Exhibit 99.1
Press Release | Source: Hana Biosciences |
HANA BIOSCIENCES ANNOUNCES SECOND QUARTER 2006 RESULTS
South San Francisco, CA (August 14, 2006) - Hana Biosciences (NASDAQ: HNAB), a biopharmaceutical company focused on advancing cancer care, announced today financial results for the second quarter of 2006.
| · | Non-GAAP net loss of $6.5 million for the three months ended June 30, 2006 compared to $2.1 million for the three months ended June 30, 2005; GAAP net loss was $20.7 million for the three months ended June 30, 2006, which included an $11.9 million one-time charge related to the company’s acquisition of product candidates from Inex Pharmaceuticals Corp. and a $2.3 million charge related to FAS 123R employee stock-based compensation expense.1,2 |
| · | Non-GAAP net loss of $9.7 million for the six months ended June 30, 2006 compared to $4.5 million for the six months ended June 30, 2005; GAAP net loss was $24.1 million for the six months ended June 30, 2006, which included an $11.9 million one-time charge related to the acquisition of product candidates from Inex and a $2.5 million charge related to FAS 123R employee stock-based compensation expense.1,2 |
| · | Cash used in operations was $5.4 million in the second quarter of 2006. Hana ended the quarter with approximately $46.6 million in cash and cash equivalents and short-term investments, compared to $17.6 million at December 31, 2005. |
“In the second quarter of 2006, we achieved a significant number of clinical, regulatory and business development milestones,” said Mark J. Ahn, Ph.D., President and CEO of Hana. “We are pleased to have completed and submitted our NDA for Zensana™ as we believe this product has the potential to offer a convenient and attractive alternative to those patients suffering from chemotherapy, radiotherapy, and post-operative induced nausea and vomiting. In addition we doubled the size of our product pipeline during the quarter as a result of our transaction with Inex, while advancing several clinical-stage programs on schedule with corporate timelines.
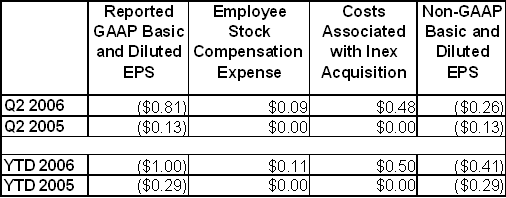
A reconciliation2 between non-GAAP and GAAP earnings per share for the second quarters of 2006 and 2005 is provided in the following table:
Note: Amounts may not sum due to rounding.
Key Achievements in Second Quarter 2006
| | · | Announced the submission of a New Drug Application (NDA) for Zensana (ondansetron HCl) Oral Spray to the U.S. Food and Drug Administration (FDA) seeking marketing approval for the prevention of nausea and vomiting associated with chemotherapy, radiotherapy and post-operative induced nausea and vomiting. If approved, Hana targets commercial launch of Zensana in the United States in the first half of 2007. |
| | · | Initiated a multicenter, multinational Phase II clinical trial with Talvesta™ (talotrexin) in relapsed or refractory non-small cell lung cancer (NSCLC). |
| | · | Completed licensing of three additional targeted cancer drug candidates from Inex Pharmaceuticals. |
| | · | Presented Phase I clinical results in solid tumors and NSCLC with Talvesta at the 2006 American Society of Clinical Oncology (ASCO) Annual Meeting. |
| | · | Presented pivotal trials confirming Zensana™ (ondansetron HCl) Oral Spray 8 mg dose is statistically bioequivalent to the current commercially available 8 mg ondansetron tablet at the ASCO Annual Meeting. |
| | · | Presented new survey results at the ASCO Annual Meeting which indicated medical oncologists, radiation oncologists and oncology nurses surveyed believe Zensana may be an attractive formulation for patients with chemotherapy and radiotherapy induced nausea and vomiting. |
| | · | Announced that the FDA granted orphan drug designation for Talvesta in patients with acute lymphoblastic leukemia (ALL). |
| | · | Announced that the FDA granted orphan drug designation for Ropidoxuridine (IPdR) for the treatment of malignant glioma, which makes up approximately half of all primary brain tumors. |
| | · | Announced that Hana joined the Russell 3000® Index and the Russell Microcap™ Index on June 30, 2006. |
| | · | On April 17, 2006 Hana’s common shares began trading on the NASDAQ Global Market (formerly the NASDAQ National Market) under the symbol HNAB. |
Hana Biosciences’ management will host a live webcast to include a discussion of earnings and a business update at 5:30 am PDT, 8:30 am EDT. Those interested in hearing management’s discussion may join the call by dialing (866) 585-6398 in the US/Canada. International participants may access the call by dialing (416) 849-9626. Participants may also access a live webcast of the conference call on Hana’s website at www.hanabiosciences.com.
