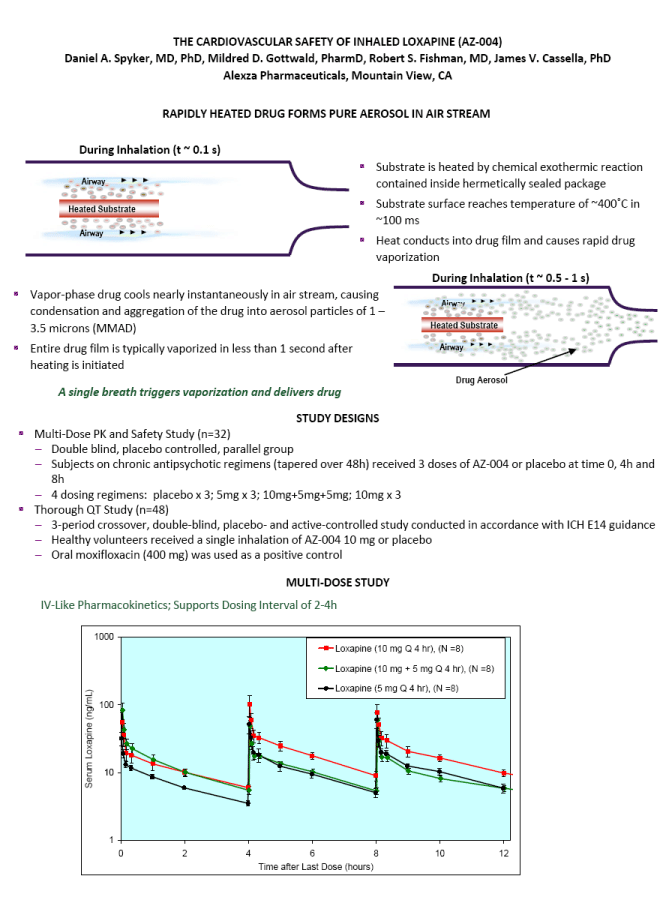
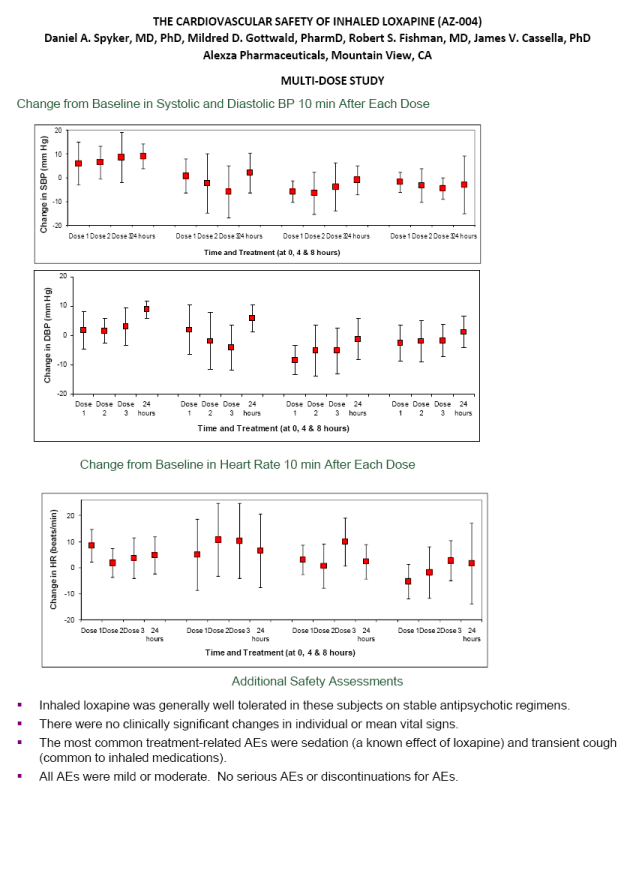
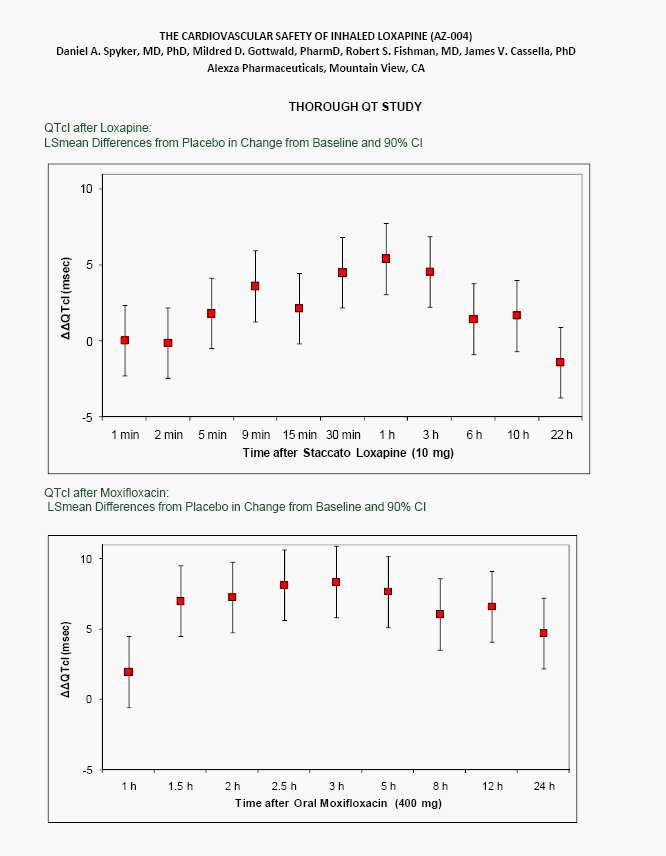
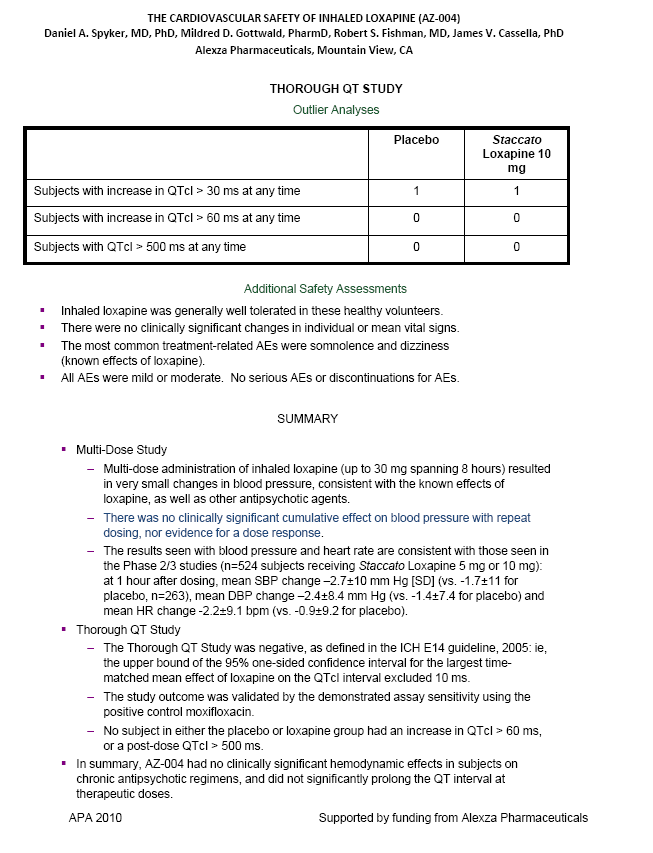
| ABSTRACT Background: AZ-004 (inhaled loxapine) is being developed for the treatment of agitation in patients with schizophrenia or bipolar disorder. Loxapine was administered via inhalation using the Staccato(r) system, which delivers thermally-generated drug aerosol to the deep lungs for rapid systemic absorption with IV-like kinetics. Objective: To describe the cardiovascular safety of inhaled loxapine from 2 separate clinical protocols: a Phase 1 study in 32 subjects on chronic antipsychotic medication; and a thorough QT (TQT) study in 48 healthy volunteers. Methods: Consenting male and female adults, 18 to 65 years of age, were enrolled in the studies and randomly assigned to treatment. The Phase 1 study was a double blind, placebo controlled, parallel group investigation of the safety of repeat doses (at time 0, 4 and 8 hours) in subjects on chronic antipsychotic medication. Each cohort received either 5 mg x 3 doses, 10 mg x 3 doses, placebo x 3 doses, or 10 mg followed by two doses of 5 mg. The TQT study was a double-blind, double-dummy, active- and placebo-controlled, 3 period crossover study investigating the effect on QT interval of single doses of 10 mg AZ-004, 400 mg oral moxifloxacin, and placebo. Results: In the Phase 1 study, there were no important changes in vital signs with repeat dosing in subjects on chronic antipsychotic medication. In the highest dose group (10 mg x 3), mean changes from baseline in systolic BP were -1.75, -3.13, and -4.38 mmHg at 10 minutes after the first, second, and third doses, respectively. In the TQT study, AZ-004 did not increase QT intervals, as demonstrated by the upper bound of the one-sided 95% CIs placed on the point estimate of the placebo-subtracted change of QTcI