UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For The Fiscal Year Ended December 31, 2011
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
COMMISSION FILE NUMBER: 001-34256
HEARTWARE INTERNATIONAL, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 26-3636023 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
205 Newbury Street, Suite 101
Framingham, Massachusetts 01701
+1 508 739 0950
(Address of principal executive offices) (Zip Code)
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class Common Stock, $0.001 Par Value Per Share | Name of Each Exchange on which Registered The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | þ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No þ
The aggregate market value of the registrant’s outstanding common stock, other than shares held by persons who may be deemed affiliates of the registrant, computed by reference to the closing sale price of the common stock reported on the NASDAQ Stock Market as of June 30, 2011, was approximately $658.9 million. For purposes of the above statement only, shares of the registrant’s common stock held by directors and executive officers and entities or persons that, to the registrant’s knowledge, owned 10% or more of the registrant’s outstanding common stock as of June 30, 2011 have been excluded from this number in that these persons may be deemed affiliates of the registrant. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 15, 2012, the registrant had 14,114,055 shares of common stock, par value $0.001, issued and outstanding.
Documents Incorporated By Reference
Portions of the registrant’s definitive proxy statement to be delivered to stockholders in connection with the registrant’s 2012 Annual Meeting of Stockholders are incorporated herein by reference in Part III of this Annual Report on Form 10-K to the extent stated herein. Such proxy statement will be filed with the Securities and Exchange Commission within 120 days of the registrant’s fiscal year ended December 31, 2011.
References
Unless the context requires otherwise, references in this Annual Report on Form 10-K to:
| • | “HeartWare,” “the Company,” “HeartWare Group,” “we,” “us” and “our” refer to HeartWare International, Inc. and its consolidated subsidiaries, HeartWare Pty. Limited, HeartWare, Inc., HeartWare GmbH, HeartWare (UK) Limited and HeartWare France. |
| • | “HeartWare International, Inc.” refers to HeartWare International, Inc., a Delaware corporation incorporated on July 29, 2008. |
| • | “HeartWare Pty. Limited” refers to HeartWare Pty. Limited (formerly known as HeartWare Limited), an Australian proprietary corporation originally incorporated on November 26, 2004. |
| • | “HeartWare, Inc.” refers to HeartWare, Inc., a Delaware corporation incorporated on April 3, 2003. HeartWare, Inc. was acquired by HeartWare Pty. Limited on January 24, 2005. |
| • | “HeartWare GmbH” refers to HeartWare GmbH, a German corporation established on February 19, 2010. |
| • | “HeartWare (UK) Limited” refers to HeartWare (UK) Limited, a limited liability corporation established in the United Kingdom on February 19, 2010. |
| • | “HeartWare France” refers to HeartWare France, a French corporation established on August 16, 2011. |
Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These forward-looking statements are based on our management’s beliefs, assumptions and expectations and on information currently available to our management. Generally, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward-looking statements, which generally are not historical in nature. All statements that address operating or financial performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements, including without limitation:
| • | our expectations with respect to regulatory submissions and approvals, such as FDA approval of our premarket approval application for our HeartWare® Ventricular Assist System for a bridge-to-transplant indication; |
| • | our expectations with respect to our clinical trials, including enrollment in or completion of our clinical trials as well as approval of new clinical trials and continued access protocols with respect to our existing clinical trials; |
| • | our expectations with respect to the integrity or capabilities of our intellectual property position; |
| • | our ability and plans to commercialize our existing products; |
| • | our ability and plans to develop and commercialize new products and the expected features and functionalities and possible benefits of these products; and |
| • | our estimates regarding our capital requirements and financial performance, including profitability. |
Our management believes that these forward-looking statements are reasonable as and when made. However, you should not place undue reliance on our forward-looking statements because they speak only as of the date when made. We do not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by federal securities laws and the rules and regulation of the Securities and Exchange Commission. We may not actually achieve the plans, projections or expectations disclosed in our forward-looking statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, including without limitation those described in Part I, Item 1A. “Risk Factors”
1
and elsewhere in this report and those described from time to time in our future reports filed with the Securities and Exchange Commission. Investors should read the entire Annual Report on Form 10-K and consult their respective financial, legal or other professional adviser in relation to the subject matter therein, especially as it pertains to our risks and uncertainties outlined in Part I, Item 1A. “Risk Factors” of this Annual Report on Form 10-K, together with the information provided in our other public filings with the Securities and Exchange Commission.
Corporate Information
HeartWare International, Inc. was incorporated in Delaware on July 29, 2008 and became the successor issuer to HeartWare Limited, an Australian corporation, on November 13, 2008, as a result of the Australian Court approved redomiciliation of HeartWare Limited from Australia to Delaware. Prior to this date, HeartWare Limited was the ultimate parent company of the HeartWare Group and, following the redomiciliation, HeartWare International, Inc. became the ultimate parent company. In January 2009, HeartWare Limited was converted to an Australian private company and was renamed HeartWare Pty. Limited.
We further discuss our corporate history under “Business—Corporate History”.
In connection with the 2008 redomiciliation referred to above, each holder of HeartWare Limited ordinary shares, share options or performance rights received one share of common stock, one stock option or one restricted stock unit, of HeartWare International, Inc., for every 35 of HeartWare Limited ordinary shares, share options or performance rights, respectively, held by such holder. Unless the context requires otherwise, all information in this Annual Report on Form 10-K regarding shares, options or other securities of HeartWare International, Inc. or HeartWare Limited, as applicable, including related data on a per unit basis, has been adjusted to give effect to the 2008 redomiciliation transaction, whether such information pertains to a date or period of time subsequent or prior to the redomiciliation transaction.
Our principal executive offices are located at 205 Newbury Street, Suite 101, Framingham, Massachusetts. Our telephone number is 1-508-739-0950. Our website address iswww.heartware.com. We make available on this website, free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports as soon as reasonably practicable after we electronically file or furnish such materials to the Securities and Exchange Commission. We have included our website address in this Annual Report on Form 10-K as an inactive textual reference only. The information on, or that can be accessed through, our website is not part of this Annual Report on Form 10-K.
Currency
Unless indicated otherwise in this Annual Report on Form 10-K, all references to “$”, “U.S.$” or “dollars” refer to United States dollars, the lawful currency of the United States of America. References to “AU$” refer to Australian dollars, the lawful currency of the Commonwealth of Australia. References to “€” or “Euros” means Euros, the single currency of Participating Member States of the European Union. References to “£” or “British Pounds” refer to British pound sterling, the lawful currency of the United Kingdom.
Trademarks
HEARTWARE®, HVAD®, MVAD®, KRITON® and various company logos are the trademarks of the Company, in the United States, Europe, Australia and other countries. All other trademarks and trade names mentioned in this Annual Report on Form 10-K are the property of their respective owners.
2
Overview
HeartWare develops and manufactures small implantable heart pumps, or ventricular assist devices, for the treatment of advanced heart failure. The HeartWare Ventricular Assist System (the “HeartWare System”), which includes a ventricular assist device (“VAD”), or blood pump, patient accessories and surgical tools, is designed to provide circulatory support for patients in the advanced stage of heart failure. The core of the HeartWare System is a proprietary continuous flow blood pump, the HVAD Pump, which is a full-output device capable of pumping up to 10 liters of blood per minute. The HeartWare System is designed to be implanted adjacent to the heart, avoiding the abdominal surgery generally required to implant similar devices.
Heart failure is a chronic disease that results in the heart’s pumping power being weaker than normal. In a healthy person, the left ventricle of the heart pumps oxygenated blood into the aorta and the blood is then circulated throughout the body until it returns through the venous system to the right side of the heart, which pumps it into the lungs where it is re-oxygenated. If the left ventricle is not working properly, the oxygenated blood is not fully cleared from the lungs and the blood is not circulated effectively. If the muscle of the left ventricle is damaged or is not working efficiently, the ventricle will tend to compensate by working harder in an effort to supply adequate blood flow into the aorta. The increased effort generally results in dilation, or enlargement, of the ventricle, rather than increased blood flow. This dilation then makes it harder for the heart to contract effectively which results in even lower blood flow and increased effort and further dilation of the ventricle. This progressive, degenerative process generally continues until the patient becomes debilitated and eventually dies from inadequate clearing of the lungs and inadequate flow of oxygenated blood throughout the body. The inadequate lung clearance or lung congestion is why the advanced stages of heart failure are called congestive heart failure.
In 2009, we received CE Marking for the HeartWare System in the European Union and in March 2011 we received approval from the Therapeutic Goods Administration in Australia allowing for commercial sale and distribution of our device for bridge-to-transplant use. In the U.S., the device is the subject of clinical trials for two indications: bridge-to-transplant and destination therapy. Our device is also available in other countries around the world under special access programs and limited commercial availability. As of December 31, 2011, the HeartWare System has been implanted outside of the U.S. in patients at over 73 health care sites in 22 countries.
Bridge-to-transplant
HeartWare's ADVANCE clinical trial is a Food and Drug Administration approved investigational device exemption, or IDE, study designed to evaluate the HeartWare® Ventricular Assist System as a bridge to heart transplantation for patients with end-stage heart failure. Between August 2008 and February 2010, 140 patients at 30 hospitals in the United States received the HeartWare investigational device. The protocol analysis includes 137 patients in the investigational device cohort.
On December 27, 2010, HeartWare submitted to the FDA a Premarket Approval, or PMA, application seeking approval of the HeartWare System for the bridge-to-transplant indication. The PMA application was supported with data from our bridge-to-transplant clinical trial, named “ADVANCE”, in the U.S. In February 2012, we were notified that a FDA Circulatory System Devices Panel of the Medical Devices Advisory Committee will review our application on April 25, 2012.
IDE Supplements have allowed us to continue to enroll patients in our ADVANCE trial under a Continued Access Protocol (“CAP”). The CAP makes the HeartWare System available to patients and clinicians while also providing additional data for the FDA to evaluate prior to determining whether or not to approve the HeartWare System. The CAP patients will be enrolled and followed under a modified protocol of the ADVANCE trial. Through December 31, 2011, 202 patients have been enrolled in the study under CAP. We have exhausted our current CAP allotment and have submitted an application to the FDA for an additional cohort of patients under CAP.
3
On November 14, 2010, data from HeartWare’s bridge to heart transplantation (“BTT”) study, ADVANCE, was presented at the 2010 Scientific Sessions of the American Heart Association by co-principal investigator Keith Aaronson, M.D. M.S., Associate Professor in the Division of Cardiovascular Medicine and Medical Director of the Heart Transplant Program and Center for Circulatory Support at the University of Michigan, on behalf of the ADVANCE investigators.
Results from the ADVANCE clinical study showed that 92% of the investigational device patients met the per protocol primary endpoint of the trial, which was defined as alive on the originally implanted device, transplanted or explanted for recovery at 180 days. Results from the ADVANCE clinical study also demonstrated that 94% of the investigational device patients enrolled in the study achieved a survival endpoint at 180 days.
Results for the comparator arm of the study, derived from 499 contemporaneous patients from the Interagency Registry for Mechanically Assisted Circulatory Support (“INTERMACS”) demonstrated 90% success of the primary endpoint at 180 days, as well as Kaplan-Meier survival at 180 days of 90%. Based on these results for the primary endpoint of the ADVANCE study, non-inferiority of the investigational device was established [p<0.0001].
In April 2011, clinical data from our ADVANCE bridge-to-transplant clinical trial was presented at The International Society of Heart and Lung Transplantation (ISHLT) 31st Annual Meeting and Scientific Sessions in San Diego. The data showing 180-day survival, using Kaplan-Meier analysis, for a combined 250 investigational device patients in the original study and the CAP was 94%.
In October 2011, we presented updated clinical data from our ADVANCE bridge-to-transplant clinical trial and the CAP at the 25th European Association for Cardio-Thoracic Surgery in Lisbon. The updated data for 241 patients enrolled in either the pivotal trial ADVANCE or CAP, and supported for at least six months, demonstrated a 180-day survival of 93%.
Destination Therapy
In June 2010, we received conditional IDE approval from the FDA to begin enrollment in our destination therapy clinical study for the HeartWare System. Designed to enroll up to 450 patients at 50 U.S. hospitals, the non-inferiority study, which is named “ENDURANCE,” is a randomized, controlled, unblinded, multi-center clinical trial to evaluate the use of the HeartWare System as a destination therapy in advanced heart failure patients. The study population will be selected from patients with end-stage heart failure who have not responded to standard medical management and who are ineligible for cardiac transplantation. Patients in the study will be randomly selected to receive either the HeartWare System or, as part of a control group they will be implanted with any alternative ventricular assist device (“VAD”) approved by the FDA for destination therapy, in a 2:1 ratio. Each patient receiving the HeartWare System or control VAD will be followed to the primary endpoint at two years, with a subsequent follow-up period extending to five years post implant. In August 2010, our first patient was implanted as part of the ENDURANCE trial and we received full IDE approval from the FDA in September 2010. As of December 31, 2011, 311 patients had been enrolled in the study.
Other Clinical Activities
As of January 15, 2011, we entered into a Public, Private Partnership Agreement with the Regents of the University of Michigan whereby we will act as industry sponsor of a study conducted by the University of Michigan Cardiovascular Center and the University of Pittsburgh exploring the potential benefits of VADs in patients who will be given earlier access to these devices under a grant awarded from the National Heart, Lung and Blood Institute. In the study, called REVIVE-IT, researchers will compare whether non-transplant eligible patients with heart failure less advanced than that of current VAD recipients do better with implanted devices than with current medical therapy. Pursuant to the terms of the agreement, we have committed to provide financial support up to $9.6 million over the five-year trial period. The REVIVE-IT study device will be HeartWare’s ventricular assist device, the HVAD pump. The pilot study of approximately 12 U.S. sites, including Michigan and Pittsburgh, will include 100 patients. Enrollment in the study is expected to commence in 2012.
4
Other Devices
Beyond the HeartWare System, we are also evaluating our new miniaturized device, known as the MVAD. The MVAD is based on the same technology platform as the HeartWare System but adopts an axial flow, rather than a centrifugal flow, configuration and is being developed in multiple designs. The MVAD designs are currently at the preclinical stage undergoing animal studies focused on less invasive implantation techniques, in preparation for first-in-man studies. We expect to enroll our first patient in an MVAD first-in-man study during 2012. Each of the MVAD configurations is approximately one-third the size of the HVAD Pump. We believe that the MVAD designs will be implantable by surgical techniques that are even less invasive than those required to implant the HVAD Pump.
Operations
We began generating revenue from sales of the HeartWare System in August 2008 and have incurred net losses in each year since our inception. We expect our losses to continue as we expand our pipeline through continued research and development into next generation products, continue our clinical trials and expand commercial markets outside of the United States.
We have financed our operations primarily through the issuance of convertible notes and the issuance of shares of our common stock. Most recently, on December 15, 2010, the Company issued Convertible Senior Notes with an aggregate principal amount of $143.75 million pursuant to the terms of an Indenture dated as of December 15, 2010. The Convertible Senior Notes are senior unsecured obligations of the Company. The Convertible Senior Notes bear interest at a rate of 3.5% per annum, payable semi-annually in arrears on June 15 and December 15 of each year. The Convertible Senior Notes will mature on December 15, 2017, unless earlier repurchased or converted. In February 2010, we closed a public offering, under a shelf registration on Form S-3 filed with the Securities and Exchange Commission on December 24, 2009, of approximately 1.77 million shares of our common stock at an offering price of $35.50 per share for aggregate gross proceeds of approximately $62.8 million. This amount includes the underwriter’s exercise of their over-allotment option to purchase an additional 230,595 shares of our common stock at the offering price. In August 2009, we sold approximately 2.74 million shares of our common stock through private placements in the United States and Australia, which raised net proceeds of approximately $58.6 million
We are headquartered in Framingham, Massachusetts. We have operations and manufacturing facilities in Miami Lakes, Florida, a development and operations facility in Sydney, Australia and a distribution and customer service facility in Hannover, Germany. As of December 31, 2011, we had 330 employees worldwide.
