UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 1-16239
ATMI, Inc.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 06-1481060 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
7 Commerce Drive, Danbury, CT | | 06810 |
| (Address of principal executive offices) | | (Zip Code) |
203-794-1100
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of each class | | Name of each exchange on which registered |
| Common Stock, par value $0.01 per share | | The NASDAQ Stock Market LLC (NASDAQ Global Select Market) |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
| | | | | | |
Large accelerated filer | | ¨ | | Accelerated filer | | þ |
| | | |
Non-accelerated filer | | ¨ | | Smaller reporting company | | ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No þ
The aggregate market value of the common stock held by non-affiliates of the registrant at June 30, 2011, was approximately $638,332,000 based on the closing sale price of such stock on the NASDAQ Global Select Market on that date.
The number of shares outstanding of the registrant’s common stock as of January 31, 2012 was 31,728,205.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of our proxy statement for the annual meeting of stockholders to be held on May 23, 2012 (are incorporated by reference in Part III of this form 10-K).
ATMI, INC.
Annual Report on Form 10-K
For the Fiscal Year Ended December 31, 2011
TABLE OF CONTENTS
PART I
References in this annual report to “the Company,” “ATMI,” “we,” “us” and “our” refer to ATMI, Inc. and our wholly-owned subsidiaries on a consolidated basis.
Item 1. Business
Cautionary Statements Under the Private Securities Litigation Reform Act of 1995
Forward-Looking Statements
Disclosures included in this Form 10-K contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements may be identified by words such as “anticipate,” “plan,” “believe,” “seek,” “estimate,” “expect,” “could,” and words of similar meanings and include, without limitation, statements about the expected future business and financial performance of ATMI such as financial projections, expectations for demand and sales of new and existing products, customer and supplier relationships, research and development programs, market and technology opportunities, international trends, business strategies, business opportunities, objectives of management for future operations, microelectronics industry (including wafer start) growth, life sciences industry growth and trends in the markets in which the Company participates. Forward-looking statements are based on management’s current expectations and assumptions, which are inherently subject to uncertainties, risks and changes in circumstances that are difficult to predict. Investors and others should consider the cautionary statements and risk factors discussed in Item 1A below. Actual outcomes and results may differ materially from these expectations and assumptions because of changes in political, economic, business, competitive, market, regulatory, and other factors. ATMI undertakes no obligation to update publicly or review any forward-looking statements, whether as a result of new information, future developments or otherwise, except as required by law.
Our Business
We believe we are among the leading suppliers of high performance materials, materials packaging and materials delivery systems used worldwide in the manufacture of microelectronics devices. Our products consist of “front-end” semiconductor performance materials, sub-atmospheric pressure gas delivery systems for safe handling and delivery of toxic and hazardous gases to semiconductor process equipment, and high-purity materials packaging and dispensing systems that allow for the reliable introduction of low volatility liquids and solids to microelectronics and biopharmaceutical processes. ATMI targets semiconductor and flat-panel display manufacturers, whose products form the foundation of microelectronics technology rapidly proliferating through the consumer products, information technology, automotive, and communications industries. The market for microelectronics devices is continually changing, which drives demand for new products and technologies that have improved performance at lower cost. ATMI’s customers include many of the leading semiconductor manufacturers in the world who target leading-edge technologies. ATMI also addresses an increasing number of critical materials handling needs for the life sciences markets. Our proprietary containment, mixing, and bioreactor technologies are sold to the biotechnology, laboratory and cell therapy markets, which we believe offer significant growth potential. ATMI’s objective is to meet the demands of our microelectronics and life sciences customers with solutions that maximize the efficiency and safety of their manufacturing processes, reduce capital or operating costs, and minimize the time to develop new products and integrate them into their processes.
ATMI’s core competencies include:
| | • | | knowledge of the science and economics of process applications for customer needs in markets served; |
| | • | | the ability to use a combinatorial science-based research approach and high-productivity development (“HPD”) capabilities to significantly shorten our new product development life cycle and develop next generation materials necessary as the semiconductor industry moves toward advanced technology generations, such as 14 nanometers; |
| | • | | the materials science of packaging, delivery, and deposition of ultra-pure semiconductor materials; |
| | • | | the ability to rapidly develop innovative technology and intellectual property that strengthens our competitive position; |
| | • | | knowledge of high-purity materials handling, containment, dispensing, mixing and bioreactor systems to address life sciences customers’ needs for drug research, development and commercialization. |
1
Our customers’ manufacturing processes are increasingly complex, resulting in rapidly changing requirements for materials and materials handling solutions. ATMI has historically capitalized on the growth of the microelectronics and life sciences industries in general through:
| | • | | a strategy of leveraging the combination of our performance materials and materials handling competence to provide greater process efficiency value to our customers; |
| | • | | an extensive research and development program that has produced a stream of proprietary and patented products for these markets; |
| | • | | a key customer focus, which has included providing applications development in order to offer materials solutions for future generation technologies and the ability to perform electrical test measurements in our development efforts to ensure our solutions meet our customers’ needs; and |
| | • | | strategic alliances and collaboration efforts that have allowed us to add complementary technologies to our product portfolio more rapidly than through internal development alone. |
The majority of ATMI’s semiconductor business generally tracks semiconductor wafer starts. Additional financial information about the Company and related geographic information can be found in Item 7: “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this Form 10-K.
We believe we have achieved a leadership position in high-performance semiconductor materials, materials packaging, and materials delivery systems for the microelectronics market by focusing on providing solutions to our customers that allow them to make faster, more advanced, or less expensive devices while improving their manufacturing asset productivity and production yields. We also focus on partnering with customers to bring new technologies into high-volume production as quickly and efficiently as possible. ATMI plans to continue to focus on leveraging our core technologies to create new high-growth product lines, including growing our leadership position in advanced interconnect applications, which today is focused on copper. We believe we also have achieved a leadership position in the small but fast growing market for one-time use mixing and bioreactor applications in the life sciences market.
In the first half of 2009, driven by the challenging repercussions of global credit and financial market disruptions which caused an economic downturn, our customers cautiously managed their inventories. In the second half of 2009, macroeconomic indicators began to recover driven by customers who had reduced their inventory levels earlier in the year and government sponsored demand generation programs. However, unemployment levels remained at historically high levels in the U.S., Europe and certain other parts of the world, which caused continued economic uncertainty. In 2010, we have seen periods of increasing momentum and then contraction as our customers respond to the global macroeconomic cycles. Through the first half of 2011 our quarterly sequential revenue continued to improve, but in the second half of 2011, global economic conditions began to soften as customers managed inventories in the face of continued uncertainty.
ATMI’s operations comprise one operating business segment.
ATMI’s Strategy
ATMI’s strategic intent is to bethe source of process efficiency solutionsto technology-driven customers by providing innovative materials and related delivery systems and technologies to:
| | • | | Focus development and application engineering initiatives with the leading manufacturers to provide next generation performance materials and process solutions; |
| | • | | Target high-growth, high-margin specialty markets that use ATMI’s core materials and packaging technologies and require products that are consumed in the production process; |
| | • | | Add value through performance materials packaging, dispensing, and process technologies designed to meet the demands of users for greater levels of purity, productivity, safety, environmental responsiveness, and speed; |
| | • | | Significantly shorten new product development time lines and the development of next generation materials through the use of a combinatorial science-based research approach and HPD capabilities; |
2
| | • | | Leverage ATMI’s technology leadership by investing extensively to develop proprietary and patented materials and process solutions, which the Company uses to quickly commercialize new offerings for customers; and |
| | • | | Form strategic alliances, including joint development programs and collaborative marketing efforts, to accelerate the introduction of ATMI’s products into existing and new markets. |
In summary, ATMI’s strategy does not encompass a “traditional” materials supplier-to-customer relationship. In those relationships, suppliers tend to provide materials to customers based solely on the cost, quantity, and quality of the materials being supplied. Instead, ATMI works to develop partnerships with its customers based on our ability to improve the process efficiency of customers’ development, scale-up, manufacturing and supply chain processes, thereby reducing customers’ total cost of ownership. ATMI seeks to provide value to its customers through the use of its technical capabilities, and applications knowledge in a manner that changes its commercial relationship with those customers to a value-sharing relationship.
Microelectronics Products and Processes
ATMI believes it is among the most innovative suppliers of high-purity materials and related delivery systems and technologies. Products serving the integrated circuit (“IC”) fabrication market represent the largest portion of ATMI’s business and development activities. The principal drivers for this market are cost, yield, speed, utilization of capital, and risk reduction. The success of an electronic component or device is driven by the increased functionality it can deliver at a lower cost. Yield and capital utilization are significant drivers for the IC industry due to the implications on throughput and financial return, and the challenge is compounded by the requirement to manufacture devices for increasingly complex advanced technology generations. In an industry where the capital infrastructure is significant and product life cycles are short, the ability to bring the next generation technology rapidly to market can make a significant difference in our customers’ success. ATMI’s ability to shorten deployment of production-ready solutions is critical to our success.
High Productivity Development. ATMI uses utilizes combinatorial-science based methods for materials discovery, unit process development and device integration in support of our customers’ needs to develop new materials in response to the challenges of decreasing node sizes. Our experience in materials development and packaging combined with these capabilities bring the unique ability to provide massively parallel experimentation for materials screening, process window characterization, device integration and performance optimization.
Collaborative Arrangements.In 2011, ATMI entered into a collaborative development agreement (“CDA”) with an advanced memory integrated circuit manufacturer for the purpose of developing molecules, including chemical precursors, and material systems for next generation semiconductor products. ATMI will use its HPD platform to collaboratively work with this customer on specific statements of work designed to develop next-generation materials and is currently working with other customers negotiating similar types of arrangements.
Ion Implantation.The primary issues for IC manufacturers are production throughput, cost, and safety because of the hazardous properties of the implant gases used. ATMI’s patented SDS® solutions use a standard gas cylinder containing an adsorbent material. The cylinder is filled with gas under conditions such that the gas is adsorbed onto the adsorbent material at sub-atmospheric pressure. Sub-atmospheric storage of hazardous gases minimizes potential leaks of gas during transportation and use, thus providing significant safety and environmental improvements over traditional high-pressure cylinders. In addition, SDS products allow more process gas to be stored in the cylinder, providing significantly higher rates of productivity than traditional methods of gas delivery used in ion implantation manufacturing processes. These advantages have led the majority of significant chip manufacturers to adopt this technology as the industry standard for dopant gas delivery. Materials packaged in SDS systems include primarily arsine, phosphine, and boron trifluoride. The third generation of SDS products, called SDS3, maintains all the inherent safety features of previous generation SDS products, but dramatically increases the gas storage capacity by using a new adsorbent. The two to three times capacity improvement over the previous SDS products allows ion implanter users to reduce tool down time, resulting in significant cost savings for our customers.
Copper Integration. ATMI believes it is a market leader in copper electroplating materials and processes in semiconductor development and manufacturing with our Viaform® product, which includes inorganic and proprietary organic molecules that provide the wiring for copper interconnects allowing manufacturers to eliminate processing steps. ATMI also focuses on the total copper integration scheme with post-chemical mechanical planarization (“CMP”) cleaning solutions developed using our HPD capability, which uses knowledge-rich, combinatorial science experiments to deliver the best possible solution. One such outcome is PlanarClean®, a new post-CMP cleaning solution which is part of our Surface Preparation product line.
Deposition. Several processes for depositing thin films such as chemical vapor deposition (“CVD”) and atomic layer deposition (“ALD”) processes are enabled by advanced liquid, gaseous and solid precursors. ATMI’s UltraPur-™ brand of materials provides solutions for pre-metal dielectric, dielectric and barrier applications. ATMI is well-positioned for the incorporation of ALD processes
3
by the semiconductor industry with its ProE-Vap® ampoule. This proprietary container allows for reliable delivery of low volatility solid precursors required for processes that demand ALD, like high-k gates. ATMI has successfully adopted the carbon adsorption technology used in SDS and introduced products for semiconductor deposition processes marketed under the SAGE® brand. These applications include: low-k plasma-enhanced deposition, or “PE-CVD”, processes using low-k materials, pre-metal dielectric high-density plasma, or “HDP-CVD”, and films using phosphine gases and thermal deposition processes using germane gases.
Surface Preparation. ATMI’s ST and AP photoresist strip cleaning materials are proprietary chemistries used for applications such as semiconductor post-etch residue removal, wafer etching, organics removal, negative resist removal, edge bead removal, and corrosion prevention.
Materials Packaging. ATMI’s NOWPak® liner technologies and container assemblies form the basis for its high-purity liquid materials packaging and dispensing system product portfolio for applications in IC fabrication and flat-panel display. This product line includes the: Bag-in-a-Bottle™ and Bag-in-a-Can ™ container systems which have their own companion dispense connection systems. Each package features a pre-cleaned collapsible inner liner, or “bag”, inside a rugged, high-density polyethylene overpack. The standard liner films are made of polytetrafluoroethylene and other polymers, which allow chemicals to be delivered to the manufacturing process without compromising their inherent purity. The empty inner liner is easily removed for waste consolidation, and the outer shell is recyclable or returnable for insertion of a new replacement liner. The dispensing system promotes full use of the chemical, chemical isolation from environmental contamination, and improved safety during dispense by sealing and isolating the chemical from the environment to further protect the chemical and the operator.
Life Sciences Products
ATMI is addressing an increasing number of critical materials handling needs for the life sciences markets. Other markets for our proprietary high-purity materials handling and dispensing systems include the biotechnology, laboratory and cell therapy markets, which we believe offer significant growth potential. The biotechnology industry has been using disposable components like filters, connectors, and disposable storage bags for several years; however, biotechnology customers have a growing need to combine these disposable components and integrate them into disposable systems. ATMI has responded to this trend by launching a complete offering of scalable disposable containment, dispensing, mixing and bioreactor systems.
ATMI’s Integrity™ line of proprietary disposable mixing technology is the market leading technology for disposable mixing applications within the life sciences market. The solutions delivered to our customers consist of a hardware system that is compatible with a one-time use mixing “bag” container; the technology delivers unique particle-free mixing and is scalable to an industrial scale. A typical biotech manufacturing process has over 20 different mixing steps starting from a cell culture process through the final-fill drug solutions. ATMI mixing technology is typically used for preparing buffers and media solutions for feeding the cell culture and chromatography applications, but also other process steps like virus inactivation and final-fill solutions.
In November 2010, we acquired the Belgian biotechnology firm Artelis S.A., an innovator in the area of highly-efficient bioprocesses and technologies for cell therapy research and manufacturing scale-up. The acquisition complements ATMI’s leadership in ultra-pure single-use films, bioreactors, and mixing systems for characterizing, developing, and manufacturing biopharmaceuticals and extends our global capabilities to a broad range of biopharmaceutical process expertise.
Raw Materials
We use a broad range of specialty and commodity chemicals and polymers in the development of our products, including parts and sub-assemblies that are obtained from outside suppliers. We seek, where possible, to have several sources of supply for all of these materials. Although we may, in some instances, rely on a single or a limited number of suppliers, or upon suppliers in a single country, for certain of these materials, we have not experienced any sustained interruption in production or the supply of these materials and do not anticipate any significant difficulties in obtaining the materials necessary to manufacture our products.
4
Working Capital
In the ordinary course of our business, we maintain an adequate level of working capital at all times to support business needs. In accordance with microelectronics, flat panel display and life sciences industry practices, we do not need to carry significant amounts of finished goods inventory to meet the delivery requirements of our customers. We generally do not provide customers with rights of return (with the exception of standard warranty provisions, which historically have not been material) and we generally do not provide customers extended payment terms beyond 90 days.
Customers, Sales, and Marketing
ATMI sells and distributes its products worldwide primarily through a direct global sales and service organization. For a breakdown of revenue by geography, see Note 16 in the Notes to the Consolidated Financial Statements (Part II, Item 8 of this Form 10-K). Also, for detail regarding revenue by product type, see Note 15 in the Notes to the Consolidated Financial Statements (Part II, Item 8 of this Form 10-K). ATMI markets and sells its materials products to end-use customers, chemical suppliers, and original equipment manufacturers (“OEMs”) through its direct sales force in North America, Europe, Taiwan, South Korea, Japan, China, and Singapore, with limited use of regional manufacturing representatives in certain parts of Asia and Europe. NOWPak containers are generally sold to chemical suppliers, who sell their high-purity chemicals in NOWPak containers at the request of end-users. ATMI’s life sciences products are sold directly and through a global distribution agreement to life sciences and certain semiconductor companies, predominately in Europe and to an increasing extent in the United States. Consistent with 2010, through October of 2011, ATMI sold its SDS products for ion implant applications directly to certain end-users and through an exclusive distribution agreement with Matheson Tri-Gas, Inc. (“Matheson”). During the years ended December 31, 2011, 2010 and 2009, respectively, ATMI recognized $65.8 million, $79.9 million, and $37.9 million of revenues from Matheson, which represented 16.9 percent, 21.8 percent, and 14.9 percent of our revenues for these periods. On October 31, 2011, ATMI entered into an agreement with Matheson which terminated their exclusive license, manufacturing and distribution rights for SDS® in exchange for a $95 million cash payment by ATMI, and payments for the repurchase by ATMI of certain inventory in the Matheson distribution channel. During the years ended December 31, 2011, 2010 and 2009, respectively, ATMI recognized revenues from a Taiwanese foundry of $48.7 million, $46.8 million, and $36.2 million, which represented 12.5 percent, 12.7 percent, and 14.2 percent of our revenues for these periods. During the years ended December 31, 2011, 2010 and 2009, respectively, ATMI recognized revenues from a leading Korean integrated circuit manufacturer of $47.6 million, $31.3 million, and $16.3 million, which represented 12.2 percent, 8.5 percent, and 6.4 percent of our revenues for these periods. There are no material seasonal effects on our business. The global nature of our business and the significance of our foreign operations expose us to certain risks in the countries in which we operate, including political and economic stability, the adequacy of country infrastructure and labor resources, currency fluctuations and controls, compliance with foreign laws and intellectual property protection. Investors and others should consider the cautionary statements and risk factors discussed in Item 1A below.
5
Manufacturing
This table summarizes the locations, products manufactured and size of ATMI’s various manufacturing facilities as of December 31, 2011.
| | | | | | |
Location | | Products | | Square Footage | |
Anseong, South Korea | | • CVD materials (Microelectronics) | | | 13,000 | |
Anseong, South Korea | | • high-purity materials packaging systems (Microelectronics) | | | 10,000 | |
Brussels, Belgium | | • high efficiency bioreactor solutions (Life sciences) | | | 22,000 | |
Burnet, TX | | • liquid materials and delivery systems (Microelectronics) | | | 77,000 | |
Bloomington, MN | | • high-purity materials packaging systems (Microelectronics / Life sciences) | | | 68,000 | |
Danbury, CT | | • gas delivery systems and liquid materials (Microelectronics) | | | 73,000 | |
Hoegaarden, Belgium | | • high-purity materials packaging and mixing systems (Life sciences) | | | 74,000 | |
Until October 31, 2011, we used an exclusive contract manufacturer, Matheson, for the manufacture and distribution of approximately 75 percent of our SDS products. Under the terms of this manufacturing agreement, ATMI retained the right to manufacture 25 percent of all SDS Products, which were manufactured in our Danbury, CT facility. We terminated this agreement on October 31, 2011, but under the terms of the termination agreement, Matheson will continue to manufacture approximately 75 percent of the SDS products for a period of up to two years. We use contract manufacturers for certain of our other materials and delivery equipment products, both in the U.S. and in Asia. For further information regarding the termination of Matheson’s license, manufacturing and distribution rights, please see Item 7: “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this Form 10-K.
6
Competition
ATMI’s primary competitors in the semiconductor materials product lines include Air Products and Chemicals (Electronics Division), DuPont Electronic Technologies, Dow Chemical Company (including Rohm and Haas), BASF and Air Liquide as well as several smaller companies that specialize in niche markets.
ATMI’s SDS products (using adsorbent-based delivery technology) face competition from Praxair, Inc., and others, who have a mechanical-based product approach to delivering gas at sub-atmospheric pressure which currently comprise a small portion of the market. Several companies compete with high-pressure gas cylinders and solid sources. There are numerous domestic and foreign companies that offer products that compete with ATMI’s materials, materials packaging and materials delivery systems.
ATMI believes that its ability to compete in the markets for containers and dispensing systems is dependent largely upon its patented NOWPak technology and its proven ability to enhance and improve its products and technologies. Our NOWPak product line primarily competes with glass bottle manufacturers.
ATMI’s primary competitors in the life sciences product line include Sartorius Stedim Biotech S.A., Thermo Scientific HyClone, Inc., Merck Millipore, and Xcellerex.
ATMI competes in established markets based on our ability to innovate and on product performance, process efficiency, safety and price. In new and emerging markets we compete based on our ability to develop innovative products that fulfill new and changing customer needs in an efficient, cost-effective manner. Increased competition has, and may continue to, affect the prices we are able to charge for our products. In addition, our competitors could own or could obtain intellectual property rights which could restrict our ability to market our existing products and/or to innovate and develop new products.
Research and Development
The Company’s R&D expenses consist of personnel and other direct and indirect costs for internally funded project development, including the use of outside service providers. ATMI also participates in joint development efforts with several key semiconductor manufacturers, advanced technology developers, and semiconductor equipment manufacturers. Total expenses for R&D for the years ended December 31, 2011, 2010 and 2009 were $53.7 million, $48.6 million, and $37.2 million, respectively. Total research and development expenditures represented 13.8 percent, 13.2 percent, and 14.6 percent of revenues in 2011, 2010 and 2009, respectively.
ATMI has made significant investments to create global HPD capabilities that incorporate high-productivity combinatorial science-based research tools to shorten the new product development life cycle and more rapidly create new materials needed by our customers for their advanced technology roadmaps. While conventional R&D methods allow for one process experiment to be conducted on a single wafer, HPD capabilities enable engineers to evaluate up to 192 different test chemistries on a wafer at once. This technique allows independent control of formulation, stir rate and exposure time at each independent site on a test surface, yielding a rich source of data regarding materials choices in a fraction of the time and at lower cost than conventional research methods. Beyond the improvements in speed and data quality during the evaluation period, results are also subjected to both automated and human experience-driven understanding of semiconductor manufacturing process flows. The union of these forces produces much faster learning cycles, and makes it possible to rapidly narrow substantial volumes of data to a most promising subset of chemical formulations. The process is iterative, concurrently assessing large numbers of precisely selected, newly designed test variations.
Since production of the earliest integrated circuits (“ICs”), a readily identifiable group of the same dielectrics, dopants and metals were applied with only limited refinements or modifications. The introduction of copper has changed that dynamic; now, virtually every new technology generation requires no fewer than a dozen new materials. Looking into the future, the industry evolution to 14 nanometer will encompass approximately 45 new materials in a process involving nearly 1,200 steps, dramatically increasing the need to have a capability which enables more rapid feedback for the determination of materials that would be appropriate for individual processes. There is a significant and growing need among our customers to have access to HPD capabilities to enable them to keep pace with these market changes. The fundamental obstacle to overcome in trying to develop optimal chemistries in this environment is that conducting experiments in the conventional serial fashion will not produce answers rapidly enough to meet technology node insertion deadlines or competitive needs. Our HPD capabilities allow us to pursue more new product opportunities, in a shorter time, and using fewer resources compared to previous methods. By performing a higher rate of experiments and identifying promising routes for integrating these materials in actual production flows, and in certain instances, learning about device characteristics, including electrical performance, much earlier in the process than traditional approaches should differentiate ATMI’s materials development capabilities from our competition. We have demonstrated reductions in cycle time of up to 70 percent in the development of certain customer solutions. We are currently using our HPD capabilities to solve customers’ materials challenges in applications such as post-CMP cleaning, high-dose implant strip, copper post-etch residue removal, advanced precursors, high-k/metal
gate cleaning and others — all areas that pose substantial development hurdles for our customers in the race to transition to the next technology node.
7
Strategic Alliances
ATMI forms strategic alliances, including joint development programs and collaborative marketing efforts, to develop new products and to accelerate the introduction of its products. These programs have led to significant technological advances, including the development of proprietary advanced materials and semiconductor manufacturing processes. In October 2011, ATMI terminated its exclusive license, manufacture, and distribution agreement with Matheson, whereby ATMI had granted licensing rights for the manufacturing and worldwide distribution of certain SDS products to Matheson. Following a transition period of up to two years, ATMI will manufacture SDS products for worldwide distribution. ATMI has a strategic alliance with Enthone, Inc. (“Enthone”), a subsidiary of Cookson Electronics, pursuant to which ATMI purchased the exclusive worldwide marketing and distribution rights to Enthone’s copper ECD products, including its ViaForm products, through 2013, subject to automatic renewal upon satisfaction of certain conditions. Under the terms of the agreement, Enthone continues to manufacture the ViaForm products for ATMI. ATMI holds a minority equity interest in Intermolecular Inc. (“Intermolecular”), has purchased HPD tools from Intermolecular, and has dedicated development resources with multiple key customers using this technology platform. ATMI has a minority equity interest in Anji Microelectronics Co., Ltd., with operations in Shanghai, China, as well as marketing agreements and licensing agreements around advanced semiconductor materials. ATMI has a minority equity investment in Lake LED Materials, Co., LTD (“Lake LED”), a South Korean company. We have also formed a strategic alliance with Lake LED focused on providing metal organic precursors to the light emitting diode (“LED”) market for customers outside of Asia. ATMI holds a minority interest in Disposable-Lab, S.A.S., a French start-up whose mission is to design and manufacture disposable lab equipment for the biopharmaceutical industry. We also hold a minority interest in ATMI Austar Lifesciences, Ltd., an early stage enterprise whose purpose is to manufacture high density polyethylene and Tyvek bags and other pharmaceutical components for the life sciences industry. We have an equity interest in Green Lyon Group, Inc., a United States company that is developing technology for the recycling of printed circuit boards. These programs enhance ATMI’s core technology base and promote the introduction of targeted products.
Backlog
Substantial portions of our business are conducted with open-ended supply contracts and / or consignment agreements that do not specify quantities. Also, the SDS gas delivery source product carries no backlog. Therefore, the Company does not believe that backlog as of any particular date is indicative of future results.
Patents and Proprietary Rights
ATMI has made, and continues to make, a significant investment in securing intellectual property protection for its technology and products. ATMI seeks to protect its technology by, among other things, filing patent applications where appropriate. The Company also relies upon trade secrets, know-how, continuing technological innovation, and licensing opportunities to help develop and maintain its competitive position.
As of December 31, 2011, ATMI owns or controls approximately 374 United States patents and has approximately 192 current United States patent applications pending. Foreign counterparts of certain of these applications have been filed, or may be filed at an appropriate time. ATMI decides on a case-by-case basis whether, and in which countries, it will file counterparts of a United States patent application. ATMI’s United States patents expire between approximately 2012 and 2029. ATMI also holds approximately 29 United States registered trademarks.
ATMI requires all employees, outside scientific collaborators, sponsored researchers, and other advisors and consultants who are provided confidential information to execute confidentiality agreements upon the commencement of employment or consulting relationships with the Company. These agreements generally provide that all confidential information developed or made known to the entity or individual during the course of the entity’s or individual’s relationship with ATMI is to be kept confidential and not disclosed to third parties except in specific circumstances. All of ATMI’s employees have entered into agreements providing for the assignment of rights to inventions made by them while employed by the Company.
Environmental Considerations
Regulation.ATMI uses hazardous materials and generates regulated waste streams as part of its manufacturing, processing and R&D activities. As a result, the Company is subject to a variety of governmental regulations related to the storage, use, transportation, and disposal of these materials. ATMI’s failure to comply with present or future laws could result in fines or other liabilities being imposed on the Company, suspension of production or a cessation of operations. Investors and others should consider the cautionary statements and risk factors discussed in Item 1A below.
8
Sustainability. ATMI considers the environmental sustainability of our products through our development process with the objective of ensuring we provide our customers with solutions that meet their needs and, to the extent possible, minimize environmental impact.
Employees and Employee Relations
As of December 31, 2011, ATMI employed 814 individuals, including 329 in sales, marketing, and administration, 324 in operations, and 161 in research and development. Approximately 9 percent of the Company’s employees are covered by collective bargaining agreements, which expire in June 2013. All of the employees covered by these agreements are based in Belgium. ATMI has never experienced any work stoppages and considers its relations with its employees to be good.
