
Intro Slide Exhibit 99.3
BACKGROUND Page PD-1 blockade has significantly improved outcomes in advanced melanoma, yet durable responses are elicited in less than half of the patients. There remains an area of unmet need in patients who are anti-PD-1 naïve or experienced. KEYTRUDA® (pembrolizumab) is an anti-PD-1 monoclonal antibody (mAb) that is approved by the FDA to treat patients with unresectable or metastatic melanoma and recurrent or metastatic HNSCC. SD-101 is a synthetic class-C, CpG-oligodeoxynucleotide, toll-like receptor nine (TLR9) agonist
BACKGROUND CONT. Page In preclinical studies of anti-PD-1 non-responder mouse tumor models, intratumoral injection of SD-101, in combination with PD-1 blockade, suppressed the growth of tumors at the injected and distant non-injected sites. Wang S, et al., PNAS, 2016. 113(46): p. E7240-e7249 In a previous phase 1b/2 study of patients with indolent non-Hodgkin’s lymphoma, treatment of a single lesion with low dose radiation and intratumoral SD-101 induced abscopal tumor shrinkage in 83% of patients. Frank, M.J., et al. Cancer Discovery 2018. DOI: 10.1158/2159-8290 In the phase 1b portion of SYNERGY-001/MEL-01, intratumoral injections of SD-101 in combination with pembrolizumab demonstrated clinical responses in both injected and distant lesions of patients with metastatic melanoma, both those who were naïve to anti-PD-1/L1 therapy or experienced. Ribas A, et al. Cancer Discovery 2018; DOI: 10.1158/2159-8290.CD-18-0280

BACKGROUND CONT. Page Here, we report the results presented at ESMO from the ongoing phase 2 expansion of SYNERGY-001 in patients with: advanced melanoma resistant/refractory (R/R) to anti-PD-1/L1 therapy; advanced melanoma naïve to anti-PD-1/L1 therapy; and advanced head and neck squamous cell carcinoma naïve to anti-PD-1/L1 therapy.
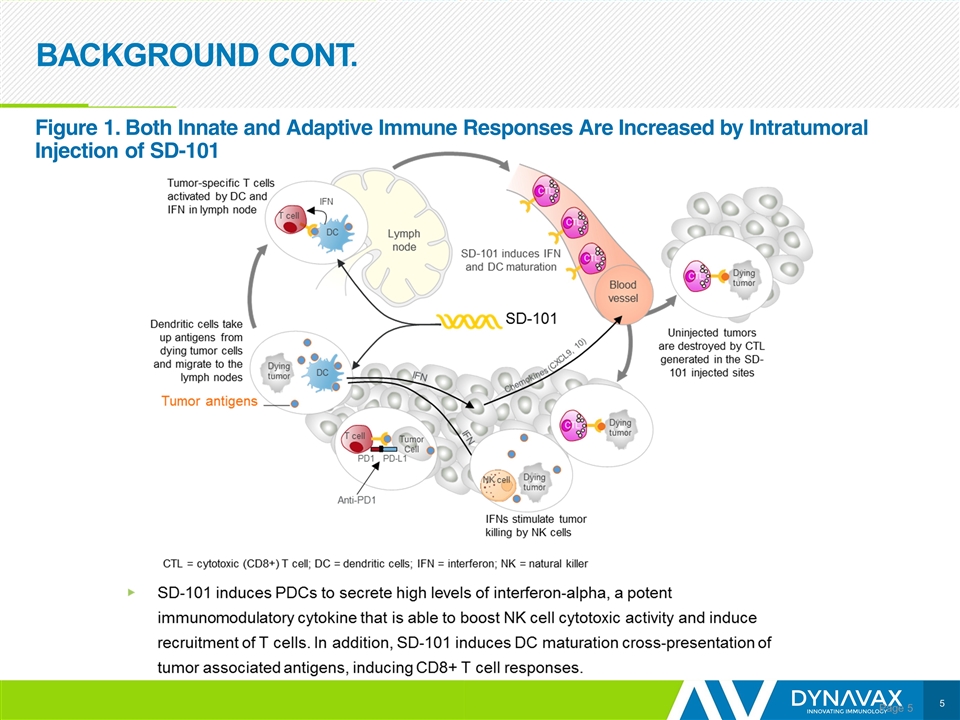
