Exhibit 99.1
Opiant Pharmaceuticals, Inc. Reports Second Quarter 2018 Financial Results and Provides Corporate Update
SANTA MONICA, Calif, August 9, 2018 – Opiant Pharmaceuticals, Inc. (“Opiant”) (NASDAQ: OPNT), a specialty pharmaceutical company developing pharmacological treatments for addictions and drug overdose, today reported financial results for the quarter and six months ended June 30, 2018, and provided a corporate update.
“Opiant is in a strong position to execute its operating plan,” said Roger Crystal, M.D., Chief Executive Officer of Opiant. “As a leader in addiction and drug overdose with an approved product and others in development targeting several substantial markets with critical unmet needs, we are well-positioned to create significant long-term shareholder value. We have a growing royalty revenue stream from net sales of NARCAN® Nasal Spray for the treatment of opioid overdose by our commercial partner, Adapt Pharmaceuticals (“Adapt”), and our successful development of this product serves to de-risk our robust and advancing pipeline.”
“Importantly, we are supported by a strong financial profile, with cash of approximately $11.2 million at the end of the second quarter,” continued Dr. Crystal. “Beyond our balance sheet, we were recently awarded a grant of approximately $7.4 million from the National Institutes of Health’s (“NIH”) National Institute on Drug Abuse for the development of OPNT003, our lead product candidate. OPNT003, nasal nalmefene, is a long-acting opioid antagonist currently in development for the treatment of opioid overdose. We believe our financial resources are sufficient to fund the completion of a registration study for OPNT003, and the results from two Phase II studies for our other product candidates, OPNT001 for Bulimia Nervosa and OPNT002 for Alcohol Use Disorder.”
“We achieved significant sequential growth of over 70% in royalty revenue in the second quarter of 2018,” said David O’Toole, Chief Financial Officer of Opiant. “These results provide a strong foundation for potential further NARCAN® success in the second half of the year.”
Recent Corporate Highlights
| • | Received first tranche of $500,000 from the previously awarded grant of approximately $7.4 million from the NIH’s National Institute on Drug Abuse for the development of OPNT003 |
| • | Participated in multiple key industry conferences |
| o | American Society of Clinical Psychopharmacology 2018 Annual Meeting |
| § | Phil Skolnick, Ph.D., Opiant’s Chief Scientific Officer, chaired a panel titled, “The Opioid Epidemic: Crisis and Solutions,” and Dr. Crystal served as a panelist |
