UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2007
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
COMMISSION FILE NUMBER: 814-00203
UTEK CORPORATION
| | |
| Delaware | | 59-3603677 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
2109 Palm Avenue
Tampa, FL 33605
(Address of principal executive offices)
(813) 754-4330
(Registrant’s telephone number)
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
| | |
TITLE OF EACH CLASS | | NAME OF EACH EXCHANGE ON WHICH REGISTERED |
| COMMON STOCK, $.01 PAR VALUE | | AMERICAN STOCK EXCHANGE |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT: NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter periods as the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ¨ Accelerated filer x Non-accelerated filer ¨ Smaller reporting company ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of the registrant’s Common Stock held by non-affiliates at June 30, 2007 was $95,278,827.
As of February 27, 2008, there were 9,165,243 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for the 2008 Annual Meeting of Shareholders are incorporated by reference into Part III of this report.
TABLE OF CONTENTS
UTEK CORPORATION
Annual Report on Form 10-K for the Fiscal Year Ended December 31, 2007
PART I
Unless the context requires otherwise, reference in this Form 10-K to “UTEK”, the “Company”, “we,” “us” and words of similar import refer to UTEK Corporation and its wholly owned subsidiaries, UTEK-Europe, Ltd. and UTEKip, Ltd.
General
UTEK Corporation (the “Company”) commenced operations in 1997 in the business of technology transfer originally incorporated under the laws of the State of Florida, and subsequently under the laws of the State of Delaware in July 1999.
UTEK is an Open Innovation service company. UTEK’s services enable clients to become stronger innovators, source externally developed technologies and create value from their intellectual property. UTEK is a business development company with operations in the United States, United Kingdom and Israel.
UTEK provides Open Innovation services for its clients, including: technology transfer, intellectual property analysis, online technology exchanges and related consulting services. Open Innovation is a process which refers to enhancing a company’s products through in-licensing external sources of innovation and the creation of additional value through out-licensing intellectual property.
The potential client benefits from UTEK Corporation’s Open Innovation services include:
| | • | | Reduced research and development expense; |
| | • | | Opportunity to leverage external research and development; and |
| | • | | Create value from intellectual property. |
Our services enable companies to acquire externally developed technologies from universities and research laboratories worldwide to augment their internal research and development efforts. In addition, UTEK provides services to help companies create value from their intellectual property. Technology transfer refers to the process by which new technologies, developed in universities, government research facilities, or similar research settings, are licensed to companies for potential commercial development and use. Our goal is to provide our clients an opportunity to acquire and commercialize innovative technologies primarily developed by universities, medical centers, federal research laboratories and select corporations.
We also provide our clients with a full range of complementary Open Innovation related services and products, including the provision of technical and business expertise to help companies analyze, assess, protect and leverage their patents and intellectual property assets to enhance their business.
We are a non-diversified, closed-end management investment company that has elected to be regulated as a business development company (“BDC”) under the Investment Company Act of 1940 (the “1940 Act”). We do not have a registered investment adviser, and our management, under the supervision of our Board of Directors, makes our investment and management decisions.
Corporate Strategy
Our strategic objective is to increase our net assets by providing Open Innovation services and by effectuating technology transfer transactions with companies pursuant to which we receive securities or cash as compensation for the sale of technology rights. Our strategy incorporates the following key elements:
Develop relationships with companies through our technology acquisition alliance program. Our technology acquisition alliances are designed to help companies enhance their new product pipeline through the
1
acquisition of proprietary technologies primarily from universities, medical centers and federal research laboratories. We normally receive either cash or unregistered shares of common stock from clients as payment for the services we render to them under our technology acquisition alliances. All of our technology transfers are completed pursuant to our technology acquisition alliance agreements with our clients.
Execute technology transfers through our U2B®process. To effectuate a technology transfer, we will typically create a newly formed company to acquire a new technology from a university, medical center or federal research laboratory and then sell this newly formed company to our client for securities or, to a lesser extent, cash. We call this technology transfer process U2B®. It is our plan that the shares we receive in these exchanges will, in the course of our business, be sold for cash or other assets.
A benefit of effectuating technology transfer transactions through our U2B® investment process is that such transactions do not result in a current taxable event for us for income tax purposes. We have not acquired, and do not currently intend to acquire a new technology from a university, medical center or federal research laboratory in connection with our U2B® process without the prior agreement of our client to subsequently acquire such new technology from us. As a result, our technology transfer service is a just-in-time transfer service that does not require maintaining an inventory of licensable technology.
Continue to build strong relationships with research institutions.In order to provide us access to new technologies, we have entered into agreements with universities and research centers worldwide. We intend to continue to build on these relationships and create new relationships with additional universities and research centers.
Provide strategic services and products to companies and research institutions.We seek to provide companies and research institutions with a full range of complementary technology transfer related services and products including the following:
| | • | | KnowledgeExpress.com Website—Used by technology transfer, intellectual property, licensing and business development professionals in universities, corporations and government agencies for access to a comprehensive information platform combining business development and technology resources with expert search and report generation. |
| | • | | Pharma-Transfer.com Website—Tracks the research and development efforts of universities, research institutions and life science companies in order to locate and deliver advanced technology acquisition opportunities to corporate subscribers in the pharmaceutical industry. |
| | • | | TechEx.com Website—Used by technology transfer and research professionals to efficiently exchange biomedical licensing opportunities and innovations available for partnering. This exchange was developed by Yale University. |
| | • | | Uventures.com Website—An Internet-based exchange primarily used for the marketing of physical science technologies developed at universities and research organizations. |
| | • | | Pharmalicensing.com Website—An online, global resource for partnering, licensing and business development within the life science and biopharmaceutical industry. |
| | • | | UTEK Intellectual Capital Consulting (ICC)—Provides strategic intellectual property consulting services to assist our client companies in assessing and leveraging their intellectual property assets. |
Pursue complementary acquisitions. Many of the products and services we offer to companies were acquired by us through strategic acquisitions. We plan to make additional acquisitions of related or synergistic businesses in the future. In considering whether to purchase a company, we evaluate a number of factors including synergy with services that we currently provide, reputation in the industry, the ability to assist our existing business units, quality of service provided, territory that the service is offered in, purchase price, and the strength of relationships with client companies.
2
Leverage the experience and relationships of our management team.We plan to utilize our experience in the field of technology transfer to grow our net assets. We have assembled a management team which has extensive experience in the field of technology transfer. Our management team has experience in identifying, negotiating, structuring and consummating technology transfers. We have approximately ten years of experience in the technology transfer business and have executed more than 100 technology transfers.
Provide new Open Innovation services to assist clients in growing their businesses and reducing R&D associated costs. Through the acquisition of Pharmalicensing.com and potentially other Open Innovation focused businesses, we believe that we will increase our ability to serve our clients in a more comprehensive manner.
According to the Aberdeen Group (www.aberdeen.com), in a December 2007 report entitledProduct Innovation Agenda 2010: Profiting from Innovation Today and Tomorrow, of the more than 300 companies surveyed, approximately 30% said that they will implement an Open Innovation program within the next 24 months. The primary reasons given for this were the desire for increased product revenue (82% of respondents) and decreased product costs (60% of respondents). We believe that the results of this report are consistent with our impression of the current marketplace opportunity as well as our current growth strategy.
Business Model
Open Innovation: Technology Transfer Process
Our technology transfer process generally follows a specific series of steps, which our management believes provides the greatest opportunity for creating value for our client companies. This process is designed to search and, when appropriate, transfer technologies from research institutions to our client companies.
Our technology transfer process generally involves the following steps:
| | (1) | We enter into technology acquisition alliance agreements with clients to learn about their technology needs and identify new discoveries that they may want to acquire. |
| | (2) | Our scientific team works with our clients to create a profile describing the new technologies they would like to acquire. |
| | (3) | Using our relationships with universities and research laboratories worldwide combined with our database of over 53,000 technologies available for immediate license, we search, filter and present to our client companies what our science team feels are the best two to three possible discoveries per month which are available for immediate license. |
| | (4) | Once we identify the technologies, we assist our technology acquisition alliance clients in conducting their own due diligence on the technologies. |
| | (5) | If a client wants to acquire the presented technology, UTEK will provide a term sheet describing the transaction. Upon mutual agreement of terms, UTEK will then seek to purchase the technology with our own capital and subsequently sell or transfer the technology to our client for unregistered stock or cash. |
| | (6) | If needed, UTEK can also provide additional resources at the time of transfer to help accelerate the commercialization of new products based on the transferred technology. These may include pre-paid sponsored research agreements to advance the technology, pre-paid consulting agreements with the inventor(s) of the technology and financing for prototype development and product launch. |
| | (7) | Often, the process does not end once the first technology transfer is complete. We continue, per our client’s needs and specifications, to present several technologies each month throughout the term of the technology acquisition alliance agreement. |
3
In the past, many of the clients with which we engage in such transactions frequently have had little or no prior operating history. When we engage in transactions with smaller capitalization companies, we seek to limit the downside potential of working with such clients by, among other things, requiring that such clients pay us a more significant premium over our cost than mid-cap and large capitalization companies for the new technologies they acquire from us in connection with the technology transfer. During 2007, we have endeavored to diversify our client base to include mid-cap and large capitalization companies. It is our hope that improved client diversification will result in more stable growth, improved cash flow and reduced volatility in future periods.
Client Technology Selection
We conduct technology transfers with clients that our management believes are positioned to benefit from the acquisition of new technology. Prior to completing a technology transfer with a client, our management and, to the extent it is deemed to be advisable by our management, our Scientific Advisory Council will review the scientific merit and risks of the potential technology transfer. For more information about our Scientific Advisory Council, see “Business—Scientific Advisory Council.”
When we assist a client in evaluating a new technology, we review the technology to make sure that it meets some or all of the following criteria:
| | • | | the technology should represent a significant advance over existing technologies; |
| | • | | there should be an existing global market for the technology once it is commercialized; and |
| | • | | the technology should be socially responsible (i.e., not intended for destructive or harmful purposes). |
In addition, we consider some or all of the following additional matters in connection with our decision to conduct a technology transfer:
| | • | | financial requirements to commercialize the technology; |
| | • | | the operating record and quality of the entrepreneurial group associated with the client. |
If, in our management’s view, a technology meets some or all of the criteria discussed above, then we will commence negotiations with the technology developer to arrange for a license of the technology. Normally, we seek to acquire a worldwide exclusive license for the fields of use that are important for our client, although non-exclusive licenses are also considered when appropriate. These licenses usually have an upfront fee, royalty provision, and minimum annual royalties. Pursuant to the terms of these license agreements, all rights to royalties remain with the university or research institute that grants the license. The term for most agreements is for the useful life of the intellectual property underlying the license. Our management will review license agreements and advise our client as to license terms and requirements.
Acquisitions
We continually investigate opportunities to grow by acquisition. In particular, we are currently actively pursuing strategic acquisitions that support Open Innovation. During the three years ended December 31, 2006, we made six acquisitions in cash and shares of UTEK stock valued at $4,835,000. We did not have any acquisitions during 2007. Our goal for 2008 is to acquire additional companies in the Open Innovation field.
When investigating a potential acquisition target, we evaluate a number of characteristics, including management strength, profitability, synergy with our existing consulting and technology strengths. We do not limit our discussions with potential target companies by size, field or geography although we target primarily those companies with a commitment to Open Innovation, that utilize technology as a core competency and that
4
are experienced in providing innovative product applications, as well as those that can help us expand our suite of client services. In making acquisitions, we seek to utilize stock rather than cash as consideration in order to leverage our capabilities and provide incentives to the management or consulting personnel who typically join UTEK as part of the transaction. Depending on the size of the company and the number of acquisitions we consummate, the value of UTEK's stock may temporarily be adversely affected. While we attempt to make acquisitions that we believe will be accretive to our shareholders, because these acquisitions represent long-term investments, there may be a short-term negative impact on our earnings per share.
Recent Developments
In January 2008, we acquired Pharmalicensing.com, a leading biopharmaceutical Open Innovation resource designed for professionals involved with partnering, licensing and business development worldwide. Actively supporting in- and out-licensing activities, Pharmalicensing provides partnering services, business development reports and industry news. Pharmalicensing is actively utilized by over 1,000,000 pharmaceutical and life science industry professionals each year. We believe that this acquisition will enable us to expand our Open Innovation strategy by increasing our range of client services, especially in the life sciences. Through Pharmalicensing, we will be able to provide clients with the following services:
| | • | | Out-licensing: Pharmalicensing offers a resource for clients to out-license their intellectual property, minimizing the time and costs associated with this activity. |
| | • | | Profiling: Designed to complement the client’s business development strategy, a Pharmalicensing profile exposes the client’s licensing requirements to a focused and targeted audience of the key decision-makers within the pharmaceutical and biotech industry. |
| | • | | Reports: Pharmalicensing publishes and distributes a range of business intelligence reports specializing in life sciences covering strategy, deal making trends and more. |
| | • | | Partnering tools: Provides clients with business critical information designed to enhance deal making effectiveness, including company and deal information and up to date industry news. |
TekScout™ Open Innovation Network (www.tekscout.com) was launched in March 2008. TekScout helps companies look outside of their internal R&D departments to address the challenge of completing R&D projects that they may not have the time or the internal capabilities to solve. The TekScout Open Innovation Network makes it easy for companies to post these projects in a secure environment and find scientists and engineers around the world who can complete them. It also helps qualified scientists and engineers find R&D challenges that need solving, and provides them both financial and intellectual rewards for their solutions. In addition, we recently hired an experienced software executive to manage this business.
Strategos, Inc., a strategic innovation consulting firm that provides consulting services, primarily to large companies has agreed to be acquired by us. The transaction is expected to close in March 2008, subject to certain conditions and approvals. Strategos is based in Chicago, IL, and was established in 1995. Under the terms of the Stock Purchase Agreement, Strategos will be acquired for 1,248,960 shares of UTEK Corporation unregistered common stock. Strategos shareholders shall receive one third of the shares at closing and the remaining UTEK shares will be held in escrow and paid upon achieving certain performance milestones over the next two years. The shareholders of Strategos have agreed not to sell any of the unregistered shares for 12 months following close of the transaction, which is expected to take place in March 2008.
Competition
The Open Innovation service business is highly competitive. We expect that if our service model proves to be successful, our current competitors in the market may duplicate our strategy, and new competitors may enter. We currently compete against other technology transfer companies, including IP Group plc, Competitive Technologies, Inc., BTG plc, Sagentia Group AG and many private firms, some of which are much larger and
5
have significantly greater financial resources than we do. In addition, these companies will be competing with us to acquire technologies from universities and government research laboratories. We also compete against large companies that seek to license university-developed technologies themselves. We cannot assure you that we will be able to successfully compete against these competitors in the acquisition of technology licenses, funding of technology development or marketing to potential client companies. We believe we have developed a competitive advantage by assembling a large, robust database of university discoveries available for license combined with our experience in technology transfer. In addition, we have developed a search engine for university discoveries that facilitate the finding of relevant new discoveries for our client companies. Also, we believe that our U2B® model makes it easier for companies of all sizes to acquire technologies from universities and research institutions.
Financial Information about Financial Segments and Geographic Areas
Information concerning sales and segment income attributable to each of the Company’s business segments and geographic areas is set forth in Note 11 of our Notes to Consolidated Financial Statements under Item 8. “Financial Statements and Supplementary Data” and is incorporated herein by reference.
Employees
As of February 19, 2008, we had 68 employees. We believe our relations with our employees are good.
Scientific Advisory Council
The principal purpose of our Scientific Advisory Council is to assist our management in the evaluation of new technologies for our clients. Our Scientific Advisory Council is comprised of more than 40 experts in a broad range of scientific, medical and engineering disciplines.
Regulation
We have elected to be regulated as a BDC under the 1940 Act. Pursuant to the 1940 Act, a BDC must be organized in the United States for the purpose of investing in or lending to private or thinly traded public companies and making managerial assistance available to them. A BDC may use capital provided by public shareholders and from other sources to make long-term, private investments in businesses.
As a BDC, we may not acquire any asset other than “qualifying assets” unless at the time we make the acquisition, the value of our qualifying assets represent at least 70% of the value of our total assets. The principal categories of qualifying assets relevant to our business are:
| | • | | Securities in an eligible portfolio company that are purchased in transactions not involving any public offering. An eligible portfolio company is defined under the 1940 Act to include any issuer that: |
| | • | | is organized and has its principal place of business in the U.S.; |
| | • | | is not an investment company or a company operating pursuant to certain exemptions under the 1940 Act, other than a small business investment company wholly owned by a BDC; and |
| | • | | does not have any class of securities listed on a national securities exchange; |
| | • | | Securities received in exchange for or distributed with respect to securities described in the bullet above or pursuant to the exercise of options, warrants, or rights relating to those securities; and |
| | • | | Cash, cash items, government securities, or high quality debt securities (as defined in the 1940 Act), maturing in one year or less from the time of investment. |
6
In October 2006, the SEC re-proposed rules providing for an additional definition of eligible portfolio company. As re-proposed, the rule would expand the definition of eligible portfolio company to include certain public companies that list their securities on a national securities exchange. The SEC is seeking comment regarding the application of this proposed rule to companies with: (1) a public float of less than $75 million; (2) a market capitalization of less than $150 million; or (3) a market capitalization of less than $250 million. There is no assurance that such proposal will be adopted or what the final proposal will entail.
To include certain securities described above as qualifying assets for the purpose of the 70% test, a business development company must offer to make available to the issuer of those securities significant managerial assistance such as providing guidance and counsel concerning the management, operations, or business objectives and policies of a portfolio company. We offer to provide managerial assistance to each of our portfolio companies.
As a BDC, we are required to meet a coverage ratio of the value of total assets to total senior securities, which include all of our borrowings and any preferred stock we may issue in the future, of at least 200%. We may also be prohibited under the 1940 Act from knowingly participating in certain transactions with our affiliates without the prior approval of our directors who are not interested persons and, in some cases, prior approval by the SEC.
Except as described below or otherwise permitted by the 1940 Act, we are not able to issue and sell our common stock at a price below our net asset value per share. We may, however, sell our common stock, warrants, options or rights to acquire our common stock, at a price below the net asset value of the common stock if our Board of Directors determines that such sale is in our best interests and that of our stockholders, and our stockholders approve such sale. In any such case, the price at which our securities are to be issued and sold may not be less than a price which, in the determination of our Board of Directors, closely approximates the market value of such securities (less any distributing commission or discount). We may also make rights offerings to our stockholders at prices per share less than the net asset value per share, subject to applicable requirements of the 1940 Act.
The 1940 Act prohibits us from acquiring (i) more than 3% of the total outstanding shares of another investment company; (ii) shares of another investment company having an aggregate value in excess of 5% of the value of our total assets; or (iii) shares of another registered investment company and all other investment companies having an aggregate value in excess of 10% of the value of our total assets.
Our Board of Directors has appointed a chief compliance officer pursuant to the requirements of the 1940 Act. We are periodically examined by the SEC for compliance with the 1940 Act.
As with other companies regulated by the 1940 Act, a BDC must adhere to certain substantive regulatory requirements. A majority of our directors must be persons who are not interested persons, as that term is defined in the 1940 Act. Additionally, we are required to provide and maintain a bond issued by a reputable fidelity insurance company to protect us against larceny and embezzlement. Furthermore, as a BDC, we are prohibited from protecting any director or officer against any liability to our company or our shareholders arising from willful misfeasance, bad faith, gross negligence or reckless disregard of the duties involved in the conduct of such person’s office.
As required by the 1940 Act, we maintain a code of ethics that establishes procedures for personal investment and restricts certain transactions by our personnel. Personnel subject to the code of ethics may invest in securities for their personal investment accounts, so long as such investments are made in accordance with the code’s requirements.
We may not change the nature of our business so as to cease to be, or withdraw our election as, a BDC unless authorized by vote of a “majority of the outstanding voting securities,” as defined in the 1940 Act.
7
Income Tax Matters
For federal and state income tax purposes, we are taxed at regular corporate rates on ordinary income and recognize gains on distributions of appreciated property. We are not entitled to the special tax treatment available to BDCs that elect to be treated as regulated investment companies under the Internal Revenue Code because, among other reasons, we do not distribute at least 90% of “investment company taxable income” as required by the Internal Revenue Code for such treatment. Distributions of cash or property by us to our stockholders, if any, will be taxable as dividends only to the extent that we have current or accumulated earnings and profits. Distributions in excess of current or accumulated earnings and profits will be treated first as a return of capital to the extent of the holder’s tax basis and then as gain from the sale or exchange of property.
Determination of Net Asset Value
Quarterly Net Asset Value Determination
We determine the net asset value per share of our common stock quarterly. We disclose these net asset values in the periodic reports we file with the SEC. The net asset value per share of our common stock is equal to the value of our total assets minus total liabilities divided by the total number of shares of common stock outstanding.
At December 31, 2007, approximately 66% of our net assets were in investments recorded at fair value. Value, as defined in Section 2(a)(41) of the 1940 Act, is (i) the market price for those securities for which a market quotation is readily available and (ii) for all other securities and assets, fair value as determined in good faith by our Board of Directors. Since there is typically no readily available market value for the investments in our portfolio, we value substantially all of our investments at fair value as determined in good faith by the Board of Directors. In making its determination, our Board of Directors considers valuation appraisals provided by independent valuation service providers. Because of the inherent uncertainty of determining the fair value of investments that do not have a readily available market value, the fair value of our investments determined in good faith by our Board of Directors may differ significantly from the values that would have been used had a ready market existed for the investments, and the differences could be material.
There is no single standard for determining fair value in good faith. As a result, determining fair value requires that judgment be applied to the specific facts and circumstances of each portfolio investment while employing a consistently applied valuation process for the types of investments we hold. We will record unrealized depreciation on investments when we believe that an investment has become impaired, including where realization of an equity security is doubtful. Conversely, we will record unrealized appreciation if we have an indication that the underlying company has appreciated in value and, therefore, our equity security has also appreciated in value, where appropriate.
With respect to equity securities in private companies, each investment is valued using industry valuation benchmarks, and then the value is assigned a discount reflecting the illiquid nature of the investment, as well as our minority, non-control position. When an external event such as a purchase transaction, public offering, or subsequent equity sale occurs, the pricing indicated by the external event is used to corroborate our valuation. Equity securities in public companies that carry certain restrictions on sale are generally valued at a discount from the public market value of the securities.
The Board of Directors bases its determination upon, among other things, applicable quantitative and qualitative factors. These factors may include, but are not limited to, type of securities, nature of business, marketability, market price of unrestricted securities of the same issue (if any), comparative valuation of securities of publicly-traded companies in the same or similar industries, current financial conditions and operating results, sales and earnings growth, operating revenues, competitive conditions and current and prospective conditions in the overall stock market.
8
We have retained an independent valuation service provider, Klaris, Thomson & Schroeder, Inc., to supply us with quarterly valuations of our portfolio of investments. Our Board of Directors uses these valuations to aid in its determination of the fair value of our investments. Our Board of Directors retains its responsibility for determining the fair value of our portfolio of investments.
Determinations in Connection with Offerings
In connection with each offering of shares of our common stock, our Board of Directors or a committee thereof is required to make the determination that we are not selling shares of our common stock at a price below the then current net asset value of our common stock at the time at which the sale is made. Our Board of Directors considers the following factors, among others, in making such determination:
| | • | | the net asset value of our common stock disclosed in the most recent periodic report we filed with the SEC; |
| | • | | our management’s assessment of whether any material change in the net asset value of our common stock has occurred (including through the realization of gains on the sale of our portfolio securities) from the period beginning on the date of the most recently disclosed net asset value of our common stock to the period ending two days prior to the date of the sale of our common stock; and |
| | • | | the magnitude of the difference between the net asset value of our common stock disclosed in the most recent periodic report we filed with the SEC and our management’s assessment of any material change in the net asset value of our common stock since the date of the most recently disclosed net asset value of our common stock in the proposed offering. |
Importantly, this determination does not require that we calculate the net asset value of our common stock in connection with each offering of shares of our common stock, but instead it involves the determination by our Board of Directors or a committee thereof that we are not selling shares of our common stock at a price below the then current net asset value of our common stock at the time at which the sale is made.
Moreover, to the extent that there is even a remote possibility that we may (i) issue shares of our common stock at a price below the then current net asset value of our common stock at the time at which the sale is made or (ii) trigger the undertaking (which we are required to provide in certain registration statements we may file from time to time with the SEC) to suspend the offering of shares of our common stock if the net asset value of our common stock fluctuates by certain amounts in certain circumstances until the prospectus relating to such offering is amended, our Board of Directors will elect, in the case of clause (i) above, either to postpone the offering until such time that there is no longer the possibility of the occurrence of such event or to undertake to determine the net asset value of our common stock within two days prior to any such sale to ensure that such sale will not be below our then current net asset value, and, in the case of clause (ii) above, to comply with such undertaking or to undertake to determine the net asset value of our common stock to ensure that such undertaking has not been triggered.
These processes and procedures are part of our compliance policies and procedures. Records will be made contemporaneously with all determinations described in this section, and these records will be maintained with other records we are required to maintain under the 1940 Act.
Corporate Offices and Available Information
Our executive offices are located at 2109 Palm Avenue, Tampa, Florida 33605 and our telephone number is (813) 754-4330. We also have offices in Pennsylvania, Israel and the United Kingdom.
Our Internet address is www.utekcorp.com. We make available free of charge through our website our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Information contained on our website is not incorporated by reference into this Annual Report on Form 10-K, and you should not consider information contained on our website to be part of this Annual Report on Form 10-K.
9
Investing in our common stock involves a high degree of risk. As a result, there can be no assurance that we will achieve our strategic objective. You should consider carefully the risks described below. In addition to the risk factors described below, other factors that could cause actual results to differ materially include:
| | • | | Changes in the economy; |
| | • | | Changes in the market for technology transfer and Open Innovation services; |
| | • | | Risk associated with possible disruption in our operations due to terrorism; |
| | • | | Future regulatory actions and conditions in our operating areas; and |
| | • | | Other risks and uncertainties as may be detailed from time to time in our public announcements and SEC filings. |
We may not be able to sell the securities we receive in connection with our technology acquisition alliance agreements and technology transfers for an amount equal to the revenue we previously recognized in connection with such transactions.
We recognize revenue in connection with our technology acquisition alliances and technology transfers in an amount equal to the fair value of the securities we receive in connection with such transactions. With respect to our technology acquisition alliance agreements, we recognize such revenue as earned in accordance with the contractual terms of each technology acquisition alliance agreement. With respect to our technology transfers, we recognize such revenue upon the consummation of each technology transfer. Because the securities we receive in connection with such transactions are subject to legal restrictions on resale and are mainly issued by thinly traded public companies or private companies, our ability to sell such securities may be limited. Given these facts as well as the subjective judgments and estimates inherent in fair value accounting, we may not be able to sell the securities we receive in connection with our technology acquisition alliances and technology transfers for an amount equal to the revenue we previously recognized in connection with such transactions.
Our quarterly and annual results fluctuate significantly.
Our quarterly and annual operating results fluctuate significantly due to a number of factors. These factors include the small number and range of values of the transactions that are completed each quarter, fluctuations in the values of our investments, the timing of the recognition of unrealized gains and losses, the degree to which we encounter competition in our markets, the volatility of the stock market and its impact on our unrealized gains and losses, as well as other general economic conditions. As a result of these factors, quarterly and annual results are not necessarily indicative of our performance in future quarters and years.
Our investment portfolio is highly concentrated in a limited number of portfolio companies and, as a result, our financial results are largely dependent upon the performance of these companies.
Our investment portfolio is highly concentrated in a limited number of portfolio companies and, as a result, our financial results are largely dependent upon the performance of these companies. If one or more of these companies fails to perform as expected, our financial results could be negatively affected.
Our portfolio companies are development stage companies dependent upon the successful commercialization of new technologies. Each of our portfolio companies is subject to a high degree of risk, and we may not be able to sell securities we receive in connection with our technology acquisition alliances or technology transfers.
We enter into technology acquisition alliance and technology transfer agreements with development stage companies that our management believes can benefit from our expertise in technology transfer. Development stage companies are subject to all of the risks associated with new businesses. In addition, our portfolio companies are
10
also subject to the risks associated with research and development of new technologies. These risks include the risk that new technologies cannot be identified, developed or commercialized, may not work, or may become obsolete. Our portfolio companies must successfully acquire licenses to new technologies, and in some cases, further develop new technologies. We cannot assure you that any of our portfolio companies will be successful. Our portfolio companies will be competing with larger, established companies with greater access to, and resources for, further development of these new technologies. We may not be able to sell the securities we receive in connection with our technology acquisition alliances or technology transfers if our portfolio companies are not successful.
Our portfolio companies depend upon the research and development activities of universities, medical research centers and federal research laboratories, over which neither our portfolio companies nor we have any control.
Our portfolio companies depend upon the research activities of universities, medical research centers and federal research laboratories. Neither we, nor our portfolio companies, have any control over the research activities of universities, medical research centers and federal research laboratories. As neither we nor our portfolio companies provide supervision of any universities, medical research centers and federal research laboratories, and we cannot ensure that the research will be done properly and that the results, which we may license, will be reproducible. In addition, we have no control over what types of technologies are presented to us by universities, medical research centers and federal research laboratories for evaluation and commercial development. Further, the licenses to technologies that our portfolio companies obtain may be non-exclusive.
Technologies acquired by our portfolio companies may become obsolete before we can sell their securities.
Neither our portfolio companies nor we have any control over the pace of technology development. There is a significant risk that a portfolio company could acquire the rights to a technology that is currently, or is subsequently made, obsolete by other technological developments. If this were to occur, we may be unable to sell or otherwise dispose of the securities we received in connection with our technology acquisition alliances and technology transfer transactions with such companies.
The patents on the technologies that our portfolio companies license may infringe upon the rights of others and patent applications that have been submitted may not be granted.
Many of our portfolio companies rely upon patents to protect the technologies that they license. If the patents on technologies that they license are found to infringe upon the rights of others, or are held to be invalid, then the licenses to such technologies will have little or no value to our portfolio companies. In addition, if a patent to a technology licensed by a portfolio company is found to infringe upon the rights of others, the portfolio company may be liable for monetary damages. Our portfolio companies are dependent upon the universities, medical centers or government research facilities to file, secure and protect patents on licensed technologies. In the event that a patent is challenged or violated, our portfolio companies may not have the financial resources to defend the patent either in the preliminary stages of litigation or in court. In addition, if our portfolio companies acquire licenses to technologies with patents pending, we cannot assure you that such patents will be granted. If any such events were to occur, we may be unable to sell or otherwise dispose of the securities we received in connection with our technology acquisition alliances and technology transfers with such companies.
Technologies that have been developed with funding from the U.S. government may have limits on their use, which could affect the value of the technology to a portfolio company.
Technologies developed with funds provided by the U.S. government have restrictions regarding where they may be sold and have limits on exclusivity. A portfolio company that acquires a technology developed with federal funding may be limited as to where it can sell the technology. The technology may only be allowed to be sold or manufactured within the U.S. In addition, the U.S. government has the right to use technologies that it has funded regardless of whether the technology has been licensed to a third party. Such regulations may limit the marketability of a technology and therefore reduce the value of the technology to our portfolio companies.
