
2013 Advaxis, Inc. | OTCQB:ADXS Ticker: ADXS September 2013 Corporate Presentation Empowering the immune system from within™

2013 Advaxis, Inc. | OTCQB:ADXS Forward Looking Statements This presentation contains forward - looking statements, including, but not limited to: statements as to the anticipated timing of clinical studies and other business developments, statements as to the development of new constructs, expectations as to the adequacy of our cash balances to support our operations for specified periods of time and as to the nature and level of cash expenditures, expectations as to market opportunities, our ability to take advantage of those opportunities, and the risk factors set forth from time to time in Advaxis' SEC filings, including but not limited to its report on Form 10 - K for the fiscal year ended October 31, 2012, available at http://www.sec.gov. The Company undertakes no obligation to publicly release the result of any revision to these forward - looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law. You are cautioned not to place undue reliance on any forward - looking statements. 2

2013 Advaxis, Inc. | OTCQB:ADXS Experienced Management Team & Board of Directors Management Team Daniel J. O ’ Connor, Esq. President and Chief Executive Officer ▪ 15 years of executive, legal, regulatory, compliance, manufacturing and quality experience in the biopharmaceutical industry ▪ Former Senior VP and General Counsel of ImClone Systems Incorporated ▪ Played a key role in development, licensing and commercialization of Erbitux ® and was the executive leader who enabled the company to be sold to Eli Lilly in 2008 for $6.5B Robert Petit, Ph.D. Executive Vice President and Chief Scientific Officer ▪ 25 years experience in oncology drug development ▪ U.S. medical strategy lead for Yervoy ® program at Bristol Myers Squibb (NYSE: BMY) as the Director of Medical Strategy for oncology products and Director of Global Clinical Research ▪ VP of Clinical Development at MGI Pharma and Aesgen, Inc. Mark Rosenblum Chief Financial Officer ▪ 25 years experience in accounting and financial leadership. VP, Chief Accounting Officer of Wellman, Inc., a $1.2B chemical company; CFO and Secretary, HemoBioTech, Inc. Chris French Vice President, Regulatory and Medical Affairs ▪ 20 years of research and pharmaceutical experience in drug development ▪ Management positions in medical affairs, regulatory affairs, business development and scientific communications, ▪ U.S. Director of Oncology Scientific Communications, Bristol Myers Squibb., Senior Director, MGI Pharma and VP Regulatory and Scientific Affairs, Aesgen. 3 Board of Directors David Sidransky, MD ▪ Co - Founder & Chairman, Champions Oncology ▪ Professor, Johns Hopkins, Oncology Medicine James Patton, MD, MBA, Chairman ▪ VP, Millennium Oncology Management ▪ Founder & Chairman, VAL Health Roni A. Appel ▪ Managing Director, LibertyView Equity Partners Richard Berman ▪ Former CEO, Easylink Services ▪ former SVP, Bankers Trust Company, ▪ Director, Lustros, Inc., and Neostem, Inc. Thomas McKearn, MD ▪ Founder, Cytogen Corporation Thomas A. Moore ▪ Former Chairman & CEO, Advaxis Daniel J. O ’ Connor, Esq. ▪ President & CEO, Advaxis

