Exhibit 99.2
In the preliminary prospectus supplement filed pursuant to Rule 424(b)(5) in connection with a public offering of common stock by Ziopharm Oncology, Inc. (the “Company”), the Company provided the following overview of the Company’s business as updates to the information provided in the Company’s previous periodic filings with the Securities and Exchange Commission.
Company Overview
We are a clinical-stage biopharmaceutical company focused on discovering, acquiring, developing and commercializing next generation immunotherapy platforms that leverage cell- and gene-based therapies to treat patients with cancer. We are developing two immuno-oncology platform technologies that utilize the immune system by employing novel, controlled gene expression and innovative cell engineering technologies designed to deliver safe, effective, andscalable non-viral cell- and viral-based gene therapies for the treatment of multiple cancer types. Our first platform is referred to as Sleeping Beauty and is based on the genetic engineering of immune cells usinga non-viral transposon/transposase system that is intended to stably reprogram T cells outside of the body for subsequent infusion. Our second platform is termedControlled IL-12, which is designed to stimulate expression of interleukin 12,or IL-12, a master regulator of the immune system, in a controlled and safe manner to focus the patient’s immune system to attack cancer cells. We believe these two platforms have the potential to provide unique and powerful solutions to address the issues associated with (1) treating solid tumors with heterogeneous and unknown antigens, and (2) providing cost-effective scalable manufacturing solutions for T cell receptor T cell, or TCR+ T, therapies for solid tumors and chimeric antigen receptor, or CAR T cell, or CAR+ T, therapies targeting CD19 on malignant B cells.
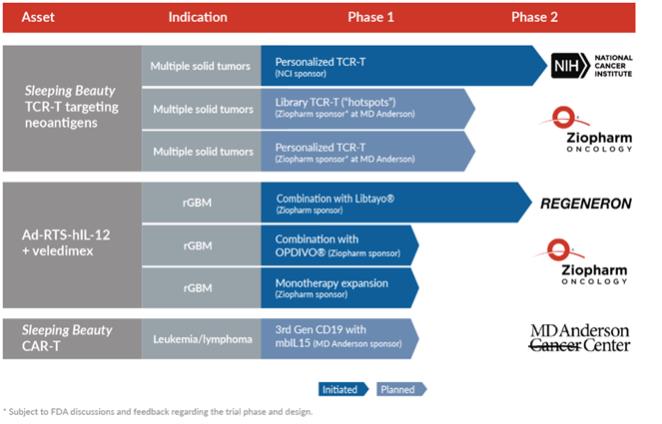
Using our Sleeping Beauty platform, we are developing TCR+ T therapies initially to target solid tumors. Our T cell receptor, or TCR, program designs and manufactures T cells that are intended to target tumor-specific antigens, thereby delivering personalized therapy that can attack an individual patient’s cancer. These antigens are referred to as neoantigens as they are only expressed by the tumor, reducing the potential for toxicity upon targeting normal cells. A minority of neoantigens are shared between patients and between classes of tumors and are referred to as “hotspots”. The Sleeping Beauty system uses DNA plasmids to reprogram T cells to express introduced TCRs ona patient-by-patient basis (addressing inter-tumor heterogeneity) and possibly to express more than one TCR for each patient (addressing intra-tumor heterogeneity). The genetic modification using the Sleeping Beauty system of recipient-derived products enables us to target neoantigens in two ways. The first recognizes that most neoantigens are unique to each patient’s tumor and we plan to infuse TCR+ T cells expressing recipient-derived (autologous) TCRs. The second is based on the finding that some neoantigens in hotspots are shared between patients and we plan to administer TCR+ T expressing allogeneic TCRs from a library derived from third parties. We havein-licensed from the National Cancer Institute, or the NCI, multiple allogeneic TCRs derived from third parties that are reactive to mutated KRAS, TP53 and EGFR and we plan to expand our TCR library as part of our commitment to advance clinical development for the treatment of patients whose solid tumors have driver mutations. These TCRs are typically obtained from tumor-infiltrating lymphocytes, or TILs.
Under our Cooperative Research and Development Agreement, the NCI is conducting a Phase 2 clinical trial to evaluate autologous peripheral blood lymphocytes genetically modified with the Sleeping Beauty system to express autologous (personalized) TCRs. The U.S. Food and Drug Administration, or FDA, has cleared the investigational new drug, or IND, application submitted by the NCI for this clinical trial. The trial was initiated in October 2019 and preparations to enable patient enrollment by the NCI are underway. We expect the trial will enroll patients with a broad range of solid tumors over the next several years.
In addition, we are currently planning a clinical program to study our TCR approach with The University of Texas MD Anderson Cancer Center, or MD Anderson. Under this program, we expect to evaluate both our personalized TCR, or autoTCR, approach and our hotspot TCR, or alloTCR, approach. Our autoTCR approach is designed to identify neoantigens and TCRs on apatient-by-patient basis, which we believe should allow it to be broadly applicable to many patients’ solid tumors. The advantage of the alloTCR approach is that a subset of patients with solid tumors may be rapidly treated based on screening them for target neoantigens (e.g., in TP53), identifying human leukocyte antigen, and matching these data to the alloTCRs in the library.