Filed Pursuant to Rule 424(b)(5)
Registration No. 333-201826
CALCULATION OF REGISTRATION FEE
| | | | | | | | |
Title of Each Class of Securities To Be Registered | | Amount to be Registered | | Proposed Maximum Offering Price Per Share | | Proposed Maximum Aggregate Offering Price | | Amount of Registration Fee(1) |
Common stock, par value $0.001 per share | | 9,708,738 | | $5.15 | | $50,000,001 | | $5,795 |
| (1) | Calculated in accordance with Rule 456(b) and Rule 457(r) of the Securities Act. This “Calculation of Registration Fee” table shall be deemed to update the “Calculation of Registration Fee” table in the registrant’s Registration Statement on Form S-3 (File No. 333-201826). |
PROSPECTUS SUPPLEMENT
(to prospectus dated February 2, 2015)
$50,000,000

9,708,738 Shares of Common Stock
We are offering 9,708,738 shares at an offering price of $5.15 per share to one institutional investor. Our common stock is listed on The NASDAQ Capital Market under the symbol “ZIOP.” On May 11, 2017, the last reported sale price of our common stock on The NASDAQ Capital Market was $7.12 per share.
Investing in our common stock involves a high degree of risk. Please read “Risk Factors” beginning on page S-5 of this prospectus supplement, on page 10 of the accompanying prospectus and in the documents incorporated by reference into this prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | | | | | | | |
| | | PER
SHARE | | | TOTAL | |
Public offering price | | $ | 5.15 | | | $ | 50,000,001 | |
Underwriting discounts and commissions(1) | | $ | 0.2575 | | | $ | 2,500,000 | |
Proceeds to us, before expenses | | $ | 4.8925 | | | $ | 47,500,001 | |
| | (1) | See “Underwriting” beginning on page S-11 of this prospectus supplement for additional information regarding the compensation payable to the underwriter. |
Delivery of the shares of common stock is expected to be made on or about May 16, 2017.
Sole Book-Running Manager
Guggenheim Securities
Prospectus Supplement dated May 11, 2017.
TABLE OF CONTENTS
S-i
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first part is this prospectus supplement, which describes the specific terms of this common stock offering and also adds to and updates information contained in the accompanying prospectus and the documents incorporated by reference herein and therein. The second part, the accompanying prospectus, provides more general information, some of which may not apply to this offering. Generally, when we refer to this prospectus, we are referring to this prospectus supplement and the accompanying prospectus combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information contained in the accompanying prospectus or any document incorporated by reference herein or therein filed prior to the date of this prospectus supplement, you should rely on the information in this prospectus supplement; provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document incorporated by reference in the accompanying prospectus—the statement in the document having the later date modifies or supersedes the earlier statement.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date specified in the relevant agreement. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
You should rely only on the information contained in or incorporated by reference in this prospectus supplement, the accompanying prospectus and in any free writing prospectus that we have authorized for use in connection with this common stock offering. We have not, and the underwriter has not, authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the underwriter is not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference herein and therein, and in any free writing prospectus that we have authorized for use in connection with this common stock offering, is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have changed since those dates. You should read this prospectus supplement, the accompanying prospectus, the documents incorporated by reference herein and therein, and any free writing prospectus that we have authorized for use in connection with this offering, in their entirety before making an investment decision. You should also read and consider the additional information in the documents to which we have referred you in the sections of this prospectus supplement and in the accompanying prospectus entitled “Where You Can Find More Information” and “Incorporation of Information by Reference.”
We and the underwriter are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the accompanying prospectus and the offering of the common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying prospectus must inform themselves about, and observe any restrictions relating to, the offering of the common stock and the distribution of this prospectus supplement and the accompanying prospectus outside the United States. This prospectus supplement and the accompanying prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the accompanying prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
Unless otherwise stated, all references in this prospectus supplement and the accompanying prospectus to “we,” “us,” “our,” “ZIOPHARM,” the “Company” and similar designations refer to ZIOPHARM Oncology, Inc.
This prospectus supplement and the accompanying prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus supplement and the accompanying prospectus, including logos, artwork and other visual displays, may appear without the® orTM symbols, but such references are not intended to indicate, in any way, that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
S-ii
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights certain information about us, this offering and selected information contained elsewhere in or incorporated by reference in this prospectus supplement. This summary is not complete and does not contain all of the information that you should consider before deciding whether to invest in our common stock. For a more complete understanding of our company and this offering, we encourage you to read and consider carefully the more detailed information in this prospectus supplement and the accompanying prospectus, including the information referred to under the heading “Risk Factors” in this prospectus supplement beginning on page S-5, the information referred to under the heading “Risk Factors” in the accompanying prospectus beginning on page 10, the information incorporated by reference in this prospectus supplement and the accompanying prospectus, which are described under “Where You Can Find More Information” and “Incorporation of Information by Reference,” and the information included in any free writing prospectus that we have authorized for use in connection with this offering.
Company Overview
ZIOPHARM Oncology, Inc. is a biopharmaceutical company seeking to develop, acquire and commercialize, on its own or with partners, a diverse portfolio of cancer therapies that address unmet medical needs. We are currently focused on developing products in immuno-oncology that employ novel gene expression, control and cell technologies to deliver safe, effective and scalable cell- and viral-based therapies for the treatment of cancer and graft-versus-host-disease, or GvHD. Pursuant to two exclusive channel partner agreements, or Channel Agreements, with Intrexon Corporation, or Intrexon, we obtained certain exclusive rights to Intrexon’s technologies for use in the fields of oncology and GvHD.
The technologies represent an industrialized engineering approach for molecular and cell biology and gene control. They employ an inducible gene-delivery system, or switch, that enables controlled in vivoexpression of genes that produce therapeutic proteins to treat cancer. We and Intrexon refer to this “switch” as the RheoSwitch Therapeutic System®, or RTS®, platform. Our initial product candidate being developed using the immuno-oncology platform is Ad-RTS-IL-12 + veledimex, a clinical-stage product that we license from Intrexon under the Channel Agreement in the field of oncology.
Ad-RTS-IL-12 + veledimex uses our gene delivery system to produce interleukin-12, or IL-12, a potent, naturally occurring anti-cancer protein. IL-12 is a potent pro-inflammatory cytokine capable of reversing immune escape mechanisms and improving the function of tumor fighting natural killer, or NK, and T cells. Additionally, expression of functional IL-12 in human subjects by direct intratumoral injection of Ad-RTS-hIL-12 + veledimex is further demonstrated by the generation of downstream interferon gamma, or IFN-g. We have completed two Phase 2 studies evaluating Ad-RTS-hIL-12 + veledimex, the first for the treatment of metastatic melanoma, and the second for the treatment of metastatic breast cancer. We have also concluded enrollment of a single-center Phase 1b/2 study, following standard chemotherapy, for the treatment of patients with locally advanced or metastatic breast cancer. We presented our Phase 1b/2 clinical study results for the treatment of metastatic breast cancer at the European Society for Medical Oncology, or ESMO, Congress in October 2016.
Our multi-center Phase 1 study of Ad-RTS-IL-12 + veledimex in patients with recurrent or progressive glioblastoma, or GBM, or Grade III malignant glioma, a form of brain cancer, is ongoing. On February 24, 2016, we announced the successful completion of the initial dosing phase of the study and the dosing of the first patient in the next succeeding cohort of the study. On June 27, 2016, we announced the successful completion of enrollment in the first and second cohorts of the study. In August 2016, we completed enrollment of the third cohort of the study. We presented updated clinical data from the study as well as nonclinical data in a pontine mouse model at the 2016 Annual Scientific Meeting of the Society for Neuro-Oncology, or SNO, in November 2016. We believe these data will support the initiation of a pediatric brain tumor clinical trial during the first half of 2017.
In addition to Ad-RTS-IL-12 + veledimex as monotherapy, we have undertaken preclinical studies that suggest we may be able to combine this viral-based immunotherapy with an immune checkpoint inhibitor, or iCPI, to improve the anti-tumor effect for GBM. In May 2016, we presented the preclinical data from these preclinical studies at the Annual Meeting of the American Society of Gene and Cell Therapy, or ASGCT, and we believe the data will lend support to the first-in-human application of combining Ad-RTS-IL-12 + veledimex with an iCPI for investigational treatment of GBM.
On July 23, 2015, the U.S. Food and Drug Administration, or the FDA, granted orphan drug designation for Ad-RTS-IL-12 + veledimex for the treatment of malignant glioma. Orphan drug designation provides eligibility for a seven-year period of market exclusivity in the United States after product approval, an accelerated review process, accelerated approval where appropriate, grant funding, tax benefits and an exemption from user fees.
Pursuant to our Channel Agreement for the cancer program, in January 2015, we and Intrexon obtained an exclusive, worldwide license to certain additional immuno-oncology technologies owned and licensed by The University of Texas MD Anderson Cancer Center, or MD Anderson, including technologies relating to novel chimeric antigen receptors, or CARs, natural killer cells, or NK cells, and T-cell receptors, or TCRs. We refer to this license as the MD Anderson License. We plan to use these technologies to develop genetically modified T cells and other immune cells that will target and kill cancer cells using viral and non-viral approaches to gene transfer. Regarding our non-viral approach, we are using the Sleeping Beauty, or SB, a transposon/transposase system under the MD Anderson License to express CAR in clinical trials to render T cells specific for CD19. The initial associated trials using the first-generation CAR with a four-week manufacturing process showed favorable progression-free survival, or PFS, and/or overall survival when patient- and donor-derived CAR T cells, or CAR+ T, were infused after hematopoietic stem-cell transplantation, or HSCT. All patients receiving autologous SB-modified T cells had non-Hodgkin lymphoma, or NHL, and most patients receiving allogeneic CAR+ T cells had acute lymphoblastic leukemia, or ALL. A summary of the initial clinical experience using SB-modified CD-19 CAR+T in these NHL and ALL patients was published in the Journal of Clinical Investigation in September 2016. In addition to survival of the recipients, these trials demonstrated that the infused T cells persisted for lengths of time that compared favorably with T cells genetically modified with virus to express CAR.
We are currently enrolling patients for an investigator-led Phase 1 study using second-generation CD19-specific CAR+ T cells with a revised CAR structure in patients with advanced lymphoid malignancies at MD Anderson. A patient with multiple-relapsed B-cell ALL received CD19-specific CAR+ T cells produced with a three-week manufacturing process and achieved a complete remission with normalization of positron emission tomography–computed tomography, or PET/CT, tumor imaging lasting approximately six months. At the 2016 Annual Meeting of the ASGCT, we presented preclinical data in a mouse tumor model showing improved survival with treatment
S-1
using CAR+ T cells with reduced time in culture (approximately two weeks as compared to the four weeks needed for previous first-generation CAR+ T process). Our second-generation CD19 trial is underway, and employs a shortened manufacturing process advancement, with processing times as short as two weeks. On January 31, 2017, we announced that a patient with triple-hit NHL, treated in January 2017, was the first to receive SB-modified CD19-specific CAR+ T cells, with the manufacturing time reduced to two weeks. We believe that further decreases to the T-cell culture time in the manufacturing process will advance our efforts to address the challenges of cost and manufacturing time associated with these therapies.
In the preclinical setting, the time to administration of third-generation SB CAR+ T cells co-expressing a membrane-bound version of IL-15, or mbIL15, has been reduced to less than two days. This shortened process, referred to as point-of-care, delivers genetically modified T cells with superior proliferative potential. We believe that data presented at the 2016 Annual Meeting of American Society of Hematology, or ASH, supported by an earlier publication in the Proceedings of the National Academy of Sciences, indicated promising results: third generation SB CAR+ T cells demonstrated that a single, low dose of T cells co-expressing a CD19-specific CAR and mbIL15 resulted in sustained in vivo persistence that produced potent anti-tumor effects and superior leukemia-free survival. These clinical and preclinical data support our point-of-care plans to rapidly infuse SB CAR+ T cells in a Phase 1 trial. With the intent to administer clinical-grade SB CAR+ T cells in less than 48 hours, this non-viral CAR-T approach has the potential to outpace viral-based methods. Together with Intrexon, we currently have research programs evaluating additional CAR targets and CARs co-expressed with cytokines, and in particular, mbIL15. Control systems are also being developed such as the RTS® for receptor and/or cytokine expression, as well as for the conditional ablation of genetically modified cells using a kill switch. We anticipate future CAR+ T programs will also utilize the point-of-care manufacturing approach.
On May 2, 2017, we announced that an investigator-initiated Investigational New Drug, or IND, application for a Phase 1 study of CD33-specific CAR+ T therapy for treatment of relapsed or refractory acute myeloid leukemia, or AML, was accepted by the FDA. The CD33-specific CAR+ T cells incorporate a kill switch designed to eliminate the modified T cells under potential adverse safety conditions. Treatment of the first patient in this Phase 1 study is expected to begin in the third quarter of 2017. We believe the expected clinical data will help us establish if CD33 is a suitable target for CAR+ T cells at which point additional clinical plans will be considered, such as transition of the program under the point-of-care manufacturing approach.
Only a minority of tumor antigens are on the surface and able to be targeted by CARs, while most tumor-derived antigens are within the cell and will likely need to be targeted by TCRs. Therefore, we are developing approaches to target solid tumors using T cells genetically modified with the SB system to express TCRs for recognition of neoantigens. Data demonstrating the ability to generate T cells with patient-specific TCRs against neoantigens utilizing the SB system were published in Molecular Therapy in 2016. We also presented further preclinical information regarding the targeting of solid tumors at the 2016 Annual Meeting of ASH. On January 10, 2017, we announced the signing of a Cooperative Research and Development Agreement, or CRADA, with the National Cancer Institute, or NCI, for the development of adoptive cell transfer, or ACT, -based immunotherapies genetically modified using the SB transposon/transposase system to express TCRs for the treatment of solid tumors. The principal goal of the CRADA is to develop and evaluate ACT for patients with advanced cancers using autologous peripheral blood lymphocytes, or PBL, genetically modified using the non-viral SB system to express TCRs that recognize specific immunogenic mutations, or neoantigens, expressed within a patient’s cancer. We will conduct clinical evaluations of the ability of these SB-engineered PBL to express TCRs reactive against cancer mutations to mediate cancer regression in patients with metastatic disease. Research conducted under the CRADA will be at the direction of Steven A. Rosenberg, M.D., Ph.D., Chief of the Surgery Branch at the NCI, in collaboration with us and the researchers at Intrexon.