2013 Advaxis, Inc. | OTCQB:ADXS Investment Highlights Immunotherapy company with cutting - edge, proprietary platform technology in the hottest area of oncology 4 Lead drug candidate, ADXS - HPV (ADXS11 - 001), targets HPV - associated cancers with multiple registration opportunities Comprehensive, single, well - tolerated easy to manufacture and administer immunotherapy ▪ Cervical cancer program advancing to registration studies ▪ Anal Cancer Phase 1 ongoing ▪ Head and Neck Cancer Phase 1 ongoing 1 Orphan Drug Designation Granted 2 Orphan Drug Designations Applications Pending ▪ Anal cancer - Granted ▪ Invasive cervical cancer - Pending ▪ Head & neck cancer - Pending Broad immunotherapy - focused IP, discovery platform and pipeline Experienced management team and board of directors 41 patents issued and 35 pending patent applications ADXS - HPV preliminary Phase 2 Best Response Data: 6 complete responses, 6 partial responses, and 33 stable disease in 110 patients
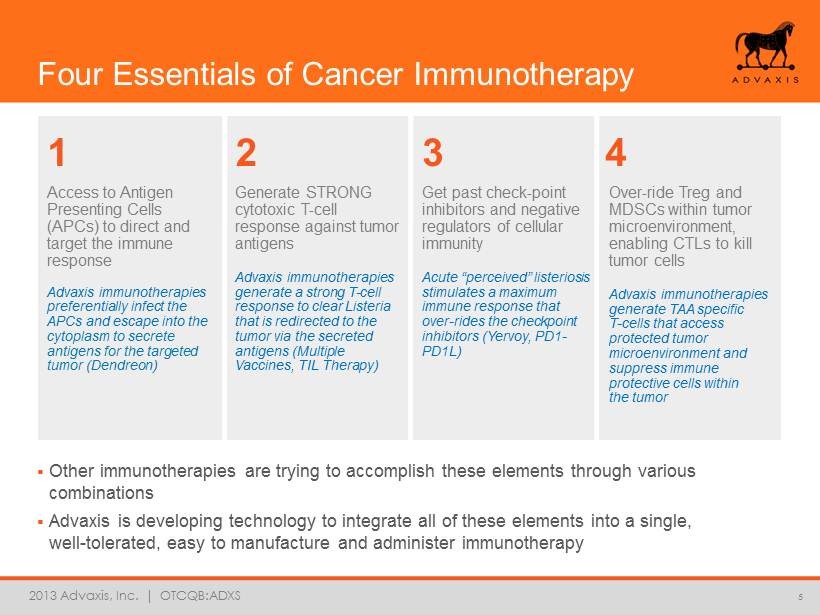
2013 Advaxis, Inc. | OTCQB:ADXS Four Essentials of Cancer Immunotherapy 5 Access to Antigen Presenting Cells (APCs) to direct and target the immune response Advaxis immunotherapies preferentially infect the APCs and escape into the cytoplasm to secrete antigens for the targeted tumor (Dendreon) ▪ Other immunotherapies are trying to accomplish these elements through various combinations ▪ Advaxis is developing technology to integrate all of these elements into a single, well - tolerated, easy to manufacture and administer immunotherapy 1 2 3 4 Generate STRONG cytotoxic T - cell response against tumor antigens Advaxis immunotherapies generate a strong T - cell response to clear Listeria that is redirected to the tumor via the secreted antigens (Multiple Vaccines, TIL Therapy) Get past check - point inhibitors and negative regulators of cellular immunity Acute “ perceived ” listeriosis stimulates a maximum immune response that over - rides the checkpoint inhibitors (Yervoy, PD1 - PD1L) Over - ride Treg and MDSCs within tumor microenvironment, enabling CTLs to kill tumor cells Advaxis immunotherapies generate TAA specific T - cells that access protected tumor microenvironment and suppress immune protective cells within the tumor

2013 Advaxis, Inc. | OTCQB:ADXS Advaxis Approach: 4 Elements in 1 6 Access APC, Secrete LLO - TAA, MHC1 CD8 T cell Expansion, Pass Checkpoints Chemokines, Tumor Infiltration Dec. Treg, MDSCs, Tumor Cell Lysis 1 2 3 4

2013 Advaxis, Inc. | OTCQB:ADXS Product Candidate Pre Phase 1 Phase 2 Phase 3 ADXS - HPV 1 ADXS - HPV 2 ADXS - HPV 3 ADXS - PSA ADXS - cHER2 Veterinary Program ADXS - cHER2 Canine Osteosarcoma Cervical Cancer, India Head & Neck Cancer Anal Cancer Prostate Cancer Breast Cancer Proprietary Technology Platform Fuels Robust Clinical Pipeline 7 1. Gynecologic Oncology Group is conducting a P2 study in this patient population in the U.S. 2. This study is being sponsored and conducted by Cancer Research UK 3. This study is being sponsored and conducted by Brown University Oncology Group

