Filed by Variagenics, Inc.
Pursuant to Rule 425 under the
Securities Act of 1933
and deemed filed pursuant to Rule 14a-12
under the Securities Exchange Act of 1934
Subject Company: Variagenics, Inc.
Commission File No.: 000-31035

| Hyseq/Variagenics Merger December 2002 |

| Statements included in this talk which are not historical in nature, including those relating to Hyseq's proposed merger with VARIAGENICS and Hyseq's future business prospects, are "forward-looking statements" within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by words such as "believe," "expect," "anticipate," "should," "may," "estimate," "goals," and "potential," among others. These statements, including statements about the proposed merger and future financial and operating results, are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties, assumptions and other factors that could cause actual results to differ materially from those in the forward-looking statements. Such factors include risk that the proposed merger may not be approved by stockholders, risk that the two companies' businesses will not be integrated successfully, costs related to the proposed merger, and other factors (such as economic, business, competitive and/or regulatory factors) affecting each company's businesses generally as set forth in Hyseq's and VARIAGENICS' filings with the SEC, including their Annual Reports on Form 10-K for the fiscal years ended 2001, their most recent Quarterly Reports on Form 10-Q and their Current Reports on Form 8-K. Hyseq expressly disclaims any duty to update information contained herein. Safe Harbor Statement |

| Agenda Transaction Overview Hyseq Highlights Variagenics Highlights Strategic Rationale & Opportunity |

| Transaction Overview |

| "NewCo" at a Glance Stock-for-stock merger Fixed exchange ratio: 1 VGNX share:1.6451 HYSQ shares Pro Forma Ownership: 63% Variagenics / 37% Hyseq 7 "NewCo" Board Members Dr. George Rathmann to serve as chairman Dr. Ted W. Love to serve as President, CEO and board member 2 additional directors from Hyseq; 3 directors from Variagenics Headquarters remains in Sunnyvale, CA Clinical operations and biotherapeutic research and discovery will reside in Sunnyvale, CA Molecular diagnostics development will reside in Cambridge, MA |

| "NewCo" at a Glance Combined company will be approximately 110-120 employees Merger Completion Proxy statement not reviewed by the SEC, expediting the close of the transaction Simultaneous shareholder meetings to approve transaction on January 28, 2003 Post-closing, combined company to be re-named with a new Nasdaq stock symbol |

| Hyseq Highlights |

| Hyseq Highlights Alfimeprase: Near-term Product Opportunity Currently in Phase I Clinical Trials Strong IP Base in Full-length Human Genes Multiple R&D Strategies to Speed Drug Discovery Highly Experienced Management Team |

| Alfimeprase Near-term Product Opportunity World class partner, Amgen Novel acting thrombolytic Targeting peripheral vascular disease Market large and unserved (>$500M) Compelling pre-clinical data-faster & more consistent clot lysis Granted orphan drug status for PAO Strong IP |
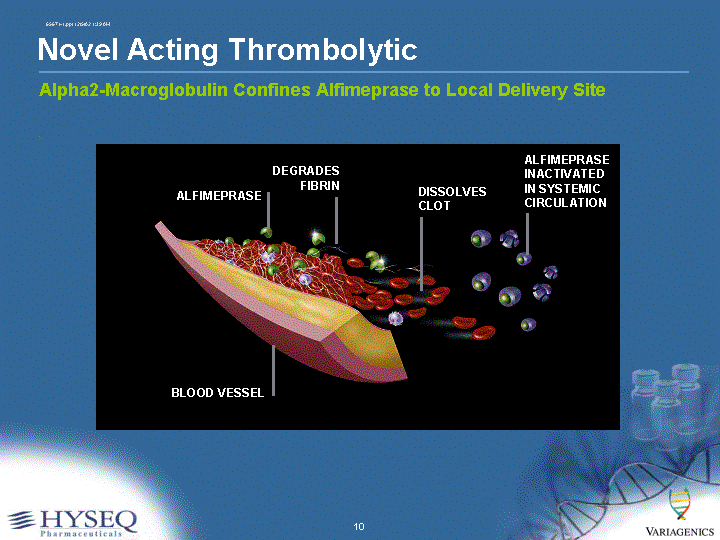
| Alpha2-Macroglobulin Confines Alfimeprase to Local Delivery Site ALFIMEPRASE BLOOD VESSEL DEGRADES FIBRIN DISSOLVES CLOT ALFIMEPRASE INACTIVATED IN SYSTEMIC CIRCULATION Novel Acting Thrombolytic |

