UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| x | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2012
OR
| o | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from_____________ to _____________.
Commission file number 000-29981
TRISTAR WELLNESS SOLUTIONS, INC.
(Exact name of registrant as specified in its charter)
| Nevada | | 91-2027724 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
10 Saugatuck Ave. Westport CT | | 06880 |
(Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code (203) 226-4449
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | | Name of each exchange on which registered |
| None | | None |
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, par value $0.001
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to and post such files). Yes o No x
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | o | Accelerated filer | o |
| Non-accelerated filer | o | Smaller reporting company | x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
Aggregate market value of the voting stock held by non-affiliates: $11,131,130 as based on last reported sales price of such stock. The voting stock held by non-affiliates on that date consisted of 4,077,338 shares of common stock.
Applicable Only to Registrants Involved in Bankruptcy Proceedings During the Preceding Five Years:
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes o No o
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. As of March 28, 2013, there were 44,452,338 shares of common stock, $0.001 par value, issued and outstanding.
Documents Incorporated by Reference
List hereunder the following documents if incorporated by reference and the Part of the Form 10-K (e.g., Part I, Part II, etc.) into which the document is incorporated: (1) Any annual report to security holders; (2) Any proxy or information statement; and (3) Any prospectus filed pursuant to rule 424(b) or (c) of the Securities Act of 1933. The listed documents should be clearly described for identification purposes (e.g., annual report to security holders for fiscal year ended December 24, 1980). None.
TriStar Wellness Solutions, Inc.
TABLE OF CONTENTS
| PART I |
| | | | | |
| ITEM 1 – | BUSINESS | | | 2 | |
| ITEM 1A – | RISK FACTORS | | | 10 | |
| ITEM 1B – | UNRESOLVED STAFF COMMENTS | | | 15 | |
| ITEM 2 ‑ | PROPERTIES | | | 15 | |
| ITEM 3 ‑ | LEGAL PROCEEDINGS | | | 15 | |
| ITEM 4 – | MINE SAFETY DISCLOSURES | | | 15 | |
| | | | | | |
| PART II |
| | | | | | |
| ITEM 5 – | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | | | 16 | |
| ITEM 6 – | SELECTED FINANCIAL DATA | | | 17 | |
| ITEM 7 – | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION | | | 18 | |
| ITEM 7A – | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | | | 22 | |
| ITEM 8 – | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | | | 22 | |
| ITEM 9 – | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | | | 22 | |
| ITEM 9A – | CONTROLS AND PROCEDURES | | 24 | |
| ITEM 9B – | OTHER INFORMATION | | | 25 | |
| | | | | | |
| PART III |
| | | | | | |
| ITEM 10 – | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNACE | | | 26 | |
| ITEM 11 – | EXECUTIVE COMPENSATION | | | 30 | |
| ITEM 12 – | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | | | 33 | |
| ITEM 13 ‑ | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | | | 35 | |
| ITEM 14 – | PRINCIPAL ACCOUNTING FEES AND SERVICES | | | 36 | |
| | | | | | |
| PART IV |
| | | | | | |
| ITEM 15 ‑ | EXHIBITS, FINANCIAL STATEMENT SCHEDULES | | | 37 | |
This Annual Report includes forward-looking statements within the meaning of the Securities Exchange Act of 1934 (the “Exchange Act”). These statements are based on management’s beliefs and assumptions, and on information currently available to management. Forward-looking statements include the information concerning possible or assumed future results of operations of the Company set forth under the heading “Management's Discussion and Analysis of Financial Condition or Plan of Operation.” Forward-looking statements also include statements in which words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “estimate,” “consider,” or similar expressions are used.
Forward-looking statements are not guarantees of future performance. They involve risks, uncertainties, and assumptions. The Company's future results and shareholder values may differ materially from those expressed in these forward-looking statements. Readers are cautioned not to put undue reliance on any forward-looking statements.
ITEM 1 – BUSINESS
Corporate History
We were incorporated on August 28, 2000 in the state of Nevada under the name “Quadric Acquisitions”. Following our incorporation we were not actively engaged in any business activities. On April 25, 2001, we were acquired by Zkid Network Company and changed our name to ZKid Network Co. As a result, we became engaged in the business of providing media content for children through the use of our proprietary software. On February 8, 2006, we announced that we would be unable to raise the necessary funds to continue with our then-existing business model and plan. Accordingly, we decided to seek an active company to acquire.
On May 8, 2006, we closed a share exchange agreement with Star Metro Group Limited, which became our wholly-owned subsidiary. Under the terms of the share exchange agreement we exchanged 60,000 shares of our company for 100% of the issued and outstanding shares of Star Metro Group at a ratio of 1 share of our common stock for each 2,000 shares of Star Metro Group Limited’s stock. As a result of the share exchange agreement, we became engaged in the development, production and sale of a line of biodegradable, single use, food and beverage containers. On March 20, 2006, we changed our name from ZKid Network Co. to Eatware Corporation.
On November 27, 2006, we changed our name from “Eatware Corporation” to “Star Metro Corp.” We were required to effect this name change by the terms of an agreement we entered into on November 13, 2006, with Glory Team Industrial Limited and Eddie Chou, an ex-director of our company. We effected this name change by merging Star Metro Corp., our newly incorporated and wholly-owned subsidiary that was created for this purpose, into our company, with our company carrying on as the surviving corporation under the name “Star Metro Corp.”
On February 26, 2007, we changed our name from “Star Metro Corp.” to “Biopack Environmental Solutions Inc.” This name change was effected by merging Biopack Environmental Solutions Inc., our newly incorporated and wholly-owned subsidiary that was created for this purpose, into our company, with our company carrying on as the surviving corporation under the name “Biopack Environmental Solutions Inc.”.
On March 27, 2007, we completed a share exchange with the shareholders of Roots Biopack Group Limited, a company formed under the laws of the British Virgin Islands. Under the terms of the share exchange agreement we acquired all of the issued and outstanding common shares of Roots Biopack Group in exchange for the issuance by our company of 90,000,000 common shares to the former shareholders of Roots Biopack Group.
On November 3, 2011, the People’s Court of Guandong Jiangmen Pengjiang District held a hearing relating to our landlord’s claim for unpaid rent for our factory plus penalty interest and other claims. The landlord had made a claim for payment of overdue rent in the amount of RMB 1,236,000, penalty interest in the amount of RMB 1,067,930 and a claim for potential loss of income in the amount of RMB 618,000, for a total amount claimed of RMB 2,921,930 (approximately $451,379). At the hearing, the Court ruled that after two unsuccessful attempts to auction the factory’s assets at the minimum level set by the Court appointed independent valuation company’s fair market assessment price, the Court set the reference value at RMB 3,613,139.20 (approximately $569,359) and transferred all the assets to the landlord. The landlord is legally responsible for settling any claims made by creditors, and the case has been closed.
On April 27, 2012, we entered into an agreement whereby Rockland Group, LLC, a Texas limited liability company (“Rockland”), purchased Six Hundred and Twenty Thousand (620,000) shares of Biopack Environmental Solutions, Inc. Series A Convertible Preferred Stock (“Series A Preferred Shares”), One Million (1,000,000) shares of Biopack Environmental Solutions, Inc. Series B Convertible Preferred Stock (“Series B Preferred Shares”) and Seven Hundred Ten Thousand (710,000) shares of Biopack Environmental Solutions, Inc. Series C Convertible Preferred Stock (“Series C Preferred Shares”) from the holders of those shares. The Series A Preferred Shares and Series B Preferred Shares were issued prior to December 31, 2011. The Series C Preferred Shares were issued in April 2012. Together the Shares represented approximately 63% of our outstanding votes on all matters brought before the holders of our common stock for approval and, therefore, represented a change of control. Each share of Series A, B and C Convertible Preferred Stock is convertible into 5 shares of common stock on the date of this transaction. The transaction closed on April 27, 2012.
After the Agreement with Rockland, during 2012, we entered into the following agreements and transactions:
· On April 27, 2012, we entered into a Subsidiary Acquisition Option Agreement (“Subsidiary Option Agreement”) with Xinghui Ltd., a Chinese entity (“Purchaser“), under which sold all the shares in the BPAC Subsidiaries to the Purchaser, effective July 11, 2012, in exchange for the Purchaser assuming all our liabilities as of April 25, 2012, which included all of the liabilities and obligations of ours except for a $400,000 principal amount convertible note that was owed to Trilane Limited as of April 27, 2012. As a result of us exercising our rights under the Subsidiary Option Agreement, we no longer own the BPAC Subsidiaries, including any of their assets or liabilities.
· On June 25, 2012, we entered into a License and Asset Purchase Option Agreement (the “Agreement”) with NorthStar Consumer Products, LLC, a Connecticut limited liability company (“NCP”), under which we, acquired the exclusive license to develop, market and sell, NCP’s Beaute de Maman™ product line, which is a line of skincare and other products specifically targeted for pregnant women. In addition, we acquired the exclusive license rights to develop, market and sell NCP’s formula being developed for itch suppression, which would be sold as an over-the-counter product, if successful. In exchange for these license rights we agreed to issue NCP 225,000 shares of our Series D Convertible Preferred Stock. This transaction closed on June 26, 2012. Additionally, under the Agreement, in connection with our license rights and to ensure we could fulfill any immediate orders timely, we purchased all existing finished product of the Beaute de Maman™ product line currently owned by NCP. In exchange for the inventory we agreed to issue NCP 25,000 shares of Series D Convertible Preferred Stock. Each share is convertible into twenty five shares of common stock.
· On July 11, 2012, we entered into a Marketing and Development Services Agreement (the “Marketing Agreement”) with InterCore Energy, Inc. (“ICE”). Under the Marketing Agreement we were retained to market and develop certain assets referred to as the Soft & Smooth Assets held by ICE. The Soft & Smooth Assets include all rights, interests and legal claims to that certain invention entitled “Delivery Device with Invertible Diaphragm” which is a novel medical applicator that is capable of delivering medicants and internal devices within the body in an atraumatic fashion (without producing injury or damage). In addition, we were also granted the exclusive option, in our sole discretion, to purchase the Soft & Smooth Assets from ICE for warrants to purchase One Hundred Fifty Thousand (150,000) shares of our common stock at One Dollar ($1) per share, with a four (4) year expiration period. On February 12, 2013, we entered into an Asset Purchase Agreement (the “HLBCDC Asset Purchase Agreement”) with HLBC Distribution Company, Inc. (“HLBCDC”), under which we exercised our option to purchase the Soft & Smooth Assets held by HLBCDC, which had acquired the Soft & Smooth Assets from ICE. In exchange for the Soft and Smooth Assets we agreed to issue to HLBCDC warrants enabling HLBCDC to purchase One Hundred Fifty Thousand (150,000) shares of our common stock at One Dollar ($1) per share, with a four (4) year expiration period.
As a result of the above transactions, as of December 31, 2012, we were a company involved in developing, marketing and selling, NCP’s Beaute de Maman™ product line, which is a line of skincare and other products specifically targeted for pregnant and nursing women, as well as developing the Soft and Smooth Assets.
Subsequent to December 31, 2012, on March 7, 2013, we entered into an Exclusive Manufacturing, Marketing and Distribution Definitive License Agreement (the “Argentum Agreement”) with Argentum Medical, LLC, a Delaware limited liability company (“Argentum”), under which we acquired an exclusive license to develop, market and sell products based on a technology called “Silverlon”, a proprietary silver coating technology providing superior performance related to OTC wound treatment. The license is for 15 years, with a possible 5 year extension. Under the Agreement, we are obligated to order a certain amount of proprietary Silverlon film each contract year and if the minimums are not met Argentum could cancel the Agreement. In exchange for these license rights we agreed to pay Argentum royalty payments based on the adjusted gross revenues generated by product sales, as well as issue Argentum a warrant to purchase up to 750,000 shares of our common stock with an exercise price of $0.50 per share. The warrant vests in two equal installments of 375,000 shares each, with the vesting based on product’s success in obtaining certain approvals from the Food and Drug Administration. The warrants expire five (5) years after they vest.
As a result of the Argentum Agreement we plan to proceed with product development during 2013, as well as locate other potential acquisition targets in the woundcare space.
Business Overview
All of our operations as of December 31, 2012 related to the operations of the Beaute de Maman™ product line and developing the Soft and Smooth Assets. Earlier in 2012, prior to the above-described transactions, we were in the business of developing, manufacturing, distributing and marketing bio-degradable food containers and disposable industrial packaging for consumer products. However, since our operations in the latter half of 2012, as well as moving forward, will focus on the high interest “Over the Counter” (OTC) packaged goods market the remainder of the disclosure in this Annual Report focuses on Company plans within the OTC industry.
Moving forward our management intends to focus on developing and marketing proprietary health and wellness products within targeted categories using in-house product development and R&D expertise combined with targeted acquisitions or licensing rights to provide access to patented technologies that support product features and performance. In all cases our plan is to own the unique formulas or processes via internal development, direct acquisition or license. As appropriate we will pursue and defend patents on each product offering to help protect from competitive incursion.
Industry Overview
We target the consumer health and wellness (CH&W) marketplace. We believe this market represents a long-term, high growth and profitable opportunity. Conservatively estimated at $29 Billion in ex-factory sales in the U.S. in 2012 ($51 Billion in retail sales), the CH&W market has consistently grown at +3-4% annually despite the recent economic downturn and is forecasted to maintain or exceed that level of growth in the future.1 We believe we have entered the market uniquely aligned to important demographic and economic drivers underlying dramatically changing consumer behavior related to personal healthcare.
Fueling market growth is a “perfect storm” of consumer, industry, and government dynamics:
| · | The exploding costs of institutional and provider-based healthcare (growing at 1.5 times the rate of the national GDP); |
| · | The growing demand for care due to epidemic levels of obesity and related conditions (such as diabetes and heart disease); |
| · | An increasing number of insured Americans resulting from the Affordable Care Act reforms, and an aging population. The aging population holds new expectations for a healthy, active long life. “Boomers” will redefine the senior market as we know it today; |
| · | The industry faces a projected shortage in physicians and a healthcare service provider community that is permanently shifting the delivery and responsibility of care to the consumer. |
The combined impact of these factors provides the fuel for the “perfect storm”.
________________________________
1 JD Ford & Company Investment Bankers: Global Health & Wellness: State of the Industry. Resilience and Continued Growth Expected in Global Health & Wellness Industry: Copyright 2009.
The “Perfect Storm” Of Consumer Healthcare Trends
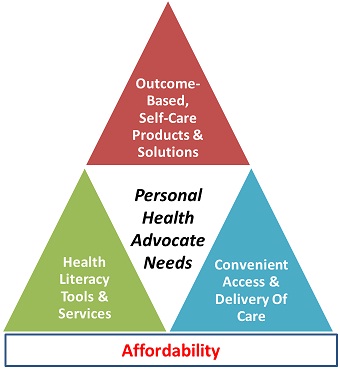
This “down-streaming” of care to the consumer is resulting in the transformation of consumer healthcare: fueling long-term growth and transforming the consumer from being a passive patient to being their own personal health advocate. With this transformation is increased demand for:
1) New outcome-based, self-care products and solutions;
2) New health literacy tools to enable the consumer to be an effective and self-directed health advocate; and
3) New and more convenient access and delivery of consumer health products, solutions, and services often avoiding the traditional “Gatekeepers” in the healthcare system.
Core needs of the new “Personal Health Advocate”
Meeting the core needs of the new personal health advocate is our management’s primary strategic focus for TriStar Wellness Solutions, Inc. We are targeting high growth and margin opportunity categories that provide a fundamental linkage back to the new consumer needs for brands and products that encourage high personal involvement/engagement and are consistent with personal health advocacy trends. More specifically, we are focused on categories and market space opportunities that represent:
| • | Outcome-based cost effective solutions with demonstrable results both in therapeutic relief and quality of life (QOL) improvement; |
| • | High personal involvement in care management (for self or caregiver); |
| • | Chronic versus episodic conditions and therapies. |
Our management is committed to the concept that companies will win with a consumer centric focus when they are able to efficiently convert consumer trends and defined gaps in product needs to actual innovation and product solutions strongly tied to a scientific foundation.
Principal Products
Initially our priority direct-to-consumer target markets are focused on women’s health and wound care.
In the year ended December 31, 2012, our focus is on women’s health. Women’s health is projected to achieve 5.9% compounded annual growth rate from 2010 to 2016.2 Our management believes the incremental pace of market expansion within this category, combined with the traditional role for women as the household-family health decision maker, supports the company’s commercial initiative. By introducing our brands to this critical wellness consumer we believe we are building a positive relationship for our future brands and product solutions.
Our first acquisition, Beauté de Maman™, represents our initial entry into the $500M category of products specifically targeted to Pregnancy & New Mothers (P&NM). We define the category as pregnancy support, nursing products, delivery products and other post-partum products. This category addresses unique needs of ~ 4 million pregnant women and over 20 million caregivers (sisters, mothers, other female relatives, friends, etc.) actively supporting each pregnant woman at any one time in the U.S.3 Beauté de Maman™ has been launched on a direct-to-consumer platform via the web, with the plan to expand in 2013 into drug and mass retail to reach a wider consuming public.
___________________________
2 Euromonitor Research & ACNielson Market Projects Expecting Mothers and Womens Health-GAGR: Trade Sources & National Statistics: Copyright June 2012.
Beaute de Maman™ represents a broad range of premium products targeted to meet the routine hygiene and unique symptomatic needs of pregnant and nursing women (e.g. Acne, nausea, stretch marks, etc.). Each product in this line is developed to deliver superior product performance while also being formulated with natural ingredients to be safe for the mother and developing baby. We conduct extensive research with leading medical databases to ensure each ingredient is proven to be safe. The composition of each product is formulated with over 95% natural ingredients. The products do not contain Parabens, BPA or Phthalates and are never tested on animals. The company is planning on important new line extensions in 2013 to extend the portfolio into new segments. Each year we plan on introducing 2-3 new items to the brand line. Ongoing product development activities are supported by extensive testing and formula optimization.
The second core product area is directed at the Direct-to-Consumer (DTC) wound care market space. We have both a detailed acquisition strategy and a comprehensive internal development program to offer significant technology-based enhancements to wound care solutions in this underserviced category.
