l
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended March 31, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-37785
Reata Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in its Charter)
| | |
Delaware | | 11-3651945 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer
Identification No.) |
| | |
5320 Legacy Drive
Plano, Texas | | 75024 |
(Address of principal executive offices) | | (Zip Code) |
(972) 865-2219
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Securities Exchange Act of 1934:
| | | | |
Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
Class A Common Stock, Par Value $0.001 Per Share | | RETA | | NASDAQ Global Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, an emerging growth company, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | |
Large accelerated filer |
| ☒ |
| Accelerated filer |
| ☐ |
Non-accelerated filer |
| ☐ |
| Smaller reporting company |
| ☐ |
Emerging growth company | | ☐ | | | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of May 5, 2023, the registrant had 33,036,126 shares of Class A common stock, $0.001 par value per share, and 4,515,316 shares of Class B common stock, $0.001 par value per share, outstanding.
TABLE OF CONTENTS
i
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking statements that involve substantial risks and uncertainties. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. In this Quarterly Report on Form 10-Q, all statements, other than statements of historical or present facts, including statements regarding our future financial condition, future revenues, projected costs, prospects, business strategy, and plans and objectives of management for future operations, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “might,” “estimate,” “continue,” “anticipate,” “intend,” “target,” “project,” “model,” “should,” “would,” “plan,” “expect,” “predict,” “could,” “seek,” “goals,” “potential,” and similar terms or expressions that concern our expectations, strategy, plans, or intentions. These forward-looking statements include, but are not limited to, statements about:
•our plans and objectives for the commercialization of SKYCLARYS® and the timing thereof; including reimbursement, marketing costs, revenues, and licenses (if any);
•the potential market size and the size of the patient population for SKYCLARYS and the market opportunities for SKYCLARYS;
•our ability to successfully build our commercial infrastructure to manufacture, market and sell SKYCLARYS, including the successful development and implementation of our sales and marketing campaigns for SKYCLARYS;
•the ability of our third-party suppliers and contract manufacturers to manufacture SKYCLARYS at the required quality and quantities and in compliance with applicable laws and regulations;
•our expectations regarding the timing, costs, conduct, and outcome of our clinical trials, including statements regarding the timing of the initiation and availability of data from such trials;
•the timing and likelihood of regulatory filings and approvals for our product candidates;
•whether regulatory authorities determine that additional trials or data are necessary in order to accept a new drug application for review and/or approval;
•our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates;
•our plans to research, develop, and commercialize our product candidates;
•the manufacturing, supply, and commercialization of our product candidates, if approved;
•the rate and degree of market acceptance of our product candidates;
•our expectations regarding the potential market size and the size of the patient populations for our product candidates, if approved for commercial use, and the potential market opportunities for commercializing our product candidates;
•the success of competing therapies that are or may become available;
•our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates;
•the ability to license additional intellectual property relating to our product candidates and to comply with our existing license agreements;
•our ability to maintain and establish relationships with third parties, such as contract research organizations (CROs), contract manufacturing organizations, suppliers, and distributors;
•our ability to maintain and establish collaborators with development, regulatory, and commercialization expertise;
•our ability to attract and retain key scientific or management personnel;
•our ability to grow our organization and increase the size of our facilities to meet our anticipated growth;
1
•the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing;
•our expectations related to the use of our available cash;
•our ability to develop, acquire, and advance product candidates into, and successfully complete, clinical trials;
•the initiation, timing, progress, and results of future preclinical studies and clinical trials, and our research and development programs;
•the impact of governmental laws and regulations and regulatory developments in the United States and foreign countries;
•developments and projections relating to our competitors and our industry;
•the impact of pandemics on our clinical trials, our supply chain, and our operations; and
•other risks and uncertainties, including those described under the heading “Risk Factors” included in our most recent Annual Report on Form 10-K for the year ended December 31, 2022, filed with the U.S. Securities and Exchange Commission (SEC) on February 24, 2023.
Any forward-looking statements in this Quarterly Report on Form 10-Q reflect our current views with respect to future events or to our future financial performance and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by these forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
You should read this Quarterly Report on Form 10-Q and the documents that we have filed as exhibits to this Quarterly Report on Form 10-Q completely and with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
2
DEFINED TERMS
Unless the context requires otherwise, references to “Reata,” “the Company,” “we,” “us,” or “our” in this Quarterly Report on Form 10-Q refer to Reata Pharmaceuticals, Inc. and its subsidiaries. We also have used several other terms in this Quarterly Report on Form 10-Q, most of which are explained or defined below.
| | |
Abbreviated Term | | Defined Term |
AbbVie | | AbbVie Inc. |
ADPKD | | Autosomal dominant polycystic kidney disease |
ALS | | Amyotrophic lateral sclerosis |
ATP | | Adenosine triphosphate |
bardoxolone | | Bardoxolone methyl |
BXLS | | Blackstone Life Sciences, LLC |
Cemdomespib | | Previously referred to as RTA 901 |
CKD | | Chronic kidney disease |
CRL | | Complete Response Letter |
CRO | | Contract research organization |
DPNP | | Diabetic peripheral neuropathic pain |
eGFR | | Estimated glomerular filtration rate |
EMA | | European Medicines Agency |
ESPP | | Reata Pharmaceuticals, Inc. 2022 Employee Stock Purchase Plan |
ESRD | | End stage renal disease |
Exchange Act | | Securities Exchange Act of 1934 |
FA | | Friedreich’s ataxia |
FDA | | United States Food and Drug Administration |
Kyowa Kirin | | Kyowa Kirin Co., Ltd. |
LTIP Plan | | Second Amended and Restated Long Term Incentive Plan |
MAA | | Marketing Authorization Application |
NDA | | New Drug Application |
PK | | Pharmacokinetic |
RSU | | Restricted Stock Unit |
SEC | | U.S. Securities and Exchange Commission |
U.S. GAAP | | Accounting principles generally accepted in the United States |
3
PART I - FINANCIAL INFORMATION
Item 1. Financial Statements.
Reata Pharmaceuticals, Inc.
Consolidated Balance Sheets
(in thousands, except share data)
| | | | | | | | |
| | March 31, 2023 | | | December 31, 2022 | |
| | (unaudited) | | | | |
Assets | | | | | | |
Cash and cash equivalents | | $ | 84,940 | | | $ | 42,312 | |
Marketable debt securities | | | 236,019 | | | | 345,202 | |
Prepaid expenses and other current assets | | | 11,583 | | | | 10,256 | |
Total current assets | | | 332,542 | | | | 397,770 | |
Property and equipment, net | | | 11,060 | | | | 11,179 | |
Operating lease right-of-use-assets | | | 103,807 | | | | 105,258 | |
Inventory, noncurrent | | | 5,713 | | | | — | |
Other assets | | | 460 | | | | 284 | |
Total assets | | $ | 453,582 | | | $ | 514,491 | |
Liabilities and stockholders’ equity | | | | | | |
Accounts payable | | $ | 14,448 | | | $ | 18,706 | |
Accrued direct research liabilities | | | 19,445 | | | | 13,836 | |
Other current liabilities | | | 17,499 | | | | 24,267 | |
Operating lease liabilities, current | | | 3,206 | | | | 2,151 | |
Total current liabilities | | | 54,598 | | | | 58,960 | |
Operating lease liabilities, noncurrent | | | 114,727 | | | | 117,313 | |
Liability related to sale of future royalties, net | | | 414,930 | | | | 403,913 | |
Total noncurrent liabilities | | | 529,657 | | | | 521,226 | |
Commitments and contingencies | | | | | | |
Stockholders’ equity: | | | | | | |
Common stock A, $0.001 par value:
500,000,000 shares authorized; issued and outstanding – 33,010,817 and
31,762,052 at March 31, 2023 and December 31, 2022, respectively | | | 33 | | | | 32 | |
Common stock B, $0.001 par value:
150,000,000 shares authorized; issued and outstanding – 4,516,934 and
4,913,348 at March 31, 2023 and December 31, 2022, respectively | | | 5 | | | | 5 | |
Additional paid-in capital | | | 1,552,938 | | | | 1,501,800 | |
Accumulated deficit | | | (1,683,649 | ) | | | (1,567,532 | ) |
Total stockholders’ equity | | | (130,673 | ) | | | (65,695 | ) |
Total liabilities and stockholders’ equity | | $ | 453,582 | | | $ | 514,491 | |
See accompanying notes.
4
Reata Pharmaceuticals, Inc.
Unaudited Consolidated Statements of Operations
(in thousands, except share and per share data)
| | | | | | | | |
| | Three Months Ended | |
| | March 31 | |
| | 2023 | | | 2022 | |
Collaboration revenue | | | | | | |
License and milestone | | $ | — | | | $ | 893 | |
Other revenue | | | 195 | | | | 21 | |
Total collaboration revenue | | | 195 | | | | 914 | |
Operating cost and expenses | | | | | | |
Research and development | | | 55,477 | | | | 39,804 | |
Selling, general and administrative | | | 54,885 | | | | 24,841 | |
Depreciation | | | 288 | | | | 308 | |
Total operating cost and expenses | | | 110,650 | | | | 64,953 | |
Other income (expense), net | | | (5,662 | ) | | | (9,772 | ) |
Loss from operations | | | (116,117 | ) | | | (73,811 | ) |
Benefit from (provision for) taxes on income | | | — | | | | (31 | ) |
Net loss | | $ | (116,117 | ) | | $ | (73,842 | ) |
Net loss per share—basic and diluted | | $ | (3.14 | ) | | $ | (2.03 | ) |
Weighted-average number of common shares used in
net loss per share basic and diluted | | | 36,948,063 | | | | 36,412,621 | |
See accompanying notes.
5
Reata Pharmaceuticals, Inc.
Unaudited Consolidated Statements of Stockholders’ Equity
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended March 31, 2023 | |
| | Common Stock A | | | Common Stock B | | | Additional
Paid-In | | | Total
Accumulated | | | Total
Stockholders’ | |
| | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Equity | |
Balance at December 31, 2022 | | | 31,762,052 | | | $ | 32 | | | | 4,913,348 | | | $ | 5 | | | $ | 1,501,800 | | | $ | (1,567,532 | ) | | $ | (65,695 | ) |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (116,117 | ) | | | (116,117 | ) |
Compensation expense
related to stock-based compensation | | | — | | | | — | | | | — | | | | — | | | | 38,057 | | | | — | | | | 38,057 | |
Exercise of options | | | 519,224 | | | | 1 | | | | | | | — | | | | 13,080 | | | | — | | | | 13,081 | |
Issuance of common stock
upon vesting of restricted
stock units | | | 333,127 | | | | | | | — | | | | — | | | | 1 | | | | — | | | | 1 | |
Conversion of common
stock Class B to Class A | | | 396,414 | | | | — | | | | (396,414 | ) | | | — | | | | — | | | | — | | | | — | |
Balance at March 31, 2023 | | | 33,010,817 | | | $ | 33 | | | | 4,516,934 | | | $ | 5 | | | $ | 1,552,938 | | | $ | (1,683,649 | ) | | $ | (130,673 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended March 31, 2022 | |
| | Common Stock A | | | Common Stock B | | | Additional
Paid-In | | | Total
Accumulated | | | Total
Stockholders’ | |
| | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Equity | |
Balance at December 31, 2021 | | | 31,478,197 | | | $ | 31 | | | | 4,919,249 | | | $ | 5 | | | $ | 1,441,584 | | | $ | (1,255,631 | ) | | $ | 185,989 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (73,842 | ) | | | (73,842 | ) |
Compensation expense
related to stock-based compensation | | | — | | | | — | | | | — | | | | — | | | | 15,444 | | | | — | | | | 15,444 | |
Exercise of options | | | — | | | | — | | | | 9,375 | | | | — | | | | 194 | | | | — | | | | 194 | |
Issuance of common stock
upon vesting of restricted
stock units | | | 35,107 | | | | | | | 2,835 | | | | — | | | | — | | | | — | | | | — | |
Conversion of common
stock Class B to Class A | | | 12,210 | | | | — | | | | (12,210 | ) | | | — | | | | — | | | | — | | | | — | |
Balance at March 31, 2022 | | | 31,525,514 | | | $ | 31 | | | | 4,919,249 | | | $ | 5 | | | $ | 1,457,222 | | | $ | (1,329,473 | ) | | $ | 127,785 | |
See accompanying notes.
6
Reata Pharmaceuticals, Inc.
