Filed by: Eyetech Pharmaceuticals, Inc.
Pursuant to Rule 425 under the Securities Act of 1933
and deemed filed pursuant to Rule 14a-12
under the Securities Exchange Act of 1934
Subject Company: Eyetech Pharmaceuticals, Inc.
Commission File No.: 000-50516 The press release and slide presentation below were furnished to the Securities and Exchange Commission on Form 8-K filed on August 22, 2005, for the purpose of complying with Regulation FD, Rule 425 under the Securities Act of 1933 and Rule 14a-12 under the Securities Exchange Act of 1934. For technical reasons relating to the SEC’s EDGAR filing system, the information is being refiled pursuant to Rule 425 of the Securities Act of 1933, and is deemed filed pursuant to Rule 14a-12 under the Exchange Act of 1934. No new information is included in this filing.
OSI Pharmaceuticals and Eyetech Pharmaceuticals Announce
Signing of Definitive Merger Agreement; Acquisition Projected to be Accretive and
Generate Combined 2006 Revenues of Over $600 Million
MELVILLE, N.Y. & NEW YORK, Aug 21, 2005 (BUSINESS WIRE) — OSI Pharmaceuticals, Inc. (NASDAQ: OSIP) and Eyetech Pharmaceuticals, Inc. (NASDAQ: EYET) today announced that they have entered into a definitive merger agreement whereby OSI has agreed to acquire Eyetech, a biopharmaceutical company that focuses on the development and commercialization of novel therapeutics to treat eye diseases. Under the merger agreement, OSI will acquire all outstanding shares of Eyetech common stock at a price of $20 per share in a combination of cash and OSI common stock, for an aggregate purchase price of approximately $935 million, representing a 43 percent premium over Eyetech’s $13.99 closing share price on August 19, 2005. The merger agreement calls for 75 percent of the purchase price, or $15 per share, to be paid in cash with the remaining 25 percent to be paid in OSI common stock using an exchange ratio of 0.12275 OSI shares for each share of Eyetech. Approximately 5.7 million OSI shares will be issued in the transaction. The acquisition is subject to a number of closing conditions, including Eyetech stockholder approval and regulatory approvals, and the parties expect to close the transaction by the end of 2005. Eyetech will seek stockholder approval of the transaction at a special meeting called to consider the merger, the date of which will be announced following completion of initial regulatory filings.
“The acquisition of Eyetech represents the rare opportunity to combine two inherently strong growth stories and create a dynamic new entity with real strength. The combination of OSI and Eyetech will create a substantial biopharmaceutical company with over $600 million of projected revenues in 2006 and strong growth prospects for the future,” commented Colin Goddard, Ph.D., Chief Executive Officer of OSI Pharmaceuticals. “We believe the proposed transaction will deliver scale to OSI’s current business, bring forward profitability and provide for double-digit revenue growth over the next five years. Moving forward, we anticipate that OSI will be a profitable enterprise with three high quality business units in the attractive commercial arenas of oncology, eye diseases and diabetes, possess a pipeline of high quality drug candidates and be led by an experienced and deep management team. We are also delighted to bring into the OSI family a dedicated and talented Eyetech team that is focused on improving the health and well being of patients afflicted with devastating eye diseases such as macular degeneration and diabetic macular edema.”
“OSI is the ideal partner for Eyetech,” said David R. Guyer, M.D., Chief Executive Officer of Eyetech Pharmaceuticals. “With OSI, we create a powerful new biopharmaceutical franchise, one with scale, depth of resources as well as strength and security of a diversified and growing revenue base. This transaction provides our shareholders with good value for their equity and our employees with an opportunity for an even more exciting future. I am thrilled by the opportunity to work with Colin Goddard and his management team, as we move into the next exciting phase of our development and growth together.”
Strong Financials Drive Accretive Growth
With combined revenues of over $600 million projected for 2006, OSI will be well positioned to accelerate profitability into 2006. Moving beyond 2006, OSI believes that revenues of the combined company will grow at a compound annual growth rate in the mid-teens for the five-year period starting in 2007, and that EBITDA and adjusted EPS (which excludes the amortization of identifiable intangible assets related to this transaction) compound annual growth rates during the same period will be greater than 30 percent and 25 percent, respectively, creating a strong growth company further supported by a dynamic product portfolio and pipeline. The proposed transaction is projected to be accretive to OSI on a cumulative basis for both adjusted and GAAP EPS over the next four years. OSI has developed these projections based on what management believes is a conservative assessment of the current and future competitive environment for neovascular age-related macular degeneration (AMD) and the science behind Macugen®, and included some assumptions for synergies and cost savings that will result as Eyetech transitions from a public company to a business unit of OSI.
A Diversified Biopharmaceutical Company Focused On Three Significant Therapeutic Areas
