UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
o REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended March 31, 2008
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission file number 0-30858
VALCENT PRODUCTS INC.
(Exact name of Registrant as specified in its charter)
Not Applicable
(Translation of Registrant's name into English)
Alberta
(Jurisdiction of incorporation or organization)
Suite 1010 -789 West Pender Street, Vancouver, British Columbia V6C 1H2
(Address of principal executive offices)
George Orr
789 West Pender Street, Suite 1010, Vancouver, B.C. Canada V6C 1H2
Phone: 1-800-877-1626
Fax: 604-606-7980
(Name, Telephone, Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
| Title of each class | | Name of each exchange |
| | | on which registered |
| | | |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
Common Stock, no par value
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
Common Stock, no par value
(Title of Class)
Indicate the number of outstanding shares of each of the issuer's classes of capital or common stock as of the close of the period covered by the annual report.
44,276,321 common shares, without par value, issued and outstanding at March 31, 2008.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
| Large Accelerated filer o | Accelerated filer o | Non Accelerated filer x |
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP | o | International Financial Reporting Standards as issued | o | Other | x |
| | | by the International Accounting Standards Board | | | |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 x Item 18 o
If this is an annual report, indicated by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
TABLE OF CONTENTS
| | | | | |
| | | | | Page Number |
| PART I | | | | 4 |
| | | | | |
| ITEM 1 | | Identity of Directors, Senior Management and Advisors | | 4 |
| | | | | |
| ITEM 2 | | Offer Statistics and Expected Timetable | | 4 |
| | | | | |
| ITEM 3 | | Key Information | | 4 |
| | | | | |
| ITEM 4 | | Information on the Company | | 15 |
| | | | | |
| ITEM 5 | | Operating and Financial Review and Prospects | | 33 |
| | | | | |
| ITEM 6 | | Directors, Senior Management and Employees | | 44 |
| | | | | |
| ITEM 7 | | Major Shareholders and Related Party Transactions | | 49 |
| | | | | |
| ITEM 8 | | Financial Information | | 53 |
| | | | | |
| ITEM 9 | | The Offer and Listing | | 58 |
| | | | | |
| ITEM 10 | | Additional Information | | 59 |
| | | | | |
| ITEM 11 | | Quantitative and Qualitative Disclosures About Market Risk | | 76 |
| | | | | |
| ITEM 12 | | Description of Securities Other than Equity Securities | | 76 |
| | | | | |
| | | | | |
| PART II | | | | 77 |
| | | | | |
| ITEM 13 | | Defaults, Dividends, Arrearages and Delinquencies | | 77 |
| | | | | |
| ITEM 14 | | Material Modifications to the Rights of Security Holders and Use of Proceeds | | 77 |
| | | | | |
| ITEM 15 | | Control and Procedures | | 77 |
| | | | |
| ITEM 16 | | [Reserved] | | |
| | | | | |
| ITEM 16A | | Audit Committee Financial Expert | | 80 |
| | | | | |
| ITEM 16B | | Code of Ethics | | 80 |
| | | | | |
| ITEM 16C | | Principal Accountant Fees and Services | | 80 |
| | | | | |
| ITEM 16D | | Exemptions from the Listing Standards for Audit Committee | | 80 |
| | | | | |
| ITEM 16E | | Purchase of Equity Securities by the Issuer and Affiliated Purchasers | | 80 |
| | | | | |
| | | | | |
| PART III | | | | 81 |
| | | | | |
| ITEM 17 | | Financial Statements | | 81 |
| | | | | |
| ITEM 18 | | Exhibits | | 82 |
CAUTIONARY NOTICE REGARDING FORWARD LOOKING STATEMENTS
Certain written and oral statements made by our Company and subsidiaries of our Company may constitute “forward-looking statements” as defined under the Private Securities Litigation Reform Act of 1995. This includes statements made in this report, in other filings with the Securities and Exchange Commission (“SEC’), in press releases, and in certain other oral and written presentations. Generally, the words “anticipates”, “believes”, “expects”, “plans”, “may”, “will”, “should”, “seeks”, “estimates”, “projects”, “predicts”, “potential”, “continues”, “intends”, and other similar words identify forward-looking statements. All statements that address operating results, events or developments that we expect or anticipate will occur in the future, including statements related to sales, earnings per share results, and statements expressing general expectations about future operating results, are forward-looking statements and are based upon the Company’s current expectations and various assumptions. The Company believes there is a reasonable basis for its expectations and assumptions, but there can be no assurance that the Company will realize its expectations or that the Company’s assumptions will prove correct. Forward-looking statements are subject to risks that could cause them to differ materially from actual results. Accordingly, the Company cautions readers not to place undue reliance on forward-looking statements. We believe that these risks include but are not limited to the risks described in this report under Part I, Item 3, Subsection D entitled “Risk Factors” and Part I, Item 5, entitled “Operating and Financial Review and Prospects” and that are otherwise described from time to time in our SEC reports filed after this report. These statements appear in a number of places in this Form 20-F and include all statements that are not statements of historical fact regarding the intent, belief or current expectations of us, our directors or our officers, with respect to, among other things: (i) our liquidity and capital resources, (ii) our financing opportunities and plans, (iii) our ability to attract customers to generate revenues, (iv) competition in our business segment, (v) market and other trends affecting our future financial condition or results of operations, (vi) our growth strategy and operating strategy, and (vii) the declaration and/or payment of dividends.
Investors and prospective investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and that actual results may differ materially from those projected in the forward-looking statements as a result of various factors. The factors that might cause such differences include, among others, those set forth in this annual report, and we undertake no obligation to update any of the forward-looking statements in this annual report on Form 20-F after the date of this report.
SELECTED FINANCIAL DATA
Corporate Summary
The Company is a life sciences company with scientific expertise in the fields of plant propagation and cultivation, crop production, plant breeding and plant biochemistry, and plant physiology to make significant contributions to the world's economy and quality of life. Valcent’s research and development programs result in new and innovative products, processes, and technologies to be commercialized and licensed internationally. As the global population grows, so does the need for quality foods, enhanced nutrition, consistent feed ingredients for livestock, alternative fuel sources, and environmentally friendly chemical alternatives.
SELECTED FINANCIAL DATA - continued
In 2007, Valcent acquired a six acre property and developed infrastructure and security; in El Paso, Texas. A laboratory facility was constructed and equipped in order to facilitate and expedite programs to effect an algae identification, characterization and cultivation technology; and to commercialize a high density vertical technology for the production of food crops. A team of senior scientists, lab technicians, engineering staff, and project managers, directed by our President, Glen Kertz, has been assembled based on their expertise in the fields of plant propagation and cultivation, plant breeding and plant biochemistry, and plant physiology, crop production with emphasis on the study, identification and characterization of algae. Dedicated project specific teams are currently focused on the development and commercialization of two unique and productive plant growing systems which address the energy and food crises facing the world.
We are, at present, a development stage company focused primarily on:
| (i) | the development of a commercial bio-diesel feed stock technology via a formalized corporate joint venture with Global Green Solutions, Inc. (“Global Green”) through 50% owned Texas subsidiary, Vertigo Algae Technologies LLC. (During the year ended March 31, 2008 and through May 1, 2008, the Company operated this initiative via a non-incorporated joint venture with Global Green); |
| (ii) | the development of our High Density Vertical Growth System designed to produce vegetables and other plant crops (“HDVG System”); |
| (iii) | the development and marketing of the Tomorrow GardenTM consumer retail product in our UK based subsidiary; |
| (iv) | ongoing research and development with tissue culture technologies, plant growth technologies, and other product and technology development initiatives; and |
| (v) | ongoing development planning and research regarding our Nova Skin Care System product line. |
As at September 15, 2008, the 49,856,980 shares were issued and outstanding, the Company had 25,400,002 warrants outstanding. In addition, the Company had substantial convertible debentures outstanding that may be converted into common share equity (see ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS Liquidity and Capital Resources).
All amounts are stated in Canadian dollars unless otherwise noted.
The following selected financial data has been derived from our audited financial statements and related notes for the preceding five years and has been prepared and presented in accordance with Canadian generally accepted accounting principles. Effective March 24, 2004, we disposed of our interest in Nettron Media Group, formerly our wholly-owned subsidiary. The comparative financial results of Nettron Media Group for the year ended March 31, 2004 have been segregated and presented as deficit from prior operations and discontinued operations on the Balance Sheets in our audited financial statements. For comparative purposes this selected financial data for the years ended March 31, 2004, 2005, 2006, 2007, and 2008 is also presented as though it were prepared under United States generally accepted accounting principles. Our audited financial statements for the year ended March 31, 2008 have been prepared in accordance with Canadian generally accepted accounting principles, which, except as noted in Notes 1 and 20, conform in all material respects with those of the United States and with the requirements of the Securities and Exchange Commission.
SELECTED FINANCIAL DATA - continued
Selected Financial Data (Expressed in Canadian Dollars) | | March 31, 2008 12 Months | | | March 31, 2007 12 Months As Restated | | | March 31, 2006 12 Months As Restated | | | March 31, 2005 12 Months | | | March 31, 2004 12 Months | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Net Operating Revenues per Canadian and U.S. GAAP | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) from operations | | $ | 12,028,222 | | | $ | 8,171,090 | | | $ | 3,442,933 | | | $ | 45,694 | | | $ | 25,885 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) from operations U.S. GAAP | | $ | 13,292,888 | | | $ | 9,749,339 | | | $ | 2,844,879 | | | $ | 45,694 | | | $ | 25,885 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) from continued operations per Canadian GAAP | | $ | 12,028,222 | | | $ | 8,171,090 | | | $ | 3,466,888 | | | $ | 45,694 | | | $ | 25,885 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) from discontinued operations per Canadian GAAP | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | (2,238 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net (Income) loss per Canadian GAAP | | $ | 12,712,358 | | | $ | 8,138,393 | | | $ | 3,466,888 | | | $ | 45,694 | | | $ | 23,647 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) from continued operations per U.S. GAAP | | $ | 13,292,888 | | | $ | 9,749,339 | | | $ | 2,844,879 | | | $ | 45,694 | | | $ | 25,885 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) from discontinued operations per U.S. GAAP | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | (2,238 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net (Income) loss per U.S. GAAP | | $ | 13,292,888 | | | $ | 9,749,339 | | | $ | 2,844,879 | | | $ | 45,694 | | | $ | 23,647 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) per share from continued operations - basic, Canadian GAAP | | $ | 0.36 | | | $ | 0.42 | | | $ | 0.354 | | | $ | 0.007 | | | $ | 0.01 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss per share from discontinued operations, Canadian GAAP | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | |
| | | | | | | | | | | | | | | | |
| Loss (Income) per share after discontinued operations, Canadian GAAP | | $ | 0.36 | | | $ | 0.42 | | | $ | 0.354 | | | $ | 0.007 | | | $ | 0.01 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) per share from continued operations, U.S. GAAP | | $ | 0.37 | | | $ | 0.51 | | | $ | 0.27 | | | $ | 0.007 | | | $ | 0.01 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) per share from discontinued operations, U.S. GAAP | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.01 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss (Income) per share after discontinued operations, U.S.GAAP | | $ | 0.37 | | | $ | 0.51 | | | $ | 0.27 | | | $ | 0.007 | | | $ | 0.01 | |
| | | | | | | | | | | | | | | | | | | | | |
| Share capital per Canadian GAAP | | $ | 16,691,282 | | | $ | 8,196,982 | | | $ | 4,099,870 | | | $ | 2,999,420 | | | $ | 2,999,420 | |
| | | | | | | | | | | | | | | | | | | | | |
| Share capital per U.S. GAAP | | $ | 16,866,115 | | | $ | 8,434,032 | | | $ | 4,391,371 | | | $ | 2,999,420 | | | $ | 2,999,420 | |
| | | | | | | | | | | | | | | | | | | | | |
| Common shares issued | | | 44,276,321 | | | | 30,666,068 | | | | 15,787,835 | | | | 6,435,374 | | | | 6,435,374 | |
| | | | | | | | | | | | | | | | | | | | | |
| Weighted average shares outstanding per Canadian GAAP | | | 35,545,740 | | | | 19,261,192 | | | | 10,548,042 | | | | 6,435,374 | | | | 6,435,374 | |
| | | | | | | | | | | | | | | | | | | | | |
| Weighted average shares outstanding per U.S. GAAP | | | 35,545,740 | | | | 19,261,192 | | | | 10,548,042 | | | | 6,435,374 | | | | 6,435,374 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total Assets per Canadian GAAP | | $ | 4,605,914 | | | $ | 4,142,485 | | | $ | 1,392,801 | | | $ | 936 | | | $ | 2,059 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total Assets per U.S. GAAP | | $ | 4,700,482 | | | $ | 4,372,786 | | | $ | 1,392,801 | | | $ | 936 | | | $ | 2,059 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net assets (liabilities) per Canadian GAAP | | $ | (3,068,935 | ) | | $ | (24,376 | ) | | $ | (441,099 | ) | | $ | (237,950 | ) | | $ | (192,256 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net assets (liabilities) per U.S. GAAP | | $ | (3,161,013 | ) | | $ | 665,904 | | | $ | (590,697 | ) | | $ | (237,950 | ) | | $ | (192,256 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Convertible debentures (current and long term portions) per U.S. GAAP) | | $ | 5,389,387 | | | $ | 2,282,483 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | |
| | | | | | | | | | | | | | | | | | | | | |
| Cash Dividends Declared per common shares, Canadian and U.S. GAAP | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | | | $ | 0.00 | |
| | | | | | | | | | | | | | | | | | | | | |
| Exchange Rates (CND to USD) Period Average | | $ | 1.03304 | | | $ | 0.8783 | | | $ | 0.8385 | | | $ | 0.7842 | | | $ | 0.7403 | |
SELECTED FINANCIAL DATA - continued
Exchange Rates (CDN to USD) For the five most recent years ended March 31, calculated on the average of 12 month end closing days | |
| | | |
| March 31, 2008 | | $ | 1.0219 | |
| March 31, 2007 | | $ | 1.1336 | |
| March 31, 2006 | | $ | 1.1905 | |
| March 31, 2005 | | $ | 1.2716 | |
| March 31, 2004 | | $ | 1.3462 | |
for six most recent months | | Period High | | | Period Low | |
| | | | | | | |
| | | | | | | |
| August 2008 | | $ | 0.9771 | | | $ | 0.9365 | |
| July 2008 | | $ | 0.9984 | | | $ | 0.9770 | |
| June 2008 | | $ | 1.0072 | | | $ | 0.9818 | |
| May 2008 | | $ | 1.0157 | | | $ | 0.9814 | |
| April 2008 | | $ | 0.9963 | | | $ | 0.9756 | |
| March 2008 | | $ | 1.0223 | | | $ | 0.9755 | |
| Exchange Rate (CDN to USD) September 15, 2008 | | $ | 0.9437 | | | | | |
CAPITALIZATION AND INDEBTEDNESS
Not Applicable.
REASONS FOR THE OFFER AND USE OF PROCEEDS
Not Applicable.
RISK FACTORS
Our business entails a significant degree of risk, and an investment in our securities should be considered highly speculative. An investment in our securities should only be undertaken by persons who can afford the loss of their entire investment. The following is a general description of material risks, which may adversely affect our business, our financial condition, including liquidity and profitability, and our results of operations, ultimately affecting the value of an investment in shares of our common stock.
GENERAL BUSINESS RISKS
We are a development stage company and based on our historical operating losses and negative cash flows from operating activities there is uncertainty as to our ability to continue as a going concern.
We have a history of operating losses and negative cash flows from operating activities, resulting in our continued dependence on external financing arrangements. In the event that we are unable to achieve or sustain profitability or are otherwise unable to secure additional external financing, we may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern. Any such inability to continue as a going concern may result in our security holders losing their entire investment. Our financial statements, which have been prepared in accordance with Canadian GAAP, contemplate that we will continue as a going concern and do not contain any adjustments that might result if we were unable to continue as a going concern. Changes in our operating plans, our existing and anticipated working capital needs, the acceleration or modification of our expansion plans, lower than anticipated revenues, increased expenses, potential acquisitions or other events will all affect our ability to continue as a going concern.
GENERAL BUSINESS RISKS - continued
From inception, we have historically generated minimal revenues while sustaining substantial operating losses and we anticipate incurring continued operating losses and negative cash flows in the foreseeable future resulting in uncertainty of future profitability and limitation on our operations.
From inception, we have generated minimal revenues and experienced negative cash flows from operating losses. We anticipate continuing to incur such operating losses and negative cash flows in the foreseeable future, and to accumulate increasing deficits as we increase our expenditures for (i) technology, (ii) infrastructure, (iii) research and development, (iv) sales and marketing, (v) interest charges and expenses related to previous equity and debt financings, and (v) general business enhancements. Any increases in our operating expenses will require us to achieve significant revenue before we can attain profitability. In the event that we are unable to achieve profitability or raise sufficient funding to cover our losses, we may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern.
We have historically had working capital shortages, even following significant financing transactions.
We have had working capital shortages in the past and, although we raised significant capital through debt and equity offerings, we have generated significant losses, which have impacted working capital. As of March 31, 2008, our balance sheet reflects a working capital deficit of ($4,153,903). This condition has continued since the date of those financial statements, and we expect that these conditions will continue for the foreseeable future unless we are able to raise a substantial amount of additional financing. In view of the matters described herein, our ability to continue to pursue our plan of operations as described herein is dependent upon our ability to raise the capital necessary to meet our financial requirements on a continuing basis.
Based on our expected negative cash flow from operations and investing activities, we anticipate that we will not be able to positively impact our working capital unless we are able to generate revenues. In connection with a financing completed in July 2008, the holder of the Company's security interest in substantially all of our assets could make it difficult for us to raise additional capital through the issuance of debt securities.
Our accumulated deficit makes it more difficult to borrow funds.
As of the year ended March 31, 2008, and as a result of historical operating losses from prior operations, we had a deficit from prior operations of $3,237,370, and a deficit of $24,317,639 from losses accumulated during our development stage resulting in a total accumulated deficit of $27,555,009. Lenders generally regard an accumulated deficit as a negative factor in assessing creditworthiness, and for this reason, the extent of our accumulated deficit, coupled with our historical operating losses will negatively impact our ability to borrow funds if and when required. Any inability to borrow funds, or a reduction in favorability of terms upon which we are able to borrow funds, including the amount available to us, the applicable interest rate and the collateralization required, may affect our ability to meet our obligations as they come due, and adversely affect on our business, financial condition, and results of operations, raising substantial doubts as to our ability to continue as a going concern.
Our debt arrangements include the provision of a secured interest in our assets and may make it more difficult to borrow funds, or default of debt provisions may impact our assets and ability to operate; certain unsecurted debt instruments are due upon demand and may cause financial liquidity difficulties for the Company.
During July 2008, the Company undertook a US$2.428 million financing convertible debt instrument whereunder part of the terms included a secured interest in the Company’s assets to the lender. Should the Company default on material terms of the debt instrument, the lenders may be able to realize on their secured interest in our assets which would have a negative affect on our ability to operate and conduct our business raising substantial doubts as to our ability to continue as a going concern.
In addition, the Company has convertible and non convertible interest bearing promissory notes that are due upon demand. A demand by the debt holder of such debt securities could cause financial hardship, litigation, and other negative influence on the financial condition of the Company that could cause disruption to our operations.
Our having recently undergone a change in business direction and significant restructuring coupled with our limited experience as a publicly traded company with substantial operations in several different industries, may increase our expenses and place significant demands on our management.
From inception we have undergone several changes in business direction and consequently have previously had only limited operations. As a result of our most recent change in business direction approximately three years ago, significant restructuring, increasing regulation, and our limited experience as a publicly traded company may make it difficult to respond to our regulatory and reporting obligations, and could increase our general, administrative, legal and auditing costs and place substantial time demands on our management. We anticipate that, due to the increased complexity of our corporate structure and increased reporting and governance obligations, and our simultaneous pursuit of various product lines in different industries, our regulatory and reporting obligations will require further expenditures to train additional personnel and retain appropriate legal and accounting professional services. In the event that these expenditures precede or are not subsequently followed by revenues or that we are unable to raise sufficient funding to cover any increase in our expenses, we may not be able to meet our obligations as they come due, and our business, financial condition, and results of operations may be negatively affected, raising substantial doubts as to our ability to continue as a going concern.
GENERAL BUSINESS RISKS - continued
We identified material weaknesses in our disclosure controls and procedures and our internal control over financial reporting.
Section 404 of the Sarbanes-Oxley Act of 2002 requires management to assess our internal control over financial reporting (“ICFR”) pursuant to a defined framework. In making that assessment, management identified a material weakness in our disclosure controls as a result of several material weaknesses identified in our ICFR as described in Item 15 below. There are inherent limitations in the effectiveness of any system of internal control, and accordingly, even effective ICFR can provide only reasonable assurance with respect to financial statement preparation and may not prevent or detect misstatements. Material weaknesses make it more likely that a material misstatement of annual or interim financial statements will not be prevented or detected. In addition, effective ICFR at any point in time may become ineffective in future periods because of changes in conditions or deterioration in the degree of compliance with our established policies and procedures.
RISKS ASSOCIATED WITH OUR BUSINESSES AND INDUSTRIES
We face serious competition in our business segments from new market entrants as well as a number of established companies with greater resources and existing customer bases.
The markets for our potential products rapidly evolve and are intensely competitive as new products are regularly introduced. Competition in our market segments is based primarily upon:
| | • | brand name recognition; |
| | | |
| | • | availability of financial resources; |
| | | |
| | • | the quality of products; |
| | | |
| | • | reviews received for products from independent reviewers who publish in magazines, websites, newspapers and other industry publications; |
| | | |
| | • | availability of access to retail shelf space; |
| | | |
| | • | the price of each product; and |
| | | |
| | • | the number of products then available. |
We face competition from other consumer retail manufacturers and industrial manufacturers, all of whom generally sell through the same combination of channels as we intend to, including retail, wholesale, direct-response and online marketing sales.
To remain competitive in the market for our products and potential products, we rely heavily upon what we believe to be our superior potential product quality, marketing and sales abilities, proprietary technology, product development capabilities and our management’s experience. However, we may not be able to effectively compete in these intensely competitive markets, as some of our competitors have longer operating histories, larger customer bases and greater financial, marketing, service, support, technical and other resources, affording them the ability to undertake more extensive marketing campaigns and adopt more aggressive pricing policies, than we can. Moreover, we believe that competition from new entrants will increase as the market for each of our potential products expands. If our potential product lines are not successful, our business, financial condition and results of operations will be negatively affected.
RISKS ASSOCIATED WITH OUR BUSINESSES AND INDUSTRIES - continued
Our intellectual property may not be adequately protected from unauthorized use by others, which could increase our litigation costs and adversely affect our sales.
Our intellectual properties are the most important assets that we possess in our ability to generate revenues and profits and we will rely very significantly on these intellectual property assets in being able to effectively compete in our markets. However, our intellectual property rights may not provide meaningful protection from unauthorized use by others, which could result in an increase in competing products and a reduction in our own sales. Moreover, if we must pursue litigation in the future to enforce or otherwise protect our intellectual property rights, or to determine the validity and scope of the proprietary rights of others, we may not prevail and will likely have to make substantial expenditures and divert valuable resources in any case. We may not have adequate remedies if our proprietary content is appropriated.
If our potential products infringe upon proprietary rights of others, lawsuits may be brought requiring us to pay large legal expenses and judgments and redesign or discontinue selling one or more of our potential products.
We are not aware of any circumstances under which our potential products infringe upon any valid existing proprietary rights of third parties. Infringement claims, however, could arise at any time, whether or not meritorious, and could result in costly litigation or require us to enter into royalty or licensing agreements. If we are found to have infringed the proprietary rights of others, we could be required to pay damages, redesign our potential products or discontinue their sale. Any of these outcomes, individually or collectively, would negatively affect our business, financial condition and results of operations.
If we are unable to successfully break into new markets, implement our growth strategy or manage our business as it does grow, our future operating results could suffer.
As a development stage company, we face several challenges in entering each of the industrial markets and consumer retail markets for our respective potential products, particularly consumers’ lack of awareness of our company and our potential product lines, competing for market share with established consumer retail product manufacturers and difficulties in competing for, hiring and retaining representative personnel in each of our respective potential markets. In addition, we face several challenges common to any new market entrant, including problems typically associated with unfamiliarity of local market conditions and market demographics. Each new market we enter may also have different competitive conditions, consumer tastes and discretionary spending patterns, which may require us to adjust our growth strategy or modify the way in which we manage our business. To the extent that we are unable to break into or meet the challenges associated with establishing ourselves in a new market, our future operating results could suffer and our financial condition and business may be negatively affected.
Changes in consumer and industrial preferences or discretionary spending may negatively affect our future operating results.
Within the consumer/retail businesses and industries in which we intend to operate, revenues are largely generated by consumer preferences and discretionary spending. Our success as a potential manufacturer and retailer of consumer and industrial products will depend, in part, on the popularity of each of our potential product offerings. Any shift in consumer sentiment away from our potential product or industrial product lines could have a negative affect on our ability to achieve future profitability. Our success also depends on a number of factors affecting levels of consumer discretionary income and spending, including, the following, among other, social and economic conditions:
| | • | general business conditions; |
| | | |
| | • | interest rates; |
| | | |
| | • | inflation; |
| | | |
| | • | consumer debt levels; |
| | | |
| | • | the availability of consumer credit; |
RISKS ASSOCIATED WITH OUR BUSINESSES AND INDUSTRIES - continued
| | • | taxation; |
| | | |
| | • | fuel prices and electrical power rates; |
| | | |
| | • | unemployment trends; |
| | | |
| | • | natural disasters; |
| | | |
| | • | terrorist attacks and acts of war; and |
| | | |
| | • | other matters that influences consumer confidence and spending. |
Consumer and end user purchases of discretionary items, including our potential products and product lines, could decline during periods in which discretionary income is lower or actual or perceived unfavorable economic conditions exist. Should this occur, and if we are unable to introduce new products and product lines that consumers find appealing, our business, financial condition and results of operations will be negatively affected.
We may be subject to adverse publicity or claims by consumers arising out of use of our potential product lines.
We may be subject to complaints from or litigation by consumers, whether or not meritorious, relating to quality, health, efficiency, efficacy, or operational aspects of our potential consumer and industrial product lines including environmental impacts. Such claims could arise at any time and, should they arise, we may not be successful in defending them. Any litigation, regardless of the outcome, would entail significant costs and use of management time, which could impair our ability to generate revenue and profit. For these reasons or, should we be found liable with regard to a claim arising out of any of our potential product lines, our business, financial condition, and results of operations would be negatively affected.
We face substantial competition in attracting and retaining qualified senior management and key personnel and may be unable to develop and grow our business if we cannot attract and retain senior management and key personnel as necessary, or if we were to lose our existing senior management and key personnel.
As a development stage company, our success, to a large extent, depends upon our ability to attract, hire and retain highly qualified and knowledgeable senior management and key personnel who possess the skills and experience necessary to satisfy our business and client service needs. Our ability to attract and retain such senior management and key personnel will depend on numerous factors, including our ability to offer salaries, benefits and professional growth opportunities that are comparable with and competitive to those offered by more established consumer retail product manufacturers and industrial product manufacturers. We may be required to invest significant time and resources in attracting and retaining, as necessary, additional senior management and key personnel, and many of the companies with which we will compete for any such individuals have greater financial and other resources which afford them the ability to undertake more extensive and aggressive hiring campaigns than we can. Furthermore, an important component to the overall compensation offered to our senior management and key personnel will be equity. If our stock prices do not appreciate over time, it may be difficult for us to attract and retain senior management and key personnel. Moreover, should we lose any member of our senior management or key personnel, we may be unable to prevent the unauthorized disclosure or use of our trade secrets, including our technical knowledge, practices, procedures or client lists by such individuals. The normal running of our operations may be interrupted, and our financial condition and results of operations negatively affected as a result of any inability on our part to attract or retain the services of qualified and experienced senior management and key personnel. If any member of our existing senior management or key personnel left and a suitable replacement was not found, or should any of our former senior management or key personnel disclose our trade secrets, the normal running of our operation may be negatively interrupted.
RISKS ASSOCIATED WITH OUR BUSINESSES AND INDUSTRIES - continued
We are highly dependent upon the success of our algae to biodiesel joint venture project, our high density vertical growth system commercialization, and the risk associated with the replication of laboratory results or prototype tests in the larger scale demonstration project and commercialization project is critical to the Company. We believe that the demonstration facility relating to algae to our biodiesel joint venture project will be ready to replicate laboratory results during 2009, and expect the majority of our commercialization of our high density vertical growth system will occur during fall and winter months this year. If the expected results can not be replicated on a commercial scale, the expected revenue from these ventures will not be realized and the investment in these pilot projects is unlikely to be recovered. This would have a very material adverse effect on the Company’s financial results.
There is no certainty that our key projects will be operationally successful or profitable.
We are highly dependent upon the success of our algae biodiesel joint venture project and our high density vertical growth system. Although we are optimistic that there is a large demand for these technologies and that we are poised to capitalize on that demand, if that demand does not materialize to the extent that we project or we are not successful in promoting and distributing our technology, this will have a material adverse effect on the Company’s financial results.
RISKS ASSOCIATED WITH AN INVESTMENT IN OUR COMMON STOCK
Unless an active trading market develops for our securities, you may not be able to sell your shares.
Although, we are a reporting company and our common shares are listed on the OTC Bulletin Board (owned and operated by the Nasdaq Stock Market, Inc.) under the symbol “VCTPF”, currently, there is not an active trading market for our common stock and an active trading market may never develop or, if it does develop, may not be maintained. Failure to develop or maintain an active trading market will have a generally negative affect on the price of our common stock, and you may be unable to sell your common stock or any attempted sale of such common stock may have the affect of lowering the market price and therefore your investment could be a partial or complete loss.
Under certain circumstances, some of our outstanding common stock purchase warrants may be exercised without our receiving any cash.
As at March 31, 2008, we have outstanding warrants to purchase up to approximately 15,752,251 shares of our common stock, most of which are exercisable on a “net cashless” basis, which means they can be exercised, without payment of the stated exercise price, solely in exchange for cancellation of that number of common shares into which the warrants are exercisable. The number of shares for which any such warrant would be cancelled under a net cashless exercise would be the number of shares having a then current market value equal to the aggregate exercise price of the warrant, in whole or in part, based on its stated exercise price. In effect, a net cashless exercise of any such warrants would mean that, even though we would receive no cash, we would have to issue additional shares, thereby diluting, potentially significantly, our reportable earnings per share. Although the circumstances under which the net cashless exercise provision may be elected by the holders of our warrants are limited, any such exercise would have a negative affect, indirectly, on the trading market price of our common stock.
Since our common stock is thinly traded it is more susceptible to extreme rises or declines in price, and you may not be able to sell your shares at or above the price paid.
Since our common stock is thinly traded its trading price is likely to be highly volatile and could be subject to extreme fluctuations in response to various factors, many of which are beyond our control, including:
| | • | the trading volume of our shares; |
| | | |
| | • | the number of securities analysts, market-makers and brokers following our common stock; |
| | | |
| | • | changes in, or failure to achieve, financial estimates by securities analysts; |
RISKS ASSOCIATED WITH AN INVESTMENT IN OUR COMMON STOCK - continued
| | • | new products introduced or announced by us or our competitors; |
| | | |
| | • | announcements of technological innovations by us or our competitors; |
| | | |
| | • | actual or anticipated variations in quarterly operating results; |
| | | |
| | • | conditions or trends in our business industries; |
| | | |
| | • | announcements by us of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| | | |
| | • | additions or departures of key personnel; |
| | | |
| | • | sales of our common stock; and |
| | | |
| | • | general stock market price and volume fluctuations of publicly-traded, and particularly microcap, companies. |
You may have difficulty reselling shares of our common stock, either at or above the price you paid, or even at fair market value. The stock markets often experience significant price and volume changes that are not related to the operating performance of individual companies, and because our common stock is thinly traded it is particularly susceptible to such changes. These broad market changes may cause the market price of our common stock to decline regardless of how well we perform as a company. In addition, securities class action litigation has often been initiated following periods of volatility in the market price of a company’s securities. A securities class action suit against us could result in substantial legal fees, potential liabilities and the diversion of management’s attention and resources from our business. Moreover, and as noted below, our shares are currently traded on the OTC Bulletin Board and, further, are subject to the penny stock regulations. Price fluctuations in such shares are particularly volatile and subject to manipulation by market-makers, short-sellers and option traders.
Our common stock is subject to the “penny stock” regulations, which are likely to make it more difficult to sell.
Our common stock is considered a “penny stock,” which generally is a stock trading under US$5.00 and not registered on a national securities exchange or quoted on the Nasdaq National Market. The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. These rules generally have the result of reducing trading in such stocks, restricting the pool of potential investors for such stocks, and making it more difficult for investors to sell their shares once acquired. Prior to a transaction in a penny stock, a broker-dealer is required to:
| | • | deliver to a prospective investor a standardized risk disclosure document that provides information about penny stocks and the nature and level of risks in the penny stock market; |
| | | |
| | • | provide the prospective investor with current bid and ask quotations for the penny stock; |
| | | |
| | • | explain to the prospective investor the compensation of the broker-dealer and its salesperson in the transaction; |
| | | |
| | • | provide investors monthly account statements showing the market value of each penny stock held in their account; and |
| | | |
| | • | make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. |
RISKS ASSOCIATED WITH AN INVESTMENT IN OUR COMMON STOCK - continued
These requirements may have the effect of reducing the level of trading activity in the secondary market for a stock that is subject to the penny stock rules. Since our common stock is subject to the penny stock rules, investors in our common stock may find it more difficult to sell their shares.
We as a Canadian Company, and must ensure compliance with Canadian as well as American securities laws. As a result, there may be securities regulations in the jurisdiction where our shareholders are resident that must be met, and such regulations may impact the ability of our shareholders to trade our securities.
As a foreign private issuer, we are exempt from certain informational requirements of the Exchange Act to which domestic United States issuers are subject.
As a foreign private issuer we are not required to comply with all of the informational requirements of the Exchange Act. As a result, there may be less information concerning our Company publicly available than if we were a domestic United States issuer. In addition, our officers, directors and principal shareholders are exempt from the reporting and short profit provisions of Section 16 of the Exchange Act and the rules promulgated thereunder. Therefore, our shareholders may not know on a timely basis when our officers, directors and principal shareholders purchase or sell shares of our common stock. See “Additional Information”.
As we are a Canadian company with much of our assets and key personnel located outside of the United States, you may have difficulty in acquiring United States jurisdiction or enforcing a United States judgment against us, our key personnel or our assets.
We are a Canadian company organized under the Business Corporations Act (Alberta). Many of our assets and certain of our key personnel, including our directors and officers, reside outside of the United States. As a result, it may be difficult or impossible for you to effect service of process within the United States upon us or any of our key personnel, or to enforce against us or any of our key personnel judgments obtained in United States’ courts, including judgments relating to United States federal securities laws. In addition, Canadian courts may not permit you to bring an original action in Canada or recognize or enforce judgments of United States’ courts obtained against us predicated upon the civil liability provisions of the federal securities laws of the United States or of any state thereof. Accordingly, you may have more difficulty in protecting your interests in the face of actions taken by our management, members of our board of directors or our controlling shareholders than you would otherwise as shareholder in a United States public company.
We do not intend to pay any common stock dividends in the foreseeable future.
We have never declared or paid a dividend on our common stock and, because we have very limited resources and a substantial accumulated deficit, we do not anticipate declaring or paying any dividends on our common stock in the foreseeable future. Rather, we intend to retain earnings, if any, for the continued operation and expansion of our business. It is unlikely, therefore, that the holders of our common stock will have an opportunity to profit from anything other than potential appreciation in the value of our common shares held by them. If you require dividend income, you should not rely on an investment in our common stock. See “Dividend Policy”.
Future issuances of our common stock may depress our stock price and dilute your interest. We have convertible debt instruments convertible into common shares, warrants and other financial instruments that may be convertible into common stock.
There are existing convertible notes and interest outstanding in excess of $5,202,741 as at March 31, 2008, and in excess of $7,500,000 as at September 15, 2008 that may be converted into an unknown number of shares causing shareholder dilution. At current market prices at the date of this annual report, this may result in at least 15-20 million shares to be issued if fully converted. Some of these notes have provisions that are “toxic” with reference to equity conversion privileges that allow a 30%+ conversion to the then trading market at the date of conversion allowing at any date an unknown amount of shares to be issued in exchange for the conversion of principal, accrued interest, and registration penalties. In addition, other provisions of some of these convertible securities limit or “cap” the exercise price at US$0.50 per share limiting the decrease in conversion equity to be issued in the event that our share price increases in the trading market. As a result, a significant risk relates to the shareholder dilution that may be experienced in consequence with securities issued and those that might be issued in the future.
RISKS ASSOCIATED WITH AN INVESTMENT IN OUR COMMON STOCK - continued
As at March 31, 2008, we have outstanding warrants to purchase up to approximately 15,752,251 shares of our common stock, and as of September 15, 2008, we had 25,785,106 warrants outstanding to purchase common shares for terms ranging from 1 to 5 years.
We may issue additional shares of our common stock in future financings or grant stock options to our employees, officers, directors and consultants under our stock incentive plan. Any such issuances could have the affect of depressing the market price of our common stock and, in any case, would dilute the percentage ownership interests in our Company by our shareholders. In addition, we could issue serial preferred stock having rights, preferences and privileges senior to those of our common stock, including the right to receive dividends and/or preferences upon liquidation, dissolution or winding-up in excess of, or prior to, the rights of the holders of our common stock. This could depress the value of our common stock and could reduce or eliminate amounts that would otherwise have been available to pay dividends on our common stock (which are unlikely in any case) or to make distributions on liquidation.
Additionally, if possible under terms that we believe to be appropriate given our financial condition and other circumstances, we will likely seek to raise additional financing during our year ending March 31, 2009. We may also issue additional shares, options, and warrants where we can to obtain necessary services.
Some of the issuances we have made in the past, and are likely to make in the future, have been issued at prices below market and at prices below our historical market prices. Consequently, our shareholders have suffered dilution in the value of their shares and can expect that we will be issuing additional securities on similar terms. Further dilution can be expected to occur when our outstanding options and warrants are exercised or debentures are converted at prices below the market.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT
By certificate of amendment dated April 15, 2005, we changed our name from Nettron.com, Inc. to Valcent Products Inc. to reflect a newly adopted business plan. On May 3, 2005 we delisted our commons stock from the TSX Venture Exchange, maintaining only its quotation on the OTC Bulletin Board and changed our common stock symbol to “VCTPF”. Effective May 3, 2005, and in order to render our capital structure more amenable to contemplated financing, we effected a consolidation of our common shares on a one-for-three-basis. Unless otherwise noted, all references to the number of common shares are stated on a post-consolidation basis.
Corporate History
We were incorporated in accordance with the provisions of the Business Corporations Act (Alberta) on January 19, 1996, as 681673 Alberta Ltd., and later changed to Ironclad Systems Inc. Beginning in 1996, following the completion of a public offering, our common shares began trading as a junior capital pool company on the Alberta Stock Exchange (later becoming part of the Canadian Venture Exchange, which was thereafter acquired and renamed the TSX Venture Exchange).
On May 8, 1999, while still operating our bicycle rental and eco-tour businesses through Bikestar Rentals Inc., we incorporated Nettron Media Group Inc., a wholly-owned subsidiary under the laws of the State of Texas, as a marketing enterprise focusing on products and services that could be effectively marketed through the internet as well as more traditional business channels. Nettron Media Group Inc.’s primary focus was Cupid’s Web, an interactive online dating and marketing service. We also changed our name from Bikestar Rentals Inc. to AdventurX.com, Inc., and later to Nettron.com, Inc.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
In 2000, and in connection with Cupid’s Web, we signed an agreement in principle to acquire all of the outstanding capital stock of a group of companies operating a worldwide dating service franchise, as well as a collection of dating magazines and websites.
On January 1, 2001, in order to fully focus on our interactive dating and marketing services, we disposed of all of the outstanding capital stock of Arizona Outback Adventures LLC and Bikestar Rentals Inc.
On February 18, 2002, due to general weakness in the equity markets, we terminated the agreement in principle to acquire the dating service franchise and related businesses originally entered into in 2000. On March 24, 2004, we disposed of our interest in Nettron Media Group Inc. and began exploring business opportunities that might allow us to restart commercial operations.
By certificate of amendment dated April 15, 2005, we changed our name from Nettron.com, Inc. to Valcent Products Inc. to reflect a newly adopted business plan. On May 3, 2005 we delisted our common stock from the TSX Venture Exchange, maintaining only its quotation on the OTC Bulletin Board and changed our symbol to “VCTPF”. Effective May 3, 2005, and in order to render our capital structure more amenable to contemplated financing, we effected a consolidation of our common shares on a one-for-three-basis. Unless otherwise noted, all references to the number of common shares are stated on a post-consolidation basis.
On August 5, 2005, we completed a licensing agreement with Pagic LP (a company affiliated with our President and director, Glen Kertz) for the exclusive worldwide marketing rights to certain potential products and a right of first offer on future potential products.
In order to facilitate the business plan, the Company formed a wholly-owned Nevada corporation, Valcent USA, Inc. to conduct operations in the United States in November 2006. In turn, Valcent USA, Inc. incorporated Valcent Management, LLC, and a wholly-owned limited liability corporation under the laws of Nevada, to serve as the general partner in Valcent Manufacturing Ltd., a limited partnership also formed by Valcent USA, Inc., under the laws of Texas, wherein Valcent USA, Inc. serves as its limited partner. Valcent Products EU Limited was incorporated by Valcent Products Inc. in the domicile of England to conduct future anticipated operations in Europe. Valcent Vertigro Algae Technologies, LLC, a Texas limited liability corporation was formed as a 50% owned subsidiary to each of Valcent, USA Inc. and Global Green to develop algae related technologies.
Fundamental Transaction
On August 5, 2005, we completed a licensing agreement with Pagic LP, formerly MK Enterprises LLC, (“Pagic”) for the exclusive worldwide marketing rights to certain potential products and a right of first offer on future potential products.
On October 19, 2005, we incorporated Valcent USA, Inc., as a wholly-owned subsidiary under the laws of the State of Nevada. In turn, Valcent USA, Inc. incorporated Valcent Management, LLC, a wholly-owned limited liability company under the laws of the State of Nevada, to serve as the general partner in Valcent Manufacturing Ltd., a limited partnership also formed by Valcent USA, Inc., under the laws of the State of Texas, wherein Valcent USA, Inc. serves as limited partner, in order to conduct operations in Texas.
During the year ended March 31, 2007, the Company incorporated Valcent Products EU Limited (“Valcent EU”) in England to conduct operations and development initiatives in Europe.
We are, at present, a development stage company focused primarily on:
| (i) | the development of a commercial bio-diesel feed stock technology via a formalized corporate joint venture with Global Green through 50% owned Texas subsidiary, Vertigo Algae Technologies LLC. (During the year ended March 31, 2008 and through May 1, 2008, the Company operated this initiative via a non-incorporated joint venture with Global Green); |
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
| (ii) | the development of our High Density Vertical Growth System designed to produce vegetables and other plant crops (“HDVG System”); |
| (iii) | the development and marketing of the Tomorrow GardenTM consumer retail product in our UK based subsidiary; |
| (iv) | ongoing research and development with tissue culture technologies, plant growth technologies, and other product and technology development initiatives; and |
| (v) | ongoing development planning and research regarding our Nova Skin Care System product line. |
From inception, we have generated minimal revenues and experienced negative cash flows from operating activities and our history of losses has resulted in our continued dependence on external financing. Any inability to achieve or sustain profitability or otherwise secure additional external financing, will negatively impact our financial condition and raises substantial doubts as to our ability to continue as a going concern.
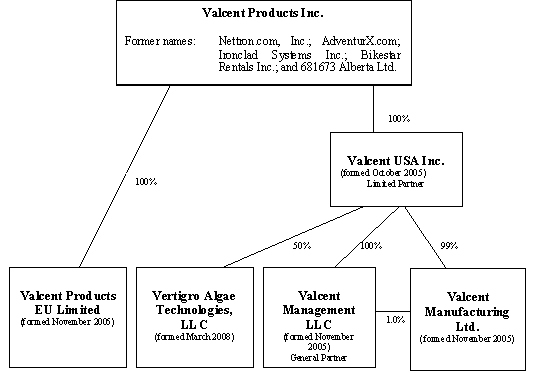
Organizational Structure
The following organizational chart sets forth our current corporate structure and reflects subsidiary interests relating to our various entities.
