UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| | |
| (Mark One) | | |
| ý | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011 |
o |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to |
COMMISSION FILE NUMBER 001-33484
|
HELICOS BIOSCIENCES CORPORATION
(Exact name of registrant as specified in its charter)
| | |
| DELAWARE | | 05-0587367 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification No.) |
One Kendall Square
Building 200
Cambridge, Massachusetts
(Address of principal executive offices) |
|
02139
(Zip Code) |
Registrant's telephone number, including area code:(617) 264-1800 |
Securities registered pursuant to Section 12(b) of the Act: |
Title of each class |
|
Name of each exchange on which registered |
| Common Stock, $0.001 par value | | OTC Bulletin Board |
Securities registered pursuant to Section 12(g) of the Act:None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| Large accelerated filer o | | Accelerated filer o | | Non-accelerated filer o
(Do not check if a
smaller reporting company) | | Smaller reporting company ý |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the registrant's Common Stock held beneficially or of record by stockholders who are not affiliates of the registrant, based upon the closing price of the Common Stock on June 30, 2011, as reported by the OTCQB Global Market, was approximately $6,119,963. For the purposes hereof, "affiliates" include all executive officers and directors of the registrant.
As of March 19, 2012, the Company had 87,196,489 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None
Table of Contents
HELICOS BIOSCIENCES CORPORATION (a development stage company)
FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2011
TABLE OF CONTENTS
2
Table of Contents
PART I
ITEM 1. BUSINESS OVERVIEW
Helicos BioSciences Corporation ("Helicos" or the "Company") is a life sciences company focused on innovative genetic analysis technologies and the monetization of that technology and related intellectual property. Helicos has developed a proprietary technology to enable the rapid analysis of large volumes of genetic material by directly sequencing single molecules of DNA or RNA. Our True Single Molecule Sequencing (tSMS)TM approach differs from current methods of sequencing DNA or RNA because it analyzes individual molecules of DNA directly instead of analyzing a large number of copies of the molecule which must be produced through complex sample preparation techniques. Our tSMS technology eliminates the need for costly, labor-intensive and time-consuming sample preparation techniques, such as amplification or cloning, which are required by other methods to produce a sufficient quantity of genetic material for analysis.
Our Helicos® Genetic Analysis Platform is designed to obtain sequencing information by repetitively performing a cycle of biochemical reactions on individual DNA or RNA molecules and imaging the results after each cycle. The platform consists of an instrument called the HeliScope™ Single Molecule Sequencer, an image analysis computer tower called the HeliScope™ Analysis Engine, associated reagents, which are chemicals used in the sequencing process, and disposable supplies. The information generated from using our tSMS products may lead to improved drug therapies, personalized medical treatments and more accurate diagnostics for cancer and other diseases.
As previously disclosed in our quarterly and periodic reports with the Securities and Exchange Commission, we have had limited operations to date and our activities have consisted primarily of raising capital, conducting research and development and recruiting personnel. Accordingly, we are considered to be in the development stage at December 31, 2011, as defined by the Financial Accounting Standards Board ("FASB"). We operate as one reportable segment. Helicos is a Delaware corporation and was incorporated on May 9, 2003. Furthermore, as of December 31, 2011 and March 23, 2012, we had $1.0 million and $34,000, respectively, in cash and cash equivalents.
As a result of several headcount reductions and restructurings during 2010, which eliminated approximately 60 positions, we entered the first quarter of 2011 with only 22 employees. In order to further conserve our limited resources, during the second quarter of 2011, we further reduced our workforce by phasing out six business and research positions. These six positions were phased out during the third quarter of 2011 and we ultimately reduced our total headcount to ten positions. In addition, during the third quarter of 2011, we entered into a new lease reducing the size and associated expense of our leased facility in Cambridge, Massachusetts which houses our headquarters and scientific laboratory.
Notwithstanding these most recent workforce reductions and despite our previous reductions, restructurings, expense saving measures, and the bridge loan facility (the "Bridge Debt Financing") contemplated by the Subordinated Secured Note Purchase Agreement (the "Note Purchase Agreement") we entered into in 2010 with investment funds affiliated with Atlas Venture and Flagship Ventures (the "Purchasers"), pursuant to which we agreed to sell to the Purchasers, and the Purchasers agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), we do not have sufficient funds to operate our business. We continue to require significant additional capital on a month-to-month basis in order to continue our operations beyond the existing $2,000,000 committed portion of the Bridge Debt Financing. As of the date of this filing, all $2,000,000 has been drawn against this committed facility. Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified, but there can be no assurance that any such funding will be available on reasonable terms, if at all. The remaining uncommitted portion of funding under the Bridge Debt Financing facility of $1,625,000, if made available by the Purchasers in their sole discretion, is not
3
Table of Contents
sufficient to fund operations and related litigation expenses through the current planned September 2012 trial date and the ultimate resolution of the pending Patent Litigation. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. No further commitments beyond the current outstanding notes with a maturity date of May 15, 2012 have been made under the uncommitted portion of the Bridge Debt Financing facility. We are currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that we will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs. As a result, we are no longer able to remain current in making payments to trade vendors and other unsecured creditors when such payments are due. Workforce reductions, as well as curtailed financial resources, will further impact our ability to operate our business. In addition, these constraints have caused us to significantly scale back service support and reagent supply to our current installed base. We have also significantly curtailed collaborative activities with other parties. Despite these measures, there cannot be any assurance that we will have sufficient resources to operate our business.
In January 2012, we made a final $1,070,000 payment on the senior debt facility pursuant to the Loan and Security Agreement among us, certain lenders and General Electric Capital Corporation, as agent, dated as of December 31, 2007, as amended (the "Loan Agreement"). As a result, our obligations under the Loan Agreement have been satisfied and the senior debt lenders no longer have a security interest in our assets, including our intellectual property assets.
We require substantial additional funding in order to continue our operations. If we are unable to successfully raise additional capital, we may have to cease operations and/or seek bankruptcy protection. If we are required to seek protection from our creditors through a bankruptcy filing, it is likely that there would be little or no assets available for payment or distribution to our stockholders, and that our stockholders will lose their entire investment in us. In addition, to seeking additional funding we are actively exploring a variety of strategic transactions, including the sale, license or other disposition of a portion of our assets in order to raise funding, or the potential sale of all or substantially all our assets or a sale of the Company. We believe it is very unlikely that stockholders would receive any significant value from any such transaction.
During 2011, we deployed our limited resources by focusing on intellectual property monetization and concentrating our research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals, which were designed to enable us to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives. With a limited number of employees and resources, however, our ability to pursue research and development efforts in targeted areas proved unsuccessful and are unlikely to resume. Going forward, we are only able to focus our limited resources on intellectual property monetization and selected research and development activities that are primarily related to grant-funded initiatives and that support our limited sequencing services. In order to defend our intellectual property rights through licensing and enforcement strategies, we have taken several actions. First, on August 27, 2010, we filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") for patent infringement. The lawsuit, filed in the United States District Court for the District of Delaware (the "Court"), accuses Pacific Biosciences of infringing four patents and seeks injunctive relief and monetary damages. Second, on October 14, 2010, we announced the launch of a technology licensing program for our Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. Third, on October 22, 2010, we filed an amended complaint against Pacific Biosciences by naming Illumina, Inc. and Life Technologies Corporation as additional defendants. The amended lawsuit alleges that the defendants willfully infringe our patents in suit by using their respective technologies, sequencing systems and
4
Table of Contents
products which we allege are within the scope of one or more claims of our patents in suit. We are seeking a permanent injunction enjoining the defendants from further infringement as well as monetary and punitive damages, costs and attorneys' fees, interests and other relief as determined by the Court. See Part I, Item 3, Legal Proceedings, for more information regarding the litigation. We believe that the proceeds generated by these litigations (the "Patent Litigation"), if we are successful, could be substantial. There is no assurance that we will be successful in the Patent Litigation, and even in the event of a favorable outcome in this litigation there can be no assurance that there will be any proceeds remaining for common stockholders, as a result of various arrangements in place with professional service providers, bridge lenders, licensors and others. As of the date of this filing, we do not have adequate financial resources and will continue to need to generate additional funding from various sources including contract sequencing service projects, government grants and yet to be identified third party financing arrangements in order to continue our intellectual property monetization strategy. There can be no assurance that we will be able to generate the funding necessary to continue our intellectual property monetization strategy. If we are unable to execute our operations according to our plans and obtain additional financing, we will be forced to cease operations and/or seek bankrupty protection.
In connection with the Bridge Debt Financing and as security for our obligations under our contingency fee arrangement with outside intellectual property litigation counsel relating to the patent infringement lawsuit we filed against Pacific Biosciences of California, Inc., Illumina, Inc. and Life Technologies Corporation, we entered into a subordinated security agreement with outside intellectual property litigation counsel. Pursuant to this agreement, we agreed to grant outside intellectual property litigation counsel a priority security interest in the patent infringement lawsuit. Under the terms of the contingency fee arrangement, we are obligated to pay outside intellectual property litigation counsel up to 40% of the proceeds paid to us in connection with the settlement, judgment or other resolution of the cause of action that is the subject of the intellectual property litigation against Pacific Biosciences, Illumina and Life Technologies (an any recovery therefrom) (the "Cause of Action") or certain business transactions resulting from or relating to the patent infringement lawsuit. To date, we, and those providing professional services in connection with this patent litigation on a contingent basis have expended significant resources in pursuit of this intellectual property monetization effort and are expected to continue to do so going forward. There is no assurance that we will be successful in this litigation, and even in the event of a favorable outcome in this litigation there can be no assurance that there will be any proceeds remaining for common stockholders, as a result of various arrangements in place with professional service providers, bridge lenders, licensors and others.
As an inducement for the Purchasers to enter into the Bridge Debt Financing, we entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") Under the terms of the Risk Premium Agreement, we have agreed to pay the Purchasers the following portions of the consideration we receive (the "Payment Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). For purposes of determining amounts payable under the Risk Premium Agreement, the Payment Consideration will not include amounts we use to first satisfy specified existing obligations, including obligations under the Loan Agreement, under professional contingency arrangements (including the contingency fee arrangement between us and Outside IP Litigation Counsel relating to the Cause of Action) and obligations that may arise under existing technology license agreements (the "Existing IP Licensing Agreements") between us and each of Arizona Technology Enterprises, Roche Diagnostics GMBH and Roche Diagnostics Corporation, and
5
Table of Contents
California Institute of Technology (as previously disclosed by us, under the Existing IP Licensing Agreements, we are obligated to pay license fees in connection with the licensing, sublicensing, legal settlements or sales of specified intellectual property or services provided to third parties ranging from a single digit percentage up to one-half of the income from those activities).
For purposes of the Risk Premium Agreement and subject to various provisions therein, the following shall constitute Liquidity Transactions: (A) the receipt by us of any payments from third parties relating to the intellectual property portfolio of the Company, including payments received as a license or other settlement in patent infringement litigation brought by us ("IP Licensing Events"); (B) a merger of the Company in which there is a change of control; and (C) the sale or exclusive license by us of all or substantially all of our assets or intellectual property
In approving the transactions relating to the Bridge Debt Financing, the Board approved a management incentive plan (the "Management Incentive Plan") pursuant to which participants in the Management Incentive Plan, including Dr. Ivan Trifunovich and Mr. Jeffrey Moore, will be entitled to receive, in the aggregate, an amount equal to 7.5% of certain payment consideration resulting from intellectual property licensing events. The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board. The Bridge Debt Financing and Risk Premium Agreement and all of the transactions contemplated by those agreements were approved by an independent committee of the Board. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, Ph.D., who is a Partner of Atlas Venture, abstained from the vote of the Board approving the transactions.
Please see Part I, Item 1A, in this Form 10-K, for a discussion of the risks related to ownership of our common stock.
Our future capital requirements will depend on many factors and we may require additional capital beyond our currently anticipated amounts. Any such required additional capital may not be available on reasonable terms, if at all, given our prospects, the current economic environment and restricted access to capital markets. The continued depletion of our resources may make future funding more difficult or expensive to attain. Additional equity financing may be dilutive to our stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict our ability to operate as a business; and strategic partnerships may result in royalties or other terms which reduce our economic potential from any adjustments to our existing long-term strategy. If we are unable to execute our operations according to our plans or to obtain additional financing, we may be forced to cease operations. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets or the amount of reclassification of liabilities, or any adjustment that might be necessary should we be unable to continue as a going concern.
Since our inception, we have incurred losses and have not generated positive cash flows from operations. We expect our losses to continue for a considerable period of time. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and equity/(deficit). As of December 31, 2011 and March 23, 2012, we had $1.0 million and $34,000, respectively, in cash and cash equivalents. We will, on a month-to-month basis during 2012, require significant additional capital to continue our operations, As of the date of this filing, all amounts available under the $2,000,000 committed facility have been drawn and no additional funding under the committed facility is available to support ongoing operations. Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified. Although we are engaged in active discussions with the Bridge Debt lenders with regard to securing funding from the $2,000,000 uncommitted portion of the Bridge Debt facility and are also exploring other funding options with the Bridge Debt lenders, there can be no assurance that any such funding or other sources of funding will
6
Table of Contents
be available to us on reasonable terms, if at all. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. We are currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that we will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs. If the Company is unable to execute its operations according to its plans and obtain additional financing, the Company will be forced to cease operations and/or seek bankruptcy protection. Because our present capital resources are not sufficient to fund our planned operations for a twelve month period as of the date of this Annual Report on Form 10-K for the year ended December 31, 2011, our current financial resources raise substantial doubt about our ability to continue as a going concern.
BACKGROUND ON DNA STRUCTURE AND FUNCTION
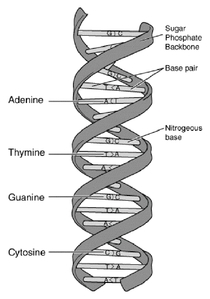
The genetic program that controls a living cell is encoded in its DNA. The diagram below shows the typical double-helix structure of DNA. The two strands are made of subunits called nucleotides, each of which contains a phosphate, a sugar and a side-chain called a base. The phosphates and sugars form the backbone of the polymer, and the bases face each other. The letters A, G, T and C represent the four types of nucleotide bases: adenine, guanine, thymine and cytosine.
| | |
The bases align with each other in a complementary structure held together by hydrogen bonds. A "T" on one strand always bonds with an "A" on the other strand, and a "G" on one strand always bonds with a "C" on the other strand. This bonding between DNA strands is called hybridization, and the resulting structure is called a base pair.
The genome of an organism is a complete DNA sequence of that organism. The human genome contains about three billion base pairs of DNA, which is represented twice in each cell. In a human, the individual acquires one version of the genome from the mother and one version from the father. |
|
 |
The human genome includes approximately 30,000 genes. Genes are segments of DNA that contain the information needed for a cell to make proteins. Each gene has one or more parts called exons including coding regions that specify the sequence of amino acids for that protein. Genes also contain regulatory elements that determine when, where and how much protein is made. While it is currently understood that approximately 97% of the human genome does not code for proteins, recent research suggests that this non-coding DNA also contains important regulatory elements which play important roles in controlling when and how much genes are expressed. In addition, new knowledge reveals a wealth of information contained within the transcribed regions of the genome that do not make up protein coding genes but yet these non-coding RNAs are becoming increasingly important in the study of biology.
The process of making proteins using the information contained within DNA involves a process called gene expression. To express a gene, enzymes called RNA polymerases transcribe the coding region into molecules of messenger RNA, or mRNA. The mRNA moves from the nucleus into the cytoplasm, where the cell's protein synthesis machinery translates the genetic sequence information and assembles a chain of amino acids into a protein.
7
Table of Contents
GENETIC ANALYSIS INDUSTRY OVERVIEW
Genomic information has become a critical tool to understand the mechanics of life, the environmental effect on biological systems, as well as diagnosis and treatment of disease. Life science tools that analyze genomic material have provided tremendous insights into the complexity and variability of the genome and have changed the methods and strategies by which scientists conduct their research. Genomic information enables the possibility and promise of personalized medicine and should bring forth a new era in patient knowledge whereby individuals now can have access to their own genetic information to make informed decisions concerning the prevention and treatment of disease in partnership with their physician.
Since the development of genetic engineering techniques in the 1970s, the analysis of genetic material has become a mainstay of biological research. The first automated DNA sequencer was invented in 1986, based on technology developed by Frederick Sanger and his colleagues in 1975, which is commonly referred to as Sanger sequencing. Subsequent versions of commercial DNA sequencers have increased the speed of DNA sequencing by 3,000 fold, making possible the Human Genome Project. In 1996 the first commercial microarray was introduced and enabled a new era of RNA analysis by measuring gene expression across many genes in a single experiment. Subsequent versions of the commercial microarrays including DNA and RNA have significantly increased the amount of information per experiment and provided selected single nucleotide polymorphisms, or SNPs of the whole human genome on a single chip, enabled large scale genome-wide SNP association studies and have been commercialized for several diagnostic applications.
Since 2007, there has been significant replacement of both of these existing genetic analysis technologies with Next Generation Sequencers (NGS) and Single-Molecule Sequencing technologies, because the limitations of Sanger sequencing technologies, such as low throughput, high cost and complexity of use restricted their use in large-scale studies. Next generation DNA and cDNA sequencing have emerged to provide the most comprehensive genome-wide information without any prior knowledge of the sequence or sequence variation contained within experimental samples.
Nevertheless, as next generation sequencing technologies continue to improve the speed and reduce the per base cost of DNA sequencing, these instruments continue to be limited by their detection sensitivity, thereby requiring DNA amplification to obtain sufficient material to adequately read the sequence. As with Sanger-based sequencing technologies, this requirement for amplification adds to the cost and complexity of these sequencing methods, and presents limits on the scalability of sample preparation and may limit the accuracy of the data produced. Moreover, many NGS technologies appear to possess biases and are hampered by their lack of absolute quantitative accuracy which may limit their applicability to the broader genetic analysis space. Additionally, the practical application of sequence data for molecular diagnostics is still heavily burdened by the costs associated with NGS sample preparation workflows.
In the past, the prohibitive cost of high-volume sequencing at the genome scale has caused scientists to use other genetic analysis technologies to examine discrete aspects of gene structure or function. For example, researchers use gene expression analysis to compare amounts of mRNA made from different genes, and genotyping to examine specific gene segments known to contain sequence variations, called single nucleotide polymorphisms, or SNPs. Technologies available for gene expression analysis and genotyping include:
- •
- chip- or bead-based microarrays, in which collections of short DNA molecules are attached to the surface of a glass chip or to beads and used to determine the identity and abundance of particular DNA or RNA molecules in a sample; and
8
Table of Contents
- •
- real-time PCR, also called RT-PCR, which is the method of biochemically copying or amplifying the DNA in a sample through a process called PCR in which the identity and quantity of amplified DNA from the sample is measured as the analysis is performed.
While these other genetic analysis technologies address the cost limitations of DNA and RNA sequencing, they generally provide only limited information and suffer from a range of technical limitations, the most important of which is the high cost of replacement as new sequence information is added and products are updated. NGS technologies that enable a broad range of applications with a relatively similar sample preparation methodology and data analysis format would greatly enable the ability to perform both broad and deep studies of important biological samples. NGS technologies that do not require significant investments in up front automation and infrastructure would also allow large scale studies to be performed in mid to small sized laboratories as well as translational and diagnostic laboratories by allowing continuous use of the same capital equipment for each of the different genetic analysis applications.
The scope and pace of much important research, and the routine application of genomic information in clinical medicine, had been limited by the shear cost of data acquisition however today we have begun to see hope for patients. Dramatic NGS sequencing performance improvements have resulted in the declining cost of whole genome sequencing and now enable large scale experimentation not previously possible. Efforts to overcome this barrier have been supported by the National Institutes of Health, whose National Human Genome Research Institute established the "Revolutionary Genome Sequencing Technologies—The $1,000 Genome," grant program to fund researchers' efforts to develop technology to enable the complete sequencing of an individual human genome at a cost of approximately $1,000 along with the more recent Sequencing Technology Development Program representing NHGRI's Signature Project for the American Recovery and Reinvestment Act. This project was specifically designed to support large-scale, high impact research projects that are expected to accelerate critical scientific breakthroughs and enable growth and investment in biomedical research. We have been supported by grants, from both of these funding initiatives, which have been applied directly to the development of our single molecule sequencing technology. In April 2010, we were awarded an R01 Research Project Grant from the NHGRI for Direct RNA Sequencing and are eligible to receive reimbursement for our research expenses of up to $1.6 million through February 2013. In June 2010, we were awarded a Fast Track Phase 1 Small Business Innovation Research grant to pursue research related to the development of methods to sequence patient DNA and RNA samples at an attomole level. The phase 1 portion of the grant provides funding for eligible research expenses of up to $146,000 through November 2010. Subsequent to December 31, 2010 we received approval for Phase II Fast Track funding for an additional two years totaling approximately $1.5 million. Additionally, we received a $150,000 subcontract award for a Department of Homeland Security grant funded to the Midwest Research Institute to interrogate rare variant detection in bacterial samples using our single molecule sequencing technology. In October 2010, we were awarded three federal grants totaling approximately $722,000 under the Patient Protection and Affordable Care Act of 2010, of which $472,000 was received during the fourth quarter of 2010 and the balance was received during the first quarter of 2011. The costs associated with these grants were incurred in prior 2009 and 2010 fiscal periods. The grants were awarded under the Qualified Therapeutic Discovery Project (QTDP) aimed towards producing products that diagnose diseases or determine molecular factors related to diseases by developing molecular diagnostics to guide therapeutic decisions.
Scientists have long realized that many of the disadvantages of ensemble based sequencing could be addressed through the direct sequencing of single molecules. The ability to directly measure individual sequences has the potential to reduce the cost and complexity of large scale experiments while increasing sensitivity. The simplicity of the sample preparation and detection would also provide the capability to combine multiple application techniques in order to get the most comprehensive view of each sample. For nearly 20 years, researchers have attempted without success to develop such a
9
Table of Contents
single molecule sequencing technology. Past efforts fell short largely due to complexity or technological hurdles in signal detection, surface materials, biochemistry, enzymology, bioinformatics, automation or engineering. In 2003, one of our co-founders, Stephen R. Quake, DPhil, demonstrated, we believe for the first time, that sequence information could be obtained from single molecules of DNA. We have replicated and improved upon Professor Quake's approach to develop our True Single Molecule Sequencing (tSMS)™ technology. (Harris, T., et al., Single molecule DNA sequencing of a viral genome.Science 2008, 320:106-109; Bowers, J., et al. Virtual terminator nucleotides for next-generation DNA sequencing.Nat Methods 2009, 6:593-595.) However, while other parties continue to innovate new sequencing and related technologies, we are no longer able to keep pace due to our lack of sufficient financial and other resources. As a result, while competing technologies emerge and gain market acceptance through rapid development and consumer adoption, we have been unable to make meaningful technological advances.
HELICOS TECHNOLOGY
Our True Single Molecule Sequencing (tSMS)™ technology is a powerful approach that directly measures single molecules. This novel approach allows our system to directly analyze billions of individual sequences in parallel and avoids the need for complex sample preparation techniques, amplification or cloning required by other methods. Our products utilizing our tSMS technology benefit from simple, scalable sample preparation techniques and automated high-throughput sequencing processes that enable sequencing with speed and provide unprecedented quantitative accuracy. This technology was designed to provide scientists and clinicians with extensive capabilities for basic and translational research, for pharmaceutical research and development, and for the development and clinical application of genomic diagnostics.
Our Helicos® Genetic Analysis System (the "Helios System") was designed to provide the following benefits:
- •
- Enhanced single-molecule throughput. Scientists measure the throughput of a DNA sequencing technology based on the number of bases analyzed per unit of time. The HeliScope™ Single Molecule Sequencer has achieved throughput rates of approximately 180 million analyzable bases per hour, depending on the application. The HeliScope Sequencer remains the highest throughput single molecule platform providing sequence information on upwards of a billion nucleic acid molecules per run. In addition, we have designed the imaging capability of the HeliScope Sequencer to accommodate a maximum throughput approaching one billion bases per hour. To achieve this additional increase in throughput, we would need to improve the efficiency and accuracy of the system's sequencing chemistry, increase the density of strands of DNA that bind to the surface of the flow cell in which the sequencing reactions take place and make corresponding enhancements to the image processing software.
- •
- Simplicity of sampling preparation. Because the sample preparation process for genome sequencing using our HeliScope Sequencer involves only small quantities of reagents and a few simple steps, we believe that it will be less costly, less time-consuming, less error prone, and require lower amounts of starting material (without amplification) than the sample preparation processes used in current NGS technologies.
- •
- Scalability. The sample preparation process is highly scalable because it does not require the need for the costly automation of complex sample preparation techniques, amplification or cloning required by existing NGS methods and thus makes genomic scale studies possible in small to mid-sized research organizations.
During the initial research market commercialization phase of our business, our business plan, which we can no longer pursue due to our lack of adequate financial resources, was based on our belief that our Helicos System could be used as a universal method of genetic analysis potentially replacing
10
Table of Contents
existing methods of gene expression analysis and genotyping. Based on the quantitative performance exhibited by the HeliScope Sequencer, our business plan was based on the belief that the Helicos System was capable of performing applications of gene expression analysis at a comparable cost per sample, and in the case of high volume analyses, at lower cost, in comparison with then-current technologies. We believe, however, that performing expression analysis using the tSMS approach provides a more unbiased and accurate measurement of expression and reveals new insight into the complexity of the transcriptome (Kapranov et al 2010. New class of gene-termini-associated human RNAs suggests a novel RNA copying mechanism.Nature. 2010, 29: 642-6). In addition, although we do not have the financial resources to explore the application of Helicos' technology in the field of molecular diagnostics, we believe that Helicos' technology is uniquely suited for applications in molecular diagnostics. In particular, we believe that our platform's quantitative accuracy, the use of small sample quantities in simple preparation methods, and high throughput, as well as lack of biases typically seen with sample amplification, can be especially useful in the field of molecular diagnostics.
Our True Single Molecule Sequencing (tSMS)™ Technology
Our True Single Molecule Sequencing (tSMS)™ technology enables the simultaneous sequencing of large numbers of strands of single DNA or RNA molecules. The first step of our single molecule sequencing approach is to cut, or shear, a sample of DNA or RNA into relatively small fragments. The double helix of each fragment is then separated into its two complementary strands. Each strand is used as a template for synthesis of a new complementary strand. This is accomplished through a series of biochemical reactions in which each of the four bases are successively introduced. If the introduced base is complementary to the next base in the template, it will be added to the new strand. Each of the added bases is tagged with a fluorescent dye, which is illuminated, imaged and then removed. The sequence of each new DNA or RNA strand is determined by collating the images of the illuminated bases from each cycle of highly specific incorporation and imaging. The raw sequencing data is then analyzed by computer algorithms.
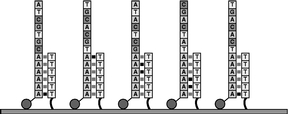
The series of figures below outlines an example of how our tSMS technology operates to sequence single molecules from genomic DNA. The actual process our HeliScope™ Single Molecule Sequencer will utilize to sequence DNA molecules will depend on the application. In September 2009 we published the first demonstration of the direct sequencing of RNA molecules which enables additional potentially significant areas of research and in 2010 published an extension of our work on direct RNA sequencing demonstrating genome surveys of polyadenylation maps in yeast and human RNAs.
| | |
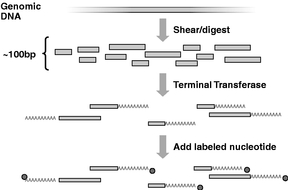
Figure 1
To prepare the sample for sequencing, the genomic DNA is first cut into small pieces of about 100 bases. The enzyme called terminal transferase is then used to add a string of "A" nucleotides to one end of each strand. Then, a nucleotide labeled with a single fluorescent dye molecule is added to the end of the strand. |
|
 |
11
Table of Contents
12
Table of Contents
| | |
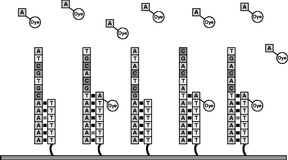
Figure 6
The process outlined in Figures 4 and 5 is repeated with each of the four types of labeled nucleotides. Repeating this cycle for a total of 120 times adds an average of more than 33-35 nucleotides to the primer strand. The number of bases added to a primer is the "read length." |
|
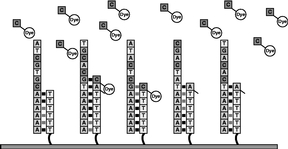 |
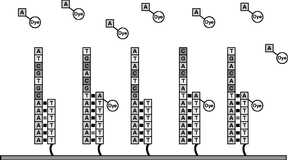
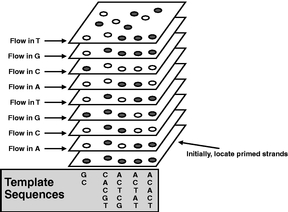
Figure 7
The system's computer analyzes the series of images from each cycle and determines the sequence of bases in the template strand. The sequence is "read" by correlating the position of a fluorescent molecule in its vertical track with the knowledge of which base was added at that cycle. Finally, the sequence data is exported to another computer system for further analysis depending on the application. |
|
 |
The Helicos® Genetic Analysis Platform
The Helicos® Genetic Analysis Platform consists of the following components:
- •
- Helicos® Genetic Analysis System—The instrument component of the Helicos Genetic Analysis Platform consists of three major components:
- •
- The HeliScope™ Single Molecule Sequencer which performs the True Single Molecule Sequencing (tSMS)™ chemistry and directly analyzes images of single molecules, producing accurate sequences of billions of templates at a time. The HeliScope Sequencer consists of a high-speed mechanical stage and a laser illumination subsystem, an image acquisition subsystem, a fluid handling subsystem and computer subsystems that control and analyze the sequencing reactions. To operate the instrument, a user loads a prepared sample of DNA, complementary DNA (cDNA) or RNA onto our flow cell using the HeliScope™ Sample Loader, places the flow cell on the mechanical stage and inserts our consumable reagent pack into the fluid handling system. From that point onward, all sequencing reactions are conducted automatically by the instrument. After each base is added, the mechanical stage moves the flow cells under a microscope lens. Four lasers illuminate the fluorescent tags of the bases, and a camera images the flow cells through the microscope lens.
- •
- HeliScope™ Analysis Engine provides computing power for near real-time image analysis and on-board data storage. The on-board data storage is appropriately sized to support two complete runs, enabling flexibility of operation and maximizing uptime. The Analysis Engine operates downstream from the HeliScope™ Sequencer in the data pipeline. It consists of the System Server, Object Finders, and an uninterruptible power supply (UPS). Components are mounted in a single enclosure for locating convenience and installation ease. Data communication between the HeliScope Sequencer and Analysis Engine is accomplished across Gigabit Ethernet (GigE) lines, providing high reliability and allowing for considerable physical distance between components.
13
Table of Contents
- •
- HeliScope™ Sample Loader facilitates and speeds the loading of samples into the Helicos® flow cells. It provides 25 discreet loading ports to ensure proper separation of samples and ease of loading.
- •
- Helicos® True Single Molecule Sequencing (tSMS)™ Reagent Kits. Reagent kits for sequencing which consist of proprietary formulations of a DNA polymerase enzyme, our proprietary fluorescently tagged bases, our proprietary imaging reagent, a proprietary formulation of a cleavage reagent and our proprietary application specific flow cells that have a proprietary surface coating with the chemical and optical properties needed for single molecule sequencing.
Consumable reagents. The biochemical sequencing reactions that occur in the HeliScope Sequencer involve the use of a proprietary formulation of a DNA polymerase enzyme, proprietary fluorescently tagged bases and proprietary imaging reagents. We have developed proprietary nucleotide triphosphates, called Virtual Terminator™ Nucleotides that allow us to add only one base at a time to each DNA strand. Our proprietary imaging reagents improve the stability of our fluorescent tags and increase their brightness. Our cleavage reagents are used to remove the fluorescent tags from the Virtual Terminators nucleotides.
Disposable supplies. The HeliScope™ Single Molecule Sequencer is designed to perform sequencing reactions inside one or two glass flow cells. When two flow cells are used, the system alternates between the flow cells, performing sequencing reactions in one flow cell while recording images from the other. Each flow cell has an active area of about 16 square centimeters and contains 25 separate channels. Our flow cells are designed to allow researchers to sequence separate samples in each channel, which will enable the simultaneous sequencing of at least 50 different DNA, cDNA or RNA samples. Our flow cell is designed to permit binding of DNA strands at an average density of approximately 100 million strands of DNA per square centimeter, equaling an average of approximately 2.8 billion strands of DNA for both flow cells. In addition, we have other reagent kit and run configurations that allow customers to choose the use of 1 or 2 flow cells and between 1 and 50 channels, adjust the number of strands of DNA imaged, and select run times between 2.5 and 8 days.
SCIENTIFIC APPLICATIONS
Our ability to build genomic knowledge from patient samples to discover and inform healthcare options will require novel, simplified methods for gene sequencing derived from patient natural DNA as well as methods for the analysis of limiting quantities of nucleic acids derived from clinical samples, including, but not limited to, fine needle aspirates, circulating tumor cells and body fluids. Further, many patient specimens with linked clinical information have been collected and stored as formalin-fixed paraffin embedded tissues. The Helicos® Genetic Analysis System provides new opportunities for genomic studies which utilize limiting nucleic acid quantities and encompass many areas of research, development and diagnostic use. Our current limited commercialization and research and development efforts are focused on research efforts that are supported by existing grant funding and that support our limited sequencing services. These include:
- •
- Analysis of Small Sample Quantities: With funding from a Phase I SBIR Grant, Helicos has demonstrated the ability to perform experimentation not possible with other commercially available NGS platforms—maintaining the basic principles of simple sample preparation involving no amplification and minimal sample manipulation enabling experiments never before possible. The ability to work with attomole level nucleic acid as well as new technologies emerging from this work remain core to our business and allow competitive distinction for Helicos at this time. By enabling the analysis of small amounts of nucleic acid material, without the requirement for amplification, a number of commercial and scientific applications can be realized including: 1) the detection and analysis of small amounts of circulating tumor cells from bodily fluids will enable diagnostics for the early detection of cancer onset, 2) the ability to perform molecular analyses on rare and precious biomedical samples without consuming large
14
Table of Contents
amounts of the sample and without skewing the results by amplification will enable heretofore impossible retrospective clinical research, 3) the ability to manipulate and analyze degraded and damaged nucleic acid material will facilitate forensic studies, and 4) the potential for direct analysis of unculturable microorganisms, which represent over 80% of the earth's microbiome and are available in limited quantities, can be analyzed for the discovery and scientific insight into novel biological functions and the elucidation of pathogenic molecular pathways.
- •
- Direct RNA Sequencing: The traditional approaches to transcriptome analyses have been typically dependent on the creation of a cDNA intermediate, an indirect measurement of the RNA in the cell. A unique feature of the Helicos System is our ability to sequence RNA directly without amplification or cDNA conversion. This method can also be adapted for experiments where sample material is limiting. We currently focus on demonstrating the unique attributes of the data derived from this technology with collaborators across the globe.
- •
- Accurate Quantitation for RNA and DNA: Digital gene expression and RNA Sequencing provides a hypothesis free, global, and quantitative analysis of the transcriptome. Helicos single molecule sequencing method allows for the quantitative measurement of virtually all genes in a sample by counting the number of individual mRNA molecules produced from each gene. This allows one to examine all the genes present in a cell or tissue in a hypothesis independent manner with no bias toward the genes believed to be expressed. We believe the end result is a highly sensitive and quantitative measurement which will allow not only for the detection of highly expressed transcripts but also for the detection of very rare transcripts represented by only a few molecules of RNA per cell. Our DNA quantitation has focused on the application of quantitative analysis of the human genome for detection of copy number differences across the genome.
- •
- Ancient DNA Sequencing: Ancient DNA sequencing benefits from the unique attributes of the Helicos single molecule direct sequencing methods. DNA derived from tissue abstracts obtained from ancient remains may be degraded, heavily modified and contaminated with larger fragments of modern DNA. Traditional sequencing methods depend on ligation and amplification steps and typically enrich for the larger, modern DNA leading to low sequence yields of the ancient DNA of interest. With SMS methods, degraded ancient DNA is simply tailed and sequenced in the presence of the modern DNA and provides significant benefit in yield and sequence output for researchers.
RESEARCH AND DEVELOPMENT
We advanced the development of our True Single Molecule Sequencing (tSMS)™ technology since we began operations in 2003. In 2004, we began to produce sequence data from single molecules of DNA and in 2005, we sequenced genomic DNA from a small virus called M13 using our tSMS technology. Also in 2005, we began to design the Helicos® Genetic Analysis System. In 2006, we received a $2 million grant from the National Human Genome Research Institute and completed the design of the critical components of the Helicos System. In 2007, we substantially finished the assembly of five commercial grade Helicos Systems and began shipping our Helicos Systems to initial customers in 2008. Our initial shipment to our first customer, Expression Analysis, Inc. ("EA"), was made on March 5, 2008. This system was an early version and did not consistently achieve our commercial specification levels at EA and, as a result, on January 27, 2009, we agreed to have EA return the Helicos System that was installed at EA. Following EA's return of this system, our commercial relationship with EA was suspended. EA did not make any payments for this Helicos System that was ultimately returned.
In 2008, we introduced a second generation Virtual Terminator™ Nucleotides with improved performance and shelf-life and second generation image analysis software with improved performance. During 2010, we invested in limited research and development activities to maintain the performance of our Helicos System and develop new applications with the existing system. We do not plan to allocate a
15
Table of Contents
significant amount of resources to research and development activities beyond selected activities that are primarily related to grant-funded initiatives and to support our limited sequencing services.
In the years ended December 31, 2009, 2010, and 2011 we incurred $18.3 million, $14.0 million and $4.9 million respectively, of research and development expenses. Research and Development expense for fiscal years 2009, 2010 and 2011, included charges of $3.1 million, $2.6 million and $2.0 million, respectively, in connection with write-offs of certain inventory or accrual of non-cancellable purchase commitments for inventory items that are no longer used in the business and we included this charge in research and development expense.
MANUFACTURING AND RAW MATERIALS
As a result of our limited resources, we only manufacture a limited supply of consumables for our installed base of customers and internal usage requirements and no longer manufacture instruments.
MARKETING, SALES, SERVICE AND SUPPORT
As a result of our limited resources, we have significantly curtailed or eliminated our sales, marketing and service efforts. We expect that any revenues in the foreseeable future will be derived primarily from conducting research under grants that have been awarded to us, the sale of sequencing services to research institutions in the academic, clinical, governmental, pharmaceutical, biotechnology and agriculture biology segments, and from the sale of consumables to our installed base of customers. To date, we have instruments in operation at several research institutions including two academic centers, a genome center, a cancer center and two commercial locations. As of the date of this filing, we do not foresee commencing any efforts to sell new instruments.
COMPETITION IN THE GENETIC ANALYSIS MARKET
Competition among entities developing or commercializing instruments, research tools or services for genetic analysis is intense. A number of companies offer DNA sequencing equipment or consumables, including Life Technologies, Inc., Beckman Coulter, Inc., the Life Sciences Division of GE Healthcare, Illumina, Inc., Complete Genomics, Inc. and Roche Applied Science. Furthermore, a number of other companies and academic groups are in the process of developing novel techniques for DNA sequencing. These companies include, among others, Ion Torrent (recently acquired by Life Technologies Corporation), Genizon BioSciences, Intelligent Bio-Systems, Lucigen, Microchip Biotechnologies, Pacific Biosciences, Shimadzu Biotech, ZS Genetics, Oxford Nanopore, NabSys and IBM. For RNA analysis and/or genotyping there are a number of companies that offer equipment and supplies including Affymetrix, Inc., Agilent Technologies, Life Technologies Corporation (formerly Applera Corporation), Bio-Rad Laboratories, Luminex and Nanostring. Three companies provide a wide range of products that span both DNA and RNA analysis—Life Technologies, Corporation, Affymetrix, Inc. and Illumina, Inc. However, the solutions that are provided by these competitors are separate applications that require different sample preparation techniques, consumables, analysis software and instrumentation with limited correlation between platforms.
Many of these companies have substantially greater capital resources, research and product development capabilities and greater financial, scientific, manufacturing, marketing, and distribution experience and resources, including human resources, than we do. These companies may develop or commercialize genetic analysis technologies before us or that are more effective than those we are developing, and may obtain patent protection or other intellectual property rights that could limit our rights to offer genetic analysis products or services.
INTELLECTUAL PROPERTY
Developing and maintaining a strong intellectual property position is an important element of our business strategy. In light of our intensified focus on intellectual property monetization through
16
Table of Contents
licensing and enforcement strategies, this aspect of our business has become even more central to our strategy and operations. See Part I, Item 3, Legal Proceedings for further discussion relating to our patent infringement enforcement actions. To that end, we have increased the resources of outside legal counsel, and established a disciplined process of growing and broadening our intellectual property assets, which involves substantial management attention. Our patent portfolio relating to our proprietary technology is comprised, on a worldwide basis, of various patents and pending patent applications, which, in either case, we own directly or for which we are the exclusive or semi-exclusive licensee. A number of these patents and patent applications are foreign counterparts of U.S. patents or patent applications. Among other things, our patent estate includes patents and/or patent applications having claims directed to:
- •
- a broad array of next generation sequencing technologies based on sequencing-by-synthesis methods;
- •
- the overall True Single Molecule Sequencing (tSMS)™ method;
- •
- certain components of the Helicos® Genetic Analysis Platform, including our laser illumination subassembly, our flow cells and various methods for using our HeliScope Sequencer;
- •
- methods for focusing our lasers and imaging our flow cell surfaces, and our use of combinations of laser optical paths;
- •
- our Virtual Terminator™ Nucleotides and other nucleotides;
- •
- various aspects of our sample preparation processes;
- •
- algorithms for analysis of our data;
- •
- reagent formulations for imaging and for sequencing; and
- •
- methods that can be used in molecular diagnostic testing for making inferences about disease state and for improving accuracy.
Within broad technological categories, our patent portfolio can be broken down as follows: most of our issued (81%) and pending (60%) patents are directed to sequencing by synthesis, primarily at the single molecule level; the smallest portion of the patent portfolio (3% of issued patents and 3% of pending applications) involves bioinformatics and data processing; the remainder of the portfolio is roughly evenly split among sample preparation (6% of issued patents and 12% of pending applications), instrumentation (10% of issued patents and 11% of pending applications), and commercial applications (14% of pending patent applications). The last-mentioned of these groups—commercial applications—encompasses wide areas of scientific and commercial interest including direct RNA sequencing, single-cell analysis, high-throughput screening, digital gene expression, and candidate region re-sequencing.
Our patents generally have terms of 20 years from their respective non-provisional priority filing dates. The first non-provisional patent applications prosecuted by Helicos were filed in 2004 and thus our issued patents are not scheduled to expire until 2024 or later. We have filed terminal disclaimers in certain later-filed patents, which means that such later-filed patents are scheduled to expire earlier than the twentieth anniversary of their respective non-provisional priority filing dates, although not earlier than 2024 with respect to patent applications prosecuted by Helicos. We have also in-licensed patents and patent applications. All of the material patents and patent applications to which we have licensed rights are scheduled to expire in 2017 or later.
Patent law relating to the scope of claims in the technology field in which we operate is still evolving. The degree to which we will be able to protect our technology with patents, therefore, is uncertain. Others may independently develop similar or alternative technologies, duplicate any of our technologies and, if patents are licensed or issued to us, design around the patented technologies
17
Table of Contents
licensed to or developed by us. In addition, we could incur substantial costs in litigation if we are required to defend ourselves in patent suits brought by third parties or if we initiate such suits.
We regard as proprietary any technology that we or our exclusive licensors have developed or discovered, including technologies disclosed in our patent estate, and that was not previously in the public domain. Aspects of our technology that we consider proprietary may be placed into the public domain by us or by our licensors, either through publication or as a result of the patent process. We may choose for strategic business reasons to make some of our proprietary technology publicly available whether or not it is protected by any patent or patent application.
With respect to proprietary know-how that is not patentable and for processes for which patents are difficult to obtain or enforce, we may rely on trade secret protection and/or confidentiality agreements to protect our interests. While we require all employees, consultants, collaborators, customers and licensees to enter into confidentiality agreements, we cannot be certain that proprietary information will not be disclosed or that others will not independently develop substantially equivalent proprietary information.
In addition to our patents, patent applications, confidential know-how, and potential trade secrets, we license technology that we consider to be material to our business.
Roche License Agreement. In June 2004, we entered into a license agreement with Roche Diagnostics (the "Roche License Agreement") which granted us a worldwide, semi-exclusive royalty-bearing license, with the right to grant sublicenses under a patent relating to sequencing methods. In connection with the Roche License Agreement, we paid an upfront fee of 175,000 Euros and committed to pay an annual license fee ranging from 10,000 to 40,000 Euros. There are no milestone payments potentially payable by us under the Roche License Agreement in addition to those described above. We have an option to convert the license to non-exclusive beginning in 2008, in which case the annual license fees would be reduced to 10,000 Euros beginning in 2008. We have the right to terminate the Roche License Agreement at any time for convenience upon 90 days prior written notice to Roche Diagnostics. We both have the right to terminate the Roche License Agreement upon breach by the other party, subject to notice and an opportunity to cure. The Roche License Agreement also terminates upon the occurrence of specified bankruptcy events. As part of the Roche License Agreement, we agreed to pay single digit royalties based on a percentage of defined net sales. We also agreed to pay half of our income amounts that we receive based on sublicenses that we grant to third parties. Our royalty obligation, if any, extends until the expiration of the last-to-expire of the licensed patents. Through December 31, 2011, no royalty payments have been made. All license fee amounts paid to date have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. The total expense recognized under the Roche License Agreement for the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) through December 31, 2011 was $54,000, $59,000, $58,000 and $538,000, respectively.
AZTE License Agreement. In March 2005, we entered into a license agreement with Arizona Technology Enterprises (the "AZTE License Agreement") that granted us a worldwide, exclusive, irrevocable, royalty-bearing license, with the right to grant sublicenses, under specified patents and patent applications exclusively licensed by AZTE from Arizona State University and the University of Alberta (the "Licensed Patents"). In connection with the AZTE License Agreement, we paid an upfront fee of $350,000, committed to an annual license fee of $50,000, which has increased to $100,000 upon the successful issuance of a U.S. patent, committed to pay a three-year maintenance fee of $50,000, payable in equal annual installments beginning in March 2006, and issued 88,888 shares of restricted common stock, which vest in two equal installments upon the achievement of separate milestones. There are no milestone payments potentially payable by us under the AZTE License Agreement in addition to those described above. We are obligated to use reasonable commercial efforts to develop, manufacture and commercialize licensed products. In addition, if we fail to use
18
Table of Contents
commercially-reasonable efforts to develop, manufacture and sell licensed products, the AZTE License Agreement converts from exclusive to non-exclusive. The AZTE License Agreement will remain in force until terminated. We have the right to terminate the AZTE License agreement at any time for convenience upon 60 days prior written notice to Arizona Technology Enterprises. We both have the right to terminate the agreement upon breach by the other party, subject to notice and an opportunity to cure. The AZTE License Agreement also terminates upon the occurrence of specified bankruptcy events.
