Filing under Rule 425 under
the U.S. Securities Act of 1933
Filing by: Sankyo Company, Limited
Subject Company: Daiichi Pharmaceutical Co., Ltd.
and Sankyo Company, Limited
SEC File No. 132-02292
(updated version of previously submitted materials)

Business Integration
of
Daiichi Pharmaceutical Co.,Ltd
and
Sankyo Co., Ltd
25 February, 2005

Background to Integration
Kiyoshi Morita
President & CEO
Daiichi Pharmaceutical Co., Ltd
2

Today’s Announcement
Basic Agreement concluded today to integrate businesses of Daiichi and Sankyo
Holding company to be established on Oct 1*
Complete integration of prescription pharmaceutical operations by April 2007*
*Dates are proposed dates only
3

Outline of Holding Company
Name DAIICHI SANKYO COMPANY, LIMITED
Timing of Integration 1 October, 2005*
Integration Process Companies to become subsidiaries of joint holding company through joint share transfer
Head Office Present Head Office of Sankyo
Delisting and Listing
End September, 2005* – Daiichi, Sankyo delisted
1 October, 2005* – Holding company to be listed
Stock Transfer Ratios
1.159 shares in holding company for each Daiichi share
1 share in holding company for each Sankyo share
*Dates are proposed dates only
4

Milestones
March 2005 Establishment of Integration Committee
May 2005 Signing of Definitive Agreement
June 2005 Shareholders’ Meeting
End Sept 2005* Delisting of existing companies
1 October 2005* Holding company established
April 2007 Complete integration of prescription pharmaceutical operations
*Dates are proposed dates only
5

DAIICHI SANKYO
Achievement of true competitiveness as
a Japan based
global pharma-innovator
DAIICHI SANKYO
6

