As filed with the Securities Exchange Commission on March 31, 2008
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
| o | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2007
OR
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| o | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
GIVEN IMAGING LTD.
(Exact name of Registrant as specified in its charter)
Israel
(Jurisdiction of incorporation or organization)
Hermon Building, New Industrial Park
Yoqneam 20692, Israel
(Address of principal executive offices)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Ordinary Shares, par value NIS 0.05 per share
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None.
The number of outstanding shares of each of the issuer’s classes of capital or common stock as of December 31, 2007:
29,241,785 Ordinary Shares, par value NIS 0.05 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer o Accelerated filer x Non-accelerated filer o
Indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 o Item 18 x
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
TABLE OF CONTENTS
| | | Page |
PART I | | |
| Item 1. | Identity of Directors, Senior Management and Advisors | 1 |
| Item 2. | Offer Statistics and Expected Timetable | 1 |
| Item 3. | Key Information | 1 |
| Item 4. | Information on the Company | 12 |
| Item 4A. | Unresolved Staff Comments | 30 |
| Item 5. | Operating and Financial Review and Prospects | 30 |
| Item 6. | Directors, Senior Management and Employees | 46 |
| Item 7. | Major Shareholders and Related Party Transactions | 54 |
| Item 8. | Financial Information | 61 |
| Item 9. | The Offer and Listing | 62 |
| Item 10. | Additional Information | 64 |
| Item 11. | Quantitative and Qualitative Disclosures About Market Risk | 75 |
| Item 12. | Description of Securities Other Than Equity Securities | 75 |
| | | |
PART II | | |
| Item 13. | Defaults, Dividend Arrearages and Delinquencies | 76 |
| Item 14. | Material Modifications to the Rights of Security Holders and Use of Proceeds | 76 |
| Item 15. | Controls and Procedures | 76 |
| Item 16. | [Reserved] | 77 |
| Item 16A. | Audit Committee Financial Expert. | 77 |
| Item 16B. | Code of Ethics. | 77 |
| Item 16C. | Principal Accountant Fees and Services | 77 |
| Item 16D. | Exemptions From the Listing Standards for Audit Committees. | 77 |
| Item 16E. | Purchases of Equity Securities by the Issuer and Affiliated Purchasers | 77 |
| | | |
PART III | | |
| Item 17. | Financial Statements | 78 |
| Item 18. | Financial Statements | 78 |
| Item 19. | Exhibits | 78 |
GIVEN, GIVEN & Design, PILLCAM, PILLCAM & Logo, PILLCAM IMAGING CAPSULE & Design, AGILE, RAPID, RAPID ACCESS, ORDERWIN, ORDER WHEN I NEED, FINGERS HOLDING A CAPSULE & Logo, FINGERS HOLDING PILLCAM CAPSULE & Logo, ICCE, ICCE Logos, and International Conference on Capsule Endoscopy are Trademarks and/or Registered Trademarks of Given Imaging Ltd. its subsidiaries and/or affiliates in the United States and/or other countries. All other company or product names are the trademarks or registered trademarks of their respective holders. All rights not expressly granted are reserved.
Not Applicable.
Not Applicable.
The selected consolidated statements of operations data for the years ended December 31, 2005, 2006 and 2007, and the selected consolidated balance sheet data as of December 31, 2006 and 2007, have been derived from our audited consolidated financial statements set forth elsewhere in this Form 20-F. These financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America. The selected consolidated statements of operations data for the years ended December 31, 2003 and 2004, and the selected consolidated balance sheet data as of December 31, 2003, 2004 and 2005, have been derived from our audited consolidated financial statements not included in this Form 20-F, which have been prepared in accordance with generally accepted accounting principles in the United States of America. You should read the selected consolidated financial information set forth below in conjunction with our consolidated financial statements and the related notes as well as Item 5 “Operating and Financial Review and Prospects” included elsewhere in this Annual Report on Form 20-F.
| | | Year ended December 31, | |
| | | 2003 | | 2004 | | 2005 | | 2006(1) | | 2007(1) | |
| | | (in thousands, except share and per share data) | |
Statements of Operations Data: | | | | | | | | | | | | | | | | |
| Revenues | | $ | 40,539 | | $ | 65,020 | | $ | 86,776 | | $ | 95,029 | | $ | 112,868 | |
| Cost of revenues | | | (13,551 | ) | | (17,734 | ) | | (22,070 | ) | | (24,154 | ) | | (29,721 | ) |
| Early repayment of royalty bearing government grants | | | — | | | — | | | — | | | — | | | (4,843 | ) |
| Gross profit | | | 26,988 | | | 47,286 | | | 64,706 | | | 70,875 | | | 78,304 | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development, gross | | | (7,037 | ) | | (7,363 | ) | | (8,833 | ) | | (12,678 | ) | | (12,847 | ) |
| Royalty-bearing government grants | | | 1,303 | | | 1,140 | | | 1,244 | | | 1,867 | | | 1,242 | |
| Research and development, net | | | (5,734 | ) | | (6,223 | ) | | (7,589 | ) | | (10,811 | ) | | (11,605 | ) |
| Sales and marketing | | | (26,804 | ) | | (33,652 | ) | | (43,281 | ) | | (50,732 | ) | | (55,446 | ) |
| General and administrative | | | (5,312 | ) | | (6,916 | ) | | (9,657 | ) | | (16,027 | ) | | (20,981 | ) |
| Termination of marketing agreement | | | — | | | — | | | — | | | — | | | 22,860 | |
| Other | | | — | | | — | | | — | | | — | | | (422 | ) |
| Total operating expenses | | | (37,850 | ) | | (46,791 | ) | | (60,527 | ) | | (77,570 | ) | | (65,594 | ) |
| Operating profit (loss) | | | (10,862 | ) | | 495 | | | 4,179 | | | (6,695 | ) | | 12,710 | |
| Financing income, net | | | 995 | | | 956 | | | 762 | | | 3,980 | | | 5,520 | |
| Profit (loss) before taxes on income and minority share | | | (9,867 | ) | | 1,451 | | | 4,941 | | | (2,715 | ) | | 18,230 | |
| Income tax benefit (expense) | | | — | | | 690 | | | 286 | | | (127 | ) | | (4,548 | ) |
| Profit (loss) before minority share | | | (9,867 | ) | | 2,141 | | | 5,227 | | | (2,842 | ) | | 13,682 | |
| Minority share in losses of subsidiary | | | 258 | | | 747 | | | 1,116 | | | 1,334 | | | 1,503 | |
| Net profit (loss) | | $ | (9,609 | ) | $ | 2,888 | | $ | 6,343 | | $ | (1,508 | ) | $ | 15,185 | |
| Basic earnings (loss) per ordinary share (2) | | $ | (0.38 | ) | $ | 0.11 | | $ | 0.23 | | $ | (0.05 | ) | $ | 0.52 | |
| Diluted earnings (loss) per ordinary share (2) | | $ | (0.38 | ) | $ | 0.10 | | $ | 0.21 | | $ | (0.05 | ) | $ | 0.49 | |
| Weighted average number of ordinary shares used in computing basic earnings (loss) per ordinary share (2) | | | 25,493,073 | | | 26,633,964 | | | 27,781,223 | | | 28,053,849 | | | 28,961,968 | |
| Weighted average number of ordinary shares used in computing diluted earnings (loss) per ordinary share (2) | | | 25,493,073 | | | 29,353,448 | | | 29,695,164 | | | 28,053,849 | | | 31,030,458 | |
| | | As of December 31, | |
| | | 2003 | | 2004 | | 2005 | | 2006 | | 2007 | |
| | | (in thousands) | |
Balance Sheet Data: | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 25,367 | | $ | 80,861 | | $ | 65,356 | | $ | 44,510 | | $ | 37,103 | |
| Short term investments | | | — | | | — | | | 288 | | | 17,245 | | | 23,191 | |
| Working capital | | | 34,970 | | | 92,987 | | | 86,217 | | | 79,015 | | | 74,996 | |
| Marketable securities | | | — | | | — | | | 21,664 | | | 34,769 | | | 41,629 | |
| Total assets | | | 55,569 | | | 124,224 | | | 149,110 | | | 158,177 | | | 177,316 | |
| Long-term liabilities | | | 1,192 | | | 10,984 | | | 24,246 | | | 22,838 | | | 3,938 | |
| Total liabilities | | | 8,847 | | | 28,430 | | | 47,005 | | | 46,892 | | | 41,725 | |
| Accumulated deficit | | | (58,635 | ) | | (55,747 | ) | | (49,404 | ) | | (50,912 | ) | | (35,727 | ) |
| Total shareholders’ equity | | | 44,798 | | | 94,617 | | | 102,044 | | | 107,786 | | | 133,595 | |
(1) | Effective January 1, 2006, we have adopted SFAS 123(R), which requires us to recognize as an expense the grant-date fair value of stock options and other equity-based compensation to employees, over their respective vesting period. Accordingly, our operating expenses in 2006 and 2007 include a total of $5.2 million and $5.7 million, respectively, of additional compensation expense allocated among research and development expenses, marketing expenses and general and administrative expenses based on the division in which the recipient of the option grant is employed. For more information on the adoption of SFAS 123(R), see Note 1K to our consolidated financial statements. |
| (2) | See Notes 1L of the notes to our consolidated financial statements for an explanation of the number of shares used in computing earning (loss) per share data. |
CAUTIONARY LANGUAGE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 20-F contains forward-looking statements within the meaning of the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. We have based these forward-looking statements on our current expectations and projections about future events. These statements include but are not limited to:
| | • | the adequacy of our cash balances and cash flow from operations to support our operations or future growth, in general or for specified periods of time; |
| | | |
| | • | statements as to the potential or expected acceptance of our current and future products by the medical community, particularly gastroenterologists; |
| | | |
| | • | statements as to expected increases in sales, operating results and certain expenses, including research and development and sales and marketing expenses; |
| | | |
| | • | statements as to anticipated reimbursement from U.S. and non-U.S. healthcare payers for our products; |
| | | |
| | • | expectations as to the development of our products and technology, and the timing of enhancements to our products and new product launches; |
| | | |
| | • | expectations as to the market opportunities for the Given System and other products, including capsule endoscopes, for visualizing other sections of the gastrointestinal tract, such as the esophagus, stomach and the colon, as well as our ability to take advantage of those opportunities; |
| | | |
| | • | expectations as to the timing, results and content of future clinical studies and publications; |
| | | |
| | • | statements as to the expected outcome of legal and patent proceedings in which we are involved; |
| | | |
| | • | statements as to the expectation for the content of future publications regarding our products; |
| | • | expectations as to the receipt and timing of regulatory clearances and approvals, and the anticipated timing of sales of the Given System in new markets or for new indications; |
| | | |
| | • | estimates of the impact of changes in currency exchange rates on our operating results; |
| | | |
| | • | expectations as to the adequacy of our inventory of critical components and finished products; |
| | | |
| | • | expectations as to the adequacy of our manufacturing facilities; and |
| | | |
| | • | statements as to our expected treatment under Israeli and U.S. federal tax legislation and the impact that new tax and corporate legislation may have on our operations. |
In addition, forward-looking statements may be, but are not necessarily, identified by the use of forward-looking terminology such as “may,” “anticipates,” “estimates,” “expects,” “intends,” “plans,” “believes,” and words and terms of similar substance. Forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual events, results, performance, circumstances or achievements of the Company to be materially different from any future events, results, performance, circumstances or achievements expressed or implied by such forward-looking statements. Factors that could cause actual events, results, performance, circumstances or achievements to differ from such forward-looking statements include, but are not limited to, the following: (1) our ability to obtain regulatory clearance for our products in one or more countries and adverse changes in regulatory environment, (2) our ability to have successful clinical trials and to provide evidence of the clinical and economic effectiveness of our products, (3) our success in implementing our sales, marketing and manufacturing plans, (4) protection and validity of patents and other intellectual property rights, (5) the impact of currency exchange rates, (6) the effect of competition by other companies, (7) the outcome of future litigation, (8) the reimbursement policies for our product from healthcare payers, (9) quarterly variations in operating results, (10) the possibility of armed conflict or civil or military unrest in Israel, and (11) other risks and factors disclosed in our filings with the U.S. Securities and Exchange Commission, including, but not limited to, risks and factors identified under such headings as “Risk Factors”, “Cautionary Language Regarding Forward-Looking Statements” and “Operating and Financial Review and Prospects” in this Annual Report on Form 20-F for the year ended December 31, 2007. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report. Except for the Company’s ongoing obligations to disclose material information under the applicable securities laws, it undertakes no obligation to release publicly any revisions to any forward-looking statements, to report events or to report the occurrence of unanticipated events.
RISK FACTORS
If we are unable to manufacture, market or sell the PillCam capsules, our revenues may decline significantly or we may not be able to maintain our expected annual growth rate.
A substantial portion of our revenues and our annual revenue growth to date has resulted from sales of the PillCam SB capsule. We expect that a substantial majority of our revenues for the foreseeable future will continue to come from sales of the PillCam SB capsule. Sales of the PillCam SB capsule contributed $62.5 million, or 72%, in 2005, $76.4 million, or 80%, in 2006 and $90.6 million, or 80% in 2007. In addition, we expect sales of other PillCam capsules, such as PillCam ESO and PillCam COLON, to increasingly contribute to our revenues in the future. If we are unable to manufacture, market or sell the PillCam capsules, and PillCam SB in particular, for any reason, including, for example, product recall, natural disaster, unavailability of components, war in Israel or as a result of a legal action against us, our revenues may decline significantly or we may not be able to maintain our expected annual growth rate.
If we fail to increase utilization of our workstations and recurring orders of our PillCam SB capsule, we may not be able to achieve the growth rate we expect.
Since sales of our PillCam SB capsule accounts for a substantial majority of our revenues, the level of recurring orders of our PillCam SB capsule by our customers is an important factor in growing our revenues. We are seeking to increase the level of recurring orders by a number of methods directed to increasing utilization of the PillCam capsules by physicians, including focused selling and marketing activities, frequent contact with our customers, improving reimbursement coverage, generating supporting clinical evidence to expand indications and educating physicians regarding the clinical benefits of the PillCam capsule, increasing operating efficiencies of our system to the benefit of physicians and collaborating with strategic industry participants. Increasing the level of recurring orders by our customers is also important to attracting new customers to purchase and use the Given System. If we are unable to increase utilization of our workstations and the level of recurring orders of our PillCam SB capsule, we may not be able to achieve the revenues necessary to maintain our growth rate.
If the number of workstations or capsules we sell declines, we may not be able to achieve broader market penetration or may choose to place systems in the market at a discounted price, and our revenues and gross margins may be negatively affected.
Our principal product is the Given System, consisting of a line of PillCam capsules and the related data recorder and computer workstation with our proprietary RAPID software. As of December 31, 2007, we had an installed base of nearly 4,250 systems worldwide. Growth in our installed base of Given Systems has contributed to our revenue growth. Our success in making or increasing sales of the Given System depends on a number of factors. First, we must demonstrate that the Given System is clinically-effective and cost-effective in diagnosing a range of disorders of the gastrointestinal tract, and we must inform and educate both physicians and the third-party payers responsible for providing reimbursement coverage about the clinical and economic benefits of using the Given System. Second, we must broaden the applications for which the use of the Given System is reimbursed, expand the number of people with such reimbursement coverage and educate healthcare providers as to the clinical and economic benefits of using the Given System for such broader indications so that they are encouraged to adopt the Given System. Third, we must continue to improve operational efficiencies of the Given System and demonstrate and convince gastroenterologists that the Given System fits within their normal practice routines. If the number of workstations or capsules we sell declines, we may not be able to achieve broader market penetration or may choose to place them in the market at a discounted price, in which case our revenues and gross margins will be negatively affected.
If we are unable to expand reimbursement coverage from third-party healthcare payers for procedures using the Given System, or if reimbursement is insufficient to create an economic benefit for purchasing or using the Given System when compared to alternative procedures, demand for the Given System and PillCam capsules may not grow at the rate we expect.
Demand for the Given System depends significantly on the eligibility of the procedures performed using the Given System for reimbursement through government-sponsored healthcare payment systems and private third-party payers. Reimbursement practices vary significantly from country to country and within some countries, by region, and we must obtain reimbursement approvals on a country-by-country and region-by-region basis. In general, the process of obtaining reimbursement coverage approvals has been longer outside of the United States. Historically, we have experienced higher sales in territories in which we have received reimbursement coverage for capsule endoscopy using the Given System and in territories in which health authorities and regulators approved the marketing or use of capsule endoscopy. We may not be able to obtain further approvals in a timely manner or at all and existing reimbursement coverage policies may be revised from time to time outside of our control by third-party payers. If physicians, hospitals and other healthcare providers are unable to obtain sufficient coverage and reimbursement from third-party payers for procedures using the Given System, or if reimbursement is, or is perceived by our customers to be, insufficient to create an economic incentive for purchasing or using our product or does not adequately compensate physicians and health care providers compared to the other procedures they offer, demand for the Given System and the PillCam capsules may not grow at the rate we expect.
Our future growth depends in part on our ability to market the PillCam SB capsule as a primary endoscopic diagnostic tool for a variety of disorders of the small intestine.
The PillCam SB capsule has been cleared for marketing by the FDA for the detection of disorders of the small intestine. In recent years, sales of PillCam SB capsules have accounted for the significant majority of our revenues. Our ability to market and sell our line of PillCam capsules is highly dependent on the availability and adequacy of third party reimbursement for the procedures performed with the Given System. Most reimbursement policies today cover the small bowel capsule endoscopy procedure only after a previous procedure, such as endoscopy or radiology, has been performed and many of them contain other requirements making coverage for indications beyond obscure gastrointestinal bleeding, or OGIB, such as suspected Crohn’s disease, more difficult to obtain for our customers. Our ability to expand the use of the PillCam SB capsule depends substantially on our ability to convince third party payers to provide reimbursement coverage for the PillCam SB capsule as a primary diagnostic tool, without the requirement to perform a prior procedure, such as endoscopy or radiology, and to provide favorable and effective reimbursement coverage for the PillCam SB capsule for small bowel indications beyond OGIB. If we are unable to obtain such reimbursement coverage, sales of the PillCam SB capsule may not increase as we expect.
If we are unable to expand the market for the PillCam ESO capsule and obtain adequate third-party reimbursement for the esophageal capsule endoscopy procedure, sales of the PillCam ESO capsule may not grow as we expect or at all.
Our ability to market and sell our PillCam ESO capsule depends significantly on our ability to expand the use of the PillCam ESO capsule. To date, PillCam ESO has been used primarily in the detection of esophageal varices, a condition prevalent in patients suffering from cirrhosis, a chronic liver disease. As of January 31, 2008, approximately 31 million individuals in the United States had reimbursement coverage for using the esophageal capsule endoscopy procedure in the detection of esophageal varices. However, due to the limited prevalence of esophageal varices in the general population, we believe the market opportunity for the use of the PillCam ESO capsule in the detection of varices is limited. We believe that the primary market opportunity for our PillCam ESO capsule may be in the detection of Gastro-Esophageal Reflux Disease, or GERD, which is more prevalent in the general population than varices. However, in order to successfully market and sell the PillCam ESO capsule in the detection of GERD we must first generate and present clinical data supporting this use and, subsequently, to obtain adequate reimbursement coverage. Our ability to expand the use of the PillCam ESO capsule depends substantially on our ability to convince additional third party payers to provide reimbursement coverage for this capsule for the varices indication, the adequacy of such coverage and our ability to generate and present clinical data supporting reimbursement for the GERD indication. If we are unable to do so, sales of the PillCam ESO capsule may not grow as we expect or at all.
If we are not successful in taking over marketing and sales of the PillCam ESO capsule in the United States following termination of our exclusive agreement with Ethicon Endo-Surgery, sales of our PillCam ESO capsule may not grow as we expect or at all.
Until recently, InScope, a division of Ethicon Endo-Surgery, a Johnson & Johnson company, held exclusive rights to market our PillCam ESO capsule in the United States since this capsule received regulatory clearance in November 2004. In November 2007, Ethicon terminated the agreement with us and we regained all rights in connection with the PillCam ESO capsule in January 2008. Consequently, our future growth depends in part on our success in transitioning sales and marketing activities from Ethicon to us. Sales of the PillCam ESO capsule in 2007 were insignificant and may continue to be lower than we expect if we are not successful in marketing the PillCam ESO capsule. This may negatively impact our revenue growth.
If we are unable to market and sell our PillCam COLON capsule we may miss a significant market opportunity and may not grow as we expect.
In 2007, we began limited sales of our new PillCam COLON capsule in Europe. We intend to market and sell this capsule as a complementary tool for traditional colonoscopy for patients who are unable or unwilling to undergo traditional colonoscopy or have incomplete colonoscopy. However, in the United States the FDA has recently determined that the PillCam COLON is not substantially equivalent to any marketed device in the United States for visualization of the colon and therefore can not be cleared for marketing in the United States, our biggest market, through the 510(k) process based on the currently available clinical data. There can be no assurance that we will be able to receive FDA clearance for this capsule in the foreseeable future or at all or that the PillCam COLON will be accepted as comparable or superior to existing technologies for visualization of the colon. Our ability to market and sell the PillCam COLON successfully depends on one or more of the following:
| | • | Receipt of FDA marketing clearance in the United States: We cannot be sure that FDA clearance or other regulatory approvals will be granted. In order to obtain FDA clearance and other regulatory approvals, we will be required to demonstrate that the PillCam COLON is safe and effective for its intended purpose. |
| | | |
| | • | The existence of clinical data sufficient to support the use of the PillCam COLON for visualization of the colon as compared to other colon visualization methods: If clinical trials indicate that PillCam COLON is not as clinically-effective as other current methods, or if the PillCam COLON procedure causes unexpected complications or other unforeseen negative effects, we may not obtain regulatory clearance to market and sell this capsule or physicians may be reluctant to use it. |
| | | |
| | • | The availability of sufficient clinical and cost-effectiveness data for the American Medical Association, or AMA, to provide a favorable permanent “current procedural terminology”, or CPT, code and for private third-party payers to make an adequate reimbursement decision to provide coverage for the PillCam COLON procedure. |
| | | |
| | • | The availability of a reliable colon cleansing and preparation procedure for the PillCam COLON capsule, which is accepted by physicians and patients. |
If we are unable to achieve one or more of the above, we may not be able to market and sell the PillCam COLON capsule or the demand for the PillCam COLON may be lower than expected and sales of PillCam COLON may not contribute to our growth at the rate we expect or at all.
We face direct competition from manufacturers of capsule endoscopy systems and may lose market share if we are unable to compete effectively in the marketplace.
Olympus Corporation has a competing capsule endoscopy system for the small bowel, which it is selling in the United States, Europe and Australia. In addition, we believe that other companies in Korea and China began selling capsule endoscopy systems for the small bowel in Asian and possibly other countries outside the United States and are selling these systems at a lower price than ours. If we are unable to compete effectively in the marketplace against the Olympus capsule endoscopy system and other competing systems, we may lose market share, experience delays in completing sales as a result of longer decision making process among potential customers, or experience erosion of our gross margins as a result of growing price pressure.
We face competition from large, well-established manufacturers of existing technologies for detecting gastrointestinal disorders, as well as from gastrointestinal products in general which compete for the limited capital expenditure budgets of customers.
Competition for the Given System also comes from existing technologies for detecting gastrointestinal disorders and diseases. Existing technologies include traditional endoscopy and radiological imaging. The principal manufacturers of gastrointestinal endoscopes are Olympus, Hoya, and Fujinon. The principal manufacturers of equipment for radiological imaging are General Electric Healthcare Systems, Siemens Medical Solutions, a division of Siemens AG, Philips Medical Systems Ltd., Toshiba Corporation and Shimadzu Corporation for x-ray equipment. These companies have substantially greater financial resources than we do, and they have established reputations as well as worldwide distribution channels for medical instruments to physicians. If we are unable to convince physicians to adopt the Given System over the current technologies marketed by our competitors, our results of operations may suffer. For details regarding our cooperation agreement with Fujinon, see “Item 4 – Business Overview – Competition.”
In addition to competition from products performing similar clinical functions to the Given System, there is also competition for the limited capital expenditure budgets of customers. Another capital equipment item for gastroenterology may compete with our system for the same capital budget, which is typically limited, and therefore the potential purchaser may be required to choose between the two items of capital equipment. If we are unable to market the Given System more effectively than other products which could be purchased using the same budget as the Given System, we may be unable to maintain our current growth rate.
Because of the importance of our patent portfolio to our business, we may lose market share to our competitors if we fail to protect our technology.
Protection of our technology is key to our future success. We rely on patent protection, as well as a combination of copyright, trade secret, design and trademark laws, nondisclosure and confidentiality agreements and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Currently, many of our patent applications are still pending and we will be able to use them to protect our technology against potential competitors only after they are issued. The process of issuing a patent may sometimes be lengthy and may not always result in issued patents in a form that will be advantageous to us or at all. Our patents and applications cover particular aspects of our products and technology and may be challenged, invalidated or circumvented by third parties. There may be other effective technologies, designs or methods relating to capsule endoscopy. If other effective methods are not covered by our patents or applications and our competitors are able to commercialize products using these methods, it could have an adverse effect on our sales. In addition, our competitors may obtain patents that will prevent us from using technologies, designs or methods we would like to integrate into our products. We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by former employees. Laws protecting intellectual property only provide protection in the jurisdiction they are enacted. The laws and judicial systems of foreign countries may not protect or enable enforcement of our intellectual property rights to the same extent as the laws of the United States. If we do not adequately protect our intellectual property, our competitors or other parties could make products similar to ours and compete more efficiently with us, which could result in a decrease in our market share. For information regarding our patent litigation with Olympus, see Item 8 – Legal Proceedings.
If we are unable to successfully manage the introduction of new or improved products into the market, our operating results may be negatively affected.
We began marketing and selling the Given System in August 2001. Since then, we have introduced new products or significant improvements to existing components of the Given System frequently. Newer products or system components may not be able to support and work with older product versions. For example, our PillCam COLON capsule only works with RAPID 5.0 or newer versions of the RAPID software. In order for as many of our customers as possible to utilize our most advanced capsule endoscopy technology, we need to manage new product introductions and installations efficiently, addressing concerns of customers regarding product upgrade costs, time constraints and training and education in light of possible short product cycles. If we are unable to cause our customers to use the most advanced technology available or effectively address compatibility issues between older and newer products, we may harm our competitive position and our sales may be negatively affected. In addition, a failure to successfully manage the transition to newer products, may result in obsolete inventory of older products, which we may be required to write off. This will negatively affect our operating results.
If we are unable to introduce new capsules and products for use in the gastrointestinal tract our growth may be negatively affected.
Our objective is to expand the use of the Given System as a platform to be used with a variety of products and indications. We intend to add to our current PillCam capsules by developing and introducing new capsules and products. There can be no assurance that we will be able to develop new products that will enjoy widespread market acceptance as superior to existing technologies for detection of abnormalities in other parts of the gastrointestinal tract or that can be used in other parts of the gastrointestinal tract. In addition, we will be required to obtain FDA clearance in the United States and other regulatory approvals outside of the United States before commercially distributing the Given System for use in other parts of the gastrointestinal tract or introducing new products for use in the gastrointestinal tract. These regulatory processes can be lengthy and expensive and we cannot be sure that FDA clearance or other regulatory approvals will be granted. In order to obtain FDA clearance and other regulatory approvals, and to obtain reimbursement coverage for use of new products, we may be required to conduct additional clinical trials to demonstrate the diagnostic and cost-effectiveness of these new products. If future clinical trials indicate that new products are not as clinically-effective or cost-effective as current methods, or that it may cause unexpected complications or other unforeseen negative effects, we may not obtain regulatory clearance to market and sell these new products or obtain reimbursement coverage, and our growth would be adversely affected.
Any disruption in the United States, our primary market for our products, may result in a material reduction in our revenues and negatively affect our operating results.
Since we began selling the Given System in 2001, most of our revenues have been generated from sales in the United States. Sales in the United States accounted for $63.9 million, or 74%, of our revenues in 2005, $66.4 million, or 70%, of our revenues in 2006 and $72.3 million, or 64%, in 2007. Any disruption to our operations or market in the United States resulting from changes in management or the sales team of our U.S. subsidiary, adverse changes in reimbursement policies, new regulatory requirements, macro-economic changes and other events, many of which are outside our control, may result in a material reduction in our revenues and negatively affect our operating results.
If we are unable to successfully market and sell our products in Japan, one of our significant growth opportunities may be materially adversely affected.
In April 2007, we received regulatory clearance to market our capital equipment and PillCam SB capsule in Japan and subsequently initial reimbursement coverage for the PillCam SB capsule was announced for indications involving obscure gastrointestinal bleeding. Suzuken Co. Ltd., one of Japan’s largest pharmaceutical and medical device distributors, has exclusive rights to distribute our products in Japan. Suzuken also owns 15% of our Japanese subsidiary. Consequently, our future growth in the Japanese market depends significantly on the success of Suzuken and on our business relationship with Suzuken. In addition, marketing our other products in Japan will require additional, product-specific regulatory clearances. In particular, the current regulatory clearance we have obtained in Japan covers an early version of our data recorder of which we now have only limited inventory remaining. We have applied for clearance for our more advanced version of data recorder, similar to the version we sell in the United States and Europe. Generally, the process for obtaining marketing clearance for medical devices in Japan could range from six months for products with only very minor modifications from previous cleared product versions to a few years in case of a completely new device. There is no assurance that we will receive regulatory clearances in Japan for any of our additional products. Finally, our main competitor in the field of capsule endoscopy is based in Japan and competition may be intense once it enters the Japanese market. If we are unable to successfully market and sell our products in Japan for any of the foregoing or other reasons, one of our significant growth opportunities will be materially adversely affected.
Because the medical device industry is litigious, we are susceptible to intellectual property suits that could cause us to incur substantial costs or pay substantial damages or prohibit us from selling our products.
There is a substantial amount of litigation over patent and other intellectual property rights in the medical device industry. We are currently a party to patent litigation in the United States against Olympus Corporation and its affiliates. For a detailed description, see “Item 8 – Financial Information – Legal Proceedings.”
Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often uncertain. While we have attempted to ensure that the Given System does not infringe other parties’ valid patents and proprietary rights, searches typically performed to identify potentially infringed patents of third parties are not always conclusive and, because patent applications can take many years to issue, there may be applications now pending of which we are unaware, which may later result in issued patents which our current or future products may infringe. In addition, our competitors or other parties may assert that our product and the methods it employs may be covered by patents held by them. If our products infringe a valid patent, we could be prevented from selling them unless we can obtain a license or redesign the product to avoid infringement. A license may not always be available or may require us to pay substantial royalties. We also may not be successful in any attempt to redesign our product to avoid any infringement. Infringement and other intellectual property claims, with or without merit, can be expensive and time-consuming to litigate and can divert management’s attention from operating our business.
We are subject to extensive regulation by the FDA which could restrict the sale and marketing of the Given System and could cause us to incur significant costs.
FDA regulations may require us to submit for clearance improvements and modifications of the Given System, including new or improved PillCam capsules and new or improved RAPID software versions, before we are allowed to market them in the United States. FDA regulations also prohibit us from promoting or advertising our cleared products for uses not within the scope of our clearances or making unsupported safety and effectiveness claims. Currently, the Given System has been cleared by the FDA for the detection of abnormalities of the small intestine and the esophagus. Noncompliance with applicable regulatory requirements can result in enforcement action which may include recalling products, ceasing product marketing, paying significant fines and penalties, and similar FDA actions which could limit product sales or delay or halt product shipment. Additionally, if we are unable to receive FDA clearance for new or improved products, such as PillCam COLON, the marketing and sale of these products will be delayed or cancelled, which in turn may materially adversely affect our growth potential. Unanticipated changes in existing regulatory requirements or adoption of new requirements could also materially adversely affect our financial condition and results of operations.
We are required to adhere to the FDA’s Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. The FDA also requires us to adhere to the Quality System Regulation, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging and shipping of the Given System. The FDA enforces the Quality System Regulation through inspections. Our quality system is also subject to the Medical Devices Directive of the European Union requiring compliance with the International Standard Organization’s standard ISO 9001:2000, ISO 13485:1996 and ISO 13485:2003, which are quality standards setting forth requirements for medical device manufacturers that are more specific than the general requirements specified in ISO 9001. If we fail a Quality System Regulation inspection by the FDA or any other regulator, our operations could be disrupted and our manufacturing delayed. Failure to take adequate corrective action in response to a Quality System Regulation inspection could force a shutdown of our manufacturing operations and a recall of the Given System, which would have a material adverse effect on our product sales, financial condition and results of operations.
If we or our distributors do not obtain and maintain the necessary regulatory approvals in a specific country or region, we will not be able to market and sell the Given System in that country or region.
In addition to the United States, Germany, France, Australia and Israel, where we market and sell the Given System directly with our own direct sales and marketing organizations, we sell the Given System in over 60 other countries. To be able to market and sell the Given System in a specific country or region, we or our distributors must comply with the regulations of that country or region. While the regulations of some countries do not impose barriers to marketing and selling the Given System or only require notification, others require that we or our distributors obtain the approval of a specified regulatory body. These regulations, including the requirements for approvals, and the time required for regulatory review vary from country to country. Obtaining regulatory approvals is expensive and time-consuming, and we cannot be certain that we or our distributors will receive regulatory approvals for all of our products in each country or region in which we plan to market our products. If we modify the Given System, we or our distributors may need to apply for new regulatory approvals before we are permitted to sell it. We may not continue to meet the quality and safety standards required to maintain the authorizations that we or our distributors have received. If we or our distributors are unable to maintain our authorizations in a particular country or region, we will no longer be able to sell our products in that country or region, and our ability to generate revenues will be materially adversely affected.
Our failure to comply with radio frequency regulations in a specific country or region could impair our ability to commercially distribute and market the Given System in that country or region.
The Given System includes a wireless radio frequency transmitter and receiver, and is therefore subject to equipment authorization requirements in a number of countries and regions. In the United States, Europe and Japan, authorities require advance clearance of all radio frequency devices before they can be sold or marketed in these jurisdictions. Modifications to the approved system design and specifications may require new or further regulatory approvals before we are permitted to market and sell a modified system. If we are unable to maintain our current approvals or obtain any additional required approvals from the authorities responsible for the radio frequency regulations in these and other jurisdictions where we sell the Given System, an enforcement action could be brought to prevent the sale or use of the Given System in these countries. Any such action could negatively affect our results of operations.
Some of our activities may subject us to risks under federal and state laws prohibiting “kickbacks” and false or fraudulent claims.
Certain U.S. federal and state laws, including but not limited to the federal anti-kickback statute, prohibit, among other things, the offer, payment, solicitation or receipt of any form of remuneration in return for the referral of healthcare items or services reimbursable by a federal or state health care program such as Medicare or Medicaid. While the federal anti-kickback statute applies only to products or services for which payment may be made in whole or in part by a federal or state health care program, state laws often also apply to private third party payers such as commercial insurance plans. Other federal and state laws, including the Federal False Claims Act, prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment to Medicare, Medicaid, or other third-party payers that are false or fraudulent, or are for items or services that were not provided as claimed.
These laws may apply to the sales, marketing and other promotional activities of manufacturers of medical devices, such as us, and may limit the kinds of financial arrangements we may have with hospitals, physicians, and other potential purchasers of medical devices. The anti-kickback statute, similar federal and state laws, and false claims laws prescribe substantial civil and criminal penalties for noncompliance. A government action against us under one of these legal authorities could result in financial and other penalties, delay or prohibit sales of some or all of our products or services and, even if unsuccessful, could cause adverse publicity and be costly to respond to, and thus could have a material adverse effect on our business, results of operations and financial condition.
We rely on local distributors to market and distribute the Given System in most of the territories in which we sell it.
With the exception of Australia, France, Germany, Israel and the United States, we rely on distributors for the marketing and distribution of the Given System. Under most of our agreements with local distributors, a distributor is granted the right to market the Given System for a specified period in a particular country or region, subject to the attainment of minimum sales targets. The distributor is required to prepare and submit to us for our approval a sales plan for the Given System and to obtain the requisite regulatory and reimbursement approvals for the Given System. Our success in generating sales in countries or regions where we have engaged local distributors depends in part on the efforts of others whom we do not control. In 2007, we derived $24.7 million, or 21.8%, of our revenues from sales to local distributors, compared to $15.7 million, or 16.5%, from sales to local distributors in 2006. To date, we have changed a number of our distributors due to a failure to meet minimum sales targets and other reasons. If a distributor is terminated by us or goes out of business, it may take us a period of time to locate an alternative distributor and to train its personnel to market the Given System and our ability to sell the Given System in that distributor’s country or region could be adversely affected.
Our reliance on single source suppliers could harm our ability to meet demand for the Given System in a timely manner or within budget.
We depend on single source suppliers for some of the components necessary for the production of the Given System. For example, we have sole suppliers for the imaging sensor and transmitter of our PillCam capsules. If the supply of these components is disrupted or terminated, or if these suppliers are unable to supply the quantities of components that we require, we may not be able to find alternative sources for these key components. Although we maintain a strategic inventory of key components, the inventory may not be sufficient to satisfy the demand for our products if supply is interrupted, and is subject to risk of loss due to catastrophic events such as fire at a storage facility. As a result, we may be unable to meet demand for our products, which could harm our ability to generate revenues, lead to customer dissatisfaction and damage our reputation. If we are required to change the manufacturer of any of these key components of the Given System, there may be a significant delay in locating a suitable alternative manufacturer. Additionally, we may be required to verify that the new manufacturer maintains facilities and procedures that comply with FDA and other applicable quality standards and with all applicable regulations and guidelines. The delays associated with the selection of a new manufacturer could delay our ability to manufacture our product in a timely manner or within budget. Furthermore, in the event that the manufacturer of a key component of our product ceases operations or otherwise ceases to do business with us, we may not have access to the information necessary to enable another supplier to manufacture the component. The occurrence of any of these events could harm our ability to meet demand for the Given System in a timely manner or within budget.
Conditions in Israel affect our operations and may limit our ability to produce and sell our product which could decrease our revenues.
Our corporate offices, manufacturing facilities (other than our PillCam SB backup production line in Ireland), and research and development facilities are located in Israel. Political, economic and military conditions in Israel may directly affect our operations. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors and a state of hostility, varying in degree and intensity, has led to security and economic problems for Israel. Any escalation in these hostilities or any future armed conflict, political instability or violence in the region, could prevent us from using our corporate offices and primary manufacturing facility in Israel, harm our ability to manufacture and sell our products.
If we lose our key personnel or are unable to attract and retain additional personnel, our business and ability to compete will be harmed.
We are dependent on the principal members of our management, scientific staff and sales team. In order to implement our business strategy, we will need to keep our key personnel with expertise in research and development, clinical testing, government regulation, manufacturing, sales, marketing and finance. Our product development plans depend in part on our ability to retain engineers with expertise in a variety of technical fields. The loss of a number of these persons or our inability to attract and retain qualified personnel could harm our business and our ability to compete.
Our operations could be disrupted as a result of the obligation of key personnel in Israel to perform military service.
Generally, all male adult citizens and permanent residents of Israel between ages 21 and 45 are obligated, unless exempt, to take part in military reserve duty annually. Many of our male employees are currently obligated to perform annual reserve duty. Additionally, all Israeli residents who perform reserve duty are subject to being called to active duty at any time under emergency circumstances. Our operations could be disrupted by the absence for a significant period of one or more of our officers or employees due to military service. Any such disruption to our operations could delay our business plans.
Our international operations will expose us to the risk of fluctuations in currency exchange rates.
In 2007, we derived 67.5% of our revenues in U.S. dollars and 23.3% in Euro, with the remainder denominated in Japanese Yen and other currencies. The currency denomination of our revenues depends on the location of the customer or the distributor used to fulfill our customers’ orders. Conversely, in 2007, in addition to our U.S. dollar and Euro-denominated liabilities, 31% of our expenses were denominated in New Israeli Shekels, or Shekels. Our Shekel-denominated liabilities consist principally of salaries and related personnel expenses. We anticipate that for the foreseeable future a material portion of our liabilities will continue to be denominated in Shekels. If the value of a currency in which our receivables are denominated devalues against the value of a currency in which our liabilities are denominated, there will be a negative impact on our operating margins, as well as on our net income. Our revenues and expenses may not always be fully hedged against our currency exposure through financial instruments. In addition, if we wish to maintain the dollar-denominated value of our products in non-U.S. markets, devaluation in the local currencies of our customers relative to the U.S. dollar could cause our customers to cancel or decrease orders or default on payment. In addition, as of December 31, 2007, 38% of our cash and cash equivalents were denominated in currencies other than the U.S. dollar and we are therefore subject to the risk of exchange rate fluctuations among the U.S. dollar, the Yen, the Shekel, the Australian dollar and the Euro.
Recent global economic market conditions may negatively affect our liquidity and financial results.
As of December 31, 2007, we had $37.1 million in cash and cash equivalents, which were held in bank accounts and deposits with maturities of three months or less located with a number of high rated banks inside and outside of Israel. In addition, as of December 31, 2007, we had $41.6 million invested in marketable securities and $23.1 million invested in short term investments. Approximately two thirds of these cash balances were invested in securities issued by the United States government or its agencies and in AAA money market funds. The remainder was held in corporate bonds and commercial paper that were highly-rated by rating agencies at the time of investment. Our cash and investments are subject to general credit, liquidity, market and interest rate risks, which may be exacerbated by the dislocation that has affected various sectors of the financial markets since the middle of 2007 and caused credit and liquidity issues for a number of reputable financial institutions. These risks associated with our investment portfolio may have a negative effect on our liquidity and financial results.
The use of the Given System, including ingestion of the PillCam capsules, could result in product liability claims that could be expensive, damage our reputation and harm our business.
Our business exposes us to an inherent risk of potential product liability claims related to the manufacturing, marketing and sale of medical devices. The medical device industry has historically been litigious, and we face financial exposure to product liability claims if the use of our product were to cause or contribute to injury or death, whether by aggravating existing patient symptoms or otherwise. There is also the possibility that defects in the design or manufacture of the Given System might necessitate a product recall. Although we maintain product liability insurance for the Given System, the coverage limits of these policies may not be adequate to cover future claims. In the future, we may be unable to maintain product liability insurance on acceptable terms or at reasonable costs and such insurance may not provide us with adequate coverage against potential liabilities. A product liability claim, regardless of merit or ultimate outcome, or any product recall could result in substantial costs to us, damage to our reputation, customer dissatisfaction and frustration and a substantial diversion of management attention. A successful claim brought against us in excess of, or outside of, our insurance coverage could have a material adverse effect on our financial condition and results of operations.
The price of our shares could fluctuate significantly as a result of a number of factors, including varying quarterly financial performance or our failure to meet our guidance or the expectations of analysts or investors, which may lead to additional volatility in our share price.
Our ordinary shares commenced trading on the Nasdaq Global Market in October 2001 and on the Tel Aviv Stock Exchange in March 2004. In 2007, the closing price of our shares has ranged from $19.25 to $31.49 per share on the Nasdaq Global Market and NIS 81.47 to NIS 135.90 on the Tel Aviv Stock Exchange. The price of our shares could fluctuate significantly for, among other things, the following reasons: future announcements concerning us or our competitors, the existence and outcome of litigation concerning our intellectual property assets, changes in third-party reimbursement practices, regulatory developments, and new clinical or economic data regarding our current or future products. In addition, it is our practice to provide guidance to the market as to our expected revenues and earnings per share based on information available to us at the time of the guidance. If our operating results do not meet our guidance or the expectations of securities analysts or investors, the price of our shares would likely decline. In addition, based on our experience to date, we believe that many of our customers delay purchasing our products until the end of the fiscal quarter because they believe this will enable them to negotiate more favorable terms. Therefore, revenues from sales are concentrated at the end of each fiscal quarter making it difficult for us to determine the success of each quarter until its end. This may result in lower than expected quarterly revenues if external or other events cause potential customers to defer their purchasing decisions even for a short period of time. Furthermore, we believe that demand for systems and capsules may be materially affected by seasonal factors during the summer months when physicians and administrators are more likely to postpone purchasing decisions due to summer vacations and patients are more likely to postpone less urgent diagnostic procedures until later in the year. Both of these factors may result in slower sales during the summer. Share price fluctuations may be exaggerated by low trading volume of our ordinary shares and changes in trading practices in our ordinary shares, such as short selling. Securities class action litigation has often been brought against companies following periods of volatility in the price of their shares. Any securities litigation claims brought against us could result in substantial expense and divert management’s attention from our business.
The largest beneficial owner of our shares, IDB Holding Corporation Ltd., has significant influence over matters requiring shareholder approval.
The largest beneficial owners of our shares, four individuals in Israel who together control IDB Holding Corporation Ltd., beneficially owned approximately 43.9% of our ordinary shares as of December 31, 2007. As a result, IDB Holding Corporation Ltd. could exercise a significant influence over our operations and business strategy and has sufficient voting power to control the outcome of matters requiring shareholder approval. These matters may include:
| • | the composition of our board of directors which has the authority to direct our business and to appoint and remove our officers; |
| | |
| • | approving or rejecting a merger, consolidation or other business combination; |
| | |
| • | raising future capital; and |
| | |
| • | amending our articles of association which govern the rights attached to our ordinary shares. |
This concentration of ownership of our ordinary shares could delay or prevent proxy contests, mergers, tender offers, open-market purchase programs or other purchases of our ordinary shares that might otherwise give our other shareholders the opportunity to realize a premium over the then-prevailing market price of our ordinary shares. This concentration of ownership may also adversely affect our share price.
Future sales of our ordinary shares in the public market and low trading volume could adversely affect our share price.
As of December 31, 2007, we had 29,241,785 million ordinary shares outstanding. Approximately 50% of these shares are “control securities” available for resale on the Nasdaq Global Market subject, however, to volume limitations under Rule 144. In addition, all of our ordinary shares are available for resale on the Tel Aviv Stock Exchange, subject to compliance with Regulation S under the Securities Act of 1933. Most of these restricted securities are held by the largest beneficial owner of our shares, IDB Holding Corporation Ltd. Future sales of these restricted shares, or the perception that these sales could occur, could adversely affect the market price of our ordinary shares. We have periodically experienced a low trading volume of our ordinary shares, and if one or a small number of parties buys or sells a large number of our ordinary shares, we may experience volatility in our share price and the price and liquidity of our shares may be adversely affected.
Our ordinary shares are traded on more than one market and this may result in price variations.
Our ordinary shares are traded on the Nasdaq Global Market and the Tel Aviv Stock Exchange. Trading in our ordinary shares on these markets is made in different currencies (dollars on the Nasdaq Global Market, and Shekels on the Tel Aviv Stock Exchange) and at different times (due to different time zones, trading days and public holidays in the United States and Israel). The trading prices of our ordinary shares on these two markets may differ due to these and other factors. Any decrease in the trading price of our ordinary shares on one of these markets could cause a decrease in the trading price of our ordinary shares on the other market.
If we are characterized as a passive foreign investment company, our U.S. shareholders may suffer adverse tax consequences.
If, for any taxable year, our passive income, or our assets which produce passive income, exceed specified levels, we may be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. This characterization could result in adverse U.S. tax consequences for our U.S. shareholders that may include having gains realized on the sale of our shares treated as ordinary income, rather than as capital gains and potential payment of additional tax which apply to such gains. Furthermore, in certain cases, long-term capital gains rates might also be denied for certain dividends that may be paid by us to our shareholders. We believe we were not a PFIC for the fiscal year ended December 31, 2007. However, the tests for determining PFIC status are applied annually and are based in part on reference to the market value of our shares and valuing our intangible assets using the methods prescribed for publicly traded corporations, and it is difficult to make accurate predictions of future income and assets, which are relevant to this decision. Accordingly, we cannot give any assurance that we will not become a PFIC, in particular, since the value of our shares is likely to fluctuate. For a more detailed discussion of the consequences of our being classified as a PFIC, see “Taxation and Government Programs—United States Federal Income Taxation—Passive Foreign Investment Company Considerations.” U.S. shareholders are urged to consult with their own U.S. tax advisors with respect to the U.S. tax consequences of investing in our ordinary shares.
The government grants we have received from the Office of the Chief Scientist in Israel for research and development expenditures limit our ability to manufacture products and transfer technologies outside of Israel and require us to satisfy specified conditions. If we fail to satisfy these conditions, we may be required to pay additional royalties to the Office of the Chief Scientist or be subject to criminal charges.
From 1998 through 2007, we received royalty-bearing grants from the government of Israel through the Office of the Chief Scientist of the Ministry of Industry and Trade for the financing of a portion of our research and development expenditures in Israel. While we have paid our entire royalty obligation resulting from these grants, the terms of the Chief Scientist grants prohibit us from manufacturing products developed using these grants outside of Israel without special approvals and completely prohibit the transfer of our technologies and related intellectual property rights outside of Israel. This restriction may impair our ability to outsource manufacturing, engage in change in control transactions or otherwise transfer our technology outside Israel. We currently hold an approval from the Office of the Chief Scientist to manufacture limited quantities of the PillCam SB capsule in a facility in Ireland and we will be required to obtain an approval before manufacturing the PillCam ESO capsule or the PillCam COLON capsule outside of Israel. If we fail to comply with any of the conditions imposed by the Office of the Chief Scientist, we may be required to pay additional royalties to the Office of the Chief Scientist or be subject to criminal charges.