Unaudited Consolidated Statements of Cash Flows
(in thousands)
| | | | | | | | |
| | Three Months Ended | |
| | March 31 | |
| | 2023 | | | 2022 | |
Operating activities | | | | | | |
Net loss | | $ | (116,117 | ) | | $ | (73,842 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | |
Depreciation | | | 288 | | | | 308 | |
Non-cash interest expense on liability related to sale of future royalty | | | 11,017 | | | | 9,871 | |
Stock-based compensation expense | | | 38,057 | | | | 15,444 | |
Amortization of discount (premium) on marketable debt securities | | | (1,954 | ) | | | — | |
Changes in operating assets and liabilities: | | | | | | |
Income tax receivable and payable | | | 9 | | | | — | |
Prepaid expenses, other current assets and other assets | | | (1,334 | ) | | | 877 | |
Inventory | | | (5,713 | ) | | | — | |
Accounts payable | | | (4,355 | ) | | | (4,525 | ) |
Accrued expenses, other current and long-term liabilities | | | (1,268 | ) | | | (8,914 | ) |
Operating lease obligations | | | (80 | ) | | | 3,489 | |
Deferred revenue | | | — | | | | (893 | ) |
Net cash used in operating activities | | | (81,449 | ) | | | (58,185 | ) |
Investing activities | | | | | | |
Purchases of property and equipment | | | (141 | ) | | | (288 | ) |
Purchases of marketable securities | | | (63,864 | ) | | | — | |
Maturity from marketable securities | | | 175,000 | | | | |
Net cash provided by (used in) investing activities | | | 110,995 | | | | (288 | ) |
Financing activities | | | | | | |
Exercise of options | | | 13,081 | | | | 194 | |
Issuance of common stock | | | 1 | | | | — | |
Net cash provided by financing activities | | | 13,082 | | | | 194 | |
Net increase (decrease) in cash and cash equivalents | | | 42,628 | | | | (58,279 | ) |
Cash and cash equivalents at beginning of year | | | 42,312 | | | | 590,258 | |
Cash and cash equivalents at end of period | | $ | 84,940 | | | $ | 531,979 | |
Non-cash activity: | | | | | | |
Right-of-use assets obtained in exchange for lease obligations | | $ | — | | | $ | 4,885 | |
Purchases of equipment in accounts payable, accrued expenses, other current, and long-term liabilities | | $ | 106 | | | $ | 2,258 | |
See accompanying notes.
7
Reata Pharmaceuticals, Inc.
Notes to Unaudited Consolidated Financial Statements
1. Description of Business
Reata Pharmaceuticals, Inc.’s (Reata, the Company, we, us, or our) mission is to identify, develop, and commercialize innovative therapies that change patients’ lives for the better. The Company focuses on small-molecule therapeutics with novel mechanisms of action for the treatment of severe, life-threatening diseases with few or no approved therapies. Our first product, SKYCLARYS® (omaveloxolone), is the first and only drug approved by U.S. Food and Drug Administration (FDA) for the treatment of Friedreich's ataxia (FA) in adults and adolescents aged 16 years and older. We have submitted a Marketing Authorization Application (MAA) for omaveloxolone for the treatment of FA to the European Medicines Agency (EMA) in Europe and the application is under review. We are also developing cemdomespib (previously referred to as RTA 901), the lead product candidate from our Hsp90 modulator program, in neurological indications.
Omaveloxolone activates the transcription factor Nrf2 to normalize mitochondrial function, restore redox balance, and resolve inflammation, in nonclinical models. Because mitochondrial dysfunction, oxidative stress, and inflammation are features of many diseases, we believe our Nrf2 activators have many potential clinical applications.
We possess exclusive, worldwide rights to develop, manufacture, and commercialize omaveloxolone. We are the exclusive licensee of cemdomespib and have worldwide commercial rights.
In May 2023, Kyowa Kirin reported results from the AYAME study, a Phase 3 trial which was conducted in Japan studying the safety and efficacy of bardoxolone in patients with diabetic kidney disease. Based on the results of AYAME and its potential regulatory impact, we and Kyowa Kirin have decided to discontinue our bardoxolone CKD programs, including the FALCON and EAGLE clinical trials. See Note 13, Subsequent Events, to our condensed consolidated financial statements for further details.
The Company’s consolidated financial statements include the accounts of all majority-owned subsidiaries. Accordingly, the Company’s share of net earnings and losses from these subsidiaries is included in the consolidated statements of operations. Intercompany profits, transactions, and balances have been eliminated in consolidation.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (U.S. GAAP) for interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all the information and notes required by U.S. GAAP for complete financial statements. In the opinion of management, all adjustments (consisting of normal recurring adjustments) considered necessary for a fair presentation have been included. Operating results for the three months ended March 31, 2023 are not necessarily indicative of the results that may be expected for the year ending December 31, 2023. The consolidated balance sheet at December 31, 2022, has been derived from the audited consolidated financial statements at that date but does not include all of the information and footnotes required by U.S. GAAP for complete financial statements. For further information, refer to the annual consolidated financial statements and footnotes thereto of the Company.
New Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies that the Company adopts as of the specified effective date. Unless otherwise discussed below, the Company does not believe that the adoption of recently issued standards have or may have a material impact on its condensed consolidated financial statements and disclosures.
8
Summary of Significant Accounting Policies
The significant accounting policies used in the preparation of these condensed consolidated financial statements for the three months ended March 31, 2023 are consistent with those discussed in Note 2 to the consolidated financial statements in the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, with the exception of the following:
Inventory
We capitalize inventory costs related to products to be sold in the ordinary course of business. We make a determination of capitalizing inventory costs for a product based on, among other factors, status of regulatory approval, information regarding safety, efficacy and expectations relating to commercial sales and recoverability of costs. Pre-launch inventory is held as an asset when there is a high probability of regulatory approval for the product and when there are probable future economic profits. Inventory may consist of raw materials, work in process and finished goods. We began capitalizing inventory related to SKYCLARYS in the quarter ended March 31, 2023, as we received approval of SKYCLARYS in February 2023, and the related costs were expected to be recoverable through the commercialization of SKYCLARYS. Prior to the start of capitalizing inventory, costs for commercially saleable product and materials of $39.2 million were incurred and included in research and development expenses. As a result, cost of product revenues related to SKYCLARYS will initially reflect a lower average per unit cost of materials over the next 35-40 months as previously expensed inventory is utilized for commercial production and sold to customers.
Inventories are valued under the specific identification method and are stated at the lower of cost or net realizable value. We measure inventory based on lot-based costing where inventory is valued at the purchase price for the specific lot. We assess recoverability of inventory each reporting period to determine any write down to net realizable value resulting from excess or obsolete inventories.
3. Collaboration Agreements
Subsequent to the 2019 reacquisition of certain rights originally licensed to AbbVie Inc. (AbbVie) (see “AbbVie,” below), the Company’s collaboration revenue and deferred revenue have been generated primarily from licensing fees and reimbursements for expenses received under our exclusive license with Kyowa Kirin (the Kyowa Kirin Agreement).
Kyowa Kirin
In December 2009, the Company entered into an exclusive license with Kyowa Kirin to develop and commercialize bardoxolone in the licensed territory. The terms of the agreement include payment to the Company of a nonrefundable, up-front license fee of $35.0 million and additional development and commercial milestone payments. As of March 31, 2023, the Company has received $50.0 million related to regulatory development milestone payments from Kyowa Kirin and has the potential in the future to achieve another $47.0 million from regulatory milestones and $140.0 million from commercial milestones. The Company also has the potential to achieve tiered royalties ranging from the low teens to the low 20 percent range, depending on the country of sale and the amount of annual net sales, on net sales by Kyowa Kirin in the licensed territory. The Company is participating on a joint steering committee with Kyowa Kirin to oversee the development and commercialization activities related to bardoxolone. Any future milestones and royalties received are subject to mid to lower single digit percent declining tiered commissions to certain consultants as compensation for negotiations of the Kyowa Kirin Agreement.
9
The Company regularly evaluates its remaining performance obligation under the Kyowa Kirin Agreement. Accordingly, revenue may fluctuate from period to period due to changes to its estimated performance obligation period and variable considerations. The Company began recognizing revenue related to the up-front payment upon execution of the Kyowa Kirin Agreement as the Company’s period of performance began. In March 2021, the Company’s performance obligation period under the Kyowa Kirin Agreement was extended to and completed in June 2022. On July 27, 2021, Kyowa Kirin submitted a New Drug Application (NDA) in Japan to the Ministry of Health, Labour and Welfare for bardoxolone for improvement of renal function in patients with Alport syndrome. Based on this submission, the Company earned a $5.0 million milestone payment, variable consideration previously considered constrained, under the Kyowa Kirin Agreement. As a result, the Company recorded $4.7 million in collaboration revenue, a cumulative catch-up for the portion of this milestone that was satisfied in prior periods, and $0.3 million in deferred revenue that was recognized over the remaining performance obligation period. Under the Kyowa Kirin Agreement, we will not recognize any deferred revenue subsequent to June 30, 2022.
In May 2023, Kyowa Kirin reported results from the AYAME study, a Phase 3 trial which was conducted in Japan studying the safety and efficacy of bardoxolone in patients with diabetic kidney disease. Based on the results of AYAME and its potential regulatory impact, we and Kyowa Kirin have decided to discontinue our bardoxolone CKD programs, including the FALCON and EAGLE clinical trials. See Note 13, Subsequent Events, to our condensed consolidated financial statements for further details.
AbbVie
In September 2010, the Company entered into a license agreement with AbbVie (the AbbVie License Agreement) for an exclusive license to develop and commercialize bardoxolone in the Licensee Territory (as defined in the AbbVie License Agreement).
In December 2011, the Company entered into a collaboration agreement with AbbVie (the Collaboration Agreement) to jointly research, develop, and commercialize the Company’s portfolio of second and later generation oral Nrf2 activators.
In October 2019, the Company and AbbVie entered into an Amended and Restated License Agreement (the Reacquisition Agreement) pursuant to which the Company reacquired the development, manufacturing, and commercialization rights concerning its proprietary Nrf2 activator product platform originally licensed to AbbVie in the AbbVie License Agreement and the Collaboration Agreement. In exchange for such rights, the Company agreed to pay AbbVie $330.0 million, all of which was subsequently paid. Additionally, the Company will pay AbbVie an escalating, low single-digit royalty on worldwide net sales, on a product-by-product basis, of omaveloxolone and certain next-generation Nrf2 activators. The execution of the Reacquisition Agreement ended our performance obligations under the Collaboration Agreement.
As of December 31, 2021, the Company has fully satisfied its payable to AbbVie, therefore no interest expense was recognized thereafter.
4. Liability Related to Sale of Future Royalties
On June 24, 2020, the Company closed on the Development and Commercialization Funding Agreement with an affiliate of Blackstone Life Sciences, LLC (BXLS), which provides funding for the development and commercialization of bardoxolone for the treatment of CKD caused by Alport syndrome, autosomal dominant polycystic kidney disease (ADPKD), and certain other rare CKD indications in return for future royalties (the Development Agreement). The Development Agreement includes a $300.0 million payment by an affiliate of BXLS in return for various percentage royalty payments on worldwide net sales of bardoxolone, once approved in the United States or certain specified European countries, by Reata and its licensees, other than Kyowa Kirin. The royalty percentage will initially be in the mid-single digits and, in future years, can vary between higher-mid single digit percentages to low-single digit percentages depending on various milestones, including indication approval dates, cumulative royalty payments, and cumulative net sales. Pursuant to the Development Agreement, we have granted BXLS a security interest in substantially all of our assets. After a bardoxolone product approval has been obtained by the Company, the Company is obligated to make certain minimum cumulative payment amounts in 2025 through 2033, but only until BXLS has achieved a certain internal rate of return target.
10
In addition, concurrent with the Development Agreement, the Company entered into a common stock purchase agreement (the Purchase Agreement) with affiliates of BXLS to sell an aggregate of 340,793 shares of the Company’s Class A common stock at $146.72 per share for a total of $50.0 million.
The Company concluded that there were two units of accounting for the consideration received, comprised of the liability related to the sale of future royalties and the common shares. The Company allocated the $300.0 million from the Development Agreement and $50.0 million from the Purchase Agreement between the two units of accounting on a relative fair value basis at the time of the transaction. The Company allocated $294.5 million, which includes $0.8 million in transaction costs incurred, in transaction consideration to the liability, and $55.5 million to the common shares. The Company determined the fair value of the common shares based on the closing stock price on June 24, 2020, the closing date of the Development Agreement. At inception, the effective interest rate under the Development Agreement, including transaction costs, was approximately 13.8%. During the first quarter of 2022, the Company reassessed the expected royalty payments and lowered our previous estimate of future sales for which royalties will be paid. Accordingly, we have prospectively adjusted and recognized lower non-cash interest expense using a 10.9% effective interest rate, as of March 31, 2023.
The following table shows the activity within the liability related to sale of future royalties for the three months ended March 31, 2023:
| | | |
| Liability Related to Sale of Future Royalties | |
| (in thousands) | |
Balance at December 31, 2022 | $ | 404,634 | |
Non-cash interest expense recognized | | 11,000 | |
Balance at March 31, 2023 | | 415,634 | |
Less: Unamortized transaction cost | | (704 | ) |
Carrying value at March 31, 2023 | $ | 414,930 | |
On May 4, 2023, we entered into an Amended and Restated Development and Commercialization Funding Agreement (the Amended Funding Agreement) with BXLS. See Note 13, Subsequent Events, to our condensed consolidated financial statements or further details.
5. Inventory
All of the Company’s inventories relate to the manufacturing of SKYCLARYS. The following table sets forth the Company’s inventories as of March 31, 2023:
| | | | |
| | Three Months Ended | |
| | March 31 | |
| | 2023 | |
| | (in thousands) | |
Raw materials | | $ | 3,446 | |
Work-in-process | | | 2,267 | |
Finished goods | | | — | |
Total | | $ | 5,713 | |
Inventory in excess of the amount expected to be sold within one year is classified as noncurrent inventory. At March 31, 2023, all of the Company’s inventories were classified as noncurrent inventory.
