
A New Day for Ziopharm Oncology October 9, 2018 Exhibit 99.2

This presentation contains certain forward-looking information about Ziopharm Oncology, Inc. that is intended to be covered by the safe harbor for "forward-looking statements" provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as "may," "will," "could," "expects," "plans," "anticipates," and "believes." These statements include, but are not limited to, statements regarding the expected benefits of the strategic transaction including entry into an exclusive license agreement with Precigen, Inc., the progress, timing and results of preclinical and clinical trials involving the Company's product candidates including the development of Sleeping Beauty-modified TCRs and CD19-specific CAR-T therapies, the expected timing for the Company's response to the U.S. Food and Drug Administration (FDA) in regards to its investigational new drug (IND) application for its third-generation Phase 1 trial to evaluate CD19-specific CAR-T therapies under technology referred to as point-of-care, the expected timing for the filings or amendments of IND applications for its other product candidates and the progress of the Company's research and development programs. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied by, the forward-looking statements. These risks and uncertainties include, but are not limited to: whether chimeric antigen receptor T cell (CAR+ T) approaches, Ad-RTS-hIL-12, TCR and NK cell-based therapies, or any of our other therapeutic candidates will advance further in the preclinical or clinical trials process and whether and when, if at all, they will receive final approval from the FDA or equivalent foreign regulatory agencies and for which indications; whether