
August 9, 2021 2nd Quarter 2021 Financial Results and Update on Clinical Development Programs Exhibit 99.2
This presentation contains certain “forward-looking” statements that are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical or present facts, are forward-looking statements, including statements regarding our future financial condition, future revenues, projected costs, prospects, business strategy, and plans and objectives of management for future operations, including our plans to submit for regulatory filings. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “might,” “estimate,” “continue,” “anticipate,” “intend,” “target,” “project,” “model,” “should,” “would,” “plan,” “expect,” “predict,” “could,” “seek,” “goal,” “potential,” or the negative of these terms or other similar terms or expressions that concern our expectations, strategy, plans, or intentions. These statements are based on our intentions, beliefs, projections, outlook, analyses, or current expectations using currently available information, and are not guarantees of future performance, and involve certain risks and uncertainties. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that our expectations will prove to be correct. Therefore, actual outcomes and results could materially differ from what is expressed, implied, or forecasted in these statements. Any differences could be caused by a number of factors including but not limited to: our expectations regarding the timing, costs, conduct, and outcome of our clinical trials, including statements regarding the timing of the initiation and availability of data from such trials; the timing and likelihood of regulatory filings and approvals for our product candidates; whether regulatory authorities determine that additional trials or data are necessary in order to obtain approval; our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; our plans to research, develop, and commercialize our product candidates; the commercialization of our product candidates, if approved; the rate and degree of market acceptance of our product candidates; our expectations regarding the potential market size and the size of the patient populations for our product candidates, if approved for commercial use, and the potential market opportunities for commercializing our product candidates; the success of competing therapies that are or may become available; our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates; the ability to license additional intellectual property relating to our product candidates and to comply with our existing license agreements; our ability to maintain and establish relationships with third parties, such as contract research organizations, contract manufacturing organizations, suppliers, and distributors; our ability to maintain and establish collaborators with development, regulatory, and commercialization expertise; our ability to attract and retain key scientific or management personnel; our ability to grow our organization and increase the size of our facilities to meet our anticipated growth; the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing; our expectations related to the use of our available cash; our ability to develop, acquire, and advance product candidates into, and successfully complete, clinical trials; the initiation, timing, progress, and results of future preclinical studies and developments and projections relating to our competitors and our industry; the impact of governmental laws and regulations and regulatory development in the United States and foreign countries; the impact of the coronavirus disease (COVID-19) on our clinical trials, our supply chain, and our operations; and other risks and uncertainties, including those described under the heading “Risk Factors” included in our most recent Annual Report on Form 10-k for the year ended December 31, 2020, filed with the U.S. Securities and Exchange Commission (SEC) on March 1, 2021. Additional factors that could cause actual results to differ materially from our expectations can be found in our Securities and Exchange Commission filings. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the effects of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. All forward-looking statements included in this presentation are expressly qualified in their entirety by these cautionary statements. The forward-looking statements speak only as of the date made and, other than as required by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. Bardoxolone methyl, omaveloxolone, and RTA 901 are investigational drugs, and their safety and efficacy have not been established by any agency.

Today’s Agenda

Regulatory Update on Omaveloxolone for Patients with Friedreich’s ataxia NDA: New Drug Application

Regulatory Update on Bardoxolone for Patients with Alport Syndrome FDA: U.S. Food and Drug Administration; BIMO: Bioresearch monitoring; PDUFA: Prescription Drug User Fee Act

Mid-Cycle Communication Meeting Overview Meeting focused on clinical and statistical topics Four significant issues identified, which we believe are addressable FDA invited us to address these issues in follow up submissions to the NDA Other review topics previously raised were not mentioned FDA did not designate any safety issues as significant issues FDA communicated that a Risk Evaluation and Mitigation Strategies (REMS) program is not needed based on its current review FDA is continuing its review of the NDA

