- CLRB Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
FWP Filing
Cellectar Biosciences (CLRB) FWPFree writing prospectus
Filed: 4 Aug 14, 12:00am
Issuer Free Writing Prospectus
Filed Pursuant to Rule 433
Registration Statement No. 333-196091
August 4, 2014

1 Cellectar Biosciences July 2014

2 2 Safe Harbor Statement This slide presentation contains forward - looking statements . Such statements are valid only as of today, and we disclaim any obligation to update this information . These statements are only estimates and predictions and are subject to known and unknown risks and uncertainties that may cause actual future experience and results to differ materially from the statements made . These statements are based on our current beliefs and expectations as to such future outcomes . Drug discovery and development involve a high degree of risk . Factors that might cause such a material difference include, among others, uncertainties related to the ability to raise additional capital required to complete the development programs described herein, the ability to attract and retain partners for our technologies, the identification of lead compounds, the successful preclinical development thereof, the completion of clinical trials, the FDA review process and other government regulation, our pharmaceutical collaborators’ ability to successfully develop and commercialize drug candidates, competition from other pharmaceutical companies, product pricing and third - party reimbursement . A complete description of risks and uncertainties related to our business is contained in our periodic reports filed with the Securities and Exchange Commission including our Form 10 - K for the year ended December 31 , 2013 . These forward looking statements are made only as of the date hereof, and we disclaim any obligation to update any such forward looking statements .

3 3 The issuer has filed a registration statement (including a prospectus) with the SEC for the offering to which this communication relates . Before you invest, you should read the prospectus in that registration statement and other documents the issuer has filed with the SEC for more complete information about the issuer and this offering . You may get these documents for free by visiting EDGAR on the SEC Web site at www . sec . gov . Alternatively, the issuer, any underwriter or any dealer participating in the offering will arrange to send you the prospectus if you request it by calling 1 - 212 - 813 - 1010 .
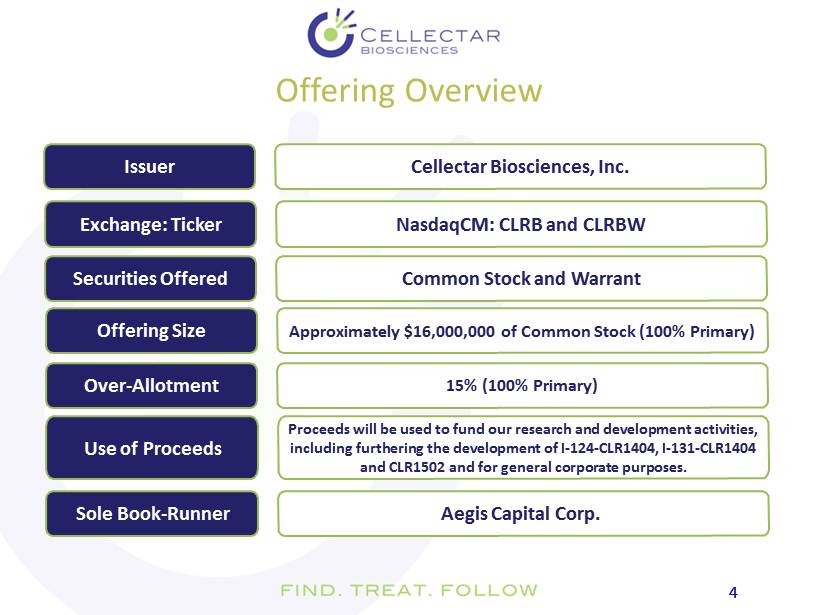
4 4 Offering Overview Issuer Cellectar Biosciences, Inc. Exchange: Ticker NasdaqCM : CLRB and CLRBW Offering Size Approximately $16,000,000 of Common Stock (100% Primary) Use of Proceeds Proceeds will be used to fund our research and development activities, including furthering the development of I - 124 - CLR1404, I - 131 - CLR1404 and CLR1502 and for general corporate purposes. Over - Allotment 15% (100% Primary) Sole Book - Runner Aegis Capital Corp. Securities Offered Common Stock and Warrant

5 5 Overview • Leveraging highly selective delivery and retention platform to develop novel agents to detect, treat and monitor a broad spectrum of cancers • Cellectar’s Phospholipid Ether ( PLE) Analog technology is based on extensive research by technology founders and independent academic research facilities • Over 25 PLEs evaluated to create optimal delivery vehicle compound • New leadership and refined d evelopment s trategy • CEO, CFO and executive leadership changes • Restructured Board • Prioritize registration enabling studies • Focus on near - term, cost - sensitive platform validating programs • Seek partnership opportunities to advance pipeline while optimizing internal resources

6 6 Management Team • Executive Team: • Simon Pedder, PhD : President, CEO and Director • Chelsea Therapeutics - CEO; Hoffmann - La Roche - Director of International Clinical Science, Director of International Clinical Operations, Global Project Leader of Pharmaceutical Development, Life Cycle Leader, PEGASYS/IFN and Head of Hepatitis Franchise, Pharma Business, and Vice President of Pharma Business Oncology • Jamey Weichert, PhD : Technology Founder, Chief Scientific Officer and Director • University of Wisconsin Associate Professor of the Departments of Radiology, Medical Physics, Pharmaceutics and member of the Comprehensive Cancer Center • Chad Kolean : Chief Financial Officer • Pioneer Surgical Technology - CFO; TomoTherapy – corporate controller • Kevin Kozak , MD, PhD : Chief Medical Officer • Mercy Regional Cancer Center - Director of Radiation Oncology • Board of Directors • Stephen Hill, Chairman: CEO, Targacep t ; Formerly: CEO, Solvay Pharmaceuticals ; President & CEO, ArQule ; Head of Global Drug Development, Hoffmann - La Roche • John Neis: Managing Director, Venture Investors • Paul Berns : President & CEO , Anacor Pharmaceuticals ; Formerly: CEO , Allos Therapeutics ; CEO, Bone Care International • Simon Pedder • Jamey Weichert