The acquisition of Eyetech by OSI creates a diversified biopharmaceutical company that focuses on three therapeutic disease areas of significant market potential: oncology, eye diseases and diabetes. The combined company will have two major marketed products (for the treatment of cancer and age-related macular degeneration) and a robust product pipeline offering both new indications for the marketed products and novel therapeutics in all three disease areas. Tarceva® and Macugen are two of the most exciting and novel biotech products launched in recent years. Tarceva is the first EGFR inhibitor to demonstrate improved survival in both non-small cell lung cancer (NSCLC) and pancreatic cancer, two of the most deadly forms of cancer. Tarceva is approved in the United States for advanced NSCLC and is the subject of a supplemental New Drug Application under review with the FDA for the treatment of pancreatic cancer. Macugen is a novel first in class therapeutic that selectively binds to the pathological isoform of Vascular Endothelial Growth Factor (VEGF) to treat all forms of neovascular AMD including occult, minimally classic and predominantly classic lesion subtypes. Both Tarceva and Macugen are expected to be launched in the European Union by early 2006.
Tarceva has ongoing or planned Phase III trials in front-line and adjuvant NSCLC and in ovarian and colorectal cancers. On the basis of strong Phase II results, Macugen is scheduled to begin Phase III trials for the treatment of Diabetic Macular Edema in 2005, and is also being studied for the treatment of Retinal Vein Occlusion. The combined company will also have five innovative product candidates in development. The clinical pipeline will include (OSI) Prosidion’s lead compound, PSN9301, a Dipeptidyl Peptidase IV (DPIV) inhibitor currently in Phase II clinical trials, the glycogen phosphorylase inhibitor (PSN357) currently in a Phase I clinical trial and (OSI) Oncology’s novel c-kit/KDR inhibitor (OSI-930) which is in Phase I trials. Eyetech’s novel PDGF inhibitor (E10030) for neovascular AMD, and (OSI) Prosidion’s glucokinase activator (PSN010) are also scheduled to enter clinical trials over the next six months.
-2-
OSI Business Model Combines Stand-Alone Business Units With Strong Corporat Operations And Core Expertise
During the last year, OSI has evolved a business model that combines separate business units with centralized corporate planning, operations, R&D technical operations and manufacturing expertise. This model allows the efficient management of independent business teams and, along with favorable geography, will facilitate a seamless integration of Eyetech following completion of the merger. Dr. David Guyer will join OSI Pharmaceuticals as Executive Vice President and CEO of the (OSI) Eyetech subsidiary with Mr. Paul Chaney and Dr. Tony Adamis also continuing in their current roles to establish a strong (OSI) Eyetech leadership team within OSI.
Bear, Stearns & Co. Inc. is acting as the exclusive financial advisor to OSI . Merrill Lynch & Co. is acting as the exclusive financial advisor to Eyetech in connection with this transaction. L.E.K. Consulting have also provided advice to OSI on the transaction. The firms of Mintz Levin Cohn Ferris Glovsky and Popeo PC and Wilmer Cutler Pickering Hale and Dorr LLP have served as counsel to OSI and Eyetech, respectively.
Conference Call / Webcast
OSI and Eyetech will host a conference call and webcast on August 22, 2005 at 8:00AM (Eastern Time). To access the live call or the seven-day archive via the Internet, log on to www.osip.com. Please connect to the website at least 15 minutes prior to the conference call to ensure adequate time for any software download that may be needed to access the webcast. Alternatively, please call 1-800-259-0251 (U.S.) or 1-617-614-3671 (international) to listen to the call. The conference ID number is 39146380. Telephone and internet replay will be available following the filing of the transcript of the call with the Securities and Exchange Commission through September 5, 2005. To access the replay, please call 1-888-286-8010 (U.S.) or 1-617-801-6888 (international) and reference ID number 82062251.
About OSI Pharmaceuticals
OSI Pharmaceuticals is committed to “shaping medicines and changing lives” by discovering, developing and commercializing high-quality and novel pharmaceutical products that extend life or improve the quality of life for cancer and diabetes patients worldwide. The company operates through two business teams, (OSI) Oncology and (OSI) Prosidion. (OSI) Oncology is focused on developing molecular targeted therapies designed to change the paradigm of cancer care. (OSI) Prosidion is committed to the generation of novel, targeted therapies for the treatment of type 2 diabetes and obesity. OSI’s flagship product, Tarceva® (erlotinib), is the first drug discovered and developed by OSI to obtain FDA approval and the only EGFR inhibitor to have demonstrated the ability to improve survival in both non-small cell lung cancer and pancreatic cancer patients. OSI markets Tarceva through partnerships with Genentech, Inc. in the U.S. and with Roche throughout the rest of the world.
In addition to Tarceva, (OSI) Oncology exclusively markets Novantrone® (mitoxantrone concentrate for injection) for its approved oncology indications and markets Gelclair® Bioadherent Oral Gel for the relief of pain associated with oral mucositis. The research and development pipeline consists of novel molecularly targeted anti-cancer agents focused on signal
-3-
transduction pathways involved in cell proliferation, apoptosis and angiogenesis. The most advanced of these programs, targeting the co-inhibition of c-kit/KDR, has two candidates in development.
(OSI) Prosidion is the diabetes and obesity business team within OSI Pharmaceuticals, dedicated to the discovery and development of novel drugs for the treatment of type 2 diabetes and obesity. (OSI) Prosidion’s lead compound, PSN9301, is a Dipeptidyl Peptidase IV (DPIV) inhibitor currently in Phase II clinical trials. Other product candidates include a glycogen phosphorylase inhibitor currently in a Phase I clinical trial and a glucokinase activator scheduled to enter clinical trials in 2005. (OSI) Prosidion owns or has licensing rights to a portfolio of DPIV medical use patents with claims covering DPIV as a target for anti-diabetes therapy and the use of combinations of DPIV inhibitors with other anti-diabetes drugs such as metformin. A number of non-exclusive licenses to the patent estate have been granted to major pharmaceutical companies. (OSI) Prosidion operates through OSI’s wholly-owned subsidiary, Prosidion Limited, in Oxford, U.K.