| Faster Clot Lysis with Alfimeprase Piglet Acute Thrombosis Piglet Acute Thrombosis Rat Acute Thrombosis Canine Acute Thrombosis FIB, ALF and tPA in mg dosages UK in Unit dosages |

| Compelling Pre-clinical Data 1.3 ? 0.6 0.7 ? 0.6 0.7 ? 0.6 0.3 ? 0.6 0.0 ? 0.0 UK 970KU (n=3) 2.7 ? 0.6 2.7 ? 0.6 2.7 ? 0.6 2.7 ? 0.6 0.0 ? 0.0 ALF 30mg (n=3) 4hr 2hr 1hr 30min 0min GROUP Angiographic Flow Score (0-3) Baseline Day 4 (pre-lysis) ALF 30mg (30 min) |
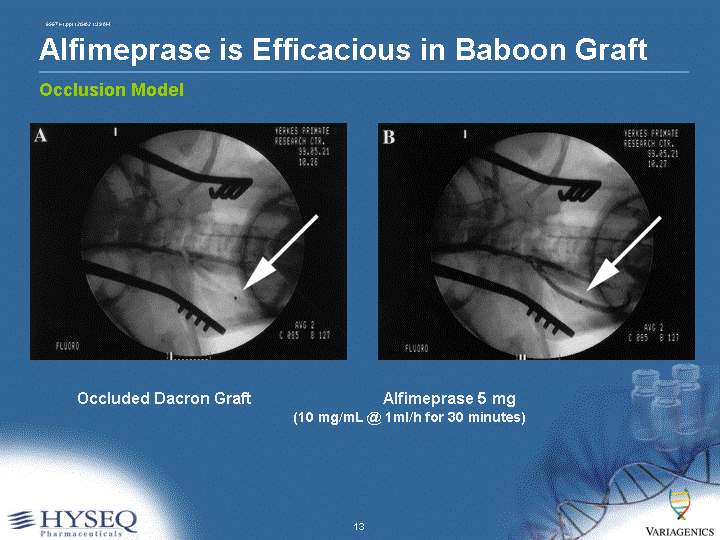
| Alfimeprase is Efficacious in Baboon Graft Occluded Dacron Graft Alfimeprase 5 mg (10 mg/mL @ 1ml/h for 30 minutes) Occlusion Model |
| >$500MM U.S. Market Peripheral Arterial Occlusion 1998 U.S. sales of Abbokinase(r) were > $200MM (off-label use) No widely used products currently on the market Catheter Occlusion U.S. central catheter occlusion cases of > 1million Sales potential for tPA estimated at $200-300MM (SG Cowen) Deep Vein Thrombosis Affects over 2 million Americans per year; 600,000 hospitalizations Third most common cardiovascular disease (behind ACS and stroke) May be treated with catheter directed lysis (infusions up to 3 days) |
| Acute Peripheral Arterial Occlusion Lead Indication: Acute obstruction of blood flow to the leg by a clot >100,000 cases reported annually Limited treatment options: bypass surgery or angioplasty Currently no widely used products on the market Major morbidity: tissue death, gangrene, amputation |