chimeric antigen receptor T cell (CAR+ T) approaches, Ad-RTS-hIL-12, TCR and NK cell-based therapies, and our other therapeutic products will be successfully marketed if approved; the strength and enforceability of our intellectual property rights; competition from other pharmaceutical and biotechnology companies; and the other risk factors contained in our periodic and interim SEC reports filed from time to time with the Securities and Exchange Commission, including but not limited to, our Annual Report on Form 10-K for the fiscal year ended December 31, 2017, and subsequent reports that the Company may file with the Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. Forward-Looking Statements New Day for Ziopharm Oncology

Agenda (beginning 8 am ET October 9, 2018) On the Call Laurence Cooper, MD, PhD, CEO David Mauney, MD, EVP, CBO and Interim COO David Connolly, VP, Corporate Communications and IR Rob Hadfield, General Counsel Introduction / Overview Laurence Cooper Terms of New Agreement David Mauney Clinical Programs Update Laurence Cooper Q&A New Day for Ziopharm Oncology

The New Ziopharm

The “New Ziopharm” with Full Autonomy New Day for Ziopharm Oncology New, simplified licensing agreement with Intrexon/Precigen Preferred stock issued to Intrexon fully retired Revised Board of Directors Strategic flexibility for corporate development, sublicensing Aligned financial incentives and resources Immunotherapy pipeline focused on solid tumors

Refined Focus on Controlled IL-12 Sleeping Beauty for All TCR Targets and Two CAR Targets New Day for Ziopharm Oncology Platform #1 Controlled IL-12 Platform #2 Sleeping Beauty Ad-RTS-hIL-12 plus veledimex for brain cancer, other solid tumors Monotherapy Combination with immune checkpoint inhibitors Optionality on next-gen delivery Exclusive worldwide rights Sleeping Beauty TCRs targeting neoantigens for solid tumors CD19-specific CAR and second unnamed CAR target