As part of the AZTE License Agreement, we agreed to pay a single digit percentage royalty based on defined net sales. We also agreed to pay a mid-teens percentage of specified sublicense income amounts that are received based on sublicenses granted to third parties which increases to 30 percent after we receive an aggregate of $50,000 of such amounts. Our royalty obligation, if any, extends until the expiration of the last-to-expire of the Licensed Patents. Through December 31, 2011, no royalty payments have been made. All license fee amounts paid to date have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. In May 2006, in accordance with the license agreement, due to the successful issuance of a U.S. patent, the committed annual license fee increased from $50,000 to $100,000 and 44,444 shares of the restricted common stock vested. The vesting of 44,444 shares of restricted common stock resulted in a charge to research and development expense of $127,000 based on the fair value of our common stock at the time the milestone was achieved. The remaining 44,444 shares of restricted common stock vested immediately upon the successful issuance of a second U.S. patent on January 12, 2010 and we recorded an expense of $54,000 associated with the vesting of these shares. The total expense recognized under the AZTE License Agreement for the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) through December 31, 2011 was $141,000, $154,000, $100,000 and $1,244,000, respectively.
On August 26, 2011, we entered into a First Amendment to the License Agreement (the "Amendment") with AZTE. The Amendment expanded the field under the AZTE License Agreement so that AZTE has now granted us the exclusive right and license under the Licensed Patents to the full extent of the entire scope of all claims within the Licensed Patents, in all fields of use. AZTE's license to us under the AZTE License Agreement (the "AZTE Licensed Rights"), gives us the rights to several issued patents covering sequencing-by-synthesis methods and a number of pending patent applications, now expressly includes detection methods that do not rely on the detection of optically-labeled nucleotides (the "Expanded Field"). Under the terms of the Amendment, we will remain obligated to pay AZTE a specified percentage of income from our-granted sublicenses or other transfers of the AZTE Licensed Rights, including as part of any sale of the Company or litigation settlement. Amounts we owe to AZTE will be a percentage of the proportionate value of the AZTE Licensed Rights in any such transaction. We also agreed to make additional payments to AZTE for Company-granted sublicenses or other such transfers of the AZTE Licensed Rights within the Expanded Field. We retain our existing first right to initiate and pursue patent infringement suits involving the AZTE Licensed Rights. There has been no expense recorded for this amendment for the period ending December 31, 2011.
Caltech License Agreement. In November 2003, we entered into a license agreement with California Institute of Technology (the "Caltech License Agreement") that granted us a worldwide, exclusive, royalty-bearing license, with the right to grant sublicenses, under specified patents and patent applications, and a worldwide, non-exclusive royalty bearing license, with the right to grant sublicenses, under specified technology outside the scope of the licensed patents. In connection with the Caltech License Agreement, we issued 46,514 shares of common stock, and recorded a charge of $20,000. In addition, we pay an annual license fee of $10,000 per year. The license fee payments are creditable against single digit royalties calculated upon sales of products covered by patents licensed under the agreement. We are also obligated to pay California Institute of Technology a single digit percentage of specified license and sublicense income, a single digit percentage of proceeds from sales of specified
19
Table of Contents
intellectual property and a single digit percentage of service revenue amounts that we receive based on licenses and sublicenses that we grant, sales of intellectual property and services that we provide to third parties. The royalty obligation with respect to any licensed product extends until the later of the expiration of the last-to-expire of the licensed patents covering the licensed product and three years after the first commercial sale of the licensed product in any country for non-patented technology covered under the agreement. Through December 31, 2011, no royalty payments have been made. In March 2007, we amended the Caltech License Agreement to provide rights under an additional patent application under the terms of the existing license in exchange for a one-time payment of $50,000 to the California Institute of Technology. There are no milestone payments potentially payable by us under the Caltech License Agreement in addition to those described above. All license fee amounts paid to date and the value of the common stock issued have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. The total expense recognized under the Caltech License Agreement for the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) through December 31, 2011 was $10,000, $10,000, $10,000 and $143,000, respectively.
PerkinElmer License Agreement. In April 2007, we entered into an agreement with PerkinElmer LAS, Inc. ("PerkinElmer"), in which PerkinElmer granted us a worldwide, non-exclusive, non-transferable, non-sublicensable, royalty bearing license under specified patents. Our license from PerkinElmer grants us rights under certain patents to produce and commercialize certain of the reagents used in some applications on the Helicos System, which contain chemicals purchased from PerkinElmer. In exchange for rights licensed from PerkinElmer, we are obligated to pay PerkinElmer a single digit percentage of our net revenue from the sale of reagents that contain chemicals covered by the patents licensed under the PerkinElmer agreement. There are no milestone payments potentially payable by us under this agreement with PerkinElmer. We have the right to terminate the agreement at any time upon 90 days written notice to PerkinElmer. Each party has the right to terminate the agreement upon breach by the other party subject to notice and an opportunity to cure. The agreement also terminates upon the occurrence of specified bankruptcy events. PerkinElmer has the sole right under the agreement to enforce the licensed patents. There has been no expense recorded for this agreement for any period from May 9, 2003 (inception) through December 31, 2011.
See Note 7 to the Consolidated Financial Statements contained in this Form 10-K for additional information on license agreements.
GOVERNMENTAL REGULATION
We are subject, both directly and indirectly, to existing and potential future government regulation of our operations and markets. The life sciences industry, which is the market for our technology, has historically been heavily regulated. Our business is also directly affected by a wide variety of government regulations applicable to business enterprises generally and to companies operating in the life science industry in particular. Given the evolving nature of this industry, legislative bodies or regulatory authorities may adopt additional regulations that adversely affect our market opportunities. In particular, if we decide to market our diagnostic assays as laboratory developed tests ("LDTs") under Clinical Laboratory Improvement Amendments ("CLIA"), we will need to seek CLIA certification, and be subject to the requirements and oversight of the Centers for Medicare and Medicaid Services. We may not receive such certification in a timely fashion or at all. Furthermore, CLIA regulations may change over time and the U.S. Food and Drug Administration ("FDA") may ultimately decide to regulate the devices and methods we will use in the delivery of our LDTs.
20
Table of Contents
COMPLIANCE WITH ENVIRONMENTAL PROVISIONS
We are not materially affected by compliance with federal, state, and local environmental provisions which have been enacted or adopted to regulate the distribution of materials into the environment.
CORPORATE INFORMATION
We were incorporated in Delaware in May 2003. In 2003, one of our co-founders, Professor Stephen R. Quake, who was then at the California Institute of Technology, demonstrated, we believe for the first time, that sequence information could be obtained from a single strand of DNA. Shortly thereafter, Noubar Afeyan, Chief Executive Officer of Flagship Ventures, and Stanley Lapidus, then a Venture Partner at Flagship Ventures, met with Professor Quake and agreed to found a company to develop and commercialize technology based on Professor Quake's single molecule approach. Combining the experience of Professor Quake in single molecule methods, Dr. Afeyan in the sequencing technology and life sciences businesses, and Mr. Lapidus in diagnostics and entrepreneurship, we focused exclusively on the technical and commercial development of technology based on Professor Quake's approach. Professor Eric Lander, Director of the Broad Institute of MIT and Harvard, and a leader in the DNA sequencing field, provided helpful guidance and advice during our founding stages.
EMPLOYEES
We had 10 full-time employees at December 31, 2011. We have never had a work stoppage and none of our employees are covered by collective bargaining agreements. We believe our employee relations are good. Our continuing operations depends in large part on our ability to retain our existing employees.
AVAILABLE INFORMATION
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, definitive proxy statements on Form 14A, current reports on Form 8-K, and any amendments to those reports are made available free of charge on our website, www.helicosbio.com, as soon as reasonably practicable after such reports are electronically filed with or furnished to the Securities and Exchange Commission (the "SEC"). Statements of changes in beneficial ownership of our securities on Form 4 by our executive officers and directors are made available on our website by the end of the business day following the submission to the SEC of such filings. In addition, the SEC's website, www.sec.gov, contains reports, proxy statements, and other information regarding reports that we file electronically with the SEC. During 2010, our securities were delisted from the NASDAQ Global Market and the trading of our common stock was suspended before the opening of business on November 16, 2010. A Form 25-NSE was subsequently filed with the SEC on January 21, 2011 which removed our securities from listing and registration on The NASDAQ Stock Market. The Company's securities are currently traded under the symbol "HLCS" on the OTCQB which is a market tier for OTC-traded companies that are registered and reporting with the SEC. Investors can now view real time stock quotes for HLCS at http://www.otcmarkets.com.
Item 1A. RISK FACTORS
The following important factors could cause our actual business, prospects, financial results or financial condition to differ materially from those contained in forward-looking statements made in this Annual Report on Form 10-K or elsewhere by management from time to time.
21
Table of Contents
RISKS RELATED TO OUR BUSINESS
Beginning in January 2012, our business will require additional funding, which may not be available on favorable terms, if at all, or without potentially very substantial dilution to our stockholders. If we do not raise the necessary funds, we will need to terminate our operations and/or file for bankruptcy.
Our business will require additional financial investment that we have not yet secured and we will need to raise additional capital on a month-to-month basis during 2012 in order to sustain our business and to pursue our new business priorities. The amount of additional capital we will need to raise depends on many factors, including:
- •
- our current or any future litigation related to defending our intellectual property or defending lawsuits that allege our actions infringe the intellectual property of third parties;
- •
- the revenue generated by future sales of our products or services;
- •
- the timeliness of payments from our customers;
- •
- the ability to structure arrangements with, and make payments to, our unsecured creditors;
- •
- the continued funding to the Bridge Debt Financing facility under the November, 2010 Note Purchase Agreement;
- •
- the emergence of competing or complementary technological developments;
- •
- the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
- •
- regulatory changes in our markets; and
- •
- the terms and timing of any collaborative, licensing or other arrangements that we may establish.
Notwithstanding these most recent workforce reductions and our previous reductions, restructurings, expense saving measures, and the Bridge Debt Financing, we do not have sufficient funds to pursue our business priorities. We continue to require significant additional capital on a month-to-month basis in order to continue our operations beyond the existing $2,000,000 committed portion of the Bridge Debt Financing. As of the date of this filing, all $2,000,000 has been drawn against this committed facility. Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified, but there can be no assurance that any such funding will be available on reasonable terms, if at all. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. No further commitments beyond the current outstanding notes with a maturity date of May 15, 2012 have been made under the uncommitted portion of the Bridge Debt Financing facility. We are currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that we will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs. As a result, we are no longer able to remain current in making payments to trade vendors and other unsecured creditors when such payments are due. Workforce reductions, as well as curtailed financial resources, will further impact our ability to operate our business. In addition, these constraints have caused us to significantly scale back service support and reagent supply to our current installed base. We have also significantly curtailed collaborative activities with other parties. Despite these measures, there cannot be any assurance that we will have sufficient resources to execute our business priorities.
We do not know whether the additional capital that we will require will be available when and as needed, on favorable terms or if at all, or that our actual cash requirements will not be greater than
22
Table of Contents
anticipated. We may seek additional capital through a combination of private and public equity offerings, debt financings and collaboration, strategic and licensing arrangements. To the extent we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of such sales may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, would involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt or making capital expenditures or that require early repayment, even with penalties, in connection with a change of control. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we would have to relinquish valuable rights to our technologies or products, or grant licenses on terms that are not favorable to us. We may not be able to complete any such capital raising transactions on acceptable terms, or at all. If adequate additional funds are not available to us when required, we will be required to terminate some or all aspects of our operations and/or seek bankruptcy protection. If we are required to seek protection from our creditors through a bankruptcy filing, it is likely that there would be little or no assets available for payment or distribution to our stockholders, and that our stockholders will lose their entire investment in us. In addition, to seeking funding we are actively exploring a variety of strategic transactions, including the sale, license or other disposition of a portion of our assets in order to raise funding, or the potential sale of all or substantially all our assets or a sale of the Company. We believe it is very unlikely that stockholders would receive any significant value from any such transaction.
We are implementing a new strategic focus. If we are unsuccessful in pursuing our new business initiatives, our business, financial condition, results of operations and prospects will be materially adversely affected and we may have to cease operations.
We are implementing a new strategic focus and plan to deploy our limited resources by focusing on intellectual property monetization. In addition, on October 14, 2010, we announced the launch of a technology licensing program for our Single Molecule Sequencing Platform. While our new business opportunities will rely upon the capabilities of our proprietary Helicos® Genetic Analysis Platform and the strength of our intellectual property portfolio, the business model will be materially different than that which we have engaged in before. We are unable to give any assurance we will be commercially successful in our new business strategy. Our failure to be commercially successful in any such new business opportunity would materially adversely impact our business, financial condition, results of operations and prospects and could ultimately require that we cease operations.
The audit report contained in our Annual Report on Form 10-K for the year ended December 31, 2011 contains an explanatory paragraph to the effect that there is substantial doubt about our ability to continue as a going concern.
This "going concern" statement may discourage some third parties from contracting with us and some investors from purchasing our stock or providing alternative capital financing. The resulting failure to establish, or the loss of existing, favorable commercial relationships or to satisfy our capital requirements would adversely affect our business, financial condition, results of operations and prospects. Unless we raise additional funds, either through public or private sales of equity, from borrowings or from strategic partners, we will likely have to cease operations. Even if we can continue operating by reducing expenses, the requisite significant reduction in our workforce and operating expenses will adversely affect our business and prospects.
We may not be able to meet our debt service obligations, or may otherwise default, under our secured loan and security agreements, which would materially adversely affect our business, financial condition, results of operations and prospects.
Our secured loan and security agreements impose restrictions and obligations on us with which we may not be able to comply. If we fail to comply with such restrictions, our lenders may declare us in
23
Table of Contents
default. The occurrence of an event of default could lead to acceleration of such debt or any instruments evidencing indebtedness that contain cross-acceleration or cross-default provisions. In such event, we may be required to sell assets, to refinance, or to obtain additional financing. Such refinancing may not be possible and additional financing may not be available on commercially acceptable terms or at all.
We have a history of operating losses, expect to continue to incur substantial losses, and might never achieve or maintain profitability.
We are a development-stage company with a limited operating history. We have incurred significant losses in each fiscal year since our inception, including net losses attributable to common stockholders of $28.0 million, $18.8 million and $5.8 million in the years ended December 31, 2009, 2010 and 2011, respectively. As of December 31, 2011, we had an accumulated deficit of $192.3 million. These losses have resulted principally from costs incurred in our research and development programs and from our selling, general and administrative expenses. In the year ended December 31, 2011, we used cash in operating activities of $0.9 million. As of December 31, 2011 and March 23, 2012, we had $1.0 million and $34,000 respectively, in cash and cash equivalents.
We expect our losses to continue for a considerable period of time as we pursue our new business priorities. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders' equity/(deficit). Because of the numerous risks and uncertainties associated with our new business priorities, we are unable to predict when we will become profitable, and we may never become profitable.
Following our reductions in force in 2010 and 2011, which resulted in the elimination of approximately 90% of our headcount, we may not be able to retain our remaining employees while we pursue our business priorities. As a result, we may be unable to achieve our goals, in which case, our business, financial conditions, results of operations and prospects will be materially adversely affected and we may have to cease operations.
In connection with our efforts to consider alternatives to our long-term strategic focus, in 2010 and 2011, we committed to reductions in headcount and restructuring plans (the "Restructuring Plans") which involved the consolidation and reorganization of our operations in order to reduce operating costs, conserve cash and direct our resources to continue advancing towards our near term goals. The Restructuring Plans involved a reduction in headcount of approximately 70 employees or 90% of the organization. We entered 2012 with only 10 employees and are substantially dependent on the performance of these employees, which include senior management and key scientific and technical personnel. We do not maintain employment contracts with any of our employees. As a result, while we begin to pursue our new business priorities, we will rely heavily on our ability to retain our remaining employees. However, depending on our circumstances, we may need to implement additional workforce reductions in the future. The loss of the service of any number of our remaining employees may significantly delay or prevent our ability to pursue our business objectives by diverting management's attention to transition matters and identification of suitable replacements, if any, and could have a material adverse effect on our business prospects, operating results and financial condition. For example, because of the complex and technical nature of our Helicos System and the dynamic market in which we compete, any failure to retain a sufficient number of our remaining, qualified employees could materially harm our ability to pursue our business priorities.
Given the magnitude of our Restructuring Plans' reductions in force and the potential uncertainty about our strategic direction and long-term roles within our organization, the morale of our remaining employees may be lowered, key employees may be distracted and our business may experience a loss of continuity while we pursue our business priorities. Each of our remaining employees could terminate his or her relationship with us at any time. These persons' expertise would be difficult to replace and the failure to do so would have a material adverse effect on our ability to achieve our business goals.
24
Table of Contents
There can be no assurance that we will have the financial resources or otherwise be successful in retaining our remaining personnel and our failure to do so would have a material adverse effect on our business, financial condition and results of operations. Our inability to retain existing personnel during this period of uncertainty or our inability to attract new personnel that fit within a new strategic focus would prevent our exploiting fully our business priorities and thereby cause a material adverse effect on our business, financial condition, results of operations and prospects.
We have limited experience.
You should evaluate us in the context of the uncertainties and complexities affecting an early stage company in the life science industry which is experiencing the challenges associated with entering into new, highly competitive markets. Based on our limited experience, we may not:
- •
- effectively execute on or focus our research and development efforts with our limited resources;
- •
- properly model new opportunities to ensure appropriate resource allocation; or
- •
- create product or service offerings that are appropriately developed to meet customer needs.
We use hazardous chemicals and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development activities involve the controlled use of hazardous materials, including chemicals and biological materials. Our operations produce hazardous waste products. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials. We do not currently maintain separate environmental liability coverage. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of hazardous materials. Compliance with environmental laws and regulations may be expensive, and current or future environmental regulations may impair our research, development and production efforts.
We rely on a single laboratory facility for our research and development activities and are occupying our space without a long term lease agreement.
We rely on a single laboratory facility in Cambridge, Massachusetts for our research and development activities. This facility and certain pieces of laboratory equipment would be difficult to replace and may require significant replacement lead-time. This facility may be affected by natural disasters such as earthquakes, floods and fires. In the event our laboratory facility or equipment is affected by man-made or natural disasters, we would be unable to develop products for a significant period of time. Although we maintain insurance on this facility, it may not be adequate to protect us from all potential losses if this facility were damaged or destroyed. In addition, the lease for our Cambridge facility expires on December 31, 2012. We are unable to enter into a longer term lease arrangement due to our limited resources and financial condition.
Because we are subject to existing and potential additional governmental regulation, we may become subject to burdens on our operations, and the markets for our products may be narrowed.
We are subject, both directly and indirectly, to the adverse impact of existing and potential future government regulation of our operations and markets. The life sciences industry, which is the market for our technology, has historically been heavily regulated. Given the evolving nature of this industry, legislative bodies or regulatory authorities may adopt additional regulation that adversely affects our market opportunities. Our business is also directly affected by a wide variety of government regulations applicable to business enterprises generally and to companies operating in the life science industry in particular. Failure to comply with these regulations or obtain or maintain necessary permits and licenses could result in a variety of fines or other censures or an interruption in our business operations which
25
Table of Contents
may have a negative impact on our ability to generate revenues and could increase the cost of operating our business. In particular, if we decide to market our diagnostic assays as laboratory developed tests ("LDTs") under Clinical Laboratory Improvement Amendments ("CLIA"), we will need to seek CLIA certification, and be subject to the requirements and oversight of the Centers for Medicare and Medicaid Services. We may not receive such certification in a timely fashion or at all. Furthermore, CLIA regulations may change over time and the U.S. Food and Drug Administration ("FDA") may ultimately decide to regulate the devices and methods we will use in the delivery of our LDTs. Although we can closely monitor the initiatives and announcements and policy changes coming from the regulatory agencies, we cannot predict the regulatory changes that may arise and we may not be able to meet the requirements of new laws or regulations enacted by the FDA pertaining to the performance and marketing of our testing services.
We have incurred, and will continue to incur, significant increased costs as a result of operating as a public company, and our management is and will continue to be required to devote substantial time to new compliance initiatives.
The Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC have imposed various requirements on public companies, including requiring changes in corporate governance practices. Our management and other personnel devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly.
In addition, the Sarbanes-Oxley Act requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting, as required by Section 404(a) of the Sarbanes-Oxley Act. However, our testing may reveal deficiencies in our internal control over financial reporting that are deemed to be material weaknesses. Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management time on compliance-related issues. We currently do not have an internal audit group and we will evaluate the need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
Our failure to establish a strong intellectual property position and enforce our intellectual property rights against others would enable competitors to develop similar or alternative technologies.
Our success depends in part on our ability to obtain and maintain intellectual property protection for our technologies. Our policy is to seek to protect our intellectual property by, among other methods, filing U.S. patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. The U.S. Congress recently passed the Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in September 2011. The America Invents Act reforms United States patent law in part by changing the standard for patent approval from a "first to invent" standard to a "first to file" standard and developing a post-grant review system. This new legislation changes United States patent law in a way that may weaken our ability to obtain or maintain patent protection for future inventions in the United States.
Our patent portfolio relating to our proprietary technology is comprised of issued patents and pending patent applications which, in either case, we own directly or for which we are the exclusive or
26
Table of Contents
semi-exclusive licensee. Some of these patents and patent applications are foreign counterparts of U.S. patents or patent applications. We may not be able to maintain and enforce existing patents or obtain further patents for our products, processes and technologies. Even if we are able to maintain our existing patents or obtain further patents, these patents may not provide us with substantial protection or be commercially beneficial. The issuance of a patent is not conclusive as to its validity or enforceability, nor does it provide the patent holder with freedom to operate unimpeded by the patent rights of others. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and the extent of future protection is highly uncertain, so there can be no assurance that the patent rights that we have or may obtain will be valuable. Others have filed patent applications that are similar in scope to ours, and in the future are likely to file patent applications that are similar or identical in scope to ours or those of our licensors. We cannot predict whether any of our competitors' pending patent applications will result in the issuance of valid patents. Moreover, we cannot assure investors that any such patent applications will not have priority or dominate over our patents or patent applications. The invalidation of key patents owned by or licensed to us or non-approval of pending patent applications could increase competition, and materially adversely affect our business, financial condition and results of operations. Furthermore, there can be no assurance that others will not independently develop similar or alternative technologies, duplicate any of our technologies, or, if patents are issued to us, design around the patented technologies developed by us.
We are involved in lawsuits and administrative proceedings to protect or enforce our patents and proprietary rights and to determine the scope and validity of others' proprietary rights, which will result in substantial costs and diversion of resources and which, if unsuccessful, could harm our competitive position and our results of operations.
Litigation and administrative proceedings are and may continue to be necessary to enforce our patent and proprietary rights and/or to determine the scope and validity of others' proprietary rights. Litigation on these matters has been prevalent in our industry and we expect that this will continue. To determine the priority of inventions, we may have to initiate and participate in interference proceedings declared by the U.S. Patent and Trademark Office that could result in substantial costs in legal fees and could substantially affect the scope of our patent protection. Also, our intellectual property is and may continue to be subject to significant administrative and litigation proceedings such as invalidity, opposition, reexamination, or reissue proceedings against our patents. The outcome of any litigation or administrative proceeding might not be favorable to us, and, in that case, we might be required to develop alternative technological approaches that we may not be able to complete successfully or require licenses from others that we may not be able to obtain. Even if such licenses are obtainable, they may not be available at a reasonable cost. We may also be held liable for money damages to third parties and could be enjoined from using technologies. In addition, legal proceedings to enforce our intellectual property rights or to determine the validity and scope of the intellectual property or other proprietary rights of others, the proceedings will be burdensome and expensive, even if we were to prevail. These types of administrative proceedings and any additional litigation that may be necessary in the future could result in our patent protection being significantly modified or reduced, and could result in substantial costs and diversion of resources and could have a material adverse effect on our business, operating results or financial condition. In August 2006, we filed an opposition against EP 1 105 529 B1 in the European Patent Office, with respect to which we received a preliminary non-binding opinion upholding the patent. In October 2008, EP 1 105 529 B1 was maintained in amended form and in January 2009 we filed a notice of appeal of the European Patent Office's decision. The patent owner filed a response brief on October 19, 2009, and we filed a reply brief on March 19, 2010.
27
Table of Contents
On August 27, 2010, we filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") in the United States District Court for the District of Delaware (the "Court") (CA. No. 10-735-SLR) alleging that Pacific Biosciences infringes U.S. Patent Nos. 7,645,596 ("'596 Patent"), 7,037,687 ("'687 Patent"), 7,169,560 ("'560 Patent"), and 7,767,400 ("'400 Patent"). On October 22, 2010, we filed an amended complaint naming Illumina, Inc. ("Illumina") and Life Technologies Corporation ("Life Technologies") as additional defendants. In the amended complaint, we further allege that Life Technologies infringes the '596 Patent, the '687 Patent, and the '560 Patent, and that Illumina infringes the '687 Patent, the '560 Patent, and U.S. Patent No. 7,593,109 ("'109 Patent"). We accuse Pacific Biosciences of willful patent infringement based at least on its manufacture, use, and sale of its Single Molecule Real Time ("SMRT™") DNA sequencing technology. We accuse Life Technologies of willful patent infringement based at least on its manufacture, use, and offer for sale of its Single Molecule Real-Time DNA Sequencing of a Quantum-dot Nanocrystal technology for single molecule sequencing of DNA. We accuse Illumina of willful patent infringement based at least on its manufacture, use, offer for sale, sale, and importation of its sequencing technology for single molecule sequencing of DNA including its Genome Analyzer, HiSeq 2000, and its Sq Module in combination with its iScan and HiScan platforms. We are seeking a permanent injunction enjoining each of the defendants from further infringement as well as compensatory and punitive damages, costs, and attorneys' fees, interest, and other relief as determined by the Court. On January 27, 2011, Pacific Biosciences submitted requests to the United States Patent and Trademark Office (the "Patent Office") for inter parties reexamination of the '687 Patent, the '560 Patent, the '596 Patent and the '400 Patent and are seeking a determination that one or more of the claims of those patents are invalid. The Patent Office granted the requests for reexamination of the '400 Patent and the '596 Patent on March 10, 2011, the request for reexamination of the '560 Patent on March 31, 2011, and the request for reexamination of the '687 Patent on April 13, 2011, and issued office actions rejecting all claims of the patents. The Patent Office issued Actions Closing Prosecution in the reexamination proceedings for the '560 Patent, the '687 Patent, the '596 Patent, and the '400 Patent on September 8, 2011; October 5, 2011; October 20, 2011; and January 5, 2012, respectively. All four reexaminations remain pending. On April 25, 2011, Pacific Biosciences moved the Court to stay the litigation pending the outcome of the patent-reexamination proceedings. Life Technologies later joined the motion. On June 23, 2011, Life Technologies moved the Court to drop or sever and stay our claims against it. The Court has denied both motions. On December 14, 2011, the defendants moved the Court to transfer the litigation to the Northern District of California. The Court has not yet entered an order on this motion. On February 15, 2011, we and Illumina filed a Stipulated Order of Dismissal Between Helicos Biosciences Corporation and Illumina, Inc. as to the '560 Patent, pursuant to which our claims against Illumina for infringement of the '560 Patent would be dismissed with prejudice, and Illumina's counterclaims regarding the '560 Patent would be dismissed without prejudice. The Court conducted a scheduling conference on February 9, 2011 and set a trial date of September 10, 2012. Fact discovery has been largely concluded and the parties have exchanged initial expert reports regarding infringement and validity.
We depend upon our ability to license technologies, and the failure to license or otherwise acquire necessary technologies could harm our ability to sell our products or defend our intellectual property position.
We hold various licenses to use certain technologies that we consider to be material to our business. Each of these licenses imposes a range of obligations on us and may be terminated if we breach the terms of any of the respective agreements. We may also be required to enter into additional licenses with third parties for other technologies that we consider to be necessary for our business. If we are unable to maintain our existing licenses or obtain additional technologies on acceptable terms, we could be required to develop alternative technologies, either alone or with others, in order to avoid infringing the intellectual property to which we no longer hold a license. This could require the tests we are developing to be re-configured, which could negatively impact the availability of our tests, once
28
Table of Contents
developed, for commercial sale and increase our development costs. Failure to license or otherwise acquire necessary technologies would harm our ability to market and sell our diagnostic products, once developed, which could materially adversely affect our business, financial condition and results of operations. In addition, any licenses we obtain from federally-funded institutions are subject to the march-in rights of the U.S. government.
We may be the subject of costly and time-consuming lawsuits brought by third parties for alleged infringement of their proprietary rights, which could limit our ability to use certain technologies in the future, force us to redesign or discontinue our products, or pay royalties to continue to sell our products.
Our success depends, in part, on us neither infringing patents or other proprietary rights of third parties nor breaching any licenses to which we are a party. We may be the subject of legal claims by third-parties that our technology or products infringe their patents or otherwise violate their intellectual property rights. In addition, the technology that we license from third parties for use in our Helicos System could become subject to similar infringement claims. Infringement claims asserted against us or our licensors may have a material adverse effect on our business, results of operations or financial condition. Any claims, either with or without merit, could be time-consuming and expensive to defend, and could divert our management's attention away from the execution of our business plan. Moreover, any settlement or adverse judgment resulting from the claim could require us to pay substantial amounts of money or obtain a license to continue to use the technology that is the subject of the claim, or otherwise restrict or prohibit our use of the technology. There can be no assurance that we would be able to obtain a license on commercially reasonable terms, if at all, from third parties asserting an infringement claim; that we would be able to develop alternative technology on a timely basis, if at all; or that we would be able to obtain a license to use a suitable alternative technology to permit us to continue offering, and our customers to continue using, our affected products. We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights. If we become involved in such litigation, it could consume a substantial portion of our managerial and financial resources.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
The measures that we use to protect the security of our intellectual property and other proprietary rights may not be adequate, which could result in the loss of legal protection for, and thereby diminish the value of, such intellectual property and other rights.
Our success depends in part on our ability to protect our intellectual property and other proprietary rights. In addition to patent protection, we also rely upon a combination of trademark, trade secret, copyright and unfair competition laws, as well as license agreements and other contractual provisions, to protect our intellectual property and other proprietary rights. In addition, we attempt to protect our intellectual property and proprietary information by requiring our employees, consultants and certain academic collaborators to enter into confidentiality and assignment of inventions agreements. There can be no assurance, however, that such measures will provide adequate protection for our patents, copyrights, trade secrets or other proprietary information. In addition, there can be no assurance that trade secrets and other proprietary information will not be disclosed, that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to or disclose our trade secrets and other proprietary information. To the extent that our intellectual property and other proprietary rights are not adequately protected, third parties might gain
29
Table of Contents
access to our proprietary information, develop and market genetic analysis systems similar to our tSMS technology, or use trademarks similar to ours, each of which could materially harm our business. Existing U.S. federal and state intellectual property laws offer only limited protection. Moreover, the laws of other countries in which we may market our technology may afford little or no effective protection of our intellectual property. The failure to adequately protect our intellectual property and other proprietary rights could materially harm our business.
RISKS RELATED TO OWNERSHIP OF OUR COMMON STOCK
Investing in our stock is highly speculative and an investor will likely lose some of, or the entire amount invested.
Our business will require additional financial investment that we have not yet secured and we will need to raise additional capital on a month-to-month basis during 2012 in order to sustain our business and to pursue our new business priorities. If we cannot secure additional financing we may be forced to cease operations and/or file for bankruptcy protection. Therefore, an investment in our stock is highly speculative. Stockholders are unlikely to realize any value with respect to their stock.
There is no assurance that we will be successful in the Patent Litigation, and even in the event of a favorable outcome in this litigation there can be no assurance that there will be any proceeds remaining for common stockholders, as a result of various arrangements in place with professional service providers, bridge lenders, licensors and others. As of the date of this filing, we do not have adequate financial resources and will continue to need to generate additional funding from various sources including contract sequencing service projects, government grants and yet to be identified third party financing arrangements in order to continue our intellectual property monetization strategy. There can be no assurance that we will be able to generate the funding necessary to continue our intellectual property monetization strategy. If we are unable to execute our operations according to our plans and obtain additional financing, we will be forced to cease operations and/or seek bankruptcy protection.
Our common stock has been delisted from The NASDAQ Global Market, which negatively impacts the price of our common stock and our ability to access the capital markets.
On June 28, 2010, we received a letter from The NASDAQ Stock Market LLC ("NASDAQ") advising that for the previous 30 consecutive business days, we failed to comply with the minimum market value of listed securities ("MVLS") requirement for continued listing on the NASDAQ Global Market pursuant to NASDAQ Marketplace Rule 5450(b)(2)(A) and that we did not otherwise satisfy the criteria for continued listing pursuant to NASDAQ Marketplace Rule 5450(b). NASDAQ stated in its letter that in accordance with NASDAQ Marketplace Rule 5810(c)(3)(C), we would be provided 180 calendar days, or until December 27, 2010, to regain compliance with the MVLS continued listing requirement. MVLS is calculated by multiplying the total shares outstanding by the daily closing bid price. On October 12, 2010, we received a delisting determination letter from NASDAQ due to our failure to regain compliance with the NASDAQ Global Market's minimum bid price requirement for continued listing as set forth in Listing Rule 5450(a)(1). In addition, we did not hold our annual meeting of stockholders for fiscal 2010 which is an additional NASDAQ continued listing requirement. Based on the unlikely ability for us to meet the NASDAQ MVLS requirements due to the state of our finances and near-term business prospects, on November 12, 2010, we withdrew our appeal to NASDAQ regarding this delisting notice. Therefore, our securities were delisted from the NASDAQ Global Market and the trading of our common stock was suspended before the opening of business on November 16, 2010, and a Form 25-NSE was subsequently filed with the SEC on January 21, 2011 which removed our securities from listing and registration on The NASDAQ Stock Market. The delisting of our common stock will significantly affect the ability of investors to trade our securities and will significantly negatively affect the value and liquidity of our common stock. In addition, the delisting of our common stock will materially adversely affect our ability to raise capital on terms acceptable to
30
Table of Contents
us or at all. Delisting from the NASDAQ Global Market will also have other negative results, including the potential loss of confidence by suppliers and employees, the loss of institutional investor interest and fewer business development opportunities.
As a result of our most recent financing activities, we are subject to various obligations to creditors and others that are likely to materially reduce or eliminate the total return on equity for our common stockholders.
Our obligations to make future Risk Premium Payments under the terms of the Risk Premium Agreement, the Note Purchase Agreement, the contingency fee arrangement with Outside IP Litigation Counsel, the Existing IP Licensing Agreements and the Management Incentive Plan, are highly dilutive to our common stockholders and materially reduce their share of any future earnings of our company. There is no assurance that we will be successful in the Patent Litigation, and even in the event of a favorable outcome in this litigation there can be no assurance that there will be any proceeds remaining for common stockholder, as a result of various arrangements in place with professional service providers, bridge lenders (including the Risk Premium Agreement), licensors and others. The agreements and obligations referenced in this risk factor are more fully described in Note 8 of the Notes to Consolidated Financial Statements, under the caption Bridge Financing and Senior Debt Restructuring, in this Form 10-K. If do not receive sufficient proceeds from the patent Litigation and/or are unable to raise additional financing, we may be required to file for bankruptcy protection. If so, it is likely that there would be little or no assets available for payment or distribution to our stockholders.
Our directors and management will exercise significant control over our company, which will limit your ability to influence corporate matters.
Certain of our directors and executive officers and their affiliates collectively control a majority of our outstanding common stock as of December 31, 2011. As a result, these stockholders, if they act together, will be able to influence our management and affairs and all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control of our company and might negatively affect the market price of our common stock.
The market price of our common stock may be volatile, which could result in substantial losses for our stockholders and subject us to securities class action litigation.
Market prices of technology and healthcare companies have been particularly volatile recently. Some of the factors that may cause the market price of our common stock to fluctuate include:
- •
- investors' perception of our ability to successfully implement our new business priorities;
- •
- fluctuations in our quarterly operating results or the operating results of companies perceived to be similar to us;
- •
- changes in estimates of our financial results;
- •
- failure of our technology to achieve or maintain market acceptance or commercial success;
- •
- changes in market valuations of similar companies;
- •
- success of competitive products and services;
- •
- changes in our capital structure, such as future issuances of securities or the incurrence of additional debt;
- •
- announcements by us or our competitors of significant products, contracts, acquisitions or strategic alliances;
- •
- regulatory developments in the United States, foreign countries or both;
31
Table of Contents
- •
- litigation involving our company, our general industry or both;
- •
- additions or departures of key personnel;
- •
- investors' general perception of us; and
- •
- changes in general economic, industry and market conditions.
In addition, if the market for biotechnology and life sciences stocks or the stock market in general experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition or results of operations. If any of the foregoing occurs, it could cause our stock price to fall and may expose us to class action lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
A significant portion of our total outstanding shares may be sold into the public market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
A majority of the shares of our common stock outstanding as of December 31, 2011 may be offered and sold by selling stockholders pursuant to effective registration statements on Forms S-3 declared effective by the SEC. In addition, up to 22,653,964 additional shares of our common stock may be offered and sold pursuant to effective registration statements by selling stockholders upon exercise of warrants. In addition, a majority of the other outstanding shares of our common stock and other warrants are eligible for resale by the holders of those shares pursuant to other effective registration statements or in exempt private transactions.
If our existing stockholders sell substantial amounts of our common stock in the public market, the market price of our common stock could decrease significantly. The perception in the public market that our stockholders might sell shares of common stock could also depress the market price of our common stock. A decline in the price of shares of our common stock might impede our ability to raise capital through the issuance of additional shares of our common stock or other equity securities, and may cause you to lose part or all of your investment in our shares of common stock.
We have registered the issuance of all shares of common stock that we have issued and may issue under our employee option plans. Having registered the issuance of these shares, they can be freely sold in the public market upon issuance. In addition, as of December 31, 2011, there were 277,777 shares of common stock reserved for future issuance as charitable contribution to the Broad Institute, Inc. that will become eligible for sale in the public market to the extent permitted by Rule 144 under the Securities Act of 1933, as amended.
Due to these factors, sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock.
Provisions in our certificate of incorporation and by-laws or Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore, depress the trading price of our common stock.
Provisions of our certificate of incorporation and by-laws and Delaware law may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares of our common stock. These provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions include:
- •
- a staggered board of directors;
- •
- limitations on the removal of directors;
32
Table of Contents
- •
- advance notice requirements for stockholder proposals and nominations;
- •
- the inability of stockholders to act by written consent or to call special meetings; and
- •
- the ability of our board of directors to make, alter or repeal our by-laws.
The affirmative vote of the holders of at least 75% of our shares of capital stock entitled to vote is necessary to amend or repeal the above provisions of our certificate of incorporation. In addition, our board of directors has the ability to designate the terms of and issue new series of preferred stock without stockholder approval. Also, absent approval of our board of directors, our by-laws may only be amended or repealed by the affirmative vote of the holders of at least 75% of our shares of capital stock entitled to vote. Accordingly, given that our executive officers, directors and their affiliates collectively own a majority of our outstanding common stock, certain of these persons acting together will have the ability to block any such amendment.
In addition, Section 203 of the Delaware General Corporation Law prohibits a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person which together with its affiliates owns, or within the last three years has owned, 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner.
The existence of the foregoing provisions and anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of our company, thereby reducing the likelihood that you could receive a premium for your common stock in an acquisition.
We do not currently intend to pay dividends on our common stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid any cash dividends on our common stock and do not currently intend to do so for the foreseeable future. We currently intend to invest our future earnings, if any, to fund our growth. Therefore, you are not likely to receive any dividends on your common stock for the foreseeable future and the success of an investment in shares of our common stock will depend upon any future appreciation in its value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 2. PROPERTIES
We lease a 6,000 square foot facility in Cambridge, Massachusetts that houses our headquarters and scientific laboratory. This lease dated September 2011 expires on December 31, 2012.
For additional information regarding obligations under operating leases see Note 7 of the Notes to the Consolidated Financial Statements contained in this Form 10-K.
ITEM 3. LEGAL PROCEEDINGS
On August 27, 2010, we filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") in the United States District Court for the District of Delaware (CA. No. 10-735-SLR) alleging that Pacific Biosciences infringes U.S. Patent Nos. 7,645,596 ("'596 Patent"), 7,037,687 ("'687 Patent"), 7,169,560 ("'560 Patent"), and 7,767,400 ("'400 Patent"). On October 22, 2010, we filed an amended complaint naming Illumina, Inc. ("Illumina") and Life Technologies Corporation ("Life Technologies") as additional defendants. In the amended complaint, we further allege that Life Technologies infringes the '596 Patent, the '687 Patent, and the '560 Patent, and that Illumina infringes
33
Table of Contents
the '687 Patent, the '560 Patent, and U.S. Patent No. 7,593,109 ("'109 Patent"). We accuse Pacific Biosciences of willful patent infringement based at least on its manufacture, use, and sale of its Single Molecule Real Time ("SMRT™") DNA sequencing technology. We accuse Life Technologies of willful patent infringement based at least on its manufacture, use, and offer for sale of its Single Molecule Real-Time DNA Sequencing of a Quantum-dot Nanocrystal technology for single molecule sequencing of DNA. We accuse Illumina of willful patent infringement based at least on its manufacture, use, offer for sale, sale, and importation of its sequencing technology for single molecule sequencing of DNA including its Genome Analyzer, HiSeq 2000, and its Sq Module in combination with its iScan and HiScan platforms. We are seeking a permanent injunction enjoining each of the defendants from further infringement as well as compensatory and punitive damages, costs, and attorneys' fees, interest, and other relief as determined by the Court. On January 27, 2011, Pacific Biosciences submitted requests to the United States Patent and Trademark Office (the "Patent Office") for inter parties reexamination of the '687 Patent, the '560 Patent, the '596 Patent and the '400 Patent and are seeking a determination that one or more of the claims of those patents are invalid. The Patent Office granted the requests for reexamination of the '400 Patent and the '596 Patent on March 10, 2011, the request for reexamination of the '560 Patent on March 31, 2011, and the request for reexamination of the '687 Patent on April 13, 2011, and issued office actions rejecting all claims of the patents. The Patent Office issued Actions Closing Prosecution in the reexamination proceedings for the '560 Patent, the '687 Patent, the '596 Patent, and the '400 Patent on September 8, 2011; October 5, 2011; October 20, 2011; and January 5, 2012, respectively. All four reexaminations remain pending. On April 25, 2011, Pacific Biosciences moved the Court to stay the litigation pending the outcome of the patent-reexamination proceedings. Life Technologies later joined the motion. On June 23, 2011, Life Technologies moved the Court to drop or sever and stay our claims against it. The Court has denied both motions. On December 14, 2011, the defendants moved the Court to transfer the litigation to the Northern District of California. The Court has not yet entered an order on this motion. On February 15, 2011, we and Illumina filed a Stipulated Order of Dismissal Between Helicos Biosciences Corporation and Illumina, Inc. as to the '560 Patent, pursuant to which our claims against Illumina for infringement of the '560 Patent would be dismissed with prejudice, and Illumina's counterclaims regarding the '560 Patent would be dismissed without prejudice. The Court conducted a scheduling conference on February 9, 2011 and set a trial date of September 10, 2012. Fact discovery has been largely concluded and the parties have exchanged initial expert reports regarding infringement and validity.
On March 21, 2012, we received an attorney demand letter from one of our major customers alleging that Helicos is in breach of the customer's system and reagent kit supply agreements in connection with the sale of multiple Helicos systems and reagent kits that were sold and installed prior to December 31, 2009. The demand letter alleges breach of warranty and that the systems did not conform to Helicos' specifications and were defective in material and workmanship. In addition, the demand letter alleges that Helicos did not provide the requisite level of customer support in connection with the system service and maintenance requirements set forth in the system supply agreement. The demand letter states that, if the parties are unable to reach an amicable settlement, the customer will terminate the supply agreements and request that Helicos refund approximately $5.0 million. The Company has deferred all revenue related to this arrangement, is in the process of evaluating the claims, and has not yet responded to the demand letter.
ITEM 4. MINE SAFETY DISCLOSURES
34
Table of Contents
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock has traded publicly under the symbol "HLCS" since our initial public offering in May 2007. Our securities were delisted from the NASDAQ Global Market and the trading of our common stock was suspended on November 16, 2010, and a Form 25-NSE was subsequently filed with the SEC on January 21, 2011 which removed our securities from listing and registration on The NASDAQ Stock Market. Our securities are currently traded under the symbol "HLCS" on the OTCQB which is a market tier for OTC traded companies that are registered and reporting with the SEC. The following table sets forth the range of quarterly high and low sales prices for our common stock in 2010 and 2011 on the NASDAQ Global Market and the OTCQB, as applicable.
| | | | | | | | | | | | | |
| | 2010 | | 2011 | |
|---|
| | High | | Low | | High | | Low | |
|---|
First quarter | | $ | 1.43 | | $ | 0.72 | | $ | 0.23 | | $ | 0.12 | |
Second quarter | | $ | 0.98 | | $ | 0.38 | | $ | 0.15 | | $ | 0.06 | |
Third quarter | | $ | 0.85 | | $ | 0.38 | | $ | 0.18 | | $ | 0.06 | |
Fourth quarter | | $ | 0.58 | | $ | 0.11 | | $ | 0.16 | | $ | 0.03 | |
Holders
As of March 19, 2012, there were approximately 48 holders of record of our common stock, one of which is Cede & Co., a nominee for Depository Trust Company ("DTC"). All of the shares of common stock held by brokerage firms, banks and other financial institutions as nominees for beneficial owners are deposited into participant accounts at DTC and therefore are considered to be held of record by Cede & Co. as one shareholder.
Dividends
We have never declared or paid any cash dividends on our capital stock and do not expect to pay any cash dividends for the foreseeable future. We intend to use future earnings, if any, in the operation and expansion of our business. Any future determination relating to our dividend policy will be made at the discretion of our board of directors, based on our financial condition, results of operations, contractual restrictions, capital requirements, business properties, restrictions imposed by applicable law and other factors our board of directors may deem relevant.
Securities Authorized for Issuance Under Equity Compensation Plans
Please see Part III, Item 12, Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters in this Form 10-K, for a discussion of our equity compensation plans.
35
Table of Contents
Issuer Purchases of Equity Securities
During 2011, we purchased 27,896 restricted shares from employees to cover withholding taxes due from the employees at the time the shares vested. The following table provides information about these purchases of restricted shares for the year ended December 31, 2011:
| | | | | | | |
Period | | Total Number of
Shares Purchased | | Average Price
Paid Per Share
($) | |
|---|
January 1 to 31, 2011 | | | 21,348 | | $ | 0.12 | |
February 1 to 28, 2011 | | | — | | | — | |
March 1 to 31, 2011 | | | — | | | — | |
April 1 to 30, 2011 | | | — | | | — | |
May 1 to 31, 2011 | | | — | | | — | |
June 1 to 30, 2011 | | | — | | | — | |
July 1 to 31, 2011 | | | 6,548 | | $ | 0.08 | |
August 1 to 31, 2011 | | | — | | | — | |
September 1 to 30, 2011 | | | — | | | — | |
October 1 to 31, 2011 | | | — | | | — | |
November 1 to 30, 2011 | | | — | | | — | |
December 1 to 31, 2011 | | | — | | | — | |
| | | | | | | |
Total | | | 27,896 | | | | |
| | | | | | | |
Upon the termination of employees during the year ended December 31, 2011, 596,436 unvested restricted shares were forfeited. The following table provides information about our forfeited restricted shares for the year ended December 31, 2011:
| | | | |
Period | | Total Number of
Shares Forfeited | |
|---|
January 1 to 31, 2011 | | | 174,561 | |
February 1 to 28, 2011 | | | — | |
March 1 to 31, 2011 | | | — | |
April 1 to 30, 2011 | | | — | |
May 1 to 31, 2011 | | | 43,750 | |
June 1 to 30, 2011 | | | 153,125 | |
July 1 to 31, 2011 | | | 37,500 | |
August 1 to 31, 2011 | | | 75,000 | |
September 1 to 30, 2011 | | | 75,000 | |
October 1 to 31, 2011 | | | 37,500 | |
November 1 to 30, 2011 | | | — | |
December 1 to 31, 2011 | | | — | |
| | | | |
Total | | | 596,436 | |
| | | | |
ITEM 6. SELECTED FINANCIAL DATA
As a smaller reporting company, we are not required to provide disclosure pursuant to this Item.