We receive significant tax benefits that may be reduced or eliminated in the future.
Our investment program in leasehold improvements and equipment at our manufacturing facility in Yoqneam, Israel has been granted “approved enterprise” status and we are therefore eligible for significant tax benefits under the Israeli Law for Encouragement of Capital Investments. From time to time, the government of Israel has considered reducing or eliminating the tax benefits available to approved enterprise programs such as ours. These tax benefits may not be continued in the future at their current levels or at all. If these tax benefits were reduced or eliminated, the amount of taxes that we pay would likely increase. In addition, our approved enterprise status imposes certain requirements on us, such as the location of our manufacturing facility, location of certain subcontractors and the extent to which we may outsource portions of our production process. If we do not meet these requirements, the law permits the authorities to cancel the tax benefits retroactively. See Item 10 “Additional Information—Taxation.”
We may not be able to enforce covenants not to compete and therefore may be unable to prevent competitors from benefiting from the expertise of some of our former employees involved in research and development activities.
We currently have non-competition agreements with substantially all of our employees who are involved in research and development, nearly all of whom are located in Israel. These agreements prohibit our employees, if they cease working for us, from directly competing with us or working for our competitors for a limited period of time following termination of employment. In many jurisdictions, courts are increasingly refusing to enforce restrictions on competition by former employees or have interpreted them narrowly. For example, in Israel, courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or its intellectual property. If we cannot demonstrate that harm would be caused to us, we may be unable to prevent our competitors from benefiting from the expertise of our former employees.
A. HISTORY AND DEVELOPMENT OF THE COMPANY
Given Imaging Ltd. was incorporated by RDC Rafael Development Corporation under the laws of the State of Israel in January 1998. We are registered with the Israeli registrar of companies in Jerusalem. Our registration number is 51-257802-2. Our address is Hermon Building, New Industrial Park, Yoqneam 20692, Israel. Our telephone number is 04-909-7777. Our agent in the United States is our subsidiary Given Imaging, Inc. Given Imaging, Inc.’s address is 3950 Shackelford Road, Suite 500, Duluth GA 30096.
See Items 5 and 18 for a description of capital expenditures by us for the past three fiscal years. We have not made any divestitures during the same time period.
We develop, manufacture and market innovative diagnostic products for the visualization and detection of disorders of the gastrointestinal tract. We pioneered capsule endoscopy, a proprietary approach to visual examination of the gastrointestinal tract through the use of a miniaturized video camera contained in a ingestible disposable capsule. Our principal product, which incorporates our core technology, is the Given System, a proprietary wireless imaging system that uses our disposable video capsules, which we refer to as the PillCam capsules. The PillCam video capsules are easily ingested by the patient and move naturally through the gastrointestinal tract without discomfort while wirelessly transmitting to a portable recorder, enabling the gastroenterologist to view high quality video, images and data on our workstation, utilizing our proprietary RAPID software. We believe that capsule endoscopy provides a patient-friendly solution that addresses a significant market opportunity and overcomes many of the shortcomings of traditional diagnostic tools for gastrointestinal disorders. We believe that each segment of the gastrointestinal tract presents meaningful opportunities for patient-friendly diagnostic procedures. In 2001, we commenced marketing the Given System, our capsule endoscopy platform, with the M2A capsule (which we re-branded in 2004 as the PillCam Small Bowel capsule, or PillCam SB), for detection of disorders of the small bowel. As of December 31, 2007, we had an installed base of nearly 4,250 Given Systems and had sold more than 650,000 PillCam SB capsules in over 60 countries worldwide. A substantial portion of our revenues and our annual revenue growth to date has resulted from sales of the PillCam SB capsule. Since November 2004, we also market and sell the PillCam ESO capsule for visualizing the esophagus. In 2007, we began selling the first generation of our PillCam COLON capsule in Europe following receipt of the CE mark. During 2007, we had limited initial sales of the PillCam COLON capsule and in 2008 we plan to continue gradual sales into specific markets following the completion of ongoing clinical trials. We have also developed the AGILE Patency Capsule and system, which is a dissolvable capsule that enables physicians to determine whether there are obstructions or strictures in the gastrointestinal tract that may prevent passage of our PillCam SB capsule. We launched the original Patency Capsule and scanner in Europe in November 2003. Following receipt of FDA clearance, we began marketing and selling the AGILE Patency System in the United States in May 2006.
Our long-term objective, subject to further development and receipt of regulatory clearances and/or approvals, is to establish the Given System as a platform for diagnosis of disorders in all parts of the gastrointestinal tract and the PillCam capsules as a primary diagnostic administered to patients with such suspected disorders.
Disorders of the Gastrointestinal Tract
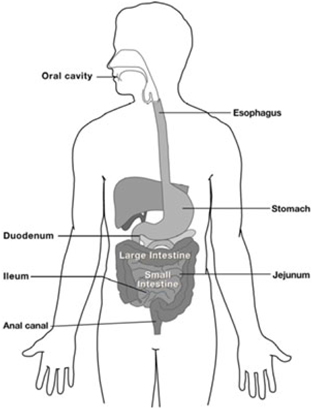
The gastrointestinal tract is a series of organs in the body responsible for digesting food. These organs principally include the mouth, esophagus, stomach, small intestine and colon. The following is an illustration of the gastrointestinal tract:
The upper gastrointestinal tract consists of the mouth, esophagus, stomach and duodenum, which is the first portion of the small intestine. The esophagus is an approximately ten inch long tube that connects the throat and the stomach. The stomach is a sac-like organ that produces enzymes to break down food. The small intestine is an approximately 21 foot long hollow organ that is primarily responsible for absorption of food components. The three parts of the small intestine are the duodenum, the jejunum and the ileum. The small intestine is located in the abdominal cavity between the stomach and the large intestine, or colon. The lower gastrointestinal tract consists of the lower two-thirds of the small intestine (the jejunum and the ileum) and the colon. The colon is the final portion of the gastrointestinal tract and is primarily responsible for absorbing water before waste is excreted.
The gastrointestinal tract is susceptible to various disorders. Typical symptoms of such disorders include heartburn, upper or lower abdominal pain, bleeding, diarrhea, constipation, anemia, weight loss and nausea. Some of these symptoms are not specific to any particular disorder, but may be common to more than one underlying disorder, often requiring the gastroenterologist to make a differential diagnosis. We believe that PillCam capsule endoscopy can have a significant role in assisting gastroenterologists in making an evidence-based diagnosis. The disorders causing the above-mentioned symptoms may include:
| | • | inflammatory bowel disease, including Crohn’s disease and ulcerative colitis, both of which inflame the lining of the digestive tract; |
| | | |
| | • | Obscure bleeding and iron deficiency anemia (IDA); |
| | | |
| | • | celiac disease, which causes damage to the intestine due to an allergic reaction to gluten in the diet; |
| | | |
| | • | Gastro-esophageal reflux disease, which is the flow-back of acidic stomach content into the esophagus, causing esophagitis, which is inflammation of the esophageal lining, or Barrett’s esophagus, a pre-cancerous condition; |
| | • | irritable bowel syndrome, which is a functional disorder characterized by abdominal pain or cramping and changes in bowel function without any organic manifestation; |
| | | |
| | • | peptic ulcer disease, which occurs when the lining of the stomach or duodenum is worn away by stomach acid; and |
| | | |
| | • | growths, such as tumor or polyps, which may be cancerous. |
| | Our products currently assist in the diagnosis of disorders of the small intestine, the esophagus and the colon. | |
| | • | Small intestine disorders. According to a study published in 2001 by the American Gastroenterology Association, or AGA, approximately 19 million Americans suffer from numerous disorders of the small intestine, including bleeding, Crohn’s disease, celiac disease, chronic diarrhea, irritable bowel syndrome and small bowel cancer. Prior to the development of capsule endoscopy, there was no convenient and effective method for visualizing the lumen of the entire small intestine. In 2006, the American Society of Gastrointestinal Endoscopy, or ASGE, published a guideline document, which recommends capsule endoscopy of the small bowel as a first line test for imaging small bowel mucosa. |
| | • | Esophageal disorders. GERD is the frequent backward flow, or reflux, of stomach contents into the esophagus due to an improperly functioning valve between the stomach and esophagus. Stomach acid, enzymes and bile irritate the esophagus and cause a wide range of symptoms and complications, most commonly persistent, severe heartburn and chest pain. According to the AGA study mentioned above, there are approximately 9 million physician office visits each year diagnosing GERD. If left untreated, GERD can lead to ulceration of the esophagus, respiratory problems or esophageal cancer. It is estimated that approximately 10% to 15% of patients with GERD symptoms have Barrett’s esophagus, a pre-cancerous condition with an associated risk for esophageal cancer. Another serious disorder of the esophagus is known as esophageal varices, a life-threatening complication caused by severe bleeding from small veins in the esophagus, usually associated with chronic liver disease. Thus, it is very important to screen patients with cirrhosis of the liver for the presence of varices and to monitor those patients with known varices. We believe that the PillCam ESO capsule may provide an effective method for screening for and detecting esophageal varices. |
| | | |
| | • | Colon Disorders Colonic disorders, including colorectal cancer, inflammatory bowel disease such as ulcerative colitis and Crohn’s colitis, diverticulosis, and lower gastrointestinal hemorrhage, account for significant mortality. According to data from the American Cancer Society, in the United States colon cancer is the third most common cancer diagnosed in both men and women and the second leading cause of death from cancer. It was recently estimated that each year approximately 150,000 Americans are diagnosed with this cancer and approximately 50,000 Americans die from colorectal cancer. Importantly, most colorectal cancer cases and deaths are thought to be preventable with screening tests that allow for the detection and removal of precancerous lesions. Despite the ever-growing body of evidence supporting the benefits of colorectal cancer screening, most eligible average-risk Americans do not undergo any form of screening, including conventional screening colonoscopy. Numerous reasons have been postulated to explain this including patient fears, presumed procedural discomfort, and embarrassment. The PillCam COLON capsule was developed for use as a safe, minimally invasive, non-sedation requiring, patient-friendly modality to visualize the colon and rectum. |
Current Detection Methods for Gastrointestinal Disorders
The most common methods for detection of gastrointestinal disorders, including disorders of the small intestine, are endoscopy and radiological imaging.
Traditional Endoscopy
A traditional endoscope is a device consisting of a flexible tube and an optical system. There are several types of endoscopic procedures used to identify disorders in the gastrointestinal tract. The basic endoscopic procedures available include:
| | • | Upper endoscopy. In upper endoscopy, the physician inserts an endoscope, which is an approximately 3.5 foot long tube, through the patient’s mouth. In esophagogastroduodenoscopy, or EGD, the gastroscope passes down the esophagus and into the stomach and duodenum for visual examination. In esophagoscopy, only the esophagus is viewed. |
| | | |
| | • | Colonoscopy. In colonoscopy, the physician inserts a colonoscope, which is an approximately 5.5 foot long endoscope, into the patient’s colon through the anus. Colonoscopy is the primary method for detecting disorders of the colon and is the standard screening tool for early detection of colon cancer. |
| | | |
| | • | Push enteroscopy. Push enteroscopy involves the insertion of an approximately six foot long push enteroscope into the mouth. Due to the length and curvature of the small intestine, push enteroscopy enables the physician to view only the first one-third of the small intestine. The procedure is lengthy and difficult to perform for both the physician and the patient. |
| | | |
| | • | Double balloon endoscopy. This technique involves the use of a balloon at the end of a special enteroscope camera and an overtube, which is a tube that fits over the endoscope, and which is also fitted with a balloon. This technique allows viewing the entire small bowel and therapeutic intervention once pathology is identified. However, it requires sedation and is skill- and time- intensive. |
A traditional endoscope can perform both diagnostic and limited treatment functions. In a traditional endoscopic procedure, the physician is able to control the movement of the endoscope through the gastrointestinal tract, to stop the endoscope and examine more closely a particular area in the gastrointestinal tract and to take a tissue sample or seal a bleeding site using the endoscope. However, traditional endoscopy has some risks and limitations, including the following:
| | • | Requires sedation. Due to the need to insert a tube through the mouth or anus, a traditional endoscopic examination typically requires sedation of the patient due to patient discomfort. |
| | | |
| | • | Involves potential complications. Potential complications of traditional endoscopic procedures include difficulty in breathing while the tube is inserted in the mouth, perforation or tearing of the intestinal wall, post-procedural infection and vomiting, abdominal swelling, sore throat, diarrhea and cross-contamination resulting from inadequate disinfection of endoscopes. |
| | | |
| | • | Causes patient anxiety, discomfort and pain. Many patients are unwilling to undergo traditional endoscopic procedures due to the pain and discomfort associated with having a tube inserted through the mouth or anus. |
| | | |
| | • | Requires substantial time commitment. Patients undergoing a traditional endoscopic procedure are required to remain in the physician’s office or hospital during the procedure. In addition, the effects of the sedation cause the patient to be inactive for several hours following the procedure. |
Radiological Imaging
Radiological imaging is a commonly used method for initial detection of the small intestine and other parts of the gastrointestinal tract. Radiological imaging is used for detection of disorders of the esophagus only in limited situations, generally where gross structural lesions are suspected. During a radiological imaging examination, the patient swallows a contrast medium (such as barium), which is a dense liquid that coats the internal organs and makes them appear on x-ray film. The procedure produces a series of black and white x-ray images of the lumen, or cavity, of the small intestine.
A more detailed examination, the double contrast small intestine enema, or enteroclysis, requires insertion of a tube through the mouth or nose, which is then pushed through the stomach and duodenum. High density barium and then methyl cellulose, a gel-like material used to expand the intestine, are injected through the tube into the patient’s small intestine prior to a series of x-ray exposures.
Radiological imaging also has risks and limitations as a diagnostic tool, including the following:
| | • | Cannot provide direct imaging of the mucosa. Radiological imaging does not provide a detailed view of soft tissue, including the mucosa, or internal layer of the gastrointestinal tract. In addition, radiological imaging does not provide clear visualization of ulcerations or flat malignant lesions. A lesion must have a certain mass and a distinguishable shape in order to be detected by radiology. |
| | | |
| | • | Has difficulty detecting small pathologies. Radiological imaging has difficulty detecting smaller-sized disorders or pathologies (typically less than five millimeters in diameter). Larger pathologies of up to ten millimeters in diameter can also remain undetected. |
| | | |
| | • | Has difficulty detecting strictures. Radiological imaging has difficulty detecting strictures - which are a three dimensional phenomena, and with a two-dimensional image this is not easily detected and frequently misdiagnosed. |
| | | |
| | • | Causes patient discomfort. Radiological imaging is uncomfortable for patients, requiring them to drink barium, which has an unpleasant chalky taste. A double contrast procedure is even more uncomfortable due to the insertion of a tube into the body through the mouth or nose. These preparatory measures can induce vomiting, particularly in a double contrast procedure if the injected contrast liquids return to the stomach. In elderly patients, the passage of barium can be difficult and can result in blockage requiring the use of disimpaction techniques. There is some morbidity associated with barium induced blockage in elderly patients. |
| | | |
| | • | Exposes patient to radiation. Radiological imaging poses increased risks of exposure to ionizing radiation for the patient. A double contrast procedure requires three to five times the amount of radiation to the patient. Tracking the progress of a disorder through repeated radiological imaging increases this risk. |
The Given Imaging Solution
The Given System features the PillCam capsule endoscope, a miniaturized video camera contained in a disposable capsule that is naturally ingested by the patient and delivers high quality color video images of the inside of the gastrointestinal tract in a painless manner. Capsule endoscopy with our PillCam capsules represents a fundamentally new approach to visual examination of the gastrointestinal tract and provides a solution to many of the shortcomings of other procedures by offering the following benefits:
| | • | Patient-friendly procedure with no sedation. Capsule endoscopy provides a patient-friendly tool for the diagnosis of patients that present symptoms of suspected disorders of the small intestine, the esophagus and the Colon. Procedures with the PillCam capsules require no sedation and the capsules are easily ingested by the patient and do not use x-rays to produce images. Procedures with PillCam SB and PillCam ESO do not require significant patient preparation. The PillCam COLON procedure requires preparation and cleansing of the colon prior to ingesting the capsule, similar to colonoscopy. While this preparation is unpleasant to the patient, the PillCam COLON procedure itself, similar to other PillCam procedures, does not require sedation or the insertion of a tube into the body. We believe that this patient-friendly solution may increase the number of patients who undergo diagnosis for gastrointestinal disorders of the small intestine, the esophagus and the colon, since existing methods have been intimidating or uncomfortable for many potential candidates. |
| | • | Improved or comparable diagnostic yield for PillCam SB and PillCam ESO. The PillCam SB capsule is the only wireless test that provides direct imaging of the entire small intestine. By comparison, double balloon endoscopy involves inserting a tube into the body, requires sedation and is skill- and time-intensive. Other methods provide access only to approximately the first third of the small intestine. As a result, clinical trials demonstrate that the PillCam SB capsule has a significantly higher diagnostic yield in detecting disorders of the small intestine when compared to other traditional modalities, including push enteroscopy and radiological imaging. Diagnostic yield means the number or percent of patients that had a diagnosis made using a specific test. In addition, as demonstrated through clinical studies and on-going experience with the Given System, a negative finding from the PillCam SB capsule, unlike conventional small bowel diagnostic techniques, has significant diagnostic value as it may allow physicians to rule out the existence of certain suspected abnormalities based on this finding thereby avoiding the need to engage in additional costly or inconvenient diagnostic procedures. Data from clinical trials reviewed by the FDA for clearing PillCam ESO demonstrated that the ability of the Given System to assist the physician in detecting disorders of the esophagus is comparable to that of traditional endoscopy. |
| | | |
| | | Results from initial clinical studies with the PillCam COLON suggest that it is a promising modality for evaluating the colon. While we believe that the PillCam COLON procedure will eventually provide a less invasive alternative to traditional colonoscopy, further capsule and procedure development as well as significant additional clinical data to support the use of this capsule as a screening tool are necessary before we can realize this market opportunity to its fullest potential. In the meantime, we market PillCam COLON in Europe for visualization of the colon and as a complementary tool to traditional colonoscopy in patients who are unable or unwilling to undergo colonoscopy or other colon visualization techniques. However, to date we have not been successful in obtaining FDA clearance to market this capsule in the United States. |
| | | |
| | • | Administered on an outpatient basis. The PillCam capsule is generally administered in an outpatient setting with a brief visit to the physician’s clinic or hospital. In the case of the PillCam SB capsule, the patient can go about his or her daily routine as the capsule transmits images and other data to the portable data recorder. In the case of the PillCam ESO capsule, the procedure can be completed in a short visit to the physician’s office. We believe the Given System offers a significant opportunity for gastroenterologists and endoscopy departments to expand their business by increasing the number of procedures performed at their office or facility. |
| | | |
| | • | Detects small pathologies. Unlike radiological imaging procedures, the PillCam video capsule provides direct visualization of the intestinal mucosa which allows detailed (up to 0.1 millimeter) visualization of small pathologies. This increases the possibility of detecting and diagnosing at an early stage, pathologies that might otherwise go undetected. |
| | | |
| | • | Natural passage requires no insufflation. Many traditional endoscopic procedures require insufflation, or the forcing of air into the gastrointestinal tract. This process can cause considerable patient discomfort. The Given System does not require insufflation because the PillCam capsule is ingested and moves with the natural contractions of the digestive tract. The absence of insufflation allows the PillCam capsule to capture images of the gastrointestinal tract in its normal physiological state. This approach, called “physiological endoscopy,” allows the physician to clearly view the mucosa. |
| | | |
| | • | Provides convenient digital reporting, storage and remote consulting capabilities. The physician can review the video produced by the Given System without seeing the patient or having him or her remain in the office or clinic during the review, thereby providing the physician with greater flexibility. In addition, the RAPID software includes various features that enhance the physician’s efficiency and productivity, such as innovative display methods for faster review, localizing findings, help in identifying anatomical landmarks for easy orientation, managing images and patient information, reporting modules and convenient options for sending still images or short video files to the patient file, to the referring physician or a colleague for consultation. Some of these features are proprietary and covered by patent applications, which we believe add an additional competitive advantage as physicians become more comfortable using these functions. |
| | | |
| | • | Provides a cost-effective diagnostic tool. We believe that the PillCam SB capsule endoscopy procedure is more cost-effective from a third party payor perspective than traditional methods for imaging the gastrointestinal tract. With respect to the PillCam SB capsule for the small bowel, two economic outcomes studies reported by the Office of Health Policy and Clinical Outcomes of the Thomas Jefferson University in Philadelphia concluded that diagnosing small intestinal bleeding or Crohn’s disease using the PillCam SB capsule procedure is cost-effective from a third party payor perspective. We believe that the Given System may result in additional cost savings due to the reduction in physician resources and facility costs permitted by the outpatient nature of the PillCam SB capsule procedure, its higher diagnostic yield – in the case of the PillCam SB capsule – and the potential for earlier diagnosis of disorders, allowing earlier therapeutic intervention or other change in patient management. |
The Given System also has some limitations. The PillCam capsule moves naturally through the gastrointestinal tract; consequently, the capsule’s passage is not controlled by the physician who cannot stop or steer the capsule for close-up detailed viewing of suspected disorders. In addition, the Given System, unlike a traditional endoscope, cannot take biopsies or be used for minor surgical procedures, such as cauterizing bleeding sites in the gastrointestinal tract. While endoscopes may be used in patients with obstructions or strictures in the gastrointestinal tract, the PillCam capsule may not pass naturally through the gastrointestinal tract of patients with obstructions or strictures, and accordingly the Given System is not recommended for use in these patients.
Our Products
The Given System consists of three components:
PillCam capsules.
The PillCam capsules are miniaturized disposable color video cameras encased in a plastic shell, which incorporate one or more specially developed imaging devices based on complementary metal oxide semiconductor, or CMOS, technology. Other components include optics, white-light emitting diodes for illumination, an application-specific integrated circuit device for control and image transmission, low-power silver oxide batteries, an antenna and other discrete electronic components.
Until their use, the PillCam capsules are stored in a hermetically sealed package. Before the patient ingests a capsule, the package is opened and the removal of the capsule from the package triggers a switch that activates the capsule. After the patient ingests the PillCam capsule with a small amount of water, the capsule passes naturally through the gastrointestinal tract. The PillCam capsules are excreted naturally from the body, usually within a day or two, without pain or discomfort.
We are currently selling the following PillCam capsules:
| | • | PillCam SB – Our initial capsule for the Given System was PillCam SB video capsule for visualization of the small intestine. The PillCam SB capsule measures 11mm x 26mm and transmits images at a rate of two images per second for approximately eight hours, resulting in approximately 50,000 images, at which time the operation is stopped and recording ceases. After ingesting the capsule at the physician’s office, during the procedure, the patient can continue his or her daily routine as the capsule transmits to a portable data recorder and then return the data recorder to the physician’s office for review and diagnosis. The PillCam SB capsule is used for a variety of conditions of the small intestine, including obscure bleeding, suspected Crohn’s disease, iron deficiency anemia and suspected small bowel tumors. In late 2007, we began marketing our newest version of the small bowel capsule, PillCam SB 2. This new capsule has improved optics, illumination and field of view and operates at a rate of two frames per second. We believe that the diagnostic capabilities of PillCam SB 2 are better than the diagnostic capabilities of the first generation PillCam SB capsule. |
| | | |
| | • | PillCam ESO – In November 2004, we received FDA clearance for our first generation of the PillCam ESO capsule for visualization of the esophageal mucosa. The PillCam ESO capsule contains an imaging device and light source at both ends of the capsule. The patient ingests the PillCam ESO capsule in the physician’s office. In early 2008, we began marketing our newest version of our esophageal capsule, PillCam ESO 2. PillCam ESO 2 has improved optics and illumination compared to the first PillCam ESO and a frame rate of up to 18 frames per second. We believe that the PillCam ESO 2 capsule provides physicians better image quality and a more precise view of the esophageal mucosa and suspected pathologies, which in turn are more likely to result in improved diagnostic capabilities compared to the first generation of the PillCam ESO capsule. |
| | | |
| | • | PillCam COLON – PillCam COLON is the third video capsule we developed. We began selling this product in Europe in the second half of 2007, following receipt of the CE mark permitting us to market this product in the European Union. Currently, this product does not have FDA clearance for marketing in the United States. We plan to increase sales of this product gradually, following completion of additional clinical trials that are underway in Europe. The PillCam COLON capsule measures 11 mm by 31 mm, slightly larger than PillCam SB and PillCam ESO. Since the lumen of the colon is wider than the small bowel and it is highly compartmentalized, we have integrated new features into PillCam COLON. The capsule has tiny cameras at each end which capture four images a second for up to 10 hours. Similar to other methods of colon visualization, the PillCam COLON capsule procedure includes a colon cleansing and preparation procedure, as well as additional prokinetic agents to enhance capsule propulsion. The platform for PillCam COLON includes the same components as PillCam SB and PillCam ESO, including a sensor array and data recorder. Upon completion of the procedure, the physician downloads the recorded data to a RAPID workstation to review the images. |
| | | |
| | • | AGILE Patency System – The AGILE Patency System consists of the AGILE Patency capsule, a dissolvable capsule the same size as the PillCam SB with a radio frequency identification (RFID) tag packed in a lactose and barium powder. The AGILE Patency capsule is ingested by the patient and allows physicians to confirm free passage of a PillCam capsule in a patient’s gastrointestinal tract. |
Data recorder and sensor array.
After ingestion by the patient, the PillCam capsule transmits information from the body to a proprietary wireless data recorder through an array of antennae, or sensor array, that are secured with adhesive pads to the abdomen, in the case of a procedure performed with a PillCam SB or a PillCam COLON capsule, or to the chest, in the case of a procedure performed with a PillCam ESO capsule. The data recorder is worn on a belt around the waist of the patient for the duration of the examination.
Computer workstation with proprietary RAPID software.
After the recording ceases, the data recorder is returned to the physician’s office at the patient’s convenience, where it is connected to the RAPID computer workstation, our capsule endoscopy platform, for transfer of the data captured during the test. Our proprietary RAPID software then processes the data received from the capsule. The RAPID software also includes a number of proprietary algorithms relating to the speed of processing imaging data, as well as to the visual presentation of this data. The Given workstation presents a video stream for viewing by the physician. The physician can review the video carefully, save specific images for the patient file, or attach the thumbnail images and the short video file to an e-mail in order to send to the referring physician or consult with a colleague. The “Multiview” feature of our RAPID software provides users with a simultaneous viewing mode of two or four consecutive images, thereby accelerating review speed. In addition, the RAPID software contains a proprietary algorithm that we developed called SBI, or suspected blood indicator, which automatically marks images that correlate with the existence of suspected bleeding. In addition, we provide our users with the RAPID Reader, which is a narrowed version of the RAPID Workstation software that can be installed on a standard personal computer, allowing the physician to review RAPID videos at any time and place as convenient.
Important features of our RAPID software include:
| • | “Automatic Mode,” which uses advanced software resulting in more efficient review of the video; |
| | |
| | |
| • | “Quick View,” which allows fast preview of the video and highlights potentially interesting images in the video stream; and |
| | |
| • | Image atlas, which compares the on-screen case image with known reference images stored in the database. The reference images can be searched by findings, diagnosis, or using Capsule Endoscopy Structured Terminology, or CEST. |
In the United States and Europe we also market our RAPID Real Time Viewing device. This is a dedicated handheld device with our RAPID Real Time software that enables real-time viewing during a capsule endoscopy procedure with our PillCam capsules. The RAPID Real Time Viewing device also allows the physician to activate the data recorder and transfer data from the data recorder to a data storage device, like a disk-on-key. These features enable remote customer sites that do not have access to a permanent workstation to administer our PillCam capsules to patients and then deliver the results to a central location for processing and interpretation.
Warranty and Service
Our standard warranty for our PillCam capsules is typically 18 months from the date of manufacture. Our standard warranty for other components of the Given System normally extends for a period of one to two years from the date of the first installation at the customer’s location with the exception of the Sensor Array, which carries a limited warranty of six months from installation or 10 months from delivery. Generally, no warranty is provided more than 16 months from the date of shipment of the Given System to the customer. During the warranty period, we are obligated to repair or replace, at our election, every defective product.
When a customer reports a problem with the Given System, first line service is normally provided by our own technical personnel in the territories in which we operate directly, such as the United States, Germany, France and Australia. In territories in which we operate through a distributor, the distributor is responsible for providing first line service. If our personnel in the field or our distributors are not able to resolve the problem, the defective part is shipped to our main facility in Israel for repair or replacement. Often, the defective part is replaced promptly out of a stock of spare parts we maintain in all of our direct territories. Occasionally, we are able to resolve service calls using remote access software that allows us to provide maintenance and support services for our products from a remote location, including our distributor’s office, through a telephone line.
When the warranty expires, our customers are offered the opportunity to sign a post-sale customer support contract with us. Under this contract, the customer pays a fixed amount per year in consideration for receiving our maintenance and support services. As of December 31, 2007, we had an installed base of nearly 4,250 systems worldwide, of which 1,999 were under warranty and 660 were covered by a service contract.
Clinical Studies
Clinical studies are an important part of our product development and market development activities. We use clinical studies during product development to optimize the development process. Most of our clinical studies are conducted for market development purposes. With these trials we strive to demonstrate the clinical and economic benefits of our products to support their use by physician and reimbursement coverage by third party payers. Many of the investigators conducting clinical studies relating to capsule endoscopy using our products have presented their results at major gastroenterology meetings or submitted them for publication in peer-reviewed medical journals. Accordingly, the number of presentations and publications of studies evaluating the Given System is constantly growing and hundreds of articles, editorials and case reports have been published in peer reviewed journals to date. These papers provide the results of clinical studies as well as accumulated experience from on-going use of the Given System for a range of indications. We believe that these presentations and publications assist our marketing and educational efforts and support our efforts to obtain new reimbursement coverage policies and expand existing policies.
Examples of our clinical studies activity include:
PillCam SB. To demonstrate the clinical and economic benefits of the Given System, the PillCam SB capsule has been tested in clinical studies sponsored by us and by independent third parties. Our clinical studies program is conducted in a number of countries, primarily Australia, France, Germany, Israel, Italy, the Netherlands, Spain, Sweden, the United Kingdom, Japan and the United States. Initially, our clinical studies program focused on patients suffering from suspected bleeding in the small intestine. Subsequent clinical trials have focused more on evaluating the performance of the PillCam SB capsule in detecting other disorders of the small intestine, primarily suspected Crohn’s disease, and also tumors, malabsorption (such as celiac disease), mucosal injuries induced by long-term use of NSAIDs (non-steroidal anti-inflammatory drugs, such as aspirin) and the performance of the PillCam SB capsule in children over the age of ten.
The results of these clinical studies to date have demonstrated that capsule endoscopy with our PillCam SB has a comparable or higher diagnostic yield in the detection of disorders of the small intestine than comparable methods, such as push-enteroscopy and radiological examinations, such as barium follow-through and computerized tomography (CT). In 2007, we initiated a clinical study that examines the efficacy of PillCam SB capsule in patients with suspected inflammatory bowel disease. We also initiated studies to examine a possible role of the PillCam SB capsule in Cystic Fibrosis and Ulcerative Colitis.
PillCam ESO. We have recently completed and submitted for publication the results of a multi-center, multinational clinical study evaluating the PillCam ESO for the detection of esophageal varices. In this study, our first generation PillCam ESO capsule was used. The authors stated that the minimal invasiveness, good tolerance and good agreement of capsule endoscopy with the traditional modality of Esophageal-Gastro-Duodonoscopy, or EGD, might increase adherence to screening programs. We believe that this conclusion suggests a more important role for PillCam ESO in the management of the treatment of cirrhotic patients and may contribute to further reimbursement coverage and increased sales of the PillCam ESO capsule. In 2008, we plan to conduct a multicenter study in the United States with our new PillCam ESO 2 capsule, to evaluate the effectiveness of this capsule in detecting Suspected Barrett’s, a pre-cancerous esophageal condition that may develop in patients suffering from Gastro Esophageal Reflux Disease, or GERD, compared to standard EGD.
PillCam COLON. Following encouraging results of two small preliminary studies in 2006, in 2007 we conducted a large multi-center, study with PillCam COLON in five European countries. This study was designed to evaluate the use of PillCam COLON in the visualization of the colon. The study concluded that PillCam COLON capsule endoscopy is a promising new modality that in the future may challenge traditional methods used today for colorectal cancer screening. We believe this is the biggest market opportunity for the PillCam COLON capsule. According to guidelines of professional associations in the United States, it is estimated that approximately 12.5 million people over the age of 50 or that are otherwise at increased risk for colon cancer need to be screened each year in the United States, yet patient compliance is only around 50 percent. Patient compliance rates in Europe are believed to be even lower than this. We believe that the PillCam COLON procedure will eventually provide a less invasive alternative to traditional colonoscopy. However, further capsule and procedure development as well as significant additional clinical data to support the use of this capsule as a screening tool are necessary before we can realize this market opportunity. Our clinical study activities relating to the colon also include trials designed to optimize the colon cleansing and preparation procedure that is an important factor in a successful examination of the colon. In the meantime, we market PillCam COLON in Europe for visualization of the colon in patients who are unable or unwilling to undergo traditional colonoscopy or in cases of incomplete colonoscopies. To date, we have not been able to receive FDA clearance to market this capsule in the United States.
The PillCam capsules are not recommended for use by patients who have known or suspected gastrointestinal obstructions, narrowing, and certain other abnormalities, such as swallowing disorders. In patients with unsuspected or unknown obstructions, narrowing or certain other abnormalities of the gastrointestinal tract, the PillCam capsules can potentially become blocked from natural excretion, requiring hospitalization, and in some cases surgery, to remove it. According to the 2005 ICCE consensus report that was published in November 2005 in the peer-review journal Endoscopy, which defines practice guidelines and protocols for the use of both PillCam SB and PillCam ESO capsules by gastroenterologists, the rate of capsule retention depends on the indication. The rate of retention in patients with obscure gastrointestinal bleeding is 1.5% (15 out of 1089 cases); in patients with known Crohn’s disease is 5% (4 out of 80 cases); and in patients with suspected Crohn’s disease is 1.4% (1 out of 71 cases). The consensus report stated that the PillCam capsule has been reported to identify areas of narrowing of the gastrointestinal tract and there is nothing to indicate that the capsule itself is creating any obstructions or narrowing. The consensus report also stated that, while there is no accepted method of completely avoiding capsule retention, it is clear that obtaining a good medical history is the best single method. Patients with abdominal pain, distension and nausea should be suspected of having a potential for capsule retention. Other risk factors include known Crohn’s disease and a history of chronic NSAID use that is not necessarily current. A history of small bowel obstruction, previous small bowel resection or previous abdominal surgery are not in and of themselves indicators of probable retention. Once retention has been diagnosed, only endoscopic and surgical intervention have been shown to be effective for removal of the capsule. Surgical intervention is also recommended by the consensus report because it allows removal and treatment of the pathology that resulted in the capsule retention in the first place. There is no available data on the success of medical therapies for retention, such as initiating a course of steroids, stopping NSAIDs, or using prokinetics to aid in passing the capsule.
The Patency System
We expect that the availability of the AGILE Patency System will contribute to an increased use of the PillCam SB capsule in the diagnosis of suspected or known gastrointestinal obstructions or narrowing, such as suspected or known Crohn’s disease.
The AGILE Patency System consists of the AGILE Patency capsule, a dissolvable capsule the same size as the PillCam with a radio frequency identification (RFID) tag packed in a lactose and barium powder. The AGILE Patency capsule is ingested by the patient and allows physicians to confirm free passage of a PillCam capsule in a patient’s gastrointestinal tract. The reusable component of the AGILE Patency System is a hand-held Patency Scanner, which detects the signal from the RFID tag. If the scanner indicates that the tag is no longer in the gastrointestinal tract, patency has been established and the patient can ingest a PillCam capsule without fear of impaction in a stricture. If the scanner indicates that the tag is located in the patient’s body, an obstruction preventing the passage of the PillCam capsule may exist. If the AGILE Patency capsule remains in the body, it starts dissolving after 30 hours into small fragments that are naturally excreted. Since the capsule contains barium, in those instances where the AGILE Patency capsule is not excreted after ingestion, the physician may detect its location within the body using fluoroscopy.
Marketing and Distribution
Beginning in 2007, our sales and marketing operations were organized in three geographical regions: Americas (United States, Canada and Latin America); EMEA (Europe, Middle East and Africa) and Asia-Pacific (Japan, Australia and the rest of Asia). This organization enables us to focus on the particular needs of each region.
We market the Given System using either direct or indirect sales, depending on the potential size of the market and local market conditions. Currently, we market the Given System directly in Australia, France, Germany, the United States and Israel. In the United States, which accounted for 70% of our revenues in 2006 and 64% of our revenues in 2007, we market our products primarily through our wholly-owned subsidiary, Given Imaging, Inc., located in Duluth, Georgia. As of December 31, 2007, we had a direct sales force in the United States of approximately 71 field sales representatives. We have approximately 16 field sales representatives in the other jurisdictions in which we directly market the Given System.
In addition to our direct markets, we market and sell our products in more than 60 other countries through local distributors or representatives. Sales to our local distributors worldwide accounted for 21.8% of our revenues in 2007. As part of the cooperation agreement we signed with Fujinon Corporation in March 2007, we began collaborating with Fujinon in the distribution of our products in a few countries. For example, Fujinon now distributes our products in China where their presence is more significant than ours. For more information on our agreement with Fujinon, see under the heading “Competition” in this Item 4. Under standard terms of our distribution agreements, we generally grant to one distributor in each particular country or region the right to market the Given System for a defined period. During the contract period, the distributor is required to meet minimum sales targets set out in each distribution agreement. We may, in our sole discretion, upon prior written notice, terminate a distribution agreement with a distributor in the event that a distributor fails to meet its minimum sales targets. To date, we have changed a number of our distributors due to failure to meet minimum sales targets and other reasons. We have the right not to renew a distribution agreement if we are unable to reach an agreement with the distributor as to minimum sales targets during any renewal period. Each distributor is responsible for obtaining and maintaining any regulatory approvals or registrations required to sell the Given System in that distributor’s sales territory. Each distributor is also responsible for preparing and submitting to us for our approval a marketing plan for the Given System in that distributor’s sales territory. After receiving our approval of the marketing plan, each distributor is responsible for implementing the marketing plan, including, participating in local and national trade shows, conducting education and training sessions and conducting sponsored marketing clinical trials in its sales territory.
In Japan, we operate through Given Imaging, K.K., our Japanese subsidiary, that was established as a joint venture with Marubeni Corporation and Suzuken Co., Ltd. to commercialize the Given System in Japan. Marubeni is one of Japan’s largest trading companies and Suzuken is one of Japan’s largest pharmaceutical distributors. To date, Marubeni and Suzuken together invested an aggregate of $9.0 million in Given Imaging K.K. in consideration of an aggregate 49% interest. We retain a 51% controlling interest. In addition, Suzuken has been appointed by Given Imaging K.K. as its exclusive distributor in Japan. We received regulatory clearance to market our RAPID workstation and PillCam SB capsule in Japan in April 2007. Additionally, effective October 1, 2007, Japanese authorities announced initial reimbursement coverage for procedures using our PillCam SB capsule for small bowel indications with obscure bleeding. This reimbursement covers the entire adult population in Japan. Following these developments, we expect that our business in Japan will significantly contribute to our expected growth in 2008. For more information regarding our business in Japan, see “Item 5 - Operating Results.”
Most of our revenues come from recurring sales of our PillCam capsules. Our marketing strategy in our mature and educated markets in the United States, Europe and Australia focuses on increasing the utilization of PillCam capsules by each account and consequently increasing capsule reorders. We seek to achieve this by providing a complete platform for capsule endoscopy that may be used for diagnostic purposes in more than one area of the gastrointestinal tract, frequently visiting our customers and maintaining a closer relationship with them, educating them about updated data on the clinical and economic benefits of our products and enhancing the operating efficiencies of the Given System. In new markets, such as Japan, our initial focus is on driving the placement of Given Systems in order to expand our market penetration in gastroenterology physician offices and gastroenterology departments within hospitals. At the same time, we do market development activities, such as, clinical trials, working with payers to obtain appropriate reimbursement and educational activities with the purpose of defining and expanding the market for the PillCam ESO and PillCam COLON capsule in territories in which these products have regulatory clearance so that we are able to have the full benefit of the Given platform.
The clinical benefits of capsule endoscopy and its place in routine practice management are demonstrated in the ICCE consensus reports. The ICCE is an annual scientific meeting dedicated to presentations and discussions of the most recent advances in the field of capsule endoscopy. This annual conference serves as a basis for establishing a community of physicians who utilize the Given System and accelerates the dissemination of new clinical data. In ICCE 2005 a group of gastroenterology opinion leaders from around the world issued consensus reports, which define practice guidelines and protocols for the use of both PillCam SB and PillCam ESO by gastroenterologists in several disease states. These consensus reports were developed in response to the need to formalize practice guidelines for using capsule endoscopy. The consensus reports focused on five areas: gastrointestinal bleeding, inflammatory bowel disease, celiac disease, use of bowel preparations and prokinetics, and esophageal capsule endoscopy. The consensus reports were published in the October 2005 issue of Endoscopy, the official, peer-reviewed publication of the European Society of Gastrointestinal Endoscopy (ESGE) and an update was published in 2007. This update includes practice guidelines for using capsule endoscopy in the detection of small bowel tumors and expanded definitions of disease states such as iron deficiency anemia and suspected Crohn’s disease. We view the consensus reports as a significant tool in our marketing activities and we believe the reports will help us establish capsule endoscopy as a standard practice worldwide. In 2007, we held one ICCE conference in the United States and a second in Europe.
In order to drive strong growth in the future, we need to demonstrate and convince gastroenterologists that the Given System fits within their normal practice routines. Our newest version, RAPID 5.0, which was cleared for marketing in the United States in 2007, includes features that we believe significantly improve physicians’ reading efficiency and diagnostic confidence, which we expect will drive physicians to incorporate capsule endoscopy into their daily routine. We intend to continue investing in training and education of our customers to help them realize the full potential of our newest technology.
We also use trade shows and scientific meetings and offer workshops, courses, videos and seminars to educate our customers. In 2007, we participated in the Digestive Disease Week (DDW) and the annual meeting of the American College of Gastroenterology (ACG) in the United States, the United European Gastro Week (UEGW) in Paris, and numerous other regional, national and local trade shows. In addition, we held numerous regional and local courses and seminars and trained hundreds of physicians and nurses on capsule endoscopy and our products. We believe that these education programs helped also to expand the knowledge of participating physicians and provide an independent endorsement of the clinical value and importance of the Given System.
A variety of special interest groups related to capsule endoscopy have been formed in the United States, Europe and Japan to provide a dynamic forum to share knowledge, encourage research, and support the advancement of capsule endoscopy. One of the first groups formed was the Capsule Endoscopy Special Interest Group, which is sponsored by the American Society of Gastrointestinal Endoscopy, or ASGE. In Europe, a group of leading gastroenterologists gathered to form the European Capsule Endoscopy Group (ECEG). A similar group, the Capsule Endoscopy Study Group (CESG), was formed to complete the first clinical trials for capsule endoscopy in Japan and to educate the Japanese physician community on the practical application of capsule endoscopy. We believe that these groups in the United States, Europe and Japan provide an important contribution to the broad adoption of capsule endoscopy within these markets.
Since 2005, we have sponsored the operation of a web portal dedicated to capsule endoscopy, www.CapsuleEndoscopy.org. This portal addresses gastroenterology professionals and provides training and educational materials and information, including a virtual image atlas, clinical papers, course information and discussion forums for a variety of clinical topics. Over 4,500 members from more than 106 countries have already enrolled at the site as of December 31, 2007.
In November 2007, we announced the termination of our exclusive sales representation, co-promotion and cooperation agreement with Ethicon Endo-Surgery, a Johnson & Johnson company, which was signed in May 2004. InScope, a business division of Ethicon Endo-Surgery had exclusive rights to market our PillCam ESO capsule in the United States during this period and performed sales and market development activities. Ethicon cited a shift in its strategic priorities within gastroenterology and other areas as the reason for ending the relationship. As a result of this termination, we regained full responsibility for the marketing and sale of the PillCam ESO capsule in the United States at the beginning of 2008. In accordance with the terms of termination, Ethicon will continue to fund existing clinical trial activities relating to this capsule until they are completed. For more information on the termination of the agreement with Ethicon and its consequences, see “Item 5 - Operating Results.”
Effective January 1, 2007, a permanent CPT Code was assigned to our PillCam ESO capsule by the American Medical Association, or AMA, and the Center for Medicare and Medicaid Services, or CMS, and, thereafter, we saw the first third-party reimbursement coverage for procedures with the PillCam ESO capsule to evaluate esophageal varices in patients diagnosed with cirrhosis of the liver, a chronic liver disease. As of December 31, 2007, approximately 31 million people in the United States had coverage for this indication. In 2007, we completed and submitted for publication the results of a large multi-center study to evaluate the use of PillCam ESO in the evaluation of esophageal varices in patients diagnosed with cirrhosis of the liver. The authors stated that the minimal invasiveness, good tolerance and good agreement of capsule endoscopy with the traditional modality of Esophageal-Gastro-Duodonoscopy, or EGD, might increase adherence to esophageal screening programs. We believe that this conclusion suggests a more important role for PillCam ESO in the management of the treatment of cirrhotic patients and may contribute to further reimbursement coverage and increased sales of the PillCam ESO capsule.
With the release of PillCam ESO 2, a growing body of positive clinical data, initial reimbursement coverage, and the benefit of a few years of market development activities, we believe we are well positioned to assume full responsibility over the marketing and sales of PillCam ESO from Ethicon and expect sales of this capsule to grow in 2008. However, sales of the PillCam ESO capsule are expected to remain low compared to sales of our PillCam SB capsule in the foreseeable future.
Manufacturing
We purchase both custom and off-the-shelf components from a number of suppliers. Except as described below, the components and services we purchase are available from more than one supplier.
PillCam Capsules
The manufacture of the PillCam capsules is a complex process involving a number of separate processes and components. Our manufacturing process consists primarily of assembling externally purchased components and sub-assemblies in an environmentally controlled area. After assembly, each PillCam capsule is inspected and packaged.
We manufacture the PillCam capsules at our facilities in Yoqneam, Israel. Two production lines are used to manufacture the PillCam SB capsule and one to manufacture the PillCam ESO capsule. We also have one production line which we use to manufacture the PillCam COLON and one production line for our AGILE Patency capsule. We have also installed at a facility in Ireland one semi-automated production line for the PillCam SB capsule for back-up purposes. We believe we have adequate capacity to manufacture capsules needed to satisfy estimated demand for the foreseeable future.
There are single-source suppliers for two key components of the PillCam capsule:
| | • | A U.S.-based supplier developed the imaging sensor that is integrated into the PillCam capsules based on our specifications and is also manufacturing and supplying this imaging sensor exclusively to us. Under our contract with this supplier, it may not offer the imaging sensor as a standard catalog part. In the event that this supplier ceases operations or enters into liquidation, we are entitled to receive all information necessary to manufacture the sensor upon the payment of reasonable royalties to be agreed upon with the supplier. We signed an amendment, effective as of June 2005, to our development and manufacturing agreement with this supplier and agreed that it will develop and manufacture an enhanced version of the imaging sensor based on specifications that we provide. This amendment also extended the initial term of the agreement until November 2012, with an option to extend that term by mutual agreement. Under this amendment, we have agreed to specified minimum purchase commitments, which we may terminate if the supplier fails to satisfy agreed-upon performance criteria. We have agreed to purchase the enhanced sensor only from this supplier and the supplier has agreed to sell the sensor exclusively to us. The agreement permits the supplier to disregard the exclusive sales requirement if we materially breach the agreement and fail to cure such breach within a specified time. |
| | | |
| | • | A Canadian company, supplies the transmitter that is integrated into the PillCam capsules. This company began supplying the transmitter to us in the fourth quarter of 2004. We currently expect that this Canadain supplier will fulfill substantially all of our future needs for transmitters for the PillCam capsules. The agreement includes non-compete provisions prohibiting this supplier from selling the transmitters to other parties and, for a certain period of time following termination of the agreement, from transferring any of the intellectual property and design specifications associated with the development of the transmitter to any potential competitors in our market. In July 2005, we agreed with this supplier that it will develop and manufacture an enhanced version of the transmitter based on specifications that we provide. In addition, the initial term of the agreement was extended until April 2012, subject to earlier termination in specified circumstances, with the option to extend annually thereafter for up to five years. |
We believe that we would be able to arrange substitute sources of supply for these two components within approximately one year of lead time. We believe that if we need to find a substitute source of supply, our inventory of components and finished products, together with our right to submit final purchase orders prior to termination of our agreements with these suppliers, are sufficient to continue sales of the Given System for all or most of the lead-time period.