About Eyetech
Eyetech Pharmaceuticals, Inc. is a biopharmaceutical company that specializes in the development and commercialization of novel therapeutics to treat diseases of the eye. Eyetech’s initial focus is on diseases affecting the back of the eye. Eyetech is commercializing and further developing Macugen® (pegaptanib sodium injection) with Pfizer Inc for the treatment of neovascular AMD. Macugen is also being studied for other indications, including DME and RVO.
Additional Information About the Merger And Where To Find It
OSI intends to file a registration statement on Form S-4 with the Securities and Exchange Commission (SEC) containing a proxy statement/prospectus in connection with the proposed merger. The proxy statement/prospectus will be mailed to the stockholders of Eyetech to consider and vote upon the proposed merger. Investors and stockholders are urged to carefully read the proxy statement/prospectus and other relevant materials filed with the SEC when they become available because they will contain important information about OSI, Eyetech, the merger, and other related matters. Investors and stockholders may obtain free copies of these documents (when they are available) and other documents filed with the SEC at the SEC’s web site at www.sec.gov. These documents can also be obtained for free from OSI by directing a request to OSI Investor Relations at 631-962-2000 and for free from Eyetech by directing a request to Eyetech Investor Relations at 212-824-3100.
Participants in the Merger
OSI, Eyetech and their respective executive officers, directors and other members of management or employees may be deemed to be participants in the solicitation of proxies from Eyetech stockholders with respect to the transactions contemplated by the merger agreement. Information regarding OSI’s executive officers and directors is available in OSI’s Annual Report on Form 10-K for the year ended September 30, 2004 and its proxy statement dated February 2, 2005 for its 2005 Annual Meeting of Stockholders, which are filed with the SEC. Information
-4-
regarding Eyetech’s officers and directors is available in Eyetech’s Annual Report on Form 10-K for the year ended December 31, 2004, its proxy statement dated April 11, 2005 for its 2005 Annual Meeting of Stockholders and its Current Report on Form 8-K dated June 15, 2005, which are filed with the SEC. You can obtain free copies of these documents from OSI and Eyetech using the contact information above. Additional information regarding interests of such participants will be included in the registration statement containing the proxy statement/prospectus that will be filed with the SEC and available free of charge as indicated above.
In addition, in connection with the execution of the merger agreement, Dr. David Guyer, Eyetech’s Chief Executive Officer, Paul G. Chaney, Eyetech’s Chief Operating Officer, and Dr. Anthony P. Adamis, Eyetech’s Chief Scientific Officer, have entered into letter agreements with OSI setting forth the terms under which these individuals will continue their employment with OSI following the merger. Furthermore, in connection with the execution of the merger agreement, Eyetech’s Board of Directors authorized the payment of transaction completion bonuses in the aggregate amount of $350,000. The recipients of these bonuses, and the amounts they may receive, will be determined by Eyetech’s Board of Directors based on the recommendation of its Compensation Committee. Such recipients may include executive officers of Eyetech. Additional information regarding these arrangements and the interests of such participants will be included in the registration statement containing the proxy statement/prospectus that will be filed with the SEC and available free of charge as indicated above.
Safe Harbor for Forward-Looking Statements
This document contains “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements are typically preceded by words such as “believes,” “ expects,” “anticipates,” “intends,” “will,” “may,” “should,” or similar expressions. These forward-looking statements are subject to risks and uncertainties that may cause actual future experience and results to differ materially from those discussed in these forward-looking statements. Important factors that might cause such a difference include, but are not limited to, the ability of Eyetech to obtain stockholder approval of the merger; the possibility that the merger will not close or that the closing will be delayed; the challenges and costs of integrating the operations and personnel of Eyetech; reaction of customers of Eyetech and OSI and related risks of maintaining pre-existing relationships of Eyetech and OSI; the impact of acquisitions and divestitures on the synergies of OSI’s programs; competitive factors, including pricing pressures; the success of research and development activities; and other events and factors disclosed previously and from time to time in OSI’s and Eyetech’s filings with the Securities and Exchange Commission, including OSI’s Annual Report onForm 10-K for the year ended September 30, 2004 and Eyetech’s Quarterly Report onForm 10-Q for the quarter ended June 30, 2005. Except for OSI’s and Eyetech’s ongoing obligations to disclose material information under the federal securities laws, OSI and Eyetech disclaim any obligation to update any forward-looking statements after the date of this document.
This document is not an offer to sell shares of OSI securities which may be issued in the proposed merger. Such OSI common stock is offered only by means of the proxy statement/prospectus referred to herein.
-5-
SOURCE: OSI Pharmaceuticals, Inc. and Eyetech Pharmaceuticals, Inc.
OSI Pharmaceuticals
Kathy Galante, 631-962-2000
or
Burns McClellan (Representing OSI)
Justin Jackson (Media), 212-213-0006 ext. 327
or
Lisa Burns (Investors) 212-213-0006
or
Eyetech
Chris Smith, 212-824-3175
or
Edelman Financial (Representing Eyetech)
Doug Donsky (Media), 212-704-4473
Judith Flynn (Investors), 212-819-4809
-6-