| § | Dr. Skolnick received the 2018 “Donald Klein Lifetime Achievement Award” for his contributions to the field of neuropsychopharmacology |
| o | BIO International Convention |
| § | Dr. Crystal served as a panelist on “Biology of Pain and Addiction – Next Generation Treatments for Pain and Addiction” |
| • | Recent national TV appearances, including ‘Fox and Friends’ and Fox Business Network’s “Mornings with Maria” – https://www.opiant.com/media/ |
| • | Appointed biotech and pharmaceutical industry veteran Richard J. Daly to Board of Directors |
Exhibit 99.1
Financial Results for the Second Quarter Ended June 30, 2018
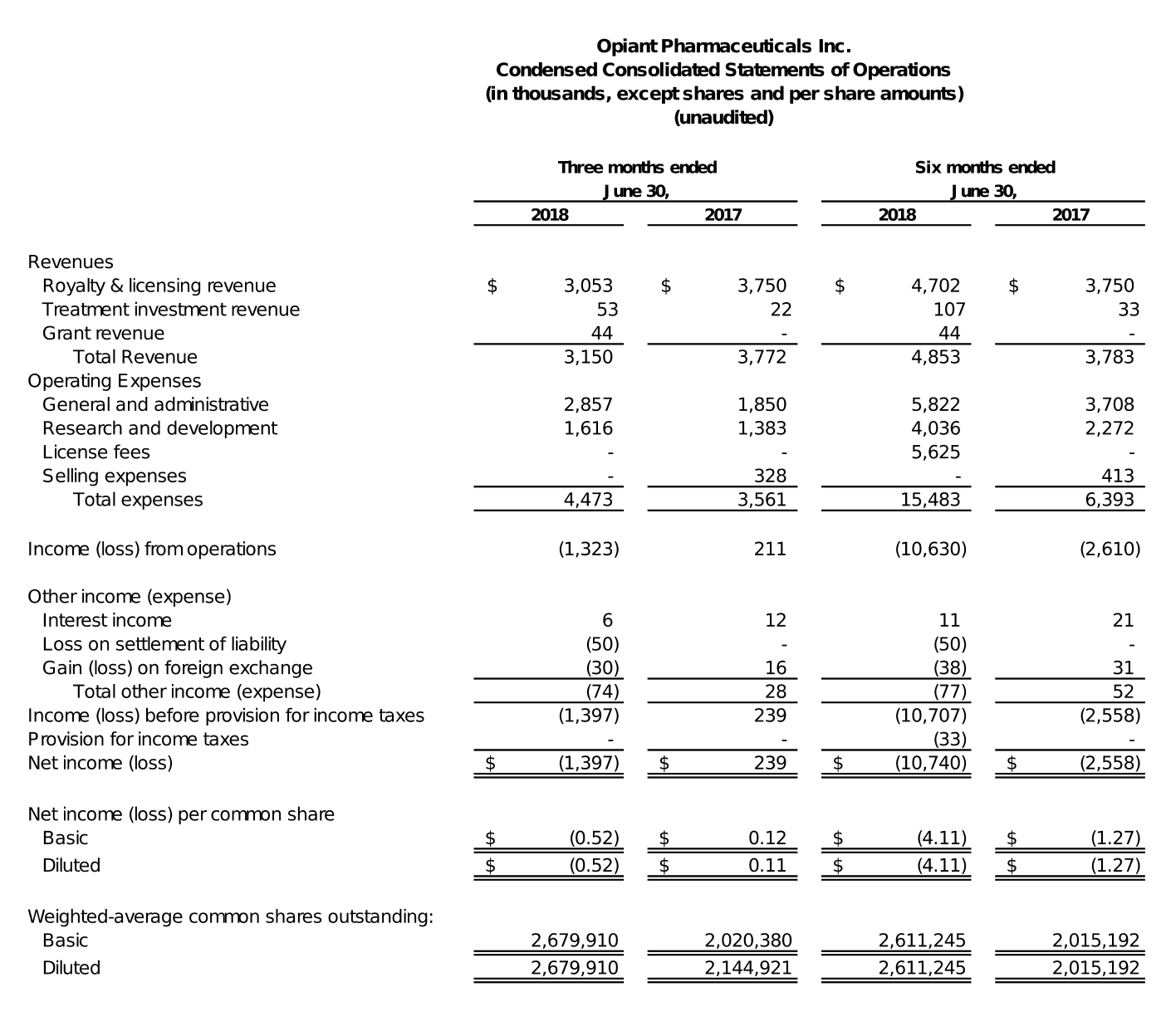
For the three months ended June 30, 2018, Opiant recorded approximately $3.2 million in revenue, compared to $3.8 million during the corresponding period of 2017. For the three months ended June 30, 2018, Opiant recognized $3.1 million of royalty revenue from the license agreement between Opiant and Adapt for the sale of NARCAN® (naloxone hydrochloride) Nasal Spray. For the three months ended June 30, 2017, Opiant recognized $3.8 million of revenue from the sale to SWK of Opiant’s right to receive royalties arising from the sale, by Adapt, of NARCAN® Nasal Spray. This $3.8 million payment from SWK was a one-time payment as provided under the royalty monetization agreement between Opiant and SWK.
General and Administrative expenses for the three months ended June 30, 2018, were approximately $2.9 million, compared to $1.9 million in the comparable period of 2017. The increase was primarily due to a $0.7 million increase associated with stock-based compensation expense and a $0.3 million increase attributed to employee salaries and related expense, during the three months ended June 30, 2018, as compared to the three months ended June 30, 2017.
Research and development expenses for the three months ended June 30, 2018, were approximately $1.6 million, compared to approximately $1.4 million in the comparable period of 2017. The increase was primarily attributable to a $0.4 million increase in stock-based compensation expense and an increase of $0.3 million in personnel and related expense, partially offset by a $0.5 million reduction in third-party expenses associated with Opiant’s research and development programs.
Net loss for the three months ended June 30, 2018, was $1.4 million, or a loss of $0.52 per basic and diluted share, compared to net income of $0.2 million, or income of $0.12 per basic share and $0.11 per diluted share, for the comparable period of 2017.