BACKGROUND CONT. Page Figure 1. Both Innate and Adaptive Immune Responses Are Increased by Intratumoral Injection of SD-101

Abstract 3781: Phase 1b/2, Open-Label, Multicenter Study of Intratumoral SD-101 in Combination With Pembrolizumab in Patients With Advanced Melanoma Resistant/Refractory to Anti-PD-1/PD-L1 Therapy (SYNERGY-001/KEYNOTE-184, NCT02521870) A. Ribas1, I. Mehmi2, T. Medina3, C. Lao4, S. Kummar5, A. Amin6, S. Deva7, A. K. Salama8, T. Tueting9, M. Milhem10, C. J. Hoimes11, G. Daniels12, M. Shaheen13, S. Jang14, M. Barve15, A. Powell16, S. Chandra17, E. V. Schmidt18, R. Janssen19, G. V. Long20 1Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, USA; 2Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, USA; 3University of Colorado Cancer Center, Aurora, USA; 4University of Michigan Health System, Ann Arbor, USA; 5Stanford University School of Medicine, Stanford, USA; 6Levine Cancer Institute, Charlotte, USA; 7Auckland City Hospital, Auckland, NZ; 8Duke University, Durham, USA; 9University Hospital Madgeburg, Madgeburg, DE; 10University of Iowa, Iowa City, USA; 11Case Western Reserve University, Cleveland, USA; 12University of California, San Diego, USA; 13University of Arizona Cancer Center North, Tucson, USA; 14Melanoma and Skin Cancer Center, Inova Char Cancer Institute, Fairfax, USA; 15Mary Crowley Cancer Research, Dallas, USA; 16Affinity Research, Nedlands, AU; 17Northwestern University, Chicago, USA; 18Merck & Co., Inc., Kenilworth, NJ, USA; 19Dynavax Technologies Corporation, Berkeley, USA; 20Melanoma Institute Australia, University of Sydney, AU
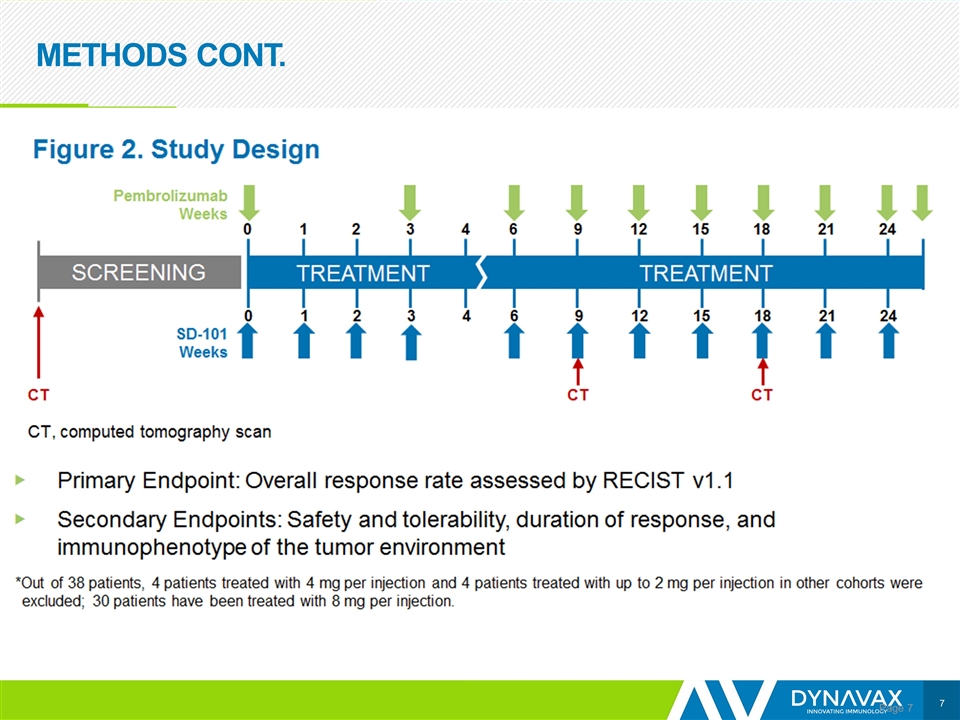
METHODS CONT. Page
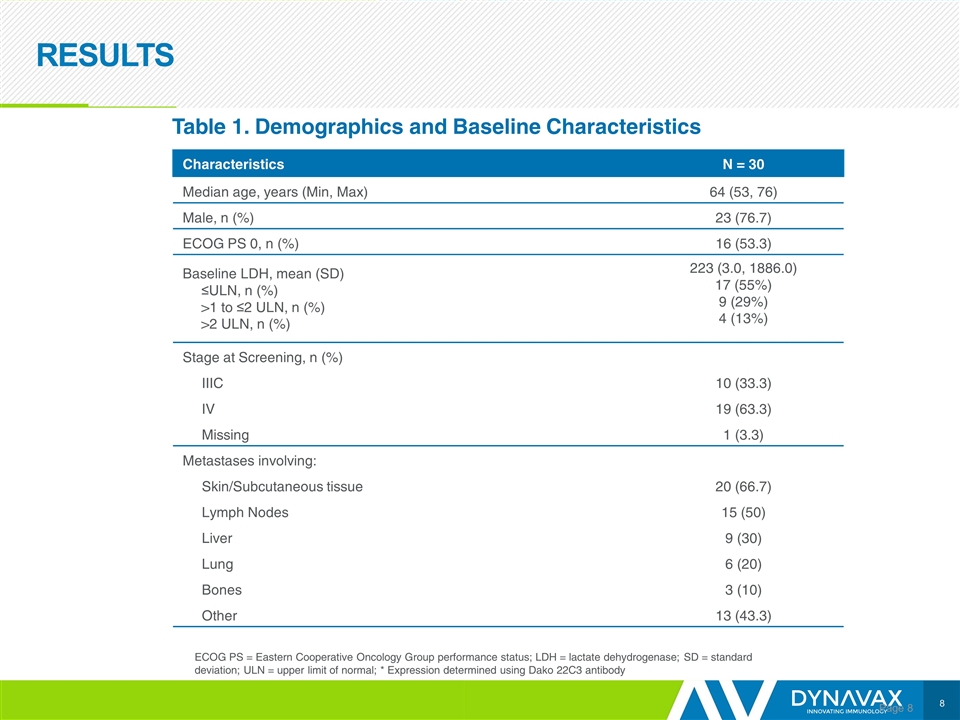