Within the wound care category we plan to target the large population of consumers suffering from nuisance bleeding, typically associated with patients on anticoagulants (blood thinners) such as Coumadin and Plavix. The special health concerns of this substantial and rapidly expanding group is not being served well by today’s highly commoditized product selection.4
Sales and Marketing
In the year ended December 31, 2012, we focused the sales and marketing resources for the Beaute de Maman™ brand on efficient internet portals via the brand website and selected web-based retailers. Going forward we plan to expand distribution into the conventional retail channels. Concurrent with this sales channel expansion we plan to invest in more traditional marketing elements that can drive important awareness and trial incentives for target consumers.
We retained the services of a nationally recognized broker organization to represent the sales of the product line to conventional retailers. Additionally the company retained the services of a recognized advertising agency to lead in the development of effective digital and conventional advertising materials and media plans.
Research and Development
Research and development has been focused on fundamental product design and testing supporting the future portfolio plans of the business. Within the Beauté de Maman™ brand we are planning to launch in 2013 a new line of hair care products for pregnant and nursing women. We currently have several additional product development initiatives underway supporting retail launches in 2014 and beyond. We rely on our internal product development team and external technology providers working under confidentiality agreements to facilitate all development initiatives. Other than the confidentiality agreements, we do not have agreements with these external technology providers and we pay them on an “as we go” basis.
_____________________________3 CDC, National Vital Statistics Report, August 28, 2012, Volume 61, Number 1
4 TriStar Wellness Solutions, Inc. Concept Test conducted November 28, 2012
Employees
As of December 31, 2012, we had no direct employees. Our core operating management team was working under individual consulting agreements. In early 2013 we executed employment contracts with our core management members. Our Chief Financial Officer is the only member of our management that continues to perform services on a consulting basis.
Available Information
We are a fully reporting issuer, subject to the Securities Exchange Act of 1934. Our Quarterly Reports, Annual Reports, and other filings can be obtained from the SEC’s Public Reference Room at 100 F Street, NE., Washington, DC 20549, on official business days during the hours of 10 a.m. to 3 p.m. You may also obtain information on the operation of the Public Reference Room by calling the Commission at 1-800-SEC-0330. The Commission maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the Commission at http://www.sec.gov.
ITEM 1A. – RISK FACTORS.
As a smaller reporting company we are not required to provide a statement of risk factors. However, we believe this information may be valuable to our shareholders for this filing. We reserve the right to not provide risk factors in our future filings. Please note that these risk factors relate to our current business of operating the business of Beaute de Maman™ pursuant to our License and Asset Purchase Option Agreement with NorthStar Consumer Products, LLC, and our development of the Soft and Smooth Assets pursuant to our Marketing and Development Services Agreement with InterCore Energy, Inc., since risk factors related to our prior operations are not relevant as of the date of this filing. Our primary risk factors and other considerations include:
We have a limited operating history and limited historical financial information upon which you may evaluate our performance.
You should consider, among other factors, our prospects for success in light of the risks and uncertainties encountered by companies that, like us, have a limited operating history. We may not successfully address these risks and uncertainties or successfully market our existing and new products. If we fail to do so, it could materially harm our business and impair the value of our common stock. Even if we accomplish these objectives, we may not generate the positive cash flows or profits we anticipate. To date we only have limited revenues from the Beaute de Maman™ operations and currently our future revenues are dependent upon the future sales of the Beaute de Maman™ product line, as well as successfully developing a marketable product based on the Soft and Smooth Assets. Unanticipated problems, expenses, and delays are frequently encountered in establishing and developing new products in the medical technology field. These include, but are not limited to, inadequate funding, lack of consumer acceptance, competition, product development setbacks, and inadequate sales and marketing. The failure by us to address satisfactorily any of these conditions could have a materially adverse effect upon us and may force us to reduce or curtail operations. No assurance can be given that we can or will ever operate profitably.
We may not be able to meet our future capital needs.
To date, we have only generated limited revenue from the Beaute de Maman™ operations and we have no cash liquidity or capital resources. Our future capital requirements will depend on many factors, including our ability to grow Beaute de Maman™’s operations, develop the Soft and Smooth Assets, identify and acquire solid companies, our ability to generate positive cash flow from operations, and the effect of competing market developments. We will need additional capital in the near future. Any equity financings will result in dilution to our then-existing stockholders. Sources of debt financing may result in high interest expense. Any financing, if available, may be on terms deemed unfavorable.
If we are unable to meet our future capital needs, we may be required to reduce or curtail operations.
Since our change of control transaction closed on April 27, 2012, we have relied on financing from investors and our officers and directors to fund operations, and we have only generated limited revenue. We have limited cash liquidity and capital resources. Our future capital requirements will depend on many factors, including our ability to market our products successfully, our ability to generate positive cash flow from operations, and our ability to obtain financing in the capital markets. Our business plan requires additional financing beyond our anticipated cash flow from current operations. Consequently, although we currently have no specific plans or arrangements for financing, we intend to raise funds through private placements, public offerings, or other such means. Any equity financings would result in dilution to our then-existing stockholders. Sources of debt financing may result in higher interest expense and may expose us to liquidity problems. Any financing, if available, may be on terms deemed unfavorable. If adequate funds are not obtained, we may be required to reduce or curtail operations.
If we are unable to attract and retain key personnel, we may not be able to compete effectively in our market.
Our success will depend, in part, on our ability to attract and retain key management, both including and beyond what we have today. We will attempt to enhance our management and technical expertise by recruiting qualified individuals who possess desired skills and experience in certain targeted areas. If we are unable to retain staff and to attract and retain sufficient additional employees, and the requisite information technology, engineering, and technical support resources, could have a material adverse effect on our business, financial condition, results of operations, and cash flows. The loss of key personnel could limit our ability to develop and market our products.
Competition could have a material adverse effect on our business.
Our current investments are in the medical device and products field. The medical products industry is a highly competitive industry and the products that we currently have an interest in may not compete well in the marketplace, which could cause our revenues to be less than expected, and/or may cause us to increase the number of our personnel or our advertising or promotional expenditures to maintain our competitive position or for other reasons.
An increase in government regulations could have a material adverse effect on our business.
The U.S. and certain other countries in which we operate impose certain federal and state or provincial regulations, and also require warning labels and signage on medical products. New or revised regulations or increased licensing fees, requirements, or taxes could also have a material adverse effect on our financial condition or results of operations.
If we fail to obtain or maintain applicable regulatory clearances or approvals for our products, or if such clearances or approvals are delayed, we will be unable to distribute our products in a timely manner, or at all, which could significantly disrupt our business and materially and adversely affect our sales and profitability.
The sale and marketing of our products are subject to regulation in the countries where we intend to conduct business. For a significant portion of our products, we need to obtain and renew licenses and registrations with the Food and Drug Administration (FDA), and its equivalent in other markets. The processes for obtaining regulatory clearances or approvals can be lengthy and expensive, and the results are unpredictable. If we are unable to obtain clearances or approvals needed to market existing or new products, or obtain such clearances or approvals in a timely fashion, our business would be significantly disrupted, and our sales and profitability could be materially and adversely affected.
In particular, as we enter foreign markets, we lack the experience and familiarity with both the regulators and the regulatory systems, which could make the process more difficult, more costly, more time consuming and less likely to succeed.
If we fail to comply with regulatory requirements, regulatory agencies may take action against us, which could significantly harm our business.
Marketed products, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for these products, are subject to continual requirements and review by the FDA and other regulatory bodies. In addition, regulatory authorities subject a marketed product, its manufacturer and the manufacturing facilities to ongoing review and periodic inspections. We will be subject to ongoing FDA requirements, including required submissions of safety and other post-market information and reports, registration requirements, current Good Manufacturing Practices (“cGMP”) regulations, requirements regarding the distribution of samples to physicians and recordkeeping requirements.
The cGMP regulations also include requirements relating to quality control and quality assurance, as well as the corresponding maintenance of records and documentation. Regulatory agencies may change existing requirements or adopt new requirements or policies. We may be slow to adapt or may not be able to adapt to these changes or new requirements.
We may be unable to adequately protect our proprietary rights.
Our ability to compete partly depends on the superiority, uniqueness, and value of our intellectual property and technology. To protect our proprietary rights, we will rely on a combination of patent, copyright, and trade secret laws, confidentiality agreements with our employees and third parties, and protective contractual provisions. Despite these efforts, any of the following occurrences may reduce the value of our intellectual property:
| | · | Our applications for patents relating to our business may not be granted or, if granted, may be challenged or invalidated; |
| | · | Issued patents may not provide us with any competitive advantages; |
| | · | Our efforts to protect our intellectual property rights may not be effective in preventing misappropriation of our technology; |
| | · | Our efforts may not prevent the development and design by others of products or technologies similar to, competitive with, or superior to, those we develop; or |
| | · | Another party may obtain a blocking patent and we would need to either obtain a license or design around the patent in order to continue to offer the contested feature or service in our products. |
We may be forced to litigate to defend our intellectual property rights, or to defend against claims by third parties against us relating to intellectual property rights.
We may be forced to litigate to enforce or defend our intellectual property rights, to protect our trade secrets, or to determine the validity and scope of other parties’ proprietary rights. Any such litigation could be expensive and could distract our management from focusing on operating our business. The existence and/or outcome of any such litigation could harm our business.
We may not be able to effectively manage our growth and operations, which could have a material adverse effect on our business.
We may experience rapid growth and development in a relatively short period of time. Should this happen, the management of this growth could require, among other things, continued development of our financial and management controls and management information systems, stringent control of costs, increased marketing activities, the ability to attract and retain qualified management personnel, and the training of new personnel. We intend to retain additional personnel as appropriate to manage our expected growth and expansion. Failure to successfully manage our possible growth and development could have a material adverse effect on our business and the value of our common stock.
Our future research and development projects may not be successful.
The successful development of products can be affected by many factors. Products that appear to be promising at their early phases of research and development may fail to be commercialized for various reasons, including the failure to obtain the necessary regulatory approvals. In addition, the research and development cycle for new products for which we may obtain such approvals certificate typically is long.
There is no assurance that any of our future research and development projects will be successful or completed within the anticipated time frame or budget or that we will receive the necessary approvals from relevant authorities for the production of these newly developed products, or that these newly developed products will achieve commercial success. Even if such products can be successfully commercialized, they may not achieve the level of market acceptance that we expect.
Any products we develop, acquire, or invest in may not achieve or maintain widespread market acceptance.
The success of any products we develop, acquire, or invest in will be highly dependent on market acceptance. We believe that market acceptance of any products will depend on many factors, including, but not limited to:
| | · | the perceived advantages of our products over competing products and the availability and success of competing products; |
| | · | the effectiveness of our sales and marketing efforts; |
| | · | our product pricing and cost effectiveness; |
| | · | the safety and efficacy of our products and the prevalence and severity of adverse side effects, if any; and |
| | · | publicity concerning our products, product candidates, or competing products. |
If our products fail to achieve or maintain market acceptance, or if new products are introduced by others that are more favorably received than our products, are more cost effective, or otherwise render our products obsolete, we may experience a decline in demand for our products. If we are unable to market and sell any products we develop successfully, our business, financial condition, results of operations, and future growth would be adversely affected.
Developments by competitors may render our products or technologies obsolete or non-competitive.
The medical device industry is intensely competitive and subject to rapid and significant technological changes. A large number of companies are pursuing the development of medical devices for markets that we are targeting. We face competition from pharmaceutical and biotechnology companies in the United States and other countries. In addition, companies pursuing different but related fields represent substantial competition. Many of the organizations competing with us have substantially greater capital resources, larger research and development staffs and facilities, longer medical device development history in obtaining regulatory approvals, and greater manufacturing and marketing capabilities than do we. These organizations also compete with us to attract qualified personnel and parties for acquisitions, joint ventures, or other collaborations.
If we fail to maintain an effective system of internal controls or discover material weaknesses in our internal controls over financial reporting, we may not be able to report our financial results accurately or timely or to detect fraud, which could have a material adverse effect on our business.
An effective internal control environment is necessary for us to produce reliable financial reports and is an important part of our effort to prevent financial fraud. We are required to periodically evaluate the effectiveness of the design and operation of our internal controls over financial reporting. Based on these evaluations, we may conclude that enhancements, modifications, or changes to internal controls are necessary or desirable. While management evaluates the effectiveness of our internal controls on a regular basis, these controls may not always be effective. There are inherent limitations on the effectiveness of internal controls, including collusion, management override, and failure of human judgment. In addition, control procedures are designed to reduce rather than eliminate business risks. If we fail to maintain an effective system of internal controls, or if management or our independent registered public accounting firm discovers material weaknesses in our internal controls, we may be unable to produce reliable financial reports or prevent fraud, which could have a material adverse effect on our business, including subjecting us to sanctions or investigation by regulatory authorities, such as the Securities and Exchange Commission. Any such actions could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements, which could cause the market price of our common stock to decline or limit our access to capital.
Our common stock may be affected by limited trading volume and may fluctuate significantly.
There has been a limited public market for our common stock and there can be no assurance that an active trading market for our common stock will develop. This could adversely affect our shareholders’ ability to sell our common stock in short time periods or possibly at all. Our common stock has experienced and is likely to continue to experience significant price and volume fluctuations that could adversely affect the market price of our common stock without regard to our operating performance. Our stock price could fluctuate significantly in the future based upon any number of factors such as: general stock market trends; announcements of developments related to our business; fluctuations in our operating results; announcements of technological innovations, new products, or enhancements by us or our competitors; general conditions in the U.S. and/or global economies; developments in patents or other intellectual property rights; and developments in our relationships with our customers and suppliers. Substantial fluctuations in our stock price could significantly reduce the price of our stock.
Our common stock is traded on the OTC Markets which may make it more difficult for investors to resell their shares due to suitability requirements.
Our common stock is currently traded on the OTC Markets (Pink Sheets) where we expect it to remain for the foreseeable future. Broker-dealers often decline to trade in OTC Markets stocks given that the market for such securities is often limited, the stocks are often more volatile, and the risk to investors is often greater. In addition, OTC Markets’ stocks are often not eligible to be purchased by mutual funds and other institutional investors. These factors may reduce the potential market for our common stock by reducing the number of potential investors. This may make it more difficult for investors in our common stock to sell shares to third parties or to otherwise dispose of their shares. This could cause our stock price to decline.
ITEM 1B – UNRESOLVED STAFF COMMENTS
This Item is not applicable to us as we are not an accelerated filer, a large accelerated filer, or a well-seasoned issuer; however, we have not received written comments from the Commission staff regarding our periodic or current reports under the Securities Exchange Act of 1934 within the last 180 days before the end of our last fiscal year.
ITEM 2 – PROPERTIES
Our office space is approximately 2,500 square feet and we lease the space rent-free from NorthStar Consumer Products, LLC, an entity controlled by John Linderman and James Barickman, two of our executive officers. Beginning in 2013 it is anticipated that we will begin paying a pro rata portion of the rent for our office space, expected to be 2,900 per month.
ITEM 3 – LEGAL PROCEEDINGS
We are not a party to any litigation. In the ordinary course of business, we may from time to time be involved in various pending or threatened legal actions. The litigation process is inherently uncertain and it is possible that the resolution of such matters might have a material adverse effect upon our financial condition and/or results of operations. However, in the opinion of our management, other than as set forth herein, matters currently pending or threatened against us are not expected to have a material adverse effect on our financial position or results of operations.
ITEM 4 – MINE SAFETY DISCLOSURES
There is no information required to be disclosed under this Item.
PART II
ITEM 5 – MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is currently listed for trading on OTC Markets (Pink Sheets), OTCQB tier, under the trading symbol “TWSI”. Our common stock was originally listed on the OTC Bulletin Board on May 28, 2002, but was delisted on September 19, 2011, due to our failure to file our Quarterly Report on Form 10-Q for June 30, 2011. We are now current in our ’34 Act reporting obligations. If management elects to attempt to re-list on the OTC Bulletin Board we must have a market maker file a 15c2-11 application on our behalf and be approved by FINRA.
The following table sets forth the high and low bid information for each quarter within the fiscal years ended December 31, 2011 and 2012, as best we could estimate from publicly-available information. The information reflects prices between dealers, and does not include retail markup, markdown, or commission, and may not represent actual transactions. On January 18, 2013, we effected a 1-for-1,000 reverse stock split. The numbers below reflect the reverse stock split.
| Fiscal Year Ended | | | | Bid Prices | |
| December 31, | | Period | | High | | | Low | |
| | | | | | | | | |
| 2011 | | First Quarter | | $ | 40.00 | | | $ | 24.00 | |
| | | Second Quarter | | $ | 27.90 | | | $ | 7.10 | |
| | | Third Quarter | | $ | 9.00 | | | $ | 2.20 | |
| | | Fourth Quarter | | $ | 6.00 | | | $ | 2.00 | |
| | | | | | | | | | | |
| 2012 | | First Quarter | | $ | 5.00 | | | $ | 0.90 | |
| | | Second Quarter | | $ | 28.50 | | | $ | 1.00 | |
| | | Third Quarter | | $ | 22.00 | | | $ | 4.00 | |
| | | Fourth Quarter | | $ | 13.00 | | | $ | 6.00 | |
The Securities Enforcement and Penny Stock Reform Act of 1990 requires additional disclosure relating to the market for penny stocks in connection with trades in any stock defined as a penny stock. The Commission has adopted regulations that generally define a penny stock to be any equity security that has a market price of less than $5.00 per share, subject to a few exceptions which we do not meet. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the risks associated therewith.
Holders
As of December 31, 2012, there were approximately 41,032 (adjusted to reflect the 1-for-1,000 reverse stock split) shares of our common stock outstanding held by 1,408 holders of record and numerous shares held in brokerage accounts. As of March 28, 2013, there were 44,452,338 shares of our common stock outstanding. Of these shares, 4,077,338 were held by non-affiliates. On the cover page of this filing we value the 4,077,338 shares held by non-affiliates at $11,131,130. These shares were valued at $2.73 per share, based on our share price on March 28, 2013.
Warrants
As of December 31, 2012, we did not have any warrants to purchase our common stock outstanding.
