Filed by: OSI Pharmaceuticals, Inc.
Pursuant to Rule 425 Under the Securities Act of 1933
Subject Company: Eyetech Pharmaceuticals, Inc.
Commission File No.: 000-50516
The slide presentation below was used by OSI Pharmaceuticals, Inc. (“OSI”) on September 29, 2005 during a presentation by Michael G. Atieh, Executive Vice President and Chief Financial Officer, of OSI, on OSI’s product portfolio and business developments.

| Shaping Medicine, Changing Lives UBS Global Life Sciences Conference NASDAQ / NMS: OSIP September 2005 |
| OSI Has Filed an S-4 Required OSI & Eyetech Merger Information Additional Information About the OSI and Eyetech Merger And Where To Find It OSI has filed a registration statement on Form S-4 with the Securities and Exchange Commission (SEC) containing a proxy statement/prospectus in connection with the proposed merger. The proxy statement/prospectus will be mailed to the stockholders of Eyetech to consider and vote upon the proposed merger. Investors and stockholders are urged to carefully read the proxy statement/prospectus and other relevant materials filed with the SEC when they become available because they will contain important information about OSI, Eyetech, the merger, and other related matters. Investors and stockholders may obtain free copies of these documents (when they are available) and other documents filed with the SEC at the SEC's web site at www.sec.gov. These documents can also be obtained for free from OSI by directing a request to OSI Investor Relations at 631-962-2000 and for free from Eyetech by directing a request to Eyetech Investor Relations at 212-824-3100. Participants in the Merger OSI, Eyetech and their respective executive officers, directors and other members of management or employees may be deemed to be participants in the solicitation of proxies from Eyetech stockholders with respect to the transactions contemplated by the merger agreement. Information regarding OSI's executive officers and directors is available in OSI's Annual Report on Form 10-K for the year ended September 30, 2004 and its proxy statement dated February 2, 2005 for its 2005 Annual Meeting of Stockholders, which are filed with the SEC. Information regarding Eyetech's executive officers and directors is available in Eyetech's Annual Report on Form 10-K for the year ended December 31, 2004, its proxy statement dated April 11, 2005 for its 2005 Annual Meeting of Stockholders and its Current Report on Form 8-K dated June 15, 2005, which are filed with the SEC. You can obtain free copies of these documents from OSI and Eyetech using the contact information above. Additional information regarding interests of such participants will be included in the registration statement containing the proxy