Background
7
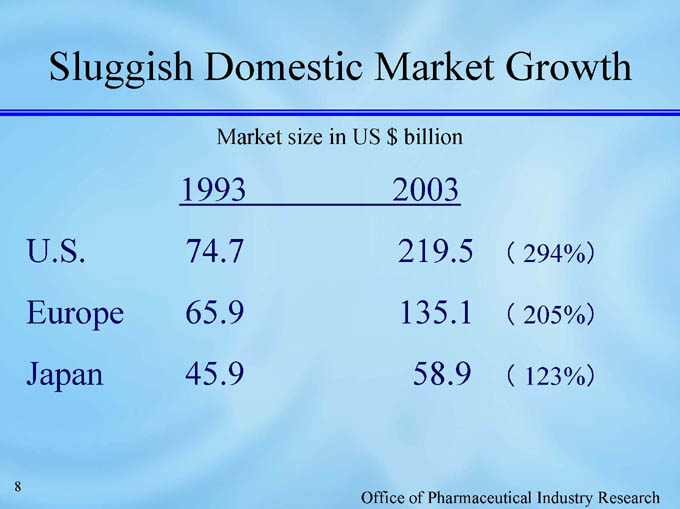
Sluggish Domestic Market Growth
Market size in US $ billion
1993 2003
U.S. 74.7 219.5 ( 294%)
Europe 65.9 135.1 ( 205%)
Japan 45.9 58.9 ( 123%)
Office of Pharmaceutical Industry Research
8
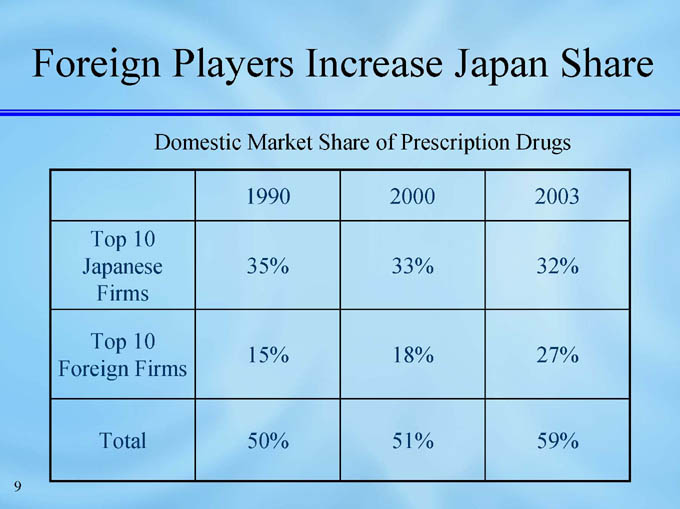
Foreign Players Increase Japan Share
Domestic Market Share of Prescription Drugs
1990
2000
2003
Top 10 Japanese Firms
35%
33%
32%
Top 10 Foreign Firms
15%
18%
27%
Total
50%
51%
59%
9
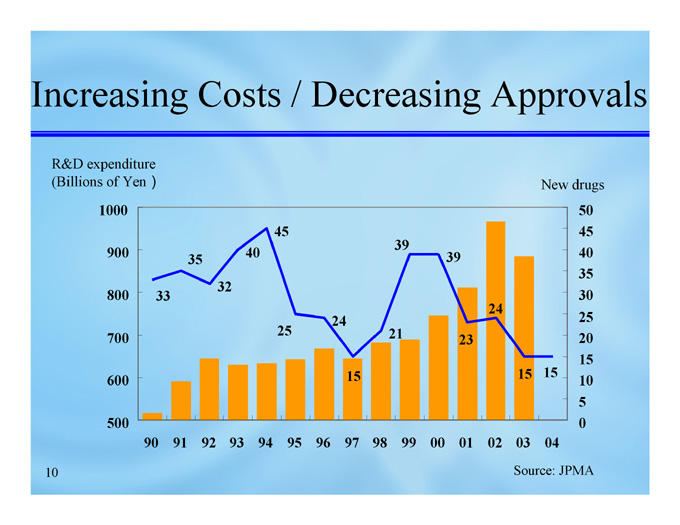
Increasing Costs / Decreasing Approvals
R&D expenditure
(Billions of Yen)
New drugs
1000
50
45
45
39
900
40
39
40
35
35
32
800
33
30
24
24
25
25
20
700
23
21
15
15
15
10
600
15
5
0
500
03
02
01
00
99
98
97
96
95
94
93
92
91
90
04
Source: JPMA
10
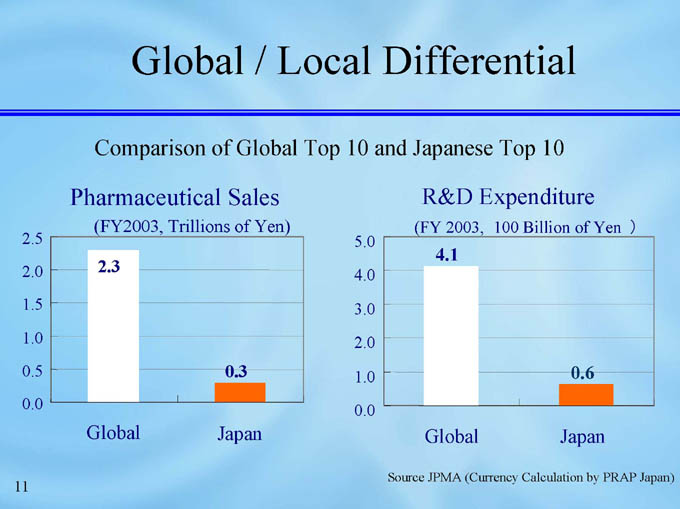
Global / Local Differential
Comparison of Global Top 10 and Japanese Top 10
Pharmaceutical Sales
R&D Expenditure
)
100 Billion of Yen
(FY 2003,
(FY2003, Trillions of Yen)
5.0
2.5
4.1
2.3
4.0
2.0
3.0
1.5
2.0
1.0
0.3
0.6
1.0
0.5
0.0
0.0
Japan
Japan
Global
Global
Source JPMA (Currency Calculation by PRAP Japan)
11

Benefits
12

Benefits of Integration
Integration provides:
Strong R&D franchise categories
Global reach and overwhelming domestic sales strength
Expansion of corporate strategy options arising from increased scale
Increased operational efficiency
Outstanding personnel resources
13