Market Opportunity
Heart Failure
Heart failure is one of the leading causes of death in the developed world. The American Heart Association estimates that heart failure affects 5.8 million people in the United States, while the European Society of Cardiology reports a prevalence of at least 10 million in European countries. Heart failure is a cardiovascular disease with both an increasing incidence and death rate worldwide. In the United States, approximately 670,000 new cases are diagnosed annually and approximately 300,000 patient deaths are attributed to advanced heart failure.
Our Target Markets—Class III and Class IV Patients
Our devices target certain classes of advanced heart failure patients, specifically Class III and IV patients as defined by the New York Heart Association (“NYHA”). It is estimated that the number of Class III and Class IV heart failure patients worldwide is approximately 7 million and that approximately 20% of these patients could benefit from a circulatory assist device. We believe that there is a significant market opportunity for ventricular assist devices, or VADs, that are smaller, easier to implant, easier to use and/or more reliable than the devices that are currently available. We also believe there is a significant market opportunity for any device that, relative to existing therapies, demonstrates superior patient outcomes at a lower cost.
It is estimated that there are approximately 5 million Class III heart failure patients worldwide. Of these five million patients, we estimate that approximately 1 million patients are severely impacted by congestive heart failure,
5
or CHF, but are not yet nearing the end stages of the disease. While these patients suffer on a daily basis, they do not need the same full support as the sicker, later-stage Class IV patients and they may be less willing to undergo the more invasive procedure required for the placement of the typical VAD. We believe that up to one-third of these one million patients could be candidates for a less invasive surgical approach because of the potential for reduced surgical risk and shorter post-operative recovery periods.
CHF Treatment Options
Although many pharmacological therapies and pacing devices that are designed to stimulate the heart have proven to be effective at prolonging the quality and duration of a patient’s life, such treatments and devices do not halt the progression of CHF. Pharmacologic management of CHF focuses primarily on increasing or stimulating the force of heart contractions. Medication regimens aim to improve the effectiveness of the heart’s contractions and slow the rate of CHF progression. For later stage Class III and Class IV patients, some investigations have suggested that the increase in patient survival rates using medical therapy is limited and that optimal medical therapy has not been demonstrated to stop or reverse the effects of CHF. Other approaches, such as devices that allow physicians to restrict or reduce the size of the heart and cell based therapy, are either in the early development stages or have not yet achieved outcomes that we believe would lead most physicians to consider these technologies as viable solutions.
Heart transplantation is the current primary therapy for refractory advanced heart failure and ultimately provides the best recovery of cardiac function. Heart transplantation is an effective and accepted surgical procedure that can result in end-stage heart failure patients resuming relatively normal lives for a period usually expected to be ten years or longer. However, the therapy is significantly constrained by the limited number of available donor hearts. Also, many patients with heart failure are ineligible for heart transplantation because of factors such as age or the presence of other diseases.
VAD Treatment for Advanced Heart Failure
Circulatory assist devices are designed to take over some or all of the pumping function of the heart by mechanically pumping blood into the aorta. Implantation of circulatory assist devices is the only therapy other than transplantation that has been shown to rehabilitate a patient from NYHA Class IV to Class I or II. A November 2001 article inThe New England Journal of Medicineon a study entitled “Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure,” or the REMATCH study, concluded that “the use of a left ventricular assist device in patients with advanced heart failure resulted in a clinically meaningful survival benefit and an improved quality of life. A left ventricular assist device is an acceptable alternative therapy in selected patients who are not candidates for cardiac transplantation.” The conclusions in this study have since been reconfirmed in a number of subsequent similar studies with VADs, including a bridge-to-transplant study and a destination therapy study, reported in the August 2007 and November 2009 articles respectively inThe New England Journal of Medicine.
A large population of end-stage heart failure patients can benefit from VAD therapy, such as our HeartWare System. Within this population there are four different indications of use of VADs: “bridge-to-transplant” therapy, “bridge-to-decision” therapy, “destination therapy” and “bridge-to-recovery” therapy.
Bridge-to-transplant therapy—Each year, the number of heart failure patients in need of a heart transplant exceeds the number of donor hearts that become available. According to the Organ Procurement and Transplantation Network , or OPTN, and Scientific Registry of Transplant Recipients, or SRTR, there were 1,853 heart transplants conducted in the United States in 2009, and as of February 3, 2012, 3,127 people are currently listed for heart transplant. The OPTN/SRTR 2010 Annual Data Report reported approximately 46% of the transplant candidates in 2009 spent one year or more on the waiting list, while nearly 40% of the patients transplanted were on a VAD as a bridge-to-transplant. Bridging the patient to transplant provides time to stabilize the patient until a suitable donor heart becomes available. We expect this percentage of patients on the waiting list who receive VAD support as a bridge-to-transplant to increase as surgeons and cardiologists become more familiar with the technology and confidence in the procedure grows in line with improving clinical data and device reliability.
6
Bridge-to-decision therapy—VADs are increasingly being used to assist physicians in determining which patients previously not eligible for a transplant should be listed. Rather than disqualify certain patients based upon their pre-VAD implant status, many patients now receive VAD implants and then the physician subsequently evaluates whether or not to list them for heart transplant in the future. The VAD “bridges” the physician’s listing decision and enables them to determine whether or not the patient will be a good transplant candidate by evaluating their overall health status after time spent on the VAD. This indication is best reflected in the National Institute of Health’s, or NIH, sponsored INTERMACS registry, which showed in June 2010 that 43% of registered patients were listed for heart transplant at the time of their implant, while 42% were listed as “bridge to candidacy”, or bridge-to-decision. Of note, 11.5% were registered as destination therapy.
Destination therapy—Circulatory assist devices can also be used as a permanent or lifetime therapy in medically refractory advanced heart failure patients who are deemed ineligible for heart transplantation due to, for example, their age or the presence of other diseases. The NIH estimates that destination therapy represents a long-term option for up to 100,000 patients in the United States. For these late stage patients, drug therapy historically has been the only alternative, with the 12-month mortality rate of approximately 75%. We believe that device durability and reliability, together with ease and perceived risk of implantation and better clinical outcomes, are important factors in determining whether destination therapy VADs will become accepted by physicians and patients.
Bridge-to-recovery therapy—Circulatory assist devices that provide prolonged unloading of the heart muscle, or myocardium, have been claimed to lead to recovery of the heart in some patients. In these patients, the combination of ventricular unloading combined with pharmaceutical therapy enables the physician to wean the patient from the pump and eventually remove it. This potential application of VADs was cited in the November 2006New England Journal of Medicinearticle that described a recovery rate of approximately 75% in the Harefield Hospital study. While there can be no certainty that these results will be replicated or occur with sufficient repeatability in similar clinical trials, we believe that if use of VADs in these circumstances achieves widespread physician acceptance, the potential market for use of our HeartWare System in bridge-to-recovery therapy could increase significantly since removal of the device reduces the potential clinical risks associated with pumps that are left in place for multiple years.
Our Solution and Products
Proprietary Pump Technology
The HeartWare System features the smallest, full-output centrifugal pump designed to be implanted in the chest, directly adjacent to the heart. At the core of our technology platform is our proprietary “hybrid” system for suspending the impeller, which is the only moving part within the pump. The impeller is suspended within the pump housing by the opposing forces of passive magnets and hydrodynamic thrust generated by the pump impeller, which circulates a cushion of blood. Once power is applied to the device and the impeller begins to rotate, there are no points of mechanical contact within the pump, thus providing a completely wearless pumping system.
We believe the hybrid suspension system has several important advantages over traditional technologies. The elimination of the internal mechanical bearings which are characteristic of second generation devices removes all points of mechanical friction or contact within the pump. We believe that this removal of contact should lead both to longer term reliability of the device and to a potential reduced risk of physical damage to blood cells as they pass through the pump. Our hybrid suspension technology also establishes a miniaturization path, which we believe will allow us to significantly downsize our pump technology without compromising clinical performance. We believe competing pump designs which rely on either active magnetic or hydrodynamic forces alone face various physical constraints that may limit their ability to downsize without sacrificing performance.
The HeartWare System
The first product in our portfolio, the HeartWare System, is comprised of the HVAD Pump, a small, permanently implantable VAD, patient accessories and surgical tools. The HVAD Pump is capable of generating up to 10 liters of blood flow per minute. With a displaced volume of only 50 cubic centimeters and a mass of 140 grams, the HVAD Pump is the only full-output pump implantable in the pericardial space, directly adjacent to the heart. It is also the only pump designed to be implanted above the diaphragm in all eligible patients. We believe the implanting in the pericardial space generally leads to significantly shorter surgery time and a less invasive procedure relative to alternative devices, which are normally implanted in the abdomen.
7
Device reliability of the HeartWare System is designed to be enhanced through the use of dual motor stators with independent drive circuitry, allowing a seamless transition between dual and single stator mode if required. The pump’s inflow cannula is integrated with the device itself, providing proximity between the heart and the pumping mechanism, facilitating ease of implant and helping to ensure optimal blood flow characteristics. The use of a wide-bladed impeller and the clear flow paths through the pump are designed to help reduce the risk of pump-induced damage to blood cells.
The HeartWare System has been approved for sale in Europe since early 2009. In the United States, we must obtain Premarket Approval, or PMA, before the HeartWare System can be commercialized. That PMA application was submitted to the FDA in late December 2010 and in February 2012, we were notified that a FDA Circulatory System Devices Panel of the Medical Devices Advisory Committee will review our application on April 25, 2012.
The HeartWare MVAD
The MVAD is a miniaturized blood pump intended for chronic heart failure patients. The device is a full-output axial flow pump with a fully suspended rotor and a displacement volume approximately one-third that of the HVAD Pump. The pre-clinical Good Laboratory Practices (GLP) in-vivo studies completed in September 2011 have shown the MVAD to have similar comparable blood flow characteristics to the HVAD Pump. The MVAD is designed for pericardial implantation and initial human clinical trials are expected to commence in summer 2012.
We believe it is likely that more patients will be willing to undergo a shorter, less invasive surgical procedure that may result in quicker recoveries and hospital discharge. We have taken advantage of the versatility of the MVAD design with multiple configurations specific to less invasive implantation procedures. This development has been supported by over 100 in-vivo studies. These devices may expand the potential pool of chronic heart failure patients.
Before the MVAD product will be available for commercial sale, we will need to achieve the following milestones:
| • | completion of the system development including next generation peripherals (e.g., controller, batteries, power adapters); |
| • | approval of and successful completion of a clinical trial; and |
| • | receipt of regulatory approvals for commercialization. |
Enhanced Quality of Life with Implantable Devices
Currently, the HeartWare System and all commercially available VADs are powered by a controller and battery packs worn external to the body. Power is transferred to the implanted pump via a thin electrical cable, called a driveline, which exits the patient’s skin in the abdominal area. We are working to develop an implantable system utilizing transcutaneous energy transfer, or TET, that will eliminate the need for a percutaneous driveline. A TET system contains a wearable power management system that is inductively coupled to implanted electronics that includes a rechargeable battery pack. The patient can remove the wearable power management system and enjoy a high quality lifestyle while the system is powered by the internal battery pack.
We are collaborating with Dualis MedTech GmbH, a subsidiary of AVRA Surgical, Inc., on the development of an implantable system. Since mid-2011, a team of HeartWare and Dualis engineers has worked to successfully demonstrate the feasibility of integrating Dualis’ proprietary wireless energy transfer technology with both HeartWare HVAD and MVAD systems. It is expected that an implantable system will be ready for GLP animal studies in 2013 and for human pre-clinical trials following successful completion of GLP studies.
8
Our Business Strategy
Our primary goal, above all else, is to focus on optimizing outcomes of patients being treated for congestive heart failure. To this end, we are leading innovation in the VAD sector and are also striving to develop and maintain a proprietary technology platform that enables the development of a pipeline of ever-smaller heart pumps that will reduce procedural invasiveness and simultaneously increase the number of patients who can benefit from our products. In addition, we intend to explore technologies and therapies.
We believe that our technology portfolio provides us with a significant competitive advantage in the market. To capitalize on that advantage, we are pursuing the following plan:
Expand Market Penetration outside of the U.S.—We sell to VAD centers and distributors throughout Europe and in other countries outside the U.S. With the receipt of CE Marking in January 2009, we began to develop the necessary infrastructure to support commercial sales in Europe. Throughout 2011 we continued to expand our infrastructure to support commercial activity, including establishing a European distribution facility, and have generated sales through 2011 from customers in 22 countries outside of the U.S. In the future, we intend to build wider distribution channels and ordering systems to deliver our products to the European market on a wider commercial scale as well as increase the number of countries where we have approval to sell our device commercially.
Obtain regulatory approval in the United States—In September 2008, we received full IDE approval from the FDA and commenced a 150 patient bridge-to-transplant clinical trial in up to 28 centers. The FDA allowed an increase to 40 centers in 2009, and in February 2010, we completed enrollment of this trial with 140 patients implanted with the HeartWare System. The remaining 10 patients were enrolled but did not receive an implant of the HeartWare System because they failed to meet the trial’s inclusion and exclusion criteria. We filed a PMA application with the FDA in late December 2010. In February 2012, we were notified that a FDA Circulatory System Devices Panel of the Medical Devices Advisory Committee will review our application on April 25, 2012.
In June 2010, we received conditional IDE approval from the FDA to begin enrollment in our destination therapy clinical study for the HeartWare System. Designed to enroll up to 450 patients at 50 U.S. hospitals, the non-inferiority study, which is named “ENDURANCE,” is a randomized, controlled, unblinded, multi-center clinical trial to evaluate the use of the HeartWare System as a destination therapy in advanced heart failure patients. Each patient receiving the HeartWare System or control VAD will be followed to the primary endpoint at two years, with a subsequent follow-up period extending to five years post implant. In August 2010, our first patient was implanted as part of the ENDURANCE trial and we received full IDE approval from the FDA in September 2010. As of December 31, 2011, 311 patients have been enrolled in the ENDURANCE trial.
Focus on continuous product development—In parallel with the clinical development of the HeartWare System, we plan to advance the development of our next generation products, such as MVAD and a TET system, and to enhance our existing HeartWare System peripheral equipment. We expect assessment and development and/or enhancement work for the MVAD, the TET system and our existing HeartWare System peripheral equipment to continue throughout 2012. We have completed GLP studies for the MVAD and the designs are currently at the preclinical stage undergoing animal studies focused on less invasive implantation techniques, in preparation for first-in-man studies. The primary objective of these projects is improved ease of implantation and use of the HeartWare System that we believe will enhance market acceptance.
Partner with leading professionals in the fields of cardiovascular surgery around the world—We have established relationships with leading professionals in the field of cardiovascular surgery and heart centers around the world and continue to expand this network. We believe these relationships are key to our growth as they help to drive clinical awareness of our products.