statement/prospectus that will be filed with the SEC and available free of charge as indicated above. In addition, in connection with the execution of the merger agreement, Dr. David Guyer, Eyetech's Chief Executive Officer, Paul G. Chaney, Eyetech's Chief Operating Officer, and Dr. Anthony P. Adamis, Eyetech's Chief Scientific Officer, have entered into letter agreements with OSI setting forth the terms under which these individuals will continue their employment with OSI following the merger. Furthermore, in connection with the execution of the merger agreement, Eyetech's Board of Directors authorized the payment of transaction completion bonuses in the aggregate amount of $350,000. The recipients of these bonuses, and the amounts they may receive, will be determined by Eyetech's Board of Directors based on the recommendation of its Compensation Committee. Such recipients may include executive officers of Eyetech. Additional information regarding these arrangements and the interests of such participants will be included in the registration statement containing the proxy statement/prospectus that will be filed with the SEC and available free of charge as indicated above. |
| OSI Has Filed an S-4 Required OSI & Eyetech Merger Information Safe Harbor for Forward-Looking Statements This document contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements are typically preceded by words such as "believes," " expects," "anticipates," "intends," "will," "may," "should," or similar expressions. These forward- looking statements are subject to risks and uncertainties that may cause actual future experience and results to differ materially from those discussed in these forward-looking statements. Important factors that might cause such a difference include, but are not limited to, the completion of clinical trials, the FDA review process and other governmental regulation, OSI's and its collaborator's abilities to successfully develop and commercialize drug candidates, competition from other pharmaceutical companies, the ability to effectively market products, and risks relating to the OSI and Eyetech merger including the ability of Eyetech to obtain stockholder approval of the merger, the possibility that the merger will not close or that the closing will be delayed, and the challenges and costs of integrating the operations and personnel of Eyetech, and other events and factors disclosed previously and from time to time in OSI's and Eyetech's filings with the Securities and Exchange Commission, including OSI's Annual Report on Form 10-K for the year ended September 30, 2004 and Eyetech's Quarterly Report on Form 10-Q for the quarter ended June 30, 2005. Except for OSI's and Eyetech's ongoing obligations to disclose material information under the federal securities laws, OSI and Eyetech disclaim any obligation to update any forward-looking statements after the date of this document. ***** This document is not an offer to sell shares of OSI securities which may be issued in the proposed merger. Such OSI common stock is offered only by means of the proxy statement/prospectus referred to herein. |