The New Ziopharm Terms of New Agreement

Historic Overview of Ziopharm and Intrexon Relationship New Day for Ziopharm Oncology January 2011 – Intrexon and Ziopharm enter into Exclusive Channel Collaboration (ECC)* agreement Established 50-50 share of net profits; Ziopharm is Intrexon’s exclusive oncology partner; Limited sub-licensing rights January 2015 – Intrexon, Ziopharm and MD Anderson announce Exclusive Licensing Agreement for CAR-T, TCR, NK Cell programs specifically for development of nonviral adoptive cell therapies March 2015 – Intrexon-Ziopharm announce global CAR-T collaboration with Merck KGaA June 2016 – Intrexon and Ziopharm renegotiate ECC 80-20 Ziopharm-Intrexon sales royalties (changed from 50-50); 50-50 on sub-licensing agreement remains; Intrexon received $120M in preferred stock plus 12% annually January 2017 – Intrexon and Ziopharm announce Collaborative Research and Development Agreement (CRADA) with National Cancer Institute (NCI) – Sleeping Beauty-modified TCRs for solid tumors March 2017 – Intrexon announces restructuring, formation of Precigen for all health care assets October 2018 – ECC terminated, replaced with new licensing agreement * Intrexon transferred rights to Precigen when it was launched in 2017 ** Now, with the exception of CD19