11
The securities we hold in our portfolio companies are subject to restriction on resale and we may not be able to sell the securities we hold for amounts equal to their recorded value, if at all.
Our portfolio companies are mainly thinly traded public companies or private companies, and we acquire securities in our portfolio companies in private transactions. As a result, substantially all of the securities we hold in our portfolio companies are subject to legal restrictions on resale. Furthermore, our ability to sell the securities in our portfolio may be limited by, and subject to, the lack of or limited nature of a trading market for such securities. Therefore, we cannot assure you that we will be able to sell our portfolio company securities for amounts equal to the values that we have ascribed to them at the time we desire to sell.
We are dependent on sales transactions structured as tax-free exchanges to sell the new companies we form to acquire new technologies. A change in the Internal Revenue Code or a negative interpretation of the Internal Revenue Code affecting tax-free exchanges could have a significant negative impact on our business and financial results.
To effectuate a technology transfer, we typically create a newly formed company to acquire a new technology from a university, medical center or federal research laboratory and then exchange the securities of such newly formed company for securities in the company that acquires such newly formed company and the technology held by such newly formed company. We call this unique technology transfer process U2B®. We anticipate that most, if not all, of such transactions will be structured as tax-free exchanges under Section 368 of the Internal Revenue Code. A benefit of structuring our technology transfers as tax-free exchanges under Section 368 is that such transactions do not result in a current taxable event for us for income tax purposes. If Section 368 were to be amended so that we were no longer able to structure our technology transfers as tax-free exchanges or the Internal Revenue Service took a negative position regarding our tax treatment of our technology transfers as tax-free exchanges, we may not be able to effectuate our technology transfers on commercially reasonable terms without suffering adverse tax consequences. In such event, we may be required to significantly alter the use of our U2B® investment process, which could have a significant negative impact on our business and financial results.
The agreements we have with universities, medical research centers and federal research laboratories do not guarantee that such entities will grant licenses to us or other companies.
The agreements that we have entered into with universities, medical research centers and federal research laboratories provide us with the ability to evaluate the commercial potential for technologies at an early stage of development. These agreements, however, do not provide us with any guarantee that following our evaluation, the university, medical research center or federal research laboratory will grant a license to us or other companies. As a result, we may expend time and resources evaluating a technology and not be able to secure a license to such technology.
We are dependent upon and have little or no control over the efforts of portfolio companies to successfully commercialize the acquired technologies or to retain the licenses to such technologies.
We receive common stock from our clients based upon the mutually agreed upon values of the new companies we form to acquire new technologies, their licensed technology and the portfolio companies. We then intend to sell the securities that we acquire at some time in the future. Therefore, our ability to profit from an investment is ultimately dependent upon the price we receive for the shares of the portfolio company. In most cases, the value of the portfolio companies will be dependent upon successfully commercializing the technologies they acquire. We do not have control over the portfolio companies. These portfolio companies may face intense competition, including competition from companies with greater financial resources, more extensive research and development, manufacturing, marketing and service capabilities and a greater number of qualified and experienced managerial and technical personnel. They may need additional financing which they are unable to secure and which we are unable or unwilling to provide, or they may be subject to adverse developments
12
unrelated to the technologies they acquire. They may lose the rights granted to them for the technology for failure to comply with the license agreement. We cannot assure you that any of the portfolio companies will be successful or that we will be able to sell the securities we receive at a profit or for sufficient amounts to even recover our cash outlay in connection with our technology acquisition alliances and technology transfers or that our portfolio companies will not take actions that could be detrimental to us.
Our investments in our portfolio companies may be concentrated in one or more industries, and if these industries should decline or fail to develop as expected, our cash outlay in connection with our technology acquisition alliances and technology transfers will be lost.
Our investments in our portfolio companies may be concentrated in one or more industries. This concentration will mean that our investments will be particularly dependent on the development and performance of those industries. Accordingly, our investments may not benefit from any advantages that might be obtained with greater diversification of the industries in which our portfolio companies operate. If those industries should decline or fail to develop as expected, our investments in our portfolio companies in those industries will be subject to loss.
Substantially all our portfolio investments are recorded at fair value as determined in good faith by our Board of Directors, and as a result, there is uncertainty regarding the value of our portfolio investments.
At December 31, 2007 and December 31, 2006, investments amounting to $28.9 million or 66% of our net assets and $33.3 million or 65% of our net assets, respectively, have been valued at fair value as determined by our Board of Directors. Pursuant to the requirements of the 1940 Act, our Board of Directors is responsible for determining in good faith the fair value of our investments for which market quotations are not readily available. Because there is typically no readily available market value for the investments in our portfolio, our Board of Directors determines in good faith the fair value of these investments pursuant to a valuation policy and a consistently applied valuation process. There is no single standard for determining fair value in good faith. As a result, determining fair value requires that judgment be applied to the specific facts and circumstances of each portfolio investment while employing a consistently applied valuation process for the types of investments we hold. If we were required to sell any such investments, there is no assurance that the fair value, as determined by the Board of Directors, would be obtained. If we were unable to obtain fair value for such investments, there would be an adverse effect on our net asset value and on the price of our common stock.
We adjust quarterly the valuation of our portfolio to reflect the Board of Directors’ determination of the fair value of each investment in our portfolio. As a result, the value of our portfolio securities and earnings change significantly from quarter to quarter. Any changes in fair value are recorded in our statements of operations as “Change in net unrealized appreciation (depreciation) of investments.”
Our business depends on key personnel.
We rely on, and will continue to be substantially dependent upon, the continued services of our management, principally our Chief Executive Officer and Chairman, Clifford M. Gross, Ph.D., our Chief Operating Officer, Douglas Schaedler, and our Chief Financial Officer, Carole R. Wright. We also depend upon our management’s key contacts with universities, medical research centers and federal research laboratories to maintain our access to new technologies and our relationships with companies in the private sector in order to effectuate the sale of technologies through our U2B® process. Therefore, a change in our executive management team could have a negative impact on the company.
Additional equity or debt financing may not be available to our portfolio companies, which could result in our losing our cash outlay in connection with our technology acquisition alliances and technology transfers with the portfolio company if the portfolio company fails.
We expect that many of our portfolio companies will require additional equity or debt financing to ensure their continued viability. The amount of additional financing needed will depend upon the maturity and objectives of the particular portfolio company. The availability of financing is generally a function of conditions
13
that are beyond the control of our portfolio companies. We cannot assure that our portfolio companies will be able to predict accurately the future financing requirements necessary for their success or that additional financing will be available to our portfolio companies from any source. If additional equity or debt financing is not available, some of our portfolio companies may be forced to cease operations, which could adversely affect our success and result in the loss of a substantial portion or all of our cash outlay in connection with our technology acquisition alliances and technology transfers with the portfolio company.
Changes in the laws or regulations that govern us could have a material impact on our operations.
Any change in the laws or regulations that govern our business could have a material impact on us or on our operations. Laws and regulations may be changed from time to time, and the interpretations of the relevant laws and regulations also are subject to change.
We are subject to certain risks associated with our foreign operations and investments.
We have operations in the United Kingdom and Israel and make investments in foreign companies. As of December 31, 2007, approximately 2% of our assets were comprised of assets in foreign operations and investments in foreign companies.
Certain risks are inherent in foreign operations, including:
| | • | | difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
| | • | | foreign customers may have longer payment cycles than customers in the U.S.; |
| | • | | tax rates in certain foreign countries may exceed those in the U.S. and foreign earnings may be subject to withholding requirements, exchange controls or other restrictions; |
| | • | | general economic and political conditions in countries where we operate may have an adverse effect on our operations; |
| | • | | exposure to risks associated with changes in foreign exchange rates; |
| | • | | difficulties associated with managing a large organization spread throughout various countries; |
| | • | | difficulties in enforcing intellectual property rights; and |
| | • | | required compliance with a variety of foreign laws and regulations. |
Investing in foreign companies may expose us to additional risks not typically associated with investing in U.S. companies. These risks include changes in foreign exchange rates, exchange control regulations, political and social instability, expropriation, imposition of foreign taxes, less liquid markets and less available information than is generally the case in the U.S., higher transaction costs, less government supervision of exchanges, brokers and issuers, less developed bankruptcy laws, difficulty in enforcing contractual obligations, lack of uniform accounting and auditing standards and greater price volatility.
As we continue to expand our business globally, our success will depend, in part, on our ability to anticipate and effectively manage these and other risks. We cannot assure you that these and other factors will not have a material adverse effect on our international operations or our business as a whole.
Our common stock may not continue to trade at a premium to our net asset value.
Our common stock continues to trade in excess of our net asset value. See Note 16 “Selected Per Share Data and Ratios” to our consolidated financial statements included in this Form 10-K. There can be no assurance, however, that our common stock will continue to trade at a premium to our net asset value. The possibility that our common stock will trade at premiums that are unsustainable over the long term is separate and distinct from the risk that our net asset value will decrease.
14
We may issue shares of our common stock at a discount to the market price for such shares, which may put downward pressure on the market price for shares of our common stock.
If we issue shares of our common stock at a discount to the market price for such shares, it may put downward pressure on the market price for shares of our common stock. Such downward pressure could in turn encourage short sales or similar trading with respect to shares of our common stock, which could in itself, place further downward pressure on the market price for shares of our common stock.
We may issue shares of our common stock in conjunction with the acquisition of other businesses, which may put downward pressure on the market price for shares of our common stock and create additional dilution of the current shares outstanding.
Consistent with our current strategy, we may seek to acquire other businesses through the issuances of common stock and or cash. If common stock is used in these transactions, it would create additional dilution of the current shares outstanding. Further, such issuances may result in downward pressure on our share price as a result of these additional shares being issued. Also, there is the potential that the market may not respond favorably to potential new acquisitions, which could also negatively affect our share price.
One of our current stockholders has significant influence over our management and affairs.
Clifford M. Gross, Ph.D., our Chief Executive Officer and Chairman, beneficially owns approximately 22% of our common stock as of December 31, 2007. Therefore, Dr. Gross may be able to exert influence over our management and policies. Dr. Gross may acquire additional equity in our company in the future. The concentration of ownership may also have the effect of delaying, preventing or deterring a change of control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of the sale of our company and might ultimately affect the market price of our common stock.
We may need additional capital in the future and it may not be available on acceptable terms.
We have historically relied on equity financing and, to a lesser extent, cash flow from the sale of our investments and debt financing to fund our operations, capital expenditures and expansion. However, we may require additional capital in the future to fund our operations or respond to competitive pressures or strategic opportunities. We cannot assure that additional financing will be available on terms favorable to us, or at all. In addition, the terms of available financing may place limits on our financial and operating flexibility. If we are unable to obtain sufficient capital in the future, we may:
| | • | | be forced to reduce our operations; |
| | • | | not be able to expand or acquire complementary businesses; and |
| | • | | not be able to develop new services or otherwise respond to changing business conditions or competitive pressures. |
Regulations governing our operation as a business development company will affect our ability to and the way in which we raise additional capital.
We may require additional capital in the future to fund our operations or respond to competitive pressures or strategic opportunities. We may acquire additional capital from the following sources:
Senior Securities and Other Indebtedness. We may issue debt securities or preferred stock and/or borrow money from banks or other financial institutions, which we refer to collectively as senior securities, up to the maximum amount permitted by the 1940 Act. If we issue senior securities, including debt or preferred stock, we will be exposed to additional risks, including the following:
| | • | | Under the provisions of the 1940 Act, we will be permitted, as a BDC, to issue senior securities only in amounts such that our asset coverage, as defined in the 1940 Act, equals at least 200% after each |
15
| | issuance of senior securities. If the value of our assets declines, we may be unable to satisfy this test. If that happens, we may be required to sell a portion of our investments and, depending on the nature of our leverage, repay a portion of our debt at a time when such sales and/or repayments may be disadvantageous; |
| | • | | It is likely that any senior securities or other indebtedness we issue will be governed by an indenture or other instrument containing covenants restricting our operating flexibility; |
| | • | | We, and indirectly, our stockholders will bear the cost of issuing and servicing such securities and other indebtedness; and |
| | • | | Preferred stock or any convertible or exchangeable securities that we issue in the future may have rights, preferences and privileges more favorable than those of our common stock, including separate voting rights and could delay or prevent a transaction or a change in control to the detriment of the holders of our common stock. |
Additional Common Stock. We are not generally able to issue and sell our common stock at a price below our net asset value per share. We may, however, sell our common stock, warrants, options or rights to acquire our common stock, at a price below our net asset value of the common stock if our Board of Directors determines that such sale is in our best interests and that of our stockholders, and our stockholders approve such sale. In any such case, the price at which our securities are to be issued and sold may not be less than a price which, in the determination of our Board of Directors, closely approximates the market value of such securities (less any distributing commission or discount). We may also make rights offerings to our stockholders at prices per share less than the net asset value per share, subject to applicable requirements of the 1940 Act. If we raise additional funds by issuing more common stock or senior securities convertible into, or exchangeable for, our common stock, the percentage ownership of our stockholders at that time would decrease and they may experience dilution. Moreover, we can offer no assurance that we will be able to issue and sell additional equity securities in the future, on favorable terms or at all.
We are focusing our business on providing Open Innovation services to our clients which is a new and uncertain trend in our industry.
We are focused on providing Open Innovation services to our clients. While these services utilize our well established technology transfer capabilities, they also incorporate additional products and services which in their entirety are as yet unproven in their ability to generate significant revenue. As a result, if our Open Innovation services are not well received by our clients or if industry changes its focus off of Open Innovation, this may result in reduced revenue and profitability for us.
Our common stock price may be volatile.
The trading price of our common stock has fluctuated significantly and may continue to fluctuate substantially, depending on many factors, many of which are beyond our control and may not be directly related to operating performance. These factors include the following:
| | • | | price and volume fluctuations in the overall stock market from time to time; |
| | • | | significant volatility in the market price and trading volume of securities of BDCs or technology transfer companies; |
| | • | | changes in regulatory policies, accounting or tax guidelines with respect to BDCs or technology transfer companies; |
| | • | | actual or anticipated changes in our sales or earnings or fluctuations in our operating results; |
| | • | | actual or anticipated changes in the value of our investments; |
16
| | • | | changes in financial reporting requirements; |
| | • | | general economic conditions and trends; |
| | • | | loss of a major funding source; |
| | • | | departures of key personnel; |
| | • | | changes to the market or shareholder’s acceptance of our unique technology transfer business; or |
| | • | | the consummation of mergers or acquisitions of related businesses. |
| Item 1B. | Unresolved Staff Comments |
Not applicable.
Our principal office is located in Tampa, Florida. We lease this office space from Ybor City Group, Inc., a subsidiary of UTEK Real Estate Holdings, Inc., one of our portfolio companies. This property is adequate for our current domestic office needs. If our office requirements increase beyond our current expectations, we believe that additional office space may be available for lease at this location.
We also lease office space in Pennsylvania, Israel and the United Kingdom. These properties are adequate for our current needs.
We may be a party from time to time in certain lawsuits in the normal course of our business. While the outcome of these legal proceedings cannot at this time be predicted with certainty, we do not expect that these proceedings will have a material effect upon our financial condition or results of operations.
| Item 4. | Submission of Matters to a Vote of Security Holders |
No matters were submitted to a vote of shareholders during the quarter ended December 31, 2007.
17
PART II
| Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our shares of common stock trade on the American Stock Exchange (AMEX) and the AIM market of the London Stock Exchange under the symbol “UTK”. Computershare Trust Company, Inc., 350 Indiana Street, Suite 800, Golden, CO 80401; 303-262-0600, serves as transfer agent for our common stock.
We had approximately 3,000 stockholders of record at February 25, 2008. The net asset value per share of our common stock at December 31, 2007 was $4.85.
Price Range of Common Stock and Dividends
The following table reflects the high and low closing prices for our common stock as reported on the AMEX and the cash dividends declared per common share for the period indicated:
| | | | | | | | | |
| | | High | | Low | | Dividends |
Fiscal year 2007 | | | | | | | | | |
First quarter | | $ | 13.84 | | $ | 10.55 | | | — |
Second quarter | | $ | 17.90 | | $ | 13.16 | | | — |
Third quarter | | $ | 17.57 | | $ | 13.00 | | | — |
Fourth quarter | | $ | 16.49 | | $ | 12.72 | | | — |
| | | |
Fiscal year 2006 | | | | | | | | | |
First quarter | | $ | 14.50 | | $ | 11.90 | | $ | 0.02 |
Second quarter | | $ | 20.40 | | $ | 12.65 | | | — |
Third quarter | | $ | 23.80 | | $ | 18.22 | | | — |
Fourth quarter | | $ | 19.99 | | $ | 10.78 | | $ | 0.02 |
Our Board of Directors has sole discretion in determining whether to declare and pay cash dividends in the future. The declaration of cash dividends will depend on our profitability, financial condition, cash requirements, future prospects and other factors deemed relevant by our Board of Directors. Our ability to pay cash dividends in the future could be limited or prohibited by regulatory requirements and the terms of financing agreements that we may enter into or by the terms of any preferred stock that we may authorize and issue.
Performance Graph
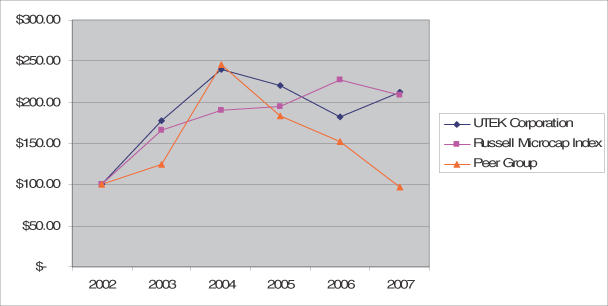
The following graph shows a comparison of the five-year cumulative total return, assuming the reinvestment of dividends, on our common stock with that of the Russell Microcap Index and the Company’s peer group including British Technology Group plc, Competitive Technologies, Inc., IP Group plc, and Sagentia Group AG. The graph assumes $100 was invested on December 31, 2002 in our common stock, the Russell Microcap Index companies, and the companies in the peer group. Note that historical stock price performance is not necessarily indicative of future stock price performance.
18
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN
AMONG UTEK, RUSSELL MICROCAP INDEX AND THE COMPANY’S PEER GROUP
| Item 6. | Selected Financial Data |
The following table presents our selected consolidated financial and other data and has been derived from our audited financial statements for the years ended December 31, 2007, 2006, 2005, 2004 and 2003. The information below should be read in conjunction with “Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the notes thereto, each of which is included in another section of this annual report on Form 10-K. Our acquisitions have not had a material effect on the trends reflected in the selected financial data.
| | | | | | | | | | | | | | | |
| | | Year Ended December 31, |
| | | 2007 | | 2006 | | 2005 | | 2004 | | 2003 |
Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | |
Income from operations | | $ | 20,300,949 | | $ | 56,952,937 | | $ | 22,743,823 | | $ | 7,188,615 | | $ | 3,805,177 |
Net income from operations | | | 3,776,742 | | | 19,944,207 | | | 5,887,830 | | | 942,722 | | | 180,568 |
Net income from operations per diluted common share | | $ | 0.42 | | $ | 2.27 | | $ | 0.80 | | $ | 0.15 | | $ | 0.04 |
Weighted average shares: | | | | | | | | | | | | | | | |
Diluted | | | 8,989,234 | | | 8,786,605 | | | 7,325,312 | | | 6,098,537 | | | 4,266,918 |
Cash dividends declared per common share | | | — | | $ | 0.04 | | | — | | | — | | | — |
| | | | | |
| | | 2007 | | 2006 | | 2005 | | 2004 | | 2003 |
Balance Sheet Data: | | | | | | | | | | | | | | | |
Total assets | | $ | 45,221,077 | | $ | 53,040,810 | | $ | 49,005,960 | | $ | 25,879,644 | | $ | 12,549,448 |
Long-term obligations | | | — | | | — | | | — | | | — | | | 847,890 |
Net asset per share | | $ | 4.85 | | $ | 5.71 | | $ | 5.58 | | $ | 3.85 | | $ | 2.33 |
19
| Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations |
Special Note Regarding Forward-Looking Statements
The following discussion should be read in conjunction with our consolidated financial statements and the notes thereto included elsewhere in this annual report on Form 10-K. This annual report on Form 10-K contains forward-looking statements regarding the plans and objectives of management for future operations. These forward-looking statements may involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by any forward-looking statements. Forward-looking statements, which involve assumptions and describe our future plans, strategies and expectations, are generally identifiable by use of the words “may,” “will,” “should,” “expect,” “anticipate,” “estimate,” “believe,” “intend” or “project” or the negative of these words or other variations on these words or comparable terminology. These forward-looking statements are based on assumptions that may be incorrect, and we cannot assure you that the projections included in these forward-looking statements will come to pass. Our actual results could differ materially from those expressed or implied by the forward-looking statements as a result of various factors.
Overview
Executive Summary
Our financial condition is dependent on a number of factors including our ability to effectuate technology transfers and the performance of the equity investments that we receive in connection with these transfers. Substantially all of our investments are in development stage and start-up companies and thinly traded public companies. These businesses are thinly capitalized, unproven, small companies that lack management depth, are dependent on new, commercially unproven technologies and have no or a limited history of operations.
Our total assets were $45.2 million and our net assets were $43.7 million at December 31, 2007, compared to $53.0 million and $51.0 million at December 31, 2006, respectively. Net asset value per share was $4.85 at December 31, 2007 and $5.71 at December 31, 2006. At the end of fiscal year 2007, we had no long-term debt outstanding and $5.3 million in cash and cash equivalents.
Income from operations for fiscal year 2007 totaled approximately $20.3 million, as compared to $57.0 million in 2006. Net income from operations for fiscal year 2007 totaled approximately $3.8 million as compared to $19.9 million in 2006. Net realized losses on investments, net of deferred tax effect, totaled approximately $1.4 million in 2007 as compared to net realized gains of $900,000 in 2006. In this regard, we received gross proceeds of $1.9 million in 2007 and $9.7 million in 2006 in connection with the sale of the securities we received in connection with our technology acquisition alliance agreements and technology transfers. Net change in unrealized depreciation of investments, net of deferred tax benefit, was $10.8 million in 2007 as compared to $25.8 million in 2006.
On June 15, 2007, the Company’s stockholders voted to approve an amendment to the Company’s Certificate of Incorporation to increase the number of authorized shares of common stock from 19,000,000 to 29,000,000. The additional 10,000,000 shares are part of the existing class of common stock and will have the same rights and privileges as the shares of common stock currently issued and outstanding. The Board of Directors deemed it desirable to increase the number of shares of common stock the Company is authorized to issue in order to provide adequate flexibility in the future. The holders of common stock are not entitled to preemptive rights or cumulative voting, and accordingly, the issuance of additional common shares will dilute the ownership and voting rights of shareholders.
20
Recently, we have taken steps to improve the efficiency of our technology transfer business model and refocused it on Open Innovation. Some of the improvements we have made include:
| | • | | Expanded our scientific review team for technology due diligence; |
| | • | | Hired an experienced head of business development formerly employed by IBM to oversee our sales efforts; |
| | • | | Hired an experienced professional in technology licensing who was formerly president of Lucent’s technology licensing business; |
| | • | | Hired an experienced manager to run our on-line Open Innovation technology sourcing service; |
| | • | | Engaged Professor Henry Chesbrough, the world’s thought leader on Open Innovation as a strategic advisor to the Company; |
| | • | | Improved our technology search capabilities through our expanded proprietary technology database and internally developed search engine; |
| | • | | Enhanced client acceptance procedures including the hiring of a manager of due diligence to conduct background checks on principal officers of prospective client companies, and the approval by a Review Committee made up of the executive officers and the director of operations prior to accepting a new client; and |
| | • | | Acquired Pharmalicensing.com, one of the world’s leading Open Innovation exchanges in the life sciences. |
As a result of these efforts, we have experienced an increase in the number of active engagements and the average market capitalization of our clients. Our goal is to increase the diversity of our clients, and thus, our portfolio companies. Transactions with larger clients require additional time to close. This has resulted in a decrease in the number of executed technology transfers as well as a decrease in gross revenues in 2007 compared to 2006.
UTEK is refining its corporate strategy for 2008 to expand our business. Changes in our strategy include the following:
| | • | | Seeking to be the first full-service provider of Open Innovation services; |
| | • | | Increasing our pricing for technology acquisition alliances; |
| | • | | Diversify our technology transfer business to include cash transactions; |
| | • | | Identifying and, when appropriate, acquiring companies that complement our Open Innovation strategy; and |
| | • | | Launch of the TekScout™ Open Innovation Network |
Technology Transfers and Technology acquisition alliances
In 2007, we increased the number and diversity of active technology acquisition alliance clients and decreased the number of completed technology transfers:
| | • | | During 2007, we signed 80 new technology acquisition alliance agreements as compared to 44 new technology acquisition alliances in 2006; and |
| | • | | During 2007, we completed 16 technology transfers valued at approximately $16.4 million as compared to 29 technology transfers valued at approximately $51.2 million in 2006. |
21
Portfolio Activity
The following is a list of significant changes in our portfolio during the year ended December 31, 2007:
| | • | | The sale of some or all of our shares in Shumate Industries, Inc., Health Sciences Group, Inc., Broadcast International, Inc., Xethanol Corporation, NetFabric Holdings, Inc. and various other portfolio companies for approximately $1.9 million, which resulted in realized losses of $1.4 million (net of income tax effect); |
| | • | | The completion of 16 technology transfers valued at approximately $16.2 million (one technology transfer was completed for $200,000 in cash); and |
| | • | | A net unrealized loss of $10.8 million (net of income tax effect) in the fair value of our investments. |
Our most significant portfolio investments at December 31, 2007 were in Material Technologies, Inc., UTEK Real Estate Holdings, Inc., Advanced Medical Isotope Corporation, World Energy Solutions, Inc. and Cyberlux Corporation. These five investments total $15.1 million in fair value and represent 50% of our investments and 35% of net assets at December 31, 2007.
Our capital investments made in our newly formed companies during the year ended December 31, 2007 totaled $3.8 million. Of the total capital invested in our newly formed companies during the year ended December 31, 2007, $762,000 was expended on license and consulting fees and $3.1 million was spent to assist our clients in commercializing their new technology. All of these items are reflected in the accompanying consolidated statement of operations as acquisition of technology rights.
The net unrealized depreciation for the year ended December 31, 2007 was primarily due to a reduction in value of the following nine investments in our portfolio: Advanced Refractive Technologies, Inc., American Soil Technologies, Inc., CytoDyn, Inc., vidShadow.com, Inc., Emission & Power Supply, Inc., Manakoa Services Corporation, Klegg Electronics, Inc., World Energy Solutions, Inc. and Industrial Biotechnology Corporation; partially offset by the appreciation of Broadcast International, Inc. and Health Sciences Group, Inc.
While these unrealized losses were significant, failures among small cap companies are not unexpected and may occur in the future. The current portfolio is comprised of approximately 60 holdings. Many of these positions are with small capitalization companies, which over time may have high failure rates due to a variety of factors. For clients that fail, UTEK may lose the entire amount of its capital spent acquiring and transferring the technology to them.
The value of our investments can fluctuate due to factors that are specific to each investment (e.g., inability to obtain additional capital, inability to execute business model, termination of technology licenses, etc.) or to general marketplace factors.
Results of Operations
Summary of Results for Years Ended December 31, 2007, 2006 and 2005
Income from Operations (Revenue)
| | | | | | | | | | | | | | | |
(in thousands, except percentages) | | 2007 | | 2006 | | 2005 | | Percent
Change 2007
versus 2006 | | | Percent
Change 2006
versus 2005 | |
Sale of Technology Rights | | $ | 16,373 | | $ | 51,191 | | $ | 18,132 | | (68 | )% | | 182 | % |
Consulting and Other Services | | | 3,343 | | | 4,950 | | | 4,179 | | (32 | )% | | 18 | % |
Investment Income, net | | | 585 | | | 812 | | | 433 | | (28 | )% | | 87 | % |
| | | | | | | | | | | | | | | |
Income from Operations | | $ | 20,301 | | $ | 56,953 | | $ | 22,744 | | (64 | )% | | 150 | % |
| | | | | | | | | | | | | | | |
22
Sale of Technology Rights
Sale of technology rights income strictly relates to the revenue generated from completed technology transfers. We completed sixteen technology transfers during the year ended December 31, 2007 compared to the twenty-nine technology transfers completed during the year ended December 31, 2006. We have experienced an increase in the average market capitalization of our clients. Transactions with these larger clients are requiring additional time to close. This has resulted in a decrease in the number of executed technology transfers. The technology transfers had an average value of $1.0 million and $1.8 million for the years ended December 31, 2007 and 2006, respectively. The lower average value of the technology transfer is a function of a decrease in the cost to acquire the technology rights partially offset by an increase in the average technology transfer multiple. In addition, there were 4 technology transfers with income per transfer of less than $500,000 in 2007 and none in 2006. The average cost of the acquisition of technology rights for the year ended December 31, 2007 was $238,000 compared to $446,000 for 2006. The technology transfer multiple is the revenue markup on the acquisition of technology rights costs. The average technology transfer multiple for the year ended December 31, 2007 was 4.29 as compared to 3.95 for the year ended December 31, 2006. With the exception of $200,000 received in the first quarter of 2007, all income from the sale of technology rights for the years ended December 31, 2007 and December 31, 2006 was received in the form of equity securities.
The increase in sale of technology rights resulted from completing twenty-nine technology transfers during the year ended December 31, 2006 compared to the fourteen technology transfers completed during the year ended December 31, 2005. All of the income from the sale of technology rights was received in the form of equity securities for both the year ended December 31, 2006 and 2005. In addition, the average value of technology transfers increased 36%. The average technology transfer multiple remained consistent from year to year.
In 2008, our revenues from the sale of technology rights are expected to increase modestly over that of 2007.
Consulting and Other Services
Income from technology acquisition alliance services was approximately $1.5 million and $3.0 million for the years ended December 31, 2007 and 2006, respectively. The decrease in income from technology acquisition alliances in 2007 resulted primarily from a change in our pricing of the technology acquisition alliances, partially offset by the increase in the number of technology acquisition alliance agreements. In January 2007, we reduced the price of our technology acquisition alliance services and changed the payment terms to primarily cash. This was done to enhance client diversification and reduce the cost of handling small equity stakes. We increased the number of new technology acquisition alliances from forty-four in 2006 to eighty in 2007. Of the eighty alliances formed in 2007, three agreements called for payment in stock and the remaining agreements called for monthly fees to be paid in cash.
Other consulting income for the year ended December 31, 2007 included income of $583,000 from our Intellectual Capital Consulting division, as compared to $644,000 for the year ended December 31, 2006. Our UTEK Information Services division comprised the balance of consulting fee and other services income of $1.3 million for 2007 and 2006. Of the total consulting and other services income received during the year ended December 31, 2007, 27% was paid in the form of equity securities in companies and the balance was paid in cash. Of such income received during the year ended December 31, 2006, 44% was paid in the form of equity securities and the balance was paid in cash.
