
Anders Cervin1, Joanne Rimmer2, Agnieszka Wrobel3, Yogen Abelak4, Lindsay Brayton5, Yina Kuang5 1University of Queensland Centre for Clinical Research, Royal Brisbane & Women’s Hospital Campus, Herston, QLD, Australia; 2Monash Health and Department of Surgery, Monash University, Melbourne, Australia; 3Centrum Medyczne ALL-MED, Krakow, Poland; 4Vistamed Sp. z o.o., Wroclaw, Poland; 5Lyra Therapeutics, Inc., Watertown, MA, USA. Presented by Robert C. Kern, MD Professor and Chairman, Department of Otolaryngology – Head & Neck Surgery Feinberg School of Medicine, Northwestern University Long-acting implantable corticosteroid matrix for chronic rhinosinusitis: Results of LANTERN Phase 2 randomized controlled study Includes data presented at the Combined Otolaryngology Spring Meetings (COSM) on April 11, 2021 Exhibit 99.1

DISCLAIMER This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including statements regarding the company’s lead product candidate LYR-210, the results relating to the Company’s Phase 2 LANTERN clinical trial for LYR-210 and the Company’s plans to initiate a pivotal Phase 3 study for LYR-210 in CRS for both non-polyp and polyp patients. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause the company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the fact that the company has incurred significant losses since inception and expects to incur losses for the foreseeable future; the company’s need for additional funding, which may not be available; the company’s limited operating history; the fact that the company has no approved products; the fact that the company’s product candidates are in various stages of development; the fact that the company may not be successful in its efforts to identify and successfully commercialize its product candidates; the fact that clinical trials required for the company’s product candidates are expensive and time-consuming, and their outcome is uncertain; the fact that the FDA may not conclude that certain of the company’s product candidates satisfy the requirements for the Section 505(b)(2) regulatory approval pathway; the company’s inability to obtain required regulatory approvals; effects of recently enacted and future legislation; the possibility of system failures or security breaches; effects of significant competition; the fact that the successful commercialization of the company’s product candidates will depend in part on the extent to which governmental authorities and health insurers establish coverage, adequate reimbursement levels and pricing policies; failure to achieve market acceptance; product liability lawsuits; the fact that the company relies on third parties for the manufacture of materials for its research programs, pre-clinical studies and clinical trials; the company’s reliance on third parties to conduct its preclinical studies and clinical trials; the company’s inability to succeed in establishing and maintaining collaborative relationships; the company’s reliance on certain suppliers critical to its production; failure to obtain and maintain or adequately protect the company’s intellectual property rights; failure to retain key personnel or to recruit qualified personnel; difficulties in managing the company’s growth; effects of natural disasters; the fact that the global pandemic caused by COVID-19 could adversely impact the company’s business and operations, including the company’s clinical trials; the fact that the price of the company’s common stock may be volatile and fluctuate substantially; significant costs and required management time as a result of operating as a public company and any securities class action litigation. These and other important factors discussed under the caption “Risk Factors” in the company’s Annual Report on Form 10-K filed with the SEC on March 9, 2021 and its other filings with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. While the company may elect to update such forward-looking statements at some point in the future, it disclaims any obligation to do so, even if subsequent events cause its views to change. This presentation also includes statistical and market data that we obtained from industry [publications and research, surveys and studies conducted by third parties or us. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. All of the market data used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. While we believe these industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data. The industry in which we operate is subject to a high degree of uncertainty, change and risk due to a variety of factors, which could cause results to differ materially from those expressed in the estimates made by the independent partners and by us.