We plan to leverage the synergy between the platforms to accelerate an immuno-oncology pipeline and programs for the development of allogeneic CAR+ T and/or NK cells that can be used as off-the-shelf, or OTS, therapies. For example, NK cells do not have endogenous TCRs, so do not require genetic editing to eliminate TCRs. Thus, NK cells may be used as an OTS therapy while minimizing concern for causing GvHD. Further, cytokines such as IL-12 are “fuel” for NK cells. In addition to developing T cells, we expect to initiate an investigator-led trial of OTS primary NK cells for elderly patients with AML who are not candidates for standard intensive chemotherapy, after completing our regulatory review during 2017. We have additional interest in OTS products, such as the development of an allogeneic CAR+ T therapy.
We plan to continue to combine Intrexon’s technology suite with our capabilities to translate science to the patient, and to identify and develop additional products to stimulate or inhibit key pathways, including those used by the body’s immune system, to treat cancer.
On March 27, 2015, we entered into a global collaboration with Intrexon focused exclusively on CAR+ T products with Ares Trading, or Ares, a biopharmaceutical division of Merck KGaA, which we refer to as the Ares Trading Agreement. Intrexon will share the economic provisions of this collaboration equally with us, including an upfront payment of $115.0 million that was received in July 2015, milestones and royalties. Under this collaboration, Ares has already selected two CAR+ T targets for which we will perform certain research activities that will, in part, be funded by Ares. Pursuant to the terms of an amendment to our Channel Agreement with Intrexon, which we refer to as the ECP Amendment, that we entered into at the time of the Ares Trading Agreement, we will be responsible for any additional research and development expenditures. Once these candidates reach investigational new drug stage, the programs will be transferred to Ares for clinical development and commercialization. We and Intrexon will also independently conduct research and development on other CAR+ T candidates, with Ares having the opportunity during clinical development to opt-in to these candidates for additional payments to us and Intrexon. We and Intrexon, in the exclusive partnership with Ares, are progressing development of the two CAR+ T targets, utilizing our key technologies for regulating cytokine expression, the proprietary RTS® platform, and mbIL15.
On September 28, 2015, we entered into a new exclusive channel collaboration agreement, or the GvHD Agreement, with Intrexon to develop therapies for the treatment and/or prevention of GvHD, a major complication of allogeneic HSCT, which significantly impairs the quality of life and survival of many recipients. Allogeneic HSCT is used for the treatment of various diseases, including hematological malignancies, immunological deficiencies as well as non-malignant conditions. Human studies have shown that administration of low-dose subcutaneous interleukin-2, or IL-2, a cytokine critical for modulation of the immune system, in patients with steroid-refractory GvHD acts via regulatory T cells, or Tregs, to ameliorate its manifestations.
S-2
We believe that the combined expertise and knowledge gained from our research programs with Intrexon in adoptive T-cell therapies and cytokine modulation for the treatment of cancer well-positions us to develop and implement therapeutic approaches addressing an area of high unmet medical need for patients with GvHD. Through the GvHD Agreement, we and Intrexon plan to pursue engineered cell therapy strategies, used either separately or in combination, for targeted prevention and/or treatment of GvHD. The first approach is expected to utilize the infusion of Tregs, such as those conditionally expressing IL-2, utilizing the RTS® platform. The second approach is expected to utilize the deployment of Intrexon’s orally delivered microbe-based ActoBiotics® therapeutics, based on Lactococcus lactis, such as to express IL-2 to modulate immune function.
Corporate information
We originally incorporated in Colorado in September 1998 (under the name Net Escapes, Inc.) and later changed our name to “EasyWeb, Inc.” in February 1999. We re-incorporated in Delaware on May 16, 2005 under the same name. On September 13, 2005, we completed a “reverse” acquisition of privately held ZIOPHARM, Inc., a Delaware corporation. To effect this transaction, we caused ZIO Acquisition Corp., our wholly-owned subsidiary, to merge with and into ZIOPHARM, Inc., with ZIOPHARM, Inc. surviving as our wholly owned subsidiary. In accordance with the terms of the merger, the outstanding common stock of ZIOPHARM, Inc. automatically converted into the right to receive an aggregate of approximately 97.3% of our outstanding common stock (after giving effect to the transaction). Following the merger, we caused ZIOPHARM, Inc. to merge with and into us and we changed our name to “ZIOPHARM Oncology, Inc.” Although EasyWeb, Inc. was the legal acquirer in the transaction, we accounted for the transaction as a reverse acquisition under generally accepted accounting principles. As a result, ZIOPHARM, Inc. became the registrant with the Securities and Exchange Commission and the historical financial statements of ZIOPHARM, Inc. became our historical financial statements.
Our principal executive offices are located at One First Avenue, Parris Building 34, Navy Yard Plaza, Boston, Massachusetts 02129, and our telephone number is (617) 259-1970. Our website is www.ziopharm.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this prospectus supplement or the accompanying prospectus, and you should not consider it part of this prospectus supplement or part of the accompanying prospectus.
S-3
THE OFFERING
Common stock offered by us in this offering | 9,708,738 shares of our common stock |
Common stock to be outstanding immediately after this offering | 142,090,408 shares |
Use of proceeds | We intend to use the net proceeds from this offering for general corporate and working capital purposes, including the advancement of all of our clinical programs. See “Use of Proceeds.” |
Risk factors | An investment in our common stock involves a high degree of risk. See “Risk Factors” beginning on page S-5 for a discussion of some of the factors you should carefully consider before deciding to invest in shares of our common stock. |
NASDAQ Capital Market symbol | ZIOP |
The number of shares of common stock to be outstanding immediately after this offering is based on 132,381,670 shares of common stock outstanding as of March 31, 2017 and excludes:
| | • | | 3,539,669 shares of our common stock issuable upon the exercise of stock options outstanding as of March 31, 2017, having a weighted average exercise price of $5.10 per share; |
| | • | | 1,698,426 shares of our common stock available as of March 31, 2017 for future issuance pursuant to our 2012 Equity Incentive Plan; and |
| | • | | shares of our common stock issuable upon the conversion of our Series 1 preferred stock. |
S-4
RISK FACTORS
An investment in our common stock involves a high degree of risk. Before deciding whether to invest in our common stock, you should consider carefully the risks described below and discussed under the section captioned “Risk Factors” contained in our Quarterly Report on Form 10-Q for the period ended March 31, 2017, which is incorporated by reference in this prospectus supplement and the accompanying prospectus, in its entirety, together with other information in this prospectus supplement, the accompanying prospectus, the information and documents incorporated by reference, and in any free writing prospectus that we have authorized for use in connection with this offering. See “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.” If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could be harmed. This could cause the trading price of our common stock to decline, resulting in a loss of all or part of your investment. The risks described below and in the documents referenced above are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also affect our business.
Risks Related to our Business
We need to raise additional capital to fund our operations. The manner in which we raise any additional funds may affect the value of your investment in our common stock.
As of March 31, 2017, we have incurred approximately $673.6 million of accumulated deficit and had approximately $66.4 million of cash and cash equivalents. Given our current development plans, and after giving effect to the proceeds of this offering, we anticipate that our current cash resources will be sufficient to fund our operations into the fourth quarter of 2018. However, changes may occur that would consume our existing capital prior to then, including expansion of the scope of, and/or slower than expected progress of, our research and development efforts and changes in governmental regulation. Actual costs may ultimately vary from our current expectations, which could materially impact our use of capital and our forecast of the period of time through which our financial resources will be adequate to support our operations. Also our estimates include the advancement of our immuno-oncology product candidates in the clinic under our Channel Agreements with Intrexon and our increased expenses as we begin to advance candidates pursuant to the MD Anderson License with MD Anderson and commence providing funding for certain research and development activities of MD Anderson pursuant to the terms of the MD Anderson License, and we expect that the costs associated with these and any additional product candidates we pursue will increase the level of our overall research and development expenses significantly going forward.
In addition to above factors, our actual cash requirements may vary materially from our current expectations for a number of other factors that may include, but are not limited to, changes in the focus and direction of our development programs, competitive and technical advances, costs associated with the development of our product candidates, our ability to secure partnering arrangements, and costs of filing, prosecuting, defending and enforcing our intellectual property rights. If we exhaust our capital reserves more quickly than anticipated, regardless of the reason, and we are unable to obtain additional financing on terms acceptable to us or at all, we will be unable to proceed with development of some or all of our product candidates on expected timelines and will be forced to prioritize among them.
The unpredictability of the capital markets may severely hinder our ability to raise capital within the time periods needed or on terms we consider acceptable, if at all. Moreover, if we fail to advance one or more of our current product candidates to later-stage clinical trials, successfully commercialize one or more of our product candidates, or acquire new product candidates for development, we may have difficulty attracting investors that might otherwise be a source of additional financing.
Our need for additional capital and limited capital resources may force us to accept financing terms that could be significantly dilutive to existing stockholders. To the extent that we raise additional capital by issuing equity securities, our stockholders may experience dilution. In addition, we may grant future investors rights superior to those of our existing stockholders. If we raise additional funds through collaborations and licensing arrangements, it may be necessary to relinquish some rights to our technologies, product candidates or products, or grant licenses on terms that are not favorable to us. If we raise additional funds by incurring debt, we could incur significant interest expense and become subject to covenants in the related transaction documentation that could affect the manner in which we conduct our business.
We report interim data on certain of our clinical trials and we cannot assure you that interim data will be predictive of either future interim results or final study results.
As part of our business, we provide updates related to the development of our product candidates, which may include updates related to interim clinical trial data. To date, our clinical trials have involved small patient populations and because of the small sample size, the interim results of these clinical trials may be subject to substantial variability and may not be indicative of either future interim results or final results.
Healthcare legislative reform measures may have a material adverse effect on our business and results of operations.
In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory enactments in recent years that change the healthcare system in ways that could impact our future ability to sell our product candidates profitably.
Furthermore, there have been and continue to be a number of initiatives at the federal and state level that seek to reduce healthcare costs. Most significantly, in March 2010, President Obama signed into law the Patient Protection and Affordable Health Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the ACA, which includes measures that significantly change the way healthcare is financed by both governmental and private insurers. Among the provisions of the ACA of importance to the pharmaceutical industry are the following:
| | • | | An annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; |
| | • | | An increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for most branded and generic drugs, respectively; |
| | • | | A new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; |
| | • | | An extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
| | • | | New methodologies by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, and for drugs that are line extensions; |
| | • | | Expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals with income at or below 133% of the Federal Poverty Level, thereby potentially increasing both the volume of sales and manufacturers’ Medicaid rebate liability; |
| | • | | Expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
| | • | | A new requirement to annually report drug samples that certain manufacturers and authorized distributors provide to physicians; |
| | • | | Expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance; |
| | • | | A licensure framework for follow-on biologic products; |
| | • | | A new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and |
| | • | | Establishment of a Center for Medicare & Medicaid Innovation at the Centers for Medicare & Medicaid Services, or CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. |
S-5
We cannot predict the full impact of the ACA, as many of the reforms require the promulgation of detailed regulations implementing the statutory provisions, some of which have not yet fully occurred. Further, since its enactment there have been judicial and Congressional challenges to certain aspects of the ACA As a result, there have been delays in the implementation of, and action taken to repeal or replace, certain aspects of the ACA. Most recently, in March 2017, the U.S. House of Representatives introduced legislation known as the American Health Care Act, which, if enacted, would amend or repeal significant portions of the ACA. The prospects for legislative action on this bill are uncertain. Congress could consider other legislation to repeal or replace certain elements of the ACA. We continue to evaluate the effect that the ACA and its possible repeal and replacement has on our business. We cannot predict the full impact of the ACA on companies in the pharmaceutical industry, as many of the ACA reforms require the promulgation of detailed regulations implementing the statutory provisions, which has not yet occurred.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. For example, in August 2011, President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint Select Committee on Deficit Reduction did not achieve its targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reductions to several government programs. These reductions include aggregate reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013, and, due to subsequent legislative amendments, will stay in effect through 2025 unless additional Congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Further, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. The full impact of these new laws, as well as laws and other reform and cost containment measures that may be proposed and adopted in the future, remains uncertain, but may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our future customers and accordingly, our ability to generate revenue, attain profitability, or commercialize our products.
Risks Related to this Offering
Management will have broad discretion as to the use of the proceeds from this offering, and we may not use the proceeds effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. Accordingly, you will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that the proceeds will be used in a way that does not yield a favorable, or any, return for us. Our failure to apply these funds effectively could have a material adverse effect on our business, delay the development of our product candidates and cause the price of our common stock to decline.
You will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase.
Since the price per share of our common stock being offered is substantially higher than the net tangible book value per share of our common stock, you will suffer substantial dilution in the net tangible book value of the common stock you purchase in this offering. Based on the public offering price of $5.15 per share, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us, and based on a net tangible book value of our common stock of $0.26 per share as of March 31, 2017, if you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of $4.58 per share in the net tangible book value of common stock. See the section entitled “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
You may experience future dilution as a result of future equity offerings and other issuances of our common stock.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share in this offering. We cannot assure you that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders, including investors who purchase shares of common stock in this offering. The price per share at which we sell additional shares of our common stock or securities convertible into common stock in future transactions may be higher or lower than the price per share in this offering.
If securities and/or industry analysts fail to continue publishing research about our business, if they change their recommendations adversely or if our results of operations do not meet their expectations, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
S-6
In addition, it is likely that in some future period our operating results will be below the expectations of securities analysts or investors. If one or more of the analysts who cover us downgrade our stock, or if our results of operations do not meet their expectations, our stock price could decline.
Our stock price is volatile and may decline regardless of our operating performance, and you may not be able to resell your shares at or above the price at which you purchased such shares.