Income from technology acquisition alliance services was approximately $3.0 million and $2.15 million for the years ended December 31, 2006 and 2005, respectively. The increase in income from technology acquisition alliances in 2006 resulted primarily from providing services under forty-four new technology acquisition alliance agreements, as compared to thirty-six new technology acquisition alliance agreements during 2005. Other consulting income for the year ended December 31, 2006 included income of $644,000 from our Intellectual
23
Capital Consulting division, as compared to $1.16 million for the year ended December 31, 2005. UTEK Information Services comprised the balance of consulting fee and other services income of $1.3 million for 2006 and $869,000 for 2005. Of the total consulting and other income received during the years ended December 31, 2006 and 2005, 51% and 34%, respectively, was paid in the form of equity securities and the balances were paid in cash.
The increase in the number of our technology acquisition alliances in 2006 and 2007 is primarily a result of a focus on Open Innovation, an increase in the overall demand for our services, as well as an increase in our business development team including a vice president of business development. We believe that we are growing our customer base as a result of increased sales and marketing efforts and better recognition in the business community regarding the value and availability of our services. Our new technology acquisition alliances have increased the diversity of our customer makeup. Our current customer base has a larger average market capitalization than in previous years. Although this is beneficial to our business, having customers with a larger market capitalization has resulted in a significant increase in the time needed to complete technology transfers. Therefore, although we have increased the number of technology acquisition alliance customers, the number of technology transfers completed for the year ended December 31, 2007 has decreased.
Revenues from consulting and other services are expected to increase as a result of the addition of Pharmalicensing, the expansion of our consulting services client base and an increase in our pricing structure for technology acquisition alliances scheduled to go into effect in 2008.
Investment Income, net
Investment income decreased in 2007 compared to 2006, due to lowered cash and cash equivalent balances. Interest income is expected to continue this decline as a result of lower cash balances and lower interest rates.
Expenses
Acquisition of Technology Rights
| | | | | | | | | | | | | | | | | | |
(in thousands, except percentages) | | 2007 | | | 2006 | | | 2005 | | | Percent
Change 2007
versus 2006 | | | Percent
Change 2006
versus 2005 | |
Acquisition of technology rights | | $ | 3,816 | | | $ | 12,945 | | | $ | 4,600 | | | (71 | )% | | 181 | % |
As a percent of sale of technology rights | | | 23 | % | | | 25 | % | | | 25 | % | | (2 | )ppt* | | -0- | ppt |
| * | The abbreviation “ppt” throughout this section denotes percentage points. |
Acquisition of technology rights costs consist of the direct costs associated with our technology transfers, which include cash to further accelerate commercialization efforts, license fees to acquire new technologies, consulting fees with the inventor of the technologies, and sponsored research fees with the university or research facility transferring the technologies. The overall decrease in acquisition of technology rights from 2007 to 2006 was due to the reduced number of technology transfers completed and reduced costs per transaction for the year ended December 31, 2007 as compared to 2006. We completed thirteen less technology transfers during 2007 than in 2006. The average cost per technology transfer decreased approximately $207,000 or 46% during the year ended December 31, 2007.
The overall increase in acquisition of technology rights from 2005 to 2006 was due to completing fifteen additional technology transfers in the year ended December 31, 2006 than in 2005. In addition, the average cost per technology transfer increased approximately $118,000 or 36% during the year ended December 31, 2006.
Acquisition of technology rights costs are directly related to sale of technology rights revenue. We expect that the acquisition of technology rights costs in 2008 will remain at approximately 25% of sale of technology rights.
24
The following table provides certain information relating to the costs of the acquisition of technology rights we incurred in connection with our technology transfers during the year ended December 31, 2007:
| | | | | | | |
Date | | Client Acquiring Newly Formed Company | | Newly Formed Company | | Dollar
Amount of
Expenses |
January 4 | | Manakoa Services Corporation | | Infinite Identification Technologies, Inc. | | $ | 400,000 |
January 11 | | Cyberlux Corporation | | Hybrid Lighting Technologies, Inc. | | | 192,455 |
January 30 | | CytoDyn, Inc. | | Advanced Genetic Technologies, Inc. | | | 167,500 |
January 31 | | Material Technologies, Inc. | | Stress Analysis Technologies, Inc. | | | 130,000 |
February 12 | | Liberty Diversified Holdings, Inc. | | Sero Tonin Solutions, Inc. | | | 70,052 |
March 12 | | Metamorphix Global, Inc. | | Flex Crete Technologies, Inc. | | | 52,884 |
March 12 | | Klegg Electronics, Inc. | | Tempo Control Technologies, Inc. | | | 135,388 |
March 28 | | Avalon Oil & Gas, Inc. | | Leak Location Technologies, Inc. | | | 155,000 |
April 30 | | Material Technologies, Inc. | | Damage Assessment Technologies, Inc. | | | 300,000 |
May 30 | | Klegg Electronics, Inc. | | Klegg Network Storage Technologies, Inc. | | | 450,000 |
June 28 | | Material Technologies, Inc. | | Non-Destructive Assessment Technologies, Inc. | | | 280,000 |
July 12 | | Pathway One Plc | | WebMed Technologies, Inc. | | | 305,215 |
July 20 | | MachineTalker, Inc. | | Wideband Detection Technologies, Inc. | | | 40,000 |
Sept 28 | | World Energy Solutions, Inc. | | Hydrogen Safe Technologies, Inc. | | | 492,350 |
November 12 | | NeoStem, Inc. | | Stem Cell Technologies, Inc. | | | 300,000 |
December 28 | | MachineTalker, Inc. | | Micro Wireless Technologies, Inc. | | | 345,000 |
| | | | | | | |
| | | | | | $ | 3,815,844 |
| | | | | | | |
The following table provides certain information relating to costs of the acquisition of technology rights we incurred in connection with our technology transfers during the year ended December 31, 2006:
| | | | | | | |
Date | | Client Acquiring Newly Formed Company | | Newly Formed Company | | Dollar
Amount of
Expenses |
January 20 | | Fuel FX International, Inc. | | Emissions Detection Technologies, Inc. | | $ | 300,000 |
January 27 | | Broadcast International, Inc. | | Video Processing Technologies, Inc. | | | 625,000 |
January 30 | | WebSky, Inc. | | Strategic Wireless Solutions, Inc | | | 265,000 |
March 6 | | Trio Industries Group, Inc. | | Ultra Fine Coating Systems, Inc. | | | 534,000 |
March 15 | | American Soil Technologies, Inc. | | Advanced Fertilizer Technologies, Inc. | | | 500,000 |
March 16 | | Advanced Refractive Technologies, Inc. | | Ocular Therapeutics, Inc. | | | 400,000 |
April 3 | | Trio Industries Group, Inc. | | Natural Adhesive Technologies, Inc. | | | 555,000 |
April 4 | | Advanced Refractive Technologies, Inc. | | Advanced Glaucoma Technologies, Inc. | | | 399,999 |
April 5 | | UBA Technology, Inc. | | Intellitouch Technologies, Inc. | | | 502,000 |
May 1 | | Industrial Biotechnology Corporation | | Bio-Repellant Technologies, Inc. | | | 400,000 |
May 12 | | Kwikpower International Plc | | Hydrocarbon Synthesis Technologies, Inc. | | | 407,500 |
May 12 | | Kwikpower International Plc | | Advanced BioEnergy Technologies, Inc. | | | 380,000 |
June 1 | | Trio Industries Group, Inc. | | Advanced Powder Coating Technologies, Inc. | | | 550,000 |
June 12 | | Kwikpower International Plc | | Advanced Biofuel Technologies, Inc. | | | 375,000 |
25
| | | | | | | |
Date | | Client Acquiring Newly Formed Company | | Newly Formed Company | | Dollar
Amount of
Expenses |
June 13 | | Xethanol Corporation | | Advanced Biomass Gasification Technologies, Inc. | | | 450,000 |
June 20 | | Klegg Electronics, Inc. | | Smart Speaker Technologies, Inc. | | | 528,628 |
July 12 | | Avalon Oil & Gas, Inc. | | Ultrasonic Mitigation Technologies, Inc. | | | 410,000 |
July 14 | | DME Interactive Holdings, Inc. | | Multimedia Control Technologies, Inc. | | | 512,650 |
July 18 | | CytoDyn, Inc. | | Advanced Influenza Technologies, Inc. | | | 934,399 |
August 11 | | NetFabric Holdings, Inc | | Intrusion Detection Technologies, Inc. | | | 523,600 |
August18 | | Material Technologies, Inc. | | Materials Monitoring Technologies, Inc. | | | 589,000 |
August 29 | | Industrial Biotechnology Corporation | | Advanced Pheromone Technologies, Inc. | | | 325,000 |
September12 | | Liberty Diversified Holdings, Inc. | | Innovative Packaging Technologies, Inc. | | | 435,000 |
September 22 | | Advanced Medical Isotope Corp. | | Neu-Hope Technologies, Inc. | | | 335,000 |
October 9 | | World Energy Solutions, Inc. | | Pure Air Technologies, Inc. | | | 593,369 |
November 1 | | Klegg Electronics, Inc. | | Universal Wireless Technologies, Inc. | | | 265,000 |
November 8 | | Avalon Oil & Gas, Inc. | | IntelliWell Technologies, Inc. | | | 250,000 |
November 10 | | Cyberlux Corporation | | SPE Technologies, Inc. | | | 375,000 |
December 6 | | Cargo Connection Logistics Holding, Inc. | | Nuclear Material Detection Technologies, Inc. | | | 225,000 |
| | | | | | | |
| | | | | | $ | 12,945,145 |
| | | | | | | |
The following table provides certain information relating to the costs of the acquisition of technology rights we incurred in connection with our technology transfers during the year ended December 31, 2005:
| | | | | | | |
Date | | Client Acquiring Newly Formed Company | | Newly Formed Company | | Dollar
Amount of
Expenses |
January 10 | | Xethanol Corporation | | Superior Separation Technologies, Inc. | | $ | 110,000 |
February 22 | | Health Sciences Group, Inc. | | Open Cell Biotechnologies, Inc. | | | 265,000 |
March 31 | | Swiss Medica, Inc. | | Anti-Depression Biohealth Solutions, Inc. | | | 196,673 |
June 30 | | Manakoa Services Corporation | | Vigilant Network Technologies, Inc. | | | 655,122 |
July 18 | | eLinear Corporation | | Secure Voice Communications, Inc. | | | 25,000 |
August 5 | | Industrial Biotechnology Corporation | | Advanced Bioscience, Inc. | | | 350,174 |
August 15 | | Xethanol Corporation | | Xylose Technologies, Inc. | | | 530,080 |
September 1 | | Fuel FX International, Inc. | | Emissions-Reduction Technologies, Inc. | | | 351,722 |
September 15 | | INSEQ Corporation | | Separation and Recovery Technologies, Inc. | | | 400,000 |
September 30 | | Kwikpower International Plc | | Biodiesel Technologies, Inc. | | | 490,799 |
October 3 | | Trio Industries | | Kenaf Core Technologies, Inc. | | | 350,000 |
October 12 | | Fuel FX International, Inc. | | Emissions Analysis, Inc. | | | 175,000 |
November 17 | | Trio Industries, Inc. | | Cornboard Technologies, Inc. | | | 425,000 |
December 13 | | Advanced Refractive Technologies, Inc. | | Optimetrix Technologies, Inc. | | | 275,000 |
| | | | | | | |
| | | | | | $ | 4,599,570 |
| | | | | | | |
26
Salaries and Wages
| | | | | | | | | | | | | | | | | | |
(in thousands, except percentages) | | 2007 | | | 2006 | | | 2005 | | | Percent
Change 2007
versus 2006 | | | Percent
Change 2006
versus 2005 | |
Salaries and wages | | $ | 3,519 | | | $ | 3,430 | | | $ | 2,675 | | | 3 | % | | 28 | % |
As a percent of revenue | | | 17 | % | | | 6 | % | | | 12 | % | | 11 | ppt | | (6 | )ppt |
Salaries and wages include non-sales employee and officer salaries and related benefits including bonuses and stock-based compensation. Salaries and wages increased $88,000 during the year ended December 31, 2007 due primarily to an increase in officers’ compensation related to the COO and CFO, as well as an increase in the stock-based compensation expense resulting from additional option grants.
The increase in salaries and wages for the year ended December 31, 2006 compared to the year ended December 31, 2005 is due to a number of factors, including: salaries related to six additional months of the Knowledge Express website (acquired July 1, 2005) employees comprised $200,000; salaries related to additional Knowledge Express website employees hired in 2006 of $60,000; additional accounting personnel salaries of $135,000; a $189,000 increase in salaries in the UK; a reduction in salaries of $360,000 related to moving EKMS business from Massachusetts to the main office; and stock-based compensation expense of $500,000 recognized in 2006 as a result of the adoption of SFAS 123(R),Share-Based Payment.
We expect salaries and wages to increase during 2008 as a result of an expected increase in officer salaries and the creation of three new management positions to strengthen the senior management team and enhance the potential for Company development.
Professional Fees
| | | | | | | | | | | | | | | | | | |
(in thousands, except percentages) | | 2007 | | | 2006 | | | 2005 | | | Percent
Change 2007
versus 2006 | | | Percent
Change 2006
versus 2005 | |
Professional fees | | $ | 1,359 | | | $ | 1,320 | | | $ | 774 | | | 3 | % | | 70 | % |
As a percent of revenue | | | 7 | % | | | 2 | % | | | 3 | % | | 5 | ppt | | (1 | )ppt |
Professional fees include accounting fees, legal fees and valuation expenses for our investments. The increase in professional fees for the year ended December 31, 2007 compared to the year ended December 31, 2006 relates to an increase in legal fees, offset by a slight decrease in the quarterly valuation fees. The Company incurred legal fees related to its registration statement filing and responses to SEC comment letters in 2007 and 2006 as well as a related SEC matter in 2007. These expenses should not be recurring in 2008.
The increase in professional fees for the year ended December 31, 2006 compared to the year ended December 31, 2005 relates primarily to additional legal and accounting fees of approximately $300,000 and $80,000, respectively, related to the 2006 registration statement filings, as well as $90,000 in additional valuation costs as a result of the increased number of our portfolio holdings.
We expect to have a decrease in professional fees for the year ended December 31, 2008 as a result of resolution of certain SEC comments related to the Company’s previously filed registration statement.
Sales and Marketing
| | | | | | | | | | | | | | | | | | |
(in thousands, except percentages) | | 2007 | | | 2006 | | | 2005 | | | Percent
Change 2007
versus 2006 | | | Percent
Change 2006
versus 2005 | |
Sales and marketing | | $ | 2,036 | | | $ | 3,128 | | | $ | 2,681 | | | (35 | )% | | 17 | % |
As a percent of revenue | | | 10 | % | | | 5 | % | | | 12 | % | | 5 | ppt | | (7 | )ppt |
27
Sales and marketing expenses include advertising, marketing, salaries and commissions paid to sales personnel, commissions paid to outside service providers, travel and other selling expenses. Commissions decreased $395,000 for the year ended December 31, 2007, compared to the year ended December 31, 2006 as a result of using less outside service providers for the purpose of selling technology acquisition alliance agreements. Salaries, wages and commissions paid to sales-related employees decreased $750,000 for the year ended December 31, 2007 compared to the year ended December 31, 2006 due to lower commissions related to the completion of thirteen fewer technology transfers. In addition, we have implemented certain changes in our sales and marketing division in an effort to increase sales leads, and eventually sales, without incurring substantial additional costs.
Sales salaries and commissions increased $340,000 for the year ended December 31, 2006 compared to the year ended December 31, 2005 as a result of the increased number of technology transfers and an increase in technology transfer values, as well as an increase in the size of the sales force. There were twenty-nine technology transfers during the year ended December 31, 2006, as compared to fourteen for the year ended December 31, 2005. Commissions paid to outside service providers used strictly to generate technology acquisition alliance agreements increased $192,000 for the year ended December 31, 2006 due to an increase in the number of technology acquisition alliances brought in by these providers. These increases were offset by a decrease in outside consultant costs associated with a decrease UTEK-EKMS revenue.
It is our intention to continue to grow our sales force by increasing the number of sales personnel in 2008.
General and Administrative
| | | | | | | | | | | | | | | | | | |
(in thousands, except percentages) | | 2007 | | | 2006 | | | 2005 | | | Percent
Change 2007
versus 2006 | | | Percent
Change 2006
versus 2005 | |
General and administrative | | $ | 2,838 | | | $ | 3,865 | | | $ | 2,562 | | | (27 | )% | | 51 | % |
As a percent of revenue | | | 14 | % | | | 7 | % | | | 11 | % | | 7 | ppt | | (4 | )ppt |
The decrease in general and administrative costs for the year ended December 31, 2007 compared to the year ended December 31, 2006 is largely due to charitable contributions, SEC filing costs and employee related costs. Charitable contributions decreased due to a non-recurring gift of 5.1 million common shares of HydroFlo, Inc. valued at $663,000 in 2006. Employee recruitment costs decreased $125,000 in 2007 because these were one-time costs incurred by the accounting department during 2006. SEC filing costs decreased $170,000 in 2007 as a result of the registration statement filings in 2006. Employee related costs decreased $110,000 due to the reduction in total compensation related to the completion of thirteen fewer technology transfers.
The increase in general and administrative costs for the year ended December 31, 2006 compared to the year ended December 31, 2005 is largely due to charitable contributions, outside services, printing and reproduction, and rent expense. Charitable contributions include a gift of 5.1 million common shares of HydroFlo, Inc. valued at $663,000. Outside services increased $90,000 during 2006 due to the cost of royalties paid related to our Knowledge Express website acquired on July 1, 2005, as well as amounts paid to employment placement agencies. Printing and reproduction increased approximately $155,000 due to costs associated with our registration statement filings in 2006. Rent increased $155,000 as a result of the buy-out of the lease at our Massachusetts location related to the relocation of the EKMS division to the corporate headquarters in Tampa, Florida and the additional cost of our new corporate headquarters.
We expect to have an increase in general and administrative expenses for the year ended December 31, 2008 as a result of planned growth for the Company, including acquisitions.
28
Goodwill Impairment
Management conducts an annual impairment analysis at the end of each year. In connection with the 2006 impairment analysis, we determined there was an impairment of the goodwill related to the Pharma Transfer, Ltd. acquisition due to a decrease in the annual revenue. We subsequently recorded a partial impairment of the goodwill in the first quarter of 2007. In connection with the 2007 impairment analysis, we determined there was another impairment of the goodwill related to the Pharma Transfer, Ltd. acquisition and an impairment of the goodwill related to the Knowledge Express acquisition due to a decrease in the annual revenue. As a result, we recorded another partial impairment of the goodwill in the fourth quarter of 2007. These write-downs resulted in an impairment charge of approximately $159,000 ($99,000 after tax) and $51,000 ($32,000 after tax) for the United States segment during 2007.
We decided during 2006 to make significant changes in strategy for UTEKip, Ltd., primarily switching the focus of operations in Israel from software to technology transfer. These changes were other-than-temporary; therefore our management determined there was an impairment of the original purchase goodwill. We recorded a total impairment of the goodwill for UTEKip (Israel segment) in 2006. Based on an analysis of the undiscounted cash flows, we determined that the goodwill for this reporting unit was impaired. This resulted in a write-down of approximately $235,000, $147,000 after tax, for the year ended December 31, 2006.
Net Realized Gains or Losses on Investments
| | | | | | | | | | | | | | | | | |
(in thousands, except percentages) | | 2007 | | | 2006 | | 2005 | | | Percent
Change 2007
versus 2006 | | | Percent
Change 2006
versus 2005 | |
Realized gains / (losses) | | $ | (1,447 | ) | | $ | 903 | | $ | (3,459 | ) | | (260 | )% | | 126 | % |
Net realized losses on investments, net of income tax effect, amounted to $1,447,380 for the year ended December 31, 2007, and were related to sales as follows:
| | | | | | |
Portfolio Company | | Number of
Shares | | Realized
Gain (Loss) | |
Advanced Refractive Technologies, Inc. | | 1,533,333 | | $ | (46,064 | ) |
American Soil Technologies, Inc. | | 87,855 | | | 96 | |
Shumate Industries, Inc. | | 171,432 | | | 127,234 | |
Health Sciences Group, Inc. | | 3,023,703 | | | (974,894 | ) |
Manakoa Services Corporation | | 578,958 | | | (72,076 | ) |
Swiss Medica, Inc. | | 1,735,000 | | | (181,851 | ) |
Xethanol Corporation | | 136,838 | | | (378,936 | ) |
Broadcast International, Inc. | | 505,798 | | | 222,543 | |
Rival Technologies, Inc. | | 120,000 | | | (224 | ) |
Power3 Medical Products, Inc. | | 221,033 | | | (106,931 | ) |
World Energy Solutions, Inc. | | 19,500 | | | (19,693 | ) |
XELR8 Holdings, Inc. | | 33,825 | | | 10,939 | |
NetFabric Holdings, Inc. | | 20,000 | | | 150 | |
IPORUSSIA, Inc. | | 258,064 | | | (1,016 | ) |
Maelor Plc | | 176,250 | | | 14,471 | |
U.S. Wireless Online, Inc. | | 750,000 | | | (41,128 | ) |
| | | | | | |
Total | | | | ($ | 1,447,380 | ) |
| | | | | | |
29
Net realized gains on investments, net of income tax effect, amounted to $903,181 for the year ended December 31, 2006 and were related to sales as follows:
| | | | | | |
Portfolio Company | | Number of
Shares | | Realized
Gain (Loss) | |
Sequiam Corporation | | 645,000 | | $ | 3,015 | |
INyX, Inc. | | 29,999 | | | 29,733 | |
Swiss Medica, Inc. | | 465,000 | | | (12,790 | ) |
Z Trim Holdings | | 973,508 | | | 395,270 | |
Israel Technology Acquisition Corporation | | 50,000 | | | 6,732 | |
Israel Technology Acquisition Corporation—warrants | | 100,000 | | | 2,739 | |
Fortress America Acquisition Corporation | | 40,000 | | | 18,971 | |
Xethanol Corporation | | 1,215,475 | | | 1,889,832 | |
eFoodSafety.com, Inc. | | 61,224 | | | (3,380 | ) |
HydroFlo, Inc.(1) | | 5,535,044 | | | (40,721 | ) |
Genethera, Inc. | | 14,796 | | | (21,583 | ) |
Magic Media Networks, Inc. | | 97,904 | | | 225 | |
E Med Future, Inc. | | 675,032 | | | (218,444 | ) |
GS CleanTech Corporation | | 295,500 | | | (36,180 | ) |
Harborlight Diversified Fund, LP | | 250,000 | | | 17,116 | |
Health Sciences Group, Inc. | | 100,000 | | | (47,511 | ) |
Manakoa Services Company | | 58,574 | | | (11,759 | ) |
NutraCea International Corporation | | 359,182 | | | 277,325 | |
Pacific Biometrics, Inc. | | 91,885 | | | 16,539 | |
Power3 Medical Products, Inc. | | 80,000 | | | (13,537 | ) |
SheerVision Inc. | | 129,835 | | | (330,063 | ) |
US Energy Initiatives | | 166,000 | | | (9,240 | ) |
American Soil Technologies, Inc. | | 25,300 | | | 104 | |
Vitacube Systems Holding, Inc. | | 54,857 | | | (40,503 | ) |
Intra-Asia Entertainment Corporation | | 720,639 | | | (968,709 | ) |
| | | | | | |
Total | | | | $ | 903,181 | |
| | | | | | |
Net realized losses on investments, net of income tax effect, amounted to $3,458,979 for the year ended December 31, 2005 and were related to sales as follows:
| | | | | | |
Portfolio Company | | Number of
Shares | | Realized
Gain (Loss) | |
Circle Group Holdings, Inc. | | 2,642,197 | | $ | 1,340,949 | |
E Med Future, Inc. | | 608,968 | | | (161,387 | ) |
Telkonet, Inc. | | 20,668 | | | 37,317 | |
Magic Media Networks, Inc. | | 477,096 | | | (41,021 | ) |
eFoodSafety.com, Inc. | | 61,224 | | | (2,355 | ) |
Full Circle Registry, Inc. | | 200,000 | | | (99,784 | ) |
HydroFlo, Inc. | | 364,956 | | | 88,510 | |
Silver Screen Studios, Inc. | | 47,615 | | | (29,137 | ) |
Peak Entertainment, Inc. | | 4,000 | | | (7,056 | ) |
Swiss Medica, Inc. | | 200,000 | | | 8,683 | |
Jenex Corporation | | 133,333 | | | (8,099 | ) |
Group of zero value shares(2) | | Various | | | (4,585,599 | ) |
| | | | | | |
Total | | | | ($ | 3,458,979 | ) |
| | | | | | |
30
| (1) | We elected to abandon our right, title and interest in and to 400,000 shares common stock of HydroFlo, Inc. See Note 2 of Notes to Consolidated Financial Statements under Item 8. “Financial Statements and Supplementary Data.” |
| (2) | Upon approval from our Board of Directors, our management made the decision to sell these investments because such investments had been carried on our financial statements with a zero value for several quarters. Maintaining these investments was costly due to expenses we incurred in connection with our independent valuation service provider’s quarterly valuation of such investments and, in the opinion of management, the likelihood of the future appreciation in value of such investments was minimal. The following investments were included in the group of zero value shares above: Provision Operation Systems, Inc., Advanced Recycling Sciences, Inc., Graphco Holdings Corp., Nubar, Inc., Assuretec Holdings, Inc., Prime Pharmaceutical Corporation, Primapharm Funding Corporation, Silver Screen Studios, Inc., Mixed Entertainment, Inc., Hydrogen Technology Applications, Inc., Zkid Network Company, GreenWorks Corporation, and Xeminex, Ltd. |
Net realized gains and losses can vary substantially due to a variety of factors and may not be indicative of future performance. As a result of the uncertainty surrounding the future values of our investments, we are unable to make any projections or estimates regarding realized gains or losses expected in 2008.
Net Changes in Unrealized Appreciation or Depreciation on Investments
We determine the value of each investment in our portfolio on a quarterly basis and changes in value result in unrealized appreciation or depreciation being recognized. At December 31, 2007, approximately 66% of our net assets represented investments recorded at fair value. Value, as defined in Section 2(a)(41) of the 1940 Act, is (i) the market price for those securities for which a market quotation is readily available and (ii) for all other securities and assets, fair value is as determined in good faith by the Board of Directors. Although many of the securities we hold in our portfolio are quoted on the OTC Bulletin Board or listed on the American Stock Exchange, our Board of Directors is required to determine the fair value of such securities if the validity of the market quotations appears to be questionable, or if the number of quotations is such as to indicate that there is a thin market in the security. The fair value of these securities is frequently less than the market quotations for such securities. Because there is typically no readily available market value for the investments in our portfolio, we value substantially all of our investments at fair value as determined in good faith by the Board of Directors. In making its determination, our Board of Directors may consider valuation appraisals provided by independent valuation service providers. Because of the inherent uncertainty of determining the fair value of investments that do not have a readily available market value, the fair value of our investments determined in good faith by the Board of Directors may differ significantly from the values that would have been used had a ready market existed for the investments, and the differences could be material.
| | | | | | | | | | | | | | | | | | |
(in thousands, except percentages) | | 2007 | | | 2006 | | | 2005 | | | Percent
Change 2007
versus 2006 | | | Percent
Change 2006
versus 2005 | |
Unrealized appreciation / (depreciation) | | $ | (10,806 | ) | | $ | (25,758 | ) | | $ | (333 | ) | | (58 | )% | | 7631 | % |
31
Net change in unrealized depreciation on investments, net of income tax effect, amounted to $10,806,048 for the year ended December 31, 2007 and was related to our investments as follows:
| | | | |
Portfolio Company | | Net Unrealized
Appreciation
(Depreciation) | |
Advanced Refractive Technologies, Inc. | | $ | (1,261,124 | ) |
American Soil Technologies, Inc. | | | (720,328 | ) |
Broadcast International, Inc. | | | 912,368 | |
CytoDyn, Inc. | | | (825,467 | ) |
Industrial Biotechnology Corporation | | | (1,243,595 | ) |
Emission & Power Supply, Inc. | | | (766,590 | ) |
Health Sciences Group, Inc. | | | 939,768 | |
Klegg Electronics, Inc. | | | (3,489,976 | ) |
Manakoa Services Corporation | | | (1,055,455 | ) |
vidShadow, Inc. | | | (703,548 | ) |
World Energy Solutions, Inc. | | | (1,436,653 | ) |
All other investments | | | (1,155,448 | ) |
| | | | |
| | $ | (10,806,048 | ) |
| | | | |
The net unrealized depreciation for the year ended December 31, 2007 was primarily due to the write down of four investments in our portfolio, including Advanced Refractive Technologies, Inc., Klegg Electronics, Inc., Industrial Biotechnology Corporation and World Energy Solutions, Inc.
While these unrealized losses were significant, reduction in values and failures among small cap companies is not unexpected and may occur in the future. The current portfolio is comprised of more than 60 holdings. Many of these positions are with small capitalization companies which over time may have high failure rates due to a variety of factors. For clients that fail, UTEK may lose the entire amount of its capital spent acquiring and transferring the technology to them.
Net unrealized depreciation on investments, net of income tax effect, amounted to $25,758,186 for the year ended December 31, 2006 and was related to our investments as follows:
| | | | |
Portfolio Company | | Net Unrealized
Appreciation
(Depreciation) | |
Health Sciences Group, Inc. | | $ | (434,227 | ) |
Xethanol Corporation | | | (425,918 | ) |
Material Technologies, Inc. | | | (545,424 | ) |
Industrial Biotechnology Corporation | | | (4,914,275 | ) |
KP Renewables, Plc | | | (3,830,809 | ) |
Advanced Refractive Technologies, Inc. | | | (1,015,456 | ) |
Fuel FX International, Inc. | | | (784,954 | ) |
Trio Industries Group, Inc. | | | (8,306,858 | ) |
UBA Technology, Inc. | | | (1,619,168 | ) |
CytoDyn, Inc. | | | (1,736,363 | ) |
Avalon Oil and Gas, Inc. | | | (700,252 | ) |
Liberty Diversified Holdings, Inc. | | | (659,661 | ) |
All other investments | | | (784,821 | ) |
| | | | |
| | $ | (25,758,186 | ) |
| | | | |
The net unrealized depreciation for the year ended December 31, 2006 was mostly due to the significant write down of five of our portfolio investments: Industrial Biotechnology Corp., Trio Industries Group, Inc., KP Renewables, Plc. (Kwikpower International Plc), UBA Technology, Inc., and CytoDyn, Inc.