RESULTS Page Characteristics N = 30 Median age, years (Min, Max) 64 (53, 76) Male, n (%) 23 (76.7) ECOG PS 0, n (%) 16 (53.3) Baseline LDH, mean (SD) ≤ULN, n (%) >1 to ≤2 ULN, n (%) >2 ULN, n (%) 223 (3.0, 1886.0) 17 (55%) 9 (29%) 4 (13%) Stage at Screening, n (%) IIIC 10 (33.3) IV 19 (63.3) Missing 1 (3.3) Metastases involving: Skin/Subcutaneous tissue 20 (66.7) Lymph Nodes 15 (50) Liver 9 (30) Lung 6 (20) Bones 3 (10) Other 13 (43.3) Table 1. Demographics and Baseline Characteristics ECOG PS = Eastern Cooperative Oncology Group performance status; LDH = lactate dehydrogenase; SD = standard deviation; ULN = upper limit of normal; * Expression determined using Dako 22C3 antibody
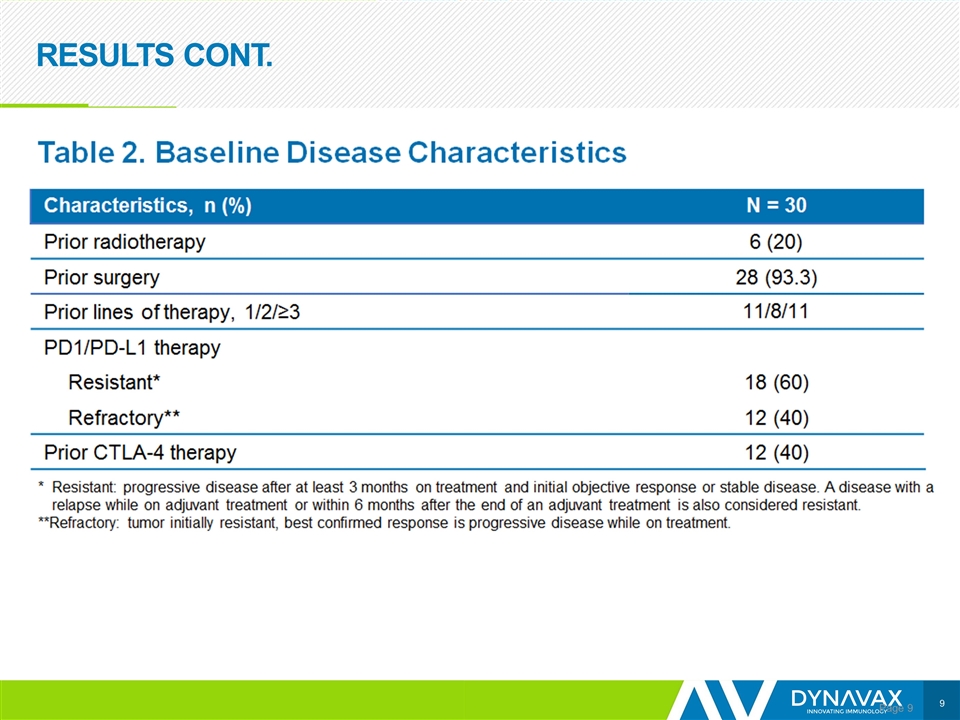
RESULTS CONT. Page
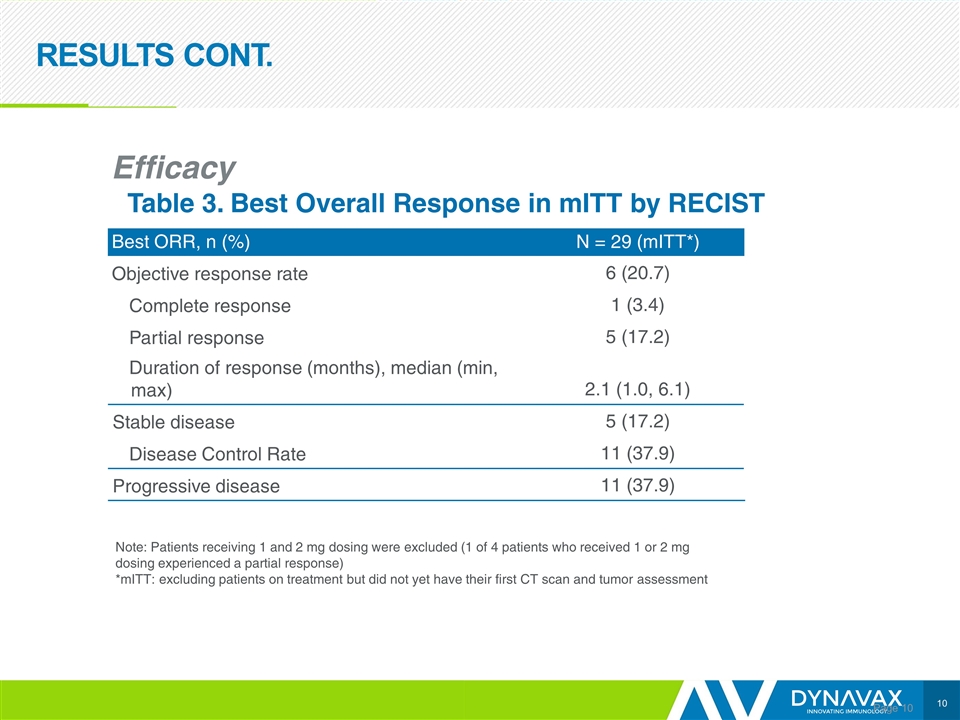
RESULTS CONT. Page Efficacy Table 3. Best Overall Response in mITT by RECIST Best ORR, n (%) N = 29 (mITT*) Objective response rate 6 (20.7) Complete response 1 (3.4) Partial response 5 (17.2) Duration of response (months), median (min, max) 2.1 (1.0, 6.1) Stable disease 5 (17.2) Disease Control Rate 11 (37.9) Progressive disease 11 (37.9) Note: Patients receiving 1 and 2 mg dosing were excluded (1 of 4 patients who received 1 or 2 mg dosing experienced a partial response) *mITT: excluding patients on treatment but did not yet have their first CT scan and tumor assessment
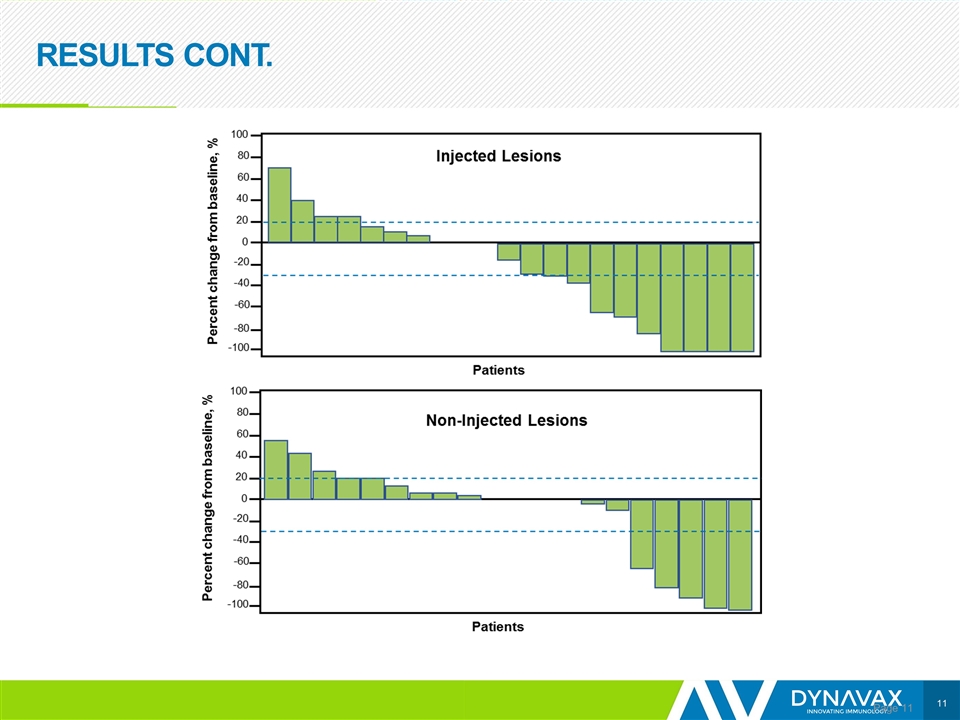
RESULTS CONT. Page
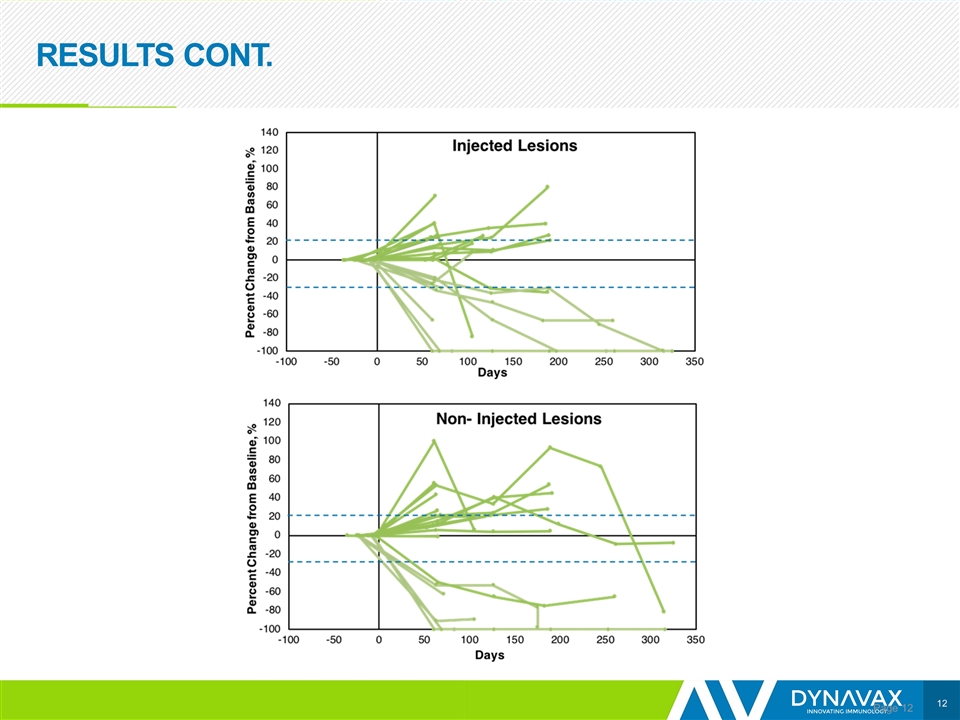
RESULTS CONT. Page
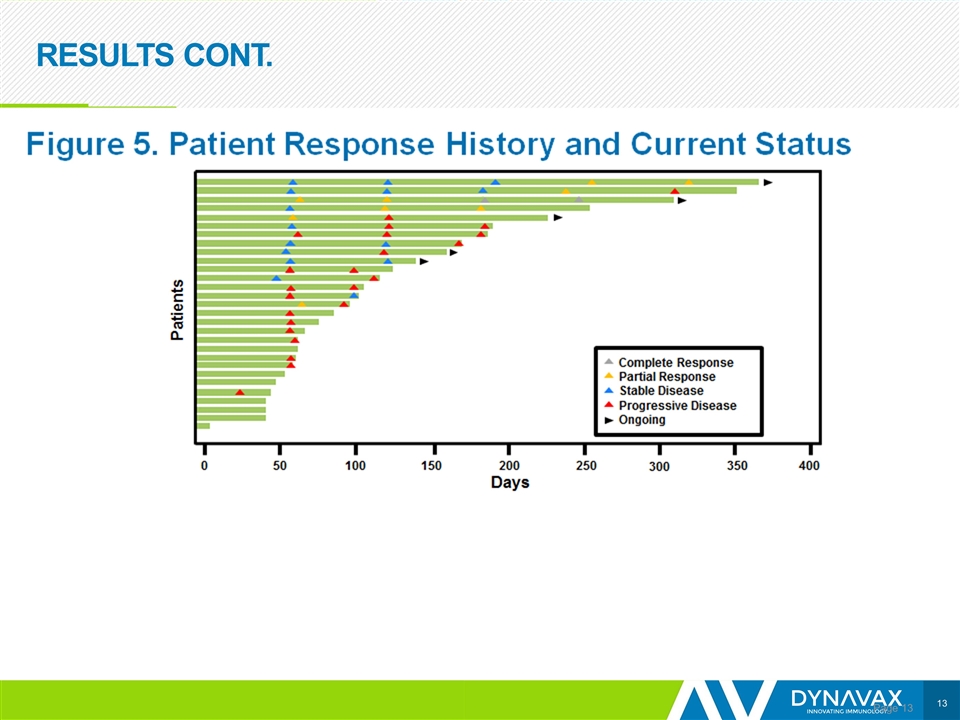
RESULTS CONT. Page
CONCLUSIONS Page Preliminary data show encouraging efficacy, with an ORR of 21% Responses were observed in both SD-101 injected and non-injected lesions; however, it is too early to determine the durability of responses The addition of SD-101 to pembrolizumab appears to restore tumor sensitivity to PD-1 inhibitors in patients who are R/R to such therapy Based on data from the anti-PD-1/L1 naïve melanoma population, 25 patients with R/R disease are being enrolled to receive 2 mg per injection