Valcent Products Inc., Alberta, Canada is the direct and or indirect parent corporation to the following:
Valcent USA Inc., Nevada, USA – 100%
Valcent Management LLC, Nevada, USA – 100%
Valcent Manufacturing Ltd., Texas, USA – 100%
Valcent Products EU Limited, UK – 100%
Vertigro Algae Technologies LLC, Texas, USA – 50%
Valcent Products, Inc. formed a wholly-owned Nevada corporation, Valcent USA, Inc. to conduct operations in the United States in November 2006. In turn, Valcent USA, Inc. formed Valcent Management, LLC, as a wholly-owned limited liability corporation under the laws of Nevada, to serve as the general partner in Valcent Manufacturing Ltd., a limited partnership also formed by Valcent USA, Inc., under the laws of Texas, wherein Valcent USA, Inc. serves as its limited partner. Valcent Products EU Limited was incorporated by Valcent Products Inc. in the domicile of England to conduct future anticipated operations in Europe. Vertigro Algae Technologies, LLC, a Texas Limited Liability Corporation was formed as a 50% owned subsidiary to each of Valcent, USA Inc. and Global Green to develop algae related technologies.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
Current License Agreements
On July 29, 2005, we entered into five related definitive agreements (the “Pagic Agreements”) with Pagic LP (formerly MK Enterprises LLC), an entity controlled by M. Glen Kertz, our current Chief Executive Officer, acting President, and Chairman of the board of directors, including:
| (i) | a master license agreement for a term continuing so long as royalty payments continue to be made as required for the exclusive worldwide marketing and distribution rights to three unrelated and proprietary potential consumer retail products that had previously been developed (the “Pagic Master License”), certain of which are patent pending by Pagic, including the Nova Skin Care System, the Dust Wolf TM, and the Tomorrow Garden TM Kit (collectively, and together with any improvements thereon, the “Initial Products”); |
| (ii) | the Pagic Master License also includes a license for a term continuing so long as royalty payments continue to be made as required for the exclusive worldwide marketing and distribution rights to any ancillary products developed and sold for use by consumers in connection with the Initial Products (the “Initial Ancillaries”); |
| (iii) | a product development agreement pursuant to which we were granted a right for an initial period of five years to acquire a license for a term continuing so long as royalty payments continue to be made as required for the exclusive worldwide marketing and distribution rights to any new products developed by Pagic (any such products, collectively, the “Additional Products”, and, the agreement itself, the “Pagic Product Development Agreement”); |
| (iv) | the Pagic Product Development Agreement also includes a license for a term continuing so long as royalty payments continue to be made as required for the exclusive worldwide marketing and distribution rights to any ancillary products developed and sold for use by consumers in connection with the Additional Products (the “Additional Ancillaries”); and |
| (v) | a related services agreement pursuant to which Pagic shall provide consulting support in connection with the Initial Products, the Initial Ancillaries, the Additional Products and the Additional Ancillaries (the “Pagic Consulting Agreement”), in exchange for the following: |
| | 1) | 20,000,000 shares of our common stock which have been issued to Pagic and its assigns; |
| | | |
| | 2) | a one-time US$125,000 license fee (paid); |
| | | |
| | 3) | reimbursement for US$125,000 in development costs associated with each of the Initial Products since March 17, 2005 (paid); |
| | | |
| | 4) | consulting fees of US$156,000 per year, payable monthly in advance, which the Company has paid to date; and |
| | | |
| | 5) | the greater of the following, payable annually beginning in the second license year (beginning April 1, 2007): |
| | (i) | US$400,000 inclusive of all consulting fees, royalty and other fees; or |
| | | |
| | (ii) | the aggregate of the following: |
| | | |
| | | subject to a minimum amount of US$37,500 per Initial Product during the second year of the Pagic Master License, and US$50,000 each year thereafter, continuing royalties payable quarterly at a rate of: |
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
| | ● | US$10.00 per Nova Skin Care System unit sold; |
| | | |
| | ● | US$2.00 per Dust WolfTM unit sold; |
| | | |
| | ● | 4.5% of annual net sales of the Tomorrow GardenTM Kit; and |
| | | |
| | ● | 3% of annual net sales of Initial Ancillaries. |
| | 6) | a one-time US$50,000 license fee for each Additional Product licensed (except for one pre-identified product); and |
| | 7) | subject to a minimum amount of US$50,000 per year commencing with the second year of each corresponding license, continuing royalties of 4.5% of annual net sales and 3% on annual net sales of any Additional Ancillaries. |
Global Green Joint Venture and License Arrangements
Beginning on October 2, 2006, we granted certain rights of joint participation to Global Green relating to the joint venture of our high density vertical bio-reactor technology named “Vertigro”, an algae based bio-diesel feedstock initiative. Refer to “PLAN OF OPERATIONS, “High Density Vertical Bio-Reactor and Global Green Joint Venture”, and “Technology License Agreement” between Pagic LP, West Peak Ventures of Canada Ltd., and Valcent Products, Inc.
Additional License Fees Incurred in the Year ended March 31, 2008
The Company has accrued US$150,000 as at March 31, 2008 in connection with three further licensing arrangements in the year ended March 31, 2008: the Vertigro Technology License Agreement, the High Density Vertical Growth System for growing vegetables, and a plant growth technology. Formal licensing documentation is in process for the High Density Vertical Growth System.
PLAN OF OPERATIONS
From inception we have generated minimal revenues from our business operations and have traditionally met our ongoing obligations by raising capital through external sources of financing.
At present, we do not believe that our current financial resources are sufficient to meet our working capital needs over the next twelve months and, accordingly, we will need to secure additional external financing to continue our operations. We anticipate raising additional capital though further private equity or debt financings and shareholder loans. If we are unable to secure such additional external financing, we may not be able to meet our obligations as they come due or to fully implement our intended plan of operations, as set forth below, raising substantial doubts as to our ability to continue as a going concern.
Our plan of operations over the course of the next twelve months is to focus primarily on the continued development of our industrial technologies and commercialization initiatives relating to out high density vertical vegetable growing systems (“HDVG System”), the development via joint venture of our high density vertical bioreactor technology named “Vertigro”, an algae based bio-diesel feedstock initiative, and development of our lines of potential consumer retail products.
The Company’s El Paso, Texas based life sciences operations employs scientific expertise in the fields of plant propagation and cultivation, crop production, plant breeding and plant biochemistry, and plant physiology to make significant contributions to the world's economy and quality of life. Valcent’s research and development programs result in new and innovative products, processes, and technologies to be commercialized and licensed internationally. As the global population grows, so does the need for quality foods, enhanced nutrition, consistent feed ingredients for livestock, alternative fuel sources, and environmentally friendly chemical alternatives.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
PLAN OF OPERATIONS - continued
Valcent Products Inc. is Green Technologies Focused:
Valcent is in the final stages of developing a commercial scale model of its high density vertical vegetable growing systems as well as reaching advanced development phases of research and development to produce algae biomass fuels and other algae products. Both systems can be sited on non-arable land, thus not competing with conventional food production. We believe both use very little water or fertilizer. Both are intended by management to be scaled up to meet specific demand.
Based on management’s testing and performance expectations, the Company’s High Density Vegetable-growing technology produces up to 10-20 times the yield of fresh, nutritious vegetables, using only 5% of the water of conventional crop practices. Deployed within an urban environment to save on transportation costs, vegetables are delivered live to the consumer, without the application of herbicides or pesticides.
The development of our Vertical Bioreactor algae production technology, at the El Paso facility, has had what management believes are encouraging early results. Management’s target is to produce vegetable oil that can receive secondary processing to biodiesel or other fuel products in line with the cost of fossil fuels or less. We own 50% of this project, however on September 26, 2008, Valcent entered into an agreement with Global Green to purchase its entire membership interest in Vertigro Algae Technologies LLC, the company responsible for developing the Company’s proprietary technologies to produce algae biomass fuels and other algae products. The price to be paid for the Membership interest is US$5 million in cash and 5 million common shares in the capital of the Company, and is subject to finance.
The Company and Global Green further agreed that Global Green, on a non-exclusive basis, may be engaged to facilitate the commercialization and development of the Company’s technologies with particular emphasis on bio mass power generation including the integration of Global Green’s “Greensteam” applications, the terms and consideration for which are to be determined on a project specific basis..
The Company’s UK subsidiary, Valcent Products EU Limited has directed efforts towards in-depth market research, developing a marketing plan, and equipping a fully functional plant tissue culture laboratory. Valcent Products EU is marketing a range of Tomorrow Garden plant products, culturing a number of more exotic plant species (such as orchids etc.), a range of culinary herbs for marketing later in the year, and is reviewing a number of options for special multi herb packaged kits. Further, Valcent Products EU is researching the development of a range of Chinese medicinal herbs, which can be sold in a “growing kit” form through “alternate medicine” outlets and to pharmaceutical companies involved in research in this field.
Key Personnel
| • | Glen Kertz, our President and CEO is responsible for overseeing all Company development initiatives while heading Vertigro and HDVG System research, design, and development as well as new product research and development initiatives including tissue culture and plant growth technologies. Mr. Glen Kertz also manages intellectual property, trademarks, and patents relating to our various technologies and products; |
| • | Forrest Ely, the Chief Operating Officer (effective January 1, 2007) of Valcent Manufacturing Ltd., under the direction of M. Glen Kertz, is responsible for overseeing all aspects of our commercial design, product development, and fulfillment activities relating to our HDVG System and Vertigro technologies. He will also aid in the design, engineering, and build out of our Company’s research facility located in El Paso; |
| • | Chris Bradford, the Managing Director of Valcent Products EU Limited is responsible for UK business operations and the Company’s "Tomorrow Garden TM" retail plant sales initiative, as well as development of European based HDVG System market development and sales rollout; and |
| • | Jack Potts the Vice President, Sales and Marketing - Consumer Products Division of Valcent Manufacturing Ltd. is responsible for formulating, managing and overseeing all aspects of our consumer products marketing strategies, including our retail sales, infomercial, internet, and other distribution strategies. He is also be responsible for working with our advertising agency contracts in facilitating our entry and sustainability in the direct-response, online and consumer retail marketing segments. |
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
PLAN OF OPERATIONS - continued
El Paso Research and Development Facility
In 2007, Valcent acquired a six acre property and developed infrastructure and security; in El Paso, Texas. A laboratory facility was constructed and equipped in order to facilitate and expedite programs to effect an algae identification, characterization and cultivation technology; and to commercialize a high density vertical technology for the production of food crops. A team of senior scientists, lab technicians and project managers, directed by our President, Glen Kertz, has been assembled based on their expertise in the fields of plant propagation and cultivation, plant breeding and plant biochemistry, and plant physiology, crop production with emphasis on the study, identification and characterization of algae. Dedicated project specific teams are currently focused on the development and commercialization of two unique and productive plant growing systems which address the energy and food crises facing the world.
More specifically, our plan of operations with respect to each of our lines of potential consumer retail products and commercial bio-diesel feed stock initiative is provided as follows:
POTENTIAL COMMERCIAL PRODUCTS
High Density Vertical Bio-Reactor and Global Green Joint Venture
We are in the development stages of creating technology for a high density vertical bioreactor in conjunction with joint venture partner, Global Green, though Vertigro Algae Technologies, LLC, a company owned 50% by each of Valcent and Global Green. The objective of this technology is to produce a renewable feed stock for the further processing of biodiesel from the growth of oil bearing algae, that in the process also sequesters carbon dioxide from the atmosphere (CO2 is generally accepted as a contributor to global warming). Our high density vertical bioreactor is configured in a manner intended to promote the rapid growth of various forms of algae which is later processed to remove volatile oils suitable for the production of bio diesel or other fuels. Owing to the vertical nature of the design, our technology allows the reactors to be stacked on a smaller foot print of land than traditional growing methods require. We believe secondary potential markets for this technology include industrial, commercial, and manufacturing businesses that produce carbon dioxide emissions that wish to gain possible credits for carbon sequestration, as well as possible pharmaceutical, fertilizers, and other use. We anticipate the launch of this technology in 2009, however, this date may be delayed for several reasons, including but not limited to the availability of financing and delays in the successful or economically viable development of the technology.
It is now generally accepted that algae is potentially a viable solution for a perpetually renewable source of biofuel; some algae species are 50% oil by weight. Our El Paso science and engineering team has researched and developed a proprietary vertical bioreactor panel and process designed for the cultivation of algae. The closed loop, re-circulating system increases yield and controls the potential of contamination, dramatically reducing the area required for cultivation unlike conventional crops such as palm, soy, and corn which do not require arable land. Further, the planned Valcent bioreactor technology provides for continuous production and harvest without the requirement to shut down the system. Most conventional cultivation procedures require a protocol of shutting down, draining and then re-starting and inoculating in order to harvest algae for processing.
Of equal importance to the development of the mechanical systems and infrastructure to cultivate algae, we are focused as a primary component of the development program, the investigation of algae- the plant. The science team has developed, and is patenting, specialized analytical equipment installed in the El Paso laboratory specifically designed to identify and characterize algae species in order to maximize efficiency in commercial production. The Company is currently optimizing the system to increase yields and finalize a commercial production prototype.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
POTENTIAL COMMERCIAL PRODUCTS - continued
Potential Markets – High Density Vertical Bio-Reactor
Research is ongoing with respect to the algae based bioreactor technologies, however it is management’s aim to have the technology compete with fossil fuels. Valcent's High Density Vertical Bioreactors algae-to-biofuel technology mass produces algae convertible to vegetable oil which is suitable for refining into a cost-effective, non-polluting biodiesel. We believe the algae derived fuel will be an energy efficient replacement for fossil fuels and can be used in any diesel powered vehicle or machinery. In addition, 90% by weight of the algae is captured carbon dioxide, which is "sequestered" by this process and so contributes significantly to the reduction of greenhouse gases.
Current data projects high yields of algae biomass, which can be harvested and processed into algal oil for biofuel feedstock at a significantly lower cost than comparable oil-producing crops such as palm and soybean. Other potential uses of the algae include ingredients in food, pharmaceutical, and health and beauty products.
The High Density Vertical Bioreactors technology was developed in recognition and response to a huge unsatisfied demand for vegetable oil feedstock by biodiesel refiners and marketers. Biodiesel, in 2000, was the only alternative fuel in the United States to have successfully completed the Environmental Protection Agency required Tier I and Tier II health effects testing under the Clean Air Act. These tests conclusively demonstrated biodiesel's significant reduction of virtually all regulated emissions. A U.S. Department of Energy study has shown that the production and use of Biodiesel, compared to petroleum diesel, resulted in a 78.5% reduction in carbon dioxide emissions.
Algae, like all plants, requires carbon dioxide, water with nutrients and sunlight for growth. The High Density Vertical Bioreactor technology is ideal for locations adjacent to heavy producers of carbon dioxide such as coal fired power plants, refineries or manufacturing facilities, as the absorption of CO2 by the algae significantly reduces greenhouse gases. These reductions represent value in the form of Certified Emission Reduction credits, so-called carbon credits, in jurisdictions that are signatories to the Kyoto Protocol. Although the carbon credit market is still small, it is growing fast, valued in 2005 at $6.6 Billion in the European Union and projected to increase to $77 Billion if the United States accepts a similar national cap-and-trade program.
Valcent's HDVB bioreactor system can be deployed on non-arable land, requires very little water due to its closed circuit process, does not incur significant labor costs and does not employ fossil fuel burning equipment.
Competition - - High Density Vertical Bio-Reactor
The Company has many competitors attempting to create biofuels from algae utilizing a number of innovative technologies. For our algae based products, we depend on technologies being developed by Pagic LP, a company in which our President has an interest, our scientific and engineering teams, and patent protection on those technologies and processes, product development capabilities, and our management’s experience to compete within the algae biofuels market segments. We may not be able to effectively compete in these intensely competitive markets. Moreover, some of our competitors have longer operating histories, greater financial resources, greater research and development support infrastructure, and technical and other resources. Furthermore, we believe that competition from new entrants will increase as the demand for green technologies and continues to increase worldwide. A partial list of competitors in the algae biofuels space follows:
| | · | Greenfuel Technologies Corporation |
| | · | Aquaflow Bionomic Corporation |
| | · | Green Star Products, Inc. |
| | · | Royal Dutch Shell and HR Biopetroleum |
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
POTENTIAL COMMERCIAL PRODUCTS - continued
| | · | International Energy, Inc . |
| | · | Circle Biodiesel & Ethanol Corporation |
High Density Vertical Growth System
We are developing a high density vertical growth system “HDVG System” that is intended to grow a wide variety of crop products. The Company is experimenting with vegetable crops utilizing the growing system within its greenhouse production plant in El Paso, Texas. We believe the HDVG System technology provides a solution to rapidly increasing food costs caused by transportation/fuel costs spiraling upwards with the cost of oil. Developed over several years by Valcent's research and development partner, Pagic LP, the system is designed to grow vegetables and other foods much more efficiently and with greater food value than in agricultural field conditions. Our research to date indicates that the HDVG System demonstrates the following characteristics:
| | ■ | Produces approximately 10-20 times the normal production volume for field crops |
| | | |
| | ■ | Requires 5% of the normal water requirements for field crops |
| | | |
| | ■ | Can be built on non-arable lands and close to major city markets |
| | | |
| | ■ | Can work in a variety of environments: urban, suburban, countryside, desert etc. |
| | | |
| | ■ | Does not use herbicides or pesticides |
| | | |
| | ■ | Expected operating and capital cost savings over field agriculture |
| | | |
| | ■ | Will likely reduce, to a large extent, transportation costs to market resulting in further savings, higher quality, fresher foods on delivery, and less transportation pollution |
| | | |
| | ■ | Will be easily scalable from small to very large food production situations |
The HDVG System grows plants in closely spaced pockets on clear, vertical panels that are moving on an overhead conveyor system. The system is designed to provide maximum sunlight and precisely correct nutrients to each plant. Ultraviolet light and filter systems exclude the need for herbicides and pesticides. Sophisticated control systems gain optimum growth performance through the correct misting of nutrients, the accurate balancing of PH and the delivery of the correct amount of heat, light and water.
Management believes advantages of the HDVG System include:
| | ■ | reduction in global transport and associated carbon emissions |
| | | |
| | ■ | food and fuel safety, security and sovereignty |
| | | |
| | ■ | local food is better for public health |
| | | |
| | ■ | building of local economies |
| | | |
| | ■ | control of externalities and true costs |
In a rapidly urbanizing world where the majority of people now live in cities, localization requires that food and fuel be produced in an urban context. At present, we are not aware of a locally sustained urban community anywhere in the world. Urban sustainability is yet to be realized primarily because urban agriculture presents a number of technological challenges. The main challenge is a lack of growing space.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
POTENTIAL COMMERCIAL PRODUCTS - continued
Vertical growing is a new idea currently emerging which offers great promise for increasing urban production. Vertical growing systems have been proposed as possible solutions for increasing urban food supplies while decreasing the ecological impact of farming. The primary advantage of vertical growing is the high density production it allows using a significantly reduced physical footprint and fewer resources relative to conventional agriculture. Vertical growing systems can be applied in combination with existing hydroponics, and greenhouse technologies which already address many aspects of the sustainable urban production challenge (i.e., soil-free, organic production, closed loop systems that maximize water and nutrient efficiencies, etc.). Vertical growing, hydroponics and greenhouse production have yet to be combined into an integrated commercial production system, but, such a system would have major potential for the realization of environmentally sustainable urban food and fuel production.
The Company is currently developing an HDVG System and specification of the commercial scale plant capable of defining final operating and capital costs to maximize sales return on cost.
Reduced Global Transport and Associated Carbon Emissions: It is widely recognized that the transportation of food over long distances – compared to locally sourced foods – causes excessive energy use, air pollution and a contributor to climate change. Today, food products typically travel between great distances between source and market. The energy used for food transport often far outweighs the human energy gained by the food. The associated need for long-haul refrigeration and packaging further increases the energy costs of food transport. Long-distance and cross-continental food trade is clearly on the rise with the tonnage of food shipped between nations having grown many times over in the last fifty years. Choosing local over global products has powerful potential to reduce energy consumption.
Food and Fuel Safety, Security and Sovereignty: We believe that the further that food is shipped from its growing source, the more vulnerable our food and energy systems become. There are many factors that can interrupt global production and distribution systems including political instability, changes in government, fluctuations in international markets, oil shortages and depletion, war and conflict, acts of terrorism, and natural disasters such as floods, earthquakes, drought, or hurricanes. Localization of food and fuel production has important potential for daily security and emergency preparedness as every community should be able to supply at least a fraction of the food and fuel required by its residents.
Food that is transferred across borders does not necessarily meet the traditional safety standards of consumer countries. Since local food is produced under tractable conditions, in adherence with local food safety standards, greater levels of food safety are ensured.
About 25,000 people die every day of hunger or hunger related causes according to the United Nations. In Africa, for example, it is agriculture itself that is in crisis; caused by years of wars, coups and civil strife, increases in population, and natural problems such as drought. Sub Saharan African soil quality is classified as degraded in about 72% of arable land and 31% of pasture land.
The High Density Vertical Growing System grows leafy lettuce, micro greens (small leafed plants of a wide variety), spinach, herbs, mints, beets, strawberries, wheatgrass, alfalfa and other grains. The HDVG System has the capability of, on average, growing up to 10-20 times the amount of vegetables per acre than conventional field production while using only 5% of the water. Field lettuce loses half its nutritional value within 24 hours and delivery to distant customers can take up to a week. Innovations inherent to the HDVG System will make it possible to deliver vegetables which are still alive to the consumer.
The Company believes that one-eighth acre replicable turnkey modules can be scaled up to meet output and crop diversity requirements. The system can be sited anywhere, in urban, suburban or even desert environments, wherever vegetables are needed. A primary advantage of vertical growing is the high density production it allows using a much reduced physical footprint and fewer resources relative to conventional agriculture. Initial production performance for the HDVG System is based on growing leafy lettuce.
Potential Markets – High Density Vertical Growth System for Vegetables
Vertical growing systems have been proposed as possible solutions for increasing urban food supplies while decreasing the ecological impact of farming, and as a result, we expect the technology to compete with traditional growing techniques Vertical growing, hydroponics and greenhouse production have yet to be combined into an integrated commercial production system, but, such a system would have major potential for the realization of environmentally sustainable urban food and fuel production.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
POTENTIAL COMMERCIAL PRODUCTS - continued
Nova Skin Care System
Developments to Date: The Nova Skin Care System features a proprietary micro-vibration technology that cleanses, exfoliates and moisturizes skin. The Nova Skin Care System uses lotions and creams with anti-oxidants to make skin look healthier and more beautiful. Users see a decrease in the appearance of fine lines and wrinkles and enhanced skin tone, along with firmer skin due to increased collagen. Featured on the nationally syndicated Montel Williams show, the Nova Skin Care System is generating critical acclaim from divergent media across the country including popular lifestyle magazines including Marie Claire who said the system “saves your skin” and “helps to cleanse all those hard-to-reach areas” and Jewel which reports the system makes one’s face “silky smooth.”
During 2007, a 28-minute infomercial created by Hawthorne Direct, a premiere direct response television advertising agency, was developed for consumer direct retail product introduction. The informational campaign, which was awarded a Direct Marketing Association 2007 Millennium Award, was featured in heavy rotation from coast to coast on national and local networks since June 2007 and is supported by a public relations campaign that generated numerous positive reviews.
The system features the NOVAtique, a unique applicator that features a patented drive system that surrounds its motor with powerful, rare-earth magnets. Also called Neodymium magnets, they create thousands of movements per use, in all directions. These movements cannot be duplicated by the human hand. Developed by Glen Kertz, the Nova Skin Care System features a proprietary micro-vibration technology that releases impurities, delivers moisture and nutrients and decreases the appearance of fine lines and wrinkles.
The three skin care products featured with the Nova Skin Care System include Purify, a purifying cleanser specially formulated for deep cleansing without stripping essential oils; Reveal, a deep pore refining scrub with micro-beads; and Quench, a rejuvenating moisturizing cream. Each kit includes the NOVAtique unit, the three skin care products (30 day size) and 20 Sterile Micro Pads.
Our Nova Skin Care System is still in test sales phases of development. During 2007, we finalized an agreement with Solid Integrations, LLC, located in the city of Ciudad Juarez, Chihuahua, Mexico, for the manufacture and assembly of our Nova Skin Care System. All of the raw material components, tooling and fixtures, as well as the packaging and the associated creams and lotions that are included with the Nova Skin Care System were procured. We retained Arizona Natural Resources, Inc., a private label and contract cosmetic manufacturing firm, located in Phoenix, Arizona, to formulate and manufacture the creams and lotions to our specifications which are included with our Nova Skin Care System; all of the finished creams and lotions and other raw materials were shipped to Jamco, our contracted warehouse and distribution points in El Paso, Texas. Solid Integrations, contract their manufacturing to assembly agent, Mack Technologies Inc. in Chihuahua, Mexico, for final assembly and packaging. During the last quarter of the year ended March 31, 2007 and the first quarter of the year ended March 31, 2008, we exported components to Solid Integrations, LLC and subsequently assembled some 16,000+ units of the Nova Skin Care Systems packaged for resale and promotion purposes. During the year ended March 31, 2008, approximately half of this inventory of finished goods were either sold during sales testing, or utilized in product introduction promotions.
We entered into a contract with InPulse Response Group of Scottsdale, Arizona to provide telemarketing services related to the Nova infomercial. We have also engaged Wells Fargo Bank, N.A. to provide merchant processing services for credit card transactions. We have also entered into an agreement with GSI Commerce, formerly Accretive Commerce of Huntersville, North Carolina to provide order entry, data processing, customer service, and product fulfillment services.
Increasing the breadth of Nova product lines are under consideration by management and the incorporation of possible second generation design changes to the current Nova Skin Care System to re-engineer ergonomics and increase performance while decreasing product costs. In conjunction with redesign efforts and product line expansion analyses, marketing and distribution planning is under consideration and analysis by management based on test sales data derived to date to provide a comprehensive strategy for the further development of the Nova brand and product line. Internet sales initiatives are being prioritized for current finished goods inventories. Due to limited management and financial resources in combination with the Company’s focus and commitment to Vertigro and HDVG System initiatives, Nova Skin Care Systems development may decrease during the 2009 fiscal year.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
POTENTIAL COMMERCIAL PRODUCTS - continued
During the year ended March 31, 2008, the Company received approximately $584,144 (2007 - - $nil) in cash from $1,079,432 (2007 - $17,484) in gross test sales relating to the Nova Skin Care System, with the difference between sales and cash included in accounts receivable. As at March 31, 2008, the Company had Nova Skin Care System related accounts receivable of $374,502 (2007 - $16,412) which is net of an allowance for doubtful accounts of $93,653 (2007 - $3,798). Cash received from the sales of the Nova Skin Care System is credited to product development costs owing to the testing nature of this stage of product development.
In anticipation of Nova related redesign initiatives, during the year ended March 31, 2008, raw materials inventories for the Nova Skin Care System was written down by $413,216 (2007 - $nil). This amount has been included in product development expense for the year. As previously stated, with the Company’s focus and commitment to Vertigro and HDVG System initiatives, budget for Nova Skin Care System developments may be limited.
Potential Markets - - The Nova Skin Care System
Based on research conducted by an employee representative of our Company, the skincare market is one of the largest and fastest growing market segments in the world today. In 1998, Johnson & Johnson estimated the annual global skincare market at approximately $48 billion dollars. Between 1996 and 2001, the facial skincare market segment itself saw a 41.1% growth rate extending across all demographic boundaries. In 2002, the United States became the single largest facial skincare market in the world by surpassing Japan. The United States and Japan alone account for well over one third of global skincare sales.
Direct-Response / Online Marketing - The Nova Skin Care System
According to the Direct Marketing Association’s DMA Statistical Fact Book 2005 , the United States electronic direct-response industry, including direct orders, lead generation and traffic generation for television, radio and internet, reached approximately US$323 billion in 2005. Of this, direct-response television accounted for approximately US$182 billion and the internet approximately US$70 billion. According to the Electronic Retailing Association, more than one-quarter of Americans have reportedly purchased products advertised via infomercials.
Initially, we intend to launch direct-response marketing programs tailored to each of our potential consumer retail product lines, focusing on infomercials that use celebrity and/or professional endorsements, home shopping channels, our own website www.valcent.net, and websites we intend to develop specifically for each of our potential product lines, as well as distribution through other internet retailers. More specifically, we have taken, or intend to take, the following steps to facilitate the direct-response and online marketing of each of our potential consumer retail products.
Presently, we have produced and tested a thirty minute infomercial featuring our Nova Skin Care System with Hawthorne Direct, Inc., which premiered in late December, 2006. We have been conducting infomercial testing and sales programs since the infomercial introduction. We are also in the beginning stages of developing contacts with and introducing our Nova Skin Care System to several cable television shopping networks in the United States. We have engaged Stanton Street Technology Group, a web development and design company, to launch a website dedicated exclusively to the promotion and sale our Nova Skin Care System.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
POTENTIAL COMMERCIAL PRODUCTS - continued
Competition - - The Nova Skin Care System
In relation to our Nova Skin Care System, we anticipate that we will face competition from other cosmetic and personal hygiene retail manufacturers, all of whom generally sell through the same combination of channels as we intend to, including retail, wholesale, direct-response and online marketing sales.
There are currently several cosmetic and personal hygiene retail manufacturers that employ various technologies intended to assist in cleansing and exfoliating the skin. The most common products utilize microdermabrasion, a procedure whereby the skin is sandblasted with aluminum oxide crystals through a wand-like devise that suctions itself to the skin’s surface. Other products make use of high-frequency vibrations or electrical impulses to stimulate, massage and exfoliate the skin.
We currently anticipate competing with the following companies and products, among others:
| | · | Neutrogena – Advanced Solutions At-Home Microdermabrasion System; |
| | · | Homedics – Facial Cleansing & Microdermabrasion Set; |
| | · | DermaNew – Microdermabrasion Total Body Experience; |
| | · | H2O Plus – Skin Renewal Microdermabrasion Kit; |
| | · | Visage Naturel – Home Microdermabrasion System; |
| | · | DermaPower – Home Microdermabrasion System; |
| | · | Artemis Woman – The Healing Gems Microdermabrasion System; |
| | · | Sharper Image – Microdermabrasion System for Professional Skin Rejuvenations; |
| | · | Yuen Mai Industrial Co., Ltd. – Ultrasonic Massager; |
| | · | Ultrasonic – Ultrasonic Face & Body Massager; |
| | · | Wedian Technology Co. Ltd. – Ultrasonic Beauty Stimulator; and |
Tomorrow GardenTM
Our Tomorrow GardenTM kit is an indoor herb garden kit, designed to offer, direct to the consumer, an easy to use kit featuring herbs and plants not otherwise readily available in the marketplace. Glen Kertz, our President, has conducted twelve years of research in the development, processes and techniques underlying the technology in the Tomorrow GardenTM and based on his research believes that the Tomorrow GardenTM kit offers an improved plant lifespan of three to six months, as opposed to the traditional shelf life of approximately seven to ten days for fresh herbs, and requires only ambient light, with no watering or other maintenance, to survive. We believe our Tomorrow GardenTM plant kit could be capable of supplying many of the standard herbs traditionally offered in grocery shops today, such as basil, mint, thyme, rosemary, parsley and cilantro, but may, in addition, supply more exotic herbs or pharmaceutical grade plants. Our Tomorrow GardenTM kit is currently in a development and test sales phase operating out of our offices located in England.
The Company’s UK subsidiary, Valcent Products EU Limited was established and registered as a UK company and wholly owned subsidiary of Valcent Products Inc. in 2007. During the fiscal year ended 2008, efforts were directed at in depth market research, developing the marketing plan, fitting out the new building and equipping a fully functional plant tissue culture laboratory. Further staff were recruited.
The official launch of the Tomorrow Garden was premiered at the BBC Gardeners’ World Exhibition at the NEC in Birmingham on June 11 – 14, 2008, with a follow-up at the Royal Horticultural Society’s Hampton Court Flower Show on July 8 -13, 2008.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
POTENTIAL COMMERCIAL PRODUCTS - continued
The launch focused on a range of ferns aimed at the Junior/Educational market sector. As ferns have been around since pre-historic times, and are known to have provided a large proportion of the diet of the herbivore dinosaurs, the emphasis will be on inviting children in the 7yrs – 14yrs age group to grow “dino food”. Growing kits have been designed in the appropriate packaging, reflecting the dinosaur theme. In conjunction with the launch, children growing these ferns will be invited to enter a competition to win a model dinosaur, the first prize being a trip for a family of four to the Natural History Science Museum in London. In addition, special school packs will be prepared in time for the autumn teacher’s conferences, so that schools can purchase the growing kits as an educational tool for culturing the ferns in the classroom.
Valcent EU is culturing a number of more exotic plant species (such as orchids) so that it can both follow-up and compliment the initial product launch. It is also developing a range of culinary herbs for marketing later in the year and are reviewing a number of options for special “Christmas” packs (e.g. the Christmas Rose). Finally, through “in house” expertise, Valcent EU is researching the development of a range of Chinese medicinal herbs, which can be sold in “growing kit” form through “alternate medicine” outlets or (ultimately) to pharmaceutical companies involved in this field of research.
Valcent EU is now fully operational with regards to the Tomorrow Garden product line and anticipates that it will commence generating test sales revenues generated from both from e-commerce through the website (www.tomorrowgarden.eu) and as a result of product launches at the BBC Gardeners’ World Exhibition and the RHS Hampton Court Flower Show.
Potential Markets - - The Tomorrow Garden™ Kit
Culinary and exotic herbs in fresh, dried, frozen, powdered and canned forms are primarily used in the retail and food services markets, where local products and imports have traditionally met demand. We believe that our Tomorrow Garden™ Kit is ideally suited to become a leader in the retail and food services markets because of its ability to offer fresh herbs in market segments where they may not otherwise be available without considerable expense.
Initially to be marketed to the UK Garden Products market valued in 2003 at approximately GB£5.8 billion, data shows that the market doubled in value in the period of 10 years to 2002. Forward projections made in 2003 indicated an annual increase of 5%, however these figures are notoriously difficult to predict when markets are largely controlled by the weather, other pressures on disposable income and slow downs in house sales. When this was coupled with aggressive price competition, it saw the market value drop to 5.1 Billion GB£ or US$9.4 billion in 2004-5. In the period of the last 10+ years, the overall improvement of UK summer weather, and the better than average temperatures in spring and summer, greatly helped to boost sales for outdoor living, including furniture, patio plants, and the like.
Direct-Response / Online Marketing - The Tomorrow Garden™ Kit
Our Tomorrow Garden™ is presently in sales testing development phase and once a final fully-functioning production-level unit is near completion we will tailor a more comprehensive sales and marketing plan for our Tomorrow Garden™. Initially, however, we plan to launch a website dedicated exclusively to the Tomorrow Garden™ through which we expect to conduct online sales.
Competition - - The Tomorrow Garden™ Kit
In relation to our Tomorrow Garden™ Kit, we anticipate that we will face competition from other manufacturers and providers of dried, frozen, powdered and canned herbs, as well as competition from local growers and distributors of fresh herbs, all of whom generally sell through the same combination of channels as we intend to, including wholesale and retail markets, and direct and online sales.
There are several other manufacturers and providers of culinary and exotic herbs, the most common products being prepackaged, dried, frozen or canned herbs or do-it-yourself kits. We currently anticipate competing with the following companies and products, among other local growers and distributors:
| | • | HerbKits.com – Indoor Herb Gardening Kits; and |
| | | |
| | • | New England Herb Company. |
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
MARKETING AND ADVERTISING
We will endeavor to create strong brands for each of our potential product lines relating to the Nova Skin Care System and the Tomorrow GardenTM initiatives and generally market our potential products through:
| | • | print advertising; |
| | | |
| | • | infomercials and home shopping television; |
| | | |
| | • | celebrity endorsements; |
| | | |
| | • | internet websites; |
| | | |
| | • | product sampling campaigns; |
| | | |
| | • | in-store promotions, displays and retailer assisted co-operative advertising; |
| | | |
| | • | publicity activities; and |
| | | |
| | • | trade shows. |
Presently, we have engaged Sanders\Wingo, a marketing, brand strategy and advertising firm with an ex-director of the Company in common, to develop sales books and marketing materials for use in presentations to possible distributors and retailers for Nova Skin Care System related potential consumer retail products, and Packaging Corporation of America, a manufacturer of containerboard and corrugated packaging products, to develop packaging materials for use in shipping our potential products to consumers. We have also secured toll-free phone numbers for use in sales and customer service, have obtained a U.P.C. company prefix from GS-1, allowing us to assign and print U.P.C. numbers for each of our potential products, and have established a merchant account with our financial institution so that we may accept and process customer credit card transactions.
Going forward, we intend to adhere to comprehensive sales and marketing plans tailored to each of our potential product lines and to use a number of venues to create product awareness and knowledge.
MANUFACTURING, FULFILLMENT AND SUPPLIERS
We are presently evaluating resources necessary to continue the development of our Nova Skin Care System and production and test marketing of initial products of our Tomorrow Garden™. Manufacture to date for Tomorrow Garden™ has been carried out in Valcent EU premises in London UK, while future support is set to come from additional facilities located in the Company’s El Paso, premises as operated by Valcent Manufacturing Ltd. Our Nova Skin Care System has been manufactured, assembled and packaged by Solid Integrations, LLC, located in the city of Ciudad Juarez, Chihuahua, Mexico, from where it will be shipped to our contract product development, warehouse and distribution center in El Paso, Texas, which, in turn, will provide product to our contract fulfillment enterprise, GSI Commerce.
We entered into a manufacturing agreement with Solid Integrations, LLC, for the manufacture and assembly of our Nova Skin Care System. Under this agreement we will provide and make available to Solid Integrations, LLC, and its subcontractor(s), the raw materials, machinery, equipment, tooling and molds necessary for Solid Integrations, LLC, and its subcontractor(s), to manufacture and assemble the Nova Skin Care System at a manufacturing facility located in Mexico.
As of the date of this annual report, we have no long-term written agreements and no intentions of entering into any such agreements with any suppliers or manufacturers, and we are not substantially dependent, nor do we anticipate becoming substantially dependent, upon any one or more suppliers, including Solid Integrations, as we believe that there are many such suppliers available with the capabilities that we will require.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
REGULATIONS
Except as described below, we are not currently subject to direct regulation by any foreign or domestic government agency, other than regulations applicable to businesses generally.
Food and Drug Administration
Because the Nova Skin Care System is not intended to treat or cure any ailment we do not believe that it is a medical device as defined by section 201(h) of the United States Federal Food, Drug and Cosmetic Act. While we do not believe that our Nova Skin Care System is a medical device as defined by section 201(h) of the United States Federal Food, Drug and Cosmetic Act, it may nevertheless be classified as such and subject to regulation by the Food and Drug Administration or other federal, state and local authorities. These regulations relate to the manufacture, labeling, sale, promotion, distribution, import, export and shipping of products that are deemed medical devices. In the United States, before a new medical device may be marketed, the manufacturer must first receive, unless there exists an applicable exemption, either clearance under section 510(k) of the Federal Food, Drug and Cosmetic Act or pre-market approval from the Food and Drug Administration. The Food and Drug Administration’s 510(k) clearance process usually takes anywhere from three to twelve months, or longer, while the process of obtaining pre-market approval is much more costly and uncertain and generally takes from one to three years, or longer. To obtain pre-market approval and, in some cases, a 510(k) clearance, a manufacturer must conduct controlled clinical trials designed to test the safety and effectiveness of the medical device. Conducting clinical trials generally entails a long, expensive and uncertain process that is subject to delays and failure at any stage. The data obtained from clinical trials may be inadequate to support approval or clearance of a submission. In addition, the occurrence of unexpected findings in connection with clinical trials may prevent or delay obtaining Food and Drug Administration approval or clearance. If we conduct clinical trials of the Nova Skin Care System, they may be delayed or halted, or be inadequate to support approval or clearance, for numerous reasons, including, among others:
| | • | the Food and Drug Administration, other regulatory authorities or an institutional review board placing a clinical trial on hold; |
| | | |
| | • | insufficient patient enrollment, higher than anticipated attrition, non-compliance with trial protocols or patient follow-up not occurring at expected rates; |
| | | |
| | • | institutional review boards and third party clinical investigators delaying or rejecting our trial protocol; |
| | | |
| | • | third party clinical investigators declining to participate in a trial or not performing a trial on our anticipated schedule or consistent with the clinical trial protocol, good clinical practices or other Food and Drug Administration requirements; |
| | | |
| | • | third party organizations not performing data collection and analysis in a timely or accurate manner; |
| | | |
| | • | regulatory inspections of our clinical trials or manufacturing facilities, among other things, requiring us to undertake corrective actions or suspend, terminate or invalidate our clinical trials; |
| | | |
| | • | changes in governmental regulations or administrative actions; and |
| | | |
| | • | the interim or final results of our clinical trials being inconclusive or unfavorable as to safety or effectiveness. |
Medical devices may be marketed only for the indications for which they are approved or cleared. The Food and Drug Administration may refuse requests for 510(k) clearance or pre-market approval or, if granted, clearances may be revoked if safety or effectiveness problems arise. As we do not believe that our Nova Skin Care System is a medical device, we are currently conducting test sales, however it may nevertheless be classified as such at a later date.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
COMPETITION
Competition in each of the industries in which we intend to sell our potential products is based primarily upon:
| | • | brand name recognition; |
| | | |
| | • | availability of financial resources; |
| | | |
| | • | the quality of products; |
| | | |
| | • | reviews received for products from independent reviewers who publish in magazines, websites, newspapers and other industry publications; |
| | | |
| | • | availability of access to retail shelf space; |
| | | |
| | • | the price of each product; and |
| | | |
| | • | the number of products then available. |
We will rely, for all of our potential product lines, on what we believe to be our superior product quality, marketing and sales abilities, proprietary technology, product development capabilities and our management’s experience to compete within each of our market segments. However, we may not be able to effectively compete in these intensely competitive markets. Moreover, some of our competitors have longer operating histories, larger customer bases and greater financial, marketing, service, support, technical and other resources, affording them the ability to undertake more extensive marketing campaigns and adopt more aggressive pricing policies, than we can. Furthermore, we believe that competition from new entrants will increase as the markets for each of our potential products expand.
INTELLECTUAL PROPERTY
Overview
We rely for our business on a combination of pending patents, trademarks and trade secrets in order to protect our intellectual property. Our pending patents, trademarks and trade secrets are among the most important assets we possess in our ability to generate revenue and profits and we will depend significantly on these intellectual property assets in being able to effectively compete in our markets.
We cannot be certain that the precautions we have taken to safeguard our pending patents, trademarks and trade secrets will provide meaningful protection from unauthorized use. If we must pursue litigation in the future to enforce or otherwise protect our intellectual property rights, or to determine the validity and scope of the proprietary rights of others, we may not prevail and will likely have to make substantial expenditures and divert valuable resources in the process. Moreover, we may not have adequate remedies if our intellectual property is appropriated or our trade secrets are disclosed.
Patents Pending
On July 29, 2005 we entered into a master license agreement with MK Enterprises LLC (subsequently named “Pagic LP” and hereinafter so named) for certain patent pending potential products and the expertise which will form the basis of the patent pending potential products, from which we intend to derive our revenue. In accordance with the terms of the agreement, and in exchange for the exclusive worldwide license to manufacture, market and sell the covered products, we agreed to (i) issue 20,000,000 shares of our common stock to Pagic LP, (ii) pay a one-time US$125,000 license fee to Pagic LP, (iii) reimburse Pagic LP for US$125,000 in product development costs incurred since March 17, 2005, and (iv) make certain ongoing royalty and consulting fee payments to Pagic LP.
While the pending patent applications covering the technology and expertise licensed under the Company’s master license with Pagic LP, patent protection may not be granted at all, and in the event that patents are issued, the title, rights and interests associated with any such patents shall remain the exclusive property of Pagic’s consultant and our Chief Executive Officer, President, Chairman and a member of our board of directors, M. Glen Kertz.
ITEM 4. INFORMATION ON THE COMPANY: CORPORATE HISTORY AND DEVELOPMENT - continued
INTELLECTUAL PROPERTY - continued
As of the date of this annual report, we have received case and/or application numbers for each of the intellectual property assets for which we are seeking patents. Patent status for each division is provided as follows:
| Division | Patents Granted | Patents Pending | Patents Published |
| High Density Vertical Bio-Reactor | | 5 | 4 |
| High Density Vertical Growth System | 2 | | |
| Nova Skin Care System | 2 | | 4 |
All of our other remaining patent applications are in the preliminary stages of the application process.
Trademarks
We have applied for registration of our trademarks in the United States in order to establish and protect our brand names as part of our intellectual property assets. We have applied for registration of Dust Wolf™, Tomorrow Garden™, “Valcent”, “Nova”, the Nova logo, as well as various designs associated with our prospective Nova Skin Care product line. As of the date of this annual report, we have received case and/or application numbers for each of the intellectual property assets for which we are seeking registration. With respect to our Dust Wolf™ application, the mark has been published and we are presently awaiting receipt of a notice of allowance, all of our other remaining registrations are in the preliminary stages of the application process and remain pending.
Trade Secrets
Whenever we deem it important for purposes of maintaining competitive advantages, we require parties with whom we share, or who otherwise are likely to become privy to, our trade secrets or other confidential information to execute and deliver to us confidentiality and/or non-disclosure agreements. Among others, this includes employees, consultants and other advisors, each of whom we require to execute such an agreement upon commencement of their employment, consulting or advisory relationships. These agreements generally provide that all confidential information developed or made known to the individual by us during the course of the individual’s relationship with us, be kept confidential and not to be disclosed to third parties except under specific circumstances.
As of the date of this annual report, we have executed a confidentiality and non-disclosure agreement with Pagic LP and are in the process of drafting confidentiality and/or non-disclosure agreements for our other key employees, consultants and advisors.
SEASONALITY
We may experience slight seasonal fluctuations in the sale of our potential consumer retail products if consumers elect to defer discretionary spending. However, we believe that the overall effects of any seasonal variations in sales activity will be insignificant.
PROPERTY, PLANT AND EQUIPMENT
Our principal executive offices are located at Suite 1010 - 789 West Pender Street, Vancouver, British Columbia V6C 1H2 (Canada). Our telephone number is (604) 606-7979.
On November 16, 2007, our wholly owned subsidiary, Valcent Products EU Limited leased office and development space in Launceston, Cornwall, UK, under a ten-year lease beginning November 15, 2007 and ending on November 15, 2017 at a quarterly cost of GB£12,550 (CDN$26,017). There were 9 years and 7.5 months remaining on the lease as at March 31, 2008. The Company has net property comprised of $340,482 comprised leasehold improvements, computer equipment, furniture and fixtures, vehicles, equipment, and other assets located at this location.
The Company acquired approximately six acres of land in El Paso, Texas for $275,240. The Company has net property comprised of $519,386 comprised leasehold improvements, computer equipment, furniture and fixtures, building, equipment, and other assets at this location.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
The following discussion and analysis should be read together with our audited financial statements for the year ended March 31, 2008 and the notes to the financial statements. Our audited financial statements have been prepared in accordance with Canadian generally accepted accounting principles which, except as noted in Notes 3 and 20 to our March 31, 2008 audited financial statements, conform in all material respects with those of United States generally accepted accounting principles and with the requirements of the U.S. Securities and Exchange Commission.
OPERATING LEGACY AND ACCUMULATED LOSSES
Valcent Products Inc. [formerly Nettron.Com, Inc.] | |
| Selected Financial Data [Annual] | |
| (Expressed in Canadian Dollars) | |
| | | 12 months ended | |
| | | 2008 | | | 2007 | | | 2006 | | | 2005 | |
| Net Operating Revenues | | $ | 0 | | | | 0 | | | | 0 | | | | 0 | |
| Loss from operations | | $ | 12,028,222 | | | | 8,171,090 | | | | 3,466,888 | | | | 45,694 | |
| Loss from prior operations | | $ | 0 | | | | 0 | | | | 0 | | | | 45,694 | |
| Loss from development stage | | $ | 12,712,358 | | | | 8,138,393 | | | | 3,442,933 | | | | 0 | |
| Net loss per Canadian GAAP | | $ | 12,712,358 | | | | 8,138,393 | | | | 3,466,888 | | | | 45,694 | |
| Loss per share | | $ | 0.36 | | | | 0.42 | | | | 0.35 | | | | 0.01 | |
| | | | | | | | | | | | | | | | | |
| Share capital | | $ | 16,691,282 | | | | 8,196,982 | | | | 4,099,870 | | | | 2,999,420 | |
| Common shares issued | | | 44,276,321 | | | | 30,666,068 | | | | 15,787,835 | | | | 6,435,374 | |
| Weighted average shares outstanding | | | 35,545,740 | | | | 19,261,192 | | | | 10,548,042 | | | | 6,435,374 | |
| Total Assets | | $ | 4,605,914 | | | | 4,142,485 | | | | 1,392,801 | | | | 936 | |
| Total Liabilities | | $ | (7,674,846 | ) | | | (4,166,861 | ) | | | (1,833,900 | ) | | | (238,886 | ) |
| | | | | | | | | | | | | | | | | |
| Cash Dividends Declared per Common Shares | | $ | 0 | | | | 0 | | | | 0 | | | | 0 | |
| | | | | | | | | | | | | | | | | |
| Exchange Rates (CDN $ to US $) yearly average | | $ | 1.03304 | | | | 0.8783 | | | | 0.8385 | | | | 0.7842 | |
| | | | | | | | | | | | | | | | | |
| Exchange Rates (CDN $ to British Pound £) yearly average | | $ | 2.07314 | | | | n/a | | | | n/a | | | | n/a | |
YEAR ENDED MARCH 31, 2008 COMPARED WITH YEAR ENDED MARCH 31, 2007
Operating Results
For the year ended March 31, 2008, the Company focused on (i) the development of a commercial bio-diesel feed stock technology via a joint venture with Global Green (the “Vertigro Project”), (ii) the development of our High Density Vertical Growth System (“HDVG System”) designed to more efficiently produce vegetables and other plant crops, (iii) the development and test marketing of the Tomorrow GardenTM consumer retail product in our UK based subsidiary, (iv) the development of direct test sales initiatives and product introduction promotion relating to our Nova Skin Care System, (v) ongoing research and development with tissue culture technologies, plant growth technologies, and other product and technology development initiatives, and (vi) a series of private offering transactions with institutional and other investors, pursuant to which we raised US$5,129,636 through private placements of equity comprised of common shares and warrants, US$367,671 from warrant and share option exercises, and US$1,291,000 from convertible debentures, promissory notes, and short term advances.