6. Marketable Debt Securities
During the quarter ended March 31, 2023, the Company invested its excess cash balances in marketable debt securities and, at each balance sheet date presented, the Company classified all of its investments in debt securities as held-to-maturity and as current assets as they mature within 12 months and represent the investment of funds available for current operations.
11
The Company considers all available evidence to evaluate if an impairment loss exists, and if so, marks the investment to market through a charge to the Company’s consolidated statements of operations and comprehensive loss. The Company did not record any impairment charges related to our marketable debt securities during the three months ended March 31, 2023.
The following tables summarize our marketable debt securities (in thousands), as of March 31, 2023:
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | |
| | Amortized
Cost | | | Gross
Unrealized
Gains | | | Gross
Unrealized
Losses | | | Fair Value (1) | |
Marketable debt securities: | | | | | | | | | | | | |
U.S. treasury securities | | | 236,019 | | | | 17 | | | | (27 | ) | | | 236,009 | |
Total | | $ | 236,019 | |
| $ | 17 | | | $ | (27 | ) | | $ | 236,009 | |
(1) The fair value was determined using the three-tier fair value hierarchy for disclosure in accordance with Accounting Standards Codification 820-10. The Company’s investments in marketable securities are classified within Level 2 of the fair value hierarchy. The Company uses quoted prices for similar assets sourced from certain third-party pricing services. The third-party pricing services generally utilize industry standard valuation models for which all significant inputs are observable, either directly or indirectly, to estimate the price or fair value of the securities. The primary input generally includes reported trades of or quotes on the same or similar securities. The Company does not make additional judgments or assumptions made to the pricing data sourced from the third-party pricing services.
7. Other Income (Expense), Net
| | | | | | | | |
| | Three Months Ended | |
| | March 31 | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Other income (expense), net | | | | | | |
Investment income | | $ | 3,435 | | | $ | 132 | |
Non-cash interest expense on liability
related to sale of future royalty | | | (11,017 | ) | | | (9,871 | ) |
Other income (expense) | | | 1,920 | | | | (33 | ) |
Total other income (expense), net | | $ | (5,662 | ) | | $ | (9,772 | ) |
| | | | | | |
Investment Income
Interest income consists primarily of interest generated from our cash and cash equivalents and marketable debt securities.
Non-Cash Interest Expense on Liability Related to Sale of Future Royalties
Non-cash interest expense consists of recognition of interest expense based on the Company’s current estimate of future royalties expensed to be paid over the estimated term of the Development Agreement.
Other Income (Expense)
Other income (expense) consists primarily of gains and losses on foreign currency exchange, sales of assets, lease termination and employee retention credit.
In the three months ended March 31, 2023, other income included $2.0 million, related to the employee retention credit (ERC) under the CARES Act.
12
8. Leases
The Company headquarters is located in Plano, Texas, where it leases approximately 122,000 square feet of office space. The Company leases additional space located in Irving, Texas, where it leases approximately 34,890 square feet of office and laboratory space.
On February 4, 2022, the Company extended the lease for the office and laboratory space in Irving, Texas, to October 31, 2024, with an option to extend for a fixed 12-month period.
On March 8, 2022, the Company extended the lease for the Plano office to December 31, 2023.
The Company has an additional lease of a single-tenant, build-to-suit building of approximately 327,400 square feet of office and laboratory space located in Plano, Texas with an initial lease term of 16 years. The Company entered into the lease agreement on October 15, 2019 (the 2019 Lease Agreement), and at the Company’s option, it may renew the lease for two consecutive five-year renewal periods or one ten-year renewal period. On December 15, 2021, the Company obtained control of the space, and, accordingly, the Company recorded related right-of-use assets and the lease liabilities during the fourth quarter of 2021. The Company recorded the liability associated with the 2019 Lease Agreement at the present value of the lease payments not yet paid, using the discount rate as of the commencement date. As the discount rate implicit in the 2019 Lease Agreement was not readily determinable, the Company utilized its incremental borrowing rate. The renewals are not assumed in the determination of the lease term, since they are not deemed to be reasonably assured at the inception of the lease. At inception, the Company recorded $124.5 million as a right-of-use asset, which represented a lease liability of $133.2 million, net of $8.7 million of lease incentives recognized.
For the three months ended March 31, 2023, the Company paid $3.9 million for amounts included in the measurement of lease liabilities. During the three months ended March 31, 2023 and 2022, the Company recorded total rent expense of $4.3 million and $4.3 million, respectively.
Supplemental balance sheet and other information related to the Company’s operating leases is as follows:
| | | | | | | | | | |
| | | | As of March 31, | |
| | | | 2023 | | | 2022 | |
Weighted-average remaining lease term (in years) | | | 15.0 | | | | 15.6 | |
Weighted-average discount rate | | | | | 6.5 | % | | | 6.5 | % |
Maturities of lease liabilities by fiscal year for the Company’s operating leases:
| | | | |
| | As of March 31, 2023 | |
| | (in thousands) | |
2023 (remaining nine months) | | $ | 5,835 | |
2024 (1) | | | 6,457 | |
2025 | | | 11,777 | |
2026 | | | 12,007 | |
2027 | | | 12,241 | |
Thereafter | | | 143,829 | |
Total lease payments (1) | | | 192,146 | |
Less: Imputed interest | | | (74,213 | ) |
Present value of lease liabilities | | $ | 117,933 | |
(1) Above table includes one year rent abatement applied beginning in June 2023 following FDA approval of SKYCLARYS
13
9. Income Taxes
The following table summarizes income tax benefit expense and effective income tax rate:
| | | | | | | | |
| | Three Months Ended | |
| | March 31 | |
| | 2023 | | | 2022 | |
| | (in thousands, except for percentage data) | |
Benefit from (provision for) taxes on income | | $ | — | | | $ | (31 | ) |
Effective income tax rate | | | 0.0 | % | | | 0.0 | % |
The Company’s effective tax rate for the three months ended March 31, 2023, varies with the statutory rate primarily due to changes in the valuation allowance related to certain deferred tax assets generated or utilized in the applicable period.
Deferred tax assets are regularly reviewed for recoverability by jurisdiction and valuation allowances are established based on historical and projected future taxable losses and the expected timing of the reversals of existing temporary differences. The Company has recorded valuation allowances against the majority of its deferred tax assets as of March 31, 2023, and the Company expects to maintain these valuation allowances until there is sufficient evidence that future earnings can be achieved, which is uncertain at this time.
10. Stock-Based Compensation
In 2022, our stockholders approved the Reata Pharmaceuticals, Inc. 2022 Employee Stock Purchase Plan (the "ESPP"), pursuant to which 500,000 shares of common stock were authorized for issuance. Under the ESPP, each offering period is twelve months, with two six-month purchase periods. At the end of each purchase period the employees may purchase shares of common stock through payroll deductions made over the term of the purchase period. The per-share purchase price at the end of each purchase period is equal to the lesser of 85% of the closing price of the Company’s common stock at the beginning of the offering period or the purchase date. As of March 31, 2023, the Company has not issued any common stock in relation to the ESPP. The Company recorded approximately $0.2 million in compensation expense related to the ESPP during the three months ended March 31, 2023.
The following table summarizes time-based and performance-based stock compensation expense reflected in the consolidated statements of operations:
| | | | | | | | |
| | Three Months Ended | |
| | March 31 | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Research and development | | $ | 14,270 | | | $ | 7,606 | |
Selling, general and administrative | | | 23,787 | | | | 7,838 | |
Total stock compensation expense | | $ | 38,057 | | | $ | 15,444 | |
Restricted Stock Units (RSUs)
The following table summarizes RSUs as of March 31, 2023, and changes during the three months ended March 31, 2023, under the Second Amended and Restated Long Term Incentive Plan (LTIP Plan):
| | | | | | | | |
| | Number of
RSUs | | | Weighted-Average
Grant Date Fair
Value | |
Outstanding at January 1, 2023 | | | 1,151,656 | | | $ | 45.45 | |
Granted | | | 453,049 | | | | 40.03 | |
Vested | | | (352,042 | ) | | | 34.64 | |
Forfeited | | | (13,113 | ) | | | 50.56 | |
Outstanding at March 31, 2023 | | | 1,239,550 | | | $ | 46.48 | |
14
As of March 31, 2023, total unrecognized compensation expense related to RSU and performance-based RSU awards that were deemed probable of vesting was approximately $41.2 million, which excludes 36,500 shares of unvested performance-based RSUs that were deemed not probable of vesting totaling unrecognized stock-based compensation expense of $4.4 million.
Stock Options
The following table summarizes stock option activity as of March 31, 2023, and changes during the three months ended March 31, 2023, under the LTIP Plan and standalone option agreements:
| | | | | | | | |
| | Number of
Options | | | Weighted-
Average
Price | |
Outstanding at January 1, 2023 | | | 6,009,199 | | | $ | 65.92 | |
Granted | | | 1,784,222 | | | | 37.69 | |
Exercised | | | (519,224 | ) | | | 25.21 | |
Forfeited | | | (248,260 | ) | | | 188.95 | |
Expired | | | (44,033 | ) | | | 111.59 | |
Outstanding at March 31, 2023 | | | 6,981,904 | | | $ | 56.57 | |
Exercisable at March 31, 2023 | | | 3,376,690 | | | $ | 64.37 | |
As of March 31, 2023, total unrecognized compensation expense related to stock options and performance- based stock options that were deemed probable of vesting was approximately $92.1 million, which excludes 172,500 shares of unvested performance-based stock options that were deemed not probable of vesting totaling unrecognized stock-based compensation expense of $13.2 million.
The total intrinsic value of all outstanding options and exercisable options as of March 31, 2023 was $313.6 million and $141.1 million, respectively.
The number of weighted average options that were not included in the diluted earnings per share calculation because the effect would have been anti-dilutive represented 8,221,454 and 6,878,671 shares as of March 31, 2023 and 2022, respectively.
11. Employee Benefit Plans
In 2010, we adopted an Employee Investment Plan, qualified under Section 401(k) of the Internal Revenue Code, which is a retirement savings plan covering substantially all of our U.S. employees (the Plan). The Plan is administered under the “safe harbor” provision of ERISA. Under the Plan, an eligible employee may elect to contribute a percentage of their salary on a pre-tax basis, subject to federal statutory limitations. Beginning in January 2019, the Company implemented a discretionary employer matching contribution of $1.00 for every $1.00 contributed by a participating employee up to $7,000 annually in 2023 and 2022, which such matching contributions become fully vested after four years of service. The Company recorded expense of $1.4 million and $1.3 million for the three months ended March 31, 2023 and 2022, which includes the Company’s contributions and administrative costs.
12. Commitments and Contingencies
Litigation
From time to time, the Company is a party to legal proceedings in the course of its business, including the matters described below. The outcome of any such legal proceedings, regardless of the merits, is inherently uncertain. In addition, litigation and related matters are costly and may divert the attention of our management and other resources that would otherwise be engaged in other activities. If the Company were unable to prevail in any such legal proceedings, its business, results of operations, liquidity and financial condition could be adversely affected. The Company recognizes accruals for litigations to the extent that it can conclude that a loss is both probable and reasonably estimable and recognizes legal expenses as incurred.
15
Bardoxolone Securities Litigation
In late 2021 and early 2022, certain putative stockholders of the Company filed complaints in the United States District Court for the Eastern District of Texas alleging violations of the federal securities laws against the Company and certain of its executives, including its Chief Executive Officer; its Chief Operating Officer, Chief Financial Officer, and President; and its Chief Innovation Officer (in one of the suits). On April 22, 2022, the suits were consolidated, and a lead plaintiff was appointed. On June 21, 2022, the lead plaintiff filed a complaint against the Company, the aforementioned executives, certain current and former member of the Company’s Board of Directors, and underwriters in connection with secondary offerings of Company stock in 2019 and 2020. The complaint alleges, among other things, that the Company made false and misleading statements regarding the sufficiency of the Phase 2 and Phase 3 CARDINAL studies to support an NDA for bardoxolone in the treatment of CKD caused by Alport syndrome, and the Company’s interactions with the FDA concerning potential approval for bardoxolone. The complaint asserts claims under the Securities Act of 1933 and the Securities Exchange Act of 1934 (Exchange Act). The plaintiffs seek, among other things, a class action designation, an award of damages, and costs and expenses, including attorney fees and expert fees. The Company believes that the allegations contained in the complaint are without merit and intends to defend the case. The Company cannot predict at this point the length of time that this action will be ongoing or the liability, if any, which may arise therefrom.
Indemnifications
Accounting Standards Codification 460, Guarantees, requires that, upon issuance of a guarantee, the guarantor must recognize a liability for the fair value of the obligations it assumes under that guarantee.
As permitted under Delaware law and in accordance with the Company’s bylaws, officers and directors are indemnified for certain events or occurrences, subject to certain limits, while the officer or director is or was serving in such capacity. The maximum amount of potential future indemnification is unlimited; however, the Company has obtained director and officer insurance that limits its exposure and may enable recoverability of a portion of any future amounts paid. The Company believes the fair value for these indemnification obligations is minimal. Accordingly, the Company has not recognized any liabilities relating to these obligations as of March 31, 2023.