Topical steroids used to treat CRS are suboptimal1,2 Limited ability to reach inflammation deep within the sinonasal passages Rapid clearance rates Poor patient compliance 40-60% of CRS patients fail medical management and become candidates for functional endoscopic sinus surgery3 ~4 million CRS patients fail medical management annually in the U.S.4 ~400,000 CRS patients undergo FESS annually in the U.S.3 Novel therapeutic modalities are needed for CRS patients that fail medical management CHRONIC RHINOSINUSITIS (CRS) 1Moeller W et al. Rhinology. Dec 2009;47(4):405-12. 2Nabi S et al. J Otolaryngol Head Neck Surg. Apr 2012;41 Suppl 1:S49-55. 3Young LC et al. Allergy Rhinol (Providence). 2012;3(1):e8-e12. 4Baguley et al. Int Forum Allergy Rhinol, 2014;4(7):525-32
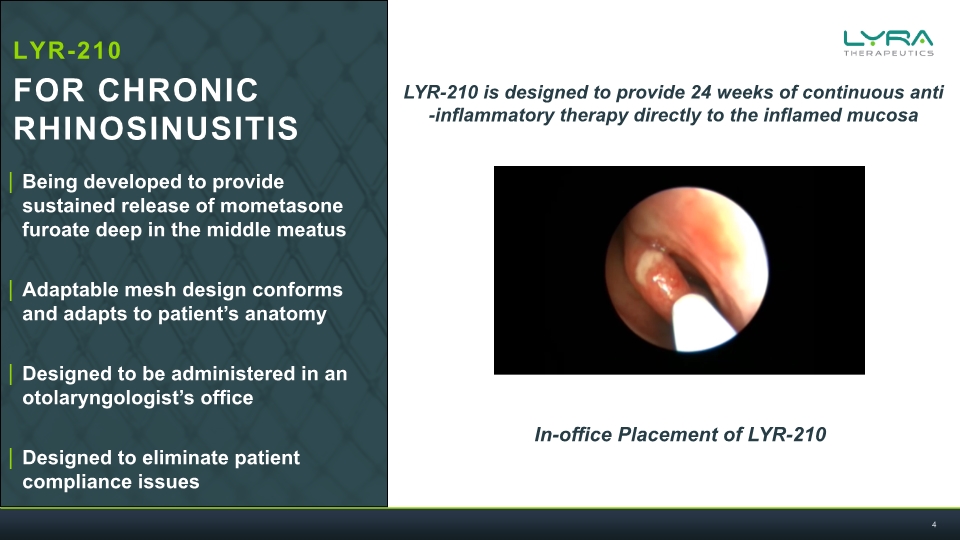
Being developed to provide sustained release of mometasone furoate deep in the middle meatus Adaptable mesh design conforms and adapts to patient’s anatomy Designed to be administered in an otolaryngologist’s office Designed to eliminate patient compliance issues LYR-210 is designed to provide 24 weeks of continuous anti-inflammatory therapy directly to the inflamed mucosa In-office Placement of LYR-210 LYR-210 FOR CHRONIC RHINOSINUSITIS
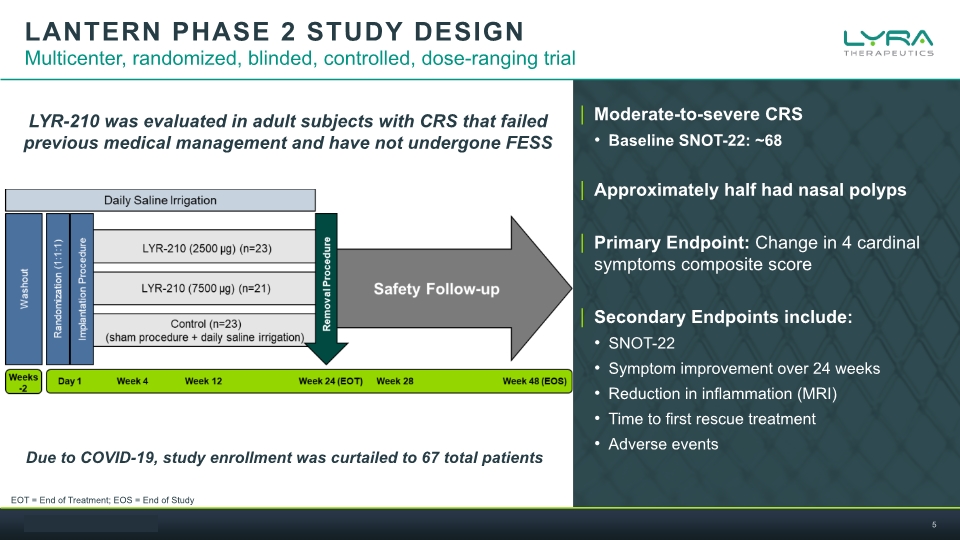
LANTERN PHASE 2 STUDY DESIGN Multicenter, randomized, blinded, controlled, dose-ranging trial Moderate-to-severe CRS Baseline SNOT-22: ~68 Approximately half had nasal polyps Primary Endpoint: Change in 4 cardinal symptoms composite score Secondary Endpoints include: SNOT-22 Symptom improvement over 24 weeks Reduction in inflammation (MRI) Time to first rescue treatment Adverse events LYR-210 was evaluated in adult subjects with CRS that failed previous medical management and have not undergone FESS Due to COVID-19, study enrollment was curtailed to 67 total patients EOT = End of Treatment; EOS = End of Study

