As filed with the Securities and Exchange Commission on March 25, 2005
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
| £ | | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | |
| OR |
| | | |
| S | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | |
| FOR THE FISCAL YEAR ENDED DECEMBER 31, 2004 |
| | | |
| OR |
| | | |
| £ | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
GIVEN IMAGING LTD.
(Exact name of Registrant as specified in its charter)
Israel
(Jurisdiction of incorporation or organization)
13 Ha'Yetzira Street
Yoqneam 20692, Israel
(Address of principal executive offices)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
None.
Securities registered or to be registered pursuant to Section 12(g) of the Act:
Ordinary Shares, par value NIS 0.05 per share
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None.
The number of outstanding shares of each of the issuer's classes of capital or
common stock as of December 31, 2004:
27,621,386 Ordinary Shares, par value NIS 0.05 per share
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes S No £
Indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 £ Item 18 S
TABLE OF CONTENTS
The GIVEN logo, GIVEN, PillCam, PILLCAM, the PillCam logo, ORDERWIN, ORDER WHEN I NEED, RAPID, PILLCAM IMAGING CAPSULE and design, FINGERS HOLDING A CAPSULE logo and, FINGERS HOLDING PILLCAM CAPSULE logo, are our trademarks or registered trademarks. All other registered trademarks appearing herein are owned by their holders.
i
PART I
Item 1. Identity of Directors, Senior Management and Advisors
Not Applicable.
Item 2. Offer Statistics and Expected Timetable
Not Applicable.
Item 3. Key Information
The selected consolidated statements of operations data for the years ended December 31, 2002, 2003 and 2004, and the selected consolidated balance sheet data as of December 31, 2003 and 2004, have been derived from our audited consolidated financial statements set forth elsewhere in this Form 20-F. These financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America. The selected consolidated statements of operations data for the years ended December 31, 2000 and 2001, and the selected consolidated balance sheet data as of December 31, 2000, 2001 and 2002, have been derived from our audited consolidated financial statements not included in this Form 20-F and have also been prepared in accordance with generally accepted accounting principles in the United States of America. You should read the selected consolidated financial information set forth below in conjunction with our consolidated financial statements and the related notes as well as Item 5 “Operating and Financial Review and Prospects” included elsewhere in this Annual Report on Form 20-F.
| | | Year ended December 31,
|
| | | 2000
| | 2001
| | 2002
| | 2003
| | 2004
|
| | | (in thousands, except share and per share data) |
Statements of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Revenues | | $ | — | | | $ | 4,733 | | | $ | 28,904 | | | $ | 40,539 | | | $ | 65,020 | |
Cost of revenues | | | — | | | | 2,476 | | | | 11,907 | | | | 13,551 | | | | 17,734 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Gross profit | | | — | | | | 2,257 | | | | 16,997 | | | | 26,988 | | | | 47,286 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development, gross | | | (4,137 | ) | | | (6,156 | ) | | | (8,609 | ) | | | (7,037 | ) | | | (7,363 | ) |
Royalty-bearing participation | | | 312 | | | | 44 | | | | — | | | | 1,303 | | | | 1,140 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Research and development, net | | | (3,825 | ) | | | (6,112 | ) | | | (8,609 | ) | | | (5,734 | ) | | | (6,223 | ) |
Sales and marketing | | | (2,923 | ) | | | (12,902 | ) | | | (22,681 | ) | | | (26,804 | ) | | | (33,652 | ) |
General and administrative | | | (1,224 | ) | | | (2,664 | ) | | | (4,749 | ) | | | (5,312 | ) | | | (6,916 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Total operating expenses | | | (7,972 | ) | | | (21,678 | ) | | | (36,039 | ) | | | (37,850 | ) | | | (46,791 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Operating profit (loss) | | | (7,972 | ) | | | (19,421 | ) | | | (19,042 | ) | | | (10,862 | ) | | | 495 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Financing income, net | | | 433 | | | | 764 | | | | 1,469 | | | | 995 | | | | 956 | |
Other expenses, net | | | — | | | | — | | | | (711 | ) | | | — | | | | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Profit (loss) before taxes on income | | | (7,539 | ) | | | (18,657 | ) | | | (18,284 | ) | | | (9,867 | ) | | | 1,451 | |
Taxes on income | | | — | | | | — | | | | — | | | | — | | | | 690 | |
Profit (loss) before minority share | | | (7,539 | ) | | | (18,657 | ) | | | (18,284 | ) | | | (9,867 | ) | | | 2,141 | |
Minority share in losses (profits) of subsidiary | | | — | | | | — | | | | (26 | ) | | | 258 | | | | 747 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Net profit (loss) | | $ | (7,539 | ) | | $ | (18,657 | ) | | $ | (18,310 | ) | | $ | (9,609 | ) | | $ | 2,888 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Basic profit (loss) per ordinary share(1) | | $ | (1.14 | ) | | $ | (1.60 | ) | | $ | (0.73 | ) | | $ | (0.38 | ) | | $ | 0.11 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Diluted profit (loss) per ordinary share(1) | | $ | (1.14 | ) | | $ | (1.60 | ) | | $ | (0.73 | ) | | $ | (0.38 | ) | | $ | 0.10 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Ordinary shares used in computing basic profit (loss) per ordinary share(1) | | | 8,804,188 | | | | 12,879,369 | | | | 25,182,563 | | | | 25,493,073 | | | | 26,633,964 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Ordinary shares used in computing diluted profit (loss) per ordinary share(1) | | | 8,804,188 | | | | 12,879,369 | | | | 25,182,563 | | | | 25,493,073 | | | | 29,353,448 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
1
| | | As of December 31,
|
| | | 2000
| | 2001
| | 2002
| | 2003
| | 2004
|
| | | (in thousands) |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 21,360 | | | $ | 61,230 | | | $ | 35,792 | | | $ | 25,367 | | | $ | 80,861 | |
Working capital | | | 20,584 | | | | 62,891 | | | | 43,972 | | | | 34,970 | | | | 92,987 | |
Total assets | | | 25,530 | | | | 75,923 | | | | 68,728 | | | | 55,569 | | | | 124,224 | |
Long-term liabilities, net of current portion | | | 317 | | | | 522 | | | | 882 | | | | 1,192 | | | | 10,984 | |
Total liabilities | | | 2,455 | | | | 6,590 | | | | 12,969 | | | | 8,847 | | | | 28,430 | |
Accumulated deficit | | | (12,059 | ) | | | (30,716 | ) | | | (49,026 | ) | | | (58,635 | ) | | | (55,747 | ) |
Total shareholders' equity | | | 23,075 | | | | 69,333 | | | | 53,577 | | | | 44,798 | | | | 94,617 | |
| | | | | | | | | | | | | | | | | | | | |
| | | |
| (1) | | See Notes 1K and 10 of the notes to our consolidated financial statements for an explanation of the number of shares used in computing per share data. |
CAUTIONARY LANGUAGE REGARDING FORWARD-LOOKING STATEMENTS
This Form 20-F contains forward-looking statements. We have based these forward-looking statements on our current expectations and projections about future events. These statements include but are not limited to:
| • | the adequacy of our cash balances and cash flow from operations to support our operations or future growth, in general or for specified periods of time; |
| |
| • | expectations as to raising additional financing; |
| |
| • | statements as to the potential or expected expansion in acceptance of our current and future products by the medical community, particularly gastroenterologists; |
| |
| • | statements as to expected increases in sales, operating results and certain expenses, including research and development and sales and marketing expenses; |
| |
| • | statements as to anticipated reimbursement from U.S. and non-U.S. healthcare payors for our products; |
| |
| • | expectations as to the development of our products and technology, and the timing of enhancements to our products and new product launches; |
| |
| • | expectations as to the market opportunities for the Given System and other products, including capsule endoscopes, for visualizing other sections of the gastrointestinal tract, such as the esophagus, stomach and the colon, as well as our ability to take advantage of those opportunities; |
| |
| • | expectations as to the timing and content of future clinical studies and publications; |
| |
| • | statements as to the expected outcome of legal and patent proceedings in which we are involved; |
| |
| • | expectations as to the receipt and timing of regulatory clearances and approvals, and the anticipated timing of sales of the Given System in new markets or for new indications; |
| |
| • | estimates of the impact of changes in currency exchange rates on our operating results; |
| |
| • | expectations as to the adequacy of our inventory of critical components and finished products; |
| |
| • | expectations as to the adequacy of our manufacturing facilities and the timing of any expansion; and |
| |
| • | statements as to our expected treatment under Israeli and U.S. federal tax legislation and the impact that new Israeli tax and corporate legislation may have on our operations. |
2
In addition, statements that use the terms “believe”, “expect”, “plan”, “intend”, “estimate”, “anticipate” and similar expressions are intended to identify forward-looking statements. All forward-looking statements in this Form 20-F reflect our current views about future events and are based on assumptions and are subject to risks and uncertainties that could cause our actual results to differ materially from future results expressed or implied by the forward-looking statements. Many of these factors are beyond our ability to control or predict. You should not put undue reliance on any forward-looking statements. Unless we are required to do so under U.S. federal securities laws or other applicable laws, we do not intend to update or revise any forward-looking statements.
3
RISK FACTORS
If we are unable to achieve broader penetration of the Given System among gastroenterologists, we may not be able to maintain our current growth rate.
Our principal product is the Given System, consisting of the PillCam capsule and the related data recorder and computer workstation. In 2004, we sold 654 Given Systems and approximately 90,400 PillCam SB capsules compared to 631 Given Systems and approximately 51,400 PillCam SB capsules in 2003. At the end of November 2004, we began marketing our new PillCam ESO capsule following receipt of marketing clearance from the U.S. Food and Drug Administration, or FDA. As of December 31, 2004, we had sold approximately 2,290 systems and 172,000 PillCam SB capsules. Growth in our installed base of Given Systems has contributed to our revenue growth. If we are unable to continue to achieve broad penetration among gastroenterologists, we may be unable to maintain our current growth rate. Our success in making sales of the Given System depends on a number of factors. First, we must demonstrate that the Given System is clinically-effective and cost-effective in diagnosing a range of disorders of the gastrointestinal tract, and we must inform and educate both physicians and the third-party payors responsible for providing reimbursement coverage about the clinical and economic benefits of using the Given System. Second, we must broaden the indications for which the use of the Given System is reimbursed, expand the number of lives with such reimbursement coverage and educate healthcare providers as to the clinical and economic benefits of using the Given System for such broader indications so that they are encouraged to adopt the Given System. Third, we must convince third party payors to provide reimbursement coverage for capsule endoscopy as a primary diagnostic tool and not only after a previous procedure, such as endoscopy or radiology, has been performed. If we are unable to increase reimbursement coverage, expand the indications for which reimbursement is granted and convince more physicians to adopt the Given System, future penetration of the Given System among gastroenterologists may not be sufficient to maintain our current growth rate.
If we are unable to increase PillCam capsule usage rates among gastroenterologists, we may not be able to maintain our current growth rate.
A substantial portion of our revenue growth to date has resulted from increased sales of the PillCam SB capsule. Sales of the PillCam SB capsule contributed $10.9 million, or 37.7%, in 2002, $22.9 million, or 56.4%, in 2003, and $41.6 million, or 64%, in 2004. While growth in the installed base of Given Systems is one factor that contributes to growth in sales of the PillCam capsules, another factor is the number of capsules that are used during a given time period on each Given System, referred to as the usage rate. The usage rate per system in 2004 was approximately 1.15 PillCam SB capsules per system per week in the United States and 0.9 PillCam SB capsules per system per week globally (including the United States). The usage rate per system during the last three years has varied slightly but has generally been in the range of 0.9 to 1.2 PillCam SB capsules per system per week in the United States and in the range of between 0.6 to 0.9 PillCam SB capsule per system per week globally (including the United States). The usage rate outside the United States has historically been lower than in the United States. We are seeking to increase the usage rate by a number of methods, including increased selling and marketing activities, more frequent contact with our customers, attaining reimbursement coverage for broader indications, educating physicians regarding the clinical benefits of the PillCam capsule, increasing operating efficiencies of our sytem to the benefit of physicians and collaborating with strategic industry participants, such as InScope, a division of Ethicon Endo-Surgery, a Johnson & Johnson company, to market our new PillCam ESO capsule. Increasing the usage rate of our customers is also important to attracting new customers to purchase and use the Given System. If we are unable to increase the usage rate of the Given System, we may not be able to generate the revenues necessary to maintain our growth.
4
We may be unable to maintain or increase our profitability.
We were formed in 1998 and recorded our initial sales of the Given System in the third quarter of 2001. We achieved positive cash flow in the fourth quarter of 2003 and became profitable in the second quarter of 2004; however, we have significant operating expenses and must continue increasing our revenues from sales of the Given System and of the PillCam capsules at a higher rate than the increase in our expenses in order to maintain and grow our profits. If we fail to do so, we may be unable to maintain or increase our profitability.
If we are unable to expand reimbursement coverage from third-party healthcare payors for procedures using the Given System, or if reimbursement is insufficient to cover the costs of purchasing or using the Given System when compared to alternative procedures, demand for the Given System may not continue to grow.
Demand for the Given System depends significantly on the eligibility of the procedures performed using the Given System for reimbursement through government-sponsored healthcare payment systems and private third-party payors. In general, the process of obtaining reimbursement coverage approvals has been slower outside of the United States. Reimbursement practices vary significantly from country to country and within some countries, by region, and we must obtain reimbursement approvals on a country-by-country and region-by-region basis. Historically, we have experienced higher sales in territories in which we have received reimbursement for capsule endoscopy using the Given System and in territories in which health authorities and regulators approved the marketing or use of capsule endoscopy. We may not be able to obtain further approvals in a timely manner or at all. If physicians, hospitals and other healthcare providers are unable to obtain sufficient coverage and reimbursement from third-party payors for procedures using the Given System, or if reimbursement is insufficient to cover the costs of purchasing or using our product or does not adequately compensate physicians and health care providers compared to the other procedures they offer, we may be unable to generate sufficient sales to continue to grow.
Our future growth depends in part on our ability to market the Given System and the PillCam SB capsule for a variety of disorders of the small intestine.
The Given System and the PillCam SB capsule have been cleared for marketing by the FDA for the detection of disorders of the small intestine. However, in order to achieve sustained long-term growth we must successfully market the Given System for detection of a variety of disorders of the small intestine with high prevalence. Use of the Given System is highly dependent on the availability of third party reimbursement. Initially, most reimbursement policies issued for the Given System in the United States by third party payors covered its use only for detection of suspected bleeding in the small intestine, which represents a small part of the total population with suspected disorders of the small intestine. Currently, however, most reimbursement policies cover other prevalent indications in the small intestine, such as suspected Crohn's disease. As of December 31, 2004, the total insured population in the United States covered for capsule endoscopy for these expanded indications was approximately 179 million. Our ability to further expand the use of the Given System depends substantially on our ability to demonstrate to additional governmental and private third-party payors the diagnostic and cost-effectiveness of the Given System for other disorders of the small intestine. If third-party payors do not accept our data as supporting coverage and reimbursement for the detection of other disorders of the small intestine, our ability to sell the Given System for the detection of these disorders would be harmed and our growth would be adversely affected.
5
We currently depend on the Given System and the PillCam SB and our new PillCam ESO capsules for substantially all of our revenues. If we are unable to manufacture, market or sell the Given System or the PillCam capsules, our financial condition and results of operations would be materially adversely affected.
Sales of the Given System and our PillCam SB capsule for visualization of the small intestine currently account for substantially all of our revenues. In addition, in the third quarter of 2004, we launched our new PillCam ESO video capsule in Europe and in the fourth quarter of 2004 we launched this product in the United States. If we are unable to manufacture, market or sell the Given System or the PillCam SB and PillCam ESO video capsules, our financial condition and results of operations would be materially adversely affected.
Our future growth also depends in part on the success of Ethicon Endo-Surgery in marketing our new PillCam ESO video capsule, particularly in the United States.
In May 2004, we entered into an exclusive sales representation and co-promotion agreement with Ethicon Endo-Surgery, a Johnson & Johnson company. InScope, a division of Ethicon Endo-Surgery established to market products to the gastroenterology market, has exclusive rights to market our PillCam ESO capsule. The alliance initially is worldwide, excluding Japan. InScope has begun marketing PillCam ESO capsule in the United States but has not yet commenced marketing activities in other territories, which may subsequently be excluded if we do not reach agreement with InScope regarding the marketing activities of InScope in these other territories by March 31, 2005 or a later date that may be agreed upon by the parties. Under the agreement, InScope is responsible for the marketing of PillCam ESO capsule, as well as for obtaining reimbursement coverage for the PillCam ESO capsule. In September 2004, we launched PillCam ESO capsule in Europe and in November 2004 we received FDA clearance. Consequently, we depend on the success of InScope in marketing the PillCam ESO capsule and obtaining reimbursement coverage for this capsule. If InScope is not successful, our sales of the PillCam ESO capsule may be lower than expected and our revenues may suffer.
Any disruption in the United States, our primary market for our products, may result in a material reduction in our revenues and negatively affect our results of operations.
Since we began selling the Given System in 2001, most of our revenues have been generated from sales in the United States. Sales in the United States accounted for 15.6 million, or 54%, of our revenues in 2002, 26.2 million, or 65%, of our revenues in 2003 and 46.7 million, or 72%, of our revenues in 2004. Any disruption to our market in the United States resulting from adverse changes in reimbursement policies, regulatory requirements, macro-economic changes and other events, many of which are outside our control, may result in a material reduction in our revenues and negatively affect our results of operations.
We may be unable to introduce other products for use in other parts of the gastrointestinal tract.
Our objective is to expand the use of the Given System by developing and introducing new capsules for visualizing other parts of the gastrointestinal tract, including the stomach and the colon. There can be no assurance of widespread market acceptance of the Given System as superior to existing technologies for detection of abnormalities in other parts of the gastrointestinal tract or that we will succeed in marketing and selling capsules for use in other parts of the gastrointestinal tract. In addition, we will be required to obtain FDA clearance in the United States and other regulatory approvals outside of the United States before commercially distributing the Given System for use in other parts of the gastrointestinal tract or introducing new products for use in the gastrointestinal tract. These regulatory processes can be lengthy and expensive and we cannot be sure that FDA clearance or other regulatory approvals will be granted. In order to obtain FDA clearance and other regulatory approvals, and to obtain reimbursement coverage for use of the Given System in other parts of the gastrointestinal tract, we will be required to conduct additional clinical trials to demonstrate the diagnostic and cost-effectiveness of the Given System.
6
If future clinical trials indicate that the Given System is not as clinically-effective or cost-effective in these parts of the gastrointestinal tract as current methods, or that it causes unexpected complications or other unforeseen negative effects, we may not obtain regulatory clearance to market and sell the Given System for use in other parts of the gastrointestinal tract or obtain reimbursement coverage, and our growth would be adversely affected.
Changes in healthcare system policies may make it difficult for physicians, hospitals and other healthcare providers to obtain adequate reimbursement for procedures using the Given System, which could adversely affect demand for the Given System.
Many healthcare payors have adopted a managed care system in which they contract to provide comprehensive healthcare for a fixed cost per person, irrespective of the amount of care actually provided. Therefore, the amount of reimbursement provided may not be sufficient to encourage physicians to purchase or utilize the Given System. We are unable to predict what changes will be made in the reimbursement policies of third-party payors. We could be adversely affected by changes in reimbursement policies of governmental or private healthcare payors to the extent any such changes affect reimbursement amounts or methods for procedures in which the Given System is used.
Because of the importance of our patent portfolio to our business, we may lose market share to our competitors if we fail to protect our intellectual property rights.
Protection of our patent portfolio is key to our future success. We rely on patent protection, as well as a combination of copyright, trade secret, design and trademark laws, nondisclosure and confidentiality agreements and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Currently, many of our patent applications are still pending and we will be able to use them to protect our technology against potential competitors only when they are issued. The process of issuing a patent may sometimes be lengthy and may not always result in issued patents in a form that will be advantageous to us or at all. In addition, once issued, patents may be challenged, invalidated or circumvented by third parties. Our patents and applications cover particular aspects of our products and technology and may be challenged, invalidated or circumvented by third parties. There may be more effective technologies, designs or methods. If the most effective method is not covered by our patents or applications and our competitors are able to commercialize products using these methods, it could have an adverse effect on our sales. We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by former employees. The laws and judiciary systems of foreign countries also may not protect or enable enforcement of our intellectual property rights to the same extent as the laws of the United States. Even if we adequately protect our intellectual property rights, litigation may be necessary to enforce these rights. This litigation could be lengthy, result in substantial costs to us and a substantial diversion of management attention and allow our competitors to gain significant market position in the meantime. If we do not adequately protect our intellectual property, our competitors or other parties could use the intellectual property that we have developed to enhance their products or make products similar to ours and compete more efficiently with us, which could result in a decrease in our market share.
Because the medical device industry is litigious, we are susceptible to intellectual property suits that could cause us to incur substantial costs or pay substantial damages or prohibit us from selling our products.
There is a substantial amount of litigation over patent and other intellectual property rights in the medical device industry. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often uncertain. While we have attempted to ensure that the Given System does not infringe other parties' valid patents and proprietary rights, searches typically performed to identify potentially infringed patents of third parties are not always conclusive and, because patent applications can take many years to issue, there may be
7
applications now pending of which we are unaware, which may later result in issued patents which our current or future products may infringe. In addition, our competitors or other parties may assert that our product and the methods it employs may be covered by patents held by them. If our products infringe a valid patent, we could be prevented from selling them unless we can obtain a license or redesign the product to avoid infringement. A license may not always be available or may require us to pay substantial royalties. We also may not be successful in any attempt to redesign our product to avoid any infringement. Infringement and other intellectual property claims, with or without merit, can be expensive and time-consuming to litigate and can divert management's attention from operating our business.
We face competition from large, well-established manufacturers of existing technologies for detecting gastrointestinal disorders, as well as from gastrointestinal products in general which compete for the limited capital expenditure budgets of customers.
We consider the current competition for the Given System to be existing technologies for detecting gastrointestinal disorders and diseases. Existing technologies include traditional endoscopy and radiological imaging. The principal manufacturers of gastrointestinal endoscopes are Olympus, Asahi Optical Co., which manufactures and markets cameras and other imaging equipment under the Pentax brand, and Fujinon. The principal manufacturers of equipment for radiological imaging are General Electric Healthcare Systems, Siemens Medical Solutions, a division of Siemens AG, Philips Medical Systems Ltd., Toshiba Corporation, Shimadzu Corporation and Trex Medical Corporation for x-ray equipment. These companies have substantially greater financial resources than we do, and they have established reputations as well as worldwide distribution channels for medical instruments to physicians. If we are unable to convince physicians to adopt the Given System over the current technologies marketed by our competitors, our results of operations will suffer. We are aware of research and development efforts by some of these companies, including Olympus, and other individuals and companies to develop and bring to market imaging capsules or other less invasive imaging techniques that may be competitive with the Given System. In addition to competition from products performing similar clinical functions to the Given System, there is also competition for the limited capital expenditure budgets of customers. Another capital equipment item for gastroenterology that is priced similarly to our system may compete with our system for the same hospital capital budget, which is typically limited, and therefore the potential purchaser may be required to choose between the two items of capital equipment. If we are unable to market the Given System more effectively than other products which could be purchased using the same budget as the Given System, we may be unable to maintain our current growth rate.
We will be required to increase manufacturing quantities of the Given System and could encounter manufacturing problems or delays that could result in lost revenue.
As of December 31, 2004, we had manufactured and sold approximately 172,000 PillCam SB capsules and approximately 3,400 PillCam ESO capsules, which we started to manufacture and sell in the fall of 2004 following FDA clearance. In 2005, we expect to increase the sales of PillCam SB capsules and will have the first full year of production of PillCam ESO capsules. We are using two semi-automatic production lines to manufacture PillCam SB capsules and one semi-automated production line to manufacture PillCam ESO capsules. We expect to complete the construction of a second production line for PillCam ESO capsules in the second quarter of 2005. These four production lines are installed at our manufacturing facilities in Yoqneam, Israel. Each semi-automatic production line has the capacity to manufacture approximately 180,000 PillCam capsules per year. However, if demand for the PillCam capsule continues to grow we may have to manufacture PillCam capsules in quantities that we have not manufactured to date. We have installed a back-up semi-automatic production line for PillCam SB capsules at the facilities of Pemstar, Inc. in Ireland and are constructing another backup line for PillCam ESO capsules in this facility. We may be unable to establish or maintain reliable, high-volume manufacturing capacity and may encounter difficulties in scaling up production of the Given System, whether by installing
8
one or more fully-automated production lines, or otherwise. If demand for the Given System exceeds our manufacturing capacity, we could develop a substantial backlog of customer orders and our ability to generate revenues will be limited and our reputation in the marketplace may be harmed.
We are subject to extensive regulation by the FDA which could restrict the sale and marketing of the Given System and could cause us to incur significant costs.
The U.S. Food and Drug Administration, or FDA, regulations prohibit us from promoting or advertising the Given System, or any other devices that the FDA may clear in the future, for uses not within the scope of our clearances or making unsupported safety and effectiveness claims. Currently, the Given System has been cleared by the FDA for the detection of abnormalities of the small intestine and the esophagus. These determinations can be subjective, and the FDA may disagree with our promotional claims. Noncompliance with applicable regulatory requirements can result in enforcement action which may include recalling products, ceasing product marketing, paying significant fines and penalties, and similar FDA actions which could limit product sales, delay or halt product shipment, delay new product clearance or approval, and materially adversely affect our financial condition and results of operations. Unanticipated changes in existing regulatory requirements or adoption of new requirements could materially adversely affect our financial condition and results of operations.
We are required to adhere to the FDA's Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. The FDA also requires us to adhere to the Quality System Regulation, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging and shipping of the Given System. The FDA enforces the Quality System Regulation through inspections. Our quality system has passed four audits, including a re-certification audit, pursuant to the Medical Devices Directive of the European Union, the International Standard Organization's standard ISO 9001:2000, ISO 13485:1996 and ISO 13485:2003, which are quality standards setting forth requirements for medical device manufacturers that are more specific than the general requirements specified in ISO 9001. While these quality standards contain requirements which are generally similar to the Quality System Regulation required by the FDA, we have never been through a Quality System Regulation inspection by the FDA and cannot assure you that we would pass. If we fail a Quality System Regulation inspection, our operations could be disrupted and our manufacturing delayed. Failure to take adequate corrective action in response to a Quality System Regulation inspection could force a shutdown of our manufacturing operations and a recall of the Given System, which would have a material adverse effect on our product sales, financial condition and results of operations.
If we or our distributors do not obtain and maintain the necessary regulatory approvals in a specific country or region, we will not be able to market and sell the Given System in that country or region.
In addition to the United States, Germany, France, Australia and Israel, where we market and sell the Given System directly with our own direct sales and marketing organizations, we sell the Given System in more than 50 other countries. To be able to market and sell the Given System in a specific country or region, we or our distributors must comply with the regulations of that country or region. While the regulations of some countries do not impose barriers to marketing and selling the Given System or only require notification, others require that we or our distributors obtain the approval of a specified regulatory body. These regulations, including the requirements for approvals, and the time required for regulatory review vary from country to country. Obtaining regulatory approvals is expensive and time-consuming, and we cannot be certain that we or our distributors will receive regulatory approvals in each country or region in which we plan to market our products. If we modify the Given System, we or our distributors may need to
9
apply for new regulatory approvals before we are permitted to sell it. We may not continue to meet the quality and safety standards required to maintain the authorizations that we or our distributors have received. If we or our distributors are unable to maintain our authorizations in a particular country or region, we will no longer be able to sell our products in that country or region, and our ability to generate revenues will be materially adversely affected.
Our failure to comply with radio frequency regulations in a specific country or region could impair our ability to commercially distribute and market the Given System in that country or region.
The Given System includes a wireless radio frequency transmitter and receiver, and is therefore subject to equipment authorization requirements in a number of countries and regions. In the United States, the Federal Communications Commission, or FCC, requires advance clearance of all radio frequency devices before they can be sold or marketed in the United States. The FCC granted our equipment certification application for the Given System in March 2001 based on the current system design and specifications. Modifications to the Given System may require new or further FCC approval before we are permitted to market and sell a modified system, and it could take several months to obtain any necessary FCC approval.
In Europe, the frequency range in which the Given System operates is subject to technical standards for radio frequency use developed by the Short Range Device Maintenance Group of the European Conference of Postal and Telecommunications Administrations. In February 2002, the Short Range Device Maintenance Group modified the technical standards for the specific radio frequency that the Given System uses to redefine the period of time during which any device can operate continuously in that frequency, known as the “duty cycle.” This change permits the Given System to transmit for more than 10% of the time that it is in operation, which is necessary for the Given System to function. On March 19, 2004, the Electronics Communications Commission of the European Conference of Postal and Telecommunications Administration adopted this technical standard as a decision. The Short Range Device Maintenance Group standards and European Conference of Postal and Telecommunications Administrations decisions generally are not legally binding, and must be implemented into national laws by European governments to become binding. As of December 31, 2004, records of the European Conference of Postal and Telecommunications Administrations indicate that only two European governments, Italy and Poland, have indicated that they will not implement the new technical standards for the duty cycle. Prior to the implementation of the new technical standards change described above or if the technical standards are not implemented in a timely manner, we will seek to obtain waivers or separate authorizations to operate our system in each individual European country, either in advance or if our compliance with spectrum requirements is questioned. If those countries do not implement the technical standards, or we are unable to receive such a waiver or a separate authorization or to redesign our transmitter to operate on a radio frequency that is not subject to a limit on the duty cycle, an enforcement action could be brought to prevent the sale or use of the Given System in these countries. Any such action could negatively affect our results of operations.
Some of our activities may subject us to risks under federal and state laws prohibiting “kickbacks” and false or fraudulent claims.
A federal law commonly known as the Medicare/Medicaid anti-kickback law, and several similar state laws, prohibit payments that are intended to induce physicians or others either to refer patients or to acquire or arrange for or recommend the acquisition of health care products or services. While the federal law applies only to referrals, products or services for which payment may be made by a federal health care program, state laws often apply regardless of whether federal funds may be involved. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices, such as us, by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians, and other potential purchasers of medical devices. Other federal and state laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare,
10
Medicaid, or other third-party payors that are false or fraudulent, or are for items or services that were not provided as claimed. Since we provide some coding and billing advice to purchasers of our products, and since we cannot be sure that the government will regard any billing errors that may be made as inadvertent, these laws are potentially applicable to us. Anti-kickback and false claims laws prescribe civil and criminal penalties for noncompliance that can be substantial. Even an unsuccessful challenge could cause adverse publicity and be costly to respond to, and thus could have a material adverse effect on our business, results of operations and financial condition.
We have experienced rapid growth and our failure to manage this growth could harm our business.
Our revenues have grown rapidly and we face significant challenges and risks in building and managing our sales and marketing team, including managing geographically dispersed sales efforts and hiring and adequately training our sales people in the use and benefits of the Given System. To succeed in the implementation of our business strategy and maintain our growth, our management team must diligently plan and rapidly execute our sales and marketing strategy, while continuing our research and development, regulatory, clinical and manufacturing activities, as well as manage our continued growth by implementing effective long term planning. If we are unable to effectively plan, manage and execute these efforts, our business may be harmed.
We rely on local distributors to market and distribute the Given System in most of the territories in which we sell it.
With the exception of Australia, France, Germany, Israel and the United States, we rely on distributors for the marketing and distribution of the Given System. Under most of our agreements with local distributors, a distributor is granted the right to market the Given System for an initial period of approximately three years in a particular country or region, subject to the attainment of minimum sales targets. In 2003, we extended the initial term of some of our distributors by an additional two years, subject to updated minimum sales commitments. The distributor is required to prepare and submit to us for our approval a sales plan for the Given System and to obtain the requisite regulatory and reimbursement approvals for the Given System. Our success in generating sales in countries or regions where we have engaged local distributors depends in part on the efforts of others whom we do not control. In 2004, we derived $9.8 million, or 15%, of our revenues from sales to local distributors, compared to $7.3 million, or 18%, from sales to local distributors in 2003. To date, we have changed a small number of our distributors due to a failure to meet minimum sales targets. If a distributor is terminated by us or goes out of business, it may take us a period of time to locate an alternative distributor and to train its personnel to market the Given System and our ability to sell the Given System in that distributor's country or region could be adversely affected.
Our reliance on single source suppliers could harm our ability to meet demand for the Given System in a timely manner or within budget.
We depend on single source suppliers for some of the components necessary for the production of the Given System. Specifically, Micron Technology, Inc., through its Micron Imaging Group and other wholly-owned subsidiaries, is currently the sole supplier of the imaging sensor and the packaging for the sensor, and Zarlink Semiconductors is currently the sole supplier of the transmitter that is integrated into the PillCam capsules. If the supply of these components is disrupted or terminated, or if these suppliers are unable to supply the quantities of components that we require, we may not be able to find alternative sources for these key components. Although we maintain a strategic inventory of key components, the inventory many not be sufficient to satisfy the demand for our products if supply is interrupted, and is subject to risk of loss due to catastrophic events such as fire at a storage facility. As a result, we may be unable to meet demand for our products, which could harm our ability to generate revenues, lead to customer dissatisfaction and damage our reputation. If we are required to change the manufacturer of any of these key components of the Given System, there may be a significant delay in locating a suitable alternative manufacturer. Additionally, we may be required to verify that the new
11
manufacturer maintains facilities and procedures that comply with FDA and other applicable quality standards and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer could delay our ability to manufacture our product in a timely manner or within budget. Furthermore, in the event that the manufacturer of a key component of our product ceases operations or otherwise ceases to do business with us, we may not have access to the information necessary to enable another supplier to manufacture the component. The occurrence of any of these events could harm our ability to meet demand for the Given System in a timely manner or within budget.
Conditions in Israel affect our operations and may limit our ability to produce and sell our product which could decrease our revenues.
Our corporate offices, our manufacturing facilities (other than our backup production lines in Ireland), and our research and development facilities are located in Israel. Political, economic and military conditions in Israel directly affect our operations. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors and a state of hostility, varying in degree and intensity, has led to security and economic problems for Israel. Since October 2000, there has been a significant increase in terrorist violence in Israel, the West Bank and the Gaza Strip, including armed hostilities between Israel, the Palestinian Authority and other groups in the West Bank and Gaza Strip. Any further escalation in these hostilities or any future armed conflict, political instability or violence in the region, could have a negative effect on our business condition, harm our results of operations and adversely affect the share price of publicly traded Israeli companies such as us.
If we lose our key personnel or are unable to attract and retain additional personnel, our business and ability to compete will be harmed.
We are dependent on the principal members of our management and scientific staff. In order to implement our business strategy, we will need to keep our key personnel with expertise in research and development, clinical testing, government regulation, manufacturing, sales, marketing and finance. Our product development plans depend in part on our ability to retain engineers with expertise in a variety of technical fields. The loss of a number of these persons or our inability to attract and retain qualified personnel could harm our business and our ability to compete.
Our operations could be disrupted as a result of the obligation of key personnel in Israel to perform military service.
Generally, all male adult citizens and permanent residents of Israel are, unless exempt, obligated to take part in up to 30 days (and sometimes as many as 36 days) of military reserve duty annually. Many of our male employees are currently obligated to perform annual reserve duty, as are two of our executive officers. Additionally, all Israeli residents who perform reserve duty are subject to being called to active duty at any time under emergency circumstances. Our operations could be disrupted by the absence for a significant period of one or more of our officers or employees due to military service. Any such disruption to our operations could adversely affect the development of our business and our financial condition.
Our international operations will expose us to the risk of fluctuations in currency exchange rates.
In 2004, we derived 75.1% of our revenues in U.S. dollars and 20.5% in the Euro, with the remainder denominated in other currencies. The currency denomination of our revenues depends on the location of the customer or the distributor used to fulfill our customers' orders. Conversely, in 2004, in addition to our U.S. dollar and Euro-denominated liabilities, 28.0% of our expenses were denominated in New Israeli Shekels, or shekels. Our shekel-denominated liabilities consist principally of salaries and related personnel expenses. We anticipate that for the foreseeable future a material portion of our liabilities will continue to be denominated in shekels. If the value of a currency in which our receivables are denominated devalues against the value of a currency in
12
which our liabilities are denominated, there will be a negative impact on our profit margin for sales of the Given System. Our revenues and expenses may not always be fully hedged against our currency exposure through financial instruments. In addition, if we wish to maintain the dollar-denominated value of our product in non-U.S. markets, devaluation in the local currencies of our customers relative to the U.S. dollar could cause our customers to cancel or decrease orders or default on payment. In addition, as of December 31, 2004, 10.1% of our cash and cash equivalents were denominated in currencies other than the U.S. dollar and we are therefore subject to the risk of exchange rate fluctuations between the Yen, the New Israeli Shekel, the dollar and the Euro.
The use of the Given System, including ingestion of the PillCam capsules, could result in product liability claims that could be expensive, damage our reputation and harm our business.
Our business exposes us to an inherent risk of potential product liability claims related to the manufacturing, marketing and sale of medical devices. The medical device industry has historically been litigious, and we face financial exposure to product liability claims if the use of our product were to cause or contribute to injury or death, whether by aggravating existing patient symptoms or otherwise. There is also the possibility that defects in the design or manufacture of the Given System might necessitate a product recall. Although we maintain product liability insurance for the Given System, the coverage limits of these policies may not be adequate to cover future claims. Particularly as sales of the Given System increase, we may be unable to maintain product liability insurance on acceptable terms or at reasonable costs and such insurance may not provide us with adequate coverage against potential liabilities. A successful claim brought against us in excess of, or outside of, our insurance coverage could have a material adverse effect on our financial condition and results of operations. A product liability claim, regardless of merit or ultimate outcome, or any product recall could result in substantial costs to us and a substantial diversion of management attention.
Our quarterly financial performance is likely to vary in the future, and may not meet our guidance or the expectations of analysts or investors, which may lead to additional volatility in our share price.
Our ordinary shares commenced trading on the Nasdaq National Market in October 2001 and on the Tel Aviv Stock Exchange in March 2004. In 2004, the closing price of our shares has ranged from $17.87 to $44.08 per share on the Nasdaq National Market and NIS 123.90 to NIS 194.60 on the Tel Aviv Stock Exchange. The price of our shares could fluctuate significantly for, among other things, the following reasons: future announcements concerning us or our competitors, changes in third-party reimbursement practices, regulatory developments, and new clinical or economic data regarding our current or future products. In addition, it is our practice to provide guidance to the market as to our expected revenues and earnings per share based on information available to us at the time of the guidance. If our quarterly operating results do not meet our guidance or the expectations of securities analysts or investors, the price of our shares would likely decline. In addition, based on our experience to date, we believe that many of our customers delay purchasing systems until the end of the fiscal quarter because they believe this will enable them to negotiate more favorable terms. Therefore, revenues from system sales may be concentrated at the end of each fiscal quarter making it difficult for us to determine the success of each quarter until its end and resulting in lower than expected quarterly revenues if external or other events cause a large number of potential customers to defer their purchasing decisions even for a short period of time. Furthermore, we believe that demand for systems and capsules may be materially affected by seasonal factors during the summer months when physicians and administrators are more likely to postpone purchasing decisions relating to systems due to summer vacations and patients are more likely to postpone less urgent diagnostic procedures until later in the year. Both of these factors may result in slower sales during the summer. Share price fluctuations may be exaggerated by low trading volume of our ordinary shares and changes in trading practices in our ordinary shares, such as short buying and selling. Securities class action litigation has often been brought against companies following periods of volatility. Any securities
13
litigation claims brought against us could result in substantial expense and divert management's attention from our business.
The two largest beneficial owners of our shares, IDB Holding Corporation Ltd., which is controlled by four individuals, and certain funds affiliated with OrbiMed Advisors, Inc., which we refer to as the OrbiMed investors and which are controlled by Samuel D. Isaly, have significant influence over matters requiring shareholder approval, which could, among other things, delay or prevent a change of control.
The largest beneficial owners of our shares, four individuals in Israel through IDB Holding Corporation Ltd., beneficially own 37.2% of our ordinary shares, and the principal of the OrbiMed investors beneficially owns approximately 13.2% of our ordinary shares. As a result, these shareholders, if they were to act together, could exercise a controlling influence over our operations and business strategy and have sufficient voting power to control the outcome of matters requiring shareholder approval. These matters may include
| • | the composition of our board of directors which has the authority to direct our business and to appoint and remove our officers; |
| |
| • | approving or rejecting a merger, consolidation or other business combination; |
| |
| • | raising future capital; and |
| |
| • | amending our articles of association which govern the rights attached to our ordinary shares. |
This concentration of ownership of our ordinary shares could delay or prevent proxy contests, mergers, tender offers, open-market purchase programs or other purchases of our ordinary shares that might otherwise give our other shareholders the opportunity to realize a premium over the then-prevailing market price of our ordinary shares. This concentration of ownership may also adversely affect our share price.
Future sales of our ordinary shares in the public market and low trading volume could adversely affect our share price.
As of December 31, 2004, we had 27.6 million ordinary shares outstanding. Approximately 50% of these shares are “restricted securities” available for resale on the Nasdaq National Market subject, however, to volume limitations under Rule 144. In addition, all of our ordinary shares are available for resale on the Tel Aviv Stock Exchange, subject to compliance with Regulation S under the Securities Act of 1933. Future sales of these restricted shares, or the perception that these sales could occur, could adversely affect the market price of our ordinary shares. We have periodically experienced a low trading volume of our ordinary shares, and if one or a small number of parties buys or sells a large number of our ordinary shares, we may experience volatility in our share price and our share price may be adversely affected. In addition, the holders of approximately 13 million ordinary shares are entitled to request that we register their ordinary shares under the Securities Act of 1933 and have the right to request that we include their shares in any registration statement that we file, in each case, subject to exclusion for marketing reasons. Registration of these shares would result in them becoming freely tradable without restriction immediately upon the effectiveness of such registration, except for shares purchased by affiliates, which may be sold subject to the volume limitations of Rule 144. These factors could also make it more difficult for us to raise additional funds through future offerings of our ordinary shares or other securities.
Our ordinary shares are traded on more than one market and this may result in price variations.
Our ordinary shares are traded on the Nasdaq National Market and the Tel Aviv Stock Exchange. Trading in our ordinary shares on these markets is made in different currencies (dollars on the Nasdaq National Market, and Israeli shekels on the Tel Aviv Stock Exchange) and at different times (due to different time zones, trading days and public holidays in the United States and Israel). The trading prices of our ordinary shares on these two markets may differ due to
14
these and other factors. Any decrease in the trading price of our ordinary shares on one of these markets could cause a decrease in the trading price of our ordinary shares on the other market.
If we are characterized as a passive foreign investment company, our U.S. shareholders may suffer adverse tax consequences.
If, for any taxable year, our passive income, or our assets which produce passive income, exceed specified levels, we may be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. This characterization could result in adverse U.S. tax consequences for our U.S. shareholders, which may include having gains realized on the sale of our ordinary shares treated as ordinary income, rather than as capital gains income, having potentially punitive interest charges apply to those gains, and the denial of the taxation of certain dividends paid by us at the lower rates applicable to long-term capital gains. We believe we were not a PFIC for the fiscal year ended December 31, 2004. However, the tests for determining PFIC status are applied annually and are based in part on reference to the market value of our shares and valuing our intangible assets using the methods prescribed for publicly traded corporations, and it is difficult to make accurate predictions of future income and assets, which are relevant to this decision. Accordingly, we cannot give any assurance that we will not become a PFIC, in particular, since the value of our shares is likely to fluctuate. For a more detailed discussion of the consequences of our being classified as a PFIC, see “Taxation and Government Programs—United States Federal Income Taxation—Passive Foreign Investment Company Considerations.” U.S. shareholders are urged to consult with their own U.S. tax advisors with respect to the U.S. tax consequences of investing in our ordinary shares.