We depend on single source suppliers with whom we do not have long term contracts for some other components necessary for the production of the Given System, such as the electrical circuit boards in our PillCam capsules and our computer workstations. For a description of the risks associated with our dependence on single source suppliers, see Item 3 - Risk Factors.
Portable Data Recorder and Sensor Array
We designed our portable data recorder, sensor array and their related accessories. Data recorders are manufactured externally and assembled and tested at our facilities. Sensor arrays are manufactured and assembled externally and are tested at our facilities. We have established a back-up facility outside Israel for testing workstations, data recorders and sensor arrays.
Computer Workstation
Our computer workstation is an off-the-shelf computer workstation pre-loaded with our proprietary RAPID software and integrated software that together allow us to service the workstation from remote locations through standard telephone connections.
Manufacturing Facilities and Disaster-Preparedness
In order to maintain our special tax benefits under our approved enterprise status, we are required to conduct our manufacturing and a majority of our subcontracting in specific locations in Israel. We currently plan to continue these practices as we increase our manufacturing capacity. For more information, see “Taxation and Government Programs-Israeli Tax Considerations and Government Programs — Taxation of Companies —Tax Benefits under the Law for the Encouragement of Capital Investments, 1959.”
We have also taken the following measures for disaster-preparedness:
| | • | We have installed one back-up semi-automatic production line at a facility in Ireland. Our backup line has the capacity to manufacture approximately 15,000 PillCam SB capsules per month. We believe that this line can be operational upon 60 days’ notice. |
| | | |
| | • | Our practice is to hold inventory of critical components, such as the imaging sensor and the transmitter, for a period of time ranging from approximately two to 18 months, depending on the risk profile we allocate to each critical component. |
| | | |
| | • | Our practice is to hold approximately six weeks’ inventory of finished products at our offices in Israel, Sydney, Hamburg, Paris and Duluth, Georgia. |
| | | |
| | • | We maintain back-up copies of all production files, original certifications and all proprietary software masters outside of our facilities in Israel. |
The FDA requires us to adhere to the Quality System Regulation which requires manufacturers to follow elaborate design, testing, control, documentation and other quality assurance procedures during the manufacturing process. In addition, we are required to comply with the Medical Devices Directive of the European Union requiring adherence to the International Standard Organization’s standard ISO 9001 and EN 46001, a European quality standard setting forth requirements for medical device manufacturers that are more specific than the general requirements specified in ISO 9001. These quality standards contain requirements that are generally similar to the Quality System Regulation required by the FDA.
Intellectual Property
An important part of our competitive strategy is to seek, when appropriate, protection for our products and proprietary technology through the use of various United States and foreign patents, trademarks, trade secrets and contractual arrangements. We intend to prosecute and defend our proprietary technology.
We acquired the rights to our first issued U.S. and Israeli patents in January 1998 under a technology purchase and license agreement with Rafael Armament Development Authority. See Item 7 “Major Shareholders and Related Party Transactions.” These patents expire in January 2014 and January 2015, respectively. In addition, we hold 89 additional issued patents in the United States, Australia, France, Germany, India, Israel, Italy, Japan, South Korea, Spain, Taiwan and the United Kingdom covering different elements of our technology.
These patents expire between 2017 and 2025. We also hold six utility models in Japan and nine utility models in Germany. As of December 31, 2007, we also had more than 500 pending patent applications worldwide based on approximately 180 priority applications relating to various elements and functions of our product and its enhancements.
We seek to protect our product names and logos through trademark use and registration in the United States and other countries. GIVEN, GIVEN & Design, PILLCAM, PILLCAM & Logo, PILLCAM IMAGING CAPSULE & Design, AGILE, RAPID, RAPID ACCESS, ORDERWIN, ORDER WHEN I NEED, FINGERS HOLDING A CAPSULE & Logo, FINGERS HOLDING PILLCAM CAPSULE & Logo, ICCE, ICCE Logos, and International Conference on Capsule Endoscopy are our trademarks or registered trademarks.
In March 2004, the U.S. Patent and Trademark Office, or USPTO, notified us that it would conduct a reexamination of some of the claims in our first U.S. patent known as the ‘531 patent, pursuant to a request submitted by Olympus Corporation. In April 2006, the USPTO issued a decision confirming the validity of 13 of the original 17 claims of the ‘531 patent. In September 2006, we appealed the rejection of the other four claims and this appeal is currently pending.
We currently are engaged in patent litigation against Olympus Corporation and its affiliates in the District Court for the Eastern District of Pennsylvania. For more information, see “Item 8 – Financial Information – Legal Proceedings.”
Competition
Olympus Corporation has been marketing and selling a competing capsule endoscopy system in Europe and Australia since October 2005. In September 2007, Olympus received FDA clearance to market its capsule endoscopy system and small bowel capsule in the United States. According to public sources, Olympus is also seeking regulatory clearance to market its capsule endoscopy system in Japan. In addition to Olympus, a Chinese company is selling its capsule endoscopy systems in China at lower prices than our system and presented its systems at industry trade shows outside Asia. Finally, according to publicly available information, in 2007 a South Korean company began selling a competing system in Korea as well as in Europe and Australia.
We believe that we have a competitive advantage compared to Olympus and other sellers of capsule endoscopes in the capsule endoscopy market. First, we were the first company to sell and market capsule endoscopes in the most mature markets in the United States, Europe and Japan and have a large installed base of customers. Second, we have been concentrating primarily on the development and sale of capsule endoscopy systems and are selling a platform enabling us to use our line of PillCam capsules for diagnosing disorders in parts of the gastrointestinal tract other than the small bowel, while Olympus and other companies have only announced the launch of a small bowel capsule at this time. Finally, we have a patent portfolio that we believe protects critical aspects of our technology and creates technological barriers for our competitors, which may force them to enter the market with inferior products or delay their entry into certain territories.
In addition, we face competition from existing technologies for detecting gastrointestinal disorders and diseases, including traditional endoscopy and radiology. Our success depends in large part on convincing physicians to adopt the Given System over current technologies.
Three companies control the major portion of the worldwide gastrointestinal traditional endoscopy market. These companies, Olympus, Hoya and Fujinon, have marketed and sold flexible endoscopic equipment for many years. These companies have substantially greater financial resources than we do, and they have established reputations as well as worldwide distribution channels for medical instruments to gastroenterologists. We are aware of research and development efforts by some of these companies and other individuals and companies to develop and bring to market imaging capsules or other minimally invasive imaging techniques.
To strengthen our competitive position, in March 2007 we signed a cooperation agreement with Fujinon Corporation. The goal of the agreement is to build closer collaboration between the companies in research and development, component sourcing, marketing and product distribution worldwide, except in Japan. Under the terms of the agreement, we and Fujinon will collaborate to develop future products for the gastrointestinal endoscopy and diagnostic field. The agreement also grants Fujinon non-exclusive rights to distribute our capital equipment and small bowel products including our RAPID workstation and data recorders, PillCam SB, and AGILE Patency capsules in certain countries worldwide, which will be determined by the two companies on a case-by-case basis. As of December 31, 2007, Fujinon distributes our products in China.
In addition, there are several companies focused on radiological diagnostics that provide x-ray machines and other imaging products used for barium series radiological examinations. These companies include but are not limited to GE Healthcare, Siemens Medical Solutions, Philips Medical Systems, Toshiba Corporation and Shimadzu Corporation.
In addition to competition from products performing similar clinical functions to the Given System, there is also competition among gastrointestinal products for the limited capital expenditure budgets of customers. For example, another capital equipment item for gastroenterology may compete with our system for the same hospital capital budget, which is typically limited, and therefore the potential purchaser may be required to choose between the various items of capital equipment.
U.S. Government Regulation
FDA Clearance and Regulation of the Given System
All the products we market and sell in the United States have received FDA clearance. Our first clearance was received in August 2001 for the first generation of the PillCam SB capsule. In November 2004, we received FDA marketing clearance for our PillCam ESO capsule and in 2006 we received clearance to market our AGILE Patency capsule and scanner. During 2007 we received clearance to market a number of new products, such as the new PillCam SB 2 and PillCam ESO 2 capsules and the newest version of our RAPID software, RAPID 5.0. All of our products that have been cleared to date by the FDA, have been cleared through such 510(k) clearance process. However, the FDA has recently determined that the PillCam COLON is not substantially equivalent to any marketed device in the United States for visualization of the colon and therefore can not be cleared for marketing in the United States through the 510(k) process that is further described below.
FDA Clearance and Regulation of the Future Products
Any new medical device that we wish to commercially distribute in the United States will likely require either 510(k) clearance or premarket application approval from the FDA prior to commercial distribution. 510(k) clearance or amendment to premarket application is also required when a change is made to a legally marketed device or to expand the product label.
510(k) Clearance Process. To obtain 510(k) clearance, an applicant must submit a premarket notification demonstrating that the proposed device is substantially equivalent in intended use and in safety and effectiveness to a “predicate device” - either a previously 510(k) cleared device or a pre-amendment device for which the FDA has not called for premarket applications. The FDA’s 510(k) clearance process usually takes from three to 12 months, but it can last longer. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could even require a premarket application approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with the determination, the agency may retroactively require the manufacturer to seek 510(k) clearance or premarket application approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or pre-market application approval is obtained. Our PillCam ESO video capsule and the advanced versions of the RAPID software and data recorder have received FDA marketing clearance under the 510(k) clearance process.
De Novo Classification. If the FDA denies 510(k) clearance of a device because it is novel and an adequate predicate device does not exist, the “de novo classification” procedure can be invoked based upon reasonable assurance that the device is safe and effective for its intended use. This procedure approximates the level of scrutiny in the 510(k) process but may add several months to the clearance process. If the FDA grants the request, the device is permitted to enter commercial distribution in the same manner as if 510(k) clearance had been granted. We received our original FDA clearance for the Given System pursuant to the de novo classification procedure which is intended for novel but low risk devices. Our application for clearance under the de novo classification procedure included clinical data from a non-significant risk study. We cannot be sure that future studies of the Given System in the United States will be eligible for the same abbreviated regulatory treatment.
Premarket Application Approval Process. If the FDA denies 510(k) clearance for a product and denies de novo classification, the product must follow the premarket application approval process, which requires proof of the safety and effectiveness of the device to the FDA’s satisfaction. A premarket application must provide extensive preclinical and clinical trial data and also information about the device and its components regarding, among other things, device design, manufacturing and labeling. After approval of a premarket application, a new premarket application or premarket application supplement is required in the event of a modification to the device, its labeling or its manufacturing process. The premarket application approval pathway is much more costly, lengthy and uncertain. It generally takes from one to three years or longer.
FCC Clearance and Regulation
Because the Given System includes a wireless radio frequency transmitter and receiver, it is subject to equipment authorization requirements in the United States. The U.S. Federal Communications Commission, or FCC, requires advance clearance of all radio frequency devices before they can be sold or marketed in the United States. These clearances ensure that the proposed products comply with FCC radio frequency emission and power level standards and will not cause interference. We received an equipment certificate from the FCC in March 2001 based on the current system design and specifications for the Given System. Any modifications to the Given System may require new or further FCC approval before we are permitted to market and sell a modified system, and it could take several months to obtain any necessary FCC approval.
Anti-Kickback and False Claims Laws
In the United States, there are federal and state anti-kickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration intended to induce the purchase or recommendation of healthcare products and services. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation in federal healthcare programs. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices such as Given, by limiting the kinds of financial arrangements (including sales programs) we may have with hospitals, physicians and other potential purchasers of the medical devices. Other provisions of state and federal law provide civil and criminal penalties for presenting, or causing to be presented, to third-party payers for reimbursement, claims that are false or fraudulent, or which are for items or services that were not provided as claimed. Since we are providing coding and billing advice to purchasers of our products, and since we cannot be sure that the government will regard any billing errors as inadvertent, we could be subject to an enforcement proceeding in a case where our advice was deemed to have caused the submission of improper claims.
Health Insurance Portability and Accountability Act of 1996 and Related Laws
U.S. Federal and state laws protect the confidentiality of certain patient health information, including patient records, and restrict the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated patient privacy rules under the Health Insurance Portability and Accountability Act of 1996, or HIPAA.
These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their protected health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. Because we are selling products and services to persons and entities subject to HIPAA and are exposed to personally-identifiable health information in the course of our operations, we also may be subject to HIPAA, as well as similar state laws. HIPAA imposes civil and criminal penalties for violations of its provisions, which could be substantial. State privacy laws have their own penalty provisions which could apply in a given case.
Regulation in Europe
Commercialization of medical devices in member countries of the European Union is regulated by directives adopted by the European Union. The European Union presently requires that all medical products bear the CE mark, an international symbol of adherence to quality assurance standards and demonstrated clinical effectiveness. Compliance with the Medical Device Directive, as certified by a recognized European Notified Body, permits the manufacturer to affix the CE mark on its products. We received our initial authorization to affix the CE mark to the Given System in May 2001 and have since received such authorization for other products. We have authority to affix the CE mark to all our products that are currently commercially available in the European Union. In 2007, we received the CE mark for our new small bowel, esophageal and colon capsules, as well as for our RAPID 5.0 software.
If we modify the Given System, we may need to apply for permission to affix the CE mark to the modified product. Additionally, we will need to apply for a CE mark for any new products that we may develop in the future. We cannot be certain that we will be able to obtain permission to affix the CE mark for new or modified products or that we will continue to meet the quality and safety standards required to maintain the permissions that we receive. If we are unable to maintain permission to affix the CE mark to our products, we will no longer be able to sell our products in member countries of the European Union.
In Europe, the frequency range in which the Given System operates is subject to technical standards for radio frequency use developed by the Short Range Device Maintenance Group of the European Conference of Postal and Telecommunications Administrations. We currently have clearance to operate our system in Europe based on the current system design and specifications; however, modifications to the Given System may require new or further approvals before we are permitted to market and sell a modified system, and it could take several months to obtain any necessary approvals.
Regulation in Other Countries
In order for us to market the Given System in countries other than the United States, we must obtain regulatory approvals and comply with extensive safety and quality regulations in these countries. These regulations, including the requirements for approvals or clearance and the time required for regulatory review, vary from country to country. Failure to obtain regulatory approval or clearance in any foreign country in which we plan to market our product may harm our ability to generate revenue and harm our business.
In all of the countries in which we are currently selling the Given System we have either received regulatory approval or clearance or been informed that approval or clearance is not required. Renewals or updates of the regulatory status of our products in all these countries are done annually.
In Japan, we received regulatory clearance to market our RAPID workstation and PillCam SB capsule in April 2007. The clearance we have received covers an early version of our data recorder of which we now have only limited inventory remaining. Marketing our other products in Japan will require additional, product-specific regulatory clearances. In 2007, we submitted an application for marketing clearance for our DR 2.0 data recorder. We intend to submit applications for at least a few additional products in 2008. Generally, the process for obtaining marketing clearance for medical device in Japan could range from six months for products with only very minor modifications from previous cleared product versions to a few years in case of a completely new device.
Third-Party Reimbursement
Reimbursement in the United States
In the United States, healthcare providers that purchase medical devices generally rely on third-party payers, such as Medicare, Medicaid, private health insurance plans and health maintenance organizations, to reimburse all or a portion of the cost of the devices, as well as any related healthcare services utilizing the devices. FDA clearance does not result in coverage and reimbursement by third-party payers.
Coding. Generally, a current procedural terminology, or CPT, code is necessary to facilitate claims for reimbursement. If a procedure is not covered by an appropriate existing code, an application for a new code can be made to the American Medical Association, or AMA. However, this process can be lengthy, typically taking two or more years before the new code is effective. In the meantime, claims may be submitted using a miscellaneous CPT code or using a temporary G-code, if one is established by the Department of Health and Human Services’ Centers for Medicare and Medicaid Services, or CMS. In December 2002, CMS established a temporary G-code specifically for capsule endoscopy. In October 2003, the American Medical Association assigned a permanent CPT code for capsule endoscopy of the small bowel, effective January 1, 2004. With the assignment of the permanent CPT code for capsule endoscopy of the small bowel, the temporary G-code was abolished. In November 2006, the AMA and CMS assigned a permanent CPT code for capsule endoscopy of the esophagus, effective January 1, 2007.
For capsule endoscopy of the small bowel performed in a physician’s office, the CPT code includes a global value for both the technical and the professional components of the procedure. In the outpatient hospital setting, claims are submitted using the CPT code and paid by Medicare under the Ambulatory Payment Classification, or APC, which covers the technical component and includes the cost of the facility and supplies related to the procedure. The physician is paid separately for the interpretation. In January 2003, CMS established a New Technology APC for capsule endoscopy of the small bowel in the hospital outpatient setting and, in November 2004, CMS reclassified the APC into a GI diagnostic category for payment in an outpatient hospital setting, effective January 1, 2005.
For capsule endoscopy of the esophagus performed in a physician’s office, the CPT code includes a global value for both the technical and the professional components of the procedure. In the outpatient hospital setting, claims are submitted using the CPT code and paid by Medicare under the APC, which covers the technical component and includes the cost of the facility and supplies related to the procedure. The physician is paid for the interpretation separately. In November 2006, CMS assigned an upper gastrointestinal (GI) diagnostic category APC for capsule endoscopy of the esophagus in the hospital outpatient setting effective January 2007.
Reimbursement Coverage. A payor’s decision to cover a device or medical procedure is independent of the coding process, although the existence of an appropriate CPT code and APC may assist in obtaining coverage. Generally, payers may deny coverage if they determine that a procedure was not reasonable or necessary as determined by the payor, was experimental or was used for an unapproved indication. During the past several years, the major third-party payers have substantially revised their reimbursement methodologies in an attempt to contain their healthcare reimbursement costs.
Third-party payers in the United States began issuing coverage policies for capsule endoscopy in early 2002. Initially, all reimbursement policies provided coverage for capsule endoscopy of the small bowel only for the diagnosis of obscure gastrointestinal bleeding. Subsequently, reimbursement coverage has been expanded to include other indications and as of December 31, 2007, most Medicare carriers and private payers, with a total insured population in the United States of approximately 209 million individuals, also cover capsule endoscopy of the small bowel for suspected Crohn’s disease, suspected small bowel tumors and other small bowel pathologies.
We continuously are making efforts to improve reimbursement coverage for the PillCam SB capsule. For example, we are working with third party payers to expand the number of International Statistical Classification of Diseases, known as ICD, diagnosis codes, for small bowel diseases. ICD diagnosis codes are used to classify diseases and a wide variety of medical symptoms, findings and complaints and to report diagnoses to payers to support medical necessity for services provided. We believe that the availability of more ICD diagnosis codes for small bowel-related conditions will enhance the ability of physicians to submit and receive payment for claims relating to small bowel diagnostic procedures using our PillCam SB capsule, which, in turn, may result in an increased usage of this capsule.
Almost all of the reimbursement policies currently in effect require that a previous procedure, such as endoscopy or radiology, be performed prior to using the Given System and some may require prior authorization. We intend to continue our efforts to establish the Given System as a primary tool administered to patients with suspected disorders of the small bowel and to convince third party payers to provide reimbursement coverage for capsule endoscopy as a primary diagnostic tool by presenting to them economic and health outcomes analyses showing the benefits of using the PillCam SB capsule as a primary diagnostic tool. To this end, the peer-reviewed journal Disease Management published in its Winter 2004 issue an economic analysis of using PillCam SB capsules as a first-line tool for diagnosing patients with suspected Crohn’s disease. The study, conducted by researchers at Thomas Jefferson University, concluded that using the PillCam SB capsule as a first line procedure for detecting small bowel Crohn’s disease is less costly from a third-party payor perspective than existing diagnostic approaches. This publication supports the company’s efforts to convince payers to revise policies to cover PillCam SB capsules when performed as a first line procedure in patients with suspected Crohn’s disease. As a result of these efforts, several third-party payers issued new or updated policies that do not require other endoscopic procedures prior to performing small bowel capsule endoscopy. As of December 31, 2007, approximately 30 million individuals in the United States now have coverage under these new or updated policies.
As of December 31, 2007, approximately 31 million individuals had reimbursement coverage for capsule endoscopy of the esophagus using our PillCam ESO capsule to evaluate esophageal varices in patients diagnosed with cirrhosis of the liver, a chronic liver disease. Numerous payers are reimbursing this procedure on a case-by-case basis. We expect that the publication of additional clinical data will facilitate coverage of esophageal capsule endoscopy by additional third-party payers during 2008. We have established a toll-free reimbursement help-line whereby reimbursement specialists assist our customers with general information about the process of submitting prior authorizations, claims and appeals in the event of a denial.
Reimbursement Rates. Even if a device or medical procedure is covered, reimbursement rates must be adequate for most providers to use it routinely. Reimbursement rates vary depending on the third-party payor and individual insurance plan involved, the procedure performed and other factors. Medicare reimbursement for inpatient hospital services is based on a fixed amount per admission based on the patient’s specific diagnosis and the procedure performed during the hospital stay. As a result, any illness to be treated or procedure to be performed in an inpatient setting will be reimbursed only at a prescribed rate set by the government. However, the Given System is not typically subject to these restrictions for hospital inpatient services because the capsule endoscopy procedure is most often performed on an outpatient basis and reimbursed by Medicare under the outpatient regulations, which allows for separate reimbursement. Medicare is covering the capsule endoscopy procedure under the outpatient regulations because the Given System is purchased for placement in an outpatient setting, where patients are not admitted to a hospital as an in-patient for the capsule endoscopy procedure.
In 2007, for procedures performed in a physician’s office, the national average global fee paid by Medicare under the CPT code for capsule endoscopy of the small bowel was $955, a decrease of 3.2% compared to 2006. For procedures performed in an outpatient hospital setting, the national average physician fee paid by Medicare was $180, a decrease of 4.8% compared to 2006, and the national average payment rate to the hospital for the technical component was $583. These payment rates are modified annually. Effective January 1, 2008, the national average global fee paid by Medicare for a procedure in a physician’s office was decreased to $944, the national average physician fee for the professional component of a hospital outpatient procedure was decreased to $178 and the national average payment rate to the hospital for the technical component increased to $607 due to an increase in the APC rate for gastrointestinal small bowel procedures classified under APC 142.
For capsule endoscopy of the esophagus, in 2007, the national average global fee paid by Medicare for a procedure in a physician’s office was $743.. The national average physician fee paid by Medicare in an outpatient hospital setting was $53 and the national average payment rate to the hospital for the technical component was $511, under the APC 141 for upper gastrointestinal procedures. Effective January 1, 2008, the national average global fee paid by Medicare for a procedure in a physician’s office increased to $756, the national average physician fee paid by Medicare in an outpatient hospital setting increased to $55 and the national average payment rate to the hospital for the technical component increased to $541 under the APC 141 for upper gastrointestinal procedures.
Coverage Outside the United States
In countries outside the United States, coverage is obtained from various sources, including governmental authorities, private health insurance plans, and labor unions. In some foreign countries, private insurance systems may also offer payments for some therapies. Although not as prevalent as in the United States, health maintenance organizations are emerging in certain European countries. Coverage systems in international markets vary significantly by country and, within some countries, by region. Coverage approvals must be obtained on a country-by-country or region-by-region basis. In general, the process of obtaining coverage approvals has been slower outside of the United States.
In Europe, the population with reimbursable access to capsule endoscopy at the end of 2007 remained largely unchanged compared to 2006 and was approximately 166 million. The number of people in Europe with reimbursement for expanded indications at the end of 2007 was also similar to 2006 at approximately 101 million. In 2008, we expect that French authorities will approve reimbursement for PillCam SB capsule endoscopy for over 60 million citizens in France.
In Japan, authorities announced initial reimbursement coverage for our PillCam SB capsule. Effective October 1, 2007, the entire adult population in Japan is eligible for reimbursement of the PillCam SB capsule procedure for small bowel indications with obscure gastrointestinal bleeding.
In Australia, reimbursement coverage for use of capsule endoscopy in the detection of gastrointestinal bleeding exists for all residents who hold Australian and/or New Zealand citizenship, providing coverage for approximately 20 million residents.
While coverage in Austria, Switzerland, Spain, Germany and Australia is generally limited to the indication of suspected small intestinal bleeding, the public healthcare systems in the Czech Republic, Denmark, Italy, Israel, Portugal, Sweden and the United Kingdom cover capsule endoscopy for broader indications, including suspected Crohn’s disease. We cannot provide any assurance that we will obtain any additional approvals in a timely manner or at all.
C. ORGANIZATIONAL STRUCTURE
Given Imaging Ltd. is organized under the laws of the State of Israel and, as of December 31, 2007, held directly and indirectly the percentage indicated of the outstanding capital stock of the following subsidiaries:
Name of Subsidiary | | Country of Incorporation | | Percentage Ownership | |
| Given Imaging Pty. Ltd. | | | Australia | | | 100 | |
| Given Imaging, Inc. | | | United States | | | 100 | |
| Given Imaging s.a.s. | | | France | | | 100 | |
| Given Imaging GmbH | | | Germany | | | 100 | |
| Given Imaging B.V. | | | Netherlands | | | 100 | |
| Given Imaging KK | | | Japan | | | 51 | |
| Given Imaging PTE (Asia-Pacific) Ltd. | | | Singapore | | | 100 | |
D. PROPERTY, PLANTS AND EQUIPMENT
We have a total of approximately 10,300 square meters (approximately 110,869 square feet) in Yoqneam, Israel, hosting our corporate headquarters and a number of production lines, under a lease that expires in December 31, 2015.
We believe that our existing facilities will be adequate to meet our needs in Israel for the foreseeable future.
Our subsidiaries are party to the following leases:
| | • | Given Imaging, Inc. leases 2,318 square meters (24,919 square feet) of office space in Duluth, Georgia under a sub-lease that expires in September 2009; |
| | | |
| | • | Given Imaging GmbH leases approximately 818 square meters (8,801 square feet) of office space in Hamburg, Germany, pursuant to a lease for an indefinite term that may be terminated by us or the landlord with an advance notice of six months; |
| | | |
| | • | Given Imaging Pty. Ltd. leases 244 square meters (2,626 square feet) of office space in North Ryde, Australia, pursuant to a lease that expires in February 2011; |
| | | |
| | • | Given Imaging s.a.s. leases a total of 241.5 square meters (2,598 square feet) of office space in Paris, France, consisting of 168 square meters (1,808 square meters) pursuant to a lease that expires in September 2010 and that may be terminated by us at the end of each three year period upon six months’ notice and additional 73.5 square meters (791 square feet) pursuant to a lease that expires in January 2012 and that may be terminated by us at the end of October 2008; |
| | | |
| | • | Given Imaging K.K. leases 443 square meters (4,765 square feet) of office space in Tokyo, pursuant to a lease that expires in August 2010; and |
| | | |
| | • | Given Imaging PTE (Asia-Pacific) Ltd. leases 102 square meters (1,101 square feet) of office space in Singapore, pursuant to a lease that expires on August 31, 2010. |
ITEM 4A. UNRESOLVED STAFF COMMENTS
Not Applicable.
Overview
We develop, manufacture and market innovative diagnostic products for disorders of the gastrointestinal tract. Our principal product, which incorporates our core technology, is the Given System, a proprietary wireless imaging system that represents a fundamentally new approach to visual examination of the gastrointestinal tract. The Given System uses a miniaturized video camera contained in a disposable capsule, which we refer to as the PillCam capsule, that is easily ingested by the patient and delivers high quality color video in a noninvasive manner. Our long-term objective, subject to further development and receipt of regulatory clearances and/or approvals, is to establish the Given System as a platform for diagnosis of disorders in all parts of the gastrointestinal tract and the PillCam capsules as a primary diagnostic tool administered to patients with such suspected disorders. We believe that each segment of the gastrointestinal tract presents meaningful opportunities for patient-friendly diagnostic procedures.
In 2001, we commenced marketing the Given System with the PillCam SB capsule for detection of disorders of the small bowel. As of December 31, 2007, we had an installed base of nearly 4,250 Given Systems and had sold over 650,000 PillCam SB capsules in more than 60 countries worldwide. Since November 2004, we also market and sell our PillCam ESO capsule for visualization of the esophagus. In the second half of 2007, we began selling the first generation of our PillCam COLON capsule in Europe following receipt of the CE mark to market this capsule throughout the European Union. To date, we have not been able to obtain FDA clearance to market the PillCam COLON capsule in the United States. We have also developed and are selling in the United States, Europe and several other jurisdictions, a patency capsule, which is a dissolvable capsule that enables physicians to determine whether there are obstructions or strictures in the gastrointestinal tract that may prevent passage of our PillCam capsules.
We were incorporated in Israel in January 1998. We raised approximately $53.2 million of net proceeds in our initial public offering and were listed on the Nasdaq Global Market in October 2001. We completed a follow-on offering in June 2004 in which we raised additional net proceeds of $44.3 million. Since March 2004, our shares have also been listed on the Tel Aviv Stock Exchange. Since our inception, we have devoted substantially all of our resources to developing the Given System, performing clinical trials and marketing and selling the Given System and PillCam capsules.
Revenues
We derive our revenues from sales of the Given System, consisting of a RAPID workstation, a portable data recorder, and disposable PillCam capsules, and from recurring sales of our PillCam capsules to our installed base. We also derive a small portion of our revenues from post-sale customer support contracts entered into by customers at the end of the warranty period for the computer workstation and data recorders. We also derive limited revenues from sales of our AGILE Patency System.
Revenue breakdown. In the early years since we commenced sales of the Given System, the majority of our revenues came from sale of the Given System to new customers. However, with nearly 4,250 systems now installed worldwide, of which nearly 2,640 are in the United States, a substantial majority of our revenues is generated from reorders of our PillCam capsules, particularly PillCam SB capsules, by existing customers. The proportion of our revenues derived from sales of capital equipment components of the Given System, such as the RAPID workstations and portable data recorders, and revenues derived from sales of the PillCam capsules, which generate recurring sales, is an important indicator of our results of operations. In recent years, we have seen a gradual increase in the share of revenues generated from recurring sale of our PillCam capsules. In 2007, we derived approximately 80.3% of our revenues from sales of the PillCam SB capsules, compared to 80.4% of our revenues in 2006 and 72.1% of our revenues in 2005. Sales of the PillCam ESO capsule represented approximately 0.9% of our revenues in 2007, compared to 1.5% of our revenues in 2006 and 5.0% of our revenues in 2005. Sales of PillCam COLON represented approximately 1.0% of our revenues in 2007. We expect that a substantial majority of our revenues in the future will continue to come from recurring sales of our PillCam capsules.
The following table sets forth information for the periods indicated regarding the breakdown of our revenues:
| | | $ | | % of annual revenues | |
| | | 2005 | | 2006 | | 2007 | | 2005 | | 2006 | | 2007 | |
| | | (U.S. dollar in thousands) | |
| Workstations and data recorders | | $ | 17,831 | | $ | 14,104 | | $ | 15,267 | | | 20.5 | % | | 14.8 | % | | 13.5 | % |
| PillCam SB capsule | | | 62,528 | | | 76,360 | | | 90,614 | | | 72.1 | | | 80.4 | | | 80.3 | |
| PillCam COLON capsule | | | N/A | | | N/A | | | 1,106 | | | N/A | | | N/A | | | 1.0 | |
| PillCam ESO capsule | | | 4,384 | | | 1,438 | | | 1,012 | | | 5.1 | | | 1.5 | | | 0.9 | |
| Patency system and capsule | | | 174 | | | 353 | | | 523 | | | 0.2 | | | 0.4 | | | 0.4 | |
| Service | | | 1,859 | | | 2,774 | | | 4,346 | | | 2.1 | | | 2.9 | | | 3.9 | |
| Total | | $ | 86,776 | | $ | 95,029 | | $ | 112,868 | | | 100.0 | % | | 100.0 | % | | 100 | % |
Workstations and data recorders. In 2007, our revenues from the sale of workstations and data recorders increased compared to our revenues from the sale of these products in 2006 due to the initial clearance we received to market our system and Pillcam SB capsule in Japan. Typically, in new markets sales of capital equipment are more significant than sales of disposables such as the PillCam capsules. In the more mature markets, such as Europe and the United States, our revenues from sale of workstations and data recorders are decreasing compared to prior periods. The decrease in revenues from sale of capital equipment in those other markets is attributable to both lower quantities of workstations and data recorders sold and lower average selling price. We believe that, except in Japan and France, sales of workstations and data recorders is not likely to grow at historical rates or at all since a majority of gastroenterologists in our main markets already have access to a capsule endoscopy system. Another important reason for this decrease in sales of capital equipment was our continued focus on increasing utilization and reorders of the PillCam SB capsule in existing accounts over capital equipment sale.
In addition, with the introduction of new products or newer versions of existing products or as part of our promotional activities, we place our capital equipment or replace older equipment of many customers with newer versions of our capital equipment at a reduced price. This resulted in a lower average selling price for our capital equipment and contributed to the decline in revenues from sales of capital equipment.
In 2008, revenues from sales of workstations and data recorders may increase further in Japan and France. Generally, however, we believe that a significant majority of our revenues in the foreseeable future will come from the sale of capsules compared to capital equipment.
PillCam SB. Substantially all of our revenues from capsule sales were attributed to sales of the PillCam SB capsule, which we began selling worldwide in the fourth quarter of 2001. We expect recurring sales of the PillCam SB capsule to continue to account for a substantial majority of our revenues from capsule sales in 2008. The primary reasons for this are:
| • | Most small bowel indications for which the PillCam SB capsule is used are covered by governmental and commercial reimbursement policies. |
| | |
| • | Several reimbursement policies cover small bowel capsule endoscopy as a primary diagnostic tool, without the requirement to perform other diagnostic procedures prior to using the PillCam SB capsule. We expect this trend to contribute to more capsule endoscopy procedures of the small bowel being performed. |
| | |
| • | We recently began selling in the United States our new small bowel capsule, PillCam SB 2, which is more advanced then previous versions. |
| | |
| • | Our sales force is primarily focused on selling capsules and the market for the PillCam SB capsule is currently a more developed market compared to the market for our PillCam ESO capsule or PillCam COLON capsule. |
| | |
| • | Receipt of regulatory clearance to market the PillCam SB capsule in Japan in April 2007. |
PillCam ESO. We have marketed the PillCam ESO in the Unites Stated since November 2004. From 2005 through the end of 2007, sales of our PillCam ESO were insignificant due primarily to the lack of reimbursement coverage, as well as limited clinical data to support widespread use of the PillCam ESO capsule.
Effective January 1, 2007, a permanent CPT Code was assigned to our PillCam ESO capsule by the American Medical Association, or AMA, and the Center for Medicare and Medicaid Services, or CMS, and during 2007 we saw the first third-party reimbursement coverage for procedures with the PillCam ESO capsule to evaluate esophageal varices in patients diagnosed with cirrhosis of the liver, a chronic liver disease. In addition, in late 2006, we completed a large multi-center study to evaluate the use of PillCam ESO in the evaluation of esophageal varices in patients diagnosed with cirrhosis of the liver. As of December 31, 2007, approximately 31 million individuals in the United States had reimbursement coverage for using the esophageal capsule endoscopy procedure in the detection of esophageal varices. Reimbursement coverage for the use of PillCam ESO in the detection of Gastro-Esophageal Reflux Disease, or GERD, which is more prevalent in the general population than varices, is not expected before additional clinical data supporting such use is available.
In the first quarter of 2008, we began marketing and selling PillCam ESO 2, our newest version of our esophageal capsule. We believe that this new capsule is a significant improvement over the prior version and will contribute to increased demand for PillCam ESO. The existence of initial reimbursement and favorable clinical data to support to use of PillCam ESO in the evaluation of esophageal varices, are also expected to gradually increase the demand for our PillCam ESO capsule, mostly for the varices indication. However, because esophageal varices is not a prevalent condition in the general population, we expect that sales of PillCam ESO in the foreseeable future will continue to be small compared to sales of PillCam SB. We expect a more significant increase in sales of PillCam ESO when and if there is clinical data and reimbursement coverage to support and cover the use of this capsule in GERD patients. In 2008, we intend to begin market development activities for the GERD market.
In November 2007, we announced the termination of our exclusive sales representation, co-promotion and cooperation agreement with Ethicon Endo-Surgery, a Johnson & Johnson company, which was signed in May 2004. InScope, a business division of Ethicon Endo-Surgery had exclusive rights to market our PillCam ESO capsule in the United States during this period and performed sales and market development activities. Ethicon cited a shift in its strategic priorities within gastroenterology and other areas as the reason for ending the relationship. As a result of this termination, we regained full responsibility for the marketing and sale of the PillCam ESO capsule in the United States at the beginning of 2008. In accordance with the terms of termination, Ethicon has paid us $1.2 million to continue to fund existing clinical trial activities in 2008 relating to the PillCam ESO capsule until they are completed. In addition, Ethicon has paid us $7.6 million in fees associated with the termination of the agreement.
PillCam COLON. PillCam COLON is the third video capsule we developed. We believe the launch of this capsule in Europe in the second half of 2007 reinforces the Given Platform as the leading product for capsule endoscopy and demonstrates our commitment to this field. Sales of PillCam COLON in 2007 were limited. This capsule became available in Europe only in the second half of 2007. In addition, this capsule is a new tool for diagnosis of colon disorders and we expect it will take time to educate physicians about its benefits and how it can fit into their practice management routines. Finally, there is only limited clinical evidence to support the use of this capsule. We are currently conducting a number of clinical trials that we believe will support our market development activities. In the meantime, we market PillCam COLON in Europe as a complementary tool for colonoscopy offering a method for visualization of the colon in patients who are unable or unwilling to undergo traditional colonoscopy or in cases of incomplete colonoscopies.
PillCam COLON has not yet received FDA marketing clearance in the United States. The FDA has recently determined that the PillCam COLON capsule is not substantially equivalent to any marketed device in the United States for visualization of the colon and therefore can not be cleared for marketing in the United States, our biggest market, through the 510(k) process, based on the currently available clinical data. There can be no assurance that we will be able to obtain FDA clearance for the PillCam COLON capsule, or even if we do, that this capsule will achieve widespread market acceptance as superior to existing technologies for visualization of the colon. We believe the following are important factors in determining the success of this product in the foreseeable future:
| | • | Receipt of FDA marketing clearance in the United States: We cannot be sure that FDA clearance or other regulatory approvals will be granted. In order to obtain FDA clearance and other regulatory approvals, we will be required to demonstrate that the PillCam COLON is safe and effective for its intended purpose. |
| | | |
| | • | Clinical data sufficient to support the use of the PillCam COLON for visualization of the colon as compared to other diagnostic modalities: If clinical trials indicate that PillCam COLON is not as clinically-effective as other current methods, or if the PillCam COLON procedure causes unexpected complications or other unforeseen negative effects, we may not obtain regulatory clearance to market and sell this capsule. |
| | | |
| | • | The availability of sufficient clinical and cost-effectiveness data for the American Medical Association, or AMA, to provide a favorable permanent “current procedural terminology”, or CPT, code, and for private third-party payers to make an adequate reimbursement decision to provide coverage for the PillCam COLON procedure. |
| | | |
| | • | The availability of a reliable colon cleansing and preparation procedure for the PillCam COLON capsule, which is accepted by physicians and patients. |
We believe that there is a significant market and a long-felt need among physicians and patients for a simple, cost-effective and non-invasive technique for colorectal cancer screening, which represents a significant market opportunity for the PillCam COLON capsule. According to guidelines of professional associations in the United States, it is estimated that approximately 12.5 million people over the age of 50 or that are otherwise at increased risk for colon cancer need to be screened each year in the United States, yet patient compliance is only around 50 percent. Patient compliance rates in Europe are believed to be even lower. We believe that the PillCam COLON procedure will eventually provide a less invasive alternative to traditional colonoscopy. However, further capsule and procedure development as well as significant additional clinical data to support the use of this capsule as a screening tool are necessary before we can realize this market opportunity. In the meantime, we market PillCam COLON in Europe for visualization of the colon in patients who are unable or unwilling to undergo traditional colonoscopy or in cases of incomplete colonoscopies.
While we expect sales of PillCam COLON to increase gradually, we do not expect that the PillCam COLON capsule will contribute significantly to our revenues in 2008 for the following reasons:
| | • | We are not likely to receive FDA clearance for the PillCam COLON capsule in 2008; |
| | | |
| | • | Clinical data for the PillCam COLON is limited; and |
| | | |
| | • | Reimbursement for the PillCam COLON procedure is not expected in 2008. |
PillCam capsule reorders. The portion of our total revenues resulting from recurring capsule sales is an important indicator for measuring our results of operations because it indicates the level of adoption by physicians of the Given System. We seek to increase the level of recurring sales of our PillCam capsule by a number of methods, including:
| | • | broadening the reimbursed indications, conducting clinical trials to prove the clinical benefits of capsule endoscopy compared to other diagnostic procedures of the gastrointestinal tract and educating physicians regarding the clinical benefits of the PillCam capsules; |
| | | |
| | • | increased selling and marketing activities and more frequent contact with our customers to inform and educate them about our technology; and |
| | | |
| | • | enhancing operating efficiencies of the system to allow physicians to incorporate capsule endoscopy into their daily practice routines. |
The following table sets forth information for the periods indicated regarding the total numbers of PillCam SB capsules sold and the percentage of revenues derived from such sales which represent reorders:
| | | 2005 | | 2006 | | 2007 | |
| Total number of PillCam SB capsules sold* | | | 135,500 | | | 165,000 | | | 191,800 | |
| Number of PillCam SB capsule sales representing reorders | | | 128,760 | | | 155,840 | | | 177,000 | |
| % of revenues from capsule sales that represent reorders | | | 95% | | | 94.4% | | | 92.3% | |
| * | Sales of PillCam ESO began in November 2004 and reorders in subsequent years were negligible primarily due to the lack of reimbursement. Accordingly, reorders of PillCam ESO are not included in the above table. |
Seasonality
We believe that demand for systems and capsules may be affected by seasonal factors during the summer months when physicians and administrators are more likely to postpone purchasing decisions relating to the Given System due to summer vacations, and patients are more likely to postpone less urgent diagnostic procedures until later in the year. We believe that the seasonal effect in the third quarter may become more pronounced if the portion of our revenues derived from reorders continues to grow.
Geographical breakdown
We derived 66% of our revenues in 2007 from the Americas, compared to 71% in 2006. The following table sets forth the geographic breakdown of our revenues for the periods indicated:
| | | 2005 | | 2006 | | 2007 | |
| Americas | | | 75% | | | 71% | | | 66% | |
| EMEA | | | 19% | | | 23% | | | 24% | |
| APAC | | | 6% | | | 6% | | | 10% | |
We expect that a significant portion of our revenues in the foreseeable future will continue to come from the United States, mainly due to our ability to leverage existing market share to generate additional sales, a favorable reimbursement system and general acceptance of new technologies among physicians. In Japan, we received marketing clearance for the Given System and our PillCam SB in April 2007 and began selling the Given System there. Since Japan is a new market, we expect to initially focus on the placement of systems in order to expand our market penetration. Behind the United States, Japan is considered one of the largest markets in the world for medical devices for use in the gastrointestinal tract. Therefore, we believe that the Japanese market represents a significant growth opportunity for our business in 2008 and beyond.
Reimbursement
We believe that the existence of reimbursement coverage and the amount of reimbursement will continue to significantly affect the proportion of our revenues that are derived from sales in the United States. Availability of reimbursement is a key factor in the decision of physicians and healthcare providers to purchase the Given System and perform capsule endoscopy procedures. Once a payor has decided to provide reimbursement for use of the Given System, the level of reimbursement coverage provided also becomes a key factor in a physician’s decision. We estimate that as of December 31, 2007, reimbursement for small bowel capsule endoscopy was available worldwide to approximately 500 million people. In the United States approximately 209 million people are covered with most reimbursement policies providing coverage for a number of small bowel indications, including obscure bleeding, suspected Crohn’s disease, suspected small bowel tumors and other small bowel pathologies. In Europe, reimbursement coverage for small bowel capsule endoscopy was available for approximately 166 million people as of December 31, 2007, and reimbursement coverage for expanded indications, such as Crohn’s disease and other small bowel disorders was available for approximately 101 million people at the end of 2007. While we initially expected that reimbursement in France will be in place in late 2007, the process with French authorities is taking longer than we expected. We now believe the reimbursement process in France could be completed in the first half of 2008, which will result in coverage for approximately 60 million people. This represents a significant growth opportunity for us in Europe.
Another significant growth opportunity stems from the decision of Japanese authorities to reimburse capsule endoscopy of the small bowel. Effective October 1, 2007, the entire adult population in Japan (approximately 105 million people) is eligible for reimbursement of the PillCam SB capsule procedure for small bowel indications with obscure gastrointestinal bleeding. We believe this reimbursement will contribute to significant growth in sales of the Given System with the PillCam SB capsule in Japan. Finally, reimbursement coverage for use of the PillCam SB capsule in the detection of gastrointestinal bleeding is available for approximately 20 million individuals in Australia and New Zealand.
In addition to continuing our efforts to expand reimbursement coverage, we have made significant efforts in educating our customers regarding coverage conditions and rates. For example, we maintain more frequent contact with existing and potential payers on the one hand, and with our customers on the other hand. We believe that increased customer awareness and knowledge is important to remove any misunderstandings that may exist among physicians regarding the availability of reimbursement or coverage rates and to allow more patients the benefit of our technology. We also maintain a reimbursement telephone support line to respond directly to inquiries from customers.
In 2007, we also continued our efforts to improve reimbursement coverage for the PillCam SB capsule. First, we are working with third party payers to expand the number of International Statistical Classification of Diseases, known as ICD, diagnosis codes, for small bowel diseases. ICD diagnosis codes are used to classify diseases and a wide variety of medical symptoms, findings and complaints and to report diagnoses to payers to support medical necessity for services provided. We believe that the availability of more ICD diagnosis codes for small bowel-related conditions will enhance the ability of physicians to submit and receive payment for claims relating to small bowel diagnostic procedures using our PillCam SB capsule, which, in turn, may result in an increased usage of this capsule. Second, we continue our efforts to establish the PillCam SB capsule as a primary diagnostic tool for patients with suspected disorders of the small bowel and to convince third party payers to provide reimbursement coverage for capsule endoscopy as a primary diagnostic tool. Coverage as a primary diagnostic tool means that physicians will be able to prescribe the PillCam SB capsule at their discretion, without the requirement to perform any previous endoscopic diagnostic procedure. We believe this could increase physicians’ use of the PillCam SB capsule. To convince the payers, we will need to present to them economic and health outcomes analyses showing the benefits of using the PillCam SB capsule as a primary diagnostic tool. As of December 31, 2007, approximately 30 million individuals in the United States had reimbursement coverage for PillCam SB as a primary diagnostic tool.
In the United States, effective January 1, 2008, the national average global fee paid by Medicare for a procedure in a physician’s office was decreased to $944 from $955 in 2007 and the physician fee for the professional component of a hospital outpatient procedure was decreased to $178 from $180 in 2007. Reimbursement rates may also be modified in the future. Based on recent history, we do not expect that modest changes to reimbursement rates will have a material effect on our business.
Effective January 1, 2007, we have a permanent CPT code for the esophageal capsule endoscopy procedure with our PillCam ESO. We have also obtained the first few reimbursement policies for this procedure with 31 million individuals in the United States covered as of December 31, 2007.
To date, there is no reimbursement coverage for procedures with our PillCam COLON capsule. This capsule is new to the market and more clinical evidence will be necessary to support reimbursement. Reimbursement coverage for this capsule is not expected in 2008.
Competition
Olympus Corporation has been marketing and selling a competing capsule endoscopy system in Europe and Australia since October 2005. In September 2007, Olympus received FDA clearance to market its capsule endoscopy system and small bowel capsule in the United States. According to public sources, Olympus is also seeking regulatory clearance to market its capsule endoscopy system in Japan. In addition to Olympus, a Chinese company is selling its capsule endoscopy systems in China at lower prices than us and presented its systems at industry trade shows outside Asia. Finally, according to publicly available information, in 2007 a South Korean company began selling a competing system in Korea as well as in Europe and Australia.
The effect of competition from Olympus or other possible direct competitors is uncertain. On the one hand, we may lose market share, be forced to reduce the price of our products or experience delays in completing sales as a result of a longer decision making process among potential customers. On the other hand, we believe that the entry of Olympus into the capsule endoscopy market further validates the market opportunity of our PillCam platform and may result in greater market acceptance of our products, which we believe have a number of advantages over competing products. First, we were the first company to sell and market capsule endoscopes in the most mature markets in the United States, Europe and Japan and have a large installed base of customers. Second, we have been concentrating primarily on the development and sale of capsule endoscopy systems and have a platform enabling us to use our line of PillCam capsules for diagnosing disorders in areas of the gastrointestinal tract in addition to the small bowel, while Olympus only has a small bowel capsule at this time and has not announced the development of other capsules. Third, we believe our technological solution, and in particular our software solution, is superior to the products introduced by competitors. Finally, we have a patent portfolio that we believe protects critical aspects of our technology and may create technological barriers for our competitors, which may force them to enter the market with inferior products or delay their entry into certain territories.