2013 Advaxis, Inc. | OTCQB:ADXS Advaxis Platform Yields Numerous Product Candidates ▪ Lm can secrete extracellular, intracellular or transmembrane regions ▪ Lm can express proteins with different functions such as enzymes, receptors, transcription factors, etc. ▪ Chimeric molecules can be created by the fusion of epitope rich fragments 8 Lm - LLO Immunotherapy Tumor Antigen Tumor model Reference ADXS11 - 001 HPV16 - E7 TC - 1 Gunn et al 2001 ADXS31 - 142 Prostate Specific antigen TRAMPC1 /PSA Shahabi et al. 2008, Wallecha et al. 2009 ADXS31 - 164 Her2/neu Chimera NT - 2 Breast/Transgenic Her2 Seavey et al 2009 Shahabi et al 2011 Lm - LLO - HMW - MAA HMW - MAA, C - terminus fragment NT - 2 Breast/Transgenic Her2/B16F10 - HMW - MAA Maciag et al 2008 Lm - LLO - ISG15 ISG15 4T1 breast tumor model Wood et al 2012 Lm - LLO - CD105 Endoglin NT - 2 Breast/Transgenic Her2 Wood et al 2011 Lm - LLO - flk VEGF NT - 2 Breast/Transgenic Her2 Seavey et al 2009 Bivalent Therapy Her - 2 - chimera/HMW - MAA - C NT - 2 Breast/Transgenic Her2 Ongoing

2013 Advaxis, Inc. | OTCQB:ADXS Multiple Phase 1/2 Clinical Trials ▪ Safety: predominately cytokine - release syndrome - Grade 1/2 transient, non - cumulative that respond to symptomatic treatment or self resolve (<3.0% related SAEs) ▪ Efficacy: improved survival, complete responses, partial responses, alone or in combination with chemotherapy in recurrent/refractory cervical cancer ▪ Activity in multiple high risk HPV strains Over 500 doses in over 200 patients and increasing *results published, ** preliminary data reported *** Q4 2013 9 Phase 1 refractory cervical cancer (15 patients)* Phase 1/2 head and neck cancer (27 patients) Phase 2 refractory cervical cancer (110 patients)** Phase 1/2 head and neck cancer (expecting 25 patients), pending IRB approval*** Phase 2 refractory cervical cancer (67 patients) Phase 1/2 anal cancer (25 patients)

2013 Advaxis, Inc. | OTCQB:ADXS ADXS - HPV – A Targeted Therapy with Multiple Registration Opportunities Immunotherapy targeting HPV - associated cancers HPV - associated cancers include: invasive cervical, HPV+ head & neck, anal, penile, vulvar, and vaginal ▪ Single agent activity observed in recurrent cervical cancer: preliminary data suggest: ▪ Improved survival and objective tumor responses including complete responses, partial responses, and stable disease ▪ Well - tolerated safety profile ▪ Exclusive world - wide rights licensed to Advaxis ▪ Applied for Orphan Drug Designation in 3 indications ▪ Anal Cancer (granted); pending in invasive cervical and head & neck cancers 10

2013 Advaxis, Inc. | OTCQB:ADXS Growth in HPV - associated Cancers Increases Global Opportunity for ADXS - HPV ▪ HPV has a causative role in 5% of cancers worldwide ▪ 80% of sexually active Americans will have contracted at least one strain of HPV by age 50 per the CDC ▪ Current vaccines prevent infection by 2 (out of 15) oncogenic HPV strains but have no effect on the millions that are already infected ▪ Vaccination has plateaued at 32% in the US 11 ~80,000 Head & Neck ~527,000 Cervical ~99,000 Anal ~27,000 Vulvar ~86,000 Penile ~13,000 Vaginal WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre). Human Papillomavirus and Related Cancers in World. Summary Report 2010.

