|
Exhibit 99.1 
|
GI Dynamics, Inc.
Q2 16 Quarterly Shareholder Brief
8/11/16
Forward-Looking Statements
Currency References
Financial amounts in this presentation are expressed in US Dollars, except where specifically noted.
Forward-Looking Statements
This presentation contains forward-looking statements concerning: our development and commercialization plans; our potential revenues and revenue growth, costs, excess inventory, profitability and financial performance; our ability to obtain reimbursement for our products; our clinical trials, and associated regulatory submissions and approvals; the number and location of commercial centers offering the EndoBarrier®; and our intellectual property position. These forward-looking statements are based on the current estimates and expectations of future events by the management of GI Dynamics, Inc. as of the date of this presentation and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those indicated in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: risks associated with the consequences of terminating the ENDO Trial and the possibility that future clinical trials will not be successful or confirm earlier results; risks associated with obtaining funding from third parties; risks relating to the timing and costs of clinical trials, the timing of regulatory submissions, the timing, receipt and maintenance of regulatory approvals, the timing and amount of other expenses, and the timing and extent of third-party reimbursement; risks associated with commercial product sales, including product performance; competition; risks related to market acceptance of products; intellectual property risks; risks related to excess inventory; risks related to assumptions regarding the size of the available market, benefits of our products, product pricing, timing of product launches, future financial results and other factors including those described in our filings with the U.S. Securities and Exchange Commission. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We do not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law.
Disclaimer
This presentation and any supplemental materials have been prepared by GI Dynamics, Inc. based on available information. The information contained in this presentation is an overview and does not contain all information necessary to make an investment decision. Although reasonable care has been taken to ensure the facts stated in this presentation are accurate and that the opinions expressed are fair and reasonable, no representation or warranty, express or implied, is made as to the fairness, accuracy, completeness, or correctness of such information and opinions and no reliance should be placed on such information or opinions. To the maximum extent permitted by law, none of GI Dynamics,
Inc., or any of its members, directors, officers, employees, or agents or advisors, nor any other person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise arising in connection with it, including, without limitation, any liability arising from fault or negligence on the part of GI Dynamics, Inc. or any of its directors, officers, employees or agents.
8/11/2016 GI Dynamics, Inc. 2
Conference Call Agenda
Q2 ‘16 results
Cash flow and cash runway update
EndoBarrier Overview
T2D Treatment Options
Safety profile update
Clinical data
Regulatory progress
Update on corporate priorities
Looking ahead
8/11/2016 GI Dynamics, Inc. 3
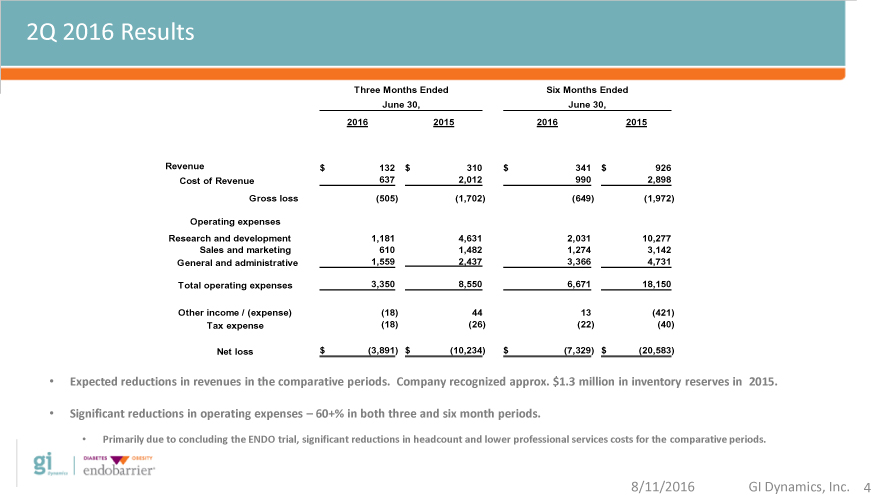
2Q 2016 Results
Three Months Ended Six Months Ended
June 30, June 30,
2Q 2016 2016 2015 2016 2015
Revenue $ 132 $ 310 $ 341 $ 926
Cost of Revenue 637 2,012 990 2,898
Gross loss(505)(1,702)(649)(1,972)
Operating expenses
Research and development 1,181 4,631 2,031 10,277
Sales and marketing 610 1,482 1,274 3,142
General and administrative 1,559 2,437 3,366 4,731
Total operating expenses 3,350 8,550 6,671 18,150
Other income / (expense)(18) 44 13(421)
Tax expense(18)(26)(22)(40)
Net loss $(3,891) $(10,234) $(7,329) $(20,583)
Expected reductions in revenues in the comparative periods. Company recognized approx. $ 1.3 million in inventory reserves in 2015.