LBA45: Phase 1b/2, Open Label, Multicenter, Study of the Combination of SD-101 and Pembrolizumab in Patients with Advanced Melanoma Who Are Naïve to Anti-PD-1/L1 Therapy (SYNERGY-001/KEYNOTE-184, NCT02521870) G. Long1, M. Milhem2, A. Amin3, C. J. Hoimes4, T. Medina5, R. Conry6, C. Lao7, G. Daniels8, S. Reddy9, I. Mehmi10, R. Andtbacka11, M. Barve12, M. Shaheen13, T. Tueting14, M. Chisamore15, B. Xing16, A. Candia16, E. Gamelin16, R. Janssen16, A. Ribas17 1Melanoma Institute Australia, The University of Sydney and Royal North Shore and Mater Hospitals, Sydney, Australia; 2University of Iowa, Iowa City, USA; 3Levine Cancer Institute, Charlotte, USA; 4Case Western Reserve University, Cleveland, USA; 5University of Colorado Cancer Center, Aurora, USA; 6University of Alabama School of Medicine, Birmingham, USA; 7University of Michigan Health System, Ann Arbor, USA; 8University of California, San Diego, USA; 9Stanford University, Stanford, USA; 10Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, USA; 11University of Utah, Salt Lake City, USA; 12Mary Crowley Cancer Research, Dallas, USA; 13University of Arizona Cancer Center North, Tucson, USA; 14University Hospital Magdeburg, Magdeburg, Germany; 15Merck & Co., Inc., Kenilworth, NJ, USA; 16Dynavax Technologies Corporation, Berkeley, USA; 17Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, USA
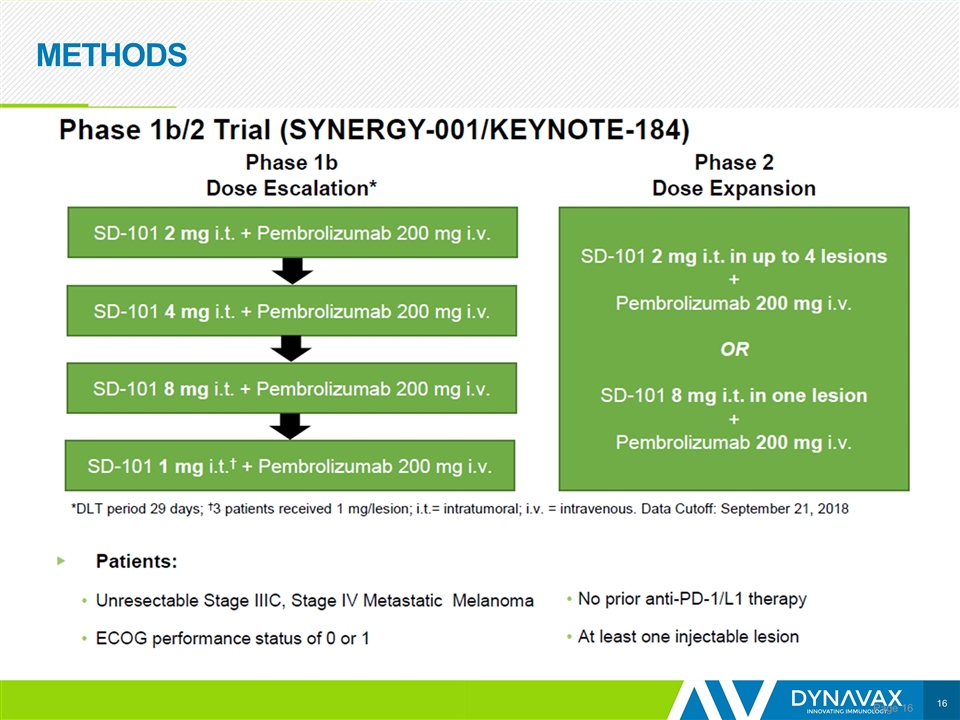
METHODS Page
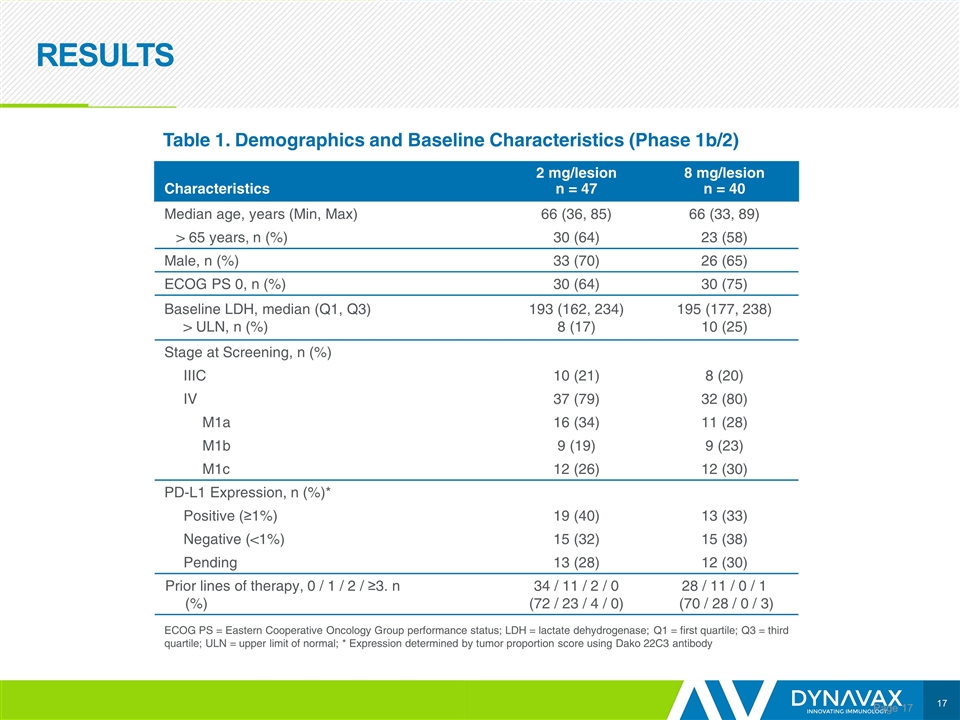
RESULTS Page Characteristics 2 mg/lesion n = 47 8 mg/lesion n = 40 Median age, years (Min, Max) 66 (36, 85) 66 (33, 89) > 65 years, n (%) 30 (64) 23 (58) Male, n (%) 33 (70) 26 (65) ECOG PS 0, n (%) 30 (64) 30 (75) Baseline LDH, median (Q1, Q3) > ULN, n (%) 193 (162, 234) 8 (17) 195 (177, 238) 10 (25) Stage at Screening, n (%) IIIC 10 (21) 8 (20) IV 37 (79) 32 (80) M1a 