being less than 10 ms at all 11 post-dose time points. Moxifloxacin significantly prolonged QTcI demonstrating assay sensitivity. Conclusions: AZ-004 had no significant hemodynamic effect in subjects on chronic antipsychotic regimens, and did not significantly prolong the QT interval at therapeutic doses. THE CARDIOVASCULAR SAFETY OF INHALED LOXAPINE (AZ-004) Daniel A. Spyker, MD, PhD, Mildred D. Gottwald, PharmD, Robert S. Fishman, MD, James V. Cassella, PhD Alexza Pharmaceuticals, Mountain View, CA INTRODUCTION AZ-004 (inhaled loxapine) is being developed to provide a rapidly acting, noninvasive alternative for the treatment of agitation in patients with schizophrenia or bipolar disorder Inhaled loxapine is administered via inhalation using the Staccato(r) system, which delivers thermally generated drug aerosol to the lungs for rapid systemic absorption with IV-like kinetics. In separate Phase 3 studies in patients with schizophrenia (n=344) or bipolar disorder (n=314), there was a highly significant reduction in agitation (assessed by Positive and Negative Syndrome Scale - Excited Component [PANSS- EC]) after administration of 5 mg or 10 mg AZ-004, compared with placebo, starting as early as 10 min after dosing. OBJECTIVE To describe the cardiovascular safety of inhaled loxapine from 2 separate clinical protocols A Phase 1 multi-dose study in 32 subjects on chronic antipsychotic medication A thorough QT study in 48 healthy volunteers METHODS Loxapine was delivered by oral inhalation using the Staccato system. The hand-held Staccato system provides rapid, reliable deep lung delivery of a drug using a thermally-generated condensation aerosol. A single inhalation actuates the controlled, rapid heating of a thin layer of excipient-free drug on a metal substrate. The time from breath-activated substrate heating to drug entry into the respiratory tract is less than 1 second. NR6-6 |