Pursuant to Rule 425 under the
Securities Act of 1933
and deemed filed pursuant to Rule 14a-12
under the Securities Exchange Act of 1934
Subject Company: Variagenics, Inc.
Commission File No.: 000-31035

| Wall Street Analyst Forum November 12, 2002 Dr. Ted W. Love, President & CEO |

| Statements included in this talk which are not historical in nature, including those relating to Hyseq's proposed merger with VARIAGENICS and Hyseq's future business prospects, are "forward-looking statements" within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by words such as "believe," "expect," "anticipate," "should," "may," "estimate," "goals," and "potential," among others. These statements, including statements about the proposed merger and future financial and operating results, are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties, assumptions and other factors that could cause actual results to differ materially from those in the forward-looking statements. Such factors include risk that the proposed merger may not be approved by stockholders, risk that the two companies' businesses will not be integrated successfully, costs related to the proposed merger, and other factors (such as economic, business, competitive and/or regulatory factors) affecting each company's businesses generally as set forth in Hyseq's and VARIAGENICS' filings with the SEC, including their Annual Reports on Form 10-K for the fiscal years ended 2001, their most recent Quarterly Reports on Form 10-Q and their Current Reports on Form 8-K. Hyseq expressly disclaims any duty to update information contained herein. Safe Harbor Statement |

| HYSEQ AND VARIAGENICS ANNOUNCE MERGER Building for the Future: New Company Will Maximize Excellent Cash Position and Experienced Management Team to Support Development of Novel Products |

| Exchange ratio: 1 VGNX share:1.6451 HYSQ share Reverse triangular stock-for-stock merger Combined company will change name and have new stock symbol traded on Nasdaq Cash resulting from the merger will fund operations through approximately Dec. 2004 Dr. Ted W. Love President, CEO and board member Dr. George Rathmann chairman of 7 member board of directors (3 from each existing board) Headquartered in Sunnyvale, CA ~ 110-120 employees Targeted to complete merger by February 2003 Bankers: Banc of America Securities and SG Cowen Securities Group "NewCo" At a Glance |

| Alfimeprase, a novel acting thrombolytic in Phase I trials Robust biotherapeutic drug development pipeline based on proprietary human proteins and associated IP Cancer diagnostics program Strong partnerships with major pharmaceutical companies including: Amgen, Novartis, Kirin and Deltagen "NewCo" At a Glance Combining significant assets to create a product-focused organization |

| Alfimeprase: Near-term Product Opportunity Currently in Phase I Clinical Trials Strong IP Base in Full-length Human Genes Multiple R&D Strategies to Speed Drug Discovery Highly Experienced Management Team Hyseq Highlights |

| Alfimeprase |
| World class partner, Amgen Novel acting thrombolytic Targeting peripheral vascular disease Market large and unserved (>$500M) Compelling pre-clinical data-faster & more consistent clot lysis Granted orphan drug status for PAO Strong IP Alfimeprase Near-term Product Opportunity About to Enter Clinic |

| ALFIMEPRASE BLOOD VESSEL DEGRADES FIBRIN DISSOLVES CLOT ALFIMEPRASE INACTIVATED IN SYSTEMIC CIRCULATION Novel Acting Thrombolytic Alpha2-Macroglobulin Confines Alfimeprase to Local Delivery Site |
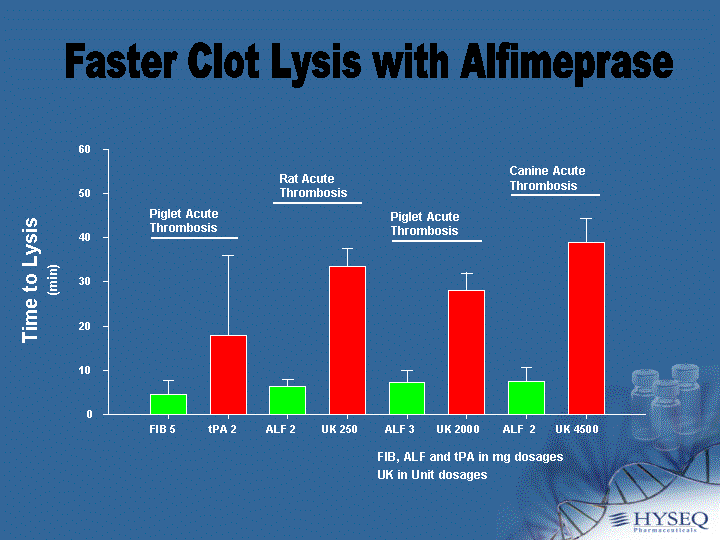
| FIB 5 tPA 2 ALF 2 UK 250 ALF 3 UK 2000 ALF 2 UK 4500 Time to Lysis (min) 0 10 20 30 40 50 60 Piglet Acute Thrombosis FIB, ALF and tPA in mg dosages UK in Unit dosages Piglet Acute Thrombosis Rat Acute Thrombosis Canine Acute Thrombosis Faster Clot Lysis with Alfimeprase |