| OSIP Exiting 2005 A Controversial Deal Clouding Perspective? On August 21st OSIP Announced an Agreement to Acquire Eyetech Pharmaceuticals Wall Street has Viewed this as a Controversial Transaction Contract Commits OSI to the Transaction Pending EYET Shareholder Approval None-the-less OSIP Board & Management has Met Twice to Re- Examine Logic of Transaction and has Re-affirmed Commitment to Proceed S-4 Filed on September 20th, HSR Clearance Received and Closing Anticipated in 2005 Focus of Today's Presentation is on the Dynamic & Exciting Biopharmaceutical Company We Believe that this Transaction Delivers |
| Establishing A Top-tier Biotech Franchise Building Upon Tarceva We Have Evolved a Bold Strategy That Recognized the Limitations and Exposure of a One-Product/One-Business Area Company and Envisioned the Creation of a Top-Tier Biotech That is Both: Scientifically Diversified and Financially Strong With Sustainable Growth Prospects Core Scientific Excellence Applied Across 2-3 Commercially Attractive Business Areas Providing Research Synergies, Risk Mitigation and Broader R&D Choice Strong Revenue Growth Allowing Re-Investment in R&D While Delivering Expected Profitability From Flagship Brand; and Financially Diversified and Scientifically Strong Diversifying Revenue Dependence on One Brand Lowers Risk & Increases Flexibility Scientific Strength and Focus Increases Odds of Success |
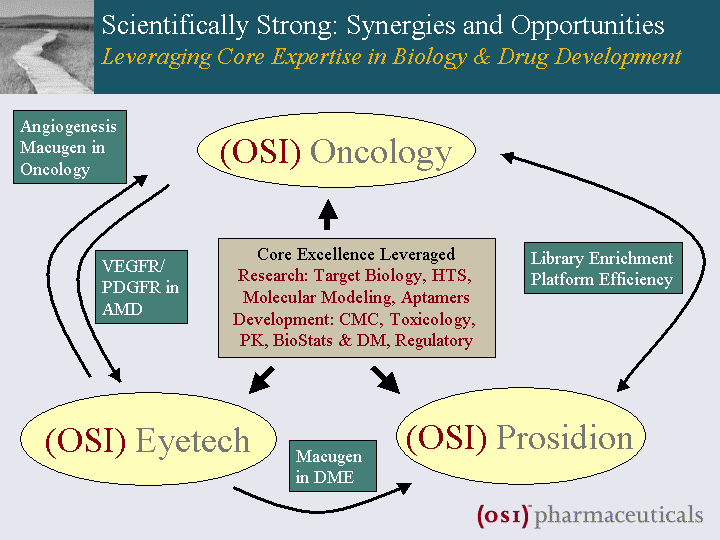
| Scientifically Strong: Synergies and Opportunities Leveraging Core Expertise in Biology & Drug Development Core Excellence Leveraged Research: Target Biology, HTS, Molecular Modeling, Aptamers Development: CMC, Toxicology, PK, BioStats & DM, Regulatory (OSI) Oncology (OSI) Prosidion (OSI) Eyetech Angiogenesis Macugen in Oncology VEGFR/ PDGFR in AMD Macugen in DME Library Enrichment Platform Efficiency |
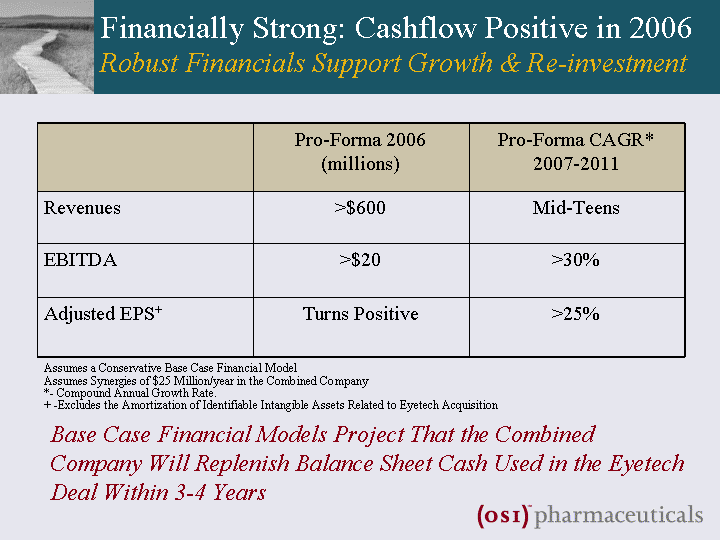
| Financially Strong: Cashflow Positive in 2006 Robust Financials Support Growth & Re-investment Pro-Forma 2006 (millions) Pro-Forma CAGR* 2007-2011 Revenues >$600 Mid-Teens EBITDA >$20 >30% Adjusted EPS+ Turns Positive >25% Assumes a Conservative Base Case Financial Model Assumes Synergies of $25 Million/year in the Combined Company *- Compound Annual Growth Rate. + -Excludes the Amortization of Identifiable Intangible Assets Related to Eyetech Acquisition Base Case Financial Models Project That the Combined Company Will Replenish Balance Sheet Cash Used in the Eyetech Deal Within 3-4 Years |