New License for Exclusive Rights to Controlled IL-12, Sleeping Beauty TCRs and Sleeping Beauty CD19-CAR New Day for Ziopharm Oncology Controlled IL-12 Platform Exclusive rights to Ad-RTS-hIL-12 plus veledimex Ziopharm gains optionality for next-generation technologies for RTS-IL-12 Sleeping Beauty Platform Exclusive rights to T cells genetically modified with Sleeping Beauty to express TCRs Exclusive rights to Sleeping Beauty CD19-specific CAR-T and rights to second unnamed CAR target Ziopharm gains broad sub-licensing rights providing flexibility to pursue partnerships with lower sub-licensing fees to Precigen, than previous ECC agreement Non-exclusive rights to pursue additional programs Milestone payments upon commencement of later stage clinical and regulatory milestones Tiered royalty payments based on net sales

Additional Terms of 2018 Licensing Agreement with Precigen New Day for Ziopharm Oncology Preferred stock valued at approximately $156.9 million* retired R.J. Kirk resigns from Ziopharm Board of Directors Ziopharm retains collaboration with MD Anderson Cancer Center and the approximately $31.7 million** available from prepayments Ziopharm will assume full control of CRADA with NCI for TCRs CAR-T development by Precigen remains subject to Merck KGaA Precigen retains worldwide rights to CD33 and all other CAR targets, excluding Ziopharm’s CD19 and 2nd unnamed CAR target with Sleeping Beauty platform Ziopharm receives capped royalties on Precigen CAR products * As of Sept. 30, 2018 ** As of June 30, 2018

The New Ziopharm Clinical Programs Update
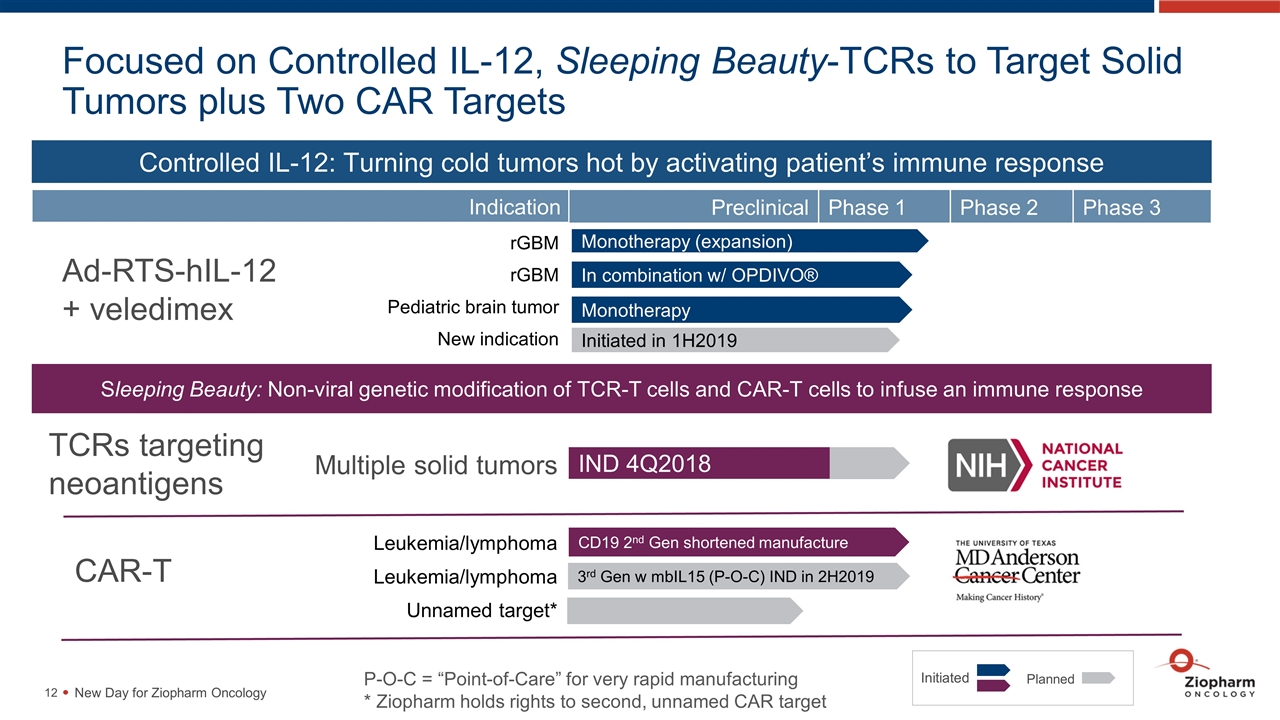
Focused on Controlled IL-12, Sleeping Beauty-TCRs to Target Solid Tumors plus Two CAR Targets New Day for Ziopharm Oncology Sleeping Beauty: Non-viral genetic modification of TCR-T cells and CAR-T cells to infuse an immune response Monotherapy (expansion) P-O-C = “Point-of-Care” for very rapid manufacturing * Ziopharm holds rights to second, unnamed CAR target Initiated Planned