Company Information
ATMI was incorporated under the laws of Delaware in 1997, and its predecessor company was incorporated under the laws of Delaware in 1987. ATMI’s headquarters is located at 7 Commerce Drive, Danbury, Connecticut 06810, and the telephone number is (203) 794-1100.
ATMI’s website can be found on the Internet atwww.atmi.com. The website contains information about the Company and its operations. We make available free of charge through our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K, and amendments to these reports, as soon as reasonably practicable after we electronically file such material with, or furnish such material to, the Securities and Exchange Commission (SEC). These reports may be accessed on our website by following the link underInvestor and then clicking onFinancial information.
Any of our reports filed or furnished with the SEC can also be obtained in print by any stockholder who requests them from our Investor Relations Department:
Investor Relations
ATMI, Inc.
7 Commerce Drive
Danbury, CT 06810
9
Item 1A. Risk Factors
Cautionary Statements Regarding Future Results of Operations
You should read the following cautionary statements in conjunction with the factors discussed elsewhere in this and other of our filings with the Securities and Exchange Commission (SEC) and in materials incorporated by reference in these filings. These cautionary statements are intended to highlight material factors that may affect our financial condition and results of operations. Like other companies, we are susceptible to macroeconomic downturns in the United States or abroad that may affect the general economic climate and our performance and the performance of our customers. Similarly, the price of our common stock is subject to volatility because of fluctuations in general market conditions, differences in our results of operations from estimates and projections generated by the investment community, and other factors beyond our control.
Our profit margins may be adversely affected by a number of factors.
Our profit margins may be adversely affected in the future by a number of factors, including price reductions, decreases in our shipment volume, reductions in, or obsolescence of, our inventory and shifts in our product mix. Many of our expenses, particularly those relating to capital equipment and manufacturing overhead, are fixed in the short term. Accordingly, reduced demand for our products and services can cause our fixed production costs to be allocated across reduced production volumes, which can adversely affect our gross margin and profitability, and reduced demand could adversely affect our performance. Our ability to reduce expenses is further constrained because we must continue to invest in research and development to maintain our competitive position and to maintain service and support for our existing global customer base.
Cyclicality in the markets we sell to may adversely affect our performance.
The semiconductor market has historically been cyclical and subject to significant and often rapid increases or decreases in demand. These changes, along with cyclical changes in the flat-panel display market, could adversely affect our results of operations and could have an adverse effect on the market price of our common stock. Our results of operations have been adversely affected, and may be further affected in the future, if demand for semiconductors, or devices that use semiconductors, or flat panels decreases or grows at a significantly slower pace than has historically occurred. Subsequent upturns in the markets which we serve have historically been characterized by sudden increased product demand and production capacity constraints. We may have difficulty reacting quickly enough to a sudden upturn in demand for our products and may incur significant expediting and manufacturing costs to meet a rapid increase in customer demand.
The recent global economic crisis and continuing uncertainty in the financial markets could materially and adversely affect our business and results of operations.
In the second half of 2009, we began to see indications of industry recovery from the global credit and financial market disruptions that began in late 2008 and continued into 2009, which caused customers to reduce their inventory levels in the face of the economic downturn and government sponsored demand generation programs. As the recovery gained momentum, our quarterly sequential revenues improved into the first half of 2011; however, as we saw with the decline in wafer starts in the second half of 2011, and the global economic uncertainty, particularly in the European Economic Community, until the general global economy demonstrates marked improvement in the form of consistent growth, uncertainties will continue to affect businesses such as ours in a number of ways, making it difficult to accurately forecast and plan our future business activities. In addition, financial difficulties experienced by our suppliers, distributors or customers could result in product delays, increased accounts receivable defaults and inventory challenges. Similarly, the price of our common stock is subject to volatility due to fluctuations in general market conditions, differences in our results of operations from estimates and projections generated by the investment community, and other factors beyond our control.
On an ongoing basis, our business may be affected by economic down-turns which could impact the volatility and liquidity of financial and credit markets, the general global economy, and factors such as inflationary or deflationary pressures yielding other market or economic challenges. There can be no assurance that there will not be deterioration in credit and financial markets affecting consumer confidence in economic conditions.
Our business could be adversely affected if we cannot protect our proprietary technology or if we infringe on the proprietary technology of others.
Our proprietary technology supports our ability to compete effectively with other companies. Although we have been awarded, have filed applications for or have been licensed under numerous patents in the United States and other countries, these patents may not
fully protect our technology or competitive position. Further, our competitors may apply for and obtain patents that will restrict our ability to make and sell our products.
10
Our competitors may intentionally infringe our patents. Third parties may also assert infringement claims against us in the future. Litigation may be necessary to enforce patents issued to us, to protect our trade secrets or know-how, to defend ourselves against claimed infringement of the rights of others or to determine the scope and validity of the proprietary rights of others. The defense and prosecution of patent suits are both costly and time consuming, even if the outcome is favorable to us. Outside the United States, in particular, such proceedings can be extremely expensive and their outcome very unpredictable. An adverse outcome in the defense of a patent suit could cause us to lose proprietary rights, subject us to significant liabilities to third parties or require us to license rights from third parties or to cease selling our products. Any of these events could have a material adverse effect on our business, operating results and financial condition. We also rely on unpatented proprietary technology that others may independently develop or otherwise obtain access to. Our inability to maintain the proprietary nature of our technologies could negatively affect our revenues and earnings.
The loss of or significant curtailment of purchases by any of our largest customers could adversely affect our results of operations.
While we generate revenue from hundreds of customers worldwide, in 2011, approximately 75 percent of our revenues were generated by 20 customers. The loss of or significant curtailment of purchases by one or more of our top customers, including curtailments due to a change in the design or manufacturing sourcing policies or practices of these customers or the timing of customer inventory adjustments may adversely affect our results of operations. Our customers and their customers aggressive management of inventory has already adversely affected our results of operations in the past and may continue to adversely affect future results of operations.
Customer driven pricing pressure may adversely affect our average selling prices.
We face aggressive cost-containment pressures from our customers. There can be no assurances that we will be able to maintain current prices in the face of continuing pricing pressures. Over time, the average price for our products may decline as the markets for these products become more competitive. Any material reduction in product prices could negatively affect both revenues and profits.
Our revenues and earnings could be negatively affected if we cannot anticipate market trends, enhance our existing products and processes, develop and commercialize new products and processes, and identify and consummate strategic acquisitions.
We believe that our future success will depend, in part, upon our ability to anticipate rapidly changing technologies and market trends, to enhance our existing products and processes, to develop and commercialize new products and processes, and to expand through selected acquisitions of technologies or businesses or other strategic alliances. The microelectronics industry markets we serve undergo frequent technological changes, which in turn create demand for new and improved products and process technologies. We may not be able to improve our existing products and process technologies or to develop and market new products and technologies that will be cost-effective or introduced in a timely manner or accepted in the marketplace. We may not be able to leverage our knowledge to make full use of combinatorial science and HPD capabilities as an effective market offering for our customers. Our failure to develop or introduce enhanced and new products and processes in a timely manner may negatively affect our revenues and earnings, and result in a potential impairment of assets. Management considers, on a continuing basis, potential acquisitions of technologies and businesses and other strategic alliances, some of which may be material to us. However, we cannot be assured that we will identify or succeed in consummating transactions with suitable acquisition candidates or alliance partners in the future.
We may have difficulty obtaining the resources or products we need for manufacturing or assembling our products or operating other aspects of our business, which could adversely affect our ability to meet demand for our products and may increase our costs.
We have hundreds of suppliers providing various materials that we use in the production of our products and other aspects of our business, and we seek, where possible, to have several sources of supply for all of these materials. However, we may rely on a single or a limited number of suppliers, or upon suppliers in a single country, for certain of these materials. The inability of such suppliers to deliver adequate supplies of reasonable quality production materials or other supplies could disrupt our production process. In addition, production could be disrupted by the unavailability of the resources used in production such as electricity, chemicals, and gases. The unavailability or reduced availability of the materials or resources we use in our business may require us to reduce production of products or may require us to incur additional costs in order to obtain an adequate supply of these materials or resources. Our life sciences business is also subject to governmental approval for products that are part of the manufacture of pharmaceuticals. The occurrence of any of these events could adversely affect our business and results of operations.
11
We face intense competition from a variety of sources, including larger companies.
The markets for our products are intensely competitive. A number of domestic and international companies engage in commercial activities in the markets we serve. Many of these companies have substantially greater financial, research and development, manufacturing and marketing resources than we do. In addition, as these industries evolve, other competitors may emerge. To remain competitive, we must continue to invest in and focus upon research and development and product and process innovation. We may not be successful if we cannot compete on: price, technical capabilities, quality, or customer service.
Our global manufacturing and sales activities subject us to risks associated with legal, political, economic or other changes.
We have facilities in eight countries worldwide and, in 2011, approximately 80 percent of our revenues came from sales to companies outside the United States. The global nature of our business and the significance of our foreign operations expose us to certain risks in the countries in which we operate, including political and economic instability, the adequacy of country infrastructure and labor resources, currency fluctuations and controls, compliance with foreign laws and intellectual property protection, changes in export controls, health conditions, and possible disruptions in transportation networks, which could result in an adverse effect on our business operations in such countries and our results of operations.
Our results of operations could be adversely affected by fluctuations in exchanges rates.
Given our current operations and large customer base outside the United States, we employ the use of forward currency exchange contracts to attempt to minimize the potentially adverse earnings effect from exchange rate fluctuations on our net balance sheet exposures. Nevertheless, in periods when the U.S. dollar significantly fluctuates in relation to the non-U.S. currencies in which we transact business, such as the Euro, Japanese Yen, the South Korean Won, and the New Taiwan Dollar, fluctuations can have an adverse effect on our revenues and results of operations.
Our results of operations could be adversely affected by climate change and natural events in the locations in which we, our customers or our suppliers operate.
We have manufacturing and other operations in locations subject to natural events such as severe weather and earthquakes that could disrupt operations. In addition, our suppliers and customers also have operations in such locations. A natural disaster that results in a prolonged disruption to our operations, or our customers’ or suppliers’ operations, may adversely affect our results of operations and financial condition. Also, climate change poses both regulatory and physical risks that could harm our results of operations or affect the way we conduct our businesses.
Incorrect forecasts of customer demand could adversely affect our results of operations.
Our ability to match inventory and production mix with the product mix needed to fill current orders and orders to be delivered may affect our ability to meet our forecasts. In addition, when responding to customers’ requests for shorter shipment lead times, we manufacture product based on forecasts of customers’ demands. These forecasts are based on multiple assumptions. If we inaccurately forecast customer demand, we may incur expedited shipping costs to deliver products to meet customer demand or hold excess or obsolete inventory that would reduce our profit margins and could adversely affect our results of operations.
We may have difficulty managing our growth and attracting and retaining highly skilled scientific, technical, managerial and marketing personnel, which could adversely affect our revenues and increase our operating expenses.
We have historically experienced periods of growth and intend to grow our business in the future. The management of our growth requires qualified personnel, systems and other resources. Our future success will depend in part on our ability to attract and retain highly skilled scientific, technical, managerial and marketing personnel. Competition for such personnel in the industries that we serve is intense, and our competitors are often larger and more established than we are. We may not be successful in attracting and retaining qualified personnel. In addition, our expansion may also significantly strain operational, management, financial, sales and marketing and other resources. To manage growth effectively, we must continue to enhance and integrate our information technology infrastructure, systems and controls and successfully expand, train and manage our employee base. We may not be able to manage this expansion effectively, including by providing satisfactory levels of customer service and technical support. Inability to manage our growth and to attract and retain skilled personnel could have a material adverse effect on our business, operating results and financial condition.
12
We engage in business combinations and asset acquisitions, and may encounter difficulties integrating acquired businesses with our current operations; therefore, we may not realize the anticipated benefits of the acquisitions.
We seek to grow, in part, through strategic business combinations and asset acquisitions. In the past several years, we have made certain acquisitions intended to complement and expand our business, and may continue to do so in the future. The success of these transactions will depend on our ability to integrate assets and personnel acquired in these transactions, apply our internal controls to these acquired businesses, and cooperate with our strategic partners. We may encounter difficulties in integrating acquisitions with our operations, applying our internal controls processes to these acquisitions, and in managing strategic investments. We may also have difficulty in integrating and managing new IT environments. Furthermore, we may not realize the degree or timing of benefits we anticipate when we first enter into a transaction. Any of the foregoing could adversely affect our business and results of operations.
In 2011, we terminated the license, manufacturing, and distribution agreements for our proprietary SDS® and VAC® products (“SDS Direct transaction”). To achieve the anticipated benefits of this transaction requires a transition period with the former license, manufacturing, and distribution partner. It will also require a certain amount of capital spending associated with expanding our manufacturing facilities and obtaining new business licenses and permits in certain countries. We may have difficulty transitioning responsibilities away from the former partner of our SDS® and VAC® products causing the transition to be more expensive than anticipated, or potentially causing business interruptions. We may also encounter issues in obtaining the necessary licenses and permits in certain countries making it difficult to achieve the full revenue and cash flow benefits of this transaction in a timely manner.
We face the risk of product liability claims.
The manufacture and sale of our products, which include thin film, other toxic materials and packages for life sciences applications, involve the risk of product liability claims. In addition, a failure of one of our products at a customer site could interrupt the business operations of the customer. Our existing insurance coverage limits may not be adequate to protect us from all liabilities that we might incur in connection with the manufacture and sale of our products if a successful product liability claim or series of product liability claims were brought against us.
We may be subject to information technology system failures, network disruptions and breaches in data security.
Information technology system failures, network disruptions and breaches of data security from cyber-attacks could disrupt our operations causing customer communication and order management issues, unintentional disclosure of customer, employee and proprietary information, and disruption in transaction processing which could affect our reputation and reporting of financial results. While management has taken steps to address these concerns by implementing sophisticated network security, hiring competent personnel and establishing prudent internal control measures, there can be no assurance that a system failure or data security breach will not have a material adverse effect on our financial condition and operating results.
Our business is potentially subject to substantial liabilities for failure to comply with environmental regulations.
We use, generate and discharge toxic or otherwise hazardous chemicals and waste in our manufacturing, processing and research and development activities. As a result, we are subject to a variety of governmental regulations related to the storage, use and disposal of these materials. Our failure to comply with present or future laws could result in fines or other liabilities being imposed on us, suspension of production or a cessation of operations.
In addition, under federal and state statutes and regulations, a government agency may seek to recover its response costs and/or require future remedial measures from both operators and owners of property where releases of hazardous substances may have occurred (including releases by prior occupants) or are ongoing, and for which only partial indemnification may be available in some cases.
Our activities may also result in our being subject to additional regulation. Such regulations could require us to acquire significant additional equipment or to incur other substantial expenses to comply with environmental laws. Our failure to control the use of hazardous substances could subject us to substantial financial liabilities.
Our financial results or financial condition could be adversely affected by changes in accounting standards and subjective assumptions, estimates and judgments by management related to complex accounting matters.
Generally accepted accounting principles and related accounting pronouncements, implementation guidelines and interpretations with regard to a wide range of matters that are relevant to our business, such as revenue recognition, business combinations, asset impairment, inventories, fair value measurements, self-insurance, tax matters and litigation, are highly complex and involve many
subjective assumptions, estimates and judgments. Changes in these rules or their interpretation or changes in underlying assumptions, estimates or judgments could significantly change our reported or expected financial performance or financial condition.
13
Our results of operations could be adversely affected by changes in taxation.
Because we are a multi-national company, we have a business presence in many countries and, as a result, are subject to taxation and audit by a number of taxing authorities. Tax rates vary among the jurisdictions in which we operate. Our results of operations could be affected by market opportunities or decisions we make that cause us to increase or decrease operations in one or more countries, or by changes in applicable tax rates or audits by the taxing authorities in countries in which we operate. In addition, we are subject to laws and regulations in various locations that govern the determination of which is the appropriate jurisdiction to decide when and how much profit has been earned and is subject to taxation in that jurisdiction. Changes in these laws and regulations could affect the locations where we are deemed to earn income, which could in turn affect our results of operations. We have deferred tax assets on our balance sheet. Changes in applicable tax laws and regulations could affect our ability to realize those deferred tax assets, which could also affect our results of operations. Each quarter we forecast our tax liability based on our forecast of our performance for the year. If that performance forecast changes, our forecasted tax liability may change.
Compliance with changing corporate governance regulations and public disclosures may result in additional risks and exposures.
Changing laws, regulations and standards relating to corporate governance and public disclosure, and new regulations from the SEC, have created uncertainty for public companies such as ours. One such example is the current ambiguity regarding the breadth and pace of adoption of IFRS whether by convergence or SEC mandate. These laws, regulations, and standards are subject to varying interpretations in many cases and as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices and our commitment to maintaining high standards with regard thereto. As a result, our efforts to comply with evolving laws, regulations, and standards have resulted in, and are likely to continue to result in, increased selling, general, and administrative expenses and significant management time and attention. In particular, our efforts to comply with Section 404 of the Sarbanes-Oxley Act of 2002 and the related regulations regarding our required assessment and, our independent registered public accounting firm’s audit, for fiscal 2011 have necessitated, and we expect such efforts to continue to necessitate, the commitment of significant financial and managerial resources. We are also monitoring the evolving establishment of SEC rules in compliance with the Dodd-Frank Wall Street Reform and Consumer Protection Act to determine the potential future effect on our disclosures and shareholders rights.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
This table summarizes the location and size of ATMI’s significant real properties as of December 31, 2011:
| | September 30, | | September 30, |
Location | | Square Footage | | Lease / Own |
Anseong, South Korea | | 13,000 | | Own |
Anseong, South Korea | | 10,000 | | Lease |
Bloomington, MN | | 68,000 | | Lease |
Burnet, TX | | 77,000 | | Own |
Brussels, Belgium | | 22,000 | | Lease |
Chutung Town, Taiwan | | 18,000 | | Lease |
Danbury, CT (1) | | 31,000 | | Lease |
Danbury, CT | | 73,000 | | Lease |
Hoegaarden, Belgium | | 74,000 | | Own |
Hsin-chu, Taiwan | | 30,000 | | Lease |
Round Rock, TX | | 15,000 | | Lease |
Shanghai, China | | 7,000 | | Lease |
Suwon-si, South Korea | | 7,000 | | Lease |
Tempe, AZ | | 11,000 | | Lease |
Tokyo, Japan | | 6,000 | | Lease |
14
ATMI also leases other sales offices throughout the world, each one of which occupies 5,000 or fewer square feet.
Our fixed assets as of December 31, 2011 include the manufacturing facilities and non-manufacturing facilities such as sales and administrative offices, including leasehold improvements made to those facilities under non-cancelable leases, set forth in the table above and a substantial quantity of machinery and equipment. The facilities, leasehold improvements, machinery and equipment in use as of December 31, 2011 are in good operating condition, are well-maintained and substantially all are in regular use.
We believe that the fixed assets capitalized and facilities in operation at December 31, 2011 for the production of our products are suitable and adequate for the business conducted therein in the current business environment and have sufficient production capacity for their present intended purposes. While we are currently assessing the required amount of capital spending necessary for the expected incremental demand for our SDS products, because of the transition services contracted from the former manufacturer as a result of the SDS Direct transaction, we do not foresee any production capacity issues. Utilization of our facilities varies based on demand for our products. We continuously review our anticipated requirements for facilities and, based on that review, may from time to time adjust our facility plans.
Item 3. Legal Proceedings
ATMI is, from time to time, involved in legal actions, governmental audits, and proceedings relating to various matters incidental to its business including contract disputes, intellectual property disputes, product liability claims, employment matters, export and trade matters, and environmental claims. While the outcome of such matters cannot be predicted with certainty, in the opinion of management, after reviewing such matters and consulting with ATMI’s counsel and considering any applicable insurance or indemnifications, any liability which may ultimately be incurred is not expected to materially affect ATMI’s consolidated financial position, cash flows or results of operations.
Item 4. (Removed and Reserved)
15
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
The following graph compares the cumulative total stockholder return on ATMI’s common stock with the return on the Total Return Index for the NASDAQ Global Select Market (NASDAQ US Index) and the NASDAQ Electronic Components Stock Index. The measurement assumes a $100 investment as of December 31, 2006 with all dividends, if any, reinvested. The data presented are on an annual basis for the five years ended December 31, 2011. The performance shown is not necessarily indicative of future performance.
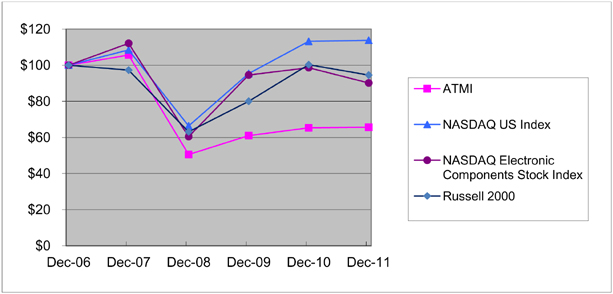
Relative Stock Performance
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | | | | NASDAQ | | | | | | | |
| | | | | | Electronic | | | | | | | |
| | | NASDAQ | | | Components | | | | | | Russell | |
Date | | US Index | | | Stock Index | | | ATMI | | | 2000 | |
12/29/06 | | | 100.00 | | | | 100.00 | | | | 100.00 | | | | 100.00 | |
12/31/07 | | | 108.47 | | | | 112.12 | | | | 105.63 | | | | 97.27 | |
12/31/08 | | | 66.35 | | | | 60.44 | | | | 50.54 | | | | 63.10 | |
12/31/09 | | | 95.38 | | | | 94.57 | | | | 60.99 | | | | 80.02 | |
12/31/10 | | | 113.19 | | | | 98.61 | | | | 65.31 | | | | 100.27 | |
12/31/11 | | | 113.81 | | | | 90.17 | | | | 65.61 | | | | 94.51 | |
The cumulative total stockholder return graph and related data provided in Part II Item 5 of this Form 10-K is not “soliciting material,” is not deemed filed with the SEC and is not to be incorporated by reference in any filing by us under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
16
The common stock of ATMI has traded on the NASDAQ Global Select Market under the symbol ATMI since October 13, 1997, and the common stock of our predecessor company traded under that symbol from 1993 until October 12, 1997. This table sets forth, for the periods indicated, the high and low sales price for the common stock as reported on the NASDAQ Global Select Market:
| | | September 30, | | | | September 30, | |
| | | High | | | Low | |
Fiscal year ended December 31, 2011 | | | | | | | | |
1st Quarter | | $ | 21.28 | | | $ | 17.01 | |
2nd Quarter | | | 20.65 | | | | 17.25 | |
3rd Quarter | | | 21.32 | | | | 15.32 | |
4th Quarter | | | 21.48 | | | | 15.15 | |
| | |
Fiscal year ended December 31, 2010 | | | | | | | | |
1st Quarter | | $ | 19.65 | | | $ | 15.13 | |
2nd Quarter | | | 22.05 | | | | 14.21 | |
3rd Quarter | | | 16.07 | | | | 12.13 | |
4th Quarter | | | 20.99 | | | | 14.47 | |
As of January 31, 2012, there were approximately 153 holders of record of our common stock.
We have never paid cash dividends on our common stock and have no current plans to do so. There are no contractual restrictions in place that currently materially limit, or are likely in the future to materially limit, us from paying dividends on our common stock, but applicable state law may limit the payment of dividends. Our present policy is to retain earnings, if any, to provide funds for the operation and expansion of our business.
The Transfer Agent and Registrar for ATMI is Continental Stock Transfer & Trust Company.
Purchases of Equity Securities – This table lists all repurchases (both open market and private transactions) during the three months ended December 31, 2011 of any of our securities registered under Section 12 of the Exchange Act, by or on behalf of us, or any affiliated purchaser.
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | Total Number
of Shares
Repurchased (2) | | | Average
Price Paid
per Share | | | Total Number of
Shares Purchased
as Part of Publicly
Announced
Programs (3) | | | Maximum Dollar
Value of Shares
that May Yet Be
Purchased Under
the Programs | |
Period (1) | | | | | | | | | | | | | | | | |
| | | | |
October 2011 | | | 1,063 | | | $ | 15.28 | | | | — | | | $ | 48,182,523 | |
November 2011 | | | 659 | | | $ | 20.16 | | | | — | | | $ | 48,182,523 | |
December 2011 | | | 663 | | | $ | 19.17 | | | | — | | | $ | 48,182,523 | |
| | | | | | | | | | | | | | | | |
Total | | | 2,385 | | | $ | 17.71 | | | | — | | | $ | 48,182,523 | |
| | | | | | | | | | | | | | | | |
| (1) | There were no other repurchases of our equity securities by or on behalf of us or any affiliated purchaser during the fiscal quarter ended December 31, 2011. |
| (2) | Share repurchases are shown on a trade-date basis. Represents shares repurchased to satisfy employee minimum tax withholding obligations on vesting of restricted stock. |
| (3) | In August 2010, the Company’s Board of Directors approved a share repurchase program for up to $50.0 million of ATMI common stock. Under the terms of the share repurchase program, repurchases are made from time to time in open market transactions at prevailing market prices or in privately negotiated transactions. Management determines the timing and amount of purchases under the programs based upon market conditions or other factors. The program, which has no expiration date, does not require the Company to purchase any specific number or amount of shares and may be suspended or reinstated at any time at the Company’s discretion and without notice. |
17
Item 6. Selected Financial Data
These selected consolidated statements of operations for the years ended December 31, 2011, 2010, 2009, 2008 and 2007 and the consolidated balance sheet data as of such dates are derived from ATMI’s audited consolidated financial statements. The data below should be read in conjunction with the consolidated financial statements and notes thereto and other financial information included elsewhere in this Form 10-K (in thousands, except per share data).