The market price for our common stock is volatile and may fluctuate significantly in response to a number of factors, most of which we cannot control, including:
| | • | | price and volume fluctuations in the overall stock market; |
| | • | | market conditions or trends in our industry or the economy as a whole; |
| | • | | changes in operating performance and stock market valuations of other biopharmaceutical companies generally, or those that develop and commercialize cancer drugs in particular; |
| | • | | the financial or operational projections we may provide to the public, any changes in these projections or our failure to meet these projections; |
| | • | | changes in financial estimates or ratings by any securities analysts who follow our common stock, our failure to meet these estimates or failure of those analysts to initiate or maintain coverage of our common stock; |
| | • | | the public’s response to press releases or other public announcements by us or third parties, including our filings with the SEC, and announcements relating to product development, litigation and intellectual property impacting us or our business; |
| | • | | the sustainability of an active trading market for our common stock; |
| | • | | future sales of our common stock by our executive officers, directors and significant stockholders; |
| | • | | announcements of mergers or acquisition transactions; |
| | • | | our inclusion or deletion from certain stock indices; |
| | • | | announcements of medical innovations or new products by our competitors; |
| | • | | announcements of changes in our senior management; |
| | • | | other events or factors, including those resulting from war, incidents of terrorism, natural disasters or responses to these events; and |
| | • | | changes in accounting principles. |
In addition, the stock markets, and in particular The NASDAQ Capital Market, have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many biopharmaceutical companies. Stock prices of many biopharmaceutical companies have fluctuated in a manner unrelated or disproportionate to the operating performance of those companies. In the past, stockholders have instituted securities class action litigation following periods of market volatility. If we were involved in securities litigation, we could incur substantial costs and our resources and the attention of management could be diverted from our business.
Our principal stockholders, executive officers and directors have substantial control over the company, which may prevent you and other stockholders from influencing significant corporate decisions and may harm the market price of our common stock.
As of March 31, 2017, our executive officers, directors and holders of five percent or more of our outstanding common stock, beneficially owned, in the aggregate, 21.1% of our outstanding common stock. These stockholders may have interests that conflict with our other stockholders and, if acting together, have the ability to influence the outcome of matters submitted to our stockholders for approval, including the election and removal of directors and any merger, consolidation or sale of all or substantially all of our assets. Accordingly, this concentration of ownership may harm the market price of our common stock by:
| | • | | delaying, deferring or preventing a change in control; |
| | • | | impeding a merger, consolidation, takeover or other business combination involving us; or |
| | • | | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
S-7
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement and the accompanying prospectus contain, and the documents incorporated by reference herein and therein and any free writing prospectus that we have authorized for use in connection with this offering may contain, forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Exchange Act. All statements other than statements of historical facts contained in this prospectus supplement, the accompanying prospectus and the information we incorporate by reference are forward-looking statements. These statements relate to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. Forward-looking statements may include, but are not limited to statements about:
| | • | | our ability to finance our operations and business initiatives; |
| | • | | the sufficiency of our cash and investments and our expected uses of cash; |
| | • | | the progress, timing and results of preclinical and clinical trials involving our product candidates; |
| | • | | the progress of our research and development programs; |
| | • | | the costs and timing of the development and commercialization of our products; |
| | • | | additional planned regulatory filings for the approval and commercialization of our immuno-oncology product candidates; |
| | • | | whether any of our other therapeutic discovery and development efforts will advance further in preclinical research or in the clinical trial process and whether and when, if at all, our product candidates will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; |
| | • | | whether any other therapeutic products we develop will be successfully marketed if approved; |
| | • | | the risk that final trial data may not support interim analysis of the viability of our product candidates; |
| | • | | our ability to achieve the results contemplated by our collaboration agreements and the benefits to be derived from relationships with collaborators; |
| | • | | competition from other pharmaceutical and biotechnology companies; |
| | • | | the development of, and our ability to take advantage of, the market for our product candidates; |
| | • | | the anticipated amount, timing and accounting of deferred revenues, milestones and other payments under licensing, collaboration or acquisition agreements, research and development costs and other expenses; |
| | • | | the strength and enforceability of our intellectual property rights; |
| | • | | our assessment of the potential impact on our future revenues of health care reform legislation in the United States; |
| | • | | the timing and impact of measures worldwide designed to reduce health care costs; |
| | • | | the uncertainty of economic conditions in certain countries in Europe and Asia such as related to the United Kingdom’s referendum in June 2016 in which voters approved an exit from the European Union, commonly referred to as “Brexit”; and general economic conditions. |
In some cases, you can identify forward-looking statements by terms such “anticipate,” “believe,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “project,” “target,” “will” and other words and terms of similar meaning. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We discuss many of these risks in greater detail under the heading “Risk Factors” in this prospectus supplement and in our SEC filings.
You should read this prospectus supplement, the accompanying prospectus, the documents we have filed with the SEC that are incorporated by reference and any free writing prospectus that we have authorized for use in connection with this offering completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in the foregoing documents by these cautionary statements. You should not place undue reliance on these statements. Forward-looking statements speak only as of the date of the document containing the applicable statement. We do not undertake any obligation to publicly update any forward-looking statements.
S-8
USE OF PROCEEDS
We estimate that the net proceeds to us from the sale of our common stock offered hereby will be approximately $47 million, after deducting the underwriting discounts and commissions and our estimated offering expenses.
We currently intend to use the net proceeds from this offering for general corporate and working capital purposes, including the advancement of all of our clinical programs.
The amount and timing of these expenditures will depend on a number of factors, including the progress of our research and development efforts, the progress of any partnering efforts, technological advances and the competitive environment for our product candidates. Accordingly, you will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that the proceeds will be used in a way that does not yield a favorable, or any, return for us. Pending application of the net proceeds as described above, we intend to invest the proceeds in investment grade interest bearing instruments.
S-9
DILUTION
Our net tangible book value as of March 31, 2017 was approximately $34.5 million, or $0.26 per share. Net tangible book value per share is determined by dividing our total tangible assets, less total liabilities, by the number of shares of our common stock outstanding as of March 31, 2017. Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers of shares of common stock in this offering and the net tangible book value per share of our common stock immediately after this offering.
After taking into account the sale by us of 9,708,738 shares of our common stock in this offering at the public offering price of $5.15 per share, less the underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of March 31, 2017 would have been approximately $81.7 million, or $0.57 per share. This would represent an immediate increase in net tangible book value of $0.31 per share to existing stockholders and in immediate dilution of $4.58 per share to investors purchasing our common stock in this offering at the public offering price. The following table illustrates this dilution on a per share basis:
| | | | | | | | |
Public offering price per share | | | | | | $ | 5.15 | |
Net tangible book value per share as of March 31, 2017 | | $ | 0.26 | | | | | |
Increase per share attributable to investors purchasing our common stock in this offering | | $ | 0.31 | | | | | |
As adjusted net tangible book value per share as of March 31, 2017, after giving effect to this offering | | | | | | $ | 0.57 | |
Dilution in net tangible book value per share to investors purchasing our common stock in this offering | | | | | | $ | 4.58 | |
The amounts above are based on 132,381,670 shares of common stock outstanding as of March 31, 2017 and excludes:
| | • | | 3,539,669 shares of our common stock issuable upon the exercise of stock options outstanding as of March 31, 2017, having a weighted average exercise price of $5.10 per share; |
| | • | | 1,698,426 shares of our common stock available as of March 31, 2017 for future issuance pursuant to our 2012 Equity Incentive Plan; and |
| | • | | shares of our common stock issuable upon the conversion of our Series 1 preferred stock. |
To the extent options outstanding as of March 31, 2017 have been or may be exercised or other shares have been issued, there may be further dilution to investors.
S-10
UNDERWRITING
Guggenheim Securities, LLC is acting as the sole book-running manager of this offering. We have entered into an underwriting agreement with Guggenheim Securities, LLC, or the underwriter. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriter, and the underwriter has agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus supplement, the number of shares of common stock listed next to its name in the following table:
| | | | |
Name | | Number of
shares | |
Guggenheim Securities, LLC | | | 9,708,738 | |
| | | | |
Total | | | 9,708,738 | |
| | | | |
The underwriting agreement provides that the obligations of the underwriter are subject to certain conditions precedent. We have agreed to indemnify the underwriter and certain of its controlling persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriter may be required to make in respect of those liabilities.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriter to us per share of common stock. The underwriting fee is $0.2575 per share and a total of $2,500,000.
We estimate that the total expenses of this offering, excluding the underwriting discounts and commissions, will be approximately $350,000.
We have agreed that we will not, subject to specified exceptions, (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, or file with SEC a registration statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exercisable or exchangeable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, or (ii) enter into any swap or other arrangement that transfers, in whole or in part, any portion of the economic consequences associated with the ownership of any shares of common stock or any such other securities (regardless of whether any of these transactions are to be settled by the delivery of shares of common stock or such other securities, in cash or otherwise), in each case without the prior written consent of Guggenheim Securities, LLC for a period of 90 days after the date of this prospectus supplement.
Our directors and executive officers have entered into lock-up agreements with the underwriter prior to the commencement of this offering pursuant to which each of these persons or entities, subject to specified exceptions, for a period of 90 days after the date of this prospectus supplement, may not, without the prior written consent of Guggenheim Securities, LLC, (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock (including, without limitation, common stock or such other securities which may be deemed to be beneficially owned by such persons or entities in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant), (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the common stock or such other securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of common stock or such other securities, in cash or otherwise, or (iii) make any demand for or exercise any right with respect to the registration of any shares of our common stock or any security convertible into or exercisable or exchangeable for our common stock.
Our common stock is listed on the Nasdaq Capital Market under the symbol “ZIOP.”
The underwriter has advised us that it, pursuant to Regulation M under the Securities Exchange Act of 1934, as amended, and certain persons participating in the offering may engage in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this offering. These activities may have the effect of stabilizing or maintaining the market price of the common stock at a level above that which might otherwise prevail in the open market.
A stabilizing bid is a bid for the purchase of shares of common stock on behalf of the underwriter for the purpose of fixing or maintaining the price of the common stock. A syndicate covering transaction is the bid for or the purchase of shares of
S-11
common stock on behalf of the underwriter to reduce a short position incurred by the underwriter in connection with the offering. Similar to other purchase transactions, the underwriter’s purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the underwriter to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the common stock originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
Neither we, nor the underwriter makes any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. The underwriter is not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
The underwriter may also engage in passive market making transactions in our common stock on The NASDAQ Capital Market in accordance with Rule 103 of Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specified purchase limits are exceeded.
The underwriter and certain of its affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriter and certain of its affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses. In the ordinary course of their various business activities, the underwriter and certain of its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates.
S-12
LEGAL MATTERS
Cooley LLP, Boston, Massachusetts, will pass upon the validity of the issuance of the common stock offered hereby. The underwriter is being represented by Proskauer Rose LLP, New York, New York.
EXPERTS
The financial statements and the effectiveness of internal control over financial reporting incorporated in this prospectus by reference to the Annual Report on Form 10-K for the year ended December 31, 2016 have been audited by RSM US LLP, an independent registered public accounting firm, as stated in their report incorporated by reference herein and have been so incorporated in reliance upon such reports and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy these reports, proxy statements and other information at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549 or at the SEC’s other public reference facilities. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the public reference rooms. You can request copies of these documents by writing to the SEC and paying a fee for the copying costs. In addition, the SEC maintains an Internet site athttp://www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Our SEC filings are available on the SEC’s Internet site. We maintain a website athttp://www.ziopharm.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this prospectus supplement or the accompanying prospectus, and you should not consider it part of this prospectus supplement or part of the accompanying prospectus.
S-13
INCORPORATION OF INFORMATION BY REFERENCE
We are allowed to incorporate by reference information contained in documents that we file with the SEC. This means that we can disclose important information to you by referring you to those documents and that the information in this prospectus supplement is not complete and you should read the information incorporated by reference for more detail. Information in this prospectus supplement supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus supplement, while information that we file later with the SEC will automatically update and supersede the information in this prospectus supplement.
We incorporate by reference the documents listed below and any future filings we will make with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act from the date of this prospectus supplement but prior to the termination of the offering of the securities covered hereby (other than Current Reports or portions thereof furnished under Item 2.02 or 7.01 of Form 8-K):
| | • | | Annual Report on Form 10-K for the fiscal year ended December 31, 2016, filed on February 16, 2017; |
| | • | | The information specifically incorporated by reference into our Annual Report on Form 10-K for the fiscal year ended December 31, 2016 from our Definitive Proxy Statement on Schedule 14A, filed on April 28, 2017; |
| | • | | Quarterly Report on Form 10-Q for the quarter ended March 31, 2017, filed on May 1, 2017; |
| | • | | Current Report on Form 8-K filed on May 2, 2017; and |
| | • | | The description of our common stock set forth in the registration statement on Form 8-A registering our common stock under Section 12 of the Exchange Act, which was filed with the SEC on September 20, 2006, including any amendments or reports filed for purposes of updating such description. |
We will provide to each person, including any beneficial owner, to whom a prospectus is delivered a copy of any or all of the documents that are incorporated by reference in this prospectus supplement but not delivered with this prospectus, including exhibits that are specifically incorporated by reference in such documents. You may request a copy of such documents at no cost, by writing or telephoning us at the following address or telephone number:
ZIOPHARM Oncology, Inc.
One First Avenue, Parris Building 34, Navy Yard Plaza
Boston, Massachusetts 02129
Attention: Chief Legal Officer
Telephone: (617) 259-1970
S-14
PROSPECTUS

Common Stock
Preferred Stock
Warrants
Debt Securities
From time to time, we may offer and sell any combination of common stock, preferred stock, warrants and debt securities, in one or more offerings. We may also offer common stock or preferred stock upon conversion of debt securities, common stock upon conversion of preferred stock, or common stock, preferred stock or debt securities upon the exercise of warrants. We may offer these securities separately or as units, which may include combinations of the securities.
This prospectus describes the general terms of these securities and the general manner in which these securities will be offered. We will provide the specific terms of these offerings and securities in one or more supplements to this prospectus. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement and any related free writing prospectus may also add, update or change information contained in this prospectus. You should carefully read this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as any documents incorporated by reference, before buying any of the securities being offered.
Our common stock is listed on the NASDAQ Capital Market under the symbol “ZIOP.” On January 30, 2015, the closing price of our common stock, as reported on the NASDAQ Capital Market, was $8.95.
This prospectus may not be used to consummate a sale of any securities unless accompanied by a prospectus supplement.