36
Table of Contents
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following "Management's Discussion and Analysis of Financial Condition and Results of Operations", as well as disclosures included elsewhere in this Form 10-K, include "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. This Act provides a safe harbor for forward-looking statements to encourage companies to provide prospective information about themselves so long as they identify these statements as forward-looking and provide meaningful cautionary statements identifying important factors that could cause actual results to differ from the projected results. All statements other than statements of historical fact we make in this Form 10-K are forward-looking. In particular, the statements herein regarding future sales and operating results; our ability to raise capital or finance our operations; Company and industry growth and trends; growth of the markets in which we participate; international events; product performance; the generation, protection and acquisition of intellectual property, and litigation related to such intellectual property; new product introductions; development of new products, technologies and markets; the acquisition of or investment in other entities; the construction of new or refurbishment of existing facilities by us; and statements preceded by, followed by or that include the words "intends", "estimates", "plans", "believes", "expects", "anticipates", "should", "could" or similar expressions, are forward-looking statements. Forward-looking statements reflect our current expectations and are inherently uncertain. Our actual results may differ significantly from our expectations. We assume no obligation to update this forward-looking information. The section entitled "Risk Factors" describes some, but not all, of the factors that could cause these differences.
The following discussion and analysis should be read in conjunction with our historical financial statements and the notes to those financial statements which are included in Item 8 of Part II of this Form 10-K.
BUSINESS OVERVIEW
Helicos BioSciences Corporation ("Helicos" or the "Company") is a life sciences company focused on innovative genetic analysis technologies. Helicos has developed a proprietary technology to enable the rapid analysis of large volumes of genetic material by directly sequencing single molecules of DNA or RNA. Our tSMS approach differs from current methods of sequencing DNA or RNA because it analyzes individual molecules of DNA directly instead of analyzing a large number of copies of the molecule which must be produced through complex sample preparation techniques. Our tSMS technology eliminates the need for costly, labor-intensive and time-consuming sample preparation techniques, such as amplification or cloning, which are required by other methods to produce a sufficient quantity of genetic material for analysis.
Our Helicos® Genetic Analysis Platform is designed to obtain sequencing information by repetitively performing a cycle of biochemical reactions on individual DNA or RNA molecules and imaging the results after each cycle. The platform consists of an instrument called the HeliScope™ Single Molecule Sequencer, an image analysis computer tower called the HeliScope™ Analysis Engine, associated reagents, which are chemicals used in the sequencing process, and disposable supplies. The information generated from using our tSMS products may lead to improved drug therapies, personalized medical treatments and more accurate diagnostics for cancer and other diseases.
As previously disclosed in our quarterly and periodic reports with the SEC, we have had limited operations to date and our activities have consisted primarily of raising capital, conducting research and development and recruiting personnel. Accordingly, we are considered to be in the development stage at December 31, 2011, as defined by the Financial Accounting Standards Board ("FASB"). We operate as one reportable segment. Helicos is a Delaware corporation and was incorporated on May 9, 2003.
37
Table of Contents
Furthermore, as of December 31, 2011 and March 23, 2012, we had $1.0 million and $34,000, respectively, in cash and cash equivalents.
As a result of several headcount reductions and restructurings during 2010, which eliminated approximately 60 positions, we entered the first quarter of 2011 with only 22 employees. In order to further conserve our limited resources, during the second quarter of 2011, we further reduced our workforce by phasing out six business and research positions. These six positions were phased out during the third quarter of 2011 and ultimately reduced our total headcount to ten positions. In addition, during the third quarter of 2011, we entered into a new lease reducing the size and associated expense of our leased facility in Cambridge, Massachusetts which houses our headquarters and scientific laboratory. We did not incur or pay out severance charges or lease exit costs in connection with the implementation of these measures. In January 2012, we made a final $1,070,000 payment on the senior debt facility pursuant to the Loan and Security Agreement among us, certain lenders and General Electric Capital Corporation, as agent, dated as of December 31, 2007, as amended (the "Loan Agreement"). As a result, our obligations under the Loan Agreement have been satisfied and the senior debt lenders no longer have a security interest in our assets, including our intellectual property assets.
Notwithstanding these most recent workforce reductions and despite our previous reductions, restructurings, expense saving measures, and the Bridge Debt Financing (see Note 8 of the Notes to the Consolidated Financial Statements), we do not have sufficient funds to operate our business. We continue to require significant additional capital on a month-to-month basis in order to continue our operations beyond the existing $2,000,000 committed portion of the Bridge Debt. As of the date of this filing, all $2,000,000 has been drawn against this committed facility (including $700,000 that we received under the Bridge Debt facility on November 30, 2011 and $633,334 that was received on December 22, 2011). Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified, but there can be no assurance that any such funding will be available on reasonable terms, if at all. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. No further commitments beyond the current outstanding notes with a maturity date of May 15, 2012, have been made under the uncommitted portion of the Bridge Debt Financing facility. We are currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that we will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs. As a result, we are no longer able to remain current in making payments to trade vendors and other unsecured creditors when such payments are due. Workforce reductions, as well as curtailed financial resources, will further impact our ability to operate our business. In addition, these constraints have caused us to significantly scale back service support and reagent supply to our current installed base. We have also significantly curtailed collaborative activities with other parties. Despite these measures, there cannot be any assurance that we will have sufficient resources to execute our business priorities.
During 2011, we deployed our limited resources by focusing on intellectual property monetization and concentrating our research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals, which were designed to enable us to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives. With a limited number of employees and resources, however, our ability to pursue research and development efforts in targeted areas that were designed to achieve tangible proof-of-concept goals connected with potential partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives proved unsuccessful and are unlikely
38
Table of Contents
to resume. Going forward, we are only able to focus our limited resources on intellectual property monetization and selected research and development activities that are primarily related to grant-funded initiatives and that support our limited sequencing services. In order to defend our intellectual property rights through licensing and enforcement strategies, we have taken several actions. First, on August 27, 2010, we filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") for patent infringement. The lawsuit, filed in the United States District Court for the District of Delaware, accuses Pacific Biosciences of infringing four patents and seeks injunctive relief and monetary damages. Second, on October 14, 2010, we announced the launch of a technology licensing program for our Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. Third, on October 22, 2010, we filed an amended complaint against Pacific Biosciences by naming Illumina, Inc. and Life Technologies Corporation as additional defendants. The amended lawsuit alleges that the defendants willfully infringe our patents in suit by using their respective technologies, sequencing systems and products which we allege are within the scope of one or more claims of our patents in suit. We are seeking a permanent injunction enjoining the defendants from further infringement as well as monetary and punitive damages, costs and attorneys' fees, interests and other relief as determined by the Court. See Part I, Item 3, Legal Proceedings, for more information regarding the litigation. We believe that the proceeds generated by these litigations, if we are successful, could be substantial. There is no assurance that we will be successful in the Patent Litigation, and even in the event of a favorable outcome in this litigation there can be no assurance that there will be any proceeds remaining for common stockholders, as a result of various arrangements in place with professional service providers, bridge lenders (including the Risk Premium Agreement with the bridge lenders), licensors and others. As of the date of this filing, we do not have adequate financial resources and will continue to need to generate additional funding from various sources including contract sequencing service projects, government grants and yet to be identified third party financing arrangements in order to continue our intellectual property monetization strategy. There can be no assurance that we will be able to generate the funding necessary to continue our intellectual property monetization strategy. If we are unable to execute our operations according to our plans and obtain additional financing, we will be forced to cease operations.
In connection with the Bridge Debt Financing and as security for our obligations under our contingency fee arrangement with outside intellectual property litigation counsel relating to the patent infringement lawsuit we filed against Pacific Biosciences of California, Inc., Illumina, Inc. and Life Technologies Corporation, we entered into a subordinated security agreement with outside intellectual property litigation counsel. Pursuant to this agreement, we agreed to grant outside intellectual property litigation counsel a priority security interest in the patent infringement lawsuit. Under the terms of the contingency fee arrangement, we are obligated to pay outside intellectual property litigation counsel up to 40% of the proceeds paid to us in connection with the settlement, judgment or other resolution of the Cause of Action (as defined in the contingency fee arrangement) or certain business transactions resulting from or relating to the patent infringement lawsuit. To date, we, and those providing professional services in connection with this patent litigation on a contingent basis have expended significant resources in pursuit of this intellectual property monetization effort and are expected to continue to do so going forward. There is no assurance that we will be successful in this litigation, and even in the event of a favorable outcome in this litigation there can be no assurance that there will be any proceeds remaining for common stockholders, as a result of various arrangements in place with professional service providers, bridge lenders (including the Risk Premium Agreement), licensors and others.
As an inducement for Atlas Venture and Flagship Ventures (the "Purchasers") to enter into the Bridge Debt Financing, we entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") (see Note 8 of the Notes to the Consolidated Financial Statements). Under the terms of the Risk Premium Agreement, we have agreed to pay the Purchasers the following portions of the consideration we receive (the "Payment
39
Table of Contents
Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). For purposes of determining amounts payable under the Risk Premium Agreement, the Payment Consideration will not include amounts we use to first satisfy specified existing obligations, including obligations under the Loan Agreement, under professional contingency arrangements (including the contingency fee arrangement between us and Outside IP Litigation Counsel relating to the Cause of Action) and obligations that may arise under existing technology license agreements (the "Existing IP Licensing Agreements") between us and each of Arizona Technology Enterprises, Roche Diagnostics GMBH and Roche Diagnostics Corporation, and California Institute of Technology (as previously disclosed by us, under the Existing IP Licensing Agreements, we are obligated to pay license fees in connection with the licensing, sublicensing, legal settlements or sales of specified intellectual property or services provided to third parties ranging from a single digit percentage up to one-half of the income from those activities). For purposes of the Risk Premium Agreement and subject to various provisions therein, the following shall constitute Liquidity Transactions: (A) the receipt by us of any payments from third parties relating to our intellectual property portfolio of the Company, including payments received as a license or other settlement in patent infringement litigation brought by us ("IP Licensing Events"); (B) a merger of the Company in which there is a change of control; and (C) the sale or exclusive license by us of all or substantially all of our assets or intellectual property.
In approving the transactions relating to the Bridge Debt Financing, the Board approved a management incentive plan (the "Management Incentive Plan") pursuant to which participants in the Management Incentive Plan, including Dr. Ivan Trifunovich and Mr. Jeffrey Moore, will be entitled to receive, in the aggregate, an amount equal to 7.5% of certain payment consideration resulting from intellectual property licensing events. The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board. The Bridge Debt Financing and Risk Premium Agreement and all of the transactions contemplated by those agreements were approved by an independent committee of the Board. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, Ph.D., who is a Partner of Atlas Venture, abstained from the vote of the Board approving the transactions.
Our future capital requirements will depend on many factors and we may require additional capital beyond our currently anticipated amounts. Any such required additional capital may not be available on reasonable terms, if at all, given our prospects, the current economic environment and restricted access to capital markets. The continued depletion of our resources may make future funding more difficult or expensive to attain. Additional equity financing may be dilutive to our stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict our ability to operate as a business; and strategic partnerships may result in royalties or other terms which reduce our economic potential from any adjustments to our existing long-term strategy. If we are unable to execute our operations according to our plans or to obtain additional financing, we may be forced to cease operations and/or seek bankruptcy protection. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets or the amount of reclassification of liabilities, or any adjustment that might be necessary should we be unable to continue as a going concern.
Since our inception, we have incurred losses and have not generated positive cash flows from operations. We expect our losses to continue for a considerable period of time. These losses, among
40
Table of Contents
other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders' equity/(deficit). As of December 31, 2011 and March 23, 2012, we had $1.0 million and $34,000, respectively, in cash and cash equivalents. We will, on a month-to-month basis during 2012, require significant additional capital to continue our operations. As of the date of this filing, all amounts available under the $2,000,000 committed facility have been drawn and no additional funding under the committed facility is available to support ongoing operations. Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified. Although we are engaged in active discussions with the Bridge Debt lenders with regard to securing funding from the $2,000,000 uncommitted portion of the Bridge Debt facility and are also exploring other funding options with the Bridge Debt lenders, there can be no assurance that any such funding or other sources of funding will be available to us on reasonable terms, if at all. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. We are currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that we will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs. If the Company is unable to execute its operations according to its plans and obtain additional financing, the Company will be forced to cease operations and/or seek bankruptcy protection. If we are required to seek protection from our creditors through a bankruptcy filing, it is likely that there would be little or no assets available for payment or distribution to our stockholders, and that our stockholders will lose their entire investment in us. In addition, if we are unable to obtain sufficient funding we may seek to sell, license or otherwise dispose of a portion of our assets in order to raise funding, or pursue the sale of all or substantially all our assets or a sale of the Company. We believe it is very unlikely that stockholders would receive any significant value from any such transaction. Because our present capital resources are not sufficient to fund our planned operations for a twelve month period as of the date of this Annual Report on Form 10-K for the year ended December 31, 2011, our current financial resources raise substantial doubt about our ability to continue as a going concern.
We were incorporated in Delaware in May 2003 under the name RareEvent Medical Corporation, renamed Newco LS6, Inc. in September 2003 and ultimately renamed Helicos BioSciences Corporation in November 2003. Our activities to date have consisted primarily of conducting research and development. Accordingly, we are considered to be in the development stage at December 31, 2011, as defined by the Financial Accounting Standards Board ("FASB"). Our fiscal year ends on December 31, and we operate as one reportable segment. Our corporate offices are located at One Kendall Square, Building 200, Cambridge, Massachusetts 02139.
FINANCIAL OVERVIEW
Product revenue
Product revenue for the year ended December 31, 2011 consists primarily of $571,000 of revenue recognized from the sale of proprietary reagents to customers. Product revenue for the year ended December 31, 2010 consists of $750,000 of revenue recognized from the sale of one Helicos Systems for which all acceptance criteria were satisfied during the period and approximately $550,000 of revenue recognized from the sale of proprietary reagents to customers. Product revenue for the year ended December 31, 2009 consists of $1.8 million of revenue recognized from the sale of two instrument systems for which all acceptance criteria were satisfied during the period and $500,000 of revenue recognized from the sale of proprietary reagents to customers for the year ended December 31, 2009.
41
Table of Contents
Service revenue
Service revenue for the year ended December 31, 2011 consists of $1.2 million of revenue recognized from the sales of DNA and RNA sequencing services. We had no significant service revenues for the years ending 2010 and 2009.
Grant revenue
In September 2006, we were awarded a grant from the National Human Genome Research Institute ("NHGRI"), a branch of the National Institutes of Health ("NIH"), pursuant to which we are eligible to receive reimbursement of our research expenses of up to $2.0 million through August 2009. We recognized revenue during the years ended December 31, 2008 and 2009 of $772,000 and $454,000, respectively, in connection with this award. We have fully expended all available funds under this grant as of December 31, 2009.
In September 2009, we were awarded another grant from the NHGRI, pursuant to which we are eligible to receive reimbursement of our research expenses of up to $2.9 million through August 2011. The two year grant is part of the Sequencing Technology Development Program, representing one of NHGRI's signature projects under the American Recovery and Reinvestment Act's effort to jumpstart the economy and create or save millions of jobs. This project, part of NIH's "Grand Opportunities" program, is designed to support large-scale, high impact research projects that are expected to accelerate critical scientific breakthroughs and enable growth and investment in biomedical research and development. In connection with this award we recognized $247,000, $2.2 million and $432,000 in revenue in the years ended December 31, 2009, 2010 and 2011, respectively. We have fully expended all available funds under this grant as of December 31, 2011.
In April 2010, we were awarded an RO1 Research Project Grant from the NHGRI, pursuant to which we are eligible to receive reimbursement for our research expenses of up to $1.6 million through February 2013. We are pursuing research related to direct RNA sequencing under this grant. In connection with this award, we recognized $346,000 and $389,000 in revenue in the years ended December 31, 2010 and 2011, respectively.
In April 2010, we completed a collaborative study that was sponsored by The Children's Oncology Group, under which we were reimbursed for our research expenses and recognized grant revenue of $199,000 in the year ended December 31, 2010. The purpose of the study was to generate large-scale data sets and obtain novel information that would allow deeper insights into cancer genomes with a focus on Ewing's Sarcoma.
In June 2010, we were awarded a Phase 1 Small Business Innovation Research grant from the NHGRI under which we will be pursuing research related to the development of methods to sequence patient DNA and RNA samples at an attomole level. The phase 1 portion of the grant provides funding for eligible research expenses of up to $146,000 through November 2010. Subsequent to December 31, 2010, we were awarded approximately $1.5 million in additional funding under this grant. In connection with this award we recognized $141,000 and $656,000 in grant revenue in the years ended December 31, 2010 and 2011, respectively.
In October 2010, we were awarded three federal grants totaling approximately $722,000 under the Patient Protection and Affordable Care Act of 2010, of which $472,000 was received during the fourth quarter of 2010 and reflected in other income and $250,000 was received during the first quarter of 2011. The costs associated with these grants were incurred in prior 2009 and 2010 fiscal periods. The grants were awarded under the Qualified Therapeutic Discovery Project (QTDP) which is aimed towards producing products that diagnose diseases or determine molecular factors related to diseases by developing molecular diagnostics to guide therapeutic decisions. No further amounts are expected to be received under these awards.
42
Table of Contents
Cost of product revenue
Cost of product revenue for the years ended December 31, 2009, 2010 and 2011 was $1.2 million, $0.7 million, and $0.2 million, respectively, and is related to the sale of instruments and proprietary reagents.
Research and development expenses
Research and development expenses consist of costs associated with scientific research activities, and engineering development efforts, including costs related to grant revenue. Such costs primarily include salaries, benefits and stock-based compensation; lab and engineering supplies; investment in equipment; consulting fees; and facility related costs, including rent and depreciation. During the years ended December 31, 2009, 2010 and 2011, research and development expenses included $1.9 million, $0.7 million, and $0 respectively, of labor and overhead costs associated with the under-utilization of our manufacturing facility. Research and development expenses for the years ended December 31, 2009, 2010 and 2011 were $18.3 million, $14.0 million and $4.9 million, respectively. In December 2008, we implemented a 30% reduction in our workforce and took other measures to reduce our future operating costs. From 2008 to 2009, our expenses decreased in connection with the reduction in force plan implemented in December 2008, as we focused more of our efforts on manufacturing activities and spent less time and resources on research and development activities. In 2010, we had two further reductions in force, the economic benefits from which started to be realized in the third quarter of fiscal year 2010 and fully realized in fiscal 2011. In 2011, we had one reduction in force which was implemented through the phase out of certain positions during the third quarter of 2011. We did not incur or payout any severance charges related to this reduction. Additionally, in fiscal years 2009, 2010 and 2011, we recorded charges of $3.1 million, $2.6 million and $2.0 million, respectively, in connection with write-offs of certain inventory or accrual of non-cancellable purchase commitments for inventory items that are no longer used in the business and we included this charge in research and development expense.
We continue to believe that the Helicos System can potentially access a wide range of genetic analysis tests useful to the basic, pharmaceutical, and biomedical research and diagnostic markets. In addition, in the past we have envisioned a series of performance enhancements to the Helicos System and chemistries and consumables used on the initial Helicos System which would have potentially served to greatly enhance the sequencing throughput. Each of these research and development projects is dependent upon achieving technical objectives, which are inherently uncertain. As a result of these uncertainties, we are unable to predict to what extent we will receive cash inflows from the sale of future tests or from the future enhanced throughput. As of the date of this Annual Report on Form 10-K, we do not have adequate resources to pursue the further development of these tests or enhanced throughput initiatives.
Impairment of Long-Lived Assets
During the third quarter of 2010, we altered our business direction as a result of our inability to raise funding to support the repositioning of our business in the genetic analysis markets. Following the reduction of our business efforts in the genetic analysis markets, we redirected our efforts to focus on a technology licensing and enforcement program to monetize the value of our intellectual property portfolio.
As a result of this altered business direction, we undertook an analysis of the ongoing carrying value of our long-lived assets. Based on this analysis, during the twelve months ended December 31, 2010, we recorded an impairment charge related to long-lived assets of approximately $1.9 million, of which approximately $1.8 million was related to assets used in research and development and
43
Table of Contents
approximately $0.1 million was related to assets used for selling, general and administrative purposes. These charges were derived from the net book value of the assets.
Selling, general and administrative expenses
Selling, general and administrative expenses consist principally of salaries, benefits and stock-based compensation, consulting and professional fees, including patent related costs, general corporate costs and facility costs not otherwise included in research and development expenses or cost of product revenue.
Selling, general and administrative expenses for the years ended December 31, 2009, 2010 and 2011 were $12.5 million, $10.5 million and $4.1 million, respectively. The decrease in selling, general and administrative expense between 2009 and 2011 is largely attributable to several reductions in force that we implemented in 2010 and 2011.
Restructuring
In December 2008, we implemented a reduction in force plan that resulted in the reduction of approximately 30% of our workforce and restructuring charges relating to one-time termination benefits of approximately $433,000. These charges represent employee severance and termination costs which were paid out during the fourth quarter of 2008 and the first quarter of 2009.
In fiscal year 2010, we implemented two additional reductions in force as part of our repositioning strategy in the diagnostics market. In May 2010, we eliminated approximately 40 positions and incurred restructuring charges relating to employee severance and termination costs as well as facility consolidation costs of approximately $549,000 in fiscal year 2010, of which $543,000 was recorded in research and development expense and $6,000 was recorded in selling, general and administrative expense. As of December 31, 2010, there were no remaining payments due pursuant to this restructuring.
In September 2010, we implemented a reduction in force of approximately 14 positions and incurred restructuring charges relating to one-time termination benefits of approximately $172,000 all of which was recorded in research and development expense. As of December 31, 2010, the balance of this restructuring cost was $11,000. This remaining restructuring cost was paid in 2011. We estimate the annualized savings from these 2010 restructurings to be approximately $8.2 million.
In June 2011, we implemented a reduction of force of approximately six business and research positions. These six positions were phased out during the third quarter of 2011. We did not incur or payout severance charges in connection with this reduction.
RESULTS OF OPERATIONS
Year ended December 31, 2010 compared to year ended December 31, 2011
Revenues. Revenue for the years ended December 31, 2010 and 2011 were as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2010 | | 2011 | | Change | | % | |
|---|
Product revenue | | $ | 1,301 | | $ | 571 | | $ | (730 | ) | | -56 | % |
Service revenue | | | — | | | 1,182 | | | 1,182 | | | — | % |
Grant revenue | | | 3,096 | | | 1,477 | | | (1,619 | ) | | -52 | % |
| | | | | | | | | | | |
| | $ | 4,397 | | $ | 3,230 | | $ | (1,167 | ) | | | |
| | | | | | | | | | | |
44
Table of Contents
Product revenue. We recognized $1.3 million of product revenue during the year ended December 31, 2010, and $0.6 million of product revenue during the year ended December 31, 2011. The $0.7 million decrease from the year ended December 31, 2010 to the year ended December 31, 2011 is due primarily to the recognition of revenue related to the sale of one Helicos System in fiscal year 2010. We had no systems sales during the year ended December 31, 2011. As of December 31, 2010, we are no longer actively engaged in the sale of Helicos Systems. As of December 31, 2011, we had $8.0 million in deferred revenue which relates to six Helicos Systems for which all revenue recognition criteria have not been met. All cash receipts related to the amounts recorded in deferred revenue have been received, and future recognition of deferred revenue will not result in any further cash receipts. The $0.9 million costs for these deferred products is recorded in inventory. On March 21, 2012, we received an attorney demand letter from one of our major customers relating to certain product shipments reflected in deferred revenue. See Part I, Item 3, Legal Proceedings, for further information regarding the attorney demand letter.
Service revenue. We recognized $1.2 million of service revenue during the year ended December 31, 2011. We had no significant service revenue during the year ended December 31, 2010. Service revenue is related primarily to DNA and RNA sequencing services.
Grant revenue. We recognized $3.1 million of grant revenue during the year ended December 31, 2010, and $1.5 million of grant revenue during the year ended December 31, 2011. We worked on four grants in fiscal year 2010 compared to three grants in fiscal year 2011. Grant revenue is related to the reimbursement of expenses in connection with our government research grants.
Cost of product revenue. Cost of product revenue for the years ended December 31, 2010 and 2011 was as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2010 | | 2011 | | Change | | % | |
|---|
Cost of product revenue | | $ | 713 | | $ | 224 | | $ | (489 | ) | | -69 | % |
Cost of product revenue for the year ended December 31, 2010 was $0.7 million compared to $0.2 million for the year ended December 31, 2011. In fiscal year 2010, cost of product revenue included the net production cost of one Helicos System recognized as sales as well as the cost of proprietary reagents shipped to customers and recognized as revenue during this period. In fiscal year 2011, cost of product revenue included the cost of proprietary reagents shipped to customers and recognized as revenue during this period. In March 2011, the Broad Institute, Inc. returned their Helicos System to us. There are no costs to be reflected in the financial statements related to the return of the Helicos System from the Broad Institute, Inc.
Research and development expenses. Research and development expenses during the years ended December 31, 2010 and 2011 were as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2010 | | 2011 | | Change | | % | |
|---|
Research and development | | $ | 14,047 | | $ | 4,930 | | $ | (9,117 | ) | | -65 | % |
Research and development expenses decreased $9.1 million from the year ended December 31, 2010 to the year ended December 31, 2011. This decrease was due primarily to a number of cost reductions implemented during fiscal years 2010 and 2011 as we shifted our strategic focus toward the molecular diagnostic market in the first half of 2010 and ultimately to a focus on a technology licensing and enforcement program to monetize the value of our intellectual property portfolio in the later part of 2010. As a result of significant reduction in the scope of our operations during fiscal years 2010 and
45
Table of Contents
2011, cash based expense reductions for fiscal year 2011 totaling $7.2 million were realized in various areas including but not limited to: labor costs totaling $3.9 million, research support costs of $0.7 million, restructuring costs of $0.6 million, facility cost of $0.8 million and other various costs totaling $1.2 million. Additional fiscal 2011 non-cash based expense reductions totaling $1.9 million included a reduction in stock based compensation of $1.1 million and a reduction in depreciation expense of $0.8 million. Included in research and development expenses are costs associated with service and grant revenue. We recorded a non-cash charge of $2.0 million to research and development expense in the fourth quarter of 2011 related to deferred inventory costs that we concluded were no longer realizable.
Selling, general and administrative expenses. Selling, general and administrative expenses during the years ended December 31, 2010 and 2011 were as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2010 | | 2011 | | Change | | % | |
|---|
Selling, general and adminstrative | | $ | 10,506 | | $ | 4,124 | | $ | (6,382 | ) | | -61 | % |
Selling, general and administrative expenses decreased $6.4 million from the year ended December 31, 2010 to the year ended December 31, 2011. This decrease was due primarily to a significant decrease in expenses related to the reduction in force that was implemented during fiscal year 2010 and fiscal year 2011 that eliminated all selling activity and significantly reduced G&A activity as we focused on developing a strategy to pursue the molecular diagnostic market and ultimately implement a strategy to pursue a focus on a technology licensing and enforcement program to monetize the value of our intellectual property portfolio. As a result of significant reduction in the scope of our operations during fiscal years 2010 and 2011, cash based expense reductions for fiscal year 2011 totaling $3.0 million were realized in various areas including but not limited to: labor costs of $1.8 million, facility costs of $0.3 million, and other various costs totaling $0.9 million. Additional fiscal 2011 non-cash based expense reductions totaling $3.4 million included a reduction stock based compensation of $3.2 million and a reduction in depreciation expense $0.2 million.
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2010 | | 2011 | | Change | | % | |
|---|
Impairment of long-lived assets | | $ | 1,894 | | $ | — | | $ | (1,894 | ) | | -100 | % |
During the third quarter of 2010, we altered our business direction as a result of our inability to raise funding to support the repositioning of our business in the genetic analysis markets. As a result of this altered business direction, we undertook an analysis of the ongoing carrying value of our long lived assets. We determined that the ongoing value of certain laboratory equipment was equivalent to our liquidation value, which we estimated was approximately ten percent of the original purchase price based on the value realized by other similarly situated life science companies that have been forced to realize value through a liquidation process. Based on this analysis, during the twelve months ended December 31, 2010, we recorded an impairment charge related to long lived assets of approximately $1.9 million, of which approximately $1.8 million was related to assets used in research and development and approximately $0.1 million was related to assets used for selling, general and administrative purposes.
46
Table of Contents
Interest income. Interest income for the years ended December 31, 2010 and 2011 was as follows:
| | | | | | | | | | | | | |
| | Year ended
December 31, | |
| |
| |
|---|
($ in thousands)
| | 2010 | | 2011 | | Change | | % | |
|---|
Interest income | | $ | 19 | | $ | — | | $ | (19 | ) | | -100 | % |
The decrease in interest income from the year ended December 31, 2010 compared to the year ended December 31, 2011 was due primarily to significantly lower cash balances.
Interest expense. Interest expense for the years ended December 31, 2010 and 2011 was as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2010 | | 2011 | | Change | | % | |
|---|
Interest expense | | $ | 614 | | $ | 680 | | $ | 66 | | | 11 | % |
The increase in interest expense from the year ended December 31, 2010 compared to the year ended December 31, 2011 is attributable to the amortization of deferred financing costs, related to the Bridge Debt and Notes in 2011.
Other income. Other income for the years ended December 31, 2010 and 2011 was as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2010 | | 2011 | | Change | | % | |
|---|
Other income | | $ | 472 | | $ | 900 | | $ | 428 | | | 91 | % |
The increase in other income from the year ended December 31, 2010 compared to the year ended December 31, 2011 is due to the receipt of the remaining $250,000 in federal government related to the Therapeutic Discovery Tax Credit pursuant to grants we received under the Patient Protection and Affordable Care Act of 2010, and the receipt of $649,000 from a business interruption claim we made in 2010. In the year ending December 31, 2010 we received $472,000 from the federal government related to the Therapeutic Discovery Tax Credit pursuant to grants we received under the Patient Protection and Affordable Care Act of 2010.
Change in fair value of warrant liability. Change in fair value of warrant liability for the years ended December 31, 2010 and 2011 was as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2010 | | 2011 | | Change | | % | |
|---|
Change in fair value of warrant liability | | $ | 4,118 | | $ | 3 | | $ | (4,115 | ) | | -100 | % |
In connection with the September and December 2009 equity offerings, we issued warrants to purchase an aggregate of 6,482,509 shares of common stock. The fair value of the warrants is recorded as a liability on the balance sheet and was estimated at $0.2 million and $25,000 at December 31, 2010 and 2011, respectively. The fair value of the warrant liability is determined at the end of each reporting period with the resulting gains and losses recorded as the change in fair value of warrant liability on the income statement. For the years ended December 31, 2010 and 2011, we realized a gain of $4.1 million and $3,000, respectively, due to the change in the fair value of the warrant liability. The gain in fiscal year 2010 was principally a result of the decrease of our stock price between the closing dates of the respective equity offerings and December 31, 2010 and the gain in fiscal year 2011 was a result of the decrease of our stock price between December 31, 2010 and December 31, 2011. Future
47
Table of Contents
changes in the fair value of the warrant liability will be due primarily to fluctuations in the value of our common stock.
Year ended December 31, 2009 compared to year ended December 31, 2010
Revenues. Revenue for the years ended December 31, 2009 and 2010 were as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2009 | | 2010 | | Change | | % | |
|---|
Product revenue | | $ | 2,325 | | $ | 1,301 | | $ | (1,024 | ) | | -44 | % |
Grant revenue | | | 701 | | | 3,096 | | | 2,395 | | | 342 | % |
| | | | | | | | | | | |
| | $ | 3,026 | | $ | 4,397 | | $ | 1,371 | | | 45 | % |
| | | | | | | | | | | |
Product revenue. We recognized $2.3 million of product revenue during the year ended December 31, 2009, and $1.3 million of product revenue during the year ended December 31, 2010. The $1.0 million decrease from the year ended December 31, 2009 to the year ended December 31, 2010 is due primarily to the recognition of revenue related to the sale of one Helicos System in fiscal year 2010 compared to the recognition of the sales of two Helicos Systems in fiscal year 2009 while sale of proprietary reagents to customers remained essentially constant in both years. As of December 31, 2010, we are no longer actively engaged in the sale of Helicos Systems. As of December 31, 2010, we had $7.2 million in deferred revenue which relates to six Helicos Systems for which all revenue recognition criteria have not been met.
Grant revenue. We recognized $0.7 million of grant revenue during the year ended December 31, 2009, and $3.1 million of grant revenue during the year ended December 31, 2010. We worked on four grants in fiscal year 2010 compared to two grants in fiscal year 2009. Grant revenue is related to the reimbursement of expenses in connection with our government research grants.
Cost of product revenue. Cost of product revenue for the years ended December 31, 2009 and 2010 was as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2009 | | 2010 | | Change | | % | |
|---|
Cost of product revenue | | $ | 1,239 | | $ | 713 | | $ | (526 | ) | | -42 | % |
Cost of product revenue for the year ended December 31, 2009 was $1.2 million compared to $713,000 for the year ended December 31, 2010. In fiscal year 2009, cost of product revenue included the net production cost of two Helicos Systems recognized as sales, the net cost of a system donated to the Broad Institute, Inc., as well as the cost of proprietary reagents shipped to customers and recognized as revenue during this period. In fiscal year 2010, cost of product revenue included the net production cost of one Helicos System recognized as sales as well as the cost of proprietary reagents shipped to customers and recognized as revenue during this period. In March 2011, the Broad Institute, Inc. returned their Helicos System to us. There are no costs to be reflected in the financial statements of the Company related to the return of the Helicos System from the Broad Institute, Inc.
48
Table of Contents
Research and development expenses. Research and development expenses during the years ended December 31, 2009 and 2010 were as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2009 | | 2010 | | Change | | % | |
|---|
Research and development | | $ | 18,256 | | $ | 14,047 | | $ | (4,209 | ) | | -23 | % |
Research and development expenses decreased $4.2 million from the year ended December 31, 2009 to the year ended December 31, 2010. This decrease was due primarily to a number of cost reductions implemented during fiscal year 2010 as we shifted our strategic focus toward the molecular diagnostic market. During fiscal year 2010, we significantly reduced research and development activities as we focused our efforts on developing molecular diagnostic tests resulting in a reduction of labor costs of approximately $2.3 million and a related reduction in research support costs of approximately $1.2 million. In connection with the change in our strategic focus, we discontinued the production of Helicos Systems in the first half of fiscal year 2010 which resulted in a reduction of pre-production variances of $1.2 million and the recognition of a further $2.6 million charge for excess and obsolete inventories during the third quarter of 2010, resulting in a decrease of $500,000 in excess and obsolete inventory charges compared to fiscal year 2009. Lastly, as a result of curtailing and ceasing our Helicos System manufacturing operations in early 2010, these costs were classified as research and development expenses for the period.
Selling, general and administrative expenses. Selling, general and administrative expenses during the years ended December 31, 2009 and 2010 were as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2009 | | 2010 | | Change | | % | |
|---|
Selling, general and adminstrative | | $ | 12,481 | | $ | 10,506 | | $ | (1,975 | ) | | -16 | % |
Selling, general and administrative expenses decreased $2.0 million from the year ended December 31, 2009 to the year ended December 31, 2010. This decrease was due primarily to a significant decrease in expenses related to the field application support group for fiscal year 2010 as a result of the curtailment of our commercial sales activity in early 2010 as we focus on developing a strategy to pursue the molecular diagnostic market. In addition during fiscal year 2010, cost reduction measures of approximately $300,000 related to the 2010 reduction in force activities were implemented and a reduction in depreciation expense of $600,000 was realized due to the expiration of useful lives of a significant portion of the fixed assets utilized by this function during the current fiscal period.
| | | | | | | | | | |
| | Year ended December 31, | |
| |
|---|
($ in thousands)
| | 2009 | | 2010 | | Change | |
|---|
Impairment of long-lived assets | | $ | — | | $ | 1,894 | | $ | 1,894 | |
During the third quarter of 2010, we altered our business direction as a result of our inability to raise funding to support the repositioning of our business in the genetic analysis markets. As a result of this altered business direction, we undertook an analysis of the ongoing carrying value of our long lived assets. We determined that the ongoing value of certain laboratory equipment was equivalent to our liquidation value, which we estimated was approximately ten percent of the original purchase price based on the value realized by other similarly situated life science companies that have been forced to realize value through a liquidation process. Based on this analysis, during the twelve months ended
49
Table of Contents
December 31, 2010, we recorded an impairment charge related to long lived assets of approximately $1.9 million, of which approximately $1.8 million was related to assets used in research and development and approximately $0.1 million was related to assets used for selling, general and administrative purposes.
Interest income. Interest income for the years ended December 31, 2009 and 2010 was as follows:
| | | | | | | | | | | | | |
| | Year ended
December 31, | |
| |
| |
|---|
($ in thousands)
| | 2009 | | 2010 | | Change | | % | |
|---|
Interest income | | $ | 75 | | $ | 19 | | $ | (56 | ) | | -75 | % |
The decrease in interest income from the year ended December 31, 2009 compared to the year ended December 31, 2010 was due primarily to significantly lower cash balances.
Interest expense. Interest expense for the years ended December 31, 2009 and 2010 was as follows:
| | | | | | | | | | | | | |
| | Year ended December 31, | |
| |
| |
|---|
($ in thousands)
| | 2009 | | 2010 | | Change | | % | |
|---|
Interest expense | | $ | 1,044 | | $ | 614 | | $ | (430 | ) | | -41 | % |
The decrease in interest expense from the year ended December 31, 2009 compared to the year ended December 31, 2010 is attributable to the year over year decrease in our outstanding senior debt obligations.
Other income. Other income for the years ended December 31, 2009 and 2010 was as follows:
| | | | | | | | | | |
| | Year ended December 31, | |
| |
|---|
($ in thousands)
| | 2009 | | 2010 | | Change | |
|---|
Other income | | $ | — | | $ | 472 | | $ | 472 | |
In the year ended December 31, 2010, we received $472,000 from the federal government related to the Therapeutic Discovery Tax Credit pursuant to grants we received under the Patient Protection and Affordable Care Act of 2010.
Change in fair value of warrant liability. Change in fair value of warrant liability for the years ended December 31, 2009 and 2010 was as follows:
| | | | | | | | | | | | | |
| | Year ended
December 31, | |
| |
| |
|---|
($ in thousands)
| | 2009 | | 2010 | | Change | | % | |
|---|
Change in fair value of warrant liability | | $ | 1,959 | | $ | 4,118 | | $ | 2,159 | | | 110 | % |
In connection with the September and December 2009 equity offerings, we issued warrants to purchase an aggregate of 6,482,509 shares of common stock. The fair value of the warrants is recorded as a liability on the balance sheet and was estimated at $5.3 million and $0.2 million at December 31, 2009 and 2010, respectively. The fair value of the warrant liability is determined at the end of each reporting period with the resulting gains and losses recorded as the change in fair value of warrant liability on the income statement. For the years ended December 31, 2009 and 2010, we realized a gain of $2.0 million and $4.1 million, respectively, due to the change in the fair value of the warrant liability. The gain in fiscal year 2009 was principally a result of the decrease of our stock price between the closing dates of the respective equity offerings and December 31, 2009 and the gain in fiscal year 2010 was a result of the decrease of our stock price between December 31, 2009 and December 31, 2010. Future changes in the fair value of the warrant liability will be due primarily to fluctuations in the value of our common stock.
50
Table of Contents
LIQUIDITY AND CAPITAL RESOURCES
We have incurred losses since our inception in May 2003 and, as of December 31, 2011, we have an accumulated deficit of $192.3 million. We have financed our operations to date principally through the sale of common stock and preferred stock, including our IPO, two private placements of common stock and warrants, an underwritten offering of common stock and warrants, debt financings and interest earned on investments and revenue. Through December 31, 2011, we have received net proceeds of $80.3 million through the issuance of common stock, including our IPO, private placements and an underwritten offering of common stock and warrants, $66.4 million from the issuance of preferred stock, $2.4 million in bridge debt financing, $20.3 million in debt financing from lenders for working capital, capital expenditures and general corporate purposes and $12.2 million of revenue. Working capital deficit as of December 31, 2010 was $(5.6) million, consisting of $6.7 million in current assets and $12.3 million in current liabilities. Working capital deficit as of December 31, 2011 was $(11.0) million, consisting of $2.5 million in current assets and $13.5 million in current liabilities. Our cash and cash equivalents are held in interest-bearing cash accounts. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily to achieve liquidity and capital preservation.
The following table summarizes our net increase (decrease) in cash and cash equivalents for the years ended December 31, 2009, 2010, 2011 and for the period from May 9, 2003 (date of inception) through December 31, 2011:
| | | | | | | | | | | | | |
| |
| |
| |
| | Period from
May 9, 2003
(date of inception)
through
December 31, 2011 | |
|---|
| | Year ended December 31, | |
|---|
($ in thousands)
| | 2009 | | 2010 | | 2011 | |
|---|
Net cash provided by (used in): | | | | | | | | | | | | | |
Operating activities | | $ | (15,488 | ) | $ | (11,967 | ) | $ | (941 | ) | $ | (138,782 | ) |
Investing activities | | | (22 | ) | | 231 | | | 75 | | | (9,433 | ) |
Financing activities | | | 11,727 | | | (1,681 | ) | | (648 | ) | | 149,214 | |
| | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | $ | (3,783 | ) | $ | (13,417 | ) | $ | (1,514 | ) | $ | 999 | |
| | | | | | | | | | |
Net cash used in operating activities was $12.0 million for the twelve months ended December 31, 2010 compared to $0.9 million for the twelve months ended December 31, 2011. The $11.1 million decrease was primarily due to a decrease in the net loss of $12.9 million from our reduction in research and development, and operating activities. An additional decrease in cash used by operating activities was $1.5 million of net changes in operating assets and liabilities, primarily comprised of a change in the use of cash of: $0.2 million related to accounts receivable and unbilled receivables, $0.9 million related to inventory, and $1.1 million related to accrued expenses and other current liabilities offset by $0.9 million in a reduction in the use of cash related to deferred revenue. These cash reductions are partially offset by the following non cash reductions : a) the reduction in various non cash costs totaling $7.6 million and including specific reductions of: $0.5 million in the provision for excess and obsolete inventory, $1.0 million in depreciation and amortization, $1.9 million for an impairment charge to long lived assets and $4.3 million in stock based compensation expense; which was further offset by b) the reduction of the non-cash benefit related to a $4.1 million decrease in the change in the fair value of the warrant liability.
Net cash used in operating activities was $15.5 million for the twelve months ended December 31, 2009 compared to $12.0 million for the twelve months ended December 31, 2010. The $3.5 million decrease was primarily due to a decrease in the net loss of $9.2 million and the $1.9 million impairment of long-lived assets in fiscal year 2010 partially offset by a $1.3 million decrease in depreciation and amortization and a $2.2 million increase related to the change in the fair value of the warrant liability. Additionally offsetting the decrease in cash used by operating activities was
51
Table of Contents
$4.8 million of net changes in operating assets and liabilities, primarily comprised of $3.6 million from deferred revenue and $3.0 million from accrued expenses and other current liabilities.
Net cash provided by investing activities of $231,000 for the twelve months ended December 31, 2010 is primarily due to a $225,000 reduction of restricted cash. Net cash provided by investing activities of $75,000 for the twelve months ended December 31, 2011 is due to the sale of fixed assets during the year.
Net cash provided from financing activities was $11.7 million for the twelve months ended December 31, 2009 compared to net cash usage of $1.7 million for the twelve months ended December 31, 2010. The $13.4 million decrease was primarily due to $15.0 million of proceeds from the issuance of common stock and warrants in fiscal year 2009, partially offset by a decrease in debt payments of $1.0 million and the Bridge Debt Financing of $667,000 in fiscal year 2010. Net cash used in financing activities was $1.7 million for the twelve months ended December 31, 2010 compared to net cash usage of $0.6 million for the twelve months ended December 31, 2011. The $1.0 million decrease in cash usage from financing activities was primarily due to $0.7 million of proceeds from Bridge Debt Financing and a decrease in debt payments of $0.3 million in fiscal year 2011.
Operating capital and capital expenditure requirements
Since our inception, we have incurred losses and have not generated positive cash flows from operations. We expect our losses to continue for a considerable period of time. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and equity/(deficit). As of December 31, 2011 and March 23, 2012, we had $1.0 million and $34,000, respectively, in cash and cash equivalents. We will, on a month-to-month basis during 2012, require significant additional capital to continue our operations, As of the date of this filing, all amounts available under the $2,000,000 committed facility have been drawn and no additional funding under the committed facility is available to support ongoing operations. Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified. Although we are engaged in active discussions with the Bridge Debt lenders with regard to securing funding from the $2,000,000 uncommitted portion of the Bridge Debt facility and are also exploring other funding options with the Bridge Debt lenders, there can be no assurance that any such funding or other sources of funding will be available to us on reasonable terms, if at all. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. We are currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that we will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs. If the Company is unable to execute its operations according to its plans and obtain additional financing, the Company will be forced to cease operations and/or seek bankruptcy protection. Because our present capital resources are not sufficient to fund our planned operations for a twelve month period as of the date of this Annual Report on Form 10-K for the year ended December 31, 2011, our current financial resources raise substantial doubt about our ability to continue as a going concern.
On June 28, 2010, we received a letter from The NASDAQ Stock Market LLC ("NASDAQ") advising that for the previous 30 consecutive business days, we failed to comply with the minimum market value of listed securities ("MVLS") requirement for continued listing on the NASDAQ Global Market pursuant to NASDAQ Marketplace Rule 5450(b)(2)(A) and that we did not otherwise satisfy the criteria for continued listing pursuant to NASDAQ Marketplace Rule 5450(b). NASDAQ stated in its letter that in accordance with NASDAQ Marketplace Rule 5810(c)(3)(C), we would be provided
52
Table of Contents
180 calendar days, or until December 27, 2010, to regain compliance with the MVLS continued listing requirement. MVLS is calculated by multiplying the total shares outstanding by the daily closing bid price.
On October 12, 2010, we received a delisting determination letter from NASDAQ due to our failure to regain compliance with the NASDAQ Global Market's minimum bid price requirement for continued listing as set forth in Listing Rule 5450(a)(1). In addition, we had not yet held our annual meeting of stockholders for fiscal 2010 which is an additional requirement for us to comply with in order to maintain our continued listing on NASDAQ. Based on the unlikely ability of us to meet the NASDAQ MLVS requirements due to the state of our finances and near-term business prospects, on November 12, 2010, we withdrew our appeal to NASDAQ regarding this delisting notice. As a result, our securities were delisted from the NASDAQ Global Market and the trading of our common stock was suspended before the opening of business on November 16, 2010, and a Form 25-NSE was subsequently filed with the SEC on January 21, 2011 which removed our securities from listing and registration on The NASDAQ Stock Market. We were advised by Pink OTC Markets Inc., which operates an electronic quotation service for securities traded over-the-counter ("OTC"), that our securities were immediately eligible for quotation on the OTCQB. The OTCQB is a market tier for OTC traded companies that are registered and reporting with the SEC.