Six Months Ended June 30, 2018 Financial Results
For the six months ended June 30, 2018, Opiant recorded approximately $4.9 million in revenue, compared $3.8 million in the corresponding period of 2017. During the six months ended June 30, 2018, Opiant recognized $4.7 million of royalty revenue from the license agreement between it and Adapt for the sale of NARCAN® (naloxone hydrochloride) Nasal Spray. During the six months ended June 30, 2017, Opiant recorded $3.8 million from the sale to SWK of Opiant’s right to receive royalties arising from the sale, by Adapt, of NARCAN® Nasal Spray. This $3.8 million payment from SWK was a one-time milestone payment, as provided under the royalty monetization agreement between Opiant and SWK.
General and administrative expenses for the six months ended June 30, 2018, were approximately $5.8 million, compared to $3.7 million for the six months ended June 30, 2017. The increase was primarily due to a $1.6 million increase associated with stock-based compensation expense, and $0.5 million increase in corporate overhead.
Research and development expenses for the six months ended June 30, 2018, were approximately $4.0 million, compared to $2.3 million in the comparable period of 2017. The increase was primarily attributable to a $0.7 million increase in stock-based compensation expense, a $0.6 million increase in personnel and related expense, and a $0.4 million increase in third-party expenses associated with Opiant’s research and development programs.
Exhibit 99.1
License fees for the six months ended June 30, 2018, were $5.6 million. The license fees relate to Opiant’s obligations under the License Agreement with Adapt. There were no license fees for the six months ended June 30, 2017.
Net loss for the six months ended June 30, 2018, was $10.7 million, or a loss of $4.11 per basic and diluted share, compared to a net loss of $2.6 million, or a loss of $1.27 per basic and diluted share, for the comparable period of 2017. The significant increase in net loss for the six months ended June 30, 2018, compared to the same period in 2017, was primarily due to the non-recurring license fee of $5.6 million paid to Adapt.
At June 30, 2018, Opiant had cash and cash equivalents of approximately $11.2 million, compared to approximately $8.1 million at December 31, 2017.
Conference Call Details
Thursday, August 9th @ 4:30pm Eastern Time/1:30pm Pacific Time
Toll Free: 800-263-0877
International: 323-794-2094
Conference ID: 5522978
Webcast: http://public.viavid.com/index.php?id=130379
Replays, Available through August 23rd:
Domestic: 844-512-2921
International: 412-317-6671
Replay PIN: 5522978
About Opiant Pharmaceuticals, Inc.
Opiant Pharmaceuticals, Inc. is a specialty pharmaceutical company developing pharmacological treatments for addictions and drug overdose. The National Institute on Drug Abuse (NIDA), a component of the NIH, describes addictive disorders as chronic relapsing brain diseases which burden society at both the individual and community levels. With its innovative opioid antagonist nasal delivery technology, Opiant is positioned to become a leader in these treatment markets. Opiant's first drug overdose product, NARCAN® Nasal Spray, is approved for marketing in the U.S. and Canada by its partner, Adapt Pharmaceuticals. For more information please visit: www.opiant.com.
Forward-Looking Statements
This press release contains forward-looking statements. These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our or our industry's actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed, implied or inferred by these forward-looking statements, and among other things, our ability to maintain cash balances and successfully commercialize or partner our product candidates currently under development. In some cases, you can identify forward-looking statements by terminology such as "may," "will," "should," "could," "would," "expects," "plans," "intends," "anticipates," "believes," "estimates," "predicts," "projects," "potential," or
Exhibit 99.1
"continue" or the negative of such terms and other comparable terminology. These statements are only predictions based on our current expectations and projections about future events. You should not place undue reliance on these statements. Actual events or results may differ materially. In evaluating these statements, you should specifically consider various factors. Additional factors that could materially affect actual results can be found in our Form 10-KT for the transition period August 1 to December 31, 2017 and Form 10-Q for the period ended March 31, 2018, filed with the Securities and Exchange Commission on March 7, 2018 and May 8, 2018, respectively, including under the caption titled "Risk Factors." These and other factors may cause our actual results to differ materially from any forward-looking statement. We undertake no obligation to update any of the forward-looking statements after the date of this press release to conform those statements to reflect the occurrence of unanticipated events, except as required by applicable law.