An audio replay will be available until August 21, 2006 following the call by dialing (888) 566-0813 US/Canada and (402) 220-2507 for International participants. Enter #4111557 when prompted for the playback access code. The webcast will be available via the company’s website until August 28, 2006.
Reconciliation of GAAP to Non-GAAP Net Loss:
GAAP refers to generally accepted accounting principles in the United States of America. Hana’s non-GAAP net loss and loss per share excludes the impact of the one-time upfront fees paid to Inex Pharmaceuticals Corp. and transaction fees paid as part of its acquisition and stock option expense related to implementation of FAS 123R. We are reporting non-GAAP results in addition to, and not as a substitute for, financial measures calculated in accordance with GAAP. The Company provides these non-GAAP numbers to facilitate a comparison of our business from period to period and to allow investors to analyze our business results using the same measures our management uses to evaluate our operating performance. We encourage investors to carefully consider our results under GAAP, as well as our non-GAAP reconciliation between these presentations to more fully understand our business.
1 The company adopted Statement of Financial Accounting Standards No. 123R (or FAS 123R) on a modified prospective basis beginning January 1, 2006. As a result, no employee stock-based compensation expense using FAS 123R has been recognized in GAAP-reported amounts in any prior period.
2 Hana’s non-GAAP net loss and non-GAAP loss per share excludes the impact of the one-time upfront fees paid to Inex Pharmaceuticals and transaction fees paid as part of its acquisition of three product candidates. The differences in non-GAAP and GAAP numbers are reconciled in the accompanying tables.
About Hana Biosciences, Inc.
Hana Biosciences, Inc. (NASDAQ: HNAB) is a South San Francisco, CA-based biopharmaceutical company that acquires, develops, and commercializes innovative products to advance cancer care. The company is committed to creating value by building a world-class team, accelerating the development of lead product candidates, expanding its pipeline by being the alliance partner of choice, and nurturing a unique company culture. Additional information on Hana Biosciences can be found at www.hanabiosciences.com.
***
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements involve risks and uncertainties that could cause Hana's actual results to differ materially from the anticipated results and expectations expressed in these forward-looking statements. These statements are based on current expectations, forecasts and assumptions that are subject to risks and uncertainties, which could cause actual outcomes and results to differ materially from these statements. Among other things, there can be no assurances that any of Hana's development efforts relating to its product candidates will be successful. Other risks that may affect forward-looking information contained in this press release include the possibility of being unable to obtain regulatory approval of Hana's product candidates, the risk that the results of clinical trials may not support Hana's claims, Hana's reliance on third-party researchers to develop its product candidates, and its lack of experience in developing pharmaceutical products. Additional risks are described in the company's most recently filed Form 10-K and Form 10-Q. Hana assumes no obligation to update these statements, except as required by law.
Contact:
Hana Biosciences
Remy Bernarda, 650-288-2769
Associate Director, Investor Relations
email: investor.relations@hanabiosciences.com
fax: 650-588-2787
HANA BIOSCIENCES, INC.
