
June, 2014

Forward - Looking Statement Statements made in this presentation stating the Company ’ s beliefs, intentions, and expectations are forward - looking statements. The company ’ s actual results could differ materially from those projected. Additional information is contained in the company ’ s SEC filings such as our Form 10 - K and Form 10 - Qs filed at www.sec.gov . 2 Protalex , Inc .
Protalex , Inc. Biotechnology company focused on autoimmune diseases Public company, ticker is PRTX, market capitalization approximately $250 mm Lead product: PRTX - 100 ▪ Staphylococcal protein A, a natural protein isolated from cultured bacteria ▪ Safe and well - tolerated in four human clinical trials ▪ Efficient and inexpensive production process 3 Protalex , Inc.

Protalex Key Team Members Arnold P Kling – President, Director; Principal of Niobe Ventures, LLC, experienced investor in and manager of early stage technology companies Kirk M Warshaw – CFO, Director; experienced in finances of development stage company James W Dowe III – Vice - Chair of SAB; active investor in biotechnology, computer software and investment management companies William E. Gannon, MD – Chief Medical Officer; more than 20 years experience in clinical development and regulatory affairs at Quintiles, PPD, and other companies; Medical Director for Capital City Technical Consulting Bruce McClain, MD – Medical Director; more than 20 years experience in clinical development and product safety; senior roles at Aeras Global and MedImmune Richard Frankovich , Ph.D . -- VP of ITP Programs; 27 years pharma experience, former Head of Hematology Franchise and Global Commercial Leader for Promacta at GSK Benjamin R Bowen, Ph.D . – Senior Advisor; background in pharma and biotech R&D, investment banking Michelle Catalina, Ph.D . – Director of Preclinical Studies; academic research background in immunology, former instructor at U Mass Medical Center 4
PRTX - 100 Background Highly purified Staphylococcus aureus protein A (SPA) SPA is a superantigen ▪ SPA acts as an immunological “disguise” and increases bacterial virulence ▪ Purified SPA binds antibodies, forming discrete immune complexes, and immune cells of monocyte lineage, attenuating their inflammatory activity ( MacClellan , et al. 2011) Demonstrated activity in cellular and animal models of disease 5 Protalex , Inc.
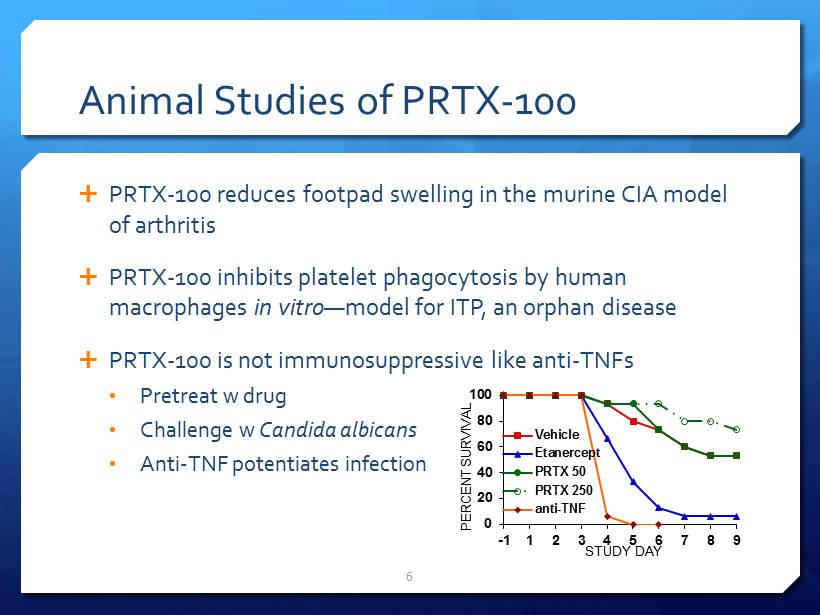
Animal Studies of PRTX - 100 PRTX - 100 reduces footpad swelling in the murine CIA model of arthritis PRTX - 100 inhibits platelet phagocytosis by human macrophages in vitro — model for ITP, an orphan disease PRTX - 100 is not immunosuppressive like anti - TNFs • Pretreat w drug • Challenge w Candida albicans • Anti - TNF potentiates infection 6 0 20 40 60 80 100 -1 1 2 3 4 5 6 7 8 9 PERCENT SURVIVAL STUDY DAY Vehicle Etanercept PRTX 50 PRTX 250 anti-TNF
Autoimmune Disease Market Opportunity RA biologics current market value exceeds $18 B and is growing RA market disruptions envisioned ▪ PFE’s Xeljanz ( tofacitinib ) oral drug recently approved ▪ Biosimilars to anti - TNFs and anti - CD20s in development Current biologics are expensive to produce and have limited use outside of US, Europe, and Japan; our cost per treatment is thousands - fold less, enabling a global strategy Other autoimmune diseases represent highly underserved indications with potential for orphan drug designation 7 Protalex , Inc .

