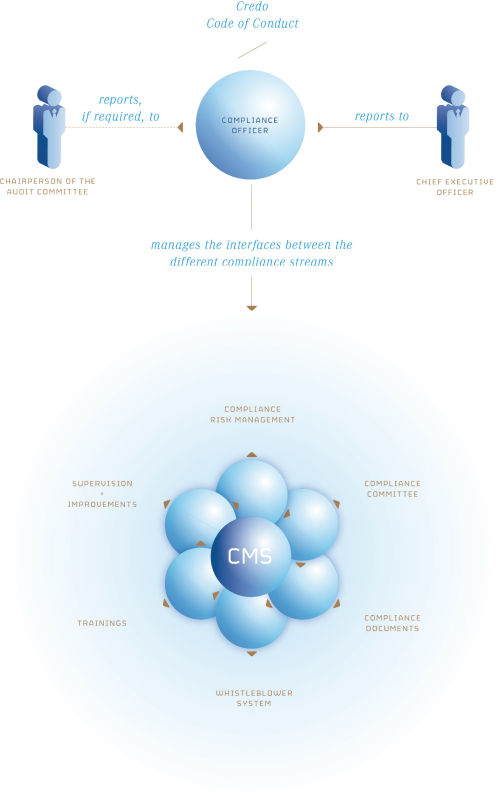
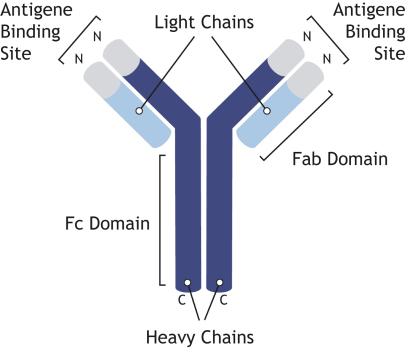
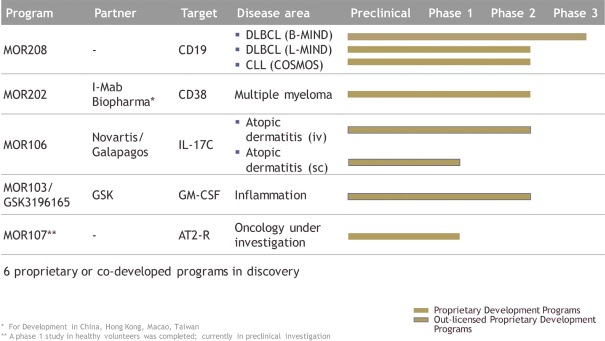
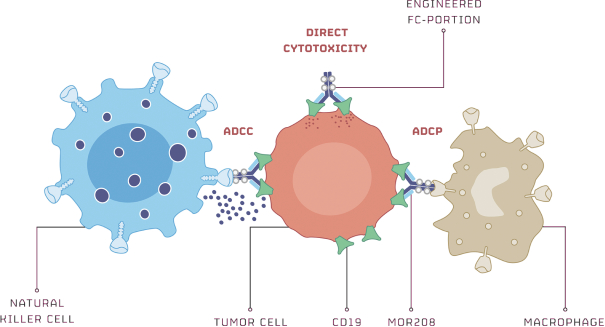
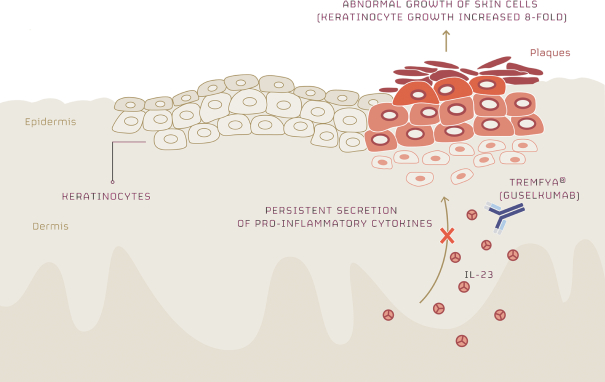
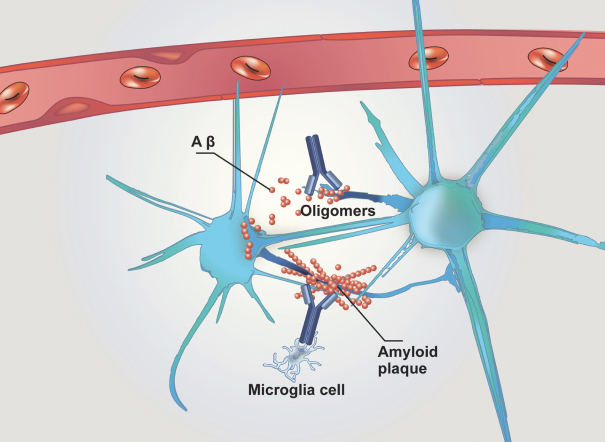
Any issued patents that we may receive or license in the future may be challenged, invalidated or circumvented. For example, we cannot be certain of the priority of our patents and patent applications over third-party patents and patent applications. In addition, because of the extensive time required for clinical development and regulatory review of a product candidate we may develop, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby limiting the protection such patent would afford the respective product and any competitive advantage such patent may provide. For more information regarding the risks related to our intellectual property, please see “Risk factors—Risks related to intellectual property”.