The government grants we have received for research and development expenditures limit our ability to manufacture products and transfer technologies outside of Israel and require us to satisfy specified conditions. If we fail to satisfy these conditions, we may be required to refund grants previously received together with interest and penalties, and may be subject to criminal charges.
From 1998 through 2004, we received grants totaling $3.6 million from the government of Israel through the Office of the Chief Scientist of the Ministry of Industry and Trade for the financing of a portion of our research and development expenditures in Israel. The terms of the Chief Scientist grants prohibit us from manufacturing products developed using these grants outside of Israel without special approvals and completely prohibit the transferring of our technologies and related intellectual property rights outside of Israel. Even if we receive approval to manufacture the Given System outside of Israel, we would be required to pay an increased total amount of royalties, which may be up to 300% of the grant amount plus interest, depending on the manufacturing volume that is performed outside of Israel, as well as a possible increased rate or royalties. This restriction may impair our ability to outsource manufacturing, engage in change in control transactions or otherwise transferring our technology outside Israel. We currently hold an approval from the Office of the Chief Scientist to manufacture limited quantities of the PillCam SB capsule in Pemstar's facilities in Ireland without an increase in royalty rates and we will be required to obtain an approval before manufacturing the PillCam ESO capsule outside of Israel. In addition, if we fail to comply with any of the conditions imposed by the Office of the Chief Scientist, we may be required to refund any grants previously received together with interest and penalties, and may be subject to criminal charges. In recent years, the government of Israel has accelerated the rate of repayment of Chief Scientist grants and may further accelerate them in the future.
We receive significant tax benefits that may be reduced or eliminated in the future.
Our investment program in leasehold improvements and equipment at our manufacturing facility in Yoqneam, Israel has been granted approved enterprise status and we are therefore eligible for significant tax benefits under the Israeli Law for Encouragement of Capital Investments. From time to time, the government of Israel has considered reducing or eliminating the tax benefits available to approved enterprise programs such as ours. These tax benefits may
15
not be continued in the future at their current levels or at all. If these tax benefits were reduced or eliminated, the amount of taxes that we pay would likely increase. In addition, our approved enterprise status imposes certain requirements on us, such as the location of our manufacturing facility, location of certain subcontractors and the extent to which we may outsource portions of our production process. If we do not meet these requirements, the law permits the authorities to cancel the tax benefits retroactively. See Item 10 “Additional Information—Taxation.”
It may be difficult to enforce a U.S. judgment against us, our officers and directors or to assert U.S. securities laws claims in Israel or serve process on our officers and directors.
We are incorporated in Israel. The majority of our executive officers and directors are not residents of the United States, and the majority of our assets and the assets of these persons are located outside the United States. Therefore, it may be difficult for an investor, or any other person or entity, to enforce a U.S. court judgment based upon the civil liability provisions of the U.S. federal securities laws in an Israeli court against us or any of these persons or to effect service of process upon these persons in the United States. Additionally, it may be difficult for an investor, or any other person or entity, to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws because Israel is not the most appropriate forum to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel addressing the matters described above.
Under current Israeli law, we may not be able to enforce covenants not to compete and therefore may be unable to prevent competitors from benefiting from the expertise of some of our former employees involved in research and development activities.
We currently have non-competition agreements with substantially all of our employees who are involved in research and development, nearly all of whom are located in Israel. These agreements prohibit our employees, if they cease working for us, from directly competing with us or working for our competitors for a limited period of time following termination of employment. Israeli courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company's confidential commercial information or its intellectual property. If we cannot demonstrate that harm would be caused to us, we may be unable to prevent our competitors from benefiting from the expertise of our former employees.
16
Item 4. Information on the Company
A. History and Development of the Company
We were incorporated by RDC Rafael Development Corporation under the laws of the State of Israel in January 1998. We are registered with the Israeli registrar of companies in Jerusalem. Our registration number is 51-257802-2. Our address is 13 Ha' Yetzira Street, Yoqneam 20692, Israel. Our telephone number is (011) 972-4-909-7777. Our agent in the United States is our subsidiary Given Imaging, Inc. Given Imaging, Inc.'s address is Oakbrook Technology Center, 5555 Oakbrook Parkway, No. 355, Norcross, GA 30093.
See Items 5 and 18 for a description of capital expenditures by us for the past three fiscal years. We have not made any divestitures during the same time period.
B. Business Overview
We develop, manufacture and market innovative diagnostic products for disorders of the gastrointestinal tract. We pioneered capsule endoscopy, a proprietary approach to visual examination of the gastrointestinal tract through the use of a miniaturized video camera contained in a disposable capsule. Our principal product, which incorporates our core technology, is the Given System, a proprietary wireless imaging system that uses our disposable video capsules, which we refer to as the PillCam capsules. The PillCam video capsules are easily ingested by the patient and move naturally through the gastrointestinal tract without discomfort while wirelessly transmitting high quality color images and data to a portable recorder. We believe that capsule endoscopy provides a patient-friendly solution that addresses a significant market opportunity and overcomes many of the shortcomings of traditional diagnostic tools for gastrointestinal disorders. In 2001, we commenced marketing the Given System, our capsule endoscopy platform, with the M2A capsule (which we rebranded in 2004 as the PillCam Small Bowel capsule, or PillCam SB), for detection of disorders of the small bowel. As of December 31, 2004, we had an installed base of approximately 2,290 Given Systems and had sold approximately 172,000 PillCam SB capsules in over 50 countries worldwide. In November 2004, we received FDA marketing clearance for our second PillCam capsule, the PillCam ESO capsule, for visualizing the esophagus. We market the PillCam ESO video capsule through a strategic marketing and sales alliance we formed in May 2004 with InScope, a division of Ethicon Endo-Surgery, a Johnson & Johnson company. We have also developed the Patency Capsule and system, which is a dissolvable capsule that enables physicians to determine whether there are obstructions or strictures in the gastrointestinal tract. We launched the Patency Capsule and system in Europe in November 2003 and are conducting clinical trials for the Patency Capsule and system in the United States and, if results are favorable, we plan to pursue FDA clearance.
We are continuing to work on enhancements to the Given System. For example, in November 2004, simultaneously with the launch of our new PillCam ESO video capsule, we launched RAPID 3.0, an advanced version of our RAPID software and DR 2.0, an advanced version of our data recorder. The new RAPID software and data recorder provide further efficiencies to the physician, including increased reliability of the data recorder, shortening the download time from the data recorder to the workstation and improving physician productivity. We believe that the efficiencies created by these improvements will increase PillCam capsule utilization by users of the Given System and provide us with a significant competitive advantage. We intend to continue developing and bringing to market enhancements to the Given System that further improve system performance and assist users in the diagnostic process.
Our long-term objective, subject to further development and receipt of regulatory clearances and/or approvals, is to establish the Given System as the first tool administered to patients with suspected disorders of the small intestine, the esophagus or other parts of the gastrointestinal tract, such as the stomach and the colon. We believe that each such segment of the gastrointestinal tract presents meaningful opportunities for patient-friendly diagnostic procedures. In furtherance of this objective, we intend to continue our research and development activities and clinical trials to
17
expand the applications for our capsule endoscopy platform, including to the stomach and the colon, and, if the results are positive, pursue regulatory clearance and/or approvals in the United States and elsewhere.
Disorders of the Gastrointestinal Tract
The gastrointestinal tract is a series of organs in the body responsible for digesting food. These organs principally include the mouth, esophagus, stomach, small intestine and colon. The following is an illustration of the gastrointestinal tract:
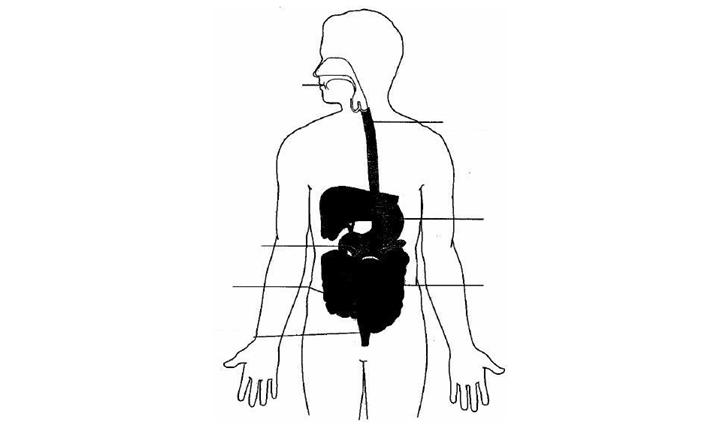
Duodenum
Ileum
Anal canal
Jejunum
Stomach
Esophagus
Oral cavity
Large Intestine
Small
Intestine
The upper gastrointestinal tract consists of the mouth, esophagus, stomach and duodenum, which is the first portion of the small intestine. The esophagus is an approximately ten inch long tube that connects the throat and the stomach. The stomach is a sac-like organ that produces enzymes to break down food. The small intestine is an approximately 21 foot long hollow organ that is primarily responsible for digesting food. The three parts of the small intestine are the duodenum, the jejunum and the ileum. The small intestine is located in the abdominal cavity between the stomach and the large intestine, or colon. The lower gastrointestinal tract consists of the lower two-thirds of the small intestine (the jejunum and the ileum) and the colon. The colon is the final portion of the gastrointestinal tract and is primarily responsible for absorbing water before waste is excreted.
The gastrointestinal tract is susceptible to various disorders. Typical symptoms of such disorders include heartburn, upper or lower abdominal pain, bleeding, diarrhea, constipation, anemia, weight loss and nausea. Some of these symptoms are not specific to any particular disorder, but may be common to more than one underlying disorder, often requiring the gastroenterologist to make a differential diagnosis. We believe that PillCam capsule endoscopy can have a significant role in assisting gastroenterologists in making an evidence-based diagnosis. The disorders causing the above-mentioned symptoms may include:
| • | inflammatory bowel disease, including Crohn's disease and ulcerative colitis, both of which inflame the lining of the digestive tract; |
| |
| • | celiac disease, which causes damage to the intestine due to an allergic reaction to gluten in the diet; |
18
| • | Gastro-esophageal reflux disease, which is the flow-back of acidic stomach content into the esophagus, causing esophagitis, which is inflammation of the esophageal lining, or Barrett's esophagus, a pre-cancerous condition; |
| |
| • | gastritis, which is inflammation of the lining of the stomach; |
| |
| • | irritable bowel syndrome, which is a functional disorder characterized by abdominal pain or cramping and changes in bowel function without any organic manifestation; |
| |
| • | peptic ulcer disease, which occurs when the lining of the stomach or duodenum is worn away by stomach acid; and |
| |
| • | growths, such as tumor or polyps, which may be cancerous. |
Our products currently assist in the diagnosis of disorders of the small intestine and the esophagus.
| • | Small intestine disorders. According to data from the American Gastroenterology Association, or AGA, approximately 19 million Americans suffer from numerous disorders of the small intestine, including bleeding, Crohn's disease, celiac disease, chronic diarrhea, irritable bowel syndrome and small bowel cancer. Based on SMG Marketing Group research, it is estimated that more than one million diagnostic procedures to examine the small intestine were performed in 2001. Prior to the development of capsule endoscopy, there was no convenient and effective method for visualizing the interior of the entire small intestine. As a result, we believe that many gastroenterologists first examine the colon or stomach before ordering an examination of the small intestine. |
| |
| • | Esophageal disorders. GERD is the frequent backward flow, or reflux, of stomach contents into the esophagus due to an improperly functioning valve between the stomach and esophagus. Stomach acid, enzymes and bile irritate the esophagus and cause a wide range of symptoms and complications, most commonly persistent, severe heartburn and chest pain. According to AGA data, an estimated 61 million Americans suffer from heartburn at least once a month and there are approximately 9 million physician office visits each year diagnosing GERD. If left untreated, GERD can lead to ulceration of the esophagus, respiratory problems or esophageal cancer. It is estimated that approximately 10% to 15% of patients with GERD symptoms have Barrett's esophagus, a pre-cancerous condition with an associated risk for esophageal cancer. In the United States alone, according to the American Cancer Society, there were expected to be approximately 14,250 new cases of esophageal cancer in 2004. Another serious disorder of the esophagus is known as esophageal varices, a life-threatening complication caused by severe bleeding from small veins in the eshophagus, usually associated with chronic liver disease. Esophageal varices are present in about 40% of patients with cirrhosis of the liver, which has been reported to affect 400,000 Americans based on data from the National Digestive Disease Information Clearinghouse. The risk of death with the first variceal bleed is approximately 20%-25%. Thus, it is very important to screen patients with cirrhosis of the liver for the presence of varices and to monitor those patients with known varices. We believe that the PillCam ESO capsule may provide an effective method for screening for and detecting esophageal varices. |
Current Detection Methods for Gastrointestinal Disorders
The most common methods for detection of gastrointestinal disorders, including disorders of the small intestine, are endoscopy and radiological imaging.
Traditional Endoscopy
A traditional endoscope is a device consisting of a flexible tube and an optical system. There are several types of endoscopic procedures used to identify disorders in the gastrointestinal tract. The basic endoscopic procedures available include:
| • | Upper endoscopy. In upper endoscopy, the physician inserts an endoscope, which is an approximately 3.5 foot long tube, through the patient's mouth. In esophagogastroduodenoscopy, or EGD, the gastroscope passes down the esophagus and into the stomach and duodenum for visual examination. In esophagoscopy, only the esophagus is viewed. |
19
| • | Colonoscopy. In colonoscopy, the physician inserts a colonoscope, which is an approximately 5.5 foot long endoscope, into the patient's colon through the anus. Colonoscopy is the primary method for detecting disorders of the colon. Because of the increasing awareness of colon cancer, colonoscopy as a screening tool for early detection of colon cancer is becoming more common. |
| |
| • | Enteroscopy. Enteroscopy involves the visualization of the small intestine using an endoscope. There are two principal methods of enteroscopy: |
| |
| |
| • | Push enteroscopy. Push enteroscopy involves the insertion of an approximately six foot long push enteroscope into the mouth. Due to the length and curvature of the small intestine, push enteroscopy enables the physician to view only the first one-third of the small intestine. The procedure is lengthy and difficult to perform for both the physician and the patient and is substantially more complicated than gastroscopy. |
| |
| • | Sonde enteroscopy. Sonde enteroscopy involves the insertion of an approximately nine foot long enteroscope through the nose. A gastroscope is simultaneously inserted into the mouth to assist in placement of the enteroscope through the pylorus, the valve connecting the stomach to the small intestine. The gastroscope is then withdrawn and the balloon at the end of the sonde enteroscope is expanded. The patient is required to stay in bed in the hospital for seven to twelve hours while the sonde enteroscope is drawn through the small intestine by the natural contractions of the gastrointestinal tract. When the tip of the enteroscope reaches the end of the small intestine, the air is released from the balloon and, as the sonde enteroscope is withdrawn through the nose of the patient, the gastroenterologist views images of the small intestine in real time. As a result of the introduction of capsule endoscopy and due to the extreme discomfort resulting from the procedure, sonde enteroscopy is no longer performed in the United States. |
A traditional endoscope can perform both diagnostic and limited treatment functions. In a traditional endoscopic procedure, the physician is able to control the movement of the endoscope through the gastrointestinal tract, to stop the endoscope and examine more closely a particular area in the gastrointestinal tract and to take a tissue sample or seal a bleeding site using the endoscope. However, traditional endoscopy has some risks and limitations, including the following:
| • | Requires sedation. Due to the need to insert a tube through the mouth or anus, a traditional endoscopic examination typically requires sedation of the patient due to patient discomfort. |
| |
| • | Involves potential complications. Potential complications of traditional endoscopic procedures include difficulty in breathing while the tube is inserted in the mouth, perforation or tearing of the intestinal wall, post-procedural infection and vomiting, abdominal swelling, sore throat, diarrhea and cross-contamination resulting from inadequate disinfection of endoscopes. Certain clinical studies have reported that perforation rates range between 0.01% and 1.9% for esophagoscopy, between 0.1% and 0.7% for esophagogastroduodenoscopy, or EGD, and between 0.01% and 0.3% for colonoscopy. These studies report that mortality rates range between zero and 0.98% for esophagoscopy, between zero and 0.07% for esophagogastroduodenoscopy, and between 0.01 and 0.02% for colonoscopy. To date, there have been no reports of perforation or mortality attributed to the capsule endoscopy procedure. |
| |
| • | Causes patient anxiety, discomfort and pain. Many patients are unwilling to undergo traditional endoscopic procedures due to the pain and discomfort associated with having a tube inserted through the mouth or anus. |
| |
| • | Requires substantial time commitment. Patients undergoing a traditional endoscopic procedure are required to remain in the physician's office or hospital during the procedure. In addition, the effects of the sedation cause the patient to be inactive for several hours following the procedure. |
20
Radiological Imaging
Radiological imaging is a commonly used method for initial detection of the small intestine and other parts of the gastrointestinal tract. Radiological imaging is used for detection of disorders of the esophagus only in limited situations, generally where gross structural lesions are suspected. During a radiological imaging examination, the patient swallows a contrast medium (such as barium), which is a dense liquid that coats the internal organs and makes them appear on x-ray film. The procedure produces a series of black and white x-ray images of the lumen, or cavity, of the small intestine.
A more detailed examination, the double contrast small intestine enema, or enteroclysis, requires insertion of a tube through the mouth or nose, which is then pushed through the stomach and duodenum. High density barium and then methyl cellulose, a gel-like material used to expand the intestine, are injected through the tube into the patient's small intestine prior to a series of x-ray exposures.
Radiological imaging also has risks and limitations as a diagnostic tool, including the following:
| • | Cannot provide direct imaging of the mucosa. Radiological imaging does not provide a detailed view of soft tissue, including the mucosa, or internal layer of the gastrointestinal tract. In addition, radiological imaging does not provide clear visualization of ulcerations or flat malignant lesions. A lesion must have a certain mass and a distinguishable shape in order to be detected by radiology. |
| |
| • | Has difficulty detecting small pathologies. Radiological imaging has difficulty detecting smaller-sized disorders or pathologies (typically less than five millimeters in diameter). Larger pathologies of up to ten millimeters in diameter can also remain undetected. |
| |
| • | Has difficulty detecting strictures. Radiological imaging has difficulty detecting strictures—which are a three dimensional phenomena, and with a two-dimensional image this is not easily detected and frequently misdiagnosed. |
| |
| • | Causes patient discomfort. Radiological imaging is uncomfortable for patients, requiring them to drink barium, which has an unpleasant chalky taste. A double contrast procedure is even more uncomfortable due to the insertion of a tube into the body through the mouth or nose. These preparatory measures can induce vomiting, particularly in a double contrast procedure if the injected contrast liquids return to the stomach. In elderly patients, the passage of barium can be difficult and can result in blockage requiring the use of disimpaction techniques. There is some morbidity associated with barium induced blockage in elderly patients. |
| |
| • | Exposes patient to radiation. Radiological imaging poses increased risks of exposure to ionizing radiation for the patient. A double contrast procedure requires three to five times the amount of radiation to the patient. Tracking the progress of a disorder through repeated radiological imaging increases this risk. |
The Given Imaging Solution
The Given System features the PillCam capsule endoscope, a miniaturized video camera contained in a disposable capsule that is naturally ingested by the patient and delivers high quality color video images of the inside of the gastrointestinal tract in a painless manner. Capsule endoscopy with our PillCam capsules represents a fundamentally new approach to visual examination of the gastrointestinal tract and provides a solution to many of the shortcomings of other procedures by offering the following benefits:
| • | Patient-friendly procedure with no sedation. Capsule endoscopy provides a patient-friendly and painless tool for the diagnosis of patients that present symptoms of suspected disorders of the small intestine and the esophagus. The PillCam capsule requires no sedation or patient preparation (other than a typical overnight fast in the case of the PillCam SB capsule), is easily ingested by the patient and does not use x-rays to produce images. We believe that this patient-friendly solution will increase the number of patients who undergo diagnosis for gastrointestinal disorders of the small intestine and the esophagus, since existing methods have been intimidating or uncomfortable for many potential candidates. |
21
| • | Improved or comparable diagnostic yield. The PillCam SB capsule is the only test that provides direct imaging of the entire small intestine. By comparison, a push enteroscope accesses only approximately the first third of the small intestine. As a result, clinical trials demonstrate that the Given System has a significantly higher diagnostic yield in detecting disorders of the small intestine when compared to all other modalities, including push enteroscopy and radiological imaging. In addition, as demonstrated through clinical studies and on-going experience with the Given System, a negative finding from the Given System, unlike conventional diagnostic techniques, has significant diagnostic value as it may allow physicians to rule out the existence of certain suspected abnormalities based on this finding thereby avoiding the need to engage in additional costly or inconvenient diagnostic procedures. Data from clinical trials reviewed by the FDA for clearing PillCam ESO demonstrated that the diagnostic efficacy of the Given System in detecting disorders of the esophagus is comparable to the diagnostic efficacy of traditional endoscopy. |
| |
| • | Administered on an outpatient basis. The PillCam capsule is generally administered in an outpatient setting with a brief visit to the physician's clinic or hospital. In the case of the PillCam SB capsule, the patient can go about his or her daily routine as the capsule transmits images and other data to the portable data recorder. In the case of the PillCam ESO capsule, the procedure takes up to twenty minutes. We believe the Given System offers a significant opportunity for gastroenterologists and endoscopy departments to expand their business by increasing the number of procedures performed at their office or facility. |
| |
| • | Detects small pathologies. Unlike radiological imaging procedures, the PillCam video capsule provides direct visualization of the intestinal mucosa which allows detailed (up to 0.1 millimeter) visualization of small pathologies. This increases the possibility of detecting and diagnosing at an early stage pathologies that might otherwise go undetected. |
| |
| • | Natural passage requires no insufflation. Many traditional endoscopic procedures require insufflation, or the forcing of air into the gastrointestinal tract. This process can cause considerable patient discomfort. The Given System does not require insufflation because the PillCam capsule is ingested and moves with the natural contractions of the digestive tract. The absence of insufflation allows the PillCam capsule to capture images of the gastrointestinal tract in its normal physiological state. This approach, called “physiological endoscopy,” allows the physician to clearly view the mucosa. |
| |
| • | Provides convenient digital reporting, storage and remote consulting capabilities. The physician can review the video produced by the Given System without seeing the patient or having him or her remain in the office or clinic during the review, thereby providing the physician with greater flexibility. In addition, the RAPID software includes various features that enhance the physician's efficiency and productivity, such as innovative display methods for faster review, localizing findings, help in identifying anatomical landmarks for easy orientation, managing images and patient information, reporting modules and convenient options for sending still images or short video files to the patient file, to the referring physician or a colleague for consultation. Some of these features are proprietary and covered by patent applications, which we believe add an additional competitive advantage as physicians become more used to using these functions. |
| |
| • | Provides a cost-effective diagnostic tool. We believe that the PillCam SB capsule endoscopy procedure is more cost-effective from a third party payor perspective than traditional methods for imaging the gastrointestinal tract. With respect to the PillCam SB capsule for the small bowel, two economic outcomes studies reported by the Office of Health Policy and Clinical Outcomes of the Thomas Jefferson University in Philadelphia concluded that diagnosing small intestinal bleeding or Crohn's disease using the PillCam SB capsule procedure is cost-effective from a third party payor perspective. We believe that the Given System may result in additional cost savings due to the reduction in physician resources and facility costs permitted by the outpatient nature of the PillCam SB capsule procedure, its higher diagnostic yield—in the case of the PillCam SB capsule—and the potential for earlier diagnosis of disorders, allowing earlier therapeutic intervention or other change in patient management. |
22
The Given System also has some limitations. The PillCam capsule moves naturally through the gastrointestinal tract; consequently, the capsule's passage is not controlled by the physician who cannot stop or steer the capsule for close-up detailed viewing of suspected disorders. In addition, the Given System, unlike a traditional endoscope, cannot take biopsies or be used for minor surgical procedures, such as cauterizing bleeding sites in the gastrointestinal tract. While endoscopes may be used in patients with obstructions or strictures in the gastrointestinal tract, the PillCam capsule may not pass naturally through the gastrointestinal tract of patients with obstructions or strictures, and accordingly the Given System is not recommended for use in these patients.
Our Products
The Given System consists of three components:
PillCam capsule
The PillCam capsules are miniaturized disposable color video cameras encased in a plastic shell approximately 11 millimeters in diameter by 26 millimeters in length which incorporates one or more specially developed imaging devices based on complementary metal oxide semiconductor, or CMOS, technology. Other components include optics, white-light emitting diodes for illumination, an application-specific integrated circuit device for control and image transmission, low-power silver oxide batteries, an antenna and other discrete electronic components.
Until their use, the PillCam capsules are stored in a hermetically sealed package. Before the patient ingests a capsule, the package is opened and the removal of the capsule from the package triggers a switch that activates the capsule. After the patient ingests the PillCam capsule with a small amount of water, the capsule passes naturally through the gastrointestinal tract. The PillCam capsules are excreted naturally from the body, usually within a day or two, without pain or discomfort. There is no need to retrieve the capsules after passage.
In May 2002, we added localization capabilities to the Given System. The RAPID software, when used with the PillCam SB capsule, creates a graphic representation of the patient's gastrointestinal tract, helping physicians assess the location of the PillCam SB capsule within the body when an image is captured and assisting them in directing treatment to the affected area.
We are currently selling PillCam capsules designed for visualization of two areas of the gastrointestinal tract, the small bowel and the esophagus:
| • | PillCam SB—Our initial capsule for the Given System was PillCam SB video capsule for visualization of the small intestine. The PillCam SB capsule transmits images at a rate of two images per second for approximately eight hours, resulting in approximately 50,000 images, at which time the battery power is depleted and recording ceases. After ingesting the capsule at the physician's office, during the procedure, the patient can continue his or her daily routine as the capsule transmits images and other data to a portable data recorder and then returns the data recorder to the physician's office for review and diagnosis. Since its launch in 2001, the PillCam SB capsule has been used primarily with indications of suspected bleeding in the small intestine. Following reimbursement for extended indications, PillCam SB capsule is increasingly being used with additional indications in the small intestine, including suspected Crohn's disease and suspected small bowel tumors. |
| |
| • | PillCam ESO—In November 2004, we received FDA clearance for our new PillCam ESO capsule for visualization of the esophageal mucosa, which we market through our strategic marketing alliance with InScope, a division of Ethicon Endo-Surgery, a Johnson & Johnson company. The PillCam ESO capsule contains an imaging device and light source at both ends of the capsule and is designed to capture up to 14 images per second as it passes down the esophagus. The patient ingests the PillCam ESO capsule in a reclining position in the physician's office. The whole procedure takes about twenty minutes. The PillCam ESO capsule can be used only with our new generation of data recorder, the DR2, and RAPID software, the RAPID 3. |
23
Data recorder and sensor array
After ingestion by the patient, the PillCam capsule transmits images and other data to a proprietary wireless data recorder through an array of antennae, or sensor array, that are secured with adhesive pads to the abdomen, in the case of a procedure performed with a PillCam SB capsule, or to the chest, in the case of a procedure performed with a PillCam ESO capsule. The data recorder is worn on a belt around the waist of the patient for the duration of the examination. Simultaneously with the launch of our new PillCam ESO video capsule in November of 2004 we introduced an advanced version of our data recorder, the DR 2.0, which is now provided as standard as part of all new Given Systems we sell. This advanced version supports PillCam SB and PillCam ESO procedures performed with our products, is completely solid-state and therefore we believe should be much less susceptible to shock, enables quicker and more reliable connection to the sensor array, weighs less and is therefore more comfortable for the patient, and has a robust storage mechanism and faster download capability.
Computer workstation with proprietary RAPID software
After the recording ceases, the data recorder is returned to the physician's office at the patient's convenience, where it is connected to the RAPID computer workstation, our capsule endoscopy platform, for downloading of the images and data captured during the test. Our proprietary RAPID software then processes the images and other data received from the capsule. The RAPID software also includes a number of proprietary algorithms relating to the speed of processing imaging data, as well as to the visual presentation of this data. After processing the images, the Given workstation generates a video stream of the images for viewing by the physician. The physician can review the video carefully, save specific images for the patient file, or attach the thumbnail images and the short video file to an e-mail in order to send to the referring physician or consult with a colleague. The “multiview” feature of our RAPID software provides users with a simultaneous viewing mode of two or four consecutive images, thereby accelerating review speed and reducing overall review time. In addition, the RAPID software contains a proprietary algorithm that we developed called SBI, or suspected blood indicator, which automatically marks images that correlate with the existence of suspected bleeding. In addition, we provide our users with the RAPID Reader, which is a narrowed version of the RAPID Workstation software that can be installed on a standard PC, allowing the physician to review RAPID videos at any time and place as convenient.
Simultaneously with the launch of our PillCam ESO video capsule, we introduced the new version of our RAPID software, the RAPID 3.0, which is now provided as standard as part of all Given Systems we sell. This advanced version supports PillCam SB and PillCam ESO capsule procedures and is compatible with the other components of the Given System. This enhanced version displays images from both imagers of the PillCam ESO video capsule simultaneously and enhances the capsule endoscopy procedure by introducing enhanced review and analysis features resulting in significant operating efficiencies for the physician, and providing improved operating interface that simplifies the administration involved with the procedure, including collecting and displaying patient information and issuing reports.
24
Upgrade of the Given System
Simultaneously with the launch of our PillCam ESO capsule, we began offering upgrade of the Given Systems already installed at our customers to the new RAPID 3.0. At the same time, we also launched a promotional campaign in the United States together with InScope in which we offered a significant discount on the combined purchase price of a DR 2.0 and ten PillCam ESO capsules. This upgrade and promotional campaign will allow our customers to perform procedures with the PillCam ESO capsule that is compatible only with the RAPID 3.0 and DR 2.0 and not with older versions of these products. In the United States, the upgrade to RAPID 3.0 is carried out through an independent contractor and in other territories through our local distributors. Customers whose systems are still under warranty or that have entered into service contracts with us, receive this upgrade free of charge. Customers whose systems are out of warranty and have not signed a service contract and wish to upgrade their systems may elect to either enter into a service contract with us or pay the list price of the upgraded products.
Warranty and Service
Our standard warranty for defective products extends for a period of one year from the date of the first installation at the customer's location with the exception of the Sensor Array and Battery Pack which carry a limited warranty of 6 months from installation or 10 months from delivery. Generally, no warranty is provided more than 16 months from the date of shipment of the Given System to the customer. During the warranty period, we are obligated to repair or replace, at our election, every defective product.
When a customer reports a problem with the Given System, first line service is normally provided by our own technical personnel in the territories in which we operate directly, such as the United States, Germany, France and Australia. In territories in which we operate through a distributor, the distributor is responsible for providing first line service. If our personnel in the field or our distributors are not able to resolve the problem, the defective part is shipped to our main facility in Israel for repair or replacement. Often, the defective part is replaced promptly out of a stock of spare parts we maintain in all of our direct territories and many of the territories in which we operate through a distributor. In 2004, we introduced a remote access software that allows us to provide maintenance and support services for our products from a remote location, including our distributor's office.
When the warranty expires, our customers are offered the opportunity to sign a service contract with us. Under this contract, the customer pays a fixed amount per year in consideration for receiving our maintenance and support services, as well as any upgrades of our products, free of charge. As of December 31, 2004, we had approximately 2,290 systems installed worldwide, of which 739 were under warranty and 323 were covered by a service contract.
Clinical Studies
To demonstrate the clinical and economic benefits of the Given System, the PillCam SB capsule has been tested in clinical studies sponsored by us and by independent third parties. Our clinical studies program is conducted in a number of countries, primarily Australia, Belgium, Canada, France, Germany, Israel, Italy, the Netherlands, Portugal, Spain, Sweden, the United Kingdom, Japan and the United States. Initially, our clinical studies program focused on patients suffering from suspected bleeding in the small intestine. Subsequent clinical trials have focused more on evaluating the performance of the PillCam SB capsule in detecting other disorders of the small intestine such as suspected Crohn's disease, tumors, malabsorption (such as celiac disease), mucosal injuries induced by long-term use of NSAIDs (non-steroidal anti-inflammatory drugs, such as aspirin) and the performance of the PillCam SB capsule in children over the age of ten.
The results of these clinical studies to date have demonstrated that the Given System has a significantly higher diagnostic yield in the detection of disorders of the small intestine than comparative methods, such as push-enteroscopy, gastroscopy and radiological examinations, such as barium follow-through, enteroclysis, computerized tomography (CT) , magnetic resonance (MR)
25
and angiography. Many of the investigators conducting these studies have presented their results at major gastroenterology meetings or submitted them for publication in peer-reviewed medical journals. Accordingly, the number of presentations and publications of studies evaluating the Given System is constantly growing. As of December 31, 2004, 253 articles, editorials and case reports had been published in peer reviewed journals. These papers provide the results of clinical studies as well as accumulated experience from on-going use of the Given System for a range of indications. We believe that these presentations and publications assist our marketing and educational efforts and support our efforts to obtain new reimbursement coverage policies and expand existing policies. We intend to expand our clinical trials program to cover other potential indications for the PillCam SB capsule.
In 2003, the FDA agreed to remove the adjunctive labeling which originally accompanied its clearance of the Given System in 2001, with the result that the PillCam SB capsule is marketed in the United States as a standalone tool for the detection of abnormalities of the small intestine. The FDA's decision was based on materials we submitted showing an analysis of the results of 32 clinical studies involving 691 patients with various suspected disorders of the small intestine. In November 2004, our new PillCam ESO capsule was cleared for marketing in the United States for visualization of the esophageal muccosa.
We have also conducted clinical trials to compare the PillCam ESO capsule to traditional endoscopy in the detection of esophageal disorders. The pivotal trial for obtaining FDA clearance was conducted in seven hospitals in the United States, Germany and Israel, and involved 106 patients with GERD symptoms. The patients were examined first using the PillCam ESO capsule followed by traditional endoscopy on the same day performed with a gastroscope. In this trial, the PillCam ESO capsule showed comparable efficacy to conventional upper-endoscopy. All patients ingested the PillCam ESO capsule without difficulty, no adverse events were reported and patients preferred the PillCam ESO capsule over conventional endoscopy in all categories. The investigators concluded that the PillCam ESO capsule is a convenient, patient-friendly, sensitive method for visualization of esophageal disorders and may provide an effective method to screen patients for Barrett's esophagus, a pre-cancerous complication of GERD.
We continue to conduct clinical trials with PillCam ESO capsules in coordination with our partner, InScope. We expect that results of clinical trials with PillCam ESO capsules will demonstrate its clinical and economic benefits in early detection of disorders of the esophagus, including esophageal varices, GERD and Barret's esophagus.
The PillCam capsules are not recommended for use by patients who have known or suspected gastrointestinal obstructions, narrowing, and certain other abnormalities, such as swallowing disorders. In patients with unsuspected or unknown obstructions, narrowing or certain other abnormalities of the gastrointestinal tract, the PillCam capsules can potentially become blocked from natural excretion, requiring hospitalization, and in some cases surgery, to remove it. In an analysis of several studies presented at the 2002 American College of Gastroenterology, there were seven cases reported from a total population of 937, or 0.75%, in which the individuals had obstructions or narrowing and, as a result, the capsule did not pass naturally. In all these cases, either the condition was known to the physician and the capsule was administered to collect information prior to a scheduled surgery or the condition had not previously been detected and eventually would have required surgery for treatment. In all cases, the capsule was removed without incident and the symptoms associated with the condition causing the blockage were resolved with the help of the data provided by the capsule. In a study of PillCam SB capsule ingestions in the United States from August 2001 to December 2003, we estimated that the total number of PillCam SB capsule ingestions in the United States was approximately 50,360. We received reports of 48 cases, or 0.09%, of these ingestions where the capsule was delayed or did not pass naturally. In 22 of these cases, the subject was diagnosed with Crohn's disease. In 16 patients the capsule was removed during surgery to treat the disease and in one patient the capsule was removed during laparoscopy, totaling surgical intervention in 17 patients, i.e., 35% of retained capsules or 0.03% of total ingestions. Six patients required minimally invasive endoscopy for capsule retrieval. Fourteen other patients excreted the capsule naturally without any treatment
26
or following administration of steroids or laxatives, nine patients demonstrated no symptoms and are under follow-up and two patients are contraindicated for intervention due to terminal disease. Therefore, 65% of patients with retained capsules did not require surgical intervention and/or remained with no symptoms for more than 12 months. To date, there have been no reports of mortality attributed to the PillCam capsule procedure. There have not been any reports of retention of the PillCam ESO capsule.
The Patency System
In November 2003, we launched our Patency System in Europe, a simple and prep-less procedure designed to enable physicians to confirm free passage in the gastrointestinal tract. The Patency System consists of the Patency capsule, a dissolvable capsule the same size as the PillCam capsule (i.e., approximately 11 millimeters in diameter by 26 millimeters in length) with a radio frequency identification (RFID) tag packed in a lactose and barium powder. The Patency capsule is ingested by the patient and allows physicians to confirm free passage in a patient's gastrointestinal tract. The reusable component of the Patency System is a hand-held Patency Scanner, which detects the signal from the RFID tag. If the scanner indicates that the tag is no longer in the gastrointestinal tract, patency has been established and the patient can ingest a PillCam capsule without fear of impaction in a stricture. If the scanner indicates that the tag is located in the patient's body, an obstruction preventing the passage of the PillCam capsule may exist. If the Patency capsule remains in the body, it starts dissolving after 40 hours into small fragments that are naturally excreted. Since the capsule contains barium, in those instances where the Patency capsule is not excreted after ingestion, the physician may detect its location within the body using fluoroscopy.
We are conducting clinical trials for the Patency System in the United States and, if results are favorable, we plan to pursue FDA clearance. We have obtained authorization to affix the CE mark to the Patency System, permitting us to market the Patency System in the member countries of the European Union, and we have also obtained regulatory clearance to market the Patency System in Australia.
Marketing and Distribution
We market and sell our products through a combination of direct sales through our marketing subsidiaries, and through independent distributors. The following table indicates the jurisdictions in which we were marketing and selling the Given System as of December 31, 2004, and the method used:
27
Country or Region
| | Method
|
Africa | | |
South Africa | | Distributor |
Asia | | |
China, India, Singapore, South Korea, Thailand, Taiwan | | Distributors |
Australia and New Zealand | | |
Australia | | Direct sales (Given Imaging Pty. Ltd.) and distributors |
New Zealand | | Distributors |
Europe | | |
France | | Direct sales (Given Imaging s.a.s.) |
Germany | | Direct sales (Given Imaging GmbH) |
Austria, Belgium, Bulgaria, Cyprus,
Czech Republic, Denmark, Estonia,
Federal Republic of Yugoslavia,
Serbia and Montenegro, Finland,
Greece, Hungary, Iceland, Ireland,
Italy, Latvia, Luxemburg,
Macedonia, Malta, Netherlands,
Norway, Poland, Portugal, Romania,
Russia, Slovenia, Spain, Sweden,
Switzerland, Turkey, Ukraine,
United Kingdom | | Distributors |
Middle East | | |
Israel | | Direct sales (Given Imaging Ltd.) |
North America | | |
United States | | Direct sales (Given Imaging, Inc.) |
Canada, Puerto Rico | | Distributors |
Latin America | | |
Argentina, Brazil, Chile, Colombia,
Costa Rica, Dominican Republic,
Mexico, Panama, Peru, Uruguay,
Venezuela | | Distributors |
Our sales and marketing operations are organized in three geographical regions: United States; Europe, Canada and Latin America; Japan and rest of the world. This organization enables us to focus more effectively on each region and to make senior individuals within the company more directly responsible for activities within each region.
We market the Given System using either direct or indirect sales, depending on the size of the market and local market conditions. Currently, we market the Given System directly in Australia, France, Germany, the United States and Israel through approximately 80 sales and marketing personnel employed by us and our subsidiaries in those jurisdictions. In the United States, which accounted for 65% of our revenues in 2003 and 72% of our revenues in 2004, we market our products through our wholly-owned subsidiary, Given Imaging, Inc., located in Norcross, Georgia. As of December 31, 2004, we had a direct sales force in the United States of approximately 45 personnel, including a director of sales, five regional managers, 31 sales representatives, five capsule endoscopy clinical specialists and an educational director. In addition, we currently employ sales managers in Portugal and Spain, who work in conjunction with our local distributor in each of those countries.
In May 2002, we entered into an agreement with Marubeni Corporation and Suzuken Co., Ltd. for the purpose of investing in Given Imaging K.K., a Japanese subsidiary established to commercialize the Given System in Japan. Marubeni is one of Japan's largest trading companies and Suzuken is one of Japan's largest pharmaceutical distributors. Marubeni and Suzuken together invested an aggregate of $4.3 million in Given Imaging K.K. in consideration for an aggregate 49% interest and we retained a 51% controlling interest. Given Imaging K.K. has conducted clinical trials and has submitted the results to the Japanese Ministry of Health, Labor and Welfare for the purpose of obtaining clearance to market the Given System in Japan. We expect to receive
28
marketing clearance for our products in Japan during 2005. Suzuken has been appointed by Given Imaging K.K. as its exclusive distributor in Japan.
Sales to our local distributors worldwide accounted for approximately 15% of our revenues in 2004. Under the terms of our distribution agreements, we generally grant to one distributor in each particular country or region the right to market the Given System for an initial period of approximately three years. During this period, the distributor is required to meet minimum sales targets set out in each distribution agreement. We may, in our sole discretion, upon prior written notice, terminate a distribution agreement with a distributor in the event that a distributor fails to meet its minimum sales targets. To date, we have changed a number of our distributors due to failure to meet minimum sales targets. During 2003, we extended the term of those distributors whose initial term had expired for an additional two years, subject to an adjustment to the minimum sales target. We have the right not to renew a distribution agreement if we are unable to reach an agreement with the distributor as to minimum sales targets during any renewal period. Each distributor is responsible for obtaining and maintaining any regulatory approvals or registrations required to sell the Given System in that distributor's sales territory. Each distributor is also responsible for preparing and submitting to us for our approval a marketing plan for the Given System in that distributor's sales territory. After receiving our approval of the marketing plan, each distributor is responsible for implementing the marketing plan and for conducting sponsored marketing clinical trials in its sales territory.
Our marketing strategy is to drive the placement of Given Systems and increase utilization of capsules per workstation. Prior to 2005, we focused primarily on expanding our market penetration of Given Systems in gastroenterology physician offices and gastroenterology departments within hospitals. Commencing in 2005, we are also focused on increasing utilization by our existing installed base, which we intend to achieve by maintaining a closer relationship with our customers, educating them about updated data on the clinical and economic benefits of our products and enhancing the operating efficiencies of the Given System. We also use trade shows and scientific meetings and offer workshops, courses, videos and seminars to educate our customers. In 2004, 585 physicians and nurses received training in 16 courses and seminars, 11 of which were organized by the American Society of Gastrointestinal Endoscopy and the American College of Gastroenterology, which we believe helped also to expand the knowledge of participating physicians and provide an independent endorsement of the clinical value and importance of the Given System.