For the year ended March 31, 2007, we focused (i) the development of our commercial bio-diesel feed stock technology via a joint venture with Global Green, (ii) the development of product inventories and direct sales initiatives relating to our Nova Skin Care System, and (iii) the development and anticipated marketing of the Tomorrow GardenTM consumer retail product in our UK based subsidiary and, (iv) on a series of related private offering transactions with institutional and other investors, pursuant to which we raised $4,817,114 through the issuance of convertible debentures and $1,028,266 from the issuance of common shares.
We incurred losses of $12,712,358 for the year ended March 31, 2008, as compared to $8,138,393 for the year ended March 31, 2007 after inclusion of a prior period correction in the amount of $3,068,889 pertaining primarily to accounting methods used to value and amortize convertible debenture instruments.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
YEAR ENDED MARCH 31, 2008 COMPARED WITH YEAR ENDED MARCH 31, 2007 - continued
The consolidated financial statements for the year ended March 31, 2007 have been restated to adjust prepaid expenses and insurance expense for amounts that relate to the 2008 fiscal year as well as to correct the bifurcation of convertible notes to record the values of the debt and the debt discounts, the amortization of the related debt discount using the effective interest method and the transfer of the conversion component of convertible notes to share capital upon conversion of these notes into common shares of the Company. This error was noted in the current year and resulted in a restatement to reduce net loss by $2,801,178 for the year ended March 31, 2007. The resulting restatement to amounts for the year-ended March 31, 2007 are as follows:
| | | As Previously Reported March 31, 2007 | | | Adjustment | | | As Restated March 31, 2007 | |
| Convertible debentures | | $ | 5,301,129 | | | $ | (2,558,667 | ) | | $ | 2,742,462 | |
| Share capital | | $ | 7,836,903 | | | $ | 360,079 | | | $ | 8,196,982 | |
| Contributed surplus | | $ | 3,253,333 | | | $ | 80,102 | | | $ | 3,333,435 | |
| Conversion component of convertible notes | | $ | 4,167,190 | | | $ | (879,332 | ) | | $ | 3,287,858 | |
| Deficit, end of year | | $ | 14,674,170 | | | $ | (3,068,889 | ) | | $ | 11,605,281 | |
| Deficit, beginning of year | | $ | 3,734,599 | | | $ | (267,711 | ) | | $ | 3,466,888 | |
| Insurance expense | | $ | 149,855 | | | $ | (71,071 | ) | | $ | 78,784 | |
| Interest, accretion and financing on convertible notes | | $ | 5,606,886 | | | $ | (2,880,819 | ) | | $ | 2,726,067 | |
| Foreign exchange gain (loss) | | $ | 110,006 | | | $ | (116,999 | ) | | $ | (6,993 | ) |
| Basic loss per share | | $ | 0.57 | | | $ | (0.15 | ) | | $ | 0.42 | |
Revenues
For the years ended March 31, 2008 and March 31, 2007, we had no revenue. The Company generated approximately US$598,775 (2007 $nil) in cash related to test sales of the Nova Skin Care system which has been netted against product development costs.
Interest income for the years ended March 31, 2008 and March 31, 2007 was $10,805 and $25,704 respectively.
The foreign exchange gain for the years ended March 31, 2008 and March 31, 2007 was $611,133 and $6,993 respectively.
Operating Expenses
Product development expenses increased by $2,519,014 to $4,081,435 for the year ended March 31, 2008 as compared with the year ended March 31, 2007. The increase is due to the larger scale and scope of a) Nova Skin Care Products marketing and product testing rollout with significant infomercial media advertising costs, b) increased Vertigro research and development costs, c) new development initiatives relating to tissue culture research, plant growth technologies, and HDVG System product development, and d) the advent of Valcent EU costs relating to the Tomorrow GardenTM and UK based HDVG System development initiatives, all pursuant to license arrangements acquired in the Pagic Agreements. Product development expenses were $1,562,421 during the year ended March 2007.
In conjunction with convertible debenture financings during the year ended 2008, the Company incurred $2,804,582 in interest, accretion and financing on convertible notes in the twelve month period ended March 31, 2008. This represents a $78,515 decrease from the $2,726,067 that had been incurred during the year ended March 31, 2007 stemming primarily from amortization of convertible debenture funding activity during the 2007 and 2008 fiscal years.
Advertising and media development was $1,953,998 during the year ended March 31, 2008 (2007 $1,092,917) that reflects the more advanced stages of marketing systems development that include infomercial media purchases in connection with test sales initiatives of our Nova Skin Care System that commenced in December, 2006.
As a result of the issuance of options to directors, officers, employees and consultants the Company incurred stock option compensation expenses of $990,305 during the year ended March 31, 2008 (2007 - $1,127,141).
Professional fees decreased by $281,188 to $431,270for the year ended March 31, 2008 from $712,458 for the year ended March 31, 2007. The decrease is primarily attributable to costs associated with decreased business activity relating to intellectual property legal services, and executive search services between the respective years.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
YEAR ENDED MARCH 31, 2008 COMPARED WITH YEAR ENDED MARCH 31, 2007 - continued
Travel expenses increased by $134,967 to $291,465 (2007 - $156,498) for the year ended March 31, 2008 as a result of increased activity in all of the Company’s operations, increased number of active development projects, as well as new costs relating to an additional subsidiary interest located in the United Kingdom.
Rent expenses increased $12,225 to $77,918 for the year ended March 31, 2008 from $65,693 for the year ended March 31, 2007. The increase in rent costs incurred relates to our new offices and operations located in the United Kingdom.
Office and miscellaneous expenses decreased $46,879 to $234,817 for the year ended March 31, 2008 from $281,696 for the year ended March 31, 2007. The decrease is due to cost streamlining and organizational emphasis on project development initiatives and research facility build out at our facility in El Paso, Texas.
Filing and transfer agent expenses increased $5,617 to $44,500 for the year ended March 31, 2008, from $38,883 for the year ended March 31, 2007. The increase is primarily attributable to costs associated with the proportionate increase of financing and business activity over the 2007 fiscal year.
Investor relations fees increased $583,708 to $871,542 (2007 - $287,834) for the year ended March 31, 2008 as a result of the Company employing an increasing number of third party consultants in advisory, business consulting services, and investor relations activities with the bulk of the increase represented by non-cash stock based compensation.
Interest and penalties inclusive of interest on long term debt expenses increased $112,446 to $127,856 (2007 - $15,410) for the year ended March 31, 2008 as a result of an increasing number of interest bearing debt instruments and minimal repayment of outstanding debt issues. The interest component included in the preceding figures relating to Long term debt increased to $15,480 (2007 - $8,500) due to a complete year of debt repayments during fiscal 2008 as compared with a partial year of debt repayments during fiscal 2007 in connection with lands acquired for research relating to the Company’s development of its algae based bio-diesel feedstock initiative.
Insurance expense decreased to $71,071 for the year ended March 31, 2008, from $78,784 for the year ended March 31, 2007. The decrease is primarily attributable to the rebate of 2007 insurance costs received in the year ended March 31, 2008 and associated decreased insurance coverage required which was prorated over both 2007 and 2008 fiscal years as adjusted by a prior period adjustment.
As a result of increasing operating capacity and existing and as new project build out at our El Paso, Texas operation, as well as the development of our new Cornwall, UK offices, the Company’s property and equipment and land were valued at a net book value of $1,135,108 (2007 - $348,487) during the year ended March 31, 2008, and incurred a depreciation and amortization charge of $47,463 (2007 - $25,288).
Due to fluctuations in the United States dollar in relation to the Canadian dollar, the Company incurred a foreign exchange gain of $611,133 (2007 - $6,993) during the year ended March 31, 2008.
Net Loss
Our reported loss increased by $4,573,965 to $12,712,358 ($0.36 basic loss per share) for the year ended March 31, 2008 as compared to $8,138,393 ($0.42 basic loss per share) for the same period ending March 31, 2007 after considering a prior period correction of $3,068,889 applied to the 2007 fiscal year. The increase during the 2008 fiscal year is largely a result of a) the prior period correction in the amount of $3,068,889 pertaining primarily to accounting methods used to value and amortize convertible debenture instruments, and to a lesser degree an adjustment to prepaid expenses and insurance expense for amounts that relate to the 2008 fiscal year-end, b) $365,021 in aggregate expenses associated with increasing scale and scope of product development and marketing initiatives relating to all our projects under development, increasing Company consulting arrangements relating to increasing scale and scope of business operations, as well as expenses our new UK operating offices, and c) the write down of our product license by $1,306,074, all of which was offset by $611,133 of gain relating to foreign exchange accounts and $10,805 of interest income. Due to uncertainty in determining future cash flows related to products under license, the Company, during the year ended March 31, 2008, wrote-down the value of the product license by $1,306,074 to $1.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
YEAR ENDED MARCH 31, 2008 COMPARED WITH YEAR ENDED MARCH 31, 2007 - continued
Liquidity and Capital Resources
Because we are organized in Canada, our March 31, 2008 financial statements have been prepared by our management in accordance with Canadian GAAP applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business.
Our accumulated losses during the development stage increased by $12,712,358 to $24,317,639 for the year ended March 31, 2008 from approximately $11,605,281 for the year ended March 31, 2007.
Our working capital deficit increased to $4,153,903 from $1,600,602 for the year ended March 31, 2008 representing a $2,553,301 increase over the year ended March 31, 2007. As described in Note 1 to our March 31, 2008 financial statements, these conditions raise substantial doubt as to our ability to continue as a going concern. We do not believe our working capital is sufficient for our present requirements, and anticipate that we will need to secure additional capital to fund our operations.
We raised US$1,041,000 in net cash proceeds from the issuance of convertible debentures during the year ended March 31, 2008, as compared to US$4,133,866 for the year ended March 31, 2007.
| | | US $ | | | CDN $ | |
| | | Balance | | | 2008 | | | 2008 | | | 2008 | | | 2008 | | | Balance | | | Balance | |
| | | March 31, | | | Issued | | | Equity | | | Interest / | | | | | | March 31, | | | March 31, | |
| Date of Issue | | 2007 | | | Principal | | | Portion | | | Penalty | | | Conversions | | | 2008 | | | 2008 | |
| | | | | | | | | | | | | | | | | | | | | | |
| July/August 2005 (Note 10(a)) | | $ | 316,957 | | | $ | 0 | | | $ | 0 | | | $ | 22,389 | | | $ | (79,521 | ) | | $ | 259,825 | | | $ | 265,583 | |
| April 2006 (Note 10(b)) | | | 495,607 | | | | 0 | | | | 0 | | | | 38,835 | | | | 0 | | | | 534,442 | | | | 546,841 | |
| April 2006 (Note 10(c)) | | | 79,115 | | | | 0 | | | | 0 | | | | 6,427 | | | | 0 | | | | 85,542 | | | | 87,527 | |
| December 2006 (Note 10(e)) | | | 670,486 | | | | 0 | | | | 0 | | | | 989,296 | | | | 0 | | | | 1,659,782 | | | | 1,698,289 | |
| January 2007 (Note 10(f)) | | | 813,084 | | | | 0 | | | | 0 | | | | 967,767 | | | | (211,668 | ) | | | 1,569,183 | | | | 1,605,558 | |
| August 2007 (Note 10(g)) | | | 0 | | | | 650,000 | | | | (230,007 | ) | | | 258,574 | | | | 0 | | | | 678,567 | | | | 694,310 | |
| September 2007 (Note 10(h)) | | | 0 | | | | 391,000 | | | | (213,249 | ) | | | 119,682 | | | | 0 | | | | 297,433 | | | | 304,633 | |
| | | $ | 2,375,249 | | | $ | 1,041,000 | | | $ | (443,256 | ) | | $ | 2,402,970 | | | $ | (291,189 | ) | | $ | 5,084,774 | | | $ | 5,202,741 | |
| | | US $ | | | CDN $ | |
| | | Balance | | | 2007 | | | 2007 | | | 2007 | | | 2007 | | | Balance | | | Balance | |
| | | March 31, | | | Issued | | | Equity | | | Interest / | | | | | | March 31, | | | March 31, | |
| Date of Issue | | 2006 | | | Principal | | | Portion | | | Penalty | | | Conversions | | | 2007 | | | 2007 | |
| | | | | | | | | | | | | | | | | | | | | | |
| July/August 2005 (Note 10(a)) | | $ | 1,429,104 | | | $ | 0 | | | $ | 0 | | | $ | 119,875 | | | $ | (1,232,022 | ) | | $ | 316,957 | | | $ | 365,959 | |
| April 2006 (Note 10(b)) | | | 0 | | | | 551,666 | | | | (388,313 | ) | | | 567,366 | | | | (235,112 | ) | | | 495,607 | | | | 572,228 | |
| April 2006 (Note 10(c)) | | | 0 | | | | 82,200 | | | | (48,776 | ) | | | 70,054 | | | | (24,363 | ) | | | 79,115 | | | | 91,346 | |
| December 2006 (Note 10(e)) | | | 0 | | | | 1,500,000 | | | | (1,067,962 | ) | | | 238,448 | | | | 0 | | | | 670,486 | | | | 774,143 | |
| January 2007 (Note 10(f)) | | | 0 | | | | 2,000,000 | | | | (1,331,232 | ) | | | 144,316 | | | | 0 | | | | 813,084 | | | | 938,786 | |
| | | $ | 1,429,104 | | | $ | 4,133,866 | | | $ | (2,836,283 | ) | | $ | 1,140,059 | | | $ | (1,491,497 | ) | | $ | 2,375,249 | | | $ | 2,742,462 | |
To provide working capital for product development, during July and August 2005, the Company issued one-year, unsecured US$1,277,200 8% per annum convertible notes and three-year Class A and B warrants to acquire: (i) up to 1,702,933 common shares of the Company at a price per share of US$0.50; and (ii) up to an additional 1,702,933 common shares of the Company at a price per share of US$1.00. The holders of the convertible notes may elect to convert the notes into common shares of the Company at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the common stock for the ten trading days prior to conversion; and (ii) US$0.55. Accrued and unpaid interest may be converted into common shares of the Company at US$0.50 per share. The Company may, subject to notice provisions and the common shares trading above US$1.50 per share for more than twenty consecutive trading days, elect to payout the notes and interest due by paying 130% of the amount due under the notes plus interest. The common stock purchase warrants carry a “net cashless” exercise feature (“Cashless Conversion Feature”) allowing the holder thereof, under certain limited circumstances, to exercise the warrants without payment of the stated exercise price, but rather solely in exchange for the cancellation of that number of common shares into which such warrants are exercisable. As a result of the issuance of the warrants in conjunction with the convertible notes, the Company recorded a non-cash financing expense of $1,328,337. These convertible notes are unsecured, and due on demand.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
YEAR ENDED MARCH 31, 2008 COMPARED WITH YEAR ENDED MARCH 31, 2007 - continued
In conjunction with this financing, the Company paid consultants an amount equal to 10% of the gross proceeds, which was included in investor relations during the year ended March 31, 2006 and issued 425,735 common shares at a deemed value of $285,242. There are 255,440 finders’ A warrants outstanding whereby the holders have the right to purchase 255,440 common shares at US$0.50 per share until August 5, 2008 and 425,733 finders’ B warrants whereby the holders shall have the right to purchase 425,733 common shares at US$0.75 per share until August 5, 2008. A total of US$82,200 in registration penalties incurred in the year ended March 31, 2007 were converted to a new convertible debenture in the same amount on April 6, 2007.
During the year ended March 31, 2008, convertible notes of US$75,000 and interest totaling US$4,521 were converted to 262,057 common shares, and interest of US$22,389 (2007 – US$119,875) was accrued on the principal balance of these convertible notes.
On April 6, 2006, the Company consummated a private offering transaction with and among a syndicated group of investors, pursuant to which the Company issued, in the aggregate, US$551,666 in 8% per annum convertible notes and three-year Class A and B warrants to acquire: (i) up to 735,544 shares of the Company’s common stock at a price per share of US$0.50; and (ii) up to an additional 735,544 shares of the Company’s common stock at a price per share of US$1.00. Subject to certain limitations, the principal amount of the notes, together with any accrued interest may be converted into shares of the Company’s common stock at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the common stock for the ten trading days prior to conversion; or (ii) US$0.55. The convertible notes carry a redemption feature, which allows the Company to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that the common shares have a closing price of US$1.50 per share for at least twenty consecutive trading days and there has otherwise been no default. The common stock purchase warrants carry a Cashless Conversion Feature. These convertible notes are unsecured, and due on demand.
In conjunction with these private offering transactions, the Company paid consultants: (i) US$55,166 cash, representing 10% of the gross proceeds realized; (ii) 183,886 shares of common stock; (iii) three-year warrants to purchase up to 110,320 shares of common stock at a price per share of US$0.50; and (iv) three-year warrants to purchase up to 183,867 shares of common stock at a price per share of US$0.75.
During the year ended March 31, 2008, the Company accrued US$38,835 (2007 – US$567,366) in interest on the principal balance of these convertible notes.
On April 6, 2006, and in conjunction with certain private placements, the Company reached a verbal agreement with the group of institutional and other investors, wherein the Company agreed to convert US$82,200 in accrued penalties associated with the July 25, 2005 through August 5, 2005 convertible notes into US$82,200 convertible penalty notes (note 10(a)) carrying terms similar to the July 25, 2005 through August 5, 2005 convertible notes and an aggregate of 109,600 warrants. These warrants carry a cashless conversion feature and each of these warrants entitles the holder to purchase additional common shares for three years at a price of US$0.75 per share. These convertible notes are unsecured, and due on demand.
During the year ended March 31, 2008, the Company accrued US$6,427 (2007 – US$70,054) in interest on the principal balance of these convertible notes.
Certain of the July and August 2005 and the April 6, 2006 convertible notes contained registration rights whereby the Company agreed to pay a penalty of 2% for every thirty days after a required filing and registration effective date plus a reduction in the warrant price of certain of the warrants issued of US$0.10. As a result of the Company not filing its registration statement until April 27, 2006, the Company incurred penalties, which have been included in interest expense. An aggregate of 3,407,372 previously issued share purchase warrants relating to certain of the July and August 2005 and the April 6, 2006 convertible notes have reduced exercise prices from US$0.50, US$0.75, and US$1.00 to US$0.40, US$0.65, and US$0.90, respectively. In 2007, the Company recognized $80,102 in interest expense with the corresponding amount to contributed surplus as a result of re-valuation of the warrants upon the change in the pricing. The registration statement has subsequently been declared effective.
On December 1, 2006, the Company accepted subscriptions of US$1,500,000 towards a private placement of 8% per annum, unsecured, convertible notes and three-year warrants to acquire: (i) up to an aggregate of 2,000,000 shares of the Company’s common stock at a price per share of US$0.50; and (ii) up to an additional 2,000,000 shares of the Company’s common stock at a price per share of US$1.00. Subject to certain limitations, the principal amount of the notes, together with any accrued interest may be converted into shares of the Company’s common stock at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the Company’s common stock for the ten trading days prior to conversion; or (ii) US$0.55. The convertible notes carry a redemption feature, which allows the Company to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that the common shares have a closing price of US$1.50 per share for at least twenty consecutive trading days and there has otherwise been no default.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
YEAR ENDED MARCH 31, 2008 COMPARED WITH YEAR ENDED MARCH 31, 2007 - continued
These convertible notes are unsecured and due on demand. The common stock purchase warrants may be exercised on a cashless basis.
During the year ended March 31, 2008, the Company accrued US$989,296 (2007 - US$238,448) in interest on the principal balance of these convertible notes.
The right of the note holders to convert into the Company’s common stock is subject to the contractual agreement between the parties that any conversion by the note holders may not lead at the date of such conversion to an aggregate equity interest in the common stock of the Company greater than 9.99% inclusive of any derivative securities including options, warrants, convertible debt, any other convertible debt securities, or any other financial instruments convertible into common equity.
On January 29, 2007, the Company completed a private placement comprised of $2,000,000 convertible notes. The convertible notes will mature on December 11, 2008, carry interest at 6% per annum and are unsecured. The notes are convertible into “Units” at the note holders’ discretion at a conversion price of US$0.50 per Unit. Each “Unit” consists of one common share and one purchase warrant to purchase an additional common share at US$0.70 per share until December 11, 2008. The notes and any accrued interest are callable by the Company at any time after December 11, 2007 by providing thirty days’ written notice to the note holders. Interest on the notes will be compounded annually and be cumulative until the earlier of either the date the Company achieves pre-tax earnings or the end of the term. At the discretion of the note holders, interest on the notes is payable in either cash or units at US$0.50 per unit. In connection with this financing, the Company has paid consultants US$108,000 in cash and issued 135,000 warrants exercisable at US$0.50 per unit, with each unit consisting of one common share and one share purchase warrant to purchase a further common share at US$0.70 per share until December 11, 2008. The Company is obligated to file a resale registration statement on the underlying securities within four months of closing, which it has failed to do.
As a result of the failure to file the registration statement, the Company recorded penalties of US$120,000 as of March 31, 2007 and a further US$289,973 during the year ended March 31, 2008. In addition, during the year ended March 31, 2008, convertible notes of US$200,000 and interest and registration penalty totaling US$11,668 were converted to 485,707 common shares, and the Company accrued US$677,794 in interest on the principal balance of these convertible notes (2007 - US$24,316).
On August 10, 2007, the Company issued an unsecured convertible term promissory note in the amount of US$650,000 to a third party. The convertible note is due on demand and bears interest at 6% with both interest and principal convertible at the option of the lender into units at US$0.60 per unit, with each unit consisting of one common share and one-half share purchase warrant with each whole share purchase warrant exercisable at US$0.75 to purchase an additional common share. After November 25, 2008, this convertible note accrues interest at the rate of 15% per annum. During the year ended March 31, 2008, the Company accrued US$230,007 (2007 – US$0) in interest on the principal balance of these convertible notes.
The Company is required to register for trading the securities underlying the conversion features of this convertible note on a best efforts basis, but has failed to do so within terms agreed. A one-time financial penalty of US$28,567 for failure to register the securities underlying this convertible note within 180 days from the date of issuance has been incurred in the year ended March 31, 2008.
The right of the note holder to convert into the Company’s common stock is subject to the contractual agreement between parties that any conversion by the note holder may not lead, at the date of such conversion, to an aggregate equity interest in the common stock of the Company greater than 9.99% inclusive of any derivative securities including options, warrants, convertible debt, any other convertible debt securities, or any other financial instruments convertible into common equity.
On September 27, 2007, the Company issued unsecured one-year term convertible notes bearing interest at 6% per annum in the amount of US$391,000 to third parties. Both interest and principal may be converted at the option of the lender at any time at US$0.60 per unit, with each unit consisting of one common share and one-half share purchase warrant, with each whole share purchase warrant exercisable at US$0.75 to purchase an additional common share for a two-year term from the date of conversion. During the year ended March 31, 2008, the Company accrued US$96,222 (2007 - US$0) in interest on the principal balance of these convertible notes.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
YEAR ENDED MARCH 31, 2008 COMPARED WITH YEAR ENDED MARCH 31, 2007 - continued
The Company is required to register for trading the securities underlying the conversion features of this convertible note on a best efforts basis, but has failed to do so within terms agreed. A one-time financial penalty of US$23,460 for failure to register the securities underlying this convertible note within 90 days from the date of issuance has been incurred during the year ended March 31, 2008.
For the year ended March 31, 2008, the Company received $5,081,103 from the issuance of common shares relating to private offering transactions with institutional and other investors. We raised a further $367,671 from the exercise of outstanding warrants and options.
We further raised $154,196 from interest bearing promissory notes during the year ended March 31, 2008 for an aggregate outstanding of $270,167 as at March 31, 2008 (2007 - $115,460). Our advances and amounts due from related parties increased by $560,341 to $1,490,516 as at March 31, 2008 (2007 - $930,175).
As a result of the Nova Skin Care System our inventories were $617,195 as at March 31, 2008 (2007 - $1,236,808).
As at March 31, 2008, accounts receivable of $462,156 consists of $374,502 (2007 $30,282) net of allowance for doubtful accounts of $90,263 (2007 $3,798) due from the Company’s product fulfillment agent, GSI Commerce, Inc. (formerly Accretive Commerce Inc.) from test sales relating to the Company’s Nova Skin Care System, ($7,806) (2007 $426,987) due from the Company to Global Green, the Company’s joint venture partner in the development of the Company’s High Density Vertical Bio-Reactor technology, $51,472 in value added tax owed to the Company’s subsidiary, Valcent EU, and goods and services tax receivable by the Company of $43,988.
Prepaid expenses as at March 31, 2008 consists of $2,015,837 (2007 - $285,690), which is the deferred portion of these agreements with the balance consisting of $103,709 (2007 - $71,071) in prepaid insurance and rental deposits.
Restricted cash as at March 31, 2008 consists of certificates of deposit and interest earned of $108,471 (2007 - $117,327) that are pledged to secure long term debt relating to our purchased lands in El Paso.
We purchased $834,084 (2007 - $310,448) in property and equipment during the year ended March 31, 2008. During the year ended March 31, 2007, we incurred long term debt in connection with a land purchase. As at March 31, 2008, we owed an aggregate of $174,862 relating to this debt (2007 - $209,114).
As at March 31, 2008, we had $163,437 in cash (2007 - $314,972) and we currently have approximately $265,000 in cash as at September 15, 2008.
Due to uncertainty in determining future cash flows related to products under license, the Company, during the year ended March 31, 2008, wrote down the value of the product license by $1,306,074 to $1.
SUBSEQUENT EVENTS TO MARCH 31, 2008
Unless otherwise noted in Management’s Discussion and Analysis, the following events occurred after March 31, 2008:
Subsequent events not disclosed elsewhere in these financial statements are as follows:
The Company entered into the following agreements on April 1, 2008 and May 1, 2008 respectively:
| (i) | A financial services agreement with an arm’s length private company for a term of seven months. Pursuant to the agreement, the Company issued 120,000 common shares valued at US$84,000; and |
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
SUBSEQUENT EVENTS TO MARCH 31, 2008 - continued
| (ii) | A financial and business consulting agreement with an individual consultant for a term of twelve months. Pursuant to the agreement, the Company issued 300,000 common shares valued at US$201,000. |
The Company completed a series of US$0.60 per unit private placements. Each unit consisted of one common share and one-half common share purchase warrant with each whole warrant exercisable at US$0.75 per share for two years. The private placements took place on May 7, June 6, and July 23, 2008, respectively, with details as follows:
| (i) | issued 2,996,666 units for gross proceeds of US$1,798,000. The Company paid US$122,577 in cash consultant’s fees with respect to this issuance; |
| (ii) | issued 195,000 units for gross proceeds of US$117,000. The Company paid no consultant’s fees respecting the issuance; and |
| (iii) | issued 61,000 units for gross proceeds of US$36,600. The Company paid US$2,562 in cash consultant’s fees with respect to this issuance. |
The Company issued the following common shares in relation to the conversion of certain notes:
| (i) | On June 2, 2008, the Company issued 245,049 common shares relating to the conversion of US$100,000 in convertible debt and US$9,403 in accumulated interest; and |
| (ii) | On July 3, 2008, the Company issued 267,221 common shares relating to the conversion of US$92,281 in convertible debt and US$9,243 in accumulated interest. |
On July 21, 2008, the Company closed a financing of zero coupon, 12% interest, senior secured convertible promissory notes in the amount of US$2,428,160 with an aggregate purchase price of US$2,168,000 with four investors, one of which was the Company’s Chief Financial Officer as to US$168,000. The debt is convertible into shares of common stock at the lesser of US$0.51 per share (unless the conversion price has been adjusted pursuant to further contract covenants) and 70% of the average of the five lowest closing bid prices for the ten preceding trading days. The Company issued each purchaser in the private placement two warrants, one warrant being redeemable by the Company and the other being non-redeemable. The non-redeemable warrants are exercisable at US$0.55 and permit the holder to purchase shares of common stock equal to 100% of the number of shares issuable upon the conversion of the notes calculated on July 21, 2008. The redeemable warrants are exercisable at US$0.75 and permit the holder to purchase common stock equal to 50% of the number of shares issuable upon the conversion of the notes issued calculated on the closing date. The Company issued a total of redeemable warrants to purchase an aggregate of 4,761,098 shares of common stock and a total of redeemable warrants to purchase an aggregate of 2,380,550 shares of common stock. Further, the Company issued 439,216 non-redeemable warrants and 219,608 redeemable warrants and $160,000 in cash fees to close the transaction. Each non-redeemable warrant is exercisable at US$0.55, and each redeemable warrant is exercisable at US$0.75; warrants carry a term of five years from the date of closing of the financing. The redeemable warrant may be redeemed by the Company only if certain conditions have been satisfied including the Company’s common stock having closed at $1.50 per share for a period of 20 consecutive trading days and the warrant holder being able to resell the shares acquired upon exercise through a resale registration statement or under Rule 144 of the Securities Act.
On July 7, 2008, the Company extended to December 31, 2008, the expiry dates of 2,872,224 warrants exercisable between US$0.75 and US$1.00 expiring May 15, 2008 through August 18, 2008.
On August 18, 2008, the Company issued 300,000 common shares to Pagic LP in relation to the Technology License agreed to by the Company, Pagic LP, and West Peak Ventures of Canada Ltd. on May 5, 2008.
On August 18, 2008, the Company issued 383,331 common shares pursuant to the exercise of 383,331 warrants for gross proceeds of US$191,666.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
SUBSEQUENT EVENTS TO MARCH 31, 2008 - continued
The Company issued the following common shares in relation to the conversion of certain notes:
On August 18, 2008, the Company issued 62,703 common shares relating to the conversion of US$25,000 in convertible debt and US$6,352 in accumulated interest and registration penalty. The Company also issued as part of converted amounts, 54,550 warrants exercisable at US$0.70 per share until December 11, 2008;
On September 10, 2008, the Company issued 549,690 common shares relating to the conversion of US$250,000 in convertible debt and US$24,845 in accumulated interest. The Company also issued as part of converted amounts, 549,690 warrants exercisable at US$0.70 per share until December 11, 2008; and
On September 19, the Company issued 417,322 common shares relating to the conversion of US$175,000 in convertible debt and US$33,662 in accumulated interest and registration penalty. The Company also issued as part of converted amounts, 385,104 warrants exercisable at US$0.70 per share until December 11, 2008.
On September 3, 2008, the Company signed a Letter of Agreement with the Hydroganics Joint Venture, an Australian Group, (“Hydroganics”) for the exclusive use of the Company’s HDVG System for growing vegetables excluding grains in Australia. Under the terms of the agreement, Hydroganics will invest US$2,500,000 by purchasing Valcent equity, Valcent will have a carried 30% equity interest in Hydroganics until Hydroganics has raised US$10,000,000 in equity for the development and commercialization of HVGS System in Australia. Thereafter, Valcent shall have anti dilution rights to purchase 30% of any further equity issued.
On September 16, 2008, the Company signed a License Agreement with a private Alberta Corporation based in Fort McMurray, Alberta, Canada (“Albertaco”) for the exclusive use of Company’s HDVG System for growing vegetables in the Province of Alberta. Under the terms of the agreement, the purchaser will invest US$1,500,000 by purchasing Valcent equity, Valcent will have a carried 30% equity interest in Albertaco until Albertaco has raised US$10,000,000 in equity for the development and commercialization of the HDVG System in Alberta. Thereafter, Valcent shall have anti dilution rights to purchase 30% of any further equity issued.
On September 26, 2008, the Company entered into an agreement with Global Green to purchase its entire membership interest in Vertigro Algae Technologies LLC, the company responsible for developing the Company’s proprietary technologies to produce algae biomass fuels and other algae products. The price to be paid for the Membership interest is US$5 million in cash and 5 million common shares in the capital of the Company. The transaction is subject to finance. The Company and Global Green further agreed that Global Green, on a non-exclusive basis, may be engaged to facilitate the commercialization and development of the Company’s technologies with particular emphasis on bio mass power generation including the integration of Global Green’s “ Greensteam” applications, the terms and consideration for which are to be determined on a project specific basis.
CAPITAL COMMITMENTS
Commitments not disclosed elsewhere in this annual report are as follows:
On November 16, 2007, Valcent EU leased office and development space in Launceston, Cornwall, UK, under a ten-year lease beginning November 15, 2007 and ending on November 15, 2017 at a quarterly cost of $26,017 (GB£12,550). There were 9 years and 7.5 months remaining on the lease as at March 31, 2008. Fiscal yearly commitments are as follows:
| 2009 | | $ | 104,068 | |
| 2010 | | | 104,068 | |
| 2011 | | | 104,068 | |
| 2012 | | | 104,068 | |
| 2013 | | | 104,068 | |
| Thereafter | | | 305,037 | |
| | | $ | 825,377 | |
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
CAPITAL COMMITMENTS - continued
The Company believes that debt and equity sources of capital from investors will provide the capital necessary for it to meet its current capital commitments.
Fluctuations in Operating Results
Our annual operating results are likely to fluctuate significantly in the future as a result of numerous factors, including, among others:
| | • | The availability of adequate financing; |
| | | |
| | • | Our ability to develop an organizational infrastructure and effective management systems; |
| | | |
| | • | The success of our advertising and marketing plans; |
| | | |
| | • | Market acceptance and general consumer demand for our products; |
| | | |
| | • | The performance of our products; |
| | | |
| | • | Our ability to effectively distribute our products; |
| | | |
| | • | The introduction of products that compete with our own; |
| | | |
| | • | The adoption of new disclosure and/or corporate governance requirements associated with the maintenance of our status as a publicly reporting issuer; |
| | | |
| | • | Potential lawsuits involving our products or other matters; and |
| | | |
| | • | General business and economic conditions; |
| | | |
| | • | Currency valuation fluctuations |
Impact of Inflation
We do not believe that inflation has had a material effect on our business.
OFF-BALANCE SHEET ARRANGEMENTS
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a material adverse affect on our financial condition or results of operations.
CONTRACTUAL OBLIGATIONS
On June 28, 2005, Valcent Manufacturing, Ltd. leased office and development space in El Paso, Texas, under a three-year lease at a cost of US$3,170 per month. There were 5 months remaining on the lease as at March 31, 2008 (2007 – 14 months).
On November 16, 2007, our wholly owned subsidiary, Valcent EU Limited leased office and development space in Launceston, Cornwall, UK, under a ten-year lease beginning November 15, 2007 and ending on November 15, 2017 at a quarterly cost of GB£12,550 (CDN$26,017). There were 9 years and 7.5 months remaining on the lease as at March 31, 2008.
On December 12, 2006, the Company entered into a Public Relations Agreement with a third party to provide public relations services to the Company. The agreement required the Company to issue 25,000 restricted common shares in advance of each quarter during the course of the agreement’s one year term for a total of 100,000 restricted common shares at a price of US$0.45 per common share, the payment of approved expenses, and monthly fees ranging from US$4,250 to US$5,250 per month. During the year ended March 31, 2007, 25,000 shares were issued pursuant to this agreement and an additional 75,000 were issued subsequently during the year ended March 31, 2008. The contract has been extended after its expiry on a month to month basis and a further 25,000 shares were issued at a value of US$0.45 per common share.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS - continued
CONTRACTUAL OBLIGATIONS - continued
On April 1, 2007, the Company entered into an agreement with a third party to provide investor relations and financial services for an eight month term from April 1, 2007 through December 31, 2007. The agreement provides for the following:
| b) | US$3,000 in compensation per month payable quarterly during the effective term; |
| c) | a further US$2,000 payable monthly in common shares (27,508 common shares were issued in the year ended March 31, 2008 for aggregate value of US$18,000); |
| d) | share options to purchase 500,000 shares were issued at US$0.60 exercise price that vest quarterly over a two year period; and |
| e) | the issuance of 12,500 common shares at a price of US$0.80 per share which were issued on April 24, 2008. |
At March 31, 2008, the Company’s long-term debt outstanding was as follows:
| | | 2008 | | | 2007 | |
| | | | | | | |
| Prime plus 0.25% (2008 – 8.00%) bank loan repayable in monthly instalments of US$2,336 including interest, due September 28, 2011, secured by a first charge on land and | | | | | | |
| $108,471 of cash | | | 174,862 | | | | 209,114 | |
| | | | | | | | | |
| Less: Current portion | | | 16,250 | | | | 13,451 | |
| | | | | | | | | |
| | | $ | 158,612 | | | $ | 195,663 | |
The portion of long-term debt repayable in each of the next four years is approximately as follows:
| To March 31, | | | |
| | | | |
| 2009 | | | 16,250 | |
| 2010 | | | 17,258 | |
| 2011 | | | 18,806 | |
| 2012 | | | 106,298 | |
A summary of the Company’s convertible notes are as follows:
| Convertible Note Continuity: | | | | |
| | | US $ | | | CDN $ | |
| | | Balance | | | 2008 | | | 2008 | | | 2008 | | | 2008 | | | Balance | | | Balance | |
| | | March 31, | | | Issued | | | Equity | | | Interest / | | | | | | March 31, | | | March 31, | |
| Date of Issue | | 2007 | | | Principal | | | Portion | | | Penalty | | | Conversions | | | 2008 | | | 2008 | |
| | | | | | | | | | | | | | | | | | | | | | |
| July/August 2005 (Note 10(a)) | | $ | 316,957 | | | $ | 0 | | | $ | 0 | | | $ | 22,389 | | | $ | (79,521 | ) | | $ | 259,825 | | | $ | 265,583 | |
| April 2006 (Note 10(b)) | | | 495,607 | | | | 0 | | | | 0 | | | | 38,835 | | | | 0 | | | | 534,442 | | | | 546,841 | |
| April 2006 (Note 10(c)) | | | 79,115 | | | | 0 | | | | 0 | | | | 6,427 | | | | 0 | | | | 85,542 | | | | 87,527 | |
| December 2006 (Note 10(e)) | | | 670,486 | | | | 0 | | | | 0 | | | | 989,296 | | | | 0 | | | | 1,659,782 | | | | 1,698,289 | |
| January 2007 (Note 10(f)) | | | 813,084 | | | | 0 | | | | 0 | | | | 967,767 | | | | (211,668 | ) | | | 1,569,183 | | | | 1,605,558 | |
| August 2007 (Note 10(g)) | | | 0 | | | | 650,000 | | | | (230,007 | ) | | | 258,574 | | | | 0 | | | | 678,567 | | | | 694,310 | |
| September 2007 (Note 10(h)) | | | 0 | | | | 391,000 | | | | (213,249 | ) | | | 119,682 | | | | 0 | | | | 297,433 | | | | 304,633 | |
| | | $ | 2,375,249 | | | $ | 1,041,000 | | | $ | (443,256 | ) | | $ | 2,402,970 | | | $ | (291,189 | ) | | $ | 5,084,774 | | | $ | 5,202,741 | |
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
DIRECTORS AND SENIOR MANAGEMENT
The following table sets forth our directors and executive officers and their ages as of the date of March 31, 2008. Unless otherwise stated, the address for each of our directors and executive officers is c/o Valcent Products Inc., Suite 1010 - - 789 West Pender Street, Vancouver, British Columbia V6C 1H2 (Canada).
| Name | | Age | | Position |
| | |
| M. Glen Kertz (1) | | 55 | | Chief Executive Officer, acting President, Chairman of the Board and Director |
| | | | | |
| F. George Orr (2)(3) | | 47 | | Chief Financial Officer, Secretary and Director |
| | | | | |
| Robert Wingo | | 62 | | Director (resigned July 29, 2008) |
| | | | | |
| Gerry Jardine | | 57 | | Director |
| | | | | |
| Naveen Aggarwal | | 50 | | Director |
| George Stapleton | | 54 | | Director |
| (1) | Appointed acting President, Chairman of the Board and a director by resolution of the board of directors, in accordance with Section 111 of the Business Corporations Act (Alberta), following the resignation of Douglas E. Ford as acting President and Secretary, on July 31, 2005. Appointed Chief Executive Officer by resolution of the board of directors in accordance with Section 121 of the Business Corporations Act (Alberta), and Section 6 of our bylaws on November 30, 2005. |
| | |
| (2) | Appointed director by resolution of the board of directors, in accordance with Section 111 of the Business Corporations Act (Alberta), following the resignation of Larry F. Robb and Malcolm E. Rogers Jr., on July 23, 2005. |
| | |
| (3) | Appointed Secretary by resolution of the board of directors, in accordance with Section 111 of the Business Corporations Act (Alberta), following the resignation of Douglas E. Ford as acting President and Secretary, on July 31, 2005. Appointed Chief Financial Officer by resolution of the board of directors in accordance with Section 121 of the Business Corporations Act (Alberta), and Section 6 of our bylaws on November 30, 2005. |
M. Glen Kertz — Chief Executive Officer, acting President, Chairman of the Board and Director
In October 1985, Glen Kertz founded Gruppe Inc., a privately held tissue culture laboratory and growing operation, which he owned until January 1987. From October 1987 to May 1991, Mr. Kertz was the President of AgriStar, a publicly traded company that produced a gas permeable membrane for cell culture laboratories, and, from June 1999 to July 2004, Pagic, Inc., a consulting business management and development company. In addition, from June 1999 to July 2004, Mr. Kertz served as the President and a director of Nettron.com, Inc., predecessor to Valcent Products Inc., in July of 2005 he was appointed acting President, Chairman of the Board and a director, and, in November of 2005, he was appointed Chief Executive Officer of Valcent Products Inc.
F. George Orr, C.A. — Chief Financial Officer, Secretary and Director
F. George Orr is a self-employed Chartered Accountant with over fifteen years of accounting and consulting experience in private and public company administration, governance, audit procedures and reporting requirements. In July of 2005, he was appointed Secretary and a director, and, in November of 2005, he was appointed Chief Financial Officer of Valcent Products Inc. Mr. Orr holds a Bachelor of Commerce from St. Mary’s University, Halifax.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES - continued
DIRECTORS AND SENIOR MANAGEMENT - continued
Robert V. Wingo - Director (resigned July 29, 2008)
Mr. Wingo joined Sanders\Wingo in 1983. Mr. Wingo’s leadership has been instrumental for clients like Farah USA, Fuddruckers, Dr. Scholl’s, AT&T, United States Postal Service, Shell Oil and the Greater El Paso Chamber of Commerce. Mr. Wingo has received countless honors, including UTEP’s 2002 Gold Nugget award and the Silver Medal award from the American Advertising Federation. Wingo was appointed by Governor Rick Perry to the Texas Economic Development Corporation. He also sits on the Holocaust Museum Board of El Paso and has been recognized by Black Enterprise in its annual B.E. 100’s list for the past three years.
Gerry Jardine — Director
Gerry Jardine is the President/Chief Executive Officer and a director of Silica Resources Corp. since January 31, 2008, a company involved in molybdenum exploration in the United States. Mr. Jardine also is a member of the Investor Relations team at Sweetwater Capital. Mr. Jardine was president and chief executive officer and a director of New Pacific Ventures from 2001 to 2006. From 1999 to 2004, he was the secretary/treasurer and a director of Prefco Enterprises Inc., a public Canadian construction/development company. From 1989 to 2003, Mr. Jardine was the president and chief executive officer and a director of Truax Venture Corporation, a Canadian public exploration company. Additionally, from 1996 to 2003, Mr. Jardine was the president of Amcan Fiscal Consultants Inc., a private consulting company. From 1986 to 1997, Mr. Jardine was the president and chief executive officer and a director of PowerTech Industries Inc., a Canadian public company involved in the manufacture and sales of HVAC equipment. Over the past twenty-three years, Mr. Jardine has also served as a director to more than half a dozen other companies, mainly operating in resource industries.
Naveen Aggarwal - Director
Naveen Aggarwal is the Senior Vice President of Sales and Marketing at Active Intelligence Corp., a company which specializes in the systems integration of Oracle Retail’s (Retek) retail solutions portfolio. As Senior Vice President of Sales & Marketing for Active Intelligence, Mr. Aggarwal established the company as the sole delivery partner for Oracle Retail (Retek) training for North America. Mr. Aggarwal has held senior level positions at many leading international companies including Sun Microsystems, Quest Software, Netscape Communications and Northern Telecom. Mr. Aggarwal received degrees in Neurophysiology and Mathematics/Computer Science at the University of Toronto.
