Exhibit 99.4
Independent expert opinion
for the determination of the equity value
as of 13. September 2006
Schering Aktiengesellschaft, Berlin
KPMG Deutsche Treuhand-Gesellschaft
Aktiengesellschaft Wirtschaftsprüfungsgesellschaft
Independent expert opinion
for the determination of the equity value
as of 13. September 2006
Schering Aktiengesellschaft, Berlin
KPMG Deutsche Treuhand-Gesellschaft
Aktiengesellschaft Wirtschaftsprüfungsgesellschaft
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page I
Contents
| | | | | | | | | | | |
Contents | | | | | | | | | I | |
| | | | | | | | | | | |
Annex | | | | | | | | | III | |
| | | | | | | | | | | |
| 1 | | Assignement and Execution | | | 1 | |
| | | 1.1 | | Assignement | | | 1 | |
| | | 1.2 | | Execution | | | 2 | |
| | | | | | | | | | | |
| 2 | | Legal and economic status | | | 4 | |
| | | 2.1 | | Legal status | | | 4 | |
| | | 2.2 | | Economic status | | | 5 | |
| | | | | 2.2.1 | | Business operations and organization | | | 5 | |
| | | | | 2.2.2 | | Market environment and competitive situation | | | 10 | |
| | | | | 2.2.3 | | Business area Gynecology & Andrology | | | 15 | |
| | | | | 2 2.4 | | Business area Diagnostic Imaging | | | 20 | |
| | | | | 2.2.5 | | Business area Specialized Therapeutics | | | 22 | |
| | | | | 2.2.6 | | Business area Oncology | | | 27 | |
| | | | | 2.2.7 | | Other Sources | | | 31 | |
| | | | | | | | | | | |
| 3 | | General valuation principles | | | 33 | |
| | | 3.1 | | Discounted earnings value | | | 33 | |
| | | 3.2 | | Special items | | | 35 | |
| | | 3.3 | | Liquidation value and net asset value | | | 35 | |
| | | 3.4 | | Stock market value | | | 35 | |
| | | | | | | | | | | |
| 4 | | Methodical Approach | | | 37 | |
| | | 4.1 | | Structure and definiton of the valuation entity | | | 37 | |
| | | 4.2 | | Effective date of the valuation | | | 37 | |
| | | 4.3 | | Analysis of profitability and derivation of future earnings | | | 37 | |
| | | | | 4.3.1 | | Analysis of historical earnings | | | 38 | |
| | | | | 4.3 2 | | Analysis of finacial planning | | | 38 | |
| | | | | 4.3.3 | | Determining sustainable earnings | | | 39 | |
| | | | | | | | | | | |
| 5 | | Planned Accounts | | | 41 | |
| | | 5.1 | | Operating Profit | | | 42 | |
| | | | | 5.1.1 | | Contribution margin of the business area | | | | |
| | | | | | | Gynecolgy & Andrology | | | 42 | |
| | | | | 5.1.2 | | Contribution margin of the business area Diagnostic Imaging | | | 47 | |
| | | | | 5.1.3 | | Contribution margin of the business area Specialized | | | | |
| | | | | | | Therapeutics | | | 50 | |
| | | | | 5.1.4 | | Contribution margin of the business area Oncology | | | 54 | |
| | | | | 5.1.5 | | Contribution margin of the business area Other Sources | | | 59 | |
| | | | | 5.1.6 | | Bridge contribution margins to the legal accounts | | | 61 | |
| | | 5.2 | | Cost of marketing and selling | | | 61 | |
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page II
| | | | | | | | | | | |
| | | 5.3 | | Cost of research & development | | | 63 | |
| | | 5.4 | | Cost of engineering and administration | | | 64 | |
| | | 5.5 | | Other Result | | | 66 | |
| | | 5.6 | | Derivation of the companies net profit | | | 67 | |
| | | | | | | | | | | |
| 6 | | Discount rate | | | 71 | |
| | | 6.1 | | Risk-free rate | | | 71 | |
| | | 6.2 | | Risk premium | | | 72 | |
| | | 6.3 | | Growth rate | | | 73 | |
| | | | | | | | | | | |
| 7 | | Equity Value | | | 75 | |
| | | 7.1 | | Discounted earnings value | | | 75 | |
| | | 7.2 | | Special items | | | 76 | |
| | | 7.3 | | Equity value | | | 78 | |
| | | | | | | | | | | |
| 8 | | Plausibility assessment using market prices | | | 80 | |
| | | 8.1 | | Analysis Schering AG’s share price | | | 80 | |
| | | 8.2 | | Comparative market valuation | | | 85 | |
| | | | | 8.2.1 | | Selection of comparable companies | | | 86 | |
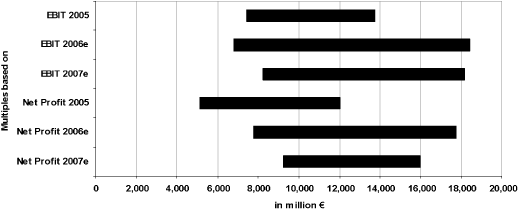
| | | | | 8.2.2 | | Determination of the multiples | | | 86 | |
| | | | | 8.2.3 | | Value range of the multiples | | | 87 | |
| | | | | | | | | | | |
| 9 | | Cash compensation and guaranteed dividend | | | 89 | |
| | | 9.1 | | Cash compensation under § 305 AktG | | | 89 | |
| | | 9.2 | | Guaranteed dividend under § 304 AktG | | | 89 | |
| | | | | | | | | | | |
| 10 | | Concluding remarks | | | 93 | |
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page III
Annex
| | |
| Annex 1 | | Bridge adjusted / unadjusted operating profit |
| | |
| Annex 2 | | General Engagement Terms for Wirtschaftsprüfer and Wirtschaftsprüfungsgesellschaften (“GET”) |
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 1
1 Assignement and Execution
1.1 Assignement
The management board of Bayer AG, Leverkusen (hereinafter, “Bayer”), the management board of Schering Aktiengesellschaft, Berlin (hereinafter, “Schering AG” or “the Company”) as well as the managing directors of Dritte BV GmbH, Leverkusen (hereinafter “DBV”) mandated us in a letter dated 18 May 2006 with the preparation of a valuation of
Schering AG, Berlin.
Thepurpose of our engagementis to provide a independent expert opinion to determine the fair market value of Schering AG, the amount of a reasonable guaranteed dividend under § 304 AktG and the amount of a reasonable compensation under § 305 AktG as a preparatory step for the Report in connection with the intended Domination and Profit and Loss Transfer Agreement pursuant to § 291 AktG. The effective valuation date is set to be 13 September 2006, the date of the intended extraordinary shareholders’ meeting in which the conclusion of the Domination and Profit and Loss Transfer Agreement is to be decided.
When carrying out the assignment, we relied on the German “Standards for performing company valuations” (IDW S 1: Grundsätze zur Durchführung von Unternehmensbewertungen) which have been established by the main specialized committee of the German Institute of Certified Public Accountants (Institut der Wirtschaftsprüfer in Deutschland e.V.) dated 18 October 2005 (IDW S 1). For the purposes of this report, we are providing our report in the function as anindependent expert(neutraler Gutachter). The value of the company is, thus, an objective amount.
The engagement is subject to the attachedGeneral Engagement Terms(“GET”) for accountants and accounting firms in the version dated 1 January 2002 (Allgemeine Auftragsbedingungen für Wirtschaftsprüfer und Wirtschaftsprüfungsgesellschaften) attached as Annex 2. The maximum amount of our liability is determined pursuant to no. 9 of the GET and supplemental written agreements. With regard to third parties no. 1 para. 2 and no. 9 of the GET are determinative.
The independent expert opinion has been prepared in connection with the intended conclusion of a Domination and Profit and Loss Transfer Agreement pursuant to § 291 AktG between DBV and Schering AG and is intended exclusively for the internal use by the clients. The internal use also includes the use of the independent expert opinion, especially the results of the valuation, in the context of the written and oral reporting to the shareholders at the and in connection with the shareholders’ meeting of Schering AG. This includes the possibility to review the independent expert opinion in connection with the shareholders’ meeting in the context of viewing the documents of Schering AG by the shareholders.
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 2
Subject to KPMG’s prior written approval, independent expert opinion may bereleasedto third parties in its full version only. This release must include a written statement setting out the purpose of the engagement, restrictions on releasing the report to other parties and liability arrangements. The release is conditional upon the third party agreeing in writing to accept the current GET and the limitations in our liability as well as confirming that it will treat the report as confidential and will not release the report to any other party.
The attorneys, auditors and advisors mandated by the clients in connection with the intended contract pursuant to § 291 AktG are not third parties within the meaning of this paragraph to the extent that they are subject to professional confidentiality and have not been released by the clients from the obligation to maintain confidentiality.
1.2 Execution
Weexecutedthe assignment from 22 May 2006 through 27 July 2006 in the offices of Schering AG as well as in our offices in Berlin and Frankfurt, Germany.
We have compiled the following list of thedocumentsupon which our activity was primarily based:
| – | | Draft of the Domination and Profit and Loss Transfer Agreement between DBV and Schering AG in the version dated 27 July 2006; |
| |
| – | | Audited annual financial statements and consolidated financial statements of Schering AG for the years 2003 through 2005 prepared and certified with an unrestricted auditors certification by BDO Deutsche Warentreuhand Aktiengesellschaft Wirtschaftsprüfungsgesell-schaft; |
| |
| – | | Schering quarterly reports for the first and second quarter 2006; |
| |
| – | | Planned accounts of Schering for the years 2006 through 2008 dated December 2005; |
| |
| – | | Documents on the strategic process at Schering in the year 2006 with the outlook up to 2015 in the version dated 20 June 2006; |
| |
| – | | Articles of Association, excerpt from the Commercial Register. |
In addition, theinformation contact personsdesignated by the client willingly provided additional information.
As a general rule, our determination of the value is based on the documents provided for the purpose of the valuation. We critically examined these documents, but we did not subject them to an audit as we would have in the case of auditing annual financial statements. The planned accounts were examined by us with regard to plausibility and were discussed with the management board of Schering AG and other responsible employees. In addition, we took into account the current legal and economic parameters, the customer and supplier structures as well as the assets, the financial conditions and the earnings positions of previous years.
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 3
We wish to point out that the following presented calculations for the determination of the value of Schering AG have generally been prepared without disclosing decimals. Since the calculation was made with the exact values, the addition or subtraction of values in the tables can lead to discrepancies with the indicated subtotals or the total sums. So far as not otherwise noted, all of the financial numbers shown for Schering AG conform to the provisions of the International Financial Reporting Standards (IFRS).
The management board of Schering AG, the management board of Bayer AG, as well as the managing directors of DBV have issued aletter of representationto us stating that all information relevant to the preparation of this independent expert opinion has been granted correctly and completely.
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 4
2 Legal and economic status
2.1 Legal status
Schering AG is a stock corporation under German law with itsregistered officein Berlin. It is registered in theCommercial Registerof the Local Court Charlottenburg, Berlin, under HRB 283 B.
Theshare capitalof Schering AG is€ 194,000,000 and is divided into 194,000,000 shares. As of 23 July 2006 the company held 3,159,000 treasury shares.
The purpose of the Company is according to the Articles of Association:
| – | | the research and development of, the purchase and sale of chemical and biotechnological products; these products include especially pharmaceuticals, pharmaceutical ingredients, diagnostics and vaccines for human and veterinary medicine as well as fine chemicals, radioactive substances, and intermediate products; |
| |
| – | | the research of, the development of, the manufacture and sales of medicines and equipment for medical and laboratory purposes and |
| |
| – | | the preparation, acquisition, and utilization of chemical, biological, and technical procedures and facilities. |
TheArticles of Associationof Schering AG are valid in the version dated 26 April 2006. The fiscal year is the calendar year.
As of 30 June 2006, Schering AG has more than 150 subsidiaries worldwide. Of particular importance from an operational point of view are the wholly owned subsidiaries Schering Oy with its registered office in Helsinki, Finland, Berlex Inc. with its registered office in Monteville, USA (hereinafter, “Berlex”), Medrad Inc. with its registered office in Indianola, USA (hereinafter, “Medrad”), Schering S.p.A. Italy with its registered office in Milan, Italy, Schering do Brasil Químíca e Farmacéutica Ltda, São Paolo/SP, Brazil, Nihon Schering K.K., Osaka, Japan, Schering Mexicana S.A. de C.V., Mexico, D.F., Mexico.
Schering AG and its affiliates are shown as fully consolidated in the Schering Group for purposes of reporting and planning in accordance with the International Financial Reporting Standards (IFRS).
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 5
2.2 Economic status
2.2.1 Business operations and organization
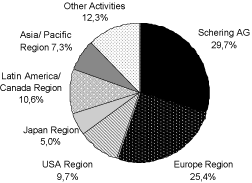
Schering is active in all important regional markets for pharmaceutical products. The business operations of Schering AG are organized in the form of a matrix with theregionsbeing the primary segment and the business areas being the second criterium. Thebusiness areasare responsible for research and development of medications and therapeutic applications and therefore share the profit responsibility with the regions. The primary responsibility for the ongoing business lies with the segments. The segments have the responsibility for adapting these to regional peculiarities and distributing them in the respective markets. Schering AG’s pharmaceutical business is specialized into the business areas Gynecology & Andrology, Diagnostic Imaging, Special Therapeutics, and Oncology. Schering AG’s worldwide pharmaceutical business is further divided on a geographical basis. The pharmaceutical business has five geographic segments: Europe Region, USA, Japan, Latin America/Canada, and Asia/Pacific. The 2005 sales distributions per segment and business area (calculated without the interest in ALK-Scherax, (hereinafter “ALK”), and the radiopharmaceutical business) are shown in the following table:
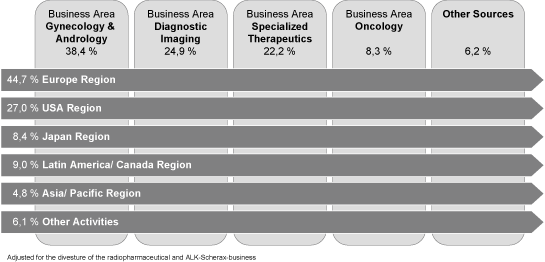
Adjusted for the divesture of the radiopharmaceutical and ALK-Scherax-business
Since 1 January 2006, Other Activities include the business of the Medrad with application technologies for contrast agents, which in fiscal year 2005 were accounted for in the segment USA.
The business areas and segments are supported by central departments in which overlapping resources in marketing, distribution, technology and administration as well as research and development are bundled.
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 6
The number of employees in the Schering Group as of 30 June 2006 was 23,098 (on a full-time basis). As shown in the following graph, these were primarily employed at Schering AG in Germany and in the European segment.
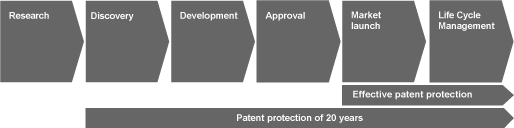
The process of researching, discovering a substance, development, approval and market introduction of a (patented) medication is of central importance for the success of a pharmaceutical company engaged in research. The discovery of a substance is followed by a complex development process in which comprehensive clinical studies prove the effectiveness and safety of the substance. Assuming that these studies run successfully and all regulatory conditions are satisfied, an innovative medication can be approved. As a result of these requirements, pharmaceutical companies engaged in research normally only have a few years in order to achieve pioneering profits. The following graph illustrates the process of research, development and market introduction of innovative medicines:
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 7
The effective patent protection lasts for a period of less then ten years1 in which successful product innovations must cover the expenses of their own research projects and those of less successful research projects. Accordingly, the duration of the effective patent protection is of particular importance for the economic development of Schering. Therefore, pharmaceutical companies engaged in research try to extend the period of effective patent protection by applying for formulation and process patents to protect themselves against generic competition.
The following table shows the expiration dates for the main Schering products and/or production processes.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Specialized | | |
| | | Gynecology & Andrology | | Diagnostic Imaging | | Therapeutics | | Oncology |
| | | Yasmin | | Mirena | | Femovan | | Magnevist | | Ultravist | | Betaferon1 | | Campath | | Leukine |
| | | | | | | Device | | | | | | | | | | | | | | | | | | |
| Patent on | | Formulation | | (Inserter) | | Process | | Process | | Product | | Formulation | | Method of use | | Product | | Product | | Product | | Product |
| |
Europe | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Germany | | | 2020 | *** | | | 2015 | | | | 2013 | | | | 2006 | | | | — | | | | 2007 | | | | — | | | | — | | | | 2008 | | | | 2009 | | | | 2006 | |
| France | | | 2020 | *** | | | 2015 | | | | 2013 | | | | 2006 | | | | — | | | | 2007 | | | | — | | | | — | | | | 2008 | | | | 2009 | | | | 2006 | |
| Italy | | | 2020 | *** | | | 2015 | | | | 2013 | | | | 2006 | | | | 2007 | | | | 2007 | | | | — | | | | 2009 | | | | 2008 | | | | 2009 | | | | 2006 | |
| Great Britain | | | 2020 | *** | | | 2015 | | | | 2013 | | | | 2006 | | | | — | | | | 2007 | | | | — | | | | — | | | | 2008 | | | | 2009 | | | | 2006 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
United States | | | 2020 | *** | | | 2015 | | | | 2013 | | | | — | | | | 2011 | | | | 2009 | | | | 2013 | | | | — | | | | 2007 | | | | 2015 | | | | 2012 | |
| | | | | | �� | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Japan | | | 2020*/ | *** | | | — | | | | 2013 | | | | 2006 | | | | — | | | | 2007 | | | | — | | | | — | | | | 2003 | ** | | | 2009 | | | | 2006 | |
| | |
| 1 | | In the USA and in the countries outside of Europe the multiple sclerosis-medicament Betaferon is marketed under the brand name Betaseron. |
| |
| * | | Patent pending |
| |
| ** | | Appeal against rejection of patent term extension pending |
| |
| *** | | Composition comprising micronized drospirenone together with ethinylestradiol |
| |
| 1 | | BPl Pharmadaten 2005, p. 15. |
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 8
The earnings position of Schering Group in the fiscal years 2003 through 2005 can be shown as follows:
Schering Group — Income Statement
| | | | | | | | | | | | | |
| | | 2003* | | 2004* | | 2005 |
| | | €m | | €m | | €m |
| |
| Net sales | | | 4,828 | | | | 4,907 | | | | 5,308 | |
| Cost of sales | | | 1,233 | | | | 1,206 | | | | 1,256 | |
| |
Gross profit | | | 3,595 | | | | 3,701 | | | | 4,052 | |
| |
in % of net sales | | | 74.5 | % | | | 75.4 | % | | | 76.3 | % |
| Cost of | | | | | | | | | | | | |
| marketing and selling | | | 1,519 | | | | 1,544 | | | | 1,687 | |
| research and development | | | 923 | | | | 918 | | | | 982 | |
| engineering and administration | | | 563 | | | | 522 | | | | 522 | |
| Other operating income | | | 399 | | | | 362 | | | | 432 | |
| Other operating expenses | | | 293 | | | | 311 | | | | 365 | |
| |
Operating profit | | | 696 | | | | 768 | | | | 928 | |
| |
in % of net sales | | | 14.4 | % | | | 15.7 | % | | | 17.5 | % |
| | | | | | | | | | | | | |
| Financial result | | | 15 | | | | -9 | | | | 42 | |
| Income taxes | | | 259 | | | | 252 | | | | 346 | |
| |
Profit for the period | | | 452 | | | | 507 | | | | 624 | |
| |
| Minority interest | | | -3 | | | | -3 | | | | -5 | |
| |
Net profit | | | 449 | | | | 504 | | | | 619 | |
| |
in % of net sales | | | 9.3 | % | | | 10.3 | % | | | 11.7 | % |
| |
| | |
| * | | previous year’s figures adjusted in accordance with the amendment “Actual Gains and Losses, Group Plans and Disclosures” to IAS 19 |
After a small increase in sales in the fiscal year 2004, Schering was able to realize an 8 % increase in net sales and a 9 % increase in profits growth in the year 2005. Economies of scale and clear savings in the area of costs were the reason for the 21 % improvement in net income. In addition, financial results in the fiscal year 2005 improved. After a 12 % increase in 2004 the corporate group profit increased by 23 % in the year 2005.
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 9
The following overview shows the Schering Group’s financial statement for the fiscal years 2003 through 2005:
Consolidated Balance Sheet of the Schering Group
| | | | | | | | | | | | | |
| | | 2003* | | 2004* | | 2005 |
| | | €m | | €m | | €m |
| |
Assets | | | | | | | | | | | | |
| Intangible assets | | | 723 | | | | 678 | | | | 694 | |
| Property, plant and equipment | | | 1,213 | | | | 1,176 | | | | 1,161 | |
| Financial assets | | | 262 | | | | 334 | | | | 309 | |
| Other non-current assets | | | 289 | | | | 350 | | | | 355 | |
| |
Non-current assets | | | 2,487 | | | | 2,538 | | | | 2,519 | |
| |
| | | | | | | | | | | | | |
| Inventories | | | 996 | | | | 992 | | | | 959 | |
| Trade receivables | | | 1,430 | | | | 1,402 | | | | 1,752 | |
| Cash and cash equivalents | | | 566 | | | | 785 | | | | 776 | |
| Assets classified as held for sale | | | 0 | | | | 0 | | | | 97 | |
| |
Current assets | | | 2,992 | | | | 3,179 | | | | 3,584 | |
| |
| | | | | | | | | | | | | |
| |
Total assets | | | 5,479 | | | | 5,717 | | | | 6,103 | |
| |
| | | | | | | | | | | | | |
Equity and Liabilities | | | | | | | | | | | | |
| Issued capital | | | 194 | | | | 194 | | | | 194 | |
| Share premium account | | | 334 | | | | 334 | | | | 334 | |
| Retained earnings | | | 2,239 | | | | 2,292 | | | | 2,741 | |
| Treasury shares | | | 0 | | | | -4 | | | | -4 | |
| Equity before minority interest | | | 2,767 | | | | 2,816 | | | | 3,265 | |
| Minority interest | | | 16 | | | | 17 | | | | 18 | |
| |
Total equity | | | 2,783 | | | | 2,833 | | | | 3,283 | |
| |
| | | | | | | | | | | | | |
| Non-current provisions | | | 1,290 | | | | l,380 | | | | 900 | |
| Non-current borrowings | | | 36 | | | | 199 | | | | 228 | |
| Other non-current liabilities | | | 24 | | | | 30 | | | | 32 | |
| |
Non-current liabilities | | | 1,350 | | | | 1,609 | | | | 1,160 | |
| |
| | | | | | | | | | | | | |
| Current provisions | | | 656 | | | | 713 | | | | 863 | |
| Trade payables | | | 354 | | | | 304 | | | | 375 | |
| Current borrowings | | | 74 | | | | 39 | | | | 27 | |
| Other current liabilities | | | 262 | | | | 219 | | | | 236 | |
| Liabilities directly associated with assets classified as held for sale | | | 0 | | | | 0 | | | | 159 | |
| |
Current liabilities | | | 1,346 | | | | 1,275 | | | | l,660 | |
| |
| | | | | | | | | | | | | |
| |
Total equity and liabilities | | | 5,479 | | | | 5,717 | | | | 6,103 | |
| |
| | |
| * | | previous year’s figures adjusted in accordance with the amendment “Actual Gains and Losses, Group Plans and Disclosures” to IAS 19; the figures were also restated to give effect to changes in the presentation of Total equity (IAS 1) and in the accounting for employee share purchase plans (IFRS 2) |
The assets of the balance sheet for the year 2005 is characterized by an increase in the short term assets which has its basis primarily in the increase in assets, accounts receivable, and other investments. The securities relate primarily to fixed interest securities having a remaining term
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 10
of less than one year. The increase of these assets by€ 196 million is substantially a consequence of the increase in liquidity as a result of retained earnings from the previous years. In addition,€ 450 million were used to finance the Schering Altersversorgung Treuhand Verein (Schering pension organization). The increase in accounts receivable for goods and services is a result of the increased sales volume of fiscal year 2005.
The assets for the sale of the radiopharmaceuticals business in 2006 were accounted for under the assets classified as held for sale in fiscal year 2005.
The development of the fixed assets in the fiscal year 2005 was influenced by the extraordinary depreciation of assets in the radiopharmaceuticals business and investments such as the new construction of the laboratory building S116 in Berlin. Furthermore, the production site Lys-Lez-Lannoy in France has been sold.
The increase in profit reserves had its basis in the retention of substantial portions of the annual net profit.
The long term provisions include provisions for pensions and similar obligations have been reduced in the fiscal year 2005 primarily as a result of funding the Schering Altersversorgung Treuhand Verein in the amount of€ 450 million. This was offset in part by an increase of existing pension reserves resulting from the lowering of the calculatory interest rate.
The increase in current liabilities essentially has its correspondent base to business development in the form of increases in the items trade payables and current provisions.
2.2.2 Market environment and competitive situation
The worldwide pharmaceutical market has grown during the period from 2000 through 2005 at an annual average rate of 11 %.2 This growth had different strengths in various regional markets, as will be explained below — especially in the most important pharmaceutical markets of USA, Europe and Japan. The market growth and the growth of Schering AG are especially dependent on the following factors:
| – | | Development of the structure of the population |
| |
| – | | (State) regulation of the health market |
| |
| – | | Portion of sales of generics of the pharmaceutical market |
| | |
| 2 | | IMS Health, Global pharmaceutical sales 2005; IMS-Data reports no information on contrasting agents, which Schering’s Diagnostic Imaging division develops and sells. |
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 11
The pharmaceutical market is characterized by six multi-national corporate groups which together achieved approximately 30 % of worldwide sales.3 These corporate groups are characterized in each case by a portfolio of patent protected medicines. If a medication achieves more than USD 1 billion per year, this is referred to as a “blockbuster”. The prerequisites for this success are research, development and market introduction of medications whose effective substances normally have an innovative nature with regard to effectiveness and safety. The importance of the few introductions of new products satisfying these requirements is of particular relevance for the earnings power of a pharmaceutical company engaged in research because the research and development costs of all projects must be covered by the few successful product innovations.
