Exhibit 99.1
Telavancin for the Treatment of Hospital-Acquired
Pneumonia in Severely Ill and Older Patients:
The ATTAIN Studies
E Rubinstein,(1) GR Corey,(2) HW Boucher,(3) MS Niederman,(4) AF Shorr,(5) A Torres,(6) SL Barriere,(7) HD Friedland(7); On behalf of the ATTAIN Study Group
(1)University of Manitoba, Winnipeg, Canada; (2)Duke University Medical Center, Durham, NC, USA; (3)Tufts Medical Center, Boston, MA, USA; (4)SUNY, Stony Brook, NY, USA; (5)Georgetown University, Washington, DC, USA; (6)Hospital Clínic de Barcelona, Barcelona, Spain; (7)Theravance, Inc., South San Francisco, CA, USA
Presented at the 48th Annual ICAAC/IDSA 46th Annual Meeting, Washington, DC, October 25-28, 2008.
1
ABSTRACT (REVISED)
Background. Telavancin (TLV) is an investigational lipoglycopeptide with potent bactericidal activity in vitro against Gram-positive pathogens including methicillin-resistant Staphylococcus aureus (MRSA). The ATTAIN program studied TLV for the treatment of hospital-acquired pneumonia (HAP). In this analysis, we compared the clinical cure rates achieved with TLV or vancomycin (VAN) for severely ill and older patients.
Methods. ATTAIN 1 and 2 were methodologically identical, randomized, double-blind, Phase 3 studies. Patients (>18 years of age) with HAP caused by suspected or confirmed Gram-positive pathogens were randomized to TLV 10 mg/kg intravenous (IV) q24 h or VAN 1 g IV q12 h for 7 to 21 days. Test-of-cure (TOC) visit was conducted 7 to 14 days after end-of-study treatment. Clinically evaluable (CE) patients were those who met prespecified criteria for evaluability.
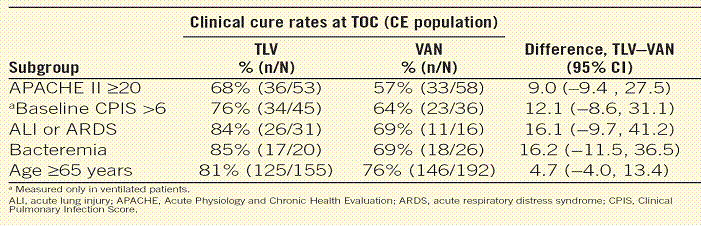
Results. Pooled clinical cure rates at TOC for several clinically relevant subgroups including the elderly as well as patients with severe HAP at baseline are presented in the Table. The overall incidence of treatment-emergent adverse events was comparable between treatment groups.
Conclusions. TLV achieved numerically higher cure rates than conventional therapy for treatment of HAP in severely ill and older patients with comparable treatment-emergent adverse events.
2
INTRODUCTION
· In the United States, pneumonia is the leading cause of death among patients with health care-associated infections.(1)
· A recent survey of culture-positive pneumonia identified Staphylococcus aureus as a dominant pathogen for all types of pneumonia, including hospital-acquired pneumonia (HAP), and the only pathogen that correlates with mortality.(2)
· Conventional treatment is associated with high mortality among patients with bacteremic HAP due to S. aureus.(3)
· Telavancin is an investigational, rapidly bactericidal lipoglycopeptide with a potent multifunctional mechanism of action against clinically relevant Gram-positive pathogens, including methicillin-resistant S. aureus.(4)
· In Phase 3 clinical trials for complicated skin and skin structure infections and HAP, telavancin achieved its objective of noninferiority in the all-treated (AT) and clinically evaluable (CE) patient populations.(5),(6)
· In this analysis, we compared telavancin and vancomycin for treatment of HAP in severely ill or older patients from the Assessment of Telavancin for Hospital-acquired Pneumonia (ATTAIN) studies.
METHODS
Patients
Major inclusion criteria
· Men and nonpregnant women >18 years of age.
· Clinical signs and symptoms consistent with pneumonia acquired after 48 hours in an inpatient acute- or chronic-care facility, or acquired within 7 days after being discharged following >3 days of hospital stay.
· Radiographic and laboratory findings consistent with bacterial pneumonia.
· Adequate respiratory specimen for Gram stain and culture.
Major exclusion criteria
· Receipt of potentially effective systemic antibiotic therapy for Gram-positive pneumonia for more than 24 hours immediately prior to randomization.
3
· Respiratory tract specimens with only Gram-negative bacteria seen on Gram stain or culture.
· An infection due to organisms known to be resistant to either of the study medication regimens or suspected pulmonary disease that precluded evaluation of therapeutic response.
· Documented or suspected meningitis, endocarditis, osteomyelitis, or evidence for compromised host immune response.
Study design
