UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
(Mark One)
| |
☐ | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| |
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2022
OR
| |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
OR
| |
☐ | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report
Commission file number: 001-39131
LIMINAL BIOSCIENCES INC.
(Exact name of Registrant as specified in its charter)
Canada
(Jurisdiction of incorporation or organization)
440 Armand-Frappier Boulevard, Suite 300
Laval, Quebec
H7V 4B4
+1 450 781 0115
(address of principal executive offices)
Bruce Pritchard
Chief Executive Officer
Liminal BioSciences Inc.
440 Armand-Frappier Boulevard,
Suite 300
Laval, Quebec
H7V 4B4
Tel: +1 450 781 0115
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered, pursuant to Section 12(b) of the Act
| | | | |
Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
Common Shares, no par value | | LMNL | | The Nasdaq Stock Market LLC |
Securities registered or to be registered pursuant to Section 12(g) of the Act. None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act. None
Indicate the number of outstanding shares of each of the issuer’s classes of capital stock or common stock as of the close of business covered by the annual report.
Common Shares, no par value: 31,042,560(1) common shares outstanding as of December 31, 2022
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ Accelerated filer ☐ Non-accelerated filer ☒ Emerging growth company ☒
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards † provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under section 404(b) of the Sarbanes-Oxley Act (15 U.S.C 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☐ No ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| | | | |
U.S. GAAP ☐ | | International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ | | Other ☐ |
If “Other” has been checked in response to the previous question indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
(1) The number of shares does not reflect the 10-for-1 consolidation of our common shares effected on February 1, 2023.
TABLE OF CONTENTS
i
ii
INTRODUCTION
Unless the context otherwise requires, all references in this Annual Report on Form 20-F, or the Annual Report, to “Liminal,” “company,” “we,” “us” and “our” refer to Liminal BioSciences Inc. and, where appropriate, our subsidiaries.
Our fiscal year ends on December 31. This Annual Report includes our audited consolidated financial statements as of December 31, 2022 and 2021 and for the years ended December 31, 2022, 2021 and 2020, which are prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, or IFRS. None of our financial statements were prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP.
Except for the executive and directors’ compensation (Item 6B) which is presented in U.S. Dollars (unless otherwise specified), our financial information is presented in Canadian Dollars and all references in this Annual Report to “$” means Canadian Dollars and all references to “US$” means U.S. Dollars.
For the convenience of the reader, in this Annual Report, unless otherwise indicated, amounts presented in USD translated from Canadian Dollars, were translated at the rate of $1.00 to US$0.7699, which is the average rate for the 2022 fiscal year, (2021 average rate: $1.00=US$ 0.7969). Translations from Great Britain Pounds or GBP into U.S. Dollars were made at the rate of 1 GPB to US$ 1.2438 which is the average rate for the 2022 fiscal year (2021 average rate: 1.00 GBP = US$ 1.3755). Such U.S. Dollar amounts are not necessarily indicative of the amounts of U.S. Dollars that could actually have been purchased upon exchange of Canadian Dollars or GBP at the dates indicated.
We have made rounding adjustments to some of the figures included in this Annual Report. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that preceded them.
Unless otherwise indicated, all financial information included in this Annual Report has been retroactively adjusted to give effect to the 1000-for-1 consolidation of our common shares effected on July 5, 2019 and the 10-for-1 consolidation of our common shares effected on February 1, 2023.
This Annual Report includes registered and unregistered trademarks such as Liminal BioSciences® which are protected under applicable intellectual property laws and are the property of Liminal. Solely for convenience, our trademarks referred to in this Annual Report and in other publicly filed documents may appear without the ® or ™ symbol, but such references are not intended to indicate, in any way, that we will not assert our rights to the fullest extent under applicable law. All other trademarks used in this Annual Report are the property of their respective owners.
We are incorporated under the laws of Canada. All of our assets are located outside the United States. In addition, the majority of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons’ assets may be located outside the United States. As a result, it may be difficult for investors to effect service of process within the United States upon us or such persons or to enforce against them or against us, judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof. In addition, investors should not assume that the courts of Canada (i) would enforce judgments of U.S. courts obtained in actions against us, our officers or directors, or other said persons, predicated upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States or (ii) would enforce, in original actions, liabilities against us or such directors, officers or experts predicated upon the United States federal securities laws or any securities or other laws of any state or jurisdiction of the United States.
In addition, there is doubt as to the applicability of the civil liability provisions of U.S. federal securities law to original actions instituted in Canada. It may be difficult for an investor, or any other person or entity, to assert U.S. securities laws claims in original actions instituted in Canada.
iii
Special Note Regarding Forward-Looking Statements
This Annual Report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, that are based on our management’s beliefs and assumptions and on information currently available to our management. These statements are “forward-looking” because they represent our expectations, intentions, plans and beliefs about our business and the markets we operate in and on various estimates and assumptions based on information available to our management at the time these statements are made. For example, forward-looking statements around financial performance and revenues are based on financial modelling undertaken by our management. This financial modelling takes into account revenues that are uncertain. It also includes forward-looking revenues from transactions based on probability. In assessing probability, management considers the status of negotiations for any revenue generating transactions, and the likelihood, based on the probability of income, that associated costs will be incurred. Management then ranks the probabilities in such a way that only those revenues deemed highly or reasonably likely to be secured are included in the projections.
All statements other than statements of historical facts may be forward-looking statements. Without limiting the generality of the foregoing, words such as “may”, “will”, “expect”, “believe”, “anticipate”, “intend”, “could”, “might”, “would”, “should”, “estimate”, “continue”, “plan” or “pursue”, “seek”, “project”, “predict”, “potential” or “targeting” or the negative of these terms, other variations thereof, comparable terminology or similar expressions, are intended to identify forward-looking statements although not all forward-looking statements contains these terms and phrases.
Forward-looking statements are provided for the purposes of assisting you in understanding us and our business, operations, prospects and risks at a point in time in the context of historical and possible future developments and therefore you are cautioned that such information may not be appropriate for other purposes. Actual events or results may differ materially from those anticipated in these forward-looking statements if known or unknown risks affect our business, or if estimates or assumptions turn out to be inaccurate. In particular, forward-looking statements included in this Annual Report include, without limitation, statements with respect to:
•our plans to develop and commercialize our product candidates;
•our ability to develop, manufacture and successfully commercialize value-added pharmaceutical products;
•our ability to reduce our cash burn;
•uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals;
•the availability of funds and resources to pursue research and development projects;
•the successful and timely completion of our drug discovery, preclinical studies and clinical trials;
•the properties of our product candidates;
•the analysis of our preclinical and clinical trial data;
•our ability to take advantage of business opportunities in the pharmaceutical industry;
•potential strategic transactions that we may pursue, including potential divestments or sale of non-core assets;
•our reliance on key personnel, collaborative partners and other third parties;
•the validity and enforceability of our patents and proprietary technology;
•expectations regarding our ability to raise capital;
iv
•the use of certain hazardous materials;
•the availability and sources of raw materials;
•our third-party manufacturing capabilities;
•the value of our intangible assets;
•negative operating cash flow;
•the outcome of any current or pending litigation against us;
•uncertainties related to the regulatory process and approvals;
•increasing data security costs;
•costs related to environmental safety regulations;
•competing drugs, as well as from current and future competitors;
•developing products for the indications we are targeting;
•market acceptance of our product candidates by patients and healthcare professionals;
•our ability to secure insurance coverage;
•general changes in economic or market conditions, including as a result of inflation or bank failures;
•the impact of the ongoing COVID-19 pandemic and other geopolitical tensions, such as Russia's ongoing invasion of Ukraine, on our business and its potential effect on the operations of third party service providers and collaborators with whom we conduct business, our industry and the economy;
•volatility of our share price;
•our ability to comply with Nasdaq Capital Market's continued listing requirements; and
•other risks and uncertainties, including those listed in the section of this Annual Report titled “Item 3.D—Risk Factors.”
You should refer to the section of this Annual Report titled “Item 3.D—Risk Factors” for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
You should read this Annual Report and the documents that we reference in this Annual Report and have filed as exhibits to this Annual Report completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
v
This Annual Report contains market data and industry forecasts that were obtained from industry publications. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified any third-party information. While we believe the market position, market opportunity and market size information included in this Annual Report is generally reliable, such information is inherently imprecise.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete. Our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
vi
PART I
Item 1. Identity of Directors, Senior Management and Advisers.
Not applicable.
Item 2. Offer Statistics and Expected Timetable.
Not applicable.
Item 3. Key Information.
A. [Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Our business faces significant risks. You should carefully consider all of the information set forth in this Annual Report and in our other filings with the United States Securities and Exchange Commission, or the SEC, including the following risk factors which we face and which are faced by our industry. Our business, financial condition or results of operations could be materially adversely affected by any of these risks. This report also contains forward-looking statements that involve risks and uncertainties. Our results could materially differ from those anticipated in these forward-looking statements, as a result of certain factors including the risks described below and elsewhere in this Annual Report and our other SEC filings. See “Special Note Regarding Forward-Looking Statements” above.
Summary of Selected Risks Associated with Our Business
•We will require additional funding to finance our operations, which may not be available on acceptable terms, if at all. Failure to obtain this necessary capital when needed may force us to delay, reduce or terminate certain of our product development programs or other operations.
•The market conditions or our business performance may prevent us from having access to the public markets in the future.
•We may not have the ability to close a transaction for our non-core assets, including a potential divestment or sale of such non-core assets.
•If we fail to implement and maintain effective internal controls over financial reporting, our ability to produce accurate financial statements on a timely basis could be impaired.
•We have identified a material weakness in our internal control over financial reporting. If we fail to remediate the material weakness and maintain an effective system of disclosure controls and internal control over financial reporting, our ability to report our results of operations and financial condition accurately and in a timely manner could be impaired.
7
•Litigation or legal proceedings could expose us to significant liabilities and have a negative impact on our reputation, financial resources, ability to secure financings or business.
•We may not be successful in our efforts to build a pipeline of product candidates.
•Our product candidates may not succeed in preclinical or clinical testing; may be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria.
•We do not know whether any of our planned clinical trials will proceed or be completed on schedule, or at all.
•We depend on our key personnel to research, discover, develop and coordinate the manufacture of our product candidates and the loss of key personnel or the inability to attract highly qualified individuals could have a material adverse effect on our business and growth potential.
•We may fail to achieve our publicly announced milestones or not achieve publicly announced milestones in due time.
•We may not be successful in partnering and/or commercializing our product candidates or obtaining royalties and milestone payments.
•Competitors may develop alternatives that render our product candidates obsolete or less attractive.
•We rely on third parties to develop, test, formulate and manufacture our product candidates and to monitor, support, conduct and oversee clinical trials of the development candidates that we are developing, and such reliance may adversely affect us if we are not able to maintain or secure agreements with such third parties on acceptable terms, or if these third parties do not perform their services as required.
•Our internal information technology systems and those of our current and any future third-party vendors, collaborators and other contractors or consultants may be vulnerable to a variety of disruptive elements, including cyber-attacks by malicious third parties, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures.
•We may not be able to protect our intellectual property rights throughout the world or not have sufficient financial resources to prepare, file and prosecute patent applications, maintain and enforce our intellectual property rights and defend any intellectual property-related claims.
•Our pending patent applications may not result in issued patents or may not be valid or enforceable or there could be unpublished applications or patent applications maintained in secrecy that may later issue with claims covering our product candidates.
•The continued listing of our common shares on Nasdaq is conditional upon our ability to maintain the applicable continued listing requirements of Nasdaq. Failure to maintain the applicable continued listing requirements of the Nasdaq could result in our common shares being delisted from Nasdaq.
•We are a “controlled company” within the meaning of the applicable Nasdaq listing rules and expect that Structured Alpha LP, or SALP, will continue to own a significant percentage of our common shares and will be able to exert significant control over matters subject to shareholder approval.
•Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and share price.
8
Risks Related to Our Financial Position
We will require additional funding to finance our operations, which may not be available on acceptable terms, if at all. Failure to obtain this necessary capital when needed may force us to delay, reduce or terminate certain of our product development programs or other operations.
As of December 31, 2022, we had approximately $37.1 million of cash and cash equivalents. As of the date of this Annual Report, our available cash is not projected to be sufficient to support our operating plan for at least the next 12 months. As such, there is substantial doubt regarding our ability to continue as a going concern.
We will require additional funding and may not be able to raise the capital necessary to continue and complete the research and development of our product candidates and their commercialization, if approved. We have historically generated revenues from now discontinued operations, but have never achieved or maintained profitability, and will need additional financing in order to continue our activities.
Our future capital requirements will depend on many factors, including:
•the progress, results and costs of laboratory testing, manufacturing, preclinical and clinical development for our current product candidates;
•the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials of other product candidates that we may pursue;
•the ability to close a transaction for our non-core assets that we may pursue, in whole or in part, including a potential divestment or sale of such non-core assets;
•the development requirements of other product candidates that we may pursue;
•the timing and amounts of any milestone or royalty payments we may be required to make under future license or royalty stream agreements;
•the costs of hiring additional personnel, including clinical, regulatory, medical, scientific and seasoned management personnel;
•the costs, timing and outcome of regulatory review of our product candidates or regulatory applications;
•the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims;
9
•the legal and financial costs required to comply with being a public company, including the costs of maintaining director and officer liability insurance; and
•the extent to which we acquire or in-license other product candidates and technologies.
In the past, we have been financed in part through debt and public equity offerings and we may effect additional debt or equity offerings to raise capital, the size of which cannot be predicted. The issuance and sales of substantial amounts of equity or other securities, or the perception that such issuances and sales may occur, could adversely affect the market price of our common shares. In addition, to the extent that we raise additional capital by issuing equity securities, our existing shareholders’ ownership may experience substantial dilution, and the terms of these securities may include liquidation or other preferences that adversely affect shareholder rights. Equity and debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as redeeming our shares, making investments, incurring additional debt, making capital expenditures or declaring dividends.
The incurrence of indebtedness could result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants therein, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights or other assets and other operating restrictions that could adversely affect our ability to conduct our business.
If we raise additional capital through collaborations, strategic alliances or third-party licensing arrangements, we may have to relinquish valuable rights to our intellectual property, future revenue streams, research programs or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional capital through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts, or grant rights to develop and market product candidates that we would otherwise develop and market ourselves.
There is no assurance that sufficient financing will be available when needed to allow us to continue as a going concern. The perception that we may not be able to continue as a going concern may also make it more difficult to operate our business due to concerns about our ability to meet our contractual obligations. Our ability to continue as a going concern is contingent upon, among other factors, obtaining alternate financing. We cannot provide any assurance that we will be able to raise additional capital on acceptable terms, if at all, reducing our ability to access capital, which could in the future negatively affect our liquidity. If we are unable to secure additional capital, we may be required to curtail our research and development initiatives and take additional measures to reduce costs in order to conserve our cash in amounts sufficient to sustain operations and meet our obligations. These measures could cause significant delays in our preclinical, clinical and regulatory efforts, which are critical to the realization of our business plan. The accompanying consolidated financial statements do not include any adjustments that may be necessary should we be unable to continue as a going concern. Such adjustments could be material. If we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our financial statements, and it is likely that investors will lose all or a part of their investment.
We are not profitable and may never achieve profitability.
We are not profitable and may never achieve profitability. We have been reporting losses since our inception. We will need to generate significant revenues to achieve profitability. There is no guarantee that we will succeed in developing and commercializing or partnering our product candidates, controlling our expenses and developing additional product candidates, and, therefore, we may never become profitable.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. These net losses will adversely impact our shareholders’ deficit and net assets and may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses will increase substantially as we:
•continue our ongoing and planned research and development of our product candidates under development;
10
•conduct preclinical studies and clinical trials for our current and potential future product candidates;
•seek to discover and develop additional product candidates and further expand our clinical product pipeline;
•seek regulatory approvals for any product candidates that successfully complete clinical trials;
•continue to scale up outsourced manufacturing capacity with the aim of securing sufficient quantities to meet our demand requirements for clinical trials;
•develop, maintain, expand and protect our intellectual property portfolio;
•acquire or in-license other product candidates and technologies;
•hire additional clinical, regulatory, medical, scientific and other support personnel, including personnel to support our product development and planned business development or partnering efforts; and
•add clinical, operational, financial and management information systems.
To become and remain profitable, we must succeed in developing and eventually commercializing or out-licensing products that generate significant revenue. This will require us to be successful in a range of challenging activities, including completing preclinical studies and clinical trials of our product candidates, obtaining regulatory approval, manufacturing, marketing and selling any products for which we may obtain regulatory approval, as well as discovering and developing additional product candidates. We may never succeed in these activities and, even if we do, may never generate revenue that is significant enough to achieve profitability.
Because of the numerous risks and uncertainties associated with the development, manufacture, delivery and commercialization of drugs, we are unable to accurately predict the timing or amount of expenses or when, or if, we will be able to achieve profitability. If we are required by regulatory authorities to perform studies in addition to those currently expected, or if there are any delays or impediments in the initiation and completion of our clinical trials or the development of any of our product candidates, our expenses could increase and profitability could be further delayed.
Even if we achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable could impair our ability to raise capital, expand our business, maintain our research and development efforts or continue our operations.
The market conditions or our business performance may prevent us from having access to the public markets in the future.
The market conditions or our business performance may prevent us from having access to the public markets in the future. In such a case, we will have to use other means of financing, such as issuing debt instruments or entering into private financing agreements, which may not be available to us on favorable terms and conditions or at all. These debt instruments may contain terms and conditions (e.g., covenants, etc.) which may be challenging or difficult for us to respect, may be breached or trigger default provisions. Accordingly, we may be required to compensate counterparties, for costs and losses incurred as a result of various events, including breaches of representations and warranties, covenants, claims that may arise during the terms of said debt instruments or as a result of litigation that may be suffered by counterparties. If adequate funding is not available to us, we may be required to delay, reduce or eliminate our research and development of new product candidates, our clinical trials or our marketing and commercialization efforts to launch and distribute products, if any.
11
If we fail to implement and maintain effective internal controls over financial reporting, our ability to produce accurate financial statements on a timely basis could be impaired.
As a public company in the United States, we are subject to reporting obligations under U.S. securities laws, including the Sarbanes-Oxley Act. Section 404(a) of the Sarbanes-Oxley Act, or Section 404(a), requires that management assess and report annually on the effectiveness of our internal control over financial reporting and identify any material weaknesses in our internal control over financial reporting. Although Section 404(b) of the Sarbanes-Oxley Act, or Section 404(b), requires our independent registered public accounting firm to issue an annual report that addresses the effectiveness of our internal control over financial reporting, we have opted to rely on the exemptions provided in the JOBS Act, and consequently will not be required to comply with SEC rules that implement Section 404(b) until such time as we are no longer an emerging growth company.
The presence of material weaknesses could result in financial statement errors which, in turn, could lead to errors in our financial reports or delays in our financial reporting, which could require us to restate our operating results or result in our auditors issuing a qualified audit report. In order to establish and maintain effective disclosure controls and procedures and internal control over financial reporting, we will need to expend significant resources and provide significant management oversight.
Developing, implementing and testing changes to our internal control may require specific compliance training of our directors and employees, entail substantial costs in order to modify our existing accounting systems, take a significant period of time to complete and divert management’s attention from other business concerns. These changes may not, however, be effective in establishing and maintaining adequate internal controls.
If either we are unable to conclude that we have effective internal control over financial reporting or, at the appropriate time, our independent auditors are unwilling or unable to provide us with an unqualified report on the effectiveness of our internal control over financial reporting as required by Section 404(b), investors may lose confidence in our operating results, the price of our common shares could decline and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404, we may not be able to remain listed on the Nasdaq.
We have identified a material weakness in our internal control over financial reporting. If we fail to remediate the material weakness and maintain an effective system of disclosure controls and internal control over financial reporting, our ability to report our results of operations and financial condition accurately and in a timely manner could be impaired.
As a public company, we are required, pursuant to Section 404(a), to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting for the fiscal year ended December 31, 2022. This assessment is required to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
As previously disclosed, during the preparation of our consolidated financial statements for the year ended December 31, 2021, management identified a material weakness in our internal control over financial reporting related to the carrying value of our held-for-sale assets at September 30, 2021 and the net loss from discontinued operations for the nine months ended September 31, 2021. As disclosed in Item 15 of the Company’s Annual Report on Form 20-F for the year ended December 31, 2021, or the 2021 Annual Report, the financial statements for the period ended September 30, 2021 contained a misstatement in the carrying value of the held-for-sale assets relating to the PBT disposal. Accordingly, as shown in the Summary of Consolidated Quarterly Results contained in Item 5A of the 2021 Annual Report, management and our audit committee concluded that it was appropriate to restate our previously issued financial results for the period ended September 30, 2021.
12
In connection with the preparation of our consolidated financial statements for the year ended December 31, 2022, management again identified a material weakness in our internal control over financial reporting relating to the accounting for complex transactions, which was in the process of being remediated by management as disclosed in the 2021 Annual Report. In this particular instance, we acquired the non-controlling interests of our subsidiary, Pathogen Removal Diagnostic Technologies Inc. In our interim financial statements for the periods ended March 31, 2022, June 30, 2022 and September 30, 2022, we incorrectly allocated net income to non-controlling shareholders. As a result, net loss attributable to the non-controlling shareholders was overstated with offsetting misclassifications between non-controlling interest and shareholders deficit.
Accordingly, as shown in the Summary of Consolidated Quarterly Results contained in Item 5A of this Annual Report, management and our audit committee concluded that it was appropriate to restate our previously issued financial results for the periods ended March 31, 2022, June 30, 2022 and September 30, 2022. The error did not result in an adjustment to previously reported net income or loss per share in any prior fiscal years.
Our remediation plan initially included reviewing our quarterly and year-end close timetables to ensure any subsequent complex accounting matters were given priority and allocated the appropriate resources. In addition, we hired a CFO to lead and build out the Finance team’s expertise and bandwidth. During the fourth quarter of fiscal 2022, our CFO restructured the Finance team to further optimize and simplify the reporting structure, which is expected to improve operating and reporting efficiencies. Management believes the Finance team is now better structured and more aligned with the Company's current operations and believes the current control environment is better suited for the size of the Company and for accounting and financial reporting practices going forward. We cannot assure you that these measures will be effective. We also cannot assure you that there will not be additional material weaknesses or significant deficiencies in our internal control over financial reporting in the future.
Any additional or sustained failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition or results of operations. If we are unable to remediate the material weakness or to conclude in the future that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have additional material weaknesses in our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by the Nasdaq Stock Exchange, the SEC or other regulatory authorities. Failure to remedy any material weaknesses in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets. For details of the controls, the identified material weakness and our remediation plan, see the section of this Annual Report entitled “Item 15. Controls and Procedures.”
13
Recent trends towards rising inflation may materially adversely affect our business and corresponding financial position and cash flows.
The recent trends towards rising inflation may materially adversely affect our business and corresponding financial position and cash flows. Inflationary factors, such as increases in the cost of our materials and supplies and overhead costs may adversely affect our operating results. Rising interest and inflation rates also present a recent challenge impacting the global economy and could make it more difficult for us to obtain traditional financing on acceptable terms, if at all, in the future. Although non-material impact on our financial position or results of operations have been experienced to date, we may experience more significant impacts in the near future (especially if inflation rates continue to rise), including increases of our operating costs, including our labor costs and research and development costs, due to supply chain constraints, and the ongoing conflict between Russia and Ukraine, and employee availability and wage increases, which may result in additional stress on our working capital resources.
Litigation or legal proceedings could expose us to significant liabilities and have a negative impact on our reputation or business.
From time to time, we may be party to various claims and litigation proceedings. We evaluate these claims and litigation proceedings to assess the likelihood of unfavorable outcomes and to estimate, if possible, the amount of potential losses. Based on these assessments and estimates, we may establish reserves, as appropriate. These assessments and estimates are based on the information available to management at the time and involve a significant amount of management judgment. Actual outcomes or losses may differ materially from our assessments and estimates. For example, on March 2, 2021, a complaint was filed against us, SALP, Thomvest Asset Management Ltd., Consonance Capital Management LP and certain of our directors and officers by multiple individual shareholder plaintiffs. For more information, see Note 29 to our consolidated financial statements included elsewhere in this Annual Report.
Even when not merited, the defense of these lawsuits may divert our management’s attention, and we may incur significant expenses in defending these lawsuits. The results of litigation and other legal proceedings are inherently uncertain, and adverse judgments or settlements in some of these legal disputes may result in adverse monetary damages, penalties or injunctive relief against us, which could have a material adverse effect on our financial position, cash flows or results of operations. Any claims or litigation, even if fully indemnified or insured, could damage our reputation and make it more difficult to compete effectively or to obtain adequate insurance in the future.
Furthermore, while we maintain insurance for certain potential liabilities, such insurance does not cover all types and amounts of potential liabilities and is subject to various exclusions as well as caps on amounts recoverable. Even if we believe a claim is covered by insurance, insurers may dispute our entitlement to recovery for a variety of potential reasons, which may affect the timing and, if the insurers prevail, the amount of our recovery.
14
Risks Related to the Development and Monetization of our Product Candidates
We may not be successful in our efforts to build a pipeline of product candidates.
We intend to develop additional product candidates. However, we may not be able to develop product candidates that are safe and effective, or which compare favorably with other commercially available alternatives. Even if we are successful in continuing to build our pipeline and developing product candidates, the potential product candidates that we identify may not be suitable for clinical development, including as a result of lack of safety, lack of tolerability, lack of efficacy or other characteristics that indicate that they are unlikely to be products that will receive marketing approval, achieve market acceptance or obtain reimbursements from third-party payors. We cannot provide any assurance that we will be able to successfully advance any of these additional product candidates through the development process. Our research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development or commercialization for many reasons, including the following:
•we may not be successful in building a platform to identify additional product candidates;
•we may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates;
•our product candidates may not succeed in preclinical or clinical testing;
•we may fail to obtain regulatory approval to initiate a clinical trial, to enter into clinical trial agreements with study sites and principal investigators, obtain the relevant ethics committee approvals to initiate the clinical trials or fail or be delayed to recruit and enroll participants;
•a product candidate may on further study be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria;
•competitors may develop alternatives that render our product candidates obsolete or less attractive;
•product candidates we develop may nevertheless be covered by third-parties’ patents or other exclusive rights;
•the market for a product candidate may change during our development program so that the continued development of that product candidate is no longer reasonable;
•we may revisit our corporate strategy and corporate priorities and no longer pursue the development, manufacture or commercialization of certain product candidates that require substantial technical, financial and human resources;
•we may be unable to find qualified and affordable contract manufacturing organizations or contract research organizations or delays or inability to obtain timely regulatory approvals;
•a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all;
•changes in methods of product candidate manufacturing or formulation may result in additional costs or delay and any of these changes could cause our product candidates to perform differently and affect the results of planned clinical trials or other future clinical trials conducted with the altered materials; and
•a product candidate may not be accepted as safe and effective by patients, the medical community or third-party payors, if applicable.
If any of these events occur, we may be forced to abandon our development efforts for a program or programs, or we may not be able to identify, discover, develop or commercialize additional product candidates, which would have a material adverse effect on our business and could potentially cause us to cease operations.
15
Even if we receive FDA, MHRA or other regulatory approval to market our product candidates, we cannot assure that any such product candidates will be commercialized, widely accepted in the marketplace or as effective or more effective than other commercially available alternatives. Further, because of our limited financial and managerial resources, we are required to focus our research programs on certain product candidates and on specific diseases. As a result, we may fail to capitalize on viable commercial products or profitable market opportunities, be required to forego or delay pursuit of opportunities with other product candidates or other diseases that may later prove to have greater commercial potential, or relinquish valuable rights to such product candidates through divestment, sale, collaboration, licensing, royalty or other strategic alternative arrangements in cases in which it would have been advantageous for us to retain sole development and/or commercialization rights.
If we do not successfully develop and commercialize product candidates or collaborate with others to do so, we will not be able to obtain product revenue in future periods, which could significantly harm our financial position.
16
We do not know whether any of our planned clinical trials will proceed or be completed on schedule, or at all.
We do not know whether any of our planned clinical trials will proceed or be completed on schedule, or at all. The commencement of our planned clinical trials could be substantially delayed or prevented by several factors, including:
•limited number of, and competition for, suitable patients with the indications required for enrollment in our clinical trials;
•limited number of, and competition for, suitable sites to conduct our clinical trials;
•delay or failure to obtain FDA or non-U.S. regulatory agencies’ approval or agreement to commence a clinical trial;
•delay or failure to obtain sufficient supplies of the product candidate for our clinical trials;
•delay or failure to reach agreement on acceptable clinical trial agreement terms or clinical trial protocols with prospective sites or investigators; and
•delay or failure to obtain IRB approval to conduct a clinical trial at a prospective site.
The completion of any clinical trial could also be substantially delayed or prevented by several factors, including:
•slower than expected rates of subject or patient recruitment and enrollment;
•failure of subjects or patients to complete the clinical trial;
•unforeseen safety issues;
•lack of efficacy evidenced during any clinical trial;
•termination of any clinical trial by one or more clinical trial sites;
•inability or unwillingness of subjects, patients or medical investigators to follow clinical trial protocols;
•failure by clinical research organization to comply with applicable laws and regulations;
•inability to monitor subjects or patients adequately during or after treatment; and
•introduction of competitive product candidates that may impede our ability to retain subjects or patients in any clinical trial.
Clinical trials may be suspended or terminated at any time by the FDA, other regulatory authorities, the IRB overseeing the clinical trial at issue, any of our clinical trial sites with respect to that site, or us. Any failure or significant delay in completing any clinical trial for our product candidates could materially harm our financial results and the commercial prospects for our product candidates. In addition, the impact of future public health crises may delay or prevent subjects or patients from enrolling or from receiving treatment in accordance with the protocol and the required timelines, which could delay our clinical trials, or otherwise prevent us from completing our clinical trials on our planned timelines or at all, and harm our ability to obtain approval for such product candidate.
17
Clinical trials may not demonstrate a clinical benefit of our product candidates.
Clinical trials may not demonstrate a clinical benefit of our product candidates. Positive results from preclinical studies and early clinical trials should not be relied upon as evidence that later stage, large scale or controlled clinical trials will succeed. We will be required to demonstrate with substantial evidence through well-controlled clinical trials that our product candidates are safe and effective for use in a diverse population before we can seek regulatory approvals for their commercial sale. Success in early clinical trials does not mean that future clinical trials will be successful because product candidates in later stage clinical trials may fail to demonstrate sufficient safety and efficacy to the satisfaction of regulatory authorities despite having progressed through initial clinical trials.
We may experience numerous unforeseen events prior to, during or as a result of clinical trials that could delay or prevent our ability to receive marketing approval or commercialize any of our product candidates, including:
•the FDA, MHRA or other comparable regulatory authority may disagree as to the number, design or implementation of our clinical trials, or may not interpret the results from clinical trials as we do;
•regulators or institutional review boards, or IRBs, may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
•we may not reach agreement on acceptable terms with prospective clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different clinical trial sites;
•clinical trials of our product candidates may produce negative or inconclusive results;
•we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs;
18
•the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate, participants may drop out of these clinical trials at a higher rate than we anticipate or we may fail to recruit eligible patients to participate in a trial;
•our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all;
•regulators may issue a clinical hold, or regulators or IRBs may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks;
•the cost of clinical trials of our product candidates may be greater than we anticipate;
•the FDA, MHRA or other comparable regulatory authorities may fail to approve our manufacturing processes or facilities;
•the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate;
•our product candidates may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators or IRBs to suspend or terminate the clinical trials; and
•the approval policies or regulations of the FDA, MHRA or other comparable regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval.
To the extent that the results of the trials are not satisfactory for the FDA, MHRA or regulatory authorities in other countries or jurisdictions to approve our new drug application, or NDA, or other comparable applications, the commercialization of our product candidates may be significantly delayed, or we may be required to expend significant additional resources, which may not be available to us, to conduct additional trials in support of potential approval of our product candidates.
Even after the completion of Phase 3 clinical trials, regulatory authorities may disagree with our clinical trial design and our interpretation of data and may require us or our partners to conduct additional clinical trials to demonstrate the efficacy of our product candidates. There is no assurance that a regulatory authority will issue a marketing authorization. Regulatory authorities may determine that our product candidates, the manufacturing process or the manufacturing facilities we use do not meet applicable requirements to ensure the continued safety, purity and potency of our product candidates.
Our product candidates could cause undesirable and potentially serious side effects during clinical trials that could delay or prevent their regulatory approval or commercialization.
Our product candidates could cause undesirable and potentially serious side effects during clinical trials that could delay or prevent their regulatory approval or commercialization. Undesirable side effects caused by any of our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by regulatory authorities for any or all targeted indications.
19
This, in turn, could prevent us from commercializing our product candidates and generating revenues from their sale. In addition, if our product candidates receive marketing approval and we or others later identify undesirable side effects caused by the product:
•regulatory authorities may withdraw their approval of the product;
•we may be required to recall the product, change the way the product is administered, conduct additional clinical trials or change the labelling of the product;
•a product may become less competitive and product sales may decrease; or
•our reputation may suffer.
Any one or a combination of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase the costs and expenses of commercializing the product, which in turn could delay or prevent us from generating revenues from the sale of the affected product.
In addition, as with the results of any statistical sampling, we cannot be sure that all side effects of our product candidates may be uncovered in clinical trials, and it may be the case that only with a significantly larger number of patients exposed to the product candidate for a longer duration, a more complete safety profile will be identified.
Failure to recruit and enroll participants for clinical trials may cause the development of our product candidates to be delayed or halted.
Failure to recruit and enroll participants for clinical trials may cause the development of our product candidates to be delayed or halted. We may encounter delays or rejections in recruiting and enrolling enough participants to complete clinical trials. Participants enrollment depends on many factors, including:
•the eligibility criteria defined in the protocol;
•the number of patients with the disease or condition being studied;
•the understanding of risks and benefits of the product candidate in the trial;
•clinicians’ and subjects or patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating or drugs that may be used off-label for these indications;
•the size and nature of the patient population who meet inclusion criteria;
•the proximity of subjects or patients to study sites;
•the design of the clinical trial;
•clinical trial investigators’ ability to recruit clinical trial investigators with the appropriate competencies and experience;
•competing clinical trials for similar therapies;
•our ability to obtain and maintain subjects or patient consents;
•the risk that subjects or patients enrolled in clinical trials will drop out of the clinical trials before completion of their treatment; and
20
•the impact of public health crises, such as the COVID-19 pandemic.
In particular, some of our clinical trials are designed to enroll subjects or patients with characteristics that are found in a very small population. In addition, since the number of qualified clinical investigators is limited, we expect to conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which could further reduce the number of subjects or patients who are available for our clinical trials in these clinical trial sites.
Delays in subjects or patient enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these clinical trials and adversely affect our ability to advance the development of our product candidates. In addition, many of the factors that may lead to a delay in the commencement, halt or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
We depend on our key personnel to research, discover, develop and coordinate the manufacture of our product candidates and the loss of key personnel or the inability to attract highly qualified individuals could have a material adverse effect on our business and growth potential.
We depend on our key personnel to research, discover, develop and coordinate the manufacture of our product candidates and the loss of key personnel or the inability to attract highly qualified individuals could have a material adverse effect on our business and growth potential. Our mission is to discover or acquire novel therapeutic product candidates targeting unmet medical needs in attractive specialty markets. The achievement of this mission requires qualified scientific and management personnel. The loss of scientific personnel or of members of management could have a material adverse effect on our business. In addition, our growth is and will continue to be dependent, in part, on our ability to retain and hire qualified personnel. There can be no guarantee that we will be able to continue to retain our current employees or will be able to attract qualified personnel and members of management to pursue our business plan.
21
We may fail to achieve our publicly-announced milestones or not achieve publicly-announced milestones in due time.
We may fail to achieve publicly announced milestones or may not achieve our publicly announced milestones in due time. From time to time, we publicly announce the timing of the occurrence of certain events. These statements are forward-looking and are based on management’s best estimate relating to the occurrence of such events. However, the events and actual timing of such events may differ from what has been publicly disclosed. These variations may occur as a result of a series of events, including the nature of the results obtained during a clinical trial or during a research phase, problems with a supplier, clinical research organization or contract manufacturing organization or any other event having the effect of delaying or otherwise impacting the milestone publicly announced. Our policy on forward-looking information does not consist in updating such information if the publicly disclosed timeline varies, unless otherwise required to do so by law. Any variation in the timing of certain events having the effect of postponing or otherwise postponing such events could have an adverse material effect on our business plan, financial condition or operating results.
Our commercial success depends largely on the development and monetization of our product candidates.
Our commercial success depends largely on the development and monetization of our product candidates. Our failure to do so will have a material adverse effect on us.
22
Our ability to generate revenues in the future is primarily dependent on the commercialization and/or partnering of our product candidates. There can be no guarantee of commercialization or monetization of these product candidates as they remain in development. Our ability to successfully commercialize or monetize any product candidates will depend on numerous factors including, without limitation:
•the willingness of the FDA, United Kingdom Medicines and Healthcare Product Regulatory Agency, or MHRA, and other regulatory agencies to grant approvals for the conduct of our clinical trials and acceptance of our preclinical studies as the basis for the review and approval of our product candidates;
•the timing, progress, costs, and results of ongoing and planned clinical trials;
•the timing, outcome and costs of seeking and obtaining regulatory approvals from the FDA, MHRA and other regulatory agencies;
•the timing and costs of maintaining supply agreements of our product candidates for clinical trials and other studies and, if approved, for commercial supply;
•an increase in the number of competitors in the same market;
•the cost to maintain, expand, defend and enforce our intellectual property portfolio and our ability to effectively prosecute and protect our intellectual property and avoid patent infringement;
•the costs of acquiring, licensing or investing in additional businesses, products or technologies; and
•any other condition, obligation or requirement that may arise, all of which may delay our capacity to generate revenues and will adversely materially affect our financial conditions and operating results.
Many of these factors are beyond our control, including the time needed to adequately initiate, conduct and complete clinical testing and the regulatory submission process. It is possible that none of our product candidates or future product candidates will ever obtain regulatory approval, even if we expend substantial time and resources seeking such approval. If we do not achieve one or more of these factors in a timely manner or at all, or any other factors impacting the successful development of biopharmaceutical products, we could experience significant delays or an inability to successfully develop our product candidates, which would materially harm our business.
We are exploring investing efforts into building a drug discovery platform but these efforts may not be successful.
We intend to continue to develop our drug discovery platform that focused on exploring structural biology and drug design by utilizing our know-how and new tools and technologies, which may, in the future, include artificial intelligence and machine learning, in the drug discovery process with the goal to identify novel biological targets that may have been previously undruggable and potentially develop product candidates for these novel targets.
However, we may not be successful in our development and integration of a drug discovery platform, as developing a drug discovery platform to identify new product candidates requires substantial technical, financial and human resources. Even if we are successful, the potential product candidates that we identify may not be suitable for clinical development, including as a result of lack of safety, lack of tolerability or other characteristics that indicate that they are unlikely to be products that will receive marketing approval, achieve market acceptance or obtain reimbursements from third-party payors. If we are unable to develop our drug discovery platform and identify suitable compounds for preclinical and clinical development, we may not be able to obtain sufficient product revenues in future periods, which could significantly harm our financial position.
23
We face competition and the development of new product candidates by other companies could materially adversely affect our business and our product candidates.
We face competition and the development of new product candidates by other companies could materially adversely affect our business and our product candidates. The biopharmaceutical and pharmaceutical industries are highly competitive, and we must compete with pharmaceutical companies, biotechnology companies, academic and research institutions as well as governmental agencies for the development and commercialization of product candidates. Some of these competitors develop product candidates in the indications in which we are involved and could be considered direct or indirect competitors.
In the indications currently being studied by us for development, there may exist companies that are at a more advanced stage of developing a product to treat those same diseases. Some of these competitors have capital resources, research and development personnel and facilities that are superior to ours. Mergers and acquisitions in the biotechnology and pharmaceutical industries may also result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical studies, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. In addition, some competitors are more experienced than we are in the commercialization of medical products and already have a sales force in place to launch new products. Consequently, they may be able to develop alternative forms of medical treatment which could compete with our product candidates and commercialize them more rapidly and effectively than we can.
If the market opportunities for any of our product candidates we may develop are smaller than we believe they are, our revenue may be adversely affected and our business may suffer. Moreover, because the target patient populations we are seeking to treat are small, and the addressable patient population even smaller, we must be able to successfully identify patients and capture a significant market share to achieve profitability and growth.
We focus our research and product development on treatments of disease which may be for rare indications. Given the small number of patients who have any such rare disease that we are targeting or may be targeting, it is critical to our ability to grow and become profitable that we continue to successfully identify patients with these rare diseases. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates we may develop, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, surveys of clinics, patient foundations or market research that we conducted, and may prove to be incorrect or contain errors. New studies may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. The effort to identify patients with diseases we seek to treat is in early stages, and we cannot accurately predict the number of patients for whom treatment might be possible. Additionally, the potentially addressable patient population for our product candidates we may develop may be limited or may not be amenable to treatment with our other product candidates we may develop, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect our results of operations and our business. Further, even if we obtain significant market share for our product candidates we may develop, because the potential target populations are very small, we may never achieve profitability despite obtaining such significant market share.
24
Interim top-line and preliminary results from our clinical trials that we announce or publish from time to time may change as more participant data become available and are subject to audit and verification procedures, which could result in material changes in the final data.
From time to time, we may publish interim top-line or preliminary results from our clinical trials. Interim results from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as participants enrollment continues and more patient data become available. Preliminary or top-line results also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim and preliminary data should be viewed with caution until the final data are available. Differences between preliminary or interim data and final data could significantly harm our business prospects and may cause the trading price of our common stock to fluctuate significantly.
If we are unable to enter into divestment, sales, marketing and distribution, licensing agreements or other strategic alternative transactions with third parties, our product candidates, if and when they are approved, may not be successfully launched or commercialized.
We currently do not have commercialization capabilities, such that we are unable to commercialize any product candidate for which we may obtain regulatory approval. To achieve commercial success for any product candidate for which we may obtain marketing approval, we will need to enter into distribution or licensing agreements or other alternative strategic transaction, which may involve divestment of certain assets, with third parties to commercialize and deliver our product candidates to patients and healthcare providers.
We would have to pursue collaborative arrangements regarding the sales and marketing of our products. However, we may not be successful in entering into arrangements with third parties to sell, market and distribute our product candidates or may be unable to do so on terms that are favorable to us, or if we are able to do so, that they would be effective and successful in commercializing our products. In addition, we would have limited control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our product candidates effectively. If we do not establish sales, marketing and distribution capabilities successfully in collaboration with third parties, we will not be successful in commercializing or launching our product candidates in the United States or elsewhere.
The development, manufacture and commercialization of product candidates could expose us to liability claims which could exceed our insurance coverage.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. In addition, a product liability claim could tarnish our reputation, whether or not such claims are covered by insurance or are with or without merit. Such liability claims may, amongst other things, result in:
•decreased demand for any product candidates or drugs that we may develop;
•injury to our reputation and significant negative media attention;
•withdrawal of clinical trial participants;
•clinical trial hold or product recall;
•substantial monetary awards paid to trial participants or patients;
25
•reduced resources of our management to pursue our business strategy; and
•the inability to commercialize any products that we may develop.
The development, manufacture and commercialization of product candidates could expose us to liability claims which could exceed our insurance coverage. A risk of product liability claims is inherent in the development and commercialization of human therapeutic products. Product liability insurance is very expensive and offers limited protection. A product liability claim against us could potentially be greater than the coverage offered and, therefore, have a material adverse effect upon us and our financial position.
If our competitors develop and market products that are safer, more effective or more convenient or less expensive than our products, if any, our commercial opportunity will be reduced or eliminated.
Our commercial opportunity with respect to our products, if approved, will be reduced or eliminated if our competitors develop and market products that are more effective, have fewer side effects, are more convenient or are less expensive than our products, if any. Our competitors include large, fully-integrated pharmaceutical companies and more established biotechnology and medical device companies, many of which have significant resources and expertise in research and development, manufacturing, testing, obtaining regulatory approvals and marketing. It is possible that competitors will succeed in developing technologies that are safer, more effective, more convenient or with a lower cost of goods and price than those in our product candidates, or that would render our technology obsolete or non-competitive. See “—Risks Related to our Intellectual Property” for additional risks relating to our intellectual property protection from generics entering into our commercial markets.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or have a greater likelihood of success.
Because we have limited financial and management resources, we focus on research programs and product candidates that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through divestment, sale, collaboration, licensing, royalty or other strategic alternative arrangements in cases in which it would have been more advantageous for us to retain sole development, manufacture and/or commercialization rights to such product candidate.
26
Risks Related to our Business Operations and our Reliance on Third Parties
The success of our product candidates is influenced by our collaborations with our partners and any adverse developments in our relationship with our partners could materially harm our business.
The success of our product candidates is influenced by our collaborations with our partners. Any adverse developments in our relationship with our partners could materially harm our business. We are subject to a number of risks associated with any collaboration that could be entered into for the development of our product candidates, including the risk that these collaborators may terminate the relevant agreement(s) upon the occurrence of certain specified events, including a material breach by us of any of our obligations under the respective agreements. If any of our partners terminate or breach our agreements with them, or otherwise fail to complete their obligations in a timely manner, it may have a detrimental effect on our financial position by reducing or eliminating the potential for us to receive technology access and license fees, milestones and royalties, reimbursement of development costs, as well as possibly requiring us to devote additional efforts and incur costs associated with pursuing internal development of product candidates. Furthermore, if our partners do not prioritize and commit sufficient resources to programs associated with our product candidates or collaboration product candidates, we or our partners may be unable to commercialize these product candidates, which would limit our ability to generate revenue and become profitable.
We may not be able successful in entering into strategic agreements with third parties for the divestment of our non-core assets, in whole or in part, which may adversely affect our short-term cash position and expose us to increased liabilities.
We have announced that we are focusing our corporate strategy and resources on our small molecule therapeutics business, to become more streamlined with a singular focus on our core research capabilities and emerging pipeline. Inability to streamline or focus on said core capabilities and emerging pipeline may adversely affect our short-term cash position and expose us to increased liabilities, including liabilities related to the early termination of contractual obligations.
27
We have entered, and may in the future enter into, collaboration agreements with third parties for the development of our product candidates, which may adversely affect our ability to generate revenue.
We have entered into and may seek to enter into collaborations or partnerships with third parties for the development, manufacture and/or potential commercialization of our product candidates. Should we seek to collaborate with a third party with respect to a prospective development program, we may not be able to locate a suitable partner or to enter into an agreement on commercially reasonable terms or at all. Even if we succeed in securing partners for the development and commercialization of our product candidates, we have limited control over the time and resources that our partners may dedicate to the development and commercialization of our product candidates. These collaborations pose a number of risks, including the following:
•partners may not have sufficient resources or decide not to devote the necessary resources due to internal constraints such as budget limitations, lack of human resources or a change in strategic focus;
•partners may believe our intellectual property is not valid or is unenforceable or the product candidate infringes on the intellectual property rights of others;
•partners may dispute their responsibility to conduct development and commercialization activities pursuant to the applicable collaboration, including the payment of related costs or the division of any revenue;
•partners may decide to pursue a competitive product developed outside of the collaboration arrangement;
•partners may not be able to obtain, or believe they cannot obtain, the necessary regulatory approvals; or
•partners may delay the development or commercialization of our product candidates in favor of developing or commercializing another party’s product candidate.
Thus, collaboration agreements may not lead to development, manufacture, regulatory approval or successful commercialization of product candidates in the most efficient manner or at all. Some collaboration agreements are terminable without cause on short notice. Once a collaboration agreement is signed, it may not lead to regulatory approval and commercialization of a product candidate. We also face competition in seeking out partners. If we are unable to secure new collaborations that achieve the collaborator’s objectives and meet our expectations, we may be unable to advance our product candidates and may not generate meaningful revenue.
28
We may rely on third-party suppliers of services to conduct preclinical studies and clinical trials and the failure by such third parties to comply with their obligations may delay the studies and trials, as applicable, and/or have an adverse effect on our development program.
We may rely on third-party suppliers of services to conduct preclinical studies and clinical trials and the failure by such third parties to comply with their obligations may delay the studies and trials, as applicable, and/or have an adverse effect on our development program. We have limited resources to conduct preclinical studies and clinical trials and may rely on third-party suppliers of services to conduct our studies and trials. If our third-party suppliers of services become unavailable for any reason, including as a result of the failure to comply with the rules and regulations governing the conduct of preclinical studies and clinical trials, operational failures, such as equipment failures or unplanned facility shutdowns, damage from any event, including fire, flood and earthquake, and impacts from public health crises, business restructuring or insolvency, or if they fail to perform their contractual obligations pursuant to the terms of the agreements entered into with us, such as failing to do the testing, compute the data or complete the reports further to the testing, we may incur delays in connection with the planned timing of our submission of an investigative new drug application, or IND, clinical trial application, or CTA, or NDA submission. For example, primarily due to COVID-19 related restrictions, we have experienced some delays in the start of certain preclinical studies with third-party contract research organizations located in China. Such restrictions, limitations or other events may, in the future, whether as a result of COVID-19 or the outbreak of another infectious disease, result in a period of business and manufacturing disruption, and in reduced operations, any of which could materially affect our business, financial condition and results of operations. If the damage to any of our third-party suppliers of services is extensive or if, for any reason, such suppliers do not operate in compliance with Good Clinical Practices or are unable or refuse to perform their contractual obligations, we will need to find alternative third-party suppliers of services.
If we are required to change or select new third-party suppliers of services, the timing of the work related to preclinical studies and/or clinical trials could be delayed since the number of competent and reliable third-party suppliers to conduct preclinical and clinical work in compliance with good laboratory practice is limited. Any selection of new third-party suppliers to carry out work related to preclinical studies and clinical trials will be time-consuming and will result in additional delays in receiving data, analysis and reports from such third-party suppliers which, in turn, will delay the obtaining of regulatory approval to commercialize our product candidates. Furthermore, such delays could increase our expenditures to develop a product and materially adversely affect our operating results and financial condition.
Our product candidates are complex and difficult to manufacture, which could cause production problems that result in delays in our development. We also rely in whole or in part on third parties for the manufacture and supply of our product candidates and such reliance may adversely affect us if the third parties are unable to fulfill their obligations.
The candidate we selected for clinical development, LMNL6511, will be manufactured by a third-party contract manufacturer. The manufacturing process we and the third parties with whom we contract use to produce our product candidates is complex, and several factors could cause production interruptions, including equipment malfunctions, facility contamination, raw material shortages or contamination, natural disasters, disruption in utility services, human error or disruptions in the operations of our suppliers.
29
If our third-party manufacturing capabilities become unavailable to us for any reason, including as a result of the failure to comply with cGMP regulations, manufacturing problems or other operational failures, such as equipment failures or unplanned facility shutdowns required to comply with cGMP, damage from any event, including fire, flood and earthquake, and impacts from public health crises, business restructuring or insolvency, we may be delayed in manufacturing product candidates and could be unable to meet the regulatory requirements of the FDA, MHRA or other regulatory agencies to obtain market approval for our product candidates or be unable to manufacture and supply product for commercialization. Any such event could delay the supply of a product to conduct clinical trials and, if a product has reached commercialization, could prevent the supply of the product and adversely affect our revenues. If the damage to manufacturing facilities is extensive, we will need to find alternative manufacturing capacity. For example, restarting our in-house manufacturing or, alternatively, the selection of an alternative third-party manufacturer will be time-consuming and costly. Qualification of a new third-party manufacturer, if available, will include an assessment of the capacity of such third-party manufacturer to produce the quantities that may be requested from time to time by us, the manufacturing process and its compliance with cGMP and typically takes approximately 18 months. In addition, the third-party manufacturer will have to familiarize itself with our technology. Any delay in finding an alternative third-party manufacturer of a product, or recommencing our in-house manufacturing of such product, could result in a shortage of the product, delay clinical trial programs and the filing for regulatory approval of a product, and deprive us of potential product revenues.
We expect that we will have to continue to rely on third parties for the manufacture and supply of our product candidates and such reliance may adversely affect us if the third parties are unable to fulfill their obligations. We do not have the resources, facilities or experience to manufacture all of our product candidates on our own, which requires us to shift more production to third-party manufacturers. We may need to rely more heavily on third parties to manufacture and supply product candidates for clinical trials and we may rely on third parties for some time to manufacture and supply large quantities of product for commercial sales. Our reliance on third-party manufacturers will expose us to a number of risks, including failure to perform their contractual obligations under agreements with us, such as a failure to deliver the quantities requested on a timely basis.
As a company based outside of the United States, our business is subject to economic, political, regulatory and other risks associated with international operations.
As a company based in Canada with operations in the United Kingdom, our business is subject to risks associated with conducting business internationally. Most of our suppliers and clinical trial relationships are located outside the United States. Accordingly, our future results could be harmed by a variety of factors, including:
•economic weakness, including inflation, or political instability in particular non-U.S. economies and markets;
•differing and changing regulatory requirements for product approvals;
•differing jurisdictions could present different issues for securing, maintaining or obtaining freedom to operate in such jurisdictions;
•potentially reduced protection for intellectual property rights;
•difficulties in compliance with different, complex and changing laws, regulations and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties and regulations;
•changes in non-U.S. regulations and customs, tariffs and trade barriers;
•changes in a specific country’s or region’s political or economic environment;
•trade protection measures, import or export licensing requirements or other restrictive actions by governments;
30
•differing reimbursement regimes in certain non-U.S. markets;
•negative consequences from changes in tax laws;
•compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
•workforce uncertainty in countries where labor unrest is more common than in the United States;
•litigation or administrative actions resulting from claims against us by current or former employees or consultants individually or as part of class actions, including claims of wrongful terminations, discrimination, misclassification or other violations of labor law or other alleged conduct;
•difficulties associated with staffing and managing international operations, including differing labor relations;
•production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
•business interruptions resulting from geo-political actions, including war, terrorism and other geopolitical tensions, such as the Russia-Ukraine war, or natural disasters and public health crises, such as COVID-19.
We may not receive the full payment of all milestones or royalty payments pursuant to the agreements entered into with third parties.
We may not receive the full payment of all milestones or royalty payments pursuant to the agreements entered or to be entered into with third parties and, consequently, our financial conditions and operating results could be adversely impacted. We have entered into license agreements and other forms of agreements with third parties regarding the development and commercialization of some of our technologies and product candidates. These agreements generally require that the third party pays to us certain amounts upon the attainment of various milestones and possibly include royalties on the sale of the developed product, if approved. There can be no guarantee that we will receive the payments described in those agreements since the development of the product candidates may be cancelled if the research does not yield positive results. Under such circumstances, we would not receive royalties as well. Even if the development of a product yields positive results, all of the risks described herein with respect to the obtaining of regulatory approval are applicable. Finally, if there occurs a disagreement between us and the third party, the payment relating to the attainment of milestones or of royalties may be delayed. The occurrence of any of those circumstances could have a material adverse effect on our financial condition and operating results.
31
If we breach any of the agreements under which we license rights to our product candidates or technology from third parties, we could lose license rights that are important to our business.
If we breach any of the agreements under which we license rights to our product candidates or technology from third parties, we could lose license rights that are important to our business. We license the development, manufacturing and commercialization rights for certain product candidates, and could, potentially, enter into similar licenses for other product candidates in the future. Under these licenses, we are subject to various obligations, including royalty and milestone payments, limits on sublicensing, insurance obligations and the obligation to use commercially reasonable best efforts to develop and exploit the licensed technology. If we fail to comply with any of these obligations or otherwise breach these agreements, our licensors may have the right to terminate the license in whole or in part or to terminate the exclusive nature of the license. Loss of any of these licenses or the exclusivity rights provided therein could harm our financial condition and operating results.
We may be subject to damages resulting from claims that we, our employees, collaborators or consultants have wrongfully used or disclosed alleged trade secrets of third parties.
We may be subject to damages resulting from claims that we, our employees, collaborators or consultants, have wrongfully used or disclosed alleged trade secrets of third parties. Many of our employees were previously employed, and certain of our consultants are currently employed, at universities, public institutions, biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that we, or these employees or consultants, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of these current or former employers. Litigation may be necessary to defend against these claims.
If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. We may be subject to claims that employees of our partners or licensors of technology licensed by us have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. We may become involved in litigation to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel.
32
Our internal information technology systems, or those of our third-party vendors, collaborators or other contractors or consultants, may fail or suffer security breaches, which could result in a significant disruption of our product development programs, give rise to significant liability, subject us to costly and protracted litigation, cause significant reputational harm and impact our ability to operate our business effectively.
We are increasingly dependent upon information technology systems, infrastructure, and data to operate our business. In the ordinary course of business, we collect, store, and transmit confidential information (including but not limited to intellectual property, proprietary business information, and personal information). It is critical that we do so in a secure manner to maintain the confidentiality and integrity of such information. We also have outsourced elements of our operations to third parties, and as a result we manage a number of third-party vendors and other contractors and consultants who have access to our confidential information. The failure of any of these third parties to provide accurate and timely service may adversely impact our business operations. In addition, if such third-party service providers were to cease operations, temporarily or permanently, face financial distress or other business disruption, or increase their fees, or if our relationships with these providers deteriorate, we could suffer increased costs until a replacement provider is secured or an alternative solution is implemented, if possible.
Our internal information technology systems and those of our current and any future third-party vendors, collaborators and other contractors or consultants may be vulnerable to a variety of disruptive elements, including cyber-attacks by malicious third parties (including the deployment of computer viruses, harmful malware, ransomware, denial-of-service attacks, social engineering, and other means to affect service reliability and threaten the confidentiality, integrity, and availability of information), unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. In particular, the risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity, and sophistication of attempted attacks and intrusions from around the world have increased, and phishing and social engineering attacks have increased in particular in connection with the COVID-19 pandemic. We have, and may continue to, experience various phishing scams. Through a recent phishing scam, an intruder was able to re-direct funds payable to an employee to a fraudulent bank account, by pretending to be the beneficiary employee. The attempt was identified and stopped before any funds were transferred to the fraudulent account. To lessen the impact of such scams, we train our employees on the risks of these phishing attempts and how to identify them and we implemented other types of employee identification measures.
We may not be able to anticipate all types of security threats, and we may not be able to implement preventive measures effective against all such security threats. The techniques used by cyber criminals change frequently, may not be recognized until launched, and can originate from a wide variety of sources, including outside groups such as external service providers, organized crime affiliates, terrorist organizations, or hostile foreign governments or agencies. While we have not experienced any significant system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations or a loss of, or damage to, our data or applications, or those of our third-party vendors and other collaborators, contractors and consultants, it could result in a disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other proprietary information, significant delays or setbacks in our research, or other similar disruptions. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur significant liability, our competitive position could be harmed, our reputation could be damaged, and the further development and commercialization of our product candidates could be delayed. In addition, any such event that leads to unauthorized access, use, or disclosure of personal information, including personal information regarding our patients or employees, could compel us to comply with federal and/or state breach notification laws and foreign law equivalents, subject us to mandatory corrective action, and otherwise subject us to liability under laws and regulations that protect the privacy and security of personal information.
33
The costs related to significant security breaches or disruptions could be material and exceed the limits of scope of the cybersecurity insurance we maintain against such risks. If the information technology systems of our third-party vendors and other collaborators, contractors and consultants become subject to disruptions or security breaches, we may be exposed to material liability and have insufficient recourse against such third parties and we may have to expend significant resources to mitigate the impact of such an event, and to develop and implement protections to prevent future events of this nature from occurring.
Any potential noncompliance with personal data protection laws and regulations could have a material adverse effect on our results of operations and financial condition.
In the ordinary course of business, we collect, store, and transmit confidential information (including but not limited to intellectual property, proprietary business information, and personal information). We operate in an environment that relies on our collection, storage, and transmission of large sets of individuals’ personal information. Also, the operation of our business requires data to flow freely across borders of numerous countries in which there are different, and potentially conflicting, frequently changing data privacy laws in effect. We are subject to various state and foreign laws governing the privacy and security of health information in certain circumstances. For example, the EU General Data Protection Regulation, or GDPR, which took effect in May 2018, and the California Consumer Privacy Act, which took effect in January 2020, impose strict requirements on how we and third parties with whom we contract collect, share, export or otherwise process personal information, and provide for significant penalties for noncompliance. Breaches of our systems or those of our third-party contractors could risk exposure of such personal information to unauthorized persons.
Any event involving the substantial loss of personal information could result in significant liability, reputational harm, damaged relationships with business partners, and monetary penalties under laws enacted or being enacted around the world, any of which may have a negative impact on our business and operations.
We may be subject to environmental remediation obligations or other obligations under environmental laws and regulations and climate change could exacerbate certain of the threats facing our business.