HUCAL
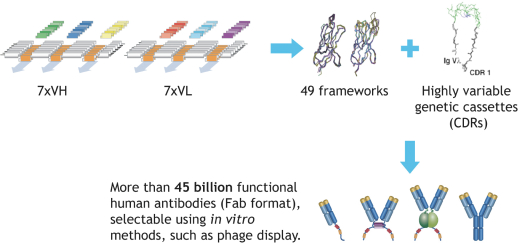
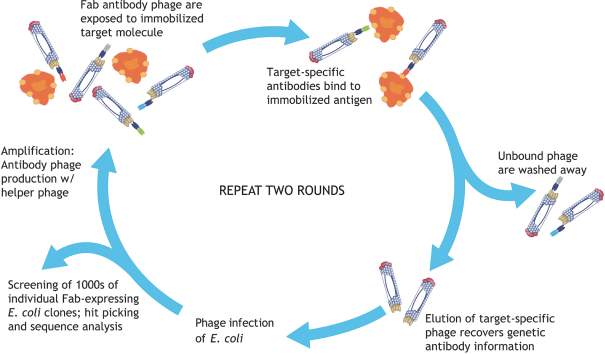
Our HuCAL platform patent portfolio is wholly owned and the platform is protected by several patent families. The basic HuCAL patents, based on WO97/08320, expired in August 2016. These patents covered the composition of the library, methods to isolate antibodies from the library and methods to diversify antibodies isolated from the library. At least four additional patent families protecting other technological aspects of the library, such as the specific CDR design (based on WO2008/053275) and certain display methods used with the library (based on WO2001/05950) as well as improvements of this method (based on WO2009/024593) are still in force in all major jurisdictions, including Australia, Canada, China, the European Union (EP2190987), Israel, Japan, New Zealand, South Africa and the United States. The last U.S. patent (US9062097) expires on February 18, 2030. Patents in other jurisdictions expire in August 2028. The HuCAL library is also protected by considerableknow-how proprietary to us.
YLANTHIA
Our Ylanthia antibody library patent portfolio is wholly owned and the platform is protected by two key patent families covering the composition of the library and nucleic acid collections encoding the library. Patent applications (based on WO2010/136598 and WO2012/066129) are filed in all major jurisdictions, including Australia, Canada, China, the European Union, Hong Kong, India, Israel, Japan, Mexico, New Zealand, Russia, Singapore, South Africa, South Korea and the United States. The patent term is expected to last at least until November 2031 (EP2640742 and US8367586, both granted). One material U.S. patent, US9541559, expires on May 6, 2032. Certain patent protected ancillary technologies are used as well, including the Slonomics technology. Like the HuCAL antibody library, the Ylanthia library encompasses considerableknow-how proprietary to us.
SLONOMICS
Our Slonomics technology is protected by five patent families. The patent family covering the key technology, being a method used for the generation of diversified libraries, such as antibody libraries, has an expiry date of at least March 2029. The most relevant U.S. patent, US9115352, has an expiry date of December 6, 2030. Counterparts in the European Union (EP2110435) and Japan expire in March 2029.
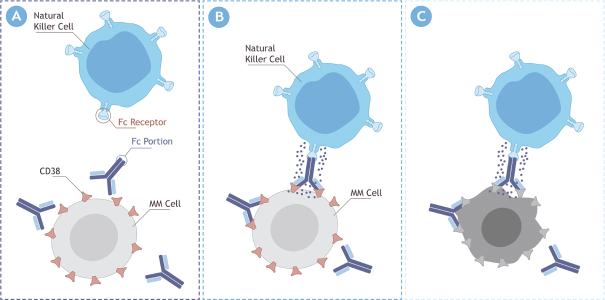
MOR208
Our MOR208 patent portfolio is fully owned and/or exclusively licensed from Xencor and the program is currently protected by at least ten different patent families covering various aspects of the molecule, its compositions, methods of use, combination treatments, and formulation, as well as other aspects. The basiccomposition-of-matter patent wasin-licensed from Xencor and was filed in Australia, Canada, the European Union, Hong Kong, India, Japan and the United States. The expiry date for the composition of matter patent is August 2029 for the United States and August 2027 for the other countries, not including any potential patent term extensions.
Other patent families were filed and are prosecuted in Australia, Brazil, Canada, China, the European Union, Israel, India, Japan, Mexico, New Zealand, Qatar, Russia, Singapore, South Africa, South Korea and the United States.
183





 Chairperson
Chairperson Member
Member