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | 2011 | | | 2010 | | | 2009 | | | 2008 | | | 2007 | |
| | | | | |
Consolidated Statements of Operations: | | | | | | | | | | | | | | | | | | | | |
Revenues | | $ | 390,087 | (1) | | $ | 367,256 | | | $ | 254,704 | | | $ | 339,063 | | | $ | 364,088 | |
Cost of revenues | | | 205,957 | (2) | | | 191,248 | | | | 152,520 | (9) | | | 172,551 | (16) | | | 182,480 | (20) |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 184,130 | | | | 176,008 | | | | 102,184 | | | | 166,512 | (17) | | | 181,608 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 53,708 | | | | 48,645 | | | | 37,162 | (10) | | | 37,809 | | | | 29,879 | |
Selling, general, and administrative | | | 83,546 | (3) | | | 85,425 | | | | 76,359 | (11) | | | 88,781 | | | | 99,227 | (21) |
Contract termination | | | 84,590 | (4) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 221,844 | | | | 134,070 | | | | 113,521 | | | | 126,590 | | | | 129,106 | |
| | | | | | | | | | | | | | | | | | | | |
Operating income (loss) | | | (37,714 | ) | | | 41,938 | | | | (11,337 | ) | | | 39,922 | | | | 52,502 | |
Interest income | | | 1,205 | | | | 949 | | | | 1,196 | | | | 3,126 | | | | 7,689 | |
Other income (expense), net | | | (2,625 | )(5) | | | 7,740 | (7) | | | (3,515 | )(12) | | | (2,902 | )(18) | | | (788 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | (39,134 | ) | | | 50,627 | | | | (13,656 | ) | | | 40,146 | | | | 59,403 | |
Provision (benefit) for income taxes | | | (19,115 | )(6) | | | 11,121 | (8) | | | (6,996 | ) | | | 6,819 | (19) | | | 18,864 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | (20,019 | ) | | $ | 39,506 | | | $ | (6,660 | ) | | $ | 33,327 | | | $ | 40,539 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Earnings (loss) per share—diluted | | $ | (0.63 | ) | | $ | 1.24 | | | $ | (0.21 | ) | | $ | 1.04 | | | $ | 1.16 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Weighted-average shares outstanding—diluted | | | 31,703 | | | | 31,895 | | | | 31,398 | | | | 32,078 | | | | 35,093 | |
| | | | | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents, and marketable securities (22) | | $ | 113,484 | | | $ | 152,572 | | | $ | 107,978 | | | $ | 96,020 | | | $ | 193,697 | |
Working capital | | | 226,232 | | | | 236,263 | | | | 203,760 | (13) | | | 190,095 | | | | 280,221 | |
Total assets | | | 513,686 | | | | 533,589 | | | | 458,963 | (14) | | | 452,449 | (23) | | | 492,076 | (23) |
Long-term obligations | | | 18,399 | | | | 28,427 | | | | 17,790 | (15) | | | 15,688 | (23) | | | 10,491 | (23) |
Total stockholders’ equity | | | 450,331 | | | | 458,425 | | | | 411,490 | | | | 408,897 | | | | 434,383 | |
18
The Company has never declared any cash dividends.
| (1) | Revenues were negatively impacted by $6.9 million due to the effects of the SDS Direct transaction which included $5.1 million of revenue reversal for inventory repurchases and an estimated $1.8 million net impact due to excess inventory in the SDS channel, which was partially offset by incremental revenues realized as a result of the transaction. Includes $2.4 million from IP settlements, which represent payments received related to periods prior to 2011. |
| (2) | The cost of revenues were favorably impacted by $1.5 million due to the effects of the SDS Direct transaction, representing a $1.1 million of cost reversals for inventory repurchased and $0.4 million estimated net effect due to excess inventory in the SDS channel, partially offset by transition service costs and incremental costs on revenues realized following the transaction. |
| (3) | Includes $1.7 million gain on remeasurement of contingent consideration, a $2.4 million benefit related to state tax credits, and distribution expenses of $1.3 million related to the SDS Direct transaction. |
| (4) | $84.6 million contract termination charge related to the SDS Direct transaction. |
| (5) | Includes $1.3 million foreign exchange loss on Euro exposures, $0.7 million of accretion related to the Artelis contingent consideration liability, partially offset by a $0.9 million investment gain. |
| (6) | Includes $31.0 million tax benefit associated with the $84.6 million pre-tax contract termination charge. |
| (7) | Includes a $0.5 million gain from the sale of a marketable security, $2.5 million gain on sale of shares of one of our equity-method investees, and a $5.9 million fair value remeasurement gain on our minority interest from our acquisition of Artelis. |
| (8) | Includes $1.8 million of tax benefits (including interest) recognized to reverse previously established reserves for uncertain tax positions due to expiration of statutes of limitation and settlements, a $2.1 million tax benefit related to a European dividend, and a $1.9 million charge related to changes in valuation allowances. |
| (9) | Includes $1.1 million charge for incremental excess and obsolete inventory related to product discontinuances and a reserve to cover expected product shelf-life issues; and a $3.1 million impairment charge for long-lived assets written down to their estimated fair values primarily related to the planned idling of manufacturing capacity of our gas products. |
| (10) | Includes a $1.6 million impairment charge for long-lived assets written down to their estimated fair values related primarily to idled equipment. |
| (11) | Includes a $1.4 million charge to increase our reserves for bad debt to cover exposures related to customer bankruptcy filings and uncertainties of collections; a $2.6 million impairment charge for long-lived assets written down to their estimated fair values primarily related to redundant enterprise management software; and a $0.6 million charge for SG&A severance costs. |
| (12) | Includes a $2.5 million impairment charge, primarily related to a write-down associated with an auction-rate security. |
| (13) | Balance has been reduced by $0.1 million related to reclassification of unrecognized tax benefits and valuation allowances. |
| (14) | Balance has been reduced by $0.6 million related to reclassification of unrecognized tax benefits and valuation allowances. |
| (15) | Balance has been reduced by $0.6 million related to reclassification of unrecognized tax benefits and valuation allowances. |
| (16) | Includes a $2.4 million business interruption claim recovery related to a fire at a contract manufacturer in Taiwan. |
| (17) | Includes a $3.1 million benefit associated with the settlement of a dispute with a distributor ($3.7 million recognized in revenues, with $0.6 million of associated costs recognized in cost of revenues). |
| (18) | Includes a $2.0 million gain from the sale of a marketable security, $1.6 million of impairment charges related to our strategic investment portfolio and a $1.8 million reserve on a convertible note, and $1.1 million representing our proportionate share of gains on sales of assets by one of our equity-method investees. |
| (19) | Includes a $3.7 million tax benefit (including interest) recognized to reverse previously established reserves for uncertain tax positions as a result of the expiration of the applicable statute of limitations. |
| (20) | Includes $1.1 million of increased customs expense on imported goods from the U.S. to an overseas affiliate. |
| (21) | Includes $1.1 million associated with a contingent legal fee arrangement. |
| (22) | Includes non-current marketable securities of $3.3 million, $25.4 million, $10.6 million, $3.7 million, and $0 million at December 31, 2011, 2010, 2009, 2008, and 2007, respectively, which includes $22.1 million reclassification of our Intermolecular investment from Other non-current assets to Marketable securities, current portion. |
| (23) | Balances have been reduced by $0.6 million and $0.2 million for years ended December 31, 2008 and December 31, 2007, respectively, related to reclassification of unrecognized tax benefits from deferred tax liabilities. |
19
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This discussion and analysis of our financial condition and results of operations should be read together with the consolidated financial statements and the related notes thereto appearing in Item 8 of this Form 10-K.
Company Overview
We believe we are among the leading suppliers of high performance materials, materials packaging and materials delivery systems used worldwide in the manufacture of microelectronics devices. Our products consist of “front-end” semiconductor performance materials, sub-atmospheric pressure gas delivery systems for safe handling and delivery of toxic and hazardous gases to semiconductor process equipment, high-purity materials packaging and dispensing systems that allow for the reliable introduction of low volatility liquids and solids to microelectronics and biopharmaceutical processes. ATMI targets both semiconductor and flat-panel display manufacturers, whose products form the foundation of microelectronics technology rapidly proliferating through the consumer products, information technology, automotive, and communications industries. The market for microelectronics devices is continually changing, which drives demand for new products and technologies at lower cost. ATMI’s customers include many of the leading semiconductor and flat-panel display manufacturers in the world who target leading-edge technologies. ATMI also addresses an increasing number of critical materials handling needs for the life sciences markets. Our proprietary containment, mixing, and bioreactor technologies are sold to the biotechnology, laboratory and cell therapy markets, which we believe offer significant growth potential. ATMI’s objective is to meet the demands of our microelectronics and life sciences customers with solutions that maximize the efficiency of their manufacturing processes, reduce capital or operating costs, and minimize the time to develop new products and integrate them into their processes.
Use of Estimates
Preparation of our financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. Note 1 to the consolidated financial statements in Item 8 of this Form 10-K describes the significant accounting policies used in preparation of the consolidated financial statements. Management believes the most complex and sensitive judgments, because of their significance to the Consolidated Financial Statements, result primarily from the need to make estimates about the effects of matters that are inherently uncertain. The most significant areas involving management judgments and estimates are described below. Actual results in these areas could differ from management’s estimates. These policies are determined by management and have been reviewed by ATMI’s Audit Committee.
Revenue Recognition
We recognize revenue when the following four criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. Revenues from product sales are generally recognized upon delivery to a common carrier when terms are equivalent to free-on-board (“FOB”) origin and upon receipt by a customer when terms are equivalent to FOB destination. In instances where final acceptance of equipment is specified by the purchase agreement, revenue is deferred until all acceptance criteria have been satisfied, except when reasonable reserves for returns can be effectively established due to substantial successful installation history for homogenous transactions. Should changes in conditions cause management to determine these criteria are not met for certain future transactions, revenue recognized for any reporting period could be adversely affected. We accrue for sales returns, warranty costs, and other allowances based on a current evaluation of our experience based on stated terms of the transactions. Should actual product failure rates or customer return experience differ from our estimates, revisions to the estimated accruals would be required.
Prior to October 31, 2011, we used Matheson as our exclusive contract manufacturer and distribution partner for the manufacture and distribution of our SDS products (the “Licensed Products”). Under the terms of the original manufacturing agreement, ATMI retained the right to manufacture 25 percent of all Licensed Products, while Matheson had the right to manufacture 75 percent of all Licensed Products. Upon completion of manufacture, ATMI purchased all Licensed Products produced by Matheson. Under the terms of the distribution agreement, we received payment from Matheson based upon a formula which was dependent on the sale price obtained by Matheson from its customers. ATMI recognized revenue from the sale of Licensed Products to Matheson when Matheson sold the Licensed Products to its customers, because that is when the sales price became fixed and determinable. On October 31, 2011, we terminated the agreements with Matheson for manufacturing, marketing, selling and distributing of our SDS products. Matheson will provide ongoing support services to ATMI during a brief transition period., and will continue to manufacture a portion of the SDS products for up to two years, for which it will be paid additional fees by ATMI. Subject to such continuing services, ATMI assumed control of all manufacturing, distribution, logistics and sales services, which Matheson had provided globally prior to execution of the Termination Agreement, other than the distribution of the SDS and VAC product lines in Japan, which services will continue to be provided by Matheson’s parent company, Taiyo Nippon Sanso Corporation (“TNSC”). In those regions where Matheson is involved in distributing products during the transition period, revenues will be recognized only after sale of the Licensed Products to the end users.
20
Accounts Receivable Allowances
The allowance for doubtful accounts is established to represent our best estimate of the net realizable value of the outstanding accounts receivable balances. We estimate our allowance for doubtful accounts based on past due amounts and historical write-off experience, as well as trends and factors surrounding the credit risk of the markets we operate in and the financial viability of specific customers. In an effort to identify adverse trends, we assess the financial health of the markets we operate in and perform periodic credit evaluations of our customers and ongoing reviews of account balances and aging of receivables. Amounts are considered past due when payment has not been received within the time frame of the credit terms extended. Write-offs are charged directly against the allowance for doubtful accounts and occur only after all collection efforts have been exhausted. Actual write-offs and adjustments could differ from the allowance estimates because of unanticipated changes in the business environment as well as factors and risks surrounding specific customers.
As of December 31, 2011 and 2010 we had $0.8 million of accounts receivable allowances recorded. Although management believes these reserves are adequate, any adverse changes in market conditions may require us to record additional reserves.
Inventory Valuation Reserves
Inventory valuation reserves are established in order to report inventories at the lower of cost or market value on our consolidated balance sheets. The determination of inventory valuation reserves requires management to make estimates and judgments on the future salability of inventories. Valuation reserves for excess, obsolete, and slow-moving inventory are estimated by comparing the inventory levels of individual parts to both future sales forecasts or production requirements and historical usage rates in order to identify inventory where the resale value or replacement value is less than inventory cost. Other factors that management considers in determining these reserves include whether individual inventory parts or chemicals meet current specifications and cannot be substituted for or reworked into a part currently being sold or used as a service part, overall market conditions, and other inventory management initiatives.
As of December 31, 2011 and 2010, we had $2.6 million and $2.4 million, respectively, of inventory valuation reserves recorded. Although management believes these reserves are adequate, any adverse changes in market conditions may require us to record additional inventory valuation reserves.
Non-marketable Equity Securities
We selectively invest in non-marketable equity securities of private companies, which range from early-stage companies that are often still defining their strategic direction to more mature companies whose products or technologies may directly support an ATMI product or initiative. Our non-marketable equity investments are recorded using cost basis or the equity method of accounting, depending on the facts and circumstances of each investment. At December 31, 2011, the carrying value of our portfolio of strategic investments in non-marketable equity securities totaled $8.0 million ($22.3 million at December 31, 2010). The main driver of the reduction from December 31, 2010 was the initial public offering of Intermolecular, which caused us to reclassify our investment from “Other non-current assets” to “Marketable securities” because the securities became publicly traded. In certain instances, we loan funds to early-stage investees at market interest rates to enable them to focus on product and technology development. Non-marketable equity and debt securities are included in the consolidated balance sheets under the caption “Other non-current assets.” We receive regular financial information from our investee’s typically on a one month lag.
Investments in non-marketable equity securities are inherently risky, and some of these companies are likely to fail. Their success (or lack thereof) is dependent on product development, market acceptance, operational efficiency, attracting and retaining talented professionals, and other key business success factors. In addition, depending on their future prospects, they may not be able to raise additional funds when needed or they may receive lower valuations, with less favorable investment terms than in previous financings, and the investments would likely become impaired.
We review our investments quarterly for indicators of impairment; however, for non-marketable equity securities, the impairment analysis may require significant judgment to identify events or circumstances that would likely have a significant adverse effect on the fair value of the investment. The indicators that we use to identify those events or circumstances include (a) the investee’s revenue and earnings trends relative to predefined milestones and overall business prospects, (b) the technological feasibility of the investee’s products and technologies, (c) the general market conditions in the investee’s industry or geographic area, including adverse regulatory or economic changes, (d) factors related to the investee’s ability to remain in business, such as the investee’s liquidity, and the rate at which the investee is using its cash, and (e) the investee’s receipt of additional funding at a lower valuation.
21
Investments identified as having an indicator of impairment are subject to further analysis to determine if the investment is other-than-temporarily impaired, in which case we write the investment down to its fair value, using the framework required by Accounting Standards Codification (“ASC”) 820 “Fair Value Measurements and Disclosures.” When an investee is not considered viable from a financial or technological point of view, we write down the entire investment since we consider the estimated fair market value to be nominal. If an investee obtains additional funding at a valuation lower than our carrying amount or requires a new round of equity funding to stay in operation and the new funding does not appear imminent, we presume that the investment is other-than-temporarily impaired, unless specific facts and circumstances indicate otherwise. We recognized an $0.8 million impairment charge in our portfolio of non-marketable equity securities in 2011 (none in 2010 and 2009).
Income Taxes
The net deferred tax asset at December 31, 2011 was $23.4 million compared to a net deferred tax liability of $1.3 million at December 31, 2010 driven by the $31.0 million benefit from the $84.6 million contract termination charge associated with the SDS Direct transaction. For our deferred future tax benefits, we believe that our earnings during the periods when the temporary differences become deductible will be sufficient to realize the related future income tax benefits. For those jurisdictions where the expiration date of tax carryforwards or the projected operating results indicate that realization is not likely, a valuation allowance is provided.
In the ordinary course of business there is inherent uncertainty in quantifying our income tax positions. We assess our income tax positions and record tax benefits for all years subject to examination based upon management’s evaluation of the facts, circumstances and information available at the reporting date. For those tax positions where it is more likely than not that a tax benefit will be sustained, we have recorded the largest amount of tax benefit with a greater than 50 percent likelihood of being realized upon ultimate settlement with a taxing authority. For those income tax positions where it is not more likely than not that a tax benefit will be sustained, no tax benefit has been recognized in the financial statements. We adjust these unrecognized tax benefits, including any impact on the related interest and penalties, in light of changing facts and circumstances. A number of years may elapse before a particular matter for which we have established an unrecognized tax benefit is audited and fully resolved. To the extent we prevail in matters for which we have recorded an unrecognized benefit or are required to pay amounts in excess of what we have recorded our effective tax rate in a given financial statement period could be materially affected. An unfavorable tax settlement might require use of our cash and/or result in an increase in our effective tax rate in the year of resolution. A favorable tax settlement would be recognized as a reduction in our effective tax rate in the year of resolution. For a discussion of current tax matters, see Note 10 to the consolidated financial statements in Item 8 of this Form 10-K.
In assessing the need for a valuation allowance, we estimate future taxable income, considering the feasibility of ongoing tax planning strategies and the realizability of tax loss carryforwards. Valuation allowances related to deferred tax assets can be affected by changes to tax laws, changes to statutory tax rates and future taxable income levels. In the event we were to determine that we would not be able to realize all or a portion of our deferred tax assets in the future, we would reduce such amounts through a charge to income in the period in which that determination is made or when tax law changes are enacted. Conversely, if we were to determine that we would be able to realize our deferred tax assets in the future in excess of the net carrying amounts, we would decrease the recorded valuation allowance through an increase to income in the period in which that determination is made. Changes in deferred tax asset valuation allowances recorded in a business combination and income tax uncertainties after the acquisition date generally will affect income tax expense.
Depreciable Lives of Property, Plant and Equipment
ATMI’s net property, plant and equipment at December 31, 2011 and 2010 was $116.3 million and $119.1 million, respectively, representing 22.6 percent and 22.3 percent, respectively, of the Company’s consolidated total assets. Depreciation expense for the years ended December 31, 2011, 2010 and 2009 was $23.3 million, $22.8 million and $22.3 million, respectively. Management judgment is required in the determination of the estimated depreciable lives that are used to calculate annual depreciation expense and accumulated depreciation.
Property, plant and equipment are recorded at cost and depreciated over the assets’ useful lives on a straight-line basis for financial reporting purposes. The estimated useful life represents the projected period of time that the asset will be productively employed by the Company and is determined by management based on many factors, including historical experience with similar assets and technological life cycles. Circumstances and events relating to these assets are monitored to ensure that changes in asset lives or impairments are identified and prospective depreciation expense or impairment expense is adjusted accordingly. The depreciation periods used are: buildings, 5 to 35 years; machinery and equipment, 3 to 15 years; software, 5 to 10 years; cylinders and canisters, 7 to 15 years; furniture and fixtures, 5 years; and leasehold improvements, over the lesser of the lease term or estimated useful life. We use accelerated depreciation methods for tax purposes where appropriate.
22
Equity-Based Compensation
We account for awards of equity-based compensation under our employee stock plans using the fair value method. Accordingly, we estimate the grant date fair value of our equity-based awards and amortize this fair value to compensation expense over the requisite service period or vesting term. To estimate the fair value of our stock option awards we currently use the Black-Scholes-Merton options-pricing model. The determination of the fair value of equity-based awards on the date of grant using an options-pricing model is affected by our then current stock price as well as assumptions regarding a number of complex and subjective variables. Management is required to make certain judgments for these variables which include the expected stock price volatility over the term of the awards, the expected term of options based on employee exercise behaviors, and the risk-free interest rate. For awards granted subsequent to January 1, 2006, expected stock price volatility is based on the historical volatility of ATMI common stock for a period shorter than the expected term of the options. We have excluded the historical stock price volatility prior to the public announcement regarding the sale of our non-core businesses in 2004, because those businesses that were sold represented a significant portion of ATMI’s consolidated business and were subject to considerable cyclicality associated with the semiconductor equipment industry, which drove increased volatility in ATMI’s stock price. The expected term of options granted represents the period of time that options are expected to be outstanding. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for a period commensurate with the estimated expected term. We recognize expense only for those awards expected to vest. If actual results are not consistent with our assumptions and judgments used in estimating key assumptions, in future periods, the stock option expense that the Company records for future grants may differ significantly from what the Company has recorded in the current period.
Equity-based compensation expense is generally recognized on a straight-line or accelerated attribution basis over the estimated service period of the awards.
Fair Value Measurements
All of our financial assets and liabilities are measured at fair value based upon Level 1 or Level 2 inputs, as defined under ASC 820, with the exception of one auction rate security, which has been measured using Level 3 inputs, because the security is illiquid. For Level 1 measurements, we use quoted prices in active markets for identical assets and liabilities. For Level 2 measurements, we use observable inputs other than Level 1 prices, such as quoted prices for similar assets and liabilities; quoted prices in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which all significant inputs are observable or can be derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities. For Level 3 measurements, we use unobservable inputs to the valuation methodology that are significant to the measurement of fair value of assets or liabilities.
The calculation of fair value for our auction-rate security requires critical judgments and estimates by management including assumptions about the anticipated term and the yield that a market participant would require to purchase such a security in the current market environment. As of December 31, 2011 we have recorded a temporary impairment charge of $0.9 million, net of tax, within the caption “accumulated other comprehensive income” on the consolidated balance sheets. We determine the fair value of our auction-rate security by incorporating assumptions about the anticipated term and the yield that a market participant would require to purchase such a security in the current market environment. We determined that there was no incremental credit loss in 2011 and 2010. In 2009, we recorded a credit loss of $0.3 million in our consolidated statement of operations. While we believe the valuation methodologies are appropriate, the use of valuation methodologies is highly judgmental and changes in methodologies or market conditions for this security can have a material impact on the values of the related assets, our financial position, and overall liquidity.
On November 2, 2010, ATMI’s Belgian subsidiary acquired the remaining 60 percent of the outstanding shares of Artelis S.A. As part of the acquisition, we recognized a liability for the fair value of contingent payments tied to future revenue performance for the fiscal years 2012 through 2014. The contingent payment tied to future revenue performance has a range of possible outcomes from zero to $23.3 million. The fair value measurements were calculated using unobservable inputs (primarily using discounted cash flow analyses, a discounted average rate of 5.3 percent, and reliance on the market and product knowledge of internal experts), classified as Level 3, requiring significant management judgment due to the absence of quoted market prices or observable inputs for assets of a similar nature.
As of December 31, 2011 and 2010, the fair value of our auction-rate security was $3.3 million and $2.9 million, respectively. As of December 31, 2011 and 2010, the fair value of our Artelis acquisition contingent performance obligations associated with future revenue was $7.2 million and $8.4 million. See Notes 1 and 5 for additional discussion regarding our auction-rate security and Notes 1, 5 and 7 for additional details regarding the Artelis acquisition.
23
Goodwill and Other Intangible Assets
The assets and liabilities of acquired businesses are recorded under the purchase method of accounting at their estimated fair values at the dates of acquisition. Goodwill represents costs in excess of fair values assigned to the underlying net assets of acquired businesses.
Goodwill and intangible assets deemed to have indefinite lives are not amortized, but are subject to annual impairment testing. In 2011, we adopted ASU 2011-08, “Intangibles – Goodwill and Other (Topic 350): Testing Goodwill for Impairment”, which simplified how entities are required to test goodwill for impairment by permitting an initial assessment of qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the two-step goodwill impairment test.
For reporting units where the qualitative determination does not indicate that it is more likely than not that the fair value of a reporting unit is more than its carrying value, the two-step test is performed. In order to perform the two-step test we estimate the fair value of a reporting unit based on the best information available as of the date of the assessment, which primarily incorporates management assumptions about expected future cash flows and contemplates other valuation techniques. Future cash flows can be affected by changes in the global economy and local economies, changes in the microelectronics and biopharmaceutical industries, changes in technology, and the execution of management’s plans. We concluded that goodwill was not impaired during 2011. A 10% decline in projected reporting unit cash flows would not impact the conclusion we reached in 2011. Although no goodwill impairment has been recorded to date, there can be no assurances that future goodwill impairments will not occur.
Other Long-Lived Amortizable Assets
We evaluate the potential impairment of other long-lived assets when appropriate. If the carrying value of assets exceeds the sum of the undiscounted expected future cash flows, the carrying value of the asset is written down to fair value.
Recent Accounting Pronouncements
See Note 1 to the consolidated financial statements in Item 8 of this Form 10-K for information concerning recently issued accounting pronouncements.
Related Party Transactions
The Company’s related parties are primarily unconsolidated equity affiliates. The Company did not engage in any material transactions involving related parties that included terms or other aspects that differ from those which would be negotiated with independent parties. In November 2011, Intermolecular completed an initial public offering, and we converted our preferred shares into common shares and exercised a warrant into common shares, which caused us to reclassify our investment from “Other Non-Current Assets” to “Marketable securities” as the securities are now publicly traded. At December 31, 2011 we own common shares with a market value of $33.1 million.
Results of Operations
This table shows the effect of pre-tax compensation cost arising from equity-based payment arrangements on the consolidated statements of operations (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
Cost of revenues | | $ | 496 | | | $ | 357 | | | $ | 315 | |
Research and development | | | 900 | | | | 866 | | | | 523 | |
Selling, general, and administrative | | | 6,143 | | | | 6,470 | | | | 4,902 | |
| | | | | | | | | | | | |
Total equity-based compensation expense | | $ | 7,539 | | | $ | 7,693 | | | $ | 5,740 | |
| | | | | | | | | | | | |
24
Year Ended December 31, 2011 Compared to Year Ended December 31, 2010
Overview
Results for the year ended December 31, 2011 include revenues of $390.1 million, an agreement for the reacquisition of license, manufacturing, & distribution rights for our Safe Delivery Source (“SDS®”) product line, and our first fee-based collaborative development arrangement with a key customer, which will allow us to more effectively demonstrate our High Productivity Development (“HPD”) capabilities.
The 2011 results include the effects of terminating a license, manufacturing and distribution agreement associated with our SDS® technology in exchange for a $95 million cash payment. As a result of this agreement, we recognized a one-time operating charge of $84.6 million in the fourth quarter of 2011. In connection with this agreement, revenues in the fourth quarter were unfavorably impacted by $6.9 million associated with a cash payment of $5.1 million to repurchase inventory due to previously recognized product shipments into the former distributor’s channel as well as an estimated $1.8 million net impact due to excess inventory in the SDS distribution channel in regions where the former distributor continues to sell product until ATMI secures the appropriate licenses to fully conduct business, which was partially offset by incremental revenues from the transaction. As a result of this agreement, beginning with the second quarter of 2012, we anticipate increased revenues of $7 million to $8 million per quarter; a 200 basis point improvement in gross margins, a $2 million to $3 million increase to selling, general and administrative (“SG&A”) expense per quarter, and an increase of $0.08 to $0.09 of diluted earnings per share per quarter. We anticipate that we will spend $2 million on integration related activities in the first quarter of 2012.
For the year ended December 31, 2011, ATMI revenues increased by 6.2 percent to $390.1 million compared to 2010 revenues of $367.3 million, including the $6.9 million unfavorable fourth quarter impact mentioned above. The 2011 growth reflects strength in the first half of 2011 in consumer electronics demand which drove higher wafer starts and increased fab utilization followed by a lower wafer start growth environment in the second half of 2011. The revenue growth from 2010 to 2011 was driven by a 17 percent increase in copper materials and a 25 percent increase in Life sciences. Our gross profit margin declined by 70 basis points to 47.2 percent in 2011 compared to 47.9 in 2010 due to the impact of fourth quarter inventory repurchases, anticipated price reductions, some unanticipated cost overruns, and product mix. Research and development expenses (“R&D”) grew 10.4 percent in 2011 compared to 2010 primarily driven by product development related spending associated with the November 2010 acquisition of Artelis. SG&A declined by 2.2 percent in 2011 from 2010 due to a $2.4 million benefit related to a capital-based state tax credit, and a $1.7 million gain from the change in fair value of a contingent consideration obligation. SG&A, as a percent of revenues, decreased to 21.4 percent in 2011 compared to 23.3 percent in 2010. The operating loss of $37.7 million in 2011 was driven by the $84.6 million, one-time contract termination charge associated with the SDS Direct transaction. The 2011 effective tax benefit rate of 48.8 percent would have been an effective tax rate of 26.2 percent, excluding the $31.0 million tax benefit from the $84.6 million one-time contract termination charge. The 2011 net loss was $20.0 million ($0.63 per diluted share) compared to net income of $39.5 million ($1.24 per diluted share) in 2010. The 2011 net loss of $20.0 million includes the after tax impact of $53.6 million due to the SDS Direct termination charge.