32
Net unrealized depreciation on investments, net of income tax effect, amounted to $333,176 for the year ended December 31, 2005 and was related to our investments as follows:
| | | | |
Portfolio Company | | Net Unrealized
Appreciation
(Depreciation) | |
UTEK Real Estate Holdings | | $ | (148,551 | ) |
Circle Group Holdings, Inc. | | | (3,369,849 | ) |
Health Sciences Group, Inc. | | | (513,009 | ) |
Swiss Medica, Inc. | | | (156,262 | ) |
Hydroflo, Inc. | | | (212,458 | ) |
Manakoa Services Company | | | (1,291,108 | ) |
Industrial Biotechnology Corporation | | | 2,197,377 | |
KP Renewables, Plc | | | (168,869 | ) |
INSEQ Corporation | | | (1,065,035 | ) |
Trio Industries Group, Inc. | | | 616,387 | |
Group of zero value shares(1) | | | 4,100,000 | |
All other investments | | | (321,799 | ) |
| | | | |
| | $ | (333,176 | ) |
| | | | |
| (1) | Upon approval from our Board of Directors, our management made the decision to sell these investments because such investments had been carried on our financial statements with a zero value for several quarters. Maintaining these investments was costly due to expenses we incurred in connection with our independent valuation service provider’s quarterly valuation of such investments and, in the opinion of management, the likelihood of the future appreciation in value of such investments was minimal. The following investments were included in the group of zero value shares above: Provision Operation Systems, Inc., Advanced Recycling Sciences, Inc., Graphco Holdings Corp., Nubar, Inc., Assuretec Holdings, Inc., Prime Pharmaceutical Corporation, Primapharm Funding Corporation, Silver Screen Studios, Inc., Mixed Entertainment, Inc., Hydrogen Technology Applications, Inc., Zkid Network Company, GreenWorks Corporation, and Xeminex, Ltd. For more information about each of these investments, see the schedule of investments included in our consolidated financial statements contained elsewhere in this annual report on Form 10-K. |
The net unrealized depreciation for the year ended December 31, 2005 was mostly due to the significant write down of three investments in our portfolio, offset by the unrealized gain that resulted from the recognition of losses on several zero value investments (see (1) above). The three investments with significant unrealized losses were Z Trim Holdings (Circle Group Holdings, Inc.), Manakoa Services Company and GS Energy Corporation (INSEQ Corporation). Because we had previously recorded the depreciated value of the zero value investments as unrealized depreciation, we had to make an accounting entry to reverse the unrealized depreciation. This accounting entry resulted in an unrealized appreciation of $4.6 million relating to these investments.
Changes in unrealized appreciation or depreciation can vary substantially due to a variety of factors and may not be indicative of future performance.
33
Liquidity and Capital Resources
At December 31, 2007, we had cash and cash equivalents of $5.3 million. We also had investments in U.S. Treasuries and certificates of deposit (CDs) of $1.5 million. We typically invest our excess cash in U.S. Treasuries and CDs, which normally have three-month to one-year maturities. These investments do not qualify as cash equivalents.
We have financed substantially all of our operations since inception through the issuance of equity securities and, to a lesser extent, sales of investments, cash received in connection with the provision of technology acquisition alliance and other consulting services and the use of funds from our investments in U.S. Treasuries and certificates of deposit. Our primary source of liquidity and capital for the year ended December 31, 2007 was $3.2 million in connection with the provision of technology acquisition alliance and other consulting services, $3.1 million in proceeds generated from the sale of U.S. Treasuries and CDs, $1.9 million in proceeds generated from the sale of shares of our portfolio companies and $543,000 in proceeds from the exercise of stock options.
Our income from operations consists primarily of the sale of technology rights and consulting income from technology acquisition alliances in exchange for equity securities rather than cash. In the year ended December 31, 2007, 84% of our income from operations was paid in the form of equity securities. Of the $20.3 million in income from operations in 2007, $3.2 million was received in the form of cash. During the year ended December 31, 2007, we used approximately $3.8 million to fund our technology transfer transactions and approximately $9.8 million for operating expenses. Looking forward to 2008, we expect our cash outflow for technology transfer transactions will be scaled to available cash and that our operating expenses should be comparable overall to those incurred in 2007.
During the year ended December 31, 2007, we loaned UTEK Real Estate Holdings, Inc., one of our portfolio companies, an additional $784,000 for real estate improvements and operations. We expect additional renovations to a separate building to begin in the first quarter of 2008 and to be completed by late spring of 2008 with the additional cash outlay to be approximately $200,000.
On December 1, 2006, our Board of Directors approved a special dividend of $0.02 per share that was paid on January 31, 2007 to shareholders of record on January 12, 2007. Our Board of Directors will have sole discretion in determining whether to declare and pay cash dividends in the future. The declaration of cash dividends will depend on our profitability, financial condition, cash requirements, future prospects and other factors deemed relevant by our Board of Directors. Our ability to pay cash dividends in the future could be limited or prohibited by regulatory requirements and the terms of financing agreements that we may enter into or by the terms of any preferred stock that we may authorize and issue.
In February 2007, we obtained a $4,000,000 line of credit with the Bank of Tampa. The advances on the line of credit accrue interest (payable monthly) at prime (7.25% as of December 30, 2007). The principal and any unpaid interest are due upon demand. This line is collateralized with commercial real estate owned by UTEK Real Estate Holdings, Inc. We have not utilized this line of credit and as of December 31, 2007 we had no outstanding debt.
We currently intend to fund our capital expenditures, as well as liquidity needs, with existing cash and cash equivalent balances, our investments in U.S. treasuries and certificates of deposit and borrowings, as well as with cash generated by operations and the sales of our investments. We believe that these sources will be sufficient to meet working capital needs, capital requirements, and current commitments for at least the next twelve months. However, our capital requirements will depend on many factors, the most important factor is our sales of technology rights. In addition, the sale of our investments is dependent on market price, which is unpredictable. We may need to scale the number of sales of technology rights to available cash resources in the near term. In addition, we may seek to raise additional funds through public or private debt or equity financing for long-term liquidity. Additional funds may not be available on favorable terms to us, if at all.
34
Contractual Obligations
The following table reflects a summary of our contractual obligations and other commercial commitments as of December 31, 2007:
Contractual Obligations
| | | | | | | | | | | | | | | | | | |
| | | Payments due by December 31, | | Payments due
more than
5 Years |
| | | 2008 | | 2009 | | 2010 | | 2011 | | 2012 | |
Operating Lease Obligations | | $ | 291,541 | | $ | 155,925 | | $ | 1,301 | | $ | -0- | | $ | -0- | | $ | -0- |
| | | | | | | | | | | | | | | | | | |
Critical Accounting Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the amounts reported in the financial statements. Critical accounting estimates are those that are both important to the presentation of our financial condition and results of operations and require management’s most difficult, complex, or subjective judgments. We consider the following accounting policies and related estimates to be critical:
Valuation Methodology
Currently, we primarily receive cash in connection with our technology acquisition alliance agreements and illiquid securities in connection with our technology transfers. Historically, we primarily received illiquid securities in connection with both our technology acquisition alliance agreements and technology transfers. The securities received are generally subject to restrictions on resale and generally are thinly traded or have no established market.
We determine fair value to be the amount for which an investment could be exchanged in an orderly disposition over a reasonable period of time between willing parties other than in a forced or liquidation sale. Our valuation process is intended to provide a consistent basis for determining the fair value of our portfolio investments. We record unrealized depreciation on investments when we believe that an investment has become impaired, including where realization of an equity security is doubtful. We record unrealized appreciation if we believe that the underlying portfolio company has appreciated in value and, therefore, our equity security has also appreciated in value. Upon the sale of our investments, the values that are ultimately realized may be different from the presently determined fair values of such securities. This difference could be material. Our equity interests in portfolio companies for which there is no liquid public market are valued using industry valuation benchmarks, and then the value is assigned a discount reflecting the illiquid nature of the investment as well as our minority, non-control position. When an external event such as a purchase transaction, public offering, or subsequent equity sale occurs, the pricing indicated by the external event is used to corroborate our valuation. The determined values are generally discounted to account for restrictions on resale and minority ownership positions. The value of our equity interests in public companies for which market quotations are readily available is based on the public market price on the balance sheet date. Securities that carry certain restrictions on resale are typically valued at a discount from the public market value of the security.
With respect to loans or debt securities we hold in our portfolio for which there is no liquid public market, the fair value of such loans or debt securities normally corresponds to cost, unless the borrower’s condition or other factors lead to a determination of fair value at a different amount.
The fair value of our investments at December 31, 2007 and December 31, 2006 was determined by our Board of Directors. At December 31, 2007 and December 31, 2006, we received valuation assistance from our independent valuation firm, Klaris, Thomson & Schroeder, Inc., on our entire portfolio of investments for which market quotations were not available.
35
Net Realized Gains/Losses and Net Change in Unrealized Appreciation/Depreciation
Realized gains or losses are measured by the difference between the net proceeds from the repayment or sale and the original cost basis of the investment without regard to unrealized appreciation or depreciation previously recognized. The original cost basis of the securities we receive in connection with our technology acquisition alliance agreements and technology transfers is equal to the amount of revenue we recognized upon the receipt of such securities.
Net change in unrealized appreciation or depreciation reflects the change in portfolio investment values during the reporting period, including the reversal of previously recorded unrealized appreciation or depreciation when gains or losses are realized.
Stock-Based Compensation
We account for stock option grants in accordance with the provisions of Statement of Financial Accounting Standards (“SFAS”) No. 123(R),Share-Based Payment. Under the modified prospective approach of SFAS 123(R), compensation cost recognized during the year ended December 31, 2007 and 2006 includes compensation cost for all share-based payments granted prior to, but not yet vested as of January 1, 2006, based on the grant date fair value estimated in accordance with the original provisions of SFAS 123, and compensation cost for all share-based payments granted subsequent to January 1, 2006, based on the grant date fair value estimated in accordance with the provisions of SFAS 123(R).
Prior to the adoption of SFAS 123(R) in 2006, we accounted for stock option grants using the intrinsic value method prescribed by APB Opinion No. 25, “Accounting for Stock Issued to Employees,” and accordingly, recognized no compensation expense for stock option grants. The impact on our income from operations before taxes, net increase in net assets, basic earnings per share and diluted earnings per share had we accounted for stock-based compensation in accordance with SFAS 123(R) in 2005 would have been approximately $329,000, $310,000, $0.04 and $0.05 lower, respectively. Prior periods were not restated to reflect the impact of adopting the new standard and there is no cumulative effect.
As a result of adopting SFAS 123(R), our income from operations before taxes, net increase in net assets, basic earnings per share and diluted earnings per share were approximately $613,000, $595,000, $0.07 and $0.07 lower, respectively, for the year ended December 31, 2007, and $505,000, $476,000, $0.06 and $0.06 lower, respectively, for the year ended December 31, 2006, than if we had continued to account for stock-based compensation under APB Opinion No. 25 for our stock options.
We use the Black-Scholes option pricing model to estimate the fair value of stock-based awards on the date of grant, using assumptions for volatility, expected term, risk-free interest rate and dividend yield. We have used one grouping for the assumptions as our option grants are primarily basic with similar characteristics. The expected term of options granted has been calculated using the simplified method as prescribed in Staff Accounting Bulletin No. 107 and represents the period of time that options granted are expected to be outstanding. Historical data was used to estimate option exercises and employee terminations. Estimated volatility is based upon our historical market price at consistent points in a period equal to the expected life of the options. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant and the dividend yield is based on the historical dividend yield.
Recently Issued Accounting Pronouncements
In February 2007, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards (“SFAS”) No. 159,The Fair Value Option for Financial Assets and Financial Liabilities—Including an Amendment of FASB Statement No. 115. SFAS No. 159 permits an entity to choose to measure many financial instruments and certain other items at fair value. SFAS No. 159 is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. The Company’s most significant financial instruments are our investments, which are currently carried at fair value. Therefore, the adoption of SFAS No. 159 will not have a significant impact, if any, on our results of operations or financial position.
36
In June 2007, the AICPA issued Statement of Position (“SOP”) 07-1, Clarification of the Scope of the Audit and Accounting Guide Investment Companies and Accounting by Parent Companies and Equity Method Investors for Investments in Investment Companies. SOP 07-1 clarifies which entities are within the scope of the AICPA Audit and Accounting Guide Investment Companies (the “Guide”). SOP 07-1 was originally due to become effective for fiscal years beginning on or after December 15, 2007, but the Financial Accounting Standards Board subsequently voted to indefinitely defer the effective date. Companies that are regulated by the Investment Company Act of 1940 are automatically within the scope of the Guide. As such, the adoption of SOP 07-1 is not expected to materially impact the Company as it already adheres to the provisions of the Guide.
Upon adoption of SOP 07-1, a company must also adopt the provisions of FASB Staff Position (“FSP”) No. FIN 46R-7 “Application of FASB Interpretation No. 46R to Investment Companies.” FSP No. FIN 46R-7 permanently exempts investment companies from applying the provisions of Interpretation 46R to investments carried at fair value.
In December 2007, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards (“SFAS”) No. 141(R),Business Combinations. SFAS No. 141(R) changes the way that companies account for business combinations. More assets and liabilities assumed will be measured at fair value as of the acquisition date; liabilities related to contingent consideration will be remeasured at fair value in each subsequent reporting period; and an acquirer will expense acquisition-related costs as opposed to capitalizing them. SFAS No. 141(R) is effective for business combinations consummated in fiscal years beginning subsequent to December 15, 2008 with no early adoption permitted. The adoption of SFAS No. 141(R) will have an impact on any acquisitions subsequent to 2008; however, we are unable to determine the impact will have on our results of operations or financial position.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk |
Market risk is the risk of loss arising from adverse changes in market rates and prices. We are primarily exposed to equity price risk and foreign exchange risk. The following is a discussion of our equity market risk and foreign exchange risk.
Equity price risk arises from exposure to securities that represent an ownership interest in our portfolio companies. The value of our equity securities and our other investments are based on quoted market prices or our Board of Directors’ good faith determination of their fair value (which is based, in part, on quoted market prices). Market prices of common equity securities, in general, are subject to fluctuations, which could cause the amount to be realized upon sale or exercise of the instruments to differ significantly from the current reported value. The fluctuations may result from perceived changes in the underlying economic characteristics of our portfolio companies, the relative price of alternative investments, general market conditions and supply and demand imbalances for a particular security.
We are also subject to risk from changes in foreign exchange rates with respect to our subsidiaries that use a foreign currency as their functional currency. Such changes could result in cumulative translation gains or losses that are included in our net assets. Revenue from foreign subsidiaries as a percentage of total revenue was 2% for the year ended December 31, 2007. Our foreign subsidiaries are based in the United Kingdom and Israel. Exchange rate fluctuations between the U.S. dollar and the currencies of these countries result in positive or negative fluctuations in the amounts relating to foreign operations reported in our consolidated financial statements. We generally do not use foreign currency options and forward contracts to hedge against the earnings effect of such fluctuations. While we do not expect to incur material losses as a result of this currency risk, there can be no assurance that losses will not result.
37
| Item 8. | Financial Statements and Supplementary Data |
UTEK CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS AND SCHEDULES
38
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors
UTEK Corporation and Subsidiaries
Tampa, Florida
We have audited the accompanying consolidated statements of assets and liabilities of UTEK Corporation and subsidiaries (the “Company”) including the schedules of investments as of December 31, 2007 and 2006 and the related consolidated statements of operations, cash flows and changes in net assets for the three years ended December 31, 2007. These consolidated financial statements and consolidated schedules of investments are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and schedules of investments based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements and schedules of investments referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2007 and 2006 and the results of its operations, cash flows and changes in net assets for the three years ended December 31, 2007 in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 1 to the consolidated financial statements, the Company adopted FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes—an interpretation of FASB 109,” effective January 1, 2007. As further discussed in Note 1 to the consolidated financial statements, the Company adopted SFAS No. 123(R), “Share-Based Payment,” effective January 1, 2006.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of the Company’s internal control over financial reporting as of December 31, 2007, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 11, 2008 expressed an unqualified opinion thereon.
|
/s/ PENDER NEWKIRK & COMPANY |
| Pender Newkirk & Company LLP |
| Certified Public Accountants |
Tampa, Florida
February 11, 2008
Except for the last paragraph of footnote 15, the
date is February 26, 2008
39
REPORT OF MANAGEMENT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
UTEK Corporation
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Rules 13a-15(f) under the Securities Exchange Act of 1934 (“Exchange Act”). The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the Consolidated Financial Statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements and even when determined to be effective, can only provide reasonable assurance with respect to financial statement preparation and presentation.
Management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2007. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), in Internal Control—Integrated Framework. Based on our assessment, management believes that the Company maintained effective internal control over financial reporting as of December 31, 2007.
Our internal control over financial reporting as of December 31, 2007 has been audited by Pender Newkirk & Company LLP, an independent registered public accounting firm, as stated in their report which is included herein.
40
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Board of Directors
UTEK Corporation and Subsidiaries
Tampa, Florida
We have audited the internal control over financial reporting of UTEK Corporation and Subsidiaries (the “Company”) as of December 31, 2007, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion the Company maintained effective internal control over financial reporting as of December 31, 2007, in all material respects, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the statements of assets and liabilities of the Company, including the schedules of investments, as of December 31, 2007 and 2006, and the related statements of operations, cash flows and changes in net assets for each of the three years in the period ended December 31, 2007 and our report dated February 11, 2008 (Except for the last paragraph of footnote 15, the date is February 26, 2008) expressed an unqualified opinion thereon.
Pender Newkirk & Company LLP
Certified Public Accountants
Tampa, Florida
February 11, 2008
41
UTEK Corporation
Consolidated Statements of Assets and Liabilities
| | | | | | | | |
| | | December 31,
2007 | | | December 31,
2006 | |
ASSETS | | | | | | | | |
Investments: | | | | | | | | |
Non-affiliate investments (cost: 2007 - $31,588,337;
2006 - $41,786,374) | | $ | 6,705,850 | | | $ | 14,578,700 | |
Affiliate investments (cost: 2007 - $43,779,616;
2006 - $22,354,448) | | | 14,429,000 | | | | 8,713,900 | |
Controlled investments (cost: 2007 - $17,231,458;
2006 - $15,485,318) | | | 7,768,561 | | | | 10,001,772 | |
U.S. Treasuries and certificates of deposit (cost: 2007- $1,498,346; 2006 - $4,584,651) | | | 1,498,346 | | | | 4,584,651 | |
| | | | | | | | |
Total investments | | | 30,401,757 | | | | 37,879,023 | |
Cash and cash equivalents | | | 5,254,576 | | | | 9,685,111 | |
Accounts receivable, net of allowance for bad debt | | | 358,338 | | | | 534,605 | |
Prepaid expenses and other assets | | | 378,248 | | | | 375,007 | |
Fixed assets, net | | | 476,578 | | | | 595,001 | |
Goodwill | | | 2,821,064 | | | | 3,021,163 | |
Intangible assets | | | 123,812 | | | | 190,100 | |
Deferred tax asset | | | 5,406,704 | | | | 760,800 | |
| | | | | | | | |
TOTAL ASSETS | | | 45,221,077 | | | | 53,040,810 | |
| | | | | | | | |
LIABILITIES | | | | | | | | |
Accrued expenses | | | 790,693 | | | | 595,764 | |
Deferred revenue | | | 755,836 | | | | 1,463,884 | |
| | | | | | | | |
TOTAL LIABILITIES | | | 1,546,529 | | | | 2,059,648 | |
| | | | | | | | |
NET ASSETS | | $ | 43,674,548 | | | $ | 50,981,162 | |
| | | | | | | | |
Commitments and Contingencies | | | | | | | | |
Composition of net assets: | | | | | | | | |
Preferred stock, $.01 par value, 1,000,000 shares authorized; none issued and outstanding | | | — | | | | — | |
Common stock, $.01 par value, 29,000,000 shares authorized; 9,011,276 and 8,936,009 shares issued and outstanding at December 31, 2007 and 2006, respectively | | $ | 90,114 | | | $ | 89,361 | |
Additional paid-in capital | | | 53,148,643 | | | | 51,993,822 | |
Accumulated income: | | | | | | | | |
Accumulated net operating income | | | 33,498,108 | | | | 29,721,366 | |
Net realized loss on investments, net of income taxes | | | (3,512,598 | ) | | | (2,065,218 | ) |
Net unrealized depreciation of investments, net of deferred income taxes | | | (39,703,173 | ) | | | (28,897,125 | ) |
Foreign currency translation adjustment | | | 153,454 | | | | 138,956 | |
| | | | | | | | |
Net assets | | $ | 43,674,548 | | | $ | 50,981,162 | |
| | | | | | | | |
Net asset value per share | | $ | 4.85 | | | $ | 5.71 | |
| | | | | | | | |
See accompanying notes
42
UTEK Corporation
Consolidated Statements of Operations
| | | | | | | | | | | | |
| | | Year ended December 31 | |
| | | 2007 | | | 2006 | | | 2005 | |
Income from operations: | | | | | | | | | | | | |
Sale of technology rights | | $ | 16,372,550 | | | $ | 51,190,576 | | | $ | 18,131,941 | |
Consulting and other services | | | 3,343,134 | | | | 4,950,036 | | | | 4,178,504 | |
Investment income, net | | | 585,265 | | | | 812,325 | | | | 433,378 | |
| | | | | | | | | | | | |
| | | 20,300,949 | | | | 56,952,937 | | | | 22,743,823 | |
| | | |
Expenses: | | | | | | | | | | | | |
Acquisition of technology rights | | | 3,815,844 | | | | 12,945,145 | | | | 4,599,570 | |
Salaries and wages | | | 3,518,769 | | | | 3,430,327 | | | | 2,675,317 | |
Professional fees | | | 1,358,668 | | | | 1,320,108 | | | | 774,434 | |
Sales and marketing | | | 2,035,860 | | | | 3,127,841 | | | | 2,680,603 | |
General and administrative | | | 2,837,909 | | | | 3,865,432 | | | | 2,561,842 | |
Goodwill impairment | | | 210,140 | | | | 234,940 | | | | — | |
| | | | | | | | | | | | |
| | | 13,777,190 | | | | 24,923,793 | | | | 13,291,766 | |
| | | | | | | | | | | | |
Income before income taxes | | | 6,523,759 | | | | 32,029,144 | | | | 9,452,057 | |
Provision for income taxes | | | 2,747,017 | | | | 12,084,937 | | | | 3,564,227 | |
| | | | | | | | | | | | |
Net income from operations | | | 3,776,742 | | | | 19,944,207 | | | | 5,887,830 | |
| | | |
Net realized and unrealized gains (losses): | | | | | | | | | | | | |
Net realized gains (losses) on investments, net of income tax expense (benefit) of $(873,256), $545,163 and $(2,086,923) for 2007, 2006 and 2005, respectively | | | (1,447,380 | ) | | | 903,181 | | | | (3,458,979 | ) |
Net change in unrealized depreciation of investments, net of deferred tax benefit of $(6,519,665), $(15,540,139) and $(201,008) for 2007, 2006 and 2005, respectively | | | (10,806,048 | ) | | | (25,758,186 | ) | | | (333,176 | ) |
| | | | | | | | | | | | |
Net increase (decrease) in net assets from operations | | $ | (8,476,686 | ) | | $ | (4,910,798 | ) | | $ | 2,095,675 | |
| | | | | | | | | | | | |
Net increase (decrease) in net assets from operations per share: | | | | | | | | | | | | |
Basic | | $ | (0.94 | ) | | $ | (0.56 | ) | | $ | 0.29 | |
Diluted | | $ | (0.94 | ) | | $ | (0.56 | ) | | $ | 0.29 | |
Weighted average shares: | | | | | | | | | | | | |
Basic | | | 8,989,234 | | | | 8,786,605 | | | | 7,194,212 | |
Diluted | | | 8,989,234 | | | | 8,786,605 | | | | 7,325,312 | |
Dividend declared or paid per share: | | | — | | | $ | 0.04 | | | | — | |
See accompanying notes
43
UTEK Corporation
Consolidated Statements of Cash Flows
| | | | | | | | | | | | |
| | | Year Ended December 31 | |
| | | 2007 | | | 2006 | | | 2005 | |
Operating Activities: | | | | | | | | | | | | |
Net increase (decrease) in net assets from operations | | $ | (8,476,686 | ) | | $ | (4,910,798 | ) | | $ | 2,095,675 | |
Adjustments to reconcile net increase (decrease) in net assets from operations to net cash flows from operating activities: | | | | | | | | | | | | |
Change in net unrealized depreciation of investments | | | 17,325,713 | | | | 41,298,325 | | | | 532,762 | |
Depreciation and amortization | | | 212,350 | | | | 211,933 | | | | 144,434 | |
Goodwill and intangible asset impairment | | | 210,140 | | | | 301,469 | | | | — | |
(Gain) loss on sale of investments | | | 2,320,637 | | | | (1,448,344 | ) | | | 5,545,902 | |
Loss on disposal of fixed assets | | | 22,403 | | | | 37,263 | | | | — | |
Bad debt expense | | | 364,586 | | | | 108,943 | | | | 179,980 | |
Stock-based compensation | | | 612,893 | | | | 504,589 | | | | — | |
Deferred income taxes | | | (4,645,904 | ) | | | (2,911,021 | ) | | | 1,276,296 | |
Investment securities received in connection with the sale of technology rights | | | (16,172,550 | ) | | | (51,190,576 | ) | | | (18,034,510 | ) |
Consulting and other services rendered in exchange for investment securities | | | (1,021,117 | ) | | | (2,104,287 | ) | | | (1,404,096 | ) |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Accounts receivable | | | 124,231 | | | | 55,859 | | | | (576,419 | ) |
Prepaid expenses and other assets | | | (3,241 | ) | | | 73,801 | | | | (88,130 | ) |
Deferred revenue | | | (222,059 | ) | | | (22,145 | ) | | | 116,052 | |
Accrued expenses | | | 363,920 | | | | 48,969 | | | | (404,832 | ) |
| | | | | | | | | | | | |
Net cash flows from operating activities | | | (8,984,684 | ) | | | (19,946,020 | ) | | | (10,616,886 | ) |
| | | | | | | | | | | | |
Investing Activities: | | | | | | | | | | | | |
Proceeds received on sale of equity investments | | | 1,923,528 | | | | 9,740,302 | | | | 3,097,496 | |
Net proceeds from sale (purchases) of short-term investments | | | 3,086,305 | | | | 7,555,540 | | | | (6,800,319 | ) |
Purchases of fixed assets | | | (50,042 | ) | | | (443,536 | ) | | | (138,402 | ) |
Investment in UTEK Real Estate Holdings, Inc. | | | (783,789 | ) | | | (1,181,472 | ) | | | — | |
Acquisition of 22nd Street of Ybor City, Inc. | | | — | | | | (1,000,000 | ) | | | — | |
Acquisition of Knowledge Express (net cash acquired $26,423) | | | — | | | | — | | | | (1,473,577 | ) |
| | | | | | | | | | | | |
Net cash flows from investing activities | | | 4,176,002 | | | | 14,670,834 | | | | (5,314,802 | ) |
| | | | | | | | | | | | |
Financing Activities: | | | | | | | | | | | | |
Net proceeds from issuance of common stock | | | — | | | | 8,955,182 | | | | 15,794,705 | |
Proceeds from exercise of stock options / warrants | | | 542,681 | | | | 755,518 | | | | 1,663,300 | |
Distributions to stockholders | | | (179,032 | ) | | | (177,865 | ) | | | — | |
Payments on bank debt | | | — | | | | — | | | | (23,516 | ) |
| | | | | | | | | | | | |
Net cash flows from financing activities | | | 363,649 | | | | 9,532,835 | | | | 17,434,489 | |
| | | | | | | | | | | | |
Foreign currency translation adjustment | | | 14,498 | | | | 151,836 | | | | (16,357 | ) |
| | | | | | | | | | | | |
Increase (decrease) in cash and cash equivalents | | | (4,430,535 | ) | | | 4,409,485 | | | | 1,486,444 | |
Cash and cash equivalents at beginning of period | | | 9,685,111 | | | | 5,275,626 | | | | 3,789,182 | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of period | | $ | 5,254,576 | | | $ | 9,685,111 | | | $ | 5,275,626 | |
| | | | | | | | | | | | |
See accompanying notes
44
UTEK Corporation
Consolidated Statements of Cash Flows (continued)
| | | | | | | | | |
| | | Year ended December 31 |
| | | 2007 | | 2006 | | 2005 |
Supplemental Disclosures of Non-Cash Investing Activities | | | | | | | | | |
Investment securities received for unearned technology acquisition alliance services (net) | | $ | 280,463 | | $ | 2,030,874 | | $ | 1,862,775 |
| | | | | | | | | |
Dividend declared not paid | | | | | $ | 179,032 | | | |
| | | | | | | | | |
The Company issued 82,919 shares of common stock to purchase 22nd Street of Ybor City Group, Inc. | | | | | $ | 1,000,000 | | | |
| | | | | | | | | |
The Company purchased all of the capital stock of INTRA-DMS, Ltd. for $300,000 in common stock. In conjunction with the acquisition, liabilities were assumed as follows: | | | | | | | | | |
Fair value of assets acquired | | | | | | | | $ | 502,035 |
Consideration given | | | | | | | | | 300,000 |
| | | | | | | | | |
Liabilities assumed | | | | | | | | $ | 202,035 |
| | | | | | | | | |
The Company issued 119,134 shares of common stock issued to purchase Ybor City Group, Inc. | | | | | | | | $ | 1,650,000 |
| | | | | | | | | |
45
UTEK Corporation
Consolidated Statements of Changes in Net Assets
| | | | | | | | | | | | |
| | | Year ended December 31 | |
| | | 2007 | | | 2006 | | | 2005 | |
Changes in net assets from operations: | | | | | | | | | | | | |
Net income from operations | | $ | 3,776,742 | | | $ | 19,944,207 | | | $ | 5,887,830 | |
Net realized gain (loss) on sale of investments, net of related income taxes | | | (1,447,380 | ) | | | 903,181 | | | | (3,458,979 | ) |
Change in net unrealized depreciation of investments, net of related deferred taxes | | | (10,806,048 | ) | | | (25,758,186 | ) | | | (333,176 | ) |