16 (34) 11 (28) M1b 9 (19) 9 (23) M1c 12 (26) 12 (30) PD-L1 Expression, n (%)* Positive (≥1%) 19 (40) 13 (33) Negative (<1%) 15 (32) 15 (38) Pending 13 (28) 12 (30) Prior lines of therapy, 0 / 1 / 2 / ≥3. n (%) 34 / 11 / 2 / 0 (72 / 23 / 4 / 0) 28 / 11 / 0 / 1 (70 / 28 / 0 / 3) Table 1. Demographics and Baseline Characteristics (Phase 1b/2) ECOG PS = Eastern Cooperative Oncology Group performance status; LDH = lactate dehydrogenase; Q1 = first quartile; Q3 = third quartile; ULN = upper limit of normal; * Expression determined by tumor proportion score using Dako 22C3 antibody
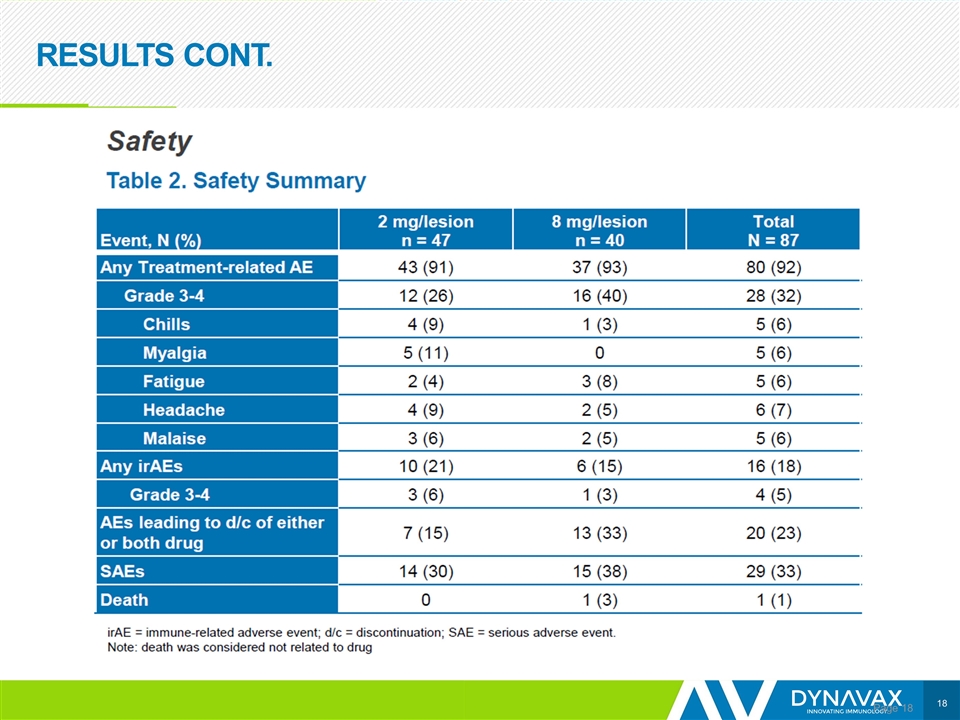
RESULTS CONT. Page
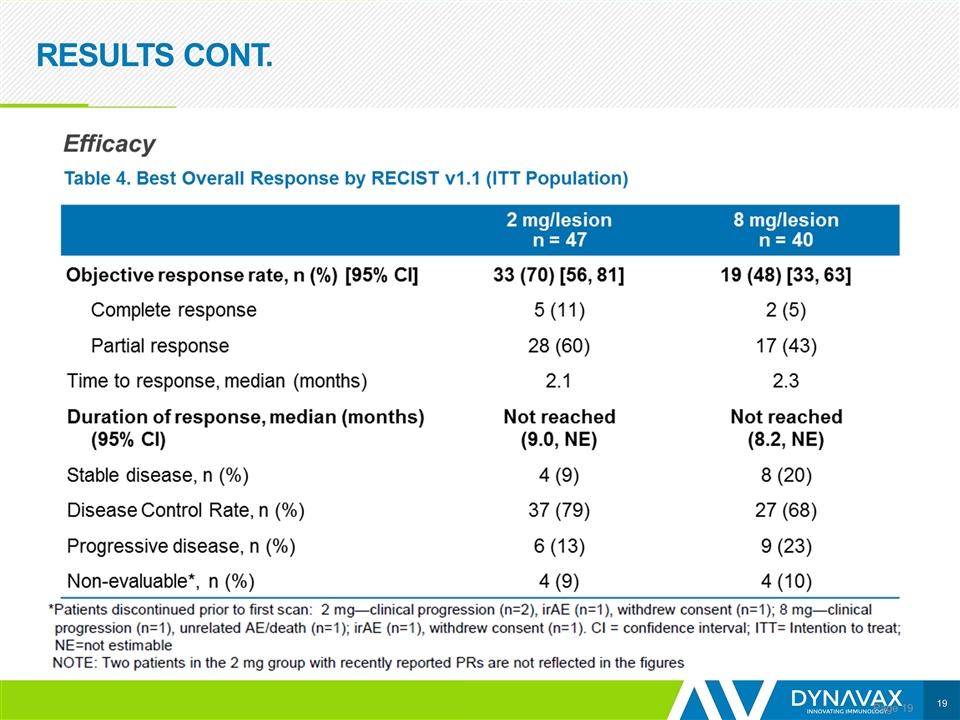
RESULTS CONT. Page
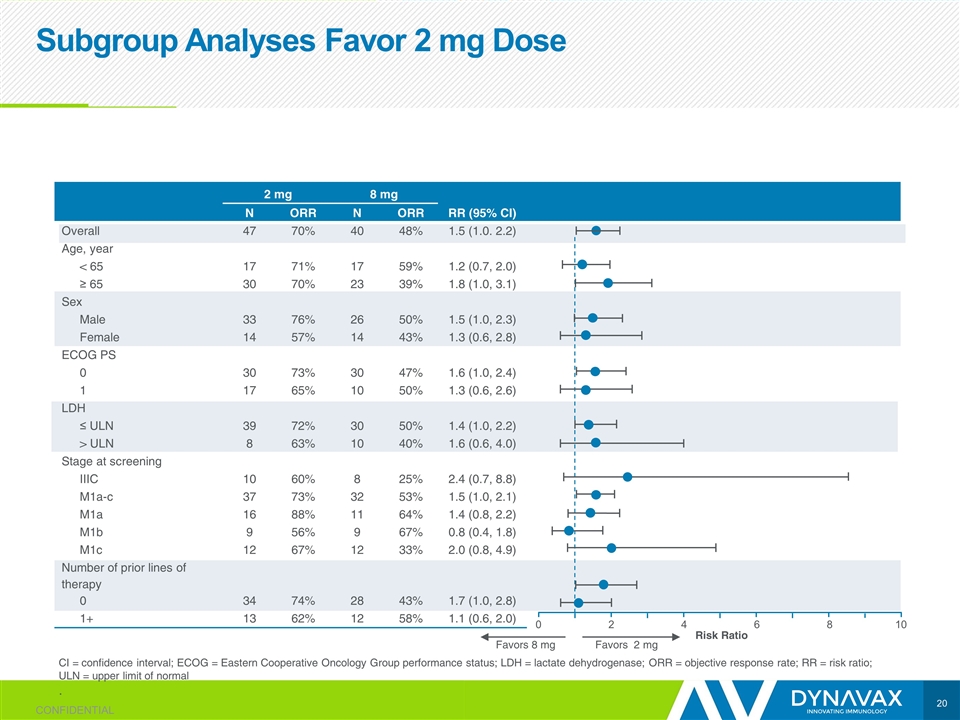
Subgroup Analyses Favor 2 mg Dose CI = confidence interval; ECOG = Eastern Cooperative Oncology Group performance status; LDH = lactate dehydrogenase; ORR = objective response rate; RR = risk ratio; ULN = upper limit of normal . 2 mg 8 mg N ORR N ORR RR (95% CI) Overall 47 70% 40 48% 1.5 (1.0. 2.2) Age, year < 65 17 71% 17 59% 1.2 (0.7, 2.0) ≥ 65 30 70% 23 39% 1.8 (1.0, 3.1) Sex Male 33 76% 26 50% 1.5 (1.0, 2.3) Female 14 57% 14 43% 1.3 (0.6, 2.8) ECOG PS 0 30 73% 30 47% 1.6 (1.0, 2.4) 1 17 65% 10 50% 1.3 (0.6, 2.6) LDH ≤ ULN 39 72% 30 50% 1.4 (1.0, 2.2) > ULN 8 63% 10 40% 1.6 (0.6, 4.0) Stage at screening IIIC 10 60% 8 25% 2.4 (0.7, 8.8) M1a-c 37 73% 32 53% 1.5 (1.0, 2.1) M1a 16 88% 11 64% 1.4 (0.8, 2.2) M1b 9 56% 9 67% 0.8 (0.4, 1.8) M1c 12 67% 12 33% 2.0 (0.8, 4.9) Number of prior lines of therapy 0 34 74% 28 43% 1.7 (1.0, 2.8) 1+ 13 62% 12 58% 1.1 (0.6, 2.0) Favors 8 mg Favors 2 mg CONFIDENTIAL 0 2 4 6 8 10 Risk Ratio
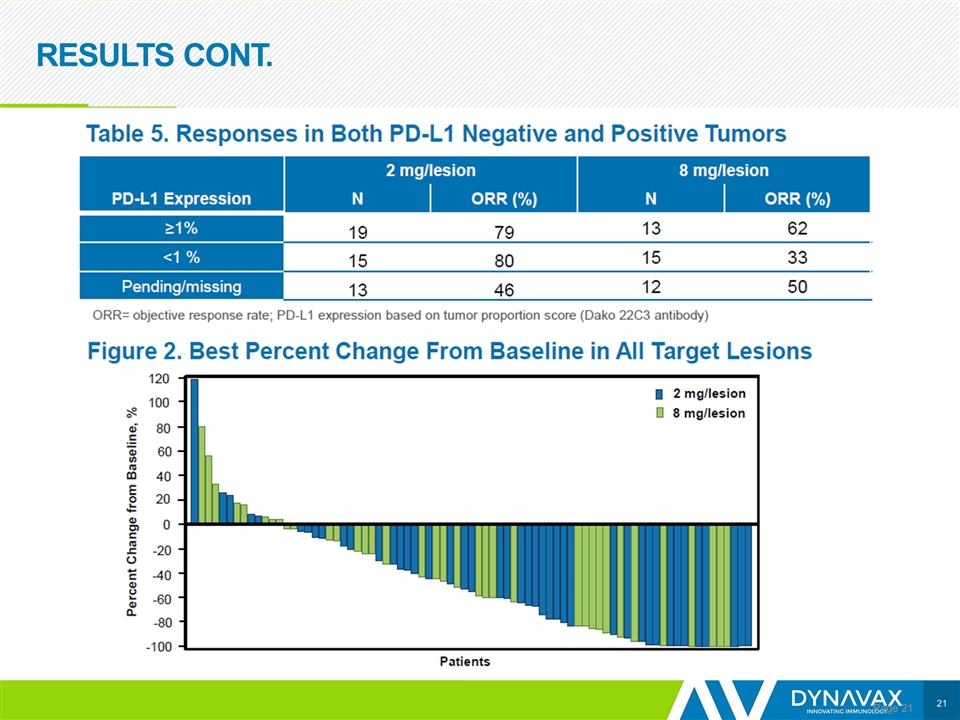
RESULTS CONT. Page
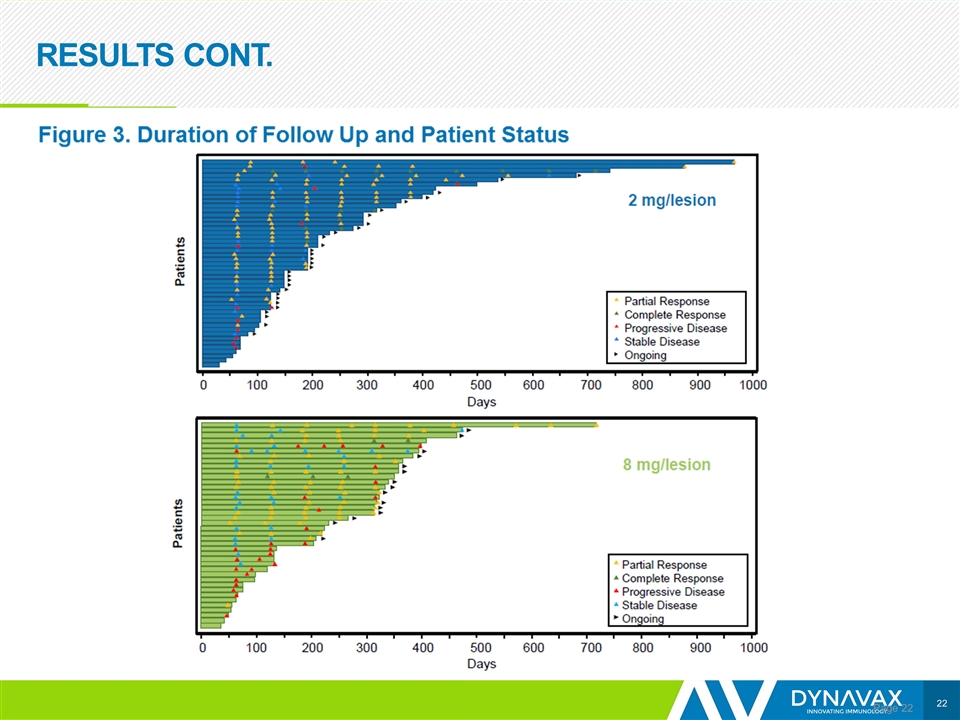
RESULTS CONT. Page
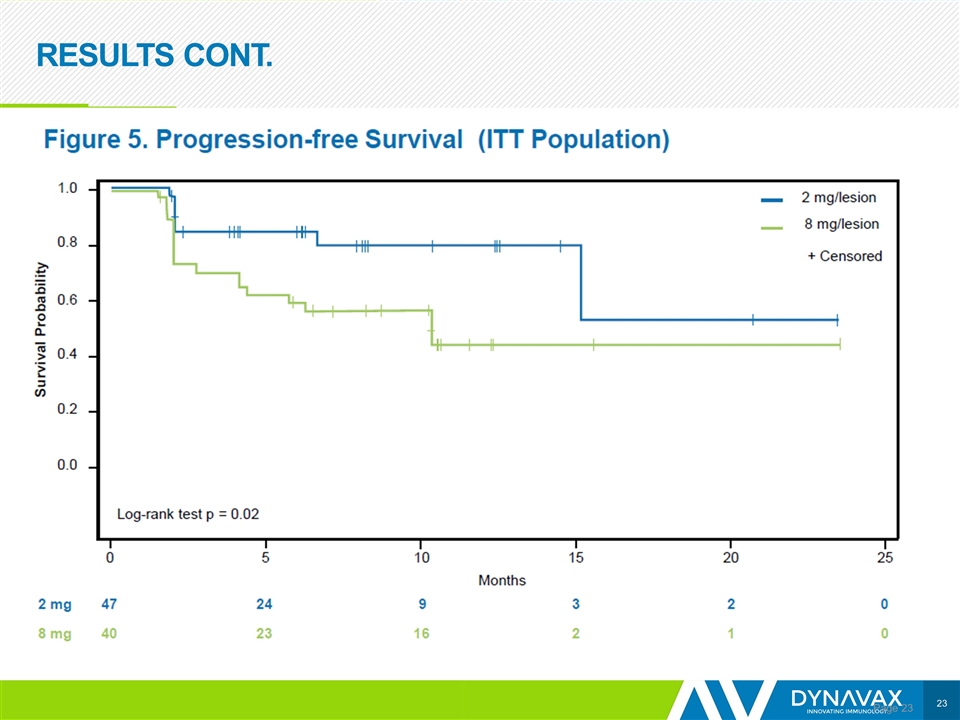
RESULTS CONT. Page