Significant reductions in operating expenses – 60+% in both three and six month periods.
Primarily due to concluding the ENDO trial, significant reductions in headcount and lower professional services costs for the comparative periods.
8/11/2016 GI Dynamics, Inc. 4
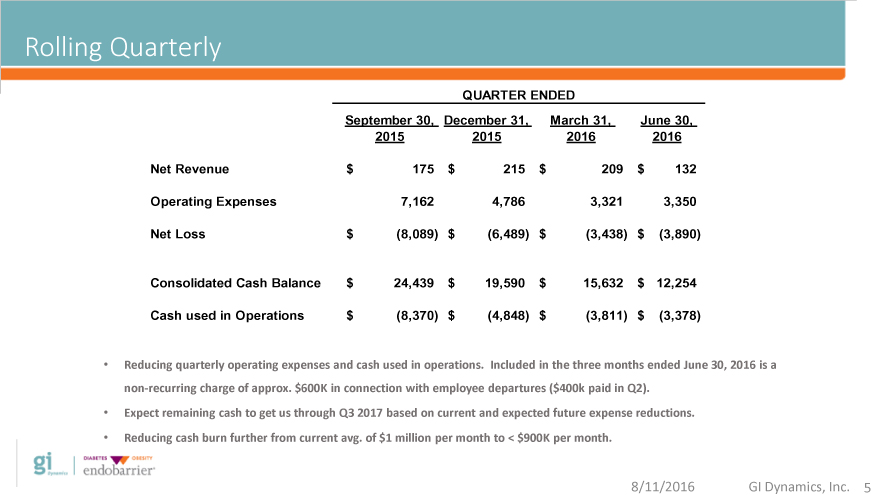
Rolling Quarterly
QUARTER ENDED
Rolling September 30, December 31, March 31, June 30,
2015 2015 2016 2016
Net Revenue $ 175 $ 215 $ 209 $ 132
Operating Expenses 7,162 4,786 3,321 3,350
Net Loss $(8,089) $(6,489) $(3,438) $(3,890)
Consolidated Cash Balance $ 24,439 $ 19,590 $ 15,632 $ 12,254
Cash used in Operations $(8,370) $(4,848) $(3,811) $(3,378)
Reducing quarterly operating expenses and cash used in operations. Included in the three months ended June 30, 2016 is a
non-recurring charge of approx. $600K in connection with employee departures ($ 400k paid in Q2).
Expect remaining cash to get us through Q3 2017 based on current and expected future expense reductions.
Reducing cash burn further from current avg. of $1 million per month to < $900K per month.