| | | | | | | | | | | | |
Net (decrease) increase in net assets from operations | | | (8,476,686 | ) | | | (4,910,798 | ) | | | 2,095,675 | |
| | | | | | | | | | | | |
Distributions to Stockholders (Paid or Declared): | | | | | | | | | | | | |
From net income from operations(1) | | | — | | | | (356,897 | ) | | | — | |
| | | | | | | | | | | | |
Capital stock transactions: | | | | | | | | | | | | |
Proceeds from issuance of common stock net of offering costs of $-, $1,044,860 and $2,036,042 for the years ended December 31, 2007, 2006 and 2005, respectively | | | — | | | | 8,955,182 | | | | 15,794,705 | |
Proceeds from the exercise of stock options | | | 542,681 | | | | 755,518 | | | | 1,663,300 | |
Issuance of stock options for compensation | | | 612,893 | | | | 504,587 | | | | — | |
Deferred tax related to stock-based compensation expense | | | — | | | | 440,616 | | | | — | |
Common stock issued in acquisition of 22nd Street of Ybor City, Inc. | | | — | | | | 1,000,000 | | | | — | |
Common stock issued in acquisition of Ybor City Group, Inc. | | | — | | | | — | | | | 1,650,000 | |
Common stock issued in acquisition of UTEKip, Ltd. | | | — | | | | — | | | | 300,000 | |
| | | | | | | | | | | | |
Net increase in net assets from stock transactions | | | 1,155,574 | | | | 11,655,903 | | | | 19,408,005 | |
| | | | | | | | | | | | |
Foreign currency translation adjustment | | | 14,498 | | | | 151,836 | | | | (155,505 | ) |
| | | | | | | | | | | | |
Net (decreased) increase in net assets | | | (7,306,614 | ) | | | 6,540,044 | | | | 21,348,175 | |
Net assets at beginning of year | | | 50,981,162 | | | | 44,441,118 | | | | 23,092,943 | |
| | | | | | | | | | | | |
Net assets at end of year | | $ | 43,674,548 | | | $ | 50,981,162 | | | $ | 44,441,118 | |
| | | | | | | | | | | | |
| (1) | Distributions to shareholders as noted in the Consolidated Statement of Cash Flows for the year ended December 31, 2007 was accrued at December 31, 2006; therefore, it is not reflected as a distribution to shareholders for purposes of this schedule. |
See accompanying notes
46
UTEK CORPORATION
Consolidated Schedule of Investments December 31, 2007
| | | | | | | | | | | | | |
Shares | | Dates of
Acquisition | | Non-Affiliate Investments(1) | | Original
Cost Basis | | Value | | Percentage
of Net
Assets | |
| | | | Advanced Medical Isotope Corporation(12)
Development of isotopes to treat diseases | | | | | | | | | |
| 95,000 | | 9/06 | | Preferred Stock | | $ | 1,803,417 | | $ | 1,750,000 | | 4.0 | % |
| 600,000 | | 5/06 | | Common Stock | | | 63,000 | | | 324,000 | | 0.7 | |
| 478,562 | | 1/06 | | Broadcast International, Inc. Telecommunications | | | 579,060 | | | 1,682,100 | | 3.9 | |
| | | | Advanced Refractive Technologies, Inc.Ophthalmic technologies | | | | | | | | | |
| 100,000 | | 4/06 | | Series D Preferred Stock | | | 1,996,176 | | | 560,000 | | 1.3 | |
| 97,000 | | 3/06 | | Series C Preferred Stock | | | 2,066,063 | | | 543,200 | | 1.2 | |
| 97,000 | | 12/05 | | Series B Preferred Stock | | | 1,032,675 | | | 282,300 | | 0.6 | |
| 4,000,000 | | 5/06 | | Common Stock | | | 76,368 | | | 400 | | <0.1 | |
| 560,000 | | 1/07 | | Mimedx, Inc. (privately held) Connective tissue technology | | | — | | | 840,000 | | 1.9 | |
| (7) | | 4/07 | | Synthetic Blood International, Inc. Biotechnology products | | | 120,000 | | | 233,700 | | 0.5 | |
| 40,000 | | 7/06 | | Bacterin International, Inc.(privately held) Bioactive coatings for medical devices | | | 120,000 | | | 120,000 | | 0.3 | |
| 60,000 | | 12/05 | | Metamorphix Global, Inc.(privately held)(13) Design and manufacture of countertops | | | 120,000 | | | 90,000 | | 0.2 | |
| 171,432 | | 10/06-9/07 | | GammaCan International, Inc. Anti-cancer immunotherapy | | | 83,016 | | | 65,100 | | 0.1 | |
| 264,333 | | 1/07 | | Ecosphere Technologies, Inc. Defense, homeland security and global ship repair | | | 97,300 | | | 47,600 | | 0.1 | |
| 109,091 | | 7/06 | | Turbine Truck Engines, Inc. Heavy-duty highway truck engines | | | 72,000 | | | 41,500 | | 0.1 | |
| 269,230 | | 8/06 | | Protocall Technologies, Inc. On-demand software and entertainment | | | 11,577 | | | 31,500 | | 0.1 | |
| 697,860 | | 8/06-11/06 | | Magnitude Information Systems, Inc. Computer ergonomics | | | 25,920 | | | 27,900 | | 0.1 | |
| 2,560,000 | | 8/04-11/06 | | TenthGate, Inc.(8) Healthcare related products and services | | | 40,000 | | | 24,200 | | 0.1 | |
| 180,000 | | 5/06 | | U.S. Starcom, Inc. Communications services and products | | | 17,181 | | | 12,000 | | <0.1 | |
| 9,748 | | 4/06-3/07 | | Xethanol Corporation(13) Bioethanol and derivative products | | | 88,836 | | | 5,850 | | <0.1 | |
| 825,852 | | 5/06-7/06 | | MM2 Group, Inc. Financial consulting for nutraceuticals | | | 32,685 | | | 5,800 | | <0.1 | |
| 1,200,000 | | 10/06 | | Laserlock Technologies, Inc. Security solutions for the gaming industry | | | 24,100 | | | 5,100 | | <0.1 | |
| 808,529 | | 12/04 | | SolarBrook Water and Power Corp. (HydroFlo, Inc.) Treatment and purification of water | | | 125,861 | | | 3,900 | | <0.1 | |
47
| | | | | | | | | | | | | |
Shares | | Dates of
Acquisition | | Non-Affiliate Investments(1) | | Original
Cost Basis | | Value | | Percentage
of Net
Assets | |
| 660,000 | | 6/05 | | BP International, Inc. Shade structures | | | 78,852 | | | 3,400 | | <0.1 | |
| 1,250,010 | | 5/06 | | In Veritas Medical Diagnostics, Inc. Medical devices designs and testing | | | 74,400 | | | 2,700 | | <0.1 | |
| 2,011,765 | | 5/06-6/06 | | KKS Venture Management, Inc (Rheologics, Inc.) Study of blood viscosity | | | 86,100 | | | 1,000 | | <0.1 | |
66,667 | | 9/05-4/06 | | Quest Minerals & Mining Corporation Coal and mineral mining | | | 69,044 | | | 800 | | <0.1 | |
| 85,714 | | 9/05 | | New Life Scientific, Inc. Pharmaceutical biotechnologies | | | 81,816 | | | 600 | | <0.1 | |
| 384,000 | | 7/06 | | aeroTelesis, Inc. Satellite and wireless bandwidth utilization | | | 33,394 | | | 600 | | <0.1 | |
| 387,097 | | 6/06 | | Tradequest International, Inc. Provider of voice over internet protocol | | | 76,092 | | | 400 | | <0.1 | |
| 122,449 | | 1/06 | | 5G Wireless Communications, Inc. Broadband wireless | | | 78,851 | | | 200 | | <0.1 | |
| 232,211 | | 5/05 | | Preservation Sciences, Inc. Green technologies and development | | | — | | | — | | 0.0 | |
| 140,000 | | 3/05 | | AdAl Group, Inc.(5) Aluminum extruded products manufacturer | | | 72,912 | | | — | | 0.0 | |
| 37,500 | | 6/05 | | Modern Technology Corporation Technology development and acquisition company | | | 82,152 | | | — | | 0.0 | |
| 6 | | 5/06-6/06 | | EFT BioTech Holdings, Inc. (HumWare Media Corp.) Media advertising | | | 14,236 | | | — | | 0.0 | |
| | | | UBA Technology, Inc. Software development | | | | | | | | | |
| 2,482,521 | | 2/06-6/06 | | Common Stock | | | 1,691,419 | | | — | | 0.0 | |
| 95,000 | | 4/06 | | Series A Convertible Preferred Stock | | | 1,619,849 | | | — | | 0.0 | |
| 7,787,565 | | 6/05-6/06 | | Trio Industries Group, Inc. Protective powder coating | | | 12,330,401 | | | — | | 0.0 | |
| | | | KP Renewables Plc (Kwikpower International Plc)(5)
Renewable energy | | | | | | | | | |
| | 5/06 | | Convertible Debenture, due 5/10/07 | | | 4,433,401 | | | — | | 0.0 | |
| | 9/05 | | Convertible Debenture, due 9/30/06 | | | 1,884,920 | | | — | | 0.0 | |
| 2,500 | | 3/05 | | Common Stock | | | 94,500 | | | — | | 0.0 | |
| 261,234 | | 7/04-7/05 | | eLinear, Inc.(5) Telecommunication security provider | | | 190,763 | | | — | | 0.0 | |
| | | | | | | | | | | | | |
| | | | Total Investments in Non-Affiliates | | $ | 31,588,337 | | $ | 6,705,850 | | 15.4 | % |
| | | | | | | | | | | | | |
48
| | | | | | | | | | | | | |
Shares | | Dates of
Acquisition | | Affiliate Investments(2) | | Original
Cost Basis | | Value | | Percentage
of Net
Assets | |
| | | | Material Technologies, Inc.(9) Metal fatigue detection | | | | | | | | | |
| 20,550,304 | | 9/04-6/07 | | Common Stock | | $ | 4,907,329 | | $ | 4,521,100 | | 10.4 | % |
| 47,500 | | 1/07 | | Series E Convertible Preferred Stock | | | 694,640 | | | 648,400 | | 1.5 | |
| | | | Cyberlux Corporation(9)
LED lighting solutions | | | | | | | | | |
| 148,000 | | 11/06-1/07 | | Series C Preferred Stock | | | 2,181,640 | | | 1,781,000 | | 4.1 | |
| 27,981,484 | | 6/06-1/07 | | Common Stock | | | 537,920 | | | 559,600 | | 1.3 | |
| | | | Emission & Power Solutions, Inc. (Fuel FX International, Inc.) (privately held) Reductional environmental emissions | | | | | | | | | |
| 100,000 | | 1/06 | | Series B Preferred Stock | | | 2,100,000 | | | 840,000 | | 1.9 | |
| 9,900,717 | | 4/05-4/06 | | Common Stock | | | 1,980,142 | | | 752,500 | | 1.7 | |
| 3,620,307 | | 5/06-9/07 | | Avalon Oil and Gas, Inc. Oil and gas producers | | | 2,854,089 | | | 868,900 | | 2.0 | |
| | | | Manakoa Services Corporation(12) Compliance analysis and monitoring | | | | | | | | | |
| 95,000 | | 1/07 | | Series B Preferred Stock | | | 2,280,000 | | | 760,000 | | 1.7 | |
| 7,799,515 | | 8/04-4/07 | | Common Stock | | | 2,122,641 | | | 58,500 | | 0.1 | |
6,000,000 | | 7/07 | | Pathway One Plc(5)(9) Sales and development licenses | | | 852,300 | | | 719,500 | | 1.6 | |
49,500,000 | | 7/07 | | MachineTalker, Inc.(9) Intelligent wireless security networks | | | 993,000 | | | 668,300 | | 1.5 | |
1,426,754 | | 9/07 | | USTelematics, Inc.(14) Broadband telecommunication for moving vehicles | | | — | | | 510,100 | | 1.2 | |
412,000 | | 9/07 | | NeoStem, Inc.(9) Stem cell banking services | | | 761,440 | | | 441,700 | | 1.0 | |
2,040,000 | | 4/06-7/06 | | CytoDyn, Inc. Development stage biotechnology company Common Stock | | | 3,640,772 | | | 118,300 | | 0.3 | |
100,000 | | 1/07 | | Series A Preferred Stock | | | 845,000 | | | 260,000 | | 0.6 | |
6,909,390 | | 9/05-6/07 | | American Soil Technologies, Inc.(9) Fertilizer innovation | | | 1,622,606 | | | 314,400 | | 0.7 | |
4,426,136 | | 7/06 | | vidShadow.com, Inc. (DME Interactive Holdings, Inc.) Multi-media entertainment | | | 752,443 | | | 199,200 | | 0.5 | |
164,495,817 | | 8/06-4/07 | | Cargo Connection Logistics Holdings, Inc. World trade logistics | | | 1,026,031 | | | 172,700 | | 0.4 | |
7,145,000 | | 5/06-8/06 | | NetFabric Holdings, Inc. Information technology services | | | 607,034 | | | 142,900 | | 0.3 | |
461,222,608 | | 5/05-9/05 | | GS Energy Corporation (INSEQ Corp.) Waste minimization | | | 2,434,783 | | | 89,900 | | 0.2 | |
5,462 | | 8/05-8/06 | | Industrial Biotechnology Corporation(10) Manufactures and markets flavors and fragrances | | | 6,351,998 | | | 2,000 | | <0.1 | |
49
| | | | | | | | | | | | | |
Shares | | Dates of
Acquisition | | Affiliate Investments(2) | | Original
Cost Basis | | Value | | Percentage
of Net
Assets | |
33,730,000 | | 4/05-1/06 | | WebSky, Inc. Broadband wireless | | | 897,750 | | | — | | 0.0 | |
4,221,165 | | 4/01-12/02 | | Stealth MediaLabs, Inc.(14) Software products | | | 1,708,000 | | | — | | 0.0 | |
5,346 | | 7/06-9/06 | | Liberty Diversified Holdings, Inc. Printing and packaging
Common Stock | | | 1,245,258 | | | — | | 0.0 | |
63,981 | | 2/07 | | Series B Preferred Stock | | | 382,800 | | | — | | 0.0 | |
| | | | | | | | | | | | | |
| | | | Total Investments in Affiliates | | $ | 43,779,616 | | $ | 14,429,000 | | 33.0 | % |
| | | | | | | | | | | | | |
| | | | | |
| | | | | Control Investments(3) | | | | | | | |
1,000 | | 11/99-11/06 | | UTEK Real Estate Holdings, Inc. (privately held) | | | | | | | | | |
| | | | Real estate development | | $ | 4,131,574 | | $ | 3,472,000 | | 7.9 | % |
16,119,672 | | 9/05-9/07 | | World Energy Solutions, Inc.(11) Energy saving technologies
Common Stock | | | 4,628,449 | | | 2,031,100 | | 4.7 | |
(6) | | 1/06-9/07 | | UTEK Real Estate Holdings, Inc. (privately held) (Demand note, interest rate @ 5%) | | | 1,965,261 | | | 1,965,261 | | 4.5 | |
15,009,402 | | 3/06-5/07 | | Klegg Electronics, Inc. Manufacturer/distributor for retail electronic products | | | 6,506,174 | | | 300,200 | | 0.7 | |
| | | | | | | | | | | | | |
| | | | Total Investments in Control Investments | | $ | 17,231,458 | | $ | 7,768,561 | | 17.8 | % |
| | | | | | | | | | | | | |
| | | | | |
| | | | | U.S. Treasuries and Certificates of Deposit(4) | | | | | | | |
| | | | U.S. Treasuries: | | | | | | | | | |
500,000 | | 11/07 | | United States Treasury Bill, maturity 2/07/08, interest rate @ 3.06% | | $ | 498,550 | | $ | 498,550 | | 1.1 | % |
| | | | | | | | | | | | | |
| | | | Total U.S. Treasuries | | $ | 498,550 | | $ | 498,550 | | 1.1 | % |
| | | | | | | | | | | | | |
| | | | Certificates of Deposit: | | | | | | | | | |
100,000 | | 8/07 | | State Bank India CD, maturity 2/22/08, interest rate @ 5.15% | | $ | 99,983 | | $ | 99,983 | | 0.2 | % |
100,000 | | 8/07 | | Indymac Bank FSB CD, maturity 2/25/08, interest rate @ 5.2% | | | 100,004 | | | 100,004 | | 0.2 | |
100,000 | | 8/07 | | First Natl Bank Arizona CD, maturity 2/27/08, interest rate @ 5.15% | | | 99,982 | | | 99,982 | | 0.2 | |
100,000 | | 8/07 | | Charter Bank West CD, maturity 2/29/08, interest rate @ 5.1% | | | 99,975 | | | 99,975 | | 0.2 | |
100,000 | | 8/07 | | Discover Bank CD, maturity 2/29/08, interest rate @ 5.15% | | | 99,982 | | | 99,982 | | 0.2 | |
100,000 | | 8/07 | | Lehman Coml Bank CD, maturity 2/29/08, interest rate @ 5.15% | | | 99,982 | | | 99,982 | | 0.2 | |
100,000 | | 8/07 | | Sterling Savings Bank CD, maturity 3/24/08, interest rate @ 5.1% | | | 99,966 | | | 99,966 | | 0.2 | |
100,000 | | 8/07 | | Capmark Bank CD, maturity 5/22/08, interest rate @ 5.15% | | | 99,986 | | | 99,986 | | 0.2 | |
100,000 | | 8/07 | | Firstcity Bank CD, maturity 5/22/08, interest rate @ 5.1% | | | 99,967 | | | 99,967 | | 0.2 | |
50
| | | | | | | | | | | | | |
Shares | | Dates of
Acquisition | | U.S. Treasuries and Certificates of Deposit(4) | | Original
Cost Basis | | Value | | Percentage
of Net
Assets | |
100,000 | | 8/07 | | Provident Bank CD, maturity 5/27/08, interest rate @ 5.1% | | | 99,969 | | | 99,969 | | 0.2 | |
| | | | | | | | | | | | | |
| | | | Total Certificates of Deposit | | $ | 999,796 | | $ | 999,796 | | 2.3 | % |
| | | | | | | | | | | | | |
| | | | Total Investments in U.S. Treasuries and CDs | | $ | 1,498,346 | | $ | 1,498,346 | | 3.4 | % |
| | | | | | | | | | | | | |
| | | | TOTAL INVESTMENTS | | $ | 94,097,757 | | $ | 30,401,757 | | 69.6 | % |
| | | | | | | | | | | | | |
| | | | Cash and other assets, less liabilities | | | | | | 13,272,791 | | 30.4 | % |
| | | | | | | | | | | | | |
| | | | Net assets at December 31, 2007 | | | | | $ | 43,674,548 | | 100.0 | % |
| | | | | | | | | | | | | |
Notes to Schedule of Investments:
| | • | | Except where otherwise noted, all of our investments listed above are in common stock of companies that are publicly quoted on the OTC Bulletin Board or listed on the American Stock Exchange or other similar markets. |
| | • | | The above investments, with the exception of the U.S. Treasuries and certificates of deposits and a demand note issued by UTEK Real Estate Holdings, Inc., are non-income producing. Equity investments that have not paid dividends within the last twelve months are considered non-income producing. |
| | • | | The value of all securities for which there is no readily available market value is determined in good faith by the Board of Directors. In making its determination, the Board of Directors has considered valuation appraisals provided by an independent valuation service provider. (See Note 2 to the Notes to the Consolidated Financial Statements.) |
| | • | | As of December 31, 2007, all of the securities that we own are subject to legal restrictions on resale. As a result, our ability to sell or otherwise transfer the securities we hold in our portfolio is limited. |
| (1) | Non-affiliate investments are generally defined under the Investment Company Act of 1940 as companies in which the Company owns less than 5% of the voting securities. |
| (2) | Affiliate investments are generally defined under the Investment Company Act of 1940 as companies in which the Company owns at least 5% but not more than 25% of the voting securities. |
(3) | Control investments are generally defined under the Investment Company Act of 1940 as companies in which the Company owns more than 25% of the voting securities. We own 100% of UTEK Real Estate Holdings, Inc. (“UREHI”), which holds four investments: Rosbon LLC, ABM of Tampa Bay, Inc., 22nd Street of Ybor City, Inc. and Ybor City Group, Inc. UREHI holds 150 equity interests of the total equity interests outstanding of Rosbon LLC and all of the outstanding shares of capital stock of ABM of Tampa Bay, Inc., 22nd Street of Ybor City, Inc. and Ybor City Group, Inc. |
| (4) | The Company invests excess cash in a number of U.S. Treasury Bills and certificates of deposit. These short-term investments normally have three month to one year maturities and do not qualify as cash or cash equivalents. |
| (5) | Non-U.S. company or the company’s principal place of business is outside the U.S. |
| (6) | Investment consists of a loan receivable with subsidiaries of UTEK Real Estate Holdings, Inc. |
| (7) | Investment consists of warrants to purchase 1,500,000 shares of Synthetic Blood International, Inc. common stock. |
| (8) | During the period ended December 31, 2007, the Company reclassified this investment from Affiliate investments to Non-affiliate investments based on the criteria in notes (1) and (2). |
51
| (9) | During the period ended December 31, 2007, the Company reclassified this investment from Non-affiliate investments to Affiliate investments based on the criteria in notes (1) and (2). |
| (10) | During the period ended December 31, 2007, the Company reclassified this investment from Control investments to Affiliate investments based on the criteria in notes (2) and (3). |
| (11) | During the period ended December 31, 2007, the Company reclassified this investment from Affiliate investments to Control investments based on the criteria in notes (2) and (3). |
| (12) | Advanced Medical Isotope Corporation and Manakoa Services Company are related through common management. |
| (13) | Xethanol Corporation and Metamorphix Global are related though common management. |
| (14) | Stealth MediaLabs, Inc. and USTelematics, Inc. are related through common management. |
See accompanying notes
52
UTEK CORPORATION
Consolidated Schedule of Investments
December 31, 2006
| | | | | | | | | | | | | |
Shares | | Dates of
Acquisition | | Non-Affiliate Investments(1) | | Original
Cost Basis | | Value | | Percentage
of Net
Assets | |
| | | | Advanced Refractive Technologies, Inc. Opthalmic technologies | | | | | | | | | |
100,000 | | | | Preferred D Stock | | $ | 1,996,176 | | $ | 1,400,000 | | 2.7 | % |
97,000 | | | | Preferred C Stock | | | 2,066,063 | | | 1,358,000 | | 2.7 | |
97,000 | | | | Preferred B Stock | | | 1,032,675 | | | 705,700 | | 1.4 | |
5,533,333 | | | | Common Stock | | | 158,364 | | | 26,200 | | 0.1 | |
| | | | World Energy Solutions, Inc. Energy saving technologies | | | | | | | | | |
100,000 | | | | Preferred A Stock | | | 2,632,500 | | | 2,430,000 | | 4.8 | |
201,672 | | | | Common Stock | | | 127,714 | | | 36,300 | | 0.1 | |
| | | | Advanced Medical Isotope Corporation(11) Development of isotopes to treat diseases | | | | | | | | | |
95,000 | | | | Preferred Stock | | | 1,803,417 | | | 1,591,300 | | 3.1 | |
600,000 | | | | Common Stock | | | 63,000 | | | 96,000 | | 0.2 | |
| | | | Cyberlux Corporation LED lighting solutions | | | | | | | | | |
98,000 | | | | Preferred C Stock | | | 1,605,240 | | | 1,605,200 | | 3.1 | |
1,481,484 | | | | Common Stock | | | 80,820 | | | 40,000 | | 0.1 | |
| | | | American Soil Technologies, Inc. Fertilizer innovation | | | | | | | | | |
4,275,000 | | | | Preferred B Stock | | | 1,571,691 | | | 1,432,100 | | 2.8 | |
317,857 | | | | Common Stock | | | 70,388 | | | 56,700 | | 0.1 | |
6,368,802 | | | | Material Technologies, Inc. Metal fatigue detection | | | 1,839,969 | | | 891,600 | | 1.7 | |
984,360 | | | | Broadcast International, Inc. Telecommunications | | | 1,223,592 | | | 863,800 | | 1.7 | |
| | | | UBA Technology, Inc(8) Software development | | | | | | | | | |
2,482,521 | | | | Common Stock | | | 1,691,419 | | | 600 | | <.1 | |
95,000 | | | | Convertible Preferred A | | | 1,619,849 | | | 714,600 | | 1.4 | |
146,586 | | | | Xethanol Corporation(8)(12) Bioethanol and derivative products | | | 964,599 | | | 299,500 | | 0.6 | |
171,432 | | | | Shumate Industries, Inc. Energy field service applications | | | 78,852 | | | 205,200 | | 0.4 | |
60,000 | | | | Metamorphix Global (privately held)(12) Design and manufacture of countertops | | | 120,000 | | | 120,000 | | 0.2 | |
40,000 | | | | Bacterin International (privately held) Bioactive coatings for medical devices | | | 120,000 | | | 120,000 | | 0.2 | |
120,000 | | | | Rival Technologies, Inc. Diesel engine technologies | | | 82,104 | | | 96,900 | | 0.2 | |
1,250,010 | | | | In Veritas Medical Diagnostics, Inc. Medical devices designs and testing | | | 74,400 | | | 63,800 | | 0.1 | |
171,432 | | | | GammaCan International, inc. Anti-cancer immunotherapy | | | 83,016 | | | 51,400 | | 0.1 | |
53
| | | | | | | | | | |
Shares | | Dates of
Acquisition | | Non-Affiliate Investments(1) | | Original
Cost Basis | | Value | | Percentage
of Net
Assets |
109,091 | | | | Turbine Truck Engines, Inc. Heavy-duty highway truck engines | | 72,000 | | 39,300 | | 0.1 |
176,250 | | | | Maelor Plc(5) Products for niche healthcare applications | | 24,147 | | 34,200 | | 0.1 |
1,411,765 | | | | MM2 Group, Inc. Financial consulting for nutraceuticals | | 56,004 | | 33,900 | | 0.1 |
2,011,765 | | | | Rheologics, Inc. (8) Study of blood viscosity | | 86,100 | | 28,200 | | 0.1 |
1,635,000 | | | | Swiss Medica, Inc. Health bioscience products | | 304,988 | | 28,000 | | 0.1 |
1,200,000 | | | | Laserlock Technologies, Inc. Security solutions for the gaming industry | | 24,100 | | 20,100 | | <.1 |
269,230 | | | | Protocall Technologies, Inc. On-demand software and entertainment | | 11,577 | | 19,500 | | <.1 |
33,825 | | | | XLER8, Inc. (Vitacube Systems, Inc.) Specialty nutraceuticals | | 16,427 | | 18,900 | | <.1 |
387,097 | | | | Tradequest International, Inc. Provider of voice over internet protocol | | 76,092 | | 18,600 | | <.1 |
697,860 | | | | Magnitude Information Systems, Inc. Computer ergonomics | | 25,920 | | 17,800 | | <.1 |
232,211 | | | | Preservation Sciences, Inc. Green technologies and development | | — | | 17,800 | | <.1 |
122,449 | | | | 5G Wireless Communications, Inc. Broadband wireless | | 78,851 | | 14,300 | | <.1 |
221,033 | | | | Power3 Medical Products, Inc. Healthcare products | | 201,599 | | 13,900 | | <.1 |
180,000 | | | | U.S. Starcom, Inc. Communications services and products | | 17,181 | | 13,800 | | <.1 |
750,000 | | | | U.S. Wireless Online, Inc. Wireless broadband networks | | 66,000 | | 9,100 | | <.1 |
258,064 | | | | IPORUSSIA, Inc. Business advisory services provider | | 24,000 | | 8,800 | | <.1 |
115,069 | | | | Inverted Paradigms Corporation Safety and security software | | 32,510 | | 6,400 | | <.1 |
105,600 | | | | HumWare Media Corporation Media advertising | | 14,236 | | 5,900 | | <.1 |
384,000 | | | | aeroTelesis, Inc. Satellite and wireless bandwidth utilization | | 33,394 | | 4,300 | | <.1 |
774,951 | | | | Universal Detection Technology Detection devices for bacterial spores | | 30,378 | | 3,600 | | <.1 |
660,000 | | | | BP International, Inc. Shade structures | | 78,852 | | 3,300 | | <.1 |
2,335,977 | | | | PracticeXpert, Inc. Operational efficiencies for medical practitioners | | 27,172 | | 3,300 | | <.1 |
808,529 | | | | HydroFlo, Inc.(6) Business development company | | 125,861 | | 3,000 | | <.1 |
85,714 | | | | New Life Scientific, Inc. Pharmaceutical biotechnologies | | 81,816 | | 2,900 | | <.1 |
54
| | | | | | | | | | | | | |
Shares | | Dates of
Acquisition | | Non-Affiliate Investments(1) | | Original Cost
Basis | | Value | | Percentage
of Net
Assets | |
666,668 | | | | Quest Minerals & Mining Corporation Coal and mineral mining | | | 69,044 | | | 1,800 | | <.1 | |
50,925 | | | | Global General Technologies, Inc. Homeland security systems | | | 13,228 | | | 1,700 | | <.1 | |
37,500 | | | | Modern Technology Corporation Technology development and acquisition company | | | 82,152 | | | 1,400 | | <.1 | |
140,000 | | | | AdAl Group, Inc.(5) Aluminum extruded products manufacturer | | | 72,912 | | | — | | 0.0 | |
7,787,565 | | | | Trio Industries Group, Inc.(8) Protective powder coating | | | 12,330,401 | | | — | | 0.0 | |
| | | | KP Renewables Plc (Kwikpower International Plc)(5) Renewable energy | | | | | | | | | |
| | | | Convertible Debenture, due 5/10/07 | | | 4,433,401 | | | — | | 0.0 | |
| | | | Convertible Debenture, due 9/30/06 | | | 1,884,920 | | | — | | 0.0 | |
50,000 | | | | Common Stock | | | 94,500 | | | — | | 0.0 | |
261,234 | | | | eLinear, Inc.(5) Telecommunication security provider | | | 190,763 | | | — | | 0.0 | |
| | | | | | | | | | | | | |
| | | | Total Investments in Non-Affiliates | | $ | 41,786,374 | | $ | 14,578,700 | | 28.6 | % |
| | | | | | | | | | | | | |
| | | | | |
| | | | | Affiliate Investments(2) | | | | | | | |
9,900,717 | | | | Fuel FX International, Inc. Reductional environmental emissions Common stock | | $ | 1,980,142 | | $ | 1,831,600 | | 3.6 | % |
100,000 | | | | Preferred Series B Stock | | | 2,100,000 | | | 990,000 | | 1.9 | |
4,444,876 | | | | DME Interactive Holdings, Inc.(9) Multi-media entertainment | | | 769,820 | | | 1,344,600 | | 2.6 | |
35,131,142 | | | | Avalon Oil and Gas, Inc. Oil and gas producers | | | 1,986,939 | | | 864,200 | | 1.7 | |
2,040,000 | | | | Cytodyn, Inc.(9) Development stage biotechnology company | | | 3,640,772 | | | 856,800 | | 1.7 | |
33,730,000 | | | | WebSky Inc. Broadband wireless | | | 897,750 | | | 674,600 | | 1.3 | |
164,951,070 | | | | Cargo Connection Logistics Holdings, Inc. World trade logistics | | | 1,033,434 | | | 643,300 | | 1.3 | |
8,512,064 | | | | Manakoa Services Company(11) Compliance analysis and monitoring | | | 2,306,893 | | | 415,000 | | 0.8 | |
7,165,000 | | | | NetFabric Holdings, Inc.(9) Information technology services | | | 608,694 | | | 394,000 | | 0.8 | |
461,222,608 | | | | GS Energy Corporation (INSEQ Corp.) Waste minimization | | | 2,434,783 | | | 269,800 | | 0.5 | |
16,037,500 | | | | Liberty Diversified Holdings, Inc. Printing and packaging | | | 1,245,258 | | | 187,600 | | 0.4 | |
4,221,165 | | | | Stealth MediaLabs, Inc. Software products | | | 1,708,000 | | | 121,600 | | 0.2 | |
55
| | | | | | | | | | | | | |
Shares | | Dates of
Acquisition | | Affiliate Investments(2) | | Original Cost
Basis | | Value | | Percentage
of Net
Assets | |
3,023,703 | | | | Health Sciences Group, Inc. Nutraceutical and pharmaceutical products | | | 1,601,963 | | | 95,200 | | 0.2 | |
2,560,000 | | | | TenthGate, Inc.(9) Healthcare related products and services | | | 40,000 | | | 25,600 | | <.1 | |
| | | | | | | | | | | | | |
| | | | Total Investments in Affiliates | | $ | 22,354,448 | | $ | 8,713,900 | | 17.1 | % |
| | | | | | | | | | | | | |
| | | | | |
| | | | | Control Investments(3) | | | | | | | |
1,000 | | | | UTEK Real Estate Holdings, Inc., (privately held) Real estate development | | $ | 4,131,574 | | $ | 3,614,500 | | 7.1 | % |
52,192,755 | | | | Klegg Electronics, Inc.(10) Manufacturer/distributor for retail electronic products | | | 3,820,274 | | | 3,209,900 | | 6.3 | |
21,933,451 | | | | Industrial Biotechnology Corporation(10) Manufactures and markets flavors and fragrances | | | 6,351,998 | | | 1,995,900 | | 3.9 | |
(10) | | | | Ybor City Group, Inc. (privately held) (Demand note, interest rate @ 5%) | | | 1,181,472 | | | 1,181,472 | | 2.3 | |
| | | | | | | | | | | | | |
| | | | Total Investments in Control Investments | | $ | 15,485,318 | | $ | 10,001,772 | | 19.6 | % |
| | | | | | | | | | | | | |
| | | | | |
| | | | | U.S. Treasuries and Certificates of Deposit(4) | | | | | | | |
Par Value | | | | U.S. Treasuries: | | | | | | | | | |
2,000,000 | | 8/06 | | United States Treasury, maturity 1/18/07, interest rate @ 5.045% | | $ | 1,996,481 | | $ | 1,996,481 | | 3.9 | % |
1,500,000 | | 8/06 | | United States Treasury, maturity 2/01/07, interest rate @ 4.952% | | | 1,494,092 | | | 1,494,092 | | 2.9 | |
1,000,000 | | 8/06 | | United States Treasury, maturity 2/15/07, interest rate @ 4.751% | | | 994,078 | | | 994,078 | | 1.9 | |
| | | | | | | | | | | | | |
| | | | Total U.S. Treasuries | | $ | 4,484,651 | | $ | 4,484,651 | | 8.8 | % |
| | | | | | | | | | | | | |
| | | | Certificates of Deposit: | | | | | | | | | |
100,000 | | 8/06 | | Washington Mutual Bank CD, maturity 1/30/07, interest rate @ 5.00% | | $ | 100,000 | | $ | 100,000 | | 0.2 | % |
| | | | | | | | | | | | | |
| | | | Total Certificates of Deposit | | $ | 100,000 | | $ | 100,000 | | 0.2 | % |
| | | | | | | | | | | | | |
| | | | Total Investments in U.S. Treasuries and CDs | | $ | 4,584,651 | | $ | 4,584,651 | | 9.0 | % |
| | | | | | | | | | | | | |
| | | | TOTAL INVESTMENTS | | $ | 84,210,791 | | $ | 37,879,023 | | 74.3 | % |
| | | | | | | | | | | | | |
| | | | Cash and other assets, less liabilities | | | | | | 13,102,139 | | 25.7 | % |
| | | | | | | | | | | | | |
| | | | Net assets at December 31, 2006 | | | | | $ | 50,981,162 | | 100.0 | % |
| | | | | | | | | | | | | |
56
Notes to Schedule of Investments:
| | • | | Except where otherwise noted, all of our investments listed above are in common stock of companies that are publicly quoted on the OTC Bulletin Board or listed on the American Stock Exchange or other similar markets. |
| | • | | The above investments, with the exception of the U.S. Treasuries and certificates of deposits and the demand not issued by Ybor City Group, Inc., are non-income producing. Equity investments that have not paid dividends within the last twelve months are considered non-income producing. |
| | • | | The value of all securities for which there is no readily available market value is determined in good faith by the Board of Directors. In making its determination, the Board of Directors has considered valuation appraisals provided by an independent valuation service provider. (See Note 2 to the Notes to Consolidated Financial Statements.) |
| | • | | As of December 31, 2006, all of the securities that we own are subject to legal restrictions on resale. As a result, our ability to sell or otherwise transfer the securities we hold in our portfolio is limited. |
| (1) | Non-affiliate investments are generally defined under the Investment Company Act of 1940 as companies in which the Company owns less than 5% of the voting securities. |
| (2) | Affiliate investments are generally defined under the Investment Company Act of 1940 as companies in which the Company owns at least 5% but not more than 25% of the voting securities. |
(3) | Control investments are generally defined under the Investment Company Act of 1940 as companies in which the Company owns more than 25% of the voting securities. We own 100% of UTEK Real Estate Holdings, Inc. (“UREHI”), which holds four investments: Rosbon LLC, ABM of Tampa Bay, Inc., 22nd Street of Ybor City, Inc. and Ybor City Group, Inc. UREHI holds 150 equity interests of the total equity interests outstanding of Rosbon LLC and all of the outstanding shares of capital stock of ABM of Tampa Bay, Inc., 22nd Street of Ybor City, Inc. and Ybor City Group, Inc. |
| (4) | The Company invests excess cash in a number of U.S. Treasury Bills and certificates of deposit. These short-term investments normally have three month to one year maturities and do not qualify as cash or cash equivalents. |
| (15) | Non-U.S. company or the company’s principal place of business is outside the U.S. |
| (16) | Closed-end management investment company that has elected to be regulated as a business development company under the Investment Act of 1940. During the nine months ended September 30, 2006, the Company made a gift of 5.1 million shares of HydroFlo, Inc. common stock to certain not-for-profit institutions and abandoned its right, title and interest in and to 400,000 shares of HydroFlo, Inc.’s common stock (See note 2 to the consolidated financial statements). |
| (17) | Investment consists of a loan receivable. |
| (18) | During the period ended December 31, 2006, the Company reclassified this investment from Affiliate investments to Non-affiliate investments based on the criteria in notes (1) and (2). |
| (19) | During the period ended December 31, 2006, the Company reclassified this investment from Non-affiliate investments to Affiliate investments based on the criteria in notes (1) and (2). |
| (20) | During the period ended December 31, 2006, the Company reclassified this investment from Affiliate investments to Control investments based on the criteria in notes (2) and (3). |
| (21) | Advanced Medical Isotope Corporation and Manakoa Services Company are related through common management. |
| (22) | Xethanol Corporation and Metamorphix Global are related though common management. |
See accompanying notes
57
UTEK Corporation
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of Business and Significant Accounting Policies
The Company
The Company provides Open Innovation services. The Company’s services enable companies to acquire externally developed technologies from universities and research laboratories worldwide to augment their internal research and development efforts. In addition, UTEK provides services to help companies develop innovation strategies and create value from their intellectual property. One component of Open Innovation is technology transfer which refers to the process by which new technologies, developed in universities, government research facilities, or similar research settings, are licensed to companies for potential commercial development and use. The Company’s goal is to provide its clients an opportunity to acquire and commercialize innovative technologies primarily developed by universities, medical centers, federal research laboratories and select corporations.
