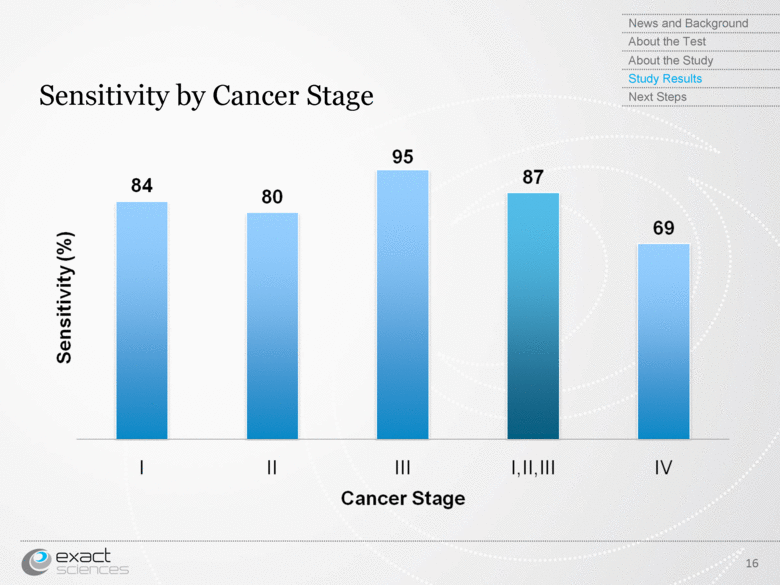Exhibit 99
| Presentation of Validation Study Results October 28, 2010 |
| Certain matters contained in this presentation, other than historical information, consist of forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 relating to, among other things, our expectations concerning the timing of potential commercial and clinical milestones, the efficacy of our technology, our commercial and FDA regulatory strategy, our available cash and cash equivalents, and our business and financial outlook. These forward-looking statements are not guarantees of future performance and are subject to a variety of risks and uncertainties that could cause actual results to differ materially from the results contemplated thereby. Any forward-looking statements we make should be considered in light of the risks and uncertainties contained in our filings with the Securities and Exchange Commission, including but not limited to those contained in our most recent Form 10-K and subsequent Forms 10-Q. We incorporate herein the discussion of those factors. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today. We undertake no obligation to update or revise the information provided herein, whether as the result of new information, future events or circumstances or otherwise. Safe Harbor Statement 1 |