Chronic Kidney Disease Pipeline

Alport Syndrome
Mid-Cycle Communication Meeting Purpose of the mid-cycle communication meeting is for the FDA to provide Sponsor with a status update on the NDA review, including any issues identified Meeting was focused on four clinical and statistical review issues that were identified as significant Acute PD effects vs. slowing of decline in kidney function Treatment duration as a covariate in Week 104 analysis Post-hoc sensitivity analysis for off-treatment analysis window duration Impact of COVID-19 FDA did not designate any safety issues as significant issues We believe each of these issues are addressable with additional data and analyses We plan to address each of the issues raised by the FDA through submission of additional data and analyses to the NDA
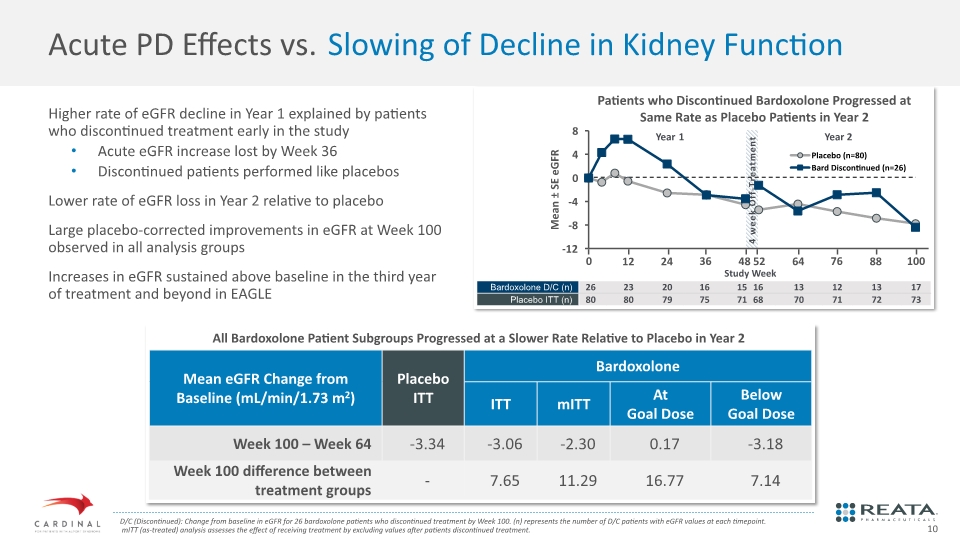
4 week Off-Treatment Patients who Discontinued Bardoxolone Progressed at Same Rate as Placebo Patients in Year 2 Study Week Higher rate of eGFR decline in Year 1 explained by patients who discontinued treatment early in the study Acute eGFR increase lost by Week 36 Discontinued patients performed like placebos Lower rate of eGFR loss in Year 2 relative to placebo Large placebo-corrected improvements in eGFR at Week 100 observed in all analysis groups Increases in eGFR sustained above baseline in the third year of treatment and beyond in EAGLE Acute PD Effects vs. Slowing of Decline in Kidney Function D/C (Discontinued): Change from baseline in eGFR for 26 bardoxolone patients who discontinued treatment by Week 100. (n) represents the number of D/C patients with eGFR values at each timepoint. mITT (as-treated) analysis assesses the effect of receiving treatment by excluding values after patients discontinued treatment. All Bardoxolone Patient Subgroups Progressed at a Slower Rate Relative to Placebo in Year 2