The Company is a non-diversified, closed-end management investment company that has elected to be treated as a business development company (“BDC”) under the Investment Company Act of 1940 (“1940 Act”). The Company’s strategic objective is to increase net assets by providing open innovation services and by effectuating technology transfer transactions with companies pursuant to which we receive securities or cash as compensation for the sale of technology rights.
Innovation and Strategic Consulting
The Company provides strategic innovation consulting services to larger clients to help them become more efficient innovators. The process involves our clients working with a handful of seasoned and experienced professionals capable of unlocking an organization's capacity for strategy and innovation.
Technology Acquisition Alliance
The Company’s technology acquisition alliance agreements are designed to help our customers enhance their new product pipeline through the acquisition of proprietary technologies primarily from universities, medical centers and federal research laboratories. The Company may receive cash or unregistered shares of common stock from companies as payment for the services we provide. Technology transfers are completed according to the terms set forth in the agreements with our client companies.
Technology Transfers
To effectuate a technology transfer, we will typically create a newly formed company to acquire a new technology from a university, medical center or federal research laboratory and then sell this newly formed company to our client for securities or cash. We call this unique technology transfer process U2B®. It is our plan that the shares we receive in these exchanges will, in the course of our business, be sold for cash or other assets. A benefit of effectuating technology transfers through our U2B® investment process is that such transactions do not result in a current taxable event for us for income tax purposes. We have not acquired, and do not currently intend to acquire a new technology from a university, medical center and federal research laboratory in connection with our U2B® process without the prior agreement of our client to subsequently acquire such new technology from us.
Patent Analytic Services
UTEK Intellectual Capital Consulting uses a team of on-call scientists and industry experts to provide technical and business knowledge to help our clients identify, assess, protect and leverage their intellectual property assets (“IP”). The Patent Analytic group helps clients identify the strengths and weaknesses of corporate IP and competitors’ IP. The group also identifies gaps in competitors’ IP portfolio that reveal white-space opportunities to pursue for our clients.
58
Online Exchanges and Databases
UTEK Information Services has a group of internet websites including Pharma Transfer, TechEx, Uventures and Knowledge Express. Pharma Transfer provides a source of research and business development opportunities for the international pharmaceutical market encompassing all areas of pipeline development, from early-stage discovery, through pre-clinical and clinical trials, to registered products that are all available for co-development or licensing. TechEx is an online searchable database for life science discoveries and Uventures is an online searchable database for physical science discoveries. Knowledge Express is a searchable database, which provides our clients with comprehensive coverage of licensing agreements, corporate profiles, clinical trials, deals, drug pipelines, drug sales, licensable technologies, patents and royalty rates.
Principles of Consolidation
UTEK Corporation commenced operations in 1997 in the business of technology transfer originally incorporated under the laws of the State of Florida, and subsequently under the laws of the State of Delaware in July 1999. The consolidated financial statements include the accounts of UTEK Corporation and its wholly owned subsidiaries; UTEK-Europe, Ltd. (Europe) and UTEKip, Ltd. (Israel). All intercompany transactions and balances are eliminated in consolidation.
Portfolio investments are held for the purpose of deriving investment income and future capital gains. The financial results of the Company’s portfolio investments are not consolidated in the Company’s financial statements.
Reclassifications
Certain reclassifications have been made to the 2006 and 2005 balances to conform to the 2007 financial statement presentation.
Cash and Cash Equivalents
The Company considers all highly liquid, fixed income investments with maturities of three months or less at the time of acquisition to be cash equivalents.
Investments
Pursuant to the requirements of the 1940 Act, UTEK Corporation’s Board of Directors is responsible for determining, in good faith, the fair value of our securities and assets for which market quotations are not readily available. In making its determination, the Board of Directors has considered valuation appraisals provided by an independent valuation service provider. With respect to equity securities in privately–owned companies, each investment is valued using industry valuation benchmarks, and then the value is assigned a discount reflecting the illiquid nature of the investment, as well as the minority, non-control position. When an external event such as a purchase transaction, public offering, or subsequent equity sale occurs, the pricing indicated by the external event is used to corroborate our private equity valuation. Equity securities in public companies that carry certain restrictions on resale are generally valued at a discount from the market value of the securities as quoted on the national securities exchange or national securities association.
The Board of Directors bases its determination upon, among other things, applicable quantitative and qualitative factors. These factors may include, but are not limited to, type of securities, nature of business, marketability, market price of unrestricted securities of the same issue (if any), comparative valuation of securities of publicly traded companies in the same or similar industries, current financial conditions and operating results, sales and earnings growth, operating revenues, competitive conditions and current and prospective conditions in the overall stock market.
59
Without a readily available market value, the value of the portfolio of equity securities may differ significantly from the values that would be placed on the portfolio if there existed a ready market for such equity securities, and the differences could be material. Substantially all of the Company’s investments owned at December 31, 2007 and December 31, 2006 (66% and 65% of net assets, respectively), are stated at fair value as determined by the Board of Directors, in the absence of readily available fair values. The Company uses the first-in, first-out (FIFO) method of accounting for sales of its investments.
Shares of stock provided by the portfolio companies in exchange for both technology acquisition alliance services and technology transfers are recorded at fair value on the day that the transactions are executed. The certificates are received subsequent to the transaction date.
Accounts Receivable
The Company provides an allowance for losses on trade receivables based on a review of the current status of existing receivables and management’s evaluation of periodic aging of accounts. The Company charges off accounts receivable against the allowance for losses when an account is deemed to be uncollectible. It is not the Company’s policy to accrue interest on past due receivables. The provision for doubtful accounts and notes was approximately $75,000 and $38,000 as of December 31, 2007 and 2006, respectively.
Fixed Assets
Fixed assets are stated at cost, less accumulated depreciation. Depreciation is recorded using the straight-line method over the estimated useful lives of the respective assets (generally five and seven years). Leasehold improvements are amortized over the shorter of the estimated useful life of the assets or lease term. The carrying amount of all long-lived assets is evaluated periodically to determine if adjustment to the depreciation and amortization period or the unamortized balance is warranted. The Company believes that no impairment of fixed assets exists at December 31, 2007.
Maintenance and repairs are charged to operations when incurred. Betterments and renewals are capitalized. When fixed assets are sold or otherwise disposed of, the asset account and related accumulated depreciation account are relieved, and any gain or loss is included in the statement of operations.
Goodwill and Intangible Assets
Goodwill represents the excess of the purchase price over the fair value of the assets acquired in connection with the Company’s acquisitions. Intangible assets represent the cost of websites, customer lists and software obtained in connection with certain of the Company’s acquisitions. The Company adheres to the Statement of Financial Accounting Standards No. 142, Goodwill and Other Intangible Assets. Accordingly, goodwill is not being amortized but is subject to annual impairment tests. Intangible assets with finite lives are amortized over their estimated useful lives.
Impairment of Long-lived Assets
We account for long-lived asset impairments under Statement of Financial Accounting Standards No. 144 (“SFAS 144”),Accounting for the Impairment or Disposal of Long Lived Assets. Consistent with prior guidance, SFAS 144 requires a three-step approach for recognizing and measuring the impairment of assets to be held and used. The Company recognizes impairment losses on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amounts. The impairment loss is measured by comparing the fair value of the asset to its carrying amount. Fair value is estimated based on discounted future cash flows. Assets to be sold are classified as Discontinued Operations, stated at the lower of the assets carrying amount or fair value and depreciation is no longer recognized.
60
Foreign Currency Translation
The Company translates the assets and liabilities of its non-U.S. functional currency subsidiaries into dollars at the current rates of exchange in effect at the end of each reporting period. Revenues and expenses are translated using rates that approximate those in effect during the period. Translation adjustments are included in the Consolidated Statements of Net Assets under the caption “foreign currency translation adjustment.”
Revenue Recognition
Sale of Technology Rights
The Company recognizes revenue from the sale of technology rights upon the exchange of the securities of our newly formed companies for securities in the portfolio company that acquires such newly formed company and the technology held by such newly formed company. The Company records revenue based on the fair value of the consideration received. In most cases, the consideration received for the rights is unregistered shares of common or preferred stock of the portfolio company.
Technology Acquisition Alliance Agreements
Technology acquisition alliance services are performed pursuant to service agreements (usually one year in length) in which UTEK provides consulting services by identifying and evaluating technology acquisition opportunities in exchange for unregistered shares of the portfolio company or cash. These agreements are typically cancelable with thirty days notice.
Revenue from technology acquisition alliance agreements in which unregistered shares of common stock are received before they are earned are deferred and recognized over the term of each agreement. For technology acquisition alliance agreements in which the stock is received ratably over the agreement, revenue is recognized as earned. The common stock received as payment is recorded as an investment at fair value.
PatentAnalytic Services
Revenue from consulting contracts is recognized ratably over the term of the contract, typically ninety days. These contracts are generally paid in the form of cash.
Online Exchanges and Databases
Revenue from the sale of subscriptions to the Company’s websites generally is received in the form of cash and initially is deferred and subsequently recognized ratably over the term of the subscription.
Acquisition of Technology Rights
The direct costs associated with the Company’s technology transfers are recorded as “acquisition of technology rights” within expenses on the accompanying statements of operations and may include cash to further accelerate commercialization efforts, license fees to acquire new technologies, consulting fees with the inventor of the technologies, and sponsored research fees with the university or research facility transferring the technologies.
Stock-Based Compensation
At December 31, 2007, the Company had two share-based equity compensation plans, which are described more fully in Note 8.
61
Effective January 1, 2006, the Company adopted the provisions of Statement of Financial Accounting Standards No. 123(R) (“SFAS 123(R)”),Share-Based Paymentand elected to use the modified prospective transition method and the Black-Scholes valuation model to account for stock option grants. Prior to the adoption of SFAS 123(R), the Company accounted for stock option grants using the intrinsic value method prescribed in APB Opinion No. 25,Accounting for Stock Issued to Employees, and accordingly, recognized no compensation expense for stock option grants.
Under the modified prospective approach, SFAS 123(R) applies to new awards granted subsequent to the date of adoption, January 1, 2006. Compensation cost recognized during the years ended December 31, 2007 and 2006 includes compensation cost for all share-based payments granted prior to, but not yet vested as of January 1, 2006, based on the grant date fair value estimated in accordance with the original provisions of SFAS 123, and compensation cost for all share-based payments granted subsequent to January 1, 2006, based on the grant date fair value estimated in accordance with the provisions of SFAS 123(R). For equity awards granted after the date of adoption, the Company amortizes share-based compensation expense on a straight-line basis over the vesting term. Compensation expense is recognized only for share-based payments expected to vest. The Company estimates forfeitures, both at the date of grant as well as throughout the vesting period, based on the Company’s historical experience and future expectations. Prior periods were not restated to reflect the impact of adopting the new standard and there is no cumulative effect.
The following table illustrates the pro forma effects on our net increase in net assets from operations (net earnings) and related per share information for the year ended December 31, 2005 had SFAS 123(R) been in effect in 2005.
| | | |
| | | 2005 |
Net increase in net assets, as reported | | $ | 2,095,675 |
Increase in net assets per share, as reported | | | |
Basic | | $ | 0.29 |
Diluted | | $ | 0.29 |
Compensation expense per SFAS 123, net of related tax effect* | | | — |
| |
Pro forma effects | | | |
Pro forma compensation expense per SFAS 123, net of related tax effect* | | | 310,472 |
Net increase in net assets, pro forma | | $ | 1,785,203 |
Increase in net assets per share, pro forma | | | |
Basic | | $ | 0.25 |
Diluted | | $ | 0.24 |
| * | Tax effect pertains only to certain non-qualified stock options. |
Income Taxes
Deferred taxes are provided on the asset and liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss and tax credit carry forwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases. Future tax benefits for net operating loss carryforwards are recognized to the extent that realization of these benefits is considered more likely than not. This determination is based on the expectation that related operations will be sufficiently profitable or various tax, business and other planning strategies will enable us to utilize the operating loss carryforwards. We cannot be assured that we will be able to realize these future tax benefits or that future valuation allowances will not be required. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
For federal and state income tax purposes, we are taxed at regular corporate rates on ordinary income and recognize gains on distributions of appreciated property. We are not entitled to the special tax treatment available
62
to BDCs that elect to be treated as regulated investment companies under the Internal Revenue Code because, among other reasons, we do not distribute at least 90% of “investment company taxable income” as required by the Internal Revenue Code for such treatment.
On January 1, 2007, the Company adopted the provisions of Financial Accounting Standards Board Interpretation No. 48 (“FIN 48”), Accounting for Uncertainty in Income Taxes, which clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements in accordance with FASB Statement No. 109, Accounting for Income Taxes. FIN 48 provides guidance on the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN 48 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosures, and transition. As of January 1, 2007, the Company had no unrecognized tax benefits and did not record any cumulative effect adjustment to net assets as a result of adopting FIN 48.
Net Realized Gains or Losses and Net Change in Unrealized Appreciation or Depreciation
Realized gains or losses are measured by the difference between the net proceeds from the repayment or sale and the original cost basis of the investment without regard to unrealized appreciation or depreciation previously recognized. The original cost basis of the securities we receive in connection with our technology acquisition alliance agreements and technology transfers is equal to the amount of revenue we recognized upon the receipt of such securities. Net change in unrealized appreciation or depreciation of investments reflects the change in portfolio investment values during the reporting period, including the reversal of previously recorded unrealized appreciation or depreciation when gains or losses are realized.
Earnings per Share (EPS)
Basic earnings per share is computed on the basis of the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share is computed on the basis of the weighted average number of shares of common stock plus the effect of dilutive potential common shares outstanding during the period using the treasury stock method. Dilutive potential common shares consist of outstanding stock options.
Components of basic and diluted per share data are as follows:
| | | | | | |
| | | 2007 | | 2006 | | 2005 |
Weighted average outstanding shares of common stock | | 8,989,234 | | 8,786,605 | | 7,194,212 |
Dilutive effect of stock options | | — | | — | | 131,100 |
| | | | | | |
Common stock and common stock equivalents | | 8,989,234 | | 8,786,605 | | 7,325,312 |
| | | | | | |
Shares excluded from calculation of diluted EPS(1) | | 652,025 | | 582,050 | | 117,500 |
| | | | | | |
| (1) | These shares attributable to outstanding stock options were excluded from the calculation of diluted EPS because their inclusion would have been anti-dilutive. The shares excluded from the calculation for the years ended December 31, 2007 and 2006 would have been anti-dilutive because there was a net decrease in net assets from operations during the period. |
Dividends to Shareholders
Dividends to shareholders are recorded on the date of declaration.
63
Financial Instruments and Concentrations of Credit Risk
The Company’s financial instruments consist of investments, cash and cash equivalents, accounts receivable, accounts payable and accrued expenses. Investments are recorded at fair value as determined by the Company’s Board of Directors and discussed previously in this note. The fair value of trade accounts receivable and payable and certain accrued expenses approximate their carrying amounts in the financial statements due to the short maturity of such instruments. The fair value of U.S. Treasuries and certificates of deposit is recorded based upon their market value.
Financial instruments with significant credit risk include investments and cash and cash equivalents. The Company invests its cash and cash equivalents and its U.S. Treasuries and certificates of deposit with high credit quality financial institutions. Certain cash and cash equivalents were in excess of FDIC insurance limits at December 31, 2007. The Company has not experienced any losses on such accounts.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. The most significant estimates relate to the valuation of the investment portfolio and the recognition of revenue in connection with the receipt of unregistered securities pursuant to technology acquisition alliance agreements and technology transfers. Actual results could differ from those estimates.
Recently Issued Accounting Pronouncements
In February 2007, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards (“SFAS”) No. 159,The Fair Value Option for Financial Assets and Financial Liabilities—Including an Amendment of FASB Statement No. 115. SFAS No. 159 permits an entity to choose to measure many financial instruments and certain other items at fair value. SFAS No. 159 is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. The Company’s most significant financial instruments are our investments, which are currently carried at fair value. Therefore, the adoption of SFAS No. 159 will not have a significant impact, if any, on our results of operations or financial position.
In June 2007, the AICPA issued Statement of Position (“SOP”) 07-1, Clarification of the Scope of the Audit and Accounting Guide Investment Companies and Accounting by Parent Companies and Equity Method Investors for Investments in Investment Companies. SOP 07-1 clarifies which entities are within the scope of the AICPA Audit and Accounting Guide Investment Companies (the “Guide”). SOP 07-1 was originally due to become effective for fiscal years beginning on or after December 15, 2007, but the Financial Accounting Standards Board subsequently voted to indefinitely defer the effective date. Companies that are regulated by the Investment Company Act of 1940 are automatically within the scope of the Guide. As such, the adoption of SOP 07-1 is not expected to materially impact the Company as it already adheres to the provisions of the Guide.
Upon adoption of SOP 07-1, a company must also adopt the provisions of FASB Staff Position (“FSP”) No. FIN 46R-7 “Application of FASB Interpretation No. 46R to Investment Companies.” FSP No. FIN 46R-7 permanently exempts investment companies from applying the provisions of Interpretation 46R to investments carried at fair value.
In December 2007, the Financial Accounting Standards Board issued Statement of Financial Accounting Standards (“SFAS”) No. 141(R),Business Combinations. SFAS No. 141(R) changes the way that companies account for business combinations. More assets and liabilities assumed will be measured at fair value as of the acquisition date; liabilities related to contingent consideration will be remeasured at fair value in each subsequent reporting period; and an acquirer will expense acquisition-related costs as opposed to capitalizing them. SFAS No. 141(R) is effective for business combinations consummated in fiscal years beginning subsequent to December 15, 2008 with no early adoption permitted. The adoption of SFAS No. 141(R) will have an impact on any acquisitions subsequent to 2008; however, we are unable to determine the impact will have on our results of operations or financial position.
64
2. Investments
Investments at December 31, 2007 and December 31, 2006 were valued at fair value as determined by the Board of Directors, with the assistance of appraisals provided by an independent valuation service provider, in the absence of readily available market values.
The values assigned to these securities are based upon available information and may not reflect amounts that could be realized if the Company found it necessary to immediately sell such securities, or amounts that ultimately may be realized. Accordingly, the fair values included in the accompanying schedule of investments may differ from the values that would have been used had a ready market existed for these securities and such differences could be material.
The 1940 Act prohibits the Company from acquiring (i) more than 3% of the total outstanding shares of another investment company; (ii) shares of another investment company having an aggregate value in excess of 5% of the value of the Company’s total assets; or (iii) shares of another registered investment company and all other investment companies having an aggregate value in excess of 10% of the value of the Company’s total assets. Subsequent to the Company’s acquisition of shares of common stock in HydroFlo, Inc., the Company became aware that HydroFlo, Inc. was a closed-end management investment company that had elected to be treated as a BDC under the 1940 Act. Because the Company’s ownership of HydroFlo, Inc. exceeded certain of the limits set forth above, the Company made a gift of 5,100,000 shares of HydroFlo, Inc.’s common stock to certain nonprofit organizations during the year ended December 31, 2006. The fair market value of such shares immediately prior to such gift was approximately $663,000. As a result, the Company recorded an expense in the amount of $663,000, which is included in general and administrative expenses in the accompanying statement of operations for the year ended December 31, 2006.
The Company also elected to abandon its right, title and interest in and to 400,000 shares of common stock of HydroFlo, Inc. because HydroFlo, Inc. may have issued such shares in violation of the 1940 Act. Such shares were originally issued by HydroFlo, Inc. to the Company pursuant to a technology acquisition alliance agreement. Such action resulted in the Company’s recognition of a $7,000 capital loss, net of income tax, during the year ended December 31, 2006.
On August 2, 2004, the Company assisted Shriners Hospitals for Children in transferring a tendon replacement technology they had developed and patented to Crystal Point Partners. As consideration for the services it rendered in connection with the transfer, the Company received 50% of the upfront consideration from the license agreement with Shriners Hospitals for Children and Myrmidon Biomaterials, Inc., including 100,000 unregistered shares of common stock from Myrmidon Biomaterials, Inc. During 2006, with approval by the Board of Directors, management determined that the investment in Myrmidon Biomaterials, Inc. was permanently impaired and the investment was written off.
On September 30, 2005, the Company entered into an agreement and plan of acquisition with Ybor City Group, Inc. to acquire all of its issued and outstanding shares of capital stock for an aggregate purchase price of $3,150,000. Ybor City Group is a real estate holding company that owns a commercial real estate property in Tampa, Florida. UTEK financed the acquisition through the issuance of 119,134 shares of its common stock and a $1,500,000 mortgage. Ybor City Group, Inc. and the associated mortgage entered into by Ybor City Group, Inc. are included within UTEK Real Estate Holdings, Inc. within the Company’s portfolio investments.
During September 2005, upon approval by the Board of Directors, management made the decision to sell some of the investments that the Company had carried on its financial statements with a zero value for several quarters. Maintaining these investments was costly and the likelihood of future value was minimal. The following investments were included in the group of zero value shares: Provision Operation Systems, Inc., Advanced Recycling Sciences, Inc., Graphco Holdings Corp., Nubar, Inc., Assuretec Holdings, Inc., Prime Pharmaceutical Corporation, Primapharm Funding Corporation, Silver Screen Studios, Inc., Mixed Entertainment, Inc., Hydrogen Technology Applications, Inc., Zkid Network Company, GreenWorks Corporation, and Xeminex, Ltd.
65
Technology acquisition alliances:
During the year ended December 31, 2007, the Company entered into 80 technology acquisition alliance agreements. Of these, 10 were terminated during the year ended December 31, 2007 and 8 were terminated subsequent to December 31, 2007. The income recognized from all technology acquisition alliance agreements for the year ended December 31, 2007 was approximately $1.5 million. At December 31, 2007, the Company had 53 active technology acquisition alliance clients.
During the year ended December 31, 2006, the Company entered into forty-four technology acquisition alliance agreements. Of these, fifteen were terminated during the year ended December 31, 2006 and two were terminated subsequent to December 31, 2006. As a result of these agreements, the fair value of the Company’s assets increased by $2.7 million for the year ended December 31, 2006. The income recognized from all technology acquisition alliance agreements for the year ended December 31, 2006 was approximately $2.2 million.
During the year ended December 31, 2005, the Company entered into thirty-six technology acquisition alliance agreements, of which, four were subsequently terminated in 2005 and four were terminated in 2006. As a result of these agreements, the fair value of the Company’s assets increased by $1.6 million at December 31, 2005. The income recognized from all technology acquisition alliance agreements for the year ended December 31, 2005 was approximately $1.4 million.
Technology Transfers:
All of our technology transfers are generally completed according to our technology acquisition alliance service agreements with our clients.