| August 22, 2005 Shaping Medicine, Changing Lives |
| OSI & Eyetech Announce Merger Safe Harbor for Forward-Looking Statements This document contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements are typically preceded by words such as "believes," " expects," "anticipates," "intends," "will," "may," "should," or similar expressions. These forward- looking statements are subject to risks and uncertainties that may cause actual future experience and results to differ materially from those discussed in these forward-looking statements. Important factors that might cause such a difference include, but are not limited to, the ability of Eyetech to obtain stockholder approval of the merger; the possibility that the merger will not close or that the closing will be delayed; the challenges and costs of integrating the operations and personnel of Eyetech; reaction of customers of Eyetech and OSI and related risks of maintaining pre-existing relationships of Eyetech and OSI; the impact of acquisitions and divestitures on the synergies of OSI's programs; competitive factors, including pricing pressures; the success of research and development activities; and other events and factors disclosed previously and from time to time in OSI's and Eyetech's filings with the Securities and Exchange Commission, including OSI's Annual Report on Form 10-K for the year ended September 30, 2004 and Eyetech's Quarterly Report on Form 10-Q for the quarter ended June 30, 2005. Except for OSI's and Eyetech's ongoing obligations to disclose material information under the federal securities laws, OSI and Eyetech disclaim any obligation to update any forward-looking statements after the date of this document. ***** This document is not an offer to sell shares of OSI securities which may be issued in the proposed merger. Such OSI common stock is offered only by means of the proxy statement/prospectus referred to herein. |