George Stapleton - Director
Mr. Stapleton received his civil engineering degree from Georgia Institute of Technology in 1975 and has 33 years of international experience in the offshore oil construction and service industry, project development, oil and gas exploration and production and energy infrastructure related projects. Mr. Stapleton is currently the Chief Executive Officer of Megawest Energy Corp and a director of PetroBeam, Inc. He was the Chief Operating Officer and a Director of Deerfield Energy LLC from 2005 and the President and a director of Trinity Sands Energy LLC. He was formerly the President and remains a director since 2004 of E-T Energy Ltd., a private Canadian company testing an electro-thermal approach to the production of bitumen from the Athabasca tar sands. From 2000 to 2002 he was responsible for the development of simulation based training packages for the energy and industrial sectors for Simulis, LLC. He was President - Products & Services for NEOppg Ltd. from 1998 to 2000 focusing on a proprietary multilateral system, heavy oil production systems and artificial lift products. Prior to that he was Vice-President, Operations for Asian Tiger Energy Company, an independent oil company developing oil and gas projects, operating primarily in Southeast Asia. Before Asian Tiger, Mr. Stapleton spent 19 years overseas with J. Ray McDermott in the Arabian Gulf, North Sea and Asia as Field Engineer, Operations Engineer, Project Manager, Fabrication Division Manager at Batam Island, Indonesia and finally as Division Manager – Business Development and Project Management where he oversaw project management of all turnkey projects for Southeast Asia and was responsible for business development activities throughout the region.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES - continued
COMPENSATION
The following table sets forth the total compensation paid to each of our directors and executive officers serving during the fiscal year ended March 31, 2008.
| | | | | | | | | | | | | | |
| | | | Annual Compensation | | | Long Term Compensation | |
| | | | | | | | |
| Name and Principal Position | Year Ended March 31, 2008 | | Salary | | | Bonus/Fees | | | Awards Restricted Stock Awards | | | Securities Underlying Options/SARs (#) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
M. Glen Kertz (1) President and Director | | | | — | | | $ | 565,135 | | | | 300,000 | | | | — | |
| | | | | | | | | | | | | | | | | | |
F. George Orr (2) CFO and Director | | | | — | | | | 35,370 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | |
Robert Wingo (3) Director | | | | — | | | | 244,861 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | |
Gerry Jardine (4) Director | | | | — | | | | 75,085 | | | | — | | | | 300,000 | |
| | | | | | | | | | | | | | | | | | |
Naveen Aggarwal (5) Director | | | | — | | | | 76,740 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | |
George Stapleton Director | | | | — | | | | — | | | | — | | | | 300,000 | |
| | | | | | | | | | | | | | | | | | |
| (1) | During the year ended March 31, 2008, Pagic LP, a company affiliated with Glen Kertz, was paid or accrued amounts as shown for product development, expenditures inclusive of service related fees and royalties under various license agreements between the Company and Pagic LP. In addition, a further 300,000 common shares were agreed to be issued under the Technology License Agreement dated May 5, 2008 between Pagic LP, West Peak Ventures of Canada Ltd., and the Company. These shares were issued subsequent to the year ended March 31, 2008. |
| | |
| (2) | Mr. F. George Orr was appointed Secretary and a director of the Board, and, in November of 2005, he was appointed Chief Financial Officer of Valcent Products Inc. He performs these duties as an independent consultant. |
| | |
| (3) | Resigned, effective July 29, 2008. Amounts were paid for advertising services performed to Sanders Wingo, a company with a common director. |
| | |
| (4) | Mr. Gerry Jardine, a director of the Company received amounts owing to consulting services and investor relations services to a related company. During the year, he also received 300,000 share options exercisable at US$0.70 per share until October 10, 2011 that were fully vested in the period ended March 31, 2008. |
| | |
| (5) | Mr. Naveen Aggarwal received amounts for consulting services performed. |
| | |
| (6) | During the year, Mr. Stapleton received 300,000 share options exercisable at US$0.70 per share until October 10, 2011 that were fully vested in the period ended March 31, 2008. |
In accordance with Section 106(3) of the Business Corporations Act (Alberta), each of our directors holds office for a term of one year or until their reappointment, unless otherwise removed or resigns, with such term expiring at the succeeding annual shareholders’ meeting. Officers are appointed by, and serve at the discretion of, our board of directors.
We currently have no arrangements in place for the provision of benefits upon the termination of our directors or officers.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES - continued
BOARD PRACTICES - continued
Board of Director Committees
We have one standing committee, the audit committee, comprised of members of our board of directors. Our audit committee was formed in 1996 and in March 2005 adopted a formal written charter. The current members of our audit committee include Gerry Jardine, Naveen Aggarwal, and F. George Orr, and we presently have, and will continue to have, one member, F. George Orr, who is a “financial expert” (as defined in accordance with Item 16A(b) of Form 20-F). Our audit committee meets with our external auditors annually, prior to completion of our audited financial statements, and with our management regularly throughout the fiscal year.
We have no formal compensation committee.
EMPLOYEES
Valcent Manufacturing Ltd., El Paso. TX. As at September 15, 2008, we had 30 full time employees, and also retained 4 consultants.
Valcent Products EU Limited, Cornwall, England - As at September 15, 2008, we had 9 full time employees/consultants.
Valcent Products Inc., Vancouver, B.C. As at September 15, 2008, we had 11 employees/consultants, all of which provided services on a part time basis.
Our key consultants and primary responsibilities are as follows:
Consultants
M. Glen Kertz, our Chief Executive Officer acting President, Chairman and a member of our board of directors, is located in El Paso, Texas, and is primarily responsible for the direction and management of all of our research and development activities, as well as oversight of all aspects of our operations and manufacturing, potential product development and sales and marketing.
Solid Integrations LLC, a design and manufacturing firm, located in the city of Ciudad Juarez, Chihuahua, Mexico, has been retained as a general and engineering consultant and manager, and will perform all of the manufacturing-related activities for our three lines of potential consumer retail products, as well as to, as needed, design prototypes and production flow processes for current and future potential consumer retail products.
Tim Brock – Mr. Brock holds a B.A. in Economics from the University of British Columbia and has in-depth experience in seed capital funding, initial public offerings and sponsorship on Canadian and American venture stock exchanges.
F. George Orr - Mr. Orr holds a Bachelor of Commerce from St. Mary’s University, Halifax. He is a self-employed Chartered Accountant with over fifteen years of accounting and consulting experience in private and public company administration, governance, audit procedures and reporting requirements. In July of 2005, he was appointed Secretary and a director, and, in November of 2005, he was appointed Chief Financial Officer of Valcent Products Inc.
So as to maintain our competitive position, we may require consultants and other advisors with whom we contract to execute, among other things, non-competition agreements upon commencement of their consulting or advisory relationships. These agreements typically provide that for a reasonable time following the conclusion of the consulting or advisory relationship, such individuals will not engage directly or indirectly in our business or solicit our customers or suppliers.
As of the date of this annual report, we have executed a non-competition agreement with Pagic LP.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES - continued
The following table sets forth, as of September 15, 2008, information regarding the share ownership of our common stock for each of our directors and executive officers serving during the fiscal year ended March 31, 2008. The number of shares beneficially owned is determined in accordance with Rule 13d-3 of the Securities and Exchange Act of 1934, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rule, beneficial ownership includes any shares as to which the shareholder has sole or shared voting power or investment power and also any shares which the shareholder has the right to acquire within 60 days.
| | Amount and Nature of Ownership | | | Percent of Class* | |
| | | | | | | |
| M. Glen Kertz (1) | | | 4,247,000 | | | | 8.52 | % |
| | | | | | | | | |
| F. George Orr (2) | | | 3,239,914 | | | | 6.32 | % |
| | | | | | | | | |
| Robert Wingo (3) (resigned July 29, 2008) | | | 375,000 | | | | ** | % |
| | | | | | | | | |
| Gerry Jardine (4) | | | 302,450 | | | | ** | % |
| | | | | | | | | |
| Naveen Aggarwal (5) | | | 1,000,000 | | | | 1.99 | % |
| | | | | | | | | |
| George Stapleton (6) | | | 1,132,924 | | | | 2.24 | % |
| | | | | | | | | |
All officers and directors as a group (6 persons) | | | 10,297,288 | | | | 19.44 | % |
| * | No director or executive officer has different voting rights from any other holder of our common stock. Based on 49,856,981 shares issued and outstanding at September 15, 2008. |
| | |
| ** | Beneficially owns less than one percent. |
| | |
| (1) | Consists of 4,247,000 shares of common stock beneficially owned through Pagic LP, previously MK Enterprises LLC. Voting and/or investment power over all of these securities is held by M. Glen Kertz. |
| | |
| (2) | Consists of (i) 950,000 shares of common stock directly owned, (ii) 778,205 unregistered shares of common stock beneficially owned through this director’s spouse, (iii) 120,401 shares of common stock and 60,200 warrants exercisable at US$0.75 per share until December 18, 2009 issued pursuant to private placement of units at US$0.60 per share all directly owned, (iv) 777,696 shares issuable on conversion of US$168,000 in convertible notes and interest accrued to September 15, 2008 of US$2,619 with interest and principal assumed converted according to the terms of the convertible note at a 30% discount to the 4 lowest volume weighted average bid prices of the stock preceding the election to convert with a share price market value assumption of US$0.55 per share, 368,941 warrants exercisable at US$0.55 until July 21, 2013, and 184,471 warrants exercisable at US$0.75 until July 21, 2013. |
| | |
| (3) | Consists of 300,000 fully vested stock options with exercise price of US$0.55 until December 13, 2011 issued under our 2006 Stock Option Plan directly owned, and (ii) 75,000 fully vested stock options with exercise price of US$1.00 until March 1, 2010 issued under our 2006 Stock Option Plan directly owned by Sanders Wingo, a company with this director in common. |
| | |
| (4) | Consists of (i) 300,000 fully vested stock options with exercise price of US$0.70 until October 10, 2011 under our 2006 Stock Option Plan directly owned, and (ii) 2,450 trading shares purchased in market transactions. |
| | |
| (5) | Consists of (i) 300,000 fully vested stock options with exercise price of US$0.55 until December 13, 2011 issued under our 2006 Stock Option Plan directly owned, and (ii) 700,000 common shares of common stock beneficially owned through a spouse. |
| | |
| (6) | Consists of (i) 300,000 fully vested stock options with exercise price of US$0.70 and an expiration date of October 11, 2011 issued under our 2006 Stock Option Plan directly owned, and (ii) 300,000 shares of common stock and 150,000 warrants exercisable at US$0.75 per share issued pursuant to private placement of units at US$0.60 per share in a private company affiliated with this director, (iii) 89,447 trading shares purchased in market transactions, (iv) 214,967 shares issuable on conversion of US$46,141 in convertible notes and interest accrued to September 15, 2008 of US$2,619 with interest converted at US$0.50 per share, and principal assumed converted according to the terms of the convertible note at a 30% discount to the 4 lowest volume weighted average bid prices of the stock preceding the election to convert with a share price market value assumption of US$0.55 per share, 36,713 warrants exercisable at US$0.40 until April 6, 2009, 36,713 warrants exercisable at US$0.90 until April 6, 2009, and 5,084 warrants exercisable at US$0.65 until April 6, 2009. |
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES - continued
On December 14, 2006, the Company replaced its existing Canadian and US stock option plans with a new single stock option plan (the “2006 Stock Option Plan”). The 2006 Stock Option Plan was adopted by the Board of Directors on December 16, 2006 and adopted as amended by the Shareholders of the Company on February 15, 2008. The 2006 Stock Option Plan allows for share options to be issued to Company employees, directors, officers, and consultants on both a qualified and non-qualified basis. The aggregate number of shares of Common Stock as to which options and bonuses may be granted from time to time under the Plan shall not exceed 20% (the “Plan Maximum”) of the Company’s issued and outstanding shares of Common Stock. In addition, the aggregate number of shares of Common Stock to which Incentive Stock Options may be granted shall not exceed 17% of the Company’s issued and outstanding shares of Common Stock . The plan is designed to encourage our directors, executive officers, consultants and other key employees to acquire a proprietary interest in the Company. Our Stock Option is Plan is administered by the Board of Directors, or a committee designated thereby, and reserve for issuance thereunder, in the aggregate, a total of 20% of our issued and outstanding common shares, on a non-diluted basis, to be increased or decreased as the number of our issued and outstanding shares change. Our Stock Option Plans provide that the terms of the options and the option prices shall be fixed by the Board or committee and subject to the requirements of the exchange on which our common shares are traded, or any other governing regulatory body, at the time of exercise. Options granted shall expire after a period of five years or terminate three months after the recipient ceases to be our employee.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
MAJOR SHAREHOLDERS
As of March 31, 2007, there were 44,276,321 common shares issued and outstanding. The table below sets forth certain information, to the extent known or reasonably ascertainable, regarding our major shareholders, including beneficial owners of more than 5% of our common stock, as of September 15, 2008. The number of shares beneficially owned is determined in accordance with Rule 13d-3 of the Securities and Exchange Act of 1934, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rule, beneficial ownership includes any shares as to which the shareholder has sole or shared voting power or investment power and also any shares which the shareholder has the right to acquire within 60 days.
| | | Shares Beneficially Owned |
| | | |
| Major Shareholders | | Number | | | Percent |
| | | | | | |
| Pagic LP (1) | | | 4,247,000 | | | | 8.52 | % |
| | | | | | | | | |
| Larry Thompson (2) | | | 5,500,000 | | | | 10.71 | % |
| | | | | | | | | |
| F. George Orr (3) | | | 3,239,914 | | | | 6.32 | % |
| | | | | | | | | |
| Agosto Corporation Limited (4) (6) | | | 7,949,020 | | | | 13.63 | % |
| | | | | | | | | |
| Woodburn Holdings Ltd. (5) (6) | | | 4,756,833 | | | | 8.84 | % |
| | | | | | | | | |
| Platinum Long Term Growth VI, LLC (7) (6) | | | 9,058,823 | | | | 15.38 | % |
| | | | | | | | | |
| Total | | | 34,751,590 | | | | 47.14 | % |
| | | | | | | | | |
| Total Shares outstanding at September 15, 2008 | | | 49,856,981 | | | | | |
| (1) | Includes 3,947,000 shares of common stock issued as of August 11, 2005, and 300,000 shares of common stock issued on August 18, 2008 pursuant to a further Technology License Agreement between Pagic LP, West Peak Ventures of Canada Ltd, and Pagic LP. Voting and/or investment power over these common shares is held by M. Glen Kertz of Pagic LP. |
| | |
| (2) | Consists of (i) 4,000,000 shares of common stock directly owned, (ii) 250,000 warrants to purchase common shares at US$0.75 per share until December 18, 2009, and (iii) 1,250,000 warrants to purchase commons shares at US$0.75 per share until May 1, 2010. |
| | |
| (3) | Consists of (i) 950,000 shares of common stock directly owned, (ii) 778,205 unregistered shares of common stock beneficially owned through this director’s spouse, (iii) 120,401 shares of common stock and 60,200 warrants exercisable at US$0.75 per share until December 18, 2009 issued pursuant to private placement of units at US$0.60 per share all directly owned, (iv) 777,696 shares issuable on conversion of US$168,000 in convertible notes and interest accrued to September 15, 2008 of US$2,619 with interest and principal assumed converted according to the terms of the convertible note at a 30% discount to the 4 lowest volume weighted average bid prices of the stock preceding the election to convert with a share price market value assumption of US$0.55 per share, 368,941 warrants exercisable at US$0.55 until July 21, 2013, and 184,471 warrants exercisable at US$0.75 until July 21, 2013. |
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS - continued
MAJOR SHAREHOLDERS - continued
| (4) | Consists of convertible debentures for stated values as follows with conversion figures (if applicable) based on a weighted average price of US$0.55 per share: (i) US$235,000 6% convertible note with accrued interest of US$22,599 at $US0.50 per unit convertible into 515,197 common shares and 515,197 warrants to purchase common shares at US$0.70 per share until December 11, 2008; (ii) US$850,000 8% (15% after December 1, 2007) convertible note with interest accrued to September 15, 2008 of US$165,459 convertible at the lesser of US$0.55 per share and 70% of the average five lowest closing bid prices for the ten trading days preceding conversion (market value of shares assumed US$0.55 for purposes herein) resulting in 2,603,645 potential common shares on conversion, and includes 1,333,333 Class A warrants convertible into common shares at US$0.50 per share until December 1, 2009 and 1,333,333 Class B warrants convertible into common shares at US$1.00 per share until December 1, 2009; (iii) $650,000 6% (15% after November 14, 2007) convertible term note with accrued interest to September 15, 2008 of US$89,326 convertible into units at the US$0.60 per unit, resulting in 1,232,210 common shares and 616,105 share purchase warrants exercisable at US$0.75 for a 3 year term from the date of conversion, and (iv) 100,000 common shares issued in connection with a debt agreement pertaining to bonus interest. Voting and/or investment power over these common shares is held by Dr. J. Gordon Murphy, a resident of Barbados. Dr. Murphy beneficially owns 2,000,000 shares in the capital of the Company but such shares are not included in the shares reported for Agosto Corporation Limited in the above table. |
| | |
| (5) | Consists of (i) 762,750 shares of common stock directly, (ii) 38,000 shares of common stock owned in the name of the Robert Baker, the President of Woodburn Holdings Ltd., and (iii) US$650,000 8% (15% after December 1, 2007) convertible note with interest accrued to September 15, 2008 of US$126,527 convertible at the lesser of US$0.55 per share and 70% of the average five lowest closing bid prices for the ten trading days preceding conversion (market value of shares assumed US$0.55 for purposes herein) resulting in 2,222,751 potential common shares on conversion, and includes 866,666 Class A warrants convertible into common shares at US$0.50 per share until December 1, 2009 and 866,666 Class B warrants convertible into common shares at US$1.00 per share until December 1, 2009. Voting and/or investment power over these common shares is held by Robert Baker of Vancouver, British Columbia. |
| | |
| (6) | This shareholder has contractually agreed with the Company to not convert any portion of certain notes or the exercise of certain warrants that would provide this shareholder with an equity interest in the Company of more than 9.99% of the Company’s issued and outstanding capital at any date while this convertible note is outstanding. |
| | |
(7) | The subject common shares are issuable upon conversion of a US$1,848,000 principal amount zero coupon senior secured convertible promissory note. The note may be converted into common shares at any time at a conversion price of US$0.51 per share. However, after January 16, 2009, the conversion price of the Note will equal the lesser of US$0.51 (unless the conversion price has been adjusted as provided in the Note) and 70% of the average of the five lowest closing bid prices of the common shares for the ten trading days prior to the conversion date. The Company also issued 3,623,529 non-redeemable warrants exercisable at US$0.55 until July 20, 2013 and 1,811,765 redeemable warrants exercisable at US$0.75 until July 20, 2013. |
At of September 9, 2008, we had one hundred seventy seven registered (177) shareholders of record holding 49,307,291 shares of common stock. United States shareholders held 22,571,703 shares of our common stock, representing approximately 45.8% of the issued and outstanding shares of our common stock. On September 15, 2008, pursuant to a convertible debenture conversion, 549,690 total shares were issued in exchange for principal and interest to a Canadian investor bringing total issued and outstanding shares to 49,856,981.
We are not directly or indirectly owned or controlled by another corporation, by any foreign government or by another natural or legal person, severally or jointly and we have no knowledge of any arrangements, the operation of which may at a subsequent date result in a change in our control.
RELATED PARTY TRANSACTIONS
The following information is provided in connection with all transactions or loans dating back to April 1, 2007 between us and (a) enterprises that directly or indirectly through one or more intermediaries, control or are controlled by, or are under common control with, us, (b) associates, (c) individuals owning, directly or indirectly, securities representing an interest in our voting power that gives them significant influence over us, and close members of any such individuals’ families, (d) key management personnel including those persons having authority and responsibility for planning, directing, and controlling our activities, such as directors and senior management and close members of any such individuals’ families, and (e) enterprises in which a substantial interest in the voting power is owned, directly or indirectly, by any person described in (c) or (d) or over which such a person is able to exercise significant influence:
Related party transactions are in the ordinary course of business and are measured at the exchange amount at the time the agreement is entered into or services are provided. Due to related parties includes the following amounts in respect to certain of the following transactions as at March 31 not otherwise noted:
| | | | 2008 | | | 2007 | |
| (a) | Pagic royalties, etc. Pagic is a company related by a common director, M. Glen Kertz, our President and director | | $ | 189,156 | | | $ | 0 | |
| (b) | CFO charges and advances. George Orr is our CFO and director. | | | 158,370 | | | | 43,500 | |
| (b) | Consulting services and unsecured loan advances | | | 73,896 | | | | 57,364 | |
| (c) | Consulting services and unsecured loan advances due to West Peak and its principal shareholder, Tim Brock, a beneficial owner of more that 5% of the Company’s common shares | | | 1,000,165 | | | | 784,362 | |
| (d) | Advertising due to Sanders Wingo, with our ex-director, Robert Wingo in common | | | 52,145 | | | | 17,319 | |
| (e) and (f) | Operational management consulting due to our directors, Naveen Aggarwal and Gerry Jardine | | | 16,784 | | | | 27,630 | |
| | | | $ | 1,490,516 | | | $ | 930,175 | |
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS - continued
RELATED PARTY TRANSACTIONS - continued
Related party transactions are in the ordinary course of business and are measured at the exchange amount. Amounts due to related parties are non-interest bearing and have no specific terms of repayment. Related party transactions not disclosed elsewhere in these financial statements are as follows:
| | | | | 2008 | | | 2007 | |
| (a) | Charges from Pagic, a company related by a common director, Glen Kertz, our President and director, for product development expenses including royalties | | $ | 565,135 | | | $ | 269,455 | |
| (b) | Charges from our CFO and director, George Orr for professional fees | | $ | 35,370 | | | $ | 33,000 | |
| | Short-term advance from CFO and director, George Orr, at 10% interest per annum | | $ | 150,000 | | | $ | 0 | |
| | Charges from private companies with this director (George Orr) in common for: | | | | | | | | |
| | (i) | Office rent paid to Sweetwater Capital Corp. | | $ | 30,000 | | | $ | 7,500 | |
| | (ii) | Consulting fee paid to Sweetwater Capital Corp. | | $ | 150,000 | | | $ | 46,745 | |
| | (iii) | Unsecured loan advances from Savi Product Marketing Group Inc. | | $ | 57,364 | | | $ | 57,364 | |
| (c) | West Peak and its principal shareholder, Tim Brock, a beneficial owner of more that 5% of the Company’s shares: | | | | | | | | |
| | (i) | The Company received unsecured loan advances, at 8% interest per annum | | $ | 977,665 | | | $ | 784,837 | |
| | (ii) | Consulting services paid to West Peak | | $ | 52,500 | | | $ | 0 | |
| (d) | Advertising services of a private company, Sanders Wingo, with our ex-director, Robert Wingo in common | | $ | 244,861 | | | $ | 337,749 | |
| (e) | Operational management consulting services of our director, Naveen Aggawal | | $ | 76,740 | | | $ | 0 | |
| (f) | Operational management consulting and investor relations services of our director, Gerry Jardine and a related company providing investor relations services | | $ | 75,085 | | | $ | 0 | |
| (g) | Bonus of 100,000 restricted common shares to a retiring director, Carlton Parfitt | | $ | 0 | | | $ | 52,889 | |
Pagic LP License and Related Transactions
On July 29, 2005, we entered into a series of related definitive agreements with Pagic LP, formerly MK Enterprises LLC (“Pagic”), an entity controlled by M. Glen Kertz, our current Chief Executive Officer, acting President, Chairman and a member of our board of directors, including:
| | (i) | a master license agreement for a term continuing so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to three unrelated and proprietary potential consumer retail products that had previously been developed (the “Pagic Master License”), certain of which are patent pending, including the Nova Skin Care System, the Dust Wolf™, and the Tomorrow Garden™ Kit (collectively, and together with any improvements thereon, the “Initial Products”) each of which are described in fuller detail elsewhere in this annual report. See “Business—Potential Products” and “Business—Intellectual Property”; |
| | | |
| | (vi) | the Pagic Master License also includes a license for a term continuing so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to any ancillary products developed and sold for use by consumers in connection with the Initial Products (the “Initial Ancillaries”); |
| | | |
| | (vii) | a product development agreement pursuant to which we were granted a right for an initial period of five years to acquire a license for a term continuing so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to any new products developed by Pagic (any such products, collectively, the “Additional Products”, and, the agreement itself, the “Pagic Product Development Agreement”); |
| | | |
| | (viii) | the Pagic Product Development Agreement also includes a license for a term continuing so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to any ancillary products developed and sold for use by consumers in connection with the Additional Products (the “Additional Ancillaries”); and |
| | | |
| | (ix) | a related services agreement pursuant to which Pagic shall provide consulting support in connection with the Initial Products, the Initial Ancillaries, the Additional Products and the Additional Ancillaries (the “Pagic Consulting Agreement”), in exchange for the following: |
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS - continued
RELATED PARTY TRANSACTIONS - continued
| | • | 20,000,000 shares of our common stock; |
| | | |
| | • | a one-time US$125,000 license fee; |
| | | |
| | • | reimbursement for US$125,000 in development costs associated with each of the Initial Products since March 17, 2005; |
| | | |
| | • | consulting fees of US$156,000 per year, payable monthly in advance; and |
| | | |
| | • | the greater of the following, payable annually beginning in the second license year: |
| | (i) | US$400,000; or |
| | | |
| | (ii) | the aggregate of the following: |
| | • | subject to a minimum amount of US$37,500 per Initial Product during the second year of the Pagic Master License, and US$50,000 each year thereafter, continuing royalties payable quarterly at a rate of: |
| | | US$10.00 per Nova Skin Care System unit sold; |
| | | |
| | | US$2.00 per Dust Wolf™ unit sold; |
| | | |
| | | 4.5% of annual net sales of the Tomorrow Garden™ Kit; |
| | | |
| | and | |
| | | |
| | | 3% of annual net sales of Initial Ancillaries. |
| | • | a one-time US$50,000 license fee for each Additional Product licensed (except for one pre-identified product); and |
| | | |
| | • | subject to a minimum amount of US$50,000 per year commencing with the second year of each corresponding license, continuing royalties of 4.5% of annual net sales and 3% on annual net sales of any Additional Ancillaries. |
Although Mr. Kertz was neither an officer nor a director at the time we entered into the Pagic Agreements, he was both an officer and a director prior to July 2004 and assumed his current positions immediately following our entering into these agreements. During the year ended March 31, 2006, we paid approximately $121,447 in product development fees to Pagic respectfully. Although we believe that the terms of the Pagic Agreements, and the fees paid to Pagic thereunder, are as fair as that which could have been obtained in an arms length transaction with unaffiliated third parties, the conflict of interest which existed as a result of Mr. Kertz’s prior involvement with us gives rise to the potential that the contractual terms may be less favorable to us than would otherwise have been the case.
Global Green Joint Venture
On October 2, 2006, the Company entered into a letter agreement replaced on July 9, 2007 by the Vertigro Algae Stakeholders Letter of Agreement, (together “LOA”) with Pagic, West Peak Ventures of Canada Limited (“West Peak”) and Global Green, whereby Global Green agreed to fund the next phase of the development of a high density vertical bio-reactor technology. Pursuant to the LOA, the Company and Global Green established a commercial joint venture named “Vertigro” in which Global Green agreed to provide up to US$3,000,000 in initial funding to continue the research and development of the bio-reactor technology, construct a working prototype of the bio-reactor and develop the technology for commercial uses. The Company is obligated to provide product support, research and development, and the non-exclusive use of the Company’s properties and lands for which Global Green has agreed to reimburse the Company as part of its US$3,000,000 initial funding commitment. Until such time as the joint venture has fully repaid to Global Green the US$3,000,000, Global Green shall receive 70% of the net cash flow generated by anticipated future operations after which each of Global Green and the Company will hold a 50% interest in the Vertigro, subject to an aggregate 4.5% royalty to Pagic and West Peak. Vertigro covers the bio-reactor technology and any subsequent related technologies for the commercial scale products of algae based biomass for all industrial commercial and retail applications including, but not limited to bio-fuel, food, health, pharmaceutical, and animal and agricultural feeds.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS - continued
RELATED PARTY TRANSACTIONS - continued
As at March 31, 2008, Global Green and the Company incurred a total of US$5,735,893 (2007 - US$2,023,379) in costs related to Vertigro. Of the costs incurred to March 31, 2008, Global Green paid US$4,338,204 (2007 - US$1,653,981) of which US$7,806 (2007 - US$369,398) is included in accounts payable.
Global Green and the Company have completed negotiations and documentation of a formal business arrangements to streamline and enhance Vertigro operating and commercial development systems via a Limited Liability Company Operating Agreement discussed more fully below, and has incorporated a business entity to advance further project development. As a result, the Company formed Vertigro Algae Technologies, LLC under the Texas Business Organizations with Global Green, and other related parties to the Vertigro joint venture. The members of the new Texas LLC are Global Green and the Company, each holding 3 million units. It is anticipated that the Vertigro Algae Technologies, LLC will continue to conduct research and development of the Vertigro growing systems, the development of a commercial scale build out of an operating unit in conjunction with increasing the yield per acre of the algae oil produced by the system.
In addition, the Company is developing extraction technologies from research and development being conducted in conjunction with certain US based university interests specializing in process extraction.
Limited Liability Company Operating Agreement for Vertigro Algae Technologies, LLC
On May 5, 2008, Valcent USA and Global Green signed a Limited Liability Company Operating Agreement (“New Operating Agreement”) replacing the LOA to form Vertigro Algae Technologies, LLC (“Vertigro Algae”), a Texas LLC, which is the formalized operating entity for the previous unincorporated operations of Vertigro. The business activities of Vertigro Algae will be the same as when the operations were under Vertigro. Under the New Operating Agreement, Global Green and Valcent will each hold a 50% stake in Vertigro Algae, and have committed to fund project development according to ownership allocation. Further, Valcent will acquire assets of Vertigro, including buildings, laboratory, and equipment. To allow for prior capital contributions, Global Green has incurred in excess of the Company’s prior aggregate capital contribution, Global Green will receive 70% of the net cash flow generated by Vertigro Algae until it has received US$3,000,000 in excess of its 50% interest in such cash flow.
Technology License Agreement
On May 7, 2008, effective May 5, 2008, Vertigo Algae executed a Technology License Agreement together with Pagic LP, West Peak Ventures of Canada Ltd., and Valcent Products, Inc. The Technology License Agreement licenses certain Algae Biomass Technology and Intellectual Property to Vertigo Algae for purposes of commercialization and exploitation for all industrial, commercial, and retail applications world-wide (“Algae Biomass Technology”). In return for the Algae Biomass Technology, both Valcent Products, Inc. and Global Green will each provide 300,000 restricted common shares to Pagic LP., and also pay a one time commercialization fee of US$50,000 upon the Algae Biomass Technology achieving commercial viability. The Technology License Agreement is subject to royalty of 4.5% of gross customer sales receipts for use of the Algae Biomass Technology; aggregate annual royalty minimum amounts are US$50,000 in 2009, US$100,000 in 2010, and US$250,000 in 2011 and each year thereafter in which the Technology License Agreement is in place.
All prior agreements between the Company and Pagic that relate to the Algae Biomass Technology will be replaced by the new Operating Agreement and the Technology License.
INTERESTS OF EXPERTS AND COUNSEL
Not applicable.
ITEM 8. FINANCIAL INFORMATION
CONSOLIDATED STATEMENTS AND OTHER FINANCIAL INFORMATION
See Item 17 and our financial statements and accompanying notes beginning on page F-1 of this Form 20-F.
Legal proceedings
There are no pending, and we are not aware of any contemplated, legal, governmental or arbitration proceedings, including those related to bankruptcy, receivership or those involving a third party which have, or may have, significant effects on our financial position or profitability.
ITEM 8. FINANCIAL INFORMATION - continued
CONSOLIDATED STATEMENTS AND OTHER FINANCIAL INFORMATION - continued
Dividend Policy
We have never paid a dividend and have no intentions of doing so in the foreseeable future.
SIGNIFICANT CHANGES
| | • | To retire certain short-term obligations and existing liabilities and to provide additional general corporate and working capital to pursue our business plan, on April 6, 2006, we consummated a follow-on private offering transaction with and among a syndicated group of institutional investors pursuant to which we issued, in the aggregate, US$551,666 in 8% per annum convertible notes and three-year warrants to acquire (i) up to 735,544 shares of our common stock at a price per share of US$0.40, and (ii) up to an additional 735,544 shares of our common stock at a price per share of US$0.90. Subject to certain limitations, the principal amount of the notes, together with any accrued interest may be converted into shares of our common stock at the lesser of (i) 70% of the average of the five lowest closing bid prices for our common stock for the ten trading days prior to conversion, or (ii) US$0.55. The convertible notes carry a redemption feature which allows us to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that our common shares have a closing price of US$1.50 per share for at least twenty consecutive trading days and there has otherwise been no default. The common stock purchase warrants carry a “net cashless” exercise feature allowing the holder thereof, under certain limited circumstances, to exercise the warrants without payment of the stated exercise price, but rather solely in exchange for the cancellation of that number of common shares into which such warrants are exercisable. |
| | | |
| | • | Also in connection with the follow-on private offering transaction, we issued 183,886 shares of our common stock and three-year warrants to purchase up to an additional 110,320 shares of our common stock at a price per share of US$0.50 and up to an additional 183,867 shares of our common stock at a price of US$0.75 as partial compensation in the form of a finder’s fee. |
| | | |
| | • | Our registration statement on Form F-1 was originally filed with the SEC on April 27, 2006, which delay resulted in a reduction in the exercise price of the Class A and Class B warrants issued as part of the July 25, 2005 through August 5, 2005 and April 6, 2006 transactions from US$0.50 and US$1.00 to US$0.40 and US$0.90, respectively. Moreover, as of the date of this annual report we are accruing penalties at a rate of 2% per thirty day period for having failed to have our registration statement declared effective by June 5, 2006. As of September 13, 2006, we have accrued up to approximately US$123,094 in penalties under the terms of the registration provisions of each of the July 25, 2005 through August 5, 2005 and April 6, 2006 transactions, exclusive of those penalties previously converted into convertible penalty notes and warrants. The penalties which we have accumulated to date, and any penalties which we may accumulate for future delays, will negatively affect our business, our financial condition and our results of operations, leading to a corresponding reduction in our net income and the likelihood of a net loss for the year in which they are incurred. The delays in meeting our registration obligations are a consequence, among other reasons, of ongoing delays resulting from our having recently undergone a significant restructuring and change in business direction, and the unavailability of certain information necessary for preparing the filing. Our management is hopeful that we will cause our registration statement to be declared effective in the near future. |
| | | |
| | • | In connection with the July 25, 2005 through August 5, 2005 transactions, on April 6, 2006 we reached a verbal agreement with the syndicated group of institutional and other investors, to convert accruing penalties associated with delays in our filing of a registration statement under the terms of the transactions, an aggregate of US$82,200, into convertible penalty notes carrying terms similar to those notes issued in the original series of private offering transactions, and in addition to issue each investor one three-year penalty warrant for each US$0.75 of penalties owed to purchase, in the aggregate, up to an additional 109,600 shares of common stock at a price per share of US$0.75. |
| | | |
| | • | Pursuant to an Investor Relations Consulting Agreement with a third party for services provided to Valcent, SmallCap Partners Ltd. received 120,000 shares of common stock at US$0.41 per share. |
| | | |
| | • | On May 31, 2006, the Company issued 786,700 shares pursuant to the license agreement. As of June 30, 2006, 7,601,325 common shares remained to be issued pursuant to the license agreement and are reflected in share subscription. |
| | | |
| | • | On May 15, 2006, the Company completed a private placement of units whereby it issued a total of 1,104,165 units at US$0.60 per unit whereby each unit consisted of one common share and one share purchase warrant to purchase an additional common share at US$0.80. Of the warrants issued, 833,332 expire on May 15, 2008 and 270,833 on June 7, 2008. The Company also paid consultants fees consisting of $58,860 plus 66,666 warrants to purchase that number of common shares at US$0.80 until May 15, 2008 and 21,666 warrants to purchase that number of common shares at US$0.80 until June 7, 2008.· |
| | | |
| | • | On August 18, 2006, the Company completed a private placement of units whereby it issued a total of 430,000 units at US$0.60 per unit for a total of US$258,000 whereby each unit consisted of one common share and one share purchase warrant to purchase an additional common share at US$0.80. The warrants issued will expire on August 18, 2008. The Company paid consultants fees consisting of $58,860 plus 24,800 warrants to purchase that number of common shares at US$0.80 until August 18, 2008. |
| | | |
| | • | We entered into a manufacturing agreement with Solid Integrations, LLC, for the manufacture and assembly of our Nova Skin Care System. Under this agreement we will provide and make available to Solid Integrations, LLC, and its subcontractor(s), the raw materials, machinery, equipment, tooling and molds necessary for Solid Integrations, LLC, and its subcontractor(s), to manufacture and assemble the Nova Skin Care System at a manufacturing facility located in Mexico. |
| | | |
| | • | During the year ended March 31, 2007, $1,491,497 of the convertible note principal and interest were converted into 3,967,157 common shares. |
ITEM 8. FINANCIAL INFORMATION - continued
SIGNIFICANT CHANGES - continued
| | • | During the quarter ended December 31, 2006, pursuant to Regulation S of the Securities Act, the Company received subscriptions of US$1,500,000 from a group of non-US accredited investors towards a private placement of 8% per annum convertible notes and three year warrants to acquire (i) up to 2,000,000 shares of our common stock at a price per share of US$0.50, and (ii) up to an additional 2,000,000 shares of our common stock at a price per share of US$1.00. Subject to certain limitations, the principal amount of the notes, together with any accrued interest may be converted into shares of our common stock at the lesser of (i) seventy percent (70%) of the average of the five lowest closing bid prices for our common stock for the ten trading days prior to conversion, or (ii) US$0.55. The convertible notes carry a redemption feature which allows us to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that our common shares have a closing price of US$1.50 per share for at least twenty consecutive trading days and there has otherwise been no default. The common stock purchase warrants carry a “net cashless” exercise feature allowing the holder thereof, under certain limited circumstances, to exercise the warrants without payment of the stated exercise price, but rather solely in exchange for the cancellation of that number of common shares into which such warrants are exercisable. |
| | | |
| | • | On January 12, 2007, pursuant to an Investor Relations Consulting Agreement with a third party for services provided to the Company, SmallCap Corporate Partners Inc. received a total of 160,000 shares of common stock at US$0.45 per share. |
| | | |
| | • | On January 12, 2007, pursuant to a Public Relations Agreement with a third party for services provided to Valcent, Vorticom Inc. received a total of 25,000 shares of restricted common stock and will receive 25,000 shares of stock quarterly for the twelve month term following the date of the agreement. |
| | | |
| | • | On January 12, 2007, the Board of Directors authorized a bonus of 100,000 restricted common shares to a retiring director of the Company. |
| | | |
| | • | On January 19, 2007, pursuant to a Consulting Services Agreement with a third party for services provided to the Company, PowerOne Capital Markets Limited received a total of 400,000 shares of common stock. |
| | | |
| | • | On January 29, 2007, the Company completed a private placement comprised of $2,000,000 convertible promissory notes with a group of non-US accredited investors, pursuant to Regulation S of the Securities Act. The convertible notes will mature on December 11, 2008 and carry interest at six percent (6%) per annum. The Notes are convertible into “Units” at the Note Holder’s discretion at a conversion price of US$0.50 per Unit. Each “Unit” consists of one Valcent common share and one purchase warrant to purchase an additional common share at US$0.70 per share until December 11, 2008. The Notes and any accrued interest are callable by the Company at any time after December 11, 2007 by providing thirty days written notice to the Note Holders. Interest on the Notes will be compounded annually and be cumulative until the earlier of either the date that the Company’s achieves pre-tax earnings or the end of the term. Pre-tax earnings are to be determined in accordance with Valcent’s quarterly and annual financial statements as filed with regulatory agencies and will be payable thirty days after filing with such regulatory agencies. At the discretion of the Note Holder, interest on the Notes is payable in either cash or Units at US$0.50 per Unit. The Company is obligated to file a resale registration statement on the underlying securities within four months of closing. |
| | | |
| | • | In connection with finders fees payable under our January 2007 $2,000,000 private placement financing of convertible promissory notes, US$108,000 in cash was paid to PowerOne Capital Markets Limited along with 135,000 warrants issued exercisable at US$0.50 per unit, with each unit consisting of one common share and one share purchase warrant to purchase a further common share at US$0.70 per share until December 11, 2008. |
| | | |
| | • | Global Green Joint Venture On October 2, 2006, the Company entered into a letter agreement with Pagic, West Peak Ventures of Canada Limited (“West Peak”) and Global Green whereby Global Green agreed to fund the next phase of the development of our High Density Vertical Bio-Reactor technology (the “GGS Agreement”). Pursuant to the GGS Agreement, the Company and Global Green established a commercial joint venture named “Vertigro”, in which Global Green has agreed to provide up to US$3,000,000 in funding to continue the research and development of the Bio-Reactor technology, construct a working prototype of the Bio-Reactor and develop the technology for commercial uses. The Company is obligated to provide product support, research and development, and the non-exclusive use of the Company’s warehouse and land near El Paso, Texas, as necessary for which Global Green has agreed to reimburse the Company as part of its US$3,000,000 commitment. Until such time as the joint venture has fully repaid the US$3,000,000, Global Green will have an 80% joint venture interest, leaving the Company with a 20% carried joint venture interest, both subject to an aggregate product license royalty of 0.9% to Pagic and West Peak. Once the joint venture has repaid Global Green the US$3,000,000, Global Green’s interest will be reduced to 70% and the Company will retain a 30% non-carried interest, both subject to an aggregate product license royalty of 4.5% to Pagic and West Peak. As at March 31, 2007, Global Green had incurred a total of US$2,023,379 in costs related to the GGS Agreement. Of the costs incurred to March 31, 2007, Global Green had paid US$1,653,981 and owed the Company $426,507 (US$369,398), which is included in accounts receivable and has been subsequently collected. On July 9, 2007, the parties to the GGS Agreement entered into the Vertigro Algae Stakeholders Letter of Agreement (the “Global Green Joint Venture”), which replaced the GGS Agreement. Pursuant to the new agreement, each of Global Green and the Company will hold a 50% interest in the Global Green Joint Venture, subject to an aggregate 4.5% royalty to Pagic and West Peak. The Global Green Joint Venture covers the Bio-Reactor and any subsequent related technologies for the commercial scale products of algae based biomass for all industrial commercial and retail applications including but not limited to bio-fuel, food, health, pharmaceutical, animal and agricultural feeds. |
ITEM 8. FINANCIAL INFORMATION - continued
SIGNIFICANT CHANGES - continued
| | • | During the year ended March 31, 2007, the Company entered into a ten year commercial real estate note totaling US$190,000 to fund the acquisition of land. The loan is secured by the land and a US$50,000 term deposit which is included in the prepaid expenses. Borrowings under the Agreement accrue interest equal to the Wall Street Journal prime rate plus 0.25 percent adjusted annually. The current interest rate is 8% per annum and the current portion of the loan is $14,041. |
| | | |
| | • | During the year ended March 31, 2007, the Company issued 8,388,025 restricted common shares as the remaining shares owed in conjunction with the Licensing Agreement with Pagic LP (formerly MK Enterprises LLC). The Company on July 29, 2005, agreed to issue Pagic and its assigns 20 million common shares at a deemed cost of $1,306,075, based on the historical cost of the license, of which 11,611,975 shares were issued during the year ended March 31, 2006 and the balance during the year ended March 31, 2007. Included in the 20 million shares issued for the license were 9,428,205 shares that were issued to parties that became related parties to the Company. |
| | | |
| | • | On April 1, 2007, the Company entered into an agreement with a third party to provide investor relations and financial services for an eight month term from April 1, 2007 through December 31, 2007. The agreement provides for the following: |
| b) | US$3,000 in compensation per month payable quarterly; |
| c) | a further US$2,000 payable monthly in common shares; |
| d) | share options to purchase 500,000 shares at US$0.60 exercise price that vest quarterly over a two year period; and |
| e) | the issuance of 12,500 common shares at a deemed price of US$0.80 per share. |
| | | |
| | • | On August 10, 2007, the Company issued a convertible term promissory note in the amount of $650,000 to a third party. The convertible note is due November 15, 2007 with interest at the rate of 6% with both interest and principal convertible at the option of the lender at the end of term into units at the US$0.60 per unit, with each unit convertible into one common share and one half share purchase warrant with each whole share purchase warrant exercisable at US$0.75 to purchase an additional common shares. The Company is required to register for trading the securities underlying the conversion features of this convertible note by February 10, 2008 on a best efforts basis without penalty. |
| | | |
| | • | During the year ended March 31, 2008, the Company completed a private placement of units whereby a total of 8,382,721 units were issued at US$0.60 per unit for gross proceeds of US$5,029,636. Each unit consists of one common share and one-half common share purchase warrant with each whole warrant exercisable at US$0.75 to purchase an additional common share of the Company. The Company paid US$88,438 cash to consultants in connection with this private placement. |
| | | |
| | • | During the year ended March 31, 2008, the Company completed a private placement of units whereby a total of 142,857 units were issued at US$0.70 per unit for gross proceeds of US$100,000. Each unit consists of one common share and one-half common share purchase warrant with each whole warrant exercisable at US$0.85 to purchase an additional common share of the Company. |
| | | |
| | • | During the year ended March 31, 2008, the Company issued 3,462,008 shares in connection with seven financial consulting services agreements for administrative, investor relations, business and financial consulting services at an average value of US$0.68 per share. |
| | | |
| | • | During the year ended March 31, 2008, the Company issued the following common shares pursuant to the exercise of warrants, options, and the conversion of convertible debt: |
| · | Issued 699,903 common shares on exercise of 699,903 share purchase warrants at an average exercise price of US$0.40 per warrant; |
| · | Issued 175,000 common shares on exercise of 175,000 stock options at an exercise price of US$0.50 per option by a past director of the Company; and |
| · | Issued 747,764 common shares upon receiving conversion notices to convert US$275,000 of convertible notes, interest of US$4,521 and a registration penalty of US$11,668 from holders. |
| | | |
| | • | On May 5, 2008, Valcent USA and Global Green signed a Limited Liability Company Operating Agreement (“New Operating Agreement”) replacing the LOA to form Vertigro Algae Technologies, LLC (“Vertigro Algae”), a Texas LLC, which is the formalized operating entity for the previous unincorporated operations of Vertigro. The business activities of Vertigro Algae will be the same as when the operations were under Vertigro. Under the New Operating Agreement, Global Green and the Company will each hold a 50% stake in Vertigro Algae, and have committed to fund project development according to ownership allocation. Further, the Company will acquire assets of Vertigro, including buildings, laboratory, and equipment. To allow for prior capital contributions, Global Green has incurred in excess of the Company’s prior aggregate capital contribution, Global Green will receive 70% of the net cash flow generated by Vertigro Algae until it has received $3,000,000 in excess of its 50% interest in such cash flow. |
| | | |
| | • | On May 5, 2008, Vertigro Algae executed a Technology License Agreement (“Technology License”) together with Pagic, West Peak and the Company. The Technology License licenses certain algae biomass technology and intellectual property to Vertigro Algae for purposes of commercialization and exploitation for all industrial, commercial, and retail applications worldwide (“Algae Biomass Technology”). In return for the Algae Biomass Technology, both the Company and Global Green will each issue 300,000 common shares to Pagic (unissued), and also pay a one-time commercialization fee of US$50,000 upon the Algae Biomass Technology achieving commercial viability. The Technology License is subject to royalty of 4.5% of gross customer sales receipts for use of the Algae Biomass Technology; and aggregate annual royalty minimum amounts of US$50,000 in 2009, US$100,000 in 2010, and US$250,000 in 2011 and each year thereafter in which the Technology License is in place. |
ITEM 8. FINANCIAL INFORMATION - continued
SIGNIFICANT CHANGES - continued
| | • | All prior agreements between the Company and Pagic that relate to the Algae Biomass Technology will be replaced by the new Operating Agreement and the Technology License. |
| | | |
| | • | On August 10, 2007, the Company issued an unsecured convertible term promissory note in the amount of US$650,000 to a third party. The convertible note is due on demand and bears interest at 6% with both interest and principal convertible at the option of the lender into units at US$0.60 per unit, with each unit consisting of one common share and one-half share purchase warrant with each whole share purchase warrant exercisable at US$0.75 to purchase an additional common share. After November 25, 2008, this convertible note accrues interest at the rate of 15% per annum. A one-time financial penalty of US$28,567 for failure to register the securities underlying this convertible note within 180 days from the date of issuance has been incurred in the year ended March 31, 2008. |
| | | |
| | • | On September 27, 2007, the Company issued unsecured one-year term convertible notes bearing interest at 6% per annum in the amount of US$391,000 to third parties. Both interest and principal may be converted at the option of the lender at any time at US$0.60 per unit, with each unit consisting of one common share and one-half share purchase warrant, with each whole share purchase warrant exercisable at US$0.75 to purchase an additional common share for a two-year term from the date of conversion. A one-time financial penalty of US$23,460 for failure to register the securities underlying this convertible note within 90 days from the date of issuance has been incurred during the year ended March 31, 2008. |
| | | |
| | • | During the year ended March 31, 2008, the Company raised $154,196 from interest bearing promissory notes during the year ended March 31, 2008 for an aggregate outstanding of $270,167 as at March 31, 2008 (2007 - $115,460). |
| | | |
| | • | As a result of the Nova Skin Care System our inventories were $617,195 as at March 31, 2008 (2007 - $1,236,808). The company wrote down raw materials inventories during the year ended March 31, 2008 due to obsolescence. |
| | | |
| | • | We purchased $834,084 (2007 - $310,448) in fixed assets during the year ended March 31, 2008 relating to the development of our various operational divisions located in El Paso, Texas and Cornwall, UK. |
| | | |
| | • | The Company entered into the following agreements on April 1, 2008 and May 1, 2008 respectively: |
| (i) | A financial services agreement with an arm’s length private company for a term of seven months. Pursuant to the agreement, the Company issued 120,000 common shares valued at US$84,000; and |
| (ii) | A financial and business consulting agreement with an individual consultant for a term of twelve months. Pursuant to the agreement, the Company issued 300,000 common shares valued at US$201,000. |
| | | |
| | • | The Company completed a series of US$0.60 per unit private placements. Each unit consisted of one common share and one-half common share purchase warrant with each whole warrant exercisable at US$0.75 per share for two years. The private placements took place on May 7, June 6, and July 23, 2008, respectively, with details as follows: |
| (i) | issued 2,996,666 units for gross proceeds of US$1,798,000. The Company paid US$122,577 in cash consultant’s fees with respect to this issuance; |
| (ii) | issued 195,000 units for gross proceeds of US$117,000. The Company paid no consultant’s fees respecting the issuance; and |
| (iii) | issued 61,000 units for gross proceeds of US$36,600. The Company paid US$2,562 cash in consultant’s fees with respect to this issuance. |
| | | |
| | • | On May 28, 2008, the Company issued 100,000 common shares in relation to a promissory note as part of the note’s interest provisions. |
| | | |
| | • | The Company issued the following common shares in relation to the conversion of certain notes: |
| (i) | On June 2, 2008, the Company issued 245,049 common shares relating to the conversion of US$100,000 in convertible debt and US$9,403 in accumulated interest; and |
| (ii) | On July 3, 2008, the Company issued 267,221 common shares relating to the conversion of US$92,281 in convertible debt and US$9,243 in accumulated interest. |
ITEM 8. FINANCIAL INFORMATION - continued
SIGNIFICANT CHANGES - continued
| | • | On July 21, 2008, the Company closed a financing of zero coupon, 12% interest, senior secured convertible promissory notes in the amount of US$2,428,160 with an aggregate purchase price of US$2,168,000 with four investors, one of which was the Company’s Chief Financial Officer as to US$168,000. The debt is convertible into shares of common stock at the lesser of US$0.51 per share (unless the conversion price has been adjusted pursuant to further contract covenants) and 70% of the average of the five lowest closing bid prices for the ten preceding trading days. The Company issued each purchaser in the private placement two warrants, one warrant being redeemable by the Company and the other being non-redeemable. The non-redeemable warrants are exercisable at US$0.55 and permit the holder to purchase shares of common stock equal to 100% of the number of shares issuable upon the conversion of the notes calculated on July 21, 2008. The redeemable warrants are exercisable at US$0.75 and permit the holder to purchase common stock equal to 50% of the number of shares issuable upon the conversion of the notes issued calculated on the closing date. The Company issued a total of redeemable warrants to purchase an aggregate of 4,761,098 shares of common stock and a total of redeemable warrants to purchase an aggregate of 2,380,550 shares of common stock. Further, the Company issued 439,216 non-redeemable warrants and 219,608 redeemable warrants and $160,000 in cash fees to close the transaction. Each non-redeemable warrant is exercisable at US$0.55, and each redeemable warrant is exercisable at US$0.75; warrants carry a term of five years from the date of closing of the financing. The redeemable warrants may be redeemed by the Company only if certain conditions have been satisfied including the Company’s common stock having closed at $1.50 per share for a period of 20 consecutive trading days and the warrant holder being able to resell the shares acquired upon exercise through a resale registration statement or under Rule 144 of the Securities Act. |
| | | |
| | • | On August 18, 2008, the Company issued 300,000 common shares to Pagic LP in relation to the Technology License agreed to by the Company, Pagic LP, and West Peak Ventures of Canada Ltd. on May 5, 2008. |
| | | |
| | • | On August 18, 2008, the Company issued 383,331 common shares pursuant to the exercise of 383,331 warrants for gross proceeds of US$191,666. |
| | | |
| | • | The Company issued the following common shares in relation to the conversion of certain notes: |
| (i) | On August 18, 2008, the Company issued 62,703 common shares relating to the conversion of US$25,000 in convertible debt and US$6,352 in accumulated interest and registration penalties. The Company also issued as part of converted amounts, 54,550 warrants exercisable at US$0.70 per share until December 11, 2008; |
| (ii) | On September 10, 2008, the Company issued 549,690 common shares relating to the conversion of US$250,000 in convertible debt and US$24,845 in accumulated interest. The Company also issued as part of converted amounts, 549,690 warrants exercisable at US$0.70 per share until December 11, 2008; and |
| (iii) | On September 19, the Company issued 417,322 common shares relating to the conversion of US$175,000 in convertible debt and US$33,662 in accumulated interest and registration penalties. The Company also issued as part of converted amounts, 385,104 warrants exercisable at US$0.70 per share until December 11, 2008. |
ITEM 9. THE OFFER AND LISTING
OFFERING AND LISTING DETAILS
The table below sets forth certain information regarding the price history of our common shares, including (i) annual high and low market prices for each of the five most recent fiscal years, (ii) quarterly high and low market prices for each full quarter of the two most recent fiscal years, and (iii) monthly high and low market prices for each month of the six most recent months. The prices have been adjusted to reflect a one-for-three common share consolidation effective May 3, 2005.