PRTX - 100 Clinical Experience 2005 – IND filed for RA 2006 – Phase 1 study completed 2007 – Second Phase 1 using PRTX - 100 with improved production/CMC processes 2010 - 11 – Phase 1b Study (PRTX - 100 - 103) in South Africa; presented at ACR Annual Meeting in November, 2012 November 2012 – Second Phase 1b Study (PRTX - 100 - 104 ), initiated in US June 2014 – Topline results of unblinded analysis of cohorts 1 through 4 of PRTX - 100 - 104 8 Protalex , Inc.
PRTX - 100 - 103: Study Objectives Primary ▪ Assess safety and tolerability of iv PRTX - 100 weekly x 4 doses Secondary ▪ Assess immunogenicity after ≥3 doses ▪ Determine PK and estimate of PRTX - 100 plasma exposure after first and fourth dose ▪ Determine whether a relationship exists between immunogenicity of PRTX - 100 and safety and PK ▪ Assess effect of PRTX - 100 on measures of disease activity, e.g., DAS28 - CRP and CDAI 9 Protalex , Inc.
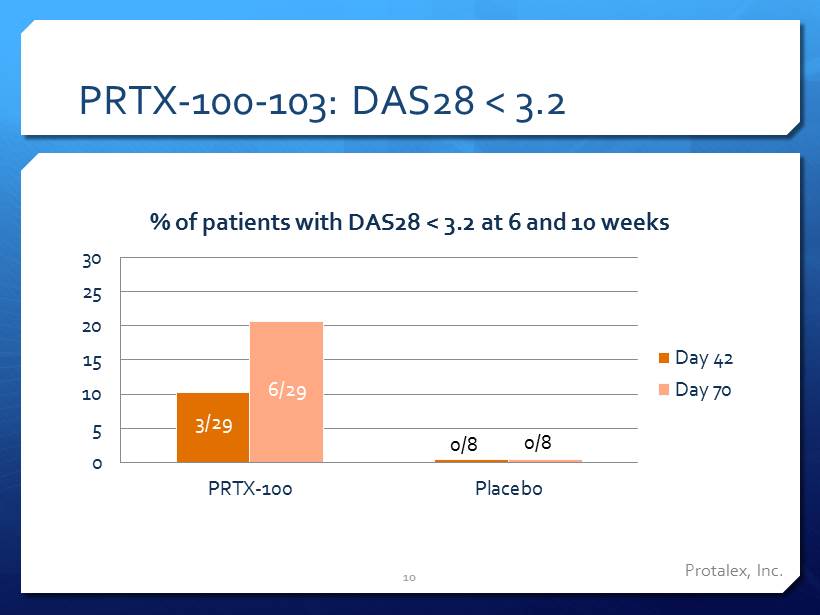
PRTX - 100 - 103: DAS28 < 3.2 0 5 10 15 20 25 30 PRTX-100 Placebo % of patients with DAS28 < 3.2 at 6 and 10 weeks Day 42 Day 70 3/29 6/29 0/8 0/8 10 Protalex , Inc.