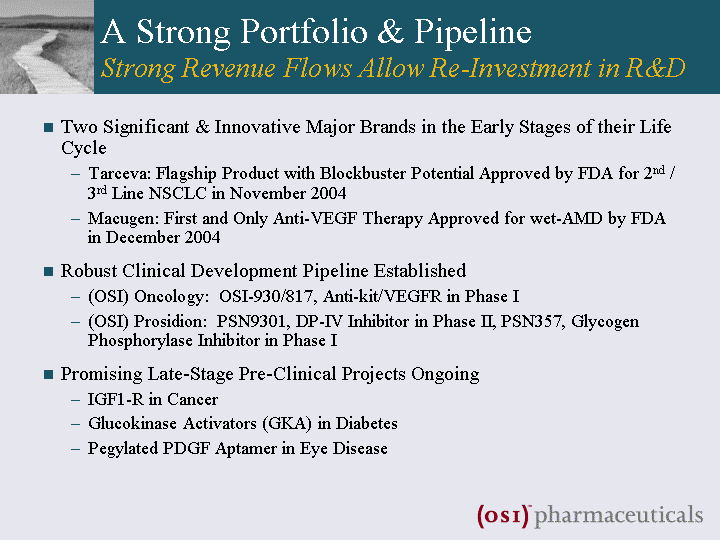
| A Strong Portfolio & Pipeline Strong Revenue Flows Allow Re-Investment in R&D Two Significant & Innovative Major Brands in the Early Stages of their Life Cycle Tarceva: Flagship Product with Blockbuster Potential Approved by FDA for 2nd / 3rd Line NSCLC in November 2004 Macugen: First and Only Anti-VEGF Therapy Approved for wet-AMD by FDA in December 2004 Robust Clinical Development Pipeline Established (OSI) Oncology: OSI-930/817, Anti-kit/VEGFR in Phase I (OSI) Prosidion: PSN9301, DP-IV Inhibitor in Phase II, PSN357, Glycogen Phosphorylase Inhibitor in Phase I Promising Late-Stage Pre-Clinical Projects Ongoing IGF1-R in Cancer Glucokinase Activators (GKA) in Diabetes Pegylated PDGF Aptamer in Eye Disease |
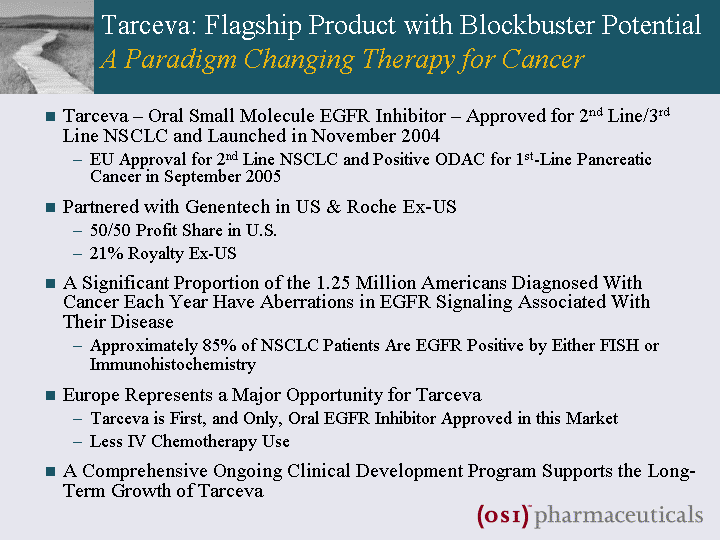
| Tarceva: Flagship Product with Blockbuster Potential A Paradigm Changing Therapy for Cancer Tarceva - Oral Small Molecule EGFR Inhibitor - Approved for 2nd Line/3rd Line NSCLC and Launched in November 2004 EU Approval for 2nd Line NSCLC and Positive ODAC for 1st-Line Pancreatic Cancer in September 2005 Partnered with Genentech in US & Roche Ex-US 50/50 Profit Share in U.S. 21% Royalty Ex-US A Significant Proportion of the 1.25 Million Americans Diagnosed With Cancer Each Year Have Aberrations in EGFR Signaling Associated With Their Disease Approximately 85% of NSCLC Patients Are EGFR Positive by Either FISH or Immunohistochemistry Europe Represents a Major Opportunity for Tarceva Tarceva is First, and Only, Oral EGFR Inhibitor Approved in this Market Less IV Chemotherapy Use A Comprehensive Ongoing Clinical Development Program Supports the Long- Term Growth of Tarceva |
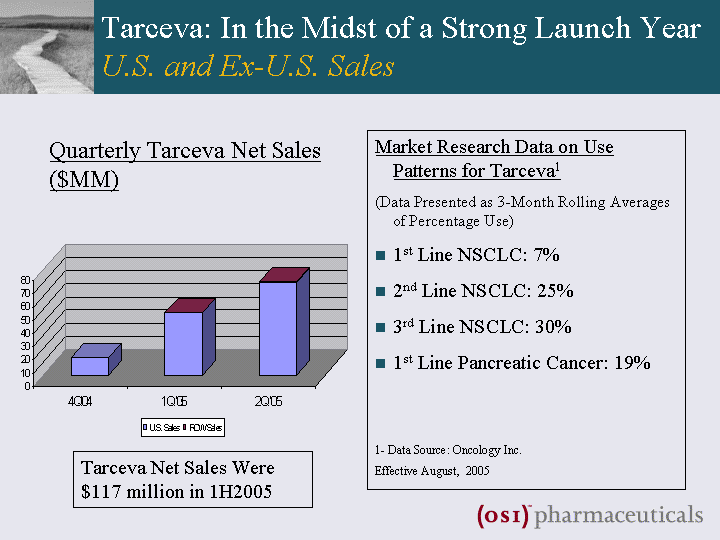
| Tarceva: In the Midst of a Strong Launch Year U.S. and Ex-U.S. Sales Market Research Data on Use Patterns for Tarceva1 (Data Presented as 3-Month Rolling Averages of Percentage Use) 1st Line NSCLC: 7% 2nd Line NSCLC: 25% 3rd Line NSCLC: 30% 1st Line Pancreatic Cancer: 19% 1- Data Source: Oncology Inc. Effective August, 2005 Quarterly Tarceva Net Sales ($MM) Tarceva Net Sales Were $117 million in 1H2005 |
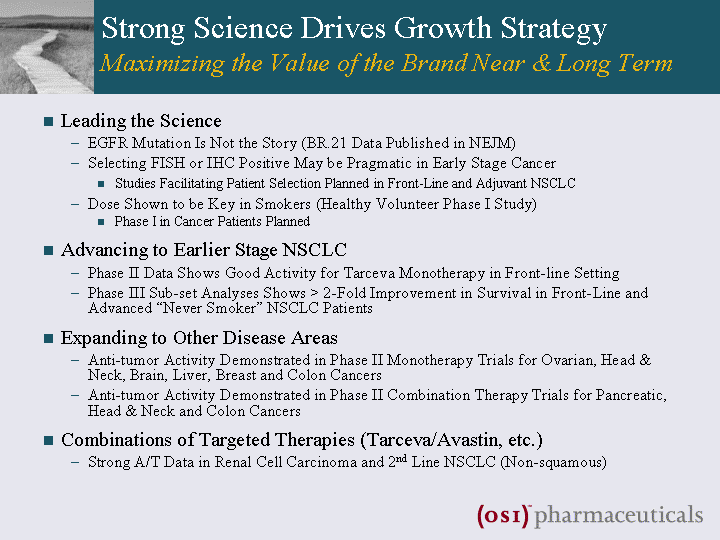
| Strong Science Drives Growth Strategy Maximizing the Value of the Brand Near & Long Term Leading the Science EGFR Mutation Is Not the Story (BR.21 Data Published in NEJM) Selecting FISH or IHC Positive May be Pragmatic in Early Stage Cancer Studies Facilitating Patient Selection Planned in Front-Line and Adjuvant NSCLC Dose Shown to be Key in Smokers (Healthy Volunteer Phase I Study) Phase I in Cancer Patients Planned Advancing to Earlier Stage NSCLC Phase II Data Shows Good