Our common shares are presently quoted on the United States OTC Bulletin Board, under the symbol “VCTPF” (formerly “NTTRF”). Effective May 3, 2005, we changed our name from Nettron.com, Inc. to Valcent Products Inc. and delisted our common shares from the TSX-Venture Exchange, Inc. (formerly The Canadian Venture Exchange), where we had been trading under the symbol “NTT.H”, maintaining only our listing only on the United States OTC Bulletin Board. Prior to our delisting from the TSX-Venture Exchange we had been quoted thereon under the symbol “NTT.H” from August 18, 2003 though May 2, 2005, “NTT.T” from February 18, 2003 to August 17, 2003 and “NTT” prior to February 18, 2003.
ITEM 9. THE OFFER AND LISTING - continued
OFFERING AND LISTING DETAILS - continued
| Period | | | OTC Bulletin Board (USD) | | | TSX Venture Exchange (CND) | |
| | | | High | | | Low | | | High | | | Low | |
| | | | | | | | | | | | | | |
| Fiscal year ended March 31, | | | | | | | | | | | | | |
| 2004 | | | $ | 0.075 | | | $ | 0.0144 | | | $ | 0.090 | | | $ | 0.030 | |
| 2005* | | | $ | 0.600 | | | $ | 0.030 | | | $ | 0.555 | | | $ | — | |
| 2006 | | | $ | 0.950 | | | $ | 0.250 | | | $ | — | | | $ | — | |
| 2007 | | | $ | 0.840 | | | $ | 0.400 | | | $ | — | | | $ | — | |
| 2008 | | | $ | 0.930 | | | $ | 0.450 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | |
| Quarter ended | | | | | | | | | | | | | | | | | |
| September 30, 2006 | | | $ | 0.750 | | | $ | 0.500 | | | $ | — | | | $ | — | |
| December 31, 2006 | | | $ | 0.700 | | | $ | 0.400 | | | $ | — | | | $ | — | |
| March 31, 2007 | | | $ | 0.740 | | | $ | 0.450 | | | $ | — | | | $ | — | |
| June 30, 2007 | | | $ | 0.670 | | | $ | 0.510 | | | $ | — | | | $ | — | |
| September 30, 2007 | | | $ | 0.770 | | | $ | 0.500 | | | $ | — | | | $ | — | |
| December 31, 2007 | | | $ | 0.930 | | | $ | 0.550 | | | $ | — | | | $ | — | |
| March 31, 2008 | | | $ | 0.900 | | | $ | 0.450 | | | $ | — | | | $ | — | |
| June 30, 2008 | | | $ | 0.740 | | | $ | 0.500 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | |
| Month ended | | | | | | | | | | | | | | | | | |
| March 31, 2008 | | | $ | 0.730 | | | $ | 0.450 | | | $ | — | | | $ | — | |
| April 30, 2008 | | | $ | 0.740 | | | $ | 0.670 | | | $ | — | | | $ | — | |
| May 31, 2008 | | | $ | 0.700 | | | $ | 0.550 | | | $ | — | | | $ | — | |
| June 30, 2008 | | | $ | 0.700 | | | $ | 0.500 | | | $ | — | | | $ | — | |
| July 31, 2008 | | | $ | 0.670 | | | $ | 0.480 | | | $ | — | | | $ | — | |
| August 31, 2008 | | | $ | 0.620 | | | $ | 0.480 | | | $ | — | | | $ | — | |
| * | We voluntarily delisted from the TSX Venture Exchange on May 3, 2005. |
MARKETS
At present, and since May 3, 2005, our common shares are quoted solely on the OTC Bulletin Board, under the symbol “VCTPF”.
SHARE CAPITAL
Not applicable.
MEMORANDUM AND ARTICLES OF ASSOCIATION
Organization, Object and Purpose
We were incorporated in Canada on January 19, 1996, in accordance with the provision of the Business Corporations Act (Alberta). Article 5 of our articles of incorporation provides that we are neither restricted from carrying on any business nor required to carry on a certain business.
MEMORANDUM AND ARTICLES OF ASSOCIATION - continued
Directors
Voting Powers
In accordance with Section 4.19 of our bylaws and Section 120 of the Business Corporations Act (Alberta) if one of our directors is party to or has a material interest in a material contract or proposed material contract with us or with one of our subsidiaries, that director must disclose the nature and extent of their interest at the first meeting at which the proposed material contract is considered, or at the first meeting after which the director becomes an interested party, either in writing or, at the director’s request, entered into the meeting minutes. All material contracts or proposed material contracts in which one of our directors has a material interest must be referred to our board or shareholders for approval, even if not one which, in the ordinary course of business, would otherwise require approval. A director who is party to or has a material interest in a material contract or proposed material contract, shall refrain from voting on any resolution to approve the material contract unless it is one for (i) an arrangement by way of security for money lent to or obligations undertaken by the director for our benefit, (ii) a transaction relating primarily to the director’s remuneration, (iii) a contract for indemnity insurance, or (iv) a contract with an affiliate.
In accordance with our bylaws and the Business Corporations Act (Alberta) there are no limitations on the power of our directors, in the absence of an independent quorum, to vote compensation to themselves.
Borrowing Powers
In accordance with Section 3.01 of our bylaws and Section 103 of the Business Corporations Act (Alberta) our directors may, without shareholder authorization, (i) borrow money on our credit, (ii) issue, reissue, sell or pledge debt obligations, (iii) give a guarantee on our behalf to secure performance of an obligation, and (iv) mortgage, hypothecate, pledge or otherwise create a security interest in all or any of our property, owned or subsequently acquired, to secure any of our obligations. These powers can be modified by means of an amendment to our articles of incorporation or bylaws, or by unanimous shareholder consent.
Indemnification of Directors and Officers and Limitation of Liability
In accordance with Section 7 of our bylaws and Section 124 of the Business Corporations Act (Alberta), each of our directors and officers is relieved from liability for the acts, receipts or defaults of any other of our directors, officers or employees, notwithstanding any loss, damage or expense caused to us, provided, however, that such director or officer discharges their own duties honestly, in good faith and with a view toward our best interests, and exercises the care, diligence and skill that a reasonably prudent person would in comparable circumstances.
Subject to certain limitations as set forth in Section 124 of the Business Corporations Act (Alberta), we have undertaken to indemnify our current or former directors and officers against all reasonable costs, charges and expenses, including amounts paid to settle an action or satisfy a judgment, incurred in respect of any civil, criminal or administrative proceedings to which such director or officer is made party to by reason of being or having been a director or officer. Our board of directors may, from time to time, purchase and maintain insurance policies for the benefit of our directors and officers as against any such liabilities incurred, except with respect to liability arising out of a director or officer’s failure to act honestly, in good faith and with a view toward our best interests. We do not presently maintain a directors’ and officers’ insurance or reimbursement policy.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or controlling persons under the forgoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable as a matter of United States law.
Qualifications
There is no mandatory retirement age required by our bylaws or the Business Corporations Act (Alberta) and no requirement that our directors be shareholders of our Company.
MEMORANDUM AND ARTICLES OF ASSOCIATION - continued
Common Shares
In accordance with Article 2 and Schedule A of our articles of incorporation filed January 19, 1996, and as amended, there are authorized an unlimited number of common shares which our directors may issue without nominal or par value.
Dividends
Holders of shares of our common stock are entitled, subject to the rights privileges, restrictions and conditions attached to our preferred shares, to receive such dividends as our directors may from time to time, by resolution, declare.
Voting Rights
Holders of shares of our common stock are entitled to receive notice of and vote, on the basis of one vote for each common share so held, at every meeting of the shareholders of our Company.
Liquidation Rights
Holders of shares of our common stock are entitled, subject to the rights, preferences and privileges, of any authorized and outstanding class and series of our preferred shares, to receive, in the event of liquidation, dissolution or winding up or upon the distribution of any of our assets among the holders of shares of our common stock, other than by way of dividends, their pro rata share of such distribution.
In accordance with Article 2 and Schedule B of our articles of incorporation filed January 19, 1996, and as amended, there are authorized an unlimited number of preferred shares, which our directors may, from time to time, issue without nominal or par value in one or more series consisting of such number of shares as our directors may determine. Currently there are no preferred shares outstanding. Our directors, by resolution, may fix the designation, rights, privileges, restrictions and conditions attached to the preferred shares of each series, including without limiting the generality of the foregoing, the rate, form, entitlement and payment of preferential dividends, the redemption price, terms, procedure and conditions for redemption, if any, voting rights and conversion rights, and sinking fund, purchase fund or other provisions attaching to the preferred shares of such series.
Dividends
Holders of shares of our preferred stock shall be entitled to preference over holders of shares of our common stock and any other of our shares ranking junior with respect to the payment of dividends as our directors may from time to time, by resolution, declare. No dividend shall at any time be declared or paid or set apart for payment on any shares ranking junior to shares of our preferred stock unless all dividends up to and including the dividends payable for the last completed period for which such dividends were payable on each series of preferred shares then issued and outstanding shall have been declared and paid or set apart for payment at the date, nor shall we call for redemption or redeem or purchase for cancellation or reduce or otherwise pay off any of our shares of preferred stock or any shares ranking junior to shares of our preferred stock unless all dividends up to and including the dividends payable for the last completed period for which such dividends were payable on each series of preferred shares then issued and outstanding shall have been declared and paid or set apart for payment as of the date of such call for redemption, purchase, reduction or other payment.
Holders of shares of our preferred stock shall rank on parity with holders of shares of all other series of our preferred stock with respect to payment of dividends, exclusive of any conversion rights, and if cumulative dividends are not paid in full in respect of a series of our preferred stock, the holders of shares of all series of our preferred stock shall participate ratably.
Voting Rights
Holders of shares of our preferred stock shall be entitled to those voting rights which our directors may, by resolution, designate with respect thereto.
MEMORANDUM AND ARTICLES OF ASSOCIATION - continued
Liquidation Rights
Holders of shares of our preferred stock shall be entitled to preference over holders of shares of our common stock and any other of our shares ranking junior with respect to the distribution of our assets in the event of liquidation, dissolution or winding up, whether voluntary or involuntary, and may also be given such other preferences as our directors may fix, by resolution, prior to issuance.
Holders of shares our of preferred stock shall rank on parity with holders of shares of all other series of our preferred stock with respect to distribution of assets in the event of liquidation, dissolution or winding up, whether voluntary or involuntary, exclusive of any conversion rights, and if return of capital is not paid in full in respect of a series of our preferred stock, the holders of shares of all series of our preferred stock shall participate ratably.
Shareholder Action
In accordance with the provisions of the Business Corporations Act (Alberta) ordinary resolutions, such as those adopting amendments to our bylaws, must be passed by a majority of shareholders voting thereon, whereas special resolutions require a majority of not less than two-thirds of the votes cast by shareholders voting thereon. Special resolutions are required in (i) authorizing share splits where more than one class of shares exist, (ii) increasing stated capital with respect to a class or series of shares where the amount to be added is not received in consideration for the issuance of shares and where more than one class of shares exist, (iii) decreasing stated capital for any purpose, (iv) making certain amendments to or restating our articles of incorporation, (v) approving an amalgamation agreement, (vi) approving a continuance into another jurisdiction, (vii) selling, leasing or exchanging of all or substantially all of our property, other than in the ordinary course of business, or (viii) liquidating our assets and dissolving our Company.
Shareholders Meetings
Annual Meetings
We normally hold our annual shareholders’ meeting shortly after completion of the preceding fiscal year at our principal executive offices in Canada with the record date begin set and notice provided to our shareholders not less than twenty-one nor more than fifty days prior to the meeting. Only those shareholders entitled to vote at the meeting, the directors, auditors and any other persons required to attend under any provision of our articles of incorporation, bylaws or the Business Corporations Act (Alberta) are entitled to attend the meeting.
Special Meetings
Our board, the chairman of the board, managing director or president, have the power to call a special meeting at anytime, and notice of any special meeting shall state the text of any special resolutions to be submitted at the meeting.
Share Ownership
Although our shareholder are subject to certain beneficial ownership reporting requirements as a result of our having a class of securities registered under Section 12 of the Securities Exchange Act of 1934, there are no provisions in our bylaws governing the ownership threshold above which shareholder ownership must be disclosed.
With the exception of the Investment Canada Act, as amended, and the potential application of the Competition Act there are no limitations on the ability or right of persons to own our securities, including the rights of non-resident or foreign shareholders to hold or exercise voting rights on our securities imposed by the Business Corporations Act (Alberta) or by our articles of incorporation or bylaws.
MEMORANDUM AND ARTICLES OF ASSOCIATION - continued
Investment Canada Act
The Investment Canada Act, as amended, generally prohibits implementation of any of a number of enumerated reviewable investments by an individual, government or agency thereof, corporation, partnership, trust or joint venture that is non-Canadian, as defined in the Investment Canada Act, unless the Minister responsible for administration of the Investment Canada Act is satisfied that the investment is likely to be of a net benefit to Canada.
In accordance with the provisions of the Investment Canada Act, an investment or series of investments to directly or indirectly acquire control of a Canadian company by a non-Canadian that is a “WTO Investor” or a Canadian company which was immediately prior to the investment controlled by a WTO Investor, is reviewable if the value of the assets of the company equal or exceed $256 million, the threshold established for the year 2006. In succeeding years, the threshold amount may be increased or decreased in accordance with the provisions of Section 14.1 of the Investment Canada Act. A WTO Investor is one who is a member of the World Trade Organization, currently comprised of one hundred forty-eight member countries, including the United States, or a WTO Investor-controlled entity, as defined in the Investment Canada Act.
An investment or series of investments to directly or indirectly acquire control of a Canadian company by a non-Canadian, other than a WTO Investor, is reviewable if the value of the assets of the company equal or exceed $5 million, or if an order for review is made by the Governor in Council on grounds that the investment is related to Canada’s cultural heritage or national identity.
Procedurally, the Investment Canada Act requires a non-Canadian who is establishing a new Canadian business or making an investment that will result in the acquisition of an existing Canadian business, to, depending on the transaction, file with the Director of Investments either notification of the transaction or an application for review.
Where an investment is reviewable in accordance with the provisions of the Investment Canada Act, an application for review must be filed prior to implementation of the investment, in response to which the non-Canadian investor will receive a receipt bearing a certified date. Within forty-five days following certified date the Minister responsible for administering the Investment Canada Act must either notify the non-Canadian investor that he is satisfied that the investment is likely to be of a net benefit to Canada or, if the Minister is unable to complete the review, notify the non-Canadian investor that the Minister shall, within thirty days unless a further period is agreed upon, complete consideration of the investment. Once the thirty day period has elapsed, the Minister will be deemed to be satisfied that the investment is likely to be of a net benefit to Canada and the investment may thereafter be implemented.
The notification procedure involves filing a brief statement of information at any time up to thirty days following implementation of the investment, in response to which the non-Canadian investor will receive a receipt bearing a certified date and advising the non-Canadian investor that the proposal is either unconditionally non-reviewable or that it will not be reviewed provided there is no additional notice of review issued within twenty-one days following the certified date.
Exempt from the notice and review provisions of the Investment Canada Act are, among other transactions, those acquisitions carried out (i) in the ordinary course of one’s business, (ii) in connection with the realization of security granted for a loan, (iii) for the purpose of facilitating financing, provided the acquirer divest himself of control within two years, and (iv) by reason of an amalgamation, merger, consolidation, or corporate reorganization, following which the ultimate control remains unchanged.
Exempt from the review provisions of the Investment Canada Act are investments to acquire control of Canadian businesses that (i) engage in the production of uranium, (ii) provide any financial services, (iii) provide transportation services, or (iv) constitute a cultural business.
MEMORANDUM AND ARTICLES OF ASSOCIATION - continued
Competition Act
The Competition Act, as amended, is designed to maintain and encourage competition while promoting efficiency and adaptability in the local Canadian economy, as well as to expand opportunities for Canadian participation in the world markets.
In accordance with the provisions of the Competition Act an investment giving rise to the acquisition or establishment, directly or indirectly, by one or more persons of control over or a significant interest in the whole or part of a business of a competitor, supplier, customer or other person may be subject to substantive review by the Commissioner of Competition, the officer responsible for administering the Competition Act.
Pre-investment notification must be made to the Commissioner of Competition by parties of a proposed transaction where certain party and transaction size thresholds are satisfied. The party size threshold requires that parties of a proposed transaction, together with their affiliates, have total assets in Canada or gross revenues from sales in, from or into Canada that exceed $400 million in the aggregate. The transaction size threshold requirements vary depending on the nature of the investment.
Transactions subject to pre-investment notification may not be completed until (i) the applicable statutory waiting periods have expired or have been earlier terminated by the Commissioner of Competition without his having taken action to prohibit the transaction’s implementation, or (ii) the Commissioner of Competition has issued an advance ruling certificate, or has waived the parties obligation to notify. Transactions involving only affiliates or those in the public interest, among others, are exempt from pre-investment notification requirements.
Regardless of whether pre-investment notification is required, the Commissioner of Competition may apply to the Competition Tribunal, a specialized tribunal established by Section 3 if the Competition Tribunal Act and empowered with the authority to hear and dispose of certain matters arising under the Competition Act, for substantive review of a transaction.
Where the Competition Tribunal finds the transaction likely to prevent or lessen competition substantially it may order the investment not proceed or, if it has been completed, may order its dissolution or the disposition of some of the assets or shares involved.
Change in Control
There are no provisions in our articles of incorporation or bylaws that would have an effect of delaying, deferring or preventing a change in our control with respect to a merger, acquisition or corporate restructuring.
MATERIAL CONTRACTS
The following is a summary of certain key economic and related provisions of all material contracts that we have entered into since March, 2006.
Pagic License and Related Transactions
On March 17, 2005, we reached an agreement in principle with Pagic LP, formerly MK Enterprises LLC (“Pagic”), an entity controlled by Glen Kertz, our current Chairman, director and acting president to obtain or otherwise secure the following:
| | (i) | A license for a period lasting so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to three unrelated and proprietary consumer retail products that had previously been developed by Pagic, including the Ultrasonic Skincare System, the Dust Wolf TM, and the Tomorrow Garden TM, (collectively, and together with any improvements thereon, the “Initial Products”); |
| | | |
| | (ii) | A license for a period lasting so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to any ancillary products developed and sold for use by consumers in connection with the Initial Products (“Initial Ancillaries”); |
MATERIAL CONTRACTS - continued
| | (iii) | A right for an initial period of five years to acquire a license for a period lasting so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to any new products developed by Pagic (collectively, “Additional Products”); |
| | | |
| | (iv) | A license for a period lasting so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to any ancillary products developed and sold for use by consumers in connection with the Additional Products (“Additional Ancillaries”); and |
| | | |
| | (v) | A related services agreement pursuant to which Pagic would provide consulting support in connection with the Initial Products, the Initial Ancillaries, the Additional Products and the Additional Ancillaries. |
On July 29, 2005, we entered into a series of related definitive agreements with Pagic (collectively, the “Pagic Agreements”), including (i) a master license agreement for a period lasting so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to the Initial Products and the Initial Ancillaries (the “Pagic Master License”), (ii) a product development agreement pursuant to which we were granted a right for an initial period of five years to acquire a license, for a period lasting so long as royalty payments continue to be made as required, for the exclusive worldwide marketing and distribution rights to any Additional Products and any Additional Ancillaries (the “Pagic Product Development Agreement”), and (iii) a consulting agreement pursuant to which we will receive consulting support from Pagic in connection with each of the Initial Products, the Initial Ancillaries, the Additional Products and the Additional Ancillaries (the “Pagic Consulting Agreement”), in exchange for the following:
| | • | 20,000,000 shares of our common stock; |
| | | |
| | • | A one-time US$125,000 license fee; |
| | | |
| | • | Reimbursement for US$125,000 in development costs associated with each of the Initial Products since March 17, 2005; |
| | | |
| | • | The greater of the following, payable annually: |
| | (i) | US$400,000; or |
| | | |
| | (ii) | The aggregate of the following: |
| | • | Subject to a minimum amount of US$37,500 per Initial Product during the second year of the Pagic Master License, and US$50,000 each year thereafter, continuing royalties payable quarterly at a rate of: |
| | : | US$10.00 per Ultrasonic Skincare System unit sold; |
| | | |
| | : | US$2.00 per Dust Wolf TM, unit sold; |
| | | |
| | : | 4.5% of annual net sales of the Tomorrow Garden Kit; and |
| | | |
| | : | 3% of annual net sales of Initial Ancillaries. |
| | • | A one-time US$50,000 license fee for each Additional Product licensed (except for a one pre-identified product); |
| | | |
| | • | Subject to a minimum amount of US$50,000 per year commencing with the second year of each corresponding license, continuing royalties of 4.5% of annual net sales and 3% on annual net sales of any Additional Ancillaries; and |
| | | |
| | • | Consulting fees of US$156,000 per year, payable monthly in advance. |
MATERIAL CONTRACTS - continued
The Pagic Agreements contain considerably more detail than the summary of key economic and related provisions set forth above. This summary does not purport to be a complete description of these agreements, and is qualified in is entirety by reference to the Pagic Master License, the Pagic Product Development Agreement, and the Pagic Consulting Agreement respectively, copies of which are included as Exhibits to this filing. Prospective investors in our common stock are strongly encouraged to read these agreements in their entirety.
During the year ended March 31, 2007, the Company issued 8,388,025 restricted common shares as the remaining shares owed in conjunction with the Licensing Agreement with Pagic LP (formerly MK Enterprises LLC). The Company on July 29, 2005, agreed to issue Pagic and its assigns 20 million common shares at a deemed cost of $1,306,075, based on the historical cost of the license, of which 11,611,975 shares were issued during the year ended March 31, 2006 and the balance during the year ended March 31, 2007. Included in the 20 million shares issued for the license were 9,428,205 shares that were issued to parties that became related parties to the Company.
July/August 2005 Private Offering Transaction
In conjunction with this financing, the Company paid consultants an amount equal to 10% of the gross proceeds, which was included in investor relations during the year ended March 31, 2006 and issued 425,735 common shares at a deemed value of $285,242. There are 255,440 finders’ A warrants outstanding whereby the holders have the right to purchase 255,440 common shares at US$0.50 per share until August 5, 2008 and 425,733 finders’ B warrants whereby the holders shall have the right to purchase 425,733 common shares at US$0.75 per share until August 5, 2008. A total of US$82,200 in registration penalties incurred in the year ended March 31, 2007 were converted to a new convertible debenture in the same amount on April 6, 2007 (Note 10(c)).
During the year ended March 31, 2008, convertible notes of US$75,000 and interest totaling US$4,521 were converted to 262,057 common shares, and interest of US$22,389 (2007 – US$119,875) was accrued on the principal balance of these convertible notes. As part of the subscription agreement that was part of the July/August 2005 Subscription Agreements, we agreed to grant certain registration rights to each of the investors and the finders, pursuant to which we became obligated to registering all of the shares issuable upon conversion of the promissory notes and under the warrants. Under the terms of the subscription agreement, a registration statement must have been filed on Form F-1 no later than sixty days following the close of the transaction and must be declared effective not later than one hundred fifty days following the close of the transaction. Thereafter, on one occasion, for a period commencing one hundred fifty-one days following the close of the transaction and not later than two years following the close of the transaction, any record holder or holders of more than fifty percent (50%) of the shares of our common stock issued or issuable upon conversion of the promissory notes or exercise of the warrants may, upon written request, demand that we prepare and file a registration statement registering shares issuable upon conversion of all sums due under the convertible promissory notes and exercisable under the warrants.
The July/August 2005 Subscription Agreements contain considerably more detail than the summary of key economic and related provisions set forth above. This summary does not purport to be a complete description of these agreements, and is qualified in its entirety by reference to the actual form of subscription agreement, convertible notes, Class A common stock purchase warrants, and Class B common stock purchase warrants, respectively, copies of which are included as Exhibits to this filing. Prospective investors in our common stock are strongly encouraged to read these agreements in their entirety.
APRIL 2006 FOLLOW-ON PRIVATE OFFERING TRANSACTION
On April 6, 2006, the Company consummated a private offering transaction with and among a syndicated group of investors, pursuant to which the Company issued, in the aggregate, US$551,666 in 8% per annum convertible notes and three-year Class A and B warrants to acquire: (i) up to 735,544 shares of the Company’s common stock at a price per share of US$0.50; and (ii) up to an additional 735,544 shares of the Company’s common stock at a price per share of US$1.00. Subject to certain limitations, the principal amount of the notes, together with any accrued interest may be converted into shares of the Company’s common stock at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the common stock for the ten trading days prior to conversion; or (ii) US$0.55.
MATERIAL CONTRACTS - continued
The convertible notes carry a redemption feature, which allows the Company to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that the common shares have a closing price of US$1.50 per share for at least twenty consecutive trading days and there has otherwise been no default. The common stock purchase warrants carry a Cashless Conversion Feature. These convertible notes are unsecured, and due on demand.
In conjunction with these private offering transactions, the Company paid consultants: (i) US$55,166 cash, representing 10% of the gross proceeds realized; (ii) 183,886 shares of common stock; (iii) three-year warrants to purchase up to 110,320 shares of common stock at a price per share of US$0.50; and (iv) three-year warrants to purchase up to 183,867 shares of common stock at a price per share of US$0.75.
During the year ended March 31, 2008, the Company accrued US$38,835 (2007 – US$567,366) in interest on the principal balance of these convertible notes. As part of the financing transaction, we also granted certain registration rights to each of the investors and finders pursuant to which we became committed to registering all of the shares issued as part of such transaction, including those issuable upon conversion of the notes and exercise of the warrants, by filing a registration statement on Form F-1 covering such shares within fourteen days of the closing date, or by April 20, 2006, and causing such registration statement to be declared effective by the SEC by June 5, 2006. In accordance with the terms of the registration provisions any delays in meeting our obligations subject us to liability in an amount, payable in cash, at a rate of 2% of the outstanding amount on the convertible notes per thirty day period for the duration of any such delay, and, furthermore, any delays in our filing of a registration statement by April 20, 2006 subject us to liability in the form of a reduction in the exercise price of the Class A and Class B warrants issued as part of such transaction in an amount of US$0.10 per week for the duration of any such delay.
The April 2006 Subscription Agreements contain considerably more detail than the summary of key economic and related provisions set forth above. This summary does not purport to be a complete description of these agreements, and is qualified in its entirety by reference to the actual form of subscription agreement, convertible notes, Class A common stock purchase warrants and Class B common stock purchase warrants, respectively, copies of which were filed with our registration statement on Form F-1 on April 27, 2006 and which are incorporated herein by reference. Prospective investors in our common stock are strongly encouraged to read these agreements in their entirety.
US$82,200 April 2006 penalty Convertible Note
On April 6, 2006, and in conjunction with certain private placements, the Company reached a verbal agreement with investors wherein the Company agreed to convert US$82,200 in accrued penalties associated with the July 25, 2005 through August 5, 2005 convertible notes into US$82,200 convertible penalty notes (Note 10(a)) carrying terms similar to the July 25, 2005 through August 5, 2005 convertible notes and an aggregate of 109,600 warrants. These warrants carry a Cashless Conversion Feature and each warrant entitles the holder to purchase additional common shares for three years at a price of US$0.75 per share. These convertible notes are unsecured and due on demand.
MAY/AUGUST 2006
On May 15, 2006, the Company completed a private placement of units whereby it issued a total of 1,104,165 units at US$0.60 per unit whereby each unit consisted of one common share and one share purchase warrant to purchase an additional common share at US$0.80. Of the warrants issued, 833,332 expire on May 15, 2008 and 270,833 on June 7, 2008. The Company also paid consultants fees consisting of $58,860 plus 66,666 warrants to purchase that number of common shares at US$0.80 until May 15, 2008 and 21,666 warrants to purchase that number of common shares at US$0.80 until June 7, 2008.
Pursuant to an Investor Relations Consulting Agreement with a third party for services provided to Valcent, SmallCap will receive a total of 120,000 shares of common stock at US$ 0.41 per share.
On August 18, 2006, the Company completed a private placement of units whereby it issued a total of 430,000 units at US$0.60 per unit for a total of US$258,000 whereby each unit consisted of one common share and one share purchase warrant to purchase an additional common share at US$0.80. The warrants issued will expire on August 18, 2008. The Company paid consultants fees consisting of $58,860 plus 24,800 warrants to purchase that number of common shares at US$0.80 until August 18, 2008.
MATERIAL CONTRACTS - continued
Nova Skin Care System – Solid Integrations, LLC Manufacturing Agreement
We entered into a manufacturing agreement with Solid Integrations, LLC, for the manufacture and assembly of our Nova Skin Care System. Under this agreement we will provide and make available to Solid Integrations, LLC, and its subcontractor(s), the raw materials, machinery, equipment, tooling and molds necessary for Solid Integrations, LLC, and its subcontractor(s), to manufacture and assemble the Nova Skin Care System at a manufacturing facility located in Mexico.
Global Green Joint Venture
On October 2, 2006, the Company entered into a letter agreement replaced on July 9, 2007 by the Vertigro Algae Stakeholders Letter of Agreement, (together “LOA”) with Pagic, West Peak Ventures of Canada Limited (“West Peak”) and Global Green, whereby Global Green agreed to fund the next phase of the development of a high density vertical bio-reactor technology. Pursuant to the LOA, the Company and Global Green established a commercial joint venture named “Vertigro” in which Global Green agreed to provide up to US$3,000,000 in initial funding to continue the research and development of the bio-reactor technology, construct a working prototype of the bio-reactor and develop the technology for commercial uses. The Company is obligated to provide product support, research and development, and the non-exclusive use of the Company’s properties and lands for which Global Green has agreed to reimburse the Company as part of its US$3,000,000 initial funding commitment. Until such time as the joint venture has fully repaid to Global Green the US$3,000,000, Global Green shall receive 70% of the net cash flow generated by anticipated future operations after which each of Global Green and the Company will hold a 50% interest in the Vertigro, subject to an aggregate 4.5% royalty to Pagic and West Peak. Vertigro covers the bio-reactor technology and any subsequent related technologies for the commercial scale products of algae based biomass for all industrial commercial and retail applications including, but not limited to bio-fuel, food, health, pharmaceutical, and animal and agricultural feeds.
As at March 31, 2008, Global Green and the Company incurred a total of US$5,735,893 (2007 - US$2,023,379) in costs related to Vertigro. Of the costs incurred to March 31, 2008, Global Green paid US$4,338,204 (2007 - US$1,653,981) of which US$7,806 is included in accounts payable. During the year ended March 31, 2007, accounts receivable included US$369,398 due from Global Green. On July 9, 2007, the parties to the GGS Agreement entered into the Vertigro Algae Stakeholders Letter of Agreement (the “Global Green Joint Venture”), which replaced the GGS Agreement. Pursuant to the new agreement, each of Global Green and the Company will hold a 50% interest in the Global Green Joint Venture, subject to an aggregate 4.5% royalty to Pagic and West Peak. The Global Green Joint Venture covers the Bio-Reactor and any subsequent related technologies for the commercial scale products of algae based biomass for all industrial commercial and retail applications including but not limited to bio-fuel, food, health, pharmaceutical, animal and agricultural feeds.
Limited Liability Company Operating Agreement for Vertigro Algae Technologies, LLC
On May 5, 2008, Valcent USA and Global Green signed a Limited Liability Company Operating Agreement (“New Operating Agreement”) replacing the LOA to form Vertigro Algae Technologies, LLC (“Vertigro Algae”), a Texas LLC, which is the formalized operating entity for the previous unincorporated operations of Vertigro. The business activities of Vertigro Algae will be the same as when the operations were under Vertigro. Under the New Operating Agreement, Global Green and the Company will each hold a 50% stake in Vertigro Algae, and have committed to fund project development according to ownership allocation. Further, the Company will acquire assets of Vertigro, including buildings, laboratory, and equipment. To allow for prior capital contributions, Global Green has incurred in excess of the Company’s prior aggregate capital contribution, Global Green will receive 70% of the net cash flow generated by Vertigro Algae until it has received US$3,000,000 in excess of its 50% interest in such cash flow.
Technology License Agreement
On May 5, 2008, Vertigro Algae executed a Technology License Agreement (“Technology License”) together with Pagic, West Peak and the Company. The Technology License licenses certain algae biomass technology and intellectual property to Vertigro Algae for purposes of commercialization and exploitation for all industrial, commercial, and retail applications worldwide (“Algae Biomass Technology”). In return for the Algae Biomass Technology, both the Company and Global Green will each issue 300,000 common shares to Pagic LP, and also pay a one-time commercialization fee of US$50,000 upon the Algae Biomass Technology achieving commercial viability. The Technology License is subject to royalty of 4.5% of gross customer sales receipts for use of the Algae Biomass Technology; and aggregate annual royalty minimum amounts of US$50,000 in 2009, US$100,000 in 2010, and US$250,000 in 2011 and each year thereafter in which the Technology License is in place.
All prior agreements between the Company and Pagic that relate to the Algae Biomass Technology will be replaced by the new Operating Agreement and the Technology License.
MATERIAL CONTRACTS - continued
Purchase Agreement
On September 26, 2008, the Company entered into an agreement with Global Green to purchase its entire membership interest in Vertigro Algae Technologies LLC, the company responsible for developing the Company’s proprietary technologies to produce algae biomass fuels and other algae products. The price to be paid for the Membership interest is US$5 million in cash and 5 million common shares in the capital of the Company. The transaction is subject to finance. The Company and Global Green further agreed that Global Green, on a non-exclusive basis, may be engaged to facilitate the commercialization and development of the Company’s technologies with particular emphasis on bio mass power generation including the integration of Global Green’s “ Greensteam” applications, the terms and consideration for which are to be determined on a project specific basis.
US$1,500,000 DECEMBER 2006 CONVERTIBLE NOTE
On December 1, 2006, the Company accepted subscriptions of US$1,500,000 towards a private placement of 8% per annum, unsecured, convertible notes and three-year warrants to acquire: (i) up to an aggregate of 2,000,000 shares of the Company’s common stock at a price per share of US$0.50; and (ii) up to an additional 2,000,000 shares of the Company’s common stock at a price per share of US$1.00. Subject to certain limitations, the principal amount of the notes, together with any accrued interest may be converted into shares of the Company’s common stock at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the Company’s common stock for the ten trading days prior to conversion; or (ii) US$0.55. The convertible notes carry a redemption feature, which allows the Company to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that the common shares have a closing price of US$1.50 per share for at least twenty consecutive trading days and there has otherwise been no default. These convertible notes are unsecured and due on demand. The common stock purchase warrants may be exercised on a cashless basis.
During the year ended March 31, 2008, the Company accrued US$989,296 (2007 - US$238,448) in interest on the principal balance of these convertible notes.
The right of the note holders to convert into the Company’s common stock is subject to the contractual agreement between the parties that any conversion by the note holders may not lead at the date of such conversion to an aggregate equity interest in the common stock of the Company greater than 9.99% inclusive of any derivative securities including options, warrants, convertible debt, any other convertible debt securities, or any other financial instruments convertible into common equity.
US$2,000,000 JANUARY 2007 CONVERTIBLE NOTE
On January 29, 2007, the Company completed a private placement comprised of US$2,000,000 convertible notes. The convertible notes will mature on December 11, 2008, carry interest at 6% per annum and are unsecured. The notes are convertible into “Units” at the note holders’ discretion at a conversion price of US$0.50 per Unit. Each “Unit” consists of one common share and one purchase warrant to purchase an additional common share at US$0.70 per share until December 11, 2008. The notes and any accrued interest are callable by the Company at any time after December 11, 2007 by providing thirty days’ written notice to the note holders. Interest on the notes will be compounded annually and be cumulative until the earlier of either the date the Company achieves pre-tax earnings or the end of the term. At the discretion of the note holders, interest on the notes is payable in either cash or units at US$0.50 per unit. In connection with this financing, the Company has paid consultants US$108,000 in cash and issued 135,000 warrants exercisable at US$0.50 per unit, with each unit consisting of one common share and one share purchase warrant to purchase a further common share at US$0.70 per share until December 11, 2008. The Company is obligated to file a resale registration statement on the underlying securities within four months of closing, which it has failed to do.
As a result of the failure to file the registration statement, the Company recorded penalties of US$120,000 as of March 31, 2007 and a further US$289,973 during the year ended March 31, 2008. In addition, during the year ended March 31, 2008, convertible notes of US$200,000 and interest and registration penalty totaling US$11,668 were converted to 485,707 common shares, and the Company accrued US$677,794 in interest on the principal balance of these convertible notes (2007 - US$24,316).
MATERIAL CONTRACTS - continued
US$391,000 SEPTEMBER 2007 CONVERTIBLE NOTE
On September 27, 2007, the Company issued unsecured one-year term convertible notes bearing interest at 6% per annum in the amount of US$391,000 to third parties. Both interest and principal may be converted at the option of the lender at any time at US$0.60 per unit, with each unit consisting of one common share and one-half share purchase warrant, with each whole share purchase warrant exercisable at US$0.75 to purchase an additional common share for a two-year term from the date of conversion. During the year ended March 31, 2008, the Company accrued US$96,222 (2007 - US$0) in interest on the principal balance of these convertible notes.
The Company is required to register for trading the securities underlying the conversion features of this convertible note on a best efforts basis, but has failed to do so within terms agreed. A one-time financial penalty of US$23,460 for failure to register the securities underlying this convertible note within 90 days from the date of issuance has been incurred during the year ended March 31, 2008.
Long Term Debt
During the year ended March 31, 2007, the Company entered into a ten year commercial real estate note totaling US$190,000 to fund the acquisition of land. The loan is secured by the land and an aggregate US$100,000 in term deposits which is included in the prepaid expenses. Borrowings under the Agreement accrue interest equal to the Wall Street Journal prime rate plus 0.25 percent adjusted annually. The current interest rate is 8.50% per annum. At March 31, 2008, remaining long term debt was $174,862 (2007 - - $209,114) of which $16,250 (2007 - $13,451) represented the current portion of aggregate long term debt.
TERM LOAN
On August 10, 2007, the Company issued an unsecured convertible term promissory note in the amount of US$650,000 to a third party. The convertible note is due on demand and bears interest at 6% with both interest and principal convertible at the option of the lender into units at US$0.60 per unit, with each unit consisting of one common share and one-half share purchase warrant with each whole share purchase warrant exercisable at US$0.75 to purchase an additional common share. After November 25, 2008, this convertible note accrues interest at the rate of 15% per annum. During the year ended March 31, 2008, the Company accrued US$230,007 (2007 – US$0) in interest on the principal balance of these convertible notes.
The Company is required to register for trading the securities underlying the conversion features of this convertible note on a best efforts basis, but has failed to do so within terms agreed. A one-time financial penalty of US$28,567 for failure to register the securities underlying this convertible note within 180 days from the date of issuance has been incurred in the year ended March 31, 2008.
The right of the note holder to convert into the Company’s common stock is subject to the contractual agreement between parties that any conversion by the note holder may not lead, at the date of such conversion, to an aggregate equity interest in the common stock of the Company greater than 9.99% inclusive of any derivative securities including options, warrants, convertible debt, any other convertible debt securities, or any other financial instruments convertible into common equity.
2008 CONVERTIBLE NOTE
On July 21, 2008, the Company closed a financing of zero coupon, 12% interest, senior secured convertible promissory notes in the amount of US$2,428,160 with an aggregate purchase price of US$2,168,000 with four investors, one of which was the Company’s Chief Financial Officer as to US$168,000. The debt is convertible into shares of common stock at the lesser of US$0.51 per share (unless the conversion price has been adjusted pursuant to further contract covenants) and 70% of the average of the five lowest closing bid prices for the ten preceding trading days. The Company issued each purchaser in the private placement two warrants, one warrant being redeemable by the Company and the other being non-redeemable. The non-redeemable warrants are exercisable at US$0.55 and permit the holder to purchase shares of common stock equal to 100% of the number of shares issuable upon the conversion of the notes calculated on July 21, 2008. The redeemable warrants are exercisable at US$0.75 and permit the holder to purchase common stock equal to 50% of the number of shares issuable upon the conversion of the notes issued calculated on the closing date. The Company issued a total of redeemable warrants to purchase an aggregate of 4,761,098 shares of common stock and a total of redeemable warrants to purchase an aggregate of 2,380,550 shares of common stock. Further, the Company issued 439,216 nonredeemable warrants and 219,608 redeemable warrants and $160,000 in cash fees to close the transaction. Each non-redeemable warrant is exercisable at US$0.55, and each redeemable warrant is exercisable at US$0.75; warrants carry a term of five years from the date of closing of the financing. The redeemable warrant may be redeemed by the Company only if certain conditions have been satisfied including the Company’s common stock having closed at $1.50 per share for a period of 20 consecutive trading days and the warrant holder being able to resell the shares acquired upon exercise through a resale registration statement or under Rule 144 of the Securities Act.