Customers and customer concentration
We market and sell the Given System through a direct sales force in the United States, Germany, France, Australia and Israel. We rely on third-party distributors in international markets outside these countries. We sell the Given System primarily to hospitals, gastroenterology offices and gastroenterology outpatient facilities. In 2007, we derived $24.7 million, or 21.8%, of our revenues from sales to local distributors, compared to $15.7 million, or 16.5% in 2006. The increase in sales to distributors is attributable primarily to increased sales to our exclusive distributor in Japan after we received regulatory clearance to market our PillCam SB in Japan. Our direct sales revenues are derived from a large number of individual customers and have higher gross margins. In 2007, no single direct sales customer accounted for more than 0.3% of our revenues and no single distributor accounted for more than 4.6% of our revenues. It is our policy to require collateral or security in connection with sales to distributors. Due to these factors and the geographical dispersion of our customers, we believe that we adequately control our exposure to credit risks associated with accounts receivable. To date, we have not experienced any material bad debts and we have collected substantially all of our receivables.
Cost of revenues and gross margins
Cost of revenues consists primarily of materials and components, as well as manufacturing costs and related depreciation of our production facilities, the salary and related costs of our technical staff assembling our products, royalties payable to the Office of the Chief Scientist, or OCS, warranty costs and product liability insurance. In December 2007, we made an early repayment of all our outstanding royalty obligation and accrued interest to the OCS. The repayment resulted in a charge of $4.8 million to cost of revenues.
The principal factors affecting our gross margins are related to the volume of PillCam capsules we sell, the sale prices, the product mix (namely, the proportion of our revenues derived from sales of workstations and portable data recorders, compared to sales of the PillCam capsules), as well as the percentage of our sales made as direct sales. In general, our gross margins from capsule sales are higher than our average gross margins from sales of capital equipment, such as workstations and data recorders. A primary reason for the lower gross margins is that from time to time, with the introduction of new products or newer versions of existing products or as part of our promotional activities, we place our capital equipment or replace older equipment of many customers with newer versions of our capital equipment at a reduced price or at no charge. In addition, our gross margins in territories in which we use our direct sales force are generally higher than our gross margins in territories in which we market and sell our products through third-party distributors. In the future, our gross margins may also be negatively affected by the emergence of direct competition in the field of capsule endoscopy.
Operating expenses
Research and development. Our research and development expenses consist primarily of costs associated with the design, development, pre-manufacture and testing of, and enhancements to, the Given System, clinical studies and obtaining regulatory approvals, patent costs, sponsored research costs and other expenses related to our product development and research program. We expense our research and development costs as they are incurred. “Research and development expenses, net” are net of grants received from the Israeli Government through the Office of the Chief Scientist of the Ministry of Industry and Trade. We plan to continue investing in research and development, as we enhance the Given System, pursue the development of new products and perform more clinical trials to drive continued expansion of reimbursement for the PillCam capsules worldwide.
Sales and marketing. Our sales and marketing expenses consist primarily of salaries, commissions to our sales force, travel and related costs for our internal sales staff and costs related to marketing activities such as medical meetings, medical training and education, trade shows, and promotional and public relations activities, as well as costs associated with development of our website. We expect that our selling and marketing expenses will increase in the future as we increase sales of PillCam capsules, further expand our sales and marketing team, expand our educational activities and expand our promotional efforts. From November 2004 until December 31, 2007, our marketing expenses included commissions we paid Ethicon Endo-Surgery under our exclusive sales representation, co-promotion and cooperation agreement for the marketing of the PillCam ESO capsule. Under this agreement, we paid Ethicon commissions on sales in the United States at a rate of 50% on the sale of the PillCam ESO capsule and 10% on sales of capital equipment parts of the Given System, including workstations and data recorders, to customers that use the PillCam ESO capsule. Under the terms of the agreement, as of December 31, 2007, we received from Ethicon milestone payments totaling $25 million. We have recorded these milestone payments as a reduction of commission expenses and recognize them ratably over the term of the agreement. Our agreement with Ethicon was terminated in January 2008. Ethicon has paid us $7.6 million in fees associated with the termination of the agreement. In addition, Ethicon has paid us $1.2 million to continue to fund existing clinical trial activities in 2008 relating to the PillCam ESO capsule until they are completed. As a result of the early termination of our agreement with Ethicon, we accelerated the effect of the milestone payments we had received from Ethicon prior to the termination and recorded additional credit to operating expenses of approximately $23 million in the fourth quarter of 2007. This credit has resulted in an increase to our net income in a similar amount. We will record additional credit to operating expenses of approximately $5.4 million as a result of this early termination during the first quarter of 2008.
General and administrative. Our general and administrative expenses consist primarily of salaries and related costs for our executive and administrative staff, insurance premiums, and legal, accounting and consulting expenses. Our general and administrative expenses increased significantly in 2007 and expect to continue at a relatively high level in 2008, primarily as a result of legal expenses associated with our patent litigation in the United States with Olympus.
Equity-based compensation. Our operating expenses also include amortization of stock-based compensation, which is allocated among research and development expenses, marketing expenses and general and administrative expenses based on the division in which the recipient of the option grant is employed. In December 2004, the Financial Accounting Standard Board, or FASB, issued a new Financial Accounting Standard, SFAS 123(R), that requires companies, including Given Imaging, to recognize as an expense the grant-date fair value of stock options and other equity-based compensation to employees. Effective January 1, 2006, we adopted SFAS 123(R) using the modified prospective method for the valuation of our equity-based compensation. The adoption of SFAS 123(R) resulted in the recognition of $5.7 million of additional compensation expense in 2007, compared to $5.2 million of additional compensation expense in 2006, the first year in which this accounting standard was in effect. The application of FAS 123(R) has a material impact on our earnings per share. In 2008, we expect to continue our practice of granting stock options and other equity awards to our directors, officers, employees and consultants. The resulting compensation expenses in 2008 will be impacted by various factors, including the number of options we grant and their fair value at the date of grant.
Financing income, net. Financing income, net consists primarily of interest earned on our cash balances, income from marketable securities and foreign exchange gains or losses, net of financing expenses. Financing expenses consist primarily of bank fees and currency fees.
Taxes. In 2007, Israeli companies were generally subject to income tax at the corporate tax rate of 29%. This tax rate is expected to be gradually reduced to a rate of 25% by 2010. However, our investment program in leasehold improvements and equipment at our manufacturing facility in Yoqneam, Israel has been granted approved enterprise status and, therefore, we are eligible for the reduced tax benefits described later in this section in “Corporate Tax.” These benefits should result in income recognized by us from our investment program being tax exempt for a specified period. However, these benefits may not be applied to reduce the tax rate for any income that is not derived from sales of our product manufactured at our facility in Yoqneam, Israel.
As of December 31, 2007, we have recorded a net deferred tax asset of $1.4 million. On a regular basis, we estimate our actual current tax exposure and assess temporary differences resulting from differing treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheet. The deferred tax asset represents our assessment of accumulated losses that could be carried forward to future years to reduce taxable income in those future years. We consider projected future taxable income and tax planning strategies in making this assessment. We must then assess the likelihood that our deferred tax assets will be recovered and, to the extent we believe that recovery is not likely, we must establish a valuation allowance. To the extent we establish a valuation allowance or increase this allowance in a period, we must include an expense within the tax provision in the statement of operations. The deferred tax asset may be realized over time, depending upon the generation of future taxable income during the periods in which those accumulated losses become deductible.
Significant management judgment is required in determining our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. Based upon our projections for future taxable income of our subsidiaries over the periods in which the deferred tax assets are deductible, we believe that it is more likely than not that we will realize the benefits of this deferred tax asset. However, the amount of the deferred tax asset considered realizable could be reduced in the near term if estimates of future taxable income during the carry-forward period are reduced. Significant management judgement is also required in determining our tax liability under FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes,” which we adopted on January 1, 2007.
Minority share in losses (profits) of subsidiary. Minority share in losses (profits) of subsidiary consists of the losses attributed to the 49% interest of minority shareholders in our 51% controlled Japanese subsidiary, Given Imaging K.K.
Critical Accounting Policies
Our significant accounting policies are more fully described in Note 1 to our consolidated financial statements. However, certain of our accounting policies are particularly important to the description of our financial position and results of operations. In applying these critical accounting policies, our management uses its judgment to determine the appropriate assumptions to be used in making certain estimates. Those estimates are based on our historical experience, the terms of existing contracts, our observation of trends in the industry, information provided by our customers and information available from other outside sources, as appropriate. These estimates are subject to an inherent degree of uncertainty. With respect to our policies on revenue recognition, warranty costs and inventories, our historical experience is based principally on our operations since we commenced selling the Given System in the second quarter of 2001. Our critical accounting policies include:
| | • | Revenue recognition. We recognize revenues from sales of the Given System upon delivery, provided that collection of payment is probable, there is persuasive evidence of an arrangement, no significant obligations in respect of installation remain and the price is fixed or determinable. Our arrangements with customers and distributors do not contain product return rights. Certain of our sales contracts include a post-contract customer support, or PCS, component. We defer recognition of the revenue attributed to the PCS component of the sale and recognize revenue based on the term of the support period, which is generally a one-year period following the sale. The fair value of the PCS component is based on the price at which we sell customer support contracts separately following the expiration of the standard warranty period for our products. |
| | | |
| | • | Inventories. Inventories are stated at the lower of cost or market, cost being determined on the basis of the average cost method for raw materials and finished goods and on the basis of actual manufacturing costs for work-in-progress and sub-contractors. We write down fully the cost of components in our inventory which we discover do not perform during the production process. As we expand and enhance our manufacturing operations, the write down of amounts of non-performing components in our inventory may change. Spare parts and raw materials that are no longer used in producing our products are written down to their fair market value. In addition, we add to the cost of finished products held in inventory the overhead from our manufacturing process. |
| | | |
| | • | Foreign currency translation. In preparing our consolidated financial statements, we are required to translate non-U.S. dollar amounts in our financial statements and the financial statements of our subsidiaries into U.S. dollars. Under the relevant accounting guidance the treatment of any gains or losses resulting from this translation is dependent upon our management’s determination of the functional currency of each subsidiary. The functional currency is determined based on management’s judgment and involves consideration of all relevant economic facts and circumstances affecting the subsidiary. Generally, the currency in which the subsidiary transacts a majority of its transactions, including billings, financing, payroll and other expenditures would be considered the functional currency. However, any dependency upon the parent and the nature of the subsidiary’s operations must also be considered. If any subsidiary’s functional currency is deemed to be the local currency, then any gain or loss associated with the translation of that subsidiary’s financial statements into U.S. dollars would be included as other comprehensive income. However, if the functional currency of a subsidiary is deemed to be the U.S. dollar, then any gain or loss associated with the translation of these financial statements would be included within statement of operations. Based on our assessment of the factors discussed above, we consider the U.S. dollar to be the functional currency for each of our subsidiaries. Therefore, all gains and losses from translations are recorded in our statement of operations and are included in determining our net income. In the event that we determine that the functional currency of these or any future subsidiaries is not the U.S. dollar, any foreign currency gains or losses would not affect our net income for the year presented. |
| | • | Accounting for income taxes. As part of the process of preparing our consolidated financial statements we are required to estimate our income taxes in each of the jurisdictions in which we operate. This process requires us to estimate our actual current tax exposure and make an assessment of temporary differences resulting from differing treatment of items for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheet. We must then assess the likelihood that our deferred tax assets will be recovered and, to the extent we believe that recovery is not likely, we must establish a valuation allowance. To the extent we establish a valuation allowance or increase this allowance in a period, we must include an expense within the tax provision in the statement of operations. Significant management judgment is required in determining our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We have recorded a deferred tax asset, net of $1.4 million as of December 31, 2007. Based upon our projections for future taxable income in our U.S. subsidiary over the periods in which the deferred tax assets are deductible, we believe that it is more likely than not that we will realize the unreserved benefits of these deductible differences. The amount of the deferred tax asset considered realizable, however, could be reduced in the near term if estimates of future taxable income during the carry forward period are reduced. Effective January 1, 2007, we adopted the provision of FASB Interpretation No. 48, “Accounting for Uncertainties in Income Taxes” which prescribes a recognition threshold and measurement process for recording in the financial statements uncertain tax positions taken or expected to be taken in a tax return. We performed our evaluation mainly for the tax years ended December 31, 2004 and thereafter and for earlier tax years which remain subject to examination by local tax authorities in some tax jurisdictions. |
| | | |
| | • | Accounting for Equity Awards. Effective January 1, 2006, we adopted SFAS No. 123(R), which supersedes Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees”. Generally, the approach in SFAS 123(R) is similar to the approach described in SFAS 123 “Accounting for Stock-Based Compensation” (“SFAS No. 123”). However, SFAS 123(R) requires all equity-based payments to employees, including grants of employee stock options, to be recognized in the statement of income based on their fair values. We elected the modified-prospective method and therefore prior periods were not restated. Under the modified-prospective method, compensation costs recognized in 2007 include also compensation costs for all share-based payments granted prior to, but not yet vested, as of December 31, 2007. In 2007, we recognized equity-based compensation expense under SFAS 123(R) in the amount of $5.7 million. When calculating this equity-based compensation expense we took into consideration awards that are ultimately expected to vest. Therefore, this expense has been reduced for estimated forfeitures. In our pro forma information required under SFAS No. 123 for the periods prior to fiscal 2007, we accounted for forfeitures as they occurred. We elected to apply the intrinsic value-based method prescribed in APB Opinion No. 25 for our equity-based compensation to employees and directors and provide the pro forma disclosure provisions of SFAS No. 123, as amended by SFAS No. 148, “Accounting for Stock-Based Compensation - Transition and Disclosure, an amendment of SFAS No. 123”. As such, we computed and recorded compensation expense for grants whose terms were fixed with respect to the number of shares and option price only if the market price on the date of grant exceeded the exercise price of the stock option. The compensation cost for the fixed plans was recorded over the period the employee performs the service to which the stock compensation relates. |
Results of Operations
Our consolidated statements of operations data for the years ended December 31, 2005, 2006 and 2007 are set forth below:
| | | Year ended December 31, | |
| | | 2005 | | 2006(*) | | 2007(*) | |
| | | (in thousands) | |
Statements of Operations Data: | | | | | | | | | | |
| Revenues | | $ | 86,776 | | $ | 95,029 | | $ | 112,868 | |
| Cost of revenues | | | (22,070 | ) | | (24,154 | ) | | (29,721 | ) |
| Early repayment of royalty bearing government grants | | | — | | | — | | | (4,843 | ) |
| Gross profit | | | 64,706 | | | 70,875 | | | 78,304 | |
| Operating expenses: | | | | | | | | | | |
| Research and development, gross | | | (8,833 | ) | | (12,678 | ) | | (12,847 | ) |
| Royalty-bearing government grants | | | 1,244 | | | 1,867 | | | 1,242 | |
| Research and development, net | | | (7,589 | ) | | (10,811 | ) | | (11,605 | ) |
| Sales and marketing | | | (43,281 | ) | | (50,732 | ) | | (55,446 | ) |
| General and administrative | | | (9,657 | ) | | (16,027 | ) | | (20,981 | ) |
| Termination of marketing agreement | | | — | | | — | | | 22,860 | |
| Other | | | — | | | — | | | (422 | ) |
| Total operating expenses | | | (60,527 | ) | | (77,570 | ) | | (65,594 | ) |
| Operating profit (loss) | | | 4,179 | | | (6,695 | ) | | 12,710 | |
| Financing income, net | | | 762 | | | 3,980 | | | 5,520 | |
| Profit (loss) before taxes on income and minority interest | | | 4,941 | | | (2,715 | ) | | 18,230 | |
| Income tax benefit (expense) | | | 286 | | | (127 | ) | | (4,548 | ) |
| Profit (loss) before minority share | | | 5,227 | | | (2,842 | ) | | 13,682 | |
| Minority share in losses of subsidiary | | | 1,116 | | | 1,334 | | | 1,503 | |
| Net profit (loss) | | $ | 6,343 | | $ | (1,508 | ) | $ | 15,185 | |
* 2006 and 2007 operating expenses include (i) $5.2 million and $5.7 million, respectively, of expenses resulting from the grant of options and the application of SFAS 123(R), and (ii) $1.1 million and $5.2 million, respectively, of legal expenses resulting from our patent litigation with Olympus.
Our historical operating results as a percentage of net revenues for the years ended December 31, 2005, 2006 and 2007 are set forth below:
| | | Year ended December 31, | |
| | | 2005 | | 2006 | | 2007 | |
Statements of Operations Data: | | | | | | | | | | |
| Revenues | | | 100.0 | % | | 100.0 | % | | 100 | % |
| Cost of revenues | | | 25.4 | | | 25.4 | | | 26.3 | |
| Early repayment of royalty bearing government grant | | | — | | | — | | | 4.3 | |
| Gross profit | | | 74.6 | | | 74.6 | | | 69.4 | |
| Operating expenses: | | | | | | | | | | |
| Research and development, gross | | | (10.2 | ) | | (13.3 | ) | | (11.4 | ) |
| Royalty-bearing government grants | | | 1.4 | | | 2.0 | | | 1.1 | |
| Research and development, net | | | (8.8 | ) | | (11.3 | ) | | (10.3 | ) |
| Sales and marketing | | | (49.9 | ) | | (53.4 | ) | | (49.1 | ) |
| General and administrative | | | (11.1 | ) | | (16.9 | ) | | (18.6 | ) |
| Termination of marketing agreement | | | — | | | — | | | 20.2 | |
| Other | | | — | | | — | | | (0.4 | ) |
| Total operating expenses | | | (69.8 | ) | | (81.6 | ) | | (58.2 | ) |
| Operating profit (loss) | | | 4.8 | | | (7.0 | ) | | 11.2 | |
| Financing income, net | | | 0.9 | | | 4.2 | | | 4.9 | |
| Profit (loss) before taxes on income and minority share | | | 5.7 | | | (2.8 | ) | | 16.1 | |
| Income tax benefit (expense) | | | 0.3 | | | (0.1 | ) | | (4.0 | ) |
| Profit (loss) before minority share | | | 6.0 | | | (2.9 | ) | | 12.1 | |
| Minority share in losses of subsidiary | | | 1.3 | | | 1.4 | | | 1.3 | |
| Net profit (loss) | | | 7.3 | % | | (1.5) | % | | 13.4 | |
Year Ended December 31, 2007 compared to Year Ended December 31, 2006
Revenues. Revenues increased by $17.8 million, or 18.8%, to $112.8 million in 2007 from $95.0 million in 2006. This increase was primarily due to an increase of $14.3 million, or 18.7%, in sales of our PillCam SB, an increase of $1.2 million, or 8.3%, in revenues from sales of capital equipment, namely workstation and data recorder, an increase of $1.6 million in revenues from service contracts and individual parts, and revenues of $1.1 million from sales of our PillCam COLON capsule. Approximately 85% of the $17.8 million increase in revenues was attributable to increases in the number of products and services sold, approximately 5% was attributable to a higher average selling price of some of our products and approximately 10% was attributable to the impact of currency fluctuation. This increase in revenues was partially offset by a decrease of $0.4 million, or 29.6%, in revenues from our PillCam ESO capsules. The increase in revenues from capital equipment sales is attributable primarily to initial sales of the Given System in Japan following receipt of regulatory clearance in Japan.
Cost of revenues and gross margins. Cost of revenues, excluding a charge related to the early repayment of royalty bearing participation to the Office of Chief Scientist in Israel, increased to $29.7 million in 2007, compared to $24.2 million in 2006. Gross margins, excluding the effect of the payment to the Office of Chief Scientist, or OCS, were 73.7%, compared to 74.6% in 2006. The decrease in gross margins was mainly due to discounts on workstations sold. Gross margins, including the effect of the charge resulted from the early repayment to OCS, were 69.4%
Research and development. Gross research and development expenses were $12.8 million, similar to 2006. Labor expenses increased by $0.8 million compared to 2006. Labor expenses in 2007 included $0.4 million of stock-option compensation expenses as a result of the adoption of SFAS 123(R), compared to $0.6 million in 2006. Expenses related to quality assurance, maintenance of patents and other general expenses relating to research and development projects increased by $0.6 million. These increases were off set by a decrease of $1.1 million in R&D projects and a decrease of $0.3 million in clinical trials.
Research and development expenses, net of grants received from the Office of the Chief Scientist in Israel, totaled $11.6 million in 2007, compared to $10.8 million in 2006. Grants totaling $1.2 million were received in 2007 compared to $1.9 million received in 2006. In both years, the grants were received for new products under development. The decrease in grants received in 2007 is attributable to our decision to end our participation in the grant program of the OCS and to accelerate repayment of our royalty obligations to the OCS.
Sales and marketing. Sales and marketing expenses increased by $4.7 million, or 9.3%, to $55.4 million in 2007 from $50.7 million in 2006. This increase consisted primarily of an increase of $4.3 million in employment-related expenses due to increased number of sales and marketing employees, an increase of $1.0 million in commissions to our sales representatives, and expenses of $0.7 million for setting up a subsidiary in Singapore. These increases were partially offset by a decrease of $1.2 million in commissions to Ethicon due to lower sales of our PillCam ESO capsule.
General and administrative. General and administrative expenses increased by $5.0 million, or 30.9%, to $21.0 million in 2007 from $16.0 million in 2006. This increase was primarily due to an increase of $4.2 million in legal expenses resulting from the patent litigation with Olympus and an increase of $0.5 million resulting from additional stock-option compensation expense.
Financing income, net. Financing income, net, increased by $1.5 million to $5.5 million in 2007 from $4.0 million in 2006. The increase was due mainly to exchange rate gains.
Taxes on income. We had a tax expense of $4.5 million in 2007 compared to a tax expense of $0.1 million in 2006. The increase in tax expense is mainly due to income resulting from the termination of our agreement with Ethicon Endo-Surgery ($3.0 million) and from taxes on increased profitability of our U.S subsidiary.
Minority share in losses of subsidiary. Minority share in losses of a subsidiary, Given Imaging K.K., was $1.5 million in 2007, compared to $1.3 million in 2006. Given Imaging K.K. started marketing activities in Japan on October 1, 2007, when national reimbursement became effective.
Year Ended December 31, 2006 compared to Year Ended December 31, 2005
Revenues. Revenues increased by $8.2 million, or 9.5%, to $95.0 million in 2005 from $86.8 million in 2005. This increase was primarily due to an increase of $13.8 million, or 22.1%, in sales of our PillCam SB capsule and an increase of $0.8 million in service contract revenues. Approximately 97% of the $13.8 million increase in sales of the PillCam SB capsule was attributable to increases in number of capsules sold, and approximately 3% was attributable to the higher average selling price of capsules compared to 2005. The increase in service contract revenues was attributable mainly to the increased number of maintenance agreements in the United States. These increases were partially offset by a decrease of $3.6 million, or 22.5%, in revenues from sales of capital equipment, namely workstation and data recorder, and by a decrease of $3.0 million in revenues from our PillCam ESO capsules. Approximately 52% of the decrease in revenues from capital equipment sales is attributable to lower quantities and 48% of the decrease is attributable to lower average selling price. We sold a smaller number of capital equipment items primarily because a majority of gastroenterologists in the United States already have access to a capsule endoscopy system. The lower selling price is mainly due to promotional activities during the year and an increase of second systems sold to existing customers, which were sold at a significant discount.
Cost of revenues and gross margins. Cost of revenues increased to $24.2 million in 2006 compared to $22.1 million in 2005, while gross margins remained at 74.6% in 2006, similar to 2005. The increase in cost of revenues was mainly due to an increase of $1.4 million in consumption of raw materials, an increase of $0.3 million in labor expenses and an increase of $0.6 in other manufacturing costs. The increase was slightly offset by a decrease of $0.2 million in royalties payable to the Office of the Chief Scientist.
Research and development. Gross research and development expenses increased by $3.9 million, or 43.5%, to $12.7 million in 2006 from $8.8 million in 2005. This increase was due mainly to a $1.4 million increase in labor expenses, $0.6 million of stock-options compensation expenses as a result of the adoption of SFAS 123(R), an increase of $0.6 million in investments in R&D projects, an increase of $0.7 million in investments in clinical trials and an increase of $0.5 million in other expenses.
Research and development expenses, net of grants received from the office of the Israeli Chief Scientist, totaled $10.8 million in 2006, compared to $7.6 million in 2005. Grants totaling $1.9 million were received in 2006 compared to $1.2 million received in 2005. In both years, the grants were received for new products under development.
Sales and marketing. Sales and marketing expenses increased by $7.4 million, or 28.6%, to $50.7 million in 2006 from $43.3 million in 2005. This increase consisted primarily of an increase of $6.1 million in employment-related expenses due to increased number of sales and marketing employees, mainly in the United States, an additional $1.8 million of stock options-related compensation expenses as a result of the adoption of SFAS 123(R), an increase of $1.0 million in expenses related to our participation in trade shows and other marketing events, an increase of $0.5 million in clinical and regulatory activities in Japan and an increase of $0.8 in travel expenses. These increases were offset by a decrease of $1.5 million in commissions to Ethicon Endo-Surgery for sales of our PillCam ESO capsule and ancillary products, and a decrease of $1.3 million in the provision for uncollectible sales tax at our U.S. subsidiary of 2005.
General and administrative. General and administrative expenses increased by $6.3 million, or 66.0%, to $16.0 million in 2006 from $9.7 million in 2005. This increase was primarily due to an expense of $2.8 million resulting from additional stock-options compensation expense following the adoption of SFAS 123(R), an increase of $2.3 million in salaries and fringe benefits, resulting mainly from changes in management, an increase of $0.7 million in legal and other professional expenses and an increase of $0.5 million in investments in information systems.
Financing income, net. Financing income, net, increased by $3.2 million to $4.0 million in 2006 from $0.8 million in 2005. The increase was due mainly to a change in our investment policy. Financing income in 2006 was generated from interest income of $1.6 million from short-term deposits, income of $1.8 million from marketable securities and $0.8 million of exchange gains, offset by bank expenses of $0.3 million.
Taxes on income. We had a tax expense of $0.1 million in 2006 compared to a tax benefit of $0.3 million in 2005.
Impact of Currency Fluctuations
Our sales to our customers in 2007 were denominated 67.5% in U.S. dollars, 23.3% in Euros and 9.2% in other currencies, depending on the location of the customer or the distributor used to fulfill our customers’ orders. In 2007, 31% of our expenses, principally salaries and related personnel expenses were denominated in Shekels, and we expect this level of Shekel expenses to continue for the foreseeable future. During 2007, the U.S. dollar weakened against the Shekel by 9.0%. In addition, 49% of our expenses were denominated in U.S. dollars, 13% were denominated in Euros and 7.0% were denominated in Yen or other currencies. If the value of a currency in which our revenues are denominated weakens against the value of a currency in which our expenses are denominated, there will be a negative impact on the profit margins for sales of our products. In addition, as of December 31, 2007, 38% of our cash and cash equivalents were denominated in currencies other than U.S. dollar and we are therefore subject to the risk of exchange rate fluctuations among U.S. dollar, Yen, Shekel, Australian dollar and Euro. In 2007, we have used different hedging tools in order to minimize the effect of currency fluctuations on our income. If we wish to maintain the dollar-denominated value of our product in non-U.S. markets, devaluation in the local currencies of our customers relative to the U.S. dollar could cause our customers to cancel or decrease orders or default on payment.
B. LIQUIDITY AND CAPITAL RESOURCES
From our inception through December 31, 2007, we raised a total of $152 million through public and private sales of our equity securities. As of December 31, 2007, we had a cash balance of approximately $101.8 million, consisting of $37.1 million in cash and cash equivalents,$41.6 million invested in marketable securities and $23.1 million in short term investments. Our working capital, which we calculate by subtracting our current liabilities from our current assets, was $75.0 million.
We believe that our cash reserves and expected cash from operations will be sufficient to meet our anticipated cash needs for working capital and capital expenditures in 2008.
The following table sets forth the components of our cash flows for the periods indicated:
| | | 2005 | | 2006 | | 2007 | |
| | | (in thousands) | |
| Net cash provided by operating activities | | $ | 13,488 | | $ | 2,861 | | $ | 11,375 | |
| Net cash used in investing activities | | | (29,883 | ) | | (30,757 | ) | | (23,958 | ) |
| Net cash provided by financing activities | | | 1,069 | | | 6,795 | | | 4,936 | |
| Effect of exchange rate changes on cash | | | (179 | ) | | 255 | | | 240 | |
| Decease in cash and cash equivalents | | $ | (15,505 | ) | $ | (20,846 | ) | $ | (7,407 | ) |
Net cash provided by operating activities was $11.4 million in 2007, compared to $2.9 million in 2006 and $13.5 million in 2005. The increase in net cash from operating activities in 2007 compared to 2006 resulted primarily from an increase in net income of $16.7 million, a decrease of $10.1 million in marketable securities, a decrease of $4.4 million in inventories and an increase of $8.0 million in accounts payable, offset by an increase of $17.5 million in trade and non-trade accounts receivable and a decrease of $13.7 million in deferred income. The decrease in net cash from operating activities in 2006 compared to 2005 resulted primarily from a reduction of $2.6 million in net income (excluding compensation expenses which do not have an effect on cash flow) to $3.7 million, compared to net income of $6.3 million in 2005, an increase of $2.0 million in inventories, a net increase in trading securities of $5.1 million and a decrease of $4.8 in accounts receivable due to $5.0 million received in 2006 from Ethicon Endo-Surgery for the milestone achieved in 2005 under our agreement with Ethicon.
Net cash used in investing activities was $24.0 million in 2007, compared to $30.8 million in 2006 and $29.9 million in 2005. Investing activities in 2007 consisted primarily of investment of $5.8 million in fixed assets and an investment of $17.8 million in marketable securities, net of proceeds from sales of such securities. Our capital expenditures in 2007 consisted primarily of investments of $2.7 million in manufacturing machinery and equipment, $1.3 million in computer hardware and software, and $1.1 million in patents. Investment activities in 2006 consisted primarily of investing $24.9 million in marketable securities and $5.9 million in capital expenditures and the capitalization of costs associated with our patents and trademarks. Our capital expenditures in 2006 consisted primarily of $1.9 million in machinery and equipment, $0.5 million in new real property leases on our facilities, $1.3 million in computers and software, $0.4 million in office furniture and equipment and $1.2 million in patents. Our capital expenditures in 2005 consisted primarily of $2.3 million in machinery and equipment, $2.8 million in new real property leases on our facilities, $0.9 million in computers and software, $0.5 million in office furniture and equipment and $0.9 million in patents. We expect to continue investing significant amounts in 2008 in order to support our growth plans.
Net cash provided by financing activities was $4.9 million in 2007, compared to $6.8 million in 2006 and $1.1 million in 2005. In 2007, net cash provided by financing activities resulted primarily from proceeds of $4.3 million received from the exercise of employee stock options. In 2006, net cash provided by financing activities resulted primarily from proceeds of $2.0 million received from the exercise of employee stock options and $4.8 million representing a minority investment in our Japanese subsidiary, Given Imaging K.K. In 2005, net cash provided by financing activities resulted primarily from proceeds from the exercise of employee stock options.
Market Risk
As of December 31, 2007, we had $37.1 million in cash and cash equivalents, which were invested in bank accounts and deposits with maturities of three months or less located with a number of highly-rated banks inside and outside of Israel. In addition, as of December 31, 2007, we had $41.6 million invested in marketable securities and $23.1 million invested in short term investments. We invest these additional amounts in longer-term financial instruments in order to seek to achieve a higher yield. Approximately two thirds of these cash balances were invested in securities issued by the United States government or its agencies and in AAA money market funds. The remainder was held in corporate bonds and commercial paper that were highly-rated by rating agencies at the time of investment. All investments are made in compliance with investment policies approved by the audit committee of our board of directors. Our cash and investments are subject to general credit, liquidity, market and interest rate risks, which may be exacerbated by the dislocation that has affected various sectors of the financial markets since the middle of 2007 and caused credit and liquidity issues for a number of reputable financial institutions. For more information about market risks, see “Item 3 – Risk Factors,” “Item 5 - Operating and Financial Review and Prospects – Impact of Currency Fluctuations” and “Item 11 – Quantitative and Qualitative Disclosures About Market Risks.”
Corporate Tax
Israeli companies were generally subject to income tax at the corporate rate of 29% in 2007. This tax rate is expected to be gradually reduced to a rate of 25% by 2010. As of December 31, 2007, our net operating loss carry-forwards for Israeli tax purposes amounted to $4.0 million. Under Israeli law, net operating losses can be carried forward indefinitely and offset against certain future taxable income.
In addition, our investment program in equipment and leasehold improvements at our manufacturing facility in Yoqneam, Israel has been granted approved enterprise status and we are, therefore, eligible for tax benefits under the Law for the Encouragement of Capital Investments, 1959 (the “Investment Law”). Subject to compliance with applicable requirements, the portion of our undistributed income derived from our approved enterprise program will be exempt from corporate tax for a period of ten years commencing in the first year in which we generate taxable income. The ten-year period may not extend beyond the later of 14 years from the year in which approval was granted or 12 years from the year in which operations or production by the enterprise began. We received approved enterprise status for investments beginning in 1999. According to a recent reform to the Investment Law, we are permitted to claim tax benefits in respect of future investments retroactively on our corporate tax returns instead of filing an application for tax benefits in advance with the Investment Center, the administrator of the Investment Law, and without prior approval and without submitting any reports to the Investment Center. Audits of any claim for tax benefits will take place by the Israeli income tax authority as part of the general tax audits it may perform from time to time. We cannot assure our shareholders that we will receive approvals in the future for approved enterprise status or that tax benefits for approved investments will continue at current levels or at all.
We expect that a substantial portion of the income we derive in the future will be from this approved enterprise program. These benefits should result in income recognized by us being tax exempt for a specified period after we begin to report taxable income and exhaust any net operating loss carry-forwards. These benefits may not be applied to reduce the tax rate for any income that is not derived from sales of our products manufactured at our facility in Yoqneam, Israel.
Our approved enterprise status imposes certain requirements on us, such as the location of our manufacturing facility, location of certain subcontractors and the extent to which we may outsource portions of our production process. These requirements limit our ability to pursue production arrangements that may otherwise be more favorable to us if we want to maintain these tax benefits. Therefore, we may be required to weigh the possible loss of these benefits against other benefits from pursuing arrangements which are not, or which may not be considered by the relevant Israeli authorities to be, in compliance with these requirements. If we do not meet these requirements, Israeli law permits the authorities to cancel such tax benefits retroactively.
As of December 31, 2007, the net operating loss carry-forwards of our subsidiaries for tax purposes amounted to $31 million. A subsidiary’s net operating loss carry-forwards for tax purposes relating to a jurisdiction are generally available to offset future taxable income of such subsidiary in that jurisdiction, subject to applicable expiration dates.
Government Grants
Our research and development efforts have been financed, in part, through grants from the Office of the Chief Scientist of the Israeli Ministry of Industry, Trade and Labor. We have received approval for grants totaling $7.5 million from the Office of the Chief Scientist, including $0.7 million which was provided by the Office of Chief Scientist to support our 2007 research and development.
Under Israeli law, royalties on the revenues derived from sales of the Given System or any part of the Given System are payable to the Israeli government, generally at the rate of 3.0% during the first three years of sales and 3.5% beginning with the fourth year. The maximum aggregate royalties paid generally cannot exceed 100% of the grants made to us. The amount bears interest equal to the 12-month London Interbank Offered Rate (LIBOR) applicable to dollar deposits that is published on the first business day of each calendar year. Royalties are paid on our consolidated revenues.
In light of our available cash position, we have recently paid all our outstanding royalty obligation and accrued interest of approximately $4.8 million to the Office of the Chief Scientist. This payment resulted in a one-time charge of approximately $4.8 million to our statement of operations in the fourth quarter of 2007. As a result of this payment, we have no further outstanding royalty obligations to the Office of the Chief Scientist in Israel.
We continue to be involved in non-royalty bearing government-funded research programs inside and outside of Israel. One example is our leadership of a European consortium that will develop an integrated imaging and bio-sensing system to screen for cancer of the gastrointestinal (GI) tract. This “Nano-based capsule-Endoscopy with Molecular Imaging and Optical biopsy,” or NEMO project, began in December 2006. In addition to Given Imaging, this consortium includes other European companies and institutions. The NEMO group will invest a total of €4.7 million over the next three years in this research, of which the European Commission will contribute €2.8 million. Our expected gross and net contribution during this period is €1.3 million and €0.6 million, respectively. The objective of the NEMO project is to increase patient compliance with currently recommended screening guidelines by developing an advanced cancer screening system that is patient-friendly, highly-sensitive and specific for early detection of cancer. To achieve this, NEMO will attempt to integrate optical technologies with Nano-technologies, bio-sensing and maneuvering technologies to create a unique PillCam capsule endoscope capable of secretion analysis and the detection of marked and deep tissue disorders. We believe that the combination of the image and molecular analysis to mark the tumor may provide a novel and effective medical device for mass screening of gastrointestinal cancer.
C. RESEARCH AND DEVELOPMENT
Our gross research and development expenditures were $12.8 million for the year ended December 31, 2007, $12.7 million for the year ended December 31, 2006 and $8.8 million for the year ended December 31, 2005. Our research and development activities are conducted by our research and development and regulatory affairs staff primarily at our headquarters in Israel. Our research and development efforts are focused primarily on developing new capsules to be used in the detection of abnormalities in the colon, improvements to our existing products and new technologies for future expansion of our product offering. In 2007, we completed the development of the newest version of our Pillcam SB and PillCam ESO capsules and received FDA clearance to market these capsules in the United States. In addition, we completed the development of our newest software, RAPID 5.0, and received FDA clearance for this product in May 2007. We view our innovation and focus on capsule endoscopy technology as an important competitive advantage and intend to continue our focus on research and development activities.
See discussion in Parts A and B of Item 5 “Operating Results and Financial Review and Prospects.”
E. OFF-BALANCE SHEET ARRANGEMENTS
N/A
F. CONTRACTUAL OBLIGATIONS
The following table of our material contractual obligations as of December 31, 2007, summarizes the aggregate effect that these obligations are expected to have on our cash flows in the periods indicated:
| | | Payments due by period | |
Contractual obligations | | Total | | 2008 | | 2009 | | 2010 | | 2011 | | Later Years | |
| | | (in thousands) | |
| Capital Leases(1) | | $ | 569 | | $ | 121 | | $ | 101 | | $ | 103 | | $ | 105 | | $ | 139 | |
| Operating Leases(2) | | | 16,831 | | | 3,580 | | | 3,021 | | | 2,268 | | | 1,731 | | | 6,231 | |
| Purchasing Obligations | | | 17,640 | | | 6,510 | | | 1,500 | | | 2,250 | | | 2,250 | | | 5,130 | |
| Tax Contingency Reserve(3) | | | 2,901 | | | 2,901 | | | - | | | - | | | - | | | - | |
| Total | | $ | 37,941 | | $ | 13,112 | | $ | 4,622 | | $ | 4,621 | | $ | 4,086 | | $ | 11,500 | |
| (1) | Consists of capital leases for motor vehicles. |
| | |
| (2) | Consists of operating leases for office and manufacturing space and motor vehicles. |
| | |
| (3) | Tax contingency resulting from the termination of our agreement with Ethicon Endo-Surgery. |
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. DIRECTORS AND SENIOR MANAGEMENT
Our executive officers and directors and their ages and positions as of the date of this annual report are as follows:
Name | | Age | | Position |
| | | | | |
Executive Officers: | | | | |
| Nachum (Homi) Shamir | | 54 | | President, Chief Executive Officer and Director |
| Kevin Rubey | | 50 | | Chief Operations Officer |
| Yuval Yanai | | 55 | | Chief Financial Officer |
| Christopher Rowland | | 46 | | President, Americas |
| Kazem Samandari | | 57 | | President, Asia-Pacific/Japan |
| Manfred Gehrtz | | 57 | | President, EMEA |
| Ori Braun | | 51 | | Senior Vice President of Business Development |
| Keith Chrzanowski | | 47 | | Senior Vice President, Global Human Resources |
| Daphna Levy | | 54 | | Senior Vice President, General Manager, Israel |
| | | | | |
Directors: | | | | |
| Israel Makov (1) | | 68 | | Chairman of the Board of Directors |
| Doron Birger(2) | | 56 | | Director |
| James M. Cornelius (1)(2)(3)(4) | | 64 | | Director |
| Michael Grobstein (1)(2)(3)(4) | | 65 | | Director |
| Dennert O. Ware (3) | | 66 | | Director |
| Arie Mientkavich | | 65 | | Director |
| Prof. Anat Loewenstein | | 48 | | Director |
| (1) | Member of our executive committee. |
| | |
| (2) | Member of our compensation and nominating committee. |
| | |
| (3) | Member of our audit committee. |
| | |
| (4) | Outside director under the Israeli Companies Law. |
Nachum (Homi) Shamir has served as our President and Chief Executive Officer and a director since April 9, 2006. Prior to joining us, Mr. Shamir served as Corporate Vice President of Eastman Kodak Company and as the President of Eastman Kodak’s Transaction and Industrial Solutions Group, which includes several business units, including Kodak Versamark, Inc. (whose operations were previously those of Scitex Digital Printing Inc.) of which Mr. Shamir was President and Chief Executive Officer. From June 2003 to January 2004, Mr. Shamir served as the President and Chief Executive Officer of Scitex Corporation. From January 2001 to January 2004, he served as the President and Chief Executive Officer of Scitex Digital Printing, having previously served as its Chief Operating Officer since July 2000. Prior thereto, Mr. Shamir was Managing Director and General Manager of Scitex Digital Printing (Asia Pacific) Pte Ltd., from the incorporation of this Singapore-based company in 1994. From 1993 until 1994 he was with the Hong Kong based Scitex Asia Pacific (H.K.) Ltd. Before joining Scitex, Mr. Shamir held senior management positions at various international companies mainly in the Asia Pacific regions. Mr. Shamir holds a B.Sc from the Hebrew University of Jerusalem and an M.P.A. from Harvard University.
Kevin Rubey has served as our Chief Operations Officer since June 2001. Prior to joining us, from 1998 to May 2001, Mr. Rubey worked at Eastman Kodak Company, where he led global manufacturing and operations for the Health Imaging Business Unit. From 1996 to 1998, Mr. Rubey was Manufacturing Director of the Medical Imaging Business Unit of Imation Corporation, a U.S. information technology company specializing in data storage and color image management. Prior to that, from 1977 to 1996, Mr. Rubey worked at the 3M Corporation in a variety of positions in the health, consumer and information technology businesses. Mr. Rubey holds a B.Sc. in mechanical engineering and an M.B.A. from the University of Minnesota.
Yuval Yanai has served as our Chief Financial Officer since September 1, 2005. From October 2000 through August 2005, he served as Senior Vice President and Chief Financial Officer of Koor Industries Ltd., one of Israel’s largest holding corporations. Prior to that, from April 1998 to September 2000 he served as Vice President and Chief Financial Officer of NICE Systems Ltd., an Israeli global provider of Insight from Interactions, and from 1991 to April 1998, he was the Vice President, Finance and Chief Financial Officer of Elscint Ltd., a former Israeli company engaged in the manufacturing of medical imaging devices that was acquired by larger companies in this field. He joined Elscint in 1985 and served as Corporate Controller and Corporate Treasurer through 1991. Previously, Mr. Yanai served as a director of Makteshim-Agan Industries Ltd., Equity One, Inc., BVR Systems Ltd., Tadiran Communication Ltd., The Elisra Group and Telrad Networks Ltd. Mr. Yanai holds a B.Sc. in Accounting and Economics from the Tel Aviv University.
Christopher Rowland has served as President of our Americas region since May 2006. He joined us in February 2006 as our Senior Vice President of Business Development and Corporate Strategy. Prior to that, Mr. Rowland worked for 17 years at Boston Scientific Corporation, a medical device company, in various sales, marketing and general management positions across all Boston Scientific divisions and in several countries. Most recently he was Vice President of Marketing for the Endovations division of Boston Scientific, where he had responsibility for all marketing, product development and launch activities of products in the gastrointestinal field. Prior to that, from 1985 until 1989 Mr. Rowland worked for Xerox Corporation in various sales positions. Mr. Rowland has a B. Sc. in Marketing from Southern Illinois University.
Kazem Samandari has served as the President of our Asia Pacific/Japan Region since July 2007. Prior to joining us, from 2004 to 2007, Mr. Samandari was Executive Vice President Global Sales & Marketing at Eastman Kodak, Inkjet Printing Solutions. From 1994 to 2004, Mr. Samandari served as the Vice President of Scitex Digital Printing operations in Asia Pacific & Japan and was responsible for the commercial, technical & legal aspects of the activities in the region. From 1986 to 1994, Mr. Samandari served as Managing Director of GMC Digital Systems, a high-tech start-up company founded in 1984, which was a pioneer in the field of high speed, high performance, digital printing equipment. Mr. Samandari holds an M.S. degree in electrical & electronics engineering and a Ph.D. in technical sciences and industrial economy from the Swiss Federal Institute of Technology.
Manfred Gehrtz has served as President, EMEA since April 2007. Prior to that, he was President, International between January 2006 and March 2007. Prior to that, from January 2004 until December 2005, Mr. Gehrtz served as our Corporate Vice President, General Manager for Europe. Prior to joining us, from 1995 to 2003, Mr. Gehrtz was Managing Director, the head of the Endoscope Division and Vice President of Medical Systems Europe at Olympus Optical Co. (Europa) GmbH, a developer of products in the fields of photography, endoscopy, microscopy, communication and diagnostics. From 1990 to 1995, Mr. Gehrtz was Managing Director and Chief Executive Officer of Aesculap Meditec GmbH, a German company that develops and manufactures medical laser systems. Prior to that, from 1986 to 1990, Mr. Gehrtz was a Specialist, Lab Manager and Production Facility Manager at IBM Deutschland GmbH. Mr. Gehrtz holds a Ph.D. in Physical Chemistry and an M.Sc. in Physics from the University of Munich, Germany. From 1983 to 1985, Mr. Gehrtz was a Post-Doctoral Fellow at the IBM Research Laboratory in San Jose, California.
Ori Braun has served as Senior Vice President - Business Development since October 2006. Prior to joining us, Mr. Braun served as President of Valor Inc., a leader in productivity increasing engineering software solutions to the PCB design, fabrication and assembly industry. From 2004 to 2005, he served as President of LifeWatch Inc., a leading cardiac monitoring services company. From 1985 to 2004 he founded and held various CEO and executive positions at 3DV Systems Inc., a company developing and selling 3D camera technology, at Helios Software Engineering Ltd., a company in the business of simulation technology that was acquired by Cadence Design Systems Inc., and at Lansoft Computing Ltd. Between 1994 and 1996, Mr. Braun held the position of Vice President Business Development at Rafael Development Corporation, or RDC, a large shareholder of Given Imaging, which is a holding company controlled by Discount Investment Corporation Ltd., or DIC, another significant shareholder of Given Imaging. Mr. Braun holds a B.Sc. in Mechanical Engineering from the Ben Gurion University in Beer Sheva, Israel.
Keith A. Chrzanowski has served as our Senior Vice President of Human Resources since January 1, 2008. Prior to that, from January 2005, until December 2007, he was Director of Human Resources of our Americas region. Prior to joining us, from July 2002 until January 2005, Mr. Chrzanowski was Senior Director/Vice President of Human Resources for McKesson Provider Technologies, a division of McKesson specializing in delivering software to include automation and robotics, business process re-engineering, analytics, and other services that connect healthcare providers, physicians, payors and patients across all care settings. From July 2000 until July 2002, Mr. Chrzanowski was Vice President of Human Resources for Spherion’s Outsourcing Group which provided services to Fortune 500 customers. From 1991 to 2000, Mr. Chrzanowski worked as a Human Resource Manager and Director of Human Resources for diagnostic and medical supply divisions which initially were a part of Baxter Healthcare and eventually acquired by Cardinal Health in 1999. Prior to joining Baxter, Mr. Chrzanowski worked for Schlumberger Industries in the United States and Canada as a Human Resources Manager from 1987 until 1991. From 1983 until 1987 Mr. Chrzanowski held a variety of Human Resources positions in support of Beecham’s Consumer Products businesses. Mr. Chrzanowski has a B.A. in Communications from Western Illinois University and an M.A. in Organizational Theory from Norwich University.
Daphna Levy has served as our Senior Vice President and General Manager, Israel, since January 2008. Prior to that, from January 2002 until December 2007, Ms. Levy served as our Vice President-Research and Development and from November 2000 until December 2001 as manager of our algorithm group. Prior to joining us, from January 1999 until November 2000, Ms. Levy was a project leader at Interspark Ltd., a subsidiary of Philips DAP. From 1996 to 1999, Ms. Levy served as manager of an image processing group at Rafael – Armament Development Authority. Ms. Levy holds an M.Sc. in electrical engineering from Santa Clara University, California, a B.Sc. in electrical engineering from the Israel Institute of Technology and an M.B.A. from Tel Aviv University, Rekanati School of Business.