| Agenda 2 News and Background About the Test About the Study Study Results Next Steps News and Background About the Test About the Study Study Results Next Steps |
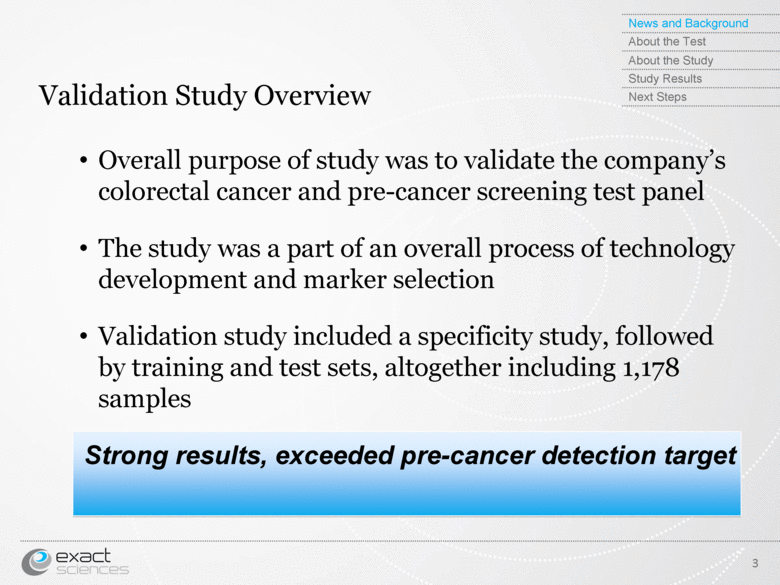
| Validation Study Overview 3 Overall purpose of study was to validate the company’s colorectal cancer and pre-cancer screening test panel The study was a part of an overall process of technology development and marker selection Validation study included a specificity study, followed by training and test sets, altogether including 1,178 samples Strong results, exceeded pre-cancer detection target News and Background About the Test About the Study Study Results Next Steps |
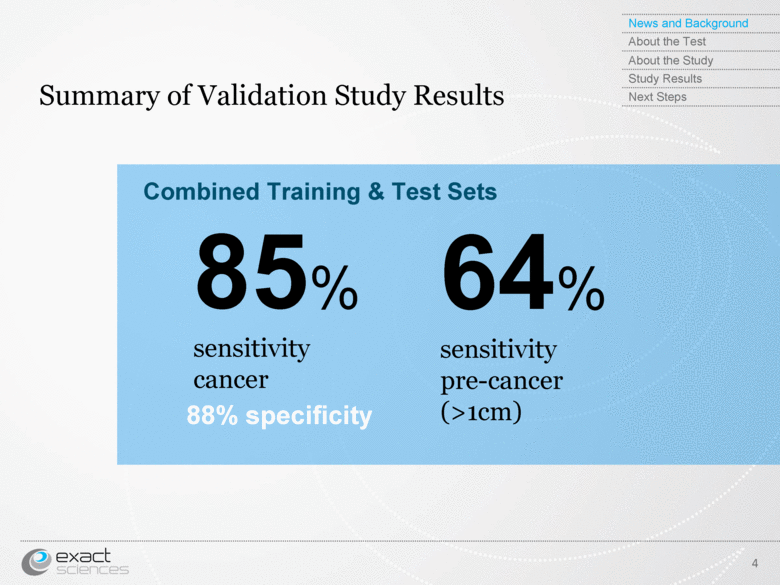
| Summary of Validation Study Results 4 85% sensitivity cancer 64% sensitivity pre-cancer (>1cm) Combined Training & Test Sets 88% specificity News and Background About the Test About the Study Study Results Next Steps |
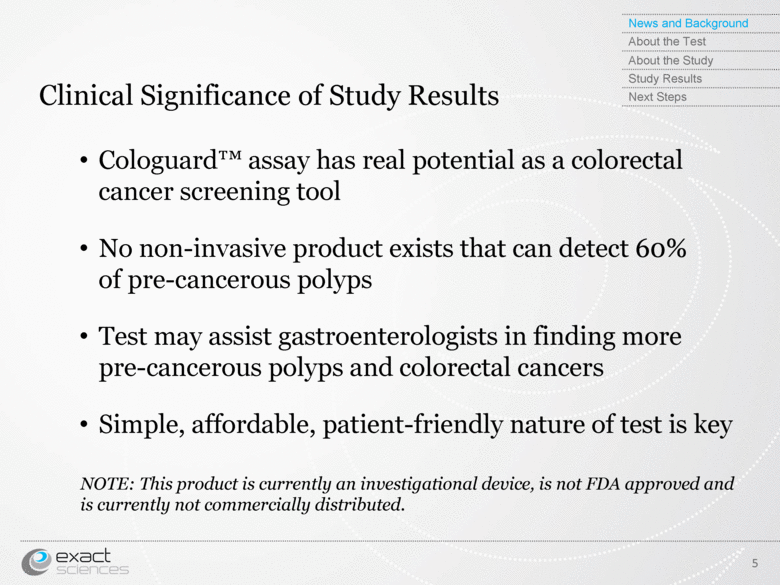
| Clinical Significance of Study Results Cologuard™ assay has real potential as a colorectal cancer screening tool No non-invasive product exists that can detect 60% of pre-cancerous polyps Test may assist gastroenterologists in finding more pre-cancerous