2013 Advaxis, Inc. | OTCQB:ADXS Confidential discussions with multiple partners for the licensing of immunotherapies Licensing of ADXS - HPV Opportunistic Business Development Strategy Initially Focusing on High Prevalence Regions 12 Asia ▪ Signed MoU for commercialization in Asia ▪ Clinical strategy to conduct pivotal program for cervical cancer Licensing of ADXS - cHER2 Diligence underway with animal health divisions of several major pharmaceutical companies Strategic markets with high HPV prevalence ▪ Established commercial terms for the exclusive license of ADXS - HPV

2013 Advaxis, Inc. | OTCQB:ADXS ADXS - HPV Phase 2 Study Overview ▪ Multi - center Phase 2 trial conducted in India ▪ Recurrent cervical cancer patients ▪ Failed previous treatments, ECOG 0 - 2, majority with aggressive disease ▪ Single agent activity with a single cycle of ADXS - HPV (3 doses) ▪ Apparent survival benefit and objective tumor responses (CR/PR) in recurrent patients with poor prognosis ▪ Well - tolerated ▪ 59% patients reported no Aes related/possibly related to ADXS - HPV; 41% reported only Grade 1 or 2; <2% reported Grade 3 ▪ Predominately cytokine - release syndrome associated with infusion - Grade 1/2 transient, non - cumulative toxicities that self - resolved or responded to symptomatic treatment 13

2013 Advaxis, Inc. | OTCQB:ADXS 3m 6m 9m 12m 18m Phase 2 Cervical Cancer ADXS - HPV +/ - Cisplatin Study Design 14 Recurrent/Refractory Cervical (N = 110) Arm A N = 55 ADXS Only Arm B N = 55 ADXS+Cisplatin Treatment Follow - up Phase Scans ADXS ADXS ADXS ADXS ADXS ADXS 3m 6m 9m 12m 18m R ADXS C C C C C 1x10 9 cfu x3 on days 0, 28, 56 as an 80 ml infusion over 15 minutes ADXS11 - 001 = 1x10 9 CFU as an 80 ml infusion over 15 minutes on days 0, 88, 106, 134 Cisplatin = 40 mg/m 2 x5 weekly on days 30, 37, 44, 51, 58

2013 Advaxis, Inc. | OTCQB:ADXS -100% -75% -50% -25% 0% 25% 50% 75% 100% 125% 150% 121006 100008 128001 124011 116007 111005 104006 109009 105007 111001 121001 107006 101007 107003 107007 110010 107005 113001 110016 107008 109004 116003 115004 112005 128002 110008 110012 113010 104001 100019 104003 107009 116006 100004 110004 103009 110003 121007 100011 105009 107002 100015 103015 121002 101008 104005 118001 115008 103011 101006 111002 110007 103003 100012 103017 103008 103012 101001 110002 103010 103014 110009 111006 115005 119005 ADXS ADXS/CIS N =65 ADXS - HPV Phase 2 Best Response Data: (as of May 17, 2013) Tumor reduction observed in patients infected with different high risk HPV strains including HPV 16, 18, 31, 33 and 45, as expected. 15 6 Complete Responses 6 Partial Responses 33 Stable Disease