7 7 Overview • Three Key Clinical Data Milestones By Year - end 2015 • H1 15: Phase II Imaging trial of I - 124 - CLR1404 in glioblastoma • YE 15 : PI/II trial of I - 131 - CLR1404 in multiple myeloma • YE: 15 PI POC trial of CLR1502 in breast cancer surgery • Established In - house Manufacturing C apabilities • Attractive Partnership Opportunities • Global/Regional licensing opportunities • Therapeutic partnership opportunities • Multiple tumor indications • Collaborations around PLE platform (payload players, life cycle management) and CLR1502 (optical imaging devices) to build the body of evidence on platform • Over $2.5 million in grants supporting research with Cellectar technology

8 8 Near Universal Cancer - Targeting Platform I - 124 - CLR1404 Potential Future Products Other imaging and therapeutic payloads Therapy Products in Development Optical imaging PET imaging Proprietary Phospholipid Ether (PLE) Analog Cancer - Targeting Vehicle Payload Cancer - Targeted Payload CLR1502 IR - 775 I - 131 - CLR1404 131 I * 124 I
9 9 Phospholipid Ether (PLE) Analogs • Five Unique Attributes: x Selectively taken up by cancer cells including cancer stem cells regardless of anatomic location within the body x Prolonged retention in cancer cells including cancer stem cells x Broad spectrum of cancers - selectively retained by >60 xenograft, orthotopic , and transgenic cancer and cancer stem cell derived models examined to date x Imaging and therapeutic agents can be attached x Diapeutic: Imaging predicts therapeutic delivery
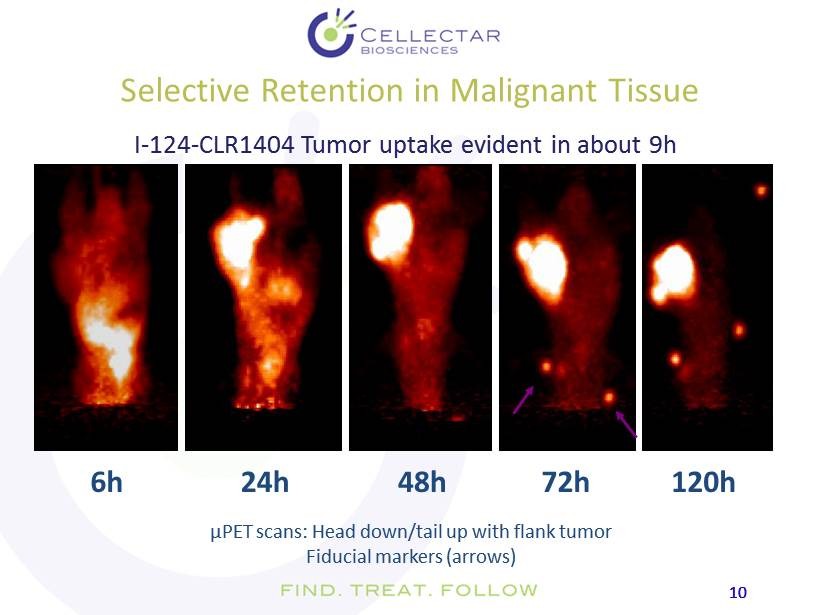
10 10 Selective Retention in Malignant Tissue µ PET scans: Head down/tail up with flank tumor Fiducial markers (arrows) 6h 24h 48h 72h 120h I - 124 - CLR1404 Tumor uptake evident in about 9h
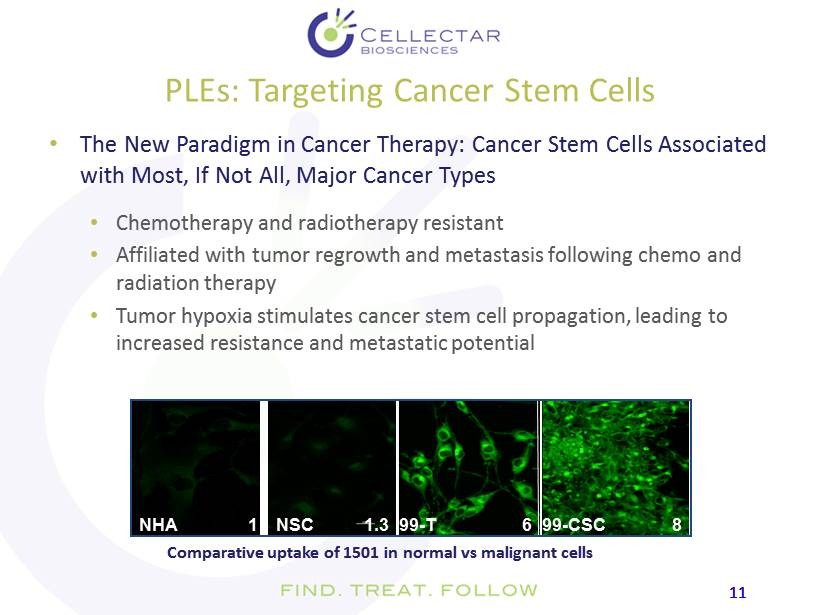
11 11 PLEs: Targeting Cancer Stem Cell s • The New Paradigm in Cancer Therapy: Cancer Stem Cells Associated with Most, If Not All, Major Cancer Types • Chemotherapy and radiotherapy resistant • Affiliated with tumor regrowth and metastasis following chemo and radiation therapy • Tumor hypoxia stimulates cancer stem cell propagation, leading to increased resistance and metastatic potential NSC NHA 99 - T 99 - CSC 1 8 6 1.3 Comparative uptake of 1501 in normal vs malignant cells
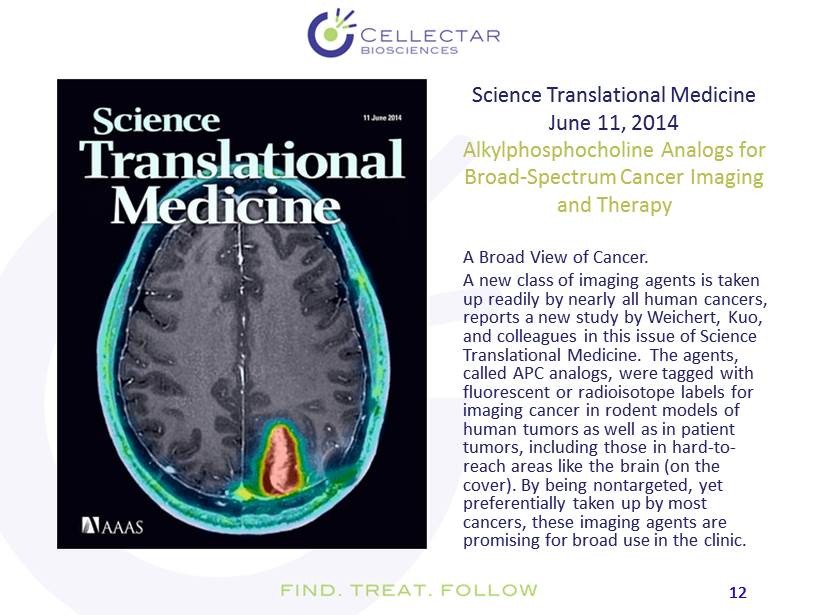
12 12 A Broad View of Cancer. A new class of imaging agents is taken up readily by nearly all human cancers, reports a new study by Weichert, Kuo, and colleagues in this issue of Science Translational Medicine. The agents, called APC analogs, were tagged with fluorescent or radioisotope labels for imaging cancer in rodent models of human tumors as well as in patient tumors, including those in hard - to - reach areas like the brain (on the cover). By being nontargeted , yet preferentially taken up by most cancers, these imaging agents are promising for broad use in the clinic. Science Translational Medicine June 11, 2014 Alkylphosphocholine Analogs for Broad - Spectrum Cancer Imaging and Therapy