Treatment Duration as a Covariate in Week 104 Analysis FDA questioned, and then acknowledged, that we followed the pre-specified analysis model, which included treatment duration as a covariate During the meeting, we discussed the imputation step, which did not pre-specify use of covariates FDA suggested that the treatment duration covariate could have been included We noted that the SAP did not specify covariates for the imputation step Due to sparse reference data for treatment duration, inclusion of this covariate would have resulted in over 30% of imputed eGFR values falling outside of the range of observed values Many would have been extreme and exceeded biological plausibility (max observed +28 mL/min vs. max imputed of +105 mL/min) Would not affect the overall treatment effect Extreme values would artificially inflate the standard error and cause loss of statistical significance
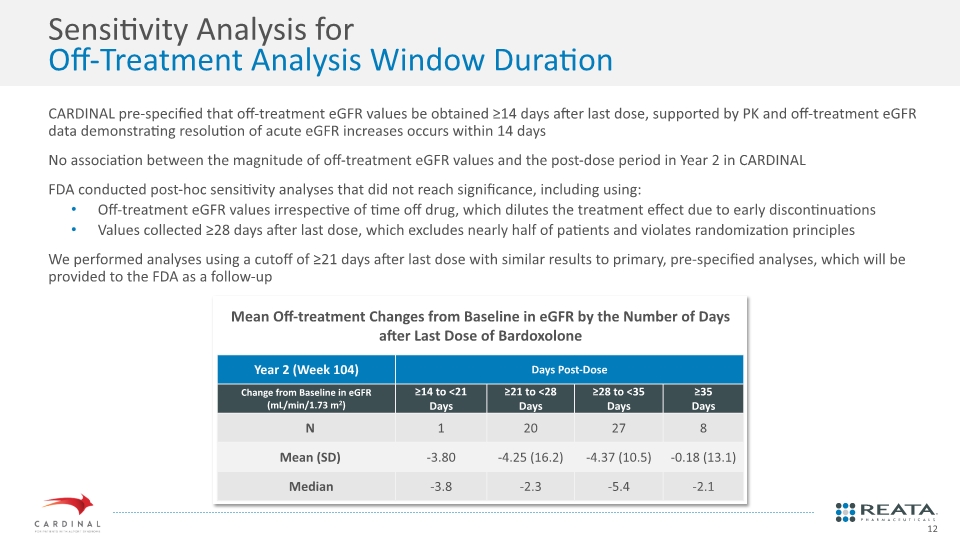
CARDINAL pre-specified that off-treatment eGFR values be obtained ≥14 days after last dose, supported by PK and off-treatment eGFR data demonstrating resolution of acute eGFR increases occurs within 14 days No association between the magnitude of off-treatment eGFR values and the post-dose period in Year 2 in CARDINAL FDA conducted post-hoc sensitivity analyses that did not reach significance, including using: Off-treatment eGFR values irrespective of time off drug, which dilutes the treatment effect due to early discontinuations Values collected ≥28 days after last dose, which excludes nearly half of patients and violates randomization principles We performed analyses using a cutoff of ≥21 days after last dose with similar results to primary, pre-specified analyses, which will be provided to the FDA as a follow-up Sensitivity Analysis for Off-Treatment Analysis Window Duration