| OSI & Eyetech Announce Merger Additional Information About the Merger And Where To Find It OSI intends to file a registration statement on Form S-4 with the Securities and Exchange Commission (SEC) containing a proxy statement/prospectus in connection with the proposed merger. The proxy statement/prospectus will be mailed to the stockholders of Eyetech to consider and vote upon the proposed merger. Investors and stockholders are urged to carefully read the proxy statement/prospectus and other relevant materials filed with the SEC when they become available because they will contain important information about OSI, Eyetech, the merger, and other related matters. Investors and stockholders may obtain free copies of these documents (when they are available) and other documents filed with the SEC at the SEC's web site at www.sec.gov. These documents can also be obtained for free from OSI by directing a request to OSI Investor Relations at 631-962-2000 and for free from Eyetech by directing a request to Eyetech Investor Relations at 212-824-3100. Participants in the Merger OSI, Eyetech and their respective executive officers, directors and other members of management or employees may be deemed to be participants in the solicitation of proxies from Eyetech stockholders with respect to the transactions contemplated by the merger agreement. Information regarding OSI's executive officers and directors is available in OSI's Annual Report on Form 10-K for the year ended September 30, 2004 and its proxy statement dated February 2, 2005 for its 2005 Annual Meeting of Stockholders, which are filed with the SEC. Information regarding Eyetech's officers and directors is available in Eyetech's Annual Report on Form 10-K for the year ended December 31, 2004, its proxy statement dated April 11, 2005 for its 2005 Annual Meeting of Stockholders and its Current Report on Form 8-K dated June 15, 2005, which are filed with the SEC. You can obtain free copies of these documents from OSI and Eyetech using the contact information above. Additional information regarding interests of such participants will be included in the registration statement containing the proxy statement/prospectus that will be filed with the SEC and available free of charge as indicated above. In addition, in connection with the execution of the merger agreement, Dr. David Guyer, Eyetech's Chief Executive Officer, Paul G. Chaney, Eyetech's Chief Operating Officer, and Dr. Anthony P. Adamis, Eyetech's Chief Scientific Officer, have entered into letter agreements with OSI setting forth the terms under which these individuals will continue their employment with OSI following the merger. Furthermore, in connection with the execution of the merger agreement, Eyetech's Board of Directors authorized the payment of transaction completion bonuses in the aggregate amount of $350,000. The recipients of these bonuses, and the amounts they may receive, will be determined by Eyetech's Board of Directors based on the recommendation of its Compensation Committee. Such recipients may include executive officers of Eyetech. Additional information regarding these arrangements and the interests of such participants will be included in the registration statement containing the proxy statement/prospectus that will be filed with the SEC and available free of charge as indicated above. |
| OSI & Eyetech Announce Merger Creating the Template for a Top 10 Biotech Company "OSI and Eyetech Announced Yesterday a Definitive Merger Agreement Creating a Financially Strong and Diversified BioPharmaceutical Company With Two Major Marketed Brands (Tarceva & Macugen), a Robust Pipeline and a Talented Management Team Operating in Three Attractive Commercial Arenas (Oncology, Eye Diseases, and Diabetes)." |