We may offer these securities in amounts, at prices and on terms determined at the time of offering. The securities may be sold directly by us to investors, through agents designated from time to time or to or through underwriters or dealers, on a continuous or delayed basis. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus. If any agents or underwriters are involved in the sale of any securities with respect to which this prospectus is being delivered, the names of such agents or underwriters and any applicable fees, commissions, discounts and options to purchase additional securities will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds that we expect to receive from such sale will also be set forth in a prospectus supplement.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” contained in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved these securities or determined if this prospectus is truthful or complete. A representation to the contrary is a criminal offense.
The date of this Prospectus is February 2, 2015.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or the Commission, using a “shelf” registration process. Under this shelf registration process, from time to time, we may sell any combination of the securities described in this prospectus in one or more offerings. This prospectus provides you with a general description of the securities we may offer. Each time we offer and sell securities under this prospectus, we will provide a prospectus supplement that will contain more specific information about the terms of the applicable offering. The prospectus supplement may include a discussion of risks or other special considerations applicable to us or the offered securities. We may also authorize one or more free writing prospectuses to be provided to you that may contain material information relating to these offerings. The prospectus supplement, and any related free writing prospectus that we may authorize to be provided to you, may also add, update or change the information contained in this prospectus or in the documents incorporated by reference into this prospectus. If there is any inconsistency between the information in this prospectus and the applicable prospectus supplement, you must rely on the information in the prospectus supplement. We urge you to carefully read this prospectus, any applicable prospectus supplement and any related free writing prospectus, together with the information incorporated herein by reference as described under the headings “Where You Can Find More Information” and “Incorporation of Information by Reference” before buying any of the securities being offered.THIS PROSPECTUS MAY NOT BE USED TO CONSUMMATE A SALE OF SECURITIES UNLESS IT IS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
You should rely only on the information contained in, or incorporated by reference into, this prospectus or any applicable prospectus supplement, along with the information contained in any related free writing prospectus that we have authorized for use in connection with a specific offering. We have not authorized anyone to provide you with different information. No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus that we may authorize to be provided to you. You must not rely on any unauthorized information or representation. This prospectus is an offer to sell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate only as of the date on the front of the document, and any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any related free writing prospectus, or any sale of a security. Our business, financial condition, results of operations and prospects may have changed since those dates.
i.
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus or incorporated by reference into this prospectus, and does not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire prospectus, the applicable prospectus supplement and any related free writing prospectus, including the risks of investing in our securities discussed under the heading “Risk Factors” contained in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference into this prospectus, including our financial statements, and the exhibits to the registration statement of which this prospectus is a part. Unless otherwise indicated, “ZIOPHARM,” our “Company,” “we,” “us,” “our” and similar terms refer to ZIOPHARM Oncology, Inc.
Company overview
ZIOPHARM Oncology, Inc. is a biopharmaceutical company that seeks to acquire, develop and commercialize, on its own or with commercial partners, a diverse portfolio of cancer therapies that can address unmet medical needs through synthetic biology. Pursuant to an exclusive channel agreement with Intrexon Corporation, or Intrexon, we obtained rights to Intrexon’s synthetic biology platform for use in the field of oncology, which included a clinical stage product candidate, Ad-RTS-IL-12 used with the oral activator veledimex. The synthetic biology platform is an industrialized engineering approach for molecular and cell biology and gene control. It employs an inducible gene-delivery system that enables controlled in vivo expression of genes that produce therapeutic proteins to treat cancer. Ad-RTS-IL-12 + veledimex uses this gene delivery system to produce Interleukin-12, or IL-12, a potent, naturally occurring anti-cancer protein. We have completed two Phase 2 studies evaluating Ad-RTS-IL-12 + veledimex, the first for the treatment of metastatic melanoma, and the second for the treatment of metastatic breast cancer; data from these Phase 2 studies was presented in December 2014. We are continuing to pursue intratumoral injection of Ad-RTS-IL-12 + veledimex in breast cancer and brain cancer.
In addition to our synthetic biology programs, we recently obtained an exclusive, worldwide license to certain immuno-oncology technologies owned and licensed by The University of Texas M.D. Anderson Cancer Center, or MD Anderson, including technologies relating to novel chimeric antigen receptors, or CARs, natural killer, or NK cells and T cell receptors, or TCRs. Combining these technologies with Intrexon’s technology suite and clinically tested RheoSwitch Therapeutic System®, or RTS®, IL-12 modules, we plan to develop CAR-T and other immune cells that will target and kill cancer cells. We plan to leverage the synergy between the platforms to accelerate a promising synthetic immuno-oncology pipeline, with up to five CAR-T therapies expected to enter the clinic in 2015 and programs for the development of allogeneic CAR-T therapies that can be used off-the-shelf expected to be initiated in 2016.
We plan to continue to combine Intrexon’s technology suite with our capabilities to translate science to the patient to identify and develop additional products to stimulate or inhibit or stimulate key pathways, including those used by the body’s immune system, to treat cancer.”
We also have a portfolio of small molecule drug candidates, which are no longer a strategic focus of our development activities and for some of which we are seeking partners to pursue further development and potential commercialization.
1.
Enabling technologies
Synthetic biology
Synthetic biology entails the application of engineering principles to biological systems for the purpose of designing and constructing new biological systems or redesigning/modifying existing biological systems. Biological systems are governed by DNA, the building block of gene programs, which control cellular processes by coding for the production of proteins and other molecules that have a functional purpose and by regulating the activities of these molecules. This regulation occurs via complex biochemical and cellular reactions working through intricate cell signaling pathways, and control over these molecules modifies the output of biological systems. Synthetic biology has been enabled by the application of information technology and advanced statistical analysis, also known as bioinformatics, to genetic engineering, as well as by improvements in DNA synthesis. Synthetic biology aims to engineer gene-based programs or codes to modify cellular function to achieve a desired biological outcome. Its application is intended to allow more precise control of drug concentration and dose, thereby improving the therapeutic index associated with the resulting drug.
On January 6, 2011, we entered into an Exclusive Channel Partner Agreement with Intrexon, which we refer to as the Channel Agreement, to develop and commercialize novel DNA-based therapeutics in the field of cancer treatment by combining Intrexon’s synthetic biology platform with our capabilities to translate science to the patient. As a result, our DNA synthetic biology platform employs an inducible gene-delivery system that enables regulated and controlled delivery of genes that produce therapeutic proteins to treat cancer. The first example of this regulated controlled delivery is achieved by producing IL-12, a potent, naturally occurring anti-cancer protein, under the control of Intrexon’s proprietary biological “switch” to turn on and off (and on and off repeatedly) the therapeutic protein expression at the tumor site. We and Intrexon refer to this “switch” as the RheoSwitch Therapeutic System® or RTS® platform. Our initial drug candidate being developed using the synthetic biology platform is Ad-RTS-IL-12 + veledimex.
More detailed descriptions of our clinical development for each are set forth in this report under the caption “—Product candidates” below.
Immuno-oncology
Immuno-oncology, which utilizes a patient’s own immune system to treat cancer, is one of the most actively pursued areas of research by biotechnology and pharmaceutical companies today. Cancer cells contain mutated proteins and may overexpress other proteins usually found in the body at low levels. The immune system typically recognizes unusual or aberrant cell protein expression and eliminates these cells in a highly efficient process known as immune surveillance. A central player in immune surveillance is a type of white blood cell known as the T cell. In healthy individuals, T cells identify and kill infected or abnormal cells, including cancer cells. Cancer cells develop the ability to evade immune surveillance, which is a key factor in their growth, spread, and persistence. In the last five years, there has been substantial scientific progress in countering these evasion mechanisms using immunotherapies, or therapies that activate the immune system.
On January 13, 2015, we, together with Intrexon, entered into a license agreement with MD Anderson, which we refer to as the MD Anderson License. Pursuant to the MD Anderson License, we and Intrexon hold an exclusive, worldwide license to certain technologies owned and licensed by MD Anderson including technologies relating to novel CAR-T cell therapies arising from the laboratory of Laurence Cooper, M.D., Ph.D., professor of pediatrics at MD Anderson, as well as either co-exclusive or non-exclusive licenses under certain related technologies.
Combining the non-viral genetic engineering technologies we licensed from MD Anderson together with Intrexon’s industrialized approach to gene engineering and cell control, we believe we can rapidly and efficiently reprogram T cells to express a particular CAR or TCR construct that will enable the T cell to recognize and target
2.
cancer cells. CAR-T cells target cell surface tumor antigens, such as CD-19, that exist on cancer cells and that are independent of human leukocyte antigens, or HLAs, and which we refer to as “public” antigens. TCR+ cells target tumor antigens that are dependent on HLAs and which we refer to as “private” antigens. Natural killer cells target tumors with loss of HLAs, or tumors with no antigens. Most CAR-T cell and TCR products currently being developed by competitors are autologous, or derived from the patient’s own blood, and gene engineered with viral technology. As a result, the patient’s blood must be harvested, shipped to a manufacturing facility where it is modified using a retrovirus to express the CAR or TCR, and then shipped back to be infused into the patient. The process can take several weeks to a month and is very labor intensive and costly. Currently, this complex technique can only be done in very sophisticated laboratories. We believe we will be able to manufacture our CAR-T cells and TCRs using non-viral methods, which we expect will enable a simpler process requiring only days or hours and result in a lower cost of manufacturing. Our non-viral methods could also potentially enable autologous point of care treatment, where a patient’s own T cells would be modified at or near the point of care, for example, utilizing a local blood bank, to express the CAR-T or TCR construct and then infused back into the patient, potentially during the same visit. In addition, we intend to use our non-viral methods to develop allogeneic treatments that can be used off-the-shelf. An allogeneic off-the-shelf treatment would enable a patient to be treated with a CAR-T or TCR construct that is created from a separate healthy donor, personalized for that patient, and then distributed to the point of care. Our non-viral methods, which we believe are nimble, fast and less costly than other approaches, together with our industrialized, scalable engineering approach are expected to enable highly efficient and less costly manufacturing approaches to gene engineered cell-based therapy. In addition, our proprietary RheoSwitch Therapeutic System® may give us the ability to control in vivo gene expression (on-off-on-off etc.) in CAR-T or TCR cells, which we believe could result in significantly lower toxicity compared to other products currently in development.
Product candidates
The following chart identifies our current synthetic biology product candidates and their stage of development, each of which are described in more detail below.
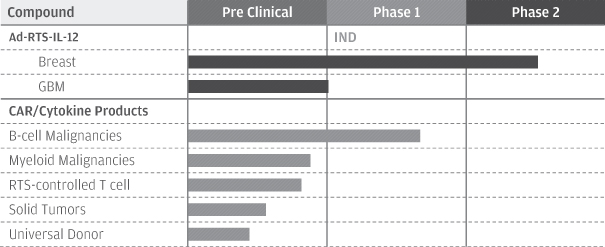
3.
Synthetic biology programs
Ad-RTS-IL-12 + veledimex
Ad-RTS-IL-12 + veledimex has been evaluated in two Phase 2 studies, the first for the treatment of metastatic melanoma, and the second for the treatment of unresectable recurrent or metastatic breast cancer. Ad-RTS-IL-12 + veledimex is our lead product candidate, which uses our gene delivery system to produce IL-12, a potent, naturally occurring anti-cancer protein.
More specifically, IL-12 is a potent immunostimulatory cytokine which activates and recruits dendritic cells that facilitate the cross-priming of tumor antigen-specific T cells. Intratumoral administration of Ad-RTS-IL-12 + veledimex, which allows for adjustment of IL-12 gene expression upon varying the dose of veledimex, is designed to reduce the toxicity elicited by systemic delivery of IL-12, and increase efficacy through high intratumoral expression.
We reported the controlled local expression of IL-12 as an immunotherapeutic treatment of glioma (brain cancer) in animal models through the use of the RTS® at the October 2013 AACR-NCI-EORTC conference. Veledimex brain penetration was demonstrated in normal mice and monkeys with intact blood brain barriers. Treatment with Ad-RTS-IL-12 + veledimex and DC-RTS-IL-12 + veledimex both demonstrated dose-related increase in survival in the mouse GL-261 glioma model with no adverse clinical signs observed. In December 2013, we announced the unanimous approval of the Recombinant DNA Advisory Committee of the National Institutes of Health, or the RAC/NIH, for the initiation of a Phase 1 study of Ad-RTS-IL-12 + veledimex, in subjects with recurrent or progressive high grade gliomas. The U.S Food and Drug Administration, or FDA, has requested additional nonclinical information to support the Phase 1 study and this data has been generated. Subject to reaching agreement with the FDA, we anticipate initiation of the Phase 1 study during the first half of 2015. Glioblastoma is by far the most frequent malignant brain tumor and is associated with a particularly aggressive course and dismal prognosis. The current standard of care is based on surgical resection to the maximum feasible extent, followed by radiotherapy and concomitant adjuvant temozolomide. Such aggressive treatment, however, is associated with only modest improvements in survival resulting in a very high unmet medical need.
At the American Association for Cancer Research, or AACR, 2014 Annual Meeting, in April 2014, we presented data from a preclinical study conducted jointly by us and Intrexon demonstrating the anti-tumor effects and tolerability of Ad-RTS-mIL-12 in a glioblastoma murine model. Veledimex was found to effectively cross the blood brain barrier, with dose-related increases in plasma and brain tissue exposure, and no accumulation in brain tissue following repeat dosing. The study data demonstrated that administration of Ad-RTS-mIL-12 + veledimex resulted in dose-related increases in survival of four- to five-fold, without exhibiting an adverse safety profile, when compared to median survival in vehicle control groups.
At the 17th Annual Meeting of the American Society of Gene and Cell Therapy, or ASGCT, in May 2014, we presented results demonstrating the potent anti-tumor and anti-cancer stem cell effects of Ad-RTS-IL-12 in a preclinical glioma model. Results from human and laboratory studies of Ad-RTS-IL-12 demonstrated that precise control of IL-12 gene expression levels can be achieved using Intrexon’s RTS®. Rapid, tight modulation of in vivo expression of IL-12 using the activator ligand, veledimex, was demonstrated across these studies. When IL-12 expression was “switched on” it rapidly led to expression and an immune response. This immune response was characterized by an increase in tumor infiltrating lymphocytes with system wide immune activation. The data presented in May 2014 further demonstrated that Ad-RTS-IL-12 has potent anti-cancer effects in a glioma model, showing both a reduction in tumor mass and prolonged survival when compared to existing treatment standards. The data also showed a significant reduction in cancer stem cells, as measured by dramatically reduced nestin levels. Cancer stem cells are thought to play a critical role in recurrence and metastasis.