During the year ended December 31, 2007, we completed the following sixteen technology transfers:
| | | | | | | | | |
Date | | Name of Company Acquiring the Newly Formed Company | | Newly Formed Company | | Consideration – Unregistered Shares or
Cash* | | Price per
Share(1) |
January 4 | | Manakoa Services Corporation | | Infinite Identification Technologies, Inc. | | 95,000 preferred(2) | | $ | 24.000 |
January 11 | | Cyberlux Corporation | | Hybrid Lighting Technologies, Inc. | | 50,000 preferred(3) 26,500,000 common | | | 11.530 0.020 |
January 30 | | CytoDyn, Inc. | | Advanced Genetic Technologies, Inc. | | 100,000 preferred(4) | | | 8.450 |
January 31 | | Material Technologies, Inc. | | Stress Analysis Technologies, Inc. | | 47,500 preferred(5) | | | 14.620 |
February 12 | | Liberty Diversified Holdings, Inc. | | Sero Tonin Solutions, Inc. | | 63,981 preferred(6) | | | 6.000 |
March 12 | | Metamorphix Global, Inc. | | Flex Crete Technologies, Inc. | | $200,000 cash | | | — |
March 12 | | Klegg Electronics, Inc. | | Tempo Control Technologies, Inc. | | 10,901,250 | | | 0.040 |
March 28 | | Avalon Oil and Gas, Inc. | | Leak Location Technologies, Inc. | | 34,875,000 | | | 0.020 |
April 30 | | Material Technologies, Inc. | | Damage Assessment Technologies, Inc. | | 7,125,000 | | | 0.215 |
May 30 | | Klegg Electronics, Inc. | | Klegg Network Storage Technologies, Inc. | | 8,550,000 | | | 0.255 |
June 28 | | Material Technologies, Inc. | | Non-Destructive Assessment Technologies, Inc. | | 6,412,500 | | | 0.240 |
July 12 | | Pathway One, Plc | | WebMed Technologies, Inc. | | 6,000,000 | | | 0.140 |
66
| | | | | | | | |
Date | | Name of Company Acquiring the Newly Formed Company | | Newly Formed Company | | Consideration – Unregistered Shares
or Cash* | | Price per
Share(1) |
July 20 | | MachineTalker, Inc. | | Wideband Detection Technologies, Inc. | | 3,000,000 | | 0.050 |
September 28 | | World Energy Solutions, Inc. | | Hydrogen Safe Technologies, Inc. | | 7,500,000 | | 0.260 |
November 12 | | NeoStem, Inc. | | Stem Cell Technologies, Inc. | | 400,000 | | 1.750 |
December 28 | | MachineTalker, Inc | | Micro Wireless Technologies, Inc. | | 46,500,000 | | 0.018 |
During the year ended December 31, 2006, we completed the following twenty-nine technology transfers:
| | | | | | | | | |
Date | | Name of Company Acquiring the Newly Formed Company | | Newly Formed Company | | Consideration – Unregistered Shares* | | Price per
Share(1) |
January 20 | | Fuel FX International, Inc. | | Emissions-Detection Technologies, Inc. | | 100,000(7) | | $ | 21.000 |
January 27 | | Broadcast International, Inc. | | Video Processing Technologies, Inc. | | 944,360 | | | 1.210 |
January 30 | | WebSky, Inc. | | Strategic Wireless Solutions, Inc. | | 33,250,000 | | | 0.027 |
March 6 | | Trio Industries Group, Inc. | | Ultra Fine Coating Systems, Inc. | | 1,805,000 | | | 1.350 |
March 15 | | American Soil Technologies, Inc. | | Advanced Fertilizer Technologies, Inc. | | 4,275,000 | | | 0.0367 |
March 16 | | Advanced Refractive Technologies, Inc. | | Ocular Therapeutics, Inc. | | 97,000(8) | | | 21.300 |
April 3 | | Trio Industries Group, Inc. | | Natural Adhesive Technologies, Inc. | | 1,566,089 | | | 2.090 |
April 4 | | Advanced Refractive Technologies, Inc. | | Advanced Glaucoma Technologies, Inc. | | 100,000(9) | | | 19.960 |
April 5 | | UBA Technology, Inc. | | Intellitouch Technologies, Inc. | | 48,614,797(10) | | | 0.034 |
May 1 | | Industrial Biotechnology Corp. | | Bio-Repellant Technologies, Inc. | | 3,865,979 | | | 0.720 |
May 12 | | Kwikpower International Plc | | Hydrocarbon Synthesis Technologies, Inc. | | (11) | | | |
May 12 | | Kwikpower International Plc | | Advanced BioEnergy Technologies, Inc. | | (12) | | | |
June 1 | | Trio Industries Group, Inc. | | Advanced Powder Coating Technologies, Inc. | | 1,470,987 | | | 1.770 |
June 12 | | Kwikpower International Plc | | Advanced Biofuel Technologies, Inc. | | (13) | | | |
June 13 | | Xethanol Corporation | | Advanced Biomass Gasification Technologies, Inc. | | 136,838 | | | 6.400 |
June 20 | | Klegg Electronics, Inc. | | Smart Speaker Technologies, Inc. | | 22,941,327 | | | 0.104 |
July 12 | | Avalon Oil and Gas, Inc. | | Ultrasonic Mitigation Technologies, Inc. | | 15,437,500 | | | 0.082 |
July 14 | | DME Interactive Holdings, Inc. | | Multimedia Control Technologies, Inc. | | 4,426,136 | | | 0.170 |
July 18 | | Cytodyn, Inc. | | Advanced Influenza Technologies, Inc. | | 2,000,000 | | | 1.780 |
August 11 | | NetFabric Holdings, Inc | | Intrusion Detection Technologies, Inc. | | 7,125,000 | | | 0.083 |
August 18 | | Material Technologies, Inc. | | Materials Monitoring Technologies, Inc. | | 35,749,213 | | | 0.047 |
67
| | | | | | | | |
Date | | Name of Company Acquiring the Newly Formed Company | | Newly Formed Company | | Consideration – Unregistered Shares* | | Price per
Share(1) |
August 29 | | Industrial Biotechnology Corp. | | Advanced Pheromone Technologies, Inc. | | 4,642,857 | | 0.290 |
September 12 | �� | Liberty Diversified Holdings, Inc. | | Innovative Packaging Technologies, Inc. | | 15,437,500 | | 0.076 |
September 22 | | Advanced Medical Isotope Corp. | | Neu-Hope Technologies, Inc. | | 95,000(14) | | 18.983 |
October 9 | | World Energy Solutions, Inc. | | Pure Air Technologies, Inc. | | 100,000(15) | | 26.325 |
November 1 | | Klegg Electronics, Inc. | | Universal Wireless Technologies, Inc. | | 28,771,428 | | 0.0469 |
November 8 | | Avalon Oil & Gas, Inc. | | IntelliWell Technologies, Inc. | | 19,000,000 | | 0.0335 |
November 10 | | Cyberlux Corporation | | SPE Technologies, Inc. | | 98,000 | | 16.380 |
December 6 | | Cargo Connection Logistics Holding, Inc. | | Nuclear Material Detection Technologies, Inc. | | 168,539,326 | | 0.00596 |
During the year ended December 31, 2005, we completed the following fourteen technology transfers:
| | | | | | | | | |
Date | | Name of Company Acquiring the Newly Formed Company | | Newly Formed Company | | Consideration –
Unregistered Shares* | | Price per
Share(1) |
January 10 | | Xethanol Corporation | | Superior Separation Technologies, Inc. | | 250,000 | | $ | 2.500 |
February 22 | | Health Sciences Group, Inc. | | Open Cell Biotechnologies, Inc. | | 822,845 | | | 0.730 |
March 31 | | Swiss Medica, Inc. | | Anti Depression Biohealth Solutions, Inc. | | 1,862,069(16) | | | 0.190 |
June 30 | | Manakoa Services Corporation | | Vigilant Network Technologies, Inc. | | 5,365,854 | | | 0.280 |
July 18 | | eLinear Corporation | | Secure Voice Communications, Inc. | | 150,528 | | | 0.690 |
August 5 | | Industrial Biotechnology Corporation | | Advanced Bioscience, Inc. | | Preferred A
10,000,000 | | | 0.200 |
August 15 | | Xethanol Corporation | | Xylose Technologies, Inc. | | 567,857 | | | 3.020 |
September 1 | | Fuel FX International, Inc. | | Emissions-Reduction Technologies, Inc. | | 5,715,000 | | | 0.200 |
September 15 | | INSEQ Corporation | | Separation and Recovery Technologies, Inc. | | 434,782,608 | | | 0.0056 |
September 30 | | Kwikpower International, Plc | | Biodiesel Technologies, Inc. | | (17) | | | 2.500 |
October 3 | | Trio Industries Group, Inc | | Kenaf Core Technologies, Inc. | | 1,153,178 | | | 1.230 |
October 12 | | Fuel FX International, Inc. | | Emissions Analysis, Inc. | | 3,500,000 | | | 0.200 |
November 17 | | Trio Industries Group, Inc. | | Cornboard Technologies, Inc. | | 1,643,500 | | | 1.530 |
December 13 | | Advanced Refractive Technologies, Inc. | | Optimetrix Technologies, Inc. | | Preferred B
97,000 | | | 10.650 |
| * | Unless otherwise noted, the Company received unregistered shares of common stock of the company acquiring the Company’s newly formed company. |
| (1) | Represents the valuation price per share at the date of acquisition. |
| (2) | Preferred A shares convertible into common shares based on a value of $3.8 million. |
| (3) | Preferred C shares convertible into common shares based on a value of $768,500. |
| (4) | Preferred A shares convertible into common shares based on a value of $1.3 million. |
| (5) | Preferred E shares convertible into common shares based on a value of $926,250. |
68
| (6) | Preferred D shares convertible into common shares based on a value of $638,000. |
| (7) | Preferred B shares convertible into common shares based on a value of $2.1 million. |
| (8) | Preferred C shares convertible into common shares based on a value of $2.8 million. |
| (9) | Preferred D shares convertible into common shares based on a value of $2.0 million. |
| (10) | The Company also received 95,000 Preferred A shares convertible into common shares based on a value of $2.5 million. |
| (11) | Consideration consisted of a £1.2 million convertible debenture. The Company has recognized the value of the investment based upon the fair value of the 2.4 million common shares underlying the convertible debenture. |
| (12) | Consideration consisted of a £1.3 million convertible debenture. The Company has recognized the value of the investment based upon the fair value of the 2.5 million common shares underlying the convertible debenture. |
| (13) | Consideration consisted of a £1.2 million convertible debenture. The Company has recognized the value of the investment based upon the fair value of the 2.3 million common shares underlying the convertible debenture. |
| (14) | 5% Preferred A shares convertible into common shares based on a value of $3,182,500. |
| (15) | Preferred A shares convertible into common shares based on a value of $4,05,000. |
| (16) | The Company also received $96,637 in cash. |
| (17) | Consideration consisted of a £1.25 million convertible debenture. The Company has recognized the value of the investment based upon the fair value of the 1,984,126 common shares underlying the convertible debenture. |
3. Fixed Assets
Fixed assets consist of the following:
| | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
Computer Equipment | | $ | 486,552 | | | $ | 456,149 | |
Furniture and Fixtures | | | 307,212 | | | | 350,655 | |
Leasehold Improvements | | | 23,883 | | | | 5,017 | |
| | | | | | | | |
| | | 817,647 | | | | 811,821 | |
Less: Accumulated Depreciation | | | (341,069 | ) | | | (216,820 | ) |
| | | | | | | | |
| | $ | 476,578 | | | $ | 595,001 | |
| | | | | | | | |
Depreciation expense was approximately $145,000, $122,000 and $43,000 for the years ended December 31, 2007, 2006, and 2005, respectively.
4. Goodwill
The Company conducts its annual impairment analysis at the end of each year. In connection with the 2006 impairment analysis, the Company determined there was an impairment of the goodwill related to the Pharma Transfer, Ltd. acquisition. In connection with the 2007 impairment analysis, the Company determined there was another impairment of the goodwill related to the Pharma Transfer, Ltd. acquisition and an impairment of the goodwill related to the Knowledge Express acquisition. As a result, the Company recorded another partial impairment of the goodwill in the fourth quarter 2007. These write-downs resulted in an impairment charge of approximately $159,000 ($99,000 after tax) for the United Kingdom segment and $51,000 ($32,000 after tax) for
69
the United States segment during 2007. These impairment charges are included in goodwill impairment in the statement of operations for the year ended December 31, 2007.
The Company decided during 2006 to make significant changes in strategy for UTEKip, Ltd., primarily switching the focus of operations in Israel from software to technology transfer, the Company’s core business. These changes were other-than-temporary; therefore management determined there was an impairment of the original purchase goodwill. The Company recorded a total impairment of the goodwill for UTEKip(Israel segment) in 2006. Based on an analysis of the undiscounted cash flows, the Company determined that the goodwill for this reporting unit was impaired. This resulted in a write-down of approximately $235,000, $147,000 after tax, which is an operating expense in the statement of operations for the year ended December 31, 2006.
The carrying amount of goodwill is summarized in the following table:
| | | | | | |
| | | December 31, |
| | | 2007 | | 2006 |
UTEK-Europe, Ltd. | | $ | 562,501 | | $ | 562,501 |
UTEK-EKMS, Inc. | | | 426,869 | | | 426,869 |
Pharma-Transfer, Ltd. | | | 372,000 | | | 528,553 |
UTEKip, Ltd. | | | — | | | — |
Knowledge Express | | | 1,437,000 | | | 1,488,110 |
Currency exchange adjustment | | | 22,694 | | | 15,130 |
| | | | | | |
| | $ | 2,821,064 | | $ | 3,021,163 |
| | | | | | |
5. Intangible Assets
During 2006, the Company decided to make significant changes in strategy for UTEKip, Ltd., primarily switching the focus of operations in Israel from software to technology transfer, the Company’s core business. In connection therewith, the Company ceased operation of the UTEKip website as of year-end and wrote-off the remaining carrying value of the related intangible asset. In addition, the Company ceased operation of the UTEK-EKMS website during 2006 as a result of duplication of website contents with other UTEK websites and the Company wrote-off the remaining carrying value of the related intangible asset. These write-offs resulted in an impairment charge of approximately $51,000 ($32,000 after tax) for the Israel segment and $16,000 ($9,000 after tax) for the United States segment during 2006. Impairment charges are included in general and administrative expenses in the statement of operations for the year ended December 31, 2006. The Company believes that no additional impairment of intangible assets exists at December 31, 2007.
The carrying amount of intangible assets is summarized in the following table:
| | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
Tech-Ex website | | $ | 70,900 | | | $ | 70,900 | |
Uventures website | | | 40,000 | | | | 40,000 | |
Pharma-Transfer website | | | 112,195 | | | | 112,195 | |
UTEKip website | | | — | | | | — | |
UTEK-EKMS website | | | — | | | | — | |
Knowledge Express customer list/website/trademarks | | | 150,000 | | | | 150,000 | |
| | | | | | | | |
| | | 373,095 | | | | 373,095 | |
Less: Accumulated amortization | | | (249,282 | ) | | | (182,995 | ) |
| | | | | | | | |
| | $ | 123,813 | | | $ | 190,100 | |
| | | | | | | | |
70
Intangible assets are being amortized over the estimated useful lives of the respective assets of five years. Amortization expense related to intangible assets was approximately $66,000, $90,000 and $80,000 for the years ended December 31, 2007, 2006 and 2005, respectively. The estimated aggregate amortization expense for intangible assets for the years 2008, 2009 and 2010 will be approximately $59,000, $50,000 and $15,000, respectively, and $0 thereafter.
6. Income Taxes
The Company accounts for income taxes under Statement of Financial Accounting Standards No. 109 (“SFAS 109”),Accounting for Income Taxes. Deferred income tax assets and liabilities are determined based upon differences between financial reporting and tax basis of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
The components of the income tax provision on operations, excluding income tax expense (benefit) on realized gains (losses) and unrealized appreciation (depreciation) of investments are as follows:
| | | | | | | | | |
| | | Year Ended December 31, |
| | | 2007 | | 2006 | | 2005 |
Current: | | | | | | | | | |
Federal | | $ | — | | $ | — | | $ | — |
State | | | — | | | — | | | — |
Foreign | | | — | | | — | | | — |
| | | | | | | | | |
| | $ | — | | $ | — | | $ | — |
| | | | | | | | | |
Deferred: | | | | | | | | | |
Federal | | $ | 2,482,024 | | $ | 10,935,888 | | $ | 3,226,692 |
State | | | 264,993 | | | 1,149,049 | | | 337,535 |
| | | | | | | | | |
| | | 2,747,017 | | | 12,084,937 | | | 3,564,227 |
| | | | | | | | | |
| | $ | 2,747,017 | | $ | 12,084,937 | | $ | 3,564,227 |
| | | | | | | | | |
A reconciliation of the differences between the effective income tax rate and the statutory federal tax rate follows:
| | | | | | | | | |
| | | Year Ended December 31, |
| | | 2007 | | 2006 | | 2005 |
Tax at U.S. statutory rate | | $ | 2,218,078 | | $ | 10,889,909 | | $ | 3,213,699 |
State taxes, net of federal benefit | | | 236,812 | | | 1,162,658 | | | 337,535 |
Stock options | | | 149,560 | | | — | | | — |
Other | | | 142,567 | | | 32,370 | | | 12,993 |
| | | | | | | | | |
| | $ | 2,747,017 | | $ | 12,084,937 | | $ | 3,564,227 |
| | | | | | | | | |
Significant components of the Company's deferred tax assets and liabilities are as follows:
| | | | | | | |
| | | As of December 31, | |
| | | 2007 | | 2006 | |
Net operating loss carryforward | | $ | 5,033,192 | | $ | 3,107,826 | |
Tax credit carryforward | | | — | | | — | |
Other | | | 125,777 | | | (15,375 | ) |
Investments | | | 247,735 | | | (2,331,651 | ) |
| | | | | | | |
Net deferred tax asset (liability) | | $ | 5,406,704 | | $ | 760,800 | |
| | | | | | | |
71
SFAS 109 requires a valuation allowance to reduce the deferred tax assets reported if, based on the weight of the evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. After consideration of all the evidence, both positive and negative, management has determined that a valuation allowance was not necessary as of December 31, 2007 and 2006.
At December 31, 2007, the Company had available U.S. net operating loss carry-forwards of approximately $11,735,000, which expire as follows: 2021—$287,000; 2022—$371,000; 2023—$1,645,000; 2024—$69,000; 2025—3,791,000; and 2027—$5,572,000.
7. Stockholders' Equity
Transactions in common stock for the three years ended December 31, 2007, were as follows:
| | | | | | | | | |
Common Stock | | Shares | | Par Value | | Paid-In Capital | |
Balance at December 31, 2004 | | 6,003,163 | | $ | 60,032 | | $ | 20,959,242 | |
| | | | | | | | | |
Employee stock options exercised | | 246,750 | | | 2,468 | | | 1,660,832 | |
Warrants exercised | | 16,993 | | | 170 | | | (170 | ) |
Acquisition of UTEKip, Ltd | | 19,895 | | | 199 | | | 299,801 | |
Acquisition of Ybor City Group, Inc. | | 119,134 | | | 1,191 | | | 1,648,809 | |
AIM Listing | | 1,224,610 | | | 12,246 | | | 12,461,671 | |
Private Placement—August 2005 | | 330,960 | | | 3,310 | | | 3,317,478 | |
| | | | | | | | | |
Balance at December 31, 2005 | | 7,961,505 | | $ | 79,616 | | $ | 40,347,663 | |
| | | | | | | | | |
Share-based compensation expense | | — | | | — | | | 504,589 | |
Deferred tax related to share-based compensation expense | | — | | | — | | | 440,616 | |
Employee stock options exercised | | 75,255 | | | 753 | | | 754,764 | |
Private placement—February 2006 | | 816,330 | | | 8,163 | | | 8,947,019 | |
Acquisition of 22nd Street of Ybor City, Inc. | | 82,919 | | | 829 | | | 999,171 | |
| | | | | | | | | |
Balance at December 31, 2006 | | 8,936,009 | | $ | 89,361 | | $ | 51,993,822 | |
| | | | | | | | | |
Share-based compensation expense | | — | | | — | | | 612,893 | |
Employee stock options exercised | | 75,267 | | | 754 | | | 541,928 | |
| | | | | | | | | |
Balance at December 31, 2007 | | 9,011,276 | | $ | 90,114 | | $ | 53,148,643 | |
| | | | | | | | | |
On April 11, 2005, the Company sold 1,224,610 shares of its common stock to certain institutional and other investors located outside the United States for net proceeds of approximately $12.5 million. In connection with this offering, the Company listed its shares of common stock for trading on the AIM market of the London Stock Exchange.
On February 8, 2006, the Company consummated a financing which raised approximately $10 million (before expenses) from the sale of 816,330 shares of common stock. Piper Jaffray, & Co. received an aggregate commission of $702,000.
On March 30, 2006, the Company declared a dividend of $0.02 per share to stockholders of record as of April 28, 2006. The dividend was paid on May 19, 2006. The Company also declared a dividend of $0.02 per share to stockholders of record as of January 8, 2007. This dividend was paid on January 31, 2007.
On June 15, 2007, the Company’s stockholders voted to approve an amendment to the Company’s Certificate of Incorporation to increase the number of authorized shares of common stock from 19,000,000 to 29,000,000. The additional 10,000,000 shares are part of the existing class of common stock and will have the same rights and privileges as the shares of common stock currently issued and outstanding. The Board of Directors deemed it desirable to increase the number of shares of common stock the Company is authorized to
72
issue in order to provide adequate flexibility in the future. The holders of common stock are not entitled to preemptive rights or cumulative voting, and accordingly, the issuance of additional common shares will dilute the ownership and voting rights of shareholders.
See Note 8 for further information on share-based compensation expense and employee stock options exercised. See Note 10 for further information on acquisitions.
8. Stock-Based Compensation
The Company had two share-based equity compensation plans at December 31, 2007. The Company adopted a stock option plan in September 1999 (the “1999 Plan”) and a non-qualified stock option plan in February 2000 (the “2000 Plan”). Under the terms of the 1999 Plan, as amended, the Company is authorized to issue options to purchase up to 1,385,000 shares of the Company's common stock. The options are intended to be incentive stock options within the meaning of Section 422 of the Internal Revenue Code (the “Code”), however, options may be issued under the 1999 Plan, as amended, that do not qualify for incentive treatment under the Code. Under the terms of the 2000 Plan, the Company is authorized to issue options to purchase up to 315,000 shares of the Company's common stock. Under the 2000 Plan, as amended, the Company may only issue options that do not qualify for incentive treatment under Section 422 of the Code. Options, under both plans, are granted at the fair market value of the stock on the date of grant, except in the case of a more than 10% shareholder for which grants are exercisable at 110% of fair market value of the stock on the date of grant. Options generally become fully vested three to four years from the date of grant and expire five years from the date of grant. At December 31, 2007, the Company had 449,203 shares available for future stock option grants under existing plans.
Effective January 1, 2006, the Company adopted the provisions of Statement of Financial Accounting Standards No. 123(R) (“SFAS 123(R)”),Share-Based Payment. Prior to the adoption of SFAS 123(R), we accounted for stock option grants using the intrinsic value method prescribed in APB Opinion No. 25,Accounting for Stock Issued to Employees, and accordingly, recognized no compensation expense for stock option grants.
Under the modified prospective approach, SFAS 123(R) applies to new awards granted subsequent to the date of adoption, January 1, 2006. Compensation cost recognized during the years ended December 31, 2007 and 2006 includes compensation cost for all share-based payments granted prior to, but not yet vested as of January 1, 2006, based on the grant date fair value estimated in accordance with the original provisions of SFAS 123, and compensation cost for all share-based payments granted subsequent to January 1, 2006, based on the grant date fair value estimated in accordance with the provisions of SFAS 123(R). Prior periods were not restated to reflect the impact of adopting the new standard and there is no cumulative effect.
The impact of SFAS 123(R) on cash used in operations was an increase of approximately $613,000 and $505,000 for the years ended December 31, 2007 and 2006, respectively. There was no impact on cash flow from investing or financing activities for either 2007 or 2006.
We use the Black-Scholes option pricing model to estimate the fair value of stock-based awards on the date of grant. The assumptions employed in the calculation of the fair value of share-based compensation expense were calculated as follows for all years presented:
| | • | | Expected dividend yield—based on the Company’s historical dividend yield. |
| | • | | Expected volatility—based on the Company’s historical market price at consistent points in a period equal to the expected life of the options. |
| | • | | Risk-free interest rate—based on the U.S. Treasury yield curve in effect at the time of grant. |
| | • | | Expected life of options—calculated using the simplified method as prescribed in Staff Accounting Bulletin No. 107, where the expected life is equal to the sum of the vesting period and the contractual term divided by two. |
73
The following table summarizes the assumptions used to estimate the fair value of stock options granted during the years ended December 31, 2007, 2006 and 2005.
| | | | | | | | | | | | |
| | | 2007 | | | 2006 | | | 2005 | |
Expected dividend yield | | | 0-0.25 | % | | | 0-0.12 | % | | | 0 | % |
Expected volatility | | | 37-40 | % | | | 54-64 | % | | | 28 | % |
Risk-free interest rate | | | 3.40-4.91 | % | | | 4.35-5.21 | % | | | 3.76-4.55 | % |
Expected life of options | | | 3.75-3.88 years | | | | 3.5-3.75 years | | | | 3.5-4.0 years | |
Weighted average grant date fair value | | $ | 4.73 | | | $ | 6.61 | | | $ | 4.12 | |
Net cash proceeds from the exercise of stock options were approximately $543,000 and $756,000 for the years ended December 31, 2007 and 2006, respectively. Total compensation cost related to stock options was approximately $613,000 and $505,000 for the years ended December 31, 2007 and 2006, respectively. The tax benefits from the exercise of common stock options and from the recognition of compensation costs were not significant during 2007 or 2006. At December 31, 2007, there was $1,789,000 of unrecognized compensation cost related to stock options which is expected to be recognized over a weighted average period of 2.9 years.
The following table represents stock option activity as of and for the three years ended December 31, 2007:
| | | | | | | | | | | |
| | | Number
of Shares | | | Weighted
Average
Exercise
Price | | Weighted
Average
Remaining
Contractual Life | | Aggregate
Intrinsic
Value |
Options Outstanding—December 31, 2004 | | 574,017 | | | $ | 6.89 | | | | | |
Granted | | 195,000 | | | | 14.07 | | | | | |
Exercised | | (246,750 | ) | | | 6.72 | | | | $ | 1,940,000 |
Forfeited/cancelled/expired | | (17,500 | ) | | | 8.01 | | | | | |
| | | | | | | | | | | |
Options Outstanding—December 31, 2005 | | 504,767 | | | $ | 11.16 | | | | | |
| | | | | | | | | | | |
Granted | | 204,000 | | | | 15.42 | | | | | |
Exercised | | (75,255 | ) | | | 10.04 | | | | $ | 528,000 |
Forfeited/cancelled/expired | | (51,462 | ) | | | 14.49 | | | | | |
| | | | | | | | | | | |
Options Outstanding—December 31, 2006 | | 582,050 | | | $ | 13.18 | | | | | |
| | | | | | | | | | | |
Granted | | 291,500 | | | | 13.80 | | | | | |
Exercised | | (75,267 | ) | | | 13.44 | | | | $ | 485,000 |
| | | | | | | | | | | |
Forfeited/cancelled/expired | | (146,258 | ) | | | 7.03 | | | | | |
| | | | | | | | | | | |
Options Outstanding—December 31, 2007 | | 652,025 | | | $ | 14.08 | | 3.25 years | | $ | 451,000 |
| | | | | | | | | | | |
Options Exercisable—December 31, 2007 | | 255,489 | | | $ | 13.25 | | 1.82 years | | $ | 431,000 |
| | | | | | | | | | | |
The total grant date fair value of options vested during the years ended December 31, 2007, 2006 and 2005 was approximately $434,000, $469,000 and $511,000, respectively.
The following table summarizes information about outstanding and exercisable stock options at December 31, 2007:
| | | | | | | | | | | | |
| | | Outstanding Options | | Exercisable Options |
Range of Exercise Prices | | Outstanding
at 12/31/07 | | Weighted
Average
Exercise Price | | Remaining
Contractual Life
in Years | | Exercisable
at 12/31/07 | | Weighted
Average
Exercise Price |
$5.64 - $6.99 | | 65,000 | | $ | 6.57 | | 0.28 | | 65,000 | | $ | 6.57 |
$12.80 - $13.95 | | 316,400 | | | 13.42 | | 4.09 | | 31,364 | | | 13.53 |
$14.00 - $15.90 | | 198,625 | | | 15.28 | | 2.81 | | 132,875 | | | 15.14 |
$18.40 - $22.04 | | 72,000 | | | 20.44 | | 3.55 | | 26,250 | | | 19.90 |
| | | | | | | | | | | | |
| | 652,025 | | $ | 14.08 | | 3.25 | | 255,489 | | $ | 13.25 |
| | | | | | | | | | | | |
74
Pursuant to an agreement we entered into in connection with our initial public offering, the Company issued 100,000 warrants on October 25, 2000, at $.0003 to the underwriter, to purchase an equal number of shares of the Company’s common stock. These warrants were exercisable at $9.90 per share. The warrant holder exercised 41,890 and 54,210 warrants in the years ended December 31, 2005 and 2004, respectively. The remaining 3,900 warrants expired on October 25, 2005.
9. Employee Benefit Plan
In 2003, the Company adopted the UTEK Corporation Simple IRA Plan (the “Plan”) for employees of the Company and its subsidiaries. The Plan allows employees who satisfy the service requirements of the Plan to contribute pre-tax wages to the Plan, subject to legal limits, $10,500 in 2007 with “catch up” deferrals of an additional $2,000 for participants age 50 and older. The Company matches 100% of the first 3% of wages contributed by employees. The Company’s matching contributions vest immediately and were approximately $74,000, $72,000 and $58,000 in 2007, 2006 and 2005, respectively.
10. Acquisitions
On March 30, 2006, the Company entered into an agreement and plan of acquisition with 22nd Street of Ybor City, Inc. to acquire all of its issued and outstanding shares of capital stock for an aggregate purchase price of $2.0 million. 22nd Street of Ybor City, Inc. is a real estate holding company that owns a commercial real estate property in Tampa, Florida. UTEK acquired 100% of the outstanding shares of 22nd Street of Ybor City, Inc. through the issuance of 82,919 shares of unregistered common stock with a market value of $1.0 million plus a cash payment of $1.0 million. The acquired business will be operated by UTEK Real Estate Holdings, Inc., one of UTEK’s portfolio companies.
On January 29, 2005, the Company purchased 100% of the outstanding stock of INTRA-DMS, Ltd. for $300,000 in unregistered shares of the Company’s common stock. Accordingly, the results of operations for INTRA-DMS, Ltd. have been included in the accompanying consolidated financial statements from that date forward. The acquisition was made for the purpose of expanding our technology acquisition alliance services with the analysis of intellectual property. As one of our wholly-owned subsidiaries, INTRA-DMS, Ltd. has changed its name to UTEKip, Ltd.
On July 7, 2005, the Company purchased 100% of the assets of Knowledge Express Data Systems (“Knowledge Express”); a U.S. based on-line IP information service, for $1,500,000 in cash. Knowledge Express is positioned to service the IP needs of UTEK, its subsidiaries and its customers. The purchase price has been allocated to the underlying assets purchased (and liabilities) based on their estimated fair values. The resulting goodwill from this transaction is approximately $1,500,000.
The value of the stock issued in the acquisitions noted above was determined based on the average market price of the shares over the five days before the terms of the acquisition agreements were agreed upon and publicly announced.
75
Following is a condensed balance sheet showing the fair values of the assets acquired and the liabilities assumed as of the respective dates of acquisition:
| | | | | | |
| | | UTEKip,
Ltd. | | Knowledge
Express |
Assets | | | | | | |
Cash and cash equivalents | | $ | — | | $ | 26,423 |
Accounts receivable net of allowance for bad debt | | | 18,780 | | | 141,100 |
Prepaid Expenses and other current assets | | | — | | | 97,477 |
Fixed assets | | | 14,788 | | | 113,000 |
Intangible assets | | | 74,396 | | | 150,000 |
Goodwill acquired in the acquisition | | | 246,773 | | | 1,488,110 |
| | | | | | |
| | | 354,737 | | | 2,016,110 |
| | | | | | |
Liabilities | | | | | | |
| | |
Accrued Expenses | | | 31,221 | | | 58,102 |
Deferred Income | | | — | | | 458,008 |
Short term debt | | | 23,516 | | | — |
| | | | | | |
| | | 54,737 | | | 516,110 |
| | | | | | |
Net assets acquired | | $ | 300,000 | | $ | 1,500,000 |
| | | | | | |
Condensed balance sheet information for the 2006 acquisition of 22nd Street of Ybor City, Inc. has not been presented because the business is operated under UTEK’s portfolio companies. Pro forma financial information for the acquisitions has not been presented as the impact would be immaterial.
11. Segment Reporting
The Company’s principal area of activity is technology transfer related services. The Company has three reportable operating segments: United Kingdom, Israel and the United States. The United Kingdom segment includes our wholly owned subsidiaries UTEK-Europe, Ltd. and Pharma Transfer, Ltd.; the Israel segment includes UTEKip, Ltd.; and the United States segment includes UTEK Corporation and UTEK-EKMS, Inc., which was dissolved into UTEK Corporation during 2006.