We may be subject to environmental remediation obligations or other obligations under environmental laws and regulations and climate change could exacerbate certain of the threats facing our business. We are subject to laws and regulations concerning the environment, safety matters, regulation of chemicals and product safety in the countries where we operate our business. These requirements include regulation of the handling, manufacture, transportation, use and disposal of materials, including the discharge of pollutants into the environment. In the normal course of our business, hazardous substances may be released into the environment, which could cause environmental or property damage or personal injuries, and which could subject us to remediation obligations regarding contaminated soil and groundwater or potential liability for damage claims.
In addition, global climate change could exacerbate certain of the threats facing our business, including the business continuity depends on how well we protect our facilities and equipment. Several areas of our operations further raise environmental considerations, such as greenhouse gas emissions and disposal of hazardous residual materials. Failure to recognize and adequately respond to changing governmental and public expectations on environmental matters could result in fines, missed opportunities, additional regulatory scrutiny or harm to our brand and reputation which could potentially have an adverse effect on our business and financial results.
34
Risks Related to our Intellectual Property
Our failure to protect our intellectual property may have a material adverse effect on our ability to develop, commercialize or partner our product candidates.
Our failure to protect our intellectual property may have a material adverse effect on our ability to develop, commercialize or partner our product candidates. We will be able to protect our intellectual property rights from unauthorized use by third parties only to the extent that our intellectual property rights are covered by valid and enforceable patents or are effectively maintained as trade secrets. We try to protect our intellectual property by filing patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. Because the patent position of pharmaceutical companies involves complex legal and factual questions, the issuance, scope and enforceability of patents cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated or circumvented. If our patents are invalidated or found to be unenforceable, we will lose the ability to exclude others from making, using or selling the inventions claimed. Moreover, an issued patent does not guarantee us the right to use the patented technology or commercialize a product using that technology. Third parties may have blocking patents that could be used to prevent us from developing our product candidates, selling our products, if any, or commercializing our patented technology. Thus, patents that we own may not allow us to exploit the rights conferred by our intellectual property protection. Moreover, our pending patent applications may not result in patents being issued. Even if issued, they may not be issued with claims sufficiently broad to protect our product candidates and technologies or may not provide us with a competitive advantage against competitors with similar product candidates or technologies. Furthermore, others may independently develop product candidates or technologies similar to those that we have developed or discover our trade secrets. In addition, the laws of many countries do not protect intellectual property rights to the same extent as the laws of Canada, Europe and the United States, and those countries may also lack adequate rules and procedures for defending intellectual property rights effectively. There can be no guarantee that we will receive patents in countries where we file patent applications for our product candidates. As a result, the validity and enforceability of our patents cannot be predicted with certainty. In addition, we cannot guarantee that:
•we or our licensors were the first to make the inventions covered by each of our issued patents and pending patent applications;
•we or our licensors were the first to file patent applications for these inventions;
•others will not independently develop similar or alternative technologies or duplicate any of our or our licensors’ technologies;
•any of our or our licensors’ pending patent applications will result in issued patents;
•that there are no prior public disclosures that could invalidate our or our licensors' patents, as the case may be;
•that there are not unpublished applications or patent applications maintained in secrecy that may later issue with claims covering our product candidates;
•that others will not circumvent our owned or in-licensed patents;
•our owned or in-licensed issued patents will provide us with any competitive advantages or that it will not be narrowed in scope or be held invalid or unenforceable as a result of legal challenges by third parties;
•any of our or our licensors’ patents will be valid or enforceable;
•any patents issued to us or our licensors and collaboration partners will provide us with any competitive advantages, or will not be challenged by third parties;
•we will develop or in-license additional proprietary technologies that are patentable; or
•the patents of others will not have an adverse effect on our business.
35
We rely on trade secrets, know-how and technology, which are not protected by patents, to maintain our competitive position.
We also rely on trade secrets, know-how and technology, which are not protected by patents, to maintain our competitive position. We try to protect this information by entering into confidentiality undertakings with parties that have access to it, such as our current and prospective suppliers, employees and consultants. Any of these parties may breach the undertakings and disclose the confidential information to our competitors. Enforcing a claim that a third party illegally obtained and is using trade secrets is expensive and time consuming and the outcome is unpredictable. In addition, it could divert management’s attention. If any trade secret, know-how or other technology not protected by a patent were to be disclosed to or independently developed by a competitor, our competitive position could be harmed.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business.
We may need to obtain additional licenses from others to advance our research and development activities or allow the commercialization of our product candidates. Our license agreements impose, and we expect that future license agreements will impose, various development, diligence, commercialization, and other obligations on us. Our licensors might conclude that we have materially breached our obligations under such license agreements and might therefore terminate the license agreements, thereby removing or limiting our ability to develop and commercialize product candidates and technology covered by these license agreements. If these in-licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors or other third parties would have the freedom to seek regulatory approval of, and to market, product candidates identical to ours and we may be required to cease our development and commercialization of any related product. In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. Disputes may arise regarding intellectual property subject to a licensing agreement, including:
•the scope of rights granted under the license agreement and other interpretation-related issues;
•the extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
•the sublicensing of patent and other rights under our collaborative development relationships;
•our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
•the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and
•the priority of invention of patented technology.
We may not be able to protect our intellectual property rights throughout the world.
We may not be able to protect our intellectual property rights throughout the world. Filing, prosecuting and defending patents on all of our product candidates and products, if any, when and if we have any, in every jurisdiction would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we or our licensors have not obtained patent protection to develop our own product candidates. These product candidates may compete with our product candidates, when and if we have any, and may not be covered by any of our or our licensors’ patent claims or other intellectual property rights.
36
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of Canada and the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of Canada and the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology and/or pharmaceuticals, which could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee’s former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm and rely on our outside counsels to pay these fees due to non-U.S. patent agencies. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
37
Patent protection for our product candidates or products, if any, may expire before we are able to maximize their commercial value, which may subject us to increased competition and reduce or eliminate our opportunity to generate product revenue.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Patent protection for our product candidates or products, if any, may expire before we are able to maximize their commercial value, which may subject us to increased competition and reduce or eliminate our opportunity to generate product revenue. The patents for our product candidates have varying expiration dates and, when these patents expire, we may be subject to increased competition and may not be able to recover our development costs. In some of the larger economic territories, such as Canada, the United States and Europe, patent term extension/restoration may be available to compensate for time taken during aspects of the product candidate’s regulatory review. However, we cannot be certain that an extension will be granted, or if granted, what the applicable time period or the scope of patent protection afforded during any extended period will be. In addition, even though some regulatory agencies may provide some other form of exclusivity for a product candidate under its own laws and regulations, we may not be able to qualify the product candidate or obtain the exclusive time period.
If we are unable to obtain patent term extension/restoration or some other exclusivity, we could be subject to increased competition and our opportunity to establish or maintain product revenue could be substantially reduced or eliminated. Furthermore, we may not have sufficient time to recover our development costs prior to the expiration of our Canadian and non-Canadian patents.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and may be unable to protect our rights to, or use of, our technology.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and may be unable to protect our rights to, or use of, our technology. If we choose to go to court to restrain a third party from using the inventions claimed in our patents or licensed patents, that individual or corporation has the right to ask the court to rule that these patents are invalid and/or should not be enforced against that third party. These lawsuits are expensive and would consume time and other resources even if we were successful in stopping the infringement of these patents.
In addition, there is a risk that the court will decide that these patents are invalid or unenforceable and that we do not have the right to refrain the third party from using the inventions. There is also the risk that, even if the validity of these patents is upheld, the court will refuse to grant a decision or judgment in our favor on the ground that such other party’s activities do not infringe our rights.
If we wish to use the technology or compound claimed in issued and unexpired patents owned by a third party, we will need to obtain a license from such third party, enter into litigation to challenge the validity or enforceability of the patents or incur the risk of litigation in the event that the owner asserts that we infringed its patents. The failure to obtain a license to technology or the failure to challenge an issued patent that we may require to develop or commercialize our product candidates may have a material adverse impact on our operating results and financial condition.
If a third party asserts that we infringed their patents or other proprietary rights, we could face a number of risks that could seriously harm our results of operations, financial condition and competitive position, including:
•patent infringement and other intellectual property claims, which would be costly and time consuming to defend, whether or not the claims have merit, and which could delay the regulatory approval process and divert management’s attention from our business;
•substantial damages for past infringement (including treble damages and attorneys’ fees for willful infringement), which we may have to pay if a court determines that our product candidates or technologies infringe a competitor’s patent or other proprietary rights;
38
•a court prohibiting us from selling or licensing our technologies or future drugs unless the third party licenses its patents or other proprietary rights to us on commercially reasonable terms, which it is not required to do; and
•if a license is available from a third party, we may have to pay substantial royalties or lump sum payments or grant cross licenses to its patents or other proprietary rights to obtain that license.
The biopharmaceutical industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our product candidates or methods of use either do not infringe the patent claims of the relevant patent and/or that the patent claims are invalid, and we may not be able to do this. Proving invalidity during a court proceeding, in particular, is difficult since it requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents.
Canadian patent laws as well as the laws of some foreign jurisdictions provide for provisional rights in published patent applications beginning on the date of publication, including the right to obtain reasonable royalties, if a patent is subsequently issued and certain other conditions are met. We cannot predict the outcome of any invalidity challenge. Alternatively, it is possible that we may determine it prudent to seek a license from the patent holder to avoid potential litigation and other potential disputes. We cannot be sure that a license would be available to us on acceptable terms, or at all.
Because some patent applications in the United States may be maintained in secrecy until the patents are issued, because patent applications in Canada and many foreign jurisdictions are typically not published until 18 months after filing, and because publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our licensors’ issued patents or our pending applications or our licensors’ pending applications, or that we or our licensors were the first to invent the technology.
39
Patent applications filed by third parties that cover technology similar to our technology may have priority over our or our licensors’ patent applications and could further require us to obtain rights to issued patents covering such technologies. If another party files a U.S. patent application on an invention similar to ours, we may elect to participate in or be drawn into an interference or derivation proceeding at the U.S. Patent and Trademark Office, or USPTO, to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in a loss of our U.S. patent position with respect to such inventions.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations. We cannot predict whether third parties will assert these claims against us or against our licensors, or whether those claims will harm our business. If we are forced to defend against these claims, whether they are with or without any merit, whether they are resolved in favor of or against us or our licensors, we may face costly litigation and diversion of management’s attention and resources. As a result of these disputes, we may have to develop costly non-infringing technology, or enter into licensing agreements. These agreements, if necessary, may be unavailable on terms acceptable to us, if at all, which could seriously harm our business or financial condition. Even if a license is offered to us, it may be non-exclusive and our competitors may gain access to the same technology.
Our commercial success depends, in part, on our ability not to infringe on third-parties’ patents and other intellectual property rights. Our capacity to commercialize our product candidates will depend, in part, on the non-infringement of third-parties’ patents and other intellectual property rights. The biopharmaceutical and pharmaceutical industries have produced a multitude of patents and it is not always clear to participants, including us, which patents cover various types of products or methods of use. The scope and breadth of patents is subject to interpretation by the courts and such interpretation may vary depending on the jurisdiction where the claim is filed and the court where such claim is litigated. The holding of patents by us for our product candidates and our applications does not guarantee that we are not infringing on other third-parties’ patents and there can be no guarantee that we will not be in violation of third-parties’ patents and other intellectual property rights. Patent analysis for non-infringement is based in part on a review of publicly available databases. Although we review from time to time certain databases to conduct patent searches, we do not have access to all databases. It is also possible that some of the information contained in the databases has not been reviewed by us or was found to be irrelevant at the time the searches were conducted. In addition, because patents take years to be issued, there may be currently pending applications that we are unaware of which may later be issued. As a result of the foregoing, there can be no guarantee that we will not violate third-party patents. Because of the difficulty in analyzing and interpreting patents, there can be no guarantee that a third party will not assert that we infringe upon any of its patents or any of its other intellectual property rights.
There is no guarantee that we will not become involved in litigation. Litigation with any third party, even if the allegations are without merit, is expensive, time-consuming and will divert management’s attention from the daily execution of our business plan. Litigation implies that a portion of our financial assets would be used to sustain the costs of litigation instead of being allocated to further the development of our business plan. If we are involved in patent infringement litigation, we will need to demonstrate that our product candidates do not infringe the patent claims of the relevant patent, that the patent claims are invalid or that the patent is unenforceable. If we were found liable for infringement of third-parties’ patents or other intellectual property rights, we could be required to enter into royalty or licensing agreements on terms and conditions that may not be favorable to us, and/or pay damages, including up to treble damages (but only if found liable of willful infringement) and/or cease the development and commercialization of our product candidates. Any finding that we are guilty of patent infringement could materially adversely affect our business, financial conditions and operating results.
40
There may be issued patents that we are unaware of that our product candidates may infringe, or patents that we believe we do not infringe but could be found to be infringing.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
Changes in either the patent laws or interpretation of the patent laws in the United States or other jurisdictions could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. For example, prior to March 2013, the first to invent a claimed invention was entitled to a patent in the United States, assuming that other requirements for patentability were met, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act (or the America Invents Act), enacted in September 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application is entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. The America Invents Act also included a number of significant changes that affect the way patent applications are prosecuted and that provide additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter parties review, and derivation proceedings. These changes have increased the uncertainties related to patent prosecution and patent challenges.
In addition, the patent positions of companies in the development and commercialization of pharmaceuticals are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. The laws and regulations governing patents could change in unpredictable ways depending on future actions by the U.S. Congress, the federal courts, the USPTO, and the legislative, judicial, and regulatory branches of other jurisdictions in which we may seek patent protection, and such changes could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
Risks Related to Legal Compliance Matters
Our current and future operations are subject to extensive healthcare laws, regulation and enforcement and failure to comply with those laws could have a material adverse effect on our results of operations and financial condition.
Healthcare providers, physicians and third-party payors will play a primary role in the recommendation and prescription of any product we may develop and any product for which we obtain marketing approval. Our current and future operations expose us to broadly applicable healthcare laws and regulations that may affect the business or financial arrangements and relationships through which we research, market, sell and distribute our products, if any. The laws that may affect our operations in the United States include, but are not limited to:
•the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs;
•federal civil and criminal false claims laws, including the False Claims Act, which can be enforced by private individuals through civil whistleblower or qui tams actions, and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent;
41
•the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which, among other things, created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations, which imposes certain requirements on certain covered healthcare providers, health plans, and healthcare clearinghouses as well as their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information, and their covered subcontractors, relating to the privacy, security, and transmission of individually identifiable health information;
•the Physician Payments Sunshine Act, enacted as part of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or, collectively, ACA, which requires certain manufacturers of drugs, devices, and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the Centers for Medicare & Medicaid Services, or CMS, information related to: (i) payments and other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), other healthcare professionals (such as physician assistants and nurse practitioners), and teaching hospitals; and (ii) ownership and investment interests held by physicians and their immediate family members; and
•foreign and state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require product manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; state and local laws that require the registration of pharmaceutical sales representatives; and state and foreign laws governing the privacy and security of health information in certain circumstances, including General Data Protection Regulations, or GDPR, many of which differ from each other in significant ways, thus complicating compliance efforts.
The scope of these laws and our lack of experience in establishing the compliance programs necessary to comply with this complex and evolving regulatory environment increases the risks that we may violate the applicable laws and regulations. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to significant penalties, including civil, criminal, and administrative penalties, damages, fines, disgorgement, imprisonment, the curtailment or restructuring of our operations, diminished profits and future earnings, the exclusion from participation in federal and state healthcare programs, additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, any of which could materially adversely affect our ability to operate our business and our financial results.
42
Third-party payors, including governmental health administration authorities, private health insurers and other organizations, may not provide coverage and adequate reimbursement for our products, if any, and related treatment.
Our ability to successfully commercialize any of our product candidates, if approved, will depend in part on the extent to which coverage and adequate reimbursement will be available from third-party payors, including government payors, such as Medicare and Medicaid, private health insurers and health maintenance organizations. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement rates, but also have their own methods and approval process apart from Medicare determinations. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us or our collaborators to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Patients who are prescribed medications for the treatment of their conditions, and their prescribing physicians, generally rely on third-party payors to reimburse all or part of the costs associated with their prescription drugs. Therefore, patients are unlikely to use our products unless coverage and reimbursement is adequate to cover all or a significant portion of the cost of our products. If coverage and reimbursement are not available or reimbursement is available only to limited levels, we may not successfully commercialize any product candidate for which we obtain marketing approval.
Healthcare legislative reform measures may have a material adverse effect on our business and results of operations.
The trend toward managed healthcare in the United States, the growth of organizations such as HMOs and MCOs, and legislative proposals to reform healthcare and government insurance programs could significantly influence the purchase of pharmaceutical products, resulting in lower prices and a reduction in product demand.
In the United States and some foreign jurisdictions there have been, and continue to be, several legislative and regulatory changes and proposed reforms of the healthcare system to contain costs, improve quality, and expand access to care. In the United States, there have been and continue to be a number of healthcare-related legislative initiatives that have significantly affected the pharmaceutical industry. For example, the ACA was passed in March 2010, and substantially changed the way healthcare is financed by both governmental and private insurers and continues to significantly impact the U.S. pharmaceutical industry.
There have been executive judicial and Congressional challenges to certain aspects of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the ACA.
For example, the Tax Cuts and Jobs Act of 2017, or the Tax Act, included a provision that repealed, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” On June 17, 2021, the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Further, on August 16, 2022, President Biden signed the Inflation Reduction Act of 2022, or IRA, into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the "donut hole" under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges and the healthcare reform measures of the Biden administration will impact the ACA.
43
Moreover, there has been heightened governmental scrutiny in the United States of pharmaceutical pricing practices in light of the rising cost of prescription drugs. Such scrutiny has resulted in several recent Congressional inquiries, Presidential executive orders, and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products.
At the federal level, the Trump administration used several means to propose or implement drug pricing reform, including through federal budget proposals, executive orders and policy initiatives. In July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to Biden’s executive order, on September 9, 2021, the U.S. Department of Health and Human Services, or HHS, released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. Further, the IRA, among other things (i) directs HHS to negotiate the price of certain high-expenditure, single-source drugs and biologics covered under Medicare and (ii) imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation. These provisions will take effect progressively starting in fiscal year 2023, although they may be subject to legal challenges. It is currently unclear how the IRA will be implemented but is likely to have a significant impact on the pharmaceutical industry. Additionally, the Biden administration released an additional executive order on October 14, 2022, directing HHS to report on how the Center for Medicare and Medicaid Innovation can be further leveraged to test new models for lowering drug costs for Medicare and Medicaid beneficiaries. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Current and future healthcare legislation could have a significant impact on our business. There is uncertainty with respect to the impact these changes, if any, may have, and any changes likely will take time to unfold. Any additional federal or state healthcare reform measures could limit the amounts that third-party payors will pay for healthcare products and services, and, in turn, could significantly reduce the projected value of certain development projects and reduce our profitability.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations, which can harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, the Foreign Corrupt Practices Act, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We may engage third parties to develop, manufacture or sell our products, if any, outside the United States, to conduct clinical trials, and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other collaborators, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences.
44
Risks Related to our Common Shares and our Status as a Public Company
If we fail to comply with the continued listing requirements of Nasdaq, we would face possible delisting, which would result in a limited public market for our common shares and make obtaining future debt or equity financing more difficult for us.
Our common shares may be delisted if we fail to maintain certain listing requirements of the Nasdaq Stock Market (“Nasdaq”). On March 4, 2022, we received notice from the Listing Qualifications Department of Nasdaq indicating that, for the last 30 consecutive business days, the bid price for the common stock had closed below the minimum $1.00 per share and as a result, we were no longer in compliance with the Nasdaq Listing Rule 5550(a)(2). We regained compliance with the minimum bid price rule on February 15, 2023.
We cannot assure you that we will continue to comply with the requirements for continued listing on the Nasdaq Capital Market in the future. If our common shares is delisted from the Nasdaq Capital Market, the common shares would likely trade in the over-the-counter market. If our shares were to trade on the over-the-counter market, selling the shares could be more difficult because smaller quantities of shares would likely be bought and sold, transactions could be delayed, and security analysts’ coverage of us may be reduced. In addition, in the event the common shares is delisted, broker-dealers have certain regulatory burdens imposed upon them, which may discourage broker-dealers from effecting transactions in the common stock, further limiting the liquidity of the common shares. These factors could result in lower prices and larger spreads in the bid and ask prices for the common shares. Such delisting from the Nasdaq Capital Market and continued or further declines in our share price could also greatly impair our ability to raise additional necessary capital through equity or debt financing and could significantly increase the ownership dilution to shareholders caused by our issuing equity in financing or other transactions.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and share price.
The global economy, including credit and financial markets, has experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates, higher interest rates, increases in inflation rates and uncertainty about economic stability. For example, the COVID-19 pandemic resulted in widespread unemployment, economic slowdown and extreme volatility in the capital markets. Similarly, the Russia-Ukraine war has created extreme volatility in the global capital markets and is expected to have further global economic consequences, including disruptions of the global supply chain and energy markets. Any such volatility and disruptions may have adverse consequences on us or the third parties on whom we rely. If the equity and credit markets deteriorate, including as a result of recent bank closures, political unrest or war, or as a result of a financial crisis, it may make any necessary debt or equity financing more difficult to obtain in a timely manner or on favorable terms, more costly or more dilutive. Inflation can adversely affect us by increasing our costs. Any significant increases in inflation and related increase in interest rates could have a material adverse effect on our business, results of operations and financial condition.
The price of our common shares historically has been volatile and fluctuates substantially, and you could lose all or part of your investment.
The market prices for the securities of biopharmaceutical companies, including us, have historically been volatile. The market has from time-to-time experienced extreme price and volume fluctuations that are unrelated to the operating performance of any particular company.
45
The trading price of our common shares could continue to be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including: the results and adequacy of our preclinical studies and clinical trials, as well as those of its collaborators, or its competitors; other evidence of the safety or effectiveness of our product candidates or those of our competitors; announcements of technological innovations or new product candidates by us or our competitors; governmental regulatory actions; developments with collaborators; developments (including litigation) concerning patent or other proprietary rights of us or our competitors; concern as to the safety of our product candidates; period-to-period fluctuations in operating results; changes in estimates of our performance by securities analysts; market conditions for biotechnology stocks in general; rising inflations and related increases in interest rates; bank failures; geo-political actions, including war and terrorism, or natural disasters and public health crises; and other factors not within our control could have a significant adverse impact on the market price of our securities, regardless of our operating performance. These and other market and industry factors may cause the market price and demand for our common shares to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from selling their shares at or above the price paid for the shares and may otherwise negatively affect the liquidity of our common shares.
In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted. A class action suit against us could result in substantial costs, potential liabilities and the diversion of management’s attention and resources. Furthermore, during the course of litigation, there could be negative public announcements of the results of hearings, motions or other interim proceedings or developments, which could have a negative effect on the market price of our common shares.
We expect to issue common shares in the future. Holders of stock options and warrants may elect to exercise their options or warrants into common shares depending on the stock price. Future issuances of common shares, or the perception that such issuances are likely to occur, could affect the prevailing trading prices of our common shares. Future issuances of our common shares could result in substantial dilution to our shareholders. In addition, the existence of warrants may encourage short selling by market participants.
Any future indebtedness we may incur, could lead to adverse consequences and will have priority over our common shares with respect to payment in the event of a liquidation, dissolution or winding up.
In any liquidation, dissolution or winding up of us, our common shares would rank below all debt claims against us. In addition, any convertible or exchangeable securities or other equity securities currently outstanding or that we may issue in the future may have rights, preferences and privileges more favorable than those of our common shares. In addition, if we are unable to repay, refinance or restructure our indebtedness when payment is due, the lenders could proceed against any collateral granted to them to secure such indebtedness or force us into bankruptcy or liquidation. As a result, holders of our common shares may not be entitled to receive any payment or other distribution of assets upon the liquidation or dissolution until after our obligations to our debt holders and holders of equity securities that rank senior to our common shares have been satisfied.
We are a “controlled company” within the meaning of the applicable Nasdaq listing rules and, as a result, qualify for exemptions from certain corporate governance requirements. If we rely on these exemptions, you will not have the same protections afforded to shareholders of companies that are subject to such requirements.
We believe SALP will continue to control a majority of the voting power of our outstanding common shares for the foreseeable future. As a result, we will be a “controlled company” within the meaning of applicable Nasdaq listing rules. Under these rules, a company of which more than 50% of the voting power for the election of directors is held by an individual, group or another company is a “controlled company.” For so long as we remain a “controlled company,” we may elect not to comply with certain corporate governance requirements, including the requirements:
•that a majority of the board of directors consists of independent directors;
46
•for an annual performance evaluation of the HR and compensation committee; and
•that we have a HR and compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities.
We may continue to use all or some of these exemptions in the future. As a result, you may not have the same protections afforded to shareholders of companies that are subject to all of the Nasdaq corporate governance requirements.
We expect that Structured Alpha LP, or SALP, will continue to own a significant percentage of our common shares and will be able to exert significant control over matters subject to shareholder approval.
SALP is currently our majority shareholder and we expect that SALP will continue to be our controlling shareholder in the future. As of the date hereof, SALP beneficially owns approximately 64.0% of the voting power of our outstanding share capital, and although we have registered for resale all of SALP’s shares, we believe SALP will continue to remain a controlling shareholder for the foreseeable future. Therefore, SALP will have the ability to substantially influence us and exert significant control through this ownership position. For example, SALP may be able to control elections of directors, issuance of equity, including to our employees under equity incentive plans, amendments of our organizational documents, or approval of any merger, amalgamation, sale of assets or other major corporate transaction. SALP’s interests may not always coincide with our corporate interests or the interests of other shareholders, and it may exercise its voting and other rights in a manner with which you may not agree or that may not be in the best interests of our other shareholders. Further, there may be changes to the management or ownership of SALP that could impact SALP’s interests in a way that may not coincide with our corporate interests or the interests of other shareholders. So long as SALP continues to own a majority of our equity, it will continue to be able to strongly influence and effectively control our decisions.
Our ability to raise capital may be limited by applicable laws and regulations.
Using a shelf registration statement on Form F-3 to raise additional capital generally takes less time and is less expensive than other means, such as conducting an offering under a Form F-1 registration statement. However, our ability to raise capital using a shelf registration statement may be limited by, among other things, SEC rules and regulations. Under SEC rules and regulations, if our public float (the market value of our common stock held by non-affiliates) is less than $75.0 million, then the aggregate market value of securities sold by us or on our behalf under our Form F-3 in any 12-month period is limited to an aggregate of one-third of our public float. As our public float is currently less than $75.0 million, we are currently subject to this limitation. If our ability to utilize a Form S-3 registration statement for a primary offering of our securities continues to be limited to one-third of our public float, we may conduct such an offering pursuant to an exemption from registration under the Securities Act or under a Form F-1 registration statement, and we would expect either of those alternatives to increase the cost of raising additional capital relative to utilizing a Form F-3 registration statement.
As a controlled company, we may not be able to sustain an active, liquid and orderly trading market for our common stock.
We have a limited number of shares that trade each day compared to many other companies, especially given our status as a controlled company, which limits the number of shares actually available to be bought and sold by shareholders in the public market. You may not be able to sell your shares quickly or at the market price if trading in shares of our common stock is not active.
Further, a lesser active market may impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic partnerships or acquire companies or products by using our shares of common stock as consideration.
47
Sales of our common stock in the public market could cause the market price of our common stock to decline.
Sales of a substantial number of shares of our common stock in the public market, including those held by SALP, or the perception that these sales might occur, could depress the market price of our common shares and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the timing of or the effect that such sales may have on the prevailing market price of our common shares.
Our organizational and ownership structure may create significant conflicts of interests.
Our organizational and ownership structure involves a number of relationships that may give rise to certain conflicts of interest between us and minority holders of our common shares, on the one hand, and SALP and its owners, on the other hand. Certain of our directors who have been nominated to our board by SALP have economic exposure to the financial performance of SALP and its investments, including our shares, and, accordingly, their interests may be aligned with SALP’s interests, which may not always coincide with our corporate interests or the interests of our other shareholders. Further, our other shareholders may not have visibility into the economic exposure of such directors to the financial performance of SALP’s investments, including our shares, which may change at any time through acquisition, disposition, dilution, or otherwise. Any change in the economic exposure of directors nominated by SALP for election to our board to the financial performance of SALP and its investments, including our shares, could impact the interests of those holders.
In addition, SALP is entitled to nominate two directors for election to our board of directors. While any directors nominated by SALP must be the subject of a favorable recommendation of our HR and compensation committee and must act in accordance with their fiduciary duties to our other shareholders, such directors’ interests may not coincide with the interests of our other shareholders.
In addition, we are party to certain related party agreements with SALP. SALP and its partners, as well as directors nominated by SALP, may have interests which differ from our interests or those of the minority holders of our common shares. These agreements are, and any future material transaction between us and SALP or any other affiliate of SALP will be, subject to our related party transaction policy and the standards of conduct contained in our Code of Ethics and Business Conduct under which a director is expected to declare his or her interest in any transaction or agreement in which he or she may have a material interest, and as circumstances warrant, abstain from voting on the approval of any such transaction or agreement. To the extent we fail to appropriately deal with any such related-party transaction or conflict of interest, it could negatively impact our reputation and ability to raise additional funds and the willingness of counterparties to do business with us, all of which could have an adverse effect on our business, financial condition, results of operations, and cash flows.
48
We will continue to incur significantly increased costs as a result of operating as a reporting company whose common shares are publicly traded in the United States and is unable to use the Multijurisdictional Disclosure System, and our management will be required to devote substantial time to new compliance initiatives.
As a public company whose shares are publicly traded in the United States and reporting under the Exchange Act and is no longer eligible to report under the Multijurisdictional Disclosure System, we incur significant legal, accounting and other expenses that we did not incur previously. These expenses will likely be even more significant after we no longer qualify as an emerging growth company. The Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of the Nasdaq and other applicable securities rules and regulations impose various requirements on public companies in the United States, including the establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our senior management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified senior management personnel or members for our board of directors.
However, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404(a), we will be required to furnish a report by our senior management on our internal control over financial reporting. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm, pursuant to Section 404(b). To prepare for eventual compliance with Section 404(b), we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404.
Compliance with laws and regulations affecting public companies may cause us to incur additional expenses and may also increase the compliance risks associated with such changes, which may have a material adverse effect on our financial condition and operating results.
The occurrence of any changes in the laws and regulations applicable to public companies, including any changes to the existing disclosure obligations under applicable Canadian securities laws and regulations and related rules and policies, may cause us to incur additional expenses associated with the assessment of the impacts of such changes as well as a result of the implementation and monitoring of its compliance obligations, including any new internal processes and controls that need to be implemented or reporting requirements as a result of such changes.
49
Any changes in the laws and regulations affecting public companies may also increase the compliance risks associated with such changes, which could result in enforcement actions, penalties or lawsuits, which may have a material adverse effect on our financial condition and operating results. Any such increased compliance risks may make it more difficult for us to comply with our indemnification obligations and to secure appropriate directors and officers’ liability insurance policies or may result in a significant increase in the cost to secure appropriate insurance coverage. We may not be able to afford a significant increase in the costs to secure appropriate directors and officers’ liability insurance coverage and may have to secure reduced coverage limits or settle for a higher retention amount for indemnifiable losses, which may not cover the claims against our past, present or future directors for which we are bound to indemnify our directors and officers. Any reduced limit in the insurance coverage or increase of the retention amount for indemnifiable losses may result in a difficulty to attract and retain experienced and qualified directors to serve on our board of directors and officers.
We are an “emerging growth company” and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common shares may be less attractive to investors.
We are an “emerging growth company” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404, exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We may take advantage of these exemptions until we are no longer an emerging growth company. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the aggregate market value of our common shares held by non-affiliates exceeds $700 million as of the end of our second fiscal quarter before that time, in which case we would no longer be an emerging growth company as of the following December 31st (the last day of our fiscal year). We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and the price of our common shares may be more volatile.
Under Section 107(b) of the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We qualify as a foreign private issuer and, as a result, we will not be subject to U.S. proxy rules and will be subject to Exchange Act reporting obligations that permit less detailed and frequent reporting than that of a U.S. domestic public company.
We report under the Exchange Act as a non-U.S. company with foreign private issuer status. Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; (ii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and (iii) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K upon the occurrence of specified significant events. In addition, foreign private issuers are not required to file their annual report until 120 days after the end of each fiscal year, while U.S. domestic issuers that are accelerated filers are required to file their annual report on Form 10-K within 75 days after the end of each fiscal year.
50
Foreign private issuers also are exempt from Regulation FD, aimed at preventing issuers from making selective disclosures of material information. As a result of the above, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
If we lose our status as a foreign private issuer, we would be required to comply with the Exchange Act reporting and other requirements applicable to U.S. domestic issuers, which are more detailed and extensive than the requirements for foreign private issuers. We may also be required to make changes in our corporate governance practices in accordance with various SEC and Nasdaq rules. The regulatory and compliance costs to us under U.S. securities laws if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer may be significantly higher than the cost we would incur as a foreign private issuer. As a result, we expect that a loss of foreign private issuer status would increase our legal and financial compliance costs and would make some activities highly time consuming and costly. We also expect that if we were required to comply with the rules and regulations applicable to U.S. domestic issuers, it would make it more difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our board of directors.
U.S. investors may be unable to enforce certain judgments.
We are a company existing under the CBCA. Most of our directors and officers are residents of Canada or otherwise reside outside the United States, and substantially all of our assets are located outside the United States. As a result, it may be difficult to effect service within the United States upon us or upon some of our directors and officers. Execution by U.S. courts of any judgment obtained against us or any of our directors or officers in U.S. courts may be limited to assets located in the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of U.S. courts predicated upon civil liability of us and our directors and executive officers under the U.S. federal securities laws. There may be doubt as to the enforceability in Canada against non-U.S. entities or their controlling persons, directors and officers who are not residents of the United States, in original actions or in actions for enforcement of judgments of U.S. courts, of liabilities predicated solely upon U.S. federal or state securities laws.
We may be a passive foreign investment company, which may result in adverse U.S. federal income tax consequences for U.S. Holders of our common shares.
Generally, if for any taxable year 75% or more of our gross income is passive income, or at least 50% of the average quarterly value of our assets are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. Although we do not believe that we were a PFIC for the year ending December 31, 2022, our determination is based on an interpretation of complex provisions of the law, which are not addressed in a significant number of administrative pronouncements or rulings by the Internal Revenue Service. Accordingly, there can be no assurance that our conclusions regarding our status as a PFIC for the 2021 taxable year will not be challenged by the Internal Revenue Service and, if challenged, upheld in appropriate proceedings. In addition, because PFIC status is determined on an annual basis and generally cannot be determined until the end of the taxable year, there can be no assurance that we will not be a PFIC for the current or future taxable years. If we are characterized as a PFIC, our shareholders who are U.S. holders may suffer adverse tax consequences, including the treatment of gains realized on the sale of our common shares as ordinary income, rather than as capital gain, the loss of the preferential rate applicable to dividends received on our common shares by individuals who are U.S. Holders, and the addition of interest charges to the tax on such gains and certain distributions. A U.S. shareholder of a PFIC generally may mitigate these adverse U.S. federal income tax consequences by making a “qualified electing fund” election, or, to a lesser extent, a “mark to market” election. However, we do not intend to provide the information necessary for U.S. Holders to make qualified electing fund elections if we are classified as a PFIC.
51
We may be impacted by certain taxes in multiple jurisdictions.
We are a multinational corporation with operations in multiple jurisdictions. As a result, we need to be compliant with the tax laws and regulations of Canadian federal, provincial and local governments, the United Kingdom and other international jurisdictions. This includes transfer pricing laws and regulations between many of these jurisdictions. Significant judgment is required in determining our provision for income taxes and claims for investment tax credits related to qualifying Scientific Research and Experimental Development expenditures, both in Canada and in foreign jurisdictions. Various internal and external factors may have favorable or unfavorable effects on future provisions for income taxes and our effective income tax rate. These factors may include, but are not limited to, changes in tax laws, regulations and/or tax rates, audits by tax authorities, changing interpretations of existing tax laws or regulations, changes in estimates of prior years’ items and changes in future levels of research and development, or R&D, spending. Furthermore, new accounting pronouncements or new interpretations of existing accounting pronouncements may have a material impact on our effective income tax rate.
We may be impacted by certain tax treatments applicable to various revenue streams in different tax jurisdictions. We are subject to withholding taxes on certain of our revenue streams. The withholding tax rates that are used are based on the interpretation of specific tax acts and related treaties. If a tax authority has a different interpretation from us, it could potentially impose additional taxes, interest and/or penalties. These additional costs would potentially reduce the amount of revenue we ultimately receive and retain. From time-to-time, we have implemented reorganization transactions to improve or simplify our overall tax structure. Challenges from a tax authority having a different interpretation of the effect of these transactions could potentially have a materially adverse impact on us, both in the form of additional taxes, interest and/or penalties that may be assessed and additional costs that we may incur in contesting such assessments or indemnifications claims from third parties pursuant to purchase and sale transactions.
We do not intend to pay dividends in the foreseeable future.
We have never declared or paid any dividends on our common shares. We intend, for the foreseeable future, to retain our future earnings, if any, to finance further research activities and the expansion of our business. As a result, the return on an investment in our common shares will likely depend upon any future appreciation in value, if any, and on a shareholder’s ability to sell our common shares. The payment of future dividends, if any, will be reviewed periodically by our board of directors and will depend upon, among other things, conditions then existing including earnings, financial conditions, cash on hand, financial requirements to fund our development activities, development and growth, and other factors that our board of directors may consider appropriate in the circumstances.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. If few securities analysts commence coverage of us, or if industry analysts cease coverage of us, the trading price for our common shares would be negatively affected. If one or more of the analysts who cover us downgrade our common shares or publish inaccurate or unfavorable research about our business, our common shares price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our common shares could decrease, which might cause the price of our common shares and trading volume to decline.
52
Item 4. Information on the Company.
A. History and Development of the Company
We were incorporated on October 14, 1994 under the Canada Business Corporations Act, or the CBCA, under the name Innovon Life Sciences Holdings Limited. We changed our name to “Prometic Life Sciences Inc.” on May 19, 1998 and subsequently changed our name to “Liminal BioSciences Inc.” on October 3, 2019. On July 28, 1998, our common shares began trading on the Toronto Stock Exchange, or the TSX, under the trading symbol “PLI” and, on October 7, 2019, began trading under the trading symbol “LMNL.” On November 18, 2019, our common shares began trading on the Nasdaq Global Market, or the Nasdaq, under the trading symbol “LMNL.” On August 5, 2020, we voluntarily delisted our common shares from the TSX.
Our head and registered office is located at 231 Dundas Street East, Belleville, Ontario, Canada K8N 0K1. The telephone number of our principal executive office is 1-450-781-0115 and our corporate website is www.liminalbiosciences.com. The information contained on our website is not incorporated by reference into this Annual Report, and you should not consider any information contained on, or that can be accessed through, our website as part of this Annual Report or in deciding whether to purchase our common shares. The SEC maintains an Internet website that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
In September 2022, we transferred our common shares from The Nasdaq Global Market to The Nasdaq Capital Market. In February 2023, we completed a consolidation of all the issued and outstanding common shares of the Company on the basis of a consolidation ratio of ten (10) pre-consolidation shares for one (1) post-consolidation share to regain compliance with Nasdaq’s minimum bid price requirement for continued listing by achieving a closing bid price on Nasdaq Capital Market of at least $1.00 per share for a minimum of 10 consecutive trading days. In February 2023, we received written notice from Nasdaq that the Company had regained compliance with Nasdaq Listing Rule 5450(a)(1).
Our actual capital expenditures for the years ended December 31, 2020, 2021 and 2022 amounted to $1.1 million, $0.1 million and $nil, respectively. These capital expenditures primarily consisted of investments in our biotherapeutics and bioproduction facilities in connection with the development of our former drug, Ryplazim® plasminogen (human) and expenses related to the opening of a new plasma collection center in the United States, of which businesses were divested in 2021. We expect our capital expenditures to be more in line with our most recent year's expenditures, as we continue to streamline our business and advance our small molecule research and development programs.
53
B. Business Overview
We are a development stage biopharmaceutical company focused on discovering and developing novel and distinctive small molecule therapeutics that modulate G protein-coupled receptors, or GPCR, pathways. We are designing proprietary novel small molecule therapeutic candidates with the intent of developing best/first in class therapeutics for the treatment of metabolic, inflammatory and fibrotic diseases with significant unmet medical needs, using our integrated drug discovery platform, medicinal chemistry expertise and deep understanding of the GPCR biology.
Our pipeline is currently made up of three development programs. The candidate we selected for clinical development, LMNL6511, a selective antagonist for the GPR84 receptor, is expected to commence a Phase 1 clinical trial in the second half of 2023. We are also developing potential OXER1 antagonists and GPR40 agonists, both of which are at the preclinical stage. In addition to these priority development programs, we continue to explore other development opportunities to add to our pipeline.
We believe that our drug discovery platform and deep understanding of GPCRs allows us to identify small molecule candidates that can accurately target GPCRs where other drug discovery approaches have been unsuccessful. Our drug discovery platform leverages a fully integrated chemistry and biology expertise supported by our broad in vivo capabilities, which allows us to investigate our preclinical drug candidates’ efficacy in a wide variety of animal models and enables us to develop small molecule therapeutic candidates for the treatment of various metabolic, inflammatory and fibrotic diseases. We aim to develop best or first-in-class therapies targeting indications with significant unmet needs, where a novel small molecule approach may be better suited using our drug discovery platform, specialized know-how and data-driven development plans.
We are led by a strong, experienced team with proven track records in the discovery, development, and approval of biopharmaceuticals. Our team’s extensive experience in clinical development, and regulatory success is backed by our data driven philosophy.
GPCRs
G proteins, also known as guanine nucleotide binding proteins, act as molecular switches inside cells and are involved in transmitting signals. GPCRs regulate numerous diverse physiological and pathological processes. Due to their abundance, broad distribution, and crucial involvement in cellular physiology and biochemistry, GPCRs represent the largest family of receptors in the human genome and hold significant importance in the development of therapeutics.
Around 35% of all approved drugs target GPCRs, yet only around 16% of GPCR pathways have an approved drug, providing us with a significant opportunity for discovery and development of novel small molecule therapeutics for GPCR pathways.
All of our development programs target seven transmembrane GPCRs (7TM GPCR’s), where the receptor protein passes through the cell membrane seven times. These receptors are easily accessible to hydrophilic drugs due to their presence on the cell surface, and their non-uniform expression enables selectivity in modulating physiological processes. Agonists and antagonists of 7TM GPCRs receptors are utilized for treating various diseases in all organ systems. An agonist is a drug that binds to a target and mimics the action of the natural ligand. An antagonist is a drug that binds to a receptor and prevents other molecules (such as the natural ligand) from binding.
54
Our Small Molecule Discovery Platform Targeting GPCRs
We believe that oral small molecule therapeutics could overcome the challenges associated with biologic and peptide drugs, which can be costly and inconvenient for patients. Improved patient access could be particularly significant for common chronic diseases involving the endocrine and pulmonary systems. We believe that our drug discovery platform can capitalize on the advantages of highly selective potent small molecule therapeutics, such as potentially enhanced clinical activity and improved safety profile due to lower and more convenient dosing requirements and lower costs.
Our development pipeline:
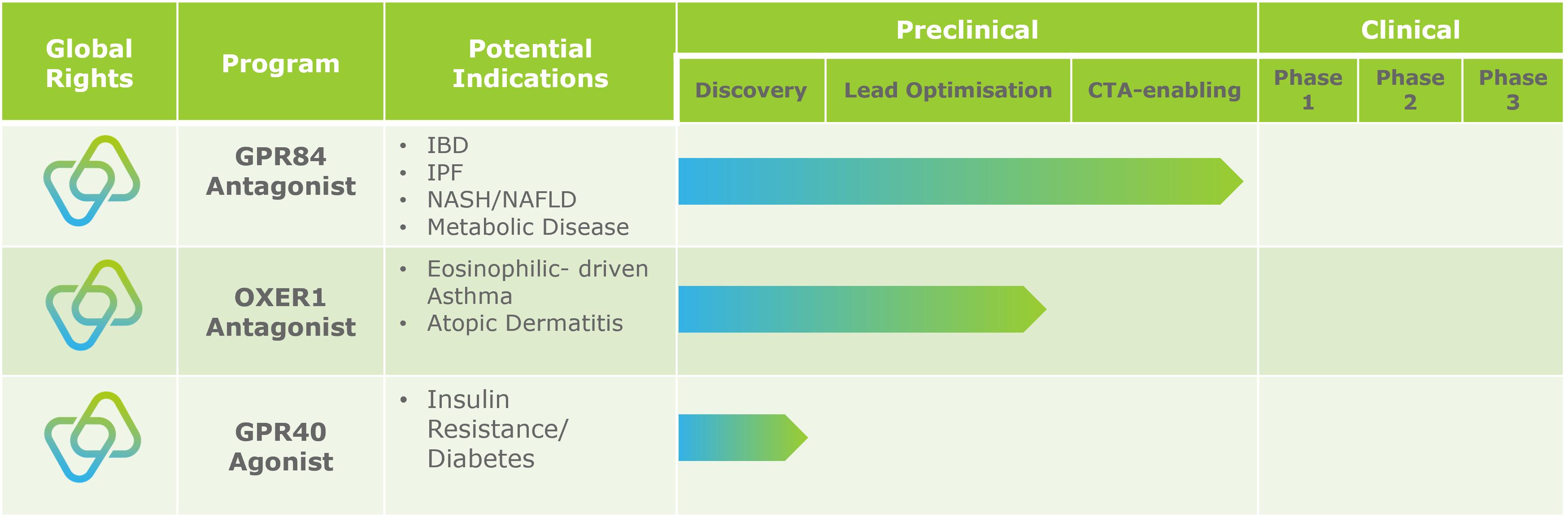
The candidate we selected for clinical development, LMNL6511, is an oral, selective antagonist of the orphan GPCR, GPR84, designed to treat metabolic diseases, inflammation and/or fibrosis in several different therapeutic categories. We believe that the GPR84 receptor may be an important biological target in several therapeutic areas by blocking a proinflammatory modulator on immune cells, including macrophages, once inflammation is established.
The GPR84 receptor itself, is primarily expressed in immune cells in addition to multiple organ systems such as the liver, lung, and gastrointestinal, or GI, tract. Its expression is upregulated in response to inflammatory stimuli. It therefore provides an attractive therapeutic target in several chronic metabolic, inflammatory and fibrosis driven disease processes.
We anticipate seeking approval to commence a first-in-human Phase 1 clinical trial of LMNL6511 during the second half of 2023, once we successfully complete CTA enabling activities.
We are also developing a selective OXER1 antagonist candidate. OXER1 is a GPCR that is highly selective for 5-oxo-ETE, believed to be one of the most potent human eosinophil chemo-attractants. Migration of eosinophils to body sites including the lungs and intestines is mediated by eosinophil chemo-attractants such as 5-oxo-ETE. Eosinophils play a key role in inflammation-driven diseases, including skin, respiratory diseases and gastro-intestinal diseases such as asthma, allergic rhinitis, chronic obstructive pulmonary disorder, atopic dermatitis, psoriasis and acne. Our OXER1 antagonist discovery program is currently at the preclinical stage. We are working towards selecting a lead candidate for further development in Eosinophilic driven diseases in the first half of 2023 and are aiming to commence a first-in-human clinical trial in 2024.
We are also developing a GPR40 agonist as a potential therapeutic treatment for type 2 diabetes. Free fatty acid receptor 1, FFAR 1 (also known as G protein coupled receptor 40, or GPR40) is a validated clinical target for the treatment of diabetes, demonstrated by the phase 2/3 trials of the GPR40 agonist, TAK875. However, the development of this compound was terminated due to a low frequency of drug induced liver injury (DILI). We believe that small molecule agonists of GPR40 can be designed to minimize the risk of DILI. Our GPR40 agonist program is currently in the discovery phase.
In July 2022, we discontinued development of our small molecule product candidate, fezagepras. The decision to discontinue the development of fezagepras was based on results from the Phase 1a single-ascending dose, or SAD, clinical trial, which indicated that fezagepras was significantly inferior compared to sodium phenylbutyrate as a nitrogen scavenger. The recommendation to stop the development program for fezagepras was not based on safety concerns.
As further described above under "Item 3.D-Risk Factors, we do not have sufficient funds to continue maintaining our operating activities, even at low spending levels, for the next 12 months and, as a result, we will require additional funding to finance our operations, which may not be available on acceptable terms, if at all. Failure to obtain this necessary capital when needed may force us to delay, reduce or terminate certain of our product development programs or other operations”. Our December 31, 2022 financial statements have been prepared on a going concern basis.
55
Our Strategy
Our goal is to leverage our drug discovery platform and to develop distinctive novel small molecule therapeutics to treat the complex biology of metabolic, inflammatory and fibrotic diseases to address a wide range of significant unmet needs.
The key activities to achieve this goal include:
•Investing in and leveraging our GPCR knowledge and drug discovery platform to develop differentiated GPCR targeted therapies for unmet medical needs;
•Advancing the candidate we selected for clinical development, LMNL6511, to clinical stage targeting the treatment of fibrosis and metabolic diseases;
•Progressing the development of our OXER1 antagonist program and nominating a lead candidate for further development in eosinophil mediated diseases;
•Pursue the development of our GPR40 agonist program aiming to identify and develop a novel liver-safe GPR40 agonist for the treatment of type 2 diabetes, or T2D; and
•Identifying potential opportunities to monetize non-core assets and to streamline our costs overall.
Our Team
We are led by a strong, experienced team with proven track records in the discovery, development, and approval of biopharmaceuticals, all driven to make a difference.
Our team is led by Bruce Pritchard. Mr. Pritchard was appointed as Chief Executive Officer, or CEO, effective 13 November 2020. Prior to his appointment as CEO, he acted as one of the two Chief Operating Officers, or COO, and as Chief Financial Officer. In his role as COO, he was responsible for the Company’s discovery, clinical development, program management and business development functions related to small molecule therapeutics. A Chartered Accountant by training, he has spent over two decades working in the life sciences and pharmaceutical industry. Mr. Pritchard brings a proven track record of success in strategic acquisitions and in raising debt and equity finance. He also brought to the role many years of experience in general management and operations. Prior to joining Liminal, Mr. Pritchard worked as a senior director for CV Therapeutics, a NASDAQ listed biopharmaceutical company. Mr. Pritchard is based out of our office in Cambridge.
In addition to our executive officers, we have assembled a team of research and development managers with extensive experience in drug discovery, drug development, corporate development and capital market activities, including Dr. Gary Bridger, our Interim Chief Scientific Officer, who has 30 years of experience and a proven track record in drug discovery and clinical development holding positions at AnorMED, Genzyme Corporation (thereafter acquired by Sanofi S.A.) and Xenon Pharmaceuticals and has held various venture capital positions.
56
Dr Bridger co-founded AnorMED Inc. in 1996 and served as Vice President of Research and Development and Chief Scientific Officer. He was responsible for research, development, and clinical programs within AnorMED; a leading company in the development of chemokine receptor inhibitors, a family of 7-transmembrane GPCR’s, for a variety of disease applications. He was responsible for the development of AMD3100 (Mozobil®(plerixafor)), a new stem cell mobilizing agent for treatment of multiple myeloma and non-hodgkins lymphoma patients, including the design and completion of two randomized Phase 3 clinical trials. In late 2006, AnorMED was acquired by Genzyme for US $600 million where Dr. Bridger also held senior positions. While at Genzyme, he was directly involved in the successful regulatory filing, approval and launch of Mozobil®(plerixafor), in the U.S., Europe and Canada. Mozobil®(plerixafor) was approved by the US FDA in 2008. From November 2006 to December 2007, Dr. Bridger served as Senior Vice President of Research and Development of Genzyme, which was acquired by Sanofi, S.A. Dr. Bridger completed his PhD in Synthetic Organic Chemistry at the University of Manchester Institute of Science and Technology (UK) and subsequently completed a post-doctoral fellowship at Boston College (USA). He has authored more than 90 peer-reviewed publications and has over 40 issued patents.
Our Preclinical Programs
Our current development programs are focused on developing small molecule therapeutics for the treatment of metabolic, inflammatory and fibrotic conditions. We currently have one clinical development candidate and two drug discovery programs, each in the preclinical development phase.
57
Our Selective G-protein coupled receptor 84 (GPR84) Antagonist Program
Our most advanced program is for the development of a selective GPR84 antagonist drug candidate designed to treat inflammation and/or fibrosis in several different therapeutic categories.
In January 2023, we nominated LMNL6511 as the candidate selected for clinical development for this program. Through our internal drug discovery and lead optimization work we have been able to develop LMNL6511, a new structural class of high potency, small molecule antagonist of GPR84.
LMNL6511 is a selective antagonist for the GPR84 receptor designed to treat inflammation and/or fibrosis in several diseases. Published research supports the role of GPR84 as an important biological target in several therapeutic areas of interest by blocking a proinflammatory modulator on immune cells, including macrophages, once inflammation is established.
GPR84 is classified as an orphan receptor since its definitive endogenous ligand is undefined. However, it can be activated by medium-chain fatty acids (MCFA) such as decanoic acid, 6-OAU, and diindolylmethane (DIM), indicating the presence of three unique binding sites, which likely correspond to different endogenous ligands. Additionally, the activation of each binding site may lead to different downstream effects, a phenomenon known as signaling bias, which is well-documented within the GPCR superfamily.
Schematic of GPR84 receptor and its ligands:
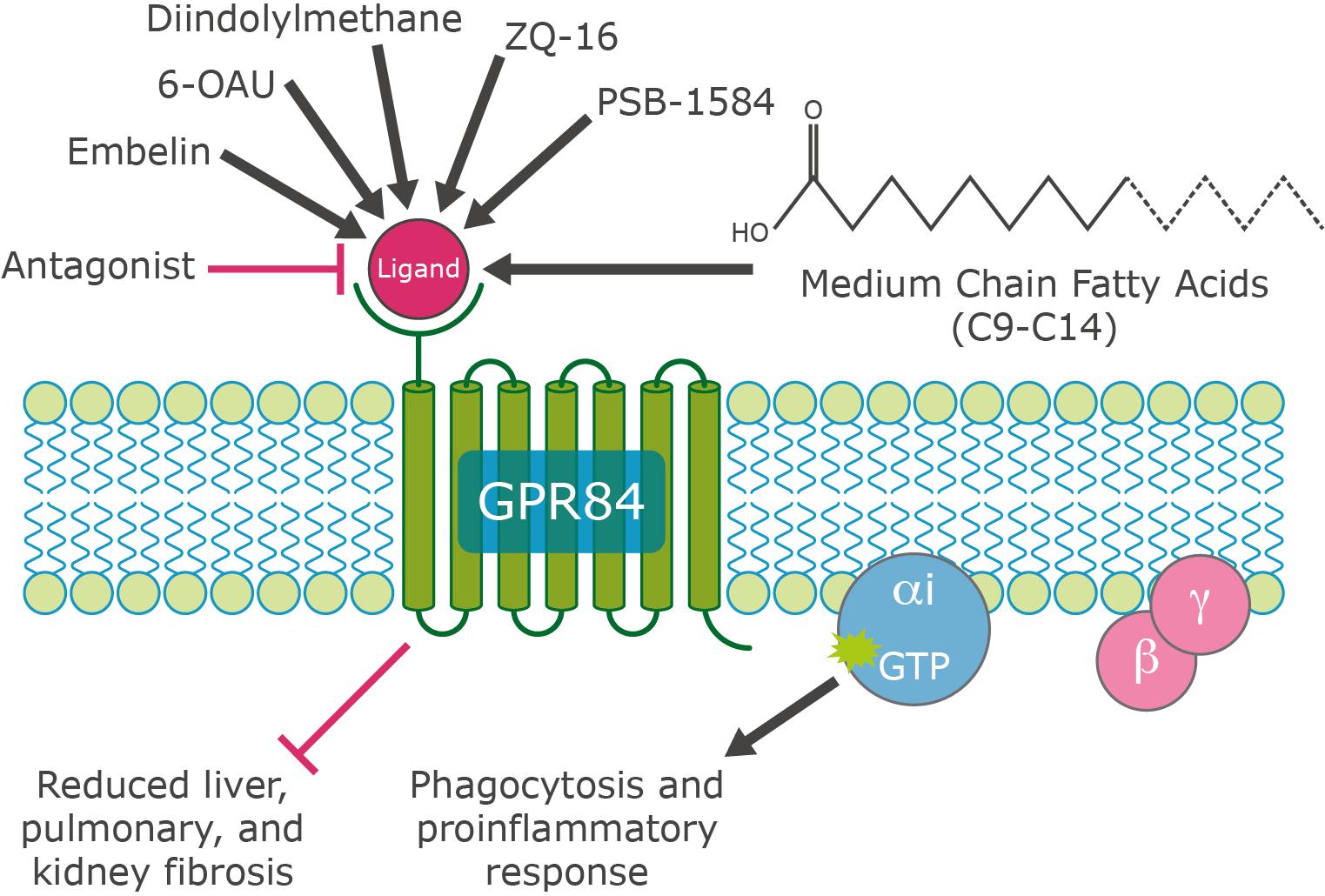
GPR84 is a pro-inflammatory target primarily expressed on cells associated with the immune system and its expression levels increase significantly during periods of inflammatory stress. Inhibition of GPR84 can inhibit neutrophil and macrophage migration and reduce cytokine release.
GPR84 expression is not restricted to cells in the immune system; it is also expressed in tissues such as the lung, brain, heart, muscle, colon, kidney, liver, intestine and adipose. Through its role in inflammation, GPR84 may be a mediator of the relationship between inflammation, obesity and diabetes. Rodent models suggest that GPR84 expression is up-regulated in adipocytes in response to TNF-α released from infiltrating macrophages, and that this in turn can lead to a down-regulation in adiponectin expression in adipocytes. Adiponectin is known to have anti-diabetic, anti-inflammatory, and anti-atherogenic effects, and it also functions as an insulin sensitizer.
More recently, 3-hydroxy decanoate, an agonist of GPR84 and a metabolite of decanoic acid was found to be enriched in the circulation of obese individuals compared to non-diabetic controls. This was associated with adipose inflammation and increased fasting insulin levels. Consequently, we intend to incorporate biomarker measurements of diabetes and lipids in our first-in-human phase 1 study of LMNL6511, with the goal of identifying an early indicator of biological activity.
We believe that the GPR84 receptor could be an important biological target in several therapeutic areas of interest. GPR84 is broadly expressed and may play an interesting role in the relationship between inflammation, obesity and diabetes. Our preclinical research, combined with published work from other groups, indicates a potential role for antagonism of GPR84 in fibrotic diseases, including Non-Alcoholic Steatohepatitis, or NASH, Inflammatory bowel disease, or IBD, and Idiopathic Pulmonary Fibrosis, or IPF.
Non-alcoholic fatty liver, or NAFLD, is a condition that is estimated to affect 79 million people in the United States, and Non-Alcoholic Steatohepatitis, or NASH, is estimated to affect 17 million people in the United States due to the obesity epidemic and is the manifestation of metabolic disease in the liver. According to Global Industry Analysts, Inc. forecasts, the combined NAFLD and NASH market is expected to be worth $12 billion in the United States by 2027.
58
NASH is a progressed state of NAFLD, where the chronic injury suffered by the liver due to the excess fatty deposits associated with NAFLD trigger inflammation and fibrosis. Though only a small percentage of NAFLD patients progress to NASH, the sheer number of NAFLD patients has made NASH the most common cause of severe liver disease worldwide. NASH and its associated co-morbidities, such as fibrosis, remain a major unmet medical need with treatment offering little recourse. Though the biologic mechanisms underlying the pathogenesis of NASH are not fully characterized, the current understanding describes excess lipids present in the liver ultimately leading to hepatoxic injury, followed by inflammation and fibrosis with an associated decline in liver function. Current research implicates multiple pathways for both the initial lipid accumulation and the dysregulated healing response.
There are currently no approved therapies for NAFLD, NASH or associated fibrosis, although one potential therapy (Ocaliva®, developed by Intercept Pharmaceuticals) is currently under FDA review. Current treatment options are limited to diet and lifestyle modifications that may control or reduce the amount of excess fat deposits in the liver. In January 2023, Madrigal Pharmaceuticals Inc. provided an update on their Phase 3 MAESTRO-NASH clinical trial of Resmetirom for the treatment of NASH with Liver Fibrosis. Results of the Phase 3 trial showed potential for the drug candidate’s impact on treatment of NASH and associated symptoms such as ballooning, steatosis and inflammation.