Benefits for Daiichi
Integration provides Daiichi:
Enhanced capabilities of global clinical studies and drug discovery
Further enhancement of domestic sales strength through doubling of MR numbers and increased share with specified distributors
Business infrastructure/opportunities in Europe
14
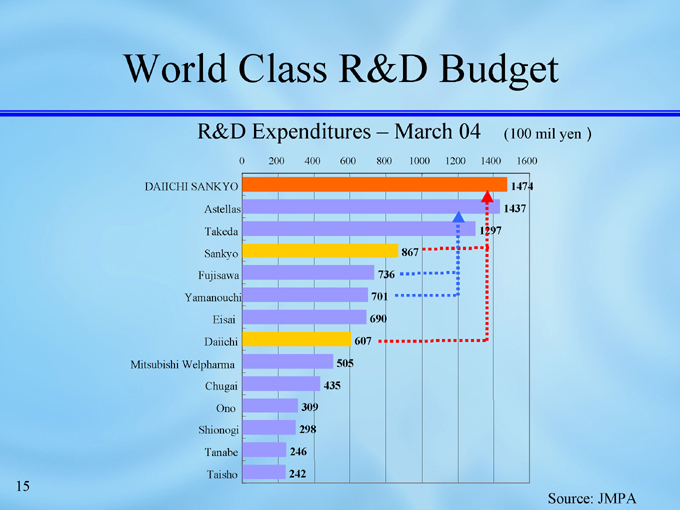
World Class R&D Budget
R&D Expenditures – March 04 (100 mil yen)
1400
1200
1000
800
600
400
200
0
1600
1474
DAIICHI SANKYO
1437
Astellas
1297
Takeda
867
Sankyo
736
Fujisawa
Yamanouchi
701
690
Eisai
607
Daiichi
505
Mitsubishi Welpharma
435
Chugai
309
Ono
298
Shionogi
246
Tanabe
242
Taisho
Source: JMPA
15
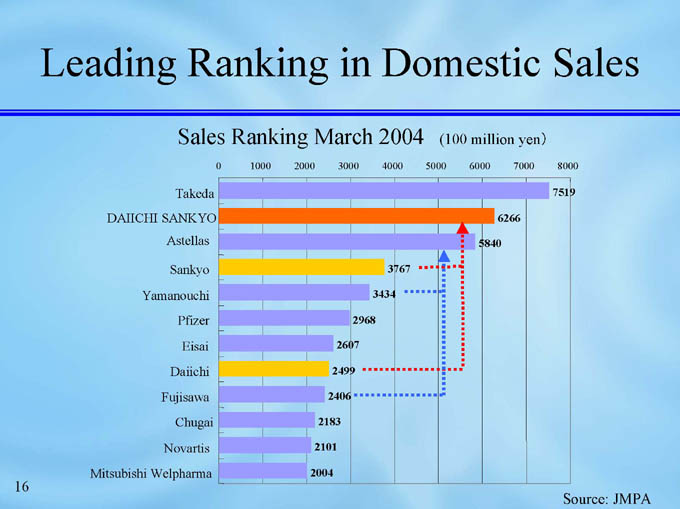
Leading Ranking in Domestic Sales
Sales Ranking March 2004 (100 million yen)
7000
6000
5000
4000
3000
2000
1000
0
8000
Takeda
7519
DAIICHI SANKYO
6266
Astellas
5840
Sankyo
3767
3434
Yamanouchi
Pfizer
2968
Eisai
2607
Daiichi
2499
Fujisawa
2406
Chugai
2183
Novartis
2101
Mitsubishi Welpharma
2004
Source: JMPA
16

A Japan based
global pharma-innovator
DAIICHI SANKYO

Synergies
Takashi Shoda
President & CEO
Sankyo Co., Ltd.
18

Benefits of Integration
Integration provides:
Strong R&D franchise categories
Global reach and overwhelming domestic sales strength
Expansion of corporate strategy options arising from increased scale
Increased operational efficiency
Outstanding personnel resources
19