polyps and colorectal cancers Simple, affordable, patient-friendly nature of test is key NOTE: This product is currently an investigational device, is not FDA approved and is currently not commercially distributed. 5 News and Background About the Test About the Study Study Results Next Steps |
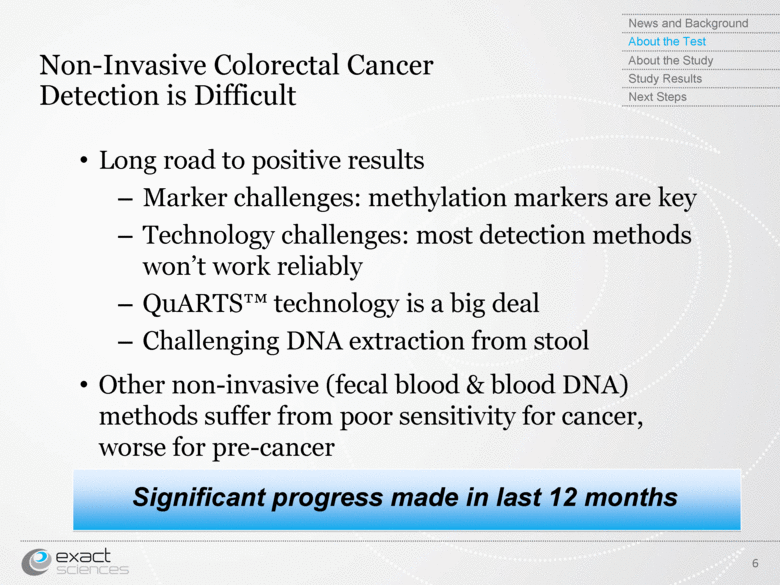
| Non-Invasive Colorectal Cancer Detection is Difficult Long road to positive results Marker challenges: methylation markers are key Technology challenges: most detection methods won’t work reliably QuARTS™ technology is a big deal Challenging DNA extraction from stool Other non-invasive (fecal blood & blood DNA) methods suffer from poor sensitivity for cancer, worse for pre-cancer Significant progress made in last 12 months 6 News and Background About the Test About the Study Study Results Next Steps |
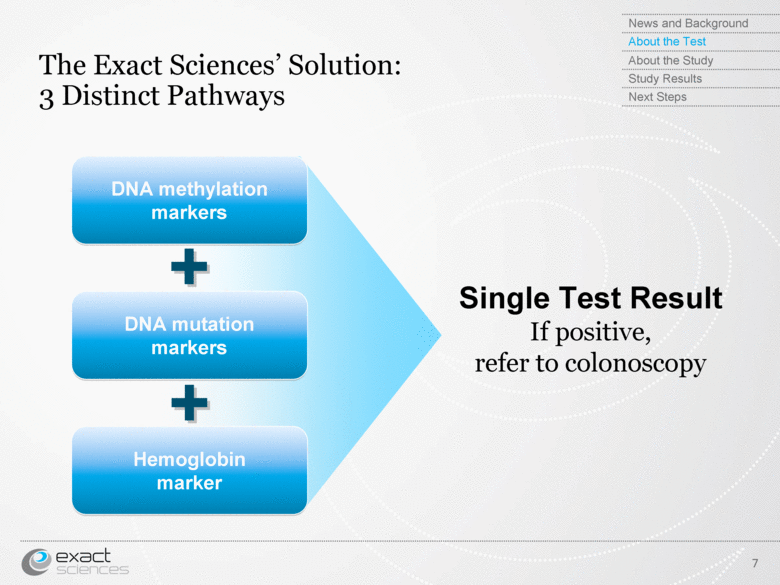
| The Exact Sciences’ Solution: 3 Distinct Pathways DNA methylation markers DNA mutation markers Hemoglobin marker Single Test Result If positive, refer to colonoscopy + + News and Background About the Test About the Study Study Results Next Steps 7 |
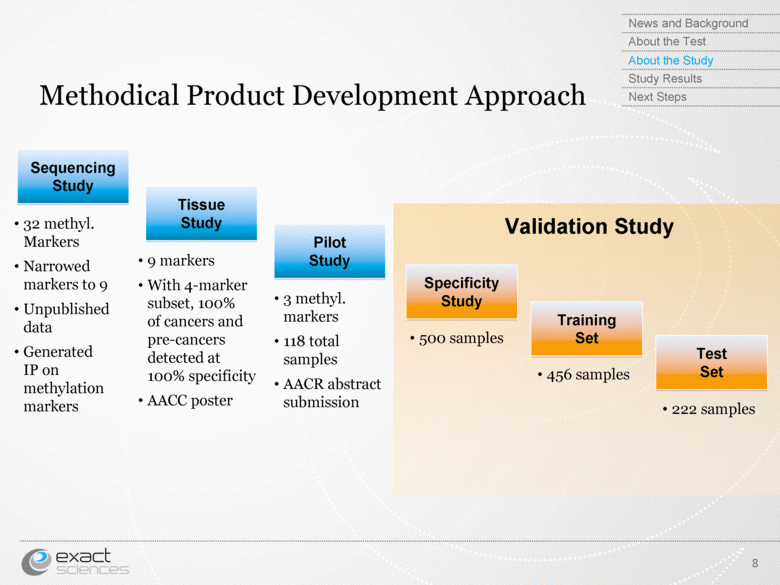
