As filed with the Securities and Exchange Commission on March 01, 2010
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
| o | | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES
EXCHANGE ACT OF 1934 |
OR
| þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934 |
| | |
For the fiscal year ended December 31, 2009 |
OR
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934 |
OR
| o | | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934 |
Commission file number 1-15170
GlaxoSmithKline plc
(Exact name of Registrant as specified in its charter)
England
(Jurisdiction of incorporation or organization)
980 Great West Road, Brentford, Middlesex TW8 9GS England
(Address of principal executive offices)
Simon Bicknell
Company Secretary
GlaxoSmithKline plc
980 Great West Road
Brentford, TW8 9GS
England
+44 20 8047 5000
company.secretary@gsk.com
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| | | |
| Title of Each Class | | Name of Each Exchange On Which Registered |
| | | |
American Depositary Shares, each representing 2 Ordinary Shares,
Par value 25 pence | | New York Stock Exchange |
| 4.850% Notes due 2013 | | New York Stock Exchange |
| 5.650% Notes due 2018 | | New York Stock Exchange |
| 6.375% Notes due 2038 | | New York Stock Exchange |
| Floating Rate Notes due 2010 | | New York Stock Exchange |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
(Title of class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
(Title of class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
| | | |
| Ordinary Shares of Par value 25 pence each | | 5,190,934,201 |
| | | |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
þ Yes o No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
o Yes þ No
Note — Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
þ Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
o Yes o No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer” and “large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | |
| Large accelerated filerþ | | Accelerated filero | | Non-accelerated filero |
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| | | | | |
| U.S. GAAPo | | International Financial Reporting Standards as issued | | Othero |
| | | by the International Accounting Standards Boardþ | | |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17o Item 18o
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
o Yes þ No
Cautionary statement regarding forward-looking statements
The Group’s reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this document and written information released, or oral statements made, to the public in the future by or on behalf of the Group, may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’ and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results. The Group undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
Forward-looking statements involve inherent risks and uncertainties. The Group cautions investors that a number of important factors, including those in this document, could cause actual results to differ materially from those contained in any forward-looking statement. Such factors include, but are not limited to, those discussed under ‘Risk factors’ on pages 43 to 47 of this Annual Report.
23
Our responsibility
Health initiatives
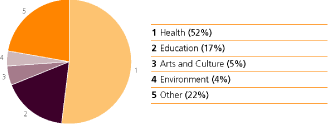
Our contribution to improve healthcare included the following grants:
| | | | | |
| Non-profit partner | | Amount in 2009 | | Purpose of grant |
| | | | | |
| Children’s Health Fund USA | | $1,461,000 | | To continue the Referral Management Initiative (RMI) which ensures continuity of specialist medical care for high-risk children who are often homeless and for general support |
| | | | | |
| GSK IMPACT Awards UK and USA | | £787,000 | | To recognise excellence in non-profit community health organisations. Charities receive unrestricted grants for their work dealing with diverse and difficult social issues and access to healthcare |
| | | | | |
Medical Research Charities
UK | | £400,000 | | To support medical research programmes |
| | | | | |
| Education initiatives |
| | | | | |
| Non-profit partner | | Amount in 2009 | | Purpose of grant |
| | | | | |
Institute for a Competitive Workforce
USA | | $100,000 | | To improve education and create a skilled workforce for the future, working in partnership with a broad business coalition and staffed by the US Chamber of Commerce |
| | | | | |
| ‘Science in the Summer’ Philadelphia, Pittsburgh and North Carolina | | $558,000 | | To teach basic scientific concepts and inspire school children through an enquiry-based science education programme |
| | | | | |
Project ENTHUSE
UK | | £200,000 | | To support Continuing Professional Development (CPD) for science teachers and ultimately encourage children to engage with science and pursue careers in science and technology |
| | | | | |
Royal Society of Chemistry
UK | | £100,000 | | To support a programme to target science teachers in the UK, who are not chemistry specialists, and provide them with the key skills and confidence to be effective in their chemistry teaching |
| | | | | |
Employee involvement
Our employees are encouraged to contribute to their local communities through employee volunteering schemes. Support includes employee time, cash donations to charities where employees volunteer and matching gift programmes.
Through the US GSK Matching Gift Program, we matched 15,000 employee and retiree gifts at a value of $4.7 million in 2009 plus over $1 million to the United Way campaign. GSK’s GIVE programme provided grants of over $314,000 to 353 organisations where US employees volunteered and £272,000 to 410 UK-based non-profit organisations via the GSK Making a Difference programme.
In 2009, our Group-wide volunteer initiative was launched to give every GSK employee one paid day off each year to volunteer for a good cause. Employees supported a wide range of charities and projects including work in local schools, shelters for the homeless, community gardens, nursing homes and aiding communities affected by natural disasters.
The GSK PULSE Volunteer Partnership is a new initiative launched in April 2009 that empowers high-performing employees to volunteer for a period of three to six months lending their professional expertise. PULSE volunteers work full-time with one of our partner non-governmental organisations (NGO) to create sustainable change for impoverished communities around the world. From our 2009 in-take, we had 58 PULSE volunteers, working in 18 different countries for 25 non-governmental organisations. Employees continue to receive their GSK salary during their placement and in 2009 this represented an in-kind donation of £428,000.
GSK Annual Report 2009
73
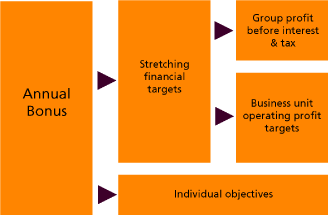
Remuneration Report
 Sir Crispin Davis
Sir Crispin DavisRemuneration Committee Chairman
Dear Shareholder
As the new Chairman of GSK’s Remuneration Committee I am pleased to present the Committee’s Remuneration Report for 2009 for which we will be seeking approval from shareholders at our AGM in May.
As you know, we made some important changes to GSK’s remuneration policy for our UK Executive Directors last year to deliver appropriately structured pay through alignment with the market and GSK’s key strategic priorities. There was a high level of shareholder engagement in relation to these changes, and we were pleased to receive such a strong vote in favour of last year’s Remuneration Report at the AGM.
Senior management alignment and competitiveness
Since then, we have made further progress in simplifying and aligning the remuneration structures across the Corporate Executive Team (CET). As a result of this, primary pay benchmarks will be based on the nature of each individual role rather than the industry benchmark previously used. Share options will normally no longer be granted; instead, CET members will receive Performance Share Plan awards, and will also be eligible to participate in GSK’s Deferred Annual Bonus Plan. There will also be a more standardised pay mix across CET roles below the Executive Directors.
The Committee would not want to reward failure and so considers that severance terms should be more limited. We have therefore determined that the contracts of any new CET appointees would normally include severance terms of one year’s base salary only, with no bonus entitlement. In addition, I am pleased to report that the CEO has agreed to remove his contractual entitlement to bonus in the event of termination of his employment and also to note the increase in his holding of GSK shares.
Strategic alignment
The introduction of a second performance measure in the Performance Share Plan has provided a clear focus on cash generation in the business. We are continuing to develop measures that further align our remuneration with the ongoing work to transform GSK. Given the importance of long term organic growth and R&D productivity to the future of GSK, we are assessing the most meaningful ways of measuring success in these areas so that they may be considered as performance measures for future awards.
Good governance
There have been a number of corporate governance developments in the past year in response to the economic turmoil, with more likely to come in 2010.
When we reviewed our arrangements last year we wanted to ensure that we did not motivate excessive risk taking. We introduced a new Deferred Annual Bonus Plan, and were one of the first companies to introduce a ‘clawback’ mechanism for annual bonuses should problems arise in the years after a bonus award has been made. We continue to monitor best practice governance developments, and commit to regular reviews of our remuneration arrangements to ensure that they continue to encourage the right behaviours from our leadership team.
The following report provides further detail on GSK’s current remuneration arrangements including the changes made and those to be implemented. The Committee believes that these changes support the future of the business and are in the best interests of shareholders.
Sir Crispin Davis
Remuneration Committee Chairman
24th February 2010
GSK Annual Report 2009
87
Remuneration Report
GSK granted share options to Executive Directors on an annual basis until 2009. The Directors hold these options under the various share option plans referred to in Note 42 to the financial statements, ‘Employee share schemes’. None of the Non-Executive Directors had an interest in any option over the company’s shares.
The table below sets out, for share options granted in respect of 2007 and 2008, the performance periods, the performance targets and whether or not the options have vested at 31st December 2009.
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Performance target |
| | | | | | | | | | | Vesting status | | | Annualised growth | | | Percentage of |
| Grant | | Footnote | | | Performance period | | | at 31.12.09 | | | in EPS | | | award vesting |
| | | | | | | | | | | | | | | |
| February 2007 | | | a | | | | 01.01.07 – 31.12.09 | | | Unvested | | | > RPI + 6% | | | | 100 | % |
| February 2008 | | | | | | | 01.01.08 – 31.12.10 | | | Unvested | | | RPI + 5% | | | | 83 | % |
| | | | | | | | | | | | | | | RPI + 4% | | | | 67 | % |
| | | | | | | | | | | | | | | RPI + 3% | | | | 50 | % |
| | | | | | | | | | | | | | | < RPI + 3% | | | | 0 | % |
| | | | | | | | | | | | | | | |
| | |
| a) | | The performance targets for these share options were not met, and as a result they lapsed on the third anniversary of the date of grant. |
The table below sets out, for share options granted in respect of 2009 the performance period and targets.
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Performance target |
| | | | | | | Vesting status | | Annualised growth | | Percentage of |
| Grant | | Performance period | | | at 31.12.09 | | in EPS | | award vesting |
| | | | | | | | | | | | | |
| February 2009 – 50% of award | | | 01.01.09 – 31.12.11 | | | Unvested | | > RPI + 6% | | | 100 | % |
| February 2009 – 50% of award | | | 01.01.09 – 31.12.12 | | | Unvested | | RPI + 5% | | | 85 | % |
| | | | | | | | | | | RPI + 4% | | | 65 | % |
| | | | | | | | | | | RPI + 3% | | | 30 | % |
| | | | | | | | | | | < RPI + 3% | | | 0 | % |
| | | | | | | | | | | | | |
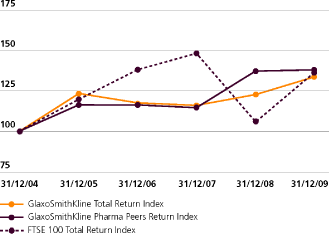
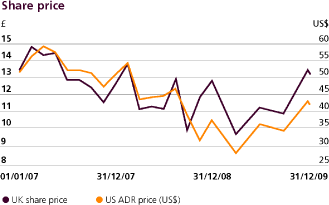
The highest and lowest closing prices during the year ended 31st December 2009 for GlaxoSmithKline shares were £13.34 and £9.87, respectively. The highest and lowest prices for GlaxoSmithKline ADS during the year ended 31st December 2009 were $42.91 and $27.27, respectively. The market price for a GlaxoSmithKline share on 31st December 2009 was £13.20 (31st December 2008 – £12.85) and for a GlaxoSmithKline ADS was $42.25 (31st December 2008 – $37.27). The prices on 19th February 2010 were £12.35 per GlaxoSmithKline share and $38.26 per GlaxoSmithKline ADS.
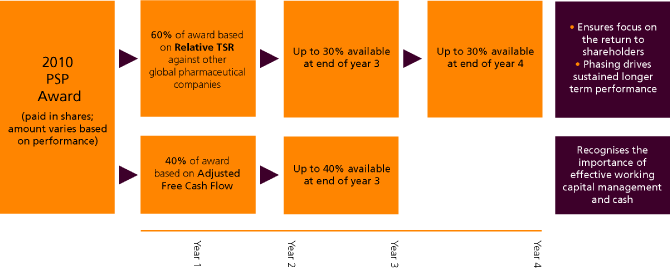
Performance Share Plan (PSP) awards
Performance share awards are made to Executive Directors on an annual basis. The Directors hold these options under the various PSP plans referred to in Note 42 to the financial statements.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Mr A Witty – Shares | | | | | | | | | | Market | | | | | | | | | | | | | | | | | | | Additional | | | | |
| | | | | | Number | | | price on | | | Vested | | | | | | | shares by | | | | |
| | | Unvested | | | granted in | | | date of | | | | | | | Market | | | | | | | | | | | dividends | | | Unvested | |
| Performance period | | at 31.12.08 | | | 2009 | | | grant | | | Number | | | price | | | Gain | | | Lapsed | | | reinvested | | | at 31.12.09 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 01.01.06 – 31.12.08 | | | 85,942 | | | | – | | | | £14.68 | | | | – | | | | – | | | | – | | | | 87,126 | | | | 1,184 | | | | – | |
| 01.01.07 – 31.12.09 | | | 91,821 | | | | – | | | | £14.88 | | | | – | | | | – | | | | – | | | | – | | | | 3,589 | | | | 95,410 | |
| 01.01.08 – 31.12.10 | | | 232,908 | | | | – | | | | £11.47 | | | | – | | | | – | | | | – | | | | – | | | | 9,102 | | | | 242,010 | |
| 01.01.08 – 31.12.10 | | | 63,443 | | | | – | | | | £12.21 | | | | – | | | | – | | | | – | | | | – | | | | 2,480 | | | | 65,923 | |
| 01.01.09 – 31.12.11 | | | – | | | | 470,809 | | | | £10.51 | | | | – | | | | – | | | | – | | | | – | | | | 5,337 | | | | 476,146 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Mr J Heslop – Shares | | | | | | | | | | Market | | | | | | | | | | | | | | | | | | | Additional | | | | |
| | | | | | Number | | | price on | | | Vested | | | | | | | shares by | | | | |
| | | Unvested | | | granted in | | | date of | | | | | | | Market | | | | | | | | | | | dividends | | | Unvested | |
| Performance period | | at 31.12.08 | | | 2009 | | | grant | | | Number | | | price | | | Gain | | | Lapsed | | | reinvested | | | at 31.12.09 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 01.01.06 – 31.12.08 | | | 111,613 | | | | – | | | | £14.68 | | | | – | | | | – | | | | – | | | | 113,150 | | | | 1,537 | | | | – | |
| 01.01.07 – 31.12.09 | | | 113,426 | | | | – | | | | £14.88 | | | | – | | | | – | | | | – | | | | – | | | | 4,433 | | | | 117,859 | |
| 01.01.08 – 31.12.10 | | | 108,690 | | | | – | | | | £11.47 | | | | – | | | | – | | | | – | | | | – | | | | 4,248 | | | | 112,938 | |
| 01.01.09 – 31.12.11 | | | – | | | | 197,740 | | | | £10.51 | | | | – | | | | – | | | | – | | | | – | | | | 2,242 | | | | 199,982 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
GSK Annual Report 2009
88
Remuneration Report
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Dr M Slaoui – Shares | | | | | | | | | | Market | | | | | | | | | | | | | | | | | | | Additional | | | | |
| | | | | | Number | | | price on | | | Vested | | | | | | | shares by | | | | |
| | | Unvested | | | granted in | | | date of | | | | | | | Market | | | | | | | | | | | dividends | | | Unvested | |
| Performance period | | at 31.12.08 | | | 2009 | | | grant | | | Number | | | price | | | Gain | | | Lapsed | | | reinvested | | | at 31.12.09 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 01.01.06 – 31.12.08 | | | 32,055 | | | | – | | | | £14.68 | | | | 16,248 | | | | 11.91 | | | | 193,518 | | | | 16,248 | | | | 441 | | | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Dr M Slaoui – ADS | | | | | | | | | | Market | | | | | | | | | | | | | | | | | | | Additional | | | | |
| | | | | | Number | | | price on | | | Vested | | | | | | | ADS by | | | | |
| | | Unvested | | | granted in | | | date of | | | | | | | Market | | | | | | | | | | | dividends | | | Unvested | |
| Performance period | | at 31.12.08 | | | 2009 | | | grant | | | Number | | | price | | | Gain | | | Lapsed | | | reinvested | | | at 31.12.09 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 01.01.07 – 31.12.09 | | | 76,284 | | | | – | | | | $58.00 | | | | – | | | | – | | | | – | | | | – | | | | 3,002 | | | | 79,286 | |
| 01.01.08 – 31.12.10 | | | 73,115 | | | | – | | | | $44.75 | | | | – | | | | – | | | | – | | | | – | | | | 2,876 | | | | 75,991 | |
| 01.01.09 – 31.12.11 | | | – | | | | 2,620 | | | | $33.42 | | | | – | | | | – | | | | – | | | | – | | | | 66 | | | | 2,686 | |
| 01.01.09 – 31.12.11 | | | – | | | | 69,000 | | | | $33.50 | | | | – | | | | – | | | | – | | | | – | | | | 804 | | | | 69,804 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
This includes those performance shares held by Dr Slaoui’s connected person, who is also an employee of GSK.
Under the terms of the PSP the number of shares actually vesting is determined following the end of the relevant measurement period and is dependent on GSK’s performance during that period as described on pages 78 to 79. The Committee adjusted the comparator group by removing Schering-Plough and Wyeth following their de-listing during 2009, and revised the vesting schedule accordingly. For outstanding and future awards, TSR performance will be measured against the revised comparator group including GSK, as set out below.
Dividends are reinvested on the performance shares awarded to Executives, throughout the performance period and up to the date of the final award. The dividend reinvestment is calculated as of the dividend payment date. Under the terms of the PSP, US participants may defer receipt of all or part of their vested awards. The total gain on vesting of PSP awards made by Executive Directors and connected persons is £193,518 (2008 – £4,826,067).
The following vesting schedules apply to PSP awards made in 2007 and 2008.
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | TSR vesting schedule |
| Award | | % of Award | | | Performance Period | | | TSR rank with 12 other companies | | | Percentage of award vesting |
| | | | | | | | | | | | |
| 2007 | | | 100 | | | | 01.01.07 – 31.12.09 | | | | 1 | | | | 100 | % |
| 2008 | | | 100 | | | | 01.01.08 – 31.12.10 | | | | 2 | | | | 100 | % |
| | | | | | | | | | | | 3 | | | | 87 | % |
| | | | | | | | | | | | 4 | | | | 74 | % |
| | | | | | | | | | | | 5 | | | | 61 | % |
| | | | | | | | | | | | 6 | | | | 48 | % |
| | | | | | | | | | | Median | | | | 35 | % |
| | | | | | | | | | | Below median | | | | 0 | % |
| | | | | | | | | | | | |
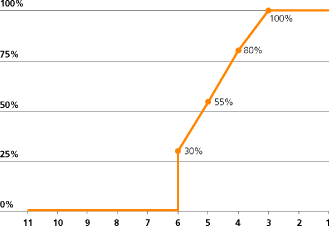
The following vesting schedules apply to PSP awards made in 2009.