Our future capital requirements will depend on many factors and we will require additional capital beyond our currently anticipated amounts. Any such required additional capital may not be available on reasonable terms, if at all, given our prospects, the current economic environment and restricted access to capital markets. The continued depletion of our resources may make future funding more difficult or expensive to attain. Additional equity financing may be dilutive to our stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict our ability to operate as a business; and strategic partnerships may result in royalties or other terms which reduce our economic potential from any adjustments to our existing long-term strategy. If we are unable to execute our operations according to our plans or to obtain additional financing, we may be forced to cease operations and/or seek bankruptcy protection. If we are required to seek protection from our creditors through a bankruptcy filing, it is likely that there would be little or no assets available for payment or distribution to our stockholders, and that our stockholders will lose their entire investment in us. In addition, if we are unable to obtain sufficient funding we may seek to sell, license or otherwise dispose of a portion of our assets in order to raise funding, or pursue the sale of all or substantially all our assets or a sale of the Company. We believe it is very unlikely that stockholders would receive any significant value from any such transaction. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets or the amount of reclassification of liabilities, or any adjustment that might be necessary should we be unable to continue as a going concern.
Failure to satisfy our capital requirements or any delay in doing so would have a material negative impact on our ability to continue as a going concern.
Through March 20, 2012, we received cumulative sales orders for ten Helicos Systems, the last unit we sold was in May 2010. During 2011 and from our inception to date, we recognized revenue on three of the ten sales orders, respectively. We have shipped six units that have not met our revenue recognition criteria and one sales order unit was returned. A system that was installed at The Broad Institute, Inc. on a no-cost basis was returned to us in March 2011. In addition, we had one Helicos System at a leading academic institution for scientific and commercial evaluation which was returned to us in March 2011.
The continued depletion of our resources make future funding more difficult or expensive to attain, if at all. Additional equity financing would be dilutive to our stockholders; debt financing, if available, would involve significant cash payment obligations and covenants that restrict our ability to operate as a business; and strategic partnerships would result in royalties or other terms which reduce
53
Table of Contents
our economic potential. If we are unable to execute our operations according to our plans or to obtain additional financing, we will be forced to cease operations.
Our forecast of the period of time through which our financial resources will be adequate to support our operations, and the costs associated with our new business priorities are forward-looking statements and involve risks and uncertainties, and actual results could vary materially and negatively as a result of a number of factors, including the factors discussed in the "Risk Factors" section of this Annual Report on Form 10-K. We have based these estimates on assumptions that may prove to be wrong, and we could consume our available capital resources sooner than we currently expect.
Because of the numerous risks and uncertainties associated with our business we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to sustain our operations. Our future capital requirements will depend on many factors, including, but not limited to, the following:
- •
- any litigation related to defending our intellectual property or defending lawsuits that allege our actions infringe intellectual property of third parties;
- •
- the success of our research and development efforts;
- •
- the revenue generated by future sales of our products or services;
- •
- the timeliness of payments from our customers;
- •
- the ability to structure arrangements with and make payments to our unsecured creditors;
- •
- the continued funding to the Bridge Debt Financing facility under the Note Purchase Agreement;
- •
- the emergence of competing or complementary technological developments;
- •
- the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
- •
- regulatory changes in our markets; and
- •
- the terms and timing of any collaborative, licensing or other arrangements that we may establish.
Notwithstanding these most recent workforce reductions and despite our previous reductions, restructurings, expense saving measures, and the Bridge Debt Financing, we do not have sufficient funds to operate our business. We continue to require significant additional capital on a month-to-month basis in order to continue our operations beyond the existing $2,000,000 committed portion of the Bridge Debt. As of the date of this filing, all $2,000,000 has been drawn against this committed facility. Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified, but there can be no assurance that any such funding will be available on reasonable terms, if at all. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. No further commitments beyond the current outstanding notes with a maturity date of May 15, 2012 have been made under the uncommitted portion of the Bridge Debt Financing facility. We are currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that we will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs. As a result, we are no longer able to remain current in making payments to trade vendors and other unsecured creditors when such payments are due. Workforce reductions, as well as curtailed financial resources, will further impact our ability to implement our business priorities. In addition, these constraints have caused us to significantly scale
54
Table of Contents
back service support and reagent supply to its current installed base. We have also significantly curtailed collaborative activities with other parties. Despite these measures, there cannot be any assurance that we will have sufficient resources to operate our business.
Contractual Obligations
The following table summarizes our outstanding obligations as of December 31, 2011 and the effect those obligations are expected to have on our liquidity and cash flows in future periods:
| | | | | | | | | | | | | | | | |
Contractual obligations | | Total | | Less than
1 year | | 1-3 years | | 3-5 years | | More than
5 years | |
|---|
| | ($ in thousands)
| |
|---|
Operating leases | | $ | 220 | | $ | 220 | | $ | — | | $ | — | | $ | — | |
Purchase commitments | | | 236 | | | 236 | | | — | | | — | | | — | |
Debt (including interest) | | | 3,276 | | | 3,276 | | | — | | | — | | | — | |
License agreements (1) | | | 920 | | | 162 | | | 324 | | | 324 | | | 110 | |
| | | | | | | | | | | | |
Total | | $ | 4,652 | | $ | 3,894 | | $ | 324 | | $ | 324 | | $ | 110 | |
| | | | | | | | | | | | |
- (1)
- Consists of fixed payments that we believe we are reasonably likely to make under the license agreements over the lives of the underlying existing patents.
The table above does not include possible royalties payable under our license agreements or contingent payments which may become due in connection with Risk Premium Payments due under the Risk Premium Agreement or proceeds derived from the current pending intellectual property litigation. Included in debt amounts are $1.0 million which was paid in January 2012. Not included in the table above is $375,000 drawn down from the uncommitted portion of the Bridge Debt in January 2012. Our current real estate lease on our offices in Cambridge, Massachusetts has a lease expiration of December 31, 2012.
License agreements and patents
We have fixed annual costs associated with license agreements into which we have entered. In addition, we may have to make contingent payments in the future upon the realization of certain milestones or royalties payable under these agreements.
Loan and security agreement
In December 2007, we entered into a loan and security agreement with two lenders including GE Capital Corporation, which is serving as agent. The loan agreement provided that we may borrow up to $20.0 million at an interest rate equal to the sum of (i) the greater of (A) an interest rate based on the Federal Reserve's three year Treasury Constant Maturities Rate and (B) 3.84% plus (ii) 6.11%. The initial term loan was made on the closing date in an aggregate principal amount equal to $10.0 million.
In June 2008, we entered into an amendment to the loan and security agreement with two lenders including GE Capital Corporation. A subsequent term loan was made upon execution of the amendment in an aggregate principal amount equal to $10.0 million. The loan amendment provided that the interest rate for the subsequent term loan is equal to the sum of (i) the greater of (A) an interest rate based on the Federal Reserve's three year Treasury Constant Maturities Rate and (B) 3.17% plus (ii) 8.33%. The loan agreement, as amended, contained affirmative and negative covenants to which we and our subsidiaries were required to adhere. Pursuant to the amendment, we were required to maintain, at all times, unrestricted cash in our bank account equal to at least $10.0 million. The borrowings under the loan agreement were collateralized by essentially all of our assets. Payments are required to be made on a monthly basis. For the initial term loan, interest-only payments were required for the first five months. Thereafter, for the following 31 months, payments of
55
Table of Contents
principal and interest will be due. For the subsequent term loan, principal and interest payments are required for the 36 month term of the loan As further discussed below under the caption "Bridge Financing and Senior Debt Restructuring (November 2010)," we restructured the loan and security agreement during the fourth quarter of 2010.
The loan and security agreement contains a subjective material adverse clause, which allows the debt holders to call the loans upon a material adverse event. Based on the terms of the loan and security agreement, the entire balance due is classified in the current liabilities section in the accompanying balance sheet at December 31, 2011.
As of December 31, 2010 and 2011, the outstanding balance on the loan agreement was $2.8 million and $1.1 million, respectively. In January 2012, we made a final $1,070,000 payment on the senior debt facility pursuant to the Loan and Security Agreement. As a result, our obligations under the Loan Agreement have been satisfied and the senior debt lenders no longer have a security interest in our assets, including our intellectual property assets.
Bridge Financing and Senior Debt Restructuring (November 2010)
We entered the fourth quarter of 2010 without any prospects of securing long-term financing from outside investors. An independent committee (the "Independent Committee") of our Board evaluated the alternatives available to us and concluded that the bridge financing led by certain inside investors was in the best interests of our stakeholders in view of our current financial and operating condition, and would best position us to continue operations for the remainder of 2010 and into 2011. The Independent Committee met multiple times to consider the alternatives and reached the conclusion that the bridge financing was the best alternative for generating value for stakeholders. As a result, we entered into an insider bridge loan facility with two of the four venture capital firms whose representatives are members of our Board. In connection with this transaction, we entered into a Subordinated Secured Note Purchase Agreement (the "Note Purchase Agreement") with investment funds affiliated with Atlas Venture and Flagship Ventures (the "Purchasers"), pursuant to which we have agreed to sell to the Purchasers, and the Purchasers have agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), $2,000,000 of which is committed and $2,000,000 of which is optional (the "Bridge Debt Financing"). We continue to require significant additional capital on a month-to-month basis in order to continue our operations beyond the existing $2,000,000 committed portion of the Bridge Debt. As of the date of this filing, all $2,000,000 has been drawn against this committed facility. Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified, but there can be no assurance that any such funding will be available on reasonable terms, if at all. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. No further commitments beyond the current outstanding notes with a maturity date of May 15, 2012 have been made under the uncommitted portion of the Bridge Debt Financing facility. We are currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that we will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs.
The Notes will accrue interest at a rate of 10% per annum plus a risk premium as described below. The outstanding principal and any unpaid accrued interest on all of the Notes shall be due and payable in full (i) upon demand by the Purchasers, which demand shall be no earlier than December 31, 2012, (ii) upon our receiving at least $10,000,000 in proceeds from a subsequent equity financing, (iii) upon a change of control of the Company or (iv) upon an event of default. A Purchaser
56
Table of Contents
may elect to convert principal and interest outstanding under any of the Notes upon the closing of any future round of equity or debt financing of the Company into shares, and at the per share price, of the securities issued at such financing. Simultaneous with execution of the Note Purchase Agreement, we and the requisite parties entered into an Amendment to the Amended and Restated Investor Rights Agreement, dated as of March 1, 2006, by and among us and the parties specified therein, including the Purchasers (the "IRA Amendment"), pursuant to which the Purchasers will be entitled to demand and piggyback registration rights consistent with the terms of the Purchasers' existing registration rights for any securities issued upon conversion of the Notes.
Our obligations under the Notes are secured by a security interest on all of our assets, including our intellectual property, that, with regard to all of our assets other than the Cause of Action, is junior to the Lenders' first priority security interest under the Loan Agreement, and with regard to the Cause of Action, is junior to the Lenders' first priority security interest under the Loan Agreement and the second priority security interest of our outside legal counsel that is representing us in the Cause of Action ("Outside IP Litigation Counsel").
In connection with the Bridge Debt Financing and as security for our obligations under our contingency fee arrangement with outside intellectual property litigation counsel relating to the patent infringement lawsuit we filed against Pacific Biosciences of California, Inc., Illumina, Inc. and Life Technologies Corporation, we entered into a subordinated security agreement with outside intellectual property litigation counsel. Pursuant to this agreement, we agreed to grant outside intellectual property litigation counsel a priority security interest in the patent infringement lawsuit. Under the terms of the contingency fee arrangement, we are obligated to pay outside intellectual property litigation counsel up to 40% of the proceeds paid to us in connection with the settlement, judgment or other resolution of the Cause of Action (as defined in the contingency fee arrangement) or certain business transactions resulting from or relating to the patent infringement lawsuit. To date, we, and those providing professional services in connection with this patent litigation on a contingent basis have expended significant resources in pursuit of this intellectual property monetization effort and are expected to continue to do so going forward. There is no assurance that we will be successful in this litigation, and even in the event of a favorable outcome in this litigation there can be no assurance that there will be any proceeds remaining for common stockholders, as a result of various arrangements in place with professional service providers, bridge lenders (including the Risk Premium Agreement with the bridge lenders), licensors and others.
As an inducement for Atlas Venture and Flagship Ventures (the "Purchasers") to enter into the Bridge Debt Financing, we entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") (see Note 8 of the Notes to the Consolidated Financial Statements). Under the terms of the Risk Premium Agreement, we have agreed to pay the Purchasers the following portions of the consideration we receive (the "Payment Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). For purposes of determining amounts payable under the Risk Premium Agreement, the Payment Consideration will not include amounts we use to first satisfy specified existing obligations, including obligations under the Loan Agreement, under professional contingency arrangements (including the contingency fee arrangement between us and Outside IP Litigation Counsel relating to the Cause of Action) and obligations that may arise under existing technology license agreements (the "Existing IP Licensing Agreements") between us and each of Arizona Technology Enterprises, Roche Diagnostics GMBH and Roche Diagnostics Corporation, and California Institute of Technology (as previously disclosed by us, under the Existing IP Licensing
57
Table of Contents
Agreements, we are obligated to pay license fees in connection with the licensing, sublicensing, legal settlements or sales of specified intellectual property or services provided to third parties ranging from a single digit percentage up to one-half of the income from those activities). For purposes of the Risk Premium Agreement and subject to various provisions therein, the following shall constitute Liquidity Transactions: (A) the receipt by us of any payments from third parties relating to the intellectual property portfolio of the Company, including payments received as a license or other settlement in patent infringement litigation brought by us ("IP Licensing Events"); (B) a merger of the Company in which there is a change of control; and (C) the sale or exclusive license by us of all or substantially all of our assets or intellectual property.
On March 29, 2012, we issued $175,000 of additional notes to the Purchasers pursuant to the Note Purchase Agreement, with a maturity date of May 15, 2012 and terms consistent with the previously issued notes (the "March 2012 Notes"). In addition, we entered into letter agreement amending certain Secured Promissory Notes in the aggregate original principal value of $200,000 issued by Helicos to the Purchasers in January 2012 (the "January 2012 Notes"). The March 29, 2012 letter agreement further extended the maturity date of the January 2012 Notes to May 15, 2012. The total original principal amount of the certain Secured Promissory Notes issued by Helicos since the beginning of calendar 2012 is $375,000 (the "Notes") and have a maturity date of May 15, 2012 (the "Maturity Date"). On February 22, 2012, we entered into a letter agreement amending the January 2012 Notes to extend the maturity date to March 31, 2012. We issued the January 2012 Notes on January 13, 2012 to the Purchasers pursuant to the Note Purchase Agreement. The January 2012 Notes were initially issued with a 12-day maturity in anticipation of closing a larger financing, which has not yet occurred. The Notes accrue interest at a rate of 10% per annum. The outstanding principal and any unpaid accrued interest on the Notes is due and payable on the earlier of (i) the Purchasers jointly demanding in writing the payment of the outstanding amounts at any time on or after the Maturity Date or (ii) the occurrence of an event of default. Our obligations under the Notes are secured by a security interest on all of our assets, including its intellectual property, that, with regard to all of our assets other than the Cause of Action and is junior to the security interest of our outside legal counsel that is representing us in the Cause of Action. We used the proceeds from the issuance of the January 2012 Notes along with available cash to repay all amounts outstanding under the Loan and Security Agreement among us, certain lenders and General Electric Capital Corporation, as agent, dated as of December 31, 2007, as amended.
In approving the transactions relating to the Bridge Debt Financing, the Board approved a management incentive plan (the "Management Incentive Plan") pursuant to which participants in the Management Incentive Plan, including Dr. Ivan Trifunovich and Mr. Jeffrey Moore, will be entitled to receive, in the aggregate, an amount equal to 7.5% of certain payment consideration resulting from intellectual property licensing events. The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board. The Bridge Debt Financing and Risk Premium Agreement and all of the transactions contemplated by those agreements were approved by an independent committee of the Board. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, Ph.D., who is a Partner of Atlas Venture, abstained from the vote of the Board approving the transactions.
OFF-BALANCE SHEET ARRANGEMENTS
During the twelve month periods ended December 31, 2010 and 2011, we did not have any special purpose entities or off-balance sheet financing arrangements.
58
Table of Contents
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our management's discussion and analysis of our financial condition and results of operations is based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported expenses during the reporting periods. We evaluate our estimates and judgments on an ongoing basis. Actual results may differ materially from these estimates under different assumptions or conditions.
Inventory
Prior to reaching technological feasibility, start-up manufacturing costs, such as those relating to the assembly, testing and performance validation of the Helicos® Genetic Analysis System, were expensed to research and development as the costs were incurred. When management determined that the Helicos System was ready for commercial launch during December 2007, we began capitalizing our manufacturing costs to inventory.
We value our inventory at the lower of cost or market on a first-in, first-out basis. Our policy is to capitalize inventory costs associated with its products when, based on management's judgment, future commercialization is considered probable and the future economic benefit is expected to be realized.
We periodically review our inventories for excess or obsolete inventory and write-down obsolete or otherwise unmarketable inventory to its estimated net realizable value. If the actual net realizable value is less than that estimated by us, or if there are any further determinations that inventory will not be marketable based on estimates of demand, additional inventory write-downs will be required.
We value inventory in accordance with FASB guidance on inventory costs and inventory pricing to clarify that abnormal amounts of idle facility expense, freight, handling costs, and wasted material (spoilage) should be recognized as current-period charges regardless of whether they meet the criterion of "so abnormal." In addition, guidance requires that allocation of fixed production overheads to the costs of conversion be based on the normal capacity of the production facilities.
We concluded that because we are not yet functioning under normal capacity, our production level is abnormally low. As such, abnormal costs such as the unfavorable labor, overhead and absorption variances were recognized as current period charges, rather than as a portion of the inventory cost. During the years ended December 31, 2009, 2010 and 2011, research and development expenses included $1.9 million, $0.7 million, and $0, respectively, of labor and overhead costs associated with the under-utilization of the manufacturing facility. During fiscal year 2010, we ceased manufacturing Helicos Systems. During the year ended December 31, 2009, we recorded a $3.1 million charge to write-off excess and obsolete inventory. This charge was recorded as a research and development expense resulted in a $1.7 million decrease to inventory and a $1.4 million increase to accrued expenses for excess non-cancellable purchase commitments. During the year ended December 31, 2010, we recorded a $2.6 million charge to research and development expense to write-off excess and obsolete inventory. We recorded a non-cash charge of $2.0 million to research and development expense in the fourth quarter of 2011 related to deferred inventory costs that we concluded were no longer realizable.
Revenue recognition
Government research grants that provide for payments to us for work performed are recognized as revenue when the related expenses are incurred.
We recognize revenue in accordance with FASB accounting guidance on revenue recognition in financial statements and accounting for multiple element revenue arrangements. This guidance requires that persuasive evidence of a sales arrangement exists; delivery of goods occurs through transfer of title
59
Table of Contents
and risk and rewards of ownership, the selling price is fixed or determinable and collectability is reasonably assured. We also follow FASB guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets.
In instances where we sell instruments with a related installation obligation, we will allocate the revenue between the instrument and the installation based on relative fair value at the time of the sale. The instrument revenue will be recognized when title and risk of loss passes. The installation revenue will be recognized when the installation is performed. If fair value is not available for any undelivered element, revenue for all elements is deferred until delivery and installation are complete.
In instances where we sell an instrument with specified acceptance criteria, we will defer revenue recognition until such acceptance has been obtained.
Revenue from service contracts sold in conjunction with the sale of equipment is recognized ratably over the service period.
Impairment of long-lived assets
Long-lived assets primarily include property and equipment. In accordance with FASB accounting guidance for the impairment or disposal of long-lived assets, we periodically review long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives are no longer appropriate. Each impairment test is based on a comparison of the undiscounted cash flows expected to result from the use and eventual disposition of the asset to the carrying amount of the asset. If impairment is indicated, the asset is written down to its estimated fair value based on a discounted cash flow analysis. Determining the fair value of long-lived assets includes significant judgment by management, and different judgments could yield different results. In fiscal year 2010, we incurred a charge for impairment of property and equipment of $1.9 million (see Note 5 of Notes to Consolidated Financial Statements).
Allowance for doubtful accounts
We plan to perform ongoing evaluations of our customers and continuously monitor collections and payments to estimate an allowance for doubtful accounts based on the aging of the underlying receivables and our experiences of specific collection issues.
Stock-based compensation
We account for stock-based compensation issued to non-employees in accordance with FASB accounting guidance accounting for equity instruments that are "Issued to Other Than Employees for Acquiring, or in Conjunction with, Selling Goods or Services." We record the expense of such services based on the estimated fair value of the equity instrument using the Black-Scholes option pricing model. The value of the equity instrument is charged to earnings over the term of the service agreement.
For stock-based compensation awards granted to both employees and non-employees, we use the fair value method of calculating stock-based compensation. Calculating the fair value of stock-based awards requires the input of highly subjective assumptions. We use the Black-Scholes option pricing model to value our stock option awards. Stock-based compensation expense is significant to our financial statements and is calculated using our best estimates which involve inherent uncertainties and the application of management's judgment. Significant estimates include the expected life of the stock option, stock price volatility, risk-free interest rate and forfeiture rates.
During the year ended December 31, 2011, we recognized approximately $0.8 million of stock-based compensation expense related to equity awards granted to employees and non-employees. Total unrecognized stock-based compensation expense for all stock-based awards was approximately $147,000
60
Table of Contents
at December 31, 2011, of which $60,000 will be recognized in 2012, $58,000 in 2013 and $29,000 thereafter. This results in these amounts being recognized over a weighted-average period of 1.0 years.
RECENT ACCOUNTING PRONOUNCEMENTS
In October 2009, the FASB issued an amendment to the accounting guidance for revenue arrangements with multiple deliverables. This guidance provides principles for allocation of consideration among its multiple-elements, allowing more flexibility in identifying and accounting for separate deliverables under an arrangement. The guidance introduces an estimated selling price method for valuing the elements of a bundled arrangement if vendor-specific objective evidence or third-party evidence of selling price is not available, and significantly expands related disclosure requirements. This guidance is effective on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. We adopted this accounting guidance on a prospective basis in the first quarter of 2011 for new and materially modified revenue arrangements. The adoption did not have a material impact on our consolidated financial statements.
In October 2009, the FASB issued an amendment to the accounting standards related to certain revenue arrangements that include software elements. This standard clarifies the existing accounting guidance such that tangible products that contain both software and non-software components that function together to deliver the product's essential functionality, shall be excluded from the scope of the software revenue recognition accounting standards. Accordingly, sales of these products may fall within the scope of other revenue recognition accounting standards or may now be within the scope of this standard and may require an allocation of the arrangement consideration for each element of the arrangement. We adopted this standard on January 1, 2011. This adoption did not have a material impact on our consolidated financial statements.
In January 2011, we adopted"Improving Disclosures About Fair Value Measurements" which requires additional disclosure about the amounts of and reasons for significant transfers in and out of Level 1 and Level 2 fair value measurements. In addition, effective for interim and annual periods beginning after December 15, 2010, which for us is January 1, 2011, this standard further requires an entity to present disaggregated information about activity in Level 3 fair value measurements on a gross basis, rather than as one net amount. As this accounting standard only requires enhanced disclosure, the adoption of this newly issued accounting standard did not impact our financial position or results of operations.
In May 2011, the FASB issued "Fair Value Measurement (Topic 820): Amendment to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs". This newly issued accounting standard clarifies the application of certain existing fair value measurement guidance and expands the disclosure for fair value measurements that are estimated using significant unobservable (Level 3) inputs. This ASU is effective on a prospective basis for annual and interim reporting periods beginning on or after December 15, 2011, which for us is January 1, 2012. We do not expect that adoption of this standard will have a material impact on our financial position or results of operations.
In June 2011, the FASB issued an amendment to the accounting guidance for presentation of comprehensive income. Under the amended guidance, a company may present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In either case, a company is required to present each component of net income along with total net income, each component of other comprehensive income along with a total for other comprehensive income, and a total amount for comprehensive income. Regardless of choice in presentation, a company is required to present on the face of the financial statements reclassification adjustments for items that are reclassified from other comprehensive income to net income in the statement(s) where the components of net income and the components of other comprehensive income
61
Table of Contents
are presented. For public companies, the amendment is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, and shall be applied retrospectively. Early adoption is permitted. Other than a change in presentation, the adoption of this update is not expected to have a material impact on our consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our exposure to market risk is limited to our cash. The goals of our investment policy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. We also seek to maximize income from our investments without assuming significant risk. To achieve our goals, we maintain our cash in interest-bearing cash accounts. As all of our investments are cash deposits in a global bank, it is subject to minimal interest rate risk.
EFFECT OF CURRENCY EXCHANGE RATES AND EXCHANGE RATE RISK MANAGEMENT
We conduct business operations outside of the United States primarily in Japan and to a lesser extent in certain European countries. The foreign denominated financial obligations that we are exposed to at this time in Japan are relatively short-term. Our practice in Europe has been to enter into US-dollar-denominated transactions with our customers. Therefore, any currency fluctuations will not have a material impact on our financial position, results of operations or cash flows.
62
Table of Contents
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Helicos BioSciences Corporation (A development stage company)
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | |
| | Page |
|---|
Report of independent registered public accounting firm | | 64 |
Consolidated balance sheets as of December 31, 2010 and December 31, 2011 | |
65 |
Consolidated statements of operations for the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) to December 31, 2011 | |
66 |
Consolidated statements of redeemable convertible preferred stock and stockholders' equity (deficit) for the period from May 9, 2003 (date of inception) to December 31, 2011, and the years ended December 31, 2009, 2010 and 2011 | |
67 |
Consolidated statements of cash flows for the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) to December 31, 2011 | |
74 |
Notes to consolidated financial statements | |
75 |
63
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Helicos BioSciences Corporation:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of redeemable convertible preferred stock and stockholders' equity (deficit) and of cash flows present fairly, in all material respects, the financial position of Helicos BioSciences Corporation and its subsidiary (a development stage enterprise) at December 31, 2010 and December 31, 2011, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2011 and, cumulatively, for the period from May 9, 2003 (date of inception) to December 31, 2011, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations and has limited available funds as of December 31, 2011, which raises substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
March 30, 2012
64
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| | | | | | | |
| | December 31, | |
|---|
| | 2010 | | 2011 | |
|---|
ASSETS | | | | | | | |
Current assets | | | | | | | |
Cash and cash equivalents | | $ | 2,513 | | $ | 999 | |
Accounts receivable | | | 267 | | | 174 | |
Unbilled receivables | | | 704 | | | 244 | |
Inventory | | | 2,935 | | | 910 | |
Prepaid expenses and other current assets | | | 306 | | | 128 | |
| | | | | | |
Total current assets | | | 6,725 | | | 2,455 | |
Property and equipment, net | | | 126 | | | 51 | |
Other assets | | | 152 | | | 58 | |
| | | | | | |
Total assets | | $ | 7,003 | | $ | 2,564 | |
| | | | | | |
LIABILITIES AND STOCKHOLDERS' DEFICIT | | | | | | | |
Current liabilities | | | | | | | |
Accounts payable | | $ | 794 | | $ | 801 | |
Accrued expenses and other current liabilities | | | 1,388 | | | 1,573 | |
Deferred revenue | | | 7,314 | | | 7,959 | |
Current portion of long-term debt | | | 2,779 | | | 3,145 | |
| | | | | | |
Total current liabilities | | | 12,275 | | | 13,478 | |
Long-term debt, net of current portion | | | 674 | | | — | |
Warrants | | | 220 | | | 25 | |
| | | | | | |
Total liabilities | | | 13,169 | | | 13,503 | |
Commitments and contingencies (Notes 7 & 8) | | | | | | | |
Stockholders' deficit | | | | | | | |
Preferred stock: par value $0.001 per share; 5,000,000 shares authorized at December 31, 2010 and December 31, 2011; no shares issued and outstanding at December 31, 2010 and December 31, 2011 | | | — | | | — | |
Common stock: par value $0.001 per share; 120,000,000 shares authorized at December 31, 2010 and December 31, 2011; 86,557,606 shares issued and outstanding at December 31, 2010 and 87,196,489 shares issued and outstanding at December 31, 2011, respectively | | | 87 | | | 87 | |
Additional paid-in capital | | | 180,180 | | | 181,232 | |
Deficit accumulated during the development stage | | | (186,433 | ) | | (192,258 | ) |
| | | | | | |
Total stockholders' deficit | | | (6,166 | ) | | (10,939 | ) |
| | | | | | |
Total liabilities and stockholders' deficit | | $ | 7,003 | | $ | 2,564 | |
| | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
65
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| | | | | | | | | | | | | |
| |
| |
| |
| | Period from
May 9, 2003
(date of inception)
through
December 31, 2011 | |
|---|
| | Year Ended December 31, | |
|---|
| | 2009 | | 2010 | | 2011 | |
|---|
Product revenue | | $ | 2,325 | | $ | 1,301 | | $ | 571 | | $ | 4,233 | |
Service revenue | | | — | | | — | | | 1,182 | | | 1,182 | |
Grant revenue | | | 701 | | | 3,096 | | | 1,477 | | | 6,787 | |
| | | | | | | | | | |
Total revenue | | | 3,026 | | | 4,397 | | | 3,230 | | | 12,202 | |
| | | | | | | | | | |
Costs and expenses | | | | | | | | | | | | | |
Cost of product revenue | | | 1,239 | | | 713 | | | 224 | | | 2,186 | |
Research and development | | | 18,256 | | | 14,047 | | | 4,930 | | | 113,593 | |
Selling, general and administrative | | | 12,481 | | | 10,506 | | | 4,124 | | | 75,066 | |
Impairment of long-lived assets | | | — | | | 1,894 | | | — | | | 1,894 | |
| | | | | | | | | | |
Total costs and expenses | | | 31,976 | | | 27,160 | | | 9,278 | | | 192,739 | |
| | | | | | | | | | |
Operating loss | | | (28,950 | ) | | (22,763 | ) | | (6,048 | ) | | (180,537 | ) |
Interest income | | | 75 | | | 19 | | | — | | | 4,153 | |
Interest expense | | | (1,044 | ) | | (614 | ) | | (680 | ) | | (5,186 | ) |
Change in fair value of warrant liability | | | 1,959 | | | 4,118 | | | 3 | | | 6,080 | |
Other income | | | — | | | 472 | | | 900 | | | 1,372 | |
| | | | | | | | | | |
Net loss | | | (27,960 | ) | | (18,768 | ) | | (5,825 | ) | | (174,118 | ) |
Beneficial conversion feature related to Series B redeemable, convertible, preferred stock | | | — | | | — | | | — | | | (18,140 | ) |
| | | | | | | | | | |
Net loss attributable to common stockholders | | $ | (27,960 | ) | $ | (18,768 | ) | $ | (5,825 | ) | $ | (192,258 | ) |
| | | | | | | | | | |
Net loss per share attributable to common stockholders—basic and diluted | | $ | (0.42 | ) | $ | (0.24 | ) | $ | (0.07 | ) | | | |
| | | | | | | | | | | |
Weighted average number of common shares used in computation—basic and diluted | | | 65,833,252 | | | 78,440,311 | | | 86,944,075 | | | | |
| | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
66
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT)
Period from May 9, 2003 (date of inception) to December 31, 2011
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A redeemable
convertible preferred stock | | Series B redeemable
convertible preferred stock | |
| |
| |
| |
| | Deficit
accumulated
during
development
stage | |
| |
| |
|---|
| | Common stock | |
| |
| |
| |
| |
|---|
| | Additional
paid-in
capital | | Subscription
receivable | | Other
accumulated
income (loss) | | Total
stockholders'
equity (deficit) | |
|---|
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | |
|---|
Balance at inception | | | — | | $ | — | | | — | | $ | — | | | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | |
Issuance of Series A redeemable convertible preferred stock in December 2003 for cash at $0.9555 per share, net of issuance costs of $59 | | | 27,815,946 | | | 26,469 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
Conversion of promissory note for shares of Series A redeemable convertible preferred stock in December 2003 at $0.9555 per share | | | 366,300 | | | 350 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
Issuance of restricted common stock in October 2003 to a founder for cash | | | — | | | — | | | — | | | — | | | 444,444 | | | | | | 2 | | | — | | | — | | | — | | | 2 | |
Issuance of restricted common stock in November and December 2003 to nonemployees | | | — | | | — | | | — | | | — | | | 618,126 | | | 1 | | | 2 | | | (3 | ) | | — | | | — | | | — | |
Issuance of common stock in December 2003 at $0.45 per share in exchange for intellectual property | | | — | | | — | | | — | | | — | | | 46,514 | | | — | | | 20 | | | — | | | — | | | — | | | 20 | |
Stock-based compensation expense | | | — | | | — | | | — | | | — | | | — | | | — | | | 23 | | | — | | | — | | | — | | | 23 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (547 | ) | | — | | | (547 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2003 | | | 28,182,246 | | | 26,819 | | | — | | | — | | | 1,109,084 | | | 1 | | | 47 | | | (3 | ) | | (547 | ) | | — | | | (502 | ) |
Exercise of a stock warrant to purchase shares of common stock in January 2004 | | | — | | | — | | | — | | | — | | | 120,123 | | | — | | | — | | | — | | | — | | | — | | | — | |
Cash received from investors in January 2004 for previously issued shares of Series A redeemable convertible preferred stock | | | — | | | 50 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
Cash received fron nonemployee in January 2004 for previously issued shares of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 3 | | | — | | | — | | | 3 | |
Issuance of restricted common stock in February, March and April 2004 to employees for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 155,555 | | | | | | 1 | | | — | | | — | | | — | | | 1 | |
Issuance of restricted common stock in September 2004 to nonemployees for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 11,888 | | | — | | | — | | | — | | | — | | | — | | | — | |
Exercise of nonemployee stock options in December 2004 for cash of $0.45 per share | | | — | | | — | | | — | | | — | | | 15,200 | | | | | | 6 | | | — | | | — | | | — | | | 6 | |
Stock-based compensation expense | | | — | | | — | | | — | | | — | | | — | | | — | | | 138 | | | — | | | — | | | — | | | 138 | |
Unrealized short-term loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (17 | ) | | (17 | ) |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (7,064 | ) | | — | | | (7,064 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2004 | | | 28,182,246 | | | 26,869 | | | — | | | — | | | 1,411,850 | | | 1 | | | 192 | | | — | | | (7,611 | ) | | (17 | ) | | (7,435 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
67
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2011
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A redeemable
convertible preferred stock | | Series B redeemable
convertible preferred stock | |
| |
| |
| |
| | Deficit
accumulated
during
development
stage | |
| |
| |
|---|
| | Common stock | |
| |
| |
| |
| |
|---|
| | Additional
paid-in
capital | | Subscription
receivable | | Other
accumulated
income (loss) | | Total
stockholders'
equity (deficit) | |
|---|
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | |
|---|
Balance at December 31, 2004 | | | 28,182,246 | | $ | 26,869 | | | — | | $ | — | | | 1,411,850 | | $ | 1 | | $ | 192 | | $ | — | | $ | (7,611 | ) | $ | (17 | ) | $ | (7,435 | ) |
Issuance of restricted common stock in March 2005 in exchange for intellectual property | | | — | | | — | | | — | | | — | | | 88,888 | | | — | | | — | | | — | | | — | | | — | | | — | |
Issuance of restricted common stock in July 2005 to employees for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 55,555 | | | 1 | | | — | | | — | | | — | | | — | | | 1 | |
Issuance of restricted common stock in April and December 2005 to nonemployees for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 1,666 | | | — | | | 1 | | | — | | | — | | | — | | | 1 | |
Exercise of employee stock options in June 2005 for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 277 | | | — | | | — | | | — | | | — | | | — | | | — | |
Exercise of nonemployee stock options in September 2005 for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 444 | | | — | | | — | | | — | | | — | | | — | | | — | |
Stock-based compensation expense | | | — | | | — | | | — | | | — | | | — | | | — | | | 55 | | | — | | | — | | | — | | | 55 | |
Vesting of previously issued shares of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | 36 | | | — | | | — | | | — | | | 36 | |
Change in unrealized short-term loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 17 | | | 17 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (10,918 | ) | | — | | | (10,918 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2005 | | | 28,182,246 | | | 26,869 | | | — | | | — | | | 1,558,680 | | | 2 | | | 284 | | | — | | | (18,529 | ) | | — | | | (18,243 | ) |
Issuance of restricted common stock in January 2006 to nonemployees for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 1,111 | | | — | | | — | | | — | | | — | | | — | | | — | |
Issuances of Series B redeemable convertible preferred stock in March 2006 for cash at $1.29 per share, net of issuance costs of $108 | | | — | | | — | | | 15,503,876 | | | 19,892 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
Issuance of common stock in July 2006 to employees for cash at $0.585 per share | | | — | | | — | | | — | | | — | | | 44,444 | | | — | | | 247 | | | — | | | — | | | — | | | 247 | |
Issuance of restricted common stock in September, November and December 2006 to employees for cash at $0.585 per share | | | — | | | — | | | — | | | — | | | 394,444 | | | — | | | 2 | | | (4 | ) | | — | | | — | | | (2 | ) |
Exercise of nonemployee stock options in November 2006 for cash at $0.585 per share | | | — | | | — | | | — | | | — | | | 4,444 | | | — | | | 2 | | | — | | | — | | | — | | | 2 | |
Exercise of employee stock options in January and December 2006 for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 48,146 | | | — | | | 22 | | | — | | | — | | | — | | | 22 | |
Stock-based compensation expense | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,058 | | | — | | | — | | | — | | | 1,058 | |
Vesting of previously issued shares of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | 157 | | | — | | | — | | | — | | | 157 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (20,580 | ) | | — | | | (20,580 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2006 | | | 28,182,246 | | | 26,869 | | | 15,503,876 | | | 19,892 | | | 2,051,269 | | | 2 | | | 1,772 | | | (4 | ) | | (39,109 | ) | | — | | | (37,339 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
68
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2011
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A redeemable
convertible preferred stock | | Series B redeemable
convertible preferred stock | |
| |
| |
| |
| | Deficit
accumulated
during
development
stage | |
| |
| |
|---|
| | Common stock | |
| |
| |
| |
| |
|---|
| | Additional
paid-in
capital | | Subscription
receivable | | Other
accumulated
income (loss) | | Total
stockholders'
equity (deficit) | |
|---|
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | |
|---|
Balance at December 31, 2006 | | | 28,182,246 | | $ | 26,869 | | | 15,503,876 | | $ | 19,892 | | | 2,051,269 | | $ | 2 | | $ | 1,772 | | $ | (4 | ) | $ | (39,109 | ) | $ | — | | $ | (37,339 | ) |
Issuances of Series B redeemable convertible preferred stock in January 2007 for cash at $1.29 per share, net of issuance costs of $6 | | | — | | | — | | | 15,503,876 | | | 19,994 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
Exercise of employee stock options in January 2007 for cash at $0.585 per share | | | — | | | — | | | — | | | — | | | 4,311 | | | — | | | 3 | | | — | | | — | | | — | | | 3 | |
Exercise of employee stock options in June, August and December 2007 for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 1,866 | | | — | | | — | | | — | | | — | | | — | | | — | |
Exercise of employee stock options in June, July and November 2007 for cash at $1.80 per share | | | — | | | — | | | — | | | — | | | 1,079 | | | — | | | 2 | | | — | | | — | | | — | | | 2 | |
Exercise of non-employee stock options in June, October and November 2007 for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 6,500 | | | — | | | 3 | | | — | | | — | | | — | | | 3 | |
Issuance of restricted common stock in July and August 2007 to employees | | | — | | | — | | | — | | | — | | | 56,757 | | | — | | | — | | | — | | | — | | | — | | | — | |
Issuance of restricted common stock in April 2007 to a non-employee | | | — | | | — | | | — | | | — | | | 2,222 | | | — | | | — | | | — | | | — | | | — | | | — | |
Cash received from employee in January 2007 for previously issued shares of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 4 | | | — | | | — | | | 4 | |
Cancellation of shares of restricted common stock | | | — | | | — | | | — | | | — | | | (88,888 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
Forfeiture of shares of unvested restricted common stock | | | — | | | — | | | — | | | — | | | (11,111 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
Stock-based compensation expense | | | — | | | — | | | — | | | — | | | — | | | — | | | 3,396 | | | — | | | — | | | — | | | 3,396 | |
Vesting of previously issued shares of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | 85 | | | — | | | — | | | — | | | 85 | |
Beneficial conversion feature related to Series B redeemable convertible preferred stock | | | — | | | — | | | — | | | — | | | — | | | — | | | 18,140 | | | — | | | (18,140 | ) | | — | | | — | |
Reclassification of amounts due to stockholders for fractional shares upon reverse stock split | | | — | | | — | | | — | | | — | | | (10 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
Issuance of common stock in initial public offering ("IPO"), net of discounts, commissions and issuance costs of $4,750 | | | — | | | — | | | — | | | — | | | 5,400,000 | | | 5 | | | 43,845 | | | — | | | — | | | — | | | 43,850 | |
Issuance of common stock in over-allotment to underwriters, net of discounts and commissions of $250 | | | — | | | — | | | — | | | — | | | 397,000 | | | 1 | | | 3,322 | | | | | | | | | | | | 3,323 | |
Conversion of preferred stock | | | (28,182,246 | ) | | (26,869 | ) | | (31,007,752 | ) | | (39,886 | ) | | 13,153,293 | | | 13 | | | 66,742 | | | — | | | — | | | — | | | 66,755 | |
Exercise of warrants to purchase common stock | | | — | | | — | | | — | | | — | | | 9,350 | | | — | | | 162 | | | — | | | — | | | — | | | 162 | |
Net loss | | | — | | | — | | | — | | | — | | | | | | | | | | | | | | | (36,805 | ) | | | | | (36,805 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2007 | | | — | | $ | — | | | — | | $ | — | | | 20,983,638 | | $ | 21 | | $ | 137,472 | | $ | — | | $ | (94,054 | ) | $ | — | | $ | 43,439 | |
The accompanying notes are an integral part of these consolidated financial statements.