The following is a summary of selected consolidated earnings information (in thousands of dollars):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | December 31, | | | | |
| | | 2011 | | | 2010 | | | % Change | |
Revenues | | $ | 390,087 | | | $ | 367,256 | | | | 6.2 | % |
Cost of revenues | | | 205,957 | | | | 191,248 | | | | 7.7 | % |
| | | | | | | | | | | | |
Gross profit | | | 184,130 | | | | 176,008 | | | | 4.6 | % |
Gross margin | | | 47.2 | % | | | 47.9 | % | | | (0.7 | %) |
Research and development | | | 53,708 | | | | 48,645 | | | | 10.4 | % |
R&D as a percent of revenues | | | 13.8 | % | | | 13.2 | % | | | 0.6 | % |
Selling, general, and administrative | | | 83,546 | | | | 85,425 | | | | (2.2 | %) |
SG&A as a percent of revenues | | | 21.4 | % | | | 23.3 | % | | | (1.9 | %) |
Contract termination | | | 84,590 | | | | — | | | | — | |
Operating income (loss) | | | (37,714 | ) | | | 41,938 | | | | (189.9 | %) |
Operating margin | | | (9.7 | %) | | | 11.4 | % | | | (21.1 | %) |
Effective tax (benefit) rate | | | (48.8 | %) | | | 22.0 | % | | | 27.0 | % |
Net income (loss) | | $ | (20,019 | ) | | $ | 39,506 | | | | (150.7 | %) |
Earnings (loss) per diluted share | | $ | (0.63 | ) | | $ | 1.24 | | | | (150.8 | %) |
25
Revenues. For the year ended December 31, 2011, ATMI revenues increased by 6.2 percent to $390.1 million compared to full year 2010 revenue of $367.3 million. The 2011 revenues were negatively impacted by $6.9 million associated with a cash payment of $5.1 million to repurchase previously recognized product shipments into the former distributor’s channel as well as an estimated $1.8 million net impact due to excess inventory in the SDS distribution channel in regions where the former distributor continues to sell product until ATMI secures the appropriate licenses to fully conduct business, which was partially offset by incremental revenues from the transaction. The 2011 revenue growth reflects strength in the first half of 2011 in consumer electronics demand, which drove higher wafer starts and increased fab utilization, followed by a lower wafer start growth environment in the second half of 2011. The growth in revenues in 2011 compared to 2010 occurred in both our microelectronics and life sciences product lines. Driven by a strong first half of 2011, revenues in our microelectronics product lines grew 4.5 percent to $351.8 million in 2011 from $336.5 million in 2010 primarily due to strong growth in our copper products associated with adoption of our materials at the advanced technology nodes. In our life sciences product lines, revenues increased 24.5 percent to $38.3 million in 2011 from $30.7 million in 2010 driven by the transition from qualification to volume production at a few customers, and the additional placements of our mixing and bioreactor platforms in the marketplace and royalties from intellectual property licensing agreements. Given the ongoing pressures to bring costs down in the consumer and microelectronics industries, we continue to experience pricing pressure with several of our legacy products. Foreign currency translation provided a 100 basis point benefit to revenue in 2011 driven by a weakening US Dollar against the Japanese Yen and the Euro.
Gross Profit. In 2011, gross profit of $184.1 million increased 4.6 percent from $176.0 million in 2010. Our gross profit margin declined by 70 basis points to 47.2 percent in 2011 compared to 47.9 in 2010 due to the impact of fourth quarter inventory repurchases, anticipated price reductions, some unanticipated cost overruns, and product mix. The SDS Direct transaction reduced gross profit by $4.7 million and gross profit margin by 60 basis points due to the reversal of previously recognized product shipments the estimated effect of excess channel inventories and incremental transition services costs. Gross profit in our microelectronics product lines increased 3.0 percent to $170.7 million in 2011 compared to $165.8 million in 2010. Gross profit margin in microelectronics was approximately 49 percent in 2011 compared to 49 percent in 2010. Gross profit in our life sciences product lines increased 31.1 percent to $13.4 million in 2011 compared to $10.2 million for the same period in 2010. Life sciences gross profit margin increased year over year to 35 percent in 2011 from 33 percent in 2010. Life sciences gross profit margin for 2011 includes the effect of the U.S. manufacturing capacity we brought online in mid-2010.
Research and Development Expenses. R&D expense increased 10.4 percent to $53.7 million in 2011 compared to $48.6 million in 2010. The increase in 2011 spending was driven by increased product development spending associated with the November 2010 Artelis acquisition ($3.3 million), consumption of material consumables ($0.7 million), intellectual property fees ($0.5 million), and rent ($0.3 million), partially offset by lower HPD licensing and maintenance ($1.8 million), and cost reimbursements related to a collaborative development agreement (“CDA”) ($0.9 million). The CDA was executed in the third quarter of 2011 with an advanced memory integrated circuit manufacturer for the purpose of developing molecules, including chemical precursors, and material systems for next generation semiconductor products. We recognized a benefit of $0.9 million in 2011 and expect to recognize up to $2.9 million of benefit in 2012.
Selling, General and Administrative Expenses. SG&A declined 2.2 percent to $83.5 million in 2011 compared to $85.4 million in 2010. The decrease is the result of a $2.4 million benefit related to a capital-based state tax credit, a $1.7 million gain from the change in fair value of a contingent consideration obligation, lower maintenance ($0.7 million), legal fees ($0.5 million) and asset impairments ($0.3 million), partially offset by expenses associated with the Artelis acquisition ($3.7 million), a 2010 bad debt reserve release of $0.8 million not repeated in 2011, $1.3 million associated with transition costs related to the SDS Direct transaction and increased employee related costs ($1.3 million).
Contract Termination Expense. In 2011, we recognized an $84.6 million charge associated with terminating a long-term license, manufacturing and distribution agreement with Matheson Tri-Gas, Inc. that gives ATMI responsibility for worldwide distribution of our SDS and VAC gas storage and delivery system.
Operating Income (Loss). For the year ended December 31, 2011, we generated an operating loss of $37.7 million compared to operating income of $41.9 million in 2010. The decrease is primarily a result of the SDS Direct contract termination charge and other items as noted above.
Interest Income. Interest income was $1.2 million for the year ended December 31, 2011 compared to $0.9 million for the year ended December 31, 2010, primarily caused by higher invested balances and slightly improved average yields.
Other income (expense), net. In 2011, we reported net other expense of $2.6 million, which was driven by a $0.8 million impairment reserve for a minority investment, losses from equity method investees of $0.6 million and losses from foreign exchange of $1.2 million partially offset by a $0.7 million gain from a previously reserved investment. In 2010, we reported net other income of $7.7 million, which was driven by a $5.9 million non-cash gain on fair value of our minority interest in Artelis coincident with the acquisition of the remaining outstanding shares and a $2.5 million gain on the sale of Anji shares.
26
Provision for Income Taxes. In 2011, we had an effective income tax benefit rate of 48.8 percent, compared to a 2010 effective income tax rate of 22.0 percent. Excluding the $31.0 million benefit from the SDS Direct contract termination charge, the 2011 effective income tax rate would have been 26.2 percent. The 2011 effective income tax rate differs from the Federal statutory rate of 35.0 percent primarily due to the benefit of the SDS Direct contract termination charge, lower income tax rates in foreign jurisdictions, an increase in certain valuation allowances, the U.S. R&D tax credit, equity based compensation, and state taxes.
ATMI has not provided for U.S. federal income and foreign withholding taxes on approximately $92.4 million of undistributed earnings from non-U.S. operations as of December 31, 2011, because such earnings are intended to be reinvested indefinitely outside of the United States. These earnings could become subject to additional tax if they are remitted as dividends, loaned to ATMI, or upon sale of subsidiary stock. It is not practicable to estimate the amount or timing of the additional tax, if any, that eventually might be paid on the foreign earnings.
South Korea has granted the Company an income tax exemption that expires in 2014, including the last two years at 50 percent of the exemption. The exemption applies only to income related to one of the Company’s product lines. The effect of the tax exemption was to reduce income tax expense by $2.8 million, $2.1 million, and $1.3 million for the years ended December 31, 2011, 2010, and 2009.
At December 31, 2011, ATMI had $3.7 million of unrecognized tax benefits, which, if recognized, would favorably affect the effective income tax rate in future periods. $0.7 million of this amount is included in deferred taxes, and the balance of $3.0 million is included in the caption “Other non-current liabilities” on the consolidated balance sheets, together with $0.4 million of accrued interest (net) on tax reserves and $0 accrued for penalties. In January 2012, the Internal Revenue Service completed a U.S. tax audit of tax years 2008 and 2009, although the refund claim for tax year 2009 is still subject to review by the Joint Committee on Taxation.
Year Ended December 31, 2010 Compared to Year Ended December 31, 2009
Overview
During the year ended December 31, 2010, ATMI’s revenues increased by 44.2 percent compared to the year ended December 31, 2009. Growth was seen across all of our product lines and was primarily the result of higher customer demand driven by higher wafer starts and fab utilization and increased demand for life sciences products. Our gross margin increased by 780 basis points to 47.9 percent in 2010 compared to 40.1 percent in 2009, primarily because of sales volume increases as a result of the improving global economy and favorable product mix. Research and development expenses (“R&D”) grew 30.9 percent in 2010 from 2009. The increase was driven primarily by increased HPD licensing/maintenance and higher employee-related spending. SG&A increased by 11.9 percent in 2010 from 2009. The increase is primarily the result of increased employee-related expenses, travel and legal expenses. SG&A, as a percent of revenues, decreased to 23.3 percent in 2010 compared to 30.0 percent in 2009. Operating income increased to $41.9 million in 2010 from a loss of $11.3 million in 2009. In 2010, we recognized $8.4 million of gains on equity-method investments, due either to the sale of shares or re-measurement as a result of an acquisition. Our effective tax rate was 22.0 percent in 2010, compared to an effective tax benefit rate of 51.2 percent in 2009. In 2010, we generated net income of $39.5 million ($1.24 per diluted share) compared to a net loss of $6.7 million ($0.21 per diluted share) in 2009.
On November 2, 2010, ATMI’s Belgian subsidiary acquired the remaining 60 percent of the outstanding shares of Artelis S.A. The total accounting purchase consideration of $21.8 million included the cash payment of $4.0 million, the fair value of contingent payments tied to future revenue performance of $8.4 million, the carrying value of $5.9 million related to our original 40 percent non-controlling ownership interest, and assumed debt of $3.5 million. The contingent payments tied to future revenue performance have a range of possible outcomes from zero to a maximum of $23.3 million. In 2010, we had an effective income tax rate of 22.0 percent, compared to a 2009 effective income tax benefit rate of 51.2 percent. The 2010 income tax rate differs from the Federal statutory rate of 35.0 percent primarily due to the benefit of lower income tax rates in foreign jurisdictions, a non-taxable investment remeasurement gain from the Artelis acquisition, tax benefits on foreign dividends, an increase in certain valuation allowances, the U.S. R&D tax credit reinstatement, state taxes, and changes in established reserves.
The following is a summary of selected consolidated earnings information (in thousands of dollars):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | December 31, | | | | |
| | | 2010 | | | 2009 | | | % Change | |
Revenues | | $ | 367,256 | | | $ | 254,704 | | | | 44.2 | % |
Cost of revenues | | | 191,248 | | | | 152,520 | | | | 25.4 | % |
| | | | | | | | | | | | |
Gross profit | | | 176,008 | | | | 102,184 | | | | 72.2 | % |
Gross margin | | | 47.9 | % | | | 40.1 | % | | | 7.8 | % |
Research and development | | | 48,645 | | | | 37,162 | | | | 30.9 | % |
27
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | December 31, | | | | |
| | | 2010 | | | 2009 | | | % Change | |
R&D as a percent of revenues | | | 13.2 | % | | | 14.6 | % | | | (1.4 | %) |
Selling, general, and administrative | | | 85,425 | | | | 76,359 | | | | 11.9 | % |
SG&A as a percent of revenues | | | 23.3 | % | | | 30.0 | % | | | (6.7 | %) |
Operating income (loss) | | | 41,938 | | | | (11,337 | ) | | | 469.9 | % |
Operating margin | | | 11.4 | % | | | (4.5 | %) | | | 15.9 | % |
Effective tax rate | | | 22.0 | % | | | (51.2 | %) | | | 73.2 | % |
Net income (loss) | | $ | 39,506 | | | $ | (6,660 | ) | | | 693.2 | % |
Earnings per diluted share | | $ | 1.24 | | | $ | (0.21 | ) | | | 690.5 | % |
Revenues. Revenues increased 44.2 percent to $367.3 million in 2010 from $254.7 million in 2009. Growth was seen across all of our product lines and was primarily the result of higher customer demand driven by increased wafer starts and increased demand for life sciences products. Growth occurred in both our microelectronics and life sciences product lines, but was more pronounced in the microelectronics product lines, primarily as a result of improved wafer starts and fab utilization rates due in large part to the macroeconomic recovery, and also driven by strength in demand for our copper-related materials, especially in memory applications. Revenues in 2009 were also negatively impacted by excess inventory in the SDS distribution channel. Revenues in our microelectronics product lines grew 45.9 percent to $336.5 million in 2010 from $230.7 million in 2009. In our life sciences product line, revenues grew 28.1 percent to $30.7 million in 2010 compared to $24.0 million in 2009. The growth in life sciences revenues was driven by a combination of the continuing industry shift to disposables, which is driving demand for our single-use mixing and containment technologies. While demand, in general, has been improving, given the pressures to bring costs down in the consumer and microelectronics industries, we continued to experience pricing pressure with several of our older products, including SDS and our photo and copper material products. The effect of foreign currency was not significant in 2010 as declines associated with the U.S. dollar’s weakness against the Japanese Yen were partially offset by strengthening against the Euro.
Gross Profit. Gross profit increased 72.2 percent to $176.0 million in 2010 from $102.2 million in 2009. The increase in gross profit was driven primarily by increased revenues across our product lines as a result of improved economic conditions and a mix benefit driven by increased sales volumes in our copper materials, SDS, and life sciences product lines, partially offset by continued pricing pressure. Gross profit in 2009 was reduced by $3.1 million from asset impairment charges and by $2.1 million of expense to increase our reserves for excess and obsolete inventories. Gross profit in our microelectronics product lines increased 74.4 percent to $165.8 million in 2010 from $95.1 million in 2009. Gross profit margin in microelectronics was approximately 49 percent in 2010 compared to approximately 41 percent in 2009. Gross profit in our life sciences product lines increased 43.5 percent to $10.2 million in 2010 compared to $7.1 million for 2009. Gross profit margins in our life sciences product lines also improved from approximately 30 percent in 2009 to approximately 33 percent in 2010 driven by increased revenue volumes.
Research and Development Expenses. R&D increased 30.9 percent to $48.6 million in 2010 compared to $37.2 million in 2009 as we continued to fund our investments to develop advanced materials and HPD capabilities. The increase in 2010 spending was driven by increased HPD licensing and maintenance contract costs ($6.6 million), higher employee-related spending ($2.8 million) driven by increased incentives of $1.3 million and higher salaries $0.6 million, and equity-based compensation of $0.3 million, increased depreciation ($0.8 million), higher outside services spending ($0.6 million), increased travel ($0.5 million), maintenance ($0.4 million), increased consumption of materials and consumables ($0.4 million), and higher intellectual property costs ($0.5 million), partially offset by significantly lower asset impairments ($1.6 million). As a percentage of revenues, spending was lower in 2010 (13.2 percent) than in 2009 (14.6 percent), because of economic improvements, growth in wafer starts, and fab utilization rates which resulted in increased revenues.
Selling, General, and Administrative Expenses. SG&A increased 11.9 percent (or $9.1 million) to $85.4 million in 2010 from $76.4 million in 2009. SG&A, as a percentage of revenues, decreased to 23.3 percent in 2010 compared to 30.0 percent in 2009. The increase in 2010 spending was due to higher employee-related costs ($8.8 million) caused primarily by higher incentive compensation of $4.6 million, higher salaries on increased payroll of $0.9 million, and equity-based compensation of $1.5 million. We also increased spending in the following areas: Travel and entertainment ($1.8 million), employee recruitment ($1.4 million), and legal expenses ($1.3 million) which were driven by both business development and litigation activities. These spending increases were partially offset by increased distributor marketing reimbursements ($1.1 million), lower depreciation ($0.8 million) and significantly lower asset impairments ($2.2 million). The results in 2009 included $2.5 million of asset impairment charges related primarily to redundant enterprise management software and a $1.4 million charge to increase bad debt expense, due to customer bankruptcies and general economic conditions.
Operating Income (Loss).In 2010, we generated operating income of $41.9 million compared to an operating loss of $11.3 million in 2009. This change is from a variety of factors, as noted above.
Interest Income. Interest income decreased to $0.9 million in 2010 from $1.2 million in 2009. The primary reason for the decrease was lower average yields on invested balances.
28
Impairment of Investments.The results for 2009 included a $2.5 million impairment charge, primarily related to a write-down associated with an auction-rate security.
Other Income (Expense), Net. In 2010, other income (expense), net of $7.7 million was driven by a $5.9 million non-cash investment gain on our minority interest in Artelis, a $2.5 million gain from the sale of a portion of our equity investment in Anji, a $0.5 million gain from the sale of a marketable equity security and the release of notes receivable reserves of $0.4 million. In 2009, we recognized $1.2 million of losses from investments accounted for by the equity method and $0.2 million of realized and unrealized losses on foreign exchange.
Provision (Benefit) for Income Taxes. In 2010, we had an effective income tax rate of 22.0 percent, compared to a 2009 effective income tax benefit rate of 51.2 percent. The 2010 income tax rate differs from the Federal statutory rate of 35.0 percent primarily due to the benefit of lower income tax rates in foreign jurisdictions, a non-taxable investment remeasurement gain from the Artelis acquisition, tax benefits on foreign dividends, an increase in certain valuation allowances, the U.S. R&D tax credit reinstatement, state taxes, and changes in established reserves. The 2009 income tax benefit rate of 51.2 percent differs from the Federal statutory rate of 35.0 percent primarily due to the benefit of lower income tax rates in foreign jurisdictions, and a net $0.7 million reversal of previously established reserves, primarily resulting from the lapse of the applicable statutes of limitations. As of December 31, 2010, the Company had a net deferred tax liability on the balance sheet of $1.3 million, primarily because of temporary differences (i.e., accrued liabilities, inventory adjustments, equity-based compensation, and depreciation and amortization), state tax credit carry forwards, foreign and state net operating loss carry forwards, and R&D tax credits in Taiwan.
Liquidity and Capital Resources
We assess liquidity in terms of our ability to generate cash to fund our operating and investing activities. Of particular importance to management are cash flows generated by operating activities, cash used for capital expenditures, and cash used for acquisitions.
Until required for use in the business, we invest our cash reserves in bank deposits, certificates of deposit, money market securities, government and government-sponsored bond obligations, and other interest bearing marketable debt instruments in accordance with our investment policy. We have contracted with investment advisers to invest our funds consistent with our investment policy. The value of our investments may be adversely affected by increases in interest rates, instability in the global financial markets that reduces the liquidity of securities included in our portfolio, and by other factors which may result in other-than-temporary declines in value of the investments, which could impact our financial position and our overall liquidity. Each of these events may cause us to record charges to reduce the carrying value of our investment portfolio or sell investments for less than our acquisition cost. We attempt to mitigate these risks with the assistance of our investment advisors by investing in high-quality securities and monitoring the overall risk profile of our portfolio. We also maintain a well-diversified portfolio that limits our credit exposure through concentration limits set within our investment policy.
We have financed our operating needs and capital expenditures through cash flows from our operations, and existing cash. We expect to continue to finance current and planned operating requirements principally through cash from operations, as well as existing cash resources. We believe that these funds will be sufficient to meet our operating requirements for the foreseeable future. However, we may, from time to time, seek additional funding through a combination of equity and debt financings or from other sources.
We continue to invest in R&D to provide future sources of revenue through the development of new products, as well as through additional uses for existing products. We consider R&D and the development of new products and technologies an integral part of our growth strategy and a core competency of the Company. Likewise, we continue to make capital expenditures in order to expand and modernize manufacturing and R&D facilities around the globe and to drive efficiencies throughout the organization. Additionally, management considers, on a continuing basis, potential acquisitions of strategic technologies and businesses complementary to the Company’s current business.
See Part I, Item 1A, “Risk Factors” of this Form 10-K for risk factors that could negatively impact our cash position and ability to fund future operations.
29
A summary of our cash flows follows (in thousands):
| | | September 30, | | | | September 30, | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | |
Cash provided by (used for): | | | | | | | | |
Operating Activities | | $ | (30,962 | ) | | $ | 65,976 | |
Investing Activities | | | (2,471 | ) | | | (58,754 | ) |
Financing Activities | | | 379 | | | | (3,691 | ) |
Effects of exchange rate changes on cash | | | (1,071 | ) | | | 379 | |
Operating Activities
In 2011, we used $31.0 million of cash for operations, which represents a change of $96.9 million from the $66.0 million generated from operations in 2010. Cash used in operations in 2011 included an $84.6 million cash charge related to the Matheson contract termination, partially offset by $31.0 million in deferred taxes associated with the charge. Cash used for operations was also the result of increases in inventories and other assets, and decreases in accrued expenses. Partially offsetting these items were a decrease in accounts receivable and an increase in other liabilities. Inventories increased by $10.8 million in 2011 driven by stronger demand, $3.0 million of SDS purchases from Matheson in the fourth quarter, and an increase to other strategic safety stocks.
Investing Activities
Net cash used for investing activities decreased by $56.3 million in 2011 to $2.5 million. The primary reason for the decrease in 2011 was that we did not reinvest funds in marketable securities as existing marketable securities matured through the second half of the year in order to ensure liquid funds were on hand for the anticipated $95.0 million contract termination payment to Matheson. Other than changes in marketable securities, our investing activities primarily relate to purchases of property, plant and equipment and acquisitions. Cash used for investing activities in 2011 was driven by $20.7 million of capital expenditures, $10.4 million associated with the acquisition of license, manufacturing, distribution rights and a non-compete agreement from Matheson, and $6.7 million for a cost-basis investment. Cash used for investing activities in the year ended 2010 was driven by the $3.9 million acquisition of Artelis, minority equity investments, cash used for net purchases of marketable securities of $40.8 million, and $16.2 million of capital expenditures; partially offset by $5.2 million of cash generated from the sale of shares of one of our equity method investments.
Financing Activities
Financing activities generated cash of $0.4 million in 2011 compared to a use of cash of $3.7 million in 2010. The cash provided in 2011 was related to the proceeds from the exercise of stock options of $1.3 million, partially offset by share repurchases of $0.9 million. The $3.7 million use of cash in 2010 was comprised of share repurchases of $2.6 million, the repayment of a loan assumed in the Artelis acquisition of $2.4 million, and net repayments of credit lines of $0.5 million, partially offset by the proceeds from the exercise of stock options of $1.8 million.
30
Summary of Contractual Obligations
The following is a summary of consolidated debt, lease, purchase and other obligations at December 31, 2011 (see Notes 3, 6, 7, 9 and 14 of the consolidated financial statements in Item 8 of this Form 10-K), in thousands:
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | Payments Due by Period | |
| | | Total | | | Less Than
1 Year | | | 1-3 Years | | | 4-5 Years | | | Thereafter | |
Contractual Obligations: | | | | | | | | | | | | | | | | | | | | |
| | | | | |
Capital leases | | $ | 205 | | | $ | 48 | | | $ | 133 | | | $ | 24 | | | $ | — | |
| | | | | |
Operating leases | | $ | 12,306 | | | | 3,579 | | | | 5,011 | | | | 929 | | | | 2,787 | |
| | | | | |
Purchase obligations: | | | | | | | | | | | | | | | | | | | | |
Inventory | | $ | 15,622 | | | | 15,622 | | | | — | | | | — | | | | — | |
Capital | | $ | 954 | | | | 954 | | | | — | | | | — | | | | — | |
Other (1) | | $ | 10,188 | | | | 10,188 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | 26,764 | | | | 26,764 | | | | — | | | | — | | | | — | |
| | | | | |
Other L/T liabilities (2) | | $ | 5,145 | | | | — | | | | 4,756 | | | | — | | | | 389 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Total debt, lease, purchase, and other long-term liability obligations | | $ | 44,420 | | | $ | 30,391 | | | $ | 9,900 | | | $ | 953 | | | $ | 3,176 | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | Includes $7.6 million commitment to purchase R&D services associated with a strategic alliance partner. |
| (2) | Includes $3.6 million of asset retirement obligations. |
See Note 9 to the consolidated financial statements in Item 8 of this Form 10-K for further discussion of leases.
Off-Balance Sheet Arrangements
In the fourth quarter of 2011, we extended the contract term of an existing commitment with Intermolecular to purchase R&D technology and processes. The extension maintains our current R&D infrastructure and expense structures over the next three years. The cash payment commitments are $11.5 million, $5.0 million, $1.3 million, and $1.3 million in fiscal years 2011, 2012, 2013 and 2014, respectively. In 2011, there was a $1.3 million increase in commitments for that year due to the contract extension. The contract extension did not include any full time equivalent resource commitments beyond 2012.
Operations Outside the United States
For the years ended December 31, 2011, 2010 and 2009, sales outside the United States, including Asia and Europe, accounted for 79.4 percent, 82.3 percent and 80.2 percent, respectively, of our revenues. Sales to Taiwan for the years ended December 31, 2011, 2010 and 2009 were 25.0 percent, 24.3 percent and 21.8 percent, respectively, of our revenues. Sales to Japan for the years ended December 31, 2011, 2010 and 2009 were 11.1 percent, 13.5 percent and 14.3 percent, respectively, of our revenues. Sales to South Korea for the years ended December 31, 2011, 2010 and 2009 were 20.3 percent, 18.4 percent and 20.2 percent, respectively, of our revenues. Management anticipates that sales outside the United States will continue to account for a significant percentage of our total revenues. ATMI has wholly-owned subsidiaries in Taiwan, Singapore, China, Japan, and Germany where the Company sells and services several product lines. ATMI also has a wholly-owned subsidiary in South Korea that manufactures, sells, and distributes materials packaging, materials delivery equipment, and thin-film materials to the semiconductor and flat-panel display markets in South Korea, and NOWPak® to the Taiwan and Japanese markets. In addition, ATMI has two wholly-owned subsidiaries in Belgium that manufacture and sell high-purity materials packaging products primarily to the life sciences industry.
31
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
As of December 31, 2011, the Company’s cash and cash equivalents and marketable securities included bank deposits, time deposits, money market securities, and government and government-sponsored bond obligations. As of December 31, 2011, an increase of 100 basis points in interest rates on securities with maturities greater than one year would reduce the fair value of the Company’s marketable securities portfolio by approximately $0.4 million. Conversely, a reduction of 100 basis points in interest rates on securities with maturities greater than one year would increase the fair value of the Company’s marketable securities portfolio by approximately $0.5 million.
Foreign Currency Exchange Risk
Most of the Company’s sales are denominated in U.S. dollars and as a result, the Company has limited exposure to foreign currency exchange risk with respect to sales made. Approximately 40 percent of the Company’s revenues for the year ended December 31, 2011 were denominated in Japanese Yen (“JPY”), Korean Won (“KRW”), and Euros (“EUR”), but a majority of the product is sourced in U.S. dollars. Management periodically reviews the Company’s exposure to currency fluctuations. This exposure may change over time as business practices evolve and could have a material effect on the Company’s financial results in the future. We use forward foreign exchange contracts to hedge specific exposures relating to intercompany payments and anticipated, but not yet committed, intercompany sales (primarily parent company export sales to subsidiaries at pre-established U.S. dollar prices). The terms of the forward foreign exchange contracts are generally matched to the underlying transaction being hedged, and are typically under one year.
Because such contracts are directly associated with identified transactions, they are an effective hedge against fluctuations in the value of the foreign currency underlying the transaction. We recognize in earnings (other income (expense), net) changes in the fair value of all derivatives designated as fair value hedges. We generally do not hedge overseas sales denominated in foreign currencies or translation exposures. Further, we do not enter into derivative instruments for trading or speculative purposes and all of our derivatives were highly effective throughout the periods reported.
At December 31, 2011, we held forward foreign currency exchange contracts as economic hedges with notional amounts totaling $22.0 million, which are being used to hedge recorded foreign denominated liabilities and which will be settled in either JPY, KRW, EUR, or New Taiwan Dollars (“NTD”). Holding other variables constant, if there were a 10 percent decline in foreign exchange rates against the US dollar for the JPY, KRW, EUR and NTD, the fair market value of the foreign exchange contracts outstanding at December 31, 2011 would increase by approximately $0.3 million, which would be expected to be fully offset by foreign exchange gains on the amounts being hedged. The effect of an immediate 10 percent change in other foreign exchange rates would not be expected to have a material effect on the Company’s future operating results or cash flows.
Changes in Market Risk
Although we have seen mixed signs of stability, the global economy continues to deal with the repercussions of the global recession, as manifest in the recent European economic crisis that caused significant credit and financial market swings. There can be no assurance that there will not be further deterioration in credit and financial markets and deterioration of confidence in economic conditions. These economic uncertainties affect businesses such as ours in a number of ways, making it difficult to accurately forecast and plan our future business activities.