PRTX - 100 - 103: Summary PRTX - 100 was well tolerated ▪ 3 mild to moderate infusion reactions, no SAEs related to study drug ▪ Anti - PRTX - 100 antibodies elicited in majority of patients but neither incidence nor titer was related to dose ▪ Patients with antibody response showed increased clearance without increase in AEs. Antibodies do not appear to preclude treatment response Relationship between dose and C max was linear but clearance and AUC were variable The higher doses of PRTX - 100 resulted in low disease activity, with maximal improvement at 10 weeks after the first dose 11 Protalex , Inc.
PRTX - 100 - 104 : Overview Multi - center US study initiated 4Q12. Phase 1b randomized, multiple - dose, placebo - controlled, dose - escalation study of PRTX - 100 in adults with active RA on MTX 41 patients dosed in four dose - escalating cohorts, starting at 1.50 m g/kg, with fifth cohort to investigate extended dosing schedule Primary objective: safety and tolerability of PRTX - 100 administered by iv injections over five weeks Secondary objectives include determining effects on measures of disease activity, assessing immunogenicity, evaluating PK, and investigating durability of response Enrollment commenced November 2012 in the US; last dose of cohorts one through four completed July 2013; expansion cohorts completed September 2013; cohort 5 initiated October 2013, last dose expected August 2014, topline cohort 5 data 4Q14 Topline data for cohorts 1 through 4 announced June 3, 2014 12 Protalex , Inc .