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | TSR vesting schedule |
| Award | | % of Award | | | Performance Period | | | TSR rank with 10 other companies | | | Percentage of award vesting |
| | | | | | | | | | | | |
| 2009 | | | 30 | | | | 01.01.09 – 31.12.11 | | | | 1 | | | | 100 | % |
| | | | 30 | | | | 01.01.09 – 31.12.12 | | | | 2 | | | | 100 | % |
| | | | | | | | | | | | 3 | | | | 100 | % |
| | | | | | | | | | | | 4 | | | | 80 | % |
| | | | | | | | | | | | 5 | | | | 55 | % |
| | | | | | | | | | | Median | | | | 30 | % |
| | | | | | | | | | | Below median | | | | 0 | % |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | Adjusted free cash flow vesting schedule |
| | | | | | | | | | | Cash flow Targets | | | | |
| Award | | % of Award | | | Performance Period | | | £bn | | | Percentage of award vesting |
| | | | | | | | | | | | |
| 2009 | | | 40 | | | | 01.01.09 – 31.12.11 | | | | 13.5 – 16.0 | | | | 25% – 100 | % |
| | | | | | | | | | | | |
GSK Annual Report 2009
89
Remuneration Report
Share Value Plan awards
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Dr M Slaoui – Shares and ADS
| | | | | | | | | | Market | | | | | | | |
| | | | | | Number | | | price on | | | Vested & deferred | | | | |
| | Unvested | | | granted in | | | date of | | | | | | | Market | | | | | | | Unvested | |
| Plan year | | at 31.12.08 | | | 2009 | | | grant | | | Number | | | price | | | Gain | | | at 31.12.09 | |
| 2006 (Shares) | | | 1,200 | | | | – | | | | £14.68 | | | | 1,200 | | | | £11.33 | | | | £13,596 | | | | – | |
| 2007 (ADS) | | | 890 | | | | – | | | | $58.00 | | | | – | | | | – | | | | – | | | | 890 | |
| 2008 (ADS) | | | 890 | | | | – | | | | $44.75 | | | | – | | | | – | | | | – | | | | 890 | |
| 2008 (ADS) | | | 2,980 | | | | – | | | | $48.55 | | | | – | | | | – | | | | – | | | | 2,980 | |
| 2009 (ADS) | | | – | | | | 1,490 | | | | $33.42 | | | | – | | | | – | | | | – | | | | 1,490 | |
| | | | | | | | | | | | | | | | | | | | |
As an Executive Director, Dr Slaoui is not eligible to receive awards under the Share Value Plan. The awards shown above reflect the holdings of Dr Slaoui’s connected person, an employee of GSK. The awards are subject to three-year vesting periods and vesting is contingent on continued employment with GSK.
Pension benefits
The accrued annual pension benefits and transfer values for Executive Directors in office on 31st December 2009 on retirement are set out below.
The Companies Act 2006 requires disclosure of the accrued benefit at the end of the year, the change in accrued benefit over the year, the transfer value at both the beginning and end of the year and the change in the transfer value over the year. The Listing Rules require additional disclosure of the change in the accrued benefit, net of inflation and the transfer value of this change. Pensions for the Executive Directors have been disclosed in the currency in which the pension is payable.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Change in | | | | |
| | | | | | | | | | | Change in | | | Personal | | | | | | | | | | | | | | | accrued | | | Transfer value | |
| | | Accrued | | | Accrued | | | accrued | | | contributions | | | Transfer | | | Transfer | | | Change | | | benefit over | | | of change | |
| | | benefit at | | | benefit at | | | benefit | | | made during | | | value at | | | value at | | | in transfer | | | year net | | | in accrued | |
| Executive Directors | | 31.12.08 | | | 31.12.09 | | | over year | | | the year | | | 31.12.08 | | | 31.12.09 | | | value | | | of inflation | | | benefit | * |
| | 000 | | | 000 | | | 000 | | | 000 | | | 000 | | | 000 | | | 000 | * | | 000 | | | 000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Mr A Witty | | | £315 | | | | £446 | | | | £131 | | | | £30 | | | | £3,848 | | | | £6,272 | | | | £2,394 | | | | £115 | | | | £1,638 | |
| Mr J Heslop | | | £170 | | | | £201 | | | | £31 | | | | £16 | | | | £2,837 | | | | £3,787 | | | | £934 | | | | £23 | | | | £471 | |
| Dr M Slaoui | | | $131 | | | | $187 | | | | $56 | | | | – | | | | $731 | | | | $1,101 | | | | $370 | | | | $54 | | | | $370 | |
| Dr M Slaoui | | | €55 | | | | €59 | | | | €4 | | | | – | | | | €608 | | | | €647 | | | | €39 | | | | €3 | | | | €39 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| * | | These are shown net of contributions made by the individual. |
Mr Witty and Mr Heslop participate in the Glaxo Wellcome Defined Benefit Plan with an accrual rate of 1/30th of final pensionable salary per annum. In 2000 all benefits accrued under the Glaxo Wellcome UK pension arrangements were augmented by the Trustees of the plans by 5% to reflect a distribution of surplus. This augmentation will apply to that element of Mr Witty and Mr Heslop’s pension earnings before 31st March 2000.
Mr Witty’s and Mr Heslop’s transfer values have been calculated on the basis of actuarial advice in accordance with pensions regulation. The transfer value represents the present value of future payments to be made under the pension plan. Mr Witty’s annual accrued benefit has increased by £130,556 (£114,770 excluding the effects of inflation), and the transfer value less personal contributions has increased by £2,394,197 over the year. Mr Heslop’s annual accrued benefit has increased by £31,040 (£22,504 excluding the effects of inflation) and the transfer value less personal contributions has increased by £934,150 over the year.
GSK Annual Report 2009
107
Notes to the financial statements
6 Segment information
GSK has implemented IFRS 8 ‘Operating segments’ with effect from 1st January 2009 and this has resulted in a change to the segmental information reported by GSK. Comparative information has been presented on a consistent basis.
GSK’s operating segments are being reported based on the financial information provided to the Chief Executive Officer and the responsibilities of the Corporate Executive Team (CET). Individual members of the CET are responsible for geographic regions of the Pharmaceuticals business and for the Consumer Healthcare business as a whole, respectively, before major restructuring.
R&D investment is essential for the sustainability of the pharmaceutical businesses. However, for segment reporting, the USA, Europe, Emerging Markets and Asia Pacific/Japan regional pharmaceutical operating profits exclude allocations of globally funded R&D as well as central costs, principally corporate functions and unallocated manufacturing costs. GSK’s management reporting process allocates all intra-Group profit on a product sale to the market in which that sale is recorded, and the profit analyses below have been presented on that basis.
The Other trading pharmaceuticals segment includes Canada, Puerto Rico, Stiefel, central vaccine tender sales and contract manufacturing sales. The Stiefel business is being integrated into GSK and with effect from 1st January 2010, results will be reported within the relevant geographical pharmaceuticals segments, in line with the way in which the business will be managed.
GSK acquired the HIV business of Pfizer with effect from 30th October 2009 in return for a 15% minority stake in the combined HIV businesses, now called ViiV Healthcare Limited. In line with the way the ViiV Healthcare business is to be managed, it will be reported as a separate segment from 1st January 2010. For 2009, the GSK HIV business is reported within the relevant Pharmaceuticals segments; incremental income and costs since the creation of ViiV Healthcare have been reported within Other trading pharmaceuticals.
The Pharmaceuticals R&D segment is the responsibility of the Chairman, Research & Development and is therefore being reported as a separate segment.
Unallocated pharmaceuticals costs include costs such as vaccines R&D and central manufacturing costs not attributed to other segments.
Corporate and other unallocated costs and disposal profits include corporate functions, costs for legal matters, fair value movements on financial instruments and investments and unallocated profits on asset disposals.
| | | | | | | | | | | | | |
| | | | | | | 2008 | | | 2007 | |
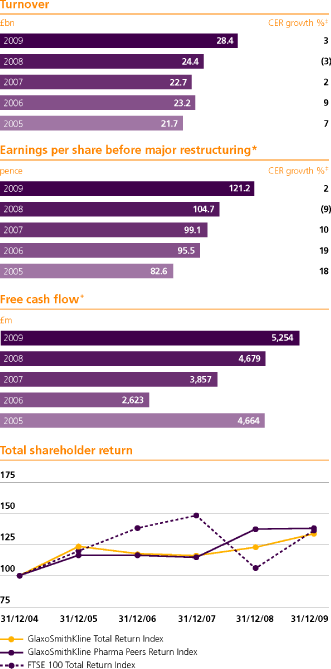
| Turnover by segment | | 2009 | | | (restated) | | | (restated) | |
| | £m | | | £m | | | £m | |
| | | | | | | | | |
| US pharmaceuticals | | | 9,180 | | | | 8,894 | | | | 9,273 | |
| Europe pharmaceuticals | | | 7,681 | | | | 6,483 | | | | 5,560 | |
| Emerging Markets pharmaceuticals | | | 2,973 | | | | 2,290 | | | | 1,895 | |
| Asia Pacific/Japan pharmaceuticals | | | 2,700 | | | | 1,918 | | | | 1,701 | |
| Other trading pharmaceuticals | | | 1,180 | | | | 796 | | | | 734 | |
| | | | | | | | | |
| Pharmaceuticals turnover | | | 23,714 | | | | 20,381 | | | | 19,163 | |
| Consumer Healthcare turnover | | | 4,654 | | | | 3,971 | | | | 3,553 | |
| | | | | | | | | |
| | | | 28,368 | | | | 24,352 | | | | 22,716 | |
| | | | | | | | | |
| |
| Pharmaceutical turnover by therapeutic area | | 2009 | | | 2008 | | | 2007 | |
| | £m | | | £m | | | £m | |
| | | | | | | | | |
| Respiratory | | | 6,977 | | | | 5,817 | | | | 5,032 | |
| Anti-virals | | | 4,150 | | | | 3,206 | | | | 3,027 | |
| Central nervous system | | | 1,870 | | | | 2,897 | | | | 3,348 | |
| Cardiovascular and urogenital | | | 2,298 | | | | 1,847 | | | | 1,554 | |
| Metabolic | | | 1,181 | | | | 1,191 | | | | 1,508 | |
| Anti-bacterials | | | 1,592 | | | | 1,429 | | | | 1,323 | |
| Oncology and emesis | | | 629 | | | | 496 | | | | 477 | |
| Vaccines | | | 3,706 | | | | 2,539 | | | | 1,993 | |
| Other | | | 1,311 | | | | 959 | | | | 901 | |
| | | | | | | | | |
| | | | 23,714 | | | | 20,381 | | | | 19,163 | |
| | | | | | | | | |
| |
| Consumer Healthcare turnover by category | | 2009 | | | 2008 | | | 2007 | |
| | £m | | | £m | | | £m | |
| | | | | | | | | |
| OTC medicines | | | 2,319 | | | | 1,935 | | | | 1,788 | |
| Oral healthcare | | | 1,484 | | | | 1,240 | | | | 1,049 | |
| Nutritional healthcare | | | 851 | | | | 796 | | | | 716 | |
| | | | | | | | | |
| | | | 4,654 | | | | 3,971 | | | | 3,553 | |
| | | | | | | | | |
GSK Annual Report 2009
110
Notes to the financial statements
6 Segment informationcontinued
| | | | | | | | | |
| | | | | | | 2008 | |
| Total assets by segment | | 2009 | | | (restated) | |
| | £m | | | £m | |
| | | | | | |
| US pharmaceuticals | | | 2,536 | | | | 2,957 | |
| Europe pharmaceuticals | | | 2,450 | | | | 2,538 | |
| Emerging Markets pharmaceuticals | | | 1,925 | | | | 1,303 | |
| Asia Pacific/Japan pharmaceuticals | | | 1,278 | | | | 1,095 | |
| Other trading pharmaceuticals | | | 3,804 | | | | 108 | |
| Pharmaceuticals R&D | | | 2,842 | | | | 3,087 | |
| Other unallocated pharmaceuticals | | | 12,956 | | | | 13,399 | |
| | | | | | |
| Pharmaceuticals operating assets | | | 27,791 | | | | 24,487 | |
| Consumer Healthcare operating assets | | | 3,799 | | | | 3,859 | |
| | | | | | |
| Segment operating assets | | | 31,590 | | | | 28,346 | |
| Corporate and other unallocated assets | | | 921 | | | | 680 | |
| | | | | | |
| Total operating assets | | | 32,511 | | | | 29,026 | |
| Investments in associates and joint ventures | | | 895 | | | | 552 | |
| Liquid investments | | | 268 | | | | 391 | |
| Derivative financial instruments | | | 197 | | | | 963 | |
| Cash and cash equivalents | | | 6,545 | | | | 5,623 | |
| Current and deferred taxation | | | 2,432 | | | | 2,836 | |
| Assets held for sale | | | 14 | | | | 2 | |
| | | | | | |
| Total assets | | | 42,862 | | | | 39,393 | |
| | | | | | |
The other unallocated pharmaceuticals segment includes assets for the centrally managed pharmaceutical and vaccine manufacturing operations, the depreciation on which, totalling £618 million (2008 – £536 million; 2007 – £475 million) is recovered through the standard cost of product charged to businesses.
7 Major restructuring programme
In October 2007, GSK announced a significant new Operational Excellence programme to improve the effectiveness and productivity of its operations. A significant expansion of the Operational Excellence programme was approved by the Board and announced in February 2009. A further expansion was approved by the Board and announced in February 2010. Total costs for the implementation of the expanded programme are expected to increase from £3.6 billion to approximately £4.5 billion, to be incurred over the period from 2007 to 2012. Approximately 50% of these costs were incurred by 31st December 2009, and approximately 30% are expected to be incurred in 2010 with the balance mostly in 2011. In total, approximately 75% of these costs are expected to be cash expenditures and 25% are expected to be asset write-downs. Uncertainties exist over the exact amount and timing of cash outflows as a result of potential future exchange rate fluctuations and as many elements of the restructuring programme are subject to employee consultation procedures, making it difficult to predict with precision when these procedures will be completed. However, the majority of the remaining cash payments are expected to be made in 2010 and 2011. The programme is now estimated to deliver total annual pre-tax savings of up to £2.2 billion by 2012, with savings realised across the business. Of the total restructuring costs of £832 million incurred in 2009, £761 million was incurred under the Operational Excellence programme in the following areas:
| • | | the closure of a number of manufacturing sites, including Dartford and Crawley in the UK and Cidra in Puerto Rico, giving rise to asset write-downs and staff reductions; |
| • | | the adoption of more customised sales approaches, leading to staff reductions in a number of sales forces, principally in France; |
| • | | cost saving projects in R&D, focused primarily on the simplification and streamlining of support infrastructure, including some site rationalisations, and |
| • | | projects to simplify or eliminate processes, leading to staff reductions in administrative and support functions. |
In addition, costs of £71 million were incurred during the year under the restructuring programme related to the integration of the Stiefel Laboratories, Inc. business in the USA, following its acquisition in July 2009.
GSK Annual Report 2009
113
Notes to the financial statements
10 Employee costs
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | £m | | | £m | | | £m | |
| | | | | | | | | |
| Wages and salaries | | | 5,387 | | | | 4,640 | | | | 4,444 | |
| Social security costs | | | 661 | | | | 653 | | | | 527 | |
| Pension and other post-employment costs, including augmentations (Note 28) | | | 491 | | | | 505 | | | | 313 | |
| Cost of share-based incentive plans | | | 179 | | | | 241 | | | | 237 | |
| Severance and other costs from integration and restructuring activities | | | 449 | | | | 485 | | | | 212 | |
| | | | | | | | | |
| | | | 7,167 | | | | 6,524 | | | | 5,733 | |
| | | | | | | | | |
In 2009, wages and salaries increased by 4% in CER terms.
The Group provides benefits to employees, commensurate with local practice in individual countries, including, in some markets, healthcare insurance, subsidised car schemes and personal life assurance.
| | | | | | | | | | | | | |
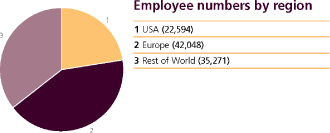
| The average number of persons employed by the Group (including Directors) during the year: | | 2009 | | | 2008 | | | 2007 | |
| | Number | | | Number | | | Number | |
| | | | | | | | | |
| Manufacturing | | | 31,467 | | | | 33,372 | | | | 33,975 | |
| Selling, general and administration | | | 53,183 | | | | 52,115 | | | | 53,707 | |
| Research and development | | | 14,204 | | | | 15,646 | | | | 15,719 | |
| | | | | | | | | |
| | | | 98,854 | | | | 101,133 | | | | 103,401 | |
| | | | | | | | | |
The average number of Group employees excludes temporary and contract staff. The numbers of Group employees at the end of each financial year are given in the financial record on page 188. The average number of persons employed by GlaxoSmithKline plc in 2009 was nil (2008 – nil).
The compensation of the Directors and Senior Management (members of the CET) in aggregate, was as follows:
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | £m | | | £m | | | £m | |
| | | | | | | | | |
| Wages and salaries | | | 23 | | | | 17 | | | | 16 | |
| Social security costs | | | 1 | | | | 1 | | | | 1 | |
| Pension and other post-employment costs | | | 3 | | | | 3 | | | | 3 | |
| Cost of share-based incentive plans | | | 4 | | | | 12 | | | | 15 | |
| | | | | | | | | |
| | | | 31 | | | | 33 | | | | 35 | |
| | | | | | | | | |
11 Finance income
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | £m | | | £m | | | £m | |
| | | | | | | | | |
| Interest income arising from: | | | | | | | | | | | | |
| cash and cash equivalents | | | 46 | | | | 107 | | | | 98 | |
| available-for-sale investments | | | 15 | | | | 31 | | | | 49 | |
| derivatives at fair value through profit or loss | | | (5 | ) | | | 159 | | | | 79 | |
| loans and receivables | | | 11 | | | | 22 | | | | 27 | |
| Realised gains on liquid investments | | | – | | | | 2 | | | | 1 | |
| Fair value adjustments on derivatives at fair value through profit or loss | | | (3 | ) | | | 4 | | | | – | |
| Net investment hedge ineffectiveness | | | 4 | | | | (13 | ) | | | 7 | |
| Unwinding of discounts on assets | | | 2 | | | | 1 | | | | 1 | |
| | | | | | | | | |
| | | | 70 | | | | 313 | | | | 262 | |
| | | | | | | | | |
All derivatives at fair value through profit or loss other than designated and effective hedging instruments (see Note 41, ‘Financial instruments and related disclosures’) are classified as held-for-trading financial instruments under IAS 39. Interest income arising from derivatives at fair value through profit or loss relates to swap interest income.