Benefits for Sankyo
Strengthening of product mix in franchise categories, centering on cardiovascular
Access to Daiichi’s expertise and know-how in cancer category
Seamless pipeline post- CS-747, CS-505
Increase in number of quality MR’s domestically
Increase in corporate strategy options through expansion of overseas operations
Enhancement of corporate strategy in Asia (China, Korea)
20
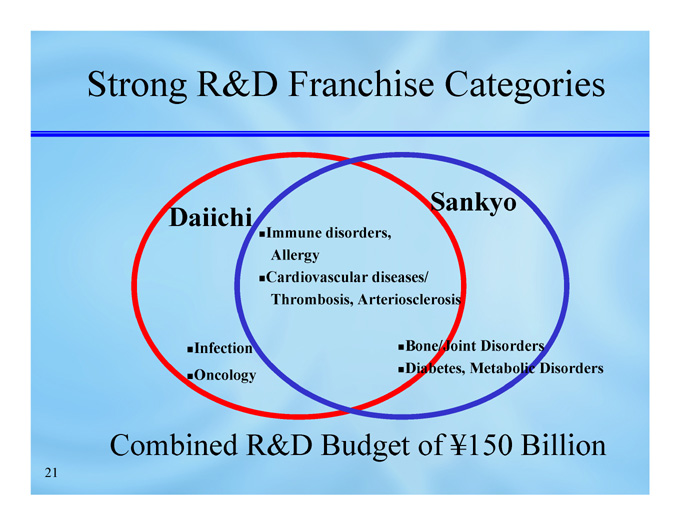
Strong R&D Franchise Categories
Sankyo
Daiichi
Immune disorders, Allergy
Cardiovascular diseases/ Thrombosis, Arteriosclerosis
Infection
Oncology
Bone/Joint Disorders
Diabetes, Metabolic Disorders
Combined R&D Budget of ¥150 Billion
21
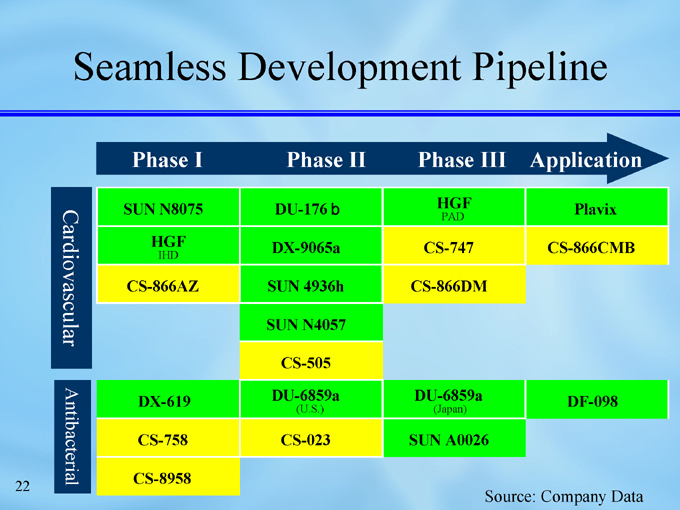
Seamless Development Pipeline
Phase III
Phase II
Phase I
Application
Cardiovascular
HGF
DU-176b
SUN N8075
Plavix
PAD
HGF
CS-747
DX-9065a
CS-866CMB
IHD
SUN 4936h
CS-866AZ
CS-866DM
SUN N4057
CS-505
Antibacterial
DU-6859a
DU-6859a
DX-619
DF-098
(U.S.)
(Japan)
CS-023
CS-758
SUN A0026
CS-8958
Source: Company Data
22
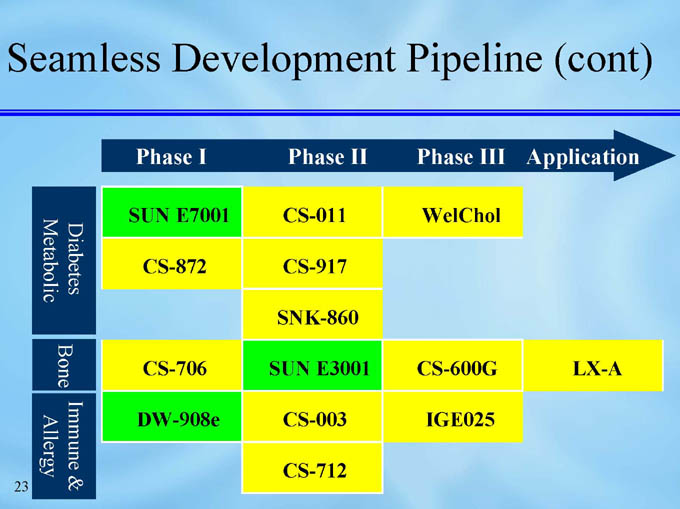
Seamless Development Pipeline (cont)
Phase III
Phase II
Phase I
Application
Diabetes Metabolic
CS-011
SUN E7001
WelChol
CS-872
CS-917
SNK-860
LX-A
CS-600G
SUN E3001
CS-706
Bone
Immune & Allergy
CS-003
DW-908e
IGE025
CS-712
23
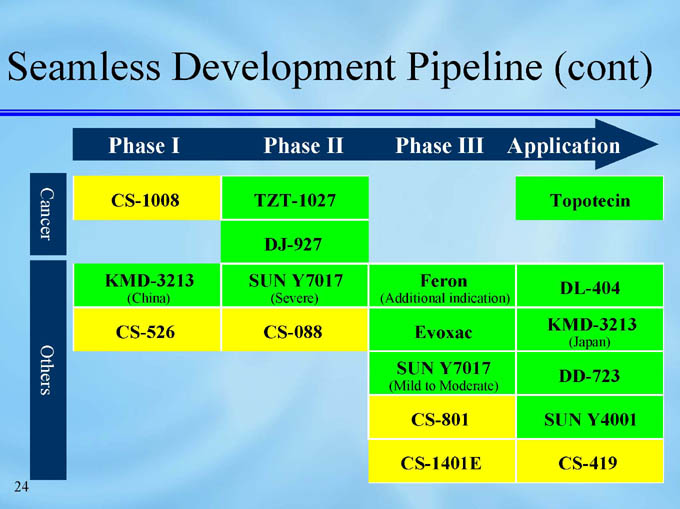
Seamless Development Pipeline (cont)
Phase III
Phase II
Phase I
Application
Cancer
TZT-1027
CS-1008
Topotecin
DJ-927
Others
SUN Y7017
KMD-3213
Feron
DL-404
(Severe)
(China)
(Additional indication)
KMD-3213