A summary of income from operations (revenue) and other financial information by reportable operating segment is shown below:
| | | | | | | | | | | | |
| | | United Kingdom | | Israel | | United States | | Consolidated |
Long-lived assets December 31, 2007 | | $ | 589,406 | | $ | 13,621 | | $ | 2,818,427 | | $ | 3,421,454 |
Total assets December 31, 2007 | | | 681,140 | | | 39,317 | | | 44,500,620 | | | 45,221,077 |
Long-lived assets December 31, 2006 | | | 580,916 | | | 18,611 | | | 3,206,737 | | | 3,806,264 |
Total assets December 31, 2006 | | | 719,238 | | | 71,771 | | | 52,249,801 | | | 53,040,810 |
| | | | | | | | | | | | | | | |
| | | For the year ended December 31, 2007 |
| | | United Kingdom | | | Israel | | | United States | | | Consolidated |
Income from Operations | | $ | 274,221 | | | $ | 123,511 | | | $ | 19,903,217 | | | $ | 20,300,949 |
Income (loss) before interest, other expenses and income taxes | | | (116,568 | ) | | | (164,481 | ) | | | 6,804,808 | (1) | | | 6,523,759 |
Depreciation and amortization | | | 1,658 | | | | 7,021 | | | | 203,671 | | | | 212,350 |
76
| | | | | | | | | | | | | | | |
| | | For the year ended December 31, 2006 |
| | | United Kingdom | | | Israel | | | United States | | | Consolidated |
Income from Operations | | $ | 398,087 | | | $ | 182,371 | | | $ | 56,372,479 | | | $ | 56,952,937 |
Income (loss) before interest, other expenses and income taxes | | | (330,263 | ) | | | (521,447 | )(2) | | | 32,880,854 | (3) | | | 32,029,144 |
Depreciation and amortization | | | 3,546 | | | | 17,274 | | | | 191,113 | | | | 211,933 |
| | | | | | | | | | | | | | |
| | | For the year ended December 31, 2005 |
| | | United Kingdom | | | Israel | | | United States | | Consolidated |
Income from Operations | | $ | 830,316 | | | $ | 204,596 | | | $ | 21,708,911 | | $ | 22,743,823 |
Income (loss) before interest, other expenses and income taxes | | | (140,017 | ) | | | (232,292 | ) | | | 9,824,366 | | | 9,452,057 |
Depreciation and amortization | | | 25,485 | | | | 17,943 | | | | 101,006 | | | 144,434 |
| (1) | The Company recorded goodwill impairment for Pharma Transfer, Ltd. of $159,030 and Knowledge Express of $51,110, which are included in the current period loss. |
| (2) | The Company recorded goodwill impairment of $234,940 and intangible asset impairment of $51,000 for UTEKip, Ltd., which is included in the 2006 loss. |
| (3) | The Company recorded intangible asset impairment of $16,000 for the UTEK-EKMS website, which is included in the 2006 loss. |
12. Commitments and Contingencies
From time to time, some of the Company’s portfolio companies may receive correspondence or other notices of alleged breach of the license agreement. Some of these correspondences and notices provide for a period of time in which to cure the alleged breach. The failure of the portfolio companies to cure the alleged breach may have a material adverse impact on the Company’s results of operations and financial position.
Effective September 1, 2004, the Company entered into a three year employment agreement with our Chief Executive Officer, Clifford M. Gross, providing for an annual base salary of $300,000 for his services. In addition to his salary, Dr. Gross will receive a reasonable allowance for an automobile and certain severance benefits. Under this employment agreement, the range of potential commitment payments given the termination of Dr. Gross or a change in the Company’s control is between $769,000 and $3,601,000. This includes compensation benefits and related taxes, as well as health benefits under certain circumstances. This employment agreement expired in September 2007 and is currently in the process of being renegotiated.
The Company entered into an employment agreement with our Chief Operating Officer and Chief Compliance Officer, Douglas Schaedler, effective March 20, 2007. The term of the agreement is for one year and provides for an annual salary of $225,000. Additionally, Mr. Schaedler will receive a bi-annual discretionary bonus, 100,000 stock options and a severance package equal to 90 days of his annual salary.
Effective November 29, 2007, the Company entered into a three-year employment agreement with our Chief Financial Officer, Carole R. Wright. Under the terms of the agreement, Ms. Wright will receive an annual salary of $170,000 for the first year, $185,000 for the second year and $200,000 for the final year. In addition, Ms. Wright will be entitled to discretionary cash bonuses to be determined by the Company’s Compensation Committee of the Board of Directors and a $100,000 severance package.
77
In February 2007, we obtained a $4,000,000 uncommitted bank line of credit with the Bank of Tampa. The advances on the line of credit accrue interest (payable monthly) at prime (7.25% as of December 30, 2007). The principal and any unpaid interest are due upon demand. This line is collateralized with commercial real estate owned by UTEK Real Estate Holdings, Inc. We have not utilized this line of credit and as of December 31, 2007 we had no outstanding debt.
The Company leases its office facilities and certain equipment for various terms under long-term, non-cancelable operating lease agreements. The leases expire at various dates through 2010 and provide for various renewal options. In the normal course of business, it is expected that these leases will be renewed or replaced by leases on other properties. The leases provide for increases in future minimum annual rental payments. Lease expense charged to operations for the years ended December 31, 2007, 2006 and 2005 was approximately $317,000, $277,000 and $122,000 respectively.
The following is a schedule by year of future minimum rental payments required under the operating lease agreements:
| | | |
2008 | | $ | 291,541 |
2009 | | | 155,925 |
2010 | | | 1,301 |
| | | |
| | $ | 448,767 |
| | | |
13. Related Party Transactions
Sam Reiber, the Company’s General Counsel and a member of the Board of Directors, is also a partner with the law firm Linsky and Reiber in Tampa, Florida. Linsky and Reiber has received approximately $54,000, $56,000 and $58,000 in compensation in 2007, 2006 and 2005, respectively, for legal services performed for the Company. Linsky and Reiber also holds 6,100 shares of the Company’s common stock as of December 31, 2007.
During the years ended December 31, 2007 and 2006, the Company loaned funds for operations and real estate improvements of approximately $784,000 and $1.2 million, respectively, to certain subsidiaries of UTEK Real Estate Holdings, Inc., one of UTEK’s portfolio companies. In addition, the Company leases space for its corporate headquarters from Ybor City Group, Inc., a subsidiary of UTEK Real Estate Holdings, Inc. The lease agreement has a 36 month term commencing July 1, 2006 through June 30, 2009. The lease agreement provides for a 3% increase in the minimum monthly rental payment effective on the lease anniversary. In 2007, the Company took on extra space in the building, which increased the lease payment. The monthly lease payment is $21,605 as December 31, 2007. The Company paid rent of approximately $258,000 and $130,000 to Ybor City Group,, Inc. during the years ended December 31, 2007 and 2006, respectively.
78
14. Interim Financial Information (Unaudited)
| | | | | | | | | | | | | | | | |
| | | March 31 | | | June 30 | | | September 30 | | | December 31 | |
Fiscal year 2007: | | | | | | | | | | | | | | | | |
Income from operations (Revenue) | | $ | 7,947,771 | | | $ | 6,388,449 | | | $ | 3,694,914 | | | $ | 2,269,815 | |
Net income from operations | | | 2,233,391 | | | | 1,935,831 | | | | 188,192 | | | | (580,672 | ) |
Net increase (decrease) in net assets from operations | | | (406,569 | ) | | | (3,584,734 | ) | | | (134,017 | ) | | | (4,351,366 | ) |
Net increase (decrease) in net assets from operations per share: | | | | | | | | | | | | | | | | |
Basic | | $ | (0.05 | ) | | $ | (0.40 | ) | | $ | (0.01 | ) | | $ | (0.48 | ) |
Diluted | | $ | (0.05 | ) | | $ | (0.40 | ) | | $ | (0.01 | ) | | $ | (0.48 | ) |
Fiscal year 2006: | | | | | | | | | | | | | | | | |
Income from operations (Revenue) | | $ | 11,807,006 | | | $ | 23,059,307 | | | $ | 13,612,176 | | | $ | 8,474,448 | |
Net income from operations | | | 3,609,574 | | | | 9,767,356 | | | | 4,026,601 | | | | 2,540,676 | |
Net increase (decrease) in net assets from operations | | | 5,557,126 | | | | 11,059,619 | | | | (4,473,588 | ) | | | (17,053,955 | ) |
Net increase (decrease) in net assets from operations per share: | | | | | | | | | | | | | | | | |
Basic | | $ | 0.66 | | | $ | 1.25 | | | $ | (0.50 | ) | | $ | (1.91 | ) |
Diluted | | $ | 0.65 | | | $ | 1.23 | | | $ | (0.50 | ) | | $ | (1.91 | ) |
Fiscal year 2005: | | | | | | | | | | | | | | | | |
Income from operations (Revenue) | | $ | 2,538,529 | | | $ | 2,542,797 | | | $ | 10,448,383 | | | $ | 7,214,114 | |
Net income from operations | | | 174,273 | | | | 28,713 | | | | 3,733,289 | | | | 1,951,555 | |
Net increase (decrease) in net assets from operations | | | (2,961,173 | ) | | | (742,038 | ) | | | 5,663,261 | | | | 135,625 | |
Net increase (decrease) in net assets from operations per share: | | | | | | | | | | | | | | | | |
Basic | | $ | (0.49 | ) | | $ | (0.10 | ) | | $ | 0.75 | | | $ | 0.02 | |
Diluted | | $ | (0.49 | ) | | $ | (0.10 | ) | | $ | 0.74 | | | $ | 0.02 | |
15. Subsequent Events
On December 20, 2007, the Company entered into a Stock Purchase Agreement with Partnering Intelligence Limited and Bridgehead International Limited, pursuant to which the Company agreed to issue 153,967 shares of unregistered common stock, valued at $2,150,000, to Partnering Intelligence in consideration for all of the shares of Pharmalicensing Limited owned by Partnering Intelligence, which represents 100% of the issued and outstanding shares of Pharmalicensing. The transaction closed and became effective on January 3, 2008. Pharmalicensing’s operations are immaterial for pro forma disclosure.
Pharmalicensing, based in York, England, is a company organized under the laws of England and Wales, and is engaged in an open innovation service for partnering, licensing and business development within the life science and biopharmaceutical industry.
The Company acquired the shares of Pharmalicensing through its subsidiary UTEK Europe, Ltd. Transfer of the 153,967 shares of UTEK common stock will be restricted for twelve months following the completion of the transaction.
On February 26, 2008, the Company entered into a Stock Purchase Agreement with Strategos, Inc. Under the terms of the Stock Purchase Agreement, Strategos will be acquired for 1,248,960 shares of UTEK Corporation unregistered common stock. The number of shares is based on the average ten-day closing price prior to execution of the stock purchase agreement. Strategos shareholders shall receive one third of the shares at closing
79
and the remaining UTEK shares will be held in escrow and paid upon achieving certain performance and employment milestones over the next two years. The shareholders of Strategos have agreed not to sell any of the unregistered shares for at least 12 months following closing of the transaction, which is expected to take place in early March 2008. Strategos, Inc. based in Chicago, Illinois is a company organized under the laws of California, and is a strategic innovation consulting firm that provides consulting services, primarily to Fortune 500 companies.
16. Selected Per Share Data and Ratios
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31 | |
| | | 2007 | | | 2006 | | | 2005 | | | 2004 | | | 2003 | |
Per share information:(1) | | | | | | | | | | | | | | | | | | | | |
Net asset value, beginning of year | | $ | 5.71 | | | $ | 5.58 | | | $ | 3.85 | | | $ | 2.33 | | | $ | 1.87 | |
Net income from operations(1) | | | 0.42 | | | | 2.27 | | | | .80 | | | | .15 | | | | 0.04 | |
Net change in realized and unrealized appreciation/depreciation on investments (after taxes)(2) | | | (1.41 | ) | | | (3.45 | ) | | | (1.70 | ) | | | (0.22 | ) | | | (0.59 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total from investment operations | | | (0.99 | ) | | | (1.18 | ) | | | (0.90 | ) | | | (0.07 | ) | | | (0.55 | ) |
Foreign currency translation adjustment(1) | | | — | | | | 0.02 | | | | (0.02 | ) | | | 0.01 | | | | 0.01 | |
Distributions to shareholders(1) | | | — | | | | (0.04 | ) | | | — | | | | — | | | | — | |
Net increase from stock transactions(1) | | | 0.13 | | | | 1.33 | | | | 2.65 | | | | 1.58 | | | | 1.00 | |
| | | | | | | | | | | | | | | | | | | | |
Net asset value, end of year | | $ | 4.85 | | | $ | 5.71 | | | $ | 5.58 | | | $ | 3.85 | | | $ | 2.33 | |
| | | | | | | | | | | | | | | | | | | | |
Per share market value, end of year | | $ | 13.20 | | | $ | 11.34 | | | $ | 13.79 | | | $ | 14.99 | | | $ | 11.10 | |
Investment return, based on market price at end of period(3) | | | 16 | % | | | (18 | )% | | | (8 | )% | | | 35 | % | | | 78 | % |
| | | | | |
Ratios/supplemental data: | | | | | | | | | | | | | | | | | | | | |
Net assets, end of year | | $ | 43,674,548 | | | $ | 50,981,162 | | | $ | 44,441,118 | | | $ | 23,092,943 | | | $ | 11,152,370 | |
Ratio of expenses to average net assets | | | 29 | % | | | 52 | % | | | 39 | % | | | 33 | % | | | 38 | % |
Ratio of net income from operations to average net assets | | | 8 | % | | | 42 | % | | | 17 | % | | | 6 | % | | | 2 | % |
Diluted weighted average number of shares outstanding during the year | | | 8,989,234 | | | | 8,786,605 | | | | 7,325,312 | | | | 6,098,537 | | | | 4,266,918 | |
| (1) | Calculated based on diluted weighted average number of shares outstanding during the year. |
| (2) | Calculated as a balancing amount necessary to reconcile the change in net assets value per share with the other per share information presented. This amount may not agree with the aggregate gains and losses for the period because the difference in the net asset value at the beginning and end of year does not inherently equal the per share changes of the line items disclosed. |
| (3) | Calculated as the change in market price during the period divided by the market price at the end of the period. |
80
UTEK CORPORATION
Schedule of Investments in and Advances to Affiliates
| | | | | | | | | | | | | | | | |
Portfolio Company and Investment | | Year Ended
December 31,
2007 Amount
of Interest or
Dividends(1) | | December 31,
2006 Value | | Gross
Additions(2) | | Gross
Reductions(3) | | | December 31,
2007 Value |
Affiliate Investments | | | | | | | | | | | | | | | | |
Emission & Power Solutions, Inc. (Fuel FX International, Inc.)(privately held) | | | | | | | | | | | | | | | | |
Common stock | | $ | — | | $ | 1,831,600 | | $ | — | | $ | (1,079,100 | ) | | $ | 752,500 |
Preferred Series B stock | | | — | | | 990,000 | | | — | | | (150,000 | ) | | | 840,000 |
Health Sciences Group, Inc. | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 95,200 | | | 1,506,763 | | | (1,601,963 | ) | | | — |
GS Energy Corporation (INSEQ Corp.) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 269,800 | | | — | | | (179,900 | ) | | | 89,900 |
Manakoa Services Company | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 415,000 | | | — | | | (356,500 | ) | | | 58,500 |
Preferred Series B stock | | | | | | — | | | 2,280,000 | | | (1,520,000 | ) | | | 760,000 |
Material Technologies, Inc.(5) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 4,894,603 | | | (373,503 | ) | | | 4,521,100 |
Preferred Series E stock | | | — | | | — | | | 694,640 | | | (46,240 | ) | | | 648,400 |
MachineTalker, Inc.(5) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 954,000 | | | (285,700 | ) | | | 668,300 |
NeoStem, Inc.(5) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 750,800 | | | (309,100 | ) | | | 441,700 |
TenthGate, Inc.(4) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 25,600 | | | — | | | (25,600 | ) | | | — |
Stealth MediaLabs, Inc. | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 121,600 | | | — | | | (121,600 | ) | | | — |
Cyberlux Corporation(5) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 559,600 | | | — | | | | 559,600 |
Preferred Series C stock | | | — | | | — | | | 2,181,640 | | | (400,640 | ) | | | 1,781,000 |
vidShadow.com, Inc. (DME Interactive Holdings) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 1,344,600 | | | | | | (1,145,400 | ) | | | 199,200 |
Cytodyn, Inc. | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 856,800 | | | — | | | (738,500 | ) | | | 118,300 |
Preferred Series A stock | | | — | | | — | | | 845,000 | | | (585,000 | ) | | | 260,000 |
Cargo Connection Logistics Holdings, Inc. | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 643,300 | | | — | | | (470,600 | ) | | | 172,700 |
World Energy Solutions, Inc.(5)(7) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 2,046,600 | | | (2,046,600 | ) | | | — |
Preferred Series A stock | | | — | | | — | | | 2,633,000 | | | (2,633,000 | ) | | | — |
Avalon Oil and Gas, Inc. | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 864,200 | | | 867,150 | | | (862,450 | ) | | | 868,900 |
Pathway One Plc(5) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 852,300 | | | (132,800 | ) | | | 719,500 |
USTelematics, Inc.(5) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 684,800 | | | (174,700 | ) | | | 510,100 |
American Soil Technologies, Inc.(5) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 1,569,800 | | | (1,255,400 | ) | | | 314,400 |
81
| | | | | | | | | | | | | | | | |
Portfolio Company and Investment | | Year Ended
December 31,
2007 Amount
of Interest or
Dividends(1) | | December 31,
2006 Value | | Gross
Additions(2) | | Gross
Reductions(3) | | | December 31,
2007 Value |
Industrial BiotechnologyCorporation(6) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 712,800 | | | (710,800 | ) | | | 2,000 |
WebSky Inc. | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 674,600 | | | — | | | (674,600 | ) | | | — |
NetFabric Holdings | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 394,000 | | | — | | | (251,100 | ) | | | 142,900 |
Liberty Diversified Holdings, Inc. | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 187,600 | | | — | | | (187,600 | ) | | | — |
Preferred Series B stock | | | — | | | — | | | 382,800 | | | (382,800 | ) | | | — |
| | | | | | | | | | | | | | | | |
Total Investments in Affiliate Investments | | $ | — | | $ | 8,713,900 | | $ | 24,416,296 | | $ | (18,701,196 | ) | | $ | 14,429,000 |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Portfolio Company and Investment | | Year Ended
December 31,
2007
Amount of
Interest or
Dividends(1) | | December 31,
2006 Value | | Gross
Additions(2) | | Gross
Reductions(3) | | | December 31,
2007 Value |
Control Investments | | | | | | | | | | | | | | | | |
UTEK Real Estate Holdings, Inc., (privately held) | | | | | | | | | | | | | | | | |
Common Stock | | $ | — | | $ | 3,614,500 | | $ | — | | $ | (142,500 | ) | | $ | 3,472,000 |
Klegg Electronics, Inc | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 3,209,900 | | | 2,685,900 | | | (5,595,600 | ) | | | 300,200 |
Industrial Biotechnology Corporation(6) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | 1,995,900 | | | — | | | (1,995,900 | ) | | | — |
World Energy Solutions, Inc.(7) | | | | | | | | | | | | | | | | |
Common stock | | | — | | | — | | | 3,419,000 | | | (1,387,900 | ) | | | 2,031,100 |
Ybor City Group, Inc. (privately held) | | | | | | | | | | | | | | | | |
Demand note, interest rate @ 5% | | | — | | | 1,181,472 | | | 783,789 | | | — | | | | 1,965,261 |
| | | | | | | | | | | | | | | | |
Total Investment in Control Investment | | | -0- | | $ | 10,001,772 | | $ | 6,888,689 | | $ | (9,121,900 | ) | | $ | 7,768,561 |
| | | | | | | | | | | | | | | | |
This schedule should be read in conjunction with the Company’s consolidated financial statements including the schedule of investments.
(1) All of the listed securities with the exception of the demand note issued by Ybor City Group, Inc. are generally non-income producing. In addition all of the listed securities are “restricted securities” within the meaning of Rule 144 of the Securities Act of 1933. In some cases, preferred stock may also be non-income producing. The principal amount for debt and the number of shares of common stock and preferred stock is shown in the Schedule of Investments as of December 31, 2007.
(2) Gross additions include increases in the cost basis of investments resulting from new portfolio investments, and the movement of an existing portfolio company into this category from a different category. Gross additions also include net increases in unrealized appreciation or net decreases in unrealized depreciation.
(3) Gross reductions include decreases in the cost basis of investments resulting from the sale of portfolio investments, and the movement of an existing portfolio company out of this category into a
82
(3) Gross reductions include decreases in the cost basis of investments resulting from the sale of portfolio investments, and the movement of an existing portfolio company out of this category into a different category. Gross reductions also include net increases in unrealized depreciation or net decreases in unrealized appreciation.
(4) During the year ended December 31, 2007, the Company reclassified this investment from Affiliate investments to Non-affiliate investments.
(5) During the year ended December 31, 2007, the Company reclassified this investment from Non-affiliated investments to Affiliate investments.
(6) During the year ended December 31, 2007, the Company reclassified this investment from Control investments to Affiliate investments.
(7) During the year ended December 31, 2007, the Company reclassified this investment from Affiliate investments to Control investments.
83
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
Not applicable.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
Our Chief Executive Officer and Chief Financial Officer have evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Securities Exchange Act of 1934) as of the end of the period covered by this report. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in reports we file or submit under the Securities Exchange Act of 1934 is (i) recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and (ii) accumulated and communicated to our management, including our Chief Executive Officer, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Securities Act of 1934. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based upon the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, our management concluded that our internal control over financial reporting is effective as of December 31, 2007.
Attestation Report of the Registered Public Accounting Firm
Our independent registered public accounting firm, Pender Newkirk & Company LLP, has issued an attestation report on the effectiveness of the Company’s internal control over financial reporting, which is set forth under the heading “Report of Independent Registered Public Accounting Firm.”
Changes in Internal Control over Financial Reporting
There was no significant change in our internal control over financial reporting (as defined in Rule 13a-15(f) of the Securities Exchange Act of 1934) that occurred during our most recently completed fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information |
Not Applicable
84
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
Information about our directors may be found under the caption “NOMINEES” in the Company’s Proxy Statement for the 2008 Annual Meeting of Stockholders (the “Proxy Statement”) to be filed pursuant to Regulation 14A under the Securities Exchange Act of 1934 within 120 days from the fiscal year end. Information about our executive officers may be found under the caption “EXECUTIVE OFFICERS” in the Proxy Statement. Information about the audit committee may be found under the captions “MEETINGS OF THE BOARD OF DIRECTORS AND COMMITTEES” and “MEMBERSHIP ON BOARD COMMITTEES” in the Proxy Statement. Information about beneficial ownership may be found under the caption “SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE” in the Proxy Statement. All of the aforementioned information is incorporated herein by reference.
Code of Business Conduct and Ethics for Directors and Employees
We have adopted a Code of Business Conduct and Ethics for all of our directors and employees, including our Chief Executive Officer and Chief Financial Officer. We have posted a copy of our Code of Business Conduct and Ethics on our Internet website atwww.utekcorp.com. Any waivers of the Code of Business Conduct and Ethics must be approved, in advance, by our full Board of Directors. Any amendments to, or waivers from the Code of Business Conduct and Ethics that apply to our executive officers and directors will be posted on our Internet website located atwww.utekcorp.com.
| Item 11. | Executive Compensation |
The information set forth under the captions “DIRECTOR COMPENSATION,” “EXECUTIVE COMPENSATION,” “COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION” and “COMPENSATION COMMITTEE REPORT” in the Proxy Statement is incorporated herein by reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information set forth under the captions “SECURITY OWNERSHIP” in the Proxy Statement is incorporated herein by reference.
Securities Authorized For Issuance under Equity Compensation Plans
As of December 31, 2007, we had two stock option plans under which shares of our common stock were authorized for issuance.
| | | | | | | |
Plan category | | Number of securities to be
issued upon exercise of
outstanding options, warrants
and rights | | Weighted-average exercise
price of outstanding options,
warrants and rights | | Number of securities
remaining available for
future issuance under equity
compensation plans
(excluding securities
reflected in column(a) |
| | | (a) | | (b) | | (c) |
Equity compensation plans approved by security holders | | 652,025 | | $ | 14.08 | | 449,203 |
Equity compensation plans not approved by security holders | | — | | | — | | — |
Total | | 652,025 | | $ | 14.08 | | 449,203 |
85
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information set forth under the captions “CERTAIN RELATIONSHIPS AND TRANSACTIONS” and “DIRECTOR INDEPENDENCE” in the Proxy Statement is incorporated herein by reference.
| Item 14. | Principal Accountant Fees and Services |
The information set forth under the captions “FEES BILLED TO THE COMPANY BY REGISTERED INDEPENDENT PUBLIC ACCOUNTING FIRM” and “POLICY ON PRE-APPROVAL OF SERVICES PROVIDED BY REGISTERED INDEPENDENT PUBLIC ACCOUNTING FIRM” in the Proxy Statement is incorporated herein by reference.
86
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
(a) Documents filed as part of this report:
1. The following Financial Statements of UTEK Corporation are contained in Item 8 of this Form 10-K:
| | • | | Consolidated Statements of Assets and Liabilities at December 31, 2007 and 2006 |
| | • | | Consolidated Statements of Operations for the years ended December 31, 2007, 2006 and 2005 |
| | • | | Consolidated Statements of Cash Flows for the years ended December 31, 2007, 2006 and 2005 |
| | • | | Consolidated Statements of Changes in Net Assets for the years ended December 31, 2007, 2006 and 2005 |
| | • | | Consolidated Schedule of Investments at December 31, 2007 and 2006 |
| | • | | Notes to Consolidated Financial Statements |
| | • | | Selected Per Share Data and Ratios for the years ended December 31, 2007, 2006, 2005, 2004, and 2003 |
| | • | | Report of Independent Registered Public Accounting Firm |
| | • | | Report of Management on Internal Control over Financial Reporting |
| | • | | Report of Independent Registered Public Accounting Firm |
2. The following financial statement schedules are filed herewith:
Schedule 12-14 of Investments in and Advances to Affiliates
In addition, there may be additional schedules not provided because (i) such schedules are not required or (ii) the information required has been presented in the aforementioned financial statements.
3. The following exhibits are filed with this report or are incorporated herein by reference to a prior filing, in accordance with Rule 12b-32 under the Securities Exchange Act of 1934:
| | |
| 3.1 | | Certificate of Incorporation, dated July 6, 1999, as filed and recorded with the Secretary of State of the State of Delaware on July 13, 1999. (Incorporated by reference to Exhibit 3.1 filed with the Company’s registration statement on Form N-2 (File No. 333-93913) filed with the Commission on December 30, 1999.) |
| |
| 3.2 | | Certificate of Amendment to Certificate of Incorporation, dated October 14, 1999, as filed and recorded with the Secretary of State of the State of Delaware on October 15, 1999. (Incorporated by reference to Exhibit 3.2 filed with the Company’s registration statement on Form N-2 (File No. 333-93913) filed with the Commission on December 30, 1999.) |
| |
| 3.3 | | By-Laws of UTEK Corporation. (Incorporated by reference to Exhibit 3.3 filed with the Company’s registration statement on Form N-2 (File No. 333-93913) filed with the Commission on December 30, 1999.) |
| |
| 3.4 | | Certificate of Amendment to Certificate of Incorporation dated July 23, 2001, as filed and recorded with the Secretary of State of the State of Delaware on July 24, 2001. (Incorporated by reference to Exhibit 3.4 to the Company’s Form 10-K filed with the Commission on April 1, 2002.) |
| |
| 10.1 | | UTEK Corporation Amended and Restated Employee Stock Option Plan. (Incorporated by reference to Exhibit A filed with the Company’s Proxy Statement filed on June 30, 2005.) |
87
| | |
| 10.2 | | UTEK Corporation Amended and Restated Non-Qualified Stock Option Plan. (Incorporated by reference to Appendix C to Schedule 14A to the Company’s Form 10-Q for the quarter ended June 30, 2005.) |
| |
| 10.3 | | Employment Agreement between UTEK Corporation and Carole R. Wright. (Incorporated by reference to Exhibit No. 10.1 filed with the Company’s Form 8-K filed on November 30, 2007.) |
| |
| 10.4 | | Employment Agreement between UTEK Corporation and Douglas C. Schaedler. (Incorporated by reference to Exhibit No. 10.1 filed with the Company’s Form 8-K filed on March 22, 2007.) |
| |
| 10.5 | | Form of Incentive Stock Option Agreement. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 10-Q for the quarter ended June 30, 2005.) |
| |
| 10.13 | | Corporate Custody Agreement dated May 29, 2003 between UTEK Corporation and Bank of Tampa. (Incorporated by reference to Exhibit 99.j filed with the Company’s Registration Statement on Form N-2 on October 26, 2005.) |
| |
| 10.14 | | Corporate Custody Agreement dated July 18, 2006 between UTEK Corporation and Gunn Allen Financial, Inc. (Incorporated by reference to Exhibit 99.i.l filed with the Company’s Registration Statement on Form N2/A on July 19, 2006.) |
| |
| 10.20 | | Note with The Bank of Tampa: Commercial Security Agreement, Business Loan Agreement and Promissory Note. (Incorporated by reference to Exhibit 99.2 to the Company’s Form 10-K for the year ended December 31, 2006.) |
| |
| 11.1 | | Computation of per share earnings is included in Item 8 of this Form 10-K. |
| |
| 14.1 | | Code of Ethics. (Incorporated by reference to Exhibit 99.1 to the Company’s Form 10-K for the year ended December 31, 2006.) |
| |
| 21.1 | | Subsidiaries of the registrant, and jurisdiction of incorporation/organization: UTEK Europe, Ltd., - United Kingdom and UTEKip, Ltd. - Israel. |
| |
| 31.1* | | Certification of the Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350. |
| |
| 31.2* | | Certification of the Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350. |
| |
| 32.1* | | Certification of the Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350. |
| |
| 32.2* | | Certification of the Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350. |
88
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized on March 4, 2008.
| | |
| UTEK CORPORATION |
| |
By: | | /s/ CLIFFORD M. GROSS |
| | Clifford M. Gross, Ph.D. |
| | Chairman and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
| | | | |
Signature | | Title(Capacity) | | Date |
/s/ CLIFFORD M. GROSS Clifford M. Gross | | Chairman and Chief Executive Officer (Principal Executive Officer) | | March 4, 2008 |
| | |
/s/ CAROLE R. WRIGHT Carole R. Wright | | Chief Financial Officer (Principal Financial and Accounting Officer) | | March 4, 2008 |
| | |
/s/ SAM REIBER Sam Reiber | | Director | | March 4, 2008 |
| | |
/s/ STUART M. BROOKS Stuart M. Brooks | | Director | | March 4, 2008 |
| | |
/s/ HOLLY CALLEN-HAMILTON Holly Callen-Hamilton | | Director | | March 4, 2008 |
| | |
/s/ KWABENA GYIMAH-BREMPONG Kwabena Gyimah-Brempong | | Director | | March 4, 2008 |
| | |
/s/ ARTHUR CHAPNIK Arthur Chapnik | | Director | | March 4, 2008 |
| | |
/s/ JOHN MICEK John Micek | | Director | | March 4, 2008 |
| | |
/s/ KEITH A. WITTER Keith A. Witter | | Director | | March 4, 2008 |
| | |
/s/ FRANCIS MAUDE Francis Maude | | Director | | March 4, 2008 |
89