The Company has certain agreements with licensors, licensees, collaborators, and vendors that contain indemnification provisions. In such provisions, the Company typically agrees to indemnify the licensor, licensee, collaborator, or vendor against certain types of third-party claims. The Company accrues for known indemnification issues when a loss is probable and can be reasonably estimated. There were no accruals for expenses related to indemnification issues for any period presented.
13. Subsequent Events
AYAME Study
In May 2023, Kyowa Kirin reported results from the AYAME study, a Phase 3 trial which was conducted in Japan to evaluate the safety and efficacy of bardoxolone in patients with diabetic kidney disease. AYAME met its primary and key secondary endpoints, and no significant safety issues were noted. However, there was no separation in the occurrence of ESRD events between the bardoxolone and placebo groups after three years of treatment. Based on the results of AYAME and its potential regulatory impact, we and Kyowa Kirin have decided to discontinue our bardoxolone CKD programs, including the FALCON and EAGLE clinical trials. We are not expecting any material costs to be incurred in connection with the discontinuance of these ongoing studies.
Liability Related to Sale of Future Royalties
With the discontinuation of bardoxolone development, on May 4, 2023, we entered into an Amended and Restated Development and Commercialization Funding Agreement (the Amended Funding Agreement) with BXLS. The Amended Funding Agreement provides that all covenants in the Development and Commercialization Funding Agreement regarding commercialization of bardoxolone, restricting the incurrence of indebtedness, and restricting license and licensing transactions are removed and all prior security interests granted to BXLS are released.
16
In addition, the Amended Funding Agreement provides for a low, single digit royalty payment to BXLS on net sales of omaveloxolone for Friedreich's ataxia. Pursuant to the Amended Funding Agreement, to secure our payment obligations to BXLS, (i) we have granted BXLS a security interest in a segregated deposit account and (ii), subject to certain limitations as set forth in the Amended Funding Agreement, have agreed to maintain in such account an initial balance and thereafter an amount equal to the aggregate amount of Omav Royalty payments made for the immediately preceding two calendar quarter period.
We are currently evaluating the net impact on the liability related to sale of future royalties and any adjustments will be recorded in second quarter financial statements.
Term Loan
On May 5, 2023, the Company, entered into an agreement (the Term Loan) with BPCR Limited Partnership and BioPharma Credit Investments V (Master) LP, as Lenders, which are funds managed by Pharmakon Advisors, LP, and BioPharma Credit PLC, as the collateral agent for the Lenders, pursuant to which the Lenders agreed to make term loans to the Company in an aggregate principal amount of up to $275 million. The initial tranche of $75 million will be funded on May 12, 2023, the second tranche of $50 million is conditioned on meeting certain regulatory or product production criteria and two final tranches of $75 million each will be made available based on the achievement of certain commercial sales milestones; with the fourth tranche being optional to the Company. The Term Loan bears interest at 7.50% plus three-month SOFR per annum, with a SOFR floor of 2.50%. Repayment terms are quarterly interest-only payments for three years, then quarterly amortization over the following two years. The Term Loan is secured by substantially all of the assets of the Company, its U.S. subsidiaries, and its U.K., Swiss, and Irish subsidiaries.
17
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and related notes and other financial information appearing in this Quarterly Report on Form 10-Q. Some of the information contained in this discussion and analysis or set forth elsewhere in this Quarterly Report on Form 10-Q, including information with respect to our plans and strategy for our business, operations, and product candidates, includes forward-looking statements that involve risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, among other things, those described under the headings “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” and discussed elsewhere in this Quarterly Report on Form 10-Q.
Overview
We are a biopharmaceutical company focused on identifying, developing, and commercializing innovative therapies that change patients’ lives for the better. We concentrate on small-molecule therapeutics with novel mechanisms of action for the treatment of serious or life-threatening diseases with few or no approved therapies and unmet need. Our first product, SKYCLARYS®(omaveloxolone), is the first and only drug approved by the U.S. Food and Drug Administration (FDA) for the treatment of Friedreich's ataxia (FA) in adults and adolescents aged 16 years and older. We have submitted a Marketing Authorization Application (MAA) for omaveloxolone for the treatment of FA to the European Medicines Agency (EMA) in Europe and the application is under review. We are also developing cemdomespib (previously referred to as RTA 901), the lead product candidate from our Hsp90 modulator program, in neurological indications and Nrf2 activators for neurological diseases.
Omaveloxolone activates the transcription factor Nrf2 to normalize mitochondrial function, restore redox balance, and resolve inflammation, in nonclinical models. Because mitochondrial dysfunction, oxidative stress, and inflammation are features of many diseases, we believe our Nrf2 activators have many potential clinical applications.
We possess exclusive, worldwide rights to develop, manufacture, and commercialize omaveloxolone and our Nrf2 activators. We are the exclusive licensee of cemdomespib and have worldwide commercial rights.
In May 2023, Kyowa Kirin reported results from the AYAME study, a Phase 3 trial which was conducted in Japan studying the safety and efficacy of bardoxolone in patients with diabetic kidney disease. Based on the results of AYAME and its potential regulatory impact, we and Kyowa Kirin have decided to discontinue our bardoxolone CKD programs, including the FALCON and EAGLE clinical trials. See Note 13, Subsequent Events, to our condensed consolidated financial statements for further details.
Recent Key Developments
Omaveloxolone for Friedreich’s Ataxia
In February 2023, we announced that the FDA approved SKYCLARYS, the first and only drug approved by the FDA for the treatment of FA in adults and adolescents 16 years of age and older. FA is a rare, inherited neurodegenerative disorder that is typically diagnosed during adolescence. Patients with FA experience progressive loss of coordination, muscle weakness, and fatigue, which commonly progresses to motor incapacitation and wheelchair reliance by their teens or early twenties, and eventually death. FA affects approximately 5,000 diagnosed patients in the U.S.
We are evaluating strategies to support global label expansion for younger pediatric patients. In the second quarter of 2023, we plan to request a meeting with the FDA to discuss possible label expansion for younger pediatric patients who are not included in the approved label. A pediatric pharmacokinetic study evaluating safety, tolerability, and pharmacokinetic profile (PK) of omaveloxolone in FA patients is planned to begin in the fourth quarter of 2023.
18
We have completed the final stages of SKYCLARYS drug product manufacturing and packaging. As previously reported, a process-related drug substance impurity above the reporting threshold was observed. This impurity is part of the omaveloxolone drug substance process chemistry that was previously at levels below the reporting threshold. An NDA supplement was submitted to the FDA to increase the drug substance specification for this impurity above the current specification. The approval of this supplement is required to release SKYCLARYs drug product to our specialty pharmacy. The FDA is reviewing the supplement as a Prior Approval Supplement under Priority Review with an approval target action date in mid-August. The FDA stated that the review of the supplement was prioritized and that the approval potentially should come earlier than the action date. We believe that the information provided in the NDA supplement, which was developed based upon FDA and international harmonized guidance, is sufficient to support the proposed change. Provided that there are no major review issues with the supplement, we believe that SKYCLARYS will be available through the specialty pharmacy no later than mid-August 2023.
We submitted an MAA for omaveloxolone for the treatment of patients with FA to the EMA in the fourth quarter of 2022 and the application is under review. We recently received the omaveloxolone MAA Day 120 List of Questions from the EMA. The EMA separates their questions into “major” and “other” categories. With respect to major questions on clinical efficacy, the EMA invited us to discuss the robustness of the efficacy results and suggested the discussion could include the impact of imbalances in baseline characteristics on the pivotal study results and extrapolation of the results to patients with advanced disease and patient groups not included in the pivotal trial. They also asked us to justify inclusion of the pes cavus population in the indication. With respect to major questions on clinical safety, the EMA asked us to discuss the similarities between Nrf2 activators with respect to chemical structure and safety and to discuss suitable contraindications and warnings for cardiac, renal, diabetic and other conditions taking into account the populations studied. With respect to major questions on drug product quality, the EMA requested us to clarify the information on the potential for nitrosamine formation and to provide further information on active substance control strategies. We believe that we have the data and analyses necessary to address the requests of the EMA, and we plan to provide responses to the EMA in the third quarter of 2023.
Cemdomespib for Neurological Indications, Including Diabetic Peripheral Neuropathic Pain
We are developing cemdomespib, a novel, once-daily, oral HSP90 modulator for patients with DPNP. We have finalized the design of a randomized, double-blind, placebo-controlled Phase 2 trial of cemdomespib in patients with diabetic peripheral neuropathic pain (DPNP) and plan to start the trial in the third quarter of 2023. We are the exclusive licensee of cemdomespib and have worldwide commercial rights.
AYAME Trial for Bardoxolone in Diabetic CKD Conducted by Kyowa Kirin
In May 2023, Kyowa Kirin reported results from the AYAME study, a Phase 3 trial which was conducted in Japan studying the safety and efficacy of bardoxolone in patients with diabetic kidney disease. AYAME met the primary and key secondary endpoints, and no significant safety issues were noted. However, there was no separation in the occurrence of ESRD events between the bardoxolone and placebo groups after three years of treatment.
Based on the results of AYAME and its potential regulatory impact, we and Kyowa Kirin have decided to discontinue our bardoxolone CKD programs, including the ongoing FALCON Phase 3 trial in patients with ADPKD and EAGLE open-label extension study in patients with Alport syndrome and ADPKD. We will be communicating these plans with regulators and our clinical trial sites immediately.
Background: Our Programs
The following chart outlines each of our programs by indication and phase of development:
19
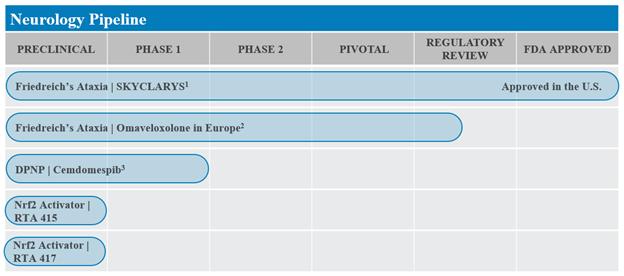
1SKYCLARYS® (omaveloxolone) is indicated for the treatment of FA in adults and adolescents aged 16 years and older.
2MAA submitted in fourth quarter of 2022 and is currently under review.
3Plan to initiate a Phase 2 trial of cemdomespib in patients with DPNP in the third quarter of 2023.
Programs in Neurological Diseases
In February 2023, the FDA approved SKYCLARYS for the treatment of FA in adults and adolescents aged 16 years and older. FA is a rare, inherited neurodegenerative disorder that is typically diagnosed during adolescence and can lead to premature death. We are seeking approval for omaveloxolone for the treatment of FA in Europe and plan to seek approval in additional countries and regions outside the U.S. Because mitochondrial dysfunction is a key feature of many neurological diseases, we believe Nrf2 activators may be broadly applicable to treat neurological diseases by activating Nrf2 to normalize and improve mitochondrial function and adenosine triphosphate (ATP) production.
We are also developing cemdomespib for the treatment of neurological diseases. Cemdomespib is a highly potent and selective C-terminal modulator of Hsp90, which has a critical role in mitochondrial function, protein folding, and inflammation. Cemdomespib has demonstrated profound efficacy in a wide range of animal models of neurological disease, including diabetic neuropathy, neuroinflammation, and neuropathic pain. We have finalized the design of a randomized, double-blind, placebo-controlled Phase 2 trial of cemdomespib in patients with DPNP and plan to start the trial in the third quarter of 2023.
Omaveloxolone in Patients with Friedreich’s Ataxia
Patients with FA experience progressive loss of coordination, muscle weakness, and fatigue, that commonly progresses to motor incapacitation and wheelchair reliance. Diagnosis of FA typically occurs by genetic testing, and most people with FA are diagnosed in their teens and early twenties. Patients with FA typically require a wheelchair in their twenties and the mean age of death for patients with FA is in the mid-thirties. Childhood-onset FA can occur as early as age five, is more common than later onset FA, and normally involves more rapid disease progression. Based on literature and proprietary research, we believe FA affects approximately 6,000 patients in the United States and 22,000 individuals globally. According to a recent insurance claim analysis, we estimate that there are approximately 5,000 patients diagnosed with FA in the United States.
In February 2023, we announced FDA approval of our first product SKYCLARYS (omaveloxolone), the first drug indicated for the treatment of FA in adults and adolescents 16 years of age and older. With this approval, the FDA granted a rare pediatric disease priority review voucher.
We are evaluating strategies to support global label expansion for younger pediatric patients. In the second quarter of 2023, we plan to request a meeting with the FDA to discuss possible label expansion for younger pediatric patients who are not included in the approved label. A pediatric pharmacokinetic study evaluating safety, tolerability, and pharmacokinetic profile (PK) of omaveloxolone in FA patients is planned to begin in the fourth quarter of 2023.
20
Additional pediatric study plans will be finalized after receiving an opinion from EMA scientific advice (see Regulatory Interactions in Europe section) and FDA feedback.
We have agreed with the FDA on multiple post-marketing requirements. The first is to conduct a clinical drug-drug interaction study to determine the effect of concomitant administration of a moderate CYP3A4 inducer on the pharmacokinetics of SKYCLARYS in healthy volunteers. Second, we have committed to conduct a thorough QT study to assess the risk of QT prolongation with SKYCLARYS. We plan to initiate these studies in the second half of 2023. Third, we have committed to conduct a study to assess the concentration of SKYCLARYS in breast milk (milk only). Fourth, we have committed to conduct a global pregnancy and lactation surveillance program to collect prospective and retrospective data in women exposed to SKYCLARYS to assess risk of maternal and fetal/neonate complications. Finally, we have agreed to conduct additional non-clinical studies. We are on track with the agreed upon milestones.