32
Item 8. Financial Statements and Supplementary Data
The Reports of Independent Registered Public Accounting Firm, the consolidated financial statements and the financial statement schedule that are listed in the Index to Consolidated Financial Statements and Financial Statement Schedule are included herein on pages F-1 through F-36.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Form 10-K. There are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures. Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives. Based upon this evaluation, our CEO and CFO concluded that, as of the end of the period covered by this Form 10-K, our disclosure controls and procedures were effective in that they provided reasonable assurance that the information we are required to disclose in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the applicable rules and forms, and that it is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with accounting principles generally accepted in the United States of America. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Our management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2011. In making its assessment, management has used the criteria set forth by the Committee of Sponsoring Organizations (COSO) of the Treadway Commission inInternal Control—Integrated Framework.Our management concluded that based on its assessment, our internal control over financial reporting was effective as of December 31, 2011. The Company’s internal control over financial reporting as of December 31, 2011 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their attestation report on the Company’s internal control over financial reporting, which is included in this annual report.
There has been no change in our internal control over financial reporting during the quarter ended December 31, 2011 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
33
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information required by Item 10 has been omitted from this report, and is incorporated by reference to the sections “Section 16(a) Beneficial Ownership Reporting Compliance,” “Election of Directors,” “Board Operations” and “Executive Officers” in our definitive Proxy Statement for the 2012 Annual Meeting of Stockholders, which we will file with the SEC pursuant to Regulation 14A within 120 days after the end of our 2011 fiscal year.
Item 11. Executive Compensation
Information required by Item 11 has been omitted from this report and is incorporated by reference to the sections “Board Operations” and “Compensation and Other Information Concerning Executive Officers and Directors” in our definitive Proxy Statement for the 2012 Annual Meeting of Stockholders, which we will file with the SEC pursuant to Regulation 14A within 120 days after the end of our 2011 fiscal year.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information required by Item 12 has been omitted from this report and is incorporated by reference to the sections “Stock Ownership” and “Equity Compensation Plan Information” in our definitive Proxy Statement for the 2012 Annual Meeting of Stockholders, which we will file with the SEC pursuant to Regulation 14A within 120 days after the end of our 2011 fiscal year.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information required by Item 13 has been omitted from this report and is incorporated by reference to the sections “Certain Relationships and Related Transactions” and “Board Operations” in our definitive Proxy Statement for the 2012 Annual Meeting of Stockholders, which we will file with the SEC pursuant to Regulation 14A within 120 days after the end of our 2011 fiscal year.
Item 14. Principal Accountant Fees and Services
Information required by Item 14 has been omitted from this report and is incorporated by reference to the section “Fees of Independent Registered Public Accounting Firm and Report of the Audit Committee” in our definitive Proxy Statement for the 2012 Annual Meeting of Stockholders, which we will file with the SEC pursuant to Regulation 14A within 120 days after the end of our 2011 fiscal year.
34
PART IV
Item 15. Exhibits and Financial Statement Schedules
The following documents are filed as part of this annual report on Form 10-K:
Reports of Independent Registered Public Accounting Firm
Consolidated Balance Sheets—December 31, 2011 and 2010
Consolidated Statements of Operations for the years ended December 31, 2011, 2010 and 2009
Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2011, 2010 and 2009
Consolidated Statements of Cash Flows for the years ended December 31, 2011, 2010 and 2009
Notes to Consolidated Financial Statements
| (2) | Financial Statement Schedule: |
Schedule II—Valuation and Qualifying Accounts
The Reports of Independent Registered Public Accounting Firm, consolidated financial statements and financial statement schedule listed in the Index to Consolidated Financial Statements and Financial Statement Schedule on page F-1 hereof are filed as part of this report, commencing on page F-2 hereof.
All other financial statement schedules are omitted as the required information is not applicable or the information is shown in the consolidated financial statements or related notes.
The documents set forth below are filed herewith or incorporated herein by reference to the location indicated.
| | |
Exhibit No. | | Description |
| |
| 3.01 | | Restated Certificate of Incorporation dated as of July 30, 2005 (Filed as Exhibit 3 (i) to ATMI’s Current Report on Form 8-K on August 3, 2005, File No. 1-16239, and incorporated herein by reference). |
| |
| 3.02 | | Amended and Restated Bylaws of ATMI dated December 17, 2008 (Filed as Exhibit 3.1 to ATMI’s Current Report on Form 8-K on December 18, 2008, File No. 1-16239, and incorporated herein by reference). |
| |
| 4.01 | | Specimen of ATMI’s Common Stock Certificate (Filed on September 10, 1997 as Exhibit 4.01 to the Company’s Registration Statement on Form S-4, Registration No. 333-35323). |
| |
| 10.01* | | Employment Agreement between Daniel P. Sharkey and ATMI, Inc. dated December 31, 2004 (Filed as Exhibit 10.1 to ATMI’s Current Report on Form 8-K on January 5, 2005, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.02* | | Employment Agreement between Douglas A. Neugold and ATMI, Inc. dated December 31, 2004 (Filed as Exhibit 10.1 to ATMI’s Current Report on Form 8-K/A on January 5, 2005, File No. 1-16239, and incorporated herein by reference). |
35
| | |
Exhibit No. | | Description |
| |
| 10.03* | | Agreement of Lease between Melvyn J. Powers and Mary P. Powers d/b/a/ M&M Realty and Advanced Technology Materials, Inc., dated December 23, 1994 (Filed as Exhibit 10.04 to ATMI’s 2008 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.04 | | First Amendment to Agreement of Lease dated as of November 22, 2000 by and between Commerce Park Realty, LLC and Advanced Technology Materials, Inc. (Filed as Exhibit 10.05 to ATMI’s 2005 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.05 | | Second Amendment to Agreement of Lease dated as of March 24, 2003 by and between Commerce Park Realty, LLC and Advanced Technology Materials, Inc. (Filed as Exhibit 10.05 to ATMI’s 2005 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.06* | | ATMI, Inc. 1995 Stock Plan (Filed as Exhibit 10.07 to ATMI’s 2008 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.07* | | ATMI, Inc. 1997 Stock Plan, dated October 10, 1997 (Filed on April 6, 1998 as Exhibit 99.1 to the Company’s Registration Statement on Form S-8, Registration No. 333-49561). |
| |
| 10.08* | | ATMI, Inc. 1998 Stock Plan, dated May 20, 1998 (Filed on June 9, 1998 as Exhibit 99.1 to the Company’s Registration Statement on Form S-8, Registration No. 333-56349). |
| |
| 10.09 | | Agreement of Lease between Seymour R. Powers, Leon Griss and Ruth Griss and Advanced Technology Materials, Inc., dated November 24, 2000 (Filed as Exhibit 10.10 to ATMI’s 2008 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.10* | | ATMI, Inc. 2000 Stock Plan, dated May 24, 2000 (Filed on September 20, 2000 as Exhibit 99.1 to the Company’s Registration Statement on Form S-8, Registration No. 333-46222). |
| |
| 10.11* | | ATMI, Inc. 2003 Stock Plan (as amended May 21, 2003), (Filed on August 1, 2003 as Exhibit 4.6 to the Company’s Registration Statement on Form S-8, Registration No. 333-107591). |
| |
| 10.12* | | ATMI, Inc. 1998 Employee Stock Purchase Plan, (as amended February 28, 2003), (Filed on August 1, 2003 as Exhibit 4.7 to the Company’s Registration Statement on Form S-8, Registration No. 333-107591). |
| |
| 10.13 | | Alliance Agreement dated as of May 16, 2003 by and between Advanced Technology Materials, Inc. on its own behalf and on behalf of its Affiliates and Enthone, Inc. (Filed as Exhibit 10.15 to ATMI’s 2003 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). (1) |
| |
| 10.14* | | Non-Employee Directors Deferred Compensation Program of ATMI, Inc. 1998 Stock Plan (Filed as Exhibit 10.19 to ATMI’s 2005 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.15* | | Form of ATMI, Inc. Executive Management Restricted Stock Grant Agreement (Filed as Exhibit 10.20 to ATMI’s 2006 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
36
| | |
Exhibit No. | | Description |
| |
| 10.16* | | Form of ATMI, Inc. Employee Non-Qualified Stock Option Agreement (Filed as Exhibit 10.21 to ATMI’s 2006 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.17 | | Agreement of Lease between West Real Estate and Management, Inc. and ATMI Packaging, Inc., and ATMI, Inc., dated October 21, 2004 (Filed as Exhibit 10.23 to ATMI’s 2004 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.18* | | Form of ATMI, Inc. Employee Restricted Stock Grant Agreement – Non-Executive Management (Filed as Exhibit 10.23 to ATMI’s 2006 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.19* | | Form of ATMI, Inc. Non-Employee Director Restricted Stock Grant (Filed as Exhibit 10.24 to ATMI’s 2006 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.20 | | First Amendment to Agreement of Lease dated as of March 24, 2003 by and between Seymour R. Powers, Trustee, and Leon Griss and Advanced Technology Materials, Inc. (Filed as Exhibit 10.25 to ATMI’s 2005 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.21* | | Amendment to ATMI’s 1998 Employee Stock Purchase Plan (amended effective February 28, 2003 and January 1, 2007), (Filed as Exhibit 99 to ATMI’s Form 10-Q for the quarter ended September 30, 2006, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.22 | | Second Amendment to Agreement of Lease dated as of January 18, 2007 by and between Seymour R. Powers, Trustee, and Carole Kolsky, Deborah A. Tauber and Stephen L. Griss (as successors to Leon Griss and Ruth Griss) and Advanced Technology Materials, Inc. (Filed as Exhibit 10.27 to ATMI’s 2006 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.23 | | Third Amendment to Agreement of Lease dated as of January 18, 2007 by and between Commerce Park Realty, LLC and Advanced Technology Materials, Inc. (Filed as Exhibit 10.28 to ATMI’s 2006 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.24* | | Form of ATMI, Inc. Non-Employee Directors Non-Qualified Stock Option Agreement (Filed as Exhibit 10.29 to ATMI’s 2006 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.25* | | Employment Agreement between Timothy C. Carlson and ATMI, Inc. dated December 31, 2004 (Filed as Exhibit 10.31 to ATMI’s 2007 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.26* | | Amendment to Employment Agreement between Timothy C. Carlson and ATMI, Inc. dated August 1, 2005 (Filed as Exhibit 10.32 to ATMI’s 2007 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.27* | | Employment Agreement between Ellen Harmon and ATMI, Inc. dated January 14, 2008 (Filed as Exhibit 10.33 to ATMI’s 2007 Annual Report Form 10-K, File No. 1-16239, and incorporated herein by reference). |
37
| | |
Exhibit No. | | Description |
| |
| 10.28* | | Amendment to Employment Agreement between Timothy C. Carlson and ATMI, Inc. dated January 31, 2008, effective as of September 7, 2007 (Filed as Exhibit 10.34 to ATMI’s 2007 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.29* | | Amendment to Employment Agreement between Daniel P. Sharkey and ATMI, Inc. dated January 31, 2008, effective as of September 7, 2007 (Filed as Exhibit 10.35 to ATMI’s 2007 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.30 | | First Amendment to Agreement of Lease between West Real Estate and Management, Inc. and ATMI Packaging, Inc., and ATMI, Inc., dated September 23, 2008 (Filed as Exhibit 10.32 to ATMI’s 2008 Annual Report on Form 10-K, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.31* | | Amendment to Employment Agreement between Douglas A. Neugold and ATMI, Inc. dated April 14, 2008 (Filed as Exhibit 10.2 to ATMI’s Current Report on Form 8-K, filed on April 16, 2008, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.32* | | Amendment to Employment Agreement between Daniel P. Sharkey and ATMI, Inc. dated April 14, 2008 (Filed as Exhibit 10.3 to ATMI’s Current Report on Form 8-K, filed on April 16, 2008, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.33* | | Amendment to Employment Agreement between Timothy C. Carlson and ATMI, Inc. dated April 14, 2008 (Filed as Exhibit 10.4 to ATMI’s Current Report on Form 8-K, filed on April 16, 2008, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.34* | | Amendment to Employment Agreement between Ellen Harmon and ATMI, Inc. dated April 14, 2008 (Filed as Exhibit 10.5 to ATMI’s Current Report on Form 8-K, filed on April 16, 2008, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.35* | | First Amendment to Non-Employee Directors Deferred Compensation Program of ATMI, Inc. 1998 Stock Plan (Filed as Exhibit 10.1 to ATMI’s Current Report on Form 8-K, filed on March 6, 2008, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.36* | | Amendment to The ATMI, Inc. 1998 Stock Plan (Filed as Exhibit 10.2 to ATMI’s Current Report on Form 8-K, filed on March 6, 2008, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.37* | | Amendment to The ATMI, Inc. 2000 Stock Plan (Filed as Exhibit 10.3 to ATMI’s Current Report on Form 8-K, filed on March 6, 2008, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.38* | | ATMI, Inc. 2003 Stock Plan (as Amended May 21, 2003 and March 2, 2008) (Filed as Exhibit 10.4 to ATMI’s Current Report on Form 8-K, filed on March 6, 2008, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.39* | | ATMI, Inc. Non-Employee Directors Deferred Compensation Program of ATMI, Inc. 2003 Stock Plan (Filed as Exhibit 10.5 to ATMI’s Current Report on Form 8-K, filed on March 6, 2008, File No. 1-16239, and incorporated herein by reference). |
38
| | |
Exhibit No. | | Description |
| |
| 10.40 | | Third Amendment to Lease dated as of October 30, 2008 by and between Seymour R. Powers, Trustee, and Carole Kolsky, Deborah A. Tauber and Stephen L. Griss (as successors to Leon Griss and Ruth Griss) and Advanced Technology Materials, Inc. (Filed as Exhibit 10.41 to ATMI’s Annual Report on Form 10-K, filed on February 18, 2011, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.41 | | Fourth Amendment to Agreement of Lease dated as of October 30, 2008 by and between Commerce Park Realty, LLC and Advanced Technology Materials, Inc. (Filed as Exhibit 10.42 to ATMI’s Annual Report on Form 10-K, filed on February 18, 2011, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.42* | | ATMI, Inc. 2010 Stock Plan, dated May 26, 2010 (Filed on June 4, 2010 as Exhibit 4.4 to the Company’s Registration Statement on Form S-8, Registration No. 333-167318). |
| |
| 10.43* | | ATMI, Inc. Non-Employee Directors Deferred Compensation Program of ATMI, Inc. 2010 Stock Plan. (Filed as Exhibit 10.44 to ATMI’s Annual Report on Form 10-K, filed on February 18, 2011, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.44 | | Notice of First Renewal Option of Agreement of Lease dated as of October 27, 2010 by and between Seymour R. Powers, Trustee, and Carole Kolsky, Deborah A. Tauber and Stephen L. Griss (as successors to Leon Griss and Ruth Griss) and Advanced Technology Materials, Inc. (Filed as Exhibit 10.45 to ATMI’s Annual Report on Form 10-K, filed on February 18, 2011, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.45 | | Notice of First Renewal Option of Agreement of Lease dated as of December 13, 2010 by and between Seymour R. Powers, Trustee, and Carole Kolsky, Deborah A. Tauber and Stephen L. Griss (as successors to Leon Griss and Ruth Griss) and Advanced Technology Materials, Inc. (Filed as Exhibit 10.46 to ATMI’s Annual Report on Form 10-K, filed on February 18, 2011, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.46* | | Amended and Restated Employment Agreement dated January 19, 2012 between ATMI, Inc. and Douglas A. Neugold (Filed as Exhibit 10.1 to ATMI’s Current Report on Form 8-K filed on January 23, 2012, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.47* | | Amended and Restated Employment Agreement dated January 19, 2012 between ATMI, Inc. and Timothy C. Carlson (Filed as Exhibit 10.2 to ATMI’s Current Report on Form 8-K filed on January 23, 2012, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.48* | | Amended and Restated Employment Agreement dated January 19, 2012 between ATMI, Inc. and Daniel P. Sharkey (Filed as Exhibit 10.3 to ATMI’s Current Report on Form 8-K filed on January 23, 2012, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.49* | | Amended and Restated Employment Agreement dated January 19, 2012 between ATMI, Inc. and Ellen T. Harmon (Filed as Exhibit 10.4 to ATMI’s Current Report on Form 8-K filed on January 23, 2012, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.50* | | Form of Total Shareholder Return Performance Restricted Stock Unit Agreement (2012 and 2013 Grants) (Filed as Exhibit 10.5 to ATMI’s Current Report on Form 8-K filed on January 23, 2012, File No. 1-16239, and incorporated herein by reference). |
| |
| 10.51* | | Form of Total Shareholder Return Performance Restricted Stock Unit Agreement (2014 and later Grants) (Filed as Exhibit 10.6 to ATMI’s Current Report on Form 8-K filed on January 23, 2012, File No. 1-16239, and incorporated herein by reference). |
39
| | |
Exhibit No. | | Description |
| |
| 10.52 | | Termination Agreement dated October 31, 2011 between Advanced Technology Materials, Inc. and Matheson Tri-Gas, Inc. |
| |
| 21 | | Subsidiaries of ATMI, Inc. |
| |
| 23 | | Consent of Independent Registered Public Accounting Firm. |
| |
| 31.1 | | Certification of Chief Executive Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended. |
| |
| 31.2 | | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as amended. |
| |
| 32 | | Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 is furnished herewith. |
| |
| 101 | | Interactive data files pursuant to Rule 405 of Regulation S-T. |
| * | Indicates a management contract or compensatory plan or arrangement. |
| (1) | Portions omitted pursuant to a request for confidential treatment. |
40
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | |
| | | | ATMI, Inc. |
| | | |
| February 17, 2012 | | | | By: | | /s/ Douglas A. Neugold |
| | | | | | Douglas A. Neugold |
| | | | | | Chairman, President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/s/ Douglas A. Neugold Douglas A. Neugold | | Chairman,President, Chief Executive Officer and Director (principal executive officer) | | February 17, 2012 |
| | |
/s/ Timothy C. Carlson Timothy C. Carlson | | Executive Vice President, Chief Financial Officer and Treasurer (principal financial officer) | | February 17, 2012 |
| | |
/s/ David M. Ward David M. Ward | | Vice President, Controller (principal accounting officer) | | February 17, 2012 |
| | |
/s/ Mark A. Adley Mark A. Adley | | Director | | February 17, 2012 |
| | |
/s/ Eugene G. Banucci, Ph.D. Eugene G. Banucci, Ph.D. | | Director | | February 17, 2012 |
| | |
/s/ Robert S. Hillas Robert S. Hillas | | Director | | February 17, 2012 |
| | |
/s/ Stephen H. Mahle Stephen H. Mahle | | Director | | February 17, 2012 |
| | |
/s/ C. Douglas Marsh C. Douglas Marsh | | Director | | February 17, 2012 |
| | |
/s/ George M. Scalise George M. Scalise | | Director | | February 17, 2012 |
| | |
/s/ Cheryl L. Shavers, Ph.D. Cheryl L. Shavers, Ph.D. | | Director | | February 17, 2012 |
41
ATMI, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS AND FINANCIAL STATEMENT
SCHEDULE
F-1
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of ATMI, Inc.
We have audited the accompanying consolidated balance sheets of ATMI, Inc. as of December 31, 2011 and 2010, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2011. Our audits also included the financial statement schedule listed in the Index at Item 15. These financial statements and schedule are the responsibility of ATMI, Inc.’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of ATMI, Inc. at December 31, 2011 and 2010, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2011, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), ATMI, Inc.’s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 17, 2012 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
Stamford, Connecticut
February 17, 2012
F-2
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of ATMI, Inc.
We have audited ATMI, Inc.’s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). ATMI, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in Item 9A, “Management’s Report on Internal Control Over Financial Reporting.” Our responsibility is to express an opinion on ATMI, Inc.’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, ATMI, Inc. maintained in all material respects, effective internal control over financial reporting as of December 31, 2011, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of ATMI, Inc. as of December 31, 2011 and 2010, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2011 and our report dated February 17, 2012 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
Stamford, Connecticut
February 17, 2012
F-3
ATMI, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except per share data)
| | | September 30, | | | | September 30, | |
| | | December 31, | | | December 31, | |
| | | 2011 | | | 2010 | |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 34,523 | | | $ | 68,648 | |
Marketable securities, current portion | | | 75,632 | | | | 58,495 | |
Accounts receivable, net of allowances of $781 and $783, respectively | | | 51,563 | | | | 54,518 | |
Inventories, net | | | 73,622 | | | | 62,832 | |
Income taxes receivable | | | 3,721 | | | | 4,627 | |
Deferred income taxes | | | 4,409 | | | | 6,801 | |
Prepaid expenses | | | 14,439 | | | | 14,384 | |
Other current assets | | | 13,279 | | | | 12,695 | |
| | | | | | | | |
Total current assets | | | 271,188 | | | | 283,000 | |
| | |
Property, plant, and equipment, net | | | 116,275 | | | | 119,053 | |
Goodwill | | | 46,546 | | | | 46,981 | |
Other intangibles, net | | | 36,571 | | | | 28,948 | |
Marketable securities, non-current | | | 3,329 | | | | 25,429 | |
Deferred income taxes, non-current | | | 19,139 | | | | 2,097 | |
Other non-current assets | | | 20,638 | | | | 28,081 | |
| | | | | | | | |
Total assets | | $ | 513,686 | | | $ | 533,589 | |
| | | | | | | | |
Liabilities and stockholders’ equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | | 20,779 | | | $ | 21,045 | |
Accrued liabilities | | | 7,940 | | | | 5,918 | |
Accrued salaries and related benefits | | | 10,469 | | | | 12,163 | |
Income taxes payable | | | 1,229 | | | | 3,700 | |
Other current liabilities | | | 4,539 | | | | 3,911 | |
| | | | | | | | |
Total current liabilities | | | 44,956 | | | | 46,737 | |
| | |
Deferred income taxes, non-current | | | 162 | | | | 10,245 | |
Other non-current liabilities | | | 18,237 | | | | 18,182 | |
Commitments and contingencies (Note 14) | | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Preferred stock, par value $.01 per share: 2,000 shares authorized; none issued | | | — | | | | — | |
Common stock, par value $.01 per share: 100,000 shares authorized; | | | | | | | | |
39,912 and 39,640 issued and 31,718 and 31,495 outstanding in 2011 and 2010, respectively | | | 399 | | | | 396 | |
Additional paid-in capital | | | 444,642 | | | | 435,840 | |
Treasury stock at cost (8,194 and 8,145 shares in 2011 and 2010, respectively) | | | (231,173 | ) | | | (230,272 | ) |
Retained earnings | | | 228,414 | | | | 248,433 | |
Accumulated other comprehensive income | | | 8,049 | | | | 4,028 | |
| | | | | | | | |
Total stockholders’ equity | | | 450,331 | | | | 458,425 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 513,686 | | | $ | 533,589 | |
| | | | | | | | |
See accompanying notes.
F-4
ATMI, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share data)
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
Revenues | | $ | 390,087 | | | $ | 367,256 | | | $ | 254,704 | |
Cost of revenues | | | 205,957 | | | | 191,248 | | | | 152,520 | |
| | | | | | | | | | | | |
Gross profit | | | 184,130 | | | | 176,008 | | | | 102,184 | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 53,708 | | | | 48,645 | | | | 37,162 | |
Selling, general and administrative | | | 83,546 | | | | 85,425 | | | | 76,359 | |
Contract termination | | | 84,590 | | | | — | | | | — | |
| | | | | | | | | | | | |
Total operating expenses | | | 221,844 | | | | 134,070 | | | | 113,521 | |
| | | | | | | | | | | | |
Operating income (loss) | | | (37,714 | ) | | | 41,938 | | | | (11,337 | ) |
Interest income | | | 1,205 | | | | 949 | | | | 1,196 | |
Impairment of investments | | | (776 | ) | | | — | | | | (2,486 | ) |
Other income (expense), net | | | (1,849 | ) | | | 7,740 | | | | (1,029 | ) |
| | | | | | | | | | | | |
Income (loss) before income taxes | | | (39,134 | ) | | | 50,627 | | | | (13,656 | ) |
Provision (benefit) for income taxes | | | (19,115 | ) | | | 11,121 | | | | (6,996 | ) |
| | | | | | | | | | | | |
Net income (loss) | | $ | (20,019 | ) | | $ | 39,506 | | | $ | (6,660 | ) |
| | | | | | | | | | | | |
| | | |
Earnings (loss) per common share—basic | | $ | (0.63 | ) | | $ | 1.26 | | | $ | (0.21 | ) |
| | | |
Weighted average shares outstanding—basic | | | 31,703 | | | | 31,413 | | | | 31,398 | |
| | | |
Earnings (loss) per common share—diluted | | $ | (0.63 | ) | | $ | 1.24 | | | $ | (0.21 | ) |
| | | |
Weighted average shares outstanding—diluted | | | 31,703 | | | | 31,895 | | | | 31,398 | |
See accompanying notes.
F-5
ATMI, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands)
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | Common
Stock | | | Additional
Paid-in
Capital | | | Treasury
Stock | | | Retained
Earnings | | | Accumulated
Other
Comprehensive
Income | | | Total | |
Balance at December 31, 2008 | | | 392 | | | | 421,040 | | | | (227,101 | ) | | | 214,300 | | | | 266 | | | | 408,897 | |
Issuance of 15 shares of common stock pursuant to the exercise of employee stock options | | | — | | | | 236 | | | | — | | | | — | | | | — | | | | 236 | |
Purchase of 35 treasury shares | | | — | | | | — | | | | (569 | ) | | | — | | | | — | | | | (569 | ) |
Equity based compensation | | | — | | | | 5,740 | | | | — | | | | — | | | | — | | | | 5,740 | |
Income tax deficiency from equity-based compensation | | | — | | | | (579 | ) | | | — | | | | — | | | | — | | | | (579 | ) |
Other | | | 1 | | | | (1 | ) | | | — | | | | — | | | | — | | | | — | |
Cumulative effect of adoption of new accounting standard | | | — | | | | — | | | | — | | | | 1,287 | | | | (1,287 | ) | | | — | |
Net loss | | | — | | | | — | | | | — | | | | (6,660 | ) | | | — | | | | (6,660 | ) |
Reclassification adjustment related to marketable securities sold in net unrealized gain position, net of $32 tax provision | | | — | | | | — | | | | — | | | | — | | | | (55 | ) | | | (55 | ) |
Change in fair value on available-for-sale securities, net of deferred income tax of $1,139 | | | — | | | | — | | | | — | | | | — | | | | 1,940 | | | | 1,940 | |
Cumulative translation adjustment | | | — | | | | — | | | | — | | | | — | | | | 2,540 | | | | 2,540 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (2,235 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2009 | | | 393 | | | | 426,436 | | | | (227,670 | ) | | | 208,927 | | | | 3,404 | | | | 411,490 | |
Issuance of 104 shares of common stock pursuant to the exercise of employee stock options | | | 1 | | | | 1,818 | | | | — | | | | — | | | | — | | | | 1,819 | |
Purchase of 179 treasury shares | | | — | | | | — | | | | (2,602 | ) | | | — | | | | — | | | | (2,602 | ) |
Equity based compensation | | | — | | | | 7,693 | | | | — | | | | — | | | | — | | | | 7,693 | |
Income tax deficiency from equity-based compensation | | | — | | | | (105 | ) | | | — | | | | — | | | | — | | | | (105 | ) |
Other | | | 2 | | | | (2 | ) | | | — | | | | — | | | | — | | | | — | |
Net income | | | — | | | | — | | | | — | | | | 39,506 | | | | — | | | | 39,506 | |
Reclassification adjustment related to marketable securities sold in net unrealized gain position, net of $282 tax provision | | | — | | | | — | | | | — | | | | — | | | | (481 | ) | | | (481 | ) |
Change in fair value on available-for-sale securities, net of deferred income tax of $201 | | | — | | | | — | | | | — | | | | — | | | | 343 | | | | 343 | |
Cumulative translation adjustment | | | — | | | | — | | | | — | | | | — | | | | 762 | | | | 762 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income | | | — | | | | — | | | | — | | | | — | | | | — | | | | 40,130 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | $ | 396 | | | $ | 435,840 | | | $ | (230,272 | ) | | $ | 248,433 | | | $ | 4,028 | | | $ | 458,425 | |
Issuance of 71 shares of common stock pursuant to the exercise of employee stock options | | | 1 | | | | 1,265 | | | | — | | | | — | | | | — | | | | 1,266 | |
Purchase of 48 treasury shares | | | — | | | | — | | | | (901 | ) | | | — | | | | — | | | | (901 | ) |
Equity based compensation | | | — | | | | 7,539 | | | | — | | | | — | | | | — | | | | 7,539 | |
Other | | | 2 | | | | (2 | ) | | | — | | | | — | | | | — | | | | — | |
Net loss | | | — | | | | — | | | | — | | | | (20,019 | ) | | | — | | | | (20,019 | ) |
Reclassification adjustment related to marketable securities sold in net unrealized gain position, net of $0 tax provision | | | — | | | | — | | | | — | | | | — | | | | (1 | ) | | | (1 | ) |
Change in fair value on available-for-sale securities, net of deferred income tax of $4,351 | | | — | | | | — | | | | — | | | | — | | | | 7,119 | | | | 7,119 | |
Cumulative translation adjustment | | | — | | | | — | | | | — | | | | — | | | | (3,097 | ) | | | (3,097 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income | | | — | | | | — | | | | — | | | | — | | | | — | | | | (15,998 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | $ | 399 | | | $ | 444,642 | | | $ | (231,173 | ) | | $ | 228,414 | | | $ | 8,049 | | | $ | 450,331 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes.