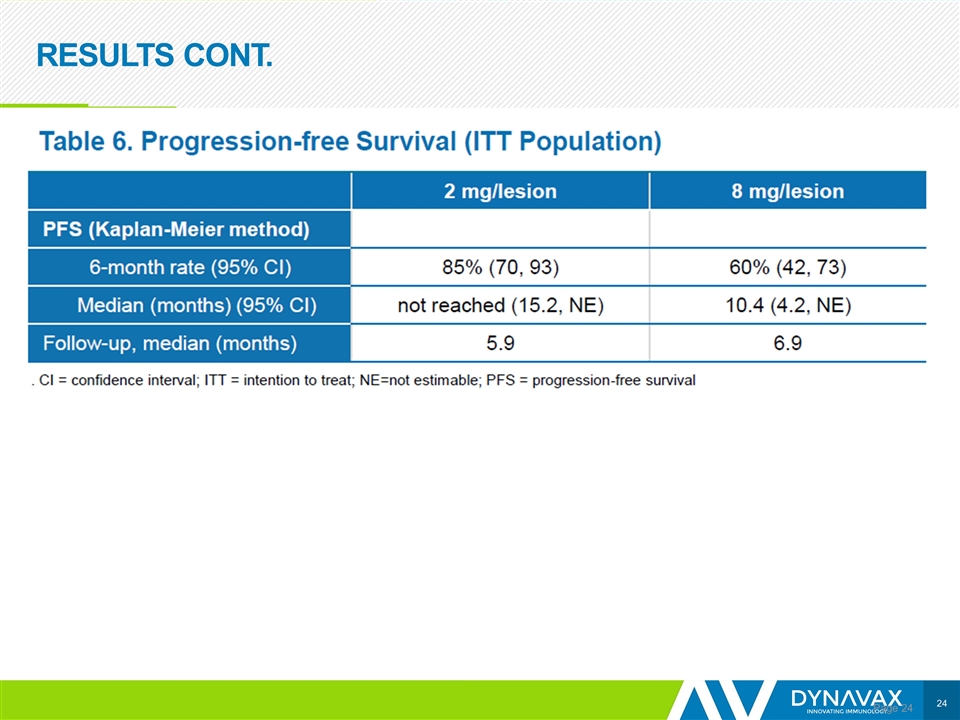
RESULTS CONT. Page
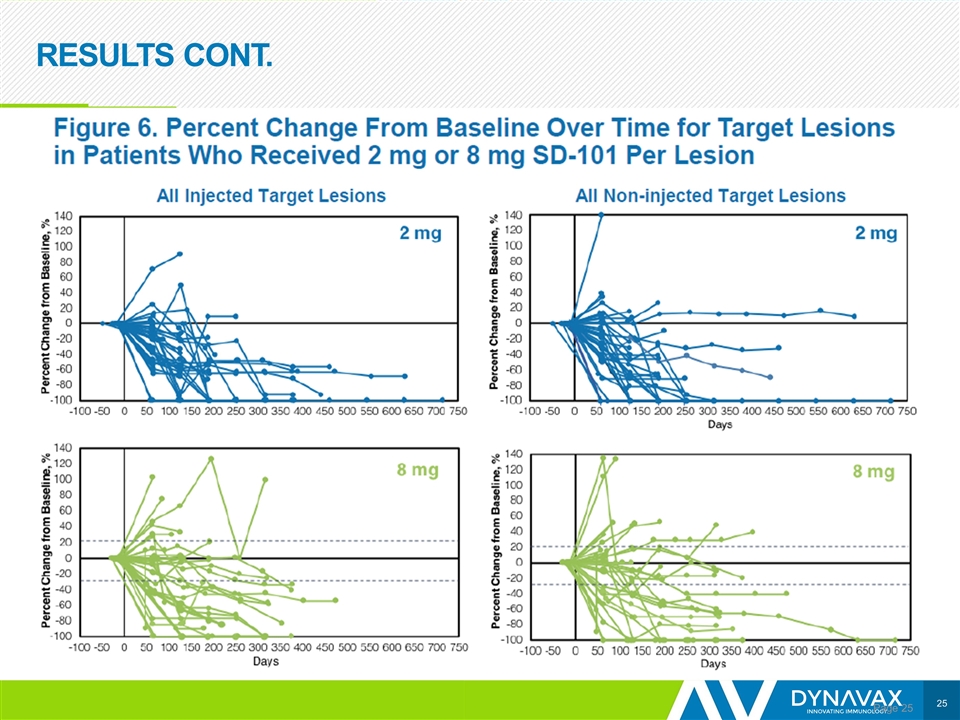
RESULTS CONT. Page
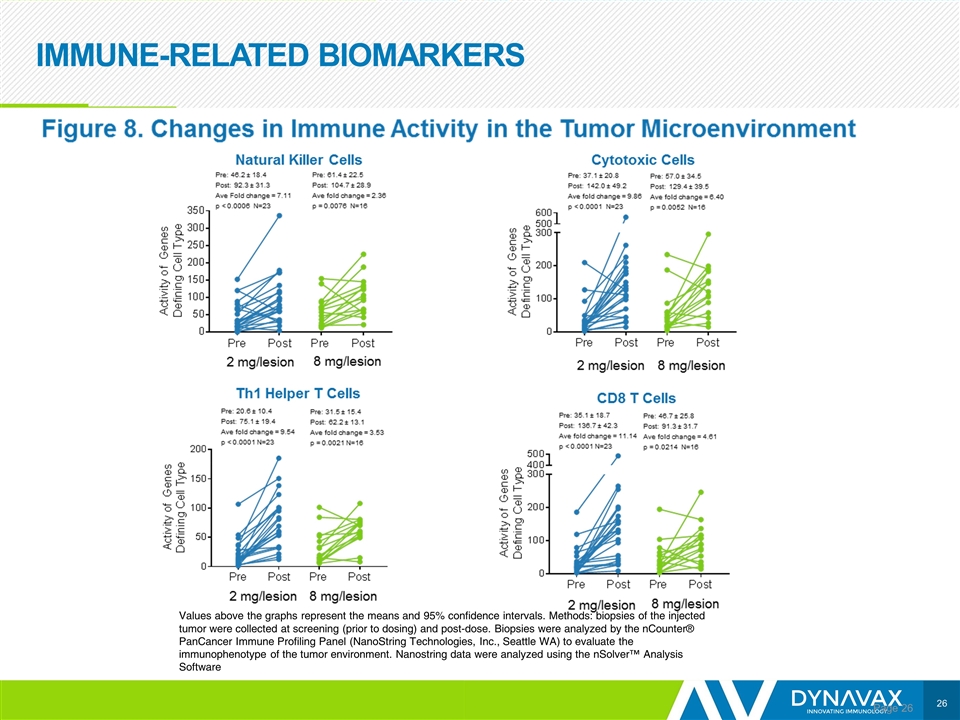
IMMUNE-RELATED BIOMARKERS Page Values above the graphs represent the means and 95% confidence intervals. Methods: biopsies of the injected tumor were collected at screening (prior to dosing) and post-dose. Biopsies were analyzed by the nCounter® PanCancer Immune Profiling Panel (NanoString Technologies, Inc., Seattle WA) to evaluate the immunophenotype of the tumor environment. Nanostring data were analyzed using the nSolver™ Analysis Software

CONCLUSIONS Page The addition of 2 mg/lesion of SD-101 to pembrolizumab appears to increase immune activity in the tumor microenvironment and efficacy compared with 8 mg/lesion in similar patient populations The ORR and PFS were significantly better in the 2 mg/lesion SD-101 group than in the 8 mg/lesion SD-101 group Responses occurred in patients with PD-L1 negative tumors and PD-L1 positive tumors Tumor shrinkage occurred in injected lesions, and non-injected visceral lesions including in the liver and lung The combination of SD-101 and pembrolizumab was well tolerated, consistent with previous reports AEs associated with SD-101 were transient, mild to moderate flu-like symptoms that were manageable with over-the-counter medications No increase in immune-related AEs over pembrolizumab monotherapy was observed Clinical responses were supported by immunologic data consistent with the mechanism of SD-101 Increases in CD8+ cells, NK cells, cytotoxic cells, and Th1 cells in the tumor microenvironment were observed in both SD-101 dose groups but were higher in the 2 mg group and appeared to correlate with enhanced clinical efficacy