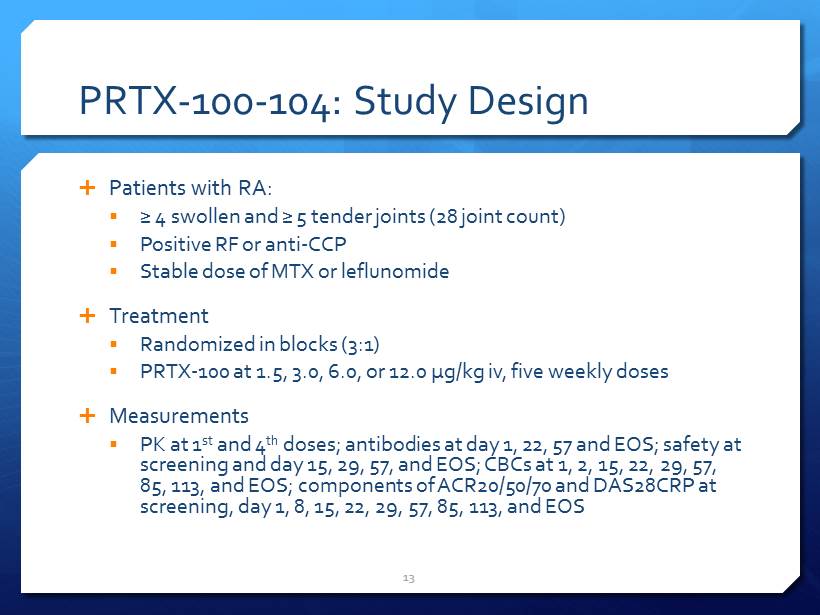
PRTX - 100 - 104: Study Design Patients with RA: ▪ ≥ 4 swollen and ≥ 5 tender joints (28 joint count) ▪ Positive RF or anti - CCP ▪ Stable dose of MTX or leflunomide Treatment ▪ Randomized in blocks (3:1) ▪ PRTX - 100 at 1.5, 3.0, 6.0, or 12.0 µg/kg iv, five weekly doses Measurements ▪ PK at 1 st and 4 th doses; antibodies at day 1, 22, 57 and EOS; safety at screening and day 15, 29, 57, and EOS; CBCs at 1, 2, 15, 22, 29, 57, 85, 113, and EOS; components of ACR20/50/70 and DAS28CRP at screening, day 1, 8, 15, 22, 29, 57, 85, 113, and EOS 13
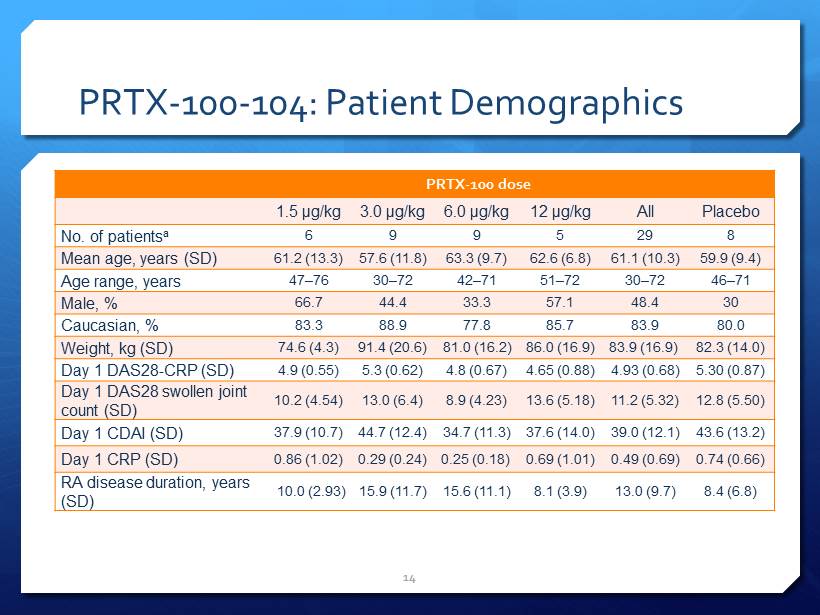
PRTX - 100 - 104: Patient Demographics 14 PRTX - 100 dose 1.5 µg/kg 3.0 µg/kg 6.0 µg/kg 12 µg/kg All Placebo No. of patients a 6 9 9 5 29 8 Mean age, years (SD) 61.2 (13.3) 57.6 (11.8) 63.3 (9.7) 62.6 (6.8) 61.1 (10.3) 59.9 (9.4) Age range, years 47 – 76 30 – 72 42 – 71 51 – 72 30 – 72 46 – 71 Male, % 66.7 44.4 33.3 57.1 48.4 30 Caucasian, % 83.3 88.9 77.8 85.7 83.9 80.0 Weight, kg (SD) 74.6 (4.3) 91.4 (20.6) 81.0 (16.2) 86.0 (16.9) 83.9 (16.9) 82.3 (14.0) Day 1 DAS28 - CRP (SD) 4.9 (0.55) 5.3 (0.62) 4.8 (0.67) 4.65 (0.88) 4.93 (0.68) 5.30 (0.87) Day 1 DAS28 swollen joint count (SD) 10.2 (4.54) 13.0 (6.4) 8.9 (4.23) 13.6 (5.18) 11.2 (5.32) 12.8 (5.50) Day 1 CDAI (SD) 37.9 (10.7) 44.7 (12.4) 34.7 (11.3) 37.6 (14.0) 39.0 (12.1) 43.6 (13.2) Day 1 CRP (SD) 0.86 (1.02) 0.29 (0.24) 0.25 (0.18) 0.69 (1.01) 0.49 (0.69) 0.74 (0.66) RA disease duration, years (SD) 10.0 (2.93) 15.9 (11.7) 15.6 (11.1) 8.1 (3.9) 13.0 (9.7) 8.4 (6.8)