| OSIP Acquisition of Eyetech (EYET) Economic and Deal Highlights OSI is Acquiring Eyetech (EYET) for $20 Per Share A 43% Premium to the $13.99 EYET Closing Price on 8/19/05 Deal Valued at $935 Million Comprised of 75% or $701 Million Cash and 25% or $234 Million in OSIP Stock Priced at $40.73 Per Share (Based on a 20 Day Trailing Average Close of OSIP) OSI Will Issue 5.7 Million OSIP Shares Representing an Approximately 10% Proforma Ownership of OSIP by Eyetech Shareholders The Transaction Will Require Eyetech Shareholder Approval and is Anticipated to Close by the End of 2005 |

| OSIP Acquisition of Eyetech (EYET) Pro-forma Combined Balance Sheet (est.) All numbers, in millions, are estimates at time of projected closing Pro-forma Estimate 12/31/05 |

| OSI Acquisition of Eyetech A Financially Driven Transforming Deal for OSI-1 A Strategic & Enabling Financial Transaction for OSI - We Believe the Transaction Positions Us To: Deliver a Cash Positive 2006 on Revenues of Over $600 Million Create an Efficient Platform to Deliver Cumulative Accretive Growth Over the Next Four Years on an Adjusted EPS* & GAAP EPS Basis Accretive Using Conservative Models of Macugen Market Share Significant Upside if Further Macugen and Lucentis Data Favors Macugen Provides Attractive Premium to Eyetech Shareholders With Opportunity to Participate in the Success of the Combined Entity Scale & Risk Diversification Sets the Stage for The Creation of a Top-ten Biotech Franchise Access to Capital, Top-line and Bottom-line Growth, Pipeline, Scientific Cache All Improved *excludes the amortization of identifiable intangible assets related to this transaction |

| OSI Acquisition of Eyetech A Financially Driven Transforming Deal for OSI-2 A Rare Opportunity to Combine Two Inherently Strong Growth Stories to Create a Dynamic New Entity With Real Strength Tarceva and Macugen Are Two of the Most Exciting Major Biotech Products of Recent Years Creates a Diversified Biopharmaceutical Company With a Focus on Three Therapeutic Disease Areas of Significant Market Potential Smooth and Efficient Integration Eyetech Fits Into OSI Organizational Structure as a 3rd Business Unit Geographic Location Allows Optimal Synergies and Creation of a Cost-effective Platform There Are Additional Strategic Tie-ins to the Rest of the Business Development of Macugen for Diabetic Complications Biological Research Expertise in the Area of Angiogenesis |

| OSI Acquisition of Eyetech Strategic Context and Background Transaction Fits in With the Strategy OSI Has Evolved to Follow Through on Tarceva Success Goal: to Capitalize on Tarceva and Avoid the Pitfalls of a Single Product Company by Creating Scale, Depth and Risk Mitigation Represents a Key Component to Three Identified Strategic Goals Over the Next Three Years Execution on Tarceva Establishment of Solid Oncology Franchise Behind Tarceva Diversification Into Other Disease Areas Multi-business Unit Model Formalized Around Acquisition of Outstanding Shares of (OSI) Prosidion in Spring of This Year Created a Template for Growth and Expansion Management Team Strengthened Around Business Unit & Core Corporate Framework |
| OSI Acquisition of Eyetech A Strategic and Enabling Financial Transaction Eyetech's Flagship Product Macugen Adds More Leverage to a Combined Platform Than a Stand-alone Situation Competitive Threat of Lucentis Is Real but Macugen Remains Viable Analyzed Multiple Financial Scenarios Financial Benefits to OSI Across All Scenarios Estimated 2006 Combined Revenues of Over $600 Million Accelerates Profitability Even in Lower Market Share Models, Adjusted EPS for Combined Company Is Accretive Anticipated Success in Diabetic Macular Edema Protects and Expands Macugen Franchise Any Positive Macugen Event or Lucentis Issue Carries Significant Upside |

| OSI Acquisition of Eyetech A Strategic and Enabling Financial Transaction Pro-Forma 2006 (millions) Pro-Forma CAGR* 2007-2011 Revenues >$600 Mid-Teens EBITDA >$20 >30% Adjusted EPS+ Turns Positive >25% Assumes a Conservative Base Case Financial Model Assumes Synergies of $25 Million/year in the Combined Company *Compound Annual Growth Rate +excludes the amortization of identifiable intangible assets related to this transaction |

| Creating Scale and Risk Diversification A Strong Portfolio Projected by End of 2006 PRODUCT IND TRACK PHASE I PHASE II PHASE III Marketed Oncology: Tarceva - NSCLC Tarceva - Pancreatic Cancer Tarceva - Other Indications Novantrone Gelclair OSI-930/OSI817 (c-kit/KDR) IGF1R Eye Disease: Macugen (AMD) Macugen (DME) Macugen (RVO) E10030 (anti-PDGF) Diabetes: PSN9301 (DP-IV Inhibitor) PSN357(GP Inhibitor) PSN010 (GKA) *Excludes VEGFR,HER-2 & PDGFR Products discovered in OSI/Pfizer alliance being developed by Pfizer. OSI will receive royalties on net sales. |