Abstract 3560: Phase 1b/2, Open-Label, Multicenter Study of Intratumoral SD-101 in Combination With Pembrolizumab in Anti-PD-1 Treatment-Naïve Patients With Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (SYNERGY-001/KEYNOTE-184, NCT02521870) Ezra Cohen,1 Alain Algazi,2 Douglas Laux,3 Deborah Wong,4 Asim Amin,5 Lisle Nabell,6 Michael Chisamore,7 Erick Gamelin,8 Robert Janssen,8 Sarwan Bishnoi9 1Moores Cancer Center, University of California San Diego, La Jolla, CA USA; 2University of California San Francisco, CA USA; 3University of Iowa, Iowa City, IA USA; 4Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA USA; 5Levine Cancer Institute, Charlotte, NC USA; 6University of Alabama at Birmingham, Birmingham, AL USA; 7Merck & Co., Inc., Kenilworth, NJ USA; 8Dynavax, Berkeley, CA USA; 9Adelaide Cancer Centre, Kurralta Park, Australia
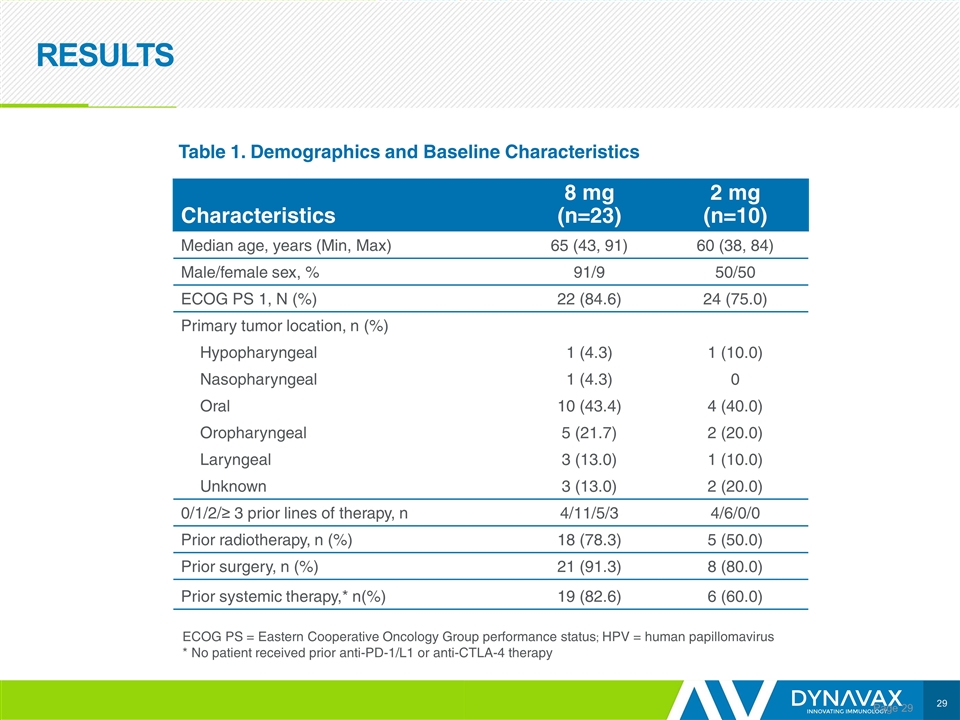
RESULTS Page Characteristics 8 mg (n=23) 2 mg (n=10) Median age, years (Min, Max) 65 (43, 91) 60 (38, 84) Male/female sex, % 91/9 50/50 ECOG PS 1, N (%) 22 (84.6) 24 (75.0) Primary tumor location, n (%) Hypopharyngeal 1 (4.3) 1 (10.0) Nasopharyngeal 1 (4.3) 0 Oral 10 (43.4) 4 (40.0) Oropharyngeal 5 (21.7) 2 (20.0) Laryngeal 3 (13.0) 1 (10.0) Unknown 3 (13.0) 2 (20.0) 0/1/2/≥ 3 prior lines of therapy, n 4/11/5/3 4/6/0/0 Prior radiotherapy, n (%) 18 (78.3) 5 (50.0) Prior surgery, n (%) 21 (91.3) 8 (80.0) Prior systemic therapy,* n(%) 19 (82.6) 6 (60.0) Table 1. Demographics and Baseline Characteristics ECOG PS = Eastern Cooperative Oncology Group performance status; HPV = human papillomavirus * No patient received prior anti-PD-1/L1 or anti-CTLA-4 therapy
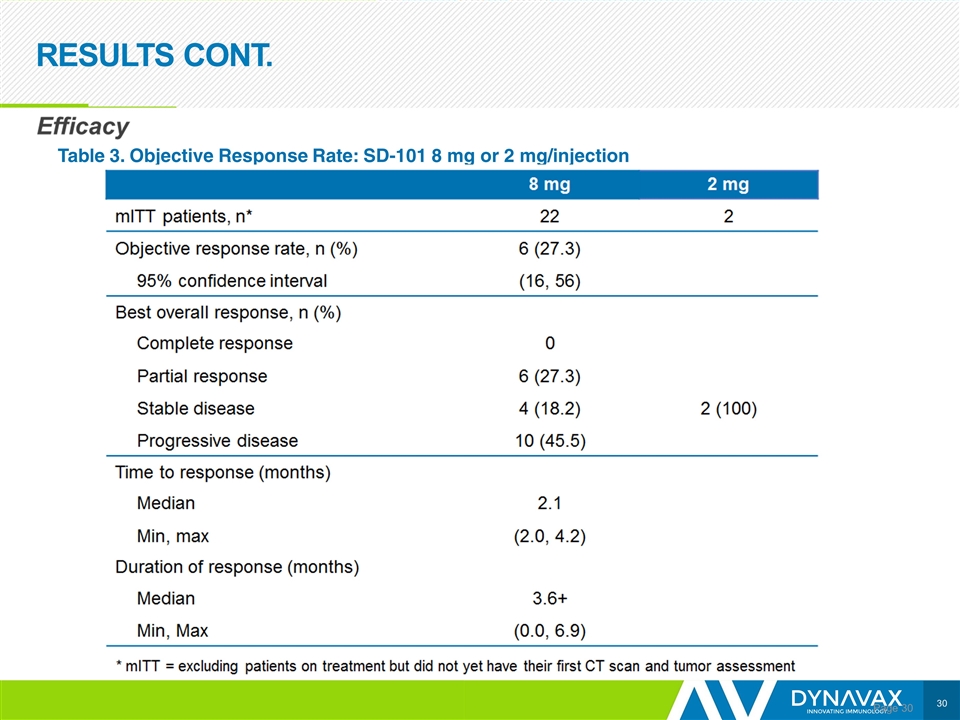
RESULTS CONT. Page Table 3. Objective Response Rate: SD-101 8 mg or 2 mg/injection
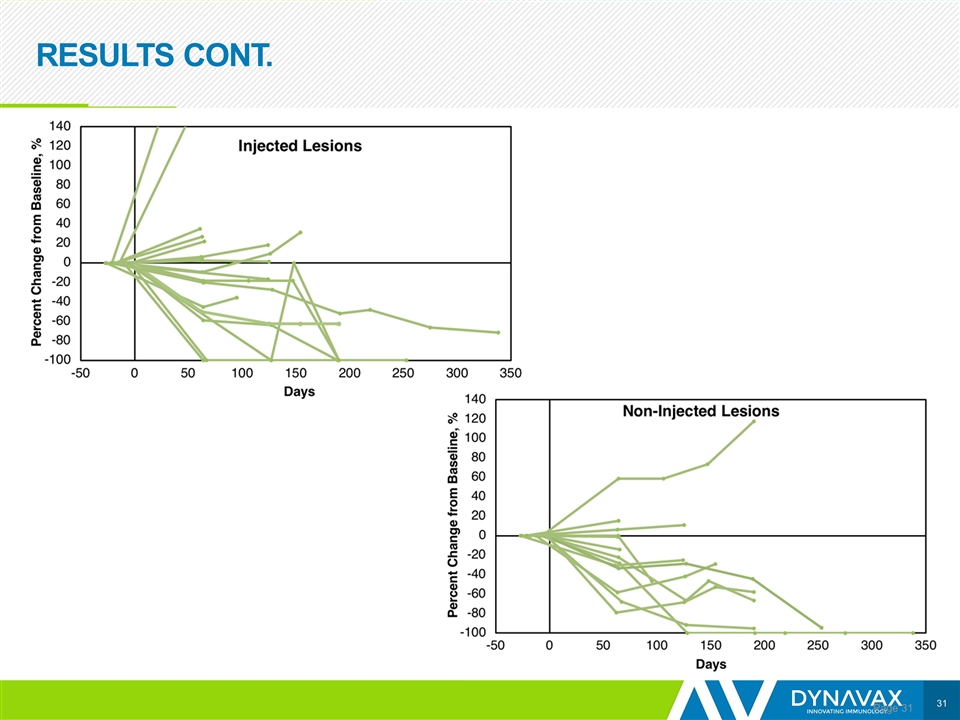
RESULTS CONT. Page
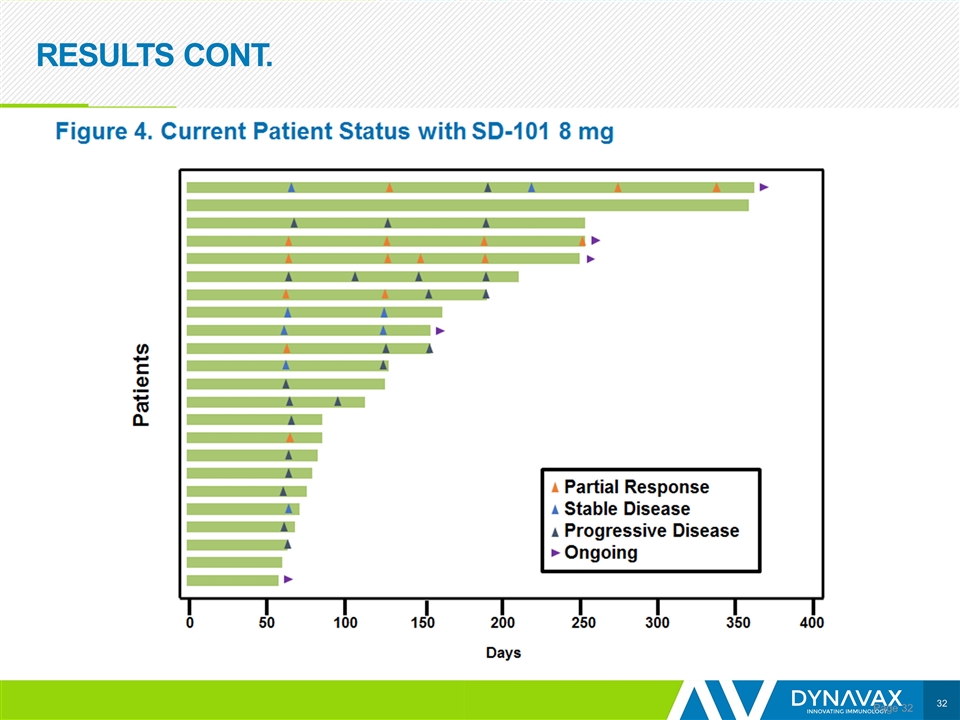
RESULTS CONT. Page