CONTACTS:
Dan Ferry
Managing Director
LifeSci Advisors, LLC
Daniel@lifesciadvisors.com
(617) 535-7746
Exhibit 99.1
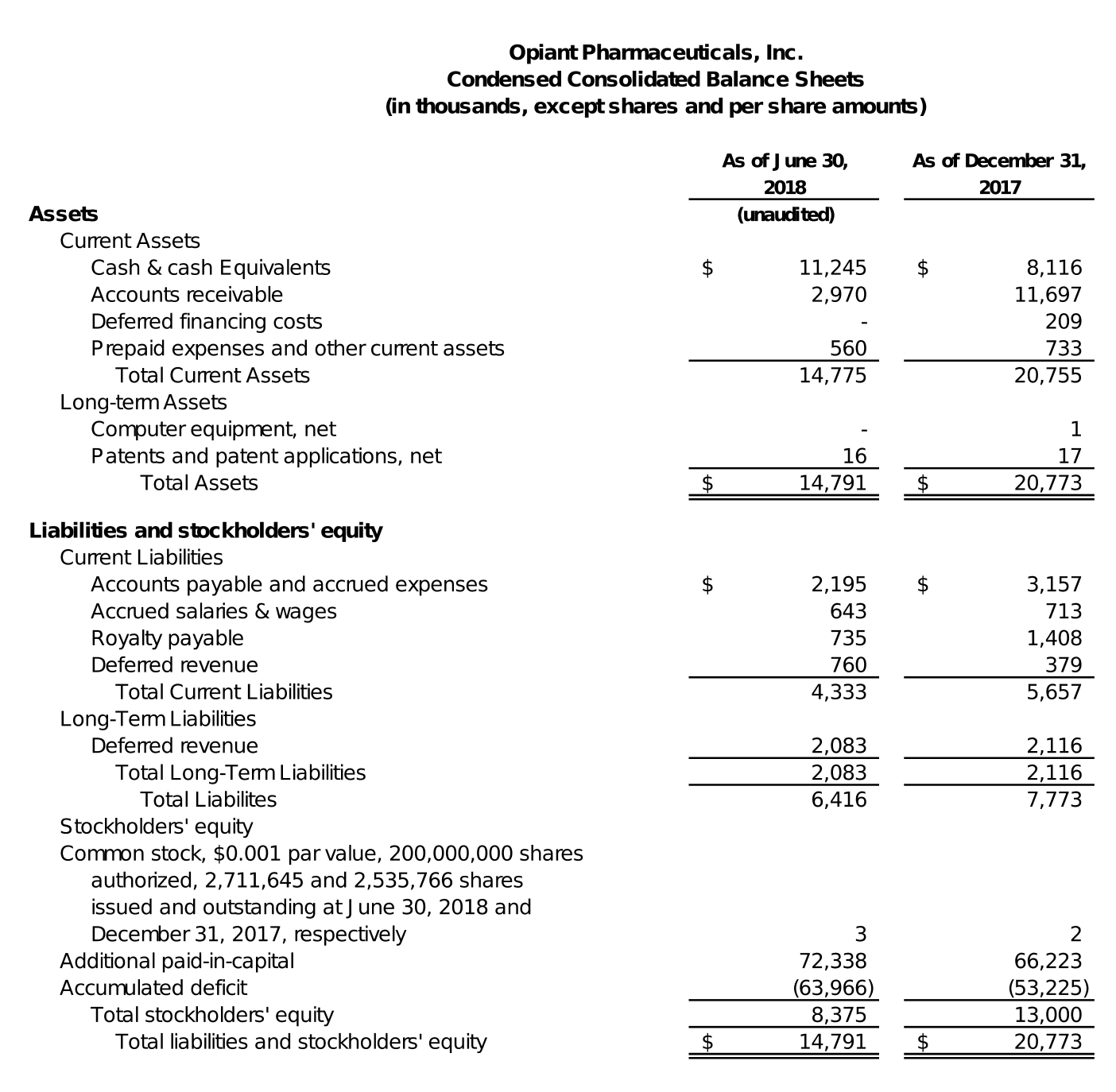
Exhibit 99.1