| Tarceva and Macugen in One Portfolio Two of the Premier Biotech Products of Recent Years Tarceva - OSI's Flagship EGFR Inhibitor - Approved for 2nd Line/3rd Line NSCLC and Launched in November 2004 Over $117MM in Sales in First Half of 2005 European Approval Anticipated in 4Q2005 Growth Strategy: Progression to Front-line and Adjuvant Settings in NSCLC Phase II Data Showed Good Activity in Front-Line Phase III Studies in First-line (SATURN & TITAN) and Adjuvant Setting Planned Growth Strategy: Progression to Other Disease Settings sNDA for Front-line Pancreatic Cancer Filed (PDUFA 11/2/05) Phase III Study in Ovarian & Colon Cancers Planned Anti-tumor Activity Demonstrated in Phase II Monotherapy Trials for Ovarian, Head & Neck, Brain, Liver, Breast and Colon Cancers Anti-tumor Activity Demonstrated in Combination Therapies for Pancreatic, Head & Neck and Colon Cancers IST and CTEP Programs (over 100 trials) Help to Pave the Way Growth Strategy: Combinations with Other Targeted Therapies Strong A+T Data in Renal Cell Carcinoma and 2nd Line NSCLC (Non-squamous) |

| Tarceva and Macugen in One Portfolio Macugen: A Breakthrough Therapy for AMD Macugen Is the Quintessential Biotech Product - a Pegylated Aptamer Representing a Novel, First-in-class Therapeutic That Selectively Binds to Vascular Endothelial Growth Factor Isoform-165 VEGF-165 is the Pathogenic Isoform Causing Choroidal Neovascularization (CNV), the Causative Event in Age Related Macular Degeneration (AMD) Neovascular AMD Afflicts 200,000 Americans/year and Leads to Blindness Macugen is the First and Only FDA-approved Treatment for all Types of Neovascular AMD Macugen Approved in December 2004 & Launched in January 2005 on Basis of Two Phase III Clinical Trials Showing Ability to Arrest Progression of Neovascular- AMD Subset Analysis Suggests Macugen Can Improve Vision in Patients with Early Disease Only Other Competitor in Market Is Visudyne/PDT, Approved for Predominantly Classic AMD Genentech's Lucentis (a Promiscuous Anti-VEGF Antibody Fragment) Has Emerged as a Strong Competitor Based on Initial Phase III Data |

| Assessing Macugen & Lucentis A Clear On-going Role for Macugen Both Macugen and Lucentis Are High Tech Products Targeting VEGF Lucentis Is More Promiscuous, Binding to All Forms of VEGF While Promiscuous Agents May Deliver Somewhat Higher Efficacy, They Generally Result in More Side-effects MARINA Efficacy Data Is Impressive MARINA Study Two Year Safety Data and Emerging Safety Information From Other Lucentis Trials Will Be Carefully Monitored In Any Scenario, With Its Exquisite Selectivity and Outstanding Side-effect Profile, We Believe There Will Be an Important Role for Macugen in the Marketplace In Combination With Visudyne-PDT for Predominately Classic Patients, Macugen's Side-effect Profile May Lend It Well to Become Anti-VEGF Therapy of Choice |

| Assessing Macugen & Lucentis Macugen is Well Positioned to Compete Market Dynamics Confer First-mover Advantage Macugen Is Having an Outstanding Launch Year Eyetech (7/25/05) Guidance for 2005 Revenue Is $175 to $190 Million Pfizer Partnership and Eyetech Commercial Presence Is Very Effective Estimated Two Year Lead Time in Marketplace Allows for Brand Loyalty and Comfort Amongst Physicians for a Safe and Efficacious Product OSI's Market Research Also Indicates: Macugen's Efficacy - Especially in Patients with Early Disease - May Be Under Appreciated Differences in Efficacy Between the Macugen and Lucentis May Be More Subtle Than Is Currently Perceived |