GSK Annual Report 2009
116
Notes to the financial statements
14 Taxationcontinued
Following its audit of the period 2001 to 2003, the IRS issued Statutory Notices of Deficiency to GSK asserting income and withholding tax deficiencies, and associated penalties, arising from the IRS’s reclassification of an intercompany financing arrangement in those years from debt to equity, and its consequent recharacterisation of the amounts paid as dividends subject to withholding tax under the US – UK treaty. All amounts due under the financing arrangement were paid on a timely basis, with the final payment made in April 2008. GSK disagreed with the IRS’s position and, in August 2008, initiated actions in the United States Tax Court to contest the Statutory Notices of Deficiency. On 19th November 2009, GSK and the IRS filed a Stipulation with the Tax Court in which the IRS conceded all asserted tax deficiencies and penalties arising from its reclassification of the above intercompany financing arrangement from debt to equity, resulting in no additional tax cost to GSK. The IRS claim had previously been estimated at $864m for 2001-2003. GSK and the IRS are now in the process of finalising the tax computations for the 2001 to 2003 tax years. It is anticipated that resolution of the issue in the years 2004 to 2008 will be reflected in a closing agreement. Resolution of the issue had no impact on the Group’s results.
In Canada, GSK is continuing to contest a court decision in respect of transfer pricing in the early 1990s. The date of the appeal hearing has been set for March 2010. GSK continues to believe that it has made adequate provision for the liabilities likely to arise from periods which are open and not yet agreed by tax authorities. The ultimate liability for such matters may vary from the amounts provided and is dependent upon the outcome of litigation where appropriate or by agreement with the relevant tax authorities.
No provision has been made for taxation which would arise on the distribution of profits retained by overseas subsidiaries, on the grounds that the Group is able to control the timing of the reversal of these temporary differences and it is probable that they will not reverse in the foreseeable future. The aggregate amount of these unremitted profits at the balance sheet date was approximately £29 billion (2008 – £28 billion). The introduction of the UK dividend exemption on 1st July 2009, now enables the reasonable quantification of the incremental liability from repatriation of profits to the UK. The deferred tax on unremitted earnings at 31st December 2009 is estimated to be approximately £500 million, which relates to taxes payable on repatriation and dividend withholding taxes levied by overseas tax jurisdictions.
| | | | | | | | | | | | | |
| Movement on current tax account | | Payable | | | Recoverable | | | Net | |
| | £m | | | £m | | | £m | |
| | | | | | | | | |
| At 1st January 2009 | | | (780 | ) | | | 76 | | | | (704 | ) |
| Exchange adjustments | | | 12 | | | | 1 | | | | 13 | |
| Charge for the year | | | (2,056 | ) | | | (358 | ) | | | (2,414 | ) |
| Cash paid | | | 1,393 | | | | 311 | | | | 1,704 | |
| Other movements | | | (20 | ) | | | 28 | | | | 8 | |
| | | | | | | | | |
| At 31st December 2009 | | | (1,451 | ) | | | 58 | | | | (1,393 | ) |
| | | | | | | | | |
Movement in deferred tax assets and liabilities
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Pensions & | | | | | | | | | | | Manu- | | | | | | | Share | | | Other | | | | | | | |
Deferred taxation
assets/(liabilities) | | Accelerated | | | | | | | Intra- | | | other post | | | | | | | Legal | | | facturing | | | Stock | | | option | | | net | | | Offset | | | | |
| | capital | | | | | | | group | | | retirement | | | Tax | | | & other | | | restruct- | | | valuation | | | and award | | | temporary | | | within | | | | |
| | allowances | | | Intangibles | | | profit | | | benefits | | | losses | | | disputes | | | uring | | | adjustments | | | schemes | | | differences | | | countries | | | Total | |
| | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Deferred tax assets at 1st January 2009 | | | 23 | | | | 152 | | | | 1,234 | | | | 1,062 | | | | 196 | | | | 249 | | | | 162 | | | | 15 | | | | 102 | | | | 830 | | | | (1,265 | ) | | | 2,760 | |
Deferred tax liabilities at 1st January 2009 | | | (726 | ) | | | (970 | ) | | | – | | | | – | | | | (23 | ) | | | – | | | | – | | | | (247 | ) | | | – | | | | (13 | ) | | | 1,265 | | | | (714 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| At 1st January 2009 | | | (703 | ) | | | (818 | ) | | | 1,234 | | | | 1,062 | | | | 173 | | | | 249 | | | | 162 | | | | (232 | ) | | | 102 | | | | 817 | | | | – | | | | 2,046 | |
| Exchange adjustments | | | 15 | | | | 36 | | | | (45 | ) | | | (87 | ) | | | (13 | ) | | | (28 | ) | | | (7 | ) | | | 22 | | | | – | | | | (59 | ) | | | – | | | | (166 | ) |
| Credit/(charge) to income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | – | | | | | |
| statement | | | 89 | | | | 74 | | | | (6 | ) | | | (113 | ) | | | (52 | ) | | | 82 | | | | (11 | ) | | | 52 | | | | 11 | | | | 66 | | | | – | | | | 192 | |
| Credit/(charge) to equity | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | 13 | | | | – | | | | – | | | | 13 | |
| Credit/(charge) to statement of comprehensive income | | | – | | | | – | | | | – | | | | 183 | | | | – | | | | – | | | | – | | | | – | | | | – | | | | (9 | ) | | | – | | | | 174 | |
| Acquisitions | | | (5 | ) | | | (591 | ) | | | – | | | | (2 | ) | | | 75 | | | | – | | | | 13 | | | | (10 | ) | | | – | | | | (10 | ) | | | – | | | | (530 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| At 31st December 2009 | | | (604 | ) | | | (1,299 | ) | | | 1,183 | | | | 1,043 | | | | 183 | | | | 303 | | | | 157 | | | | (168 | ) | | | 126 | | | | 805 | | | | – | | | | 1,729 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Deferred tax assets at 31st December 2009 | | | 24 | | | | 177 | | | | 1,183 | | | | 1,043 | | | | 211 | | | | 303 | | | | 157 | | | | 30 | | | | 126 | | | | 822 | | | | (1,702 | ) | | | 2,374 | |
Deferred tax liabilities at 31st December 2009 | | | (628 | ) | | | (1,476 | ) | | | – | | | | – | | | | (28 | ) | | | – | | | | – | | | | (198 | ) | | | – | | | | (17 | ) | | | 1,702 | | | | (645 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | (604 | ) | | | (1,299 | ) | | | 1,183 | | | | 1,043 | | | | 183 | | | | 303 | | | | 157 | | | | (168 | ) | | | 126 | | | | 805 | | | | – | | | | 1,729 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
GSK Annual Report 2009
117
Notes to the financial statements
14 Taxationcontinued
The deferred tax charge to income relating to changes in tax rates is £9 million. All other deferred tax movements arise from the origination and reversal of temporary differences. Other net temporary differences include accrued expenses and other provisions.
At 31st December 2009, the Group had recognised a deferred tax asset of £183 million (2008 – £173 million) in respect of income tax losses of approximately £617 million (2008 – £566 million). Of these losses, £76 million (2008 – £142 million) are due to expire between 2010-2019, £445 million (2008 – £357 million) are due to expire between 2020 – 2029 and £96 million (2008 – £67 million) are available indefinitely. At 31st December 2009, the Group had not recognised any deferred tax asset in respect of income tax losses of approximately £4,397 million (2008 – £4,526 million), of which £34 million (2008 – £37 million) are due to expire between 2010-2019, £159 million (2008 – £66 million) are due to expire between 2020 – 2029 and £4,204 million (2008 – £4,423 million) which are available indefinitely. The Group had capital losses at 31st December 2009 of approximately £4.3 billion in respect of which no deferred tax asset has been recognised. Deferred tax assets are recognised where it is probable that future taxable profit will be available to utilise losses.
Factors affecting the tax charge in future years
As a global organisation there are many factors which could affect the future effective tax rate of the Group. The mix of profits across different territories, transfer pricing and other disputes with tax authorities and the location of research and development activity can all have a significant impact on the Group’s effective tax rate.
Changes to tax legislation in territories where GSK has business operations could also impact the Group’s effective tax rate. The UK tax authorities have proposed some significant changes to the UK taxation system. In December 2009 the UK Government announced that it intends to introduce a Patent Box regime applying a reduced rate of corporation tax to income from patents. The changes are expected to have effect from April 2013, following a period of consultation. The UK Government also continues to consult with business on proposed changes to the Controlled Foreign Company regime. These changes are expected to be enacted in 2011.
15 Earnings per share
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | pence | | | pence | | | pence | |
| | | | | | | | | |
| Basic earnings per share | | | 109.1 | | | | 88.6 | | | | 94.4 | |
| Adjustment for major restructuring | | | 12.1 | | | | 16.1 | | | | 4.7 | |
| | | | | | | | | |
| Basic earnings per share before major restructuring | | | 121.2 | | | | 104.7 | | | | 99.1 | |
| | | | | | | |
| Diluted earnings per share | | | 108.2 | | | | 88.1 | | | | 93.7 | |
| Adjustment for major restructuring | | | 12.1 | | | | 16.0 | | | | 4.6 | |
| | | | | | | | | |
| Diluted earnings per share before major restructuring | | | 120.3 | | | | 104.1 | | | | 98.3 | |
| | | | | | | | | |
Basic and adjusted earnings per share have been calculated by dividing the profit attributable to shareholders by the weighted average number of shares in issue during the period after deducting shares held by the ESOP Trusts and Treasury shares.
Adjusted earnings per share is calculated using results before major restructuring earnings. The calculation of results before major restructuring is described in Note 1 ‘Presentation of the financial statements’.
Diluted earnings per share have been calculated after adjusting the weighted average number of shares used in the basic calculation to assume the conversion of all potentially dilutive shares. A potentially dilutive share forms part of the employee share schemes where its exercise price is below the average market price of GSK shares during the period and any performance conditions attaching to the scheme have been met at the balance sheet date.
The numbers of shares used in calculating basic and diluted earnings per share are reconciled below.
| | | | | | | | | | | | | |
| Weighted average number of shares in issue | | 2009 | | | 2008 | | | 2007 | |
| | millions | | | millions | | | millions | |
| | | | | | | | | |
| Basic | | | 5,069 | | | | 5,195 | | | | 5,524 | |
| Dilution for share options | | | 39 | | | | 31 | | | | 43 | |
| | | | | | | | | |
| Diluted | | | 5,108 | | | | 5,226 | | | | 5,567 | |
| | | | | | | | | |
Shares held by the ESOP Trusts are excluded. The trustees have waived their rights to dividends on the shares held by the ESOP Trusts.
GSK Annual Report 2009
119
Notes to the financial statements
17 Property, plant and equipmentcontinued
| | | | | | | | | | | | | | | | | |
| | | | | | | Plant, | | | | | | | |
| | | Land and | | | equipment | | | Assets in | | | | |
| | | buildings | | | and vehicles | | | construction | | | Total | |
| | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | |
| Depreciation at 1st January 2008 | | | (1,534 | ) | | | (5,290 | ) | | | – | | | | (6,824 | ) |
| Exchange adjustments | | | (385 | ) | | | (914 | ) | | | – | | | | (1,299 | ) |
| Provision for the year | | | (228 | ) | | | (692 | ) | | | – | | | | (920 | ) |
| Disposals and write-offs | | | 85 | | | | 265 | | | | – | | | | 350 | |
| Transfer to assets held for sale | | | – | | | | 1 | | | | – | | | | 1 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Depreciation at 31st December 2008 | | | (2,062 | ) | | | (6,630 | ) | | | – | | | | (8,692 | ) |
| Exchange adjustments | | | 128 | | | | 312 | | | | – | | | | 440 | |
| Provision for the year | | | (283 | ) | | | (847 | ) | | | – | | | | (1,130 | ) |
| Disposals and write-offs | | | 129 | | | | 478 | | | | – | | | | 607 | |
| Transfer to assets held for sale | | | 1 | | | | 1 | | | | – | | | | 2 | |
| | | | | | | | | | | | |
| Depreciation at 31st December 2009 | | | (2,087 | ) | | | (6,686 | ) | | | – | | | | (8,773 | ) |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Impairment at 1st January 2008 | | | (122 | ) | | | (239 | ) | | | (81 | ) | | | (442 | ) |
| Exchange adjustments | | | (22 | ) | | | (27 | ) | | | (14 | ) | | | (63 | ) |
| Disposals and write-offs | | | 50 | | | | 67 | | | | 27 | | | | 144 | |
| Impairment losses | | | (70 | ) | | | (176 | ) | | | (20 | ) | | | (266 | ) |
| Reclassifications | | | – | | | | (44 | ) | | | 44 | | | | - | |
| Reversal of impairments | | | 3 | | | | 7 | | | | – | | | | 10 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Impairment at 31st December 2008 | | | (161 | ) | | | (412 | ) | | | (44 | ) | | | (617 | ) |
| Exchange adjustments | | | 6 | | | | 10 | | | | 4 | | | | 20 | |
| Disposals and write-offs | | | 28 | | | | 104 | | | | 4 | | | | 136 | |
| Impairment losses | | | (27 | ) | | | (108 | ) | | | (25 | ) | | | (160 | ) |
| Reversal of impairments | | | 1 | | | | 10 | | | | – | | | | 11 | |
| | | | | | | | | | | | |
| Impairment at 31st December 2009 | | | (153 | ) | | | (396 | ) | | | (61 | ) | | | (610 | ) |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Total depreciation and impairment at 31st December 2008 | | | (2,223 | ) | | | (7,042 | ) | | | (44 | ) | | | (9,309 | ) |
| | | | | | | | | | | | | | | | | |
| Total depreciation and impairment at 31st December 2009 | | | (2,240 | ) | | | (7,082 | ) | | | (61 | ) | | | (9,383 | ) |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net book value at 1st January 2008 | | | 2,978 | | | | 2,968 | | | | 1,875 | | | | 7,821 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net book value at 31st December 2008 | | | 3,756 | | | | 3,644 | | | | 2,278 | | | | 9,678 | |
| | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Net book value at 31st December 2009 | | | 3,762 | | | | 3,433 | | | | 2,179 | | | | 9,374 | |
| | | | | | | | | | | | |
The net book value at 31st December 2009 of the Group’s land and buildings comprises freehold properties £3,462 million (2008 – £3,510 million), properties with leases of 50 years or more £239 million (2008 – £185 million) and properties with leases of less than 50 years £61 million (2008 – £61 million).
Included in land and buildings at 31st December 2009 are leased assets with a cost of £561 million (2008 – £519 million), accumulated depreciation of £261 million (2008 – £263 million), impairment of £nil (2008 – £8 million) and a net book value of £300 million (2008 – £248 million). Included in plant, equipment and vehicles at 31st December 2009 are leased assets with a cost of £126 million (2008 – £77 million), accumulated depreciation of £44 million (2008 – £36 million), and a net book value of £82 million (2008 – £41 million). Some lease agreements include renewal or purchase options or escalation clauses.
The impairment losses principally arise from decisions to rationalise facilities and are calculated based on either fair value less costs to sell or value in use. The value in use calculations determine the net present value of the projected risk-adjusted, post-tax cash flows of the relevant asset or cash generating unit, applying a discount rate of the Group post-tax weighted average cost of capital (WACC) of 8%, adjusted where appropriate for country specific risks. Where an impairment is indicated and a pre-tax cash flow calculation is expected to give a materially different result, the test would be reperformed using pre-tax cash flows and a pre-tax discount rate. The Group WACC is equivalent to a pre-tax discount rate of approximately 11%. The impairment losses have been charged to cost of sales (£95 million), R&D (£47 million) and SG&A (£18 million), and include £57 million (2008 – £197 million) arising from the major restructuring programmes.
Reversals of impairment arise from subsequent reviews of the impaired assets where the conditions which gave rise to the original impairments are deemed no longer to apply. All of the reversals have been credited to cost of sales.
GSK Annual Report 2009
121
Notes to the financial statements
18 Goodwillcontinued
Goodwill is allocated to cash generating units which are tested for impairment at least annually. Following the implementation of IFRS 8 ‘Operating segments’ in 2009 the cash generating units to which some of the goodwill balances are allocated have changed. The goodwill arising on the acquisitions of ID Biomedical, Sirtris Pharmaceuticals and Domantis has been split between the five pharmaceutical segments (USA, Europe, Emerging Markets, Asia Pacific/Japan and Other) for impairment testing purposes as either the benefit of the acquired businesses is split among the five pharmaceutical segments or they do not generate independent cash flows.
The valuation of the US pharmaceuticals cash generating unit for Reliant Pharmaceuticals has been prepared on a fair value less costs to sell basis, using turnover and earnings multiples derived from observed market data. The value of goodwill inherent in the US pharmaceuticals cash generating unit is considerably in excess of the book value of the acquired goodwill.
The recoverable amounts of the other cash generating units are assessed using either a value in use or a fair value less costs to sell model. Value in use is calculated as the net present value of the projected risk-adjusted post-tax cash flows plus a terminal value of the cash generating unit to which the goodwill is allocated. Initially a post-tax discount rate is applied to calculate the net present value of the post-tax cash flows. The post-tax discount rate used is based on the Group WACC of 8%, as most cash generating units have integrated operations across large parts of the Group. The Group WACC is equivalent to a pre-tax discount rate of approximately 11%. The discount rate is increased where specific country risks are sufficiently significant to have a material impact on the outcome of the impairment test. Where the impairment test indicates that the recoverable value of the unit is close to or below its carrying value, the test is reperformed using a pre-tax discount rate and pre-tax cash flows in order to determine if an impairment exists and to establish its magnitude.
Fair value is calculated using a similar discounted cash flow approach based on the Group’s acquisition valuation model. A post-tax discount rate is applied to the projected risk-adjusted post-tax cash flows and terminal value.
Details relating to the discounted cash flow models used in the impairment tests of the other significant goodwill balances are as follows:
| | | | | | | |
| | | Stiefel Laboratories CGU | | ViiV Healthcare CGU | | Five pharmaceutical segments CGUs |
| | | | | | | |
| Valuation basis | | Fair value less costs to sell | | Fair value less costs to sell | | Value in use |
| | | | | | | |
| Key assumptions | | Sales growth rates | | Sales growth rates | | Sales growth rates |
| | | Profit margins | | Profit margins | | Profit margins |
| | | Achievement of synergy targets | | Discount rate | | Discount rate |
| | | Discount rate | | | | |
| | | | | | | |
| Determination of assumptions | | Growth rates are internal forecasts based on both internal and external market information. Margins reflect past experience, adjusted for expected changes. Post-acquisition synergy targets reflect management expectations of cost savings that can be achieved.