On February 12, 2013, we entered into an Asset Purchase Agreement with HLBC Distribution Company, Inc. (“HLBCDC”), under which we exercised our option to purchase the Soft & Smooth Assets held by HLBCDC. In exchange for the Soft and Smooth Assets we agreed to issue to HLBCDC warrants enabling HLBCDC to purchase One Hundred Fifty Thousand (150,000) shares of our common stock at One Dollar ($1) per share, with a four (4) year expiration period.
Dividends
There have been no cash dividends declared on our common stock, and we do not anticipate paying cash dividends in the foreseeable future. Dividends are declared at the sole discretion of our Board of Directors.
Securities Authorized for Issuance Under Equity Compensation Plans
There are no outstanding options or warrants to purchase shares of our common stock under any equity compensation plans.
Currently, we do not have any equity compensation plans. As a result, we did not have any options, warrants or rights outstanding as of December 31, 2012.
Recent Issuance of Unregistered Securities
We did not have any recent issuances of unregistered securities as of December 31, 2012.
If our stock is listed on an exchange we will be subject to the Securities Enforcement and Penny Stock Reform Act of 1990 requires additional disclosure relating to the market for penny stocks in connection with trades in any stock defined as a penny stock. The Commission has adopted regulations that generally define a penny stock to be any equity security that has a market price of less than $5.00 per share, subject to a few exceptions which we do not meet. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the risks associated therewith.
ITEM 6 – SELECTED FINANCIAL DATA
As a smaller reporting company we are not required to provide the information required by this Item.
ITEM 7 – MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
Forward-Looking Statements
This Annual Report on Form 10-K of TriStar Wellness Solutions, Inc. for the period ended December 31, 2012 contains forward-looking statements, principally in this Section and “Business.” Generally, you can identify these statements because they use words like “anticipates,” “believes,” “expects,” “future,” “intends,” “plans,” and similar terms. These statements reflect only our current expectations. Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy and actual results may differ materially from those we anticipated due to a number of uncertainties, many of which are unforeseen, including, among others, the risks we face as described in this filing. You should not place undue reliance on these forward-looking statements which apply only as of the date of this annual report. To the extent that such statements are not recitations of historical fact, such statements constitute forward-looking statements that, by definition, involve risks and uncertainties. In any forward-looking statement where we express an expectation or belief as to future results or events, such expectation or belief is expressed in good faith and believed to have a reasonable basis, but there can be no assurance that the statement of expectation of belief will be accomplished.
We believe it is important to communicate our expectations to our investors. There may be events in the future; however, that we are unable to predict accurately or over which we have no control. The risk factors listed in this filing, as well as any cautionary language in this annual report, provide examples of risks, uncertainties and events that may cause our actual results to differ materially from the expectations we describe in our forward-looking statements. Factors that could cause actual results or events to differ materially from those anticipated, include, but are not limited to: our ability to successfully obtain financing for product acquisition; changes in product strategies; general economic, financial and business conditions; changes in and compliance with governmental regulations; changes in various tax laws; and the availability of key management and other personnel.
Overview
We were incorporated on August 28, 2000 in the state of Nevada under the name “Quadric Acquisitions”. Following our incorporation we were not actively engaged in any business activities. On April 25, 2001, we were acquired by Zkid Network Company and changed our name to ZKid Network Co. As a result, we became engaged in the business of providing media content for children through the use of our proprietary software. On February 8, 2006, we announced that we would be unable to raise the necessary funds to continue with our then-existing business model and plan. Accordingly, we decided to seek an active company to acquire.
On May 8, 2006, we closed a share exchange agreement with Star Metro Group Limited, which became our wholly-owned subsidiary. Under the terms of the share exchange agreement we exchanged 60,000,000 shares of our company for 100% of the issued and outstanding shares of Star Metro Group at a ratio of 1 share of our common stock for each 2,000 shares of Star Metro Group Limited’s stock. As a result of the share exchange agreement, we became engaged in the development, production and sale of a line of biodegradable, single use, food and beverage containers. On March 20, 2006, we changed our name from ZKid Network Co. to Eatware Corporation.
On November 27, 2006, we changed our name from “Eatware Corporation” to “Star Metro Corp.” We were required to effect this name change by the terms of an agreement we entered into on November 13, 2006, with Glory Team Industrial Limited and Eddie Chou, an ex-director of our company. We effected this name change by merging Star Metro Corp., our newly incorporated and wholly-owned subsidiary that was created for this purpose, into our company, with our company carrying on as the surviving corporation under the name “Star Metro Corp.”
On February 26, 2007, we changed our name from “Star Metro Corp.” to “Biopack Environmental Solutions Inc.” This name change was effected by merging Biopack Environmental Solutions Inc., our newly incorporated and wholly-owned subsidiary that was created for this purpose, into our company, with our company carrying on as the surviving corporation under the name “Biopack Environmental Solutions Inc.”.
On March 27, 2007, we completed a share exchange with the shareholders of Roots Biopack Group Limited, a company formed under the laws of the British Virgin Islands. Under the terms of the share exchange agreement we acquired all of the issued and outstanding common shares of Roots Biopack Group in exchange for the issuance by our company of 90,000,000 common shares to the former shareholders of Roots Biopack Group.
On November 3, 2011, the People’s Court of Guandong Jiangmen Pengjiang District held a hearing relating to our landlord’s claim for unpaid rent for our factory plus penalty interest and other claims. The landlord had made a claim for payment of overdue rent in the amount of RMB 1,236,000, penalty interest in the amount of RMB 1,067,930 and a claim for potential loss of income in the amount of RMB 618,000, for a total amount claimed of RMB 2,921,930 (approximately $451,379). At the hearing, the Court ruled that after two unsuccessful attempts to auction the factory’s assets at the minimum level set by the Court appointed independent valuation company’s fair market assessment price, the Court set the reference value at RMB 3,613,139.20 (approximately $569,359) and transferred all the assets to the landlord. The landlord is legally responsible for settling any claims made by creditors, and the case has been closed.
On April 27, 2012, the holders of our preferred stock, which account for the voting control of the company, entered into an Agreement for the Purchase of Preferred Stock (the “Agreement”) with Rockland Group, LLC, a Texas limited liability company (“Rockland”), under which Rockland purchased Six Hundred Twenty Thousand (620,000) shares of TriStar Wellness Solutions, Inc. Series A Convertible Preferred Stock (the “Series A Preferred Shares”), One Million Shares (1,000,000) shares of TriStar Wellness Solutions, Inc. Series B Convertible Preferred Stock (the “Series B Preferred Shares”) and Seven Hundred Ten Thousand (710,000) shares of TriStar Wellness Solutions, Inc. Series C Convertible Preferred Stock (the “Series C Preferred Shares”, and together with the Series A Preferred Shares and the Series B Preferred Shares, the “Shares”). These shares represent approximately 63% of our outstanding votes on all matters brought before the holders of our common stock for approval and, therefore, represented a change of control. The transaction closed on April 27, 2012.
After the Agreement with Rockland, during 2012, we entered into the following agreements and transactions:
· On April 27, 2012, we entered into a Subsidiary Acquisition Option Agreement (“Subsidiary Option Agreement”) with Xinghui Ltd., a Chinese entity (“Purchaser“), under which sold all the shares in the BPAC Subsidiaries to the Purchaser, effective July 11, 2012, in exchange for the Purchaser assuming all our liabilities as of April 25, 2012, which included all of the liabilities and obligations of ours except for a $400,000 principal amount convertible note that was owed to Trilane Limited as of April 27, 2012. As a result of us exercising our rights under the Subsidiary Option Agreement, we no longer own the BPAC Subsidiaries, including any of their assets or liabilities.
· On June 25, 2012, we entered into a License and Asset Purchase Option Agreement (the “Agreement”) with NorthStar Consumer Products, LLC, a Connecticut limited liability company (“NCP”), under which we, acquired the exclusive license to develop, market and sell, NCP’s Beaute de Maman™ product line, which is a line of skincare and other products specifically targeted for pregnant women. In addition, we acquired the exclusive license rights to develop, market and sell NCP’s formula being developed for itch suppression, which would be sold as an over-the-counter product, if successful. In exchange for these license rights we agreed to issue NCP 225,000 shares of our Series D Convertible Preferred Stock. This transaction closed on June 26, 2012. Additionally, under the Agreement, in connection with our license rights and to ensure we could fulfill any immediate orders timely, we purchased all existing finished product of the Beaute de Maman™ product line currently owned by NCP. In exchange for the inventory we agreed to issue NCP 25,000 shares of Series D Convertible Preferred Stock. Each share is convertible into twenty five shares of common stock.
· On July 11, 2012, we entered into a Marketing and Development Services Agreement (the “Marketing Agreement”) with InterCore Energy, Inc. (“ICE”). Under the Marketing Agreement we were retained to market and develop certain assets referred to as the Soft & Smooth Assets held by ICE. The Soft & Smooth Assets include all rights, interests and legal claims to that certain invention entitled “Delivery Device with Invertible Diaphragm” which is a novel medical applicator that is capable of delivering medicants and internal devices within the body in an atraumatic fashion (without producing injury or damage). In additiona, we were also granted the exclusive option, in our sole discretion, to purchase the Soft & Smooth Assets from ICE for warrants to purchase One Hundred Fifty Thousand (150,000) shares of our common stock at One Dollar ($1) per share, with a four (4) year expiration period. On February 12, 2013, we entered into an Asset Purchase Agreement (the “HLBCDC Asset Purchase Agreement”) with HLBC Distribution Company, Inc. (“HLBCDC”), under which we exercised our option to purchase the Soft & Smooth Assets held by HLBCDC, which had acquired the Soft & Smooth Assets from ICE. In exchange for the Soft and Smooth Assets we agreed to issue to HLBCDC warrants enabling HLBCDC to purchase One Hundred Fifty Thousand (150,000) shares of our common stock at One Dollar ($1) per share, with a four (4) year expiration period.
As a result of the above transactions, as of December 31, 2012, we were a company involved in developing, marketing and selling, NCP’s Beaute de Maman™ product line, which is a line of skincare and other products specifically targeted for pregnant women, as well developing the Soft and Smooth Assets.
Discontinued Operations
As a result of the successful action by our prior landlord, as well as our election under the Subsidiary Acquisition Option Agreement, our operations during the year ended December 31, 2011 are included in the accompanying financial statements as discontinued operations. It is important to note that although we did have operations during 2011, we are required to classify those operations as discontinued operations in the attached financial statements due to the subsequent actions taken by us and others. The details related to our operations during these periods can be found in Note 8 to the attached financial statements.
Results of Operations for the Years Ended December 31, 2012 and 2011
Net profit (loss)
Our loss for the year ended December 31, 2012 totaled $2,248,519, with $1,818,357 derived from continuing operations and $430,162 attributed to discontinued operations, compared to a net loss for the year ended December 31, 2011 of $288,546, with a loss of $81,711 derived from continuing operations and a loss of $206,835 derived from operations that are now discontinued. Our loss of $2,248,519 for the year ended December 31, 2012 was primarily the result of $700,001 of research and development expenses recorded due to the purchase of the Soft & Smooth products on July 11, 2012, $383,750 of general and administrative expenses related to related party consulting contracts, and $400,000 related to the beneficial conversion feature on convertible debt issued as part of the recapitalization of our assets and liabilities.
Research and Development
For the year ended December 31, 2012 and 2011, we had research and development expenses of $700,001 and $0, respectively. The increase in research and development expenses is related to the purchase of the Soft & Smooth products on July 11, 2012 in exchange for 2,000,000 shares of our Series D Convertible Preferred Stock. The fair value of the shares amounted to $700,001 and was based on the closing stock price on the date of this transaction. This transaction was recorded as a component of research and development expenses during 2012 because the viability of an alternative future use was uncertain.
General and Administrative
Our general and administrative expenses for the year ended December 31, 2012 were $999,348, compared to $90,502 for the year ended December 31, 2011. The increase in operating expenses from the period ended December 31, 2012 compared to the prior year period was due to increased related party consulting expense and professional fees incurred related to the Change of Control transaction on April 27, 2012.
Loss from Discontinued Operations
Loss from discontinued operations for the year ended December 31, 2012 were $430,162, compared to $206,835 for the year ended December 31, 2011. The increase in loss from discontinued operations from the period ended December 31, 2012 compared to the prior year period was due to $400,000 related to the beneficial conversion feature on convertible debt issued as part of the recapitalization of our assets and liabilities, offset, by expenses related to general and administrative and impairment, depreciation and amortization.
Liquidity and Capital Resources
The following is a summary of our cash flows provided by (used in) operating, investing and financing activities from continuing operations during the periods indicated:
| | | Year Ended December 31, | |
| | | 2012 | | | 2011 | |
| | | | | | | |
| Cash at beginning of period | | $ | 0 | | | $ | 0 | |
| Net cash used in operating activities | | | (341,963 | ) | | | (68,835 | ) |
| Net cash provided by (used in) discontinued operations | | | (30,162 | ) | | | 110,171 | |
| Net cash (used in) investing activities | | | (1,057 | ) | | | 0 | |
| Net cash provided by (used in) financing activities | | | 384,352 | | | | (29,628 | ) |
| Effect of exchange rate on the balance of cash | | | 0 | | | | (11,708 | ) |
| Cash at end of period | | $ | 11,170 | | | $ | 0 | |
Cash Flows from Operating Activities – For the year ended December 31, 2012, net cash used in continuing operations was $341,963 compared to net cash used in continuing operations of $68,835 for the year ended December 31, 2011. Net cash used in continuing operations for the year ended December 31, 2012 was primarily related to start-up related professional fees as well as payments to related parties.
Cash Flows from Discontinued Operations – For the year ended December 31, 2012, net cash used in discontinued operations was $30,162 as compared to $110,171 in cash provided by discontinued operations.
Cash Flows from Investing Activities –Net cash used in investing activities from continuing operations for the year ended December 31, 2012 was due to the purchase of computer equipment for $1,057.
Cash Flows from Financing Activities – Net cash flows provided by financing activities from continuing operations in the year ended December 31, 2012 was $384,352, compared to net cash used in financing activities of $29,628 in the same period in 2011. For the twelve months in 2012, the cash flows provided by financing activities from continuing operations were from $175,000 in proceeds from notes payable and $209,352 in proceeds from issuance of our Series D Convertible Preferred Stock. For the twelve months in 2011, the cash flows used in financing activities from continuing operations were entirely from debt redemptions.
Our existing liquidity is not sufficient to fund our operations, anticipated capital expenditures, working capital and other financing requirements for the foreseeable future. We will need to seek to obtain additional debt or equity financing, especially if we experience downturns or cyclical fluctuations in our business that are more severe or longer than anticipated, or if we experience significant increases in the cost of raw material and manufacturing, lose a significant customer, or increases in our expense levels resulting from being a publicly-traded company. If we attempt to obtain additional debt or equity financing, we cannot assure you that such financing will be available to us on favorable terms, or at all.
Our financial statements for the year ended December 31, 2012 indicate there is substantial doubt about our ability to continue as a going concern as we are dependent on our ability to retain short term financing and ultimately to generate sufficient cash flow to meet our obligations on a timely basis in order to attain profitability, as well as successfully obtain financing on favorable terms to fund the company’s long term plans. We can give no assurance that our plans and efforts to achieve the above steps will be successful.
Contractual Obligations
The following table summarizes our contractual obligations as of December 31, 2012:
| | | 2012 | | | 2013 | | | 2014 | | | 2015 | | | 2016 | | | Total | |
| Debt obligations | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ - | | | $ | - | |
| Service contracts | | $ | - | | | $ | - | | | $ | - | | | $ - | | | $ | - | | | $ | - | |
| Operating leases | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Off Balance Sheet Arrangements
We have no off balance sheet arrangements.
ITEM 7A – QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a smaller reporting company we are not required to provide the information required by this Item.
ITEM 8 – FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
For a list of financial statements and supplementary data filed as part of this Annual Report, see the Index to Financial Statements beginning at page F-1 of this Annual Report.
ITEM 9 – CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Dismissal of Previous Independent Registered Public Accounting Firm
On February 22, 2013, our Board of Directors approved the dismissal of Wong Lam Leung & Kwok CPA Limited as our independent auditor, effective immediately, and notified them of such dismissal.
Wong Lam Leung & Kwok CPA Limited audited our financial statements, including our balance sheets as of December 31, 2011, 2010 and 2009, and our related statements of operations, changes in stockholders’ equity, and statements of cash flows for the annual periods then ended. The audit report of Wong Lam Leung & Kwok CPA Limited on our financial statements for the period stated above (the “Audit Period”) did not contain any adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles, but the reports of Wong Lam Leung & Kwok CPA Limited, for the Audit Period contained an emphasis of a matter paragraph which indicated conditions existed which raised substantial doubt about our ability to continue as a going concern.
During the fiscal years ended December 31, 2011, 2010 and 2009, and through Wong Lam Leung & Kwok CPA Limited’s dismissal on February 22, 2013, there were (1) no disagreements with Wong Lam Leung & Kwok CPA Limited on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedures, which disagreements, if not resolved to the satisfaction of Wong Lam Leung & Kwok CPA Limited, would have caused Wong Lam Leung & Kwok CPA Limited to make reference to the subject matter of the disagreements in connection with its reports, and (2) no events of the type listed in paragraphs (A) through (D) of Item 304(a)(1)(v) of Regulation S-K.
We furnished Wong Lam Leung & Kwok CPA Limited with a copy of this disclosure on February 22, 2013, providing Wong Lam Leung & Kwok CPA Limited with the opportunity to furnish the Company with a letter addressed to the Commission stating whether it agrees with the statements made by us herein in response to Item 304(a) of Regulation S-K and, if not, stating the respect in which it does not agree. A copy of Wong Lam Leung & Kwok CPA Limited’s letter to the SEC is filed as Exhibit 16.1 to our Current Report on Form 8-K filed with Commission on February 26, 2013.
Engagement of New Independent Registered Public Accounting Firm
Concurrent with the decision to dismiss Wong Lam Leung & Kwok CPA Limited as our independent auditor, the Board of Directors appointed M&K CPAS, PLLC (“M&K”) as our independent auditor.