Beyond our post-marketing requirements, we will sponsor a voluntary, post-marketing, prospective, observational, multinational registry study of patients treated commercially with SKYCLARYS. The primary objective of the registry is to evaluate the long-term safety of SKYCLARYS in FA patients in the real-world setting.
We have completed the final stages of SKYCLARYS drug product manufacturing and packaging. As previously reported, a process-related drug substance impurity above the reporting threshold was observed. This impurity is part of the omaveloxolone drug substance process chemistry that was previously at levels below the reporting threshold. An NDA supplement was submitted to the FDA to increase the drug substance specification for this impurity above the current specification. The approval of this supplement is required to release SKYCLARYs drug product to our specialty pharmacy. The FDA is reviewing the supplement as a Prior Approval Supplement under Priority Review with an approval target action date in mid-August. The FDA stated that the review of the supplement was prioritized and that the approval potentially should come earlier than the action date. We believe that the information provided in the NDA supplement, which was developed based upon FDA and international harmonized guidance, is sufficient to support the proposed change. Provided that there are no major review issues with the supplement, we believe that SKYCLARYS will be available through the specialty pharmacy no later than mid-August 2023.
Regulatory Interactions in Europe
We submitted an MAA for omaveloxolone for the treatment of patients with FA to the EMA in the fourth quarter of 2022 and the application is under review. We recently received the omaveloxolone MAA Day 120 List of Questions from the EMA. The EMA separates their questions into “major” and “other” categories. With respect to major questions on clinical efficacy, the EMA invited us to discuss the robustness of the efficacy results and suggested the discussion could include the impact of imbalances in baseline characteristics on the pivotal study results and extrapolation of the results to patients with advanced disease and patient groups not included in the pivotal trial. They also asked us to justify inclusion of the pes cavus population in the indication. With respect to major questions on clinical safety, the EMA asked us to discuss the similarities between Nrf2 activators with respect to chemical structure and safety and to discuss suitable contraindications and warnings for cardiac, renal, diabetic and other conditions taking into account the populations studied. With respect to major questions on drug product quality, the EMA requested us to clarify the information on the potential for nitrosamine formation and to provide further information on active substance control strategies. We believe that we have the data and analyses necessary to address the requests of the EMA, and we plan to provide responses to the EMA in the third quarter of 2023.
Prior to submission of the MAA, we received a positive opinion from the Pediatric Committee on our Pediatric Investigation Plan, and, as agreed in this plan, we have submitted a request for scientific advice seeking additional input on the protocol design.
Omaveloxolone and Our Other Nrf2 Activators for Other Neurological Indications
Because mitochondrial dysfunction is a key feature of many neurological and neuromuscular diseases, we believe Nrf2 activators may be broadly applicable to treat such diseases by activating Nrf2 to normalize and improve mitochondrial function and ATP production.
21
Based on our understanding of the pathophysiology of neurological diseases, characterized by mitochondrial dysfunction, inflammation, and oxidative stress, we believe our Nrf2 activators may be applicable to both rare diseases such as Huntington’s disease, progressive supranuclear palsy, frontotemporal dementia, primary progressive multiple sclerosis, and others, as well as non-rare diseases such as Parkinson’s disease, Alzheimer’s disease, epilepsy, and others. Consistent with this, we have observed promising activity of omaveloxolone and our other Nrf2 activators in preclinical models of many of these diseases. Our Nrf2 activators reduced seizure frequency in refractory, progressive epilepsy models and restored mitochondrial function in patient biopsy samples and preclinical models of FA, amyotrophic lateral sclerosis (ALS), familial and sporadic Parkinson’s disease, and frontotemporal dementia. In clinical trials, improvements in neuromuscular function have been observed in FA patients treated with omaveloxolone as assessed by the modified Friedreich’s Ataxia Rating Scale, and improvements in mitochondrial function, as measured by reductions in blood lactate and heart rate, have been observed in patients with primary mitochondrial disease. We have developed additional small molecule activators of Nrf2, including RTA 415 and RTA 417, which are currently in preclinical development. The non-clinical profiles of these to molecules support advancement to clinical studies and have shown broad activity in non-clinical models of inflammation, autoimmune disease, and neurodegeneration. Late Investigational New Drug (IND)-directed studies are currently underway or planned, and we anticipate IND filings in 2024 for both molecules. We are currently evaluating the impact of provisions of the Inflation Reduction Act (IRA) on our future development plans for omaveloxolone and our other Nrf2 activators.
Cemdomespib in Neurological Diseases
Cemdomespib is the lead product candidate from our Hsp90 modulator program, which includes highly potent and selective C-terminal modulators of Hsp90. We have observed favorable activity of cemdomespib in a range of preclinical models of neurological disease, including models of diabetic neuropathy, neuroinflammation, and neuropathic pain.
Historically, other companies have explored N-terminal Hsp90 inhibitors for cancer therapeutics; however, this approach has been associated with multiple adverse effects including peripheral neuropathy and ocular toxicity. Binding at the C-terminus of Hsp90 leads to increased transcription of Hsp70, a cytoprotective and molecular chaperone gene, which facilitates cell survival in response to stress without the deleterious activities of N-terminal inhibition.
In preclinical rodent disease models, we observed that cemdomespib administered orally once-daily rescued compromised nerve function, restored thermal and mechanical sensitivity, and improved nerve conductance velocity and mitochondrial function. These effects are dose-dependent, reversible, and HSP70-dependent.
We completed a Phase 1 SAD/MAD trial of oral, once daily cemdomespib in healthy adult volunteers to evaluate the safety, tolerability, and pharmacokinetic (PK) profile. The PK was approximately dose-proportional up to the highest doses evaluated with a half-life ranging from two to nine hours. Human exposures easily exceeded the exposures necessary for efficacy in multiple animal models. No safety or tolerability concerns were reported. In the third quarter of 2022, we completed additional Phase 1 clinical pharmacology studies of cemdomespib, including a drug-drug interaction study which demonstrated an acceptable profile.
We have finalized the design of a randomized, double-blind, placebo-controlled, two-part, 12-week Phase 2 trial of cemdomespib in patients with DPNP. The trial will assess pain using the numeric pain rating scale (NPRS). We plan to enroll 192 patients randomized in each part for a total of 384 patients. Patients are allowed to take up to one standard of care medication. In order for patients to enroll, they must not have achieved adequate pain control upon entry with a NPRS pain intensity score of at least 4 on a 0 to 10 point scale at screening. We will conduct an exposure-response analysis using Part 1 data to select doses for Part 2. The primary endpoint is the change in average pain intensity assessed by NPRS at Week 12. We plan to initiate the trial in the third quarter of 2023.
There are about four million patients with moderate to severe DPNP in the United States, and about two million adult patients diagnosed with DPNP seek treatment annually. We are the exclusive licensee of cemdomespib and have worldwide commercial rights.
Programs in Chronic Kidney Disease
We and Kyowa Kirin, our strategic collaborator in CKD in Japan, were developing bardoxolone for the treatment of CKD in multiple indications, including ADPKD, CKD caused by Alport syndrome, and type 1 and 2 diabetic CKD.
22
On February 25, 2022, we received a Complete Response Letter (CRL) from the FDA with respect to its review of our NDA for bardoxolone in the treatment of patients with CKD caused by Alport syndrome. The CRL indicated that the FDA cannot approve the NDA in its present form. Based on its review, the FDA concluded that it does not believe the submitted data demonstrates that bardoxolone is effective in slowing the loss of kidney function in patients with Alport syndrome and reducing the risk of progression to kidney failure and requested additional data to support the efficacy and safety of bardoxolone. Their conclusion was based on efficacy and safety concerns primarily set forth in the FDA’s briefing book and discussed at the Cardiovascular and Renal Drugs Advisory Committee meeting held on December 8, 2021.
We have been sponsoring FALCON, a Phase 3, international, multi-center, randomized, double-blind, placebo-controlled trial studying the safety and efficacy of bardoxolone in patients with ADPKD, a rare and serious hereditary form of CKD. In fourth quarter of 2022, we had received scientific advice from the EMA that the proposed Phase 3 clinical study cannot be used as a standalone study to support the indication of bardoxolone in ADPKD. The EMA recommended that we evaluate, in addition to the traditional endpoints, the effect of bardoxolone on total kidney volume in these patients and requested that we seek additional guidance on our program.
In May 2023, Kyowa Kirin reported results from the AYAME study, Phase 3 trial of bardoxolone in patients with diabetic kidney disease. The primary endpoint is time to onset of a ≥ 30% decrease in eGFR from baseline or ESRD. The secondary endpoints are time to onset of a ≥ 40% decrease in eGFR from baseline or ESRD, time to onset of a ≥ 53% decrease in eGFR from baseline or ESRD, time to onset of ESRD, and change in eGFR from baseline at each evaluation time point.
AYAME met the primary and key secondary endpoints and no significant safety issues were observed in patients receiving bardoxolone. However, there was no separation in the occurrence of ESRD events between the bardoxolone and placebo groups after three years of treatment.
Based on the results of AYAME and its potential regulatory impact, we and Kyowa Kirin have decided to discontinue our bardoxolone CKD programs, including the ongoing FALCON Phase 3 trial in patients with ADPKD and EAGLE open-label extension study in patients with Alport syndrome and ADPKD. We will be communicating these plans with regulators and our clinical trial sites immediately.
United States Commercial Launch
On February 28, 2023, we received FDA approval of SKYCLARYS for the treatment of FA and launched SKYCLARYS in the United States as the first approved product for this disease. Commercialization efforts began immediately following approval with launch strategies that support physician education and utilization, patient activation, and access to therapy. Reata field sales representatives began physician engagements on March 6th. The sales organization consists of a team of Region Business Directors and Neurology Account Managers responsible for educating healthcare providers (HCPs) currently treating FA. Our Field Access Team was also deployed at approval to help facilitate access to SKYCLARYS. The Access team consists of National Account Directors focused on SKYCLARYS coverage by top national and regional payers, and a Patient Access Liaison Team, hired to support practice-level access needs. Together, this team’s primary responsibility is to facilitate patient access to the drug by working to minimize and navigate payer criteria. Coverage by most national and regional payers is expected to take approximately four to eight weeks or longer via medical exception while formulary review progresses over the next three to six months. Patient access services through Reata REACH went live and were operational at approval, including the REACH call center and website. SKYCLARYS patient enrollment forms became available for download at approval. Reata REACH has been accepting patient enrollment forms submitted by HCPs and is working with payers for coverage authorization through prior authorization and medical exceptions.
In the United States, there are approximately 5,000 diagnosed FA patients today that can be linked HCPs, of which an estimated 4,500 represent our total on-label addressable market and excludes patients under the age of 16. Through the evaluation of ICD-10 claims data, we have identified approximately 2,500 target HCPs treating patients with FA today.
23
Ex-United States Commercial Readiness
Our ability to launch omaveloxolone is dependent on the successful filing and defense of an MAA and approval by the EMA or other regulatory agencies. Outside of the United States, where appropriate and depending on the terms of our contractual arrangements, we plan, either alone, or with new collaboration partners, to commercialize our products.
With regards to omaveloxolone, if approved, we plan to launch in the EU in the second quarter of 2024. In parallel to our regulatory activities to respond to the EMA’s questions concerning our regulatory submission, we have been setting up our commercial launch infrastructure and preparing our reimbursement dossiers for submission in target countries. Beyond the EU, we continue to evaluate market opportunities for our products in other markets.
Corporate Overview
To date, we have focused most of our efforts and resources on developing our product candidates, conducting preclinical studies and clinical trials, and build-out of commercial infrastructure for SKYCLARYS. In February 2023, we announced that the FDA approved SKYCLARYS, for the treatment of FA in adults and adolescents 16 years of age and older in the U.S. We believe SKYCLARYS commercial drug product will be available through the specialty pharmacy no later than mid-August 2023, at which time we will start to generate product revenue. We have historically financed our operations primarily through revenue generated from our collaborations with AbbVie and Kyowa Kirin, from sales of our securities, secured loans, and a strategic financing from BXLS. We have not received any payments or revenue from collaborations other than nonrefundable upfront, milestone, and cost sharing payments from our collaborations with AbbVie and Kyowa Kirin, from the Development Agreement with BXLS, and from reimbursements of expenses under the terms of our agreement with Kyowa Kirin. We have incurred losses in each year since our inception, other than in 2014. As of March 31, 2023, we had $84.9 million of cash and cash equivalents, marketable debt securities of $236.0 million and an accumulated deficit of $1,683.6 million. We continue to incur significant research and development and other expenses related to our ongoing operations. We anticipate that we will continue to incur losses. As we successfully launch SKYCLARYS, and generate commercial product revenue, we anticipate that our losses will decrease in the foreseeable future, before we reach profitability. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders’ equity and working capital. Our failure to become and remain profitable could depress the market price of our Class A common stock and could impair our ability to raise capital, expand our business, diversify our product offerings, or continue our operations.