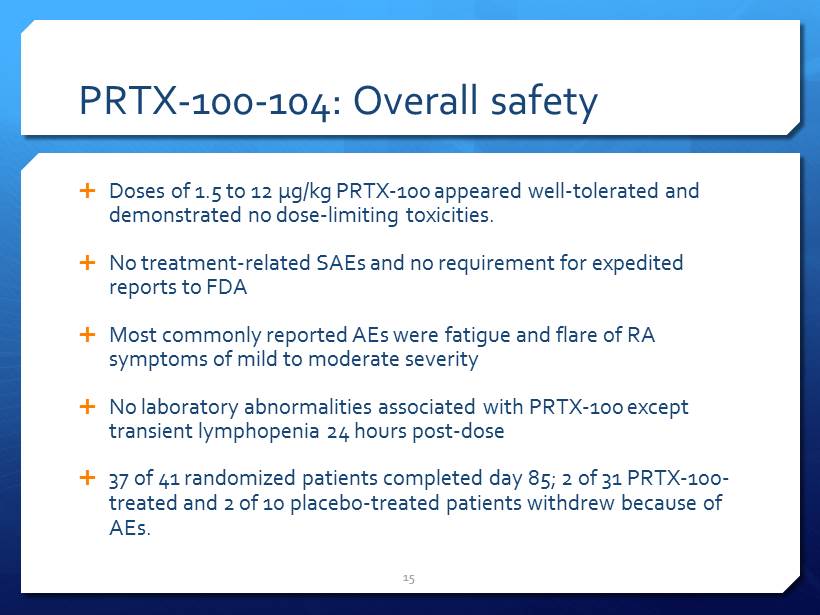
PRTX - 100 - 104: Overall safety Doses of 1.5 to 12 µg/kg PRTX - 100 appeared well - tolerated and demonstrated no dose - limiting toxicities. No treatment - related SAEs and no requirement for expedited reports to FDA Most commonly reported AEs were fatigue and flare of RA symptoms of mild to moderate severity No laboratory abnormalities associated with PRTX - 100 except transient lymphopenia 24 hours post - dose 37 of 41 randomized patients completed day 85; 2 of 31 PRTX - 100 - treated and 2 of 10 placebo - treated patients withdrew because of AEs. 15
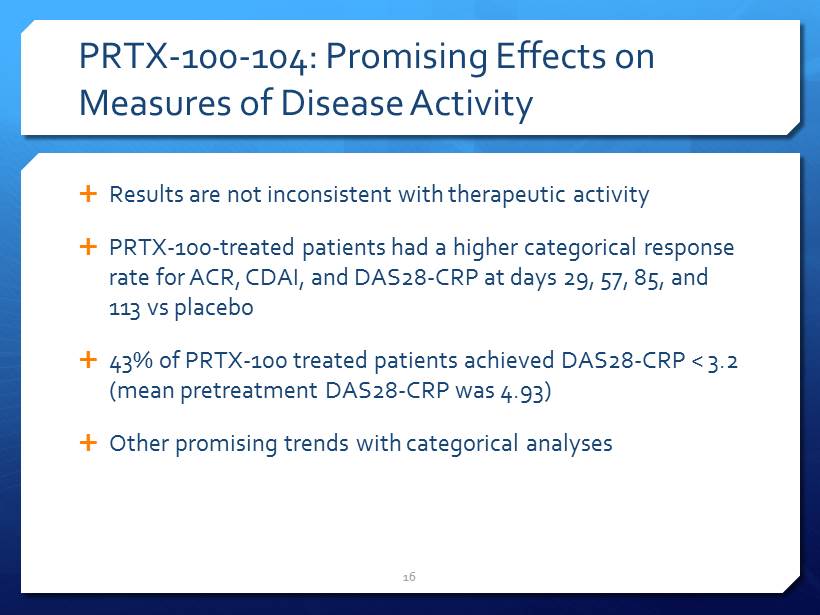
PRTX - 100 - 104: Promising Effects on Measures of Disease Activity Results are not inconsistent with therapeutic activity PRTX - 100 - treated patients had a higher categorical response rate for ACR, CDAI, and DAS28 - CRP at days 29, 57, 85, and 113 vs placebo 43% of PRTX - 100 treated patients achieved DAS28 - CRP < 3.2 (mean pretreatment DAS28 - CRP was 4.93) Other promising trends with categorical analyses 16
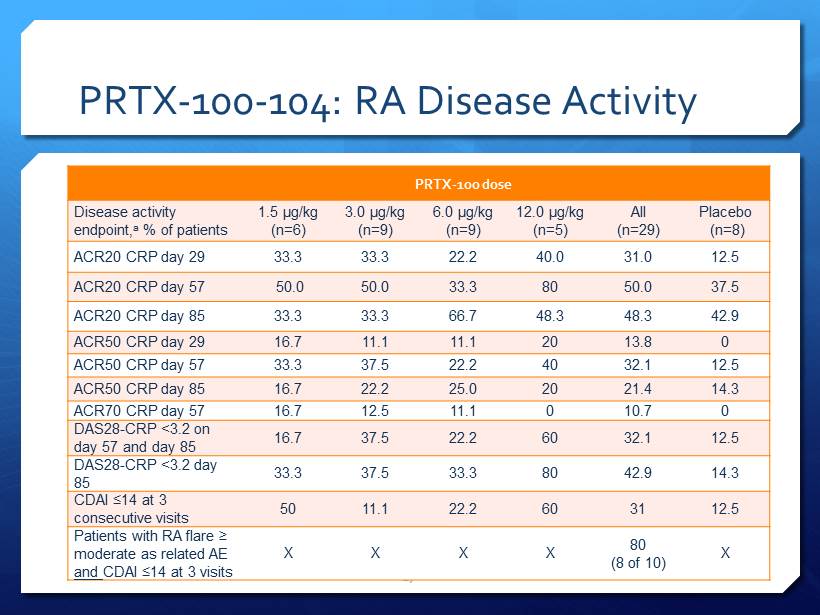
PRTX - 100 - 104: RA Disease Activity 17 PRTX - 100 dose Disease activity endpoint, a % of patients 1.5 µg/kg (n=6) 3.0 µg/kg (n=9) 6.0 µg/kg (n=9) 12.0 µg/kg (n=5) All (n=29) Placebo ( n =8) ACR20 CRP day 29 33.3 33.3 22.2 40.0 31.0 12.5 ACR20 CRP day 57 50.0 50.0 33.3 80 50.0 37.5 ACR20 CRP day 85 33.3 33.3 66.7 48.3 48.3 42.9 ACR50 CRP day 29 16.7 11.1 11.1 20 13.8 0 ACR50 CRP day 57 33.3 37.5 22.2 40 32.1 12.5 ACR50 CRP day 