Well-tolerated throughout the 24-week treatment period at both 2500µg and 7500µg doses No treatment-related SAEs Treatment-related AEs in Control and LYR-210 (7500µg) groups occurred at comparable rates Treatment-related AEs in more than 1 subject: Epistaxis: 3 subjects in LYR-210 (2500µg) group Rhinitis: 3 subjects in LYR-210 (7500µg) group Rhinorrhea: 2 subjects in LYR-210 (2500µg) group Headache: 2 subjects in Control group All treatment-related AEs were mild or moderate apart from 1 event in LYR-210 (2500µg) group: Increased viscosity of upper respiratory secretion LYR-210 LANTERN Phase 2 Study
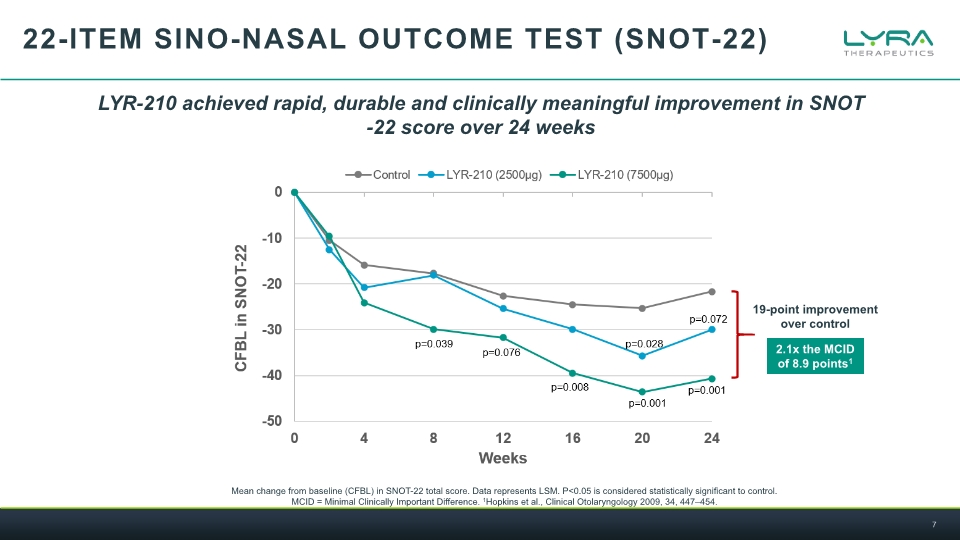
22-ITEM SINO-NASAL OUTCOME TEST (SNOT-22) Mean change from baseline (CFBL) in SNOT-22 total score. Data represents LSM. P<0.05 is considered statistically significant to control. MCID = Minimal Clinically Important Difference. 1Hopkins et al., Clinical Otolaryngology 2009, 34, 447–454. LYR-210 achieved rapid, durable and clinically meaningful improvement in SNOT-22 score over 24 weeks 19-point improvement over control 2.1x the MCID of 8.9 points1
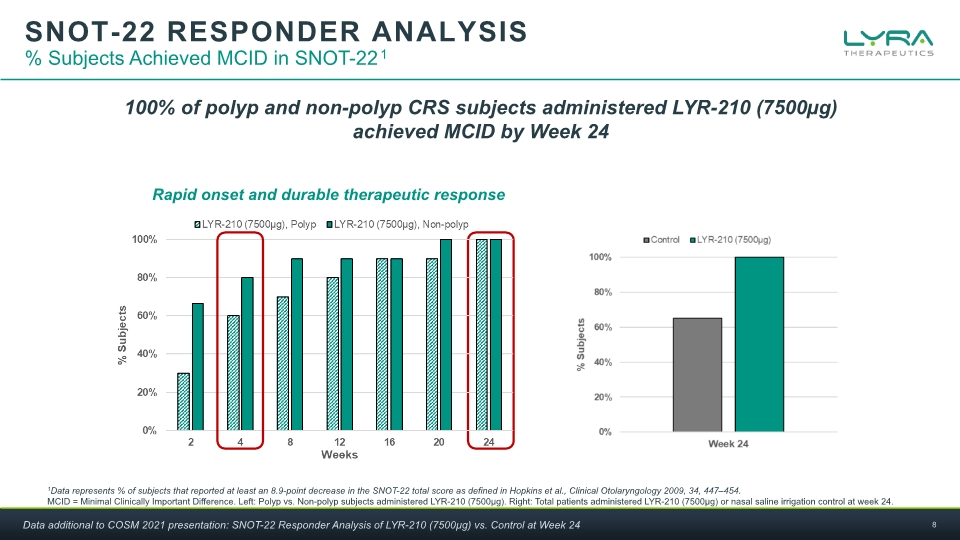
SNOT-22 RESPONDER ANALYSIS % Subjects Achieved MCID in SNOT-221 1Data represents % of subjects that reported at least an 8.9-point decrease in the SNOT-22 total score as defined in Hopkins et al., Clinical Otolaryngology 2009, 34, 447–454. MCID = Minimal Clinically Important Difference. Left: Polyp vs. Non-polyp subjects administered LYR-210 (7500µg). Right: Total patients administered LYR-210 (7500µg) or nasal saline irrigation control at week 24. Rapid onset and durable therapeutic response 100% of polyp and non-polyp CRS subjects administered LYR-210 (7500µg) achieved MCID by Week 24 Data additional to COSM 2021 presentation: SNOT-22 Responder Analysis of LYR-210 (7500µg) vs. Control at Week 24