��During the United European Gastro Week (UEGW) in September 2004, a group of leading gastroenterologists gathered to form the European Capsule Endoscopy Group (ECEG). This group has developed clinical forums on a variety of special interest topics related to capsule endoscopy. The goal of the ECEG is to provide a dynamic forum to share knowledge, encourage research, and support the advancement of capsule endoscopy. A similar group, the Capsule Endoscopy Study Group (CESG), formed to complete the first clinical trials for capsule endoscopy in Japan and to educate the Japanese physician community on the practical application of capsule endoscopy. We believe that these groups in Europe and Japan provide an important contribution to the broad adoption of capsule endoscopy within these markets.
In 2002, we initiated the International Conference on Capsule Endoscopy, or ICCE, an annual scientific meeting dedicated to presentations and discussions of the most recent advances in the field of capsule endoscopy. The interest and activity surrounding this conference has grown rapidly since its launch. At the first ICCE, which took place in March 2002 in Rome, 60 papers were presented to approximately 90 physicians from 12 countries, which represented early adopters of the Given System. By comparison, at the fourth ICCE which took place in March 2005 in Miami, more than 100 papers were presented before more than 400 physicians from 33 countries. These conferences serve as a basis for establishing a community of physicians who utilize the Given System and accelerates the dissemination of new clinical data.
In order to drive strong growth in the future, we intend to continue investing in our customers, expand our field sales force in the United States and focus on strengthening relationships with our customers. In addition, we have recently sponsored the creation of a new
29
web site, www.CapsuleEndoscopy.org, addressed to gastroenterology professionals, through which we intend to provide training and educational materials and information, including a virtual image atlas, clinical papers, course information and discussion forums for a variety of clinical topics.
Strategic Marketing and Sales Alliance with Ethicon Endo-Surgery
On May 10, 2004, we entered into an exclusive sales representation, co-promotion and cooperation agreement with Ethicon Endo-Surgery, a Johnson & Johnson company. InScope, a business division of Ethicon Endo-Surgery established to market products to the gastroenterology market, has exclusive rights to market our PillCam ESO capsule. Under the agreement, we retain responsibility for regulatory approvals, manufacturing, supply chain management, order invoicing and processing, collections and technical support for the PillCam ESO capsule. We retain sole ownership of the intellectual property associated with the Given System, the PillCam capsules and any future innovations that we make. InScope is responsible for sales, marketing and promotion, funding mutually agreed upon clinical trials in territories covered by the agreement, as well as efforts to establish reimbursement policies for the PillCam ESO capsule. InScope will also collaborate with us to expand the installed base of the Given System, which is our platform for the PillCam ESO capsule, the PillCam SB capsule and other capsules which we are developing.
Under the terms of the agreement, InScope committed to pay us up to $50 million, pending achievement of specified milestones by both parties. Out of this amount, we received a milestone payment of $10 million in December 2004 following FDA clearance of the PillCam ESO capsule and an additional $5.0 million in February 2005 as a result of completing the upgrade of 90% of our installed base in the United States to include RAPID 3.0 that our customers need in order to perform capsule endoscopy procedures with the PillCam ESO capsule. Additional milestone payments of up to $35 million may become payable to us by February 2007. To market and promote the PillCam ESO capsule, InScope created a sales force, which has more than doubled the number of sales representatives promoting our PillCam technology in the United States. This InScope sales force markets the PillCam ESO and will also market other InScope products as they are launched. We pay InScope commissions on sales in the United States at a rate of 50% on sales of PillCam ESO capsules and 10% on sales of other parts of the Given System, including workstations and portable data recorders. Subject to achieving specified minimum sales targets and meeting certain conditions, the exclusive term of the agreement will continue for up to 11 years followed by a four-year transition period during which InScope's marketing rights to market the PillCam ESO capsule will be co-exclusive with ours. The alliance initially is worldwide, excluding Japan. InScope began marketing PillCam ESO capsules in the United States in November 2004 following FDA clearance for the PillCam ESO capsule. InScope has not yet commenced marketing activities in territories other than the United States, which territories may subsequently be excluded if we do not reach an agreement with InScope regarding the marketing activities of InScope in these other territories by March 31, 2005 or a later date that may be agreed upon by the parties.
We believe that, by collaborating with a leading healthcare company, we will significantly improve our ability to market and grow our installed base, increase sales of PillCam capsules and reduce the risks inherent in launching a new product targeted at a different area of the gastrointestinal tract. As a result of the agreement, we will benefit from the significant increase in the number of sales representatives marketing our products in the United States. In addition, we believe that the alliance with InScope will accelerate acceptance of the Given System among gastroenterologists, primary care physicians who refer patients with GERD symptoms to gastroenterologists, and third party payors.
Manufacturing
We purchase both custom and off-the-shelf components from a number of suppliers. Except as described below, the components and services we purchase are available from more than one supplier.
30
PillCam Capsules
The manufacture of the PillCam capsules is a complex process involving a number of separate processes and components. Our manufacturing process consists primarily of assembling externally purchased components and sub-assemblies in an environmentally controlled area. After assembly, each PillCam capsule is inspected and packaged.
We manufacture the PillCam capsules at our facilities in Yoqneam, Israel using three semi-automated production lines. We purchased our first production line from Pemstar, Inc., a U.S. electronics manufacturing service and equipment company, and it became operational in 2002. We installed two additional semi-automated production lines, which became operational in the second half of 2003 and the second half of 2004, respectively. Two of these production lines are used to manufacture the PillCam SB capsule and one to manufacture the PillCam ESO capsule. We are currently constructing a fourth semi-automated production line, which we plan to use for manufacturing the PillCam ESO capsule. We expect that this fourth production line will become operational in the second quarter of 2005. Each semi-automatic production line in our Yoqneam, Israel facility has a capacity to manufacture approximately 180,000 PillCam capsules per year. As of December 30, 2004, we had manufactured and sold approximately 172,000 PillCam SB capsules and approximately 3,400 PillCam ESO capsules, substantially all of which were produced on these semi-automated production lines. We have also installed at Pemstar's facilities in Ireland a semi-automated production line of the PillCam SB capsule for backup purposes. We expect to install another production line at Pemstar's facilities during the second quarter of 2005 to be used as a backup for the manufacture of our new PillCam ESO capsule. Each of these backup production lines has a capacity to manufacture an additional 180,000 PillCam capsules per year. We believe we have adequate capacity to manufacture capsules needed to satisfy estimated demand for the foreseeable future.
In June 2002, we also entered into a one year non-exclusive technical services agreement with Pemstar, pursuant to which Pemstar is providing us with technical services relating to the manufacture of the PillCam capsules on the single semi-automated production line we purchased from Pemstar in 2001. These services include supervising the operation and maintenance of the production lines for the PillCam capsules and purchasing the components necessary to produce the PillCam capsules, either through us or directly from the suppliers. Pemstar is required to deliver to us each month the number of PillCam capsules that we specify in a purchase order to be provided to it three months in advance. In consideration for providing these services to us, we pay monthly to Pemstar a fixed amount for each functional PillCam capsule that is delivered to us. This amount decreases as the volume of PillCam capsules produced increases. In June 2004, we extended this agreement until December 31, 2005 and thereafter we have an option to extend the agreement for two additional terms of two years each.
We perform all supervisory, operational and maintenance functions for the two other semi-automated lines we currently operate in our manufacturing facility in Yoqneam, Israel, without Pemstar's assistance.
There are single-source suppliers for two key components of the PillCam capsule:
| • | Micron Technology, Inc., by and through its Micron Imaging Group and other wholly-owned subsidiaries, supplies the imaging sensor that is integrated into the PillCam capsules. Under our contract with Micron , Micron may not offer the imaging sensor as a standard catalog part. In the event that Micron ceases operations or enters into liquidation, we are entitled to receive all information necessary to manufacture the sensor upon the payment of reasonable royalties to be agreed upon with Micron Technology. In November 2002, we entered into a development and manufacturing agreement with Micron with respect to enhancements to our existing imaging sensor. The agreement is for an initial term of five years until November 2007, with an option to extend that term by mutual agreement. Pursuant to this agreement, Micron has agreed to develop enhancements to the sensor based on specifications that we provide. We have agreed to purchase the enhanced sensor only from Micron and Micron has agreed to sell the sensor exclusively to us. The agreement permits Micron to disregard the exclusive sales requirement if we fail to purchase agreed-upon minimum quantities of Micron's sensors. Pursuant to the agreement, we have also agreed with Micron to cooperate in our mutual efforts to market the product. |
31
| • | Zarlink Semiconductors AB, a Canadian company, supplies the transmitter that is integrated into the PillCam capsules. Zarlink began supplying the transmitter to us in the fourth quarter of 2004, replacing our previous supplier, Asicom Technologies. We currently expect that Zarlink Semiconductor will fulfill substantially all of our future needs for transmitters for the PillCam capsules. Under our agreement, we have agreed to purchase a minimum quantity of transmitters during the first 36 months following the development and testing phase, and if we fail to do so we must make certain payments to Zarlink Semiconductor in respect of the shortfall. After this initial 36-month period, Zarlink Semiconductor may terminate the agreement on six months notice if we do not order a minimum quantity of transmitters in each subsequent 12-month period, subject to our right to submit a final purchase order. The agreement also includes non-compete provisions prohibiting Zarlink Semiconductor from selling the transmitters to other parties and, for a certain period of time following termination of the agreement, from transferring any of the intellectual property and design specifications associated with the development of the transmitter to any potential competitors in our market. The initial term of the agreement expires in April 2007, subject to earlier termination in specified circumstances, with the option to extend annually thereafter for up to five years. |
We believe that we would be able to arrange substitute sources of supply for these two components in approximately a one-year period of lead time. We believe that if we need to find a substitute source of supply, our inventory of components and finished products, together with our right to submit final purchase orders prior to termination of our agreements with Micron Technology and Zarlink Semiconductors, is sufficient to continue sales of the Given System for all or most of the lead-time period.
Portable Data Recorder and Sensor Array
We designed our portable data recorder, sensor array and their related accessories. Data recorders are manufactured externally and assembled and tested at our facilities. Sensor arrays are manufactured and assembled externally and are tested at our facilities. We have established a back-up facility at Pemstar's facilities in Ireland for testing workstations, data recorders and sensor arrays.
Computer Workstation
Our computer workstation is an off-the-shelf computer workstation pre-loaded with our proprietary RAPID software and integrated software that together allow us to service the workstation from remote locations through standard telephone connections.
Manufacturing facilities and disaster-preparedness
In order to maintain our special tax benefits under our approved enterprise status, we are required to conduct our manufacturing and a majority of our subcontracting in specific locations in Israel. We currently plan to continue these practices as we increase our manufacturing capacity. For more information, see “Taxation and Government Programs—Israeli Tax Considerations and Government Programs—Taxation of Companies—Tax Benefits Under the Law for the Encouragement of Capital Investments, 1959.”
We have also taken the following measures for disaster-preparedness:
| • | We have installed a back-up semi-automatic production line at the facilities of Pemstar, Inc. in Ireland, and have a second semi-automated line under construction. Each of these backup lines has a capacity to manufacture approximately 15,000 PillCam capsules per month. We believe that these lines can be operational upon 60 days notice. |
32
| • | Our policy is to hold approximately six weeks inventory of finished products at our offices in Israel, Sydney, Hamburg, Paris and Norcross, Georgia. |
| |
| • | We maintain back-up copies of all production files, original certifications and all proprietary software masters outside of our facilities in Israel. |
The FDA requires us to adhere to the Quality System Regulation which requires manufacturers to follow elaborate design, testing, control, documentation and other quality assurance procedures during the manufacturing process. Our quality system has passed three audits pursuant to the Medical Devices Directive of the European Union, the International Standard Organization's standard ISO 9001 and EN 46001, a European quality standard setting forth requirements for medical device manufacturers that are more specific than the general requirements specified in ISO 9001. These quality standards contain requirements that are generally similar to the Quality System Regulation required by the FDA. However, we have never been through a Quality System Regulation inspection by the FDA and cannot assure you we would pass such an inspection.
Intellectual Property
An important part of our competitive strategy is to seek, when appropriate, protection for our products and proprietary technology through the use of various United States and foreign patents, trademarks, trade secrets and contractual arrangements. We intend to prosecute and enforce our proprietary technology against any violator.
We acquired the rights to our basic issued U.S. and Israeli patents in January 1998 under a technology purchase and license agreement with Rafael Armament Development Authority. See Item 7 “Major Shareholders and Related Party Transactions.” These patents expire in January 2014 and January 2015, respectively. We hold 15 additional issued patents in Australia, France, Israel, South Korea and the United States covering different elements of our technology. These patents expire between July 2014 and August 2022. In addition, as of December 31, 2004, we had 285 patent applications worldwide based on 157 priority applications relating to various elements and functions of our product and its enhancements.
We seek to protect our product names and logos through trademark use and registration in the United States and other countries. In 2004 we re-branded our line of video capsule from “M2A” to “PillCam.” The GIVEN logo, GIVEN, PillCam, PILLCAM, thePillCam logo, ORDERWIN, ORDER WHEN I NEED, RAPID, PILLCAM IMAGING CAPSULE and design, FINGERS HOLDING A CAPSULE logo and, FINGERS HOLDING PILLCAM CAPSULE logo, are our trademarks or registered trademarks.
In March 2004, the U.S. Patent and Trademark Office notified us that it would conduct a reexamination of some of the claims in one of our U.S. patents in the U.S. Patent and Trademark Office pursuant to a request submitted by Olympus Corporation. For more information, see Item 8 “Financial Information—Legal Proceedings” in Part I of this report.
33
Competition
We currently consider the competition for our product to be existing technologies for detecting gastrointestinal disorders and diseases, including traditional endoscopy and radiology. Our success depends in large part on convincing physicians to adopt the Given System over current technologies.
Three companies control the major portion of the worldwide gastrointestinal endoscopy market. These companies, Olympus, Asahi Optical (which manufactures and sells cameras and endoscopes under the Pentax brand) and Fujinon, have marketed and sold flexible endoscopic equipment for many years and may be developing capsule technology that competes with ours. These companies have substantially greater financial resources than we do, and they have established reputations as well as worldwide distribution channels for medical instruments to gastroenterologists. We are aware of research and development efforts by some of these companies, including Olympus, and other individuals and companies to develop and bring to market imaging capsules or other minimally invasive imaging techniques.
In addition, there are several companies focused on radiological diagnostics that provide x-ray machines and other imaging products used for barium series radiological examinations. These companies include but are not limited to GE Healthcare, Siemens Medical Solutions, Philips Medical Systems, Toshiba Corporation and Shimadzu Corporation.
In addition to competition from products performing similar clinical functions to the Given System, there is also competition among gastrointestinal products for the limited capital expenditure budgets of customers. For example, another capital equipment item for gastroenterology that is priced similarly to our system may compete with our system for the same hospital capital budget, which is typically limited, and therefore the potential purchaser may be required to choose between the two items of capital equipment.
U.S. Government Regulation
FDA Clearance and Regulation of the Given System
In August 2001, we received clearance from the FDA to market the Given System in the United States for adjunctive use in the detection of abnormalities of the small intestine. In 2003, based on review of a meta-analysis of data from 32 studies in a total of 691 patients, the FDA approved the removal of the adjunctive labeling, with the result that the Given System can now be marketed in the United States as a standalone tool for the detection of abnormalities of the small intestine. In 2003, we received two further clearances from the FDA. First for a suspected blood indicator, a new feature of the Given System which automatically marks images that contain the color red and which therefore correlate with the existence of suspected bleeding, and, second, for use of the PillCam capsule in children aged 10 through 18. In November 2004, we received FDA marketing clearance for our PillCam ESO video capsule and for the advanced RAPID software and data recorder.
FDA Clearance and Regulation of the Future Products
Any new medical device that we wish to commercially distribute in the United States will likely require either 510(k) clearance or premarket application approval from the FDA prior to commercial distribution. 510(k) clearance or amendment to premarket application is also required when a change is made to a legally marketed device or to expand the product label.
510(k) Clearance Process. To obtain 510(k) clearance, an applicant must submit a premarket notification demonstrating that the proposed device is substantially equivalent in intended use and in safety and effectiveness to a “predicate device”—either a previously 510(k) cleared device or a preamendment device for which the FDA has not called for premarket applications. The FDA's 510(k) clearance process usually takes from four to 12 months, but it can last longer. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k)
34
clearance or could even require a premarket application approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with the determination, the agency may retroactively require the manufacturer to seek 510(k) clearance or premarket application approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket application approval is obtained. Our PillCam ESO video capsule and the advanced versions of the RAPID software and data recorder have received FDA marketing clearance under the 510(k) clearance process.
De Novo Classification. If the FDA denies 510(k) clearance of a device because it is novel and an adequate predicate device does not exist, the “de novo classification” procedure can be invoked based upon reasonable assurance that the device is safe and effective for its intended use. This procedure approximates the level of scrutiny in the 510(k) process but may add several months to the clearance process. If the FDA grants the request, the device is permitted to enter commercial distribution in the same manner as if 510(k) clearance had been granted. We received our original FDA clearance for the Given System pursuant to the de novo classification procedure which is intended for novel but low risk devices. Our application for clearance under the de novo classification procedure included clinical data from a nonsignificant risk study. We cannot assure you that future studies of the Given System in the United States will be eligible for the same abbreviated regulatory treatment.
Premarket Application Approval Process. If the FDA denies 510(k) clearance for a product, and denies de novo classification, the product must follow the premarket application approval process, which requires proof of the safety and effectiveness of the device to the FDA's satisfaction. A premarket application must provide extensive preclinical and clinical trial data and also information about the device and its components regarding, among other things, device design, manufacturing and labeling. After approval of a premarket application, a new premarket application or premarket application supplement is required in the event of a modification to the device, its labeling or its manufacturing process. The premarket application approval pathway is much more costly, lengthy and uncertain. It generally takes from one to three years or longer.
FCC Clearance and Regulation
Because the Given System includes a wireless radio frequency transmitter and receiver, it is subject to equipment authorization requirements in the United States. The U.S. Federal Communications Commission, or FCC, requires advance clearance of all radio frequency devices before they can be sold or marketed in the United States. These clearances ensure that the proposed products comply with FCC radio frequency emission and power level standards and will not cause interference. We received an equipment certificate from the FCC in March 2001 based on the current system design and specifications for the Given System. Any modifications to the Given System may require new or further FCC approval before we are permitted to market and sell a modified system, and it could take several months to obtain any necessary FCC approval.
Anti-Kickback and False Claims Laws
In the United States, there are federal and state antikickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration intended to induce the purchase or recommendation of healthcare products and services. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation in federal healthcare programs. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices such as us, by limiting the kinds of financial arrangements (including sales programs) we may have with hospitals, physicians and other potential purchasers of the medical devices. Other provisions of state and federal law provide civil and criminal penalties for presenting, or causing to be presented, to third-party payors for reimbursement, claims that are false or fraudulent, or which are for items or services that were not provided as claimed. Since we are providing coding and billing advice to purchasers of our products, and since we cannot be sure that the government will
35
regard any billing errors as inadvertent, we could be subject to an enforcement proceeding in a case where our advice was deemed to have caused the submission of improper claims.
Health Insurance Portability and Accountability Act of 1996 and Related Laws
U.S. Federal and state laws protect the confidentiality of certain patient health information, including patient records, and restrict the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated patient privacy rules under the Health Insurance Portability and Accountability Act of 1996, or HIPAA. These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their protected health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. Because we are selling products and services to persons and entities subject to HIPAA and are exposed to personally-identifiable health information in the course of our operations, we also may be subject to HIPAA, as well as similar state laws. HIPAA imposes civil and criminal penalties for violations of its provisions, which could be substantial. State privacy laws have their own penalty provisions which could apply in a given case.
Regulation in Europe
Commercialization of medical devices in member countries of the European Union is regulated by directives adopted by the European Union. The European Union presently requires that all medical products bear the CE mark, an international symbol of adherence to quality assurance standards and demonstrated clinical effectiveness. Compliance with the Medical Device Directive, as certified by a recognized European Notified Body, permits the manufacturer to affix the CE mark on its products. We received authorization to affix the CE mark to the Given System in May 2001 and successfully passed the 2004 annual re-certification for compliance with ISO 9001:2000, ISO 13485:1996 and ISO 13485:2003 and the CE mark. In September 2004, our new PillCam ESO video capsule was added to our CE mark certification.
If we modify the Given System, we may need to apply for permission to affix the CE mark to it. Additionally, we will need to apply for a CE mark for any new products that we may develop in the future. We cannot be certain that we will be able to obtain permission to affix the CE mark for new or modified products or that we will continue to meet the quality and safety standards required to maintain the permissions that we receive. If we are unable to maintain permission to affix the CE mark to our products, we will no longer be able to sell our products in member countries of the European Union.
In Europe, the frequency range in which the Given System operates is subject to technical standards for radio frequency use developed by the Short Range Device Maintenance Group of the European Conference of Postal and Telecommunications Administrations. In February 2002, the Short Range Device Maintenance Group modified the technical standards for the specific radio frequency that the Given System uses to redefine the period of time during which any device can operate continuously in that frequency, known as the “duty cycle.” This change permits the Given System to transmit for more than 10% of the time that it is in operation, which is necessary for the Given System to function. The Short Range Device Maintenance Group standards and European Conference of Postal and Telecommunications Administrations rules generally are not legally binding, and must be implemented into national laws by European governments to become binding. As of December 31, 2004, records of the European Conference of Postal and Telecommunications Administrations indicate that only two European governments, Italy and Poland, have indicated that they will not implement the new technical standards for the duty cycle. Prior to the implementation of the new technical standards change described above or if the technical standards are not implemented in a timely manner, we will seek to obtain waivers or separate authorizations to operate our system in each individual European country, either in advance or if our compliance with spectrum requirements is questioned. If those countries do not implement the technical standards, or we are unable to receive such a waiver or a separate
36
authorization or to redesign our transmitter to operate on a radio frequency that is not subject to a limit on the duty cycle, an enforcement action could be brought to prevent the sale or use of the Given System in these countries.
Regulation in Other Countries
In order for us to market the Given System in countries other than the United States, we must obtain regulatory approvals and comply with extensive safety and quality regulations in these countries. These regulations, including the requirements for approvals or clearance and the time required for regulatory review, vary from country to country. Failure to obtain regulatory approval or clearance in any foreign country in which we plan to market our product may harm our ability to generate revenue and harm our business.
In all of the countries in which we are currently selling the Given System we have either received regulatory approval or been informed that approval is not required. Renewals or updates of the regulatory status of our products in all these countries are done annually.
In Japan, we are in the process of obtaining clearance to market the Given System after submitting the results of a clinical trial conducted in Japan together with results of clinical trials conducted in other countries to the Japanese Ministry of Health, Labor and Welfare. We are also in the process of testing our products for compliance with the “extremely low power radio station” category of the Japanese Radio Law, which does not require a license.
Third-Party Reimbursement
Reimbursement in the United States
In the United States, healthcare providers that purchase medical devices generally rely on third-party payors, such as Medicare, Medicaid, private health insurance plans and health maintenance organizations, to reimburse all or a portion of the cost of the devices, as well as any related healthcare services utilizing the devices. FDA clearance does not result in coverage and reimbursement by third-party payors.
Coding. Generally, a current procedural terminology, or CPT, code is necessary to facilitate claims for reimbursement. If a procedure is not covered by an appropriate existing code, an application for a new code can be made to the American Medical Association. This process can be lengthy, however, typically taking two or more years before the new code is effective. In the meantime, claims may be submitted using a miscellaneous CPT code or using a temporary G-code, if one is established by the Department of Health and Human Services' Centers for Medicare and Medicaid Services, or CMS. In December 2002, CMS established a temporary G-code specifically for capsule endoscopy. In October 2003, the American Medical Association assigned a permanent CPT code for capsule endoscopy of the small bowel, effective January 1, 2004. With the assignment of the permanent CPT code for capsule endoscopy of the small bowel, the temporary G-code was abolished.
For capsule endoscopy procedures of the small bowel performed in a physicians office, the CPT code covers a global fee for both the technical and the professional components. In the outpatient hospital setting, claims are submitted using the CPT code and paid by Medicare using an Ambulatory Payment Classification, or APC, code, which covers the technical component, which includes the cost of the facility and supplies related to the procedure. The physician is paid separately. In January 2003, CMS established a New Technology APC for capsule endoscopy of the small bowel in the hospital outpatient setting and in November 2004, CMS reclassified the APC into a GI diagnostic category for payment in an outpatient hospital setting, effective January 1, 2005.
Under our exclusive sales representation, co-promotion and cooperation agreement with Ethicon Endo-Surgery, Ethicon is responsible to obtain a CPT code for capsule endoscopy of the esophagus using our PillCam ESO video capsule. We do not expect this CPT code to be effective before January 2007. We expect that the assignment of a permanent CPT code for capsule
37
endoscopy of the esophagus will significantly improve the potential for physicians to receive reimbursement for use of the Given System. However, the assignment of this CPT code does not require either Medicare carriers or private payors to cover capsule endoscopy of the esophagus.
Reimbursement Coverage. A payor's decision to cover a device or medical procedure is independent of the coding process, although the existence of an appropriate CPT code and APC may assist in obtaining coverage. Generally, payors may deny coverage if they determine that a procedure was not reasonable or necessary as determined by the payor, was experimental or was used for an unapproved indication. During the past several years, the major third-party payors have substantially revised their reimbursement methodologies in an attempt to contain their healthcare reimbursement costs.
Third-party payors in the U.S. began issuing coverage policies for capsule endoscopy in early 2002. Initially, all reimbursement policies issued covered capsule endoscopy of the small bowel only for diagnosing suspected small intestinal bleeding. Subsequently, reimbursement coverage has been expanded to include other indications and as of December 31, 2004, 36 Medicare carriers and 43 private payors, with a total insured population of more than 167 million individuals, also cover capsule endoscopy of the small bowel for suspected Crohn's disease, suspected small bowel tumors and other small bowel pathologies.
As of December 31, 2002, the total population with reimbursement coverage in the United States was approximately 62 million individuals. As of December 31, 2003, the total population with reimbursement coverage for capsule endoscopy of the small bowel was approximately 151 million individuals, representing an increase of 144%, and consisting of approximately 37 million individuals covered by Medicare carriers in 41 local jurisdictions and approximately 114 million individuals covered by private payors, including three national and 31 local payors. As of December 31, 2004, the total U.S. population with reimbursement coverage for capsule endoscopy of the small bowel was approximately 206 million individuals, representing an increase of 36.4% over 2003, and consisting of approximately 39 million individuals covered by Medicare carriers in 50 local jurisdictions and approximately 167 million individuals covered by private payors, including three national and 63 local payors.
Almost all of the reimbursement policies currently in effect require that a previous procedure, such as endoscopy or radiology, be performed prior to using the Given System and some may require prior authorization. We intend to continue our efforts to establish the Given System as the first tool administered to patients with suspected disorders of the small bowel and to convince third party payors to provide reimbursement coverage for capsule endoscopy as a primary diagnostic tool by presenting to them economic and health outcomes analyses showing the benefits of using the PillCam SB capsule as a first line tool. To this end, the peer-reviewed journal Disease Management published in its Winter 2004 issue an economic analysis of using PillCam SB capsules as a first-line tool for diagnosing patients with suspected Crohn's disease. The study, conducted by researchers at Thomas Jefferson University, concluded that using the PillCam SB capsule as a first line procedure for detecting small bowel Crohn's disease is less costly from a third-party payor perspective than existing diagnostic approaches. This publication supports the company's efforts to convince payors to revise policies to cover PillCam SB capsules when performed as a first line procedure in patients with suspected Crohn's disease.
As of December 31, 2004, no third party payor had established coverage policy for capsule endoscopy of the esophagus using our PillCam ESO capsule. While some third party payors may provide reimbursement coverage for this procedure before a dedicated CPT code for capsule endoscopy of the esophagus is assigned, we do not expect broad coverage for this procedure prior to assignment of such code, which we expect to occur in late 2006, effective January 2007. We have established a toll-free reimbursement help-line whereby reimbursement specialists assist our customers in submitting prior authorizations, claims and appeals in the event of a denial.
Reimbursement Rates. Even if a device or medical procedure is covered, reimbursement rates must be adequate for most providers to use it routinely. Reimbursement rates vary depending on the third-party payor and individual insurance plan involved, the procedure performed and other factors. Medicare reimbursement for inpatient hospital services is based on a fixed amount per
38
admission based on the patient's specific diagnosis. As a result, any illness to be treated or procedure to be performed in an inpatient setting will be reimbursed only at a prescribed rate set by the government that is known in advance to the hospital. If the treatment cost is less, the hospital is still reimbursed for the entire fixed amount; if it costs more, the hospital cannot bill the patient for the difference. However, the Given System is not subject to these restrictions for hospital inpatients as the Given System is reimbursed by Medicare under the outpatient regulations, which allows for more separate reimbursement for individual services than the inpatient system. Medicare is covering the Given System under the outpatient regulations because the Given System is purchased for placement in an outpatient setting, where patients are not admitted to a hospital or department for the capsule endoscopy procedure.
Under the G-code issued by CMS in December 2002, which was effective until it was replaced by the CPT code in January 1, 2004, Medicare carriers paid a global fee of $768 on a national average for capsule endoscopy with the PillCam SB capsule performed in private physician offices and approximately $109 on a national average for the professional fee for procedures performed in hospital outpatient facilities. Under the applicable APC, Medicare paid an additional $625 on a national average for the technical component for procedures performed in a hospital outpatient setting, which covers the cost of the facility and the capsule. At the time the new CPT code was made effective, CMS abolished the G-code and increased the payment rates for the global fee and physician fee for capsule endoscopy with the PillCam SB capsule, effective January 1, 2004. The global fee paid by Medicare for procedures in a physician's office was increased 22% to $938 on a national average and the professional fee for hospital outpatient procedures was increased 69% to $184 on a national average. The payment rate to the hospital for the technical component also increased effective January 1, 2004, to $650 on a national average, reflecting a 4% increase. These payment rates are modified periodically. Effective January 1, 2005, the global fee paid by Medicare for procedures in a physician's office was increased to $985 on a national average and the professional fee for hospital outpatient procedures was increased to $190 on a national average. The payment rate to the hospital for the technical component decreased to $496 on a national average due to APC reclassification of the capsule endoscopy procedure with the PillCam SB capsule.
Coverage Outside the United States
In countries outside the United States, coverage is obtained from various sources, including governmental authorities, private health insurance plans, and labor unions. In some foreign countries, private insurance systems may also offer payments for some therapies. Although not as prevalent as in the United States, health maintenance organizations are emerging in certain European countries. Coverage systems in international markets vary significantly by country and, within some countries, by region. Coverage approvals must be obtained on a country-by-country or region-by-region basis. In general, the process of obtaining coverage approvals has been slower outside of the United States. In Europe, prior to 2004, the public healthcare systems in Austria, Denmark, Portugal, Sweden, Switzerland approved budgets for capsule endoscopy procedures performed in public hospitals, providing coverage for the entire populations of approximately 40 million people of those countries. The population with reimbursable access to capsule endoscopy in Europe grew to 102 million at the end of 2004, and the number of lives with reimbursement for expanded indications grew from 24 million at the end of 2003 to 87 million at the end of 2004. This increase was mainly the result of guidance issued in December 2004 by the National Institute of Clinical Excellence (NICE) of the United Kingdom National Health Service (NHS), allowing 58 million U.K. residents free access to capsule endoscopy under the NHS' public health system. Also in 2004, a regional government in Italy has approved reimbursement for capsule endoscopy using PillCam SB capsules for approximately 4.3 million individuals in the region and in Germany, the Doctors' Chambers in two states have released recommendations for coverage of privately insured patients. In Australia, reimbursement has been approved for all residents in Australia who hold Australian and/or New Zealand citizenship, providing coverage for the approximately 20 million residents. While coverage in Switzerland and Australia is generally limited to the indication of suspected small intestinal bleeding, the public healthcare systems in Denmark, Portugal, Sweden
39
and the United Kingdom cover capsule endoscopy for broader indications, including suspected Crohn's disease. We cannot provide any assurance that we will obtain any additional approvals in a timely manner or at all.
Patency System
As of the date of this annual report, we have not received any reimbursement approvals for the Patency System.
C. Organizational Structure
Given Imaging Ltd. is organized under the laws of the State of Israel and, as of December 31, 2004, held directly and indirectly the percentage indicated of the outstanding capital stock of the following subsidiaries:
| | Name of Subsidiary
| | Country of Incorporation
| | Percentage Ownership
|
| | Given Imaging Pty. Ltd. | | Australia | | 100 |
| | Given Imaging, Inc. | | United States | | 100 |
| | Given Imaging s.a.s. | | France | | 100 |
| | Given Imaging GmbH | | Germany | | 100 |
| | Given Imaging B.V. | | Netherlands | | 100 |
| | Given Imaging K.K. | | Japan | | 51 |
D. Property, Plants and Equipment
Our principal administrative and research and development activities occupy a 3,250 square meter (34,983 square foot) facility in Yoqneam, Israel. We lease this facility pursuant to a lease that expires in July 2005. In March 2005 we began moving our corporate headquarter, including our principal administrative and research and development activities, to another office building in Yoqneam, the same building in which we currently lease space for three of our production lines for the PillCam capsules. Following this relocation of our corporate headquarters, we will have a total of approximately 10,300 square meters (approximately 110,869 square feet) in Yoqneam under a lease that expires in December 31, 2015, including 1,750 square meters (18,837 square feet) in an adjacent building that currently hosts one semi-automated production line that we use for the manufacture of the PillCam ESO video capsule. For information regarding our agreement with Pemstar, Inc., in connection with the automated and semi-automated production lines, please see Item 4 “Information on the Company—Business Overview—Manufacturing.”
We believe that our existing facilities will be adequate to meet our needs in Israel for the next 12 months.
Our subsidiaries are party to the following leases:
| • | Given Imaging, Inc. leases 1,536 square meters (16,526 square feet) of office space in Norcross, Georgia pursuant to a five-year lease that expires in May 2006; |
| |
| • | Given Imaging GmbH leases approximately 590 square meters (6,348 square feet) of office space in Hamburg, Germany, pursuant to a lease that may not be terminated by us or our landlord prior to May 2008 without the payment of a specified early termination fee; |
| |
| • | Given Imaging Pty. Ltd. leases 244 square meters (2,626 square feet) of office space in North Ryde, Australia, pursuant to a five-year lease that expires in February 2006 with an option to extend the term of the lease for an additional five years; |
| |
| • | Given Imaging s.a.s. leases 168 square meters (1,808 square feet) of office space in Paris, France, pursuant to a nine-year lease that expires in September 2010 and that may be terminated by us at the end of each three year period upon six months notice; and |
40
| • | Given Imaging K.K. leases 103 squares meters (1,114 square feet) of office space in Tokyo, pursuant to a four-year lease that expires on December 31, 2007, subject to our right to terminate the lease on December 31, 2005 upon six months prior notice. |
Item 5. Operating and Financial Review and Prospects
A. Operating Results
Overview
We develop, manufacture and market innovative diagnostic products for disorders of the gastrointestinal tract. Our principal product, which incorporates our core technology, is the Given System, a proprietary wireless imaging system that represents a fundamentally new approach to visual examination of the gastrointestinal tract. The Given System uses a miniaturized video camera contained in a disposable capsule, which we refer to as the PillCam capsule, that is easily ingested by the patient and delivers high quality color images in a painless and noninvasive manner. In 2001, we commenced marketing the Given System with the PillCam SB capsule for detection of disorders of the small bowel. As of December 31, 2004, we had an installed base of approximately 2,290 Given Systems and had sold approximately 172,000 PillCam SB capsules in more than 50 countries worldwide. At the end of November 2004, we received clearance to market our PillCam ESO capsule. We market the PillCam ESO video capsule through a strategic marketing and sales alliance formed in May 2004 with InScope, a division of Ethicon Endo-Surgery, a Johnson & Johnson company. During December 2004, we sold approximately 3,400 PillCam ESO capsules, most of them as part of a promotional campaign in which customers received a significant discount on the combined purchase of 10 PillCam ESO capsules and the new DR 2.0. In addition, in November 2003, we launched our Patency System in Europe which allows physicians to detect the location of obstructions or strictures in a patient's gastrointestinal tract. We are also conducting clinical trials for the Patency System in the United States and intend to pursue FDA marketing clearance, if the results are favorable. The Patency System has received the CE-Mark which allows it to be marketed throughout the European Union.
We were incorporated in Israel in January 1998. Our initial public offering and listing on the Nasdaq National Market occurred in October 2001 and we completed a follow-on offering in June 2004 in which we raised net proceeds of $44.3 million. Since March 2004, our shares have also been listed on the Tel Aviv Stock Exchange. Since our inception, we have devoted substantially all of our efforts to developing the Given System, raising capital, recruiting personnel and marketing the Given System and PillCam capsules. We recorded our initial sales of the Given System in the third quarter of 2001.
Revenues
We derive virtually all of our revenues from sales of the Given System, consisting of a Rapid workstation, a portable data recorder, and disposable PillCam capsules. Since 2002, we have derived a small portion of our revenues from service contracts entered into by customers at the end of the warranty period for the computer workstation and data recorders. As more of the systems that we sold initially approach the end of their warranty period, we expect the amount and proportion of our revenues derived from service contracts to increase, although we expect the proportion to remain small. Since the fourth quarter of 2003, we have derived limited revenues from sales of our Patency System in Europe.
In November 2004 we received FDA marketing clearance for our new PillCam ESO video capsule, used for visualization of the esophagus, following receipt of the CE-Mark in September 2004, permitting us to market the product in Europe. On May 10, 2004, we entered into an exclusive sales representation, co-promotion and cooperation agreement with Ethicon Endo-Surgery, a Johnson & Johnson company. InScope, a business division of Ethicon Endo-Surgery established to market products to the gastroenterology market, has exclusive rights to market our PillCam ESO capsule. Under the agreement, we retain responsibility for regulatory approvals, manufacturing, supply chain, order invoicing and processing, collections and technical support for the PillCam ESO capsule. We retain sole ownership of the intellectual property associated with the Given System, the
41
PillCam capsules and any future innovations that we make. InScope is responsible for sales, marketing and promotion, performing clinical trials in territories covered by the agreement, as well as efforts to establish reimbursement policies for the PillCam ESO capsule. InScope will collaborate with us to expand the installed base of the Given System, which is our platform for the PillCam ESO capsule, the PillCam SB capsule and other capsules which we are developing.
To market and promote PillCam ESO, InScope created a sales force which has more than doubled the number of sales representatives promoting our PillCam technology in the United States. Subject to achieving specified minimum sales targets and meeting certain conditions, the exclusive term of the agreement will continue for up to 11 years followed by a four-year transition period during which InScope's marketing rights will be co-exclusive with ours. The alliance initially is worldwide, excluding Japan. InScope has begun marketing PillCam ESO capsules in the United States in November 2004 following FDA clearance for the PillCam ESO capsule. InScope has not yet commenced marketing activities in territories other than the United States, which territories may subsequently be excluded if we do not reach agreement with InScope regarding the marketing activities of InScope in these other territories by March 31, 2005 or a later date that may be agreed upon by the parties. We expect to generate a significant portion of our revenues in the future from sales of the PillCam ESO capsules, although there are uncertainties in the adoption rate until reimbursement is available.
Revenue trends and drivers
Since we commenced sales of the Given System, we have focused on growing our revenues both through sales of more systems and through capsule sales. We analyze our revenues using a number of different indicators described below:
Revenue breakdown. We consider the proportion of our revenues derived from sales of the Rapid workstations and portable data recorders, which are one-time payments, compared to the proportion of revenues derived from sales of the PillCam capsules, which generate recurring sales, to be an important tool for analyzing our results of operations. In 2002, we derived 37.7% of our revenues from sales of the PillCam SB capsule compared to 56.4% of our revenues in 2003 and 64.0% of our revenues in 2004. Sales of the PillCam ESO capsule, which began at the end of November 2004, represented an additional 2.8% of our revenues from capsule sales in 2004. As we have increased the installed base of Rapid workstations, the proportion of our revenues derived from sales of PillCam capsules has increased. An additional factor that drives the proportion of our revenues derived from sales of PillCam capsules is the usage rate per workstation. The usage rate per system during the last three years has varied slightly but has generally been in the range of 0.9 to 1.2 PillCam capsules per system per week in the United States and between 0.6 to 0.9 PillCam capsules per system per week globally (including the United States). We seek to increase the utilization of the Given System by a number of methods, including broadening the reimbursed indications, increased selling and marketing activities, more frequent contact with our customers, educating physicians regarding the clinical benefits of the PillCam capsules and enhancing operating efficiencies of the system. The increase in the usage rate is also key to attracting new customers to purchase and use the Given System and to maintaining our growth in revenues, as more potential customers are likely to purchase the Given System if they believe they will utilize it more frequently.
The following table sets forth information for the periods indicated regarding the breakdown of our revenues:
| | | $
| | % of annual revenues
|
| | | 2002
| | 2003
| | 2004
| | 2002
| | 2003
| | 2004
|
| | | (in thousands) | | | | | | | | | | | | |
Workstations and data recorders | | | 17,230 | | | | 15,879 | | | | 18,669 | | | | 59.6 | | | | 39.2 | | | | 28.7 | |
PillCam SB capsule | | | 10,889 | | | | 22,865 | | | | 41,622 | | | | 37.7 | | | | 56.4 | | | | 64.0 | |
PillCam ESO capsule | | | — | | | | — | | | | 1,829 | | | | — | | | | — | | | | 2.8 | |
Patency system and capsule | | | — | | | | 15 | | | | 188 | | | | — | | | | * | | | | * | |
Service | | | 785 | | | | 1,780 | | | | 2,712 | | | | 2.7 | | | | 4.4 | | | | 4.2 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Total | | | 28,904 | | | | 40,539 | | | | 65,020 | | | | 100.0 | % | | | 100.0 | % | | | 100.0 | % |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | | | | |
* Less than 1%.
42
PillCam capsule reorders. We consider the proportion of our revenues from PillCam capsule sales that are derived from reorders to be an important tool for analyzing our business. In 2002, 69.7% of PillCam capsule unit sales resulted from reorders compared to 87.7% in 2003 and 92.7% in 2004. We believe that reorders represent a potentially stable source of revenue provided that capsule usage per installed system remains the same or increases.
The following table sets forth information for the periods indicated regarding the total numbers of PillCam SB capsules sold and the percentage of revenues derived from such sales which represent reorders:
| | | | 2002
| | 2003
| | 2004
|
| | Total number of PillCam SB capsules sold* | | | 26,340 | | | | 51,400 | | | | 90,400 | |
| | Number of PillCam SB capsule sales representing reorders | | | 18,350 | | | | 45,100 | | | | 83,850 | |
| | % of revenues from capsule sales that represent reorders | | | 69.7 | % | | | 87.7 | % | | | 92.7 | % |
| | | | | | | | | | | | | | |
* Sales of PillCam ESO began only in November 2004 and are not included in the above table.
Seasonality
We believe that demand for systems and capsules may be affected by seasonal factors during the summer months when physicians and administrators are more likely to postpone purchasing decisions relating to the Given System due to summer vacations, and patients are more likely to postpone less urgent diagnostic procedures until later in the year. We believe that the seasonal effect in the third quarter may become more pronounced if the portion of our revenues derived from reorders continues to grow.