Activity for Tarceva Monotherapy in Front-line Setting Phase III Sub-set Analyses Shows > 2-Fold Improvement in Survival in Front-Line and Advanced "Never Smoker" NSCLC Patients Expanding to Other Disease Areas Anti-tumor Activity Demonstrated in Phase II Monotherapy Trials for Ovarian, Head & Neck, Brain, Liver, Breast and Colon Cancers Anti-tumor Activity Demonstrated in Phase II Combination Therapy Trials for Pancreatic, Head & Neck and Colon Cancers Combinations of Targeted Therapies (Tarceva/Avastin, etc.) Strong A/T Data in Renal Cell Carcinoma and 2nd Line NSCLC (Non-squamous) |
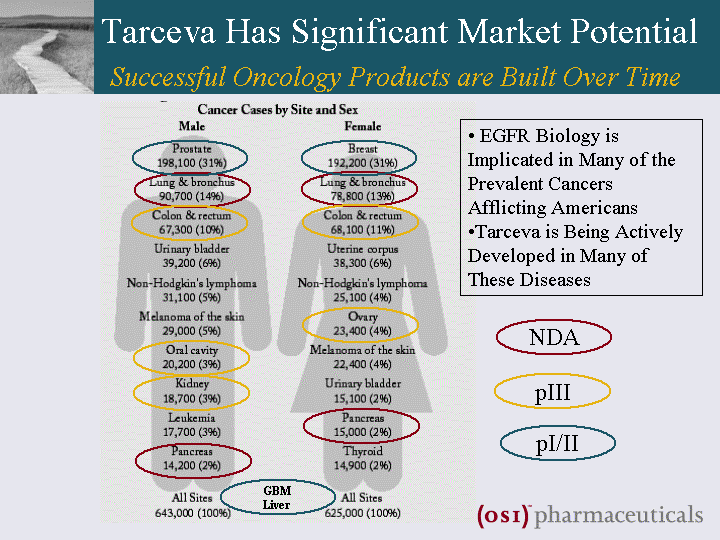
| Tarceva Has Significant Market Potential Successful Oncology Products are Built Over Time NDA pIII pI/II GBM Liver EGFR Biology is Implicated in Many of the Prevalent Cancers Afflicting Americans Tarceva is Being Actively Developed in Many of These Diseases |
| Salvage 1st Line Adv. Adjuvant Prevention Other Pancreatic NSCLC GU Breast GYN Head & Neck Other Uses CRC GI GBM Tarceva Tarceva Life Cycle Plan Other Key Indications 16 15 13 14 18 17 19 20 21 4 2 23 5 11 10 9 12 7 24 22 3 1 6 8 24 30 26 12 4 1 38 7 39 31 29 27 28 9 23 17 33 34 35 42 48 49 25 2 15 36 13 37 8 16 22 11 46 43 41 40 54 57 61 62 3 63 65 66 32 51 50 44 6 52 5 55 53 58 60 59 64 19 20 21 18 56 45 47 10 14 1 3 2 12 20 28 35 8 10 13 7 42 4 60 27 25 23 24 37 19 17 29 30 33 21 40 43 11 31 59 15 32 9 62 38 16 18 14 58 41 36 61 45 44 39 48 50 51 46 47 49 52 53 55 56 57 54 26 68 69 70 71 72 64 66 67 65 73 74 63 34 22 5 6 Randomized Studies P I/II Development Efforts 2005 IST's + Chemo 2005 IST's Single-Agent |
| Growing Tarceva Beyond Launch Achieving Tarceva's Blockbuster Potential Growth Strategy: Establishing Scientific Principles for Tarceva Use Publication Strategy Focused on Mutation Story, Patient Selection, Dose and Tumor Biology Growth Strategy: Progression to Front-line and Adjuvant Settings in NSCLC Phase III SATURN and TITAN Trials Set to Begin Phase III Study in Adjuvant Setting Planned Growth Strategy: Progression to Other Disease Settings Pancreatic Cancer Should Provide First sNDA and Validates Broad Utility Phase III Study in Colorectal Cancer Underway Phase III Studies in Ovarian and Head & Neck Cancer Planned IST and CTEP Programs Help to Pave the Way and Drive Broad Publication Strategy Growth Strategy: Combinations with Other Targeted Therapies Phase II Combination Studies with Avastin in NSCLC and Renal Cell Carcinoma (RCC) All Oral Combinations with Other Targeted Therapies |