IND 4Q2018 Controlled IL-12: Turning cold tumors hot by activating patient’s immune response Ad-RTS-hIL-12 + veledimex Preclinical Phase 1 Phase 2 Phase 3 Indication rGBM rGBM Pediatric brain tumor New indication In combination w/ OPDIVO® Monotherapy Initiated in 1H2019 TCRs targeting neoantigens Multiple solid tumors CAR-T Leukemia/lymphoma Leukemia/lymphoma Unnamed target* CD19 2nd Gen shortened manufacture 3rd Gen w mbIL15 (P-O-C) IND in 2H2019
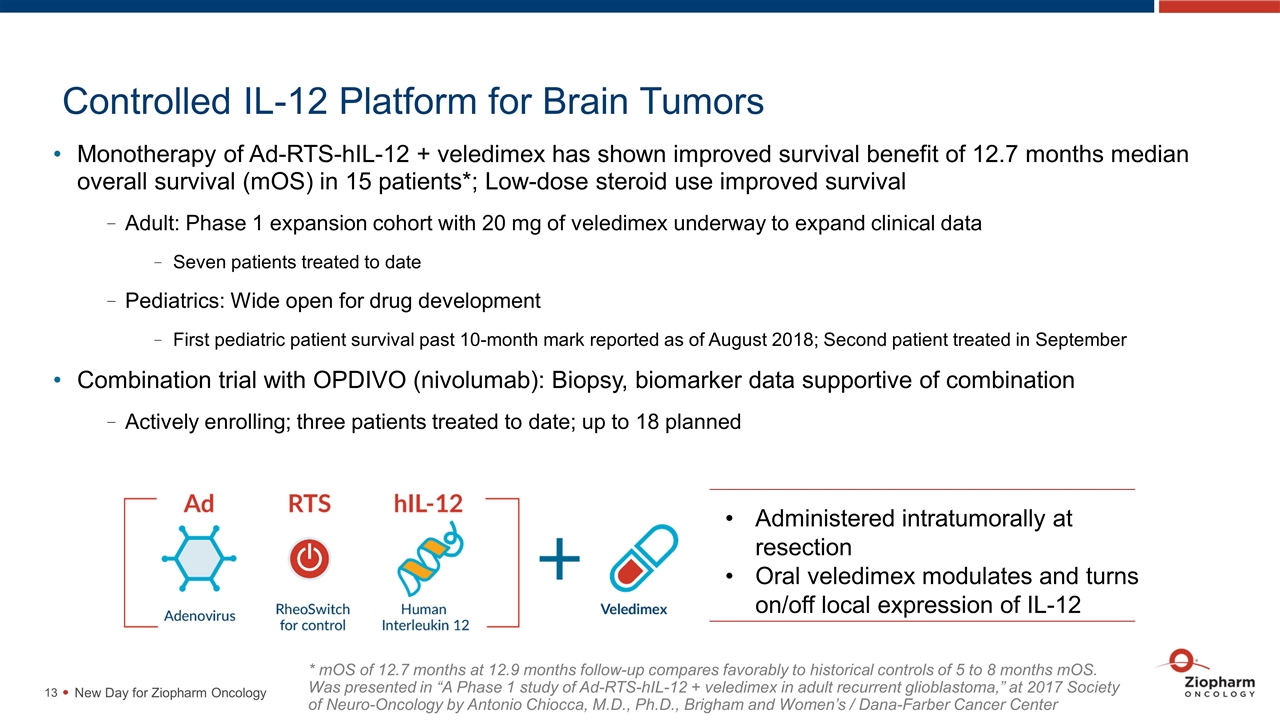
Controlled IL-12 Platform for Brain Tumors Monotherapy of Ad-RTS-hIL-12 + veledimex has shown improved survival benefit of 12.7 months median overall survival (mOS) in 15 patients*; Low-dose steroid use improved survival Adult: Phase 1 expansion cohort with 20 mg of veledimex underway to expand clinical data Seven patients treated to date Pediatrics: Wide open for drug development First pediatric patient survival past 10-month mark reported as of August 2018; Second patient treated in September Combination trial with OPDIVO (nivolumab): Biopsy, biomarker data supportive of combination Actively enrolling; three patients treated to date; up to 18 planned New Day for Ziopharm Oncology Administered intratumorally at resection Oral veledimex modulates and turns on/off local expression of IL-12 * mOS of 12.7 months at 12.9 months follow-up compares favorably to historical controls of 5 to 8 months mOS. Was presented in “A Phase 1 study of Ad-RTS-hIL-12 + veledimex in adult recurrent glioblastoma,” at 2017 Society of Neuro-Oncology by Antonio Chiocca, M.D., Ph.D., Brigham and Women’s / Dana-Farber Cancer Center
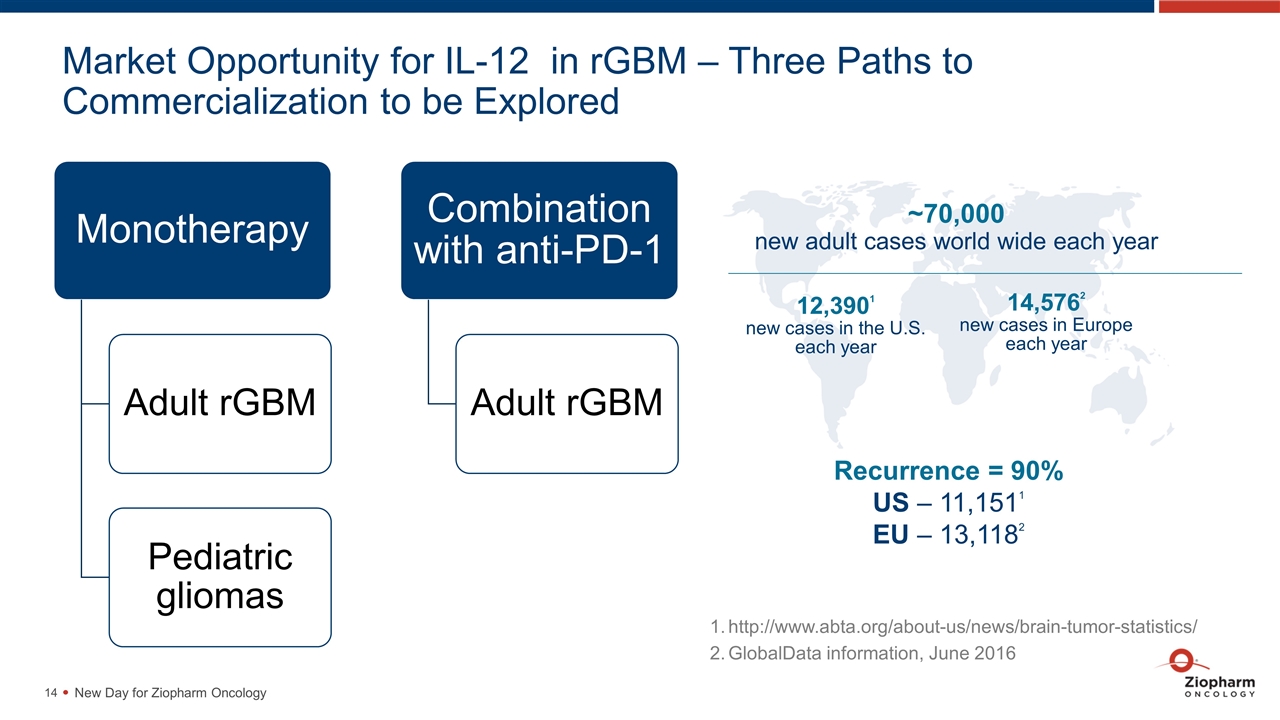