| IND Transferred to Hyseq H1 '02 Phase II Clinical Trials to Begin H1 '03 Phase III Clinical Trials to Begin H2 '03 NDA Targeted for H1 '05 Phase I Clinical Trials Began H1 '02 2002 2003 2004 2005 2006 Alfimeprase Timeline |
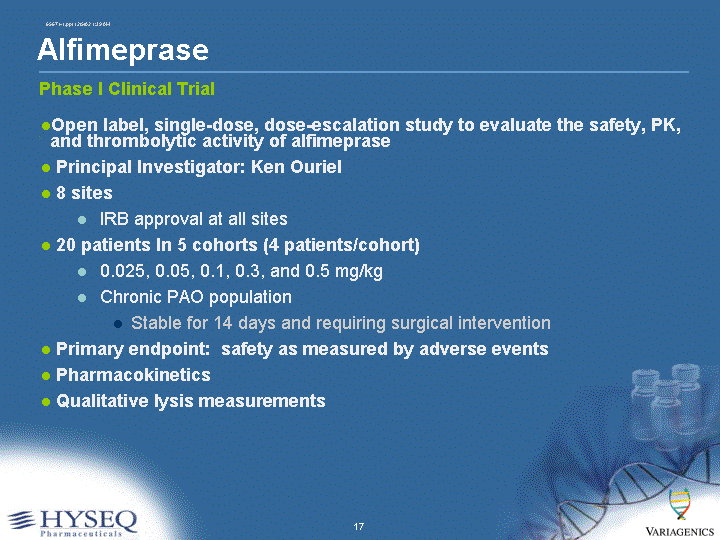
| Alfimeprase Open label, single-dose, dose-escalation study to evaluate the safety, PK, and thrombolytic activity of alfimeprase Principal Investigator: Ken Ouriel 8 sites IRB approval at all sites 20 patients In 5 cohorts (4 patients/cohort) 0.025, 0.05, 0.1, 0.3, and 0.5 mg/kg Chronic PAO population Stable for 14 days and requiring surgical intervention Primary endpoint: safety as measured by adverse events Pharmacokinetics Qualitative lysis measurements Phase I Clinical Trial |
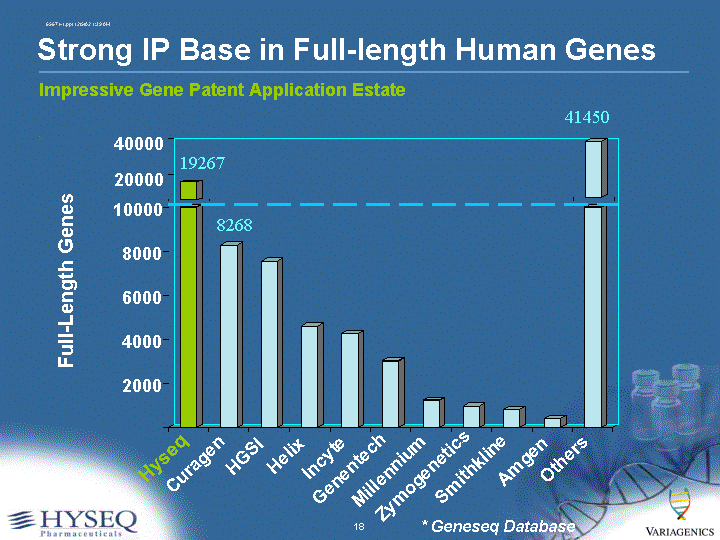
| Impressive Gene Patent Application Estate Strong IP Base in Full-length Human Genes Full-Length Genes 2000 4000 6000 8000 10000 Hyseq Curagen HGSI Helix Incyte Genentech Millennium Zymogenetics Smithkline Amgen Others 20000 40000 19267 8268 41450 * Geneseq Database |
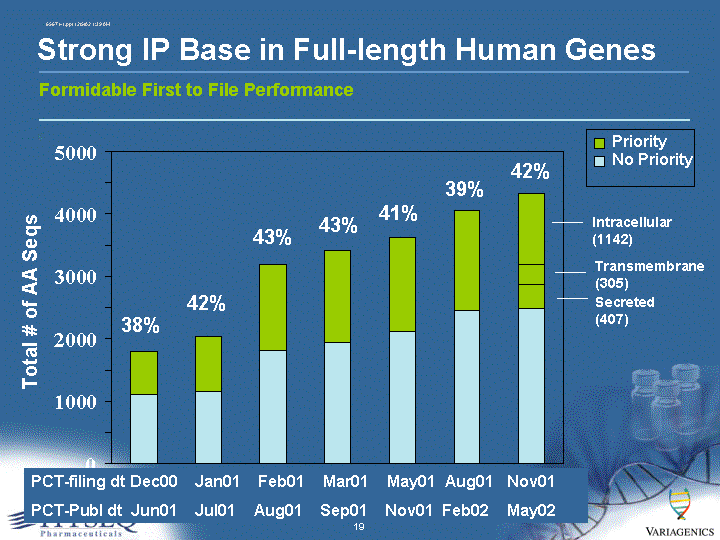
| Formidable First to File Performance Strong IP Base in Full-length Human Genes 0 1000 2000 3000 4000 5000 Total # of AA Seqs Priority No Priority PCT-filing dt Dec00 Jan01 Feb01 Mar01 May01 Aug01 Nov01 PCT-Publ dt Jun01 Jul01 Aug01 Sep01 Nov01 Feb02 May02 Intracellular (1142) Transmembrane (305) Secreted (407) 38% 42% 43% 43% 41% 39% 42% |
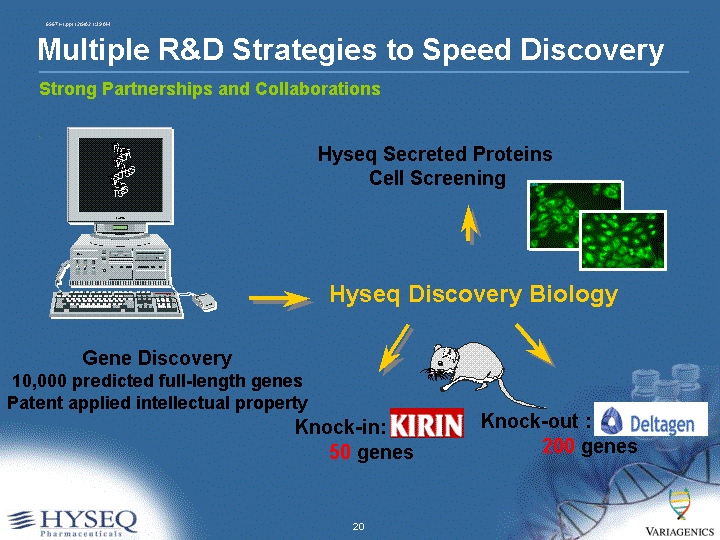
| Strong Partnerships and Collaborations Multiple R&D Strategies to Speed Discovery A T C G G T A T C G G T T C A C G C G T C G G T T C A C G C G T Gene Discovery 10,000 predicted full-length genes Patent applied intellectual property Knock-in: Kirin 50 genes Knock-out : Deltagen 200 genes Hyseq Discovery Biology Hyseq Secreted Proteins Cell Screening |