COMPOSITE OF 4 CARDINAL SYMPTOMS (4CS) Nasal Blockage, Facial Pain/Pressure, Nasal Discharge, and Loss of Smell Mean change from baseline (CFBL) in the 7-day average score in the 4CS composite score (nasal blockage, facial pain/pressure, nasal discharge (anterior/posterior), and loss of smell). 4CS scale: 0-12. Data represents LSM. P<0.05 is considered statistically significant to control. LYR-210 (7500µg) achieved statistically significant improvement in the 4CS at 24 weeks and earlier compared to control

LYR-210 achieved dose-dependent improvement in the cardinal symptoms of CRS Mean change from baseline (CFBL) in the 7-day average score in nasal blockage, facial pain/pressure, or nasal discharge (anterior/posterior) for all patients. Data represents LSM. P<0.05 is considered statistically significant to control. Nasal Blockage Nasal Discharge Facial Pain/Pressure Cardinal symptoms of crs Data additional to COSM 2021 presentation
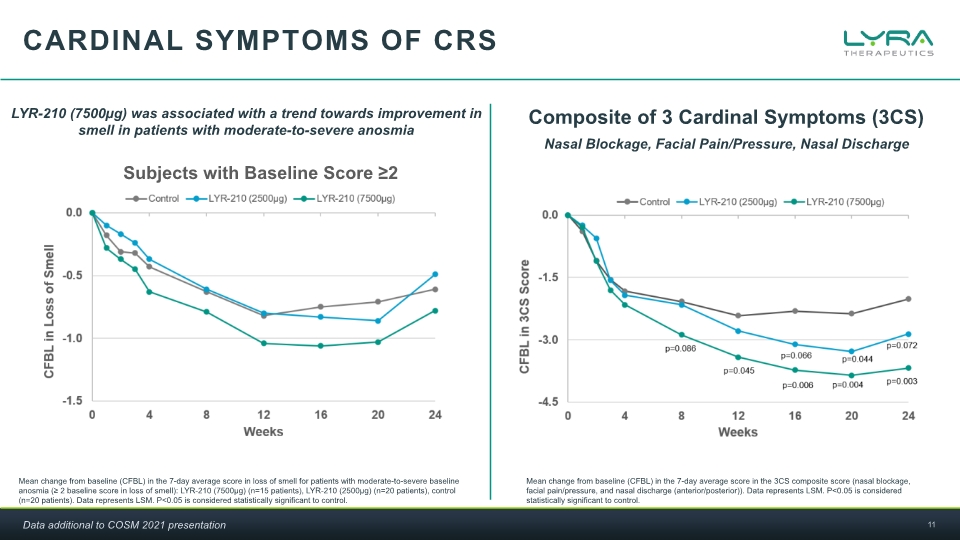
Cardinal symptoms of crs Mean change from baseline (CFBL) in the 7-day average score in loss of smell for patients with moderate-to-severe baseline anosmia (≥ 2 baseline score in loss of smell): LYR-210 (7500µg) (n=15 patients), LYR-210 (2500µg) (n=20 patients), control (n=20 patients). Data represents LSM. P<0.05 is considered statistically significant to control. LYR-210 (7500µg) was associated with a trend towards improvement in smell in patients with moderate-to-severe anosmia Composite of 3 Cardinal Symptoms (3CS) Mean change from baseline (CFBL) in the 7-day average score in the 3CS composite score (nasal blockage, facial