Discount rate based on Group WACC. | | Growth rates are internal forecasts based on both internal and external market information. Margins reflect past experience, adjusted for expected changes. Discount rate based on Group WACC. | | Growth rates are internal forecasts based on both internal and external market information. Margins reflect past experience, adjusted for expected changes. Discount rate based on Group WACC. |
| | | | | | | |
Period of specific
projected cash flows | | 10 years | | 20 years | | 5 years |
| | | | | | | |
| Discount rate | | 8% | | 8% | | 8% |
| | | | | | | |
| Terminal growth rate | | 2% p.a. | | 2% p.a. | | 2% p.a. |
| | | | | | | |
GSK Annual Report 2009
124
Notes to the financial statements
19 Other intangible assetscontinued
Amortisation and impairment losses, net of reversals, have been charged in the income statement as follows:
| | | | | | | | | | | | | | | | | |
| | | Amortisation | | | Net impairment losses | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | |
| Cost of sales | | | 29 | | | | 34 | | | | 1 | | | | – | |
| Selling, general and administration | | | 270 | | | | 181 | | | | 1 | | | | 25 | |
| Research and development | | | 133 | | | | 96 | | | | 170 | | | | 90 | |
| | | | | | | | | | | | |
| | | | 432 | | | | 311 | | | | 172 | | | | 115 | |
| | | | | | | | | | | | |
The net book value of computer software includes £80 million (2008 – £125 million) of internally generated costs.
Licences, patents, etc. includes a large number of acquired licences, patents, know-how agreements and marketing rights, which are either marketed or in use, or still in development. The net book value includes £6 million (2008 – £7 million) of internally generated costs. Impairment losses of £168 million (2008 – £118 million) principally arise on assets in development that are no longer being actively pursued. Note 38, ‘Acquisitions and disposals’ gives details of additions through business combinations in the year. The book values of the largest individual items are as follows:
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
Fluviral | | | 648 | | | | 654 | |
Lovaza | | | 637 | | | | 781 | |
Selzentry | | | 337 | | | | – | |
Arzerra | | | 191 | | | | 156 | |
Duac | | | 165 | | | | – | |
Fraxiparine | | | 158 | | | | 180 | |
| Others | | | 3,040 | | | | 1,724 | |
| | | | | | |
| | | | 5,176 | | | | 3,495 | |
| | | | | | |
Amortised brands include OTC rights relating toalli, with a book value at 31st December 2009 of £260 million (2008 – £294 million).
Indefinite life brands comprise a portfolio of Consumer Healthcare products primarily acquired with the acquisitions of Sterling Winthrop, Inc. in 1994, Block Drug Company, Inc. in 2001 and CNS, Inc. in 2006, together with a number of pharmaceutical brands from the acquisition of Stiefel Laboratories, Inc. in 2009. The book values of the major brands are as follows:
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
Panadol | | | 399 | | | | 411 | |
Sensodyne | | | 271 | | | | 289 | |
| Stiefel trade name | | | 209 | | | | – | |
Breathe Right | | | 193 | | | | 216 | |
Physiogel | | | 176 | | | | – | |
Polident | | | 115 | | | | 123 | |
Corega | | | 102 | | | | 109 | |
Biotene | | | 108 | | | | 99 | |
Poligrip | | | 71 | | | | 75 | |
Solpadeine | | | 59 | | | | 60 | |
| Others | | | 753 | | | | 412 | |
| | | | | | |
| | | | 2,456 | | | | 1,794 | |
| | | | | | |
Each of these brands is considered to have an indefinite life, given the strength and durability of the brand and the level of marketing support. The brands are in relatively similar stable and profitable market sectors, with similar risk profiles, and their size, diversification and market shares mean that the risk of market-related factors causing a reduction in the lives of the brands is considered to be relatively low. The Group is not aware of any material legal, regulatory, contractual, competitive, economic or other factor which could limit their useful lives. Accordingly, they are not amortised.
Each brand is tested annually for impairment applying a fair value less costs to sell methodology, generally using four year post-tax cash flow forecasts with a terminal value calculation and a discount rate equal to the Group post-tax WACC of 8%, adjusted where appropriate for country-specific risks. The main assumptions include future sales price and volume growth, product contribution and the future expenditure required to maintain the product’s marketability and registration in the relevant jurisdictions. These assumptions are based on past experience and are reviewed as part of management’s budgeting and strategic planning cycle for changes in market conditions and sales erosion through competition. The terminal growth rates applied of between 2% and 3% are management’s estimates of future long-term average growth rates of the relevant markets. In each case the valuations indicate sufficient headroom such that a reasonably possible change to key assumptions is unlikely to result in an impairment of these brands.
GSK Annual Report 2009
125
Notes to the financial statements
20 Investments in associates and joint ventures
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Joint | | | Associated | | | 2009 | | | Joint | | | Associated | | | 2008 | |
| | | ventures | | | undertakings | | | Total | | | ventures | | | undertakings | | | Total | |
| | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | | | | |
| At 1st January | | | 28 | | | | 524 | | | | 552 | | | | 15 | | | | 314 | | | | 329 | |
| Exchange adjustments | | | (3 | ) | | | (44 | ) | | | (47 | ) | | | 6 | | | | 131 | | | | 137 | |
| Additions | | | 36 | | | | 312 | | | | 348 | | | | 6 | | | | 3 | | | | 9 | |
| Disposals | | | – | | | | (69 | ) | | | (69 | ) | | | – | | | | – | | | | – | |
| Transfer from other investments | | | – | | | | 56 | | | | 56 | | | | – | | | | 39 | | | | 39 | |
| Fair value adjustment | | | – | | | | 8 | | | | 8 | | | | – | | | | 3 | | | | 3 | |
| Retained (loss)/profit for the year | | | (15 | ) | | | 62 | | | | 47 | | | | 1 | | | | 34 | | | | 35 | |
| | | | | | | | | | | | | | | | | | |
| At 31st December | | | 46 | | | | 849 | | | | 895 | | | | 28 | | | | 524 | | | | 552 | |
| | | | | | | | | | | | | | | | | | |
The Group held two significant associated undertakings at 31st December 2009.
Quest Diagnostics Inc., a US clinical laboratory business listed on the New York Stock Exchange. The investment had a book value at 31st December 2009 of £410 million (2008 – £463 million) and a market value of £1,153 million (2008 – £1,316 million). At 31st December 2009, the Group owned 16.8% of Quest (2008 – 18.7%). During the year, the Group sold 5.7 million shares in Quest, realising a profit of £115 million. Although the Group holds less than 20% of the ownership interest and voting control in Quest, the Group has the ability to exercise significant influence through both its significant shareholding and its nominated director’s active participation on the Quest Board of Directors and Board sub-committees.
In November 2009, GSK increased its shareholding in Aspen Pharmacare Holdings Limited by acquiring 68.5 million shares in consideration for the transfer of certain assets. GSK’s shareholding in Aspen on 31st December 2009 was 81.7 million shares or 19%. Aspen, listed on the Johannesburg Stock Exchange, is Africa’s largest pharmaceutical manufacturer and a major supplier of branded and generic pharmaceutical, healthcare and nutritional products to the southern African and selected international markets. After elimination of unrealised gains, the investment had a book value at 31st December 2009 of £372 million, including estimated goodwill of £259 million. The market value of the shares held by GSK at 31st December 2009 was £505 million. Although the Group holds less than 20% of the ownership interest and voting control of Aspen, the Group has the ability to exercise significant influence through both its shareholding and its nominated director’s active participation on the Aspen Board of Directors.
The transfer from other investments in 2009 relates to the Group’s holding in Aspen, previously classified within Other investments.
In August 2009, GSK invested £20 million to establish a 40% interest in Shenzhen GlaxoSmithKline – Neptunus Biologicals Co., Ltd, a new joint venture primarily operating in the fields of research, development and manufacture of flu vaccines.
During 2009, GSK made additional capital contributions totalling £16 million to Shionogi-GlaxoSmithKline Holdings, L.P.
Summarised balance sheet information in respect of the Group’s associates is set out below:
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
| Total assets: | | | | | | | | |
| Quest Diagnostics Inc. | | | 5,319 | | | | 5,836 | |
| Aspen Pharmacare Holdings Limited | | | 1,318 | | | | – | |
| Others | | | 121 | | | | 115 | |
| | | | | | |
| | | | 6,758 | | | | 5,951 | |
| | | | | | |
| | | | | | | | | |
| Total liabilities: | | | | | | | | |
| Quest Diagnostics Inc. | | | (2,828 | ) | | | (3,333 | ) |
| Aspen Pharmacare Holdings Limited | | | (689 | ) | | | – | |
| Others | | | (19 | ) | | | (20 | ) |
| | | | | | |
| | | | (3,536 | ) | | | (3,353 | ) |
| | | | | | |
| | | | | | | | | |
| Net assets | | | 3,222 | | | | 2,598 | |
| | | | | | |
The summarised balance sheet information in respect of Aspen Pharmacare Holdings Limited is based on analysts forecasts available at 31st December 2009.
Investments in joint ventures comprise £57 million share of gross assets (2008 – £36 million) and £11 million share of gross liabilities (2008 – £8 million). These principally arise from 50% interests in two joint ventures, Shionogi-GlaxoSmithKline Holdings, L.P., which is developing specified chemical compounds, and GlaxoSmithKline Shire Canada, which primarily co-marketsCombivir,TrizivirandEpivirin certain territories, both of which are now part of the ViiV Healthcare business. Investments in joint ventures also include a 28% interest in Pharmaceutical Insurance Limited, which is a mutual insurance company covering pharmaceutical business risk, and a 40% interest in GlaxoSmithKline – NeptunusBio, which is a flu vaccine research, development and manufacturing venture.
GSK Annual Report 2009
126
Notes to the financial statements
21 Other investments
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
| At 1st January | | | 478 | | | | 517 | |
| Exchange adjustments | | | (48 | ) | | | 129 | |
| Additions | | | 175 | | | | 87 | |
| Net fair value movements | | | 57 | | | | (94 | ) |
| Impairment losses | | | (95 | ) | | | (65 | ) |
| Transfer to investments in associates and joint ventures | | | (56 | ) | | | (39 | ) |
| Disposals | | | (57 | ) | | | (57 | ) |
| | | | | | |
| At 31st December | | | 454 | | | | 478 | |
| | | | | | |
Other investments comprise non-current equity investments which are available-for-sale investments recorded at fair value at each balance sheet date. For investments traded in an active market, the fair value is determined by reference to the relevant stock exchange quoted bid price. For other investments, the fair value is estimated by reference to the current market value of similar instruments or by reference to the discounted cash flows of the underlying net assets. The Group holds a number of equity investments in entities where the Group has entered into research collaborations. Other investments include listed investments of £245 million (2008 – £319 million).
On disposal of investments, fair value movements are reclassified from reserves to the income statement based on average cost for shares acquired at different times.
The impairment losses recorded in the tables above have been recognised in the income statement for the year within other operating income, together with amounts reclassified from the fair value reserve on recognition of the impairments. These impairments initially result from prolonged or significant declines in the fair value of the equity investments below acquisition cost, subsequent to which any further declines in fair value are immediately taken to the income statement. At 31st December 2009 impaired assets with a fair value of £105 million (2008 – £118 million) are included in other investments.
The transfer to associates relates to the Group’s holding in Aspen Pharmacare Holdings Limited, which increased during the year to 19%.
22 Other non-current assets
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
| Amounts recoverable under insurance contracts | | | 299 | | | | 293 | |
| Pension schemes in surplus | | | 23 | | | | 39 | |
| Other receivables | | | 261 | | | | 247 | |
| | | | | | |
| | | | 583 | | | | 579 | |
| | | | | | |
23 Inventories
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
| Raw materials and consumables | | | 1,153 | | | | 1,127 | |
| Work in progress | | | 1,437 | | | | 1,295 | |
| Finished goods | | | 1,474 | | | | 1,634 | |
| | | | | | |
| | | | 4,064 | | | | 4,056 | |
| | | | | | |
GSK Annual Report 2009
128
Notes to the financial statements
27 Trade and other payables
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
| Trade payables | | | 1,855 | | | | 1,153 | |
| Wages and salaries | | | 1,089 | | | | 946 | |
| Social security | | | 125 | | | | 148 | |
| Other payables | | | 280 | | | | 233 | |
| Deferred income | | | 156 | | | | 103 | |
| Customer return and rebate accruals | | | 1,379 | | | | 1,337 | |
| Other accruals | | | 1,888 | | | | 2,155 | |
| | | | | | |
| | | | 6,772 | | | | 6,075 | |
| | | | | | |
Customer return and rebate accruals are provided for by the Group at the point of sale in respect of the estimated rebates, discounts or allowances payable to customers, principally in the USA. Provisions are made at the time of sale but the actual amounts paid are based on claims made some time after the initial recognition of the sale. As the amounts are estimated they may not fully reflect the final outcome and the amounts are subject to change dependent upon, amongst other things, the types of buying group and product sales mix. The level of provision is reviewed and adjusted quarterly in the light of historical experience of actual rebates, discounts or allowances given and returns made and any changes in arrangements. Future events could cause the assumptions on which the provisions are based to change, which could affect the future results of the Group.
28 Pensions and other post-employment benefits
| | | | | | | | | | | | | |
| Pension and other post-employment costs | | 2009 | | | 2008 | | | 2007 | |
| | £m | | | £m | | | £m | |
| | | | | | | | | |
| UK pension schemes | | | 206 | | | | 236 | | | | 108 | |
| US pension schemes | | | 94 | | | | 60 | | | | 24 | |
| Other overseas pensions schemes | | | 101 | | | | 87 | | | | 89 | |
| Unfunded post-retirement healthcare schemes | | | 90 | | | | 118 | | | | 90 | |
| Other post-employment costs | | | – | | | | 4 | | | | 2 | |
| | | | | | | | | |
| | | | 491 | | | | 505 | | | | 313 | |
| | | | | | | |
| Analysed as: | | | | | | | | | | | | |
| Funded defined benefit/hybrid pension schemes | | | 338 | | | | 318 | | | | 171 | |
| Unfunded defined benefit pension schemes | | | 25 | | | | 23 | | | | 17 | |
| Unfunded post-retirement healthcare schemes | | | 90 | | | | 118 | | | | 90 | |
| | | | | | | | | |
| Defined benefit schemes | | | 453 | | | | 459 | | | | 278 | |
| Defined contribution pension schemes | | | 38 | | | | 42 | | | | 33 | |
| Other post-employment costs | | | – | | | | 4 | | | | 2 | |
| | | | | | | | | |
| | | | 491 | | | | 505 | | | | 313 | |
| | | | | | | | | |
The costs of the defined benefit pension and post-retirement healthcare schemes are charged in the income statement as follows:
| | | | | | | | | | | | | |
| | | | | | | | | |
| Cost of sales | | | 121 | | | | 179 | | | | 72 | |
| Selling, general and administration | | | 195 | | | | 160 | | | | 129 | |
| Research and development | | | 137 | | | | 120 | | | | 77 | |
| | | | | | | | | |
| | | | 453 | | | | 459 | | | | 278 | |
| | | | | | | | | |
GSK entities operate pension arrangements which cover the Group’s material obligations to provide pensions to retired employees. These arrangements have been developed in accordance with local practices in the countries concerned. Pension benefits can be provided by state schemes; by defined contribution schemes, whereby retirement benefits are determined by the value of funds arising from contributions paid in respect of each employee; or by defined benefit schemes, whereby retirement benefits are based on employee pensionable remuneration and length of service. Some ‘hybrid’ defined benefit schemes also include defined contribution sections.
129
Notes to the financial statements
28Pensions and other post-employment benefits continued
Pension costs of defined benefit schemes for accounting purposes have been calculated using the projected unit method. In certain countries pension benefits are provided on an unfunded basis, some administered by trustee companies. Formal, independent, actuarial valuations of the Group’s main plans are undertaken regularly, normally at least every three years.
Actuarial movements in the year are recognised through the statement of comprehensive income. Discount rates are derived from AA rated corporate bond yields except in countries where there is no deep market in corporate bonds where government bond yields are used. Discount rates are selected to reflect the term of the expected benefit payments. The expected rate of return on bonds reflects the portfolio mix of index-linked, government and corporate bonds. An equity risk premium of between 3% and 4% is added to longer term government bond yields to give the expected rate of return on equities. Projected inflation rate and pension increases are long-term predictions based on the yield gap between long-term index-linked and fixed interest Gilts. In the UK, mortality rates are determined by adjusting the PCA00 standard mortality tables to reflect recent scheme experience. These rates are then projected to reflect improvements in life expectancy in line with the medium cohort (i.e. improvements at recently observed higher levels which are assumed to continue to 2020) with minimum improvements thereafter of 1% per year for males and 0.5% for females. In the USA, mortality rates are calculated using the RP2000 fully generational table, projected using scale AA, with the white collar adjustment.
The average life expectancy assumed now for an individual at the age of 60 and projected to apply in 2029 for an individual then at the age of 60 is as follows:
| | | | | | | | | | | | | | | | | |
| | | UK | | | USA | |
| | | Male | | | Female | | | Male | | | Female | |
| | | Years | | | Years | | | Years | | | Years | |
| | | | |
| Current | | | 27.3 | | | | 28.2 | | | | 24.5 | | | | 26.2 | |
| Projected for 2029 | | | 29.6 | | | | 29.5 | | | | 26.4 | | | | 27.3 | |
| | | | |
The assets of funded schemes are generally held in separately administered trusts, either as specific assets or as a proportion of a general fund, or are insurance contracts. Assets are invested in different classes in order to maintain a balance between risk and return. Investments are diversified to limit the financial effect of the failure of any individual investment. Following an asset liability study in 2007, the Group decided to adopt a strategy to reduce gradually the allocation of investment in equities. During 2009, it was agreed that the pace of reallocation would be increased primarily through investment of the deficit reduction contributions in bonds. The target allocation of equities and property in the US scheme has been reduced to 50% of the total.
In the UK the defined benefit pension schemes operated for the benefit of former Glaxo Wellcome employees and former SmithKline Beecham employees remain separate. These schemes were closed to new entrants in 2001 and subsequent UK employees are entitled to join a defined contribution scheme. In the USA the former Glaxo Wellcome and SmithKline Beecham defined benefit schemes were merged during 2001. In addition, the Group operates a number of post-retirement healthcare schemes, the principal one of which is in the USA.