Explore complimentary or alternative therapies and technologies—We intend to explore business development opportunities including strategic alliances, joint ventures, and acquisitions that might complement or expand our market opportunities. Recently, we entered into a development agreement with Dualis MedTech to develop ventricular assist devices with wireless transcutaneous energy transfer (TET) system technology exclusively for HeartWare.
9
Sales and Marketing
Our sales and marketing strategy is to educate and promote the benefits of ventricular assist devices for the treatment of clinical heart failure among a variety of health care professionals. Outside of the U.S., we market directly to cardiac centers and hospitals that perform heart transplants as well as through medical device distributors with experience in local markets. In the U.S., until we receive the necessary regulatory approvals, our device is not marketed but the device is available through clinical studies.
We work with a broad spectrum of health care industry participants to promote the clinical benefits of our device, including hospital administrators, cardiologists, surgeons, nurses, perfusionists, insurers and government and industry representatives. Key to the development of our business is optimizing patient outcomes via effective training and clinical end-user support programs and resources.
More than 1,800 implants have been performed globally with the HeartWare Ventricular Assist System. At December 31, 2011, the HeartWare System had been implanted in patients outside of the U.S. at over 74 health care sites in 22 countries. To support commercial sales and enrollments in clinical trials we have created field teams including sales and marketing personnel and clinical specialists to educate and service this larger and rapidly growing patient base. In addition, we partner with leading physicians in the field to proctor and preceptor new physicians on the use of our devices in their centers and to present clinical and technical data about our system at scientific symposia, congresses, and trade shows, as well as publish in peer reviewed cardiovascular journals.
Our product management team conducts market research on end-user preferences and unmet needs, indentifies ways to evergreen our HVAD technology with new enhancements, and works with research and development on new technologies that meet newly identified needs that are not currently addressed with our current platform of products.
Intellectual Property
We rely on a combination of patents, trade secrets, trademarks and copyrights, together with non-disclosure and confidentiality agreements, to protect our proprietary rights in our technologies.
As of December 31, 2011, we have 27 issued U.S. patents, 10 issued Australian patents, 5 issued patents in each of Japan, Germany, the United Kingdom and France, as well as patents issued in the Netherlands, Spain, Italy, Korea, Canada and Israel. We also have 29 pending U.S. non-provisional patent applications and a number of international patent applications filed under the Patent Cooperation Treaty, as well as in Japan, Europe, Australia, China, Canada, India, Korea and Israel.
Our U.S. and foreign issued patents and patent applications cover fundamental technologies underlying our hemodynamically and physiologically compatible full-output, long-term circulatory assist devices. The main technologies claimed in patents and patent applications include:
| • | use of dual stators in a blood pump; |
| • | the combination of passive magnetic bearings and hydrodynamic thrust bearings; |
| • | channels or wide-bladed impellers in a blood pump; |
| • | the use of ceramic between an impeller and motor stator; |
| • | flow estimation based on impeller speed and viscosity; and |
| • | use of platinum alloy for blood pump impellers. |
Major patents and pending patent applications covering technologies for our HeartWare System are scheduled to expire at various times between 2016 and 2027. Patents and patent applications covering technologies for our MVAD pump system are scheduled to expire at various times between 2024 and 2030.
We actively monitor our intellectual property position and periodically review new developments to identify prudent extensions to our patent portfolio. We plan to file additional patent applications on inventions that we believe are patentable and important to our business. We may also license or acquire patents from third parties that may enhance or expand our development activities. Accordingly, we intend to pursue and defend aggressively patent protection on our proprietary technologies. We have previously asserted claims and responded to counterclaims
10
relating to our intellectual property. In connection with such processes, we have entered into and may in the future enter into settlement agreements pursuant to which third parties or their respective successors or assigns may commercialize competing technologies or products that would have otherwise been precluded by our patents subject to the agreement. See Item 1A. “Risk Factors”.
Despite our efforts, we may be subject to challenges, with or without merit, regarding our patents or other intellectual property. The medical device industry is characterized by a large number of patents and by frequent and substantial intellectual property litigation. Our products and technologies could infringe, or other persons could allege that our products and technologies infringe, upon the proprietary rights of third parties. If third parties successfully assert infringement or other claims against us, we may not be able to sell our products. In addition, patent or intellectual property disputes or litigation may be costly, result in product development delays or divert the efforts and attention of our management and technical personnel. If any such disputes or litigation arise, we may seek to enter into a royalty or licensing arrangement. However, such an arrangement may not be available on commercially acceptable terms, if at all. We may decide, in the alternative, to litigate the claims or to design around the patented or otherwise proprietary technology. At this time we are not party to any material legal proceedings that relate to patents or proprietary rights. We have had communication with various parties regarding certain of our patents which are material to our business and these are discussed in Item 1A. “Risk Factors.”
Our intellectual property also includes non-patented technology, processes and procedures, and technical knowledge and know-how accumulated or acquired since inception, all of which are significant to our competitive position. It is our policy to enter into confidentiality, non-disclosure and intellectual property assignment agreements with employees and consultants to help ensure that we can protect our rights in developed proprietary technology and prohibit the disclosure of any confidential information or trade secrets.
Government Regulation
United States
Our products are regulated by the FDA as a Class III medical device under the U.S. Food, Drug, and Cosmetic Act. FDA regulations govern:
| • | product design and development; |
| • | product testing; |
| • | product manufacturing; |
| • | product safety and effectiveness; |
| • | product labeling; |
| • | product storage; |
| • | record keeping; |
| • | premarket approval; |
| • | advertising and promotion; |
| • | distribution; |
| • | product sales and post-market activities; |
| • | import and export; |
| • | medical device (adverse event) reporting; and |
| • | field corrective actions (e.g., recalls). |
Premarket Approval
Each of our devices will be regulated as a Class III medical device. PMA approval from the FDA is required before marketing of a Class III medical device in the United States can commence. The process of obtaining PMA can be costly, lengthy and uncertain. A PMA application must be supported by extensive data including, but not limited to, technical, preclinical and clinical trials to demonstrate the safety and effectiveness of the device to the FDA’s satisfaction. Among other information, the PMA application must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed device and patient labeling.
11
If the FDA determines that a PMA application is complete, the FDA accepts the application and then begins an in-depth review of the submitted information. The FDA, by statute and regulation, has 180 days to review an accepted PMA application, although the review and response process generally occurs over a significantly longer period of time, typically one year, and can take up to several years. During this review period, the FDA may request additional information or clarification of information already provided. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a pre-approval inspection of our and our key suppliers’ facilities to evaluate compliance with the quality system regulation. They will also conduct a Bioresearch Monitoring (“BIMO”) inspection of the clinical trial including some of the clinical data sites. To help assure the continued safety and effectiveness of an approved device, the FDA may also require a post-approval study as a condition of approval. A post-approval study may be a clinical or non-clinical study required in the PMA approval order and is intended to gather specific information to address questions about the post-market performance and physician experience with an approved medical device. Under the Medical Device User Fee and Modernization Act of 2002, the fee to submit a PMA application can be up to $200,775. User fees are expected to rise over time. We qualified for a small business exemption that allowed us to file our first PMA application at no charge. PMA supplements are required for modifications to the manufacturing process, labeling, use and design of a device that is approved through the premarket approval process. PMA supplements often require submission of the same type of information as a PMA application except that the supplement is limited to information needed to support any changes from the device covered by the original PMA.
After completing enrollment in our ADVANCE trial, we implanted additional patients under a Continued Access to Investigational Devices program (“Continued Access”). We may request approval for additional cohorts of continued access patients in the future until the device is approved. Once enrollment in our ENDURANCE trial is completed, we expect to request approval from the FDA of a Continued Access Protocol of the device for the destination therapy indication.
Pervasive and Continuing FDA Regulation
Clinical trials require extensive recordkeeping and reporting requirements. Our clinical trials must be conducted under the oversight of an institutional review board at the relevant clinical trial site and in accordance with applicable regulations and policies including, but not limited to, the FDA’s good clinical practice, or GCP, requirements. We, the trial data safety monitoring board, the FDA or the institutional review board at each site at which a clinical trial is being performed may suspend a clinical trial at any time for various reasons, including a belief that the risks to study patients outweigh the anticipated benefits.
Both before and after FDA approval, numerous regulatory requirements apply. These include:
| • | quality system regulation, which requires manufacturers to follow design, testing, control, documentation and other quality assurance procedures during the design, manufacturing and commercialization phases; |
| • | regulations which govern product labels and labeling, prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling and promotional activities; |
| • | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and |
| • | notices of correction or removal and recall regulations. |
Advertising and promotion of medical devices are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Recently, some promotional activities for FDA-regulated products have resulted in enforcement actions brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act, competitors and others can initiate litigation relating to advertising claims.
Compliance with regulatory requirements is enforced through periodic, unannounced facility inspections by the FDA. Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions against us:
| • | warning letters or untitled letters; |
12
| • | fines, injunction and civil penalties; |
| • | recall or seizure of our products; |
| • | customer notification, or orders for repair, replacement or refund; |
| • | operating restrictions, partial suspension or total shutdown of production or clinical trials; |
| • | refusing our request for pre-market approval of new products; |
| • | withdrawing pre-market approvals that are already granted; and |
| • | criminal prosecution. |
European Union
The primary regulatory environment in Europe is that of the European Union, or EU which consists of 27 member states in Europe. The EU has adopted two directives that cover medical devices—Directive 93/42/EEC covering medical devices and Directive 90/385/EEC for active implantable medical devices, as well as numerous standards that govern and harmonize the national laws and standards regulating the design, manufacture, clinical trials, labeling, adverse event reporting and post market surveillance activities for medical devices that are marketed in member states. Medical devices that comply with the requirements of the national law of the member state in which they are first marketed will be entitled to bear CE Marking, indicating that the device conforms to applicable regulatory requirements, and, accordingly, can be commercially marketed within EEC states and other countries that recognize this mark for regulatory purposes. We received CE Marking for the HeartWare System in January 2009.
Australia
In Australia, the Therapeutic Goods Administration, or TGA, is responsible for administering the Australian Therapeutics Goods Act. The Office of Devices, Blood and Tissues is the department within the TGA responsible for devices. The TGA recognizes five classes of medical devices and HeartWare’s circulatory assist device falls under the category of “active implantable medical devices.”
The Australian Register of Therapeutic Goods, or ARTG, controls the legal supply of therapeutic goods in Australia. The ARTG is the register of information about therapeutic goods for human use that may be imported, supplied in, or exported from Australia. Any use of an unapproved medical device in humans, even in pilot trials, requires an exemption from the requirement for inclusion on the ARTG.
In March 2011 we received approval from the TGA to sell the HeartWare System commercially in Australia.
Other International Regulations
We are also subject to international regulations in other countries where our products are sold. We currently have limited sales to customers in countries outside of the EU, U.S. and Australia including Malaysia and New Zealand. These regulations relate to product standards, packaging and labeling requirements, import restrictions, tariff regulations, duties and tax requirements. Many of the regulations applicable to our products in these countries are similar to those of the FDA. The national health or social security organizations of certain countries require our products to be qualified before they can be marketed in those countries.
In order to be positioned for access to European and other international markets, we sought and obtained certification under the International Standards Organization (“ISO”) 13485 standards. ISO 13485 is a set of integrated requirements, which when implemented, form the foundation and framework for an effective quality management system. These standards were developed and published by the ISO, a worldwide federation of national bodies, founded in Geneva, Switzerland in 1947. ISO has more than 90 member countries and ISO certification is widely regarded as essential to enter Western European markets.
Healthcare Regulation
Recent healthcare policy changes
In response to perceived increases in health care costs in recent years, there have been and continue to be proposals by the federal government, state governments, regulators and third-party payors to control these costs and, more generally, to reform the U.S. healthcare system. For example, on March 23, 2010, the Patient Protection and
13
Affordable Care Act (“PPACA”) was signed into law. On March 30, 2010, a companion bill, the Health Care and Education Reconciliation Act of 2010 (the “Reconciliation Act”) was also signed into law. Among other things, the PPACA and the Reconciliation Act (collectively, the “Acts”), when taken together, impose a 2.3% excise tax on the sale of certain medical devices that will take effect in 2013. In addition, it is possible that standard setters or regulators may address certain unique aspects of the accounting for the Acts in the future.
Regulations related to prohibiting “kickbacks” and false claims and protecting patient confidentiality
A federal law commonly known as the Medicare/Medicaid anti-kickback law, and several similar state and foreign laws, prohibit payments that are intended to induce physicians or others either to refer patients or to acquire or arrange for or recommend the acquisition of healthcare products or services. These laws constrain our sales, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians or other potential purchasers of medical devices. Other federal and state laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent, or for items or services that were not provided as claimed. Because we may provide some coding and billing information to purchasers of the HeartWare System and our other products, and because we cannot assure that the government will regard any billing errors that may be made as inadvertent, these laws are potentially applicable to us. Anti-kickback and false claims laws prescribe civil and criminal penalties for noncompliance, which can be substantial.
There are a number of federal and state and foreign laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated patient privacy rules under the Health Insurance Portability and Accountability Act of 1996, or HIPAA. These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. If we are found to be in violation of the privacy rules under HIPAA or similar laws, we could be subject to civil or criminal penalties.
Transparency of certain payments
A section of the PPACA known as the Sunshine Act requires applicable manufacturers of drugs and devices to report annually for publication certain payments and other transfers of value to physicians and teaching hospitals as well as certain ownership interests held by physicians. Applicable manufacturers, including the Company, must begin to submit reports in 2013 with respect to payments and transfers occurring in 2012. We are establishing processes and procedures to capture and report payments to physicians and teaching hospitals.
Third Party Reimbursement
In the United States, hospitals and doctors generally rely on third-party payers, such as Medicare, Medicaid, private health insurance plans and self funded employers to pay or reimburse for all or part of the cost of medical devices and the related surgical procedures. In the United States, heart failure represents Medicare’s greatest area of spending.
In 2011, the Center for Medicare and Medicaid Services, or CMS, established reimbursement rates for the treatment of patients with LVADS, with major complications and comorbidities (“MS-DRG 1”) and without major complications and comorbidities (“MS-DRG 2”). Most patients that receive VADs and all patients that receive heart transplants are eligible for MD-DRG 1 reimbursement. Using the 2011 published payment rates, the national average Medicare payment to CMS-certified centers for MS-DRG 1 procedures is approximately $140,000. Actual payments are subject to other variables such as an application of a wage index, indirect medical education costs, cost outliers, and disproportionate share payments for each institution. In addition, when VAD patients are discharged from the hospital and then readmitted for transplantation, hospitals may qualify for 2 separate MS-DRG 1 or 2 payments.
We believe that our products will be Medicare-eligible and therefore that they should be entitled to reimbursement. Several insurance providers have also implemented U.S. policies for circulatory assist devices,
14
including Blue Cross and Blue Shield Plans, Aetna, Cigna, United Healthcare and others but such coverage may not be available if insurance providers refuse to cover Medicare approved IDE Category B2 clinical trials. We believe that many private insurers will cover our devices if they are also covered by Medicare. All of our sites in the U.S. bridge-to-transplant and destination therapy clinical trials received Medicare and third party reimbursement to some extent. In 2010, we added a reimbursement and government policy professional to our staff and will continue to build this team in 2012 with a view to improving insurance reimbursement outcomes for the HeartWare System.