| Assessing Macugen & Lucentis Macugen Has a Number of Potential Upsides Established Safety Profile Macugen: Potential Upsides from Conservative Assumptions Subgroup Efficacy Data Analysis Dosing Potential better safety profile in comparison to Lucentis, especially in combination with Visudyne Potential inflammation risk with Lucentis Long-term Safety not yet Reported for Lucentis Trial design for Macugen and Lucentis are different, making it difficult to make apples to apples comparison Underappreciated Macugen efficacy in sub-group patient analysis In a subgroup of patients with early disease, 20% of Macugen patients gained three lines of vision on the eye chart, compared to zero in the control group Macugen is administered less frequently than Lucentis Drug delivery research program underway to reduce frequency of dosing Established Position in AMD Market Physicians are becoming comfortable with Macugen First mover advantage in an emerging therapeutic class Pfizer/Eyetech have established a strong commercial infrastructure |
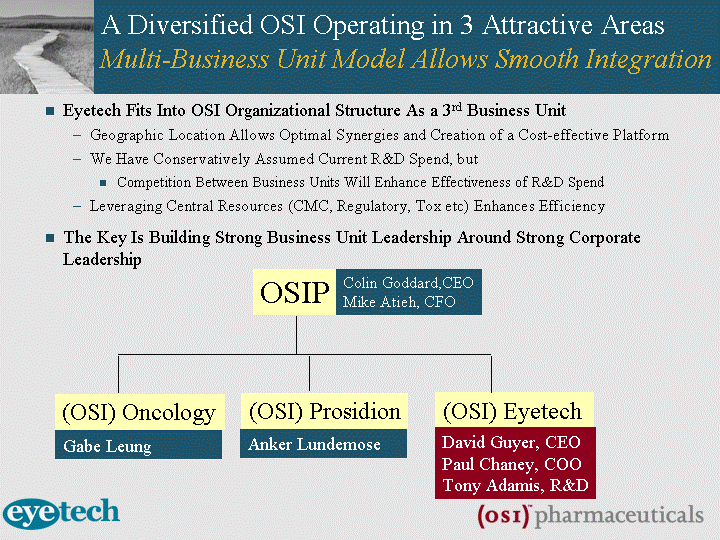
| A Diversified OSI Operating in 3 Attractive Areas Multi-Business Unit Model Allows Smooth Integration Eyetech Fits Into OSI Organizational Structure As a 3rd Business Unit Geographic Location Allows Optimal Synergies and Creation of a Cost-effective Platform We Have Conservatively Assumed Current R&D Spend, but Competition Between Business Units Will Enhance Effectiveness of R&D Spend Leveraging Central Resources (CMC, Regulatory, Tox etc) Enhances Efficiency The Key Is Building Strong Business Unit Leadership Around Strong Corporate Leadership OSIP (OSI) Oncology (OSI) Prosidion (OSI) Eyetech Colin Goddard,CEO Mike Atieh, CFO Gabe Leung Anker Lundemose David Guyer, CEO Paul Chaney, COO Tony Adamis, R&D |

| Why OSI? Makes Financial and Strategic Sense Attractive Premium for Eyetech Shareholders With Opportunity to Participate in the Success of the Combined Company Allows Enhanced Scale, Growth and Diversification Combines Two of the Most Successful Recent Biotech Product Launches Tarceva Represents Real Innovation Complementary Research and Development Platforms Allows Pursuit of Eyetech R&D Pipeline in More Robust Platform Macugen Potential in Diabetic Complications Eyetech Partnership With Archemix Provides Access to Aptamers Strong Long-term Commitment of Eyetech Management Team Expect a Smooth Integration and Transition |

| Macugen (AMD) Approved in US, Canada and Brazil EU Approval Expected Q1'06 Japan Approval Expected 2007 Macugen + Visudyne Ph IIIb/IV (AMD) Started Q2'05 Macugen PhIII (DME) Trial to Start 2005 Macugen PhII (RVO) Trial to Be Fully Enrolled 2H'05 Macugen + anti-PDGF Aptamer PhI (AMD) to Start 1H'06 Expanding the Macugen Franchise Maximizing Macugen in the Near Term |

| AMD AMD AMD Macugen Target ICAM Signaling +PDT for AMD Diabetic Retinopathy Glaucoma Scarring Retinal Vein Occlusion Oncology Drug Delivery Pre-Clinical Development Phase I Phase II Phase III/IIIb/IV Commercialized Lead Optimization Exploratory Diabetic Macular Edema Target TGF-^ E10030 (Anti- PDGF) Proliferative Vitreoretinopathy AMD Scarring AMD Diabetic Retinopathy Glaucoma PVR A Robust Pipeline Capitalizing on Drug Delivery and Aptamers |

| OSIP Acquisition of Eyetech Summary Creates a Substantial Biotech Company Resulting From a Fundamentally Sound Financial Transaction The Deal Works Financially Even With Our Conservative Models Over $600 Million in Projected '06 Revenue Delivers Real Scale to Business, Brings Forward Profitability, and Projected Double Digit Revenue Growth for the Next 5 Years Moving Forward, OSI Will Be a Profitable Organization With Three High Quality Business Units in Oncology, Eye Care and Diabetes, a Pipeline Comprising Multiple High Quality Candidates in Each Arena and a Talented and Deep Management Team |

| August 22, 2005 Shaping Medicine, Changing Lives |






