Published research also supports the role of the GPR84 receptor in the potential treatment of IBD. Such research proposes that GPR84 is highly upregulated in inflamed colon tissues of active Ulcerative Colitis or UC, patients and DSS-induced colitis mice, and infiltrating GPR84+ macrophages are significantly increased in the colonic mucosa of both the UC patients and the mice with colitis. GPR84 activation imposes pro-inflammatory properties in colonic macrophages by enhancing NLRP3 inflammasome activation. Genetic deletion or chemical blockade of GPR84 attenuates DSS-induced colitis in mice by reducing the M1 polarization and function of pro-inflammatory macrophages.
UC is typically first point of entry in IBD with Crohn’s Disease, or CD, as a potential follow-on indication. In the USA alone, the prevalence of UC is 730,000 and CD is 709,000 with the market expected to reach $37 billion by 2029.
An antagonist of the GPR84 receptor has also been studied in a clinical setting as a potential therapy for IPF. In a phase IIa clinical trial for IPF, patients receiving the GPR84 antagonist GLPG1205 (100 mg, orally once daily) for 26 weeks on top of standard of care had a smaller forced vital capacity (FVC) decline compared with placebo (GLPG1205: −31.29 mL; placebo: −79.47 mL). The change in pulmonary lobar volume, as measured by FRI, correlates with the FVC decline observed. However, it is important to note that this study was not powered to show statistical significance.
IPF is considered a rare, sporadic disease. According to the National Institutes of Health (NIH), about 100,000 people in the United States have IPF. Approximately 30,000 to 40,000 new cases are diagnosed each year. Worldwide, IPF affects 13 to 20 out of every 100,000 people.
Subject to continued satisfactory results in ongoing clinical trial application (CTA)-enabling work, we expect to seek approval to commence a first-in-human Phase 1 clinical trial of LMNL6511, in healthy volunteers, in the second half of 2023. We also expect that on-going in-vivo experiments in animal models of IPF, IBD and metabolic diseases will allow us to select a lead clinical indication in the coming months.
Our Selective Oxo-eicosanoid receptor 1 (OXER1) Antagonist Program
Our second preclinical program is for the development of a selective OXER1 antagonist aimed at treating eosinophilic driven diseases, or EDDs. Our OXER1 antagonist discovery program is currently at the preclinical stage. We are targeting selecting a lead candidate for further development in the first half of 2023.
59
OXER1 is a GPCR that is highly selective for 5-oxo-eicosatetraenioc acid (5-oxo-ETE), a potent human eosinophil chemo-attractant known to be involved in EDDs. EDDs are inflammatory illnesses, generally in skin, respiratory and GI disease in which elevated levels of activated eosinophils are a direct cause or thought to play a pivotal role. Interaction of 5-oxo-ETE with OXER1 is an important mediator of migration of eosinophils. Selective OXER1 antagonists may function as therapeutic or prophylactic agents for the above-mentioned disease. OXER-1 may represent a promising target in a novel pathway for the treatment of eosinophilic driven diseases.
Migration of eosinophils to body sites including the lungs and intestines is mediated by eosinophil chemo-attractants such as 5-oxo-ETE. Eosinophils are involved in acute and chronic inflammation and play an important role in a large number of allergic, inflammatory and proliferative diseases, such as skin, respiratory diseases and gastro-intestinal diseases, such as asthma, allergic rhinitis, chronic obstructive pulmonary disorder, atopic dermatitis, psoriasis and acne.
Eosinophils are major effector cells in the immune system. They are part of the innate immune system; traditionally recognized as the first line of defense against parasitic infections. Eosinophils themselves are key in mounting an appropriate immune response against pathogens. When activated, they release a cocktail of toxic proteins (to damage the parasite), along with cytokines to attract other immune cells. However, when eosinophils are chronically activated, these toxic proteins can also damage normal tissue and promote inflammation. Eosinophils are also recruited from the blood into the tissues at the sites of inflammation. They also play a role in tissue repair and resolution of inflammation.
5-oxo-ETE binds to OXER1 and activates intracellular signaling pathways: Gαi response leading to inhibition of cyclic AMP (cAMP) formation (in green); βγ response leading to phospholipase C (PLC)/calcium mobilization/protein kinase C (PKC), phosphatidylinositol 3-kinase (PI3K)/Akt, and p38 signaling (in red); and β-arrestin response leading to MAP kinase signaling and cytosolic phospholipase A2 (cPLA2) activation (in blue). The βγ and β-arrestin signaling pathways lead to the indicated cellular responses in granulocytes, which could be blocked by an OXER1 antagonist.
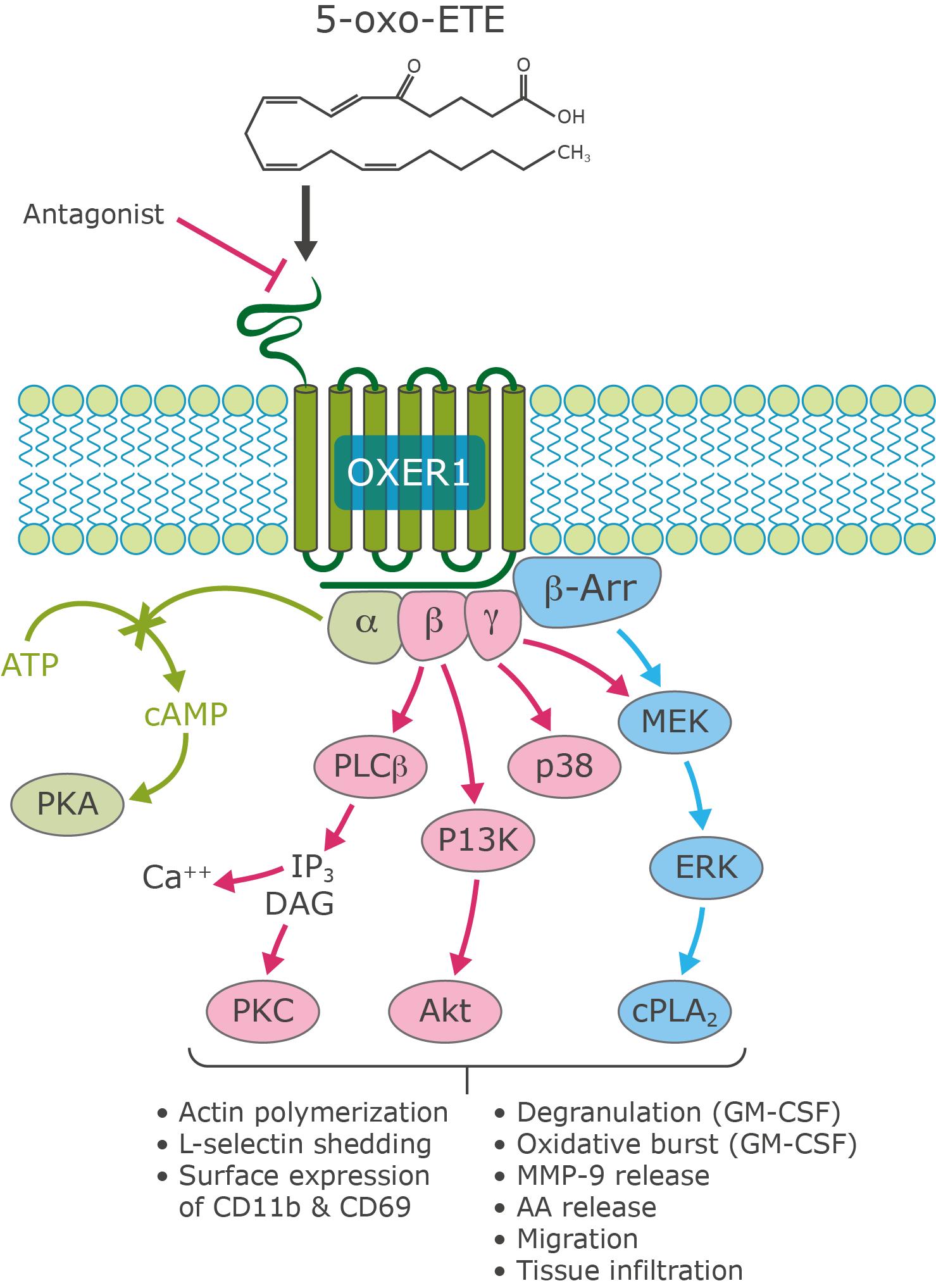
EDDs are inflammatory illnesses, generally in skin, respiratory, gastrointestinal, or GI conditions, in which elevated levels of activated eosinophils are a direct cause or thought to play a critical role. Eosinophils are involved in acute and chronic inflammation and play an important role in a large number of allergic, inflammatory and proliferative diseases.
EDD such as Eosinophilic Asthma and Atopic Dermatitis, or AD, represent a significant area of unmet need in global health. Asthma is a heterogeneous disease; there are multiple clinical sub-types. The two primary asthma phenotypes, Type 2 high and Type 2 low, are defined by eosinophilic and neutrophilic pattern of inflammation respectively. The Type 2 high subtype is associated with the cytokines IL-5 and IL-13. Most drugs have focused on Type 2 high asthma, including four of the biologics approved for treatment of uncontrolled asthma target IL-5, IL-5R or IL-4/IL-13. Globally, Asthma affected an estimated 262 million people in 2019 and caused 455 000 deaths. The global Eosinophilic and severe asthma markets are expected to reach $127 million by 2029 with approved drugs already producing sales of $1.3 billion (Fasendra® 2021 sales) and $1.6 billion (Nucala 2021 sales).
The immune response observed during the course of AD is characterized by a biphasic inflammation. A Th2-biased immune response (IL-4, IL-13, TSLP and eosinophils) is predominant in the initial and acute phase of AD, while in chronic AD skin lesions, a Th1/Th0 dominance has been described (IFN-γ, IL-12, IL-5 and GM-CSF). The prevalence of AD is estimated to be 15-20% in children and 1-3% in adults, and the incidence has in-creased by 2 to 3-fold during the past decades in industrialized countries. It is expected that the growing global Atopic Dermatitis market may reach 7.6 billion by 2029 with approved drugs already producing sales of $6.4 billion (Dupixent®) and an additional $1.1 billion by 2027 (CIBINQOTM).
Several biologics have been approved for the treatment of eosinophil-related diseases. Several of the approved monoclonal antibody treatments for severe eosinophilic asthma are currently in clinical trials aimed at expanding their indications to a broader range of eosinophilic disorders. Compared to approved biologics, small molecule OXE receptor antagonists may offer a promising and potentially more cost-effective option for treatment of eosinophilic-driven disorders.
60
Our OXER1 antagonist program is currently at the pre-clinical stage. Pending the outcome of our preclinical research, we plan to nominate a lead candidate for our OXER1 antagonist program for further development of our OXER1 antagonist program in Eosinophilic driven diseases in the first half of 2023.
Our GPR40 Agonist Program
Our third preclinical program is a drug discovery program for the development of a liver safe GPR40 agonist, compared to other compounds in development, as a possible treatment for T2D.
GPR40, a G protein-coupled receptor for free fatty acids, also known as FFA1 or FFAR1, is a seven transmembrane GPCR highly expressed in pancreatic β-cells, intestinal L, K, and I cells and in neurons and responds to medium and long chain unsaturated fatty acids, resulting in increased insulin secretion only in the presence of elevated glucose levels. The receptor's glucose dependency makes it an attractive target for developing potentially safe and effective therapies for type 2 diabetes. While the mechanism of action is not fully understood, previous published research suggests that GPR40 is mainly coupled with the G protein α-subunit of the Gq family (Gαq), which triggers an increase in phospholipase C (PLC) activity, leading to intracellular calcium mobilization and protein kinase C (PKC) activation. GPR40 is also expressed in enteroendocrine cells of the gastrointestinal tract, with activation resulting in the secretion of incretins such as GLP-1 and GIP, which can indirectly regulate insulin secretion.
Several companies have focused on developing small molecule GPR40 agonists. Based on published preclinical and clinical studies, activating GPR40 can enhance glycemic control by promoting insulin secretion that is dependent on glucose levels. The initial evidence for the potential of GPR40 as a target for treating type 2 diabetes came from phase II results of TAK-875, which demonstrated significant reductions in HbA1c and fasting blood glucose levels without causing hypoglycemia in patients with T2D. However, in phase 3 trials, TAK-875 (Takeda), which was the most clinically advanced GPR40 agonist, exhibited a low frequency of drug-induced liver injury (DILI). Takeda subsequently discontinued development of TAK-875 due to liver safety concerns. Since the GPR40 receptor is not expressed in the liver, toxicity is not considered a hallmark of GPR40 agonism but rather an intrinsic property if an individual agonist, stimulating the search for new agonists that minimize the risk of liver toxicity. The most advanced clinical candidate of the second-generation GPR40 agonists is CPL280 from Celon Pharma, a compound that minimizes the formation of a reactive Acyl glucuronide metabolite in vivo, a potential cause of liver toxicity. We believe that identification of a GPR40 agonist that does not exhibit liver toxicity is achievable and discovery medicinal chemistry is ongoing.
GPR40 membrane receptor model includes three characterized binding sites and multiple signaling pathways:
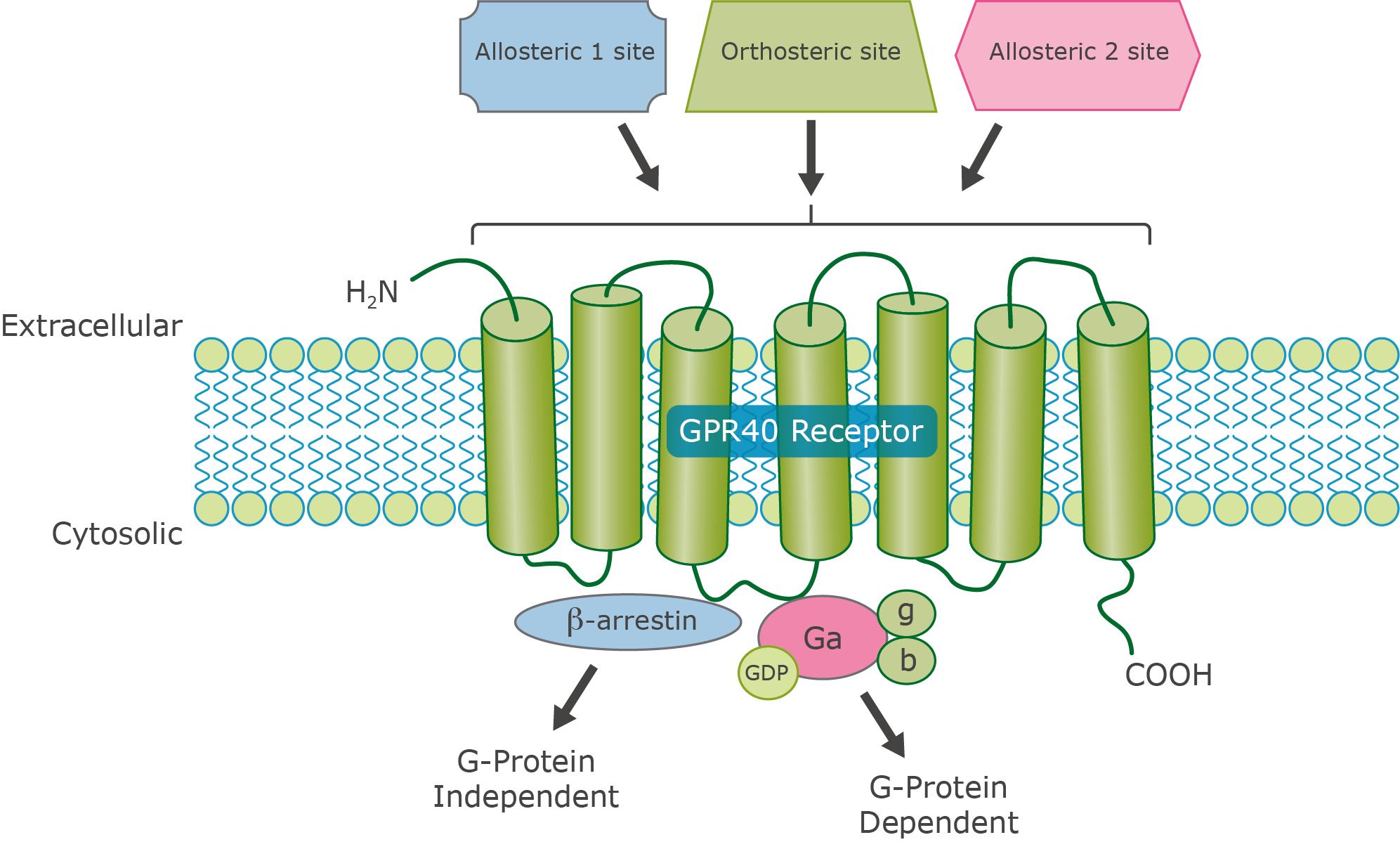
Diabetes is a chronic, metabolic disease characterized by elevated levels of blood glucose (or blood sugar), which leads over time to serious damage to the heart, blood vessels, eyes, kidneys and nerves. The most common is T2D, usually in adults, which occurs when the body becomes resistant to insulin or doesn't make enough insulin. In the past 3 decades, the prevalence of T2D has risen dramatically in countries of all income levels. For people living with diabetes, access to affordable treatment, including insulin, is critical to their survival. There is a globally agreed target to halt the rise in diabetes and obesity by 2025.
Obesity and diabetes are global epidemics with more than 1.9 billion people being obese or overweight and 463 million adults having diabetes. Obesity is the most common cause of several metabolic defects and is associated with complications such as T2D and cardiovascular disease. Both the number of cases and the prevalence of diabetes have been steadily increasing over the past few decades.
Our GPR40 agonist development program is currently at the discovery stage.
61
Manufacturing
We rely and expect to continue to rely for the foreseeable future, on third-party contract development manufacturing organizations, or CDMOs, to synthesize, optimize and produce our small molecule product candidates for preclinical and clinical testing. We require that our CDMOs produce our drug substances and finished drug products for clinical trials in accordance with current Good Manufacturing Practices, or cGMPs, and all other applicable laws and regulations. We maintain agreements with our manufacturers that include confidentiality and intellectual property provisions to protect our proprietary rights related to our product candidates.
We do not currently have arrangements in place for redundant supply. As our development programs expand and we build new process efficiencies, we expect to continually evaluate this strategy with the objective of satisfying demand for preclinical studies, clinical trials and, if approved, the manufacture, sale and distribution of commercial products.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary rights. While we believe that our product candidates, technology, knowledge, experience and scientific resources provide us with competitive advantages, we compete in the highly competitive markets and face significant competition from many sources, including pharmaceutical and biotechnology companies, as well as academic institutions, governmental agencies and private and public research institutions.
We compete in the segments of the biotechnology, pharmaceutical and other related industries that develop and market drugs and therapies for the treatment of inflammation, metabolic and respiratory disorders.
There are many other companies, including large biotechnology companies, that are developing therapies for the same therapeutic areas that our product candidates target.
The first GPR84 antagonist to enter clinical trials was GLPG1205 from Galapagos. In published work, GLPG1205 demonstrated activity in pre-clinical models of IPF, UC and NASH/NAFLD and subsequently entered a phase 2 clinical trial in patients with IPF. Galapagos discontinued development of drugs that target fibrotic diseases, including GLPG1205, in 2021. The current development status of GLPG1205 is unknown.
Several biologics have been approved for the treatment of eosinophil-related diseases. There are currently no small molecule OXE receptor antagonists in clinical trials or approved for treatment of eosinophilic-driven disorders.
Many of the companies against which we are competing or against which we may compete in the future, either alone or with their strategic collaborators, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved drugs than we do. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies or universities and research institutions. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and enrolling patients for our clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
62
We could see a reduction or elimination of our commercial opportunity if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. The availability of reimbursement from government and other third-party payors will also significantly affect the pricing and competitiveness of our products. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
License Agreements
Amended and Restated License Agreement with The Royal Institution for the Advancement of Learning/ McGill University and Florida Institute of Technology, Inc.-
Concurrent with the acquisition of Fairhaven Pharmaceuticals Inc., or Fairhaven, in July 2020, Fairhaven entered into an amended and restated license agreement with The Royal Institution for the Advancement of Learning/McGill University and Florida Institute of Technology, Inc. (the “Licensors”) effective as of May 16, 2018. Pursuant to the amended and restated license agreement, the Licensors granted us worldwide, exclusive royalty-bearing right to use and practice certain licensed patents covering 5-oxo-ETE receptor antagonist compounds and indole analogs as 5-oxo-ETE receptor antagonists and methods of use thereof (the “Licensed Patents”) and know-how, with the exclusive right to develop, modify, adapt, improve and customize certain tangible devices, materials, services or processes, in the course of exploitation which, in the absence of the amended and restated license agreement, would infringe one or more of the issued or pending claims of Licensed Patents (the “Licensed Products”), and the right to make, have made, commercialize, exploit, reproduce, market, sell, rent, distribute, lease or otherwise transfer, import and export certain Licensed Products, contingent upon our material compliance with the terms of the Amended and Restated License Agreement. We expect to utilize the Licensed Patents and know-how licensed from the Licensors in connection with the development of our pre-clinical OXER1 antagonist program. Under the Amended and Restated License Agreement, we are obliged to pay a low single digit royalty to the Licensors based on a percentage of net sales of the Licensed Products. The Amended and Restated License Agreement terminates upon the last to expire, or become abandoned, Licensed Patent(s), whether by statute or otherwise, unless it earlier terminates by operation of law or by acts of the parties in accordance with the terms of the Amended and Restated License Agreement.
Intellectual Property
We own and control the intellectual property utilized in most of our technologies, products and potential product candidates, giving us the option to develop and eventually commercialize these products in various geographies, to develop new formulations, and to select CDMOs and clinical research organizations, or CROs, of our choice. Our intellectual property rights include our trademarks, patents and patent applications, regulatory dossiers, manufacturing, and process know-how. Our intellectual property portfolio has been built in large part from in-house technology and product research and development over the past 20 years as well as strategic relationships.
Our approach regarding our intellectual property portfolio is to file and/or license patents and patent applications as appropriate and to seek to obtain patent protection in at least the major pharmaceutical markets, including the United States, Canada, major European countries and Japan. We also rely on trade secrets, proprietary unpatented information, trademarks, and contractual arrangements to protect our technology and enhance our competitive position. We currently have a patent estate comprised of owned and in-licensed patents and patent applications. Our patent portfolio includes patents and patent applications claiming compounds, pharmaceutical compositions, nutraceuticals, processes, and methods for treating diseases, disorders, or conditions.
63
GPR84 Antagonist Program
For our GPR84 antagonist program, we wholly own five patent families comprised of pending patent applications directed to compositions of matter and methods of their medical use. Two of the patent families each comprise three pending patent applications which relate to compounds and methods useful for antagonizing G-protein coupled receptor, for use in the treatment of various disorders, whereby patents, if granted based on these pending patent applications, would be expected to expire no sooner than 2042, excluding any patent term adjustments or other forms of extensions. The remaining three patent families each comprise one pending provisional patent application which relate to compounds and methods useful for antagonizing G-protein coupled receptor, for use in the treatment of various disorders, whereby patents, if granted based on non-provisional patent applications filed in the future based on these pending provisional patent applications, would be expected to expire no sooner than 2043, excluding any patent term adjustments or other forms of extensions.
OXER1 Antagonist Program
With respect to our OXER1 antagonist program, we have in-licensed inventions that relate to novel pharmaceutically useful compounds which are antagonists of a G-protein coupled eicosanoid receptor, to methods for their preparation and to pharmaceutical compositions and therapeutic methods for treating eicosanoid-mediated disorders, such as inflammatory and allergic conditions.
Furthermore, we wholly own a patent family which relates to compounds and methods which are useful for antagonizing G-protein coupled receptor OXER1, useful in treating various disorders. If granted, patents based on pending applications in this family would be expected to expire no sooner than 2042, excluding any patent term adjustments or other possible extensions.
Our Trademark Portfolio
LIMINAL BIOSCIENCES is our trademark currently registered in Australia, Brazil, Switzerland, China, European Union, Israel, Japan, Mexico, Norway, New Zealand, Turkey, United Kingdom as well as in the United States and pending registration in Canada. This mark is our name and is used in association with several goods and services, namely pharmaceutical research and development, and pharmaceutical preparations and compositions being developed for the treatment of multiple indications.
We also have unregistered trademark rights in certain countries in which we operate, where trademark rights arise from use, rather than registration.
Other Intellectual Property Portfolio
Our portfolio of intellectual property also contains registrations and domain names associated with our trademarks and pending trademark application.
Our Policy on Intellectual Property
Our intellectual property practice is to keep all information relating to proprietary compounds, inventions, improvements, trade secrets, know-how and continuing technological innovation confidential and, where practicable, file patent and trademark applications. In particular, as part of our intellectual property protection practice, we, where we deem practicable and commercially reasonable:
•file patent applications for any new and patentable invention, development or improvement in the United States and in other countries;
•prosecute pending patent applications in conformity with applicable patent laws and in a manner that covers our activities;
•file trademark applications in countries of interest for our trademarks;
•register domain names whose addresses include our trademark names; and
64
•maintain our intellectual property rights by paying government fees as may be necessary to ensure such rights remain in force.
Government Regulation
In the United States, pharmaceutical products are subject to extensive regulation by the FDA under the Federal Food, Drug, and Cosmetic Act, or FDCA. The FDCA and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications, or NDAs, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
We cannot market a drug product in the United States until the product candidate has received FDA approval. The steps required before a new drug may be marketed in the United States generally include the following:
•completion of extensive nonclinical laboratory tests, animal studies, and formulation studies in accordance with the FDA's Good Laboratory Practice, or GLP, regulations;
•submission to the FDA of an Investigational New Drug, or IND, for human clinical testing, which must become effective before human clinical trials may begin;
•performance of adequate and well-controlled human clinical trials in accordance with Good Clinical Practice, or GCP, requirements to establish the safety and efficacy of the product for each proposed indication;
•submission to the FDA of a New Drug Application, or NDA, after completion of all pivotal clinical trials;
•satisfactory completion of an FDA inspection of sites involved in our clinical trials;
•satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the active pharmaceutical ingredient, or API, and finished product are produced and tested to assess compliance with cGMPs; and
•FDA review and approval of the NDA prior to any commercial marketing or sale of the product in the United States.
Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Clinical trials involve the administration of the investigational new biologic to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted in compliance with federal regulations, including GCP requirements, as well as under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval at each site at which the clinical trial will be conducted. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB's requirements, or may impose other conditions.
65
U.S. Biopharmaceutical Products Development Process
Before testing any product candidate, the product candidate enters the nonclinical testing stage. Nonclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies to assess the potential safety and activity of the product candidate. The conduct of the nonclinical tests must comply with federal regulations and requirements including GLPs.
The clinical study sponsor must submit the results of the nonclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. Some nonclinical testing typically continues after the IND is submitted. An IND is an exemption from the FDCA that allows an unapproved product to be shipped in interstate commerce for use in an investigational clinical trial and a request for FDA authorization to administer an investigational product to humans. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA requests certain changes to a protocol before the study can begin, or the FDA places the clinical study on a clinical hold within that 30-day time period. The FDA may also impose clinical holds on a product candidate at any time before or during clinical trials due to safety concerns or non-compliance. If the FDA imposes a clinical hold, studies may not recommence without FDA authorization and then only under terms authorized by the FDA. Accordingly, we cannot be sure that submission of an IND will result in the FDA allowing clinical trials to begin, or that, once begun, issues will not arise that suspend or terminate such studies.
Clinical trials involve the administration of the product candidate to healthy volunteers or subjects under the supervision of qualified investigators, generally physicians not employed by or under the study sponsor’s control. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical study, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety, including stopping rules that assure a clinical study will be stopped if certain adverse events should occur. Each protocol and any amendments to the protocol must be submitted to the FDA as part of the IND. Clinical trials must be conducted and monitored in accordance with the FDA’s regulations comprising the GCP requirements, including the requirement that all research subjects provide informed consent. Further, each clinical study must be reviewed and approved by an independent IRB, at or servicing each institution at which the clinical study will be conducted. An IRB is charged with protecting the welfare and rights of study participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the form and content of the informed consent that must be signed by each clinical study subject or his or her legal representative and must monitor the clinical study until completed. Additionally, some trials are overseen by an independent group of qualified experts organized by the trial sponsor, known as a data safety monitoring board or committee.
66
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
•Phase 1. The product is initially introduced into healthy human subjects and tested for safety. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients.
•Phase 2. The product is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage and dosing schedule.
•Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy, potency and safety in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling.
Post-approval clinical trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These clinical trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication, particularly for long-term safety follow-up.
During all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, and clinical trial investigators. Annual progress reports detailing the results of the clinical trials must be submitted to the FDA. Written IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events, any findings from other studies, tests in laboratory animals or in vitro testing that suggest a significant risk for human subjects, or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor’s initial receipt of the information. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsor or its data safety monitoring board may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the investigational product has been associated with unexpected serious harm to patients.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the characteristics of the product as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
67
After the completion of clinical trials, FDA approval of a NDA must be obtained before commercial marketing of the product. The NDA must include results of product development, laboratory and animal studies, human studies, information on the manufacture and composition of the product, proposed labeling and other relevant information. In addition, under the Pediatric Research Equity Act, or PREA, a NDA or supplement must contain data to assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers. Unless otherwise required by regulation, PREA does not apply to any product for an indication for which orphan designation has been granted. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the NDA for filing and, even if filed, that any approval will be granted on a timely basis, if at all.
Under the Prescription Drug User Fee Act, as amended, or PDUFA, each NDA must be accompanied by a significant user fee. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application submitted by a small business. Additionally, no user fees are assessed on NDAs for product candidates designated as orphan drugs, unless the product candidate also includes a non-orphan indication.
Within 60 days following submission of the application, the FDA reviews the application submitted to determine if it is substantially complete before the agency accepts it for filing. The FDA may refuse to file any application that it deems incomplete or not properly reviewable at the time of submission and may request additional information. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review to determine, among other things, whether the proposed product is safe and effective, for its intended use, and whether the product is being manufactured in accordance with cGMP. The FDA may refer applications for novel products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. FDA also will determine whether a Risk Evaluation and Mitigation Strategy, or REMS, is necessary to assure the safe use of the product. If the FDA concludes a REMS is needed, the sponsor must submit a proposed REMS; the FDA will not approve the application without a REMS, if required.
Before approving an application, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, the FDA will typically inspect one or more clinical trial sites to assure that the clinical trials were conducted in compliance with IND study requirements and GCP requirements. To assure cGMP and GCP compliance, an applicant must incur significant expenditure of time, money and effort in the areas of training, record keeping, production, and quality control.
68
Notwithstanding the submission of relevant data and information, the FDA may ultimately decide that the NDA does not satisfy its regulatory criteria for approval and deny approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data. If the agency decides not to approve the NDA in its present form, the FDA will issue a complete response letter that usually describes all of the specific deficiencies identified by the FDA. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical trials. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit, addressing all of the deficiencies identified in the letter, or withdraw the application.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. The FDA may impose restrictions and conditions on product distribution, prescribing, or dispensing in the form of a risk management plan, or otherwise limit the scope of any approval. In addition, the FDA may require post marketing clinical trials, sometimes referred to as Phase 4 clinical trials, designed to further assess a product’s safety and effectiveness, and testing and surveillance programs to monitor the safety of approved products that have been commercialized. As a condition for approval, the FDA may also require additional nonclinical testing as a Phase 4 commitment.
One of the performance goals agreed to by the FDA under the PDUFA is to review standard NDAs in ten months from filing and priority NDAs in six months from filing, whereupon a review decision is to be made. The FDA does not always meet its PDUFA goal dates and its review goals are subject to change from time to time. The review process and the PDUFA goal date may be extended by three months if the FDA requests or the sponsor otherwise provides additional information or clarification regarding information already provided in the submission within the last three months before the PDUFA goal date.
Maintaining substantial compliance with applicable federal, state, and local statutes and regulations requires the expenditure of substantial time and financial resources. Rigorous and extensive FDA regulation of biopharmaceutical products continues after approval, particularly with respect to cGMP. Manufacturers of our products are required to comply with applicable requirements in the cGMP regulations, including quality control and quality assurance and maintenance of records and documentation. Following approval, the manufacturing facilities are subject to biennial inspections and such inspections may result in an issuance of FDA Form 483 deficiency observations or a warning letter, which can lead to plant shutdown and other more serious penalties and fines. Prior to the institution of any manufacturing changes, a determination needs to be made whether FDA approval is required in advance. If not done in accordance with FDA expectations, the FDA may restrict supply and may take further action. Annual product reports are required to be submitted.
69
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan designation, or ODD, to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition with either a patient population of fewer than 200,000 individuals in the United States, or a patient population greater of than 200,000 individuals in the United States when there is no reasonable expectation that the cost of developing and making available the drug or biologic in the United States will be recovered from sales in the United States for that drug or biologic. ODD must be requested before submitting a BLA or NDA. After the FDA grants ODD, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
If a product that has received ODD and subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full NDA, to market the same biologic or drug for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or if FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Orphan drug exclusivity does not prevent the FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of ODD are tax credits for certain research and a waiver of the application user fee.
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received ODD. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Other Healthcare Laws and Compliance Requirements
Pharmaceutical companies are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which they conduct their business. Such laws include, without limitation: the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering or paying remuneration, to induce, or in return for, either the referral of an individual, or the purchase or recommendation of an item or service for which payment may be made under any federal healthcare program; federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment to the federal government, including federal healthcare programs, that are false or fraudulent; the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal statutes which prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters, and which, as amended by the Health Information for Economic and Clinical Health Act of 2009, or HITECH, also imposes certain requirements on HIPAA covered entities and their business associates, and their covered subcontractors relating to the privacy, security and transmission of individually identifiable health information; the U.S. federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to annually report to the federal government, information related to (i) payments and other "transfers of value" made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), other healthcare professionals (such as physician assistants and nurse practitioners), and teaching hospitals, and (ii) ownership and investment interests held by physicians and their immediate family members; and U.S. state and foreign law equivalents of each of the above federal laws, which, in some cases, differ from each other in significant ways, and may not have the same effect, thus complicating compliance efforts.
70
If their operations are found to be in violation of any of such laws or any other governmental regulations that apply, they may be subject to significant penalties, including, without limitation, civil, criminal and administrative penalties, damages, fines, exclusion from government-funded healthcare programs, such as Medicare and Medicaid or similar programs in other countries or jurisdictions, integrity oversight and reporting obligations to resolve allegations of non-compliance, disgorgement, imprisonment, contractual damages, reputational harm, diminished profits and the curtailment or restructuring of our operations.
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any pharmaceutical or biological product for which we obtain regulatory approval. Sales of any product depend, in part, on the extent to which such product will be covered by third-party payors, such as federal, state, and foreign government healthcare programs, commercial insurance and managed healthcare organizations, and the level of reimbursement for such product by third-party payors. Decisions regarding the extent of coverage and amount of reimbursement to be provided are made on a plan-by-plan basis. As there is no uniform policy of coverage and reimbursement for drug products among third-party payors in the United States, coverage and reimbursement policies for drug products can differ significantly from payor to payor. There may be significant delays in obtaining coverage and reimbursement as the process of determining coverage and reimbursement is often time-consuming and costly which will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage or adequate reimbursement will be obtained. It is difficult to predict at this time what government authorities and third-party payors will decide with respect to coverage and reimbursement for our drug products. For products administered under the supervision of a physician, obtaining coverage and adequate reimbursement may be particularly difficult because of the higher prices often associated with such drugs. Additionally, separate reimbursement for the product itself or the treatment or procedure in which the product is used may not be available, which may impact physician utilization.
In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Third-party payors are increasingly challenging the prices charged for medical products and services, examining the medical necessity and reviewing the cost effectiveness of pharmaceutical or biological products, medical devices and medical services, in addition to questioning safety and efficacy. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit sales of any product. Decreases in third-party reimbursement for any product or a decision by a third-party payor not to cover a product could reduce physician usage and patient demand for the product.
Healthcare Reform
The United States and some foreign jurisdictions are considering or have enacted a number of reform proposals to change the healthcare system. There is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by federal and state legislative initiatives, including those designed to limit the pricing, coverage, and reimbursement of pharmaceutical and biopharmaceutical products, especially under government-funded health care programs, and increased governmental control of drug pricing.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or, collectively, ACA, was signed into law, which substantially changed the way healthcare is financed by both governmental and private insurers in the United States, and significantly affected the pharmaceutical industry. The ACA contains a number of other provisions of particular import to the pharmaceutical and biotechnology industries, including, but not limited to, those governing enrollment in federal healthcare programs, a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, and annual fees based on pharmaceutical companies’ share of sales to federal health care programs.
71
Since its enactment, there have been executive, judicial, Congressional, and executive branch challenges to certain aspects of the ACA. For example, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the ACA-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminated the health insurer tax. In addition, the Tax Cuts and Jobs Act of 2017, or the Tax Act, was enacted, which, among other things, removed penalties for not complying with ACA’s individual mandate to carry health insurance. On June 17, 2021, the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the "individual mandate" was repealed by Congress. Since the enactment of the Tax Act, there have been additional amendments to certain provisions of the ACA. For example, on August 16, 2022, President Biden signed the Inflation Reduction Act of 2022, or IRA, into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the "donut hole" under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges and the healthcare reform measures of the Biden administration will impact the ACA.
Other legislative changes have been proposed and adopted since the ACA was enacted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year and reduced payments to several types of Medicare providers, which will remain in effect until 2031 unless additional Congressional action is taken. Under current legislation, the actual reduction in Medicare payments will vary from 1% in 2022 to up to 4% in the final fiscal year of this sequester. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries, Presidential executive orders, and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products.
At the federal level, the Trump administration used several means to propose or implement drug pricing reform, including through federal budget proposals, executive orders and policy initiatives. For example, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs.
In response to Biden’s executive order, on September 9, 2021, the U.S. Department of Health and Human Services, or HHS, released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. Further, the IRA, among other things (i) directs HHS to negotiate the price of certain high-expenditure, single-source drugs and biologics covered under Medicare and (ii) imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation. These provisions will take effect progressively starting in fiscal year 2023, although they may be subject to legal challenges. It is currently unclear how the IRA will be implemented but is likely to have a significant impact on the pharmaceutical industry. Additionally, the Biden administration released an additional executive order on October 14, 2022, directing HHS to report on how the Center for Medicare and Medicaid Innovation can be further leveraged to test new models for lowering drug costs for Medicare and Medicaid beneficiaries. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
72
Product Development
We have made significant investments over the last twenty years in the development of our proprietary technologies, our small molecule and plasma-derived therapeutics platforms and the product candidates arising therefrom. These investments and in-house development strategy have allowed us to be flexible in our approaches and adaptive when needed as well as retaining control over intellectual property rights and the potential commercial upside thereon. Furthermore, it allows us to develop the necessary skill sets internally on both the development of manufacturing processes as well as product development (preclinical and clinical) in various disease indications. Notwithstanding the foregoing, we believe that it is important to have a balance between in-house product development and outsourcing same or partnering such activities. Finally, pursuing the development and commercialization phase in partnership with other companies (especially for specific indications and/or geographic regions) is also interesting for us because it provides continuous external validation of our technology and possibilities of short and long term revenues from fees collected at the initiation of the partnership as well as via milestones payments and royalty streams.
Environmental Protection
We produce a certain amount of chemical waste in our R&D and manufacturing activities that is managed in accordance with applicable environmental protection standards by companies that specialize in hazardous waste management. Our research laboratories generate hazardous waste that is also removed by companies that specialize in hazardous waste management, in accordance with strict internal procedures and applicable regulatory requirements. Compliance with such requirements is not expected to have a significant effect on our competitive position.
73
C. Organizational Structure
We are structured as a parent company with separate operating entities which all are directly or indirectly controlled by us.
The following chart indicates:
(i)the name of the operating subsidiaries and their acronym;
(ii)jurisdiction of incorporation of our operating subsidiaries; and
(iii)voting interest (expressed as a percentage) beneficially owned, controlled or directed by us in each subsidiary.
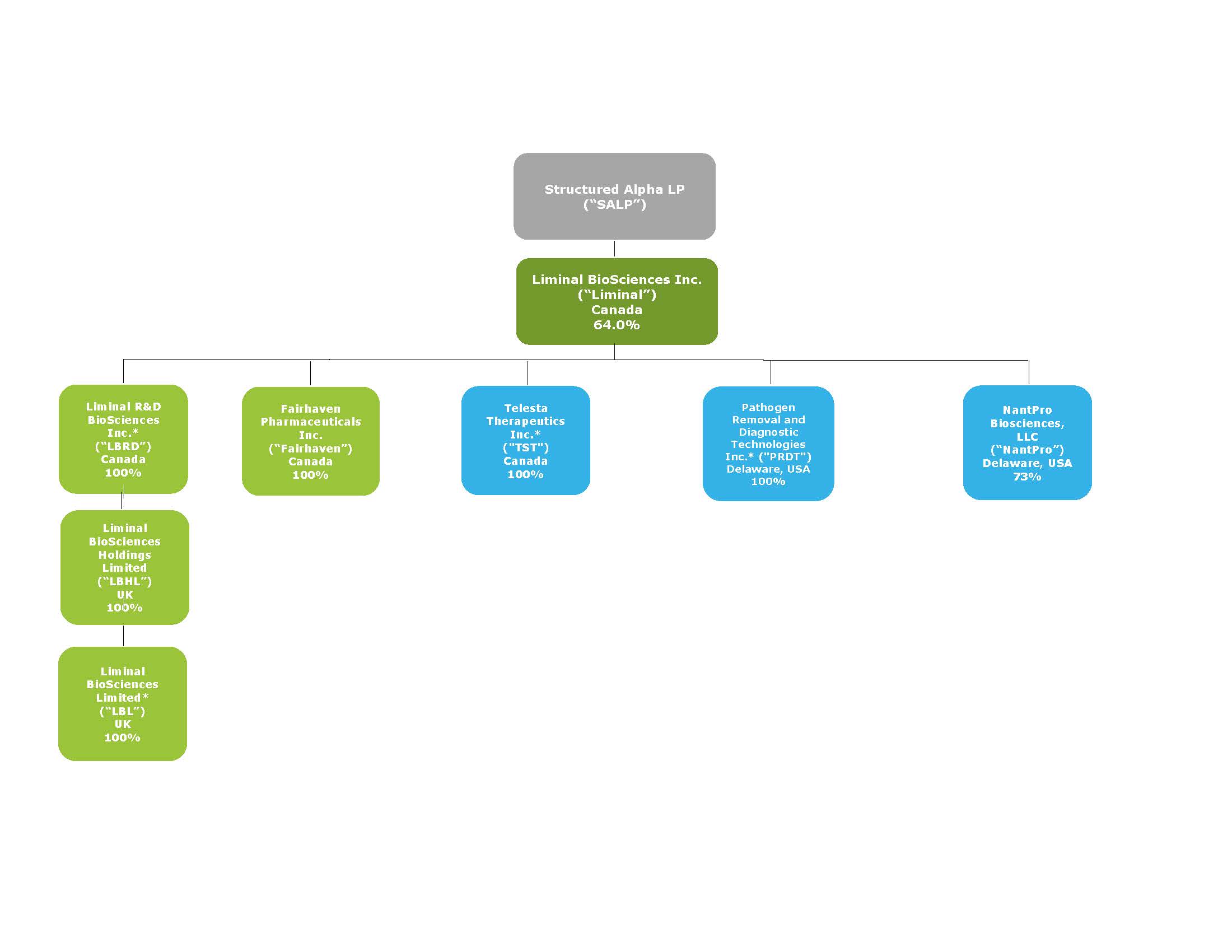
* Subsidiary that meet the definition of significant subsidiaries for the financial year ended December 31, 2022.
D. Property, Plant and Equipment.
We own our registered office space in Belleville, Ontario. We lease our executive office and laboratory space in Laval, Québec, Canada, which respectively consists of approximately 1,555 and 12,167 square meters, and office space in Sawston, United Kingdom which consists of approximately 3,985 square feet. The lease for our principal executive office in Laval expires on October 31, 2025. The lease for our office in Sawston expires on November 12, 2024. We believe our current facilities are sufficient to meet our needs. If we need to add new facilities or expand existing facilities as we add employees, we believe that suitable additional space will be available to accommodate any such expansion of our operations.
74
Not applicable.
75
Item 5. Operating and Financial Review and Prospects.
Overview
Program Overview
We are a development stage biopharmaceutical company focused on discovering and developing novel and distinctive small molecule therapeutics that modulate G protein-coupled receptors, or GPCR, pathways. We are designing proprietary novel small molecule therapeutic candidates with the intent of developing best/first in class therapeutics for the treatment of metabolic, inflammatory and fibrotic diseases with significant unmet medical needs, using our integrated drug discovery platform, medicinal chemistry expertise and deep understanding of the GPCR biology.
Our pipeline is currently made up of three development programs. The candidate we selected for clinical development, LMNL6511, a selective antagonist for the GPR84 receptor, is expected to commence a Phase 1 clinical trial in the second half of 2023. We are also developing potential OXER1 antagonists and GPR40 agonists, both of which are at the preclinical stage. In addition to these priority development programs, we continue to explore other development opportunities to add to our pipeline.
The candidate we selected for clinical development, LMNL6511, is an oral, selective antagonist of the orphan GPCR, GPR84, designed to treat metabolic diseases, inflammation and/or fibrosis in several different therapeutic categories. We believe that the GPR84 receptor may be an important biological target in several therapeutic areas by blocking a proinflammatory modulator on immune cells, including macrophages, once inflammation is established.
The GPR84 receptor itself, is primarily expressed in immune cells in addition to multiple organ systems such as the liver, lung, and gastrointestinal, or GI, tract. Its expression is upregulated in response to inflammatory stimuli. It therefore provides an attractive therapeutic target in several chronic metabolic, inflammatory and fibrosis driven disease processes. We anticipate seeking approval to commence a first-in-human Phase 1 clinical trial of LMNL6511 during the second half of 2023, once we successfully complete CTA enabling activities.
We are also developing a selective OXER1 antagonist candidate. OXER1 is a GPCR that is highly selective for 5-oxo-ETE, believed to be one of the most potent human eosinophil chemo-attractants. Migration of eosinophils to body sites including the lungs and intestines is mediated by eosinophil chemo-attractants such as 5-oxo-ETE. Eosinophils play a key role in inflammation-driven diseases, including skin, respiratory diseases and gastro-intestinal diseases such as asthma, allergic rhinitis, chronic obstructive pulmonary disorder, atopic dermatitis, psoriasis and acne. Our OXER1 antagonist discovery program is currently at the preclinical stage. We are working towards selecting a lead candidate for further development in Eosinophilic driven diseases in the first half of 2023 and are aiming to commence a first-in-human clinical trial in 2024.
76
We are also developing a GPR40 agonist as a potential therapeutic treatment for type 2 diabetes. Free fatty acid receptor 1, FFAR 1 (also known as G protein coupled receptor 40, or GPR40) is a validated clinical target for the treatment of diabetes, demonstrated by the phase 2/3 trials of the GPR40 agonist, TAK875. However, the development of this compound was terminated due to a low frequency of drug induced liver injury (DILI). We believe that small molecule agonists of GPR40 can be designed to minimize the risk of DILI. Our GPR40 agonist program is currently in the discovery phase.
Recent developments
•In September 2022, upon approval from the Listing Qualifications Department of The Nasdaq Stock Market ("Nasdaq"), we transferred the listing of our common shares from The Nasdaq Global Market to The Nasdaq Capital Market, as allowed under Listing Rule 5810(c)(3)(A). Our common shares began trading on The Nasdaq Capital Market effective at the start of trading on September 6, 2022. In connection with the transfer to the Nasdaq Capital Market, Nasdaq granted us a second period of 180 calendar days, or until February 27, 2023, to regain compliance with the minimum bid price requirement for continued listing by achieving a closing bid price on Nasdaq of at least $1.00 per share for a minimum of 10 consecutive trading days.
•In February 2023, we completed a consolidation of all the issued and outstanding common shares of the Company on the basis of a consolidation ratio of ten pre-consolidation shares for one post-consolidation share to regain compliance with the minimum bid price requirement for continued listing by achieving a closing bid price on Nasdaq Capital Market of at least $1.00 per share for a minimum of 10 consecutive trading days. In February 2023, we received written notice from Nasdaq that we regained compliance with the Nasdaq Listing Rule 5450(a)(1) in regard to the minimum bid price requirement for continued listing on the Nasdaq Capital Market.
77
•In October 2022, the non-binding Letter of Intent, or LOI, for the sale of our dormant manufacturing facility located in Belleville, Ontario, or Belleville facility, entered into with a third party in January 2022 was terminated as a result of certain requirements not having been satisfied. Subsequent to the year ended December 31, 2022, the Belleville facility, formerly part of the plasma-derived therapeutics segment and previously classified as property, plant and equipment, met the criteria to be classified as held for sale and will be classified accordingly as of March 31, 2023.
•In November 2022, we made changes to our executive leadership team. Nicole Rusaw, who served as interim Chief Financial Officer since March 2, 2022, was appointed as Chief Financial Officer. Dr. Gary Bridger, a member of our Board of Directors and Strategic Advisor to the Company, was named Interim Chief Scientific Officer.
•In December 2022, we sold our previously owned facility located in Pointe-Claire, Quebec, or Labrosse facility, formerly part of the plasma-derived therapeutics segment and previously classified as property, plant and equipment, which met the criteria to be classified as held for sale during the quarter ended March 31, 2022 at a carrying amount of $0.8 million. We received $3.2 million in net proceeds from this sale.
Financial Performance
Under item 5, amounts in tables are expressed in thousands of CAD, except per share amounts which are in full Canadian dollars.
On February 1, 2023, we performed a 10 to 1 share consolidation of our issued equity instruments including common shares, warrants and options. The quantities and per unit prices presented in the 20-F have been retroactively adjusted to give effect to this share consolidation.
We entered into two share purchase agreements, or SPAs, with Kedrion S.p.A., or Kedrion, during the quarter ended June 30, 2021: the first for the sale of Prometic Plasma Resources Inc. (PPR) and Prometic Plasma Resources USA Inc. (PPR USA), operating the plasma collection centers, which dispositions were completed on May 21, 2021, and the second for the sale of our former Ryplazim® business operated through its subsidiaries Prometic Bioproduction Inc. (PBP), which was disposed on July 9, 2021, and our former plasma-derived therapeutics manufacturing facility, Prometic Biotherapeutics Inc. (PBT), the holder of the biologicals license application or BLA and intellectual property rights for Ryplazim® which was disposed on October 15, 2021.. Additionally, our former subsidiary PBT entered into an agreement with another party for the sale of the Priority Review Voucher, or PRV, it received on June 4, 2021, in conjunction with FDA approval of its BLA. This sale closed on September 28, 2021. These disposals cover the majority of Liminal’s plasma-derived therapeutics segment.
We have ceased to consolidate these entities in our consolidated financial statements as of the date of the disposal. Our interest in PPR, PPR USA, PBP and PBT has been presented separately as “Discontinued Operations” in the comparative results in accordance with the guidance under IFRS 5, Non-Current Asset Held for Sale and Discontinued Operations.
78
Financial operations overview
Revenues
Revenues include royalty revenues and rental revenues.
Research and development expenses
Research and development, or R&D, expenses comprise the costs to have a contract development and manufacturing organization manufacture the product candidate used in pre-clinical studies and clinical trials. It also includes the cost of external consultants supporting the clinical trials and pre-clinical studies, employee compensation and other operating expenses involved in research and development activities. Government grant credits for eligible R&D salaries and rent in Canada reduce the R&D expenses.
Administration expenses
Administration expenses mainly consist of salaries and benefits related to our executive, finance, human resources, business development, legal, intellectual property, and information technology support functions. Professional fees reported under administrative expenses mainly include legal fees, accounting fees, audit fees and fees for taxation advisors. It also includes operating expenses such as insurance costs, office expenses, and travel costs pertaining to administration. Government grant credits for eligible administrative salaries and rent in Canada are also included in administration expenses.
Gain on foreign exchange
Gain on foreign exchange includes the effects of foreign exchange variations on monetary assets and liabilities denominated in foreign currencies between the rates at which they were initially recorded at in the functional currency at the date of the transaction and when they are retranslated at the functional currency spot rate of exchange at the reporting date. All differences are included in the consolidated statement of operations.
Finance costs
Finance costs mainly includes interest expense from long-term debt, lease liabilities and banking charges. Finance costs also includes financing transaction cost associated with financial instruments carried at fair value through profit or loss. Finance costs are presented net of interest income which primarily results from the interest earned on the cash we hold.
Loss (gain) on extinguishments of liabilities
When the terms of our long-term debt are modified significantly, the then existing debt is considered extinguished and the carrying amount of the debt before modification is derecognized, and the fair value of the modified debt is recognized. The difference is recorded as a loss (gain) on extinguishment of liabilities. Similarly, when a debt agreement is terminated resulting in a cash payment, the difference between the carried amount of the debt and the amount paid is recorded as a loss (gain) on extinguishment of liabilities.
Change in fair value of financial instruments measured at fair value through profit or loss
Fair value increases and decreases on financial instruments measured at fair value through profit or loss are presented here. Over the last two years, this caption includes the changes in fair values of the warrant liability.
79
Impairment losses
Impairment losses include impairments recorded on long-lived assets, including but not limited to capital assets, right-of-use assets and intangible assets.
Income tax expense
Income tax expense includes the current tax expense that will be payable to or collectable from the taxation authorities in the various jurisdictions in which we operate. Income tax expense also includes deferred income tax expense and recoveries. Deferred income tax assets are recognized to the extent that it is probable that future tax profits will allow the deferred tax assets to be recovered.
Discontinued operations
Discontinued operations comprise the revenues and expenses of operations and the gains/losses on disposition and transaction expenses related thereto, for the following activities and assets:
•two subsidiaries, Prometic Bioseparations Ltd. and Prometic Manufacturing Inc., that were previously included in our bioseparation segment, were sold on November 25, 2019 and were reflected in discontinued operations in 2020;
•four subsidiaries namely Prometic Plasma Resources Inc. (PPR), Prometic Plasma Resources USA Inc. (PPR USA), Prometic Bioproduction Inc. (PBP) and Prometic Biotherapeutics Inc. (PBT) which were formerly part of the plasma-derived therapeutics segment were sold together with the Priority Review Voucher (PRV) in a series of transactions in 2021, and another subsidiary, Prometic Biotherapeutics Ltd, also part of the same segment, for which operations have ceased and the entity has been dissolved. These impact discontinued operations in all periods presented in this MD&A;
•variations in the portions of a Contract Manufacturing Organization, or CDMO agreement, which was terminated in August 2022, were accounted for as a provision and a lease liability in regard to our former plasma-derived therapeutics segment, due to changes in payment estimates or discount rates; and
•the operating costs of our previously owned Labrosse facility, formerly part of the plasma-derived therapeutics segment are included in discontinued operations for all periods presented in this Item 5 of this Annual Report. The Labrosse facility was classified as held for sale at March 31, 2022 and was sold in December 2022.
All amounts relating to the activities above have been presented as discontinued operations in the current and prior periods. More specifically, we have restated the prior periods to remove the impact of those operations from all lines in the financial statements (revenues, cost of sales and production costs, R&D and administration, selling and marketing being the lines most impacted) and have reclassified those results to the income (loss) from discontinued operations lines in the consolidated financial statements. The proceeds and expenses pertaining to the sale of the businesses and assets are included as part of the gain on sale of discontinued operations.
80
Comparison of years ended December 31, 2022, 2021 and 2020
The consolidated statements of operations for the year ended December 31, 2022 compared to the corresponding periods in 2021 and 2020 are presented in the following tables:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | Change | |
| | 2022 | | | 2021 | | | 2020 | | | 2022 vs 2021 | | | 2021 vs 2020 | |
Revenues | | | 401 | | | | 643 | | | | 724 | | | | (242 | ) | | | (81 | ) |
| | | | | | | | | | | | | | | |
Expenses | | | | | | | | | | | | | | | |
Research and development expenses | | | 15,298 | | | | 18,347 | | | | 14,234 | | | | (3,049 | ) | | | 4,113 | |
Administration expenses | | | 17,866 | | | | 31,928 | | | | 32,619 | | | | (14,062 | ) | | | (691 | ) |
Gain on foreign exchange | | | (2,964 | ) | | | (1,397 | ) | | | (35 | ) | | | (1,567 | ) | | | (1,362 | ) |
Finance costs | | | 1,078 | | | | 6,330 | | | | 2,899 | | | | (5,252 | ) | | | 3,431 | |
Loss (gain) on extinguishments of liabilities | | | 212 | | | | (75 | ) | | | — | | | | 287 | | | | (75 | ) |
Change in fair value of financial instruments
measured at fair value through profit or loss | | | (1,648 | ) | | | (9,886 | ) | | | (850 | ) | | | 8,238 | | | | (9,036 | ) |
Impairment losses | | | — | | | | 341 | | | | 1,087 | | | | (341 | ) | | | (746 | ) |
Net loss from continuing operations
before income taxes | | $ | (29,441 | ) | | $ | (44,945 | ) | | $ | (49,230 | ) | | $ | 15,504 | | | $ | 4,285 | |
| | | | | | | | | | | | | | | |
Current income tax | | | (811 | ) | | | — | | | | (144 | ) | | | (811 | ) | | | 144 | |
Deferred income tax | | | 286 | | | | 118 | | | | (65 | ) | | | 168 | | | | 183 | |
Income tax expense (recovery) on continuing
operations | | | (525 | ) | | | 118 | | | | (209 | ) | | | (643 | ) | | | 327 | |
Net loss from continuing operations | | $ | (28,916 | ) | | $ | (45,063 | ) | | $ | (49,021 | ) | | $ | 16,147 | | | $ | 3,958 | |
| | | | | | | | | | | | | | | |
Discontinued operations | | | | | | | | | | | | | | | |
Gain (loss) on sale of discontinued operations,
net of income taxes of $nil | | | (600 | ) | | | 140,403 | | | | 3,380 | | | | (141,003 | ) | | | 137,023 | |
Income (loss) from discontinued operations,
net of income taxes | | | 30,138 | | | | (83,127 | ) | | | (73,116 | ) | | | 113,265 | | | | (10,011 | ) |
Total income (loss) from discontinued
operations | | $ | 29,538 | | | $ | 57,276 | | | $ | (69,736 | ) | | $ | (27,738 | ) | | $ | 127,012 | |
Net income | | $ | 622 | | | $ | 12,213 | | | $ | (118,757 | ) | | $ | (11,591 | ) | | $ | 130,970 | |
| | | | | | | | | | | | | | | |
Net income (loss) attributable to: | | | | | | | | | | | | | | | |
Non-controlling interests in continuing operations | | | 122 | | | | (669 | ) | | | (832 | ) | | | 791 | | | | 163 | |
Owners of the parent | | | | | | | | | | | | | | | |
- Continuing operations | | | (29,038 | ) | | | (44,394 | ) | | | (48,189 | ) | | | 15,356 | | | | 3,795 | |
- Discontinued operations | | | 29,538 | | | | 57,276 | | | | (69,736 | ) | | | (27,738 | ) | | | 127,012 | |
| | | 500 | | | | 12,882 | | | | (117,925 | ) | | | (12,382 | ) | | | 130,807 | |
Net income | | $ | 622 | | | $ | 12,213 | | | $ | (118,757 | ) | | $ | (11,591 | ) | | $ | 130,970 | |
| | | | | | | | | | | | | | | |
Income (loss) per share attributable to
the owners of the parent
basic and diluted: | | | | | | | | | | | | | | | |
From continuing operations | | $ | (9.36 | ) | | $ | (14.72 | ) | | $ | (19.72 | ) | | $ | 5.36 | | | $ | 5.00 | |
From discontinued operations | | | 9.52 | | | | 18.99 | | | $ | (28.53 | ) | | $ | (9.47 | ) | | | 47.52 | |
Total income per share | | $ | 0.16 | | | $ | 4.27 | | | $ | (48.25 | ) | | $ | (4.11 | ) | | $ | 52.52 | |
Weighted average number of outstanding shares
(in thousands) | | | 3,104 | | | | 3,016 | | | | 2,444 | | | | 88 | | | | 572 | |
81
Continuing Operations analysis
Revenues
The following table provides the breakdown of total revenues from continuing operations by source of revenue for the year ended December 31, 2022 compared to the corresponding periods in 2021 and 2020:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | Change | |
| | 2022 | | | 2021 | | | 2020 | | | 2022 vs 2021 | | | 2021 vs 2020 | |
Royalty revenues | | $ | 401 | | | $ | 565 | | | $ | 572 | | | $ | (164 | ) | | $ | (7 | ) |
Rental revenue | | | — | | | | 78 | | | | 152 | | | | (78 | ) | | | (74 | ) |
| | $ | 401 | | | $ | 643 | | | $ | 724 | | | $ | (242 | ) | | $ | (81 | ) |
Revenues include nominal amounts of royalty and rental revenues. Royalty revenues are dependent on sales made by a third party. There were no rental revenues during the year ended December 31, 2022.