Producers of generics are clearly different from pharmaceutical enterprises engaged in research because the producers of generics put pharmaceuticals based on formerly patented substances on the market without any substantial research. Since these chemically equivalent medicines are offered normally much cheaper than the original medication, the sales and margins of the original medication rapidly fall upon market introduction of generic competitive products.
Generic producers achieve approximately 50 % of the sales in the generic pharmaceutical market. The producers of generics worldwide share of sales for pharmaceuticals were 9 % in the year 2004.4
As a consequence of the savings of public organizations (social insurance) on the spending side, generics will continue to gain in importance. These savings in benefit cuts result in price pressure on the original medications. This price pressure in a market which is subject to generics could also lead to an increasing substitution of original products by generics. This development is also promoted by the fact that patients with health insurance must in the future themselves bear a greater portion of their health costs. Finally, pharmaceutical enterprises engaged in research can only protect themselves against generic competition after expiration of the patent protection by establishing strong (product) brands.
As a result of the planned product introductions and the demographic developments, it is anticipated that the worldwide pharmaceutical market, starting at a base of USD 602 billion in the year 2005, will grow in the next 5 years annually at rates of 5 to 8 %5. This market growth is expected to have its basis in innovative products, therapeutic progress and new applications for existing products.
| | |
| 3 | | Pharmaceuticals & Biotechnology, Datamonitor May 2005, p. 13. The six companies are Pfizer, Sanofi-Aventis, GlaxoSmith-Kline, Merck & Co., J & J, AstraZeneca. |
| |
| 4 | | Generics, Datamonitor August 2005, p. 9 |
| |
| 5 | | IMS Global Pharmaceutical market 2005. |
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 12
The increased sale of medicines is also attributed to two additional factors. In the first place, the increasing average age of the population and the observation that older people require more medicines. Secondly, increasing purchasing power worldwide enables patients in developing countries to increasingly resort to modern therapies.6
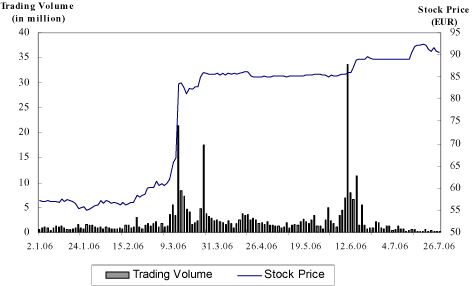
Schering’s most important markets continue to be in Europe, despite Schering’s worldwide presence. The segments Europe, USA and Japan represent 80.7 % of the net sales of Schering. The following graph summarizes Schering net sales according to segments in the fiscal year 2005, adjusted for the net sales of the radiopharmaceuticals business and the ALK-participation. To clarify, we would like to point out that the European segment contains regions outside the EU and that the USA segment also includes net sales of Medrad outside the USA.
Segment Europe Region
Segment Europe is the most important region for Schering, representing 44.7 % of the Group sales in the fiscal year 2005. It includes the countries in the European Union and the other European countries including Russia and Turkey. In addition, this segment includes the countries in the Caucasus, Central Asia and the Middle East, the Indian sub-continent and the entire African Continent. This segment also includes the business activities of Shering Oy, Helsinki, Finland, Jenapharm GmbH & Co. KG, Jena, Cis bio Int., Gif/Yvette, France, (hereinafter “Cis bio Int.”) as well as Justesa Imagen, Madrid, Spain.
The strongest pharmaceutical markets in terms of net sales in Europe are Germany, France and Italy, representing 47 % of the net sales in the segment Europe.
| | |
| 6 | | Pharmaceutical industry pulse, SG Cowen, March 2006, p. 16. |
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 13
The tension between innovation and ability to finance is approached in different manners in the individual countries in Europe: There are binding price negotiations in France for all pharmaceuticals; Sweden is characterized by clear regulations on reimbursement, while in The Netherlands, the regulation of prices by the state is delegated mostly to the insurers.
Positive developments in the pharmaceutical markets are seen in the progress of medicine and technology for developments relating to diseases which are not yet subject to therapy. In addition, the expansion of the European market influences the growth potential: Ten countries joining the EU represented only 6 % of the European pharmaceutical market in 2004 and are expected to grow in the following five years in high one digit or low two digit percentage rates.7
As a result of this development, the European pharmaceutical market grew in 2005 by 7.1 % to USD 169.5 billion.8 In the European segment, Schering had growth in net sales in the fiscal year 2005 of 5 %.
Segment USA Region
This segment includes the pharmaceutical activities in the geographic region of the United States of America as well as Puerto Rico. Additionally, included in the USA segment until 2005 are the foreign sales of application systems (injection systems and accessories) for contrast agents sold by the Medrad.9 The rights to the name Schering are held by the independent American pharmaceutical company Schering-Plough, Corporation, Kenilworth, USA (hereinafter “Schering-Plough”), due to historic reasons.
The USA segment is the second most important segment for Schering AG providing 27.0 % of group sales in fiscal year 2005. The enormous importance of the American pharmaceutical market (world market share in year 2005: 47 %10) is not reflected in the sales distribution of Scher-ing. At the same time, this market grew 10.1 % per year from 2001 to 2004. Producers of generic pharmaceuticals participated strongly in the growth of this market (CAGR 2000 to 2004 13.2 %)11. In fiscal year 2005, it achieved growth in net sales in the amount of 13 % after adjustment for currency exchange effects (unadjusted 14 %).
National approval for pharmaceuticals is carried out in the USA by the Food and Drug Administration (hereinafter, the “FDA”). The FDA controls the safety and effectiveness of medical products, human and veterinary pharmaceuticals. The approval of a medication in the US mar-
| | |
| 7 | | BPI: Pharmadaten 2005, p. 27 |
| |
| 8 | | IMS Health Reports dated 20 June 2006. |
| |
| 9 | | Since 1 January 2006 the sales of the Medrad have been included under Other Activities. |
| |
| 10 | | Arzneimittelmarkt, VFA 2005; Umsatz zu Herstellabgabepreisen im Apothekenmarkt. |
| |
| 11 | | Generics Datamonitor, August 2005, p. 8. |
Unauthorized translation
of the legally binding
German original
Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 14
ket requires compliance with certain requirements for production facilities and production processes, which are formulated and controlled by the FDA.
Segment Japan
Schering AG achieved 8.4 % of their net sales in the Japanese pharmaceutical market. This high priced market is characterized by very restrictive approval processes. The public health authority (National Health Insurance, NHI) has taken steps to enact a two year cycle of price reductions for approved medicines. Due to these regulatory actions, Schering expects regular future price reductions.
However, Schering had growth in net sales in the local currency of 1 % as a result of increased sales volume.
Segment Latin America / Canada Region
The segment Latin America / Canada is managed by the regional center in Mexico and is responsible for distribution through more than 15 subsidiaries in Latin America, Canada as well as in the Caribbean region 9.0 % of the net sales of Schering AG were achieved in this segment in the fiscal year 2005. The strongest markets in terms of sales are Brazil, Mexico and Canada.
The market environment in Latin American is characterized by a strong presence of generic Pharmaceuticals and the distribution of black market products. This is enabled by the lack of reimbursement of pharmaceuticals, very limited enforcement of patent legislation as well as the approval policy of the regulating authorities.
Yasmin is the strongest product in terms of sales in virtually all countries in this segment. Contraceptives in general achieve 70 % of the net sales proceeds of Schering AG in this segment.
The development in the Latin American segment is strongly dependent on the rate of exchange compared to the Euro. Adjusted for these effects, Schering achieved a 9 % growth rate in fiscal year 2005 (unadjusted 19 %).
Segment Asia/Pacific Region
The Asian/Pacific segment contributed 4.8 % of Schering Group sales in fiscal year 2005.
The segment includes the countries in Southeast Asia and Eastern Asia (without Japan) as well as Australia and New Zealand. In the fiscal year 2005, Australia, South Korea and China were the strongest markets in terms of sales which achieved 74 % of the net sales in this segment.
A common characteristic in all sub-markets is the lack of reimbursement of costs which has the consequence of less value being placed on brands and a preference for generic products. An
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 15 |
exception is China where the pharmaceutical market is dominated by individual products. Therefore, the focus on strong (product) brands represents a good marketing strategy there.
Taiwan, South Korea, Hong Kong and Singapore are characterized by an advanced development of the pharmaceutical market. Contrary to this, China and Indonesia have great potential for growth as a result of the interplay between a strong economy and increasing rates of diagnosis and treatment.
The Chinese pharmaceutical market in 2005 was USD 11.7 billion. China will be the seventh largest pharmaceutical market in the world in 2009 as the result of anticipated two digit growth rates.12
2.2.3 Business area Gynecology & Andrology
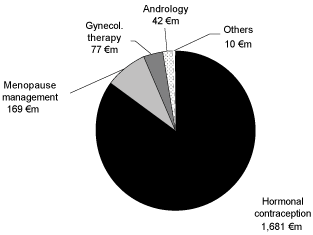
The Gynecology&Andrology business area consists of four indication areas: hormonal contraception, menopause management, gynecological therapy and andrology. The net sales in the fiscal year 2005 in these indications are are shown as follows:
The relevant genital-urinary market for the division Gynecology&Andrology generated a worldwide sales volume of USD 19.7 billion in the year 2005. A market volume of USD 23.7 billion is anticipated for the year 2010 based on moderate growth rates averaging 3.8%.13 In the fiscal year 2005, the Gynecology and Andrology business area achieved net sales of€ 1,979 million. The strongest segment in terms of growth was Europe with a net sales share of 54.2 %, followed by the segment USA with 22.1 % and the segment Latin America /
| | |
| 12 | | IMS Health Reports, 20 June 2006. |
| |
| 13 | | Wood Mackenzie’s Productview, March 2006. |
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 16 |
Canada with 16.6 %. The strongest field in terms of sales was hormonal contraception with a net sales share of 85.0 %, followed by menopause management with 8.5 % of business area net sales.
Hormonal Contraception
Oral and non-oral contraceptive medications for women are developed and marketed in the field relating to hormonal contraception. Since the year 2005, Schering has been the market leader in this indication area with a share of the global market of approximately 27 %. The market share in the segment Europe was 47 % in the fiscal year 2005. The market share of Schering, in the segment USA, in which the highest rate of growth is anticipated in the future, was 14 % as a result of the active appearance on the market which only occurred recently. In addition to pure contraception, the product focus is on health relevant additional uses for the treatment of premenstrual disphory, dysmenorrhorea and acne.
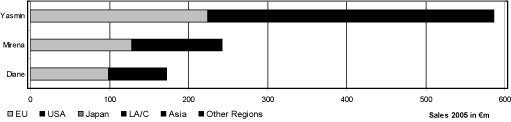
The three strongest products of the gynecology and andrology business area in terms of sales involve the indication area of hormonal contraception and make up more than 50 % of the net sales in this business area.
The strongest product in terms of sales in the fiscal year 2005 wasYasminwith net sales of€ 586 million, making up 29.6 % of the net sales of the business area. This product was introduced into the market in the year 2000 and has patent protection until the year 2020. It is based on the innovative progestin drospirenone developed by Schering AG itself and is, at the present time, the most successful oral contraceptive worldwide. Yasmin had growth in net sales of 36.4 % in the fiscal year 2005 representing a worldwide market share of more than 13 % and, thus, contributed substantially to securing the market leadership in this indication area. The largest portion of net sales of Yasmin was in the USA with 48.2 %.
Schering has an additional innovative product inMirena,which was introduced into the market in 199014 and achieved in the fiscal year 2005 net sales of€243 million. This corresponds to 12.3 % of the net sales in the business area and a growth in net sales of 22.1 % compared to the
| | |
| 14 | | Market introduction in Finland by Leiras, Helsinki, Finland. |
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 17 |
year 2004. The largest portion of net sales of Mirena was 52.7 % in Europe. Mirena is a progestin releasing intra-uterine system and is a medication in the indication area of non-oral contraceptives which guarantees an effective contraception for five years after being placed in the uterus. At the present time, more than seven million women worldwide use this system. The patent protection for the device for Mirena continues until the year 2015, and the patent protection for the production process lasts until the year 2013.
The third strongest product in terms of sales in the fiscal year 2005 wasDiane,a no longer patent protected therapy for acne having additionally a contraceptive effect, which made a contribution of€ 172 million or 8.7 % to the net sales in this business area while having a reduction in net sales of -8.0 %. The reduction in net sales was primarily the result of the increasing competition from generics. They threaten as a general rule the sales of the product portfolio as when the patent protection expires they offer much cheaper medications.
The company has the strategic goal of concentrating on patent protected, innovative products and substances in order to secure the future growth in net sales of the business area and its market leadership. In the self developed progestin drospirenone, Schering has a patent protected product as the basis for the further expansion of a broad product family with blockbuster potential (the Yasmin family).
Yasminelle,a low dosage variety of Yasmin which received approval in The Netherlands as the reference country for the EU in August 2005 and European wide approval in May 2006, as well as YAZ, a low-dosage oral contraceptive with an innovative dosage scheme and positive effects on premenstrual dysphory, provides the company with two medications which are intended to continue the positive trend in the sales of Yasmin in the future.
A moderate, average growth of 4.3 % annually in the period up to 2010 is anticipated in the market for hormonal contraceptives.15 The main competitors for the Company are Johnson&Johnson, New Brunswick, USA (hereinafter “J&J”), having a market share of approximately 21 %, Barr Pharmaceutical Inc., Pomona, USA (hereinafter “Barr”) with a market share of approximately 10%, Akzo Nobel NV, Arnhem, The Netherlands with a market share of approximately 11 %, Watson Pharmaceutical Inc., Corona, USA with a market share of approximately 7 % and Wyeth Pharmaceuticals, Madison, USA (hereinafter “Wyeth”) with a market share of approximately 9 %.
The market in developed segments such as Europe and the USA is increasingly saturated and overall characterized by a high concentration of competitors, an existing broad range of products and the increased entry of suppliers of generic products. In addition, the demographic development in these segments indicates that there will be an additional flattening of market
| | |
| 15 | | Wood Mackenzie’s Productview, March 2006. |
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 18 |
growth. Growth above market average will only be possible in the future for companies having unique characteristics as a result of innovative and patented products and by increasing the application rate, especially in the USA. Over the mid-term, future growth is anticipated as a result of the expansion of the product range in products with lower dosages of effective substances, new types of dosage regimens, additional medical uses and forms of applications. Thus, research and development activities are focusing on the development of low dosage oral and alternative contraceptives (e.g. contraceptive patches, vaginal rings). In addition, emphasis is currently being placed in the field of non-hormonal medications. The product pipeline of Schering AG with DUB OC, LCS and FC patch contains future products with the above described characteristics which correspond to the developing trends in the case of contraceptive products and are able to generate future growth potential in excess of market growth.
Menopause Management
The Menopause Management indication area is concentrated on medications for the hormonal treatment of menopause symptoms. After the collapse of the market as a result of a Woman’s Health Initiative study of the FDA in the year 2003, which involved the negative after-effects of hormonal applications, the market is now in a slow recovery phase. The competitive environment in the Menopause Management market is characterized by the market leadership of Wyeth having a market share of approximately 40 %.
Approximately half of the net sales in the field were achieved in the fiscal year 2005 with products whose patent protection already expired. WithAngeliq,Schering has introduced into the worldwide market a hormonal medication which was approved in July 2003 in Europe and in September 2005 in the USA and which should be a future driver of growth in this field. Angeliq contributed 0.6 % to the net sales of the business area with net sales of around almost€ 11 million in the fiscal year 2005, whereby the net sales compared to the previous year were almost six times higher. Angeliq contains the progestin drospirenone in addition to estradiol, which emphasizes the strategy of Schering in expanding a portfolio of patented drospirenone based products. Schering’s strategic goal is a significant increase in the market share in the menopause management market which is intended to be supported by the worldwide product introduction of Angeliq as well as the expansion of the Angeliq family.
The market for menopause management in the year 2005 had a moderate average growth worldwide sales volume of approximately€ 2.3 billion, which is supposed to increase to approximately€ 2.7 billion in the year 2010 with moderate annual average growth of 3.3 % annually.16 The anticipated growth in the market for menopause products will be strengthened by 2 % annual growth in the target group of women between the ages of 45 and 64 in the USA.17
| | |
| 16 | | Wood Mackenzie’s Productview, March 2006. |
| |
| 17 | | Analysis by Schering AG. |
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 19 |
The long term oriented trend towards non-hormonal medications also indicates that there will be a further increase in the rate of acceptance.
Gynecological Therapy
The indication area of Gynecological Therapy achieves at the present time low net sales with old products which are solely advertised as low value sales and only contribute to a minor degree to the net sales of the business area. The future importance of this field consists in the expansion of a new platform for products for the treatment of uterine fibroids and endometriosis. In the case of the treatment of uterine fibroids, medications are involved which relate to the treatment of benign tumors in the uterine musculature which until now have normally had to be surgically removed. Endometriosis treatment involves medications to ease the symptoms arising as a consequence of the collection of uterine mucus membrane outside of the inner part of the uterus. Future products are still in an early development phase.
Andrology
The indication area of Andrology involves the indication of hypogonadism (deficiency in the function of the testicles) (treatment for testosterone deficiency) and fertility control (development of reliable, reversible methods to control fertility in men). Schering AG is the market leader in Europe in the field of testosterone therapy with the productsNebido(net sales in 2005,€ 7 million) andTestogel(net sales in 2005,€ 12 million); Testogel is licensed for 15 European countries. Nebido is a depository testosterone medication for the treatment of male testosterone deficiency which only has to be injected four times annually and, thus, is user friendly. Nebido was approved in Europe in July 2004. Testogel is another testosterone product but as a gel it has another method of application for short-term treatment. Testogel is marketed by Solvay SA, Brussels, Belgium (hereinafter “Solvay” and in the USA by Icos Corp., Bothell, USA and is known as Androgel).
The market for hypogonadism therapy (HGT) in the regions of the USA, Europe, Latin America, and Asia is different. Solvay is the market leader in the USA with the project Androgel having 67 % of the market share. The total market in the USA has a volume of€ 384 million. Schering will not be represented in this market during the planning period. The HGT market in Europe is much smaller as a result of the lower price level compared to the USA, and has a total volume of€ 57 million. Schering achieved a market share in the fiscal year 2005 in Europe of 46 %.
Schering has the goal of continuing to expand the market leadership position in Europe with Testogel as the primary (short-term therapy) and Nebido as the secondary therapy (mid-term application). In addition, expansion in the treatment field of hypogonadism for aging men will strengthen the growth of sales over the mid-term. The demographic changes in recent years have been leading to an increased number of men over the age of 40 years who are potential
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 20 |
users. The increased life expectancy for men and an increasingly higher acceptance rate can positively influence the moderate future market growth.
2.2.4 Business area Diagnostic Imaging
The business area Diagnostic Imaging is divided into the fields x-ray contrast agents, magnetic resonance imaging contrast agents and application technologies. The radiopharmaceuticals business mainly of CIS bio Int. was sold by Schering in the first half of 2006 to a Belgium syndicate.
The contrast agents business includes diagnostics for imaging procedures such as x-rays, computer tomography (CT) scan and magnetic resonance imaging (MRI). Schering is one of the world’s leading suppliers in the field of in-vivo diagnostics. The business with applications systems (injection systems and accessories) for contrast agents is conducted by Medrad group. This company is the worldwide market leader in its field of business, having a market share of currently more than 70 % in the USA and more than 50 % in Europe.
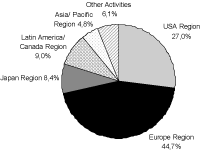
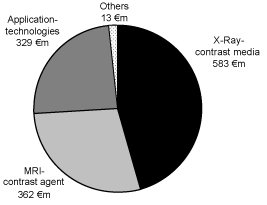
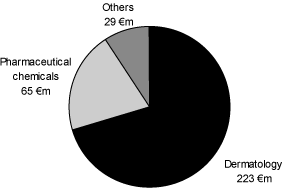
The net sales in the amount of€ 1.287 million of the fiscal year 2005, adjusted for the 2006 divestiture of the radiopharmaceuticals business (net sales 2005:€ 117 million), were allocated as follows:
Financial Year 2005
adjusted*
*adjusted for the divesture of the radiopharmaceuticals business
The business with x-ray contrast agents involved primarily the products Ultravist and lopamiron, licensed from Bracco S.p.A, Milan, Italy, (hereinafter also “Bracco”). The MRI contrast agents business was based primarily on the sale of the product Magnevist which was the first
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 21 |
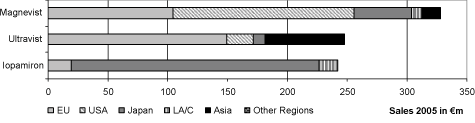
MRI contrast agent on the market. The distribution of the sales of these three contrast agent products in the segments in the fiscal year 2005 was as follows:
MRI Contrast Agents
The commercial situation in the MRI contrast agent field is currently characterized by the product Magnevist. Magnevist is an extra cellular contrast agent for the diagnosis in the central nervous system and throughout the body. It contributed€ 328 million net sales in the fiscal year 2005 which was 90.6 % of the net sales in the field of MRI contrast agents. The net sales are made primarily in the segments USA, Europe and Japan. The product portfolio in the field of MRI contrast agents also includes: Primovist and Resovist for liver specific imaging, Gadovist for imaging of the brain and the spinal cord as well as Vasovist (not yet approved for use in the USA) for imaging the circulatory system. These products in the contrast agents market are supposed to create the basis in order to satisfy the increasing demand for organ specific MRI contrast agents and in order to secure sales even after expiration of the patents for Magnevist in the year 2007 in Europe and Japan as well as in the year 2013 in the USA.
X-ray Contrast Agents
The primary products in the field of x-ray contrast agents are Ultravist and lopamiron. Ultravist and lopamiron are approved for all common x-ray examinations including CT scans.
Net sales of the x-ray contrast agent Ultravist in the fiscal year 2005 were€ 248 million or 42.5 % of the net sales of the x-ray contrast agent’s field. The net sales of Ultravist were primarily in Europe and Asia. As a result of the late market entry of Schering into the market in the USA, only below average net sales are achieved there compared to the other segments.
The x-ray contrast agent lopamiron is produced by Schering under a license from Bracco, and is distributed mostly in Japan as well as in Latin America and France. Accordingly, there are only sales with this product in the segments Europe, Japan and Latin America / Canada, whereby approximately 85.5 % of the net sales were realized in Japan in the fiscal year 2005. lopamiron had net sales of€ 242 million in the fiscal year 2005 or 18.8 % of the total net sales in the field of Diagnostic Imaging, as well as 41.5 % in the field of x-ray contrast agents.
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 22 |
The contrast agents market (x-ray and MRI contrast agents) is dominated by six market participants. In addition to Schering, these are Bracco, General Electric Company, Fairfield, USA, Daiichi Pharmaceuticals, Tokyo, Japan, Guerbet S.A., Villepinte, France and Tyco/Mallinckrodt, St. Louis, USA. The market for diagnostic imaging will grow in the next five years at an average annual rate of 2.9 % according to estimates by EvaluatePharma. The growth will be primarily influenced by MRI contrast agents with an average growth of 9.9 % annually.18 The growth in the contrast agents market is driven by the increasing number of examinations with imaging procedures as a result of the increased number of installed devices and the increasingly aging population because typically diseases relating to age such as heart disease are examined by imaging procedures. This quantity related growth will be partially offset in markets with currently above average price levels by the ongoing price competition between original suppliers and, to a limited degree, by generic suppliers, as well as by increasing measures to slow down costs in the field of health services, such as by state ordered price reductions, especially in Japan.
Application Technologies
The Medrad group distributes as a supplier of application technologies primarily injection systems and accessories for contrast agents which, for example, enable a precise application of contrast agents in the fields of CT scans and MRI. In addition to this, infusion pumps are distributed which secure a continuous application of the medication even in the strong magnetic field of a nuclear spin tomography. In addition, Medrad generates net sales from services for the installation and maintenance of its own and third party injection systems.
Medrad group achieved 68.3 % of its net sales in the USA in the fiscal year 2005 and 18.2 % in Europe. Medrad had growth in net sales in the fiscal year 2005 of 21.4 %. The growth of Medrad is driven by the increasing number of examinations with imaging procedures supported by contrast agents, the switch to injection systems from one-head to two-head injection systems and the resulting additional need for sterile consumables (cartridges, tubes, etc.) as well as the increasing installation of computer imaging machines and magnetic resonance imaging machines in Medrad’s markets which include the installation of an injection systems.
2.2.5 Business area Specialized Therapeutics
The business area Specialized Therapeutics includes the indication areas central nervous system (multiple sclerosis and Parkinson’s disease), cardiovascular system (heart disease, throm-bangiitis obliterans and thrombosis) and gastrointestinal disease (Colitis Ulcerosa and Morbus Crohn).
| | |
| 18 | | EvaluatePharma 8 June 2006. |
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 23 |
The indication area central nervous system includes products for the disease multiple sclerosis, in which Schering is a worldwide leading supplier, as well as Parkinson’s disease. In the indication area cardiovascular system, Schering primarily has products for thrombangiitis obliterans as well as thrombosis. In the indication area gastrointestinal diseases, the business development with the development project Sargramostim (distributed in the business area oncology under the product name Leukine) for Morbus Crohn is being pursued.
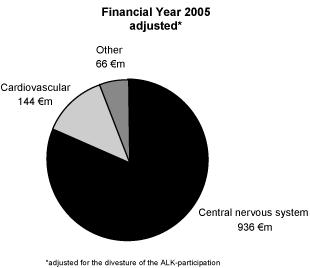
The following graph illustrates the share of net sales in the business area in the year 2005 after adjustment for the 2006 sale of the investment in ALK-Scherax (net sales in 2005:€ 34 million):
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 24 |
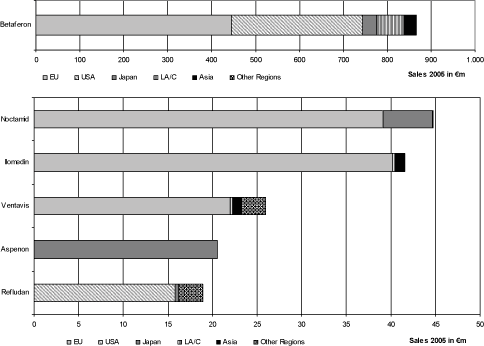
The net sales in the year 2005 for the six strongest products of the division in terms of net sales can be shown when subdividing according to segments as follows:
The multiple sclerosis medication Betaferon19 was in 2005 Schering’s only blockbuster, with€ 867 million net sales or a 16.8 % portion of the Group sales. The net sales of the top 2 to the top 6 products of the business area are allocated in an amount of 66.8 % to Europe in fiscal year 2005, despite products with a strong regional relation such as Refludan (USA) and Aspenon (Japan).