MATERIAL CONTRACTS - continued
LICENSE AGREEMENTS
On September 3, 2008, the Company signed a Letter of Agreement with the Hydroganics Joint Venture, an Australian Group, (“Hydroganics”) for the exclusive use of the Company’s HDVG System for growing vegetables excluding grains in Australia. Under the terms of the agreement, Hydroganics will invest US$2,500,000 by purchasing Valcent equity, Valcent will have a carried 30% equity interest in Hydroganics until Hydroganics has raised US$10,000,000 in equity for the development and commercialization of HVGS System in Australia. Thereafter, Valcent shall have anti dilution rights to purchase 30% of any further equity issued.
On September 16, 2008, the Company signed a License Agreement with a private Alberta Corporation based in Fort McMurray, Alberta, Canada (“Albertaco”) for the exclusive use of Company’s HDVG System for growing vegetables in the Province of Alberta. Under the terms of the agreement, the purchaser will invest US$1,500,000 by purchasing Valcent equity, Valcent will have a carried 30% equity interest in Albertaco until Albertaco has raised US$10,000,000 in equity for the development and commercialization of the HDVG System in Alberta. Thereafter, Valcent shall have anti dilution rights to purchase 30% of any further equity issued.
Vertigro Algae Technologies, LLC Purchase Agreement
On September 26, 2008, the Company entered into an agreement with Global Green to purchase its entire membership interest in Vertigro Algae Technologies LLC, the company responsible for developing the Company’s proprietary technologies to produce algae biomass fuels and other algae products. The price to be paid for the Membership interest is US$5 million in cash and 5 million common shares in the capital of the Company. The transaction is subject to finance. The Company and Global Green further agreed that Global Green, on a non-exclusive basis, may be engaged to facilitate the commercialization and development of the Company’s technologies with particular emphasis on bio mass power generation including the integration of Global Green’s “ Greensteam” applications, the terms and consideration for which are to be determined on a project specific basis.
EXCHANGE CONTROLS
There are no governmental laws, decrees, regulations or other legislation in Canada which restrict the import or export of capital, including the availability of cash and cash equivalents. With the exception of withholding tax requirements, there are no governmental laws, decrees, regulations or other legislation in Canada which affect the remittance of dividends, interest or other payments, to non-Canadian resident holders of shares of our securities.
TAXATION
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSEQUENCES
The following is a general discussion of material United States federal income tax consequences that may apply to holders and prospective holders of shares of our common stock. This discussion is based on the Internal Revenue Code of 1986, as amended, (the “Code”), Treasury Department regulations promulgated thereunder, published Internal Revenue Service (“IRS”) rulings and administrative pronouncements and court decisions of current applicability, any or all of which may materially and adversely change at any time, possibly on a retroactive basis. In addition, this discussion does not consider the potential effects, adverse or beneficial, of any proposed changes in the governing tax law, whether by judicial, executive or legislative action, which, if effected, may be applied, possibly on a retroactive basis. Further, no opinion was requested by us, or has been provided by our counsel or independent registered public accounting firm, with respect to the United States income tax consequences described in the following discussion. Accordingly, we urge holders and prospective holders of shares of our common stock to consult with, and rely upon, their own tax advisors in analyzing the potential United States federal, state, local and non-United States tax consequences associated with purchasing, owning and disposing of shares of our common stock.
As used in this section the term “U.S. Holder” denotes the beneficial owner of one or more shares of our common stock who is:
| | • | an individual citizen or resident of the United States; |
| | | |
| | • | a corporation, or entity taxable as a corporation, that is created or organized under the laws of the United States or of any political subdivision thereof or the District of Columbia; |
| | | |
| | • | an estate whose income is taxable in the United States irrespective of source; or |
| | | |
| | • | a trust if, (i) a court within the United States is able to exercise primary supervision over the administration of the trust, and (ii) one or more United States persons have the authority to control all substantial decisions of the trust. |
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSEQUENCES - continued
This discussion does not address the tax consequences peculiar to, and the term U.S. Holder does not include, persons subject to special provisions of United States federal income tax law, such as tax-exempt organizations, qualified retirement plans, individual retirement accounts and other tax-deferred accounts, financial institutions, insurance companies, real estate investment trusts, regulated investment companies, dealers in securities, persons or entities that have a functional currency other than the United States dollar, shareholders subject to the alternative minimum tax, shareholders who hold our common shares as part of a straddle, hedging or a conversion transaction, constructive sale or other arrangement involving more than one position, partners and other pass-through entities and persons holding an interest in such entities, and shareholders who acquired our common shares through the exercise of employee stock options or otherwise as compensation for services. This discussion is limited to U.S. Holders who own shares of our common stock as capital assets within the meaning of Section 1221 of the Code. This discussion does not address the consequences peculiar to persons or entities holding an interest in a shareholder or the consequences peculiar to a person of the ownership, exercise or disposition of any options, warrants or other rights to acquire shares of our common stock. If a partnership (including an entity treated as a partnership for United States federal income tax purposes) holds shares of our common stock, the tax treatment of a partner will generally depend upon the status of the partner and upon the activities of the partnership. If you are a partnership, or a partner in a partnership, holding shares of our common stock, we urge you to consult with, and rely upon, your own tax advisor in analyzing the potential United States federal, state, local and non-United States tax consequences associated with purchasing, owning and disposing of shares of our common stock.
Dividends
Though we do not anticipate paying any cash dividends in the foreseeable future, should U.S. Holders of shares of our common stock receive dividend distributions:
| | • | to the extent such distributions are paid out of our current or accumulated earnings and profits, as determined for United States federal income tax purposes, subject to any applicable foreign tax credit, and without a reduction for any Canadian income tax withheld from such distributions, such holders would generally be required to include, in their gross income for United States federal income tax purposes, an amount equal to the United States Dollar value of such distributions on the date of receipt (based on the exchange rate on such date). |
| | | |
| | • | to the extent such dividend distributions exceed our current or accumulated earnings and profits, as determined for United States federal income tax purposes, the amount of any distribution so received will (i) first be applied to reduce a U.S. Holder’s adjusted basis in our common shares, and, as a return of basis, will not be subject to United States federal income tax, and (ii) thereafter, any residual amount of such distribution will be treated as a gain from the sale or exchange of our common shares, which amount is taxable as a capital gain. |
Any taxable dividends received on shares of our common stock by a U.S. Holder who is an individual, a trust or an estate (an “Individual Holder”) will be treated as qualified dividend income, which is subject to tax at preferential rates through December 31, 2008, provided that:
| | • | we are a qualified foreign corporation—one eligible for the benefits of a comprehensive income tax treaty with the United States, which the United States Treasury Department has determined to be satisfactory for this purpose and which includes an exchange of information program—for which purposes the Canada-US Income Tax Convention (1980) qualifies; |
| | | |
| | • | we are not a passive foreign investment company for the taxable year during which the dividend is paid or the immediately preceding taxable year (which we do not believe we are, have been or anticipate that we will be); |
| | | |
| | • | the Individual Holder has held the common shares for more than 60 days during the 121-day period beginning 60 days prior to the date on which the common shares become ex-dividend; and |
| | | |
| | • | the Individual Holder is not obligated to make related payments with respect to positions in substantially similar or related property. |
Special rules may apply to any extraordinary dividend—a dividend equal to or in excess of 10% of a shareholder’s adjusted basis, or, under certain circumstances, the fair market value, of a share of common stock—requiring that such a distribution be treated as qualified dividend income. Any loss from the sale or exchange of shares of our common stock with respect to which an Individual Holder has received an extraordinary dividend treated as qualified dividend income will be deemed a long-term capital loss to the extent of such dividend. Taxable extraordinary dividends not treated as qualified dividend income will be taxed as ordinary income, rather than as capital gains.
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSEQUENCES - continued
Foreign Tax Credit
A U.S. Holder who pays, or has withheld from distributions, Canadian income tax with respect to the ownership of shares of our common stock may be entitled, at his or her election, to either a deduction or a tax credit for such foreign tax paid or withheld. Furthermore, a U.S. Holder that is a domestic corporation owning 10% or more of our voting stock may be eligible to claim a deemed paid foreign tax credit based on any underlying non-United States income taxes paid by us. The election to credit or deduct foreign taxes is made on a yearly basis and applies to all foreign income taxes paid by, or withheld from, the U.S. Holder during the year.
There are significant and complex limitations applicable to the foreign tax credit and as such we urge you to consult with, and rely upon, your own tax advisor regarding the availability of the foreign tax credit, the deemed paid foreign tax credit available to certain corporations and the application of limitations on the credit in light of your particular circumstances.
Sale, Exchange or other Disposition of Common Shares
Generally, a U.S. Holder will recognize a taxable gain or loss upon the sale, exchange or other disposition of shares of our common stock in an amount equal to the difference between the amount realized by the U.S. Holder from such sale, exchange or other disposition and the U.S. Holder’s tax basis in such shares. Subject to certain qualifications and limitations, such gain or loss will be treated as a long-term capital gain or loss if the U.S. Holder has held the common shares for more than one year at the time of the sale, exchange or other disposition. Preferential tax rates for long-term capital gains may apply to certain U.S. Holders who satisfy this minimum holding period. There are presently no preferential tax rates for long-term capital gains recognized by a corporation.
Special Rules
Special United States federal income tax rules apply to shareholders of passive foreign investment companies and controlled foreign corporations. The preceding discussions do not address the United States federal income tax consequences to a U.S. Holder in the event that we were treated as a passive foreign investment company or controlled foreign corporation. While our management does not believe that we are, or that we are likely to become, a passive foreign investment company or a controlled foreign corporation, the circumstances which would result in our becoming such are not entirely within our control and as such there always exists the possibility, however remote, that we may become a passive foreign investment company or controlled foreign corporation in the future.
Passive Foreign Investment Company
A non-United States entity treated as a corporation for United States federal income tax purposes will be a passive foreign investment company in any taxable year in which, after taking into account the income and assets of the corporation and certain subsidiaries pursuant to a “look through” rule, either (i) 75% or more of its gross income is passive income, such as interest, dividends and certain rents and royalties, or (ii) at least 50% of the average value of its assets are attributable to assets that produce passive income or are held for the production of passive income. Our management does not believe that we are a passive foreign investment company, or that we will become a passive foreign investment company in the future, because we are primarily engaged in the refinement and manufacture of three lines of unrelated potential consumer retail products. Moreover, we have not received 75% or more of our gross income from passive sources, nor has 50% or more of the fair market value of our assets been held for the production of passive income, for any taxable year. Nonetheless, in the event we were treated as a passive foreign investment company for any taxable year, U.S. Holders of shares of our common stock may be subject to United States federal income taxes in excess of those previously described on distributions paid with respect to, or dispositions of, our common shares.
There are significant and complex issues associated with the taxation of a U.S. Holder of shares of a passive foreign investment company and as such we urge you to consult with, and rely upon, your own tax advisor regarding the impact of the passive foreign investment company rules and the advisability and availability of certain United States federal income tax elections that may alleviate adverse tax consequences that may result if we are or were to become a passive foreign investment company.
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSEQUENCES - continued
Controlled Foreign Corporation
A controlled foreign corporation is a foreign corporation of which more than 50% of the stock, by vote or value, is owned, directly, indirectly or constructively, by U.S. Holders each of whom, directly, indirectly or constructively, owns 10% or more of the total combined voting power of all classes of stock of the foreign corporation (each a “CFC Shareholder”). If we are a controlled foreign corporation, a CFC Shareholder would be treated as receiving current distributions of an allocable share of certain types of our income at the end of each year. Additionally, such a CFC Shareholder would recognize ordinary income to the extent of an allocable share of our earnings and profits, rather than capital gain, on the sale of his or her common shares. Our management does not believe that we are a controlled foreign corporation, because U.S. Holders who directly, indirectly or constructively own 10% or more of the total voting power of our outstanding common shares do not own more than 50% of shares of our common stock.
United States Federal Income Taxation of Non-U.S. Holders
For purposes of this discussion, a beneficial owner of our common shares that is not a U.S. Holder (other than a partnership or entity treated as a partnership for United States federal income tax purposes) is a “Non-U.S. Holder”. If you are a partnership, or a partner in a partnership, holding shares of our common stock, we urge you to consult with, and rely upon, your own tax advisor in analyzing the potential United States federal, state, local and non-United States tax consequences associated with purchasing, owning and disposing of shares of our common stock.
Distributions on our Common Shares
Any distributions we pay to a Non-U.S. Holder will not be subject to United States federal income tax or withholding tax if the Non-U.S. Holder is not engaged in a United States trade or business. If the Non-U.S. Holder is engaged in a United States trade or business, any distributions we pay will be subject to United States federal income tax at regular graduated rates if (i) those distributions are treated as dividends or capital gains (as previously described with respect to U.S. Holders); (ii) are effectively connected with the Non-U.S. Holder’s United States trade or business; and, (iii) if an income tax treaty applies, are attributable to a permanent establishment maintained by the Non-U.S. Holder in the United States. In addition, a branch profits tax may be imposed at a 30% rate, or at such lower rate under an applicable income tax treaty, on the effectively connected earnings of a Non-U.S. Holder that is a corporation.
Sale, Exchange or other Disposition of Common Shares
Non-U.S. Holders generally will not be subject to United States federal income tax on any gain recognized on the disposition of shares of our common stock unless the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States and, if an income tax treaty applies, is attributable to a permanent establishment maintained by the Non-U.S. Holder in the United States. If the Non-U.S. Holder is engaged in a United States trade or business and a gain recognized on the disposition of shares of our common stock is effectively connected with that trade or business, and, if a tax treaty applies, is attributable to a permanent establishment maintained by such Non-U.S. Holder in the United States, such gain will be subject to United States federal income tax at regular graduated rates and, if the Non-U.S. Holder is a corporation, a branch profits tax may be imposed at a 30% rate, or at such lower rate established under an applicable income tax treaty. A Non-U.S. Holder who is (i) an individual (ii) present in the United States for 183 days or more in a taxable year, and (iii) who meets certain other requirements will also be subject to United States federal income tax on capital gains recognized during such year, including such gains realized from a disposition of our stock.
Information Reporting and Backup Withholding Tax
Generally, dividend payments or other taxable distributions made within the United States will be subject to information reporting requirements and a United States backup withholding tax if a U.S Holder fails to provide an accurate taxpayer identification number certified under penalties of perjury, as well as certain other information, or otherwise establish an exemption from backup withholding.
Non-U.S. Holders may be required to establish their exemption from information reporting and backup withholding by certifying their status on an IRS Form W-8BEN, W-8ECI or W-8IMY as applicable.
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSEQUENCES - continued
If a Non-U.S. Holder sells shares to or through the United States office of a United States or foreign broker, the payment of proceeds generally will be subject to information reporting requirements and a backup withholding tax unless the Non-U.S. Holder properly certifies its non-United States status under penalties of perjury or otherwise establishes an exemption and the payer or broker does not have actual knowledge or reason to know that the holder is a United States person. Information reporting requirements and backup withholding taxes generally will not apply to the payment of proceeds of the sale of common shares effected outside the United States by a foreign office of a broker. Information reporting requirements (but not backup tax withholding requirements) will apply, however, to the payment of the sale proceeds if the broker is a United States person or has certain other contacts with the United States.
Backup withholding is not an additional tax. Rather, a holder generally may obtain a refund of any amounts withheld under the backup withholding rules that exceed such holder’s United States federal income tax liability by timely filing a properly completed claim for refund with the IRS.
MATERIAL CANADIAN FEDERAL INCOME TAX CONSEQUENCES
The following summary is a general discussion of material Canadian federal income tax consequences that may apply to holders and prospective holders of shares of our common stock that are non-residents of Canada (“Non-Canadian Holders”). This discussion is based on the Income Tax Act (Canada), the regulations promulgated thereunder, all amendments thereto publicly proposed by or on behalf of the Minster of Finance (Canada) prior to the date hereof, the published administrative practices of the Canada Revenue Agency and on the current provisions Canada-United States Income Tax Convention (1980), as amended (the “Canada-US Treaty”), any or all of which may materially and adversely change at any time, possibly on a retroactive basis. In addition, this discussion does not consider the potential effects, adverse or beneficial, of any proposed changes in the governing tax law, whether by judicial, executive or legislative action, which, if effected, may be applied, possibly on a retroactive basis. No opinion was requested by us, or has been provided by our counsel or independent registered public accounting firm, with respect to the Canadian income tax consequences described in the following discussion. Accordingly, we urge holders and prospective holders of shares of our common stock to consult with, and rely upon, their own tax advisors in analyzing the potential Canadian federal, provincial, local and non-Canadian tax consequences associated with purchasing, owning and disposing of shares of our common stock.
As used in this section the term “Non-Canadian Holder” denoted the beneficial owner of one or more shares of our common stock who is:
| | • | a non-resident of Canada; |
| | | |
| | • | holds our common shares as capital property; |
| | | |
| | • | deals with us at arm’s length; and |
| | | |
| | • | does not use or hold our common shares in or in the course of carrying on business in Canada. |
Dividends
Though we do not anticipate paying any cash dividends in the foreseeable future, should a Non-Canadian Holder of shares of our common stock receive dividend distributions, such holder will be subject to a Canadian withholding tax equal to 25%, subject to such lower rate as may be available under an applicable tax treaty, of the gross amount of any distributions paid or deemed to be paid.
In accordance with the Canada-US Treaty the rate of withholding tax applicable to distributions paid to a Non-Canadian Holder who is a resident of the United States is:
| | • | to the extent such holder beneficially owns at least 10% of our voting stock, 5% of the gross amount of such distribution paid; or |
| | | |
| | • | to the extent such holder beneficially owns less than 10% of our voting stock, 15% of the gross amount of such distribution paid. |
MATERIAL CANADIAN FEDERAL INCOME TAX CONSEQUENCES - continued
We are required to withhold the applicable amount from each distribution paid and remit the withheld amount directly to the Receiver General for Canada for the account of the Non-Canadian Holder.
In accordance with the Canada-US Treaty, certain tax-exempt entities residing in the United States may be exempt from Canadian withholding taxes, including any withholding taxes levied in respect of dividends received on our common shares.
Sale, Exchange or other Disposition of Common Shares
A Non-Canadian Holder who disposes of shares of our common stock, including by deemed disposition upon death, will not be subject to Canadian federal income tax on any capital gain (or capital loss) thereby realized unless the common shares constitute “taxable Canadian property” as defined by the Income Tax Act (Canada) and no relief is afforded under any applicable tax treaty. Generally, our common shares will not constitute taxable Canadian property of a Non-Canadian Holder unless (i) the common shares are held as capital property used in carrying on a business (other than an insurance business) in Canada, or (ii) the Non-Canadian Holder or persons with whom the Non-Canadian Holder did not deal at arm’s-length alone or together held or held options to acquire, at any time within the five years preceding the disposition, 25% or more of the shares of any class of our capital stock.
A Non-Canadian Holder who is resident of the United States and realizes a capital gain on disposition of common shares that are taxable Canadian property will nevertheless, by virtue of the Canada-US Treaty, generally be exempt from Canadian tax thereon unless (i) more than 50% of the value of the common shares are derived from, or forms an interest in, Canadian real estate, including Canadian mineral resource properties, (ii) the common shares formed part of the business property of a permanent establishment that the Non-Canadian Holder has or had in Canada within the twelve months preceding disposition, or (iii) the Non-Canadian (a) was a resident of Canada at any time within the ten years immediately preceding the disposition and for a total of 120 months during the twenty years, preceding the disposition, and (b) owned the common share when he ceased to be a resident of Canada.
A Non-Canadian Holder who is subject to Canadian tax in respect of a capital gain on disposition of common shares must include one-half of the capital gain in computing his taxable income earned in Canada. The Non-Canadian Holder may, subject to certain limitations, deduct one half of any capital loss arising on disposition of taxable Canadian property from taxable capital gains realized in the year of disposition in respect of taxable Canadian property and, to the extent not so deductible, from such taxable capital gains of any of the three preceding years or any subsequent year.
A Non-Canadian Holder whose common shares constitute taxable Canadian property and who is a resident of the United States and entitled to benefits under the Canada-US Treaty generally would be exempt from Canadian tax on any capital gain realized on a disposition of those shares in any event, provided such shares do not derive their value principally from Canadian real property (including Canadian resource properties). Our management is of the view that our common shares do not derive their value principally from Canadian real property. A Non-Canadian Holder claiming exemption under the Treaty will, however, have Canadian tax filing obligations with respect to a disposition of common shares.
DOCUMENTS ON DISPLAY
All documents referred to in this annual report may be inspected at our offices during regular business hours located at Suite 1010 - 789 West Pender Street, Vancouver, British Columbia V6C 1H2 (Canada), telephone (604) 606-7979.
Not applicable.
ATTESTATION REPORT OF THE REGISTERED PUBLIC ACCOUNTING FIRM
Not applicable.
DISCLOSURE CONTROLS AND PROCEDURES
As required by Rule 13a-15 under the Securities Exchange Act of 1934 (the “1934 Act”), as of March 31, 2008, we carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures. This evaluation was carried out under the supervision and with the participation of our CEO (our principal executive officer) and our CFO (our principal financial officer), who concluded, that because of the material weakness in our internal control over financial reporting (“ICFR”) described below, our disclosure controls and procedures were not effective as of March 31, 2008.
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and our principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Internal Control Over Financial Reporting
Our management is also responsible for establishing ICFR as defined in Rules 13a-15(f) and 15(d)-15(f) under the 1934 Act. Our ICFR are intended to be designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Our ICFR are expected to include those policies and procedures that management believes are necessary that:
| (i) | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| (ii) | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and our directors; and |
| (iii) | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
DISCLOSURE CONTROLS AND PROCEDURES - continued
Management recognizes that there are inherent limitations in the effectiveness of any system of internal control, and accordingly, even effective internal control can provide only reasonable assurance with respect of financial statement preparation and may not prevent or detect misstatements. In addition, effective internal control at a point in time may become ineffective in future periods because of changes in conditions or due to deterioration in the degree of compliance with our established policies and procedures.
As of March 31, 2008, management assessed the effectiveness of the Company's internal control over financial reporting (ICFR) based on the criteria for effective ICFR established in Internal Control--Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and SEC guidance on conducting such assessments by smaller reporting companies and non-accelerated filers.
Based on that assessment, management concluded that, during the period covered by this report, such internal controls and procedures were not effective as of March 31, 2008 and that material weaknesses in ICFR existed as more fully described below.
As defined by Auditing Standard No. 5, “An Audit of Internal Control Over Financial Reporting that is Integrated with an Audit of Financial Statements and Related Independence Rule and Conforming Amendments,” established by the Public Company Accounting Oversight Board ("PCAOB"), a material weakness is a deficiency or combination of deficiencies that results more than a remote likelihood that a material misstatement of annual or interim financial statements will not be prevented or detected. In connection with the assessment described above, management identified the following control deficiencies that represent material weaknesses as of March 31, 2008:
| (1) | Lack of an independent audit committee or audit committee financial expert, and no independent directors. Although our audit committee functions with one officer/director and two other directors who are not corporate officers, the two non-officer directors are paid consultants to the Company and as such may not be deemed truly independent. These factors may be counter to corporate governance practices as defined by the various stock exchanges and may lead to less supervision over management; |
| (2) | Inadequate staffing and supervision within our bookkeeping operations. We have three employees or consultants involved in bookkeeping functions in three separate operating subsidiaries where transactional volumes require such services, and we utilize independent consultants on a part-time basis to supplement our accounting staff. The relatively small number of people who are responsible for bookkeeping functions prevents us from segregating duties within our internal control system. The inadequate segregation of duties is a weakness because it could lead to the untimely identification and resolution of accounting and disclosure matters or could lead to a failure to perform timely and effective reviews which may result in a failure to detect errors in spreadsheets, calculations, or assumptions used to compile the financial statements and related disclosures as filed with the SEC; |
| (3) | Outsourcing of significant portions of the accounting operations of our Company. Because there are few employees in our administration, we have outsourced a portion of the accounting functions of our Company to an independent accounting firm where particular skills are required due to the nature of complex financial transactions especially relating to convertible debenture financings. The employees of this accounting firm are managed by supervisors within the accounting firm, and are not answerable to the Company's management. This is a material weakness because it could result in a disjunction between the accounting policies adopted by our Board of Directors and the accounting practices applied by the accounting firm; |
| (4) | Insufficient installation of information technology to assist in our accounting functions. Because of a lack of working capital and personnel, we have been unable to upgrade our information technology software and hardware to assist in providing effective controls; |
| (5) | Insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of US GAAP and SEC disclosure requirements; |
| (6) | Ineffective controls over period end financial disclosure and reporting processes. |
DISCLOSURE CONTROLS AND PROCEDURES - continued
As of March 31, 2008, management assessed the effectiveness of our internal control over financial reporting and based on that evaluation, they concluded that, during the year ended March 31, 2008 and to date, the internal controls and procedures were not effective due to deficiencies that existed in the design or operation of our internal controls over financial reporting. However, management believes these weaknesses did not have an effect on our financial results. During the course of their evaluation, we did not discover any fraud involving management or any other personnel who play a significant role in our disclosure controls and procedures or internal controls over financial reporting.
Due to a lack of financial and personnel resources, we are not able to, and do not intend to, immediately take any action to remediate these material weaknesses. We will not be able to do so until, if ever, we acquire sufficient financing and staff to do so. We will implement further controls as circumstances, cash flow, and working capital permit. Notwithstanding the assessment that our ICFR was not effective and that there were material weaknesses as identified in this report, we believe that our consolidated financial statements contained in our Annual Report on Form 20-F for the fiscal year ended March 31, 2008, fairly presents our financial position, results of operations and cash flows for the years covered thereby in all material respects.
The Company relies on the part time involvement of its Chief Financial Officer and consultants for period end financial disclosure and the reporting process. This risk is mitigated by the active involvement of the audit committee and the board of directors in reviewing the financial statements. However, the lack of full time personnel who have technical experience and knowledge is an internal control weakness and may result in the failure to timely report financial results.
It is unlikely that the above noted internal control weakness can be properly addressed until the Company grows to a significant size to warrant the expense, such as the hiring of additional personnel, associated with implementing additional segregation of duties. We are committed to improving our financial organization and internal controls. As part of this commitment, we intend to hire an additional person on a full time basis, when sufficient funds are available to us, with technical experience and knowledge in accounting to better segregate duties consistent with control objectives and will increase our personnel resources and technical accounting expertise within the accounting function.
Management believes that the material weaknesses set forth above were the result of the scale of our operations and are intrinsic to our small size. Management believes these weaknesses did not have an effect on our financial results.
We are committed to improving our financial organization. As part of this commitment, we will, as soon as funds are available to the Company (1) appoint one or more outside directors to our board of directors who shall be appointed to the audit committee of the Company resulting in a fully functioning audit committee who will undertake the oversight in the establishment and monitoring of required internal controls and procedures; (2) create a position to segregate duties consistent with control objectives and will increase our personnel resources; and (3) hire independent third parties to perform expert advice. We will continue to monitor and evaluate the effectiveness of our internal controls and procedures and our internal controls over financial reporting on an ongoing basis and are committed to taking further action and implementing additional enhancements or improvements, as necessary and as funds allow. This annual report does not include an attestation report of the Company's independent registered public accounting firm regarding internal control over financial reporting. Management's report is not subject to attestation by the Company's independent registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit us to provide only management's report in this annual report.
This Annual Report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our independent registered public accounting firm pursuant to temporary rules of the SEC that permit us to provide only management’s report in this Annual Report.
There were no changes in our internal control over financial reporting during the quarter ended June 30, 2008, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
We presently have, and will continue to have, one audit committee member who is a “financial expert” (as defined in accordance with Item 16A(b) of Form 20-F), F. George Orr. Mr. Orr is not “independent” as that term is defined in Section 803A of the American Stock Exchange Company Guide and SEC Rule 10A-3 under the Exchange Act of 1934.
As of the date of the filing of this annual report on Form 20-F we have not adopted a code of ethics (as defined in accordance with Item 16B(a) of Form 20-F), however, we intend to, as soon as practicable, form a committee to advise on the content and adoption of a code of ethics.
Our independent auditors for the fiscal years ended March 31, 2008 and 2007 were Smythe Ratcliffe, Chartered Accountants of Vancouver, British Columbia.
AUDIT FEES
The aggregate audit fees billed for the fiscal years ended March 31, 2007 and March 31, 2008 for professional services rendered by Smythe Ratcliffe, Chartered Accountants, for the audit of our annual financial statements were $66,100 and $118,440 respectively.
AUDIT COMMITTEE PRE-APPROVAL POLICIES AND PROCEDURES
Our audit committee charter requires us to request the prior approval of our audit committee on every occasion for which we engage our principal accountants or their associated entities to render any audit or non-audit services.
TAX FEES
The aggregate fees billed in the last two fiscal years for professional tax compliance advice and planning was US$0 (2008) (fees not yet determined), and US$0 (2007) (fees not yet determined).
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEE
Not applicable.
Valcent Products Inc.
(A Development Stage Company)
Consolidated Financial Statements
(Expressed in Canadian Dollars)
March 31, 2008 and 2007
| Index | Page |
| | |
| Auditors' Report to the Shareholders | F-1 |
| | |
| Consolidated Financial Statements | |
| | |
| Consolidated Balance Sheets | F-2 |
| | |
| Consolidated Statements of Operations and Deficit | F-3 |
| | |
| Consolidated Statements of Cash Flows | F-4 |
| | |
| Notes to the Consolidated Financial Statements | F-5 to F-39 |
AUDITORS' REPORT
TO THE SHAREHOLDERS OF VALCENT PRODUCTS INC.
(A Development Stage Company)
We have audited the consolidated balance sheets of Valcent Products Inc. (a development stage company) as at March 31, 2008 and 2007 and the consolidated statements of operations and deficit and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board of the United States of America. Those standards require that we plan and perform an audit to obtain reasonable assurance whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation.
In our opinion, these consolidated financial statements present fairly, in all material respects, the financial position of the Company as at March 31, 2008 and 2007 and the results of its operations and its cash flows for the years then ended in accordance with Canadian generally accepted accounting principles.
“Smythe Ratcliffe LLP” (signed)
Chartered Accountants
Vancouver, Canada
August 6, 2008, except as to note 2
which is as of September 25, 2008.
COMMENTS BY AUDITORS FOR US READERS ON CANADA-US REPORTING DIFFERENCES
In the United States, reporting standards of the Public Company Accounting Oversight Board for auditors require the addition of an explanatory paragraph (following the opinion paragraph) when the financial statements are affected by conditions and events that cast substantial doubt on the Company’s ability to continue as a going-concern, such as those described in note 1 to the financial statements. Our report to the shareholders dated August 6, 2008 is expressed in accordance with Canadian reporting standards, which do not permit a reference to such events and conditions in the auditors’ report when these are adequately disclosed in the financial statements.
As discussed in note 2 to the financial statements, an error resulting in an overstatement of previously reported net loss for the year ended March 31, 2007 was discovered during the 2008 year. Accordingly, the 2007 financial statements have been restated and an adjustment has been made to deficit as of April 1, 2007 to correct the error.
“Smythe Ratcliffe LLP” (signed)
Chartered Accountants
Vancouver, Canada
August 6, 2008, except as to note 2
which is as of September 25, 2008.
Valcent Products Inc.
(A Development Stage Company)
Consolidated Balance Sheets
(Expressed in Canadian Dollars)
| | | 2008 | | | 2007 | |
| | | | | | (Note 2) | |
| Assets | | | | | | |
| Current | | | | | | |
| Cash and cash equivalents | | $ | 163,437 | | | $ | 314,972 | |
| Accounts receivable (Notes 8 and 16) | | | 462,156 | | | | 462,055 | |
| Prepaid expenses (Note 7) | | | 2,119,546 | | | | 356,761 | |
| Inventories (Note 9) | | | 617,195 | | | | 1,236,808 | |
| | | | | | | | | |
| | | | 3,362,334 | | | | 2,370,596 | |
Restricted Cash (Note 13) | | | 108,471 | | | | 117,327 | |
Property and Equipment (Notes 5 and 13) | | | 1,135,108 | | | | 348,487 | |
Product License (Note 6) | | | 1 | | | | 1,306,075 | |
| | | | | | | | | |
| | | $ | 4,605,914 | | | $ | 4,142,485 | |
| | | | | | | | | |
| Liabilities and Shareholders' Deficiency | |
| | | | | | | | | |
| Current | | | | | | | | |
| Accounts payable | | $ | 382,605 | | | $ | 115,744 | |
| Accrued liabilities | | | 153,958 | | | | 53,906 | |
| Current portion of long-term debt (Note 13) | | | 16,250 | | | | 13,451 | |
| Promissory notes payable (Note 18) | | | 270,167 | | | | 115,460 | |
| Due to related parties (Note 11) | | | 1,490,516 | | | | 930,175 | |
| Convertible notes (Note 10) | | | 5,202,741 | | | | 2,742,462 | |
| | | | | | | | | |
| | | | 7,516,237 | | | | 3,971,198 | |
| Long-term debt (Note 13) | | | 158,612 | | | | 195,663 | |
| | | | | | | | | |
| | | | 7,674,849 | | | | 4,166,861 | |
| Shareholders' Deficiency | | | | | | | | |
| Share capital (Note 14) | | | 16,691,282 | | | | 8,196,982 | |
| Contributed surplus (Note 14(e)) | | | 4,203,394 | | | | 3,333,435 | |
| Conversion component of convertible notes (Notes 10 and 14(f)) | | | 3,591,398 | | | | 3,287,858 | |
| Accumulated deficit from prior operations | | | (3,237,370 | ) | | | (3,237,370 | ) |
| Accumulated deficit during the development stage | | | (24,317,639 | ) | | | (11,605,281 | ) |
| | | | | | | | | |
| | | | (3,068,935 | ) | | | (24,376 | ) |
| | | | | | | | | |
| | | $ | 4,605,914 | | | $ | 4,142,485 | |
Going-concern (Note 1) and Commitments (Notes 6, 8, 10, 12, 13 and 18)
On behalf of the board
"Glen Kertz" Director F. George Orr" Director
See notes to the consolidated financial statements.
Valcent Products Inc.
(A Development Stage Company)
Consolidated Statements of Operations and Deficit
(Expressed in Canadian Dollars)
| | | 2008 | | | 2007 | | | 2006 | |
| | | | | | (Note 2) | | | (Note 2) | |
| | | | | | (as restated) | | | (as restated) | |
| Expenses | | | | | | | | | |
| Product development (Note 16) | | $ | 4,081,435 | | | $ | 1,562,421 | | | $ | 685,432 | |
| Interest, accretion and financing on convertible notes | | | 2,804,582 | | | | 2,726,067 | | | | 1,630,852 | |
| Advertising and media development | | | 1,953,998 | | | | 1,092,917 | | | | 0 | |
| Stock-based compensation | | | 990,305 | | | | 1,127,141 | | | | 533,664 | |
| Investor relations | | | 871,542 | | | | 287,834 | | | | 19,037 | |
| Professional fees | | | 431,270 | | | | 712,458 | | | | 175,833 | |
| Travel | | | 291,465 | | | | 156,498 | | | | 69,600 | |
| Office and miscellaneous | | | 234,817 | | | | 281,696 | | | | 38,125 | |
| Interest and penalties | | | 112,376 | | | | 6,910 | | | | 193,987 | |
| Rent | | | 77,918 | | | | 65,693 | | | | 56,771 | |
| Insurance | | | 71,071 | | | | 78,784 | | | | 0 | |
| Amortization | | | 47,463 | | | | 25,288 | | | | 9,382 | |
| Filing and transfer agent fees | | | 44,500 | | | | 38,883 | | | | 26,250 | |
| Interest on long-term debt | | | 15,480 | | | | 8,500 | | | | 0 | |
| Management fees | | | 0 | | | | 0 | | | | 4,000 | |
| | | | | | | | | | | | | |
| Loss from Operations | | | 12,028,222 | | | | 8,171,090 | | | | 3,442,933 | |
| Other (Income) Expense | | | | | | | | | | | | |
| Write-down on product license (Note 6) | | | 1,306,074 | | | | 0 | | | | 0 | |
| Interest income | | | (10,805 | ) | | | (25,704 | ) | | | 0 | |
| Foreign exchange gain (loss) | | | (611,133 | ) | | | (6,993 | ) | | | 23,955 | |
| | | | | | | | | | | | | |
| Net Loss and Comprehensive Loss for Year | | | 12,712,358 | | | | 8,138,393 | | | | 3,466,888 | |
| Deficit During Development Stage, Beginning of Year, | | | | | | | | | | | | |
| As previously reported | | | 14,674,170 | | | | 3,734,599 | | | | 0 | |
| Prior period correction (Note 2) | | | (3,068,889 | ) | | | (267,711 | ) | | | 0 | |
| | | | | | | | | | | | | |
| As restated | | | 11,605,281 | | | | 3,466,888 | | | | 0 | |
| | | | | | | | | | | | | |
| Deficit During Development Stage, End of Year | | $ | 24,317,639 | | | $ | 11,605,281 | | | $ | 3,466,888 | |
| | | | | | | | | | | | | |
Loss Per Share - Basic (Note 21) | | $ | 0.36 | | | $ | 0.42 | | | $ | 0.329 | |
| | | | | | | | | | | | | |
| Weighted Average Number of Common Shares Outstanding | | �� | 35,545,740 | | | | 19,261,192 | | | | 10,548,042 | |
See notes to the consolidated financial statements.
Valcent Products Inc.
(A Development Stage Company)
Consolidated Statements of Cash Flows
(Expressed in Canadian Dollars)
| | | 2008 | | | 2007 | | | 2006 | |
| | | | | | (Note 2) | | | (Note 2) | |
| | | | | | (as restated) | | | (as restated) | |
| Cash Provided By (Used In) | | | | | | | | | |
| Operating Activities | | | | | | | | | |
Net loss for year | | $ | (12,712,358 | ) | | $ | (8,138,393 | ) | | $ | (3,466,888 | ) |
Items not involving cash | | | | | | | | | | | | |
| Interest, accretion and financing on convertible notes | | | 2,839,430 | | | | 2,327,985 | | | | 1,345,868 | |
| Stock-based compensation (Note 14) | | | 990,305 | | | | 1,127,141 | | | | 533,664 | |
| Write-down on product license (Note 6) | | | 1,306,074 | | | | 0 | | | | 0 | |
| Product development | | | 506,869 | | | | 0 | | | | 0 | |
| Consulting | | | 198,493 | | | | 52,708 | | | | 0 | |
| Investor relations | | | 199,904 | | | | 174,924 | | | | 0 | |
| Interest and penalties | | | 4,195 | | | | 0 | | | | 0 | |
| Amortization | | | 47,463 | | | | 25,288 | | | | 9,382 | |
| Foreign exchange gain | | | (630,984 | ) | | | 0 | | | | | |
| Changes in non-cash working capital items | | | 732,607 | | | | (1,870,635 | ) | | | 104,109 | |
| | | | (6,518,002 | ) | | | (6,300,982 | ) | | | (1,473,865 | ) |
| Investing Activities | | | | | | | | | | | | |
Product license | | | 0 | | | | 0 | | | | (306,075 | ) |
Property and equipment | | | (834,084 | ) | | | (310,448 | ) | | | (72,709 | ) |
| | | | (834,084 | | | | (310,448 | ) | | | (378,784 | ) |
| | | | | | | | | | | | | |
| Financing Activities | | | | | | | | | | | | |
| Advances from (repayment to) related parties | | | 560,341 | | | | 860,902 | | | | (84,076 | ) |
| Restricted cash | | | 0 | | | | 117,327 | | | | 0 | |
| Proceeds from issuance of common shares, net | | | 5,448,774 | | | | 1,028,266 | | | | 0 | |
| Promissory notes payable | | | 150,512 | | | | 115,460 | | | | 0 | |
| Repayments of long-term debt | | | (34,252 | ) | | | (25,440 | ) | | | 0 | |
| Proceeds from issuance of convertible notes | | | 1,100,108 | | | | 4,817,114 | | | | 1,565,069 | |
| Shares issued for settlement of debt | | | 0 | | | | 0 | | | | 234,609 | |
| Debt discount | | | 0 | | | | 0 | | | | 149,598 | |
| | | | 7,225,483 | | | | 6,913,629 | | | | 1,865,200 | |
| | | | | | | | | | | | | |
| Foreign Exchange on Cash Held in Foreign Currency | | | (24,932 | ) | | | 0 | | | | 0 | |
| (Decrease) Increase in Cash During Year | | | (151,535 | ) | | | 302,199 | | | | 12,551 | |
| Cash and Cash Equivalents, Beginning of Year | | | 314,972 | | | | 12,773 | | | | 222 | |
| | | | | | | | | | | | | |
| Cash and Cash Equivalents, End of Year | | $ | 163,437 | | | $ | 314,972 | | | $ | 12,773 | |
| | | | | | | | | | | | | |
| Supplemental Cash Flow Information | | | | | | | | | | | | |
| Shares issued for product license | | $ | 0 | | | $ | 419,401 | | | $ | 0 | |
| Shares issued for investor relations/public relations | | $ | 303,804 | | | $ | 460,614 | | | $ | 0 | |
| Shares issued as bonus to director | | $ | 0 | | | $ | 52,708 | | | $ | 0 | |
| Shares issued for consulting services | | $ | 2,110,429 | | | $ | 107,681 | | | $ | 0 | |
| Shares issued on conversion of convertible notes | | $ | 532,295 | | | $ | 1,668,363 | | | $ | 0 | |
| Interest paid | | $ | 15,480 | | | $ | 0 | | | $ | 0 | |
| Income taxes paid | | $ | 0 | | | $ | 0 | | | $ | 0 | |
| | | | | | | | | | | | | |
See notes to the consolidated financial statements.