13 13 In - House Manufacturing Capabilities • Our investment in manufacturing potentially allows complete control through clinical trials and registration • GMP manufacturing fully operational • Reliability for dosimetry dose demonstrated • Exceptionally high purity and radiochemical purity • Meets FDA standards for clinical trials • Easy , fast and inexpensive distribution • D oes NOT need extensive radiopharmacy formulation • Shipped as a patient ready product only requiring individual dose calibration • Can be shipped fully stable overnight at ambient temperature • Working toward bringing all elements of I - 124 - CLR1404 and CLR1502 manufacturing for I - 124 - CLR1404 and CLR1502
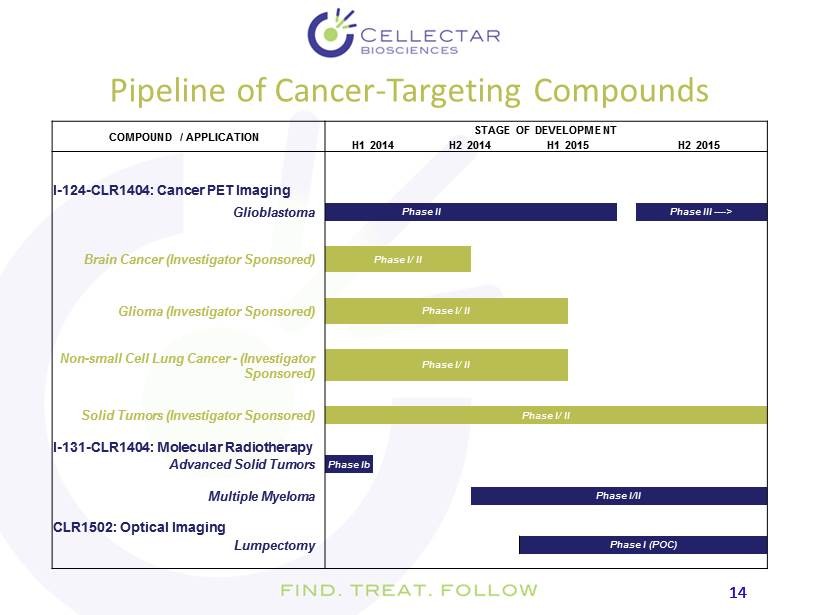
14 14 COMPOUND / APPLICATION STAGE OF DEVELOPMENT H1 2014 H2 2014 H1 2015 H2 2015 I - 124 - CLR1404: Cancer PET Imaging Glioblastoma Phase II Phase III ---- > Brain Cancer (Investigator Sponsored) Phase I/ II Glioma (Investigator Sponsored) Phase I/ II Non - small Cell Lung Cancer - (Investigator Sponsored) Phase I/ II Solid Tumors (Investigator Sponsored) Phase I/ II I - 131 - CLR1404: Molecular Radiotherapy Advanced Solid Tumors Phase Ib Multiple Myeloma Phase I/II CLR1502: Optical Imaging Lumpectomy Phase I (POC) Pipeline of Cancer - Targeting Compounds
15 15 PLE + PET Imaging Isotope = I - 124 - CLR1404 More Accurate Tumor Imaging for Better Patient Management