International reimbursement varies from country to country and often hospital to hospital. The European system is more effective at focusing resource intensive procedures in a small number of centers within each country and LVAD’s fall into that category of resource intensive procedures. In those hospitals that perform VAD implantation, we believe that there are adequate budgets to purchase circulatory assist devices. As in the United States, we believe that in Europe certain groups of physicians will drive the decision as to which VAD to purchase.
Competition
Competition in the VAD industry is expected to increase as better devices become available. We believe that our products compete primarily on their safety and efficacy as a treatment for congestive heart failure as compared to other devices and other treatments. Other factors that affect our ability to effectively compete in the VAD market is our ability to obtain necessary regulatory approvals to market the device in the U.S., the price of our device and the ability of healthcare providers to secure reasonable reimbursement rates. We believe that only smaller, less invasive, reliable and durable devices will remain as viable alternatives for the treatment of congestive heart failure. In the long run, we believe our continued competitive success will depend on our ability to enhance patient outcomes and develop innovate products.
Our principal competitors in the implantable VAD space include Thoratec Corporation, World Heart Corporation, Jarvik Heart, MicroMed Technology, Inc, Berlin Heart AG, and Terumo Heart, Inc., and a range of other smaller, specialized medical device companies with devices at varying stages of development.
We believe that the key features of the HeartWare System that provide us with certain advantages over our competitors’ known products include:
| • | small device size which allows for implantation in the pericardial space immediately surrounding the heart in all patients unlike other full-output VADs that are currently available; |
| • | our proprietary, hybrid technology system for suspending the pump impellers, providing a wearless pumping system; and |
| • | a design that includes a wide-bladed impeller and integrated inflow cannula, which is to designed to optimize blood flow characteristics. |
Although we believe the HeartWare System provides us with competitive advantages over our competitors’ known products, we note that:
| • | our product’s success is dependent on our clinical trials demonstrating the safety and efficacy in the U.S.; |
| • | our market is an emerging market and is reliant upon acceptance of VAD technology. |
See Item 1A. “Risk Factors.” for additional information.
Research and Development
Research and development costs include activities related to the research, development, design, testing, and manufacturing of prototypes of our products as well as costs associated with certain clinical and regulatory activities. We expect our research and development expenses to continue to increase as we continue to research and develop improvements to the HeartWare System, research the application of, and develop our miniaturized heart pump technology, conduct additional clinical trials and hire additional employees. For the years ended December 31, 2009, 2010 and 2011 we incurred research and development expenses of $15.1 million, $33.1 million and $50.1 million, respectively.
15
Manufacturing and Assembly
Our manufacturing activities to date,and for the foreseeable future, will continue to consist primarily of process development, component assembly, quality control testing and sustaining engineering. Most of the components of the HeartWare System are manufactured by third parties, including the center post, pump housing and impeller. Some critical components, including the controller, are manufactured solely by an outside supplier and are essentially provided to us as a finished good ready-for-sale as part of our HeartWare System.
In order to sell our product commercially in the European Union, we are required to meet certain regulatory standards. In October 2008, we received a Certificate of Registration from British Standard Institution (BSI) certifying that the Company’s Quality Management System complies with the requirements of ISO 13485:2003. It signifies that HeartWare has established a comprehensive quality system that conforms to the International Organization for Standardization (“ISO”) 13485:2003 requirements. The ISO 13485:2003 standard is fully recognized in many countries as a measure of quality. In January 2009, we received a Full Quality Assurance Certificate, CE 540273 from BSI. It signifies that the HeartWare Ventricular Assist System designed and manufactured by HeartWare conforms with the provisions of Council Directive for Active Implantable Medical Devices, 90/385/EEC, Annex 2, Section 3.2 at every stage, from design to final controls. In order to maintain these certifications we must show through annual surveillance audits conducted by the British Standard Institute (BSI) that HeartWare’s Quality System remains compliant with the requirements of ISO 13485 and applicable standards.
We do not presently have supply agreements with some of our key suppliers and we have not secured second source suppliers for all of our supplies. See Item 1A. ”Risk Factors” for additional information.
Employees
As of December 31, 2011, we had 330 employees, of whom 229 employees are engaged in operations activities including research and development, quality assurance and manufacturing activities, 65 are engaged in marketing, clinical and regulatory activities and 36 are engaged in finance, legal and other administrative functions. None of our employees are represented by a labor union or covered by a collective bargaining agreement other than employees in France that are subject to national, collective bargaining agreements. We consider our relations with our employees to be good.
Corporate History
HeartWare International, Inc. was incorporated in Delaware on July 29, 2008 as a wholly-owned subsidiary of HeartWare Limited, a corporation incorporated in Australia on November 26, 2004. On November 13, 2008, HeartWare Limited completed its redomiciliation from Australia to Delaware pursuant to certain schemes of arrangement approved by an Australian court. Subsequent to the redomiciliation, HeartWare Limited was renamed HeartWare Pty. Limited. In connection with this redomiciliation, each holder of HeartWare Limited ordinary shares was issued one share of HeartWare International, Inc. common stock in exchange for every 35 ordinary shares of HeartWare Limited. As a result, HeartWare Limited became a wholly-owned subsidiary of HeartWare International, Inc., and HeartWare International, Inc. became the parent company of the HeartWare Group.
HeartWare Limited acquired our operating subsidiary, HeartWare, Inc., on January 24, 2005. HeartWare, Inc. is a Delaware corporation which was incorporated on April 8, 2003 under the name Perpetual Medical, Inc., and which changed its name to HeartWare, Inc. on July 10, 2003. Since July 10, 2003, HeartWare, Inc. has operated the business formerly owned and operated by Kriton Medical, Inc., which had been developing the HeartWare VAD System since approximately 1995.
In May 2003, Kriton filed for protection from creditors under Chapter 11 of the United States Bankruptcy Code. On May 20, 2003, Kriton and its lead investor Apple Tree Partners I, L.P., proposed a joint plan of liquidation for Kriton. On June 20, 2003, the United States Bankruptcy Court of the Southern District of Florida issued a court order confirming the plan of liquidation. This court order, together with a supplemental court order approving a settlement between Apple Tree Partners and various stockholders of Kriton issued on July 3, 2003, approved the sale of substantially all the assets of Kriton to HeartWare, Inc. On July 10, 2003, HeartWare, Inc. purchased substantially all of the assets of Kriton free and clear of any and all liens, security interests, encumbrances and claims. The assets included all of Kriton’s patents and other intellectual property which were assigned to HeartWare, Inc.
16
In connection with the asset purchase, HeartWare, Inc. issued Series A-1 and Series A-2 Preferred Stock to certain creditors of Kriton. The Series A-1 and Series A-2 Preferred Stock do not have any voting rights or the right to receive dividends but entitle the holders thereof to receive upon certain liquidation events of HeartWare, Inc. (but not the liquidation of or change of control of the parent of HeartWare, Inc.) an amount equal to $10 per share of Series A-1 and an amount of $21 per share of Series A-2. HeartWare, Inc. continued to operate as an independent entity until January 24, 2005, when HeartWare Limited acquired all of the voting stock of HeartWare, Inc. in exchange for the issuance by HeartWare Limited of 2.5 million shares (as adjusted for a reverse split in the ratio of 35 to 1) and a convertible note in the principal amount of $1.1 million. The convertible note was redeemed during the third quarter of 2008.
Legal Proceedings
On February 24, 2010, we received a letter from two holders of Series A Preferred Stock in HeartWare, Inc., an indirect subsidiary of HeartWare International, Inc. These holders requested various financial and other information regarding HeartWare, Inc. for the purpose of determining the Company’s compliance with their rights as holders of Series A Preferred Stock, including whether a liquidation event has occurred since inception in 2003. HeartWare, Inc. issued Series A-1 and Series A-2 Preferred Stock to certain equity holders of Kriton Medical, Inc. when HeartWare, Inc. purchased out of bankruptcy substantially all of the assets of Kriton in July 2003. The Series A-1 and Series A-2 Preferred Stock do not have voting or dividend rights but entitle the holders thereof to receive, upon certain liquidation events of HeartWare, Inc. (but not the liquidation of or change of control of HeartWare International, Inc.), an amount equal to $10 per share of Series A-1 and $21 per share of Series A-2. The aggregate liquidation preference payment obligation totals approximately $15 million.
On June 27, 2011, HeartWare International, Inc. and HeartWare, Inc., along with HeartWare’s directors, certain officers and a significant stockholder, were named as defendants in a putative class action lawsuit filed in Massachusetts state court by two other Series A Preferred Stockholders on behalf of all holders of Series A Preferred Stock. The complaint alleges that the defendants breached their fiduciary and contractual obligations to Series A Preferred Stockholders by preventing them from receiving a payment of the liquidation preference in connection with certain corporate transactions, including a transaction in 2005 in which HeartWare, Inc. was acquired by HeartWare Limited, a subsidiary of HeartWare International, Inc. The plaintiffs seek monetary damages, interest, costs and limited equitable relief. We do not believe HeartWare International, Inc., HeartWare, Inc. or any of our directors, officers or stockholders have abrogated the rights, or in any way failed to satisfy obligations owed to, any of our stockholders, including holders of Series A Preferred Stock. On September 12, 2011, the defendants served on plaintiffs a motion to dismiss the complaint with prejudice. On February 3, 2012, counsel for plaintiffs and defendants entered into a Memorandum of Understanding to settle the matter. Defendants have agreed to pay up to $1.125 million to participating putative class members in exchange for a full and unconditional release of all claims asserted in the litigation, including any and all claims arising from any right to receive a payment upon any liquidation or deemed liquidation event that has arisen or may arise in the future. We expect insurance to fund a significant portion of the settlement amount, although coverage is not assured. The settlement must be finally approved by the court following notice to putative class members.
Our business faces many risks. We believe the risks described below are material risks facing the Company. However, the risks described below may not be the only risks we face. Additional unknown risks or risks that we currently consider immaterial, may also impair our business operations. If any of the events or circumstances described below actually occurs, our business, financial condition or results of operations could suffer, and the trading price of our shares could decline significantly. Investors should consider the specific risk factors discussed below, together with the cautionary statements under the caption “Forward-Looking Statements” and the other information and documents that we file from time to time with the Securities and Exchange Commission.
Risks Related to Our Business and Industry
17
We have incurred operating losses since our inception and anticipate that we will continue to incur operating losses for the foreseeable future.
We have incurred net losses since our inception, including net losses of $55.1 million, $29.4 million and $23.8 million for the fiscal years ended December 31, 2011, 2010 and 2009, respectively. As of December 31, 2011, our accumulated deficit was $182.3 million. Currently, we only have one product approved for sale outside of the U.S.. None of our products are approved for commercial sale in the U.S. although we presently derive revenue from reimbursed sales of the HeartWare system for use in clinical trials in the United States. We continue to incur substantial clinical trial expenditures, significant research and development costs and costs related to our operations. We expect to continue to incur significant operating losses for the foreseeable future as we incur costs associated with:
| • | manufacturing product; |
| • | continuing to conduct multiple clinical trials; |
| • | further product research and development for next generation products and peripherals and efforts to sustain and maintain incremental improvements to existing products and peripherals; |
| • | building our service capabilities to meet growing customer demand; |
| • | growing, maintaining and protecting our intellectual property; |
| • | seeking regulatory approvals; |
| • | expanding our sales and marketing capabilities on a global basis, including building a team to support U.S. commercialization should the FDA approve our device for marketing in the U.S.; |
| • | increasing our manufacturing operations to meet increasing demand; |
| • | broadening our infrastructure in order to meet the needs of our growing operations; and |
| • | complying with the requirements related to being a public company in both the United States and Australia. |
To become and remain profitable, we must succeed in developing and commercializing products with significant market potential. This will require us to succeed in a range of challenging activities, including all of the activities listed above. We may never succeed in these activities, and we may never obtain regulatory approvals in the markets in which we expect to operate or otherwise generate revenue sufficient to achieve profitability. Further, the markets in which we operate may contract or we may not obtain significant market share so as to support our ongoing business operations. If we do achieve profitability, we may not be able to sustain it.
We have a significant amount of indebtedness consisting currently of our convertible senior notes. We may not be able to generate enough cash flow from our operations to service or pay principal on our indebtedness, and we may incur additional indebtedness in the future, which could adversely affect our business, financial condition and results of operations. Upon conversion of our convertible senior notes at the election of the holders, to the extent we settle such conversion in cash it could impact our liquidity; to the extent we settle in stock it may dilute our existing stockholders.
As of December 31, 2011, our total consolidated indebtedness totaled $94.3 million, net of discounts, all of which constituted indebtedness under our 3.5% Convertible Senior Notes due 2017 in the principal amount of $143.75 million. Our ability to make payments on, and to refinance, our convertible senior notes, any future indebtedness, and to fund planned capital expenditures, research and development efforts, working capital, acquisitions and other general corporate purposes depends on our ability to generate cash in the future. This, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory, clinical and other factors, some of which are beyond our control. If we do not generate sufficient cash flow from operations or if future borrowings are not available to us in an amount sufficient to pay our indebtedness, including payments of principal upon conversion of the convertible senior notes or on their maturity, or to fund our liquidity needs, we may be forced to refinance all or a portion of our indebtedness, including the convertible senior notes, on or before the maturity thereof, sell assets, reduce or delay capital expenditures, seek to raise additional capital or take other similar actions. We may not be able to affect any of these actions on commercially reasonable terms or at all. Our ability to refinance our indebtedness will depend on our financial condition at the time, the restrictions in the instruments governing our indebtedness and other factors, including market conditions. In addition, in the event of a default with respect to the convertible senior notes, the holders of the convertible senior notes and/or the trustee under the indenture governing these notes may accelerate the payment of our obligations under these notes, which could have a material adverse effect on our business, financial condition and results of operations. Our inability to generate sufficient cash flow to satisfy our debt service obligations, or to refinance or restructure our obligations on commercially reasonable terms or at all, would likely have an adverse effect, which could be material, on our business, financial condition and results of operations.
18
In addition, our significant indebtedness combined with our other financial obligations and contractual commitments could have other important consequences. For example, it could:
| • | make us more vulnerable to adverse changes in general U.S. and worldwide economic, industry and competitive conditions and adverse changes in government regulation; |
| • | limit our flexibility in planning for, or reacting to, changes in our business and our industry; |
| • | place us at a competitive disadvantage compared to our competitors who have less debt; and |
| • | limit our ability to borrow additional amounts for working capital, capital expenditures, acquisitions, debt service requirements, execution of our business strategy or other purposes. |
Any of these factors could materially and adversely affect our business, financial condition and results of operations. In addition, if we incur additional indebtedness, which we are not prohibited from doing under the terms of the indenture governing the convertible senior notes, the risks related to our business and our ability to service our indebtedness would increase.
In the event the conditional conversion feature of the notes is triggered, holders of notes will be entitled to convert the notes at any time during specified periods at their option. If one or more holders elect to convert their notes, unless we elect to satisfy our conversion obligation by delivering solely shares of our common stock (other than cash in lieu of any fractional share), we would be required to settle a portion or all of our conversion obligation through the payment of cash, which could adversely affect our liquidity. In addition, even if holders do not elect to convert their notes, we could be required under applicable accounting rules to reclassify all or a portion of the outstanding principal of the notes as a current rather than long-term liability, which would result in a material reduction of our net working capital. The terms of our convertible senior notes permit us to settle them, upon conversion by the holders thereof, in cash, stock, or a combination thereof. To the extent we use stock for settlement, our existing stockholders may be diluted.