(A DEVELOPMENT STAGE COMPANY)
CONDENSED BALANCE SHEETS
| | | June 30, | | December 31, | |
| | | 2006 | | 2005 | |
ASSETS | | (Unaudited) | | | | |
| Current assets: | | | | | |
| Cash and cash equivalents | | $ | 44,266,298 | | $ | 17,082,521 | |
| Short term investments | | | 2,332,051 | | | 472,000 | |
| Prepaid expenses and other current assets | | | 69,744 | | | 74,729 | |
| Total current assets | | | 46,668,093 | | | 17,629,250 | |
| | | | | | | | |
| Property and equipment, net | | | 323,899 | | | 76,496 | |
| Other assets | | | -- | | | 20,453 | |
| Restricted cash | | | 125,166 | | | -- | |
| Total assets | | $ | 47,117,158 | | $ | 17,726,199 | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | |
| Current liabilities: | | | | | | | |
| Accounts payable | | $ | 1,091,750 | | $ | 671,491 | |
| Accrued expenses | | | 2,742,488 | | | 865,135 | |
| Total liabilities | | | 3,834,238 | | | 1,536,626 | |
| | | | | | | | |
| Commitments and contingencies | | | | | | | |
| | | | | | | | |
| Stockholders' equity: | | | | | | | |
| | | | | | | | |
| Common stock to be issued | | | 185,841 | | | -- | |
Common stock; $0.001 par value 100,000,000 shares authorized; 28,742,784 and 22,348,655 shares issued and outstanding at June 30, 2006 and December 31, 2005, respectively | | | 28,743 | | | 22,349 | |
| Additional paid-in capital | | | 85,372,186 | | | 34,400,345 | |
| Accumulated other comprehensive loss | | | (96,000 | ) | | (164,000 | ) |
| Deficit accumulated during the development stage | | | (42,207,850 | ) | | (18,069,121 | ) |
| Total stockholders' equity | | | 43,282,920 | | | 16,189,573 | |
| Total liabilities and stockholders' equity | | $ | 47,117,158 | | $ | 17,726,199 | |
HANA BIOSCIENCES, INC.
(A DEVELOPMENT STAGE COMPANY)
CONDENSED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Unaudited)
| | | | | | | Cumulative Period from December 6, 2002 (date of inception) | |
| | | Three Months Ended June 30, | | Six Months Ended June 30, | | to June 30, | |
| | 2006 | | 2005 | | 2006 | | 2005 | | 2006 | |
| Operating expenses: | | | | | | | | | | | |
| General and administrative | | $ | 2,576,308 | | $ | 963,270 | | $ | 3,560,283 | | $ | 1,670,483 | | $ | 10,393,865 | |
| Research and development | | | 18,368,374 | | | 1,150,447 | | | 20,946,506 | | | 2,872,172 | | | 32,360,602 | |
| | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 20,944,682 | | | 2,113,717 | | | 24,506,789 | | | 4,542,655 | | | 42,754,467 | |
| | | | | | | | | | | | | | | | | |
| Loss from operations | | | (20,944,682 | ) | | (2,113,717 | ) | | (24,506,789 | ) | | (4,542,655 | ) | | (42,754,467 | ) |
| | | | | | | | | | | | | | | | | |
| Other income (expense): | | | | | | | | | | | | | | | | |
| Interest income, net | | | 249,426 | | | 30,495 | | | 384,752 | | | 50,062 | | | 583,533 | |
| Other expense, net | | | (8,800 | ) | | (2,633 | ) | | (16,692 | ) | | (10,137 | ) | | (36,916 | ) |
| Total other income (expense) | | | 240,626 | | | 27,862 | | | 368,060 | | | 39,925 | | | 546,617 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net loss | | $ | (20,704,056 | ) | $ | (2,085,855 | ) | $ | (24,138,729 | ) | $ | (4,502,730 | ) | $ | (42,207,850 | ) |
| Net loss per share, basic and diluted | | $ | (0.81 | ) | $ | (0.13 | ) | $ | (1.00 | ) | $ | (0.29 | ) | | | |
| | | | | | | | | | | | | | | | | |
Shares used in computing net loss per share, basic and diluted | | | 25,640,398 | | | | | | 24,037,103 | | | 15,402,248 | | | | |
| | | | | | | | | | | | | | | | | |
| Comprehensive loss: | | | | | | | | | | | | | | | | |
| Net loss | | $ | (20,704,056 | ) | $ | (2,085,855 | ) | $ | (24,138,729 | ) | $ | (4,502,730 | ) | | | |
| Unrealized gain (loss) | | | (156,000 | ) | | - | | | 68,000 | | | - | | | | |
| | | | | | | | | | | | | | | | | |
| Comprehensive loss | | $ | (20,860,056 | ) | $ | (2,085,855 | ) | $ | (24,070,729 | ) | $ | (4,502,730 | ) | | | |