8/11/2016 GI Dynamics, Inc. 5
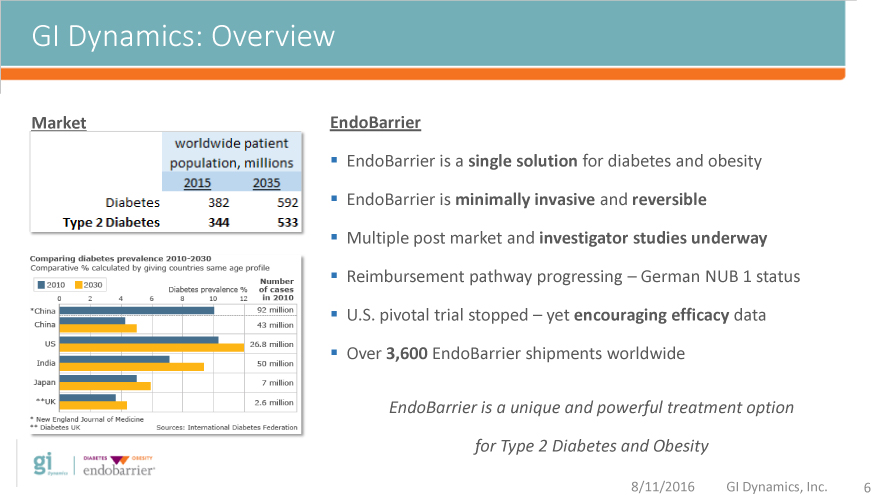
GI Dynamics: Overview
Market EndoBarrier
EndoBarrier is a single solution for diabetes and obesity
EndoBarrier is minimally invasive and reversible
Multiple post market and investigator studies underway
Reimbursement pathway progressing – German NUB 1 status
U.S. pivotal trial stopped – yet encouraging efficacy data
Over 3,600 EndoBarrier shipments worldwide
EndoBarrier is a unique and powerful treatment option
for Type 2 Diabetes and Obesity
8/11/2016 GI Dynamics, Inc. 6
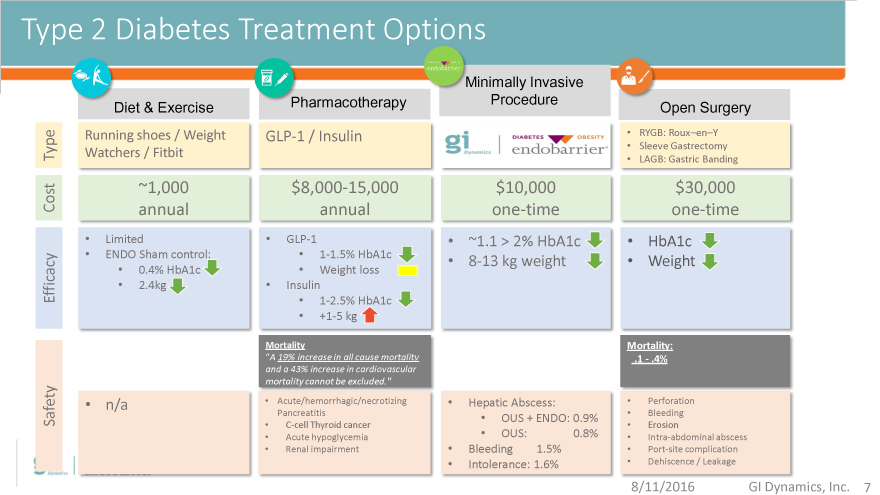
Type 2 Diabetes Treatment Options
Minimally Invasive
Diet & Exercise Pharmacotherapy Procedure Open Surgery
Running shoes / Weight GLP-1 / Insulin RYGB: Roux–en–Y
Type Watchers / Fitbit Sleeve Gastrectomy
LAGB: Gastric Banding
~1,000 $8,000-15,000 $10,000
Cost annual annual one-time one-time
Limited GLP-1 ~1.1 > 2% HbA1c HbA1c
ENDO Sham control: 1-1.5% HbA1c 8-13 kg weight Weight
0.4% HbA1c Weight loss
Efficacy 2.4kg Insulin
1-2.5% HbA1c
+1-5 kg
Mortality Mortality:
“A 19% increase in all cause mortality .1—.4%
and a 43% increase in cardiovascular
mortality cannot be excluded.”