At the AACR 2014 Immunology and Immunotherapy Meeting in December 2014, together with Intrexon, we presented clinical results from the Ad-RTS-hIL-12 + veledimex studies in patients with advanced breast cancer and
4.
melanoma demonstrating local and systemic IL-12-mediated anti-cancer activity, as well as safety and control of both immune- and IL-12-mediated toxicity with use of the RTS® gene switch. In two open-label Phase 2 clinical studies, twelve patients with metastatic advanced stage breast cancer and twenty-six patients with metastatic melanoma were administered Ad-RTS-hIL-12. Following intra-tumoral injection of Ad-RTS-hIL-12, expression of IL-12 within patients was controlled by the RTS® gene switch using the oral activator ligand, veledimex, at doses ranging from 5mg to 160mg. All subjects had heavy tumor burden and disease progression at the time of enrollment, with mean number of prior therapies at 14 and 10 for breast cancer and melanoma patients, respectively. Treatment with Ad-RTS-hIL-12 + veledimex resulted in an increase in the immune cytokine IL-12 and downstream cytokines, IFN-g, IP-10 and IL-10, resulting in a significant increase in the number of CD8+ T-cells. Among seven evaluable subjects in the Phase 2 clinical study of Ad-RTS-IL-12 + veledimex in patients with recurrent or metastatic breast cancer, three had stable disease, including one triple negative breast cancer subject who crossed the primary endpoint of 16 week progression free survival, for a disease control rate (stable disease or better) of 43%. Target lesions and tumor burden were significantly reduced in approximately 40% of patients. In the Phase 1/2 study of Ad-RTS-hIL-12 + veledimex in subjects with unresectable stage III/IV melanoma, of eighteen evaluable subjects, one had a partial response and six had stable disease, for a disease control rate of 39%. In melanoma patients for whom a response was observed, there was evidence of local and systemic anti-cancer activity. The adverse event profile of Ad-RTS-hIL-12 + veledimex in both melanoma and breast cancer was predictable, reversible and characteristic of immune activation. The most common³ Grade 3 treatment emergent adverse events, or TEAEs, in breast cancer and melanoma included neutropenia and electrolyte abnormalities (21%) each, LFTs increased (16%), leukopenia (13%) and pyrexia, hypotension, lymphopenia, anemia, and cytokine release syndrome (11%) each. Importantly, all TEAEs and SAEs³ Grade 3 reversed rapidly upon discontinuation of veledimex oral dosing.
Also at the AACR 2014 Immunology and Immunotherapy Meeting in December 2014, together with Intrexon, we presented preclinical data supporting the potential for cytolytic activity against solid tumor targets with allogeneic, genetically-modified stem cells enabled for controlled release of cell-linking moieties, or CLMs, within the tumor micro-environment and preclinical data describing the development of a novel, high-throughput screening technology for rapidly identifying bi-specific antibodies capable of inducing targeted immunologic activity through the activation of T-cells or other immune cells against tumors. CLMs are small bi-specific antibody fragments capable of directing potent T-cell mediated tumor lysis by bridging the immunologic synapses of T-cells and surface targets on tumor cells. Previous studies have shown that the systemic distribution and pharmacokinetic profile of bi-specific antibodies limit their utility for many target/effector combinations. In two preclinical studies, Intrexon and Ziopharm researchers interrogated a large number of CLM-based effectors for their ability to activate white blood cells from peripheral blood and lyse receptor target-positive tumor cells. Allogeneic, tumor targeting stem cells were then genetically modified to express CLMs within the tumor microenvironment using the RTS® platform as a mechanism for providing spatial and temporal control. The first study demonstrated the ability of Intrexon’s proprietary image-based screening systems and rapid DNA assembly to screen a large number of EGFR and HER2 receptor-targeted CLM variants for their ability to recruit CD3+ T-cells and mediate selective cell killing against target positive cells in peripheral blood co-cultures. The image-based screening platform allowed for real time target cell killing information to be obtained, as well as kinetic cell morphologic analyses to understand the dynamics of killing activity, thereby shortening the developmental timeline to lead candidate selection. The second study validated these CLM candidates in scalable, allogeneic endometrial regenerative cells, or ERCs, genetically modified to express an anti-CD3-anti-EGFR CLM under RTS® ligand inducible control. Expression of CLMs under the RTS® inducible promoter provided effective control of CLM secretion and modulation of killing activity, with veledimex-dependent cytotoxicity of greater than 80% against an EGFR+ KRAS mutant lung cancer cell model. CLM-expressing ERCs were found to be effective in co-culture killing assays at cellular doses as low as 1% of target cells. These data supported the feasibility of localized cytolytic activity of CLM-secreting allogeneic cell therapy products against EGFR+ KRAS mutant solid tumor malignancies.
We have completed the Phase 2 monotherapy studies in melanoma and breast cancer using Ad-RTS-IL-12 + veledimex. Additionally, we expect a future trial with IL-12 in combination therapies with standard of care for
5.
breast cancer. As the treatment of advanced melanoma has undergone and continues to undergo a rapid evolution with the introduction and approval of highly promising new single and combination agents, the standard of care in this indication has become uncertain, resulting in a much more competitive and commercially unpredictable environment. As a result, we are pursuing intratumoral injection of Ad-RTS-IL-12 + veledimex in brain cancer and breast cancer, and will pause further development of Ad-RTS-IL-12 + veledimex in melanoma with intratumoral injection. However, through current strategic initiatives, we expect to utilize RTS-IL-12 + veledimex in cell based immunotherapy of melanoma and other cancers. We plan to initiate a Phase 1 trial to evaluate Ad-RTS-IL-12 + veledimex as a single agent in the treatment of patients with brain cancer in the first half of 2015.
CAR-T/cytokine programs
We are actively pursuing non-viral, genetic engineering technologies to develop novel CAR-T, NK and TCR cells. Combining this technology with Intrexon’s industrialized synthetic biologic engineering and clinically tested and validated RTS IL-12 modules, represents a differentiated approach to genetically modified CAR-T cell and other immune cells. Employing novel cell engineering techniques and multigenic gene programs, we expect to implement next-generation non-viral adoptive cellular therapies based on designer cytokines and CARs under
control of RTS® technology targeting both hematologic malignancies and solid tumors. We plan to leverage the synergy between the platforms to accelerate a promising synthetic immuno-oncology pipeline, with up to five CAR-T therapies expected to enter the clinic in 2015 and programs for the development of allogeneic CAR-T therapies that can be used off-the-shelf expected to be initiated in 2016.
Research suggests that T cells can be re-programmed to have a very strong anti-cancer therapeutic effect through the expression of CARs to redirect specificity to tumors without HLA restrictions. The signature event within this field has resulted from the infusion of T cells expressing CARs into patients with B cells leukemias and lymphomas. Many of these patients have responded to these new therapies with a durable and dramatic anti-tumor effect after infusing CD19-specific T cells. Despite the highly promising results that have been demonstrated by early researchers in the field, current technologies and approaches have shown a number of serious drawbacks, including toxicity, manufacturing complexity and expense. A particular problem is that infusions of T cells into patients with large amounts of disease have invariably led to significant issues of toxicity for recipient patients. These toxicities primarily involve three major, potentially catastrophic side-effects:
| | 1. | The rapid killing of tumor cells releases a large number intracellular constituents that are very toxic to various organs and is called “tumor lysis syndrome” that can be fatal, |
| | 2. | the supra-physiologic release of cytokines (“cytokine storm”) that causes fever, instability of blood pressure, mental status changes and on occasion, death, and |
| | 3. | on-target, and off-tissue toxicity represented by the concomitant damage of normal B cells and loss of humoral (antibody) immunity. |
We expect to be able to tightly control expansion and activation of CAR-T cells in the body, which has the potential to alleviate or abrogate these toxicities.
MD Anderson’s platform, which uses the exclusive Sleeping Beauty system, or SB system, generates and characterizes new CAR-T designs, which enables a high throughput approach to evaluate the CAR-T. This “EZ-CAR-T” non-viral system is used to fashion immuno-receptors that differ in specificity and ability to activate T cells. These CAR-T molecules are evaluated in a “go/no go” system based on serial killing and protection of T cells from activation-induced cell death. Non-viral gene transfer using the SB system is unique in the field of oncology. Examples of cell engineering techniques that we expect to employ with the SB system are induced pluripotent stem cell (iPSC) processing technologies combined with Laser-enabled Analysis and Processing, or LEAP®, which consists of computerized image-based selection and laser processing for very rapid cell identification and purification as well as AttSite® Recombinases, which involves stable, targeted gene integration
6.
and expression with proprietary serine recombinases. We believe the advanced DNA vectors derived from SB can be used to avoid the expense and manufacturing difficulty associated with creating CAR-T cells using viral vectors. After electroporation, the transposon/transposase improves the efficiency of integration of plasmids used to express CAR and other transgenes in T cells. Propagation of genetically modified T cells on activating and propagating cells, or AaPC, provide a competitive advantage over other non-viral methods of modification. The SB system combined with artificial antigen-presenting cells can selectively propagate and thus retrieve CAR+ T cells suitable for human application. T cells can also be genetically modified using these technologies to target a panel (several) cancer antigen targets. We are on the verge of implementing technology to manufacture “minimally-manipulated” T cells within days of gene transfer by electroporation.
We expect this platform will rapidly integrate with Intrexon’s RTS® and multigenic control gene programs. The programs are also designed and built for rapid transition to universal donor products or minimally manipulated point of care products. Dr. Laurence Cooper and colleagues at MD Anderson recently published research which demonstrated that transformed, primary, and pluripotent stem cells can be permanently modified to eliminate HLA-A expression, demonstrating how to generatea prioricells from one allogeneic donor for infusion into multiple recipients representing a significant step towards our goal of on-demand therapy that can bepre-deployed at multiple sites and infused when needed. The primary factor limiting the development of a universal donor product is the existence of graft-vs-host response, or GVHD. GVHD occurs because the newly transplanted cells regard the recipient’s body as foreign. When this happens, the newly transplanted cells attack the recipient’s body. Additional research from Dr. Cooper and colleagues at MD Anderson suggests that “universal” allogeneic T cells generated from one donor could be administered to multiple recipients. This is achieved by genetically editing CD19-specific CAR+ T cells to eliminate expression of the endogenousaß TCR, the gene responsible for triggering GVHD, without compromising CAR-dependent effector functions. Genetically modified T cells are generated using the SB system to stably introduce the CD19-specific CAR with subsequent permanent deletion ofa orb TCR chains with nucleases. The translation of the SB system and AaPC for use in clinical trials highlights how a nimble and cost-effective approach to developing genetically modified T cells can be used to implement clinical trials infusing next-generation T cells with improved therapeutic potential. We are expanding our initial trials targeting CD19 and planning to conduct additional trials with re-designed CAR-Ts expanding beyond CD19+ tumor cells.
Anticipated Milestones
We expect the following milestones to occur in 2015 and 2016:
| | • | | Intra-tumoral IL-12 RheoSwitch® programs: |
| | • | | Early data is expected in the fourth quarter of 2015 for our Phase 1/2 study in Breast Cancer with standard of care. |
| | • | | Early data is expected in the fourth quarter of 2015 for our Phase 1 study of Glioblastoma multiforme (GBM). |
| | • | | We expect to initiate two Phase 1 studies of next-generation CD19 CARs in the second quarter of 2015. |
| | • | | We expect to initiate a Phase 1 study of next-generation CAR with an inducible cytokine in the fourth quarter of 2015. |
| | • | | We expect to initiate a novel CAR for myeloid malignancies in the fourth quarter of 2015. |
| | • | | We expect to receive interim data on two Phase 1 CARs studies in advanced leukemia and lymphomas in the fourth quarter of 2015. |
7.
| | • | | We expect to initiate other leukemia and solid tumor CAR-T cell studies in 2016. |
| | • | | We expect to initiate allogeneic, off-the-shelf T-cell studies in 2016. |
Data from all programs is expected in 2015 and 2016. We are also evaluating additional potential preclinical candidates and continuing discovery efforts aimed at identifying other potential product candidates under our Channel Agreement with Intrexon. In addition, we may seek to enhance our pipeline in synthetic biology through focused strategic transactions, which may include acquisitions, partnerships and in-licensing activities. We are actively seeking to out-license some or all of our small molecule programs to further support our synthetic biology efforts.
Small molecule programs
In addition to our synthetic biology programs discussed above, we have certain rights to three small molecule programs, palifosfamide (or isophosphoramide mustard), darinaparsin and indibulin, all of which we are no longer actively pursuing. With respect to palifosfamide, in March 2013, we announced that the pivotal Phase 3 study, PICASSO 3, did not meet its primary endpoint of progression-free survival, and that we would terminate our development program in metastatic soft tissue sarcoma. In addition, we recently received the overall survival endpoint data from our study of palifosfamide in combination with carboplatin and etoposide chemotherapy versus carboplatin and etoposide alone in chemotherapy naïve patients with metastatic small cell lung cancer, which we refer to as MATISSE, which data will be submitted for presentation at a scientific forum during the first half of 2015.
We are seeking transactions with third parties for the possible out-license of palifosfamide. With respect to darinaparsin, we have entered into an amended and restated global licensing agreement with Solasia Pharma K.K., or Solasia, on July 31, 2014 granting Solasia an exclusive worldwide license to develop and commercialize darinaparsin, and related organoarsenic molecules, in both intravenous and oral forms in all indications for human use. In exchange, we will be eligible to receive from Solasia development-and sales-based milestones, a royalty on net sales of darinaparsin, once commercialized, and a percentage of any sublicense revenues generated by Solasia. During 2014, we determined to no longer pursue clinical development of indibulin.
Recent developments
Expected cash as of December 31, 2014
Based upon preliminary estimates, we expect to have approximately $43 million in cash and cash equivalents as of December 31, 2014. We have not yet completed our year-end financial close process for the year ended December 31, 2014. This estimate of our cash and cash equivalents as of December 31, 2014 is based on preliminary estimates of our financial results that we expect to report for the period. These estimates are subject to completion of our financial closing procedures. Our independent registered public accounting firm, McGladrey LLP, has not audited, reviewed or compiled these estimates. These estimates are not a comprehensive statement of our financial results for the year ended December 31, 2014 and our actual results may differ materially from these estimates as a result of the completion of our financial closing procedures, final adjustments and other developments arising between now and the time that our financial results for this period are finalized.