HNSCC CONCLUSIONS Page The combination therapy showed encouraging efficacy in patients with HNSCC, with an ORR of 27% Median DOR has not been reached. Some prolonged responses Responses observed in both SD-101 injected and non-injected lesions
SD-101 + PEMBROLIZUMAB CONCLUSIONS Page Why we think SD-101 adds meaningful clinical benefit to pembrolizumab therapy SD-101 in combination with local low dose radiation induced abscopal responses in 80% of patients with indolent non-Hodgkin’s lymphoma 8 mg of SD-101 induces responses in anti-PD-1/L1 refractory and resistant melanoma tumors in 21% of patients In 2 similar patient populations with melanoma naïve to anti-PD-1/L1 therapy treated with the same dose of pembrolizumab, the difference in the groups was the dose of SD-101 they received. In this setting, 2 mg/lesion of SD-101 induces a significantly higher ORR and longer PFS than 8 mg/lesion With responses in PD-L1 negative tumors And biomarker activity that suggests 2 mg is the biologically optimal dose in melanoma compared with 8 mg. In HNSCC, 8 mg of SD-101 appears to induce a higher ORR than pembrolizumab monotherapy SD-101 adds little additional toxicity to pembrolizumab
SD-101 + PEMBROLIZUMAB CONCLUSIONS Page Moving forward: Currently recruiting patients to receive 2 mg in 1 to 4 lesions: Advanced melanoma who have tumors that are refractory or resistant to anti-PD-1/L1 therapy Recurrent or metastatic HNSCC with no prior anti-PD-1/L1 therapy

ACKNOWLEDGEMENTS Page This study was sponsored by Dynavax Technologies Corporation in collaboration with Merck & Co., Inc., Kenilworth, NJ USA. We thank the patients and their families and caregivers for participating in the study; the participating principal investigators and their study teams; Albert Candia, Kavya Kazipeta and Tripta Dahiya for contributions to the analysis of the data (Dynavax Technologies Corporation).



