The probability of success for each of our product candidates and clinical programs and our ability to generate product revenue and become profitable depend upon a variety of factors, including the quality of the product candidate, clinical results, investment in the program, competition, manufacturing capability, commercial viability, and our collaborators’ ability to successfully execute our development and commercialization plans. We will also require additional capital through equity, debt, or royalty financings or collaboration arrangements in order to fund our operations and execute on our business plans, and there is no assurance that such financing or arrangements will be available to us on commercially reasonable terms or at all. For a description of the numerous risks and uncertainties associated with product development and raising additional capital, see “Risk Factors” included in our Annual Report on Form 10-K for the year ended December 31, 2022.
Financial Operations Overview
Revenue
Our revenue to date has been generated primarily from licensing fees received under our collaborative license agreements and reimbursements for expenses. We currently have not generated any revenue from the sale of products to date. In February 2023, we announced that the FDA approved SKYCLARYS, for the treatment of FA in adults and adolescents 16 years of age and older in the U.S. We believe SKYCLARYS commercial drug product will be available through the specialty pharmacy no later than mid-August 2023, at which time we will start to generate product revenue. In the future, we may generate revenue from product sales, royalties on product sales, reimbursements for collaboration services under our current collaboration agreements, or license fees, milestones, or upfront payments if we enter into any new collaborations or license agreements. We expect that our future revenue will fluctuate from quarter to quarter for many reasons, including the uncertain timing and amount of any such payments and sales.
24
Our license and milestone revenue has been generated primarily from the Kyowa Kirin Agreement, the AbbVie License Agreement, and the Collaboration Agreement and consists of upfront payments and milestone payments. License revenue recorded with respect to the Kyowa Kirin Agreement, the AbbVie License Agreement, and the Collaboration Agreement consists solely of the recognition of deferred revenue. Under our revenue recognition policy, collaboration revenue associated with upfront, non-refundable license payments received under our license and collaboration agreements are deferred and recognized ratably over the expected term of the performance obligations under each agreement. Under the Reacquisition Agreement, we no longer have performance obligations under the AbbVie License Agreement and the Collaboration Agreement. Under the Kyowa Kirin Agreement, we will not recognize any deferred revenue subsequent to June 30, 2022.
In May 2023, Kyowa Kirin reported results from the AYAME study, a Phase 3 trial which was conducted in Japan studying the safety and efficacy of bardoxolone in patients with diabetic kidney disease. Based on the results of AYAME and its potential regulatory impact, we and Kyowa Kirin have decided to discontinue our bardoxolone CKD programs, including the FALCON and EAGLE clinical trials. See Note 13, Subsequent Events, to our condensed consolidated financial statements for further details.
Research and Development Expenses
The largest component of our total operating expenses has historically been our investment in research and development activities, including the clinical development of our product candidates. From our inception through March 31, 2023, we have incurred a total of $1,315.2 million in research and development expense, a majority of which relates to the development of bardoxolone and omaveloxolone. We expect our research and development expense to continue to increase in the future as we advance our product candidates through clinical trials and expand our product candidates portfolio. The process of conducting the necessary preclinical and clinical research to obtain regulatory approval is costly and time-consuming, and we consider the active management and development of our clinical pipeline to be crucial to our long-term success. The actual probability of success for each product candidate and preclinical program may be affected by a variety of factors, including the safety and efficacy data for product candidates, investment in the program, competition, manufacturing capability, and commercial viability.
Research and development expenses include:
•expenses incurred under agreements with clinical trial sites that conduct research and development activities on our behalf;
•expenses incurred under contract research agreements and other agreements with third parties;
•employee and consultant-related expenses, which include salaries, benefits, travel, and stock-based compensation;
•laboratory and vendor expenses related to the execution of preclinical studies and clinical trials;
•the cost of acquiring, developing, manufacturing, and distributing clinical trial materials;
•the cost of development, scale up, and process validation activities to support product registration; and
•facilities, depreciation, and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance, and other supply costs.
Research and development costs are expensed as incurred. Costs for certain development activities such as clinical trials are highly judgmental and are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and our clinical sites.
We base our expense accruals related to clinical trials on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical trials and preclinical studies on our behalf. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing costs, we estimate the time period over which services will be performed and the level of effort to be expended in each period.
To date, we have not experienced material changes in our estimates of accrued research and development expenses after a reporting period. However, due to the nature of estimates, we cannot assure you that we will not make changes to our estimates in the future as we become aware of additional information about the status or conduct of our clinical trials and other research activities.
25
Kyowa Kirin has allowed us to conduct clinical studies of bardoxolone in certain rare forms of kidney diseases in Japan and has reimbursed us the majority of the costs for our CARDINAL study in Japan. Kyowa Kirin is the in-country caretaker in our FALCON study in Japan and we are reimbursing Kyowa Kirin for the costs of a certain number of patients in the study.
In May 2023, Kyowa Kirin reported results from the AYAME study, a Phase 3 trial which was conducted in Japan studying the safety and efficacy of bardoxolone in patients with diabetic kidney disease. Based on the results of AYAME and its potential regulatory impact, we and Kyowa Kirin have decided to discontinue our bardoxolone CKD programs, including the FALCON and EAGLE clinical trials. See Note 13, Subsequent Events, to our condensed consolidated financial statements or further details.
The following table summarizes our research and development expenses incurred during the three months ended March 31, 2023:
| | | | | | | | |
| | Three Months Ended | |
| | March 31 | |
| | 2023 | | | 2022 | |
| | (in thousands) | |
Bardoxolone | | $ | 10,910 | | | $ | 6,462 | |
Omaveloxolone | | | 7,481 | | | | 7,597 | |
Cemdomespib (previously referred to as RTA 901) | | | 2,448 | | | | 1,407 | |
Other research and development expenses | | | 34,638 | | | | 24,338 | |
Total research and development expenses | | $ | 55,477 | | | $ | 39,804 | |
The program-specific expenses summarized in the table above include costs that we directly allocate to our product candidates. Our other research and development expenses include salaries, benefits, stock-based compensation and preclinical, research, and discovery costs, which we do not allocate on a program-specific basis.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist primarily of employee-related expenses for executive, operational, finance, legal, compliance, commercial, and human resource functions. Selling, general and administrative expenses also include facility-related costs, professional fees, accounting and legal services, depreciation expense, other external services, and expenses associated with obtaining and maintaining our intellectual property rights.
We anticipate that our selling, general and administrative expenses will increase in the future as we continue to build-out our global commercial and compliance infrastructure and field team to support the launch of SKYCLARYS in the U.S., as well as the potential launch of SKYCLARYS into additional markets, assuming regulatory approvals, and continue to advance and develop our pipeline and advance our product candidates, including partnered programs, into later-stage development, and prepare regulatory submissions. However, we expect that certain expenses will be variable depending on the timing of manufacturing batches, clinical trial enrollment and results, regulatory review of our product candidates and programs, and stock-based compensation expenses due to our determination regarding the probability of vesting for performance-based awards.
Other Income (Expense), Net
Other income (expense) includes interest and gains earned on our cash and cash equivalents, and marketable debt securities, amortization of debt issuance costs, imputed interest on long term payables, foreign currency exchange gains and losses, and non-cash interest expense on liability related to the sale of future royalties.
On May 4, 2023, we entered into the Amended Funding Agreement with BXLS. See Note 13, Subsequent Events, to our condensed consolidated financial statements or further details.
On May 5, 2023, we entered into a Term Loan arrangement. See Note 13, Subsequent Events, to our condensed consolidated financial statements or further details.
26
Benefit from (Provision for) Taxes on Income
Provision for taxes on income consists of net loss, taxed at federal tax rates and adjusted for certain permanent differences. Realization of deferred tax assets is generally dependent upon future earnings by jurisdiction, of which the timing and amount are uncertain for the majority of our deferred tax assets, and valuation allowances are maintained against them. Changes in valuation allowances also affect the tax provision.
Results of Operations
Comparison of the Three Months Ended March 31, 2023 and 2022 (unaudited)
The following table sets forth our results of operations for the three months ended March 31:
| | | | | | | | | | | | | | | | |
| | 2023 | | | 2022 | | | Change $ | | | Change % | |
| | (in thousands, except for percentage data) | |
Collaboration revenue | | | | | | | | | | | | |
License and milestone | | $ | — | | | $ | 893 | | | $ | (893 | ) | | | (100 | ) |
Other revenue | | | 195 | | | | 21 | | | | 174 | | | | 829 | |
Total collaboration revenue | | | 195 | | | | 914 | | | | (719 | ) | | | (79 | ) |
Operating cost and expenses | | | | | | | | | | | | |
Research and development | | | 55,477 | | | | 39,804 | | | | 15,673 | | | | 39 | |
Selling, general and administrative | | | 54,885 | | | | 24,841 | | | | 30,044 | | | | 121 | |
Depreciation | | | 288 | | | | 308 | | | | (20 | ) | | | (6 | ) |
Total operating cost and expenses | | | 110,650 | | | | 64,953 | | | | 45,697 | | | | 70 | |
Other income (expense), net | | | (5,662 | ) | | | (9,772 | ) | | | 4,110 | | | | 42 | |
Loss from operations | | | (116,117 | ) | | | (73,811 | ) | | | (42,306 | ) | | | 57 | |
Benefit from (provision for) taxes on income | | | — | | | | (31 | ) | | | 31 | | | | 100 | |
Net loss | | $ | (116,117 | ) | | $ | (73,842 | ) | | $ | (42,275 | ) | | | 57 | |
Revenue
License and milestone revenue represented approximately 0% and 98% of total revenue for the three months ended March 31, 2023 and 2022, respectively. License and milestone revenue decreased by 100%, primarily due to the Company reaching the end of the performance obligation of the Kyowa Kirin Agreement as of June 30, 2022.
Other revenue was immaterial for the three months ended March 31, 2023, and 2022.
On February 28, 2023, the FDA approved SKYCLARYS for the treatment of FA in the U.S. No product revenue was recognized for the three months ended March 31, 2023 or for the three months ended March 31, 2022.
Expenses
The following table summarizes our expenses, including as a percentage of total expenses, for the three months ended March 31:
| | | | | | | | | | | | | | | | |
| | 2023 | | | % of Total
Expenses | | | 2022 | | | % of Total
Expenses | |
| | (in thousands, except for percentage data) | |
Research and development | | $ | 55,477 | | | | 50 | % | | $ | 39,804 | | | | 61 | % |
Selling, general and administrative | | | 54,885 | | | | 49 | % | | | 24,841 | | | | 38 | % |
Depreciation | | | 288 | | | | 1 | % | | | 308 | | | | 1 | % |
Total expenses | | $ | 110,650 | | | | | | $ | 64,953 | | | | |
27
Research and Development Expenses
Research and development expenses increased by $15.7 million, or 39%, for the three months ended March 31, 2023, compared to the three months ended March 31, 2022. The increase is due to an increase in stock-based compensation due to the vesting of certain performance-based equity grants related to the receipt of FDA approval of SKYCLARYS on February 28, 2023, and certain ongoing clinical study costs.
Research and development expenses, as a percentage of total expenses, was 50% and 61% for the three months ended March 31, 2023 and 2022, respectively. The decrease of 11% was due to the proportionately larger increase in selling, general and administrative expenses, compared to research and development expenses.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased by $30.0 million, or 121%, for the three months ended March 31, 2023, compared to the three months ended March 31, 2022. The increase was primarily due to an increase in stock-based compensation due to the vesting of certain performance-based equity grants related to the receipt of FDA approval of SKYCLARYS on February 28, 2023, and increased commercial activities for SKYCLARYS.
Selling, general and administrative expenses, as a percentage of total expenses, was 49% and 38%, for the three months ended March 31, 2023 and 2022, respectively. The increase of 11% was due to the proportionately larger increase in selling, general and administrative expenses, compared to research and development expenses.
Other Income (Expense), Net
Other income (expense), net decreased by $4.1 million for the three months ended March 31, 2023, compared to the three months ended March 31, 2022. The decrease was primarily due to a $3.3 million increase in interest income generated from marketable debt securities, a $2.0 million increase related to the employee retention credit under the CARES Act, and a $1.1 million increase of interest expense attributable to the payable to BXLS under the Development Agreement.
Benefit from (Provision for) Taxes on Income
Benefit from (Provision for) taxes on income was immaterial for the three months ended March 31, 2023 and 2022.
28
Liquidity and Capital Resources
Since our inception, we have funded our operations primarily through collaboration and license agreements, the sale of preferred and common stock, the sale of royalty interests, and secured loans. Through March 31, 2023, we have raised gross cash proceeds of $476.6 million through the sale of convertible preferred stock and $785.0 million from payments under license and collaboration agreements. We also obtained $1,222.1 million in net proceeds from our initial public offering, follow-on offerings, and the sale of our Class A common stock under the Purchase Agreement, and $299.0 million in net proceeds from the sale of future royalties under the Development Agreement. We have not generated any revenue from the sale of any products. As of March 31, 2023, we had available cash and cash equivalents of approximately $84.9 million and marketable debt securities of $236.0 million. Our cash and cash equivalents and marketable debt securities are invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation.