| Macugen: A Valuable Second Brand A Breakthrough Therapy for Wet AMD Macugen - a Pegylated Aptamer - Is a Novel, First-in-class Therapeutic That Selectively Binds to Vascular Endothelial Growth Factor Isoform-165, the Pathogenic Isoform Causing Choroidal Neovascularization (CNV) Wet AMD Afflicts Over 200,000 Americans/year and Leads to Blindness Macugen, Approved in December 2004, is the First and Only FDA-approved Treatment for all Types of Wet AMD (Predominantly Classic, Minimally Classic and Occult) EU Approval is Pending (Positive CHMP Opinion Received in September 2005) Macugen is Partnered With Pfizer 50% Gross Profit Share in US (Eyetech Books Sales) & Mid-Teens Royalty Ex-US Only Other Competitor in Market Is Visudyne/PDT, Approved for Predominantly Classic AMD Genentech's Lucentis (a Promiscuous Anti-VEGF Antibody Fragment) Has Emerged as a Strong Future Competitor Based on Initial Phase III Data Genentech's Avastin is Attracting Some Off-label Use We Were Aware of Anecdotal Information Regarding Use When We Executed Merger Agreement |
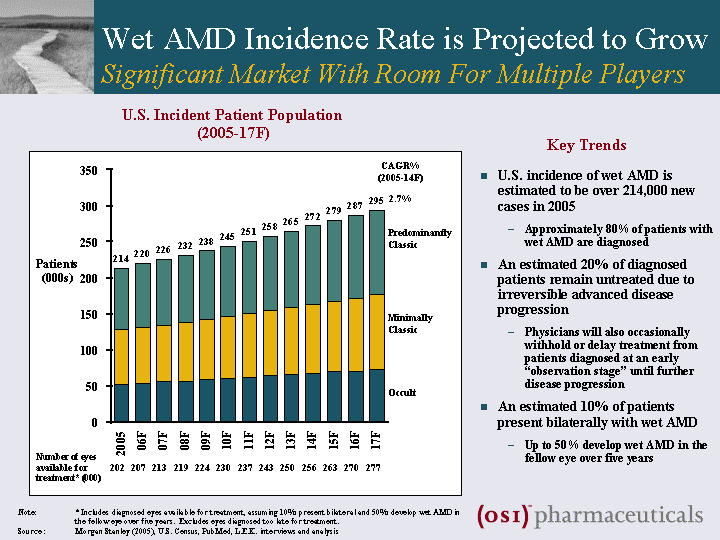
| Wet AMD Incidence Rate is Projected to Grow Significant Market With Room For Multiple Players Patients (000s) U.S. Incident Patient Population (2005-17F) Predominantly Classic Minimally Classic U.S. incidence of wet AMD is estimated to be over 214,000 new cases in 2005 Approximately 80% of patients with wet AMD are diagnosed An estimated 20% of diagnosed patients remain untreated due to irreversible advanced disease progression Physicians will also occasionally withhold or delay treatment from patients diagnosed at an early "observation stage" until further disease progression An estimated 10% of patients present bilaterally with wet AMD Up to 50% develop wet AMD in the fellow eye over five years Note: * Includes diagnosed eyes available for treatment, assuming 10% present bilateral and 50% develop wet AMD in the fellow eye over five years. Excludes eyes diagnosed too late for treatment. Source : Morgan Stanley (2005), U.S. Census, PubMed, L.E.K. interviews and analysis Occult 2.7% CAGR% (2005-14F) Number of eyes available for treatment* (000) 202 207 213 219 224 230 237 243 250 256 263 270 277 Key Trends |
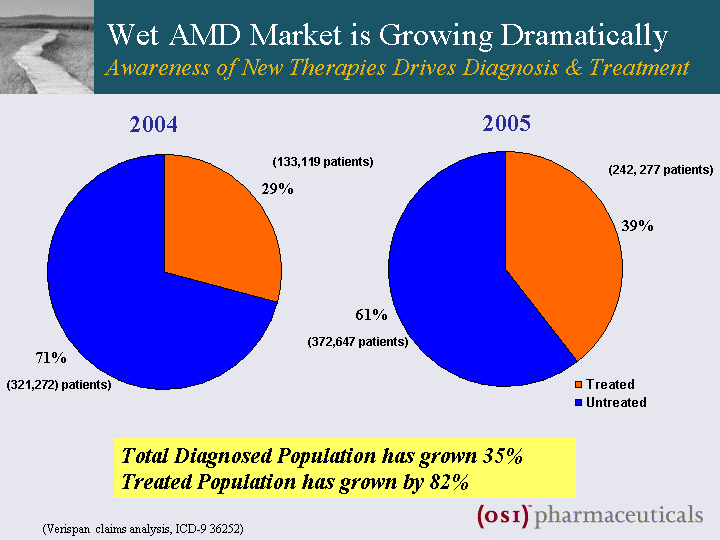
| 2005 2004 (242, 277 patients) (372,647 patients) (133,119 patients) (321,272) patients) Wet AMD Market is Growing Dramatically Awareness of New Therapies Drives Diagnosis & Treatment Total Diagnosed Population has grown 35% Treated Population has grown by 82% (Verispan claims analysis, ICD-9 36252) |
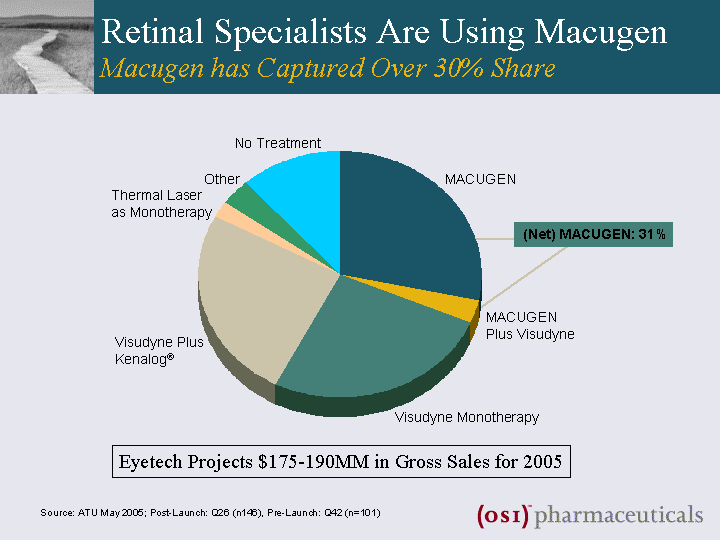
| Retinal Specialists Are Using Macugen Macugen has Captured Over 30% Share (Net) MACUGEN: 31% 28% 26% 25% 3% 2% 5% 3% MACUGEN Visudyne Monotherapy Thermal Laser as Monotherapy No Treatment MACUGEN Plus Visudyne Visudyne Plus Kenalog(r) Other Source: ATU May 2005; Post-Launch: Q26 (n146), Pre-Launch: Q42 (n=101) Eyetech Projects $175-190MM in Gross Sales for 2005 |