Market Opportunity for IL-12 in rGBM – Three Paths to Commercialization to be Explored New Day for Ziopharm Oncology ~70,000 new adult cases world wide each year 12,3901 new cases in the U.S. each year 14,5762 new cases in Europe each year Recurrence = 90% US – 11,1511 EU – 13,1182 http://www.abta.org/about-us/news/brain-tumor-statistics/ GlobalData information, June 2016 Monotherapy Adult rGBM Pediatric gliomas Combination with anti-PD-1 Adult rGBM

Status of Investigational New Drug Application for Third-Generation Trial to Evaluate CD19-specific CAR-T Therapy Per disclosures in June and August 2018, FDA requested additional pre-clinical process development, placed clinical hold on IND Guidance update on “point-of-care” (“P-O-C”): FDA mandated a 70% cell viability threshold Ziopharm and MD Anderson executing on cell viability improvements Anticipate filing amended IND in 2H2019 Second-generation Sleeping Beauty trial at MD Anderson Cancer Center ongoing Dosing, CAR design, reduced manufacturing and release testing time Encouraging clinical data New Day for Ziopharm Oncology Genetic modification of T cells with Sleeping Beauty system to produce T cells in < 2 days mbIL15 improves T cell persistence Potential advantage over off-the-shelf Third-party OTS cells require lymphodepletion, but mbIL15 may enable avoidance
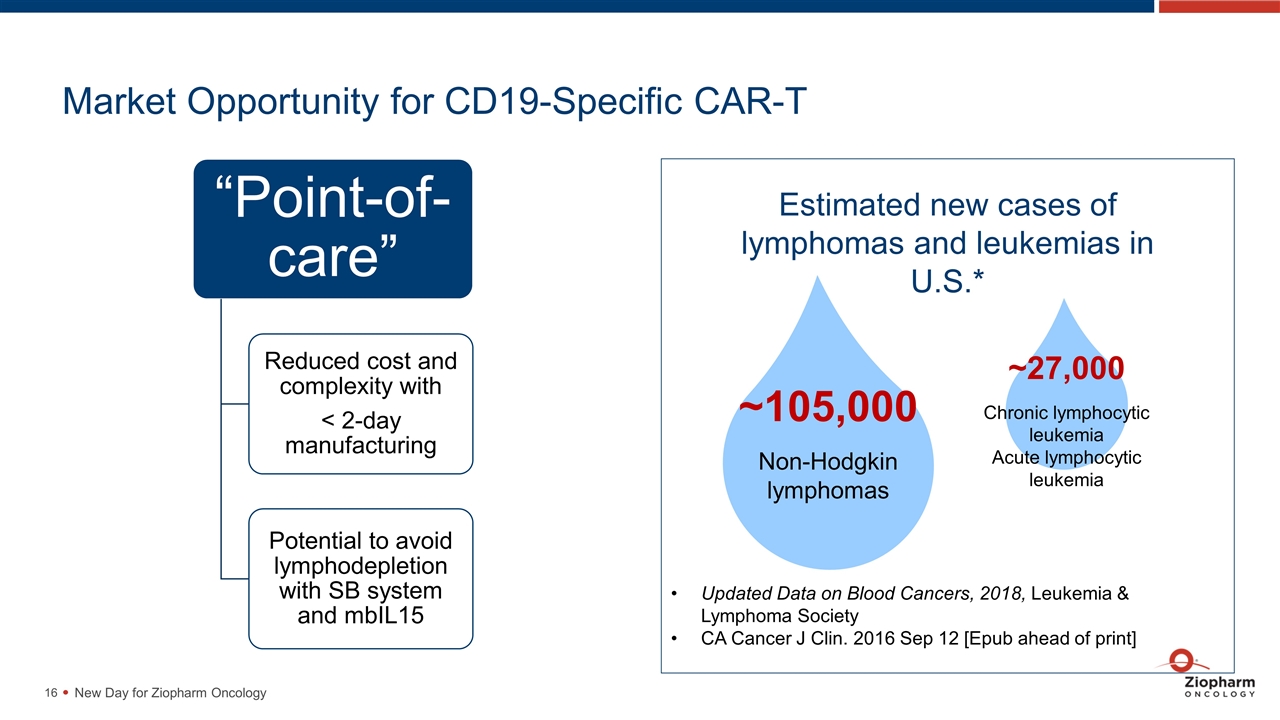
Market Opportunity for CD19-Specific CAR-T New Day for Ziopharm Oncology Estimated new cases of lymphomas and leukemias in U.S.* Updated Data on Blood Cancers, 2018, Leukemia & Lymphoma Society CA Cancer J Clin. 2016 Sep 12 [Epub ahead of print] Non-Hodgkin lymphomas ~105,000 ~27,000 Chronic lymphocytic leukemia Acute lymphocytic leukemia “Point-of-care” Reduced cost and complexity with < 2-day manufacturing Potential to avoid lymphodepletion with SB system and mbIL15