Cash Flows
The following table sets forth the primary sources and uses of cash for each of the three months ended March 31:
| | | | | | | | |
| | 2023 | | | 2022 | |
| | (in thousands) | | | | |
| | | | | | |
Net cash provided by (used in) : | | | | | | |
Operating activities | | $ | (81,449 | ) | | $ | (58,185 | ) |
Investing activities | | | 110,995 | | | | (288 | ) |
Financing activities | | | 13,082 | | | | 194 | |
Net change in cash and cash equivalents | | $ | 42,628 | | | $ | (58,279 | ) |
Operating Activities
Net cash used in operating activities was $81.5 million for the three months ended March 31, 2023, consisting primarily of a net loss of $116.1 million adjusted for non-cash items including stock-based compensation expense of $38.1 million, non-cash interest expense on liability related to sale of future royalty of $11.0 million, amortization of premium on marketable debt securities of $2.0 million, and a net change in operating assets and liabilities of $12.7 million. The change in operating assets and liabilities that impacted our use of cash in operations include a decrease of $4.4 million in accounts payable due to timing of payments, an increase of $5.7 million in inventory and an increase of $1.3 million in prepaids and other assets, and a decrease of $1.3 million in direct research and other current and long-term liabilities.
Investing Activities
Net cash provided by investing activities was $111.0 million and net cash used in investing activities was $0.3 million for the three months ended March 31, 2023 and 2022, respectively. The change is due to the purchase of $63.9 million in marketable debt securities during the period, offset by cash received of $175.0 million from maturities of marketable securities.
Financing Activities
Net cash provided by financing activities was $13.1 million and $0.2 million for the three months ended March 31, 2023 and 2022, respectively, primarily consisting of stock options exercises.
29
Operating Capital Requirements
To date, we have not generated any revenue from product sales. We believe SKYCLARYS commercial drug product will be available through the specialty pharmacy no later than mid-August 2023, at which time we will start to generate product revenue. We anticipate that we will continue to incur losses in the future periods. As we launch SKYCLARYS and generate commercial product revenue, we anticipate that our losses will decrease in the foreseeable future, before we reach profitability. We are subject to all the risks related to the development and commercialization of novel therapeutics, including those described under the heading “Risk Factors” included in our most recent Annual Report on Form 10-K, and we may encounter unforeseen expenses, difficulties, complications, delays, and other unknown factors that may adversely affect our business. We anticipate that we will need substantial additional funding in connection with our continuing operations.
In October 2019, we entered into the 2019 Lease Agreement, relating to a new headquarter building lease of approximately 327,400 square feet of office and laboratory space located in Plano, Texas.
•In December 2021, we obtained control of the building, and, accordingly, we recorded related right-of-use assets and the lease liabilities during the fourth quarter of 2021.
•In December 2021, we paused the tenant improvement activities for the new headquarter building. If at a future date, we determine to move into the building, capital expenditures will need to be incurred based on our occupancy requirements at that time.
•The initial term of the lease is 16 years, with up to ten years of extension at our option. The annual base rent payment, which began in June 2022, will be determined based on the project cost, subject to an initial annual cap of approximately $13.3 million. Beginning in the third lease year, the base rent will increase 1.95% per annum each year. In addition to the annual base rent, we will pay for taxes, insurance, utilities, operating expenses, assessments under private covenants, maintenance and repairs, certain capital repairs and replacements, and building management fees.
In June 2020, we closed on the Development Agreement and Purchase Agreement, each dated June 10, 2020, under which certain BXLS entities paid us an aggregate of $350.0 million in exchange for future royalties on bardoxolone and an aggregate of 340,793 shares of our Class A common stock at $146.72 per share.
In December 2020, we closed a follow-on underwritten public offering of 2,000,000 shares of our Class A common stock for gross proceeds of $281.7 million. Net proceeds to us from the offering were approximately $277.5 million, after deducting underwriting discounts and commissions and offering expenses.
In July 2021, Kyowa Kirin announced the submission of an NDA in Japan for bardoxolone for improvement of renal function in patients with Alport syndrome. We earned a $5.0 million milestone related to this event that was received and began to be recognized in the third quarter of 2021.
On May 4, 2023, we entered into an Amended Funding Agreement (the “Amended Funding Agreement”) with BXLS. See Note 13, Subsequent Events, to our condensed consolidated financial statements or further details.
On May 5, 2023, we entered into a Term Loan arrangement. See Note 13, Subsequent Events, to our condensed consolidated financial statements or further details.
30
Our longer-term liquidity requirements will require us to raise additional capital, such as through additional equity, debt, or royalty financings or collaboration arrangements. Our future capital requirements will depend on many factors, including product revenue and the timing of our expenditures related to clinical trials. We believe our existing cash and cash equivalents and marketable debt securities will be sufficient to enable us to fund our operations through the end of 2026. However, we anticipate opportunistically raising additional capital before that time through equity offerings, collaboration or license agreements, additional debt financings, or royalty financings in order to maintain adequate capital reserves. In addition, we may choose to raise additional capital at any time for the further development of our existing product candidates and may also need to raise additional funds sooner to pursue other development activities related to additional product candidates. Decisions about the timing or nature of any financing will be based on, among other things, our perception of our liquidity and of the market opportunity to raise equity, debt, or royalty financing. Additional securities may include common stock, preferred stock, or debt securities. We may explore strategic collaborations or license arrangements for any of our product candidates. If we do explore any arrangements, there can be no assurance that any agreement will be reached, and we may determine to cease exploring a potential transaction for any or all of the assets at any time. If an agreement is reached, there can be no assurance that any such transaction would provide us with a material amount of additional capital resources.
Until we can generate a sufficient amount of revenue from SKYCLARYS and our other product candidates, we expect to finance future cash needs through public or private equity or debt offerings, loans, royalty financings, and collaboration or license transactions. Additional capital may not be available on reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back, or discontinue the development or commercialization of one or more of our product candidates. If we raise additional funds through the issuance of additional equity or debt securities, it could result in dilution to our existing stockholders or increased fixed payment obligations, and any such securities may have rights senior to those of our common stock. If we incur indebtedness or obtain royalty financing, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell, or license intellectual property rights, and other operating restrictions that could adversely affect our ability to conduct our business, and any such debt or royalty financing could be secured by some or all of our assets. Any of these events could significantly harm our business, financial condition, and prospects. For a description of the numerous risks and uncertainties associated with product development and raising additional capital, see “Risk Factors” included in our Annual Report on Form 10-K for the year ended December 31, 2022.
31
Our forecast of the period through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. Our future funding requirements, both near- and long-term, will depend on many factors, including, but not limited to:
•the time and unreimbursed costs necessary to commercialize products in territories in which our product candidates are approved for sale;
•the level of reimbursement or third-party payor pricing available to our products;
•the costs of obtaining third-party commercial supplies of our products, if any, manufactured in accordance with regulatory requirements;
•the scope, rate of progress, results, and cost of our clinical trials, preclinical testing, and other activities related to the development of our product candidates;
•the number and characteristics of product candidates that we pursue;
•the costs necessary to obtain regulatory approvals, if any, for our product candidates in the United States and other jurisdictions, and the costs of post-marketing studies that could be required by regulatory authorities in jurisdictions where approval is obtained;
•the revenue from any future sales of our products or for which we are entitled to a profit share, royalties, and milestones;
•the costs associated with any potential loss or corruption of our information or data in a cyberattack on our computer systems or those of our suppliers, vendors, or collaborators who store or transmit our data;
•the costs associated with being a public company;
•any additional costs we incur, or delays in clinical trials we experience, associated with the pandemic; and
•the costs we incur in the filing, prosecution, maintenance, and defense of our patent portfolio and other intellectual property rights.
If we cannot expand our operations or otherwise capitalize on our business opportunities because we lack sufficient capital, our business, financial condition, and results of operations could be materially adversely affected.
Contractual Obligations and Commitments
We have various contractual obligations and other commitments that require payments at certain specified periods. The following table summarizes our contractual obligations and commitments as of March 31, 2023 (unaudited):
| | | | | | | | | | | | | | | | | | | | |
| | Payments due by period | |
| | Less than
1 year | | | 1 to 3
years | | | 4 to 5
years | | | 6 years and beyond | | | Total | |
| | (unaudited, in thousands) | |
Operating lease obligations(1) | | $ | 5,961 | | | $ | 20,090 | | | $ | 24,325 | | | $ | 141,770 | | | $ | 192,146 | |
Total contractual obligations | | $ | 5,961 | | | $ | 20,090 | | | $ | 24,325 | | | $ | 141,770 | | | $ | 192,146 | |
(1) Above table includes one year rent abatement applied beginning in June 2023 following FDA approval of SKYCLARYS
The terms of the Development Agreement require us to potentially to pay future royalty payments based on product development success. The above table excludes such obligations as the amount and timing of such obligations are unknown or uncertain, which are further described in Note 4, Liability Related to Sale of Future Royalties, to Consolidated Financial Statements contained in this Quarterly Report on Form 10-Q.
On May 4, 2023, we entered into an Amended Funding Agreement with BXLS. See Note 13, Subsequent Events, to our condensed consolidated financial statements for further details.
On May 5, 2023, we entered into a Term Loan arrangement. See Note 13, Subsequent Events, to our condensed consolidated financial statements for further details.
32
Clinical Trials
As of March 31, 2023, we have several on-going clinical trials in various stages. Under agreements with various CROs and clinical trial sites, we incur expenses related to clinical trials of our product candidates and potential other clinical candidates. The timing and amounts of these disbursements are contingent upon the achievement of certain milestones, patient enrollment, and services rendered or as expenses are incurred by the CROs or clinical trial sites. Therefore, we cannot estimate the potential timing and amount of these payments, and they have been excluded from the table above.
In May 2023, Kyowa Kirin reported results from the AYAME study, a Phase 3 trial which was conducted in Japan studying the safety and efficacy of bardoxolone in patients with diabetic kidney disease. Based on the results of AYAME and its potential regulatory impact, we and Kyowa Kirin have decided to discontinue our bardoxolone CKD programs, including the FALCON and EAGLE clinical trials. See Note 13, Subsequent Events, to our condensed consolidated financial statements for further details.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to revenue recognition, accrued research and development expenses, income taxes, and stock-based compensation. We base our estimates on historical experience, known trends and events, and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are described in Note 2 of Part I, Item 1 of this Quarterly Report on Form 10-Q and in Part I, Item 7, “Critical Accounting Policies and Significant Judgments and Estimates” in our Annual Report on Form 10-K. There have been no changes to our critical accounting policies and estimates since our Annual Report on Form 10-K for the year ended December 31, 2022.
Off-Balance Sheet Arrangements
Since our inception, we have not had any relationships with unconsolidated organizations or financial partnerships, such as structured finance or special purpose entities, that would have been established for the purpose of facilitating off-balance sheet arrangements, and we have not engaged in any other off-balance sheet arrangements, as defined in the rules and regulations of the Securities and Exchange Commission (SEC). Please refer to any changes to significant accounting policies during the quarter ended March 31, 2023 in Note 2 of Part I, Item 1 of this Quarterly Report on Form 10-Q.
Recent Accounting Pronouncements
For a discussion of recent accounting pronouncements, please see Note 2, Summary of Significant Accounting Policies, of Notes to Consolidated Financial Statements contained in this Quarterly Report on Form 10-Q.
33
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risks in the ordinary course of our business. These market risks are principally limited to interest rate fluctuations. We had cash and cash equivalents of $84.9 million and marketable debt securities of $236.0 million at March 31, 2023, primarily invested in U.S. government treasuries. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general U.S. interest rates, particularly if our investments are in short-term securities. The primary objective of our investment activities is to preserve principal and liquidity while maximizing income without significantly increasing risk. We do not enter into investments for trading or speculative purposes. Due to the short-term nature of our investment portfolio, we do not believe an immediate and hypothetical increase of 100 basis points in interest rates would have a material effect on the fair market value of our portfolio, and accordingly we do not expect a sudden change in market interest rates to affect materially our operating results or cash flows.
We contract with research, development, and manufacturing organizations and investigational sites globally. Generally, these contracts are denominated in United States dollars. However, we may be subject to fluctuations in foreign currency rates in connection with agreements not denominated in United States dollars. We do not hedge our foreign currency exchange rate risk.
We will pay interest under our Loan Agreement at a floating rate. See Note 13, Subsequent Events, to our consolidated financial statements for further details.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of March 31, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized, and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of March 31, 2023, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
34
Changes in Internal Control Over Financial Reporting
During the three months ended March 31, 2023, in connection with the approval of SKYCLARYS, we designed and implemented new procedures and controls around our inventory processes. No other change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the quarter ended March 31, 2023 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II — OTHER INFORMATION
Item 1. Legal Proceedings.
For a discussion of material pending legal proceedings, please read Note 12, Commitments and Contingencies – Litigation, to our condensed consolidated financial statements included in Part I, Item I, “Financial Statements (Unaudited),” of this Quarterly Report on Form 10-Q, which is incorporated into this item by reference.
Item 1A. Risk Factors.
In addition to other information set forth in this Quarterly Report on Form 10-Q, you should carefully consider the risk factors and other cautionary statements described under the heading “Risk Factors” included in our Annual Report on Form 10-K for the year ended December 31, 2022, which could materially affect our businesses, financial condition, or future results. Additional risks and uncertainties currently unknown to us, or that we currently deem to be immaterial, also may materially adversely affect our business, financial condition, or future results. There have been no material changes in our risk factors from those described in the Annual Report on Form 10-K for the year ended December 31, 2022.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
None.