The Group has applied the following financial assumptions in assessing the defined benefit liabilities:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | UK | | | USA | | | Rest of World | |
| | | 2009 | | | 2008 | | | 2007 | | | 2009 | | | 2008 | | | 2007 | | | 2009 | | | 2008 | | | 2007 | |
| | | % pa | | | % pa | | | % pa | | | % pa | | | % pa | | | % pa | | | % pa | | | % pa | | | % pa | |
| | | | | | | |
| Rate of increase of future earnings | | | 4.60 | | | | 3.90 | | | | 4.25 | | | | 4.50 | | | | 4.50 | | | | 5.00 | | | | 3.00 | | | | 3.10 | | | | 3.25 | |
| Discount rate | | | 5.70 | | | | 6.20 | | | | 5.75 | | | | 5.75 | | | | 6.00 | | | | 6.00 | | | | 4.70 | | | | 5.00 | | | | 4.75 | |
| Expected pension increases | | | 3.60 | | | | 2.90 | | | | 3.25 | | | | n/a | | | | n/a | | | | n/a | | | | 2.20 | | | | 2.10 | | | | 2.00 | |
| Cash balance credit/conversion rate | | | n/a | | | | n/a | | | | n/a | | | | 4.75 | | | | 4.50 | | | | 4.75 | | | | 1.60 | | | | 1.20 | | | | 1.60 | |
| Inflation rate | | | 3.60 | | | | 2.70 | | | | 3.25 | | | | 2.50 | | | | 2.50 | | | | 2.50 | | | | 1.70 | | | | 1.70 | | | | 1.75 | |
| | | | | | | |
GSK Annual Report 2009
134
Notes to the financial statements
28Pensions and other post-employment benefits continued
The defined benefit pension obligation is analysed as follows:
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | £m | | | £m | | | £m | |
| | | | | | | | | |
| Funded | | | (12,126 | ) | | | (10,662 | ) | | | (10,079 | ) |
| Unfunded | | | (312 | ) | | | (318 | ) | | | (259 | ) |
| | | | | | | | | |
| | | | (12,438 | ) | | | (10,980 | ) | | | (10,338 | ) |
| | | | | | | | | |
Post-retirement benefits are unfunded.
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | Post-retirement | |
| | | Pensions | | | benefits | |
| Movements in fair values of assets | | UK | | | USA | | | Rest of World | | | Group | | | Group | |
| | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | |
| Assets at 1st January 2007 | | | 6,554 | | | | 1,953 | | | | 741 | | | | 9,248 | | | | – | |
| Exchange adjustments | | | – | | | | (29 | ) | | | 68 | | | | 39 | | | | – | |
| Expected return on assets | | | 389 | | | | 141 | | | | 37 | | | | 567 | | | | – | |
| Settlements and curtailments | | | – | | | | – | | | | 2 | | | | 2 | | | | – | |
| Actuarial gains/(losses) | | | 168 | | | | 46 | | | | (18 | ) | | | 196 | | | | – | |
| Employer contributions | | | 397 | | | | 8 | | | | 99 | | | | 504 | | | | 41 | |
| Scheme participants’ contributions | | | 38 | | | | – | | | | 5 | | | | 43 | | | | 3 | |
| Benefits paid | | | (253 | ) | | | (115 | ) | | | (49 | ) | | | (417 | ) | | | (44 | ) |
| | | | | | | | | | | | | | | |
| Assets at 31st December 2007 | | | 7,293 | | | | 2,004 | | | | 885 | | | | 10,182 | | | | – | |
| Exchange adjustments | | | – | | | | 598 | | | | 298 | | | | 896 | | | | – | |
| Expected return on assets | | | 442 | | | | 144 | | | | 47 | | | | 633 | | | | – | |
| Settlements and curtailments | | | – | | | | – | | | | 3 | | | | 3 | | | | – | |
| Actuarial losses | | | (1,691 | ) | | | (614 | ) | | | (134 | ) | | | (2,439 | ) | | | – | |
| Employer contributions | | | 340 | | | | 10 | | | | 93 | | | | 443 | | | | 44 | |
| Scheme participants’ contributions | | | 33 | | | | – | | | | 5 | | | | 38 | | | | 9 | |
| Benefits paid | | | (282 | ) | | | (126 | ) | | | (60 | ) | | | (468 | ) | | | (53 | ) |
| | | | | | | | | | | | | | | |
| Assets at 31st December 2008 | | | 6,135 | | | | 2,016 | | | | 1,137 | | | | 9,288 | | | | – | |
| Exchange adjustments | | | – | | | | (221 | ) | | | (93 | ) | | | (314 | ) | | | – | |
| Expected return on assets | | | 347 | | | | 121 | | | | 46 | | | | 514 | | | | – | |
| Settlements and curtailments | | | – | | | | – | | | | (51 | ) | | | (51 | ) | | | – | |
| Actuarial gains | | | 729 | | | | 122 | | | | 19 | | | | 870 | | | | – | |
| Employer contributions | | | 594 | | | | 190 | | | | 110 | | | | 894 | | | | 58 | |
| Scheme participants’ contributions | | | 17 | | | | – | | | | 8 | | | | 25 | | | | 11 | |
| Benefits paid | | | (345 | ) | | | (156 | ) | | | (71 | ) | | | (572 | ) | | | (69 | ) |
| Acquisitions | | | 22 | | | | – | | | | 18 | | | | 40 | | | | – | |
| | | | | | | | | | | | | | | |
| Assets at 31st December 2009 | | | 7,499 | | | | 2,072 | | | | 1,123 | | | | 10,694 | | | | – | |
| | | | | | | | | | | | | | | |
The UK defined benefit schemes include defined contribution sections with account balances totalling £765 million at 31st December 2009 (2008 – £553 million; 2007 – £693 million).
During 2009, the Group made special funding contributions to the UK pension schemes totalling £332 million and £95 million to the US scheme (2008 – £200 million to the UK pension schemes). In 2009, GSK reached an agreement with the trustees of the UK pension schemes to make additional contributions to eliminate the pension deficit identified at the 31st December 2008 actuarial funding valuation. The additional contributions are expected to be £365 million per year for 2010 to 2013. The contributions are based on a discount rate of 5.25% and an inflation assumption of 2.8%. The next review of contribution levels is expected to be at the 31st December 2011 actuarial valuation although the Group has agreed to review mortality assumptions before then which could result in an earlier revision to contributions.
Employer contributions for 2010, including special funding contributions, are estimated to be approximately £800 million in respect of defined benefit pension schemes and £60 million in respect of post-retirement benefits.
GSK Annual Report 2009
139
Notes to the financial statements
32Net debt continued
Current assets
Liquid investments are classified as available-for-sale investments. At 31st December 2009, they included US Treasury notes and other government bonds. The effective interest rate on liquid investments at 31st December 2009 was approximately 4.6% (2008 – approximately 5.5%). Liquid investment balances at 31st December 2009 earning interest at floating and fixed rates amount to £1 million and £267 million, respectively (2008 – £1 million and £390 million).
The effective interest rate on cash and cash equivalents at 31st December 2009 was approximately 0.7% (2008 – approximately 1.8%). Cash and cash equivalents balances at 31st December 2009 earning interest at floating and fixed rates amount to £6,372 million and £17 million, respectively (2008 – £5,520 million and £4 million).
GSK’s policy regarding the credit quality of cash and cash equivalents is referred to in Note 41, ‘Financial instruments and related disclosures’.
Short-term borrowings
Commercial paper comprises a US $10 billion programme, of which $1 billion (£621 million) was in issue at 31st December 2009 (2008 – $nil (£nil)), backed up by committed facilities of 364 days duration of $3.9 billion (£2.4 billion) (2008 – $3.9 billion (£2.7 billion)) renewable annually, and liquid investments, cash and cash equivalents as shown in the table above.
The weighted average interest rate on current bank loans and overdrafts at 31st December 2009 was 4.8% (2008 – 1.59%).
Long-term borrowings
At the year-end, GSK had long-term borrowings of £14.8 billion (2008 – £15.2 billion) of which £9.5 billion (2008 – £9.8 billion) falls due in more than five years.
Long-term borrowings repayable after five years carry interest at effective rates between 3.88% and 6.38%. The repayment dates range from 2015 to 2042. The average effective interest rate of all notes at 31st December 2009 was approximately 4.9% (2008 – approximately 5.0%).
Secured liabilities
GSK had no loans secured by charges on non-current and current assets in the year (2008 – £nil). The Group has pledged investments in US Treasury Notes with a par value of $103 million (2008 – $198 million) as security against irrevocable letters of credit issued on the Group’s behalf in respect of the Group’s self-insurance activity. Provisions in respect of self-insurance are included within the provisions for legal and other disputes discussed in Note 29, ‘Other provisions’.
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| Finance lease obligations | | £m | | | £m | |
| | | | | | |
| Rental payments due within one year | | | 44 | | | | 53 | |
| Rental payments due between one and two years | | | 38 | | | | 39 | |
| Rental payments due between two and three years | | | 26 | | | | 30 | |
| Rental payments due between three and four years | | | 16 | | | | 17 | |
| Rental payments due between four and five years | | | 6 | | | | 6 | |
| Rental payments due after five years | | | 16 | | | | 9 | |
| | | | | | |
| Total future rental payments | | | 146 | | | | 154 | |
| Future finance charges | | | (16 | ) | | | (18 | ) |
| | | | | | |
| Total finance lease obligations | | | 130 | | | | 136 | |
| | | | | | |
Finance lease obligations at 31st December 2009 bearing interest at floating and fixed rates amount to £89 million and £41 million, respectively (2008 – £98 million and £38 million).
GSK Annual Report 2009
140
Notes to the financial statements
33Share capital and share premium account
| | | | | | | | | | | | | |
| | | | | | | | | | | Share | |
| | | | Ordinary Shares of 25p each | | | premium | |
| | | Number | | | £m | | | £m | |
| | | | | | | | | |
| |
Share capital authorised | | | | | | | | | | | | |
At 31st December 2007 | | | 10,000,000,000 | | | | 2,500 | | | | | |
At 31st December 2008 | | | 10,000,000,000 | | | | 2,500 | | | | | |
At 31st December 2009 | | | 10,000,000,000 | | | | 2,500 | | | | | |
| | | | | | | |
| |
Share capital issued and fully paid | | | | | | | | | | | | |
At 1st January 2007 | | | 5,991,601,848 | | | | 1,498 | | | | 858 | |
| Issued under share option schemes | | | 37,307,678 | | | | 9 | | | | 408 | |
| Share capital purchased and cancelled | | | (16,322,500 | ) | | | (4 | ) | | | – | |
| | | | | | | | | |
| |
At 31st December 2007 | | | 6,012,587,026 | | | | 1,503 | | | | 1,266 | |
| Issued under share option schemes | | | 5,640,119 | | | | 2 | | | | 60 | |
| Share capital purchased and cancelled | | | (356,910,908 | ) | | | (90 | ) | | | – | |
| | | | | | | | | |
| |
At 31st December 2008 | | | 5,661,316,237 | | | | 1,415 | | | | 1,326 | |
| Issued under share option schemes | | | 3,812,482 | | | | 1 | | | | 42 | |
| | | | | | | | | |
At 31st December 2009 | | | 5,665,128,719 | | | | 1,416 | | | | 1,368 | |
| | | | | | | | | |
| | | | | | | | | |
| | | 31st December | | | 31st December | |
| | | 2009 | | | 2008 | |
| | | | | | |
| Number (‘000) of shares issuable under outstanding options (Note 42) | | | 213,110 | | | | 220,459 | |
| | | | | | |
| Number (‘000) of unissued shares not under option | | | 4,121,761 | | | | 4,118,225 | |
| | | | | | |
At 31st December 2009, of the issued share capital, 117,735,257 shares were held in the ESOP Trust, 474,194,158 shares were held as Treasury shares and 5,073,199,304 shares were in free issue. All issued shares are fully paid. The nominal, carrying and market values of the shares held in the ESOP Trust are disclosed in Note 42, ‘Employee share schemes’.
The company did not make any purchases of its own shares in 2009. There have been no purchases since 31st December 2009. GSK does not expect to make significant share repurchases in 2010.
GSK Annual Report 2009
142
Notes to the financial statements
35Related party transactions
GSK held a 16.8% interest in Quest Diagnostics Inc. at 31st December 2009 (2008 – 18.7%). The Group and Quest Diagnostics are parties to a long-term contractual relationship under which Quest Diagnostics is the primary provider of clinical laboratory testing to support the Group’s clinical trials testing requirements worldwide. During 2009, Quest Diagnostics provided services of £47 million (2008 – £42 million) to the Group. At 31st December 2009, the balance payable by GSK to Quest Diagnostics was £10 million (2008 – £nil).
In March 2009, 5,749,157 shares in the Group’s associate Quest Diagnostics Inc. were sold for a cash consideration of £178 million, the majority of the shares being sold direct to Quest Diagnostics Inc. with the remainder being sold in the market.
On 30th November 2009, GSK completed the extension of its strategic relationship with Aspen Pharmacare Holdings Limited by the acquisition of a minority shareholding in the South African based pharmaceutical company. The transaction resulted in GSK acquiring 68.5 million shares in Aspen in consideration for the transfer of certain assets and in Aspen becoming an associate. A gain of £183 million on the transaction is included within other operating income. At 31st December 2009, GSK held 81.7 million shares, a 19% interest in Aspen.
During December 2009, GSK distributed £18 million of its products through Aspen’s extensive distribution network. At 31st December 2009, the balance due to GSK from Aspen was £18 million (2008 – £nil) and the balance payable by GSK to Aspen was £13 million (2008 – £nil).
In 2009, both the Group and Shionogi & Co. Ltd. entered into transactions with their 50/50 US joint venture company in support of the research and development activities conducted by that joint venture company. During 2009, GSK provided services to the joint venture of £15 million (2008 – £7 million). At 31st December 2009, the balance due to GSK from the joint venture was £14 million (2008 – £5 million).
The aggregate compensation of the Directors and CET is given in Note 10, ‘Employee Costs’.
36Adjustments reconciling profit after tax to operating cash flows
| | | | | | | | | | | | | |
| | | 2009 | | | 2008 | | | 2007 | |
| | | £m | | | £m | | | £m | |
| | | | | | | | | |
| Profit after tax | | | 5,669 | | | | 4,712 | | | | 5,310 | |
| Tax on profits | | | 2,222 | | | | 1,947 | | | | 2,142 | |
| Share of after tax profits of associates and joint ventures | | | (64 | ) | | | (48 | ) | | | (50 | ) |
| Finance income net of finance costs | | | 713 | | | | 530 | | | | 191 | |
| Depreciation | | | 1,130 | | | | 920 | | | | 796 | |
| Amortisation of intangible assets | | | 432 | | | | 311 | | | | 226 | |
| Impairment and assets written off | | | 445 | | | | 436 | | | | 206 | |
| Profit on sale of intangible assets | | | (835 | ) | | | (170 | ) | | | (5 | ) |
| Profit on sale of investments in associates | | | (115 | ) | | | – | | | | – | |
| Profit on sale of equity investments | | | (40 | ) | | | (33 | ) | | | (32 | ) |
| Changes in working capital: | | | | | | | | | | | | |
| Increase in inventories | | | (132 | ) | | | (411 | ) | | | (457 | ) |
| (Increase)/decrease in trade receivables | | | (473 | ) | | | 519 | | | | (77 | ) |
| (Increase)/decrease in other receivables | | | (134 | ) | | | 22 | | | | (2 | ) |
| Increase/(decrease) in trade payables | | | 499 | | | | (39 | ) | | | 9 | |
| Increase/(decrease) in other payables | | | 409 | | | | (162 | ) | | | (196 | ) |
| (Decrease)/increase in pension and other provisions | | | (320 | ) | | | 548 | | | | (123 | ) |
| Share-based incentive plans | | | 179 | | | | 241 | | | | 237 | |
| Other | | | (40 | ) | | | (268 | ) | | | (95 | ) |
| | | | | | | | | |
| | | | 3,876 | | | | 4,343 | | | | 2,770 | |
| | | | | | | | | |
| | | | | | | | | | | | | |
| Cash generated from operations | | | 9,545 | | | | 9,055 | | | | 8,080 | |
| | | | | | | | | |
GSK Annual Report 2009
143
Notes to the financial statements
37Reconciliation of net cash flow to movement in net debt
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | 2009 | | | 2008 | | | 2007 | |
| | | | | | | | | | | | | | | | | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net debt at beginning of year | | | | | | | | | | | | | | | | | | | (10,173 | ) | | | (6,039 | ) | | | (2,450 | ) |
| Increase in cash and bank overdrafts | | | | | | | | | | | 1,054 | | | | 1,148 | | | | 1,411 | |
| Cash (inflow)/outflow from liquid investments | | | | | | | | | | | | | | | | | | | (87 | ) | | | (905 | ) | | | 39 | |
| Net increase in long-term loans | | | | | | | | | | | | | | | | | | | (1,358 | ) | | | (5,523 | ) | | | (3,276 | ) |
| Net repayment of/(increase in) short-term loans | | | | | | | | | | | 102 | | | | 3,059 | | | | (1,632 | ) |
| Net repayment of obligations under finance leases | | | | | | | | | | | 48 | | | | 48 | | | | 39 | |
| Debt of subsidiary undertakings acquired | | | | | | | | | | | (9 | ) | | | – | | | | – | |
| Exchange adjustments | | | | | | | | | | | | | | | | | | | 1,041 | | | | (1,918 | ) | | | (88 | ) |
| Other non-cash movements | | | | | | | | | | | | | | | | | | | (62 | ) | | | (43 | ) | | | (82 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Movement in net debt | | | | | | | | | | | | | | | | | | | 729 | | | | (4,134 | ) | | | (3,589 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net debt at end of year | | | | | | | | | | | | | | | | | | | (9,444 | ) | | | (10,173 | ) | | | (6,039 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Analysis of changes in net debt | | At 31.12.08 | | | Exchange | | | Other | | | Reclassifications | | | Acquisitions | | | Cash flow | | | At 31.12.09 | |
| | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | | | | | | | |
| Liquid investments | | | 391 | | | | (36 | ) | | | – | | | | – | | | | – | | | | (87 | ) | | | 268 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | | 5,623 | | | | (171 | ) | | | – | | | | – | | | | 94 | | | | 999 | | | | 6,545 | |
| Overdrafts | | | (151 | ) | | | 13 | | | | – | | | | – | | | | – | | | | (39 | ) | | | (177 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| | | | 5,472 | | | | (158 | ) | | | – | | | | – | | | | 94 | | | | 960 | | | | 6,368 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Debt due within one year: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Commercial paper | | | – | | | | – | | | | – | | | | – | | | | – | | | | (621 | ) | | | (621 | ) |
| Eurobonds and Medium-Term Notes | | | (481 | ) | | | 69 | | | | (38 | ) | | | (641 | ) | | | – | | | | 470 | | | | (621 | ) |
| Other | | | (324 | ) | | | 33 | | | | (20 | ) | | | (25 | ) | | | (9 | ) | | | 293 | | | | (52 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| | | | (805 | ) | | | 102 | | | | (58 | ) | | | (666 | ) | | | (9 | ) | | | 142 | | | | (1,294 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Debt due after one year: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Eurobonds, Medium-Term Notes and private financing | | | (15,131 | ) | | | 1,128 | | | | 24 | | | | 641 | | | | – | | | | (1,358 | ) | | | (14,696 | ) |
| Other | | | (100 | ) | | | 5 | | | | (28 | ) | | | 25 | | | | – | | | | 8 | | | | (90 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| | | | (15,231 | ) | | | 1,133 | | | | (4 | ) | | | 666 | | | | – | | | | (1,350 | ) | | | (14,786 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net debt | | | (10,173 | ) | | | 1,041 | | | | (62 | ) | | | – | | | | 85 | | | | (335 | ) | | | (9,444 | ) |
| | | | | | | | | | | | | | | | | | | | | |
For further information on significant changes in net debt see Note 32 ‘Net debt’.
