MGL-3196
(resmetirom) NASH Phase 2 and 3 Clinical Studies
In October 2016, we initiated a Phase 2 proof of concept clinical trial in patients with liver biopsy documented NASH, including those with
type-2
diabetes, dyslipidemia and hypertension. In the study we randomized 125 NASH patients 2:1, resmetirom or placebo QD in a double-blind, placebo-controlled, study of once-daily resmetirom versus placebo in patients with NASH, including those with
type-2
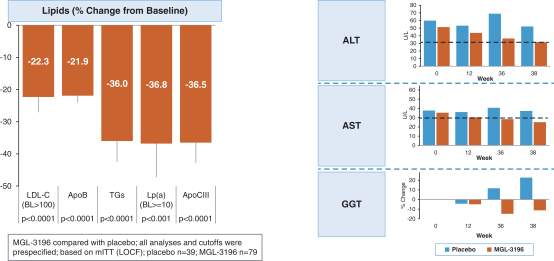
diabetes. Patients continued treatment through 36 weeks. The study was conducted in the United States. The primary endpoint was to evaluate the efficacy of resmetirom as measured by the reduction of liver fat at 12 weeks, and the secondary endpoint was to evaluate the efficacy of resmetirom as measured by a reduction of NASH, which was assessed by liver biopsy, at 36 weeks. Other secondary and exploratory endpoints included safety and tolerability, and effects on serum biomarkers at 12 and 36 weeks, lipid parameters, and biomarker measures of insulin sensitivity. We reached our
top-line
analysis of the primary endpoint in December 2017, and we reached our
top-line
analysis of the secondary endpoint (NASH assessment on liver biopsy) in May 2018. There was an extension study in a subset of the patients that completed the main
36-week
study which was completed in 2019.
In September 2013, the American Association for the Study of Liver Disease and the FDA conducted a joint workshop focused on trial designs and endpoints in drug and diagnostic development for liver disease secondary to NAFLD, including NASH. In December 2014, the journal Hepatology accepted for publication a manuscript summarizing the workshop output, including potentially acceptable surrogate endpoints for clinical studies supporting the approval of agents for NASH and liver fibrosis. We believe that our Phase 2 NASH study design incorporated surrogate secondary endpoints consistent with the current FDA requirements for demonstration of efficacy in registrational trials. Following completion of our Phase 2 clinical trial of resmetirom in NASH patients, we held an end of Phase 2 meeting with the FDA, received positive feedback.
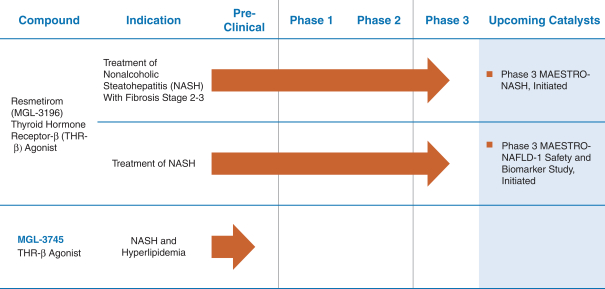
In March 2019 the Company announced that it had initiated a Phase 3 trial in NASH with its once daily, oral thyroid hormone receptor beta selective agonist,
MGL-3196
(resmetirom). This double-blind, placebo-controlled study will be conducted at more than 150 sites in the United States and the rest of the world. Patients with liver biopsy confirmed NASH with stage 2 or 3 fibrosis will be randomized 1:1:1 to receive a single oral daily dose of placebo, resmetirom 80 mg or resmetirom 100 mg. A second liver biopsy at week 52 in the first 900 patients will be the basis of filing for subpart
H-accelerated
approval; the primary endpoint will be the percent of patients treated with either dose of resmetirom as compared with placebo who achieve NASH resolution on the week 52 liver biopsy, defined as the absence of hepatocyte ballooning (score=0), and minimal lobular inflammation (score
0-1),
associated with at least a 2 point reduction in NAS, and no worsening of fibrosis stage. Two key secondary endpoints are reduction in
LDL-cholesterol
and a
1-point
or more improvement in fibrosis stage on the week 52 biopsy with no worsening of NASH. Patients will continue in the study for a total of approximately 54 months and will be evaluated for a composite clinical outcome including cirrhosis on liver biopsy, or a liver related event such a hepatic decompensation. The total anticipated enrollment is approximately 2,000 patients and will include up to 15% high risk F1 fibrosis stage NASH patients whose efficacy responses will be evaluated as exploratory endpoints.
In December 2019 the Company announced it had opened for enrollment
MAESTRO-NAFLD-1,
a
52-week,
double-blind, placebo controlled Phase 3 clinical study in 700 patients with biopsy-confirmed or presumed NASH recruited from sites in the U.S. Key endpoints are safety, including safety biomarkers, LDL cholesterol, lipid biomarkers, and fibrosis biomarkers. Except for serial liver biopsies, the study protocol is similar to the MAESTRO-NASH study with resmetirom doses of 80 mg or 100 mg or placebo and includes key secondary lipid,
MRI-PDFF
and NASH biomarker endpoints. In addition,
MAESTRO-NAFLD-1
includes an open label arm in which up to 100 patients will be dosed with 100 mg resmetirom. The MAESTRO
-NAFLD-1
study will help support the adequacy of the safety database at the time of NDA submission for subpart H approval for treatment of NASH in patients with F2 or F3 fibrosis (MAESTRO-NASH, NASH resolution surrogate endpoint).
Madrigal has agreement on liver biopsy endpoints with the FDA, and long term liver related benefit endpoints for the treatment of NASH. Given that NASH is an evolving field, and FDA evaluates risk versus