Central Nervous System
As a result of the dominance of Betaferon in the Specialized Therapeutics business area with a share of 92.6 % of net sales in the Central Nervous System indication area in the year 2005, the commercial development of the business area primarily depends on this product. The primary sales markets for Betaferon are the segments Europe and USA which had 85.9 % of the net sales of Betaferon in the year 2005. Multiple sclerosis (MS) is a chronic inflammatory progressive disease of the central nervous system for which the treatment involves high recurring costs. Worldwide, approximately 1.5 million people are affected.
| | |
| 19 | | The multiple sclerosis medication in marketed in the USA and the countries outside of Europe under the name Betaseron. |
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 25 |
Betaferon is based on the protein inferon BETA-1b which was developed in cooperation with Cetus and which was approved in 1993 as the first medication for treatment of MS with recurring symptoms and was introduced into the market. This pioneering work enabled Schering to conduct long term studies on the effectiveness and safety and established the basis for the high customer loyalty of the patients. As a protein, Betaferon in the original form of dosage had to be permanently cooled both in storage and transport. Since 2003, Schering offers an improved formula with which patients can also inject uncooled Betaferon.
In addition to Schering, three other competitors supply medications for the treatment of multiple sclerosis. These are Biogen Idec, Inc., Cambridge, USA (hereinafter “Biogen Idec”), with the medicationAvonex,Serono S.A., Genf, Switzerland, withRebifas well as jointly Sanofi-Aventis S.A., Paris, France (hereinafter “Sanofi-Aventis”) and Teva Pharmaceutical Industries Ltd., Jerusalem, Israel, withCopaxone.Biogen Idec. had also obtained approval from the FDA for Tysabri in the year 2004, but this approval was withdrawn again in 2005 due to safety concerns. In June 2006,Tysabriwas again approved by the EMEA as well as by the FDA for a limited group of patients. This medication, which was previously known as Antegren, is based on the substance Natalizumab; it is anticipated that this medication will be primarily used for patients who do not react to treatment with Beta-Interferonen or Copaxone.
The following graph shows the market shares of the main medications in the MS treatment20according to net sales in the year 2005:
| | |
| 20 | | Furthermore, medication made with Mitoxantrone had sales of EUR 44.5 million and Natalizumab had sales of EUR 21.9 million in 2005. |
| | |
| | | Schering AG |
| Unauthorized translation | | Independent expert opinion |
| of the legally binding | | Equity value as of 13 September 2006 |
| German original | | Page 26 |
With a market share of approximately 24.3 % in the year 2005 in Europe,21 which represents the most important market in terms of sales, Betaferon is almost at the level of the market leader Rebif.
EvaluatePharma estimates that the market for multiple sclerosis medications will grow at an annual rate of 10.7 % from 2005 to 2008 as a result of increased income (for example, in Eastern Europe and Asia) and increasing spread of health insurance.22
The indication area central nervous system also includes Noctamid, with which net sales in the amount of€45 million were achieved in the year 2005. This corresponds to 4.8% of the net sales in the indication area of the central nervous system. Noctamid is used as a sleep agent and as initial medication for anesthesia and is distributed with a share of 87.5 % of the net sales primarily in the segment Europe, but not in the USA.
Furthermore, Schering intends to expand the product portfolio in the Specialized Therapeutics business area in the indication area central nervous system by products for Parkinson’s disease as well as new treatment methods for multiple sclerosis.
Cardiovascular system
Schering offers in the cardiovascular system indication area, among others, Ilomedin for the treatment of thrombosis and Ventavis for improving physical activity in the case of pulmonary hypertonia. These products had net sales in the year 2005 amounting to 47.2 % of the net sales in the indication area cardiovascular system. Of the net sales of these products, 92.0 % were achieved in the segment Europe.
Schering’s product portfolio also contains the medicine Aspenon, which is only marketed in Japan. This drug seeks to restore heart rhythms and produced despite its regional limitations net sales of€ 21 million in fiscal year 2005.
Beyond maintaining the existing assortment of products, Schering is not devoting any further funds for development in this field.
Gastrointestinal disease/ Other
Schering is working on the development of the use of Sargramostim from the business area Oncology for the treatment of Morbus Chron as part of a clear expansion into the gastrointestinal disease indication area. The main competitors in the indication area gastrointestinal disease are Pfizer Inc., New York, USA (hereinafter “Pfizer”), AstraZeneca Plc., London, Great Britain
| | |
| 21 | | According to Decision Resources, Multiple Sclerosis, p. 204. |
| |
| 22 | | EvaluatePharma, 8 June 2006. |
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 27 |
| | | |
(hereinafter “AstraZeneca”), UCB, S.A. Brüssels, Belgium, Abbot Laboratories, Chicago, USA, Schering-Plough, J&J, and GlaxoSmithKline plc, Brentford, Great Britain (hereinafter “GlaxoSmithKline”).
2.2.6 Business area Oncology
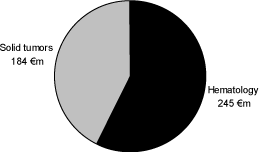
The business area Oncology is divided into two indication areas: hematological oncology and solid tumors.
Hematological oncology includes products for therapies for the targeted treatment of various forms of leukemia and lymphoma. Solid tumors include all types of cancer in the tissue of the body except for the blood, the bone marrow and the lymphatic system. This includes prostate cancer as the most common type of cancer in men as well as breast cancer in women. Other solid tumors are, among others, lung cancer, brain tumors or colorectal cancer. The current product portfolio of Schering in the indication area of oncology is primarily in the indication area of hematological oncology. Schering has two products in the market in the field of therapies for solid tumors. Schering wants to further expand this existing presence in the field of solid tumors.
The following graph shows the portions of net sales in the indication areas in the year 2005:
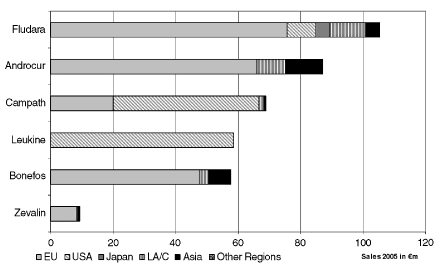
Hematological oncology involves primarily the products Fludara, Campath, Zevalin in Europe and Leukine in the USA. In the year 2004, the product Zevalin was approved and introduced into the first markets in Europe. The products Androcur and Bonefos were the main products distributed for therapy for the treatment of solid tumors in the fiscal year 2005. Bonefos is currently approved for the treatment of hypercalcaemia and osteolysis which are caused by malignant breast cancers and multiple myelomes.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 28 |
| | | |
The allocation of net sales to these products in the segments in the fiscal year 2005 can be shown as follows:
Hematological Oncology
The productFludarais used as a first line therapy and as a second line therapy for the treatment of patients with chronic lymphocytic leukemia (CLL). Fludara is approved in Europe, Canada and many other countries both as an intravenous injection as well as in the form of tablets. Flu-dara contributed€ 105 million in net sales in the fiscal year 2005 which was 42.8 % of the net sales in hematological oncology. The net sales of Fludara are achieved primarily in the segments Europe and the USA as well as Latin America/Canada. Fludara (intravenous injection) is subject to competition from generics in the USA, and also only approved for second line therapy.
In addition the productCampath(Mabcampath in Europe) is approved as the first antibody for the treatment of patients with CLL. Campath is the only approved medication for patients who have been previously treated with Alkylanzien and Fludara and also for patients who are no longer responding to treatment with Fludara. CLL normally appears after the age of 50 and is the most common form of leukemia in adults with approximately 180,000 new cases annually worldwide. Additional studies are examining the role of Campath both as a mono-therapy as well as in combination with Fludara, taking into account first line and second line therapeutic approache. These studies are being conducted in cooperation with Genzyme Corp., Cambridge, USA (hereinafter “Genzyme”). The product Campath contributed€ 69 million in net sales in the
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 29 |
| | | |
fiscal year 2005 which was 28.2 % of the net sales in the field of hematological oncology. The net sales were realized primarily in the segments USA and Europe.
Leukineis a growth factor protein (GM-CSF) produced using genetic engineering. It is used in hematological oncology to strengthen and rebuild the immune system, for example, after chemotherapy or a bone marrow transplant (immune system activator). The medication is the only colony stimulating factor which is approved in the USA for the treatment after initial chemotherapy in older patients with acute myeloisic leukemia (AML). The net sales are realized exclusively in the USA. The product Leukine contributed 23.9 % to the net sales in hematological oncology in the fiscal year 2005.
Zevalinwas introduced into the market in the fiscal year 2004 for the treatment of adult patients with follicular non-Hodgkins lymphoma (NHL) who do not respond to the treatment with or have a relapse after such a treatment. Zevalin is the first approved radio-immuno therapy, a special anti-body directed against lymphocytes which is attached to a radio-nuclide and can target the tumor cells by attacking them in a combination of radiation and immuno therapy.
NHL is a tumor of the lymphatic system and is the fifth most common form of cancer after breast cancer, prostate cancer, lung cancer and colorectal cancer. NHL occurs in the lymphocytes, a form of white blood cells. The share of net sales of Zevalin was approximately 3.8 % of the hematological oncology net sales in the fiscal year 2005 after being introduced in the market in the previous year. The product is distributed in Europe by Schering AG; Biogen Idec holds the marketing rights for this product in the USA.
The main competitors in the hematology market are Roche Holding Ltd., Basel, Switzerland (hereinafter “Roche”), Sanofi-Aventis, Amgen Inc., Thousand Oaks, USA (hereinafter “Amgen”), Astra Zeneca, Novartis AG, Basel, Switzerland (hereinafter “Novartis”), J & J, Bristol-Myers Squibb Company, New York, USA (hereinafter “BMS”), Schering-Plough and Pfizer.
Solid Tumors
The productAndrocuris used in the indication area of solid tumors for the treatment of prostate cancer if the carcinoma can no longer be surgically removed. Androcur has been subject to competition from generics after the patent protection expired a long time ago. The product contributed net sales of€ 87 million or 47.1 % to the net sales in the indication area of solid tumors. The sales are allocated to Europe, Asia as well as Latin America/Canada.
Bonefosis a bisphosphonate which is used for the treatment of breast cancer patients with bone metastases. Bonefos reduces the accompanying symptoms caused by such malignant breast cancer diseases, such as hypercalcaemia and osteolysis. Net sales of€ 58 million and, thus,
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 30 |
| | | |
31.5 % of the net sales in the indication area of solid tumors were realized in the fiscal year 2005 with the product Bonefos. The product is distributed primarily in Europe.
The main competitors in the indication area of solid tumors are Roche Holding Ltd., Sanofi-Aventis, Amgen, Astra Zeneca, Novartis AG, J & J, BMS, Schering-Plough und Pfizer.
The market for cancer medications is estimated by EvaluatePharma to grow at an average annual rate of 17 % in the next five years.23 The basis for this forecast is a market volume of€ 24.1 billion in the year 2005. The growth is driven primarily by new and further developed therapeutic approaches based on antineoplastics, cytostatics as well as innovative targeted therapies. These new therapies use new knowledge about the regulation of tumor growth and the causes of tumors, for example, by targeted inhibition of receptors for certain growth or message substances, prevention of the growth of blood vessels which are necessary for tumor growth, etc.
The market volume of antineoplastics and cytostatics is growing of an above average annual rate of approximately 29 % and 30 % respectively, which is clearly above the anticipated rate of growth of the other therapeutic approaches in the oncology market. EvaluatePharma anticipates that the growth of these cancer medications in the oncology market will increase from almost two fifths to three fifths.
Additional growth in the oncology market is expected as a result of the increased number of cases of cancer worldwide. This is, in turn, the result of higher life expectancy and the increased number of old people in the general population because most cases of cancer occur in older people. The targeted new therapeutic approaches continue to make it possible to treat patients for whom the current therapeutic forms (chemotherapy) are too toxic. Finally, higher periods of survival of the patients, especially in the case of tumors which were difficult to treat until now, and which result from the new forms of therapy, contribute to market growth.
As a result of the anticipated market growth, oncology constitutes the largest portion of the present development activities in the pharmaceutical industry and has the most development projects in later phases.24 The main emphasis of the development projects is directed towards instances of colorectal tumors, lung cancer, breast cancer and ovarian cancer. As a result of the focus of the emphasis in research of the pharmaceutical industry on oncology, it can be assumed that there will be stronger competition in the future. Since in part considerably higher costs for treatment can be assumed for new forms of therapy compared to the present price level, stricter regulatory measures, e.g. restrictions on approvals and price controls, can be anticipated. It can be assumed that the institutions in the health market will establish high requirements for the
| | |
| 23 | | EvaluatePharma, 8 June 2006 |
| |
| 24 | | Fox-Tucker, James, 2005, The Cancer Market Outlook to 2010, p. 13. |
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 31 |
| | | |
innovative level of new oncology products. This applies especially with regard to their improved effectiveness compared to competitive products.
In recent years, Schering has considerably strengthened its research and development activities for the resolved strategic expansion of the presence in the indication areas of solid tumors. The development projects are in medium to late development phases (phase II and phase III projects). The approaches in research and development indicate a high potential for innovation from the point of view of the company, among other indication areas, in the field of inhibiting the growth of blood vessels and tumors (antiangiogenesis), targeted cell cycle inhibition of tumor cells with possible protection of surrounding healthy tissue, modern immuno therapies as well as endocrinal therapies.
2.2.7 Other Sources
The Other Sources of Schering consist of the dermatology business, the pharmaceutical chemicals business with other pharmaceutical producers as well as the remaining Other Activities.
Dermatology includes products for the treatment of non-infectious and infectious diseases of the skin, including eczema, psoriasis, acne, rosacea, hemorrhoids and fungal infections.
The pharmaceutical chemicals business involves the production of basic pharmaceutical chemicals and substances for the own fields of business and other pharmaceutical producers.
The remaining Other Activities involve cooperation in the field of distribution, insurance and publications.
The following chart shows the net sales of the Other Activities for the year 2005:
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 32 |
| | | |
The dermatology business has been conducted by the subsidiary Intendis GmbH with its registered office in Berlin (hereinafter referred to as “Intendis”). Intendis contributed€ 223 million or 71.0 % net sales in the fiscal year 2005 to the Other Sources. The strongest products in the dermatology business in terms of sales areAdvantanwith€ 41 million net sales andSkinorenwith€ 40 million net sales. Advantan is a topical corticoid for the treatment of eczema, and Skinoren25 is a topical product for the treatment of rosacea.
25 The trade name in the USA is Finacea.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 33 |
| | | |
3 General valuation principles
The valuation principles described below are considered today, both in theory and in practice, as well-established and are reflected in professional journals and in the official bulletins published by the German Institute of Certified Public Accountants (IDW) and specifically in the IDW standard “Principles for performing company valuations” (Grundsatze zur Durchfuhrung von Unternehmensbewertungen, “IDW S 1”).
Based on prevailing case law and customary valuation practices, which are also consistent with the valuation described below, the compensation and guaranteed dividend must be derived on the basis of objectively determined equity value. An objective equity value represents a typical, inter-subjective and verifiable present value of future earnings of a company from the perspective of a domestic, tax-resident owner under the assumption that the company will continue to operate under the same corporate strategy in the future.
3.1 Discounted earnings value
In business management academia, case law and valuation practice, it is generally recognized that the discounted earnings value is a reliable criterion for establishing the value of a company.
According to IDW S 1, Section 2.1, an equity value may be determined according to the discounted earnings methodology or the discounted cash flow method. In the present case, the equity value was calculated using the discounted earnings methodology, the most widely used approach in German valuation practice and according to German case law. Since both methods — relying on the same assumption, particularly concerning the financing (see IDW S 1, Section 7.1) — lead to the same equity values, a presentation of the equity values according to the discounted cash flow method has not been provided in this report.
The discounted earnings value of a company assumes that a company seeks purely financial goals and is based on the present value of the company owner’s incoming net cash flow as a result of owning the company (present value of future earnings). In order to derive the present value of such net income, a discount rate is used, which represents the return on an alternative investment that is equivalent to an investment in the valued company (see, IDW S 1, section 2.1).
The company owners’ discounted net income, which is the basis for determining the discounted earnings value, is derived primarily from the dividends paid out from the financial net income generated by the company. Thus, a company valuation requires a projection of the company’s future distributable financial net income. In order to determine the net income distributable to the company owners, it must be taken into account that some of the company’s financial net income will be retained and that the undistributed net income will be used for other purposes.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 34 |
| | | |
These amounts may be used to make investments, for repaying debt capital or for returning equity capital to the owners (such as by redeeming shares) (see, IDW S 1, Section 4.4.1.1).
In calculating an objective equity value, the distribution of the financial net income that is available must be considered, taking into account the documented corporate strategy. If the financial planning is divided into two phases, then the budget itself will indicate any dividend payments and use of retained earnings in the first phase (the so-called “detailed financial planning phase”). In connection with the second phase (the so-called “sustainable earnings phase”), an assumption is typically made that in general the dividend policy of the valued company will be the equivalent of the dividend policy of an alternative investment. For purposes of calculating how any retained earnings are reinvested, it is assumed that either the amounts will yield an amount equal to the discount rate (before taxes on income at company level) — ensuring that their nominal value equals their net present value — or is equal in value to a fictitious value attributing those retained earnings directly to the shareholders who would ultimately benefit from any share price gains (see IDW S 1, Section 4.4.2.3) For this reason, a shareholder’s net income for purposes of this report includes the dividends which are payable to them as well as the retained earnings directly attributable to them.
Since an equity value is to be calculated from the perspective of the company owners, the shareholders’ personal income taxes on any dividends paid to them by the company must be taken into account (unlike the tax exempt imputation of retained earnings). Applying an average typified tax rate of 35% avoids linking an objective equity value to the individual tax situation of a given company owner. Since the half-credit method must be factored into the valuation of corporations, a typified personal tax rate of 17.5% on any distributed profits is applied in calculating the financial net income, whereas the retained earnings do not trigger any personal income taxes (see, IDW S 1, Section 4.4.2.5).
Consistent with the prevailing opinion in case law and among business management academics, the company was valued on a “stand-alone basis”, meaning that all positive and negative synergistic effects, which can be achieved only after the Domination and Profit and Loss Transfer Agreement is consummated, were not taken into account. The same applies with respect to any investments and divestitures as well as other measures which would only be made if the Domination and Profit and Loss Transfer Agreement comes into effect. Synergies which can be realized at Schering arising in the context of ade factocorporate group, that is before the Domination and Profit and Loss Transfer Agreement takes effect, were included when determining the value. This covers false synergistic effects.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 35 |
| | | |
3.2 Special items
Any matters, which cannot be factored into the calculation of the discounted earnings value, either in whole or in part, will generally be assessed separately and added to the discounted earnings value. These special items include not only non-operating assets, but also certain financial assets and real estate. Non-operating assets are any assets which may be sold without affecting the actual business operations of the company. (please refer to section 7.2)
The value of these special items is not reduced by typified shareholder income tax, as the value contribution of the special items is part of retained earnings due to the actually planned dividends.
3.3 Liquidation value and net asset value
If it seems generally more advantageous for the company to separately sell its individual assets or its self-contained operating units than to continue operating the business, then the sum of any realizable net proceeds from such sale — the liquidation value — must be taken into account.
Since the company being valued in the present case will continue to be operated indefinitely and since it may also be assumed that, given the costs connected with any liquidation (e.g., redundancy programs, employee severance packages) and a high share of intangible assets, the discounted earnings value should be higher than the relevant liquidation value in the event assets are stripped, the calculation of the liquidation values was waived for the purposes of this report. A rough approximation of the liquidation value yielded the result that the liquidation value is lower than the discounted earnings value.
The valuation of net assets from a repurchasing viewpoint yields the so-called net asset value (Rekonstruktionswert) of the company, which is merely a partial net asset value because intangible assets are generally not included. This valuation has no independent significance for calculating the overall value of an ongoing company. Thus, no net asset value was computed in the present case.
3.4 Stock market value
Since the share of Schering AG is listed on various stock exchanges, it is conceivable that the value of the company could be calculated on the basis of the relevant market capitalization of Schering AG as derived from the respective stock price. Nevertheless, there are weighty arguments against using a valuation based on stock market prices since such prices could be affected by a number of special factors such as the size and narrowness of the market, sales triggered by accidental events or speculative and other arbitrary influences, and could therefore become subject to unpredictable developments and volatility.
| | | |
| | | Schering AC |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 36 |
| | | |
The use of stock market prices (market capitalization) as a basis for determining a cash compensation and a guaranteed dividend is no substitute for a company valuation under the aforementioned principles, in as much as the latter valuation method uses a better and more extensive basis of information than the capital markets and accepts a valuation method that incorporates the calculus used in the capital markets. The valuation described hereinafter is based on an analysis of historical data and long-term financial planning, the level of detail and scope of which are not available to the public.
In its decision of 27 April 1999, the German Federal Constitutional Court (Bundesverfassungsgericht) held that for a number of special situations involving a company valuation (e.g., for purposes of making severance payments and providing compensation under §§ 304, 305 AktG, § 320b AktG or §§ 327a et seq. AktG ), the stock market price must be considered the minimum value when calculating the cash compensation for dissenting minority shareholders (the decision is published under 1 BvR 1613/94). The requirement to take the stock market prices into account when determining reasonable cash compensation does not mean, that it must always be the sole determinative factor according to the view of the German Federal Constitutional Court. There are no concerns under constitutional law about exceeding this amount. However serious constitutional law issues can arise, when the amount falls under this value.
Please refer to section 8.1 for details concerning the development and importance of the market price of Schering’s shares.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 37 |
| | | |
The general valuation principles explained in the section above represent merely abstract parameters which must now be more specifically defined for their application to the specific objective of the valuation. Accordingly, the modification of the general valuation principles into a specific methodical approach for valuing Schering, is explained below.
4.1 Structure and definiton of the valuation entity
The object of the valuation is Schering AG. Schering AG was valued on the basis of its consolidated planned accounts. The planned accounts of Schering are presented and explained in chapter 5.
The planned accounts include all consolidated affiliated companies. Associated companies or companies for which there was no plan data in the planned accounts because they are not operative or which are of minor importance as well as participations which are not necessary for the business were taken into account as special items. Furthermore, Schering’s available cash and cash equivalents and non-operating real estate, as well as the dilution effects of stock options were taken into account as additional special items.
The value of Schering AG is computed from the discounted earnings value plus the special items.
4.2 Effective date of the valuation
The valuation date for the equity value of Schering AG is 13 September 2006, the projected date of the extraordinary shareholders meeting of Schering AG.
1 January 2006 has been set as the technical effective date of the valuation. All projected future earnings were discounted to this date. The equity value established as of 1 January 2006 was compounded to 13 September 2006.
4.3 Analysis of profitability and derivation of future earnings
When estimating the future earnings, the uncertainty of the future earnings forecasts must be taken into account. Both risks and opportunities must be evaluated equally. Actual historical earnings serve here as an initial guideline.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 38 |
| | | |
4.3.1 Analysis of historical earnings
The analysis of past financial years, which was conducted in connection with calculating the equity value of Schering AG applying the discounted earnings methodology, along with the adjustments of selected items in the company’s income statement, serve the purpose of allowing — as a first step — a more accurate assessment of the basis for the respective plannings.
The adjustments carried out in connection with the historical analysis (adjustment of one-time nonrecurring influences, elimination of activities sold in the meanwhile) do not affect the calculation of the equity value since the respective equity value computations are based on the results of future financial years. Thus, any adjustments made to historical results serve merely plausibility purposes. Therefore, only the major extraordinary effects are taken into account when making adjustments for the past.
4.3.2 Analysis of financial planning
The planned accounts consist of projected income statements and projected balance sheets. The detailed financial planning phase covers the years 2006 through 2008. The sustainable earnings period covers the financial years 2009 and thereafter.
The planned accounts have been prepared according to the International Financial Reporting Standards (IFRS). As a result of the strategic new focus of Schering AG completed in the fiscal year 2005 and the related change in the definition of business areas and indication areas, the adjusted presentation covers only the past years 2004 and 2005.
The preparation of the planned accounts is part of the regular internal financial planning and reporting procedure within Schering AG. This also serves as the basis for performance based salaries in the Schering Group. The planned accounts used for the valuation are based on the planning prepared in fall 2005 and were passed by the Management Board and approved by the Supervisory Board in December 2005. The operational planning of Schering takes account of the explicitly expected changes concerning prices and costs. It is a nominal planning.
As a result of the divestment of the radiopharmaceuticals business and the 50 % participation in ALK-Scherax, the sales and earnings of the business areas Diagnostic Imaging and Specialized Therapeutics have been adjusted.
The planned accounts were adjusted in order to take into account current knowledge and the developments in the first half of 2006. The adjustments relate primarily to the following sets of facts:
Based on the business development in the first half of 2006 an actualization of the estimated operating profit for the fiscal year 2006 was made by the company. The effects of a slightly
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 39 |
| | | |
more positive estimation of the business development as well as the nonrecurring expenses due to take over bids for Schering AG was taken account of in the amount of the difference to the planned volumes of the approved planned accounts. In this context, the effects of the divestments of the participation in ALK-Scherax and the radiopharmaceutical business were taken into account. The adjustments resulting for the following planned years were considered accordingly.
Besides that, synergies to be realized by Schering as the result of the successful take over bid by Dritte BV GmbH as part of Bayer group in ade factocorporate group (prior to conclusion of the Domination and Profit and Loss Transfer Agreement) were separately assessed.
The planning of the activities of Schering AG related to the US dollar is based on a constant rate of exchange of the€ to the US dollar during the planning period. In order to take into account the anticipated changes in the level of the exchange rate, adjustments in the operating net income were made for activities in the regions affected by the US dollar (primarily in regard to USA, Latin America and Canada). The basis for the anticipated change in the level of the exchange-rate was provided by the current€/USD forward rates.