38Acquisitions and disposals
Details of the acquisition and disposal of subsidiary and associated undertakings, joint ventures and other businesses are given below:
2009
Acquisitions
Genelabs Technologies Inc.
On 7th January 2009, the Group acquired all of the share capital of Genelabs Technologies Inc, a California biotechnology company with a strong and focused portfolio in hepatitis C vaccines. The purchase price of £42 million included £12 million of cash and cash equivalents, with the remainder represented by preliminary net asset valuations of £30 million. This transaction has been accounted for by the purchase method of accounting. Genelabs Technologies Inc. had turnover of £nil and a loss after tax of £8 million for the year, of which turnover of £nil and £8 million of loss after tax related to the period since acquisition and are included in the Group accounts.
GSK Annual Report 2009
144
Notes to the financial statements
38Acquisitions and disposals continued
2009
Acquisitions continued
Genelabs Technologies Inc. continued
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | – | | | | 1 | | | | 1 | |
| Property, plant and equipment | | | 2 | | | | – | | | | 2 | |
| Other assets including cash and cash equivalents | | | 14 | | | | – | | | | 14 | |
| Deferred tax asset | | | – | | | | 26 | | | | 26 | |
| Other liabilities | | | (2 | ) | | | – | | | | (2 | ) |
| | | | | | | |
| | | | 14 | | | | 27 | | | | 41 | |
| Goodwill | | | – | | | | 1 | | | | 1 | |
| | | | | | | |
| Total consideration | | | 14 | | | | 28 | | | | 42 | |
| | | | | | | |
Bristol Myers Squibb Pakistan (Private) Limited
On 30th January 2009, the Group acquired all of the share capital of Bristol Myers Squibb Pakistan (Private) Limited and certain associated trademarks for a consideration of £25 million. As a result, the Group has acquired a portfolio of over 30 well-established pharmaceutical brands, many of which occupy leading market positions in key therapeutic disease areas in Pakistan. The purchase price of £25 million was represented by provisional valuations of intangible assets of £8 million, goodwill of £10 million and other net assets of £7 million. The goodwill arising on the acquisition reflects the potential for product growth throughout the region and the expected synergies for the Group. This transaction has been accounted for by the purchase method of accounting. Bristol Myers Squibb Pakistan (Private) Limited had a turnover of £15 million and a profit after tax of £0.3 million for the year, of which £14 million of turnover and £0.4 million of profit after tax related to the period since acquisition and are included in the Group accounts.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | 7 | | | | 1 | | | | 8 | |
| Property, plant and equipment | | | 5 | | | | 3 | | | | 8 | |
| Other assets including cash and cash equivalents | | | 6 | | | | – | | | | 6 | |
| Deferred tax provision | | | (1 | ) | | | – | | | | (1 | ) |
| Other liabilities | | | (5 | ) | | | (1 | ) | | | (6 | ) |
| | | | | | | |
| | | | 12 | | | | 3 | | | | 15 | |
| Goodwill | | | – | | | | 10 | | | | 10 | |
| | | | | | | |
| Total consideration | | | 12 | | | | 13 | | | | 25 | |
| | | | | | | |
Certain businesses from UCB S.A.
On 31st March 2009, the Group acquired from UCB S.A. its marketed product portfolio across certain territories in Africa, the Middle East, Asia Pacific and Latin America which includes several leading pharmaceutical brands in a number of disease areas. Subsequent to this date the Group completed further country acquisitions which formed part of the original transaction. The purchase price of £477 million included £5 million of net cash, £445 million of intangible assets, £87 million of goodwill and £60 million of other net liabilities. These are provisional valuations and may change in the future. The goodwill arising on the acquisition of this business reflects the potential for product growth throughout the regions and the expected synergies for the Group. This transaction has been accounted for by the purchase method of accounting.
The transaction included acquisition of both a number of legal entities and product rights that had been previously marketed outside of those entities. The product portfolio acquired has been integrated into the GSK business in the period since acquisition and it is not therefore practicable to identify the result after tax arising as a result of this transaction for the period after acquisition.
Prior to acquisition it is estimated that the product portfolio recorded turnover of £26 million. Since acquisition GSK has recorded turnover of £77 million from the products acquired.
GSK Annual Report 2009
145
Notes to the financial statements
38Acquisitions and disposals continued
2009
Certain businesses from UCB S.A. continued
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | 417 | | | | 28 | | | | 445 | |
| Property, plant and equipment | | | 1 | | | | – | | | | 1 | |
| Cash and cash equivalents | | | 5 | | | | – | | | | 5 | |
| Deferred tax provision | | | – | | | | (56 | ) | | | (56 | ) |
| Other liabilities | | | (5 | ) | | | – | | | | (5 | ) |
| | | | | | | |
| | | | 418 | | | | (28 | ) | | | 390 | |
| Goodwill | | | – | | | | 87 | | | | 87 | |
| | | | | | | |
| Total consideration | | | 418 | | | | 59 | | | | 477 | |
| | | | | | | |
AZ Tika
On 21st April 2009, the Group acquired all of the share capital of AZ Tika, a wholly owned subsidiary of Astra Zeneca plc for a cash consideration of £146 million. As a result, the Group has acquired a number of leading over-the-counter products, predominantly sold in Sweden, includingAlvedon, the country’s leading analgesic treatment. The purchase price of £146 million was represented by intangible assets of £109 million, goodwill of £50 million and other net liabilities of £13 million. The goodwill arising on the acquisition reflects the potential for product growth and the expected synergies for the Group. This transaction has been accounted for by the purchase method of accounting. Prior to acquisition the products acquired were marketed outside the entity acquired. The products acquired have been integrated into the GSK business in the period since acquisition and it is not therefore practicable to identify the result after tax arising as a result of the transaction for the period after acquisition.
Prior to acquisition it is estimated that the product portfolio recorded turnover of £7 million. Since acquisition GSK has recorded turnover of £24 million from the products acquired.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | 72 | | | | 37 | | | | 109 | |
| Other assets including cash and cash equivalents | | | – | | | | 1 | | | | 1 | |
| Deferred tax provision | | | – | | | | (14 | ) | | | (14 | ) |
| | | | | | | |
| | | | 72 | | | | 24 | | | | 96 | |
| Goodwill | | | – | | | | 50 | | | | 50 | |
| | | | | | | |
| Total consideration | | | 72 | | | | 74 | | | | 146 | |
| | | | | | | |
Stiefel Laboratories, Inc.
On 22nd July 2009, the Group acquired all of the share capital of Stiefel Laboratories, Inc., the world’s largest private dermatological company for a cash consideration of £1,993 million net of cash acquired and including £326 million of debt repaid on acquisition. The purchase price of £2,219 million (including contingent cash consideration of £152 million payable upon certain criteria being met by specified dates in the future) included £74 million of cash and cash equivalents, £1,513 million of intangible assets, £885 million of goodwill, representing the potential for additional growth from the combination of the Stiefel business and GSK’s existing dermatology portfolio, and £253 million of other net liabilities. The purchase price includes potential obligations to make additional payments of up to $300 million (£183 million) depending on the future performance of certain products. These are provisional valuations and may change in the future. Stiefel Laboratories Inc. had a turnover of £547 million and a loss after tax (including restructuring costs) of £103 million for the year ended 31st December 2009, of which £248 million of turnover and £78 million of loss after tax (including restructuring costs) related to the period since acquisition and are included in the Group accounts. Since acquisition, Stiefel made an operating profit of £35 million before restructuring costs and intangible assets amortisation.
The new business will provide significant opportunities for both sales and cost synergies. Stiefel’s products will benefit from GSK’s global distribution and commercial organisations, particularly in markets such as Brazil, Russia, India, China and Japan. GSK’s products will benefit from Stiefel’s speciality sales force relationships and experienced management in dermatology.
Cost synergies for the new business are expected primarily from combining manufacturing and administrative functions. As previously reported, GSK expects to deliver annual pre-tax cost savings of up to £155 million by 2012 with restructuring costs of approximately £205 million, of which £71 million was charged in 2009 and the remainder will be incurred over the next two years. Excluding restructuring costs, the Stiefel acquisition resulted in a dilution of GSK’s earnings per share of less than 1% in 2009 and is expected to result in an improvement of 1-2% in 2010.
GSK Annual Report 2009
146
Notes to the financial statements
38Acquisitions and disposals continued
2009
Stiefel Laboratories, Inc. continued
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | 274 | | | | 1,239 | | | | 1,513 | |
| Property, plant and equipment | | | 111 | | | | – | | | | 111 | |
| Other assets including cash and cash equivalents | | | 210 | | | | 47 | | | | 257 | |
| Deferred tax provision | | | 35 | | | | (331 | ) | | | (296 | ) |
| Other liabilities | | | (251 | ) | | | – | | | | (251 | ) |
| | | | | | | |
| | | | 379 | | | | 955 | | | | 1,334 | |
| Goodwill | | | – | | | | 885 | | | | 885 | |
| | | | | | | |
| Total consideration | | | 379 | | | | 1,840 | | | | 2,219 | |
| | | | | | | |
ViiV Healthcare Limited
On 30th October 2009, GSK acquired Pfizer Inc.’s HIV business and combined it with its own HIV business to form ViiV Healthcare Limited, a sub-group owned 85% by GSK and 15% by Pfizer. The consideration given by GSK, representing 15% of the net value of GSK’s HIV business, contingent consideration and transaction costs, was valued at £383 million. This was represented by £595 million of intangible assets, £172 million of deferred tax liability, £21 million of other net assets, £316 million increase in minority interests and £255 million of goodwill representing the economies of scale gained from the combination of the two businesses and the potential for growth of both partners’ HIV products within ViiV Healthcare. These are provisional valuations and may change in the future. The minority interest represents Pfizer’s interest in ViiV Healthcare including the right to preferential dividends based on the sales performance of certain products.
GSK has recognised an accounting gain of £296 million on this transaction arising on the disposal of a 15% interest in GSK’s HIV business to Pfizer recorded at book value, in return for 85% of Pfizer’s HIV business recorded at fair value.
The acquired Pfizer HIV business had a turnover of £89 million and a loss after tax of £39 million for the year, of which, after taking account of the transition status in various territories, £1 million of turnover and £23 million of loss after tax has been recognised in the Group accounts, including restructuring costs.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | 13 | | | | 582 | | | | 595 | |
| Other assets including cash and cash equivalents | | | 10 | | | | 11 | | | | 21 | |
| Deferred tax provision | | | – | | | | (172 | ) | | | (172 | ) |
| | | | | | | |
| | | | 23 | | | | 421 | | | | 444 | |
| Minority interests | | | – | | | | (316 | ) | | | (316 | ) |
| Goodwill | | | – | | | | 255 | | | | 255 | |
| | | | | | | |
| Total consideration | | | 23 | | | | 360 | | | | 383 | |
| | | | | | | |
| | | | | | | | | | | | | |
| Consideration | | | | | | | | | | | | |
| Fair value of assets contributed by GSK | | | | | | | | | | | 328 | |
| Fair value of contingent equity contributed by GSK | | | | | | | | | | | 37 | |
| Direct costs | | | | | | | | | | | 18 | |
| | | | | | | |
| Total consideration | | | | | | | | | | | 383 | |
| | | | | | | |
GSK Annual Report 2009
147
Notes to the financial statements
38Acquisitions and disposals continued
2009
Acquisitions continued
Laboratoire Pharmaceutique Algérien
On 10th November 2009, GSK acquired 100% of the share capital of the Algerian pharmaceutical, manufacturing and distribution group, Laboratoire Pharmaceutique Algérien, for a cash consideration of £26 million net of cash acquired. The purchase price of £29 million included £3 million of cash and cash equivalents, £35 million of goodwill, £15 million of other net liabilities, and a £6 million reduction in the value of an existing investment. These are provisional valuations and may change in the future. The goodwill reflects the potential for business synergies and further sales growth through the increase in GSK’s market presence following the acquisition of an established market participant. This transaction has been accounted for by the purchase method of accounting. Laboratoire Pharmaceutique Algérien had a turnover of £61 million for the year ended 31st December 2009. The result for the year has not yet been determined but is estimated to be a loss of £25 million. Turnover of £6 million and £1 million of loss related to the period after acquisition are recorded in the Group accounts.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Property, plant and equipment | | | 29 | | | | – | | | | 29 | |
| Cash and cash equivalents | | | 3 | | | | – | | | | 3 | |
| Other liabilities | | | (44 | ) | | | – | | | | (44 | ) |
| | | | | | | |
| | | | (12 | ) | | | – | | | | (12 | ) |
| Goodwill | | | – | | | | 35 | | | | 35 | |
| Fair value loss arising on increased investment in LPA Distribution | | | – | | | | 6 | | | | 6 | |
| | | | | | | |
| Total consideration | | | (12 | ) | | | 41 | | | | 29 | |
| | | | | | | |
NovaMin Technology Inc.
On 18th December 2009, GSK acquired 100% of the share capital of NovaMin Technology Inc., a privately held US company for a cash consideration of £87 million. The purchase price included £51 million of intangible assets, £53 million of goodwill and £17 million of net liabilities. These are provisional valuations and may change in the future. The company has a specialty oral care ingredient for the treatment of dentine hypersensitivity and the goodwill arising from the acquisition represents the potential for additional growth from the combination of the company’s technology with specific GSK oral care products. This transaction has been accounted for by the purchase method of accounting. NovaMin Technology Inc. had a turnover of £0.1 million and a loss after tax of £0.5 million for the year, of which £nil of turnover and £nil of loss after tax related to the period since acquisition and are included in the Group accounts.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | 1 | | | | 50 | | | | 51 | |
| Deferred tax provision | | | – | | | | (17 | ) | | | (17 | ) |
| | | | | | | |
| | | | 1 | | | | 33 | | | | 34 | |
| Goodwill | | | – | | | | 53 | | | | 53 | |
| | | | | | | |
| Total consideration | | | 1 | | | | 86 | | | | 87 | |
| | | | | | | |
If the above acquisitions had been made at the beginning of the year, it is estimated that Group turnover would have increased by £477 million for the year. As some of the acquisitions have been fully integrated into the GSK business it is not practicable to separately identify the impact of the acquisitions on the Group profit for the year.
Other acquisitions in the year include £16 million invested in Shionogi-GlaxoSmithKline Holdings, L.P., a joint venture in which the Group has a 50% share and £20 million invested in Shenzhen GlaxoSmithKline – Neptunus Biologicals Co., Ltd, an associate in which the Group has an initial 40% share.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Certain | | | | | | | Stiefel | | | Laboratoire | | | NovaMin | | | | | | | |
| Cash flows | | | | | | BMS | | | businesses | | | | | | | Laboratories, | | | Pharmaceutique | | | Technology | | | | | | | |
| | Genelabs | | | (Pakistan) | | | of UCB S.A. | | | AZ Tika | | | Inc. | | | Algérien | | | Inc | | | Other | | | Total | |
| | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash consideration | | | 42 | | | | 23 | | | | 477 | | | | 146 | | | | 2,067 | | | | 29 | | | | 87 | | | | 44 | | | | 2,915 | |
| Cash and cash equivalents acquired | | | (12 | ) | | | – | | | | (5 | ) | | | – | | | | (74 | ) | | | (3 | ) | | | – | | | | – | | | | (94 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net cash consideration | | | 30 | | | | 23 | | | | 472 | | | | 146 | | | | 1,993 | | | | 26 | | | | 87 | | | | 44 | | | | 2,821 | |
| Contingent consideration | | | – | | | | 2 | | | | – | | | | – | | | | 152 | | | | – | | | | – | | | | – | | | | 154 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net purchase consideration | | | 30 | | | | 25 | | | | 472 | | | | 146 | | | | 2,145 | | | | 26 | | | | 87 | | | | 44 | | | | 2,975 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
GSK Annual Report 2009
148
Notes to the financial statements
38Acquisitions and disposals continued
2008
Acquisitions continued
Sirtris Pharmaceuticals Inc.