Israel Makov has served as the Chairman of our board of directors since July 2007. Prior to joining us, he served as President and Chief Executive Officer of Teva Pharmaceutical Industries Ltd. from April 2002 until March 2007 and is currently serving as Advisor to the Board of Directors of Teva. Previously, he served as Teva’s Chief Operating Officer from January 2001, Executive Vice President from 1999 and Vice President for Business Development from 1995 until 1999. Prior to joining Teva, Mr. Makov was Chief Executive Officer of Gottex from 1993-1995, Chief Executive Officer of Yachin Hakal Ltd. from 1991 until 1993 and Chairman of Axiom Ltd. from 1987 until 1991. Mr. Makov was also a director of Bank Hapoalim Ltd. from October 2002 until February 2006, a director of Ramot at Tel Aviv University from 2001 until January 2006, and one of the founders and a director of the INNI-Israel National Nanotechnology Initiative since 2003. Mr. Makov has also served on the Board of Governors of the Technion – Israel Institute of Technology since 2006 and on the Board of Governors of the Weizmann Institute of Science since 2007. Mr. Makov holds a B.Sc. in Agriculture and M.Sc. in Economics from the Hebrew University Jerusalem.
Doron Birger has served as a director since June 2000. From August 2002 until July 2007, Mr. Birger was the Chairman of our board of directors. Mr. Birger has served as Chief Executive Officer of Elron Electronic Industries since August 2002, President since 2001, Chief Financial Officer from 1994 to August 2002, and Corporate Secretary from 1994 to 2001. Mr. Birger is a director of RDC Rafael Development Corporation and a director or chairman of the board of directors of many privately held companies in the Elron group in the fields of medical devices, semiconductors, communication and advanced materials. From 1991 to 1994, Mr. Birger was Chief Financial Officer at North Hills Electronics Ltd., an advanced electronics company. From 1990 to 1991, Mr. Birger served as Chief Financial Officer of Middle-East Pipes Ltd., a manufacturer in the metal industry. From 1988 to 1990, Mr. Birger served as Chief Financial Officer of Maquette Ltd., a manufacturer and exporter of fashion items. From 1981 to 1988, Mr. Birger was Chief Financial Officer and director at Bateman Engineering Ltd. and I.D.C. Industrial Development Company Ltd. Mr. Birger holds a B.A. and an M.A. in economics from the Hebrew University, Jerusalem.
James M. Cornelius has served as a director since October 2001 and was elected as an outside director in December 2001. Mr. Cornelius was elected Chief Executive Officer of Bristol-Myers Squibb (BMS) on April 30, 2007 and also became Chairman of BMS in February 2008. Prior to that, he served as interim Chief Executive Officer of BMS from September 12, 2006. He has been as a member of the BMS Board since January 2005. From November 15, 2005 to April 21, 2006, Mr. Cornelius was Chairman of the Board and Chief Executive Officer of Guidant Corporation (a leading cardiac and vascular medical device company listed on the NYSE), until the sale of Guidant to Boston Scientific Corporation. From 2000 until 2006, Mr. Cornelius served as the non-executive Chairman of the board of directors of Guidant Corporation. From 1994 until 2000, Mr. Cornelius served as the Senior Executive and Chairman of Guidant Corporation. From 1983 to 1994, Mr. Cornelius was a director, member of the Executive Committee, and Chief Financial Officer of Eli Lilly and Company. From 1980 to 1982, Mr. Cornelius served as President and Chief Executive Officer of IVAC Corporation, formerly part of Eli Lilly’s Medical Device and Diagnostics Division. Mr. Cornelius also serves as a director for The DIRECTV Group, Inc. Mr. Cornelius holds a B.A. in accounting and an M.B.A from Michigan State University.
Michael Grobstein has served as a director since October 2001 and was elected as an outside director in December 2001. Mr. Grobstein serves as a director of Bristol-Myers Squibb, as chairman of its audit committee and a member of its compensation and management development committee. Mr. Grobstein served as a director of Guidant Corporation from 1999 to 2006, at which time Guidant was acquired by Boston Scientific Corporation. During that period, he was chairman of Guidant’s audit committee and a member of its corporate governance committee. Mr. Grobstein worked with Ernst & Young LLP from 1964 to 1998, and was admitted as a partner in 1975. At Ernst & Young, Mr. Grobstein served as a Vice Chairman-International Operations from 1993 to 1998, as Vice Chairman-Planning, Marketing and Industry Services from 1987 to 1993, and Vice Chairman-Accounting and Auditing Services from 1984 to 1987. In these positions, Mr. Grobstein, among other things, oversaw the global strategic planning of the firm, was responsible for developing and implementing the firm’s worldwide audit service delivery process and consulted with multinational corporations on a wide variety of financial reporting matters. Mr. Grobstein is a certified public accountant in the United States and holds a B.Sc. in accounting from the University of Illinois.
Arie Mientkavich has served as a director since July 2007. In addition, he has served as Chairman of the Board of Directors of Elron Electronic Industries since January 2007 and in parallel as Chairman of the Boards of Rafael Development Corporation Ltd., or RDC, and Clal Tourism Ltd. Elron and RDC are our affiliates. Mr. Mientkavich has also been a director of Medingo Ltd. since November 2007, Chairman of the Board of Directors of Gazit Globe (Development) Ltd. and Deputy Chairman of IDB Holding Corporation Ltd. since 2006 and Deputy Chairman of Gazit Globe Ltd. since 2005. Prior to this, from 1997 through January 2006, Mr. Mientkavich served as Chairman of the Board of Directors of Israel Discount Bank Ltd. and its major subsidiaries, Israel Discount Bank of New York, Mercantile Discount Bank Ltd. and Discount Management Provident Funds. Between 1987 and 1997, Mr. Mientkavich served as Active Chairman of the Board of the Israel Securities Authority—the Israeli equivalent of the United States Securities and Exchange Commission. Prior to that, from 1979 through 1987, he was the General Counsel of the Israeli Ministry of Finance. Mr. Mientkavich holds degrees in Political Science and Law from the Hebrew University, Jerusalem and is a member of the Israeli Bar Association.
Dennert (Denny) O. Ware has served as a director since July 2007. Prior to joining our board of directors, he served as a Director, President and Chief Executive Officer of Kinetic Concepts, Inc., or KCI, from April 2000 to December 2006. Before joining KCI, he served as President and Chief Executive Officer of Boehringer Mannheim Corporation, a market leader in medical diagnostic equipment. He joined Boehringer in 1972 as Vice President of Technical Affairs of DePuy, the company’s Orthopedic Division. He later held senior management positions in the Diagnostic Division and became President and Chief Executive Officer of Boehringer Mannheim’s North American operations in 1997 a position he held until the acquisition of Boehringer Mannheim Group by Hoffman La Roche in 1998. After this acquisition, Mr. Ware continued on as President and Chief Executive Officer of Roche Diagnostics Corp until the end of 1999. Mr. Ware holds a BSChE degree from Purdue University and an M.B.A from Indiana University.
Prof. Anat Loewenstein has served as a director since August 2005. Prof. Loewenstein completed her training in Johns Hopkins University Hospital in Baltimore in 1996. She has been the Director of the Department of Ophthalmology, Tel Aviv Medical Center since January 2000, Vice Dean of the Sackler School of Medicine, Tel Aviv University since September 2006, and a Professor at the Sackler School of Medicine since April 1999. In addition, since 2000 Prof. Loewenstein has been a member of the Advisory Board of Notal Vision Ltd., a medical device company in the area of diagnostic ophthalmology, and from 1996 until 1997 she served as an advisor to Talia Technologies Ltd., which developed an instrument in diagnostic ophthalmology. She is the principal investigator in multiple multicenter drug and device studies for Pfizer, Novartis, Roche and Zeiss. She is a member of the IRB committee of the Israeli Ministry of Health. Prof. Loewenstein holds an M.D. from the Hebrew University of Jerusalem and Masters Degree in Health Administration from Tel Aviv University.
The aggregate compensation paid by us and our subsidiaries to our directors and executive officers for the year ended December 31, 2007 was $7.9 million (excluding director fees detailed below). This amount includes $3.7 million of stock based-compensation as described below. This amount also includes approximately $0.2 million set aside or accrued to provide pension, severance, retirement or similar benefits or expenses, but does not include business travel, relocation, professional and business association dues and expenses reimbursed to office holders, and other benefits commonly reimbursed or paid by companies in Israel.
In 2007, we granted our directors and executive officer a total of 1,178,742 options to purchase our ordinary shares and 6,000 restricted shares. This number includes 580,742 options granted to Mr. Makov in consideration for his engagement as Chairman of our board of directors and 100,000 options granted to Mr. Shamir, our President and Chief Executive Officer. The exercise price of these options ranges between $21.77 and $29.42 with a weighted average exercise price of $27.52. Equity awards granted to executive officers typically vest ratably over a four-year period. Equity awards granted to non-employee directors typically vest after one year of continued service on the board of directors or a committee thereof. The exercise price of these options was equal to the fair market value of our ordinary shares on the date of grant. These options will expire five years from the date of grant.
The regular fees for each non-employee director include a quarterly fee of $6,250 for their service on our board of directors, and a $1,500 fee for attending and participating in each meeting of the board of directors or any committee of the board of directors. The total amount of these payments in 2007 was $0.4 million. The directors’ fees for service by our director, Doron Birger, are paid to Elron Electronic Industries, where he serves as President and Chief Executive Officer. In addition, all of our directors were reimbursed for their expenses for each board of directors meeting attended.
Please see Item 7 “Major Shareholders and Related Party Transactions—Agreements with Directors and Officers — Employment Agreements” for information regarding the employment agreements and compensation of Nachum Shamir, our President and Chief Executive Officer and Mr. Israel Makov, the Chairman of our board of directors.
Board of Directors and Officers
Our articles of association provide that we may have up to 12 directors. Each of our directors is elected at an annual general meeting of our shareholders by a vote of the holders of a majority of the voting power present and voting at that meeting, except the outside directors under the Israeli Companies Law, whose election process is described below. Our board of directors currently consists of eight directors. Each director listed above will hold office until the next annual general meeting of our shareholders, except for our outside directors who were initially elected in December 2001 for a three-year term and are now serving their third three-year term, which will expire December 31, 2010. Other than Nachum Shamir, our President and Chief Executive Officer, and Mr. Israel Makov, our Chairman, none of our directors are our employees or are party to a service contract with us. Messrs. Chen Barir and Eyal Lifschitz left our board of directors in July 2007.
A simple majority of our shareholders at a general meeting may remove any of our directors from office, except the outside directors nominated under the Israeli Companies Law, and elect directors in their stead or fill any vacancy, however created. In addition, vacancies on the board of directors, other than vacancies created by an outside director, may be filled by a vote of a majority of the directors then in office. Our board of directors may also appoint additional directors up to the maximum number permitted under our articles of association. A director so chosen or appointed will hold office until the next annual meeting of our shareholders.
Each of our executive officers serves at the discretion of the board of directors and holds office until his or her successor is elected or until his or her earlier resignation or removal. In 2007, we hired Mr. Kazem Samandari as president of our Asia-Pacific/Japan region. Effective January 1, 2008, Mr. Keith Chrzanowski became our Senior Vice President of Human Resources and Ms. Daphna Levy was appointed Senior Vice President and General Manager, Israel. Mr. Mark Gilreath, our former Chief Marketing Officer, left us at the end of 2007.
There are no family relationships among any of our directors or executive officers. All of our executive officers have signed employment agreements.
Outside and Independent Directors
Under Israel’s Companies Law, companies incorporated under the laws of the State of Israel whose shares are listed on an exchange, including the Nasdaq Global Market, are required to appoint at least two outside directors. Outside directors are required to meet standards of independence set forth in the Israeli Companies Law. Outside directors are elected by a majority vote at a shareholders’ meeting, provided that either (1) the majority of shares voted at the meeting, including at least one-third of the shares of non-controlling shareholders voted at the meeting, vote in favor of the election of the outside director, or (2) the total number of shares voted against the election of the outside director does not exceed one percent of the aggregate voting rights in the company. The initial term of an outside director is three years. Under a recent amendment to the Israeli Companies Law, an outside director of a company whose shares are dually listed on an Israeli exchange and on a foreign exchange, including the Nasdaq Global Market, may be re-elected to one or more additional three-year terms, subject to the conditions described above for election of outside directors, if the audit committee and the board of directors have determined that these additional terms benefit the company in light of the outside director’s expertise and contribution to the company and the reasons for this determination have been presented to the shareholders prior to their approval of the re-election. Previously, an outside director could serve a maximum of two terms as an outside director. Outside directors may only be removed by the same percentage of shareholders as is required for their election, or by a court, and then only if the outside directors cease to meet the statutory requirements for their appointment or if they violate their duty of loyalty to the company. If an outside directorship becomes vacant and there are no other two serving outside directors, a company’s board of directors is required under the Companies Law to call a shareholders’ meeting immediately to appoint a new outside director. Our two outside directors are James Cornelius and Michael Grobstein. At our annual shareholders meeting in July 2007, they were elected to a third term as outside directors, which will expire in December 2010.
Each committee of a company’s board of directors is required to include at least one outside director and our audit committee is required to include both outside directors. An outside director is entitled to compensation as provided in regulations adopted under the Companies Law and is otherwise prohibited from receiving any other compensation, directly or indirectly, in connection with services provided as an outside director.
An amendment to the Companies Law that became effective on January 19, 2006 provides that every outside director appointed to the board of directors of an Israeli company must qualify as a “financial and accounting expert” or as “professionally competent,” as such terms are defined in the applicable regulations under the Companies Law, and that at least one outside director must qualify as a “financial and accounting expert.” We comply with these requirements. In addition, this amendment requires Israeli companies to determine how many directors, in addition to the outside directors, qualify as “financial and accounting experts” taking into account the nature of the company’s business, the complexity of the activities carried out by the company and the size of its board of directors. In February 2006, our board of directors determined that at least one of our directors will be a “financial and accounting expert,” in addition to the outside directors. Currently, Doron Birger qualifies as a “financial and accounting expert,” as defined in the applicable regulations.
In addition to the requirements of the Companies Law, we comply with the Nasdaq Global Market listing requirements, under which a majority of the members of our board of directors (including all members of our audit committee) are required to be independent, as that term is defined in the rules of the Nasdaq Global Market. Our board of directors has determined that all of our directors, except Messrs. Makov and Shamir, qualify as independent directors in accordance with the applicable rules.
Audit Committee
Under the Companies Law, the board of directors of any company whose shares are listed on any exchange must also appoint an audit committee comprised of at least three directors including all of the outside directors. The audit committee may not include the chairman of the board of directors, the general manager, the chief executive officer, a director employed by the company or who provides services to the company on a regular basis, or a controlling shareholder or a relative of a controlling shareholder. Under the Nasdaq Global Market listing requirements, we are required to have an audit committee consisting of at least three members, each of whom must be “independent”, as defined under the rules of the Nasdaq Global Market and the Securities Exchange Act of 1934 (the “Exchange Act”), and each of whom must be able to read and understand fundamental financial statements. In addition, one member of the audit committee must have past employment experience in finance or accounting or other comparable experience which results in the individual’s financial sophistication. We believe that each of the current members of our audit committee, Michael Grobstein, James Cornelius and Dennert Ware, meets these independence and financial literacy requirements. In addition, the board of directors has determined that Mr. Grobstein has the requisite experience and is the financial expert serving on our audit committee.
Under the Companies Law, the role of the audit committee is to identify irregularities in the business management of the company, in consultation with the internal auditor and the company’s independent accountants, and suggest an appropriate course of action. In addition, it is the role of the audit committee to approve transactions with related parties and other corporate actions specified in the Companies Law. Under the Nasdaq Global Market listing requirements, our audit committee has adopted an audit committee charter setting forth its responsibilities. The audit committee charter governing the actions of our audit committee states that in fulfilling this role the committee is entitled to rely on interviews and consultations with our management, our internal auditor and our independent public accountants, and is not obligated to conduct any independent investigation or verification. The charter also states that the audit committee is required to nominate the company’s independent accountants, which the shareholders subsequently are required to approve.
Internal Auditor
Under the Companies Law, the board of directors must appoint an internal auditor nominated by the audit committee. The role of the internal auditor is to examine whether a company’s actions comply with the law and orderly business procedure. Under the Companies Law, the internal auditor may be an employee of the company but not an interested party or an office holder, or affiliate, or a relative of an interested party or an office holder, nor may the internal auditor be the company’s independent accountant or its representative. An interested party is defined in the Companies Law as any person or entity holding 5% or more of the outstanding shares or the voting rights in the company, any person or entity who has the right to designate one director or more or the chief executive officer of the company or any person who serves as a director or as a chief executive officer. Our internal auditor is an employee of the Israeli member firm of Deloitte Touche Tohmatsu.
Compensation and Nominating Committee
Our compensation and nominating committee consists of our directors James Cornelius, Doron Birger and Michael Grobstein. In accordance with the rules of the Nasdaq Global Market, our compensation and nominating committee adopted a charter, which sets forth its responsibilities. Pursuant to the charter, the compensation and nominating committee is authorized to make decisions regarding executive compensation and terms and conditions of employment, as well as to recommend that the board of directors issue options under our stock option plans. The compensation and nominating committee is also responsible for recommending to the board of directors nominees for board membership. The composition of the committee satisfies the Nasdaq Global Market’s independent director requirements.
Executive Committee
We have an executive committee, consisting of three directors: Michael Grobstein (Chairman), Israel Makov and James Cornelius. According to the committee’s charter:
| | • | the executive committee must be comprised of at least three directors and the majority of the committee’s members must qualify as “independent directors” under applicable law and the Nasdaq Global Market independent director requirements (and one member must qualify as an “outside director” under the Israeli Companies Law); |
| | | |
| | • | the Chairman of our board of directors must serve as a member of the executive committee; |
| | | |
| | • | the executive committee shall meet as needed to conduct and oversee our affairs and fulfill its responsibilities and not less than once each quarter; |
| | | |
| | • | the executive committee may exercise the full powers of our board of directors between board meetings where, at the discretion of the chairman of the board, timely action is required or warranted, provided that the committee may not act in lieu of our board in any matter in respect of which the delegation of powers is prohibited under applicable law or that requires the approval of our shareholders; and |
| | | |
| | • | the committee is otherwise granted, among other things, the mandate to review the details of our business strategy and make recommendations to the board, to oversee and assist our chief executive officer as necessary, to monitor organization and management changes in our company and take any other action delegated or assigned from time to time by our board of directors. |
As of December 31, 2007, we and our subsidiaries had 458 employees of whom 232 were based in Israel, 150 in the United States, 50 in Europe, 13 in Japan and 13 in Asia-Pacific. This reflects an increase of 14.8 % in the number of employees since December 31, 2006. This increase was mainly in research and development employees in Israel and as a result of hiring new employees to our Japanese subsidiary to support the increasing business in Japan following regulatory clearance for our products there in April 2007. We expect our headcount to increase further in 2008, primarily in research and development, manufacturing and sales and marketing.
Under Israeli law, we and our employees in Israel are subject to protective labor provisions, including restrictions on working hours, minimum wages, minimum vacation, minimum termination notice, sick pay, severance pay and social security as well as equal opportunity and anti-discrimination laws. Orders issued by the Israeli Ministry of Labor and Welfare make certain industry-wide collective bargaining agreements and collective bargaining agreements in the electricity, steel and electronics industries applicable to us. These agreements affect matters such as cost of living adjustments to salaries, length of working hours and week, recuperation, travel expenses, and pension rights. Our employees are not represented by a labor union. We provide our employees with benefits and working conditions above the required minimum and which we believe are competitive with benefits and working conditions provided by similar companies in Israel. We have written employment agreements with all of our Israeli employees and with our senior non-Israeli employees. Competition for qualified personnel in our industry is intense and we dedicate significant resources to employee retention. We have never experienced labor-related work stoppages and believe that our relations with our employees are good.
Share Ownership by Directors and Executive Officers
For information regarding ownership of our ordinary shares by our directors and executive officers, and regarding options to purchase our ordinary shares granted to Nachum Shamir, our President and Chief Executive Officer and to Israel Makov, the Chairman of our board of directors, see Item 7 “Major Shareholders and Related Party Transactions.”
Stock Option Plans
2006 Equity Incentive Plan
Our 2006 Equity Incentive Plan provides for the grant of options to purchase our ordinary shares or the grant of restricted stock to eligible employees, directors and consultants of us or our subsidiaries. The 2006 equity plan is administered by our Board of Directors and Compensation and Nominating Committee. The plan contains provisions concerning the vesting, price, exercise and other terms of awards; however, in many cases our Compensation and Nominating Committee has authority to grant awards under different terms at its discretion. We have reserved for issuance a total of 4,000,000 authorized but unissued ordinary shares under the 2006 equity plan. As of December 31, 2007, we had outstanding under this plan options to purchase 1,968,392 shares and 106,000 shares of restricted stock.
The 2006 equity plan permits us to grant a number of equity instruments, such as options, restricted stock, restricted stock units and stock appreciation rights. Our previous plans only permitted the grant of options. Option awards under this plan must be granted at no less than the fair market value of our ordinary shares on the date of the grant and the term of the awards may not exceed ten years. Our current policy is that options granted under the 2006 equity plan expire five years following the date of the grant.
Generally, where a grant of an award under the plan is the first grant of equity to a particular person, 50% of the award is exercisable on the second anniversary of the date of grant, and 25% becomes exercisable on each of the third and fourth anniversaries of the date of the grant. In cases of subsequent grants, awards vest in four equal installments beginning with the first anniversary of the grant. To the extent the awards have vested, they may be exercised in whole or in part from time to time until their expiration.
In case of participating employees and consultants, upon the termination of their employment or service, all unvested awards are cancelled. All vested awards may be exercised within 180 days following termination. All vested awards not exercised within this period are automatically forfeited and cancelled. Unvested awards to non-employee directors whose service is terminated or discontinued for any reason other than for cause after more than five years of service on our board of directors, will automatically vest and become exercisable immediately prior to termination or discontinuation of service. These vested awards may be exercised within 180 days following termination of service, except in cases of where termination or discontinuation of service is a result of statutory requirements, death, disability or other circumstances of forced cessation of service, in which case awards may be exercised at any time until their expiration date. In a case of termination for cause of a plan participant, all awards, whether vested or unvested, are automatically forfeited and cancelled.
Under this plan, in the event of an acquisition or merger in which we are not the surviving entity and the acquiring entity does not agree to assume the awards, all outstanding, but unvested, awards will be accelerated and exercisable, ten days prior to the acquisition or merger. In addition, if the employment of a holder of outstanding awards is terminated in anticipation of or during the 12 month period following an acquisition or merger, all awards that are scheduled to vest within two years of such acquisition or merger, will be automatically accelerated and exercisable, subject to certain adjustments and exceptions.
Awards granted under the 2006 equity plan to Israeli residents may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the awards or the ordinary shares issued upon their exercise must be deposited with a trustee for at least two years following the date of the grant. Under Section 102, any tax payable by an employee from the grant or exercise of the awards is deferred until the transfer of the awards or ordinary shares by the trustee to the employee or upon the sale of the awards or ordinary shares. Gains on awards granted under the 2006 equity plan are subject to a mixed tax rate as follows: First, any profit derived from the excess of the average price of the ordinary shares of the company during the 30 trading days prior to the date of grant over the exercise price of the options and other tax deductible expenses concerning the sale, referred to as the average profit at grant date, is subject to regular income tax based on the individual tax rate of the exercising person. Second, any profit resulting from the excess of the selling price of the shares underlying the options over the average profit at grant date is subject to capital gains tax at a rate of 25%, if all the conditions and requirements under Section 102 are fulfilled. Options granted under the plan to U.S. residents may also qualify as incentive stock options within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986. The exercise price for incentive stock options must not be not less than the fair market value on the date the option is granted, or 110% of the fair market value if the option holder holds more than 10% of our share capital.
2003 Stock Option Plan
Our 2003 stock option plan provides for a grant of options to our directors, employees and consultants, including members of our medical advisory committee, and to the directors, employees or consultants of our subsidiaries. We have reserved a total of 2,500,000 ordinary shares for issuance under the plan. In addition, we have reserved for issuance under the plan any ordinary shares underlying unvested options granted under our 1998 and 2000 stock option plans that expired without exercise. As of December 31, 2007, we had outstanding options to purchase 2,132,655 shares under the 2003 stock option plan
The plan is substantially similar to our 2000 stock option plan. Generally, where a grant of options under the plan is our first grant of options to that person, the options are not exercisable before the second anniversary of the date of grant, at which time 50% of the options become exercisable with 25% becoming exercisable on each of the third and fourth anniversaries of the date of the grant. However, in cases of subsequent grants, options vest in four equal installments beginning with the first anniversary of the grant. Our Compensation and Nominating Committee has the authority to accelerate the time periods for the vesting of options. Unexercised options expire ten years after the date of grant. To the extent the options have been vested, they may be exercised in whole or in part from time to time until their expiration. Upon the termination of the employment of an employee other than for cause, the employee may exercise all vested options. Our Compensation and Nominating Committee determined that in respect of grants made beginning in 2006, optionees will have 180 days to exercise vested options following termination. If the employment of an employee is terminated for cause, all of his or her vested and unvested options will be automatically forfeited and cancelled.
In the event of an acquisition or merger, we will endeavor to ensure that the rights of the holders of outstanding options are maintained. If we are unable to do so or if our board of directors resolves otherwise, all outstanding, but unvested, stock options will be accelerated and exercisable, ten days prior to the acquisition or merger. In addition, if the employment of a holder of outstanding options is terminated in anticipation of or during the 12 month period following an acquisition or merger, all outstanding but unvested stock options will be accelerated and exercisable, subject to certain adjustments and exceptions.
Options granted under the 2003 plan to Israeli residents may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the options or the ordinary shares issued upon their exercise must be deposited with a trustee for a minimum period equal to the shorter of 30 months commencing on the date of grant or 24 months commencing on the end of the year in which the grant was made. Under Section 102, any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or ordinary shares by the trustee to the employee or upon the sale of the options or ordinary shares. Gains on options granted in 2003 and later are subject to a mixed tax rate as follows: First, any profit derived from the excess of the average price of the ordinary shares of the company during the 30 trading days prior to the date of grant over the exercise price of the options and other tax deductible expenses concerning the sale, referred to as the average profit at grant date, is subject to regular income tax based on the individual tax rate of the exercising person. Second, any profit resulting from the excess of the selling price of the shares underlying the options over the average profit at grant date is subject to capital gains tax at a rate of 25%, if all the conditions and requirements under Section 102 are fulfilled. Options granted under the plan to U.S. residents may also qualify as incentive stock options within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986. The exercise price for incentive stock options must not be not less than the fair market value on the date the option is granted, or 110% of the fair market value if the option holder holds more than 10% of our share capital.
2000 Stock Option Plan
Our 2000 stock option plan provides for the grant of options to our directors, employees or consultants, including members of our medical advisory committee, and to the directors, employees or consultants of our subsidiaries. As of December 31, 2007, we had outstanding options to purchase 568,137 shares under the 2000 stock option plan. Ordinary shares underlying options which expire without exercise under the 2000 stock option plan become available for issuance under the 2003 stock option plan.
The plan is administered by our Compensation and Nominating Committee which makes recommendations to our board of directors regarding grantees of options and the terms of the grant, including exercise prices, vesting schedules, acceleration of vesting and other matters necessary in the administration of the plan. Upon the recommendation of our Compensation and Nominating Committee, options granted under the plan to Israeli residents may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the options or the ordinary shares issued upon their exercise must be deposited with a trustee for at least two years. Any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or ordinary shares by the trustee to the employee or upon the sale of the options or ordinary shares. Options granted under the plan to U.S. residents may also qualify as incentive stock options within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986. The exercise price for incentive stock options must not be not less than the fair market value on the date the option is granted, or 110% of the fair market value if the option holder holds more than 10% of our share capital.
Under the 2000 stock option plan, options issued under the plan are not exercisable before the second anniversary of the date of grant at which time 50% of the options become exercisable with 25% becoming exercisable on each of the third and fourth anniversaries of the date of grant. Unexercised options expire ten years after the date of grant. If the employment of an employee is terminated for cause, all of his or her vested and unvested options will automatically be forfeited and cancelled.
In the event of an acquisition or merger, we will endeavor to ensure that the rights of the holders of outstanding options are maintained. If we are unable to do so or if our board of directors resolves otherwise, all outstanding, but unvested, stock options will be accelerated and exercisable, ten days prior to the acquisition or merger.
1998 Stock Option Plan
Our 1998 stock option provides for the grant of options to our directors, employees, or consultants, including members of our medical advisory committee. Our 1998 stock option plan has been superseded by our 2000 stock option plan and we have ceased issuing options under our 1998 stock option plan. As of December 31, 2007, we had outstanding options to purchase 37,500 shares under the 1998 stock option plan. Ordinary shares underlying options which expire without exercise under the 1998 stock option plan become available for issuance under the 2003 stock option plan.
Under the 1998 stock option plan, options issued under the plan are not exercisable before the second anniversary of the date of grant at which time 50% of the options become exercisable with 25% becoming exercisable on each of the third and fourth anniversaries of the date of grant. Unexercised options expire ten years after the date of grant. If the employment of an employee is terminated for cause, all of his or her vested and unvested options will automatically be forfeited and cancelled.
The following table sets forth certain information regarding the beneficial ownership of our outstanding ordinary shares as of December 31, 2007 for: (1) each person who we believe beneficially owns 5% or more of the outstanding ordinary shares, (2) each of our directors individually, (3) each of our executive officers individually, and (4) all of our directors and executive officers as a group. Beneficial ownership of shares is determined under rules of the Securities and Exchange Commission and generally includes any shares over which a person exercises sole or shared voting or investment power. The table also includes the number of shares underlying options that are exercisable within 60 days of December 31, 2007. Ordinary shares subject to these options are deemed to be outstanding for the purpose of computing the ownership percentage of the person holding these options, but are not deemed to be outstanding for the purpose of computing the ownership percentage of any other person.
The shareholders listed below do not have any different voting rights from our other shareholders. Unless otherwise noted below, each shareholder’s address is c/o Given Imaging Ltd., P.O. Box 258, Yoqneam 20692, Israel.
Name and Address | | Number of Shares Beneficially Owned | | Percentage of Shares Beneficially Owned | |
Principal shareholders: | | | | | | | |
| IDB Holding Corporation Ltd. (1) | | | 12,724,808 | | | 43.9 | % |
| AXA Assurances I.A.R.D. Mutuelle, AXA Assurances Vie Mutuelle, AXA Courtage Assurance Mutuelle, AXA, AXA Financial, Inc.(2) | | | 3,347,839 | | | 11.4 | |
Directors and executive officers: | | | | | | | |
| Nachum Shamir (3) | | | 180,000 | | | * | |
| Yuval Yanai (4) | | | 47,500 | | | * | |
| Kevin Rubey (5) | | | 225,000 | | | * | |
| Christopher Rowland (6) | | | 38,000 | | | * | |
| Mark Gilreath (7) | | | 173,900 | | | * | |
| Kazem Samandari (8) | | | 6,000 | | | * | |
| Manfred Gehrtz (9) | | | 88,500 | | | * | |
| Ori Braun | | | — | | | — | |
| Keith Chrzanowski (10) | | | 3,505 | | | * | |
| Daphna Levy (11) | | | 6,550 | | | * | |
| Israel Makov | | | — | | | — | |
| Doron Birger (12) | | | 8,044,058 | | | 27.5 | |
| James M. Cornelius (13) | | | 254,038 | | | * | |
| Michael Grobstein (14) | | | 206,000 | | | * | |
| Arie Mientkavich | | | — | | | — | |
| Dennert O. Ware | | | — | | | — | |
| Anat Loewenstein (15) | | | 33,000 | | | * | |
| All directors and executive officers as a group (16) | | | 14,067,261 | | | 48.1 | % |
| (1) | Based on a Schedule 13D/A filed on June 4, 2007 and on information provided to us supplementally, this number consists of 5,340,070 ordinary shares owned by Elron Electronic Industries Ltd., or Elron, 4,719,528 ordinary shares owned by Discount Investment Corporation Ltd., or DIC, 2,662,110 ordinary shares owned by RDC Rafael Development Corporation Ltd., or RDC, and 3,100 ordinary shares held by subsidiaries of Clal Insurance Enterprises Holdings Ltd., or CIEH, for their own account. This number does not include 277,114 Ordinary Shares held for members of the public through mutual funds, provident funds, pension funds, exchange traded funds and insurance policies which are managed by companies controlled by CIEH, which disclaims beneficial ownership of these shares. Based on information contained in that Schedule 13D/A and on information provided to us supplementally, Elron owns all of the outstanding shares of DEP Technology Holdings Ltd. which, in turn, holds 50.1% of the voting power of RDC. As a result, Elron may be deemed to be the beneficial owner of the ordinary shares owned by RDC. As of June 4, 2007, DIC owned approximately 48.6% of the outstanding shares of Elron and, as a result, DIC may be deemed to be the beneficial owner of the ordinary shares owned by RDC and by Elron. IDB Holding Corporation Ltd., or IDBH, owns the majority of the outstanding shares of IDB Development Corporation Ltd., or IDBD, which, in turn, owns the majority of the outstanding shares of DIC and CIEH. As a result, IDBH may be deemed to be the beneficial owner of the ordinary shares owned by DIC, RDC and Elron, and the ordinary shares held by subsidiaries of CIEH for their own account. The address of each of DIC, IDBH and IDBD is The Triangular Tower, 44th Floor, 3 Azrieli Center, Tel Aviv 67023, Israel. The address of RDC is Building 7, New Industrial Park, Yoqneam 20692, Israel. The address of Elron is The Triangular Tower, 42nd Floor, 3 Azrieli Center, Tel Aviv 67023, Israel. IDBH, IDBD and DIC are public companies traded on the Tel Aviv Stock Exchange. Elron is a public company traded on the Tel Aviv Stock Exchange and on the Nasdaq Global Select Market. |
| | |
| | As of December 31, 2007, (i) Ganden Holdings Ltd., or Ganden, a private Israeli company controlled by Nochi Dankner and his sister Shelly Bergman, held, directly and through a wholly-owned subsidiary, approximately 50.04% of the outstanding shares of IDBH; (ii) Shelly Bergman, through a wholly-owned company, held approximately 4.23% of the outstanding shares of IDBH; (iii) Ytzhak Dankner (the father of Shelly Bergman and Nochi Dankner), through a wholly-owned subsidiary, held 3.0% of IDBH's shares; (iv) Avraham Livnat Ltd., or Livnat, a private Israeli company controlled by Avraham Livnat, held, directly and through a wholly-owned subsidiary, approximately 11.69% of the outstanding shares of IDBH; and (v) Manor Holdings BA Ltd., or Manor, a private company controlled by Ruth Manor and Isaac Manor, held, directly and through a majority-owned subsidiary, approximately 11.68% of the outstanding shares of IDBH. |
| | Subsidiaries of Ganden, Livnat and Manor have entered into a shareholders agreement with respect to their shares of IDBH constituting, respectively, 31.02%, 10.34% and 10.34% of the outstanding shares of IDBH, for the purpose of maintaining and exercising control of IDBH as a group. Their additional holdings in IDBH are not subject to the shareholders agreement. The term of the shareholders agreement expires in May 2023. Based on the foregoing, Ganden, Manor and Livnat (by reason of their control of IDBH) and Nochi Dankner, Shelly Bergman, Ruth Manor, Isaac Manor and Avraham Livnat (by reason of their control of Ganden, Manor and Livnat, respectively) may be deemed to share with DIC, Elron and RDC the power to vote and dispose of our ordinary shares held by these entities, and also to share with subsidiaries of CIEH the power to vote and dispose of our ordinary shares held by these subsidiaries for their own account. |
| | |
| | Nochi Dankner is Chairman and Chief Executive Officer of IDBH, the Chairman of IDBD and DIC and a director of Elron. Zehava Dankner, the mother of Nochi Dankner and Shelly Bergman and the wife of Yitzhak Dankner, is a director of IDBH, IDBD and DIC. Isaac Manor and Zvi Livnat (a son of Avraham Livnat) are directors of IDBH, IDBD and DIC. Shai Livnat (another son of Avraham Livnat) is a director of IDBD. Dori Manor (the son of Isaac and Ruth Manor) is a director of IDBH, IDBD, DIC and Elron. The address of Nochi Dankner is The Triangular Tower, 44th Floor, 3 Azrieli Center, Tel Aviv 67023, Israel. The address of Shelly Bergman is 9 Mishmar Ezrehi Street, Afeka, Tel Aviv 69697, Israel. The address of Ruth Manor and Isaac Manor is 26 Hagderot Street, Savyon 56526, Israel. The address of Avraham Livnat is Taavura Junction, Ramle 72102, Israel. These individuals disclaim beneficial ownership of the shares owned by the foregoing entities except to the extent of their pecuniary interest therein. On September 29, 2003, Elron and DIC entered into a voting agreement pursuant to which, among other things, DIC agreed to vote all of its ordinary shares in favor of nominees to our board of directors proposed by Elron. The voting agreement is for a term of one year and renews automatically each year thereafter unless terminated by notice of either party to the other party no later than August 30 in each year, or unless earlier terminated by agreement of both parties thereto. |
| | |
| (2) | Based on a Schedule 13G/A filed on February 14, 2008, consists of 3,231223 ordinary shares owned by Alliance Bernstein L.P., or Alliance, and 116,616 ordinary shares owned by AXA Equitable Life Insurance Company, or AXA Equitable. Based on information contained in the Schedule 13G/A, Alliance and AXA Equitable are subsidiaries of AXA Financial, Inc. AXA Financial, Inc. is a parent holding company with respect to the holdings of Alliance and AXA Equitable. Each of Alliance and AXA Equitable operates under independent management and makes independent decisions. AXA Financial, Inc. is controlled by AXA Assurances I.A.R.D Mutuelle, AXA Assurances Vie Mutuelle and AXA Courtage Assurance Mutuelle (collectively, the “Mutuelles AXA”), as a group. Based on information contained in the Schedule 13G/A, the majority of the shares reported in such schedule are held for investment purposes on behalf of client accounts unaffiliated with the reporting entities. The address of AXA Assurances I.A.R.D Mutuelle and AXA Assurances Vie Mutuelle is 26, rue Drouot, 75009 Paris, France. The address of AXA Courtage Assurance Mutuelle is 26, rue Drouot, 75009 Paris, France. The address of AXA is 25, avenue Matignon, 75008 Paris, France. The address of AXA Financial, Inc. is 1290 Avenue of the Americas, New York, NY 10104. Each of the Mutuelles AXA, as a group, expressly disclaims beneficial ownership of any securities owned by the foregoing entities. |
| | |
| (3) | Consists of 5,000 ordinary shares, 100,000 shares of restricted stock and options to purchase 75,000 ordinary shares. |
| | |
| (4) | Consists of 5,000 ordinary shares and options to purchase 42,500 ordinary shares. |
| | |
| (5) | Consists of options to purchase 225,000 ordinary shares. |
| | |
| (6) | Consists of 3,000 ordinary shares and options to purchase 35,000 ordinary shares. |
| | |
| (7) | Consists of 1,400 ordinary shares and options to purchase 172,500 ordinary shares. Mr. Gilreath left Given Imaging at the end of 2007. |
| | |
| (8) | Consists of 6,000 restricted shares. |
| | |
| (9) | Consists of 1,000 ordinary shares and options to purchase 87,500 ordinary shares. |
| | |
| (10) | Consists of 380 ordinary shares and options to purchase 3,125 ordinary shares. |
| | |
| (11) | Consists of options to purchase 6,550 ordinary shares. |
| | |
| (12) | Consists of 2,878 ordinary shares and options to purchase 39,000 ordinary shares owned by Mr. Birger personally, 2,662,110 ordinary shares owned by RDC Rafael Development Corporation and 5,340,070 ordinary shares owned by Elron Electronic Industries. Mr. Birger is a director of RDC Rafael Development Corporation and President and Chief Executive Officer of Elron Electronic Industries and by virtue of his position may be deemed to have voting and investment power, and thus beneficial ownership with respect to the shares that Elron Electronic Industries and RDC Rafael Development Corporation own. Mr. Birger disclaims such beneficial ownership except to the extent of his pecuniary interest therein. |
| | |
| (13) | Consists of 121,038 ordinary shares and options to purchase 133,000 ordinary shares. |
| | |
| (14) | Consists of 30,000 ordinary shares and options to purchase 176,000 ordinary shares. |
| | |
| (15) | Consists of options to purchase 33,000 ordinary shares. |
| | |
| (16) | Includes 12,724,808 ordinary shares beneficially owned by IDB Holding Corporation, as well as ordinary shares and options to purchase ordinary shares beneficially held by directors and officers in their personal capacities or by their nominees. Our directors and officers disclaim beneficial ownership of the shares owned by the foregoing entities except to the extent of their pecuniary interest therein. |
B. RELATED PARTY TRANSACTIONS
Registration Rights Agreement
In July 2007, our shareholders approved a Registration Rights Agreement among us, Elron, DIC and RDC. Elron, DIC and RDC own an aggregate of 43.9% of our ordinary shares and are collectively referred to as the “affiliated shareholders.” This Registration Rights Agreement has replaced earlier registration rights granted by us to Elron, DIC, RDC, entities affiliated with OrbiMed Capital LLC and other shareholders in connection with a private placement completed in September 2000, before our initial public offering. These earlier registration rights expired in October 2006.
Our board of directors believes that this agreement is necessary to protect the market for our ordinary shares. During 2006, the affiliated shareholders demonstrated their commitment to our business by increasing their ownership level by purchasing additional shares in the open market. The Registration Rights Agreement provides the affiliated shareholders means for liquidity that are otherwise not available to them under applicable law given their ownership level. At the same time, it increases the likelihood that a sale by the affiliated shareholders will be coordinated with us and not disrupt the ordinary activity in the market for our shares due to the sale of a large number of shares at one time or during a short period.
The main terms of the proposed registration rights agreement are as follows:
Demand Registration Rights
At the request of one or more of the affiliated shareholders holding at least 5% of our then outstanding ordinary shares, we must use our best efforts to register any or all of these shareholders’ ordinary shares on the condition that the minimum aggregate offering price of the shares to be registered is at least $15 million. We must also give notice of the registration to other affiliated shareholders and include in the registration any ordinary shares that they request to include. This registration also may include ordinary shares offered by us for our own account and by our directors and officers. We may only be requested to carry out two of these demand registrations.
In connection with any such demand registration, the managing underwriter may limit the number of shares offered for marketing reasons. In such case, the managing underwriter must exclude first any shares to be registered by us for the company’s own account and, second, any shares to be registered by our directors and officers. Thereafter, the shares to be registered by the affiliated shareholders would be reduced pro rata among the affiliated shareholders requesting inclusion of their shares according to the number of shares held by each of them.
Incidental Registration Rights
The affiliated shareholders also have the right to request that we include their ordinary shares in any registration statements filed by us in the future for the purposes of a public offering, subject to specified limitations. The managing underwriter may limit the number of shares offered for marketing reasons. In this case, the managing underwriter must exclude first any shares to be registered by us, unless we initiated the registration, second the shares that the affiliated shareholders have requested to include in the registration, and third the shares of the party initiating the registration.
Form F-3 Registration Rights
At the request of an affiliated shareholder, we must make our best efforts to register such shareholder’s ordinary shares on Form F-3. We must also give notice of the registration to other affiliated shareholders to whom we have granted registration rights and include in the registration any ordinary shares they request to include. These demand rights may only be exercised if nine months have passed since the last registration that we filed in which the affiliated shareholder requesting registration was entitled to include its shares. The minimum aggregate offering price of the shares to be registered is $15 million, in case of an underwritten offering, or $5.0 million, in case of a non-underwritten offering. The managing underwriter may limit the number of shares offered for marketing reasons. In such case, the rights of each shareholder to include its ordinary shares in the registration are allocated in the same manner as in a demand registration described above.
Termination
All registration rights will expire on the fifth anniversary of the agreement. With respect to any shareholder, registration rights will expire if that shareholder can sell all of its ordinary shares within a 90 day period under Rule 144 under the United States Securities Act of 1933, as amended.
Expenses
Generally, we will pay all expenses incurred in carrying out the above registrations, as well as the fees and expenses of one legal counsel for the selling shareholders in each registration.
Agreement for Travel Services
Diesenhaus is a provider of travel services in Israel and is controlled by IDB Holding Corporation Ltd., which beneficially owns approximately 43.9% of our ordinary shares. The terms of our engagement with Diesenhaus are similar to the terms we have with other providers of travel services to us. In 2007, we paid Diesenhaus a total of $71,000, or 8.5% of our travel expenses. This agreement was approved by our audit committee and board of directors in accordance with Israeli law.
Agreement for Mobile Communication Services.
In December 2005, we entered into an agreement with Cellcom Ltd., an Israeli provider of mobile communication services. Cellcom is controlled by IDB Holding Corporation Ltd., which beneficially owns approximately 43.9% of our ordinary shares. Under the agreement, Cellcom will be the sole provider of mobile communication to us during the term of the agreement. This agreement was approved by our audit committee and Board of Directors in accordance with Israeli law. In 2007, we paid Cellcom a total of $178,000.
Directors Fees
We pay directors fees in respect of service by our directors (other than our President and Chief Executive Officer, Nachum Shamir, and Chairman, Israel Makov). See Item 6 “Directors, Senior Management and Employees — Compensation.”
Agreements with Directors and Officers
We maintain written employment agreements with all of our officers. These agreements all contain provisions standard for a company in our industry regarding noncompetition, confidentiality of information and assignment of inventions. The enforceability of covenants not to compete in Israel is limited.
Employment Agreement with the Chairman of the Board of Directors
In June 2007, we entered into an employment agreement with Mr. Israel Makov, who serves as the Chairman of our board of directors. This agreement was approved by our shareholders in the annual shareholders meeting held in July 2007. In consideration for his service as a director and Chairman of the Board of Directors, Mr. Makov receives a monthly payment of NIS 60,000 (approximately $16,500 as of the date of this annual report), subject to adjustment based on the Israeli Consumer Price Index, in lieu of any statutory or other typical adjustments. In addition, Mr. Makov is entitled to pension, disability, study fund, health insurance and other benefits in accordance with standard terms of employment in Israel, including a monthly deduction to severance fund in lieu of statutory severance. The total monthly contributions required to be made by us under all these items is 23.3% of base salary and the total contribution by Mr. Makov is 7.5% of base salary. Mr. Makov is entitled to vacation and sick leave in accordance with standard practices in Israel. We reimburse Mr. Makov for all business-related expenses and provide him with directors’ and officers’ insurance coverage and indemnification, in accordance with the terms approved by our shareholders.
In addition, we granted Mr. Makov options to purchase 580,742 of our ordinary shares. The exercise price of these options is $29.42, equal to the closing price of the ordinary shares on the NASDAQ Global Market on July 18, 2007, the date of the annual meeting of our shareholders approving this grant. These options will vest in three installments - 50% on the second anniversary of the grant and 25% on each of the third and fourth anniversary of the grant. These options may be exercised by Mr. Makov at any time during a period beginning with the vesting date of each installment and ending four years thereafter. Any options not exercised within the exercise period will be forfeited and cancelled. In the event of a “change of control” of Given Imaging, Mr. Makov will be entitled to full acceleration of his options, which will then terminate if not exercised within 12 months. “Change of control” is defined as a change in share ownership of our company resulting in a person who is not holding, personally or on his own behalf, at least 5% of our ordinary shares on the effective date of the agreement owning a number of shares giving such person effective control over our business.
Mr. Makov’s employment agreement contains provisions standard for a firm in our industry regarding non-competition and confidentiality of information.
Either we or Mr. Makov may terminate his employment for any reason upon three months’ prior written notice, in which case Mr. Makov is entitled to receive his base salary and benefits payable during the notice period and continued vesting of options during a period of six months following termination. Vested options will terminate if not exercised within 12 months after the latest vesting date. Mr. Makov’s employment may be terminated by us for cause immediately and without any termination-related payments.
The compensation of Mr. Makov as described above represents the entire compensation that Mr. Makov is entitled to receive from us. All terms described above are in lieu of the fees (in cash and equity) ordinarily paid to our non-employee directors and any payments, including bonus payments, typically paid to our officers and employees.
Employment Agreement with our President and Chief Executive Officer
We have an employment agreement with Mr. Shamir, which we signed in April 2006. The employment agreement contains provisions standard for a firm in our industry regarding non-competition, confidentiality of information and assignment of inventions. Since Mr. Shamir is also a director, under Israeli law his employment agreement and terms, as well as any changes to the agreement, are subject to approval by our shareholders. Our shareholders approved the employment agreement with Mr. Shamir in May 2006. In July 2007, our shareholders approved an amendment to Mr. Shamir’s employment contract in light of our improved performance during Mr. Shamir’s first year in office as Chief Executive Officer.