F-6
ATMI, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
Operating activities | | | | | | | | | | | | |
Net income (loss) | | $ | (20,019 | ) | | $ | 39,506 | | | $ | (6,660 | ) |
Adjustments to reconcile net income (loss) to cash (used for) provided by operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 27,162 | | | | 26,718 | | | | 26,799 | |
Deferred income taxes | | | (29,443 | ) | | | 4,587 | | | | (1,880 | ) |
Stock-based compensation expense | | | 7,539 | | | | 7,693 | | | | 5,740 | |
Gain on remeasurement of contingent consideration | | | (1,733 | ) | | | — | | | | — | |
Gain on remeasurement of non-controlling interest | | | — | | | | (5,923 | ) | | | — | |
Long-lived asset impairments | | | 594 | | | | 544 | | | | 7,298 | |
Impairment of investments | | | 776 | | | | — | | | | 2,486 | |
Other | | | 2,337 | | | | (1,050 | ) | | | 4,343 | |
Changes in operating assets and liabilities, net of effect of acquisitions: | | | | | | | | | | | | |
Accounts receivable | | | 3,986 | | | | (9,034 | ) | | | (2,790 | ) |
Inventories | | | (12,390 | ) | | | (9,197 | ) | | | 400 | |
Other assets | | | (8,233 | ) | | | (4,680 | ) | | | (3,767 | ) |
Accounts payable | | | (161 | ) | | | 5,169 | | | | 1,808 | |
Accrued expenses, income taxes and other liabilities | | | (1,377 | ) | | | 11,643 | | | | (5,016 | ) |
| | | | | | | | | | | | |
Net cash (used for) provided by operating activities | | | (30,962 | ) | | | 65,976 | | | | 28,761 | |
| | | | | | | | | | | | |
Investing activities | | | | | | | | | | | | |
Capital expenditures | | | (20,703 | ) | | | (16,173 | ) | | | (17,318 | ) |
Purchases of marketable securities | | | (86,448 | ) | | | (101,235 | ) | | | (66,540 | ) |
Proceeds from sales or maturities of marketable securities | | | 121,755 | | | | 60,432 | | | | 65,243 | |
Acquisitions of cost-basis and equity-basis investments | | | (6,746 | ) | | | (3,144 | ) | | | — | |
Acquisitions, net of cash acquired | | | — | | | | (3,928 | ) | | | — | |
Intangible rights acquired due to contract termination, net | | | (10,410 | ) | | | — | | | | — | |
Proceeds from sale of cost-basis and equity-basis investments | | | — | | | | 5,175 | | | | — | |
Other | | | 81 | | | | 119 | | | | 19 | |
| | | | | | | | | | | | |
Net cash used for investing activities | | | (2,471 | ) | | | (58,754 | ) | | | (18,596 | ) |
| | | | | | | | | | | | |
Financing activities | | | | | | | | | | | | |
Purchases of treasury stock | | | (901 | ) | | | (2,602 | ) | | | (569 | ) |
Proceeds from exercise of stock options | | | 1,266 | | | | 1,819 | | | | 236 | |
Other | | | 14 | | | | (2,908 | ) | | | (662 | ) |
| | | | | | | | | | | | |
Net cash provided by (used for) financing activities | | | 379 | | | | (3,691 | ) | | | (995 | ) |
| | | | | | | | | | | | |
Effects of exchange rate changes on cash and cash equivalents | | | (1,071 | ) | | | 379 | | | | 942 | |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | (34,125 | ) | | | 3,910 | | | | 10,112 | |
| | | | | | | | | | | | |
Cash and cash equivalents, beginning of period | | | 68,648 | | | | 64,738 | | | | 54,626 | |
| | | | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 34,523 | | | $ | 68,648 | | | $ | 64,738 | |
| | | | | | | | | | | | |
| | | |
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | | | | | | | | | | | | |
Cash interest paid | | $ | 45 | | | $ | 82 | | | $ | 188 | |
Cash income taxes paid | | $ | 11,343 | | | $ | 12,237 | | | $ | 1,552 | |
See accompanying notes.
F-7
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Summary of Significant Accounting Policies
Description of Business
ATMI, Inc. (together with its subsidiaries, collectively referred to as the “Company,” “ATMI,” or “we”) believes it is among the leading suppliers of high performance materials, materials packaging and materials delivery systems used worldwide in the manufacture of microelectronics devices. Our products consist of “front-end” semiconductor performance materials, sub-atmospheric pressure gas delivery systems for safe handling and delivery of toxic and hazardous gases to semiconductor process equipment, high-purity materials packaging and dispensing systems that allow for the reliable introduction of low volatility liquids and solids to microelectronics and biopharmaceutical processes. ATMI targets semiconductor and flat-panel display manufacturers, whose products form the foundation of microelectronics technology rapidly proliferating through the consumer products, information technology, automotive, and communications industries. The market for microelectronics devices is continually changing, which drives demand for new products and technologies at lower cost. ATMI’s customers include many of the leading semiconductor manufacturers in the world who target leading edge technologies. ATMI also addresses an increasing number of critical materials handling needs for the life sciences markets. Our proprietary containment, mixing, and bioreactor technologies are sold to the biotechnology, laboratory and cell therapy markets, which we believe offer significant growth potential. ATMI’s objective is to meet the demands of our microelectronics and life sciences customers with solutions that maximize the efficiency of their manufacturing processes, reduce capital or operating costs, and minimize the time to develop new products and integrate them into their processes.
Consolidation
The consolidated financial statements include the accounts of all subsidiaries where control exists. Equity investments generally consist of 20 percent to 50 percent owned operations which by definition demonstrate significant influence and instances where the Company through voting and similar rights can exercise significant influence. Operations less than 20 percent owned, where the Company does not exercise significant influence, are generally carried at cost. Earnings from equity investments are reported, net of income taxes, within the caption, “Other income (expense), net” on the consolidated statements of operations. Intercompany transactions have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, and expenses. While actual results could differ, management believes such estimates to be reasonable.
Revenue Recognition and Accounts Receivable
We recognize revenue when the following four criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. Revenues from product sales are generally recognized upon delivery to a common carrier when terms are equivalent to free-on-board (“FOB”) origin and upon receipt by a customer when terms are equivalent to FOB destination. In instances where final acceptance of equipment is specified by the purchase agreement, revenue is deferred until all acceptance criteria have been satisfied, except when reasonable reserves for returns can be effectively established due to substantial successful installation history for homogenous transactions. Should changes in conditions cause management to determine these criteria are not met for certain future transactions, revenue recognized for any reporting period could be adversely affected. We accrue for sales returns, warranty costs, and other allowances based on a current evaluation of our experience based on stated terms of the transactions.
Prior to October 31, 2011, we used Matheson as our exclusive contract manufacturer and distribution partner for the manufacture and distribution of our Safe Delivery Source® (“SDS”) products (the “Licensed Products”). Under the terms of the original manufacturing agreement, ATMI retained the right to manufacture 25 percent of all Licensed Products, while Matheson had the right to manufacture 75 percent of all Licensed Products. Upon completion of manufacture, ATMI purchased all Licensed Products produced by Matheson. Under the terms of the distribution agreement, we received payment from Matheson based upon a formula which was dependent on the sale price obtained by Matheson from its customers. ATMI recognized revenue from the sale of Licensed Products to Matheson when Matheson sold the Licensed Products to its customers, because that is when the sales price became fixed and determinable. On October 31, 2011, we terminated the agreements with Matheson for manufacturing, marketing, selling and/or distributing of our SDS products (“SDS Direct transaction”). Matheson will provide ongoing support services to ATMI during a transition period. Subject to such continuing services, ATMI assumed control of all manufacturing, distribution, logistics and sales services, which Matheson had provided globally prior to execution of the Termination Agreement, other than the distribution of the SDS and VAC product lines in Japan, which services will continue to be provided by Matheson’s parent company, Taiyo Nippon Sanso Corporation (“TNSC”). In those regions where Matheson is involved in distributing products during the transition period, revenues will be recognized only after sale of the Licensed Products to the end users.
F-8
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
During the years ended December 31, 2011, 2010 and 2009, ATMI recognized $65.8 million, $79.9 million, and $37.9 million of revenues from Matheson, respectively. During the years ended December 31, 2011, 2010 and 2009, ATMI recognized revenues from a Taiwanese foundry of $48.7 million, $46.8 million, and $36.2 million, respectively, and revenues of $47.6 million, $31.3 million, and $16.3 million, respectively, from a leading South Korea-based integrated circuit manufacturer.
Billings to customers for shipping and handling are included in revenues. Costs incurred for shipping and handling of products are charged to cost of revenues. Credit is extended to customers based on an evaluation of each customer’s financial condition; generally, collateral is not required. Revenues are presented in the consolidated financial statements net of sales allowances and discounts. Accounts receivable are presented in the consolidated financial statements net of the allowance for doubtful accounts. Taxes collected from customers and remitted to governmental authorities are presented on a net basis; and they are excluded from revenues.
Accounts Receivable Allowances
The allowance for doubtful accounts is established to represent our best estimate of the net realizable value of the outstanding accounts receivable balances. We estimate our allowance for doubtful accounts based on past due amounts and historical write-off experience, as well as trends and factors surrounding the credit risk of the markets we operate in and the financial viability of specific customers. In an effort to identify adverse trends, we assess the financial health of the markets we operate in and perform periodic credit evaluations of our customers and ongoing reviews of account balances and aging of receivables. Amounts are considered past due when payment has not been received within the time frame of the credit terms extended. Write-offs are charged directly against the allowance for doubtful accounts and occur only after all collection efforts have been exhausted.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents, marketable securities, accounts receivable, and currency forward exchange contracts. We invest our cash and cash equivalents and marketable securities in U.S. Government and municipal debt obligations, and time deposits. The Company had amounts due from two customers that accounted for approximately 34 percent and 42 percent of accounts receivable at December 31, 2011 and 2010, respectively.
Research and Development
Costs associated with the development of new products and improvements to existing products are charged to expense as incurred.
F-9
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Cash and Cash Equivalents and Marketable Securities
Highly liquid investments with maturities of three months or less, when acquired, are classified as cash and cash equivalents. Investments in publicly traded securities with maturities greater than three months, when acquired, are classified as marketable securities.
All of the Company’s marketable securities are classified as available-for-sale as of the balance sheet date and are reported at fair value, with unrealized gains and losses included in stockholders’ equity as a component of accumulated other comprehensive income, net of applicable taxes. We regularly review the fair value of marketable security declines below amortized cost to evaluate whether the decline is other-than-temporary. In making this determination, the Company considers all available evidence including, among other things, considering the duration and extent of the decline and the economic factors influencing the market to determine if the fair value will recover to equal or exceed the amortized cost. If we determine that the fair value will not recover, an other-than-temporary impairment is recognized, net of applicable taxes.
In April 2009, the guidance on the recognition and presentation of other-than-temporary impairments was amended for debt securities, like our Massachusetts Educational Financing Authority (“MEFA”) auction-rate security, requiring us to determine both the credit and non-credit components when we conclude an investment has an other-than-temporary impairment. The change resulted in a second quarter 2009 recognition of a cumulative-effect adjustment to retained earnings, with a corresponding adjustment to accumulated other comprehensive income because we recognized a non-cash other-than-temporary impairment of $2.4 million for our auction rate security in the first quarter of 2009. As part of the transition of adoption we determined the amount of the impairment related to credit loss and the amount related to all other factors. Since we concluded that we had no current intent to sell this security and it was not more likely than not that we will be required to sell the security before anticipated recovery of its remaining amortized cost, we adjusted accumulated other comprehensive income (loss) for the component of the impairment loss due to all other factors, net of tax. Of the total $2.4 million pre-tax loss recognized in the first quarter of 2009, we determined the credit loss was $0.3 million and the loss due to other factors was $2.1 million. The credit loss of $0.3 million is being accreted to the cost basis of the security ratably over the expected term of the security, currently estimated to be 15 years. The estimated fair value of the auction-rate security was $3.3 million at December 31, 2011 and $2.9 million at December 31, 2010. See Note 5 for further discussion regarding the auction-rate security.
Marketable securities that are in a temporarily impaired position, where management has the ability and intent to hold until anticipated recovery or maturity, are classified as either current or non-current based on the remaining contractual maturity of the security. Those securities in a temporarily impaired position with contractual maturities greater than one year are classified as non-current.
As part of our ongoing cash management optimization efforts during 2011, the Company purchased South Korean time deposits which were classified as marketable securities. At December 31, 2011 and 2010, the Company had $6.9 million and $5.4 million, respectively, of time deposits in South Korea.
As of December 31, 2011, we had $13.1 million of cash and cash equivalents in South Korea, $4.1 million of cash and cash equivalents in Taiwan and $4.2 million in cash and cash equivalents in Japan, which were not available to fund domestic US operations without repatriation. These funds could become subject to additional tax if they are repatriated. It is not practicable to estimate the amount or timing of the additional tax, if any, that eventually might be paid on these foreign funds. We do not anticipate repatriating these funds in the foreseeable future.
Non-marketable Equity and Debt Securities
We selectively invest in non-marketable equity securities of private companies, which range from early-stage companies that are often still defining their strategic direction to more mature companies whose products or technologies may directly support an ATMI product or initiative. Our non-marketable equity investments are recorded using cost basis or the equity method of accounting, depending on the facts and circumstances of each
F-10
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
investment. At December 31, 2011, the carrying value of our portfolio of strategic investments in non-marketable equity securities totaled $8.0 million ($22.3 million at December 31, 2010). The main driver of the reduction from December 31, 2010 was the initial public offering of Intermolecular, Inc., which caused us to reclassify our investment from “Other non-current assets” to “Marketable securities” because the securities became publicly traded. In certain instances, we loan funds to early-stage investees at market interest rates to enable them to focus on product and technology development. Non-marketable equity and debt securities are included in the consolidated balance sheets under the caption “Other non-current assets.” ATMI’s share of the income or losses of all equity-method investees, using the most current financial information available, which is typically one month behind ATMI’s normal closing date, is included in our results of operations from the investment date forward.
Investments in non-marketable equity securities are inherently risky, and some of these companies are likely to fail. Their success (or lack thereof) is dependent on product development, market acceptance, operational efficiency, attracting and retaining talented professionals, and other key business success factors. In addition, depending on their future prospects, they may not be able to raise additional funds when needed or they may receive lower valuations, with less favorable investment terms than in previous financings, and the investments would likely become impaired.
We review our investments quarterly for indicators of impairment; however, for non-marketable equity securities, the impairment analysis may require significant judgment to identify events or circumstances that would likely have a significant adverse effect on the fair value of the investment. The indicators that we use to identify those events or circumstances include (a) the investee’s revenue and earnings trends relative to predefined milestones and overall business prospects, (b) the technological feasibility of the investee’s products and technologies, (c) the general market conditions in the investee’s industry or geographic area, including adverse regulatory or economic changes, (d) factors related to the investee’s ability to remain in business, such as the investee’s liquidity, and the rate at which the investee is using its cash, and (e) the investee’s receipt of additional funding at a lower valuation.
Investments identified as having an indicator of impairment are subject to further analysis to determine if the investment is other-than-temporarily impaired, in which case we write the investment down to its fair value, using the framework required by Accounting Standards Codification (“ASC”) 820 “Fair Value Measurements and Disclosures.” When an investee is not considered viable from a financial or technological point of view, we write down the entire investment balance to zero since we consider the estimated fair market value to be nominal. If an investee obtains additional funding at a valuation lower than our carrying amount or requires a new round of equity funding to stay in operation and the new funding does not appear imminent, we presume that the investment is other-than-temporarily impaired, unless specific facts and circumstances indicate otherwise. We recognized an $0.8 million impairment charge in our portfolio of non-marketable equity securities in 2011 (none in 2010 and 2009).
In July 2005, ATMI made an investment in Anji Microelectronics Co., Ltd. (“Anji”), an entity in the development stage of researching and developing advanced semiconductor materials, with primary operations in Shanghai, China. We have determined that Anji is a variable interest entity. However, we have determined that we are not the primary beneficiary of Anji because we do not have the power, through voting or similar rights, to direct the activities of Anji that most significantly impact the entity’s economic performance, and we are also not expected to absorb significant losses or gains from Anji. ATMI’s carrying value of this cost basis investment is $3.9 million at December 31, 2011. The carrying value of our investment in Anji represents the cash paid, less our share of the cumulative losses during the period we used the equity-method of accounting. At December 31, 2011, our maximum exposure to loss is $4.3 million, which consists of $3.9 million of our carrying value in this investment, plus a $0.4 million reserve for a put option.
Inventories
Inventories are stated at the lower of cost or market, using the first-in, first-out (“FIFO”) method. Inventory valuation reserves are established in order to report inventories at the lower of cost or market value on our consolidated balance sheets. The determination of inventory valuation reserves requires management to make estimates and judgments on the future salability of inventories. Valuation reserves for excess, obsolete, and slow-moving inventory are estimated by comparing the inventory levels of individual parts to both future sales forecasts
F-11
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
or production requirements and historical usage rates in order to identify inventory where the resale value or replacement value is less than inventory cost. Other factors that management considers in determining these reserves include whether individual inventory parts or chemicals meet current specifications and cannot be substituted for or reworked into a part currently being sold or used as a service part, overall market conditions, and other inventory management initiatives.
As of December 31, 2011 and 2010, we had $2.6 million and $2.4 million, respectively, of inventory valuation reserves recorded.
Property, Plant, and Equipment, net
Property, plant, and equipment are carried at cost, net of accumulated depreciation. Depreciation is calculated on the straight-line method based on the estimated useful lives of the assets, which range from 3 to 35 years (see Note 6). The estimated useful life represents the projected period of time that the asset will be productively employed by the Company and is determined by management based on many factors, including historical experience with similar assets and technological life cycles. Circumstances and events relating to these assets are monitored to ensure that changes in asset lives or impairments are identified and prospective depreciation expense or impairment expense is adjusted accordingly. The depreciation periods used are: buildings, 5 to 35 years; machinery and equipment, 3 to 15 years; software, 5 to 10 years; cylinders and canisters, 7 to 15 years; furniture and fixtures, 5 years; and leasehold improvements, over the lesser of the lease term or estimated useful life. We use accelerated depreciation methods for tax purposes where appropriate.
Asset-Retirement Obligations
An asset-retirement obligation (“ARO”) is recognized in the period in which sufficient information exists to determine the fair value of the liability with a corresponding increase to the carrying amount of the related property, plant, and equipment which is then depreciated over its useful life. The liability is initially measured at fair value and then accretion expense is recorded in each subsequent period. The Company’s AROs are primarily associated with five leased facilities where we have made substantial modifications to the leased property and we are obligated to restore the facilities at the end of the contractual term of each lease. See Note 9 for further discussion on leases.
Income Taxes
Current income taxes are determined based on estimated taxes payable or refundable on tax returns for the current year. Deferred income taxes are determined using enacted tax rates for the effect of temporary differences between the book and tax bases of recorded assets and liabilities. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company recognizes the benefit of an income tax position only if it is more likely than not (greater than 50 percent) that the tax position will be sustained upon tax examination, based solely on the technical merits of the tax position. Otherwise, no benefit can be recognized. The tax benefits recognized are measured based on the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement. We adjust these unrecognized tax benefits, including any impact on the related interest and penalties, in light of changing facts and circumstances. A number of years may elapse before a particular matter for which we have established an unrecognized tax benefit is audited and fully resolved. Additionally, the Company accrues interest and related penalties, if applicable, on all tax exposures for which reserves have been established consistent with jurisdictional tax laws, which is included in income tax expense. See Note 10 for more information and disclosures on income taxes.
F-12
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Fair Value of Financial Instruments
The Company measures and reports financial assets and financial liabilities on a fair value basis, consistent with ASC 820 “Fair Value Measurements and Disclosures,” using the following three categories for classification and disclosure purposes:
Level 1 — Quoted prices in active markets for identical assets and liabilities. Level 1 assets and liabilities consist of cash, money market fund deposits, time deposits, certain of our marketable equity instruments, and forward foreign currency exchange contracts that are traded in an active market with sufficient volume and frequency of transactions.
Level 2 — Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which all significant inputs are observable or can be derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities. Level 2 assets include certain of our marketable debt instruments with quoted market prices that are traded in less active markets or priced using a quoted market price for similar instruments.
Level 3 — Unobservable inputs to the valuation methodology that are significant to the measurement of fair value of assets or liabilities. At December 31, 2011, our auction-rate-security and the Artelis acquisition contingent consideration payment liability are the only items reflected in this category.
See Note 5 for more information regarding the details, methods and assumptions used to estimate the fair value of our other financial instruments.
Foreign Currency Exchange Contracts
We use forward foreign currency exchange contracts to hedge specific or anticipated exposures relating to intercompany payments (primarily U.S. export sales to subsidiaries at pre-established U.S. dollar prices), intercompany loans and other specific and identified exposures. The terms of the forward foreign currency exchange contracts are matched to the underlying transaction being hedged, and are typically under one year. Because such contracts are directly associated with identified transactions, they are an effective hedge against fluctuations in the value of the foreign currency underlying the transaction.
Changes in the fair value of economic hedges are recognized in earnings as an offset to the change in the fair value of the underlying exposures being hedged. We do not enter into derivative instruments for trading or speculative purposes. At December 31, 2011, we did not have any cash flow hedges outstanding.
Counterparties to forward foreign currency exchange contracts are major banking institutions with credit ratings of investment grade or better and no collateral is required. There are no significant risk concentrations. We believe the risk of incurring losses on derivative contracts related to credit risk is remote.
Goodwill and Other Indefinite-Lived Intangible Assets
The assets and liabilities of acquired businesses are recorded under the purchase method of accounting at their estimated fair values at the dates of acquisition. Goodwill represents costs in excess of fair values assigned to the underlying net assets of acquired businesses.
Goodwill and intangible assets deemed to have indefinite lives are not amortized, but are subject to annual impairment testing. In 2011, we adopted ASU 2011-08, “Intangibles – Goodwill and other (Topic 350): Testing Goodwill for Impairment,” which simplified how entities are required to test goodwill for impairment by permitting an initial assessment of qualitative factors to determine whether it is more likely than not that the fair value of the reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the two-step goodwill impairment test.
F-13
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For reporting units where the qualitative determination does not indicate that it is more likely than not that the fair value of a reporting unit is more than its carrying value, the two step test is performed. In order to perform the two-step test we estimate the fair value of a reporting unit based on the best information available as of October 31, 2011, which primarily incorporates management assumptions about expected future cash flows and contemplates other valuation techniques. No goodwill impairment has been recorded to date.
Other Long-Lived Assets
We evaluate the potential impairment of other long-lived assets when indicators of impairment are present. If the carrying value of assets exceeds the sum of the undiscounted expected future cash flows, the carrying value of the asset is written down to fair value. We amortize acquired patents and other amortizable intangible assets over their estimated useful lives. All amortizable intangible assets are amortized using the straight-line method over the estimated useful lives of the assets, ranging from 3 to 15 years.
Intercompany Loans
In certain circumstances, the Company maintains intercompany agreements with and among our wholly-owned subsidiaries under which funds are provided to subsidiaries to finance general business activities. Since settlement of these agreements is not expected in the foreseeable future, and there is no repayment schedule as part of the agreements, we treat these loans as permanent advances. Therefore, any associated foreign exchange gains and losses are deferred in Accumulated other comprehensive income in the period in which they arise.
Translation of Foreign Currencies
We conduct business in many different currencies and, accordingly, are subject to the inherent risks associated with foreign exchange rate movements. The financial position and results of operations of many of our foreign subsidiaries are measured using the local currency as the functional currency. Foreign currency denominated assets and liabilities are translated into U.S. dollars at the exchange rates existing at the respective balance sheet dates, and income and expense items are translated at the average exchange rates during the respective periods. The aggregate effects of translating the balance sheets of these subsidiaries are deferred as a separate component of Stockholders’ equity.
Equity-based Compensation
The Company recognizes compensation expense in its consolidated financial statements for all share-based payments granted based on the fair value on the date of grant. For share-based payments granted with a service period vesting restriction, compensation expense is recognized on a straight-line basis or accelerated attribution method over the awards’ respective vesting period. For share-based payments granted with a performance condition, we accrue compensation expense when we determine it is probable that the awards will be earned.
Recently Issued Accounting Pronouncements
In June 2011, the FASB issued Accounting Standards Update (“ASU”, or “Update”) 2011-4, “Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRS.” This Update provides guidance which is expected to result in common fair value measurement and disclosure requirements between U.S. GAAP and IFRS and should be applied prospectively. It is not intended for this Update to result in a change in the application of the requirements in Topic 820. For public entities, the amendments are effective during interim and annual periods beginning after December 15, 2011. Early application by public entities is not permitted. We do not anticipate any material impact from this Update.
In June 2011, the FASB issued ASU 2011-5, “Comprehensive Income (Topic 220)” and in December 2011, the FASB issued ASU 2011-12 – “Comprehensive Income (Topic 220): Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income.” In ASU 2011-05, an entity has the option to present the total of comprehensive income, the components of net income, and the
F-14
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. The amendments do not change the items, portrayal or timing of what must be reported in other comprehensive income. The amendments in this Update should be applied retrospectively. In ASU 2011-12, the FASB deferred the effective date pertaining to reclassification adjustments out of accumulated other comprehensive income (“AOCI”) until further notice. Entities should continue to report reclassifications out of AOCI consistent with the presentation requirements in effect prior to Update 2011-05. These amendments are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. Early adoption is permitted. We do not anticipate any material impact from these Updates.
In December 2011, the FASB issued ASU 2011-11—”Balance Sheet (Topic 210): Disclosures about Offsetting Assets and Liabilities.” In this update, entities are required to disclose both gross information and net information about both instruments and transactions eligible for offset in the statement of financial position and instruments and transactions subject to an agreement similar to a master netting arrangement. An entity is required to apply the amendments retrospectively for annual reporting periods beginning on or after January 1, 2013, and interim periods within those annual periods. We do not anticipate any material impact from this Update.
Collaborative Arrangements
ATMI entered into a collaborative development agreement (“CDA”) with an advanced memory integrated circuit manufacturer for the purpose of developing molecules, including chemical precursors, and material systems for next generation semiconductor products. ATMI will use its High Productivity Development platform to collaboratively work with this customer on specific statements of work designed to develop next-generation materials. The agreement, which was signed in September 2011 and expires in August 2012, requires the customer to make quarterly payments to ATMI over the contract term. The arrangement has been determined to be a reimbursement of research and development costs and will be recognized as a reduction of expense under the caption, “Research and development” in the Consolidated Statements of Operations. At December 31, 2011, we recognized $0.9 million related to the CDA.
Each CDA we execute will be reviewed based on the unique terms and conditions associated with such arrangement to determine, using applicable accounting guidance, the timing of recognition, whether the arrangement is revenue producing or represents a reimbursement of research and development costs, and the appropriate amounts to be recognized. If an arrangement is determined to be revenue producing, we apply applicable accounting standards to ensure proper timing and amounts of revenue recognition. If an arrangement is determined to represent a reimbursement of research and development costs, we apply accounting standards for reimbursements to ensure proper timing, amounts and classification as an offset to research and development expenses.