| 2002 Research Goals/Progress Kirin: Goal: Enter 50 secreted protein genes into knock-in mouse model Progress: >50 genes entered Deltagen: Goal: Enter 200 secreted protein genes into knock-out mouse model Progress: 147 entered Hyseq secreted protein genes: Goal: Complete cloning and cell assay tests of all secreted protein genes Progress: Over 800 secreted protein genes cloned, over 300 tested through multiplex cell-based assays Hyseq immunotherapeutic target genes: Goal: Identify cell surface protein targets for cancer-specific antibody development. Progress: Multiple target proteins identified and validated in B-cell cancers, breast and prostate cancer. |
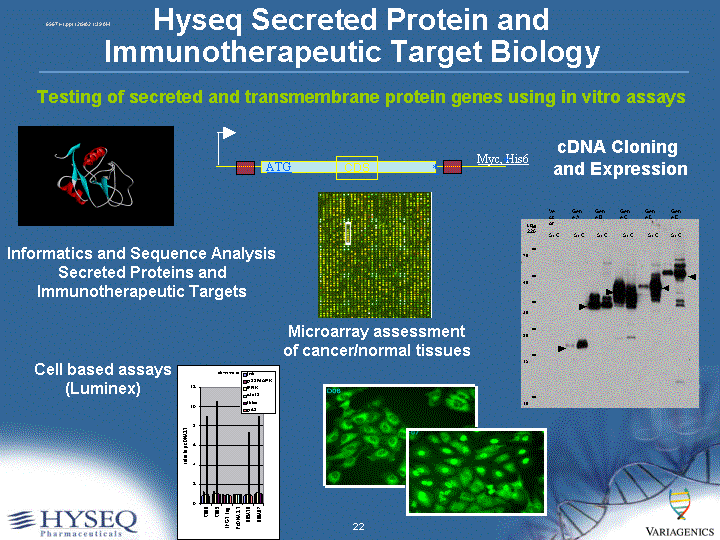
| Hyseq Secreted Protein and Immunotherapeutic Target Biology Testing of secreted and transmembrane protein genes using in vitro assays CDS ATG * Myc, His6 * Informatics and Sequence Analysis Secreted Proteins and Immunotherapeutic Targets cDNA Cloning and Expression Cell based assays (Luminex) Microarray assessment of cancer/normal tissues |
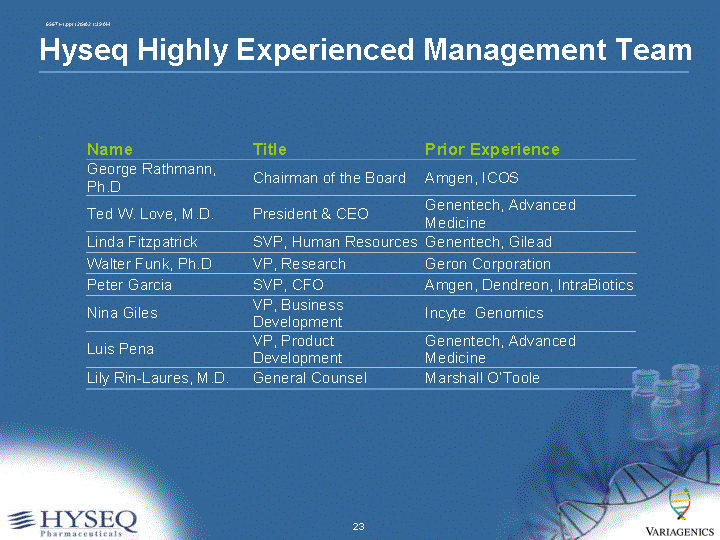
| Hyseq Highly Experienced Management Team |