We may need substantial additional funding and may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs or commercialization efforts.
Revenue generated from the HeartWare System is currently limited to commercial sales outside of the U.S. (particularly in the EU where we enjoy CE Marking), clinical trials within the United States, and through special access programs in other countries. Depending on a range of outcomes, especially our achievement of regulatory approval of our products and the growth of revenue, we may need to seek additional funding in the future. Additional funding may not be available on terms favorable to us, or at all. If we raise additional funding through the issuance of equity securities, our shares may suffer dilution. If we are unable to secure additional funding, our product development programs and our commercialization efforts would be delayed or reduced or may cease entirely.
Our products have not yet been approved for commercial sale within the United States, and our success will depend heavily on our ability to obtain FDA approval to market our HeartWare System in the U.S. for our initial and any future indications. If we are unable to complete successfully, or experience significant delays in the successful completion of, our U.S. trials, our ability to obtain regulatory approval to commercialize our products within the United States, the largest medical device market in the world, and our ability to generate revenue, will be materially adversely affected. Delays or inability to successfully complete trials outside of the U.S. can also negatively impact our business.
On January 30, 2009, we received approval for CE Marking and subsequently began generating net sales in Europe. However, future revenue will be limited if we do not receive regulatory approval to commercially sell our products in the United States. We submitted a premarket approval application to the U.S. FDA for the bridge-to-transplant indication in December 2010, and are currently conducting a U.S. clinical trial for the destination therapy indication.
19
Completion of any of our clinical trials could be delayed or adverse events during a trial could cause us to amend, repeat or terminate the trial. If this were to happen our costs associated with the trial will increase, and it will take us longer to obtain regulatory approvals and commercialize the product or we may never obtain such regulatory approvals. Our clinical trials may also be suspended or terminated at any time by regulatory authorities, the data safety and monitoring board, site investigational review boards, or by us including during the closing stages of enrollment of the trial and the subsequent patient data follow-up period in the event that, for example, there should be a series of adverse clinical events such as stroke, bleeding or pump exchanges. Any failure or significant delay in completing clinical trials for our products will harm our financial results and the commercial prospects for our products.
The completion of any of our clinical trials could be substantially delayed or prevented by several factors, including:
| • | slower than expected rates of patient recruitment and enrollment, including as a result of study inclusion and exclusion criteria and of our competitors undertaking similar clinical trials or having functionally comparable products that have received approval for sale; |
| • | failure of patients to complete the clinical trial; |
| • | physicians or patients preferring to use approved devices or other experimental treatments or devices rather than our HeartWare System; |
| • | prevalence and severity of adverse events and other unforeseen safety issues; |
| • | inability or unwillingness of patients or medical investigators to follow our clinical trial protocols; |
| • | inability to monitor patients adequately during or after treatment; |
| • | risks associated with trial design, which may result in a failure of the trial to show statistically significant results even if the product is effective; |
| • | governmental and regulatory delays or changes in regulatory requirements, policies or guidelines; |
| • | varying interpretation of data by regulatory agencies; and |
| • | perceived lack of product efficacy following clinical trials. |
The process of obtaining marketing approval or clearance from the FDA for our HeartWare System, or any future products or enhancements or modifications to any products, could:
| • | take a significant period of time; |
| • | require the expenditure of substantial resources; |
| • | involve rigorous pre-clinical and clinical testing; |
| • | require changes to our products; and |
| • | result in limitations on the indicated uses of the products. |
Assuming we are successful at filing the required FDA regulatory premarket approval applications for indications for our HeartWare System, there can be no assurance that we will receive the required approvals from the FDA or, if we do receive the required approvals, that we will receive them on a timely basis or that we will otherwise be able to satisfy the conditions of such approval, if any. The failure to receive product approval by the FDA, or any significant delay in receipt, will have a material adverse effect on our business, financial condition or results of operations. For example, we submitted a premarket approval application to the U.S. FDA for the bridge-to-transplant indication in December 2010, and any failure to obtain an approval of that application, or a delay in receiving approval, could have an adverse impact on our business.
While the U.S. is the largest medical device market in the world, the risks described above concerning U.S. trials and regulatory approval also apply to our foreign clinical trials and regulatory filings. If we cannot timely conduct foreign trials in our major target markets (to the extent required in order to market our device in such locations) and receive timely approval in such jurisdictions to market our device for a variety of indications, our business will suffer.
20
We currently rely entirely on sales of our sole product, the HeartWare System, to generate revenue. Our existing and future products may not achieve or sustain market acceptance. In addition, any factors that negatively impact sales of this product will adversely affect our business, financial condition and results of operations.
Our sole product is the HeartWare System, which we introduced to the European market in January 2009 and which does not have regulatory approval in the United States. We expect to continue to derive substantially all of our revenue for several years from the sale of this product and its related devices. Accordingly, our ability to generate revenue is entirely reliant on our ability to market and sell this product.
Even if we obtain the necessary regulatory approvals in all jurisdictions to commercialize the HeartWare System or any other product that we may develop, our products may not gain or sustain market acceptance among physicians, patients, health care payers or the medical community.
The degree of market acceptance of any of the devices that we may develop and commercialize will depend on a number of factors, including:
| • | the perceived effectiveness of the product; |
| • | the prevalence and severity of any adverse events or side effects especially as it relates to survival, quality of life, stroke, thrombus and bleeding; |
| • | potential advantages over alternative treatments or competitive products; |
| • | the strength of our marketing and distribution support; |
| • | the strength and perceived advantages of our peripherals such as the monitor, controller and batteries; and |
| • | sufficient third party coverage or reimbursement. |
If the HeartWare System, or any other product that we may develop, does not achieve an adequate level of acceptance by physicians, patients, health care payers and the medical community, we may not generate or maintain positive gross margins and we may not become profitable or be able to sustain profitability. If we do achieve market acceptance of our products, we may not be able to sustain it or otherwise achieve it to a degree which would support the ongoing viability of our operations.
If we are unable to manage our expected growth, we may not be able to meet market demand, generate expected benefits from the opportunities available to us, satisfy quality regulations or commercialize our product candidates.
We expect to continue to expand our operations and grow our research and development, product development, quality, regulatory, manufacturing, sales, marketing and administrative operations. This expansion has placed, and is expected to continue to place, a significant strain on our management, infrastructure and operational and financial resources. To manage continued growth and to commercialize our products, we will be required to improve existing operational, quality and financial systems, procedures and controls and expand, train and manage our growing employee base. Specifically, our information technology and back-up systems will need to be upgraded to accommodate our growth. In addition, we will need to manage relationships with various persons and entities participating in our clinical trials, quality systems, manufacturers, suppliers and other organizations, including various regulatory bodies in the United States and other jurisdictions. We may not be able to implement needed improvements in an efficient and timely manner and may discover deficiencies in existing systems and controls. Our failure to accomplish any of these tasks could materially harm our business.
Our ability to achieve profitability from a current net loss level will depend on our ability to increase gross revenue and reduce the per unit cost of producing the HeartWare System by increasing our customer orders and manufacturing volume.
Currently, gross sales and the gross profit from sales of the HeartWare System are not sufficient to cover our operating expenses. To achieve profitability, we need to, among other things, substantially reduce the per unit cost of our products. We believe this can be achieved by decreasing our product assembly costs and increasing our manufacturing volume, which may allow for volume purchase discounts to reduce our raw material and component costs and improve absorption of manufacturing overhead costs. If we are unable to increase sales and simultaneously reduce assembly, raw material, component and manufacturing overhead costs, our ability to achieve profitability
21
will continue to be severely constrained. Any increase in manufacturing volumes must be accompanied by a concomitant increase in customer orders. As part of our efforts to prepare for commercialization in the U.S. and expanded sales globally, we have added an additional manufacturing facility in Miami Lakes, Florida. The lease for this facility will increase our operating expenses. The occurrence of one or more factors that negatively impact sales of our products or our ability to forecast future sales may prevent us from achieving our desired increase in manufacturing efficiency, which would prevent us from attaining profitability.
We compete against companies that have longer operating histories, more established or approved products and greater resources than we do, which may prevent us from achieving further market penetration or improving operating results.
Competition in the medical device industry is intense. Our products will compete against products offered by public companies, such as Thoratec Corporation and World Heart Corporation, as well as several private companies, such as Jarvik Heart, Inc., Circulite, Evaheart and Terumo Heart, Inc. Some of these competitors have significantly greater financial and human resources than we do and have established reputations or approved products or significantly greater name recognition, as well as distribution channels and sales and marketing capabilities that are significantly larger and more established than ours. For example, Thoratec Corporation has received marketing approval in the United States for HeartMate II for both destination and bridge-to-transplant indications. Additional competitors may enter the market, and we are likely to compete with new companies in the future. We also face competition from other medical therapies which may focus on our target market as well as competition from manufacturers of pharmaceutical treatments and other devices that have not yet been developed. Competition from these companies could adversely affect our business.
In addition, in Europe our customers are geographically dispersed and, at this stage, a significant portion of our revenue is sourced in Germany among a small number of clinical sites, which also use other competing products. If these sites were to cease using our products or use our products on a reduced or inconsistent basis, such events would have a material adverse effect on our financial condition and results of operations.
Our ability to compete effectively depends upon our ability to distinguish our company and our products from our competitors and their products. Factors affecting our competitive position include:
| • | the availability of other products and procedures, such as heart transplants; |
| • | product performance and design; |
| • | product safety; |
| • | sales, marketing and distribution capabilities; |
| • | comparable clinical outcomes; |
| • | success and timing of new product development and introductions; and |
| • | intellectual property protection. |
We are still building our sales, marketing and distribution experience.
To develop and increase sales, distribution and marketing capabilities, we will continue to invest significant amounts of financial and management resources. In developing these sales, marketing and distribution functions ourselves, we will face a number of risks, including:
| • | we may not be able to attract and build a significant, successful or qualified marketing or sales force; |
| • | the cost of establishing, training and providing regulatory oversight for a marketing or sales force may be substantial; and |
| • | there are significant legal and regulatory risks in medical device marketing and sales, and any failure to comply with all legal and regulatory requirements for sales, marketing and distribution could result in enforcement action by the FDA or other authorities that could jeopardize our ability to market the product or could subject us to substantial liability. |
22
We have limited manufacturing capabilities and personnel, and if our manufacturing facilities are unable to provide an adequate supply of products, our growth could be limited and our business could be harmed.
We currently manufacture our HeartWare System at our facilities in Miami Lakes, Florida. If there were a disruption to our manufacturing facilities or the surrounding area, for example, due to a hurricane or climate change, we would have no other means of manufacturing our HeartWare System until we were able to restore the manufacturing capability at our facility or develop alternative manufacturing facilities.
If we are unable to produce sufficient quantities of our HeartWare System for sale or for use in our current and planned clinical trials, or if our manufacturing process yields substandard product, our development and commercialization efforts would be delayed. Further, even if we are able to produce sufficient quantities of our products we may not be able to attain sufficient profitability on that production or any resultant sales.
We currently have limited resources, facilities and experience to commercially manufacture our products. In order to produce our products in the quantities that we anticipate will be required to meet anticipated market demand, we will need to increase the production process and efficiency over the current level of production. There are significant technical and regulatory challenges to increasing manufacturing capacity and efficiency, and developing commercial-scale manufacturing facilities will require the investment of additional funds and hiring and retaining additional management and technical personnel who have the necessary manufacturing experience. We may not successfully complete any required increase in a timely or economically viable manner or at all. If we are unable to do so, we may not be able to produce the HeartWare System in sufficient quantities to meet future demand.
If we are unable to manufacture a sufficient or consistent supply of the HeartWare System or any other product we are developing, or if we cannot do so efficiently, our revenue, business and financial prospects would be adversely affected.
Fluctuations in foreign currency exchange rates could adversely affect our financial results.
Changes in foreign currency exchange rates can affect the value of our assets, liabilities, costs and revenue. To date, the majority of our revenue have been sourced from international sales, mainly in Europe and denominated in Euros, while most of our expenditures are incurred in U.S. dollars. We presently derive revenue in the United States from our clinical trials but until our products receive regulatory approval in the United States, if ever, our United States sourced revenue will likely constitute less than half of our net revenue.
With limited exceptions our international sales will be denominated in Euros or in local currencies, not U.S. dollars, and fluctuations in foreign currency exchange rates, especially an appreciation of the U.S. dollar against major international currencies, will materially impact our revenue and earnings. Due to the size and stage of development of our operations and revenue, we do not presently mitigate our exposure to exchange risk to a significant extent other than by holding the majority of our funds in U.S. dollars or U.S. dollar denominated investments.
We manufacture a Class III device implanted in the heart that subjects us to numerous risks.
There are risks associated with implanting our device in end stage heart failure patients, including, but not limited to, death, bleeding, stroke, device malfunction and other adverse events; should our customers experience an increase in adverse events they may reduce their usage or purchase of our device; should our patients experience injury due to these events, they may sue us. Any of these occurrences could have an adverse impact on our operations and financial results and condition.
Our manufacturing facilities and the manufacturing facilities of our suppliers must comply with applicable regulatory requirements. If we fail to achieve regulatory approval for these manufacturing facilities, our business and our results of operations would be harmed.
Completion of our clinical trials and commercialization of our products require access to, or the development of, manufacturing facilities that meet and maintain applicable regulatory standards to manufacture a sufficient
23
supply of our products. In addition, the FDA must approve facilities that manufacture our products for U.S. commercial purposes, as well as the manufacturing processes and specifications for the product, with similar, additional, approvals required in order to achieve CE marking in Europe. Suppliers of components, and products used to manufacture, our products must also comply with FDA and foreign regulatory requirements, which often require significant time, money, resources and record-keeping and quality assurance efforts and subject us and our suppliers to potential regulatory inspections and stoppages. If we or our suppliers fail to comply with the regulatory requirements for our manufacturing operations, our commercialization efforts could be delayed, which would harm our business and our results of operations.
We may not meet regulatory quality standards applicable to our manufacturing and quality processes, which could have an adverse effect on our business, financial condition or results of operations.
Even after products have received marketing approval or clearance, product approvals and clearances by the FDA or other regulatory bodies can be withdrawn due to failure to comply with regulatory standards or the occurrence of problems following initial approval whether identified through a required post-approval study or through medical device reporting. As a device manufacturer, we are required to demonstrate and maintain compliance with a variety of regulatory requirements, including the FDA’s Quality System Regulation, or “QSR.” The QSR is a complex regulatory scheme that covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of our products, including trend analysis and corrective and preventative actions. The FDA enforces the QSR through periodic unannounced site inspections. In addition, the U.S. federal medical device reporting regulations require us to provide information to the FDA whenever there is evidence that reasonably suggests that a device may have caused or contributed to a death or serious injury or, if a malfunction were to occur, could cause or contribute to a death or serious injury. Compliance with applicable regulatory requirements is subject to continual review and is rigorously monitored through periodic inspections by the FDA. Our failure to comply with the QSR or to take satisfactory corrective action in response to an adverse QSR inspection could result in enforcement actions, including a public warning letter, a shutdown of or restrictions on our manufacturing operations, delays in approving or clearing a product, refusal to permit the import or export of our products, a recall or seizure of our products, fines, injunctions, civil or criminal penalties, or other sanctions, any of which could cause our business and operating results to materially suffer.