CS-088
CS-526
Evoxac
(Japan)
SUN Y7017
DD-723
(Mild to Moderate)
CS-801
SUN Y4001
CS-1401E
CS-419
24
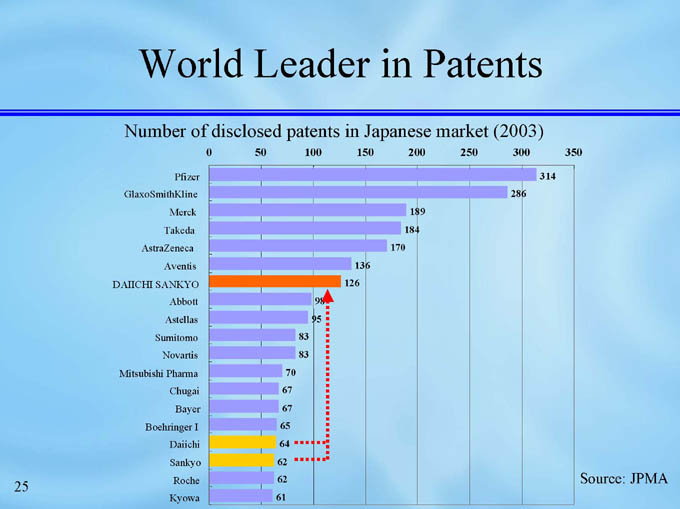
World Leader in Patents
Number of disclosed patents in Japanese market (2003)
300
250
200
150
100
50
0
350
314
Pfizer
286
GlaxoSmithKline
189
Merck
184
Takeda
170
AstraZeneca
136
Aventis
126
DAIICHI SANKYO
98
Abbott
95
Astellas
83
Sumitomo
83
Novartis
70
Mitsubishi Pharma
67
Chugai
67
Bayer
65
Boehringer I
64
Daiichi
62
Sankyo
62
Source: JPMA
Roche
61
Kyowa
25
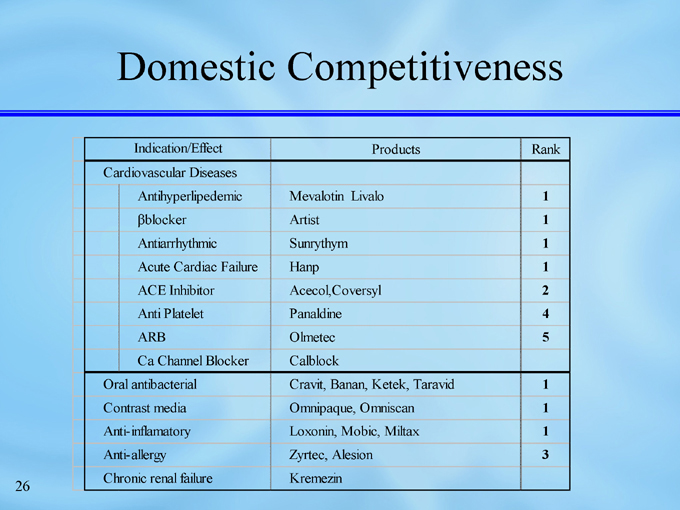
Domestic Competitiveness
Indication/Effect
Rank
Products
Cardiovascular Diseases
1
Mevalotin Livalo
Antihyperlipedemic
1
Artist
ßblocker
1
Sunrythym
Antiarrhythmic
1
Hanp
Acute Cardiac Failure
2
Acecol,Coversyl
ACE Inhibitor
4
Panaldine
Anti Platelet
5
Olmetec
ARB
Calblock
Ca Channel Blocker
1
Cravit, Banan, Ketek, Taravid
Oral antibacterial
1
Omnipaque, Omniscan
Contrast media
1
Loxonin, Mobic, Miltax
Anti-inflamatory
3
Zyrtec, Alesion
Anti-allergy
Kremezin
Chronic renal failure
26
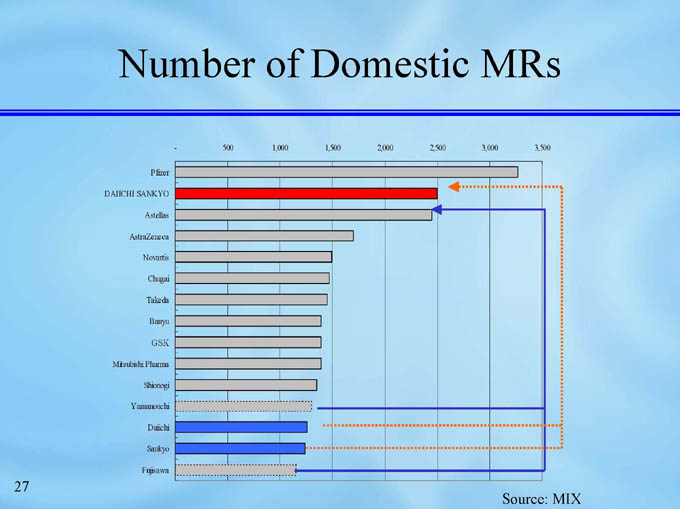
Number of Domestic MRs
3,000
2,500
2,000
1,500
1,000
500
-
3,500
Pfizer
DAIICHI SANKYO
Astellas
AstraZeneca
Novartis
Chugai
Takeda
Banyu
GSK
Mitsubishi Pharma
Shionogi
Yamanouchi
Daiichi
Sankyo
Fujisawa
Source: MIX
27
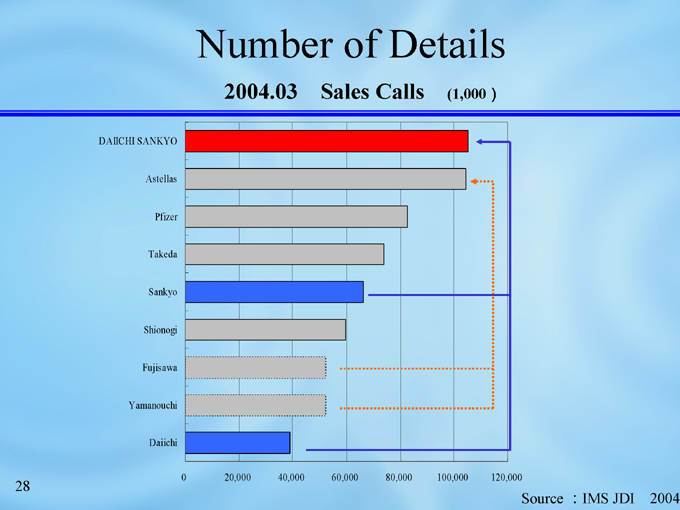
Number of Details
2004.03 Sales Calls (1,000)
DAIICHI SANKYO
Astellas
Pfizer
Takeda
Sankyo
Shionogi
Fujisawa
Yamanouchi
Daiichi
100,000
80,000
60,000
40,000
20,000
0
120,000
Source :IMS JDI 2004
28