16 16 I - 124 - CLR1404: PLE + PET Imaging Isotope • Small - molecule imaging agent for primary tumors and metastases • Proprietary PLE attached to Iodine - 124 PET imaging isotope • Robust series of i nvestigator s ponsored Phase I/II trials underway at UW Carbone Cancer Center • Lung Cancer (Traynor/Perlman) • Glioma/ Brain tumors or Mets (Hall) • Multiple Tumor Protocol ( Liu) • Prostate, Pancreas, Breast, Head and Neck and Others • Initial proof - of - concept in brain cancer • Orphan d esignation as diagnostic for management of glioma

17 17 Initial Target: Glioblastoma • MRI is not able to reliably distinguish recurrent disease from pseudo - progression or radiation necrosis • MRI shows early post - treatment changes in ~50 % of glioblastoma patients • Nearly half of the time, early changes are non - malignant pseudo - progression • Radiation necrosis seen in a variety of clinical settings • Significant patient benefit derived from earlier and definitive diagnosis of tumor progression • Avoid unnecessary therapies and interventions • Avoid discontinuation of effective therapy • Detect progression earlier, minimize critical tissue infiltration
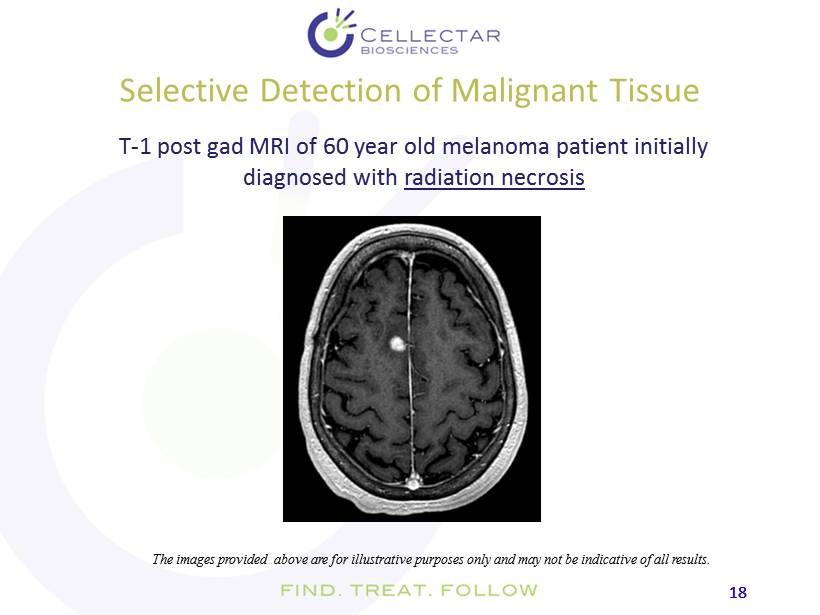
18 18 Selective Detection of Malignant Tissue T - 1 post gad MRI of 60 year old melanoma patient initially diagnosed with radiation necrosis The images provided above are for illustrative purposes only and may not be indicative of all results.
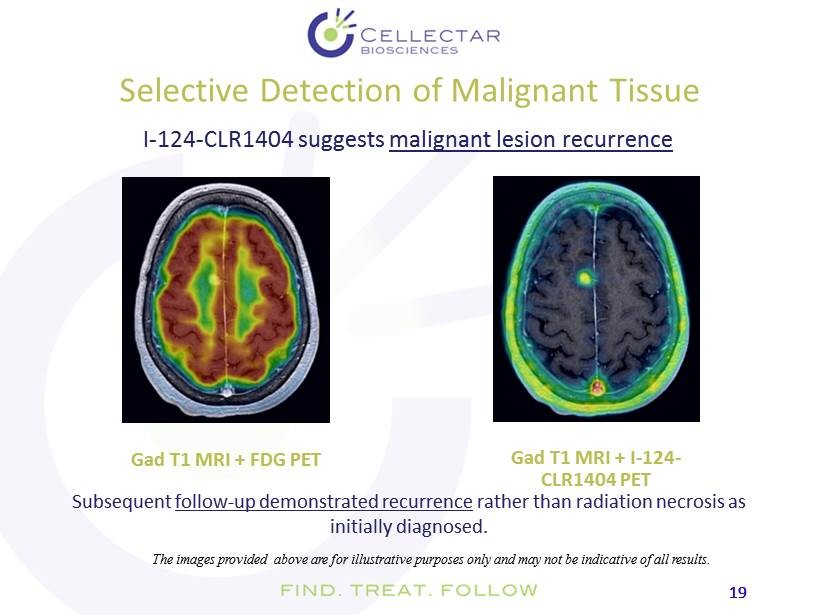
19 19 Selective Detection of Malignant Tissue Gad T1 MRI + FDG PET I - 124 - CLR1404 suggests malignant lesion recurrence The images provided above are for illustrative purposes only and may not be indicative of all results. Subsequent follow - up demonstrated recurrence rather than radiation necrosis as initially diagnosed. Gad T1 MRI + I - 124 - CLR1404 PET