In the European Union, we are required to maintain certain ISO certifications in order to sell our products and must undergo periodic inspections by notified bodies to obtain and maintain these certifications. If we fail to continue to comply with ISO regulations, European Union organizations may withdraw clearance to market, require a product recall or take other enforcement action.
Product issues could result in recalls, substantial costs and write-downs; this could also lead to delay or termination of ongoing trials.
Our products are subject to various regulatory guidelines, involve complex technologies and are approved for a specified life. Identified quality problems, such as failure of critical components such as batteries or controllers, or the failure of third parties to supply us with sufficient quantities of these products or components, could lead to adverse clinical events that could cause us to amend, repeat or terminate clinical trials, or impact the availability of our product in the marketplace. In addition, product improvements, product redundancies or failure to sell product before it expires could result in scrapping or expensive rework of product and our business, financial or results of operations could suffer. Quality issues could result in the scrapping, rework, recall or replacement of entire lots of products, substantial costs and write-offs and harm to our business reputation and financial results. Further such activities could adversely affect our relationships with our customers or affect our reputation which could materially adversely affect our earnings, results and financial viability.
We plan to operate in multiple regulatory environments that require costly and time consuming approvals.
Even if we obtain regulatory approvals in specific jurisdictions to commercialize the HeartWare System or any other product that we may develop, sales of our products in other jurisdictions will be subject to regulatory requirements that vary from country to country. The time and cost required to obtain approvals from these countries may be longer or shorter than that required for FDA approval, and requirements for licensing may differ from those of the FDA. Some jurisdictions may even require additional trials be conducted. Laws and regulations regarding the
24
manufacture and sale of our products are subject to future changes, as are administrative interpretations and policies of regulatory agencies. If we fail to comply with applicable foreign, federal, state or local market laws or regulations, we could be subject to enforcement actions. Enforcement actions could include product seizures, recalls, withdrawal of clearances or approvals, and civil and criminal penalties, which in each case would harm our business.
If we fail to obtain and maintain adequate level of reimbursement for our products by third party payers, there may be no commercially viable markets for our products or the markets may be much smaller than expected.
Although our customers have generally achieved reimbursement for the purchase of our products to date, the availability and levels of reimbursement by governmental and other third party payers affect the market for our products. Reimbursement and health care payment systems vary significantly by country, and include both government sponsored health care and private insurance. Payers may attempt to limit coverage and the level of reimbursement of new therapeutic products or experimental devices. Government and other third party payers also continually attempt to contain or reduce the costs of health care by challenging prices charged for health care products and services. Often, reimbursement is determined independently of and only following product approval.
To obtain reimbursement or pricing approval in some countries, we may be required to produce clinical data, which may involve one or more clinical trials, that compares the cost-effectiveness of our products to other available therapies. In addition, the efficacy, safety, performance and cost-effectiveness of our products in comparison to any competing products may determine the availability and level of reimbursement for our products.
We believe that future reimbursement may be subject to increased restrictions both in the United States and in international markets. Future legislation, regulation or reimbursement policies of third party payers may adversely affect the demand for our existing products as well as products currently under development and limit our ability to sell our products on a profitable basis. We cannot predict how pending or future legislative and regulatory proposals would influence the manner in which medical devices, including ours, are purchased or covered and reimbursed.
If reimbursement for our products is unavailable or limited in scope or amount or if pricing is set at unsatisfactory levels, market acceptance of our products would be impaired and our future revenue would be materially adversely affected. Historically, we have been unable to implant a significant number of patients under our IDE in the United States as the relevant insurance providers refused to provide reimbursement for our products on the basis that our products are “experimental” or “investigational” and do not have the requisite regulatory approval in the United States. Until approval of our products in the United States, this requirement will continue to materially adversely affect our revenue, earnings, business and stock price.
Destination therapy procedures represent an increasing share of ventricular assist device implants. Although we are currently conducting a destination therapy trial, we will be unable to submit for approval of a destination therapy indication for several years.
Hospitals must meet specific regulatory or reimbursement requirements in order to perform destination therapy procedures. If physicians and hospitals do not enroll patients in our current U.S. destination therapy trial, or use our products in the future when we have an approved destination therapy indication, our market opportunities will be diminished. The number of destination therapy procedures actually performed depends on many factors, most of which are out of our direct control, including:
| • | the number of sites approved for destination therapy by relevant regulatory agencies; |
| • | the clinical outcomes of destination therapy procedures; |
| • | cardiology and referring physician education, and their commitment to destination therapy; |
| • | the economics of the destination therapy procedure for individual hospitals, which includes the costs of the VAD and related pre- and post-operative procedures and their reimbursement; and |
| • | the economics of hospitals not conducting a destination therapy procedure, including the costs and related reimbursements of long-term hospitalization and alternative therapies. |
The different outcomes of these and other factors, and their timing, may have a material and adverse effect on our future results.
25
In addition, our primary competitor has received a destination therapy indication for its product. If physicians grow accustomed to that device for destination therapy and become unwilling to use our device for this indication once approved, our ability to participate in and benefit from this opportunity may suffer.
The long and variable sales and deployment cycles for our ventricular assist device, or VAD, systems may cause our product sales and operating results to vary significantly from quarter-to-quarter.
Our VAD systems have lengthy sales cycles and we may incur substantial sales and marketing expenses and expend significant effort without making a sale. Even after making the decision to purchase our VAD systems, our customers often deploy our products slowly, and this time period may be extended if our products are acquired on a consignment basis, as is the case for most of our customers. In addition, cardiac centers that buy the majority of our products are usually led by cardiac surgeons who are heavily recruited by competing centers or by centers looking to increase their profiles. When one of these surgeons moves to a new center we sometimes experience a temporary but significant reduction in purchases by the departed center while it replaces its lead surgeon. As a result, it is difficult for us to predict the quarter in which customers may purchase our VAD systems and our product sales and operating results may vary significantly from quarter to quarter.
Adverse changes in general economic conditions in the United States and overseas could adversely affect us.
We are subject to the risks arising from adverse changes in general economic market conditions. Many global economies remain sluggish as they recover from a severe recession and unprecedented turmoil. The U.S. and other developed economies continue to suffer from market volatility, difficulties in the financial services sector, tight credit markets, softness in the housing markets, concerns of inflation, reduced corporate profits and capital spending, significant job losses or slower than expected job creation, reduced consumer spending, and continuing economic uncertainties. The turmoil and the uncertainty about future economic conditions could negatively impact our current and prospective customers, adversely affect the financial ability of governments and health insurers to pay claims, adversely impact our expenses and ability to obtain financing of our operations, cause delays or other problems with key suppliers and increase the risk of counterparty failures. We cannot predict the timing, strength or duration of the lingering effects of the severe global economic downturn or the timing or strength of the subsequent recovery. Healthcare spending in the United States and foreign jurisdictions has been, and is expected to continue to be, negatively affected by these economic trends. For example, patients who have lost their jobs may no longer be covered by an employee-sponsored health insurance plan and patients reducing their overall spending may eliminate purchases requiring co-payments. Since the sale of the HeartWare System to a new patient is generally dependent on the availability of third-party reimbursement and normally requires the patient to make a significant co-payment, the impacts of the effects of the recession on our potential customers may reduce the referrals generated and thereby reduce our customer orders. Similarly, the impacts of the challenging economy on our existing customers may cause some of them to cease purchasing HeartWare Systems and this will reduce our revenue, which in turn will make it more difficult to achieve the per unit cost-savings which are expected to be attained through increases in our manufacturing volume.
The severe recession has impacted the financial stability of many private health insurers. As a result, it has been reported that some insurers are scrutinizing claims more rigorously and delaying or denying reimbursement more often. Although VAD procedures occur in relatively limited numbers, the per procedure reimbursement levels may draw the attention of third party payers. Since the sale of the HeartWare System is generally dependent on the availability of third-party reimbursement, any delay or decline in such reimbursement will adversely affect our revenue.
Healthcare policy changes, including recent federal legislation to reform the U.S. healthcare system, may have a material adverse effect on us.
In response to perceived increases in health care costs in recent years, there have been and continue to be proposals by the federal government, state governments, regulators and third-party payors to control these costs and, more generally, to reform the U.S. healthcare system. Certain of these proposals could limit the prices we are able to charge for our products or the amounts of reimbursement available for our products and could limit the acceptance
26
and availability of our products. Moreover, as discussed below, recent federal legislation would impose significant new taxes on medical device makers such as us. The adoption of some or all of these proposals, including the recent federal legislation, could have a material adverse effect on our financial position and results of operations.
On March 23, 2010, the Patient Protection and Affordable Care Act (“PPACA”) was signed into law by President Obama. On March 30, 2010, a companion bill, the Health Care and Education Reconciliation Act of 2010 (the “Reconciliation Act”) was also signed into law by President Obama. Among other things, the PPACA and the Reconciliation Act (collectively, the “Acts”), when taken together, impose a 2.3% excise tax on the sale of certain medical devices that will take effect in 2013. In addition, it is possible that standard setters or regulators may address certain unique aspects of the accounting for the Acts in the future. In light of the inherent uncertainty of how these Acts and other companion legislation, if any, will be implemented and applied, we are unable to fully predict the actual impact on our financial statements. Other elements of this legislation such as comparative effectiveness research, an independent payment advisory board, transparency requirements, payment system reforms including shared savings pilots and other provisions could meaningfully change the way healthcare is developed and delivered, and may materially impact numerous aspects of our business.
We rely on specialized suppliers for certain components and materials, and we do not have second-source suppliers for all of our components.
We depend on a number of suppliers to successfully manufacture sufficient quantities of the components we use in our products, both our existing commercial products and our products in development. We rely on suppliers for various critical components including the center post, housing and impeller that are assembled into our primary product, the HeartWare System, as well as finished products that comprise our peripheral and external equipment that is included in the HeartWare System. Lead times for our components are significant and can be up to as long as sixteen weeks and many of our components are manufactured to very tight tolerances and specifications. We do not presently have supply agreements with the vast majority of our key suppliers but have extensive purchase orders in place with these vendors.
We have second-source suppliers for some, but not all, of our components. In particular, we do not have second-source suppliers for our controllers, battery chargers and monitors. Our reliance on third-party suppliers also subjects us to other risks that could harm our business, including:
| • | we do not believe that we are a major customer of many of our suppliers, in terms of the volume of components and materials that we purchase, and these suppliers may therefore give other customers’ needs higher priority than ours; |
| • | we may not be able to obtain adequate supply in a timely manner or on commercially reasonable terms; |
| • | some of our components are extraordinarily complex and must be manufactured to extremely tight tolerances and specifications with the result that our suppliers, especially new suppliers, may make errors in manufacturing that could negatively affect the efficacy or safety of our products or cause our components not to be delivered on time or at all or to be delivered outside of specifications; |
| • | the availability of second-source suppliers may be extremely limited or their implementation as a supplier may be lengthy due to the tight tolerances and specifications in which we typically operate; |
| • | switching components or changes to our components, specifications or designs may require product redesign and submission to the FDA or a PMA supplement, which can lead to production interruptions; |
| • | our suppliers manufacture products for a range of customers, and fluctuations in demand for the products these suppliers manufacture for others may affect their ability to deliver products to us in a timely manner; and |
| • | our suppliers may encounter financial hardships unrelated to our demand, which could inhibit their ability to fulfill our orders and meet our requirements. |
Any interruption or delay in obtaining products from our third-party suppliers, or our inability to obtain products from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and cause them to cancel orders or switch to competing products.
While we have identified second-source suppliers for other key components, we have not entered into written agreements with these suppliers and we cannot assure you that we will be able to maintain our manufacturing schedule without undue delay or substantial cost if any of these arrangements is terminated.
27
Additionally, we may experience problems or delays in our own manufacturing and assembly processes, which may be harmful to our financial status or reputation and therefore make it more difficult or expensive for us to continue with or enter into relationships with specialized suppliers. Our business plan is predicated on maintaining strong relationships and favorable supply arrangements with a series of external parties to manufacture components of our HeartWare System. If we are unsuccessful in this regard or are unable to secure or maintain agreements with these manufacturers on favorable terms or at all, then our ability to commercialize our technology and expand our operations will be dramatically impaired.
We may not be able to effectively protect our intellectual property rights which could have an adverse effect on our business, financial condition or results of operations.
Our success depends in part on our ability to obtain and maintain protection in the United States and other countries of the intellectual property relating to or incorporated into our technology and products. Our patent portfolio consists of internally developed technology as well as patents and patent applications which we acquired in 2003 in connection with our purchase in bankruptcy of substantially all the assets of Kriton Medical, Inc. and which pertain to technology used in the HeartWare System. As a result, we may have less complete knowledge and records with respect to the development and ownership of the Kriton technology, patents and intellectual property than we would otherwise have for technology, patents and intellectual property developed internally by us.
Our pending and future patent applications may not issue as patents or, if issued, may not issue in a form that will provide us with any meaningful protection or any competitive advantage. Even if issued, existing or future patents may be challenged, including with respect to the development and ownership thereof, or narrowed, invalidated or circumvented, which could limit our ability to stop competitors from developing and marketing similar products or limit the length of terms of patent protection we may have for our products. Further, other companies may design around technologies we have patented, licensed or developed. Moreover, changes in patent laws or their interpretation in the United States and other countries could also diminish the value of our intellectual property or narrow the scope of our patent protection. In addition, the legal systems of certain countries do not favor the aggressive enforcement of patents, and the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours. In addition, in November 2005, we entered into a settlement agreement with Ventrassist Pty., Limited, Ventracor Limited (collectively “Ventracor”) and the University of Technology, Sydney, under which the parties resolved all of the claims and counterclaims filed by the parties in the United States District Court for the Southern District of Florida in 2004 and 2005, and agreed to mutual non-assertion covenants. As part of that agreement, we agreed not to sue Ventracor or the University of Technology, Sydney, or any of their respective successors, assigns, affiliates, customers or suppliers for infringement of 29 of our issued U.S. and worldwide patents existing as of the date of the agreement or any patents that issue from any patent applications existing as of such date (including any type of patent that claims priority or shares common priority to such patents). We also agreed not to sue such parties for infringement of all of our issued patents existing as of September 30, 2005, or any patents that issue from any patent applications existing as of such date, in respect of Ventracor’s blood pump devices existing as of the date of the agreement or any device embodying a modification of such devices which does not give rise to a new independent claim for patent infringement. As a result, Ventracor, the University of Technology, Sydney, or their respective successors or assigns may commercialize competing technology or products that would have otherwise been precluded by our patents subject to the agreement. We understand that Ventracor’s patent portfolio, or certain elements therein, have since been acquired by Thoratec Corporation.
In order to preserve and enforce our patent and other intellectual property rights, we may need to make claims or file lawsuits against third parties. This can entail significant costs to us and divert our management’s attention from developing and commercializing our products. The occurrence of these events may have a material adverse effect on our business, financial condition or results of operations.
In 2011, the America Invents Act was signed into law. The act in part seeks to more closely align patent law in the U.S. with similar laws around the world. The impact of the law is not yet clear and may alter the relative priority of our inventions and require us to act more quickly to seek intellectual property protection.
28
Claims that our current or future products infringe or misappropriate the proprietary rights of others could adversely affect our ability to sell those products and cause us to incur additional costs.