Reclassifications of Financial Statements
In the presentation of the Statements of Cash Flows, for the years ended December 31, 2010 and 2009, respectively, we have regrouped the following categories within Operating Activities under the caption “Other”: provision for (reversal of) bad debt, provision for inventory obsolescence, income tax benefit (deficiency) from share-based payment arrangements, loss from equity-method investments, and gain on sale of equity investment. For the years ended December 31, 2010 and 2009, respectively, we have regrouped the following categories within Financing Activities under the caption “Other”: credit line borrowings, credit line repayments, and payments of loans.
F-15
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
2. Marketable Securities
Marketable securities include at December 31, (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | 2011 | | | 2010 | |
| | | Cost | | | Gross
Unrealized
Gain (Loss) | | | Estimated
Fair
Value | | | Cost | | | Gross
Unrealized
Gain (Loss) | | | Estimated
Fair Value | |
Securities in unrealized gain position: | | | | | | | | | | | | | | | | | | | | | | | | |
Common stock | | $ | 22,376 | | | $ | 12,241 | | | $ | 34,617 | | | $ | 251 | | | $ | 1,545 | | | $ | 1,796 | |
Government debt obligations (1) | | | 22,612 | | | | 69 | | | | 22,681 | | | | 16,661 | | | | 87 | | | | 16,748 | |
GS (2) debt obligations | | | 7,000 | | | | 6 | | | | 7,006 | | | | 15,004 | | | | 11 | | | | 15,015 | |
U.S. Treasury obligations (3) | | | — | | | | — | | | | — | | | | 8,045 | | | | 2 | | | | 8,047 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Subtotal | | | 51,988 | | | | 12,316 | | | | 64,304 | | | | 39,961 | | | | 1,645 | | | | 41,606 | |
| | | | | | |
Securities in unrealized loss position: | | | | | | | | | | | | | | | | | | | | | | | | |
Government debt obligations (1) | | | 4,437 | | | | (2 | ) | | | 4,435 | | | | 26,138 | | | | (63 | ) | | | 26,075 | |
GS (2) debt obligations | | | — | | | | — | | | | — | | | | 8,000 | | | | (9 | ) | | | 7,991 | |
Auction-rate security (4) | | | 4,720 | | | | (1,391 | ) | | | 3,329 | | | | 4,695 | | | | (1,794 | ) | | | 2,901 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Subtotal | | | 9,157 | | | | (1,393 | ) | | | 7,764 | | | | 38,833 | | | | (1,866 | ) | | | 36,967 | |
| | | | | | |
Securities at amortized cost: | | | | | | | | | | | | | | | | | | | | | | | | |
Time deposits | | | 6,893 | | | | — | | | | 6,893 | | | | 5,351 | | | | — | | | | 5,351 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Subtotal | | | 6,893 | | | | — | | | | 6,893 | | | | 5,351 | | | | — | | | | 5,351 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
Total marketable securities | | $ | 68,038 | | | $ | 10,923 | | | $ | 78,961 | | | $ | 84,145 | | | $ | (221 | ) | | $ | 83,924 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| (1) | State and municipal government debt obligations. |
| (3) | U.S. Treasury obligations were included as part of U.S Government Sponsored securities in the Company’s Annual Report on Form 10-K for the year ended December 31, 2010. |
| (4) | The cost basis of the Massachusetts Educational Financing Authority (MEFA) auction rate security is equal to the par value of $5,000,000 less unaccreted non-cash credit losses of $280,000 and $305,000 at December 31, 2011 and December 31, 2010, respectively. |
The amortized cost and estimated fair value of available-for-sale securities, by contractual maturity, as of December 31, 2011 are shown below (in thousands); expected maturities may differ from contractual maturities because the issuers of the securities may exercise the right to prepay obligations without prepayment penalties.
F-16
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| | | September 30, | | | | September 30, | |
| | | Cost | | | Estimated
Fair Value | |
| | |
Due in one year or less | | $ | 29,276 | | | $ | 29,306 | |
Due between one and three years | | $ | 11,666 | | | $ | 11,709 | |
Auction-rate security (due in 2038) | | $ | 4,720 | | | $ | 3,329 | |
| | | | | | | | |
| | | 45,662 | | | | 44,344 | |
| | |
Common stock | | | 22,376 | | | | 34,617 | |
| | | | | | | | |
| | $ | 68,038 | | | $ | 78,961 | |
| | | | | | | | |
As of December 31, 2011, 8 out of the 42 marketable securities currently held were in an unrealized loss position. This table shows the Company’s marketable securities that were in an unrealized loss position at December 31, 2011, and also shows the duration of time the security had been in an unrealized loss position (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | Less Than 12 Months | | | 12 Months or Greater | | | Total | |
| | | Fair
Value | | | Unrealized
Losses | | | Fair
Value | | | Unrealized
Losses | | | Fair
Value | | | Unrealized
Losses | |
| | | | | | |
Government debt obligations | | $ | 4,435 | | | $ | (2 | ) | | $ | 0 | | | $ | 0 | | | $ | 4,435 | | | $ | (2 | ) |
Auction-rate security | | | — | | | | — | | | | 3,329 | | | | (1,391 | ) | | | 3,329 | | | | (1,391 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | $ | 4,435 | | | $ | (2 | ) | | $ | 3,329 | | | $ | (1,391 | ) | | $ | 7,764 | | | $ | (1,393 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
See Note 5 for further discussion.
3. Inventories
Inventories include at December 31, (in thousands):
| | | September 30, | | | | September 30, | |
| | | 2011 | | | 2010 | |
Raw materials | | $ | 20,508 | | | $ | 16,499 | |
Work in process | | | 1,681 | | | | 2,133 | |
Finished goods | | | 53,986 | | | | 46,575 | |
| | | | | | | | |
| | | 76,175 | | | | 65,207 | |
Excess and obsolescence reserve | | | (2,553 | ) | | | (2,375 | ) |
| | | | | | | | |
Inventories, net | | $ | 73,622 | | | $ | 62,832 | |
| | | | | | | | |
As of December 31, 2011, the Company had commitments for inventory purchases of $15.6 million.
As December 31, 2011 and 2010, respectively, we had $5.0 million and $3.8 million of finished goods inventory residing at non-ATMI consignment locations.
F-17
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
4. Foreign Currency Exchange Contracts
At December 31, 2011, we held foreign currency exchange contracts that are economic hedges with notional amounts totaling $22.0 million, of which $10.4 million will be settled in Euros, $1.9 million will be settled in Taiwan Dollars, $0.6 million will be settled in Japanese Yen and $9.1 million will be settled in Korean Won. Changes in the fair market value (gain or loss) on these contracts were not significant as of December 31, 2011.
At December 31, 2010, we held foreign currency exchange contracts that were economic hedges with notional amounts totaling $32.7 million, of which $12.6 million were settled in Euros, $1.8 million were settled in Taiwan Dollars, $1.9 million were settled in Japanese Yen, and $16.4 million were settled in Korean Won. Changes in the fair market value (gain or loss) on these contracts were not significant as of December 31, 2010.
5. Fair Value Measurements
At December 31, 2011 and 2010, we have included the fair value of our auction-rate security under the caption “Marketable securities, non-current” in the consolidated balance sheets.
Assets / Liabilities Measured at Fair Value on a Recurring Basis
This table summarizes the Company’s assets (liabilities) measured at fair value on a recurring basis at December 31, 2011 (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | Fair Value Measured Using | |
| | | Total | | | Quoted Prices
in Active
Markets for
Identical
Assets
(Level 1) | | | Significant
Other
Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | |
Cash & cash equivalents | | $ | 34,523 | | | | 34,523 | | | | — | | | | — | |
| | | | |
Available-for-sale marketable securities | | | | | | | | | | | | | | | | |
Common stock (1) | | $ | 34,617 | | | | 34,617 | | | | — | | | | — | |
Time deposits | | $ | 6,893 | | | | 6,893 | | | | — | | | | — | |
Government debt obligations | | $ | 27,116 | | | | — | | | | 27,116 | | | | — | |
GS debt obligations | | $ | 7,006 | | | | — | | | | 7,006 | | | | — | |
Auction-rate security | | $ | 3,329 | | | | — | | | | — | | | | 3,329 | |
| | | | |
Foreign currency exchange contract asset | | $ | 187 | | | | 187 | | | | | | | | | |
Foreign currency exchange contract liability | | $ | (212 | ) | | | (212 | ) | | | — | | | | — | |
| (1) | Includes reclassification of our Intermolecular investment from non-marketable equity securities which previously was classified as a Level 3 non-recurring measurement. |
F-18
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Company recorded losses of $2.0 million and $0.8 million for the years ended December 31, 2011 and 2010, respectively, and a gain of $0.2 million for the year ended 2009, under the caption “Other income (expense), net” in the consolidated statements of operations, related to changes in the fair value of its financial instruments for forward foreign currency exchange contracts accounted for as fair value hedges.
During 2011, our valuation methodologies were consistent with previous years and there were no transfers into or out of Level 3 based on changes in observable inputs.
There were no transfers between Level 1 and Level 2 during 2011. We reclassified our investment in Intermolecular from a non-recurring fair value measurement to a recurring fair value measurement as a result of their initial public offering in November 2011.
This table presents a reconciliation for all assets and liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) for the year ended December 31, 2011 (in thousands).
| | | September 30, | | | | September 30, | |
| | | Fair Value Measurements Using Significant
Unobservable Inputs (Level 3) | |
| | | Available-For-
Sale Marketable
Securities | | | Total | |
Balance at December 31, 2010 | | $ | 2,901 | | | $ | 2,901 | |
Total gains (losses), realized and unrealized: | | | | | | | | |
Included in net income | | | — | | | | — | |
Included in accumulated other comprehensive income | | | 428 | | | | 428 | |
Purchases, issuances, and settlements, net | | | — | | | | — | |
Transfers into (out of) Level 3 | | | — | | | | — | |
| | | | | | | | |
Balance at December 31, 2011 | | $ | 3,329 | | | $ | 3,329 | |
| | | | | | | | |
| | |
See Note 2 for further discussion. | | | | | | | | |
Assets / Liabilities Measured at Fair Value on a Nonrecurring Basis
All assets and liabilities measured at fair value on a nonrecurring basis are categorized as Level 3, requiring significant management judgment due to the absence of quoted market prices or observable inputs for assets of a similar nature. The individual amounts of assets and liabilities measured on a non-recurring basis are discussed below.
On November 2, 2010, ATMI’s Belgian subsidiary acquired the remaining 60 percent of the outstanding shares of Artelis S.A. The total accounting purchase consideration of $21.8 million included a cash payment of $4.0 million, the fair value of contingent payments tied to future revenue performance of $8.4 million, the carrying value of $5.9 million related to our original 40 percent non-controlling ownership interest, and assumed debt of $3.5 million. The contingent payments tied to future revenue performance, for the years 2012 through 2014, have a range of possible outcomes from zero to $23.3 million. The gain has been recorded in our Statements of Operations under the caption “Other income (expense), net.” The acquisition was recorded under the purchase method of accounting and, accordingly, Artelis’ results of operations are included in the Company’s financial statements from the date of acquisition.
F-19
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Consistent with prior quarters, the fair value of the Artelis contingent consideration liability was estimated using a discounted cash flow methodology. Since the value is primarily based on revenues achieved in the measurement period, our estimate this quarter included a simulation of revenues undertaken in a Monte Carlo simulation framework. We risk adjusted the revenue estimates in the simulation in accordance with their market related risks. The amounts calculated based on the simulated revenues were then discounted to present value at an average rate of 5.3 percent using a term appropriate risk-free rate plus a spread commensurate to the Company’s credit rating as of the valuation date.
The fair value of the contingent payments as of December 31, 2011 was $7.2 million compared to $8.4 million as of December 31, 2010. The decline was driven by a decline in our assessment of future sales due to the current economic uncertainty in conjunction with a reduction in value due to translation of Euro denominated balances into U.S. Dollar through 2011. We will continue to use a term appropriate risk-free rate plus a spread commensurate to our credit rating going forward.
During 2009, long-lived assets held and used with a carrying amount of $8.4 million were written down to their estimated fair values of $1.1 million, resulting in an impairment charge of $7.3 million. The fair value measurements were calculated using unobservable inputs (primarily using discounted cash flow analyses and reliance on market knowledge of internal experts), classified as Level 3, requiring significant management judgment due to the absence of quoted market prices or observable inputs for assets of a similar nature. $3.1 million of the impairment charge is included in cost of revenues and is primarily related to the planned idling of manufacturing capacity of our gas products. $1.6 million of the impairment charge is included in research and development expense and is related primarily to idled equipment used in our research and development efforts. $2.6 million of the impairment charge is included in selling, general and administrative expense and is primarily related to redundant enterprise management software.
Due to their nature, the carrying value of cash, receivables, and payables approximates fair value.
F-20
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
6. Property, Plant and Equipment, Net
Property, plant, and equipment, net, consists of the following (in thousands):
| | | September 30, | | | | September 30, | |
| | | December 31, | |
| | | 2011 | | | 2010 | |
Land | | $ | 1,104 | | | $ | 1,123 | |
Buildings | | | 26,129 | | | | 25,739 | |
Machinery and equipment | | | 139,294 | | | | 138,664 | |
Software | | | 24,955 | | | | 28,252 | |
Cylinders and canisters | | | 49,844 | | | | 43,535 | |
Furniture and fixtures | | | 3,042 | | | | 2,751 | |
Leasehold improvements | | | 24,841 | | | | 26,488 | |
Construction in progress | | | 6,486 | | | | 2,105 | |
| | | | | | | | |
| | | 275,695 | | | | 268,657 | |
Accumulated depreciation and amortization | | | (159,420 | ) | | | (149,604 | ) |
| | | | | | | | |
| | $ | 116,275 | | | $ | 119,053 | |
| | | | | | | | |
Depreciation and amortization expense for property, plant, and equipment for the years ended December 31, 2011, 2010 and 2009 was $23.3 million, $22.8 million, and $22.3 million, respectively.
Fully depreciated assets, which were no longer in use, of approximately $12.7 million (primarily software and machinery and equipment) and $2.3 million were written off in the years ended December 31, 2011 and 2010, respectively.
We recognized impairment losses from property, plant, and equipment of $0.6 million, $0.5 million, and $7.3 million, in the years ended December 31, 2011, 2010 and 2009, respectively. Refer to Note 5 for additional information.
As of December 31, 2011, the Company had commitments for capital expenditures of $1.0 million.
This table shows amounts recorded in the consolidated statements of operations related to depreciation expense for property, plant, and equipment (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
Cost of revenues | | $ | 10,480 | | | $ | 10,531 | | | $ | 10,633 | |
Research and development | | | 7,913 | | | | 5,219 | | | | 4,381 | |
Selling, general, and administrative | | | 4,928 | | | | 7,006 | | | | 7,314 | |
| | | | | | | | | | | | |
Total depreciation and amortization | | $ | 23,321 | | | $ | 22,756 | | | $ | 22,328 | |
| | | | | | | | | | | | |
F-21
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
7. Goodwill and Other Intangibles
In conjunction with the SDS Direct transaction, we recognized intangible assets of $11.8 million for the reacquired rights ($8.8 million) and a non-competition agreement ($3.0 million) net of liabilities assumed. The useful lives of the reacquired rights and the non-competition agreement are 10 years and 9 years, respectively.
The license for our distribution rights to Enthone’s copper ECD products, including its ViaForm products, is subject to automatic renewal upon satisfaction of certain conditions in 2013, which we expect to meet.
Changes in carrying amounts of goodwill and other intangibles for the years ended December 31, 2011 and 2010, respectively, were (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | Goodwill | | | Patents &
Trademarks | | | Other | | | Total Other
Intangibles | |
| | | | |
Balance at December 31, 2009 | | $ | 33,394 | | | $ | 21,760 | | | $ | 1,442 | | | $ | 23,202 | |
Acquisitions | | | 13,566 | | | | 10,028 | | | | — | | | | 10,028 | |
Amortization expense | | | — | | | | (3,502 | ) | | | (460 | ) | | | (3,962 | ) |
Other, including foreign currency translation | | | 21 | | | | (360 | ) | | | 40 | | | | (320 | ) |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | $ | 46,981 | | | $ | 27,926 | | | $ | 1,022 | | | $ | 28,948 | |
Acquisitions | | | — | | | | — | | | | 11,802 | | | | 11,802 | |
Amortization expense | | | — | | | | (3,522 | ) | | | (319 | ) | | | (3,841 | ) |
Other, including foreign currency translation | | | (435 | ) | | | (338 | ) | | | — | | | | (338 | ) |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | $ | 46,546 | | | $ | 24,066 | | | $ | 12,505 | | | $ | 36,571 | |
| | | | | | | | | | | | | | | | |
Goodwill and other intangibles balances at December 31, 2011 and 2010 were (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | Goodwill | | | Patents &
Trademarks | | | Other | | | Total Other
Intangibles | |
| | | | |
Gross amount as of December 31, 2010 | | $ | 46,981 | | | $ | 49,869 | | | $ | 1,396 | | | $ | 51,265 | |
Accumulated amortization | | | — | | | | (21,943 | ) | | | (374 | ) | | | (22,317 | ) |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | $ | 46,981 | | | $ | 27,926 | | | $ | 1,022 | | | $ | 28,948 | |
| | | | | | | | | | | | | | | | |
| | | | |
Gross amount as of December 31, 2011 | | $ | 46,546 | | | $ | 49,404 | | | $ | 13,195 | | | $ | 62,599 | |
Accumulated amortization | | | — | | | | (25,338 | ) | | | (690 | ) | | | (26,028 | ) |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | $ | 46,546 | | | $ | 24,066 | | | $ | 12,505 | | | $ | 36,571 | |
| | | | | | | | | | | | | | | | |
This table shows amounts recorded in the consolidated statements of operations related to amortization expense for intangibles (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
| | | |
Cost of revenues | | $ | 334 | | | $ | 313 | | | $ | 296 | |
Selling, general, and administrative | | | 3,507 | | | | 3,649 | | | | 4,175 | |
| | | | | | | | | | | | |
Total amortization | | $ | 3,841 | | | $ | 3,962 | | | $ | 4,471 | |
| | | | | | | | | | | | |
F-22
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The approximate amortization expense expected to be recognized related to intangible assets is (in thousands):
| | | September 30, | |
Year | | Amount | |
2012 | | $ | 4,716 | |
2013 | | | 4,716 | |
2014 | | | 4,716 | |
2015 | | | 4,201 | |
2016 | | | 4,201 | |
Thereafter | | | 14,021 | |
| | | | |
Total | | $ | 36,571 | |
| | | | |
In 2010, the Artelis acquisition was recorded under the purchase method of accounting and, accordingly, the Artelis results of operations are included in the Company’s financial statements from the date of acquisition.
The purchase consideration was allocated as summarized below (in thousands).
| | | September 30, | |
Identified intangible assets | | $ | 10,028 | |
Net liabilities acquired | | | (1,770 | ) |
Goodwill | | | 13,566 | |
| | | | |
Purchase price, net of cash acquired | | $ | 21,824 | |
| | | | |
The excess of the purchase price over the estimated fair value of identifiable net assets acquired has been recorded as goodwill. The identified intangible assets identified were patents of $9.3 million and a trademark of $0.7 million. Patents are being amortized over 15 years while the trademark has been assigned an indefinite life. Goodwill acquired is not deductible for income tax purposes.
8. Other Non-Current Assets
In 2007, we entered into a purchase agreement with Intermolecular, Inc. (“Intermolecular”). As part of the initial agreement, we purchased HPD capabilities from Intermolecular, which expanded upon an existing alliance agreement. We have since purchased additional HPD capabilities (including tool sets), as well as services related to the use of these tools, including the use of dedicated research personnel. In December 2007, we made a $10.0 million royalty prepayment to Intermolecular, which is being applied to guaranteed royalties associated with products developed using the HPD capabilities.
In November 2011, Intermolecular completed an initial public offering, and we converted our preferred shares into common shares and exercised a warrant to purchase additional common shares, which caused us to reclassify our investment from “Other Non-Current Assets” to “Marketable securities” as the securities are now publicly traded. At December 31, 2011 we own common shares with a market value of $33.1 million.
In 2011, we expensed $5.0 million of prepaid royalties and we expect to expense $2.5 million in 2012. The 2012 amount is included in the consolidated balance sheets under the caption, “Other current assets.”
In the fourth quarter of 2011, we extended the contract term of an existing commitment with Intermolecular to purchase R&D technology and processes. The extension maintains our current R&D infrastructure and expense structures over the next three years. The cash payment commitments are $11.5 million, $5.0 million, $1.3 million, and $1.3 million in fiscal years 2011, 2012, 2013 and 2014, respectively. In 2011, there was a $1.3 million increase in commitments for that year due to the contract extension. The contract extension did not include any full time equivalent resource commitments beyond 2012.
F-23
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
9. Leases
The Company leases office and manufacturing facilities, and certain manufacturing equipment under several operating leases expiring between 2012 and 2034. Rental expense was $3.7 million, 3.4 million, and $4.3 million, for the years ended December 31, 2011, 2010 and 2009, respectively.
Below is a schedule of future minimum lease payments for operating leases as of December 31, 2011 (in thousands):
| | | September 30, | |
| | | Operating
Leases | |
2012 | | $ | 3,579 | |
2013 | | | 2,761 | |
2014 | | | 1,233 | |
2015 | | | 1,017 | |
2016 | | | 929 | |
Thereafter | | | 2,787 | |
| | | | |
Total minimum lease payments | | $ | 12,306 | |
| | | | |
We lease two facilities in Danbury, CT. One facility houses our research and development activities and certain of our microelectronics manufacturing capabilities, and contains approximately 73,000 square feet of space. In December 2010 we exercised a renewal option for the period January 1, 2012 to December 31, 2016. For the period January 1, 2011 to December 31, 2011, the monthly base rent is $42,094. For the period January 1, 2012 to December 31, 2016, the monthly base rent is $47,500. There is one additional five-year renewal period available to ATMI under this lease. We have agreed to certain restoration obligations associated with this facility, which we are accounting for as an asset retirement obligation (“ARO”), associated with the leasehold improvements made to this facility. The discounted fair value of the ARO at December 31, 2011 is $2.7 million.
The other facility in Danbury, CT is our headquarters, and contains approximately 31,000 square feet of space. In October 2010, we exercised a renewal option for the period January 1, 2012 to December 31, 2016. For the period January 1, 2011 to December 31, 2016, the monthly base rent is $17,606. There is one additional five-year renewal period available to ATMI under this lease.
We lease a facility in Bloomington, MN where we manufacture high-purity materials packaging and dispensing systems, within our microelectronics and life sciences product lines. This facility contains approximately 68,000 square feet of space. We entered into an amendment of this lease on September 23, 2008, which extended the lease term to August 31, 2013. For the period January 1, 2011 to August 31, 2013, the monthly base rent is $26,706. There are two successive three-year renewal periods available to ATMI under this lease. We have agreed to certain restoration obligations associated with this facility, which we are accounting for as an ARO, associated with the leasehold improvements made to this facility. The discounted fair value of the ARO as of December 31, 2011 is $0.3 million.
F-24
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Changes in the carrying amounts of the Company’s ARO’s at December 31, 2011 are shown below (in thousands):
| | | September 30, | |
Balance as of December 31, 2010 | | $ | 3,506 | |
| |
Liabilities incurred | | | 73 | |
Liabilities settled | | | — | |
Accretion expense | | | 63 | |
| | | | |
Balance as of December 31, 2011 | | $ | 3,642 | |
| | | | |
The ARO liability is included in the consolidated balance sheets under the caption, “Other non-current liabilities.”
10. Income Taxes
Pre-tax income (loss) from continuing operations was taxed in these jurisdictions (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
| | | |
Domestic | | $ | (54,437 | ) | | $ | 32,644 | | | $ | (20,784 | ) |
Foreign | | | 15,303 | | | | 17,983 | | | | 7,128 | |
| | | | | | | | | | | | |
Total pre-tax income (loss) | | $ | (39,134 | ) | | $ | 50,627 | | | $ | (13,656 | ) |
| | | | | | | | | | | | |
Significant components of the provision (benefit) for income taxes for the following years ended are (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
| | | |
Current: | | | | | | | | | | | | |
Federal | | $ | 7,326 | | | $ | 4,144 | | | $ | (7,317 | ) |
State | | | 320 | | | | 74 | | | | 79 | |
Foreign | | | 2,682 | | | | 2,316 | | | | 2,154 | |
| | | | | | | | | | | | |
Total current | | | 10,328 | | | | 6,534 | | | | (5,084 | ) |
| | | | | | | | | | | | |
Deferred: | | | | | | | | | | | | |
Federal | | | (27,049 | ) | | | 4,174 | | | | (117 | ) |
State | | | (2,102 | ) | | | 739 | | | | (392 | ) |
Foreign | | | (292 | ) | | | (326 | ) | | | (1,403 | ) |
| | | | | | | | | | | | |
Total deferred | | | (29,443 | ) | | | 4,587 | | | | (1,912 | ) |
| | | | | | | | | | | | |
| | $ | (19,115 | ) | | $ | 11,121 | | | $ | (6,996 | ) |
| | | | | | | | | | | | |
F-25
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Significant components of the Company’s deferred tax assets and liabilities are (in thousands):
| | | September 30, | | | | September 30, | |
| | | December 31, | |
| | | 2011 | | | 2010 | |
Deferred tax assets: | | | | | | | | |
Accrued liabilities | | $ | 5,516 | | | $ | 3,647 | |
Inventory reserves | | | 1,797 | | | | 1,699 | |
Net operating loss and tax credit carryforwards | | | 12,463 | | | | 10,758 | |
Equity-based compensation | | | 8,338 | | | | 7,890 | |
Reacquired rights on contract termination | | | 30,691 | | | | — | |
Other, net | | | 525 | | | | 560 | |
| | | | | | | | |
| | | 59,330 | | | | 24,554 | |
Valuation allowance | | | (5,305 | ) | | | (3,178 | ) |
| | | | | | | | |
| | | 54,025 | | | | 21,376 | |
| | |
Deferred tax liabilities: | | | | | | | | |
Depreciation and amortization | | | (23,978 | ) | | | (21,835 | ) |
Unrealized gain on marketable securities | | | (4,333 | ) | | | (651 | ) |
Other, net | | | (2,328 | ) | | | (237 | ) |
| | | | | | | | |
| | | (30,639 | ) | | | (22,723 | ) |
| | | | | | | | |
Net deferred tax assets (liabilities) | | $ | 23,386 | | | $ | (1,347 | ) |
| | | | | | | | |
The valuation allowance relates to the realizability of certain U.S. state and foreign net operating losses, and certain foreign tax credits.
As of December 31, 2011, the Company had the following deferred tax assets related to net operating loss (“NOLs”) and tax credit carryforwards (in thousands):
| | | September 30, | | | | September 30, | |
| | | | | | Expiration | |
Federal | | | | | | | | |
-Capital Loss | | $ | 938 | | | | 2015 | |
| | | | | | | | |
| | $ | 938 | | | | | |
| | | | | | | | |
State | | | | | | | | |
-NOLs | | | 525 | | | | 2017-2031 | |
-Credits | | | 38 | | | | 2017-2024 | |
-Credits | | | 143 | | | | None | |
| | | | | | | | |
| | $ | 706 | | | | | |
| | | | | | | | |
| | |
Foreign | | | | | | | | |
- NOLs | | | 9,771 | | | | None | |
- NOLs | | | 40 | | | | 2016 | |
- Credits | | | 1,008 | | | | 2012-2013 | |
| | | | | | | | |
| | $ | 10,819 | | | | | |
| | | | | | | | |
Total | | $ | 12,463 | | | | | |
| | | | | | | | |
F-26
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The reconciliation of income tax expense (benefit) from operations computed at the U.S. federal statutory tax rate to the Company’s tax expense (benefit) is (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | For the Year Ended December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
U.S. statutory rate | | $ | (13,698 | ) | | $ | 17,719 | | | $ | (4,779 | ) |
State income taxes | | | (1,158 | ) | | | 529 | | | | (196 | ) |
Foreign income taxes | | | (5,948 | ) | | | (8,322 | ) | | | (2,375 | ) |
Tax exempt income | | | (115 | ) | | | (112 | ) | | | (213 | ) |
Change in valuation allowance of deferred tax assets | | | 2,445 | | | | 1,854 | | | | 133 | |
Adjustment to tax liabilities | | | (393 | ) | | | (213 | ) | | | 457 | |
Research & development credits | | | (681 | ) | | | (846 | ) | | | (284 | ) |
Other, net | | | 433 | | | | 512 | | | | 261 | |
| | | | | | | | | | | | |
| | $ | (19,115 | ) | | $ | 11,121 | | | $ | (6,996 | ) |
| | | | | | | | | | | | |
ATMI has not provided for U.S. federal income and foreign withholding taxes on approximately $92.4 million of undistributed earnings from non-U.S. operations as of December 31, 2011, because such earnings are intended to be reinvested indefinitely outside of the United States. These earnings could become subject to additional tax if they are remitted as dividends, loaned to ATMI, or upon sale of subsidiary stock. It is not practicable to estimate the amount or timing of the additional tax, if any, that eventually might be paid on the foreign earnings.