Geographical breakdown
We derived 72% of our revenues in 2004 from the United States compared to 65% in 2003. In 2004, we sold 479 systems in the United States, 112 systems in Europe and 63 in the rest of the world. The following table sets forth the geographic breakdown of our revenues for the periods indicated:
| | | | 2002
| | 2003
| | 2004
|
| | United States | | | 54% | | | | 65% | | | | 72% | |
| | Europe | | | 29% | | | | 29% | | | | 21% | |
| | Rest of World | | | 17% | | | | 6% | | | | 7% | |
| | | | | | | | | | | | | | |
We expect that a significant portion of our revenues in the foreseeable future will continue to come from the United States, mainly due to our ability to leverage existing market share to generate additional sales, our new strategic alliance with Ethicon Endo-Surgery for the marketing of the PillCam ESO, favorable reimbursement policies and general acceptance of new technologies among physicians.
We expect to experience increased sales of the Given System in Europe, primarily due to the expansion of reimbursement coverage. We do not, however, expect a significant increase in the percentage that revenues generated in Europe represent of our total revenues. In addition, we expect to begin selling the Given System in Japan following receipt of marketing clearance for the Given System in Japan, which we expect to receive in 2005. We do not expect to generate significant revenues from sales in Japan in 2005.
Reimbursement
We believe that availability of reimbursement is a key factor in the decision of physicians and healthcare providers to purchase the Given System and perform capsule endoscopy procedures. Once a payor has decided to provide reimbursement for use of the Given System, the level of reimbursement coverage provided also becomes an important key factor in a physician's decision. We estimate that as of December 31, 2004, reimbursement for the Given System was available
43
worldwide to approximately 327 million people of which approximately 206 million are located in the United States. In general, the process of obtaining coverage approvals has been slower outside of the United States. Prior to the beginning of 2003, all of the policies in the United States covered capsule endoscopy with PillCam SB capsules for suspected small intestinal bleeding. The number of policies covering other small bowel pathologies and indications has grown steadily since then and as of December 31, 2004 approximately 167 million people in the United States have expanded coverage for suspected Crohn's disease, suspected small bowel tumors and other small bowel pathologies. In Europe, reimbursement for capsule endoscopy was available for approximately 102 million people as of December 31, 2004 compared to approximately 40 million people at the end of 2003, and the number of lives with reimbursement for expanded indications, such as Crohn's disease and other small bowel disorders grew from 24 million at the end of 2003 to 87 million at the end of 2004. These increases were mainly the result of guidance issued in December 2004 by the National Institute of Clinical Excellence (NICE) of the United Kingdom National Health Service (NHS), allowing 58 million U.K. residents access to capsule endoscopy under the NHS' public health system.
Almost all of the reimbursement policies currently in effect for the PillCam SB capsule require that a previous procedure, such as endoscopy or radiology, be performed prior to using the Given System and some may require prior authorization. We intend to continue our efforts to establish the Given System as the first tool administered to patients with suspected disorders of the small bowel and to convince third party payors to provide reimbursement coverage for capsule endoscopy as a primary diagnostic tool by presenting to them economic and health outcomes analyses showing the benefits of using the PillCam SB capsule as a first line tool. The peer-reviewed journal Disease Management published in its Winter 2004 issue an economic analysis of using PillCam SB capsules as a first-line tool for diagnosing patients with suspected Crohn's disease. The study, conducted by researchers at Thomas Jefferson University, concluded that using the PillCam SB capsule as a first line procedure for detecting small bowel Crohn's disease is less costly from a third-party payor perspective than existing diagnostic approaches. This publication supports the company's efforts to convince payors to revise policies to cover PillCam SB capsules when performed as a first line procedure in patients with suspected Crohn's disease.
Under our exclusive sales representation and co-promotion agreement, Ethicon Endo-Surgery is responsible for obtaining reimbursement coverage for procedures performed with our PillCam ESO capsule. We do not expect a formal CPT code for the PillCam ESO capsule to be assigned before late 2006, effective January 2007. We believe that the existence of reimbursement coverage and the amount of reimbursement will continue to significantly affect the proportion of our revenues that are derived from sales in the United States.
Customers and customer concentration
We market and sell the Given System through a direct sales force in the United States, Germany, France, Australia and Israel. We rely on third-party distributors in international markets outside these countries. We sell the Given System primarily to hospitals, gastroenterology offices and gastroenterology outpatient facilities. In 2004, we derived $9.8 million, or 15%, of our revenues from sales to local distributors, compared to $7.3 million, or 18% in 2003. Our direct sales revenues are derived from a large number of individual customers and have a higher gross margin. In 2004, no single direct sales customer accounted for more than 1.2% of our revenues and no single distributor accounted for more than 2.5% of our revenues. It is our policy to require collateral or security in connection with sales to distributors. Due to these factors and the geographical dispersion of our customers, we believe that we adequately control our exposure to credit risks associated with accounts receivable. To date, we have not experienced any material bad debts and we have collected substantially all of our receivables.
Cost of revenues and gross margin
Cost of revenues consists primarily of materials, as well as manufacturing costs and related depreciation of our production facilities, the salary and related costs of our technical staff who
44
assemble our products, royalties payable to the Office of the Chief Scientist, warranty costs and product liability insurance.
The principal factors affecting our gross margin are related to the volume of PillCam capsules we sell and the product mix, namely the proportion of our revenues derived from sales of workstations and portable data recorders, compared to sales of the PillCam capsules, as well as the percentage of our sales made by direct sales. To date, our gross margins from sales of workstations and portable data recorders have been higher than our gross margins from sales of the PillCam capsules, although we expect that our gross margins from sales of the PillCam capsule will increase due to planned cost reductions from production efficiencies and reduced costs per capsule as we manufacture larger volumes of the PillCam capsule and spread the fixed costs associated with manufacturing over a larger base of sales. In addition, our gross margin in territories in which we use our direct sales force is higher than our gross margin in territories in which we market and sell our products through third-party distributors.
Operating expenses
Research and development. Our research and development expenses consist primarily of costs associated with the design, development, pre-manufacture and testing of, and enhancements to, the Given System, salaries and related personnel costs, clinical studies and obtaining regulatory approvals, patent costs, sponsored research costs and other expenses related to our product development and research program. We expense our research and development costs as they are incurred. “Research and development expenses, net” are net of grants received from the Israeli Government through the Office of the Chief Scientist of the Ministry of Industry and Trade. We plan to continue investing in research and development, as we enhance the Given System, pursue the development of new products and perform clinical trials to drive continued expansion of reimbursement for the PillCam capsules worldwide.
Sales and marketing. Our sales and marketing expenses consist primarily of salaries, commissions to our sales force, travel and related costs for our internal sales staff and costs related to marketing activities such as medical meetings, medical training and education, trade shows, and promotional and public relations activities, as well as costs associated with development of our website. We expect that our selling and marketing expenses will increase in the future as we increase sales of the PillCam ESO video capsule, further expand our sales and marketing team, expand our educational activities and expand our promotional efforts. A significant portion of this increase is attributable to the commissions we pay InScope under our exclusive sales representation, co-promotion and cooperation agreement for the marketing of the PillCam ESO video capsule. Under this agreement, we pay InScope commissions on sales in the United States at a rate of 50% commission on the sale of the PillCam ESO capsule and 10% on sales of other parts of the Given System, including workstations and data recorders. Under the terms of the agreement, we received a milestone payment of $10.0 million in December 2004 following FDA clearance of the PillCam ESO capsule and an additional $5.0 million in February 2005 as a result of completing an upgrade of 90% of our installed base in the United States to include RAPID 3.0 that our customers need in order to perform capsule endoscopy procedures with our PillCam ESO capsule. We may receive additional payments of up to $35.0 million by February 2007, contingent on achieving additional performance milestones applicable to both parties. We record these milestone payments as a reduction of marketing expenses and will recognize them ratably over the term of the agreement, which may be up to 15 years. Subject to achieving specified minimum sales targets and meeting certain conditions, the exclusive term of the agreement will continue for up to 11 years followed by a four-year transition period during which InScope's marketing rights will be co-exclusive with ours.
General and administrative. Our general and administrative expenses consist primarily of salaries and related costs for our executive and administrative staff, insurance premiums, and legal, accounting and consulting expenses. We expect general and administrative expenses to increase for the foreseeable future as our operations continue to expand.
45
Equity-based compensation. Our operating expenses also include amortization of stock-based compensation, which is allocated among research and development expenses, marketing expenses and general and administrative expenses based on the division in which the recipient of the option grant is employed. Amortization of stock-based compensation results from the granting of options to employees at below market value (calculated as the difference between the exercise price and the trading price of our ordinary shares) and to consultants at below fair market value (calculated using the Black-Scholes model) per share of our ordinary shares on the dates of grant. The stock-based compensation is being amortized to operating expenses over the vesting periods of the individual options. We incurred stock-based compensation expense in the amount of $78,000 in 2004, $105,000 in 2003, and $227,000 in 2002.
In December 2004, the Financial Accounting Standard Board, or FASB, issued a new Financial Accounting Standard, FAS 123R, that requires companies, including Given Imaging, to recognize as an expense the grant-date fair value of stock options and other equity-based compensation to employees. Under this new rule we will be required to recognize such expenses as of the third quarter of 2005. Based upon our projection of unvested stock options at the implementation date, we expect the adoption of FAS 123R to result in the recognition of material additional compensation expense in 2005 and thereafter and, consequently, we expect it will have a material impact on our projected earnings per share. We currently expect to adopt FAS 123R using the modified prospective method for the valuation of our equity-based compensation, although we continue to review alternative valuation methods permissible under this new pronouncement.
Financing income, net. Financing income, net consists primarily of interest earned on our cash balances and other financial investments and foreign exchange gains, net of financing expenses. Financing expenses consist primarily of bank fees, currency fees and credit card commissions.
Taxes. In 2004, Israeli companies were generally subject to income tax at the corporate tax rate of 35%. This tax rate is expected to be reduced to 30% by 2007. However, our investment program in leasehold improvements and equipment at our manufacturing facility in Yoqneam, Israel has been granted approved enterprise status and, therefore, we are eligible for the reduced tax benefits described later in this section in “—Corporate Tax.” These benefits should result in income recognized by us from our investment program being tax exempt for a specified period after we begin to report taxable income and exhaust any net operating loss carry-forwards. However, these benefits may not be applied to reduce the tax rate for any income that is not derived from sales of our product manufactured at our facility in Yoqneam, Israel. We recorded a deferred tax asset of $737,000 as of December 31, 2004. Based upon our projections for future taxable income in our U.S. subsidiary over the periods in which the deferred tax assets are deductible, we believe that we will realize the benefits of these deductible differences. The amount of the deferred tax asset considered realizable, however, could be reduced in the near term if estimates of future taxable income during the carry forward period are reduced.
Minority share in losses (profits) of subsidiary. Minority share in losses (profits) of subsidiary consists of the losses attributed to the 49% interest of minority shareholders in our 51% controlled Japanese subsidiary, Given Imaging K.K.
Critical Accounting Policies
Our significant accounting policies are more fully described in Note 1 to our consolidated financial statements. However, certain of our accounting policies are particularly important to the portrayal of our financial position and results of operations. In applying these critical accounting policies, our management uses its judgment to determine the appropriate assumptions to be used in making certain estimates. Those estimates are based on our historical experience, the terms of existing contracts, our observance of trends in the industry, information provided by our customers and information available from other outside sources, as appropriate. These estimates are subject to an inherent degree of uncertainty. With respect to our policies on revenue recognition, warranty costs and inventories, our historical experience is based principally on our operations since we
46
commenced selling the Given System in the second quarter of 2001. Our critical accounting policies include:
| • | Revenue recognition. We recognize revenues from sales of the Given System upon delivery, provided that collection of payment is probable, there is persuasive evidence of an arrangement, no significant obligations in respect of installation remain and the price is fixed or determinable. Our arrangements with customers and distributors do not contain product return rights. Certain of our sales contracts include a post-contract customer support, or PCS, component. We defer recognition of the revenue attributed to the PCS component of the sale and recognize based on the term of the support period, which is generally one-year period following the sale. We determine the fair value of the PCS component based on the fair value of the sale contract as a whole. Currently, the fair value of the PCS component is based on the price at which we sell customer support contracts separately following the expiration of the PCS component included in the initial sale of the Given System. |
| |
| • | Warranty costs. Our products are usually covered by a one-year warranty following sale. We accrue estimated warranty costs at the time of shipment. Our warranty reserve is based on our best estimate of the amounts necessary to settle future claims on products sold as of the balance sheet date based on contractual warranty rights and our historical experience of the frequency of failures of our products which are not covered by warranties that our suppliers give to us. The amount of our estimated warranty liability is currently approximately 3.0% of the sales of such products and may change if the costs incurred due to product failures increase in the future. In 2004, our warranty costs did not exceed our reserve of $39,000. In the event of any future problems with our products, we will need to increase the amount of our reserves. |
| |
| • | Inventories. Inventories are stated at the lower of cost or market, cost being determined on the basis of the average cost method for raw materials and finished goods and on the basis of actual manufacturing costs for work-in-progress and sub-contractors. We write down fully the cost of components in our inventory which we discover do not perform during the production process. As we expand and enhance our manufacturing operations, the write down of amounts of non-performing components in our inventory may change. Spare parts and raw materials that are no longer used in producing our product are written down to their fair market value. In addition, we add to the cost of finished products held in inventory the overhead from our manufacturing process. If these estimates change in the future, the amount of overhead allocated to cost of revenues would change. |
| |
| • | Foreign currency translation. In preparing our consolidated financial statements, we are required to translate non-U.S. dollar amounts in our financial statements and the financial statements of our subsidiaries into U.S. dollars. Under the relevant accounting guidance the treatment of any gains or losses resulting from this translation is dependent upon our management's determination of the functional currency of each subsidiary. The functional currency is determined based on management's judgment and involves consideration of all relevant economic facts and circumstances affecting the subsidiary. Generally, the currency in which the subsidiary transacts a majority of its transactions, including billings, financing, payroll and other expenditures would be considered the functional currency. However, any dependency upon the parent and the nature of the subsidiary's operations must also be considered. If any subsidiary's functional currency is deemed to be the local currency, then any gain or loss associated with the translation of that subsidiary's financial statements into U.S. dollars would be included as other comprehensive income. However, if the functional currency of a subsidiary is deemed to be the U.S. dollar then any gain or loss associated with the translation of these financial statements would be included within our statement of operations. Based on our assessment of the factors discussed above, we consider the U.S. dollar to be the functional currency for each of our subsidiaries. Therefore, all gains and losses from translations are recorded in our statement of operations and are included in determining our net income. In the event that we determine that the functional currency of these or any future subsidiaries is not the U.S. dollar, any foreign currency gains or losses would not affect our net income for the year presented. |
47
| • | Accounting for income taxes. As part of the process of preparing our consolidated financial statements we are required to estimate our income taxes in each of the jurisdictions in which we operate. This process requires us to estimate our actual current tax exposure and make an assessment of temporary differences resulting from differing treatment of items, for tax and accounting purposes. These differences result in deferred tax assets and liabilities, which are included within our consolidated balance sheet. We must then assess the likelihood that our deferred tax assets will be recovered and, to the extent we believe that recovery is not likely, we must establish a valuation allowance. To the extent we establish a valuation allowance or increase this allowance in a period, we must include an expense within the tax provision in the statement of operations. Significant management judgment is required in determining our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We have recorded a deferred tax asset of $737,000 as of December 31, 2004. Based upon our projections for future taxable income in our US subsidiary over the periods in which the deferred tax assets are deductible, we believe that we will realize the benefits of these deductible differences. The amount of the deferred tax asset considered realizable, however, could be reduced in the near term if estimates of future taxable income during the carry forward period are reduced. |
Results of Operations
Our consolidated statements of operations data for the years ended December 31, 2002, 2003 and 2004 are set forth below:
| | | Year ended December 31,
|
| | | 2002
| | 2003
| | 2004
|
| | | (in thousands) |
Statements of Operations Data: | | | | | | | | | | | | |
Revenues | | $ | 28,904 | | | $ | 40,539 | | | $ | 65,020 | |
Cost of revenues | | | 11,907 | | | | 13,551 | | | | 17,734 | |
| | | |
| | | |
| | | |
| |
Gross profit | | | 16,997 | | | | 26,988 | | | | 47,286 | |
Operating expenses: | | | | | | | | | | | | |
Research and development, gross | | | (8,609 | ) | | | (7,037 | ) | | | (7,363 | ) |
Royalty-bearing participation | | | — | | | | 1,303 | | | | 1,140 | |
| | | |
| | | |
| | | |
| |
Research and development, net | | | (8,609 | ) | | | (5,734 | ) | | | (6,223 | ) |
Sales and marketing | | | (22,681 | ) | | | (26,804 | ) | | | (33,652 | ) |
General and administrative | | | (4,749 | ) | | | (5,312 | ) | | | (6,916 | ) |
| | | |
| | | |
| | | |
| |
Total operating expenses | | | (36,039 | ) | | | (37,850 | ) | | | (46,791 | ) |
| | | |
| | | |
| | | |
| |
Operating profit (loss) | | | (19,042 | ) | | | (10,862 | ) | | | 495 | |
| | | |
| | | |
| | | |
| |
Financing income, net | | | 1,469 | | | | 995 | | | | 956 | |
Other expenses, net | | | (711 | ) | | | — | | | | — | |
| | | |
| | | |
| | | |
| |
Profit (loss) before taxes on income | | | (18,284 | ) | | | (9,867 | ) | | | 1,451 | |
Taxes on income | | | — | | | | — | | | | 690 | |
| | | |
| | | |
| | | |
| |
Profit (loss) before minority share | | | (18,284 | ) | | | (9,867 | ) | | | 2,141 | |
Minority share in (profits) losses of subsidiary | | | (26 | ) | | | 258 | | | | 747 | |
| | | |
| | | |
| | | |
| |
Net profit (loss) | | $ | (18,310 | ) | | $ | (9,609 | ) | | $ | 2,888 | |
| | | |
| | | |
| | | |
| |
48
Our historical operating results as a percentage of net revenues for the years ended December 31, 2002, 2003 and 2004 are set forth below:
| | | Year ended December 31,
|
| | | 2002
| | 2003
| | 2004
|
Statements of Operations Data: | | | | | | | | | | | | |
Revenues | | | 100.0 | % | | | 100.0 | % | | | 100.0 | % |
Cost of revenues | | | 41.2 | | | | 33.4 | | | | 27.3 | |
| | | |
| | | |
| | | |
| |
Gross profit | | | 58.8 | | | | 66.6 | | | | 72.7 | |
Operating expenses: | | | | | | | | | | | | |
Research and development, gross | | | 29.8 | | | | 17.4 | | | | 11.3 | |
Royalty-bearing participation | | | — | | | | 3.2 | | | | 1.7 | |
| | | |
| | | |
| | | |
| |
Research and development, net | | | 29.8 | | | | 14.2 | | | | 9.6 | |
Sales and marketing | | | 78.5 | | | | 66.1 | | | | 51.8 | |
General and administrative | | | 16.4 | | | | 13.1 | | | | 10.6 | |
| | | |
| | | |
| | | |
| |
Total operating expenses | | | 124.7 | | | | 93.4 | | | | 72.0 | |
| | | |
| | | |
| | | |
| |
Operating profit (loss) | | | (65.9 | ) | | | (26.8 | ) | | | 0.7 | |
Financing income, net | | | 5.1 | | | | 2.5 | | | | 1.5 | |
| | | |
| | | |
| | | |
| |
Other expenses, net | | | 2.5 | | | | — | | | | — | |
| | | |
| | | |
| | | |
| |
Profit (loss) before taxes on income | | | (63.3 | ) | | | (24.3 | ) | | | 2.2 | |
Taxes on income | | | — | | | | — | | | | 1.1 | |
| | | |
| | | |
| | | |
| |
Profit (loss) before minority share | | | (63.3 | ) | | | (24.3 | ) | | | 3.3 | |
Minority share in (profits) losses of subsidiary | | | (0.0 | ) | | | 0.6 | | | | 1.1 | |
| | | |
| | | |
| | | |
| |
Net profit (loss) | | | (63.3 | ) | | | (23.7 | ) | | | 4.4 | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | |
Year Ended December 31, 2004 compared to Year Ended December 31, 2003
Revenues. Revenues increased by $24.5 million, or 60.4%, to $65 million in 2004 from $40.5 million in 2003. This increase was primarily due to an increase of $20.7 million, or 91%, in capsule sales, an increase of $2.8 million, or 17.6%, in workstation and data recorder sales, and an increase of $0.9 million, or 52.4%, in service contract revenues. We estimate that approximately 92.6% of the increase of $24.5 million in revenues was attributable to the increase in product sales, approximately 6.2% of the increase was attributable to currency gains due to the strengthening of the Euro and the Australian dollar against the U.S. dollar, and approximately 1.1% of the increase was attributable to higher average selling prices resulting from a higher percentage of direct sales compared to sales through distributors and an increase in selling prices during the year.
Cost of revenues and gross margin. Cost of revenues increased by $4.2 million, or 30.9%, to $17.7 million in 2004 from $13.6 million in 2003, compared to an increase of 60.4% in revenues during the same period. This increase occurred due to the increase in sales of the PillCam SB capsule in 2004 and primarily resulted from an increase of $3.1 million related to the cost of materials and components, an increase of $1.2 related to manufacturing expenses, including depreciation, offset by a decrease of $0.1 in royalties payable to the Office of the Chief Scientist. The smaller increase in cost of revenues compared to revenues resulted primarily from a decrease in the cost of components for the Given System, an increase in manufacturing volumes from the smaller quantities we had manufactured in 2003 and an increase in manufacturing efficiencies. Gross margin was 72.7% in 2004 compared to 66.6% in 2003.
Research and development. Research and development expenses increased by $0.4 million, or 4.6%, to $7.4 million in 2004 from $7.0 million in 2003. This increase was primarily due to $0.3 million related to regulatory compliance and quality assurance processes related to the launch of our new products in 2004.
49
Research and development expenses, net of grants received from the Office of the Chief Scientist, increased by $0.5 million, or 8.5%, to $6.2 million in 2004 from $5.7 million in 2003. Grants totaling $1.1 million were received in 2004 compared to grants totaling $1.3 million received in 2003. In both years, the grants were received for new products under development.
Sales and marketing. Sales and marketing expenses increased by $6.9 million, or 25.5%, to $33.7 million in 2004 from $26.8 million in 2003. This increase primarily consists of an increase of $3.0 million due to the hiring of additional sales and marketing personnel, principally in the United States, and an increase in commissions paid to these personnel, the initial payment of $0.8 million paid as commission to Ethicon Endo-Surgery for sales of our new PillCam ESO capsule, an increase of $0.7 million due to participation in trade shows and other marketing events, an increase of $0.6 million due to clinical, regulatory and marketing activities in Japan of our Japanese subsidiary, Given Imaging K.K. and a $0.4 million provision for the misappropriation of funds at our U.S. subsidiary.
General and administrative. General and administrative expenses increased by $1.6 million, or 30.2%, to $6.9 million in 2004 from $5.3 million in 2003. This increase was primarily due to an increase of $0.7 million related to consultants and professional fees expenses, $0.3 million related to salaries and related expenses, $0.4 million related to increased D&O insurance costs and other miscellaneous general and administrative expenses and a $0.2 million provision for the investigation costs related to the misappropriation of funds at our U.S. subsidiary.
Financing income, net. Financing income, net, remained at the same level as in 2003 at about $1.0 million. Financing income in 2004 was derived primarily from interest income of $0.7 million and currency translation gains of $0.4 million, offset by bank expenses of $0.2 million. Financing income in 2003 was derived primarily from currency translation gains of $0.8 million, interest income of $0.3 million offset by bank expenses of $0.1 million.
Taxes on income. We had taxable income of $0.7 million in 2004, compared to no taxable income in 2003. This tax income was derived primarily from the release of part of the valuation allowance on deferred taxes assets in our US subsidiary due to our belief that it is more likely than not that this benefit will be realized by this subsidiary.
Minority share in losses (profits) of subsidiary. Minority share in losses of subsidiary was $0.7 million in 2004 compared to minority share in losses of subsidiary of $0.3 million in 2003. Given Imaging K.K. did not derive any revenues in 2004.
Misappropriation of funds in U.S. subsidiary. In connection with the preparation and audit of our 2004 financial statements, we discovered that a former employee of our U.S. subsidiary had misappropriated approximately $0.4 million during the period 2002 through 2004, with the bulk of the misappropriation taking place in 2004. We undertook an investigation, which was completed in February 2005, with the assistance of independent legal counsel and forensic accountants. The investigators did not uncover any evidence that any other employee knowingly participated in the misappropriation. Our financial statements for the year ended December 31, 2004 include a provision of $0.6 million, which we estimated would be adequate to cover substantially all of the misappropriated amount and the costs of investigation. Based on the amount and nature of the misappropriation, we believe that it did not have a material effect on our results of operations for the years ended December 31, 2002 and 2003. In March 2005, we received $0.3 million in partial restitution of the misappropriated funds.
Year Ended December 31, 2003 compared to Year Ended December 31, 2002.
Revenues. Revenues increased by $11.6 million, or 40.3%, to $40.5 million in 2003 from $28.9 million in 2002. This increase was primarily due to an increase of $12.0 million, or 110%, in capsule sales and an increase of $1.0 million, or 126.7%, in service contract revenues, offset by a decrease of $1.4 million, or 7.8%, in workstation and data recorder sales. We estimate that approximately 70% of the increase of $11.6 million in revenues was attributable to the increase in product sales, approximately 17% of the increase was attributable to currency gains due to the strengthening of the Euro and the Australian dollar against the U.S. dollar, and approximately
50
13% of the increase was attributable to higher average selling prices resulting from a higher percentage of direct sales compared to sales through distributors and an increase in selling prices during the year. We believe that the decrease in workstation and data recorder sales resulted primarily from our decision to permit our distributors, principally in the Far East, not to comply with minimum purchase requirements in order to reduce their inventory levels.
Cost of revenues and gross margin. Cost of revenues increased by $1.6 million, or 13.8%, to $13.6 million in 2003 from $11.9 million in 2002, compared to an increase of 40.3% in revenues during the same period. This increase occurred due to the increase in sales of the PillCam SB capsule in 2003 and primarily resulted from an increase of $2.1 million related to the cost of materials and components, an increase of $130,000 related to manufacturing expenses, including depreciation, offset by a decrease of $0.5 million in royalties payable to the Office of the Chief Scientist. The smaller increase in cost of revenues compared to revenues resulted primarily from a decrease in the cost of components for the Given System, an increase in manufacturing volumes from the smaller quantities we had manufactured in 2002 and an increase in manufacturing efficiencies achieved by moving to a semi-automated production line. Gross margin was 66.6% in 2003 compared to 58.8% in 2002.
During 2003, we implemented a plan to reduce our operating costs in order to achieve our profitability targets. This plan included a reduction in force of approximately 40 employees, or 15% percent of our workforce at the time, principally from our manufacturing facilities, as well as from other departments. It also included deferring non-essential expenditures. The implementation of this plan contributed to the smaller increase in our cost of revenues and operating expenses compared to the increase in our revenues.
Research and development. Research and development expenses decreased by $1.6 million, or 18.3%, to $7.0 million in 2003 from $8.6 million in 2002. This decrease was primarily due to a write down of obsolete components in connection with production modifications and redesign in 2002, which did not recur in 2003.
Research and development expenses, net of grants received from the Office of the Chief Scientist, decreased by $2.9 million, or 33.4%, to $5.7 million in 2003 from $8.6 million in 2002. Grants totaling $1.3 million were received in 2003 compared to no grants in 2002. In 2003, we received grants for new products under development. In 2002, we did not receive any grants due to completion of our program to develop the Given System for which we had previously received grants from the Office of the Chief Scientist.
Sales and marketing. Sales and marketing expenses increased by $4.1 million, or 18.2%, to $26.8 million in 2003 from $22.7 million in 2002. This increase was primarily due to an increase of $3.5 million due to the hiring of additional sales and marketing personnel, principally in the United States and Europe, and an increase of $0.8 million due to sales and marketing activities in Japan of our Japanese subsidiary, Given Imaging K.K.
General and administrative. General and administrative expenses increased by $563,000, or 11.9%, to $5.3 million in 2003 from $4.7 million in 2002. This increase was primarily due to an increase of $0.2 million related to insurance expenses, $0.1 million related to depreciation expenses and $0.2 million related to other miscellaneous general and administrative expenses.
Financing income, net. Financing income, net, decreased by $474,000, or 32.3%, to $1.0 million in 2003 from $1.5 million in 2002. Financing income in 2003 was derived primarily from currency translation gains of $0.8 million, interest income of $0.3 million offset by bank expenses of $0.1 million. Financing income in 2002 was derived primarily from interest income of $0.8 million and currency translation gains of $0.8 million. The decrease was primarily due to reduction of $0.5 million in interest income.
Minority share in losses (profits) of subsidiary. Minority share in losses of subsidiary was $0.3 million in 2003 compared to minority share in profits of subsidiary of $26,000 in 2002. Given Imaging K.K. did not derive any revenues in 2003.
51
Quarterly Results of Operations
We believe that many of our customers delay purchasing systems until the end of the fiscal quarter because they believe this will enable them to negotiate more favorable terms. Therefore, revenues from system sales are frequently concentrated at the end of each fiscal quarter making it difficult for us to determine the revenues for each quarter until its end and resulting in lower than expected quarterly revenues if external or other events cause a large number of potential customers to defer their purchasing decisions even for a short period of time. To date, revenues from sales of the PillCam capsules have been spread more evenly throughout the quarter. In addition, we believe that demand for systems and capsules is affected by seasonal factors during the summer months when physicians and administrators are more likely to postpone purchasing decisions relating to the Given System due to summer vacations, and patients are more likely to postpone less urgent diagnostic procedures until later in the year. We believe that the seasonal effect in the third quarter may become more pronounced assuming the portion of our revenues derived from reorders continues to grow.
The tables below set forth unaudited consolidated statement of operations data in dollars for each of the eight consecutive quarters ended December 31, 2004. In management's opinion, the unaudited consolidated financial statements have been prepared on the same basis as our audited consolidated financial statements contained elsewhere in this Form 20-F and include all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of such financial information.
| | | Three months ended
|
| | | March 31,
2003
| | June 30,
2003
| | Sept. 30,
2003
| | Dec. 31,
2003
| | March 31,
2004
| | June 30,
2004
| | Sept. 30,
2004
| | Dec. 31,
2004
|
| | | (unaudited)
(in thousands) |
Revenue | | $ | 8,629 | | | $ | 9,694 | | | $ | 9,682 | | | $ | 12,534 | | | $ | 12,751 | | | $ | 15,462 | | | $ | 14,594 | | | $ | 22,213 | |
Cost of revenues | | | (2,827 | ) | | | (3,363 | ) | | | (3,581 | ) | | | (3,780 | ) | | | (3,736 | ) | | | (3,782 | ) | | | (3,636 | ) | | | (6,580 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Gross profit | | | 5,802 | | | | 6,331 | | | | 6,101 | | | | 8,754 | | | | 9,015 | | | | 11,680 | | | | 10,958 | | | | 15,633 | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development, net | | | (1,834 | ) | | | (1,213 | ) | | | (1,212 | ) | | | (1,475 | ) | | | (1,621 | ) | | | (1,302 | ) | | | (1,831 | ) | | | (1,469 | ) |
Sales and marketing | | | (6,521 | ) | | | (6,998 | ) | | | (6,219 | ) | | | (7,066 | ) | | | (7,019 | ) | | | (7,864 | ) | | | (7,705 | ) | | | (11,064 | ) |
General and administrative | | | (1,366 | ) | | | (1,425 | ) | | | (1,233 | ) | | | (1,288 | ) | | | (1,208 | ) | | | (1,758 | ) | | | (1,678 | ) | | | (2,272 | ) |
Total operating expenses | | | (9,721 | ) | | | (9,636 | ) | | | (8,664 | ) | | | (9,829 | ) | | | (9,848 | ) | | | (10,924 | ) | | | (11,214 | ) | | | (14,805 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Operating profit (loss) | | | (3,919 | ) | | | (3,305 | ) | | | (2,563 | ) | | | (1,075 | ) | | | (833 | ) | | | 756 | | | | (256 | ) | | | 828 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Financing income (expenses), net | | | 228 | | | | (174 | ) | | | 573 | | | | 368 | | | | 61 | | | | (124 | ) | | | 165 | | | | 854 | |
Taxes on income | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 690 | |
Minority share in (profits) losses of subsidiary | | | 68 | | | | 129 | | | | (67 | ) | | | 128 | | | | 187 | | | | 289 | | | | 205 | | | | 66 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Net profit (loss) | | $ | (3,623 | ) | | $ | (3,350 | ) | | $ | (2,057 | ) | | $ | (579 | ) | | $ | (585 | ) | | $ | 921 | | | $ | 114 | | | $ | 2,438 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Impact of Currency Fluctuations
Currency Risk. Our sales to our customers in 2004 were denominated 75.1% in U.S. dollars, 20.5% in Euros and 4.4% in other currencies, depending on the location of the customer or the distributor used to fulfill our customers' orders. In 2004, 28.2% of our expenses, principally salaries and related personnel expenses were denominated in shekels, and we expect this level of shekel expenses to continue for the foreseeable future. If the value of a currency in which our revenues are denominated weakens against the value of a currency in which our expenses are denominated, there will be a negative impact on the profit margin for sales of the Given System. In addition, as of December 31, 2004, 10.1% of our cash and cash equivalents were denominated in currencies other than U.S. dollar and we are therefore subject to the risk of exchange rate fluctuations between the Yen, the New Israeli Shekel, the U.S. dollar and the Euro. We have covered an
52
expected surplus of Euros during 2005 against the devaluation of the Euro compared to the U.S. dollar by purchasing options to sell Euros and buy U.S. dollars at a pre-determined price. In addition, if we wish to maintain the dollar-denominated value of our product in non-U.S. markets, devaluation in the local currencies of our customers relative to the U.S. dollar could cause our customers to cancel or decrease orders or default on payment.
Translation Effect. The U.S. dollar is our functional currency, and our consolidated financial statements included elsewhere in this annual report are prepared in U.S. dollars.
B. Liquidity and Capital Resources
Since our inception and until we commenced sales in 2001, we financed our operations primarily with the proceeds of sales of our equity securities. From our inception through December 31, 2004, we raised a total of $144.5 million through public and private sales of our equity securities. Since we commenced sales in 2001, we have collected more than $134 million in receivables. As of December 31, 2004, we had $80.9 million in cash and cash equivalents, and our working capital, which we calculate by subtracting our current liabilities from our current assets, was $93 million.
In June 2004, we completed a follow-on offering of our ordinary shares in the United States in which we raised net proceeds of $44.3 million, after deducting underwriting discount and offering expenses. We intend to use the proceeds from this offering to fund business growth and expansion, provide additional working capital and for general corporate purposes. In addition, we achieved profitability in the second quarter of 2004 and as of December 31, 2004 we had $2.9 million in net profits. We believe that our cash reserves and expected cash from operations will be sufficient to meet our anticipated cash needs for working capital and capital expenditures in 2005. We have also applied to the Office of Chief Scientist for a grant to support our research and development activities in 2005.
The following table sets forth the components of our cash flows for the periods indicated:
| | | | Year ended December 31,
|
| | | | 2002
| | 2003
| | 2004
|
| | | | (in thousands) |
| | Net cash provided by (used in) operating activities | | $ | (22,233 | ) | | $ | (8,548 | ) | | $ | 11,868 | |
| | Net cash used in investing activities | | | (7,560 | ) | | | (2,647 | ) | | | (3,230 | ) |
| | Net cash provided by financing activities | | | 4,419 | | | | 653 | | | | 46,816 | |
| | Effect of exchange rate changes on cash | | | (64 | ) | | | 117 | | | | 40 | |
| | | | |
| | | |
| | | |
| |
| | Increase (decease) in cash and cash equivalents | | $ | (25,438 | ) | | $ | (10,425 | ) | | $ | 55,494 | |
| | | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | |
Net cash derived from operating activities was $11.9 million in 2004, compared to $8.5 million used in 2003 and $22.2 million used in 2002. The transition from decrease in cash in 2003 to increase in cash in 2004 resulted primarily from net income of $2.9 million, a $12.5 million improvement compared to the $9.6 million loss in 2003, the receipt of a $10 million milestone payment from Ethicon Endo-Surgery following FDA clearance for our new PillCam ESO capsule, and an increase of $7.1 million in our accounts payable, compared to a decrease in our account payables of $4.7 million in 2003. The increase in net cash derived from operations was offset by an increase of $5.6 million in inventories, compared to a decrease in inventories of $1.9 million in 2003, and an increase of $5.3 in our accounts receivable. The increase in inventories was primarily as a result of the build up of inventory to support increased sales of our products and the launch of our new PillCam ESO, DR 2.0 and Rapid 3.0 and our increase of inventory of strategic components of our products. The decrease in net cash used in operating activities from 2002 to 2003 resulted primarily from the decrease of $8.7 million in our loss, a decrease of $1.8 million in inventories, compared to an increase of $7.4 million in inventories in 2002, an increase of $0.1 million in accounts receivable, compared to an increase in account receivables of $4.4 million in 2002, a decrease of $1.0 in other accounts receivables, compared to an increase of $0.6 million in 2002, partially offset by a decrease of $4.7 million in accounts payable, compared to an increase of $5.5 million in 2002. The decrease in accounts payable resulted principally from our decision in
53
2003 to decrease our inventories during 2003. We expect that net cash provided by operating activities will increase during 2005.
Net cash used in investing activities was $3.2 million in 2004, compared to $2.6 million in 2003 and $7.6 million in 2002. Investing activities consist primarily of capital expenditures and the capitalization of costs associated with our patents and trademarks . Our principal capital expenditures in 2004 were $1.4 million for machinery and equipment, $0.3 million for the new lease of our facilities and $0.8 million for patents. Our principal capital expenditures in 2003 were $1.5 million for machinery and equipment and $0.5 million for patents. We expect that net cash used in investing activities will increase in 2005 to support our growth plans.
Net cash provided by financing activities was $46.8 million in 2004, compared to $0.7 million in 2003 and $4.4 million in 2002. The increase from 2003 to 2004 was primarily attributable to the completion of our follow-on public equity offering in June 2004 in which we raised net proceeds of $44.3 million. In 2003, net cash provided by financing activities resulted primarily from proceeds from the exercise of employee stock options.
Cash provided by operating activities net of cash used in investing activities, or net cash surplus, in 2004 was approximately $8.6 million. We expect to increase net cash surplus as our sales develop and we increase profitability.
The following table of our material contractual obligations as of December 31, 2004, summarizes the aggregate effect that these obligations are expected to have on our cash flows in the periods indicated:
| | | Payments due by period
|
Contractual obligations
| | Total
| | 2005
| | 2006
| | 2007
| | 2008
| | Later Years
|
| | | (in thousands) |
Capital leases(1) | | $ | 80 | | | $ | 14 | | | $ | 14 | | | $ | 14 | | | $ | 20 | | | $ | 18 | |
Operating leases(2) | | | 16,044 | | | | 2,231 | | | | 2,042 | | | | 1,724 | | | | 1,342 | | | | 8,705 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Total | | $ | 16,124 | | | $ | 2,245 | | | $ | 2,056 | | | $ | 1,738 | | | | 1,362 | | | $ | 8,723 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | |
| (1) | | Consists of capital leases for motor vehicles. |
| | | |
| (2) | | Consists of operating leases for office, manufacturing space and motor vehicles. |
See Note 7 to our consolidated financial statements included in this annual report for our royalty commitments and our obligations in connection with our supply agreement with Zarlink Semiconductor.
Market Risk
We invest our excess cash in bank accounts and deposits located with a number of banks inside and outside of Israel, principally in Europe. These instruments have maturities of three months or less when acquired. Due to the short-term nature of these investments, we believe that there is no material exposure to interest rate risk arising from our investments.
Corporate Tax
Israeli companies were generally subject to income tax at the corporate rate of 35% in 2004. This tax rate is expected to be reduced to 30% by 2007. As of December 31, 2004, our net operating loss carry-forwards for Israeli tax purposes amounted to $25 million. Under Israeli law, net operating losses can be carried forward indefinitely and offset against certain future taxable income.
In addition, our investment program in equipment and leasehold improvements at our manufacturing facility in Yoqneam, Israel has been granted approved enterprise status and we are, therefore, eligible for tax benefits under the Law for the Encouragement of Capital Investments, 1959. Subject to compliance with applicable requirements, the portion of our undistributed income derived from our approved enterprise program will be exempt from corporate tax for a period of
54
ten years commencing in the first year in which we generate taxable income. The ten-year period may not extend beyond the later of 14 years from the year in which approval was granted or 12 years from the year in which operations or production by the enterprise began. We received our approved enterprise status in 1999. As we continue to expand our production facilities, we have applied, and received, in 2002, an approval for the expansion of our production facilities during the subsequent two years under the alternative benefits track. The investments are expected to amount to $4.9 million and will be invested in Yoqneam, Israel. The plan is subject to certain terms set forth in the approval letter. In October 2004, we submitted an additional application in an amount of $10.1 million for additional expansion of our production facilities in the foreseeable future. We cannot assure you that we will receive approvals in the future for approved enterprise status or that tax benefits for approved enterprises will continue at current levels.
The period of tax benefits for our approved enterprise programs has not yet commenced because we are yet to realize taxable income. We expect that a substantial portion of the income we derive in the future will be from this approved enterprise program. These benefits should result in income recognized by us being tax exempt for a specified period after we begin to report taxable income and exhaust any net operating loss carry-forwards. These benefits may not be applied to reduce the tax rate for any income that is not derived from sales of our product manufactured at our facility in Yoqneam, Israel.
Our approved enterprise status imposes certain requirements on us, such as the location of our manufacturing facility, location of certain subcontractors and the extent to which we may outsource portions of our production process. These requirements limit our freedom to pursue production arrangements that may otherwise be more favorable to us if we want to maintain these tax benefits. Therefore, we may be required to weigh the possible loss of these benefits against other benefits from pursuing arrangements which are not, or which may not be considered by the relevant Israeli authorities to be, in compliance with these requirements. If we do not meet these requirements, the law permits the authorities to cancel the tax benefits retroactively.
As of December 31, 2004, the net operating loss carry-forwards of our subsidiaries for tax purposes amounted to $22.5 million. A subsidiary's net operating loss carry-forwards for tax purposes relating to a jurisdiction are generally available to offset future taxable income of such subsidiary in that jurisdiction, subject to applicable expiration dates.
Government Grants
Our research and development efforts have been financed, in part, through grants from the Office of the Chief Scientist of the Israeli Ministry of Industry, Trade and Labor. We have applied and received approval for grants totaling $3.6 million from the Office of the Chief Scientist, including $1.1 million, which was provided by the Office of Chief Scientist to support our 2004 research and development.
Under Israeli law, royalties on the revenues derived from sales of the Given System or any part of the Given System are payable to the Israeli government, generally at the rate of 3.0% during the first three years and 3.5% beginning with the fourth year. The maximum aggregate royalties paid generally cannot exceed 100%. The amount bears interest equal to the 12-month London Interbank Offered Rate (LIBOR) applicable to dollar deposits that is published on the first business day of each calendar year. Royalties are paid on our consolidated revenues.