During the year ended December 31, 2011 and through the date hereof, neither the Company nor anyone acting on its behalf consulted M&K with respect to (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements, and neither a written report was provided to the Company or oral advice was provided that M&K concluded was an important factor considered by the Company in reaching a decision as to the accounting, auditing or financial reporting issue; or (ii) any matter that was the subject of a disagreement or reportable events set forth in Item 304(a)(1)(iv) and (v), respectively, of Regulation S-K.
ITEM 9A – CONTROLS AND PROCEDURES
(a) Evaluation of Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined) in Exchange Act Rules 13a – 15(c) and 15d – 15(e)). Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer, who are our principal executive officer and principal financial officers, respectively, concluded that, as of the end of the period ended December 31, 2012, our disclosure controls and procedures were not effective (1) to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and (2) to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to us, including our chief executive and chief financial officers, as appropriate to allow timely decisions regarding required disclosure.
Our Chief Executive Officer and Chief Financial Officer do not expect that our disclosure controls or internal controls will prevent all error and all fraud. No matter how well conceived and operated, our disclosure controls and procedures can provide only a reasonable level of assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented if there exists in an individual a desire to do so. There can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
Furthermore, smaller reporting companies face additional limitations. Smaller reporting companies employ fewer individuals and find it difficult to properly segregate duties. Often, one or two individuals control every aspect of the company's operation and are in a position to override any system of internal control. Additionally, smaller reporting companies tend to utilize general accounting software packages that lack a rigorous set of software controls.
(b) Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act, as amended, as a process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States and includes those policies and procedures that:
| • | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and any disposition of our assets; |
| | |
| • | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| | |
| • | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2012. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on this assessment, Management has identified the following four material weaknesses that have caused management to conclude that, as of December 31, 2012, our disclosure controls and procedures, and our internal control over financial reporting, were not effective at the reasonable assurance level:
1. We do not have sufficient segregation of duties within accounting functions, which is a basic internal control. Due to our size and nature, segregation of all conflicting duties may not always be possible and may not be economically feasible. However, to the extent possible, the initiation of transactions, the custody of assets and the recording of transactions should be performed by separate individuals. Management evaluated the impact of our failure to have segregation of duties on our assessment of our disclosure controls and procedures and has concluded that the control deficiency that resulted represented a material weakness.
2. We have not documented our internal controls. We have limited policies and procedures that cover the recording and reporting of financial transactions and accounting provisions. As a result we may be delayed in our ability to calculate certain accounting provisions. While we believe these provisions are accounted for correctly in the attached audited financial statements our lack of internal controls could lead to a delay in our reporting obligations. We were required to provide written documentation of key internal controls over financial reporting beginning with our fiscal year ending December 31, 2009. Management evaluated the impact of our failure to have written documentation of our internal controls and procedures on our assessment of our disclosure controls and procedures and has concluded that the control deficiency that resulted represented a material weakness.
3. Effective controls over the control environment were not maintained. Specifically, a formally adopted written code of business conduct and ethics that governs our employees, officers, and directors was not in place. Additionally, management has not developed and effectively communicated to our employees its accounting policies and procedures. This has resulted in inconsistent practices. Further, our Board of Directors does not currently have any independent members and no director qualifies as an audit committee financial expert as defined in Item 407(d)(5)(ii) of Regulation S-K. Since these entity level programs have a pervasive effect across the organization, management has determined that these circumstances constitute a material weakness.
4. We maintain “disclosure controls and procedures,” as such term is defined in Rule 13a-15(e) under the Securities Exchange Act of 1934 (the “Exchange Act”), that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. We conducted an evaluation (the “Evaluation”), under the supervision and with the participation of our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), of the effectiveness of the design and operation of our disclosure controls and procedures (“Disclosure Controls”) as of the end of the period covered by this report pursuant to Rule 13a-15 of the Exchange Act. Based on this Evaluation, our CEO and CFO concluded that our Disclosure Controls were not effective as of the end of the period covered by this report due to lack of segregation of duties in financial reporting and presence of adjusting journal entries during the audit.
To address these material weaknesses, management performed additional analyses and other procedures to ensure that the financial statements included herein fairly present, in all material respects, our financial position, results of operations and cash flows for the periods presented. Accordingly, we believe that the financial statements included in this report fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented.
(c) Remediation of Material Weaknesses
In order to remediate the material weakness in our documentation, evaluation and testing of internal controls, we hope to hire additional qualified and experienced personnel to assist us in remedying this material weakness.
(d) Changes in Internal Control over Financial Reporting
There are no changes to report during our fiscal quarter ended December 31, 2012.
ITEM 9B – OTHER INFORMATION
There are no events required to be disclosed by the Item.
PART III
ITEM 10 – DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors and Executive Officers
The following table sets forth the names and ages of our directors, director nominees, and executive officers as of December 31, 2012, the principal offices and positions with the Company held by each person and the date such person became a director or executive officer of the Company. The executive officers of the Company are elected annually by the Board of Directors. The directors serve one-year terms until their successors are elected. The executive officers serve terms of one year or until their death, resignation, or removal by the Board of Directors. Unless described below, there are no family relationships among any of the directors and officers.
| Name | | Age | | Position(s) |
| | | | | |
| Harry Pond | | 62 | | Chief Executive Officer and Director |
| | | | | |
| David Horin | | 44 | | Chief Financial Officer, Chief Accounting Officer and Secretary |
Business Experience
The following is a brief account of the education and business experience of each director and executive officer during at least the past five years, indicating each person’s principal occupation during the period, and the name and principal business of the organization by which he was employed.
Harry Pond, was appointed as our President and Secretary on April 27, 2012. From 2005 to present, Mr. Pond has served as Managing Director of Rockland Group, LLC, which is a real estate development company active in the Houston, Texas real estate market. From 2008 to present, Mr. Pond has served as a senior business executive for Rockland Insurance Agency, Inc. In this position he is actively involved with the management of loss prevention, marketing, and recruiting to ensure the company’s profitability and productivity. From 1979 to present, Mr. Pond has owned and operated The Harry Pond Insurance Agency, a company that he is currently in the process of merging with Rockland Insurance Agency, Inc. Mr. Pond received his BS in mathematics and education from Texas State University. Mr. Pond serves on the Board of Directors of InterCore Energy, Inc., a public company listed on The OTC Bulletin Board.
David Horin, age 44, was appointed as our Chief Financial Officer on October 25, 2012. Mr. Horin is currently the President of Chord Advisors, LLC, an advisory firm that provides targeted financial solutions to public (small-cap and mid-cap) and private small and mid-sized companies. From March 2008 to June 2012, Mr. Horin was the Chief Financial Officer of Rodman & Renshaw Capital Group, Inc., a full-service investment bank dedicated to providing corporate finance, strategic advisory, sales and trading and related services to public and private companies across multiple sectors and regions. From March 2003 through March 2008, Mr. Horin was the Managing Director of Accounting Policy and Financial Reporting at Jefferies Group, Inc., a full-service global investment bank and institutional securities firm focused on growth and middle-market companies and their investors. Prior to his employment at Jefferies Group, Inc., from 2000 to 2003, Mr. Horin was a Senior Manager in KPMG’s Department of Professional Practice in New York, where he advised firm members and clients on technical accounting and risk management matters for a variety of public, international and early growth stage entities. Mr. Horin has a Bachelor of Science degree in Accounting from Baruch College, City University of New York. Mr. Horin is also a Certified Public Accountant.
Term of Office
Our directors hold office until the next annual meeting or until their successors have been elected and qualified, or until they resign or are removed. Our board of directors appoints our officers, and our officers hold office until their successors are chosen and qualify, or until their resignation or their removal.
Family Relationships
There are no family relationships among our directors or officers.
Involvement in Certain Legal Proceedings
Our directors and executive officers have not been involved in any of the following events during the past ten years:
| | 1. | any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| | | |
| | 2. | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| | | |
| | 3. | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; |
| | | |
| | 4. | being found by a court of competent jurisdiction (in a civil action), the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| | | |
| | 5. | being the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of: (i) any federal or state securities or commodities law or regulation; or (ii) any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or (iii) any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| | 6. | being the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Securities Exchange Act of 1934), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
All proceedings of the board of directors for the year ended December 31, 2012 were conducted by resolutions consented to in writing by the board of directors and filed with the minutes of the proceedings of our board of directors. Our company currently does not have nominating, compensation or audit committees or committees performing similar functions nor does our company have a written nominating, compensation or audit committee charter. Our board of directors does not believe that it is necessary to have such committees because it believes that the functions of such committees can be adequately performed by the board of directors.
We do not have any defined policy or procedural requirements for shareholders to submit recommendations or nominations for directors. The board of directors believes that, given the stage of our development, a specific nominating policy would be premature and of little assistance until our business operations develop to a more advanced level. Our company does not currently have any specific or minimum criteria for the election of nominees to the board of directors and we do not have any specific process or procedure for evaluating such nominees. The board of directors will assess all candidates, whether submitted by management or shareholders, and make recommendations for election or appointment.
A shareholder who wishes to communicate with our board of directors may do so by directing a written request addressed to our president at the address appearing on the first page of this annual report.
Audit Committee Financial Expert
Our board of directors has determined that it does not have an audit committee member that qualifies as an “audit committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K. We believe that the audit committee members are collectively capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting. In addition, we believe that retaining an independent director who would qualify as an “audit committee financial expert” would be overly costly and burdensome and is not warranted in our circumstances given the early stages of our development and the fact that we have not generated revenues to date.
Nomination Procedures For Appointment of Directors
As of December 31, 2012, we did not effect any material changes to the procedures by which our stockholders may recommend nominees to our board of directors.
Code of Ethics
Effective March 26, 2003, our company’s board of directors adopted a Code of Business Conduct and Ethics that applies to, among other persons, members of our board of directors, our company’s officers, contractors, consultants and advisors. As adopted, our Code of Business Conduct and Ethics sets forth written standards that are designed to deter wrongdoing and to promote:
| | (1) | honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; |
| | | |
| | (2) | full, fair, accurate, timely, and understandable disclosure in reports and documents that we file with, or submit to, the Securities and Exchange Commission and in other public communications made by us; |
| | (3) | compliance with applicable governmental laws, rules and regulations; |
| | | |
| | (4) | the prompt internal reporting of violations of the Code of Business Conduct and Ethics to an appropriate person or persons identified in the Code of Business Conduct and Ethics; and |
| | | |
| | (5) | accountability for adherence to the Code of Business Conduct and Ethics. |
Our Code of Business Conduct and Ethics requires, among other things, that all of our company's senior officers commit to timely, accurate and consistent disclosure of information; that they maintain confidential information; and that they act with honesty and integrity
In addition, our Code of Business Conduct and Ethics emphasizes that all employees, and particularly senior officers, have a responsibility for maintaining financial integrity within our company, consistent with generally accepted accounting principles, and federal and state securities laws. Any senior officer who becomes aware of any incidents involving financial or accounting manipulation or other irregularities, whether by witnessing the incident or being told of it, must report it to our company. Any failure to report such inappropriate or irregular conduct of others is to be treated as a severe disciplinary matter. It is against our company policy to retaliate against any individual who reports in good faith the violation or potential violation of our company's Code of Business Conduct and Ethics by another.
Our Code of Business Conduct and Ethics was filed with the Securities and Exchange Commission on April 15, 2003 as Exhibit 20.1 to our annual report for the fiscal year ended December 31, 2002. We will provide a copy of the Code of Business Conduct and Ethics to any person without charge, upon request.
Section 16(a) Beneficial Ownership Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires the Company’s directors and executive officers and persons who own more than ten percent of a registered class of the Company’s equity securities to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of the Company. Officers, directors and greater than ten percent shareholders are required by SEC regulations to furnish the Company with copies of all Section 16(a) forms they file.
During the most recent fiscal year, to the Company’s knowledge, the following delinquencies occurred:
Name | | No. of Late Reports | | | No. of Transactions Reported Late | | | No. of Failures to File | |
| | | | | | | | | | | | | |
| Harry Pond | | | 2 | | | | 1 | | | | 0 | |
| | | | | | | | | | | | | |
| David Horin | | | 0 | | | | 0 | | | | 1 | |
ITEM 11 – EXECUTIVE COMPENSATION
The particulars of compensation paid to the following persons:
| | (a) | all individuals serving as our principal executive officer during the year ended December 31, 2012; |
| | | |
| | (b) | each of our two most highly compensated executive officers other than our principal executive officer who were serving as executive officers at December 31, 2012 who had total compensation exceeding $100,000; and |
| | | |
| | (c) | up to two additional individuals for whom disclosure would have been provided under (b) but for the fact that the individual was not serving as our executive officer at December 31, 2012, |
who we will collectively refer to as the named executive officers, for the years ended December 31, 2012 and 2011, are set out in the following summary compensation table:
Summary Compensation
The following table provides a summary of the compensation received by the persons set out therein for each of our last two fiscal years:
| SUMMARY COMPENSATION TABLE |
Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) (4) | Non-Equity Incentive Plan Compensa- tion ($) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | All Other Compensa -tion ($) | Total ($) |
Harry Pond(1) Chief Executive Officer and Director | 2012 2011 2010 | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- |
David Horin(1) Chief Financial Officer and Secretary | 2012 2011 2010 | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- |
Gerald Lau(3) Former President, Chief Executive Officer | 2012 2011 2010 | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- |
Sean Webster(4) Former Secretary, Treasurer, Chief Financial Officer | 2012 2011 2010 | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- | -0- -0- -0- |
James Yiu Yeung Loong (5) Former Director | 2011 2010 | -0- -0- | -0- -0- | -0- -0- | -0- -0- | -0- -0- | -0- -0- | -0- -0- | -0- -0- |
Ma Cheng Ji(6) Former Director | 2011 2010 | N/A Nil | N/A Nil | N/A Nil | N/A Nil | N/A Nil | N/A Nil | N/A Nil | N/A Nil |
Michael Forster(7) Former Director | 2011 2010 | Nil Nil | Nil Nil | Nil Nil | Nil Nil | Nil Nil | Nil Nil | Nil Nil | Nil Nil |
(1) Harry Pond was appointed our President and Secretary on April 27, 2012.
(2) David Horin was appointed our Chief Financial Officer on October 25, 2012.
(3) Gerald Lau was appointed our President and Chief Executive Officer on March 27, 2007. Mr. Lau resigned from these positions effective April 27, 2012. Mr. Lau resigned as one of our directors effective November 19, 2012.
(4) Sean Webster was appointed as a director of our company on August 6, 2008 and as our Secretary, Treasurer and Chief Financial Officer on October 6, 2008. Mr. Webster resigned from these positions effective April 27, 2012.
(5) James Yiu Yeung Loong was appointed as a director of our company on April 1, 2008 and resigned on April 6, 2011.
(6) Ma Cheng Ji was appointed as a director of our company on April 1, 2008. Mr. Ma resigned as our director on July 7, 2010
(7) Michael Forster was appointed as a director of our company on April 29, 2008. Mr. Forster resigned as our director on May 2, 2011.
Employment Contracts
We currently do not have written employment agreements with our executive officers.
Director Compensation
The following table sets forth director compensation for 2012:
Name | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) |
| | | | | | | | |
| Harry Pond | -0- | -0- | -0- | -0- | -0- | -0- | -0- |
| | | | | | | | |
| Gerald Lau | -0- | -0- | -0- | -0- | -0- | -0- | -0- |
| | | | | | | | |
| Sean Webster | -0- | -0- | -0- | -0- | -0- | -0- | -0- |
No director received compensation for the fiscal years December 31, 2012 and December 31, 2011. We have no formal plan for compensating our directors for their service in their capacity as directors, although such directors are expected in the future to receive stock options to purchase common shares as awarded by our board of directors or (as to future stock options) a compensation committee which may be established. Directors are entitled to reimbursement for reasonable travel and other out-of-pocket expenses incurred in connection with attendance at meetings of our board of directors. Our board of directors may award special remuneration to any director undertaking any special services on our behalf other than services ordinarily required of a director.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth certain information concerning outstanding stock awards held by the Named Executive Officers on December 31, 2012:
| | Option Awards | Stock Awards |
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
| | | | | | | | | | |
| Harry Pond | -0- | -0- | -0- | N/A | N/A | -0- | -0- | -0- | -0- |
| | | | | | | | | | |
| David Horin | -0- | -0- | -0- | N/A | N/A | -0- | -0- | -0- | -0- |
| | | | | | | | | | |
| Gerald Lau | -0- | -0- | -0- | N/A | N/A | -0- | -0- | -0- | -0- |
| | | | | | | | | | |
| Sean Webster | -0- | -0- | -0- | N/A | N/A | -0- | -0- | -0- | -0- |
Outstanding Equity Awards at Fiscal Year-End
There were no outstanding stock options or stock appreciation rights granted to our executive officers and directors at December 31, 2012.
Aggregated Option Exercises
There were no options exercised by any officer or director of our company during our twelve month period ended December 31, 2012.
Long-Term Incentive Plan
Currently, our company does not have a long-term incentive plan in favor of any director, officer, consultant or employee of our company.