Research and development expenses
The decrease of $3.1 million in R&D expenses during the year ended December 31, 2022 compared to the corresponding period in 2021 was mainly attributable to a decrease of $2.0 million in clinical trial costs relating to fezagepras, which included a Phase 1 MAD clinical trial of fezagepras which was completed during the quarter ended March 31, 2022, and the Phase 1a SAD clinical trial of fezagepras designed as a head-to-head comparison with sodium phenylbutyrate which started in May 2022 and was terminated in July 2022. The intangible assets depreciation expense and consulting fees were also lower by $2.3 million and $0.6 million over the same period, respectively.
These decreases were partially offset by the recognition of an upfront payment of $0.4 million in regard to a royalty stream agreement, the absence of government grants from the Canada Emergency Wage Subsidy, or CEWS, and the Canada Emergency Rent Subsidy, or CERS, programs in 2022, compared to the recognition of $0.6 million in grant credits during the year ended December 31, 2021, and an increase in third party preclinical studies expense of $0.9 million during the year ended December 31, 2022 compared to the corresponding period in 2021, reflecting the increase in resources allocated to our GPR84 and OXER1 antagonist programs.
R&D expenses increased by $4.1 million during the year ended December 31, 2021 compared to the corresponding period in 2020. The increase was mainly due to an increase in third party clinical trial expenses of $2.4 million, mostly related to the MAD Phase 1 clinical trial of fezagepras, an increase in third-party preclinical studies expenses of $0.8 million, as well as increases in consulting fees of $0.6 million and intangible amortization expense of $1.1 million. Additionally, we recorded a reduction in grant credits of $0.6 million. These increases in R&D expenses were partially offset by a decrease in share-based compensation expense of $1.5 million explained below under Share-based payments expense.
Administration expenses
The decrease of $14.1 million in administration expenses during the year ended December 31, 2022 compared to the corresponding period in 2021 was mainly attributable to a decrease of $9.2 million in insurance expense as a result of reduced directors’ and officers’ insurance premiums resulting from the change in the Company’s registered office from Québec to Ontario in the later part of 2021. The decrease is also due to reductions in share-based payments expense and salaries and other benefits of $2.7 million and $1.7 million, respectively.
82
The decrease of $0.7 million in administration expenses during the year ended December 31, 2021 compared to the corresponding period in 2020 was attributable in part to a reduction in professional fees of $1.7 million and a reduction of $0.4 million in office expenses. The decrease is also due to the fact that in 2020 there was a $2.2 million expense recognized in conjunction with the additional warrants issued following an amendment to the private placement agreement completed in November of that year with no equivalent cost in 2021. These decreases in expenses were partially offset by a decrease of $1.1 million in the recognition of credits pertaining to the CEWS government grant, increases in bonus and termination benefit expenses, and an increase of $0.5 million in share-based payment expenses explained below.
Share-based payments expense
Share-based payments expense represents the compensation expense recorded as a result of stock options and RSU issued to employees and board members. The table below shows the share-based payments expense recorded in the continuing and discontinuing operations results. This expense has been recorded as follows in the consolidated statements of operations:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | Change | |
| | 2022 | | | 2021 | | | 2020 | | | 2022 vs 2021 | | | 2021 vs 2020 | |
Research and development expenses | | $ | 821 | | | $ | 936 | | | $ | 2,430 | | | $ | (115 | ) | | $ | (1,494 | ) |
Administration expenses | | | 1,043 | | | | 3,760 | | | | 3,248 | | | | (2,717 | ) | | | 512 | |
Loss from discontinued operations | | | — | | | | (444 | ) | | | 556 | | | | 444 | | | | (1,000 | ) |
| | $ | 1,864 | | | $ | 4,252 | | | $ | 6,234 | | | $ | (2,388 | ) | | $ | (1,982 | ) |
The decrease in share-based payments expense of $2.4 million during the year ended December 31, 2022 compared to the corresponding period in 2021, is mainly due to a reduction in stock option expense as a result of the reduction in senior executives in the stock option plan from 2021 to 2022 and because the fair value of stock option grants has decreased in the recent years given our lower share price.
Share-based payments expense for the year ended December 31, 2021 decreased by $2.0 million compared to the corresponding period in 2020, mainly due to the general reduction in the number of employees that are part of the Liminal group, mainly as a result of the sale of our former subsidiaries in 2021 that were part of our former plasma-derived therapeutics segment. This led to an increase in stock option forfeitures which resulted in the reversal of the share-based payment expense pertaining to unvested stock options as well as a reduction in the number of stock options granted in 2021. Share-based payments expense also declined because the average grant date fair value of a stock option has declined over the two-year period. In addition, the impact of the repricing of stock options that took place during the second quarter of 2020 was higher in 2020 since some of the repriced stock options were vested immediately and the repricing expense related to those vested options was immediately recognized. Also due to the general expensing pattern of graded vesting stock options, where the yearly expense of a given grant declines over the years of vesting, the impact of the 2020 repriced options is lower in 2021.
83
Finance costs
Finance costs decreased by $5.3 million for the year ended December 31, 2022 compared to the corresponding period in 2021, mainly attributable to a decrease in interest expense on long-term debt of $3.6 million because our loans with SALP pursuant to the consolidated loan agreement dated April 23, 2019, as subsequently amended, or SALP loans were only outstanding until February 15, 2022 compared to the prior period where they were outstanding for the entire period and because we also incurred interest on our convertible debt in 2021, until its conversion in August 2021. Also, interest income earned was higher by $0.6 million during the year ended December 31, 2022 compared to the corresponding period in 2021 due to higher average cash balances.
Our finance costs increased by $3.4 million during the year ended December 31, 2021 compared to the corresponding period in 2020 reflecting the increase in our level of indebtedness following (i) the issuance of the secured convertible debentures, or SCD, in July 2020, which remained outstanding until the SCD was converted into our common shares in October 2021, and (ii) the second term loan in September 2020, as we drew down our full line of credit with SALP.
Loss on extinguishment of liabilities
In February 2022, we repaid our outstanding long-term debt of $39.1 million and derecognized the royalty obligation of $0.1 million, both with SALP. On the repayment of the loans, we recognized a loss of $0.3 million which was partially offset by the gain of $0.1 million resulting from the derecognition of the royalty obligation.
Change in fair value of financial instruments measured at fair value through profit or loss
We recorded gains on the variation in fair value of the warrant liability that is measured at FVPL during the years ended December 31, 2022, 2021 and 2020. The main driver for these gains are the decrease in the value of the underlying shares over those periods.
Net loss from continuing operations
The net loss from continuing operations decreased by $16.4 million during the year ended December 31, 2022 compared to the corresponding period in 2021. This was mainly driven by the reductions in administration expenses of $14.1 million, reflecting the reduction in insurance expense, a decrease in finance costs of $5.3 million due to the termination of all long-term debt and an increase in foreign exchange gains of $1.6 million. These decreases were partially offset by a lower gain resulting in change in fair value of financial instruments measured at fair value through profit and loss of $8.2 million.
The net loss from continuing operations, net of taxes, decreased by $4.0 million during the year ended December 31, 2021 compared to the corresponding period of 2020 mainly due to a favorable change in fair value of financial instruments measured at fair value through profit and loss of $9.0 million and a favorable foreign exchange variance of $1.4 million. These decreases were partially offset by an increase in R&D and finance costs of $4.1 million and $3.4 million, respectively, as explained above.
84
Discontinued Operations analysis
The net income (loss) from discontinued operations is made up of the gain we recognized on the sale of our plasma-derived therapeutics and bioseparation businesses.
Loss on sale of discontinued operations
The table below provides the details of the computation of the gain (loss) on sale of our former subsidiaries for the years ended December 31, 2022, 2021 and 2020.
| | | | | | | | | | | | | | |
Year ended December 31 | | | | 2022 | | | 2021 | | | 2020 | |
Sale of bioseparation business | | | | | | | | | |
Proceeds received | | $ | — | | | $ | — | | | $ | 3,380 | |
Less: | | | | | | | | | |
Carrying amount of net assets sold | | | — | | | | — | | | | — | |
Transaction costs | | | — | | | | — | | | | — | |
Reclassification of foreign currency translation reserve from
other comprehensive income into
the statement of operations | | | — | | | | — | | | | — | |
Gain on sale of bioseparation business | | | — | | | | — | | | | 3,380 | |
| | | | | | | | | |
Sale of plasma collection centers | | | | | | | | | |
Proceeds received | | | — | | | | 13,570 | | | | — | |
Less: | | | | | | | | | |
Carrying amount of net assets sold | | | — | | | | 10,849 | | | | — | |
Transaction costs | | | — | | | | 204 | | | | — | |
Reclassification of foreign currency translation reserve
from other comprehensive income
into the statement of operations | | | — | | | | (44 | ) | | | — | |
Gain on sale of plasma collection centers | | | — | | | | 2,561 | | | | — | |
| | | | | | | | | |
Sale of Ryplazim® business | | | | | | | | | |
Proceeds received | | | — | | | | 159,787 | | | | — | |
Less: | | | | | | | | | |
Carrying amount of net assets sold | | | — | | | | 19,541 | | | | — | |
Indemnification adjustments | | | 600 | | | | 116 | | | | — | |
Transaction costs | | | — | | | | 2,288 | | | | — | |
Gain (loss) on sale of Ryplazim business | | | (600 | ) | | | 137,842 | | | | — | |
Gain (loss) on sale of subsidiaries, net of income taxes $nil | | $ | (600 | ) | | $ | 140,403 | | | $ | 3,380 | |
During the year ended December 31, 2022, an indemnification adjustment expense of $0.6 million was recorded as we received notice that a research and development tax credit claim for a former subsidiary would be disallowed. We are in the process of disputing the issue with the tax authority. Comparatively, during the year ended December 31, 2021, we had a gain of $140.4 million as multiple steps included in the various agreements leading towards the sale of our plasma-derived therapeutics segment had been executed by December 31, 2021, namely the sale of the plasma collection activities, the sale of PBP and the sale of the PRV, the last of which was sold for $131.0 million (net of selling cost of $1.9 million).
Following the sale of our interests in PBL and PMI in November 2019, we generated a gain of $26.3 million in the year ended December 31, 2019, the year of the sale, and a gain of $3.4 million in the year ended December 31, 2020 as an additional amount of proceeds was received upon resolution of a taxation matter.
85
Results from discontinued operations
The following table summarizes the results of the activities that are presented as discontinued operations in the consolidated statements of operations for the years ended December 31, 2022, 2021 and 2020.
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31 | | | Change | |
| | 2022 | | | 2021 | | | 2020 | | | 2022 vs 2021 | | | 2021 vs 2020 | |
Revenues | | $ | 15 | | | $ | 949 | | | $ | 2,593 | | | $ | (934 | ) | | $ | (1,644 | ) |
Expenses | | | | | | | | | | | | | | | |
Cost of sales and other production expenses | | | — | | | | 1,465 | | | | 1,868 | | | | (1,465 | ) | | | (403 | ) |
Research and development expenses | | | (11,825 | ) | | | 76,733 | | | | 42,757 | | | | (88,558 | ) | | | 33,976 | |
Administration expenses | | | 83 | | | | 2,360 | | | | 5,933 | | | | (2,277 | ) | | | (3,573 | ) |
Impairment losses | | | — | | | | 1,411 | | | | 19,772 | | | | (1,411 | ) | | | (18,361 | ) |
Gain on foreign exchange | | | (17 | ) | | | (136 | ) | | | (633 | ) | | | 119 | | | | 497 | |
Finance costs | | | (16,019 | ) | | | 2,242 | | | | 6,083 | | | | (18,261 | ) | | | (3,841 | ) |
Gain on disposal of capital assets | | | (2,345 | ) | | | — | | | | — | | | | (2,345 | ) | | | — | |
Gain on extinguishment of liabilities | | | — | | | | — | | | | (79 | ) | | | — | | | | 79 | |
Income (loss) from discontinued operations,
net of income taxes | | $ | 30,138 | | | $ | (83,126 | ) | | $ | (73,108 | ) | | $ | 113,264 | | | $ | (10,018 | ) |
Current income taxes | | | — | | | | 1 | | | | 8 | | | | (1 | ) | | | (7 | ) |
Net loss from discontinued operations | | $ | 30,138 | | | $ | (83,127 | ) | | $ | (73,116 | ) | | $ | 113,265 | | | $ | (10,011 | ) |
Revenues and cost of sales and other production expenses
Revenues from discontinued operations included revenues from the sale of plasma up until May 21, 2021, the date of the sale of the plasma collection centers and for the entire year for 2020. During the year ended December 31, 2022, revenues from discontinued operations included solely rental revenues from our previously owned Labrosse facility.
Research and development expenses
R&D expenses decreased by $88.6 million during the year ended December 31, 2022 compared to the corresponding period in 2021. This decrease was mainly due to a first payment made from PBT to PBP on September 29, 2021, PBP then owned by Kedrion S.p.A., for $39.5 million, representing 30% of the net proceeds it received from the sale of the PRV, as compensation for past research and development services, and a second payment of $6.4 million made in prepayment for future R&D services on the same date was recognized as R&D expense since this amount would not be recoverable upon the sale of PBT. The decrease in R&D expenses was also due to the termination of the CDMO agreement in August 2022. The termination agreement resulted in a reversal in the CDMO onerous provision of $10.7 million, included in research and development expenses from discontinued operations.
R&D expenses increased by $34.0 million during the year ended December 31, 2021 compared to the corresponding period in 2020. The increase was mainly due to the payment PBT made to PBP mentioned above and a provision for an onerous contract of $22.1 million that was recognized relating to the CDMO, which is no longer required as a result of the plasma-derived therapeutic segment divestment. This increase was partially offset by a gain of $2.5 million recognized on the reduction of our lease liability. The reduction in lease liability has arisen from the term of the lease having been reduced since we gave a notice of early termination of a master agreement entered with the CDMO, reducing the term of the contract by 3.8 years. In addition, the R&D expenses for the year ended December 31, 2021 included less than one year of operations of PBP and PBT, since they were sold on July 9 and October 15, 2021, respectively. In the year ended December 31, 2020, we had R&D operations in PBP, PBT and also some R&D costs in the plasma collection centers for the entire year.
86
Administration expenses
Administration expenses decreased by $3.6 million during the year ended December 31, 2021 compared to the corresponding period of 2020 and this is mainly due to the fact that the administration expenses for the plasma collection centers and for the Ryplazim® business ceased to be included in our 2021 results as those activities were sold during the year, whereas in 2020, we have a full year of these expenses.
Finance costs
Finance costs decreased by $18.3 million during the year ended December 31, 2022 compared to the corresponding period of 2021. This decrease was mainly driven by the termination of the CDMO agreement in August 2022, resulting in the extinguishment of the entire CDMO lease liability and the recognition of a gain on modification of a liability, included in financing costs from discontinued operations of $16.0 million.
Impairments
During the year ended December 31, 2020, we recorded an impairment of $18.6 million on right of use assets, $0.7 million on capital assets and $0.5 million on intangible assets related to the Ryplazim® cash generating unit, which was part of the plasma-derived therapeutic segment, representing an aggregate impairment of $19.8 million.
Gain on disposal of capital assets
During the year ended December 31, 2022, we recorded a gain of $2.4 million on disposal of capital assets, following the sale of our previously owned Labrosse facility, formerly part of the plasma-derived therapeutics segment and previously classified as property, plant and equipment, which met the criteria to be classified as held for sale during the quarter ended March 31, 2022 at a carrying amount of $0.8 million. As a result, we received $3.2 million in net proceeds from this sale.
87
Comparison of quarters ended December 31, 2022, 2021 and 2020
The consolidated statements of operations for the quarter ended December 31, 2022 compared to the same periods in 2021 and 2020 are presented in the following tables:
| | | | | | | | | | | | | | | | | | | | |
| | Quarter ended December 31, | | | Change | |
| | 2022 | | | 2021 | | | 2020 | | | 2022 vs 2021 | | | 2021 vs 2020 | |
Revenues | | $ | 241 | | | $ | 238 | | | 284 | | | $ | 3 | | | $ | (46 | ) |
| | | | | | | | | | | | | | | |
Expenses | | | | | | | | | | | | | | | |
Research and development expenses | | | 3,462 | | | | 4,539 | | | | 2,953 | | | | (1,077 | ) | | | 1,586 | |
Administration expenses | | | 3,939 | | | | 5,820 | | | | 7,505 | | | | (1,881 | ) | | | (1,685 | ) |
Loss on foreign exchange | | | 255 | | | | 226 | | | | 654 | | | | 29 | | | | (428 | ) |
Finance costs | | | (192 | ) | | | 1,621 | | | | 1,621 | | | | (1,813 | ) | | | — | |
Gain on extinguishment of liabilities | | | — | | | | (86 | ) | | | — | | | | 86 | | | | (86 | ) |
Change in fair value of financial instruments
measured at fair value through profit or loss | | | (150 | ) | | | (3,250 | ) | | | (850 | ) | | | 3,100 | | | | (2,400 | ) |
Impairment losses | | | — | | | | — | | | | 1,087 | | | | — | | | | (1,087 | ) |
Net loss from continuing operations
before income taxes | | $ | (7,073 | ) | | $ | (8,632 | ) | | $ | (12,686 | ) | | $ | 1,559 | | | $ | 4,054 | |
| | | | | | | | | | | | | | | |
Current income tax | | | (811 | ) | | | — | | | | — | | | | (811 | ) | | | — | |
Deferred income tax | | | 286 | | | | 118 | | | | (65 | ) | | | 168 | | | | 183 | |
Income tax expense (recovery) on continuing
operations | | | (525 | ) | | | 118 | | | | (65 | ) | | | (643 | ) | | | 183 | |
Net loss from continuing operations | | $ | (6,548 | ) | | $ | (8,750 | ) | | $ | (12,621 | ) | | $ | 2,202 | | | $ | 3,871 | |
| | | | | | | | | | | | | | | |
Discontinued operations | | | | | | | | | | | | | | | |
Gain (loss) on sale of discontinued operations,
net of income taxes of $nil | | $ | — | | | $ | (134 | ) | | | 3,380 | | | $ | 134 | | | $ | (3,514 | ) |
Income (loss) from discontinued operations,
net of income taxes of $nil | | | 2,304 | | | | (435 | ) | | | (30,750 | ) | | | 2,739 | | | | 30,315 | |
Total income (loss) from discontinued
operations | | $ | 2,304 | | | $ | (569 | ) | | | (27,370 | ) | | $ | 2,873 | | | $ | 26,801 | |
Net loss | | $ | (4,244 | ) | | $ | (9,319 | ) | | $ | (39,991 | ) | | $ | 5,075 | | | $ | 30,672 | |
| | | | | | | | | | | | | | | |
Net (loss) income attributable to: | | | | | | | | | | | | | | | |
Non-controlling interests - continuing operations | | | 635 | | | | (75 | ) | | | (268 | ) | | | 710 | | | | 193 | |
Owners of the parent | | | | | | | | | | | | | | | |
- Continuing operations | | | (7,183 | ) | | | (8,675 | ) | | | (12,353 | ) | | | 1,492 | | | | 3,678 | |
- Discontinued operations | | | 2,304 | | | | (569 | ) | | | (27,370 | ) | | | 2,873 | | | | 26,801 | |
| | | (4,879 | ) | | | (9,244 | ) | | | (39,723 | ) | | | 4,365 | | | | 30,479 | |
Net loss | | $ | (4,244 | ) | | $ | (9,319 | ) | | $ | (39,991 | ) | | $ | 5,075 | | | $ | 30,672 | |
| | | | | | | | | | | | | | | |
Income (loss) per share attributable to
the owners of the parent
basic and diluted: | | | | | | | | | | | | | | | |
From continuing operations | | $ | (2.31 | ) | | $ | (2.79 | ) | | $ | (4.52 | ) | | $ | 0.48 | | | $ | 1.73 | |
From discontinued operations | | | 0.74 | | | | (0.19 | ) | | | (10.01 | ) | | | 0.93 | | | | 9.82 | |
Total loss per share | | $ | (1.57 | ) | | $ | (2.98 | ) | | $ | (14.53 | ) | | $ | 1.41 | | | $ | 11.55 | |
Weighted average number of outstanding shares
(in thousands) | | | 3,104 | | | | 3,104 | | | | 2,733 | | | | — | | | | 371 | |
Revenues from continuing operations
The following table provides the breakdown of total revenues from continuing operations by source for the quarter ended December 31, 2022 compared to the corresponding periods in 2021 and 2020:
| | | | | | | | | | | | | | | | | | | | |
| | Quarter ended December 31, | | | Change | |
| | 2022 | | | 2021 | | | 2020 | | | 2022 vs 2021 | | | 2021 vs 2020 | |
Royalty revenues | | $ | 241 | | | $ | 229 | | | $ | 241 | | | $ | 12 | | | $ | (12 | ) |
Rental revenue | | | — | | | | 9 | | | | 43 | | | | (9 | ) | | | (34 | ) |
| | $ | 241 | | | $ | 238 | | | $ | 284 | | | $ | 3 | | | $ | (46 | ) |
88
Research and development expenses
The decrease of $1.1 million in R&D expenses during the quarter ended December 31, 2022 compared to the corresponding period in 2021 was mainly attributable to decreases in clinical trial costs, intangible assets depreciation expense and professional fees of $0.5 million, $0.4 million and $0.2 million, respectively, and a general reduction in operating expenses. These decreases were partially offset by an increase in pre-clinical trial costs of $0.4 million following the beginning of new pre-clinical studies that took place in 2022.
R&D expenses increased by $1.6 million during the quarter ended December 31, 2021 compared to the corresponding period in 2020 mainly due to increases in share-based payments expense, payroll and related expenses and intangible amortization expenses of $0.4 million, $0.9 million and $0.6 million, respectively, and a decrease in the CEWS and CERS government grants and R&D tax credits of $0.4 million each. These increases were partially offset by a decrease in laboratory consumables expense of $1.0 million.
Administration expenses
The decrease of $1.9 million in administration expenses during the quarter ended December 31, 2022 compared to the corresponding period in 2021 was mainly attributable to a decrease of $1.5 million in expense as a result of reduced directors’ and officers’ insurance premiums resulting from the change in the Company’s registered office from Québec to Ontario in the later part of 2021, a reduction in professional fees of $0.5 million and a reduction in share-based payments expense of $0.3 million. These decreases were partially offset by an increase in salaries and other benefits of $0.7 million.
The decrease of $1.7 million in administration expenses during the quarter ended December 31, 2021 compared to the corresponding period in 2020 was mainly attributable to lower directors' and officers' insurance cost of $1.0 million, as mentioned above. The decrease is also due to the fact that in 2020 there was a $2.2 million expense pertaining to the additional warrants issued following an amendment to the private placement agreement completed in November of that year with no equivalent cost in 2021. These decreases were partially offset by an increase in the share-based payments expense of $1.3 million (explained below) and a reduction in the CEWS and CERS government grants of $0.5 million.
Share-based payments expense
Share-based payments expense represents the expense recorded as a result of stock options and RSU issued to employees and board members. This expense has been recorded as follows in the consolidated statements of operations:
| | | | | | | | | | | | | | | | | | | | |
| | Quarter ended December 31, | | | Change | |
| | 2022 | | | 2021 | | | 2020 | | | 2022 vs 2021 | | | 2021 vs 2020 | |
Research and development expenses | | $ | 132 | | | $ | 209 | | | $ | (232 | ) | | $ | (77 | ) | | $ | 441 | |
Administration expenses | | | 163 | | | | 415 | | | | (903 | ) | | | (252 | ) | | | 1,318 | |
Loss from discontinued operations | | | — | | | | — | | | | 173 | | | | — | | | | (173 | ) |
| | $ | 295 | | | $ | 624 | | | $ | (962 | ) | | $ | (329 | ) | | $ | 1,586 | |
89
The decrease in share-based payments expense of $0.3 million during the quarter ended December 31, 2022 compared to the corresponding period in 2021 was mainly due to a reduction in stock option expense as a result of the reduction in senior executives in the stock option plan from 2021 to 2022 and because the fair value of stock option grants has decreased in the recent years given our lower share price.
Share-based payments expense increased by $1.6 million during the quarter ended December 31, 2021 compared to the corresponding period in 2020 principally because there were no significant stock option forfeitures in the current period as there was in 2020 when recognized the impact of the estimated forfeitures of the unvested stock options held by the former CEO following his resignation in the fourth quarter of 2020. This was partially offset by a lower stock option expense in the current quarter since we have less employees but also the fair value of the stock options have gone down reflecting the lower share price.
Finance costs
Finance costs decreased by $1.8 million for the quarter ended December 31, 2022 compared to the corresponding period in 2021, reflecting lower interest expense on long-term debt because our SALP loans were only outstanding until February 15, 2022 compared to the prior period where they were outstanding for the entire period and because we also incurred interest on our convertible debt in 2021, until its conversion in August 2021.
Financing costs remained at the same level during the fourth quarter of 2021 and 2020.
Change in fair value of financial instruments measured at fair value through profit or loss
We recorded gains on the variation in fair value of the warrant liability that is measured at FVPL during the quarters ended December 31, 2022, 2021 and 2020. The main driver for these gains was the decrease in the value of the underlying shares over those periods.
Net loss from continuing operations
The net loss from continuing operations decreased by $2.2 million during the quarter ended December 31, 2022 compared to the corresponding period in 2021. This was mainly driven by the reductions in administration expenses of $1.9 million, reflecting the reduction in insurance expense, a decrease in finance costs of $1.8 million due to the termination of all long-term debt and a decrease in R&D expenses of $1.1 million as explained above. These decreases were partially offset by a decrease in the gain on change in fair value of financial instruments measured at fair value through profit or loss of $3.1 million.
The net loss from continuing operations decreased by $3.9 million during the quarter ended December 31, 2021 compared to the corresponding period in 2020. This was mainly driven by the reduction in administration expenses of $1.7 million and the increase in the gain on the change in fair value of the warrant liability that is measured at FVPL losses of $2.4 million. These decreases were partially offset by an increase in R&D expenses of $1.6 million as explained above.
90
Income (loss) from discontinued operations
We had an income from discontinued operations, net of income taxes, of $2.3 million during the quarter ended December 31, 2022 compared to a loss of $0.4 million for the corresponding period in 2021. This was mainly due to the recognition of a gain of $2.3 million on disposal of capital assets, following the sale of our Labrosse facility in December 2022, formerly part of the plasma-derived therapeutics segment and previously classified as property, plant and equipment, met the criteria to be classified as held for sale during the quarter ended March 31, 2022 at a carrying amount of $0.8 million. We received $3.2 million in net proceeds from this sale.
The net losses from discontinued operations, net of taxes, decreased by $30.3 million during the quarter ended December 31, 2021 compared to the corresponding period in 2020. This decrease was mainly due to the recognition of an impairment of $19.7 million during the fourth quarter of 2020 and due to the fact that we only had a small loss from discontinued operations of $0.4 million since there were only minor expenses incurred from October 1 to October 15, 2021 when PBT was sold compared to having the full operations of the plasma-derived therapeutics segment during the quarter ended December 31, 2020. The gain on sale of subsidiaries declined by $3.5 million during the quarter ended December 31, 2021 compared to the corresponding period in 2020 as an additional gain of $3.4 million was recognized upon the resolution of a tax uncertainty in relation to the sale of our bioseparation business in 2020.
Selected annual information
The following table presents selected audited annual information for the years ended December 31, 2022, 2021 and 2020.
| | | | | | | | | | | | |
| | 2022 | | | 2021 | | | 2020 | |
Revenues | | $ | 401 | | | | 643 | | | | 724 | |
Net loss from continuing operations attributable to
owners of the parent | | | (29,038 | ) | | | (44,394 | ) | | | (48,189 | ) |
Net loss from continuing operations per share
attributable to owners of the parent
(basic and diluted) | | | (9.36 | ) | | | (14.72 | ) | | | (19.72 | ) |
Total assets | | | 50,459 | | | | 126,053 | | | | 117,784 | |
Total long-term financial liabilities | | $ | 4,148 | | | $ | 73,678 | | | $ | 78,785 | |
Revenues include nominal amounts of royalty and rental revenues. Royalty revenues are dependent on sales made by a third party. There were no rental revenues during the year ended December 31, 2022.
The net loss from continuing operations attributable to the owners of the parent decreased by $15.6 million during the year ended December 31, 2022 compared to the corresponding period of 2021 mainly driven by the reductions in administration expenses of $14.1 million, reflecting the reduction in insurance expense, a decrease in finance costs of $5.3 million due to the termination of all long-term debt and an increase in foreign exchange gains of $1.6 million. These decreases were partially offset by a lower gain resulting in change in fair value of financial instruments measured at fair value through profit and loss of $8.2 million.
The net loss from continuing operations attributable to the owners of the parent decreased by $3.8 million during the year ended December 31, 2021 compared to the corresponding period of 2020 mainly due to a reduction in the fair value of the warrant liability, presented in the profit and loss as a change in fair value of financial instruments measured at fair value through profit and loss of $9.0 million and a favorable foreign exchange variance of $1.4 million. These were partially offset by an increase in R&D and finance costs of $4.1 million and $3.4 million, respectively, as explained above.
91
The net loss from continuing operations per share attributable to the owners of the parent on a basic and diluted basis reflects the changes in the net loss from continuing operations attributable to the owners of the parent but also the increase in the number of common shares outstanding from year to year. The number of common shares increased in 2020 following a private placement in November 2020 and in 2021 and following the conversion of secured convertible debentures into shares in October 2021. The weighted average number of shares increased from 2,444 thousand common shares in 2020 to 3,016 thousand common shares in 2021 and to 3,104 thousand common shares in 2022.
Total assets decreased by $75.6 million from $126.1 million at December 31, 2021 to $50.5 million at December 31, 2022 reflecting the repayment of our long-term debt for an aggregate amount of $39.1 million and the payment of $11.2 million made towards the termination of the CDMO agreement as explained above.
Total assets increased by $8.3 million from $117.8 million at December 31, 2020 to $126.1 million at December 31, 2021 reflecting the increase in cash of $63.4 million as a result of the proceeds we received from the sale of our plasma-derived therapeutic business which was mostly offset by the decrease in all of the assets sold.
Long-term financial liabilities decreased by $69.5 million from $73.7 million at December 31, 2021 to $4.2 million at December 31, 2022. This decrease was mainly due to the repayment of our long-term debt for an aggregate amount of $39.1 million and the termination of the CDMO agreement as explained above.
Long-term financial liabilities decreased by $5.1 million from $78.8 million at December 31, 2020 to $73.7 million at December 31, 2021. This decrease was mainly due to the liabilities disposed of with the sale of the plasma-derived therapeutic business of $5.9 million, the decrease of the warrant liability of $9.9 million and the conversion of the secured convertible debt, in 2021, that had a balance of $2.5 million at December 31, 2020. The decreases were mostly offset by the recognition of a provision for onerous contract of $18.2 million.
Summary of consolidated quarterly results
The following table presents selected quarterly financial information for the last eight quarters:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2022 | | | 2021 | |
| | Q4 | | | Q3 (restated) | | | Q2 (restated) | | | Q1 (restated) | | | Q4 | | | Q3 | | | Q2 | | | Q1 | |
Revenues | | $ | 241 | | | $ | 3 | | | $ | 157 | | | $ | — | | | $ | 238 | | | $ | 170 | | | $ | 25 | | | $ | 210 | |
R&D expenses | | | 3,462 | | | | 3,519 | | | | 3,942 | | | | 4,375 | | | | 4,539 | | | | 4,973 | | | | 3,951 | | | | 4,884 | |
Administration expenses | | | 3,939 | | | | 4,386 | | | | 4,647 | | | | 4,894 | | | | 5,820 | | | | 9,420 | | | | 8,551 | | | | 8,137 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Element attributable to
the owners of
the parent: | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss from continuing
operations | | | (7,183 | ) | | | (4,785 | ) | | | (6,782 | ) | | | (10,924 | ) | | | (8,675 | ) | | | (9,797 | ) | | | (12,504 | ) | | | (13,418 | ) |
Net income (loss) from
discontinued operations | | | 2,304 | | | | 26,749 | | | | 266 | | | | 219 | | | | (569 | ) | | | 84,228 | | | | (19,536 | ) | | | (6,847 | ) |
Basic and diluted earnings
per share from
continuing operations | | | (2.31 | ) | | | (0.15 | ) | | | (0.22 | ) | | | (0.35 | ) | | | (0.29 | ) | | | (0.33 | ) | | | (0.42 | ) | | | (0.45 | ) |
Basic and diluted earnings
per share from
discontinuing operations | | | 0.74 | | | | 0.86 | | | | 0.01 | | | | 0.01 | | | | (0.02 | ) | | | 2.82 | | | | (0.65 | ) | | | (0.23 | ) |
92
Restatement of the first, second and third quarters of 2022 financial statements for the quarter ended March 31, 2022, the quarter and six months ended June 30, 2022 and the quarter and nine months ended September 30, 2022
During the preparation of our consolidated financial statements for the year ended December 31, 2022, we noted that in our interim financial statements for the periods ended March 31, 2022, June 30, 2022 and September 30, 2022, we incorrectly allocated net income to non-controlling shareholders. As a result, net loss attributable to the non-controlling shareholders was overstated with offsetting misclassifications between non-controlling interest and shareholders' deficit within equity. The error did not result in an adjustment to previously reported net income or loss per share in any prior fiscal year. We have proceeded to restate our interim financial statements for the periods ended March 31, 2022, June 30, 2022 and September 30, 2022:
•by recording a reduction in the net loss attributable to the NCI and increasing the net loss attributable to the owner of the parent for an amount of $0.1 million in the first quarter interim financial statements for the period ended March 31, 2022;
•by recording a reduction in the net loss attributable to the NCI and increasing the net loss attributable to the owner of the parent for an amount of $0.6 million in the second quarter interim financial statements for the period ended June 30, 2022; and
•by increasing the net loss attributable to the NCI and recording a reduction in the net loss attributable to the owner of the parent for an amount of $0.1 million in the third quarter interim financial statements for the period ended September 30, 2022.
Analysis of the quarterly results
Following the reclassification of the results of the plasma collection centers and the Ryplazim® business as discontinued operations, the revenues include nominal amounts of royalty and rental revenues.
R&D expenses were higher starting from the first quarter of 2021 up until the first quarter of 2022. This was mainly due to a general increase in clinical trial expenses as we were conducting our Phase 1 MAD clinical trial of fezagepras. This was partially offset due to the fact that we were benefiting from the CEWS and CERS government grants from the second quarter of 2020 up until the middle of the second quarter of 2021. In general, payroll and related expenses recorded in R&D expenses declined from the fourth quarter of 2020 to the fourth quarter of 2021 due to a reduction in the number of employees.
Administration expenses where higher during the third quarter of 2021 due to an acceleration of the share-based payments expense following the departure of two of our executive members, and the higher payroll and related expenses due to recognition of termination benefits resulting from a reduction in employees and a transaction bonus following the divestment of our former plasma-derived therapeutics segment. The administration expense for the fourth quarter of 2021 is lower compared to the previous quarter reflecting the lower staff level and lower directors' and officers' insurance expense by $1.3 million, as a result of a decrease in insurance premiums following a change in the province of our registered office, which changed from Québec to Ontario, during the fourth quarter of 2021. The full impact of the decrease in premiums is $2.5 million per quarter. Recent cost cutting exercises have also resulted in reduced administration expenses throughout fiscal 2022.
Both R&D and administration expenses are affected by fluctuations in share-based payment expenses from quarter to quarter.
93
The variations in the net loss from continuing operations attributable to the owners of the parent over the last eight quarters were affected by R&D and administration expense variations as explained above. In addition, the following quarters were notably impacted by gains on the changes in fair value of the warrant liability that is measured at FVPL, which reduced the net loss from continuing operations each quarter but notably by $5.1 million and $3.3 million during the third quarter of 2021 and the fourth quarter of 2021, respectively.
The variations in the foreign exchange gains and losses for the quarters presented in the above table, caused the net loss from continuing operations to fluctuate up or down by up to $2.5 million per quarter.
Following the repayment of our long-term debt of $39.1 million in the middle of the first quarter of 2022, the related finance costs decreased by approximately $1.1 million per quarter.
Net losses from discontinued operations attributable to the owners of the parent fluctuated significantly in 2021 and 2022 in part due to the results of operations of the plasma-derived therapeutics segments and the gains on disposal of its former subsidiaries. The variations are in part due to the varying R&D and administration expenses but the main variations are due to significant events impacting the results, including the recognition of 1) an expense for an onerous contract provision of $21.9 million during the second quarter of 2021, 2) a compensation expense for R&D services of $45.8 million that became payable during the third quarter of 2021 upon receipt of the PRV proceeds, 3) gains on the sale of the PRV during the third quarter of 2021 for $131.0 million (net of selling cost of $1.9 million) and from the sale of the former plasma-derived therapeutics entities that happened in during the second, third and fourth quarters of 2021, 4) gains on a lease modification and from the partial reversal of a provision, totaling $26.7 million, resulting from the termination of the CDMO agreement in the third quarter of 2022 and, 5) gains on disposition of capital assets from discontinued operations of $2.3 million resulting from the sale of the Labrosse facility in the fourth quarter of 2022. The net loss from discontinued operations during the first two quarters of 2022 comes from adjustments to residual liabilities of these former businesses.
The basic and diluted loss per share from continuing operations declined over the last eight quarters, particularly during the third quarter and the fourth quarter of 2021 principally reflecting the lower losses from continuing operations while the basic and diluted loss per share from discontinued operations varied in accordance principally with the loss from discontinued operations for each period. In addition, during the fourth quarter of 2021, we issued shares which ultimately reduce the basic and diluted loss per share from their date of issuance and for the following quarters because they increase the weighted average number of shares.
Acquisition of Fairhaven Pharmaceuticals Inc.
Pursuant to a share purchase agreement, or SPA, dated July 17, 2020, we acquired 100% of the issued and outstanding common shares of Fairhaven, a company with a preclinical research program of small molecule antagonists. In payment of the initial amount of $3.6 million due upon closing of the acquisition, we issued 202,308 common shares recorded at a fair value of $3.4 million based on the closing price of our common shares at the date of the transaction. Upon achievement of certain pre-determined research and development milestones prior to the fifth anniversary of the closing date of the acquisition, additional payments in the form of common shares totaling up to $4.4 million may become due.
As Fairhaven did not meet the definition of a business under IFRS 3, "Business Combinations," the acquisition has been accounted for as an asset acquisition essentially resulting in the recognition of an intangible asset representing the licensing rights acquired. Refer to note 5 to the consolidated financial statements for the year ended December 31, 2020 for the complete details regarding the accounting for this transaction.
94
Outstanding share data
We are authorized to issue an unlimited number of common shares. At March 7, 2023, 3,104,222 common shares, 230,273 options to purchase common shares and 789,472 warrants to purchase common shares were issued and outstanding.
Transactions between related parties (as defined per IAS 24)
Balances and transactions between our subsidiaries, which are related parties, have been eliminated on consolidation and are not reported. These transactions have been recorded at the exchange amount, meaning the amount agreed to between the parties.
At December 31, 2022 and 2021, a former CEO had a balance of $197 and $283, respectively, pursuant to a tax equalization program. The amounts are required to be repaid to us following the receipt of a refund by the former employee from the taxation authority for each of the two years covered by the program. At December 31, 2022, we received the reimbursement of the first year. The remaining amount is expected to be received once the tax return of the second and final year of the program has been assessed by the appropriate government agencies.
SALP became our majority shareholder, or our parent entity, following a debt restructuring completed on April 23, 2019.
All material transactions with SALP are disclosed in notes 15 and 16 in the consolidated financial statements for the year ended December 31, 2022. The key transactions with our parent entity mainly pertain to financing transactions and are for significant amounts. Related party transactions with SALP include:
•the recording and payment of interest on the loans with SALP with cash;
•the reimbursement of the loans;
•the payment of a fixed quarterly royalty;
•the issuance of common shares, with warrants in exchange for cash; and
•the reimbursement of professional fee expenses.
In addition to the above, we revalue our warrant liability, pertaining to warrants that are partly held by SALP, at each reporting period, which results in variations of the liability on the consolidated statement of financial position and in the consolidated statement of operations.
95
Changes in accounting policies
The accounting policies used in our annual consolidated financial statements are consistent with those we applied in our December 31, 2021 and 2020 audited annual consolidated financial statements except for the amendments to certain accounting standards which were adopted since January 1, 2021 as described below.
Amendments to IFRS 3, Business Combinations or IFRS 3 - The amendments to IFRS 3 clarify the definition of a business and includes an optional concentration test to determine whether an acquired set of activities and assets is a business. These amendments were adopted on January 1, 2020 and are applied prospectively to acquisitions made on or after this date.
Amendment to IFRS 16, Leases or IFRS 16 for COVID-19-Related Rent Concessions - IFRS 16 has been revised to incorporate an amendment issued by the IASB in May 2020. The amendment permits lessees not to assess whether particular COVID-19-related rent concessions are lease modifications and, instead, account for those rent concessions as if they were not lease modifications. In addition, the amendment to IFRS 16 provides specific disclosure requirements regarding COVID-19-related rent concessions. The amendment was adopted by us as of January 1, 2021 and had no impact on the financial statements for the year ended December 31, 2021 since we have not benefited from COVID-19 related rent concessions.
Amendment to IAS 1, Presentation of Financial statements or IAS 1 - IAS 1 has been revised to require the disclosure of material accounting policies rather than significant accounting policies and provides guidance to apply materiality judgments to accounting policy disclosure. We early adopted these amendments, and consequential amendments to other standards, for our annual audited financial statements for the year ended December 31, 2021 resulting in a reduction in our accounting policy disclosures.
Amendments to IAS 37, Provisions, Contingent Liabilities and Contingent Assets (IAS 37) - IAS 37 has been revised to specify which costs an entity includes in determining the cost of fulfilling a contract for the purpose of assessing whether the contract is onerous. The amendments are effective for annual reporting periods beginning on or after January 1, 2022. The adoption of these amendments had no impact on the computation of the provision.
Amendment to IFRS 9 Financial Instruments (IFRS 9) - IFRS 9 has been revised to clarify the fees an entity includes when assessing whether the terms of a new or modified financial liability are substantially different from the terms of the original financial liability. The adoption of the amendment had no impact on our financial statements and will be applied to financial liabilities that are modified after the date of adoption.
Amendments to IAS 12, Income taxes or IAS 12 - The amendments to IAS 12 clarify the accounting for deferred tax assets or liabilities arising from a single transaction such as leases, namely that the scope of the recognition exemption no longer applies to transactions that, on initial recognition, give rise to equal taxable and deductible temporary differences. The Company early adopted these amendments for its annual financial statements for the year ended December 31, 2022, which mainly affect the Company’s accounting for deferred tax assets and deferred tax liabilities pertaining to right of use assets and lease liabilities. There will be little to no effect on the amounts reported in the consolidated statements of financial position or the consolidated statements of operations, since only a small portion of the Company’s deferred tax assets are being recognized based on their current potential to be recovered with future tax profits. Note 26 - Income taxes note disclosures will be affected as the deferred tax assets and liabilities pertaining to the leases will no longer be presented on a net basis.
96
New Standards and interpretations not yet adopted
The IFRS accounting standards, amendments, and interpretations that we reasonably expect may have a material impact on our disclosures, financial position or results of operations when applied at a future date are as follows:
Amendments to IAS 8, Accounting policies, Changes in Accounting Estimates and Errors (IAS 8) - The amendments to IAS 8 introduce a definition of accounting estimates and provide clarifications to distinguish accounting policies from accounting estimates. The amendments are applicable retrospectively and are effective for annual reporting periods beginning on or after January 1, 2023 with earlier application permitted. We concluded that these amendments will not have an impact on our financial statements at the date of adoption and for the comparative periods.
IAS 1, Presentation of Financial Statements (IAS 1) - IAS 1 has been revised to clarify how to classify debt and other liabilities as current or non-current. The amendments help to determine whether, in the statement of financial position, debt and other liabilities with an uncertain settlement date should be classified as current (due or potentially due to be settled within one year) or non-current. The amendments also include clarifying the classification requirements for debt an entity might settle by converting it into equity. The amendments are applicable retrospectively and are effective for annual reporting periods beginning on or after January 1, 2023 with earlier application permitted. We concluded that these amendments will not have an impact on our financial statements at the date of adoption and for the comparative periods.
Significant judgments and estimates
Our management’s discussion and analysis of financial condition and results of operations are based on our financial statements, which have been prepared in accordance with IFRS. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities in our financial statements. We base our estimates on historical experience, known trends and events and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. In making estimates and judgments, management employs material accounting policies. See Note 2 to our consolidated financial statements for the year ended December 31, 2022 for a description of our material accounting policies.
We believe that the most significant management judgments and assumptions in the preparation of our consolidated financial statements are described below.
Going concern - In assessing whether the going concern assumption is appropriate and whether there are material uncertainties that may cast significant doubt about our ability to continue as a going concern, management must estimate future cash flows for a period of at least twelve months following the end of the reporting period by considering relevant available information about the future. Management has considered a wide range of factors relating to expected cash inflows such as whether we will earn other significant revenues, what will be the next steps in our research and development programs and the related expenditures as well as the financing strategy we would like to pursue and the potential sources of debt and equity financing available to us in case further financing is desired. Management has also estimated expected cash outflows such as operating and capital expenditures and debt repayment schedules. These cash flow estimates are subject to uncertainty.
97
Functional currency – We review the functional currency of foreign subsidiaries on an ongoing basis to assess if changes in the underlying transactions, events and conditions have resulted in a change. This assessment is also performed for new subsidiaries. When assessing the functional currency of a foreign subsidiary, we apply our judgment in order to determine, amongst other things, the primary economic environment in which an entity operates, the currency in which the activities are funded and the degree of autonomy of the foreign subsidiary from the reporting entity in its operations and financially. Judgment is also applied in determining whether the inter-company loans denominated in foreign currencies form part of our net investment in the foreign subsidiary. Considering such loans as part of the net investment in the foreign subsidiary results in foreign currency translation gains or losses from the translation of these loans being recorded in other comprehensive loss instead of the consolidated statement of operations.
Share-based compensation - On March 23, 2020, our board of directors approved a plan to seek shareholder approval to modify the exercise price of certain stock options as disclosed in note 17b in the consolidated financial statements. In order to determine when the expense related to this modification is recognized in our consolidated statement of operations, we evaluated the timing of notification to option holders, the timing and method of determining the exercise price and the service period. We further considered whether the holders of the stock options had sufficient understanding of the terms and conditions of the potentially revised awards, the degree of certainty of the approval for the repricing and whether the service period for earning the rights to the awards had commenced. We concluded that the definition of the grant date was not met but that the service period had commenced and therefore a preliminary calculation of the incremental fair value of the repricing of the awards was performed using assumptions as of March 31, 2020. On May 26, 2020, the conditions for a grant date were met and the options exercise price was revised to $152.10 and a final calculation to determine the incremental fair value of the repriced options was performed.
COVID-19 – The impact of the COVID-19 pandemic on our financial statements for year ended December 31, 2022 and 2021 has been limited. During a portion of those two years, we were eligible for salary and rent subsidy programs from the Government of Canada under which we submitted claims. As of the date of this 20-F, there are no subsidy programs to which we are eligible.
Fair value of financial instruments – The individual fair values attributed to the different components of a financing transaction, are determined using valuation techniques. We use judgment to select the methods used to determine certain inputs/assumptions used in the models and the models used to perform the fair value calculations in order to determine, 1) the values attributed to each component of a transaction at the time of their issuance, 2) the fair value measurements for certain instruments that require subsequent measurement at fair value on a recurring basis and 3) the fair value of financial instruments subsequently carried at amortized cost. When the determination of the fair value of a new loan is required, discounted cash flow techniques which include inputs that are not based on observable market data and inputs that are derived from observable market data are used. When determining the appropriate discount rates to use, we seek comparable interest rates where available. If unavailable, we use those considered appropriate for the risk profile of a Company in the industry.
In determining the fair value of the warrants issued in November 2020 presented as a warrant liability in the consolidated statements of financial position at December 31, 2022 and 2021, and considered to be a level 3 measurement, we made assumptions on unobservable inputs used in the valuation model that have an important impact on the resulting fair value computed.
98
Notably, we estimated the timing and the amounts of equity financings we expect to complete before the expiry of those warrants. The fair value computed could be higher if our actual equity financing needs are higher than those expected. We also estimated the future volatility of the common shares of Liminal for the contractual life of the warrants. To do so, we used the historical volatility of our shares and of comparable companies in the same industry as a starting basis for this estimate and also considered whether there are factors that would indicate that the historical volatility is not indicative of the future. In addition, we applied an illiquidity discount rate on the resulting Black-Scholes pricing model to reflect that the November 2020 warrants are not publicly traded instruments and therefore the ability to sell them is limited. In establishing the illiquidity discount rate, we considered the remaining life of the warrants and the volatility assumption for the underlying shares. The fair value of the warrants could be higher if we had selected a higher volatility assumption and/or a lower illiquidity discount rate.
The fair value estimates could be significantly different because of the use of judgment and the inherent uncertainty in estimating the fair value of these instruments that are not quoted in an active market.
Uncertainty over income tax treatments - We measure R&D tax credits for the current and prior periods at the amount we expect to recover, based on our best estimate and judgment, of the amounts we expect to receive from the tax authorities as at the reporting date, either in the form of income tax refunds or refundable grants. However, there are uncertainties as to the interpretation of the tax legislation and regulations, in particular regarding what constitutes eligible R&D activities and expenditures, as well the amount and timing of recovery of these tax credits. In order to determine whether the expenses we incur are eligible for R&D tax credits, we must use judgment in determining whether our complex R&D activities qualify for available tax credits, which makes the recovery of tax credits uncertain. As a result, there may be a significant difference between the estimated timing and amount recognized in the consolidated financial statements in respect of tax credits receivable and the actual amount of tax credits received as a result of the tax administrations' reviews of matters that were subject to interpretation. These uncertainties, relating to entities we have sold may still affect Liminal as certain indemnification obligations may be called upon, subject to contractual limitations, when these entities may be subjected to the tax administrations' reviews for taxation periods prior to the sale. The amounts recognized in the consolidated financial statements are based on our best estimates and in our best possible judgment, as noted above.
Assessing the recoverable amount of long-lived assets - We evaluate the recoverable value of long-lived assets when indicators of impairment arise or as part of the annual impairment test, if they are intangible assets not yet available for use. The recoverable value is the higher of the value in use and the fair value less costs of disposal, or FVLCD.
Long-lived assets include capital assets, ROU assets and intangible assets such as patents and licenses and other rights. Some of these rights are considered not available for use until regulatory approval to commercialize the product candidate is obtained.
When calculating the net recoverable amounts for the impairments, we make estimates and assumptions regarding the outcome of certain future events, future cash flows and their timing.
99
When determining the FVLCD for its Ryplazim® CGU in 2020, significant estimates we made include amongst others, the outcome of the exercise we had undertaken in evaluating the potential alternatives for the Ryplazim® CGU, including the probability of completing a sale or closing those activities; the operating cash outflows to support those operations until one of the alternative strategies was executed; the outcome of the FDA review of the BLA for our previously-owned Ryplazim® product candidate and the timing of completion of this review; if we were to be able to benefit from the monetization of a Priority Review Voucher, if received, and what would be the amount received upon its monetization; and whether some assets, liabilities and commitments could potentially be excluded from the activities sold and for those commitments that could be retained, the possibility of reducing those commitments and what would be their settlement amount.
A plus or minus 10% change in the probability weighted terminal value would have impacted the impairment we recorded on the Ryplazim® CGU by $3,638.
In addition, when calculating the FVLCD of an asset or a group of assets for which selling price information for comparable assets are not readily available, we also must make assumptions regarding the value it may recuperate from its sale.
Share-based compensation – To determine the fair value of stock options on a given date, we must determine the assumptions that will be used as inputs to the Black-Scholes option pricing model, including the assumption regarding the future volatility of our common shares for the expected life of the stock options. We use the historical volatility as a starting basis for the estimate and also consider whether there are factors that would indicate that the past volatility is not indicative of the future volatility. In making this assessment, we consider changes in our activities and other factors such as a significant share consolidation. As the volatility is an assumption that has a significant impact on the calculated value of a stock option, the impact of this estimate can significantly impact the share-based payment expense over the vesting period of an award.
Valuation of deferred income tax assets – To determine the extent to which deferred income tax assets can be recognized, we estimate the amount of probable future taxable profits that will be available against which deductible temporary differences and unused tax losses can be utilized. We exercise judgment to determine the extent to which realization of future taxable benefits is probable, considering the history of taxable profits, budgets and forecasts and availability of tax strategies.
Estimates and assumptions about future events and their effects cannot be determined with certainty and therefore require the exercise of judgment. As of the date of issuance of this Annual Report, we are unaware of any specific event or circumstance that would require us to update our estimates, assumptions and judgments or revise the carrying value of our assets or liabilities, and we are unable to estimate the potential impact on our future business or our financial results as of the date of this filing. These estimates may change as new events occur and additional information is obtained and changes in those estimates are recognized in the consolidated financial statements as soon as they become known.
100
Financial instruments
Use of financial instruments
The financial instruments that we use result from our operating and investing activities, namely in the form of accounts receivables and payables, and from our financing activities resulting usually in the issuance of long‑term debt. We do not use financial instruments for trading purposes and have not issued or acquired derivative financial instruments for hedging purposes. The following table presents the carrying amounts of our financial instruments at December 31, 2022 and 2021.
| | | | | | | | | | |
| | | | December 31 | | | December 31 | |
| | | | 2022 | | | 2021 | |
Financial assets | | | | | | | | |
Cash & cash equivalents | | | | $ | 37,144 | | | $ | 108,490 | |
Accounts receivable | | | | | 597 | | | | 788 | |
Long-term deposits | | | | | 30 | | | | 30 | |
Financial liabilities | | | | | | | | |
Accounts payable and accrued liabilities | | | | $ | 5,054 | | | $ | 7,059 | |
Royalty payment obligations | | | | | — | | | | 123 | |
Provisions | | | | | 6,690 | | | | 22,195 | |
Warrant liability | | | | | 106 | | | | 1,754 | |
Long-term debt | | | | | — | | | | 38,311 | |
Impact of financial instruments in the consolidated statements of operations
The following line items in the consolidated statement of operations for the quarter and the year ended December 31, 2022 include income, expense, gains and losses relating to financial instruments:
•change in fair value of financial instruments measured at fair value through profit or loss;
JOBS Act Exemptions and Foreign Private Issuer Status
We qualify as an “emerging growth company” as defined in the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. This includes an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act. We may take advantage of this exemption for up to five years or such earlier time that we are no longer an emerging growth company. We will cease to be an emerging growth company if we have more than $1.235 billion in total annual gross revenue, have more than $700.0 million in market value of our common shares held by non-affiliates or issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some but not all of these reduced burdens.
We will not take advantage of the extended transition period provided under Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Since IFRS makes no distinction between public and private companies for purposes of compliance with new or revised accounting standards, the requirements for our compliance as a private company and as a public company are the same.
101
Additionally, we report under the Exchange Act as a non-U.S. company with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
•the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act;
•the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time;
•the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events; and
•Regulation FD, which regulates selective disclosures of material information by issuers.
B. Liquidity and Capital Resources
Overview
Since completing the divestment of our former plasma collection centers and Ryplazim® business, the repayment of the long-term debt (discussed below), the termination of the CDMO agreement which has given us clarity on the remaining payments (discussed below) and the sale of our former Labrosse facility (discussed below), our funding needs for our operations are solely focused on our small molecules business.
In February 2022, we repaid the entirety of the first and second term loan, for an aggregate amount of $39.1 million, thus terminating the consolidated loan agreement with SALP and releasing the security interests granted over our assets pursuant to the loan agreement and related documents. The repayment also terminated the royalty stream agreement with SALP resulting in the derecognition of the royalty payment obligation of $0.1 million due to SALP and the cancellation of the 16,873 warrants held by SALP, issued pursuant to the restructuring agreement entered into with SALP in April 2019, having an exercise price of $152.10 per common share. The repayment, despite not being due for another two years, saved us $9.1 million in aggregate interest payments over the remaining term of the loan agreement.
In August 2022, we terminated an agreement we had retained from our involvement in the Ryplazim® business towards a CDMO as previously mentioned. A portion of the CDMO obligations were accounted for as a provision, and payments toward the provision affect the computation of the cash flows used in operating activities, while the remainder of the obligations were accounted for as a lease liability, and payments thereto impact the cash flows from financing activities. The termination agreement resulted in a significant reduction in the disbursements required under the contract. An initial payment of $11.2 million was made at the time of the execution of the agreement. We paid $3.4 million in January 2023 and the remaining $3.4 million will be payable in January 2024. The agreement contains customary releases and resulted in go-forward cash savings of approximately $33.1 million.
In December 2022, we sold the Labrosse facility, formerly part of the plasma-derived therapeutics segment resulting in a recognition of a gain of $2.3 million on disposition of capital assets from discontinued operations.
102
In regard to our small molecule research and development activities, we expect our ongoing funding requirements to increase over time as we continue the research and development of our portfolio of compounds and continue or initiate potential clinical trials. Furthermore, we expect to continue to incur costs associated with operating as a public company.
Accordingly, until we can generate sufficient and recurring revenues to finance future cash requirements, it is likely that we will need to secure additional external financing which may include public or private equity offerings, debt financings, strategic collaborations, alliances and licensing arrangements, grant funding or other sources. Despite our efforts to obtain the necessary funding and further reduce the costs of our operations, there can be no assurance of our access to further funding on acceptable terms, if at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, shareholder ownership interest may be diluted, and the terms of any additional securities may include liquidation or other preferences that adversely affect the rights of shareholders.
Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends.
If we raise funds through additional collaborations, strategic alliances, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates, or to grant licenses on terms that may not be favorable to us. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our R&D programs, clinical trials or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Liquidity position at December 31, 2022 and analysis of going concern
For the year ended December 31, 2022, we incurred a net loss from continuing operations of $28.9 million. Our working capital position, which comprised $37.1 million of cash and cash equivalents, was $31.2 million, at December 31, 2022. The decrease in our liquidity since December 31, 2021 is principally due to the repayment of the long-term debt of $39.1 million, the $11.2 million paid to the CDMO and the funds used in our ongoing operations.
With the discontinuation of the development of our small molecule product candidate, fezagepras, in July 2022, our main activities relate to the development of small molecule product candidates. Our cash runway is dependent on the research programs currently underway, the pace of their progression and their outcome, as well as those planned to be undertaken in the short term. As such, there is always a degree of uncertainty in regard to the outcome or cost of those programs. The cash runway is also dependent on decisions we make in terms of managing our capital, including raising capital through the issuance of debt or equity, and our ability to conclude such financing transactions at an acceptable cost. The need to complete financing transactions in the future is likely to continue until we can generate sufficient product revenues to finance our cash requirements. Management may revert to a variety of sources for financing future cash needs including public or private equity offerings, debt financings, strategic collaborations, alliances and licensing arrangements, grant funding, selling non-core assets or other sources.
Despite our efforts to obtain the necessary funding and improve profitability of its operations, there can be no assurance of its success in doing so, especially with respect to its access to further funding on acceptable terms, if at all.
We continue to diligently manage our spending while we focus our R&D efforts on the development of our small molecule product candidates and drug discovery programs.
103
As of December 31, 2022, we had approximately $37.1 million of cash and cash equivalents. As of the date of this Annual Report, our available cash is not projected to be sufficient to support our operating plan for at least the next 12 months. These circumstances indicate the existence of a material uncertainty that may cast substantial doubt about our ability to continue as a going concern. If we are unable to secure additional capital, it may be required to curtail our research and development initiatives and take additional measures to reduce costs in order to conserve our cash in amounts sufficient to sustain operations and meet our obligations. These measures could cause significant delays in our preclinical, clinical and regulatory efforts, which are critical to the realization of our business plan. See “Item 3.D—Risk Factors” in our Annual Report.
The audited consolidated financial statements as of December 31, 2022 do not include any adjustments to the amounts and classification of assets and liabilities that might be necessary should we be unable to continue as a going concern. Such adjustments could be material.