2013 Advaxis, Inc. | OTCQB:ADXS Patient 110 - 002: Major Tumors Eliminated 16 Resolution of lung metastasis on CT Resolution of liver metastasis on CT Resolution of para - aortic lymph node metastasis on CT* Patient # First Line Tx Stage Tx Arm Tumor Burden (mm) Tumor Decrease Baseline 3 mo. 6 mo. 9 mo. 12 mo. 18 mo. 110 - 002 RT IVB ADXS + CIS 284 84 56 34 20 36 93% *5 other lymph nodes resolved Initial Screening 9 Months ▪ Patient 110 - 002 enrolled with 284mm (sum of linear measures) of disease at 10 sites, including liver, lung, and peri - aortic nodes. The patient was previously treated with surgery and radiation (EBRTx25), and recurred within 1 year with metastatic disease. She was randomized to receive ADXS/Cis. At 3 months, she had 84mm of tumor at 5 sites, at 6 months 56mm at 3 sites, at 9 months 34mm at 2 sites, and at 12 months 20mm in a single peri - aortic node not amenable to biopsy.
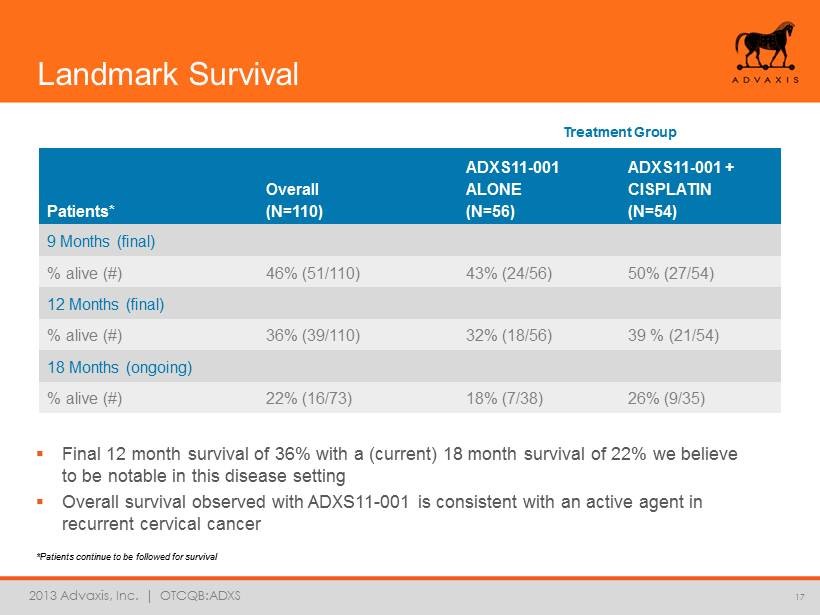
2013 Advaxis, Inc. | OTCQB:ADXS *Patients continue to be followed for survival 17 ▪ Final 12 month survival of 36% with a (current) 18 month survival of 22% we believe to be notable in this disease setting ▪ Overall survival observed with ADXS11 - 001 is consistent with an active agent in recurrent cervical cancer Treatment Group Patients* Overall (N=110) ADXS11 - 001 ALONE (N=56) ADXS11 - 001 + CISPLATIN (N=54) 9 Months (final) % alive (#) 46% (51/110) 43% (24/56) 50% (27/54) 12 Months (final) % alive (#) 36% (39/110) 32% (18/56) 39 % (21/54) 18 Months (ongoing) % alive (#) 22% (16/73) 18% (7/38) 26% (9/35) Landmark Survival

2013 Advaxis, Inc. | OTCQB:ADXS Trial Regimen N PS (%) 1 st line Prior Chem Prior RT %SAE Reported % CR Med. Surv. Resp. Durat. 12 Mo. Surv. 18 Mo. Surv. 24 Mo. Surv. Moore 2004 Cisplatin 134 0 - 2 (48/44/8) 6% 24% 91% 134% 0 deaths 8% 9M 4.5M ~36% ~12% ~5% Cis+ Taxol 130 (45/42/13) 177% 0 deaths 15% ~32% ~21% ~16% Monk 2009 Cis + Taxol 103 0 - 1 (55/45) 16 % 70% 86% 364% 2 deaths 3% 12M 5M ~50% ~36% ~15% Tewari 2013 GOG 240 (ASCO Plenary #3) Cis + Taxol 114 0 - 1 (58/42) 17 % 0% 83% ~372% 4 deaths 15% 14.3M 5M ~53% ~37% ~22% Cis + Taxol + Bev 115 4 deaths 28% 17.0M 7M ~62% ~46% ~34% Prognostic Factors for Overall Survival in Cervical Cancer ▪ Most important prognostic factors for overall survival and response rate are: ▪ ECOG performance status, ▪ Number of prior therapies, ▪ Interval from initial therapy to time of recurrence, and ▪ Local recurrence vs. distant metastases* 18 * Monk 2009, JCO