Development plans
Our current plan is to raise additional capital to support further development activities for our strategic product candidates. Based upon our current plans and without taking into account the net proceeds of this offering, we anticipate that our cash resources will be sufficient to fund our operations into the third quarter of 2015. This forecast of cash resources is forward-looking information that involves risks and uncertainties, and the actual
8.
amount of our expenses could vary materially and adversely as a result of a number of factors, including the factors discussed in the “Risk Factors” section of the applicable prospectus supplement and the uncertainties applicable to our forecast for the overall sufficiency of our capital resources. We have based our estimates on assumptions that may prove to be wrong, and our expenses could prove to be significantly higher than we currently anticipate. In particular, pursuant to the MD Anderson License, MD Anderson agreed to transfer to us certain existing research programs described in the MD Anderson License and we, together with Intrexon, agreed to enter into a research and development agreement pursuant to which we will provide funding for certain research and development activities of MD Anderson for a period of three years from the date of the MD Anderson License, in an amount between $15 and $20 million per year. In addition, we also expect to enter into additional collaboration and technology transfer agreements with MD Anderson and Intrexon to accelerate technology and clinical development of these product candidates. We expect to increase the level of our overall research and development expenses significantly going forward as a result of each of these items. Further, in light of our entry into the MD Anderson License, we expect to establish operations in Houston, Texas that will enable us to join and collaborate with the MD Anderson academic and medical community, which may require that we add headcount in the future, and which could add to our general and administrative expenses going forward. Although our forecasts for expenses and the sufficiency of our capital resources takes into account our plans to develop the technology licensed from MD Anderson and our obligations under the MD Anderson License, the MD Anderson License was entered into on January 13, 2015 and is only beginning to be implemented, therefore the actual costs associated therewith may be significantly in excess of forecasted amounts.
Corporate Information
We originally incorporated in Colorado in September 1998 (under the name Net Escapes, Inc.) and later changed our name to “EasyWeb, Inc.” in February 1999. We re-incorporated in Delaware on May 16, 2005 under the same name. On September 13, 2005, we completed a “reverse” acquisition of privately held ZIOPHARM, Inc., a Delaware corporation. To effect this transaction, we caused ZIO Acquisition Corp., our wholly-owned subsidiary, to merge with and into ZIOPHARM, Inc., with ZIOPHARM, Inc. surviving as our wholly owned subsidiary. In accordance with the terms of the merger, the outstanding common stock of ZIOPHARM, Inc. automatically converted into the right to receive an aggregate of approximately 97.3% of our outstanding common stock (after giving effect to the transaction). Following the merger, we caused ZIOPHARM, Inc. to merge with and into us and we changed our name to “ZIOPHARM Oncology, Inc.” Although EasyWeb, Inc. was the legal acquirer in the transaction, we accounted for the transaction as a reverse acquisition under generally accepted accounting principles. As a result, ZIOPHARM, Inc. became the registrant with the Commission and the historical financial statements of ZIOPHARM, Inc. became our historical financial statements.
Our principal executive offices are located at One First Avenue, Parris Building 34, Navy Yard Plaza, Boston, Massachusetts 02129, and our telephone number is (617) 259-1970. Our internet site is www.ziopharm.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this prospectus, and you should not consider it part of this prospectus or part of any prospectus supplement.
9.
RISK FACTORS
Investing in our securities involves a high degree of risk. You should carefully review the risks and uncertainties described under the heading “Risk Factors” contained in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus and the applicable prospectus supplement, before deciding whether to purchase any of the securities being offered. Each of the risk factors could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities, and the occurrence of any of these risks might cause you to lose all or part of your investment. Moreover, the risks described are not the only ones that we face. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations.
10.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference herein contain, and any prospectus supplement or free writing prospectus may contain, forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. All statements other than statements of historical facts contained in this prospectus and the documents incorporated by reference herein contain, and any prospectus supplement or free writing prospectus are forward-looking statements. These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. Forward-looking statements may include, but are not limited to statements about:
| | • | | the progress, timing and results of preclinical and clinical trials involving our drug candidates; |
| | • | | the progress of our research and development programs; |
| | • | | our plans or others’ plans to conduct future clinical trials or research and development efforts; |
| | • | | the risk that final trial data may not support interim analysis of the viability of our drug candidates; |
| | • | | our plans and expectations regarding partnering our drug candidates; |
| | • | | the benefits to be derived from relationships with our collaborators; |
| | • | | the receipt or anticipated receipt of regulatory clearances and approvals; |
| | • | | estimates of the potential markets for our drug candidates; |
| | • | | our ability to adequately protect our intellectual property rights; |
| | • | | the use of proceeds from this offering; |
| | • | | our estimates of future revenues and profitability; |
| | • | | completive risks in our industry; |
| | • | | our estimates regarding our capital requirements and our ability to control costs; and |
| | • | | our need for additional funding and the period through which we anticipate our resources will sufficient to fund operations. |
In some cases, you can identify forward-looking statements by terms such as “may”, “will”, “should”, “could”, “would”, “expects”, “plans”, “anticipates”, “believes”, “estimates”, “projects”, “predicts”, “potential” and similar expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We discuss many of these risks in greater detail under the heading “Risk Factors” in the applicable prospectus supplement or free writing prospectus and in our reports filed from time to time under the Securities Act and/or the Exchange Act. We encourage you to read these filings as they are made. Also, these forward-looking statements represent our estimates and assumptions only as of the date of the document containing the applicable statement.
You should read this prospectus, the documents incorporated by reference herein, and any prospectus supplement or free writing prospectus that we have authorized for use in connection with this offering completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in the foregoing documents by these cautionary statements.
Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments. Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements.
11.
RATIO OF EARNINGS TO FIXED CHARGES AND TO
COMBINED FIXED CHARGES AND PREFERENCE DIVIDENDS
The following table shows our ratio of earnings to fixed charges and the ratio of earnings to combined fixed charges and preference dividends for the periods indicated.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Nine Months
Ended
September 30,
2014 | |
| | | 2009 | | | 2010 | | | 2011 | | | 2012 | | | 2013 | | |
Ratio of earnings to fixed charges | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Ratio of earnings to combined fixed charges and preference dividends | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
For purposes of computing the ratio of earnings to fixed charges and the ratio of earnings to our combined fixed charges and preference dividends, earnings consist of our net income (loss) before tax benefit (provision) for the period plus fixed charges. Fixed charges consist of interest expense and amortized premiums, discounts and capitalized expenses related to indebtedness. The ratio of earnings to fixed charges and the ratio of earnings to combined fixed charges and preference dividends were less than one-to-one for each of the periods presented. Earnings were insufficient to cover fixed charges by $7.6 million in 2009, $32.7 million in 2010, $63.8 million in 2011, $96.1 million in 2012, $57.1 million in 2013 and $21.4 million in the nine months ended September 30, 2014.
12.
USE OF PROCEEDS
We will retain broad discretion over the use of the net proceeds from the sale of our securities offered by this prospectus. Unless we indicate otherwise in the applicable prospectus supplement or in any related free writing prospectus we have authorized for use in connection with a specific offering, we anticipate that any net proceeds will be used for working capital and general corporate purposes. We will set forth in the applicable prospectus supplement or free writing prospectus our intended use for the net proceeds received from the sale of securities sold pursuant to that prospectus supplement or free writing prospectus.
DILUTION
We will set forth in a prospectus supplement the following information regarding any material dilution of the equity interests of investors purchasing securities in an offering under this prospectus:
| | • | | the net tangible book value per share of our equity securities before and after the offering; |
| | • | | the amount of the increase in such net tangible book value per share attributable to the cash payments made by purchasers in the offering; and |
| | • | | the amount of the immediate dilution from the public offering price which will be absorbed by such purchasers. |
13.
DESCRIPTION OF CAPITAL STOCK
As of the date of this prospectus, our authorized capital stock consists of 280,000,000 shares, comprised of 250,000,000 shares of common stock, par value $.001 per share, and 30,000,000 shares of preferred stock, par value $.001 per share. As of January 30, 2015, there were 104,428,495 shares of common stock and no shares of preferred stock issued and outstanding. Our common stock is traded on the NASDAQ Capital Market under the symbol “ZIOP”.
The following description summarizes the material terms of our capital stock. This summary is, however, subject to the provisions of our certificate of incorporation and bylaws. For greater detail about our capital stock, please refer to our certificate of incorporation and bylaws.
Common Stock
Voting. The holders of our common stock are entitled to one vote for each outstanding share of common stock owned by such stockholder on every matter properly submitted to the stockholders for their vote. Stockholders are not entitled to vote cumulatively for the election of directors. Because of this, the holders of a majority of the shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they should so choose. At any meeting of the stockholders, a quorum as to any matter shall consist of a majority of the votes entitled to be cast on the matter, except where a larger quorum is required by law, by our certificate of incorporation or by our bylaws.
Dividend Rights. Holders of our common stock are entitled to receive ratably dividends and other distributions of cash or any other right or property as may be declared by our board of directors out of our assets or funds legally available for such dividends or distributions. The dividend rights of holders of common stock are subject to the dividend rights of the holders of any series of preferred stock that may be issued and outstanding from time to time.
Liquidation Rights. In the event of any voluntary or involuntary liquidation, dissolution or winding up of our affairs, holders of our common stock would be entitled to share ratably in our assets that are legally available for distribution to stockholders after payment of liabilities. If we have any preferred stock outstanding at such time, the holders of such preferred stock may be entitled to distribution and/or liquidation preferences that require us to pay the applicable distribution to the holders of preferred stock before paying distributions to the holders of common stock.
Rights and Preferences. Holders of our common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that we may designate and issue in the future.
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company.
See “Certain Provisions of Delaware Law, the Certificate of Incorporation and Bylaws” for a description of provisions of our certificate of incorporation and bylaws which may have the effect of delaying, deferring or preventing changes in the our control.
Preferred Stock
Pursuant to our amended and restated certificate of incorporation, our board of directors has the authority, without stockholder approval, subject to limitations prescribed by law, to provide for the issuance of up to 30,000,000 shares of preferred stock in one or more series, and by filing a certificate pursuant to the applicable law of the State of Delaware, to establish from time to time the number of shares to be included in each such series, and to fix the designation, powers, preferences and rights of the shares of each series and any qualifications, limitations or restrictions thereof, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
14.
We will fix the designations, voting powers, preferences and rights of the preferred stock of each series, as well as the qualifications, limitations or restrictions thereof, in the certificate of designation relating to that series. We will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the Commission, the form of any certificate of designation that describes the terms of the series of preferred stock we are offering before the issuance of that series of preferred stock. This description will include:
| | • | | the title and stated value; |
| | • | | the number of shares offered; |
| | • | | the liquidation preference per share; |
| | • | | the purchase price per share; |
| | • | | the dividend rate(s), period(s) and/or payment date(s) or method(s) of calculation for dividends; |
| | • | | whether dividends are cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate; |
| | • | | our right, if any, to defer payment of dividends and the maximum length of any such deferral period; |
| | • | | the procedures for any auction and remarketing, if any; |
| | • | | the provisions for a sinking fund, if any; |
| | • | | the provision for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights; |
| | • | | any listing of the preferred stock on any securities exchange or market; |
| | • | | the terms and conditions, if applicable, upon which the preferred stock will be convertible into common stock, including the conversion price (or manner of calculation) and conversion period; |
| | • | | whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange price, or how it will be calculated, and the exchange period; |
| | • | | voting rights, if any, of the preferred stock; |
| | • | | preemptive rights, if any; |
| | • | | restrictions on transfer, sale or other assignment, if any; |
| | • | | whether interests in the preferred stock will be represented by depositary shares; |
| | • | | a discussion of any material and/or special U.S. federal income tax considerations applicable to the preferred stock; |
| | • | | the relative ranking and preferences of the preferred stock as to dividend rights and rights upon the liquidation, dissolution or winding up of our affairs; |
| | • | | any limitations on issuance of any class or series of preferred stock ranking senior to or on a parity with the class or series of preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of our affairs; and |
| | • | | any other specific terms, preferences, rights, limitations or restrictions of the preferred stock. |
Our board of directors could authorize the issuance of shares of preferred stock with terms and conditions that could have the effect of discouraging a takeover or other transaction that might involve a premium price for holders of the shares or which holders might believe to be in their best interests. The issuance of preferred stock could adversely affect the voting power, conversion or other rights of holders of common stock and reduce the likelihood that common stockholders will receive dividend payments and payments upon liquidation.
15.
The laws of the state of Delaware, the state of our incorporation, provide that the holders of preferred stock will have the right to vote separately as a class on any proposal involving fundamental changes in the rights of holders of such preferred stock. This right is in addition to any voting rights that may be provided for in the applicable certificate of designation.
The transfer agent and registrar for any series of preferred stock will be set forth in the applicable prospectus supplement.
16.
DESCRIPTION OF DEBT SECURITIES
We may issue debt securities from time to time, in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. While the terms we have summarized below will apply generally to any debt securities that we may offer under this prospectus, we will describe the particular terms of any debt securities that we may offer in more detail in the applicable prospectus supplement. The terms of any debt securities offered under a prospectus supplement may differ from the terms described below. Unless the context requires otherwise, whenever we refer to the indenture, we also are referring to any supplemental indentures that specify the terms of a particular series of debt securities.
We will issue the debt securities under the indenture that we will enter into with the trustee named in the indenture. The indenture will be qualified under the Trust Indenture Act of 1939, as amended, or the Trust Indenture Act. We have filed the form of indenture as an exhibit to the registration statement of which this prospectus is a part, and supplemental indentures and forms of debt securities containing the terms of the debt securities being offered will be filed as exhibits to the registration statement of which this prospectus is a part or will be incorporated by reference from reports that we file with the SEC.
The following summary of material provisions of the debt securities and the indenture is subject to, and qualified in its entirety by reference to, all of the provisions of the indenture applicable to a particular series of debt securities. We urge you to read the applicable prospectus supplements and any related free writing prospectuses related to the debt securities that we may offer under this prospectus, as well as the complete indenture that contains the terms of the debt securities.