In connection with the valuation of Schering AG, the planned profits in the detailed planning phase are directly attributable to the shareholders in the amounts of the dividend payments and retained earnings expected.
4.3.3 Determining sustainable earnings
The determination of sustainable earnings is of considerable importance when evaluating a pharmaceutical company because the dependency of the company on individual products, especially their phase in the life cycle, as well as the estimate of the products in the process of being developed (pipeline) determine the future development to a substantial degree.
In order to estimate the development of Schering AG after the end of the planning horizon of the operative plan, we have therefore developed a forecast model to represent the further development of Schering AG, taking into account both existing products but also the products currently in the course of development for the period up to the year 2015.
The forecast model reflects the development of net sales and contribution margins (as to the definition of “contribution margin” cf. section 5.1) for all essential products in the business area and, thus, enables a long term estimate of net sales and profitability for the business area. The contribution margins have been transferred to the gross profit (cf. section 5.1.6).
With regard to the product related analysis, the effects on quantities and prices, resulting from the expiration of patent protection and the anticipated generic competition, were reflected. The potential of the products in the pipeline were included in the total estimate for the respective
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 40 |
| | | |
business areas using a probability model with probabilities for realization based on the phase of development.
In addition to the analysis of the expectations for net sales, the influence of a change in the product mix during the forecast period within the business areas on the anticipated gross profit and, thus, the observable changes in profitability were individually taken into account.
In order to estimate the development of the other functional costs, we analyzed the cost ratios of the individual types of costs in the past and in the planning period of the operative planning, taking into account the factors exercising influence at that time. These ratios establish the basis for estimating the future development of cost ratios. In doing this, influences from the forecast development of the company, structural changes, economies of scale as well as price increases were taken into account. The results were also checked for plausibility using current estimates by analysts for Schering.
Our forecast is based on the company’s internal information and external data. A major basis for estimating net sales and profitability at the product level consisted of the results of the strategic process which regularly takes place at Schering and which compares the opportunities and risks of existing and future products in a qualitative and partly quantitative manner, as well as the net present value models for important development projects prepared by Schering. In addition, we referred to market studies and estimates by analyst for the forecast.
Finally, for purposes of this valuation we computed present values equivalent sustainable earnings for the years 2009ff on the basis of this forecast taking into account the projected sustainable growth rate (please refer to section 6.3) which represent a present value equivalent amount.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 41 |
| | | |
5 Planned Accounts
The planned consolidated income statement of the Schering Group for the fiscal years 2006 through 2008 is, including a comparisof the adjusted results for the fiscal years 2004 through 2005 (see Section 2.2 and 4.3.1) shown in the following spreadsheet. The transfer from adjusted to unadjusted results is explained in Annex 1 to this report:
Consolidated Income statements of the Schering Group
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Adjusted historical* | | | | Planning | | |
| | | | 2004 | | | | 2005 | | | | 2006 | | | | 2007 | | | | 2008 | | |
| | | | €m | | | | €m | | | | €m | | | | €m | | | | €m | | |
| | | | | | | | | | | | | | | | | |
Net Sales | | | | 4,767 | | | | | 5,157 | | | | | 5,543 | | | | | 5,936 | | | | | 6,35l | | |
Growth | | | | n/a | | | | | 8.2 | % | | | | 7.5 | % | | | | 7.1 | % | | | | 7.0 | % | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Gynecology & Andrology | | | | 1 ,766 | | | | | 1,979 | | | | | 2,180 | | | | | 2,354 | | | | | 2,539 | | |
| Diagnostic Imaging | | | | 1,194 | | | | | 1,287 | | | | | 1,347 | | | | | 1,428 | | | | | 1,524 | | |
| Specialized Therapeutics | | | | 1,100 | | | | | 1,146 | | | | | 1,239 | | | | | 1,322 | | | | | 1,414 | | |
| Oncology | | | | 420 | | | | | 429 | | | | | 471 | | | | | 507 | | | | | 540 | | |
| Other Sources | | | | 287 | | | | | 317 | | | | | 306 | | | | | 325 | | | | | 334 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross Profit | | | | 3,661 | | | | | 4,000 | | | | | 4,342 | | | | | 4,683 | | | | | 5,023 | | |
as a % of net sales | | | | 76.8 | % | | | | 77.6 | % | | | | 78.3 | % | | | | 78.9 | % | | | | 79.1 | % | |
| | | | | | | | | | | | | | | | | |
| Cost of | | | | | | | | | | | | | | | | | | | | | | | | | | |
| marketing and selling | | | | 1,501 | | | | | 1,637 | | | | | 1,794 | | | | | 1,879 | | | | | 1,963 | | |
| research and development | | | | 903 | | | | | 966 | | | | | 1,021 | | | | | 1,095 | | | | | 1,180 | | |
| engineering and administration | | | | 526 | | | | | 525 | | | | | 521 | | | | | 531 | | | | | 537 | | |
| Other operating income / expenses | | | | 20 | | | | | 2 | | | | | 29 | | | | | -27 | | | | | -37 | | |
| | | | | | | | | | | | | | | | | |
Operating profit after adjustments | | | | 751 | | | | | 874 | | | | | 1,036 | | | | | 1,151 | | | | | 1,306 | | |
| | | | | | | | | | | | | | | | | |
as a % of net sales | | | | 15.8 | % | | | | 16.9 | % | | | | 18.7 | % | | | | 19.4 | % | | | | 20.6 | % | |
| | | | | | | | | | | | | | | | | |
Adjustments | | | | 17 | | | | | 54 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Operating profit according annual report | | | | 768 | | | | | 928 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | |
| * | | effects from divestment ALK, radiopharmaecuticals business and other non-recurring items |
The net sales and gross profit of the fiscal years 2004 in 2005 were presented after adjustments for the sale of the radiopharmaceuticals business and the participation in ALK as well as for extraordinary items (see 4.3.2). The adjustments shown in the above table constitute a balanced factor which is the result of the adjustments described in the following.
The adjustments applied to the net sales due to the sale of the radiopharmaceutical business and the participation in ALK amount to roughly€ 140 million in fiscal year 2004 and to roughly€ 251 million in fiscal year 2005. At the level of operating profits, this resulted in adjustments adding up to a total of€ -15 million in 2004 EUR,€ -67 million in 2005 respectively. The adjustments in the other results for the radiopharmaceuticals business in 2005 include especially the expenses for the divestiture indication area in 2005 (€ 54 million).
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 42 |
| | | |
Furthermore, the other operating result (balance of other operating income and costs) of these years has been adjusted for significant extraordinary items of the years in question. In 2005 these include the income from the dissolution of a provision for warranty claims in connection with the sale of the participation in Aventis CropScience in fiscal year 2002 (€ 88 million), income from the dissolution of provisions (€ 63 million in 2004,€ 33 million in 2005), as well as the expense for financing the pension fund in Japan in fiscal year 2004 (€ 31 million).
5.1 Operating Profit
For purposes of this report, the deduction of the operating profit is, in a first step, based on the contribution margins of the business area. The contribution margin is defined as the net sales, reduced by the production costs expressed in terms of standardized production costs, royalties for licenses as well as costs of production variances and valuation differences. Paid royalties do not reduce the gross income; these costs are shown under marketing and selling costs in the profit and loss statement. After the presentation of the contribution margins of the business areas, the bridge to operating profit is carried out. Therefore, the operating profit of Schering will be presented.
5.1.1 Contribution margin of the business area Gynecolgy & Andrology
The development of the net sales and the contribution margin in the business area Gynecology & Andrology is as follows:
Business Area Gynecology & Andrology
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Adjusted historical* | | | | Planning | | | | sustainable | | |
| | | | 2004 | | | | 2005 | | | | 2006 | | | | 2007 | | | | 2008 | | | | 2009ff. | | |
| | | | €m | | | | €m | | | | €m | | | | €m | | | | €m | | | | €m | | |
| | | | | | | | | | | | | | | | | | | | |
Net Sales | | | | 1,766 | | | | | 1,979 | | | | | 2,180 | | | | | 2,354 | | | | | 2,539 | | | | | 2,743 | | |
Growth | | | | n/a | | | | | 12.1 | % | | | | 10.2 | % | | | | 8.0 | % | | | | 7.9 | % | | | | | | |
| Female Contraception | | | | 1,473 | | | | | 1,681 | | | | | 1,843 | | | | | 1,972 | | | | | 2,113 | | | | | | | |
| Menopause Management | | | | 167 | | | | | 169 | | | | | 188 | | | | | 212 | | | | | 240 | | | | | | | |
| Gynecological Therapy | | | | 79 | | | | | 77 | | | | | 78 | | | | | 84 | | | | | 88 | | | | | | | |
| Andrology | | | | 33 | | | | | 42 | | | | | 62 | | | | | 78 | | | | | 94 | | | | | | | |
| G&A Others | | | | 14 | | | | | 10 | | | | | 9 | | | | | 8 | | | | | 4 | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Contribution Margin | | | | 1,507 | | | | | 1,703 | | | | | 1,907 | | | | | 2,078 | | | | | 2,235 | | | | | 2,479 | | |
as a % of net sales | | | | 85.3 | % | | | | 86.1 | % | | | | 87.5 | % | | | | 88.3 | % | | | | 88.0 | % | | | | 90.4 | % | |
| | | | | | | | | | | | | | | | | | | | |
| | |
| * | | no adjustments necessary |
Net Sales
Schering realized net sales in the Gynecology & Andrology business area in the fiscal year 2004 in the amount of€ 1,766 million, of which approximately€ 1 billion was generated in the segment Europe. The strongest segment in terms of sales after Europe was the USA with a share in the net sales of 20.2 %. The strongest indication area in terms of sales was hormonal contraception which achieved 83.4 % of the net sales and which distributes the three strongest products in terms of sales, Yasmin, Diane and Mirena. The growth in net sales in the fiscal year 2004 was
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 43 |
| | | |
primarily borne by an increase in net sales in the amount of 36.4 % for Yasmin. Yasmin was able to expand its share in the global market to more than 13 % in this fiscal year. Most of the growth was achieved in particular in the US American market.
Schering AG anticipates an average growth in net sales of 8.7 % in the business area Gynecology & Andrology in the planning period 2006 through 2008, which is above the anticipated growth in sales in the genital/urinary market which is relevant for this business area, and where sales increases are primarily driven by volumes. The main driver of net sales are the products in the Yasmin family and Mirena so that the indication area hormonal contraception has the largest part in the net sales of the business area with a only slightly reduced portion of 83.2 % in the net sales of the business area in the planning year 2008. The growth of net sales in the planning period is, thus, based on an active life cycle management of the existing product portfolio.
Hormonal contraception
The indication area intends to counter the future potential generic competition for Yasmin and the resulting reduction in net sales by expanding the Yasmin family with the products Yasminelle and YEZ. A number of further regionally staggered market introductions of different specifications of YAZ and Yasminelle shows the focus of the company on the Yasmin family through the resulting increase in the portion of the Yasmin family from 29.6 % in the year 2005 to 36.8 % of net sales of the business area in the year 2008. Thus, the plan is that YAC OC will be introduced into the market in the planning year 2006, YAZ PMDD and YAZ Acne will be introduced initially in the USA in the planning year 2007, Yasminelle will be introduced in the planning years 2006 and 2007 and YAZ OC will be introduced in the planning year 2008 in the segment Europe.
The business area sees the growth potential in net sales going beyond the growth in the market during the planning, using the worldwide most successful contraceptives in the Yasmin family based on the progestin drospirenone. As a result of the planned market introductions, the largest growth in sales is anticipated in the segment USA. Because the market share of Schering AG in the USA with approximately 14% is relatively small compared to the segment Europe with approximately 47 % and in light of the past excess of Yasmin, the company plans to significantly increase its market share in this segment. Furthermore, because the acceptance rate for hormonal contraceptives with approximately 14 % (in comparison, this is 26 % in the five largest countries in Europe) is also relatively small, a planned increase of the acceptance rate will result in an expected increase of net sales in the USA.
By increasing the acceptance rate for Mirena in the segment USA (approximately 1 %), which is also significantly below the comparable rate in the five largest European markets (approximately 4 %), the plan is to generate additional annual growth in sales for this product. The products in the Yasmin family and Mirena combine to realize approximately 53 % of the net
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 44 |
| | | |
sales of the business area in the year 2008. The planned share of the no longer patent protected products in the planning year 2008 is still 47 %.
The existing, but until now relatively small influence of generic products of competitors, is apparent in the relatively low degree of the erosion of non-patent protected products compared to the other business areas. Schering AG considers a risk with regard to the planned achievement of future growth in net sales to consist primarily of the market introductions by Barr Pharmaceutical Inc. with the medications Seasonique and applications based on this in the year 2007 and 2008 in the USA which will increase the generic pressure on the products of the business area. Furthermore, Wyeth is planning the market introduction of its medication Lybrel in the USA in the year 2007 and in Europe in the year 2008.
Menopause management
The net sales in the indication area menopause management stagnated at approximately€ 170 million as a result of the WHI study in the fiscal years 2004 in 2005; this amounted in the fiscal year 2005 to 8.5 % of the net sales of the business area. In the planning period, an average growth in net sales of 12.4 % is planned, which lies significantly above the anticipated growth in the market of approximately 3 %. The growth will be borne by the new product Angeliq. While the products Climara, Climen and Cliane made up half of the net sales in this indication area, their planned share of sales in the planning year 2008 is one half of this amount. Schering AG has a worldwide approved hormonal medication in Angeliq (approval in the Europe in the fiscal year 2004 and in the USA in the fiscal year 2005) which should more than offset the reduction in sales of the past products without patent protection in this indication area and which should secure growth above the market expectations during the planning period as a result of the active ingredient drospirenone in a manner comparable to the indication area of hormonal contraception.
Gynecological therapy
The net sales in this indication area as well as the net sales in the planning years 2006 through 2008 result from sales of old products which are marketed with only minimal efforts. Accordingly, the net sales in the planning period will remain at a low level. The future importance of this indication area lies in its new strategic direction, consisting of the development of a new platform for products to treat uterine fibroids and endometriosis. Potential products, however, are still only in early development stages.
Andrology
Schering AG is the market leader in Europe in the field of testosterone therapy with its products Nebido and Testogel. Management plans to significantly increase the net sales of Nebido on the basis of Testogel as the door opener from€ 7 million in the fiscal year 2005 to€ 53 million, and
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 45 |
| | | |
thus to increase the portion of net sales in this indication area within the net sales of the business area to 3.7 % in the year 2008. The strategic goal lies in achieving market leadership in Europe, Latin America and Asia which is supposed to be advanced by expanding the portfolio of products containing or being based on testosterone.
Contribution margin
As a result of the shift in the product mix to a greater portion of the strongest products in the indication area hormonal contraception, the contribution margin could be increased by 0.8 % to 86.1 % in comparison to the previous year. In addition, plans Schering AG an increase of the contribution margin by 3 % to 88 % in the year 2008. Schering will profit from changes in the product portfolio mix which will expand the sales share of higher margin products such as YAZ and Mirena which will lead to an increase in the contribution margin in the planning period. Furthermore, the increase in the portion of sales in the segment USA, in which higher gross margins can be realized compared to other segments as a result of the high price level for products, will have a positive effect on the contribution margin. The highest contribution margin is anticipated during the planning period, therefore, in the year 2007, which has its basis, among other reasons, in a significant improvement of the contribution margin for Mirena which has its basis in the cessation of the contract of license royalties which have been paid to the institution Population Council. The slight reduction in the contribution margin in the planning year 2008 compared to the previous year is primarily a result of the market introduction of YAZ OC in the segment Europe, where lower margins, compared to the USA, are realized as a result of lower sales prices.
The main development projects in the business area are as follows:
| | | | | |
| Project/ Indication | | Description | | Status |
| |
| FC-Patch | | Low-dose patch containing ethinylestradiol and gestodene for female contraception, which is applied once a week | | Phase III |
| |
| YAZ — Extended Regimen | | Low-dose oral contraceptive containing drospirenone for extended
use | | Phase III |
| |
| DUB-OC | | OC containing natural estradiol and dienogest, which is also being evaluated for the treatment of prolonged, frequent and excessive bleeding | | Phase III |
| |
Visanne
(formerly Endometrion) | | Oral dienogest-containing product for the treatment of endometriosis (proliferation of endometrial tissue outside the uterus) | | Phase III |
| |
| CCR1 | | A new, non-hormonal and oral treatment of endometriosis | | Phase II |
| |
| LCS | | Ultra low-dose LNG releasing contraceptive intrauterine system | | Phase II |
| |
| Hormonal male contraception | | Combination of progestin with testosterone for the suppression of sperm concentration | | Phase II |
| |
| eF-Ment | | HGT with androgen-metobolites for avoidence of the impact on the prostate | | Phase I |
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 46 |
| | | |
Sustainable earnings
The determination of the sustainable earnings was made taking into account the development of the company in the planning period as well as the anticipated development of the genital/urinary market. Therefore we have projected the future earnings potential of the products already placed in the market as well as the earnings potential of the products in the process of development on the valuation date.
The main driver of net sales for the years following the planning period consists of the products in the Yasmin family with the planned introductions of YAZ-extended (2010) and YAZ Plus (2011) as well as Mirena with the new applications LCS (2012). A continued growth in net sales is initially anticipated for the patent protected products in the Yasmin, Mirena and Angeliq family which, however, will then stabilize at a lower level as a result of the anticipated increase in generic competing products near the expiration of the patents (Yasmin family around 2020, Mirena administration system in the year 2015, process in the year 2013). The products currently in the product pipeline are, primarily, further developments of existing products in the course of the lifecycle management.
Further foreseeable, innovative products (e.g., DUB-OC, medication in the indication area hormonal contraceptives on the basis of natural estradiol, CCR1 in the field of gynecological therapy, a medication for the hormonal treatment of endriometriosis or eF-Ment in the indication area of andrology, an innovative drug for hypogonadism therapy), so-called new chemical entities, are at this time partially in very early phases of development; the forecast for achieving suitability for the market and market introduction involves a great deal of uncertainty, as is typical for the industry. Potential future sales were taken into account, adjusted for risks common in the market.
The high degree of market saturation in the strongest segment in terms of net sales, i.e. Europe, in which Schering already has a high market share, will make it difficult for the company to achieve the same growth in sales over the long term as in the past. Given the small market share in the segment USA in comparison to the segment Europe, a long term significant increase of the market share in this segment is required for an additional sales growth. Furthermore, the demographic trend in the developed sales regions of Europe and the USA will lead over the long term to a further flattening of the high rate of growth in net sales shown for the planning period. In consideration of the aforementioned developments in regard to the deduction sustainable results we have taken into account the net sales of the Gynecology & Andrology business area amounting to€ 2.743 million.
On the basis of the increased share in net sales of high margin products of the Yasmin family and a planned increase of share in net sales in the segment USA, a further increase in contribution margins can be anticipated. Against this background we have presumed a sustainable contribution margin of€ 2.479 million, which corresponds to a sustainable margin of 90.4%.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 47 |
| | | |
5.1.2 Contribution margin of the business area Diagnostic Imaging
The net sales in the business area Diagnostic Imaging and the contribution margin are shown in the following table adjusted for the radiopharmaceuticals business:
Business Area Diagnostic Imaging
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Adjusted historical* | | | | Planning | | | | sustainable | | |
| | | | 2004 | | | | 2005 | | | | 2006 | | | | 2007 | | | | 2008 | | | | 2009ff. | | |
| | | | €m | | | | €m | | | | €m | | | | €m | | | | €m | | | | €m | | |
| | | | | | | | | | | | | | | | | | | | |
Net Sales | | | | 1,194 | | | | | 1,287 | | | | | 1,347 | | | | | 1,428 | | | | | 1,524 | | | | | 1,859 | | |
Growth | | | | n/a | | | | | 7.8 | % | | | | 4.6 | % | | | | 6.1 | % | | | | 6.7 | % | | | | | | |
| X-Ray Contrast Media | | | | 574 | | | | | 583 | | | | | 584 | | | | | 590 | | | | | 594 | | | | | | | |
| MRI Contrast Agents | | | | 332 | | | | | 362 | | | | | 378 | | | | | 397 | | | | | 423 | | | | | | | |
| Medrad | | | | 271 | | | | | 329 | | | | | 374 | | | | | 429 | | | | | 495 | | | | | | | |
| Diagnostics Others | | | | 17 | | | | | 13 | | | | | 11 | | | | | 12 | | | | | 12 | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Contribution Margin | | | | 824 | | | | | 892 | | | | | 925 | | | | | 978 | | | | | 1,039 | | | | | 1,233 | | |
as a % of net sales | | | | 69.0 | % | | | | 69.3 | % | | | | 68.7 | % | | | | 68.5 | % | | | | 68.1 | % | | | | 66.3 | % | |
| | | | | | | | | | | | | | | | | | | | |
| | |
| * | | adjusted for the disposal of the radiopharmaceuticals business |
X-ray contrast media
The increase in net sales in the indication area from€ 9 million or 1.5 % in the fiscal year 2005 is based primarily on the increase in net sales of the product Ultravist and is the result of increased volumes of sales, especially in the segment Europe and Asia/Pacific which overcom-pensated for price reductions. The price reductions for the product Iopamiron were compensated for increased sales volumes in the fiscal year 2005. The increased volumes of sales for both products compared to the year 2004 are primarily based on an increased number of examinations with imaging procedures.
The assumption is, that there will be a growth in net sales of 0.7 % annually on average during the planning period for the indication area x-ray contrast agents. The expected reductions in sales of Iopamiron, which are primarily the result of price reductions in Japan, will be more than offset by increases in net sales of Ultravist. The increases in net sales for Ultravist are based on growth in volume which is supposed to more than compensate for the price reductions for Iopamiron und Ultravist. The growth in the sales volume for Ultravist is especially intended to be achieved in the segments Europe and the USA. In Europe Ultravist will be used for new x-ray devices and their expanded scope of application (e.g., heart imaging) and in the United States the expansion of market share will be primarily based on new contracts with large purchasing organizations of hospitals.
MRI contrast agents
The net sales in this indication area were increased in the fiscal year 2005 by€ 30 million or 9.1 %. The increases in net sales are the result of a clear increase in the sales volumes for Magnevist and Gadovist. This increase was, however, partially offset by price reductions.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 48 |
| | | |
The anticipated increase in net sales for the indication area MRI contrast agents is an average of 5.3 % annually during the planning period. It is primarily the result of a change in products from Magnevist to Gadovist and the increased use of the organ specific contrast agents Primovist and Vasovist. Accordingly, reduced net sales of Magnevist are expected. The increased volumes for Magnevist will be particularly offset by price reductions resulting from generic competition after expiration of major patents for Magnevist in Europe and Japan after 2007. The growth in net sales for the organ specific MRI contrast agents is the result of higher volumes which overcompensate for reductions in prices. The basis for the growth in the volume of these products is the anticipated continued increase in the number of MRI examinations.
Medrad
The growth in net sales in the analyzed period is primarily caused by an increase in the sales volumes of automatic injection systems resulting from a higher number of examination devices and increasing volumes for injection accessories and services as a result of an increased number of examinations. Contrary to the business with contrast agents, it is not assumed that there will be such a degree of offsetting price effects at Medrad so that Medrad shows higher rates of growth in net sales than for the business with contrast agents.
Contribution margin
The increase of the contribution to earnings in the Diagnostic Imaging business area in the fiscal year 2004 and 2005 was the result of growth in net sales of MRI contrast agents and growth at Medrad.
Further sales based increase in the contribution margin is planned for the planning period. As a result of the increasing portion of the Medrad business, which has a smaller contribution margin compared to the contrast agents business, as well as a result of the slightly reduced margins in the contrast agent business caused by price reductions, a decreasing margin is assumed for the planning period.
The main development projects in the business with contrast agents are:
| | | | | |
| Project indication | | Description | | Status |
| |
| Magnevist | | MRI contrast agent; development projects in the U.S.: | | |
| | | -for magnetic resonance angiography (MRA) | | Phase III |
| | | - for the detection of viable myocardial tissue | | Phase II |
| |
| Gadovist | | MRI contrast agent for the detection and characterization of brain lesions; development project in the U.S. | | Phase III |
| |
| Paccocath | | Application of PTCA-balloon catheter with the agent Paclitaxel by the, treatment of In-Stent-Restenosen (ISR) | | Phase III |
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 49 |
| | | |
Sustainable earnings
When determining the sustainable earnings, we took into account on the one hand the development of the product portfolio existing in the fiscal year 2008 including the respective product life cycles and on the other hand the potential earnings from projects which are currently in the process of being developed.
In the case of x-ray contrast agents, continued growth in volumes for Ultravist is assumed which will be partially offset by lower prices. In the case of Iopamiron, reductions in net sales primarily in the segment Japan are assumed also after 2008.
The assumption is that there will initially be a stable development in MRI contrast agents with regard to the net sales for Magnevist where increasing sales volumes are offset by decreasing prices. After major patents for Magnevist expire in Europe in the year 2007, the patent protection for Magnevist in the USA will end in the year 2013. Since increased pressure on prices can then also be anticipated, the assumption is that there will be reduced sales commencing in the year 2013. Compared to this, there should be increasing proceeds for the successor products, especially Gadovist and Vasovist, whose net sales were also taken into account for the anticipated further specific regional approvals. This will more than offset the reductions in net sales for Magnevist.
In the case of Medrad, we assume that the growth rates will continue to be higher than the growth rates in the other business of Schering even after the year 2008. Medrad will continue to profit from the increased number of examination devices which increasingly include the installation of an injection system and from contrast agents supported examinations as well as the change in injection systems from one-head to two-head injection systems and the resulting additional need for sterile consumables.
On the basis of the above remarks, net sales in the Diagnostic Imaging business area have been assumed in the amount of€ 1,859 million when determining the sustainable earnings.
When determining the contribution margin for the sustainable earnings, higher contribution margin can be assumed on the basis of the described growth in net sales. However, it must be taken into account that there will be lower margins for the contribution margin as the result of the continuing pressure on prices in the contrast agent business caused by comparably lower margins for Vasovist due to license royalties to the development partner EPIX Pharmaceuticals, Inc., Cambridge, USA, as well as due to the increasing portion of sales in the Medrad business in the business area Diagnostic Imaging.