| Scientific Diligence Validates Macugen Room for a Safe & Efficacious Anti-VEGF Therapy Macugen Efficacy Is Under-Appreciated Patient Populations in VISION and MARINA Trials Were Very Different Macugen Trials Included Predominantly Classic Patients and Patients With More Chronic Lesions Peer-Reviewed Publication Shows Vision Gain in Occult Patient Sub- Sets (In press "Retina", Oct. '05) 20% of Macugen Patients Had Three-Line Vision Gain vs. 0% Sham Prospective Multi-Center, Randomized, Phase II Study Underway Macugen Should Emerge as Combination Anti-VEGF of Choice Safety Profile an Advantage in Combination Therapy 360 Patient Randomized Phase IIIb/IV Macugen/Visudyne-PDT Trial Underway Based on Encouraging Phase IIA data |
| Scientific Diligence Validates Macugen Room for a Safe & Efficacious Anti-VEGF Therapy Macugen's VEGF-165 Selectivity & Excellent Side-Effect Profile is an Advantage Over Emerging Pan-VEGF Competitors Biology and Scientific Data Suggest Likely Issues With Sustained Use of Pan-VEGF Agents VEGF is an Important Neuroprotectant Published Data Shows that pan-VEGF Blockade Kills Retinal Cells Likely to Lead to Vision Loss Over Time Peripheral Vision Should Also be Monitored in Long-Term Anti-VEGF Therapy VEGF is Important to Normal Blood Vessel Remodeling & Function Cerebral Vascular Events Remain an Unanswered Question For Pan-VEGF Inhibitors Aptamers Have Advantages Over Proteins Immuno-Inflammatory Response Likely for Proteins |
| Expanding Macugen Use Beyond AMD New Opportunities Will Aid Revenues in Face of Competition Major Opportunity Exists for Macugen in Diabetic Macular Edema (DME) and Progression of Diabetic Retinopathy 500,000 Americans Have DME and 5.3 Million Have Diabetic Retinopathy Safety Likely Carries an Enhanced Premium in These Settings Randomized Phase II Data for Macugen in DME is Impressive (In press "Ophthalmology", October 2005) In the 169 Patient Study 18% of Patients Had 3-line Vision Gain and 73% of Patients Had Vision Preservation at 1-Year 900 Patient Randomized Phase III Study Set to Begin Retinal Vein Occlusion (RVO) 130,000 New Patients Per Year in the US 90 Patient Phase II Study Recruitment Complete (Results 2Q'06) Cancer Pre-Clinical Proof-of-Principle Established for Macugen Microspheres in Orthotopic Models of Glioblastoma Multiforme (GBM) |
| A Robust Pipeline Behind Tarceva & Macugen Clinical & Late Pre-Clinical Assets Across the Business (OSI)Oncology OSI-930/OSI-817: c-kit/VEGFR Program Oral PTK Inhibitors With Unique Efficacy/Safety Footprint Between Kinase Selectivity & Promiscuity OSI-930 in Phase I Trials IGF1R Receptor Tyrosine Kinase Inhibitor Program SBD Led Project Has Yielded Selective Oral Inhibitors (OSI)Eyetech E10030, Anti-PDGF Aptamer for AMD Should Enter Clinic in 1H2006 (OSI)Prosidion PSN9301, Oral DP-IV Inhibitor in Phase II Trials PSN357, Oral Glycogen Phosphorylase Inhibitor (GPI) in Phase I PSN010, Oral Glucokinase Activator (GKA) Should Enter Clinic in 1Q2006 |