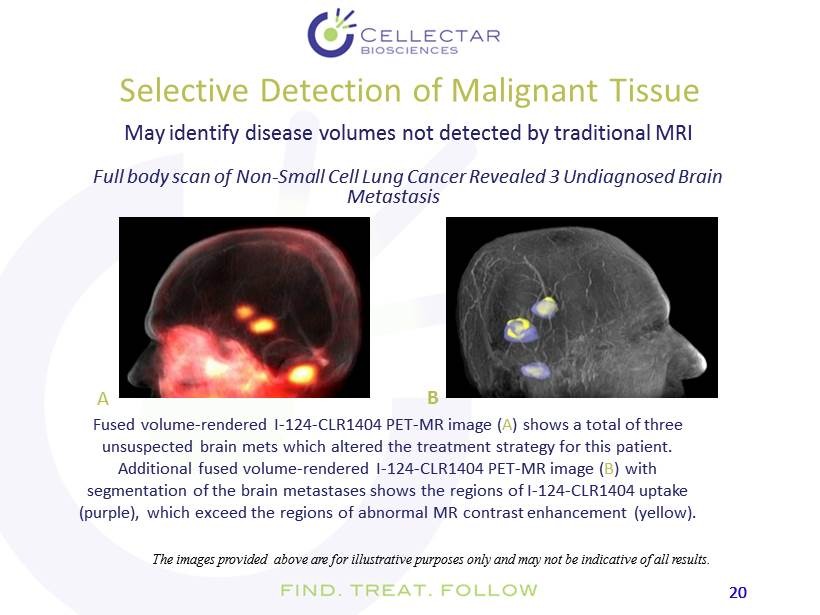
20 20 Selective Detection of Malignant Tissue May identify disease volumes not detected by traditional MRI Full body scan of Non - Small Cell Lung Cancer Revealed 3 Undiagnosed Brain Metastasis The images provided above are for illustrative purposes only and may not be indicative of all results. Fused volume - rendered I - 124 - CLR1404 PET - MR image ( A ) shows a total of three unsuspected brain mets which altered the treatment strategy for this patient. Additional fused volume - rendered I - 124 - CLR1404 PET - MR image ( B ) with segmentation of the brain metastases shows the regions of I - 124 - CLR1404 uptake (purple), which exceed the regions of abnormal MR contrast enhancement (yellow). A B
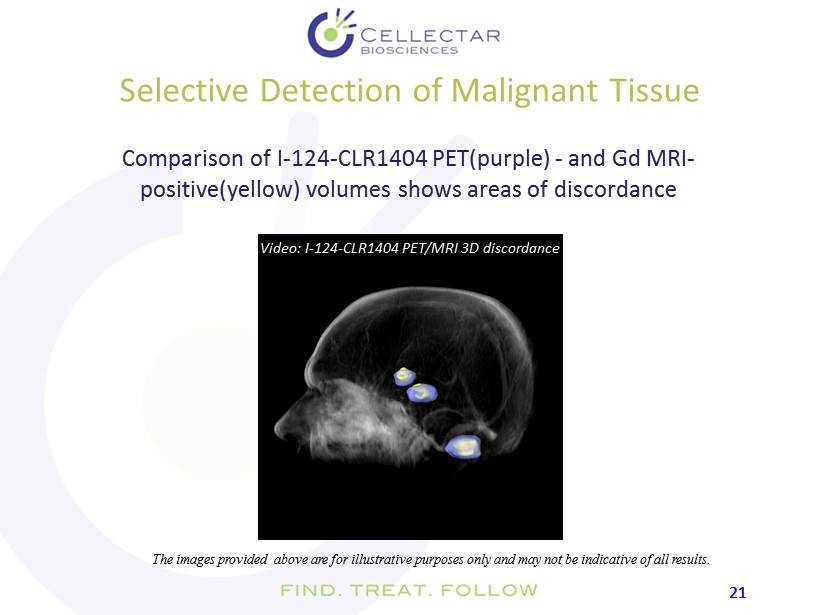
21 21 Selective Detection of Malignant Tissue Comparison of I - 124 - CLR1404 PET(purple) - and Gd MRI - positive(yellow) volumes shows areas of discordance The images provided above are for illustrative purposes only and may not be indicative of all results. Video: I - 124 - CLR1404 PET/MRI 3D discordance

22 22 Phase II Imaging Trial in Glioblastoma • Initiated Phase II trial March 2014 • Targeting 10 centers to enroll ~36 patients • Newly diagnosed or recurrent glioblastoma where SOC includes resection and/or biopsy • Compare performance of I - 124 - CLR1404 PET/CT to MRI with pathology confirmation • Approval based on more accurate determination of malignant vs. non - malignant tissue • Trial Objectives • Compare the efficacy of I - 124 - CLR1404 PET/CT imaging in detecting glioblastoma with standard of care MRI based on pathology confirmation • Confirm optimal dose and imaging time points for Phase III pivotal trial » Initiated: Q1 2014 » Data Anticipated: H1 2015