Considering these factors, a contribution margin of€ 1.233 million has been applied when determining the sustainable earnings.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 50 |
| | | |
5.1.3 Contribution margin of the business area Specialized Therapeutics
The net sales and the contribution margin of the Specialized Therapeutics business area are as follows for the various indication areas:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Adjusted historical* | | | | Planning | | | | sustainable | | |
| | | | 2004 | | | | 2005 | | | | 2006 | | | | 2007 | | | | 2008 | | | | 2009ff. | | |
| Business Area Specialized Therapeutics | | | €m | | | | €m | | | | €m | | | | €m | | | | €m | | | | €m | | |
| | | | | | | | | | | | | | | | | | | | |
Net Sales | | | | 1,100 | | | | | 1,146 | | | | | 1,239 | | | | | 1,322 | | | | | 1,414 | | | | | 1,317 | | |
Growth | | | n/a | | | | 4.2 | % | | | | 8.1 | % | | | | 6.7 | % | | | | 7.0 | % | | | | | | |
| Central Nervous System | | | | 859 | | | | | 936 | | | | | 1,033 | | | | | 1,131 | | | | | 1,220 | | | | | | | |
| Cardiovascular System | | | | 137 | | | | | 144 | | | | | 138 | | | | | 131 | | | | | 128 | | | | | | | |
| Gastrointenstinal Disease | | | | 0 | | | | | 0 | | | | | 0 | | | | | 0 | | | | | 8 | | | | | | | |
| STH Others | | | | 104 | | | | | 66 | | | | | 68 | | | | | 60 | | | | | 57 | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Contribution Margin | | | | 749 | | | | | 788 | | | | | 860 | | | | | 933 | | | | | 1,017 | | | | | 847 | | |
as a % of net sales | | | | 68.1 | % | | | | 68.7 | % | | | | 69.4 | % | | | | 70.6 | % | | | | 71.9 | % | | | | 64.3 | % | |
| | | | | | | | | | | | | | | | | | | | |
| | |
| * | | adjusted for the disposal of the interest in ALK-Scherax |
Central Nervous System
Especially as a result of the increase in net sales of Betaferon, which had net sales in the year 2005 of€ 867 million and a total of 75.7 % of the net sales in the Specialized Therapeutics business area, the net sales in the indication area Central Nervous System increased by€ 77 million. This increase results both from a growth in volumes by 3.0 % as well as from an increase in prices by 6.7 % which were able to be achieved in the market due to the continuing improvement of the product, for example with regard to the application of Betaferon. Furthermore, the cancellation of the competitor’s product approval for Tysabri in the year 2005 had a positive effect on the development of sales.
The planning of the Specialized Therapeutics business area takes into account an average growth of net sales by 7.3 % annually. This is primarily due to the continuing increases in the volumes of Betaferon in the segments Asia/Pacific, USA and Europe, which are based primarily on measures in the life cycle management, the product having been introduced to the market in 1993 and possessing a broad patient base. These measures include clinical studies to expand the use of Betaferon in early stages of multiple sclerosis (BENEFIT study) as well as in higher dosages (BEYOND study) with which the Specialized Therapeutics business area intends to reach new patient groups.
In addition to these measures to differentiate the products, the planned increase in net sales is to be achieved by activities to gain customer loyalty, such as providing information on the course of the disease and the correct use of Betaferon.
During the planning of the sales, it was taken into account that in 2006, the competitive product Tysabri of Biogen Idec, was again given restricted approval for the treatment of multiple sclerosis.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 51 |
| | | |
As a result of the increased competition in the MS market, price increases are only expected to a limited degree in the years 2006 through 2008 after the significant increase in the year 2005.
The agent patents for Betaferon will expire in the EU in the year 2008 as well as in the USA in the year 2007. It is anticipated that bio similar producers will take steps to receive approval for bio similars of the substance Inferon B-lb in Betaferon. Since the approval of proteins of such complexity is subject to high demands, however, there is a market entry barrier, especially in the form of the necessary clinical equivalency studies. This means that there will probably be delays in the market entry by bio similar suppliers. Therefore, effects from competition with bio similar suppliers are not anticipated until the year 2009.
Cardiovascular System
The net sales in the indication area of the cardiovascular system were able to be increased in the fiscal year 2005 by€ 7 million or 5.1 %. This development was based especially on the significant increase in net sales for the product Ventavis from€ 14 million to€ 26 million which led to an overcompensation of the reduction in net sales by€ 3 million to€ 42 million of the product Ilomedin. The reduction in the net sales of Ilomedin was caused by strong competition in the indication area and a patient-unfriendly provision for the application of Ilomedin. As a result, the sales volume compared to the year 2004 was 5.2 % lower and this decrease could not be compensated by a slight price increase. As a result of the expiring patent protection, a continued slight reduction in sales volumes and prices for Ilomedin is anticipated in the years 2006 and 2007, which is expected to lead to a reduction in net sales totalling€ 9 million.
During the detailed planning period, a further increase in net sales for Ventavis is anticipated driven by increases in sales volumes especially in the segment Europe. In this context, an increase compared to the year 2005 is anticipated from€ 19 million to€ 45 million up to the year 2008.
As it was already observed in the year 2005, a reduction in net sales of Refludan compared to the year 2005 is anticipated during the planning period, especially as a result of the planned reduction in volumes in the USA caused by reduced distribution activities for this product, of€ 10 million to€ 9 million in the year 2008.
Gastrointestinal
The gastrointestinal indication area is anticipated to see the first net sales in the year 2008 after the lauch of the marketing in the USA of the development project Sargramostim in the indication of Morbus Crohn, which is currently in clinical phase III. Schering expects that the marketing outside of the USA may commence in the year 2009. Sargramostim is a colony stimulating factor which is intended to be used in the gastrointestinal field for the chronic disease Morbus Crohn.
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 52 |
| | | |
Other
In addition to the above mentioned indication areas, the Specialized Therapeutics business area has various medications for different indication areas with comparably low net sales. The reduction in the fiscal year 2005 by€ 39 million resulted, among other items, from the end of distribution cooperations as well as the partial sale of the product portfolio of an affiliated company of Schering in Germany.
Contribution Margin
The increase in the contribution margin in the fiscal year 2005 is the result of the growth in sales of Betaferon. The margin is relatively low compared to other divisions, which has its cause in the fact that Schering must pay royalties notably to Chiron Corp., Emeryville (hereinafter, “Chiron”) for the worldwide use of the patents as well as for the production for the markets in the USA and parts of Europe. Furthermore, the production of the products sold outside the USA is accomplished in commissioned production by Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, (hereinafter, “Boehringer Ingelheim”).
The net sales based increase in the contribution margin during the planning period has its cause especially in a reduction of the costs of goods sold as a result of the expiration of royalty agreements for Betaferon, entailing a reduction of royalties from 2007 onwards.
The main development projects in the Specialized Therapeutics business area are as follows:
| | | | | |
| Project/Indication | | Description | | Status |
| |
| Sargramostin | | Hematopoetic growth factor (GM-CSF), Sagramostin is currently in clinical development for the treatment of Crohn’s disease | | Phase III |
| |
Betaferon/Betaseron
500 mg
(BEYOND-Studie) | | Double-dose formulation of Betaferon/Betaseron | | Phase III |
| |
| Alemtuzumab | | The approved leukemia medicament Alemtuzumab were developed for therapy application in MS treatment. | | Phase II |
| |
| Spheramine | | Dopamin producing human cells that are attached to microcarriers; used for cell therapy in Parkinson’s disease | | Phase II |
Sustainable earnings
The sustainable earnings have been determined on the basis of the development of the product portfolio in the fiscal year 2008, taking into accout the life cycles of the respektive products as well as the market potentials of projects which are currently developed or improved.
The long term development of the product Betaferon is of high relevance to this determination. Following the expiration of the patent of the agent both in the USA in the year 2007 as well as in the EU in the year 2008, the competition by bio similars will continually increase after their probable approval in the year 2009, and this will probably erode the sales. New user friendly forms of application, especially oral application, are supposed to lead to a noticeable reduction
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 53 |
in the market share of existing multiple sclerosis medicaments which must be injected, commencing upon the expected market introduction in the year 2011. A market share of approximately 40 % to 60 % for these MS therapy products with oral application is forecasted by the company for the year 2015.
In addition, we have taken into account the earnings potential of new products which are currently under development. This involves especially the development projects Alemtuzumab and Spheramine as well as the marketing of the product Sargramostim for Morbus Crohn which is expected to be introduced in the year 2008 in the USA and 2009 in Europe.
The development project Alemtuzumab which is currently in clinical phase II, an agent which is already approved in the business area Oncology for the therapy of chronic lymphocytic leukemia under the product name “Campath”, is intended to expand the product portfolio for the treatment of MS commencing in the year 2011 and will contribute substantially to offset the reductions in the sales of Betaferon. Alemtuzumab is being developed in cooperation with Genzyme.
The development project Spheramine, an innovative treatment for Parkinson’s disease, is being developed in cooperation with Titan Pharmaceuticals, Inc., San Francisco, USA. The cooperation agreement provides for a license royalty for the use of patents and the production of the product which is dependent on sales. The development project is currently in clinical phase II and is to be introduced to the market in the years 2010/2011.
When determining the sustainable earnings, we also considered the financial effects of the expected result of the exercise of the purchase option agreed with Chiron by Schering on 27 February 2006 with regard to the purchse of the production facilities as well as patents for the production of Betaferon by Schering. The negotiations between the contract partners with regard to the conditions for the acquisition have been commenced, but they are still in an early stage. The result of the negotiations and the effects on the economic position of Schering are, therefore, afflicted with uncertainty.
Recapitulating it can be stated that the above described development projects as well as additional development projects of Schering in the Specialized Therapeutics business area will only permit a partial compensation for the long term anticipated reduction in the net sales of Betaferon.
On the basis of the above described developments, net sales of the business area amount to€ 1.317 million when determining the sustainable earnings.
In the course of determining the contribution margin for the sustainable earnings, it must be taken into account that the change in the product mix towards licensed products, especially Alemtuzumab, will lead to a reduction in the sustainable contribution margin which can be
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 54 |
achieved. In addition, the erosion in prices for Betaferon as a result of competition by bio similars influences the determination of the contribution margin for the sustainable earnings.
Based on the above described developments, we have concluded a contribution margin within the sustainable earnings totalling€ 847 million for the Specialized Therapeutics business area.
5.1.4 Contribution margin of the business area Oncology
The net sales and contribution margins in the business area oncology are shown as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Business Area Oncology | | | | | | |
| | | Adjusted historical* | | Planning | | sustainable |
| | | 2004 | | 2005 | | 2006 | | 2007 | | 2008 | | 2009ff. |
| | | €m | | €m | | €m | | €m | | €m | | €m |
Net Sales | | | 420 | | | | 429 | | | | 471 | | | | 507 | | | | 540 | | | | 1,276 | |
Growth | | | n/a | | | | 2.3 | % | | | 9.8 | % | | | 7.6 | % | | | 6.4 | % | | | | |
| Hematology | | | 235 | | | | 245 | | | | 278 | | | | 315 | | | | 346 | | | | | |
| Solid Tumors | | | 185 | | | | 184 | | | | 193 | | | | 192 | | | | 194 | | | | | |
Contribution Margin | | | 323 | | | | 326 | | | | 362 | | | | 386 | | | | 409 | | | | 954 | |
as a % of net sales | | | 77.0 | % | | | 75.9 | % | | | 76.8 | % | | | 76.1 | % | | | 75.8 | % | | | 74.8 | % |
| | |
| * | | no adjustments nessecary |
Hematology
The net sales in hematological oncology in the fiscal year 2005 increased slightly due to volumes. During the planning period, growth in net sales in the indication area hematology are anticipated also primarily on the basis of volume.
The strongest product in terms of sales in hematological oncology is Fludara. There is no patent protection anymore in the USA for Fludara, and the loss of further patents can be expected in the coming years for the intravenous form of application. Nonetheless, Fludara had a slight growth in net sales (2 %) in the fiscal year 2005. This increase for Fludara is the result of growth in sales volumes and positive currency exchange effects which more than offset the moderate price reductions (net sales 2005:€ 105 million). The sales volumes increased primarily in the segment Europe because a generic product is currently not offered in Europe. In contrast, there were further reductions in net sales of the product in the USA, which resulted from the loss of patent protection in the USA in 2003 and the increasing competition from generics. The main portion of the increase in net sales in hematology in the fiscal year 2005 is based on the growth in net sales in the products Campath (€ 5 million) and Zevalin (€ 7 million). The increase in net sales for Zevalin has its basis in increased sales volumes which result from the market introduction of the product in the fiscal year 2004 in the segment Europe and in the fiscal year 2005 in the segment Asia/Pacific. Growth in volume of sales in the segments USA and Europe were the cause for the growth in net sales of Campath in the fiscal year 2005.
Further increases in net sales for Fludara, Zevalin and Campath are expected in the planning years 2006 through 2008. The growth in net sales for Fludara is above all the result of the an-
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 55 |
ticipated market introduction of Fludara in the oral application form (Fludara Oral) in the fiscal year 2006 in the segments Asia/Pacific and Japan as well as the market introduction of Fludara with an indication for transplantation (Fludara Transplantation) in the fiscal year 2007 in Japan as well as the anticipated growth in sales volume in Europe outside of the five most important countries in this segment. In addition, increasing market shares in first line therapy for chronic lymphocytic leukemia (CLL) are expected because of the positive result of studies. Compared to this, the existing and expected competition from generics in the USA and in the five most important countries in Europe in the fiscal year 2007 can be expected to lead to a reduction in sales volumes in the planning period. Despite lower sales volumes in the USA as well as in the five most important countries in Europe, the increase in the sales volumes for Fludara in the fiscal years 2006 through 2008 can probably more than compensate for the losses in net sales resulting from price reductions.
In the case of Campath, increases in volumes of sales in the planning years 2006 through 2007 are expected as a result of measures in the course of the life cycle management which have already occurred or which are expected, above all in the segments USA, Europe and Latin America/Canada. Based on current results of studies, for example, it is expected that Campath will be submitted for approval as a first line therapy. The approval is expected to be granted in the second quarter of 2007. In addition to volume based growth in net sales, a price based growth in net sales is also expected in the planning years 2006 through 2008.
The product Zevalin is still in the phase of being introduced into the market in the fiscal year 2006. As a result of the anticipated increasing acceptance in the market, increased sales volumes for Zevalin are anticipated in the planning years 2006 through 2008, but with a tendency towards decreasing rates of growth. The use of marketing instruments to promote sales was a contributing factor to a negative effect on prices in the year of the product introduction of Zevalin, and this effect will probably no longer apply after successful market introduction in the respective countries. Thus, in addition to increases in sales volumes, overall positive price effects are assumed in the planning period.
Campath and Zevalin are intended to be a platform for the future marketing of products in Schering’s business area oncology which are in the process of being developed.
Solid Tumors
The net sales of products in the indication area solid tumors were at the level of the previous year during fiscal year 2005. The primarily volume and price based decrease in the sales of Androcur were offset by positive volumes and currency exchange effects relating to the product Bonefos (€ 6 million). As a result of the expired patent protection, the life cycle of the product Androcur has already been exceeded for a long time with regard to the development of sales in the mature phase. Marketing instruments to promote sales are no longer being used. In addition
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 56 |
to the volume based reductions in sales, the anticipated price reductions are the result of generic competition as well as regulatory price control measures for older products.
Increasing net sales for Bonefos and other products (Mittoval, Tamofen) are assumed for the planning years 2006 through 2008, which increases will more than offset the anticipated further erosion of net sales due to reduced volumes and lower prices for Androcur.
The increase in net sales for the product Bonefos is especially the result of the anticipated growth in sales volumes in fiscal year 2006 which will more than compensate for the anticipated price reductions. The anticipated volume based increase in sales will be primarily in the segments Europe and Asia/Pacific, with the largest anticipated growth in sales in Great Britain respectively Australia. The cause for this anticipated development is the transfer of the distribution rights in these countries to Schering in the fiscal year 2005.
The growth in net sales for the other products in the indication area solid tumors (Mittoval, Tamofen) is primarily founded in anticipated increased sales volume). The marketing of Mittoval is limited to Italy. The net sales of Tamofen are realized in Japan. Since Tamofen serves as a platform in Japan for the oncology products currently being developed, marketing instruments are being used to support the planned volume based growth in sales. As a result of new types of hormonal forms of treatment, a progressively smaller market share of older forms of treatment, which also includes Tamofen, is anticipated, and this will lead to decreasing rates of growth in the development of sales volumes. In addition, regulatory price control measures of the national health institution in Japan (NHI) are expected to result in a decrease in net sales due to price reductions.
Contribution margin
The contribution margin in the fiscal year 2005 compared to the net sales increased underpro-portionally. The growth in net sales in the indication area hematological oncology did not result in a comparable increase in the contribution margin. The reduced margin in hematology was primarily the result of the small margins for Campath and Zevalin. The reduction in margins has its basis in regional product mixes of form of application with different prices and especially in the marketing instruments used for Zevalin in order to support the market introduction in the fiscal year 2005 which led to a decrease in margin due to price reductions. Fludara is a non-biologically produced small molecule with low production costs. This nature of the product permits the realization of a comparably high margin. Furthermore, it must be taken into account that there are participations in the profits and licensing fees to the cooperation partner Genzyme Corp., Cambridge, USA under the existing cooperation agreements with regard to Campath. For this reason, Campath is a less profitable product in the business area oncology compared to the other products. The increased share of Campath in overall net sales in hematological oncology in fiscal year 2005, together with the overall lower margins for the products Fludara, Campath and Zevalin, led to overall comparatively lower profitability in this indication area.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 57 |
The decreasing contribution margins in fiscal year 2005 also have their basis in slightly decreasing contribution margins for the products in the indication area solid tumors. On the one hand, price reductions resulting from generic competition for Androcur had a negative effect on the development of the margins. Furthermore, the increasing portion of net sales of Bonefos compared to Androcur, which has comparably higher profitability, lead to lower margins in the indication area solid tumors.
In fiscal year 2006, an increasing contribution margin is anticipated. The increase is primarily based on an anticipated higher profitability of the products in the indication area solid tumors as well as in slightly higher margins for hematological oncology. In the indication area solid tumors, the anticipated increase in the margins for Bonefos and Tamofen is responsible for the increase in margins. The slightly higher margin in the indication area hematological oncology in 2006 results, on the one hand, from anticipated changes in the regional distribution of sales for Fludara and resulting positive effects on prices, and, on the other hand, a price based increase in margins for Zevalin is anticipated as compared to slightly negative price effects in the course of the market introduction in the fiscal year 2005.
The anticipated change of the product mix in the years 2007 and 2008 resulting from the decreasing share in net sales of the product Androcur as well as anticipated price reductions for this product will lead to a reduction in the contribution margin in the indication area solid tumors. A decrease in this margin is also anticipated in the planning years 2007 and 2008 for the indication area hematological oncology. The anticipated negative development of prices for the product Fludara contributed to this. The lower margin for Campath has its basis in measures in the course of the life cycle management. In addition, the product mix will change in the planning years 2007 and 2008 as a result of the increasing share in net sales for Zevalin. The decreasing development in the margins in the indication area hematological oncology will be partially compensated by anticipated priced base increases in the margin for Zevalin.
The main development projects of Schering AG in the business area oncology are as follows:
| | | | | |
| Project/Indication | | Description | | Status |
| TOCOSOL Paclitaxel | | Novel formulation of paclitaxel for the treatment of metastatic breast cancer | | Phase III |
| | | | | |
| Bonefos | | Reduction of occurrence of bone metastases in early stage breast cancer | | Phase III |
| | | | | |
| PTK/ ZK | | Oral VEGF receptor tyrosine kinase inhibitor; anti-angiogenic small molecule designed to inhibit the formation of blood vessels that support tumor growth; is being developed for metastatic colorectal cancer | | Phase III |
| | | | | |
| ZK-EPO | | Fully synthetic and highly potent microtubule stabilizer for the treatment of prostate, ovarian, breast cancer, NSCLC and SCLC | | Phase II |
| | | | | |
| ZK-PRA | | Substance that blocks the effect of certain receptors (progesteron receptor antagonist). Potential indication: breast cancer | | Phase II |
| | | | | |
| PTK/ZK | | Oral VEGF receptor tyrosine kinase inhibitor for the treatment of glioblastoma and NSCLC | | Phase II |
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 58 |
Sustainable earnings
The products in the indication area hematological oncology currently situated in the pipeline primarily involve further developments of existing products in the course of life cycle management. Innovative products which are currently anticipated, so-called new chemical entities are in middle to late phases of development in the indication area solid tumors (phase 11 and phase III projects; see Section 4.3.3). The anticipated growth rate for the oncology business area resulting from the products currently in development for the indication area of solid tumors will contribute to oncology being a growth factor for the future development of Schering after the year 2008. We assume that Schering will profit as a result of the development portfolio from the anticipated above average annual growth rates for anti-tumor therapies, especially in the field of anti-cancer medications which inhibit the growth of cells.
The development projects which will contribute to the sustainable earnings are especially the projects TOCOSOL Paclitaxel and ZK-EPO. Furthermore, we estimate the future potential for successful development of the project PTK-ZK to be uncertain due to the negative results of the studies in the past.
Market introduction of the product involved in the project TOCOSOL Paclitaxel in the field of breast cancer is expected after the year 2009 and for further indication areas after the year 2010. It must be taken into account that TOCOSOL Paclitaxel is a licensed product which will have lower profitability compared to the existing product portfolio as a result of having to make license payments.
We have taken into account the anticipated market introduction of the project involved in the project ZK-EPO for five indication areas after the year 2010. These include especially the indication areas of breast cancer, non-small cell cancer and uterine cancer.
In addition, we have taken into account that the planned measures in life cycle management of the existing product portfolio will have a positive effect on the growth of the oncology business. This includes in the field of hematological oncology especially the anticipated market introductions of the further development for Campath and Zevalin for first line and second line therapy commencing in the fiscal year 2009 as well as the increasing contributions in net sales of Fludara Oral and Fludara Transplantation introduced in 2006 and 2007, which will more than compensate for the anticipated decrease of the existing product portfolio resulting from the increase in generic competition and anticipated price control measures by the institutions in the health market. In the indication area solid tumors, we have also taken into account the earnings potential from the anticipated market introduction of a further development of Bonefos as a preventative medicine.
Overall, a clear increase in net sales in the business area oncology is anticipated as a result of the above described factors. We have used the sales potential in the fiscal year 2009 and the
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 59 |
following years as a basis for determining a value equivalent amount of€ 1,276 million for the sustainable earnings.
The anticipated change in the product mix resulting from the increased share in sales of therapies against solid tumors will lead to lower contribution margins during the phase of introducing the new products because the anticipated market introductions of licensed products in the development portfolio and products involving life cycle management measures have lower profitability. The long term achievable contribution margin, however, is still at the level of the margin planned for the fiscal year 2008.
Based on the above described higher net sales and the change in the development of the margin, we assume a contribution margin in the sustainable earnings for the business area oncology in the amount of€ 954 million.
5.1.5 Contribution margin of the business area Other Sources
The net sales of the business area Other Sources are shown in the following table:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Business Area other Sources | | | | | | |
| | | Adjusted historical* | | Planning | | sustainable |
| | | 2004 | | 2005 | | 2006 | | 2007 | | 2008 | | 2009ff. |
| | | €m | | €m | | €m | | €m | | €m | | €m |
Net Sales | | | 287 | | | | 317 | | | | 306 | | | | 325 | | | | 334 | | | | 298 | |
Growth | | | n/a | | | | 10.3 | % | | | -3.2 | % | | | 6.1 | % | | | 2.9 | % | | | | |
| Dermatology | | | 202 | | | | 223 | | | | 245 | | | | 268 | | | | 286 | | | | | |
| Pharmaceutical Chemicals | | | 61 | | | | 65 | | | | 47 | | | | 42 | | | | 33 | | | | | |
| Other | | | 24 | | | | 29 | | | | 13 | | | | 15 | | | | 16 | | | | | |
| | | | | | | | | | | | | | | | | | | |
Contribution Margin | | | 180 | | | | 191 | | | | 204 | | | | 221 | | | | 235 | | | | 208 | |
as a % of net sales | | | 62.6 | % | | | 60.5 | % | | | 66.6 | % | | | 68.1 | % | | | 70.2 | % | | | 69.7 | % |
| | | | | | | | | | | | | | | | | | | |
* no adjustments necessary
The net sales for Other Sources increased in the fiscal year 2005 primarily as a result of higher sales volumes in the dermatology business which is bundled in the subsidiary Intendis. The anticipated increase in net sales in the indication area dermatology in the planned years 2006 through 2008 is primarily the result of the introduction of new products to new markets. The largest part of the market introductions took place in the segments USA and Japan as well as in several countries in the segment Europe. The growth in sales volumes is offset in part by negative currency exchange effects, primarily as a result of activities which depend on the US Dollar. The growth is supported by the expansion of the distribution structure in other countries in the segment Europe.
The business in the indication area pharmaceutical chemicals by Schering for other pharmaceutical producers is characterized by a reduction in margins as a result of the erosion of prices, especially as a result of the increased production of chemicals in China. Additionally, the importance of a large customer of Schering in the past years has continuously decreased. Against this background, Schering AG will increasingly withdraw from the business with pharmaceutical
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 60 |
chemicals. Accordingly, a successive decrease in net sales from the business with pharmaceutical chemical years is planned, commencing in the planned year 2006.
The reduction in other sales revenues in the business area Other Sources in the fiscal year 2006 is due to the fact that specialized therapeutics for third parties are no longer produced at the production site in Weimar. The remaining net sales from distribution cooperation relationships, insurance brokering and publishing are extrapolated in the planned years 2007 and 2008 in the range used in the planned year 2006.
Contribution Margins
Negative price effects in the dermatology business are primarily the cause of the reduction in margins of Other Sources in the fiscal year 2005, and this resulted from market developments for dermatological products in Turkey, Spain and Japan. There will be a successive increase in margins in the planned years 2006 through 2008 which results from the sinking portion of sales in the indication area pharmaceutical chemicals for which lower profitability will be achieved compared to the indication area dermatology. During the planning years 2006 through 2008, the contribution margins in the dermatological business will be in the range of the fiscal year 2005.