| Peripheral Arterial Occlusion 1998 U.S. sales of Abbokinase(r) were > $200MM (off-label use) No widely used products currently on the market Catheter Occlusion U.S. central catheter occlusion cases of > 1million Sales potential for tPA estimated at $200-300MM (SG Cowen) Deep Vein Thrombosis Affects over 2 million Americans per year; 600,000 hospitalizations Third most common cardiovascular disease (behind ACS and stroke) May be treated with catheter directed lysis (infusions up to 3 days) > $500MM U.S. Market |

| Acute obstruction of blood flow to the leg by a clot >100,000 cases reported annually Limited treatment options: bypass surgery or angioplasty Currently no widely used products on the market Major morbidity: tissue death, gangrene, amputation Lead Indication: Acute Peripheral Arterial Occlusion |

| IND transferred to Hyseq H1 '02 Currently in Phase I Clinical Trials Phase II Clinical Trials to begin H1 '03 Phase III Clinical Trials to begin H2 '03 NDA targeted for H1 '05 Alfimeprase Timeline |

| Open label, single-dose, dose-escalation study to evaluate the safety, PK, and thrombolytic activity of alfimeprase Principal Investigator: Ken Ouriel 7sites IRB approval at all sites 20 patients In 5 cohorts (4 patients/cohort) 0.025, 0.05, 0.1, 0.3, and 0.5 mg/kg Chronic PAO population Stable for 14 days and requiring surgical intervention Category I and IIa Primary endpoint: safety as measured by adverse events Pharmacokinetics Qualitative lysis measurements Alfimeprase Phase I Clinical Trial |
| Intellectual Property |

| Full-Length Genes 2000 4000 6000 8000 10000 Hyseq Curagen HGSI Helix Incyte Genentech Millennium Zymogenetics Smithkline Amgen Others 20000 40000 19267 8268 41450 Strong IP Base in Full-length Human Genes Impressive Gene Patent Application Estate * Geneseq Database |

| 0 1000 2000 3000 4000 5000 Total # of AA Seqs Priority No Priority PCT-filing dt Dec00 Jan01 Feb01 Mar01 May01 Aug01 Nov01 PCT-Publ dt Jun01 Jul01 Aug01 Sep01 Nov01 Feb02 May02 Intracellular (1142) Transmembrane (305) Secreted (407) 38% 42% 43% 43% 41% 39% 42% Strong IP Base in Full-length Human Genes Formidable First to File Performance |

| Research & Development |

| A T C G G T A T C G G T T C A C G C G T C G G T T C A C G C G T Gene Discovery 10,000 predicted full-length genes Patent applied intellectual property Knock-in: Kirin 50 genes Knock-out : Deltagen 200 genes HYSQ Discovery Biology Multiple R&D Strategies to Speed Discovery Powerful Gene Identification Technologies, World-class Biology and Development Pipeline HYSQ Secreted Proteins Cell Screening |

| Kirin: Goal: Enter 50 secreted protein genes into knock-in mouse model Progress: 40 genes entered Deltagen: Goal: Enter 200 secreted protein genes into knock-out mouse model Progress: 140 entered HYSQ secreted protein genes: Goal: Complete cloning and cell assay tests of all secreted protein genes Progress: Over 800 secreted protein genes cloned, over 300 tested through multiplex cell-based assays 2002 Research Goals/Progress |

| HYSQ Collaborations 50 100 150 200 250 Genes Analyzed Deltagen Knock-Out Kirin Knock-In Q4'02 Q1'03 Q2'03 Q3'03 Q4'03 Q1'04 Q2'04 |
| Put Lead Product Candidate into Phase I Clinical Trials Increased Patent Filings on Over 10,000 Genes-Currently have 17 gene-related patents issued Major reductions in net burn (costs + revenues) Completed Two PIPE Financings Entered 3 Major R&D Collaborations to Speed R&D Machine Refined Focus on Novel Biopharmaceuticals Recruited Highly Experienced New Management Team Favorably Resolved Affymetrix Litigation Substantial Progress in Past Year |

| Advance alfimeprase through Phase I studies and begin Phase II in the first half of 2003 Identify additional clinical candidates from Hyseq genes Continue to build & strengthen patent estate Leverage Business Development Opportunities Revenue generation R&D collaborations Strategic alliances (U.S. & Europe) Near-Term Milestones |

| Nasdaq: HYSQ |
ADDITIONAL INFORMATION
Hyseq, VARIAGENICS and their respective directors, executive officers, certain members of management and employees, may be deemed to be participants in the solicitation of proxies in connection with the proposed merger. Information regarding the persons who may, under the rules of the SEC, be considered to be participants in the solicitation of Hyseq’s stockholders in connection with the proposed merger is set forth in Hyseq’s proxy statement for its 2002 annual meeting of stockholders, dated June 28, 2002 and filed with the SEC on June 13, 2002. Information regarding the persons who may, under the rules of the SEC, be considered to be participants in the solicitation of VARIAGENICS’ stockholders in connection with the proposed merger is set forth in VARIAGENICS’ proxy statement for its 2002 annual meeting, dated April 30, 2002 and filed with the SEC on April 29, 2002. Additional information will be set forth in the joint proxy statement/prospectus when it is filed with the SEC.
FORWARD-LOOKING STATEMENTS