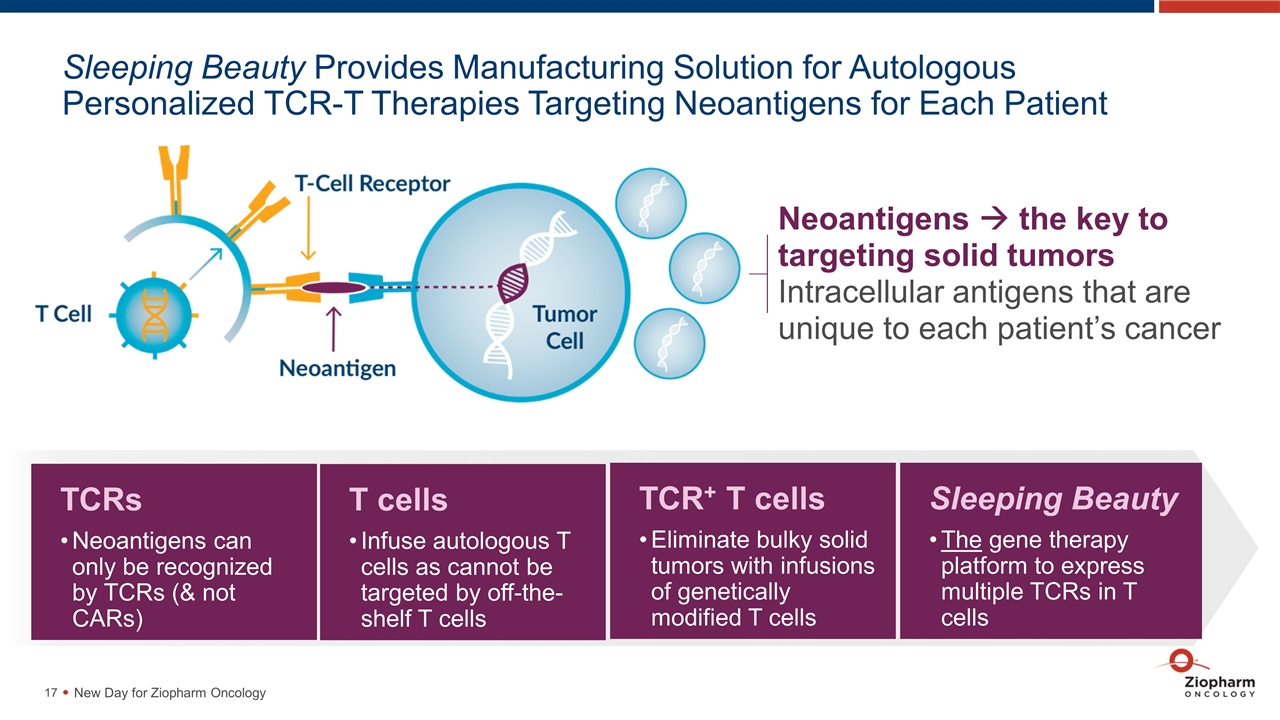
Sleeping Beauty Provides Manufacturing Solution for Autologous Personalized TCR-T Therapies Targeting Neoantigens for Each Patient Neoantigens à the key to targeting solid tumors Intracellular antigens that are unique to each patient’s cancer TCRs Neoantigens can only be recognized by TCRs (& not CARs) New Day for Ziopharm Oncology T cells Infuse autologous T cells as cannot be targeted by off-the-shelf T cells TCR+ T cells Eliminate bulky solid tumors with infusions of genetically modified T cells Sleeping Beauty The gene therapy platform to express multiple TCRs in T cells

T cells Targeting Neoantigens has Demonstrated Clinical Success in Solid Tumors (History on our Side) New Day for Ziopharm Oncology Clockwise from top left: Science. 2014 May 9;344(6184):641-5 Nat Med. 2018 Jun;24(6):724-730 N Engl J Med. 2016, Dec 8;375(23):2255-2262 J Immunol. 2013 Jun 15;190(12):6034-42 Ziopharm provides technology to commercialize T-cell targeting of TCRs Four steps to success
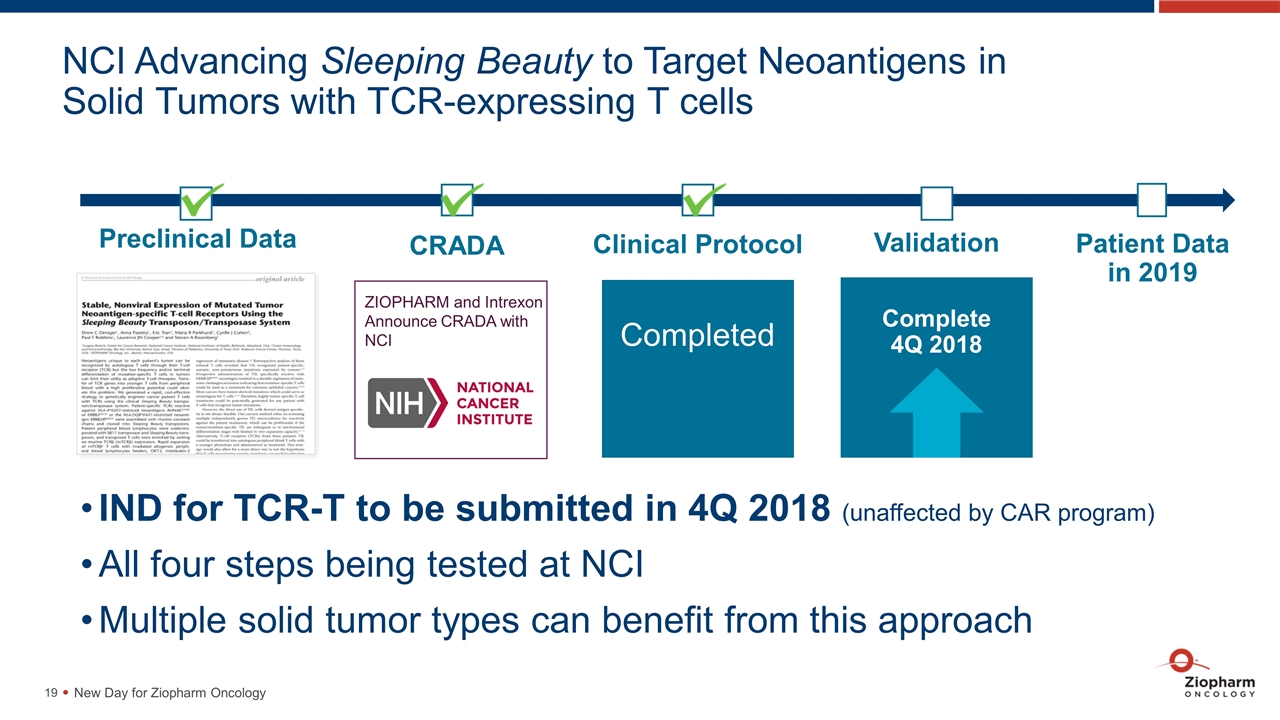
NCI Advancing Sleeping Beauty to Target Neoantigens in Solid Tumors with TCR-expressing T cells Preclinical Data Patient Data in 2019 Completed Clinical Protocol Validation Complete 4Q 2018 ZIOPHARM and Intrexon Announce CRADA with NCI CRADA IND for TCR-T to be submitted in 4Q 2018 (unaffected by CAR program) All four steps being tested at NCI Multiple solid tumor types can benefit from this approach New Day for Ziopharm Oncology
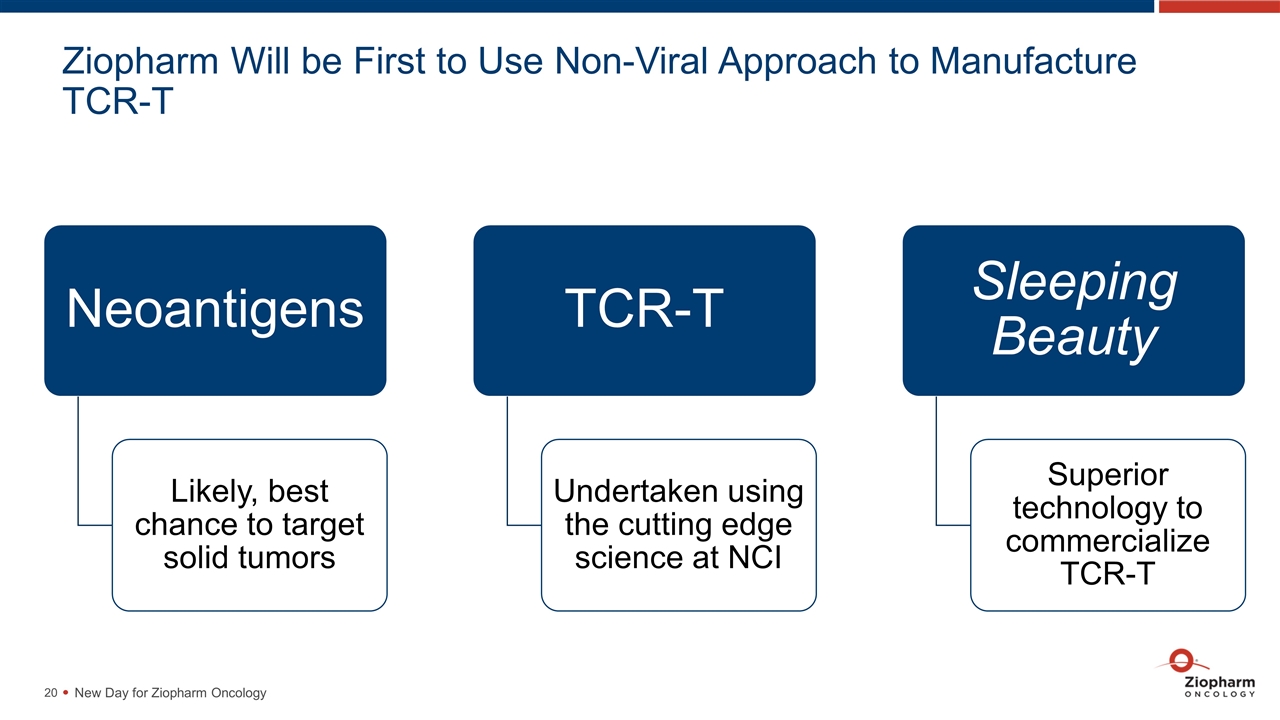
Ziopharm Will be First to Use Non-Viral Approach to Manufacture TCR-T New Day for Ziopharm Oncology Neoantigens Likely, best chance to target solid tumors TCR-T Sleeping Beauty Superior technology to commercialize TCR-T Undertaken using the cutting edge science at NCI