| Methodical Product Development Approach 8 Sequencing Study Tissue Study Pilot Study Specificity Study Training Set Test Set 32 methyl. Markers Narrowed markers to 9 Unpublished data Generated IP on methylation markers 9 markers With 4-marker subset, 100% of cancers and pre-cancers detected at 100% specificity AACC poster 3 methyl. markers 118 total samples AACR abstract submission Validation Study 500 samples 456 samples 222 samples News and Background About the Test About the Study Study Results Next Steps |
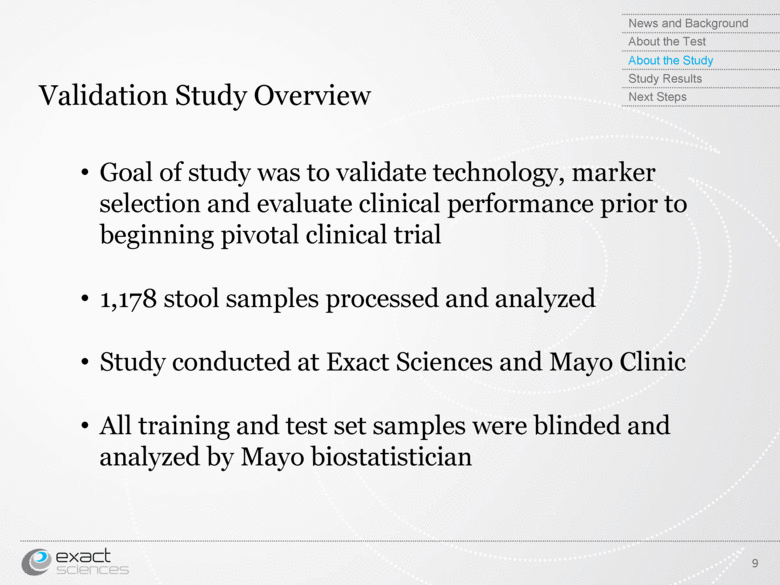
| Validation Study Overview 9 Goal of study was to validate technology, marker selection and evaluate clinical performance prior to beginning pivotal clinical trial 1,178 stool samples processed and analyzed Study conducted at Exact Sciences and Mayo Clinic All training and test set samples were blinded and analyzed by Mayo biostatistician News and Background About the Test About the Study Study Results Next Steps |
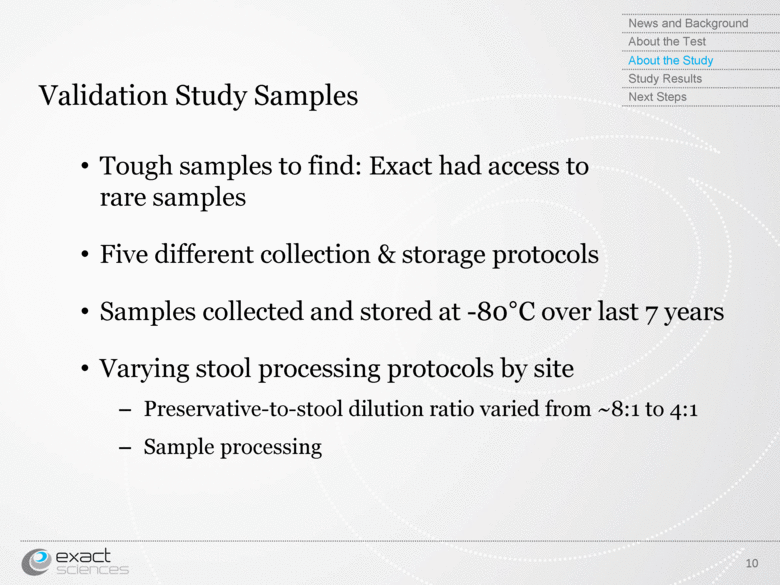
| Validation Study Samples 10 Tough samples to find: Exact had access to rare samples Five different collection & storage protocols Samples collected and stored at -80°C over last 7 years Varying stool processing protocols by site Preservative-to-stool dilution ratio varied from ~8:1 to 4:1 Sample processing News and Background About the Test About the Study Study Results Next Steps |