Material cash requirements
The timing and expected contractual outflows required to settle our financial obligations recognized in the consolidated statement of financial position at December 31, 2022 and unrecognized purchase obligations and commitments are presented in the table below:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Contractual Cash flows | |
| | Carrying
amount | | | Less than
1 year | | | 1 - 3
years | | | 3 - 5
years | | | More than
5 years | | | Total | |
Accounts payable and
accrued liabilities | | $ | 5,968 | | | $ | 5,968 | | | $ | — | | | $ | — | | | $ | — | | | $ | 5,968 | |
Lease liabilities | | | 1,487 | | | | 790 | | | | 1,022 | | | | — | | | | — | | | | 1,812 | |
Provision | | | 6,690 | | | | 3,400 | | | | 3,400 | | | | — | | | | — | | | | 6,800 | |
| | $ | 14,145 | | | $ | 10,158 | | | $ | 4,422 | | | $ | — | | | $ | — | | | $ | 14,580 | |
104
Cash flow analysis
The following major cash flow components are presented on a total company basis, inclusive of continuing and discontinued operations.
The summarized consolidated statements of cash flows for continuing and discontinued operations in aggregate, for the year ended December 31, 2022 and the corresponding periods in 2021 and 2020 are presented below.
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31 | |
| | 2022 | | | 2021 | | | 2020 | | | 2022 vs 2021 | | | 2021 vs 2020 | |
Cash flows used in operating activities | | $ | (31,820 | ) | | $ | (99,603 | ) | | $ | (75,917 | ) | | $ | 67,783 | | | $ | (23,686 | ) |
Cash flows (used in) from financing activities | | | (45,446 | ) | | | (8,424 | ) | | | 57,405 | | | | (37,022 | ) | | | (65,829 | ) |
Cash flows from investing activities | | | 3,819 | | | | 170,692 | | | | 2,305 | | | | (166,873 | ) | | | 168,387 | |
Net change in cash and cash equivalents
during the year | | | (73,447 | ) | | | 62,665 | | | | (16,207 | ) | | | (136,112 | ) | | | 78,872 | |
Net effect of currency exchange rate on
cash and cash equivalents | | | 2,101 | | | | 750 | | | | (3 | ) | | | 1,351 | | | | 753 | |
Cash and cash equivalents, beginning of the year | | | 108,490 | | | | 45,075 | | | | 61,285 | | | | 63,415 | | | | (16,210 | ) |
Cash and cash equivalents, end of the year | | $ | 37,144 | | | $ | 108,490 | | | $ | 45,075 | | | $ | (71,346 | ) | | $ | 63,415 | |
Cash flows used in operating activities decreased by $67.8 million during the year ended December 31, 2022 compared to the corresponding period in 2021. The operating cash burn during the year ended December 31, 2022 essentially reflects the cost of our small molecules business while in the comparative period, we were also operating the plasma-derived therapeutics segment as we only started to dispose of certain entities from May 2021 and onwards. Included in the operating expenses of our former segment for the year ended December 31, 2021 were payments made by PBT to PBP 2021 for R&D services of $45.8 million. The 2022 outflows include $5.2 million in payments made towards the provision portion of the CDMO agreement, upon termination of the agreement, plus refundable taxes of $0.5 million, while in 2021, outflows include payments made towards the provision portion of $2.0 million.
Cash flows used in operating activities increased by $23.7 million during the year ended December 31, 2021 compared to the same period in 2020. The increase is mainly due to the payments made by PBT, to PBP after ownership transferred to Kedrion for past and future research and development services totaling $45.8 million. This was partially offset by lower operating expenses mainly because the cost of operation for the different entities sold were only there for the portion of 2021, when they were under our ownership. In addition, those operating expenses were reduced while we were awaiting the outcome of the FDA review of the BLA.
Cash flows used in financing activities increased by $37.0 million during the year ended December 31, 2022 compared to the corresponding period in 2021 essentially due to the repayment of the principal of the SALP loans totaling $39.2 million, including transaction costs, and the payment of $5.5 million made towards the lease portion of a CDMO agreement, upon termination of the agreement. Interest paid on the loans decreased since there were no interest payments during the year ended December 31, 2022 compared to $4.0 million in the comparative period.
Cash flows from financing activities increased by $65.8 million during the year ended December 31, 2021 compared to the same period in 2020 since we did not receive any proceeds from the issuance of long-term debt in 2021 when in 2020 we received $31.5 million from the combined issuance of the second term loan with SALP and the secured convertible debentures. Similarly, in 2020 we had proceeds from the issuance of shares and warrants of $40.0 million while there were no equity financings in 2021.
105
Cash flows from investing activities increased by $166.9 million during the year ended December 31, 2022 compared to the corresponding period in 2021. The increase is mainly due to the proceeds, net of selling costs of $170.1 million we received in 2021 in connection with the divestiture of the plasma-derived therapeutics segment compared to the proceeds from the sale of our former Labrosse facility of net of selling costs of $3.2 million received in 2022.
Cash flows from investing activities decreased by $168.4 million during the year ended December 31, 2021 compared to the same period in 2020 mainly due to the proceeds, net of selling costs of $170.1 million we received in connection with the divestiture of the plasma-derived therapeutics segment compared to the proceeds we received in 2020 in relation to the sale of our former bioseparation business.
C.Research and Development, Patents and Licenses
For a discussion of our research and development activities, see “Item 4.B—Business Overview” and “Item 5.A—Operating Results.”
Other than as disclosed elsewhere in this Item 5 of our Annual Report, the recent trends towards rising inflation has had a non-material impact on our financial position or results of operations to date but may materially adversely affect our future business and corresponding financial position and cash flows. Inflationary factors, such as increases in the cost of our materials and supplies and overhead costs may adversely affect our operating results. Rising interest and inflation rates also present a recent challenge impacting the global economy and could make it more difficult for us to obtain traditional financing on acceptable terms, if at all, in the future. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, we may experience increases in the near future (especially if inflation and interest rates continue to rise) on our operating costs, including our labor costs and research and development costs, due to supply chain constraints, consequences associated with the ongoing Russia-Ukraine war, and employee availability and wage increases, which may result in additional stress on the Company’s working capital resources. For further discussions of trends, see “Item 4.B.—Business overview,” “Item 5.A.—Operating results,” and “Item 5.B.—Liquidity and capital resources.” of our Annual Report.
E.Critical Accounting Estimates
Not applicable.
106
Item 6. Directors, Senior Management and Employees.
A. Directors and Senior Management.
The following table sets forth certain information with respect to our executive officers and directors, including their ages as of March 7, 2023:
| | | | |
Name of Executive Officers | | Age | | Position(s) |
Bruce Pritchard | | 50 | | Chief Executive Officer |
Patrick Sartore | | 49 | | President |
Marie Iskra | | 47 | | General Counsel |
Nicole Rusaw | | 49 | | Chief Financial Officer |
| | | | |
Name of Non-Employee Directors | | Age | | Position(s) |
Simon Best(1)(2) | | 66 | | Lead Independent Director |
Gary J. Bridger(3) | | 60 | | Director |
Neil A. Klompas(1) | | 51 | | Director |
Alek Krstajic(2) | | 59 | | Chair |
Eugene Siklos | | 59 | | Director |
Timothy Steven Wach(1)(2) | | 62 | | Director |
(1) Member of the audit, risk and finance committee.
(2) Member of the HR and corporate governance committee.
(3) Member of the science and technology committee.
Unless otherwise indicated, the current business addresses for our executive officers and directors is 231 Dundas Street East, Belleville, Ontario, Canada, K8N 0K1. The current business address for Mr. Patrick Sartore and Ms. Marie Iskra is 440 Armand-Frappier Blvd., Suite 300, Laval, Québec, Canada H7V 4B4 and the current business address for Bruce Pritchard is Ground Floor, Unit 1, Iconix Park, London Road, Sawston, Cambridge CB22 3EG.
Each executive officer serves at the discretion of our board of directors and holds office until his or her successor is duly elected or qualified or until his or her earlier resignation or removal.
Executive Officers
Bruce Pritchard, Chief Executive Officer. Mr. Bruce Pritchard was appointed as our Chief Executive Officer effective November 13, 2020. From August 2014 to November 2020, he was one of two Chief Operating Officers. In his role as Chief Operating Officer, he was responsible for the company’s discovery, clinical development, program management, CMC and business development functions related to Small Molecule Therapeutics. Mr. Pritchard joined us as Chief Financial Officer of PBL in 2006 and was promoted to our Chief Financial Officer in July 2008, relinquishing that post in November 2015. Mr. Pritchard also served as Interim Chief Financial Officer from August 2017 to September 2019. A Heriot-Watt University graduate, Mr. Pritchard holds a Bachelor of Arts in Accountancy and Computer Science and is qualified as a Member of the Institute of Chartered Accountants of Scotland. Mr. Pritchard was appointed a Fellow of the Institute of Directors in 2014.
107
Patrick Sartore, President. Mr. Patrick Sartore was appointed as President, effective November 13, 2020. Prior to that, he was one of two Chief Operating Officers. In this role, he was responsible for the company’s commercial, marketing, clinical development, manufacturing, and corporate & business development functions, related to Plasma-Derived Therapeutics. Mr. Patrick Sartore served as our Chief Operating Officer, North America from May 2019 to November 2020. Mr. Sartore joined us in November 2006 as Senior Legal Counsel – Intellectual Property and was nominated as our Corporate Secretary in December 2007. Mr. Sartore held the position of General Counsel and Corporate Secretary from May 2013 to May 2015, at which date he was appointed Chief Legal Officer and Corporate Secretary. Mr. Sartore was previously employed by Univalor Inc. as Legal Counsel and Leger Robic Richard, L.L.P., a firm specializing in Intellectual Property, Corporate and Commercial Law, as an associate attorney. Mr. Sartore also holds a Bachelor of Science, with Distinction, from Concordia University.
Marie Iskra, General Counsel. Ms. Marie Iskra has served as our General Counsel and Secretary since September 2019. As General Counsel, Ms. Iskra oversees the legal, compliance, intellectual property and corporate governance functions. Ms. Iskra is also our Corporate Secretary. Ms. Iskra has served in various roles since September 2013, including as Associate General Counsel and Senior Legal Counsel. Prior to joining the Company, Ms. Iskra was the Director of Legal Affairs of Jubilant Draximage Inc. and Jubilant HollisterStier General Partnership from 2009 to 2013 and Legal Counsel for Draxis Health Inc., a publicly-traded company, from 2005 to 2009. Ms. Iskra holds an L.L.B. from the Université de Montreal and is a member of the Québec Bar. Ms. Iskra also holds a Certificate Arts and Sciences from the Université de Montreal.
N. Nicole Rusaw, Chief Financial Officer. Ms. Nicole Rusaw was appointed as Chief Financial Officer effective November 9, 2022. Prior to that, Ms. Rusaw served as Interim Chief Financial Officer starting March 2, 2022. Prior to joining Liminal, Ms. Rusaw was the Chief Financial Officer of two publicly traded companies, Ionic Brands Corp. from June to December 2021, and Nabis Holdings Inc. from October 1, 2019 to January 27, 2021. Prior thereto, Ms. Rusaw acted as the Interim Chief Financial Officer of Nuvo Pharmaceuticals Inc. from August 2017 to September 2018 and Interim VP Operations and Administration from October 2018 to June 2019. Prior thereto, Ms. Rusaw was the Chief Financial Officer of Transition Therapeutics Inc. from December 2011 to October 2016. Ms. Rusaw graduated from Brock University with First Class Honours Co-op and has obtained her Chartered Accountant professional designations, CPA, CA.
Non-Employee Directors
Simon Best. Prof. Best has served as a member of our board of directors since May 2014 and as our Lead Independent Director ("LID") since April 23, 2019. Prof. Best also served as our interim President and Chief Executive Office from December 2018 to April 2019. Prof. Best has experience as the founder and/or Chief Executive Officer of four biotechnology companies between 1992 and 2012, and as a Chairman or board member of major industry bodies including the UK BioIndustry Association (BIA) and the US Biotechnology Industry Organization (BIO). Since 2012, he has played a pivotal role in building pioneering Digital Healthcare companies – in Pathology with PathXL which was acquired by Philips Healthcare in May 2016 – subsequently with Cumulus Neuroscience (previously BrainWaveBank), which develops EEG tools and analytics for at-home use to validate digital biomarkers, stratify patients for clinical trials and monitor them to facilitate the development of novel drugs for neuro-degenerative disorders.
He also served as a director of EvoFem Inc. from November 2015 to December 2017. He was previously an Entrepreneur-In-Residence and Venture Partner with TVM Capital in Munich and Dubai from 2007-2010 and co-founded Par Equity LLP in Edinburgh in 2007. Prof. Best holds an M.B.A. from the London Business School and an Honorary Doctorate and B.Mus from York University. In 2007, he was elected a Fellow of the Royal Society of Edinburgh. In 2008, he was awarded an OBE by Queen Elizabeth II and appointed a Visiting Professor of Medicine by the University of Edinburgh. We believe that Prof. Best’s extensive experience in the life science and investment industries qualifies him to serve as a member of our board of directors.
108
Gary J. Bridger. Dr. Bridger has served as a member of our board of directors since May 2019. Dr. Bridger has also served as a member of the board of directors of Expansion Therapeutics since January 2022 and as a member of the board of directors of X4 Pharmaceuticals, Inc. since October 2018. From February 2015 to December 2017, Dr. Bridger served as a consultant to Xenon Pharmaceuticals Inc., a biopharmaceutical company, where he previously served as the Executive Vice President of Research and Development from January 2013 to February 2015. From October 2013 to October 2015, Dr. Bridger served as a Managing Director at Five Corners Capital Inc. From June 2010 to June 2012, Dr. Bridger served as a venture partner at Venture West Capital Management, a venture capital firm. From November 2006 to December 2007, Dr. Bridger served as Senior Vice President of Research and Development of Genzyme Corporation, a biotechnology company, which was acquired by Sanofi, S.A. In June 1996, Dr. Bridger co-founded AnorMED Inc., a biopharmaceutical company, and was its Vice President of Research and Development and Chief Scientific Officer from 2000 until its acquisition by Genzyme in November 2006. Dr. Bridger has served on the board of directors of Aquinox Pharmaceuticals, Inc. from 2013 until August 2019. Dr. Bridger holds a Ph.D. in Organic Chemistry from the University of Manchester Institute of Science and Technology. We believe that Dr. Bridger’s experience as a director and executive officer at numerous biopharmaceutical companies qualifies him to serve as a member of our board of directors.
Neil A. Klompas. Mr. Klompas has served as a member of our board of directors since May 2019. Mr. Klompas joined Zymeworks Inc. in March 2007 where he currently serves as President and Chief Operating Officer. Mr. Klompas served as Chief Financial Officer of Zymeworks Inc. from 2007 until 2022 and also as its Executive Vice President, Business Operations from September 2019 to January 2022. Prior to joining Zymeworks, he worked with KPMG LLP in Canada and the United States, and from 2005 to 2007, with KPMG's Pharmaceuticals, Biotechnology and Medical Device M&A Transaction Services practice in Princeton, New Jersey. Prior to that, Mr. Klompas worked with KPMG's Canadian Biotechnology and Pharmaceuticals practice. Mr. Klompas is a Chartered Professional Accountant and is a member of Chartered Professional Accountants of British Columbia. Mr. Klompas also holds a degree in Microbiology & Immunology from the University of British Columbia. We believe that Mr. Klompas’ experience as a financial executive in the biopharmaceutical industry qualifies him to serve as a member of our board of directors.
Alek Krstajic. Mr. Krstajic is the former Chief Executive Officer of Wind Mobile, which was sold to Shaw Communications in 2016 for $1.6 Billion. Previously, he was the founder and Chief Executive Officer of Public Mobile, which was purchased by Telus in 2013. Mr. Krstajic has also served as the President of Bell Mobility and a Senior Vice President of Rogers Communications. In the process, Mr. Krstajic and his team fundamentally changed the wireless market in Canada from a three-player market with high prices to a sustainable four player market, providing Canadians with an alternative and competitive pricing and a potential path to better value. As well as being an operator, Mr. Krstajic has shown a level of expertise managing regulatory processes to positive outcomes. Mr. Krstajic sits on a number of boards, and has received numerous awards and recognitions, including Canada’s Top 40 under 40 and a Queen Elizabeth II Diamond Jubilee Medal for his service to Canada. We believe that Mr. Krstajic's extensive business and commercial experience qualifies him to serve as a member of our board of directors.
Eugene Siklos. Mr. Siklos is the President of Thomvest Asset Management. Formerly he was the Vice President, Head of Investments for Export Development Canada (“EDC”), which he joined in 2009. In 2012, Mr. Siklos was appointed head of its direct investing activities and in 2017, was appointed head of EDC’s overall investing activities. During his time at EDC, the capital committed to fund direct, private equity and venture capital investments doubled in size to over C$2 billion, while achieving both market returns and mandate objectives for its shareholder, the Government of Canada. Mr. Siklos holds an MBA degree from Harvard Business School and has over 30 years of financial and business experience, including as a corporate finance professional with Morgan Stanley and Merrill Lynch. Mr. Siklos has served on numerous Boards of Directors, including Paragon Pharmacies (TSX), Copperleaf Technologies, BTI Systems, and ecobee. We believe that Mr. Siklos' extensive financial and investment experience qualifies him to serve as a member of our board of directors.
109
Timothy Steven Wach. Mr. Wach has served as a member of our board of directors since May 2019. Since January 2015 until February 2020, Mr. Wach served as Managing Director and Board Member of Taxand. In 1989, Mr. Wach joined Gowling Lafleur Henderson LLP as an associate and was a partner from 1992 to 2014. Mr. Wach also has experience in government relations and tax policy, having twice served in the Tax Policy Branch of the Canadian Department of Finance in Ottawa, most recently as Director of Legislative Development and Chief Legislative Counsel from 2009 to 2011. In that role, he chaired the Interdepartmental Legislation Review Committee, the committee responsible for the preparation of Canada's federal income tax legislation for submission to Parliament. We believe that Mr. Wach’s experience with respect to tax and finance qualifies him to serve as a member of our board of directors.
Family Relationships
There are no family relationships among any of our executive officers or directors.
110
B. Compensation.
Executive Compensation
Summary Compensation Table
The following table provides a summary of compensation earned during each of the financial years ended on December 31, 2022 and 2021 by the Named Executive Officers, or NEOs, in accordance with applicable rules and regulations. Unless otherwise specified, the executive and directors’ compensation is presented in U.S. Dollars. For the convenience of the reader, in this Annual Report, unless otherwise indicated, translations from Canadian Dollars into U.S. Dollars were made at the rate of $1.00 to USD0.7699, which is the average rate for the 2022 fiscal year, (2021 average rate: $1.00=USD0.7969). Translations from Great Britain Pounds or GBP into U.S. Dollars were made at the rate of 1 GPB to USD1.2438 which is the average rate for the 2022 fiscal year, (2021 average rate: 1 GBP = USD1.3755). Such U.S. Dollar amounts are not necessarily indicative of the amounts of U.S. Dollars that could have been purchased upon exchange of Canadian Dollars or GBP at the dates indicated. The number of stock options awarded has been adjusted to give effect to the 10-for-1 consolidation of our common shares effected on February 1, 2023.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Summary Compensation Table | |
Name and Principal Position | | Year | | Salary
(USD) | | | | Option Awards(1)
(USD) | | | | Non-Equity Incentive Plan Compensation(2) (USD) | | | | All Other Compensation(3)
(USD) | | | | Total Compensation
(USD) | |
Bruce Pritchard | | 2022 | | | 456,723 | | (4) | | | 134,164 | | | | | 182,689 | | (4) | | 59,532 | | (4) | | 833,108 | |
CEO | | 2021 | | | 467,670 | | (4) | | — | | | | | 289,521 | | (4) | | 48,305 | | (4) | | 805,496 | |
Patrick Sartore | | 2022 | | | 423,445 | | (5) | | | 25,852 | | | | | 167,261 | | (5) | | 23,117 | | (5) | | 639,675 | |
President | | 2021 | | | 438,295 | | (5) | | — | | | | | 275,429 | | (5) | | 23,972 | | (5) | | 737,696 | |
Marie Iskra | | 2022 | | | 300,261 | | (5) | | | 34,914 | | | | | 118,603 | | (5) | | 16,958 | | (5) | | 470,736 | |
General Counsel | | 2021 | | | 310,791 | | (5) | | — | | | | | 219,646 | | (5) | | 17,596 | | (5) | | 548,033 | |
(1)In accordance with SEC rules, this column reflects the aggregate grant date fair value of the option awards granted during the fiscal year. The fair value of the option awards was estimated using the Black Scholes option pricing model, a common valuation methodology which uses the same assumptions for determining the equity-based compensation expense in the Company's financial statements for the year-ended December 31, 2022 in accordance with IFRS. See Note 17b of the Notes to Consolidated Financial Statements in this Annual Report for a discussion of assumptions made by us in determining the aggregate grant date fair value of our option awards. The amounts in this column do not represent the actual amounts paid to or realized by our directors during the year ended December 31, 2022.
(2)Represents the cash bonus earned for each financial year, pursuant to our Short-Term Incentive Plan, or STIP. For additional information, see the “Narrative Disclosure to Summary Compensation Table – Short-Term Incentive Plan” section below.
(3)The following table provides the details for the amounts reported for 2022 for each NEO:
| | | | | | | | | | | | | | | | | | | |
Name | | | Pension Contributions
(USD) | | | Professional & Membership Dues
(USD) | | | | Car Allowance (USD) | | | | Total
(USD) | |
Bruce Pritchard | | | | 45,672 | | | | 1,422 | | | | | 12,438 | | | | | 59,532 | |
Patrick Sartore | | | | 21,172 | | | | 1,945 | | | | — | | | | | 23,117 | |
Marie Iskra | | | | 15,013 | | | | 1,945 | | | | — | | | | | 16,958 | |
(4)Paid in pounds sterling (GBP) and converted at the following exchange rate: 1 GBP=USD1.2438 (2022 average); 1 GBP=USD1.3755 (2021 average).
(5)Paid in Canadian dollars (CAD) and converted at the following exchange rate: $1.00=USD0.7699 (2022 average); $1=USD0.7969 (2021 average).
111
Narrative Disclosure to Summary Compensation Table
Base Salary
We aim to provide a salary established based on the level of responsibility relative to other positions in our company and relative to base salaries paid by the organizations in our peer group as well as the performance of the Company and of the NEOs. It is an important component of our ability to attract and retain executives who have the leadership and management skills to drive the further growth and success of our business.
In light of the significant changes to the organization that took place over the last few years, a full revision of our compensation policy was undertaken in 2022, leading to adjustments to Mr. Pritchard's base salary for better alignment with the reference market. The base salaries of the other NEOs were not adjusted in 2022.
Short-Term Incentive Plan
We provide our NEOs with the STIP, which aims to engage, recognize and reward their contributions to reaching our annual objectives. Having a significant portion of all executives’ annual incentive determined on the basis of the same corporate performance objectives reinforces the teamwork of the members of our executive team and motivates them to achieve widespread success for the whole organization. The corporate short-term performance objectives against which our annual performance is evaluated are established each year by the Board's HR and corporate governance committee, in conjunction with the CEO.
The STIP targets for the NEOs were reviewed in 2022 as part of a benchmarking review to ensure that they remain competitive with those of our peer group. Target incentive levels (as a percentage of base salary) for the NEOs were the following in 2022:
| | |
Name | | Short-Term Incentive Target |
Bruce Pritchard | | 50% |
Patrick Sartore | | 50% |
Marie Iskra | | 50% |
Corporate objectives for 2022 were established at the beginning of the year, with each objective being assigned a relative weight. The board of directors, with the support of the HR and corporate governance committee, was responsible for determining and approving a payout factor that would recognize the Company’s performance against the corporate objectives for the year. As a result of this assessment, the overall payout factor for 2022 was 80%.
In addition to the corporate objectives for 2022, individual objectives were also determined by the CEO or the other NEOs. For NEOs other than the CEO, the overall payout factors were established using weightings of 80% for performance against corporate objectives and 20% for performance against individual objectives. Mr. Pritchard's overall payout factor was determined entirely on the basis of performance against corporate objectives.
STIP
| | | | | | | | | | | | | | | | | | | | |
Name | | STIP Weightings | | | Payout Factors | | | | | | |
| | Corporate Objectives | | Individual Objectives | | | Corporate Objectives | | Individual Objectives | | | | | STIP Bonus (USD) | |
Bruce Pritchard | | | 100 | % | — | | | | 80 | % | — | | | | | | 182,689 | |
Patrick Sartore | | | 80 | % | | 20 | % | | | 80 | % | | 75 | % | | | | | 167,261 | |
Marie Iskra | | | 80 | % | | 20 | % | | | 80 | % | | 75 | % | | | | | 118,603 | |
112
Long-Term Incentives
Long-term incentive awards are designed to reward the creation of value for our shareholders while providing a vehicle to attract and retain talented and skilled executives. Under the Omnibus Incentive Plan, adopted in 2019 and replacing the previous Long Term Incentive Plan, or LTIP, we may grant stock options to our executives to ensure alignment of their interests with those of our shareholders, by creating focus on activities and development projects, which will increase share price performance over time. It is also our practice to award an initial grant of stock options to new hires to attract qualified and skilled executives. Outstanding awards granted under the previous LTIP will continue to be governed by the terms of the LTIP until such awards are exercised, expire, or are otherwise terminated or cancelled.
Stock Options
We granted stock options in 2022 to the NEOs at a level reflecting the executive’s performance and the desired positioning of their total compensation in accordance with our compensation policy and the benchmarking review conducted in 2022. In light of the changes to the organization which took place during 2021, as well as in consideration of stock option grants made in prior years, no stock options were granted to NEOs in 2021.
Perquisites and Other Benefits
We provide our NEOs with a competitive executive benefits package which is designed to attract and retain qualified executives. This package includes a capital accumulation retirement plan, including matching company contributions equal to up to 5% of each NEO’s base salary (10% for Bruce Pritchard), group insurance, car allowances (Bruce Pritchard only), reimbursement of annual medical examinations and annual professional memberships. Variations to these programs exist between NEOs due to local market conditions. The value of all such benefits conferred to the NEOs in 2022 is described in the Summary Compensation Table above.
Employment Agreements
Below are descriptions of the employment agreements with our NEOs. See the section titled “—Termination and Change of Control Benefits” for information about the benefits each NEO is entitled to in the event that such executive’s employment is terminated by us without cause or in the event that the executive’s employment is terminated by us between six months before and 12 months after a change of control.
Bruce Pritchard. We entered into an employment agreement with Bruce Pritchard that became effective on July 31, 2019. Mr. Pritchard’s employment agreement provided for his employment as Chief Operating Officer, International for an unspecified duration. This employment agreement was not modified upon his appointment as CEO on November 13, 2020. Mr. Pritchard’s July 31, 2019 employment agreement established his base salary at GBP 340,000, which was increased to GBP 367,200 as of January 1, 2022. Mr. Pritchard’s employment agreement further provides that he is eligible to earn an annual target performance bonus upon attainment of objectives to be determined by our board of directors, with a bonus target of 50%, with the potential of reaching a maximum of 100% of base salary. Mr. Pritchard also received a one-time grant of 25,354 stock options in connection with the entering into of the employment agreement. Mr. Pritchard is entitled to reimbursement for all necessary and reasonable business expenses incurred in connection with his duties in accordance with our generally applicable policies.
113
Patrick Sartore. We entered into an employment agreement with Patrick Sartore that became effective on July 22, 2019. Mr. Sartore’s employment agreement provides for his employment as Chief Operating Officer, North America, for an unspecified duration. This employment agreement has not been modified upon his appointment as President on November 13, 2020. Mr. Sartore’s employment agreement established his base salary at $465,000, which was increased to $550,000 as of September 1, 2019. Mr. Sartore’s employment agreement further provides that he is eligible to earn an annual target performance bonus upon attainment of objectives to be determined by our board of directors, with a bonus target of 50%, with the potential of reaching a maximum of 100% of base salary. Mr. Sartore also received a one-time grant of 25,354 stock options in connection with the entering into of the employment agreement. Mr. Sartore is entitled to reimbursement for all necessary and reasonable business expenses incurred in connection with his duties in accordance with our generally applicable policies.
Marie Iskra. We entered into an employment agreement with Marie Iskra that became effective on August 12, 2019. Mrs. Iskra’s employment agreement provides for her employment as General Counsel for an unspecified duration. Mrs. Iskra’s employment agreement established her base salary at $350,000, which was increased to $390,000 as of April 1, 2020. Mrs. Iskra’s employment agreement further provides that she is eligible to earn an annual target performance bonus upon attainment of objectives to be determined by our board of directors, with a bonus target of 50%, with the potential of reaching a maximum of 100% of base salary. Mrs. Iskra also received a one-time grant of 3,000 stock options in connection with the entering into of the employment agreement. Mrs. Iskra is entitled to reimbursement for all necessary and reasonable business expenses incurred in connection with her duties in accordance with our generally applicable policies.
Termination and Change of Control Benefits
Our employment agreements with our NEOs include termination and change of control benefits in the event that the NEO’s employment is terminated by us without cause or in the event that the NEO’s employment is terminated by us between six months before and 12 months after a change of control. A change of control is deemed to occur upon a change of ownership resulting from the acquisition of a majority of our common shares.
The employment agreements provide for the payment of a lump sum equivalent to the value of 12 months of salary and benefit coverage (excluding disability) and 12 months of accelerated vesting of unvested stock options for termination without cause.
If the NEO’s employment is terminated by us between six months before and 12 months after a change of control, the NEOs’ employment agreements provide for the payment of a lump sum equivalent to the value of 18 months of salary and benefit coverage (excluding disability), 150% of the NEO’s then current annual target bonus under the short-term incentive plan and accelerated vesting of all unvested stock options.
Clawback Policy
In the event that we are required to prepare an accounting restatement due to a material non-compliance of the Company, as a result of misconduct, with any financial reporting requirement under the securities laws and the NEO knowingly engaged in the misconduct, was grossly negligent in engaging in the misconduct, knowingly failed to prevent the misconduct or was grossly negligent in failing to prevent the misconduct, the NEO shall reimburse us the amount of any payment in settlement of the award earned or accrued by him during the 12-month period following the first public issuance or filing with the Autorité des Marchés Financiers (whichever first occurred) of the financial document that contained such material noncompliance.
114
Indemnification
By-law No. 1 provides for indemnification of each of our directors and executive officers to the fullest extent permitted by the Canada Business Corporations Act, or CBCA.
We have entered into indemnity agreements with each director and officer providing that if such director or officer is or was involved in any threatened, pending or completed proceeding by reason of the fact that such director or officer is or was a director or officer of the Company or is or was serving at our request as a director or officer of another entity, including service with respect to employee benefit plans, whether the basis of such proceeding is an alleged action in an official capacity while serving as a director or officer, such director or officer will be indemnified and held harmless by us to the fullest extent authorized by and in the manner set forth in the CBCA against all expense, liability and loss reasonably incurred or suffered by such director or officer in connection therewith. Under such indemnity agreements, we may indemnify any of our directors or officers in connection with a proceeding (or part thereof) initiated by such director or officer only if such proceeding (or part thereof) is authorized by the board of directors or if such proceeding is a successful proceeding, in whole or in part, by a director or officer for claims under an indemnity agreement.
Outstanding Equity Awards at Fiscal Year End
The following table indicates, for each NEO, stock option grants outstanding as of December 31, 2022. The numbers in the table below have been adjusted to give effect to the 10-for-1 consolidation of our common shares effected on February 1, 2023.
| | | | | | | | | | | | | | | | | | |
Outstanding Equity Awards at Fiscal Year End |
| | Option Awards |
Name | | Grant Date | | Number of securities underlying unexercised options (#) (Exercisable) | | | Number of securities underlying unexercised options (#) (Unexercisable) | | | | Option exercise price (USD/sh) | | | | Option expiration date |
Bruce Pritchard | | June 4, 2019 | | | 18,172 | | | | 7,182 | | (1) | | 152.10 | | (2) | June 4, 2029 |
| | December 7, 2020 | | | 11,666 | | | | 3,334 | | (3) | | 42.70 | | | | December 7, 2030 |
| | March 29, 2022 | | | 0 | | | | 7,500 | | (7) | | 10.20 | | | | March 29, 2032 |
| | June 7, 2022 | | | 0 | | | | 13,500 | | (7) | | 5.40 | | | | June 7, 2032 |
Patrick Sartore | | June 4, 2019 | | | 18,172 | | | | 7,182 | | (1) | | 152.10 | | (2) | June 4, 2029 |
| | December 7, 2020 | | | 5,833 | | | | 1,667 | | (3) | | 42.70 | | | | December 7, 2030 |
| | March 29, 2022 | | | 0 | | | | 1,500 | | (7) | | 10.20 | | | | March 29, 2032 |
| | June 7, 2022 | | | 0 | | | | 2,500 | | (7) | | 5.40 | | | | June 7, 2032 |
Marie Iskra | | June 19, 2019 | | | 456 | | | | 41 | | (4) | | 152.10 | | (2) | June 19, 2029 |
| | September 3, 2019 | | | 2,437 | | | | 563 | | (5) | | 119.90 | | (2) | September 3,2029 |
| | June 8, 2020 | | | 3,562 | | | | 2,138 | | (6) | | 140.60 | | (2) | June 8, 2030 |
| | December 7, 2020 | | | 1,500 | | | | 0 | | | | | 42.70 | | | | December 7, 2030 |
| | March 29, 2022 | | | 0 | | | | 2,500 | | (7) | | 10.20 | | | March 29, 2032 |
| | June 7, 2022 | | | 0 | | | | 2,500 | | (7) | | 4.40 | | | | June 7, 2032 |
(1)These stock options vest monthly over the 6-year period following the grant date in accordance with the following vesting schedule, based on the number of months of service completed after the grant date.
115
| | | | | | | | | | |
Months | | Vesting % | | Months | | Vesting % | | Months | | Vesting % |
1 | | 0.93% | | 25 | | 32.18% | | 49 | | 76.39% |
2 | | 1.85% | | 26 | | 33.80% | | 50 | | 77.78% |
3 | | 2.78% | | 27 | | 35.42% | | 51 | | 79.17% |
4 | | 3.70% | | 28 | | 37.04% | | 52 | | 80.56% |
5 | | 4.63% | | 29 | | 38.66% | | 53 | | 81.94% |
6 | | 5.56% | | 30 | | 40.28% | | 54 | | 83.33% |
7 | | 6.48% | | 31 | | 41.90% | | 55 | | 84.72% |
8 | | 7.41% | | 32 | | 43.52% | | 56 | | 86.11% |
9 | | 8.33% | | 33 | | 45.14% | | 57 | | 87.50% |
10 | | 9.26% | | 34 | | 46.76% | | 58 | | 88.89% |
11 | | 10.19% | | 35 | | 48.38% | | 59 | | 90.28% |
12 | | 11.11% | | 36 | | 58.33% | | 60 | | 91.67% |
13 | | 12.04% | | 37 | | 59.72% | | 61 | | 92.36% |
14 | | 12.96% | | 38 | | 61.11% | | 62 | | 93.06% |
15 | | 13.89% | | 39 | | 62.50% | | 63 | | 93.75% |
16 | | 14.81% | | 40 | | 63.89% | | 64 | | 94.44% |
17 | | 15.74% | | 41 | | 65.28% | | 65 | | 95.14% |
18 | | 16.67% | | 42 | | 66.67% | | 66 | | 95.83% |
19 | | 17.59% | | 43 | | 68.06% | | 67 | | 96.53% |
20 | | 18.52% | | 44 | | 69.44% | | 68 | | 97.22% |
21 | | 19.44% | | 45 | | 70.83% | | 69 | | 97.92% |
22 | | 20.37% | | 46 | | 72.22% | | 70 | | 98.61% |
23 | | 21.30% | | 47 | | 73.61% | | 71 | | 99.31% |
24 | | 30.56% | | 48 | | 75.00% | | 72 | | 100.00% |
(2)In Canadian dollars. Prior to October 8, 2020, the exercise prices of stock options were determined in Canadian dollar.
(3)These stock options vest monthly over the 3-year period spanning from December 7, 2020 to December 7, 2023.
(4)These stock options vest monthly over 3-year period spanning from June 19, 2020 to June 19, 2023.
(5)These stock options vest monthly over 3-year period spanning from September 3, 2020 to September 3, 2023.
(6)These stock options vest monthly over 3-year period spanning from June 8, 2021 to June 8, 2024.
(7)These stock options vest 25% upon the grant's first anniversary, and then monthly over the following 3-year period.
For information on vesting acceleration of the equity awards described above upon a NEO's termination of employment, see the "Executive Compensation – Executive Compensation Elements – Termination and Change of Control Benefits” section above.
116
Director Compensation
Director Compensation Program
The following table describes the director compensation program that is currently in effect for the 12-month period starting June 6, 2022, or the 2022-2023 Mandate, and the director compensation program that was in effect for the period spanning from June 4, 2021 through June 6, 2022, or the 2021-2022 Mandate. Cash retainers are paid on a quarterly basis and no attendance fee is paid to the members for attending the meetings of our board of directors and meetings of our standing committees. Under the director compensation program for the 2022-2023 Mandate, 26,000 stock options are granted to newly appointed directors and vest over a 3-year period. 13,000 stock options are granted for subsequent grants and vest over one-year period.
117
| | | | | | | | |
Director Compensation Program | |
| | 2022-2023 Mandate
(USD) | | | 2021-2022 Mandate
(USD) | |
Chair of Board | | | | | | |
Annual Retainer (inclusive of committee retainers/fees) | | $ | 119,335 | | | $ | 123,520 | |
| | 1,300 stock options(1)(4) | | | 1,000 stock options(2)(4) | |
Lead Independent Director | | | | | | |
Annual Retainer (inclusive of committee retainers/fees) | | $ | 103,937 | | | $ | 107,582 | |
| | 1,300 stock options(1)(4) | | | 1,000 stock options(2)(4) | |
Non-Executive Directors | | | | | | |
Annual Retainer | | $ | 57,743 | | | $ | 59,768 | |
| | 1,300 stock options(1)(4) | | | 1,000 stock options(2)(4) | |
Committee Chairs | | | | | | |
Additional Annual Retainer | | | | | | |
Audit, Risk and Finance | | $ | 38,495 | | | $ | 27,892 | |
HR and Corporate Governance(3) | | — | | | — | |
Science and Technology | | $ | 26,947 | | | $ | 27,892 | |
Attendance Fees | | No attendance fees | | | No attendance fees | |
Non-Chair Committee Members | | | | | | |
Additional Annual Retainer | | | | | | |
Audit, Risk and Finance | | $ | 13,473 | | | $ | 13,946 | |
HR and Corporate Governance(3) | | $ | 11,549 | | | $ | 11,954 | |
Science and Technology | | — | | | — | |
Attendance Fees | | No attendance fees | | | No attendance fees | |
(1)Options vest quarterly over 1-year period spanning from July 1, 2022 to July 1, 2023.
(2)Options vest quarterly over 1-year period spanning from May 1, 2021 to May 1, 2022.
(3)Included in Lead Independent Director and Board Chair annual retainers.
(4)The number of stock options awarded has been adjusted to give effect to the 10-for-1 consolidation of our common shares effected on February 1, 2023.
Director Compensation Table
The following table sets forth information concerning compensation accrued or paid to our non-employee directors during the year ended December 31, 2022 for their service on our Board.
| | | | | | | | | | | | | | | | | | | | | | | |
Director Compensation Table | |
| | Fees earned or paid in cash | | | | | | | | | | |
Name | | Board Annual Cash Retainer
(USD) | | | Committee Annual Cash Retainer
(USD) | | | Attendance Fee
(USD) | | Option awards(1)(2)(8)
(USD) | | | All other compensation
(USD) | | | | Total Compensation
(USD) | |
Current Members | | | | | | | | | | | | | | | | | | |
Simon Best(3) | | | 103,937 | | | | 13,473 | | | — | | | 6,358 | | | | | | | 123,768 | |
Gary Bridger | | | 57,743 | | | | 26,947 | | | — | | | 51,666 | | | | 180,000 | | (4) | | 316,356 | |
Neil A. Klompas(5) | | | 57,743 | | | | 33,559 | | | — | | | 6,358 | | | — | | | | | 97,660 | |
Alek Krstajic(6) | | | 119,335 | | | | 5,774 | | | — | | | 6,358 | | | — | | | | | 131,467 | |
Eugene Siklos(7) | | — | | | — | | | — | | — | | | — | | | | — | |
Timothy Steven Wach | | | 57,743 | | | | 25,022 | | | — | | | 6,358 | | | — | | | | | 89,123 | |
(1)In accordance with SEC rules, this column reflects the aggregate grant date fair value of the option awards granted during the fiscal year. The fair value of the option awards was estimated using the Black Scholes option pricing model, a common valuation methodology which uses the same assumptions for determining the equity-based compensation expense in the Company's financial statements for the year-ended December 31, 2022 in accordance with IFRS. See Note 17b of the Notes to Consolidated Financial Statements in this Annual Report for a discussion of assumptions made by us in determining the aggregate grant date fair value of our option awards. The amounts in this column do not represent the actual amounts paid to or realized by our directors during the year ended December 31, 2022.
118
(2)This amount represents the value of special stock option grants made to Dr. Bridger ($174,378) in 2022 in recognition for work performed beyond his duties as non-employee director.
(3)Prof. Best is our Lead Independent Director and Chair of the HR and corporate governance committee.
(4)This amount, paid in U.S. dollars, represents the compensation Dr. Bridger received pursuant to his consultancy agreement entered into in October 2019 with us, as amended from time to time.
(5)Mr. Klompas is Chair of the audit, risk and finance committee.
(6)Mr. Krstajic is the Chair of our board of directors and a member of the HR and corporate governance committee.
(7)Mr. Siklos waived the compensation he is entitled to receive as director on our board of directors.
(8)The following table indicates the number of option awards outstanding for each current and former director as of December 31, 2022. No share awards were provided to directors during the year ended December 31, 2022.
| | | | |
Name | | Options Outstanding(i)
(#) | |
Simon Best | | | 9,072 | |
Gary Bridger | | | 14,885 | |
Neil Klompas | | | 4,885 | |
Alek Krstajic | | | 4,300 | |
Eugene Siklos | | — | |
Timothy Steven Wach | | | 4,885 | |
(i)The number of stock options has been adjusted to give effect to the 10-for-1 consolidation of our common shares effected on February 1, 2023.
C. Board Practices.
Our board of directors is responsible for our stewardship and strategic direction. It does not actively manage but rather supervises the management of our business and affairs, to ensure a consistent focus on increasing shareholder value. In exercising their powers and discharging their duties, our directors shall (a) act honestly and in good faith with a view to the best interests of the company; and (b) exercise the care, diligence and skill that a reasonably prudent person would exercise in comparable circumstances.
Our board of directors currently consists of six members. The following table sets forth the names of our directors, the years of their initial appointment as directors and the expiration dates of their current term.
| | | | | | |
Name | | Current
Position | | Year of
Initial
Appointment | | Term
Expiration
Year |
Simon Best | | Lead Independent Director | | 2014 | | n/a |
Gary J. Bridger | | Director | | 2019 | | n/a |
Neil A. Klompas | | Director | | 2019 | | n/a |
Alek Krstajic | | Chair | | 2020 | | n/a |
Eugene Siklos | | Director | | 2020 | | n/a |
Timothy Steven Wach | | Director | | 2019 | | n/a |
119
Director Independence
As a foreign private issuer, under the listing requirements and rules of Nasdaq, we are not required to have independent directors on our board of directors, except to the extent that our audit committee is required to consist of independent requirements, subject to certain phase-in schedules. However, our board of directors has determined that, under current listing requirements and rules of Nasdaq (which we are not subject to) and taking account any applicable committee independence standards, four of our six directors, Prof. Best, Mr. Klompas, Mr. Krstajic and Mr. Wach, are independent directors. In accordance with the Nasdaq Listing Rules, a director shall be considered independent if she/he does not have any relationship which, in the opinion of the board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In addition, in accordance with Nasdaq Listing Rules, to be considered independent, a director shall not be subject to any of the mandatory bars to independence set forth in Rule 5605(a) of the Nasdaq Listing Rules.
In making such determination, our board of directors considered the relationships that each non-employee director has with us and all other facts and circumstances our board of directors deemed relevant in determining the director’s independence, including the number of ordinary shares beneficially owned by the director and his or her affiliated entities (if any).
On an annual basis, each director has to declare whether he is independent, and all such declarations are reviewed by the HR and corporate governance committee, which then makes recommendations to our board of directors in respect of our board of director’s determination on the independence of the directors. We have not instigated a board tenure policy given the length of tenure of the directors that currently serve on our board of directors, which is well below the average tenure of directors of Canadian reporting issuers.
Board Diversity
The following chart summarizes certain self-identified personal characteristics of our directors, in accordance with Nasdaq Listing Rule 5605(f). Our previous year's disclosure can be found in our Annual Report on Form 20-F filed with the SEC on March 18, 2022. Each term used in the table has the meaning given to it in the rule and related instructions:
| | | | | | | |
Board Diversity Matrix (as of December 31, 2022) |
Total Number of Directors | # |
| Female | | Male | | Non-Binary | | Did not Disclose |
Part I: Gender Identity | | | | | | | |
Directors | | | 5 | | | | 1 |
Part II: Demographic Background | | | | | | | |
African American or Black | | | | | | | |
Alaskan Native or Native American | | | | | | | |
Asian | | | | | | | |
Hispanic or Latinx | | | | | | | |
Native Hawaiian or Pacific Islander | | | | | | | |
White | | | 5 | | | | |
Two or More Races or Ethnicities | | | | | | | |
LGBTQ+ | | | | | | | |
Did Not Disclose Demographic Background | | | | | | | 1 |
120
The Company currently has no member of its board of directors who is Diverse within the meaning of Nasdaq Rule 5605(f)(2)(B). The Company believes that drawing from a broad range and variety of perspectives is beneficial to the Company's success and helps it achieve its objectives in terms of efficiency for the benefit of its shareholders. Therefore, the Company is committed to increasing the range and variety of perspectives of its directors and senior management over time and, as such, supports initiatives aimed at identifying candidates with a broad range and variety of skills, qualifications, capabilities, talents, insights and professional and life experiences. The Company has not adopted a written diversity policy or target quota relating to the identification and nomination of Diverse directors and members of senior management. Diversity encompasses gender, age, experience, education, ethnicity, religious and cultural backgrounds as well as other dimensions such as lifestyle choices and family responsibilities (the "Designated Groups"). The Company focuses on factors such as skills, qualifications, capabilities, insights, talents, personal attributes and professional and life experiences in the director and senior management identification and selection process. Recommendations for election and appointment are made on merit, in light of the skills, experience, independence and knowledge that the Company requires to be most effective with regards to the Company's current and future plans and objectives, as well as anticipated regulatory and market developments. The Company must retain the flexibility to add qualified directors and senior management. While consideration of the number of individuals from Designated Groups who are members of the Board and members of senior management will continue to be a component of the selection process, the necessity of obtaining the right synergy and balance among directors and senior management so as to optimize the Company's ability to meet the challenges it faces is paramount.
Although there are currently no Diverse directors on our board of directors, the Company has always encouraged the representation of women on the Board. In the last eight years, the Company has had between one and three women on its Board. There are currently two women among the four senior executive officers of the Company (50%) [and 63% of our leadership positions are filled by women.
The Company’s commitment to diversity is further reflected in the Equal Opportunities Policy the Company adopted, which recognizes the Company’s commitment to equality of opportunity and to the recruitment, retention, development and promotion of qualified female candidates among its workforce, including at the highest level.
Board Committees
Our board of directors has two standing committees: the audit, risk and finance committee and the HR and corporate governance committee.
Audit, risk and finance committee
The audit, risk and finance committee currently consists of Messrs. Best, Klompas and Wach.
Mr. Klompas serves as chair of the audit, risk and finance committee. All of the members of the audit, risk and finance committee are independent directors and financially literate within the meaning of the Canadian Securities Administrators rules. The members also qualify as “audit committee financial experts” as defined by the SEC.
The audit, risk and finance committee is mainly responsible for the four following fundamental matters:
•our financial reporting process and internal control systems;
•our process to identify and manage risks;
•the internal and external audit process; and
121
•our communication system to provide an open avenue of communication among the external auditors, our financial and senior management, our internal auditing department (if any), and our board of directors.
The audit, risk and finance committee reviews the interim and annual consolidated financial statements, management's discussion and analysis and other legally required public disclosure documents containing financial information. In its oversight of the services rendered by the external auditors, the audit, risk and finance committee (i) discusses with the external auditor its responsibilities in performing the audit, its determination of areas of significant audit risk and related risk mitigation procedures, and reviews and approves its annual audit plan and associated fees; (ii) discusses with the external auditor the key accounting risks and significant judgments made by management; (iii) receives written confirmation from the external auditor of its independence; (iv) pre-approves all additional engagements with the external auditor (including any non-audit services); and (v) assesses, annually, the external auditor’s performance. The audit, risk and finance committee is also involved in the assessment and selection of our external auditor.
The audit, risk and finance committee also oversees the financial reporting processes and internal controls. This comprises keeping abreast of how our management's addresses changes in the operations and transactions to ensure that they are appropriately reflected in the financial disclosure documents and the internal controls put in place to address these changes. As part of this, the audit, risk and finance committee reviews the activities of the internal audit department, including its organizational structure, resources and budget, the internal audit plan for the year and their report on their review and testing of ICFR and disclosure controls. It also considers any recommendations made by the external auditors regarding internal controls. In addition, the audit, risk and finance committee reviews the quarterly internal reporting package prepared by management, understanding the key variances from budget and the impact on the financial condition.
Finally, in the accomplishment of its financial oversight, the audit, risk and finance committee reviews and discusses our short and long-term financial plans, including financial results and the management of tax matters.
The audit, risk and finance committee meets as often as one or more members of the audit, risk and finance committee deems necessary, but in any event meets at least four scheduled times per year.
The HR and corporate governance committee
The HR and corporate governance committee consists of Messrs. Best, Krstajic and Wach. Prof. Best serves as the chair of the HR and corporate governance committee. The primary responsibility of the HR and corporate governance committee with respect to human resources and compensation are mainly to:
•review short and long-term corporate objectives relevant to the compensation of the chief executive officer and evaluate chief executive officer performance in meeting these objectives;
•review the compensation of the chief executive officer, other senior executive officers and director compensation;
•review the design and ensure implementation of incentive-compensation and equity-based plans;
•ensure that appropriate mechanisms are in place regarding succession planning for the chief executive officer; and
•perform any other activities delegated by our board of directors consistent with the responsibilities and duties relating to compensation.
The primary responsibility of the HR and corporate governance committee with respect to corporate governance will be mainly to:
•assess and establish the size and composition of our board of directors and its committees, and identify candidates with the right skills for the nomination of directors;
122
•develop and review an appropriate procedure to periodically evaluate the performance of our board of directors, its committees and their members;
•develop and monitor the continuing education programs for directors;
•review the mandate of our board of directors and its committees, of their chairs and of the lead independent director;
•review annually our Code of Ethics and Business Conduct as well as any other of our policies as deemed appropriate; and
•perform any other activities delegated by our board of directors consistent with the responsibilities and duties relating to corporate governance.
The HR and corporate governance committee meets regularly throughout the year. At the end of each meeting or whenever deemed necessary, the HR and corporate governance committee may hold an informal in-camera session without the presence of management.
Other Corporate Governance Matters
The Sarbanes-Oxley Act, as well as related rules subsequently implemented by the SEC, requires foreign private issuers, including our company, to comply with various corporate governance practices. In addition, Nasdaq rules provide that foreign private issuers may follow home country practice in lieu of the Nasdaq corporate governance standards, subject to certain exceptions and except to the extent that such exemptions would be contrary to U.S. federal securities laws.
Because we are a foreign private issuer, our members of our board of directors, executive board members and senior management are not subject to short-swing profit and insider trading reporting obligations under Section 16 of the Exchange Act. They will, however, be subject to the obligations to report changes in share ownership under Section 13 of the Exchange Act and related SEC rules.
D. Employees.
As of December 31, 2022, we had 43 full-time employees. None of our employees are represented by collective bargaining agreements. We believe that we maintain good relations with our employees. At each date shown, we had the following number of full time employees, broken out by function.
| | | | | | | | | | | | |
| | At December 31, | |
| | 2022 | | | 2021 | | | 2020 | |
Function: | | | | | | | | | |
Research and development | | | 26 | | | | 25 | | | | 163 | |
Plasma collection centers | | | — | | | | — | | | | 34 | |
General and administrative | | | 17 | | | | 23 | | | | 54 | |
Total | | | 43 | | | | 48 | | | | 251 | |
E. Share Ownership.
For information regarding the share ownership of our directors and executive officers, see “Item 6.B.—Compensation” and “Item 7.A.—Major Shareholders.”
123
Item 7. Major Shareholders and Related Party Transactions.
A. Major shareholders.
The following table sets forth information with respect to the beneficial ownership of our common shares as of March 7, 2023 for:
•each beneficial owner of more than five percent (5%) of our outstanding common shares;
•each of our executive officers;
•each of our directors; and
•all of our directors and executive officers as a group.
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include common shares that can be acquired within 60 days of March 7, 2023. The percentage ownership information shown in the table is based upon 3,104,222 common shares outstanding as of March 7, 2023.
Except as otherwise indicated, all of the shares reflected in the table are common shares and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose.
In computing the number of common shares beneficially owned by a person and the percentage ownership of that person, we deemed outstanding common shares subject to options and warrants held by that person that are immediately exercisable or exercisable within 60 days of March 7, 2023. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person. The information in the table below is based on information known to us or ascertained by us from public filings made by the shareholders. Except as otherwise indicated, addresses of the directors, executive officers and named beneficial owners are in care of Liminal BioSciences Inc., 440 Armand-Frappier Blvd., Suite 300, Laval, Québec, Canada H7V 4B4. The current address for Bruce Pritchard is Park Ground Floor, Unit 1, Iconix Park, London Road, Sawston, Cambridge CB22 3EG and the current address for Nicole Rusaw is 231 Dundas Street East, Belleville, Ontario, Canada, K8N 0K1.
124
| | | | | | | | | |
Name and Address | | Number of
Shares
Beneficially
Owned | | | Percentage of
Shares
Beneficially
Owned | | |
5% or Greater Shareholders | | | | | | | |
Structured Alpha LP(1) | | | 2,382,349 | | | 68.09 | | |
Executive Officers and Directors | | | | | | | |
Bruce Pritchard | | * | | | * | | |
Patrick Sartore | | * | | | * | | |
Marie Iskra | | * | | | * | | |
Nicole Rusaw | | * | | | * | | |
Simon Best | | * | | | * | | |
Gary J. Bridger | | * | | | * | | |
Neil A. Klompas | | * | | | * | | |
Alek Krstajic | | * | | | * | | |
Eugene Siklos | | | 2,382,349 | | (3) | 68.09 | | (3) |
Timothy Wach | | * | | | * | | |
All current executive officers and directors as a group (9 persons)(2)(4) | | | 100,892 | | | | 3.3 | | |
___________________
* Represents beneficial ownership of less than 1%.
(1)Consists of (a) 1,987,613 common shares and (b) 394,736 common shares that SALP has the right to acquire within 60 days following March 7, 2023 pursuant to the exercise of warrants. Thomvest Asset Management Ltd. is the general partner of SALP and may be deemed to beneficially own, have sole power to vote and sole power to dispose of these common shares. The address for SALP and Thomvest Asset Management Ltd. is 65 Queen Street West, Suite 2400, Toronto Ontario M5H 2M8.
(2)Consists of (a) 4,710 common shares and (b) 96,182 common shares that all officers and directors as a group have the right to acquire within 60 days following March 7, 2023 pursuant to the exercise of stock options.
(3)The amount corresponds to the shares beneficially owned by SALP as listed in footnote 1. Mr. Siklos is the President of Thomvest Asset Management Ltd. and may be deemed to have sole voting and investment power of the securities held by SALP. The address for SALP and Thomvest Asset Management Ltd. is 65 Queen Street West, Suite 2400, Toronto Ontario M5H 2M8.
(4)The aggregate amount and percentage of shares beneficially owned by the directors and officers exclude the shares beneficially owned by SALP as listed in footnote 1 for which Mr. Siklos may be deemed to have sole voting and investment power of the securities held by SALP. The address for SALP and Thomvest Asset Management Ltd. is 65 Queen Street West, Suite 2400, Toronto Ontario M5H 2M8.
As of March 7, 2023, approximately 10.44% of our outstanding common shares were held in the United States by 7 holders of record. The actual number of holders is greater than these numbers of record holders and includes beneficial owners whose common shares are held in street name by brokers and other nominees. This number of holders of record also does not include holders whose shares may be held in trust by other entities.
B. Related Party Transactions.
Since January 1, 2022, we have engaged in the following transactions with our directors, executive officers and holders of more than five percent (5%) of our outstanding voting securities and their affiliates, which we refer to as our related-parties. All of the transactions have been reviewed and approved by our board of directors or another independent committee of the board.
125
Tolling Agreement with Thomvest Asset Management Ltd and SALP
On October 1, 2021, we and SALP entered into a forbearance agreement whereby SALP agreed to forbear from seeking immediate payment of certain indemnity obligations pursuant to a subscription agreement entered into in 2019 (the "Indemnity Obligations") and from exercising certain of its rights and remedies relating thereto during the forbearance period ending in June 2022 on the terms and conditions set out in the Agreement (the "Forbearance Agreement"). The Forbearance Agreement expired on June 30, 2022 and the parties entered into a Tolling Agreement dated September 29, 2022 (the “Tolling Agreement”) to suspend the operation and avoid the expiry of any applicable limitation or prescription periods, whether statutory, equitable, contractual, under common or civil law or otherwise (the “Limitation Periods”), in respect of the Indemnity Obligations.
Consulting Services Agreement with Dr. Gary Bridger
On October 2, 2019, we entered into a Consulting Services Agreement with Dr. Gary Bridger, a member of our Board, to provide consulting services in addition to his duties as a Board director. The agreement was further extended until June 30, 2023.
Employment Agreements
We have entered into compensation, including grants of stock options and other equity awards, termination and change of control arrangements with our named executive officers, which are described under the sections entitled “Item 6.B.—Compensation—Executive Compensation—Employment Agreements” and “Item 6.B.—Compensation—Executive Compensation—Termination and Change of Control Benefits.”
126
Directors and Officers Liability Insurance
We maintain directors’ and officers’ liability insurance policies for the liability of our directors and officers arising out of the performance of their duties and for our liability arising out of securities claims. The policies provide coverage in respect of a maximum total liability of CAD20,000,000 (primary and excess liability insurance), subject to a general retention of CAD5,000,000 per loss, and includes specific exclusions, which are usually contained in insurance policies of this nature. We also maintain a concurrent excess liability insurance for the liability of our directors and officers arising out of the performance of their duties, with limits of liability of CAD7,500,000 and subject to specific exclusions which are usually contained in insurance policies of this nature.
D&O Indemnification Agreements
By-law No. 1 provides for indemnification of each of our directors and executive officers to the fullest extent permitted by the Canada Business Corporations Act, or CBCA.
We have entered into indemnity agreements with each director and officer providing that if such director or officer is or was involved in any threatened, pending or completed proceeding by reason of the fact that such director or officer is or was a director or officer of the Company or is or was serving at our request as a director or officer of another entity, including service with respect to employee benefit plans, whether the basis of such proceeding is an alleged action in an official capacity while serving as a director or officer, such director or officer will be indemnified and held harmless by us to the fullest extent authorized by and in the manner set forth in the CBCA against all expense, liability and loss reasonably incurred or suffered by such director or officer in connection therewith. Under such indemnity agreements, we may indemnify any of our directors or officers in connection with a proceeding (or part thereof) initiated by such director or officer only if such proceeding (or part thereof) is authorized by the board of directors or if such proceeding is a successful proceeding, in whole or in part, by a director or officer for claims under an indemnity agreement.
C. Interests of Experts and Counsel.
Not applicable.
127
Item 8. Financial Information.
A. Consolidated Statements and Other Financial Information.
Consolidated Financial Statements
Our consolidated financial statements are appended at the end of this Annual Report, starting at page F-1, and incorporated herein by reference.
Dividend Distribution Policy
We have not paid any dividends on our outstanding common shares, and we have no current intention to declare dividends on our common shares in the foreseeable future. Any decision to pay dividends on our common shares in the future will be at the discretion of our board of directors and will depend on, among other things, our results of operations, current and anticipated cash requirements and surplus, financial condition, any future contractual restrictions and financing agreement covenants, solvency tests imposed by corporate law and other factors that our board of directors may deem relevant. We are subject to covenants under our non-revolving LOC with SALP that places restrictions on our ability to pay dividends.