Sustainable earnings
The assumption with regard to the indication area pharmaceutical chemicals, is that the contribution to revenue remaining in the fiscal year 2008 from cooperation agreements with other pharmaceutical producers will also be realized over the long term despite the high intensity of competition and the downward tendencies which can be observed. This also applies to the remaining Other Sources. In the dermatology business, no substantially higher result potential can be expected from the development of the existing product portfolio.
Taking into account these influencing factors, Other Sources contributes to the sustainable earnings net sales in the amount of€ 298 million, its contribution margin totalling€ 208 million.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 61 |
5.1.6 Bridge contribution margins to the legal accounts
The transfer of the contribution margins to the gross operating profit during the analyzed period is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Schering Group - Reconciliation Operating Profit | | | | | | |
| | | Adjusted historical* | | Planning | | sustainable |
| | | 2004 | | 2005 | | 2006 | | 2007 | | 2008 | | 2009ff. |
| | | €m | | €m | | €m | | €m | | €m | | €m |
Net Sales | | | 4,767 | | | | 5,157 | | | | 5,543 | | | | 5,936 | | | | 6,351 | | | | 7,493 | |
Growth | | | n/a | | | | 8.2 | % | | | 7.5 | % | | | 7.1 | % | | | 7.0 | % | | | | |
Gynecology&Andrology | | | 1,766 | | | | 1,979 | | | | 2,180 | | | | 2,354 | | | | 2,539 | | | | 2,743 | |
| Diagnostic Imaging | | | 1,194 | | | | 1,287 | | | | 1,347 | | | | 1,428 | | | | 1,524 | | | | 1,859 | |
| Specialized Therapeutics | | | 1,100 | | | | 1,146 | | | | 1,239 | | | | 1,322 | | | | 1,414 | | | | 1,317 | |
| Oncology | | | 420 | | | | 429 | | | | 471 | | | | 507 | | | | 540 | | | | 1,276 | |
| Other Sources | | | 287 | | | | 317 | | | | 306 | | | | 325 | | | | 334 | | | | 298 | |
Contribution Margin | | | 3,583 | | | | 3,900 | | | | 4,257 | | | | 4,596 | | | | 4,935 | | | | 5,722 | |
as a % of net sales | | | n/a | | | | 8.9 | % | | | 9.1 | % | | | 8.0 | % | | | 7.4 | % | | | | |
| Gynecology&Andrology | | | 1,507 | | | | 1,703 | | | | 1,907 | | | | 2,078 | | | | 2,235 | | | | 2,479 | |
| Diagnostic Imaging | | | 824 | | | | 892 | | | | 925 | | | | 978 | | | | 1,039 | | | | 1,233 | |
| Specialized Therapeutics | | | 749 | | | | 788 | | | | 860 | | | | 933 | | | | 1,017 | | | | 847 | |
| Oncology | | | 323 | | | | 326 | | | | 362 | | | | 386 | | | | 409 | | | | 954 | |
| Other Sources | | | 180 | | | | 191 | | | | 204 | | | | 221 | | | | 235 | | | | 208 | |
Adjustments | | | 79 | | | | 100 | | | | 85 | | | | 87 | | | | 88 | | | | 112 | |
Gross Operating Profit | | | 3,661 | | | | 4,000 | | | | 4,342 | | | | 4,683 | | | | 5,023 | | | | 5,834 | |
as a % of net sales | | | 76.8 | % | | | 77.6 | % | | | 78.3 | % | | | 78.9 | % | | | 79.1 | % | | | 77.9 | % |
| | |
| * adjusted for the disposal of the interest in ALK and the radiopharmaceuticals business |
In the item adjustments mainly licence payments were neutralized, as they are disclosed in the income statement as cost of marketing and selling. However costs of the production variances have been added back in as they are not included in the contribution margin.
5.2 Cost of marketing and selling
The costs for marketing and selling are the most significant type of costs within the Schering group, having a share of 31.7 % of net sales in the year 2005. This reflects the high value placed on the distribution organization for the commercial success of Schering. In addition to the costs for marketing existing products, the costs for marketing and selling are characterized by the costs for introductions of new products which in part require the use of considerable resources.
The costs for marketing and selling include the costs of the subsidiaries located in the most important sales markets of the Schering Group, the local marketing organizations to the extent that these maintain their own outside sales force and the supporting regional centers in Berlin (segment Europe), Mexico (segment Latin America/Canada) and Singapore (segment Asia/Pacific). The pharmaceutical products in the USA are distributed through Berlex, and the injection systems and accessories are distributed through Medrad. In addition, the costs for marketing and selling include the royalties for distribution licenses. The other cost of selling include primarily the costs for the division global marketing and selling, which consist among other
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 62 |
items of the costs for liability insurance, global brand management as well as global market analysis.
The costs for marketing and selling can be summarized as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of marketing and setting | | | | | | |
| | | Adjusted historical* | | Planning | | sustainable |
| | | 2004 | | 2005 | | 2006 | | 2007 | | 2008 | | 2009ff. |
| | | €m | | €m | | €m | | €m | | €m | | €m |
| Cost of marketing/selling in the closer sence | | | 1,379 | | | | 1,503 | | | | 1,657 | | | | 1,765 | | | | 1,858 | | | | | |
| Cost of sales license | | | 122 | | | | 134 | | | | 136 | | | | 113 | | | | 105 | | | | | |
Cost of marketing and selling | | | 1,501 | | | | l,637 | | | | 1,794 | | | | 1,879 | | | | 1,963 | | | | 2,292 | |
Growth | | | n/a | | | | 9.1 | % | | | 9.6 | % | | | 4.7 | % | | | 4.5 | % | | | | |
as a % of net sales | | | 31.5 | % | | | 31.7 | % | | | 32.4 | % | | | 31.6 | % | | | 30.9 | % | | | 30.6 | % |
| | |
| * | | adjusted for the disposal of the interest in ALK and the radiopharmaceuticals business |
The costs of marketing and selling have increased in the year 2005, primarily as a result of costs for implementing modified global marketing processes in the context of the ASPIRE program and by currency exchange rates. During the course of adjusting the marketing and selling structure, a separate selling structure for the Oncology business area was established in the year 2005. Prior to this time, these activities were located in the marketing and distribution organization of the Specialized Therapeutics business area.
The costs of marketing and selling in the year 2006 increased compared to 2005 by 0.7 % to 32.4%. This development results from increased costs in the Oncology business area due to the preparation of the market launch of the products Fludara Oral and Fludara Transplant, as well as increased marketing and selling activities in the closer sense of the term to promote the sale of the products Campath and Zevalin. These costs for establishing the selling organization are only offset by comparably low net sales in the year 2006. In addition, the market introduction of the product YAZ in the Gynecology & Andrology business area in the USA as well as increased marketing and selling activities in the closer sense of the term for the markets in China, Latin America and individual countries in East Europe result in an increase in the costs for marketing and selling in the amount of€ 157 million.
The increasing costs of marketing and selling in the planned years 2007 and 2008 result from further anticipated market introductions in the business areas Diagnostic Imaging (Vasovist in the year 2007) and Specialized Therapeutics (Sargramostim for Morbus Crohn in the year 2008).
In these years the decrease in costs for distribution licenses is due to the fact that no more royalties have to be paid for Mirena after the agreement with Population Council has expired, 2007 being the first fiscal year fully affected by this change.
Overall, the costs of marketing and selling compared to net sales are intended to decrease from 32.4 % in the year 2006 to 30.9 % in the year 2008 as a result of economies of scale, especially
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 63 |
in the segments Asia/Pacific, Latin America/Canada and the USA as well as due to a relatively small number of product introductions.
The determination of the basis for the sustainable earnings 2009ff. was generally based on the forecast of the future business development. Over the long term, we expect that there will be a slight decrease in the cost ratio of marketing and selling. The costs of marketing and selling were extrapolated on the basis of the anticipated long term growth rate for Schering AG.
5.3 Cost of research & development
The cost of research and development consist of the costs for research activities in order to find effective agents, optimization of effective agents as well as in the pre-clinical field (up to commencement of phase I), costs for overhead, administration, infrastructure, international project management and for compliance with provisions on approvals as well as project related and local development costs (phase I up to product approval).
The following table shows the planned costs of research and development:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of research and development | | | | | | |
| | | Adjusted historical* | | Planning | | sustainable |
| | | 2004 | | 2005 | | 2006 | | 2007 | | 2008 | | 2009ff. |
| | | €m | | €m | | €m | | €m | | €m | | €m |
| Cost of reasearch | | | 216 | | | | 230 | | | | 232 | | | | 243 | | | | 252 | | | | | |
as a % of net sales | | | 4.5 | % | | | 4.5 | % | | | 4.2 | % | | | 4.1 | % | | | 4.0 | % | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of development | | | 687 | | | | 736 | | | | 789 | | | | 852 | | | | 928 | | | | | |
as a % of net sales | | | 14.4 | % | | | 14.3 | % | | | 14.2 | % | | | 14.4 | % | | | 14.6 | % | | | | |
Cost of research and development | | | 903 | | | | 966 | | | | 1,021 | | | | 1,095 | | | | 1,180 | | | | 1,386 | |
growth | | | n/a | | | | 7.0 | % | | | 5.7 | % | | | 7.2 | % | | | 7.8 | % | | | | |
as a % of net sales | | | 18.9 | % | | | 18.7 | % | | | 18.4 | % | | | 18.5 | % | | | 18.6 | % | | | 18.5 | % |
| | |
| * | | adjusted for the disposal of the interest in ALK and the radiopharmaceuticals business |
During the analyzed period from 2004 through 2008, the costs for research and development are in the range between 18.4 % and 18.9 % of net sales.
The increase in costs of research and development in the year 2005 by€ 63 million or 7%compared to the year 2004 is primarily the result of higher costs for researching effective substances and project related development costs in the Specialized Therapeutics business area, especially for the projects Sargramostim, in the indications Morbus Crohn and Betaferon BEYOND as well as in the Oncology business area for the project Epothilone.
Increasing costs of research and development in a total of€ 214 million compared to 2005 have been budgeted for the years up to 2008. These budgeted amounts are based on increasing project related development costs, especially in the Specialized Therapeutics business area for the projects Spheramine and Lipoxin and in the Oncology business area for the projects Epothilone, TOCOSOL Paclitaxel and ZK-PRA.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 64 |
The basis for the determination of sustainable earnings 2009ff. is generally determined on the basis of the passed experience as well as the amount of costs observed in the detailed planning period for the costs of research and development. The observed development of growth in net sales of Schering requires continuous research and development activities at least in the amount of during the years 2004 through 2008 actual and planned costs for research and development compared to net sales. In the light of the deduction of sustainable costs of research and development, we applied a quota in relation to forecast sales revenues of 18.5 % necessary to ensure a long term development of innovative products and to sustain the development of growth potentials considered in the sustainable earnings.
The basis in the amount of 18.5 % of the net sales is within the normal scale in the industry for costs of research and development which are in the range of between 14.1 % and 25.0 % for comparable pharmaceutical companies engaged in research based on our analysis.
5.4 Cost of engineering and administration
The costs of engineering and administration include in the first place non passed on costs of the department Industrial Operations and Environment, which covers the entire supply chain including purchasing, production, logistics, quality assurance, technology as well as energy production, supply and waste disposal and environmental protection including the function Compliance & Infrastructure Support. It must be taken into account that the cost of goods sold are to the largest part passed on to the segments and business areas as an internal cost allocation within the scope of the assessment of cost of goods sold and are shown accordingly in the cost of goods sold of the respective business areas. In addition to the costs from the department Industrial Operations and Environment, the costs of engineering and administration also include the costs of administration in the closer sense for the segments and the central functions as well as for initial and advanced training. The costs for the central functions also include the costs for the Group headquarters.
The following table shows the development of the costs for engineering and administration:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of engineering and administration | | | | | | |
| | | Adjusted historical* | | Planning | | sustainable |
| | | 2004 | | 2005 | | 2006 | | 2007 | | 2008 | | 2009ff. |
| | | €m | | €m | | €m | | €m | | €m | | €m |
Cost of engineering and administration | | | 526 | | | | 525 | | | | 521 | | | | 531 | | | | 537 | | | | 589 | |
Growth | | | n/a | | | | -0.2 | % | | | -0.9 | % | | | 2.1 | % | | | 1.1 | % | | | | |
as a % of net sales | | | 11.0 | % | | | 10.2 | % | | | 9.4 | % | | | 9.0 | % | | | 8.5 | % | | | 7.9 | % |
| | |
| * | | adjusted for the disposal of the interest in ALK and the radiopharmaceuticals business |
The costs of engineering and administration in the fiscal year 2005 were at the level of the previous year with€ 525 million. It must be taken into account that the measures introduced in previous years to increase efficiency and adapt the production capacities have had a positive effect on the development of the costs of engineering and administration. This is reflected by the cost ratio related to net sales which sank by 0.8 %.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 65 |
The planning for the fiscal year 2006 takes into consideration that there is an increase of expenses in the field of Industrial Operations and Environment in the amount of€ 16 million. This increase is especially a result of the increased logistic costs due to the reduction of regional production locations in the context of the reorganization of production locations, which costs cannot be passed on, as well as an increase of the overhead costs as the result of an expansion of management resources in the field of Compliance & Infrastructure Support which cannot be passed on, either. The increase of the costs in the field of Industrial Operations and Environment will be slightly more than offset by further planned savings in general administrative costs in the head office functions (€ 11 million) and in the area of local production costs (€ 5 million).
Schering has three locations for the production of pharmaceutical agents. In the course of measures to increase efficiency, the plan for the year 2006 is to sell a production location in Mexico.
The number of production locations for the formulation and packaging of pharmaceutical and bio-technological products is supposed to be reduced in the planning period by seven to 12.
The increase in costs of engineering and administration in the planning years 2007 and 2008 is primarily the result of the growth of the Gynecology & Andrology business area. Additional planned cost savings due to increases in efficiency as a result of measures in the context of the FOCUS initiative which have already been commenced have been taken into account in the planning period. These measures are reflected in the reduction of the cost ratio relating to net sales by approximately 1.7 % through the planning year 2008 compared to the fiscal year 2005.
Investments in the planning years are primarily intended for fixed assets in the amount of approximately€ 220 million annually, and this includes both further investments in already commenced measures as well as investments in future planned projects. These accrue to a great extent for the expected completion of the new research building at the location in Berlin, the planned concentration of the production locations, the increase in efficiency in the production of effective agents at the plant in Bergkamen as well as the likely completion of the new building for the bio-technological production location for Leukine in Seattle.
As a result of the measures to increase efficiency in the field of engineering and administration during the period up to the year 2008, no further material increases in efficiency are to be expected for the purpose of determining sustainable earnings. However, economies of scale and improving the use of capacities will compensate the increase in costs of engineering and administration which is the result of inflation as well as corporate growth.
On the basis of the explained effects, we have assumed a lower than proportional increase of the sustainable costs of engineering and administration compared to the forecast sales as shown in a cost ratio of 7.9%.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 66 |
5.5 Other Result
The other result includes profit and losses from hedging transactions and processing payments as well as expenses for services which have been charged on and the income received for these services. The passed on services include providing technical infrastructure to third parties as well as other services rendered to third parties. In addition, the balance of the other result includes income from licenses and commissions and expenses in the context of the FOCUS initiative.
The other result was lower in the fiscal year 2005 compared to the previous year primarily as a result of higher expenses in the context of the FOCUS initiative involving primarily costs in connection with the re-organization of the global production network.
The planned improvements in income resulting from improvements in efficiency in the fiscal year 2006, which have not yet been reflected as improvements in the expense items for the fiscal year 2006 at the level of the business areas, are taken into account as targets under other result. The reduction in the year 2007 compared to the previous year is generally caused by the budget requirements taken into account in the fiscal year 2006. In addition costs for the intended measures in the context of the FOCUS initiative are planned to be lower in the fiscal years 2006 and 2007 compared to the fiscal year 2005. On the other hand, increased costs for restructuring in the course of the FOCUS initiative are anticipated in the fiscal year 2008 which will lead to a further reduction of other result. Furthermore, the probable decrease of the other result will be a result of the reduction of the license income received for Betaferon.
We applied other result to the sustainable earnings on the basis of the planning year 2008. In doing so, the cost relief from restructuring expenses under the FOCUS initiative was taken into account because these will no longer apply after the fiscal year 2008. Accordingly, there is an overall balanced result when applying other result to the sustainable earnings. The resulting improvement in profitability from the restructuring costs planned for the fiscal year 2008 were considered by us in setting the cost ratio related to net sales for the corresponding items of income statement of Schering AG.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 67 |
5.6 Derivation of the companies net profit
The following table, based on the planned operating income in the fiscal years 2006 through 2008, shows the determination of the company’s net profit in these fiscal years as well as the determination of the sustainable earnings commencing in the year 2009 and continuing in subsequent years:
| | | | | | | | | | | | | | | | | |
| Schering Group - Income Statement | | | | |
| | | Planning | | sustainable |
| | | 2006 | | 2007 | | 2008 | | 2009ff. |
| | | €m | | €m | | €m | | €m |
Net Sales | | | 5,543 | | | | 5,936 | | | | 6,351 | | | | 7,493 | |
| Gynecology & Andrology | | | 2,180 | | | | 2,354 | | | | 2,539 | | | | 2,743 | |
| Diagnostic Imaging | | | 1,347 | | | | 1,428 | | | | 1,524 | | | | 1,859 | |
| Specialized Therapeutics | | | 1,239 | | | | 1,322 | | | | 1,414 | | | | 1,317 | |
| Oncology | | | 471 | | | | 507 | | | | 540 | | | | 1,276 | |
| Other Sources | | | 306 | | | | 325 | | | | 334 | | | | 298 | |
Contribution Margin | | | 4,257 | | | | 4,596 | | | | 4,935 | | | | 5,722 | |
| Gynecology & Andrology | | | 1,907 | | | | 2,078 | | | | 2,235 | | | | 2,479 | |
| Diagnostic Imaging | | | 925 | | | | 978 | | | | 1,039 | | | | 1,233 | |
| Specialized Therapeutics | | | 860 | | | | 933 | | | | 1,017 | | | | 847 | |
| Oncology | | | 362 | | | | 386 | | | | 409 | | | | 954 | |
| Other Sources | | | 204 | | | | 221 | | | | 235 | | | | 208 | |
| Adjustments | | | 85 | | | | 87 | | | | 88 | | | | 112 | |
Gross Operating Profit | | | 4,342 | | | | 4,683 | | | | 5,023 | | | | 5,834 | |
as a % of net sales | | | 78.3 | % | | | 78.9 | % | | | 79.1 | % | | | 77.9 | % |
| |
| Costs of | | | | | | | | | | | | | | | | |
| marketing and selling | | | 1,794 | | | | 1,879 | | | | 1,963 | | | | 2,292 | |
| engineering and administration | | | 1,021 | | | | 1,095 | | | | 1,180 | | | | 1,386 | |
| research and development | | | 521 | | | | 531 | | | | 537 | | | | 589 | |
| Other Results | | | 29 | | | | -27 | | | | -37 | | | | 0 | |
Operating Profit before Plan Adjustments | | | 1,036 | | | | 1,151 | | | | 1,306 | | | | 1,567 | |
| Plan Adjustments | | | | | | | | | | | | | | | | |
| Exchange Rate Trend | | | 0 | | | | -17 | | | | -29 | | | | -87 | |
| Synergy | | | 0 | | | | 18 | | | | 32 | | | | 51 | |
| Others | | | -132 | | | | 9 | | | | -9 | | | | -1 | |
Operating Profit for Valuation Purposes | | | 904 | | | | 1,160 | | | | 1,300 | | | | 1,530 | |
| Financial Result | | | -6 | | | | -4 | | | | -6 | | | | -8 | |
| Income Taxes | | | 319 | | | | 410 | | | | 456 | | | | 538 | |
Profit for the period | | | 579 | | | | 747 | | | | 837 | | | | 985 | |
| Minority Interest | | | -4 | | | | -4 | | | | -3 | | | | -3 | |
Net profit | | | 575 | | | | 743 | | | | 834 | | | | 982 | |
as a%of net sales | | | 10.4 | % | | | 12.5 | % | | | 13.1 | % | | | 13.1 | % |
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 68 |
Based on Schering’s planned accounts for the fiscal years 2006 through 2008 (adjusted for the sales of the interest in ALK and the radiopharmaceutical business), the adjustments described under Section 4.3.2 were made. These included specifically:
The planning of the activities of Schering AG dependent on the US Dollar are based on a constant level of the currency exchange rate during the planning period of EUR/USD 1.25. In order to take into account the anticipated changes in the level of the currency exchange rate, adjustments were made to the operating income for activities in the USD influenced region (concerns basically USA, Latin America and Canada). Adjustments in the fiscal year 2006 were not made because currency exchange rate based effects on the profits are already contained in the adjustments to the development of profits in the first half of the year 2006.
As of the valuation date, Schering AG is a de facto corporate group company of Bayer. Since synergy effects can be realized not only after conclusion of a corporate group agreement, but already in a de facto corporate group, an analysis was conducted with regard to possible measures by Schering and Bayer. In this examination, all integration projects currently planned were examined with regard to whether they can be realized also without the existence of a corporate group agreement under the existing legal restrictions and which economic advantages can be realized as a result for the respective companies. The anticipated pre-contractual synergies for Schering were taken into account by us in the context of the forecast of earnings and in the determination of the value.
As a result of this work, it was apparent that pre-contractual synergies can be achieved especially in the field of joint purchasing and in mutual joint distribution of products, in production as well as in the field of administration. The positive effects are anticipated for the first time in the fiscal year 2007 and should develop their full effect in 2009. The planned contribution to profits is contained in the adjustments of the planning for the respective planned years.
The profit estimate for 2006 updated on the basis of the development in the first half of the year 2006 was incorporated in the amount of the difference to the plan estimate of the previous year and is shown in the other adjustments of the planning in the fiscal year of 2006. These are concerned with a more positive estimation of the operational business development improving the operating profits. Expenses at Schering AG due to former take-over bids had the effect of lowering the profits. Those are primarily concerned with consultancy fees and personnel costs.
The resulting effects for the following periods were also taken into account.
Furthermore, there was an adjustment of earnings and expenditures on non operating real estate, which were taken into account as special items (please refer to section 7.2).
The determination of the sustainable earnings was made in accordance with the process described in section 4.3.3, taking into account the earnings potential of Schering for the years
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 69 |
commencing with 2009 applying an assumed sustainable growth rate. The resulting effects on the operating profits have been illustrated in detail in sections 5.1 through 5.5.
The operating income this way determined for purposes of valuation for the fiscal years 2006 through 2008 as well as the sustainable earnings was reduced when determining the net profit of the group by the negative interest result and the taxes on income.
The financial result was determined on the basis of planned balance sheets and planned cash flow accounts in an integrated manner. The available cash identified as of 1 January 2006 in the amount of€ 200 million was applied separately (see on this, Section 7.2) and was, thus, not taken into account in the planning period for the purposes of determining the financial result.
The financial result includes the interest expense in the detailed planning period for the variable interest rate loan which was taken up in the fiscal year 2004 in order to increase the liquidity reserve and which ends in the fiscal year 2009, and the interest expense for pension provisions as well as the interest income for liquid funds after adjustment of the available cash. The effects of the repayment of the variable interest rate loan in fiscal year 2009 in accordance with the agreement the financial result commencing in the year 2009 were taken into account accordingly. The interest expense for pension provisions was determined on the basis of the pension provisions existing as of 30 June 2006 in order to take into account the market value changes of the pension provisions as of 30 June 2006, which result from the calculatory interest as adjusted for the development of interest. The remaining liquid funds had an interest rate applied to them which is equal to the anticipated rate of interest on investments.
When determining the financial result, the assumptions made for distributions were also taken into account. Schering AG assumes for purposes of the planning that there will be a partial distribution of net profit. The remaining portions of the net profit will be retained. Since the reinvestment at the discount rate prior to the taxes accruing at the level of the company can also be shown at an equivalent value by a fictitious direct allocation of the retained amounts to the shareholders, we have taken into account for purposes of simplification that the retentions commencing with the fiscal year 2006 will be directly distributed as net earnings received by the shareholders, so that they are no longer taken into account with regard to financial result.
For the purposes of calculating taxes on income, trade tax, corporate income tax and the solidarity surcharge as well as foreign taxes on income were taken into account. When determining the taxable basis, material differences between the pre-tax profits under the IFRS and the income pursuant to the tax balance sheet as well as regional tax regulations were considered if existent. The determination of the tax expense in the planning period involved the application of a corporate group tax rate of 35.5 % in the fiscal year 2006 is reduced during the period up to the planned year 2008 and for the purpose of determining the sustainable tax expense to 35.3 % as a result of regional shifts in income as a result of different growth in the regions.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 70 |
The minority interests are primarily shareholdings of third parties in Justesa Imagen S.A. having its registered office in Madrid and Justesa do Brasil having its registered office in Barra da Tijuca.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 71 |
6 Discount rate
In order to value the company, the anticipated future net income is discounted back to the effective date of the valuation at a reasonable discount rate. This discount rate consists of the (projected) earnings and the price for the best alternative use of capital in an investment comparable to the valuation object. Economically, the discount rate reflects an investor’s alternative, which entails comparing the investor’s return on an investment in a specific company with a return on investing the same capital in an alternative equity investment. The discount rate therefore reflects the return on an alternative investment, which is comparable to an investment in the valuation object, provided that the two investments are equivalent with respect to the discounted cash flows regarding terms to maturity, risk and taxation (IDW S 1, section 7.2.4.1).
As a quantitative benchmark for determining alternative investment returns, the capital market yields on corporate equities (in the form of stock portfolios) provide the most appropriate criteria. These yields may be expressed generally as a combination of a risk-free rate and a risk premium which shareholders would demand based on the business risk.
6.1 Risk-free rate
The risk-free rate represents a risk free and maturity equivalent alternative investment compared to an investment in the enterprise being valued. In order to determine the risk-free interest rate for the purpose of determining an objective value of a business, the interest structure curve for government bonds can be used as the basis because the zero bond factors which are equivalent in terms of time derived from the interest structure curve ensures compliance with the equivalent term. The interest structure shows the relationship between the interest rates and the terms of zero bonds without a default risk.