The government of Israel does not own proprietary rights in technology developed using its funding and there is no restriction on the export of products manufactured using the technology. The technology is, however, subject to other legal restrictions, including the obligation to manufacture the product based on this technology in Israel and to obtain the Office of the Chief Scientist's consent to transfer the technology or product rights to a third party. These restrictions may impair our ability to outsource manufacturing or enter into similar arrangements for those products or technologies and they continue to apply even after we have paid the full amount of royalties payable for the grants. If the Office of the Chief Scientist consents to the manufacture of the products outside Israel, the regulations allow the Office of the Chief Scientist to require the
55
payment of increased royalties, ranging from 120% to 300% of the amount of the grant plus interest, depending on the percentage of foreign manufacture. If the manufacturing is performed outside of Israel by us, the rate of royalties payable by us on revenues from the sale of products manufactured outside of Israel will increase by 1% over the regular rates. If the manufacturing is performed outside of Israel by a third party, the rate of royalties payable by us on those revenues will be a percentage equal to the percentage of our total investment in the Given System that was funded by grants. Following our request, the Office of the Chief Scientist has approved the manufacture of limited quantities of the PillCam capsule using the back-up production line that we have established at Pemstar's facilities in Ireland without increasing royalty rates.
C. Research and Development
Our research and development expenditures, excluding grants received from the Office of the Chief Scientist, were $7.4 million for the year ended December 31, 2004, $7.0 million for the year ended December 31, 2003, and $8.6 million for the year ended December 31, 2002. Our research and development activities are conducted by our research and development and regulatory affairs staff primarily at our headquarters in Israel. As of December 31, 2004, our research and development, clinical and regulatory and engineering staff consisted of 50 employees, as well as consultants and subcontractors. Our research and development efforts are focused primarily on developing new capsules to be used in the detection of abnormalities in the stomach and the colon, improvements to our existing products and new technologies for future expansion of our product offering.
D. Trend Information
See discussion in Parts A and B of Item 5 “Operating Results and Financial Review and Prospects.”
56
Item 6. Directors, Senior Management and Employees
A. Directors and Senior Management
Our executive officers and directors and their ages and positions as of the date of this annual report are as follows:
Name
| | Age
| | Position
|
Executive Officers: | | | | | | |
Gavriel D. Meron | | | 52 | | | President, Chief Executive Officer and Director |
Kevin Rubey | | | 47 | | | Chief Operations Officer |
Zvi Ben David | | | 44 | | | Corporate Vice President and Chief Financial Officer |
Yoram Ashery | | | 39 | | | Corporate Vice President—Business Development |
Shoshana Friedman | | | 51 | | | Corporate Vice President—Regulatory and Medical Affairs |
Mark Gilreath | | | 39 | | | Corporate Vice President—Marketing Strategy |
Nancy L. S. Sousa | | | 48 | | | Corporate Vice President and President of Given Imaging, Inc. |
Manfred Gehrtz | | | 54 | | | Corporate Vice President, General Manager for Europe |
Sharon Koninksy | | | 33 | | | Corporate Director, Human Resources |
Directors: | | | | | | |
Doron Birger(1) | | | 53 | | | Chairman of the Board of Directors |
James M. Cornelius(1)(2)(3) | | | 61 | | | Director |
Michael Grobstein(1)(2)(3) | | | 62 | | | Director |
Jonathan Silverstein(1) | | | 38 | | | Director |
Reuven Baron | | | 61 | | | Director |
Dr. Dalia Megiddo(2) | | | 53 | | | Director |
Chen Barir | | | 46 | | | Director |
Eyal Lifschitz | | | 37 | | | Director |
| | | |
| (1) | | Member of our compensation and nominating committee. |
| | | |
| (2) | | Member of our audit committee. |
| | | |
| (3) | | Outside director under the Israeli Companies Law. |
Gavriel D. Meron founded Given Imaging in 1998 and has served as our President and Chief Executive Officer since that time. Mr. Meron became a director in August 2002. Prior to founding us, from 1995 to 1997, Mr. Meron served as Chief Executive Officer of APPLItec Ltd., an Israeli designer and manufacturer of video cameras and systems primarily for the medical endoscopy market. From 1993 to 1995, Mr. Meron was General Manager and Chief Operating Officer of Optibase Ltd., an Israeli manufacturer of hardware and software products that compress and playback digital video and sound for multimedia applications. From 1988 to 1993, Mr. Meron was Chief Financial Officer of InterPharm Laboratories Ltd., an Israeli company formerly listed on the Nasdaq National Market and a subsidiary of the Ares-Serono Group, a Swiss ethical pharmaceutical company. From 1983 to 1987, Mr. Meron held various management positions at the Tadiran Group, an Israeli telecommunications and semiconductor manufacturer. From 1973 to 1983, Mr. Meron served as an officer in the Israeli army overseeing the budgets of a range of military industries. Mr. Meron holds an M.B.A. from Tel Aviv University and a B.A. in economics and statistics from the Hebrew University, Jerusalem.
Kevin Rubey has served as our Chief Operations Officer since June 2001. Prior to joining us, from 1998 to May 2001, Mr. Rubey worked at Eastman Kodak Company, a consumer and professional photographic and information imaging company where he led global manufacturing
57
and operations for the Health Imaging Business Unit. From 1996 to 1998, Mr. Rubey was Manufacturing Director of the Medical Imaging Business Unit of Imation Corporation, a U.S. information technology company specializing in data storage and color image management. Prior to that, from 1977 to 1996, Mr. Rubey worked at the 3M Corporation in a variety of positions in the health, consumer and information technology businesses. Mr. Rubey holds a B.Sc. in mechanical engineering and an M.B.A. from the University of Minnesota.
Zvi Ben David has served as our Corporate Vice President and Chief Financial Officer since July 2000 and served as a director since our establishment in 1998 to June 2000. From 1995 to June 2000, Mr. Ben David was Vice President and Chief Financial Officer of RDC Rafael Development Corporation, one of our principal shareholders. From 1994 to 1995, Mr. Ben David was manager of the finance division of Electrochemical Industries (Frutarom) Ltd., an Israeli company traded on the Tel-Aviv Stock Exchange and American Stock Exchange that produces petrochemical products. Prior to that, from 1989 to 1993, Mr. Ben David was manager of the economy and control department of Electrochemical Industries (Frutarom) Ltd. From 1984 to 1988, Mr. Ben David worked at Avigosh & Kerbs, an accounting firm in Haifa, Israel. Mr. Ben David is a certified public accountant in Israel and holds a B.A. in economics and accounting from the University of Haifa.
Yoram Ashery has served as our Corporate Vice President—Business Development since April 2001. Prior to joining us, from 1995 to April 2001, Mr. Ashery was a corporate attorney, practicing in the fields of technology and medical devices and privatizations as an associate and, from January 2000, as a partner, with the law firm of Zellermayer, Pelossof & Co., who are also our legal counsel. Prior to that, Mr. Ashery served at the Office of the Attorney General of the Government of Israel, from 1993 to 1994, during which period he also completed his internship under the direct supervision of the Israeli Attorney General. From 1992 to 1996, Mr. Ashery also served as a junior lecturer in Tax Law at the Buchman Faculty of Law of the Tel-Aviv University. Mr. Ashery holds an LL.B. from the Buchman Faculty of Law of the Tel-Aviv University and a B.A. in Economics from the School of Social Sciences of the Tel-Aviv University.
Shoshana Friedman has served as our Corporate Vice President-Regulatory and Medical Affairs since January 2002. Ms. Friedman served as a consultant to us and as a member of our advisory board to our Chief Executive Officer from 1998 to January 2002. Prior to joining us, from 1996 to 2002, Ms. Friedman served as the Managing Director of PuSH-med Ltd., a regulatory consulting firm that she founded, which provided consulting services to medical device and biotechnology companies. From 1993 to 1996, Ms. Friedman was Regulatory, Clinical and Quality Assurance Director at InStent Ltd., an Israeli medical device company that was acquired by Medtronic, Inc. in 1996. Ms. Friedman holds a Quality Assurance Lead Assessor certificate from Bywater, Inc. and a Regulatory Affairs Certificate from the Regulatory Affairs Professional Society in the United States. Ms. Friedman holds a B.Sc. in mathematics and physics from Haifa University and an M.B.A. from the Hebrew University, Jerusalem.
Mark Gilreath has served as Corporate Vice President—Marketing Strategy since January 2003. He previously served as President of Given Imaging, Inc. since April 2001. Prior to that, he served as our Vice President—Global Business Development since September 2000 and as a consultant to us from October 1999 to September 2000. In 1999, Mr. Gilreath founded VortexMed, Inc., a developer of internet sites for healthcare professionals, where he served as Chief Executive Officer until September 2000. From 1992 to 1999, Mr. Gilreath held various management positions with PENTAX Precision Instrument Corporation including Product Manager, Director of Marketing, Area Sales Manager and Director of Business Development. Prior to joining PENTAX, from 1989 to 1992, Mr. Gilreath served as an officer in the U.S. Navy. He holds a B.Sc. in business finance from Winthrop University and a M.B.A. from the Fuqua School of Business at Duke University.
Nancy L. S. Sousa has served as Corporate Vice President since May 2003 and President, Given Imaging, Inc. since January 2003. Prior to joining us, she held several positions at Eastman Kodak Company from 1980 through 2002, including Director, System Concepts Center, and prior to that, as Vice President, New Business Development, Health Imaging. In addition, to those
58
positions, she previously served as Vice President, OEM Products, Consumer Imaging, Director, Strategic Business Planning, Consumer Imaging, and Product Manager, Projectors and Cameras. Ms. Sousa holds an M.Sc. in mathematics from University of Notre Dame and an M.A. in mathematics from The State University of New York.
Manfred Gehrtz has served as our Corporate Vice President, General Manager for Europe since January 2004. From 1995 to January 2004, Mr. Gehrtz was Managing Director, the head of the Endoscope Division and Vice President of Medical Systems Europe at Olympus Optical Co. (Europa) GmbH, a developer of products in the fields of photography, endoscopy, microscopy, communication and diagnostics. From 1990 to 1995, Mr. Gehrtz was Managing Director and CEO of Aesculap Meditec GmbH, a German company that develops and manufactures medical laser systems. Prior to that, from 1986 to 1990, Mr. Gehrtz was a Specialist, Lab Manager and Production Facility Manager at IBM Deutschland GmbH. Mr. Gehrtz holds a Ph. D. in Physical Chemistry and a M.Sc. in Physics from the University of Munich, Germany. From 1983 to 1985 Mr. Gehrtz was a Post-Doctoral Fellow at the IBM Research Laboratory in San Jose, California.
Sharon Koninsky has served as Corporate Director, Human Resources since 2002 and, prior to that, as Human Resources Manager. She has served as Executive Assistant to the President and Chief Executive Officer since 1998. Prior to joining us, from 1997 until 1998, Ms. Koninsky worked in the marketing department of Geotek Technologies Ltd., an Israeli telecommunications company. In 1996 Ms. Koninsky was responsible for placing personnel in administrative positions while employed at a manpower organization in Haifa. Ms. Koninsky holds a B.A. in social work and an M.A. in public administration, both from Haifa University.
Doron Birger has served as Chairman of the board of directors since August 2002 and as a director since June 2000. Mr. Birger has served as Chief Executive Officer of Elron Electronic Industries since August 2002, President since 2001, Chief Financial Officer from 1994 to August 2002, and Corporate Secretary from 1994 to 2001. Mr. Birger is a director of RDC Rafael Development Corporation and a director or chairman of the board of directors of many privately held companies in the Elron group in the fields of medical devices, semiconductors, communication and advanced materials. From 1991 to 1994, Mr. Birger was Vice President-Finance at North Hills Electronics Ltd., an advanced electronics company. From 1990 to 1991, Mr. Birger served as Chief Financial Officer of Middle-East Pipes Ltd., a manufacturer in the metal industry. From 1988 to 1990, Mr. Birger served as Chief Financial Officer of Maquette Ltd., a manufacturer and exporter of fashion items. From 1981 to 1988, Mr. Birger was Chief Financial Officer and director at Bateman Engineering Ltd. and I.D.C. Industrial Development Company Ltd. Mr. Birger holds a B.A. and an M.A. in economics from the Hebrew University, Jerusalem.
James M. Cornelius has served as a director since October 2001 and was elected as an outside director in December 2001. Mr. Cornelius currently serves as the non-executive Chairman of the board of directors of Guidant Corporation, a U.S. cardiac and vascular medical device company. From 1994 until 2000, Mr. Cornelius served as the Senior Executive and Chairman of Guidant Corporation. From 1983 to 1994, Mr. Cornelius was a director, a member of the Executive Committee and Chief Financial Officer of Eli Lilly and Company. From 1980 to 1982, Mr. Cornelius served as President and Chief Executive Officer of IVAC Corporation, formerly part of Eli Lilly's Medical Device and Diagnostics Division, and from 1978 to 1980, Mr. Cornelius was Director of Acquisitions for Eli Lilly's Medical Device and Diagnostics Division. Mr. Cornelius currently also serves as a director of Bristol-Myers Squibb Company, The Chubb Corporation, Hughes Electronics and The National Bank of Indianapolis. Mr. Cornelius holds an M.B.A. and a B.A. in accounting from Michigan State University.
Michael Grobstein has served as a director since October 2001 and was elected as an outside director in December 2001. Mr. Grobstein has served as a director of Guidant Corporation since 1999, and is currently chairman of Guidant's audit committee and a member of its corporate governance committee. Mr. Grobstein worked with Ernst & Young LLP from 1964 to 1998, and was admitted as a partner in 1975. At Ernst & Young, Mr. Grobstein served as a Vice Chairman-International Operations from 1993 to 1998, as Vice Chairman-Planning, Marketing and Industry Services from 1987 to 1993, and Vice Chairman-Accounting and Auditing Services from 1984 to
59
1987. In these positions, Mr. Grobstein, among other things, oversaw the global strategic planning of the firm, was responsible for developing and implementing the firm's worldwide audit service delivery process and consulted with multinational corporations on a wide variety of financial reporting matters. Mr. Grobstein is a certified public accountant in the United States and holds a B.Sc. in accounting from the University of Illinois.
Jonathan Silverstein has served as a director since September 2000. Mr. Silverstein is a General Partner at OrbiMed Advisors LLC, a global healthcare asset management firm. Mr. Silverstein joined OrbiMed in 1999. From 1996 to 1999, Mr. Silverstein was the Director of Life Sciences in the Investment Banking Department at the Sumitomo Bank Limited. From 1994 to 1996, Mr. Silverstein was an associate at Hambro Resource Development. Mr. Silverstein serves as a director of DOV Pharmaceuticals, Avanir Pharmaceuticals, Emphasys Medical Corporation and Predix Pharmaceuticals and is a former director for Auxilium Pharmaceuticals, Orthovita, Inc. and LifeCell Corporation. Mr. Silverstein has a B.A. in economics from Denison University and a J.D. and an M.B.A. from the University of San Diego.
Reuven Baron has served as a director since July 2000. Mr. Baron has served as President and Chief Executive Officer of RDC Rafael Development Corporation since June 2000. Prior to that, from 1993 to 2000, he was President of Galram Technology Industries, the business division of Rafael Armament Development Authority, and from 1994 to 2000, he was Chairman of Opgal Industries Ltd., an Israeli manufacturer of thermal imaging products. From 1990 to 1993, Mr. Baron was Director of Business Development for the Electronics Systems Division of Rafael. Mr. Baron holds a B.Sc. and an M.Sc. in electrical engineering from the Israel Institute of Technology.
Dr. Dalia Megiddo has served as a director since October 2001. Dr. Megiddo is Managing Partner of InnoMed Ventures, LLP, a venture capital fund in the field of medical devices and the life sciences. Dr. Megiddo also currently serves as a director of Elron Electronic Industries, one of our shareholders. From 1994 until 2002, Dr. Megiddo served a Chief Editor of Academia Medica, a multimedia medical teaching program in Israel and from 1996 until 2002 as Editor of the Israeli medical audio magazine, The Journal Club. From 1981 to 1986, Dr. Megiddo practiced family medicine at the Hebrew University Hadassah Medical Health Center. Dr. Megiddo holds an M.D. in medicine from Hebrew University, Jerusalem and an M.B.A. from Kellogg Recanati International Executive M.B.A. program of Tel Aviv University and North Western University.
Chen Barir has served as director since August 2002. Mr. Barir is Chairman of Berman & Co. Trading and Investments Ltd. and subsidiaries and affiliates, a private investment company specializing in seed and early stage venture investments and management focusing primarily on medical devices, investments in emerging markets and real estate. Mr. Barir is Chairman of Galil Medical Ltd. and Sunlight Medical Ltd., a Vice-Chairman of Oncura Inc. and a director of Optonol, Inc. and Elron Electronic Industries Ltd. Mr. Barir holds an M.B.A. from the European Institute of Business Administration (INSEAD) in Fontainebleau, France, and a Doctorate in Law and Economics from Harvard Law School, Cambridge, Massachusetts.
Eyal Lifschitz has served as a director since December 2003. Since 2001, Mr. Lifschitz has served as Chief Executive Officer of Peregrine Ventures, a venture capital fund focused on the medical device industry. Prior to that, Mr. Lifschitz was co-founder of a number of medical technology companies, including PharmaSys (acquired by Elan Corp. NYSE:ELN), where he served as Director of Business Development from 1990 to 1994, VisionCare Ophthalmic Technologies, Inc., where he served as Director of Business Development from 1997 to 2001, BioControl, Ltd., where he served as Director of Business Development from 1999 to 2001 and ECR Ltd.(acquired by AVX Corp. NYSE:AVX), where he served as General Manager from 1994 to 2001. Mr. Lifschitz completed academic studies in Philosophy and Economics at Bar-Ilan University, Israel in 1990 and holds an LL.B degree from Shearei Mishpat College in Israel.
B. Compensation
The aggregate compensation paid by us and our subsidiaries to our directors and executive officers, including stock-based compensation, for the year ended December 31, 2004 was $2.4
60
million (excluding director fees detailed below). This amount includes approximately $290,000 set aside or accrued to provide pension, severance, retirement or similar benefits or expenses, but does not include business travel, relocation, professional and business association dues and expenses reimbursed to office holders, and other benefits commonly reimbursed or paid by companies in Israel.
During the 2004, we paid each director, excluding Gavriel Meron, a quarterly fee of $3,750 for their service on our board of directors, and a $1,500 fee for attending and participating in each meeting of the board of directors or any committee of the Board of Directors. We paid an additional quarterly fee of $1,250 to the chairman of the audit committee. The total amount of these payments in 2004 was $315,000. The directors fees for service by our director, Doron Birger, are paid to Elron Electronics Industries, where he serves as President and Chief Executive Officer, and for service by our director, Reuben Baron, are paid to RDC Rafael Development Corporation, where he serves as President and Chief Executive Officer. In addition, all of our directors were reimbursed for their expenses for each board of directors meeting attended. In addition, in 2004, we granted options to purchase 25,000 ordinary shares to each of our directors, Michael Grobstein and James Cornelius, options to purchase 11,000 ordinary shares to each of our directors, Jonathan Silverstein and Dalia Megiddo and options to purchase 8,000 ordinary shares to each of our directors Chen Barir and Eyal Lifschitz. The exercise price of these options was equal to the fair market value of our ordinary shares on the date of grant.
Beginning April 2004, the base salary of Gavriel Meron, our President and Chief Executive Officer, who is also a director, was increased to $250,000 from $220,000 in 2003. Mr. Meron's base salary from July 2003 until April 2004, was subject to a 17.4% reduction as part of company-wide measures that we took in July 2003 to reduce our operating costs during that period. In 2004, we granted Mr. Meron options to purchase 120,000 ordinary shares at an exercise price equal to the fair market value of our ordinary shares on the date of grant, vesting in four equal annual installments commencing on the date of the grant.
In December 2004, our compensation committee and our board of directors approved (1) an increase of Mr. Meron's base salary from $250,000 to $271,750 commencing in 2005, (2) a grant of options to purchase 70,000 ordinary shares at an exercise price equal to the closing price of our ordinary shares on the Nasdaq National Market on the date of the grant, and (3) a bonus of between zero and $543,500 subject to us meeting certain performance targets defined by the Board of Directors. This increase in base salary, the option grant and the bonus are subject to the approval of our shareholders at the next general meeting of our shareholders.
Mr. Meron also has the use of an automobile and 22 days of paid vacation per year, as well as other benefits commonly paid by companies in Israel. Please see Item 7 “Major Shareholders and Related Party Transactions—Agreements with Directors and Officers—Employment Agreements” for information regarding Mr. Meron's employment agreement and compensation subject to shareholder approval.
C. Board Practice
Board of Directors and Officers
Our articles of association provide that we may have up to 12 directors, each of whom is elected at an annual general meeting of our shareholders by a vote of the holders of a majority of the voting power present and voting at that meeting. Our board of directors currently consists of nine directors. Each director listed above will hold office until the next annual general meeting of our shareholders, except for our outside directors who were elected in December 2001 for an initial three-year term and were reelected for one additional three year term in May 2004, which will expire in December 31, 2007. Other than Gavriel Meron, our President and Chief Executive Officer, none of our directors are our employees or are party to a service contract with us.
A simple majority of our shareholders at a general meeting may remove any of our directors from office, elect directors in their stead or fill any vacancy, however created, in our board of directors. In addition, vacancies on the board of directors, other than vacancies created by an
61
outside director, may be filled by a vote of a majority of the directors then in office. Our board of directors may also appoint additional directors up to the maximum number permitted under our articles of association. A director so chosen or appointed will hold office until the next general meeting of our shareholders.
Each of our executive officers serves at the discretion of the board of directors and holds office until his or her successor is elected or until his or her earlier resignation or removal. There are no family relationships among any of our directors or executive officers. All of our executive officers have signed employment agreements.
Outside and Independent Directors
Under Israel's Companies Law, companies incorporated under the laws of the State of Israel whose shares are listed on an exchange including the Nasdaq National Market are required to appoint at least two outside directors. Outside directors are required to meet standards of independence set forth in the Israeli companies law. Outside directors are elected by a majority vote at a shareholders' meeting, provided that either (1) the majority of shares voted at the meeting, including at least one-third of the shares of non-controlling shareholders voted at the meeting, vote in favor of the election of the outside director, or (2) the total number of shares voted against the election of the outside director does not exceed one percent of the aggregate voting rights in the company. The initial term of an outside director is three years and he or she may be reelected to one additional term of three years by a majority vote at a shareholders' meeting, subject to the conditions described above for election of outside directors. Outside directors may only be removed by the same percentage of shareholders as is required for their election, or by a court, and then only if the outside directors cease to meet the statutory requirements for their appointment or if they violate their duty of loyalty to the company. If an outside directorship becomes vacant, a company's board of directors is required under the Companies Law to call a shareholders' meeting immediately to appoint a new outside director. Our two outside directors are James Cornelius and Michael Grobstein.
Each committee of a company's board of directors is required to include at least one outside director and our audit committee is required to include both outside directors. An outside director is entitled to compensation as provided in regulations adopted under the Companies Law and is otherwise prohibited from receiving any other compensation, directly or indirectly, in connection with services provided as an outside director.
In addition to the requirements of the Companies Law, we must comply with the Nasdaq National Market listing requirements, under which our board of directors must have at least three independent directors as defined in those rules. Commencing on July 31, 2005, a majority of the members of our board of directors (including all members of our audit committee) is required to be independent under the rules of the Nasdaq National Market.
Audit Committee
Under the Companies Law, the board of directors of any company whose shares are listed on any exchange must also appoint an audit committee comprised of at least three directors including all of the outside directors. The audit committee may not include the chairman of the board of directors, the general manager, the chief executive officer, a director employed by the company or who provides services to the company on a regular basis, or a controlling shareholder or a relative of a controlling shareholder. Under the rules of the Nasdaq National Market listing requirements, we are required to have an audit committee consisting of at least three independent directors, all of whom are financially literate and one of whom has accounting or related financial management expertise. We believe that our directors, Michael Grobstein, James Cornelius and Dalia Megiddo, meet these independence and other requirements. Commencing on July 31, 2005, the members of our audit committee will be required to meet more stringent independence requirements under Nasdaq National Market rules, including minimum standards set forth in rules of the Securities and Exchange Commission and adopted by the Nasdaq National Market.
Under the Companies Law, the role of the audit committee is to identify irregularities in the business management of the company, in consultation with the internal auditor and the company's
62
independent accountants, and suggest an appropriate course of action. Under the Nasdaq National Market listing requirements, our audit committee has adopted an audit committee charter setting forth its responsibilities. In December 2003, we amended our audit committee charter to reflect new rules of the Nasdaq National Market, which will become effective with respect to us on July 31, 2005. The audit committee charter governing the actions of our audit committee states that in fulfilling this role the committee is entitled to rely on interviews and consultations with our management, our internal auditor and our independent public accountant, and is not obligated to conduct any independent investigation or verification. The charter also states that the audit committee is required to nominate the company's independent accountants, which the shareholders subsequently are required to approve.
Internal Auditor
Under the Companies Law, the board of directors must appoint an internal auditor nominated by the audit committee. The role of the internal auditor is to examine whether a company's actions comply with the law and orderly business procedure. Under the Companies Law, the internal auditor may be an employee of the company but not an interested party or an office holder, or affiliate, or a relative of an interested party or an office holder, nor may the internal auditor be the company's independent accountant or its representative. An interested party is defined in the Companies Law as a 5% or greater shareholder, any person or entity who has the right to designate one director or more or the chief executive officer of the company or any person who serves as a director or as a chief executive officer. In February 2005, our internal auditor announced his decision not to continue his engagement as Given Imaging's internal auditor in order to preserve the independence of his accounting firm that is engaged as the independent auditor of one of our affiliates. As a result, we are currently seeking to fill the position of the internal auditor.
Compensation and Nominating Committee
Our compensation and nominating committee consists of our directors James Cornelius, Doron Birger, Michael Grobstein and Jonathan Silverstein. In December 2003, in accordance with new Nasdaq National Market rules which will become effective with respect to us on July 31, 2005, our compensation and nominating committee adopted a charter, which sets forth its responsibilities. Pursuant to the charter, the compensation and nominating committee is authorized to make decisions regarding executive compensation and terms and conditions of employment, as well as to recommend that the board of directors issue options under our stock option plans. The compensation and nominating committee is also responsible for recommending to the board of directors nominees for board membership. The charter requires that the composition of the committee must satisfy the Nasdaq National Market's independent director requirements.
D. Employees
As of December 31, 2004, we and our subsidiaries had 282 employees of whom 163 were based in Israel, 82 in the United States, 31 in Europe, three in Japan and three in Australia. This reflects a 22% increase in the number of employees since December 31, 2003. This increase is mainly in production employees in Israel and sales people in our U.S. subsidiary and is attributable to the growth of our business in 2004. By comparison, in 2003, we reduced our workforce by 15% compared to 2002, principally from our manufacturing facilities in Israel. This reduction in our workforce was the result of efficiency measures initiated to reduce our operating costs in order to achieve our profitability targets.
Under law, we and our employees are subject to protective labor provisions, including restrictions on working hours, minimum wages, minimum vacation, minimal termination notice, sick pay, severance pay and social security as well as equal opportunity and anti-discrimination laws. Orders issued by the Israeli Ministry of Labor and Welfare make certain industry-wide collective bargaining agreements and collective bargaining agreements in the electricity, steel and electronics industries applicable to us. These agreements affect matters such as cost of living adjustments to
63
salaries, length of working hours and week, recuperation, travel expenses, and pension rights. Our employees are not represented by a labor union. We provide our employees with benefits and working conditions above the required minimum and which we believe are competitive with benefits and working conditions provided by similar companies in Israel. We have written employment agreements with all of our Israeli employees and with our senior non-Israeli employees. Competition for qualified personnel in our industry is intense and we dedicate significant resources to employee retention. We have never experienced labor-related work stoppages and believe that our relations with our employees are good.
E. Share Ownership
Share Ownership by Directors and Executive Officers
For information regarding ownership of our ordinary shares by our directors and executive officers, and regarding options to purchase our ordinary shares granted to Gavriel Meron, our President and Chief Executive Officer, see Item 7 “Major Shareholders and Related Party Transactions.”
Stock Option Plans
2003 Stock Option Plan
Our 2003 stock option plan provides for a grant of options to our directors, employees and consultants, including members of our medical advisory committee, and to the directors, employees or consultants of our subsidiaries. We initially reserved a total of 1,500,000 ordinary shares for issuance under the plan. In addition, we have reserved for issuance under the plan any ordinary shares underlying unvested options granted under our 1998 and 2000 Stock Option Plans that expire without exercise. As of December 31, 2004, we had outstanding options to purchase 2,756,020 shares under the 2003 stock option plan. On February 10, 2004, our board of directors approved an increase of 1,000,000 ordinary shares available under the 2003 stock option plan, which was subsequently approved by our shareholders.
The plan is substantially similar to our 2000 Stock Option Plan. Generally, where a grant of options under the plan is our first grant of options to that person, the options are not exercisable before the second anniversary of the date of grant, at which time 50% of the options become exercisable with 25% to become exercisable on each of the third and fourth anniversaries of the date of the grant. However, under the 2003 Stock Option Plan, in cases where we have granted options to a grantee under previous plans, 25% of the options will be exercisable at the time of the grant and 25% will be exercisable on the first, second and third anniversaries of the date of the grant. Our compensation and nominating committee has the authority to accelerate the time periods for exercising options. Unexercised options expire ten years after the date of grant. To the extent the options have been vested, they may be exercised in whole or in part from time to time until their expiry. Upon the termination of the employment of an employee other than for cause the employee may exercise all vested options. If the employment of an employee is terminated for cause, all of his or her vested and unvested options will expire.
In the event of an acquisition or merger, we will endeavor to ensure that the rights of the holders of outstanding options are maintained. If we are unable to do so or if our board of directors resolves otherwise, all outstanding, but unvested, stock options will be accelerated and exercisable, ten days prior to the acquisition or merger. In addition, if the employment of a holder of outstanding options is terminated in anticipation of or during the 12 month period following an acquisition or merger, all outstanding but unvested stock options will be accelerated and exercisable, subject to certain adjustments and exceptions.
Options granted under the plan to Israeli residents may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the options or the ordinary shares issued upon their exercise must be deposited with a trustee for at least two years following the end of the calendar year in which the options are granted. Under Section 102. Any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or
64
ordinary shares by the trustee to the employee or upon the sale of the options or ordinary shares. Gains on options granted in 2003 and later are subject to capital gains tax of 25%. Options granted under the plan to U.S. residents may also qualify as incentive stock options within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986. The exercise price for incentive stock options must not be not less than the fair market value on the date the option is granted, or 110% of the fair market value if the option holder holds more than 10% of our share capital.
2000 Stock Option Plan
Our 2000 stock option plan provides for the grant of options to our directors, employees or consultants, including members of our medical advisory committee, and to the directors, employees or consultants of our subsidiaries. As of December 31, 2004, we had outstanding options to purchase 1,180,440 shares under the 2000 stock option plan. Ordinary shares underlying options which expire without exercise under the 2000 stock option plan become available for issuance under the 2003 stock option plan.
The plan is administered by our compensation and nominating committee which makes recommendations to our board of directors regarding grantees of options and the terms of the grant, including exercise prices, vesting schedules, acceleration of vesting and other matters necessary in the administration of the plan. Upon the recommendation of our compensation and nominating committee, options granted under the plan to Israeli residents may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the options or the ordinary shares issued upon their exercise must be deposited with a trustee for at least two years. Any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or ordinary shares by the trustee to the employee or upon the sale of the options or ordinary shares. Options granted under the plan to U.S. residents may also qualify as incentive stock options within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986. The exercise price for incentive stock options must not be not less than the fair market value on the date the option is granted, or 110% of the fair market value if the option holder holds more than 10% of our share capital.
Under the 2000 stock option plan, options issued under the plan are not exercisable before the second anniversary of the date of grant at which time 50% of the options become exercisable and 25% on each of the third and fourth anniversaries of the date of grant. Unexercised options expire ten years after the date of grant. If the employment of an employee is terminated for cause, all of his or her vested and unvested options will expire.
In the event of an acquisition or merger, we will endeavor to ensure that the rights of the holders of outstanding options are maintained. If we are unable to do so or if our board of directors resolves otherwise, all outstanding, but unvested, stock options will be accelerated and exercisable, ten days prior to the acquisition or merger.
1998 Stock Option Plan
Our 1998 stock option provides for the grant of options to our directors, employees, or consultants, including members of our medical advisory committee. Our 1998 stock option plan has been superseded by our 2000 stock option plan and we have ceased issuing options under our 1998 stock option plan. As of December 31, 2004, we had outstanding options to purchase 365,078 shares under the 1998 stock option plan. Ordinary shares underlying options which expire without exercise under the 1998 stock option plan become available for issuance under the 2003 stock option plan.
Under the 1998 stock option plan, options issued under the plan are not exercisable before the second anniversary of the date of grant at which time 50% of the options become exercisable and 25% on each of the third and fourth anniversaries of the date of grant. Unexercised options expire ten years after the date of grant. If the employment of an employee is terminated for cause, all of his or her vested and unvested options will expire.
65
Item 7. Major Shareholders and Related Party Transactions
A. Major Shareholders
The following table sets forth certain information regarding the beneficial ownership of our outstanding ordinary shares as of the date of this Form 20-F for: (1) each person who we believe beneficially owns 5% or more of the outstanding ordinary shares, (2) each of our directors individually, (3) each of our executive officers individually, and (4) all of our directors and executive officers as a group. Beneficial ownership of shares is determined under rules of the Securities and Exchange Commission and generally includes any shares over which a person exercises sole or shared voting or investment power. The table also includes the number of shares underlying options that are exercisable within 60 days of the date of this annual report. Ordinary shares subject to these options are deemed to be outstanding for the purpose of computing the ownership percentage of the person holding these options, but are not deemed to be outstanding for the purpose of computing the ownership percentage of any other person.
The shareholders listed below do not have any different voting rights from our other shareholders. Unless otherwise noted below, each shareholder's address is c/o Given Imaging Ltd., P.O. Box 258, Yoqneam 20692, Israel.
Name and Address
| | Number of Shares
Beneficially
Owned
| | Percentage
of Shares
Beneficially Owned
|
Principal shareholders: | | | | | | | | |
IDB Holding Corporation Ltd.(1) | | | 10,277,733 | | | | 37.2 | % |
OrbiMed Advisors LLC, OrbiMed Capital LLC
and Samuel D. Isaly(2) | | | 3,636,747 | | | | 13.2 | |
Directors and executive officers: | | | | | | | | |
Gavriel D. Meron(3) | | | 844,209 | | | | 3.0 | |
Zvi Ben David(4) | | | 250,476 | | | | * | |
Kevin Rubey(5) | | | 160,250 | | | | * | |
Yoram Ashery(6) | | | 141,665 | | | | * | |
Shoshana Friedman(7) | | | 59,582 | | | | * | |
Mark Gilreath(8) | | | 129,513 | | | | * | |
Nancy L. S. Sousa(9) | | | 90,000 | | | | * | |
Manfred Gehrtz(10) | | | 11,000 | | | | — | |
Sharon Koninsky(11) | | | 38,891 | | | | * | |
Doron Birger(12) | | | 6,815,292 | | | | 24.6 | |
James M. Cornelius(13) | | | 208,500 | | | | * | |
Michael Grobstein(14) | | | 113,500 | | | | * | |
Jonathan Silverstein(15) | | | 3,704,769 | | | | 13.4 | |
Reuven Baron(16) | | | 2,733,310 | | | | 9.9 | |
Dr. Dalia Megiddo(17) | | | 11,000 | | | | * | |
Chen Barir(18) | | | 33,000 | | | | * | |
Eyal Lifshitz(19) | | | 12,500 | | | | — | |
All directors and executive officers as a group(20) | | | 16,017,388 | | | | 58.0 | % |
| | | | | | | | |
* Less than 1%
| | | |
| (1) | | Based on a Schedule 13D/A filed on November 4, 2004 and on information provided to us supplementally, consists of 4,082,982 ordinary shares owned by Elron Electronic Industries Ltd., or Elron, 3,462,441 ordinary shares owned by Discount Investment Corporation Ltd., or DIC, and 2,732,310 ordinary shares owned by RDC Rafael Development Corporation Ltd., or RDC. Based on information contained in that Schedule 13D/A and on information provided to us supplementally, Elron owns all of the outstanding shares of DEP Technology Holdings |
(footnotes continued on next page)
66
(footnotes continued from previous page)
| | | Ltd. which, in turn, holds 50.1% of the voting power of RDC. As a result, Elron may be deemed to be the beneficial owner of the ordinary shares owned by RDC. As of February 16, 2005, DIC owned approximately 45.7% of the outstanding shares of Elron and, as a result, DIC may be deemed to be the beneficial owner of the ordinary shares owned by RDC and by Elron. The address of DIC is The Triangular Tower, 44th Floor, 3 Azrieli Center, Tel Aviv 67023, Israel. The address of RDC is Building 7, New Industrial Park, Yoqneam 20692, Israel. The address of Elron is The Triangular Tower, 42nd Floor, 3 Azrieli Center, Tel Aviv 67023, Israel. As of February 16, 2005, IDB Holding Corporation, or IDBH, owned approximately 63% of the outstanding shares of IDB Development Corporation Ltd., or IDBD, which, in turn, owned approximately 67% of the outstanding shares of DIC. As a result, IDBH may be deemed to be the beneficial owner of the ordinary shares owned by DIC, RDC and Elron. The address of each of IDBH and IDBD is The Triangular Tower, 44th Floor, 3 Azrieli Center, Tel Aviv 67023, Israel. IDBH is a public company traded on the Tel Aviv Stock Exchange. Approximately 51.7% of the outstanding share capital of IDBH is owned by a group comprised of (i) Ganden Investments I.D.B. Ltd., or Ganden, a private Israeli company controlled by Nochi Dankner and his sister, Shelly Bergman, which holds approximately 31.02% of the equity of and voting power in IDBH; (ii) Manor Investments-IDB Ltd., or “Manor”, a private Israeli company controlled by Ruth Manor, which holds approximately 10.34% of the equity of and voting power in IDBH; and (iii) Avraham Livnat Investments (2002) Ltd., or Livnat, a private Israeli company controlled by Avraham Livnat, which holds approximately 10.34% of the equity of and voting power in IDBH. Ganden, Manor and Livnat, owning in the aggregate approximately 51.7% of the equity of and voting power in IDBH, entered into a Shareholders Agreement relating, among other things, to their joint control of IDBH, the term of which is until May 19, 2023. In addition, as of February 16, 2005, (a) Shelly Bergman held approximately 7.2% of the equity of and voting power in IDBH; (b) another private Israeli company controlled by Nochi Dankner and his sister, Shelly Bergman, which is the parent company of Ganden, held approximately 6.44% of the equity and voting power of IDBH; (c) another private Israeli company controlled by Ruth Manor, which is the parent company of Manor, held approximately 0.03% of the equity and voting power in IDBH and (d) another private Israeli company controlled by Avraham Livnat, which is the parent company of Livnat, held approximately 0.04% of the equity and voting power in IDBH. Nochi Dankner is Chairman of IDBH, IDBD and DIC. Shelly Bergman, Isaac Manor (the husband of Ruth Manor) and Zvi Livnat (a son of Avraham Livnat) are directors of IDBH, IDBD and DIC. Shai Livnat (another son of Avraham Livnat) is a director of IDBD. Dori Manor (the son of Isaac and Ruth Manor) is a director of IDBH, IDBD, DIC and Elron. The address of Nochi Dankner is The Triangular Tower, 44th Floor, 3 Azrieli Center, Tel Aviv 67023, Israel. The address of Shelly Bergman is 12 Recanati Street, Ramat Aviv Gimmel, Tel Aviv, Israel. The address of Ruth Manor is 26 Hagderot Street, Savyon, Israel. The address of Avraham Livnat is Taavura Junction, Ramle, Israel. These individuals disclaim beneficial ownership of the shares owned by the foregoing entities except to the extent of their pecuniary interest therein. On September 29, 2003, Elron and DIC entered into a voting agreement pursuant to which, among other things, DIC agreed to vote all of its ordinary shares in favor of nominees to our board of directors proposed by Elron. The voting agreement is for a term of one year and renews automatically each year thereafter unless terminated by notice of either party to the other party no later than August 30 in each year, or unless earlier terminated by agreement of both parties thereto. |
| | | |
| (2) | | Based on a Schedule 13D/A filed on September 29, 2004 and on information provided to us supplementally, consists of 2,250,733 ordinary shares and options to purchase 2,157 ordinary shares owned by Caduceus Private Investments L.P., or Caduceus; 485,000 ordinary shares and options to purchase 771 ordinary shares owned by Eaton Vance Worldwide Health Sciences Portfolio, or Eaton Vance; 485,000 ordinary shares and options to purchase 771 ordinary shares owned by Finsbury Worldwide Pharmaceutical Trust, or Finsbury; 330,000 ordinary |
(footnotes continued on next page)
67
(footnotes continued from previous page)
| | | shares owned by UBS Juniper Crossover Fund LLC, or Juniper; 82,171 ordinary shares and options to purchase 144 ordinary shares owned by OrbiMed Associates LLC, or OrbiMed Associates; 2,157 options to purchase ordinary shares owned by OrbiMed Advisors LLC, or OrbiMed Advisors. OrbiMed Capital is the general partner of Caduceus, OrbiMed Advisors acts as managing member of OrbiMed Associates, OrbiMed Advisors acts as investment manager of Juniper, OrbiMed Advisors acts as investment advisor to Finsbury and OrbiMed Advisors acts as investment adviser to Eaton Vance. As a result, OrbiMed Advisors and OrbiMed Capital have discretionary investment management authority with respect to the assets of Caduceus, Eaton Vance, Finsbury, OrbiMed Associates and Juniper. Samual D. Isaly owns a controlling interest in OrbiMed Advisors and OrbiMed Capital. Samuel D. Isaly, Sven Borho, Michael Sheffery and Carl L. Gordon, share ownership and control of OrbiMed Associates, OrbiMed Capital and OrbiMed Advisors. The address of OrbiMed Advisors, OrbiMed Capital, OrbiMed Advisors, Inc., Samuel D. Isaly, Sven Borho, Michael Sheffery and Carl L. Gordon is 767 Third Avenue, 30th Floor, New York, NY 10017. These individuals each disclaim beneficial ownership of the shares owned by the foregoing entities except to the extent of his pecuniary interest therein. |
| | | |
| (3) | | Consists of 11,416 ordinary shares and options to purchase 832,793 ordinary shares. |
| | | |
| (4) | | Consists of 19,026 ordinary shares, options to purchase 161,250 ordinary shares from Given Imaging and options to purchase 70,200 ordinary shares from RDC Rafael Development Corporation. |
| | | |
| (5) | | Consists of options to purchase 160,250 ordinary shares. |
| | | |
| (6) | | Consists of 5,415 ordinary shares and options to purchase 136,250 ordinary shares. |
| | | |
| (7) | | Consists of 19,026 ordinary shares owned by Ms. Friedman, options to purchase 37,500 ordinary shares owned by Ms. Friedman and options to purchase 3,056 ordinary shares owned by PuSH. J. Sh. Holding Ltd., a company owned and controlled by Ms. Friedman. |
| | | |
| (8) | | Consists of 9,513 ordinary shares and options to purchase 120,000 ordinary shares. |
| | | |
| (9) | | Consists of options to purchase 90,000 ordinary shares. |
| | | |
| (10) | | Consists of 1,000 ordinary shares and options to purchase 10,000 ordinary shares. |
| | | |
| (11) | | Consists of 9,891 ordinary shares and options to purchase 29,000 ordinary shares. |
| | | |
| (12) | | Consists of 2,732,310 ordinary shares owned by RDC Rafael Development Corporation and 4,082,982 ordinary shares owned by Elron Electronic Industries. Mr. Birger is a director of RDC Rafael Development Corporation and President and Chief Executive Officer of Elron Electronic Industries and by virtue of his position may be deemed to have voting and investment power, and thus beneficial ownership with respect to the shares that Elron Electronic Industries and RDC Rafael Development Corporation own. Mr. Birger disclaims such beneficial ownership except to the extent of his pecuniary interest therein. |
| | | |
| (13) | | Consists of 145,000 ordinary shares and options to purchase 63,500 ordinary shares. |
| | | |
| (14) | | Consists of 25,000 ordinary shares and options to purchase 88,500 ordinary shares. |
| | | |
| (15) | | Consists of 522 ordinary shares and options to purchase 67,500 ordinary shares owned by Mr. Silverstein and 3,637,255 ordinary shares beneficially owned by the OrbiMed investors. Mr. Silverstein is a principal of OrbiMed Advisors LLC and by virtue of his position may be deemed to have voting and investment power, and thus beneficial ownership with respect to the shares which the OrbiMed investors owns or over which they exercise voting and investment power. Mr. Silverstein disclaims such beneficial ownership except to the extent of his pecuniary interest therein. |
| | | |
| (16) | | Consists of 1,000 ordinary shares owned by Mr. Baron and 2,732,310 ordinary shares beneficially owned by RDC Rafael Development Corporation. Mr. Baron is the President and Chief Executive Officer of RDC Rafael Development Corporation and by virtue of his |
(footnotes continued on next page)
68
(footnotes continued from previous page)
| | | position may be deemed to have voting and investment power, and thus beneficial ownership with respect to the shares beneficially owned by RDC Rafael Development Corporation. Mr. Baron disclaims such beneficial ownership except to the extent of his pecuniary interest therein. |
| | | |
| (17) | | Consists of options to purchase 11,000 ordinary shares. |
| | | |
| (18) | | Consists of options to purchase 33,000 ordinary shares. |
| | | |
| (19) | | Consists of options to purchase 12,500 ordinary shares. |
| | | |
| (20) | | Includes 10,277,733 ordinary shares beneficially owned by IDB Holding Corporation and 3,636,747 ordinary shares beneficially owned by the OrbiMed entities, as well as ordinary shares and options to purchase ordinary shares held by directors and officers in their personal capacities or by their nominees. Our directors and officers disclaim beneficial ownership of the shares owned by the foregoing entities except to the extent of their pecuniary interest therein. |
B. Related Party Transactions
Agreement with Rafael
In January 1998, we entered into a technology purchase and license agreement with Rafael Armament Development Authority, or Rafael, which is a division of the Israeli Ministry of Defense. Rafael beneficially owns 47.8% of the outstanding shares of our shareholder, RDC Rafael Development Corporation. Pursuant to this agreement, we purchased from Rafael, for $30,000 in cash, all of Rafael's rights to the technology associated with an in vivo video camera and an autonomous video endoscope, including a swallowable capsule with a camera and an optical system, a transmitter and a reception system, which was then being developed by Rafael, which we refer to as the PillCam capsule and system. Rafael transferred to us the patents that it had been issued in connection with the PillCam capsule and system technology. Rafael also granted us an exclusive, perpetual, worldwide, royalty-free license to use other technology and know-how of Rafael which was used at Rafael for the development of the PillCam capsule and system, provided that we use this technology and know-how solely for commercial exploitation of the PillCam capsule and system. We are permitted to transfer or sublicense this other technology to third parties in connection with the commercial exploitation of the PillCam capsule and system provided that we receive Rafael's consent which is not to be unreasonably withheld. We have not received any technology from Rafael pursuant to this license. In connection with our commercial exploitation of the PillCam capsule and system, Rafael agreed to provide us with technical support in the form of equipment and use of its laboratories, subject to their availability, and access to its existing documentation. We agreed to pay to Rafael its costs plus 10% for any of Rafael's manpower that we use. Rafael also agreed to provide us with research and development and prototype manufacturing services in connection with the PillCam capsule and system at customary commercial rates. Rafael has the right to provide such services to us if it is competitive in terms of quality, quantity, timing and price. In connection with this technical support, we paid an aggregate amount to Rafael of $12,000 in 1998 only.