85 16.7 22.2 25.0 20 21.4 14.3 ACR70 CRP day 57 16.7 12.5 11.1 0 10.7 0 DAS28 - CRP <3.2 on day 57 and day 85 16.7 37.5 22.2 60 32.1 12.5 DAS28 - CRP <3.2 day 85 33.3 37.5 33.3 80 42.9 14.3 CDAI ≤14 at 3 consecutive visits 50 11.1 22.2 60 31 12.5 Patients with RA flare ≥ moderate as related AE and CDAI ≤14 at 3 visits X X X X 80 (8 of 10) X
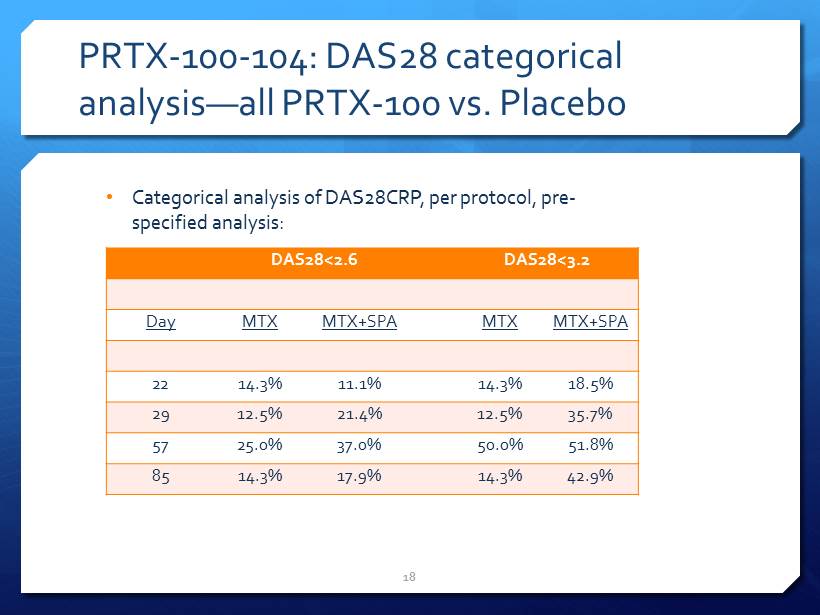
PRTX - 100 - 104: DAS28 categorical analysis — all PRTX - 100 vs. Placebo 18 DAS28<2.6 DAS28<3.2 Day MTX MTX+SPA MTX MTX+SPA 22 14.3% 11.1% 14.3% 18.5% 29 12.5% 21.4% 12.5% 35.7% 57 25.0% 37.0% 50.0% 51.8% 85 14.3% 17.9% 14.3% 42.9% • Categorical analysis of DAS28CRP, per protocol, pre - specified analysis:
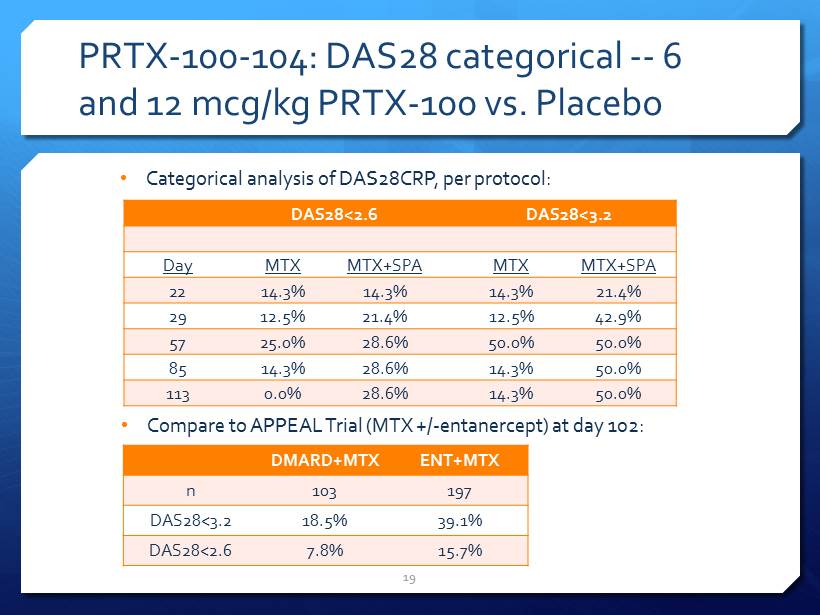
PRTX - 100 - 104: DAS28 categorical -- 6 and 12 mcg/kg PRTX - 100 vs. Placebo 19 DAS28<2.6 DAS28<3.2 Day MTX MTX+SPA MTX MTX+SPA 22 14.3% 14.3% 14.3% 21.4% 29 12.5% 21.4% 12.5% 42.9% 57 25.0% 28.6% 50.0% 50.0% 85 14.3% 28.6% 14.3% 50.0% 113 0.0% 28.6% 14.3% 50.0% • Categorical analysis of DAS28CRP, per protocol: • Compare to APPEAL Trial (MTX +/ - entanercept ) at day 102: DMARD+MTX ENT+MTX n 103 197 DAS28<3.2 18.5% 39.1% DAS28<2.6 7.8% 15.7%
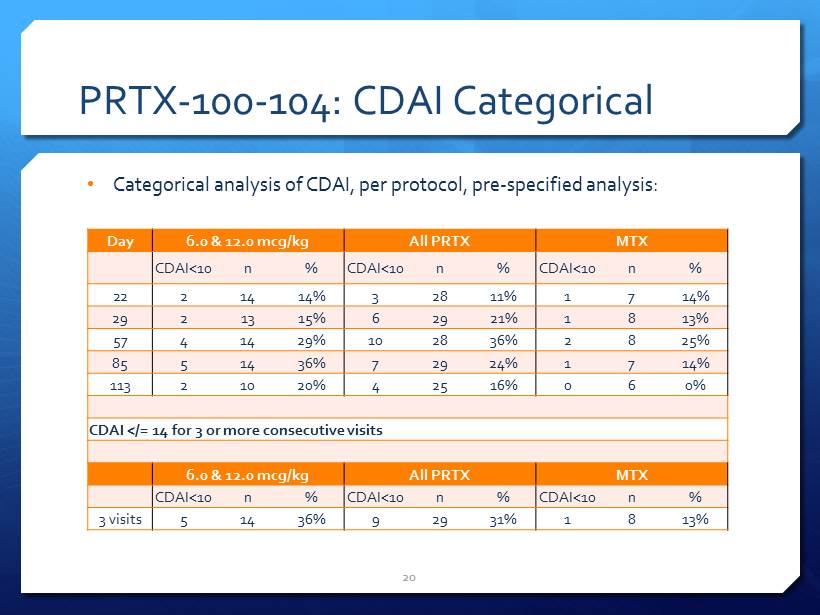
PRTX - 100 - 104: CDAI Categorical 20 Day 6.0 & 12.0 mcg/kg All PRTX MTX CDAI <10 n % CDAI <10 n % CDAI <10 n % 22 2 14 14% 3 28 11% 1 7 14% 29 2 13 15% 6 29 21% 1 8 13% 57 4 14 29% 10 28 36% 2 8 25% 85 5 14 36% 7 29 24% 1 7 14% 113 2 10 20% 4 25 16% 0 6 0% CDAI </= 14 for 3 or more consecutive visits 6.0 & 12.0 mcg/kg All PRTX MTX CDAI <10 n % CDAI <10 n % CDAI <10 n % 3 visits 5 14 36% 9 29 31% 1 8 13% • Categorical analysis of CDAI, per protocol, pre - specified analysis:
Potential of PRTX - 100 as treatment for Immune Thrombocytopenia (ITP) Unmet needs exist in ITP ▪ Immune mediated therapies that do not increase risk of infection ▪ Therapies that provide durable responses without continuous treatment ▪ Therapies that are effective in refractory ITP ▪ More convenient therapies ( i.e., no food restrictions, less frequent dosing) Efficient clinical development ▪ Rapid & objective response (platelet counts) for primary endpoint ▪ Attractive cost of clinical development as phase 3 program may require only a few hundred patients ▪ Limited competition for clinical trial patients Attractive commercial market that is growing 21
PRTX - 100 in ITP: Study Overview A proposed Phase 1/2, Open - Label, Single Arm Dose Ranging Study Patient Population: ▪ Adult patients with persistent/chronic immune thrombocytopenia (ITP) ▪ Failed at least 1 prior ITP treatment ▪ Platelet count < 30,000/µL including patients on corticosteroid, immune - suppressive medications, or a TPO - RA Doses being tested ▪ 125, 250, 500, 1000, and 2000 µg Treatment: 4 weekly doses followed by 2 monthly doses for total of 3 months treatment Additional cohort dosing Day 1 & Day 3 in the 1 st week to look for accelerated response 22
Patents and Intellectual Property Patents (five issued in US and one in Japan) ▪ Initial US patent 7,211,258, “Protein A compositions and methods of use” filed 2002 and issued 2007 for RA, juvenile RA, and systemic lupus erythematosus ▪ Continuation patents expanding use were issued for: o ITP or autoimmune TP in 2008 o Acute inflammatory response or inflammation in 2012 o Psoriasis and scleroderma in 2012 o MS in 2013 ▪ Japanese patent issued with 2023 expiration date o April 2014 notice of allowance for psoriasis, scleroderma, Crohn’s Disease ▪ Additional patent applications pending in Europe, Canada, Japan, and US Other Intellectual Property ▪ Considerable know - how in the manufacture and QA of highly purified SPA expected to remain trade secret 23 Protalex , Inc.
Protalex Objectives x 2Q13 Safety data from first three cohorts of PRTX - 100 - 104 x 3Q13 Initiation of cohort 5 extension study, to investigate monthly maintenance doses x 2Q14 Top - line results of PRTX - 100 - 104 trial □ 3Q14 Filing IND for PRTX - 100 in orphan indication □ 4Q14 Top - line results from cohort 5 24 Protalex , Inc .
Protalex Investment Highlights PRTX - 100 is a novel immunomodulatory biological drug candidate that may be useful in the treatment of a variety of autoimmune diseases PRTX - 100 is safe and well - tolerated in humans Validated manufacturing process; low cost of goods relative to other biologics Strong and growing IP position Potential efficacy in a number of orphan disease indications, with new IND to be filed 3Q14 Proof - of - concept data from ongoing phase 1b trial in rheumatoid arthritis patients announced June 2014 25 Protalex , Inc.
