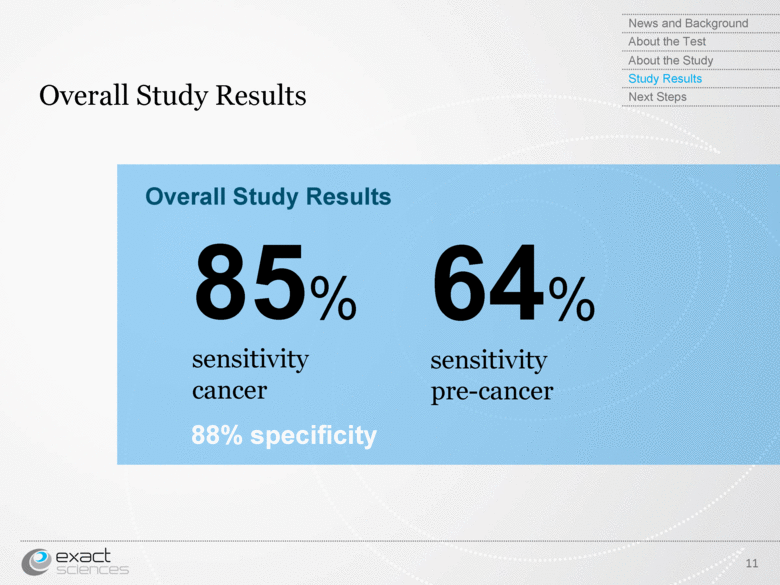
| Overall Study Results 11 85% sensitivity cancer 64% sensitivity pre-cancer Overall Study Results 88% specificity News and Background About the Test About the Study Study Results Next Steps |
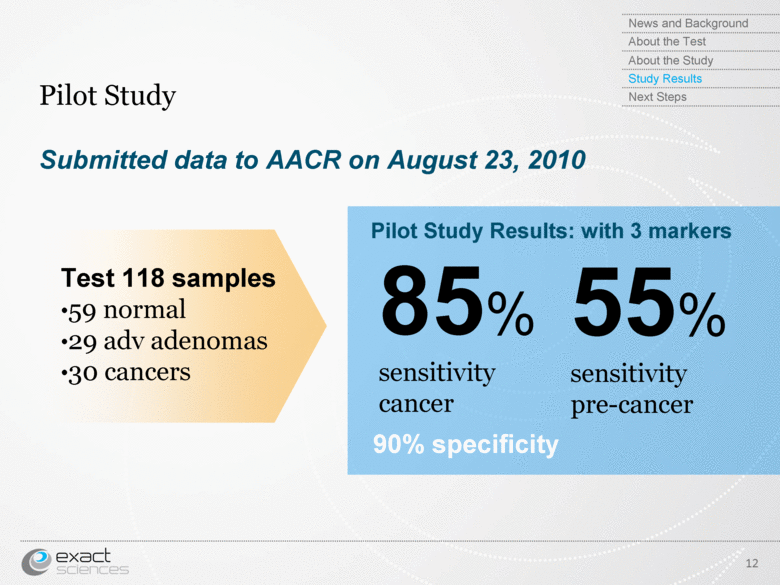
| Submitted data to AACR on August 23, 2010 Pilot Study 12 85% sensitivity cancer 55% sensitivity pre-cancer Pilot Study Results: with 3 markers 90% specificity News and Background About the Test About the Study Study Results Next Steps Test 118 samples 59 normal 29 adv adenomas 30 cancers |
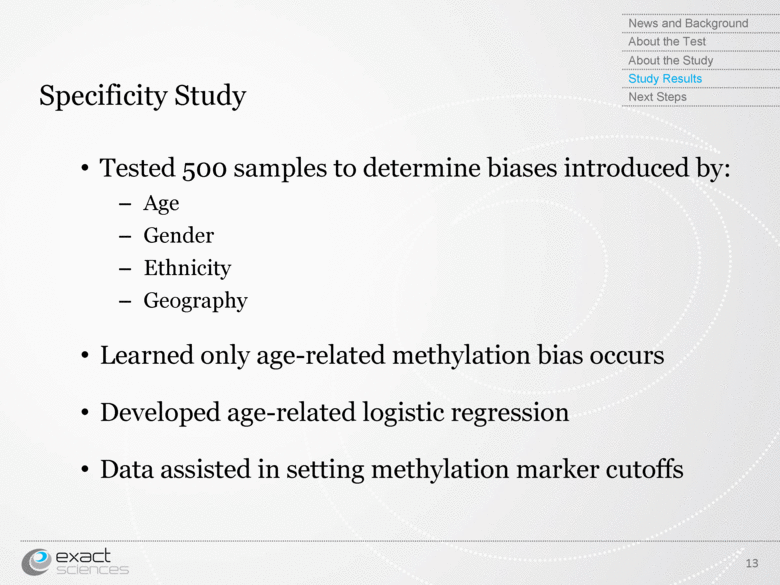
| Specificity Study Tested 500 samples to determine biases introduced by: Age Gender Ethnicity Geography Learned only age-related methylation bias occurs Developed age-related logistic regression Data assisted in setting methylation marker cutoffs 13 News and Background About the Test About the Study Study Results Next Steps |
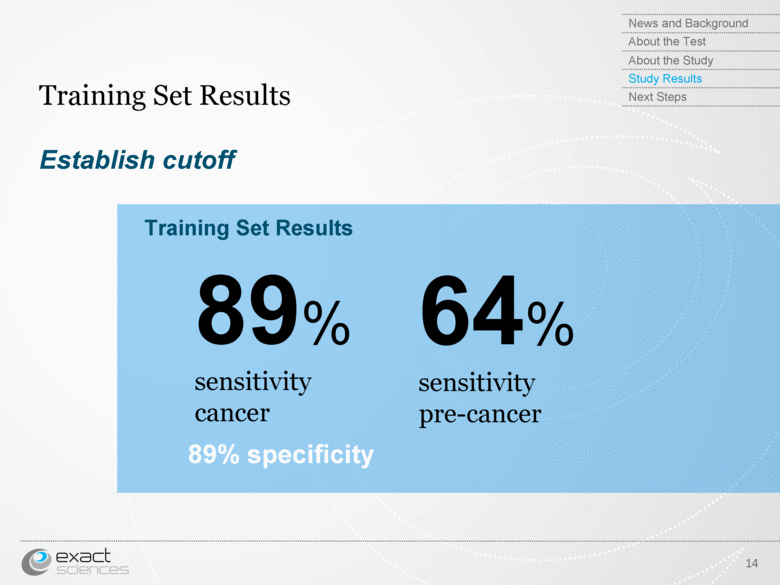
| Establish cutoff Training Set Results 14 89% sensitivity cancer 64% sensitivity pre-cancer Training Set Results 89% specificity News and Background About the Test About the Study Study Results Next Steps |
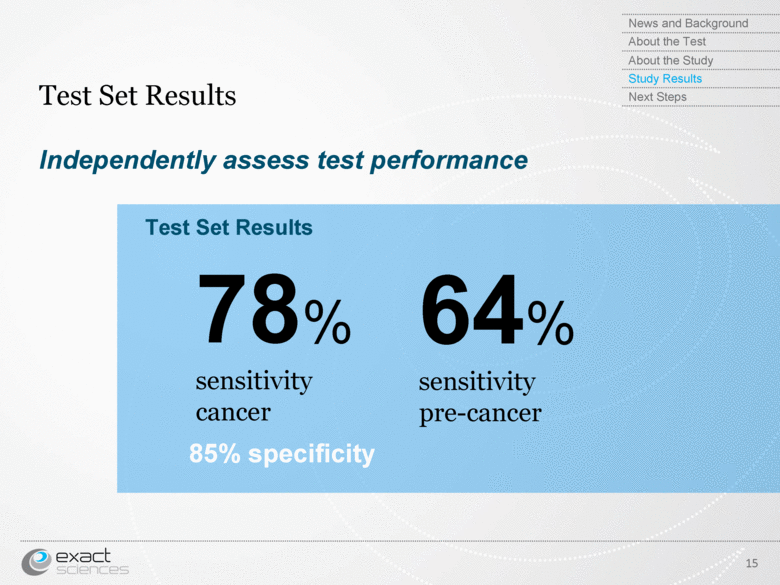