69
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2011
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A redeemable
convertible preferred stock | | Series B redeemable
convertible preferred stock | |
| |
| |
| |
| | Deficit
accumulated
during
development
stage | |
| |
| |
|---|
| | Common stock | |
| |
| |
| |
| |
|---|
| | Additional
paid-in
capital | | Subscription
receivable | | Other
accumulated
income (loss) | | Total
stockholders'
equity (deficit) | |
|---|
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | |
|---|
Balance at December 31, 2007 | | | — | | $ | — | | | — | | $ | — | | | 20,983,638 | | $ | 21 | | $ | 137,472 | | $ | — | | $ | (94,054 | ) | $ | — | | $ | 43,439 | |
Exercise of employee stock options in January, February and July 2008 for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 15,861 | | | — | | | 7 | | | — | | | — | | | — | | | 7 | |
Exercise of employee stock options in January, July and August 2008 for cash at $1.80 per share | | | — | | | — | | | — | | | — | | | 1,201 | | | — | | | 2 | | | — | | | — | | | — | | | 2 | |
Exercise of non-employee stock options in January and April 2008 for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 5,790 | | | — | | | 3 | | | — | | | — | | | — | | | 3 | |
Issuance of restricted common stock in June and July 2008 to employees | | | — | | | — | | | — | | | — | | | 190,000 | | | — | | | — | | | — | | | — | | | — | | | — | |
Forfeiture of shares of unvested restricted common stock | | | — | | | — | | | — | | | — | | | (142,077 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
Stock-based compensation expense | | | — | | | — | | | — | | | — | | | — | | | — | | | 4,538 | | | — | | | — | | | — | | | 4,538 | |
Vesting of previously issued shares of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | 43 | | | — | | | — | | | — | | | 43 | |
Issuance of common stock warrants in June 2008 in connection with debt issuance | | | — | | | — | | | — | | | — | | | — | | | — | | | 337 | | | — | | | — | | | — | | | 337 | |
Issuance of common stock and common stock warrants in securities offering, net of discounts, commissions and issuance costs of $813 | | | — | | | — | | | — | | | — | | | 42,753,869 | | | 43 | | | 17,742 | | | | | | | | | | | | 17,785 | |
Net loss | | | — | | | — | | | — | | | — | | | | | | | | | | | | | | | (45,651 | ) | | | | | (45,651 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2008 | | | — | | $ | — | | | — | | $ | — | | | 63,808,282 | | $ | 64 | | $ | 160,144 | | $ | — | | $ | (139,705 | ) | $ | — | | $ | 20,503 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
70
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2011
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A redeemable
convertible preferred stock | | Series B redeemable
convertible preferred stock | |
| |
| |
| |
| | Deficit
accumulated
during
development
stage | |
| |
| |
|---|
| | Common stock | |
| |
| |
| |
| |
|---|
| | Additional
paid-in
capital | | Subscription
receivable | | Other
accumulated
income (loss) | | Total
stockholders'
equity (deficit) | |
|---|
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | |
|---|
Balance at December 31, 2008 | | | — | | $ | — | | | — | | $ | — | | | 63,808,282 | | $ | 64 | | $ | 160,144 | | $ | — | | $ | (139,705 | ) | $ | — | | $ | 20,503 | |
Exercise of employee stock options in January 2009 for cash at $0.45 per share | | | — | | | — | | | — | | | — | | | 5,555 | | | — | | | 2 | | | — | | | — | | | — | | | 2 | |
Exercise of employee stock options in September and November 2009 for cash at $1.04 per share | | | — | | | — | | | — | | | — | | | 50,000 | | | — | | | 52 | | | — | | | — | | | — | | | 52 | |
Issuance of common stock in April and October 2009 to employees | | | — | | | — | | | — | | | — | | | 35,633 | | | — | | | — | | | — | | | — | | | — | | | — | |
Issuance of restricted common stock in January, February, April, August, September and October 2009 to employees | | | — | | | — | | | — | | | — | | | 1,662,091 | | | 2 | | | (2 | ) | | — | | | — | | | — | | | — | |
Issuance of restricted common stock in April and June 2009 to non-employees | | | — | | | — | | | — | | | — | | | 95,000 | | | — | | | — | | | — | | | — | | | — | | | — | |
Forfeiture of shares of unvested restricted common stock | | | — | | | — | | | — | | | — | | | (107,415 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
Stock-based compensation expense | | | — | | | — | | | — | | | — | | | — | | | — | | | 5,281 | | | — | | | — | | | — | | | 5,281 | |
Vesting of previously issuedshares of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | 25 | | | — | | | — | | | — | | | 25 | |
Issuance of common stock and common stock warrants in securities offering, net of discounts, commissions and issuance costs of $1,364 | | | — | | | — | | | — | | | — | | | 10,711,280 | | | 10 | | | 7,773 | | | | | | | | | | | | 7,783 | |
Exercise of warrants to purchase common stock | | | — | | | — | | | — | | | — | | | 3,110,184 | | | 3 | | | (3 | ) | | — | | | — | | | — | | | — | |
Net loss | | | — | | | — | | | — | | | — | | | | | | | | | | | | | | | (27,960 | ) | | | | | (27,960 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2009 | | | — | | $ | — | | | — | | $ | — | | | 79,370,610 | | $ | 79 | | $ | 173,272 | | $ | — | | $ | (167,665 | ) | $ | — | | $ | 5,686 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
71
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2011
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A redeemable
convertible preferred stock | | Series B redeemable
convertible preferred stock | |
| |
| |
| |
| | Deficit
accumulated
during
development
stage | |
| |
| |
|---|
| | Common stock | |
| |
| |
| |
| |
|---|
| | Additional
paid-in
capital | | Subscription
receivable | | Other
accumulated
income (loss) | | Total
stockholders'
equity (deficit) | |
|---|
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | |
|---|
Balance at December 31, 2009 | | | — | | $ | — | | | — | | $ | — | | | 79,370,610 | | $ | 79 | | $ | 173,272 | | $ | — | | $ | (167,665 | ) | $ | — | | $ | 5,686 | |
Forfeiture of shares of restricted common stock in lieu of cash paid for taxes in January, April, June, July, and October 2010 | | | — | | | — | | | — | | | — | | | (608,151 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
Issuance of common stock to Directors for fees in lieu of cash in January and April 2010 | | | — | | | — | | | — | | | — | | | 48,356 | | | — | | | — | | | — | | | — | | | — | | | — | |
Forfeiture of shares of unvested restrcited stock in January, April, June, July, and October 2010 | | | — | | | — | | | — | | | — | | | (1,282,223 | ) | | (1 | ) | | 1 | | | — | | | — | | | — | | | — | |
Stock-based compensation expense | | | — | | | — | | | — | | | — | | | — | | | — | | | 5,104 | | | — | | | — | | | — | | | 5,104 | |
Issuance of restricted common stock to employees in January, April, July, August, and October 2010 | | | — | | | — | | | — | | | — | | | 3,831,381 | | | 4 | | | (4 | ) | | — | | | — | | | — | | | — | |
Issuance of restricted common stock in March, May and June 2010 to employees in lieu of cash for 2009 bonuses | | | — | | | — | | | — | | | — | | | 655,871 | | | — | | | 584 | | | — | | | — | | | — | | | 584 | |
Re-pricing of warrants in connection with November 2010 Debt Amendment | | | — | | | — | | | — | | | — | | | — | | | — | | | 47 | | | — | | | — | | | — | | | 47 | |
Exercise of employee stock options in August 2010 for cash at $0.71 per share | | | — | | | — | | | — | | | — | | | 4,352 | | | — | | | 2 | | | — | | | — | | | — | | | 2 | |
Exercise of warrants to purchase common stock | | | — | | | — | | | — | | | — | | | 4,537,410 | | | 5 | | | 1,107 | | | — | | | — | | | — | | | 1,112 | |
Vesting of previously issued shares of restricted common stock | | | — | | | — | | | — | | | — | | | — | | | — | | | 67 | | | — | | | — | | | — | | | 67 | |
Net loss | | | — | | | — | | | — | | | — | | | | | | | | | | | | | | | (18,768 | ) | | | | | (18,768 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | | — | | $ | — | | | — | | $ | — | | | 86,557,606 | | $ | 87 | | $ | 180,180 | | $ | — | | $ | (186,433 | ) | $ | — | | $ | (6,166 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
72
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
Period from May 9, 2003 (date of inception) to December 31, 2011
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A redeemable
convertible preferred stock | | Series B redeemable
convertible preferred stock | |
| |
| |
| |
| | Deficit
accumulated
during
development
stage | |
| |
| |
|---|
| | Common stock | |
| |
| |
| |
| |
|---|
| | Additional
paid-in
capital | | Subscription
receivable | | Other
accumulated
income (loss) | | Total
stockholders'
equity (deficit) | |
|---|
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | |
|---|
Balance at December 31, 2010 | | | — | | $ | — | | | — | | $ | — | | | 86,557,606 | | $ | 87 | | $ | 180,180 | | $ | — | | $ | (186,433 | ) | $ | — | | $ | (6,166 | ) |
Forfeiture of shares of restricted common stock in lieu of cash paid for taxes in January, and July 2011 | | | — | | | — | | | — | | | — | | | (27,896 | ) | | — | | | — | | | — | | | — | | | — | | | — | |
Forfeiture of shares of unvested restricted stock in January, May, June, July, August, September, and October 2011 | | | — | | | — | | | — | | | — | | | (596,436 | ) | | (1 | ) | | 1 | | | — | | | — | | | — | | | — | |
Stock-based compensation expense | | | — | | | — | | | — | | | — | | | — | | | — | | | 847 | | | — | | | — | | | — | | | 847 | |
Exercise of warrants to purchase common stock | | | — | | | — | | | — | | | — | | | 1,263,215 | | | 1 | | | 204 | | | — | | | — | | | — | | | 205 | |
Net loss | | | — | | | — | | | — | | | — | | | | | | | | | | | | | | | (5,825 | ) | | | | | (5,825 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | | — | | $ | — | | | — | | $ | — | | | 87,196,489 | | $ | 87 | | $ | 181,232 | | $ | — | | $ | (192,258 | ) | $ | — | | $ | (10,939 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
73
Table of Contents
Helicos BioSciences Corporation (a development stage company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | | |
| | Year Ended December 31, | | Period from May 9, 2003
(date of inception)
through
December 31, 2011 | |
|---|
| | 2009 | | 2010 | | 2011 | |
|---|
Cash flows from operating activities: | | | | | | | | | | | | | |
Net loss | | $ | (27,960 | ) | $ | (18,768 | ) | $ | (5,825 | ) | $ | (174,118 | ) |
Adjustments to reconcile net loss to cash used in operating activities: | | | | | | | | | | | | | |
Depreciation and amortization | | | 2,308 | | | 1,019 | | | 10 | | | 9,068 | |
Amortization of lease incentive | | | (105 | ) | | (4 | ) | | — | | | (474 | ) |
Common stock issued for licenses | | | — | | | — | | | — | | | 147 | |
Stock-based compensation expense | | | 5,281 | | | 5,104 | | | 847 | | | 20,661 | |
Noncash interest expense related to debt and warrants | | | 325 | | | 260 | | | 502 | | | 1,639 | |
Noncash license fee | | | — | | | 54 | | | — | | | 54 | |
Noncash income related to change in fair value of warrant liability | | | (1,959 | ) | | (4,118 | ) | | (3 | ) | | (6,080 | ) |
Provisions on inventory | | | 1,699 | | | 2,570 | | | 2,025 | | | 7,871 | |
Loss on disposal of property and equipment | | | 26 | | | (33 | ) | | — | | | (7 | ) |
Impairment of long-lived assets | | | — | | | 1,894 | | | — | | | 1,894 | |
Changes in operating assets and liabilities: | | | | | | | | | | | | | |
Accounts receivable | | | (625 | ) | | 581 | | | 93 | | | (174 | ) |
Unbilled receivables | | | (41 | ) | | (208 | ) | | 460 | | | 6 | |
Inventory | | | (1,696 | ) | | (868 | ) | | — | | | (11,226 | ) |
Prepaid expenses and other current assets | | | (514 | ) | | 117 | | | 113 | | | (519 | ) |
Deferred revenue | | | 5,082 | | | 1,522 | | | 645 | | | 7,872 | |
Accounts payable | | | 216 | | | (211 | ) | | 7 | | | 801 | |
Accrued expenses and other current liabilities | | | 2,110 | | | (878 | ) | | 185 | | | 3,246 | |
Other long-term liabilities | | | 365 | | | — | | | — | | | 557 | |
| | | | | | | | | | |
Net cash used in operating activities | | | (15,488 | ) | | (11,967 | ) | | (941 | ) | | (138,782 | ) |
| | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | | |
Purchases of property and equipment | | | (22 | ) | | (27 | ) | | — | | | (9,541 | ) |
Proceeds from sale of property and equipment | | | — | | | 33 | | | 75 | | | 108 | |
Decrease (Increase) in restricted cash | | | — | | | 225 | | | — | | | — | |
Purchases of short-term investments | | | — | | | — | | | — | | | (34,709 | ) |
Maturities of short-term investments | | | — | | | — | | | — | | | 34,709 | |
| | | | | | | | | | |
Net cash (used in) provided by investing activities | | | (22 | ) | | 231 | | | 75 | | | (9,433 | ) |
| | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | | |
Proceeds from debt issuances | | | — | | | — | | | — | | | 22,256 | |
Payments on debt | | | (3,322 | ) | | (2,288 | ) | | (1,994 | ) | | (21,874 | ) |
Payments of debt issuance costs | | | — | | | (259 | ) | | — | | | (453 | ) |
Proceeds from initial public offering | | | — | | | — | | | — | | | 49,011 | |
Deferred initial public offering costs | | | — | | | — | | | — | | | (1,838 | ) |
Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs | | | — | | | — | | | — | | | 66,405 | |
Proceeds from bridge loan | | | — | | | 667 | | | 1,333 | | | 2,350 | |
Proceeds from issuance of common stock and common stock warrants | | | 14,996 | | | — | | | — | | | 32,807 | |
Proceeds from issuance of restricted common stock | | | (1 | ) | | — | | | — | | | 338 | |
Payments to employees for cancelled restricted common stock | | | — | | | — | | | — | | | (104 | ) |
Proceeds from exercise of warrants | | | — | | | 197 | | | 13 | | | 210 | |
Proceeds from exercise of stock options | | | 54 | | | 2 | | | — | | | 106 | |
| | | | | | | | | | |
Net cash provided by (used in) financing activities | | | 11,727 | | | (1,681 | ) | | (648 | ) | | 149,214 | |
| | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | (3,783 | ) | | (13,417 | ) | | (1,514 | ) | | 999 | |
Cash and cash equivalents, beginning of period | | | 19,713 | | | 15,930 | | | 2,513 | | | — | |
| | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 15,930 | | $ | 2,513 | | $ | 999 | | $ | 999 | |
| | | | | | | | | | |
Supplemental disclosure of cash flow information | | | | | | | | | | | | | |
Cash paid during the year for interest | | $ | 820 | | $ | 360 | | $ | 215 | | $ | 3,176 | |
Transfers from inventory to property and equipment | | $ | — | | $ | 1,297 | | $ | — | | $ | 1,552 | |
Noncash financing activities: | | | | | | | | | | | | | |
Issuance of redeemable convertible preferred stock warrants | | $ | — | | $ | — | | $ | — | | $ | 95 | |
Issuance of common stock warrants | | $ | 7,212 | | $ | — | | $ | — | | $ | 13,233 | |
Number of common stock shares issued upon exercise of warrants | | | 3,110,184 | | | 4,537,410 | | | 1,263,215 | | | 8,910,809 | |
Change in warrant valuation | | $ | (1,959 | ) | $ | (4,118 | ) | $ | (3 | ) | $ | (6,080 | ) |
Conversion of bridge loan to equity | | $ | — | | $ | — | | $ | — | | $ | 350 | |
Beneficial conversion feature related to Series B redeemable convertible preferred stock | | $ | — | | $ | — | | $ | — | | $ | 18,140 | |
Conversion of preferred stock to common stock | | $ | — | | $ | — | | $ | — | | $ | 66,755 | |
Reclassification of preferred stock warrants to common stock warrants | | $ | — | | $ | — | | $ | — | | $ | 162 | |
The accompanying notes are an integral part of these consolidated financial statements.
74
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Business Description
Helicos BioSciences Corporation ("Helicos" or the "Company") is a life sciences company focused on innovative genetic analysis technologies. Helicos has developed a proprietary technology to enable the rapid analysis of large volumes of genetic material by directly sequencing single molecules of DNA or RNA. The Company's tSMS approach differs from current methods of sequencing DNA or RNA because it analyzes individual molecules of DNA directly instead of analyzing a large number of copies of the molecule which must be produced through complex sample preparation techniques. The Company's tSMS technology eliminates the need for costly, labor-intensive and time-consuming sample preparation techniques, such as amplification or cloning, which are required by other methods to produce a sufficient quantity of genetic material for analysis.
The Company's Helicos® Genetic Analysis Platform is designed to obtain sequencing information by repetitively performing a cycle of biochemical reactions on individual DNA or RNA molecules and imaging the results after each cycle. The platform consists of an instrument called the HeliScope™ Single Molecule Sequencer, an image analysis computer tower called the HeliScope™ Analysis Engine, associated reagents, which are chemicals used in the sequencing process, and disposable supplies. The information generated from using our tSMS products may lead to improved drug therapies, personalized medical treatments and more accurate diagnostics for cancer and other diseases.
As previously disclosed in the Company's quarterly and periodic reports with the SEC, the Company has had limited operations to date and its activities have consisted primarily of raising capital, conducting research and development and recruiting personnel. Accordingly, the Company is considered to be in the development stage at December 31, 2011, as defined by the Financial Accounting Standards Board ("FASB"). The Company's fiscal year ends on December 31. The Company operates as one reportable segment. Helicos is a Delaware corporation and was incorporated on May 9, 2003. Furthermore, as of December 31, 2011 and March 23, 2012, the Company only had $1.0 million and $34,000, respectively, in cash and cash equivalents.
As a result of several headcount reductions and restructurings during 2010, which eliminated approximately 60 positions, the Company entered the first quarter of 2011 with 22 employees. In order to further conserve the Company's limited resources, during the second quarter of 2011, the Company further reduced its workforce by phasing out six business and research positions and we ultimately reduced the Company's total headcount to ten positions. In addition, during the third quarter of 2011, the Company entered into a new lease reducing the size and associated expense of its leased facility in Cambridge, Massachusetts which houses the Company's headquarters and scientific laboratory.
Notwithstanding these most recent workforce reductions and despite the Company's previous reductions, restructurings, expense saving measures, and the Bridge Debt Financing, the Company does not have sufficient funds to operate its business. The Company continues to require significant additional capital on a month-to-month basis in order to continue its operations beyond the existing $2,000,000 committed portion of the Bridge Debt. As of the date of this filing, all $2,000,000 has been drawn against this committed facility. Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified, but there can be no assurance that any such funding will be available on reasonable terms, if at all. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. No further commitments beyond the current outstanding notes with a maturity date of May 15, 2012 have been made under the uncommitted portion of the Bridge Debt Financing facility. The Company is
75
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Business Description (Continued)
currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that the Company will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs. As a result, the Company is no longer able to remain current in making payments to trade vendors and other unsecured creditors when such payments are due. Workforce reductions, as well as curtailed financial resources, will further impact the Company's ability to implement its business priorities. In addition, these constraints have caused the Company to significantly scale back service support and reagent supply to its current installed base. The Company has also significantly curtailed collaborative activities with other parties. Despite these measures, there cannot be any assurance that the Company will have sufficient resources to operate its business.
In January 2012, the Company made a final $1,070,000 payment on the senior debt facility pursuant to the Loan and Security Agreement among the Company, certain lenders and General Electric Capital Corporation, as agent, dated as of December 31, 2007, as amended (the "Loan Agreement"). As a result, the Company's obligations under the Loan Agreement have been satisfied and the senior debt lenders no longer have a security interest in the Company's assets, including its intellectual property assets.
During 2011, the Company deployed its limited resources by focusing on intellectual property monetization and concentrating our research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals, which were designed to enable the Company to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives and to meet its obligations under an NIH grant. With a limited number of employees and resources, however, the Company's ability to pursue research and development efforts in targeted areas that were designed to achieve tangible proof-of-concept goals connected with potential partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives proved unsuccessful and are unlikely to resume. Going forward, the Company will only be able to focus its limited resources on intellectual property monetization and selected research and development activities that are primarily related to grant-funded initiatives and that support its limited sequencing services. In order to defend the Company's intellectual property rights through licensing and enforcement strategies, the Company has taken several actions. First, on August 27, 2010, the Company filed a lawsuit against Pacific Biosciences of California, Inc. ("Pacific Biosciences") for patent infringement. The lawsuit, filed in the United States District Court for the District of Delaware, accuses Pacific Biosciences of infringing four patents and seeks injunctive relief and monetary damages. Second, on October 14, 2010, the Company announced the launch of a technology licensing program for its Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. Third, on October 22, 2010, the Company filed an amended complaint against Pacific Biosciences by naming Illumina, Inc. and Life Technologies Corporation as additional defendants. The amended lawsuit alleges that the defendants willfully infringe the Company's patents in suit by using their respective technologies, sequencing systems and products which the Company allege are within the scope of one or more claims of its patents in suit. The Company is seeking a permanent injunction enjoining the defendants from further infringement as well as monetary and punitive damages, costs and attorneys' fees, interests and other relief as determined by the Court. The Company believes that the proceeds generated by this litigation, if it is successful, could be substantial. There is no assurance that the Company will be successful in this
76
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Business Description (Continued)
litigation, and even in the event of a favorable outcome in this litigation there can be no assurance that there will be any proceeds remaining for common shareholders, as a result of various arrangements in place with professional service providers, bridge lenders (including the Risk Premium Agreement with the bridge lenders), licensors and others. As of the date of this filing, the Company does not have adequate financial resources and will continue to need to generate additional funding from various sources including contract sequencing service projects, government grants and yet to be identified third party financing arrangements in order to continue its intellectual property monetization strategy. There can be no assurance that the Company will be able to generate the funding necessary to continue its intellectual property monetization strategy. If the Company is unable to execute its operations according to its plans and obtain additional financing, the Company will be forced to cease operations and/or seek bankruptcy protection. If the Company is required to seek protection from its creditors through a bankruptcy filing, it is likely that there would be little or no assets available for payment or distribution to its stockholders, and that its stockholders will lose their entire investment in the Company. In addition, to seeking funding the Company is actively exploring a variety of strategic transactions, including the sale, license or other disposition of a portion of its assets in order to raise funding, or the potential sale of all or substantially all its assets or a sale of the Company. The Company believes it is very unlikely that stockholders would receive any significant value from any such transaction.
In connection with the Bridge Debt Financing and as security for its obligations under the Company's contingency fee arrangement with outside intellectual property litigation counsel relating to the patent infringement lawsuit the Company filed against Pacific Biosciences of California, Inc., Illumina, Inc. and Life Technologies Corporation, the Company entered into a subordinated security agreement with outside intellectual property litigation counsel. Pursuant to this agreement, the Company agreed to grant outside intellectual property litigation counsel a priority security interest in the patent infringement lawsuit. Under the terms of the contingency fee arrangement, the Company is obligated to pay outside intellectual property litigation counsel up to 40% of the proceeds paid to the Company in connection with the settlement, judgment or other resolution of the Cause of Action (as defined in the contingency fee arrangement) or certain business transactions resulting from or relating to the patent infringement lawsuit. To date, the Company, and those providing professional services in connection with this patent litigation on a contingent basis have expended significant resources in pursuit of this intellectual property monetization effort and are expected to continue to do so going forward. There is no assurance that the Company will be successful in this litigation, and even in the event of a favorable outcome in this litigation there can be no assurance that there will be any proceeds remaining for common stockholders, as a result of various arrangements in place with professional service providers, bridge lenders (including the Risk Premium Agreement with the bridge lenders), licensors and others.
As an inducement for Atlas Ventures and Flagship Ventures (the Purchasers) to enter into the Bridge Debt Financing, the Company entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement"). Under the terms of the Risk Premium Agreement, the Company agreed to pay the Purchasers the following portions of the consideration it receives (the "Payment Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30%
77
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Business Description (Continued)
of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). See Note 8 of Notes to Consolidated Financial Statements for additional terms of the Risk Premium Agreement.
In approving the transactions relating to the Bridge Debt Financing and the Fourth Amendment, the Board approved a management incentive plan (the "Management Incentive Plan") pursuant to which participants in the Management Incentive Plan, including Dr. Ivan Trifunovich and Mr. Jeffrey Moore, will be entitled to receive in the aggregate, an amount equal to 7.5% of certain payment consideration resulting from intellectual property licensing events. The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board.
The Bridge Debt Financing and Risk Premium Agreement and all of the transactions contemplated by those agreements were approved by an independent committee of the Board. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, Ph.D., who is a Partner of Atlas Venture, abstained from the vote of the Board approving the transactions.
On June 28, 2010, the Company received a letter from The NASDAQ Stock Market LLC ("NASDAQ") advising that for the previous 30 consecutive business days, the Company failed to comply with the minimum market value of listed securities ("MVLS") requirement for continued listing on the NASDAQ Global Market pursuant to NASDAQ Marketplace Rule 5450(b)(2)(A) and that the Company did not otherwise satisfy the criteria for continued listing pursuant to NASDAQ Marketplace Rule 5450(b). NASDAQ stated in its letter that in accordance with NASDAQ Marketplace Rule 5810(c)(3)(C), the Company would be provided 180 calendar days, or until December 27, 2010, to regain compliance with the MVLS continued listing requirement. MVLS is calculated by multiplying the total shares outstanding by the daily closing bid price. On October 12, 2010, the Company received a delisting determination letter from NASDAQ due to its failure to regain compliance with the NASDAQ Global Market's minimum bid price requirement for continued listing as set forth in Listing Rule 5450(a)(1). In addition, the Company did not hold its annual meeting of stockholders for fiscal 2010 which is an additional requirement for the Company to comply with in order to maintain its continued listing on NASDAQ. Based on the unlikely ability of the Company to meet the NASDAQ MLVS requirements due to the state of its finances and near-term business prospects, on November 12, 2010, the Company withdrew its appeal to NASDAQ regarding this delisting notice. As a result, the Company's securities were delisted from the NASDAQ Global Market and the trading of its common stock was suspended before the opening of business on November 16, 2010, and a Form 25-NSE was subsequently filed with the SEC on January 21, 2011 which removed the Company's securities from listing and registration on The NASDAQ Stock Market. The Company was advised by Pink OTC Markets Inc., which operates an electronic quotation service for securities traded over-the-counter ("OTC"), that its securities were immediately eligible for quotation on the OTCQB. The OTCQB is a market tier for OTC traded companies that are registered and reporting with the SEC. The Company's shares continue to trade under the symbol HLCS.
The Company's future capital requirements will depend on many factors and it may require additional capital beyond its currently anticipated amounts. Any such required additional capital may not be available on reasonable terms, if at all, given its prospects, the current economic environment and restricted access to capital markets. The continued depletion of its resources may make future funding more difficult or expensive to attain. Additional equity financing may be dilutive to the
78
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Business Description (Continued)
Company's stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict its ability to operate as a business; and strategic partnerships may result in royalties or other terms which reduce its economic potential from any adjustments to its existing long-term strategy. If the Company is unable to execute its operations according to its plans or to obtain additional financing, the Company may be forced to cease operations and/or seek bankruptcy protection. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets or the amount of reclassification of liabilities, or any adjustment that might be necessary should the Company be unable to continue as a going concern.
Since inception, the Company has incurred losses and has not generated positive cash flows from operations. The Company expects its losses to continue for a considerable period of time. These losses, among other things, have had and will continue to have an adverse effect on its working capital, total assets and stockholders' deficit. The Company on a month-to-month basis during 2012, will require significant additional capital to continue its operations. As of the date of this filing, all amounts available under the $2,000,000 committed facility have been drawn and no additional funding under the committed facility is available to support ongoing operations. Potential sources of additional funding may include funding from the $2,000,000 uncommitted portion of the Bridge Debt Financing facility and/or from other sources that have not yet been identified, but there can be no assurance that any such funding or other sources of funding will be available to the Company on reasonable terms, if at all. As of the date of this filing, $375,000 has been advanced under the uncommitted portion of the Bridge Debt Financing facility on a short term basis. The maturity date of the notes issued under the uncommitted portion of the Bridge Debt Financing facility is May 15, 2012. The Company is currently in active discussions with the Purchasers regarding the possibility of additional short-term financing to support Helicos' continued operations. The Company and Purchasers have not yet agreed on such financing and there can be no guarantee that the Company will obtain such financing or any other sources of funding or that Helicos can obtain any additional financing on terms favorable to Helicos or in amounts sufficient to meet Helicos' needs. If the Company is unable to execute its operations according to its plans and obtain additional financing, the Company will be forced to cease operations and/or seek bankruptcy protection. If the Company is required to seek protection from its creditors through a bankruptcy filing, it is likely that there would be little or no assets available for payment or distribution to its stockholders, and that its stockholders will lose their entire investment in the Company. In addition, to seeking funding the Company is actively exploring a variety of strategic transactions, including the sale, license or other disposition of a portion of its assets in order to raise funding, or the potential sale of all or substantially all its assets or a sale of the Company. The Company believes it is very unlikely that stockholders would receive any significant value from any such transaction. Because the Company's present capital resources are not sufficient to fund its planned operations for a twelve month period as of the date of this Annual Report on Form 10-K for the year ended December 31, 2011, the Company's current financial resources raise substantial doubt about its ability to continue as a going concern.
The preparation of financial statements in conformity with GAAP requires the Company to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent liabilities at the dates of the financial statements. Actual amounts may differ from these estimates because of different assumptions or conditions. The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All intercompany accounts and transactions have been eliminated.
79
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Initial Public Offering
On May 24, 2007, the Company completed its initial public offering ("IPO") of 5,400,000 shares of common stock at an initial public offering price of $9.00 per share. Net proceeds were approximately $43.9 million after deducting underwriting discounts and commissions and offering expenses paid by the Company. Total fees and expenses paid by the Company, excluding underwriting discounts and commissions were approximately $1.8 million which includes legal, accounting and printing costs and various other fees associated with registration and listing of the Company's common stock.
On May 24, 2007, upon completion of the Company's IPO, all of the Company's 59,189,998 shares of redeemable convertible preferred stock outstanding on that date were automatically converted into 13,153,293 shares of common stock. In addition, the outstanding warrants to purchase 81,184 shares of Series B redeemable convertible preferred stock were converted into warrants to purchase 18,040 shares of common stock. From January 1, 2007 through the date of the Company's IPO, the estimated fair value of the warrants to purchase 81,184 shares of Series B redeemable convertible preferred stock decreased by $42,000 to $162,000. Upon conversion on the date of the Company's IPO, the warrants to purchase 18,040 shares of the Company's common stock were reclassified to additional paid-in capital.
On June 27, 2007, the underwriters exercised their over-allotment option and purchased an additional 397,000 shares of the Company's common stock, and the net proceeds after deducting underwriters' discounts and commissions related to the offering were approximately $3.3 million.
3. Summary of Significant Accounting Policies
Basis of presentation and consolidation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All intercompany accounts and transactions have been eliminated.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, the Company evaluates its estimates, including those related to revenue recognition, inventory valuation, contingencies, excess and obsolescence of inventory, impairment of long-lived assets, allowance for doubtful accounts and stock-based compensation. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual amounts may differ from these estimates under different assumptions or conditions. Changes in estimates are recorded in the period in which they become known.
Cash and cash equivalents
The Company considers all highly liquid investments with original maturities of generally three months or less at the time of acquisition to be cash equivalents. Cash equivalents are stated at cost, which approximates fair market value.
80
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
Short-term investments
The Company classifies marketable securities as available-for-sale. These securities are carried at fair market value with unrealized gains and losses reported, if material, as a component of other comprehensive gain or loss in stockholders' deficit. There were no short-term investments at December 31, 2010 or 2011.
Concentration of credit risk
The Company has no significant off-balance sheet concentrations of credit risk such as foreign currency exchange contracts, option contracts or other hedging arrangements. Financial instruments that subject the Company to credit risk consist primarily of cash and cash equivalents. The Company maintains all of its cash in two accredited financial institutions. Management believes these accounts are subject to minimal credit and market risk and are of high credit quality.
The following table summarizes the number of trade customers that individually comprise greater than 10% of product revenues and their respective percentage of the Company's total product revenues on a gross basis:
| | | | | | | | | | | | | | | | |
| |
| | Percentage of Total Product
Revenues by Customer | |
|---|
| | Number of
Significant
Customers | |
|---|
As of: | | A | | B | | C | | D | |
|---|
December 31, 2009 | | | 2 | | | 46 | % | | 45 | % | | — | % | | — | % |
December 31, 2010 | | | 2 | | | 58 | % | | 15 | % | | — | % | | — | % |
December 31, 2011 | | | 4 | | | 32 | % | | 27 | % | | 13 | % | | 11 | % |
The table above excludes grant revenues and revenues from systems recognized from deferred revenue.
The following table summarizes the number of customers that individually comprise greater than 10% of total accounts receivable and their respective percentage of the Company's total accounts receivable:
| | | | | | | | | | | | | |
| |
| | Percentage of
Total Accounts
Receivable
by Customer | |
|---|
| | Number of
Significant
Customers | |
|---|
As of: | | A | | B | | C | |
|---|
December 31, 2010 | | | 2 | | | 28 | % | | 19 | % | | — | % |
December 31, 2011 | | | 3 | | | 33 | % | | 17 | % | | 29 | % |
The table above excludes unbilled receivables.
Inventory
Prior to reaching technological feasibility, start-up manufacturing costs, such as those relating to the assembly, testing and performance validation of the Helicos® Genetic Analysis System, were expensed to research and development as the costs were incurred. When management determined that the Helicos System was ready for commercial launch during December 2007, the Company began capitalizing its manufacturing costs to inventory.
81
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
The Company values its inventory at the lower of cost or market on a first-in, first-out basis. The Company's policy is to capitalize inventory costs associated with its products when, based on management's judgment, future commercialization is considered probable and the future economic benefit is expected to be realized.
The Company periodically reviews its inventories for excess or obsolete inventory and writes-down obsolete or otherwise unmarketable inventory to its estimated net realizable value. If the actual net realizable value is less than that estimated by the Company, or if there are any further determinations that inventory will not be marketable based on estimates of demand, additional inventory write-downs will be required.
The Company values inventory in accordance with FASB guidance on inventory costs and inventory pricing to clarify that abnormal amounts of idle facility expense, freight, handling costs, and wasted material (spoilage) should be recognized as current-period charges regardless of whether they meet the criterion of "so abnormal." In addition, guidance requires that allocation of fixed production overheads to the costs of conversion be based on the normal capacity of the production facilities.
The Company concluded that because it is not yet functioning under normal capacity, its production level is abnormally low. As such, abnormal costs such as the unfavorable labor, overhead and absorption variances were recognized as current period charges, rather than as a portion of the inventory cost. During the years ended December 31, 2009, 2010 and 2011, research and development expense included $1.9 million, $0.7 million, and $0, respectively, of labor and overhead costs associated with the under-utilization of the manufacturing facility. In fiscal year 2010 the Company ceased production of Helicos Systems. During the year ended December 31, 2009, the Company recorded a $3.1 million charge to write-off excess and obsolete inventory. This charge was recorded as a research and development expense which resulted in a $1.7 million decrease to inventory and a $1.4 million increase to accrued expenses for excess non-cancellable purchase commitments. During the year ended December 31, 2010, the Company recorded a $2.6 million charge to research and development expense to write-off excess and obsolete inventory. The Company recorded a non-cash charge of $2.0 million to research and development expense in the fourth quarter of 2011 related to deferred inventory costs that the Company concluded were no longer realizable. As of December 31, 2010 and 2011, inventory includes $2.9 million and $0.9 million respectively, related to the capitalized costs of sold HeliScopes for which the recognition of revenue has been deferred until recognition criteria have been met.
Property and equipment
Property and equipment are recorded at cost. Expenditures for maintenance and repairs are charged to expense while the costs of significant improvements are capitalized. Depreciation is provided using the straight-line method over the following estimated useful lives: Machinery and equipment—three to five years, office furniture and equipment—three years, leasehold improvements—the shorter of three years or the life of lease. Upon retirement or sale, the cost of the assets disposed of and the related accumulated depreciation are eliminated from the consolidated balance sheets and related gains or losses are reflected in the consolidated statements of operations. There have been no material retirements or sale of assets since May 9, 2003 (date of inception) through December 31, 2010. The Company sold $75,000 of property and equipment during the year ended December 31, 2011.
82
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
Long-lived assets
The Company evaluates the recoverability of property and equipment and other long-lived assets when circumstances indicate that an event of impairment may have occurred in accordance with FASB guidance. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written-down to their estimated fair values. Long-lived assets and certain identifiable intangible assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell. The Company recorded an impairment charge related to property and equipment of approximately $1.9 million during the year ended December 31, 2010. (See Note 5.)
Revenue recognition
Government research grants that provide for payments to the Company for work performed are recognized as revenue when the related expenses are incurred.
The Company recognizes revenue in accordance with FASB accounting guidance on revenue recognition in financial statements and accounting for multiple element revenue arrangements. This guidance requires that persuasive evidence of a sales arrangement exists; delivery of goods occurs through transfer of title and risk and rewards of ownership, the selling price is fixed or determinable and collectability is reasonably assured. The Company also follows FASB guidance on how to account for arrangements that involve the delivery or performance of multiple products, services and/or rights to use assets.
In instances where the Company sells instruments with a related installation obligation, the Company will allocate the revenue between the instrument and the installation based on relative fair value at the time of the sale. The instrument revenue will be recognized when title and risk of loss passes. The installation revenue will be recognized when the installation is performed. If fair value is not available for any undelivered element, revenue for all elements is deferred until delivery and installation are complete.
In instances where the Company sells an instrument with specified acceptance criteria, the Company will defer revenue recognition until such acceptance has been obtained.
Revenue from service contracts sold in conjunction with the sale of equipment is recognized ratably over the service period.
Research and development
Research and development expenditures are charged to the consolidated statement of operations as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, clinical trial and related supply costs, contract services, depreciation and amortization expense and other related costs. During the years ended December 31, 2009, 2010 and 2011 research and development expenses also included $1.9 million, $0.7 million, and $0, respectively, of labor and overhead costs associated with the under-utilization of the manufacturing facility.
83
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
Income taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, and operating loss and tax credit carryforwards. The Company's consolidated financial statements contain certain deferred tax assets, which have arisen primarily as a result of operating losses, as well as other temporary differences between financial and tax accounting. FASB guidance on accounting for income taxes requires the Company to establish a valuation allowance if the likelihood of realization of the deferred tax assets is reduced based on an evaluation of objective verifiable evidence.
Significant management judgment is required in determining the Company's provision for income taxes, the Company's deferred tax assets and liabilities and any valuation allowance recorded against those net deferred tax assets. The Company evaluates the weight of all available evidence to determine whether it is more likely than not that some portion or all of the net deferred income tax assets will not be realized.
Effective January 1, 2007, the Company adopted FASB guidance on accounting for uncertainty in income taxes. This guidance prescribes the methodology by which a company must measure, report, present and disclose in its financial statements the effects of any uncertain tax return reporting positions that a company has taken or expects to take. See Note 11, "Income Taxes" for additional disclosure.
Stock-based compensation
The Company accounts for stock-based compensation issued to non-employees in accordance with FASB guidance on accounting for equity instruments that are issued to other than employees for acquiring, or in conjunction with, selling goods or services. The Company records the expense of such services based on the estimated fair value of the equity instrument using the Black-Scholes option pricing model. The value of the equity instrument is charged to earnings over the term of the service agreement.
Other Income
Other income of $472,000 in the year ended December 31, 2010 and $250,000 in the year ended December 31, 2011 is from the receipt of a cash grant for qualifying therapeutic discovery projects that were awarded under the Patient Protection and Affordable Care Act of 2010. In addition in the year ended December 31, 2011 the Company received $649,000 from a business interruption insurance claim the Company made in fiscal 2010.
Net loss per share
Basic net loss per share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. The Company's potential dilutive shares, which include outstanding common stock options, unvested restricted stock, and common stock warrants, have not been included in the computation of diluted net loss per share for all periods as the result would be antidilutive. Such potentially dilutive shares are excluded when the effect would be to reduce a net loss per share.
84
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
Other comprehensive income (loss)
FASB guidance on reporting comprehensive income establishes standards for reporting and displaying comprehensive income and its components in a full set of general-purpose financial statements. For each of the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) to December 31, 2011, there was no material difference between the net loss and comprehensive loss.
Segment reporting
FASB guidance on disclosure about segments of an enterprise and related information establishes standards for reporting information about operating segments in annual financial statements and in interim financial reports issued to stockholders. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated on a regular basis by the chief operating decision-maker, or decision making group, in deciding how to allocate resources to an individual segment and in assessing performance of the segment. The Company believes that it operates in one segment.
Recent accounting pronouncements
In October 2009, the FASB issued an amendment to the accounting guidance for revenue arrangements with multiple deliverables. This guidance provides principles for allocation of consideration among its multiple-elements, allowing more flexibility in identifying and accounting for separate deliverables under an arrangement. The guidance introduces an estimated selling price method for valuing the elements of a bundled arrangement if vendor-specific objective evidence or third-party evidence of selling price is not available, and significantly expands related disclosure requirements. This guidance is effective on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. The Company adopted this accounting guidance on a prospective basis in the first quarter of 2011 for new and materially modified revenue arrangements. The adoption did not have a material impact on the Company's consolidated financial statements.
In October 2009, the FASB issued an amendment to the accounting standards related to certain revenue arrangements that include software elements. This standard clarifies the existing accounting guidance such that tangible products that contain both software and non-software components that function together to deliver the product's essential functionality, shall be excluded from the scope of the software revenue recognition accounting standards. Accordingly, sales of these products may fall within the scope of other revenue recognition accounting standards or may now be within the scope of this standard and may require an allocation of the arrangement consideration for each element of the arrangement. The Company adopted this standard on January 1, 2011. This adoption did not have a material impact on the Company's consolidated financial statements.
In January 2011, the Company adopted"Improving Disclosures About Fair Value Measurements" which requires additional disclosure about the amounts of and reasons for significant transfers in and out of Level 1 and Level 2 fair value measurements. In addition, effective for interim and annual periods beginning after December 15, 2010, which for the Company is January 1, 2011, this standard further requires an entity to present disaggregated information about activity in Level 3 fair value measurements on a gross basis, rather than as one net amount. As this accounting standard only
85
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
3. Summary of Significant Accounting Policies (Continued)
requires enhanced disclosure, the adoption of this newly issued accounting standard did not impact the Company's financial position or results of operations.
In May 2011, the FASB issued "Fair Value Measurement (Topic 820): Amendment to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs". This newly issued accounting standard clarifies the application of certain existing fair value measurement guidance and expands the disclosure for fair value measurements that are estimated using significant unobservable (Level 3) inputs. This ASU is effective on a prospective basis for annual and interim reporting periods beginning on or after December 15, 2011, which for the Company is January 1, 2012. The Company does not expect that adoption of this standard will have a material impact on the Company's financial position or results of operations.
In June 2011, the FASB issued an amendment to the accounting guidance for presentation of comprehensive income. Under the amended guidance, a company may present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In either case, a company is required to present each component of net income along with total net income, each component of other comprehensive income along with a total for other comprehensive income, and a total amount for comprehensive income. Regardless of choice in presentation, a company is required to present on the face of the financial statements reclassification adjustments for items that are reclassified from other comprehensive income to net income in the statement(s) where the components of net income and the components of other comprehensive income are presented. For public companies, the amendment is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, and shall be applied retrospectively. Early adoption is permitted. Other than a change in presentation, the adoption of this update is not expected to have a material impact on the Company's consolidated financial statements.
4. Inventory
The components of inventory are as follows (in thousands):
| | | | | | | |
| | December 31, | |
|---|
| | 2010 | | 2011 | |
|---|
Raw materials | | $ | — | | $ | — | |
Work in process | | | — | | | — | |
Finished goods | | | 2,935 | | | 910 | |
| | | | | | |
Inventory | | $ | 2,935 | | $ | 910 | |
| | | | | | |
The Company periodically reviews its inventories for excess or obsolete inventory and writes-down obsolete or otherwise unmarketable inventory to its estimated net realizable value. If the actual net realizable value is less than that estimated by the Company, or if there are any further determinations that inventory will not be marketable based on estimates of demand, additional inventory write-downs will be required. Based on management's estimates of demand, the Company determined that some of its inventory was in excess of its estimated net realizable value. During the year ended December 31, 2009, the Company recorded a $3.1 million charge to write-off excess and obsolete inventory. This charge was recorded as a research and development expense resulted in a $1.7 million decrease to
86
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Inventory (Continued)
inventory and a $1.4 million increase to accrued expenses for excess non-cancellable purchase commitments. During the year ended December 31, 2010, the Company recorded a $2.6 million charge to research and development expense to write-off excess and obsolete inventory. The Company recorded a non-cash charge of $2.0 million to research and development expense in the fourth quarter of 2011 related to deferred inventory costs that the Company concluded were no longer realizable. As of December 31, 2010 and 2011, inventory includes $2.9 million and $0.9 million, respectively related to the capitalized costs of sold HeliScopes for which the recognition of revenue has been deferred until recognition criteria have been met.
5. Property and Equipment, net
Property and equipment, net consist of the following (in thousands):
| | | | | | | |
| | December 31, | |
|---|
| | 2010 | | 2011 | |
|---|
Machinery and equipment | | $ | 126 | | $ | 51 | |
Leasehold improvements | | | — | | | — | |
Software | | | — | | | — | |
Office furniture | | | — | | | — | |
| | | | | | |
| | | 126 | | | 51 | |
Less accumulated depreciation and amortization | | | — | | | — | |
| | | | | | |
Property and equipment, net | | $ | 126 | | $ | 51 | |
| | | | | | |
Depreciation and amortization charged to the consolidated statements of operations for the years ended December 31, 2009, 2010, and 2011 and from May 9, 2003 (date of inception) to December 31, 2011 was $2.3 million, $1.0 million, $0 and $9.0 million, respectively.
The Company assesses its long-lived assets for impairment whenever events or changes in circumstances (a "triggering event") indicate that the carrying value of a group of long-lived assets may not be recoverable in accordance with FASB accounting guidance on accounting for the impairment or disposal of long-lived assets. During the third quarter of 2010, the Company altered its business direction as a result of its inability to raise funding to support the repositioning of the Company in the genetic analysis markets. As a result, the Company redirected its efforts to focus on a technology licensing and enforcement program to monetize the value of its intellectual property portfolio and to deploy its limited resources by concentrating its research and development efforts in targeted areas designed to achieve tangible proof-of-concept goals, which may enable the Company to seek partnership opportunities with companies interested in next-generation sequencing, or to pursue other funding and strategic alternatives.
As a result of this altered business direction, the Company undertook an analysis of the ongoing carrying value of its long-lived assets. Based on this analysis, in fiscal year 2010 the Company recorded an impairment charge related to property and equipment of approximately $1.9 million, of which approximately $1.8 million was related to assets used in research and development activities and approximately $0.1 million was related to assets used for selling, general and administrative purposes. These charges were derived from the net book value of the assets. The Company sold $75,000 of
87
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. Property and Equipment, net (Continued)
property and equipment during the year ended December 31, 2011. This equipment had a book value of $75,000 and no gain or loss was recognized.
6. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
| | | | | | | |
| | December 31, | |
|---|
| | 2010 | | 2011 | |
|---|
Professional fees | | $ | 396 | | $ | 974 | |
Compensation and benefits | | | 130 | | | 55 | |
Purchase commitments | | | 539 | | | 236 | |
Accrued interest | | | 22 | | | 2 | |
License fees | | | 90 | | | 74 | |
Other | | | 211 | | | 232 | |
| | | | | | |
Accrued expenses and other current liabilities | | $ | 1,388 | | $ | 1,573 | |
| | | | | | |
7. Commitments and Contingencies
License agreements and patents
In November 2003, the Company entered into a license agreement with California Institute of Technology (the "Caltech License Agreement") that granted the Company a worldwide, exclusive, royalty-bearing license, with the right to grant sublicenses, under specified patents and patent applications, and a worldwide, non-exclusive royalty bearing license, with the right to grant sublicenses, under specified technology outside the scope of the licensed patents. In connection with the Caltech License Agreement, the Company issued 46,514 shares of common stock, and recorded a charge of $20,000. In addition, the Company pays an annual license fee of $10,000 per year. The license fee payments are creditable against royalties based upon sales of products covered by patents licensed under the agreement. The up to single digit royalties are calculated based on a percentage of defined net sales. The Company is also obligated to pay California Institute of Technology up to a single digit percentage of specified license and sublicense income, a single digit percentage of proceeds from sales of specified intellectual property and a single digit percentage of service revenue amounts that it receives based on licenses and sublicenses that the Company grants, sales of intellectual property and services that are provided to third parties. The royalty obligation with respect to any licensed product extends until the later of the expiration of the last-to-expire of the licensed patents covering the licensed product or three years after the first commercial sale of the licensed product in any country for non-patented technology covered under the agreement. Through December 31, 2011, no royalty payments were made. In March 2007, the Company amended the Caltech License Agreement to provide rights under an additional patent application under the terms of the existing license in exchange for a one-time payment of $50,000 to the California Institute of Technology. There are no milestone payments potentially payable by the Company under the Caltech License Agreement in addition to those described above. All license fee amounts paid to date and the value of the common stock issued have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. The total expense recognized under the Caltech License Agreement for the years ended December 31, 2009, 2010 and 2011, and the
88
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Commitments and Contingencies (Continued)
period from May 9, 2003 (date of inception) through December 31, 2011 was $10,000, $10,000, $10,000 and $143,000, respectively.
In June 2004, the Company entered into a license agreement with Roche Diagnostics (the "Roche License Agreement") that granted the Company a worldwide, semi-exclusive royalty-bearing license, with the right to grant sublicenses under a patent relating to sequencing methods. In connection with the Roche License Agreement, the Company paid an upfront fee of 175,000 Euros and committed to pay an annual license fee ranging from 10,000 to 40,000 Euros. There are no milestone payments potentially payable by the Company under the Roche License Agreement in addition to those described above. The Company has an option to convert the license to non-exclusive license beginning in 2008, in which case the annual license fees would be reduced to 10,000 Euros beginning in 2008. The Company has the right to terminate the Roche License Agreement at any time for convenience upon 90 days prior written notice to Roche Diagnostics. Both the Company and Roche Diagnostics have the right to terminate the Roche License Agreement upon breach by the other party, subject to notice and an opportunity to cure. The Roche License Agreement also terminates upon the occurrence of specified bankruptcy events. As part of the Roche License Agreement, the Company agrees to pay single digit royalties based on a percentage of defined net sales. The Company also agrees to pay half of income amounts that are received based on sublicenses that the Company grants to third parties. The Company's royalty obligation, if any, extends until the expiration of the last-to-expire of the licensed patents. Through December 31, 2011, no royalty payments were made. All license fee amounts paid to date have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. The total expense recognized under the Roche License Agreement for the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) through December 31, 2011 was $54,000, $59,000, $58,000 and $538,000, respectively.
In March 2005, the Company entered into a license agreement with Arizona Technology Enterprises (the "AZTE License Agreement") that granted the Company a worldwide, exclusive, irrevocable, royalty-bearing license, with the right to grant sublicenses, under specified patents and patent applications exclusively licensed by AZTE from Arizona State University and the University of Alberta. In connection with the AZTE License Agreement, the Company paid an upfront fee of $350,000, committed to an annual license fee of $50,000, which has increased to $100,000 upon the successful issuance of a U.S. patent, committed to pay a three-year maintenance fee of $50,000, payable in equal annual installments beginning in March 2006, and issued 88,888 shares of restricted common stock, which vested in two equal installments upon the achievement of separate milestones. There are no milestone payments potentially payable by the Company under the AZTE License Agreement in addition to those described above. The Company is obligated to use reasonable commercial efforts to develop, manufacture and commercialize licensed products. In addition, if the Company fails to use commercially-reasonable efforts to develop, manufacture and sell licensed products, the AZTE License Agreement converts from exclusive to non-exclusive. The AZTE License Agreement will remain in force until terminated. The Company has the right to terminate the AZTE License Agreement at any time for convenience upon 60 days prior written notice to Arizona Technology Enterprises. Both the Company and Arizona Technology Enterprises have the right to terminate the agreement upon breach by the other party, subject to notice and an opportunity to cure. The AZTE License Agreement also terminates upon the occurrence of specified bankruptcy events.