Impact of COVID-19 FDA stated that COVID-19 did not significantly impact treatment visits, data collection, or study drug dispensation 31% of Week 104 assessments were conducted before COVID-19 and 69% of Week 104 assessments were conducted after COVID-19 FDA noted differences for the pre- and post-COVID results for the Week 104 endpoint The differences are driven by small sample set in pre-COVID arm resulting in a difference in eGFR in the pre- and post-COVID placebo arms Larger number of pediatric patients in the post-COVID subgroup This group had more rapid disease progression Point estimate for change from baseline in eGFR at Week 104 is similar across both periods for bardoxolone-treated patients
Safety FDA did not communicate significant issues about any safety topics FDA communicated that currently no REMS is expected

Bardoxolone in Other Forms of CKD

FALCON Phase 3 Study of Bardoxolone in Patients with ADPKD Phase 3 similar in design to CARDINAL with two-year treatment duration Based on experience with CARDINAL NDA submission and recent FDA feedback, we will not unblind FALCON until after study completion We noted that we may not be able to submit Year 1 data prior to Year 2 data availability FDA recommended to not unblind at Year 1 We are revising primary endpoint: from Year 1 off-treatment (Week 52) to Year 2 off-treatment (Week 104) The FDA recommended an alternate approach to handle discontinued patients in Week 104 analysis Suggested using eGFR values obtained at specified timepoints (i.e., 104 weeks after randomization) instead of within a specified window post-discontinuation Assessing impact on sample size, which may need to be increased >370 patients currently enrolled
Phase 2 trial to evaluate the safety and efficacy of bardoxolone in patients at risk of rapidly progressing CKD due to multiple etiologies Multi-center, double-blind, placebo-controlled eGFR 20-60 mL/min One of the following UACR ≥ 300 mg/g Annual eGFR decline rate ≥ 4 mL/min Hematuria Primary endpoint: eGFR change from baseline at Week 12 Secondary endpoint: eGFR change from baseline at Week 12 by CKD etiology Completed enrollment Top-line data expected in the fourth quarter of 2021 MERLIN Phase 2 Study of Bardoxolone in Patients with CKD at Risk for Rapid Progression

Financial & Operation Update
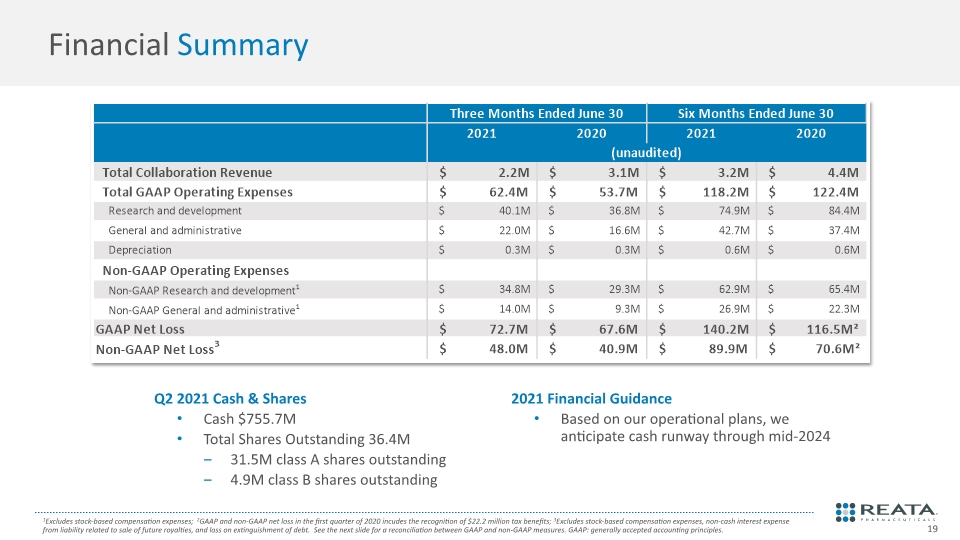
Q2 2021 Cash & Shares Cash $755.7M Total Shares Outstanding 36.4M 31.5M class A shares outstanding 4.9M class B shares outstanding 2021 Financial Guidance Based on our operational plans, we anticipate cash runway through mid-2024 Financial Summary 1Excludes stock-based compensation expenses; 2GAAP and non-GAAP net loss in the first quarter of 2020 incudes the recognition of $22.2 million tax benefits; 3Excludes stock-based compensation expenses, non-cash interest expense from liability related to sale of future royalties, and loss on extinguishment of debt. See the next slide for a reconciliation between GAAP and non-GAAP measures. GAAP: generally accepted accounting principles.
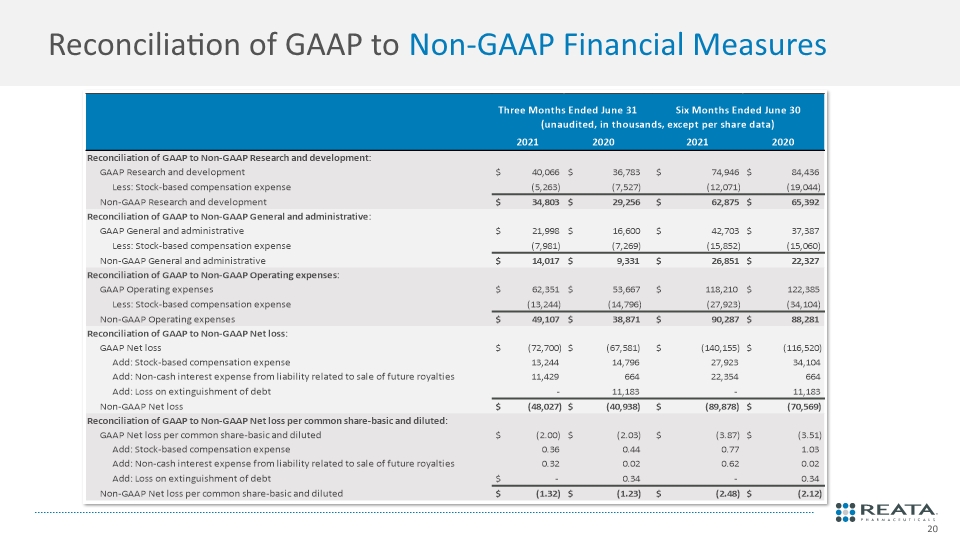
Reconciliation of GAAP to Non-GAAP Financial Measures