Substantial litigation over intellectual property rights exists in the medical device industry. We expect that we could be increasingly subject to third-party infringement claims as our revenue increases, the number of competitors grows and the functionality of products and technology in different industry segments overlaps. Third parties may currently have, or may eventually be issued, patents on which our current or future products or technologies may allegedly infringe. For example, we are aware of certain patents and patent applications owned by third parties that cover different aspects of mechanical circulatory support, methodologies for the pumping of blood and other fluids and the related devices and technologies. Any of these third parties might assert a claim of infringement against us.
In particular, in an August 2008 letter, Jarvik Heart invited us to discuss “an exclusive license” as it relates to a Jarvik patent concerning hybrid blood pumps. The patent referenced by this letter relates to technology that is material to our business. We have not had any substantive discussions with Jarvik Heart concerning this matter since our receipt of this letter and we do not believe that our blood pump infringes this patent. In addition, we received a letter from Abiomed, Inc. in September 2009 in which Abiomed suggested that we “may be interested in licensing Abiomed’s technology” as it relates to an Abiomed patent concerning bearingless blood pumps. Further, in a subsequent letter received in February 2010, it was stated that Abiomed was “concerned that HeartWare’s left ventricular assist rotary blood pump infringes one or more claims” of an Abiomed patent. We have had limited communications with Abiomed, Inc. since receipt of the initial letter. The patent referenced by these letters relates to technology that is potentially material to our business and any litigation in this regard, irrespective of the outcome, may have a material adverse effect on our financial position, liquidity or results of operations. We believe the HeartWare System does not infringe this patent.
There can be no certainty that litigation will not arise in relation to the above or other matters or, if it does arise, whether or not it will be determined in a manner which is favorable to us. Any litigation, regardless of its outcome, would likely result in the expenditure of significant financial resources and the diversion of management’s time and resources. In addition, litigation in which we are accused of infringement may cause negative publicity, adversely impact prospective customers, cause product shipment delays, prohibit us from manufacturing, marketing or selling our current or future products, require us to develop non-infringing technology, make substantial payments to third parties or enter into royalty or license agreements, which may not be available on acceptable terms or at all. If a successful claim of infringement were made against us and we could not develop non-infringing technology or license the infringed or similar technology on a timely and cost-effective basis, our revenue may decrease substantially and we could be exposed to significant liability. A court could enter orders that temporarily, preliminarily or permanently enjoin us or our customers from making, using, selling, offering to sell or importing our current or future products, or could enter an order mandating that we undertake certain remedial activities. Claims that we have misappropriated the confidential information or trade secrets of third parties can have a similar negative impact on our reputation, business, financial condition or results of operations.
We may need to initiate lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive and, if we lose, could cause us to lose some of our intellectual property rights, which would harm our ability to compete in the market.
We rely on patents to protect a portion of our intellectual property and our competitive position. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and, consequently, patent positions in the medical device industry are generally uncertain. In order to protect or enforce our patent rights, we may initiate patent litigation against third parties, such as infringement suits or interference proceedings. Litigation may be necessary to:
| • | assert claims of infringement; |
| • | enforce our patents; |
| • | protect our trade secrets or know-how; or |
| • | determine the enforceability, scope and validity of the proprietary rights of others. |
Any lawsuits that we initiate could be expensive, take significant time and divert management’s attention from other business concerns. Litigation also puts our patents at risk of being invalidated or interpreted narrowly and our
29
patent applications at risk of not issuing. Additionally, we may provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially valuable. The occurrence of any of these events may have a material adverse effect on our business, financial condition and results of operations.
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely on a combination of non-patented proprietary technology, trade secrets, processes and procedures, technical knowledge and know-how accumulated or acquired since inception. Despite these measures, any of our intellectual property rights could, however, be challenged, invalidated, circumvented or misappropriated. We generally seek to protect this information by confidentiality, non-disclosure and assignment of invention agreements with our employees, consultants, scientific advisors and third parties. These agreements may be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may be disclosed to or otherwise become known or be independently developed by competitors. To the extent that our employees, consultants or contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. If our intellectual property is disclosed or misappropriated, it would harm our ability to protect our rights and have a material adverse effect on our business, financial condition and results of operations.
We may be subject to claims that our employees or we have inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of former employers of our employees.
We employ individuals who were previously employed at other medical device companies, including our competitors or potential competitors. To the extent that our employees are involved in research areas that are similar to those in which they were involved with their former employers, we may be subject to claims that such employees have inadvertently or otherwise used or disclosed the alleged trade secrets or other proprietary information of the former employers. Litigation may be necessary to defend against such claims.
We are subject to federal and state laws prohibiting “kickbacks” and false or fraudulent claims, which, if violated, could subject us to substantial penalties. Foreign jurisdictions in which we operate may have similar laws. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business. We can be liable for our distributors’ failure to comply with these laws as well.
A federal law commonly known as the Medicare/Medicaid anti-kickback law, and several similar state and foreign laws, prohibit payments that are intended to induce physicians or others either to refer patients or to acquire or arrange for or recommend the acquisition of healthcare products or services. These laws constrain our sales, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians or other potential purchasers of medical devices. Other federal and state laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent, or for items or services that were not provided as claimed. Because we may provide some coding and billing information to purchasers of the HeartWare System and our other products, and because we cannot assure that the government will regard any billing errors that may be made as inadvertent, these laws are potentially applicable to us. In addition, these laws are potentially applicable to us because we provide reimbursement to healthcare professionals for training patients on the use of the HeartWare System and our other products. Anti-kickback and false claims laws prescribe civil and criminal penalties for noncompliance, which can be substantial. Even an unsuccessful challenge or investigation into our practices could cause adverse publicity, and be costly to respond to, and thus could have a material adverse effect on our business, financial condition or results of operations. In addition, under certain circumstances, we may be liable for the actions of our distributors to the extent they do not comply with the laws described above.
If we are found to have violated laws protecting the confidentiality of patient health information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
30
There are a number of federal and state and foreign laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated patient privacy rules under the Health Insurance Portability and Accountability Act of 1996, or HIPAA. These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. If we are found to be in violation of the privacy rules under HIPAA or similar laws (to the extent applicable to us), we could be subject to civil or criminal penalties, which could increase our liabilities, harm our reputation and have a material adverse effect on our business, financial condition and results of operations. European privacy laws are generally more stringent than similar laws in the United States. Since a majority of our revenue arises in Europe, we may be at risk should we fail to comply with local requirements even if we have complied with United States regulations.
If we fail to successfully introduce next generation products and improvements to our existing product, our future growth may suffer.
As part of our strategy, we intend to develop and introduce a number of next generation products and make enhancements to our existing product. We also intend to develop new indications for our existing products. If we are slow in bringing new products to market or otherwise fail to successfully develop, manufacture, design clinical trials for, introduce or commercialize any of these new products, product improvements and new indications on a timely basis, or if they are not well accepted by the market, our future growth may suffer. For example, we are developing a next generation pump based on our MVAD technology, designing a new and improved controller and working on a clinical strategy for a bi-ventricular indication, among others. If we are not successful in these efforts, among others, our future business opportunities and growth potential will suffer.
If we choose to license, invest in or acquire new businesses, products or technologies, in addition to or instead of developing them ourselves, these licenses, investments or acquisitions could disrupt our business and could result in the use of significant amounts of equity, cash or a combination of both.
From time to time we may seek to license, invest in or acquire new businesses, products or technologies, in addition to or instead of developing similar technologies them ourselves. Licenses, investments and acquisitions involve numerous risks, including:
| • | the inability to complete the license, investment or acquisition; |
| • | disruption of our ongoing businesses and diversion of the attention of management and other personnel; |
| • | difficulties in integrating the acquired entities, products or technologies; |
| • | risks associated with acquiring intellectual property; |
| • | difficulties in operating the acquired business profitably; |
| • | the inability to achieve anticipated synergies, cost savings or growth; |
| • | potential loss of key employees, particularly those of the acquired business; |
| • | difficulties in transitioning and maintaining key customer, distributor and supplier relationships; |
| • | risks associated with entering markets in which we have no or limited prior experience; and |
| • | unanticipated costs. |
In addition, any future licenses, investments or acquisitions may result in one or more of the following:
| • | dilutive issuances of equity securities, which may be sold at a discount to market price; |
| • | the use of significant amounts of cash; |
| • | the incurrence of debt; |
| • | the assumption of significant liabilities; |
| • | increased operating costs or reduced earnings; |
| • | financing obtained on unfavorable terms; |
| • | large one-time expenses; |
| • | sharing of revenue or profits with third parties; and |
| • | the creation of certain intangible assets, including goodwill, the write-down of which in future periods may result in significant charges to earnings. |
31
Any of these factors could materially harm our stock price, business, financial condition and results of operations.
If we cannot successfully manage the additional business and regulatory risks that result from our expansion into multiple foreign markets, we may experience an adverse impact to our business, financial condition and results of operations.
We have aggressively expanded, and expect to continue to expand, into additional foreign markets. This expansion will subject us to new business and regulatory risks, including, but not limited to:
| • | failure to fulfill foreign regulatory requirements on a timely basis or at all to market the HeartWare System or other future products; |
| • | availability of, and changes in, reimbursement within prevailing foreign health care payment systems; |
| • | adapting to the differing laws and regulations, business and clinical practices, and patient preferences in foreign countries; |
| • | difficulties in managing foreign relationships and operations, including any relationships that we may establish with foreign partners, distributors or sales or marketing agents as well as compliance with the Foreign Corrupt Practices Act and the United Kingdom’s Bribery Act; |
| • | differing levels of protection for intellectual property rights in some countries; |
| • | difficulty in collecting accounts receivable and longer collection periods; |
| • | costs of enforcing contractual obligations in foreign jurisdictions; |
| • | recessions in economies outside of the United States; |
| • | political instability and unexpected changes in diplomatic and trade relationships; |
| • | currency exchange rate fluctuations; and |
| • | potentially adverse tax consequences, including our ability to interpret local tax rules and implement appropriate tax treatment/collection. |
We will be impacted by these additional business risks, which may adversely impact our business, financial condition and results of operations. In addition, expansion into additional foreign markets imposes additional burdens on our small executive and administrative personnel, research and sales department and generally limited managerial resources. Our efforts to introduce our current or future products into additional foreign markets may not be successful, in which case we may have expended significant resources without realizing the expected benefit. Ultimately, the investments required for expansion into additional foreign markets could exceed the returns, if any, generated from this expansion.
The taxation and customs requirements, together with other applicable laws and regulations of certain foreign jurisdictions, can be inherently complex and subject to differing interpretation by local authorities. We are subject to the risk that either we have misinterpreted applicable laws and regulations, or that foreign authorities may take inconsistent, unclear or changing positions on local law, customs practices or rules. In the event that we have misinterpreted any of the above, or that foreign authorities take positions contrary to ours, we may incur liabilities that may differ materially from the amounts accrued in our financial statements.
The competition for qualified personnel is particularly intense in our industry. In addition, we have added or made changes to executive personnel during 2011 and may continue to do so as our needs evolve. If we are unable to retain or hire executive and other key personnel, we may not be able to sustain or grow our business.
Our ability to operate successfully and manage our potential future growth depends significantly upon our ability to attract, retain and motivate highly skilled and qualified research, technical, clinical, regulatory, sales, marketing, managerial and financial personnel. During 2011, we hired and expect to continue to hire a substantial number of employees in these areas and others in order to prepare for U.S. commercialization and the expected growth in our global business. During 2012, we expect to fill key open positions, including Chief Financial Officer and Vice President, Operations. However, we face intense competition for qualified personnel, and we may not be able to attract, retain and motivate these individuals. We compete for talent with numerous companies, as well as universities and non-profit research organizations. Our future success also depends on the personal efforts and
32
abilities of the principal members of our senior management and scientific staff to provide strategic direction, manage our operations and maintain a cohesive and stable environment. Although we have employment and incentive compensation agreements with all of our executive officers and incentive and compensation plans for our other personnel providing them with various economic incentives to remain employed with us, these incentives may not be sufficient to retain them. We do not maintain key man life insurance on the lives of any of the members of our senior management. The loss of key personnel for any reason or our inability to hire, retain and motivate additional qualified personnel in the future could prevent us from sustaining or growing our business.
Product liability claims could damage our reputation or adversely affect our business.
The design, manufacture and marketing of human medical devices, particularly implantable life-sustaining medical devices, carries an inherent risk of product liability claims and other damage claims. Such liability claims may be expensive to defend and may result in large judgments against us. A product liability or other damages claim, product recall or product misuse, regardless of the ultimate outcome, could require us to spend significant time and money in litigation or to pay significant damages and could seriously harm our business. We maintain clinical trial insurance and limited product liability insurance. We cannot be certain that such insurance will be sufficient to cover all claims that may be made against us. Our insurance policies generally must be renewed on an annual basis. We may not be able to maintain or increase such insurance on acceptable terms or at reasonable costs. A successful claim brought against us in excess, or outside, of our insurance coverage could seriously harm our financial condition and results of operations. Generally, our clinical trials will be conducted in (and our commercial sales will be made to sites in respect of) patients with serious life-threatening diseases for whom conventional treatments have been unsuccessful or for whom no conventional treatment exists, and, during the course of treatment, these patients could suffer adverse medical effects or die for reasons that may or may not be related to our medical devices. Any of these events could result in a claim of liability. For example, in 2009 we received a claim in connection with the death of a patient from multiple organ failure participating in our clinical trial in Germany. We may receive similar claims from time to time in the future. Such claims against us, regardless of their merit, could result in significant awards against us that could materially adversely harm our business, financial condition, results of operations and prospects. A product liability or other damages claim, product recall or product misuse involving any type of VAD, but especially involving one of ours, could also materially and adversely damage our reputation and the perception of VADs generally and affect our ability to attract and retain customers, irrespective of whether or not the claim or recall was meritorious.
Investors could lose confidence in our financial reports, and the value of our shares may be adversely affected, if our internal controls over financial reporting are found not to be effective by management or by our independent registered public accounting firm or if we make disclosure of existing or potential significant deficiencies or material weaknesses in those controls.
Management’s assessment of our internal controls over financial reporting is discussed in Item 9A of our Annual Report on Form 10-K for the year ended December 31, 2011. The Company’s management, with the participation of the Company’s Chief Executive Officer and Vice President of Finance, evaluated the effectiveness of the Company’s disclosure controls and procedures, and internal control over financial reporting as of December 31, 2011. Based on that evaluation, the Company’s Chief Executive Officer and Vice President of Finance concluded that the Company’s disclosure controls and procedures, and internal control over financial reporting are effective as of December 31, 2011. Our independent registered public accounting firm has issued their attestation report on our internal control over financial reporting, which is also included in Item 9A of our Annual Report on Form 10-K for the year ended December 31, 2011.
We continue to evaluate our existing internal controls over financial reporting against the standards adopted by the Public Company Accounting Oversight Board, or PCAOB. During the course of our ongoing evaluation of the internal controls, we may identify areas requiring improvement and will design enhanced processes and controls to address any issues identified through this review. As we continue to commercialize our products, we will need to enhance our accounting and financial controls functions, particularly as they relate to accounting for revenue and inventory, and we will need to add more personnel to our financial reporting group. Remediating any deficiencies, significant deficiencies or material weaknesses that have been or could be identified by us or our independent registered public accounting firm may require us to incur significant costs and expend significant time and management resources. We cannot assure you that any of the measures we implement to remedy any deficiencies
33
will effectively mitigate or remedy deficiencies. The existence of one or more deficiencies or weaknesses could affect the accuracy and timing of our financial reporting. Investors could lose confidence in our financial reports, and the value of our shares may be adversely affected if our internal controls over financial reporting are found not to be effective by management or by our independent registered public accounting firm or if we make disclosure of existing or potential significant deficiencies or material weaknesses in those controls.