| Independently assess test performance Test Set Results 15 78% sensitivity cancer 64% sensitivity pre-cancer Test Set Results 85% specificity News and Background About the Test About the Study Study Results Next Steps |
| Sensitivity by Cancer Stage 16 News and Background About the Test About the Study Study Results Next Steps Sensitivity (%) Cancer Stage |
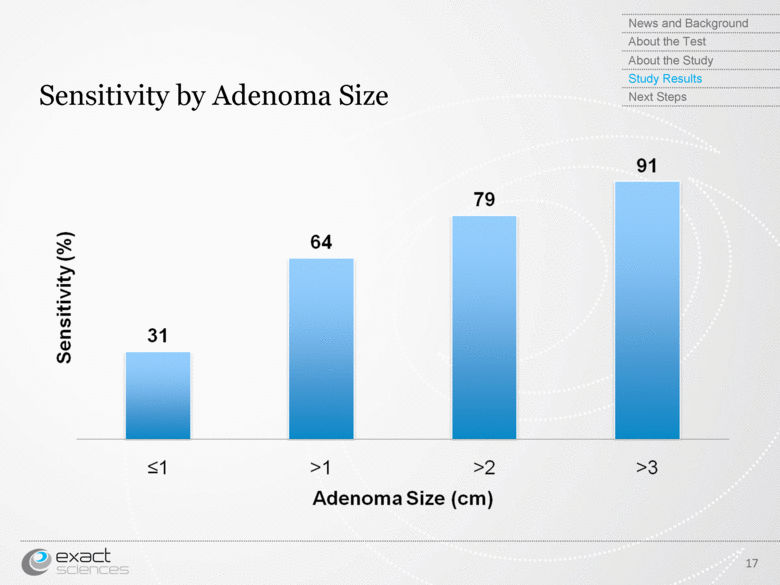
| Sensitivity by Adenoma Size 17 News and Background About the Test About the Study Study Results Next Steps Sensitivity (%) Adenoma Size (cm) |
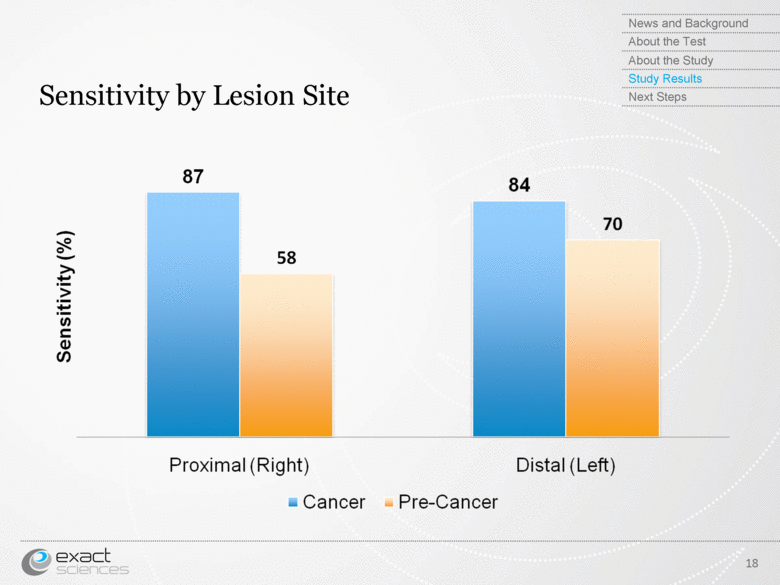
| Sensitivity by Lesion Site 18 News and Background About the Test About the Study Study Results Next Steps Sensitivity (%) Proximal (Right) Distal (Left) Cancer Pre-Cancer |
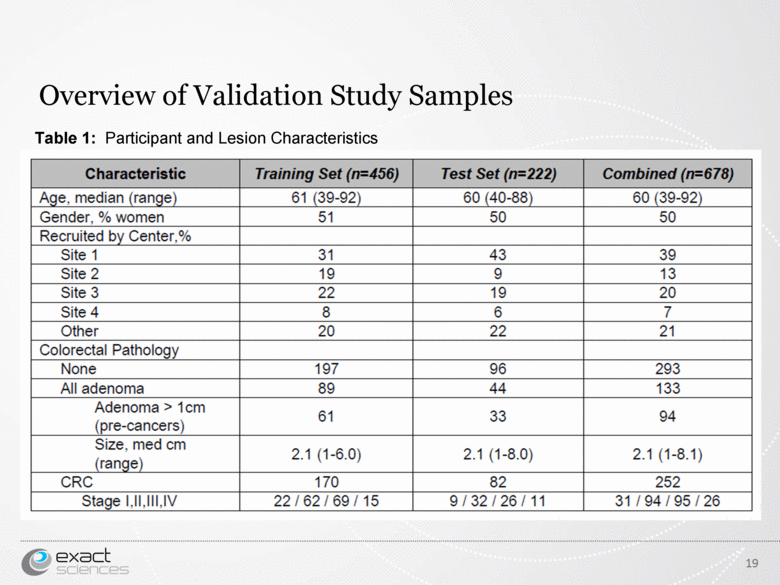
| Overview of Validation Study Samples 19 Table 1: Participant and Lesion Characteristics |
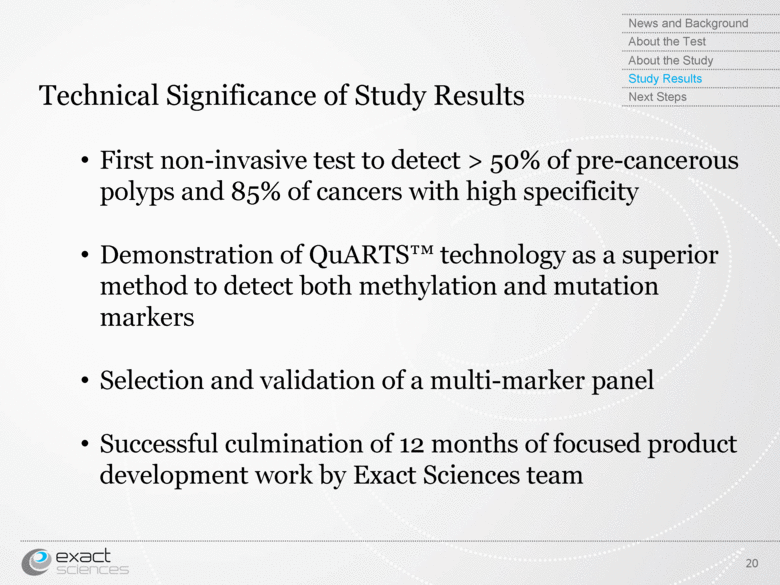
| Technical Significance of Study Results First non-invasive test to detect > 50% of pre-cancerous polyps and 85% of cancers with high specificity Demonstration of QuARTS™ technology as a superior method to detect both methylation and mutation markers Selection and validation of a multi-marker panel Successful culmination of 12 months of focused product development work by Exact Sciences team 20 News and Background About the Test About the Study Study Results Next Steps |