Under the amended agreement, Mr. Shamir was entitled to (1) an annual base salary of $400,000, effective July 1, 2007, compared to $354,750 annual base salary prior to that date, (2) a cash bonus of up to $800,000, or 200% of the annual base salary, subject to meeting certain personal and company performance targets determined from time to time at the discretion of the board of directors, and (3) a grant of options to purchase 100,000 of our ordinary shares at an exercise price of $29.42, which is equal to the closing price of the ordinary shares on the Nasdaq Global Market on the date the shareholders approved this grant. This grant will vest in four equal installments beginning on the first anniversary of the date of the grant. Mr. Shamir is also entitled to other standard benefits, such as a car allowance, medical and life insurance benefits and reimbursement of expenses. In respect of 2007, our audit committee and board of directors approved a bonus to Mr. Shamir of $450,000. This bonus is subject to approval by our shareholders at our next annual shareholders’ meeting expected in June 2008.
Mr. Shamir’s employment may be terminated by either side without cause upon three months’ prior written notice. Mr. Shamir’s employment may also be terminated by us immediately for cause or by Mr. Shamir immediately for “good reason,” which consists of an uncured breach of the employment agreement by us or a continuous and material reduction in the scope or conditions of Mr. Shamir’s employment. Under the amended employment agreement, in the event of a termination of Mr. Shamir by us without cause or by Mr. Shamir for “good reason” (with the definition expanded to include relocation outside the Atlanta, Georgia area) , Mr. Shamir will be entitled to (A) a lump sum payment equal to two times the sum of (1) his annual base salary, plus (2) his target bonus for the year of termination (assuming for this purpose that all relevant performance objectives had been met); (B) acceleration of vesting of any equity award scheduled to vest during a period of twenty-four (24) months following termination; and (C) the value of his accrued and unused vacation days and continuation of benefits, such as health and life insurance policies and car allowance, for a period of twenty-four (24) months following termination.
The amended employment agreement provides that we will gross-up any excise tax that may be imposed on Mr. Shamir under Section 4999 of the Internal Revenue Code of 1986, as amended, in case payments to him resulting from his termination trigger this tax provision.
In addition, the amended agreement provides for full acceleration of all unvested options upon a termination by us without cause or by Mr. Shamir for “good reason” in connection with a change of control event. “Change of control” is as defined in the Company’s 2006 Equity Incentive Plan, as amended, that was approved by the shareholders in May 2006.
For fiscal year 2008, our audit committee and board of directors approved for Mr. Shamir (1) an annual base salary of $416,000, effective July 1, 2008, (2) a cash bonus of up to 200% of Mr. Shamir’s annual base salary, subject to meeting certain personal and company performance targets determined from time to time at the discretion of the board of directors, and (3) a grant of options to purchase 100,000 of our ordinary shares, the exercise price of which will be equal to the closing price of the ordinary shares on the Nasdaq Global Market on the date the shareholders approve the grant, vesting in four equal installments beginning on the first anniversary of the date of the grant. The compensation of Mr. Shamir in 2008 as described above is subject to approval by our shareholders at our next annual shareholders’ meeting expected in June 2008.
Exculpation, Insurance and Indemnification
Under the Companies Law, an Israeli company may not exculpate an office holder from liability for a breach of the duty of loyalty of the office holder. However, a company may approve an act performed in breach of the duty of loyalty of an office holder provided that the office holder acted in good faith, the act or its approval does not harm the company, and the office holder discloses the nature of his or her personal interest in the act and all material facts and documents a reasonable time before discussion of the approval. An Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for a breach of duty of care, subject to specified exceptions, but only if a provision authorizing such exculpation is inserted in its articles of association. Our articles of association include such a provision.
An Israeli company may indemnify an office holder in respect of certain liabilities either in advance of an event or following an event provided a provision authorizing such indemnification is inserted in its articles of association. Our articles of association contain such an authorization. An undertaking by an Israeli company to indemnify an office holder must be limited to foreseeable liabilities and reasonable amounts determined by the board of directors. A company may indemnify an office holder against the following liabilities incurred for acts performed as an office holder:
| | • | a financial liability imposed on him or her in favor of another person pursuant to a judgment, settlement or arbitrator’s award approved by a court; |
| | | |
| | • | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf or by a third party, or in connection with criminal proceedings in which the office holder was acquitted or as a result of a conviction for a crime that does not require proof of criminal intent; and |
| | | |
| | • | reasonable expenses incurred by the office holder in connection with an investigation or other proceeding by a governmental authority, if such proceeding did not result in an indictment of the office holder, or if such proceeding did not result in an indictment of the office holder and the office holder was requested to pay a fine for a crime that does not require proof of criminal intent. |
An Israeli company may insure an office holder against the following liabilities incurred for acts performed as an office holder:
| | • | a breach of duty of loyalty to the company, to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| | | |
| | • | a breach of duty of care to the company or to a third party; and |
| | | |
| | • | a financial liability imposed on the office holder in favor of a third party. |
An Israeli company may not indemnify, insure or exculpate an office holder against any of the following:
| | • | a breach of duty of loyalty, except for insurance and indemnification where the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| | | |
| | • | a breach of duty of care committed intentionally or recklessly; |
| | | |
| | • | an act or omission committed with intent to derive illegal personal benefit; or |
| | | |
| | • | a fine levied against the office holder. |
Under the Companies Law, exculpation, indemnification and insurance of office holders must be approved by our audit committee and our board of directors and, in respect of our directors, by our shareholders as well.
Our articles of association allow us to exculpate, indemnify and insure our office holders to the fullest extent permitted by the Companies Law. Our office holders are currently covered by a directors and officers’ liability insurance policy. To date, no claims for directors and officers’ liability insurance have been filed under this policy.
We have entered into agreements with each of our office holders undertaking to exculpate, indemnify and insure them to the fullest extent permitted by law. We may enter into additional agreements to indemnify or insure our directors and officers when circumstances change or when new directors and officers join us. This indemnification is limited to events and amounts determined as foreseeable by the board of directors, and the insurance is subject to our discretion depending on its availability, effectiveness and cost. In the opinion of the U.S. Securities and Exchange Commission, however, indemnification of directors and office holders for liabilities arising under the Securities Act is against public policy and therefore unenforceable.
C. INTERESTS OF EXPERTS AND COUNSEL
Not applicable.
ITEM 8. FINANCIAL INFORMATION
A. CONSOLIDATED FINANCIAL STATEMENTS AND OTHER INFORMATION
Financial Statements
See Item 18 for audited consolidated financial statements.
Export Sales
Our manufacturing facilities for the data recorder and the PillCam capsules forming part of the Given System are located in Israel. Most of our products are exported out of Israel. For information regarding our revenues by geographic market, see Item 5: “Operating and Financial Review and Prospects.”
Legal Proceedings
Patent Disputes
On May 19, 2006, Olympus Corporation, Olympus Medical Systems Corp. and Olympus America Inc., collectively referred to in this section as “Olympus,” filed a complaint against us in the District Court for the Eastern District of Pennsylvania. In the complaint, Olympus alleged that our capsule endoscopes infringe U.S. Patent No. 5,010,412, or the ‘412 patent, entitled “High Frequency, Low Power Light Source for Video Camera.” Olympus contended that the ‘412 patent covers low power light sources of the kind used by us in our PillCam capsules. According to the complaint, Olympus did not develop this technology. Instead, in July 2005, Olympus acquired the ‘412 patent from The Boeing Company. The ‘412 patent will expire in December 2008. In addition, Olympus sought a declaratory judgment that a capsule endoscope product for which it was then seeking FDA clearance would not infringe our ‘531 patent and that the ‘531 patent is invalid. The ‘531 patent is our first patent that we acquired from Rafael Armament Development Authority, or Rafael, which is a division of the Israeli Defense Ministry. Olympus also requested an injunction preventing us from selling in the United States any product that infringes the ‘412 patent, as well as damages in an unspecified amount.
We filed our answer and counterclaim on October 20, 2006. In our answer and counterclaim we denied infringement of the ‘412 patent, and asserted that the ‘412 patent is invalid. In addition, we alleged that the ‘531 patent is valid and will be infringed by Olympus once it begins offering to sell its capsule endoscopy product in the United States. We also alleged that the Olympus product will infringe our U.S. Patent Nos. 6,934,093, entitled “Optical System,” 7,022,067, entitled “System and Method For Controlling In Vivo Camera Capture and Display Rate,” and 7,119,814, entitled “System and Method for Annotation on a Moving Image.” We also requested an injunction to prevent Olympus from selling in the United States any product that infringes our patents. We also stated that if Olympus sells its capsule endoscopy system in the United States, we may request the assessment of damages.
Olympus filed its reply and “counterclaims in response” on November 13, 2006, denying that Olympus will infringe our ‘531, ‘093, ‘067 and ‘814 patents, and alleging that our patents are invalid. We filed our reply to Olympus’s counterclaims on December 6, 2006, denying Olympus’s counterclaim.
On March 30, 2007, Olympus filed an amended complaint asserting that our capsule endoscopes infringe three additional patents owned by Olympus: U.S. Patent #5,794,226 entitled “Image Manipulation System Including Means For Assigning a File Name,” U.S. Patent #6,269,379 entitled “Medical Image Filing System Enabling Registration and Retrieval of a Plurality of Medical Images,” and U.S. Patent #6,939,292 entitled “Capsule Type Endoscope.” We filed our answer to the amended complaint in April 2007 denying infringement of these patents and asserting their invalidity.
In September 2007, Olympus received FDA clearance to market its competing capsule endoscope system in the United States and it has since commenced selling activities. As a result, we amended our pleadings to add a request for damages in an unspecified amount. In the ongoing litigation, fact discovery ended in January 2008 and expert discovery is ongoing. Trial is set for November 2008.
The outcome of this litigation, like all patent litigation, is uncertain. The ongoing litigation and any unfavorable outcome in the litigation will a material adverse effect on our business and our operating results and financial condition.
From time to time we may be involved in legal proceedings. Other than described above, currently we are not party to any legal proceedings whose outcome we expect will be material to our financial condition or results of operations.
Dividend Policy
We have never declared or paid any cash dividends on our ordinary shares and we do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. We currently intend to retain all future earnings to finance our operations and to expand our business. Any future determination relating to our dividend policy will be made at the discretion of our board of directors and will depend on a number of factors, including future earnings, capital requirements, legal restrictions, financial condition and future prospects and other factors the board of directors may deem relevant.
Significant Changes
Except as otherwise disclosed in this Form 20-F, there has been no significant change in our financial position since December 31, 2007.
A. OFFER AND LISTING DETAILS
Nasdaq Global Market
The following table lists the high and low closing sale prices of our ordinary shares for the periods indicated as reported by the Nasdaq Global Market:
Annual Highs and Lows | | High | | Low | |
| | | | | | |
| 2007 | | $ | 31.49 | | $ | 19.25 | |
| 2006 | | | 28.37 | | | 14.46 | |
| 2005 | | | 36.04 | | | 20.39 | |
| 2004 | | | 44.08 | | | 17.87 | |
| 2003 | | $ | 18.80 | | $ | 6.65 | |
Quarterly Highs and Lows | | High | | Low | |
| | | | | | |
| 4th quarter 2007 | | $ | 30.19 | | $ | 22.43 | |
| 3rd quarter 2007 | | | 31.49 | | | 25.02 | |
| 2nd quarter 2007 | | | 31.42 | | | 21.22 | |
| 1st quarter 2007 | | | 22.04 | | | 19.25 | |
| | | | | | | | |
| 1st quarter 2006 | | | 28.37 | | | 21.65 | |
| 2nd quarter 2006 | | | 24.07 | | | 15.06 | |
| 3rd quarter 2006 | | | 19.85 | | | 14.46 | |
| 4th quarter 2006 | | $ | 22.76 | | $ | 18.85 | |
| | | | | | | | |
Most Recent Six Months | | High | | Low | |
| | | | | | |
| February 2008 | | $ | 17.06 | | $ | 14.36 | |
| January 2008 | | | 23.29 | | | 15.59 | |
| December 2007 | | | 23.75 | | | 22.43 | |
| November 2007 | | | 27.20 | | | 23.12 | |
| October 2007 | | | 30.19 | | | 28.25 | |
| September 2007 | | $ | 27.76 | | $ | 25.47 | |
On February 29, 2008, the closing price of our ordinary shares on the Nasdaq Global Market was $15.86 per share. We believe that as of February 29, 2008, there were approximately 9,000 holders of record of our ordinary shares.
Tel Aviv Stock Exchange
The following table lists the high and low closing sale prices of our ordinary shares for the periods indicated as reported by the Tel Aviv Stock Exchange:
Annual Highs and Lows | | High | | Low | |
| | | | | | |
| 2007 | | NIS | 135.90 | | NIS | 81.47 | |
| 2006 | | | 132.60 | | | 65.02 | |
| 2005 | | NIS | 155.30 | | NIS | 95.79 | |
Quarterly Highs and Lows | | High | | Low | |
| | | | | | |
| 4th quarter 2007 | | NIS | 123.60 | | NIS | 47.17 | |
| 3rd quarter 2007 | | | 135.90 | | | 102.70 | |
| 2nd quarter 2007 | | | 125.10 | | | 86.14 | |
| 1st quarter 2007 | | | 92.74 | | | 81.47 | |
| | | | | | | | |
| 1st quarter 2006 | | | 132.60 | | | 101.60 | |
| 2nd quarter 2006 | | | 109.80 | | | 67.36 | |
| 3rd quarter 2006 | | | 86.50 | | | 65.02 | |
| 4th quarter 2006 | | NIS | 98.27 | | NIS | 80.54 | |
Most Recent Six Months | | High | | Low | |
| | | | | | |
| February 2008 | | NIS | 61.98 | | NIS | 47.17 | |
| January 2008 | | | 90.58 | | | 58.99 | |
| December 2007 | | | 91.50 | | | 87.53 | |
| November 2007 | | | 108.60 | | | 90.26 | |
| October 2007 | | | 123.60 | | | 111.40 | |
| September 2007 | | NIS | 112.80 | | NIS | 102.70 | |
The average exchange ratio of NIS to U.S. dollar in 2007 was NIS 4.1081 to $1.00.
B. PLAN OF DISTRIBUTION
Not applicable.
C. MARKETS
Our ordinary shares have traded publicly on the Nasdaq Global Market under the symbol “GIVN” since October 2001 and on the Tel Aviv Stock Exchange under the symbol “GIVN” since March 2004. Our ordinary shares trade publicly only on the Nasdaq Global Market and the Tel Aviv Stock Exchange.
D. SELLING SHAREHOLDERS
Not applicable.
E. DILUTION
Not applicable.
F. EXPENSES OF THE ISSUE
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. SHARE CAPITAL
Not applicable.
B. MEMORANDUM AND ARTICLES OF ASSOCIATION
Objects
Our objects under our memorandum of association are to engage in any type of manufacturing, trade, production, labor, agriculture, and professional and business services in all branches and areas of economic activity, to advance trade, importing and exporting, and any other object determined by our board of directors from time to time. Our objects under our articles of association are to engage in any lawful business.
Transfer of Shares and Notices
Fully paid ordinary shares are issued in registered form and may be freely transferred under our articles of association unless the transfer is restricted or prohibited by another instrument, Israeli law or the rules of a stock exchange on which the shares are traded. Our articles of association provide that each shareholder of record is entitled to receive at least 21 days’ prior notice of any shareholders’ meeting.
Election of Directors
Our ordinary shares do not have cumulative voting rights for the election of directors. Rather, under our articles of association our directors are appointed by the holders of a simple majority of our ordinary shares at a general shareholder meeting. As a result, the holders of our ordinary shares that represent more than 50% of the voting power represented at a shareholder meeting have the power to elect or remove any or all of our directors, subject to the special approval requirements for outside directors described under “Management-Outside Directors.” Under the Companies Law, the procedures for the appointment and removal and the term of office of directors, other than outside directors, may be contained in the articles of association of a company. Our articles of association currently do not contain provisions for staggered terms for directors. However, our articles of association may be amended in the future by a majority of our shareholders at a general shareholder meeting to provide for a staggered board or other method of electing our directors, other than with respect to our outside directors.
Dividend and Liquidation Rights
Our board of directors may declare a dividend to be paid to the holders of ordinary shares in proportion to the paid up capital attributable to the shares that they hold. Dividends may only be paid out of our profits and other surplus funds, as defined in the Companies Law, or as accrued over a period of eight quarters, whichever is higher, provided that there is no reasonable concern that a payment of a dividend will prevent us from satisfying out existing and foreseeable obligations as they become due. In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of ordinary shares in proportion to the paid up capital attributable to the shares that they hold. This right may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Shareholder Meetings
We are required to convene an annual general meeting of our shareholders once every calendar year within a period of not more than 15 months following the preceding annual general meeting. Our board of directors is required to convene a special general meeting of our shareholders at the request of two directors or one quarter of the members of our board of directors or at the request of one or more holders of 5% or more of our share capital and 1% of our voting power or the holder or holders of 5% or more of our voting power. All shareholder meetings require prior notice of at least 21 days. The chairperson of our board of directors or any other person appointed by the board presides over our general meetings. Under the Companies Law, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which may be between four and 40 days prior to the date of the meeting.
Quorum
The quorum required for an ordinary meeting of shareholders consists of at least two shareholders present, in person or by proxy, who hold or represent between them at least one-third of our issued share capital. A meeting adjourned for lack of a quorum generally is adjourned to the same day in the following week at the same time and place or any time and place as the directors designate in a notice to the shareholders. At the reconvened meeting, the required quorum consists of one or more shareholders present in person or by proxy, unless the meeting was called pursuant to a request by our shareholders in which case the quorum required is the number of shareholders holding the minimum number of voting shares necessary to make such requisition as described under “—Shareholder Meetings.”
Voting
Holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote of shareholders at a shareholder meeting. Shareholders may vote at shareholder meetings either in person or by proxy. Israeli law does not provide for public companies such as us to have shareholder resolutions adopted by means of a written consent in lieu of a shareholder meeting. Shareholder voting rights may be affected by the grant of any special voting rights to the holders of a class of shares with preferential rights that may be authorized in the future. The Companies Law provides that a shareholder, in exercising his or her rights and performing his or her obligations toward the company and its other shareholders, must act in good faith and in an acceptable manner, and avoid abusing his or her powers. This is required when voting at general meetings on matters such as changes to the articles of association, increasing the company’s registered capital, mergers and approval of related party transactions. A shareholder must also avoid oppression of other shareholders. In addition, any controlling shareholder, any shareholder who knows that its vote can determine the outcome of a shareholder vote and any shareholder who, under the company’s articles of association, can appoint or prevent the appointment of an office holder, is required to act with fairness towards the company. The Companies Law does not describe the substance of this duty and there is no binding case law that addresses this subject directly.
Resolutions
An ordinary resolution requires approval by the holders of a simple majority of the voting rights represented at the meeting, in person, by proxy or by voting instrument, and voting on the resolution.
Under the Companies Law, unless otherwise provided in the articles of association or applicable law, approval of all resolutions of the shareholders requires a simple majority. A resolution for the voluntary winding up of the company requires approval by holders of 75% of the voting rights represented at the meeting, in person, by proxy or by voting instrument and voting on the resolution.
Access to Corporate Records
Under the Companies Law, all shareholders generally have the right to review minutes of our general meetings, our shareholder register, our articles of association and any document we are required by law to file publicly with the Israeli Companies Registrar. Any shareholder who specifies the purpose of its request may request to review any document in our possession that relates to any action or transaction with a related party which requires shareholder approval under the Companies Law. We may deny a request to review a document if we determine that the request was not made in good faith, that the document contains a commercial secret or a patent or that the document’s disclosure may otherwise harm our interests.
Acquisitions under Israeli Law
Tender Offer. A person wishing to acquire shares or any class of shares of a publicly traded Israeli company and who would as a result hold over 90% of the company’s issued and outstanding share capital or of a class of shares is required by the Companies Law to make a tender offer to all of the company’s shareholders or all shareholders of such class of shares, as applicable, for the purchase of all of the issued and outstanding shares of the company or of that class of shares, as applicable. If the shareholders who do not respond to the offer hold less than 5% of the issued share capital of the company or of that class of shares, as applicable, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. However, the shareholders may petition the court to alter the consideration for the acquisition. If the shareholders who did not agree to the offer hold more than 5% of the issued and outstanding share capital of the company or of such class of shares, as applicable, the acquirer may not acquire additional shares of the company or of such class of shares, as applicable, from shareholders who accepted the tender offer if following such acquisition the acquirer would then own over 90% of the company’s issued and outstanding share capital or of the shares comprising such class, as applicable.
The Companies Law provides that, except in specified circumstances, an acquisition of shares of a public company must be made by means of a tender offer if as a result of the acquisition a person becomes the owner of 25% or more of the voting rights. This rule does not apply if there is already another 25% shareholder of the company. We believe that IDB Holding Corporation Ltd., which is controlled by four individuals in Israel, currently holds more than 25% of our outstanding ordinary shares as determined in accordance with the Companies Law. Similarly, the Companies Law provides that, except in specified circumstances, an acquisition of shares in a public company must be made by means of a tender offer if as a result of the acquisition the purchaser becomes the owner of more than 45% of the voting rights, if at such time there is no 45% or greater shareholder of the company.
Merger. The Companies Law permits merger transactions if approved by each party’s board of directors and the majority of each party’s shares voted on the proposed merger at a shareholders’ meeting. Shareholder approval is not required in certain specified circumstances, such as a merger between a company and its wholly-owned subsidiary. Under the Companies Law, merger transactions may be approved by holders of a simple majority of our shares present, in person or by proxy, at a general meeting and voting on the transaction. In determining whether the required majority has approved the merger, if our shares are held by the other party to the merger, or by any person holding at least 25% of the outstanding voting shares or 25% of the means of appointing directors of the other party to the merger, then a vote against the merger by holders of the majority of the voting shares present and voting, excluding shares abstaining and shares held by the other party or by such person, or anyone acting on behalf of either of them, is sufficient to reject the merger transaction. In certain circumstances, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the value of the parties to the merger and the consideration offered to the shareholders. Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of any of the merging companies. In addition, a merger may not be executed unless at least 50 days have passed from the time that a proposal for approval of the merger has been filed with the Israeli Registrar of Companies and at least 30 days have passed from the approval of the shareholders of each of the parties.
Anti-Takeover Measures
The Companies Law allows us to create and issue shares having rights different to those attached to our ordinary shares, including shares providing certain preferred or additional rights to voting, distributions or other matters and shares having preemptive rights. In the future, if we do create and issue a class of shares other than ordinary shares, such class of shares, depending on the specific rights that may be attached to them, may delay or prevent a takeover or otherwise prevent our shareholders from realizing a potential premium over the market value of their ordinary shares. The authorization of a new class of shares will require an amendment to our articles of association which requires the prior approval of a majority of our shareholders at a general meeting. Shareholders voting at such a meeting will be subject to the restrictions under the Companies Law described in “Voting.”
Transfer Agent and Registrar
The transfer agent and registrar for our ordinary shares is American Stock Transfer & Trust Company. Its address is 59 Maiden Lane, New York, New York 10038 and its telephone number at this location is (212) 936-5100.
Summaries of certain material contracts and amendments to these contracts are included in this Form 20-F in Item 4 - Information on the Company – Part B: Business Overview.
Israeli residents generally may freely deal in foreign currency and foreign assets, and non-residents may freely deal in Israeli currency and Israeli assets. There are currently no Israeli currency control restrictions on remittances of dividends on the ordinary shares or the proceeds from the sale of the shares provided that all taxes were paid or withheld; however, legislation remains in effect pursuant to which currency controls can be imposed by administrative action at any time.
Non-residents of Israel may freely hold and trade our securities. Neither our memorandum of association nor our articles of association nor the laws of the State of Israel restrict in any way the ownership or voting of ordinary shares by non-residents, except that such restrictions may exist with respect to citizens of countries which are in a state of war with Israel. Israeli residents are allowed to purchase our ordinary shares.
Certain Material Israeli Tax Considerations and Government Programs
The following is a description of the material Israeli income tax consequences of the ownership of our ordinary shares. The following also contains a description of material relevant provisions of the current Israeli income tax structure applicable to companies in Israel, with special reference to its effect on us. To the extent that the discussion is based on new tax legislation which has not been subject to judicial or administrative interpretation, we cannot assure shareholders that the tax authorities will accept the views expressed in the discussion in question. The discussion is not intended, and should not be taken, as legal or professional tax advice and is not exhaustive of all possible tax considerations.
The following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition of our ordinary shares. Shareholders should consult their own tax advisors concerning the tax consequences of their particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Taxation of Companies in Israel
General Corporate Tax Structure. In 2007, Israeli companies were subject to corporate tax at the rate of 29% of taxable income. This tax rate is expected to decrease gradually to 25% by 2010. However, the effective tax rate payable by a company that derives income from an approved enterprise, as discussed further below, may be considerably less.
Tax Benefits Under the Law for the Encouragement of Capital Investments, 1959. The Law for the Encouragement of Capital Investments, 1959, commonly referred to as the Investment Law, provides that a proposed capital investment in eligible facilities may, upon application to the Investment Center of the Ministry of Industry, Trade and Labor of the State of Israel, be designated as an approved enterprise. Each certificate of approval for an approved enterprise relates to a specific investment program delineated both by its financial scope, including its capital sources, and by its physical characteristics, for example, the equipment to be purchased and utilized under the program. The tax benefits derived from any certificate of approval relate only to taxable income attributable to the specific approved enterprise. If a company has more than one approval or only a portion of its capital investments is approved, its effective tax rate is the result of a weighted average of the applicable rates.
Generally, taxable income of a company derived from an approved enterprise is subject to company tax at the maximum rate of 25%, rather than the regular corporate tax rate, for a period of seven years, or ten years if the company qualifies as a foreign investors’ company as described below, commencing with the year in which the approved enterprise first generates taxable income. However, the ten-year period may not extend beyond the later of 14 years from the year in which approval was granted or 12 years from the year in which operations or production by the enterprise began. A company’s undistributed income derived from an approved enterprise in top priority locations (commonly known as “Zone A”) will be exempt from corporate tax for a period of ten years.
A company having an approved enterprise status may elect to receive an alternative package of benefits. Under the alternative package of benefits, a company’s undistributed income derived from an approved enterprise will be exempt from company tax for a period of between two and ten years from the first year of taxable income, depending on the geographic location of the approved enterprise within Israel, and the company will be eligible for a reduced tax rate for the remainder of the benefits period.
A company that has an approved enterprise in Zone A or that has elected the alternative package of benefits and that subsequently pays a dividend out of income derived from the approved enterprise during the tax exemption period will be subject to corporation tax on the gross amount of dividends distributed. The rate of the tax will be the rate which would have been applicable had the company not been tax exempt. This corporation tax rate ranges from 10% to 25%, depending on the percentage of the company’s shares held by foreign shareholders. The recipient of dividends distributed from such income is taxed at the rate applicable to dividends from approved enterprises which is 15%, or less under certain anti double-taxation treaties, if the dividend is distributed during the tax benefit period or within 12 years after the period and there is no time limit with respect to dividend distributed from an exempt income of foreign investors’ company. The company must withhold this tax at source.
Subject to applicable provisions concerning income under the alternative package of benefits, all dividends are considered to be attributable to the entire enterprise and their effective tax rate is the result of a weighted average of the various applicable tax rates. Under the Investment Law, a company that has elected the alternative package of benefits is not obliged to distribute exempt retained profits, and may generally decide from which year’s profits to declare dividends. We currently intend to reinvest any income derived from our approved enterprise programs and not to distribute the income as a dividend.
The Investment Center bases its decision whether or not to approve an application on the criteria in the Investment Law and regulations, the then prevailing policy of the Investment Center and the specific objectives and financial criteria of the applicant. In addition, the benefits available to an approved enterprise are conditional upon the fulfillment of conditions stipulated in the Investment Law and its regulations and the criteria in the specific certificate of approval, as described above. If a company does not meet these conditions, it would be required to refund the amount of tax benefits, with the addition of a consumer price index linkage adjustment and interest. There can be no assurance that any future approved enterprises that we may be awarded will be entitled to the same package of benefits as we currently have.
The Investment Center of the Ministry of Industry and Trade granted our manufacturing facility approved enterprise status under the Investment Law for investments beginning in 1999. We have elected the alternative package of benefits under these approved enterprise programs. Since our manufacturing facility is located in a “Zone A”, the portion of our income derived from this approved enterprise program will be exempt from tax for a period of ten years, commencing when we begin to realize net income from these programs, but such period may not extend beyond the later of 14 years from the year in which approval was granted or 12 years from the year in which operations or production by the enterprise began. We expect to derive a substantial portion of our income from our approved enterprise program. The benefits available to an approved enterprise program are dependent upon the fulfillment of conditions stipulated in applicable law and in the certificate of approval.
The Investment Law and the criteria for receiving an “approved enterprise” status may be amended from time to time and there is no assurance that we will be able to obtain additional benefits under the Investment Law when we apply for such benefits.
A reform in the Investment Law was made in March 2005. According to this reform, instead of filing application for tax benefits with the Investment Center, companies are now allowed to claim the tax benefits on their corporate tax returns subject to fulfilling certain conditions, without prior approval and without submitting any reports to the Investment Center. Audit of any claim for tax benefits will take place by the Israeli income tax authority as part of general tax audits it may perform from time to time.
Grants Under the Law for the Encouragement of Industrial Research and Development, 1984. Under the Law for the Encouragement of Industrial Research and Development, 1984, commonly referred to as the Research Law, research and development programs which meet specified criteria and are approved by a governmental committee of the Office of the Chief Scientist are eligible for grants of up to 50% of the project’s expenditure, as determined by the research committee, in exchange for the payment of royalties from the sale of products developed under the program. Regulations under the Research Law generally provide for the payment of royalties to the Chief Scientist of 3.0% to 3.5% on sales of products and services derived from our technology developed using these grants until 100% of the dollar-linked grant is repaid, together with interest equal to the 12 month London Interbank Offered Rate applicable to dollar deposits that is published on the first business day of each calendar year. Following the full repayment of the grant, there is no further liability for repayment.
The terms of the Israeli government participation also require that the manufacture of products developed with government grants be performed in Israel. However, under the regulations of the Research Law, if any of the manufacturing is performed outside Israel by any entity other than us, assuming we receive approval from the Chief Scientist for the foreign manufacturing, we may be required to pay increased royalties. The increase in royalties depends upon the manufacturing volume that is performed outside of Israel follows:
Manufacturing Volume Outside of Israel | | Royalties to the Chief Scientist as a Percentage of Grant | |
| | | | |
| Less than 10% | | | 100 | % |
| Between 10% and 50% | | | 120 | % |
| between 50% and 90% | | | 150 | % |
| 90% and more | | | 300 | % |
If the manufacturing is performed outside of Israel by us, the rate of royalties payable by us on revenues from the sale of products manufactured outside of Israel will increase by 1% over the regular rates. If the manufacturing is performed outside of Israel by a third party, the rate of royalties payable by us on those revenues will be a percentage equal to the percentage of our total investment in the Given System that was funded by grants. In addition, in recent years the government of Israel has accelerated the repayment of Chief Scientist grants, and may further accelerate them in the future. Following our request, the Office of the Chief Scientist has approved the manufacture of limited quantities of the PillCam capsule using the back-up production line that we have established in Ireland without increasing royalty rates.
The technology developed with Chief Scientist grants may not be transferred to third parties without the prior approval of a governmental committee under the Research Law, and may not be transferred to non-residents of Israel. The approval, however, is not required for the export of any products developed using the grants. Approval of the transfer of technology to residents of Israel may be granted in specific circumstances, only if the recipient abides by the provisions of the Research Law and related regulations, including the restrictions on the transfer of know-how and the obligation to pay royalties in an amount that may be increased. We cannot provide any assurance that any consent, if requested, will be granted.
The funds available for grants from the Chief Scientist depend on several criteria and prevailing government policy and budget, and may be reduced or eliminated in the future. Even if these grants are maintained, there is no assurance that we will receive Chief Scientist grants in the future. In addition, each application to the Chief Scientist is reviewed separately, and grants are based on the program approved by the research committee. Expenditures supported under other incentive programs of the State of Israel are not eligible for grants from the Chief Scientist. We cannot provide any assurance that applications to the Chief Scientist will be approved and, until approved, the amounts of any grants are not determinable.
Tax Benefits and Grants for Research and Development. Israeli tax law allows, under specific conditions, a tax deduction in the year incurred for expenditures that were paid in cash, including capital expenditures, relating to scientific research and development projects, if:
| | • | The expenditures are approved by the relevant Israeli government ministry, determined by the field of research; |
| | | |
| | • | The research and development is for the promotion of the company; and |
| | | |
| | • | The research and development is carried out by or on behalf of the company seeking the deduction. |
Expenditures not so approved are deductible over a three-year period. However, the amounts of any government grant made available to us are subtracted from the amount of the deductible expenses according to Israeli law.
Tax Benefits Under the Law for the Encouragement of Industry (Taxes), 1969. According to the Law for the Encouragement of Industry (Taxes), 1969, generally referred to as the Industry Encouragement Law, an industrial company is a company resident in Israel, at least 90% of the income of which, in a given tax year, determined in Israeli currency exclusive of income from specified government loans, capital gains, interest and dividends which are not classified for such company as business income, is derived from an industrial enterprise owned by it. An industrial enterprise is defined as an enterprise whose major activity in a given tax year is industrial production activity.
Under the Industry Encouragement Law, industrial companies are entitled to certain preferred corporate tax benefits, including the following:
| | • | deduction of purchases of know-how and patents over an eight-year period for tax purposes; |
| | | |
| | • | claiming expenses in connection with the issuance and listing of shares on the Tel Aviv Stock Exchange or, on or after January 1, 2003, on a recognized stock market outside of Israel, over a period of three years; and |
| | | |
| | • | Accelerated depreciation rates on equipment and buildings. |
Eligibility for benefits under the Industry Encouragement Law is not subject to receipt of prior approval from any governmental authority.
We believe that in 2007 we qualified as an Industrial Company under the Law for Encouragement of Industry (Taxes) 1969. We cannot provide any assurance that the Israeli tax authorities will agree with the determination that we qualified as an industrial company in the past or that we will maintain this qualification or our status as an industrial company. If we are delisted, gains from the sale of our ordinary shares will be subject to capital gains tax at a rate of 20%, or 25% if the seller holds 10% or more of the “means of control” of our company, as such term is defined by the law, unless an exemption or other lower tax rate applies in accordance with a tax treaty between Israel and the shareholder’s country of residence.
If we qualify as an industrial company within the definition of the Industry Encouragement Law, we are entitled to the benefits described above. We believe that in 2007 we qualified as an Industrial Company under the Industry Encouragement Law. We cannot provide any assurance that the Israeli tax authorities will agree with the determination that we qualified as an industrial company in the past or that we will maintain this qualification or our status as an industrial company.
Special Provisions Relating to Taxation Under Inflationary Conditions. The Income Tax Law (Inflationary Adjustments), 1985, generally referred to as the Inflationary Adjustments Law, represents an attempt to overcome the problems presented to a traditional tax system by an economy undergoing rapid inflation. The Inflationary Adjustments Law is highly complex. Its features which are material to us can generally be described as follows:
| | • | Where a company’s equity, as calculated under the Inflationary Adjustments Law, exceeds the depreciated cost of fixed assets, a deduction from taxable income is permitted equal to the excess multiplied by the applicable annual rate of inflation. The maximum deduction permitted in any single tax year is 70% of taxable income, with the unused portion permitted to be carried forward linked to the Israeli consumer price index. The unused portion that was carried forward may be deducted in full in the following year. |
| | | |
| | • | Where a company’s depreciated cost of fixed assets exceeds its equity, then the excess multiplied by the applicable annual rate of inflation is added to taxable income. |
| | | |
| | • | Depreciation deductions on fixed assets and losses carried forward are adjusted for inflation based on the increase in the consumer price index. |
| | | |
| | • | Gains on traded securities are taxable at the regular corporate tax rate in specified circumstances. |
Taxation of Our Shareholders
Capital Gains on Sales of Our Ordinary Shares. Israeli law imposes a capital gains tax on the sale of capital assets. The law distinguishes between real gain and inflationary surplus. The inflationary surplus is the portion of the total capital gain that is equivalent to the increase of the relevant asset’s purchase price which is attributable to the increase in the Israeli consumer price index between the date of purchase and the date of sale. Foreign residents who purchased an asset in foreign currency may request that the inflationary surplus be computed on the basis of the devaluation of the Shekel against such foreign currency. The real gain is the excess of the total capital gain over the inflationary surplus. The inflationary surplus accumulated from and after December 31, 1993, is exempt from any capital gains tax in Israel while the real gain is taxed at the applicable rate discussed below. Under an amendment to the Inflationary Adjustments Law, non-Israeli corporations might be subject to Israeli taxes on the sale of shares in an Israeli company which are traded on certain stock markets, including The Nasdaq Global Market, subject to the provisions of any applicable double taxation treaty.
Individual and corporate shareholders dealing in securities in Israel are taxed at the tax rates applicable to business income. In 2007, these regular tax rates were 29% for corporations and up to 48% for individuals.
Non-Israeli residents. Under Israeli law, the capital gain from the sale of shares by non Israeli residents is tax exempt in Israel as long as our shares are listed on the Nasdaq Global Market or any other stock exchange recognized by the Israeli Ministry of Finance, and provided certain other conditions are met, the most relevant of which are: (A) the capital gain is not attributed to the foreign resident’s permanent establishment in Israel, (B) the shares were acquired by the foreign resident after the company’s shares had been listed for trading on the foreign exchange, and (C) if the seller is a corporation, less than 25% of its means of control are held by Israeli residents. In addition, capital gains from the sale of our ordinary shares by non-Israeli residents who purchased or will have purchased the shares between July 1, 2005 and December 31, 2008 and whose state of residence has a double tax treaty with Israel will generally be tax exempt irrespective of whether our shares are listed on any stock exchange. If the shares were sold by Israeli residents, then (A) for the period ending December 31, 2002, their sale would be tax exempt so long as (1) the shares are listed on a stock exchange, such as the Nasdaq Global Market, which is recognized by the Israeli Ministry of Finance, and (2) we qualified as an industrial company or industrial holding company under the Law for Encouragement of Industry (Taxes) 1969, and (B) for the period commencing January 1, 2003, the capital gain accrued by individuals on the sale of shares will be taxed at the rate of 20%. However, if the individual shareholder is a "Controlling Shareholder,” namely, a person who holds, directly or indirectly, alone or together with other, 10% or more of one of the Israeli resident company's means of control at the time of distribution or at any time during the preceding 12 month period, such gain will be taxed at the rate of 25%. In addition, capital gain derived by an individual claiming deduction of financing expenses in respect of such gain will be taxed at the rate of 25%. The real capital gain derived by corporations will be generally subject to tax at the rate of 25%. However, the real capital gain derived from sale of securities, as defined in Section 6 of the Inflationary Adjustment Law, by a corporation, which was subject upon December 31, 2005 to the provisions of Section 6 of the Inflationary Adjustment Law, will be taxed at the corporate tax rate.
In addition, under the Convention between the Government of the United States of America and the Government of Israel with Respect to Taxes on Income, as amended, or the U.S.-Israel Tax Treaty, Israeli capital gains tax will not apply to the sale, exchange or disposition of ordinary shares by a person:
| • | who holds such shares as a capital asset; |
| • | who qualifies as a resident of the United States within the meaning of the U.S.-Israel tax treaty; and |
| • | who is entitled to claim the benefits available to the person by the U.S.-Israel Tax Treaty. |
However, this exemption does not apply, among other cases, if the gain is attributable to a permanent establishment of such person in Israel, or if the holder is a resident of the United States within the meaning of the U.S.-Israeli Tax Treaty who holds, directly or indirectly, shares representing 10% or more of our voting power during any part of the 12-month period preceding the sale, exchange or disposition, subject to certain conditions, or the capital gains can be allocated to a permanent establishment of such U.S. resident in Israel. Under these circumstances, the sale, exchange or disposition would be subject to Israeli tax, to the extent applicable. However, under the U.S.-Israel Tax Treaty, such U.S. resident generally will be permitted to claim a credit for the Israeli taxes paid against the U.S. federal income tax imposed on the sale, exchange or disposition, subject to the limitations under U.S. law applicable to foreign tax credits. The U.S.-Israel Tax Treaty does not relate to U.S. state or local taxes.
Dividends. A distribution of dividends from income attributed to an “approved enterprise” is subject to tax in Israel at the rate of 15%, subject to a reduced rate under any applicable double tax treaty. A distribution of dividend from income, which is not attributed to an “approved enterprise,” to an Israeli resident individual, will generally be subject to income tax at a rate of 20%. However, a 25% tax rate will apply if the dividend recipient is a “Controlling Shareholder,” namely, a person who holds, directly or indirectly, alone or together with other, 10% or more of one of the Israeli resident company's means of control at the time of distribution or at any time during the preceding 12 month period. If the recipient of the dividend is an Israeli resident corporation, such dividend will be exempt from income tax provided the income from which such dividend is distributed was derived or accrued within Israel.
A distribution of dividends which is not attributed to an “approved enterprise” to a non-Israeli resident is generally subject to an Israeli income tax on the receipt of dividends at the rate of 20%, or 25% if the dividend recipient is a “Controlling Shareholder,” as defined above. Those rates may be subject to a reduced tax rate under an applicable double tax treaty.
Under the U.S.-Israel Tax Treaty, the tax rate in respect of dividends distributed by an Israeli resident company to a U.S. resident corporation may be 12.5%, 15% or 25%, depending on the percentage of holding in the resident company and the resident company's type of income distributed. Dividends distributed to a U.S. individual resident are taxed at a rate of 20%, or 25% if this individual is considered to be a “controlling shareholder.”
Upon the distribution of a dividend attributed to an Approved Enterprise's income, an Israeli resident company whose shares are listed on a stock exchange is obligated to withhold tax from the amount distributed at the rate of 15%, subject to a reduced tax rate under an applicable double tax treaty If the dividend is distributed from income not attributed to the approved enterprise, the following withholding tax rates will apply: (i) Israeli resident corporation – 0%, (ii) Israeli resident individual – 20%, and (iii) non-Israeli resident – 20%, subject to a reduced tax rate under an applicable double tax treaty.
Israeli Withholding Tax. Non-residents of Israel are subject to tax on income accrued or derived from sources in Israel. These sources of income include passive income such as dividends, royalties and interest, as well as non-passive income, such as income received for services rendered in Israel. We are required to withhold income tax at the rate of 25% with respect to passive income (or 15% for dividends distributed from income generated by an approved enterprise) unless a different rate or an exemption is provided in a tax treaty between Israel and the shareholder’s country of residence. For residents of countries other than the United States, the purchaser of shares may be required to withhold 25% capital gains tax on all amounts received upon the sale of our ordinary shares, provided the capital gain from such a sale is not exempt from Israeli capital gains tax, and unless a different rate is provided in a treaty between Israel and the seller’s country of residence.
The foregoing discussion is intended only as a summary and does not purport to be a complete analysis or listing of all potential Israeli tax effects of holding our shares. We recommend that shareholders consult their tax advisors concerning the Israeli and non-Israeli tax consequences to them of holding our shares.
Certain Material U.S. Federal Income Tax Considerations
U.S. Shareholders. The following is a description of the material U.S. federal income tax consequences of the ownership of our ordinary shares. This description does not purport to address all of the tax considerations that may be relevant to a decision to purchase, own or dispose of our ordinary shares. This description assumes that holders of our ordinary shares will hold the ordinary shares as capital assets within the meaning of Section 1221 of the U.S. Internal Revenue Code of 1986, as amended, referred to as the Code. This discussion does not address all of the tax considerations that may be relevant to shareholders in light of their particular circumstances or certain types of shareholders subject to special tax treatment, including, without limitation:
| | · | regulated investment companies; |
| | · | broker-dealers (including in securities or foreign currency) or insurance companies; |
| | · | persons who have elected to apply a mark-to-market method of accounting; |
| | · | tax-exempt organizations or retirement plans; |
| | · | certain former citizens or former long-term residents of the U.S.; |
| | · | persons subject to the alternative minimum tax; |
| | · | banks and other financial institutions; |
| | · | persons who hold their shares as part of a position in a “straddle” or as part of a “hedging,” “conversion,” “constructive sale,” synthetic security, or other integrated investment; |
| | · | holders who received their shares through the exercise of employee stock options or otherwise as compensation; |
| | · | partnerships or other pass-through entities or persons who hold their shares through partnerships or other pass-through entities; |
| | · | holders who own directly, indirectly or by attribution at least 10.0% of the voting power of our shares or holders who within the past five year period owned at least 10.0% of the voting power of our shares; and |
| | · | persons whose functional currency is not the U.S. dollar. |
Further, this description does not address any U.S. federal estate and gift or alternative minimum tax consequences, nor any state, local, or foreign tax consequences relating to the acquisition, ownership and disposition of our ordinary shares.
This discussion is based on current provisions of the Code, current and proposed Treasury regulations promulgated under the Code, administrative pronouncements and judicial decisions and interpretations as of the date hereof, all of which are subject to differing interpretations or change, which change may apply retroactively and could materially affect the continued validity of this summary and the tax considerations described herein. Subject to the discussion set forth below under the heading entitled “Passive Foreign Investment Company Considerations,” this discussion assumes that the Company is not and has never been a “passive foreign investment company,” “controlled foreign corporation,” “foreign investment company” or “foreign personal holding company” for U.S. federal income tax purposes.
Unless specifically noted below, the following description applies only to owners of our ordinary shares that are U.S. Holders, as defined below, for U.S. federal income tax purposes.
For purposes of this description, a “U.S. Holder” is a beneficial owner of ordinary shares that, for U.S. federal income tax purposes, is:
| | • | citizen or resident of the United States; |
| | | |
| | • | a corporation or other entity taxable as a corporation for U.S. federal income tax purposes created or organized in or under the laws of the United States or any state thereof, or the District of Columbia; |
| | | |
| | • | an estate if its income is subject to U.S. federal income taxation regardless of its source; or |
| | | |
| | • | a trust (A) if a court within the United States is able to exercise primary jurisdiction over its administration and one or more U.S. persons have authority to control all of its substantial decisions, or (B) if, in general, it was in existence on August 20, 1996, was created as a U.S. person under the Code on the previous day and made a valid election to continue to be so treated. |
A non-U.S. Holder is a beneficial owner of ordinary shares that is not a U.S. Holder.
If a partnership (or any other entity treated as a partnership for U.S. federal income tax purposes) holds our ordinary shares, the tax treatment of the partnership and a partner in such partnership generally will depend on the status of the partner and the activities of the partnership. Such a partner or partnership should consult its own tax advisor as to its consequences.
Shareholders should consult their tax advisors with respect to the U.S. federal, state, local and foreign tax consequences of acquiring, owning or disposing of our ordinary shares.
Distributions
Subject to the discussion below under “Passive Foreign Investment Company Considerations”, the entire amount of any distribution made to you with respect to ordinary shares, other than any distributions of our ordinary shares made to all our shareholders, will constitute dividends to the extent of our current or accumulated earnings and profits as determined under U.S. federal income tax principles. For these purposes, the amount of the distribution will not be reduced by the amount of any Israeli tax withheld from the distribution. Subject to the discussion below under “Passive Foreign Investment Company Considerations”, non-corporate U.S. Holders may be taxed on the dividend distributions made in taxable years beginning on or before December 31, 2010 at the lower rates applicable to long-term capital gains (i.e., gains with respect to capital assets held for more than one year), provided that certain conditions are met, including certain holding period requirements and the absence of certain risk reduction transactions. In addition, the dividends will not be eligible for the dividends received deduction generally allowed to corporate U.S. holders. Subject to the discussion below under “Passive Foreign Investment Company Considerations”, if distributions with respect to our ordinary shares exceed our current and accumulated earnings and profits as determined under U.S. federal income tax principles, the excess distributed with respect to any ordinary share would be treated first as a tax-free return of capital to the extent of your adjusted basis in that ordinary share and thereafter as capital gain. We do not maintain calculations of our earnings and profits under U.S. federal income tax principles.
If we pay a dividend or distribution in Shekels, any such dividend or distribution will be included in your gross income in an amount equal to the U.S. dollar value of Shekels on the date of receipt. You will have a tax basis for U.S. federal income tax purposes in the Shekels received equal to that dollar value, and any subsequent gain or loss in respect of the Shekels arising from exchange rate fluctuations will generally be taxable as U.S. source ordinary income or loss.