23 23 Scalable Commercial Opportunity for I - 124 - CLR1404 • Establish efficacy in primary glioma • Pursue additional solid tumors with initial positive images: • Prostate ~239k patients • Breast ~235k patients • Lung ~228k patients • Colorectal ~102k patients • Head & Neck ~54k patients • Pancreatic ~45k patients Metastatic Brain Tumors Lung Cancer Other Solid Tumors Core Indications Label Expansion Primary Brain/ Glioma Tumors

24 24 PLE + Radiotherapeutic = I - 131 - CLR1404 Better targeted delivery to cancer cells and cancer stem cells

25 25 Better Targeting=Better Therapeutic Delivery • Proprietary cancer - targeting delivery vehicle (PLE) attached to proven radiotherapeutic • Iodine - 131, well - established as a cancer therapeutic – FDA familiarity • Preclinical single - dose data demonstrates remarkable in vivo efficacy coupled with excellent safety profile • Phase Ib dose - escalation trial: Data presented at ASCO 2014 • Evidence of anti - tumor activity with 4 patients with stable disease • Evidence of sustained uptake/retention in tumors demonstrated by SPECT imaging • Demonstrated uptake and prolonged retention • O nly in tumors not normal tissue
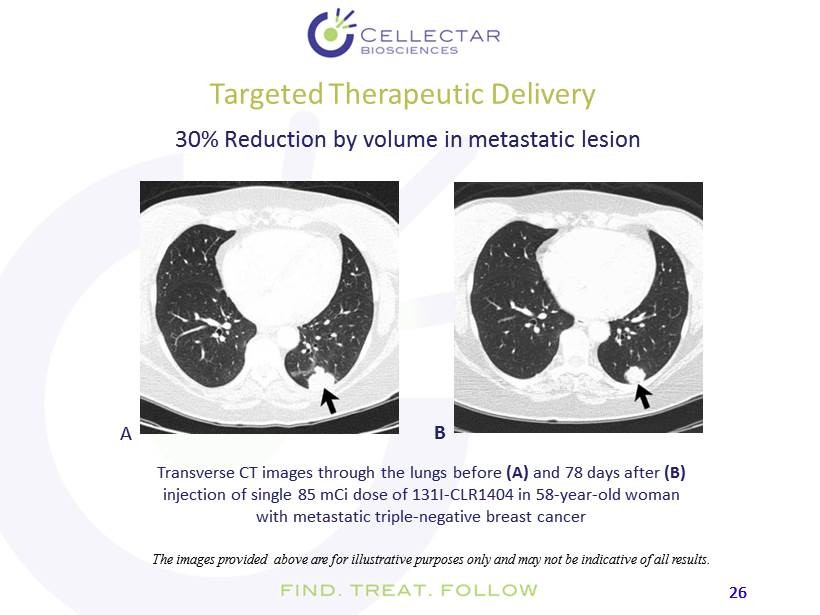
26 26 Targeted Therapeutic Delivery Transverse CT images through the lungs before (A) and 78 days after (B) injection of single 85 mCi dose of 131I - CLR1404 in 58 - year - old woman with metastatic triple - negative breast cancer A B 30% Reduction by volume in metastatic lesion The images provided above are for illustrative purposes only and may not be indicative of all results.

27 27 Multiple Myeloma • Attractive initial indication • Radiosensitivity • Uptake • Novel mechanism of action • Quantitative non - RECIST response criteria • Clear Go/No Go criteria; low cost, fast path to approval • Market Opportunity • High un - met need • Not cost - sensitive • Regulatory opportunities • Orphan drug designation • Accelerated approval • Breakthrough therapy and fast t rack designations • Foundation for additional opportunities/expanded indications