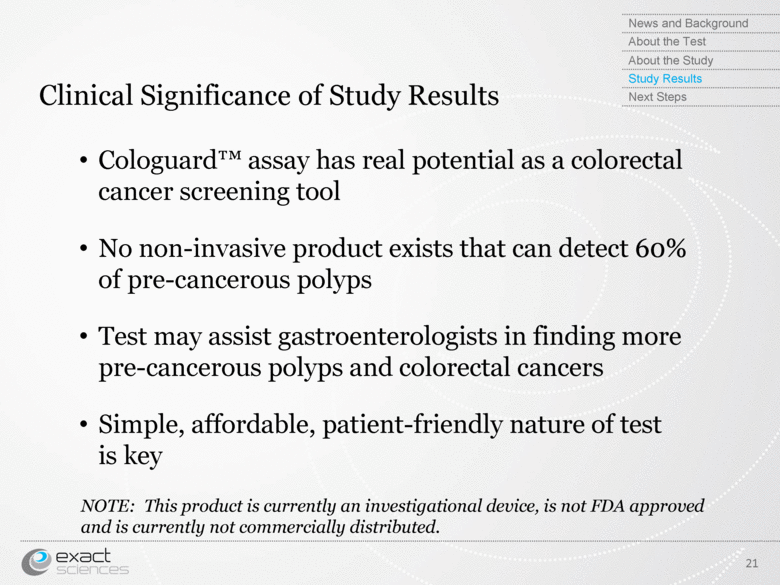
| Clinical Significance of Study Results Cologuard™ assay has real potential as a colorectal cancer screening tool No non-invasive product exists that can detect 60% of pre-cancerous polyps Test may assist gastroenterologists in finding more pre-cancerous polyps and colorectal cancers Simple, affordable, patient-friendly nature of test is key NOTE: This product is currently an investigational device, is not FDA approved and is currently not commercially distributed. 21 News and Background About the Test About the Study Study Results Next Steps |
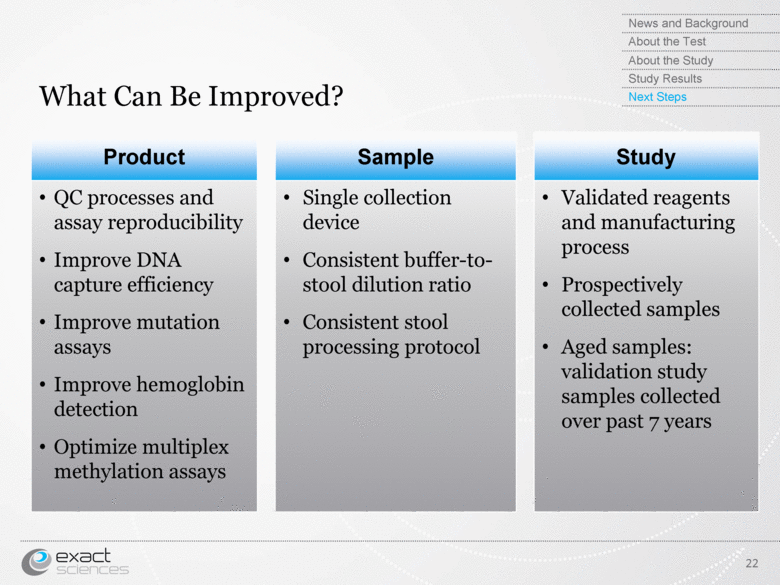
| What Can Be Improved? Product Sample Study QC processes and assay reproducibility Improve DNA capture efficiency Improve mutation assays Improve hemoglobin detection Optimize multiplex methylation assays Single collection device Consistent buffer-to-stool dilution ratio Consistent stool processing protocol Validated reagents and manufacturing process Prospectively collected samples Aged samples: validation study samples collected over past 7 years 22 News and Background About the Test About the Study Study Results Next Steps |
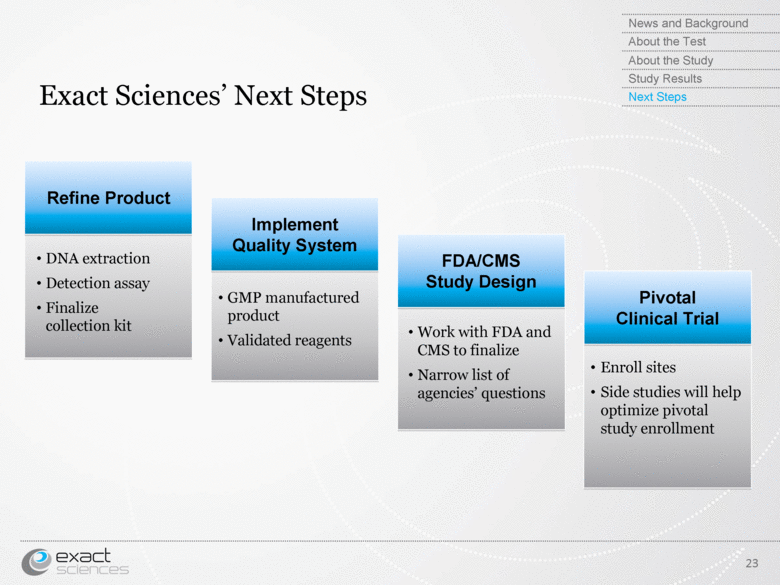