| Recent Accomplishments Signed Merger Agreement with Variagenics Put Lead Product Candidate into Phase I Clinical Trials Completed Two PIPE Financings Secured Amgen Agreement - Hyseq Leads Clinical Development Major Reductions in Net Burn (costs + revenues) Entered 3 Major R&D Collaborations to Speed R&D Machine Increased Patent Filings on Over 10,000 Full Length Genes Recruited Highly Experienced New Management Team Refined Focus on Novel Biopharmaceuticals (created Callida joint venture with Affymetrix) |

| Near-Term Milestones Initiate Alfimeprase Phase II program in the first half of 2003 Identify additional clinical candidates from Hyseq genes Continue to build & strengthen patent estate Leverage Business Development Opportunities Revenue generation R&D collaborations Strategic alliances (U.S. & Europe) |

| Variagenics Highlights |

| Variagenics Highlights Novel, high value molecular diagnostic products for colorectal cancer Expedited regulatory pathway Sustainable revenue stream Scaleable Short-term R&D funding, long-term royalties First-rate partnerships validate Variagenics' pharmacogenomics platform Continue to leverage preeminent corporate partners for value creation Strong scientific expertise Capability of identifying clinically important diagnostic markers Intellectual property estate and technology with revenue potential Biomarkers Cutting-edge bioinformatics tools and systems |

| Focus on Oncology Large, growing patient population across all indications Diagnostics potential to inform critical medical decisions Strong biology of disease Numerous oncology drug candidates in late-stage development will drive development of molecular diagnostics New generation of cytotoxic and molecular targeted drugs in late stage development Highly competitive landscape Poor to moderate therapeutic index Pharmacogenomics key into development, approval and differentiation, commercialization |

| Molecular Diagnostics Market Opportunity Molecular diagnostics market is small ( $ 2 billion+ ) but growing rapidly (40% CAGR ) Majority homebrew format - rapid availability of new tests with little direct regulatory oversight 90% infectious disease; cancer segment embryonic - few precedents exist for VGNX tests Non - infectious disease segment growing at more than 20% per year Opportunity to develop and own attractive emerging market. VGNX positioned as high value content provider. |