89
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Commitments and Contingencies (Continued)
As part of the AZTE License Agreement, the Company agreed to pay a single digit percentage royalty based on defined net sales. The Company also agrees to pay a mid-teens percentage of specified sublicense income amounts that are received based on sublicenses granted to third parties which increases to 30 percent after the Company receives an aggregate of $50,000 of such amounts. The Company's royalty obligation, if any, extends until the expiration of the last-to-expire of the licensed patents. Through December 31, 2010, no royalty payments have been made. All license fee amounts paid to date have been expensed to research and development expense as technological feasibility had not been established and the technology had no alternative future use. In May 2006, in accordance with the license agreement, due to the successful issuance of a U.S. patent, the committed annual license fee increased from $50,000 to $100,000 and 44,444 shares of the restricted common stock vested. The vesting of 44,444 shares of restricted common stock resulted in a charge to research and development expense of $127,000 based on the fair value of the Company's common stock at the time the milestone was achieved. The remaining 44,444 shares of restricted common stock vested immediately upon the successful issuance of a second U.S. patent on January 12, 2010 and resulted in a charge to research and development expense in fiscal 2010 of $54,000. The total expense recognized under the AZTE License Agreement for the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) through December 31, 2011 was $141,000, $154,000, $100,000 and $1,244,000, respectively.
On August 26, 2011, the Company entered into a First Amendment to the License Agreement (the "Amendment") with AZTE. The Amendment expanded the field under the AZTE License Agreement so that AZTE has now granted the Company the exclusive right and license under the Licensed Patents to the full extent of the entire scope of all claims within the Licensed Patents, in all fields of use. AZTE's license to the Company under the AZTE License Agreement (the "AZTE Licensed Rights"), gives the Company the rights to several issued patents covering sequencing-by-synthesis methods and a number of pending patent applications, now expressly includes detection methods that do not rely on the detection of optically-labeled nucleotides (the "Expanded Field"). Under the terms of the Amendment, the Company will remain obligated to pay AZTE a specified percentage of income from our-granted sublicenses or other transfers of the AZTE Licensed Rights, including as part of any sale of the Company or litigation settlement. Amounts the Company owes to AZTE will be a percentage of the proportionate value of the AZTE Licensed Rights in any such transaction. The Company also agreed to make additional payments to AZTE for Company-granted sublicenses or other such transfers of the AZTE Licensed Rights within the Expanded Field. The Company retains its existing first right to initiate and pursue patent infringement suits involving the AZTE Licensed Rights. There has been no expense recorded for this amendment for the period ending December 31, 2011.
In April 2007, the Company entered into an agreement with PerkinElmer LAS, Inc. ("PerkinElmer"), in which PerkinElmer granted the Company a worldwide, non-exclusive, non-transferable, non-sublicensable, royalty bearing license under specified patents. The license from PerkinElmer grants the Company rights under certain patents to produce and commercialize certain of the reagents used in some applications on the Helicos System, which contain chemicals purchased from PerkinElmer. In exchange for rights licensed from PerkinElmer, the Company is obligated to pay PerkinElmer a single digit percentage of the Company's net revenue from the sale of reagents that contain chemicals covered by the patents licensed under the PerkinElmer agreement. There are no milestone payments potentially payable by the Company under this agreement with PerkinElmer. The Company has the right to terminate the agreement at any time upon 90 days written notice to
90
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Commitments and Contingencies (Continued)
PerkinElmer. Each party has the right to terminate the agreement upon breach by the other party subject to notice and an opportunity to cure. The agreement also terminates upon the occurrence of specified bankruptcy events. PerkinElmer has the sole right under the agreement to enforce the licensed patents. There has been no expense recorded for this agreement for any period from May 9, 2003 (inception) through December 31, 2011.
Operating leases
In December 2005, the Company entered into an operating lease for new office and laboratory space. The lease expired in March 2010. In connection with this lease agreement, the Company entered into a letter of credit in the amount of $450,000, naming the Company's landlord as beneficiary. In October 2008, the letter of credit was decreased to $225,000 pursuant to the lease agreement which allows for a fifty percent reduction provided that the Company was not in default and that the lease was in full force and effect. As of December 31, 2009, the Company classified the $225,000 letter of credit as restricted cash on the consolidated balance sheet. As of December 31, 2010, the landlord fully utilized the letter of credit in lieu of rent payments from the Company. Additionally, in connection with the lease agreement, the Company received lease incentives from the landlord of certain leasehold improvements. The Company recorded the lease incentives as a liability and is amortizing them over the lease term as a reduction in rent expense. For the years ended December 31, 2009, 2010 and 2011, the Company recorded $105,000, $17,000 and $0, respectively, as a reduction in rent expense for this amortization. In March 2006, February and December 2007, July 2008, October 2009 and June 2010, the Company amended its operating lease for office and laboratory space to include additional office space in the same building. This lease expired as of December 31, 2010 and the Company consolidated its operations into a single space which is leased on a tenant-at-will basis. In fiscal year 2010, under the terms of the lease agreement, the Company realized a rent abatement of $115,000 from the lessor in connection with a casualty loss. In September 2011 the Company amended the lease further reducing its leased premises and fixing the expiration of the lease to December 31, 2012. Total rent expense was $2.0 million, $1.3 million, $0.4 million and $8.4 million for the years ended December 31, 2009, 2010, and 2011, and the period from May 9, 2003 (date of inception) through December 31, 2011, respectively.
Debt facilities
Pursuant to the bridge financing and senior debt restructuring which occurred in November 2010, the Company may be liable for certain contingent payments based upon potential future events. (See "Bridge Financing and Senior Debt Restructuring" in Note 8.)
Management Incentive Plan
In approving the transactions relating to the Bridge Debt Financing and the Fourth Amendment (see Note 8), the Board approved a management incentive plan (the "Management Incentive Plan") pursuant to which participants in the plan will be entitled to receive, in the aggregate, an amount equal to 7.5% of the Payment Consideration resulting from IP Licensing Events (gross proceeds from IP Licensing Events less various contractual and contingent payments). The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board. As of December 31, 2011, no amounts have been accrued and no payments have been made pursuant to the Management Incentive Plan.
91
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
7. Commitments and Contingencies (Continued)
Litigation
On October 14, 2010, Helicos announced the launch of a technology licensing program for its Single Molecule Sequencing and Sequencing-by-Synthesis intellectual property. The Company plans to defend its intellectual property rights through licensing and enforcement strategies. In this regard, on October 22, 2010, the Company filed an amended complaint against Pacific Biosciences by naming Illumina, Inc. and Life Technologies Corporation as additional defendants. The amended lawsuit alleges that the defendants willfully infringe the Company's patents in suit by using their respective technologies, sequencing systems and products which the Company alleges are within the scope of one or more claims of the Company's patents in suit. (See Note 1 of Notes to Consolidated Financial Statements.) Helicos is seeking a permanent injunction enjoining the defendants from further infringement as well as monetary and punitive damages, costs and attorneys' fees, interest and other relief as determined by the Court. A portion of any proceeds received by the Company pursuant to this amended lawsuit is due to counsel representing the Company in the action as counsel's only fees relating to their representation and to the Purchasers on the Notes issued pursuant to the Risk Premium Agreement entered into between the Company and the Purchasers of the Notes in connection with the Bridge Debt Financing. (See Note 8.)
On March 21, 2012, Helicos received an attorney demand letter from one of its major customers alleging that Helicos is in breach of the customer's system and reagent kit supply agreements in connection with the sale of multiple Helicos systems and reagent kits that were sold and installed prior to December 31, 2009. The demand letter alleges breach of warranty and that the systems did not conform to Helicos' specifications and were defective in material and workmanship. In addition, the demand letter alleges that Helicos did not provide the requisite level of customer support in connection with the system service and maintenance requirements set forth in the system supply agreement. The demand letter states that, if the parties are unable to reach an amicable settlement, the customer will terminate the supply agreements and request that Helicos refund approximately $5.0 million. The Company has deferred all revenue related to this arrangement, and it is in the process of evaluating the claims, and has not yet responded to the demand letter.
8. Debt
Loan and security agreement
In December 2007, the Company entered into a loan and security agreement with two lenders including GE Capital Corporation, which is serving as agent. The loan agreement provided that the Company may borrow up to $20.0 million at an interest rate equal to the sum of (i) the greater of (A) an interest rate based on the Federal Reserve's three year Treasury Constant Maturities Rate and (B) 3.84% plus (ii) 6.11%. The initial term loan was made in December 2007 in an aggregate principal amount equal to $10.0 million.
In June 2008, the Company entered into a second amendment to the loan and security agreement with two lenders including GE Capital Corporation. A subsequent term loan was made upon execution of the amendment in an aggregate principal amount equal to $10.0 million. The loan amendment provided that the interest rate for the subsequent term loan is equal to the sum of (i) the greater of (A) an interest rate based on the Federal Reserve's three year Treasury Constant Maturities Rate and (B) 3.17% plus (ii) 8.33%. The loan agreement, as amended, contained affirmative and negative covenants to which the Company and its subsidiaries must adhere. Pursuant to the amendment, the
92
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt (Continued)
Company was required to maintain, at all times, unrestricted cash in its bank account equal to at least $10.0 million. The proceeds of the loan agreement were collateralized by essentially all of the Company's assets. Payments are required to be made on a monthly basis. For the initial term loan, interest-only payments were required for the first five months. Thereafter, for the following 31 months, payments of principal and interest will be due. For the subsequent term loan, principal and interest payments are required for the 36 month term of the loan. In connection with the execution of the June 2008 amendment to the loan and security agreement with two lenders including GE Capital Corporation, the Company issued warrants to the two lenders to purchase an aggregate of 110,000 shares of common stock. The warrants have an exercise price of $4.80 per share and expire in June 2014. The fair value of the warrants was estimated at $337,000 using a Black-Scholes model with the following assumptions: expected volatility of 65.4%, risk free interest rate of 3.4%, expected life of six years and no dividends. Expected volatility was based on the volatility of similar entities in the life sciences industry of comparable size of market capitalization and financial position that completed initial public offerings within the last ten years. The fair value of the warrants was recorded as equity and a debt discount and is being amortized to interest expense over the term of the loan. In connection with the senior debt restructuring in November 2010, the exercise price of the warrants was reduced to $0.01 per share and the Company recorded $47,000 of additional debt discount to reflect the change in the value of the warrants and is amortizing this amount over the term of the loan.
In December 2008, the Company entered into an additional amendment to the loan and security agreement with certain lenders including GE Capital Corporation. The amendment amended the prepayment provisions of the loan and security agreement to allow the Company to make a prepayment of $10.0 million (the "Pay Down Amount") without incurring any prepayment penalties. Pursuant to the amendment, the Company made a prepayment, equal to the Pay Down Amount, in December 2008. In connection with such prepayment, and in lieu of the 4% final payment fee with respect to the Pay Down Amount, the amendment provides that the Company will pay a fee equal to 2% of the initial $10.0 million term loan, payable on the earlier of (a) January 31, 2011 and (b) the maturity date for the subsequent term loan. In connection with the senior debt restructuring in November 2010, the due date for this fee was deferred to the amended maturity date for the subsequent term loan.
The amendment further provides that the Company's obligations under the loan agreement, as amended, are no longer secured by a cash amount of $10.0 million. As such, this amount is no longer classified as restricted cash as of December 31, 2008. Such obligations continue to be secured under various collateral documents by interests in substantially all of the Company's personal property, including the pledge of the stock of its wholly-owned subsidiary, and proceeds of any intellectual property, but not by its intellectual property. In connection with the Senior Debt Restructuring in November 2010, the collateral securing this facility was expanded to include the Company's intellectual property.
The loan and security agreement contains a subjective material adverse clause, which allows the debt holders to call the loans upon a material adverse event. Based on an updated assessment of the terms of the loan and security agreement, the entire balance due is classified in the current liabilities section in the accompanying balance sheet at December 31, 2010.
As of December 31, 2009, the loan agreement did not require the Company to comply with any financial covenants. Through December 31, 2010, advances on the loan agreement, as amended, were $20.0 million at an interest rate of 11.5%. As of December 31, 2010 and 2011, the outstanding balance
93
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt (Continued)
on the loan agreement was $2.8 million and $1.0 million, respectively. This loan agreement was amended in November 2010 (see "Bridge Financing and Senior Debt Restructuring" below).
In January 2012, the Company made a final $1,070,000 payment on the senior debt facility pursuant to the Loan and Security Agreement among the Company, certain lenders and General Electric Capital Corporation, as agent, dated as of December 31, 2007, as amended (the "Loan Agreement"). As a result, the Company obligations under the Loan Agreement have been satisfied and the senior debt lenders no longer have a security interest in the Company assets, including the Company's intellectual property assets.
Bridge Financing and Senior Debt Restructuring
The Company entered the fourth quarter of 2010 without any prospects of securing long-term financing from outside investors. An independent committee ("Independent Committee") of the Board evaluated the alternatives available to the Company and determined that a bridge financing led by certain inside investors was in the best interests of its stakeholders in view of its current financial and operating condition, and would best position the Company to continue operations for the remainder of 2010 and into 2011. The Independent Committee met multiple times to consider the alternatives and concluded that the bridge financing was the best alternative for generating value for stakeholders. As a result, the Company entered into an insider bridge loan facility with two of the four venture capital firms whose representatives are members of the Board. In connection with this transaction, the Company entered into a Subordinated Secured Note Purchase Agreement (the "Note Purchase Agreement") with investment funds affiliated with Atlas Venture and Flagship Ventures (the "Purchasers"), pursuant to which the Company agreed to sell to the Purchasers, and the Purchasers have agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), a portion of which is committed and a portion of which is optional (the "Bridge Debt Financing"). On November 16, 2010, the Purchasers purchased Notes having an aggregate principal amount of $333,333. Subject to the Company's continued compliance with the terms of the Note Purchase Agreement, including that there not be an event of default thereunder, upon notice from the Company the Purchasers are committed to purchase an aggregate of an additional $333,333 of Notes in each of five subsequent closings, for a total aggregate committed amount of $2,000,000. The Purchasers also have the right, but not the obligation, upon the Company's request to purchase up to an additional $2,000,000 of Notes on or before December 31, 2012. As of December 31, 2011 the Company has borrowed $2.0 million against the committed portion of the Bridge Debt Financing facility. In addition, as of the date of this filing the Company has borrowed an additional $375,000 under the uncommitted portion of the Bridge Debt Financing facility on a short term basis with a maturity date of May 15, 2012.
The Notes will accrue interest at a rate of 10% per annum plus a risk premium as described below. The outstanding principal and any unpaid accrued interest on all of the Notes shall be due and payable in full (i) upon demand by the Purchasers, which demand shall be no earlier than December 31, 2012, (ii) upon the Company receiving at least $10,000,000 in proceeds from a subsequent equity financing, (iii) upon a change of control of the Company or (iv) upon an event of default. A Purchaser may elect to convert principal and interest outstanding under any of the Notes upon the closing of any future round of equity or debt financing of the Company into shares, and at the per share price, of the securities issued at such financing. Simultaneous with execution of the Note Purchase Agreement, the Company and the requisite parties entered into an Amendment to the
94
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt (Continued)
Amended and Restated Investor Rights Agreement, dated as of March 1, 2006, by and among the Company and the parties specified therein, including the Purchasers (the "IRA Amendment"), pursuant to which the Purchasers will be entitled to demand and piggyback registration rights consistent with the terms of the Purchasers' existing registration rights for any securities issued upon conversion of the Notes.
The Company's obligations under the Notes are subordinated to the indebtedness incurred by the Company under the Loan and Security Agreement among the Company, certain lenders (the "Lenders") and General Electric Capital Corporation ("GE"), as agent, dated as of December 31, 2007, as amended (the "Loan Agreement"). The Company's obligations under the Notes are secured by a security interest on all of the Company's assets, including its intellectual property, that, with regard to all of the Company's assets other than the Cause of Action, is junior to the Lenders' first priority security interest under the Loan Agreement, and with regard to the Cause of Action, is junior to the Lenders' first priority security interest under the Loan Agreement and the second priority security interest of its outside legal counsel that is representing the Company in the Cause of Action (the "Outside IP Litigation Counsel").
As an inducement for the Purchasers to purchase the Notes, the Company entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") simultaneous with the execution of, and as required by, the Note Purchase Agreement. Under the terms of the Risk Premium Agreement, the Company agreed to pay the Purchasers the following portions of the consideration it receives (the "Payment Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). For purposes of determining amounts payable under the Risk Premium Agreement, the Payment Consideration will not include amounts the Company uses to first satisfy specified existing obligations, including obligations under the Loan Agreement, under professional contingency arrangements (including the contingency fee arrangement between the Company and Outside IP Litigation Counsel relating to the Cause of Action) and obligations that may arise under existing technology license agreements (the "Existing IP Licensing Agreements") between the Company and each of Arizona Technology Enterprises, Roche Diagnostics GMBH and Roche Diagnostics Corporation, and California Institute of Technology (as previously disclosed by the Company, under the Existing IP Licensing Agreements, the Company is obligated to pay license fees in connection with the licensing, sublicensing, legal settlements or sales of specified intellectual property or services provided to third parties ranging from a single digit percentage up to one-half of the income from those activities). For purposes of the Risk Premium Agreement and subject to various provisions therein, the following shall constitute Liquidity Transactions: (A) the receipt by the Company of any payments from third parties relating to its intellectual property portfolio of the Company, including payments received as a license or other settlement in patent infringement litigation brought by the Company ("IP Licensing Events"); (B) a merger of the Company in which there is a change of control; and (C) the sale or exclusive license by the Company of all or substantially all of its assets or intellectual property.
95
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt (Continued)
In connection with the Bridge Debt Financing, on November 16, 2010, the Company, GE and the Lenders executed the Fourth Amendment to the Loan and Security Agreement, dated November 16, 2010 (the "Fourth Amendment"). Pursuant to the Fourth Amendment, GE and the Lenders waived various existing events of default and consented to the Company's incurrence of additional indebtedness and the grant of a junior security interest to the Purchasers under the Note Purchase Agreement. The Company amended warrants previously issued to the Lenders to re-price the Warrants from $4.80 to $0.01 and GE and the Lenders agreed to defer a $200,000 payment originally due January 31, 2011 to the January 1, 2012 term loan maturity date. In addition, subject to the Company's continued compliance with the terms of the Loan Agreement, including that there not be an event of default thereunder, GE and the Lenders agreed to defer up to five additional principal monthly payments with respect to the term loan. Also pursuant to the Fourth Amendment, the Company agreed to grant the Lenders a first priority security interest in all of its intellectual property and to pay the Lenders an additional $50,000 to $200,000 (depending upon how many principal payments are deferred) upon the full repayment of all outstanding amounts due with respect to the term loans. Under the terms of the Loan and Security Agreement prior to its amendment by the Fourth Amendment, the scope of the Lenders' first priority security interest included all of its assets other than intellectual property. As of the date of this filing, the Lenders have agreed to defer the additional five monthly principal payments referenced above.
In connection with the Bridge Debt Financing and the Fourth Amendment and as security for the Company's obligations under its contingency fee arrangement with Outside IP Litigation Counsel relating to the Cause of Action, the Company and Outside IP Litigation Counsel entered into a Subordinated Security Agreement pursuant to which the Company agreed to grant Outside IP Litigation Counsel a second priority security interest in the Cause of Action. Under the terms of the contingency fee arrangement, the Company is obligated to pay Outside IP Litigation Counsel up to 40% of the proceeds paid to the Company in connection with the settlement, judgment or other resolution of the Cause of Action or certain business transactions resulting from or relating to the Cause of Action.
The agreements and arrangements described above and all of the transactions contemplated by those agreements were approved by an independent committee of the Board of Directors of the Company. In addition, Noubar B. Afeyan, PhD, who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, who is a Partner of Atlas Venture, abstained from the vote of the Board of Directors of the Company approving the transactions.
96
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Debt (Continued)
As of December 31, 2011, payments relating to the senior debt and the Notes are due as follows (in thousands):
| | | | | | | | | | |
| | Senior
Debt | | Notes | | Total | |
|---|
2012 | | $ | 1,060 | | $ | 2,216 | | $ | 3,276 | |
Thereafter | | | — | | | — | | | — | |
| | | | | | | | |
Total future minimum payments | | | 1,060 | | | 2,216 | | | 3,276 | |
Less: amount representing interest | | | — | | | (216 | ) | | (216 | ) |
Less: debt discount | | | (800 | ) | | — | | | (800 | ) |
Add: interest accrued | | | — | | | 85 | | | 85 | |
Add: amortization of debt discount | | | 800 | | | — | | | 800 | |
| | | | | | | | |
Carrying value of debt | | | 1,060 | | | 2,085 | | | 3,145 | |
Less: current portion | | | (1,060 | ) | | (2,085 | ) | | (3,145 | ) |
| | | | | | | | |
Long-term obligations | | $ | — | | $ | — | | $ | — | |
| | | | | | | | |
The above table does not reflect any Risk Premium Payments potentially due pursuant to the Risk Premium Agreement.
9. Corporate Restructuring
On May 11, 2010, the Board of Directors of the Company approved a reduction in headcount and restructuring plan (the "May 2010 Restructuring Plan") which involved the consolidation and reorganization of its operations in order to reduce operating costs. The May 2010 Restructuring Plan resulted in the elimination of approximately 40 positions during the second fiscal quarter of 2010. Employees directly affected by the May 2010 Restructuring Plan were notified on May 13, 2010, and were provided with severance arrangements including outplacement assistance. These actions were approved as part of the Company's repositioning strategy in the diagnostics market. As of December 31, 2010, there were no remaining payments due pursuant to this restructuring.
The Company incurred restructuring charges relating to one-time termination benefits of approximately $549,000 in fiscal year 2010, of which $543,000 was recorded in research and development expense and $6,000 was recorded in selling, general and administrative expense. These charges represent employee severance and termination costs as well as facility consolidation costs, which were paid during 2010. As of December 31, 2010, there were no remaining payments due pursuant to this restructuring.
On September 15, 2010, the Board of Directors of the Company approved a reduction in headcount and restructuring plan (the "September 2010 Restructuring Plan") which involved the consolidation and reorganization of its operations in order to reduce operating costs. The September 2010 Restructuring Plan resulted in the elimination of approximately 14 positions during the third fiscal quarter of 2010. Employees directly affected by the September 2010 Restructuring Plan were notified on September 16, 2010, and have been provided with severance arrangements including outplacement assistance. These actions were approved as part of the Company's repositioning strategy in the diagnostics market.
97
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Corporate Restructuring (Continued)
The Company incurred restructuring charges relating to one-time termination benefits of approximately $172,000 in fiscal year 2010, all of which was recorded in research and development expense. These charges represent employee severance and termination costs. As of December 31, 2010, $11,000 of such restructurings costs remained to be paid. All of these remaining termination costs were paid during 2011.
During the second quarter of 2011, the Company further reduced its workforce by phasing out six business and research positions. These six positions were phased out during the third quarter of 2011 and ultimately reduced the Company's total headcount to ten positions. The Company did not incur or pay out severance charges or lease exit costs in connection with the implementation of these measures.
10. Common Stock and Warrant Liability
As of December 31, 2010 and 2011, the Company had 120,000,000 shares of common stock authorized. As of December 31, 2010 and 2011, the Company had 86,557,606 and 87,196,489 shares issued and outstanding, respectively. As of December 31, 2011, the Company had reserved 22,653,964 shares of common stock for issuance to common stockholders upon exercise of common stock warrants. As of December 31, 2010 and 2011, the Company has reserved 5,757,926 and 9,871,770 shares of common stock, respectively, for future issuance upon exercise of outstanding and future grants of common stock options and future issuances of restricted stock awards pursuant to the Company's 2003 and 2007 stock option plans.
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company's stockholders. Common stockholders are not entitled to receive dividends unless declared by the Company's Board of Directors.
December 2008 Financing
In December 2008, the Company entered into a securities purchase agreement with certain investors pursuant to which it sold a total of 42,753,869 units (the "Units"), each Unit consisting of (i) one share of common stock (collectively, the "Shares") and (ii) one warrant (collectively, the "Warrants") to purchase 0.6 shares of common stock at an exercise price of $0.45 per share, for a purchase price of $0.435 per unit (representing the closing bid price plus an additional amount for the warrants) (the "Offering"). The closing of the transaction occurred on December 23, 2008. In connection with the Offering, the Company raised approximately $18.6 million in gross proceeds. After paying $813,000 in placement agent fees and offering expenses, the net proceeds were $17.8 million.
In connection with the Offering, the Company issued warrants to purchase an aggregate of 25,652,333 shares of common stock which are exercisable immediately. The warrants have an exercise price of $0.45 per share and have a five year term. The relative fair value of the warrants was estimated at $4,449,397 using a Black-Scholes model with the following assumptions: expected volatility of 66.15%, risk free interest rate of 1.53%, expected life of five years and no dividends. Expected volatility was based on the volatility of similar entities in the life sciences industry of comparable size of market capitalization and financial position that completed initial public offerings within the last ten years. The relative fair value of the warrants was recorded in the equity section of the balance sheet.
In connection with the Offering, the Company entered into a registration rights agreement (the "Registration Rights Agreement") with each of the investors. The Registration Rights Agreement
98
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Common Stock and Warrant Liability (Continued)
provides that the Company file a "resale" registration statement (the "Registration Statement") covering all of the Shares and the shares issuable upon exercise of the Warrants (the "Warrant Shares"), up to the maximum number of shares able to be registered pursuant to applicable Securities and Exchange Commission ("SEC") regulations, within 30 days of the closing of the Offering. The Company filed the Registration Statement with the SEC on January 22, 2009 (File No. 333-156885), which was amended on February 13, 2009. Under the terms of the Registration Rights Agreement, the Company is obligated to maintain the effectiveness of the "resale" registration statement until all securities therein are sold or can otherwise be sold pursuant to Rule 144, without any restrictions.
A cash penalty at the rate of 2% per month will be triggered for any filing or effectiveness failures or if, at any time after six months following the closing of the Offering, the Company ceases to be current in periodic reports with the SEC. The aggregate penalty accrued with respect to each investor may not exceed 12% of the original purchase price paid by that investor.
In December 2006, the FASB issued guidance on accounting for registration payment arrangements, which addresses an issuer's accounting for registration payment arrangements. This guidance specifies that the contingent obligation to make future payments or otherwise transfer consideration under a registration payment arrangement, whether issued as a separate agreement or included as a provision of a financial instrument or other agreement, should be separately recognized and measured in accordance with FASB guidance on accounting for contingencies. This guidance further clarifies that a financial instrument subject to a registration payment arrangement should be accounted for in accordance with US GAAP without regard to the contingent obligation to transfer consideration pursuant to the registration payment arrangement. The Company applied the recognition and measurement provisions of the FASB guidance to the registration rights associated with the Registration Rights Agreement. As a result, the Company believes that the contingent obligation to make future payments is not probable under the guidance and as such has recorded no liability associated with these registration rights.
September 2009 Financing
In September 2009, the Company entered into a securities purchase agreement with certain investors pursuant to which it (i) sold to certain existing investors a total of 1,030,028 units, each unit consisting of (a) one share of common stock (collectively, the "Shares") and (b) one warrant (collectively, the "Warrants") to purchase 0.662 shares of common stock at an exercise price equal to $2.61 per share (105% of the closing bid price of the common stock on September 15, 2009), at a purchase price equal to $2.57 per unit, and (ii) sold to certain new investors a total of 3,281,252 units, each unit consisting of (a) one share of common stock and (b) one warrant to purchase 0.50 shares of common stock at an exercise price equal to $2.61 per share, at a purchase price equal to $2.24 per unit (together, the "September 2009 Offering"). The closing of the transaction occurred on September 18, 2009. In connection with the September 2009 Offering, the Company raised approximately $10.0 million in gross proceeds. After paying $646,000 in placement agent fees and offering expenses, the net proceeds were $9.4 million.
In connection with the September 2009 Offering, the Company issued warrants to purchase an aggregate of 2,322,504 shares of common stock which become exercisable on or after the six month anniversary following the closing of the transaction. The warrants had an exercise price of $2.61 per share and have a five and a half year term. The fair value of the warrants was estimated at $4.5 million
99
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Common Stock and Warrant Liability (Continued)
using a Black-Scholes model with the following assumptions: expected volatility of 100%, risk free interest rate of 2.49%, expected life of five and a half years and no dividends. Expected volatility was based on the volatility contractually specified in the warrant agreements. Due to a price adjustment clause included in the warrant agreements, the warrants were deemed to be a liability and, therefore, the fair value of the warrants was recorded in the liability section of the balance sheet. As such, the warrants will be revalued each reporting period, with the resulting gains and losses recorded as the change in fair value of warrant liability on the statement of operations. In connection with the December 2009 Offering, the exercise price for the warrants issued in this transaction was subsequently reduced to $0.31 per share in accordance with the terms of the warrant. The Company recorded a gain in the amount of $2.4 million for the change in the fair value of the warrant liability from the closing date of September 18, 2009 through the end of the year December 31, 2009. For the twelve month period ended December 31, 2010 the Company recorded a gain in the amount of $1.9 million for the change in the fair value of the warrant liability. In connection with the Bridge Debt Financing and senior debt restructuring (see Note 8), the exercise price for the warrants held by non-related parties was further reduced to $0.01 per share. At December 31, 2011, related parties held 681,884 warrants at an exercise of $2.61 per share. Such warrant holders are entitled to a reduction in the exercise price to $0.01 per share following an affirmative approval from the Company's Stockholders. During the twelve months ended December 31, 2010, 1,417,410 warrant shares from the September 2009 Offering were exercised into common stock at an exercise price ranging from $0.01 to $0.3124 per share. During the twelve months ended December 31, 2011, 223,215 warrant shares from the September 2009 Offering were exercised into common stock at an exercise price of $0.01 per share.
In connection with the September 2009 Offering, the Company entered into a registration rights agreement (the "September 2009 Registration Rights Agreement") with each of the investors. The Registration Rights Agreement provides that the Company will file a "resale" registration statement covering all of the shares of common stock and the shares of common stock issuable upon exercise of the warrants, up to the maximum number of shares able to be registered pursuant to applicable SEC regulations, within 15 days of the closing of the September 2009 Offering. The Company currently maintains an effective registration statement relating to these shares (File No. 333-162240). Under the terms of the September 2009 Registration Rights Agreement, the Company is obligated to maintain the effectiveness of the "resale" registration statement until all securities therein are sold or can otherwise be sold pursuant to Rule 144, without any restrictions. A cash penalty at the rate of 2% per month will be triggered for any filing or effectiveness failures or 1% in the event of suspensions of prospectus delivery or if, at any time after six months following the closing of the September 2009 Offering, the Company ceases to be current in its periodic reports with the SEC. The aggregate penalty accrued with respect to each investor may not exceed 12% of the original purchase price paid by that investor. The Company has not recorded an amount associated with the contingent obligation regarding the cash penalties as of December 31, 2010 or 2011 as the Company believes that the contingent obligation to make future payments is not probable.
December 2009 Financing
In December 2009, the Company entered into an Underwriting Agreement (the "Underwriting Agreement") with Thomas Weisel Partners LLC (the "Underwriter"), pursuant to which it sold to the Underwriter 6,400,000 shares of common stock and warrants to purchase up to 4,160,000 shares of common stock sold in units, at a price of $1.00 per unit ("December 2009 Offering"). Each unit
100
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Common Stock and Warrant Liability (Continued)
consists of one share of common stock and a warrant to buy 0.65 of a share of common stock. The warrants will be exercisable for a period of five years, beginning six months after issuance, at an exercise price of $1.4385 per share. The closing of the transaction occurred on December 15, 2009. The Company received approximately $6.4 million in gross proceeds from the December 2009 Offering. After paying $718,000 in underwriting discounts, commissions and offering expenses, the net proceeds were $5.6 million.
In connection with the December 2009 Offering, the Company issued warrants to purchase an aggregate of 4,160,000 shares of common stock. The warrants had an exercise price of $1.44 per share and have a five and a half year term. The fair value of the warrants was estimated at $2.7 million using a Black-Scholes model with the following assumptions: expected volatility of 100%, risk free interest rate of 2.43%, expected life of five and a half years and no dividends. Expected volatility was based on the volatility contractually specified in the warrant agreements. Due to a price adjustment clause included in the warrant agreements, the warrants were deemed to be a liability and, therefore, the fair value of the warrants was recorded in the liability section of the balance sheet. As such, the warrants will be revalued each reporting period, with the resulting gains and losses recorded as the change in fair value of warrant liability on the income statement. The Company recorded a gain in the amount of $0.4 million for the change in the fair value of the warrant liability from the closing date of September 18, 2009 through the end of the year December 31, 2009. For the twelve month period ended December 31, 2010 the Company recorded a gain in the amount of $2.2 million for the change in the fair value of the warrant liability. For the twelve month period ended December 31, 2011 the Company recorded a gain in the amount of $3,000 for the change in the fair value of the warrant liability. In connection with the Bridge Debt Financing and senior debt restructuring (see Note 8), the exercise price for the warrants was reduced to $0.01 per share. During the twelve months ended December 31, 2010, 3,120,000 warrant shares from the December 2009 Offering were exercised into common stock at an exercise price of $0.01 per share. During the twelve months ended December 31, 2011, 1,040,000 warrant shares from the December 2009 Offering were exercised into common stock at an exercise price of $0.01 per share.
11. Income Taxes
There is no provision for income taxes because the Company has incurred operating losses since inception. The reported amount of income tax expense for the years differs from the amount that would result from applying domestic federal statutory tax rates to pretax losses primarily because of the
101
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Income Taxes (Continued)
changes in the valuation allowance. Significant components of the Company's deferred tax assets at December 31, 2010 and 2011 are as follows (in thousands):
| | | | | | | |
| | December 31, | |
|---|
| | 2010 | | 2011 | |
|---|
Deferred tax assets: | | | | | | | |
Net operating loss carryforwards | | $ | 47,695 | | $ | 50,539 | |
Research and development credit carryforwards | | | 5,082 | | | 5,186 | |
Depreciation and amortization | | | 11,164 | | | 9,513 | |
Allowances and reserves | | | 2,490 | | | 2,241 | |
| | | | | | |
| | | 66,431 | | | 67,479 | |
Less: Valuation allowance | | | (66,431 | ) | | (67,479 | ) |
| | | | | | |
Net deferred tax assets | | $ | — | | $ | — | |
| | | | | | |
As of December 31, 2011, the Company has federal and state net operating losses ("NOL") of approximately $132.2 million and $106.1 million, respectively, as well as federal and state research and development credits of approximately $3.5 million and $2.6 million, respectively, which may be available to reduce future taxable income and taxes. Federal NOLs and research and development credits each begin to expire in 2024. State NOLs and research and development credits begin to expire in 2010 and 2019, respectively. As required by FASB accounting guidance on accounting for income taxes, the Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets, which are comprised principally of NOLs. Management has determined that is it more likely than not that the Company will not recognize the benefits of the federal and state deferred tax assets and, as a result, a valuation allowance of $66.4 million and $67.5 million has been established at December 31, 2010 and 2011, respectively.
A reconciliation of income tax expense (benefit) at the statutory federal income tax rate and income taxes as reflected in the financial statements is as follows:
| | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2009 | | 2010 | | 2011 | |
|---|
Federal income tax at statutory rate | | | 34.0 | % | | 34.0 | % | | 34.0 | % |
State income tax, net of federal tax benefit | | | 4.2 | % | | 2.5 | % | | 4.0 | % |
Research and development credits | | | 1.9 | % | | 1.1 | % | | 1.7 | % |
Warrant revaluation | | | 2.4 | % | | 7.5 | % | | — | % |
Book compensation related to stock options | | | (4.1 | )% | | (9.6 | )% | | (0.5 | )% |
Other | | | — | % | | (0.3 | )% | | 12.9 | % |
Increase in valuation allowance | | | (38.4 | )% | | (35.2 | )% | | (52.1 | )% |
| | | | | | | | |
Effective tax rate | | | — | % | | — | % | | — | % |
| | | | | | | | |
102
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Income Taxes (Continued)
On January 1, 2007, the Company adopted the FASB accounting guidance on uncertain tax provisions. The Company has no amounts recorded for any unrecognized tax benefits as of December 31, 2010 and December 31, 2011. In addition, the Company did not record any amount for the implementation of this guidance. The Company's policy is to record estimated interest and penalties related to the underpayment of income taxes as a component of its income tax provision. As of December 31, 2010 and December 31, 2011, the Company had no accrued interest or tax penalties recorded. Each of the Company's income tax return reporting periods since May 9, 2003 (date of inception) are open to income tax audit examination by the federal and state tax authorities.
Utilization of NOL and research and development credit carryforwards may be subject to a substantial annual limitation due to ownership changes that have occurred previously or that could occur in the future as provided by Section 382 of the Internal Revenue Code of 1986, as well as similar state provisions. These ownership changes may limit the amount of NOL and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain stockholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. Since the Company's formation, the Company has raised capital through the issuance of common stock and preferred stock, which, combined with the purchasing stockholders' subsequent disposition of those shares, may have resulted in a change of control, as defined by Section 382, or could result in a change of control in the future upon subsequent disposition. The Company has not currently completed a study to assess whether a change of control has occurred or whether there have been multiple changes of control since the Company's formation due to the significant complexity and cost associated with such study because there could be additional changes in control in the future. If the Company has experienced a change of control at any time since the Company's formation, utilization of NOL or research and development credit carryforwards would be subject to an annual limitation under Section 382. Any limitation may result in expiration of a portion of the NOL or research and development credit carryforwards before utilization. Further, until a study is completed and any limitation known, no amounts are being presented as an uncertain tax position.
The change in the valuation allowance against the deferred tax assets in the three years ended December 31, 2009, 2010 and 2011 was as follows ($ in thousands):
| | | | | | | | | | |
Fiscal Year | | Balance at
Beginning of Period | | Additions | | Balance at
End of Period | |
|---|
2009 | | $ | 50,320 | | $ | 9,528 | | $ | 59,848 | |
2010 | | | 59,848 | | | 6,583 | | | 66,431 | |
2011 | | | 66,431 | | | 1,048 | | | 67,479 | |
12. Stock-Based Compensation
In 2003, the Company's Board of Directors adopted the 2003 Stock Option and Incentive Plan (the "2003 Stock Plan"). The 2003 Stock Plan provides for the granting of incentive and non-qualified stock options, restricted stock and other equity awards to employees, officers, directors, consultants and advisors of the Company. Provisions such as vesting, repurchase and exercise conditions and limitations are determined by the Board of Directors on the grant date. The Company's 2007 Stock Option and Incentive Plan (the "2007 Stock Plan") was adopted by the Company's Board of Directors in April
103
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Stock-Based Compensation (Continued)
2007 and approved by the Company's stockholders in May 2007. The 2007 Stock Plan permits the Company to make grants of incentive stock options, non-qualified stock options, stock appreciation rights, deferred stock awards, restricted stock awards, unrestricted stock awards and dividend equivalent rights. The 2007 Stock Plan provides that the number of shares reserved and available for issuance under the plan will be automatically increased each January 1, beginning in 2008, by 4.5% of the outstanding number of shares of common stock on the immediately preceding December 31 or such lower number of shares of common stock as determined by the Board of Directors. This number is subject to adjustment in the event of a stock split, stock dividend or other change in the Company's capitalization. Generally, shares that are forfeited or canceled from awards under the 2007 Stock Plan also will be available for future awards. In addition, available shares under the Company's 2003 Stock Plan, including as a result of the forfeiture, expiration, cancellation, termination or net issuances of awards, are automatically made available for issuance under the 2007 Stock Plan. The total maximum number of shares of common stock that may be issued pursuant to the 2007 Stock Plan after December 31, 2011 is 9,871,770. As of December 31, 2011, 9,272,469 shares of common stock are available for issuance under the 2007 Stock Plan.
Stock options
During the years ended December 31, 2009, 2010, 2011 and the period from May 9, 2003 (date of inception) to December 31, 2011, the Company granted 2,633,807, 10,750, 0 and 6,120,821 stock options, respectively to certain employees and directors. The vesting of these awards is time-based and the restrictions typically lapse 25% after one year and monthly thereafter for the next 36 months.
During the years ended December 31, 2009, 2010, 2011 the Company did not grant any stock options to nonemployees. During the period from May 9, 2003 (date of inception) to December 31, 2011, the Company granted 97,530 stock options, respectively to certain nonemployees in exchange for certain consulting services. Vesting on these awards is time-based and the vesting periods range from immediate vesting on the grant date to a four-year period.
The exercise price of each stock option shall be specified by the Board of Directors at the time of grant. The vesting period for each stock option is specified by the Board of Directors at the time of grant and is generally over a four-year period. The stock options expire ten years after the grant date.
The fair value of each stock option grant was estimated on the date of grant using the Black-Scholes option-pricing model. The expected life assumption is based on the expected life assumptions of similar entities. Expected volatility is based on a blend of the volatility of Helicos and similar entities in the life sciences industry of comparable market capitalization and financial position that have completed initial public offerings within the last ten years. The risk-free interest rate is the yield currently available on U.S. Treasury zero-coupon issues with a remaining term approximating the expected term used as the input to the Black-Scholes model. FASB accounting guidance requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods as options vest, if actual forfeitures differ from those estimates. During the years ended December 31, 2009, and 2010, because substantially all of the Company's stock option grants vest monthly, stock-based employee compensation expense includes the actual impact of forfeitures. The relevant data used
104
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Stock-Based Compensation (Continued)
to determine the value of the stock option grants is as follows (the Company did not grant options in 2011):
| | | | | | | |
| | December 31, | |
|---|
| | 2009 | | 2010 | |
|---|
Weighted average risk-free interest rate | | | 2.2 | % | | 2.5 | % |
Expected life in years | | | 6.0 | | | 6.0 | |
Expected volatility | | | 63.1 | % | | 92.5 | % |
Expected dividends | | | 0.0 | % | | 0.0 | % |
A summary of stock option activity for the year ended December 31, 2011 is as follows:
| | | | | | | | | | | | | |
| | Number of
Shares | | Weighted average
exercise price | | Weighted average
remaining
contractual term
(in years) | | Aggregate
intrinsic
(in thousands) | |
|---|
Balance at December 31, 2010 | | | 2,120,061 | | $ | 3.45 | | | | | | | |
Granted | | | — | | $ | — | | | | | | | |
Exercised | | | — | | $ | — | | | | | | | |
Forfeited | | | — | | $ | — | | | | | | | |
Expired | | | (1,520,760 | ) | $ | 4.01 | | | | | | | |
| | | | | | | | | | | | | |
Balance at December 31, 2011 | | | 599,301 | | $ | 2.03 | | | 7.0 | | $ | — | |
| | | | | | | | | | | | | |
Exercisable at December 31, 2011 | | | 597,991 | | $ | 2.02 | | | 7.0 | | $ | — | |
| | | | | | | | | | | | | |
Vested and unvested expected to vest at December 31, 2011 | | | 599,173 | | $ | 2.02 | | | 7.0 | | $ | — | |
| | | | | | | | | | | | | |
From May 9, 2003 (date of inception) through December 31, 2011, there were 6,218,298 stock options granted, of which 165,022 were exercised and 5,453,975 were cancelled. The weighted average exercise prices of stock option grants, exercises, and forfeitures from May 9, 2003 (date of inception) through December 31, 2011 was $4.67 per share, $0.66 per share, $5.08 per share, respectively.
The aggregate intrinsic value is calculated based on the positive difference between the fair value of the Company's common stock on December 31, 2011 of $0.04 per share and the exercise price of the underlying options. Because all outstanding option exercise prices exceeded the fair value of the Company's common stock on December 31, 2011, there was no intrinsic value of the Company's options outstanding at December 31, 2011.
The weighted-average grant-date fair value of grants of stock options was $0.89 per share and $0.96 per share for the years ended December 31, 2009 and 2010, respectively. The Company did not grant any stock options in 2011.
The total intrinsic value of stock options exercised was $76,000 and $1,000 for the years ended December 31, 2009 and 2010, respectively. The Company had no stock option exercises in 2011.
105
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Stock-Based Compensation (Continued)
The following table summarizes information about stock options outstanding at December 31, 2011:
| | | | | | | | | | | | | | | | |
| Options outstanding | | Options exercisable | |
|---|
Range of
exercise prices | | Number of
stock options | | Weighted average
remaining life
(years) | | Number of
stock options | | Weighted average
exercise price | | Weighted average
remaining life
(years) | |
|---|
| $ 0.45–$ 0.78 | | | 90,468 | | | 6.7 | | | 90,468 | | $ | 0.74 | | | 6.7 | |
| $ 0.79–$ 0.79 | | | 216,665 | | | 7.6 | | | 216,665 | | $ | 0.79 | | | 7.6 | |
| $ 1.00–$ 1.00 | | | 1,000 | | | 7.9 | | | 520 | | $ | 1.00 | | | 7.9 | |
| $ 1.04–$ 1.04 | | | 190,771 | | | 7.0 | | | 190,771 | | $ | 1.04 | | | 7.0 | |
| $ 1.80–$11.79 | | | 100,397 | | | 5.8 | | | 99,567 | | $ | 7.70 | | | 5.8 | |
| | | | | | | | | | | | | | | |
| | | | 599,301 | | | 7.0 | | | 597,991 | | $ | 2.02 | | | 7.0 | |
| | | | | | | | | | | | | | | |
Restricted stock
During the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) to December 31, 2011, the Company granted 1,697,724, 4,535,608, 0 and 7,441,195 shares of restricted stock, respectively to certain employees. The vesting of these awards is time-based. For restricted stock granted prior to December 31, 2007, the restrictions typically lapse 25% after one year and quarterly thereafter for the next 3 years. For restricted stock granted during 2010, the vesting periods range from immediate vesting to quarterly vesting over six fiscal quarters to a two-year vesting period, with 50% vesting on each anniversary of the grant date. The Company did not grant any restricted stock during 2011.
During the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) to December 31, 2011, the Company granted 95,000, 0, 0 and 730,009 shares of restricted stock, respectively to certain nonemployees in exchange for certain consulting services. Vesting on these awards is time-based and the vesting periods range from immediate vesting on the grant date to a four-year period. The Company recorded a stock-based compensation charge on these awards following the FASB accounting guidance for accounting for stock appreciation rights and other variable stock option or award plans. In fiscal year 2010, the Company awarded 48,356 shares to directors in lieu of cash for board fees.
For employee and nonemployee restricted stock awards granted before January 1, 2007, the employee or nonemployee paid the Company cash in an amount up to the fair market value of the award. If the employee ceases employment with the Company, or if the nonemployee terminates the service arrangement, the employee or nonemployee is automatically entitled to be refunded the cash paid for any unvested awards. At the time the cash is received, the Company records the cash as subscription payable in the consolidated balance sheet, and the amount is reclassified to additional paid-in capital over the vesting period. At December 31, 2010 and 2011, the Company had no subscription payable.