ITEM 12 – SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth, as of March 28, 2013, certain information with respect to our equity securities owned of record or beneficially by (i) each Officer and Director of the Company; (ii) each person who owns beneficially more than 5% of each class of the Company’s outstanding equity securities; and (iii) all Directors and Executive Officers as a group.
| Title of Class | | Name and Address of Beneficial Owner(2) | | Nature of Beneficial Ownership | | Amount | | | Percent of Class (1) | |
| | | | | | | | | | | |
| Common Stock | | John R. Linderman (3) | | President, Chief Executive Officer and Director | | | 12,500,000 | (4) | | | 29.4 | %(4) |
| | | | | | | | | | | | | |
| Common Stock | | David Horin (3) | | Chief Financial Officer and Secretary | | | 25,000 | (5) | | | <1 | %(5) |
| | | | | | | | | | | | | |
| Common Stock | | James H. Barickman (3) | | Chief Marketing Officer and Director | | | 12,500,000 | (6) | | | 29.4 | %(6) |
| | | | | | | | | | | | | |
| Common Stock | | Frederick A. Voight (3) | | Chief Investment Officer and Director | | | 25,000,000 | (7) | | | 36 | %(7) |
| | | | | | | | | | | | | |
| Common Stock | | Michael Wax (3) | | Chief Development Officer and Director | | | 0 | | | | 0 | % |
| | | | | | | | | | | | | |
| Common Stock | | Rockland Group, LLC | | 5% Shareholder | | | 48,750,000 | (8) | | | 52.3 | % |
| | | | | | | | | | | | | |
| Common Stock | | M&K Family Limited Partnership | | 5% Shareholder | | | 25,000,000 | (9) | | | 36 | % |
| | | | | | | | | | | | | |
| | | All Officers and Directors as a Group (5 persons) | | | | | 50,000,000 | (4) – (7) | | | 63.9 | % |
| (1) | Based on 44,452,338 shares of common stock outstanding as of March 28, 2013. Shares of common stock subject to options or warrants currently exercisable, or exercisable within 60 days, are deemed outstanding for purposes of computing the percentage of the person holding such options or warrants, but are not deemed outstanding for the purposes of computing the percentage of any other person. |
| (2) | Unless indicated otherwise, the address of the shareholder is 10 Saugatuck Ave., Westport CT 06880. |
| (3) | Indicates an officer and/or director of the Company. |
| (4) | Includes 3,125,000 shares of common stock and 375,000 shares of Series D Convertible Preferred Stock, which is one-half of the 6,250,000 shares of our common stock and 750,000 shares of our Series D Convertible Preferred Stock held by Northstar Consumer Products, LLC, an entity managed by Mr. Linderman and Mr. Barickman (the other half of Northstar’s ownership has been attributed to Mr. Barickman for the purposes of this table). The 375,000 shares of Series D Preferred Shares are convertible into 9,375,000 shares of our common stock. Northstar has agreed not to convert the Series D Preferred Shares until we have sufficient authorized common stock to permit the conversion. |
| (5) | Includes warrants to purchase 25,000 shares of our common stock at $0.45 per share. The warrants vested immediately upon granting. |
| (6) | Includes 3,125,000 shares of common stock and 375,000 shares of Series D Convertible Preferred Stock, which is one-half of the 6,250,000 shares of our common stock and 750,000 shares of our Series D Convertible Preferred Stock held by Northstar Consumer Products, LLC, an entity managed by Mr. Barickman and Mr. Linderman (the other half of Northstar’s ownership has been attributed to Mr. Linderman for the purposes of this table). The 375,000 shares of Series D Preferred Shares are convertible into 9,375,000 shares of our common stock. Northstar has agreed not to convert the Series D Preferred Shares until we have sufficient authorized common stock to permit the conversion. |
| (7) | Includes 10,000,000 shares of our common stock and 600,000 shares of our Series D Convertible Preferred Stock held by Rivercoach Partners, LP, an entity managed by Mr. Voight. The Series D Preferred Shares are convertible into 15,000,000 shares of our common stock. Rivercoach has agreed not to convert the Series D Preferred Shares until we have sufficient authorized common stock to permit the conversion. |
| (8) | Includes 4,625,000 shares of our common stock, 205,000 shares of our Series A Convertible Preferred Stock, 710,000 shares of our Series B Convertible Preferred Stock, and 1,540,000 shares of our Series D Convertible Preferred Stock. Rockland Group, LLC is an entity controlled by Mr. Harry Pond, one of our former officers and directors. The Series D Preferred Shares are convertible into 5,500,000 shares of our common stock. Rockland Group has agreed not to convert the Series D Preferred Shares until we have sufficient authorized common stock to permit the conversion. |
| (9) | Includes 19,500,000 shares of our common stock and 220,000 shares of our Series D Convertible Preferred Stock. The M&K Family Partnership has agreed not to convert the Series D Preferred Shares until we have sufficient authorized common stock to permit the conversion. |
Change of Control Transaction
On April 27, 2012, the holders of our preferred stock, which account for the voting control of the company, entered into an Agreement for the Purchase of Preferred Stock (the “Agreement”) with Rockland Group, LLC, a Texas limited liability company (“Rockland”), under which Rockland purchased Six Hundred Twenty Thousand (620,000) shares of TriStar Wellness Solutions, Inc. Series A Convertible Preferred Stock, One Million Shares (1,000,000) shares of TriStar Wellness Solutions, Inc. Series B Convertible Preferred Stock, and Seven Hundred Ten Thousand (710,000) shares of TriStar Wellness Solutions, Inc. Series C Convertible Preferred Stock. At the closing, these shares represented approximately 63% of our outstanding votes on all matters brought before the holders of our common stock for approval. The transaction closed on April 27, 2012. This transaction resulted in a change of control as Rockland owned a majority of our outstanding voting securities at the close of the transaction.
ITEM 13 – CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Other than as set out below, as of December 31, 2012, we had not been a party to any transaction, proposed transaction, or series of transactions during the last two years in which the amount involved exceeded the lesser of $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years, and in which, to our knowledge, any of the following persons had, or is to have, a direct or indirect material interest: a director or executive officer of our company; a nominee for election as a director of our company; a beneficial owner of more than five percent of the outstanding shares of our common stock; or any member of the immediate family of any such person.
As of December 31, 2012, $325,970 was due to Sean Webster, our former Chief Financial Officer, Secretary, Treasurer, and a former director, and $564,320 was due to Gerald Lau, our former Chief Executive Officer, a former director of our company. This amount represents money advanced to us which had not been repaid back to them as of December 31, 2012. The amount was unsecured and bears no interest. On September 14, 2012, Rockland Group, LLC, an entity controlled by Harry Pond, one of our former officers and directors and one of our current shareholders, entered into an Assistance and Settlement Agreement with Mr. Webster and Mr. Lau under which they agreed to settle the amounts we owed to the for $32,000, which amount was due to Mr. Webster and Mr. Lau when the company got current in its periodic reporting obligations under the Securities Exchange Act of 1934, as amended. The $32,000 was paid to Mr. Webster and Mr. Lau on or about January 25, 2013, and, as a result, no further amounts are owed to Mr. Lau or Mr. Webster and they agreed to release the company from any additional amounts owed.
On April 27, 2012, the holders of our preferred stock, which accounted for the voting control of the company at the time, entered into an Agreement for the Purchase of Preferred Stock (the “Agreement”) with Rockland Group, LLC, a Texas limited liability company (“Rockland”), under which Rockland purchased Six Hundred Twenty Thousand (620,000) shares of TriStar Wellness Solutions, Inc. Series A Convertible Preferred Stock, One Million Shares (1,000,000) shares of TriStar Wellness Solutions, Inc. Series B Convertible Preferred Stock, and Seven Hundred Ten Thousand (710,000) shares of TriStar Wellness Solutions, Inc. Series C Convertible Preferred Stock. At the closing of the transaction, these shares represented approximately 63% of our outstanding votes on all matters brought before the holders of our common stock for approval. The transaction closed on April 27, 2012.
Corporate Governance
As of December 31, 2012, our Board of Directors consisted of Mr. Harry Pond. We did not have a board member that qualifies as “independent” as the term is used in NASDAQ rule 5605(a)(2).
ITEM 14 – PRINCIPAL ACCOUNTING FEES AND SERVICES
Audit fees
The aggregate fees billed for the two most recently completed fiscal periods ended December 31, 2012 and December 31, 2011 for professional services rendered by M&K CPAS, LLC and Wong Lam Leung & Kwok CPA Limited, respectively, for the audit of our annual financial statements, quarterly reviews of our interim financial statements and services normally provided by the independent accountant in connection with statutory and regulatory filings or engagements for these fiscal periods were as follows:
| | Year Ended December 31, 2012 | Year Ended December 31, 2011 |
| Audit Fees and Audit Related Fees | $22,000 | $37,100 |
| Tax Fees | $0 | |
| All Other Fees | $0 | |
| Total | $22,000 | $37,100 |
In the above table, “audit fees” are fees billed by our company’s external auditor for services provided in auditing our company’s annual financial statements for the subject year. “Audit-related fees” are fees not included in audit fees that are billed by the auditor for assurance and related services that are reasonably related to the performance of the audit review of our company’s financial statements. “Tax fees” are fees billed by the auditor for professional services rendered for tax compliance, tax advice and tax planning. “All other fees” are fees billed by the auditor for products and services not included in the foregoing categories.
Policy on Pre-Approval by Audit Committee of Services Performed by Independent Auditors
The board of directors pre-approves all services provided by our independent auditors. All of the above services and fees were reviewed and approved by the board of directors before the respective services were rendered.
The board of directors has considered the nature and amount of fees billed by M&K CPAS, LLC and Wong Lam Leung & Kwok CPA Limited and believes that the provision of services for activities unrelated to the audit is compatible with maintaining M&K CPAS LLC and Wong Lam Leung & Kwok CPA Limited’s independence.
PART IV
ITEM 15 - EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements
For a list of financial statements and supplementary data filed as part of this Annual Report, see the Index to Financial Statements beginning at page F-1 of this Annual Report.
(a)(2) Financial Statement Schedules
We do not have any financial statement schedules required to be supplied under this Item.
(a)(3) Exhibits
Refer to (b) below.
(b) Exhibits
| Item No. | | Description |
| | | |
| (3) | | Articles of Incorporation and Bylaws |
| 3.1 | | Articles of Incorporation (incorporated by reference from our Current Report on Form 8-K filed on May 9, 2001) |
| 3.2 | | Bylaws (incorporated by reference from our Current Report on Form 8-K filed on May 9, 2001) |
| 3.3 | | Certificate of Amendment of Articles of Incorporation (incorporated by reference from our Current Report on Form 8-K filed on May 9, 2001) |
| 3.4 | | Articles of Merger (incorporated by reference from our Current Report on Form 8-K filed on May 9, 2001) |
| 3.5 | | Certificate of Designation (incorporated by reference from our Current Report on Form 8-K filed on May 9, 2001) |
| 3.6 | | Articles of Merger filed with the Secretary of State of Nevada on November 21, 2006 effective on November 26, 2006 (incorporated by reference from our Current Report on Form 8-K filed on November 28, 2006) |
| 3.7 | | Articles of Merger filed with the Secretary of State of Nevada on February 21, 2007 effective on February 26, 2007 (incorporated by reference from our Current Report on Form 8-K filed on February 27, 2007) |
| 3.8 | | Certificate of Correction filed with the Secretary of State of Nevada on June 27, 2007 (incorporated by reference from our Annual Report on Form 10-KSB filed on April 15, 2009) |
| 3.9 | | Certificate of Designation filed with the Secretary of State of Nevada on July 27, 2007 (incorporated by reference from our Annual Report on Form 10-KSB filed on April 15, 2009) |
| 3.10 | | Certificate of Change filed with the Secretary of State of Nevada on June 6, 2009 (incorporated by reference from our Current Report on Form 8-K filed on June 11, 2009) |
| 3.11* | | Certificate of Designation for Series D Convertible Preferred Stock filed with the Secretary of State of Nevada on June 19, 2012 |
| 3.12* | | Certificate of Amendment to Articles of Incorporation filed with the Secretary of State of Nevada on August 29, 2012 |
| 3.13* | | Certificate of Amendment to Articles of Incorporation filed with the Secretary of State of Nevada on January 7, 2013 |
| (10) | | Material Contracts |
| 10.1 | | Agreement for the Purchase of Preferred Stock (the “Agreement”) with Rockland Group, LLC dated April 27, 2012 (incorporated by reference from our Current Report on Form 8-K filed on May 11, 2012) |
| 10.2 | | License and Asset Option Purchase Agreement with NorthStar Consumer Products, LLC dated June 25, 2012 (incorporated by reference from our Current Report on Form 8-K filed on July 2, 2012) |
| 10.3 | | Agreement for the Purchase of Preferred Stock with Rockland Group, LLC dated June 29, 2012 (incorporated by reference from our Current Report on Form 8-K filed on July 2, 2012) |
| | Subsidiary Acquisition Option Agreement with Xinghui Ltd. dated April 25, 2012 (incorporated by reference from our Current Report on Form 8-K filed on November 30, 2012) |
| 10.5 | | Marketing and Development Services Agreement with InterCore Energy, Inc. dated July 11, 2012 (incorporated by reference from our Current Report on Form 8-K filed on November 30, 2012) |
| 10.6 | | Purchase and Assignment of Rights Agreement with RWIP, LLC dated July 11, 2012 (incorporated by reference from our Current Report on Form 8-K filed on November 30, 2012) |
| 10.8 | | Asset Purchase Agreement with Northstar Consumer Products, LLC, dated February 4, 2013 (incorporated by reference from our Current Report on Form 8-K filed on February 19, 2013) |
| 10.9 | | Asset Purchase Agreement with HLBC Distribution Company, Inc., dated February 12, 2013 (incorporated by reference from our Current Report on Form 8-K filed on February 19, 2013) |
| 10.10 | | Employment Agreement with John R. Linderman dated February 1, 2013 (incorporated by reference from our Current Report on Form 8-K filed on February 19, 2013) |
| 10.11 | | Employment Agreement with James Barickman dated February 1, 2013 (incorporated by reference from our Current Report on Form 8-K filed on February 19, 2013) |
| 10.12 | | Employment Agreement with Fredrick A. Voight dated February 1, 2013 (incorporated by reference from our Current Report on Form 8-K filed on February 19, 2013) |
| 10.13 | | Employment Agreement with Michael S. Wax dated February 1, 2013 (incorporated by reference from our Current Report on Form 8-K filed on February 19, 2013) |
| 10.14 | | Exclusive Manufacturing, Marketing and Distribution Definitive License Agreement with Argentum Medical, LLC dated March 7, 2013 (Incorporated by reference from our Current Report on Form 8-K filed with the Commission on March 14, 2013) |
| (31) | | Rule 13a-14(a)/15d-14(a) Certifications |
| 31.1 | | Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer (filed herewith). |
| 31.2 | | Rule 13a-14(a)/15d-14(a) Certification of Chief Accounting Officer (filed herewith). |
| (32) | | Section 1350 Certifications |
| 32.1 | | Section 1350 Certification of Chief Executive Officer (filed herewith). |
| 32.2 | | Section 1350 Certification of Chief Accounting Officer (filed herewith). |
| 101.INS ** | | XBRL Instance Document |
| 101.SCH ** | | XBRL Taxonomy Extension Schema Document |
| 101.CAL ** | | XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.DEF ** | | XBRL Taxonomy Extension Definition Linkbase Document |
| 101.LAB ** | | XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE ** | | XBRL Taxonomy Extension Presentation Linkbase Document |
* Filed herewith.
** XBRL (Extensible Business Reporting Language) information is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections.
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | TriStar Wellness Solutions, Inc. | |
| | | | |
| Dated: April 16, 2013 | By: | /s/ John R. Linderman | |
| | | John R. Linderman | |
| | | Chief Executive Officer, President, and a Director | |
| | | | |
| Dated: April 16, 2013 | By: | /s/ David Horin | |
| | | David Horin | |
| | | Chief Financial Officer and Secretary | |
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Dated: April 16, 2013 | By: | /s/ John R. Linderman | |
| | | John R. Linderman | |
| | | Chief Executive Officer, President, and a Director | |
| | | | |
| Dated: April 16, 2013 | By: | /s/ David Horin | |
| | | David Horin | |
| | | Chief Financial Officer and Secretary | |
| | | | |
| Dated: April 16, 2013 | By: | /s/ James H. Barickman | |
| | | James H. Barickman | |
| | | Chief Marketing Officer and a Director | |
| | | | |
| Dated: April 16, 2013 | By: | /s/ Frederick A. Voight | |
| | | Frederick A. Voight | |
| | | Chief Investment Officer and a Director | |
| | | | |
| Dated: April 16, 2013 | By: | /s/ Michael Wax | |
| | | Michael Wax | |
| | | Chief Development Officer and a Director | |
ITEM 8 – FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX
| | | Page | |
| Financial Statements: | | | |
| Report of Independent Registered Public Accounting Firm - 2012 | | | F-2 | |
| Report of Independent Registered Public Accounting Firm - 2011 | | | F-3 | |
| Balance Sheet | | | F-4 | |
| Statement of Operations | | | F-5 | |
| Statements of Changes in Stockholders' Equity | | | F-6 | |
| Statement of Cash Flows | | | F-7 | |
| Notes to Financial Statements | | | F-8 | |
| | | | | |
| Supplementary Data | | | | |
| Not applicable | | | | |
Report of Independent Registered Public Accounting Firm
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
TriStar Wellness Solutions, Inc.
We have audited the accompanying balance sheet of TriStar Wellness Solutions, Inc. as of December 31, 2012, and the related statement of operations and comprehensive loss, stockholders' equity (deficit), and cash flows for the year then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit. The financial statements for the year ended December 31, 2011 were audited by other auditors whose report expressed as unqualified opinion on those statements.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of TriStar Wellness Solutions, Inc. as of December 31, 2012, and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations, has a working capital deficit and is dependent on financing to continue operations. These issues raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are described in Note 2. These financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ M&K CPAS, PLLC
April 16, 2013
Houston, TX
www.mkacpas.com
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
TO THE BOARD OF DIRECTORS AND SHAREHOLDERS OF
TRISTAR WELLNESS SOLUTIONS, INC.
(INCORPORATED IN THE UNITED STATES OF AMERICA WITH LIMITED LIABILITY)
We have audited the accompanying consolidated balance sheet of TriStar Wellness Solutions Inc. and its subsidiaries, as of 31 December 2011, and the related consolidated statements of operations, change in shareholders’ equity and cash flows for each of the years in the period ended 31 December 2011. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe our audit provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of TriStar Wellness Solutions, Inc. and its subsidiaries as of 31 December 2011, and the consolidated results of its operations and its cash flows for each of the years in the period ended 31 December 2011, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As disclosed in the consolidated financial statements, the Company has suffered substantial net losses in recent years, and has an accumulated deficit at 31 December 2011, which raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans regarding these matters are also described in Note 5. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
WONG LAM LEUNG & KWOK C.P.A. LIMITED
黃林梁郭會計師事務所有限公司
Certified Public Accountants
劉旭明,香港執業會計師,英國特許會計師
LAU YUK MING HAROLD
Certified Public Accountant (Practising), Hong Kong
Practising Certificate Number: P05468
TRISTAR WELLNESS SOLUTIONS, INC.