| Novartis Cancer Diagnostic Partnership Variagenics to identify potential markers of drug efficacy for high profile compound in Phase II development for prostate cancer Gleevec(r) (STI 571) Full integration of Variagenics' molecular epidemiology technologies Variagenics receives R&D funding; retains exclusive rights to develop any resulting DNA diagnostic tests Significant potential to expand collaboration in the near-term |
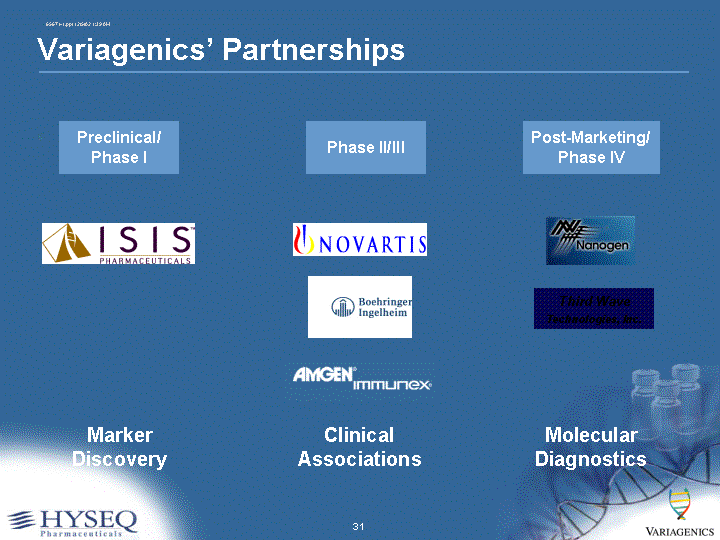
| Preclinical/ Phase I Marker Discovery Phase II/III Clinical Associations Post-Marketing/ Phase IV Molecular Diagnostics Third Wave Technologies, Inc. Variagenics' Partnerships |

| Recent Accomplishments Signed Merger Agreement with Hyseq Established collaboration with Novartis in Oncology Initiated proprietary cancer diagnostics product development program Monetized core intellectual property Restructured organization for focus |

| Strategic Rationale & Opportunity |

| Reasons for Merger - Hyseq Rationale for Proposed Transaction Merger strengthens Hyseq's ability to expedite product development Facilitates continued development of alfimeprase and additional Hyseq development programs Combined company to have sufficient cash to fund operations and development through December 2004 Significant cost savings, via some reduction in workforce, consolidation of corporate and administrative infrastructure and rationalization of product programs Both Hyseq and Variagenics product programs will be managed against strict development milestones |

| Reasons for Merger - Variagenics Rationale for Proposed Transaction Forward integration of Variagenics with a biotherapeutic product- focused company such as Hyseq Allows Variagenics' stockholders opportunity to participate in a larger, more diversified company After an extensive search for a business partner, Hyseq presented the most favorable business combination and acquisition proposal Ability to participate in Hyseq's development of alfimeprase Currently in Phase I clinical trials in eight U.S. centers Represents a significant U.S. market opportunity Rapid integration into a combined company that is expected to implement steps to ensure operational efficiencies The combined company is expected to have cash sufficient to fund its operations through December 2004 Placement of three Variagenics board members on the "NewCo" board of directors will help to ensure Variagenics will realize the above benefits |

| Medical Technology Stock Letter Monday, November 11, 2002 "This appears to be a nice fit for the two companies. For starters, VARIAGENICS brings with it a cash position of about $60 million (as of September 30). Thus, the combined entity will have enough to fund operations through late 2004. Combined, they also have a nice set of partners, with HYSQ bringing Amgen to the table, and VARIAGENICS bringing in a partnership with Novartis. We like the fact that HYSQ's management has proactively addressed their cash problem." THE WALL STREET JOURNAL (c) 2002 Dow Jones & Company. All Rights Reserved Friday, November 29, 2002 - VOL. CCXL NO. "Then there is the Hyseq-Variagenics deal. It is aimed at helping Hyseq manage a potential cash crunch; the proposed acquisition is expected to extend its life span through the end of 2004, compared with Hyseq's previous projections that it would run out of cash in the first quarter of 2003. The new company will cut expenses partly by reducing combined employment roughly 40% and by throttling back commitment to various research-and-development projects ... although its most advanced drug candidate, a clot-busting medication called alfimeprase, isn't in danger, says Chief Executive Ted Love." Public Commentary Rationale for Proposed Transaction |