Dividends received by you with respect to your ordinary shares generally will be treated as foreign source income, which may be relevant in calculating your foreign tax credit limitation. You may generally elect to claim the Israeli income tax withheld from dividends and distributions you receive with respect to your ordinary shares as a foreign tax credit against your U.S. federal income tax liability, subject to a number of limitations. Among the limitations, the foreign tax credits allowable with respect to specific classes of income cannot exceed the U.S. federal income tax payable with respect to each such class. Dividends we pay generally will be included in the “passive income” class for these purposes, or, in the case of certain financial services entity holders, “general category income.”
Subject to the discussion below under “Backup Withholding and Information Reporting,” if you are a non-U.S. Holder of our ordinary shares, you generally will not be subject to U.S. federal income or withholding tax on dividends you receive on your ordinary shares, unless the dividends are effectively connected with your conduct of a trade or business in the United States.
Sale or Exchange of Our Ordinary Shares
Subject to the discussion below under “Passive Foreign Investment Company Considerations”, if you are a U.S. Holder, you generally will recognize capital gain or loss for U.S. federal income tax purposes when you sell, exchange or otherwise dispose of our ordinary shares equal to the difference between your adjusted tax basis in the ordinary shares and the amount realized on their disposition. If you are a non-corporate U.S. Holder, the maximum marginal U.S. federal income tax rate applicable to such gain will be lower than the maximum marginal U.S. federal income tax rate applicable to ordinary income (other than certain dividends) if your holding period for our ordinary shares exceeds one year (i.e., such gain is long-term capital gain). Any gain or loss recognized by you generally will be treated as U.S. source income or loss for U.S. foreign tax credit purposes. The deductibility of capital losses is subject to limitations.
Subject to the discussion below under “Backup Withholding and Information Reporting,” if you are a non-U.S. Holder of our ordinary shares, you generally will not be subject to U.S. federal income or withholding tax on gain realized on the sale or exchange of such ordinary shares unless (1) such gain is effectively connected with your conduct of a trade or business in the United States, or (2) in the case of gain realized by an individual non-United States holder, you are present in the United States for 183 days or more in the taxable year of the sale or exchange and certain other conditions are met.
Passive Foreign Investment Company Considerations
A non-U.S. corporation will be classified as a “passive foreign investment company” or a PFIC, for U.S. federal income tax purposes in any taxable year in which, after applying applicable look-through rules, either (1) at least 75% of its gross income is “passive income,” or (2) at least 50% of the value of its gross assets is attributable to assets that produce passive income or are held for the production of passive income. Passive income for this purpose includes items such as dividends, interest, royalties, rents and gains from commodities and securities transactions.
Based on our estimated gross income, the average value of our gross assets (determined by reference to the market value of our shares and valuing our intangible assets using the methods prescribed for publicly traded corporations) and the nature of our business, we believe that we will not be classified as a PFIC for the taxable year ended December 31, 2007. Our status in future years will depend on our assets and activities in those years, although you will be treated as continuing to own an interest in a PFIC if we are a PFIC in any year while you own your shares unless you make certain elections. We have no reason to believe that our assets or activities will change in a manner that would cause us to be classified as a PFIC, but because the market price of our ordinary shares is likely to fluctuate, we cannot assure you that we will not be considered a PFIC for any taxable year. In general, if we were characterized as a PFIC for any taxable year, any gain recognized by a U.S. Holder who sells our ordinary shares would be treated as ordinary income and would be subject to tax as if the gain had been realized ratably over the holding period of such ordinary shares. The amount allocated to the current taxable year and any taxable year with respect to which were not a PFIC would be taxed as ordinary income (rather than capital gain) earned in the current taxable year. The amount allocated to other taxable years would be taxed at the highest marginal rates applicable to ordinary income for such taxable years, and the U.S. Holder also would be liable for an additional tax equal to the interest on such tax liability for such years.
If we were a PFIC, you could make a variety of elections that may alleviate the tax consequences referred to above, and one of these elections may be made retroactively. However, it is expected that the conditions necessary for making certain of such elections will not apply in the case of our ordinary shares. You should consult your own tax advisor regarding our potential status as a PFIC and the tax consequences that would arise if we were treated as a PFIC.
Backup Withholding and Information Reporting
United States backup withholding taxes and information reporting requirements generally apply to certain payments to certain non-corporate holders of stock. Information reporting requirements will, and a backup withholding tax may, apply to payments of dividends on, and to proceeds from the sale, exchange or redemption of, our ordinary shares made within the United States, or by a U.S. payor or U.S. middleman, to a holder of our ordinary shares, other than an exempt recipient, including a corporation, a payee that is not a U.S. person that provides an appropriate certification and certain other persons. Backup withholding is not an additional tax and may be claimed as a credit against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing an appropriate claim for refund with the IRS and furnishing any required information. The backup withholding tax rate is 28% for years through 2010.
The above description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership or disposition of our ordinary shares. Shareholders should consult their own tax advisors concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
F. DIVIDENDS AND PAYING AGENTS
Not applicable.
G. STATEMENTS BY EXPERTS
Not applicable.
We are currently subject to the information and periodic reporting requirements of the Exchange Act, and file periodic reports and other information with the Securities and Exchange Commission through its electronic data gathering, analysis and retrieval (EDGAR) system. Our securities filings, including this Annual Report and the exhibits thereto, are available for inspection and copying at the public reference facilities of the Securities and Exchange Commission located at 1580 100 F Street, N.E., Washington, D.C. 20549. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the Securities and Exchange Commission at 100 F Street, N.E., Washington, DC 20549. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the public reference room. The Commission also maintains a website at http://www.sec.gov from which certain filings may be accessed.
As a foreign private issuer, we are exempt from the rules under the Exchange Act relating to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the Securities and Exchange Commission as frequently or as promptly as United States companies whose securities are registered under the Exchange Act.
I. SUBSIDIARY INFORMATION
Not applicable.
Currency fluctuations. Our sales in 2007 were denominated 67.5% in U.S. dollars, 23.3% in Euros and 9.2% in other currencies, depending on the location of the customer or the distributor used to fulfill our customers’ orders. As most of our sales are made in U.S. dollars, a strengthening of the U.S. dollar could make our products less competitive in foreign markets and could cause our non-U.S. customers to cancel or decrease orders or default on payment.
We develop and manufacture products primarily in Israel and sell the majority of the products in the United States, and to a lesser extent in other countries. If the value of a currency in which our revenues are denominated weakens against the value of a currency in which our expenses are denominated, there will be a negative impact on the profit margins for sales of our products. In 2007, 49% of our expenses were denominated in U.S. dollars and 31% of our expenses were denominated in Shekels, principally consisting of salaries and related personnel expenses. We expect this level of Shekel expenses to continue for the foreseeable future. During 2007, the U.S. dollar weakened against the Shekel by approximately 9.0%. We estimate that a change of 1% in the exchange rate between the Shekel and the U.S. dollar has an impact of approximately $170,000 on our operating expenses. In addition, 13% of our expenses were denominated in Euros and 7.0% were denominated in Yen or other currencies. However, since we also generate revenues in these currencies the net effect on our business of exchange rate fluctuations of these currencies against the U.S. dollars is not material.
As of December 31, 2007, 38% of our cash and cash equivalents were denominated in currencies other than the U.S. dollar and we are therefore subject to the risk of exchange rate fluctuations among the U.S. dollar, Yen, Shekel, Australian dollar and Euro. In 2007, we have used a variety of hedging tools to seek to minimize the effect of currency fluctuations on our income.
Interest rate fluctuations. We invest some of our cash in bank accounts and deposits with maturities of three months or less located with a number of highly-rated banks inside and outside of Israel. We invest the majority of our cash in longer-term financial instruments in order to seek to achieve a higher yield. As of December 31, 2007, approximately 50% of our investments were subject to the risk of changes in interest rates. We estimate that a change of 1% in interest rates would have an impact of up to approximately $500,000 on our finance income.
General market risks. As of December 31, 2007, approximately two thirds of our cash balances were invested in securities issued by the United States government or its agencies and in AAA money market funds. The remainder was held in corporate bonds and commercial paper that were highly-rated by rating agencies at the time of investment. Nonetheless, these investments are subject to general credit, liquidity and market risks, which may be exacerbated by the dislocation that has affected various sectors of the financial markets since the middle of 2007 and caused credit and liquidity issues for a number of reputable financial institutions.
PART II
Disclosure Controls and Procedures. We maintain disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in our periodic filings with the SEC is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer (CEO) and Chief Financial Officer (CFO), as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Furthermore, management necessarily was required to use its judgment in evaluating the cost to benefit relationship of possible disclosure controls and procedures.
As of the end of the period covered by this report, we performed an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures. The evaluation was performed with the participation of senior management of each business segment and key corporate functions, and under the supervision of our CEO and CFO. Based on the evaluation, our management, including the CEO and CFO, concluded that our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Annual Report on Internal Control Over Financial Reporting. Management of the company is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act, as a process designed by, or under the supervision of, the company’s principal executive and principal financial officers and effected by the company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| | • | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the company; |
| | | |
| | • | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
| | | |
| | • | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of the company’s internal control over financial reporting as of December 31, 2007. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on our assessment, management believes that, as of December 31, 2007, the company’s internal control over financial reporting is effective.
Auditors’ Attestation Report. Our independent auditor, Somekh Chaikin, a member firm of KPMG has issued a combined report on the company's consolidated financial statements and management’s assessment of the company’s internal control over financial reporting. This report is contained in Item 18 – Financial Statements, on page F-2.
Changes in Internal Controls Over Financial Reporting. There were no changes to our internal control over financial reporting that occurred during the period covered by this Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
The board of directors has determined that Michael Grobstein is the financial expert serving on its audit committee and that Mr. Grobstein is independent as that term is defined under the Nasdaq Global Market listing requirements.
We have adopted a code of ethics applicable to our chief executive officer, chief financial officer, controller and persons performing similar functions. The code of ethics was filed with the SEC as Exhibit 11.1 to our Annual Report on Form 20-F for the fiscal year ended December 31, 2003 and is also available on our website, www.givenimaging.com. A hard copy of our code of ethics may be obtained by sending an e-mail request to info@givenimaging.com or by a written request to the corporate secretary at 2 Hacarmel Street, 20692, Yoqneam, Israel.
The following table sets forth fees for professional audit services rendered by Somekh Chaikin, a member firm of KPMG International, for the audit of our financial statements for the years ended December 31, 2006 and 2007, and fees billed for other services rendered by Somekh Chaikin, including through other offices of KPMG worldwide:
| | | 2006 | | 2007 | |
| | | (in thousands) | |
| | | | | | |
| Audit fees | | $ | 316 | | $ | 465 | |
| Audit-related fees | | | 24 | | | 283 | |
| Tax fees | | | 67 | | | 233 | |
| All other fees | | | 162 | | | 3 | |
| Total | | $ | 569 | | $ | 984 | |
| | (1) | “Audit-related fees” in 2006 are related principally to advice regarding U.S. sales tax and fees in 2007 are related principally to analysis of U.S. income tax. |
| | | |
| | (2) | “Tax fees” includes fees for professional services rendered by our auditors for tax compliance and tax advice on actual or contemplated transactions. Tax fees in 2007 are related principally to work regarding transfer prices. |
| | | |
| | (3) | “All other fees” in 2006 included fees related to advice on compliance with Sarbanes-Oxley Act requirements. |
In accordance with our pre-approval policy, our audit committee pre-approved all audit and non-audit services provided to us and to our subsidiaries during the periods listed above. Audit services must be pre-approved by the full audit committee. The authority to pre-approve non-audit services has been delegated to the Chairman of the audit committee. Any services pre-approved by the Chairman are reported to the full committee at its next scheduled meeting.
None.
None.
PART III
Not applicable.
See pages F-1 to F-37.
Exhibit | | Description |
| | | |
| 1.1 | | Articles of Association, incorporated by reference to Exhibit 3.2 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | | |
| 4.1 | | Production Development, Manufacturing and Sales Agreement, dated as of November 26, 2002, by and between Micron Technology, Inc. and the Registrant, incorporated by reference to Exhibit 4.3 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004.† |
| | | |
| 4.2 | | Addendum, dated as of June 10, 2005, to Production Development, Manufacturing and Sales Agreement, dated as of November 26, 2002, by and between Micron Technology, Inc. and the Registrant. † |
| | | |
| 4.3 | | Form of Indemnification Agreement between directors and officers of the Registrant and the Registrant, incorporated by reference to Exhibit 10.15 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | | |
| 4.4 | | Lease Agreement, dated as of November 26, 2001, by and between Sha’ar Yoqneam L.P. and the Registrant, incorporated by reference to Exhibit 10.20 of the Registration Statement on Form F-1 (File No. 333-81514) filed with the Commission on February 1, 2002. |
| | | |
| 4.5 | | Summary of Material Terms of Addendum, dated December 2002, to Lease Agreement, dated as of November 26, 2001, by and between Sha’ar Yoqneam and the Registrant, incorporated by reference to Exhibit 4.11 of the Annual Report on Form 20-F for the year ended December 31, 2002, filed with the Commission on April 10, 2003. |
| | | |
| 4.6 | | Second Addendum, dated July 5, 2004, to the Lease Agreement, dated as of November 26, 2001, by and between Sha’ar Yoqneam L.P. and the Registrant, incorporated by reference to Exhibit 4.15 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005. |
| | | |
| 4.7 | | Development and Supply Agreement, dated April 8, 2002, by and between Zarlink Semiconductor AB and the Registrant, incorporated by reference to Exhibit 4.11 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005.† |
| | | |
| 4.8 | | Addendum to Development and Supply Agreement, dated July 2005, by and between Zarlink Semiconductor AB and the Registrant, incorporated by reference to Exhibit 4.12 of the Annual Report on Form 20-F for the year ended December 31, 2005, filed with the Commission on April 7, 2006.† |
| | | |
| 4.9 | | Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.12 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005.† |
| | | |
| 4.10 | | Amendment No. 1, dated June 2004, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.13 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005.† |
| | | |
| 4.11 | | Amendment No. 2, dated October 4, 2004, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.14 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005.† |
| | | |
| 4.12 | | Amendment No. 3, dated October 27, 2005, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.16 of the Annual Report on Form 20-F for the year ended December 31, 2005, filed with the Commission on April 7, 2006. |
| 4.13 | Amendment No. 4, dated November 14, 2005, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.17 of the Annual Report on Form 20-F for the year ended December 31, 2005, filed with the Commission on April 7, 2006. |
| | |
| 4.14 | Amendment No. 5, dated September 6, 2006, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.19 of the Annual Report on Form 20-F for the year ended December 31, 2006, filed with the Commission on May 16, 2007. † |
| | |
| 4.15 | Amendment No. 6, dated February 16, 2007, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.20 of the Annual Report on Form 20-F for the year ended December 31, 2006, filed with the Commission on May 16, 2007. |
| | |
| 4.16 | Termination Agreement, dated as of December 18, 2007, by and between Ethicon Endo-Surgery, Inc. and the Registrant. |
| | |
| 4.17 | Registration Rights Agreement, dated as of July 18, 2007, by and among the Registrant and Discount Investment Corporation Ltd., Elron Electronic Industries Ltd. and RDC Rafael Development Corporation Ltd. |
| | |
| 4.18 | Distribution Agreement, dated May 9, 2002, by and between Given Imaging K.K. and Suzuken Co., Ltd. † |
| | |
| 8.1 | List of subsidiaries of the Registrant, incorporated by reference to Exhibit 8.1 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005. |
| | |
| 11.1 | Code of Ethics adopted on December 9, 2003, incorporated by reference to Exhibit 11.1 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004. |
| | |
| 12.1 | Certification of Chief Executive Officer of the Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | |
| 12.2 | Certification of Chief Financial Officer of the Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | |
| 13.1 | Certification of Chief Executive Officer of the Registrant pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
| | |
| 13.2 | Certification of Chief Financial Officer of the Registrant pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
| | |
| 15.1 | Consent of Somekh Chaikin, a member firm of KPMG International, independent registered public accountants. |
| † | Portions of this exhibit were omitted and have been filed separately with the Secretary of the Securities and Exchange Commission pursuant to an application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934. |
| | |
| * | This document is being furnished in accordance with SEC Release Nos. 33-8212 and 34-47551. |
SIGNATURES
Pursuant to the requirements of Section 12 of the Securities Exchange Act of 1934, the registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | | GIVEN IMAGING LTD. |
| | | |
| | By: | /s/ Nachum Shamir |
| | | Name: Nachum Shamir |
| | | Title: President and Chief Executive Officer |
| | | |
| | By: | /s/ Yuval Yanai |
| | | Name: Yuval Yanai |
| | | Title: Chief Financial Officer |
| | | |
| Date: March 31, 2008 | | |
ANNUAL REPORT ON FORM 20-F
INDEX OF EXHIBITS
Exhibit | | Description |
| | | |
| 1.1 | | Articles of Association, incorporated by reference to Exhibit 3.2 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | | |
| 4.1 | | Production Development, Manufacturing and Sales Agreement, dated as of November 26, 2002, by and between Micron Technology, Inc. and the Registrant, incorporated by reference to Exhibit 4.3 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004.† |
| | | |
| 4.2 | | Addendum, dated as of June 10, 2005, to Production Development, Manufacturing and Sales Agreement, dated as of November 26, 2002, by and between Micron Technology, Inc. and the Registrant. † |
| | | |
| 4.3 | | Form of Indemnification Agreement between directors and officers of the Registrant and the Registrant, incorporated by reference to Exhibit 10.15 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | | |
| 4.4 | | Lease Agreement, dated as of November 26, 2001, by and between Sha’ar Yoqneam L.P. and the Registrant, incorporated by reference to Exhibit 10.20 of the Registration Statement on Form F-1 (File No. 333-81514) filed with the Commission on February 1, 2002. |
| | | |
| 4.5 | | Summary of Material Terms of Addendum, dated December 2002, to Lease Agreement, dated as of November 26, 2001, by and between Sha’ar Yoqneam and the Registrant, incorporated by reference to Exhibit 4.11 of the Annual Report on Form 20-F for the year ended December 31, 2002, filed with the Commission on April 10, 2003. |
| | | |
| 4.6 | | Second Addendum, dated July 5, 2004, to the Lease Agreement, dated as of November 26, 2001, by and between Sha’ar Yoqneam L.P. and the Registrant, incorporated by reference to Exhibit 4.15 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005. |
| | | |
| 4.7 | | Development and Supply Agreement, dated April 8, 2002, by and between Zarlink Semiconductor AB and the Registrant, incorporated by reference to Exhibit 4.11 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005.† |
| | | |
| 4.8 | | Addendum to Development and Supply Agreement, dated July 2005, by and between Zarlink Semiconductor AB and the Registrant, incorporated by reference to Exhibit 4.12 of the Annual Report on Form 20-F for the year ended December 31, 2005, filed with the Commission on April 7, 2006.† |
| | | |
| 4.9 | | Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.12 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005.† |
| | | |
| 4.10 | | Amendment No. 1, dated June 2004, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.13 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005.† |
| | | |
| 4.11 | | Amendment No. 2, dated October 4, 2004, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.14 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005.† |
| | | |
| 4.12 | | Amendment No. 3, dated October 27, 2005, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.16 of the Annual Report on Form 20-F for the year ended December 31, 2005, filed with the Commission on April 7, 2006. |
| | | |
| 4.13 | | Amendment No. 4, dated November 14, 2005, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.17 of the Annual Report on Form 20-F for the year ended December 31, 2005, filed with the Commission on April 7, 2006. |
| 4.14 | | Amendment No. 5, dated September 6, 2006, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.19 of the Annual Report on Form 20-F for the year ended December 31, 2006, filed with the Commission on May 16, 2007. † |
| | | |
| 4.15 | | Amendment No. 6, dated February 16, 2007, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant, incorporated by reference to Exhibit 4.20 of the Annual Report on Form 20-F for the year ended December 31, 2006, filed with the Commission on May 16, 2007. |
| | | |
| 4.16 | | Termination Agreement, dated as of December 18, 2007, by and between Ethicon Endo-Surgery, Inc. and the Registrant. |
| | | |
| 4.17 | | Registration Rights Agreement, dated as of July 18, 2007, by and among the Registrant and Discount Investment Corporation Ltd., Elron Electronic Industries Ltd. and RDC Rafael Development Corporation Ltd. |
| | | |
| 4.18 | | Distribution Agreement, dated May 9, 2002, by and between Given Imaging K.K. and Suzuken Co., Ltd. † |
| | | |
| 8.1 | | List of subsidiaries of the Registrant, incorporated by reference to Exhibit 8.1 of the Annual Report on Form 20-F for the year ended December 31, 2004, filed with the Commission on March 25, 2005. |
| | | |
| 11.1 | | Code of Ethics adopted on December 9, 2003, incorporated by reference to Exhibit 11.1 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004. |
| | | |
| 12.1 | | Certification of Chief Executive Officer of the Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 12.2 | | Certification of Chief Financial Officer of the Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 13.1 | | Certification of Chief Executive Officer of the Registrant pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
| | | |
| 13.2 | | Certification of Chief Financial Officer of the Registrant pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
| | | |
| 15.1 | | Consent of Somekh Chaikin, a member of KPMG International, independent registered public accountants. |
| † | Portions of this exhibit were omitted and have been filed separately with the Secretary of the Securities and Exchange Commission pursuant to an application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934. |
| | |
| * | This document is being furnished in accordance with SEC Release Nos. 33-8212 and 34-47551. |
Given Imaging Ltd.
and its Subsidiaries
Consolidated
Financial Statements
As of and for the
Year Ended December 31, 2007
Given Imaging Ltd. and its subsidiaries
Index to Consolidated Financial Statements
| | Page |
| | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| | |
| Consolidated Balance Sheets | F-4 |
| | |
| Consolidated Statements of Operations | F-6 |
| | |
| Consolidated Statements of Changes in Shareholders’ Equity | F-7 |
| | |
| Consolidated Statements of Cash Flows | F-8 |
| | |
| Notes to the Consolidated Financial Statements | F-9 |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of
Given Imaging Ltd:
We have audited the accompanying consolidated balance sheets of Given Imaging Ltd. as of December 31, 2007 and 2006, and the related consolidated statements of operations, changes in shareholders' equity, and cash flows for each of the years in the three-year period ended December 31, 2007. We also have audited Given Imaging Ltd’s internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO)". Given Imaging Ltd's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting appearing under Item15 of Part II of this Form.20-F Our responsibility is to express an opinion on these consolidated financial statements and an opinion on the Company's internal control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the consolidated financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Given Imaging Ltd. as of December 31, 2007 and 2006, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2007, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, Given Imaging Ltd. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.”
As discussed in Note 1K to the consolidated financial statements, effective January 1, 2006, the Company has adopted Statement of Financial Accounting Standard No. 123 (revised 2004), “Share-Based Payment.”
As discussed in Note 1P to the consolidated financial statements, effective January 1, 2007, the Company has adopted FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes.”
/s/ Somekh Chaikin
Somekh Chaikin
Certified Public Accountants (Israel)
Member Firm of KPMG International
Tel - Aviv, Israel
March 31, 2008
Given Imaging Ltd. and its subsidiaries
Consolidated Balance Sheets
(In thousands except per share data)
| | | | | December 31 | |
| | | Note | | 2006 | | 2007 | |
Assets | | | | | | | |
| | | | | | | | |
| Current assets | | | | | | | | | | |
| Cash and cash equivalents | | | 1D; 2 | | $ | 44,510 | | $ | 37,103 | |
| Short-term investments | | | 1H; 5 | | | 17,245 | | | 23,191 | |
| Accounts receivable: | | | | | | | | | | |
| Trade, net | | | 1E | | | 18,887 | | | 23,315 | |
| Other | | | 3 | | | 1,463 | | | 10,385 | |
| Inventories | | | 1F; 4 | | | 18,168 | | | 15,960 | |
| Advances to suppliers | | | | | | 82 | | | 190 | |
| Deferred tax assets | | | 1P; 14D | | | 1,374 | | | 1,350 | |
| Prepaid expenses | | | | | | 1,340 | | | 1,289 | |
| | | | | | | | | | | |
| Total current assets | | | | | | 103,069 | | | 112,783 | |
| | | | | | | | | | | |
Deposits | | | | | | 469 | | | 892 | |
| | | | | | | | | | | |
Assets held for employees’ severance payments | | | 1G; 10 | | | 1,984 | | | 3,007 | |
| | | | | | | | | | | |
Marketable securities | | | 1H; 5 | | | 34,769 | | | 41,629 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Fixed assets, at cost, less accumulated depreciation | | | 1I; 6 | | | 14,811 | | | 15,422 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Intangible assets, at cost, less accumulated amortization | | | 1J; 7 | | | 3,075 | | | 3,583 | |
| | | | | | | | | | | |
Total Assets | | | | | $ | 158,177 | | $ | 177,316 | |
The accompanying notes are an integral part of these consolidated financial statements.
Given Imaging Ltd. and its subsidiaries
Consolidated Balance Sheets
(In thousands except share data)
| | | | | December 31 | |
| | | Note | | 2006 | | 2007 | |
Liabilities and shareholders' equity | | | | | | | | | | |
| | | | | | | | | | | |
| Current liabilities | | | | | | | | | | |
| | | | | | | | | | | |
| Current installments of obligation under capital lease | | | 8B | | $ | 13 | | $ | 121 | |
| Accounts payable: | | | | | | | | | | |
| Trade | | | | | | 5,550 | | | 7,275 | |
| Other | | | 9 | | | 14,620 | | | 21,012 | |
| Deferred income | | | 1N; 8C | | | 3,871 | | | 9,379 | |
| | | | | | | | | | | |
| Total current liabilities | | | | | | 24,054 | | | 37,787 | |
| | | | | | | | | | | |
Long-term liabilities | | | | | | | | | | |
| Deferred income | | | 8C | | | 20,411 | | | - | |
| Obligation under capital lease | | | 8B | | | 20 | | | 448 | |
| Liability in respect of employees’ severance payments | | | 10 | | | 2,407 | | | 3,490 | |
| | | | | | | | | | | |
Total long-term liabilities | | | | | | 22,838 | | | 3,938 | |
| | | | | | | | | | | |
Total liabilities | | | | | | 46,892 | | | 41,725 | |
| | | | | | | | | | | |
Commitments and contingencies | | | 8 | | | | | | | |
| | | | | | | | | | | |
Minority interest | | | | | | 3,499 | | | 1,996 | |
| | | | | | | | | | | |
Shareholders’ equity | | | | | | | | | | |
Share capital: | | | 11 | | | | | | | |
| Ordinary Shares, NIS 0.05 par value each (90,000,000 shares authorized as of December 31, 2006 and 2007, 28,641,291 and 29,241,785 shares issued and fully paid as of December 31, 2006 and 2007, respectively) | | | | | | 335 | | | 343 | |
| Additional paid-in capital | | | | | | 156,197 | | | 166,813 | |
| Capital reserve | | | | | | 2,166 | | | 2,166 | |
| Accumulated deficit | | | | | | (50,912 | ) | | (35,727 | ) |
| Total shareholders' equity | | | | | | 107,786 | | | 133,595 | |
| | | | | | | | | | | |
Total liabilities and shareholders' equity | | | | | $ | 158,177 | | $ | 177,316 | |
The accompanying notes are an integral part of these consolidated financial statements.
Given Imaging Ltd. and its subsidiaries
Consolidated Statements of Operations
(In thousands except share and per share data)
| | | | | Year ended December 31, | |
| | | Note | | 2005 | | 2006 | | 2007 | |
Revenues | | | 1N; 12 | | $ | 86,776 | | $ | 95,029 | | $ | 112,868 | |
| Cost of revenues | | | | | | (22,070 | ) | | (24,154 | ) | | (29,721 | ) |
| Early repayment of royalty bearing government grants | | | 10; 8A | | | - | | | - | | | (4,843 | ) |
| | | | | | | | | | | | | | |
Gross profit | | | | | | 64,706 | | | 70,875 | | | 78,304 | |
| | | | | | | | | | | | | | |
Operating expenses | | | | | | | | | | | | | |
| Research and development, gross | | | 1Q | | | (8,833 | ) | | (12,678 | ) | | (12,847 | ) |
| Royalty bearing government grants | | | 1O; 8A | | | 1,244 | | | 1,867 | | | 1,242 | |
| | | | | | | | | | | | | | |
| Research and development, net | | | | | | (7,589 | ) | | (10,811 | ) | | (11,605 | ) |
| | | | | | | | | | | | | | |
| Sales and marketing | | | | | | (43,281 | ) | | (50,732 | ) | | (55,446 | ) |
| General and administrative | | | | | | (9,657 | ) | | (16,027 | ) | | (20,981 | ) |
| Termination of marketing agreement | | | 8C | | | - | | | - | | | 22,860 | |
| Other | | | 6, 7 | | | - | | | - | | | (422 | ) |
| | | | | | | | | | | | | | |
Total operating expenses | | | | | | (60,527 | ) | | (77,570 | ) | | (65,594 | ) |
| | | | | | | | | | | | | | |
Operating profit (loss) | | | | | | 4,179 | | | (6,695 | ) | | 12,710 | |
| | | | | | | | | | | | | | |
| Financial income, net | | | 13 | | | 762 | | | 3,980 | | | 5,520 | |
| | | | | | | | | | | | | | |
Profit (loss) before taxes on income and minority share | | | | | | 4,941 | | | (2,715 | ) | | 18,230 | |
| | | | | | | | | | | | | | |
| Income tax benefit (expense) | | | 1P, 14 | | | 286 | | | (127 | ) | | (4,548 | ) |
| | | | | | | | | | | | | | |
Profit (loss) before minority share | | | | | | 5,227 | | | (2,842 | ) | | 13,682 | |
| | | | | | | | | | | | | | |
| Minority share in losses of subsidiary | | | | | | 1,116 | | | 1,334 | | | 1,503 | |
| | | | | | | | | | | | | | |
Net profit (loss) | | | | | $ | 6,343 | | $ | (1,508 | ) | $ | 15,185 | |
| | | | | | | | | | | | | | |
Earnings (loss) per share | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| Basic Earnings (loss) per Ordinary Share | | | 1L | | $ | 0.23 | | $ | (0.05 | ) | $ | 0.52 | |
| | | | | | | | | | | | | | |
| Diluted Earnings (loss) per Ordinary Share | | | | | $ | 0.21 | | $ | (0.05 | ) | $ | 0.49 | |
| | | | | | | | | | | | | | |
| Weighted average number of Ordinary Shares used to compute basic profit (loss) per Ordinary Share | | | 1L | | | 27,781,223 | | | 28,053,849 | | | 28,961,968 | |
| | | | | | | | | | | | | | |
| Weighted average number of Ordinary Shares used to compute diluted Earnings (loss) per Ordinary Share | | | 1L | | | 29,695,164 | | | 28,053,849 | | | 31,030,458 | |
The accompanying notes are an integral part of these consolidated financial statements.
Given Imaging Ltd. and its subsidiaries
Consolidated Statements of Changes in Shareholders' Equity
(In thousands except share data)
| | | | | | | Additional | | | | | | | | | |
| | Ordinary shares | | Paid-In | | | | | | | | Total | |
| | Shares | | Amount | | | | | | | | | | | |
Balance as of December 31,2004 | | | 27,621,386 | | $ | 323 | | $ | 147,878 | | $ | 2,166 | | $ | (3 | ) | $ | (55,747 | ) | $ | 94,617 | |
| | | | | | | | | | | | | | | | | | | | | | | |
Changes during the year 2005: | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options | | | 328,895 | | | 4 | | | 1,077 | | | - | | | - | | | - | | | 1,081 | |
| Amortization of unearned | | | | | | | | | | | | | | | | | | | | | | |
| compensation | | | - | | | - | | | - | | | - | | | 3 | | | - | | | 3 | |
| Net profit | | | - | | | - | | | - | | | - | | | - | | | 6,343 | | | 6,343 | |
| | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2005 | | | 27,950,281 | | | 327 | | | 148,955 | | | 2,166 | | | - | | | (49,404 | ) | | 102,044 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Changes during the year 2006: | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options | | | 591,010 | | | 7 | | | 2,029 | | | - | | | - | | | - | | | 2,036 | |
| Restricted shares issued | | | 100,000 | | | 1 | | | - | | | - | | | - | | | - | | | 1 | |
| Stock based compensation | | | - | | | - | | | 5,213 | | | - | | | - | | | - | | | 5,213 | |
| Net loss | | | - | | | - | | | - | | | - | | | - | | | (1,508 | ) | | (1,508 | ) |
| | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2006 | | | 28,641,291 | | | 335 | | | 156,197 | | | 2,166 | | | - | | | (50,912 | ) | | 107,786 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Changes during the year 2007: | | | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options | | | 589,262 | | | 8 | | | 4,272 | | | - | | | - | | | - | | | 4,280 | |
| Excess tax benefits related to stock based compensation | | | - | | | - | | | 693 | | | - | | | - | | | - | | | 693 | |
| Exercise of warrants | | | 5,232 | | | - | | | - | | | - | | | - | | | - | | | - | |
| Restricted shares issued | | | 6,000 | | | - | | | - | | | - | | | - | | | - | | | - | |
| Stock based compensation | | | - | | | - | | | 5,651 | | | - | | | - | | | - | | | 5,651 | |
| Net profit | | | - | | | - | | | - | | | - | | | - | | | 15,185 | | | 15,185 | |
| | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2007 | | | 29,241,785 | | $ | 343 | | $ | 166,813 | | $ | 2,166 | | | - | | $ | (35,727 | ) | $ | 133,595 | |
The accompanying notes are an integral part of these consolidated financial statements.
Given Imaging Ltd. and its subsidiaries
Consolidated Statements of Cash Flows
(In thousands)
| | | Year ended December 31, | |
| | | 2005 | | 2006 | | 2007 | |
Cash flows from operating activities: | | | | | | | | | | |
| Net profit (loss) | | $ | 6,343 | | $ | (1,508 | ) | $ | 15,185 | |
| | | | | | | | | | | |
Adjustments required to reconcile net profit (loss) to net cash provided by operating activities: | | | | | | | | | | |
| | | | | | | | | | | |
| Minority share in losses of subsidiary | | | (1,116 | ) | | (1,334 | ) | | (1,503 | ) |
| Depreciation and amortization | | | 3,596 | | | 4,237 | | | 4,771 | |
| Deferred tax assets | | | (482 | ) | | (155 | ) | | 24 | |
| Stock based compensation | | | 3 | | | 5,213 | | | 5,651 | |
| Excess tax benefits related to stock based compensation | | | - | | | - | | | (693 | ) |
| Other | | | 98 | | | 18 | | | 380 | |
| Net decrease (increase) in trading securities | | | - | | | (5,060 | ) | | 5,092 | |
| Increase in accounts receivable - trade | | | (6,064 | ) | | (562 | ) | | (4,428 | ) |
| Decrease (increase) in other accounts receivable | | | (4,993 | ) | | 4,801 | | | (8,922 | ) |
| Decrease (increase) in prepaid expenses | | | (66 | ) | | (320 | ) | | 51 | |
| Decrease (increase) in advances to suppliers | | | 223 | | | 250 | | | (108 | ) |
| Decrease (increase) in inventories | | | (2,378 | ) | | (1,996 | ) | | 2,208 | |
| Increase in accounts payable | | | 5,769 | | | 500 | | | 8,570 | |
| Increase (decrease) in deferred income | | | 12,555 | | | (1,223 | ) | | (14,903 | ) |
Net cash provided by operating activities | | | 13,488 | | | 2,861 | | | 11,375 | |
| | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | |
| Purchase of fixed assets and intangible assets | | | (7,948 | ) | | (5,876 | ) | | (5,772 | ) |
| Deposits | | | (16 | ) | | (41 | ) | | (355 | ) |
| Proceeds from sale of marketable securities | | | - | | | 13,120 | | | 18,753 | |
| Investments in marketable securities | | | (21,919 | ) | | (37,960 | ) | | (36,584 | ) |
Net cash used in investing activities | | | (29,883 | ) | | (30,757 | ) | | (23,958 | ) |
| | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | |
| Principal payments on capital lease obligation | | | (12 | ) | | (14 | ) | | (37 | ) |
| Proceeds from the issuance of Ordinary Shares | | | 1,081 | | | 2,037 | | | 4,280 | |
| Excess tax benefits related to stock based compensation | | | - | | | - | | | 693 | |
| Issuance of shares by consolidated company | | | - | | | 4,772 | | | - | |
Net cash provided by financing activities | | | 1,069 | | | 6,795 | | | 4,936 | |
| | | | | | | | | | | |
Effect of exchange rate changes on cash | | | (179 | ) | | 255 | | | 240 | |
| | | | | | | | | | | |
Decrease in cash and cash equivalents | | | (15,505 | ) | | (20,846 | ) | | (7,407 | ) |
Cash and cash equivalents at beginning of year | | | 80,861 | | | 65,356 | | | 44,510 | |
Cash and cash equivalents at end of year | | | 65,356 | | | 44,510 | | | 37,103 | |
Supplementary cash flow information
| | | Year ended December 31, | |
| | | 2005 | | 2006 | | 2007 | |
| Income taxes paid | | $ | 163 | | $ | 300 | | $ | 1,098 | |
| Assets acquired under capital lease | | | - | | | - | | $ | 569 | |
The accompanying notes are an integral part of these consolidated financial statements.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 1 - Organization and Summary of Significant Accounting Policies
A. General
Given Imaging Ltd. (the "Company") was incorporated in Israel in January 1998.
The Company has developed the Given System, a proprietary wireless imaging system that represents a new approach to visual examination of the gastrointestinal tract. The system uses a miniaturized video camera contained in a capsule, referred to as the PillCam™ capsule, which is ingested by the patient and delivers high quality color images in a painless and noninvasive manner.
The Given System consists of three principal components:
| • | a single-use, disposable PillCam color-imaging capsule that is ingested by the patient; |
| • | a portable data recorder and array of sensors that are worn by the patient; and |
| | • | a computer workstation with a proprietary RAPID software for downloading, processing and analyzing recorded data. |
After receiving marketing clearance from the United States Food and Drug Administration (“FDA”) in August of 2001, the Company commenced the marketing of the Given System with its first video capsule, the PillCam Small Bowel Capsule, or PillCam SB, for detection of disorders of the small bowel. In November 2004, following receipt of FDA marketing clearance, the Company began marketing and sales of its second video capsule, PillCam ESO, for detection of disorders in the esophagus. In late 2006, the Company completed the development of its third video capsule, PillCam Colon, for visual examination of the colon and received the regulatory clearance that permits the Company to market and sell this capsule in Europe. In April 2007, the Company received marketing clearance for the PillCam SB capsule from the Ministry of Health, Labor and Welfare in Japan.
The medical device industry in which the Company is involved is characterized by the risks of regulatory barriers and reimbursement issues. Penetration into the world market requires the investment of considerable resources and continuous development efforts. The Company’s future success is dependent upon several factors including technological quality, regulatory approvals, sufficient reimbursement for its products and the cost and diagnostic-effectiveness of its products compared to other methods for the examination of the gastrointestinal tract.
B. Basis of presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America and include the accounts of the Company and its wholly-owned subsidiaries in the United States, Germany, France, the Netherlands, Singapore and Australia and its 51% owned subsidiary in Japan. The accounts of its subsidiaries are consolidated from the date of their inception. All the subsidiaries were established for the purpose of marketing and selling the Given System. All intercompany balances and transactions have been eliminated in consolidation. The Company considers that it operates in only one segment.
Prior periods’ financial statement presentations in note 12A and 12B have been conformed to the current period’s presentation.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 1 - Organization and Summary of Significant Accounting Policies (cont'd)
C. Functional and reporting currency
The Company and all its subsidiaries’ functional and reporting currency is the U.S. dollar.
Transactions denominated in foreign currencies other than the U.S. dollar are translated into the functional currency using the prevailing exchange rates at the date of the transactions. Gains and losses from the translation of foreign currency transactions are recorded in other income or expenses.
D. Cash and cash equivalents
All highly-liquid investments with original maturity of three months or less from the date of deposit are considered to be cash equivalents.
E. Allowance for doubtful accounts receivable - trade
The allowance for doubtful accounts receivable is calculated on the basis of specific identification of balances, the collection of which, in management's opinion, is doubtful. In determining the adequacy of the allowance, management bases its opinion on the estimated risk, in reliance on available information with respect to the debtor's financial position and an evaluation of the collateral received.
The activity in the allowance for doubtful accounts for the three years ended December 31, 2007 is as follows:
| | | Year ended December 31, | |
| | | 2005 | | 2006 | | 2007 | |
| Opening balance | | $ | 115 | | $ | 431 | | $ | 787 | |
| Provision | | | 316 | | | 356 | | | (79 | ) |
| Write-offs | | | - | | | - | | | (379 | ) |
| | | | | | | | | | | |
| Closing balance | | $ | 431 | | $ | 787 | | $ | 329 | |
F. Inventories
Inventories are stated at lower of cost or market. Cost is determined using the average cost method for raw materials, components and finished goods and on the basis of actual manufacturing costs for work in progress.
G. Assets held for employees’ severance payments
Assets held for employees’ severance payments represent contributions to insurance policies that are recorded at their current redemption value.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 1 - Organization and Summary of Significant Accounting Policies (cont'd)
The Company accounts for marketable securities under Statement of Financial Accounting Standards (SFAS) No. 115 “Accounting for Certain Investments in Debt and Equity Securities (“SFAS 115”). Marketable securities consist of U.S. government bonds and corporate bonds, which the Company classified as “held to maturity” as of December 31, 2007 and 2006. As of December 31, 2006, the Company also held auction rate securities and money market funds, which the Company classified as “trading.”
Held-to-maturity debt securities are securities that the Company has the ability and intent to hold until maturity and are recorded at amortized cost, adjusted for the amortization or accretion of premiums or discounts. Premiums and discounts are amortized or accreted over the life of the related held-to-maturity security as an adjustment to yield using the effective-interest method.
Trading securities are bought and held principally for the purpose of selling them in the near term. Trading securities are recorded at fair value and changes in the fair value, based on closing market prices of the securities at the balance sheet date, represent unrealized gains and losses which are included in earnings.
Decline in the market value of any “held-to-maturity” security below cost, that is deemed to be other than temporary, results a reduction in the carrying amount to fair value and a corresponding impairment. The impairment is charged to earnings and a new cost basis for the security is established. During 2006 and 2007 no impairment charge was recognized.
I. Fixed assets
Fixed assets are stated at cost. Depreciation is computed by the straight-line method over the estimated useful lives of the assets at the following annual rates:
| | % | |
| Computers and software | | | 33 | |
| Instruments and laboratory equipment | | | 15 | |
| Leasehold improvements | | | 10 | |
| Motor vehicles | | | 15 | |
| Machinery and equipment | | | 15 | |
| Communication equipment | | | 15 | |
| Office furniture and equipment | | | 10-15 | |
Motor vehicles purchased under capital lease arrangements are recorded at the present value of the minimum lease payments at lease inception. Such assets and leasehold improvements are depreciated and amortized respectively, using the straight-line method over the shorter of the lease term or estimated useful life of the asset.
The Company evaluates long-lived assets and certain intangible assets for impairment in accordance with the provisions of SFAS No. 144, “Accounting for the Impairment of or Disposal of Long-Lived Assets” (“Statement 144”). This Statement requires that long-lived assets and certain identifiable intangible assets be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to undiscounted future net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 1 - Organization and Summary of Significant Accounting Policies (cont'd)
J. Intangible assets
| a. | Legal expenses related to patents and trademarks registration have been capitalized and amortized over the remaining life of the assets, which is generally eight years, and are evaluated for recoverability whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. |
| b. | Technology and content costs are generally expensed as incurred, except for certain costs relating to the development of the Company’s website that are capitalized and amortized in accordance with EITF 00-2 “Accounting for website development costs” over their estimated useful life which is generally three years. |
K. Stock compensation plans
Employees and Directors
Effective January 1, 2006, the Company adopted the fair value recognition provisions of SFAS No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123R”). This Statement requires compensation expense relating to share-based payments to be recognized in net income using a fair-value measurement method. Under the fair value method, the estimated fair value of awards is charged to income on a straight-line basis over the requisite service period, which is generally the vesting period. The Company elected the modified-prospective method and therefore prior periods were not restated. Under the modified-prospective method, compensation costs recognized in 2006 include also compensation costs for all share-based payments granted prior to, but not yet vested, as of December 31, 2005.
Stock-based compensation recognized in the consolidated statements of operations for the years ended December 31, 2007 and 2006 is based on awards ultimately expected to vest. As a result the expense has been reduced for estimated forfeitures. SFAS No. 123R required forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. In the Company’s pro forma information required under SFAS No. 123, “Accounting for Stock-Based Compensation” (“SFAS No. 123”) for the periods prior to fiscal 2006, the Company accounted for forfeitures as they occurred.
For the year ended December 31, 2006, the effect of the implementation of SFAS No. 123R was to increase expenses by $ 5,213 which changed the (loss) before taxes and net (loss) by the same amounts. Consequently, the basic and diluted loss per share for the year ended December 31, 2006 was further reduced by $0.19 .
Prior to January 1, 2006, the Company has followed SFAS No. 123, which permitted entities to recognize as an expense over the vesting period, the fair value on the date of grant of all stock-based awards. Alternatively, Statement 123 allowed entities to continue to apply the provisions of Accounting Principles Board Opinion No.25, “Accounting for Stock Issued to Employees and related interpretations” (“APB Opinion No.25”) and provide pro forma net income and proforma earnings per share disclosures for employee stock option grants as if the fair-value based method defined in Statement 123 had been applied.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 1 - Organization and Summary of Significant Accounting Policies (cont'd)
K. Stock compensation plans (cont’d)
The Company elected to apply the intrinsic value-based method prescribed in APB Opinion No. 25 for its stock compensation to employees and directors and provide the pro forma disclosure provisions of SFAS No. 123, as amended by SFAS No. 148, “Accounting for Stock-Based Compensation - Transition and Disclosure, an amendment of SFAS No. 123.”
As such, the Company computed and recorded compensation expense for grants whose terms were fixed with respect to the number of shares and option price only if the market price on the date of grant exceeded the exercise price of the stock option. The compensation cost for the fixed plans was recorded over the period the employee performs the service to which the stock compensation relates.
The following table shows the effect on net profit and earnings per Ordinary Share if the Company had applied the fair value recognition provisions of Statement 123 for the year ended December 31, 2005.
| Net profit as reported | | $ | 6,343 | |
- Compensation expenses according to | | | | |
APB 25 included in the reported net profit | | | 3 | |
- Application of compensation expenses | | | | |
according to SFAS 123 | | | (10,327 | ) |
| Pro forma net loss | | $ | (3,981 | ) |
| | | | | |
| Basic earnings (loss) per Ordinary Share: | | | | |
| As reported | | $ | 0.23 | |
| Pro forma | | $ | (0.14 | ) |
| | | | | |
| Diluted earnings (loss) per ordinary share: | | | | |
| As reported | | $ | 0.21 | |
| Pro forma | | $ | (0.14 | ) |
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 1 - Organization and Summary of Significant Accounting Policies (cont'd)
L. Earnings (loss) per Ordinary Share
Basic and diluted Earnings (loss) per Ordinary Share is presented in conformity with SFAS No. 128, “Earnings Per Share”, for all years presented. Basic Earnings (loss) per Ordinary Share is calculated by dividing the net Earnings (loss) attributable to Ordinary Shares, by the weighted average number of Ordinary Shares outstanding. Diluted Earnings (loss) per Ordinary share calculation is similar to Basic Earnings Per Share except that the weighed average of common shares outstanding is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares from options had been exercised.
The following table summarizes information related to the computation of basic and diluted Earnings (loss) per Ordinary Share for the years indicated.
| | | Year ended December 31, | |
| | | 2005 | | 2006 | | 2007 | |
| Net profit (loss) attributable to Ordinary Shares | | $ | 6,343 | | $ | (1,508 | ) | $ | 15,185 | |
| | | | | | | | | | | |
Weighted average number of Ordinary Shares outstanding used in basic Earnings (loss) per Ordinary Share calculation | | | 27,781,223 | | | 28,053,849 | | | 28,962,968 | |
| | | | | | | | | | | |
Add assumed exercise of outstanding dilutive potential Ordinary Shares | | | | | | | | | | |
| | | | 1,913,941 | | | - | | | 2,068,491 | |
| | | | | | | | | | | |
Weighted average number of Ordinary Shares outstanding used in diluted Earnings (loss) per Ordinary Share calculation | | | | | | | | | | |
| | | | 29,695,164 | | | 28,053,849 | | | 31,030,459 | |
| | | | | | | | | | | |
| Basic Earnings (loss) per Ordinary Share | | $ | 0.23 | | $ | (0.05 | ) | $ | 0.52 | |
| | | | | | | | | | | |
| Diluted Earnings (loss) per Ordinary Share | | $ | 0.21 | | $ | (0.05 | ) | $ | 0.49 | |
| | | | | | | | | | | |
Number of options excluded from the diluted Earnings per share calculation because of anti-dilutive effect | | |
2,448,114 | | |
4,114,604 | | |
1,881,033 | |
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 1 - Organization and Summary of Significant Accounting Policies (cont'd)
M. Use of estimates
The preparation of the consolidated financial statements requires management of the Company to make a number of estimates and assumptions relating to the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the period. Actual results could differ from these estimates.
