4.3) 0 Malaise 1 (4.3) 0 Cellulitis 1 (4.3) 0 AEs leading to d/c of either or both drugs 5 (21.7) 1 (14.3) SAEs 6 (26.1) 1 (14.3) Treatment-related SAEs 2 (8.7) 0 Death (treatment-related) 0 0 Table 6. Immune-Related Adverse Events: 8 mg and 2 mg/Injection* d/c = discontinuation; SAE = Serious adverse event * Three patients in the 2 mg cohort have insufficient follow-up for safety assessment Efficacy Table 3. Objective Response Rate: SD-101 8 mg or 2 mg/injection 8 mg 2 mg mITT patients, n* 22 2 Objective response rate, n (%) 6 (27.3) 95% confidence interval (16, 56) Best overall response, n (%) Complete response 0 Partial response 6 (27.3) Stable disease 4 (18.2) 2 (100) Progressive disease 10 (45.5) Time to response (months) Median 2.1 Min, max (2.0, 4.2) Duration of response (months) Median 3.6+ Min, Max (0.0, 6.9) Tumor IFN DC T cell SD-101 induces IFN and DC maturation Dendritic cells take up antigens from dying tumor cells and migrate to the lymph nodes Chemokines IFN IFN SD-101 (CXCL9, 10) Tumor antigens Lymph node DC Dying tumor T cell PD-L1 PD1 Anti-PD1 Dying tumor NK cell IFNs stimulate tumor killing by NK cells Tumor Tumor-specific T cells activated by DC and IFN in lymph node Uninjected tumors are destroyed by CTL generated in the SD-101 injected sites Blood vessel CTL CTL CTL Dying tumor CTL Dying tumor CTL CTL = cytotoxic (CD8+) T cell; DC = dendritic cells; IFN = interferon; NK = natural killer CONCLUSIONS REFERENCES Subject Best Response TPS 1 PD 0 2 PD 0 3 PR <1 4 SD 2 5 PD 5 6 PD 10 7 PD 10 8 PD 15 9 PR 30 10 PR 40 11 PD 60 12 PR 90 13 PD 95 Table 4. PD-L1 Expression Data and Efficacy: SD-101 8 mg Figure 3. Percent Change From Baseline for Target Lesions: SD-101 8 mg Figure 4. Current Patient Status with SD-101 8 mg The combination of SD-101 and pembrolizumab was well tolerated, consistent with previous reports No evidence of an increased incidence or severity of AEs over pembrolizumab monotherapy No increase in immune-related AEs over pembrolizumab monotherapy AEs associated with SD-101 were transient, mainly mild to moderate injection-site reactions and flu-like symptoms that were manageable with over-the-counter medications The combination therapy showed promising efficacy in patients with HNSCC, with an ORR of 27.3% Responses were observed in both SD-101 injected and non-injected lesions Responses were observed in both PD-L1 negative and positive tumors Lala M, Chirovsky D, Cheng JD, Mayawala K. Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC): A systematic literature review. Oral Oncol 2018;84:108-20. Keytruda (pembrolizumab) for injection, for intravenous use [package insert]. Merck Sharp & Dohme Corp, Whitehouse Station, NJ, USA. 2014. Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer 2018;119:153-9.Guiducci C, et al. J Exp Med, 2006. 203(8): p. 1999-2008. Guiducci C, Ott G, Chan JH, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med 2006;203:1999-2008. Sato-Kaneko F, Yao S, Ahmadi A, et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017;2. Ribas A, Milhem MM, Hoimes CJ, et al. Phase 1b/2, open label, multicenter, study of the combination of SD-101 and pembrolizumab in patients with advanced melanoma who are naïve to anti-PD-1 therapy. Journal of Clinical Oncology 2018;36 (suppl; abstract 9513). CT, computed tomography scan. SCREENING TREATMENT TREATMENT 0 1 2 3 4 6 9 12 15 18 21 24 0 1 2 3 4 6 9 12 15 18 21 24 CT CT CT Biopsy Biopsy Biopsy Biopsy Pembrolizumab Weeks SD-101 Weeks * mITT = excluding patients on treatment but did not yet have their first CT scan and tumor assessment Patients 0 50 100 150 200 250 300 350 400 Partial Response Stable Disease Progressive Disease Ongoing Days TPS: tumor proportion score; additional PD-L1 expression data pending PD-L1 negative PD-L1 positive METHODS Ongoing Phase 1b/2, Open-label, Multicenter, Expansion Study of Intratumoral SD-101 in Combination With Pembrolizumab (NCT02521870, SYNERGY-001 DV3-MEL-01/Keynote-184) Patients: Advanced/metastatic head and neck squamous cell carcinoma Prior anti-PD-1/PD-L1 naïve ECOG performance status of 0 or 1 At least one injectable lesion Study Treatment: Two dose levels were assessed: 8 mg one lesion and 2 mg per lesion up to 4 lesions Pembrolizumab was administered IV (200 mg Q3W) Figure 2. Study Design Primary Endpoint: Overall response rate assessed by RECIST v1.1 and reported for the modified intent-to-treat (mITT) population that excludes patients on study who have not yet reached the first CT scan. Secondary Endpoints: Safety and tolerability, DOR, time to relapse, pharmacodynamics, immunophenotype of the tumor environment Data cutoff date: August 16, 2018 RESULTS irAE = immune-related adverse event * Three patients in the 2 mg cohort have insufficient follow-up for safety assessment This study was sponsored by Dynavax Technologies Corporation in collaboration with Merck & Co., Inc., Kenilworth, NJ USA. We thank the patients and their families and caregivers for participating in the study; the participating study teams and Albert Candia, Brit Harvey, Kavya Kazipeta and Tripta Dahiya for contributions to the analysis of the data (Dynavax Technologies Corporation). Copies of this poster obtained through QR (Quick Response) and/or text key codes are for personal use only and may not be reproduced without written permission of the authors. Corresponding Author: Ezra Cohen (ecohen@ucsd.edu) SD-101 induces PDCs to secrete high levels of interferon-alpha, a potent immunomodulatory cytokine that is able to boost NK cell cytotoxic activity and induce recruitment of T cells. In addition, SD-101 induces DC maturation cross-presentation of tumor associated antigens, inducing CD8+ T cell responses. * No patients received prior PD-1/PD-L1/CTLA-4 therapy