| Expanded Partnering Opportunities Hyseq Secreted Gene Collection Immunotherapeutic Antibody Target Program Small Molecule Targets R & D Capabilities Informatics and Sequence Analysis cDNA Cloning and Expression Cell Based Assays Variagenics Molecular Epidemiology Programs Pharmacogenomic Association Studies SNP Assets SNP Patents (Pending) SNP/Haplotype Assets Combination of Individual Asset Bases and Capabilities conversations have moved from lower value "one-off" discussions to significant higher value partnering opportunities |

| "NewCo" Opportunity "NewCo" will maximize excellent cash position and experienced management team to support development of novel products Product-focused organization Strong cash position expected to fund operations for at least two years (Dec 2004) Near-term product opportunity in alfimeprase High value diagnostics program with near term revenue potential Strong partnerships with major pharmaceutical companies Amgen, Novartis, Kirin, Deltagen Significant combined intellectual property and technology assets Combined entity creates a stronger, more competitive company than either could on its own |
ADDITIONAL INFORMATION
In connection with the proposed merger, Hyseq and VARIAGENICS have filed a joint proxy statement/prospectus with the Securities and Exchange Commission (SEC). INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE JOINT PROXY STATEMENT/PROSPECTUS AS IT WILL CONTAIN IMPORTANT INFORMATION ABOUT HYSEQ, VARIAGENICS, THE MERGER AND RELATED MATTERS. INVESTORS AND SECURITY HOLDERS HAVE ACCESS TO FREE COPIES OF THE JOINT PROXY STATEMENT/PROSPECTUS AND OTHER DOCUMENTS FILED WITH THE SEC BY HYSEQ AND VARIAGENICS THROUGH THE SEC WEB SITE AT WWW.SEC.GOV. THE JOINT PROXY STATEMENT/PROSPECTUS AND RELATED MATERIALS MAY ALSO BE OBTAINED FOR FREE FROM HYSEQ AND VARIAGENICS BY CALLING THE CONTACT LISTED BELOW.
Hyseq, VARIAGENICS and their respective directors, executive officers, certain members of management and employees, may be deemed to be participants in the solicitation of proxies in connection with the proposed merger. Information regarding the persons who may, under the rules of the SEC, be considered to be participants in the solicitation of Hyseq’s stockholders in connection with the proposed merger is set forth in Hyseq’s proxy statement for its 2002 annual meeting of stockholders, dated June 28, 2002 and filed with the SEC on June 13, 2002. Information regarding the persons who may, under the rules of the SEC, be considered to be participants in the solicitation of VARIAGENICS’ stockholders in connection with the proposed merger is set forth in VARIAGENICS’ proxy statement for its 2002 annual meeting, dated April 30, 2002 and filed with the SEC on April 29, 2002. Additional information will be set forth in the joint proxy statement/prospectus when it is filed with the SEC.
FORWARD-LOOKING STATEMENTS
This document contains forward-looking statements within the meaning of the ‘safe harbor’ provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by words such as ‘believe,’ ‘expect,’ ‘anticipate,’ ‘should,’ ‘may,’ ‘estimate,’ ‘goals,’ and ‘potential,’ among others. These statements, including statements about the proposed merger, the reasons for timing of and benefits of the proposed merger, and future financial and operating goals and results, are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties, assumptions and other factors that could cause actual results to differ materially from those in the forward-looking statements. Such factors include risk that the proposed merger may not be approved by stockholders, Hyseq’s or VARIAGENICS’ inability to satisfy the closing conditions of the merger, risk that the two companies’ businesses will not be integrated successfully, costs related to the proposed merger, the termination of existing pharmaceutical and biotechnology collaborations, the combined company’s inability to further identify, develop and achieve commercial success for new products and technologies, the risk that the combined company may be unable to successfully finance and secure regulatory approval of and market its drug candidates, risks associated with VARIAGENICS’ technology, the combined company’s ability to protect its proprietary technologies, risk of new, changing and competitive technologies, and regulations in the U.S. and internationally, and other factors (such as economic, business, competitive and/or regulatory factors) affecting each company’s businesses generally as set forth in HYSEQ’s and VARIAGENICS’ filings with the SEC, including their Annual Reports on Form 10-K for the fiscal years ended 2001, their most recent Quarterly Reports on Form 10-Q and their Current Reports on Form 8-K. HYSEQ and VARIAGENICS each expressly disclaim any duty to update information contained herein.