BALANCE SHEETS
As of December 31, 2012 and 2011
| | | December 31, | | | December 31, | |
| | | 2012 | | | 2011 | |
| Assets | | | | | | |
| Current assets | | | | | | |
| Cash and cash equivalents | | $ | 11,170 | | | $ | 0 | |
| Inventory | | | 12,027 | | | | 0 | |
| Total current assets | | | 23,197 | | | | 0 | |
| | | | | | | | | |
| Non-current assets | | | | | | | | |
| Property and equipment | | | 1,057 | | | | 0 | |
| | | | 1,057 | | | | 0 | |
| | | | | | | | | |
| Assets classified as held for sale | | | 0 | | | | 5,701 | |
| | | | 1,057 | | | | 5,701 | |
| | | | | | | | | |
| Total assets | | $ | 24,254 | | | $ | 5,701 | |
| | | | | | | | | |
| Liabilities and stockholders' equity | | | | | | | | |
| Current liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 32,758 | | | $ | 272,928 | |
| Accounts payable and accrued expenses due to related parties | | | 285,420 | | | | 0 | |
| Short term debts - related party | | | 525,000 | | | | 0 | |
| Short term debts | | | 50,000 | | | | 833,195 | |
| Total current liabilities | | | 893,178 | | | | 1,106,123 | |
| | | | | | | | | |
| Liabilities classified as held for sale | | | 0 | | | | 892,243 | |
| | | | 893,178 | | | | 1,998,366 | |
| Long term liabilities | | | | | | | | |
| Long term debt | | | 0 | | | | 60,000 | |
| Due to a related party | | | 0 | | | | 250,000 | |
| Total long term liabilities | | | 0 | | | | 310,000 | |
| | | | | | | | | |
| Stockholders' deficit | | | | | | | | |
| Convertible preferred stock, $0.001 par value; 10,000,000 shares authorized; | | | | | | | | |
| 6,120,000 and 1,620,000 shares issued and outstanding as of | | | | | | | | |
| December 31, 2012 and December 31, 2011, respectively | | | 6,120 | | | | 1,620 | |
| Common stock; $0.0001 par value; 50,000,000 shares authorized; | | | | | | | | |
| 41,032 shares issued and outstanding | | | 4 | | | | 4 | |
| Additional paid-in capital | | | 8,941,623 | | | | 5,036,014 | |
| Stock issued at less than par value | | | (2,683 | ) | | | (2,683 | ) |
| Accumulated other comprehensive income | | | 0 | | | | 227,849 | |
| Accumulated deficit | | | (9,813,988 | ) | | | (7,565,469 | ) |
| | | | | | | | | |
| Total stockholders' deficit | | | (868,924 | ) | | | (2,302,665 | ) |
| | | | | | | | | |
| Total liabilities and stockholders' deficit | | $ | 24,254 | | | $ | 5,701 | |
See accompanying notes to the financial statements
TRISTAR WELLNESS SOLUTIONS, INC.
STATEMENTS OF OPERATIONS
For the Years Ended December 31, 2012 and 2011
| | | During the Years Ended | |
| | | December 31, | |
| | | 2012 | | | 2011 | |
| Continuing operations | | | | | | |
| | | | | | | |
| Operating expenses: | | | | | | |
| General and administrative | | $ | 999,348 | | | $ | 90,502 | |
| Research and development | | | 700,001 | | | | 0 | |
| Amortization of intangible assets | | | 84,375 | | | | 0 | |
| Total operating expenses | | | 1,783,724 | | | | 90,502 | |
| | | | | | | | | |
| Loss from operations | | | (1,783,724 | ) | | | (90,502 | ) |
| | | | | | | | | |
| Other income (expense): | | | | | | | | |
| Gain on sale of assets and liabilities | | | 0 | | | | 53,778 | |
| Interest expense | | | (34,633 | ) | | | (44,987 | ) |
| Loss for the period from continuing operations | | | (1,818,357 | ) | | | (81,711 | ) |
| Loss for the period from discontinued operations | | | (430,162 | ) | | | (206,835 | ) |
| | | | | | | | | |
| Net loss | | $ | (2,248,519 | ) | | $ | (288,546 | ) |
| | | | | | | | | |
| Continuing operation | | | | | | | | |
| Basic and diluted loss per share | | $ | (44.32 | ) | | $ | (1.99 | ) |
| | | | | | | | | |
| Discontinued operations | | | | | | | | |
| Basic and diluted loss per share | | $ | (10.48 | ) | | $ | (5.04 | ) |
| | | | | | | | | |
| Basic and diluted weighted average common shares outstanding | | | 41,032 | | | | 41,032 | |
See accompanying notes to the financial statements
TRISTAR WELLNESS SOLUTIONS, INC.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
For the Period from December 31, 2010 to December 31, 2012
| | | Preferred Stock | | | Common Stock | | | Accumulated | | | | | | | | | | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Deficit | | | Par | | | Translation | | | Capital | | | Deficit | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2010 | | | 1,620,000 | | | $ | 1,620 | | | | 41,032 | | | $ | 4 | | | $ | (7,276,923 | ) | | $ | (2,683 | ) | | $ | 239,557 | | | $ | 5,036,014 | | | $ | (2,002,411 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Effect of foreign currency translation | | | | | | | | | | | | | | | | | | | | | | | | | | | (11,708 | ) | | | | | | | (11,708 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | (288,546 | ) | | | - | | | | - | | | | | | | | (288,546 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2011 | | | 1,620,000 | | | $ | 1,620 | | | | 41,032 | | | $ | 4 | | | $ | (7,565,469 | ) | | $ | (2,683 | ) | | $ | 227,849 | | | $ | 5,036,014 | | | $ | (2,302,665 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2nd Quarter - Series C Preferred issued to founders in connection with the change of control transaction | | | 710,000 | | | | 710 | | | | - | | | | - | | | | | | | | - | | | | - | | | | (710 | ) | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2nd Quarter - Issuance of Series D Convertible Preferred for the acquisition of an intangible asset | | | 225,000 | | | | 225 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 84,150 | | | | 84,375 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2nd Quarter - Issuance of Series D Convertible Preferred for the acquisition of inventory | | | 25,000 | | | | 25 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 4,975 | | | | 5,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 3rd Quarter - Issuance of Series D Convertible Preferred for research and development | | | 2,000,000 | | | | 2,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 698,001 | | | | 700,001 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 3rd Quarter - Issuance of Series D Convertible Preferred for cash | | | 1,046,760 | | | | 1,047 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 208,305 | | | | 209,352 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 3rd Quarter - Issuance of Series D Convertible Preferred for services | | | 493,240 | | | | 493 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 354,490 | | | | 354,983 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Beneficial conversion charge on short term convertible debt | | | | | | | | | | | | | | | | | | | | | | | | | | | | 400,000 | | | | 400,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Imputed interest on note payable | | | | | | | | | | | | | | | | | | | | | | | | | | | - | | | | 33,672 | | | | 33,672 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Spin-off of Subsidiaries | | | | | | | | | | | | | | | | | | | | | | | | | | | (227,849 | ) | | | 2,122,726 | | | | 1,894,877 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net Loss | | | - | | | | - | | | | - | | | | - | | | | (2,248,519 | ) | | | - | | | | - | | | | - | | | | (2,248,519 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | - | |
| Balance at December 31, 2012 | | | 6,120,000 | | | $ | 6,120 | | | | 41,032 | | | $ | 4 | | | $ | (9,813,988 | ) | | $ | (2,683 | ) | | $ | - | | | $ | 8,941,623 | | | $ | (868,924 | ) |
See accompanying notes to the financial statements
TRISTAR WELLNESS SOLUTIONS, INC.
STATEMENTS OF CASH FLOWS
For the Years Ended December 31, 2012 and 2011
| | | Twelve Months ended | |
| | | December 31, | |
| | | 2012 | | | 2011 | |
| Cash flows from operating activities | | | | | | |
| Loss for the period from continuing operations | | $ | (1,818,357 | ) | | | (81,711 | ) |
| Loss for the period from discontinued operations | | | (430,162 | ) | | | (206,835 | ) |
| | | | | | | | | |
| Adjustments to reconcile net profit/loss to net cash provided by operating activities: | | | | | | | | |
| Non-cash research and development expenses | | | 700,001 | | | | 0 | |
| Imputed interest on note payable | | | 33,672 | | | | 0 | |
| Write-down/gain on sale on assets and liabilities | | | 0 | | | | (53,778 | ) |
| Interest expenses accrual | | | 0 | | | | 44,987 | |
| Issuance of series D convertible preferred stock for services | | | 354,983 | | | | 0 | |
| Amortization of Intangible assets | | | 84,375 | | | | 0 | |
| | | | | | | | | |
| Changes in working capital: | | | | | | | | |
| Inventory | | | (7,027 | ) | | | 0 | |
| Accounts payable and accruals | | | 24,970 | | | | 21,667 | |
| Accounts payable and accrued expenses - related party | | | 285,420 | | | | 0 | |
| Net cash (used in) operating activities from continuing operations | | | (341,963 | ) | | | (68,835 | ) |
| Net cash (used in) generated from operating activities from discontinued operations | | | (30,162 | ) | | | 110,171 | |
| | | | | | | | | |
| Cash flow from investing activities | | | | | | | | |
| Purchase of computer equipment | | | (1,057 | ) | | | 0 | |
| | | | | | | | | |
| Cash flow from financing activities | | | | | | | | |
| Proceeds from issuance of notes payable | | | 175,000 | | | | 0 | |
| Debts redemption | | | 0 | | | | (29,628 | ) |
| Proceeds from issuance of series D convertible preferred stock | | | 209,352 | | | | 0 | |
| Net cash generated from/(used in) financing activities from continuing operation | | | 384,352 | | | | (29,628 | ) |
| | | | | | | | | |
| Effects of exchange rate on the Balance of cash held in foreign currency | | | 0 | | | | (11,708 | ) |
| | | | | | | | | |
| Net change in cash | | | 11,170 | | | | 0 | |
| | | | | | | | | |
| Cash, beginning | | | 0 | | | | 0 | |
| | | | | | | | | |
| Cash, ending | | $ | 11,170 | | | | 0 | |
| | | | | | | | | |
| Supplemental schedule of non-cash activities | | | | | | | | |
| Beneficial conversion feature on convertible debentures | | $ | 400,000 | | | $ | - | |
| Spinoff on assets and liabilities | | $ | 2,122,726 | | | $ | - | |
| Issuance of preferred shares for asset purchase option | | $ | 784,375 | | | $ | - | |
| Issuance of preferred shares for inventory | | $ | 5,000 | | | $ | - | |
See accompanying notes to the financial statements
TRISTAR WELLNESS SOLUTIONS, INC.
NOTES TO FINANCIAL STATEMENTS
Corporate History
Tristar Wellness Solutions, Inc. (“the Company”) was incorporated on August 28, 2000 in the state of Nevada under the name “Quadric Acquisitions”. Following our incorporation the Company not actively engaged in any business activities. On April 25, 2001, the Company was acquired by Zkid Network Company and changed its name to ZKid Network Co. As a result, the Company became engaged in the business of providing media content for children through the use of our proprietary software. On February 8, 2006, the Company announced that it would be unable to raise the necessary funds to continue with the then-existing business model and plan. Accordingly, the Company decided to seek an active company to acquire.
On May 8, 2006, the Company closed a share exchange agreement with Star Metro Group Limited, which became a wholly-owned subsidiary. Under the terms of the share exchange agreement the Company exchanged 60,000 shares of company for 100% of the issued and outstanding shares of Star Metro Group at a ratio of 1 share of common stock for each 2,000 shares of Star Metro Group Limited’s stock. As a result of the share exchange agreement, the Company became engaged in the development, production and sale of a line of biodegradable, single use, food and beverage containers. On March 20, 2006, the Company changed its name from ZKid Network Co. to Eatware Corporation.
On November 27, 2006, the Company changed its name from “Eatware Corporation” to “Star Metro Corp.” The Company was required to effect this name change by the terms of an agreement entered into on November 13, 2006, with Glory Team Industrial Limited and Eddie Chou, an ex-director of the Company. The Company effected this name change by merging Star Metro Corp., a newly incorporated and wholly-owned subsidiary that was created for this purpose, into the Company, with the Company carrying on as the surviving corporation under the name “Star Metro Corp.”
On February 26, 2007, the Company changed the name from “Star Metro Corp.” to “Biopack Environmental Solutions Inc.” This name change was effected by merging Biopack Environmental Solutions Inc., a newly incorporated and wholly-owned subsidiary that was created for this purpose, into the Company, with the Company carrying on as the surviving corporation under the name “Biopack Environmental Solutions Inc.”.
On March 27, 2007, the Company completed a share exchange with the shareholders of Roots Biopack Group Limited, a company formed under the laws of the British Virgin Islands. Under the terms of the share exchange agreement the Company acquired all of the issued and outstanding common shares of Roots Biopack Group in exchange for the issuance by the Company of 90,000,000 common shares to the former shareholders of Roots Biopack Group.
Effective January 2013 all shares of the Company's common stock issued and outstanding were combined and reclassified on a one-for-one thousand basis. The effect of this reverse stock split has been retroactively applied to all periods presented.
TriStar Wellness Solutions, Inc. formerly (Biopack Environmental Solutions Inc.) is a consumer health and wellness company that targets opportunities in the self-care and quality of life marketplace. All of the Company’s operations as of December 31, 2012 related to the operations of the Beaute de Maman product line and developing the Soft and Smooth product. Prior to July 11, 2012, the Company’s primary business was the development, manufacturing, distribution and marketing of bio-degradable food containers and disposable industrial packaging for consumer products. The Company supplied biodegradable food containers and industrial packaging products to multinational corporations, supermarket chains and restaurants located across North America, Europe and Asia. The Company’s operations were conducted through the following wholly-owned subsidiaries: Roots Biopack (Intellectual Property) Limited, incorporated in Hong Kong, Roots Biopark Limited, incorporated in Hong Kong, Jiangmen Roots Biopack Ltd., incorporated in the People’s Republic of China, Starmetro Group Limited, incorporated in the British Virgin Islands and Biopack Environmental Limited (fka E-ware Corporation Limited), incorporated in Hong Kong (together the “BPAC Subsidiaries”).
2. Going Concern and Management's Plans
The Company's financial statements are prepared using accounting principles generally accepted in the United States of America (GAAP) applicable to a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company has not yet established an ongoing source of revenue sufficient to cover its operating costs and allow it to continue as a going concern. In addition, as of December 31, 2012, the Company had an accumulated deficit of $9,813,988, had incurred a net loss for the year ended December 31, 2012 of $2,248,519 and had negative working capital of $869,981. Funding has been provided by related parties as well as new investors committed to make it possible to maintain, expand, and ensure the advancement of the TriStar Wellness products.
The independent registered public accounting firm’s report on the financial statements for the fiscal year ended December 31, 2012 states that because the Company has suffered recurring operating losses from operations, there is substantial doubt about the Company’s ability to continue as a going concern. A “going concern” opinion indicates that the financial statements have been prepared assuming the Company will continue as a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Strategy
The Company intends to focus on purchasing or licensing rights to various health and wellness products using in-house expertise to develop, test, bring to market, and market a variety of health and wellness products. The Company is currently aggressively completing development of product lines that the Company believes address important market needs in target categories. The Company plans to primarily rely on internal R&D expertise complimented by an extensive network of product development specialists to optimize the product development and testing in advance of market launch. In all cases the Company’s plan is to own the unique formulas or processes via direct acquisition or license. As appropriate the Company will pursue and defend patents on each product offering to help protect from competitive incursion.
3. Summary of Significant Accounting Policies
The financial statements include the accounts of the Company and its wholly owned subsidiaries. All material inter-company balances and transactions have been eliminated from the financial statements.
In preparing financial statements in conformity with US GAAP, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and revenues and expenses during the reported periods. Significant estimates include depreciation. Actual results could differ from those estimates.
| (c) | Cash and Cash Equivalents |
For purposes of the statement of cash flows, the Company considers financial instruments with maturities of less than three months when purchased to cash equivalents. There are no cash equivalents as of the balance sheet date.
Inventories are stated at the lower of cost or market. Cost is computed on a weighted-average basis, which approximates the first-in, first-out method; market is based upon estimated replacement costs.
The Company intends to account for purchases of intangible assets in accordance with the provisions of ASC 350 “Intangibles” (“ASC 350”) and ASC 360 “Fixed Assets” (“ASC 360”). The useful lives of intangible assets will be determined at the date of purchase and periodically evaluated for reasonableness. The assets will be tested for impairment at least once annually, if determined to have an indefinite life, or whenever events or changes in circumstances indicate that the carrying amount may no longer be recoverable.
| (f) | Property, Plant and Equipment |
Property, plant and equipment are stated at cost. Depreciation is provided principally by use of the straight-line method over the useful lives of the related assets, except for leasehold properties, which are depreciated over the terms of their related leases or their estimated useful lives, whichever is less. Expenditures for maintenance and repairs, which do not improve or extend the expected useful life of the assets, are expensed to operations while major repairs are capitalized.
| (g) | Foreign Currency Transactions |
In 2011 and through June 2012, the Company’s functional currency is Hong Kong Dollars (“HKD”) and Renminbi (“RMB”) and its reporting currency is U.S. dollars. The Company’s balance sheet accounts are translated into U.S. dollars at the year-end exchange rates and all revenue and expenses are translated into U.S. dollars at the average exchange rates prevailing during the periods in which these items arise. Translation gains and losses are deferred and accumulated as a component of other comprehensive income in stockholders’ equity. Transaction gains and losses that arise from exchange rate fluctuations from transactions denominated in a currency other than the functional currency are included in the statement of operations as incurred.
Basic and diluted earnings per share is calculated using the weighted-average number of common shares outstanding during the period without consideration of the dilutive effect of convertible notes and convertible preferred shares.
| (i) | Research and Development Costs |
Expenditures for research and development are expensed as incurred. Such costs are required to be expensed until the point that technological feasibility is established.
| (j) | Concentration of Credit Risk |
The Company has no significant off-balance-sheet concentrations of credit risk such as foreign exchange contracts, options contracts or other foreign hedging arrangements. The Company maintains the majority of its cash balances with one financial institution in the form of demand deposits.