28 28 PLE + Near - Infrared Fluorophore = CLR1502 Intraoperative Optical Imaging for Better Outcomes
29 29 CLR1502: Improving Surgical Outcomes • Optical imaging agent for intraoperative tumor margin illumination • Proprietary cancer - targeting delivery vehicle (PLE) attached to near - infrared fluorophore • More accurate visualization of tumor margins during surgery for more complete malignant tissue removal • Better patient management and outcomes from fewer repeat surgeries and reduced recurrence • Potential for meaningful healthcare savings
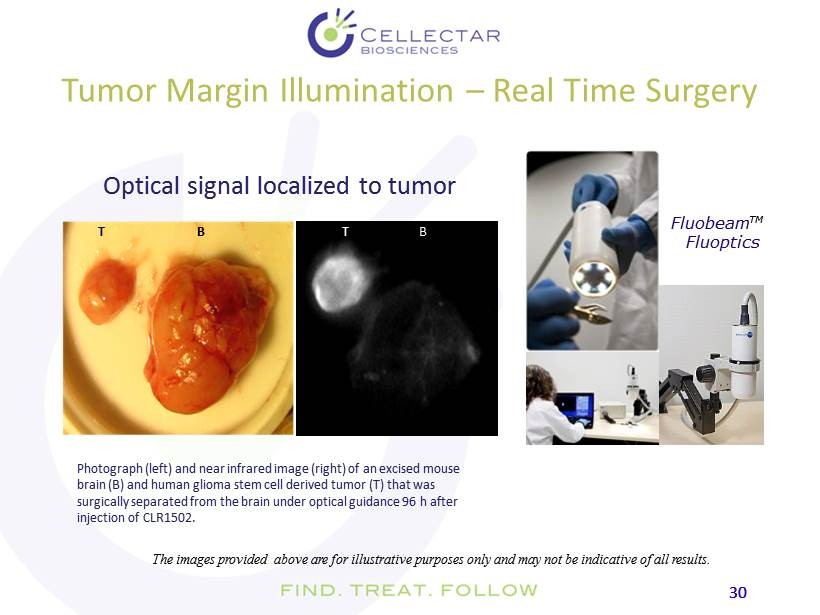
30 30 Tumor Margin Illumination – Real Time Surgery Fluobeam TM ) Fluoptics The images provided above are for illustrative purposes only and may not be indicative of all results. T T B B Photograph (left) and near infrared image (right) of an excised mouse brain (B) and human glioma stem cell derived tumor (T) that was surgically separated from the brain under optical guidance 96 h after injection of CLR1502. O ptical signal localized to tumor
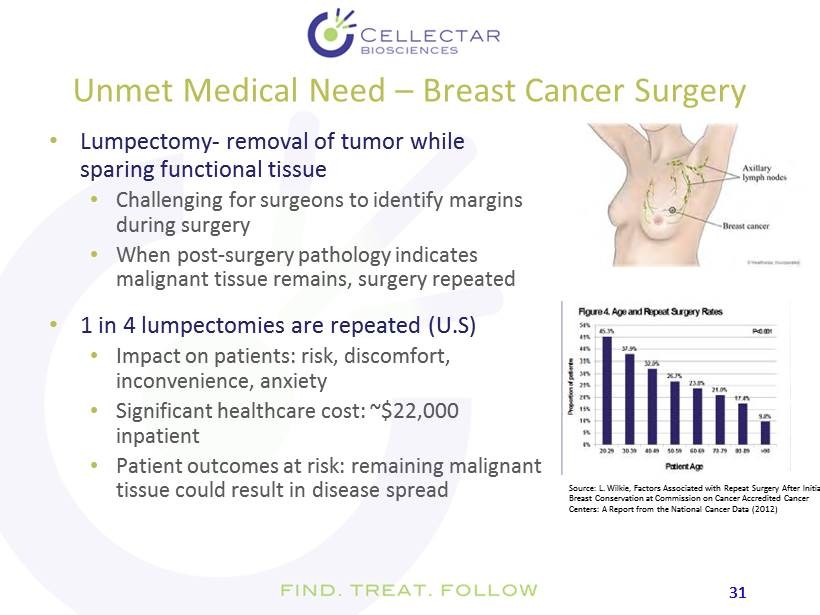
31 31 Unmet Medical Need – Breast Cancer Surgery • Lumpectomy - removal of tumor while sparing functional tissue • Challenging for surgeons to identify margins during surgery • When post - surgery pathology indicates malignant tissue remains, surgery repeated • 1 in 4 lumpectomies are repeated (U.S ) • Impact on patients: risk, discomfort, inconvenience, anxiety • Significant healthcare cost: ~$22,000 inpatient • Patient outcomes at risk: remaining malignant tissue could result in disease spread Source: L. Wilkie , Factors Associated with Repeat Surgery After Initial Breast Conservation at Commission on Cancer Accredited Cancer Centers: A Report from the National Cancer Data (2012)

32 32 CLR1502 Phase I: Breast Cancer Surgery • Seeking US IND Q4 2014 • Trial Initiation 2015 • Expect data by year - end 2015 • Trial design • Dose - ranging multi - site, ~20 patients undergoing lumpectomy • Compatible with standard of care (SOC) – only addition is CLR1502 administration and non - invasive optical imaging • Primary tumor and sentinel node resected according to SOC • Optical imaging used to assess CLR1502 illumination of any remaining tumor margin or nodal involvement • Trial objective • Safety • Determine image sensitive dose level • E stablish sensitivity and specificity of CLR1502 in the identification of malignant tissue

33 33 Summary Financial Outlook • $1.6 M cash at June 30, 2014 Capitalization Common Stock Outstanding 2,869,739 Warrants (exercisable: $10.00 - $25.00) 1,564,085 Options 619,664 Convertible Debentures Shares ($10.00) 400,000 Warrants ($20.00) 400,000 Fully Diluted 5,853,488 Authorized 20,000,000

34 34 Upcoming Milestones • Q3 14: File IND for PI/II trial of I - 131 - CLR1404 in multiple myeloma • Q4 14: Seek orphan designation for I - 131 - CLR1404 in multiple myeloma • Q4 14: File IND for PI POC trial of CLR1502 in breast cancer surgery • Q4 14: Initiate PI/II trial of I - 131 - CLR1404 in multiple myeloma • H1 15: Results of Phase II Imaging trial of I - 124 - CLR1404 in glioblastoma • H1 15: Initiate PI POC trial of CLR1502 in breast cancer surgery • YE 15 : Results of I/II trial of I - 131 - CLR1404 in multiple myeloma • YE 15 : Results of PI POC trial of CLR1502 in breast cancer surgery • Q4 15 : Initiate Phase III Imaging trial of I - 124 - CLR104 in glioblastoma