The objective estimate of the interest structure curve can be based on the data in the published interest structure data of the German Federal Bank [Deutsche Bundesbank].When using an interest structure curve, the interest rate which is equivalent to the term must be used when discounting each year. In the case of valuing an enterprise, however, this results in the practical difficulty that depending on the length of the planning period and the assumed growth rate for the following phase the so called sutainable earnings period, a present value equivalent uniform interest rate must be newly calculated using the interest structure curve. Due to reasons of practicability, a uniform risk free rate can be calculated and used on the basis of the interest structure curve for the entire period, i.e. commencing with the first year.
Using this basis, a uniform risk-free rate of 4.50 % was set.
The risk-free rate was reduced by a typified shareholder income tax rate of 35% to arrive, for computation purposes, at an after-tax rate
of 2.925%.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 72 |
6.2 Risk premium
In calculating an objective equity value, the basis for deriving the risk premium is the general market behavior, and not the subjective risk propensities of individual company owners. It may be assumed, however, that investors assume that there is a certain risk when investing cash in companies (investor risk). The risk premium may be derived using the capital market pricing models (CAPM, Tax-CAPM) from the equity yields in capital markets, calculated empirically.
The standard form of the Capital Asset Pricing Model (CAPM) is a capital market model in which the costs of capital and risk premiums are stated without factoring in the effects of any personal income taxes. The calculation of the capital market based risk premium is made by establishing the difference in yields between investments in corporate ownership interests (stocks) and investments in risk-free securities. Since equity yields and risk premiums are generally influenced by income tax, a more realistic reflection of the empirically observed equity yields is the Tax-CAPM, which expands the CAPM by specifically incorporating the effects of personal income tax; the Tax-CAPM reflects, in particular, the different tax treatment of interest income, dividend income and capital gains in German tax law.
Under the Tax-CAPM, the discount rate consists of the risk-free rate reduced by the typified shareholder income tax together with the risk premium calculated on the basis of the Tax-CAPM after income tax.
Capital market studies have indicated that investments in stocks have historically generated higher returns than investments in low risk debt instruments and that the risk premium for a market portfolio ranges between 5% and 6% in the long-term (depending also on the reporting period). This range is consistent with the current recommendations of the IDW (see IDW Fachnachrichten Nos. 1-2/2005). Since there are no indications that investors in the future would demand a different risk premium, a market risk premium of 5.5% after personal income taxes was applied for this valuation.
Given the special risk structure of each company to be valued, the aforementioned average risk premium must be modified. This company-specific and industry-specific risk is expressed as a so-called “beta factor” under the CAPM and the Tax-CAPM.
As Schering AG is listed on the stock exchange, beta factors which can be observed in the capital markets exist for Schering AG. The beta factor of Schering AG observed in the market as measured against the CDAX during the period from 31 December 2003 to 31 December 2005 was between 0.60 and 0.85; the average was 0.72. In order to achieve a high statistical validity of the empirical data, the determination of the period for the analysis to determine the beta factors was chosenasa period of 12 months each.
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 73 |
In order to factor in the changed financing structure of the company during the financial planning period, the historically observed beta factors of the leveraged (indebted) company was first converted into an unlevered beta factor (so-called “un-leveraging”). This unlevered beta factor was on average approximately 0.7. On the basis of the market risk premium of 5.5 % and the beta factor for the unlevered company, a risk premium for the operating risk was calculated at 3.85 % for Schering AG (prior to adjustment to the period specific financing structure).
Finally, the unlevered beta factor of Schering AG was calculated back into the period-specific cost of capital on the basis of the future financing structures according to the projections and the level of indebtness (the so-called “re-leveraging”).
We have compared the unlevered beta factor of Schering AG to a group of comparable businesses in the pharmaceutical sector (peer group). The beta factor of Schering AG was far below the average beta factor in the peer group. The company has two business areas with very mature product portfolios in the fields of Gynecology & Andrology and Diagnostic Imaging which, compared to other pharmaceutical enterprises, are apparently assessed by the capital markets as making a risk reducing contribution to the overall corporate risk.
6.3 Growth rate
The achievable growth of the company is reflected in the development of the projected income and expenditures as well as the balance sheet items, financial planning for the years 2006 through 2008 so that a consideration of a growth rate was not necessary. Furthermore, in the years 2009ff and thereafter (sustainable earnings), the balance sheet and income statement items and, thus, the shareholders’ projected net earnings receivable will develop further; these sustainable earnings can be considered as a growth discount in determining the discount rate. To finance the associated growth in the sustainable earnings phase, earnings in the amount of the growth rate multiplied by equity at the end of the detailed planning phase must be retained.
We included in the deduction of the sustainable earnings by the use of a forecast model the anticipated growth of the business areas after the planning period of the operative plan. The further development was explicitly incorporated in this by presenting the existing products as well as the products under development. The long term growth beyond the examination period was derived on the basis of the average economic growth of the pharmaceutical market which is above the growth of the overall economy and the anticipated long term growth perspective of each business area respectively. It was taken into account that for a long term assessment the growth rates in the pharmaceutical market will converge in the long run with a long term growth trend which in turn will converge with the growth trend of average company revenues.
Taking into consideration mid-term growth expectations of the business areas of Schering which substantiate in the long term profits, a further long term growth rate of 1,75 % has been assumed; this is above the anticipated, continuously parallel to the macroecononomic growth
| | | |
Unauthorized translationof the legally binding
German original | | Schering AG
Independent expert opinion
Equity value as of 13 September 2006
Page 74 |
developing long term average growth of company results, and thus reflects the at present above the average growth of company profits lying, but in time restricted growth of the pharmaceutical industry.
| | | | | | | |
| | | | | | | Schering AG |
| | | Unauthorized translation | | | | Independent expert opinion |
| | | of the legally binding | | | | Equity value as of 13 September 2006 |
| | | German original | | | | Page 75 |
| | | | | | | |
7 Equity Value
7.1 Discounted earnings value
On the basis of the net profits of Schering AG, and applying the relevant period specific discount rates, the discounted earnings value of Schering AG’s operating assets as of 1 January 2006 is as set forth below:
Schering AG
| | | | | | | | | | | | | | | | | |
| | | | | | | Planning | | | | | | Sustainable |
| | | 2006 | | 2007 | | 2008 | | 2009ff. |
| | | €m | | €m | | €m | | €m |
| |
Net Income | | | 575 | | | | 743 | | | | 834 | | | | 982 | |
| Retention for sustainable growth | | | — | | | | — | | | | — | | | | (52 | ) |
| Value impact of retention | | | 363 | | | | 469 | | | | 526 | | | | 586 | |
| Value impact of distribution | | | 212 | | | | 274 | | | | 308 | | | | 343 | |
| Typified income tax on distribution | | | 37 | | | | 48 | | | | 54 | | | | 60 | |
| |
Net earnings received | | | 538 | | | | 695 | | | | 780 | | | | 869 | |
| |
| | | | | | | | | | | | | | | | | |
| Net earnings received | | | 538 | | | | 695 | | | | 780 | | | | 869 | |
| Present value as of 31.12 | | | 16,239 | | | | 16,666 | | | | 17,036 | | | | — | |
| Capitalization subtotal | | | 16,777 | | | | 17,361 | | | | 17,817 | | | | 869 | |
| Discount rate | | | 6.95 | % | | | 6.91 | % | | | 6.90 | % | | | 5.10 | % |
| Present value factor applicable to the year | | | 0.9350 | | | | 0.9354 | | | | 0.9354 | | | | 19.6023 | |
| Applicable present value as of 01.01 | | | 15,687 | | | | 16,239 | | | | 16,666 | | | | 17,036 | |
| |
| | | | | | | | | | | | | | | | | |
Discounted earnings as of 01.01.2006 | | | 15,687 | | | | | | | | | | | | | |
Schering AG
| | | | | | | | | | | | | | | | | |
| | | 2006 | | 2007 | | 2008 | | 2009ff. |
| |
| Risk-free rate before income tax | | | 4.50 | % | | | 4.50 | % | | | 4.50 | % | | | 4.50 | % |
| Typified income tax | | | 1.58 | % | | | 1.58 | % | | | 1.58 | % | | | 1.58 | % |
| Risk-free rate after typified income tax | | | 2.93 | % | | | 2.93 | % | | | 2.93 | % | | | 2.93 | % |
| Market risk premium after typified income tax | | | 5.50 | % | | | 5.50 | % | | | 5.50 | % | | | 5.50 | % |
| Unleveraged beta factor | | | 0.70 | | | | 0.70 | | | | 0.70 | | | | 0.70 | |
Applicable present value as of 01.01 | | | 15,687 | | | | 16,239 | | | | 16,666 | | | | 17,036 | |
Interest-bearing debt | | | 652 | | | | 579 | | | | 579 | | | | 394 | |
| Debt-equity ratio | | | 4.16 | % | | | 3.56 | % | | | 3.47 | % | | | 3.40 | % |
| Leveraged beta factor | | | 0.73 | | | | 0.72 | | | | 0.72 | | | | 0.71 | |
| Risk premium | | | 4.02 | % | | | 3.99 | % | | | 3.98 | % | | | 3.93 | % |
| Growth rate | | | — | | | | — | | | | — | | | | 1.75 | % |
| |
Discount rate | | | 6.95 | % | | | 6.91 | % | | | 6.90 | % | | | 5.10 | % |
| |
| | | | | | | |
| | | | | | | Schering AG |
| | | Unauthorized translation | | | | Independent expert opinion |
| | | of the legally binding | | | | Equity value as of 13 September 2006 |
| | | German original | | | | Page 76 |
| | | | | | | |
A distribution ratio (dividends related to net income) in an amount of 37 % was applied for the detailed planning phase in accordance with the planning of the company. According to information which was provided from Schering, the shareholders of the company are supposed to also participate in the future growth of the company which is shown using a constant distribution ratio based on the distribution ratio for the fiscal year 2005. The distribution ratio for the sustainable earnings 2009ff. was determined at 35 %, taking into account the distribution ratios of Schering for the years 2003 through 2005 as well as the years 2006 though 2008 of the detailed planning phase. It was finally compared with the distribution ratio for a group of comparable pharmaceutical enterprises with own research and development (peer group) for the years 2003 through 2005.26 The distribution ratio of Schering corresponds almost exactly to the average distribution ratio of the peer group in the selected period in the past.
The retentions of the sustainable earnings — in the amount of growth rate related to the balance sheet equity at the end of the detailed planning phase — take into account that the long term anticipated growth of the items in the profit and loss statement and the balance sheet will have to be financed. This necessary retention for the purpose of financing the growth in the future distributions is, thus, not to be included in the amount of value impact of retentions to the shareholders and reduces the amount which is available for distributions.
A re-investment of the retentions at the discount rate before corporate taxes is value equivalent to a direct attribution of the retentions to the shareholders. Therefore the retentions were simplifying directly attributed to the shareholders as value impact of retention.
Only actual distributions — unlike retentions or the retained amounts directly attributed to the shareholders — are subject to typified shareholder income tax of 17.5% (half-credit method).
The net earnings received resulting from the value impacts of retention and of distribution (less typified shareholder income tax) were capitalised using the period-specific discount rate.
7.2 Special items
Available cash and cash equivalents
Schering AG has non-operating cash and cash equivalents totalling€ 200 million. For the purpose of defining cash and cash equivalents, it had to be taken into account that planned investments and distributions remain funded and a reasonable cash reserve remains available for managing business operations. In this connection, an analysis of cash needs of Schering AG throughout the year was conducted, resulting in a minimum cash reserve of€ 250 million. When determining the available cash, we have taken into account the cash effects from the sale of the radio-pharmacology business and the 50 % ALK-Scherax participation.
| | |
| 26 | | We refer to our discussion in Section 8.2 on compiling the peer group. |
| | | | | | | |
| | | | | | | Schering AG |
| | | Unauthorized translation | | | | Independent expert opinion |
| | | of the legally binding | | | | Equity value as of 13 September 2006 |
| | | German original | | | | Page 77 |
| | | | | | | |
Participating interests and non-operating real estate
The participating interests taken into account as special items relate primarily to the shares in Morphosys AG, Martinsried/Planegg (hereinafter, “Morphosys AG”), Sonus Pharmaceuticals, Inc., Bothell (Washington), USA (hereinafter, “Sonus”) and Astex Technology Ltd., Cambridge, Great Britain. The value of the shares in Morphosys AG was determined on the basis of the purchase price realized in the first half of 2006
(€ 16.3 million). The shares in Sonus were taken into account at their current market value (€ 14.1 million). The shares in Astex as well as the other participations of ancillary significance were valued simplified at their current book value of a total of€ 11.7 million.
The market value of the real estate which was identified as non-operating assets (relates primarily to the property in the Friedrichstrasse/Behrenstrasse) was determined at€ 27.9 million and was taken into account as a special item with the amount of capital gains after corporate taxes (€ 25.3 million).
Dilution effects of stock options
Schering AG has granted stock options for the acquisition of stock in Schering AG to employees in the context of various stock option plans. As a result of the takeover of Schering AG by Bayer AG, all stock options of the employees can be exercised as a result of change of control clauses. Schering AG can pay cash compensation instead of issuing shares under almost all of the stock option plans. In the case of the stock option plans for which this right existed, Schering AG has exercised this right. In the case of stock option plans for key managers for the years 2001 and 2002, the right to pay cash compensation on the part of Schering does not exist. Schering AG must offer stock. Under both of these stock option plans, there were still 41,100 outstanding and exercisable options as of 20 July 2006.
The exercise of option rights leads to a reduction in the equity value of the company per share which must be taken into account if the equity value is higher than the exercise price of the options prior to exercise of the option right (dilution effect).
| | | | | | | |
| | | | | | | Schering AG |
| | | Unauthorized translation | | | | Independent expert opinion |
| | | of the legally binding | | | | Equity value as of 13 September 2006 |
| | | German original | | | | Page 78 |
| | | | | | | |
The dilution effect under these stock options can be calculated as follows:
Dilution effect from stock options Schering AG
| | | | | | | |
| Preliminary equity value (discounted earnings value plus special items) as of 01. 01 .2006 | | €m | | | 15,954 | |
| Number of shares outstanding (without own shares) | | units | | | 190,841,000 | |
| Preliminary equity value per share | | € | | | 83.599 | |
| | | | | | | |
| Number of options outstanding | | units | | | 41,100 | |
| Average exercise price | | € | | | 68.80 | |
| Cash flow | | €m | | | 2.828 | |
| | | | | | | |
| Equity value after excercising the option | | €m | | | 15,957 | |
| Number of shares after option exercise | | units | | | 190,882,100 | |
| Value per share after dilution | | € | | | 83.595 | |
| | | | | | | |
| Dilution effect per share | | € | | | 0.003 | |
| |
| | | | | | | |
Dilution effect as of 01.01.2006 | | €m | | | 0.6 | |
| |
7.3 Equity value
The equity value of the Schering AG as of 13 September 2006 is calculated as follows:
Deviation equity value Schering AG
| | | €m |
| |
| Discounted earnings value | | | 15,687 | |
| Special items | | | | |
| Participating interests | | | 42 | |
| Real estate | | | 25 | |
| Available Cash and cash equivalents | | | 200 | |
| Dilution effects of stock options | | | -1 | |
| Equity value as of 01.01.2006 | | | 15,953 | |
| Accumulation factor | | | 1.0482 | |
| | | | | |
| |
Equity value as of 13.09.2006 | | | 16,723 | |
| |
The equity value of Schering AG as of 1 January 2006 in the amount of€ 15,953 million has to be cumulated with the discount rate as of 13 September 2006. The resulting equity value of Schering AG as of to the valuation date is€ 16,723 million.
| | | | | | | |
| | | | | | | Schering AG |
| | | Unauthorized translation | | | | Independent expert opinion |
| | | of the legally binding | | | | Equity value as of 13 September 2006 |
| | | German original | | | | Page 79 |
| | | | | | | |
The value of each share of Schering AG is calculated on the basis of the computed equity value and the relevant number of shares as follows:
Deviation of price per share Schering AG
| | | | | |
Equity value as of 13.09.2006 (in€ million) | | | 16,723 | |
| Number of shares outstanding (in units) | | | 190,841,000 | |
| |
| | | | | |
Price per share in € | | | 87.63 | |
| |
The share capital of Schering AG as of 13 September 2006 consisted of 194,000,000 registered no-par value shares at 1.00 Euro per share. Deducting the treasury stock consisting of 3,159,000 shares as of 23 July 2006, the relevant number of shares is 190,841,000.
| | | | | | | |
| | | | | | | Schering AG |
| | | Unauthorized translation | | | | Independent expert opinion |
| | | of the legally binding | | | | Equity value as of 13 September 2006 |
| | | German original | | | | Page 80 |
| | | | | | | |
8 Plausibility assessment using market prices
8.1 Analysis Schering AG’s share price
With regard to the decision of the Federal Constitutional Court [Bundesverfassungsgericht, “BVerfG”] dated 27 April 199927, the stock exchange price of the shares of the company to be evaluated must be compared to the value per share determined according to the discounted earnings value method. According to the order of the BVerfG, an existing stock exchange price cannot be ignored when determining the compensation. Accordingly, the stock exchange price is as a general rule the lowest level for the reasonable compensation. It is possible, however, to go below this in exceptional circumstances if the stock exchange price does not reflect the fair market value of the stock. The case law of the BVerfG was specified in the judgement of the Federal Supreme Civil Court [Bundesgerichtshof, “BGH”] dated 12 March 200128. This decision again clearly states that the stock exchange price does not have to be the lowest level for the reasonable compensation if the stock exchange price does not reflect the fair market value. According to the judgement of the BGH, this can be the case if no trade has been conducted over a long period of time with the shares if an outside shareholder was not able to sell his stock at the stock exchange price due to low trading or if the stock exchange price has been manipulated. In the present case, there are no indications that such exceptional circumstances exist.
According to the order of the BGH dated 12 March 2001, a reference price must be used as a basis when determining the stock exchange price as a general rule, i.e. to the extent that certain circumstances do not stand against this, and the reference price is calculated using the average price over a period of three months. The average is intended to eliminate possible manipulative influences and short term distortions. The three month reference period should extend up to directly before the effective date, the date of the shareholders meeting (in this case, 13 September 2006).
With regard to the issue of whether the prices must be weighted according to the daily volumes or whether a simple average should be calculated, different views exist. According to the view of the Court of Appeals [Oberlandesgericht, “OLG”] Düsseldorf (8 November 2004 —I 19 W 9/03, AG 2005, p. 538, 541), a simple average of the daily stock exchange prices must be determined. The OLG Frankfurt on the other hand (9 January 2003 — 20 W 434/93, AG 2003, p. 581, 582) refers to the Offering Regulation to the Securities Acquisition and Takeover Act [Wertpapierübernahmegesetz — Angebotsverordnung, “WpüG — Angebotsverordnung”] that for purposes of determining the compensation the average stock exchange price weighted according to volumes within the meaning of § 5 para. I and 3 WpÜG — Angebotsverordnung must be used as the basis.
| | |
| 27 | | File No. I BvR 1805/94 |
| |
| 28 | | File No. II ZB 15/00 |
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 81 |
| | | |
Since the valuation work must be completed several weeks before the shareholders meeting adopting the resolution and the joint report of the contracting parties must be made available pursuant to § 293 para. 1 AktG commencing with calling the shareholders meeting, the three month average stock exchange price cannot be determined at the time of calculating and determining the cash compensation as of the date of the shareholders meeting.
According to the predominant view in the writings on this topic (cp., Wilm, NZG 2000, p. 239; Bungert, BB 2001, p. 1165; Meilicke/Heidel, DB 2001, p. 974; Beckmann, WPg 2004, p. 624; Krieger, BB 2002, p. 56; Großfeld,Unternehmens- und Anteilsbewertung im Gesellschaftsrecht [Valuation of Companies and Shares in Corporate Law], 4th ed., 2002, p. 196; Hüffer, AktG, 6th ed., 2004, § 305, note 24e; Krieger,Münchener Handbuch des Gesellschaftsrechts[Munich Handbook of Corporate Law], vol. 4Aktiengesellschaft[Stock Corporation], 2nd ed., 1999, § 70, note 106; Koppensteiner,Kölner Kommentar zum Aktiengesetz [Cologne Commentary to the Stock Corporations Act], vol. 6, 3rd ed., 2004, § 305, note 104; Gude,Strukturänderungen und Unternehmensbewertung zum Börsenkurs[Structural Changes and Corporate Valuation at the Stock Exchange Price], 2004, p. 387; Emmerich/Habersack,Aktien- und GmbH-Konzernrecht[Corporate Group Law for Stock Corporations and GmbHs], 4th ed., 2005, § 305, note 47c; Hüffer/Schmidt-Aßmann/Weber,Anteilseigentum, Unternehmenswert und Börsenkurs [Ownership of Shares, Corporate Valuation and Stock Exchange Price], 2005, p. 38), the reference period ends on the date of publication of the takeover offers or corporate group measures (cp, District Court[Landgericht,“LG”] Frankfurt, 6 February 2002 — 3/3 O 150/94; AG 2002, p. 358, 360). This view has also been reflected in § 5 para. 1 of the Offering Regulation for the WpüG which states that the compensation offered in the case of a takeover offer must correspond to at least the average stock exchange price of the stock of the target company during the last three months prior to publication of the decision to issue an offer. The OLG Stuttgart (8 March 2006 — 20 W 5/05, ZIP 2006, p. 764) also considers the use of a reference period of three months prior to the announcement of the measure on the basis of the regulation in the capital market law and takeover law instead of the three month period prior to the shareholders meeting used to date by the BGH.
Despite the criticism expressed in legal writings, at the present times several courts follow the decision of the BGH from the year 2001 (cp, OLG Düsseldorf, 31 January 2003 — 19 W 9/00, AG 2003, p. 329, 331; OLG Hamburg, 7 August 2002 — 12 W 12/01, AG 2003, p. 583, 584). Concerns that the stock exchange price can be influenced by speculation relating to the compensation and no longer is established by normal offer and supply mechanisms after the time a publication of the intended structural measure are supposed to be of less importance compared to the effective date principle and the “free disinvestment decision”.
Doubts continue to exist, however, about whether the strict fixing of the reference period on the effective date of the corporate group measure under the preceding case law adequately takes into account the behavior of the market participants after the decision of the BVerfG in 1999 continued to exist. This applies even more so since in the present case the stock exchange prices
| | | |
| | | Schering AG |
| | | |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 82 |
| | | |
of Schering AG since March 2006 have been obviously marked by the takeover offers announced by Merck and Bayer at€ 77.00 and€ 86.00/89.00 per share respectively. Takeover offers are normally considered by the market to be a signal for subsequent measures leading to a corporate group.
The following graph shows the development of the stock exchange price of Schering AG since the beginning of the year up to 26 July 2006:
Trend of the stock price Schering AG
Source: Bloomberg, KPMG-analysis
On 13 March 2006, Merck KGaA, Darmstadt, announced its intent to submit a voluntary public takeover offer directed towards the shareholders of Schering AG in exchange for payment in the amount of€ 77.00 per Schering share in cash. The background of this involved considerations on a merger of Merck KGaA and Schering AG to a global pharmaceutical and chemical company. The price of the Schering stock increased on 13 March 2006 as a result of high volume trading of approximately 21.4 million pieces by€16.81 compared to the previous day to€ 83.55 and, thus, significantly above the takeover offer of€ 77.00, which corresponds to a gain in the stock price of approximately 25 %. On 23 March 2006, Bayer AG announced its intention to submit a voluntary public takeover offer through its 100 % subsidiary DBV to the shareholders of Schering AG for a payment of€ 86.00 per Schering share. The purpose of the takeover offer was the incorporation of Schering AG and the pharmaceuticals division of Bayer to an independent division in the sub-group Bayer HealthCare. The stock exchange price of the Schering stock increased on 24 March 2006 again on the basis of high volumes of approximately 17.5 million shares by€ 1.12 in comparison to the previous day to€ 86.07 and, thus,
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 83 |
slightly over the takeover offer of€ 86.00. The presentation of the official takeover document occurred on 13 April 2006 which, however, did not have any influence on the stock exchange price. A material condition of the takeover offer involved achieving a minimum acceptance ratio of 75 %. In addition, the goal of the acquisition was stated to be the acquisition of all stock in Schering AG. The deadline for acceptance ran from 13 April 2006 until 31 May 2006. During this period, the stock exchange price remained virtually unchanged at low volumes.
On 30 May 2006, the deadline for the offer was extended by Bayer until 14 June 2006. The conditions with regard to the minimum acceptance ratio and the takeover offer in the amount of€ 86.00 remained unchanged. During this new period, it became publicly known in the market that Merck KGaA had increased its holdings in Schering AG by additional purchases through the stock exchange to 21.4 %, which explained the high trading volumes in this period. DBV also acquired additional Schering stock through the stock exchange in this period. After Bayer announced the preparation of a mandatory offer in the course of anad hocnotice under § 15 German Securities Trading Act [Wertpapierhandelsgesetz,“WpHG”] on 14 June 2006, the commitment of Merck KGaA was made shortly before expiration of the acceptance period to sell its Schering stock to DBV at a price of€ 89.00 per share. In a furtherad hocnotice dated 14 June 2006, Bayer announced that according to the regulations in the takeover law all Schering stockholders who had already offered to sell their stock in the course of the offer process and those who would still do so prior to expiration of the offer deadline would also receive the price of€ 89.00. As a result of this, the stock price of the Schering stock increased on 15 June 2006 to€ 89.07 and remained almost unchanged at this level until 10 July 2006. In thead hocnotice under § 15 WpHG on 20 June 2006, Bayer announced that it had obtained control over 88 % of the outstanding Schering stock and, thus, the conditions for the takeover offer had been satisfied. The goal of complete takeover of Schering AG was again emphasized. After the end of the statutory acceptance deadline, Bayer announced on 12 July 2006 that it controlled 92.4 % of the outstanding Schering stock. The stock exchange price of Schering stock increased up to 26 July 2006 to€ 90.56 per share, whereby the highest stock exchange price in this period was observed to be on 18 July 2006 at a price of€ 92.48 per share.
Bayer announced on 26 July 2006 details on the intended Domination and Profit and Loss Transfer Agreement in the context of a press statement, as did Schering in the context of anad hocnotice. In particular, the offered compensation amount pursuant to § 305 AktG, the guaranteed dividend under § 304 AktG as well as the equity value of Schering AG as of 13 September 2006, the date of the intended extraordinary shareholders meeting of the Schering AG, were announced.