In connection with our acquisition of this technology, we granted to Rafael an exclusive, perpetual, worldwide, royalty-free license to use all technology that we acquired from Rafael solely in the military and security fields. Rafael has the exclusive right to file patent applications for inventions developed by Rafael in connection with the use of the PillCam capsule and system in these fields. We are required to assist Rafael in connection with these patent applications. Rafael has granted to us an exclusive, perpetual, worldwide, royalty-free license to use outside of the military and security fields any technology covered by these patents. We and Rafael have also agreed to notify each other if either of us develops any improvements to the PillCam capsule and system technology provided that doing so does not breach any confidentiality undertaking to a third party, or in the case of Rafael, security requirements. We have each agreed to grant to the other exclusive rights to use any improvement in our case in the commercial field and in Rafael's
69
case in the military and security fields, in consideration for payment of royalties in an amount to be agreed upon.
Registration Rights
Demand Registration Rights
Pursuant to an investor rights agreement, at the request of one or more of our former Series A preferred shareholders that hold at least 20% of our then outstanding ordinary shares held by our former Series A preferred shareholders, we must use our best efforts to register any or all of these shareholders' ordinary shares. We must also give notice of the registration to our other former Series A preferred shareholders and to some of our ordinary shareholders, including Discount Investment Corporation and its affiliates, and Elron Electronic Industries, and include in the registration any ordinary shares that they request to include. We are also permitted to include our shares in the registration. The minimum aggregate offering price of the shares to be registered is $5.0 million. We may only be requested to carry out two of these demand registrations.
In addition to the registrations described above that may be requested by our former Series A preferred shareholders, at the request of some of our ordinary shareholders, including Discount Investment Corporation and its affiliates, Elron Electronic Industries and PW Juniper Crossover Fund, holding at least 20% of our ordinary shares then outstanding, we must use our best efforts to register any or all of these shareholders' ordinary shares. We must also give notice of the registration to all other shareholders to whom we have granted registration rights and include in the registration any ordinary shares that they request to include. We are also permitted to include our shares in the registration. The minimum aggregate offering price of the shares to be registered is $5.0 million. We may only be requested to carry out three of these demand registrations.
In connection with each of the above registrations, the managing underwriter may limit the number of shares offered for marketing reasons. In such case, the managing underwriter must exclude first any shares to be registered by us and second, any shares to be registered by our directors and officers. Thereafter, the shares to be registered by our former Series A preferred shareholders or ordinary shareholders would be reduced pro rata among the shareholders requesting inclusion of their shares according to the number of shares held by each shareholder.
Piggyback Registration Rights
Our former Series A preferred shareholders also have the right to request that we include their ordinary shares which were issued upon conversion of our Series A preferred shares in any registration statements filed by us in the future for the purposes of a public offering, subject to specified limitations. Some of our ordinary shareholders, including Discount Investment Corporation and its affiliates, Elron Electronic Industries and PW Juniper Crossover Fund, have similar rights with respect to the ordinary shares they currently hold. The managing underwriter may limit the number of shares offered for marketing reasons. In this case, the managing underwriter must exclude first any shares to be registered by us, unless we initiated the registration, second the shares that our shareholders have requested to include in the registration, and third the shares of the party initiating the registration.
Form F-3 Registration Rights
At the request of some of our shareholders including Discount Investment Corporation and its affiliates, Elron Electronic Industries and PW Juniper Crossover Fund, or any of our former Series A preferred shareholders, we must register such shareholder's ordinary shares on Form F-3. We must also give notice of the registration to our other shareholders to whom we have granted registration rights, and include in the registration any ordinary shares they request to include. These demand rights may only be exercised if nine months have passed since the last registration that we filed in which the shareholder requesting registration was entitled to include its shares. The minimum aggregate offering price of the shares to be registered is $5.0 million. The managing
70
underwriter may limit the number of shares offered for marketing reasons. In such case, the rights of each shareholder to include its ordinary shares in the registration are allocated in the same manner as in a demand registration described above.
Termination
All registration rights will expire on October 10, 2006. With respect to any shareholder, registration rights will expire if that shareholder can sell all of its ordinary shares within a 90 day period under Rule 144 of the Securities Act.
Expenses
Generally, we will pay all expenses incurred in carrying out the above registrations, as well as the fees and expenses of one legal counsel for the selling shareholders in each registration.
Directors Fees
We pay directors fees in respect of service by our directors (other than our President and Chief Executive Officer, Gaviel Meron). See Item 6 “Directors, Senior Management and Employees—Compensation.”
Agreements with Directors and Officers
Employment Agreements
We maintain written employment agreements with all of our officers. These agreements all contain provisions standard for a company in our industry regarding noncompetition, confidentiality of information and assignment of inventions. The enforceability of covenants not to compete in Israel is limited.
In August 2003, we extended our employment agreement with Gavriel Meron, our President and Chief Executive Officer, until August 1, 2006, unless earlier terminated by either Mr. Meron or us upon one year notice. We may also terminate this agreement immediately for cause. Upon extension of Mr. Meron's employment agreement, we granted Mr. Meron options to purchase 100,000 ordinary shares at an exercise price equal to the closing price of our ordinary shares on the Nasdaq National Market on the date of the grant, vesting in four annual installments commencing on the date of the grant.
Mr. Meron's employment agreement provides, among other things, that upon the termination of Mr. Meron's employment (except when such termination is due to the breach by Mr. Meron of his duty of loyalty, willful misrepresentation of a material fact, conviction of any crime or offense involving the us or moral turpitude, or for similar causes), Mr. Meron will be entitled to prior notice of (1) one year; or (2) two years, if we terminate his employment immediately preceding or following (A) the merger or acquisition of us by another entity, if, following such transaction, the holders of the outstanding voting power of us prior to the transaction cease to hold a majority of the outstanding voting power of the surviving entity; or (B) a sale of all or substantially all of our assets (each an “M&A Event”). Should we terminate Mr. Meron's employment with immediate effect, or with a shorter notice, we are required to continue to pay to Mr. Meron his salary and the associated benefits during the remainder of the applicable notice period. Upon the occurrence of any M&A Event, and provided the M&A Event takes place during Mr. Meron's employment, all options previously granted to Mr. Meron will be accelerated and become immediately vested.
See the discussion in Part B of Item 6 “Directors, Senior Management and Employees” for a discussion regarding Mr. Meron's compensation.
In September 2004, we amended the employment agreement of each of our executive officers, except our Chief Executive Officer, which had previously been amended in 2003. The amendments provide that if the employment of an executive officer is terminated by us, other than for “cause,”
71
or by the executive officer due to specified reasons, at any time following a change in control of the company, each executive officer will be entitled to continue to receive his or her compensation and benefits for a specified period and that a portion of any unvested options at the time the change of control occurs that would have vested had the executive officer's employment continued for an additional two years from the effective date of termination, will become vested and immediately exercisable. These amendments were approved by our compensation and nominating committee and by our board of directors.
Exculpation, Insurance and Indemnification
Under the Companies Law, an Israeli company may not exculpate an office holder from liability for a breach of the duty of loyalty of the office holder. However, a company may approve an act performed in breach of the duty of loyalty of an office holder provided that the office holder acted in good faith, the act or its approval does not harm the company, and the office holder discloses the nature of his or her personal interest in the act and all material facts and documents a reasonable time before discussion of the approval. Generally, an Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for a breach of duty of care but only if a provision authorizing such exculpation is inserted in its articles of association. Our articles of association include such a provision.
An Israeli company may indemnify an office holder in respect of certain liabilities either in advance of an event or following an event provided a provision authorizing such indemnification is inserted in its articles of association. Our articles of association contain such an authorization. An undertaking by an Israeli company to indemnify an office holder must be limited to foreseeable liabilities and reasonable amounts determined by the board of directors. A company may indemnify an office holder against the following liabilities incurred for acts performed as an office holder:
| • | a financial liability imposed on him or her in favor of another person pursuant to a judgment, settlement or arbitrator's award approved by court; and |
| |
| • | reasonable litigation expenses, including attorneys' fees, incurred by the office holder or imposed by a court or other authorized authority in proceedings instituted against him or her by the company, on its behalf or by a third party, in connection with criminal proceedings in which the office holder was acquitted or as a result of a conviction for a crime or the payment of a fine that does not require proof of criminal intent. |
An Israeli company may insure an office holder against the following liabilities incurred for acts performed as an office holder:
| • | a breach of duty of loyalty to the company, to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| |
| • | a breach of duty of care to the company or to a third party; and |
| |
| • | a financial liability imposed on the office holder in favor of a third party. |
An Israeli company may not indemnify, insure or exculpate an office holder against any of the following:
| • | a breach of duty of loyalty, except to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| |
| • | a breach of duty of care committed intentionally or recklessly; |
| |
| • | an act or omission committed with intent to derive illegal personal benefit; or |
| |
| • | a fine levied against the office holder. |
Under the Companies Law, exculpation, indemnification and insurance of office holders must be approved by our audit committee and our board of directors and, in respect of our directors, by our shareholders as well.
72
Our articles of association allow us to exculpate, indemnify and insure our office holders to the fullest extent permitted by the Companies Law. Our office holders are currently covered by a directors and officers' liability insurance policy. To date, no claims for directors and officers' liability insurance have been filed under this policy.
We have entered into agreements with each of our office holders undertaking to exculpate, indemnify and insure them to the fullest extent permitted by law. This indemnification is limited to events and amounts determined as foreseeable by the board of directors, and the insurance is subject to our discretion depending on its availability, effectiveness and cost. In the opinion of the U.S. Securities and Exchange Commission, however, indemnification of directors and office holders for liabilities arising under the Securities Act is against public policy and therefore unenforceable.
C. Interests of Experts and Counsel
Not applicable.
73
Item 8. Financial Information
A. Consolidated Financial Statements and Other Information
Financial Statements
See Item 18 for audited consolidated financial statements.
Export Sales
Our manufacturing facilities for the data recorder and the PillCam capsules forming part of the Given System are located in Israel. The majority of our products are exported out of Israel. For information regarding our revenues by geographic market, see Item 5: “Operating and Financial Review and Prospects.”
Legal Proceedings
On November 25, 2002, we filed a lawsuit in the District Court in Haifa, Israel against a former employee and two companies which the former employee founded. Our claim was filed to protect our intellectual property rights following the defendants' improper use of patent applications covering technology developed at Given Imaging. The defendants filed a counterclaim on January 13, 2003, alleging that we abused legal proceedings, filed a frivolous lawsuit, acted in bad faith, and damaged the defendants' reputation and business. On January 25, 2003, our directors received correspondence from investors in the defendant companies demanding that we withdraw the lawsuit and compensate the investors. In August 2004, we settled this dispute and the settlement has been approved by the court. Under the terms of the settlement, we and one of the defendant companies will co-own the patents covered by the patent applications. We obtained an exclusive, irrevocable, perpetual and royalty free license and right to use the technology covered by these patent applications in the field of tetherless gastroenterology and the defendant company obtained an exclusive, irrevocable, perpetual and royalty free license and right to use the technology covered by these patent applications in fields unrelated to gastroenterology. In addition, the parties may both use the technology in tethered gastroenterology applications; however, the defendants agreed to time limitations on their entry into this field.
On November 14, 2002, we received correspondence from a U.S. corporation offering to grant us a license under reasonable terms for a U.S. patent for a technology allegedly utilized in the PillCam capsules. We believe that we are not utilizing any invention claimed under any valid independent claims in the patent and have responded accordingly. We have not received any correspondence from this U.S. corporation since the first quarter of 2004.
In March 2004, the U.S. Patent and Trademark Office notified us that it would conduct a reexamination of some of the claims in one of our U.S. patents in the U.S. Patent and Trademark Office pursuant to a request submitted by Olympus Corporation. We expect that the reexamination process will be completed during 2005. We do not believe that the outcome of this reexamination proceeding will affect our ability to market and sell the Given System.
On August 9, 2004, a former employee of our U.S. subsidiary filed a complaint against the U.S. subsidiary and two other individual defendants for, among other things, wrongful termination, and alleged discrimination and harassment based on age. The former employee claims he is entitled to damages, in an unstated amount, as well as interest and attorneys' fees. All of the defendants have moved to dismiss the complaint as to the individual defendants and the majority of claims as to our U.S. subsidiary. The hearing on the defendants' motion to dismiss was heard on February 15 2005. The motion was granted with 30 days leave for plaintiff to amend the complaint. The parties are also currently engaged in the discovery process and no trial date has been set. We are vigorously contesting this claim and believe that we have meritorious defenses. Since the amount of the claim is unstated, at this stage it is not possible to evaluate the likelihood or the amount of any potential loss, although we do not currently expect that an adverse outcome would have a material impact on our financial condition or results of operations.
74
We are not party to any other legal proceedings whose outcome may be material to our financial condition or results of operations.
Dividend Policy
We have never declared or paid any cash dividends on our ordinary shares and we do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. We currently intend to retain all future earnings to finance our operations and to expand our business. Any future determination relating to our dividend policy will be made at the discretion of our board of directors and will depend on a number of factors, including future earnings, capital requirements, financial condition and future prospects and other factors the board of directors may deem relevant.
Significant Changes
Except as otherwise disclosed in this Form 20-F, there has been no significant change in our financial position since December 31, 2004.
Item 9. The Offer and Listing
A. Offer and Listing Details
Nasdaq National Market
The following table lists the high and low closing sale prices of our ordinary shares for the periods indicated as reported by the Nasdaq National Market:
| | Annual Highs and Lows
| | High
| | Low
|
| | 2004 | | $ | 44.08 | | | $ | 17.87 | |
| | 2003 | | $ | 18.80 | | | $ | 6.65 | |
| | Quarterly Highs and Lows
| | High
| | Low
|
| | 1st quarter 2004 | | $ | 34.25 | | | $ | 17.87 | |
| | 2nd quarter 2004 | | $ | 38.51 | | | $ | 30.30 | |
| | 3rd quarter 2004 | | $ | 39.35 | | | $ | 31.35 | |
| | 4th quarter 2004 | | $ | 44.08 | | | $ | 27.10 | |
| | 1st quarter 2003 | | $ | 10.00 | | | $ | 8.21 | |
| | 2nd quarter 2003 | | $ | 9.20 | | | $ | 6.65 | |
| | 3rd quarter 2003 | | $ | 13.23 | | | $ | 8.41 | |
| | 4th quarter 2003 | | $ | 18.80 | | | $ | 10.58 | |
| | Most Recent Six Months
| | High
| | Low
|
| | February 2005 | | $ | 34.45 | | | $ | 31.48 | |
| | January 2005 | | $ | 36.04 | | | $ | 33.38 | |
| | December 2004 | | $ | 36.70 | | | $ | 31.55 | |
| | November 2004 | | $ | 33.31 | | | $ | 27.10 | |
| | October 2004 | | $ | 44.08 | | | $ | 32.24 | |
| | September 2004 | | $ | 39.35 | | | $ | 36.80 | |
| | | | | | | | | | |
On March 14, 2005, the last reported sale price of our ordinary shares on the Nasdaq National Market was $31.23 per share. According to our transfer agent, as of March 14, 2005, there were approximately 141 holders of record of our ordinary shares.
75
Tel Aviv Stock Exchange
The following table lists the high and low closing sale prices of our ordinary shares for the periods indicated as reported by the Tel Aviv Stock Exchange
| | Annual Highs and Lows
| | High
| | Low
|
| | 2004 | | | NIS194.60 | | | | NIS123.90 | |
| | Quarterly Highs and Lows
| | High
| | Low
|
| | 1st quarter 2004 | | | NIS155.90 | | | | NIS140.40 | |
| | 2nd quarter 2004 | | | NIS181.00 | | | | NIS140.30 | |
| | 3rd quarter 2004 | | | NIS175.40 | | | | NIS141.10 | |
| | 3rd quarter 2004 | | | NIS194.60 | | | | NIS123.90 | |
| | Most Recent Six Months
| | High
| | Low
|
| | February 2005 | | | NIS152.40 | | | | NIS138.20 | |
| | January 2005 | | | NIS155.30 | | | | NIS144.90 | |
| | December 2004 | | | NIS160.50 | | | | NIS139.60 | |
| | November 2004 | | | NIS149.00 | | | | NIS123.90 | |
| | October 2004 | | | NIS194.60 | | | | NIS146.50 | |
| | September 2004 | | | NIS175.40 | | | | NIS159.20 | |
| | | | | | | | | | |
The average exchange ratio of NIS to U.S. dollar in 2004 was NIS 4.478 to $1.0.
B. Plan of Distribution
Not applicable.
C. Markets
Our ordinary shares have traded publicly on the Nasdaq National Market under the symbol “GIVN” since October 2001 and on the Tel Aviv Stock Exchange under the symbol “GIVN” since March 2004. Our ordinary shares trade publicly only on the Nasdaq National Market and the Tel Aviv Stock Exchange.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
76
Item 10. Additional Information
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
Objects
Our objects under our memorandum of association are to engage in any type of manufacturing, trade, production, labor, agriculture, and professional and business services in all branches and areas of economic activity, to advance trade, importing and exporting, and any other object determined by our board of directors from time to time. Our objects under our articles of association are to engage in any lawful business.
Transfer of Shares and Notices
Fully paid ordinary shares are issued in registered form and may be freely transferred under our articles of association unless the transfer is restricted or prohibited by another instrument, Israeli law or the rules of a stock exchange on which the shares are traded. Our articles of association provide that each shareholder of record is entitled to receive at least 21 days' prior notice of any shareholders' meeting.
Election of Directors
Our ordinary shares do not have cumulative voting rights for the election of directors. Rather, under our articles of association our directors are appointed by the holders of a simple majority of our ordinary shares at a general shareholder meeting. As a result, the holders of our ordinary shares that represent more than 50% of the voting power represented at a shareholder meeting have the power to elect or remove any or all of our directors, subject to the special approval requirements for outside directors described under “Management—Outside Directors.” Under the Companies Law, the procedures for the appointment and removal and the term of office of directors, other than outside directors, may be contained in the articles of association of a company. Our articles of association currently do not contain provisions for staggered terms for directors. However, our articles of association may be amended in the future by a majority of our shareholders at a general shareholder meeting to provide for a staggered board or other method of electing our directors, other than with respect to our outside directors.
Dividend and Liquidation Rights
Our board of directors may declare a dividend to be paid to the holders of ordinary shares in proportion to the paid up capital attributable to the shares that they hold. Dividends may only be paid out of our profits and other surplus funds, as defined in the Companies Law, as of the end of the most recent fiscal year or as accrued over a period of two years, whichever is higher, provided that there is no reasonable concern that a payment of a dividend will prevent us from satisfying out existing and foreseeable obligations as they become due. In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of ordinary shares in proportion to the paid up capital attributable to the shares that they hold. This right may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Shareholder Meetings
We are required to convene an annual general meeting of our shareholders once every calendar year within a period of not more than 15 months following the preceding annual general meeting. Our board of directors is required to convene a special general meeting of our shareholders at the request of two directors or one quarter of the members of our board of
77
directors or at the request of one or more holders of 5% or more of our share capital and 1% of our voting power or the holder or holders of 5% or more of our voting power. All shareholder meetings require prior notice of at least 21 days. The chairperson of our board of directors presides over our general meetings. Shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which may be between four and 40 days prior to the date of the meeting.
Quorum
The quorum required for an ordinary meeting of shareholders consists of at least two shareholders present, in person or by proxy, who hold or represent between them at least 331⁄3% of our issued share capital. A meeting adjourned for lack of a quorum generally is adjourned to the same day in the following week at the same time and place or any time and place as the directors designate in a notice to the shareholders. At the reconvened meeting, the required quorum consists of one or more shareholders present in person or by proxy, unless the meeting was called pursuant to a request by our shareholders in which case the quorum required is the number of shareholders required to call the meeting as described under “—Shareholder Meetings.”
Voting
Holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote of shareholders at a shareholder meeting. Shareholders may vote at shareholder meetings either in person or by proxy. Israeli law does not provide for public companies such as us to have shareholder resolutions adopted by means of a written consent in lieu of a shareholder meeting. Shareholder voting rights may be affected by the grant of any special voting rights to the holders of a class of shares with preferential rights that may be authorized in the future. The Companies Law provides that a shareholder, in exercising his or her rights and performing his or her obligations toward the company and its other shareholders, must act in good faith and in an acceptable manner, and avoid abusing his or her powers. This is required when voting at general meetings on matters such as changes to the articles of association, increasing the company's registered capital, mergers and approval of related party transactions. A shareholder must also avoid oppression of other shareholders. In addition, any controlling shareholder, any shareholder who knows that its vote can determine the outcome of a shareholder vote and any shareholder who, under the company's articles of association, can appoint or prevent the appointment of an office holder, is required to act with fairness towards the company. The Companies Law does not describe the substance of this duty and there is no binding case law that addresses this subject directly. Any voting agreement is also subject to observance of these duties.
Resolutions
An ordinary resolution requires approval by the holders of a simple majority of the voting rights represented at the meeting, in person, by proxy or by written ballot, and voting on the resolution.
Under the Companies Law, unless otherwise provided in the articles of association or applicable law, all resolutions of the shareholders require a simple majority. A resolution for the voluntary winding up of the company requires the approval by holders of 75% of the voting rights represented at the meeting, in person, by proxy or by written ballot and voting on the resolution.
Access to Corporate Records
Under the Companies Law, all shareholders generally have the right to review minutes of our general meetings, our shareholder register, our articles of association and any document we are required by law to file publicly with the Israeli Companies Registrar. Any shareholder who specifies the purpose of its request may request to review any document in our possession that relates to any action or transaction with a related party which requires shareholder approval under the Companies Law. We may deny a request to review a document if we determine that the
78
request was not made in good faith, that the document contains a commercial secret or a patent or that the document's disclosure may otherwise harm our interests.
Acquisitions under Israeli Law
Tender Offer. A person wishing to acquire shares or any class of shares of a publicly traded Israeli company and who would as a result hold over 90% of the company's issued and outstanding share capital or of a class of shares which are listed is required by the Companies Law to make a tender offer to all of the company's shareholders or all shareholders of such class of shares, as applicable, for the purchase of all of the issued and outstanding shares of the company or of that class of shares, as applicable. If the shareholders who do not respond to the offer hold less than 5% of the issued share capital of the company or of that class of shares, as applicable, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. However, the shareholders may petition the court to alter the consideration for the acquisition. If the dissenting shareholders hold more than 5% of the issued and outstanding share capital of the company or of such class of shares, as applicable, the acquirer may not acquire additional shares of the company or of such class of shares, as applicable, from shareholders who accepted the tender offer if following such acquisition the acquirer would then own over 90% of the company's issued and outstanding share capital or of the shares comprising such class, as applicable.
The Companies Law provides that an acquisition of shares of a public company must be made by means of a tender offer if as a result of the acquisition the purchaser would become a 25% or greater shareholder of the company, subject to certain exceptions, including if there is already another 25% shareholder of the company. We believe that IDB Holding Corporation Ltd., which is controlled by four individuals in Israel, currently holds more than 25% of our outstanding ordinary shares as determined in accordance with the Companies Law. Similarly, the Companies Law provides that an acquisition of shares in a public company must be made by means of a tender offer if as a result of the acquisition the purchaser would become a 45% or greater shareholder of the company, if there is no 45% or greater shareholder of the company.
Merger. The Companies Law permits merger transactions if approved by each party's board of directors and the majority of each party's shares voted on the proposed merger at a shareholders' meeting called on at least 21 days' prior notice. Under the Companies Law, merger transactions may be approved by holders of a simple majority of our shares present, in person or by proxy, at a general meeting and voting on the transaction. In determining whether the required majority has approved the merger, if our shares are held by the other party to the merger, or by any person holding at least 25% of the outstanding voting shares or 25% of the means of appointing directors of the other party to the merger, then a vote against the merger by holders of the majority of the shares present and voting, excluding shares held by the other party or by such person, or anyone acting on behalf of either of them, is sufficient to reject the merger transaction. If the transaction would have been approved but for the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the value of the parties to the merger and the consideration offered to the shareholders. Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of any of the parties to the merger. In addition, a merger may not be executed unless at least 50 days have passed following the filing of a proposal for approval of the merger with the Israeli Registrar of Companies and at least 30 days following the approval of the shareholders of each of the parties.
Anti-Takeover Measures
The Companies Law allows us to create and issue shares having rights different to those attached to our ordinary shares, including shares providing certain preferred or additional rights to voting, distributions or other matters and shares having preemptive rights. In the future, if we do
79
create and issue a class of shares other than ordinary shares, such class of shares, depending on the specific rights that may be attached to them, may delay or prevent a takeover or otherwise prevent our shareholders from realizing a potential premium over the market value of their ordinary shares. The authorization of a new class of shares will require an amendment to our articles of association which requires the prior approval of a majority of our shareholders at a general meeting. Shareholders voting at such a meeting will be subject to the restrictions under the Companies Law decribed in “Voting.”
Transfer Agent and Registrar
The transfer agent and registrar for our ordinary shares is American Stock Transfer & Trust Company. Its address is 59 Maiden Lane, New York, New York 10038 and its telephone number at this location is (212) 936-5100.
C. Material Contracts
Summaries of the following material contracts are included in this Form 20-F in the places indicated:
| | Material Contract
| | Location
|
| | Standard Distribution Agreement | | Item 4.B: “Information on the
Company—Part B: Business Overview—Marketing and Distribution.” |
| | Agreements with Micron Technology, Inc. | | Item 4.B: “Information on the Company—Part B: Business Overview—Manufacturing.” |
| | Agreement with Zarlink Semiconductors AB | | Item 4.B: “Information on the Company—Part B: Business Overview—Manufacturing.” |
| | Agreements with Pemstar, Inc. | | Item 4.B: “Information on the Company—Part B: Business Overview—Manufacturing.” |
| | Agreement with Ethicon Endo-Surgery Inc. | | Item 4.B: “Information on the Company—Part B: Business Overview—Marketing and Distribution.” |
| | Agreement with Rafael Armament
Development Authority | | Item 7: “Major Shareholder and Related Party Transactions.” |
| | Investor Rights Agreement | | Item 7: “Major Shareholder and Related Party Transactions.” |
D. Exchange Controls
In 1998, Israeli currency control regulations were liberalized significantly, so that Israeli residents generally may freely deal in foreign currency and foreign assets, and non-residents may freely deal in Israeli currency and Israeli assets. There are currently no Israeli currency control restrictions on remittances of dividends on the ordinary shares or the proceeds from the sale of the shares provided that all taxes were paid or withheld; however, legislation remains in effect pursuant to which currency controls can be imposed by administrative action at any time.
Non-residents of Israel may freely hold and trade our securities. Neither our memorandum of association nor our articles of association nor the laws of the State of Israel restrict in any way the ownership or voting of ordinary shares by non-residents, except that such restrictions may exist with respect to citizens of countries which are in a state of war with Israel. Israeli residents are allowed to purchase our ordinary shares.
80
E. Taxation
Israeli Tax Considerations and Government Programs
The following is a description of the material Israeli income tax consequences of the ownership of our ordinary shares. The following also contains a description of material relevant provisions of the current Israeli income tax structure applicable to companies in Israel, with special reference to its effect on us. To the extent that the discussion is based on new tax legislation which has not been subject to judicial or administrative interpretation, we cannot assure shareholders that the tax authorities will accept the views expressed in the discussion in question. The discussion is not intended, and should not be taken, as legal or professional tax advice and is not exhaustive of all possible tax considerations.
The following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition of our ordinary shares. Shareholders should consult their own tax advisors concerning the tax consequences of their particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Taxation of Companies
General Corporate Tax Structure. In 2004, Israeli companies were subject to corporate tax at the rate of 35% of taxable income. This tax rate is expected to decrease gradually to 34% in 2005, 32% in 2006 and 30% from 2007. However, the effective tax rate payable by a company that derives income from an approved enterprise, as discussed further below, may be considerably less.
Tax Benefits Under the Law for the Encouragement of Capital Investments, 1959. The Law for the Encouragement of Capital Investments, 1959, commonly referred to as the Investment Law, provides that a proposed capital investment in eligible facilities may, upon application to the Investment Center of the Ministry of Industry, Trade and Labor of the State of Israel, be designated as an approved enterprise. Each certificate of approval for an approved enterprise relates to a specific investment program delineated both by its financial scope, including its capital sources, and by its physical characteristics, for example, the equipment to be purchased and utilized under the program. The tax benefits derived from any certificate of approval relate only to taxable income attributable to the specific approved enterprise. If a company has more than one approval or only a portion of its capital investments is approved, its effective tax rate is the result of a weighted average of the applicable rates.
Generally, taxable income of a company derived from an approved enterprise is subject to company tax at the maximum rate of 25%, rather than the regular corporate tax rate, for a period of seven years, or ten years if the company qualifies as a foreign investors' company as described below, commencing with the year in which the approved enterprise first generates taxable income. However, the ten-year period may not extend beyond the later of 14 years from the year in which approval was granted or 12 years from the year in which operations or production by the enterprise began. A company's undistributed income derived from an approved enterprise in top priority locations (commonly known as “Zone A”) will be exempt from corporate tax for a period of ten years.
A company having an approved enterprise status may elect to receive an alternative package of benefits. Under the alternative package of benefits, a company's undistributed income derived from an approved enterprise will be exempt from company tax for a period of between two and ten years from the first year of taxable income, depending on the geographic location of the approved enterprise within Israel, and the company will be eligible for a reduced tax rate for the remainder of the benefits period.
A company that has an approved enterprise in Zone A or that has elected the alternative package of benefits and that subsequently pays a dividend out of income derived from the approved enterprise during the tax exemption period will be subject to corporation tax on of the amount distributed. The rate of the tax will be the rate which would have been applicable had the
81
company not been tax exempt. This corporation tax rate is 10% to 25%, depending on the percentage of the company's shares held by foreign shareholders. The recipient of dividend distributed from such income is taxed at the rate applicable to dividends from approved enterprises which is 15%, or less under certain anti double-taxation treaties, if the dividend is distributed during the tax benefit period or within 12 years after the period and there is no time limit with respect to dividend distributed from an exempt income of foreign investors' company. The company must withhold this tax at source.
Subject to applicable provisions concerning income under the alternative package of benefits, all dividends are considered to be attributable to the entire enterprise and their effective tax rate is the result of a weighted average of the various applicable tax rates. Under the Investment Law, a company that has elected the alternative package of benefits is not obliged to distribute exempt retained profits, and may generally decide from which year's profits to declare dividends. We currently intend to reinvest any income derived from our approved enterprise programs and not to distribute the income as a dividend.
The Investment Center bases its decision whether or not to approve an application on the criteria in the Investment Law and regulations, the then prevailing policy of the Investment Center and the specific objectives and financial criteria of the applicant. In addition, the benefits available to an approved enterprise are conditional upon the fulfillment of conditions stipulated in the Investment Law and its regulations and the criteria in the specific certificate of approval, as described above. If a company does not meet these conditions, it would be required to refund the amount of tax benefits, with the addition of the consumer price index linkage adjustment and interest. There can be no assurance that any future approved enterprises that we may be awarded will be entitled to the same package of benefits as we currently have.
The Investment Center of the Ministry of Industry and Trade granted our manufacturing facility approved enterprise status under the Investment Law in 1999. We have elected the alternative package of benefits under these approved enterprise programs. Since our manufacturing facility is located in a “Zone A”, the portion of our income derived from this approved enterprise program will be exempt from tax for a period of ten years, commencing when we begin to realize net income from these programs, but such period may not extend beyond the later of 14 years from the year in which approval was granted or 12 years from the year in which operations or production by the enterprise began. The period of tax benefits for our approved enterprise programs has not yet commenced, because we have yet to realize taxable income. We expect to derive a substantial portion of our income from our approved enterprise program. The benefits available to an approved enterprise program are dependent upon the fulfillment of conditions stipulated in applicable law and in the certificate of approval.
Grants Under the Law for the Encouragement of Industrial Research and Development, 1984. Under the Law for the Encouragement of Industrial Research and Development, 1984, commonly referred to as the Research Law, research and development programs which meet specified criteria and are approved by a governmental committee of the Office of the Chief Scientist are eligible for grants of up to 50% of the project's expenditure, as determined by the research committee, in exchange for the payment of royalties from the sale of products developed under the program. Regulations under the Research Law generally provide for the payment of royalties to the Chief Scientist of 3.0% to 3.5% on sales of products and services derived from our technology developed using these grants until 100% of the dollar-linked grant is repaid, together with interest equal to the 12 month London Interbank Offered Rate applicable to dollar deposits that is published on the first business day of each calendar year. Following the full repayment of the grant, there is no further liability for repayment.
The terms of the Israeli government participation also require that the manufacture of products developed with government grants be performed in Israel. However, under the regulations of the Research Law, if any of the manufacturing is performed outside Israel by any entity other than us, assuming we receive approval from the Chief Scientist for the foreign manufacturing, we
82
may be required to pay increased royalties. The increase in royalties depends upon the manufacturing volume that is performed outside of Israel follows:
| | Manufacturing Volume Outside of Israel
| | Royalties to the Chief Scientist
as a Percentage of Grant
|
| | less than 50% | | | 120% | |
| | between 50% and less than 90% | | | 150% | |
| | 90% and more | | | 300% | |
| | | | | | |
If the manufacturing is performed outside of Israel by us, the rate of royalties payable by us on revenues from the sale of products manufactured outside of Israel will increase by 1% over the regular rates. If the manufacturing is performed outside of Israel by a third party, the rate of royalties payable by us on those revenues will be a percentage equal to the percentage of our total investment in the Given System that was funded by grants. In addition, in recent years the government of Israel has accelerated the repayment of Chief Scientist grants, and may further accelerate them in the future. Following our request, the Office of the Chief Scientist has approved the manufacture of limited quantities of the PillCam capsule using the back-up production line that we have established at Pemstar's facilities in Ireland without increasing royalty rates.
The technology developed with Chief Scientist grants may not be transferred to third parties without the prior approval of a governmental committee under the Research Law, and may not be transferred to non-residents of Israel. The approval, however, is not required for the export of any products developed using the grants. Approval of the transfer of technology to residents of Israel may be granted in specific circumstances, only if the recipient abides by the provisions of the Research Law and related regulations, including the restrictions on the transfer of know-how and the obligation to pay royalties in an amount that may be increased. We cannot provide any assurance that any consent, if requested, will be granted.
The funds available for grants from the Chief Scientist depend on several criteria and prevailing government policy and budget, and may be reduced or eliminated in the future. Even if these grants are maintained, there is no assurance that we will receive Chief Scientist grants in the future. In addition, each application to the Chief Scientist is reviewed separately, and grants are based on the program approved by the research committee. Expenditures supported under other incentive programs of the State of Israel are not eligible for grants from the Chief Scientist. We cannot provide any assurance that applications to the Chief Scientist will be approved and, until approved, the amounts of any grants are not determinable.
Tax Benefits and Grants for Research and Development. Israeli tax law allows, under specific conditions, a tax deduction in the year incurred for expenditures that were paid in cash, including capital expenditures, relating to scientific research and development projects, if:
| • | the expenditures are approved by the relevant Israeli government ministry, determined by the field of research; |
| |
| • | the research and development is for the promotion of the company; and |
| |
| • | the research and development is carried out by or on behalf of the company seeking the deduction. |
Expenditures not so approved are deductible over a three-year period. However, the amounts of any government grant made available to us are subtracted from the amount of the deductible expenses according to Israeli law.
Tax Benefits Under the Law for the Encouragement of Industry (Taxes), 1969. According to the Law for the Encouragement of Industry (Taxes), 1969, generally referred to as the Industry Encouragement Law, an industrial company is a company resident in Israel, at least 90% of the income of which, in a given tax year, determined in Israeli currency exclusive of income from specified government loans, capital gains, interest and dividends which are not classified for such company as business income, is derived from an industrial enterprise owned by it. An industrial
83
enterprise is defined as an enterprise whose major activity in a given tax year is industrial production activity.
Under the Industry Encouragement Law, industrial companies are entitled to certain preferred corporate tax benefits, including the following:
| • | deduction of purchases of know-how and patents over an eight-year period for tax purposes; and |
| |
| • | claiming of stock exchange issuance expenses over three years. |
Eligibility for benefits under the Industry Encouragement Law is not subject to receipt of prior approval from any governmental authority.
If we qualify as an industrial company within the definition of the Industry Encouragement Law, we are entitled to the benefits described above. We believe that in 2004 we qualified as an Industrial Company under the law for Encouragement of Industry (Taxes) 1969. We cannot provide any assurance that the Israeli tax authorities will agree with the determination that we qualified as an industrial company in the past or that we will maintain this qualification or our status as an industrial company.
Special Provisions Relating to Taxation Under Inflationary Conditions. The Income Tax Law (Inflationary Adjustments), 1985, generally referred to as the Inflationary Adjustments Law, represents an attempt to overcome the problems presented to a traditional tax system by an economy undergoing rapid inflation. The Inflationary Adjustments Law is highly complex. Its features which are material to us can generally be described as follows:
| • | Where a company's equity, as calculated under the Inflationary Adjustments Law, exceeds the depreciated cost of fixed assets, a deduction from taxable income is permitted equal to the excess multiplied by the applicable annual rate of inflation. The maximum deduction permitted in any single tax year is 70% of taxable income, with the unused portion permitted to be carried forward. |
| |
| • | Where a company's depreciated cost of fixed assets exceeds its equity, then the excess multiplied by the applicable annual rate of inflation is added to taxable income. |
| |
| • | Depreciation deductions on fixed assets and losses carried forward are adjusted for inflation based on the increase in the consumer price index. |
| |
| • | Gains on traded securities are taxable in specified circumstances. |
Taxation of Our Shareholders
Capital Gains on Sales of Our Ordinary Shares. Israeli law imposes a capital gains tax on the sale of capital assets. The law distinguishes between real gain and inflationary surplus. The inflationary surplus is the portion of the total capital gain that is equivalent to the increase of the relevant asset's purchase price which is attributable to the increase in the Israeli consumer price index between the date of purchase and the date of sale. Foreign residents who purchased an asset in foreign currency may request that the inflationary surplus be computed on the basis of the devaluation of the shekel against such foreign currency. The real gain is the excess of the total capital gain over the inflationary surplus. The inflationary surplus accumulated from and after December 31, 1993, is exempt from any capital gains tax in Israel while the real gain is taxed at the applicable rate discussed below.
Dealers in securities in Israel are taxed at regular tax rates applicable to business income.
Under the convention between the United States and Israel concerning taxes on income, Israeli capital gains tax will not apply to the sale, exchange or disposition of ordinary shares by a person:
| • | who qualifies as a resident of the United States within the meaning of the U.S.-Israel tax treaty; and |
| |
| • | who is entitled to claim the benefits available to the person by the U.S.-Israel tax treaty. |
84
However, this exemption does not apply, among other cases, if the gain is attributable to a permanent establishment of such person in Israel, or if the holder is a resident of the United States within the meaning of the U.S.-Israeli tax treaty who holds, directly or indirectly, shares representing 10% or more of our voting power during any part of the 12-month period preceding the sale, exchange or disposition, subject to specified conditions. Under these circumstances, the sale, exchange or disposition would be subject to Israeli tax, to the extent applicable. However, under the U.S.-Israel tax treaty, a U.S. resident generally would be permitted to claim a credit for the Israeli taxes paid against the U.S. federal income tax imposed on the sale, exchange or disposition, subject to the limitations under U.S. law applicable to foreign tax credits. The U.S.-Israel tax treaty does not relate to U.S. state or local taxes.