Increased Presence with Wholesalers
Share at Top 20 Wholesalers
Top 9 companies
Second 1 company
Third 2 companies
Fourth 3 companies
Fifth 1 company
Sixth or lower 4 companies
Source: Crecon
Top 20 wholesalers cover 79% of market
29
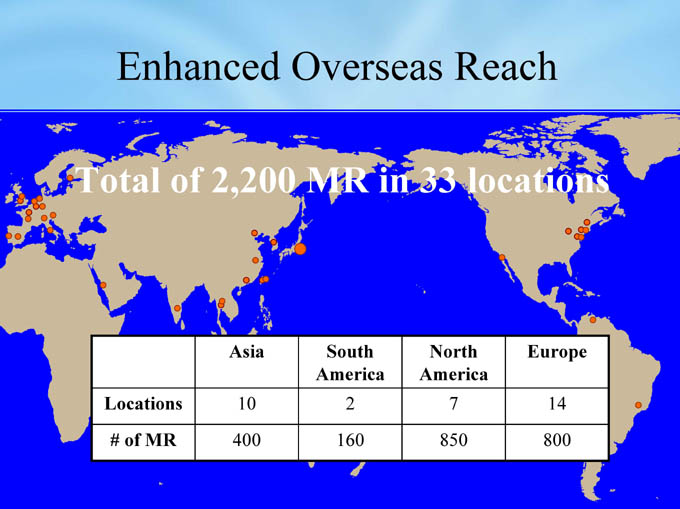
Enhanced Overseas Reach
Total of 2,200 MR in 33 locations
Asia
South
North
Europe
South America
America
Locations
10
2
7
14
10
2
7
14
of MR
#
of MR
#
400
160
850
800
30