n/a Acute/hemorrhagic/necrotizing Hepatic Abscess: Perforation
Safety Pancreatitis OUS + ENDO: 0.9% Bleeding
C-cell Thyroid cancer Erosion
Acute hypoglycemia OUS: 0.8% Intra-abdominal abscess
Renal impairment Bleeding 1.5% Port-site complication
Intolerance: 1.6% Dehiscence / Leakage
8/11/2016 GI Dynamics, Inc. 7
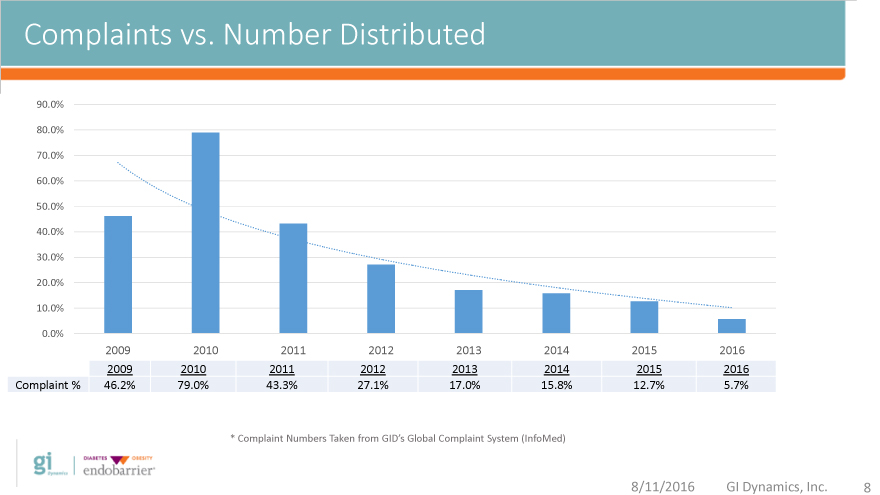
Complaints vs. Number Distributed
90.0%
80.0%
70.0%
60.0%
50.0%
40.0%
30.0%
20.0%
10.0%
0.0%
2009 2010 2011 2012 2013 2014 2015 2016
2009 2010 2011 2012 2013 2014 2015 2016
Complaint % 46.2% 79.0% 43.3% 27.1% 17.0% 15.8% 12.7% 5.7%
* Complaint Numbers Taken from GID’s Global Complaint System (InfoMed)
8/11/2016 GI Dynamics, Inc. 8
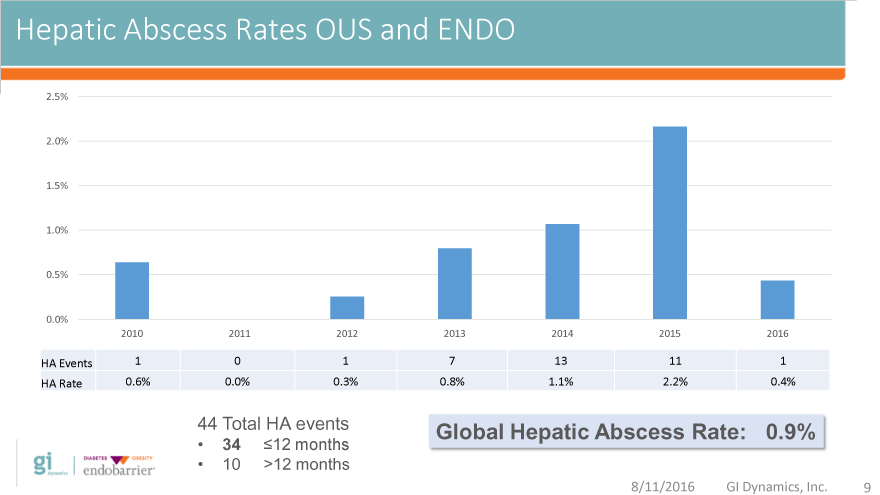
Hepatic Abscess Rates OUS and ENDO
2.5%
2.0%
1.5%
1.0%
0.5%
0.0%
2010 2011 2012 2013 2014 2015 2016
HA Events 1 0 1 7 13 11 1
HA Rate 0.6% 0.0% 0.3% 0.8% 1.1% 2.2% 0.4%
44 Total HA events Global Hepatic Abscess Rate: 0.9%
34 12 months
10 >12 months
8/11/2016 GI Dynamics, Inc. 9
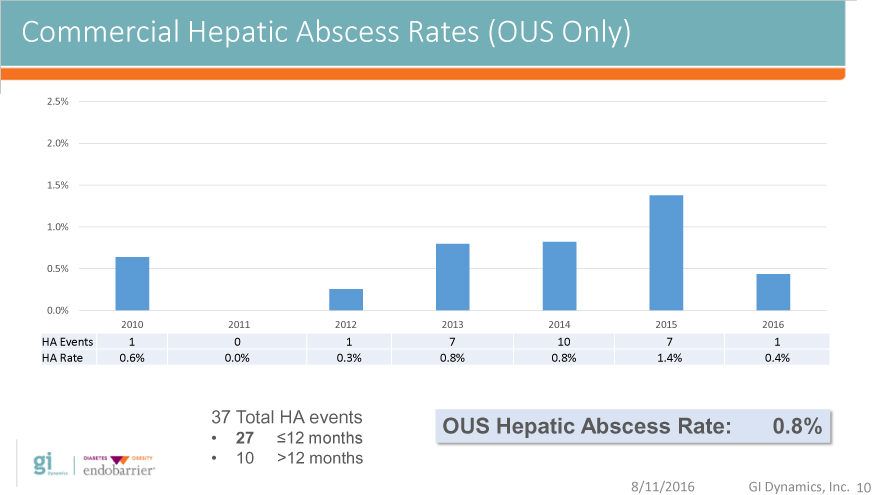
Commercial Hepatic Abscess Rates (OUS Only)
2.5%
2.0%
1.5%
1.0%
0.5%
0.0%
2010 2011 2012 2013 2014 2015 2016
HA Events 1 0 1 7 10 7 1
HA Rate 0.6% 0.0% 0.3% 0.8% 0.8% 1.4% 0.4%
37 Total HA events OUS Hepatic Abscess Rate: 0.8%
27 12 months
10 >12 months
8/11/2016 GI Dynamics, Inc. 10
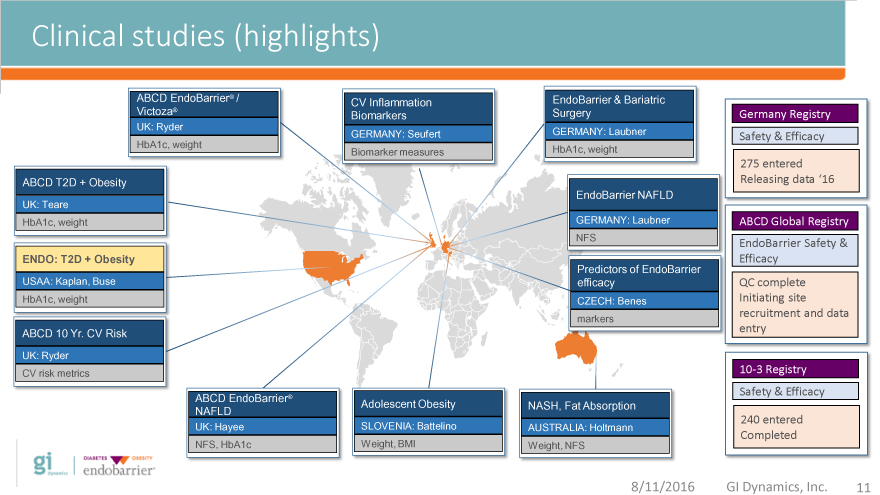
Clinical studies (highlights)
ABCD EndoBarrier® / CV Inflammation EndoBarrier & Bariatric
Victoza® Biomarkers Surgery Germany Registry
UK: Ryder GERMANY: Seufert GERMANY: Laubner Safety & Efficacy
HbA1c, weight Biomarker measures HbA1c, weight