Certain items in the prior year financial statements have been reclassified for comparative purposes to conform to the presentation in the current period’s presentation. These reclassifications have no effect on the previously reported income (loss).
| (l) | Recently Issued Accounting Pronouncements |
In October 2012, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2012-04, ''Technical Corrections and Improvements" in Accounting Standards Update No. 2012-04. The amendments in this update cover a wide range of Topics in the Accounting Standards Codification. These amendments include technical corrections and improvements to the Accounting Standards Codification and conforming amendments related to fair value measurements. The amendments in this update will be effective for fiscal periods beginning after December 15, 2012. The adoption of ASU 2012-04 is not expected to have a material impact on our financial position or results of operations.
In August 2012, the FASB issued ASU 2012-03, "Technical Amendments and Corrections to SEC Sections: Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin (SAB) No. 114, Technical Amendments Pursuant to SEC Release No. 33-9250, and Corrections Related to FASB Accounting Standards Update 2010-22 (SEC Update)" in Accounting Standards Update No. 2012-03. This update amends various SEC paragraphs pursuant to the issuance of SAB No. 114. The adoption of ASU 2012-03 is not expected to have a material impact on our financial position or results of operations.
In July 2012, the FASB issued ASU 2012-02, "Intangibles -Goodwill and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment" in Accounting Standards Update No. 2012-02. This update amends ASU 2011-08, Intangibles -Goodwill and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment and permits an entity first to assess qualitative factors to determine whether it is more likely than not that an indefinite-lived intangible asset is impaired as a basis for determining whether it is necessary to perform the quantitative impairment test in accordance with Subtopic 350-30, Intangibles -Goodwill and Other -General Intangibles Other than Goodwill. The amendments are effective for annual and interim impairment tests performed for fiscal years beginning after September 15, 2012. Early adoption is permitted, including for annual and interim impairment tests performed as of a date before July 27, 2012, if a public entity's financial statements for the most recent annual or interim period have not yet been issued or, for nonpublic entities, have not yet been made available for issuance. The adoption of ASU 2012-02 is not expected to have a material impact on our financial position or results of operations.
| | | December 31, | | | December 31, | |
| | | 2012 | | | 2011 | |
| | | | | | | |
| Short term loans | | $ | 50,000 | | | | 833,195 | |
| Short term loans - related party | | | 125,000 | | | | - | |
| | | $ | 175,000 | | | $ | 833,195 | |
| | | December 31, | | | December 31, | |
| | | 2012 | | | 2011 | |
| | | | | | | |
| Convertible debentures issued to a non related party | | $ | - | | | $ | 60,000 | |
| Convertible debenture issued to a related party | | | 400,000 | | | | 250,000 | |
| | | $ | 400,000 | | | $ | 310,000 | |
During July 2012, The Company retained short term loans on demand of $400,000 to a related party as a result of the sale of the Company’s BPAC Subsidiaries. The stated interest rate is 0.5%. The note was modified in July 2012 to include a beneficial conversion feature. The conversion price of the modified note is $0.008 per share. The Company recorded a $400,000 beneficial conversion feature as a component of discontinued operations related to this modification. The note holder confirmed to the Company that she does not intend to convert such shares as of December 31, 2012. The Company recorded accrued interest and interest expense of $32,444 ($33,672 was imputed interest and $961 was contractual) related to this note based upon a market interest rate of 8%.
During the 4th quarter of 2012, the Company issued non-convertible demand notes (formal agreements were not executed) to a related party for $125,000. The notes do not bear interest and were subsequently amended and converted into 33,334 shares of Series D Convertible Preferred Stock in February 2013. The Company recorded accrued interest and interest expense of $1,511 related to this note based upon a market interest rate of 8%.
During the 4th quarter of 2012, the Company issued non-convertible demand notes (formal agreements were not executed) to a third party for $50,000. The notes do not bear interest and were subsequently amended and converted into 13,334 shares of Series D Convertible Preferred Stock in February 2013. The Company recorded accrued interest and interest expense of $678 related to this note based upon a market interest rate of 8%.
Series A, B and C Convertible Preferred Stock – Change of Control Transaction
On April 27, 2012, the Company was party to an agreement whereby Rockland Group, LLC, a Texas limited liability company (“Rockland”), purchased six hundred and twenty thousand (620,000) shares of Biopack Environmental Solutions, Inc. Series A Convertible Preferred Stock (“Series A Preferred Shares”), one million (1,000,000) shares of Biopack Environmental Solutions, Inc. Series B Convertible Preferred Stock (“Series B Preferred Shares”) and seven hundred ten thousand (710,000) shares of Biopack Environmental Solutions, Inc. Series C Convertible Preferred Stock (“Series C Preferred Shares”) from the holders of those shares. The Series A Preferred Shares and Series B Preferred Shares were issued prior to December 31, 2011. The Series C Preferred Shares were issued in April 2012. Together the Shares represented approximately 63% of our outstanding votes on all matters brought before the holders of our common stock for approval and, therefore, represented a change of control. Each share of Series A, B and C Convertible Preferred Stock is convertible into 5 shares of common stock on the date of this transaction. The transaction closed on April 27, 2012.
Intangible Asset and Inventory Acquisition in Exchange for Series D Convertible Preferred Stock
On June 25, 2012, the Company entered into a License and Asset Purchase Option Agreement (the “Agreement”) with NorthStar Consumer Products, LLC, a Connecticut limited liability company (“NCP”), under which TriStar Consumer Products, Inc., acquired the exclusive license to develop, market and sell, NCP’s Beaute de Maman product line, which is a line of skincare and other products specifically targeted for pregnant women. In addition, the Company acquired the exclusive license rights to develop, market and sell NCP’s formula being developed for itch suppression, which would be sold as an over-the-counter product, if successful. These licenses are for a period of up to one year, subject to earlier termination upon specified events. During the term of the license, the assets and being licensed will be run by management of NCP pursuant to a consulting agreement. As a result of these license rights the Company are now responsible for developing, marketing and selling the “Beaute de Maman” products, as well as NCP’s anti-itch formula, including all expenses, contractual arrangements, etc., related to product development, manufacturing, marketing, selling, bottling and packaging, and shipping. The Company will also receive all proceeds derived from sales of the products, other than the amounts owed to Dr. Michelle Brown, from whom NCP purchased the “Beaute de Maman” assets. Such proceeds were recorded as a reduction of general and administrative expenses. Under the arrangement with Dr. Brown she is entitled to approximately seven percent (7%) of net revenue for all products sold under the Beaute de Maman brand name and derived from formulas transferred under the agreement with NCP for a 20 year period ending December 31, 2031. In exchange for these license rights the Company agreed to issue NCP 225,000 shares of our Series D Convertible Preferred Stock. This transaction closed on June 26, 2012. Additionally, under the Agreement, in connection with the Company’s license rights and to ensure it can fulfill any immediate orders timely, the Company purchased all existing finished product of the Beaute de Maman product line currently owned by NCP. In exchange for the inventory the Company agreed to issue NCP 25,000 shares of Series D Convertible Preferred Stock. Each share is convertible into twenty five shares of common stock. The fair value of the shares amounted to $89,375 and was based on the closing common stock price of common stock and the conversion ratio (each share of Series D Convertible Preferred Stock is convertible into 25 shares of common stock) on the date of this transaction. The Company recorded $84,375 as an intangible asset and $5,000 as inventory on the date of the transaction.
Issuance of Series D Convertible Preferred Stock for Cash and Services
On June 29, 2012, the Company entered into a Stock Purchase Agreement with Rockland Group, LLC, an entity owned and controlled by Harry Pond, one the Company’s officers and directors (the “Stock Purchase Agreement”). Under the Stock Purchase Agreement, Rockland Group agreed to purchase 1,540,000 shares of our Series D Convertible Preferred Stock. The value of the shares was based upon the cash received and expenses contributed.
The Company issued 1,046,760 shares of Series D Convertible Preferred Stock in exchange for $209,352 in cash. In addition, the Company issued 493,240 shares of Series D Convertible Preferred Stock in exchange for $354,983 of expenses contributed by Rockland on behalf of the Company (total Series D Preferred shares issued is 1,540,000). The value of the expenses contributed was based on the closing common stock price of common stock and the conversion ratio (each share of Series D Convertible Preferred Stock is convertible into 25 shares of common stock) the date of this transaction.
Issuance of Series D Convertible Preferred Stock for Research and Development
On July 11, 2012, the Company, entered into a Purchase and Assignment of Rights Agreement (the “Agreement”) with RWIP, LLC, an Oregon limited liability company (“RWIP”), under which the Company was assigned rights to receive certain royalty payments under previously executed agreements between RWIP and a third party. The royalty payments are equal to Twenty Percent (20%) of all net income (revenue minus expenses) received by the third party InterCore Energy, Inc., a Delaware corporation (fka. I-Web Media, Inc.) (“ICE”) from certain assets owned by ICE, as set forth in that certain asset purchase agreement between RWIP and ICE dated December 10, 2010. In exchange for these rights we agreed to issue RWIP Two Million (2,000,000) shares of our Series D Convertible Preferred Stock. The transaction closed on July 11, 2012. The fair value of the shares amounted to $700,001 and was based on the closing common stock price of common stock and the conversion ratio (each share of Series D Convertible Preferred Stock is convertible into 25 shares of common stock) on the date of this transaction. This transaction was recorded as a component of research and development expenses during 2012 because the viability of an alternative future use was uncertain.
Recapitalization of Assets and Liabilities
On July 11, 2012, the Company exercised its rights under the Subsidiary Option Agreement by sending a signed Notice of Exercise to the Escrow Agent, pursuant to the terms of the Subsidiary Acquisition Agreement. The Company also sent a copy of the Notice of Exercise directly to the Purchaser as well. As a result of the Company exercising its rights under the Subsidiary Option Agreement, the Company no longer owned the Subsidiary Shares or the Subsidiaries, including any of their assets or liabilities. The Company recorded this transaction as a recapitalization and according recorded such assets and liabilities as well accumulated other comprehensive income as a $1,894,877 adjustment to additional paid in capital due to the related party nature of the spin off.
Diluted Shares
Each share of Preferred A, B and C is convertible into five shares of common stock. Each share of Preferred D is convertible into twenty five shares of common stock. Convertible preferred stock was considered anti-dilutive for the twelve months ended December 31, 2012, due to net losses. As of December 31, 2012, there are 3,790,000 Series D Convertible Preferred Shares which are convertible into 94,750,000 of common shares. All Series D Convertible Preferred Stock voting rights are on an “as converted to common stock” basis. Dividend are not mandatory. If declared by the Board Series D Preferred Stock shall have preference over common stock and equal to other series of preferred stock. As of December 31, 2012, there are 2,330,000 Series A, B and C Convertible Preferred Shares which are convertible into 11,650,000 of common shares.
The Company has determined that common stock equivalents in excess of available authorized common shares are not derivative instruments due to the fact that an increase in authorized shares is within the Company’s control.
6. Deferred Income Taxes
The Company uses the asset and liability approach to account for income taxes. Under this method, deferred tax assets and liabilities are recognized for the expected future tax consequences of differences between the carrying amounts of assets and liabilities and their respective tax bases using tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period when the change is enacted.
On January 1, 2007, the Company adopted an accounting standard which clarifies the accounting for uncertainty in income taxes recognized in financial statements. This standard provides guidance on recognizing, measuring, presenting and disclosing in the financial statements uncertain tax positions that a company has taken or expects to take on a tax return.
During both 2012 and 2011, the Company incurred a net loss and therefore had no tax liability. The Company does not have any material uncertain income tax positions. As a result of significant losses and uncertainty of future profit, the net deferred tax asset generated by the loss carry forward has been fully reserved. The cumulative net operating loss carry forward is approximately $730,000 at December 31, 2012, and will expire in the year ended 2032. All tax losses prior to the year ended December 31, 2012 have been eliminated due to the change in control during the year ended December 31, 2012.
| | | | 2012 | |
| Deferred Tax Asset | | $ | 255,400 | |
| Less: Valuation Allowance | | | (255,400 | ) |
| Net Deferred Tax Asset | | | -0- | |
7. Discontinued Operations
On November 3, 2011, the People’s Court of Guandong Jiangmen Pengjiang District held a hearing relating to the Company’s landlord’s claim for unpaid rent for its factory plus penalty interest and other claims. The landlord had made a claim for payment of overdue rent in the amount of RMB 1,236,000, penalty interest in the amount of RMB 1,067,930 and a claim for potential loss of income in the amount of RMB 618,000, for a total amount claimed of RMB 2,921,930 (approximately $451,379). At the hearing, the Court ruled that after two unsuccessful attempts to auction the factory’s assets at the minimum level set by the Court appointed independent valuation company’s fair market assessment price, the Court set the reference value at RMB 3,613,139.20 (approximately $569,359) and transferred all the assets to the landlord. The landlord is legally responsible for settling any claims made by creditors, and the case has been closed.
On April 27, 2012, the Company entered into a Subsidiary Acquisition Option Agreement (“Subsidiary Option Agreement”) with Xinghui Ltd., a Chinese entity (“Purchaser“), under which the Company may, in our sole discretion, sell the Subsidiary Shares to Purchaser. The “Subsidiary Shares” consists of 100% ownership of the following wholly-owned subsidiaries: Roots Biopack (Intellectual Property) Limited, incorporated in Hong Kong, Roots Biopark Limited, incorporated in Hong Kong, Jiangmen Roots Biopack Ltd., incorporated in the People’s Republic of China, Starmetro Group Limited, incorporated in the British Virgin Islands and Biopack Environmental Limited (fka E-ware Corporation Limited), incorporated in Hong Kong (together the “BPAC Subsidiaries”).
On July 11, 2012, the Company exercised its rights under the Subsidiary Option Agreement by sending a signed Notice of Exercise to the Escrow Agent, pursuant to the terms of the Subsidiary Acquisition Agreement. The Company also sent a copy of the Notice of Exercise directly to the Purchaser as well. As a result of the Company exercising its rights under the Subsidiary Option Agreement, the Company no longer owned the Subsidiary Shares or the BPAC Subsidiaries, including any of their assets or liabilities. The Company recorded this transaction as a recapitalization and according recorded such assets and liabilities as well accumulated other comprehensive income as a $1,899,121 adjustment to additional paid in capital.
The liabilities assumed by the Purchaser included, but were not be limited to, Purchaser assuming and agreeing to fully perform and satisfy and be liable for all of the liabilities and obligations of the Company’s except for a $400,000 principal amount convertible note that was owed to Trilane Limited as of April 27, 2012. The note was modified in July 2012 to include a beneficial conversion feature. The conversion price of the modified note is $0.008 per share. The Company recorded a $400,000 beneficial conversion feature as a component of discontinued operations related to this modification. The note holder confirmed to the Company that she does not intend convert such shares as of December 31, 2012.
A summarized statement of operations for the discontinued operations for the comparable twelve month periods ended December 31, 2012 and December 31, 2011 is as follows:
| | | December 31, | | | December 31, | |
| | | 2012 | | | 2011 | |
| | | | | | | |
| Revenues | | $ | - | | | $ | 3,594 | |
| Cost of sales | | | - | | | | 5,486 | |
| Gross loss | | | - | | | | (1,892 | ) |
| | | | | | | | | |
| General and administrative | | | 7,717 | | | | 710,750 | |
| Depreciation and amortization | | | - | | | | 8,309 | |
| Total operating expenses | | | 7,717 | | | | 719,059 | |
| | | | | | | | | |
| Net gain on assets and liabilities written off | | | - | | | | 514,217 | |
| Interest expense | | | 22,445 | | | | - | |
| Beneficial conversion/finance cost | | | 400,000 | | | | 101 | |
| Loss from discontinued opertations | | $ | 430,162 | | | $ | 206,835 | |
Assets classified as held for sale:
| | | December 31, | | | December 31, | |
| | | 2012 | | | 2011 | |
| | | | | | | |
| Assets | | | | | | |
| Cash and cash equivalents | | $ | - | | | $ | 330 | |
| Deposits | | | - | | | | 5,371 | |
| Total assets classified as held for sale | | $ | - | | | $ | 5,701 | |
Liability classified as held for sale:
| | | December 31, | | | December 31, | |
| | | 2012 | | | 2011 | |
| | | | | | | |
| Liabilities | | | | | | |
| Accounts payable and accrued expenses | | $ | - | | | $ | 1,953 | |
| Lau Kin Chung, Gerald - former director | | | - | | | | 564,320 | |
| Sean Webster - former director | | | - | | | | 325,970 | |
| Total liabilities classified as held for sale | | $ | - | | | $ | 892,243 | |
8. Related Party Transactions
Consulting Agreements
On May 15, 2012, the Company entered into a consulting agreement with Highpeak, LLC (“Highpeak”). The two year agreement is effective from April 1, 2012 through March 31, 2014. The Company has agreed to pay Highpeak a monthly consulting fee of $10,000. The Company incurred $90,000 for the twelve months ended December 31, 2012 and has an accounts payable balance of $60,000 as of December 31, 2012.
On May 15, 2012, the Company entered into a consulting agreement with Rivercoach Partners LP (“Rivercoach”). The two year agreement is effective from April 1, 2012 through March 31, 2014. The Company has agreed to pay Rivercoach a monthly consulting fee of $10,000. The Company incurred $90,000 for the twelve months ended December 31, 2012 and has an accounts payable balance of $60,000 as of December 31, 2012.
On June 1, 2012, the Company entered into a consulting agreement with NorthStar Consumer Products, LLC (“NCP”). The two year agreement is effective from April 1, 2012 through March 31, 2014. The Company has agreed to pay NCP a monthly consulting fee of $15,000. The Company incurred $135,000 for the twelve months ended December 31, 2012 and has an accounts payable balance of $90,000 as of December 31, 2012.