| Exact Sciences’ Next Steps 23 DNA extraction Detection assay Finalize collection kit GMP manufactured product Validated reagents News and Background About the Test About the Study Study Results Next Steps Work with FDA and CMS to finalize Narrow list of agencies’ questions Enroll sites Side studies will help optimize pivotal study enrollment Implement Quality System Refine Product FDA/CMS Study Design Pivotal Clinical Trial |
| Conclusions Tremendous results in achieving targets Strong pre-cancer detection Identified areas to improve product and clinical trial design Potential to improve clinical outcomes Achieved goals of validating assay, technology and markers 24 News and Background About the Test About the Study Study Results Next Steps |
| Thank you to the Mayo Clinic and Exact Sciences employees. Thanks also to Dr. Bert Vogelstein, Dr. Sandy Markowitz, Rob Cascella and Stan Lapidus. This company’s progress would not be possible without them. |
| Questions & Answers |
| Appendix |
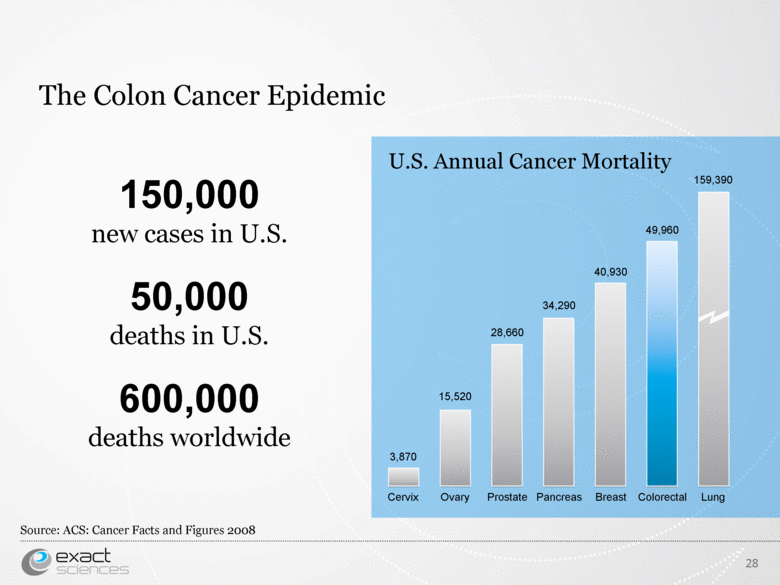
| The Colon Cancer Epidemic 28 150,000 new cases in U.S. 50,000 deaths in U.S. 600,000 deaths worldwide Source: ACS: Cancer Facts and Figures 2008 Ovary Prostate Pancreas Breast Colorectal Lung Cervix 15,520 28,660 34,290 40,930 49,960 159,390 3,870 U.S. Annual Cancer Mortality |