For residents of other countries, the purchaser of shares may be required to withhold 25% capital gains tax on all amounts received for the sale of our ordinary shares, for so long as the capital gain from such a sale is not exempt from Israeli capital gains tax, and unless a different rate is provided in a treaty between Israel and the seller's country of residence.
Under new legislation, which became effective on January 1, 2003, the capital gain from the sale of shares by non Israeli residents would be tax exempt in Israel as long as our shares are listed on the Nasdaq National Market or any other stock exchange recognized by the Israeli Ministry of Finance, and provided certain other conditions are met, the most relevant of which are: (A) the capital gain is not attributed to the foreign resident's permanent establishment in Israel, and (B) the shares were acquired by the foreign resident after the company's shares had been listed for trading on the foreign Exchange. If the shares were sold by Israeli residents, then (A) for the period ending December 31, 2002 their sale would be tax exempt so long as (1) the shares are listed on a stock exchange, such as the Nasdaq National Market, which is recognized by the Israeli Ministry of Finance, and (2) we qualified as an industrial company or industrial holding company under the law for Encouragement of Industry (Taxes) 1969, and (B) for the period commencing January 1, 2003, the sale of the shares would be subject to a 15% tax if the shares are listed on a stock exchange recognized by the Israeli Ministry of Finance. We believe that in 2004 we qualified as an Industrial Company under the law for Encouragement of Industry (Taxes) 1969. We cannot provide any assurance that the Israeli tax authorities will agree with the determination that we qualified as an industrial company in the past or that we will maintain this qualification or our status as an industrial company. If we are delisted, gains from the sale of our ordinary shares will be subject to capital gains tax at a rate of 25% unless an exemption or other tax rate applies in accordance with a tax treaty between Israel and the shareholder's country of residence.
Non-residents of Israel are subject to tax on income accrued or derived from sources in Israel. These sources of income include passive income such as dividends, royalties and interest, as well as non-passive income, such as income received for services rendered in Israel. We are required to withhold income tax at the rate of 25% with respect to passive income (or 15% for dividends distributed from income generated by an approved enterprise) unless a different rate or an exemption is provided in a tax treaty between Israel and the shareholder's country of residence.
Under an amendment to the Inflationary Adjustments Law, non-Israeli corporations might be subject to Israeli taxes on the sale of shares in an Israeli company which are traded on certain stock markets, including The Nasdaq National Market, subject to the provisions of any applicable double taxation treaty.
Stamp Duty
Stamp duty applies in Israel to various types of documents at various rates, depending primarily on the type of the document and the amount specified, or not, therein. In June 2003, the Israeli statute imposing the stamp duty was amended in a manner believed by many to significantly expand the tax basis. Following this amendment, the Israeli Tax Authority has increased enforcement of this statute. The amendment to the statue and the enforcement actions taken by the Israeli Tax Authority are in legal dispute before the Israeli Supreme Court, which has not yet ruled on this matter. In addition, under current legislation the stamp duty statute is
85
expected to be gradually phased out until its complete cancellation in 2008. To date, we have not received a demand for payment of stamp duty following this amendment. We currently do not believe that any stamp duty that may be imposed on us as a result of this amendment would materially adversely affect our financial condition.
United States Federal Income Taxation
The following is a description of the material United States federal income tax consequences of the ownership of our ordinary shares. This summary does not purport to address all of the tax considerations that may be relevant to a decision to purchase, own or dispose of our ordinary shares. This description assumes that holders of our ordinary shares will hold the ordinary shares as capital assets. This summary does not address tax considerations applicable to holders who may be subject to special tax rules, including:
| • | dealers or traders in securities or currencies; |
| |
| • | tax-exempt entities; |
| |
| • | banks, financial institutions or insurance companies; |
| |
| • | real estate investment trusts, regulated investment companies or grantor trusts; |
| |
| • | persons who received ordinary shares as compensation for the performance of services; |
| |
| • | holders who own, or are deemed to own, at least 10% or more, by voting power or value, of our shares; |
| |
| • | investors whose functional currency is not the United States dollar; or |
| |
| • | holders who hold our ordinary shares as part of a position in a straddle or as part of a hedging or conversion transaction for United States federal income tax purposes. |
Further, this description does not address any United States federal estate and gift or alternative minimum tax consequences, nor any state, local, or foreign tax consequences relating to the ownership and disposition of our ordinary shares.
This description is based on the Internal Revenue Code of 1986, as amended, United States Treasury Regulations and judicial and administrative interpretations thereof, in each case as in effect and available on the date of this annual report. The United States tax laws and the interpretation thereof are subject to change, which change could apply retroactively and could affect the tax consequences described below.
Unless specifically noted below, the following description applies only to owners of our ordinary shares that are United States holders for United States federal income tax purposes.
For purposes of this description, a United States holder is a beneficial owner of ordinary shares that, for United States federal income tax purposes, is:
| • | citizen or resident of the United States; |
| |
| • | a corporation or partnership created or organized in or under the laws of the United States or any state, including the District of Columbia; |
| |
| • | an estate if its income is subject to United States federal income taxation regardless of its source; or |
| |
| • | a trust if such trust validly has elected to be treated as a United States person for United States federal income tax purposes or if a United States court can exercise primary supervision over its administration and one or more United States persons have the authority to control all of its substantial decisions. |
A non-United States holder is a beneficial owner of ordinary shares that is not a United States holder.
Shareholders should consult their own tax advisors with respect to the United States federal, state, local and foreign tax consequences of acquiring, owning or disposing of our ordinary shares.
86
Distributions
Subject to the discussion below under “Passive Foreign Investment Company Considerations”, the entire amount of any distribution made to you with respect to ordinary shares, other than any distributions of our ordinary shares made to all our shareholders, will constitute dividends to the extent of our current or accumulated earnings and profits as determined under United States federal income tax principles. For these purposes, the amount of the distribution will not be reduced by the amount of any Israeli tax withheld from the distribution. Non-corporate U.S. Holders may be taxed on the dividend distributions made in taxable years beginning before December 31, 2008 at the lower rates applicable to long-term capital gains (i.e., gains with respect to capital assets held for more than one year). However, non-corporate U.S. Holders that do not meet a minimum holding period requirement during which they are not protected from the risk of loss, that elect to treat the dividend income as “investment income” pursuant to Section 163(d)(4) of the Code or that receive dividends with respect to which they are obligated to make related payments, will not be eligible for the reduced rates of taxation. In addition, the dividends will be included in your gross income as ordinary income and will not be eligible for the dividends received deduction generally allowed to corporate United States holders. We do not maintain calculations of our earnings and profits under United States federal income tax principles.
If distributions with respect to our ordinary shares exceed our current and accumulated earnings and profits, the excess distributed with respect to any ordinary share would be treated first as a tax-free return of capital to the extent of your adjusted basis in that ordinary share. Subject to the discussion below under “Passive Foreign Investment Company Considerations”, any amount in excess of the amount of the dividend and the return of capital would be treated as capital gain, subject to the rules described under “Sale or Exchange of our Ordinary Shares.”
If we pay a dividend or distribution in NIS, you will be required to take the dividend or distribution into account at its dollar amount based on the spot rate of exchange in effect on the distribution date. You will have a tax basis for United States federal income tax purposes in the NIS received equal to that dollar value, and any subsequent gain or loss in respect of the NIS arising from exchange rate fluctuations will generally be taxable as U.S. source ordinary income or loss.
You may generally elect to claim the Israeli income tax withheld from dividends and distributions you receive with respect to ordinary shares as a foreign tax credit against your United States federal income tax liability, subject to a number of limitations. Among the limitations, the foreign tax credits allowable with respect to specific classes of income cannot exceed the U.S. federal income tax payable with respect to each such class. Dividends we pay generally will be included in the “passive income” class for these purposes, or, in the case of certain financial services entity holders, “financial services income.” U.S. Holders should note, however, that recently enacted legislation eliminates the “financial services income” category for taxable years beginning after December 31, 2006. Under the recently enacted legislation, the foreign tax credit limitation categories are limited to “passive category income” and “general category income.” In lieu of claiming a foreign tax credit, you may claim a deduction for the withholding taxes if you itemize your deductions. Dividends received by you with respect to ordinary shares generally will be treated as foreign source income, which may be relevant in calculating your foreign tax credit limitation.
Subject to the discussion below under “Backup Withholding and Information Reporting,” if you are a non-United States holder of our ordinary shares, you will not be subject to United States federal income or withholding tax on dividends you receive on ordinary shares, unless the dividends are effectively connected with the conduct by such non-United States holder of a trade or business in the United States.
Sale or Exchange of Our Ordinary Shares
Subject to the discussion below under “Passive Foreign Investment Company Considerations”, you will recognize capital gain or loss for United States federal income tax purposes when you sell
87
or exchange our ordinary shares. The amount of gain or loss will be equal to the difference between your adjusted tax basis in the ordinary shares and the amount realized on their disposition. If you are a noncorporate United States holder, the maximum marginal United States federal income tax rate applicable to such gain will be lower than the maximum marginal United States federal income tax rate applicable to ordinary income (other than certain dividends) if your holding period for our ordinary shares exceeds one year. Any gain or loss recognized by you generally will be treated as United States source income or loss for United States foreign tax credit purposes. Capital losses may only be used to offset capital gains, except that non-corporate U.S. holders are entitled to deduct capital losses in excess of capital gains not to exceed $3,000 per taxable year.
Subject to the discussion below under “Backup Withholding and Information Reporting,” if you are a non-United States holder of our ordinary shares, we expect that you will not be subject to United States federal income or withholding tax on gain realized on the sale or exchange of such ordinary shares unless (1) such gain is effectively connected with the conduct by you of a trade or business in the United States, (2) in the case of gain realized by an individual non-United States holder, you are present in the United States for 183 days or more in the taxable year of the sale or exchange and certain other conditions are met, or (3) you are subject to the rules applicable to certain United States expatriates.
Passive Foreign Investment Company Considerations
A non-United States corporation will be classified as a “passive foreign investment company” (a “PFIC”) for United States federal income tax purposes in any taxable year in which, after applying applicable look-through rules with respect to a 25% or more owned subsidiary, either (1) at least 75% of its gross income is “passive income,” or (2) at least 50% of the gross value of its assets is attributable to assets that produce passive income or are held for the production of passive income. Passive income for this purpose includes items such as dividends, interest, royalties, rents and gains from commodities and securities transactions.
Based on our estimated gross income, the average value of our gross assets (determined by reference to the market value of our shares and valuing our intangible assets using the methods prescribed for publicly traded corporations) and the nature of our business, we believe that we will not be classified as a PFIC for the taxable year ended December 31, 2004. Our status in future years will depend on our assets and activities in those years, although you will be treated as continuing to own an interest in a PFIC if we are a PFIC in any year while you own your shares unless you make certain elections. We have no reason to believe that our assets or activities will change in a manner that would cause us to be classified as a PFIC, but because the market price of our ordinary shares is likely to fluctuate, we cannot assure you that we will not be considered a PFIC for any taxable year. If we were a PFIC, you generally would be subject to imputed interest charges and other disadvantageous tax treatment (including the denial of the taxation of such dividends at the lower rates applicable to long-term capital gains, as discussed above under “Distributions”) with respect to any gain from the sale or exchange of, and excess distributions with respect to, the ordinary shares.
If we were a PFIC, you could make a variety of elections that may alleviate the tax consequences referred to above, and one of these elections may be made retroactively. However, it is expected that the conditions necessary for making certain of such elections will not apply in the case of our ordinary shares. You should consult your own tax advisor regarding our potential status as a PFIC and the tax consequences that would arise if we were treated as a PFIC.
Backup Withholding and Information Reporting
United States backup withholding taxes and information reporting requirements apply to certain payments to noncorporate holders of stock. Information reporting requirements will, and a backup withholding tax may, apply to payments of dividends on, and to proceeds from the sale, exchange or redemption of, our ordinary shares made within the United States, or by a U.S. payor
88
or U.S. middleman, to a holder of our ordinary shares, other than an exempt recipient, including a corporation, a payee that is a non-United States person that provides an appropriate certification and certain other persons. Backup withholding is not an additional tax and may be claimed as a credit against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing an appropriate claim for refund with the IRS and furnishing any required information. The backup withholding tax rate is 28% for years 2003 through 2010.
In the case of such payments by a payor within the United States, or by a U.S. payor or U.S. middleman, to a foreign simple trust, foreign grantor trust or foreign partnership, other than payments to a foreign simple trust, foreign grantor trust or foreign partnership that qualifies as a withholding foreign trust or a withholding foreign partnership within the meaning of such income tax regulations and payments to a foreign simple trust, foreign grantor trust or foreign partnership that are effectively connected with the conduct of a trade or business in the United States, the beneficiaries of the foreign simple trust, the person treated as the owner of the foreign grantor trust or the partners of the foreign partnership, as the case may be, will be required to provide the certification discussed above in order to establish an exemption from backup withholding tax and information reporting requirements.
The above description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition of our ordinary shares. Shareholders should consult their own tax advisors concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
F. Dividends and Paying Agents
Not applicable.
G. Statements by Experts
Not applicable.
H. Documents on Display
We are currently subject to the information and periodic reporting requirements of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and file periodic reports and other information with the Securities and Exchange Commission through its electronic data gathering, analysis and retrieval (EDGAR) system. Our securities filings, including this Annual Report and the exhibits thereto, are available for inspection and copying at the public reference facilities of the Securities and Exchange Commission located at Room 1024, 450 Fifth Street, N.W., Washington, D.C. 20549 and at the Securities and Exchange Commission's regional office at Citicorp Center, 500 West Madison Street, Suite 1400, Chicago, Illinois 60661. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the Securities and Exchange Commission at 450 Fifth Street, NW, Washington, DC 20549. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the public reference room. The Commission also maintains a website at http://www.sec.gov from which certain filings may be accessed.
As a foreign private issuer, we are exempt from the rules under the Exchange Act relating to the furnishing and content of proxy statements, and our officers, directors and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the Securities and Exchange Commission as frequently or as promptly as United States companies whose securities are registered under the Exchange Act.
89
I. Subsidiary Information
Not applicable.
Item 11. Quantitative and Qualitative Disclosures about Market Risk
We do not use derivative financial instruments for trading purposes and, as of year end, we had no derivative financial instruments of any kind outstanding. Accordingly, we have concluded that there is no material market risk exposure of the type contemplated by Item 11, and that no quantitative tabular disclosures are required. We are exposed to certain other types of market risks. See Item 5.
Item 12. Description of Securities other than Equity Securities
Not applicable.
90
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies
None.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
There are no material modifications to the rights of security holders that are required to be disclosed.
Our initial public offering of our ordinary shares commenced on October 4, 2001, and terminated after the sale of all the securities registered. The co-lead managing underwriters for the offering were Lehman Brothers Inc. and Credit Suisse First Boston Corporation. We registered 5,750,000 ordinary shares in the offering, including shares issued pursuant to the exercise of the underwriters' over-allotment option. We sold 5,000,000 ordinary shares at an aggregate offering price of $60 million representing a price per share of $12.00. Under the terms of the offering,
we incurred aggregate underwriting discounts of $4.2 million. We also incurred expenses of $2.6 million in connection with the offering. The net proceeds that we received as a result of the offering were $53.2 million. As of December 31, 2004, the net proceeds had been used in their entirety as follows.
Use of Proceeds
| | Description
| | Amount
|
| | | | | (in thousands) |
Construction of plant, buildings and facilities | | — | | $ | — | |
Purchase and installation of machinery and equipment | | Computers, office equipment, installment for semi-automated production lines | | | 15,168 | |
Purchase of real estate | | — | | | — | |
Repayment of indebtedness | | Capital lease obligation for motor vehicles | | | 186 | |
Working capital | | Operating expenses | | | 37,846 | |
Temporary investments | | — | | | — | |
Any other use involving $100,000 of the proceeds | | — | | | — | |
| | | | | |
| |
Total: | | | | $ | 53,200 | |
| | | | | |
| |
| | | | | | |
None of the net proceeds of the offering was paid directly or indirectly to any director, officer, general partner of ours or to their associates, persons owning ten percent or more of any class of our equity securities, or to any of our affiliates.
We completed an additional offering of our ordinary shares on June 23, 2004, in which we sold 1,500,000 ordinary shares and raised net proceeds of $44.3 million.
Item 15. Controls and Procedures
We maintain disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in our periodic filings with the SEC is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer (CEO) and Chief Financial Officer (CFO), as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Furthermore, management necessarily was required to use its judgment in evaluating the cost to benefit relationship of possible disclosure controls and procedures.
91
As of the end of the period covered by this report, we performed an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures. The evaluation was performed with the participation of senior management of each business segment and key corporate functions, and under the supervision of our CEO and CFO. Based on the evaluation, our management, including the CEO and CFO, concluded that our disclosure controls and procedures were effective at the reasonable assurance level. During the period covered by this annual report, there have not been any changes in our internal control over financial reporting that have materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting.
In connection with the preparation and audit of our 2004 financial statements, we discovered that a former employee of our U.S. subsidiary had misappropriated approximately $0.4 million during the period 2002 through 2004, with the bulk of the misappropriation taking place in 2004. See Part A “Operating Results” under Item 5 “Operating and Financial Review and Prospectus.” We undertook an investigation, which was completed in February 2005, with the assistance of independent legal counsel and forensic accountants. The investigators concluded that insufficient supervision and a lack of segregation of duties regarding cash and cash processing at our U.S. subsidiary may have facilitated the misappropriation. Our independent auditors informed us that they did not consider these issues to constitute material weaknesses with respect to our internal control over financial reporting. We have subsequently commenced remedial actions to address these issues, including implementing a whistleblower policy, in accordance with legal requirements that become applicable to us in July 2005, to enable employees to report anonymously and confidentially any accounting, financial and other events of which they believe management should be aware.
Item 16. [Reserved]
Item 16A. Audit Committee Financial Expert
The board of directors has determined that Michael Grobstein is the financial expert serving on its audit committee.
Item 16B. Code of Ethics
We have adopted a code of ethics applicable to our chief executive officer, chief financial officer, controller and persons performing similar functions. The code of ethics was filed with the SEC as Exhibit 11.1 to our Annual Report on Form 20-F for the fiscal year ended December 31, 2003 and is also available on our website, www.givenimaging.com.
Item 16C. Principal Accountant Fees and Services
The following table sets forth the total remuneration that was paid by us and our subsidiaries to our independent accountants, KPMG, in each of our previous two fiscal years:
| | | | 2003
| | 2004
|
| | | | (in thousands)
(unaudited) |
| | Audit fees | | $ | 175 | | | $ | 225 | |
| | Audit-related fees | | | 21 | | | | 24 | |
| | Tax fees | | | 36 | | | | 64 | |
| | All other fees | | | 27 | | | | 146 | |
| | | | |
| | | |
| |
| | Total | | $ | 259 | | | $ | 459 | |
| | | | |
| | | |
| |
| | | | | | | | | | |
| | | |
| (1) | | “Audit-related fees” includes fees related to the review of the annual report we file with the SEC. |
(footnotes continued on next page)
92
(footnotes continued from previous page)
| | | |
| (2) | | “Tax fees” includes fees for professional services rendered by our auditors for tax compliance and tax advice on actual or contemplated transactions. |
| | | |
| (3) | | “All other fees” includes fees related to advice on international transfer prices, compliance with Sarbanes-Oxley Act requirements, information technology and support services and review of the prospectus distributed by us in June 2004 in connection with our follow-on offering. |
Our audit committee pre-approved all audit and non-audit services provided to us and to our subsidiaries during the periods listed above.
Item 16D. Exemptions From the Listing Standards for Audit Committees
None.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
The following table provides information regarding purchases of our ordinary shares by Elron Electronic Industries Ltd., which may be deemed to be an affiliated purchaser of us, during the fiscal year ended December 31, 2004:
|
| Period | | (a) Total Number of
Shares (or Units)
Purchased | | (b) Average Price
Paid per Share
(or Units) in US$ | | (c) Total Number of
Shares (or Units)
Purchased as
Part of Publicly
Announced
Plans or Programs | | (d) Maximum
Number (or
Approximate
Dollar Value) of
Shares (or Units)
that May Yet Be
Purchased Under the
Plans or Programs | |
|
Aug 1-31, 2004 | | | 550,000 | | | $34.88 | | 0 | | N/A | |
|
| Nov 1-30, 2004 | | | 823,513 | | | $30.04 | | 0 | | N/A | |
|
| Total | | | 1,373,513 | | | $31.98 | | 0 | | N/A | |
|
Note to column (a)
The shares purchased in August and November 2004 were purchased in open market transactions on the Nasdaq National Market and the Tel Aviv Stock Exchange, and not as part of a publicly-announced repurchase program.
93
PART III
Item 17. Financial Statements
Not applicable.
Item 18. Financial Statements
See pages F-1 to F-23 incorporated herein by reference.
Item 19. Exhibits
Exhibit
| | Description
|
| | 1.1 | | — | Articles of Association, incorporated by reference to Exhibit 3.2 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.1 | | — | Investor Rights Agreement, dated as of September 15, 2000, by and among the Registrant and the parties thereto, incorporated by reference to Exhibit 10.10 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.2 | | — | Amendment No. 1 to Investor Rights Agreement, dated as of July 16, 2001, by and among the Registrant and the parties thereto, incorporated by reference to Exhibit 10.17 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.3 | | — | Production Development, Manufacturing and Sales Agreement, dated as of November 26, 2002, by and between Micron Technology, Inc. and the Registrant, incorporated by reference to Exhibit 4.3 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004.† |
| | 4.4 | | — | Technology Purchase and License Agreement, dated as of January 29, 1998, by and between Rafael Armament Development Authority of the Israeli Ministry of Defense and the Registrant, incorporated by reference to Exhibit 10.12 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.5 | | — | Form of Indemnification Agreement between directors and officers of the Registrant and the Registrant, incorporated by reference to Exhibit 10.15 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.6 | | — | Lease Agreement, dated as of December 15, 1999, by and between B. A. T. M. Lands Ltd. and the Registrant, incorporated by reference to Exhibit 10.16 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.7 | | — | Purchase and Sale Contract, dated as of August 25, 2001, by and between Pemstar, Inc. and the Registrant, incorporated by reference to Exhibit 10.18 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.8 | | — | Form of Standard Distribution Agreement of the Registration, incorporated by reference to Exhibit 10.19 of the Registration Statement on Form F-1 (File No. 333-81514) filed with the Commission on February 1, 2002. |
| | 4.9 | | — | Lease Agreement, dated as of November 26, 2001, by and between Sha'ar Yoqneam L.P. and the Registrant, incorporated by reference to Exhibit 10.20 of the Registration Statement on Form F-1 (File No. 333-81514) filed with the Commission on February 1, 2002. |
| | 4.10 | | — | Summary of Material Terms of Addendum, dated December 2002, to Lease Agreement, dated as of November 26, 2001, by and between Sha'ar Yoqneam and the Registrant, incorporated by reference to Exhibit 4.11 of the Annual Report on Form 20-F for the year ended December 31, 2002, filed with the Commission on April 10, 2003. |
94
| | 4.11 | | — | Development and Supply Agreement, dated April 8, 2002, by and between Zarlink Semiconductor AB and the Registrant.†† |
| | 4.12 | | — | Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant.†† |
| | 4.13 | | — | Amendment No. 1, dated June 2004, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant.†† |
| | 4.14 | | — | Amendment No. 2, dated October 4, 2004, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant.†† |
| | 4.15 | | — | Summary of Material Terms of Second Addendum, dated July 5, 2004, to the Lease Agreement, dated as of November 26, 2001, by and between Sha'ar Yoqneam L.P. and the Registrant. |
| | 8.1 | | — | List of subsidiaries of the Registrant, incorporated by reference to Exhibit 8.1 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004. |
| | 9.1 | | — | Consent of KPMG Somekh Chaikin, independent registered public accountants. |
| | 11.1 | | — | Code of Ethics adopted on December 9, 2003, incorporated by reference to Exhibit 11.1 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004. |
| | 12.1 | | — | Certification of Chief Executive Officer of the Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 12.2 | | — | Certification of Chief Financial Officer of the Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 13.1 | | — | Certification of Chief Executive Officer of the Registrant pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
| | 13.2 | | — | Certification of Chief Financial Officer of the Registrant pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
| † | | Portions of this exhibit were omitted and have been filed separately with the Secretary of the Commission on March 17, 2004, pursuant to the Registrant's application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934. |
| †† | | Portions of this exhibit were omitted and have been filed separately with the Secretary of the Commission on March 25, 2005, pursuant to an application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934. |
| * | | This document is being furnished in accordance with SEC Release Nos. 33-8212 and 34-47551. |
95
SIGNATURES
Pursuant to the requirements of Section 12 of the Securities Exchange Act of 1934, the registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | GIVEN IMAGING LTD. |
| | |
| | By: /s/ GAVRIEL MERON |
| | Name: Gavriel Meron
Title: President, Chief Executive Officer and Director |
| | By: /s/ ZVI BEN DAVID |
| | Name: Zvi Ben David
Title: Vice President and Chief Financial Officer |
Date: March 25, 2005
96
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders
of Given Imaging Ltd.
We have audited the accompanying consolidated balance sheets of Given Imaging Ltd. and its subsidiaries (the “Company”) as of December 31, 2004 and 2003, and the related consolidated statements of operations, changes in shareholders' equity and cash flows for each of the years in the three year period ended December 31, 2004. These consolidated financial statements are the responsibility of the Company's Board of Directors and of its management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statements presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2004 and 2003, and the consolidated results of its operations, changes in shareholders' equity and cash flows for each of the years in the three year period ended December 31, 2004, in conformity with generally accepted accounting principles in the United States of America.
/S/ | SOMEKH CHAIKIN
Certified Public Accountants (Israel)
Member of KPMG International |
February 16, 2005 | |
F-2
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands except per share data)
| | | | | December 31
|
| | | Note
| | 2003
| | 2004
|
ASSETS | | | | | | | | | | |
Current assets | | | | | | | | | | |
Cash and cash equivalents | | 1D; 2 | | $ | 25,367 | | | $ | 80,861 | |
Accounts receivable: | | | | | | | | | | |
Trade (net of provision for doubtful debts of $115 as of December 31, 2004 and 2003) | | 1E | | | 6,945 | | | | 12,261 | |
Other | | 3 | | | 467 | | | | 1,271 | |
Inventories | | 1F; 4 | | | 8,485 | | | | 13,794 | |
Advances to suppliers | | | | | 47 | | | | 555 | |
Deferred taxes | | 1O; 13C | | | — | | | | 737 | |
Prepaid expenses | | | | | 1,314 | | | | 954 | |
| | | | | |
| | | |
| |
Total current assets | | | | | 42,625 | | | | 110,433 | |
Deposits | | | | | 361 | | | | 425 | |
Assets held for severance benefits | | 1G; 9 | | | 1,008 | | | | 1,339 | |
Fixed assets, at cost, less accumulated depreciation | | 1H; 5 | | | 9,595 | | | | 9,862 | |
Other assets, at cost, less accumulated amortization | | 1I; 6 | | | 1,980 | | | | 2,165 | |
| | | | | |
| | | |
| |
Total assets | | | | $ | 55,569 | | | $ | 124,224 | |
| | | | | |
| | | |
| |
LIABILITIES AND SHAREHOLDERS' EQUITY | | | | | | | | | | |
Current liabilities | | | | | | | | | | |
Current installments of obligation under capital lease | | 7B | | $ | 27 | | | $ | 11 | |
Accounts payable: | | | | | | | | | | |
Trade | | | | | 2,216 | | | | 5,147 | |
Other | | 8 | | | 4,462 | | | | 8,678 | |
Deferred income | | 1M; 7D | | | 950 | | | | 3,610 | |
| | | | | |
| | | |
| |
Total current liabilities | | | | | 7,655 | | | | 17,446 | |
| | | | | |
| | | |
| |
Long-term liabilities | | | | | | | | | | |
Deferred income | | 7D | | | — | | | | 9,340 | |
Obligation under capital lease, net | | 7B | | | 4 | | | | 48 | |
Liability for employee severance benefits | | 9 | | | 1,188 | | | | 1,596 | |
| | | | | |
| | | |
| |
Total long-term liabilities | | | | | 1,192 | | | | 10,984 | |
| | | | | |
| | | |
| |
Total liabilities | | | | | 8,847 | | | | 28,430 | |
| | | | | |
| | | |
| |
Commitments and contingencies | | 7 | | | | | | | | |
Minority interest | | | | | 1,924 | | | | 1,177 | |
Shareholders' equity | | | | | | | | | | |
Share capital: | | 10 | | | | | | | | |
Ordinary Shares, NIS 0.05 par value each (90,000,000 and 60,000,000 shares authorized as of December 31, 2004 and 2003, respectively) 27,621,386 and 25,649,188 shares issued and fully paid as of December 31, 2004 and 2003, respectively) | | | | | 301 | | | | 323 | |
Additional paid-in capital | | | | | 100,996 | | | | 147,878 | |
Capital reserve | | | | | 2,166 | | | | 2,166 | |
Unearned compensation | | | | | (30 | ) | | | (3 | ) |
Accumulated deficit | | | | | (58,635 | ) | | | (55,747 | ) |
| | | | | |
| | | |
| |
Total shareholders' equity | | | | | 44,798 | | | | 94,617 | |
| | | | | |
| | | |
| |
Total liabilities and shareholders' equity | | | | $ | 55,569 | | | $ | 124,224 | |
| | | | | |
| | | |
| |
| | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands except per share data)
| | | | | Year ended December 31,
|
| | | Note
| | 2002
| | 2003
| | 2004
|
Revenues | | 1M; 11 | | $ | 28,904 | | | $ | 40,539 | | | $ | 65,020 | |
Cost of revenues | | | | | 11,907 | | | | 13,551 | | | | 17,734 | |
| | | | | |
| | | |
| | | |
| |
Gross profit | | | | | 16,997 | | | | 26,988 | | | | 47,286 | |
| | | | | |
| | | |
| | | |
| |
Operating expenses | | | | | | | | | | | | | | |
Research and development, gross | | 1P | | | (8,609 | ) | | | (7,037 | ) | | | (7,363 | ) |
Royalty bearing participation | | 1N | | | — | | | | 1,303 | | | | 1,140 | |
| | | | | |
| | | |
| | | |
| |
Research and development, net | | | | | (8,609 | ) | | | (5,734 | ) | | | (6,223 | ) |
Sales and marketing | | | | | (22,681 | ) | | | (26,804 | ) | | | (33,652 | ) |
General and administrative | | | | | (4,749 | ) | | | (5,312 | ) | | | (6,916 | ) |
| | | | | |
| | | |
| | | |
| |
Total operating expenses | | | | | (36,039 | ) | | | (37,850 | ) | | | (46,791 | ) |
| | | | | |
| | | |
| | | |
| |
Operating profit (loss) | | | | | (19,042 | ) | | | (10,862 | ) | | | 495 | |
Financing income, net | | 12 | | | 1,469 | | | | 995 | | | | 956 | |
Other expenses, net | | | | | (711 | ) | | | — | | | | — | |
| | | | | |
| | | |
| | | |
| |
Profit (loss) before taxes on income | | | | | (18,284 | ) | | | (9,867 | ) | | | 1,451 | |
Taxes on income | | 1O, 13 | | | — | | | | — | | | | 690 | |
| | | | | |
| | | |
| | | |
| |
Profit (loss) before minority share | | | | | (18,284 | ) | | | (9,867 | ) | | | 2,141 | |
Minority share in losses (profits) of subsidiary | | | | | (26 | ) | | | 258 | | | | 747 | |
| | | | | |
| | | |
| | | |
| |
Net profit (loss) | | | | $ | (18,310 | ) | | $ | (9,609 | ) | | $ | 2,888 | |
| | | | | |
| | | |
| | | |
| |
Profit (loss) per share | | | | | | | | | | | | | | |
Basic profit (loss) per Ordinary Share | | 1K | | $ | (0.73 | ) | | $ | (0.38 | ) | | $ | 0.11 | |
| | | | | |
| | | |
| | | |
| |
Diluted profit (loss) per Ordinary Share | | | | $ | (0.73 | ) | | $ | (0.38 | ) | | $ | 0.10 | |
| | | | | |
| | | |
| | | |
| |
Weighted average number of Ordinary Shares used to compute basic profit (loss) per Ordinary Share | | 1K | | | 25,182,563 | | | | 25,493,073 | | | | 26,633,964 | |
| | | | | |
| | | |
| | | |
| |
Weighted average number of Ordinary Shares used to compute diluted profit (loss) per Ordinary Share | | 1K | | | 25,182,563 | | | | 25,493,073 | | | | 29,353,448 | |
| | | | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS' EQUITY
(in thousands except share data)
| | | Ordinary Shares
| | | | | | | | | | | | | | | | | | | | |
| | | Shares
| | Amount
| | Additional
Paid-In
Capital
| | Capital
Reserve
| | Unearned
Compensation
| | Accumulated
Deficit
| | Total
|
Balance as of January 1, 2002 | | | 25,104,913 | | | $ | 296 | | | $ | 100,103 | | | $ | — | | | $ | (350 | ) | | $ | (30,716 | ) | | $ | 69,333 | |
Changes during the year 2002: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exercise of stock options | | | 268,600 | | | | 2 | | | | 159 | | | | — | | | | — | | | | — | | | | 161 | |
Amortization of unearned compensation | | | — | | | | — | | | | — | | | | — | | | | 227 | | | | — | | | | 227 | |
Capital reserve from issuance of shares in a newly formed subsidiary | | | — | | | | — | | | | — | | | | 2,166 | | | | — | | | | — | | | | 2,166 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (18,310 | ) | | | (18,310 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance as of December 31, 2002 | | | 25,373,513 | | | $ | 298 | | | $ | 100,262 | | | $ | 2,166 | | | $ | (123 | ) | | $ | (49,026 | ) | | $ | 53,577 | |
Changes during the year 2003: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exercise of stock options | | | 275,675 | | | | 3 | | | | 722 | | | | — | | | | — | | | | — | | | | 725 | |
Forfeiture of stock options | | | — | | | | — | | | | (78 | ) | | | — | | | | 78 | | | | — | | | | — | |
Acceleration of vesting | | | — | | | | — | | | | 31 | | | | — | | | | — | | | | — | | | | 31 | |
Non-employees' stock options | | | — | | | | — | | | | 59 | | | | — | | | | — | | | | — | | | | 59 | |
Amortization of unearned compensation | | | — | | | | — | | | | — | | | | — | | | | 15 | | | | — | | | | 15 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (9,609 | ) | | | (9,609 | ) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance as of December 31, 2003 | | | 25,649,188 | | | $ | 301 | | | $ | 100,996 | | | $ | 2,166 | | | $ | (30 | ) | | $ | (58,635 | ) | | $ | 44,798 | |
Changes during the year 2004: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Ordinary shares issued | | | 1,500,000 | | | | 17 | | | | 44,250 | | | | — | | | | — | | | | — | | | | 44,267 | |
Exercise of stock options | | | 472,198 | | | | 5 | | | | 2,581 | | | | — | | | | — | | | | — | | | | 2,586 | |
Forfeiture of stock options | | | — | | | | — | | | | (11 | ) | | | — | | | | 3 | | | | — | | | | (8 | ) |
Non-employees' stock options | | | — | | | | — | | | | 62 | | | | — | | | | — | | | | — | | | | 62 | |
Amortization of unearned compensation | | | — | | | | — | | | | — | | | | — | | | | 24 | | | | — | | | | 24 | |
Net profit | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,888 | | | | 2,888 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
Balance as of December 31, 2004 | | | 27,621,386 | | | $ | 323 | | | $ | 147,878 | | | $ | 2,166 | | | $ | (3 | ) | | $ | (55,747 | ) | | $ | 94,617 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | Year ended December 31,
|
| | | 2002
| | 2003
| | 2004
|
| | | | | | | | | | | | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net profit (loss) | | $ | (18,310 | ) | | $ | (9,609 | ) | | $ | 2,888 | |
Adjustments required to reconcile net profit (loss) to net cash provided by (used in) operating activities: | | | | | | | | | | | | |
Minority share in profits (losses) of subsidiary | | | 26 | | | | (258 | ) | | | (747 | ) |
Depreciation and amortization | | | 2,176 | | | | 3,030 | | | | 3,147 | |
Deferred taxes | | | — | | | | — | | | | (737 | ) |
Employees' stock option compensation | | | 227 | | | | 46 | | | | 16 | |
Non-employees' stock option compensation | | | — | | | | 59 | | | | 62 | |
Other | | | 97 | | | | 9 | | | | 48 | |
Increase in accounts receivable—trade | | | (4,395 | ) | | | (80 | ) | | | (5,316 | ) |
Decrease (increase) in other accounts receivable | | | (633 | ) | | | 1,018 | | | | (804 | ) |
Decrease (increase) in prepaid expenses | | | (85 | ) | | | (80 | ) | | | 360 | |
Increase in advances to suppliers | | | (24 | ) | | | (23 | ) | | | (508 | ) |
Decrease (increase) in inventories | | | (7,402 | ) | | | 1,860 | | | | (5,648 | ) |
Increase (decrease) in accounts payable | | | 5,537 | | | | (4,708 | ) | | | 7,107 | |
Increase in deferred income | | | 534 | | | | 223 | | | | 12,000 | |
Increase (decrease) in payable to related parties | | | 19 | | | | (35 | ) | | | — | |
| | | |
| | | |
| | | |
| |
Net cash provided by (used in) operating activities | | $ | (22,233 | ) | | $ | (8,548 | ) | | $ | 11,868 | |
| | | |
| | | |
| | | |
| |
Cash flows from investing activities: | | | | | | | | | | | | |
Purchase of fixed assets and other assets | | $ | (7,472 | ) | | $ | (2,550 | ) | | $ | (3,245 | ) |
Proceeds from sales of fixed assets | | | — | | | | 60 | | | | 57 | |
Deposits, net | | | (88 | ) | | | (157 | ) | | | (42 | ) |
| | | |
| | | |
| | | |
| |
Net cash used in investing activities | | $ | (7,560 | ) | | $ | (2,647 | ) | | $ | (3,230 | ) |
| | | |
| | | |
| | | |
| |
Cash flows from financing activities: | | | | | | | | | | | | |
Principal payments on capital lease obligation | | $ | (64 | ) | | $ | (72 | ) | | $ | (37 | ) |
Proceeds from the issuance of Ordinary Shares | | | 161 | | | | 725 | | | | 46,853 | |
Proceeds from issuance of shares by consolidated company | | | 4,322 | | | | — | | | | — | |
| | | |
| | | |
| | | |
| |
Net cash provided by financing activities | | $ | 4,419 | | | $ | 653 | | | $ | 46,816 | |
| | | |
| | | |
| | | |
| |
Effect of exchange rate changes on cash | | $ | (64 | ) | | $ | 117 | | | $ | 40 | |
| | | |
| | | |
| | | |
| |
Increase (decrease) in cash and cash equivalents | | $ | (25,438 | ) | | $ | (10,425 | ) | | $ | 55,494 | |
Cash and cash equivalents at beginning of year | | | 61,230 | | | | 35,792 | | | | 25,367 | |
| | | |
| | | |
| | | |
| |
Cash and cash equivalents at end of year | | $ | 35,792 | | | $ | 25,367 | | | $ | 80,861 | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | |
Supplementary cash flow information
| | | Year ended December 31,
|
| | | 2002
| | 2003
| | 2004
|
| | | | | | | | | | | | |
Interest paid | | $ | 7 | | | $ | 3 | | | $ | — | |
Income taxes paid | | $ | 79 | | | $ | 68 | | | $ | 107 | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(in thousands except per share data)
Note 1—Organization and Summary of Significant Accounting Policies
A. General
Given Imaging Ltd. (the “Company”) was incorporated in Israel in January 1998.
The Company has developed the Given System, a proprietary wireless imaging system that represents a new approach to visual examination of the gastrointestinal tract. The system uses a miniaturized video camera contained in a capsule, referred to as PillCam™ capsule, that is ingested by the patient and delivers high quality color images in a painless and noninvasive manner.
The Given System consists of three principal components:
| • | a single-use, disposable PillCam color-imaging capsule that is ingested by the patient; |
| |
| • | a portable data recorder and array of sensors that are worn by the patient; and |
| |
| • | a computer workstation with a proprietary RAPID software for downloading, processing and analyzing recorded data. |
After receiving marketing clearance from the U.S. Food and Drug Administration (“FDA”) in August of 2001, the Company commenced the marketing of the Given System with its first video capsule, the PillCam Small Bowel Capsule, or PillCam SB, for detection of disorders of the small bowel. As of December 31, 2004, the Company had an installed base of more than 2,290 Given Systems and had sold approximately 171,800 PillCam SB capsules in over 50 countries worldwide. In November 2004, following receipt of FDA marketing clearance, the Company began marketing and sales of its second video capsule, PillCam ESO, for detection of disorders in the esophagus. The Company markets the PillCam ESO capsule through a strategic marketing alliance with Inscope, a division of Ethicon Endo-Surgery, a Johnson & Johnson company (see Note 7D).
The medical device industry in which the Company is involved is characterized by the risks of regulatory barriers and reimbursement issues. Penetration into the world market requires the investment of considerable resources and continuous development efforts. The Company's future success is dependent upon several factors including the technological quality, regulatory approvals and sufficient reimbursement for its products.
B. Basis of presentation
The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries in the USA, Germany, France, the Netherlands and Australia and its 51% owned subsidiary in Japan. The accounts of its subsidiaries are consolidated from the date of their inception. All significant intercompany balances and transactions have been eliminated in consolidation.
All the subsidiaries were established for the purpose of marketing and selling the Given System.
C. Functional and reporting currency
The accounting records of the Company are maintained in New Israeli Shekels (“NIS”) and U.S. dollars. The Company's functional and reporting currency is the U.S. dollar.
Transactions denominated in foreign currencies other than the U.S. dollar are translated into the reporting currency using current exchange rates. Gains and losses from the translation of foreign currency balances are recorded in the statement of operations.
The Company currently has no plans to pay dividends and has not determined the currency in which any dividends would be paid.
F-7
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
D. Cash and cash equivalents
All highly-liquid investments with original maturity of three months or less from the date of deposit are considered to be cash equivalents.
E. Provision for doubtful debts
The provision for doubtful debts is calculated on the basis of specific identification of balances, the collection of which, in management's opinion, is doubtful. In determining the adequacy of the provision, management bases its opinion, inter alia, on the estimated risk, in reliance on available information with respect to the debtor's financial position and an evaluation of the collateral received.
F. Inventories
Inventories are stated at lower of cost or market. Cost is determined using the average cost method for raw materials and finished goods, and on the basis of actual manufacturing costs for work in progress and sub-contractors.
G. Assets held for severance benefits
Assets held for employee severance benefits represent contributions to severance pay funds and cash surrender value of life insurance policies that are recorded at their current redemption value.
H. Fixed assets
Fixed assets are stated at cost. Depreciation is computed by the straight-line method over the estimated useful lives of the assets at the following annual rates:
| | | | %
|
| | Office furniture, leasehold improvements, laboratory equipment and other equipment | | 6–15 |
| | Computers, software and related equipment | | 20–33 |
| | Machinery and equipment | | 15 |
| | Motor vehicles | | 15 |
| | | | |
Motor vehicles purchased under capital lease arrangements are recorded at the present value of the minimum lease payments. Such assets and leasehold improvements are amortized using the straight-line method over the shorter of the lease term or estimated useful life of the asset.