106
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Stock-Based Compensation (Continued)
A summary of restricted stock activity during the year ended December 31, 2011 is as follows:
| | | | | | | | | | | | | |
| | Number of
shares | | Weighted average
grant date
fair value | | Weighted average
remaining
contractual term
(in years) | | Aggregate
intrinsic
value
(in thousands) | |
|---|
Balance of unvested restricted stock at
December 31, 2010 | | | 2,816,588 | | $ | 0.45 | | | | | | | |
Granted | | | — | | $ | — | | | | | | | |
Vested | | | (1,807,652 | ) | $ | 0.44 | | | | | | | |
Forfeited | | | (596,436 | ) | $ | 0.50 | | | | | | | |
| | | | | | | | | | | | | |
Balance of unvested restricted stock at December 31, 2011 | | | 412,500 | | $ | 0.42 | | | 2.5 | | $ | 17 | |
| | | | | | | | | | | | | |
From May 9, 2003 (date of inception) through December 31, 2011, there were 8,171,204 shares of restricted stock granted, at a weighted average grant date fair value of $0.96, of which 5,690,979 shares had fully vested at December 31, 2011, with a weighted average grant date fair value of $1.08.
The aggregate intrinsic value is calculated based on the positive difference between the fair value of the Company's common stock on December 31, 2011 of $0.04 per share and the estimated fair value of the Company's common stock at the date of grant. The total intrinsic value of restricted stock vested was $2.1 million, $1.3 million and $0.2 million for the years ended December 31, 2009, 2010 and 2011, respectively.
In March 2005, in connection with the AZTE License Agreement, the Company issued 88,888 shares of restricted common stock, which vested in two equal installments upon the achievement of separate milestones. In May 2006, due to the successful issuance of a U.S. patent, 44,444 shares of the restricted common stock vested. The vesting of 44,444 shares of restricted common stock resulted in a charge to research and development expense of $127,000 based on the fair value of the Company's common stock at the time the milestone was achieved. The remaining 44,444 shares of restricted common stock vested immediately upon the successful issuance of a second U.S. patent on January 12, 2010 and resulted in a charge to research and development expense of $54,000 based on the fair value of the Company's common stock at the time the milestone was achieved.
The Company recorded stock-based compensation expense to the extent that the fair value of the Company's common stock at the date of the grant exceeded the exercise price of the equity awards.
The Company recognized stock-based compensation expense on all employee and nonemployee awards as follows (in thousands):
| | | | | | | | | | | | | |
| |
| |
| |
| | Period from
May 9, 2003
(date of inception)
through
December 31, 2011 | |
|---|
| | Year Ended December 31, | |
|---|
| | 2009 | | 2010 | | 2011 | |
|---|
Selling, general and administrative | | $ | 3,660 | | $ | 3,796 | | $ | 612 | | $ | 15,039 | |
Research and development | | | 1,621 | | | 1,308 | | | 235 | | | 5,622 | |
| | | | | | | | | | |
Total | | $ | 5,281 | | $ | 5,104 | | $ | 847 | | $ | 20,661 | |
| | | | | | | | | | |
107
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
12. Stock-Based Compensation (Continued)
Total unrecognized stock-based compensation expense for all stock-based awards was approximately $147,000 at December 31, 2011, of which $60,000 will be recognized in 2012, $58,000 in 2013 and $29,000 thereafter. This results in these amounts being recognized over a weighted-average period of 1.0 years.
13. Fair Value Measurement
The Company considers all highly liquid investments with original maturities of generally three months or less at the time of acquisition to be cash equivalents. Cash equivalents are stated at cost, which approximates fair market value. The Company classifies marketable securities as available-for-sale. These securities are carried at fair market value with unrealized gains and, to the extent deemed temporary, unrealized losses, reported as a component of other comprehensive gain or loss in stockholders' equity/(deficit). Realized gains or losses on securities sold are based on the specific identification method.
Marketable securities are considered to be impaired when a decline in fair value below cost basis is determined to be other-than-temporary. The Company evaluates whether a decline in fair value below cost basis is other-than-temporary using available evidence regarding its investments. In the event that the cost basis of a security significantly exceeds its fair value, the Company evaluates, among other factors, the duration of the period that, and extent to which, the fair value is less than cost basis, the financial health of and business outlook for the issuer, including industry and sector performance, and operational and financing cash flow factors, overall market conditions and trends, the Company's intent to sell the investment and if it is more likely than not that the Company would be required to sell the investment before its anticipated recovery. Once a decline in fair value is determined to be other-than-temporary, a write-down is recorded in the consolidated statements of operations and a new cost basis in the security is established.
The Company's assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2010 and December 31, 2011 are measured in accordance with FASB standards. Fair values determined by Level 1 inputs utilize observable data such as quoted prices in active markets. Fair values determined by Level 2 inputs utilize data points other than quoted prices in active markets that are observable either directly or indirectly. Fair values determined by Level 3 inputs utilize unobservable data points in which there is little or no market data, which require the reporting entity to develop its own assumptions.
The following table represents the Company's assets and liabilities measured at fair value on a recurring basis at December 31, 2010:
| | | | | | | | | | | | | |
($ in thousands)
| | Quoted Prices
in Active
Markets
(Level 1) | | Other
Observable
Inputs
(Level 2) | | Unobservable
Inputs
(Level 3) | | Total | |
|---|
Money market funds | | $ | — | | $ | 53 | | $ | — | | $ | 53 | |
Warrant liability | | | — | | | — | | | 220 | | | 220 | |
108
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
13. Fair Value Measurement (Continued)
The following table represents the Company's assets and liabilities measured at fair value on a recurring basis at December 31, 2011:
| | | | | | | | | | | | | |
($ in thousands)
| | Quoted Prices
in Active
Markets
(Level 1) | | Other
Observable
Inputs
(Level 2) | | Unobservable
Inputs
(Level 3) | | Total | |
|---|
Money market funds | | $ | — | | $ | — | | $ | — | | $ | — | |
Warrant liability | | | — | | | — | | | 25 | | | 25 | |
The Company's warrant liability was valued at December 31, 2010 and 2011 using a Black-Scholes model and is therefore classified as Level 3. The valuation of the warrant liability is discussed further in Note 10.
The following tables roll forward the fair value of the warrant liability, whose fair value is determined by Level 3:
| | | | |
Balance at December 31, 2009 | | $ | 5,253 | |
Warrant exercises in 2010 | | | (915 | ) |
Change in fair value in 2010 | | | (4,118 | ) |
| | | | |
Balance at December 31, 2010 | | | 220 | |
Warrant exercises in 2011 | | | (192 | ) |
Change in fair value in 2011 | | | (3 | ) |
| | | | |
Balance at December 31, 2011 | | $ | 25 | |
| | | | |
The carrying amount of the Company's debt approximates its fair value at December 31, 2010 and December 31, 2011.
14. Net Loss per Share
Basic net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding for the period. The Company's potential dilutive shares, which include outstanding common stock options, unvested restricted stock, common stock warrants have not been included in the computation of diluted net loss per share for all periods as the result would be antidilutive. Such potentially dilutive shares are excluded when the effect would be to reduce net loss per share. Because the Company reported a net loss for the years ended December 31, 2009, 2010 and 2011, all potential common shares have been excluded from the computation of the dilutive net loss per share for all periods presented because the effect would have been antidilutive. Such potential common shares consist of the following:
| | | | | | | | | | |
| | December 31, | |
|---|
| | 2009 | | 2010 | | 2011 | |
|---|
Stock options | | | 4,761,135 | | | 2,120,061 | | | 599,301 | |
Unvested restricted stock | | | 1,495,533 | | | 2,816,588 | | | 412,500 | |
Warrants | | | 28,454,589 | | | 23,917,179 | | | 22,653,964 | |
| | | | | | | | |
| | | 34,711,257 | | | 28,853,828 | | | 23,665,765 | |
| | | | | | | | |
109
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
15. Related Party Transactions
As discussed above in Note 8 under the caption "Bridge Financing and Senior Debt Restructuring," in November 2010, the Company entered into a note purchase agreement (the "Note Purchase Agreement") with investment funds affiliated with Atlas Venture and Flagship Ventures, whose representatives are members of the Company's Board of Directors (the "Purchasers"), pursuant to which the Company agreed to sell to the Purchasers, and the Purchasers have agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), a portion of which is committed and a portion of which is optional (the "Bridge Debt Financing"). On November 16, 2010, the Purchasers purchased Notes having an aggregate principal amount of $333,333. Subject to the Company's continued compliance with the terms of the Note Purchase Agreement, including that there not be an event of default thereunder, upon notice from the Company, the Purchasers are committed to purchase an additional $333,333 of Notes in each of five subsequent closings, for a total aggregate committed amount of $2,000,000. The Purchasers also have the right, but not the obligation, upon the Company's request to purchase up to an additional $2,000,000 of Notes on or before December 31, 2012. As of December 31, 2011 the Company has borrowed $2,000,000 against the committed portion of the Bridge Debt Financing facility. In addition, as of the date of this filing the Company has borrowed an additional $375,000 under the uncommitted portion of the Bridge Debt Financing facility on a short term basis with a maturity date of May��15, 2012. The Bridge Debt Financing was approved by an independent committee of our Board of Directors.
16. 401(k) Plan
The Company has a 401(k) income deferral plan (the "Plan") for employees. According to the terms of the Plan, the Company may make discretionary matching contributions to the Plan. The Company made no discretionary contributions during the years ended December 31, 2009, 2010 and 2011.
110
Table of Contents
Helicos BioSciences Corporation (a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
17. Unaudited Quarterly Results
The Company's unaudited quarterly results are summarized below (in thousands, except share and per share data):
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended | |
|---|
| | March 31,
2010 | | June 30,
2010 | | September 30,
2010 | | December 31,
2010 | | March 31,
2011 | | June 30,
2011 | | September 30,
2011 | | December 31,
2011 | |
|---|
| | (unaudited)
| |
|---|
Product revenue | | $ | 42 | | $ | 85 | | $ | 214 | | $ | 960 | | $ | 229 | | $ | 109 | | $ | 116 | | $ | 117 | |
Service revenue | | | — | | | — | | | — | | | — | | | 371 | | | 273 | | | 385 | | | 153 | |
Grant revenue | | | 527 | | | 576 | | | 422 | | | 1,571 | | | 582 | | | 427 | | | 224 | | | 244 | |
| | | | | | | | | | | | | | | | | | |
Total revenue | | | 569 | | | 661 | | | 636 | | | 2,531 | | | 1,182 | | | 809 | | | 725 | | | 514 | |
| | | | | | | | | | | | | | | | | | |
Costs and expenses | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of product revenue | | | 72 | | | 104 | | | 94 | | | 443 | | | 98 | | | 33 | | | 70 | | | 23 | |
Research and development | | | 4,046 | | | 4,216 | | | 4,945 | | | 840 | | | 1,153 | | | 982 | | | 490 | | | 2,305 | |
General and administrative | | | 4,072 | | | 2,693 | | | 2,347 | | | 1,394 | | | 982 | | | 1,410 | | | 785 | | | 947 | |
Impairment of long-lived assets | | | — | | | — | | | 1,894 | | | — | | | — | | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | |
Total costs and expenses | | | 8,190 | | | 7,013 | | | 9,280 | | | 2,677 | | | 2,233 | | | 2,425 | | | 1,345 | | | 3,275 | |
| | | | | | | | | | | | | | | | | | |
Operating loss | | | (7,621 | ) | | (6,352 | ) | | (8,644 | ) | | (146 | ) | | (1,051 | ) | | (1,616 | ) | | (620 | ) | | (2,761 | ) |
Interest income | | | 12 | | | 4 | | | 1 | | | 2 | | | — | | | — | | | — | | | — | |
Interest expense | | | (183 | ) | | (162 | ) | | (106 | ) | | (163 | ) | | (197 | ) | | (196 | ) | | (151 | ) | | (136 | ) |
Change in fair value of warrant liability | | | 1,452 | | | 1,796 | | | (130 | ) | | 1,000 | | | (56 | ) | | 41 | | | (27 | ) | | 45 | �� |
Other income | | | — | | | — | | | — | | | 472 | | | 900 | | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | |
Net loss | | | (6,340 | ) | | (4,714 | ) | | (8,879 | ) | | 1,165 | | | (404 | ) | | (1,771 | ) | | (798 | ) | | (2,852 | ) |
| | | | | | | | | | | | | | | | | | |
Net loss attributable to common stockholders | | $ | (6,340 | ) | $ | (4,714 | ) | $ | (8,879 | ) | $ | 1,165 | | $ | (404 | ) | $ | (1,771 | ) | $ | (798 | ) | $ | (2,852 | ) |
| | | | | | | | | | | | | | | | | | |
Net loss attributable to common stockholders per share—basic and diluted | | $ | (0.08 | ) | $ | (0.06 | ) | $ | (0.11 | ) | $ | 0.01 | | $ | (0.00 | ) | $ | (0.02 | ) | $ | (0.01 | ) | $ | (0.03 | ) |
| | | | | | | | | | | | | | | | | | |
Weighted average number of common shares used in computation—basic and diluted | | | 79,259,874 | | | 79,859,226 | | | 79,459,909 | | | 85,597,281 | | | 84,915,414 | | | 85,350,069 | | | 85,418,524 | | | 86,786,842 | |
| | | | | | | | | | | | | | | | | | |
111
Table of Contents
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer (PEO) and principal financial officer (PFO), has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended (the "Exchange Act")), as of December 31, 2011. In designing and evaluating our disclosure controls and procedures, we and our management recognize that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and our management necessarily was required to apply its judgment in evaluating and implementing possible controls and procedures. Based on that evaluation, our PEO and PFO have concluded that our disclosure controls and procedures were effective at the reasonable level of assurance.
Management's Report on Internal Control over Financial Reporting
The Company's management is responsible for establishing and maintaining adequate internal control over financial reporting for the Company as defined in Rule 13a-15(f) under the Exchange Act. The Company's management assessed the effectiveness of the Company's internal control over financial reporting as of December 31, 2011. In making this assessment, the Company's management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO") inInternal Control—Integrated Framework. Based on this assessment, the Company's management concluded that, as of December 31, 2011, the Company's internal control over financial reporting is effective based on those criteria.
This annual report does not include an attestation report of the Company's independent registered public accounting firm regarding the Company's internal control over financial reporting. We were not required to have, nor have we engaged, our independent registered public accounting firm to perform an audit of internal control over financial reporting pursuant to the rules of the SEC that permit us to provide only management's report in this annual report.
Changes in Internal Control over Financial Reporting
No change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the quarter ended December 31, 2011 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
112
Table of Contents
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors
Our Board of Directors consists of seven directors and is divided into three classes with members of each class initially serving for three-year terms. Each of Dr. Afeyan and Mr. Lowy serve as Class III directors, with a term of office that had been scheduled to expire at the 2011 Annual Meeting of Stockholders. In order to conserve our limited resources, we did not hold an Annual Meeting of Stockholders in 2011 and, as a result, Dr. Afeyan and Mr. Lowy continue to serve as Class III directors. Although we have no present intention to do so, if we hold an Annual Meeting of Stockholders in 2012, the terms of Dr. Afeyan and Mr. Lowy will expire at that meeting and, subsequently, the terms of our Class I and Class II directors will expire in 2013 and 2014, respectively.
On February 25, 2011, Elisabeth K. Allison, Ph.D. and Robert F. Higgins each resigned from our Board of Directors. On April 28,2011, Theo Melas-Kyriazi resigned from our Board of Directors. On June 26,2011, Stanley Lapidus resigned from our Board of Directors. On September 9, 2011, Brian Atwood resigned from our Board of Directors.
On January 4, 2012, the Company fixed the size of its Board of Directors at five (5) members and appointed Bruce C. Ginsberg as a director of the Company. Mr. Ginsberg has been appointed to a committee of the Board of Directors formed to review certain financing matters. Mr. Ginsberg will receive an annual cash retainer in accordance with the Company's current non-employee director compensation policy.
The names of the directors, and certain information about them as of March 1, 2012, are set forth below.
| | | | | |
Name | | Age | | Position(s) |
|---|
Ivan Trifunovich, Ph.D. | | | 48 | | Chairman and Class I Director, President and Chief Executive Officer |
Peter Barrett, Ph.D. | | | 59 | | Class II Director |
Noubar B. Afeyan, Ph.D. | | | 49 | | Lead Independent Director, Class III Director |
Ronald A. Lowy | | | 56 | | Class III Director |
Bruce C. Ginsberg | | | 53 | | Class I Director |
Ivan Trifunovich, Ph.D. On September 15, 2010, the Board elected Ivan Trifunovich to serve as a member of the Company's Board and simultaneously appointed him Executive Chairman of the Board. Effective as of October 14, 2010, the Company appointed Dr. Ivan Trifunovich as its President and Chief Executive Officer. From August 17, 2010 until such appointment, Dr. Trifunovich has provided executive level consulting services to the Company at the direction of the Board in various areas, including business development, financing initiatives, strategic direction, strategic transactions and operational matters. Since August 2008, Dr. Trifunovich has served as a strategic consultant to global companies in the life sciences industry. Previously, Dr. Trifunovich served as the Senior Vice President of Third Wave Technologies, Inc., a molecular diagnostics company, from December 2001 through August 2008. Prior to joining Third Wave Technologies, Inc., Dr. Trifunovich held successive positions as Vice President of e-Business and Vice President of Research Strategy and Operations at Pharmacia Corp. Prior to joining Pharmacia, Dr. Trifunovich was a Director of New Product Marketing at Johnson & Johnson, Inc. He began his career at Bristol-Meyers Squibb, Inc. as a bench scientist, where he held several positions of increasing responsibility. Dr. Trifunovich received his Ph.D. in organic chemistry at UCLA and an M.B.A. at the University of Pennsylvania's Wharton School of Business. We believe Dr. Trifunovich's qualifications to serve on the Board of Directors include his extensive executive knowledge and experience in scientific and business management in the life sciences and
113
Table of Contents
molecular diagnostics industries. Effective September 1, 2011 Dr. Trifunovich continued to serve in his present capacity but on a reduced part time schedule. As previously announced, Dr. Trifunovich has decided to accept the additional position of President and CEO of WaferGen Biosystems, Inc. Dr. Trifunovich will continue, to serve as Chief Executive Officer and President of the Company on a part-time basis.
Noubar B. Afeyan, Ph.D. Dr. Afeyan, one of our co-founders, has served as Lead Independent Director of our Board since October 2007. Previously, he served as Chairman of our Board of Directors from 2003 to October 2007. In 1999, Dr. Afeyan founded Flagship Ventures, a venture capital firm, where he serves as Managing Partner and Chief Executive Officer. Dr. Afeyan is also a Senior Lecturer at the Massachusetts Institute of Technology's Sloan School of Management as well as the Biological Engineering Division. Dr. Afeyan serves on the Board of Directors of BG Medicine, a life sciences company focused on the discovery, development, and commercialization of novel diagnostic tests based on biomarkers, since 2000. Dr. Afeyan served on the Board of Directors of Color Kinetics, a leading LED-lighting company, until its recent acquisition by Philips in August 2007. Dr. Afeyan also served on the Board of Directors of EXACT Science Corporation and Antigenics, Inc. Dr. Afeyan received a BS in chemical engineering from McGill University and a Ph.D. in biochemical engineering from the Massachusetts Institute of Technology. We believe Dr. Afeyan's qualifications to serve on the Board of Directors include his substantial leadership experience and success in life sciences and technology entrepreneurship and venture capitalism.
Peter Barrett, Ph.D. Dr. Barrett has served as a member of our Board of Directors since 2003. Dr. Barrett has served as a Partner of Atlas Venture, a venture capital firm, since January 2003. From August 1998 to December 2001, he served as Executive Vice President and Chief Business Officer of Celera Genomics, a biopharmaceutical company, which he co-founded. Dr. Barrett serves on the board of Atlas Venture investments and several private companies. Dr. Barrett has also served on the Board of Directors of Alnylam Pharmaceuticals, Inc., Momenta Pharmaceuticals, Inc. and Akela Pharma, Inc. He is also the President of the Autism Consortium Board of Directors and is Vice Chairman of the Advisory Council of the Barnett Institute of Chemical and Biological Analysis at Northeastern University. Dr. Barrett received his BS in chemistry from Lowell Technological Institute (now known as the University of Massachusetts, Lowell) and his Ph.D. in Analytical Chemistry from Northeastern University. He also completed Harvard Business School's Management Development Program. We believe Dr. Barrett's qualifications to serve on the Board of Directors include his considerable experience in management and business development in the life sciences industry.
Ronald A. Lowy. Mr. Lowy has been a member of our Board of Directors since October 2007. He served as our Chief Executive Officer from December 2008 to October 2010 and as our President from February 2010 to October 2010. Mr. Lowy served as president and chief executive officer of Thermo/Fisher Biosciences, a division of Fisher Scientific, from 2004 to 2007. Before joining Fisher Biosciences, Mr. Lowy was president of Global Connectivity Solutions for ADC Telecommunications from April 2004 to October 2004 and as president and chief operating officer at KRONE Group from 2000 to 2004. Prior to KRONE Group, Mr. Lowy was vice president and general manager of the Automotive and Industrial Products Group of GenTek. Mr. Lowy received a BS in mechanical engineering from the University of New Hampshire and an MBA from the University of Wisconsin. We believe Mr. Lowy's qualifications to serve on the Board of Directors include his substantial business operations and strategic planning skills and experience.
Bruce C. Ginsberg. Mr. Ginsberg has been a member of our Board of Directors since January 4, 2012. Mr. Ginsberg has been appointed to a committee of the Board of Directors formed to review certain financing matters. Mr. Ginsberg is currently the President and Chief Executive Officer of MooBella, Inc., a food service technology company, and is a member of the Board of Directors of Mac-Gray Corporation (NYSE: TUC) where he serves on the Audit Committee.
114
Table of Contents
Executive Officers
Our executive officers, and their ages and positions are as follows:
| | | | | |
Name | | Age | | Position(s) |
|---|
Ivan Trifunovich, Ph.D. | | | 48 | | Chairman, President and Chief Executive Officer |
Jeffrey R. Moore | | | 51 | | Senior Vice President and Chief Financial Officer |
Ivan Trifunovich, Ph.D. See biography above in "Directors."
Jeffrey R. Moore. Mr. Moore joined us in August 2009 and serves as our Senior Vice President and Chief Financial Officer. Prior to joining us, Mr. Moore served as managing member and founder of Fiske Pond Advisors LLC, a financial advisory services firm, since 2008. Mr. Moore served as Chief Financial Officer of Affinova, Inc., a privately-held innovation technology company, from 2004 to 2008 and Vice President of Finance at Flagship Ventures from 2000 to 2006. Prior to that, Mr. Moore was with Celera Genomics and PerSpective Biosystems, where he served as the Director of Business Analysis and Planning and Vice President of Treasury Operations, respectively. As of the date of this filing Mr. Moore continues to serve in his present role at the Company in a reduced part time schedule.
There are no family relationships among our directors or executive officers.
None of the Company's directors or executive officers has been involved in any legal proceedings during the past ten years of the type described in paragraph (f) of Item 401 of Regulation S-K that are material to an evaluation of their ability or integrity.
Corporate Governance
Effective as of the date of filing of this Annual Report on Form 10-K, the Board has determined that it is in our best interest to streamline our corporate governance practices and assist our ongoing efforts to conserve our limited resources by eliminating our Nominating and Corporate Governance Committee and Compensation Committee and reconstituting the membership of our Audit Committee. As a result, effective as of such filing date, the Board has one standing Committee, the Audit Committee, which is comprised of all non-employee Board members. In addition, our entire Board has assumed responsibility for the roles and duties previously performed by our Nominating and Corporate Governance Committee and our Compensation Committee. The Audit Committee has a written charter approved by the Board which is available on our website (www.helicosbio.com). Following the resignations of Dr. Allison and Mr. Higgins in February 2011, the Company did not reconstitute the membership of its Compensation Committee or its Nominating and Corporate Governance Committee in order to comply with the requirements of each committee's respective charters.
Audit Committee. Effective as of the date of filing of this Annual Report on Form 10-K, all non-employee Board members will serve on our Audit Committee. The Audit Committee held five meetings in 2011. The Audit Committee's responsibilities include, but are not limited to:
- •
- appointing, approving the compensation of, and assessing the independence of our independent registered public accounting firm;
- •
- pre-approving auditing and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm;
- •
- reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures;
- •
- coordinating the oversight and reviewing the adequacy of our internal control over financial reporting;
115
Table of Contents
- •
- establishing policies and procedures for the receipt and retention of accounting related complaints and concerns; and
- •
- preparing the audit committee report required by SEC rules to be included in our annual report.
Our board of directors has determined that Mr. Ronald A. Lowy, who meets the independence requirement of the SEC rules, qualifies as an "audit committee financial expert" as defined under the Securities Exchange Act of 1934.
Compensation Committee Responsibilities Assumed by the Entire Board. The responsibilities of our previously constituted Compensation Committee which now have been assumed by our entire Board include, but are not limited to:
- •
- annually reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer;
- •
- evaluating the performance of our chief executive officer in light of such corporate goals and objectives and reviewing and approving the compensation of our chief executive officer;
- •
- reviewing and approving the compensation of our other executive officers;
- •
- overseeing and administering our compensation, welfare, benefit and pension plans and similar plans; and
- •
- reviewing and making recommendations to the board with respect to director compensation.
During the entire fiscal year 2010 and prior to the February 25, 2011 resignations of Dr. Allison and Mr. Higgins from our Board of Directors, the Compensation Committee was comprised of three members: Mr. Higgins (chairman), Dr. Allison and Dr. Barrett. The Company did not hold Compensation Committee meetings in 2011.
Nominating and Corporate Governance Committee Responsibilities Assumed by the Entire Board. The responsibilities of our previously constituted Nominating and Corporate Governance Committee which now have been assumed by our entire Board include, but are not limited to:
- •
- developing and recommending to the board criteria for board and committee membership;
- •
- establishing procedures for identifying and evaluating director candidates including nominees recommended by stockholders;
- •
- identifying individuals qualified to become board members;
- •
- recommending to the board the persons to be nominated for election as directors and to each of the board's committees;
- •
- developing and recommending to the board a code of business conduct and ethics and a set of corporate governance guidelines; and
- •
- overseeing the evaluation of the board and management.
During the entire fiscal year 2010 and prior to the February 25, 2011 resignation of Mr. Higgins from our Board of Directors, the Nominating and Corporate Governance Committee was comprised of two members: Dr. Afeyan (chairman) and Mr. Higgins. The Company did not hold Nominating and Corporate Governance Committee meetings in 2011.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires our executive officers and directors and persons who beneficially own more than 10% of our common stock to file with the SEC initial reports of beneficial ownership and reports of changes in beneficial ownership of common stock.
116
Table of Contents
Executive officers, directors and 10% stockholders are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file. To the best of our knowledge, based on a review of (i) Forms 3 and 4 and amendments thereto during the year ended December 31, 2011 and (ii) Forms 5 and amendments thereto furnished with respect to the year ended December 31, 2011, each director, executive officer, and 10% stockholder complied with all Section 16(a) filing requirements.
Code of Ethics
Certain documents relating to our corporate governance, including our Code of Business Conduct and Ethics, which is applicable to our directors, officers and employees, and the charter of the Audit Committee of our Board of Directors, are available on our website athttp://www.helicosbio.com. In addition, as noted above, the responsibilities of our previously constituted Compensation Committee and Nominating and Corporate Governance Committee have been assumed by the entire Board of Directors. For reference, the Compensation and Nominating and Corporate Governance roles and responsibilities are described in the charters of these former committees which remain available on our website athttp://www.helicosbio.com. We intend to disclose substantive amendments to or waivers (including implicit waivers) of any provision of the Code of Business Conduct and Ethics that apply to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, by posting such information on our website available athttp://www.helicosbio.com.
117
Table of Contents
REPORT OF THE AUDIT COMMITTEE
The following Report of the Audit Committee does not constitute soliciting material and is not deemed to be filed with the Securities and Exchange Commission, or the SEC, and is not to be incorporated by reference in any filing of the Company under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this annual report and irrespective of any general incorporation language in such filing.
The Audit Committee selects the Company's independent registered public accounting firm to audit the Company's financial statements and to perform services related to the audit, reviews the scope and results of the audit with the independent registered public accounting firm, reviews with management and the independent registered public accounting firm the Company's quarterly and annual results, reviews the periodic disclosures related to the Company's financial statements, considers the adequacy of the Company's internal accounting procedures, and compliance with the Sarbanes-Oxley Act of 2002.
With respect to the fiscal year ended December 31, 2011, the Audit Committee:
- •
- Reviewed and discussed the audited financial statements with the Company's management;
- •
- Discussed with PricewaterhouseCoopers LLP, the Company's independent registered public accounting firm, the matters required to be discussed by the Statement on Auditing Standards No. 61, as amended (Communications with Audit Committees), and SEC Rule 2-07 of Regulation S-X; and
- •
- Received the written disclosures and the letter from PricewaterhouseCoopers LLP required by applicable requirements of the Public Company Accounting Oversight Board, and has discussed with PricewaterhouseCoopers LLP its independence.
Based on these reviews and discussions, our Audit Committee has recommended to our Board of Directors that the audited financial statements be included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2011 for filing with the SEC.
Respectfully submitted by the members of the Audit Committee of the Board of Directors:
Ronald A. Lowy,Chairman
Noubar B. Afeyan
Peter Barrett
118
Table of Contents
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation
The following table sets forth certain information with respect to compensation for the year ended December 31, 2011 earned by or paid to our principal executive officers which are referred to as the named executive officers.
SUMMARY COMPENSATION TABLE
| | | | | | | | | | | | | | | | | | | |
Name and principal position | | Year | | Salary | | Stock
awards(1) | | Option
awards(1) | | All other
compensation(2) | | Total | |
|---|
Ivan Trifunovich | | | 2011 | | $ | 281,538 | (3) | $ | — | | $ | — | | $ | — | | $ | 281,538 | |
Chairman, President, and | | | 2010 | | $ | 76,154 | (3) | $ | 611,680 | | $ | — | | $ | 85,500 | (4) | $ | 773,334 | |
Chief Executive Officer | | | | | | | | | | | | | | | | | | | |
Jeffrey R. Moore | | |
2011 | |
$ |
270,000 | |
$ |
— | |
$ |
— | |
$ |
— | |
$ |
270,000 | |
Senior Vice President and | | | 2010 | | $ | 270,000 | | $ | — | | $ | — | | $ | — | | $ | 270,000 | |
Chief Financial Officer | | | | | | | | | | | | | | | | | | | |
- (1)
- Based on the dollar amount recognized for financial statement reporting purposes in accordance with FAS 123(R), the assumptions we used for calculating the grant date fair values are set forth in Footnote 12 to the Consolidated Financial Statements presented in our 2010 Form 10-K, for 2010, and in Footnote 12 to the Consolidated Financial Statements presented in this 2011 Form 10-K, for 2011.
- (2)
- Excludes medical, disability and certain other benefits received by the named executive officers that are available generally to all of our employees and certain perquisites and other personal benefits received by the named executive officers which do not exceed $10,000 in the aggregate.
- (3)
- Dr. Trifunovich was appointed as our President and Chief Executive Officer in October 2010 at a salary of $30,000 per month. Effective September 1, 2011, Dr. Trifunovich's salary was reduced to $10,000 per month. Effective March 9, 2012, Dr. Trifunovich's salary was reduced to $5,000 per month.
- (4)
- Represents consulting fees and fees paid to Dr. Trifunovich as Chairman of the Board prior to his appointment as our President and Chief Executive Officer.
Discussion of Executive Compensation
A summary of certain material terms of our compensation plans and arrangements is set forth below. For 2011, the Compensation Committee, prior to its responsibilities being assumed by the entire Board decided not to increase the salaries of any of our executive officers and during 2011 the Board reduced the salary and time commitment of the CEO as a result of our need to conserve cash and reduce expenses.
Effective as of October 14, 2010, we appointed Dr. Ivan Trifunovich as our new President and Chief Executive Officer. Under the terms of his employment arrangement with us, Dr. Trifunovich will receive: (i) a salary of $30,000 per month (reduced to $10,000 per month effective September 1, 2011, and again reduced to $5,000 per month effective March 9, 2012), subject to a potential annual increase by the then existing Compensation Committee of our Board of Directors; (ii) a bonus opportunity of up to 30% of Dr. Trifunovich's annual base salary, subject to the discretion of the then existing Compensation Committee of our Board of Directors; and (iii) an equity award in the form of restricted stock equal to 5% of our fully diluted capital stock (the "Award"). In 2010 we awarded Dr. Trifunovich 25% of the Award, or 1.25% of our fully diluted capital stock. No additional stock grants have been
119
Table of Contents
made to Dr. Trifunovich subsequent to the initial 2010 stock grant. During 2011 no cash bonuses were awarded to Dr. Trifunovich.
The Award will be subject to a time-based vesting schedule with 25% of the Award vesting on the first anniversary of the Award date (the "First Anniversary") and 6.25% of the remaining Award vesting each subsequent quarter following the First Anniversary, subject to Dr. Trifunovich's continued employment with us on each vesting date. The Award is subject to anti-dilution protection which will be triggered in the event that we effect an equity or equity-linked financing, whether strategic or otherwise, beginning with and including our next equity or equity-linked financing and continuing during the twenty-four (24) month period after such next financing until we have raised an aggregate of twenty-five million dollars ($25,000,000) in net proceeds in one or more such financings, subject to Dr. Trifunovich's continued employment with us at the consummation of such financing. If Dr. Trifunovich is terminated without cause or he resigns for good reason, 50% of the then-unvested shares of the Award will accelerate and become non-forfeitable. The Award will fully vest upon the consummation of: (i) a Sale Event (as such term is defined in the 2007 Plan); (ii) the exclusive license of substantially all of our assets; or (iii) a liquidation of our company.
Upon any distribution to our stockholders (whether by dividend, return of capital, liquidation or otherwise) or a Sale Event (as defined in the 2007 Plan), Dr. Trifunovich will receive a cash payment (the "Transaction Bonuses") from us in an amount reflecting the payment he would have received based on his stockholdings if we had actually issued him the entire Award. Dr. Trifunovich will not be entitled to any of the Transaction Bonuses once Dr. Trifunovich actually receives the entire Award. If Dr. Trifunovich is terminated by us without cause or he resigns for good reason before issuance of the entire Award, Dr. Trifunovich will remain eligible for the Transaction Bonuses, but only up to the portion of the Award vested as of his termination (after accounting for any acceleration of the Award triggered by Dr. Trifunovich's conclusion of employment with us), reduced by the amount of his actual distribution based on his current stockholdings.
From August 17, 2010 to his October 14, 2010 appointment as President and Chief Executive Officer, Dr. Trifunovich provided executive level consulting services to us at the direction of the Board in various areas, including business development, financing initiatives, strategic direction, strategic transactions and operational matters. Dr. Trifunovich earned $45,000 for these services. For his service as Executive Chairman of the Board which began on September 15, 2010, Dr. Trifunovich was paid $40,500 until his October 14, 2010 appointment as President and Chief Executive Officer.
We hired Jeffrey R. Moore as our Chief Financial Officer in August 2009 and established his base salary at $270,000 per year. In addition, Mr. Moore was eligible for an annual bonus of up to 30% of his base salary. During 2010 and 2011, no adjustments were made to Mr. Moore's base salary and no cash bonuses were awarded. Mr. Moore is also eligible to participate in our standard benefit programs.
Retention Agreements
On June 21, 2010, the Compensation Committee approved a retention program ("Retention Program") which was designed to retain and motivate the Company's employees, including members of its executive management team. The Retention Program provided for restricted stock awards ("Awards") of the Company's common stock to be granted under the 2007 Plan to all employees including executive officers Jeffrey R. Moore (in the amount of 1,100,000 shares). The Awards were subject to a vesting schedule with 12.5% vesting semi-annually on each of January 1 and July 1 over the next four (4) years. If a management participant in the Retention Program is terminated without cause or resigns for good reason during the twelve months after a change in control of the Company, any unvested shares subject to such management participant's Awards will immediately vest and such management participant will be entitled to the economic benefit of any unissued portion of his Award. The Awards to members of executive management, including Mr. Moore, were contingent and shall be
120
Table of Contents
granted upon (i) there being sufficient shares available under the 2007 Plan, or if there are not, (ii) receipt of shareholder approval of the requisite subsequent increase in shares reserved under the 2007 Plan. As of the date of this filing no shares have been granted to the Retention Program participants.
Management Incentive Plan
In connection with approving a certain financing transaction in November 2010, the Board approved a Management Incentive Plan (the "Management Incentive Plan") pursuant to which participants in the Management Incentive Plan, including Dr. Trifunovich and Mr. Moore, will be entitled to receive, in the aggregate, an amount equal to 7.5% of certain payment consideration resulting from intellectual property licensing events. The specific terms of the Management Incentive Plan, administration and all payments to be made to management thereunder will be under the control and direction of the Board. As of the date of this filing the details of the Management Incentive Plan have not been finalized and is subject to further modification at the discretion of the Board and the Company's senior debt lenders.
Other Compensation
All of our executive officers are eligible for benefits offered to employees generally, including parking or commuting passes, life, health, disability and dental insurance and our 401(k) plan. Our President and Chief Executive Officer also received an allowance for commuting expenses, including a tax gross-up for such amounts paid to him.
2007 Stock Option and Incentive Plan
The 2007 Plan was adopted by our Board of Directors in April 2007 and approved by our stockholders in May 2007. The 2007 Plan permits us to make grants of incentive stock options, non-qualified stock options, stock appreciation rights, deferred stock awards, restricted stock awards, unrestricted stock awards and dividend equivalent rights. We reserved 1,440,266 shares of our common stock for the issuance of awards under the 2007 Plan as of December 31, 2007. The 2007 Plan provides that the number of shares reserved and available for issuance under the plan will be automatically increased each January 1, beginning in 2008, by 4.5% of the outstanding number of shares of common stock on the immediately preceding December 31 or such lower number of shares of common stock as determined by the Board of Directors. In January 2008, pursuant to this provision, the number of shares of our common stock reserved for the issuance of awards under the 2007 Plan was increased by 944,263, or 4.5% of the outstanding number of shares of common stock outstanding as of December 31, 2007. In January 2009, pursuant to this provision, the number of shares of our common stock reserved for the issuance of awards under the 2007 Plan was increased by 2,871,372, or 4.5% of the outstanding number of shares of common stock outstanding as of December 31, 2008. In January 2010, pursuant to this provision, the number of shares of our common stock reserved for the issuance of awards under the 2007 Plan was increased by 3,571,677, or 4.5% of the outstanding number of shares of common stock outstanding as of December 31, 2009. In March 2011, pursuant to this provision, the number of shares of our common stock reserved for the issuance of awards under the 2007 Plan was increased by 3,489,512, or approximately 4.0% of the outstanding number of shares of common stock outstanding as of December 31, 2010. This number of reserved shares is subject to adjustment in the event of a stock split, stock dividend or other change in our capitalization. Generally, shares that are forfeited or canceled from awards under the 2007 Plan also will be available for future awards. In addition, available shares under our 2003 Stock Option and Incentive Plan, including as a result of the forfeiture, expiration, cancellation, termination or net issuances of awards, are automatically made available for issuance under the 2007 Plan.
121
Table of Contents
The 2007 Plan may be administered by either a committee of at least two non-employee directors or by our full Board of Directors, in either case acting as the administrator. The administrator has full power and authority to select the participants to whom awards will be granted, to make any combination of awards to participants, to accelerate the exercisability or vesting of any award and to determine the specific terms and conditions of each award, subject to the provisions of the 2007 Plan.
All full-time and part-time officers, employees, non-employee directors and other key persons (including consultants and prospective employees) are eligible to participate in the 2007 Plan, subject to the discretion of the administrator. There are certain limits on the number of awards that may be granted under the 2007 Plan. For example, no more than 1,444,444 shares of common stock may be granted in the form of stock options or stock appreciation rights to any one individual during any one-calendar-year period.
The exercise price of stock options awarded under the 2007 Plan may not be less than the fair market value of our common stock on the date of the option grant and the term of each option may not exceed ten years from the date of grant. The administrator will determine at what time or times each option may be exercised and, subject to the provisions of the 2007 Plan, the period of time, if any, after retirement, death, disability or other termination of employment during which options may be exercised.
To qualify as incentive options, stock options must meet additional federal tax requirements, including a $100,000 limit on the value of shares subject to incentive options which first become exercisable in any one calendar year, and a shorter term and higher minimum exercise price in the case of certain large stockholders.
- •
- Stock appreciation rights may be granted under our 2007 Plan. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the exercise date and the date of grant. The administrator determines the terms of stock appreciation rights, including when such rights become exercisable and whether to pay the increased appreciation in cash or with shares of our common stock, or a combination thereof.
- •
- Restricted stock may be granted under our 2007 Plan. Restricted stock awards are shares of our common stock that vest in accordance with terms and conditions established by the administrator. The administrator will determine the number of shares of restricted stock granted to any employee. The administrator may impose whatever conditions to vesting it determines to be appropriate. For example, the administrator may set restrictions based on the achievement of specific performance goals. Shares of restricted stock that do not vest are subject to our right of repurchase or forfeiture.
- •
- Dividend equivalent rights may be granted under our 2007 Plan. Dividend equivalent rights are awards entitling the grantee to current or deferred payments equal to dividends on a specified number of shares of stock. Dividend equivalent rights may be settled in cash or shares and are subject to other conditions as the administrator shall determine.
- •
- Cash-based awards may be granted under our 2007 Plan. Each cash-based award shall specify a cash-denominated payment amount, formula or payment ranges as determined by the administrator. Payment, if any, with respect to a cash-based award may be made in cash or in shares of stock, as the administrator determines.
Unless the administrator provides otherwise, our 2007 Plan does not allow for the transfer of awards and only the recipient of an award may exercise an award during his or her lifetime.
In the event of a merger, sale or dissolution, or a similar "sale event" in which all awards are not assumed or substituted by the successor entity, all stock options may be terminated upon the effective
122
Table of Contents
time of such sale event following an exercise period, in which case all such stock options shall first become fully exercisable.
No awards may be granted under the 2007 Plan after May 6, 2017. In addition, our Board of Directors may amend or discontinue the 2007 Plan at any time and the administrator may amend or cancel any outstanding award for the purpose of satisfying changes in law or for any other lawful purpose.
No such amendment may adversely affect the rights under any outstanding award without the holder's consent. Other than in the event of a necessary adjustment in connection with a change in the Company's stock or a merger or similar transaction, the administrator may not "reprice" or otherwise reduce the exercise price of outstanding stock options or stock appreciation rights. Further, amendments to the 2007 Plan will be subject to approval by our stockholders if the amendment (i) increases the number of shares available for issuance under the 2007 Plan, (ii) expands the types of awards available under, the eligibility to participate in, or the duration of, the plan, (iii) materially changes the method of determining fair market value for purposes of the 2007 Plan or (iv) is required by the Internal Revenue Code of 1986, as amended, or the Code, to ensure that incentive options are tax-qualified.
Stock option agreements. All stock option awards that are granted to the named executive officers pursuant to the 2007 Plan are covered by a Stock Option Agreement. Generally, under the Stock Option Agreements, 25% of the shares vest on the first anniversary of the grant date and the remaining shares vest monthly over the following three years. Our Board of Directors may accelerate the vesting schedule in its discretion.
Restricted stock award agreements. All restricted stock awards that are granted to the named executive officers pursuant to the 2007 Plan are covered by a Restricted Stock Award Agreement. Generally, under the Restricted Stock Award Agreements, 25% of the shares vest on the first anniversary of the grant date and the remaining shares vest in equal installments on the first day of each fiscal quarter over the following three years. Our Board of Directors may accelerate the vesting schedule in its discretion. The Restricted Stock Award Agreements provide that the named executive officer may not sell, transfer, pledge or otherwise encumber or dispose of any unvested shares. Upon the termination of employment, including upon death, disability, retirement or discharge or resignation for any reason, whether voluntary or involuntary or upon a sale event, any unvested shares of restricted stock are deemed to have been reacquired by the Corporation.
2003 Stock Option and Incentive Plan
Until April 2007 certain option and restricted stock purchase awards were made pursuant to our 2003 Plan. The 2003 Plan was adopted by our Board of Directors and approved by our stockholders in November 2003. Upon the adoption of our 2007 Plan, in April 2007, our Board of Directors determined not to grant any further awards under our 2003 Plan.
Our 2003 Plan is administered by either our Board of Directors or the Compensation Committee. The administrator of the 2003 Plan has full power and authority to grant and amend awards and to adopt, amend and repeal rules relating to the 2003 Plan.
Upon a sale event in which all awards are not assumed or substituted by the successor entity, all stock options may be terminated upon the effective time of such sale event following an exercise period, in which case all such stock options shall first become fully exercisable. Restricted stock shall be treated as provided in the relevant award agreement. Under the 2003 Plan, a sale event is defined as the consummation of (i) a sale of all or substantially all of the assets, (ii) a sale of the Company by merger in which the stockholders of the Company do not own a majority of the outstanding voting power of the successor entity or (iii) any other acquisition of the business of the Company, as determined by the Board of Directors.
123
Table of Contents
The following table sets forth certain information with respect to outstanding equity awards at December 31, 2011 with respect to the named executive officers.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END 2011
| | | | | | | | | | | | | | | | | | | |
| | Option awards | | Stock awards | |
|---|
Name | | Number of
securities
underlying
unexercised
options
exercisable | | Number of
securities
underlying
unexercised
options
unexercisable | | Option
exercise
price | | Option
expiration
date | | Number of
shares or
units of
stock that
have not
vested | | Market value
of shares or
units of
stock that
have not
vested(1) | |
|---|
Ivan Trifunovich(2) | | | — | | | — | | | — | | | — | | | — | | $ | — | |
Jeffrey R. Moore(3) | | |
200,000 | | |
— | |
$ |
0.79 | | |
08/06/2019 | | |
— | |
$ |
— | |
- (1)
- Based upon the fair market value of $0.04 of our common stock on December 31, 2011, the last trading day of the fiscal year.
- (2)
- Stock awards do not include unissued commitments of 4,369,143 of restricted common shares that have not yet been granted to Dr. Trifunovich.
- (3)
- Stock awards do not include unissued commitments of 1,100,000 of restricted common shares that have not yet been granted to Mr. Moore.
Potential payments upon termination or change in control
Change of Control Agreements
As discussed above, our named executive officers may be entitled to various payments under the Management Incentive and the Retention Program in connection with a change of control. In addition, we have entered into the following additional arrangements with Dr. Trifunovich and Mr. Moore.
We entered into an arrangement with Dr. Trifunovich that provides that the vesting of his stock awards shall fully accelerate upon the consummation of a (i) "Sale Event" (as such term is defined in our 2007 Stock Option and Incentive Plan), (ii) the exclusive license of substantially all of our assets or (iii) a liquidation of our Company.
We have entered into a change in control agreement with Jeffrey R. Moore. Under the change in control agreement, we will have an obligation to make payments to Mr. Moore upon a termination event following a change in control. A termination event under the agreement includes, among other things, termination of Mr. Moore's employment by the Company without cause or a termination by Mr. Moore as a result of a reduction in his annual compensation or benefits, a significant diminution of his responsibilities or a relocation of his primary business location more than 50 miles from his residence in Cambridge, Massachusetts.
The change in control agreement with Mr. Moore provides for a payment equal to (i) three-fourths of his annual base salary in effect immediately prior to the termination event, or prior to the change in control if higher, and (ii) the average annual bonus paid to him over the two fiscal years (or such shorter period to reflect actual length of service) immediately prior to the change in control. Under the change in control agreement, Mr. Moore would continue to participate in such group health and dental programs for nine months in such circumstance under his change of control agreement. The change of control agreement also provides for full acceleration of any outstanding stock options or stock-based awards upon a termination event within 12 months of a change in control. All payments under the change in control agreement are subject to reduction as may be necessary to avoid certain tax consequences.