General
The indenture does not limit the amount of debt securities that we may issue. It provides that we may issue debt securities up to the principal amount that we may authorize and may be in any currency or currency unit that we may designate. Except for the limitations on consolidation, merger and sale of all or substantially all of our assets contained in the indenture, the terms of the indenture do not contain any covenants or other provisions designed to give holders of any debt securities protection against changes in our operations, financial condition or transactions involving us.
We may issue the debt securities issued under the indenture as “discount securities,” which means they may be sold at a discount below their stated principal amount. These debt securities, as well as other debt securities that are not issued at a discount, may be issued with “original issue discount,” or OID, for U.S. federal income tax purposes because of interest payment and other characteristics or terms of the debt securities. Material U.S. federal income tax considerations applicable to debt securities issued with OID will be described in more detail in any applicable prospectus supplement.
We will describe in the applicable prospectus supplement the terms of the series of debt securities being offered, including:
| | • | | the title of the series of debt securities; |
| | • | | any limit upon the aggregate principal amount that may be issued; |
| | • | | the maturity date or dates; |
| | • | | the form of the debt securities of the series; |
| | • | | the applicability of any guarantees; |
| | • | | whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; |
| | • | | whether the debt securities rank as senior debt, senior subordinated debt, subordinated debt or any combination thereof, and the terms of any subordination; |
17.
| | • | | if the price (expressed as a percentage of the aggregate principal amount thereof) at which such debt securities will be issued is a price other than the principal amount thereof, the portion of the principal amount thereof payable upon declaration of acceleration of the maturity thereof, or if applicable, the portion of the principal amount of such debt securities that is convertible into another security or the method by which any such portion shall be determined; |
| | • | | the interest rate or rates, which may be fixed or variable, or the method for determining the rate and the date interest will begin to accrue, the dates interest will be payable and the regular record dates for interest payment dates or the method for determining such dates; |
| | • | | our right, if any, to defer payment of interest and the maximum length of any such deferral period; |
| | • | | if applicable, the date or dates after which, or the period or periods during which, and the price or prices at which, we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions and the terms of those redemption provisions; |
| | • | | the date or dates, if any, on which, and the price or prices at which we are obligated, pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option to purchase, the series of debt securities and the currency or currency unit in which the debt securities are payable; |
| | • | | the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral multiple thereof; |
| | • | | any and all terms, if applicable, relating to any auction or remarketing of the debt securities of that series and any security for our obligations with respect to such debt securities and any other terms which may be advisable in connection with the marketing of debt securities of that series; |
| | • | | whether the debt securities of the series shall be issued in whole or in part in the form of a global security or securities; the terms and conditions, if any, upon which such global security or securities may be exchanged in whole or in part for other individual securities; and the depositary for such global security or securities; |
| | • | | if applicable, the provisions relating to conversion or exchange of any debt securities of the series and the terms and conditions upon which such debt securities will be so convertible or exchangeable, including the conversion or exchange price, as applicable, or how it will be calculated and may be adjusted, any mandatory or optional (at our option or the holders’ option) conversion or exchange features, the applicable conversion or exchange period and the manner of settlement for any conversion or exchange; |
| | • | | if other than the full principal amount thereof, the portion of the principal amount of debt securities of the series which shall be payable upon declaration of acceleration of the maturity thereof; |
| | • | | additions to or changes in the covenants applicable to the particular debt securities being issued, including, among others, the consolidation, merger or sale covenant; |
| | • | | additions to or changes in the events of default with respect to the securities and any change in the right of the trustee or the holders to declare the principal, premium, if any, and interest, if any, with respect to such securities to be due and payable; |
| | • | | additions to or changes in or deletions of the provisions relating to covenant defeasance and legal defeasance; |
| | • | | additions to or changes in the provisions relating to satisfaction and discharge of the indenture; |
| | • | | additions to or changes in the provisions relating to the modification of the indenture both with and without the consent of holders of debt securities issued under the indenture; |
| | • | | the currency of payment of debt securities if other than U.S. dollars and the manner of determining the equivalent amount in U.S. dollars; |
18.
| | • | | whether interest will be payable in cash or additional debt securities at our or the holders’ option and the terms and conditions upon which the election may be made; |
| | • | | the terms and conditions, if any, upon which we will pay amounts in addition to the stated interest, premium, if any and principal amounts of the debt securities of the series to any holder that is not a “United States person” for federal tax purposes; |
| | • | | any restrictions on transfer, sale or assignment of the debt securities of the series; and |
| | • | | any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities, any other additions or changes in the provisions of the indenture, and any terms that may be required by us or advisable under applicable laws or regulations. |
Conversion or Exchange Rights
We will set forth in the applicable prospectus supplement the terms on which a series of debt securities may be convertible into or exchangeable for our common stock or our other securities. We will include provisions as to settlement upon conversion or exchange and whether conversion or exchange is mandatory, at the option of the holder or at our option. We may include provisions pursuant to which the number of shares of our common stock or our other securities that the holders of the series of debt securities receive would be subject to adjustment.
Consolidation, Merger or Sale
Unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, the indenture will not contain any covenant that restricts our ability to merge or consolidate, or sell, convey, transfer or otherwise dispose of our assets as an entirety or substantially as an entirety. However, any successor to or acquirer of such assets (other than a subsidiary of ours) must assume all of our obligations under the indenture or the debt securities, as appropriate.
Events of Default under the Indenture
Unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, the following are events of default under the indenture with respect to any series of debt securities that we may issue:
| | • | | if we fail to pay any installment of interest on any series of debt securities, as and when the same shall become due and payable, and such default continues for a period of 90 days; provided, however, that a valid extension of an interest payment period by us in accordance with the terms of any indenture supplemental thereto shall not constitute a default in the payment of interest for this purpose; |
| | • | | if we fail to pay the principal of, or premium, if any, on any series of debt securities as and when the same shall become due and payable whether at maturity, upon redemption, by declaration or otherwise, or in any payment required by any sinking or analogous fund established with respect to such series; provided, however, that a valid extension of the maturity of such debt securities in accordance with the terms of any indenture supplemental thereto shall not constitute a default in the payment of principal or premium, if any; |
| | • | | if we fail to observe or perform any other covenant or agreement contained in the debt securities or the indenture, other than a covenant specifically relating to another series of debt securities, and our failure continues for 90 days after we receive written notice of such failure, requiring the same to be remedied and stating that such is a notice of default thereunder, from the trustee or holders of at least 25% in aggregate principal amount of the outstanding debt securities of the applicable series; and |
| | • | | if specified events of bankruptcy, insolvency or reorganization occur. |
If an event of default with respect to debt securities of any series occurs and is continuing, other than an event of default specified in the last bullet point above, the trustee or the holders of at least 25% in aggregate
19.
principal amount of the outstanding debt securities of that series, by notice to us in writing, and to the trustee if notice is given by such holders, may declare the unpaid principal of, premium, if any, and accrued interest, if any, due and payable immediately. If an event of default specified in the last bullet point above occurs with respect to us, the principal amount of and accrued interest, if any, of each issue of debt securities then outstanding shall be due and payable without any notice or other action on the part of the trustee or any holder.
The holders of a majority in principal amount of the outstanding debt securities of an affected series may waive any default or event of default with respect to the series and its consequences, except defaults or events of default regarding payment of principal, premium, if any, or interest, unless we have cured the default or event of default in accordance with the indenture. Any waiver shall cure the default or event of default.
Subject to the terms of the indenture, if an event of default under an indenture shall occur and be continuing, the trustee will be under no obligation to exercise any of its rights or powers under such indenture at the request or direction of any of the holders of the applicable series of debt securities, unless such holders have offered the trustee reasonable indemnity. The holders of a majority in principal amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting any proceeding for any remedy available to the trustee, or exercising any trust or power conferred on the trustee, with respect to the debt securities of that series, provided that:
| | • | | the direction so given by the holder is not in conflict with any law or the applicable indenture; and |
| | • | | subject to its duties under the Trust Indenture Act, the trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved in the proceeding. |
A holder of the debt securities of any series will have the right to institute a proceeding under the indenture or to appoint a receiver or trustee, or to seek other remedies only if:
| | • | | the holder has given written notice to the trustee of a continuing event of default with respect to that series; |
| | • | | the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request, |
| | • | | such holders have offered to the trustee indemnity satisfactory to it against the costs, expenses and liabilities to be incurred by the trustee in compliance with the request; and |
| | • | | the trustee does not institute the proceeding, and does not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series other conflicting directions within 90 days after the notice, request and offer. |
These limitations do not apply to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the debt securities.
We will periodically file statements with the trustee regarding our compliance with specified covenants in the indenture.
Modification of Indenture; Waiver
We and the trustee may change an indenture without the consent of any holders with respect to specific matters:
| | • | | to cure any ambiguity, defect or inconsistency in the indenture or in the debt securities of any series; |
| | • | | to comply with the provisions described above under “Description of Debt Securities — Consolidation, Merger or Sale;” |
20.
| | • | | to provide for uncertificated debt securities in addition to or in place of certificated debt securities; |
| | • | | to add to our covenants, restrictions, conditions or provisions such new covenants, restrictions, conditions or provisions for the benefit of the holders of all or any series of debt securities, to make the occurrence, or the occurrence and the continuance, of a default in any such additional covenants, restrictions, conditions or provisions an event of default or to surrender any right or power conferred upon us in the indenture; |
| | • | | to add to, delete from or revise the conditions, limitations, and restrictions on the authorized amount, terms, or purposes of issue, authentication and delivery of debt securities, as set forth in the indenture; |
| | • | | to make any change that does not adversely affect the interests of any holder of debt securities of any series in any material respect; |
| | • | | to provide for the issuance of and establish the form and terms and conditions of the debt securities of any series as provided above under “Description of Debt Securities — General” to establish the form of any certifications required to be furnished pursuant to the terms of the indenture or any series of debt securities, or to add to the rights of the holders of any series of debt securities; |
| | • | | to evidence and provide for the acceptance of appointment under any indenture by a successor trustee; or |
| | • | | to comply with any requirements of the SEC in connection with the qualification of any indenture under the Trust Indenture Act. |
In addition, under the indenture, the rights of holders of a series of debt securities may be changed by us and the trustee with the written consent of the holders of at least a majority in aggregate principal amount of the outstanding debt securities of each series that is affected. However, unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, we and the trustee may make the following changes only with the consent of each holder of any outstanding debt securities affected:
| | • | | extending the fixed maturity of any debt securities of any series; |
| | • | | reducing the principal amount, reducing the rate of or extending the time of payment of interest, or reducing any premium payable upon the redemption of any series of any debt securities; or |
| | • | | reducing the percentage of debt securities, the holders of which are required to consent to any amendment, supplement, modification or waiver. |
Discharge
Each indenture provides that we can elect to be discharged from our obligations with respect to one or more series of debt securities, except for specified obligations, including obligations to:
| | • | | register the transfer or exchange of debt securities of the series; |
| | • | | replace stolen, lost or mutilated debt securities of the series; |
| | • | | pay principal of and premium and interest on any debt securities of the series; |
| | • | | maintain paying agencies; |
| | • | | hold monies for payment in trust; |
| | • | | recover excess money held by the trustee; |
| | • | | compensate and indemnify the trustee; and |
| | • | | appoint any successor trustee. |
21.
In order to exercise our rights to be discharged, we must deposit with the trustee money or government obligations sufficient to pay all the principal of, any premium, if any, and interest on, the debt securities of the series on the dates payments are due.
Form, Exchange and Transfer
We will issue the debt securities of each series only in fully registered form without coupons and, unless we provide otherwise in the applicable prospectus supplement, in denominations of $1,000 and any integral multiple thereof. The indenture provides that we may issue debt securities of a series in temporary or permanent global form and as book-entry securities that will be deposited with, or on behalf of, The Depository Trust Company, or DTC, or another depositary named by us and identified in the applicable prospectus supplement with respect to that series. To the extent the debt securities of a series are issued in global form and as book-entry, a description of terms relating to any book-entry securities will be set forth in the applicable prospectus supplement.
At the option of the holder, subject to the terms of the indenture and the limitations applicable to global securities described in the applicable prospectus supplement, the holder of the debt securities of any series can exchange the debt securities for other debt securities of the same series, in any authorized denomination and of like tenor and aggregate principal amount.
Subject to the terms of the indenture and the limitations applicable to global securities set forth in the applicable prospectus supplement, holders of the debt securities may present the debt securities for exchange or for registration of transfer, duly endorsed or with the form of transfer endorsed thereon duly executed if so required by us or the security registrar, at the office of the security registrar or at the office of any transfer agent designated by us for this purpose. Unless otherwise provided in the debt securities that the holder presents for transfer or exchange, we will impose no service charge for any registration of transfer or exchange, but we may require payment of any taxes or other governmental charges.
We will name in the applicable prospectus supplement the security registrar, and any transfer agent in addition to the security registrar, that we initially designate for any debt securities. We may at any time designate additional transfer agents or rescind the designation of any transfer agent or approve a change in the office through which any transfer agent acts, except that we will be required to maintain a transfer agent in each place of payment for the debt securities of each series.
If we elect to redeem the debt securities of any series, we will not be required to:
| | • | | issue, register the transfer of, or exchange any debt securities of that series during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption of any debt securities that may be selected for redemption and ending at the close of business on the day of the mailing; or |
| | • | | register the transfer of or exchange any debt securities so selected for redemption, in whole or in part, except the unredeemed portion of any debt securities we are redeeming in part. |
Information Concerning the Trustee
The trustee, other than during the occurrence and continuance of an event of default under an indenture, undertakes to perform only those duties as are specifically set forth in the applicable indenture. Upon an event of default under an indenture, the trustee must use the same degree of care as a prudent person would exercise or use in the conduct of his or her own affairs. Subject to this provision, the trustee is under no obligation to exercise any of the powers given it by the indenture at the request of any holder of debt securities unless it is offered reasonable security and indemnity against the costs, expenses and liabilities that it might incur.
22.
Payment and Paying Agents
Unless we otherwise indicate in the applicable prospectus supplement, we will make payment of the interest on any debt securities on any interest payment date to the person in whose name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular record date for the interest.