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 1. | Nature of Business and Ability to Continue as a Going-Concern Valcent Products Inc. (the “Company”) was incorporated under the Alberta Business Corporations Act on January 19, 1996. The Company is in the development stage and is engaged principally in the development and marketing of consumer and industrial products and processes for global markets. These consolidated financial statements have been prepared in accordance with Canadian generally accepted accounting principles (“GAAP”) applicable to a going-concern, which assumes the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. As at March 31, 2008, the Company had an accumulated deficit of $27,555,009 (2007 - $14,839,651) and a working capital deficiency of $4,153,903 (2007 - $1,600,602). The Company’s ability to continue as a going-concern is dependent upon the economic development of its products, the attainment of profitable operations, and the Company’s ability to obtain financing. These consolidated financial statements do not include any adjustments that would be necessary to the carrying values and classification of assets and liabilities should the Company be unable to continue as a going-concern. |
| 2. | Prior Period Adjustment and Comparative Figures The consolidated financial statements for the year ended March 31, 2007 have been restated to adjust prepaid expenses and insurance expense for amounts that relate to the 2008 fiscal year as well as to correct the bifurcation of convertible notes, the amortization thereof using the effective interest method and the transfer of the conversion component of convertible notes to share capital upon conversion of these notes into common shares of the Company. This error was noted in the current year and resulted in a restatement to reduce net loss by $2,801,178 and reduce deficit at the beginning of the year by $267,711 for the year ended March 31, 2007. The resulting restatement to amounts for the year-ended March 31, 2007 are as follows: |
| | | As Previously Reported March 31, 2007 | | | Adjustment | | | As Restated March 31, 2007 | |
| | | | | | | | | | |
| Convertible debentures | | $ | 5,301,129 | | | $ | (2,558,667 | ) | | $ | 2,742,462 | |
| Share capital | | $ | 7,836,903 | | | $ | 360,079 | | | $ | 8,196,982 | |
| Contributed surplus | | $ | 3,253,333 | | | $ | 80,102 | | | $ | 3,333,435 | |
| Conversion component of convertible notes | | $ | 4,167,190 | | | $ | (879,332 | ) | | $ | 3,287,858 | |
| Deficit, end of year | | $ | 14,674,170 | | | $ | (3,068,889 | ) | | $ | 11,605,281 | |
| Deficit, beginning of year | | $ | 3,734,599 | | | $ | (267,711 | ) | | $ | 3,466,888 | |
| Insurance expense | | $ | 149,855 | | | $ | (71,071 | ) | | $ | 78,784 | |
| Interest, accretion and financing on convertible notes | | $ | 5,606,886 | | | $ | (2,880,819 | ) | | $ | 2,726,067 | |
| Foreign exchange gain (loss) | | $ | 110,006 | | | $ | (116,999 | ) | | $ | (6,993 | ) |
| Basic loss per share | | $ | 0.57 | | | $ | (0.15 | ) | | $ | 0.42 | |
| | Certain of the comparative figures for the year ended March 31, 2007 have been reclassified to conform to the current year’s presentation. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 3. | Significant Accounting Policies These financial statements have been prepared in accordance with Canadian GAAP. The significant accounting policies used in these consolidated financial statements are as follows: |
| (a) | Principles of consolidation These financial statements include the accounts of Valcent Products Inc. and its wholly owned, integrated subsidiaries, Valcent Products EU Limited (“Valcent EU”) and Valcent USA, Inc. (“Valcent USA”), and Valcent USA’s wholly-owned, integrated subsidiaries, Valcent Management LLC (“Valcent Management”) and Valcent Manufacturing Ltd. (“Valcent Manufacturing”), in which Valcent Management is the general partner and Valcent USA is the limited partner. All significant inter-company transactions and balances have been eliminated. These consolidated financial statements also include the Company’s proportionate share of the assets, liabilities, income and expenses of the Vertigro Joint Venture, as described in Note 8. |
| (b) | Research and product development costs Research costs are expensed as incurred. At this time, development costs, which meet Canadian GAAP criteria including reasonable assurance regarding recoverability, are capitalized and amortized over the expected economic useful life of the product. No development costs have been deferred to date. As the Company is in the development stage, any revenue derived from the test marketing and development of products is considered to be an expense recovery and is, therefore, netted against product development costs. |
| (c) | Cash and cash equivalents The Company considers all highly liquid financial instruments with a maturity of 90 days or less from the date purchased to be cash equivalents. At March 31, 2008 and 2007, cash and cash equivalents consisted of cash on deposit and money market securities. |
| (d) | Property and equipment The Company amortizes its leasehold improvements on a straight-line basis over the life of the lease. Other assets are amortized using the declining balance method at an annual rate of 10% for building, 20% for equipment, 30% for computer equipment, 20% for furniture and fixtures, and 20% for automobiles. During the year of acquisition, amortization is 50% of amounts otherwise determinable. |
| (e) | Foreign currency transactions and translation The Company’s functional and reporting currency is the Canadian dollar. Amounts recorded in foreign currencies are translated into Canadian dollars as follows: |
| | (i) | Monetary assets and liabilities, at the year-end exchange rates; |
| | (ii) | Non-monetary assets and liabilities, at historical exchange rates; and |
| | (iii) | Revenue and expense items, at the average rate of exchange by quarter except for amortization of property and equipment, which is translated at the same rate as the related asset. |
| | Gains and losses arising from the translation of foreign currency are included in operations for the year. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 3. | Significant Accounting Policies (Continued) |
| | (f) | Use of estimates The preparation of financial statements in conformity with Canadian GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Significant areas requiring the use of estimates include allowance for doubtful accounts, the net realizable value of inventories, impairment of property and equipment, rates of amortization, fair value of product license, amount of accrued liabilities, the variables used to calculate interest, accretion and financing costs on convertible notes, the variables used to calculate the fair value of stock-based compensation, and the determination of the valuation allowance for future income tax assets. While management believes the estimates are reasonable, actual results could differ from those estimates and could impact future results of operations and cash flows. |
| (g) | Loss per share Loss per share is calculated using the weighted average number of common shares outstanding during the year. The Company uses the treasury stock method for calculating diluted earnings per share. Under this method, the dilutive effect on earnings per share is recognized on the use of the proceeds that could be obtained upon exercise of options, warrants and similar instruments. It assumes that the proceeds would be used to purchase common shares at the average market price during the period. Diluted loss per share is not presented where the effect of conversion on exercise of options and warrants and similar instruments would be anti-dilutive. |
| (h) | Stock-based compensation The Company accounts for stock-based compensation expense using the fair value based method with respect to all stock-based payments to directors, employees and non-employees, including awards that are direct awards of stock and call for settlement in cash or other assets, or stock appreciation rights that call for settlement by the issuance of equity instruments. Under this method, stock-based payments are recorded as an expense over the vesting period or when the awards or rights are granted, with a corresponding increase to contributed surplus. When stock options are exercised, the corresponding fair value is transferred from contributed surplus to share capital. |
| | (i) | Income taxes The Company follows the asset and liability method of accounting for income taxes. Under this method of tax allocation, future income tax assets and liabilities are determined based on differences between the financial statement carrying values and their respective income tax basis (temporary differences). Future income tax assets and liabilities are measured using the tax rates expected to be in effect when the temporary differences are likely to reverse. The effect on future income tax assets and liabilities of a change in tax rates is included in operations in the period in which the change is enacted or substantially assured. The amount of future income tax assets recognized is limited to the amount of the benefit that is more likely than not to be realized. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 3. | Significant Accounting Policies (Continued) |
| (j) | Non-monetary consideration In situations where common shares are issued and the fair value of the goods or services received is not readily determinable, the fair value of the common shares is used to measure and record the transaction. The fair value of the common shares issued in exchange for the receipt of goods and services is based on the stock price as of the earliest of: |
| | (i) | the date at which the counterparty’s performance is complete; |
| | (ii) | the date at which a commitment for performance by the counterparty to earn the common shares is reached; or |
| | (iii) | the date at which the common shares are issued if they are fully vested and non-forfeitable at that date. |
| (k) | Impairment of long-lived assets Long-lived assets are reviewed whenever events or changes in circumstances indicate that the carrying value may not be recoverable. An impairment loss would be recognized when the carrying amount of a long-lived asset is not recoverable and exceeds its fair value, with fair value generally based on future discounted cash flows. |
| | (l) | Revenue recognition Revenue from the sales of products is recognized when the product has been delivered to the purchaser, the price is fixed or determinable, and when collectability is reasonably assured. Sales during the development stage are netted against product development costs (Note 3(b)). Interest income is recognized on an accrual basis as earned at the stated rate of interest of the investment over the term to maturity. |
| (m) | Changes in accounting policies The following changes in accounting policies were applied in accordance with the transitional provisions contained in each of the Canadian Institute of Chartered Accountants’ (“CICA”) Handbook sections, and their adoption had no impact on the Company’s consolidated financial statements. |
| | (i) | Financial instruments Effective April 1, 2007, the Company adopted the CICA Handbook Section 3855, “Financial Instruments – Recognition and Measurement”, which establishes standards for recognizing and measuring financial assets, financial liabilities and non-financial derivatives. The standard requires the Company to account for certain financial assets and liabilities at fair value at each balance sheet date. Financial instruments must be classified into one of these five categories: held-for-trading, held-to-maturity, loans and receivables, available-for-sale financial assets or other financial liabilities. All financial instruments are measured in the balance sheet at fair value except for loans and receivables, held-to-maturity investments and other financial liabilities, which are measured at amortized cost. Subsequent measurement and changes in fair value will depend on their initial classification as follows: held-for-trading financial assets are measured at fair value and changes in fair value are recognized in net income; available-for-sale financial instruments are measured at fair value with changes in fair value recorded in other comprehensive income until the investment is no longer recognized or impaired, at which time the amounts would be recorded in net income. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 3. | Significant Accounting Policies (Continued) |
| (m) | Changes in accounting policies (Continued) |
| (i) | Financial instruments (Continued) The adoption of this section did not impact the Company’s consolidated financial statements. |
| | (ii) | Comprehensive income (loss) Effective April 1, 2007, the Company adopted the CICA Handbook Section 1530, “Comprehensive Income”, which establishes standards for presentation and disclosure of comprehensive income (loss). Comprehensive income (loss) is the overall change in the net assets of the Company for the period, other than changes attributed to transactions with shareholders. It is made up of net income and other comprehensive income (loss). The historical make up of net income (loss) has not changed. Other comprehensive income (loss) includes gains or losses, which Canadian GAAP requires to be recognized in a period but excluded from net income (loss) for that period. The Company has no items of other comprehensive income (loss) in any period presented. Accordingly, net loss as presented in the Company’s statement of operations equals comprehensive loss. |
| | (iii) | Inventories During the year ended March 31, 2008, the Company adopted CICA Handbook Section 3031, “Inventories”. This section requires that inventory be recorded at the lower of cost or net realizable value. This section also clarifies that the allocation of fixed production overhead requires the consistent use of either first-in, first-out or the weighted average method to measure inventory, and requires that any previous write-downs be reversed when the value of the inventory increases. The amount of the reversal is limited to the amount of the original write-down. Inventories are valued at the lower of cost and net realizable value, with cost being determined on an average cost basis. |
| | (iv) | Goodwill and intangible assets During the year ended March 31, 2008, the Company adopted CICA Handbook Section 3064, “Goodwill and Intangible Assets”, which replaced Section 3062, “Goodwill and Other Intangible Assets”, and Section 3450, “Research and Development Costs”. This section establishes standards for the recognition, measurement, presentation and disclosure of goodwill subsequent to its initial recognition and of intangible assets by profit-oriented enterprises. Standards concerning goodwill are unchanged from the standards included in the previous Section 3062. The Company’s intangible assets are amortized over their useful lives, unless the life is determined to be indefinite, in which case, no amortization is taken. The Company reviews the estimated useful lives and carrying values of its intangible assets as part of its periodic assessment for impairment of long-lived assets. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 3. | Significant Accounting Policies (Continued) |
| (n) | Future accounting changes |
| | (i) | Capital disclosures In February 2007, the CICA issued Handbook Section 1535, “Capital Disclosures”, which requires the disclosure of both qualitative and quantitative information that provides users of financial statements with information to evaluate the entity’s objectives, policies and procedures for managing capital. The new section is effective for the Company for the year beginning April 1, 2008. The Company is in the process of assessing the impact of this new section on its consolidated financial statements. |
| | (ii) | Financial instruments In February 2007, the CICA issued two new standards, Section 3862, “Financial Instruments Disclosures”, and Section 3863, “Financial Instruments Presentation”. These sections will replace the existing Section 3861, “Financial Instruments Disclosure and Presentation”. Section 3862 provides users with information to evaluate the significance of the financial instruments of the entity’s financial position and performance, nature and extent of risks arising from financial instruments, and how the entity manages those risks. Section 3863 deals with the classification of financial instruments, related interest, dividends, losses and gains, and the circumstances where financial assets and financial liabilities are offset. These new sections are effective for the Company for the year beginning April 1, 2008. The Company is in the process of assessing the impact of these new sections on its consolidated financial statements. |
| | (iii) | International Financial Reporting Standards ("IFRS") In 2006, the Canadian Accounting Standards Board ("AcSB") published a new strategic plan that will significantly affect financial reporting requirements for Canadian companies. The AcSB strategic plan outlines the convergence of Canadian GAAP with IFRS over an expected five year transitional period. In February 2008, the AcSB announced that 2011 is the changeover date for publicly-listed companies to use IFRS, replacing Canada's own GAAP. The date is for interim and annual financial statements relating to fiscal years beginning on or after January 1, 2011. The transition date of January 1, 2011 will require the restatement for comparative purposes of amounts reported by the Company for the year ended March 31, 2011 and earlier where applicable. While the Company has begun assessing the adoption of IFRS for 2011, the financial reporting impact of the transition to IFRS cannot be reasonably estimated at this time. |
| 4. | Financial Instruments The Company has designated its cash and cash equivalents and restricted cash as held-for-trading; accounts receivable as loans and receivables; and its accounts payable, accrued liabilities, promissory note payable, amounts due to related parties, convertible notes and long-term debt as other liabilities. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 4. | Financial Instruments (Continued) |
| (a) | Fair value The carrying values of cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities, promissory note payable, amounts due to related parties and convertible notes approximated their fair values because of the short-term maturity of these financial instruments. The carrying values of restricted cash and long-term debt approximated their fair values as these financial instruments bore interest at approximate market rates of interest. |
| (b) | Interest rate risk Interest rate risk consists of two components: |
| | (i) | To the extent that payments made or received on the Company’s monetary assets and liabilities are affected by changes in prevailing market interest rates, the Company is exposed to interest rate cash flow risk. |
| | (ii) | To the extent that changes in prevailing market interest rates differ from the interest rates in the Company’s monetary assets and liabilities, the Company is exposed to interest rate price risk. |
| | The Company is not exposed to significant interest rate risk due to the short-term maturity of its monetary current assets and current liabilities. The Company is exposed to interest rate cash flow risk on its long-term debt with variable interest rates as the payments on the loan will fluctuate as interest rates fluctuate during the term of the debt. The Company is exposed to interest rate price risk on its promissory and convertible notes with fixed interest rates as the market rate of interest differs from the interest rate of the convertible notes. |
| (c) | Credit risk The Company's financial assets that are exposed to credit risk consist of cash and cash equivalents, accounts receivable and restricted cash. This risk is minimized as cash and cash equivalents and restricted cash are placed with well capitalized, high quality financial institutions. Accounts receivable primarily consists of receivables arising in connection with test sales of the Nova Skincare System and amounts due to the Company in connection with the Company’s joint venture partner, Global Green. |
| (d) | Currency risk The Company undertakes certain transactions in foreign currencies and as such is subject to risk due to fluctuations in exchange rates. Currently the Company is exposed to currency risk with respect to approximately US$728,000 and GBP£32,000 in cash and accounts receivable and approximately US$5,519,000 and GBP£19,000 in accounts payable and convertible debt. The Company does not use derivatives or other techniques to manage foreign currency risk. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 5. | Property and Equipment |
| | | 2008 | |
| | | Cost | | | Accumulated Amortization | | | Net Book Value | |
| | | | | | | | | | |
| Land | | $ | 275,240 | | | $ | 0 | | | $ | 275,240 | |
| Building | | | 389,348 | | | | 4,951 | | | | 384,397 | |
| Equipment | | | 134,895 | | | | 3,351 | | | | 131,544 | |
| Computer equipment | | | 121,320 | | | | 31,605 | | | | 89,715 | |
| Furniture and fixtures | | | 89,134 | | | | 20,684 | | | | 68,450 | |
| Automobiles | | | 39,711 | | | | 811 | | | | 38,900 | |
| Leasehold improvements | | | 167,593 | | | | 20,731 | | | | 146,862 | |
| | | $ | 1,217,241 | | | $ | 82,133 | | | $ | 1,135,108 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | 2007 | |
| | | Cost | | | Accumulated Amortization | | | Net Book Value | |
| | | | | | | | | | | | | |
| Land | | $ | 275,240 | | | $ | 0 | | | $ | 275,240 | |
| Furniture and fixtures | | | 51,941 | | | | 9,692 | | | | 42,249 | |
| Computer equipment | | | 34,985 | | | | 13,798 | | | | 21,187 | |
| Leasehold improvements | | | 20,991 | | | | 11,180 | | | | 9,811 | |
| | | $ | 383,157 | | | $ | 34,670 | | | $ | 348,487 | |
| 6. | Product License On July 29, 2005, the Company completed a licensing agreement (“Agreement”) for the exclusive worldwide marketing rights to the Nova Skincare System, a Duster, the Tomorrow Garden Kit and a right of first offer on future products developed by Pagic LP (“Pagic”), formerly MK Enterprises LLC, a private company with a director in common. In conjunction with the Agreement, the Company agreed to issue Pagic and its assignees 20,000,000 common shares at a deemed cost of $1,000,000, based on the historical cost of the license, of which 11,611,975 shares were issued during the year ended March 31, 2006 and the balance during the year ended March 31, 2007. Of the 20,000,000 common shares issued, 9,428,205 common shares were issued to parties that became related parties to the Company. As part of the Agreement, the Company is committed to pay Pagic and its assignees, royalties of US$10 per Nova Skincare System unit sold, US$2 per Duster unit sold and 4.5% of Tomorrow Garden Kit net sales. In addition, the Company must pay a royalty of 3% of net sales related to ancillary product sales from these products. If the Company elects to acquire the rights to future products developed by Pagic, they must pay a royalty of 4.5% of net sales of the new product plus 3% of net sales from ancillary product sales. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 6. | Product License (Continued) In order to keep the products under license, the Company must pay a minimum royalty for each of the Nova Skincare System, Duster and Tomorrow Garden Kit and their related ancillary products of US$37,500 beginning April 1, 2007 and US$50,000 per year thereafter. For any new products acquired they will be subject to minimum royalties of US$50,000 per year beginning April 1, 2007. To keep the overall master license in good standing, the total of royalties and all other fees paid to Pagic shall be at least US$400,000 per year beginning April 1, 2007. During the year ended March 31, 2008, $565,135 (2007 - $269,745) was paid or accrued to Pagic under the terms of the license. Due to uncertainty in determining future cash flows related to products under license, the Company, during the year ended March 31, 2008, wrote-down the value of the product license by $1,306,074 to $1. |
| 7. | Prepaid Expenses Prepaid expenses as at March 31, 2008 consists of $2,015,837 (2007 - $285,690), which is the deferred portion of investor relations and business consulting services agreements with the balance consisting of $103,709 (2007 - $71,071) in prepaid insurance and rental deposits. |
| 8. | Vertigro Joint Venture On October 2, 2006, the Company entered into a letter agreement replaced on July 9, 2007 by the Vertigro Algae Stakeholders Letter of Agreement, (together “LOA”) with Pagic, West Peak Ventures of Canada Limited (“West Peak”) and Global Green, whereby Global Green agreed to fund the next phase of the development of a high density vertical bio-reactor technology. Pursuant to the LOA, the Company and Global Green established a commercial joint venture named “Vertigro” in which Global Green agreed to provide up to US$3,000,000 in initial funding to continue the research and development of the bio-reactor technology, construct a working prototype of the bio-reactor and develop the technology for commercial uses. The Company is obligated to provide product support, research and development, and the non-exclusive use of the Company’s properties and lands for which Global Green has agreed to reimburse the Company as part of its US$3,000,000 initial funding commitment. Until such time as the joint venture has fully repaid to Global Green the US$3,000,000, Global Green shall receive 70% of the net cash flow generated by anticipated future operations after which each of Global Green and the Company will hold a 50% interest in the Vertigro, subject to an aggregate 4.5% royalty to Pagic and West Peak. Vertigro covers the bio-reactor technology and any subsequent related technologies for the commercial scale products of algae based biomass for all industrial commercial and retail applications including, but not limited to bio-fuel, food, health, pharmaceutical, and animal and agricultural feeds. As at March 31, 2008, Global Green and the Company incurred a total of US$5,735,893 (2007 - US$2,023,379) in costs related to Vertigro. Of the costs incurred to March 31, 2008, Global Green paid US$4,338,204 (2007 - US$1,653,981) of which US$7,806 is included in accounts payable. During the year ended March 31, 2007, accounts receivable included US$369,398 due from Global Green. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 8. | Vertigro Joint Venture (Continued) Limited Liability Company Operating Agreement for Vertigro Algae Technologies, LLC On May 5, 2008, Valcent USA and Global Green signed a Limited Liability Company Operating Agreement (“New Operating Agreement”) replacing the LOA to form Vertigro Algae Technologies, LLC (“Vertigro Algae”), a Texas LLC, which is the formalized operating entity for the previous unincorporated operations of Vertigro. The business activities of Vertigro Algae will be the same as when the operations were under Vertigro. Under the New Operating Agreement, Global Green and the Company will each hold a 50% stake in Vertigro Algae, and have committed to fund project development according to ownership allocation. Further, the Company will acquire assets of Vertigro, including buildings, laboratory, and equipment. To allow for prior capital contributions, Global Green has incurred in excess of the Company’s prior aggregate capital contribution, Global Green will receive 70% of the net cash flow generated by Vertigro Algae until it has received $3,000,000 in excess of its 50% interest in such cash flow. Technology License Agreement On May 5, 2008, Vertigro Algae executed a Technology License Agreement (“Technology License”) together with Pagic, West Peak and the Company. The Technology License licenses certain algae biomass technology and intellectual property to Vertigro Algae for purposes of commercialization and exploitation for all industrial, commercial, and retail applications worldwide (“Algae Biomass Technology”). In return for the Algae Biomass Technology, both the Company and Global Green will each issue 300,000 common shares to Pagic (unissued), and also pay a one-time commercialization fee of US$50,000 upon the Algae Biomass Technology achieving commercial viability. The Technology License is subject to royalty of 4.5% of gross customer sales receipts for use of the Algae Biomass Technology; and aggregate annual royalty minimum amounts of US$50,000 in 2009, US$100,000 in 2010, and US$250,000 in 2011 and each year thereafter in which the Technology License is in place. All prior agreements between the Company and Pagic that relate to the Algae Biomass Technology will be replaced by the new Operating Agreement and the Technology License. |
| | | 2008 | | | 2007 | |
| Finished goods: | | | | | | |
| Nova Skincare Systems, 7,896 units (2007 – 10,877) | | $ | 291,703 | | | $ | 442,066 | |
| Raw materials | | | 325,492 | | | | 794,742 | |
| | | $ | 617,195 | | | $ | 1,236,808 | |
| | During the year ended March 31, 2008, raw materials inventory was written down by $413,216 (2007 - $nil) due to obsolescence. This amount has been included in product development expense for the year (Note 16). |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 10. | Convertible Notes Details of the convertible notes are as follows: Convertible note continuity: |
| | | US $ | | | CDN $ | |
| | | Balance | | | 2008 | | | 2008 | | | 2008 | | | 2008 | | | Balance | | | Balance | |
| | | March 31, | | | Issued | | | Equity | | | Interest / | | | | | | March 31, | | | March 31, | |
| Date of Issue | | 2007 | | | Principal | | | Portion | | | Penalty | | | Conversions | | | 2008 | | | 2008 | |
| | | | | | | | | | | | | | | | | | | | | | |
| July/August 2005 (Note 10(a)) | | $ | 316,957 | | | $ | 0 | | | $ | 0 | | | $ | 22,389 | | | $ | (79,521 | ) | | $ | 259,825 | | | $ | 265,583 | |
| April 2006 (Note 10(b)) | | | 495,607 | | | | 0 | | | | 0 | | | | 38,835 | | | | 0 | | | | 534,442 | | | | 546,841 | |
| April 2006 (Note 10(c)) | | | 79,115 | | | | 0 | | | | 0 | | | | 6,427 | | | | 0 | | | | 85,542 | | | | 87,527 | |
| December 2006 (Note 10(e)) | | | 670,486 | | | | 0 | | | | 0 | | | | 989,296 | | | | 0 | | | | 1,659,782 | | | | 1,698,289 | |
| January 2007 (Note 10(f)) | | | 813,084 | | | | 0 | | | | 0 | | | | 967,767 | | | | (211,668 | ) | | | 1,569,183 | | | | 1,605,558 | |
| August 2007 (Note 10(g)) | | | 0 | | | | 650,000 | | | | (230,007 | ) | | | 258,574 | | | | 0 | | | | 678,567 | | | | 694,310 | |
| September 2007 (Note 10(h)) | | | 0 | | | | 391,000 | | | | (213,249 | ) | | | 119,682 | | | | 0 | | | | 297,433 | | | | 304,633 | |
| | | $ | 2,375,249 | | | $ | 1,041,000 | | | $ | (443,256 | ) | | $ | 2,402,970 | | | $ | (291,189 | ) | | $ | 5,084,774 | | | $ | 5,202,741 | |
| | | US $ | | | CDN $ | |
| | | Balance | | | 2007 | | | 2007 | | | 2007 | | | 2007 | | | Balance | | | Balance | |
| | | March 31, | | | Issued | | | Equity | | | Interest / | | | | | | March 31, | | | March 31, | |
| Date of Issue | | 2006 | | | Principal | | | Portion | | | Penalty | | | Conversions | | | 2007 | | | 2007 | |
| | | | | | | | | | | | | | | | | | | | | | |
| July/August 2005 (Note 10(a)) | | $ | 1,429,104 | | | $ | 0 | | | $ | 0 | | | $ | 119,875 | | | $ | (1,232,022 | ) | | $ | 316,957 | | | $ | 365,959 | |
| April 2006 (Note 10(b)) | | | 0 | | | | 551,666 | | | | (388,313 | ) | | | 567,366 | | | | (235,112 | ) | | | 495,607 | | | | 572,228 | |
| April 2006 (Note 10(c)) | | | 0 | | | | 82,200 | | | | (48,776 | ) | | | 70,054 | | | | (24,363 | ) | | | 79,115 | | | | 91,346 | |
| December 2006 (Note 10(e)) | | | 0 | | | | 1,500,000 | | | | (1,067,962 | ) | | | 238,448 | | | | 0 | | | | 670,486 | | | | 774,143 | |
| January 2007 (Note 10(f)) | | | 0 | | | | 2,000,000 | | | | (1,331,232 | ) | | | 144,316 | | | | 0 | | | | 813,084 | | | | 938,786 | |
| | | $ | 1,429,104 | | | $ | 4,133,866 | | | $ | (2,836,283 | ) | | $ | 1,140,059 | | | $ | (1,491,497 | ) | | $ | 2,375,249 | | | $ | 2,742,462 | |
| | (a) | US$1,277,200 July – August 2005 Convertible Note To provide working capital for product development, during July and August 2005, the Company issued one-year, unsecured US$1,277,200 8% per annum convertible notes and three-year Class A and B warrants to acquire: (i) up to 913,332 common shares of the Company at a price per share of US$0.50; and (ii) up to an additional 913,332 common shares of the Company at a price per share of US$1.00. The holders of the convertible notes may elect to convert the notes into common shares of the Company at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the common stock for the ten trading days prior to conversion; and (ii) US$0.55. Accrued and unpaid interest may be converted into common shares of the Company at US$0.50 per share. The Company may, subject to notice provisions and the common shares trading above US$1.50 per share for more than twenty consecutive trading days, elect to payout the notes and interest due by paying 130% of the amount due under the notes plus interest. The common stock purchase warrants carry a “net cashless” exercise feature (“Cashless Conversion Feature”) allowing the holder thereof, under certain limited circumstances, to exercise the warrants without payment of the stated exercise price, but rather solely in exchange for the cancellation of that number of common shares into which such warrants are exercisable. As a result of the issuance of the warrants in conjunction with the convertible notes, the Company recorded a non-cash financing expense of $1,328,337. These convertible notes are unsecured, and due on demand. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 10. | Convertible Notes (Continued) |
| (a) | US$1,277,200 July – August 2005 Convertible Note (Continued) In conjunction with this financing, the Company paid consultants an amount equal to 10% of the gross proceeds, which was included in investor relations during the year ended March 31, 2006 and issued 425,735 common shares at a deemed value of $285,242. There are 255,440 finders’ A warrants outstanding whereby the holders have the right to purchase 255,440 common shares at US$0.50 per share until August 5, 2008 and 425,733 finders’ B warrants whereby the holders shall have the right to purchase 425,733 common shares at US$0.75 per share until August 5, 2008. A total of US$82,200 in registration penalties incurred in the year ended March 31, 2007 were converted to a new convertible debenture in the same amount on April 6, 2007 (Note 10(c)). During the year ended March 31, 2008, convertible notes of US$75,000 and interest totaling US$4,521 were converted to 262,057 common shares, and interest of US$22,389 (2007 – US$119,875) was accrued on the principal balance of these convertible notes. |
| | (b) | US$551,666 April 2006 Convertible Note On April 6, 2006, the Company consummated a private offering transaction with and among a syndicated group of investors, pursuant to which the Company issued, in the aggregate, US$551,666 in 8% per annum convertible notes and three-year Class A and B warrants to acquire: (i) up to 735,544 shares of the Company’s common stock at a price per share of US$0.50; and (ii) up to an additional 735,544 shares of the Company’s common stock at a price per share of US$1.00. Subject to certain limitations, the principal amount of the notes, together with any accrued interest may be converted into shares of the Company’s common stock at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the common stock for the ten trading days prior to conversion; or (ii) US$0.55. The convertible notes carry a redemption feature, which allows the Company to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that the common shares have a closing price of US$1.50 per share for at least twenty consecutive trading days and there has otherwise been no default. The common stock purchase warrants carry a Cashless Conversion Feature. These convertible notes are unsecured, and due on demand. In conjunction with these private offering transactions, the Company paid consultants: (i) US$55,166 cash, representing 10% of the gross proceeds realized; (ii) 183,886 shares of common stock; (iii) three-year warrants to purchase up to 110,320 shares of common stock at a price per share of US$0.50; and (iv) three-year warrants to purchase up to 183,867 shares of common stock at a price per share of US$0.75. During the year ended March 31, 2008, the Company accrued US$38,835 (2007 – US$567,366) in interest on the principal balance of these convertible notes. |
| | (c) | US$82,200 April 2006 penalty Convertible Note On April 6, 2006, and in conjunction with certain private placements, the Company reached a verbal agreement with investors wherein the Company agreed to convert US$82,200 in accrued penalties associated with the July 25, 2005 through August 5, 2005 convertible notes into US$82,200 convertible penalty notes (Note 10(a)) carrying terms similar to the July 25, 2005 through August 5, 2005 convertible notes and an aggregate of 109,600 warrants. These warrants carry a Cashless Conversion Feature and each warrant entitles the holder to purchase additional common shares for three years at a price of US$0.75 per share. These convertible notes are unsecured and due on demand. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 10. | Convertible Notes (Continued) |
| (c) | US$82,200 April 2006 penalty Convertible Note (Continued) During the year ended March 31, 2008, the Company accrued US$6,427 (2007 – US$70,054) in interest on the principal balance of these convertible notes. |
| | (d) | Warrant Exercise Price Reduction and Registration Penalty Interest Certain of the July and August 2005 and the April 6, 2006 convertible notes contained registration rights whereby the Company agreed to pay a penalty of 2% for every thirty days after a required filing and registration effective date plus a reduction in the warrant price of certain of the warrants issued of US$0.10. As a result of the Company not filing its registration statement until April 27, 2006, the Company incurred penalties, which have been included in interest expense. An aggregate of 3,407,372 previously issued share purchase warrants relating to certain of the July and August 2005 and the April 6, 2006 convertible notes have reduced exercise prices from US$0.50, US$0.75, and US$1.00 to US$0.40, US$0.65, and US$0.90, respectively. In 2007, the Company recognized $80,102 in interest expense with the corresponding amount to contributed surplus as a result of re-valuation of the warrants upon the change in the pricing. The registration statement has subsequently been declared effective. |
| | (e) | US$1,500,000 December 2006 Convertible Note On December 1, 2006, the Company accepted subscriptions of US$1,500,000 towards a private placement of 8% per annum, unsecured, convertible notes and three-year warrants to acquire: (i) up to an aggregate of 2,000,000 shares of the Company’s common stock at a price per share of US$0.50; and (ii) up to an additional 2,000,000 shares of the Company’s common stock at a price per share of US$1.00. Subject to certain limitations, the principal amount of the notes, together with any accrued interest may be converted into shares of the Company’s common stock at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the Company’s common stock for the ten trading days prior to conversion; or (ii) US$0.55. The convertible notes carry a redemption feature, which allows the Company to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that the common shares have a closing price of US$1.50 per share for at least twenty consecutive trading days and there has otherwise been no default. These convertible notes are unsecured and due on demand. The common stock purchase warrants may be exercised on a cashless basis. During the year ended March 31, 2008, the Company accrued US$989,296 (2007 - US$238,448) in interest on the principal balance of these convertible notes. The right of the note holders to convert into the Company’s common stock is subject to the contractual agreement between the parties that any conversion by the note holders may not lead at the date of such conversion to an aggregate equity interest in the common stock of the Company greater than 9.99% inclusive of any derivative securities including options, warrants, convertible debt, any other convertible debt securities, or any other financial instruments convertible into common equity. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 10. | Convertible Notes (Continued) |
| | (f) | US$2,000,000 January 2007 Convertible Note On January 29, 2007, the Company completed a private placement comprised of $2,000,000 convertible notes. The convertible notes will mature on December 11, 2008, carry interest at 6% per annum and are unsecured. The notes are convertible into “Units” at the note holders’ discretion at a conversion price of US$0.50 per Unit. Each “Unit” consists of one common share and one purchase warrant to purchase an additional common share at US$0.70 per share until December 11, 2008. The notes and any accrued interest are callable by the Company at any time after December 11, 2007 by providing thirty days’ written notice to the note holders. Interest on the notes will be compounded annually and be cumulative until the earlier of either the date the Company achieves pre-tax earnings or the end of the term. At the discretion of the note holders, interest on the notes is payable in either cash or units at US$0.50 per unit. In connection with this financing, the Company has paid consultants US$108,000 in cash and issued 135,000 warrants exercisable at US$0.50 per unit, with each unit consisting of one common share and one share purchase warrant to purchase a further common share at US$0.70 per share until December 11, 2008. The Company is obligated to file a resale registration statement on the underlying securities within four months of closing, which it has failed to do. As a result of the failure to file the registration statement, the Company recorded penalties of US$120,000 as of March 31, 2007 and a further US$289,973 during the year ended March 31, 2008. In addition, during the year ended March 31, 2008, convertible notes of US$200,000 and interest and registration penalty totaling US$11,668 were converted to 485,707 common shares, and the Company accrued US$677,794 in interest on the principal balance of these convertible notes (2007 - US$24,316). |
| | (g) | US$650,000 August 2007 Convertible Note On August 10, 2007, the Company issued an unsecured convertible term promissory note in the amount of US$650,000 to a third party. The convertible note is due on demand and bears interest at 6% with both interest and principal convertible at the option of the lender into units at US$0.60 per unit, with each unit consisting of one common share and one-half share purchase warrant with each whole share purchase warrant exercisable at US$0.75 to purchase an additional common share. After November 25, 2008, this convertible note accrues interest at the rate of 15% per annum. During the year ended March 31, 2008, the Company accrued US$230,007 (2007 – US$0) in interest on the principal balance of these convertible notes. The Company is required to register for trading the securities underlying the conversion features of this convertible note on a best efforts basis, but has failed to do so within terms agreed. A one-time financial penalty of US$28,567 for failure to register the securities underlying this convertible note within 180 days from the date of issuance has been incurred in the year ended March 31, 2008. The right of the note holder to convert into the Company’s common stock is subject to the contractual agreement between parties that any conversion by the note holder may not lead, at the date of such conversion, to an aggregate equity interest in the common stock of the Company greater than 9.99% inclusive of any derivative securities including options, warrants, convertible debt, any other convertible debt securities, or any other financial instruments convertible into common equity. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 10. | Convertible Notes (Continued) |
| | (h) | US$391,000 September 2007 Convertible Note On September 27, 2007, the Company issued unsecured one-year term convertible notes bearing interest at 6% per annum in the amount of US$391,000 to third parties. Both interest and principal may be converted at the option of the lender at any time at US$0.60 per unit, with each unit consisting of one common share and one-half share purchase warrant, with each whole share purchase warrant exercisable at US$0.75 to purchase an additional common share for a two-year term from the date of conversion. During the year ended March 31, 2008, the Company accrued US$96,222 (2007 - US$0) in interest on the principal balance of these convertible notes. The Company is required to register for trading the securities underlying the conversion features of this convertible note on a best efforts basis, but has failed to do so within terms agreed. A one-time financial penalty of US$23,460 for failure to register the securities underlying this convertible note within 90 days from the date of issuance has been incurred during the year ended March 31, 2008. |
| 11. | Related Party Transactions Related party transactions are in the ordinary course of business and are measured at the exchange amount at the time the agreement is entered into or services are provided. Related party transactions not disclosed elsewhere in these financial statements are as follows: |
| | | | | 2008 | | | 2007 | |
| (a) | Charges from Pagic, a company related by a common officer and director, for product development expenses including royalties | | $ | 565,135 | | | $ | 269,455 | |
| (b) | Charges from CFO and director for professional fees | | $ | 35,370 | | | $ | 33,000 | |
| | Short-term advance from CFO, at 10% interest per annum | | $ | 150,000 | | | $ | 0 | |
| | Charges from private companies with this director in common for: | | | | | | | | |
| | (i) | Office rent | | $ | 30,000 | | | $ | 7,500 | |
| | (ii) | Consulting fee | | $ | 150,000 | | | $ | 46,745 | |
| | (iii) | Unsecured loan advances | | $ | 57,364 | | | $ | 57,364 | |
| (c) | West Peak and its principal shareholder, a beneficial owner of more than 5% of the Company’s common shares: | | | | | | | | |
| | (i) | Unsecured loan advances, at 8% interest per annum | | $ | 977,665 | | | $ | 784,837 | |
| | (ii) | Consulting services | | $ | 52,500 | | | $ | 0 | |
| (d) | Advertising services of a private company with a director in common | | $ | 244,861 | | | $ | 337,749 | |
| (e) | Operational management consulting services of a director | | $ | 76,740 | | | $ | 0 | |
| (f) | Operational management consulting and investor relations services of a director and a related company | | $ | 75,085 | | | $ | 0 | |
| (g) | Bonus of 100,000 restricted common shares to a retiring director | | $ | 0 | | | $ | 52,889 | |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 11. | Related Party Transactions (Continued) Amounts due to related parties are non-interest bearing and have no specific terms of repayment. Due to related parties includes the following amounts at March 31 in respect to certain of the above transactions: |
| | | | 2008 | | | 2007 | |
| (a) | Pagic royalties, etc. | | $ | 189,156 | | | $ | 0 | |
| (b) | CFO charges and advances | | | 158,370 | | | | 43,500 | |
| (b) | Consulting services and unsecured loan advances | | | 73,896 | | | | 57,364 | |
| (c) | Consulting services and unsecured loan advances | | | 1,000,165 | | | | 784,362 | |
| (d) | Advertising | | | 52,145 | | | | 17,319 | |
| (e) and (f) | Operational management consulting | | | 16,784 | | | | 27,630 | |
| | | | $ | 1,490,516 | | | $ | 930,175 | |
| 12. | Commitments Commitments not disclosed elsewhere in these financial statements are as follows: On June 28, 2005, Valcent Manufacturing leased office and development space in El Paso, Texas, under a three-year lease at a cost of US$3,170 per month. There were five months remaining on the lease as at March 31, 2008. On November 16, 2007, Valcent EU leased office and development space in Launceston, Cornwall, UK, under a ten-year lease beginning November 15, 2007 and ending on November 15, 2017 at a quarterly cost of $26,017 (GB£12,550). There were 9 years and 7.5 months remaining on the lease as at March 31, 2008. Fiscal yearly commitments are as follows: |
| 2009 | | $ | 104,068 | |
| 2010 | | | 104,068 | |
| 2011 | | | 104,068 | |
| 2012 | | | 104,068 | |
| 2013 | | | 104,068 | |
| Thereafter | | | 305,037 | |
| | | $ | 825,377 | |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 13. | Long-Term Debt At March 31, the Company’s long-term debt outstanding was as follows: |
| | | 2008 | | | 2007 | |
| | | | | | | |
| Prime plus 0.25% (2007 – 8.50%) bank loan repayable in monthly instalments of US$2,336 including interest, due September 28, 2011, secured by a first charge on land and $108,471 of cash (2007 - $117,327 cash) | | $ | 174,862 | | | $ | 209,114 | |
| Less: Current portion | | | 16,250 | | | | 13,451 | |
| | | | | | | | | |
| | | $ | 158,612 | | | $ | 195,663 | |
| | The portion of long-term debt repayable in each of the next four fiscal years is as follows: |
| 2009 | | $ | 16,250 | |
| 2010 | | $ | 17,258 | |
| 2011 | | $ | 18,806 | |
| 2012 | | $ | 106,298 | |
| | Subsequent to March 31, 2008, the Company repaid this debt in its entirety. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| | (a) | Authorized: Unlimited number of common shares without par value Unlimited number of preferred shares without par value – None issued |
| | (b) | Issued and outstanding: The common share issuances consisted of the following transactions: |
| | | Number of Shares | | | Amount | |
| | | | | | (Note 2) | |
| | | | | | | |
| Balance, as at March 31, 2006 | | | 15,787,835 | | | $ | 4,099,870 | |
| Issued pursuant to product license purchase agreement (i) | | | 8,388,025 | | | | 419,401 | |
| Issued for investor and public relations services (iv) to (vii) | | | 705,000 | | | | 460,614 | |
| Issued as bonus (viii) | | | 100,000 | | | | 52,708 | |
| Private placement, net of share issue costs (ii) | | | 1,534,165 | | | | 1,028,266 | |
| Issued for financial consulting services (iii) | | | 183,886 | | | | 107,681 | |
| Conversion of convertible notes (ix) | | | 3,967,157 | | | | 1,668,363 | |
| Reallocation of equity upon conversion | | | | | | | | |
| of debentures | | | 0 | | | | 360,079 | |
Balance, as at March 31, 2007 | | | 30,666,068 | | | | 8,196,982 | |
Private placement, net of share issue costs (x) and (xi) | | | 8,525,578 | | | | 5,081,103 | |
| Issued for financial consulting services (xii) | | | 3,462,008 | | | | 2,414,233 | |
| Conversion of convertible notes (xv) | | | 747,764 | | | | 323,392 | |
| Reallocation of equity upon conversion | | | | | | | | |
| of debentures | | | 0 | | | | 232,808 | |
| Stock options exercised (xiv) | | | 175,000 | | | | 83,834 | |
| Stock-based compensation for options exercised | | | 0 | | | | 75,093 | |
| Warrants exercised (xiii) | | | 699,903 | | | | 283,837 | |
| | | | | | | | | |
| | | | 13,610,253 | | | | 8,494,300 | |
| Balance, as at March 31, 2008 | | | 44,276,321 | | | $ | 16,691,282 | |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 14. | Share Capital (Continued) |
| | (b) | Issued and outstanding (Continued): During the year ended March 31, 2007, the Company: |
| | (i) | Issued 8,388,025 shares pursuant to the Agreement (Note 6); |
| | (ii) | Completed a private placement of units whereby a total of 1,534,165 units were issued at US$0.60 per unit. Each unit consists of one common share and one common share purchase warrant to purchase an additional common share at US$0.80. Of the warrants issued, 833,332 expire on May 15, 2008, 270,833 on June 7, 2008 and 430,000 on August 18, 2008. The Company paid consultants $65,843 cash, 66,666 finders’ A warrants to purchase that number of common shares at US$0.80 until May 15, 2008, 21,666 finders’ A warrants to purchase that number of common shares at US$0.80 until June 7, 2008 and 34,400 finders’ A warrants to purchase that number of common shares at US$0.80 until August 18, 2008; |
| | (iii) | In conjunction with the April 6, 2006 convertible note offering, the Company issued 183,886 common shares to consultants; |
| | (iv) | Issued 120,000 common shares at US$0.45 per common share for investor relations services; |
| | (v) | Issued 400,000 common shares at US$0.64 per common share pursuant to a contract whereby a company is to provide investor relations services for a term of one year; |
| | (vi) | Issued 160,000 common shares at US$0.45 per common share pursuant to a contract whereby a company is to provide investor relations services for a term of one year; |
| | (vii) | Issued 25,000 common shares at US$0.45 per common share pursuant to a contract whereby a company is to provide public relations services for a term of one year; |