Others
Integration provides:
Expansion of corporate strategy options arising from increased scale
Increased operational efficiency
Outstanding personnel resources
31

Outline of Holding Company
32
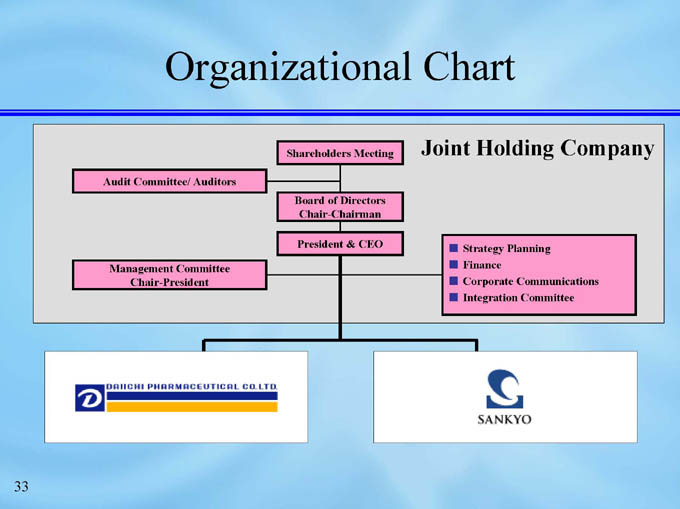
Organizational Chart
Joint Holding Company
Shareholders Meeting
Audit Committee/ Auditors
Board of Directors
Chair-Chairman
President & CEO
Strategy Planning
Finance
Corporate Communications
Integration Committee
Management Committee
Chair-President
33

Directors and Officers
Representative Director & Chairman
Kiyoshi Morita
Representative Director & President CEO
Takashi Shoda
Executive Director Hiroyuki Nagasako
Executive Director Hideho Kawamura
Executive Director Yasuhiro Ikegami
Executive Director Tsutomu Une
Non-executive Director 4 (to be appointed)
Corporate Auditor 2 (to be appointed)
External Corporate Auditor 2 (to be appointed)
34
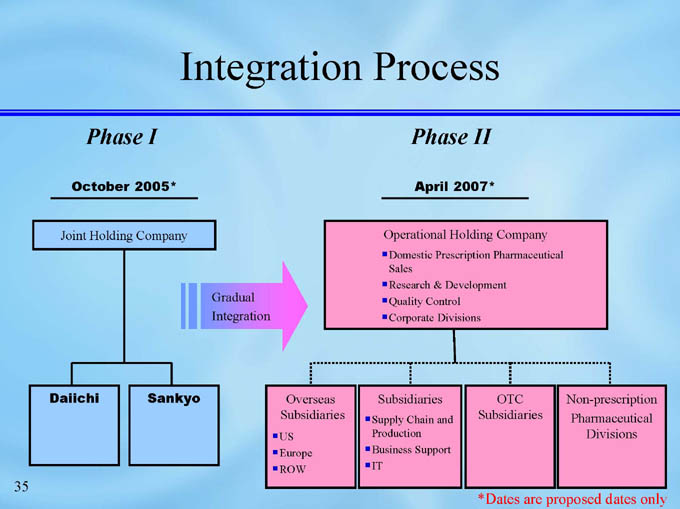
Integration Process
Phase I
Phase II
October 2005*
April 2007*
Operational Holding Company
Joint Holding Company
Domestic Prescription Pharmaceutical Sales
Research & Development
Quality Control
Corporate Divisions
Gradual
Integration
Daiichi
Non-prescription
Pharmaceutical Divisions
OTC Subsidiaries
Subsidiaries
Overseas
Subsidiaries
Sankyo
Supply Chain and Production
Business Support
IT
US
Europe
ROW
*Dates are proposed dates only
35