Legal Proceedings
We are, in the course of our business, subject to lawsuits and other claims. From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. Although the results of litigation and claims cannot be predicted with certainty, we currently believe that the final outcome of these ordinary course matters will not have a material adverse effect on our business. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Refinancing Proceedings
On April 15, 2019, we announced our intention to enter into a series of related arrangements to restructure its outstanding indebtedness, reduce our interest and certain other payment obligations, and raise sufficient cash to build a robust balance-sheet for the next phase of our development (collectively, the “Refinancing Transactions”).
On March 2, 2021, we were served with an action instituted by multiple individual shareholder plaintiffs (the “Plaintiffs”) against the Company, SALP, Thomvest Asset Management (“Thomvest”), Consonance Capital Management LP (“Consonance”), as well as the directors (the “Directors”) that were on the Company’s Board on March 31, 2019 or on April 15, 2019 and certain officers of the Company (the “D&Os”, together with Liminal, SALP, Thomvest and Consonance, the “Defendants”). The Plaintiffs allege, among other things, that, as part of the Refinancing Transactions, the Defendants (i) undervalued certain products, (ii) reduced certain of their operational activities, (iii) artificially devalued certain assets in order for them to be written off the balance sheet, (iv) conducted their business in a manner that prevented them from obtaining financing from certain parties and (v) never properly disclosed their financial difficulties, the alleged collective result of which was, among other things, that SALP and Thomvest were able to take control of the Company to the detriment of the minority shareholders. On November 2, 2021, we received service of an amended proceeding.
The Plaintiffs’s request in damages has gone from almost $700 million initially to almost $950 million in damages, approximately $905 million of which is based on the loss of future value of the Company’s shares.
128
The Company believes that the Plaintiffs’ claims are completely without merit and intends to vigorously defend itself. Defense and settlement costs associated with such lawsuits and claims can be substantial, even when these lawsuits and claims have no merit. Due to the inherent uncertainty of the litigation process, the resolution of any particular legal proceeding could have an adverse effect on our operating results or financial performance. No provisions have been recorded in the consolidated financial statements in regard to these claims.
Liminal Former Employee Dispute
On June 6, 2019, a former employee of ours filed a suit in the United States District Court, District of Connecticut alleging claims for declaratory judgment, breach of contract, breach of the covenant of good faith and fair dealing, unjust enrichment, and violation of Conn. Gen. Stat. § 31-72 for unpaid wages and benefits, based upon the former employee’s employment contract and the terms of employee benefit programs. The matter has been resolved and the District of Connecticut action was dismissed with prejudice on August 13, 2020.
B. Significant Changes
Not applicable.
Item 9. The Offer and Listing.
A. Offer and Listing Details
On July 28, 1998, our common shares began trading on the TSX under the trading symbol “PLI” and, on October 7, 2019, began trading under the trading symbol “LMNL.” Prior to July 28, 1998, there was no public trading market for our common shares. On November 18, 2019, our common shares began trading on Nasdaq under the trading symbol “LMNL.” On August 5, 2020, we voluntarily delisted our common shares from the TSX.
B. Plan of Distribution.
Not applicable.
C. Markets.
On July 28, 1998, our common shares began trading on the TSX under the trading symbol “PLI” and, on October 7, 2019, began trading under the trading symbol “LMNL.” On November 18, 2019, our common shares began trading on Nasdaq under the trading symbol “LMNL.” On August 5, 2020, we voluntarily delisted our common shares from the TSX.
D. Selling Shareholders.
Not applicable.
E. Dilution.
Not applicable.
F. Expenses of the Issue.
Not applicable.
129
Item 10. Additional Information.
A. Share Capital.
Not applicable.
B. Memorandum and Articles of Association.
See Exhibit 2.1 to this Annual Report for a summary of certain material provisions of our articles of incorporation, as amended, bylaws, as amended, and certain related sections of the Canada Business Corporations Act. See Exhibit 1.1, 1.2 and 1.3 to this Annual Report for our articles of incorporation, as amended, and Exhibits 1.4, 1.5 and 1.6 for our bylaws, as amended.
C. Material Contracts.
For information on our material contracts, please see “Item 4.—Information on the Company,” and “Item 7.B.—Related Party Transactions” of this Annual Report.
D. Exchange Controls.
Competition Act
Limitations on the ability to acquire and hold our common shares may be imposed by the Competition Act (Canada). This legislation establishes a pre-merger notification regime for certain types of merger transactions that exceed certain statutory shareholding and financial thresholds. Transactions that are subject to notification cannot be closed until the required materials are filed and the applicable statutory waiting period has expired or been waived by the Commissioner of Competition, or the Commissioner. Further, the Competition Act (Canada) permits the Commissioner to review any acquisition of control over or of a significant interest in us, whether or not it is subject to mandatory notification. This legislation grants the Commissioner jurisdiction, for up to one year, to challenge this type of acquisition before the Canadian Competition Tribunal if it would, or would be likely to, substantially prevent or lessen competition in any market in Canada.
Investment Canada Act
The Investment Canada Act requires notification and, in certain cases, advance review and approval by the Government of Canada of an investment to establish a new Canadian business by a non-Canadian or of the acquisition by a non-Canadian of “control” of a “Canadian business”, all as defined in the Investment Canada Act. Generally, the threshold for advance review and approval will be higher in monetary terms for a member of the World Trade Organization. The Investment Canada Act generally prohibits the implementation of such a reviewable transaction unless, after review, the relevant minister is satisfied that the investment is likely to be of net benefit to Canada.
The Investment Canada Act contains various rules to determine if there has been an acquisition of control. For example, for purposes of determining whether an investor has acquired control of a corporation by acquiring shares, the following general rules apply, subject to certain exceptions. The acquisition of a majority of the voting shares of a corporation is deemed to be acquisition of control of that corporation. The acquisition of less than a majority but one-third or more of the voting shares of a corporation is presumed to be an acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquiror through the ownership of voting shares. The acquisition of less than one-third of the voting shares of a corporation is deemed not to be acquisition of control of that corporation.
130
In addition, under the Investment Canada Act, national security review on a discretionary basis may also be undertaken by the federal government in respect of a much broader range of investments by a non-Canadian to “acquire, in whole or in part, or to establish an entity carrying on all or any part of its operations in Canada, with the relevant test being whether such an investment by a non-Canadian could be “injurious to national security.” The Minister of Industry has broad discretion to determine whether an investor is a non-Canadian and therefore may be subject to national security review. Review on national security grounds is at the discretion of the federal government and may occur on a pre- or post-closing basis.
See “Item 10.E.—Taxation” for additional information regarding the material U.S. federal income tax consequences relating to the ownership and disposition of our common shares by U.S. Holders (as defined thereto).
Any of these provisions may discourage a potential acquirer from proposing or completing a transaction that may have otherwise presented a premium to our shareholders. We cannot predict whether investors will find our company and our common shares less attractive because we are governed by foreign laws.
E. Taxation.
Certain Material U.S. Federal Income Tax Considerations
The following discussion describes the material U.S. federal income tax consequences relating to the ownership and disposition of common shares by U.S. Holders (as defined below). This discussion applies to U.S. Holders that hold our common shares as capital assets. This discussion is based on the U.S. Internal Revenue Code of 1986, as amended, or the Code, U.S. Treasury regulations promulgated thereunder and administrative and judicial interpretations thereof, all as in effect on the date hereof and all of which are subject to change, possibly with retroactive effect. This discussion does not address all of the U.S. federal income tax consequences that may be relevant to specific U.S. Holders in light of their particular circumstances or to U.S. Holders subject to special treatment under U.S. federal income tax law (such as certain financial institutions, insurance companies, broker-dealers and traders in securities or other persons that generally mark their securities to market for U.S. federal income tax purposes, tax-exempt entities, retirement plans, regulated investment companies, real estate investment trusts, certain former citizens or residents of the United States, persons who hold our common shares as part of a “straddle”, “hedge”, “conversion transaction”, “synthetic security” or integrated investment, persons that have a “functional currency” other than the U.S. dollar, persons that own directly, indirectly or through attribution 10% or more of the voting power of our shares, corporations that accumulate earnings to avoid U.S. federal income tax, persons subject to special tax accounting rules under Section 451(b) of the Code, persons subject to special tax accounting rules under Section 451(b) of the Code, partnerships and other pass-through entities, and investors in such pass-through entities). This discussion does not address any U.S. state or local or non-U.S. tax consequences or any U.S. federal estate, gift or alternative minimum tax consequences.
As used in this discussion, the term “U.S. Holder” means a beneficial owner of our common shares that is, for U.S. federal income tax purposes, (1) an individual who is a citizen or resident of the United States, (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, (3) an estate the income of which is subject to U.S. federal income tax regardless of its source or (4) a trust (x) with respect to which a court within the United States is able to exercise primary supervision over its administration and one or more United States persons have the authority to control all of its substantial decisions or (y) that has elected under applicable U.S. Treasury regulations to be treated as a domestic trust for U.S. federal income tax purposes.
131
If an entity treated as a partnership for U.S. federal income tax purposes holds our common shares, the U.S. federal income tax consequences relating to an investment in our common shares will depend in part upon the status and activities of such entity and the particular partner. Any such entity should consult its own tax advisor regarding the U.S. federal income tax consequences applicable to it and its partners of the purchase, ownership and disposition of our common shares. Persons considering an investment in our common shares should consult their own tax advisors as to the particular tax consequences applicable to them relating to the purchase, ownership and disposition of our common shares, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
Passive Foreign Investment Company Consequences
In general, a corporation organized outside the United States will be treated as a passive foreign investment company, or PFIC, for any taxable year in which either (1) at least 75% of its gross income is “passive income”, or the “PFIC income test”, or (2) on average at least 50% of its assets, determined on a quarterly basis, are assets that produce passive income or are held for the production of passive income, the “PFIC asset test”. Passive income for this purpose generally includes, among other things, dividends, interest, royalties, rents, and gains from the sale or exchange of property that gives rise to passive income. Assets that produce or are held for the production of passive income generally include cash, even if held as working capital or raised in a public offering, marketable securities, and other assets that may produce passive income. Generally, in determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
Although we do not believe that we were a PFIC for the year ending December 31, 2021, our determination is based on an interpretation of complex provisions of the law, which are not addressed in a significant number of administrative pronouncements or rulings by the Internal Revenue Service. Accordingly, there can be no assurance that our conclusions regarding our status as a PFIC for the 2021 taxable year will not be challenged by the Internal Revenue Service and, if challenged, upheld in appropriate proceedings. In addition, because PFIC status is determined on an annual basis and generally cannot be determined until the end of the taxable year, there can be no assurance that we will not be a PFIC for the current taxable year. Because we may continue to hold a substantial amount of cash and cash equivalents, and because the calculation of the value of our assets may be based in part on the value of our common shares, which may fluctuate considerably, we may be a PFIC in future taxable years. Even if we determine that we are not a PFIC for a taxable year, there can be no assurance that the IRS will agree with our conclusion and that the IRS would not successfully challenge our position. Our status as a PFIC is a fact-intensive determination made on an annual basis. Accordingly, our U.S. counsel expresses no opinion with respect to our PFIC status and also expresses no opinion with regard to our expectations regarding our PFIC status.
If we are a PFIC in any taxable year during which a U.S. Holder owns our common shares, the U.S. Holder could be liable for additional taxes and interest charges under the “PFIC excess distribution regime” upon (1) a distribution paid during a taxable year that is greater than 125% of the average annual distributions paid in the three preceding taxable years, or, if shorter, the U.S. Holder’s holding period for our common shares, and (2) any gain recognized on a sale, exchange or other disposition, including a pledge, of our common shares, whether or not we continue to be a PFIC. Under the PFIC excess distribution regime, the tax on such distribution or gain would be determined by allocating the distribution or gain ratably over the U.S. Holder’s holding period for our common shares. The amount allocated to the current taxable year (i.e., the year in which the distribution occurs or the gain is recognized) and any year prior to the first taxable year in which we are a PFIC will be taxed as ordinary income earned in the current taxable year. The amount allocated to other taxable years will be taxed at the highest marginal rates in effect for individuals or corporations, as applicable, to ordinary income for each such taxable year, and an interest charge, generally applicable to underpayments of tax, will be added to the tax.
132
If we are a PFIC for any year during which a U.S. Holder holds our common shares, we must generally continue to be treated as a PFIC by that holder for all succeeding years during which the U.S. Holder holds our common shares, unless we cease to meet the requirements for PFIC status and the U.S. Holder makes a “deemed sale” election with respect to our common shares. If the election is made, the U.S. Holder will be deemed to sell our common shares it holds at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain recognized from such deemed sale would be taxed under the PFIC excess distribution regime. After the deemed sale election, the U.S. Holder’s common shares would not be treated as shares of a PFIC unless we subsequently become a PFIC.
If we are a PFIC for any taxable year during which a U.S. Holder holds our common shares and one of our non-U.S. corporate subsidiaries is also a PFIC (i.e., a lower-tier PFIC), such U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC and would be taxed under the PFIC excess distribution regime on distributions by the lower-tier PFIC and on gain from the disposition of shares of the lower-tier PFIC even though such U.S. Holder would not receive the proceeds of those distributions or dispositions.
Each U.S. Holder is advised to consult its tax advisors regarding the application of the PFIC rules to our non-U.S. subsidiaries. If we are a PFIC, a U.S. Holder will not be subject to tax under the PFIC excess distribution regime on distributions or gain recognized on our common shares if such U.S. Holder makes a valid “mark-to-market” election for our common shares. A mark-to-market election is available to a U.S. Holder only for “marketable stock”.
Our common shares will be marketable stock as long as they remain listed on the Nasdaq and are regularly traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. If a mark-to-market election is in effect, a U.S. Holder generally would take into account, as ordinary income each year, the excess of the fair market value of our common shares held at the end of such taxable year over the adjusted tax basis of such common shares. The U.S. Holder would also take into account, as an ordinary loss each year, the excess of the adjusted tax basis of such our common shares over their fair market value at the end of the taxable year, but only to the extent of the excess of amounts previously included in income over ordinary losses deducted as a result of the mark-to-market election. The U.S. Holder’s tax basis in our common shares would be adjusted to reflect any income or loss recognized as a result of the mark-to-market election. Any gain from a sale, exchange or other disposition of our common shares in any taxable year in which we are a PFIC would be treated as ordinary income and any loss from such sale, exchange or other disposition would be treated first as ordinary loss (to the extent of any net mark-to-market gains previously included in income) and thereafter as capital loss.
A mark-to-market election will not apply to our common shares for any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC. Such election will not apply to any non-U.S. subsidiaries that we may organize or acquire in the future. Accordingly, a U.S. Holder may continue to be subject to tax under the PFIC excess distribution regime with respect to any lower-tier PFICs that we may organize or acquire in the future notwithstanding the U.S. Holder’s mark-to-market election for our common shares.
The tax consequences that would apply if we are a PFIC would also be different from those described above if a U.S. Holder were able to make a valid qualified electing fund, or QEF, election. At this time, we do not expect to provide U.S. Holders with the information necessary for a U.S. Holder to make a QEF election, prospective investors should assume that a QEF election will not be available.
Each U.S. person that is an investor of a PFIC is generally required to file an annual information return on IRS Form 8621 containing such information as the U.S. Treasury Department may require. The failure to file IRS Form 8621 could result in the imposition of penalties and the extension of the statute of limitations with respect to U.S. federal income tax.
133
The U.S. federal income tax rules relating to PFICs are very complex. Prospective U.S. investors are strongly urged to consult their own tax advisors with respect to the impact of PFIC status on the purchase, ownership and disposition of our common shares, the consequences to them of an investment in a PFIC, any elections available with respect to our common shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of the common shares of a PFIC.
Distributions
Subject to the discussion above under “Passive Foreign Investment Company Consequences”, a U.S. Holder that receives a distribution with respect to our common shares generally will be required to include the gross amount of such distribution in gross income as a dividend when actually or constructively received to the extent of the U.S. Holder’s pro rata share of our current and/or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent a distribution received by a U.S. Holder is not a dividend because it exceeds the U.S. Holder’s pro rata share of our current and accumulated earnings and profits, it will be treated first as a tax-free return of capital and reduce (but not below zero) the adjusted tax basis of the U.S. Holder’s common shares.
To the extent the distribution exceeds the adjusted tax basis of the U.S. Holder’s common shares, the remainder will be taxed as capital gain. Because we may not account for our earnings and profits in accordance with U.S. federal income tax principles, U.S. Holders should expect all distributions to be reported to them as dividends. Distributions on our common shares that are treated as dividends generally will constitute income from sources outside the United States for foreign tax credit purposes and generally will constitute passive category income. Such dividends will not be eligible for the “dividends received” deduction generally allowed to corporate shareholders with respect to dividends received from U.S. corporations.
Dividends paid by a “qualified foreign corporation” are eligible for taxation for certain non-corporate U.S. Holders at a reduced capital gains rate rather than the marginal tax rates generally applicable to ordinary income provided that certain requirements are met. However, if we are a PFIC for the taxable year in which the dividend is paid or the preceding taxable year (see discussion above under “Passive Foreign Investment Company Consequences”), we will not be treated as a qualified foreign corporation, and therefore the reduced capital gains tax rate described above will not apply. Each U.S. Holder is advised to consult its tax advisors regarding the availability of the reduced tax rate on dividends with regard to its particular circumstances.
A non-United States corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (a) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information provision, or (b) with respect to any dividend it pays on our common shares that are readily tradable on an established securities market in the United States. We believe that we qualify as a resident of Canada for purposes of, and are eligible for the benefits of, the U.S.-Canada Treaty, although there can be no assurance in this regard. Further, the IRS has determined that the U.S.-Canada Treaty is satisfactory for purposes of the qualified dividend rules and that it includes an exchange of information provision. Therefore, subject to the discussion above under “Passive Foreign Investment Company Consequences”, if the U.S.-Canada Treaty is applicable, such dividends will generally be “qualified dividend income” in the hands of individual U.S. Holders, provided that certain conditions are met, including holding period and the absence of certain risk reduction transactions.
134
Sale, Exchange or Other Disposition of our common shares
Subject to the discussion above under “Passive Foreign Investment Company Consequences”, a U.S. Holder generally will recognize capital gain or loss for U.S. federal income tax purposes upon the sale, exchange or other disposition of our common shares in an amount equal to the difference, if any, between the amount realized (i.e., the amount of cash plus the fair market value of any property received) on the sale, exchange or other disposition and such U.S. Holder’s adjusted tax basis in our common shares. Such capital gain or loss generally will be long-term capital gain taxable at a reduced rate for noncorporate U.S. Holders or long-term capital loss if, on the date of sale, exchange or other disposition, our common shares were held by the U.S. Holder for more than one year. Any capital gain of a non-corporate U.S. Holder that is not long-term capital gain is taxed at ordinary income rates. The deductibility of capital losses is subject to limitations. Any gain or loss recognized from the sale or other disposition of our common shares will generally be gain or loss from sources within the United States for U.S. foreign tax credit purposes.
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts and whose income exceeds certain thresholds generally are subject to a 3.8% tax on all or a portion of their net investment income, which may include their gross dividend income and net gains from the disposition of our common shares. If you are a United States person that is an individual, estate or trust, you are encouraged to consult your tax advisors regarding the applicability of this Medicare tax to your income and gains in respect of your investment in our common shares.
Information Reporting and Backup Withholding
U.S. Holders may be required to file certain U.S. information reporting returns with the IRS with respect to an investment in our common shares, including, among others, IRS Form 8938 (Statement of Specified Foreign Financial Assets). As described above under “Passive Foreign Investment Company Consequences”, each U.S. Holder who is a shareholder of a PFIC must file an annual report containing certain information. U.S. Holders paying more than US$100,000 for our common shares may be required to file IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) reporting this payment. Substantial penalties may be imposed upon a U.S. Holder that fails to comply with the required information reporting.
Dividends on and proceeds from the sale or other disposition of our common shares may be reported to the IRS unless the U.S. Holder establishes a basis for exemption. Backup withholding may apply to amounts subject to reporting if the holder (1) fails to provide an accurate United States taxpayer identification number or otherwise establish a basis for exemption, or (2) is described in certain other categories of persons. However, U.S. Holders that are corporations generally are excluded from these information reporting and backup withholding tax rules. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules generally will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
U.S. Holders should consult their own tax advisors regarding the backup withholding tax and information reporting rules.
135
Certain Canadian Federal Income Tax Considerations
The following summary describes, as of the date hereof, the material Canadian federal income tax considerations generally applicable to shareholders who are a beneficial owner of our common shares and who, at all relevant times, for the purposes of the application of the Income Tax Act (Canada) and the Income Tax Regulations (collectively, the “Canadian Tax Act”), (1) is not, and is not deemed to be, resident in Canada for purposes of the Canadian Tax Act and any applicable income tax treaty or convention; (2) deals at arm’s length with us; (3) is not affiliated with us; (4) does not use or hold, and is not deemed to use or hold, common shares in a business or part of a business carried on in Canada; (5) has not entered into, with respect to the common shares, a “derivative forward agreement”, as that term is defined in the Canadian Tax Act and (6) holds the common shares as capital property (a “Non-Canadian Holder”). This summary does not apply to a Non-Canadian Holder that is an insurer carrying on an insurance business in Canada and elsewhere or an “authorized foreign bank”, as that term is defined in the Canadian Tax Act. Such Non-Canadian Holders should consult their tax advisors for advice having regards to their particular circumstances.
This summary is based on the current provisions of the Canadian Tax Act, and an understanding of the current administrative policies of the Canada Revenue Agency published in writing prior to the date hereof. It takes into account all specific proposals to amend the Canadian Tax Act and the Canada-United States Tax Convention (1980), as amended (the “Canada-U.S. Tax Treaty”), publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Proposed Amendments”) and assumes that all Proposed Amendments will be enacted in the form proposed. However, no assurances can be given that the Proposed Amendments will be enacted as proposed, or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative policy or assessing practice whether by legislative, regulatory, administrative or judicial action nor does it take into account tax legislation or considerations of any province, territory or foreign jurisdiction, which may differ from those discussed herein.
This summary is of a general nature only and is not, and is not intended to be, legal or tax advice to any particular shareholder, and no representations with respect to the income tax consequences to any particular shareholder are made. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, you should consult your own tax advisor with respect to your particular circumstances.
Generally, for purposes of the Canadian Tax Act, all amounts relating to the acquisition, holding or disposition of the common shares must be converted into Canadian dollars based on the exchange rates as determined in accordance with the Canadian Tax Act. The amount of any dividends, capital gains or capital losses realized by a Non-Canadian Holder may be affected by fluctuations in the Canadian exchange rate.
Dividends
Dividends paid or credited on the common shares or deemed to be paid or credited on the common shares to a Non-Canadian Holder will be subject to Canadian withholding tax at the rate of 25%, subject to any reduction in the rate of withholding to which the Non-Canadian Holder is entitled under any applicable income tax treaty or convention between Canada and the country in which the Non-Canadian Holder is resident. For example, under the Canada-U.S. Tax Treaty, where dividends on the common shares are considered to be paid to or derived by a Non-Canadian Holder that is a beneficial owner of the dividends and is a U.S. resident for the purposes of, and is entitled to benefits of, the Canada-U.S. Tax Treaty, the applicable rate of Canadian withholding tax is generally reduced to 15%. We will be required to withhold the applicable withholding tax from any dividend and remit it to the Canadian government for the Non-Canadian Holder’s account.
136
Dispositions
A Non-Canadian Holder will not be subject to tax under the Canadian Tax Act on any capital gain realized on a disposition or deemed disposition of a common share, unless the common shares are “taxable Canadian property” to the Non-Canadian Holder for purposes of the Canadian Tax Act at the time of disposition and the Non-Canadian Holder is not entitled to relief under an applicable income tax treaty or convention between Canada and the country in which the Non-Canadian Holder is resident.
Generally, the common shares will not constitute “taxable Canadian property” to a Non-Canadian Holder at a particular time provided that the common shares are listed at that time on a “designated stock exchange” (as defined in the Canadian Tax Act), which includes the TSX and the Nasdaq, unless at any particular time during the 60-month period that ends at that time.
•at least 25% of the issued shares of any class or series of our capital stock was owned by or belonged to any combination of (a) the Non-Canadian Holder, (b) persons with whom the Non-Canadian Holder does not deal at arm’s length, and (c) partnerships in which the Non-Canadian Holder or a person described in (b) holds a membership interest directly or indirectly through one or more partnerships, and
•more than 50% of the fair market value of the common shares was derived, directly or indirectly, from one or any combination of: (i) real or immoveable property situated in Canada, (ii) “Canadian resource properties” (as that term is defined in the Canadian Tax Act), (iii) “timber resource properties” (as that term is defined in the Canadian Tax Act) and (iv) options in respect of, or interests in, or for civil law rights in, property in any of the foregoing whether or not the property exists.
Notwithstanding the foregoing, in certain circumstances, common shares could be deemed to be “taxable Canadian property.” Non-Canadian Holders whose common shares may constitute “taxable Canadian property” should consult their own tax advisors.
F. Dividends and Paying Agents.
Not applicable.
G. Statement by Experts.
Not applicable.
137
H. Documents on Display.
We are subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers and under those requirements will file reports with the SEC. Those reports may be inspected without charge at the locations described below. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. Nevertheless, we will file with the SEC an Annual Report on Form 20-F containing financial statements that have been examined and reported on, with and opinion expressed by an independent registered public accounting firm.
We maintain a corporate website at www.liminalbiosciences.com. We intend to post our Annual Report on Form 20-F on our website promptly following it being filed with the SEC. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report. We have included our website address in this Annual Report solely as an inactive textual reference.
The SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements and other information regarding registrants, such as us, that file electronically with the SEC.
With respect to references made in this Annual Report to any contract or other document of our company, such references are not necessarily complete and you should refer to the exhibits attached or incorporated by reference to this Annual Report for copies of the actual contract or document.
I. Subsidiary Information
Not required.
Item 11. Quantitative and Qualitative Disclosures About Market Risk.
Financial risk management
We have exposure to credit risk, liquidity risk and market risk. Our Board of Directors has the overall responsibility for the oversight of these risks and reviews our policies on an ongoing basis to ensure that these risks are appropriately managed.
i) Credit risk:
Credit risk is the risk of financial loss to our company if a customer, partner or counterparty to a financial instrument fails to meet its contractual obligations, and arises principally from the Company’s cash and receivables. The carrying amount of the financial assets represents the maximum credit exposure.
Our exposure to credit risk is generally limited since we have limited revenues and thus limited accounts receivable. We mitigate credit risk through a credit risk assessment, when credit is granted and subsequently at each reporting period.
ii) Liquidity risk:
Liquidity risk is the risk that we will not be able to meet financial obligations as they come due. We manage our liquidity risk by continuously monitoring forecasts and actual cash flows. Our current liquidity situation is discussed in the liquidity and contractual obligation section of this MD&A.
138
iii) Market risk:
Market risk is the risk that changes in market prices, such as interest rates and foreign exchange rates, will affect our income or the value of its financial instruments.
a) Interest risk:
Our interest-bearing financial liabilities have fixed rates and as such there is limited exposure to changes in interest payments as a result of interest rate risk. In February 2022, our loans were repaid in full.
b) Foreign exchange risk:
We are exposed to the financial risk related to the fluctuation of foreign exchange rates. We have had operations and suppliers in the U.S. and the U.K. during the past years and therefore a portion of our expenses are in USD and in GBP. The majority of the revenues from the sale of products in 2021 and 2020, that are part of its discontinued operations were in USD which served to mitigate a portion of the U.S. foreign exchange risk relating to the expenditures. In 2021, the proceeds received from the divestment of our discontinued operations were in USD resulting in an increased exposure to the USD which is partially mitigated by expenditures denominated in USD from our continuing operations. Financial instruments that have exposed us to foreign exchange risk have been cash, receivables, trade and other payables, lease liabilities, license payment obligations. We manage foreign exchange risk by holding foreign currencies we receive to support forecasted cash outflows in foreign currencies.
Item 12. Description of Securities Other than Equity Securities.
A. Debt Securities.
Not applicable.
B. Warrants and Rights.
Not applicable.
C. Other Securities.
Not applicable.
D. American Depositary Shares.
Not applicable.
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies.
Not applicable.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds.
Not applicable.
139
Item 15. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our chief executive officer and chief financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Exchange Act) as of December 31, 2022, have concluded that, as of such date, our disclosure controls and procedures were not effective due to a material weakness in internal control over financial reporting, as described below.
Our disclosure controls and procedures are designed to provide reasonable assurance of achieving the desired control objectives. Our management recognizes that any control system, no matter how well designed and operated, is based upon certain judgments and assumptions and cannot provide absolute assurance that its objectives will be met. Similarly, an evaluation of controls cannot provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal controls over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) and for the assessment of the effectiveness of our internal control over financial reporting. Under the supervision and with the participation of our chief executive officer (principal executive officer) and our chief financial officer (principal financial officer), management assessed our internal control over financial reporting based upon the framework in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this assessment, our management concluded that, as of December 31, 2022, our internal control over financial reporting was not effective because a material weakness in internal control over financial reporting existed as of that date, as described below.
Material Weakness in Internal Control over Financial Reporting
In connection with the preparation of our consolidated financial statements as of and for the year ended December 31, 2022, we identified a material weakness in our internal control over financial reporting relating to the accounting for complex transactions. In our interim financial statements for the period ended March 31, 2022, June 30, 2022 and September 30, 2022, we incorrectly allocated net income to non-controlling shareholders. As a result, net loss attributable to the non-controlling shareholders was overstated with offsetting misclassifications between non-controlling interest and shareholders' deficit. The error did not result in an adjustment to previously reported net income or loss per share in any prior fiscal year.
This deficiency in internal controls relates to our controls regarding the accounting analysis of complex transactions, which was in the process of being remediated as disclosed in the 2021 Annual Report (as defined below). In this particular instance, we acquired the non-controlling interests of our subsidiary, Pathogen Removal Diagnostic Technologies Inc. This acquisition was not in the ordinary course of business and required significant analysis and research by the Finance team. Due to the then-complex structure of the Finance team, the accounting analysis for this transaction was incomplete and did not go through an exhaustive internal review. As shown in the Summary of Consolidated Quarterly Results contained in Item 5A of this Annual Report, management and our audit committee concluded that it was appropriate to restate our previously issued financial results for the periods ended March 31, 2022, June 30, 2022 and September 30, 2022.
140
As previously disclosed, during the preparation of our consolidated financial statements for the year ended December 31, 2021, management identified a material weakness in our internal control over financial reporting related to the carrying value of our held-for-sale assets at September 30, 2021 and the net loss from discontinued operations for the nine months ended September 31, 2021. As disclosed in Item 15 of the Company’s Annual Report on Form 20-F for the year ended December 31, 2021 (the “2021 Annual Report”), the financial statements for the period ended September 30, 2021 contained a misstatement in the carrying value of the held-for-sale assets relating to the sale of PBT, a transaction that was not in the ordinary course of business and required significant analysis and research by the Finance team, in addition to the extra workload required for presenting the divested or soon to be divested subsidiaries as discontinued operations. This situation, coupled with the fact that the third quarter financial reporting process was conducted without a CFO from September 3, 2021, exacerbated the resource challenge within the Finance team. Consequently, the accounting analysis for this complex transaction was incomplete and did not go through an exhaustive internal review. Accordingly, as shown in the Summary of Consolidated Quarterly Results contained in Item 5A of the 2021 Annual Report, management and our audit committee concluded that it was appropriate to restate our previously issued financial results for the period ended September 30, 2021.
Remediation Plan
Our remediation plan initially included reviewing our quarterly and year-end close timetables to ensure any subsequent complex accounting matters were given priority and allocated the appropriate resources. In addition, we hired a CFO to lead and build out the Finance team’s expertise and bandwidth. During the fourth quarter of fiscal 2022, our CFO restructured the Finance team to further optimize and simplify the reporting structure, which is expected to improve operating and reporting efficiencies. Management believes the Finance team is now better structured and more aligned with the Company's current operations and believes the current control environment is better suited for the size of the Company and for accounting and financial reporting practices going forward.
Attestation Report of the Registered Public Accounting Firm
Not applicable to emerging growth companies.
Changes in Internal Control Over Financial Reporting
Except as described above under Material Weakness in Internal Controls over Financial Reporting and under remediation Plan, there were no changes in our internal control over financial reporting that occurred during the period covered by this annual report that have materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting.
Item 16. Reserved.
Not applicable.
Item 16A. Audit Committee Financial Expert.
Our board of directors has determined that all the members of our audit, risk and finance committee qualify as “audit committee financial experts” as defined by SEC rules and have the requisite financial sophistication under the applicable rules and regulations of the Nasdaq. All of the members of the audit, risk and finance committee are independent as such term is defined in Rule 10A-3 under the Exchange Act and under the listing standards of the Nasdaq Capital Market.
Item 16B. Code of Ethics.
We have adopted a Code of Ethics and Business Conduct, or the Code of Conduct, that is applicable to all of our employees, executive officers and directors. A copy of the Code of Conduct is available on our website at www.liminalbiosciences.com.
141
Item 16C. Principal Accountant Fees and Services.
PricewaterhouseCoopers LLP, from Oakville, Canada, (PCAOB identification #271) has served as our independent registered public accounting firm for the 2022 and 2021 under PCAOB standards.
Our accountant billed the following fees to us for professional services in each of those fiscal years:
| | | | | | | | |
| | Year Ended December 31, | |
| | (thousands of Canadian dollars) | |
| | 2022 | 2021 | |
Audit Fees | | $ | 455,000 | | | $ | 647,719 | |
Audit-Related Fees | | | 31,150 | | | | 131,066 | |
Tax Fees | | | — | | | | — | |
Other Fees | | | — | | | | 39,900 | |
Total | | $ | 486,150 | | | $ | 818,685 | |
“Audit Fees” are the aggregate fees billed for the audit of our annual financial statements. This category also includes services that only our independent external auditor can reasonably provide, such as consents and assistance with and review of documents filed with the SEC.
“Audit-Related Fees” are the aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit and are not reported under Audit Fees.
“All Tax Fees” consist of tax consultations, such as advice in connection with employees’ taxation arising from share-based compensation.
“Other Fees” consist of advisory services relating to the adoption or application of IFRS and translation services.
Audit and Non-Audit Services Pre-Approval Policy
To ensure the independence and objectivity of our external auditors, the provision of all non-audit services by the external auditors are pre-approved by our audit, risk and finance committee, other than non-audit services: (i) that were not recognized as non-audit services at the time of the engagement, (ii) that are promptly brought to the attention of our audit, risk and finance committee and approved, prior to the completion of the audit, by our audit, risk and finance committee or by one or more of its members to whom authority to grant such approvals has been delegated by the audit, risk and finance committee, and (iii) the aggregate amount of which is reasonably expected to constitute no more than 5% of the total amount of fees paid by us to out external auditor during the fiscal year in which the services are provided.
Item 16D. Exemptions from the Listing Standards for Audit Committees.
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers.
Not applicable.
Item 16F. Change in Registrant’s Certifying Accountant.
Not applicable.
142
Item 16G. Corporate Governance.
As a “foreign private issuer,” as defined by the SEC, we are permitted to follow home country corporate governance practices, instead of certain corporate governance practices required by the Nasdaq for U.S. domestic issuers. While we intend to follow most Nasdaq corporate governance listing standards, we intend to follow Canadian corporate governance practices of in lieu of Nasdaq corporate governance listing standards as follows:
•Exemption from quorum requirements applicable to meetings of shareholders. Such quorum requirements are not required under Canadian law. In accordance with generally accepted business practice, our by-laws provide alternative quorum requirements that are generally applicable to meetings of shareholders.
•Exemption from the requirement to obtain shareholder approval for certain issuances of securities pursuant to Nasdaq Rule 5635, including in connection with an acquisition of the stock or assets of another company.
•Exemption from requirement to adopt a formal written audit committee charter specifying the items enumerated in Nasdaq Rule 5605(c)(1). Under Nasdaq Rule 5605(c)(1), an audit committee must be directly responsible for the appointment, compensation, retention and oversight of the work of any registered public accounting firm engaged. Our audit committee charter provides that the auditors be appointed by, and their compensation be approved by, our board of directors and be ratified by our shareholders, as required under Canadian law.
Although we currently intend to comply with the Nasdaq corporate governance rules applicable other than as noted above, we may in the future decide to use the foreign private issuer exemption with respect to some or all the other Nasdaq corporate governance rules.
We intend to take all actions necessary for us to maintain compliance as a foreign private issuer under the applicable corporate governance requirements of the Sarbanes-Oxley Act, the rules adopted by the SEC and the Nasdaq corporate governance rules and listing standards.
As a foreign private issuer, our directors and senior management are not subject to short-swing profit and insider trading reporting obligations under Section 16 of the Exchange Act. They will, however, be subject to the obligations to report changes in share ownership under Section 13 of the Exchange Act and related SEC rules.
Item 16H. Mine Safety Disclosure.
Not applicable.
Item 16I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
PART III
Item 17. Financial Statements.
See pages F-1 through F-56 of this Annual Report on Form 20-F.
Item 18. Financial Statements.
Not applicable.
143
Item 19. Exhibits.
| | | | | | | | | | |
| | | | Incorporated by Reference |
Exhibit | | Description | | Schedule/ Form | | File Number | | Exhibit | | File Date |
1.1 | | Articles of Incorporation of Liminal BioSciences Inc., as amended. | | Form S-8 | | 333-235692 | | 4.1 | | 12/23/2019 |
1.2 | | Certificate of Amendment of Liminal BioSciences Inc. dated October 12, 2021 changing its Registered Office Address | | Form 6-K | | 001-39131 | | 99.1 | | 10/13/2021 |
1.3 | | Certificate of Amendment of Liminal BioSciences Inc. dated January 25, 2023 | | Form 6-K | | 001-39131 | | 99.1 | | 02/02/2023 |
1.4 | | By-Law No. 1 of Liminal BioSciences Inc., as amended and currently in effect. | | Form S-8 | | 333-235692 | | 4.2 | | 12/23/2019 |
1.5 | | By-Law No. 2 of Liminal BioSciences Inc., as currently in effect. | | Form S-8 | | 333-235692 | | 4.3 | | 12/23/2019 |
1.6 | | By-Law No. 3 of Liminal BioSciences Inc., as currently in effect. | | Form S-8 | | 333-235692 | | 4.4 | | 12/23/2019 |
2.1* | | Description of Securities | | | | | | | | |
4.1† | | Amended and Restated Stock Option Plan, dated May 10, 2017 | | Form 40-F | | 001-39131 | | 99.59 | | 11/12/2019 |
4.2† | | Amended and Restated Stock Option Plan, dated June 7, 2018 | | Form 40-F | | 001-39131 | | 99.61 | | 11/12/2019 |
4.3† | | Restricted Share Unit Plan, dated May 6, 2009 | | Form 40-F | | 001-39131 | | 99.60 | | 11/12/2019 |
4.4† | | Restricted Share Unit Plan, dated May 9, 2018 | | Form 40-F | | 001-39131 | | 99.62 | | 11/12/2019 |
4.5† | | Omnibus Incentive Plan, dated May 7, 2019 | | Form 40-F | | 001-39131 | | 99.125 | | 11/12/2019 |
4.6 | | Restructuring Agreement between Structured Alpha LP, Prometic Life Sciences Inc., Prometic Biotherapeutics Inc., Prometic Bioseparations Ltd, Prometic Biosciences Inc., Prometic Bioproduction Inc., NantPro Biosciences, LLC, Prometic Plasma Resources Inc., Prometic Pharma SMT Holdings Limited, Prometic Pharma SMT Limited, Prometic Biotherapeutics Ltd, Telesta Therapeutics Inc. and Prometic Plasma Resources (USA) Inc., dated April 15, 2019 | | Form 40-F | | 001-39131 | | 99.70 | | 11/12/2019 |
4.7 | | Private Placement Subscription Agreement, dated April 15, 2019 | | Form 40-F | | 001-39131 | | 99.71 | | 11/12/2019 |
4.8 | | Private Placement Subscription Agreement, dated April 15, 2019 | | Form 40-F | | 001-39131 | | 99.67 | | 11/12/2019 |
4.9 | | Share Purchase Agreement, among Liminal BioSciences Inc. and Gamma Biosciences GP LLC, dated November 3, 2019 | | Form 6-K | | 001-39131 | | 99.1 | | 11/18/2019 |
4.10# | | Share Purchase Agreement, dated as of July 17, 2020, among the Registrant and the investors listed therein. | | Form F-1/A | | 001-39131 | | 4.6 | | 09/14/2020 |
4.11 | | First Amending Agreement dated November 10, 2021 to the Share Purchase Agreement dated July 17, 2020, among Liminal BioSciences Inc. and the investors listed therein | | Form 6-K | | 001-39131 | | 99.1 | | 11/10/2021 |
4.12# | | Amended and Restated License Agreement between Fairhaven Pharmaceuticals Inc., The Royal Institution of the Advancement of Learning/McGill University and Florida Institute of Technology, Inc., dated May 16, 2018 | | Form 20-F | | 001-39131 | | 4.36 | | 03/24/2021 |
4.13 | | Securities Purchase Agreement dated October 29, 2020, by and among the Company and the Purchasers | | Form 6-K | | 001-39131 | | 99.1 | | 11/02/2020 |
| | | | | | | | | | |
4.14 | | Amendment No.1 to Securities Purchase Agreement dated November 25, 2020, by and among the company and the Purchasers | | Form 6-K | | 001-39131 | | 99.1 | | 11/27/2020 |
4.15 | | Registration Rights Agreement, dated October 29, 2020, by and among the Company and the Purchasers | | Form 6-K | | 001-39131 | | 99.2 | | 11/02/2020 |
4.16 | | Form of Warrant to Purchase Common Shares | | Form 6-K | | 001-39131 | | 99.3 | | 11/02/2020 |
4.17# | | Share Purchase Agreement between Liminal BioSciences Inc. and Kedrion S.p.A. dated May 14, 2021 | | Form 6-K | | 001-39131 | | 99.2 | | 05/21/2021 |
4.18# | | Share Purchase Agreement between Liminal BioSciences Inc. and Kedrion S.p.A. dated June 22, 2021 | | Form 6-K | | 001-39131 | | 99.2 | | 06/25/2021 |
4.19# | | First Amending Agreement dated September 29, 2021 to the Share Purchase Agreement between Liminal BioSciences Inc. and Kedrion S.p.A. dated June 22, 2021 | | Form 6-K | | 001-39131 | | 99.1 | | 10/04/2021 |
4.20# | | Royalty Stream Agreement between Liminal BioSciences Inc. and Innovon Pharmaceutiques Inc. dated January 26, 2022 | | Form 20-F | | 001-39131 | | 4.20 | | 03/18/2022 |
8.1* | | List of subsidiaries of the Registrant | | | | | | | | |
12.1* | | Certification by the Principal Executive Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | | | | | | | |
12.2* | | Certification by the Principal Financial Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | | | | | | | |
13.1** | | Certification by the Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | | | | | | | |
13.2** | | Certification by the Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | | | | | | | |
15.1* | | Consent of PricewaterhouseCoopers LLP | | | | | | | | |
101.INS* | | Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | | | | | | | | |
101.SCH* | | Inline XBRL Taxonomy Extension Schema Document | | | | | | | | |
101.CAL* | | Inline XBRL Taxonomy Extension Calculation Linkbase Document | | | | | | | | |
101.LAB* | | Inline XBRL Taxonomy Extension Label Linkbase Document | | | | | | | | |
101.PRE* | | Inline XBRL Taxonomy Extension Presentation Linkbase Document | | | | | | | | |
101.DEF* | | Inline XBRL Taxonomy Extension Definition Linkbase Document | | | | | | | | |
104 | | The cover page for the Company’s Annual Report on Form 20-F for the year ended December 31, 2022, has been formatted in Inline XBRL and contained in Exhibit 101 | | | | | | | | |
| | |
* | Filed herewith. |
** | Furnished herewith. |
† | Indicates a management contract or any compensatory plan, contract or arrangement. |
# | Certain portions of this exhibit (indicated by asterisks) have been omitted because they are not material and would likely cause competitive harm to Liminal BioSciences Inc. if publicly disclosed. |

Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Liminal BioSciences Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of financial position of Liminal BioSciences Inc. and its subsidiaries (together, the Company) as of December 31, 2022 and 2021, and the related consolidated statements of operations, statements of comprehensive income (loss), statements of changes in equity, and statements of cash flows for each of the three years in the period ended December 31, 2022, including the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and its financial performance and its cash flows for each of the three years in the period ended December 31, 2022 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Substantial Doubt About the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from continuing operations and has a net deficit, and negative cash outflows from operating activities, and has stated that these events or conditions indicate that a material uncertainty exists that may cast substantial doubt on the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
PricewaterhouseCoopers LLP
PwC Centre, 354 Davis Road, Suite 600, Oakville, Ontario, Canada L6J 0C5
T: +1 905 815 6300, F: +1 905 815 6499
“PwC” refers to PricewaterhouseCoopers LLP, an Ontario limited liability partnership.
F-1

Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/PricewaterhouseCoopers LLP
Chartered Professional Accountants, Licensed Public Accountants
|
Oakville, Canada March 14, 2023 |
We have served as the Company’s auditor since 2019.
F-2
LIMINAL BIOSCIENCES INC.
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
(In thousands of Canadian dollars)
| | | | | | | | |
| | December 31, | | | December 31, | |
| | 2022 | | | 2021 | |
| | | | | | |
ASSETS | | | | | | |
Current assets | | | | | | |
Cash and cash equivalents | | $ | 37,144 | | | $ | 108,490 | |
Accounts receivable | | | 1,177 | | | | 1,068 | |
Prepaids | | | 2,997 | | | | 5,071 | |
Total current assets | | | 41,318 | | | | 114,629 | |
| | | | | | |
Other long-term assets (note 8) | | | 243 | | | | 362 | |
Capital assets (note 9) | | | 4,344 | | | | 5,483 | |
Right-of-use assets (note 10) | | | 1,146 | | | | 1,609 | |
Intangible assets (note 11) | | | 3,240 | | | | 3,516 | |
Deferred tax assets (note 24) | | | 168 | | | | 454 | |
Total assets | | $ | 50,459 | | | $ | 126,053 | |
| | | | | | |
LIABILITIES | | | | | | |
Current liabilities | | | | | | |
Accounts payable and accrued liabilities (note 12) | | $ | 5,968 | | | $ | 7,343 | |
Current portion of lease liabilities (note 13) | | | 735 | | | | 7,194 | |
Current portion of provision (note 14) | | | 3,400 | | | | 3,957 | |
Total current liabilities | | | 10,103 | | | | 18,494 | |
| | | | | | |
Long-term portion of lease liabilities (note 13) | | | 752 | | | | 15,277 | |
Long-term portion of provision (note 14) | | | 3,290 | | | | 18,238 | |
Warrant liability (note 15) | | | 106 | | | | 1,754 | |
Long-term debt (note 16) | | | — | | | | 38,311 | |
Other long-term liabilities | | | — | | | | 98 | |
Total liabilities | | $ | 14,251 | | | $ | 92,172 | |
| | | | | | |
EQUITY | | | | | | |
Share capital (note 17a) | | $ | 979,849 | | | $ | 979,849 | |
Contributed surplus (note 17b) | | | 45,973 | | | | 44,109 | |
Warrants (note 17c) | | | 95,856 | | | | 95,856 | |
Accumulated other comprehensive loss | | | (3,169 | ) | | | (3,010 | ) |
Deficit | | | (1,082,301 | ) | | | (1,074,167 | ) |
Equity attributable to owners of the parent | | | 36,208 | | | | 42,637 | |
Non-controlling interests (note 18) | | | — | | | | (8,756 | ) |
Total equity | | $ | 36,208 | | | $ | 33,881 | |
Total liabilities and equity | | $ | 50,459 | | | $ | 126,053 | |
Going concern (note 1), Commitments (note 28), Subsequent event (note 31)
The accompanying notes are an integral part of the consolidated financial statements.
F-3
LIMINAL BIOSCIENCES INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands of Canadian dollars except for per share amounts)
| | | | | | | | | | | | |
Year ended December 31 | | | 2022 | | | 2021 | | | 2020 | |
Revenues | | $ | 401 | | | $ | 643 | | | $ | 724 | |
| | | | | | | | | |
Expenses | | | | | | | | | |
Research and development expenses | | | 15,298 | | | | 18,347 | | | | 14,234 | |
Administration expenses | | | 17,866 | | | | 31,928 | | | | 32,619 | |
Gain on foreign exchange | | | (2,964 | ) | | | (1,397 | ) | | | (35 | ) |
Finance costs (note 21) | | | 1,078 | | | | 6,330 | | | | 2,899 | |
Loss (gain) on extinguishment of liabilities (note 16) | | | 212 | | | | (75 | ) | | | — | |
Change in fair value of financial instruments
measured at fair value through
profit or loss (note 15) | | | (1,648 | ) | | | (9,886 | ) | | | (850 | ) |
Impairment losses (note 23) | | | — | | | | 341 | | | | 1,087 | |
Loss from continuing operations
before income taxes | | $ | (29,441 | ) | | $ | (44,945 | ) | | $ | (49,230 | ) |
| | | | | | | | | |
Current income tax | | $ | (811 | ) | | $ | — | | | $ | (144 | ) |
Deferred income tax | | | 286 | | | | 118 | | | | (65 | ) |
Income tax expense (recovery) on continuing
operations (note 24) | | | (525 | ) | | | 118 | | | | (209 | ) |
Net loss from continuing operations | | $ | (28,916 | ) | | $ | (45,063 | ) | | $ | (49,021 | ) |
| | | | | | | | | |
Discontinued operations | | | | | | | | | |
Gain (loss) on sale of discontinued operations,
net of income taxes $nil (note 6) | | | (600 | ) | | | 140,403 | | | | 3,380 | |
Income (loss) from discontinued operations,
net of taxes (note 6) | | | 30,138 | | | | (83,127 | ) | | | (73,116 | ) |
Total income (loss) from discontinued operations | | | 29,538 | | | | 57,276 | | | | (69,736 | ) |
Net income (loss) | | $ | 622 | | | $ | 12,213 | | | $ | (118,757 | ) |
| | | | | | | | | |
Net income (loss) attributable to: | | | | | | | | | |
Non-controlling interests in
continuing operations (note 18) | | $ | 122 | | | $ | (669 | ) | | $ | (832 | ) |
Owners of the parent | | | | | | | | | |
- Continuing operations | | | (29,038 | ) | | | (44,394 | ) | | | (48,189 | ) |
- Discontinued operations | | | 29,538 | | | | 57,276 | | | | (69,736 | ) |
| | $ | 500 | | | $ | 12,882 | | | $ | (117,925 | ) |
Net income | | $ | 622 | | | $ | 12,213 | | | $ | (118,757 | ) |
| | | | | | | | | |
Income (loss) per share attributable to
the owners of the parent
basic and diluted: | | | | | | | | | |
From continuing operations | | $ | (9.36 | ) | | $ | (14.72 | ) | | $ | (19.72 | ) |
From discontinued operations | | | 9.52 | | | | 18.99 | | | | (28.53 | ) |
Total income per share | | $ | 0.16 | | | $ | 4.27 | | | $ | (48.25 | ) |
Weighted average number of outstanding shares
(in thousands) | | | 3,104 | | | | 3,016 | | | | 2,444 | |
The accompanying notes are an integral part of the consolidated financial statements.
F-4
LIMINAL BIOSCIENCES INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(In thousands of Canadian dollars except for per share amounts)
| | | | | | | | | | | | |
Year ended December 31 | | | 2022 | | | 2021 | | | 2020 | |
Net income (loss) | | $ | 622 | | | $ | 12,213 | | | $ | (118,757 | ) |
| | | | | | | | | |
Other comprehensive income (loss) | | | | | | | | | |
Items that may be subsequently reclassified
to profit and loss: | | | | | | | | | |
Exchange differences on translation of
foreign operations from
continuing operations | | | (159 | ) | | | 20 | | | | 104 | |
Exchange differences on translation of
foreign operations from
discontinued operations | | | — | | | | (140 | ) | | | 149 | |
Reclassification of exchange differences on
translation of foreign operations sold to
consolidated statement of operations (note 6) | | | — | | | | (44 | ) | | | — | |
Total other comprehensive income (loss) | | $ | (159 | ) | | $ | (164 | ) | | $ | 253 | |
Total comprehensive income (loss) | | $ | 463 | | | $ | 12,049 | | | $ | (118,504 | ) |
| | | | | | | | | |
Total comprehensive income (loss)
attributable to: | | | | | | | | | |
Non-controlling interests | | $ | 122 | | | $ | (669 | ) | | $ | (832 | ) |
Owners of the parent | | | | | | | | | |
- Continuing operations | | | (29,197 | ) | | | (44,374 | ) | | | (48,085 | ) |
- Discontinued operations | | | 29,538 | | | | 57,092 | | | | (69,587 | ) |
Total comprehensive income (loss) | | $ | 463 | | | $ | 12,049 | | | $ | (118,504 | ) |
The accompanying notes are an integral part of the consolidated financial statements.
F-5
LIMINAL BIOSCIENCES INC.
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
(In thousands of Canadian dollars)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Equity (deficiency) attributable to owners of the parent | | | | | | | |
| | Share
capital | | | Contributed
surplus | | | Warrants | | | Foreign
currency
translation
reserve | | | Deficit | | | Total | | | Non-
controlling
interests | | | Total equity | |
| | $ | | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | |
Balance at January 1, 2020 | | | 932,951 | | | | 43,532 | | | | 95,856 | | | | (3,099 | ) | | | (967,051 | ) | | | 102,189 | | | | (7,255 | ) | | | 94,934 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (117,925 | ) | | | (117,925 | ) | | | (832 | ) | | | (118,757 | ) |
Foreign currency translation reserve | | | — | | | | — | | | | — | | | | 253 | | | | — | | | | 253 | | | | — | | | | 253 | |
Issuance of shares (note 17a) | | | 31,755 | | | | — | | | | — | | | | — | | | | — | | | | 31,755 | | | | — | | | | 31,755 | |
Share-based payments expense (note 17b) | | | — | | | | 6,234 | | | | — | | | | — | | | | — | | | | 6,234 | | | | — | | | | 6,234 | |
Exercise of stock options (note 17b) | | | 167 | | | | (85 | ) | | | — | | | | — | | | | — | | | | 82 | | | | — | | | | 82 | |
Shares issued pursuant to restricted share units
plan (note 17b) | | | 9,764 | | | | (9,764 | ) | | | — | | | | — | | | | — | | | | — | | | | | | | — | |
Share-based compensation paid in cash
(note 17b) | | | — | | | | (40 | ) | | | — | | | | — | | | | — | | | | (40 | ) | | | — | | | | (40 | ) |
Issuance of warrants (note 17c) | | | — | | | | — | | | | 2,623 | | | | — | | | | — | | | | 2,623 | | | | — | | | | 2,623 | |
Share issuance cost | | | — | | | | — | | | | — | | | | — | | | | (2,073 | ) | | | (2,073 | ) | | | — | | | | (2,073 | ) |
Exercise of warrants (note 17c) | | | 2,624 | | | | — | | | | (2,623 | ) | | | — | | | | — | | | | 1 | | | | — | | | | 1 | |
Balance at December 31, 2020 | | | 977,261 | | | | 39,877 | | | | 95,856 | | | | (2,846 | ) | | | (1,087,049 | ) | | | 23,099 | | | | (8,087 | ) | | | 15,012 | |
Net income (loss) | | | — | | | | — | | | | — | | | | — | | | | 12,882 | | | | 12,882 | | | | (669 | ) | | | 12,213 | |
Foreign currency translation
reserve | | | — | | | | — | | | | — | | | | (120 | ) | | | — | | | | (120 | ) | | | — | | | | (120 | ) |
Reclassification of exchange
differences on translation
of foreign operations to
consolidated statement of
operations (note 6) | | | — | | | | — | | | | — | | | | (44 | ) | | | — | | | | (44 | ) | | | — | | | | (44 | ) |
Share-based payments
expense (note 17b) | | | — | | | | 4,252 | | | | — | | | | — | | | | — | | | | 4,252 | | | | — | | | | 4,252 | |
Share-based compensation
paid in cash (note 17b) | | | — | | | | (20 | ) | | | — | | | | — | | | | — | | | | (20 | ) | | | — | | | | (20 | ) |
Shares issued upon conversion of debt (note 16) | | | 2,588 | | | | — | | | | — | | | | — | | | | — | | | | 2,588 | | | | — | | | | 2,588 | |
Balance at December 31, 2021 | | | 979,849 | | | | 44,109 | | | | 95,856 | | | | (3,010 | ) | | | (1,074,167 | ) | | | 42,637 | | | | (8,756 | ) | | | 33,881 | |
Net income | | | — | | | | — | | | | — | | | | — | | | | 500 | | | | 500 | | | | 122 | | | | 622 | |
Foreign currency translation
reserve | | | — | | | | — | | | | — | | | | (159 | ) | | | — | | | | (159 | ) | | | — | | | | (159 | ) |
Share-based payments
expense (note 17b) | | | — | | | | 1,864 | | | | — | | | | — | | | | — | | | | 1,864 | | | | — | | | | 1,864 | |
Effect of changes in the ownership
of a subsidiary on non-controlling
interests (note 18) | | | — | | | | — | | | | — | | | | — | | | | (8,634 | ) | | | (8,634 | ) | | | 8,634 | | | | — | |
Balance at December 31, 2022 | | | 979,849 | | | | 45,973 | | | | 95,856 | | | | (3,169 | ) | | | (1,082,301 | ) | | | 36,208 | | | | — | | | | 36,208 | |
The accompanying notes are an integral part of the consolidated financial statements
F-6
LIMINAL BIOSCIENCES INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands of Canadian dollars)
| | | | | | | | | | | | |
Years ended December 31 | | 2022 | | | 2021 | | | 2020 | |
Cash flows used in operating activities | | | | | | | | | |
Net loss from continuing operations during the year | | $ | (28,916 | ) | | $ | (45,063 | ) | | $ | (49,021 | ) |
Net income (loss) from discontinued operations during the year | | | 29,538 | | | | 57,276 | | | | (69,736 | ) |
| | | | | | | | | |
Adjustments to reconcile net loss to cash flows used in
operating activities: | | | | | | | | | |
Finance costs and foreign exchange | | | (16,984 | ) | | | 4,317 | | | | 8,307 | |
Gain from disposition of capital and intangible assets | | | (2,345 | ) | | | (6 | ) | | | (15 | ) |
Non-cash issuance of warrants (note 16) | | | — | | | | — | | | | 2,228 | |
Loss (gain) on sale of discontinued operations (note 6) | | | 600 | | | | (140,403 | ) | | | (3,380 | ) |
Change in fair value of financial instruments measured at
fair value through profit or loss (note 15) | | | (1,648 | ) | | | (9,886 | ) | | | (850 | ) |
Impairment losses (note 23) | | | — | | | | 1,752 | | | | 20,859 | |
Deferred income taxes (note 24) | | | 286 | | | | 118 | | | | — | |
Loss on extinguishment of liabilities (note 16) | | | 212 | | | | (75 | ) | | | (79 | ) |
Provision expense (note 14) | | | (15,505 | ) | | | 22,367 | | | | — | |
Share-based payments expense (note 17b) | | | 1,864 | | | | 4,232 | | | | 6,194 | |
Depreciation of capital assets | | | 325 | | | | 1,368 | | | | 2,779 | |
Depreciation of right-of-use assets | | | 508 | | | | 1,013 | | | | 4,578 | |
Amortization of intangible assets | | | 287 | | | | 1,963 | | | | 1,090 | |
| | | (31,778 | ) | | | (101,027 | ) | | | (77,046 | ) |
Change in non-cash working capital items | | | (42 | ) | | | 1,424 | | | | 1,129 | |
| | $ | (31,820 | ) | | $ | (99,603 | ) | | $ | (75,917 | ) |
Cash flows (used in) from financing activities | | | | | | | | | |
Proceeds from share issuances (with or without warrants) (note 17a) | | | — | | | | — | | | | 39,960 | |
Proceeds from long-term debt (with or without warrants) (note 16) | | | — | | | | — | | | | 31,533 | |
Repayment of principal on long-term debt (note 16) | | | (39,123 | ) | | | — | | | | (165 | ) |
Repayment of interest on long-term debt (note16) | | | — | | | | (3,945 | ) | | | (1,879 | ) |
Exercise of options (note 17b) | | | — | | | | — | | | | 82 | |
Proceeds from exercise of pre-funded warrants (note 17c) | | | — | | | | — | | | | 1 | |
Payments of principal on lease liabilities (note 13) | | | (4,711 | ) | | | (3,241 | ) | | | (7,069 | ) |
Payment of interest on lease liabilities (note 13) | | | (1,571 | ) | | | (1,080 | ) | | | (2,098 | ) |
Debt, share and warrants issuance and repayment costs | | | (41 | ) | | | (158 | ) | | | (2,960 | ) |
| | $ | (45,446 | ) | | $ | (8,424 | ) | | $ | 57,405 | |
Cash flows from investing activities | | | | | | | | | |
Additions to capital assets | | | (13 | ) | | | (293 | ) | | | (966 | ) |
Additions to intangible assets | | | (6 | ) | | | (170 | ) | | | (1,080 | ) |
Proceeds from sale of discontinued operations business (note 6) | | | — | | | | 173,357 | | | | 4,555 | |
Proceeds from disposal of capital assets from
discontinued operations (note 6) | | | 3,179 | | | | — | | | | — | |
Transaction costs paid relating to the sale of
discontinued operation business | | | — | | | | (2,492 | ) | | | (787 | ) |
Proceeds from disposal of capital assets | | | — | | | | 52 | | | | 133 | |
Release of restricted cash | | | — | | | | 165 | | | | — | |
Interest received | | | 659 | | | | 73 | | | | 450 | |
| | $ | 3,819 | | | $ | 170,692 | | | $ | 2,305 | |
| | | | | | | | | |
Net change in cash and cash equivalent during the year | | | (73,447 | ) | | | 62,665 | | | | (16,207 | ) |
Net effect of currency exchange rate on
cash and cash equivalents | | | 2,101 | | | | 750 | | | | (3 | ) |
Cash, beginning of year | | | 108,490 | | | | 45,075 | | | | 61,285 | |
Cash and cash equivalents, end of the period | | $ | 37,144 | | | $ | 108,490 | | | $ | 45,075 | |
Comprising of: | | | | | | | | | |
Cash | | | 37,143 | | | | 108,490 | | | | 45,075 | |
Cash equivalents | | | 1 | | | | — | | | | — | |
| | $ | 37,144 | | | $ | 108,490 | | | $ | 45,075 | |
Cash flows from discontinued operations presented in note 6
The accompanying notes are an integral part of the consolidated financial statements.