| (viii) | Issued 100,000 common shares at US$0.45 per common share to a retiring director of the Company in recognition of services rendered; and |
| | (ix) | Issued 3,967,157 common shares upon receiving conversion notices to convert US$1,424,369 in principal and interest from holders of convertible notes. |
| | | During the year ended March 31, 2008, the Company: |
| | (x) | Completed a private placement of units whereby a total of 8,382,721 units were issued at US$0.60 per unit for gross proceeds of US$5,029,636. Each unit consists of one common share and one-half common share purchase warrant with each whole warrant exercisable at US$0.75 to purchase an additional common share of the Company. The Company paid US$88,438 cash to consultants in connection with this private placement; |
| | (xi) | Completed a private placement of units whereby a total of 142,857 units were issued at US$0.70 per unit for gross proceeds of US$100,000. Each unit consist of one common share and one-half common share purchase warrant with each whole warrant exercisable at US$0.85 to purchase an additional common share of the Company; |
| | (xii) | Issued 3,462,008 shares in connection with seven financial consulting services agreements for administrative, investor relations, business and financial consulting services at an average deemed value of US$0.68 per share; |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 14. | Share Capital (Continued) |
| | (b) | Issued and outstanding (Continued): |
| | (xiii) | Issued 699,903 common shares on exercise of 699,903 share purchase warrants at an average exercise price of US$0.40 per warrant; |
| (xiv) | Issued 175,000 common shares on exercise of 175,000 stock options at an exercise price of US$0.50 per option by a past director of the Company; and |
| | (xv) | Issued 747,764 common shares upon receiving conversion notices to convert US$275,000 of convertible notes, interest of US$4,521 and a registration penalty of US$11,668 from holders (Note 10). |
| | (c) | Stock options The Company has a stock option plan (“Plan”) that allows for share options to be issued to employees, directors, officers, and consultants on both a qualified and non-qualified basis. The aggregate number of shares of common stock as to which options and bonuses may be granted from time to time under the Plan shall not exceed 20% of the Company’s issued and outstanding shares of common stock and the number of shares of common stock as to which options and bonuses may be granted under the US incentive portion of the Plan shall not exceed 17% of the Company’s issued and outstanding shares of common stock. The Company’s Plan provides that the terms of the options and the option prices shall be fixed by the board of directors or committee, and subject to the requirements of the exchange on which the Company’s common shares are traded, or any other governing regulatory body, at the time of exercise. Options granted shall expire after a period of five years or terminate three months after the recipient ceases to be an employee of the Company. Details of the status of the Company's stock options as at March 31, 2008 and 2007 and changes during the years then ended are as follows: |
| | | | | | Weighted | |
| | | | | | Average | |
| | | Number | | | US Exercise | |
| | | of Options | | | Price | |
| | | | | | | |
| Options outstanding, March 31, 2006 | | | 1,425,000 | | | $ 0.67 | |
| Granted | | | 3,010,000 | | | $ 0.59 | |
| Expired or forfeited | | | (600,000 | ) | | $ 0.61 | |
| Options outstanding, March 31, 2007 | | | 3,835,000 | | | $ 0.58 | |
| Granted | | | 2,515,000 | | | $ 0.69 | |
| Forfeited | | | (235,000 | ) | | $ 0.60 | |
| Exercised | | | (175,000 | ) | | $ 0.50 | |
| Options outstanding, March 31, 2008 | | | 5,940,000 | | | $ 0.63 | |
| Options exercisable, March 31, 2008 | | | 4,740,000 | | | $ 0.61 | |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 14. | Share Capital (Continued) |
| | (c) | Stock options (Continued) As at March 31, 2008 and 2007, the following stock options were outstanding: |
| | | | | | 2008 | | | 2007 | |
| Expiry Date | | US Exercise Price | | | Number of Options | | | Exercisable | | | Number of Options | | | Exercisable | |
| December 31, 2008 | | $ 0.50 | | | | 125,000 | | | | 125,000 | | | | 0 | | | | 0 | |
| March 22, 2009 | | $ 0.60 | | | | 500,000 | | | | 300,000 | | | | 500,000 | | | | 0 | |
| November 10, 2009 | | $ 0.60 | | | | 650,000 | | | | 450,000 | | | | 650,000 | | | | 300,000 | |
| March 1, 2010 | | $ 1.00 | | | | 75,000 | | | | 75,000 | | | | 75,000 | | | | 75,000 | |
| April 1, 2010 | | $ 0.70 | | | | 600,000 | | | | 0 | | | | 0 | | | | 0 | |
| April 9, 2010 | | $ 0.80 | | | | 50,000 | | | | 50,000 | | | | 50,000 | | | | 50,000 | |
| June 30, 2010 | | $ 0.60 | | | | 200,000 | | | | 200,000 | | | | 260,000 | | | | 260,000 | |
| October 2, 2010 | | $ 0.70 | | | | 200,000 | | | | 0 | | | | 0 | | | | 0 | |
| October 10, 2010 | | $ 0.70 | | | | 300,000 | | | | 300,000 | | | | 0 | | | | 0 | |
| December 20, 2010 | | $ 0.60 | | | | 300,000 | | | | 300,000 | | | | 0 | | | | 0 | |
| October 10, 2011 | | $ 0.70 | | | | 750,000 | | | | 750,000 | | | | 0 | | | | 0 | |
| February 21, 2011 | | $ 0.50 | | | | - | | | | - | | | | 300,000 | | | | 300,000 | |
| December 13, 2011 | | $ 0.55 | | | | 1,650,000 | | | | 1,650,000 | | | | 1,650,000 | | | | 1,325,000 | |
| December 20, 2011 | | $ 0.70 | | | | 155,000 | | | | 155,000 | | | | 0 | | | | 0 | |
| March 21, 2012 | | $ 0.60 | | | | 300,000 | | | | 300,000 | | | | 350,000 | | | | 350,000 | |
| March 31, 2012 | | $ 0.70 | | | | 85,000 | | | | 85,000 | | | | 0 | | | | 0 | |
| | | | | | | | 5,940,000 | | | | 4,740,000 | | | | 3,835,000 | | | | 2,660,000 | |
| | | The weighted average remaining contractual life is 2.77 years (2007 – 3.64 years). During the year ended March 31, 2008, 2,515,000 (2007 – 3,010,000) stock options were granted to directors, officers, employees and consultants. The fair value of these stock options is recognized as stock-based compensation expense over the vesting period of the options. The total fair value of these stock options was calculated at $1,020,687 (2007 - $1,385,759), of which $807,687 was recognized in 2008 (2007 - $1,127,141) and $213,003 will be recognized in 2009. Stock-based compensation expense includes $182,618 for the fair value of options issued in 2007, which vested in 2008. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 14. | Share Capital (Continued) |
| | (c) | Stock options (Continued) The stock-based compensation expense was $990,305 (2007 - $1,127,141). The fair value of stock options granted and the issue of finders’ warrants are calculated using the following weighted average assumptions: |
| | | 2008 | | | 2007 | |
| | | | | | | |
| Expected life (years) | | | 2 - 5 | | | | 2 - 4 | |
| Interest rate | | | 4.17 | % | | | 4.64 | % |
| Volatility | | | 94.91 | % | | | 111.90 | % |
| Estimated forfeitures | | | 0.00 | % | | | 0.00 | % |
| Dividend yield | | | 0.00 | % | | | 0.00 | % |
| | (d) | Warrants Each of the Company’s share purchase warrants are exercisable into one common share. As at March 31, 2008 and 2007 and the changes during the years then ended are as follows: |
| | | Number of Warrants | | | Weighted Average US Price | |
| | | | | | | |
| Warrants outstanding and exercisable, | | | | | | |
March 31, 2006 | | | 4,087,021 | | | $ | 0.69 | |
Granted | | | 7,666,795 | | | $ | 0.74 | |
| | | | | | | | | |
| Warrants outstanding and exercisable, | | | | | | | | |
March 31, 2007 | | | 11,753,816 | | | $ | 0.73 | |
Granted | | | 4,698,338 | | | $ | 0.90 | |
Exercised | | | (699,903 | ) | | $ | 0.41 | |
| | | | | | | | | |
| Warrants outstanding and exercisable, | | | | | | | | |
March 31, 2008 | | | 15,752,251 | | | $ | 0.74 | |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 14. | Share Capital (Continued) |
| | (d) | Warrants (Continued) As at March 31 the following share purchase warrants were outstanding: |
| Expiry Date | | US Exercise Price | | | 2008 Number of Warrants | | | 2007 Number of Warrants | |
| May 15, 2008 (i) | | $ 0.80 | | | | 833,332 | | | | 833,332 | |
| May 15, 2008 (i) | | $ 0.80 | | | | 66,666 | | | | 66,666 | |
| June 7, 2008 (i) | | $ 0.80 | | | | 270,833 | | | | 270,833 | |
| June 7, 2008 (i) | | $ 0.80 | | | | 21,666 | | | | 21,666 | |
| July 25, 2008 | | $ 0.50 | | | | 526,660 | | | | 526,660 | |
| July 25, 2008 (ii) | | $ 0.40 | | | | 480,650 | | | | 913,332 | |
| July 25, 2008 (i) | | $ 1.00 | | | | 526,660 | | | | 526,660 | |
| July 25, 2008 (ii) | | $ 0.90 | | | | 913,332 | | | | 913,332 | |
| July 25, 2008 | | $ 0.50 | | | | 216,000 | | | | 216,000 | |
| July 25, 2008 (i) | | $ 0.75 | | | | 360,001 | | | | 360,001 | |
| August 5, 2008 (i) | | $ 0.50 | | | | 262,932 | | | | 262,932 | |
| August 5, 2008 (i) | | $ 1.00 | | | | 262,932 | | | | 262,932 | |
| August 5, 2008 | | $ 0.50 | | | | 39,440 | | | | 39,440 | |
| August 5, 2008 (i) | | $ 0.75 | | | | 65,733 | | | | 65,733 | |
| August 18, 2008 | | $ 0.80 | | | | 430,000 | | | | 430,000 | |
| August 18, 2008 (i) | | $ 0.80 | | | | 34,400 | | | | 34,400 | |
| December 11, 2008 (i) | | $ 0.70 | | | | 435,554 | | | | 0 | |
| December 11, 2008 | | $ 0.50 | | | | 135,000 | | | | 135,000 | |
| April 6, 2009 | | $ 0.40 | | | | 513,334 | | | | 735,555 | |
| April 6, 2009 | | $ 0.90 | | | | 735,555 | | | | 735,555 | |
| April 6, 2009 | | $ 0.50 | | | | 65,320 | | | | 110,320 | |
| April 6, 2009 | | $ 0.75 | | | | 183,867 | | | | 183,867 | |
| April 6, 2009 | | $ 0.65 | | | | 109,600 | | | | 109,600 | |
| April 23, 2009 | | $ 0.75 | | | | 833,333 | | | | 0 | |
| May 24, 2009 | | $ 0.75 | | | | 223,333 | | | | 0 | |
| July 10, 2009 | | $ 0.75 | | | | 52,500 | | | | 0 | |
| October 9, 2009 | | $ 0.75 | | | | 608,333 | | | | 0 | |
| December 1, 2009 | | $ 0.50 | | | | 2,000,000 | | | | 2,000,000 | |
| December 1, 2009 | | $ 1.00 | | | | 2,000,000 | | | | 2,000,000 | |
| December 18, 2009 | | $ 0.75 | | | | 2,348,857 | | | | 0 | |
| February 19, 2010 | | $ 0.75 | | | | 125,000 | | | | 0 | |
| March 21, 2010 | | $ 0.85 | | | | 71,428 | | | | 0 | |
| | | | | | | | | | | | | |
| Total at March 31, 2008 | | | | | | | 15,752,251 | | | | 11,753,816 | |
| | (i) | Subsequent to March 31, 2008, the Company extended the expiry dates of these 2,877,776 warrants to December 31, 2008. No changes were made to the exercise prices. |
| | (ii) | Subsequent to March 31, 2008, the Company extended the expiry date of these 1,393,982 warrants to April 9, 2009. No changes were made to the exercise prices. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 14. | Share Capital (Continued) |
| | | 2008 | | | 2007 | |
| | | | | | | |
| Contributed surplus, beginning of year | | $ | 3,253,333 | | | $ | 1,663,066 | |
| Non-cash financing expense | | | 0 | | | | 463,126 | |
| Issuance of warrants on conversion of debentures | | | 34,849 | | | | 0 | |
| Re-pricing of warrants | | | 0 | | | | 80,102 | |
| Stock option expense for year | | | 990,305 | | | | 1,127,141 | |
| Stock options exercised during the year | | | (75,093 | ) | | | 0 | |
| | | | | | | | | |
| Contributed surplus, end of year | | $ | 4,203,394 | | | $ | 3,333,435 | |
| | (f) | Conversion component of convertible notes |
| | | | |
| Balance, March 31, 2006 | | $ | 348,532 | |
| Issuance of convertible debt | | | 3,299,405 | |
| Conversion of debentures | | | (360,079 | ) |
| | | | | |
| Balance, March 31, 2007 | | | 3,287,858 | |
| Issuance of convertible debt | | | 456,246 | |
| Re-pricing of warrants | | | 80,102 | |
| Conversion of debentures | | | (232,808 | ) |
| | | | | |
| Balance, March 31, 2008 | | $ | 3,591,398 | |
| 15. | Deferred Income Taxes The tax effects of the temporary differences that give rise to the Company’s future tax assets and liabilities are as follows: |
| | | 2008 | | | 2007 | |
| | | | | | | |
| Future income tax assets | | | | | | |
Non-capital losses carried forward | | $ | 4,593,497 | | | $ | 2,731,027 | |
Net capital losses carried forward | | | 186,646 | | | | 244,794 | |
Temporary differences on assets | | | 47,417 | | | | 0 | |
| | | | | | | | | |
| | | | 4,827,560 | | | | 2,975,821 | |
| Valuation allowance for future income tax assets | | | (4,827,560 | ) | | | (2,975,821 | ) |
| Future income tax assets, net | | $ | 0 | | | $ | 0 | |
| | The Company evaluates its valuation allowance requirements based on projected future operations. When circumstances change and this causes a change in management’s judgment about the recoverability of future tax assets, the impact of the change in the valuation allowance will be reflected in current income. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 15. | Deferred Income Taxes (Continued) The Company has non-capital losses available for income tax purposes in Canada, the United Kingdom and the United States totaling $15,305,017 (2007 - $8,008,876), which expire in various amounts from 2009 to 2028. This amount can be used to reduce taxable income of future years. Additionally, the Company has capital losses of $1,435,740 (2007 - $1,435,740), which are available to reduce capital gains in future periods. The reconciliation of income tax provision computed at statutory rates to the reported income tax provision is as follows: |
| | | 2008 | | | 2007 | |
| | | | | | | |
| | | | 33.33 | % | | | 34.12 | % |
| | | | | | | | | |
| Income tax benefit computed at Canadian statutory rates | | $ | 4,071,130 | | | $ | 2,731,027 | |
| Temporary differences not recognized | | | (7,665 | ) | | | 0 | |
| Permanent differences not recognized | | | (1,499,462 | ) | | | (1,692,480 | ) |
| Income tax rate changes | | | (37,655 | ) | | | 0 | |
| Unrecognized tax losses | | | (2,526,348 | ) | | | (1,038,547 | ) |
| | | | | | | | | |
| | | $ | 0 | | | $ | 0 | |
| 16. | Product Development Costs As the Company is in the development stage, test sales, bad debts and write-down of inventory related to the Nova Skincare System are included in product development costs as follows: |
| | | 2008 | | | 2007 | |
| | | | | | | |
| Product development costs | | $ | 4,653,998 | | | $ | 1,576,107 | |
| Test sales, Nova Skincare System | | | (1,079,432 | ) | | | (17,484 | ) |
| Bad debts | | | 93,653 | | | | 3,798 | |
| Write-down of inventory | | | 413,216 | | | | 0 | |
| | | | (572,563 | ) | | | (13,686 | ) |
| | | | | | | | | |
| Product development costs, net | | $ | 4,081,435 | | | $ | 1,562,421 | |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 17. | Segmented Information Operating segments are defined as components of an enterprise about which separate financial information is available, which is evaluated regularly by management in deciding how to allocate resources and in assessing performance. All of the Company’s operations are in the research, product development, and resale sectors. The Company’s product development, research, and resale operations are focused in the United States and, on a secondary basis, in the United Kingdom whereby management of the Company is responsible for business results and the operational decision making. Canadian operations are core to administration and finance functions. The Company’s operations are, therefore, segmented on a geographic basis. |
| | | United States | | | United Kingdom | | | Canada | | | Consolidated | |
| March 31, 2008 | | | | | | | | | | | | |
| Segmented revenue | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 0 | |
| Segmented loss | | $ | 5,432,464 | | | $ | 757,819 | | | $ | 6,120,463 | | | $ | 12,310,746 | |
| Identifiable assets | | $ | 1,979,342 | | | $ | 518,932 | | | $ | 2,107,640 | | | $ | 4,605,914 | |
| | | | | | | | | | | | | | | | | |
| March 31, 2007 | | | | | | | | | | | | | | | | |
| Segmented revenue | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 0 | |
| Segmented loss | | $ | 2,937,034 | | | $ | 0 | | | $ | 4,923,413 | | | $ | 7,860,447 | |
| Identifiable assets | | $ | 2,364,342 | | | $ | 0 | | | $ | 1,778,143 | | | $ | 4,142,485 | |
| 18. | Promissory Notes Payable As at March 31, promissory notes payable consists of the following due to a third party lender: |
| | | 2008 | | | 2007 | |
| | | | | | | |
| 7% simple interest, US$100,000 promissory note payable, unsecured, no terms of repayment | | $ | 115,971 | | | $ | 115,460 | |
| 10% simple interest, US$150,000 promissory note payable, unsecured, due May 15, 2008 (*) | | | 154,196 | | | | 0 | |
| | | | | | | | | |
| | | $ | 270,167 | | | $ | 115,460 | |
| (*) | Pursuant to the promissory note agreement, the Company is obligated to issue 100,000 common shares of the Company at maturity. Subsequent to March 31, 2008, the Company repaid the principal and interest and issued the 100,000 common shares at US$0.66 per common share. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 19. | Subsequent Events Subsequent events not disclosed elsewhere in these financial statements are as follows: |
| (a) | The Company entered into the following agreements on April 1, 2008 and May 1, 2008 respectively: |
| | (i) | A financial services agreement with an arm’s length private company for a term of seven months. Pursuant to the agreement, the Company issued 120,000 common shares valued at US$84,000; and |
| | (ii) | A financial and business consulting agreement with an individual consultant for a term of twelve months. Pursuant to the agreement, the Company issued 300,000 common shares valued at US$201,000. |
| (b) | The Company completed a series of US$0.60 per unit private placements. Each unit consisted of one common share and one-half common share purchase warrant with each whole warrant exercisable at US$0.75 per share for two years. The private placements took place on May 7, June 6, and July 23, 2008, respectively, with details as follows: |
| | (i) | issued 2,996,666 units for gross proceeds of US$1,798,000. The Company paid US$122,577 in cash consultant’s fees with respect to this issuance; |
| | (ii) | issued 195,000 units for gross proceeds of US$117,000. The Company paid no consultant’s fees respecting the issuance; and |
| | (iii) | issued 61,000 units for gross proceeds of US$36,600. The Company paid US$2,562 in cash consultant’s fees with respect to this issuance. |
| (c) | The Company issued the following common shares in relation to the conversion of certain notes: |
| | (i) | On June 2, 2008, the Company issued 245,049 common shares relating to the conversion of US$100,000 in convertible debt and US$9,403 in accumulated interest; and |
| | (ii) | On July 3, 2008, the Company issued 267,221 common shares relating to the conversion of US$92,281 in convertible debt and US$9,243 in accumulated interest. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 19. | Subsequent Events (Continued) |
| (d) | On July 21, 2008, the Company closed a financing of zero coupon, 12% interest, senior secured convertible promissory notes in the amount of US$2,428,160 with an aggregate purchase price of US$2,168,000 with four investors, one of which was the Company’s Chief Financial Officer as to US$168,000. The debt is convertible into shares of common stock at the lesser of US$0.51 per share (unless the conversion price has been adjusted pursuant to further contract covenants) and 70% of the average of the five lowest closing bid prices for the ten preceding trading days. The Company issued each purchaser in the private placement two warrants, one warrant being redeemable by the Company and the other being non-redeemable. The non-redeemable warrants are exercisable at US$0.55 and permit the holder to purchase shares of common stock equal to 100% of the number of shares issuable upon the conversion of the notes calculated on July 21, 2008. The redeemable warrants are exercisable at US$0.75 and permit the holder to purchase common stock equal to 50% of the number of shares issuable upon the conversion of the notes issued calculated on the closing date. The Company issued a total of redeemable warrants to purchase an aggregate of 4,761,098 shares of common stock and a total of redeemable warrants to purchase an aggregate of 2,380,550 shares of common stock. Further, the Company issued 439,216 non-redeemable warrants and 219,608 redeemable warrants and $160,000 in cash fees to close the transaction. Each non-redeemable warrant is exercisable at US$0.55, and each redeemable warrant is exercisable at US$0.75; warrants carry a term of five years from the date of closing of the financing. The redeemable warrants may be redeemed by the Company only if certain conditions have been satisfied including the Company’s common stock having closed at $1.50 per share for a period of 20 consecutive trading days and the warrant holder being able to resell the shares acquired upon exercise through a resale registration statement or under Rule 144 of the Securities Act. |
| 20. | Generally Accepted Accounting Principles in Canada and the United States Reconciliation of Net Loss Under Canadian GAAP to US GAAP The effect of the material measurement differences between generally accepted accounting principles in Canada and the United States on the Company’s net loss is summarized as follows: |
| | | 2008 | | | 2007 | | | 2006 | |
| | | | | | | | | | |
| Net loss under Canadian GAAP (Note 2) | | $ | 12,712,358 | | | $ | 8,138,393 | | | $ | 3,466,888 | |
| Add (deduct): | | | | | | | | | | | | |
| Interest and accretion expense (Note 20(b)) | | | 399,496 | | | | 1,463,628 | | | | (416,266 | ) |
| Debt issue costs (Note 20(c)) | | | 135,733 | | | | 108,696 | | | | (338,997 | ) |
| Product development (Note 20(d)) | | | (1,878,438 | ) | | | (420,670 | ) | | | – | |
| Net loss of interest in joint venture (Note 20(d)) | | | 1,878,438 | | | | 420,670 | | | | – | |
| Foreign exchange gain/loss and other (Note 20(b)) | | | 45,301 | | | | 38,622 | | | | 133,254 | |
| | | | | | | | | | | | | |
| Net loss and comprehensive loss under US GAAP | | $ | 13,292,888 | | | $ | 9,749,339 | | | $ | 2,844,879 | |
| | | | | | | | | | | | | |
| Loss per share under US GAAP – basic and diluted | | $ | 0.37 | | | $ | 0.51 | | | $ | 0.27 | |
| | | | | | | | | | | | | |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 20. | Generally Accepted Accounting Principles in Canada and the United States (Continued) Presentation of Balance Sheets Under Canadian and United States GAAP Balance sheets under Canadian GAAP and US GAAP are as follows: |
| | | 2008 | | | 2007 | |
| | | Canadian GAAP | | | US GAAP | | | Canadian GAAP | | | US GAAP | |
| | | | | | | | | (Note 2) | | | (Note 20(f)) | |
| | | | | | | | | | | | | |
| Assets | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Current | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 163,437 | | | $ | 163,437 | | | $ | 314,972 | | | $ | 314,972 | |
| Accounts receivable | | | 462,156 | | | | 462,156 | | | | 462,055 | | | | 462,055 | |
| Prepaid expenses | | | 2,119,546 | | | | 2,119,546 | | | | 356,761 | | | | 356,761 | |
| Inventories | | | 617,195 | | | | 617,195 | | | | 1,236,808 | | | | 1,236,808 | |
| | | | | | | | | | | | | | | | | |
| | | | 3,362,334 | | | | 3,362,334 | | | | 2,370,596 | | | | 2,370,596 | |
| | | | | | | | | | | | | | | | | |
| Restricted cash | | | 108,471 | | | | 108,471 | | | | 117,327 | | | | 117,327 | |
| Debt issue costs (Note 20(c)) | | | – | | | | 94,568 | | | | – | | | | 230,301 | |
| Property and equipment | | | 1,135,108 | | | | 1,135,108 | | | | 348,487 | | | | 348,487 | |
| Product license | | | 1 | | | | 1 | | | | 1,306,075 | | | | 1,306,075 | |
| | | | | | | | | | | | | | | | | |
| | | $ | 4,605,914 | | | $ | 4,700,482 | | | $ | 4,142,485 | | | $ | 4,372,786 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Liabilities and Shareholders' Deficiency | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Current | | | | | | | | | | | | | | | | |
| Accounts payable | | $ | 382,605 | | | $ | 382,605 | | | $ | 115,744 | | | $ | 115,744 | |
| Accrued liabilities | | | 153,958 | | | | 153,958 | | | | 53,906 | | | | 53,906 | |
| Current portion of long-term debt | | | 16,250 | | | | 16,250 | | | | 13,451 | | | | 13,451 | |
| Promissory notes payable | | | 270,167 | | | | 270,167 | | | | 115,460 | | | | 115,460 | |
| Due to related parties | | | 1,490,516 | | | | 1,490,516 | | | | 930,175 | | | | 930,175 | |
| Convertible notes (Note 20(b)) | | | 5,202,741 | | | | 5,389,387 | | | | 2,742,462 | | | | 2,282,483 | |
| | | | | | | | | | | | | | | | | |
| | | | 7,516,237 | | | | 7,702,883 | | | | 3,971,198 | | | | 3,511,219 | |
| | | | | | | | | | | | | | | | | |
| Long-term debt | | | 158,612 | | | | 158,612 | | | | 195,663 | | | | 195,663 | |
| | | | | | | | | | | | | | | | | |
| | | | 7,674,849 | | | | 7,861,495 | | | | 4,166,861 | | | | 3,706,882 | |
| | | | | | | | | | | | | | | | | |
| Shareholders' Equity (Deficiency) | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Share capital (Note 20(b)) | | | 16,691,282 | | | | 16,866,115 | | | | 8,196,982 | | | | 8,434,032 | |
| Contributed surplus (Note 20(b)) | | | 4,203,394 | | | | 9,097,348 | | | | 3,333,435 | | | | 8,063,460 | |
| Conversion component of convertible notes (Note 20(b)) | | | 3,591,398 | | | | – | | | | 3,287,858 | | | | – | |
| Accumulated deficit from prior operations | | | (3,237,370 | ) | | | (3,237,370 | ) | | | (3,237,370 | ) | | | (3,237,370 | ) |
| Accumulated deficit during the development stage | | | (24,317,639 | ) | | | (25,887,106 | ) | | | (11,605,281 | ) | | | (12,594,218 | ) |
| | | | | | | | | | | | | | | | | |
| | | | (3,068,935 | ) | | | (3,161,013 | ) | | | (24,376 | ) | | | 665,904 | |
| | | | | | | | | | | | | | | | | |
| | | $ | 4,605,914 | | | $ | 4,700,482 | | | $ | 4,142,485 | | | $ | 4,372,786 | |
| | | | | | | | | | | | | | | | | |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 20. | Generally Accepted Accounting Principles in Canada and the United States (Continued) |
| (a) | Recently Adopted Accounting Pronouncements |
| (i) | Accounting for Uncertainty in Income Taxes In June 2006, the Financial Accounting Standards Board (‘FASB’) issued Interpretation No. 48, “Accounting for Uncertainty in Income Taxes, an interpretation of FASB Statements No. 109” (“FIN 48”). FIN 48 clarifies the accounting for uncertainty in income taxes by prescribing a two-step method of first evaluating whether a tax position has met a more-likely-than-not recognition threshold and second, measuring that tax position to determine the amount of benefit to be recognized in the financial statements. FIN 48 provides guidance on the presentation of such positions within a classified balance sheet as well as on derecognition, interest and penalties, accounting in interim periods, disclosure, and transition. FIN 48 is effective for fiscal years beginning after December 15, 2006. The adoption of this statement during the year did not have a material effect on the Company's consolidated financial statements. |
| (ii) | Accounting for Servicing of Financial Assets In March 2006, the FASB issued SFAS No. 156, "Accounting for Servicing of Financial Assets, an amendment of FASB Statement No. 140, Accounting for Transfers and Servicing of Financial Assets and Extinguishments of Liabilities". This statement requires all separately recognized servicing assets and servicing liabilities be initially measured at fair value, if practicable, and permits for subsequent measurement using either fair value measurement with changes in fair value reflected in earnings or the amortization and impairment requirements of Statement No. 140. The subsequent measurement of separately recognized servicing assets and servicing liabilities at fair value eliminates the necessity for entities that manage the risks inherent in servicing assets and servicing liabilities with derivatives to qualify for hedge accounting treatment and eliminates the characterization of declines in fair value as impairments or direct write-downs. SFAS No. 156 is effective for an entity's first fiscal year beginning after September 15, 2006. The adoption of this statement during the year did not have a material effect on the Company's consolidated financial statements. |
| (iii) | Accounting for Certain Hybrid Financial Instruments In February 2006, the FASB issued SFAS No. 155, "Accounting for Certain Hybrid Financial Instruments-an amendment of FASB Statements No. 133 and 140", to simplify and make more consistent the accounting for certain financial instruments. SFAS No. 155 amends SFAS No. 133, "Accounting for Derivative Instruments and Hedging Activities"; to permit fair value re-measurement for any hybrid financial instrument with an embedded derivative that otherwise would require bifurcation, provided that the whole instrument is accounted for on a fair value basis. SFAS No. 155 amends SFAS No. 140, "Accounting for the Impairment or Disposal of Long-Lived Assets", to allow a qualifying special-purpose entity to hold a derivative financial instrument that pertains to a beneficial interest other than another derivative financial instrument. SFAS No. 155 applies to all financial instruments acquired or issued after the beginning of an entity's first fiscal year that begins after September 15, 2006, with earlier application allowed. The adoption of this statement during the year did not have a material effect on the Company's consolidated financial statements. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 20. | Generally Accepted Accounting Principles in Canada and the United States (Continued) |
| (b) | Convertible Notes Under Canadian GAAP, the principal amount of financial liabilities convertible in common shares of an entity are bifurcated between its liability and equity components. The liability component commonly consists of an entity’s obligation to make principal and interest payments while the equity component consists of the debtor’s option to convert the financial liability into common shares of the entity. The Company follows the residual value method of bifurcation under which the difference between the principal amount and the fair value of the liability component is allocated to the equity component. The liability component of the financial liability is accreted over its estimated life to the principal amount using the effective interest method. Upon conversion of the financial liability to common shares of the entity, the difference between the carrying value of the liability component and the principal amount, if any, is recognized in income as interest expense and the principal amount and equity component are transferred to share capital. Should the debtor’s option to convert the financial liability into common shares of the entity expire unexercised, the equity component is transferred to contributed surplus. Under US GAAP, the principal amount of financial liabilities convertible in common shares of an entity are not bifurcated between its liability and equity components unless the debtor’s option to convert the financial liability into common shares of the entity is in-the-money on the date of issuance, which results in a beneficial conversion feature. The value of the beneficial conversion feature is classified in equity and presented as additional paid-in-capital, the US GAAP equivalent of contributed surplus. The difference between the principal amount of a financial liability and the value of the beneficial conversion feature is classified in liabilities. The discounted financial liability is accreted over its estimated life to the principal amount using the effective interest method. Upon conversion of the financial liability to common shares of the entity, the difference between the carrying value of the financial liability and the principal amount, if any, is recognized in income as interest expense and the principal amount and amount of the beneficial conversion feature are transferred to share capital. |
| (c) | Debt Issue Costs Under Canadian GAAP, an entity may make an accounting policy choice to either defer transaction costs that are directly attributable to the issue of a financial liability classified as other than held-for-trading or to recognize all transaction costs in net income as incurred. The Company’s accounting policy is to recognize all transaction costs in net income as incurred. Under US GAAP, transaction costs that are directly attributable to the issue of a financial liability are deferred and amortized on a straight-line basis over the life of the financial liability. |
| (d) | Proportionate Consolidation Under Canadian GAAP, the accounts of joint ventures are proportionately consolidated while US GAAP requires investments in joint ventures to be accounted for under the equity method. Under Canadian GAAP, the Company has classified amounts advanced to and expended by its Vertigro joint venture of $1,878,438 (2007 - $420,670; 2006 - $nil) as product development in its statement of operations. Under US GAAP, these amounts are classified as net loss from interest in joint venture. This difference does not have material effect on the Company's consolidated balance sheet. Additional information concerning the Company’s interests in a joint venture is presented in Note 8. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 20. | Generally Accepted Accounting Principles in Canada and the United States (Continued) |
| (e) | Recently Issued Accounting Pronouncements |
| (i) | Accounting for Financial Guarantee Insurance Contracts In May 2008, the FASB issued SFAS No. 163, “Accounting for Financial Guarantee Insurance Contracts – An interpretation of FASB Statement No. 60”. SFAS 163 requires that an insurance enterprise recognize a claim liability prior to an event of default when there is evidence that credit deterioration has occurred in an insured financial obligation. It also clarifies how Statement 60 applies to financial guarantee insurance contracts, including the recognition and measurement to be used to account for premium revenue and claim liabilities, and requires expanded disclosures about financial guarantee insurance contracts. It is effective for financial statements issued for fiscal years beginning after December 15, 2008, except for some disclosures about the insurance enterprise’s risk-management activities. SFAS 163 requires that disclosures about the risk-management activities of the insurance enterprise be effective for the first period beginning after issuance. Except for those disclosures, earlier application is not permitted. The adoption of this statement is not expected to have a material effect on the Company’s consolidated financial statements. |
| (ii) | Hierarchy of GAAP In May 2008, the FASB issued SFAS No. 162, “The Hierarchy of Generally Accepted Accounting Principles”. SFAS 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements of non-governmental entities that are presented in conformity with GAAP in the United States. It is effective 60 days following the SEC’s approval of the Public Company Accounting Oversight Board amendments to AU Section 411, “The Meaning of Present Fairly in Conformity With Generally Accepted Accounting Principles”. The adoption of this statement is not expected to have a material effect on the Company’s consolidated financial statements. |
| (iii) | Disclosures about Derivative Instruments and Hedging Activities In March 2008, the FASB issued SFAS No. 161, “Disclosures about Derivative Instruments and Hedging Activities – an amendment to FASB Statement No. 133”. SFAS No. 161 is intended to improve financial standards for derivative instruments and hedging activities by requiring enhanced disclosures to enable investors to better understand their effects on an entity's financial position, financial performance and cash flows. Entities are required to provide enhanced disclosures about: (a) how and why an entity uses derivative instruments; (b) how derivative instruments and related hedged items are accounted for under Statement 133 and its related interpretations; and (c) how derivative instruments and related hedged items affect an entity’s financial position, financial performance and cash flows. It is effective for financial statements issued for fiscal years beginning after November 15, 2008, with early adoption encouraged. The Company is currently assessing the impact of the adoption of this statement. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 20. | Generally Accepted Accounting Principles in Canada and the United States (Continued) |
| (e) | Recently Issued Accounting Pronouncements (Continued) |
| | (iv) | Business Combinations In December 2007, the FASB issued SFAS No. 141R, “Business Combinations”. This statement replaces SFAS 141 and defines the acquirer in a business combination as the entity that obtains control of one or more businesses in a business combination and establishes the acquisition date as the date that the acquirer achieves control. SFAS 141R requires an acquirer to recognize the assets acquired, the liabilities assumed and any non-controlling interest in the acquiree at the acquisition date, measured at their fair values as of that date. SFAS 141R also requires the acquirer to recognize contingent consideration at the acquisition date, measured at its fair value at that date. This statement is effective for fiscal years and interim periods within those fiscal years, beginning on or after December 15, 2008 and earlier adoption is prohibited. The adoption of this statement is not expected to have a material effect on the Company's consolidated financial statements. |
| (v) | Non-controlling Interests in Consolidated Financial Statements Liabilities In December 2007, the FASB issued SFAS No. 160, “Non-controlling Interests in Consolidated Financial Statements Liabilities – an Amendment of ARB No. 51”. This statement amends ARB 51 to establish accounting and reporting standards for the non-controlling interest in a subsidiary and for the deconsolidation of a subsidiary. This statement is effective for fiscal years and interim periods within those fiscal years, beginning on or after December 15, 2008 and earlier adoption is prohibited. The adoption of this statement is not expected to have a material effect on the Company's consolidated financial statements. |
| (vi) | The Fair Value Option for Financial Assets and Financial Liabilities In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities – Including an Amendment of FASB Statement No. 115”. This statement permits entities to choose to measure many financial instruments and certain other items at fair value. Most of the provisions of SFAS No. 159 apply only to entities that elect the fair value option. However, the amendment to SFAS No. 115, “Accounting for Certain Investments in Debt and Equity Securities”, applies to all entities with available-for-sale and trading securities. SFAS No. 159 is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. Early adoption is permitted as of the beginning of a fiscal year that begins on or before November 15, 2007, provided the entity also elects to apply the provision of SFAS No. 157, “Fair Value Measurements”. The Company is currently assessing the impact of the adoption of this statement. |
| (vii) | Fair Value Measurements In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements”. The objective of SFAS No. 157 is to increase consistency and comparability in fair value measurements and to expand disclosures about fair value measurements. SFAS No. 157 defines fair value, establishes a framework for measuring fair value in GAAP, and expands disclosures about fair value measurements. SFAS No. 157 applies under other accounting pronouncements that require or permit fair value measurements and does not require any new fair value measurements. The provisions of SFAS No. 157 are effective for fair value measurements made in fiscal years beginning after November 15, 2007. The Company is currently assessing the impact of the adoption of this statement. |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| 20. | Generally Accepted Accounting Principles in Canada and the United States (Continued) |
| | (f) | Restatement of Prior Year Figures During the year ended March 31, 2008, the Company determined that it had inappropriately accounted for insurance expense and convertible notes under Canadian GAAP (refer to Note 2) and convertible notes, debt issue costs and its joint venture under US GAAP (refer to Notes 20(b), (c) and (d)) during the years ended March 31, 2007 and 2006. The following tables summarize the restatement of the affected balances: |
Year Ended March 31, 2007 | | Previously Reported | | | Change | | | Restated | |
| | | | | | | | | | |
| Statement of Operations: | | | | | | | | | |
Interest and accretion expense | | $ | 5,606,886 | | | $ | (1,149,480 | ) | | $ | 4,457,406 | |
Debt issue costs | | $ | 512,573 | | | $ | 108,696 | | | $ | 621,269 | |
Product development | | $ | 1,562,421 | | | $ | (420,670 | ) | | $ | 1,141,751 | |
Net loss of interest in joint venture | | $ | – | | | $ | 420,670 | | | $ | 420,670 | |
Insurance expense | | $ | 149,855 | | | $ | (71,071 | ) | | $ | 78,784 | |
Foreign exchange loss (gain) | | $ | 110,006 | | | $ | (78,377 | ) | | $ | 31,629 | |
Net loss and comprehensive loss under USGAAP | | $ | 10,939,571 | | | $ | (1,190,232 | ) | | $ | 9,749,339 | |
Loss per share under US GAAP – basic anddiluted | | $ | 0.57 | | | $ | (0.06 | ) | | $ | 0.51 | |
| | | | | | | | | | | | | |
| Balance Sheet: | | | | | | | | | | | | |
Debt issue costs | | $ | – | | | $ | 230,301 | | | $ | 230,301 | |
Convertible notes | | $ | 5,301,129 | | | $ | (3,018,646 | ) | | $ | 2,282,483 | |
Share capital | | $ | 7,836,903 | | | $ | 597,129 | | | $ | 8,434,032 | |
Contributed surplus | | $ | 3,253,333 | | | $ | 4,810,127 | | | $ | 8,063,460 | |
Conversion component of convertible notes | | $ | 4,167,190 | | | $ | (4,167,190 | ) | | $ | – | |
Deficit, beginning of year | | $ | 3,734,599 | | | $ | (889,720 | ) | | $ | 2,844,879 | |
Deficit, end of year | | $ | 14,674,170 | | | $ | (2,079,952 | ) | | $ | 12,594,218 | |
Year Ended March 31, 2006 | | Previously Reported | | | Change | | | Restated | |
| | | | | | | | | | |
| Statement of Operations: | | | | | | | | | |
Financing expense | | $ | 1,328,337 | | | $ | (683,977 | ) | | $ | 644,360 | |
Convertible note issuance costs | | $ | 570,226 | | | $ | (338,997 | ) | | $ | 231,229 | |
Foreign exchange loss | | $ | 23,955 | | | $ | 133,254 | | | $ | 157,209 | |
Net loss and comprehensive loss under USGAAP | | $ | 3,734,599 | | | $ | (889,720 | ) | | $ | 2,844,879 | |
Loss per share under US GAAP – basic anddiluted | | $ | 0.35 | | | $ | (0.08 | ) | | $ | 0.27 | |
Valcent Products Inc.
Notes to the Consolidated Financial Statements
(A Development Stage Company)
(Expressed in Canadian Dollars)
Years Ended March 31, 2008 and 2007
| | | Income (Numerator) | | | Shares (Denominator) | | | Per Share Amount | |
| | | | | | | | | | |
| 2008 | | | | | | | | | |
| Loss attributable to common shareholders | | $ | (12,712,358 | ) | | | 35,545,740 | | | $ | (0.36 | ) |
| | | | | | | | | | | | | |
| 2007 | | | | | | | | | | | | |
| Loss attributable to common shareholders | | $ | (8,138,393 | ) | | | 19,261,192 | | | $ | (0.42 | ) |
| | | | | | | | | | | | | |
| 2006 | | | | | | | | | | | | |
| Loss attributable to common shareholders | | $ | (3,466,888 | ) | | | 42,814,988 | | | $ | (0.33 | ) |
| | Common share equivalents consisting of 4,740,000 (2007 – 3,660,000) stock options and 15,752,251 (2007 – 11,753,816) share purchase warrants are not considered in the computation of diluted loss per share because their effect would be anti-dilutive. |
| No. | Description of Exhibit |
| | |
| 1(i)(1) | Articles of Incorporation of Valcent Products Inc., dated January 19, 1996, incorporated by reference to Exhibit 1(a)(i) on Form 20-F filed October 13, 2005. |
| | |
| 1(i)(2) | Amendment to Articles of Incorporation of Valcent Products Inc., dated March 12, 1996, incorporated by reference to Exhibit 1(a)(ii) on Form 20-F filed October 13, 2005. |
| | |
| 1(i)(3) | Amendment to Articles of Incorporation of Valcent Products Inc., dated August 19, 1996, incorporated by reference to Exhibit 1(a)(i) on Form 20-F filed October 13, 2005. |
| | |
| 1(i)(4) | Amendment to Articles of Incorporation of Valcent Products Inc., dated June 30, 1998, incorporated by reference to Exhibit 1(a)(iv) on Form 20-F filed October 13, 2005. |
| | |
| 1(i)(5) | Amendment to Articles of Incorporation of Valcent Products Inc., dated July 8, 1999, incorporated by reference to Exhibit 1(a)(v) on Form 20-F filed October 13, 2005. |
| | |
| 1(i)(6) | Amendment to Articles of Incorporation of Valcent Products Inc., dated September 28, 1999, incorporated by reference to Exhibit 1(a)(vi) on Form 20-F filed October 13, 2005. |
| | |
| 1(i)(7) | Amendment to Articles of Incorporation of Valcent Products Inc., dated April 15, 2005, incorporated by reference to Exhibit 1(a)(vii) on Form 20-F filed October 13, 2005. |
| | |
| 1(ii) | By-laws of Valcent Products Inc., dated March 26, 1996, incorporated by reference to Exhibit 1(b) on Form 20-F filed October 13, 2005. |
| | |
| 1 (iii) | Audit Committee charter of Valcent Products, Inc., dated March 2005, incorporated by reference to Exhibit 15.A on Form 20-F filed October 31, 2005. |
| | |
| 4.1 | Master license agreement between Valcent Products Inc. and MK Enterprises LLC, dated July 29, 2005, incorporated by reference to Exhibit 4(a)(i) on Form 20-F filed October 13, 2005. |
| | |
| 4.2 | Product development agreement between Valcent Products Inc. and MK Enterprises LLC, dated July 29, 2005, incorporated by reference to Exhibit 4(a)(ii) on Form 20-F filed October 13, 2005. |
| | |
| 4.3 | Invention license agreement between Valcent Products Inc. and MK Enterprises LLC, dated July 29, 2005, incorporated by reference to Exhibit 4(a)(iii) on Form 20-F filed October 13, 2005. |
| | |
| 4.4 | Consulting agreement between Valcent Products Inc. and M. Glen Kertz, dated July 29, 2005, incorporated by reference to Exhibit 4(a)(iv) on Form 20-F filed October 13, 2005. |
| | |
| 4.5 | Consulting agreement between Valcent Products Inc. and MK Enterprises LLC, dated July 29, 2005, incorporated by reference to Exhibit 4(a)(v) on Form 20-F filed October 13, 2005. |
| | |
| 4.6 | Form of liquidated damages convertible note agreement between Valcent Products Inc., and certain institutional note holders, dated April 6, 2006, incorporated by reference to Exhibit 10.12 on Form F-1/A filed June 30, 2006. |
| | |
| 4.7 | Form of liquidated damages warrant agreement between Valcent Products Inc., and certain institutional note holders, dated April 6, 2006, incorporated by reference to Exhibit 10.13 on Form F-1/A filed June 30, 2006. |
| | |
| 4.8 | Creative and production services agreement between Valcent Products Inc., and Hawthorne Direct, dated June 14, 2006, incorporated by reference to Exhibit 10.14 on Form F-1/A filed June 30, 2006. |
| | |
| 4.9 | Media services agreement between Valcent Products Inc., and Hawthorne Direct, dated June 14, 2006, incorporated by reference to Exhibit 10.15 on Form F-1/A filed June 30, 2006. |
| | |
| 4.10 | Manufacturing agreement between Valcent Manufacturing Ltd. and Solid Integrations LLC dated September 22, 2006, incorporated by reference to Exhibit 10.16 on Form F-1/A filed October 2, 2006. |
| | |
| 4.11 | Farm and Ranch Contract between Valcent Products, Inc. C/O Gordon & Mott, P.C., and Roberto H. Silvas, dated October 2, 2006, incorporated by reference to Exhibit 10.19 on Form F-1/A filed February 7, 2007. |
| | |
| 4.12 | Letter of Agreement between Valcent Products, Inc., Global Green Solutions Inc., Pagic LP, and West Peak Ventures of Canada Limited, dated October 2, 2006, incorporated by reference to Exhibit 10.17 on Form F-1/A filed February 7, 2007. |
| 4.13 | Direct Response Services Agreement between Valcent Products, Inc. and InPulse Response Group, dated October 23, 2006, incorporated by reference to Exhibit 10.22 on Form F-1/A filed February 7, 2007. |
| | |
| 4.14 | Corporate Support Agreement between Valcent Products, Inc., and Sweetwater Capital Corporation dated November 1, 2006, FILED HEREWITH. |
| | |
| 4.15 | Master Services Agreement between Valcent Products, Inc. and Accretive Commerce, Inc., dated November 30, 2006, incorporated by reference to Exhibit 10.20 on Form F-1/A filed February 7, 2007. |
| | |
| 4.16 | Stakeholders Letter of Agreement between Valcent Products, Inc., Global Green Solutions, Inc., Pagic LP, and West Peak Ventures of Canada Limited, dated June 25, 2007, FILED HEREWITH. |
| | |
| 4.17 | Consulting Services Agreement between Valcent Products, Inc., and MB Pics Capital Corporation, dated November 2, 2007, FILED HEREWITH. |
| | |
| 4.18 | Consulting Services Agreement between Valcent Products, Inc., and Yellow Rose Ltd., dated February 1, 2008, FILED HEREWITH. |
| | |
| 4.19 | Consulting Agreement between Valcent Products, Inc., and J. Gordon Murphy, dated February 29, 2008, FILED HEREWITH. |
| | |
| 4.20 | Financial Advisory Consulting Services Agreement, between Valcent Products, Inc., and Mark T. Cox, dated May 1, 2008, FILED HEREWITH. |
| | |
| 4.21 | Limited Liability Company Operating Agreement of Vertigro Algae Technologies, LLC, between Valcent USA, Inc., and Global Green Solutions Inc., dated May 5, 2008, incorporated by reference to Exhibit 10.1 on Form 6-K filed May 14, 2008. |
| | |
| 4.22 | Vertigro Algae Technologies LLC Technology License Agreement, between Valcent Products, Inc., Pagic LP, and West Peak Ventures of Canada Ltd., dated May 7, 2008, incorporated by reference to Exhibit 10.2 on Form 6-K filed May 14, 2008. |
| | |
| 4.23 | Vertigro Algae Technologies LLC Amendment to the Technology License Agreement, between Valcent Products, Inc., Pagic LP, and West Peak Ventures of Canada Ltd., dated May 5, 2008 FILED HEREWITH. |
| | |
| 4.24 | Form of convertible note agreement between Valcent Products Inc., and certain institutional note holders, dated July 16, 2008, incorporated by reference to Exhibit 4.1 on Form 6-K filed July 25, 2008. |
| | |
| 4.25 | Form of Series A Warrant between Valcent Products Inc., and certain institutional note holders, dated July 16, 2008, incorporated by reference to Exhibit 4.2 on Form 6-K filed July 25, 2008. |
| | |
| 4.26 | Form of Series B Warrant between Valcent Products Inc., and certain institutional note holders, dated July 16, 2008, incorporated by reference to Exhibit 4.3 on Form 6-K filed July 25, 2008. |
| | |
| 4.27 | Note and Warrant Purchase Agreement, between Valcent Products, Inc., and certain institutional note holders, dated July 16, 2008, incorporated by reference to Exhibit 10.1 on Form 6-K filed July 25, 2008. |
| | |
| 4.28 | Security Agreement, between Valcent Products, Inc., and Platinum Long Term Growth VI, LLC, dated July 16, 2008, incorporated by reference to Exhibit 10.2 on Form 6-K filed July 25, 2008. |
| | |
| 4.29 | Patent Trademark and Copyright Security Agreement, between Valcent Products, Inc., Valcent USA Inc., Valcent Manufacturing, Ltd., Vertigro Algae Technologies LLC, Valcent Products EU Limited, and Platinum Long Term Growth VI, LLC, dated July 16, 2008 and FILED HEREWITH. |
| | |
| 4.30 | Deed of Trust, Assignment of Rents and Leases, Security Agreement and Fixture Filing, between, Valcent Products, Inc., Valcent Manufacturing, Ltd., Deborah P. Everett, and Platinum Long Term Growth VI, LLC, dated July 16, 2008, and FILED HEREWITH. |
| | |
| 4.31 | Hazardous Substances Indemnity Agreement between Valcent Products, Inc., Valcent USA Inc., Valcent Manufacturing, Ltd., Valcent Management LLC, Vertigro Algae Technologies LLC, Valcent Products EU Limited, and Platinum Long Term Growth VI, LLC, dated July 16, 2008, and FILED HEREWITH. |
| | |
| 4.32 | Letter of Intent between Valcent Products, Inc., and Hydroganics JV, dated September 3, 2008, FILED HEREWITH. |
| | |
| 4.33 | Letter of Intent between Valcent Products, Inc., Mark Spencer, and Mike Geleta, dated September 16, 2008, and FILED HEREWITH. |
| | |
| 4.34 | Purchase and Sale Agreement between Valcent USA Inc., Global Green Solutions, Inc., and Vertigro Algae Technologies LLC, dated September 26, 2008, incorporated by reference to Exhibit 10.1 on Form 6-K filed October 1, 2008. |
| | |
| 5.1 | Subsidiaries of Valcent Products Inc., as of September 15, 2008 FILED HEREWITH. |
| | |
| 12(a)(iii) | Certification pursuant to Rule 13A-14(A) in Accordance with Section 302 of the Sarbanes-Oxley Act of 2002 FILED HEREWITH. |
| | |
| 12(a)(iiii) | Certification pursuant to Rule 13A-14(A) in Accordance with Section 302 of the Sarbanes-Oxley Act of 2002 FILED HEREWITH. |
| | |
| 13(a)(iii) | Certification pursuant to 18 U.S.C. Section 1350 as Adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 FILED HEREWITH. |
| | |
| 13(a)(iiii) | Certification pursuant to 18 U.S.C. Section 1350 as Adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 FILED HEREWITH. |
SIGNATURES
The registrant here certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | | |
| | VALCENT PRODUCTS, INC. |
| | | |
| | | |
| Date: October 10, 2008 | By: | /s/ M. Glen Kertz |
| | | |
| | | M. Glen Kertz |
| | | Chief Executive Officer and Acting President |