pain/pressure, and nasal discharge (anterior/posterior)). Data represents LSM. P<0.05 is considered statistically significant to control. Nasal Blockage, Facial Pain/Pressure, Nasal Discharge Subjects with Baseline Score ≥2 Data additional to COSM 2021 presentation
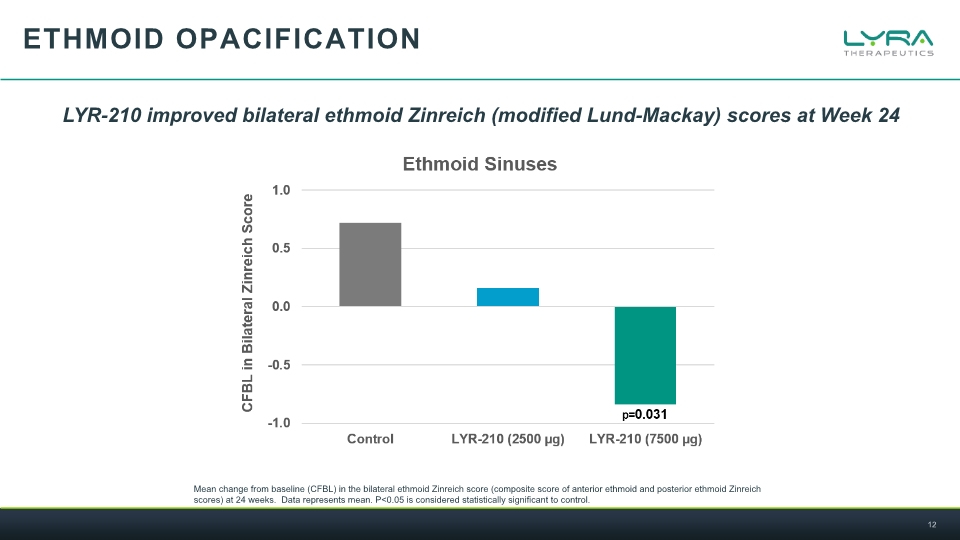
Ethmoid opacification Mean change from baseline (CFBL) in the bilateral ethmoid Zinreich score (composite score of anterior ethmoid and posterior ethmoid Zinreich scores) at 24 weeks. Data represents mean. P<0.05 is considered statistically significant to control. LYR-210 improved bilateral ethmoid Zinreich (modified Lund-Mackay) scores at Week 24
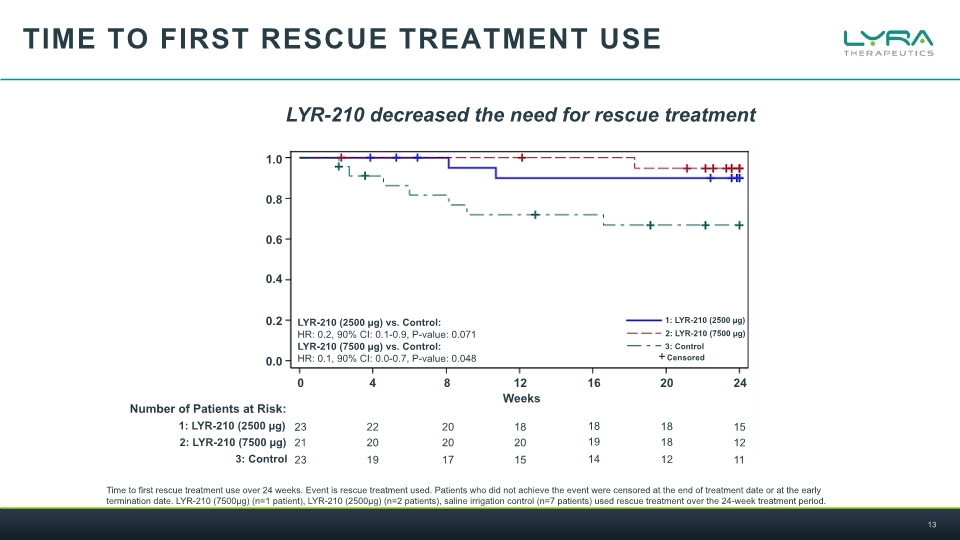
TIME TO First rescue treatment use Time to first rescue treatment use over 24 weeks. Event is rescue treatment used. Patients who did not achieve the event were censored at the end of treatment date or at the early termination date. LYR-210 (7500µg) (n=1 patient), LYR-210 (2500µg) (n=2 patients), saline irrigation control (n=7 patients) used rescue treatment over the 24-week treatment period. LYR-210 decreased the need for rescue treatment
LYR-210 achieved a rapid and durable dose-dependent improvement in the cardinal symptoms of CRS and SNOT-22 throughout 24 weeks in subjects independent of polyp status from a single administration LYR-210 decreased ethmoid opacification and need for rescue treatment LYR-210 provided up to 24 weeks of continuous local steroid treatment, eliminating issues of patient compliance in the clinical trial LYR-210 has the potential to represent a major step forward in the care for CRS patients who are facing surgery as their next treatment option Lyra Therapeutics, Inc. is advancing LYR-210 into a pivotal Phase 3 study CONCLUSIONS