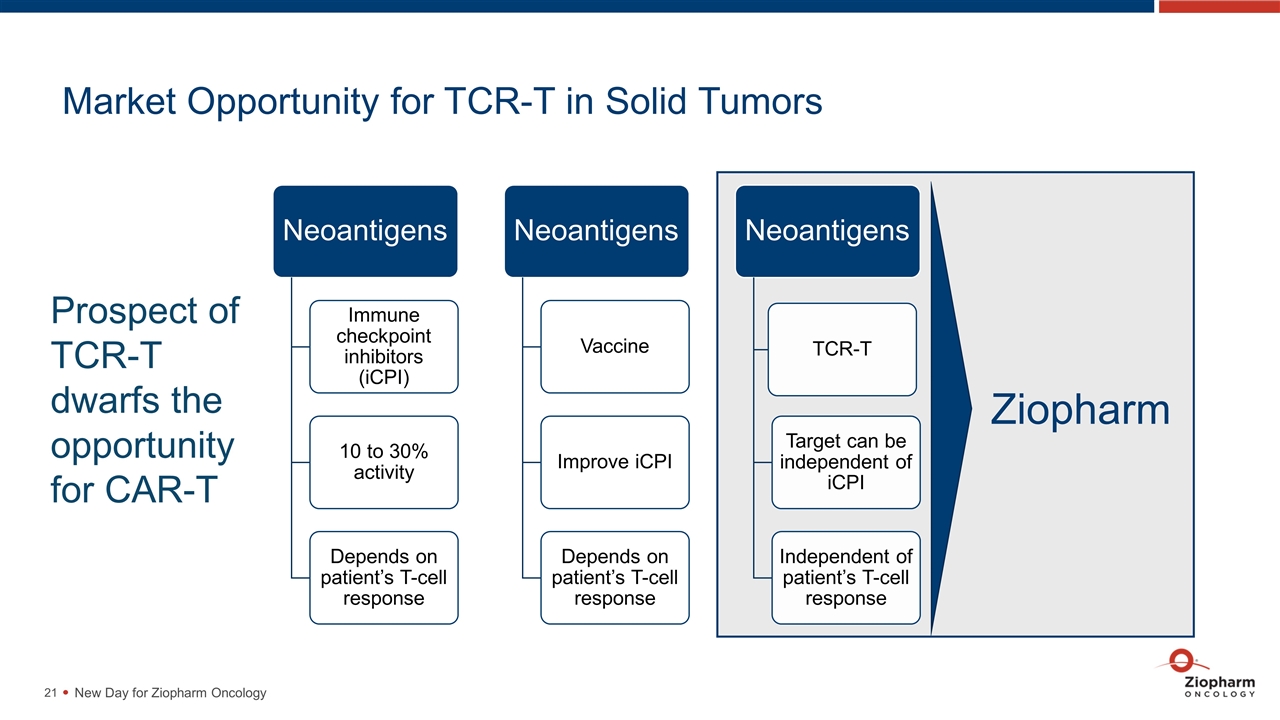
Market Opportunity for TCR-T in Solid Tumors New Day for Ziopharm Oncology Prospect of TCR-T dwarfs the opportunity for CAR-T Ziopharm Neoantigens Immune checkpoint inhibitors ( iCPI ) 10 to 30% activity Neoantigens Vaccine Improve iCPI Neoantigens TCR-T Target can be independent of iCPI Depends on patient’s T-cell response Depends on patient’s T-cell response Independent of patient’s T-cell response

The New Ziopharm New Day
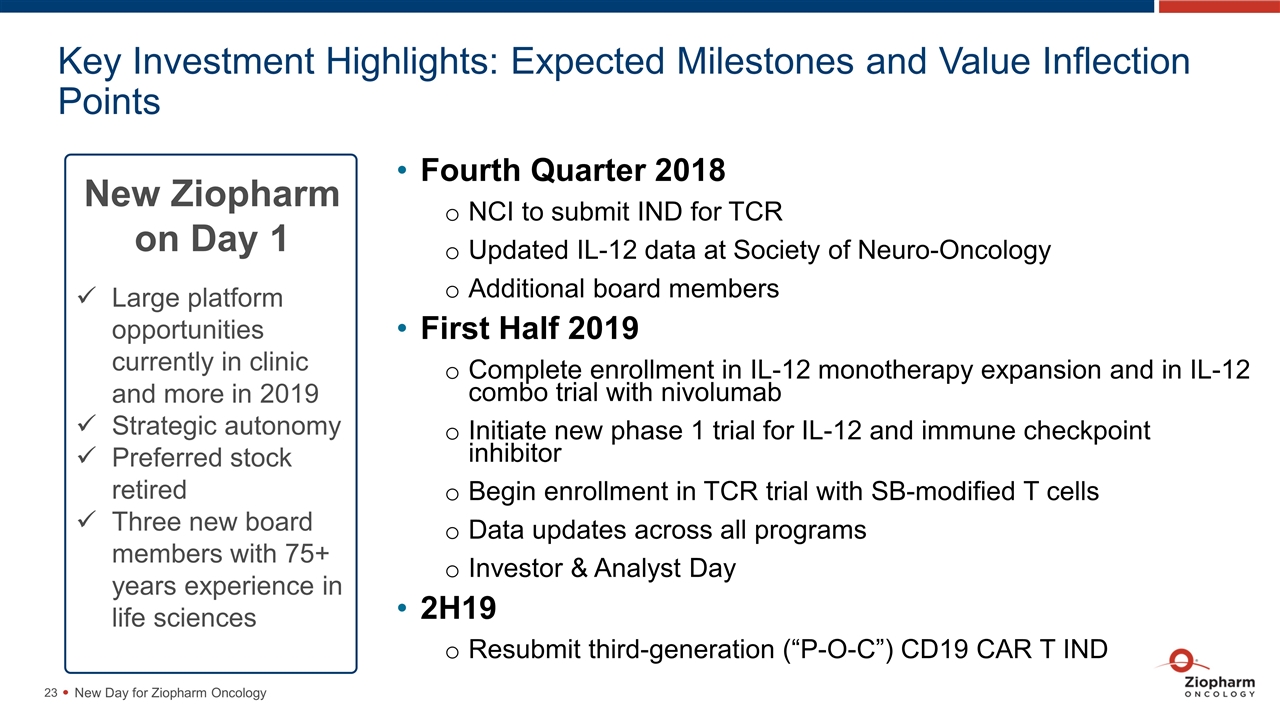
Key Investment Highlights: Expected Milestones and Value Inflection Points New Day for Ziopharm Oncology Large platform opportunities currently in clinic and more in 2019 Strategic autonomy Preferred stock retired Three new board members with 75+ years experience in life sciences New Ziopharm on Day 1 1H18 Fourth Quarter 2018 NCI to submit IND for TCR Updated IL-12 data at Society of Neuro-Oncology Additional board members First Half 2019 Complete enrollment in IL-12 monotherapy expansion and in IL-12 combo trial with nivolumab Initiate new phase 1 trial for IL-12 and immune checkpoint inhibitor Begin enrollment in TCR trial with SB-modified T cells Data updates across all programs Investor & Analyst Day 2H19 Resubmit third-generation (“P-O-C”) CD19 CAR T IND
Corporate Contacts David Connolly Vice President, Corporate Communications / Investor Relations Tel: +1 (617) 502-1881 Email: dconnolly@ziopharm.com Mike Moyer Vice President, Portfolio Strategy Tel : +1 (617) 765-3770 Email : mmoyer@ziopharm.com Brennan Doyle Managing Director SOLEBURY TROUT Tel: +1 (617) 221-9005 Email: bdoyle@troutgroup.com New Day for Ziopharm Oncology

