Concluding Remarks
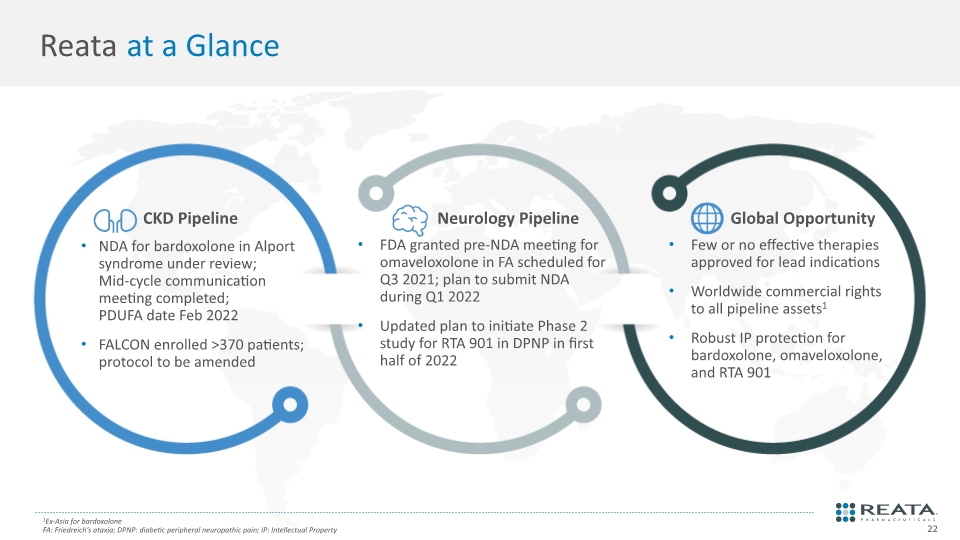
Reata at a Glance NDA for bardoxolone in Alport syndrome under review; Mid-cycle communication meeting completed; PDUFA date Feb 2022 FALCON enrolled >370 patients; protocol to be amended FDA granted pre-NDA meeting for omaveloxolone in FA scheduled for Q3 2021; plan to submit NDA during Q1 2022 Updated plan to initiate Phase 2 study for RTA 901 in DPNP in first half of 2022 Few or no effective therapies approved for lead indications Worldwide commercial rights to all pipeline assets1 Robust IP protection for bardoxolone, omaveloxolone, and RTA 901 CKD Pipeline Neurology Pipeline Global Opportunity 1Ex-Asia for bardoxolone FA: Friedreich’s ataxia; DPNP: diabetic peripheral neuropathic pain; IP: Intellectual Property

Thank you






