35 35 Appendix
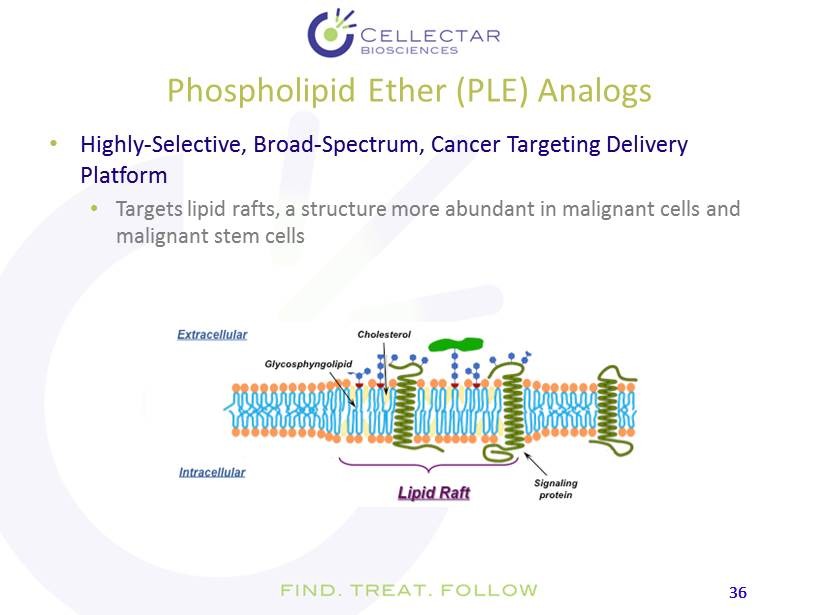
36 36 Phospholipid Ether (PLE) Analogs • Highly - Selective, Broad - Spectrum, Cancer Targeting Delivery Platform • Targets lipid rafts, a structure more abundant in malignant cells and malignant stem cells

37 37 Global Nuclear Medicine Market • P rojected to grow an average of 11% annually and reach $24 billion in 2030 • Diagnostic radiopharmaceuticals market: 5 % growth per year • Therapeutic radiopharmaceuticals: annual 30 % growth from 2014 to 2030 • Key growth driver is the aging demographic creating demand for nuclear medicine procedures • Logistical issues surrounding radiopharmaceuticals with a very short half - life continue to be one of the greatest challenges in the industry Opportunities in Nuclear Medicine, MEDraysintell , March 2014
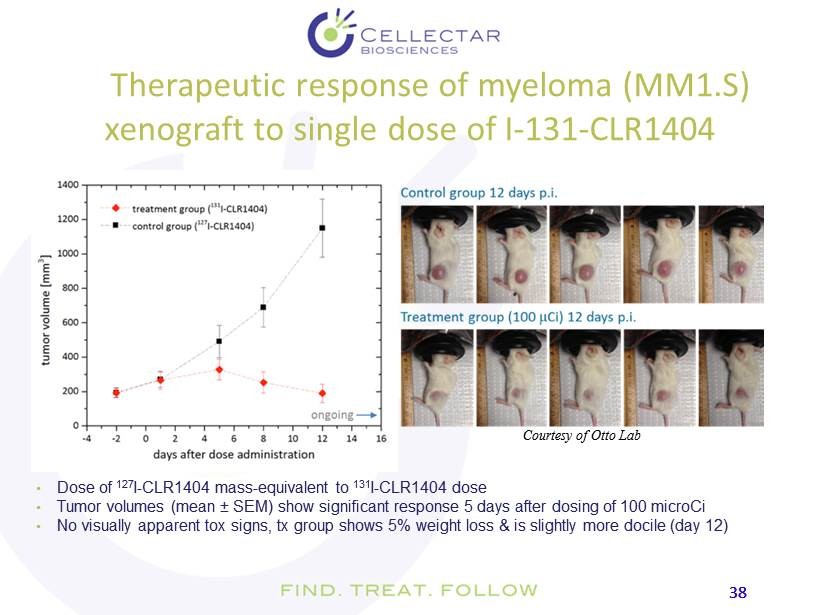
38 38 Therapeutic response of myeloma (MM1.S) xenograft to single dose of I - 131 - CLR1404 • Dose of 127 I - CLR1404 mass - equivalent to 131 I - CLR1404 dose • T umor volumes (mean ± SEM) show significant response 5 days after dosing of 100 microCi • No visually apparent tox signs, tx group shows 5% weight loss & is slightly more docile (day 12) Courtesy of Otto Lab

39 39 Building Robust Intellectual Property • PLE Platform Patents/Applications: • Different forms of phospholipid ethers • M ethods of manufacturing of phospholipid ethers • Freedom to Operate Analysis • Recently completed FTO analysis for I - 124 - CLR1404, I - 131 - CLR1404 and CLR1502 and found no FTO issues • I - 124 - CLR1404 • Orphan drug designation as diagnostic for the management of glioma: 7 years exclusivity from US approval in this indication • Composition of matter: 2016 (University of Michigan) • 3 additional method of use U.S. Patents: 2025 • Treating certain cancers • Virtual Colonoscopy • In vitro diagnostics • Pending U.S., Japanese and European patent applications: • In vivo diagnostics: 2025 • Cancer stem cell diagnostics: 2030
40 40 Building Robust Intellectual Property • I - 131 - CLR1404 • Plan to request orphan designation for multiple myeloma: 7 years exclusivity from US approval in this indication • Composition of matter: 2016 (University of Michigan) • Method of use: • Cancer therapy (1 issued, 1 pending US patents; Pending in Europe and Japan): 2025 • Stem cell therapy (pending US, Europe and Japan): 2030 • CLR1502 • Composition of matter, U.S., EU, Japan pending (expiry 2029+) • Methods of use, U.S., EU, Japan pending (expiry 2029+) • Methods of manufacture, U.S., EU, Japan pending (expiry 2029+ ) • Patent applications directed to the compound, methods of use and method of manufacture that have been filed in U.S., Europe and Japan