Under a strict economic view and a narrow interpretation of the requirements on the reference stock exchange price set by the BVerfG as a comparable, undistorted amount, the period for determining the reference price would be the three month period prior to 13 March 2006 because after 13 March 2006 the stock exchange price of Schering AG was influenced to a considerable degree by the announcement of the takeover offer, the intent to merge with Merck KGaA
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 84 |
as well as the statement about the likely amount of the takeover offer of€ 77. Rationally acting market participants increasingly acquired Schering stock after this time because the takeover offer was significantly above the stock exchange price prior to announcement of the takeover offer. Furthermore, the increase in the stock exchange price to€ 83.55 on 13 March 2006 and the resulting market reaction showed a potential improvement of the offer as had been anticipated by a number of analysts. The course of the stock exchange price for the Schering stock in light of this background does not reflect the undistorted result of offer and demand free of special effects on the basis of anticipated fundamental corporate data of Schering AG and resulting expectations of price by the market participants on that basis; rather, the course of the stock exchange price reflects the expectations on the demand side resulting after 13 March 2006 with regard to the actual takeover offer and a potential improvement. Thus, the stock exchange price of Schering stock after 13 March 2006 can already be considered to have been distorted by speculation about the takeover offer of Merck KGaA. The three month period from 13 December 2005 to 12 March 2006 would be the relevant three month period for determining the reference stock exchange price.
In its judgement dated 12 March 2001, the BGH does not consider the above described “speculation on compensation” as a basis for distortion of the stock exchange price; rather, the BGH considers this also to be behavior of the market participants which establishes the stock exchange price on the basis of corresponding supply and demand in expectation of a more beneficial compensation resulting from the affiliation agreement. In addition, it must also be taken into account, contrary to the above described primary economically motivated procedure, that it was not the takeover offer of Merck KGaA but the takeover offer of Bayer AG which is the reason for the Domination and Profit and Loss Transfer Agreement about which a resolution is to be adopted at the shareholders meeting on 13 September 2006.
It can be assumed in this regard that the share price for Schering AG was substantially influenced by thead hocnotices of Bayer AG on 14 June 2006 with the notification of Bayer AG that it was willing to pay all Schering shareholders also the price of€ 89.00 per share in Schering AG who had already offered their shares in the context of the offer process or would do so prior to expiration of the offer deadline, which resulted in an increase in the stock price for the share to approximately€ 89 after this date.
The share price for the Schering stock fluctuated around€ 89 until 12 July 2006, the date on which Bayer AG announced that it had control over 92.4 % of the Schering stock. The share price increased steadily to€ 92 in the following days. Since at that time no further information on the future integration of Schering AG and the pharmaceutical division of Bayer to an independent division of the sub-group Bayer HealthCare was known in the market, it can be assumed that as a result of the previous announcement of Bayer AG on a complete takeover, considerations by market participants about a possible procedure to exclude minority shareholders in the near future also contributed to the price building process.
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 85 |
At the latest after the press statement of Bayer as well as thead hocnotice by Schering on 26 July 2006 and the related announcement of a guaranteed dividend pursuant to § 304 AktG and the compensation under § 305 AktG in connection with the planned Domination and Profit and Loss Transfer Agreement, it can no longer be assumed as of 27 July 2006 that the price was characterized by the expectations of the market participants about the consequences of the announced Domination and Profit and Loss Transfer Agreement as the price being established as a result of undistorted supply and demand because the offered price by DBV was already fixed by the guaranteed dividend and the compensation offer.
Although much earlier time periods can be considered in the case of a strictly economic approach and a narrow interpretation of the requirements placed on the reference price by the BVerfG as a comparable, undistorted level, we are determining the average stock exchange price in accordance with the predominant opinion in the writings as the three month period prior to notice of the planned conclusion of a Domination and Profit and Loss Transfer Agreement. This was 26 July 2006, the date on which the intake of the offer submitted by Bayer to Schering AG to pay cash compensation in the amount of€ 89.00 per share in the planned contract was announced.
We are basing our method on the decision of the legislature which uses the three month period prior to publication on issuing a public offer set forth in § 5 WpÜG Offer Regulation about how to determine the minimum stock exchange price in the case of takeover offers and mandatory offers. For the same reason, the weighted average of the domestic stock exchange prices must be used as the basis. This corresponds to the provisions in § 5 para. 1 and para. 3 WpÜG Offer Regulation for the comparable case of minimum compensation in the case of takeover offers and mandatory offers.
On this basis, the period from 27 April 2006 through 26 July 2006 (included) is determinative for identifying the average share price of the Schering stock. The average weighted stock exchange price for this period determined pursuant to the above rules is€ 86.61 per share (source: Bloomberg).
Since this stock exchange price is lower than the proportionate equity value, it is not to be considered to be the minimum value for the amount of compensation offered under § 305 AktG according to the case law of the higher courts.
8.2 Comparative market valuation
The practice of valuations also knows the so called multiple method in addition to the calculation based on the present value methods. This capital markets oriented method of valuation follows the principle of an earnings based valuation, just as does the discounted earnings value method, but the value of the company is determined in a very simplified manner on the basis of a multiple applied to a financial figure. The multiple method is based on a comparable determi-
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 86 |
nation of price in such a manner that suitable multiples are determined by reference to capital market data of listed comparable companies and are then applied to the company to be valued.
The value of the company determined on the basis of this process for identifying the price by referring to key data of comparable listed companies is determined as the product of a representative financial figure of the company multiplied by the multiple for comparable businesses. The multiplier is determined on the basis of the relationship of value levels to financial figures in the comparable company.
In order to determine the plausibility of the fundamental equtiy value of Schering AG which was calculated applying economic principles, the observed market prices for comparable companies (peer group companies) and the multiples derived from them are considered.
8.2.1 Selection of comparable companies
We have looked at the industry and a comparable business model (pharmaceutical companies with own research and development) as the starting point for analyzing the comparable companies and as selection criteria in order to identify the potential comparable companies as a first step. Based on this, we examined the selected enterprises with regard to their comparability with Schering according to relevant criteria. These criteria are the strongest areas in terms of net sales as well as the regional composition and allocation of the realized net sales.
On the basis of the above criteria, the peer group consists of six companies.
8.2.2 Determination of the multiples
In order to calculate the multiples, we have used the earnings before interest and tax (EBIT) and the net profits realized at the selected comparable companies in the year 2005 and the annual EBIT both for the years 2006 and 2007 according to current IBES estimates which can be obtained from the financial services company Bloomberg. The entity value of the companies for the comparable companies was calculated using the market capitalization and adding the interest bearing financial debt.
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 87 |
The following table lists the multiples for the comparable companies:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Enterprise value / EBIT | | Equity value / Net profit |
| Multiples of comparable companies | | 2005 | | 2006e | | 2007e | | 2005 | | 2006e | | 2007e |
| |
| UCB SA | | | 15,7x | | | | 16,1 x | | | | 14,7x | | | | 8,3 x | | | | 19,4x | | | | 18,4x | |
| Serono SA | | | n/a | | | | 13,4 x | | | | 12,6 x | | | | n/a | | | | 15, 5 x | | | | 14,4x | |
| Altana AG | | | 8,9 x | | | | 8,2 x | | | | 7,6 x | | | | 14,5 x | | | | 13,4x | | | | 12,4 x | |
| Schering-Plough Corp. | | | n/a | | | | 20,9 x | | | | 16,1 x | | | | n/a | | | | 28,6 x | | | | 21,4x | |
| Merck KGaA | | | 13,9x | | | | 11,6x | | | | 9,7 x | | | | 19,4x | | | | 16,6x | | | | 15,3 x | |
| H Lundbeck A/S | | | 13,6x | | | | 18,3x | | | | 10,6x | | | | 19,3x | | | | 30,5 x | | | | 16,0 x | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| High | | | 8,9 x | | | | 8,2 x | | | | 7,6 x | | | | 8,3 x | | | | 13,4x | | | | 12,4 x | |
| Mean | | | 13,0 x | | | | 14,7 x | | | | 11, 9x | | | | 15,4x | | | | 20,7 x | | | | 16,3x | |
| Low | | | 15,7 x | | | | 20,9 x | | | | 16,1 x | | | | 19,4x | | | | 30,5 x | | | | 21,4x | |
| |
Source: Bloomberg, KPMG Analysis
The average multiples on the basis of the EBIT are between 11.9 and 14.7, and on the basis of the net profits they are between 15.4 and 20.7. The identified multpiles were then applied to the equivalent levels of financial figures at Schering AG.
8.2.3 Value range of the multiples
The following table provides an overview of the market values for the equity value of Schering AG determined using the multiples of the comparable companies. In doing this, initially the entity value of Schering AG was determined using the EBIT multiples of the comparable companies and deducting the interest bearing debt as well as the minority shares which resulted in the market value of the equity of Schering AG. When applying the multiple on the basis of the net profits of the comparable enterprises to the net profit of Schering, the value of Schering directly results.
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 88 |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | based on EBIT-Multiples | | based on Net Profit-Multiples |
| Equity Value | | 2005 | | 2006e | | 2007e | | 2005 | | 2006e | | 2007e |
EBIT Schering Group | | | 928 | | | | 904 | | | | 1,160 | | | | | | | | | | | | | |
Net Income Schering Group | | | | | | | | | | | | | | | 619 | | | | 575 | | | | 743 | |
| Enterprise Value | | | | | | | | | | | | | | | | | | | | | | | | |
| High | | | 8,259 | | | | 7,413 | | | | 8,816 | | | | | | | | | | | | | |
| Mean | | | 12,064 | | | | 13,289 | | | | 13,804 | | | | | | | | | | | | | |
| Low | | | 14,570 | | | | 18,894 | | | | 18,676 | | | | | | | | | | | | | |
| |
| Interest bearing debt* | | | 823 | | | | 652 | | | | 579 | | | | | | | | | | | | | |
| Minority Interest | | | 18 | | | | 18 | | | | 19 | | | | | | | | | | | | | |
| |
Equity Market Value | | | | | | | | | | | | | | | | | | | | | | | | |
| High | | | 7,418 | | | | 6,743 | | | | 8,218 | | | | 5,138 | | | | 7,705 | | | | 9,213 | |
Mean | | | 11,223 | | | | 12,619 | | | | 13,206 | | | | 9,533 | | | | 11,903 | | | | 12,111 | |
| Low | | | 13,729 | | | | 18,224 | | | | 18,078 | | | | 12,009 | | | | 17,538 | | | | 15,900 | |
| | |
| * | | including provisions for pensions |
On the basis of average market multiples, a range for the market value of the equity of Schering AG results which is between€ 9.5 billion and€ 13.2 billion; the maximum in the range is between€ 12.0 billion and€ 18.2 billion. On the basis of the multiple method, it can be determined that the fundamental equity value according to IDW S 1 is at the upper end of the range for comparable companies, depending on the selected multiples and the period, and in many cases the value is also above the range. Thus, there are no indications that the determined equity value compared to the present capital market environment is too low under the comparable observation of the market.
The following overview shows the complete range of the identified market multipliers as well as the equity value of Schering AG.
Margin market value of equity Schering AG
| | | |
| | | Schering AC |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 89 |
9 Cash compensation and guaranteed dividend
9.1 Cash compensation under § 305 AktG
Pursuant to § 305 AktG, a domination or profit and loss transfer agreement must contain the obligation of the controlling company to acquire the shares of an outside shareholder upon demand in exchange for reasonable cash compensation set forth in the agreement. According to the case law of the BVerfG, the stock exchange price constitutes the lower limit for the compensation. Since the equity value per share of€ 87.63 is above the average stock exchange price of Schering AG, the cash compensation must be set on the basis of the proportionate equity value in the present case.
9.2 Guaranteed dividend under § 304 AktG
Pursuant to § 304 AktG, a domination and profit and loss transfer agreement must provide for reasonable compensation for the outside shareholders by way of a recurring payment (guaranteed dividend) for the shares of the proportional share capital. The guaranteed dividend under § 304 para. 2 sentence 1 AktG must be guaranteed at least in the amount of an annual payment could be distributed according to the past profit of the company and its future outlook for profit taking into account reasonable depreciation and valuation allowances, but without establishing retained earnings, which amount could probably be distributed as an average proportion in the profits to the individual shares.
The following table shows the net profit of Schering AG as well as the profit per share for the fiscal years 2001 through 2005.
| | | | | | | | | | | | | | | | | | | | | |
| | | 2001 | | 2002 | | 2003 | | 2004 | | 2005 |
Net profit after minority interest (in million€) | | | 419 | | | | 466 | | | | 449 | | | | 504 | | | | 619 | |
| Weighted average number of shares outstanding | | | 198.0 | | | | 197.3 | | | | 194.4 | | | | 191.2 | | | | 190.0 | |
Earnings per share (in€) | | | 2.12 | | | | 2.36 | | | | 2.31 | | | | 2.64 | | | | 3.26 | |
Dividend per share (in€) | | | 0.83 | | | | 0.93 | | | | 0.93 | | | | 1.00 | | | | 1.20 | |
| |
Weighted average Earnings per share 2001 - 2005 (in€) | | | 2.54 | | | | | | | | | | | | | | | | | |
Weighted average Dividend per share 2001 - 2005 (in€) | | | 0.98 | | | | | | | | | | | | | | | | | |
In a judgement dated 21 July 2003, the German Federal Supreme Civil Court decided that the outside shareholders must be guaranteed as a (fixed) compensation the probable, distributable, average profit per share minus the German corporate income tax relating to dividends at the respectively applicable tax rate to be paid by the company. The judgement states in detail that the earned profits consist of the profit prior to German corporate income tax from which the burden with German corporate income tax must be deducted in the respective amount provided by law. Furthermore, according to the view of the German Federal Supreme Civil Court, the guaranteed dividend must be calculated using the discount rate fully adjusted for risk for the capitalisation of equity value. The special items, especially the assets not necessary for the business, are not supposed to be taken into account when determining the guaranteed dividend.
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 90 |
In our view, parts of this judgement which go beyond the specifics of the case decided, for which the offset procedure for German corporate income tax and personal income tax was applicable until 31 December 2000, do not apply. The (normally fluctuating) future earnings of Schering AG are represented in a concentrated manner in the equity value of the company which represents the payments between the company and the shareholders taking into account the timing of when the payments accrue and include special items and assets which are not necessary for the business. Contrary to the method outlined by the Federal Supreme Civil Court described above, we do not consider it reasonable to permanently withhold assets from the shareholdes by not including the assets which are not necessary for business operations. In order to determine the guaranteed dividend required by the legislature the guaranteed dividend was, therefore, calculated by way of an annuity for the equity value of Schering AG as of 13 September 2006. This procedure ensures that the assets which are not necessary for the business are covered when determining the guaranteed dividend.
The above judgement of the Federal Supreme Civil Court also does not take into account that the guaranteed dividend, at least during the course of the Agreement, is secured so that an annuity with the discount rate (pre-tax) fully adjusted for risk is not appropriate. In the case of a domination and profit and loss transfer agreement there is, of course, normally the risk that the agreement can be terminated by the dominant enterprise, so that the future guaranteed dividends are not completely free of risk. The risk also exists that the company will be reduced in its earnings potential during the course of the agreement and that the shareholder will have a participation after the agreement in an enterprise whose value has been reduced. In order to avoid these risks, the Domination and Profit and Loss Transfer Agreement between Schering AG and DBV provides in § 5. para 6 that upon termination of the affiliation agreement, the claim of the minority shareholders for cash compensation in the amount agreed in the contract (€ 89 per share) will again come into existence. Thus, in this specific case the conclusion of the affiliation agreement completely removes the entrepreneurial risk for the minority shareholders. At the time the affiliation agreement is terminated, the shareholders have the option to demand the offered cash compensation in the same amount as at the time the Domination and Profit and Loss Transfer Agreement was concluded or, in the alternative, they can remain shareholders and again participate in the entrepreneurial risk of Schering AG. The risk position of the minority shareholders, therefore, corresponds to that of a holder of a corporate bond, specifically a convertible/option bond after conclusion of the Domination and Profit and Loss Transfer Agreement. Since the recipient of the guaranteed dividend only has to bear the risk that the obligor for the guaranteed dividend — indirectly Bayer AG — may not be able to pay, it is appropriate to determine the factor for the annuity taking into account the credit risk (credit rating) of Bayer.
The interest rate used to calculate the annuity (annuity factor) results from the sum of the risk free rate and a reasonable risk premium for the credit risk (credit rating).
In order to determine the credit risk of Bayer, various documents were analyzed. These included especially the risk assessment by rating agencies (Standard & Poor’s and Moody’s) and by
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 91 |
banks as well as the current conditions of the syndicated loans to finance the acquisition of Schering as well as estimates by the Bayer treasury of conditions which could be obtained. All of these approaches are generally suitable to estimate the credit risk of Bayer, but all of these approaches have the common characteristic that they contain subjective elements, only represent a very specific situation or require other considerations and/or calculations in order to reach a quantification. In order to objectively determine the risk premium, therefore, we have referred to the bond issued in Euro by Bayer AG in the year 2006 which has a term until 2013. This shows as of 25 July 2006 a credit risk premium compared to Federal securities having the same term in an amount of 0.7340 %. In order to avoid risks for the outside shareholders from daily fluctuations of the exchange, we have rounded up the risk and calculated it at a rate of 0.75 %.
Since the interest payments from a corporate bond are subject to the full income tax rate of the recipient, the risk premium is reduced by a typified shareholder income tax burden in the amount of 35 % in order to determine a discount rate after income tax which is equivalent to the bond.
Taking into account the above circumstances, an annuity factor (after typified shareholder income tax) in an amount of 3.413 % was determined which consists of the present value equivalent interest rate derived from the valuation of the company (after typified shareholder income tax) in the amount of 2.925 % and a credit risk premium of 0.488 % (0.75 % after typified shareholder income tax).
As a result of tax free income, not all of the future profit of Schering AG is subject to German corporate income tax. In light of this, the likely average before German Corporate income tax profit available for distribution is to be divided, according to the requirements of the Federal Supreme Civil Court, in a portion burdened with German corporate income tax and a component which is not burdened with German corporate income tax. This allocation was made using an alternative determination of the equity value of Schering AG taking into account/not taking into account German corporate income tax plus the resulting solidarity surcharge and the resulting division of the equity value of Schering AG in a portion which is burdened with German corporate income tax and the solidarity surcharge and a portion which is not burdened with this.
Since the guaranteed dividend at the level of the minority shareholder is subject to half the income tax rate, the determined guaranteed dividend (after typified shareholder income tax) is increased by half of the typified shareholder income tax of 17.5 %. The resulting net guaranteed dividend (before typified shareholder income tax) is finally increased by the German corporate income tax plus solidarity surcharge in order to determine the guaranteed dividend. In doing this, the increase was only made for the part of the gross guaranteed dividend which would be burdened with German corporate income tax and the solidarity surcharge.
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 92 |
The calculation of the reasonable annual guaranteed dividend is shown in the following overview:
| | | | | | | | | | | | | | | | | |
| Derivation of the gross cash compensation (before income taxes) |
| | | | | | | Share in the cash compensation |
| | | | | | | burdened | | not burdened | | |
| | | | | | | with income | | with income | | |
| | | | | | | tax | | tax | | together |
Equity value as of 13 September 2006 (in million€) | | | | | | | 12,554.1 | | | | 4,168.9 | | | | 16,723.0 | |
multiplied with the factor of allocation 3,413% (after personal taxes) (in million€) | | | | | | | 428.4 | | | | 142.3 | | | | 570.7 | |
| Number of shares (in million) | | | | | | | 190.8 | | | | 190.8 | | | | 190.8 | |
| |
Annual cash compensation per share (after personel taxes, income taxes and solidarity surcharge) (in€) | | | | | | | 2.24 | | | | 0.75 | | | | 2.99 | |
| |
| plus personal taxes | | | 17.50 | % | | | 0.48 | | | | 0.13 | | | | 0.61 | |
| |
Annual net cash compensation per share (before personal taxes, after income taxes and solidarity surcharge) (in€) | | | | | | | 2.72 | | | | 0.90 | | | | 3.62 | |
| |
| plus income taxes and solidarity surcharge | | | 26.375 | % | | | 0.98 | | | | — | | | | 0.98 | |
| |
Annual gross cash compensation per share (before personel taxes, income taxes and solidarity surcharge) (in€) | | | | | | | 3.70 | | | | 0.90 | | | | 4.60 | |
The reasonable guaranteed dividend under § 304 AktG amounts to€ 4.60 per share (portion of profit before German corporate income tax per share) minus an amount to be paid by Schering AG German corporate income tax. This amount is calculated using the portion of the profit in the amount of€ 3.70 per share which is burdened by German corporate income tax contained in the profit German corporate tax of Schering AG per share, taken into account the respectively applicable German corporate income tax rate for the relevant fiscal year. The applicable German corporate income tax rate at the time of conclusion of the Agreement, including solidarity surcharge, is 26.375 %; this results in a deduction for German corporate income tax from the portion of the profit burdened by German corporate income tax in the amount of€ 0.98.
Assuming an unchanged German corporate income tax rate of 25.0 % and a solidarity surcharge of 5.5 %, the total net guaranteed dividend per share is€ 3.62 is despite of the higher level of safety clearly above the amounts realized in the past when compared to the dividends due to the expected positive development of Schering AG.
| | | |
| | | Schering AG |
Unauthorized translation | | Independent expert opinion |
of the legally binding | | Equity value as of 13 September 2006 |
German original | | Page 93 |
10 Concluding remarks
The result of the independent expert opinion on the determination of the equity value in the context of the planned Domination and Profit and Loss Transfer Agreement between DBV and Schering AG can be summarized as follows:
The equity value of Schering AG as of 13 September 2006 is approximately€ 16,723 million.
| • | | Based on 190,841,000 issued shares, this constitutes a value per share of€ 87.63. |
| |
| • | | The average stock exchange price during the three month period from 27 April 2006 through 26 July 2006 was€ 86.61 and is, thus, not relevant for determining the value of the cash compensation. |
| |
| • | | The reasonable guaranteed dividend under § 304 AktG amounts to€ 4.60 (gross profit per share) for each share, minus the German corporate income tax and solidarity surcharge to be rendered by Schering AG on the portion of profit in the amount of€ 3.70 per share included in the gross profit per share which is subject to German corporate income tax. In the case of the burden with German corporate income tax applicable at the time the Agreement is concluded (including solidarity surcharge) of 26.375 %, an amount of€ 0.98 results which must be deducted for taxes, so that there is a guaranteed dividend per share of€ 3.62. |
We have prepared this report on the basis of the documents provided to us as well as the information given and the results of our own examinations.
| | | |
| Frankfurt am Main, 27 July 2006 | | |
| | | |
KPMG Deutsche Treuhand-Gesellschaft | | |
Aktiengesellschaft | | |
Wirtschaftsprüfungsgesellschaft | | |
| | | |
| | | |
| | | |
| Zeidler | | Paulus |
| | | |
Certified Accountant | | Certified Accountant |
2004 2005
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Schering Group – Transfer adjusted / non-adjusted Operating Profits |
| | | | | | | | | | | 2004 | | | | | | | | | | | | | | | | | | 2005 | | | | |
| | | non-adjusted | | | | | | Adjustments | | | | | | Adjusted | | Non-adjusted | | | | | | Adjustments | | | | |
| | | | | | | ALK- | | | | | | Sub-total | | | | Other | | | | | | | | | | | ALK- | | | | | Sub-total | | Other | | Adjusted |
| | | | | | | participation | | Radiopharmaceuticals | | | | | | | | | | | | | | | | | | participation | | Radiopharmaceuticals | | | | | | |
| | | €m | | €m | | €m | | €m | | €m | | €m | | €m | | €m | | €m | | €m | | €m | | €m |
Net Sales | | | 4,907 | | | | 27 | | | | 113 | | | | 140 | | | | 0 | | | | 4,767 | | | | 5,308 | | | | 34 | | | | 117 | | | | 151 | | | | 0 | | | | 5,157 | |
Gross Profit | | | 3,701 | | | | 15 | | | | 25 | | | | 40 | | | | 0 | | | | 3,661 | | | | 4,052 | | | | 20 | | | | 32 | | | | 52 | | | | 0 | | | | 4,000 | |
as a % of net sales | | | 75.4 | % | | | 55.6 | % | | | 22.0 | % | | | 28.5 | % | | | n/a | | | | 76.8 | % | | | 76.3 | % | | | 58.8 | % | | | 27.4 | % | | | 34.5 | % | | | n/a | | | | 77.6 | % |
| Cost of | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| marketing and selling | | | 1,544 | | | | 9 | | | | 34 | | | | 43 | | | | 0 | | | | 1,501 | | | | 1,687 | | | | 9 | | | | 41 | | | | 50 | | | | 0 | | | | 1,637 | |
| research and development | | | 522 | | | | 0 | | | | -4 | | | | -4 | | | | 0 | | | | 526 | | | | 522 | | | | 1 | | | | -4 | | | | -3 | | | | 0 | | | | 525 | |
| engineering and administration | | | 918 | | | | 0 | | | | 15 | | | | 15 | | | | 0 | | | | 903 | | | | 982 | | | | 0 | | | | 16 | | | | 16 | | | | 0 | | | | 966 | |
| Other operating income / expenses | | | 51 | | | | 0 | | | | -1 | | | | -1 | | | | 32 | | | | 20 | | | | 67 | | | | 0 | | | | -56 | | | | -56 | | | | 121 | | | | 2 | |
including: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Expenses from the prefaced divestiture of the radiopharmaceuticals business | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 54 | | | | | | | | | | | | | |
Income from dissolution of a provision for warranty claims | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Aventis CropScience | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 88 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income from dissolusion of provisions | | | | | | | | | | | | | | | | | | | 63 | | | | | | | | | | | | | | | | | | | | | | | | 33 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Expenses for pension fund in Japan | | | | | | | | | | | | | | | | | | | 31 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating Profit | | | 768 | | | | 6 | | | | -21 | | | | -15 | | | | 32 | | | | 751 | | | | 928 | | | | 10 | | | | -77 | | | | -67 | | | | 121 | | | | 874 | |