Item 5. Other Information.
Loan Agreement
On May 5, 2023, we, as the borrower, and certain of our direct and indirect subsidiaries party thereto from time to time, as the guarantors (the Guarantors and, collectively with the us, the Credit Parties), entered into a loan agreement (the Loan Agreement) with BPCR Limited Partnership (as a Lender), BioPharma Credit Investments V (Master) LP (as a Lenders), and BioPharma Credit PLC, as collateral agent for the Lenders (in such capacity, the Collateral Agent), pursuant to which the Lenders agreed to make term loans to the Borrower in an aggregate principal amount of up to $275 million. The proceeds of the loans will be used to fund our general corporate and working capital requirements.
Pursuant to the terms of the Loan Agreement, the Term Loans will be advanced in up to four tranches. The first tranche (the Tranche A Loan) is expected to be advanced in the amount of $75 million on May 12, 2023, five (5) business days following the effective date of the Loan Agreement (the “Tranche A Closing Date”), subject to entering into a customary security agreement and the satisfaction of other customary conditions precedent. The second tranche (the Tranche B Loan) of $50 million will be advanced following the earlier to occur of (i) the approval of our prior approval submission by the FDA and (ii) the production of the fourth batch of omaveloxolone capsules within the
35
parameters and subject to the requirements of the approval of omaveloxolone by the FDA received in February 2023 (the Tranche B Trigger Date), subject to the satisfaction of customary conditions precedent, including that the Tranche B Trigger Date must occur on or prior to March 31, 2024; provided, however, that if the Tranche B Loan borrowing conditions are not satisfied on or prior to March 31, 2024, then no Tranche B Loan or other loans shall be extended. The third tranche (the Tranche C Loan) of $75 million will be advanced following our achieving a trailing twelve-month net product sales and royalty revenues milestone ranging from $40 million to $55 million based on the date of the first commercial sale of our omaveloxolone product, subject the prior funding of the Tranche B Loan and to the satisfaction of customary conditions precedent; provided, however, that if the Tranche C Loan borrowing conditions are not satisfied on or prior to March 31, 2024, then no Tranche C Loan shall be extended. The fourth tranche (the Tranche D Loan, and together with the Tranche A Loan, the Tranche B Loan and the Tranche C Loan, the Term Loans) of $75 million will be advanced following our achieving a trailing twelve-month net product sales and royalty revenues milestone of $100 million for any trailing twelve month period ending on or prior to December 31, 2024, subject to the prior funding of the Tranche B Loan and the Tranche C Loan and subject to the satisfaction of customary conditions precedent; provided, however, that if the Tranche D Loan borrowing conditions are not satisfied on or prior to December 31, 2024, then no Tranche D Loan shall be extended. The Tranche A Loan, Tranche B Loan and Tranche C Loan are mandatory and the failure of the Company to borrow either the Tranche B Loan or Tranche C Loan following satisfaction of the applicable conditions precedent shall be an Event of Default. Funding of the Tranche D Loan, subject to satisfaction of the applicable conditions precedent, is at our option.
The Term Loans will mature on the 5th year anniversary of the Tranche A Closing Date (Maturity Date), provided that if we do not satisfy the Tranche B Loan borrowing conditions on or prior to March 31, 2024, the Maturity Date shall be March 31, 2024. Repayment of outstanding principal of the Term Loans will be made in eight equal quarterly payments of principal commencing on June 30, 2026; provided however, that if we achieve a trailing twelve month net product sales and royalty revenues milestone of $250 million as of March 31, 2027, at our option, repayment of outstanding principal of the Term Loans will be made in four equal quarterly payments of principal commencing on June 30, 2027.
The Term Loans bear interest at 7.50% plus three-month SOFR per annum with a SOFR floor of 2.50%. We will pay to the Lenders an additional consideration (the Additional Consideration) equal to 2.00% of the Lenders’ total committed amount to fund the Term Loans, payable with respect to each Term Loan on the funding date of such Term Loan; provided however, if we achieve the Tranche B Loan and Tranche C Loan net product sales and royalty revenues milestones noted above but fail to timely borrow the Tranche B Loan or Tranche C Loan (including as a result of the prior prepayment in full of the Term Loans), then upon such failure any unpaid Additional Consideration with respect to the Tranche B Loan or Tranche C Loan shall become immediately due and payable. We may elect to prepay the Term Loans in whole prior to the Maturity Date with such prepayments being subject to a prepayment premium equal to the principal amount so prepaid multiplied by 3% if made prior to the 3rd anniversary of the funding date of the applicable Term Loan, 2% if made on or after the 3rd anniversary of the funding date of the applicable Term Loan but prior to the 4th anniversary of the funding date of the applicable Term Loan, and 1% if made on or after the 4th anniversary of the funding date of the applicable Term Loan but prior to the Maturity Date. In addition to the prepayment premium, prepayments of any Term Loan prior to the 2nd anniversary of the funding date of such Term Loan are subject to a makewhole amount equal to the sum of all interest that would have accrued through such 2nd anniversary. If the Term Loans are accelerated following the occurrence of an event of default or the Maturity Date occurs on March 31, 2024, as described above, we shall immediately pay to the Lenders the sum of all obligations for principal, interest, and the applicable makewhole and prepayment premium and the Tranche B Loan and Tranche C Loan Additional Consideration. We are required to prepay the Term Loans, together with any applicable make-whole and prepayment premiums and any unpaid Additional Consideration in respect of the Tranche B Loan and Tranche C Loan, upon a change of control and prior to certain prepayments or redemptions of permitted convertible debt, subject to exceptions for refinancings and conversions or exchanges for equity.
Our obligations under the Loan Agreement are guaranteed on a full and unconditional basis by the Guarantors, consisting of our US and certain of our foreign subsidiaries, and are secured by substantially all of the respective Credit Parties’ assets, including intellectual property, subject to certain exceptions.
The Loan Agreement contains customary affirmative and restrictive covenants and representations and warranties. We and our subsidiaries are bound by certain affirmative covenants setting forth actions that are required during the term of the Loan Agreement, including, without limitation, certain information delivery requirements, obligations to maintain certain insurance, and certain notice requirements. Additionally, we and our subsidiaries are bound by certain restrictive covenants setting forth actions that are not permitted to be taken during the term of the
36
Loan Agreement without the Lenders’ prior written consent, including, without limitation, (i) selling or disposing of assets, including certain intellectual property, (ii) amending, modifying or waiving certain material agreements or organizational documents, (iii) consummating certain change in control transactions, (iv) incurring certain additional indebtedness, (v) incurring any non-permitted lien or other encumbrance on our or our subsidiaries’ assets, (vi) paying dividends or making any distribution or payment on or redeeming, retiring or purchasing any equity interests, and (vii) making payments of certain subordinated indebtedness, in each case, subject to certain exceptions. The Loan Agreement also contains a financial covenant requiring that we achieve certain trailing twelve month net product sales and royalty revenues, measured and tested on a quarterly basis. The Loan Agreement also contains the following events of default: (i) our failure to pay principal, interest and other amounts when due, (ii) the breach of the covenants under the Loan Agreement, (iii) the occurrence of a material adverse change or a withdrawal event in respect of our omaveloxolone product, (iv) certain attachments of the Credit Parties assets and restraints on their business, (v) certain bankruptcy or insolvency events, (vi) cross-default of third-party indebtedness, (vii) the failure to pay judgements rendered against them, (viii) material misrepresentations, (ix) the loan documents ceasing to create a valid security interest in a material portion of the collateral, and (x) the occurrence of certain ERISA events. Upon the occurrence of an event of default, the Lenders may, among other things, accelerate our obligations under the Loan Agreement (including all obligations for principal, interest and any applicable make-whole and prepayment premiums); provided that upon an event of default relating to certain bankruptcy or insolvency events, all obligations will be immediately accelerated.
The foregoing description of the terms of the Loan Agreement does not purport to be complete and is subject to, and is qualified in its entirety by, reference to the Loan Agreement, which the Company has filed as an exhibit to this Quarterly Report.
Amended and Restated Development and Commercialization Funding Agreement
On May 4, 2023, we entered into an Amended Funding Agreement with an affiliate of Blackstone Life Sciences, LLC (BXLS). The Amended Funding Agreement amends and restates in its entirety our existing Development and Commercialization Funding Agreement, dated as of June 10, 2020 (the Original Funding Agreement) between us and BXLS.
The Amended Funding Agreement provides for (i) a low, single digit royalty on net sales of our omaveloxolone product for FA (subject to a fixed dollar cap and limited in time to the twenty years following commercial launch of the omaveloxolone product) (the Omav Royalty); and (ii) a single digit royalty on net sales of our bardoxolone product (limited in time to the fifteen years following the commercial launch of the bardoxolone product).
The Amended Funding Agreement also provides that, with respect to any change of control of the Company prior to January 1, 2028, we will pay to BXLS a change of control payment in an amount equal to (x) if the date of such change of control is prior to June 10, 2023, $375 million and (y) if the date of such change of control is on or after June 10, 2023, $300 million, or, in each case, potentially a lesser amount based on the sum of certain payments to BXLS, including the Omav Licensing Payments (as defined below), prior to the change of control.
The Amended Funding Agreement also provides that, in the event that prior to the earlier of a change of control of the Company or January 1, 2028, we or one of our affiliates enters into certain material transactions with respect to commercialization of our omaveloxolone product in France, Germany, Italy, Spain, the United Kingdom or the United States (collectively, Omav Licensing Transactions), then, with respect to any payment received in connection with an Omav Licensing Transaction, we shall pay to BXLS an amount equal to a certain percentage of each such payment (the Omav Licensing Payments) until such time as (i) BXLS has received $300 million, or potentially a lesser amount based on the sum of certain payments previously made to BXLS, or (ii) BXLS has received a change of control payment.
Pursuant to the Amended Development Agreement, to secure our payment obligations to BXLS we have (i) granted BXLS a security interest in a segregated deposit account and (ii), subject to certain limitations as set forth in the Amended Funding Agreement, agreed to maintain in such account an initial balance and thereafter an amount equal to the aggregate amount of Omav Royalty payments made for the immediately preceding two calendar quarter period. In addition, substantially all covenants regarding commercialization of bardoxolone and restricting the incurrence of indebtedness and license and licensing transactions, as well as termination events providing for liquidated damages, in the Original Funding Agreement were removed and all prior security interests granted to BXLS were released.
37
The foregoing description of the terms of the Amended Funding Agreement does not purport to be complete and is subject to, and is qualified in its entirety by, reference to the Amended Funding Agreement, which the Company has filed as an exhibit to this Quarterly Report.
38
Item 6. Exhibits.
| | |
Exhibit Number |
| Description |
| | |
3.1 | | Thirteenth Amended and Restated Certificate of Incorporation, dated May 11, 2016 (incorporated by reference to Exhibit 3.7 to the Company’s Form S-1 (File No. 333-208843), filed with the SEC on May 16, 2016). |
| | |
3.2 | | Third Amended and Restated Bylaws, dated as of March 1, 2023 (incorporated by reference to Exhibit 3.1 to the Company’s Form 8-K (File No. 001-37785), filed with the SEC on March 1, 2023). |
| | |
10.1# | | Amendment No. 2 to Exclusive License Agreement, dated as of April 5, 2022, by and between the KU Center for Technology Commercialization, Inc. and the Registrant, as amended. |
| | |
10.2* | | Loan Agreement, dated May 5, 2023, by and among BPCR Limited Partnership and BioPharma Credit Investments V (Master) LP, as Lenders, BioPharma Credit PLC, as Collateral Agent, and Reata Pharmaceuticals, Inc. |
| | |
10.3*# | | Amended and Restated Development and Commercialization Funding Agreement between the Company and BXLS V – River L.P, dated as of May 4, 2023. |
| | |
31.1* |
| Certification of Principal Executive Officer Pursuant to Rules 13a-1d4(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | |
31.2* |
| Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | |
32.1** |
| Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | |
32.2** |
| Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | |
101.INS* |
| Inline XBRL Instance Document - The cover page interactive data file does not appear in the interactive data file because its XBRL tags are embedded within the inline XBRL document |
| | |
101.SCH* | | Inline XBRL Taxonomy Extension Schema Document |
| | |
101.CAL* | | Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| | |
101.DEF* | | Inline XBRL Taxonomy Extension Definition Linkbase Document |
| | |
101.LAB* | | Inline XBRL Taxonomy Extension Label Linkbase Document |
| | |
101.PRE* | | Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| | |
104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
| | |
* Filed herewith.
** Furnished herewith.
# Information in this exhibit identified by brackets is confidential and has been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K because it is not material and is the type of information that the Company customarily treats as private or confidential. An unredacted copy of this exhibit will be furnished to the Securities and Exchange Commission on a supplemental basis upon request.
39
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | |
Date: May 10, 2023 | REATA PHARMACEUTICALS, INC. |
| | |
|
| By: | | /s/ J. Warren Huff |
| Name: | | J. Warren Huff |
| Title: | | Chief Executive Officer |
| | | |
| By: | | /s/ Manmeet S. Soni |
| Name: | | Manmeet S. Soni |
| Title: | | Chief Operating Officer, Chief Financial Officer, and President |
40