124
Table of Contents
The following table outlines the post-employment payments that would be made, assuming termination following a change in control on December 31, 2011 (assuming the change in control agreements were effective at that time):
| | | | |
Payments and Benefits | | Termination
without cause or
for good reason
following change in
control(1)(2) | |
|---|
Ivan Trifunovich, Ph.D. | | | | |
Accelerated vesting restricted stock commitment not yet granted | | $ | 174,766 | |
Jeffrey R. Moore | | | | |
Severance | | $ | 202,500 | |
Accelerated vesting restricted stock commitment not yet granted | | $ | 44,000 | |
Health benefits | | $ | 22,125 | |
- (1)
- If the post-employment payments described in this table would result in taxes payable by the executive officer under Section 4999 of the Internal Revenue Code of 1986, as amended, then such payment will be automatically reduced in order to avoid incurring such tax liability, unless the reduced post-employment payment would be less than the post-employment payment net of the payable taxes, in which case the executive officer is entitled to receive the full amount under the agreement.
- (2)
- The amounts reported for accelerated vesting of stock options and restricted stock awards has been calculated by multiplying the number of unvested shares by $0.04, the fair market value of our common stock on December 31, 2011, the last trading day of the fiscal year, less the applicable per share exercise or purchase prices.
Director Compensation
We do not pay any compensation to our employee directors for serving on our Board of Directors, including Ivan Trifunovich, our current President and Chief Executive Officer. We reimburse all non-employee directors for their reasonable out-of-pocket expenses incurred in attending meetings of our Board of Directors or any committees thereof.
Effective January 1, 2008, the Company adopted a revised Non-Employee Director Compensation Policy (the "Director Compensation Policy"). The Director Compensation Policy is designed to ensure that director compensation aligns the directors' interests with the long-term interests of the stockholders, that the structure of the compensation is simple, transparent and easy for stockholders to understand and that our directors are fairly compensated. Employee directors do not receive additional compensation for their services as directors. The revised policy sets the retainer at a level to more adequately compensate for director responsibilities, provide per meeting compensation for meetings held outside the original schedule and differentiate compensation for in-person versus telephonic attendance. In determining the adequate compensation in 2008, the Board of Directors looked at 30 companies in the biotechnology and pharmaceutical industry with market capitalization of $150-$400 million. The cash compensation payable to our directors is targeted to be in the 50th percentile of the cash compensation paid by these companies.
Under the Director Compensation Policy, upon initial election or appointment to the Board of Directors, new non-employee directors receive a non-qualified stock option to purchase 11,111 shares of common stock at an exercise price equal to the fair market value on the date of grant that vests one year from the date of grant. Each year of a non-employee director's tenure, the director will receive a non-qualified stock option to purchase 15,000 shares of common stock at an exercise price equal to the fair market value on the date of the grant that vests one year from the date of grant.
125
Table of Contents
Under the Director Compensation Policy, each non-employee director is paid an annual retainer of $25,000 ($40,000 for any non-employee Chairman or, as appropriate, the Lead Independent Director) for their services. For each Board of Directors meeting that a non-employee director attends in person in excess of six meetings in a single calendar year, such non-employee director shall be paid $1,500, if attended in person, and $750, if attended via telephone.
Committee members receive additional annual retainers in accordance with the following:
| | | | | | | |
Committee | | Non-employee
chairman | | Non-employee
director | |
|---|
Audit Committee | | $ | 15,000 | | $ | 10,000 | |
Compensation Committee | | $ | 10,000 | | $ | 7,500 | |
Nominating and Corporate Governance Committee | | $ | 7,500 | | $ | 5,000 | |
For each committee meeting a non-employee director attends in excess of nine meetings, for members of the Audit Committee, twelve meetings, for members of the Compensation Committee, or six meetings, for members of the Nominating and Corporate Governance Committee, such non-employee director will receive $1,000, if attended in person, and $500, if attended via telephone. These additional payments for service on a committee are due to the workload and broad-based responsibilities of the committees.
2010 Director Compensation
Effective January 1, 2010, four members of our Board of Directors, whose investment funds are currently investors in the Company, waived payment of their cash retainer and per meeting compensation for 2010 as they had done previously for 2009 in an effort to assist the Company in preserving cash and managing expenses. In addition, for 2010, the other non-employee members of our Board of Directors, including Stanley N. Lapidus, beginning in the second quarter of 2010, may receive unrestricted stock awards in the event that the trading price of the Company's common stock equaled or exceeded $0.50 per share on the date such award was earned, in lieu of receiving a cash retainer and per meeting compensation. Because our director compensation plan contemplates making these annual equity awards following our annual stockholders meeting, which we did not hold in 2010, no annual equity award was made to our directors.
2011 Director Compensation
In 2011, the Board evaluated its non-employee director compensation plan and determined to simplify our board compensation practices and to conserve resources by paying our non-employee directors only an annual retainer of $25,000 per year with no additional per meeting or audit committee compensation fees. In addition, members of our Board of Directors, whose investment funds are currently investors in the Company, waived payment of their cash retainer. During the fiscal year 2011 the Company only paid one quarter of this amount to its one eligible non investor director.
126
Table of Contents
Director Summary Compensation Table
The table below summarizes the compensation paid to non-employee Directors for the fiscal year ended December 31, 2011. Directors who are employees receive no additional compensation for Board service.
DIRECTOR COMPENSATION(1)
| | | | | | | | | | | | | |
Name | | Fees Earned or
Paid in Cash
($)(2) | | Option
Awards
($)(3) | | Stock
Awards
($)(3) | | Total
($) | |
|---|
Noubar B. Afeyan, Ph.D. | | $ | — | | | | | | | | $ | — | |
Peter Barrett, Ph.D. | | $ | — | | | | | | | | $ | — | |
Ronald A. Lowy | | $ | 6,250 | | | | | | | | $ | 6,250 | |
- (1)
- We do not maintain any non-equity incentive plans, pension plans, or non-qualified deferred compensation plans in which the directors participate. No directors received any other compensation other than what is listed above.
- (2)
- Total reflects fees and retainers earned.
- (3)
- Amount listed reflects the dollar amount recognized for financial statement reporting purposes in 2010 in accordance with FASB accounting guidance on stock option awards and thus includes amounts from awards granted in and prior to 2010. Information related to the financial reporting of stock options are presented in Footnote 12 to the Consolidated Financial Statements presented in this annual report.
Compensation Committee Interlocks and Insider Participation
Our entire Board has assumed responsibility for the roles and duties previously performed by our Compensation Committee.
Compensation Committee Report
The Board establishes general executive compensation policies and establishes the salaries and bonuses of the Company's executive officers. The Board: (i) has reviewed and discussed with management the discussion and analysis of compensation contained herein; and (ii) based on this review and discussion, the Board recommended and approved the inclusion of such discussion and analysis of compensation in our Annual Report on Form 10-K for the fiscal year ended December 31, 2011 for filing with the Securities and Exchange Commission.
Respectfully submitted by the Board of Directors:
Ivan Trifunovich,Chairman
Peter Barrett
Noubar B. Afeyan
Ronald A. Lowy
Bruce C. Ginsberg
The Compensation Committee Report does not constitute soliciting material, and shall not be deemed to be filed or incorporated by reference into any other Company filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent that the Company specifically incorporates the Compensation Committee Report by reference therein.
127
Table of Contents
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Equity Compensation Plan Information
The following table summarizes, as of December 31, 2011, the number of options issued under our stock option plans and the number of options available for future issuance under these plans.
| | | | | | | | | | |
| | (a) | | (b) | | (c) | |
|---|
Plan Category | | Number of securities
to be issued upon
exercise of
outstanding options,
warrants and rights | | Weighted-average
exercise price of
outstanding options,
warrants and rights | | Number of securities
remaining available
for future issuance
under equity
compensation plans
(excluding securities
reflected in column(a)) | |
|---|
Equity compensation plans approved by security holders(1) | | | 599,301 | | $ | 2.02 | | | 9,272,469 | |
Equity compensation plans not approved by security holders | | | — | | | — | | | — | |
| | | | | | | | |
Total | | | 599,301 | | $ | 2.02 | | | 9,272,469 | |
| | | | | | | | |
- (1)
- Includes the 2003 Stock Option and Incentive Plan and the 2007 Stock Option and Incentive Plan.
128
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding beneficial ownership of the Company's Common Stock as of March 1, 2012: (i) by each person who is known by the Company to beneficially own more than 5% of the outstanding shares of Common Stock; (ii) by each director of the Company; (iii) by each of the then named officers named in the Summary Compensation Table, excluding the Company's former employee Mr. Levine; and (iv) by all directors and executive officers of the Company as a group. Unless otherwise indicated below, each person listed below maintains a business address in the care of Helicos BioSciences Corporation, One Kendall Square, Building 200, Cambridge, MA 02139 and has sole voting and investment power with respect to all shares of Common Stock owned.
| | | | | | | |
Name of Beneficial Owner | | Shares
Beneficially Owned(1) | | Percentage of Shares
Beneficially Owned(1)(2) | |
|---|
Atlas Venture(3)
25 First Street, Suite 303
Cambridge, MA 02141 | | | 17,201,291 | | | 19.7 | % |
Flagship Ventures(4)
c/o Flagship Ventures
One Memorial Drive, 7th Floor
Cambridge, Massachusetts 02142 | | | 17,542,548 | | | 20.1 | % |
Ivan Trifunovich, Ph.D. | | | — | | | — | % |
Ronald A. Lowy(5) | | | 1,047,307 | | | 1.2 | % |
Jeffrey R. Moore(6) | | | 233,579 | | | * | |
Noubar B. Afeyan, Ph.D.(4) | | | 17,542,548 | | | 20.1 | % |
Peter Barrett, Ph.D.(3) | | | 17,201,291 | | | 19.7 | % |
All executive officers, directors and nominees as a group(7) (5 persons) | | | 36,523,725 | | | 41.9 | % |
- *
- Represents less than 1% of the outstanding Common Stock.
- (1)
- Beneficial ownership is determined in accordance with the rules of the SEC and includes voting and investment power with respect to shares. Pursuant to the rules of the SEC, the number of shares of Common Stock deemed outstanding includes shares issuable pursuant to options and warrants held by the respective person or group that may be exercised within 60 days of March 1, 2012.
- (2)
- Applicable percentage of ownership is based upon 87,196,489 shares of Common Stock outstanding as of March 19, 2012.
- (3)
- With respect to information relating to Atlas Venture, the Company has relied, in part, on information supplied on their Schedule 13D filing with the SEC dated December 10, 2009, by Atlas Venture Fund V, L.P. ("Atlas V"), Atlas Venture Entrepreneurs' Fund V, L.P. ("AVE V" and together with Atlas V, the "Atlas V Funds"), Atlas Venture Fund VI, L.P. ("Atlas VI"), Atlas Venture Entrepreneurs' Fund VI, L.P. ("AVE VI"), Atlas Venture Fund VI GmbH & Co. KG ("Atlas VI GmbH" and together with Atlas VI and AVE VI, the "Atlas VI Funds"), Atlas Venture Associates V, L.P. ("AVA V LP"), Atlas Venture Associates V, Inc. ("AVA V Inc."), Atlas Venture Associates VI, L.P. ("AVA VI LP"), Atlas Venture Associates VI, Inc. ("AVA VI Inc."), Jean-Francois Formela ("Formela"), (the "Atlas Directors"). AVA V Inc. is the sole general partner of AVA V LP. AVA V LP is the sole general partner of the Atlas V Funds. The Atlas Directors are directors of AVA V Inc. As a result, the Atlas Directors may be deemed to have beneficial ownership with respect to all shares held by AVA V Inc. AVA VI Inc. is the sole general partner of AVA VI LP. AVA VI LP is the sole general partner of Atlas VI and AVE VI and the
129
Table of Contents
managing limited partner of Atlas VI GmbH. The Atlas Directors are directors of AVA VI Inc. As a result, the Atlas Directors may be deemed to have beneficial ownership with respect to all shares held by AVA VI Inc. Each of the foregoing disclaims beneficial ownership of these shares except to the extent of their pecuniary interest therein. The shares represented herein include 5,333,238 shares issuable to Atlas Venture upon the exercise of warrants and 16,666 shares issuable to Dr. Barrett upon the exercise of stock options.
- (4)
- With respect to information relating to Flagship Ventures, the Company has relied, in part, on information supplied on their Schedule 13D filing with the SEC dated December 9, 2010, by AGTC Advisors Fund L.P. ("AGTC"), Applied Genomic Technology Capital Fund, L.P. ("AGTC Fund" and together with AGTC, the "AGTC Funds"), Flagship Ventures Fund 2004, L.P. ("Flagship"), NewcoGen Elan LLC ("NewcoGen Elan"), NewcoGen Equity Investors LLC ("NewcoGen Equity"), NewcoGen Group, LLC ("NewcoGen Group"), NewcoGen PE LLC ("NewcoGen PE"), NewcoGen Long Reign Holdings LLC ("NewcoGen Long Reign"), and ST NewcoGen LLC ("ST NewcoGen" and together with NewcoGen Elan, NewcoGen Equity, NewcoGen Group, NewcoGen PE and NewcoGen Long Reign, the "NewcoGen Funds"). NewcoGen Group Inc. ("NewcoGen Inc.") is the manager of each of the NewcoGen Funds and the general partner of AGTC Partners, L.P., which is the general partner of each of the AGTC Funds. NewcoGen Inc. is a wholly owned subsidiary of Flagship Ventures Management, Inc. ("Flagship Inc."). Flagship Ventures General Partner LLC ("Flagship LLC") is the general partner of Flagship. Noubar B. Afeyan, PhD and Edwin M. Kania, Jr. are directors of Flagship Inc. and managers of Flagship LLC. As a result, Messrs. Afeyan and Kania may be deemed to have beneficial ownership with respect to all shares held by the NewcoGen Funds, Flagship, and the AGTC Funds. The shares represented herein include 5,333,239 shares issuable to Flagship Ventures upon the exercise of warrants and 16,666 shares issuable to Dr. Afeyan upon the exercise of stock options.
- (5)
- Includes 254,558 shares issuable to Ronald A. Lowy upon exercise of stock options.
- (6)
- Includes 200,000 shares issuable to Jeffrey R. Moore upon exercise of stock options.
- (7)
- Includes an aggregate of 499,000 shares issuable upon exercise of stock options.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
Certain Relationships and Related Transactions
The Board conducts an appropriate review of all related party transactions required to be disclosed for potential conflicts of interest situations on an ongoing basis and, all such transactions are approved by the Audit Committee.
November 2010 Bridge Financing
We entered the fourth quarter of 2010 without any prospects of securing long-term financing from outside investors. An independent committee (the "Independent Committee") of our Board evaluated the alternatives available to us and determined that a bridge financing led by certain inside investors was in the best interests of our stakeholders in view of our current financial and operating condition, and would best position us to continue operations for the remainder of 2010 and into 2011. The Independent Committee met multiple times to consider the alternatives and concluded that the bridge financing was the best alternative for generating value for stakeholders. As a result, we entered into an insider bridge loan facility with two of the four venture capital firms whose representatives, Noubar B. Afeyan, Ph.D. and Peter Barrett, Ph.D. are members of the Board. In connection with this transaction, we entered into a Subordinated Secured Note Purchase Agreement (the "Note Purchase Agreement")
130
Table of Contents
with investment funds affiliated with Atlas Venture and Flagship Ventures (the "Purchasers"), pursuant to which we have agreed to sell to the Purchasers, and the Purchasers have agreed to purchase, up to an aggregate of $4,000,000 of convertible promissory notes (the "Notes"), a portion of which is committed and a portion of which is optional (the "Bridge Debt Financing"). On November 16, 2010, the Purchasers purchased Notes having an aggregate principal amount of $333,333. Subject to our continued compliance with the terms of the Note Purchase Agreement, including that there not be an event of default thereunder, upon notice from us, the Purchasers are committed to purchase an aggregate of an additional $333,333 of Notes in each of five subsequent closings, for a total aggregate committed amount of $2,000,000. The Purchasers also have the right, but not the obligation, upon our request to purchase up to an additional $2,000,000 of Notes on or before December 31, 2012. As of December 31, 2010 and as of the date of this filing, we have borrowed $666,667 against the Bridge Debt Financing facility.
The Notes will accrue interest at a rate of 10% per annum plus a risk premium as described below. The outstanding principal and any unpaid accrued interest on all of the Notes shall be due and payable in full (i) upon demand by the Purchasers, which demand shall be no earlier than December 31, 2012, (ii) upon our receiving at least $10,000,000 in proceeds from a subsequent equity financing, (iii) upon a change of control of the Company or (iv) upon an event of default. A Purchaser may elect to convert principal and interest outstanding under any of the Notes upon the closing of any future round of equity or debt financing of the Company into shares, and at the per share price, of the securities issued at such financing. Simultaneously with the execution of the Note Purchase Agreement, we and the requisite parties entered into an Amendment to the Amended and Restated Investor Rights Agreement, dated as of March 1, 2006, by and among us and the parties specified therein, including the Purchasers (the "IRA Amendment"), pursuant to which the Purchasers will be entitled to demand and piggyback registration rights consistent with the terms of the Purchasers' existing registration rights for any securities issued upon conversion of the Notes.
As an inducement for the Purchasers to purchase the Notes, we entered into a Risk Premium Payment Agreement, dated as of November 16, 2010, with the Purchasers (the "Risk Premium Agreement") simultaneously with the execution of, and as required by, the Note Purchase Agreement. Under the terms of the Risk Premium Agreement, we have agreed to pay the Purchasers the following portions of the consideration we receive (the "Payment Consideration") in Liquidity Transactions, as defined below: (i) until the aggregate Payment Consideration equals $10 million, 60% of the Payment Consideration; (ii) for aggregate Payment Consideration from $10 million to $20 million, 50% of the Payment Consideration; (iii) for aggregate Payment Consideration from $20 million to $30 million, 40% of the Payment Consideration; (iv) for aggregate Payment Consideration from $30 million to $40 million, 30% of the Payment Consideration; and (v) for aggregate Payment Consideration in excess of $40 million, 10% of the Payment Consideration (any such payments, "Risk Premium Payments"). For purposes of determining amounts payable under the Risk Premium Agreement, the Payment Consideration will not include amounts we use to first satisfy specified existing obligations, including obligations under the Loan Agreement, under professional contingency arrangements (including the contingency fee arrangement between us and Outside IP Litigation Counsel relating to the Cause of Action) and obligations that may arise under existing technology license agreements (the "Existing IP Licensing Agreements") between us and each of Arizona Technology Enterprises, Roche Diagnostics GMBH and Roche Diagnostics Corporation, and California Institute of Technology (as previously disclosed by us, under the Existing IP Licensing Agreements, we are obligated to pay license fees in connection with the licensing, sublicensing, legal settlements or sales of specified intellectual property or services provided to third parties ranging from a single digit percentage up to one-half of the income from those activities). For purposes of the Risk Premium Agreement and subject to various provisions therein, the following shall constitute Liquidity Transactions: (A) the receipt by us of any payments from third parties relating to our intellectual property portfolio, including payments received as a license or other settlement in patent infringement litigation brought by us ("IP Licensing Events");
131
Table of Contents
(B) a merger of the Company in which there is a change of control; and (C) the sale or exclusive license by us of all or substantially all of our assets or intellectual property.
In addition, Noubar B. Afeyan, Ph.D., who is the founder, a Managing Partner and the Chief Executive Officer of Flagship Ventures, and Peter Barrett, Ph.D., who is a Partner of Atlas Venture, abstained from the vote of our Board of Directors approving the Bridge Debt Financing.
See Part I, Item 7, Management's Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Bridge Financing and Senior Debt Restructuring (November 2010) for additional discussion of the Bridge Debt Financing.
Director Indemnification Agreements
We have entered into indemnification agreements with each of our non-management directors. These agreements require us to indemnify our directors to the fullest extent permitted by Delaware law.
Director Independence
Although no longer required subsequent to our delisting from the NASDAQ Global Market, a majority of the Board is "independent" as defined by the listing standards of the NASDAQ Stock Market Inc. All members of our Audit Committee satisfy the same independence criteria and, prior to their dissolution, all members of our Compensation Committee and Nominating and Corporate Governance Committee satisfied such criteria. The independent directors hold regularly scheduled meetings at which only independent directors are present.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES [UPDATE]
During fiscal year 2011 and fiscal year 2010, the aggregate fees billed by PricewaterhouseCoopers LLP for professional services were as follows:
| | | | | | | |
| | Fiscal Year Ended | |
|---|
| | December 31,
2011 | | December 31,
2010 | |
|---|
Audit Fees(1) | | $ | 150,000 | | $ | 225,000 | |
Audit-Related Fees(2) | | | — | | | 24,000 | |
Tax Fees(3) | | | — | | | — | |
All Other Fees(4) | | | | | | 1,500 | |
| | | | | | |
Total | | $ | 150,000 | | $ | 249,000 | |
| | | | | | |
- (1)
- Fees for audit services include fees associated with the audits of our consolidated financial statements. Audit fees also include amounts associated with SEC registration statements and consents.
- (2)
- Audit-related fees for 2010 consisted of fees for the audit of the Company's research and development program funded by the National Institutes of Health (NIH).
- (3)
- There were no fees billed by PricewaterhouseCoopers LLP for tax services in 2011 or 2010.
- (4)
- The amount listed as "All Other Fees" consists of fees for products and services other than those services reported above.
132
Table of Contents
Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Registered Public Accounting Firm
As required by the Audit Committee charter, the Audit Committee pre-approves the engagement of PricewaterhouseCoopers LLP for all audit and permissible non-audit services. All of the audit-related fees and other non-audit fees disclosed above, were pre-approved by the Audit Committee. The Audit Committee annually reviews the audit and permissible non-audit services performed by PricewaterhouseCoopers LLP and reviews and approves the fees charged by PricewaterhouseCoopers LLP. The Audit Committee has considered the role of PricewaterhouseCoopers LLP in providing audit services and other permissible non-audit services to Helicos and has concluded that the provision of such services was compatible with the maintenance of PricewaterhouseCoopers LLP's independence in the conduct of its auditing functions.
133
Table of Contents
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
- (a)
- The following documents are filed as part of this Form 10-K:
(1) Consolidated Financial Statements:
| | |
| | Page |
|---|
Report of independent registered public accounting firm | | 64 |
Consolidated balance sheets as of December 31, 2010 and December 31, 2011 | | 65 |
Consolidated statements of operations for the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) to December 31, 2011 | | 66 |
Consolidated statements of redeemable convertible preferred stock and stockholders' equity (deficit) for the period from May 9, 2003 (date of inception) to December 31, 2011, and the years ended December 31, 2009, 2010 and 2011 | | 67 |
Consolidated statements of cash flows for the years ended December 31, 2009, 2010 and 2011, and the period from May 9, 2003 (date of inception) to December 31, 2011 | | 74 |
Notes to consolidated financial statements | | 75 |
(3) Exhibits:
INDEX TO EXHIBITS
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 3.1 | | Form of Fourth Amended and Restated Certificate of Incorporation of the Registrant (Incorporated by reference herein to Exhibit 3.3 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 3.2 | | Amended and Restated By-laws of the Registrant (Incorporated by reference herein to Exhibit 3.2 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 4.1 | | Specimen Stock Certificate (Incorporated by reference herein to Exhibit 4.1 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 4.2+ | | Form of Warrant between the Registrant and the Lenders (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on June 30, 2008). |
| | 4.3 | | Form of Warrant (Incorporated by reference herein to Exhibit 4.1 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on December 22, 2008). |
| | 4.4+ | | Form of Warrant (Incorporated by reference herein to exhibit 4.1 to Form 8-K, filed with the Securities and Exchange Commission on September 17, 2009). |
| | 4.5+ | | Form of Warrant (Incorporated by reference herein to exhibit 4.1 to Form 8-K, filed with the Securities and Exchange Commission on December 18, 2009). |
| | 10.1 | | Master Loan Agreement by and between the Registrant and General Electric Capital Corporation, dated June 9, 2006 (Incorporated by reference herein to Exhibit 10.3 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
134
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 10.2 | | Lease Agreement by and between the Registrant and Lincoln Property Company, dated December 30, 2005 (Incorporated by reference herein to Exhibit 10.4 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.3 | | Lease Agreement by and between the Registrant and Cummings Properties, LLC, dated February 1, 2006 (Incorporated by reference herein to Exhibit 10.5 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.4 | | 2003 Stock Option and Incentive Plan and forms of agreements thereunder (Incorporated by reference herein to Exhibit 10.6 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.5† | | License Agreement between the Registrant and California Institute of Technology, dated November 30, 2003 (Incorporated by reference herein to Exhibit 10.7 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.6 | | License Agreement between the Registrant, Roche Diagnostics GMBH and Roche Diagnostics Corporation, dated June 7, 2004 (Incorporated by reference herein to Exhibit 10.8 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.7† | | License Agreement between the Registrant and Arizona Technology Enterprises, dated March 16, 2005 (Incorporated by reference herein to Exhibit 10.9 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.8 | | Form of Indemnification Agreement (Incorporated by reference herein to Exhibit 10.10 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.9 | | Amended and Restated Investor Rights Agreement by and among the Registrant and the Investors named therein, dated as of March 1, 2006 (Incorporated by reference herein to Exhibit 10.11 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.10† | | Amendment to License Agreement Having an Effective Date of March 7, 2007 between California Institute of Technology and the Registrant (Incorporated by reference herein to Exhibit 10.12 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.11+ | | Employee Offer Letter, dated as of October 15, 2003, by and between Stanley N. Lapidus and the Registrant (Incorporated by reference herein to Exhibit 10.13 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.12+ | | Amended and Restated Management Incentive Bonus Plan of the Registrant as of December 11, 2008 (Incorporated by reference herein to Exhibit 10.12 to the Company's Annual Report on Form 10-K, filed w/the Securities and Exchange Commission on March 30, 2009). |
135
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 10.13† | | License and Supply Agreement, having an effective date of April 23, 2007 between PerkinElmer LAS, Inc. and the Registrant (Incorporated by reference herein to Exhibit 10.15 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.14 | | Amendment to the Amended and Restated Investor Rights Agreement dated as of May 7, 2007 (Incorporated by reference herein to Exhibit 10.16 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.15+ | | Change in Control Agreement, dated as of May 2, 2007, by and between Stanley N. Lapidus and the Registrant (Incorporated by reference herein to Exhibit 10.17 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.16+ | | Change in Control Agreement, dated as of May 7, 2007, by and between Stephen J. Lombardi and the Registrant (Incorporated by reference herein to Exhibit 10.18 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.17+ | | Non-Employee Director Compensation Policy (Incorporated by reference herein to Exhibit 10.20 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.18+ | | 2007 Stock Option and Incentive Plan and forms of agreement thereunder, as amended (Incorporated by reference herein to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed on August 14, 2008). |
| | 10.19+ | | Change in Control Agreement between the Company and J. William Efcavitch, dated August 8, 2007 (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on August 10, 2007). |
| | 10.20† | | Loan and Security Agreement by and between the Registrant and General Electric Capital Corporation, dated December 31, 2007 (Incorporated by reference herein to Exhibit 10.23 to the Company's Annual Report on Form 10-K, filed with the Securities and Exchange Commission on March 17, 2008). |
| | 10.21+ | | Offer Letter between the Registrant and Stephen P. Hall dated April 25, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on May 1, 2008). |
| | 10.22+ | | Change in Control Agreement between the Registrant and Stephen P. Hall dated April 25, 2008 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on May 1, 2008). |
| | 10.23+ | | Second Amendment to Loan and Security Agreement by and between the Registrant, the Lenders and General Electric Capital Corporation, dated June 27, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on June 30, 2008). |
| | 10.24+ | | Offer Letter between the Registrant and Stephen J. Lombardi, dated August 19, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 20, 2008). |
136
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 10.25+ | | Amended and Restated Change in Control Agreement between the Registrant and Stephen J. Lombardi, dated August 19, 2008 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 20, 2008). |
| | 10.26+ | | Severance and Consulting Services Agreement, by and between the Registrant and Stanley N. Lapidus, dated as of September 12, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on September 12, 2008). |
| | 10.27+ | | Consultant Agreement between the Registrant and Stanley N. Lapidus, dated as of December 5, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 8, 2008). |
| | 10.28+ | | Corporate Officer Severance Plan (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 17, 2008). |
| | 10.29+ | | Securities Purchase Agreement between the Registrant and each of the Purchasers identified therein, dated December 19, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 22, 2008). |
| | 10.30+ | | Registration Rights Agreement between the Registrant and each of the Purchasers identified therein, dated December 19, 2008 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 22, 2008). |
| | 10.31+ | | Third Amendment to the Loan and Security Agreement among the Registrant, the Lenders and General Electric Capital Corporation, dated as of December 29, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 30, 2008). |
| | 10.32+ | | Change in Control Agreement by and between the Registrant and Ronald A. Lowy, dated as of January 28, 2009 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on January 30, 2009). |
| | 10.33+ | | Change in Control Agreement by and between the Company and Ronald A. Lowy, dated as of January 28, 2009 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on January 30, 2009). |
| | 10.34+ | | Form of Unrestricted Stock Award Agreement under the 2007 Stock Option and Incentive Plan (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on May 14, 2009). |
| | 10.35 | | Letter Agreement, dated August 6, 2009, by and between Stephen P. Hall and Helicos BioSciences Corporation (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 11, 2009). |
| | 10.36+ | | Offer Letter between the Company and Jeffrey R. Moore dated August 6, 2009 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 11, 2009). |
| | 10.37+ | | Change in Control Agreement between the Company and Jeffrey R. Moore dated August 6, 2009 (Incorporated by reference herein to Exhibit 10.3 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 11, 2009). |
137
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 10.38 | | Securities Purchase Agreement, between Helicos and each of the Purchasers identified therein, dated September 15, 2009 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on September 17, 2009). |
| | 10.39 | | Registration Rights Agreement, between Helicos and each of the Holders identified therein, dated September 15, 2009 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on September 17, 2009). |
| | 10.40+ | | First Amendment to Change in Control Agreement, dated as of September 28, 2009, by and between Ronald A. Lowy and Helicos BioSciences Corporation (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on September 28, 2009). |
| | 10.41 | | Fifth Amendment to Lease, dated as of October 8, 2009 Corporation (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on October 13, 2009). |
| | 10.42+ | | First Amendment to the Company's Amended and Restated Management Incentive Bonus Plan of the Registrant dated as of September 8, 2009 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on November 9, 2009). |
| | 10.43 | | Form of Unrestricted Stock Award Agreement to Employees Pursuant to the Helicos BioSciences Corporation Amended and Restated Management Incentive Plan, as amended, and the Helicos BioSciences Corporation 2nd Half Fiscal 2009 Employee Incentive Bonus Plan (Incorporated by reference herein to Exhibit 10.43 to the Company's Annual Report on Form 10-K, filed with the Securities and Exchange Commission on April 15, 2010). |
| | 10.44 | | Letter Agreement, dated February 11, 2010, by and between Stephen J. Lombardi and Helicos BioSciences Corporation (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on February 12, 2010). |
| | 10.45 | | Sixth Amendment to Lease by and between the Company and RB Kendall Fee, LLC dated June 9, 2010 (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on June 14, 2010). |
| | 10.46+ | | Form of Retention Agreement with Management dated August 6, 2010 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on November 18, 2010). |
| | 10.47 | | Letter Agreement, dated September 8, 2010, by and between, J. William Efcavitch and Helicos BioSciences Corporation (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on November 18, 2010). |
| | 10.48 | | Subordinated Secured Note Purchase Agreement, dated November 16, 2010, between the Company and each of the Purchasers identified therein (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on November 19, 2010). |
138
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 10.49 | | Risk Premium Payment Agreement, dated November 16, 2010, between the Company and each of the Purchasers identified therein (Incorporated by reference herein to Exhibit 10.2 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on November 19, 2010). |
| | 10.50 | | Waiver, Consent and Fourth Amendment to the Loan and Security Agreement, dated November 16, 2010, among the Company, the Lenders and General Electric Capital Corporation (Incorporated by reference herein to Exhibit 10.3 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on November 19, 2010). |
| | 10.51† | | First Amendment to the License Agreement dated August 26, 2011, between the Company and Arizona Technologies Enterprises (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on November 14, 2011). |
| | 10.52 | | Seventh Amendment to Lease, by and between the Company and RB Kendall Fee, LLC dated September 20, 2011 (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on September 22, 2011). |
| | 10.53 | | First Amendment to Subordinated Secured Note Purchase Agreement, dated as of November 30, 2011, between the Company and each of the Purchasers identified therein (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on December 1, 2011). |
| | 21.1 | | Subsidiary of the Registrant (Incorporated by reference herein to Exhibit 21.1 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 23.1* | | Consent of PricewaterhouseCoopers LLP, an independent registered public accounting firm. |
| | 31.1* | | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 31.2* | | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 32.1* | | Certifications pursuant to Section 320 of the Sarbanes-Oxley Act of 2002. |
| | 101 | | The following financial statements from the Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2011 are furnished herewith, formatted in XBRL (eXtensible Business Reporting Language): (i) the Consolidated Statements of Operations for the years ended December 31, 2011, 2010, and 2009, (ii) the Consolidated Balance Sheets at December 31, 2011 and December 31, 2010, (iii) the Consolidated Statement of Equity for the years ended December 31, 2011, 2010, and 2009, (iv) the Consolidated Statements of Cash Flows for the years ended December 31, 2011, 2010, and 2009, and (v) the Notes to the Consolidated Financial Statements. |
- *
- Filed herewith
- †
- Confidential treatment has been requested for certain provisions of this Exhibit pursuant to Rule 406 promulgated under the Securities Act.
- +
- Indicates a management contract or any compensatory plan, contract or arrangement.
139
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or Section 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
| | | HELICOS BIOSCIENCES CORPORATION |
Dated: March 30, 2012 |
|
By: |
|
/s/ IVAN TRIFUNOVICH
Name: Ivan Trifunovich
Title: Chairman, President and Chief Executive Officer (Principal Executive Officer) |
Dated: March 30, 2012 |
|
By: |
|
/s/ JEFFREY R. MOORE
Name: Jeffrey R. Moore
Title: Senior Vice President and Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
POWER OF ATTORNEY AND SIGNATURES
We, the undersigned officers and directors of Helicos BioSciences Corporation, hereby severally constitute and appoint Ivan Trifunovich and Jeffrey R. Moore, our true and lawful attorneys, with full power to them to sign for us and in our names in the capacities indicated below, any amendments to this Annual Report on Form 10-K, and generally to do all things in our names and on our behalf in such capacities to enable Helicos BioSciences Corporation to comply with the provisions of the Securities Exchange Act of 1934, as amended, and all the requirements of the Securities Exchange Commission.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
| | | | | | |
Signature | | Title | | Date |
|---|
|
|
|
|
|
|
|
| By: | | /s/ IVAN TRIFUNOVICH
Ivan Trifunovich | | Chairman of the Board of Directors, President, Chief Executive Officer and Director (Principal Executive Officer) | | March 30, 2012 |
By: |
|
/s/ JEFFREY R. MOORE
Jeffrey R. Moore |
|
Senior Vice President, Chief Financial Officer, Treasurer and Secretary (Principal Financial Officer and Principal Accounting Officer) |
|
March 30, 2012 |
140
Table of Contents
| | | | | | |
Signature | | Title | | Date |
|---|
|
|
|
|
|
|
|
| By: | | /s/ NOUBAR B. AFEYAN, PHD
Noubar B. Afeyan, PhD | | Director | | March 30, 2012 |
By: |
|
/s/ PETER BARRETT, PHD
Peter Barrett, PhD |
|
Director |
|
March 30, 2012 |
By: |
|
/s/ RONALD A. LOWY
Ronald A. Lowy |
|
Director |
|
March 30, 2012 |
By: |
|
/s/ BRUCE C. GINSBERG
Bruce C. Ginsberg |
|
Director |
|
March 30, 2012 |
141
Table of Contents
INDEX TO EXHIBITS
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 3.1 | | Form of Fourth Amended and Restated Certificate of Incorporation of the Registrant (Incorporated by reference herein to Exhibit 3.3 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 3.2 | | Amended and Restated By-laws of the Registrant (Incorporated by reference herein to Exhibit 3.2 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 4.1 | | Specimen Stock Certificate (Incorporated by reference herein to Exhibit 4.1 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 4.2+ | | Form of Warrant between the Registrant and the Lenders (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on June 30, 2008). |
| | 4.3 | | Form of Warrant (Incorporated by reference herein to Exhibit 4.1 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on December 22, 2008). |
| | 4.4+ | | Form of Warrant (Incorporated by reference herein to exhibit 4.1 to Form 8-K, filed with the Securities and Exchange Commission on September 17, 2009). |
| | 4.5+ | | Form of Warrant (Incorporated by reference herein to exhibit 4.1 to Form 8-K, filed with the Securities and Exchange Commission on December 18, 2009). |
| | 10.1 | | Master Loan Agreement by and between the Registrant and General Electric Capital Corporation, dated June 9, 2006 (Incorporated by reference herein to Exhibit 10.3 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.2 | | Lease Agreement by and between the Registrant and Lincoln Property Company, dated December 30, 2005 (Incorporated by reference herein to Exhibit 10.4 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.3 | | Lease Agreement by and between the Registrant and Cummings Properties, LLC, dated February 1, 2006 (Incorporated by reference herein to Exhibit 10.5 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.4 | | 2003 Stock Option and Incentive Plan and forms of agreements thereunder (Incorporated by reference herein to Exhibit 10.6 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.5† | | License Agreement between the Registrant and California Institute of Technology, dated November 30, 2003 (Incorporated by reference herein to Exhibit 10.7 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.6 | | License Agreement between the Registrant, Roche Diagnostics GMBH and Roche Diagnostics Corporation, dated June 7, 2004 (Incorporated by reference herein to Exhibit 10.8 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
142
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 10.7† | | License Agreement between the Registrant and Arizona Technology Enterprises, dated March 16, 2005 (Incorporated by reference herein to Exhibit 10.9 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.8 | | Form of Indemnification Agreement (Incorporated by reference herein to Exhibit 10.10 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.9 | | Amended and Restated Investor Rights Agreement by and among the Registrant and the Investors named therein, dated as of March 1, 2006 (Incorporated by reference herein to Exhibit 10.11 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.10† | | Amendment to License Agreement Having an Effective Date of March 7, 2007 between California Institute of Technology and the Registrant (Incorporated by reference herein to Exhibit 10.12 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.11+ | | Employee Offer Letter, dated as of October 15, 2003, by and between Stanley N. Lapidus and the Registrant (Incorporated by reference herein to Exhibit 10.13 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.12+ | | Amended and Restated Management Incentive Bonus Plan of the Registrant as of December 11, 2008 (Incorporated by reference herein to Exhibit 10.12 to the Company's Annual Report on Form 10-K, filed w/the Securities and Exchange Commission on March 30, 2009). |
| | 10.13† | | License and Supply Agreement, having an effective date of April 23, 2007 between PerkinElmer LAS, Inc. and the Registrant (Incorporated by reference herein to Exhibit 10.15 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.14 | | Amendment to the Amended and Restated Investor Rights Agreement dated as of May 7, 2007 (Incorporated by reference herein to Exhibit 10.16 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.15+ | | Change in Control Agreement, dated as of May 2, 2007, by and between Stanley N. Lapidus and the Registrant (Incorporated by reference herein to Exhibit 10.17 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.16+ | | Change in Control Agreement, dated as of May 7, 2007, by and between Stephen J. Lombardi and the Registrant (Incorporated by reference herein to Exhibit 10.18 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.17+ | | Non-Employee Director Compensation Policy (Incorporated by reference herein to Exhibit 10.20 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 10.18+ | | 2007 Stock Option and Incentive Plan and forms of agreement thereunder, as amended (Incorporated by reference herein to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed on August 14, 2008). |
143
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 10.19+ | | Change in Control Agreement between the Company and J. William Efcavitch, dated August 8, 2007 (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on August 10, 2007). |
| | 10.20† | | Loan and Security Agreement by and between the Registrant and General Electric Capital Corporation, dated December 31, 2007 (Incorporated by reference herein to Exhibit 10.23 to the Company's Annual Report on Form 10-K, filed with the Securities and Exchange Commission on March 17, 2008). |
| | 10.21+ | | Offer Letter between the Registrant and Stephen P. Hall dated April 25, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on May 1, 2008). |
| | 10.22+ | | Change in Control Agreement between the Registrant and Stephen P. Hall dated April 25, 2008 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on May 1, 2008). |
| | 10.23+ | | Second Amendment to Loan and Security Agreement by and between the Registrant, the Lenders and General Electric Capital Corporation, dated June 27, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on June 30, 2008). |
| | 10.24+ | | Offer Letter between the Registrant and Stephen J. Lombardi, dated August 19, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 20, 2008). |
| | 10.25+ | | Amended and Restated Change in Control Agreement between the Registrant and Stephen J. Lombardi, dated August 19, 2008 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 20, 2008). |
| | 10.26+ | | Severance and Consulting Services Agreement, by and between the Registrant and Stanley N. Lapidus, dated as of September 12, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on September 12, 2008). |
| | 10.27+ | | Consultant Agreement between the Registrant and Stanley N. Lapidus, dated as of December 5, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 8, 2008). |
| | 10.28+ | | Corporate Officer Severance Plan (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 17, 2008). |
| | 10.29+ | | Securities Purchase Agreement between the Registrant and each of the Purchasers identified therein, dated December 19, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 22, 2008). |
| | 10.30+ | | Registration Rights Agreement between the Registrant and each of the Purchasers identified therein, dated December 19, 2008 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 22, 2008). |
144
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 10.31+ | | Third Amendment to the Loan and Security Agreement among the Registrant, the Lenders and General Electric Capital Corporation, dated as of December 29, 2008 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on December 30, 2008). |
| | 10.32+ | | Change in Control Agreement by and between the Registrant and Ronald A. Lowy, dated as of January 28, 2009 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on January 30, 2009). |
| | 10.33+ | | Change in Control Agreement by and between the Company and Ronald A. Lowy, dated as of January 28, 2009 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on January 30, 2009). |
| | 10.34+ | | Form of Unrestricted Stock Award Agreement under the 2007 Stock Option and Incentive Plan (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on May 14, 2009). |
| | 10.35 | | Letter Agreement, dated August 6, 2009, by and between Stephen P. Hall and Helicos BioSciences Corporation (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 11, 2009). |
| | 10.36+ | | Offer Letter between the Company and Jeffrey R. Moore dated August 6, 2009 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 11, 2009). |
| | 10.37+ | | Change in Control Agreement between the Company and Jeffrey R. Moore dated August 6, 2009 (Incorporated by reference herein to Exhibit 10.3 to the Company's Form 8-K, filed with the Securities and Exchange Commission on August 11, 2009). |
| | 10.38 | | Securities Purchase Agreement, between Helicos and each of the Purchasers identified therein, dated September 15, 2009 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on September 17, 2009). |
| | 10.39 | | Registration Rights Agreement, between Helicos and each of the Holders identified therein, dated September 15, 2009 (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 8-K, filed with the Securities and Exchange Commission on September 17, 2009). |
| | 10.40+ | | First Amendment to Change in Control Agreement, dated as of September 28, 2009, by and between Ronald A. Lowy and Helicos BioSciences Corporation (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on September 28, 2009). |
| | 10.41 | | Fifth Amendment to Lease, dated as of October 8, 2009 Corporation (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on October 13, 2009). |
| | 10.42+ | | First Amendment to the Company's Amended and Restated Management Incentive Bonus Plan of the Registrant dated as of September 8, 2009 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on November 9, 2009). |
145
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 10.43 | | Form of Unrestricted Stock Award Agreement to Employees Pursuant to the Helicos BioSciences Corporation Amended and Restated Management Incentive Plan, as amended, and the Helicos BioSciences Corporation 2nd Half Fiscal 2009 Employee Incentive Bonus Plan (Incorporated by reference herein to Exhibit 10.43 to the Company's Annual Report on Form 10-K, filed with the Securities and Exchange Commission on April 15, 2010). |
| | 10.44 | | Letter Agreement, dated February 11, 2010, by and between Stephen J. Lombardi and Helicos BioSciences Corporation (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 8-K, filed with the Securities and Exchange Commission on February 12, 2010). |
| | 10.45 | | Sixth Amendment to Lease by and between the Company and RB Kendall Fee, LLC dated June 9, 2010 (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on June 14, 2010). |
| | 10.46+ | | Form of Retention Agreement with Management dated August 6, 2010 (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on November 18, 2010). |
| | 10.47 | | Letter Agreement, dated September 8, 2010, by and between, J. William Efcavitch and Helicos BioSciences Corporation (Incorporated by reference herein to Exhibit 10.2 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on November 18, 2010). |
| | 10.48 | | Subordinated Secured Note Purchase Agreement, dated November 16, 2010, between the Company and each of the Purchasers identified therein (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on November 19, 2010). |
| | 10.49 | | Risk Premium Payment Agreement, dated November 16, 2010, between the Company and each of the Purchasers identified therein (Incorporated by reference herein to Exhibit 10.2 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on November 19, 2010). |
| | 10.50 | | Waiver, Consent and Fourth Amendment to the Loan and Security Agreement, dated November 16, 2010, among the Company, the Lenders and General Electric Capital Corporation (Incorporated by reference herein to Exhibit 10.3 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on November 19, 2010). |
| | 10.51† | | First Amendment to the License Agreement dated August 26, 2011, between the Company and Arizona Technologies Enterprises (Incorporated by reference herein to Exhibit 10.1 to the Company's Form 10-Q, filed with the Securities and Exchange Commission on November 14, 2011). |
| | 10.52 | | Seventh Amendment to Lease, by and between the Company and RB Kendall Fee, LLC dated September 20, 2011 (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on September 22, 2011). |
| | 10.53 | | First Amendment to Subordinated Secured Note Purchase Agreement, dated as of November 30, 2011, between the Company and each of the Purchasers identified therein (Incorporated by reference herein to Exhibit 10.1 to the Company's Current Report on Form 8-K, filed with the Securities and Exchange Commission on December 1, 2011). |
146
Table of Contents
| | | |
Exhibit
Number | | Description of Document |
|---|
| | 21.1 | | Subsidiary of the Registrant (Incorporated by reference herein to Exhibit 21.1 to the Company's Registration Statement on Form S-1 (File No. 333-140973) which became effective on May 24, 2007). |
| | 23.1* | | Consent of PricewaterhouseCoopers LLP, an independent registered public accounting firm. |
| | 31.1* | | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 31.2* | | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | 32.1* | | Certifications pursuant to Section 320 of the Sarbanes-Oxley Act of 2002. |
| | 101 | | The following financial statements from the Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2011 are furnished herewith, formatted in XBRL (eXtensible Business Reporting Language): (i) the Consolidated Statements of Operations for the years ended December 31, 2011, 2010, and 2009, (ii) the Consolidated Balance Sheets at December 31, 2011 and December 31, 2010, (iii) the Consolidated Statement of Equity for the years ended December 31, 2011, 2010, and 2009, (iv) the Consolidated Statements of Cash Flows for the years ended December 31, 2011, 2010, and 2009, and (v) the Notes to the Consolidated Financial Statements. |
- *
- Filed herewith
- †
- Confidential treatment has been requested for certain provisions of this Exhibit pursuant to Rule 406 promulgated under the Securities Act.
- +
- Indicates a management contract or any compensatory plan, contract or arrangement.
147