2013 Advaxis, Inc. | OTCQB:ADXS Next steps towards registration in this highly aggressive malignancy ▪ Report final results from Phase 2 study in India at SITC annual meeting in November ▪ Orphan designation pending ▪ Conduct Phase 1/2 study with high dose, immunology endpoints and repeat cycles ▪ Conduct EOP2 meeting with FDA; draft Phase 3 protocols; submit SPA ▪ Complete Gynecologic Oncology Group (GOG) NCI Phase 2 study of ADXS - HPV in 67 patients with recurrent/refractory cervical cancer ▪ Conduct 2 pivotal Phase 3 trials Rationale for registering ADXS - HPV in cervical cancer ▪ 99% caused by HPV ▪ ADXS - HPV (single course) induced CR s in already treated cervical cancer patients ▪ Exclusive world - wide rights A highly aggressive malignancy ▪ Poor prognosis ▪ No standard of care ▪ Traditional cancer therapy ineffective Registration Opportunity for ADXS - HPV in Cervical Cancer 19
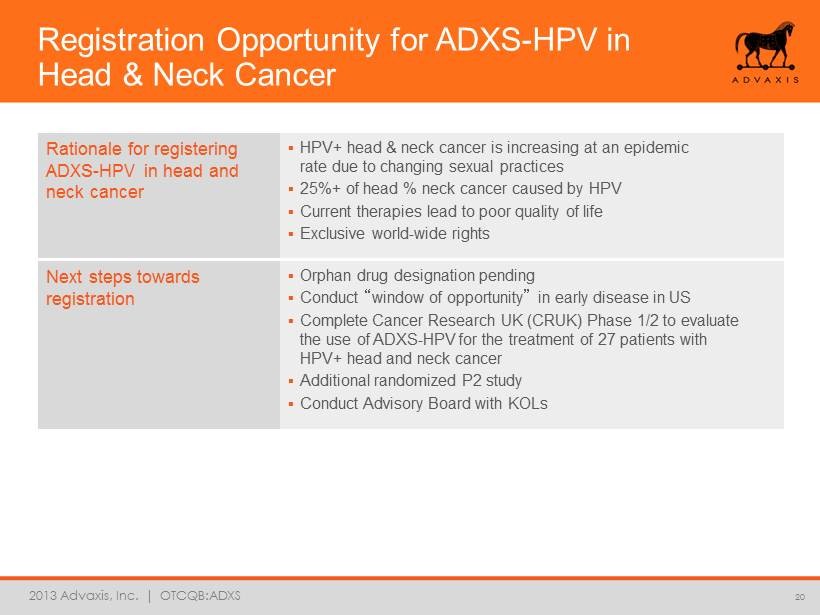
2013 Advaxis, Inc. | OTCQB:ADXS Registration Opportunity for ADXS - HPV in Head & Neck Cancer 20 Rationale for registering ADXS - HPV in head and neck cancer ▪ HPV+ head & neck cancer is increasing at an epidemic rate due to changing sexual practices ▪ 25%+ of head % neck cancer caused by HPV ▪ Current therapies lead to poor quality of life ▪ Exclusive world - wide rights Next steps towards registration ▪ Orphan drug designation pending ▪ Conduct “ window of opportunity ” in early disease in US ▪ Complete Cancer Research UK (CRUK) Phase 1/2 to evaluate the use of ADXS - HPV for the treatment of 27 patients with HPV+ head and neck cancer ▪ Additional randomized P2 study ▪ Conduct Advisory Board with KOLs

2013 Advaxis, Inc. | OTCQB:ADXS Registration Opportunity for ADXS - HPV in Anal Cancer Orphan designation granted 21 Rationale for registering ADXS - HPV in anal cancer ▪ 80 - 100% caused by HPV ▪ Current therapies are toxic and have long term side effects ▪ No therapy for recurrent disease ▪ Exclusive world - wide rights Next steps towards registration ▪ Discuss development plan with the FDA under ODD ▪ Complete Brown University Oncology Group (BrUOG) Phase 1/2 study of ADXS - HPV in 25 patients with HPV - associated anal cancer