On 5th June 2008, the Group acquired 100% of the issued share capital of Sirtris Pharmaceuticals Inc., a biopharmaceutical company based in Massachusetts, USA for a cash consideration of £376 million. The company is focused on discovering and developing proprietary, orally available, small molecule drugs with the potential to treat diseases associated with ageing, including metabolic diseases such as Type 2 diabetes. Sirtris’ drug candidates are designed to mimic certain beneficial health effects of calorie restriction by activation of sirtuins, a recently discovered class of enzymes that Sirtris believes control the ageing process. This transaction has been accounted for by the purchase method of accounting. The goodwill arising on the acquisition reflects the potential for enabling GSK to enhance its metabolic, neurology, and immuno-inflammation research efforts by establishing a world-leading presence in the sirtuin field, aided by the existence in the company of a highly experienced development team that encompasses all aspects of sirtuin biology. Sirtris Pharmaceuticals Inc. had a turnover of £nil and a loss after tax of £25 million for the year, of which £nil of turnover and £14 million of loss after tax related to the period since acquisition and are included in the Group accounts.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | – | | | | 106 | | | | 106 | |
| Property, plant and equipment | | | 2 | | | | – | | | | 2 | |
| Other assets including cash and cash equivalents | | | 86 | | | | – | | | | 86 | |
| Deferred tax provision | | | – | | | | (21 | ) | | | (21 | ) |
| Other liabilities | | | (39 | ) | | | – | | | | (39 | ) |
| | | | | | | |
| | | | 49 | | | | 85 | | | | 134 | |
| Goodwill | | | – | | | | 242 | | | | 242 | |
| | | | | | | |
| Total consideration | | | 49 | | | | 327 | | | | 376 | |
| | | | | | | |
Bristol Myers Squibb (Egypt)
On 14th October 2008, the Group acquired the Egyptian mature products business of Bristol Myers Squibb (BMS) for a cash consideration of £140 million of this amount £10 million is deferred with payment being made when alternative supply arrangements are established. The Group acquired 20 branded products that occupy leading market positions in four therapeutic disease areas in Egypt, includingDuricef(antibiotic);CapozideandCapoten(ACE inhibitors);Theragran-H(iron supplement) andKenacomb(topical steroid). Total sales of this combined mature products pharmaceuticals business in 2007 were $48.5 million. The Group will also take ownership of BMS’s high quality manufacturing facility in Giza (Greater Cairo) that will continue to supply the acquired products. The Group will have the ability to export generic versions of the acquired products to markets outside of Egypt, thereby creating a further opportunity to drive sales growth in the Middle East and North Africa region and this fact is reflected in the goodwill arising on the acquisition. The business had a turnover of £25 million and a profit after tax of £4 million for the year, of which £4 million of turnover and £0.2 million of profit after tax are related to the period since acquisition and are included in the Group accounts.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | – | | | | 65 | | | | 65 | |
| Property, plant and equipment | | | 9 | | | | 9 | | | | 18 | |
| Inventory | | | 5 | | | | – | | | | 5 | |
| | | | | | | |
| | | | 14 | | | | 74 | | | | 88 | |
| Goodwill | | | – | | | | 52 | | | | 52 | |
| | | | | | | |
| Total consideration | | | 14 | | | | 126 | | | | 140 | |
| | | | | | | |
GSK Annual Report 2009
149
Notes to the financial statements
38Acquisitions and disposals continued
If Sirtris and BMS (Egypt) had been acquired at the beginning of 2008, combined Group turnover for the year would have been £24,373 million and combined Group profit for the year would have been £4,705 million.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Shionogi- | | | | | | | |
| Cash flows | | | | | | | | | | Euclid SR | | | GlaxoSmithKline | | | | | | | |
| | Sirtris | | | BMS (Egypt) | | | Partners LP | | | Holdings, L.P. | | | Other | | | Total | |
| | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | | | | |
| Cash consideration | | | 376 | | | | 130 | | | | 2 | | | | 6 | | | | 1 | | | | 515 | |
| Cash and cash equivalents acquired | | | (52 | ) | | | – | | | | – | | | | – | | | | – | | | | (52 | ) |
| | | | | | | | | | | | | | | | | | |
| Net cash payment on acquisitions | | | 324 | | | | 130 | | | | 2 | | | | 6 | | | | 1 | | | | 463 | |
| | | | | | | | | | | | | | | | | | |
Euclid SR Partners, LP
During 2008, an additional £2 million was invested in Euclid SR Partners, LP, an associate in which the Group has a 38.6% share.
Shionogi-GlaxoSmithKline Holdings, L.P.
During 2008, an additional £6 million was invested in Shionogi-GlaxoSmithKline Holdings, L.P., a joint venture in which the Group has a 50% share.
2007
Acquisitions
Reliant Pharmaceuticals Inc.
On 18th December 2007, the Group acquired 100% of the issued share capital of Reliant Pharmaceuticals Inc., a pharmaceutical company based in the USA for a cash consideration of £814 million. The company specialises in the development and marketing of speciality medicines to combat heart disease which includes the US rights toLovaza, a treatment for adult patients with very high levels of triglycerides. This transaction has been accounted for by the purchase method of accounting. The goodwill arising on the acquisition reflects the potential for product growth throughout the USA and Puerto Rico and the expected synergies for the Group. Reliant Pharmaceuticals Inc. had a turnover of £276 million and a profit after tax of £8 million for the year, of which £8 million of turnover and £1 million of profit after tax related to the period since acquisition and are included in the Group accounts.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | 13 | | | | 600 | | | | 613 | |
| Property, plant and equipment | | | 2 | | | | 4 | | | | 6 | |
| Other assets including cash and cash equivalents | | | 80 | | | | 16 | | | | 96 | |
| Deferred tax provision | | | – | | | | (175 | ) | | | (175 | ) |
| Other liabilities | | | (75 | ) | | | (1 | ) | | | (76 | ) |
| | | | | | | |
| | | | 20 | | | | 444 | | | | 464 | |
| Goodwill | | | – | | | | 350 | | | | 350 | |
| | | | | | | |
| Total consideration | | | 20 | | | | 794 | | | | 814 | |
| | | | | | | |
GSK Annual Report 2009
150
Notes to the financial statements
38Acquisitions and disposals continued
Domantis Limited
On 5th January 2007, the Group acquired 100% of the issued share capital of Domantis Limited, a drug discovery company based in the UK for a cash consideration of £234 million. The company is developing the next generation of antibody therapies. This transaction has been accounted for by the purchase method of accounting. The goodwill arising on the acquisition reflects the potential for combining the world-leading technology of Domantis with the development programme already in place within GSK to put the Group at the forefront of biotechnology. Domantis Limited had a turnover of £nil and a loss after tax of £10 million for the year, of which £nil of turnover and £9 million of loss after tax related to the period since acquisition and are included in the Group accounts.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | – | | | | 51 | | | | 51 | |
| Property, plant and equipment | | | 1 | | | | – | | | | 1 | |
| Other assets including cash and cash equivalents | | | 19 | | | | – | | | | 19 | |
| Deferred tax provision | | | – | | | | (14 | ) | | | (14 | ) |
| Other liabilities | | | (4 | ) | | | – | | | | (4 | ) |
| | | | | | | |
| | | | 16 | | | | 37 | | | | 53 | |
| Goodwill | | | – | | | | 181 | | | | 181 | |
| | | | | | | |
| Total consideration | | | 16 | | | | 218 | | | | 234 | |
| | | | | | | |
Praecis Pharmaceuticals Inc.
On 16th February 2007, the Group acquired 100% of the issued share capital of Praecis Pharmaceuticals, Inc., a biopharmaceutical company based in the USA, for a cash consideration of £39 million. The company has developed a more efficient method of identifying drug leads targeting human disease using proprietary technology. This transaction has been accounted for by the purchase method of accounting. Praecis Pharmaceuticals Inc. had a turnover of £nil and a loss after tax of £11 million for the year, of which £nil of turnover and £9 million of loss after tax related to the period since acquisition and are included in the Group accounts.
| | | | | | | | | | | | | |
| | | Book | | | Fair value | | | Fair | |
| | | value | | | adjustment | | | value | |
| | | £m | | | £m | | | £m | |
| | | | | | | |
| Net assets acquired | | | | | | | | | | | | |
| Intangible assets | | | – | | | | 7 | | | | 7 | |
| Property, plant and equipment | | | 1 | | | | – | | | | 1 | |
| Other assets including cash and cash equivalents | | | 25 | | | | – | | | | 25 | |
| Deferred tax asset | | | – | | | | 10 | | | | 10 | |
| Other liabilities | | | (6 | ) | | | – | | | | (6 | ) |
| | | | | | | |
| | | | 20 | | | | 17 | | | | 37 | |
| Goodwill | | | – | | | | 2 | | | | 2 | |
| | | | | | | |
| Total consideration | | | 20 | | | | 19 | | | | 39 | |
| | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Cash flows | | Reliant | | | Domantis | | | Praecis | | | Other | | | Total | |
| | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | |
| Cash consideration | | | 814 | | | | 234 | | | | 39 | | | | 1 | | | | 1,088 | |
| Cash and cash equivalents acquired | | | (20 | ) | | | (16 | ) | | | (24 | ) | | | – | | | | (60 | ) |
| | | | | | | | | | | | | | | |
| Net cash payment on acquisitions | | | 794 | | | | 218 | | | | 15 | | | | 1 | | | | 1,028 | |
| | | | | | | | | | | | | | | |
If Reliant, Domantis and Praecis had been acquired at the beginning of the year, combined Group turnover for the year would have been £22,984 million and combined Group profit for the year would have been £5,314 million.
GSK Annual Report 2009
151
Notes to the financial statements
39Commitments
| | | | | | | | | |
| Contractual obligations and commitments | | 2009 | | | 2008 | |
| | £m | | | £m | |
| | | | | | |
| Contracted for but not provided in the financial statements: | | | | | | | | |
| Intangible assets | | | 12,280 | | | | 13,048 | |
| Property, plant and equipment | | | 416 | | | | 489 | |
| Investments | | | 86 | | | | 56 | |
| Purchase commitments | | | 82 | | | | 145 | |
| Business combinations | | | – | | | | 227 | |
| Pensions | | | 1,460 | | | | 597 | |
| Other commitments | | | 52 | | | | 46 | |
| Interest on loans | | | 10,733 | | | | 11,868 | |
| Finance lease charges | | | 16 | | | | 18 | |
| | | | | | |
| | | | 25,125 | | | | 26,494 | |
| | | | | | |
The commitments related to intangible assets include milestone payments, which are dependent on successful clinical development or on meeting specified sales targets, and which represent the maximum that would be paid if all milestones, however unlikely, are achieved. The amounts are not risk-adjusted or discounted. As the majority of the intangible commitments are denominated in US dollars, the weakening of foreign currencies during the year has led to an decrease in the commitments reported above. A number of commitments were made in 2009 under licensing and other agreements, including arrangements with Chroma Therapeutics Limited, Concert Pharmaceuticals, Inc., Idenix Pharmaceuticals, Inc., Prosensa B.V. and Seattle Genetics, Inc.
In 2009, GSK reached an agreement with the trustees of the UK pension schemes to make additional contributions to eliminate the pension deficit identified at the 31st December 2008 actuarial funding valuation. The table above shows this commitment, but excludes the normal ongoing annual funding requirement of approximately £150 million.
The Group also has other commitments which principally relate to revenue payments to be made under licences and other alliances.
Commitments in respect of future interest payable on loans are disclosed before taking into account the effect of interest rate swaps.
| | | | | | | | | |
| Commitments under non-cancellable operating leases | | 2009 | | | 2008 | |
| | £m | | | £m | |
| | | | | | |
| Rental payments due within one year | | | 111 | | | | 140 | |
| Rental payments due between one and two years | | | 72 | | | | 109 | |
| Rental payments due between two and three years | | | 50 | | | | 76 | |
| Rental payments due between three and four years | | | 21 | | | | 54 | |
| Rental payments due between four and five years | | | 14 | | | | 22 | |
| Rental payments due after five years | | | 69 | | | | 47 | |
| | | | | | |
| Total commitments under non-cancellable operating leases | | | 337 | | | | 448 | |
| | | | | | |
40Post balance sheet events
On 17th February 2010, GSK received a Complete Response letter from the FDA regarding the new drug application forHorizantExtended Release tablets for restless legs syndrome. The letter indicated that questions remained that precluded the approval ofHorizantfor restless legs syndrome at that time. GSK is evaluating the letter and considering the appropriate next steps. The Group’s intangible assets include £85 million in relation to this compound. It is not yet possible to determine the amount, if any, of any impairment that may be recorded in future periods, pending completion of a full analysis of the situation.
GSK Annual Report 2009
153
Notes to the financial statements
41Financial instruments and related disclosures continued
Market risk
Interest rate risk management
The policy on interest rate risk management limits the amount of floating interest payments to a prescribed percentage of trading profit.
We use an interest rate swap to redenominate one of our external borrowings into the interest rate coupon required by GSK. The duration of this swap matches the duration of the principal instrument. Interest rate derivative instruments are accounted for as fair value or cash flow hedges of the relevant assets or liabilities.
Foreign exchange risk management
Foreign currency transaction exposures arising on internal and external trade flows are not hedged. The exposure of overseas operating subsidiaries to transaction risk is minimised by matching local currency income with local currency costs. For this purpose, our internal trading transactions are matched centrally and we manage intercompany payment terms to reduce foreign currency risk. Exceptional foreign currency cash flows are hedged selectively under the management of Corporate Treasury. We manage the cash surpluses or borrowing requirements of subsidiary companies centrally using forward contracts to hedge future repayments back into the originating currency.
We seek to denominate borrowings in the currencies of our principal assets and cash flows. These are primarily denominated in US dollars, Euros and Sterling. Certain borrowings are swapped into other currencies as required. Borrowings denominated in, or swapped into, foreign currencies that match investments in overseas Group assets may be treated as a hedge against the relevant assets. Forward contracts are also used in major currencies to reduce our exposure to our investment in overseas Group assets (see ‘Net investment hedges’ section of this note for further details). The TMG reviews the ratio of borrowings to assets for major currencies monthly.
Credit risk
The Group considers its maximum credit risk to be £13,434 million (2008 – £13,265 million) which is the total of the Group’s financial assets with the exception of ’Other investments’ which do not bear credit risk. See page 155 for details on the Group’s total financial assets.
GSK’s greatest concentration of credit risk is £1.3 billion (2008 – £1.9 billion) invested in US Treasury and Treasury repo only money market funds which bear credit exposure to the US Government.
Treasury-related credit risk
In 2009, credit risk remained high during the global credit crisis. GSK has continued to maintain its conservative approach to counterparty risk throughout this period. A report on relationship banks and their credit ratings is presented annually to the TMG for approval.
The aggregate credit risk in respect of financial instruments the Group may have with one counterparty is limited by reference to the long-term credit ratings assigned for that counterparty by Moody’s and Standard and Poor’s. The table below sets out the credit ratings of counterparties for liquid investments, cash and cash equivalents and derivatives. The gross asset position on each derivative contract is considered for the purpose of this table, though, under the ISDA contracts, the amount at risk is the net asset position with each counterparty.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Credit rating of counterparty | | | | |
| 2009 | | Aaa/AAA | | | Aa2/AA | | | Aa3/AA- | | | A1/A+ | | | A2/A | | | Baa2/BBB | | | Baa3/BBB- | | | Total | |
| | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | |
| Bank balances and deposits | | | 793 | | | | 1,385 | | | | 1,359 | | | | 1,467 | | | | 102 | | | | 27 | | | | 73 | | | | 5,206 | |
| US Treasury and Treasury repo only money market funds | | | 1,305 | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | 1,305 | |
| Corporate debt instruments | | | – | | | | – | | | | 10 | | | | – | | | | – | | | | – | | | | – | | | | 10 | |
| Government securities | | | 237 | | | | – | | | | – | | | | 43 | | | | – | | | | – | | | | 12 | | | | 292 | |
| 3rd party financial derivatives | | | – | | | | 48 | | | | 32 | | | | 106 | | | | – | | | | – | | | | – | | | | 186 | |
| | | | |
| Total | | | 2,335 | | | | 1,433 | | | | 1,401 | | | | 1,616 | | | | 102 | | | | 27 | | | | 85 | | | | 6,999 | |
| | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Credit rating of counterparty | | | | |
| 2008 | | Aaa/AAA | | | Aa2/AA | | | Aa3/AA- | | | A1/A+ | | | A2/A | | | Baa2/BBB | | | Baa3/BBB- | | | Total | |
| | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | |
| Bank balances and deposits | | | 64 | | | | 1,025 | | | | 646 | | | | 1,981 | | | | 32 | | | | – | | | | 27 | | | | 3,775 | |
| US Treasury and Treasury repo only money market funds | | | 1,852 | | | | – | | | | – | | | | – | | | | – | | | | – | | | | – | | | | 1,852 | |
| Corporate debt instruments | | | – | | | | – | | | | 75 | | | | – | | | | – | | | | – | | | | – | | | | 75 | |
| Government securities | | | 231 | | | | – | | | | – | | | | 49 | | | | – | | | | – | | | | 32 | | | | 312 | |
| 3rd party financial derivatives | | | – | | | | 160 | | | | 210 | | | | 540 | | | | – | | | | – | | | | – | | | | 910 | |
| | | | |
| Total | | | 2,147 | | | | 1,185 | | | | 931 | | | | 2,570 | | | | 32 | | | | – | | | | 59 | | | | 6,924 | |
| | | | |
The credit ratings in the above tables are as assigned by Moody’s Investor Services and Standard and Poor’s respectively. Where the opinion of the two rating agencies differ, GSK assigns the lower rating of the two to the counterparty. Where local rating agency data is the only source available, the ratings are converted to global ratings equivalent to those of Moody’s Investor Services or Standard and Poor’s using published conversion tables. 2008 figures have been restated to reflect equivalent global or sovereign ratings where appropriate rather than those of local ratings providers.
GSK Annual Report 2009
157
Notes to the financial statements
41Financial instruments and related disclosures continued
Trade and other receivables and other non-current assets in scope of IAS 39
The following table reconciles financial assets within Trade and other receivables and Other non-current assets which fall within the scope of IAS 39 to the relevant balance sheet amounts. The financial assets are predominantly non-interest earning. Other assets include tax receivables, pension surplus balances and prepayments, which are outside the scope of IAS 39.
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
| Trade and other receivables (Note 24) | | | 6,492 | | | | 6,265 | |
| Other non-current assets (Note 22) | | | 583 | | | | 579 | |
| | | | | | |
| | | | 7,075 | | | | 6,844 | |
| | | | |
| | | | | | | | | |
| Analysed as: | | | | | | | | |
| Financial assets in scope of IAS 39 | | | 6,424 | | | | 6,288 | |
| Other assets | | | 651 | | | | 556 | |
| | | | | | |
| | | | 7,075 | | | | 6,844 | |
| | | | | | |
The following table shows the age of such financial assets which are past due and for which no provision for bad or doubtful debts has been made:
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
| Past due by 1–30 days | | | 262 | | | | 310 | |
| Past due by 31–90 days | | | 105 | | | | 154 | |
| Past due by 91–180 days | | | 60 | | | | 115 | |
| Past due by 181–365 days | | | 54 | | | | 89 | |
| Past due by more than 365 days | | | 78 | | | | 117 | |
| | | | | | |
| | | | 559 | | | | 785 | |
| | | | | | |
Amounts past due by greater than 90 days total £192 million (2008 – £321 million). Of this balance £132 million (2008 – £227 million) relates to receivables due from state hospital authorities in certain European countries. Given the profile of our customers, including large wholesalers and government backed agencies, no further credit risk has been identified with the trade receivables not past due other than those balances for which an allowance has been made.
Trade and other payables and other non-current liabilities in scope of IAS 39
The following table reconciles financial liabilities within Trade and other payables and Other non-current liabilities which fall within the scope of IAS 39 to the relevant balance sheet amounts. The financial liabilities are predominantly non-interest bearing. Accrued wages and salaries are included within financial liabilities. Other liabilities include payments on account and tax and social security payables, which are outside the scope of IAS 39.