275 entered
ABCD T2D + Obesity Releasing data ‘16
EndoBarrier NAFLD
UK: Teare
HbA1c, weight GERMANY: Laubner ABCD Global Registry
NFS EndoBarrier Safety &
ENDO: T2D + Obesity Efficacy
Predictors of EndoBarrier
USAA: Kaplan, Buse efficacy QC complete
HbA1c, weight CZECH: Benes Initiating site
markers recruitment and data
ABCD 10 Yr. CV Risk entry
UK: Ryder
CV risk metrics 10-3 Registry
Safety & Efficacy
ABCD EndoBarrier® Adolescent Obesity NASH, Fat Absorption
NAFLD
UK: Hayee SLOVENIA: Battelino AUSTRALIA: Holtmann 240 entered
Completed
NFS, HbA1c Weight, BMI Weight, NFS
8/11/2016 GI Dynamics, Inc. 11
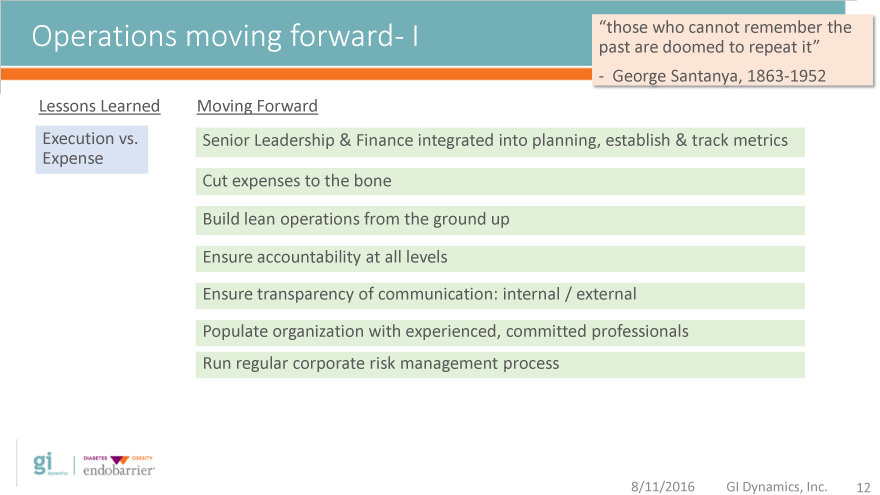
Operations moving forward- I past “those are who doomed cannot to remember repeat it” the
—George Santanya, 1863-1952
Lessons Learned Moving Forward
Execution vs. Senior Leadership & Finance integrated into planning, establish & track metrics
Expense
Cut expenses to the bone
Build lean operations from the ground up
Ensure accountability at all levels
Ensure transparency of communication: internal / external
Populate organization with experienced, committed professionals
Run regular corporate risk management process
8/11/2016 GI Dynamics, Inc. 12
Operations moving forward- II “those who cannot remember the
past are doomed to repeat it”
—George Santanya, 1863-1952
Lessons Learned Moving Forward
Regulatory Engage with individuals at regulatory bodies in proactive, transparent, respectful manner
Relationships
ENDO Clinical Maintain control over execution & decisions made during next trial
Trial