Corporate Mission
It is our mission to satisfy the medical needs of people around the globe through the ongoing creation of innovative pharmaceuticals and services
In designing all operational processes, functions and organizational structure we will leverage the strengths of each company, learn from each other with an open mind and objectively appraise and adopt the best of best, placing the interests of the integrated company first
This integration will be on equal footing, with fair, and non-discriminatory policies in job assignment and opportunities, merit based appraisal and remuneration , regardless of previous affiliation, nationality, age or gender.
36

A Japan based
global pharma-innovator
DAIICHI SANKYO
37

Filings with the U.S. SEC
Sankyo Co., Ltd, and Daiichi Pharmaceutical Co., Ltd may file a registration statement on Form F-4 with the U.S. SEC in connection with the proposed business combination of Daiichi Pharmaceutical Co., Ltd and Sankyo Co., Ltd, under a new holding company by way of a joint share transfer. The Form F-4 (if filed) will contain a prospectus and other documents. If a Form F-4 is filed and declared effective, Sankyo Co., Ltd. and Daiichi Pharmaceutical Co., Ltd plan to mail the prospectus contained in the Form F-4 to their U.S. shareholders prior to the shareholders meetings at which the share exchange will be voted upon. The Form F-4 (if filed) and prospectus will contain important information about Sankyo Co., Ltd and Daiichi Pharmaceutical Co., Ltd, the joint share transfer and related matters. U.S. shareholders of Sankyo Co., Ltd are urged to read the Form F-4, the prospectus and the other documents that may be filed with the U.S. SEC in connection with the joint share transfer carefully before they make any decision at the shareholders meeting with respect to the joint share transfer. The Form F-4 (if filed), the prospectus and all other documents filed with the U.S. SEC in connection with the joint share transfer will be available when filed, free of charge, on the U.S. SEC’s web site at www.sec.gov. In addition, the prospectus and all other documents filed with the U.S. SEC in connection with the business combination will be made available to shareholders, free of charge, by calling, writing or e-mailing:
Sankyo Co., Ltd: Daiichi Pharmaceutical Co., Ltd;
Mr Shigemichi Kondo Mr Toshio Takahashi
Corporate Communications Dept Corporate Communications Dept
3-5-1 Nihonbashi-honcho, Chuo-ku, Tokyo 14-10 Nihonbashi, 3-chome, Chuo-ku, Tokyo,
Tel: +813-5255-7034 Tel: +813-3273-7107
shige-k@sankyo.co.jp andokb5o@daiichipharm.co.jp
You may read and copy any reports and other information filed with, or submitted to, the U.S. SEC at the U.S. SEC’s public reference rooms at 450 Fifth Street, N.W., Washington, D.C. 20549 or at the other public reference rooms in New York, New York and Chicago, Illinois. Please call the U.S. SEC at 1-800-SEC-0330 for further information on public reference rooms. Filings with the U.S. SEC also are available to the public from commercial document-retrieval services and at the web site maintained by the U.S. SEC at http//www.sec.gov.
38

Forward-Looking Statements
This communication contains forward-looking information and statements about Sankyo Co.,Ltd and Daiichi Pharmaceutical Co., Ltd and their combined businesses after completion of the joint share transfer. Forward-looking statements are statements that are not historical facts. These statements include financial projections and estimates and their underlying assumptions, statements regarding plans, objectives and expectations with respect to future operations, products and services, and statements regarding future performance. Forward-looking statements are generally identified by the words “expect,” “anticipates,” “believes,” “intends,” “estimates” and similar expressions. Although the management of Sankyo Co., Ltd and Daiichi Pharmaceutical Co,. Ltd believe that the expectations reflected in such forward-looking statements are reasonable, investors and holders of Sankyo Co., Ltd and Daiichi Pharmaceutical Co., Ltd, securities are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sankyo Co., Ltd and Daiichi Pharmaceutical Co., Ltd, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include those discussed or identified in the public filings with the SEC and the local filings made by Sankyo Co., Ltd and Daiichi Pharmaceutical Co., Ltd. including those listed under “Cautionary Statement Concerning Forward-Looking Statements” and “Risk Factors” in the prospectus included in the registration statement on Form F-4 that Sankyo Co., Ltd and Daiichi Pharmaceutical Co., Ltd. may file with the U.S. SEC. Other than as required by applicable law, neither Sankyo Co, Ltd nor Daiichi Pharmaceutical Co., Ltd undertakes any obligation to update or revise any forward-looking information or statements.
39






