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | £m | | | £m | |
| | | | | | |
| Trade and other payables (Note 27) | | | (6,772 | ) | | | (6,075 | ) |
| Other non-current liabilities (Note 30) | | | (605 | ) | | | (427 | ) |
| | | | | | |
| | | | (7,377 | ) | | | (6,502 | ) |
| | | | |
| | | | | | | | | |
| Analysed as: | | | | | | | | |
| Financial liabilities in scope of IAS 39 | | | (6,051 | ) | | | (5,452 | ) |
| Other liabilities | | | (1,326 | ) | | | (1,050 | ) |
| | | | | | |
| | | | (7,377 | ) | | | (6,502 | ) |
| | | | | | |
GSK Annual Report 2009
158
Notes to the financial statements
41Financial instruments and related disclosures continued
Debt interest rate repricing table
The following table sets out the exposure of the Group to interest rates on debt before and after the effect of interest rate swaps. The maturity analysis of fixed rate debt is stated by contractual maturity and of floating rate debt by interest rate repricing dates. For the purpose of this table, debt is defined as all classes of borrowings other than obligations under finance leases.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | | | | | Effect of | | | | | | | | | | | Effect of | | | | |
| | | | | | | interest | | | | | | | | | | | interest | | | | |
| | | Debt | | | rate swaps | | | Total | | | Debt | | | rate swaps | | | Total | |
| | | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | | | | |
| Floating and fixed rate debt less than one year | | | (1,431 | ) | | | (990 | ) | | | (2,421 | ) | | | (901 | ) | | | (1,146 | ) | | | (2,047 | ) |
| Between one and two years | | | – | | | | – | | | | – | | | | (703 | ) | | | – | | | | (703 | ) |
| Between two and three years | | | (2,647 | ) | | | – | | | | (2,647 | ) | | | – | | | | – | | | | – | |
| Between three and four years | | | (1,548 | ) | | | – | | | | (1,548 | ) | | | (2,872 | ) | | | – | | | | (2,872 | ) |
| Between four and five years | | | (990 | ) | | | 990 | | | | – | | | | (1,728 | ) | | | – | | | | (1,728 | ) |
| Between five and ten years | | | (4,205 | ) | | | – | | | | (4,205 | ) | | | (4,240 | ) | | | 1,146 | | | | (3,094 | ) |
| Greater than ten years | | | (5,306 | ) | | | – | | | | (5,306 | ) | | | (5,597 | ) | | | – | | | | (5,597 | ) |
| | | | | | | | | | | | | | | | | | |
| Total | | | (16,127 | ) | | | – | | | | (16,127 | ) | | | (16,041 | ) | | | – | | | | (16,041 | ) |
| | | | | | | | | | | | | | | | |
| Original issuance profile: | | | | | | | | | | | | | | | | | | | | | | | | |
| Fixed rate interest | | | (14,696 | ) | | | 990 | | | | (13,706 | ) | | | (14,922 | ) | | | 1,146 | | | | (13,776 | ) |
| Floating rate interest | | | (1,430 | ) | | | (990 | ) | | | (2,420 | ) | | | (1,119 | ) | | | (1,146 | ) | | | (2,265 | ) |
| | | | | | | | | | | | | | | | | | |
| Total interest bearing | | | (16,126 | ) | | | – | | | | (16,126 | ) | | | (16,041 | ) | | | – | | | | (16,041 | ) |
| Non-interest bearing | | | (1 | ) | | | – | | | | (1 | ) | | | (10 | ) | | | – | | | | (10 | ) |
| | | | | | | | | | | | | | | | | | |
| | | | (16,127 | ) | | | – | | | | (16,127 | ) | | | (16,051 | ) | | | – | | | | (16,051 | ) |
| | | | | | | | | | | | | | | | | | |
Sensitivity analysis
The sensitivity analysis has been prepared on the assumption that the amount of net debt, the ratio of fixed to floating interest rates of the debt and derivatives portfolio and the proportion of financial instruments in foreign currencies are all constant and on the basis of the hedge designations in place at 31st December.
Financial instruments affected by market risk include borrowings, deposits and derivative financial instruments. The following analyses are intended to illustrate the sensitivity of such financial instruments to changes in relevant foreign exchange and interest rates.
Foreign exchange sensitivity
The table below shows the Group’s sensitivity to foreign exchange rates on its US dollar, Euro and Yen financial instruments excluding obligations under finance leases and certain non-derivative financial instruments not in net debt and which do not present a material exposure. These three currencies are the major foreign currencies in which GSK’s financial instruments are denominated. GSK has considered movements in these currencies over the last three years and has concluded that a 20% movement in rates is a reasonable benchmark. In this table, financial instruments are only considered sensitive to foreign exchange rates where they are not in the functional currency of the entity that holds them. Intercompany loans which are fully hedged to maturity with a currency swap have been excluded from this analysis.
| | | | | | | | | | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | Increase/(decrease) | | | Reduction | | | Increase/(decrease) | | | Reduction | |
| | | in income | | | in equity | | | in income | | | in equity | |
| | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | |
| 20% appreciation of the US dollar | | | 251 | | | | 755 | | | | 210 | | | | 991 | |
| 20% appreciation of the Euro | | | 8 | | | | 1,779 | | | | (20 | ) | | | 1,760 | |
| 20% appreciation of the Yen | | | – | | | | 45 | | | | 1 | | | | 52 | |
| | | | | | | | | | | | |
A 20% depreciation of the stated currencies would have an equal and opposite effect. The movements in the income statement relate primarily to hedging instruments for US dollar legal provisions, and to trade payables and trade receivables. Whilst the hedging instruments provide economic hedges, the related provisions are not financial instruments and therefore are not included in the table above. The combined sensitivity of these hedging instruments and the provisions would be insignificant if the provisions were included. The movements in equity relate to foreign exchange positions used to hedge Group assets denominated in US dollar, Euro and Yen. Therefore, a depreciation on the currency swap would give rise to a corresponding appreciation on the Group asset. Foreign exchange sensitivity on Group assets other than financial instruments is not included above.
GSK Annual Report 2009
159
Notes to the financial statements
41 Financial instruments and related disclosures continued
Interest rate sensitivity
The table below shows the Group’s sensitivity to interest rates on its floating rate Sterling, US dollar and Euro financial instruments, being the currencies in which GSK has historically issued debt and held investments. GSK has considered movements in these interest rates over the last three years and has concluded that a 2% increase is a reasonable benchmark. Debt with a maturity of less than one year is floating rate for this calculation. A 2% movement in interest rates is not deemed to have a material effect on equity.
| | | | | | | | | |
| | | 2009 | | | 2008 | |
| | | Increase/(decrease) | | | Increase/(decrease) | |
| | | in income | | | in income | |
| | | £m | | | £m | |
| | | | |
| 2% increase in Sterling interest rates | | | (2 | ) | | | 16 | |
| 2% increase in US dollar interest rates | | | 38 | | | | 13 | |
| 2% increase in Euro interest rates | | | 18 | | | | 4 | |
| | | | | | |
These interest rates could not be decreased by 2% as they are currently less than 1.0%. The maximum increase/(decrease) in income would therefore be limited to £1 million, (£4 million) and (£2 million) for Sterling, US Dollar and Euro interest rates respectively (2008 – (£16 million), (£1 million) and (£4 million)). Interest rate movements on obligations under finance leases, foreign currency derivatives, trade payables, trade receivables and other financial instruments not in net debt do not present a material exposure to the Group’s balance sheet based on a 2% increase or decrease in these interest rates.
Contractual cash flows for non-derivative financial liabilities and derivative instruments
The following is an analysis of the anticipated contractual cash flows including interest payable for the Group’s non-derivative financial liabilities on an undiscounted basis. For the purpose of this table, debt is defined as all classes of borrowings except for obligations under finance leases. Interest is calculated based on debt held at 31st December without taking account of future issuance. Floating rate interest is estimated using the prevailing interest rate at the balance sheet date. Cash flows in foreign currencies are translated using spot rates at 31st December.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Finance charge | | | Trade and | | | | |
| | | | | | | | | | | Obligations | | | on obligations | | | other | | | | |
| | | | | | | Interest on | | | under finance | | | under finance | | | payables not | | | | |
| At 31st December 2009 | | Debt | | | debt | | | leases | | | leases | | | in net debt | | | Total | |
| | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | | |
| Due in less than one year | | | (1,431 | ) | | | (757 | ) | | | (40 | ) | | | (4 | ) | | | (5,828 | ) | | | (8,060 | ) |
| Between one and two years | | | – | | | | (753 | ) | | | (32 | ) | | | (6 | ) | | | (161 | ) | | | (952 | ) |
| Between two and three years | | | (2,655 | ) | | | (754 | ) | | | (24 | ) | | | (2 | ) | | | (28 | ) | | | (3,463 | ) |
| Between three and four years | | | (1,553 | ) | | | (594 | ) | | | (14 | ) | | | (2 | ) | | | (14 | ) | | | (2,177 | ) |
| Between four and five years | | | (932 | ) | | | (536 | ) | | | (5 | ) | | | (1 | ) | | | (5 | ) | | | (1,479 | ) |
| Between five and ten years | | | (4,230 | ) | | | (2,088 | ) | | | (15 | ) | | | (1 | ) | | | (15 | ) | | | (6,349 | ) |
| Greater than ten years | | | (5,382 | ) | | | (5,251 | ) | | | – | | | | – | | | | – | | | | (10,633 | ) |
| | | | | | | | | | | | | | | | |
| Gross contractual cash flows | | | (16,183 | ) | | | (10,733 | ) | | | (130 | ) | | | (16 | ) | | | (6,051 | ) | | | (33,113 | ) |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Finance charge | | | Trade and | | | | |
| | | | | | | | | | | Obligations | | | on obligations | | | other | | | | |
| | | | | | | Interest on | | | under finance | | | under finance | | | payables not | | | | |
| At 31st December 2008 | | Debt | | | debt | | | leases | | | leases | | | in net debt | | | Total | |
| | £m | | | £m | | | £m | | | £m | | | £m | | | £m | |
| | | | | | | | | | | | | | | | |
| Due in less than one year | | | (907 | ) | | | (790 | ) | | | (48 | ) | | | (5 | ) | | | (5,246 | ) | | | (6,996 | ) |
| Between one and two years | | | (704 | ) | | | (767 | ) | | | (35 | ) | | | (4 | ) | | | (68 | ) | | | (1,578 | ) |
| Between two and three years | | | – | | | | (757 | ) | | | (27 | ) | | | (3 | ) | | | (25 | ) | | | (812 | ) |
| Between three and four years | | | (2,885 | ) | | | (757 | ) | | | (14 | ) | | | (2 | ) | | | (32 | ) | | | (3,690 | ) |
| Between four and five years | | | (1,736 | ) | | | (582 | ) | | | (4 | ) | | | (2 | ) | | | (5 | ) | | | (2,329 | ) |
| Between five and ten years | | | (4,156 | ) | | | (2,373 | ) | | | (8 | ) | | | (2 | ) | | | (76 | ) | | | (6,615 | ) |
| Greater than ten years | | | (5,678 | ) | | | (5,850 | ) | | | – | | | | – | | | | – | | | | (11,528 | ) |
| | | | | | | | | | | | | | | | |
| Gross contractual cash flows | | | (16,066 | ) | | | (11,876 | ) | | | (136 | ) | | | (18 | ) | | | (5,452 | ) | | | (33,548 | ) |
| | | | | | | | | | | | | | | | | | |
GSK Annual Report 2009
164
Notes to the financial statements
42 Employee share schemescontinued
GlaxoSmithKline share award schemes
Performance Share Plan
The Group operates a Performance Share Plan whereby awards are granted to Directors and senior executives at no cost. The percentage of each award that vests is based upon the performance of the Group over a three year measurement period. Awards granted to Directors and members of the CET prior to 2009 are subject to a single performance condition which compares GSK’s TSR over the period with the TSR of companies in the comparator group over the same period. For awards granted from 2009 onwards to Directors and members of the CET, 40% of the award will be based on the achievement of adjusted free cash flow targets over a three year measurement period. The remaining 60% of the award will be based on relative TSR performance against a comparator group as described on page 78. Half of the TSR element of each award will be measured over three years and half over four years.
For those awards made to all other eligible employees prior to 2009 the performance conditions consist of two parts, each of which applies to 50% of the award. The first part of the performance condition compares GSK’s EPS growth to the increase in the UK Retail Prices Index over the three year measurement period. The second part of the performance condition compares GSK’s TSR over the period with the TSR of companies in the comparator group over the same period. For awards granted from 2009 onwards, the first part of the performance condition continues to be based on EPS. The second part of the performance condition is based on strategic or operational business measures, over a three year measurement period, specific to the employee’s business area.
| | | | | | | | | | | | | | | | | |
| Number of shares and ADS issuable | | Shares | | | Weighted | | | ADS | | | Weighted | |
| | Number (000) | | | fair value | | | Number (000) | | | fair value | |
| At 1st January 2007 | 4,756 | | | | | | | | 4,034 | | | | |
| Awards granted | | | 2,071 | | | | £10.26 | | | | 1,501 | | | | $34.87 | |
| Awards exercised | | | (147 | ) | | | | | | | (77 | ) | | | | |
| Awards cancelled | | | (949 | ) | | | | | | | (1,131 | ) | | | | |
| | | | | | | | | | |
| At 31st December 2007 | | | 5,731 | | | | | | | | 4,327 | | | | | |
| Awards granted | | | 2,834 | | | | £7.77 | | | | 1,467 | | | | $27.99 | |
| Awards exercised | | | (1,519 | ) | | | | | | | (1,516 | ) | | | | |
| Awards cancelled | | | (511 | ) | | | | | | | (420 | ) | | | | |
| | | | | | | | | | |
| At 31st December 2008 | | | 6,535 | | | | | | | | 3,858 | | | | | |
| Awards granted | | | 3,365 | | | | £8.80 | | | | 1,392 | | | | $29.45 | |
| Awards exercised | | | (1,270 | ) | | | | | | | (21 | ) | | | | |
| Awards cancelled | | | (1,024 | ) | | | | | | | (1,497 | ) | | | | |
| | | | | | | | | | |
| At 31st December 2009 | | | 7,606 | | | | | | | | 3,732 | | | | | |
| | | | | | | | | | |
Share Value Plan
The Group operates a Share Value Plan whereby awards are granted, in the form of shares, to certain employees at no cost. The awards vest after three years. There are no performance criteria attached.
| | | | | | | | | | | | | | | | | |
| | | Shares | | | Weighted | | | ADS | | | Weighted | |
| | | Number (000) | | | fair value | | | Number (000) | | | fair value | |
| | | | | | | | | | |
| At 1st January 2007 | | | 8,794 | | | | | | | | 7,629 | | | | | |
| Awards granted | | | 5,155 | | | | £13.22 | | | | 4,231 | | | | $52.08 | |
| Awards exercised | | | (3,643 | ) | | | | | | | (3,038 | ) | | | | |
| Awards cancelled | | | (672 | ) | | | | | | | (539 | ) | | | | |
| | | | | | | | | | |
| At 31st December 2007 | | | 9,634 | | | | | | | | 8,283 | | | | | |
| Awards granted | | | 5,572 | | | | £9.85 | | | | 4,640 | | | | $36.46 | |
| Awards exercised | | | (926 | ) | | | | | | | (931 | ) | | | | |
| Awards cancelled | | | (592 | ) | | | | | | | (630 | ) | | | | |
| | | | | | | | | | |
| At 31st December 2008 | | | 13,688 | | | | | | | | 11,362 | | | | | |
| Awards granted | | | 5,572 | | | | £9.86 | | | | 4,291 | | | | $30.53 | |
| Awards exercised | | | (4,345 | ) | | | | | | | (3,783 | ) | | | | |
| Awards cancelled | | | (680 | ) | | | | | | | (561 | ) | | | | |
| | | | | | | | | | |
| At 31st December 2009 | | | 14,235 | | | | | | | | 11,309 | | | | | |
| | | | | | | | | | |
GSK Annual Report 2009
208
American Depositary Shares
Fees and charges payable by ADR holders
The Bank of New York Mellon serves as the depositary (the ‘Depositary’) for GlaxoSmithKline plc’s American Depositary Receipt (‘ADR’) programme. Pursuant to the deposit agreement between GSK, the Depositary and owners and holders of ADR (the ‘Deposit Agreement’), ADR holders may be required to pay various fees to the Depositary, and the Depositary may refuse to provide any service for which a fee is assessed until the applicable fee has been paid. In particular, the Depositary, under the terms of the Deposit Agreement, shall charge a fee of $0.05 or less per ADR (or portion thereof) for (i) the issuance, execution and delivery of ADRs or (ii) the withdrawal of shares underlying the ADRs. In addition, ADR holders may be required under the Deposit Agreement to pay the Depositary (i) any tax, duty, governmental charge or fee or stock transfer or registration fee arising in connection with the foregoing transactions or otherwise, (ii) any expense resulting from the conversion of a foreign currency into U.S. dollars and (iii) the expense of certain communications made, at the request of the ADR holder, by cable, telex or facsimile. The Depositary may (i) withhold dividends or other distributions or sell any or all of the shares underlying the ADRs in order to satisfy any tax or governmental charge and (ii) deduct from any cash distribution any tax payable thereon or the cost of any currency conversion.
Direct and indirect payments by the Depositary
The Depositary reimburses GSK for certain expenses it incurs in connection with the ADR programme, subject to a ceiling agreed between GSK and the Depositary from time to time. The Depositary has also agreed to waive certain standard fees associated with the administration of the programme.
The table below sets forth the amount of such payments received and claimed but not yet received in respect of the year ended 31st December 2009 as well as such payments received during 2009 in respect of the year ended 31st December 2008. GSK was also reimbursed £35,100 in 2009 for legal fees claimed with respect to 2007.
| | | | | | | | | | | | | |
| | | Received in respect | | | Received in respect | | | Claimed in respect of 2009 | |
| Direct and indirect payments by the Depositary | | of 2008 | | | of 2009 | | | but not yet received | |
| | | | | | | | | |
| Reimbursement of NYSE listing fees | | | – | | | | $387,787 | | | | – | |
| Reimbursement of legal fees claimed in US dollars | | | $162,284 | | | | – | | | | $333,735 | |
| Reimbursement of legal fees claimed in Sterling | | | £30,661 | | | | £34,173 | | | | £9,782 | |
| Reimbursement of PCAOB fees | | | – | | | | $161,700 | | | | – | |
Reimbursement of Annual Report production costs1 | | | – | | | | £10,000 | | | | £290,347 | |
Reimbursement of Investor Relations expenses2 | | | $232,118 | | | | $321,108 | | | | $108,078 | |
| Distribution of annual general meeting materials | | | – | | | | $409,114 | | | | – | |
| Tabulation of voting instructions cards | | | – | | | | $40,202 | | | | – | |
Reimbursement of other programme-related expenditures
claimed in US dollars | | | – | | | | – | | | | $16,050 | |
Reimbursement of other programme-related expenditures
claimed in Sterling | | | – | | | | £22,500 | | | | £9,780 | |
| | | | | | | | | |
| | |
| 1 | | Annual report production costs include SEC filing fees. |
| |
| 2 | | Investor relations expenses include travel expenses, fees of investor relations consultants, expenses involved in arranging investor relations meetings and telephone expenses. |
GSK Annual Report 2009