F-7
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
1. Nature of operations and going concern
Liminal BioSciences Inc., or Liminal or the Company, is incorporated under the Canada Business Corporations Act and is a publicly traded development stage biopharmaceutical company (Nasdaq symbol: LMNL) focused on discovering and developing distinctive novel small molecule therapeutics for inflammatory, fibrotic and metabolic diseases using its drug discovery platform and data driven approach. The Company currently has two development programs: a GPR84 antagonist program for which a lead product candidate, LMNL 6511 has been nominated and for which an Investigational New Drug or IND and Clinical Trial Application or CTA enabling work has commenced, and an OXER1 antagonist program, both of which are each at the preclinical stage.
The Company previously operated a segment devoted to the development of plasma-derived therapeutics, leveraging Liminal’s experience in bioseparation technologies used to isolate and purify biopharmaceuticals from human plasma and received approval, from the U.S. Food and Drug Administration or FDA in June 2021 for its plasma-derived product Ryplazim® (plasminogen) or Ryplazim®, a highly purified glu-plasminogen derived from human plasma that acts as a plasminogen replacement therapy for patients deficient in plasminogen protein. The Company has completed the divestment of this segment in October 2021. These activities are also presented as discontinued operations in the audited annual consolidated financial statements for the years ended December 31, 2021 and 2020 (note 6).
On February 1, 2023, the Company performed a share consolidation of all its issued and outstanding common shares, stock options and warrants on the basis of a consolidation ratio of ten pre-consolidation shares to one post consolidation share. The quantities and per unit prices of the Company's common shares, stock options and warrants presented in these audited annual consolidated financial statements have been retroactively adjusted to give effect to the share consolidation.
The Company’s registered office is located at 231 Dundas Street East, Belleville, Ontario, K8N 1E2 and its principal executive office is located at 440, Boul. Armand-Frappier, suite 300, Laval, Québec, Canada, H7V 4B4. Liminal has active business operations in Canada and the United Kingdom.
Structured Alpha LP, or SALP, is Liminal’s majority and controlling shareholder and is considered Liminal’s parent entity for accounting purposes. Thomvest Asset Management Ltd., or Thomvest, is the general partner of SALP and the ultimate controlling parent, for accounting purposes, of Liminal is The 2003 TIL Settlement.
The consolidated financial statements for the year ended December 31, 2022 are presented in Canadian dollars, $ or CAD, and have been prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting standards Board, or IASB, on a going concern basis, which presumes the Company will continue its operations for the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the ordinary course of business.
During the year ended December 31, 2022, the Company incurred a net loss from continuing operations of $28.9 million ($45.1 million for the year ended December 31, 2021) and had negative operating cash flows, including continuing and discontinued operations, of $31.8 million ($99.6 million for the year ended December 31, 2021). At December 31, 2022, the Company had an accumulated deficit of $1,082.3 million ($1,074.2 million at December 31, 2021) and a working capital of $31.2 million ($96.1 million at December 31, 2021).
F-8
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
With Liminal’s discontinuation of the development of its small molecule product candidate, fezagepras, in July 2022, Liminal’s main activities relate to the development of small molecule product candidates. The Company’s cash runway is dependent on the research programs currently underway, the pace of their progression and their outcome, as well as those planned to be undertaken in the short term. As such, there is always a degree of uncertainty in regard to the outcome or cost of those programs. The cash runway is also dependent on decisions the Company makes in terms of managing its capital, including raising capital through the issuance of debt or equity, and the Company's ability to conclude such financing transactions at an acceptable cost. The need to complete financing transactions in the future is likely to continue until the Company can generate sufficient product revenues to finance its cash requirements. Management may revert to a variety of sources for financing future cash needs including public or private equity offerings, debt financings, strategic collaborations, alliances and licensing arrangements, grant funding, selling non-core assets or other sources.
Despite the Company’s efforts to obtain the necessary funding and improve profitability of its operations, there can be no assurance of its success in doing so, especially with respect to its access to further funding on acceptable terms, if at all.
The Company currently expects that its existing resources will be sufficient to fund its planned operations and expenditures into the first quarter of Fiscal 2024.
These circumstances indicate the existence of a material uncertainty that may cast substantial doubt about the Company’s ability to continue as a going concern. If the Company is unable to secure additional capital, it may be required to curtail its research and development initiatives and take additional measures to reduce costs in order to conserve its cash in amounts sufficient to sustain operations and meet its obligations. These measures could cause significant delays in the Company’s preclinical, clinical and regulatory efforts, which are critical to the realization of its business plan. These consolidated financial statements do not include any adjustments to the amounts and classification of assets and liabilities that might be necessary should the Company be unable to continue as a going concern. Such adjustments could be material.
2. Material accounting policies
Statement of compliance
These audited annual consolidated financial statements for the year ended December 31, 2022, or consolidated financial statements, have been prepared in accordance with IFRS as issued by the IASB and were approved by the Board of Directors on March 14, 2023.
Functional and presentation currency
The consolidated financial statements are presented in Canadian dollars, ($ or CAD) which is also the Company’s functional currency. The use of other currencies will be specified.
F-9
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Basis of consolidation
The consolidated financial statements include the accounts of Liminal BioSciences Inc., and those of its subsidiaries. The Company’s subsidiaries at December 31, 2022, 2021 and 2020 are as follows:
| | | | | |
Name of subsidiary | Segment activity | Place of incorporation and
operation | Proportion of ownership interest held
by group |
| | | 2022 | 2021 | 2020 |
Fairhaven Pharmaceuticals Inc. | Small molecule therapeutics | Quebec, Canada | 100% | 100% | 100% |
Liminal R&D BioSciences Inc. | Small molecule therapeutics | Quebec, Canada | 100% | 100% | 100% |
Liminal BioSciences Holdings Limited | Small molecule therapeutics | Cambridge, United Kingdom | 100% | 100% | 100% |
Liminal BioSciences Limited | Small molecule therapeutics | Cambridge, United Kingdom | 100% | 100% | 100% |
Prometic Pharma SMT B.V | Small molecule therapeutics | Amsterdam, Netherlands | nil2) | 100% | 100% |
Prometic Bioproduction Inc. | Plasma-derived therapeutics | Quebec, Canada | nil1) | nil1) | 100% |
Prometic Plasma Resources Inc. | Plasma-derived therapeutics | Winnipeg, Canada | nil1) | nil1) | 100% |
Telesta Therapeutics Inc. | Plasma-derived therapeutics | Quebec, Canada | 100% | 100% | 100% |
NantPro Biosciences, LLC | Plasma-derived therapeutics | Delaware, U.S. | 73% | 73% | 73% |
Prometic Biotherapeutics Inc. | Plasma-derived therapeutics | Delaware, U.S. | nil1) | nil1) | 100% |
Prometic Plasma Resources USA Inc. | Plasma-derived therapeutics | Delaware, U.S. | nil1) | nil1) | 100% |
Prometic Biotherapeutics Ltd | Plasma-derived therapeutics | Cambridge, United Kingdom | 100% | 100% | 100% |
Prometic Biotherapeutics B.V. | Plasma-derived therapeutics | Amsterdam, Netherlands | nil2) | 100% | 100% |
Pathogen Removal and Diagnostic
Technologies Inc. | Corporate | Delaware, U.S. | 100% | 77% | 77% |
1) Entity sold in 2021 as part of our divestment of the plasma-derived therapeutics segment.
2) Entity deregistered from the Business Register in 2022.
The Company consolidates investees when, based on the evaluation of the substance of the relationship with the Company, it concludes that it controls the investees. The financial statements of the subsidiaries are prepared for the same reporting period as the parent Company, using consistent accounting policies. All intra-group transactions, balances, income and expenses are eliminated in full upon consolidation.
When a subsidiary is not wholly-owned the Company recognizes the non-controlling interests’ share of the net assets and results of operations in the subsidiary.
F-10
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Financial instruments
Recognition and derecognition
Financial instruments are recognized in the consolidated statement of financial position when the Company becomes a party to the contractual obligations of the instrument. On initial recognition, financial instruments are recognized at their fair value plus, in the case of financial instruments not at fair value through profit or loss, or FVPL, transaction costs that are directly attributable to the acquisition or issue of financial instruments. Financial assets are subsequently derecognized when payment is received in cash or other financial assets or if the debtor is discharged of its liability.
A financial liability is derecognized when the obligation under the liability is discharged or cancelled or expires. When an existing liability is replaced by another from the same creditor on substantially different terms, or the terms of the liability are substantially modified, such an exchange or modification is treated as the derecognition of the original liability and the recognition of a new liability. The difference in the respective carrying amounts is recognized in the consolidated statement of operations.
Classification
Subsequent to initial recognition, financial instruments are measured according to the category to which they are classified. Financial instruments are measured at amortized cost unless they are classified as fair value through other comprehensive income, or FVOCI, classified as FVPL or designated as FVPL, in which case they are subsequently measured at fair value.
The classification of financial asset debt instruments is driven by the Company’s business model for managing the financial assets and their contractual cash flow characteristics. Assets that are held to collect contractual cash flows where those cash flows solely represent payments of principal and interest are measured at amortized cost. Equity instruments that are held for trading (including all equity derivative instruments) are classified as FVPL. Financial liabilities are measured at amortized cost, unless they are required to be measured at FVPL (such as instruments held for trading or derivatives) or the Company has opted to measure them at FVPL.
The Company classifies cash, trade receivables, other receivables, restricted cash, and long-term deposits as financial assets measured at amortized cost and trade payables, wages and benefits payable, royalty payment obligations and long-term debt as financial liabilities measured at amortized cost.
The Company classifies the warrant liability as a financial liability at FVPL for which the variation in fair value is recorded in consolidated statement of operations.
Inventories
Inventories of raw materials and finished goods are valued at the lower of cost and net realizable value. Cost is determined on a first in, first out basis. The cost of manufactured inventories comprises all costs that are directly attributable to the manufacturing process, such as raw materials, direct labour and manufacturing overhead based on normal operating capacity. Net realizable value is the estimated selling price in the ordinary course of business and the estimated selling costs except for raw materials for which it is determined using replacement cost.
F-11
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Capital assets
Capital assets are recorded at cost less any government assistance, accumulated depreciation and accumulated impairment losses, if any. Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets, as described below.
| | | | | | | | | |
Capital asset | | | | | | | | | Period |
Buildings and improvements | | | | | | | 20 years |
Leasehold improvements | | | The lower of the lease term and the useful life |
Production and laboratory equipment | | | | | | | 5 - 20 years |
Furniture | | | | | | | 5 - 10 years |
Computer equipment | | | | | | | 3 - 5 years |
Government assistance
Government assistance programs, including investment tax credits on research and development expenses and salary and rent subsidies are reflected as reductions to the cost of the assets or to the expenses to which they relate and are recognized when there is reasonable assurance that the assistance will be received and all attached conditions are complied with.
Right-of-use assets
The Company recognizes a right-of-use, or ROU, asset at the commencement date of a lease which is when the date at which the underlying asset is available for use. Right-of-use assets are measured at cost, less any accumulated depreciation and impairment losses, and adjusted for any remeasurement of lease liabilities. Unless the Company is reasonably certain to obtain ownership of the leased asset at the end of the lease term, the recognized right-of-use asset is depreciated on a straight-line basis over the shorter of its estimated useful life and the lease term.
Intangible assets
Intangible assets are carried at cost less accumulated amortization. Amortization commences when the intangible asset is available for use and is calculated over the estimated useful lives of the intangible assets acquired using the straight-line method. The maximum period used for each category of intangible asset are presented in the table below.
| | | | | | | | | |
Intangible asset | | | | | | | | | Period |
Licenses and other rights | | | | | | | | | 30 years |
Donor lists | | | | | | | | | 10 years |
Patents | | | | | | | | | 20 years |
Software | | | | | | | | | 5 years |
Impairment of long-lived assets
At the end of each reporting period, the Company reviews the carrying amounts of its capital, ROU and intangible assets to determine whether there is any indication that those assets have suffered an impairment loss. If impairment indicators exist, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss, if any. For intangible assets not yet available for use, an impairment test is performed annually at November 30, until amortization commences, whether or not there are impairment indicators.
F-12
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
The recoverable amount is the higher of the fair value less costs to sell and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset for which the estimates of future cash flows have not been adjusted.
An impairment loss is recognized when the carrying amount of an asset or a cash-generating unit, or CGU, exceeds its recoverable amount by the amount of this excess. An impairment loss is recognized immediately in profit or loss in the period during which the loss is incurred. An impairment loss can be subsequently reversed, if certain conditions are met and the amount of the reversal will not exceed the carrying amount that would have been determined had an impairment loss not been recognized for the asset or CGU in prior periods. A reversal of an impairment loss is recognized immediately in profit or loss.
Lease liabilities
At the commencement date of a lease, the Company recognizes a lease liability measured at the present value of lease payments to be made over the lease term. The lease payments include fixed payments (including in-substance fixed payments) less any lease incentives, variable lease payments that depend on an index or a rate, and amounts expected to be paid under residual value guarantees. The lease payments also include the exercise price of a purchase option reasonably certain to be exercised by the Company and payments of penalties for terminating a lease, if the lease term reflects the Company exercising the option to terminate. The variable lease payments that do not depend on an index or a rate are recognized as expense in the period on which the event or condition that triggers the payment occurs.
In calculating the present value of lease payments, the Company uses the incremental borrowing rate at the lease commencement date if the interest rate implicit in the lease is not readily determinable. After the commencement date, the amount of a lease liability is increased to reflect the accretion of interest and reduced for the lease payments made. In addition, the carrying amount of a lease liability is remeasured if there is a modification, a change in the lease term, a change in the in-substance fixed lease payments or a change in the assessment whether the underlying asset will be purchased.
The Company applies the short-term lease recognition exemption to leases of 12 months or less, as well as the lease of low-value assets recognition exemption. Lease payments on short-term leases and leases of low-value assets are recognized as expense on a straight-line basis over the lease term.
The Company has elected, for the class of assets related to the lease of building space, not to separate non-lease components from lease components, and instead account for each lease component and any associated non-lease components as a single lease component.
Provisions
If changes in circumstances render a contract to be onerous, meaning the unavoidable cost of meeting the contract exceeds the economic benefits expected under it, the Company recognizes the present value of the obligations as a provision. Before a separate provision for an onerous contract is established, an entity recognizes any impairment loss that has occurred on assets dedicated to that contract. The amount recognized as a provision is the best estimate of the cash disbursements required to settle the present obligation at the end of a reporting period and as such, a provision will change if the estimate changes. The discount rate used is the pre-tax rate that reflects the market assessment of the time value of money and the risks specific to the liability.
F-13
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Revenue recognition
Sale of goods
Revenue from sale of goods is recognized when the terms of a contract with a customer have been satisfied. This occurs when:
•The control over the product has been transferred to the customer; and
•The product is received by the customer or transfer of title to the customer occurs upon shipment.
Following delivery, the customer bears the risks of obsolescence and loss in relation to the goods. Revenue is recognized based on the price specified in the contract, net of estimated sales discounts and returns. Revenue from the sale of goods is presented as part of the results from discontinued operations.
Royalty revenue
Royalty revenues are recognized once the sale of products to which the royalties give rise occurs.
Rental revenue
The Company accounts for the lease or sub-lease with its tenant as an operating lease when the Company has not transferred substantially all of the risks and benefits of ownership of its property or leased property. Revenue recognition under an operating lease commences when the tenant has a right to use the leased asset, and the total amount of contractual rent to be received from the operating lease is recognized on a straight-line basis over the term of the lease. Rental revenue also includes recoveries of operating expenses and property taxes.
Research and development expenses
Expenditure on research activities is recognized as an expense in the period during which it is incurred. An internally generated intangible asset arising from development (or from the development phase of an internal project) is recognized if, and only if, all of the following have been demonstrated:
•the technical feasibility of completing the intangible asset so that it will be available for use or sale;
•the intention to complete the intangible asset and use or sell it;
•the ability to use or sell the intangible asset;
•how the intangible asset will generate probable future economic benefits;
•the availability of adequate technical, financial and other resources to complete the development and to use or sell the intangible asset; and
•the ability to measure reliably the expenditures attributable to the intangible asset during its development.
To date, the Company has not capitalized any development costs.
F-14
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Foreign currency translation
Transactions and balances
Transactions in foreign currencies are initially recorded by the Company and its entities at their respective functional currency rates prevailing at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies are retranslated at the functional currency spot rate of exchange at the reporting date. All differences are taken to the consolidated statement of operations. Non-monetary items that are measured in terms of historical cost in a foreign currency are translated using the exchange rates at the dates when the initial transactions took place.
Group companies
The assets and liabilities of foreign operations are translated into Canadian dollars at the rate of exchange prevailing at the reporting date and their statements of operations are translated at exchange rates prevailing at the dates of the transactions. The exchange differences arising on the translation are recognized in other comprehensive loss. On disposal of a foreign operation, the component of other comprehensive loss relating to that particular foreign operation is reclassified from the consolidated statement of comprehensive loss to the consolidated statement of operations as part of the gain or loss on the disposal of the foreign operation.
Share-based payments
The fair value of stock options granted by the Company is determined at the grant date using the Black-Scholes option pricing model and is expensed over the vesting period of the options. Grants with graded vesting are considered to be multiple awards for fair value measurement. An estimate of the number of awards that are expected to be forfeited is also made at the time of grant and revised periodically if actual forfeitures differ from those estimates.
The Company also made use of a restricted share unit plan as part of its long-term incentive plan up until the end of 2020. The fair value of Restricted Share Units, or RSU, is determined using the market value of the Company’s shares on the grant date. The expense associated with RSU awards that vest over time are recognized over the vesting period. When the vesting of RSU is dependent on meeting performance targets as well as a service requirement, the Company will estimate the outcome of the performance targets to determine the expense to recognize over the vesting period, and revise those estimates until the final outcome is determined.
An estimate of the number of awards that are expected to be forfeited is also made at the time of grant and revised periodically if actual forfeitures differ from those estimates.
The Company’s policy is to issue new shares upon the exercise of stock options and the release of RSU for which conditions have been met.
F-15
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Assets held for sale and discontinued operations
The Company classifies non-current assets and disposal groups as held for sale at the end of a given reporting period if their carrying amounts will be recovered principally through a sale rather than through continuing use. Such non-current assets and disposal groups classified as held for sale are measured at the lower of their carrying amount and their fair value less cost to sell. Costs to sell are the incremental costs directly attributable to the sale, excluding finance costs and income tax expense. Such assets are only presented as held for sale when the sale is highly probable and the assets or disposal group are available for immediate sale in their present condition. Actions required to complete the sale should indicate that it is unlikely that significant changes to the sale will be made or that the sale will be withdrawn. Management must be committed to the sale, which should be expected to qualify for recognition as a completed sale within one year from the date of classification.
Capital assets included as part of the assets held for sale are not depreciated once classified as held for sale. Assets and liabilities classified as held for sale are presented separately as current items in the consolidated statement of financial position.
The results of discontinued operations are presented net of tax in the consolidated statement of operations. Incremental cost related to the disposition and income taxes are allocated to discontinued operations. The discontinued operations also include the gain or loss on the disposal, which will also include the reclassification of historical exchange differences on translation of foreign operations sold. The results of discontinued operations exclude the allocation of the corporate finance costs and general corporate overhead in the form of management fees if the costs will continue to be incurred by Liminal following the disposition. The prior period results from discontinued operations are reclassified and presented in the consolidated statements of operations.
Share and warrant issue expenses
The Company records share and warrant issue expenses as an increase to the deficit.
3. Significant accounting judgements and estimation uncertainty
The preparation of these consolidated financial statements requires the use of judgments, estimates and assumptions that affect the reported amounts of revenues, expenses, assets and liabilities and the accompanying disclosures. The uncertainty that is often inherent in estimates and assumptions could result in material adjustments to assets or liabilities affected in future periods. The significant judgments made and estimates used in the preparation of these consolidated financial statements are explained below.
Significant judgments
Going concern - In assessing whether the going concern assumption is appropriate and whether there are material uncertainties that may cast significant doubt about the Company’s ability to continue as a going concern, management must estimate future cash flows for a period of at least twelve months following the end of the reporting period by considering relevant available information about the future. Management has considered a wide range of factors relating to expected cash inflows such as whether the Company will earn other significant revenues, what will be the next steps in its research and development programs and the related expenditures as well as the financing strategy it would like to pursue and the potential sources of debt and equity financing available to it in case further financing is desired. Management has also estimated expected cash outflows such as operating and capital expenditures and debt repayment schedules. These cash flow estimates are subject to uncertainty.
F-16
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Functional currency – The functional currency of foreign subsidiaries is reviewed on an ongoing basis to assess if changes in the underlying transactions, events and conditions have resulted in a change. This assessment is also performed for new subsidiaries. When assessing the functional currency of a foreign subsidiary, management’s judgment is applied in order to determine, amongst other things, the primary economic environment in which an entity operates, the currency in which the activities are funded and the degree of autonomy of the foreign subsidiary from the reporting entity in its operations and financially. Judgment is also applied in determining whether the inter-company loans denominated in foreign currencies form part of the parent Company’s net investment in the foreign subsidiary. Considering such loans as part of the net investment in the foreign subsidiary results in foreign currency translation gains or losses from the translation of these loans being recorded in other comprehensive loss instead of the consolidated statement of operations.
Share-based compensation - On March 23, 2020, the board of directors of the Company approved a plan to seek shareholder approval to modify the exercise price of certain stock options as disclosed in note 17b. In order to determine when the expense related to this modification is recognized in the consolidated statement of operations, management evaluated the timing of notification to option holders, the timing and method of determining the exercise price and the service period. Management further considered whether the holders of the stock options had sufficient understanding of the terms and conditions of the potentially revised awards, the degree of certainty of the approval for the repricing and whether the service period for earning the rights to the awards had commenced. Management concluded that the definition of the grant date was not met but that the service period had commenced and therefore a preliminary calculation of the incremental fair value of the repricing of the awards was performed using assumptions as of March 31, 2020. On May 26, 2020, the conditions for a grant date were met and the options exercise price was revised to $152.10 and a final calculation to determine the incremental fair value of the repriced options was performed.
Estimates and assumptions
COVID-19 – The impact of the COVID-19 pandemic on the financial statements for years ended December 31, 2022 and 2021 has been limited. During a portion of 2021, the Company was eligible for salary and rent subsidy programs from the Government of Canada under which it submitted claims (note 21). As of the date of these consolidated financial statements, there are no subsidy programs to which the Company is eligible.
Fair value of financial instruments – The individual fair values attributed to the different components of a financing transaction, are determined using valuation techniques. Management uses judgment to select the methods used to determine certain inputs/assumptions used in the models and the models used to perform the fair value calculations in order to determine, 1) the values attributed to each component of a transaction at the time of their issuance, 2) the fair value measurements for certain instruments that require subsequent measurement at fair value on a recurring basis and 3) the fair value of financial instruments subsequently carried at amortized cost. When the determination of the fair value of a new loan is required, discounted cash flow techniques which includes inputs that are not based on observable market data and inputs that are derived from observable market data are used.
When determining the appropriate discount rates to use, Management seeks comparable interest rates, where available. If unavailable, it uses those considered appropriate for the risk profile of a Company in the industry.
In determining the fair value of the warrants issued in November 2020 (note 15), which are presented as a warrant liability in the consolidated statement of financial position and considered to be a level 3 measurement, the Company made assumptions on unobservable inputs used in the valuation model that have an important impact on the resulting fair value computed.
F-17
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Notably, the Company estimated the timing and the amounts of equity financings it expects to complete before the expiry of those warrants. The fair value computed could be higher if the actual equity financing needs of the Company are higher than those expected. The Company also estimated the future volatility of the common shares of Liminal for the contractual life of the warrants. To do so, the Company used the historical volatility of its own shares and of comparable companies in the same industry as a starting basis for this estimate and also considered whether there are factors that would indicate that the historical volatility is not indicative of the future. In addition, the Company applied an illiquidity discount rate on the resulting Black-Scholes pricing model to reflect that the November 2020 warrants are not publicly traded instruments and therefore the ability to sell them is limited. In establishing the illiquidity discount rate, the Company considered the remaining life of the warrants and the volatility assumption for the underlying shares. Had the Company selected a higher volatility rate and/or a lower illiquidity discount rate, the fair value of the warrant liability would have been higher.
The fair value estimates could be significantly different because of the use of judgment and the inherent uncertainty in estimating the fair value of these instruments that are not quoted in an active market.
Uncertainty over income tax treatments - R&D tax credits for the current and prior periods are measured at the amount the Company expects to recover, based on its best estimate and judgment, of the amounts it expects to receive from the tax authorities as at the reporting date, either in the form of income tax refunds or refundable grants. However, there are uncertainties as to the interpretation of the tax legislation and regulations, in particular regarding what constitutes eligible R&D activities and expenditures, as well as the amount and timing of recovery of these tax credits. In order to determine whether the expenses it incurs are eligible for R&D tax credits, the Company must use judgment in determining whether its complex R&D activities qualify for available tax credits, which makes the recovery of tax credits uncertain. As a result, there may be a significant difference between the estimated timing and amount recognized in the consolidated financial statements in respect of tax credits receivable and the actual amount of tax credits received as a result of the tax administrations' review of matters that were subject to interpretation. These uncertainties, relating to entities the Company has sold may still affect Liminal as certain indemnification obligations may be called upon, subject to contractual limitations, when these entities may be subjected to the tax administrations reviews for taxation periods prior to the sale. The amounts recognized in the consolidated financial statements are based on the best estimates of the Company and in its best possible judgment, as noted above.
Assessing the recoverable amount of long-lived assets - The Company evaluates the recoverable value of long-lived assets when indicators of impairment arise or as part of the annual impairment test, if there are intangible assets not yet available for use. The recoverable value is the higher of the value in use and the fair value less costs of disposal, or FVLCD.
Long-lived assets include capital assets, ROU assets and intangible assets such as patents and licenses and other rights. Some of these rights are considered not available for use until regulatory approval to commercialize the product candidate is obtained.
When calculating the net recoverable amounts for the impairments on continuing operations (note 24) and discontinued operations (note 6), management made estimates and assumptions regarding the outcome of certain future events, future cash flows and their timing.
F-18
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
When determining the FVLCD for its CGU plasma-derived product Ryplazim® (plasminogen) or Ryplazim® (note 6), significant estimates made included amongst others, the outcome of the exercise it had undertaken in evaluating the potential alternatives for the Ryplazim® CGU, including the probability of completing a sale or closing those activities; the operating cash outflows to support those operations until one of the alternative strategies was executed; the outcome of the FDA review of the Company’s Biological License Application, or BLA for its Ryplazim® product candidate and the timing of completion of the review; if the Company would be able to benefit from the monetization of a Priority Review Voucher, if received, and what would be the amount received upon its monetization; and whether some assets, liabilities and commitments could potentially be excluded from the activities sold and for those commitments that could be retained, the possibility of reducing those commitments and what would be their settlement amount. A 10% change in the probability weighted terminal value would have impacted the impairment recorded on the Ryplazim® CGU by $ 3,638.
When calculating the FVLCD of an asset or a group of assets for which selling price information for comparable assets are not readily available, management also must make assumptions regarding the value it may recuperate from its sale.
Share-based compensation - To determine the fair value of stock options on a given date, the Company must determine the assumptions that will be used as inputs to the Black-Scholes option pricing model, including the assumption regarding the future volatility of the common shares of Liminal for the expected life of the stock options. The Company uses the historical volatility as a starting basis for the estimate and also considers whether there are factors that would indicate that the past volatility is not indicative of the future volatility. In making this assessment, management considers changes in the Company's activities and other factors such as a significant share consolidation. As the volatility is an assumption that has a significant impact on the calculated value of a stock option, the impact of this estimate can significantly impact the share-based payment expense over the vesting period of an award.
Valuation of deferred income tax assets – To determine the extent to which deferred income tax assets can be recognized, management estimates the amount of probable future taxable profits that will be available against which deductible temporary differences and unused tax losses can be utilized. Management exercises judgment to determine the extent to which realization of future taxable benefits is probable, considering the history of taxable profits, budgets and forecasts and availability of tax strategies.
Estimates and assumptions about future events and their effects cannot be determined with certainty and therefore require the exercise of judgment. As of the date of issuance of these financial statements, the Company is not aware of any specific event or circumstance that would require it to update its estimates, assumptions and judgments or revise the carrying value of its assets or liabilities, and the Company is unable to estimate the potential impact on its future business or its financial results as of the date of this filing. These estimates may change as new events occur and additional information is obtained and changes in those estimates are recognized in the consolidated financial statements as soon as they become known.
4. Change in standards, interpretations and accounting policies
a) Adoption of new accounting standards
The accounting policies used in these annual consolidated financial statements are consistent with those applied by the Company in its December 31, 2021 and 2020 audited annual consolidated financial statements except for the amendments to certain accounting standards which are relevant to the Company and were adopted by the Company since January 1, 2021 as described below.
F-19
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Amendments to IFRS 3, Business Combinations or IFRS 3
The amendments to IFRS 3 clarify the definition of a business and includes an optional concentration test to determine whether an acquired set of activities and assets is a business. These amendments were adopted on January 1, 2020 and are applied prospectively to acquisitions made on or after this date.
Amendment to IFRS 16, Leases or IFRS 16 for COVID-19-Related Rent Concessions - IFRS 16 has been revised to incorporate an amendment issued by the IASB in May 2020. The amendment permits lessees not to assess whether particular COVID-19-related rent concessions are lease modifications and, instead, account for those rent concessions as if they were not lease modifications. In addition, the amendment to IFRS 16 provides specific disclosure requirements regarding COVID-19-related rent concessions. The amendment was adopted as of January 1, 2021 and had no impact on the financial statements for the years ended December 31, 2022 and 2021 since the Company has not benefited from COVID-19 related rent concessions.
Amendment to IAS 1, Presentation of Financial statements or IAS 1 - IAS 1 has been revised to require the disclosure of material accounting policies rather than significant accounting policies and provides guidance to apply materiality judgments to accounting policy disclosure. The Company early adopted these amendments, and consequential amendments to other standards, for its annual audited financial statements for the year ended December 31, 2022 resulting in a reduction of its accounting policy disclosure in note 2 - Material accounting policies.
Amendments to IAS 37, Provisions, Contingent Liabilities and Contingent Assets (IAS 37) - IAS 37 has been revised to specify which costs an entity includes in determining the cost of fulfilling a contract for the purpose of assessing whether the contract is onerous. The amendments are effective for annual reporting periods beginning on or after January 1, 2022. The adoption of these amendments had no impact on the computation of the provision.
Amendment to IFRS 9 Financial Instruments (IFRS 9) - IFRS 9 has been revised to clarify the fees an entity includes when assessing whether the terms of a new or modified financial liability are substantially different from the terms of the original financial liability. The adoption of the amendment had no impact on the financial statements and will be applied to financial liabilities that are modified after the date of adoption.
Amendments to IAS 12, Income taxes (IAS 12) - The amendments to IAS 12 clarify the accounting for deferred tax assets or liabilities arising from a single transaction such as leases, namely that the scope of the recognition exemption no longer applies to transactions that, on initial recognition, give rise to equal taxable and deductible temporary differences. The Company early adopted these amendments for its annual financial statements for the year ended December 31, 2022, which mainly affect the Company’s accounting for deferred tax assets and deferred tax liabilities pertaining to right of use assets and lease liabilities. There will be little to no effect on the amounts reported in the consolidated statements of financial position or the consolidated statements of operations, since only a small portion of the Company’s deferred tax assets are being recognized based on their current potential to be recovered with future tax profits. Note 24 - Income taxes note disclosures will be affected as the deferred tax assets and liabilities pertaining to the leases will no longer be presented on a net basis.
F-20
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
b) New Standards and interpretations not yet adopted
The IFRS accounting standards, amendments, and interpretations that the Company reasonably expects may have a material impact on the disclosures, the financial position or results of operations of the Company when applied at a future date are as follows:
Amendments to IAS 8, Accounting policies, Changes in Accounting Estimates and Errors (IAS 8) - The amendments to IAS 8 introduce a definition of accounting estimates and provide clarifications to distinguish accounting policies from accounting estimates. The amendments are applicable retrospectively and are effective for annual reporting periods beginning on or after January 1, 2023 with earlier application permitted. The Company concluded that these amendments will not have an impact on its financial statements at the date of adoption and for the comparative periods.
Amendments IAS 1, Presentation of Financial Statements (IAS 1) - IAS 1 has been revised to clarify how to classify debt and other liabilities as current or non-current. The amendments help to determine whether, in the statement of financial position, debt and other liabilities with an uncertain settlement date should be classified as current (due or potentially due to be settled within one year) or non-current. The amendments also include clarifying the classification requirements for debt an entity might settle by converting it into equity. The amendments are applicable retrospectively and are effective for annual reporting periods beginning on or after January 1, 2023 with earlier application permitted. The Company concluded that these amendments will not have an impact on its financial statements at the date of adoption and for the comparative periods.
5. Acquisition of Fairhaven Pharmaceuticals Inc.
Pursuant to a share purchase agreement, or SPA, dated July 17, 2020, the Company acquired 100% of the issued and outstanding common shares of Fairhaven Pharmaceuticals Inc., or Fairhaven, a company with a preclinical research program of small molecule antagonists. As consideration for the acquisition, the Company issued 202,308 common shares. Upon achievement of certain pre-determined research and development milestones prior to July 17, 2025, the Company may be obligated to make additional payments in the form of common shares totaling up to $4,374. The number of shares to be issued, if any, upon completion of a milestone, will be calculated using the five-trading day volume weighted average trading price, or VWAP, of the Company’s common shares on Nasdaq prior to the achievement of such milestone events.
As Fairhaven did not meet the definition of a business under IFRS 3, the acquisition has been accounted for as an asset acquisition, the total cost of the net assets acquired being the fair value of the consideration paid. The shares issued were recorded at a fair value of $3,441, based on the closing price of Liminal’s common shares at the date of the transaction. The transaction costs of $308 incurred by the Company were capitalized and allocated to the net assets acquired. Any future milestone payments would be recognized if and when the triggering event occurs.
F-21
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
The consideration paid and the allocation thereof to the net assets acquired were as follows:
| | | | |
Cost of acquisition | | | |
Fair value of common shares issued | | $ | 3,441 | |
Cash payment | | | 50 | |
Total consideration paid | | $ | 3,491 | |
Transaction fees | | | 308 | |
Total cost of acquisition | | $ | 3,799 | |
| | | |
Net assets acquired | | | |
Current assets | | $ | 217 | |
Licenses and other rights (note 11) | | | 3,796 | |
Current liabilities | | | (214 | ) |
Total net assets acquired | | $ | 3,799 | |
6. Discontinued operations
The Company entered into various agreements, including two share purchase agreement(s), or SPA(s), in 2021 for the sale of businesses and assets that were no longer part of its core strategy. In 2022, the Company has terminated a legacy agreement with a Contract Development Manufacturing Organization, of CDMO, relating to its previously owned plasma-derived therapeutics business, and continues to take steps towards the divestment of non-core assets.
Discontinued operations for the periods presented in the consolidated financial statements comprise the revenues and expenses of operations and the gains and transaction expenses related thereto, for the following activities, assets and liabilities:
•four subsidiaries namely Prometic Plasma Resources Inc. (PPR), Prometic Plasma Resources USA Inc. (PPR USA), Prometic Bioproduction Inc. (PBP) and Prometic Biotherapeutics Inc. (PBT), which were formerly part of the plasma-derived therapeutics segment and which were sold, together with the Priority Review Voucher (PRV), in a series of transactions in 2021, and another subsidiary, Prometic Biotherapeutics Ltd, also part of the same segment, which operations have ceased;
•the obligations towards a CDMO it retained upon the sale of the plasma derived business; and
•the operating costs and the gain on disposition of the Labrosse facility, located in Pointe-Claire, Québec in December 2022.
All amounts relating to the activities above have been presented as discontinued operations in the current and prior periods. More specifically, we have restated the prior periods to remove the impact of those operations from all lines in the financial statements (revenues, cost of sales and production cost, R&D and administration, selling and marketing being the lines most impacted) and have reclassified those results to the income (loss) from discontinued operations lines in the interim financial statements. The proceeds and expenses pertaining to the sale of the businesses and assets are included as part of the gain (loss) on sale of discontinued operations.
F-22
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
The sale of PPR and PPR USA, the plasma collection centers, to Kedrion S.p.A., or Kedrion, closed on May 21, 2021. Concurrently with the closing of this transaction, the Company entered into an option agreement, or Option, which granted Kedrion the right to acquire the Ryplazim® business by June 15, 2021 which was subsequently extended to June 22, 2021. The SPA for the Ryplazim® business was signed on June 2, 2021, with the sale of PBP subsequently closing on July 9, 2021. Between the original expiry date of the Option and the sale of PBP, the Company received additional proceeds compensating the Company for the extension of the Option and the operating costs of PBP until that date. On August 6, 2021, PBT entered into a definitive agreement for the sale of the PRV for proceeds of USD 105 million. The sale of the PRV closed on September 28, 2021 with Liminal receiving $130,966 (net of selling costs of $1,891). The sale of PBT closed on October 15, 2021.
As part of the SPAs signed with Kedrion, the Company may be required to indemnify the buyer if certain events occur following the closing of each sale transaction, subject to contractual limitations on such indemnifications. The buyer has notified us of potential claims for indemnification since the transactions originally closed which are still under evaluation. Estimates of potential payments and actual payments, if any, are/will be recorded against the gain on sale of discontinued operations, in the period an indemnification obligation becomes known.
F-23
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
In August 2022, Liminal entered into an agreement for the termination of its legacy CDMO agreement which resulted in the immediate termination of the agreement, and all obligations associated therewith in return for payments totaling $18,000. A first payment of $11,200 was made upon execution of the agreement and covered past sums due to the date of termination. The Company paid $3,400 in January 2023 and the remaining $3,400 will be payable in January 2024. The CDMO agreement is accounted for in part as a lease (note 13) and an onerous contract provision (note 14) and the termination agreement resulted in the extinguishment of the entire lease liability and the recognition of a gain on modification of a liability, included in financing costs from discontinued operations of $16,019 and a reversal in the provision of $10,741, included in research and development expenses from discontinued operations.
The Labrosse facility, formerly part of the plasma-derived therapeutics segment and previously classified as property, plant and equipment, met the criteria to be classified as held for sale during the quarter ended March 31, 2022 at a carrying amount of $829. The Labrosse facility was sold in December 2022 resulting in a recognition of a gain of $2,345 on disposition of capital assets from discontinued operations.
Gain on sale of subsidiaries
The details of the gain on sale of subsidiaries during the years ended December 31, 2022, 2021 and 2020 is provided in the table below:
| | | | | | | | | | | | |
Year ended December 31 | | | 2022 | | | | 2021 | | | | 2020 | |
Sale of bioseparation business | | | | | | | | | |
Proceeds received | | $ | — | | | $ | — | | | $ | 3,380 | |
Less: | | | | | | | | | |
Carrying amount of net assets sold | | | — | | | | — | | | | — | |
Transaction costs | | | — | | | | — | | | | — | |
Reclassification of foreign currency translation reserve
from other comprehensive income into the
statement of operations | | | — | | | | — | | | | — | |
Gain on sale of bioseparation business | | $ | — | | | $ | — | | | $ | 3,380 | |
| | | | | | | | | |
Sale of plasma collection centers | | | | | | | | | |
Proceeds received | | $ | — | | | $ | 13,570 | | | $ | — | |
Less: | | | | | | | | | |
Carrying amount of net assets sold | | | — | | | | 10,849 | | | | — | |
Transaction costs | | | — | | | | 204 | | | | — | |
Reclassification of foreign currency
translation reserve from other
comprehensive income into
the statement of operations | | | — | | | | (44 | ) | | | — | |
Gain on sale of plasma collection centers | | $ | — | | | $ | 2,561 | | | $ | — | |
| | | | | | | | | |
Sale of Ryplazim business | | | | | | | | | |
Proceeds received | | $ | — | | | $ | 159,787 | | | $ | — | |
Less: | | | | | | | | | |
Carrying amount of net assets sold | | | — | | | | 19,541 | | | | — | |
Indemnification adjustments | | | 600 | | | | 116 | | | | — | |
Transaction costs | | | — | | | | 2,288 | | | | — | |
Gain (loss) on sale of Ryplazim business | | $ | (600 | ) | | $ | 137,842 | | | $ | — | |
Gain (loss) on sale of discontinued
operations, net of income
taxes $nil | | $ | (600 | ) | | $ | 140,403 | | | $ | 3,380 | |
F-24
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
During the year ended December 31, 2022, the Company recorded an indemnification adjustment expense of $600, in connection with a disputed research and development tax credit claim with a taxation authority.
The carrying amounts of the assets and liabilities of the entities sold during the year ending December 31, 2021, as part of the sale of the plasma collection centers and the Ryplazim® business, on the dates control of the entities were transferred to the buyer, are as follows:
| | | | | | | | | | |
| | | | Plasma collection centers | | | Ryplazim business | |
Accounts receivable | | | | | 137 | | | | 1,879 | |
Inventories | | | | | 8,441 | | | | 4,640 | |
Prepaids | | | | | 21 | | | | 399 | |
Other long-term assets | | | | | 54 | | | | 50 | |
Capital assets | | | | | 2,376 | | | | 9,304 | |
Right-of-use assets | | | | | 2,000 | | | | 3,795 | |
Intangible assets | | | | | 1,092 | | | | 7,277 | |
Total assets | | | | $ | 14,121 | | | $ | 27,344 | |
| | | | | | | | |
Accounts payable and accrued liabilities | | | | | 639 | | | | 2,887 | |
Current portion of lease liabilities | | | | | 665 | | | | 986 | |
Long-term portion of lease liabilities | | | | | 1,968 | | | | 3,930 | |
Total liabilities | | | | $ | 3,272 | | | $ | 7,803 | |
Net assets sold | | | | $ | 10,849 | | | $ | 19,541 | |
Results and cash flows from discontinued operations
The net loss from discontinued operations for the years ended December 31, 2022, 2021 and 2020 are presented below:
| | | | | | | | | | | | |
Year ended December 31 | | | 2022 | | | | 2021 | | | | 2020 | |
Revenues | | $ | 15 | | | $ | 949 | | | $ | 2,593 | |
| | | | | | | | | |
Expenses | | | | | | | | | |
Cost of sales and other production expenses | | | — | | | | 1,465 | | | | 1,868 | |
Research and development expenses 1) 2) | | | (11,825 | ) | | | 76,733 | | | | 42,757 | |
Administration expenses | | | 83 | | | | 2,360 | | | | 5,933 | |
Impairments | | | — | | | | 1,411 | | | | 19,772 | |
Gain on foreign exchange | | | (17 | ) | | | (136 | ) | | | (633 | ) |
Finance costs | | | (16,019 | ) | | | 2,242 | | | | 6,083 | |
Gain on disposal of capital assets | | | (2,345 | ) | | | — | | | | — | |
Gain on extinguishment of liabilities | | | — | | | | — | | | | (79 | ) |
Income (loss) from discontinued
operations, net of income
taxes | | $ | 30,138 | | | $ | (83,126 | ) | | $ | (73,108 | ) |
Current income tax (note 24) | | — | | | | 1 | | | | 8 | |
Net loss from discontinued operations | $ | 30,138 | | | $ | (83,127 | ) | | $ | (73,116 | ) |
1) Research and development expense (income) includes expenses (income) recognized in regard to a provision relating to an agreement with a CDMO. The expense (income) results from changes to the discounted value of the provision caused by increases/decreases in expected payments, including the impact of the termination agreement, and increases in the annual inflation rate that exceed 3%, and by changes in the discount rates used to calculate the net present value of the provision.
F-25
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
2) On September 29, 2021 prior to the closing of the sale of PBT, PBT paid PBP, which ownership had already passed to Kedrion, $39,457 representing 30% of the net proceeds it received from the sale of the PRV, as compensation for past research and development services. A second payment made by PBT to PBP of $6,357 in prepayment for future R&D services on the same date was recognized as R&D expense since this amount would not be recoverable upon the sale of PBT.
At September 30, 2021, the carrying amount of the net assets of PBT, presented as assets of disposal group held for sale, exceeded the amount of the proceeds to be received upon the closing of the sale transaction that occurred on October 15, 2021. As assets held for sale must be carried at the lower of their carrying amount or their fair value less cost to sell, an impairment of $1,389 was recorded on the intangible assets during the quarter ended September 30, 2021 (note 12).
At the beginning of 2021, the Company announced it had undertaken to evaluate potential alternatives aimed at minimizing the plasma-derived therapeutics segment cash burn which may result in divestment in whole or part of this business, or other courses of action including but not limited to the closure of the Ryplazim® related operations, in order to focus our resources on the small molecules segment. As the capital, intangible and ROU assets in the Ryplazim® CGU were no longer to be used as originally planned, management proceeded to review them for impairment and writing them down to their net recoverable value determined as the FVLCD using a market approach. The Ryplazim® CGU includes the assets involved in production, R&D and commercialization activities relating to the Ryplazim® product candidate that was yet to receive regulatory approval for commercialization. The Ryplazim® CGU evaluated excluded the assets pertaining to the plasma collection activities since these could generate distinct cash inflows and could have been potentially divested separately from the Ryplazim® assets. The plasma collection assets were not considered impaired.
The FVLCD was calculated using a discounted cash flow model for one year and a terminal value of $58.1 million using a post-tax discount rate of 7.75%. The fair value computed by management is considered as a level 3 computation in the fair value hierarchy under IFRS 13, Fair value measurement. As part of this valuation exercise, management needed to make several key assumptions which affected the cash inflows and outflows considered in the model. The significant estimates used in determining the FVLCD are disclosed in note 3.
As a result of this exercise, the Company recorded impairment of $665 on capital assets (note 10), $18,553 on ROU assets (note 11) and $480 on intangible assets (note 12), respectively, representing an aggregate impairment of $19,698 on these plasma-derived therapeutic assets for the year ended December 31, 2020. Also during the year, the Company recorded other impairments on ROU assets amounting to $74.
F-26
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
The consolidated statements of cash flows for the years ended December 31, 2022, 2021 and 2020 were not restated to present the cash flows from the discontinued operations separately as the Company selected to provide this information in the present note. The cash flows from the discontinued operations and the gain on sale of subsidiaries for the years ended December 31, 2022, 2021 and 2020 are presented in the following table:
| | | | | | | | | | | | |
Year ended December 31 | | 2022 | | | 2021 | | | 2020 | |
Cash flows from (used in) operating activities 1) | | $ | (5,482 | ) | | $ | (43,089 | ) | | $ | 8,015 | |
Cash flows used in financing activities | | | (5,500 | ) | | | (3,470 | ) | | | (7,943 | ) |
Cash flows from (used in) investing activities | | | 3,179 | | | | 171,225 | | | | (729 | ) |
Net effect of currency exchange rate on cash | | | — | | | | (30 | ) | | | 76 | |
Cash flows (used) generated during the year | | $ | (7,803 | ) | | $ | 124,636 | | | $ | (581 | ) |
1)When compiling the cash flows from discontinued operations which include only certain entities from the Liminal group of companies, intra-group cash transfers between entities in the discontinued operations group and those part of continuing activities, for example the funding provided by Liminal to the discontinued operations, have been classified as part of the operating activities cash flows.
7. Accounts receivable and others
| | | | | | | | | | | | | |
| | | | | | | December 31, | | | December 31, | |
| | | | | | | | 2022 | | | | 2021 | |
Trade receivables | | | | | | | $ | 249 | | | $ | 229 | |
Tax credits and government grants
receivable | | | | | | | | 330 | | | | — | |
Sales taxes receivable | | | | | | | | 250 | | | | 280 | |
Other receivables | | | | | | | | 348 | | | | 559 | |
| | | | | | | $ | 1,177 | | | $ | 1,068 | |
8. Other long-term assets
| | | | | | | | | | | | | |
| | | | | | | December 31, | | | December 31, | |
| | | | | | | | 2022 | | | | 2021 | |
Long-term deposits | | | | | | | $ | 30 | | | $ | 30 | |
Tax credits receivable | | | | | | | | 213 | | | | 332 | |
| | | | | | | $ | 243 | | | $ | 362 | |
F-27
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
9. Capital assets
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | Production | | | Furniture and | | | | |
| | Land and | | | Leasehold | | | and laboratory | | | computer | | | | |
| | Buildings | | | improvements | | | equipment | | | equipment | | | Total | |
Cost | | | | | | | | | | | | | | | |
Balance at January 1, 2021 | | $ | 4,567 | | | $ | 7,349 | | | $ | 29,904 | | | $ | 3,365 | | | $ | 45,185 | |
Additions | | | — | | | | — | | | | 99 | | | | 13 | | | | 112 | |
Disposals | | | — | | | | (399 | ) | | | (1,516 | ) | | | (306 | ) | | | (2,221 | ) |
Sold - discontinued operations (note 6) | | | — | | | | (6,324 | ) | | | (21,525 | ) | | | (2,256 | ) | | | (30,105 | ) |
Effect of foreign exchange differences | | | — | | | | (107 | ) | | | (45 | ) | | | (9 | ) | | | (161 | ) |
Balance at December 31, 2021 | | $ | 4,567 | | | $ | 519 | | | $ | 6,917 | | | $ | 807 | | | $ | 12,810 | |
Additions | | | — | | | | — | | | | — | | | | 13 | | | | 13 | |
Disposals | | | (982 | ) | | | — | | | | (22 | ) | | | (73 | ) | | | (1,077 | ) |
Effect of foreign exchange differences | | | 1 | | | | — | | | | — | | | | — | | | | 1 | |
Balance at December 31, 2022 | | $ | 3,586 | | | $ | 519 | | | $ | 6,895 | | | $ | 747 | | | $ | 11,747 | |
| | | | | | | | | | | | | | | |
Accumulated depreciation | | | | | | | | | | | | | | | |
Balance at January 1, 2021 | | $ | 804 | | | $ | 2,901 | | | $ | 20,208 | | | $ | 2,481 | | | $ | 26,394 | |
Depreciation expense | | | 195 | | | | 268 | | | | 662 | | | | 243 | | | | 1,368 | |
Disposals | | | — | | | | (400 | ) | | | (1,301 | ) | | | (306 | ) | | | (2,007 | ) |
Impairments (note 6) | | | — | | | | — | | | | 22 | | | | — | | | | 22 | |
Sold - discontinued operations (note 6) | | | — | | | | (2,400 | ) | | | (14,281 | ) | | | (1,744 | ) | | | (18,425 | ) |
Effect of foreign exchange differences | | | — | | | | (12 | ) | | | (7 | ) | | | (6 | ) | | | (25 | ) |
Balance at December 31, 2021 | | $ | 999 | | | $ | 357 | | | $ | 5,303 | | | $ | 668 | | | $ | 7,327 | |
Depreciation expense | | | 170 | | | | 33 | | | | 59 | | | | 63 | | | | 325 | |
Disposals | | | (152 | ) | | | — | | | | (25 | ) | | | (73 | ) | | | (250 | ) |
Effect of foreign exchange differences | | | 1 | | | | — | | | | — | | | | — | | | | 1 | |
Balance at December 31, 2022 | | $ | 1,018 | | | $ | 390 | | | $ | 5,337 | | | $ | 658 | | | $ | 7,403 | |
| | | | | | | | | | | | | | | |
Carrying amounts | | | | | | | | | | | | | | | |
At December 31, 2022 | | $ | 2,568 | | | $ | 129 | | | $ | 1,558 | | | $ | 89 | | | $ | 4,344 | |
At December 31, 2021 | | | 3,568 | | | | 162 | | | | 1,614 | | | | 139 | | | | 5,483 | |
Impairment losses of $nil, $22 and $665 were recorded on capital assets that were part of the discontinued operations (note 6) during the years ended December 31, 2022, 2021 and 2020, respectively.
The depreciation expense for the year ended December 31, 2020 was $2,779.
F-28
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
10. Right-of-use assets
| | | | | | | | | | | | | | | | |
| | | | | Production | | | | | | | |
| | | | | and laboratory | | | | | | | |
| | Buildings | | | equipment | | | Other | | | Total | |
Balance at January 1, 2021 | | $ | 8,086 | | | $ | 426 | | | $ | 45 | | | $ | 8,557 | |
Lease modifications and other remeasurements | | | 3 | | | | (53 | ) | | | (2 | ) | | | (52 | ) |
Depreciation expense | | | (906 | ) | | | (95 | ) | | | (12 | ) | | | (1,013 | ) |
Sold - discontinued operations (note 6) | | | (5,497 | ) | | | (272 | ) | | | (26 | ) | | | (5,795 | ) |
Effect of foreign exchange differences | | | (77 | ) | | | (6 | ) | | | (5 | ) | | | (88 | ) |
Net book value at December 31, 2021 | | $ | 1,609 | | | $ | — | | | $ | — | | | $ | 1,609 | |
Lease modifications and other remeasurements | | | 45 | | | | — | | | | — | | | | 45 | |
Depreciation expense | | | (508 | ) | | | — | | | | — | | | | (508 | ) |
Net book value at December 31, 2022 | | $ | 1,146 | | | $ | — | | | $ | — | | | $ | 1,146 | |
The depreciation expense for the year ended December 31, 2020 was $4,578.
F-29
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
11. Intangible assets
| | | | | | | | | | | | | | | | |
| | Licenses and
other rights | | | Patents | | | Software | | | Total | |
Cost | | | | | | | | | | | | |
Balance at January 1, 2021 | | $ | 162,064 | | | $ | 6,783 | | | $ | 3,306 | | | $ | 172,153 | |
Additions (note 5) | | | — | | | | 114 | | | | (1 | ) | | | 113 | |
Sold - discontinued operations (note 6) | | | (15,006 | ) | | | (1,403 | ) | | | (2,963 | ) | | | (19,372 | ) |
Disposals | | | (1,300 | ) | | | (61 | ) | | | (33 | ) | | | (1,394 | ) |
Effect of foreign exchange differences | | | 1 | | | | (2 | ) | | | (19 | ) | | | (20 | ) |
Balance at December 31, 2021 | | $ | 145,759 | | | $ | 5,431 | | | $ | 290 | | | $ | 151,480 | |
Additions | | | — | | | | — | | | | 6 | | | | 6 | |
Disposals | | | — | | | | — | | | | (31 | ) | | | (31 | ) |
Sold - discontinued operations (note 6) | | | — | | | | — | | | | — | | | | — | |
Effect of foreign exchange differences | | | — | | | | 46 | | | | — | | | | 46 | |
Balance at December 31, 2022 | | $ | 145,759 | | | $ | 5,477 | | | $ | 265 | | | $ | 151,501 | |
| | | | | | | | | | | | |
Accumulated amortization | | | | | | | | | | | | |
Balance at January 1, 2021 | | $ | 150,592 | | | $ | 4,365 | | | $ | 1,704 | | | $ | 156,661 | |
Amortization expense | | | 316 | | | | 1,248 | | | | 399 | | | | 1,963 | |
Disposals | | | (1,298 | ) | | | (51 | ) | | | (34 | ) | | | (1,383 | ) |
Impairments (notes 6, 23) | | | 1,389 | | | | 341 | | | | — | | | | 1,730 | |
Sold - discontinued operations (note 6) | | | (8,698 | ) | | | (533 | ) | | | (1,772 | ) | | | (11,003 | ) |
Effect of foreign exchange differences | | | 16 | | | | (13 | ) | | | (7 | ) | | | (4 | ) |
Balance at December 31, 2021 | | $ | 142,317 | | | $ | 5,357 | | | $ | 290 | | | $ | 147,964 | |
Amortization expense | | | 245 | | | | 41 | | | | 1 | | | | 287 | |
Disposals | | | — | | | | — | | | | (31 | ) | | | (31 | ) |
Effect of foreign exchange differences | | | — | | | | 41 | | | | | | | 41 | |
Balance at December 31, 2022 | | $ | 142,562 | | | $ | 5,439 | | | $ | 260 | | | $ | 148,261 | |
| | | | | | | | | | | | |
Carrying amounts | | | | | | | | | | | | |
At December 31, 2022 | | $ | 3,197 | | | $ | 38 | | | $ | 5 | | | $ | 3,240 | |
At December 31, 2021 | | | 3,442 | | | | 74 | | | | — | | | | 3,516 | |
Impairment losses of $nil, $341 and $1,087 were recorded on certain licenses and patents pertaining to continuing operations (note 6) during the years ended December 31, 2022, 2021 and 2020, respectively, while impairment losses of $nil, $1,389 and $480 were recorded on intangible assets pertaining to discontinued operations during the years ended December 31, 2022, 2021 and 2020, respectively.
The amortization expense for the year ended December 31, 2020 was $1,090.
F-30
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
12. Accounts payable and accrued liabilities
| | | | | | | | | |
| | | | | | |
| | | December 31,
2022 | | | December 31,
2021 | |
Trade payables | | $ | 3,403 | | | $ | 5,762 | |
Wages and benefits payable | | | 1,651 | | | | 1,297 | |
Indemnification payable (note 6) | | | 716 | | | | 113 | |
Current portion of royalty payment obligation (note 17) | | | — | | | | 25 | |
Current portion of other employee benefit liabilities (note 17) | | | 198 | | | | 146 | |
| | | $ | 5,968 | | | $ | 7,343 | |
13. Lease liabilities
The transactions affecting the lease liabilities during the years ended December 31, 2022 and 2021 were as follows:
| | | | | | | | | |
| | | 2022 | | | 2021 | |
Balance at January 1 | | $ | 22,471 | | | $ | 33,452 | |
Interest expense | | | 1,081 | | | | 3,754 | |
Payments | | | (6,282 | ) | | | (4,321 | ) |
Derecognized - discontinued
operations (note 6) | | | — | | | | (7,549 | ) |
Lease modification and other remeasurements | | | (15,974 | ) | | | (2,588 | ) |
Effect of foreign exchange differences | | | 191 | | | | (277 | ) |
Balance at December 31 | | $ | 1,487 | | | $ | 22,471 | |
Less current portion of lease liabilities | | | (735 | ) | | | (7,194 | ) |
Long-term portion of lease liabilities | | $ | 752 | | | $ | 15,277 | |
Interest expense on lease liabilities is included as part of finance costs in the consolidated statement of operations. Interest on the lease liabilities was $6,030 for the year ended December 31, 2020.
On August 12, 2021, the Company gave a notice of early termination of a master services agreement entered into with a CDMO with whom it has a contract pertaining to its former plasma-derived therapeutics business, using the available 5-year early cancellation notification period set forth under the CDMO contract which resulted in a decrease in the term of the contract by 3.8 years. A portion of the commitments under this CDMO contract are accounted for as a lease liability while the non-lease commitment is being accounted for as an onerous contract provision since June 2021 (note 14). The financial impact of revising the lease term to reflect the effect of exercising the termination option was a decrease in lease liabilities of $2,529 and the gain on this transaction was recorded as part of the net loss from discontinued operations for the year ended December 31, 2021.