| PSN9301: The (OSI) Prosidion Flagship A Differentiated DP-IV Inhibitor for Type 2 Diabetes DP-IV Inhibitors: Prominent Emerging Class of Novel Anti-diabetic Drugs OSI Non-Exclusively Licenses Target IP DP-IV Inhibitors Prevent the Breakdown of GLP-1 GLP-1 is an Important Regulator of Blood Glucose DP-IV Inhibitors Are Oral Small-molecule Competitors of GLP-1 Analogues Data Suggests DP-IV Inhibitors to Be Beta-Cell Sparing Highly Competitive Arena Recent Positive Novartis Phase III Trial Validates Class PSN9301 is in Phase II Trials Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Fast-Acting, Oral DP-IV Inhibitor Once daily DP-IV inhibitor exposure Breakfast Lunch Dinner Mealtime Dosing Inter-Prandial Sparing May Offer Competitive Advantages Avoids Potential Toxicities From Chronic Inhibition of Other DP-IV Substrates (e.g. Substance-P, PYY) PSN9301 is Rapidly Absorbed and Readily Cleared Key: DP-IV Inhibitor Exposure |
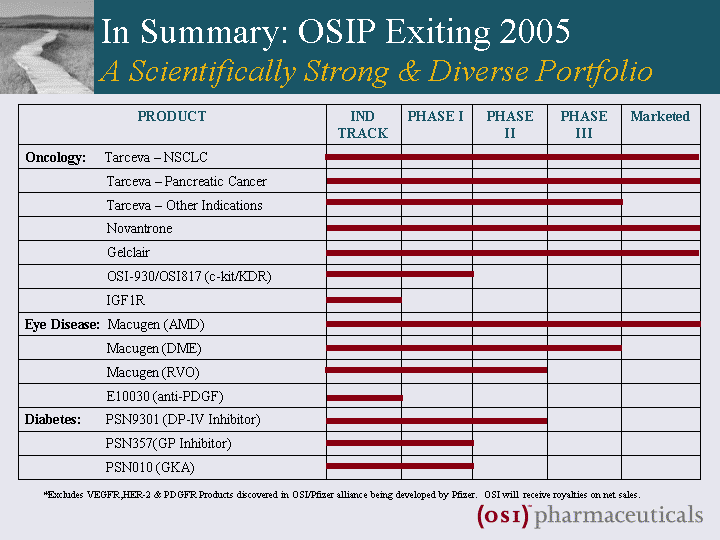
| In Summary: OSIP Exiting 2005 A Scientifically Strong & Diverse Portfolio PRODUCT IND TRACK PHASE I PHASE II PHASE III Marketed Oncology: Tarceva - NSCLC Tarceva - Pancreatic Cancer Tarceva - Other Indications Novantrone Gelclair OSI-930/OSI817 (c-kit/KDR) IGF1R Eye Disease: Macugen (AMD) Macugen (DME) Macugen (RVO) E10030 (anti-PDGF) Diabetes: PSN9301 (DP-IV Inhibitor) PSN357(GP Inhibitor) PSN010 (GKA) *Excludes VEGFR,HER-2 & PDGFR Products discovered in OSI/Pfizer alliance being developed by Pfizer. OSI will receive royalties on net sales. |
| In Summary: OSIP Exiting 2005 A Framework For an Outstanding Future In Closing the Eyetech Deal We'll Have Established the Basis for a Top-Tier Biotech Franchise A Scientifically Diversified and Financially Strong Biopharmaceutical Company Focused in Three Commercially Attractive Areas: Oncology, Eye Disease and Diabetes We Expect to be Profitable in 2006 Off of a Respectable $600MM-plus Revenue Base and Project Solid Year-on-Year Growth Thereafter Our Two Leading Products Will be the Result of Innovative, Quality Science Tarceva, an Outstanding Flagship Brand With Blockbuster Potential Macugen Faces Competition but is a Good Second Source of Revenues We Have Organized Around Focused Operating Teams Supported by Strong Core Expertise Our Science is Enhanced Not Compromised Our Pipeline is Strong Our Core Expertise is Better Leveraged and Higher Quality We Have the Management Expertise to Execute |

| Shaping Medicine, Changing Lives UBS Global Life Sciences Conference NASDAQ / NMS: OSIP September 2005 |

