Inclusion/exclusion criteria to optimize: risk avoidance, clinical outcome, enrollment
Choose sites for study compliance and focus on enrollment: quality v quantity of sites
Employ site monitors; will not delegate all major tasks to CRO or other contractors
Commercial Integrate sales operations
Execution
Leverage global reimbursement strategy
8/11/2016 GI Dynamics, Inc. 13
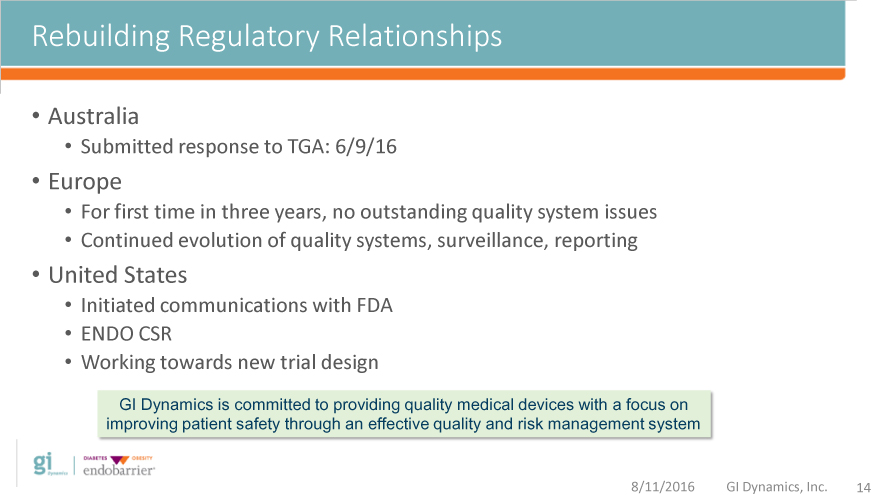
Rebuilding Regulatory Relationships
Australia
Submitted response to TGA: 6/9/16
Europe
For first time in three years, no outstanding quality system issues
Continued evolution of quality systems, surveillance, reporting
United States
Initiated communications with FDA
ENDO CSR
Working towards new trial design
GI Dynamics is committed to providing quality medical devices with a focus on
improving patient safety through an effective quality and risk management system
8/11/2016 GI Dynamics, Inc. 14
Corporate Priorities- update
1. Modify Cost Structure. We will implement a leaner, more efficient Completed; will always be ongoing
cost structure by cutting expenses to extend our cash runway. Extended cash runway by ~12 months
2. Rebuild Team. I will appoint a new chief financial officer and
chief compliance officer (responsible for clinical, regulatory, and Completed, will look at new hires moving
quality), in addition to adding other experienced team
members. forward
3. Develop Clinical Data and Core Science. We will continue to
support investigator-initiated studies around the world along with Solid work completed and continues
internal analyses of the safety and efficacy of EndoBarrier
Therapy.