In August 2022, the Company terminated a CDMO agreement (note 6) which resulted in the payment of $5,500 towards the portion of the agreement that was accounted for as a lease liability and a lease modification gain of $16,000 from this particular transaction, as the remainder of the lease liability for this agreement was reduced to $nil.
F-31
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
14. Provisions
| | | | | | | |
Initial recognition of provisions at June 30, 2021 | | | | $ | 21,928 | |
Increase to provisions during the period | | | | | 439 | |
Effect of foreign exchange difference | | | | | (262 | ) |
Interest expense | | | | | 90 | |
Balance at January 1, 2022 | | | | | $ | 22,195 | |
Decrease to provision during the period | | | | | (10,741 | ) |
Effect of foreign exchange difference | | | | | (226 | ) |
Interest expense | | | | | 393 | |
Payments | | | | | | (3,930 | ) |
Effect of changes in the discount rate | | | | | | (1,001 | ) |
Balance at December 31, 2022 | | | | | $ | 6,690 | |
Less current portion of provision | | | | | (3,400 | ) |
Long-term portion of provision | | | | $ | 3,290 | |
The Company had a long-term contract with a CDMO for which it had no use following its decision to exit the plasma-derived therapeutics business (note 13). As such, the Company recorded in June 2021, an initial provision for onerous contract for the non-lease portion of the contract calculated as the discounted value of the estimated purchase commitment set forth under the contract using the available 5-year early cancellation notification period. In August 2021, the Company sent the CDMO an early termination notice and the provision was adjusted to reflect the revised maturity date of the contract. The payments under the lease and non-lease portions are variable since there is a CAD/USD foreign exchange variation component that affects each portion, however the total purchase commitment under the lease remains the same at $9,000 per year. As such, the effects of the foreign exchange differences have on the computation of the carrying value of the provision from period to period essentially offset by the opposite variation of the foreign exchange differences on the lease portion, or the lease liability, of this same contract (note 13).
In August 2022, the Company terminated the CDMO agreement (note 6) which resulted in the payment of $3,930 towards the portion of the agreement accounted for as an onerous contract provision and the adjustment of the provision to reflect the remaining $6,800 of future disbursements. This resulted in the reversal of the provision of $10,741 at the termination date. Subsequent to December 31, 2022, the Company paid $3,400 representing the current portion of the provision. The final and remaining payment of $3,400 will be paid in January, 2024.
F-32
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
15. Warrant liability
On February 1, 2023, the Company performed a ten-to-one share consolidation of its common shares, stock options and warrants. The quantities and per unit prices presented throughout the consolidated financial statements, including this note, have been retroactively adjusted to give effect to the share consolidation.
2020
As part of the consideration for the private placement completed on November 3, 2020 (note 17a) where SALP and another investor participated equally, and a subsequent amendment to this private placement agreement made on November 25, 2020, the Company issued a total of 789,472 warrants that expire on November 3, 2025. Both of these issuances combined are referred to as the November 2020 warrants. Each warrant can be exercised to acquire one common share at an exercise price initially set at USD 55.0 and that can be reduced if equity financings are completed at a lower price before its expiry. The November 2020 warrants do not meet the definition of an equity instrument since the exercise price is denominated in USD which is different than the functional currency of Liminal which is the CAD. Consequently, they are accounted for as a financial instrument, presented as a warrant liability in the consolidated statement of financial position and carried at fair value through profit or loss.
The fair value of the warrants issued on November 3, 2020 and November 25, 2020 were $10,263 and $2,227, respectively. The portion of the total issuance cost pertaining to the private placement allocated to the issuance of the November 3, 2020 warrants of $709 and the fair value of the additional warrants issued on November 25, 2020 were recorded in the consolidated statement of operation transactions in financing costs and administration, selling and marketing expenses respectively. The fair value of the warrant liability of the November 2020 warrants was $11,640 at December 31, 2020. The gain of $850 resulting from the change in fair value of the warrants since their issuance was recognized in the statement of operations for the year ended December 31, 2020.
2021 and 2022
The fair value of the November 2020 warrants was $106 at December 31, 2022 ($1,754 at December 31, 2021) and the gain of $1,648, resulting from the change in fair value of the November 2020 warrants during the year ended December 31, 2022 ($9,886 during the year ended December 31, 2021) was recognized in the consolidated statement of operations. The fair value for the November 2020 warrants held by SALP was $53 and $877 at December 31, 2022 and 2021.
The fair value of the November 2020 warrants on the various dates discussed above was calculated using a Black-Scholes option pricing model in a Monte Carlo simulation in order to evaluate the downward adjustment mechanism to the exercise price. The assumptions used at the different valuation dates are provided in the table below:
| | | | | | | | | | | |
| | | | | December 31, | | | December 31, | |
| | | | | | 2022 | | | | 2021 | |
Underlying common share fair value (in USD) | | | | | $ | 3.25 | | | $ | 10.90 | |
Remaining life until expiry | | | | | | 2.8 | | | | 3.8 | |
Volatility | | | | | | 51.0 | % | | | 56.0 | % |
Risk-free interest rate | | | | | | 4.26 | % | | | 1.13 | % |
Expected dividend rate | | | | | | — | | | | — | |
Fair value of a warrant calculated using a
Black-Sholes pricing model (in USD) | | | | | $ | — | | | $ | 0.80 | |
Fair value of exercise price adjustment mechanism
(in USD) | | | | | $ | 0.12 | | | $ | 1.63 | |
Illiquidity discount | | | | | | 20.0 | % | | | 28.0 | % |
Fair value of a warrant (in USD) | | | | | $ | 0.10 | | | $ | 1.75 | |
Fair value of a warrant (in CAD) | | | | | $ | 0.13 | | | $ | 2.22 | |
F-33
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
16. Long-term debt
| | | | | | | | | | | | | |
| | | | | | | | 2022 | | | | 2021 | |
Balance at January 1 | | | | $ | 38,311 | | | $ | 40,532 | |
Stated and accreted interest | | | | | 520 | | | | 4,388 | |
Conversion of secured convertible debt into shares | | | | | — | | | | (2,664 | ) |
Repayment of principal | | | | | (39,123 | ) | | | — | |
Repayment of stated interest | | | | | — | | | | (3,945 | ) |
Loss on extinguishments of liabilities | | | | | | | | 292 | | | | — | |
Balance at December 31 | | | | | | | $ | — | | | $ | 38,311 | |
At December 31, 2022 and 2021, the carrying amount of the debt comprised the following loans:
| | | | | | | | | | | | | |
| | | | | | | December 31, | | | December 31, | |
| | | | | | | | 2022 | | | | 2021 | |
First term loan having a principal of $10,000 maturing
on April 23, 2024 bearing stated interest of 10% per annum
(effective interest rate of 15.05%) 1) | | | | | | $ | — | | | $ | 9,188 | |
Second term loan having a principal of $29,123 maturing on
April 23, 2024 bearing stated interest of 10% per annum
(effective interest rate of 10.47%) 1) | | | | | | | — | | | | 29,123 | |
| | | | | | | $ | — | | | $ | 38,311 | |
Less current portion of long-term debt | | | | | | | — | | | | — | |
Long-term portion of long-term debt | | | | | | $ | — | | | $ | 38,311 | |
1) The first and second term loans issued under the consolidated loan agreement with SALP were secured by all the assets of the Company and required that certain covenants be respected including maintaining an adjusted working capital ratio. In February 2022, these loans were repaid in full and the related security interests were released.
F-34
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
2022
On February 15, 2022, the Company repaid the entirety of the first and second term loans, representing an aggregate principal amount of $39,123 and the stated interest accrued of $484, for an amount of $39,123, thus terminating the consolidated loan agreement with SALP and releasing the security interests granted by the Company over its assets pursuant to the loan agreement and related documents. The difference of $292 between the carrying amount of the loans extinguished and the cash payment was recorded as a loss on extinguishment of liabilities. The Company incurred $40 in legal fees in relation to this transaction, which were recorded against the loss on extinguishment of liabilities.
The repayment also terminated the royalty stream agreement with SALP resulting in the derecognition of the royalty payment obligation to SALP included in other long-term liabilities and the recognition of a gain on extinguishment of liabilities of $120.
In concurrence with the repayment, the 16,873 warrants held by SALP and having an exercise price of $152.10 per common share (note 17c) were cancelled.
2021
Concurrently with the Fairhaven acquisition that closed on July 17, 2020, the Company issued secured convertible debentures, or SCD, to certain former Fairhaven shareholders, for an aggregate principal amount of $2,410 and bearing an interest rate of 8% per annum, compounded quarterly. The SCD were due on the earlier of i) March 31, 2022, the maturity date, unless converted into common shares of the Company prior to the maturity date or ii) upon a change of control event. At any time prior to the maturity date, the SCD holders have the right to convert the SCD into common shares of the Company.
The Company and the parties to the share purchase agreement dated July 17, 2020 entered into an amendment to this agreement in November 2021 to terminate 1) the collective rights of certain sellers to purchase additional SCD issued by the Company for an aggregate principal amount of up to $5,740 with substantially the same terms and conditions as set out in the original SCD and 2) the Company’s right, if the pre-determined events allowing the Company to trigger the conversion of the SCD occur prior to the maturity date, to require certain sellers to purchase additional SCD for an aggregate principal amount of up to $5,740, which would then be converted into common shares.
On October 20, 2021, the Company exercised its right to convert the entirety of its SCD, having a balance of $2,664 on the conversion date into 109,857 common shares of Liminal, using a conversion price of $24.20 (USD 19.60) calculated as the volume weighted average trading price of the shares in the five trading days immediately preceding the conversion. Liminal’s conversion right became exercisable upon the occurrence of an event which resulted in the Company having a cash balance over $75,000. The difference between the carrying value of the SCD and the fair value of the common shares issued and recorded in share capital of $2,589, calculated using the closing trading price on the conversion date, was $75 and was recorded as a gain on extinguishment of a liability.
F-35
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
17. Share capital and other equity instruments
On February 1, 2023, the Company performed a share consolidation of all its issued and outstanding common shares, stock options and warrants on the basis of a consolidation ratio of ten pre-consolidation shares to one post-consolidation share. The quantities and per unit prices of the Company's common shares, stock options and warrants presented in these audited annual consolidated financial statements have been retroactively adjusted to give effect to the share consolidation.
a) Share capital
Authorized and without par value
Common shares: unlimited number authorized, participating, carrying one vote per share, entitled to dividends.
Preferred shares: unlimited number authorized, issuable in one or more series.
-Series A preferred shares: unlimited number authorized, no par value, non-voting, ranking in priority to the common shares, entitled to the same dividends as the common shares, non-transferable, redeemable at the redemption amount offered for the common shares upon a change in control event.
No preferred shares have been issued. Apart from the share consolidation, there were no changes in the issued and outstanding common shares during the year ended December 31, 2022. At December 2022 and 2021, the number of common shares outstanding were as follows:
| | | | | | | | | | | | | | | | |
| | | | | | 2022 | | | | | | | 2021 | |
| | Number | | | Amount | | | Number | | | Amount | |
Balance - beginning of year | | | 3,104,222 | | | $ | 979,849 | | | | 2,994,350 | | | $ | 977,261 | |
Shares issued pursuant to a restricted share
units plan (note 17b) | | | — | | | | — | | | | 14 | | | | — | |
Shares issued upon conversion of debt | | | — | | | | — | | | | 109,858 | | | | 2,588 | |
Balance - end of year | | | 3,104,222 | | | $ | 979,849 | | | | 3,104,222 | | | $ | 979,849 | |
2021
On October 20, 2021, the Company exercised its right to convert, the entirety of its secured convertible debt (note 16) into 109,858 of its common shares.
2020
On January 29, 2020, the Company issued 9,683 common shares as a consideration for the final payment for a license acquired in January 2018. This transaction was accounted for as an extinguishment of the license acquisition payment obligation and the difference between the carrying value of the liability of $1,319 and the amount recorded for the shares issued of $1,240, which were valued at the market price of the shares on their date of issuance, was recorded as a gain on extinguishment of liabilities of $79 during the year ended December 31, 2020.
F-36
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
b) Contributed surplus (Share-based payments)
Stock options
The Company has established a stock option plan for its directors, officers, employees and service providers. The plan provides that the aggregate number of shares reserved for issuance at any time under the plan may not exceed 374,971 common shares and the maximum number of common shares, which may be reserved for issuance to any individual, may not exceed 5% of the outstanding common shares. The stock options issued under the plan may be exercised over a period not exceeding ten years from the date they were granted. Most of the stock options outstanding have a contractual life of 10 years.
The vesting period of the stock options varies from immediate vesting to vesting over a period not exceeding six years, most of them vesting over four years. Participants meeting certain service and age requirements may see the vesting of certain awards accelerate upon retirement. The vesting conditions are established by the Board of Directors on the grant date. The exercise price is based on the weighted average share price for the five business days prior to the grant.
For stock options having a CAD exercise price, the changes in the number of stock options outstanding during the years ended December 31, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | | | |
| | | | | | | 2022 | | | | | | | 2021 | |
| | | | | | Weighted | | | | | | Weighted | |
| | | | | | average | | | | | | average | |
| | | | | | exercise price | | | | | | exercise price | |
| | | Number | | | ($) | | | Number | | | ($) | |
Balance - beginning of year | | | | 102,805 | | | $ | 215.29 | | | | 248,591 | | | $ | 187.11 | |
Forfeited | | | | (1,168 | ) | | | 143.47 | | | | (132,178 | ) | | | 143.32 | |
Expired | | | | (8 | ) | | | 21,225.00 | | | | (13,608 | ) | | | 399.56 | |
Balance - end of year | | | | 101,629 | | | $ | 214.46 | | | | 102,805 | | | $ | 215.29 | |
For options having a USD exercise price, the changes in the number of stock options outstanding during the years ended December 31, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | | | |
| | | | | | | 2022 | | | | | | | 2021 | |
| | | | | | Weighted | | | | | | Weighted | |
| | | | | | average | | | | | | average | |
| | | | | | exercise price | | | | | | exercise price | |
| | | Number | | | (USD) | | | Number | | | (USD) | |
Balance - beginning of year | | | | 74,900 | | | $ | 34.88 | | | | 30,500 | | | $ | 46.98 | |
Granted | | | | 70,850 | | | | 6.33 | | | | 49,200 | | | | 28.02 | |
Forfeited | | | | (14,834 | ) | | | 16.43 | | | | (3,800 | ) | | | 41.04 | |
Expired | | | | — | | | | — | | | | (1,000 | ) | | | 42.70 | |
Balance - end of year | | | | 130,916 | | | $ | 21.52 | | | | 74,900 | | | $ | 34.88 | |
F-37
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
2022
In March 2022, 16,500 stock options having an exercise price of USD 10.20 and vesting over a period of up to four years were issued to executives and a member of the Board of Directors. In June 2022, 44,350 stock options having an exercise price of USD 5.40 and vesting over a period of up to four years, were issued to executives, members of the Board of Directors and employees. Finally, in December, 2022, 10,000 stock options having an exercise price of USD 4.10 and vesting over a period up to four years, were issued to a member of the Company's key management.
2021
In January 2021, 4,000 stock options having an exercise price of USD 53.40, of which 2,000 stock options vested immediately and the remaining stock options vest over a period up to one year, were issued to a member of the Board of Directors. In June 2021, 5,000 stock options having an exercise price of USD 40.90, of which 2,500 stock options vested immediately and the remaining stock options vest over a period up to one year, were issued to a member of the Board of Directors. In July 2021, 5,000 stock options having an exercise price of USD 39.30, of which 1,250 stock options vested on October 1, 2021 and the remaining stock options vest over a period up to one year, were issued to members of the Board of Directors. In October 2021, 35,200 stock options, having an exercise price of USD 21.70 and vesting over a period of up to four years, were issued to employees.
2020
In March 2020, Liminal’s board of directors approved a plan to reduce the exercise price of the stock options issued in June 2019, held by active employees and directors at the time of the repricing. On May 26, 2020, a revised exercise price, pending approval, of $152.10 was determined, changing the exercise price to the higher of (i) $152.10 and (ii) the five trading-day VWAP of Liminal common shares on the repricing date. On June 8, 2020, the repricing of 192,990 of the outstanding stock options having exercise prices of $270.00 and $360.00 to the revised exercise price was approved at the Company’s annual shareholder meeting.
Although the stock options were not repriced until May 26 2020, management concluded that the service period for employees and directors to earn the modified awards had commenced from the date the Company informed the holders of these stock options of the repricing proposal and the expense resulting from the repricing plan should be recognized starting from that date. Using the revised exercise price of $152.10, the Company calculated the final incremental fair value of the repricing on the grant date of May 26, 2020 to be $2,998. The incremental grant date fair value of the repriced options was estimated based on the Black-Scholes option-pricing model calculated before and after the effect of the repricing. The following Black-Scholes assumption were used:
| | | |
Expected dividend rate | | — | |
Expected volatility of share price | | 93.2 | % |
Risk-free interest rate | | 0.4 | % |
Expected life in years | | 6.3 | |
Weighted average grant date incremental fair value | $ | 15.54 | |
F-38
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
In June 2020, 43,657 stock options, having an exercise price of $140.60 and vesting over a period of up to four years, were issued to employees and directors. In October 2020, 2,000 stock options, having an exercise price of US$108.00 and vesting over a period of three years were issued to a new director. In December 2020, 28,500 stock options having an exercise price of US$42.70, of which 9,500 stock options vested immediately and the remaining stock options vest over a period up to three years, were issued to key management.
During the year ended December 31, 2020, 539 stock options were exercised resulting in cash proceeds of $82 and a transfer from contributed surplus to share capital of $85. The weighted average share price on the date of exercise of the stock options during the year ended December 31, 2020 was $184.70.
The Company uses the Black-Scholes option pricing model to calculate the fair value of stock options at the date of grant. The weighted average inputs into the model and the resulting grant date fair values during the years ended December 31, 2022, 2021 and 2020 were as follows:
| | | | | | | | | | | | | | | |
| | | | | | 2022 | | | | 2021 | | | | 2020 | |
Expected dividend rate | | | | | | — | | | | — | | | | — | |
Expected volatility of share price | | | | | | 116.0 | % | | | 115.0 | % | | | 100.5 | % |
Risk-free interest rate | | | | | | 2.97 | % | | | 1.21 | % | | | 0.5 | % |
Expected life in years | | | | | | 6.8 | | | | 6.7 | | | | 6.7 | |
Weighted average grant date fair value | | | | | $ | 7.18 | | | $ | 28.52 | | | $ | 85.08 | |
At December 31, 2022, stock options issued and outstanding denominated in CAD and USD by range of exercise price are as follows:
| | | | | | | | | | | | | | | | | | | |
| | | | Weighted
average | | | Weighted | | | | | | Weighted | |
Range of exercise | | | | remaining | | | average | | | | | | average | |
price for stock option | Number | | | contractual life | | | exercise price | | | Number | | | exercise price | |
issued in CAD | outstanding | | | (in years) | | | ($) | | | exercisable | | | ($) | |
$119.90-$146.35 | | 16,288 | | | | 7.30 | | | $ | 136.79 | | | | 12,390 | | | $ | 136.53 | |
$146.36-$211.05 | | 79,793 | | | | 6.43 | | | | 152.10 | | | | 65,168 | | | | 152.10 | |
$211.06 - $2,130.00 | | 5,025 | | | | 6.43 | | | | 358.66 | | | | 5,025 | | | | 358.66 | |
$2,130.01-$20,700.00 | | 523 | | | | 5.48 | | | | 10,761.57 | | | | 523 | | | | 10,761.57 | |
| | 101,629 | | | | 6.56 | | | $ | 214.46 | | | | 83,106 | | | $ | 229.04 | |
| | | | Weighted
average | | | Weighted | | | | | | Weighted | |
Range of exercise | | | | remaining | | | average | | | | | | average | |
price for stock option | Number | | | contractual life | | | exercise price | | | Number | | | exercise price | |
issued in USD | outstanding | | | (in years) | | | (USD) | | | exercisable | | | (USD) | |
$4.10-$4.75 | | 10,000 | | | | 9.95 | | | $ | 4.10 | | | | — | | | $ | — | |
$4.76-$7.80 | | 39,550 | | | | 9.43 | | | | 5.40 | | | | 3,250 | | | | 5.40 | |
$7.81 - $15.95 | | 16,500 | | | | 9.24 | | | | 10.20 | | | | — | | | | — | |
$15.96-$41.80 | | 34,866 | | | | 8.69 | | | | 26.98 | | | | 18,292 | | | | 31.76 | |
$41.81 - $108.00 | | 30,000 | | | | 7.94 | | | | 48.48 | | | | 24,332 | | | | 48.04 | |
| | 130,916 | | | | 8.91 | | | $ | 21.52 | | | | 45,874 | | | $ | 38.53 | |
A share-based payment compensation expense of $1,864 was recorded for the stock options for the year ended December 31, 2022 ($4,252 and $6,169 for the year ended December 31, 2021 and 2020 respectively).
F-39
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Restricted share units
The Company has established an equity-settled RSU plan for executive officers of the Company, as part of its incentive program designed to align the interests of its executives with those of its shareholders, and in accordance with its long-term incentive plan. The vesting conditions are established by the Board of Directors on the grant date. Participants meeting certain service and age requirements may see the vesting of certain awards accelerate upon retirement. Each vested RSU gives the right to receive a common share. There have been no RSU grants since 2018 and all the RSU that were earned have since been settled.
Changes in the number of RSU outstanding during the years ended December 31, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | |
| | | | | | 2022 | | | | 2021 | | | | 2020 | |
Balance - beginning of year | | | | | | — | | | | 421 | | | | 1,757 | |
Forfeited | | | | | | — | | | | (5 | ) | | | (5 | ) |
Released | | | | | | — | | | | (14 | ) | | | (1,036 | ) |
Paid in cash | | | | | | — | | | | (402 | ) | | | (295 | ) |
Balance - end of year | | | | | | — | | | | — | | | | 421 | |
2022 and 2021
There have been no expenses recorded in 2022 or in 2021 for RSU.
During the year ended December 31, 2021, 402 RSU were paid in cash resulting in a reduction to contributed surplus of $20.
2020
During the year ended December 31, 2020, 295 RSU were paid in cash resulting in a reduction to contributed surplus of $40. As at December 31, 2020, all 421 outstanding RSU were vested. A share-based payment compensation expense of $65 was recorded during the year ended December 31, 2020.
Share-based payments expense
The total share-based payments compensation expense, comprising the above-mentioned expenses for stock options and RSU, has been included in the consolidated statements of operations for the years ended December 31, 2022, 2021 and 2020 as indicated in the following table:
| | | | | | | | | | | | | | | |
| | | | | 2022 | | | 2021 | | | 2020 | |
Research and development expenses | | | | | $ | 821 | | | $ | 936 | | | $ | 2,430 | |
Administration expenses | | | | | | 1,043 | | | | 3,760 | | | | 3,248 | |
Loss from discontinued operations | | | | | | — | | | | (444 | ) | | | 556 | |
| | | | | $ | 1,864 | | | $ | 4,252 | | | $ | 6,234 | |
c) Warrants
The following table presents the number of warrants outstanding with an exercise price in CAD during the years ended December 31, 2022 and 2021:
| | | | | | | | | | | | | | | | | |
| | | | | | | 2022 | | | | | | | 2021 | |
| | | | | | Weighted | | | | | | Weighted | |
| | | | | | average | | | | | | average | |
| | | | | | exercise price | | | | | | exercise price | |
| | | Number | | | ($) | | | Number | | | ($) | |
Balance of warrants - beginning of year | | | 17,273 | | | $ | 843.30 | | | | 17,273 | | | $ | 843.30 | |
Cancelled (note 16) | | | (16,873 | ) | | | 152.10 | | | | — | | | | — | |
Balance of warrants - end of year | | | 400 | | | $ | 29,999.84 | | | | 17,273 | | | $ | 843.30 | |
F-40
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
The following table presents the changes in the number of warrants outstanding with an exercise price in USD during the years ended December 31, 2022 and 2021:
| | | | | | | | | | | | | | | | | |
| | | | | | | 2022 | | | | | | | 2021 | |
| | | | | | Weighted | | | | | | Weighted | |
| | | | | | average | | | | | | average | |
| | | | | | exercise price | | | | | | exercise price | |
| | | Number | | | (USD) | | | Number | | | (USD) | |
Balance of warrants - end of year | | | 789,472 | | | $ | 55.00 | | | | 789,472 | | | $ | 55.00 | |
The 789,472 warrants shown in the table above, are those accounted for as a warrant liability (note 16) and are included in this note in order that all the outstanding warrants are presented in aggregate in the tables above.
The warrants outstanding as at December 31, 2022, their exercise price in CAD or in USD, expiry rate and the overall weighted average exercise price in both currency are as follows:
| | | | | | | | | | | | | |
| | | | | Number | | | Expiry
date | | Exercise
price
(CAD) | |
Warrants outstanding with an exercise price in CAD | | | 400 | | | January 2023 | | $ | 30,000.00 | |
| | | | | Number | | | Expiry
date | | Exercise
price
(USD) | |
Warrants outstanding with an exercise price in USD | | | 789,472 | | | November 2025 | | $ | 55.00 | |
On February 15, 2022, the 16,873 warrants having an exercise price of $152.10 were cancelled.
F-41
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
18. Non-controlling interests
The proportion of ownership the Company had in subsidiaries not wholly-owned at December 31, 2022 and December 31, 2021 were as follows:
| | | | | | | | |
Name of subsidiary | Place of incorporation | Proportion of ownership interest held by group | |
| | December 31,
2022 | | | December 31,
2021 | |
Pathogen Removal and Diagnostic
Technologies Inc. | Delaware, U.S. | | 100 | % | | | 77 | % |
NantPro Biosciences, LLC | Delaware, U.S. | | 73 | % | | | 73 | % |
NantPro Biosciences, LLC is a dormant partnership, previously part of the plasma-derived therapeutics segment and had no operations in 2022 and 2021.
On August 8, 2022, the Company acquired for USD 30, 100% of the outstanding preferred and common shares, resulting in Liminal now having a 100% ownership of our subsidiary Pathogen Removal Diagnostic Technologies Inc., or PRDT. As a result of the acquisition of the non-controlling interests, or NCI, in PRDT, the NCI share in the results of PRDT will be nil from the transaction date onwards, in the consolidated statement of operations. The balance of the NCI on the statement of financial position on August 8, 2022, a debit of $8,634, was reclassified against the deficit account during the year ended December 31, 2022.
The results of operations of PRDT in 2022 up until the transaction date and for the comparative periods in 2021 and 2020 and the portion of the net and comprehensive loss attributed to the non-controlling interests are presented below:
| | | | | | | | | | | | | | | |
Year ended December 31 | | | | | | 2022 | | | | 2021 | | | | 2020 | |
Royalty revenues | | | | $ | 157 | | | $ | 565 | | | $ | 572 | |
Royalty expenses | | | | | 308 | | | | (88 | ) | | | (128 | ) |
Research and development expenses | | | | | (84 | ) | | | (244 | ) | | | (196 | ) |
Administration expenses and foreign
exchange (gains) losses | | | | | 2,665 | | | | (946 | ) | | | (1,506 | ) |
Net income (loss) and comprehensive income (loss) | | | | $ | 3,046 | | | $ | (713 | ) | | $ | (1,258 | ) |
Income (loss) attributable to non-controlling interests | | | | $ | 122 | | | $ | (669 | ) | | $ | (832 | ) |
F-42
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
19. Capital management
The Company defines its capital as shareholders’ equity including warrants presented as a liability and financial instruments of a long-term nature (including the current portion) less cash.
| | | | | | | | | | | | | |
| | | | | | | December 31, | | | December 31, | |
| | | | | | | 2022 | | | 2021 | |
Warrant liability | | | | | | | $ | 106 | | | $ | 1,754 | |
Lease liabilities | | | | | | | | 1,487 | | | | 22,471 | |
Provisions | | | | | | | | 6,690 | | | | 22,195 | |
Long-term debt | | | | | | | | - | | | | 38,311 | |
Total equity | | | | | | | | 36,208 | | | | 33,881 | |
Cash | | | | | | | | (37,144 | ) | | | (108,490 | ) |
Total capital | | | | | | | $ | 7,347 | | | $ | 10,122 | |
The Company manages its capital resources to fund the growth and development of its business and to ensure it has sufficient liquidities to support the working capital required to maintain its ability to continue as a going concern and to pay long-term obligations upon maturity. The Company monitors its ability to meet its financial obligations and evaluates funding requirements by forecasting cash requirements.
At the present time, the Company favors financing by issuing equity instruments in order to minimize future financial obligations, however it considers all sources of financing reasonably available, including but not limited to the issuance of equity instruments, debt and the sale of assets. The Company considers the cost of capital, the terms and conditions and the dilutive effect on shareholders when considering the different forms financings that it may prevail upon.
20. Revenues from continuing operations
| | | | | | | | | | | | | | |
| | | 2022 | | | | 2021 | | | | 2020 | |
Royalty revenues | | | | $ | 401 | | | $ | 565 | | | $ | 572 | |
Rental revenue | | | | | — | | | | 78 | | | | 152 | |
| | | | $ | 401 | | | $ | 643 | | | $ | 724 | |
F-43
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
21. Supplemental information
a) Supplemental information regarding the consolidated statements of operations
i) Government assistance
For the years ended December 31, 2021 and 2020, the Company recognized, government grants in connection with the Canada Emergency Wage Subsidy program and the Canada Emergency Rent Subsidy program, two subsidies program created by the Government of Canada in 2020 in response to the COVID-19 pandemic that the Company benefits from. Following the sale of its plasma-derived business in the middle of the second quarter of 2021, the Company was no longer eligible to these programs.
The Company also recognized research and development tax credits during the years ended December 31, 2022, 2021 and 2020. These grants were recorded as a reduction of salary expenses and other related charges and are recognized as follows in the consolidated statement of operations:
| | | | | | | | | | | | | | | |
Year ended December 31 | | | 2022 | | | | 2021 | | | | 2020 | |
Government grants recognized in research
and development expenses, continuing operations: | | | | | | | | | | | |
Salary subsidy | | | | | $ | — | | | $ | 372 | | | $ | 1,017 | |
Rent subsidy | | | | | | — | | | | 140 | | | | 108 | |
Research and development tax credits | | | | | 135 | | | | 124 | | | | 426 | |
| | | | $ | 135 | | | $ | 636 | | | $ | 1,551 | |
Government grants recognized in
administration expenses, continuing operations: | | | | | | | | | | | |
Salary subsidy | | | | | $ | 14 | | | $ | 325 | | | $ | 1,457 | |
Rent subsidy | | | | | | — | | | | 86 | | | | 63 | |
| | | | $ | 14 | | | $ | 411 | | | $ | 1,520 | |
Government grants recognized in loss from
discontinued operations: | | | | | | | | | | | |
Salary subsidy | | | | | $ | — | | | $ | 2,502 | | | $ | 4,758 | |
Rent subsidy | | | | | | — | | | | 682 | | | | 426 | |
Research and development tax credits | | | | | — | | | | 116 | | | | 1,332 | |
| | | | $ | — | | | $ | 3,300 | | | $ | 6,516 | |
ii) Finance costs
| | | | | | | | | | | | | | | |
Year ended December 31 | | | | | 2022 | | | | 2021 | | | | 2020 | |
Interest accretion on long-term debt | | | | $ | 521 | | | $ | 4,388 | | | $ | 2,209 | |
Financing fees on warrant liability | | | — | | | | — | | | | 709 | |
Interest expense on provisions | | | | | 393 | | | | 90 | | | | — | |
Other interest expense, transaction and bank fees | | | | | (142 | ) | | | 413 | | | | 485 | |
Interest expense on lease liabilities | | | | | 1,081 | | | | 3,754 | | | | 6,030 | |
Lease modification and other remeasurements | | | | | | (16,019 | ) | | | — | | | | — | |
Interest income | | | | | (775 | ) | | | (73 | ) | | | (451 | ) |
| | | | $ | (14,941 | ) | | $ | 8,572 | | | $ | 8,982 | |
The table above includes financing costs from continuing and discontinued operations. Financing costs from discontinued operations for the years ended December 31, 2022, 2021 and 2020 were $16,019, $2,242, and $6,083, respectively, and mainly represented interest expense on lease liabilities and a gain on modification of a liability (note 6).
F-44
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
iii) Employee compensation expense
| | | | | | | | | | | | | | |
Year ended December 31 | | | | | 2022 | | | | 2021 | | | | 2020 | |
Wages and salaries | | | | $ | 8,336 | | | $ | 19,731 | | | $ | 32,410 | |
Employer's benefits | | | | | 1,762 | | | | 3,201 | | | | 5,443 | |
Share-based payments expense | | | | | 1,864 | | | | 4,252 | | | | 6,234 | |
| | | | $ | 11,962 | | | $ | 27,184 | | | $ | 44,087 | |
The table above includes employee compensation expense from continuing and discontinued operations. Employee compensation expenses from discontinued operations for the year ended December 31, 2022, 2021 and 2020 were $nil, $9,958 and $23,146, respectively.
b) Information by geographic area
i) Capital, intangible and right-of-use assets by geographic area
| | | | | | | | | | | | | |
| | | 2022 | | | 2021 | |
Canada | | | | | | | $ | 8,345 | | | $ | 10,041 | |
United Kingdom | | | | | | | | 385 | | | | 567 | |
| | | $ | 8,730 | | | $ | 10,608 | |
ii) Revenues by location from continuing operations
| | | | | | | | | | | | | | | |
| | | | | 2022 | | | | 2021 | | | | 2020 | |
Canada | | | | | | 3 | | | | 78 | | | | 152 | |
United Kingdom | | | | | | 398 | | | | 565 | | | | 572 | |
| | | | | $ | 401 | | | $ | 643 | | | $ | 724 | |
Revenues are attributed to countries based on the location of the third party.
22. Pension Plan
The Company maintains a defined contribution pension plan for its permanent employees. The Company matches the contributions made by employees who elect to participate in the plan up to a maximum percentage of their annual salary. The Company’s contributions recognized as an expense, for continuing and discontinued operations in aggregate, for the year ended December 31, 2022 amounted to $299 ($598 and $1,055 for the years ended December 31, 2021 and 2020, respectively).
F-45
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
23. Impairment losses
2022
During the year ended December 31, 2022, impairment losses were $nil.
2021
During the quarter ended June 30, 2021, the Company decided it would not be moving fezagepras into a Phase 2 clinical study in Idiopathic Pulmonary Fibrosis or IPF, and a phase 1a/2b study in hypertriglyceridemia following its analysis of the interim PK results from the ongoing fezagepras phase 1 MAD clinical trial. As a result of these decisions which were considered impairment indicators, the Company recorded an impairment on the carrying value of the intangible assets for the related patents of $341 reducing their value to their estimate recoverable value of $nil. Other fezagepras patents were unaffected by the above decisions.
2020
At the end of 2020, in reviewing its portfolio of compounds in the small molecule therapeutics segment, the Company identified impairment indicators for certain patents. One of the patent families impaired concerned a molecule that had entered into a phase 1 clinical trial in 2019 that was subsequently discontinued after the review of the pharmacokinetic data for the first three cohorts obtained. Following additional pre-clinical studies conducted in 2020 to further the Company’s understanding of the mechanism of action, or MOA, lead to findings that the MOA included engaging a receptor which has been known in other products which engage the same receptor to occasionally cause undesirable side effects. Subsequently, management decided that the preclinical and clinical development activities associated with demonstrating that such molecule did not induce such side effects would be both time-consuming and costly and therefore the future development has been suspended. Another patent family impaired concerned another molecule that is licensed for development with a third party, whose research and development work we believe to be delayed from the agreed upon timelines and is unlikely to perform significant development in the near future. Further, the development of another compound was deprioritized, as the Company wishes to prioritize development of its lead compound fezagepras, as well as GPR84 and OXER1 drug candidates, which led to the impairment of the related patents. These small molecules patents were written down to their net recoverable amount of $nil, as both the FVLCD and the value in use were determined to be insignificant, resulting in an impairment of $1,072 for the year ended December 31, 2020 (note 11). During the year, the Company also recorded software impairments amounting to $15 (note 11).
24. Income taxes
The income tax expense (recovery) reported in the consolidated statement of operations for the years ended December 31, 2022, 2021 and 2020 are as follows:
| | | | | | | | | | | | | | | |
| | 2022 | | | 2021 | | | 2020 | |
Current income tax recovery | | $ | (811 | ) | | $ | — | | | $ | (144 | ) |
Deferred income tax expense | | | 286 | | | | 118 | | | | (65 | ) |
Income tax expense (recovery) from continuing operations | | | (525 | ) | | | 118 | | | | (209 | ) |
Current income tax expense from
discontinued operations (note 6) | | | | | | — | | | | 1 | | | | 8 | |
Total income tax expense (recovery) | | $ | (525 | ) | | $ | 119 | | | $ | (201 | ) |
F-46
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
The following table provides a reconciliation of the income tax expense (recovery) calculated at the combined statutory income tax rate to the income tax expense (recovery) for both continuing and discontinued operations, recognized in the consolidated statements of operations.
| | | | | | | | | | | | |
| | 2022 | | | 2021 | | | 2020 | |
Net loss before tax from continuing operations | | $ | (29,441 | ) | | $ | (44,945 | ) | | $ | (49,230 | ) |
Net income before tax from discontinued operations | | | 29,538 | | | | 57,277 | | | | (69,728 | ) |
Combined Canadian statutory income tax rate | | | 26.5 | % | | | 26.5 | % | | | 26.5 | % |
Income tax expense (recovery) at combined income tax rate | | | 26 | | | | 3,268 | | | | (31,524 | ) |
| | | | | | | | | |
Increase (decrease) in income taxes resulting from: | | | | | | | | | |
Unrecorded potential tax benefit arising from
current-period losses and other deductible
temporary differences | | | (2,330 | ) | | | 1,658 | | | | 33,238 | |
Effect of tax rate differences in foreign subsidiaries | | | 406 | | | | 9,756 | | | | 1,101 | |
Non-deductible or taxable items | | | 3,141 | | | | (6,149 | ) | | | (157 | ) |
Change in future tax rate | | | (1,008 | ) | | | (5,354 | ) | | | (1,455 | ) |
Research and development tax credit | | | 65 | | | | (3,012 | ) | | | (494 | ) |
Foreign withholding tax | | | (811 | ) | | | — | | | | — | |
Non-taxable gain on disposition of subsidiary (note 6) | | | — | | | | — | | | | (896 | ) |
Other | | | (14 | ) | | | (48 | ) | | | (14 | ) |
Income tax expense (recovery) | | $ | (525 | ) | | $ | 119 | | | $ | (201 | ) |
F-47
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Deferred tax relates to the following in the consolidated statement of financial position:
| | | | | | | | | | | | | |
Year ended December 31 | | | | | | | 2022 | | | 2021 | |
Deferred tax assets | | | | | | | | | | | |
Tax losses (non capital) | | | | | | | $ | 133,917 | | | $ | 118,276 | |
Tax losses (capital) | | | | | | | | 49,896 | | | | 7,393 | |
Research and development expenses | | | | | | | | 6,818 | | | | 7,124 | |
Unrealized loss on exchange rate | | | | | | | | 2 | | | | — | |
Undeducted financing expenses | | | | | | | | 695 | | | | 1,180 | |
Right of use liabilities | | | | | | | | 335 | | | | 5,606 | |
Share based payments | | | | | | | | 1 | | | | 1 | |
Penalty Prepayment | | | | | | | | 3,611 | | | | 5,993 | |
Interest expenses carried forward | | | | | | | | 191 | | | | 2,646 | |
Non-deductible provisions | | | | | | | | 1,968 | | | | 5,986 | |
Donations | | | | | | | | 7 | | | | 270 | |
Intangible assets | | | | | | | | 266 | | | | 683 | |
Capital Assets | | | | | | | | 1,363 | | | | 1,212 | |
Start-up expense | | | | | | | | 272 | | | | 401 | |
Total deferred tax assets | | | | | | | $ | 199,342 | | | $ | 156,771 | |
| | | | | | | | | | | |
Deferred tax liabilities: | | | | | | | | | | | |
Capital assets | | | | | | | $ | (11 | ) | | $ | (200 | ) |
Intangible Assets | | | | | | | | (42 | ) | | | — | |
Right of use assets | | | | | | | | (259 | ) | | | (143 | ) |
Unrealized gain on exchange rate | | | | | | | | (1,602 | ) | | | (436 | ) |
Other | | | | | | | | — | | | | (8 | ) |
Total deferred tax liabilities | | | | | | | $ | (1,914 | ) | | $ | (787 | ) |
| | | | | | | | | | | |
Deferred tax assets | | | | | | | $ | 197,428 | | | $ | 155,984 | |
Unrecognized deferred tax assets | | | | | | | | (197,260 | ) | | | (155,530 | ) |
| | | | | | | $ | 168 | | | $ | 454 | |
Reflected in the consolidated statement of financial position for the years ended December 31, 2022 and 2021 as follows:
| | | | | | | | | | | | | |
| | | | | | | 2022 | | | 2021 | |
Balance - beginning of year | | | | | | | $ | 454 | | | $ | 572 | |
Credited to profit and loss | | | | | | | | (286 | ) | | | (118 | ) |
Balance - end of year | | | | | | | $ | 168 | | | $ | 454 | |
F-48
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Deferred tax relates to the following in the consolidated statement of operations:
| | | | | | | | | | | | | | | |
Year ended December 31 | | | | | 2022 | | | 2021 | | | 2020 | |
Deferred tax assets | | | | | | | | | | | | |
Tax losses (non capital) | | | | | $ | (15,640 | ) | | $ | 42,309 | | | $ | (38,535 | ) |
Tax losses (capital) | | | | | | (42,503 | ) | | | (7,312 | ) | | | (81 | ) |
Research and development expenses | | | | | | 306 | | | | 15,381 | | | | 8,607 | |
Unrealized loss on exchange rate | | | | | | (2 | ) | | | 1,434 | | | | (568 | ) |
Undeducted financing expenses | | | | | | 484 | | | | 1,017 | | | | 327 | |
Right of use liabilities | | | | | | 5,271 | | | | 3,007 | | | | 709 | |
Share based payments | | | | | | — | | | | 350 | | | | 11 | |
Penalty Prepayment | | | | | | 2,382 | | | | 2,613 | | | | (7,186 | ) |
Interest expenses carried forward | | | | | | 2,455 | | | | 1,424 | | | | (1,592 | ) |
Non-deductible provisions | | | | | | 4,018 | | | | (5,958 | ) | | | 625 | |
Donations | | | | | | 263 | | | | (270 | ) | | | — | |
Intangible assets | | | | | | 418 | | | | 12,544 | | | | 1,037 | |
Capital Assets | | | | | | (151 | ) | | | (317 | ) | | | 435 | |
Start-up expense | | | | | | 128 | | | | 825 | | | | 274 | |
Other | | | | | | — | | | | 246 | | | | (50 | ) |
Total deferred tax assets | | | | | $ | (42,571 | ) | | $ | 67,293 | | | $ | (35,987 | ) |
| | | | | | | | | | | | |
Deferred tax liabilities: | | | | | | | | | | | | |
Capital assets | | | | | $ | (189 | ) | | $ | (2,607 | ) | | $ | (2,528 | ) |
Intangible Assets | | | | | | 42 | | | | (5 | ) | | | (5 | ) |
Right of use assets | | | | | | 116 | | | | (1,463 | ) | | | (3,877 | ) |
Trade and other payables | | | | | | — | | | | (892 | ) | | | (12 | ) |
Unrealized gain on exchange rate | | | | | | 1,166 | | | | 40 | | | | (127 | ) |
Other | | | | | | (8 | ) | | | 8 | | | | — | |
Total deferred tax liabilities | | | | | $ | 1,127 | | | $ | (4,919 | ) | | $ | (6,549 | ) |
| | | | | | | | | | | | |
Deferred tax assets | | | | | $ | (41,444 | ) | | $ | 62,374 | | | $ | (42,540 | ) |
Unrecognized deferred tax assets | | | | | | 41,730 | | | | (62,256 | ) | | | 42,475 | |
| | | | | $ | 286 | | | $ | 118 | | | $ | (65 | ) |
At December 31, 2022, the Corporation has non-capital losses of $543,495 ($439,706 for the year ended December 31, 2021) of which $426,005 ($371,146 for the year ended December 31, 2021) are available to reduce future taxable income for which the benefits have not been recognized. These non capital losses expire at various dates from 2023 to 2041 (except for the non-capital losses in the United Kingdom and US losses that arose after 2017 which do not expire as they have an indefinite life).
Capital losses arising in Canada can only be utilized to shelter future capital gains.
At December 31, 2022, the Corporation also has federal unused research and development expenses of $33,925 (provincial $15,035), of which $33,291 are available to reduce future taxable income for which the benefits have not been recognized. These expenses can be carried forward indefinitely.
At December 31, 2022, the Corporation also had unused federal tax credits available to reduce future income tax in the amount of $9,826 ($8,980 for the year ended December 31, 2021), expiring between 2024 and 2042. These credits have not been recorded nor have any deferred income tax assets been recognized in respect to those tax credits.
F-49
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
The unused non-capital losses expire as indicated in the table below:
| | | | | | | | | | | | | | | |
| | | | | Canada | | | Foreign | |
At December 31, 2022 | | | | | Federal | | | Provincial | | | Countries | |
Losses carried forward expiring in: | | | | | | | | | | | | |
2027 | | | | | $ | 3,510 | | | $ | 3,495 | | | $ | — | |
2028 | | | | | | — | | | | — | | | $ | — | |
2029 | | | | | | — | | | | — | | | | — | |
2030 | | | | | | — | | | | — | | | | — | |
2031 | | | | | | — | | | | — | | | | — | |
2032 | | | | | | 1,001 | | | | 995 | | | | — | |
2033 | | | | | | 4,610 | | | | 4,606 | | | | — | |
2034 | | | | | | 7,760 | | | | 9,807 | | | | — | |
2035 | | | | | | 12,565 | | | | 12,563 | | | | — | |
2036 | | | | | | 14,468 | | | | 14,467 | | | | — | |
2037 | | | | | | 7,393 | | | | 7,394 | | | | — | |
2038 | | | | | | 6,594 | | | | 6,595 | | | | — | |
2039 | | | | | | 26,330 | | | | 26,305 | | | | — | |
2040 | | | | | | 37,884 | | | | 37,751 | | | | — | |
2041 | | | | | | 40,013 | | | | 40,061 | | | | — | |
2042 | | | | | | 31,415 | | | | 32,494 | | | | — | |
| | | | | $ | 193,543 | | | $ | 196,533 | | | $ | — | |
Not expiring - UK | | | | | | — | | | | — | | | | 225,566 | |
Not expiring - US (post 2017) 1) | | | | | | — | | | | — | | | | 4,800 | |
| | | | | $ | 193,543 | | | $ | 196,533 | | | $ | 230,366 | |
1) As a result of the conversion of the parent's debt into Liminal shares on April 23, 2019, more than 50% of the issued shares of Liminal were owned by a single shareholder at December 31, 2022. US tax rules impose restrictions that will impact how $122,290 of losses are available to shelter income in future taxation years. As a result of the US restrictions, approximately $117,490 of losses will no longer be available to the company and are not presented in the available tax loss table presented above. The Company has $4,800 of U.S. tax loss carryforwards which arose after April 23, 2019 not subject to these limitations. A deferred tax asset has not been recognized for any loss carryforwards at December 31, 2022.
25. Basic and diluted earnings per share
The Company presents basic and diluted earnings per share, or EPS, data for its common shares. Basic EPS is calculated by dividing the profit or loss attributable to common shareholders of the Company by the weighted average number of common shares outstanding during the period.
For the years ended December 31, 2022, 2021 and 2020, all warrants, stock options and RSU were anti-dilutive since the Company reported net losses from continuing operations. The secured convertible debentures issued in 2020 and subsequently converted into common shares in 2021 were also anti-dilutive during the period they were outstanding.
The numbers for the average basic and diluted shares outstanding presented in the consolidated statements of operations for the year ended December 31, 2022 have been adjusted in order to reflect the effect of the share consolidation that took place on February 1, 2023 (note 17a).
F-50
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
26. Related party transactions
Balances and transactions between the Company and its subsidiaries, which are related parties of the Company, have been eliminated on consolidation and are not disclosed in this note. Details of transactions between the Company and other related parties are disclosed below and in other notes according to the nature of the transactions. These transactions have been recorded at the exchange amount, meaning the amount agreed to between the parties.
At December 31, 2022 and 2021, a former CEO had a balance of $197 and $283, respectively, owing to the Company under a tax equalization program. The amounts are required to be repaid to the Company following the receipt of a refund by the former employee from the taxation authority for each of the two years covered by the program. At December 31, 2022, the Company received the reimbursement of the first year. The remaining amount is expected to be received once the tax return of the second and final year of the program has been assessed by the appropriate government agencies.
All material transactions with SALP are disclosed in notes 15 and 16 where the particular transactions are disclosed, and otherwise in this note.
During the year ended December 31, 2022, the Company also recorded and paid legal expenses of $416 and $349 respectively ($326 and of $181 respectively in 2021), incurred by SALP that it is required to reimburse pursuant to the subscription agreement signed with SALP on April 14, 2019.
F-51
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
27. Compensation of key management personnel
The Company’s key management personnel comprise the external directors, officers and executives which included 11 individuals in 2022, 16 individuals in 2021 and 20 individuals in 2020. The remuneration of the key management personnel during the years ended December 31, 2022, 2021 and 2020 was as follows:
| | | | | | | | | | | | | |
| | | 2022 | | | 2021 | | | 2020 | |
Current employee benefits1) | | | $ | 3,865 | | | $ | 5,466 | | | $ | 6,153 | |
Pension costs | | | | 34 | | | | 78 | | | | 115 | |
Share-based payments | | | | 1,521 | | | | 4,351 | | | | 4,917 | |
Termination benefits | | | | — | | | | 406 | | | | 319 | |
| | | $ | 5,420 | | | $ | 10,301 | | | $ | 11,504 | |
1) Current employee benefits include salaries, bonuses, other employee benefits, other than those listed in the table and director fees paid in cash. In 2022, it also includes the fees paid to a consultant for the salary of the interim CFO for an amount of $407 covering the period from February 2022 to November 2022.
28. Commitments
In the normal course of business, the Company enters into license agreements for the market launching or commercialization of products. Under a royalty stream agreement entered into January 2022, the Company has made a minimum upfront payment of $400 which can be used to reduce future royalty payments calculated as a low-single digit percentage of net sales on products and a low-single digit percentage of license revenues in regard to certain small molecule product candidates targeted by the agreement. A licensing agreement requires a royalty calculated as a low-single digit percentage of net sales on products for a certain small molecule drug candidate. Under licensing agreements pertaining to the Company's former bioseparation business, the Company must pay royalties ranging from a low-single digit to a mid-double digit percentage on the royalty revenues it earns from a sub-licensing agreement.
At December 31, 2021, SALP had the right to receive, under a royalty stream agreement, minimum royalty payments and a 2% royalty on future revenues relating to patents of a specified small molecule product candidate and analogues. The obligations under this royalty agreement were secured by all the assets of the Company until the expiry of the last patent covered by this agreement. In the case where royalties based on revenues became payable, the minimum royalty previously paid would be deducted from future remittances. In February 2022, the royalty stream agreement was terminated.
F-52
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
29. Legal proceedings
The Company is, in the course of its business, subject to lawsuits and other claims. On April 15, 2019, the Company announced its intention to enter into a series of related arrangements to restructure its outstanding indebtedness, reduce its interest and certain other payment obligations, and raise sufficient cash to build a robust balance-sheet for the next phase of its development (collectively, the “Refinancing Transactions”).
On March 2, 2021, Liminal was served with an action instituted by multiple individual shareholder plaintiffs (the “Plaintiffs”) against Liminal, SALP, Thomvest, Consonance Capital Management LP (“Consonance”), as well as the directors (the “Directors”) that were on the Company’s Board on March 31, 2019 or on April 15, 2019 and certain officers of the Company (the “D&Os”, together with Liminal, SALP, Thomvest and Consonance, the “Defendants”). Such action was publicly disclosed on March 24, 2021. On November 2, 2021, Liminal received service of an amended proceeding.
The Plaintiffs’ request in damages has gone from almost $700 million initially to almost $950 million in damages, approximately $905 million of which is based on the loss of future value of the Company’s shares.
The Company believes that the Plaintiffs’ claims are completely without merit and intends to vigorously defend itself. Defense and settlement costs associated with such lawsuits and claims can be substantial, even when these lawsuits and claims have no merit. Due to the inherent uncertainty of the litigation process, the resolution of any particular legal proceeding could have an adverse effect on the Company’s operating results or financial performance. No provisions have been recorded in the interim financial statements in regard to these claims.
30. Financial instruments and financial risk management
a) Fair value
At December 31, 2022 and 2021, the fair value of financial liabilities for which fair value disclosure is required include the royalty payment obligation, the license acquisition payment obligations and the long-term debt. The fair value of those liabilities approximate the carrying amount of such instruments, except for the long-term debt at December 31, 2021, where the fair value of the debt was estimated at $39,132 while the carrying amount was $38,311 (at December 31, 2022 the fair value and carrying amount was $nil as a result of the repayment of the long term debt in February 2022).
The fair value of the long-term debt at December 31, 2021 was calculated using a discounted cash flow model and the market interest rates specific to the term of the debt instruments of 10.47% (2020 - 10%).
Fair value hierarchy
Financial instruments recorded at fair value on the consolidated statements of financial position are classified using a fair value hierarchy that reflects the significance of the inputs used in making the measurements. The fair value hierarchy has the following levels:
Level 1 – valuation based on quoted prices observed in active markets for identical assets or liabilities.
F-53
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
Level 2 – valuation techniques based on inputs that are quoted prices of similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; inputs other than quoted prices used in a valuation model that are observable for that instrument; and inputs that are derived principally from or corroborated by observable market data by correlation or other means.
Level 3 – valuation techniques with significant unobservable market inputs.
A financial instrument is classified to the lowest level of the hierarchy for which a significant input has been considered in measuring fair value.
Cash and restricted cash are considered to be level 1 fair value measurements.
The long-term deposits, royalty payment obligation, license acquisition payment obligations, provisions and long-term debt are level 2 measurements.
The warrant liability is considered to be a level 3 measurements. Further discussion regarding assumptions used in determining its fair value are discussed in notes 3 and 15.
b) Financial risk management
The Company has exposure to credit risk, liquidity risk and market risk. The Company’s Board of Directors has the overall responsibility for the oversight of these risks and reviews the Company’s policies on an ongoing basis to ensure that these risks are appropriately managed.
Credit risk:
Credit risk is the risk of financial loss to the Company if a customer, partner or counterparty to a financial instrument fails to meet its contractual obligations, and arises principally from the Company’s cash and receivables. The carrying amount of the financial assets represents the maximum credit exposure.
The Company's exposure to credit risk is generally limited since it has limited revenues and thus limited accounts receivable. Liminal mitigates credit risk through a credit risk assessment, when credit is granted and subsequently at each reporting period.
Following the sale of its bioseparation business and its plasma-derived business, the Company no longer has product sales and as such the Company’s exposure to customer credit risk is limited.
Liquidity risk:
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they come due. The Company manages its liquidity risk by continuously monitoring forecasts and actual cash flows. The Company’s current liquidity situation is discussed in note 1.
F-54
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
The following table presents the contractual maturities of the financial liabilities as of December 31, 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Contractual Cash flows | |
| | | | Carrying amount | | | Less than
1 year | | | 2-3
years | | | 4 - 5
years | | | More than
5 years | | | Total | |
Accounts payable and accrued
liabilities 1) | | | | $ | 5,968 | | | $ | 5,968 | | | $ | — | | | $ | — | | | $ | — | | | $ | 5,968 | |
Lease liabilities | | | | | 1,487 | | | | 790 | | | | 1,022 | | | | — | | | | — | | | | 1,812 | |
Provisions | | | | | 6,690 | | | | 3,400 | | | | 3,400 | | | | — | | | | — | | | | 6,800 | |
| | | | $ | 14,145 | | | $ | 10,158 | | | $ | 4,422 | | | $ | — | | | $ | — | | | $ | 14,580 | |
1) Short-term portions of the royalty payment obligations and of other employee benefit liabilities are included in the account payable and accrued liabilities.
2) The Company has fully repaid its long-term debt in February 2022 (note 16).
Market risk:
Market risk is the risk that changes in market prices, such as interest rates and foreign exchange rates, will affect the Company’s income or the value of its financial instruments.
i) Interest risk
The Company’s interest-bearing financial liabilities have fixed rates and as such, there is limited exposure to changes in interest payments as a result of interest rate risk. In February 2022, the Company fully repaid its interest bearing long-term debt (note 16).
ii) Foreign exchange risk:
The Company is exposed to the financial risk related to the fluctuation of foreign exchange rates. The Company has had operations and suppliers in the U.S. and the U.K. during the past years and therefore a portion of its expenses incurred are in USD and in GBP. The majority of the Company’s revenues from the sale of products in 2021 are part of its discontinued operations, were in USD, which served to mitigate a portion of the U.S. foreign exchange risk relating to the expenditures. In 2021, the proceeds received from the divestment of its discontinued operations (note 6) were in USD resulting in an increased exposure to the USD which is partially mitigated by expenditures denominated in USD from its continuing operations. Financial instruments that have exposed the Company to foreign exchange risk have been cash, receivables, trade and other payables, lease liabilities, license payment obligations. The Company manages foreign exchange risk by holding foreign currencies it received to support forecasted cash outflows in foreign currencies.
As at December 31, 2022 and 2021, the Company’s net exposure to currency risk through financial assets and financial liabilities denominated respectively in USD and GBP was as follows:
| | | | | | | | | | | | | | | | | |
| | | 2022 | | | 2021 | |
| | | Amount | | | Equivalent in | | | Amount | | | Equivalent in | |
Exposure in USD | | | in USD | | | full CAD | | | in USD | | | full CAD | |
Cash | | | | 2,026,962 | | | | 2,744,710 | | | | 78,045,915 | | | | 99,094,899 | |
Accounts receivable | | | | 18,787 | | | | 25,440 | | | | 185,954 | | | | 236,106 | |
Accounts payable and accrued liabilities | | | | (1,388,225 | ) | | | (1,879,795 | ) | | | (951,284 | ) | | | (1,207,845 | ) |
Other long-term liabilities | | | | — | | | | — | | | | (77,075 | ) | | | (97,862 | ) |
Long-term derivatives | | | | (78,284 | ) | | | (106,005 | ) | | | (1,381,603 | ) | | | (1,754,221 | ) |
Net exposure | | | | 579,240 | | | | 784,350 | | | | 75,821,907 | | | | 96,271,077 | |
F-55
LIMINAL BIOSCIENCES INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
(In thousands of Canadian dollars, except for per share amounts)
| | | | | | | | | | | | | | | | | |
| | | 2022 | | | 2021 | |
| | | Amount | | | Equivalent in | | | Amount | | | Equivalent in | |
Exposure in GBP | | | in GBP | | | full CAD | | | in GBP | | | full CAD | |
Cash | | | | 170,845 | | | | 279,673 | | | | 2,832,439 | | | | 4,859,049 | |
Accounts receivable | | | | 183,386 | | | | 300,203 | | | | 134,605 | | | | 230,914 | |
Accounts payable and accrued liabilities | | | | (320,774 | ) | | | (525,108 | ) | | | (1,026,984 | ) | | | (1,761,791 | ) |
Lease liabilities | | | | (135,675 | ) | | | (222,100 | ) | | | (203,186 | ) | | | (348,566 | ) |
Net exposure | | | | (102,218 | ) | | | (167,332 | ) | | | 1,736,874 | | | | 2,979,606 | |
Based on the above net exposures as at December 31, 2022, and assuming that all other variables remain constant, a 10% depreciation or appreciation of the CAD against the USD would result in a decrease or an increase of the consolidated total comprehensive loss of approximately $78 while a 10% depreciation or appreciation of the CAD against the GBP would result in a decrease or an increase of the consolidated total comprehensive loss of approximately $17. The Company has not hedged its exposure to currency fluctuations.
31. Subsequent event
In February 2023, the Company's dormant manufacturing facility located in Belleville, Ontario, or Belleville facility, formerly part of the plasma-derived therapeutics segment and previously classified as property, plant and equipment, met the criteria to be classified as held for sale. The carrying amount of the Belleville facility was $3,958 at December 31, 2022.
F-56
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
Liminal BioSciences Inc.
/s/ Bruce Pritchard
Name: Bruce Pritchard
Title: Chief Executive Officer
(Principal Executive Officer)
Date: March 15, 2023
F-57