On July 17, 2012, the Company entered into a consulting agreement with Chord Advisors, LLC (“Chord”). 50% of Chord is owned by David Horin, the Company’s Chief Financial Officer. The one year agreement is effective from July 15, 2012 through July 15, 2013. The Company has agreed to pay Chord a monthly consulting fee of $12,500. The Company incurred $68,750 for the twelve months ended December 31, 2012 and has an accounts payable balance of $62,500 as of December 31, 2012.
The Company will recognize cash consulting expenses over the requisite service period pursuant to the provisions of each specific agreement.
Series A, B and C Convertible Preferred Stock – Change of Control Transaction
On April 27, 2012, the Company was party to an agreement whereby Rockland Group, LLC, a Texas limited liability company (“Rockland”), purchased six hundred and twenty thousand (620,000) shares of Biopack Environmental Solutions, Inc. Series A Convertible Preferred Stock (“Series A Preferred Shares”), one million (1,000,000) shares of Biopack Environmental Solutions, Inc. Series B Convertible Preferred Stock (“Series B Preferred Shares”) and seven hundred ten thousand (710,000) shares of Biopack Environmental Solutions, Inc. Series C Convertible Preferred Stock (“Series C Preferred Shares”) from the holders of those shares. The Series A Preferred Shares and Series B Preferred Shares were issued prior to December 31, 2011. The Series C Preferred Shares were issued in April 2012. Together the Shares represented approximately 63% of our outstanding votes on all matters brought before the holders of our common stock for approval and, therefore, represented a change of control. Each share of Series A, B and C Convertible Preferred Stock is convertible into 5 shares of common stock on the date of this transaction. The transaction closed on April 27, 2012.
Intangible Asset Acquisition in Exchange for Series D Convertible Preferred Stock
On June 25, 2012, the Company entered into a License and Asset Purchase Option Agreement (the “Agreement”) with NorthStar Consumer Products, LLC, a Connecticut limited liability company (“NCP”), under which TriStar Consumer Products, Inc., acquired the exclusive license to develop, market and sell, NCP’s Beaute de Maman product line, which is a line of skincare and other products specifically targeted for pregnant women. In addition, the Company acquired the exclusive license rights to develop, market and sell NCP’s formula being developed for itch suppression, which would be sold as an over-the-counter product, if successful. These licenses are for a period of up to one year, subject to earlier termination upon specified events. During the term of the license, the assets and being licensed will be run by management of NCP pursuant to a consulting agreement. As a result of these license rights the Company are now responsible for developing, marketing and selling the “Beaute de Maman” products, as well as NCP’s anti-itch formula, including all expenses, contractual arrangements, etc., related to product development, manufacturing, marketing, selling, bottling and packaging, and shipping. The Company will also receive all proceeds derived from sales of the products, other than the amounts owed to Dr. Michelle Brown, from whom NCP purchased the “Beaute de Maman” assets. Such proceeds were recorded as a reduction of general and administrative expenses. Under the arrangement with Dr. Brown she is entitled to approximately seven percent (7%) of net revenue for all products sold under the Beaute de Maman brand name and derived from formulas transferred under the agreement with NCP for a 20 year period ending December 31, 2031. In exchange for these license rights the Company agreed to issue NCP 225,000 shares of our Series D Convertible Preferred Stock. This transaction closed on June 26, 2012. Additionally, under the Agreement, in connection with the Company’s license rights and to ensure it can fulfill any immediate orders timely, the Company purchased all existing finished product of the Beaute de Maman product line currently owned by NCP. In exchange for the inventory the Company agreed to issue NCP 25,000 shares of Series D Convertible Preferred Stock. Each share is convertible into twenty five shares of common stock. The fair value of the shares amounted to $89,375 and was based on the closing common stock price of common stock and the conversion ratio (each share of Series D Convertible Preferred Stock is convertible into 25 shares of common stock) on the date of this transaction. The Company recorded $84,375 as an intangible asset and $5,000 as inventory on the date of the transaction.
Issuance of Series D Convertible Preferred Stock for Cash and Services
On June 29, 2012, the Company entered into a Stock Purchase Agreement with Rockland Group, LLC, an entity owned and controlled by Harry Pond, one the Company’s officers and directors (the “Stock Purchase Agreement”). Under the Stock Purchase Agreement, Rockland Group agreed to purchase 1,540,000 shares of our Series D Convertible Preferred Stock. The value of the shares was based upon the cash received and expenses contributed.
The Company issued 1,046,760 shares of Series D Convertible Preferred Stock in exchange for $209,352 in cash. In addition, the Company issued 493,240 shares of Series D Convertible Preferred Stock in exchange for $354,983 of expenses contributed by Rockland on behalf of the Company (total Series D Preferred shares issued is 1,540,000). The value of the expenses contributed was based on the closing common stock price of common stock and the conversion ratio (each share of Series D Convertible Preferred Stock is convertible into 25 shares of common stock) the date of this transaction.
Issuance of Series D Convertible Preferred Stock for Research and Development
On July 11, 2012, the Company, entered into a Purchase and Assignment of Rights Agreement (the “Agreement”) with RWIP, LLC, an Oregon limited liability company (“RWIP”), under which the Company was assigned rights to receive certain royalty payments under previously executed agreements between RWIP and a third party. The royalty payments are equal to Twenty Percent (20%) of all net income (revenue minus expenses) received by the third party InterCore Energy, Inc., a Delaware corporation (fka. I-Web Media, Inc.) (“ICE”) from certain assets owned by ICE, as set forth in that certain asset purchase agreement between RWIP and ICE dated December 10, 2010. In exchange for these rights we agreed to issue RWIP Two Million (2,000,000) shares of our Series D Convertible Preferred Stock. The transaction closed on July 11, 2012. The fair value of the shares amounted to $700,001 and was based on the common stock price of common stock and the conversion ratio (each share of Series D Convertible Preferred Stock is convertible into 25 shares of common stock) on the date of this transaction. This transaction was recorded as a component of research and development expenses during 2012 because the viability of an alternative future use was uncertain.
Related Party Notes
During July 2012, The Company retained short term loans on demand of $400,000 to a related party as a result of the sale of the Company’s BPAC Subsidiaries. The stated interest rate is 0.5%. The note was modified in July 2012 to include a beneficial conversion feature. The conversion price of the modified note is $0.008 per share. The Company recorded a $400,000 beneficial conversion feature as a component of discontinued operations related to this modification. The note holder confirmed to the Company that she does not intend to convert such shares as of December 31, 2012. The Company recorded accrued interest and interest expense of $32,444 ($33,672 was imputed interest and $961 was contractual) related to this note based upon a market interest rate of 8%.
During the 4th quarter of 2012, the Company issued non-convertible demand notes (formal agreements were not executed) to a related party for $125,000. The notes do not bear interest and were subsequently amended and converted into 33,334 shares of Series D Convertible Preferred Stock in February 2013. The Company recorded accrued interest and interest expense of $1,511 related to this note based upon a market interest rate of 8%.
9. Intangible Assets, Net
On June 25, 2012, the Company entered into a License and Asset Purchase Option Agreement with NCP, under which TriStar Consumer Products, Inc., a wholly-owned subsidiary, acquired the exclusive license to develop, market and sell NCP’s Beaute de Maman product line of skincare and other products specifically targeted for pregnant women. In addition, the Company acquired the exclusive license rights to develop, market and sell NCP’s formula for an over-the-counter itch suppression product. Pursuant to its June 25, 2012 agreement with NCP, the Company received an exclusive licensing rights in exchange for an aggregate of 225,000 shares of Series D Convertible Preferred Stock. The Company valued the exclusive license rights as an intangible asset for $84,375 as of June 25, 2012. Each share of Preferred D is convertible into twenty five shares of common stock. The fair value of the shares amounted to $84,375 and was based on the closing common stock price on the date of this transaction. The license rights intangible assets were amortized over the estimated useful life which was six months.
| | | | |
| | | | |
| Beginning Balance | | $ | 84,375 | |
| Additions | | | - | |
| Amortization | | | (84,375 | ) |
| Balance, December 31, 2012 | | $ | 0 | |
10. Litigation
The Company recognizes a liability for a contingency when it is probable that liability has been incurred and when the amount of loss can be reasonably estimated. When a range of probable loss can be estimated, the Company accrues the most likely amount of such loss, and if such amount is not determinable, then the Company accrues the minimum of the range of probable loss. As of December 31, 2012, there was no litigation against the Company and therefore the litigation accrual was zero.
11. Fair Value Measurements
On July 1, 2008, the Company adopted Accounting Standards Codification Topic 820, Fair Value Measurements and Disclosures (“Topic 820”). Topic 820 defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosure requirements for fair value measures. The three levels are defined as follows:
Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument.
Level 3 inputs to valuation methodology are unobservable and significant to the fair measurement.
The following table represents our assets and liabilities by level measured at fair value on a recurring basis at December 31, 2012:
| Description | | Level 1 | | Level 2 | | Level 3 |
| | | None | | None | | none |
The following table represents our assets and liabilities by level measured at fair value on a recurring basis at December 31, 2011:
| Description | | Level 1 | | Level 2 | | Level 3 |
| | | None | | None | | none |
The adoption of this standard did not have a material effect on the Company’s financial position, results of operations or cash flows.
12. Subsequent Events
Material Related Party Transactions
In February, 2013, the Company closed an Asset Purchase Agreement (the “Asset Purchase Agreement”) with NorthStar Consumer Products, LLC, a Connecticut limited liability company (“NCP”), and John Linderman and James Barickman, individuals (the “Shareholders”), under which the Company exercised an option to purchase a brand of skincare and other products specifically targeted for pregnant women (the Beaute de Maman product line), in addition to an over-the-counter itch suppression formula (together, the “Business”). Previously, the Company entered into to that certain License and Asset Purchase Option Agreement dated June 25, 2012 with NCP (the “License Agreement”), under with the Company licensed the Business from NCP and had the option to purchase the Business from NCP upon certain conditions being satisfied. As set forth in the Asset Purchase Agreement those conditions were either satisfied or renegotiated to the satisfaction of the parties and the Company exercised an option, and purchased, the Business from NCP. As consideration for the purchase of the Business the Company agreed to issue NCP, or its assignees, Seven Hundred Fifty Thousand (750,000) shares of Series D Convertible Preferred Stock.
In connection with the Asset Purchase Agreement the Company entered into employment agreements with Mr. John Linderman to serve as President and Chief Executive Officer, Mr. James Barickman to serve as Chief Marketing Officer, Mr. Frederick A. Voight to serve as Chief Investment Officer, and Mr. Michael S. Wax to serve as Chief Development Officer.
Under the terms of the employment agreement with Mr. Linderman, he will serve as President and Chief Executive Officer until January 31, 2018. Unless notice is given by either party of its or his intent to terminate the agreement not later than thirty (30) days prior to the end of the initial term and the end of any successive term, the agreement shall automatically renew for successive two year periods; provided however, in no event shall the term of his employment extend beyond January 31, 2023. Mr. Linderman’s duties and responsibilities will be those generally associated with a Chief Executive Officer. His compensation will be $300,000 per year with any additional cash or equity bonuses to be determined by the Board of Directors.
Under the terms of the employment agreement with Mr. Barickman, he will serve as our Chief Marketing Officer until January 31, 2018. Unless notice is given by either party of its or his intent to terminate the agreement not later than thirty (30) days prior to the end of the initial term and the end of any successive term, the agreement shall automatically renew for successive two year periods; provided however, in no event shall the term of his employment extend beyond January 31, 2023. Mr. Barickman’s duties and responsibilities will be those generally associated with a Chief Marketing Officer. His compensation will be $300,000 per year with any additional cash or equity bonuses to be determined by our Board of Directors.
Under the terms of the employment agreement with Mr. Voight, he will serve as our Chief Investment Officer until January 31, 2018. Unless notice is given by either party of its or his intent to terminate the agreement not later than thirty (30) days prior to the end of the initial term and the end of any successive term, the agreement shall automatically renew for successive two year periods; provided however, in no event shall the term of his employment extend beyond January 31, 2023. Mr. Voight’s duties and responsibilities will be those generally associated with a Chief Investment Officer. His compensation will be $300,000 per year with any additional cash or equity bonuses to be determined by our Board of Directors.
Under the terms of the employment agreement with Mr. Wax, he will serve as our Chief Development Officer until January 31, 2018. Unless notice is given by either party of its or his intent to terminate the agreement not later than thirty (30) days prior to the end of the initial term and the end of any successive term, the agreement shall automatically renew for successive two year periods; provided however, in no event shall the term of his employment extend beyond January 31, 2023. Mr. Wax’s duties and responsibilities will be those generally associated with a Chief Development Officer. His compensation will be $300,000 per year with any additional cash or equity bonuses to be determined by our Board of Directors. .
In February 2013, the Company entered into an Asset Purchase Agreement (the “HLBCDC Asset Purchase Agreement”) with HLBC Distribution Company, Inc. (“HLBCDC”), under which the Company exercised an option to purchase the Soft & Smooth Assets held by HLBCDC. The Soft & Smooth Assets include all rights, interests and legal claims to that certain inventions entitled “Delivery Devise with Invertible Diaphragm” as further defined in the Marketing Agreement (the “Soft and Smooth Assets”). Previously, we entered into a Marketing and Development Services Agreement (the “Marketing Agreement”) with InterCore Energy, Inc., a Delaware corporation (“ICOR”), under which the Company was retained to market and develop the Soft and Smooth Assets and were granted the exclusive option, in the Company’s sole discretion, to purchase the Soft & Smooth Assets from ICOR. Subsequently, ICOR sold the Soft and Smooth Assets to HLBCDC, transferring the rights to purchase the Soft and Smooth Assets from ICOR to HLBCDC. In exchange for the Soft and Smooth Assets the Company agreed to issue to HLBCDC warrants enabling HLBCDC to purchase One Hundred Fifty Thousand (150,000) shares of common stock at One Dollar ($1) per share, with a four (4) year expiration period.
Preferred Stock and Note Conversions
In January 2013, the Company received a notice of conversion from Sue E. Alter, notifying the Company that she wished to convert $33.60 of principal and interest due under that certain Tristar Wellness Solutions, Inc. Convertible Promissory Note dated April 27, 2012 into 4,200 shares of our common stock. The shares were issued to Ms. Alter, without a restrictive legend.
In January 2013, the Company received a notice of conversion from a noteholder, notifying the Company that he wished to convert $33.60 of principal and interest due under that certain Tristar Wellness Solutions, Inc. Convertible Promissory Note dated January 28, 2013 into 4,200 shares of common stock. The shares were issued to Mr. Kent C Chisman on or about January 31, 2013, without a restrictive legend.
In February 2013, the Company received a notice of conversion from a noteholder, notifying the Company that they wished to convert $32,000 of principal and interest due under that certain Tristar Wellness Solutions, Inc. Convertible Promissory Note dated February 21, 2013 into 4,000,000 shares of common stock. The shares were issued to a third party on or about March 4, 2013, without a restrictive legend.
In February 2013, the Company received a notice of conversion from Rockland Group, LLC, one of our largest shareholders and an entity controlled by Mr. Harry Pond, one of our officers and our sole director, notifying the Company that Rockland Group, LLC wished to convert 215,000 shares of Series A Convertible Preferred Stock into 1,075,000 shares of common stock. These shares were issued to Rockland Group, LLC, with a restrictive legend.
In February 2013, the Company received a notice of conversion from Rockland Group, LLC, one of the largest shareholders and an entity controlled by Mr. Harry Pond, one of the Company’s officers and our sole director, notifying us that Rockland Group, LLC wished to convert 710,000 shares of Series C Convertible Preferred Stock into 3,550,000 shares of our common stock. These shares were issued to Rockland Group, LLC, with a restrictive legend.
In February 2013, the Company received a notice of conversion from Rivercoach Partners, LP, one of the largest shareholders and an entity controlled by Mr. Frederick A. Voight, notifying the Company that Rivercoach Partners, LP wished to convert 400,000 shares of Series D Preferred Stock into 10,000,000 shares of common stock. These shares were issued to Rivercoach Partner, LP, with a restrictive legend.
In February 2013, the Company received a notice of conversion from Highpeak, LLC, one of the largest shareholders and an entity controlled by Mr. Michael S. Wax, notifying the Company that Highpeak, LLC wished to convert 780,000 shares of Series D Preferred Stock into 19,500,000 shares of our common stock. These shares were issued to Highpeak, LLC, with a restrictive legend.
In February 2013, the Company received a notice of conversion from NorthStar Consumer Products, LLC, one of the largest shareholders and an entity controlled by Mr. John Linderman and Mr. Jamie Barickman, notifying us that NorthStar Consumer Products, LLC wished to convert 250,000 shares of Series D Preferred Stock into 6,250,000 shares of our common stock. These shares were issued to NorthStar Consumer Products, LLC, with a restrictive legend.
In February 2013, the Company received a notice of conversion from a noteholder, notifying the Company that he wished to convert $166.40 of principal and interest due under that certain Tristar Wellness Solutions, Inc. Convertible Promissory Note dated January 28, 2013 into 20,800 shares of common stock. The shares were issued to Mr. Kent C Chisman on or about February 14, 2013, without a restrictive legend.
As a result of the above stock issuances, the Company currently has approximately 44.6 million shares of common stock outstanding.
Licensing Agreement
On March 7, 2013, the Company entered into an Exclusive Manufacturing, Marketing and Distribution Definitive License Agreement (the “Agreement”) with Argentum Medical, LLC, a Delaware limited liability company (“Argentum”), under which the Company acquired an exclusive license to develop, market and sell products based on a technology called “Silverlon”, a proprietary silver coating technology providing superior performance related to OTC wound treatment. The license is for 15 years, with a possible 5 year extension. Under the Agreement, the Company is obligated to order a certain amount of proprietary Silverlon film each contract year and if the minimums are not met Argentum could cancel the Agreement. In exchange for these license rights the Company agreed to pay Argentum royalty payments based on the adjusted gross revenues generated by product sales, as well as issue Argentum a warrant to purchase up to 750,000 shares of our common stock with an exercise price of $0.50 per share. The warrant vests in two equal installments of 375,000 shares each, with the vesting based on product’s success in obtaining certain approvals from the Food and Drug Administration. The warrants expire five (5) years after they vest.
F-22