I. Other assets
| | | 1. | | The Company developed proprietary software for its computer workstations that permits downloading and viewing recorded data from the portable data recorder. The costs of developing this software were capitalized in accordance with Statement of Financial Accounting Standards No. 86, “Accounting for Costs of Computer Software to be Sold, Leased or Otherwise Marketed” (“Statement 86”). As such, capitalization of software development costs begins upon the establishment of technological feasibility as defined in Statement 86 and continues up to the time the software is available for general release to customers, at which time capitalized software costs are amortized on a straight-line basis over the expected life of the related product, which is generally three years. |
F-8
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
| | | 2. | | Legal expenses related to patent and trademark registration have been capitalized and depreciated over the remaining life of the asset, which is generally eight years. |
| | | 3. | | Technology and content costs are generally expensed as incurred, except for certain costs relating to the development of the Company's web site that are capitalized and depreciated over their estimated useful lives which are generally three years. |
J. Stock compensation plans
Employees and directors
The Company has adopted Financial Accounting Standards Board's Statement No. 123, “Accounting for Stock-Based Compensation” (“Statement 123”) which permits entities to recognize as an expense over the vesting period, the fair value on the date of grant of all stock-based awards. Alternatively, Statement 123 allows entities to continue to apply the provisions of Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees and related interpretations” (“APB Opinion No. 25”) and provide pro forma net income and pro forma earnings per share disclosures for employee stock option grants as if the fair-value based method defined in Statement 123 had been applied.
The Company has elected to continue to apply the provisions of APB Opinion No. 25 and provide the pro forma disclosure provisions of Statement 123, as amended by Statement 148, “Accounting for Stock-Based Compensation—Transition and Disclosure, an amendment of Statement No. 123” (“Statement 148”).
The Company applies the intrinsic value-based method prescribed in APB Opinion No. 25 for its stock compensation to employees and directors. As such, the Company computes and records compensation expense for grants whose terms are fixed with respect to the number of shares and option price only if the market price on the date of grant exceeds the exercise price of the stock option. The compensation cost for the fixed plans is recorded over the period the employee performs the service to which the stock compensation relates.
Non-Employees
The Company applies the fair value-based method of accounting set forth in Statement 123 to account for stock based compensation to non-employees. Using the fair value method, the total compensation expense is computed based on the fair value of the options on the date the options are granted to the non-employees since the options are fully vested.
F-9
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
The following table shows the effect on net profit (loss) and profit (loss) per Ordinary Share if the Company had applied the fair value recognition provisions of Statement 123:
| | | | Year ended December 31,
|
| | | | 2002
| | 2003
| | 2004
|
| | Net profit (loss) as reported | | $ | (18,310 | ) | | $ | (9,609 | ) | | $ | 2,888 | |
| | Deduct: Compensation expenses according to APB 25 included in the reported net profit (loss) | | | 227 | | | | 105 | | | | 78 | |
| | Add: Application of compensation expenses according to Statement 123 | | | (4,324 | ) | | | (7,012 | ) | | | (13,432 | ) |
| | | | |
| | | |
| | | |
| |
| | Pro forma net loss | | $ | (22,407 | ) | | $ | (16,516 | ) | | $ | (10,466 | ) |
| | | | |
| | | |
| | | |
| |
| | Basic profit (loss) per Ordinary Share: | | | | | | | | | | | | |
| | As reported | | $ | (0.73 | ) | | $ | (0.38 | ) | | $ | 0.11 | |
| | | | |
| | | |
| | | |
| |
| | Pro forma | | $ | (0.89 | ) | | $ | (0.65 | ) | | $ | (0.39 | ) |
| | | | |
| | | |
| | | |
| |
| | Diluted profit (loss) per ordinary share: | | | | | | | | | | | | |
| | As reported | | $ | (0.73 | ) | | $ | (0.38 | ) | | $ | 0.10 | |
| | | | |
| | | |
| | | |
| |
| | Pro forma | | $ | (0.89 | ) | | $ | (0.65 | ) | | $ | (0.39 | ) |
| | | | |
| | | |
| | | |
| |
| | Number of options excluded from the diluted earning per share calculation because of anti-dilutive effect | | | 2,977,463 | | | | 3,881,396 | | | | 165,500 | |
| | | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | |
K. Profit (loss) per Ordinary Share
Basic and diluted profit (loss) per Ordinary Share is presented in conformity with Statement of Financial Accounting Standard No. 128, “Earnings Per Share”, for all years presented. Basic profit (loss) per Ordinary Share is calculated by dividing the net profit (loss) attributable to Ordinary Shares, by the weighted average number of Ordinary Shares outstanding. Diluted profit (loss) per Ordinary share calculation is similar to Basic Earnings Per Share except that the weighed average of common shares outstanding is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares such as options had been exercised.
L. Use of estimates
Management of the Company has made a number of estimates and assumptions relating to the reporting of assets and liabilities and the disclosure of contingent assets and liabilities to prepare these consolidated financial statements in accordance with accounting principles generally accepted in the United States of America. Actual results could differ from these estimates.
M. Revenue recognition
Revenues from sales of products are recognized in accordance with Staff Accounting Bulletin No. 101 “Revenue Recognition in Financial Statements” (“SAB No. 101”), as amended by Staff Accounting Bulletin No. 104, upon delivery provided that the collection of the resulting receivable is probable, there is persuasive evidence of an arrangement, no significant obligations in respect of installation remain and the price is fixed or determinable.
For sales contracts, which include a Post Contract Customer Support (“PCS”) component, revenues from PCS are deferred and recognized ratably over the term of the support period,
F-10
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
which is generally one year, according to EITF 00-21 “Revenue Arrangements with Multiple Deliverables”.
The Company accrues estimated warranty costs at time of shipment based on contractual rights and historical experience. The Company's policy is not to grant return rights.
N. Government-Sponsored Research and Development
The Company records grants received from the Office of the Chief Scientist of the Israeli Ministry of Industry and Trade (the “OCS”) as a reduction of research and development expenses. Royalties payable to OCS are classified as cost of revenues.
O. Taxes on income
The Company accounts for income taxes under Statement of Financial Accounting Standards No. 109 “Accounting for Income Taxes” (“Statement 109”).
Under Statement 109 deferred tax assets or liabilities are recognized in respect of temporary differences between the tax bases of assets and liabilities and their financial reporting amounts as well as in respect of tax losses and other deductions which may be deductible for tax purposes in future years, based on enacted statutory tax rates applicable to the periods in which such deferred taxes will be realized. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
P. Research and development costs
Research and development costs are expensed as incurred.
Q. Concentration of credit risk
Financial instruments that may subject the Company to significant concentrations of credit risk consist principally of cash and cash equivalents and trade accounts receivable. Cash and cash equivalents are deposited with major financial institutions in Europe, the United States, Japan and Israel.
The Company performs ongoing credit evaluations of the financial condition of its customers. The risk of collection associated with trade receivables is reduced by the large number and geographical dispersion of the Company's customer base and the Company's policy of requiring collateral or security on sales to distributors.
R. Recent accounting pronouncements
The Company examined the recently issued accounting standards Statement No. 151, Statement No. 152, Statement No. 153 and EITF 03-1 and believes that the adoption of these standards will not have a significant impact on the Company's financial position or results of operations.
In December 2004, the FASB issued Statement No. 123 (revised 2004), “Share-Based Payment” (“Statement 123R”), which requires all companies to measure compensation cost for all share-based payments (including employee stock options) at fair value. Statement 123R is effective for interim or annual periods beginning after June 15, 2005. Statement 123R provides two alternative adoption methods. The first method is a modified prospective transition method whereby a company would recognize share-based employee costs from the beginning of the fiscal period in which the recognition provisions are first applied as if the fair-value-based accounting
F-11
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
method had been used to account for all employee awards granted, modified, or settled after the effective date and to any awards that were not fully vested as of the effective date.
Measurement and attribution of compensation cost for awards that are unvested as of the effective date of Statement 123R would be based on the same estimate of the grant-date fair value and the same attribution method used previously under Statement No. 123, “Accounting for Stock-Based Compensation” (“Statement 123”). The second adoption method is a modified retrospective transition method whereby a company would recognize employee compensation cost for periods presented prior to the adoption of Statement 123R in accordance with the original provisions of Statement 123; that is, an entity would recognize employee compensation costs in the amounts reported in the pro forma disclosures provided in accordance with Statement 123. A company would not be permitted to make any changes to those amounts upon adoption of Statement 123R unless those changes represent a correction of an error. For periods after the date of adoption of Statement 123R, the modified prospective transition method described above would be applied.
The Company currently expects to adopt Statement 123R in the quarter ending September 30, 2005, using the modified prospective method, although it continues to review its alternatives for adoption under this new pronouncement. Based upon its projection of unvested stock options at the implementation date, the Company expects the adoption of Statement 123R to result in the recognition of material additional compensation expense in 2005 and thereafter.
Note 2—Cash and Cash Equivalents
| | | | | | | | December 31
|
| | | | Interest rate
as of
December 31
2004
| | 2003
| | 2004
|
| | | | % | | | | | | | | |
| | Denominated in U.S. dollars | | | 2.2–2.35 | | | $ | 17,364 | | | $ | 72,650 | |
| | Denominated in New Israeli Shekels | | | 3.64–3.85 | | | | 2,413 | | | | 2,538 | |
| | Denominated in Euro | | | 1.8 | | | | 1,321 | | | | 2,508 | |
| | Denominated in Australian dollars | | | — | | | | 485 | | | | 648 | |
| | Denominated in Japanese Yen | | | — | | | | 3,784 | | | | 2,517 | |
| | | | | | | | |
| | | |
| |
| | | | | | | | $ | 25,367 | | | $ | 80,861 | |
| | | | | | | | |
| | | |
| |
| | | | | | | | | | | | | | |
Note 3—Accounts Receivable—Other
| | | | | | | | December 31
|
| | | | | | | | 2003
| | 2004
|
| |
Government institutions
| | | | | | $ | 443 | | | $ | 1,204 | |
| |
Other
| | | | | | | 24 | | | | 67 | |
| | | | | | | | |
| | | |
| |
| | | | | | | | $ | 467 | | | $ | 1,271 | |
| | | | | | | | |
| | | |
| |
| | | | | | | | | | | | | | |
Note 4—Inventories
| | | | December 31
|
| | | | 2003
| | 2004
|
| | Raw materials and components | | $ | 4,248 | | | $ | 5,058 | |
| | Work in progress | | | 1,011 | | | | 3,891 | |
| | Finished goods | | | 3,226 | | | | 4,845 | |
| | | | |
| | | |
| |
| | | | $ | 8,485 | | | $ | 13,794 | |
| | | | |
| | | |
| |
F-12
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
Note 5—Fixed Assets, at Cost, Less Accumulated Depreciation
| | | | December 31
|
| | | | 2003
| | 2004
|
| | Computers and software | | $ | 3,648 | | | $ | 3,988 | |
| | Instruments and laboratory equipment | | | 543 | | | | 587 | |
| | Leasehold improvements | | | 1,120 | | | | 1,554 | |
| | Motor vehicles | | | 144 | | | | 55 | |
| | Machinery and equipment | | | 8,351 | | | | 10,167 | |
| | Communication equipment | | | 328 | | | | 375 | |
| | Office furniture and equipment | | | 813 | | | | 927 | |
| | | | |
| | | |
| |
| | Fixed assets | | | 14,947 | | | | 17,653 | |
| | Accumulated depreciation | | | (5,352 | ) | | | (7,791 | ) |
| | | | |
| | | |
| |
| | Fixed assets less accumulated depreciation | | $ | 9,595 | | | $ | 9,862 | |
| | | | |
| | | |
| |
| | | | | | | | | | |
Depreciation expense for the years ended December 31, 2002, 2003 and 2004 are $1,757, $2,475 and $ 2,561, respectively.
Note 6—Other Assets, at Cost, Less Accumulated Amortization
| | | | December 31
|
| | | | 2003
| | 2004
|
| | Software development costs | | $ | 647 | | | $ | 647 | |
| | Patents and trademarks | | | 1,714 | | | | 2,485 | |
| | Web site application and infrastructure | | | 823 | | | | 823 | |
| | | | |
| | | |
| |
| | Other assets | | | 3,184 | | | | 3,955 | |
| | Accumulated amortization | | | (1,204 | ) | | | (1,790 | ) |
| | | | |
| | | |
| |
| | Other assets, net | | $ | 1,980 | | | $ | 2,165 | |
| | | | |
| | | |
| |
| | | | | | | | | | |
Amortization expense for the years ended December31, 2002, 2003 and 2004 are $419, $555 and $586, respectively.
Note 7—Commitments and Contingencies
A. Office of the Chief Scientist Grants
The Company's research and development efforts have been partially financed through grants from the Office of the Chief Scientist of the Israeli Ministry of Industry and Trade (the “OCS”). In return for the OCS's participation, the Company is committed to pay royalties to the Israeli Government at the rate of 3% for each of the first three years and, from the fourth year onwards, at the rate of 3.5% of the sales of its product, up to 100% of the amount of the grants received. The grants are deducted from research and development expenses. Grants received in advance of the corresponding expenditures incurred are recorded as a liability. The Company is entitled to the grants only upon incurring research and development expenditures. There are no future performance obligations related to the grants received from the OCS. However, under certain limited circumstances, the OCS may withdraw its approval of a research program or amend the terms of its approval. Upon withdrawal of approval, the grant recipient may be required to refund the grant, in whole or in part, with or without interest, as the OCS determines. The Company received from the OCS office a total cumulative amount of $3,640 of which it already repaid $1,443. The total outstanding obligation for royalties, based on royalty-bearing government
F-13
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
participation totaled approximately $2,197 as of December 31, 2004. Royalties payable to the OCS are classified in cost of revenues.
B. Leases
Capital lease for motor vehicles
The capital lease is to be repaid in four years and bear interest of 7.47%. The vehicles are pledged as collateral.
Operating leases
The Company and its subsidiaries currently lease office space and manufacturing space for periods of up to ten years (including options to extend the terms of the leases). The current lease for the Company's headquarters in Yoqneam, Israel, is scheduled to expire in July 2005. To secure its obligation under the existing leases, the Company has provided the lessor with a bank guarantee of $243.
In July 2004, the Company entered into a lease of an approximately 10,300 square meter (110,869 square feet) facility in Yoqneam, Israel. This facility will host the Company's corporate headquarters, research & development and manufacturing facilities commencing July 2005. Under this new lease agreement, the Company will pay approximately $1.0 million a year in rent and management fee. These payments are subject to adjustments based on changes in the Israeli Consumer Price Index. In addition, to secure its obligations under the lease, the Company provided a bank guaranty in the amount of approximately $700 in favor of the lessor. The lease expires on December 31, 2015. The Company has an option to extend the lease until December 31, 2020.
The Company and its subsidiaries signed several motor vehicle lease agreements. The companies deposited a total amount of $279 to guarantee their performance under the terms of the lease agreements.
Future minimum capital lease payments and future minimum operating lease payments:
| | | | December 31, 2004
|
| | | | Capital
Leases
| | Operating
Leases
|
| | 2005 | | $ | 14 | | | $ | 2,231 | |
| | 2006 | | | 14 | | | | 2,042 | |
| | 2007 | | | 14 | | | | 1,724 | |
| | 2008 | | | 20 | | | | 1,342 | |
| | 2009 and thereafter | | | 18 | | | | 8,705 | |
| | | | |
| | | |
| |
| | | | $ | 80 | | | $ | 16,044 | |
| | | | |
| | | |
| |
| | | | | | | | | | |
Office and manufacturing rental expense under the lease agreements for the years ended December 31, 2002, 2003 and 2004 are $950, $1,140 and $1,999 respectively.
C. Agreement with Pemstar Inc.
The Company has entered into a non-exclusive technical services agreement with Pemstar Inc., a U.S. electronics manufacturing service and equipment company (Pemstar), pursuant to which Pemstar provides the Company with technical services relating to the manufacture of the PillCam capsule on the semi-automated production lines the Company purchased from Pemstar.
F-14
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
Pemstar built for the Company two semi-automated production lines. One is fully operated in the Company's facilities in Yoqneam and one serves as a back-up production line outside of Israel and ready for operation on sixty days notice.
In consideration of providing these services to the Company, the Company pays Pemstar a specified amount for each functional PillCam capsule that is produced using the production line purchased from Pemstar. This amount will decrease as the volume of PillCam capsules produced increases. In addition, the Company pays Pemstar a monthly fixed amount for hosting and maintaining the back-up production line.
D. Agreement with Ethicon Endo-Surgery
On May 10, 2004, the Company entered into an exclusive sales representation, co-promotion and cooperation agreement with InScope, a division of Ethicon Endo-Surgery, a Johnson & Johnson company. InScope has exclusive rights to market the Company's PillCam ESO capsule for visualization of the esophagus. Under the terms of the agreement, the Company received a milestone payment of $10 million in December 2004 following FDA clearance of the PillCam ESO capsule and may receive additional payments of up to $40 million by February 2007, contingent on performance criteria applicable to both parties. The Company pays InScope a commission of 50% on sales of PillCam ESO capsules and a 10% commission on sales of other parts of the Given System, such as workstations and portable data recorders. Subject to achieving specified minimum sales targets and meeting certain conditions, the exclusive term of the agreement will continue for up to 11 years followed by a four-year co-exclusive transition period with the Company. The alliance initially is worldwide, excluding Japan. InScope began marketing PillCam ESO in the United States in November 2004 following FDA clearance for the PillCam ESO capsule.
InScope has not yet commenced its operations in territories other than the United States, which territories may subsequently be excluded if InScope and the Company do not reach agreement regarding the activities of InScope in these other territories by March 31, 2005.
The initial milestone payment of $10 million has been, and as any subsequent milestone payments will be deferred and systematically recognized by the Company as a reduction of commission expense over the 15 years term of the agreement.
The $10 million milestone payment is included in deferred income in the Consolidated Balance Sheets.
E. Agreement with a Canadian supplier
The Company entered into an agreement with a Canadian company (hereinafter—the supplier) that supplies the transmitter that is integrated into the PillCam capsules. The supplier began supplying the transmitter to the Company in the fourth quarter of 2004, replacing its previous supplier. Under the agreement, the Company has agreed to purchase a minimum quantity of transmitters during the first 36 months following the development and testing phase, and if it fails to do so it must make certain payments to the supplier in respect of the shortfall. After this initial 36-month period, the supplier may terminate the agreement on six months notice if the Company does not order a minimum quantity of transmitters in each subsequent 12-month period, subject to the right of the Company to submit a final purchase order. The agreement also includes non-compete provisions prohibiting the supplier from selling the transmitters to other parties and, for a certain period of time following termination of the agreement, from transferring any of the intellectual property and design specifications associated with the development of the transmitter to any potential competitors in the Company's market. The initial term of the agreement expires in April 2007, subject to earlier termination in specified circumstances, with the option to extend annually thereafter for up to five years
F-15
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
F. Legal claim
A former employee of the Company's U.S. subsidiary filed a complaint in August 2004 for, among other things, wrongful termination, and alleged discrimination and harassment based on age. The complaint does not specify the amount of damages sought. All of the defendants have moved to dismiss the complaint as to the individual defendants and the majority of claims as to the subsidiary. The hearing on the defendants' motion to dismiss is expected to be heard in February 2005. The Company is vigorously contesting this claim and believes that it has meritorious defenses. No provision in this respect has been recorded.
G. Patent reexamination
On December 30, 2003, a Japanese corporation filed a request for ex parte reexamination (hereinafter—“Reexamination Request”) of some claims in one of the Company's U.S. patents (hereinafter—the “Patent”) in the United States Patent and Trademark Office (hereinafter —the “USPTO”). The USPTO granted the Reexamination Request on March 5, 2004. The Company will defend the validity of the claims upon receiving the USPTO's Office Action.
The Company is not accused of infringing a third party's patent and its ability to sell its products is not contested.
H. Stamp Duty
Stamp duty applies in Israel to various types of documents at various rates, depending primarily on the type of the document and the amount specified, or not, therein. In June 2003, the statute imposing stamp duty was amended. Following this amendment, the Israeli Tax Authority has increased enforcement of this statute. The amendment to the statute and the enforcement actions taken by the Israeli Tax Authority are in legal dispute before the Israeli Supreme Court, which has not yet ruled on this matter. In addition, under current legislation the stamp duty statute is expected to be gradually abolished until its complete cancellation in 2008. To date, the Company has not received a demand for payment of stamp duty following this amendment. The Company does not believe that any stamp duty that may be imposed on it as a result of this amendment will be material to the Company's financial position or results of operation.
Note 8—Accounts Payable—Other
| | | | December 31,
|
| | | | 2003
| | 2004
|
| | Government institutions | | $ | 439 | | | $ | 861 | |
| | Liabilities to employees | | | 2,078 | | | | 3,795 | |
| | Advances from customers | | | 40 | | | | 35 | |
| | Warranty | | | 68 | | | | 39 | |
| | Royalties to the OCS | | | — | | | | 246 | |
| | Commissions | | | 884 | | | | 1,923 | |
| | Accrued expenses | | | 953 | | | | 1,779 | |
| | | | |
| | | |
| |
| | | | $ | 4,462 | | | $ | 8,678 | |
| | | | |
| | | |
| |
| | | | | | | | | | |
Note 9—Liability in Respect of Employee Severance Payments
Under Israeli law and labor agreements the Company is required to pay severance payments to each employee who was employed by the Company for over one year and has been terminated by the Company or resigned under certain specified circumstances. The Company's liability for
F-16
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
severance payment is covered mainly by deposits with insurance companies in the name of the employee and/or by purchase of insurance policies. The liability is calculated on the basis of the latest salary of the employee multiplied by the number of years of employment as of the balance sheet date. The provision for employee severance payment included in the balance sheet represents the total liability for such severance payment, while the assets held for severance benefits included in the balance sheet represents the Company's contributions to severance pay funds and to insurance policies. The Company may make withdrawals from the funds only upon complying with the Israeli severance pay law or labor agreements.
The U.S. subsidiary has a defined contribution retirement plan for its employees. Employees are allowed to contribute a percentage of their salary in any one year, subject to a regulatory limit. The Company makes matching contributions of up to 3% of an employee's salary. Employees are immediately vested in the Company's contributions.
Expenses recorded in respect of severance pay for the years ended December 31, 2002, 2003 and 2004 are $418, $489 and $525, respectively.
Note 10—Share Capital
A. Ordinary shares
All of the issued and outstanding Ordinary Shares of the Company are duly authorized, validly issued, fully paid and non-assessable. The Ordinary Shares of the Company are not redeemable and have no preemptive rights. The ownership or voting of Ordinary Shares by non-residents of Israel is not restricted in any way by the Company's memorandum of association, its articles of association or the laws of the State of Israel, except that citizens of countries which are, or have been, in a state of war with Israel may not be recognized as owners of Ordinary Shares.
B. Issuance of Ordinary Shares
On June 23, 2004, the Company issued in a follow on offering 1,500,000 of its ordinary shares at a price of $32 per share.
The net proceeds, after deducting underwriter's fees and issuance expenses, amounted to $44.3 million.
The Company also increased its authorized share capital by NIS 1.5 million bringing the Company's authorized share capital to NIS 4,500,000 consisting of 90 million ordinary shares, par value NIS 0.05 each. The Articles and the Company's Memorandum of Association were amended to reflect this increase.
C. Employees' and non employees' stock options
In 2003, the Company adopted a stock option plan for directors, employees and consultants. The 2003 Plan replaced and superseded previous option plans adopted by the Company in 1998 and 2000. Under these plans, the Board of Directors (or a compensation committee appointed by the board) (the “Board”) has the authority to grant options to employees of the Company and its subsidiaries, directors or consultants. Each option entitles the holder to purchase one Ordinary Share of par value of NIS 0.05 and expires after 10 years from the date of grant.
The purchase price of each share pursuant to the options granted under the 2003 Plan shall be the fair market value on the date the Board approves the grant of the option or as otherwise determined by the Board.
F-17
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
As of December 31, 2004, 4,831,590 Ordinary Shares of the Company, of a par value of NIS 0.05 each, are reserved for the exercise of options under the Plans.
Unless otherwise determined by the Board, where a grant of options under the 2003 Plan is the first grant of options made to a person, 50% of the options vest and become exercisable on the second anniversary of the date of grant. An additional 25% of the options vest and become exercisable on each of the third and fourth anniversaries of the date of the grant. If, however, a grant under the 2003 Plan is made to a person who previously received stock options under the 2003 Plan or a previous plan of the Company, 25% of the options granted are immediately vested and exercisable and an additional 25% of the options vest and become exercisable on each of the first, second and third anniversaries of the date of the grant.
Options granted to Israeli optionees are designated as Section 102 options or Section 3(i) options within the meaning of the Israeli Income Tax Ordinance and related regulations as amended under the tax reform and are held in trust on behalf of the Israeli employees, as required by such legislation.
Options granted to non-Israeli optionees may or may not contain such terms that will qualify such options as Incentive Stock Options (“ISOs”) within the meaning of Section 422(b) of the United States Internal Revenue Code of 1986, as amended (the “Code”). Options that do not contain terms that will qualify them as ISOs are referred to herein as Non-Qualified Stock Options. Each option agreement is required to state whether such option will or will not be treated as an ISO. No ISO is granted unless such option, when granted, qualifies as an “incentive stock option” under Section 422 of the Code.
Compensation expense related to options issued has been calculated based on the difference between the market price of the underlying shares and the option's exercise price. Prior to the IPO date (October 4, 2001), the market price of the underlying shares was estimated on the basis of the price that was paid by various third parties, as well as increases in value attributable to the achievement of milestones in the Company's business plan. The share price paid by third parties has been adjusted upwards between the dates of the various private offerings. Following the IPO date (October 4, 2001), the market price represents the underlying listing price on the Nasdaq National Market.
The Company applies APB Opinion No. 25 in respect of options granted to employees and directors, and recorded compensation expense of $227, $46 and $16 in the years ended December 31, 2002, 2003 and 2004, respectively, according to the intrinsic value of the above options.
The Company applies Statement 123 in respect of options granted to consultants and recorded compensation expense of $0, $59 and $62 in the years ended December 31, 2002, 2003 and 2004, respectively, related to the above options according to the Black-Scholes model.
F-18
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
The following table illustrates the effect on net profit (loss) and profit (loss) per Ordinary Share if the Company had applied the fair value recognition provisions of Statement 123:
| | | | Year ended December 31,
|
| | | | 2002
| | 2003
| | 2004
|
| | Net profit (loss) as reported | | $ | (18,310 | ) | | $ | (9,609 | ) | | $ | 2,888 | |
| | Deduct: Compensation expenses according to APB 25 included in the reported net loss | | | 227 | | | | 105 | | | | 78 | |
| | Add: Application of compensation expenses according to Statement 123 | | | (4,324 | ) | | | (7,012 | ) | | | (13,432 | ) |
| | | | |
| | | |
| | | |
| |
| | Pro forma net loss | | $ | (22,407 | ) | | $ | (16,516 | ) | | $ | (10,466 | ) |
| | | | |
| | | |
| | | |
| |
| | Basic profit (loss) per Ordinary Share | | | | | | | | | | | | |
| | As reported | | $ | (0.73 | ) | | $ | (0.38 | ) | | $ | 0.11 | |
| | | | |
| | | |
| | | |
| |
| | Pro forma | | $ | (0.89 | ) | | $ | (0.65 | ) | | $ | (0.39 | ) |
| | | | |
| | | |
| | | |
| |
| | Diluted profit (loss) per Ordinary Share | | | | | | | | | | | | |
| | As reported | | $ | (0.73 | ) | | $ | (0.38 | ) | | $ | 0.10 | |
| | | | |
| | | |
| | | |
| |
| | Pro forma | | $ | (0.89 | ) | | $ | (0.65 | ) | | $ | (0.39 | ) |
| | | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | |
The fair value of each option granted is estimated on the date of grant, using the Black-Scholes model using the following assumptions:
| | 1. | | Dividend yield of zero percent. |
| | 2. | | Risk-free average interest rate of 2.00%, 1.5% and 1.8% for December 31, 2002, 2003 and 2004, respectively, which represents the risk free interest rates to dollar-linked financial instruments as published by the Israeli Stock Exchange. |
| | 3. | | Estimated expected lives of seven years as of the date of grant in regard to options granted until the end of 2003. Options granted during 2004 have an estimated expected lives of five years. |
| | 4. | | Expected average volatility of 100%, 81% and 74%, for December 31, 2002, 2003 and 2004, respectively which represents a weighted average standard deviation rate for the price of the Company's Ordinary Shares on the NASDAQ National Market. |
| | | | The following table summarizes information relating to stock options for Ordinary Shares outstanding as of the years indicated: |
| | | | Options Outstanding
| | Options Exercisable
|
| | Exercise Price
| | Number Outstanding at
December 31, 2005
| | Weighted Average
Remaining Contractual Life
(in years)
| | Number Exercisable at
December 31, 2005
|
| | NIS 0.05 | | | 279,578 | | | | 3.5 | | | | 279,578 | |
| | $1.–$10 | | | 1,459,170 | | | | 6.7 | | | | 1,190,340 | |
| | $10.01–$20 | | | 1,621,150 | | | | 8.1 | | | | 739,700 | |
| | $20.01–$30 | | | 218,600 | | | | 9.1 | | | | 46,800 | |
| | $30.01–$40 | | | 673,040 | | | | 9.9 | | | | — | |
| | $40.01–$50 | | | 50,000 | | | | 9.8 | | | | — | |
| | | | |
| | | | | | | |
| |
| | | | | 4,301,538 | | | | | | | | 2,256,418 | |
| | | | |
| | | | | | | |
| |
F-19
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
The option allotments are as follows:
| | | | Number of Shares
| | Weighted Average
Exercise Price
| | Weighted Average Grant
Date Fair Value
|
| | | | | | | | | | | | | | |
| | Balance at January 1, 2002 | | | 2,649,663 | | | | | | | | | |
| | Granted | | | 679,000 | | | | 12.06 | | | | 11.60 | |
| | Forfeited | | | (82,600 | ) | | | 9.02 | | | | 9.42 | |
| | Exercised | | | (268,600 | ) | | | 0.60 | | | | 1.91 | |
| | | | |
| | | | | | | | | |
| | Balance at December 31, 2002 | | | 2,977,463 | | | | | | | | | |
| | Granted | | | 1,331,500 | | | | 13.78 | | | | 13.61 | |
| | Forfeited | | | (151,892 | ) | | | 9.08 | | | | 8.92 | |
| | Exercised | | | (275,675 | ) | | | 2.63 | | | | 3.34 | |
| | | | |
| | | | | | | | | |
| | Balance at December 31, 2003 | | | 3,881,396 | | | | | | | | | |
| | Granted | | | 1,011,340 | | | | 34.09 | | | | 34.09 | |
| | Forfeited | | | (119,000 | ) | | | 14.54 | | | | 14.64 | |
| | Exercised | | | (472,198 | ) | | | 5.45 | | | | 6.32 | |
| | | | |
| | | | | | | | | |
| | Balance at December 31, 2004 | | | 4,301,538 | | | | | | | | | |
| | | | |
| | | | | | | | | |
| | | | | | | | | | | | | | |
The following summarizes the departmental allocation of the stock-based compensation charge:
| | | | Year ended December 31,
|
| | | | 2002
| | 2003
| | 2004
|
| | Research and development costs | | $ | 73 | | | $ | (18 | ) | | $ | 63 | |
| | Selling and marketing expenses | | | 77 | | | | 94 | | | | 1 | |
| | General and administrative expenses | | | 77 | | | | 29 | | | | 14 | |
| | | | |
| | | |
| | | |
| |
| | | | $ | 227 | | | $ | 105 | | | $ | 78 | |
| | | | |
| | | |
| | | |
| |
| | | | | | | | | | | | | | |
Note 11—Revenues
A. Revenues by activities
| | | Year ended December 31,
|
| | | 2002
| | 2003
| | 2004
|
| | | | | | | | | | | | |
Workstations and recorders | | $ | 17,230 | | | $ | 15,879 | | | $ | 18,669 | |
PillCam SB capsule | | | 10,889 | | | | 22,865 | | | | 41,622 | |
PillCam ESO capsule | | | — | | | | — | | | | 1,829 | |
Patency capsules and scanners | | | — | | | | 15 | | | | 188 | |
Service | | | 785 | | | | 1,780 | | | | 2,712 | |
| | | |
| | | |
| | | |
| |
| | $ | 28,904 | | | $ | 40,539 | | | $ | 65,020 | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | |
B. Revenues by geographic areas
| | | Year ended December 31,
|
| | | 2002
| | 2003
| | 2004
|
| | | | | | | | | | | | |
United States | | $ | 15,648 | | | $ | 26,162 | | | $ | 46,694 | |
Europe | | | 8,334 | | | | 11,838 | | | | 13,447 | |
Rest of the world | | | 4,922 | | | | 2,539 | | | | 4,879 | |
| | | |
| | | |
| | | |
| |
| | $ | 28,904 | | | $ | 40,539 | | | $ | 65,020 | |
| | | |
| | | |
| | | |
| |
F-20
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
Note 12—Financial Income, net
| | | Year ended December 31,
|
| | | 2002
| | 2003
| | 2004
|
Currency gains | | $ | 746 | | | $ | 840 | | | $ | 444 | |
Interest income | | | 762 | | | | 272 | | | | 685 | |
Other | | | (39 | ) | | | (117 | ) | | | (173 | ) |
| | | |
| | | |
| | | |
| |
| | $ | 1,469 | | | $ | 995 | | | $ | 956 | |
| | | |
| | | |
| | | |
| |
| | | | | | | | | | | | |
Note 13—Taxes on Income
A. Company
| | (1) | | Israeli income tax is computed on the basis of the Company's results in nominal NIS determined for statutory purposes. The Company is assessed for tax purposes under the Income Tax Law (Inflationary Adjustments 1985), the purpose of which is to prevent taxation on inflationary profits. |
| | | | Pursuant to the Israeli tax law, the Company was awarded “Approved Enterprise” status under the government alternative benefits track. The program is for investments in the development of infrastructure and for investments in locally produced and imported equipment. The main benefits to which the Company will be entitled, if it implements all the terms of an approved program, are the exemption from tax on income deriving from an approved enterprise, and reduced tax rates on dividends originating from this income. |
| | | | The income derived from an approved enterprise will be exempt from tax for a ten year period, commencing on the date that taxable income is first generated by the approved enterprise (limited to the earlier of a maximum period of 12 years from the year of commencement of operations or 14 years from the year the approval letter was received). As of December 31, 2004, the benefit term had not commenced. |
| | | | On December 30, 2002, the Company received an approval from the Investment Center for the expansion of its production capacity during the next two years under the alternative benefits track. The investments are expected to amount to $4.9 million, which is being invested in its facilities in Yoqneam. The plan is subject to certain terms set forth in the approval letter. |
| | | | Dividend distributions originating from the income of the Approved Enterprise will be subject to tax at the rate of 15%, provided that the dividend is distributed during the period stipulated under Israeli law. |
| | | | In the event of a dividend distribution (including withdrawals and charges that are deemed to be dividends) out of the income originating from the approved enterprise, and on which the Company received a tax exemption, will be subject to corporate taxes at rates varying from 10%–25% depending on the percentage of foreign investment holding in the Company as defined by the Law. |
| | | | If the Company derives income from sources other than the approved enterprise during the relevant period of benefits, such income will be taxable at regular corporate tax rates (see (4) below). |
| | (2) | | The Company has net operating loss carryforwards in Israel of approximately $25 million as of December 31, 2004. These net operating loss carryforwards are linked to the Israeli Consumer Price Index and are available to offset future taxable income, if any, indefinitely. |
F-21
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
| | (3) | | The Israeli Company is exempt from tax for a ten-year period. Therefore, the Israeli Company has not recorded deferred tax assets and liabilities. |
| | (4) | | On June 29, 2004 the Knesset passed Income Tax Ordinance Amendment (No. 140 and Temporary Order), 2004 (hereinafter—the Amendment). |
| | | | The Amendment provides a gradual reduction in the company tax rate from 36% to 30% in the following manner: in 2004 the tax rate will be 35%, in 2005 the tax rate will be 34%, in 2006 the tax rate will be 32% and from 2007 onward the tax rate will be 30%. |
This change has no effect on the financial statements of the Company.
B. Subsidiaries
Because of operating losses, the Company's subsidiaries incurred no income tax expense for the period from inception through December 31, 2004. At December 31, 2004, the subsidiaries had local, federal and state net operating loss carryforwards of approximately $22,450.
C. Deferred Taxes
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers projected future taxable income and tax planning strategies in making this assessment.
Based upon projections for future taxable income over the periods in which the deferred tax assets are deductible, management believes it is more likely than not that the Company will realize the benefits of these deductible differences, net of the existing valuation allowances at December 31, 2004. The amount of the deferred tax asset considered realizable, however, could be reduced in the near term if estimates of future taxable income during the carryforward period are reduced.
The tax effects of significant items comprising the Company's deferred taxes:
| | | December 31
|
| | | 2003
| | 2004
|
Net operating tax assets regarding carryforward losses of subsidiaries | | $ | 8,730 | | | $ | 8,349 | |
Start up costs and other timing difference | | | 526 | | | | 5,484 | |
| | | |
| | | |
| |
Deferred tax asset | | | 9,256 | | | | 13,833 | |
Valuation allowance | | | (9,256 | ) | | | (13,096 | ) |
| | | |
| | | |
| |
| | $ | — | | | $ | 737 | |
| | | |
| | | |
| |
| | | | | | | | |
The net change in the total valuation allowance for the year ended December 31, 2004 was $6,243.
F-22
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
Note 14—Related Parties Transactions and Balances
A. The Company carried out transactions with companies considered to be “related parties”. All of these transactions are carried out under normal business conditions.
B. Agreement with Rafael
On January 29, 1998, the Company entered into a technology purchase and license agreement (the “Technology Purchase Agreement”) with Rafael Armament Development Authority (“Rafael”) which is a division of the Israeli Ministry of Defense.
Rafael is a related party because it beneficially owns 47.8% of the outstanding shares of RDC Rafael Development Corporation, and they both hold approximately 9.96% of the Company's shares. Pursuant to the Technology Purchase Agreement, the Company purchased from Rafael for $30 all of Rafael's rights to the technology associated with and the patent issued in connection with the prototype PillCam capsule and system. Rafael and the Company each granted each other certain rights in connection with the know-how and technology associated with the PillCam capsule and system, in the case of Rafael, solely for use in the military and security fields and, in the case of the Company, solely for commercial exploitation of the PillCam capsule.
In consideration for payment of royalties in an amount to be agreed upon, the Company and Rafael have agreed to grant to the other the exclusive right to use any improvements to the PillCam capsule and system technology developed by the other party in these fields. None of the parties has exercised these certain rights to date.
Note 15—Fair Value of Financial Instruments
The Company's financial instruments include cash and cash equivalents, accounts receivable, deposits, assets held for severance benefits and accounts payable. The carrying amounts of these financial instruments approximate fair value.
F-23
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
ANNUAL REPORT ON FORM 20-F
INDEX OF EXHIBITS
Exhibit
| | Description
|
| | 1.1 | | — | Articles of Association, incorporated by reference to Exhibit 3.2 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.1 | | — | Investor Rights Agreement, dated as of September 15, 2000, by and among the Registrant and the parties thereto, incorporated by reference to Exhibit 10.10 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.2 | | — | Amendment No. 1 to Investor Rights Agreement, dated as of July 16, 2001, by and among the Registrant and the parties thereto, incorporated by reference to Exhibit 10.17 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.3 | | — | Production Development, Manufacturing and Sales Agreement, dated as of November 26, 2002, by and between Micron Technology, Inc. and the Registrant, incorporated by reference to Exhibit 4.3 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004.† |
| | 4.4 | | — | Technology Purchase and License Agreement, dated as of January 29, 1998, by and between Rafael Armament Development Authority of the Israeli Ministry of Defense and the Registrant, incorporated by reference to Exhibit 10.12 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.5 | | — | Form of Indemnification Agreement between directors and officers of the Registrant and the Registrant, incorporated by reference to Exhibit 10.15 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.6 | | — | Lease Agreement, dated as of December 15, 1999, by and between B. A. T. M. Lands Ltd. and the Registrant, incorporated by reference to Exhibit 10.16 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.7 | | — | Purchase and Sale Contract, dated as of August 25, 2001, by and between Pemstar, Inc. and the Registrant, incorporated by reference to Exhibit 10.18 of the Registration Statement on Form F-1 (File No. 333-68142) filed with the Commission on August 22, 2001. |
| | 4.8 | | — | Form of Standard Distribution Agreement of the Registration, incorporated by reference to Exhibit 10.19 of the Registration Statement on Form F-1 (File No. 333-81514) filed with the Commission on February 1, 2002. |
| | 4.9 | | — | Lease Agreement, dated as of November 26, 2001, by and between Sha'ar Yoqneam L.P. and the Registrant, incorporated by reference to Exhibit 10.20 of the Registration Statement on Form F-1 (File No. 333-81514) filed with the Commission on February 1, 2002. |
| | 4.10 | | — | Summary of Material Terms of Addendum, dated December 2002, to Lease Agreement, dated as of November 26, 2001, by and between Sha'ar Yoqneam and the Registrant, incorporated by reference to Exhibit 4.11 of the Annual Report on Form 20-F for the year ended December 31, 2002, filed with the Commission on April 10, 2003. |
| | 4.11 | | — | Development and Supply Agreement, dated April 8, 2002, by and between Zarlink Semiconductor AB and the Registrant.†† |
| | 4.12 | | — | Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant.†† |
| | 4.13 | | — | Amendment No. 1, dated June 2004, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant.†† |
GIVEN IMAGING LTD. AND ITS CONSOLIDATED SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
(in thousands except per share data)
| | 4.14 | | — | Amendment No. 2, dated October 4, 2004, to the Exclusive Sales, Representation, Co-Promotion and Cooperation Agreement, dated May 10, 2004, by and between Ethicon Endo-Surgery, Inc. and the Registrant.†† |
| | 4.15 | | — | Summary of Material Terms of Second Addendum, dated July 5, 2004, to the Lease Agreement, dated as of November 26, 2001, by and between Sha'ar Yoqneam L.P. and the Registrant. |
| | 8.1 | | — | List of subsidiaries of the Registrant, incorporated by reference to Exhibit 8.1 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004. |
| | 9.1 | | — | Consent of KPMG Somekh Chaikin, independent registered public accountants. |
| | 11.1 | | — | Code of Ethics adopted on December 9, 2003, incorporated by reference to Exhibit 11.1 of the Annual Report on Form 20-F for the year ended December 31, 2003, filed with the Commission on March 17, 2004. |
| | 12.1 | | — | Certification of Chief Executive Officer of the Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 12.2 | | — | Certification of Chief Financial Officer of the Registrant pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 13.1 | | — | Certification of Chief Executive Officer of the Registrant pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
| | 13.2 | | — | Certification of Chief Financial Officer of the Registrant pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
| † | | Portions of this exhibit were omitted and have been filed separately with the Secretary of the Commission on March 17, 2004, pursuant to the Registrant's application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934. |
| †† | | Portions of this exhibit were omitted and have been filed separately with the Secretary of the Commission on March 25, 2005, pursuant to an application requesting confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934. |
| * | | This document is being furnished in accordance with SEC Release Nos. 33-8212 and 34-47551. |