We will pay principal of and any premium and interest on the debt securities of a particular series at the office of the paying agents designated by us, except that unless we otherwise indicate in the applicable prospectus supplement, we will make interest payments by check that we will mail to the holder or by wire transfer to certain holders. Unless we otherwise indicate in the applicable prospectus supplement, we will designate the corporate trust office of the trustee as our sole paying agent for payments with respect to debt securities of each series. We will name in the applicable prospectus supplement any other paying agents that we initially designate for the debt securities of a particular series. We will maintain a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying agent or the trustee for the payment of the principal of or any premium or interest on any debt securities that remains unclaimed at the end of two years after such principal, premium or interest has become due and payable will be repaid to us, and the holder of the debt security thereafter may look only to us for payment thereof.
Governing Law
The indenture and the debt securities will be governed by and construed in accordance with the internal laws of the State of New York, except to the extent that the Trust Indenture Act of 1939 is applicable.
23.
DESCRIPTION OF WARRANTS
The following description, together with the additional information we may include in any applicable prospectus supplements and in any related free writing prospectuses that we may authorize to be distributed to you, summarizes the material terms and provisions of the warrants that we may offer under this prospectus, which may consist of warrants to purchase common stock, preferred stock or debt securities and be issued in one or more series. Warrants may be offered independently or in combination with common stock, preferred stock or debt securities offered by any prospectus supplement. While the terms we have summarized below will apply generally to any warrants that we may offer under this prospectus, we will describe the particular terms of any series of warrants in more detail in the applicable prospectus supplement. The following description of warrants will apply to the warrants offered by this prospectus unless we provide otherwise in the applicable prospectus supplement. The applicable prospectus supplement for a particular series of warrants may specify different or additional terms.
We have filed forms of the warrant agreements and forms of warrant certificates containing the terms of the warrants that may be offered as exhibits to the registration statement of which this prospectus is a part. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of warrant and/or the warrant agreement and warrant certificate, as applicable, that describe the terms of the particular series of warrants we are offering, and any supplemental agreements, before the issuance of such warrants. The following summaries of material terms and provisions of the warrants are subject to, and qualified in their entirety by reference to, all the provisions of the form of warrant and/or the warrant agreement and warrant certificate, as applicable, and any supplemental agreements applicable to a particular series of warrants that we may offer under this prospectus. We urge you to read the applicable prospectus supplement related to the particular series of warrants that we may offer under this prospectus, as well as any related free writing prospectuses, and the complete form of warrant and/or the warrant agreement and warrant certificate, as applicable, and any supplemental agreements, that contain the terms of the warrants.
General
We will describe in the applicable prospectus supplement the terms of the series of warrants being offered, including:
| | • | | the offering price and aggregate number of warrants offered; |
| | • | | the currency for which the warrants may be purchased; |
| | • | | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| | • | | in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at, and currency in which, this principal amount of debt securities may be purchased upon such exercise; |
| | • | | in the case of warrants to purchase common stock or preferred stock, the number of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which these shares may be purchased upon such exercise; |
| | • | | the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreements and the warrants; |
| | • | | the terms of any rights to redeem or call the warrants; |
| | • | | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
| | • | | the dates on which the right to exercise the warrants will commence and expire; |
| | • | | the manner in which the warrant agreements and warrants may be modified; |
24.
| | • | | a discussion of any material or special U.S. federal income tax considerations of holding or exercising the warrants; |
| | • | | the terms of the securities issuable upon exercise of the warrants; and |
| | • | | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including:
| | • | | in the case of warrants to purchase debt securities, the right to receive payments of principal of, or premium, if any, or interest on, the debt securities purchasable upon exercise or to enforce covenants in the applicable indenture; or |
| | • | | in the case of warrants to purchase common stock or preferred stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any. |
Exercise of Warrants
Each warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable prospectus supplement. The warrants may be exercised as set forth in the prospectus supplement relating to the warrants offered. Unless we otherwise specify in the applicable prospectus supplement, warrants may be exercised at any time up to the close of business on the expiration date set forth in the prospectus supplement relating to the warrants offered thereby. After the close of business on the expiration date, unexercised warrants will become void.
Upon receipt of payment and the warrant or warrant certificate, as applicable, properly completed and duly executed at the corporate trust office of the warrant agent, if any, or any other office, including ours, indicated in the prospectus supplement, we will, as soon as practicable, issue and deliver the securities purchasable upon such exercise. If less than all of the warrants (or the warrants represented by such warrant certificate) are exercised, a new warrant or a new warrant certificate, as applicable, will be issued for the remaining warrants.
Governing Law
Unless we otherwise specify in the applicable prospectus supplement, the warrants and any warrant agreements will be governed by and construed in accordance with the laws of the State of New York.
Enforceability of Rights by Holders of Warrants
Each warrant agent, if any, will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action its right to exercise, and receive the securities purchasable upon exercise of, its warrants.
25.
CERTAIN PROVISIONS OF DELAWARE LAW,
THE CERTIFICATE OF INCORPORATION AND BYLAWS
Limitations on Directors’ Liability
Our amended and restated certificate of incorporation and our bylaws contain provisions indemnifying our directors and officers to the fullest extent permitted by law. In addition, as permitted by Delaware law, our amended and restated certificate of incorporation provides that no director will be liable to us or our stockholders for monetary damages for breach of certain fiduciary duties as a director. The effect of this provision is to restrict our rights and the rights of our stockholders in derivative suits to recover monetary damages against a director for breach of certain fiduciary duties as a director, except that a director will be personally liable for:
| | • | | the benefits to be derived from relationships with our collaborators; |
| | • | | any breach of his or her duty of loyalty to the registrant or its stockholders; |
| | • | | acts or omissions not in good faith which involve intentional misconduct or a knowing violation of law; |
| | • | | the payment of dividends or the redemption or purchase of stock in violation of Delaware law; or |
| | • | | any transaction from which the director derived an improper personal benefit. |
This provision does not affect a director’s liability under the federal securities laws.
To the extent that our directors, officers and controlling persons are indemnified under the provisions contained in our amended and restated certificate of incorporation, Delaware law or contractual arrangements against liabilities arising under the Securities Act, we have been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act, and is therefore unenforceable.
Provisions that May Have an Anti-Takeover Effect
Certain provisions set forth in our amended and restated certificate of incorporation, bylaws and in Delaware law, which are summarized below, are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in the policies formulated by our board of directors and to discourage certain types of transactions that may involve an actual or threatened change of control. In that regard, these provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our common stock that could result from actual or rumored takeover attempts. Such provisions also may have the effect of preventing changes in our management.
Blank Check Preferred Stock. Our amended and restated certificate of incorporation contains provisions that permit our board of directors to issue, without any further vote or action by the stockholders, up to 30,000,000 shares of preferred stock in one or more series and, with respect to each such series, to fix the number of shares constituting the series and the designation of the series, the voting powers (if any) of the shares of the series, and the preferences and relative, participating, optional and other special rights, if any, and any qualifications, limitations or restrictions, of the shares of such series. As a result, our board of directors could authorize the issuance of shares of preferred stock with terms and conditions that could have the effect of delaying, deferring or preventing a transaction or a change in control that might involve a premium price for holders of the registrant’s common stock or otherwise be in their best interest.
Special Meetings of Stockholders. Our bylaws provide that special meetings of stockholders may be called only by the board of directors. Stockholders are not permitted to call a special meeting of stockholders or to require that the board of directors call such a special meeting.
26.
Delaware Takeover Statute.
We are subject to Section 203 of the Delaware General Corporation Law, or DGCL, which regulates acquisitions of some Delaware corporations. In general, Section 203 prohibits, with some exceptions, a Delaware corporation that is a public company from engaging in any “business combination” with any “interested stockholder” for a period of three years following the date that such stockholder became an interested stockholder, unless:
| | • | | prior to such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| | • | | on consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding those shares owned (x) by persons who are directors and also officers and (y) by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| | • | | on or subsequent to such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
Section 203 of the DGCL defines “business combination” to include:
| | • | | any merger or consolidation involving the corporation and the interested stockholder; |
| | • | | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| | • | | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| | • | | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or |
| | • | | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an “interested shareholder” as an entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with such entity or person.
27.
PLAN OF DISTRIBUTION
We may sell the securities from time to time pursuant to underwritten public offerings, negotiated transactions, block trades or a combination of these methods. We may sell the securities to or through underwriters or dealers, through agents, or directly to one or more purchasers. We may distribute securities from time to time in one or more transactions:
| | • | | at a fixed price or prices, which may be changed; |
| | • | | at market prices prevailing at the times of sale; |
| | • | | at prices related to such prevailing market prices; or |
A prospectus supplement or supplements (and any related free writing prospectus that we may authorize to be provided to you) will describe the terms of the offering of the securities, including, to the extent applicable:
| | • | | the name or names of the underwriters, if any; |
| | • | | the purchase price of the securities or other consideration therefor, and the proceeds, if any, we will receive from the sale; |
| | • | | any options under which underwriters may purchase additional securities from us; |
| | • | | any agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation; |
| | • | | any public offering price; |
| | • | | any discounts or concessions allowed or reallowed or paid to dealers; and |
| | • | | any securities exchange or market on which the securities may be listed. |
Only underwriters named in the prospectus supplement will be underwriters of the securities offered by the prospectus supplement.
If underwriters are used in the sale, they will acquire the securities for their own account and may resell the securities from time to time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement. We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Subject to certain conditions, the underwriters will be obligated to purchase all of the securities offered by the prospectus supplement, other than securities covered by any option to purchase additional securities. Any public offering price and any discounts or concessions allowed or reallowed or paid to dealers may change from time to time. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, the nature of any such relationship.
We may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and we will describe any commissions we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
28.
We may provide agents and underwriters with indemnification against civil liabilities, including liabilities under the Securities Act, or contribution with respect to payments that the agents or underwriters may make with respect to these liabilities. Agents and underwriters may engage in transactions with, or perform services for, us in the ordinary course of business.
All securities we may offer, other than common stock, will be new issues of securities with no established trading market. Any underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market making at any time without notice. We cannot guarantee the liquidity of the trading markets for any securities.
Any underwriter may engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the Exchange Act. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum price. Syndicate-covering or other short-covering transactions involve purchases of the securities, either through exercise of the option to purchase additional securities or in the open market after the distribution is completed, to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
29.
LEGAL MATTERS
The validity of the securities offered hereby will be passed upon by Cooley LLP, Boston, Massachusetts.
EXPERTS
The financial statements and the effectiveness of internal control over financial reporting incorporated in this Prospectus by reference to the Annual Report on Form 10-K for the year ended December 31, 2013 and for the period from September 9, 2003 (date of inception) through December 31, 2013, have been audited by McGladrey LLP, an independent registered public accounting firm, as stated in their report incorporated by reference herein and have been so incorporated in reliance upon such reports and upon the authority of such firm as experts in accounting and auditing.
WHERE YOUCAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the Commission. You may read and copy these reports, proxy statements and other information at the Commission’s public reference room at 100 F Street, N.E., Washington, D.C. 20549 or at the Commission’s other public reference facilities. Please call the Commission at 1-800-SEC-0330 for more information about the operation of the public reference rooms. You can request copies of these documents by writing to the Commission and paying a fee for the copying costs. In addition, the Commission maintains an Internet site at http://www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the Commission. Our Commission filings are available on the Commission’s Internet site. We maintain a website at http://www.ziopharm.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this prospectus, and you should not consider it part of this prospectus or part of any prospectus supplement.
30.
INCORPORATION OF INFORMATION BY REFERENCE
We are allowed to incorporate by reference information contained in documents that we file with the Commission. This means that we can disclose important information to you by referring you to those documents and that the information in this prospectus is not complete and you should read the information incorporated by reference for more detail. Information in this prospectus supersedes information incorporated by reference that we filed with the Commission prior to the date of this prospectus, while information that we file later with the Commission will automatically update and supersede the information in this prospectus.
We incorporate by reference the documents listed below and any future filings we will make with the Commission under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act (i) after the date of the initial filing of the registration statement of which this prospectus is a part and prior to effectiveness of such registration statement, and (ii) from the date of this prospectus but prior to the termination of the offering of the securities covered by this prospectus (other than Current Reports or portions thereof furnished under Item 2.02 or 7.01 of Form 8-K):
| | • | | Annual Report on Form 10-K for the fiscal year ended December 31, 2013, filed on March 3, 2014; |
| | • | | The information specifically incorporated by reference into our Annual Report on Form 10-K for the fiscal year ended December 31, 2013 from our Definitive Proxy Statement on Schedule 14A, filed on April 30, 2014; |
| | • | | Quarterly Reports on Form 10-Q for the quarters ended March 31, 2014, June 30, 2014, and September 30, 2014, filed on May 8, 2014, August 7, 2014, and October 30, 2014, respectively; |
| | • | | Current Reports on Form 8-K filed on January 8, 2014, January 15, 2014, February 10, 2014, March 3, 2014 (excluding Item 2.02), March 28, 2014, April 8, 2014, May 8, 2014 (excluding Item 2.02), May 22, 2014 (excluding Item 7.01), June 19, 2014, July 31, 2014, August 7, 2014 (excluding Item 2.02), September 16, 2014, October 30, 2014 (excluding Item 2.02), December 3, 2014 (excluding Item 7.01), January 14, 2015, second filing on January 14, 2015 (excluding Item 7.01), and January 28, 2015; and |
| | • | | The description of our common stock set forth in the registration statement on Form 8-A registering our common stock under Section 12 of the Exchange Act, which was filed with the Commission on September 20, 2006, including any amendments or reports filed for purposes of updating such description. |
We will provide to each person, including any beneficial owner, to whom a prospectus is delivered a copy of any or all of the documents that are incorporated by reference in this prospectus but not delivered with this prospectus, including exhibits that are specifically incorporated by reference in such documents. You may request a copy of such documents at no cost, by writing or telephoning us at the following address or telephone number:
ZIOPHARM Oncology, Inc.
One First Avenue, Parris Building 34, Navy Yard Plaza
Boston, Massachusetts 02129
Attention: Chief Legal Officer
(617) 259-1970
31.
9,708,738 Shares

Common Stock
PROSPECTUS SUPPLEMENT
Sole Book-Running Manager
Guggenheim Securities
May 11, 2017