N. Revenue recognition
Revenues from sales of products are recognized upon delivery provided that the collection of the resulting receivable is reasonably assured, there is persuasive evidence of an arrangement, no significant obligations in respect of installation remain and the price is fixed or determinable.
For sales contracts, which include a Post Contract Customer Support (“PCS”) component, revenues allocated to PCS in accordance with EITF 00-21 “Revenue Arrangements with Multiple Deliverables”, are deferred and recognized ratably over the term of the support period, which is generally one year.
The Company accrues estimated warranty costs at time of shipment based on contractual rights and historical experience. The Company's policy is not to grant return rights.
Taxes collected from customers and remitted to Governmental Authorities are presented in the financial statements on a net basis.
The Company routinely evaluates its products for inclusion of any embedded software that is more than incidental thereby requiring consideration of AICPA Statement of Position 97-2, “Software Revenue Recognition”. Based on such evaluation, the Company has concluded that none of its products have such embedded software.
O. Government-Sponsored Research and Development
The Company records grants received from the Office of the Chief Scientist of the Israeli Ministry of Industry and Trade (the “OCS”) as a reduction of research and development expenses.
Royalties payable to OCS are recognized pursuant to sale of related products and are classified under cost of revenues. (See also Note 8 a.).
P. Taxes on income
The Company accounts for income taxes under SFAS No. 109 “Accounting for Income Taxes” (“Statement 109”). Under Statement 109, deferred tax assets or liabilities are recognized in respect of temporary differences between the tax bases of assets and liabilities and their financial reporting amounts as well as in respect of tax loss and credit carryforwards, based on enacted statutory tax rates applicable to the periods in which such deferred taxes will be realized. The tax effect resulting from a change in tax rates is recognized in the period that includes the enactment date. Valuation allowances are established when necessary to reduce deferred tax assets to the amount that is more likely than not to be realized.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 1 - Organization and Summary of Significant Accounting Policies (cont'd)
P. Taxes on income
In July 2006, the FASB issued FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes” (“FIN 48”), which prescribes a recognition threshold and measurement process for recording in the financial statements uncertain tax positions taken or expected to be taken in a tax return. FIN 48 also provides guidance on de-recognition of tax benefits, classification on the balance sheet, interest and penalties, accounting in interim periods, disclosure and transition. The accounting provisions of FIN 48 became effective for the Company beginning January 1, 2007. The effect of adoption on the Company’s consolidated results of operations, financial position and cash flows based on Company management's evaluation is presented in note 14 F.
The Company’s accounting policy is to accrue interest related to unrecognized tax benefits as a component of interest expense while penalties as a part of general and administrative expenses in the consolidated statements of operations.
Q. Research and development costs
Research and development costs are expensed as incurred.
R. Allowance for product warranty
It is the Company's policy to grant a warranty for certain products. The balance sheet provision for warranties is determined based upon the Company’s experience regarding the relationship between sales and warranty expenses.
S. Concentration of credit risk
Financial instruments that may subject the Company to significant concentrations of credit risk consist principally of cash and cash equivalents, trade accounts receivable and marketable securities.
Cash and cash equivalents are deposited with major financial institutions in Europe, the United States, Japan, Australia, Singapore and Israel.
The Company performs ongoing credit evaluations of the financial condition of its customers. The risk of collection associated with trade receivables is reduced by the large number and geographical dispersion of the Company's customer base and the Company's policy of requiring collateral or security with respect to receivables due from distributors.
T. Other Comprehensive Income
For the years ended December 31, 2005, 2006 and 2007, other comprehensive income (loss) equals net profit (loss).
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 1 - Organization and Summary of Significant Accounting Policies (cont'd)
U. Recent accounting pronouncements
In September 2006, the FASB issued FASB Statement No. 157, "Fair Value Measurement" (Statement 157). Statement 157 defines fair value, establishes a framework for the measurement of fair value, and enhances disclosures about fair value measurements. The statement does not require any new fair value measures. The statement is effective for fair value measures already required or permitted by other standards for fiscal years beginning after November 15, 2007. The Company is required to adopt Statement 157 beginning on January 1, 2008. Statement 157 is required to be applied prospectively, except for certain financial instruments. Any transition adjustment will be recognized as an adjustment to opening retained earnings in the year of adoption. The FASB announced a one- year deferral of Statement 157's fair- value measurement requirements for non-financial assets and liabilities that are not required or permitted to be measured at fair value on a recurring basis. The Company believes that the adoption of Statement 157 will not have a material impact on the Company's financial condition, results of operations and cash flows.
In February 2007, the FASB issued FASB Statement No. 159, "The Fair Value Option for Financial Assets and Financial Liabilities- including an amendment of FASB No. 115" (Statement 159). Statement 159 gives the Company the irrevocable option to carry most financial assets and liabilities at fair value that are not currently required to be measured at fair value. If the fair value is elected, changes in fair value would be recorded in earnings at each subsequent reporting date. SFAS 159 is effective for the Company's 2008 fiscal year. The Company believes that the adoption of Statement 159 will not have a material impact on the Company's financial condition, results of operations and cash flow.
In December 2007, the FASB issued FASB Statement No. 141R, "Business Combinations" (Statement 141R) and FASB Statement No. 160, "Non-controlling Interests in Consolidated Financial Statements- an amendment to ARB No. 51" (Statement 160). Statements 141R and 160 require most identifiable assets, liabilities, non-controlling interests, and goodwill acquired in a business combination to be recorded at "full fair value" and require non-controlling interests (previously referred to as minority interests) to be reported as a component of equity, which changes the accounting for transactions with non-controlling interest holders. Both Statements are effective for periods beginning on or after December 15, 2008, and earlier adoption is prohibited. Statement 141R will be applied to business combinations occurring after the effective date. Statement 160 will be applied prospectively to all non-controlling interests, including any that arose before the effective date. The Company is currently evaluating the impact of adopting statement 141R and 160 on its results of operations and financial position.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note-2 - Cash and Cash Equivalents
| | | Interest rate | | | | | |
| | as of | | | | | |
| | December 31 | | December 31 | |
| | 2007 | | 2006 | | 2007 | |
| | % | | | | | |
| Denominated in U.S. dollars | | | 4.2-5.11 | | $ | 25,794 | | $ | 22,877 | |
| Denominated in New Israeli Shekels | | | 3.4-3.6 | | | 3,099 | | | 2,022 | |
| Denominated in Euros | | | 3.8-3.9 | | | 7,766 | | | 6,642 | |
| Denominated in Australian dollars | | | | | | 469 | | | 1,102 | |
| Denominated in Singapore dollars | | | | | | - | | | 168 | |
| Denominated in Japanese Yen | | | | | | 7,382 | | | 4,292 | |
| | | | | | | | | | | |
| | | | | | $ | 44,510 | | $ | 37,103 | |
Note 3 - Accounts Receivable - Other
| | | December 31 | |
| | | 2006 | | 2007 | |
| Government institutions | | $ | 1,389 | | $ | 2,118 | |
| InScope (Note 8C) | | | - | | | 7,620 | |
| Other | | | 74 | | | 647 | |
| | | | | | | | |
| | | $ | 1,463 | | $ | 10,385 | |
Note 4 - Inventories
| | | December 31 | |
| | | 2006 | | 2007 | |
| Raw materials and components | | $ | 7,721 | | $ | 7,733 | |
| Work in progress | | | 3,533 | | | 2,941 | |
| Finished goods | | | 6,914 | | | 5,286 | |
| | | | | | | | |
| | | $ | 18,168 | | $ | 15,960 | |
Note 5 - Marketable Securities
As of December 31, 2007 and 2006, marketable securities consist U.S. government bonds and corporate bonds, which the Company classified as “held-to-maturity” (“the Bonds”). As of December 31, 2006, marketable securities also included auction rate securities and money market funds, which are classified as “trading”.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 5 - Marketable Securities (cont’d)
The amortized cost, gross unrealized losses and fair value of the “held-to-maturity” bonds by major interest type were as follows:
| | | December 31, 2007 | |
| | Amortized | | Gross unrealized | | Fair | |
| | cost | | holding losses | | Value | |
| Up to 5% | | $ | 43,015 | | $ | (553 | ) | $ | 42,462 | |
| 5.1% - 6%, 8.125% | | | 21,805 | | | (249 | ) | | 21,556 | |
| | | | | | | | | | | |
| | | $ | 64,820 | | $ | (802 | ) | $ | 64,018 | |
| | | December 31, 2006 | |
| | Amortized | | Gross unrealized | | Fair | |
| | cost | | holding losses | | Value | |
| Up to 5% | | $ | 33,574 | | $ | (601 | ) | $ | 32,973 | |
| 5.1% - 6%, 8.125% | | | 13,380 | | | (212 | ) | | 13,168 | |
| | | | | | | | | | | |
| | | $ | 46,954 | | $ | (813 | ) | $ | 46,141 | |
Maturities of the “held-to-maturity” bonds were as follows at December 31, 2007 and 2006:
| | | Amortized cost | | Fair value | |
| | | 2007 | | 2007 | |
| Current maturities | | $ | 23,191 | | $ | 22,290 | |
| Due after one year through five years | | | 41,629 | | | 41,728 | |
| | | | | | | | |
| | | $ | 64,820 | | $ | 64,018 | |
| | | Amortized cost | | Fair value | |
| | | 2006 | | 2006 | |
| Current maturities | | $ | 12,185 | | $ | 12,063 | |
| Due after one year through five years | | | 34,769 | | | 34,078 | |
| | | | | | | | |
| | | $ | 46,954 | | $ | 46,141 | |
As of December 31, 2006, marketable securities also included $5,060 in bonds classified as “trading” ($0 as of December 31, 2007). These investments are subject to price volatility associated with any interest-bearing instrument. Net realized gains and net unrealized losses on trading securities during the year ended December 31, 2006 were $133 and $15 respectively, and net realized gains during the year ended December 31, 2007 were $32 and are included in financial income, net.
Short-term investments are comprised of:
| | | December 31 | |
| | | 2006 | | 2007 | |
Current maturities of “held-to-maturity” securities | | $ | 12,185 | | $ | 23,191 | |
| Trading securities | | | 5,060 | | | - | |
| | | | | | | | |
| | | $ | 17,245 | | $ | 23,191 | |
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 6 - Fixed Assets, at Cost, Less Accumulated Depreciation
| | | December 31 | |
| | | 2006 | | 2007 | |
| Computers and software | | $ | 6,234 | | $ | 6,957 | |
| Instruments and laboratory equipment | | | 791 | | | 1,077 | |
| Leasehold improvements | | | 4,259 | | | 4,600 | |
| Motor vehicles | | | 155 | | | 186 | |
| Machinery and equipment | | | 14,952 | | | 16,060 | |
| Communication equipment | | | 418 | | | 492 | |
| Office furniture and equipment | | | 1,248 | | | 1,797 | |
| | | | | | | | |
| Fixed assets, at cost | | | 28,057 | | | 31,169 | |
| | | | | | | | |
| Accumulated depreciation | | | (13,246 | ) | | (15,747 | ) |
| | | | | | | | |
| Fixed assets at cost, less accumulated depreciation | | $ | 14,811 | | $ | 15,422 | |
Depreciation expenses for the years ended December 31, 2005, 2006 and 2007 were $2,936, $3,599, and $4,055, respectively. During 2007, the Company wrote off manufacturing equipment which was no longer in use. This resulted in a charge of $365 recorded in operating expenses- other on the consolidated statement of operations.
As of December 31 2006 and 2007, the cost of fixed assets under capital lease was $55 and $571 respectively, and the accumulated depreciation was $24 and $49 respectively.
Note 7 - Intangible Assets, at Cost, Less Accumulated Amortization
| | | December 31 | |
| | | 2006 | | 2007 | |
| | | | | | |
| Patents and trademarks | | $ | 4,594 | | $ | 5,545 | |
| Web site application | | | 922 | | | 1,165 | |
| | | | | | | | |
| Intangible assets at cost | | | 5,516 | | | 6,710 | |
| | | | | | | | |
| Accumulated amortization | | | (2,441 | ) | | (3,127 | ) |
| | | | | | | | |
| Intangible assets, at cost, less accumulated amortization | | $ | 3,075 | | $ | 3,583 | |
Amortization expenses for the years ended December 31, 2005, 2006 and 2007 were $660, $638 and 716, respectively. Estimated amortization expenses for the next five years are: $719 in 2008, $669 in 2009, $557 in 2010, $449 in 2011, and $369 in 2012. During 2007, the Company wrote off patent and trademarks which are no longer expected to be used. This resulted in a charge of $57 recorded in operating expenses - other on the consolidated statement of operations.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 8 - Commitments and Contingencies
| | A. | Office of the Chief Scientist Grants |
Until December 2007, the Company’s research and development efforts had been partially financed through grants from the Office of the Chief Scientist of the Israeli Ministry of Industry and Trade (the “OCS”). In return for the OCS’s participation, the Company was committed to pay royalties to the Israeli Government at the rate of 3% of the sales of its products for each of the first three years of the launch of the related product and, from the fourth year onwards, at the rate of 3.5% up to 100% of the amount of the grants received, plus LIBOR interest. The Company was entitled to the grants only upon incurring research and development expenditure. There were no future performance obligations related to the grants received from the OCS.
During December 2007, the Company made an early repayment of all its outstanding royalty obligation and accrued interest of $4,843 to the OCS. This repayment resulted in a one-time charge of $4,843 million presented as an early repayment of royalty bearing government grants in the Company’s consolidated statement of operations.
The Company continues to participate in other non-royalty bearing programs of the OCS.
B. Leases
Capital lease of equipment
During the year ended December 31, 2007 the Company’s subsidiary in Japan entered into a lease agreement for certain equipment. The obligation under this capital lease is to be repaid in five years and bears interest of 1.97%.
Operating leases
The Company and its subsidiaries currently lease office space and manufacturing space for periods of up to 15 years (including options to extend the terms of the leases). The current lease for the Company’s headquarters is in Yoqneam, Israel. This facility houses the Company’s corporate headquarters, research and development and manufacturing facilities. Under this lease agreement, the Company will pay approximately $1,500 a year in rent and management fee. These payments are subject to adjustments based on changes in the Israeli Consumer Price Index. In addition, to secure its obligations under the lease, the Company provided a bank guaranty in the amount of approximately $750 in favor of the lessor. The lease expires on December 31, 2015. The Company has an option to extend the lease until December 31, 2020.
The Company and its subsidiaries signed several motor vehicle lease agreements. The companies deposited a total amount of $ 193 to guarantee their performance under the terms of the lease agreements.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 8 - Commitments and Contingencies (cont’d)
B. Leases (cont’d)
The Company is committed to minimum annual payments over the next five years as follows:
| | | Capital leases | | Operating leases | |
| 2008 | | $ | 121 | | $ | 3,580 | |
| 2009 | | | 101 | | | 3,021 | |
| 2010 | | | 103 | | | 2,268 | |
| 2011 | | | 105 | | | 1,731 | |
| 2012 and thereafter | | | 139 | | | 6,231 | |
| | | $ | 569 | | $ | 16,831 | |
Depreciation of vehicles and equipment under capital lease for the years ended December 31, 2005, 2006 and 2007 was $ 9, $ 9 and $ 25, respectively.
Rental expenses under the lease agreements for the years ended December 31, 2005, 2006 and 2007 were $2,353, $2,914 and $3,484, respectively.
C. Agreement with InScope
On May 10, 2004, the Company entered into an exclusive sales representation, co-promotion and cooperation Agreement (the “Agreement”) with InScope, a division of Ethicon Endo-Surgery, a Johnson & Johnson company, providing InScope with the exclusive rights to market the Company’s PillCam ESO capsule for visual examination of the esophagus in the United States. Under the terms of the Agreement, the Company received milestone payments totaling $25,000 in 2004 and 2005 and was paying InScope a commission of 50% on sales of PillCam ESO capsules and a 10% commission on sales of capital equipment parts of the Given System, such as workstations and portable data recorders. Milestone payments received were deferred and were being systematically recognized, on a straight-line basis, by the Company as a reduction of sales and marketing expenses over the 15 year term of the Agreement.
In November 2007 InScope advised the Company that it had decided to terminate the Agreement. Under the terms of termination agreed to between the parties in December 2007, InScope agreed to pay the Company an amount of $8,820, comprising of the following:
| Termination payments | | $ | 7,620 | |
| Reimbursement for certain clinical trials | | | 1,200 | |
| | | $ | 8,820 | |
The Agreement and each party’s rights and obligations thereunder terminated in January, 2008.
The termination payments of $7,620 were recognized in the Company’s consolidated financial statements for the year ended December 31, 2007.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 8 - Commitments and Contingencies (cont’d)
C. Agreement with InScope (cont’d)
The reimbursement for certain clinical trials relate to 2008, and will be recorded in such year.
Pursuant to the early termination and the change in the remaining life of the Agreement, the Company is amortizing the remaining deferred income balance at the date of termination of $20,683 over the remaining term of the Agreement of two months. Accordingly, the Company has recognized a one time income of $22,860 in its consolidated statement of operations for the year ended December 31, 2007 under “Termination of marketing agreement” comprised of additional accelerated amortization of $15,240 of previously received milestones, and $7,620 in termination payments.
InScope paid $1,200 during December 2007, and the remaining $7,620 was paid during January 2008.
As of December 31, 2007, deferred income includes the following unamortized amounts relating to the Agreement:
D. Agreements with key single - source suppliers and commitments to suppliers
| | (1) | In 2002, the Company entered into an agreement with a Canadian company (“Canadian Company”) that supplies a component that is integrated into the PillCam capsules. The agreement also includes non-compete provisions prohibiting the Canadian company from selling the component to other parties and, for a certain period of time following termination of the agreement, from transferring any of the intellectual property and design specifications associated with the development of the component to any potential competitors in the Company’s market. |
In July 2005, the Company agreed with the Canadian Company that it will develop and manufacture an additional version of the component. In addition, the initial term of the agreement was extended until April 2012, subject to earlier termination in specified circumstances, with the option to extend annually thereafter for up to five years.
The Company is a party to a development, manufacturing and supply agreement with another supplier (“Supplier”), under which the Supplier has developed a component, that is integrated into the PillCam capsules and is also manufacturing and supplying this component exclusively for the Company. Under this contract, the Supplier may not offer the component as a standard catalog part. In the event that the Supplier ceases operations or enters into liquidation, the Company is entitled to receive all information necessary to manufacture the component upon the payment of reasonable royalties to be agreed upon with the Supplier. The Company has agreed to purchase the enhanced component only from the Supplier and the Supplier has agreed to sell the component exclusively to the Company. The agreement permits the Supplier to disregard the exclusive sales requirement if the Company materially breaches the agreement and fails to cure such breach within a specified time.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 8 - Commitments and Contingencies (cont’d)
D. Agreements with key single - source suppliers and commitments to suppliers (cont’d)
In February 2006, the Company signed an amendment to this agreement and agreed that the Supplier will develop and manufacture an enhanced version of the component. This amendment also extended the initial term of the agreement until November 2012, with an option to extend that term by mutual agreement, thereafter. Under this amendment, the Company has agreed to specified minimum purchase commitments, which the Company may terminate if the supplier fails to satisfy agreed-upon performance criteria.
| | (3) | The Company’s annual commitments under agreements with these two suppliers for the next 5 years are as follows: |
| 2008 | | $ | 6,510 | |
| 2009 | | | 1,500 | |
| 2010 | | | 2,250 | |
| 2011 | | | 2,250 | |
| 2012 | | | 5,130 | |
| | | $ | 17,640 | |
Payments under such agreements with these two suppliers for the years ended December 31, 2005, 2006 and 2007 were $8,568, $8,875 and $6,042, respectively.
E. Patent Litigation
On May 19, 2006, Olympus Corporation, Olympus Medical Systems Corp. and Olympus America Inc., collectively referred to in this section as “Olympus”, filed a complaint against the Company in the District Court for the Eastern District of Pennsylvania. In the complaint, Olympus alleged that the Company’s capsule endoscopes infringe a patent acquired by Olympus from The Boeing Company (the “Boeing Patent”). The Boeing Patent will expire in December 2008. In addition, Olympus sought a declaratory judgment that a capsule endoscope product for which it was then seeking FDA clearance would not infringe the Company’s’ first U.S. Patent, known as the 531 patent, and that the 531 patent is invalid. Olympus also requested an injunction preventing the Company from selling in the United States any product that infringes the Boeing Patent, as well as damages in an unspecified amount.
The Company filed its answer and counterclaim on October 20, 2006. In this answer and counterclaim the Company denied infringement of the Boeing Patent and asserted that the Boeing Patent is invalid. In addition, the Company alleged that the 531 patent is valid and will be infringed by Olympus once it begins offering to sell and selling its capsule endoscopy product in the United States. The Company also alleged that the Olympus product will infringe three other Company patents. The Company also requested an injunction to prevent Olympus from selling in the United States any product that infringes any of the Company’s patents. The Company also stated that, if Olympus sells its capsule endoscopy system in the United States, the Company may request the assessment of damages.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 8 - Commitments and Contingencies (cont’d)
E. Patent Litigation (cont’d)
On March 30, 2007 Olympus filed an amended complaint asserting that the Company’s capsule endoscopes infringe three additional patents owned by Olympus. The Company filed an answer to the amended complaint in April 2007 denying infringement of these patents and alleging their invalidity.
Olympus has recently received FDA clearance to market its competing capsule endoscope system in the United States and has commenced selling activities. As a result, Given has amended its pleadings to add a request for damages in an unspecified amount. In the ongoing litigation, fact discovery ended in January 2008 and expert discovery commenced in February 2008. Trial is set for November 2008.
The outcome of this litigation is uncertain and the Company can not express any opinion as to the outcome at this time. The cost of the ongoing litigation may have a material adverse effect on results of operations, and an unfavorable outcome of this litigation will have a material adverse effect on the Company’s financial position and results of operations.
| | F. | Investment in the Japanese Subsidiary |
In 2006, the Company and its Japanese partners completed additional equity financing of approximately $9,600 to finance the operations of Given Imaging K.K., the Company’s Japanese subsidiary, until it starts generating enough cash to finance its operations. The Company’s portion of the funding of approximately $4,900 was paid out of its cash reserves. Following completion of this additional equity financing, the Company continues to have a controlling interest of 51% of its Japanese subsidiary. Suzuken Co. Ltd., one of Japan’s largest pharmaceutical and medical device distributors and a 15% shareholder of Given Imaging K.K., has exclusive rights to distribute the Company's products in Japan.
G. Provision for Sales Tax
During the year ended December 31, 2005, the Company made a provision of $1,800 for potential uncollectible sales tax, interest and penalties resulting from the failure of the Company’s U.S. subsidiary to appropriately collect and remit sales tax on sales in the U.S. since the fourth quarter of 2001. The provision represented the Company’s estimate of the amounts it might not collect from its customers for remittance to the different jurisdictions, and any interest and penalties the Company may have to pay for failure to timely remit the sales tax. During 2005, 2006 and 2007 the Company has filed all required sales and use tax returns and collected tax amounts from its customers. As of December 31, 2006, and 2007 accounts payable included $702 and $456, respectively, representing sales and use taxes, interest and penalties remaining to be resolved.
H. Registration Rights Agreement
In July 2007, the Company's shareholders approved a Registration Rights Agreement between the Company and its major shareholders holding together an aggregate of 43.9% of the Company's ordinary shares (“affiliated shareholders”). This Registration Rights Agreement has replaced earlier registration rights, which expired in October 2006, granted by the Company in connection with a private placement completed in September 2000, before the Company's initial public offering.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 8 - Commitments and Contingencies (cont’d)
H. Registration Rights Agreement
Under this agreement, at the request of one or more of the affiliated shareholders holding at least 5% of the Company's then outstanding ordinary shares, the Company must use its best efforts to register any or all of these shareholders’ ordinary shares to the extent that the aggregate offering price of the shares to be registered is at least $15 million. In addition, the affiliated shareholders also have the right to request that the Company includes their ordinary shares in any registration statements filed by the Company in the future for the purposes of a public offering, subject to specified limitations. All registration rights will expire on the fifth anniversary of the agreement. With respect to any shareholder, registration rights will expire if that shareholder can sell all of its ordinary shares within a 90 day period under Rule 144 under the United States Securities Act of 1933, as amended. Generally, the Company is obligated to pay all expenses incurred in carrying out the above registrations, as well as the fees and expenses of one legal counsel for the selling shareholders in each registration.
Note 9 - Accounts Payable - Other
| | | December 31 | |
| | | 2006 | | 2007 | |
| Government institutions | | $ | 2,137 | | $ | 4,766 | |
| Liabilities relating to employees | | | 6,863 | | | 8,348 | |
| Advances from customers | | | 58 | | | 21 | |
| Warranty | | | 102 | | | 338 | |
| Royalties to the OCS | | | 214 | | | 0 | |
| Commissions | | | 2,057 | | | 1,659 | |
| Accrued expenses | | | 3,189 | | | 5,880 | |
| | | | | | | | |
| | | $ | 14,620 | | $ | 21,012 | |
Note 10 - Liability in Respect of Employee Severance Payments
Under Israeli law and labor agreements the Company is required to pay severance payments to each employee who was employed by the Company for over one year and has been terminated by the Company or resigned under certain specified circumstances. The Company's liability for severance payments is covered mainly by deposits with insurance companies in the name of the employee and/or by purchase of insurance policies. The liability is calculated on the basis of the latest salary of the employee multiplied by the number of years of employment as of the balance sheet date. The liability for employee severance payments included in the balance sheet represents the total amount due for such severance payment, while the assets held for severance benefits included in the balance sheet represents the Company’s contributions to insurance policies. The Company may make withdrawals from the funds only upon complying with the Israeli severance pay law or labor agreements.
Expenses recorded in respect of employee severance payments for the years ended December 31, 2005, 2006 and 2007 are $664, $862 and $ 806, respectively.
The U.S. subsidiary has a defined contribution retirement plan for its employees. Employees are allowed to contribute up to 18% of their salary in any one year, subject to a regulatory limit.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 10 - Liability in Respect of Employee Severance Payments (cont’d)
The Company contributes 3% of an employee's salary subject to regulatory limits. Employees are vested in the Company's contributions after 30 days of employment.
Expenses recorded in respect of the defined contribution retirement plan in the U.S for the years ended December 31, 2005, 2006 and 2007 are $356 , $603 and $604, respectively.
Note 11 - Share Capital
A. Ordinary shares
All of the issued and outstanding Ordinary Shares of the Company are authorized, issued, fully paid and non-assessable. The Ordinary Shares of the Company are not redeemable and have no preemptive rights. The ownership or voting of Ordinary Shares by non-residents of Israel is not restricted in any way by the Company’s memorandum or articles of association or the laws of the State of Israel, except that citizens of countries which are, or have been, in a state of war with Israel may not be recognized as owners of Ordinary Shares.
| | B. | Employees’ and non employees’ stock options |
In 2003, the Company adopted a stock option plan for directors, employees and consultants. The 2003 Plan replaced and superseded previous option plans adopted by the Company in 1998 and 2000. Under these plans, the Board of Directors (or a compensation committee appointed by the board) (the “Board”) has the authority to grant options to employees of the Company and its subsidiaries, directors or consultants. Each option entitles the holder to purchase one Ordinary Share of par value of NIS 0.05 and expires after 10 years from the date of grant. The company has reserved for issuance a total of 2,500,000 ordinary shares under the plan. As of December 31, 2007, 367,345 options out of this plan had not been granted.
The purchase price of each share pursuant to the options granted under the 2003 Plan shall be the fair market value on the date the Board approves the grant of the option or as otherwise determined by the Board.
Unless otherwise determined by the Board, where a grant of options under the 2003 Plan is the first grant of options made to a person, 50% of the options vest and become exercisable on the second anniversary of the date of grant. An additional 25% of the options vest and become exercisable on each of the third and fourth anniversaries of the date of the grant. If, however, a grant under the 2003 Plan is made to a person who previously received stock options under the 2003 Plan or a previous plan of the Company, 25% of the options granted are immediately vested and exercisable and an additional 25% of the options vest and become exercisable on each of the first, second and third anniversaries of the date of the grant.
In 2006, the Company adopted the 2006 Equity Incentive Plan (“the Plan”) permitting the grant of equity awards, including options and restricted stock of the Company, to eligible employees, directors and consultants of the Company and its subsidiaries. The Plan is administered by the Company’s Board of Directors and Compensation and Nominating Committee. The Plan contains provisions concerning the vesting, price, exercise and other terms of awards; however, the Compensation and Nominating Committee has authority to grant awards under different terms at its discretion. The Company has reserved for issuance a total of 4,000,000 Ordinary Shares under the Plan. As of December 31, 2007 there were 1,968,392 options outstanding under this plan, and 106,000 shares of restricted stock has been issued.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 11 - Share Capital (cont’d)
| | B. | Employees’ and non employees’ stock options (cont’d) |
Equity awards under this plan must be granted at no less than the fair market value of the Company’s ordinary shares on the date of the grant and the term of the awards may not exceed ten years. The Company’s current policy is that options granted under the Plan expire five years following the date of the grant.
Generally, where a grant of an award under the plan is the first grant of equity to an employee or consultant, 50% of the award is exercisable on the second anniversary of the date of grant, and 25% becomes exercisable on each of the third and fourth anniversaries of the date of the grant. In cases of subsequent grants, awards vest in four equal installments beginning with the first anniversary of the grant. To the extent the awards have vested, they may be exercised in whole or in part from time to time until their expiration.
In case of participating employees and consultants, all unvested awards are cancelled upon the termination of their employment or service. All vested awards may be exercised within 180 days following termination. All vested awards not exercised within this period are automatically forfeited and cancelled. Unvested awards to non-employee directors whose service is terminated or discontinued for any reason other than for cause after more than five years of service on the Company’s board of directors, will automatically vest and become exercisable immediately prior to termination or discontinuation of service. These vested awards may be exercised within 180 days following termination or discontinuation of service, except in cases where termination or discontinuation of service is a result of statutory requirements, death, disability or other circumstances of forced cessation of service, in which case awards may be exercised at any time until their expiration date. In a case of termination for cause of a plan participant, all awards, whether vested or unvested, are automatically forfeited and cancelled.
Under this plan, in the event of an acquisition or merger in which the Company is not the surviving entity and the acquiring entity does not agree to assume the awards, all outstanding, but unvested, awards will be accelerated and exercisable, ten days prior to the acquisition or merger. In addition, if the employment of a holder of outstanding awards is terminated in anticipation of or during the 12 month period following an acquisition or merger, all awards that are scheduled to vest within two years of such acquisition or merger, will be automatically accelerated and exercisable, subject to certain adjustments and exceptions.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 11 - Share Capital (cont’d)
| | B. | Employees’ and non employees’ stock options (cont’d) |
Awards granted under the 2006 equity plan to Israeli residents may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the awards or the Ordinary Shares issued upon their exercise must be deposited with a trustee for at least two years following the date of the grant. Under Section 102, any tax payable by an employee from the grant or exercise of the awards is deferred until the transfer of the awards or ordinary shares by the trustee to the employee or upon the sale of the awards or ordinary shares. Gains on awards granted under the plan are subject to capital gains tax of 25% and the Company is not entitled to a tax deduction. Options granted under the plan to U.S. residents may also qualify as incentive stock options (ISO) within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986. Options that do not contain terms that will qualify them as ISOs are treated as Non-Qualified Stock Options.
For the year ended December 31, 2005, the fair value of options granted is estimated on the date of grant, using the Black-Scholes model with the following assumptions:
| 1. | Dividend yield of zero percent. |
| | 2. | Risk-free average interest rate of 3% - 4.3% which represents the risk free rate of US$ zero - coupon Government Bonds. |
| | 3. | Estimated expected life of five years as of the date of grant. |
| | 4. | Expected average volatility of 62%, which represents a weighted average standard deviation rate for the price of the Company’s Ordinary Shares on the NASDAQ National Market. |
The fair value of options granted during the years ended December 31, 2006 and 2007 was estimated on the date of grant using the Black - Scholes model, with the following assumptions:
| 1. | Dividend yield of zero percent. |
| | 2. | Risk free average interest rate of 4.89% and 4.75/% for the years ended December 31, 2006 and 2007, respectively, which represents the risk free rate of US$ zero - coupon Government Bonds. |
| | 3. | Weighted average expected life of 3.69 and 3.75 years for the years ended December 31, 2006 and 2007, respectively, which represents the period for which the options granted are expected to be outstanding. |
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 11 - Share Capital (cont’d)
| | B. | Employees’ and non employees’ stock options (cont’d) |
| | | The expected life of the options granted to employees and directors, is calculated based on the Simplified Method as allowed under Staff Accounting Bulletin No. 107 (SAB 107), giving consideration to the contractual term of the options and their vesting schedules. |
| | 4. | Expected average volatility of 53.17% and 43.17% for the years ended December 31, 2006 and 2007 respectively, which represents a weighted average standard deviation rate for the price of the Company's Ordinary Shares in the NASDAQ National Market. |
The following table summarizes information relating to stock options for Ordinary Shares outstanding and exercisable, as of December 31, 2007 and 2006:
| | Options outstanding | |
Exercise price | | Number outstanding at | | Weighted average remaining | |
| | December 31, 2007 | | contractual life (in years) | |
| $1 - $10 | | | 438,217 | | | 3.73 | |
| $10.01-$20 | | | 1,687,900 | | | 4.64 | |
| $20.01-$30 | | | 2,308,687 | | | 4.81 | |
| $30.01-$40 | | | 499,066 | | | 6.90 | |
| | | | 4,933,870 | | | | |
| | | Options outstanding | |
Exercise price | | Number outstanding at | | Weighted average remaining | |
| | | December 31, 2006 | | contractual life (in years) | |
| $1 - $10 | | | 855,368 | | | 4.86 | |
| $10.01-$20 | | | 1,862,025 | | | 5.65 | |
| $20.01-$30 | | | 889,920 | | | 6.45 | |
| $30.01-$40 | | | 507,291 | | | 7.90 | |
| | | | 4,114,604 | | | | |
The stock option activity under the Plans is as follows:
| | | | | Weighted average | | Weighted average | |
| | Number of shares | | exercise price | | grant date fair value | |
Balance at December 31, 2005 | | | 4,033,160 | | | | | | | |
| Granted | | | 1,077,070 | | $ | 18.75 | | $ | 10.21 | |
| Forfeited | | | (404,616 | ) | | 25.87 | | | 15.29 | |
| Exercised | | | (591,010 | ) | | 3.45 | | | 2.95 | |
Balance at December 31, 2006 | | | 4,114,604 | | | | | | | |
| | | | | | | | | | | |
Balance at December 31, 2006 | | | 4,114,604 | | | | | | | |
| Granted | | | 1,453,392 | | | 26.08 | | | 10.22 | |
| Forfeited | | | (44,864 | ) | | 24.40 | | | 12.26 | |
| Exercised | | | (589,262 | ) | | 7.26 | | | 5.12 | |
Balance at December 31, 2007 | | | 4,933,870 | | | | | | | |
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 11 - Share Capital (cont’d)
B. Employees’ and non employees’ stock options (cont’d)
The following table summarizes information relating to non-vested stock options for Ordinary Shares as of December 31, 2007 and related changes during the year ended December 31, 2007:
| | | | | Weighted average grant | |
| | | Number of Shares | | Date fair value | |
Balance at January 1, 2007 | | | 1,328,787 | | $ | 10.68 | |
| Granted | | | 1,453,392 | | | 10.22 | |
| Vested | | | (534,849 | ) | | 12.69 | |
| Forfeited | | | (44,864 | ) | | 12.26 | |
Balance at December 31, 2007 | | | 2,202,466 | | $ | 9.81 | |
As of December 31, 2007, unrecognized compensation costs related to non-vested options aggregated $16,200 to be recognized over a weighted average period of 1.93 years.
The aggregate intrinsic value of options outstanding as of December 31, 2007 and 2006 is $22,442 and $19,673, respectively. The aggregate intrinsic value of options excisable as of December 31, 2007 and 2006, is $19,293 and $18,778, respectively.
The total intrinsic value of options exercised during the year ended December 31, 2007 and 2006, is $11,039 and $10,610, respectively.
On May 30, 2006, the Company issued 100,000 restricted shares to its CEO. The restricted shares will vest in four installments over a period of four years, beginning on May 30, 2007. On June 15, 2007 the Company issued 6,000 restricted shares to another one of its officers. These restricted shares will vest in three installments over a period of four years, beginning on June 15, 2009. The fair value of the restricted shares as of the date of issue is being amortized over the vesting period. Unrecognized compensation costs related to the restricted shares, as of December 31, 2006 and 2007, to be recognized over 3.4 years, were $1,516 and $1,211 respectively, and compensation expenses of $262 and $466 were recognized for the years ended December, 31 2006 and 2007, respectively.
The following table summarizes the allocation of the stock-based compensation charge for both employee and non-employee stock option grants:
| | | Year ended December 31, | |
| | | 2005 | | 2006 | | 2007 | |
| Research and development costs | | $ | - | | $ | 569 | | $ | 406 | |
| Selling and marketing expenses | | | - | | | 1,839 | | | 1,889 | |
| General and administrative expenses | | | 3 | | | 2,805 | | | 3,356 | |
| | | | | | | | | | | |
| | | $ | 3 | | $ | 5,213 | | $ | 5,651 | |
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 12 - Revenues
A. Revenues by activities
| | | Year ended December 31, | |
| | 2005 | | 2006 | | 2007 | |
| Workstations and recorders | | $ | 17,831 | | $ | 14,104 | | $ | 15,267 | |
| PillCam SB capsule | | | 62,528 | | | 76,360 | | | 90,614 | |
| PillCam ESO capsule | | | 4,384 | | | 1,438 | | | 1,012 | |
| PillCam Colon capsule | | | - | | | - | | | 1,106 | |
| Patency capsules and scanners | | | 174 | | | 353 | | | 523 | |
| Service | | | 1,859 | | | 2,774 | | | 4,346 | |
| | | | | | | | | | | |
| | | $ | 86,776 | | $ | 95,029 | | $ | 112,868 | |
B. Revenues by geographic areas
| | | Year ended December 31, | |
| | | 2005 | | 2006 | | 2007 | |
| Americas | | $ | 64,876 | | $ | 67,648 | | $ | 73,849 | |
| Europe, Middle East and Asia | | | 16,874 | | | 21,642 | | | 27,299 | |
| Asia Pacific | | | 5,026 | | | 5,739 | | | 11,720 | |
| | | | | | | | | | | |
| | | $ | 86,776 | | $ | 95,029 | | $ | 112,868 | |
Note 13 - Financial Income, net
| | | Year ended December 31, | |
| | 2005 | | 2006 | | 2007 | |
| Currency gains (losses) | | $ | (837 | ) | $ | 778 | | $ | 1,456 | |
| Interest income | | | 1,963 | | | 1,639 | | | 1,069 | |
| Income from marketable securities | | | 422 | | | 1,849 | | | 3,109 | |
| Other | | | (786 | ) | | (286 | ) | | (114 | ) |
| | | | | | | | | | | |
| | | $ | 762 | | $ | 3,980 | | $ | 5,520 | |
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 14 - Taxes on Income
A. Company
| | (1) | Israeli income tax is computed on the basis of the Company’s results in New Israeli Shekels (“NIS”) determined for statutory purposes. The Company is assessed for tax purposes under the Income Tax Law (Inflationary Adjustments 1985), the purpose of which is to prevent taxation on inflationary profits. |
Pursuant to the Encouragement Capital Investments Law -1959 (the “law”), the Company was awarded “Approved Enterprise” status under the government alternative benefits track beginning in 1999. The program is for investments in the development of infrastructure and for investments in locally produced and imported equipment. The main benefits to which the Company will be entitled, if it implements all the terms of an approved program, are the exemption from tax on income deriving from an Approved Enterprise, and reduced tax rates on dividends originating from this income.
Under the alternative benefits track, the income derived from an Approved Enterprise will be exempt from tax for a ten-year period, commencing on the date that taxable income is first generated by the Approved Enterprise (limited to the earlier of a maximum period of 12 years from the year of commencement of operations or 14 years from the year the approval letter was received).
Dividend distributions originating from income of an Approved Enterprise will be subject to tax at the rate of 15%, provided that the dividend is distributed during the period stipulated under Israeli law.
In the event of a dividend distribution (including withdrawals and charges that are deemed to be dividends) out of the income originating from the Approved Enterprise, and on which the Company received a tax exemption, the distribution is subject to corporate taxes at rates varying from 10% - 25% depending on the percentage of foreign investment holding in the Company as defined by the Law.
If the Company derives income from sources other than the Approved Enterprise during the relevant period of benefits, such income will be taxable at regular corporate tax rates (see (4) below).
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 14 - Taxes on Income (cont'd)
A. Company (cont'd)
On March 30, 2005, the Knesset approved a reform of the Encouragement of Capital Investments Law - 1959. The primary changes are as follows:
| | a. | Companies that meet the criteria of the Alternate Path of tax benefits will receive those benefits without prior approval. In addition, there will be no requirement to file reports with the Investment Center. Companies will be required to notify the Israeli Income Tax Authorities regarding the implementation of the Alternative Path. Audit will take place via the Income Tax Authorities as part of the tax audits. Request for pre-ruling is possible. |
| | b. | Tax benefits of the Alternate Path include lower tax rates or zero tax depending on the investment zone and the path chosen, lower tax rates on dividends and accelerated depreciation. |
| | c. | In order to receive benefits in the Grant Path or the Alternate Path, the Industrial Enterprise must contribute to the economic independence of Israel’s economy in one of the following ways: |
| | 1. | Its primary activity is in the Biotechnology or Nanotechnology fields and pre-approval is received from the head of research and development at the Office of the Chief Scientist; |
| | 2. | Its revenue from a specific country is not greater than 75% of its total revenues that year; |
| | 3. | 25% or more of its revenues are derived from a specific foreign market of at least 12 million residents. |
The amendments to the Law do not retroactively apply for investment programs having an Approved Enterprise approval certificate from the Investment Center issued up to December 31, 2004 (even when investments under these programs are conducted after January 1, 2005). Consequently, the amendments should not impact an existing Approved Enterprise, which received prior written approval. The new tax regime shall apply for a new Approved Enterprise and for an Approved Enterprise expansion for which the first year of benefits may be as early as 2004.
| | (2) | The Company has net operating loss carryforwards in Israel of approximately $ 4,000 as of December 31, 2007. These net operating loss carryforwards are linked to the Israeli Consumer Price Index and are available to offset future taxable income, if any, indefinitely. |
| | (3) | As explained above, the Israeli Company is exempt from tax for a ten-year period. Therefore, the Israeli Company has not recorded deferred tax assets and liabilities. |
| | (4) | On July 25, 2005, the Knesset passed the Law for the Amendment of the Income Tax Ordinance (No. 147 and Temporary Order) - 2005 (“the Amendment”). |
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 14 - Taxes on Income (cont’d)
The Amendment provides for a gradual reduction in corporate tax rate in the following manner: in 2006 - 31%, 2007 - 29%, 2008 - 27%, 2009 - 26% and from 2010 onward 25%. Furthermore, as from 2010, upon reduction of the corporate tax rate to 25%, capital gains will be subject to tax of 25%.
At December 31, 2007, the subsidiaries had local, federal and state net operating losses carryforwards of approximately $31,144 Federal and state tax loss carryforwards in the U.S subsidiary, totaling $ 10,197 will expire through 2023. Operating loss carryforwards in the Japanese subsidiary, totaling $9,709 will also expire through 2014. Operating loss carryforwards in the German, French and Australian subsidiaries amounted to $5,297, $4,868 and $1,073, respectively, can be carried forward indefinitely.
| | C. | Profit (loss) before tax and income tax benefit (expense) included in the consolidated statements of operations |
| | | Year ended December 31 | |
| | | 2005 | | 2006 | | 2007 | |
Profit (loss) before taxes on income and minority share: | | | | | | | | | | |
| Israel | | $ | 5,011 | | $ | 3,459 | | $ | 13,438 | |
| Foreign jurisdiction | | | (70 | ) | | (6,174 | ) | | 4,792 | |
| | | $ | 4,941 | | $ | (2,715 | ) | $ | 18,230 | |
| | | | | | | | | | | |
Current taxes: | | | | | | | | | | |
| Israel | | $ | - | | $ | (200 | ) | $ | (3,669 | ) |
| Foreign jurisdiction | | | (196 | ) | | (82 | ) | | (855 | ) |
| | | $ | (196 | ) | $ | (282 | ) | $ | (4,524 | ) |
| | | | | | | | | | | |
Deferred taxes: | | | | | | | | | | |
| Israel | | $ | - | | $ | - | | $ | - | |
| Foreign jurisdiction | | | 482 | | | 155 | | | (24 | ) |
| | | $ | 482 | | $ | 155 | | $ | (24 | ) |
| | | | | | | | | | | |
| Income tax benefit (expense) | | $ | 286 | | $ | (127 | ) | $ | (4,548 | ) |
D. Deferred taxes
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible.
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 14 - Taxes on Income (cont’d)
D. Deferred taxes (cont’d)
Management considers projected future taxable income and tax planning strategies in making this assessment.
Based upon projections for future taxable income over the periods in which the deferred tax assets are deductible, management believes it is more likely than not that the Company will realize the benefits of these deductible differences, net of the existing valuation allowances at December 31, 2007. The amount of the deferred tax asset considered realizable, however, could be reduced in the near term if estimates of future taxable income during the carryforward period are reduced.
The tax effects of significant items comprising the Company’s deferred taxes:
| | | December 31 | |
| | | 2006 | | 2007 | |
| | | | | | |
| Tax loss carryforwards of subsidiaries | | $ | 10,137 | | $ | 11,506 | |
| Other timing differences | | | 2,343 | | | 2,397 | |
| | | | | | | | |
| Total gross deferred tax assets | | | 12,480 | | | 13,903 | |
| Valuation allowance | | | (11,106 | ) | | (12,553 | ) |
| | | | | | | | |
| Net deferred tax asset | | $ | 1,374 | | $ | 1,350 | |
The net changes in the total valuation allowance for the years ended December 31, 2005, 2006 and 2007 are ($2,779), $789 and $1,446, respectively.
E. Reconciliation of the statutory tax benefit (expense) to actual income tax benefit (expense)
| | | Year ended December 31, | |
| | | 2005 | | 2006 | | 2007 | |
| Profit (loss) before taxes on income and minority share | | $ | 4,941 | | $ | (2,715 | ) | $ | 18,230 | |
| Tax rate | | | 0 | % | | 0 | % | | 0 | % |
| Computed expected tax | | | - | | | - | | | - | |
| Increase in unrecognized tax benefits associated with current year positions | | | - | | | - | | | (2,901 | ) |
| Differences between the definition of | | | | | | | | | | |
| capital and assets for tax purposes and other | | | - | | | (200 | ) | | 1,256 | |
| Change in valuation allowance | | | 2,779 | | | (789 | ) | | (1,446 | ) |
| Foreign tax rate differential | | | (2,493 | ) | | 862 | | | (1,457 | ) |
| | | | | | | | | | | |
| Income tax benefit (expense) | | $ | 286 | | $ | (127 | ) | $ | (4,548 | ) |
Given Imaging Ltd. and its subsidiaries
Notes To The Consolidated Financial Statements
(In thousands except share and per share data)
Note 14 - Taxes on Income (cont’d)
F. Accounting for income tax uncertainties
The Company and its subsidiaries file income tax returns in Israel, the U.S and other foreign jurisdictions. The U.S. subsidiary files income tax returns in federal jurisdictions, and various states within the U.S. With few exceptions, the Company is no longer subject to Israeli, U.S. federal, state and local, or non-U.S. income tax examinations by tax authorities for years before 2003. The German tax authorities commenced an examination of the Company's subsidiary income tax returns in Germany for the years 2001 through 2004 that is anticipated to be completed by the end of 2008 with no material tax adjustments.
The Company adopted the provisions of FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes, on January 1, 2007. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Balance at January 1, 2007 | | $ | - | |
| Additions based on tax positions related to the current year | | | 2,901 | |
| Additions for tax positions of prior years | | | - | |
| Reductions for tax positions of prior years | | | - | |
| Settlements | | | - | |
| Balance at December 31, 2007 | | $ | 2,901 | |
The unrecognized tax benefits in the amount of $2,901, if recognized, would affect the effective tax rate of the Company.
During the year ended December 31, 2007, the Company recorded approximately $99 in interest relating to unrecognized tax benefits in the consolidated statements of operations and accrued the same amount in the balance sheet as of December 31, 2007.
Note 15 - Fair Value of Financial Instruments
The Company's financial instruments include cash and cash equivalents, accounts receivable, deposits, assets held for severance benefits, marketable securities and accounts payable. Except for marketable securities considering the short term nature of these financial instruments, their carrying amounts approximate fair value. The fair value of the Company’s marketable securities is disclosed in Note 5.