2013 Advaxis, Inc. | OTCQB:ADXS ADXS - PSA for the Treatment of Prostate Cancer 22 ADXS - PSA ▪ Immunotherapy targeting cells expressing PSA ▪ Conducted a pre - IND meeting with the FDA to discuss the CMC, pharmacology, toxicology, and clinical plans for ADXS - PSA ▪ Exclusive world - wide rights Next steps ▪ Required toxicology studies completed and GMP drug product manufactured for the Phase 1 clinical study ▪ IND to be filed with the FDA for ADXS - PSA in the treatment of prostate cancer in the first half of 2014 ▪ Phase 1 study to be initiated in first half of 2014 by Advaxis in US

2013 Advaxis, Inc. | OTCQB:ADXS Immunotherapy targeting cancers overexpressing HER2, including breast cancer in addition to others ADXS - cHER2 For the Treatment of HER2 Overexpressing Cancers Veterinary Program ▪ We believe canine osteosarcoma (CO) provides excellent animal model of naturally occurring HER2 driven cancer ▪ Preliminary Phase 1 data in CO have shown encouraging survival in 9 dogs treated with ADXS - cHER2 vs. 11 untreated dogs, appearing to validate activity of the platform 23 Human Program ▪ Preliminary canine data may provide rationale to advance into human clinical trials Licensing of ADXS - cHER2 Diligence underway with animal health divisions of several major pharmaceutical companies

2013 Advaxis, Inc. | OTCQB:ADXS Key Milestones Expected to Continue to Build Momentum 24 Reporting of final Phase 2 data from India cervical cancer trial at SITC Filing IND application with the FDA for ADXS - PSA for the treatment of prostate cancer Initiation of dialogue with the FDA to establish clear path to registration for ADXS - HPV in the treatment of cervical cancer Q4 2013 H1 2014 Upcoming Response from FDA on Orphan Drug Designations for ADXS - HPV in: invasive cervical cancer and head and neck cancer

2013 Advaxis, Inc. | OTCQB:ADXS Investment Highlights Immunotherapy company with cutting - edge, proprietary platform technology in the hottest area of oncology 25 Lead drug candidate, ADXS - HPV (ADXS11 - 001), targets HPV - associated cancers with multiple registration opportunities Comprehensive, single, well - tolerated easy to manufacture and administer immunotherapy ▪ Cervical cancer program advancing to registration studies ▪ Anal Cancer Phase 1 ongoing ▪ Head and Neck Cancer Phase 1 ongoing 1 Orphan Drug Designation Granted 2 Orphan Drug Designations Applications Pending ▪ Anal cancer - Granted ▪ Invasive cervical cancer - Pending ▪ Head & neck cancer - Pending Broad immunotherapy - focused IP, discovery platform and pipeline Experienced management team and board of directors 41 patents issued and 35 pending patent applications ADXS - HPV preliminary Phase 2 Best Response Data: 6 complete responses, 6 partial responses, and 33 stable disease in 110 patients

2013 Advaxis, Inc. | OTCQB:ADXS 26 Advaxis , Inc. 305 College Road East Princeton, NJ 08540 T: 609.452.9813 ir@advaxis.com www.advaxis.com

2013 Advaxis, Inc. | OTCQB:ADXS Strong Patent Portfolio 41 patents issued ▪ Composition of matter, methods and uses covering: ▪ Live Lm ▪ Four (4) different Listeria species for human use ▪ LLO - antigen & ActA - antigen fusion proteins ▪ Delivered by Lm or stand alone ▪ Two (2) different families of adjuvant fusions ▪ Nontoxic, modified LLO 35 patents pending IP successfully defended in European Patent Court (Munich) ▪ No additional challenge permitted 27