South Korea has granted the Company an income tax exemption that expires in 2014, including the last two years at 50 percent of the exemption. The exemption applies only to income related to one of the Company’s product lines. The effect of the tax exemption was to reduce income tax expense by $2.8 million, $2.1 million, and $1.3 million for the years ended December 31, 2011, 2010, and 2009.
At December 31, 2011, ATMI had $3.7 million of unrecognized tax benefits, which, if recognized, would favorably affect the effective income tax rate in future periods. $0.7 million of this amount is included in deferred taxes, and the balance of $3.0 million is included in the caption “Other non-current liabilities” on the consolidated balance sheets, together with $0.4 million of accrued interest (net) on tax reserves and $0 accrued for penalties. At December 31, 2010, the amount of unrecognized tax benefits, which, if recognized, would have favorably affected the effective income tax rate in future periods, was $5.6 million, $0.7 million of accrued interest (net) on tax reserves and $0 accrued for penalties.
The reconciliation of the unrecognized tax benefits (exclusive of interest) at the beginning of 2010 through the end of 2011 is (in thousands):
| | | September 30, | |
Ending Balance – December 31, 2009 | | $ | 6,182 | |
Increases from prior period positions | | | 241 | |
Decreases from prior period positions | | | (586 | ) |
Increases from current period positions | | | 1,293 | |
Decreases related to settlements with taxing authorities | | | (1,130 | ) |
Decreases from lapse of statute of limitations | | | (409 | ) |
| | | | |
Ending Balance – December 31, 2010 | | $ | 5,591 | |
Reclasses out of non-income tax based positions | | | (1,350 | ) |
Increases from prior period positions | | | 59 | |
Decreases from prior period positions | | | (259 | ) |
Increases from current period positions | | | 469 | |
Decreases related to settlements with taxing authorities | | | (482 | ) |
Decreases from lapse of statute of limitations | | | (372 | ) |
| | | | |
Ending Balance – December 31, 2011 | | $ | 3,656 | |
| | | | |
F-27
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In the second quarter of 2011, based on facts and circumstances and recent interpretation of tax law, we determined that $1.4 million of unrecognized tax benefits will not impact income taxes; as a result, the classification of this amount was changed from unrecognized tax benefits to a loss contingency within the same caption “Other non-current liabilities.” It is reasonably possible that in the next 12 months, because of changes in facts and circumstances, the unrecognized tax benefits for tax positions taken related to previously filed tax returns may decrease. The range of possible decrease is $0 million to $0.2 million. In January 2012, the Internal Revenue Service completed a U.S. tax audit of tax years 2008 and 2009, although the refund claim for tax year 2009 is still subject to review by the Joint Committee on Taxation.
11. Defined Contribution Plan
The Company maintains a defined contribution plan (401(k) Plan) covering substantially all of its U.S. employees that is subject to the provisions of the Employee Retirement Income Security Act of 1974. The Company’s matching contributions are discretionary by plan year and were approximately $1.8 million, $1.5 million, and $1.1 million for the years ended December 31, 2011, 2010 and 2009, respectively. On June 5, 2009, the Company discontinued the matching of contributions, but the Company match was reinstated on October 9, 2009. The Plan provides for discretionary matching contributions of 100 percent of the first 3 percent of each participant’s eligible compensation plus 50 percent on the next 2 percent of each participant’s eligible compensation, up to statutory limitations. There is no matching contribution above 5 percent of each participant’s eligible compensation.
12. Stockholders’ Equity
This table shows the effect of pre-tax compensation cost arising from equity-based payment arrangements recognized in the consolidated statements of operations (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | December 31, | |
| | | 2011 | | | 2010 | | | 2009 | |
| | | |
Cost of revenues | | $ | 496 | | | $ | 357 | | | $ | 315 | |
Research and development | | | 900 | | | | 866 | | | | 523 | |
Selling, general, and administrative | | | 6,143 | | | | 6,470 | | | | 4,902 | |
| | | | | | | | | | | | |
Total equity-based compensation expense | | $ | 7,539 | | | $ | 7,693 | | | $ | 5,740 | |
| | | | | | | | | | | | |
No equity-based compensation cost was capitalized.
Summary of Plans
We currently have three equity-based compensation plans which provide for the granting of up to 7,000,000 shares of common stock pursuant to nonqualified stock options, incentive stock options (“ISOs”), stock appreciation rights and restricted stock awards to employees, directors and consultants of the Company. Stock options typically vest over periods ranging from one to four years with a maximum term of ten years. Restricted stock awards typically vest over periods ranging from three to five years. Shares issued as a result of stock option exercises are primarily settled by the issuance of new shares.
F-28
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
This table shows the number of shares approved by shareholders for each plan and the number of shares that remain available for equity awards at December 31, 2011 (in thousands):
| | | September 30, | | | | September 30, | |
| | | | | | # of | |
| | | # of Shares | | | Shares | |
Stock Plan | | Approved | | | Available | |
| | |
2003 Stock Plan (1) | | | 3,000 | | | | 74 | |
2010 Stock Plan (1) | | | 3,000 | | | | 2,895 | |
Employee Stock Purchase Plan (2) | | | 1,000 | | | | 245 | |
| | | | | | | | |
Totals | | | 7,000 | | | | 3,214 | |
| | | | | | | | |
| (1) | Exercise prices for ISOs and non-qualified stock options granted under this plan may not be less than 100 percent of the fair market value for the Company’s common stock on the date of grant. |
| (2) | Employees may purchase shares at 95 percent of the closing price on the day previous to the last day of each six-month offering period. This plan is not considered to be compensatory. |
Fair Value
The Company uses the Black-Scholes-Merton options-pricing model to determine the fair value of stock options under ASC 718 “Compensation – Stock Compensation.” Management is required to make certain assumptions with respect to selected model inputs, including anticipated changes in the underlying stock price (i.e., expected volatility) and option exercise activity (i.e., expected term). For awards granted subsequent to January 1, 2006, expected volatility is based on the historical volatility of ATMI common stock for a period shorter than the expected term of the options. We have excluded the historical volatility prior to the public announcement regarding the sale of our non-core businesses in 2004, because those businesses that were sold represented a significant portion of ATMI’s consolidated business and were subject to considerable cyclicality associated with the semiconductor equipment industry, which drove increased volatility in ATMI’s stock price. The expected term of options granted is derived using historical exercise patterns which represent the period of time that options granted are expected to be outstanding. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for a period commensurate with the estimated expected term. In accordance with ASC 718, in the determination of equity-based compensation cost, the Company estimates the total number of instruments that will be forfeited as a result of a failure to provide the requisite service to earn the award.
The weighted-average fair value of options granted during the years ended December 31, 2011, 2010 and 2009 was $9.66, $8.62 and $6.44, respectively, based on the Black-Scholes-Merton options-pricing model. These weighted-average assumptions were used for grants in the periods indicated:
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | 2011 | | | 2010 | | | 2009 | |
Stock option grants: | | | | | | | | | | | | |
Risk free interest rate | | | 3.15 | % | | | 3.00 | % | | | 2.15 | % |
Expected term, in years | | | 7.85 | | | | 7.40 | | | | 7.20 | |
Expected volatility | | | 43.3 | % | | | 44.0 | % | | | 41.4 | % |
Dividend yield | | | 0 | % | | | 0 | % | | | 0 | % |
F-29
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Company uses historical data to estimate forfeitures of awards from employee terminations in order to estimate compensation cost for awards expected to vest. In addition, we separate employees into groups that have similar characteristics for purposes of making forfeiture estimates.
Stock Option and Restricted Stock Activity
This table shows the option activity under the plans as of December 31, 2011 and changes during the year then ended (options are expressed in thousands; averages are calculated on a weighted basis; life in years; intrinsic value expressed in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | Number | | | Average | | | Average | | | Aggregate | |
| | | of | | | Exercise | | | Remaining | | | Intrinsic | |
| | | Options | | | Price | | | Life | | | Value | |
| | | | |
Outstanding at December 31, 2010 | | | 1,931 | | | $ | 21.87 | | | | | | | | | |
Granted | | | 211 | | | $ | 18.48 | | | | | | | | | |
Exercised | | | (59 | ) | | $ | 17.65 | | | | | | | | | |
Forfeited | | | (164 | ) | | $ | 23.84 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at December 31, 2011 | | | 1,919 | | | $ | 21.47 | | | | 4.5 | | | $ | 2,940 | |
| | | | | | | | | | | | | | | | |
| | | | |
Exercisable at December 31, 2011 | | | 1,451 | | | $ | 22.66 | | | | 3.2 | | | $ | 1,574 | |
| | | | | | | | | | | | | | | | |
The aggregate intrinsic value represents the difference between the Company’s closing stock price of $20.03 as of December 31, 2011 and the exercise price of the dilutive options at that date, multiplied by the number of dilutive options outstanding at that date. The total intrinsic value of stock options exercised during the years ended December 31, 2011, 2010 and 2009 was $0.1 million, $0.2 million, and $0 million, respectively. The total fair value of options which vested during the years ended December 31, 2011, 2010 and 2009 was $1.7 million (184,000 shares), $1.7 million (189,000 shares), and $2.1 million (167,000 shares) respectively.
An income tax deficiency was recognized in additional paid-in capital from equity-based compensation totaling $0, $0.1 million, and $0.6 million for the years ended December 31, 2011, 2010 and 2009, respectively. An income tax deficiency was recognized in income tax expense from equity-based compensation totaling $0.9 million, $0.4 million for the years ended December 31, 2011, and December 31, 2010, respectively. There was no income tax deficiency in 2009.
This table shows restricted stock activity as of December 31, 2011 and changes during the year then ended (shares are expressed in thousands; averages are calculated on a weighted basis):
| | | September 30, | | | | September 30, | |
| | | | | | Average | |
| | | Number | | | Grant | |
| | | of | | | Date Fair | |
| | | Shares | | | Value | |
| | |
Nonvested at December 31, 2010 | | | 1,184 | | | $ | 19.28 | |
Granted | | | 470 | | | $ | 18.34 | |
Vested | | | (200 | ) | | $ | 26.81 | |
Forfeited | | | (191 | ) | | $ | 17.86 | |
| | | | | | | | |
Nonvested at December 31, 2011 | | | 1,263 | | | $ | 17.97 | |
| | | | | | | | |
F-30
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The total fair value of restricted stock which vested during the years ended December 31, 2011, 2010 and 2009 was $5.3 million, $5.0 million and $3.8 million, respectively.
As of December 31, 2011, $2.4 million of unrecognized compensation cost related to non-vested stock options is expected to be recognized over a weighted-average period of approximately 1.6 years. As of December 31, 2011, $9.6 million of unrecognized compensation cost related to restricted stock is expected to be recognized over a weighted-average period of approximately 2.1 years.
Earnings Per Share
This table shows the computation of basic and diluted earnings per share (in thousands, except per share data):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | 2011 | | | 2010 | | | 2009 | |
Numerator: | | | | | | | | | | | | |
Net income (loss) | | $ | (20,019 | ) | | $ | 39,506 | | | $ | (6,660 | ) |
| | | |
Denominator: | | | | | | | | | | | | |
| | | |
Denominator for basic earnings (loss) per share—weighted average shares | | | 31,703 | | | | 31,413 | | | | 31,398 | |
Dilutive effect of employee stock options | | | — | | | | 36 | | | | — | |
Dilutive effect of restricted stock | | | — | | | | 446 | | | | — | |
| | | | | | | | | | | | |
| | | |
Denominator for diluted earnings (loss) per common share—weighted average shares | | | 31,703 | | | | 31,895 | | | | 31,398 | |
| | | | | | | | | | | | |
| | | |
Earnings (loss) per share-basic | | $ | (0.63 | ) | | $ | 1.26 | | | $ | (0.21 | ) |
Earnings (loss) per share-diluted | | $ | (0.63 | ) | | $ | 1.24 | | | $ | (0.21 | ) |
This table shows the potential common shares excluded from the calculation of weighted-average shares outstanding because their effect was considered to be antidilutive (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | 2011 | | | 2010 | | | 2009 | |
| | | |
Antidilutive shares | | | 2,144 | | | | 1,450 | | | | 2,340 | |
The Company has never declared or paid cash dividends on its capital stock.
In August 2010, the Company’s Board of Directors approved a share repurchase program for up to $50.0 million of ATMI common stock. The program, which has no expiration date, does not require the Company to purchase any specific number or amount of shares and may be suspended or reinstated at any time at the Company’s discretion and without notice. Under the terms of the share repurchase program, repurchases can be made from time to time in open market transactions at prevailing market prices or in privately negotiated transactions. Management determined the timing and amount of purchases under the Repurchase Programs based upon market conditions or other factors.
Under all the repurchase programs approved by the Company’s Board of Directors, the Company purchased a total of 8,001,000 shares of its common stock at an average price of $28.37 per share. There were no shares repurchased under these programs in 2011.
F-31
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
13. Accumulated Other Comprehensive Income
The components of accumulated other comprehensive income are (in thousands):
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | Currency
Translation
Adjustments | | | Unrealized
Gain (Loss)
on
Available-
for-Sale
Securities | | | Total | |
Balance at December 31, 2008 | | $ | 865 | | | $ | (599 | ) | | $ | 266 | |
| | | |
Cumulative effect of adoption of new accounting standard | | | — | | | | (1,287 | ) | | | (1,287 | ) |
Reclassification adjustment related to marketable securities in unrealized gain position at prior period end, net of $32 tax benefit (1) | | | — | | | | (55 | ) | | | (55 | ) |
Change in fair value of available-for-sale securities, net of deferred income tax of $1,139 | | | — | | | | 1,940 | | | | 1,940 | |
Cumulative translation adjustment | | | 2,540 | | | | — | | | | 2,540 | |
| | | | | | | | | | | | |
Balance at December 31, 2009 | | $ | 3,405 | | | $ | (1 | ) | | $ | 3,404 | |
| | | |
Reclassification adjustment related to marketable securities in unrealized gain position at prior period end, net of $282 tax provision (1) | | | — | | | | (481 | ) | | | (481 | ) |
Change in fair value of available-for-sale securities, net of deferred income tax of $201 | | | — | | | | 343 | | | | 343 | |
Cumulative translation adjustment | | | 762 | | | | — | | | | 762 | |
| | | | | | | | | | | | |
Balance at December 31, 2010 | | $ | 4,167 | | | $ | (139 | ) | | $ | 4,028 | |
| | | |
Reclassification adjustment related to marketable securities in net unrealized gain position at prior period end, net of $0 tax provision (1) | | | — | | | | (1 | ) | | | (1 | ) |
Change in fair value of available-for-sale securities, net of deferred income tax of $4,351 | | | — | | | | 7,119 | | | | 7,119 | |
Cumulative translation adjustment | | | (3,097 | ) | | | — | | | | (3,097 | ) |
| | | | | | | | | | | | |
Balance at December 31, 2011 | | $ | 1,070 | | | $ | 6,979 | | | $ | 8,049 | |
| | | | | | | | | | | | |
| (1) | Determined based on the specific identification method |
F-32
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
14. Commitments, Contingencies, and Other
ATMI is, from time to time involved in legal actions, governmental audits, and proceedings relating to various matters incidental to its business including contract disputes, intellectual property disputes, product liability claims, employment matters, export and trade matters, and environmental claims. While the outcome of such matters cannot be predicted with certainty, in the opinion of management, after reviewing such matters and consulting with ATMI’s counsel and considering any applicable insurance or indemnifications, any liability which may ultimately be incurred is not expected to materially affect ATMI’s consolidated financial position, cash flows or results of operations.
In the fourth quarter of 2011, we extended the contract term of an existing commitment with Intermolecular to purchase R&D technology and processes. The extension maintains our current R&D infrastructure and expense structures over the next three years. The cash payment commitments are $11.5 million, $5.0 million, $1.3 million, and $1.3 million in fiscal years 2011, 2012, 2013 and 2014, respectively. In 2011, there was a $1.3 million increase in commitments for that year due to the contract extension. The contract extension did not include any full time equivalent resource commitments beyond 2012.
As part of the Artelis acquisition, we recognized a liability for the fair value of contingent payments tied to future revenue performance for the fiscal years 2012 through 2014 of $8.4 million as of the acquisition date. As of December 31, 2011, the fair value of the contingent consideration was $7.2 million. The contingent payment tied to future revenue performance has a range of possible outcomes from zero to $23.3 million.
ATMI currently has self-insurance limits for U.S. employee medical claims. The medical plan for U.S. employees has a stop-loss of $0.2 million per individual occurrence and an annual aggregate stop-loss of $4.9 million.
Other
Approximately 9 percent of the Company’s employees are covered by collective bargaining agreements that will expire in June 2013. All of the employees covered by these agreements are based in Belgium. The net assets of the Company’s Belgian subsidiary represent approximately 7 percent of the Company’s consolidated net assets.
15. Segments
ATMI is organized along functional lines of responsibility, whereby each member of the Company’s executive team has global responsibility for each respective functional area, such as supply chain operations, sales, marketing, finance, and research and development. The executive team is the chief operating decision maker of ATMI. Discrete financial information is only prepared at the product-line level for revenues and certain direct costs. Functional results are reviewed at the consolidated level. ATMI’s operations comprise one operating segment. Our organizational structure has been consistent from 2009 to 2011.
ATMI derives virtually all its revenues from providing materials and packaging products and related integrated process solutions to microelectronics and life sciences manufacturers. ATMI’s products are consumed or used in the front-end manufacturing process. They span many different technology applications at various stages of maturity and in many cases are inter-related in their application to a customer’s process.
F-33
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Revenues from external customers, by product type, were as follows:
| | | September 30, | | | | September 30, | | | | September 30, | |
| | | For the Year Ended December 31, | |
(In thousands) | | 2011 | | | 2010 | | | 2009 | |
Microelectronics | | $ | 351,823 | | | $ | 336,519 | | | $ | 230,707 | |
Life sciences | | $ | 38,264 | | | $ | 30,737 | | | $ | 23,997 | |
| | | | | | | | | | | | |
Total | | $ | 390,087 | | | $ | 367,256 | | | $ | 254,704 | |
| | | | | | | | | | | | |
16. Geographic Data
The Company’s geographic data for the years ended December 31, 2011, 2010 and 2009 are (in thousands):
| | | Septem | | | | Septem | | | | Septem | | | | Septem | | | | Septem | | | | Septem | | | | Septem | | | | Septem | |
| | | | | | | | | | | | | | | Other | | | | | | Europe and | | | | |
(In thousands) | | U.S. | | | Taiwan | | | Japan | | | South Korea | | | Pacific Rim | | | Belgium | | | Other | | | Total | |
December 31, 2011 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Revenues | | $ | 80,288 | | | $ | 97,324 | | | $ | 43,416 | | | $ | 79,332 | | | $ | 50,010 | | | $ | 3,269 | | | $ | 36,448 | | | $ | 390,087 | |
Long-lived assets | | | 157,536 | | | | 6,037 | | | | 7,872 | | | | 6,440 | | | | 492 | | | | 41,376 | | | | 277 | | | | 220,030 | |
| | | | | | | | |
December 31, 2010 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Revenues | | $ | 64,824 | | | $ | 89,415 | | | $ | 49,539 | | | $ | 67,743 | | | $ | 56,377 | | | $ | 2,050 | | | $ | 37,308 | | | $ | 367,256 | |
Long-lived assets | | | 159,267 | | | | 7,581 | | | | 8,473 | | | | 6,118 | | | | 285 | | | | 41,013 | | | | 326 | | | | 223,063 | |
| | | | | | | | |
December 31, 2009 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Revenues | | $ | 50,347 | | | $ | 55,454 | | | $ | 36,482 | | | $ | 51,555 | | | $ | 31,347 | | | $ | 2,759 | | | $ | 26,760 | | | $ | 254,704 | |
Long-lived assets | | | 168,678 | | | | 8,513 | | | | 7,354 | | | | 3,274 | | | | 265 | | | | 24,216 | | | | 392 | | | | 212,692 | |
Revenues are attributed to countries based on the location of the customer. Long-lived assets are located in the respective geographic regions, as shown above. Other than Taiwan, Japan, Belgium, and South Korea, no one specific country within the Pacific Rim, Europe, South America, and other regions accounted for greater than 10 percent of consolidated revenues and long-lived assets in 2011, 2010 and 2009.
F-34
ATMI, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
17. Quarterly Results of Operations (Unaudited)
Summarized quarterly results of operations data is as follows (in thousands, except per share amounts):
| | | September 30, | | | | September 30, | | | | September 30, | | | | September 30, | |
| | | Quarter | |
| | | First | | | Second | | | Third | | | Fourth | |
2011 | | | | | | | | | | | | | | | | |
| | | | |
Revenues | | $ | 100,724 | (a) | | $ | 104,027 | | | $ | 95,006 | | | $ | 90,330 | (d) |
| | | | |
Gross profit | | | 48,091 | | | | 49,462 | | | | 44,051 | | | | 42,526 | (e) |
| | | | |
Operating income (loss) | | | 11,658 | | | | 15,448 | (b) | | | 11,816 | | | | (76,636 | )(f) |
| | | | | | | | | | | | | | | | |
Net income (loss) | | $ | 8,007 | | | $ | 11,134 | | | $ | 8,041 | (c) | | ($ | 47,201 | )(g) |
| | | | | | | | | | | | | | | | |
| | | | |
Basic income (loss) per common share: | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Earnings (loss) per common share | | $ | 0.25 | | | $ | 0.35 | | | $ | 0.25 | | | ($ | 1.49 | ) |
| | | | | | | | | | | | | | | | |
| | | | |
Diluted income (loss) per common share: | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Earnings (loss) per common share | | $ | 0.25 | | | $ | 0.34 | | | $ | 0.25 | | | ($ | 1.49 | ) |
| | | | | | | | | | | | | | | | |
| | | | |
2010 | | | | | | | | | | | | | | | | |
| | | | |
Revenues | | $ | 85,311 | | | $ | 90,996 | | | $ | 94,960 | | | $ | 95,989 | |
| | | | |
Gross profit | | | 41,689 | | | | 43,555 | | | | 45,661 | | | | 45,103 | |
| | | | |
Operating income | | | 11,994 | | | | 10,644 | | | | 10,688 | | | | 8,612 | |
| | | | | | | | | | | | | | | | |
Net income | | $ | 8,666 | (h) | | $ | 7,598 | | | $ | 9,477 | (i) | | $ | 13,765 | (j) |
| | | | | | | | | | | | | | | | |
| | | | |
Basic income per common share: | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Earnings per common share | | $ | 0.27 | | | $ | 0.24 | | | $ | 0.30 | | | $ | 0.44 | |
| | | | | | | | | | | | | | | | |
| | | | |
Diluted income per common share: | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Earnings per common share | | $ | 0.27 | | | $ | 0.24 | | | $ | 0.30 | | | $ | 0.43 | |
| | | | | | | | | | | | | | | | |
| (a) | Includes $1.7 million from IP settlements, which represents the payment of royalties for periods prior to 2011. |
| (b) | Includes a $0.9 million gain on remeasurement of contingent consideration and a $1.2 million benefit related to state tax credits. |
| (c) | Includes $0.7 million of pre-tax foreign exchange losses. |
| (d) | Revenues were negatively impacted by the effects of the SDS Direct transaction which included inventory repurchases and excess inventory in the SDS channel, partially offset by incremental revenues realized from the transaction. Includes $0.7 million from IP settlements, which represents the payment of royalties for periods prior to 2011. |
| (e) | The cost of revenues were favorably impacted by the effects of the SDS Direct transaction, representing cost reversals for inventory repurchased and the effects of excess inventory in the SDS channel, partially offset by transition service costs and incremental costs on revenues realized from the transaction. |
| (f) | Includes an $84.6 million contract termination charge related to the SDS Direct transaction, transition related distribution expenses of $1.3 million, and an $0.8 million gain on remeasurement of contingent consideration and a $1.2 million benefit related to state tax credits. |
| (g) | Includes $31.0 million of tax benefit associated with the contract termination charge, and a $0.8 million pre-tax impairment loss on an equity investment. |
| (h) | Includes a $0.5 million gain from the sale of a marketable security, and a $0.5 million tax benefit (including interest) recognized to reverse previously established reserves for uncertain tax positions. |
| (i) | Includes a $2.5 million pre-tax gain on sale of shares of one of our equity-method investees. |
| (j) | Includes a $5.9 million fair value remeasurement gain related to acquisition of Artelis, a $1.2 million tax benefit (including interest) recognized to reverse previously established reserves for uncertain tax positions, a $2.1 tax benefit related to a European dividend, and a $1.9 million charge related to changes in valuation allowances. |
F-35
ATMI, Inc.
Schedule II—Valuation and Qualifying Accounts
Three Years Ended December 31, 2011
(In thousands)
Allowances for Doubtful Accounts and Sales Returns:
| | | September 30, | |
Balance December 31, 2008 | | | 958 | |
Provision charged to income | | | 1,381 | |
Doubtful accounts written off (net) | | | (68 | ) |
Other adjustments | | | 16 | |
| | | | |
Balance December 31, 2009 | | | 2,287 | |
Provision charged to income | | | (798 | ) |
Doubtful accounts written off (net) | | | (799 | ) |
Other adjustments | | | 93 | |
| | | | |
Balance December 31, 2010 | | | 783 | |
Provision charged to income | | | 0 | |
Doubtful accounts written off (net) | | | 0 | |
Other adjustments | | | (2 | ) |
| | | | |
Balance December 31, 2011 | | $ | 781 | |
| | | | |
Allowance for Excess and Obsolete Inventories:
| | | September 30, | |
Balance December 31, 2008 | | | 2,369 | |
Provision charged to income | | | 2,093 | |
Disposals of inventory written off | | | (1,798 | ) |
Other adjustments | | | (70 | ) |
| | | | |
Balance December 31, 2009 | | | 2,594 | |
Provision charged to income | | | 625 | |
Disposals of inventory written off | | | (648 | ) |
Other adjustments | | | (196 | ) |
| | | | |
Balance December 31, 2010 | | | 2,375 | |
Provision charged to income | | | 1,104 | |
Disposals of inventory written off | | | (432 | ) |
Other adjustments | | | (494 | ) |
| | | | |
Balance December 31, 2011 | | $ | 2,553 | |
| | | | |
Future Income Tax Benefits – Valuation Allowance:
| | | September 30, | |
Balance December 31, 2008 | | | 446 | |
Additions charged to income tax expense | | | 147 | |
Reductions credited to income tax expense | | | (14 | ) |
| | | | |
Balance December 31, 2009 | | | 579 | |
Additions charged to income tax expense | | | 1,904 | |
Reductions credited to income tax expense | | | (50 | ) |
Other adjustments | | | 745 | |
| | | | |
Balance December 31, 2010 | | | 3,178 | |
Additions charged to income tax expense | | | 3,076 | |
Reductions credited to income tax expense | | | (631 | ) |
Other adjustments | | | (318 | ) |
| | | | |
Balance December 31, 2011 | | $ | 5,305 | |
| | | | |
F-36