4. Focus Revenue Efforts. We will focus on strategic commercial Working towards reimbursement, supporting
centers outside the United States. clinical studies, positioning for future revenue
5. Improve Regulatory Relationships. We will work with the FDA to
review lessons learned from the ENDO Trial to design our next Multiple legacy issues addressed
EndoBarrier Therapy trial, and collaborate with our European
Notified Body and the TGA in Australia to refine systems and post- Focused on new FDA study design within
market surveillance. current financing
8/11/2016 GI Dynamics, Inc. 15
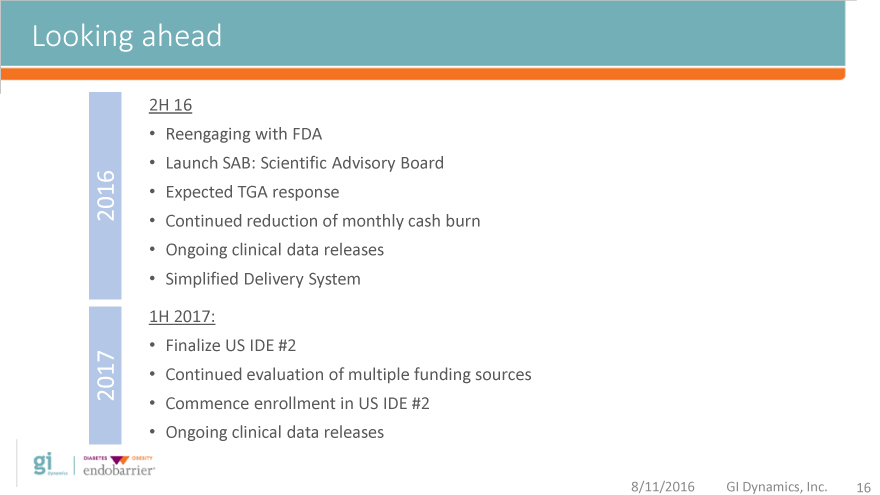
Looking ahead
2H 16
Reengaging with FDA
Launch SAB: Scientific Advisory Board
2016 Expected TGA response
Continued reduction of monthly cash burn
Ongoing clinical data releases
Simplified Delivery System
1H 2017:
Finalize US IDE #2
2017 Continued evaluation of multiple funding sources
Commence enrollment in US IDE #2
Ongoing clinical data releases
8/11/2016 GI Dynamics, Inc. 16
Thank you
Investor Inquiries: Media Inquiries:
Investor relations Media relations
United States: United States/Europe/Australia:
James Murphy investor@gidynamics.com
Chief Financial Officer +1 (781) 357-3250
+1 (781) 357-3281
Australia: United States/Australia:
David Allen or John Granger Catie Corcoran
Hawkesbury Partners Pty Limited WE Buchan
+61 2 9325 9046 +1 (813) 895-4575
8/11/2016 GI Dynamics, Inc. 17