· ATTAIN 1 and 2 were methodologically identical, randomized, double-blind, comparator-controlled, parallel-group Phase 3 studies conducted at 275 sites in 38 countries.
· Enrollment took place between January 2005 and June 2007.
· Patients were randomized (1:1) to receive telavancin 10 mg/kg intravenous (IV) every 24 hours or vancomycin 1 g IV every 12 hours for 7 to 21 consecutive days.
· Telavancin dose was adjusted in patients with creatinine clearance of <50 mL/min.
· Vancomycin dose was adjusted per site-specific guidelines.
· Switching to antistaphylococcal penicillin was allowed for infections with methicillin-susceptible S. aureus.
· Concomitant treatment with aztreonam and/or metronidazole was permitted for polymicrobial infections.
· Baseline clinical assessments included medical history, physical examination, chest radiograph or chest computed tomography, arterial blood gas in patients on mechanical ventilation or with an arterial line, creatinine clearance calculation, and electrocardiogram.
· Components of the Acute Physiology and Chronic Health Evaluation (APACHE) II score were collected for all patients. Calculations were performed by imputing zeroes for missing components.
· Severity of illness was determined based on each of the following: APACHE II score >20, baseline Clinical Pulmonary Infection Score >6, presence or absence of acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), presence or absence of bacteremia, and age >65 years.
· Safety assessments included vital signs, adverse events, electrocardiograms, and laboratory parameters.
4
Analysis populations
· AT: Randomized patients who received >1 dose of study medication.
· Modified all-treated (MAT): AT patients with baseline pathogen.
· CE: Compliant AT patients with a clinical response of “cure” after receiving study medication >5 days and at least 80% of the intended dose, or “failure” following >3 days on study medication at test-of-cure (TOC) 7 to 14 days after last dose of study drug.
· Microbiologically evaluable (ME): CE patients with a Gram-positive pathogen recovered from baseline respiratory specimens or blood cultures.
Study end points
· Clinical response at TOC:
· Cure: Signs and symptoms of pneumonia resolved
· Failure: Persistence/progression of pneumonia or relapse after end of therapy, termination of study medication due to lack of efficacy, death after >3 days attributable to the primary infection
· Indeterminate: Inability to determine outcome.
Statistics
· Descriptive statistics were calculated for patient demographics and safety data.
· Two-sided, 95% confidence intervals were constructed on the difference in cure rates (telavancin minus vancomycin).
5
RESULTS
Disposition and outcomes
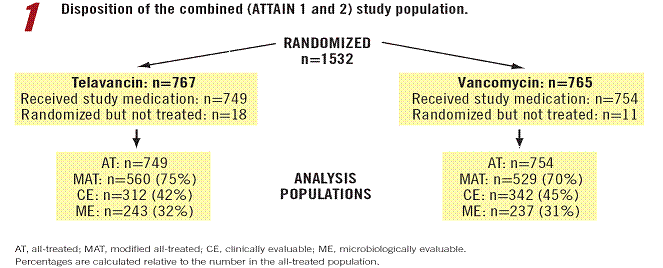
· A total of 1532 individuals were randomized to receive study medication and 1503 received at least 1 dose of study medication (Figure 1).
· In the combined CE population of ATTAIN, the proportions of severely ill patients and older patients (>65 years) were mostly balanced between treatment groups, although more patients in the telavancin group had ALI or ARDS (Table 1).

· The cure rates obtained with telavancin were comparable to those achieved with conventional therapy across all severity subgroups. The cure rates were numerically higher in the APACHE II >20, ALI or ARDS, bacteremia, and age >65 years severity subgroups (Figure 2).
6

Safety
· The incidence of treatment-emergent adverse events in patients meeting at least 1 of the criteria for more severe HAP or underlying illness were similar for both treatment groups (Table 2).

CONCLUSIONS
· Telavancin achieved numerically higher cure rates than vancomycin for treatment of HAP in severely ill and older patients.
· The comparable incidence of treatment-emergent adverse events between telavancin and vancomycin suggest that, if approved, telavancin may be an important option for the treatment of these vulnerable populations.
7
REFERENCES
(1) Klevens RM et al. Public Health Rep 2007;122:160–166.
(2) Kollef MH et al. Chest 2005;128:3854–3862.
(3) Gonzalez C et al. Clin Infect Dis 1999;29:1171–1177.
(4) Attwood RJ, LaPlante KL. Am J Health Syst Pharm 2007;64:2335–2348.
(5) Stryjewski ME et al. Clin Infect Dis 2008;46:1683–1693.
(6) Rubinstein E et al. Clin Microbiol Infect 2008;14:S14.
8