Risks Related to Our Common Stock
The price of our common stock may fluctuate significantly.
The ordinary shares of HeartWare Limited had been traded on the ASX from January 31, 2005 until November 13, 2008 when the shares of common stock of HeartWare International, Inc. started trading on the ASX in the form of CHESS Depositary Interests, or CDIs, each representing one thirty-fifth of a share of our common stock. The trading price of the common stock and the CDIs, as applicable, has been, and is likely to continue to be, volatile, which means that it could decline substantially within a short period of time. In addition, our shares of common stock began trading on The NASDAQ Stock Market LLC on February 24, 2009. Prior to that time, there had been no public market for our common stock in the United States. The closing price of our shares of common stock traded on The NASDAQ Stock Market LLC has ranged from U.S. $54.90 to U.S. $97.69 in the period from January 1, 2011 to December 31, 2011. The price of our common shares, whether traded in the form of common stock or CDIs, could fluctuate significantly for many reasons, including the following:
| • | future announcements or new information concerning us or our competitors reimbursement, or the potential market for our products; |
| • | regulatory developments (such as the status of FDA approval of our device for the bridge-to-transplant indication), enforcement actions bearing on advertising, marketing or sales, and disclosure regarding completed, ongoing or future clinical trials; |
| • | quarterly variations in operating results and our liquidity, which we have experienced in the past and expect to experience in the future; |
| • | introduction of new products or changes in product pricing policies by us or our competitors; |
| • | acquisition or loss of significant customers, distributors or suppliers; |
| • | technology acquisitions or divestitures; |
| • | changes in third party reimbursement practices; |
| • | fluctuations of investor interest in the medical device sector; and |
| • | fluctuations in the economy, world political events, foreign currency movements or general market conditions. |
In addition, stock markets in general and the market for shares of health care stocks in particular, have experienced extreme price and volume fluctuations in recent years, fluctuations that frequently have been unrelated to the operating performance of the affected companies. These broad market fluctuations may adversely affect the market price of our shares. The market price of our shares could decline below its current price and the market price of our shares may fluctuate significantly in the future. These fluctuations may be unrelated to our performance.
We do not intend to pay cash dividends on our common stock in the foreseeable future.
We have never declared or paid any cash dividends on our shares, and we currently do not anticipate paying any cash dividends in the foreseeable future. We intend to retain any earnings to finance the development and expansion of our products and business. Accordingly, our stockholders will not realize a return on their investment unless the trading price of our shares appreciates.
Anti-takeover provisions in our charter documents and Delaware law may discourage a third party from acquiring us, which could limit our stockholders’ opportunities to sell their shares at a premium.
Certain provisions of our Certificate of Incorporation and By-laws may be considered as having an anti-takeover effect, such as those provisions establishing a classified board of directors, consisting of three classes of directors, and requiring that directors be removed only for cause, authorizing the board of directors to issue from time to time any series of preferred stock and fix the designation, powers, preferences and rights of the shares of
34
such series of preferred stock, prohibiting stockholders from acting by written consent in lieu of a meeting, requiring advance notice of stockholder intention to put forth director nominees or bring up other business at a stockholders’ meeting, and prohibiting stockholders from calling a special meeting of stockholders. We are also subject to Section 203 of the Delaware General Corporation Law, which in general prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that the stockholder became an interested stockholder, unless certain conditions specified therein are satisfied. These provisions could have the effect of depriving our stockholders of an opportunity to sell their shares at a premium over prevailing market prices by discouraging third parties from seeking to obtain control of us in a tender offer or similar transaction.
We may be subject to arbitrage risks.
Investors may seek to profit by exploiting the difference, if any, in the price of our shares of common stock as reflected by the trading price of our CDIs, each representing one thirty-fifth of a share of our common stock, on the ASX and the trading price of our shares of common stock on the NASDAQ Stock Market. Such arbitrage activities could cause the price of our securities (as adjusted to reflect the fact that each CDI represents one thirty-fifth of a share of common stock) in the market with the higher value to decrease to the price set by the market with the lower value.
We are currently considering the possibility of delisting our CHESS Depositary Interests from the Australian Securities Exchange. If we should decide to pursue delisting, the market for our common stock in the United States could be disrupted, which would have an adverse affect on our stock price.
We are currently considering delisting our CHESS Depositary Interests from the Australian Securities Exchange. Any decision will take into account, among other things, due notification, and a desire to minimize the disruption, to stockholders. The delisting of our CHESS Depositary Interests from the ASX could disrupt the market for our common stock listed on the NASDAQ Stock Market in the United States as a result of the sudden influx onto the NASDAQ Stock Market of the shares of our common stock previously represented by CHESS Depositary Interests, which could have an adverse affect on our stock price. In addition, the delisting of our CHESS Depositary Interests from the ASX could have an adverse effect on our ability to raise capital, if needed, on terms acceptable to us.
We may undergo an “ownership change” for U.S. federal income tax purposes, which would limit our ability to utilize net operating losses from prior tax years.
For U.S. federal income tax purposes, we have incurred net losses since our inception. If we undergo an “ownership change” for U.S. federal income tax purposes, our ability to utilize net operating loss carry-forwards from prior years to reduce taxable income in future tax years might be limited by operation of the Internal Revenue Code, either by limiting the amount of net operating losses that can be utilized to offset taxable income in a given year, or in total over the entire carry-forward period. Certain changes in the ownership of our common stock, including the sale of our common stock by Apple Tree Partners I, L.P., one of our largest holders, may result in such an ownership change.
Item 1B. Unresolved Staff Comments
None.
Our corporate headquarters are located in Framingham, Massachusetts. We have operations and manufacturing facilities in Miami Lakes, Florida, a development and operations facility in Sydney, Australia and a distribution and customer service facility in Hannover, Germany.
Our office in Framingham, Massachusetts consists of approximately 17,800 square feet and is primarily used for administrative functions. The lease expires on December 31, 2014 and we have an option to renew the lease for one additional four-year term. We also have an option to expand with an additional 3,002 square foot space in the building.
35
One of our facilities in Miami Lakes, Florida consists of approximately 59,000 square feet and includes office space, laboratories, research and development space and three clean rooms which are ISO Class 100,000 compliant. The facility is used primarily for manufacturing, research and development and administrative functions. The lease expires on June 30, 2013 and we have an option to renew the lease for two additional, three-year terms.
On December 9, 2010, we entered into a lease for a second facility in Miami Lakes, Florida as part of our planned expansion to support our efforts to prepare for U.S. commercialization. During 2011, we performed significant improvements on the facility and improvements will continue in the first quarter of 2012. Once completed, the facility will be used primarily for manufacturing, research and development and administrative functions. Under the lease, we rent approximately 131,000 square feet for a period ending February 28, 2022, with an option to renew for two five-year terms.
One of our facilities in Sydney, Australia consists of approximately 2,600 square feet of manufacturing space. We originally leased the space for a two-year period commencing on August 31, 2009. We did not exercise our option to renew the lease for an additional three-year term. We are currently occupying the space on a month-to-month basis.
On November 1, 2011, we entered into a lease for a new facility in Sydney, Australia. This facility will replace our current facility noted above and will be used primarily for manufacturing and administrative functions. Under the lease, we rent approximately 15,100 square feet for a period ending October 31, 2014, with an option to renew for two three-year terms.
Our facility in Hannover, Germany is approximately 3,900 square feet. The lease commenced on October 11, 2010 with an initial term of two years. We have an option to renew the lease for one additional three-year term.
We believe that the facilities noted above are suitable and adequate for our needs now and for the foreseeable future.
Except as described in Note 14 to the accompanying consolidated financial statements, the Company is not a party to any material legal proceedings at the date of filing of this Annual Report on Form 10-K.
Item 4. Mine Safety Disclosures
Not applicable
36
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
On February 24, 2009 we listed on the NASDAQ Stock Market with trading commencing on February 25, 2009 under the symbol “HTWR”. Our shares of common stock also trade in the form of CHESS Depositary Interests (“CDIs”), each CDI representing one thirty-fifth of a share of our common stock, on the Australian Securities Exchange (“ASX”) under the symbol “HIN” since November 13, 2008. Prior to that date, our ordinary shares of HeartWare Limited (since renamed HeartWare Pty. Limited), of which we are the successor issuer, were traded on the ASX under the symbol “HTW”.
The following table sets forth, for the periods indicated, the high and low closing prices for our common stock on the NASDAQ.
| September 30, | September 30, | |||||||
Period | High | Low | ||||||
Fiscal Year 2011: | ||||||||
First Quarter | $ | 97.69 | $ | 81.19 | ||||
Second Quarter | 84.32 | 67.00 | ||||||
Third Quarter | 74.58 | 54.90 | ||||||
Fourth Quarter | 70.56 | 58.78 | ||||||
Fiscal Year 2010: | ||||||||
First Quarter | $ | 44.47 | $ | 33.96 | ||||
Second Quarter | 74.65 | 45.14 | ||||||
Third Quarter | 74.67 | 60.49 | ||||||
Fourth Quarter | 93.76 | 61.90 | ||||||
As of February 15, 2012, we had 14,114,055 shares of common stock issued and outstanding and there were approximately 7 holders of record of our common stock. In addition, as of that date, there were approximately 690 registered owners of our CDIs.
We have not declared or paid any cash dividends on our shares, and we currently do not anticipate paying any cash dividends in the foreseeable future. Our convertible notes were issued pursuant to the terms of an Indenture dated December 15, 2010. The Indenture does not contain any covenants or restrictions on the payments of dividends. We intend to retain any earnings to finance the development and expansion of our products and business.
37
Stock Price Performance Graph
The graph below compares the cumulative total stockholder return on an investment in our CDI’s, the NASDAQ Composite Index (U.S. companies only) and the Morningstar Medical Devices Index for the five-year period ended December 31, 2011. The graph assumes the value of an investment in our CDI’s traded on the ASX and each index was $100 on December 31, 2006 and the reinvestment of all dividends, if any.
The graph also presents the cumulative total stockholder return on an investment in our common stock for the period from February 25, 2009 to December 31, 2011. The graph assumes the value of an investment in our common stock was $100 on February 25, 2009, the date our common stock commenced trading on the NASDAQ Stock Market, and the reinvestment of all dividends, if any.
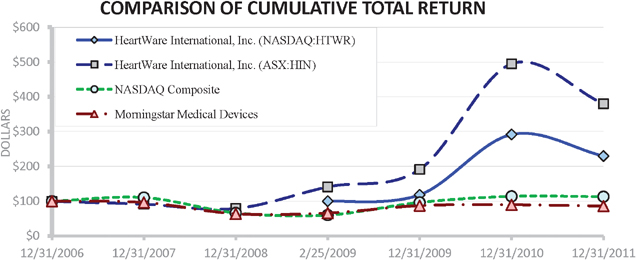
| Septe 30, | Septe 30, | Septe 30, | Septe 30, | Septe 30, | Septe 30, | Septe 30, | ||||||||||||||||||||||
Company/Market/Peer Group | 12/31/2006 | 12/31/2007 | 12/31/2008 | 2/25/2009 | 12/31/2009 | 12/31/2010 | 12/31/2011 | |||||||||||||||||||||
HeartWare International, Inc. (NASDAQ:HTWR) | $ | 100.00 | $ | 118.23 | $ | 291.90 | $ | 230.00 | ||||||||||||||||||||
HeartWare International, Inc. (ASX:HIN) | $ | 100.00 | $ | 91.45 | $ | 79.21 | $ | 141.31 | $ | 191.59 | $ | 494.96 | $ | 380.05 | ||||||||||||||
NASDAQ Composite | $ | 100.00 | $ | 110.66 | $ | 66.41 | $ | 60.16 | $ | 96.54 | $ | 114.06 | $ | 113.16 | ||||||||||||||
Morningstar Medical Devices | $ | 100.00 | $ | 96.38 | $ | 63.78 | $ | 65.88 | $ | 87.31 | $ | 89.69 | $ | 86.11 | ||||||||||||||
38
Equity Compensation Plans
The following table sets forth information regarding the Company’s Equity Compensation Plans as of December 31, 2011:
| September 30, | September 30, | September 30, | ||||||||||
Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||||
Equity compensation plans approved by security holders: | ||||||||||||
HeartWare International, Inc. Employee Stock Option Plan | 296,817 | $ | 31.87 | (1) | — | (2) | ||||||
HeartWare International, Inc. Restricted Stock Unit Plan | 47,685 | $ | 0.00 | 16,976 | (2) | |||||||
HeartWare International, Inc. 2008 Stock Incentive Plan (3) | 665,947 | $ | 5.51 | 368,982 | (2)(4) | |||||||
Equity compensation plans not approved by security holders: | ||||||||||||
Non-Plan options | 5,142 | $ | 26.71 | (1) | N/A | |||||||
| (1) | The exercise price has been converted to U.S. dollars using the spot rate at December 31, 2011. |
| (2) | Future awards to employees and directors are expected to be made under the 2008 Stock Incentive Plan as any grants under the other plans reduce the availability of grants under the 2008 Stock Incentive Plan. |
| (3) | Outstanding awards under the 2008 Stock Incentive Plan include 586,723 restricted stock units outstanding with exercise prices of $0 and 79,224 stock options outstanding with exercise prices equal to the fair value of our common stock on the date of grant. The weighted average exercise price of the outstanding stock options was $46.28 at December 31, 2011. |
| (4) | The 2008 Stock Incentive Plan includes an annual adjustment to shares available for future issuance at each January 1 based on the prior number of weighted average shares outstanding in the prior year. As of January 1, 2012, the number of shares available for future issuance under the 2008 Stock Incentive Plan was approximately 460,000. |
Item 6. Selected Financial Data
The following selected consolidated statement of operations data for the years ended December 31, 2011, 2010 and 2009 and the selected consolidated balance sheet data as of December 31, 2011 and 2010 have been derived from our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. The following selected consolidated statement of operations data for the years ended December 31, 2008 and 2007 and balance sheet data as of December 31, 2009, 2008 and 2007 have been derived from our audited consolidated financial statements which are not included in this Annual Report on Form 10-K. The selected consolidated financial data set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” below and our audited consolidated financial statements and notes thereto appearing elsewhere in this Annual Report on Form 10-K.
In connection with the 2008 redomiciliation of our corporate parent entity from Australia to the United States, each holder of HeartWare Limited ordinary shares received one share of common stock of HeartWare International, Inc., for every 35 of HeartWare Limited ordinary shares held by such holder. The 2007 per share information listed below has been adjusted to give effect to the 2008 redomiciliation transaction, whether such information pertains to a date or period of time subsequent or prior to the redomiciliation transaction.
39
| September 30, | September 30, | September 30, | September 30, | September 30, | ||||||||||||||||
| Years Ended December 31, | ||||||||||||||||||||
(In thousands, except per share data) | 2011 | 2010 | 2009 | 2008 | 2007 | |||||||||||||||
Consolidated Statement of Operations Data: |
| |||||||||||||||||||
Revenue, net | $ | 82,764 | $ | 55,164 | $ | 24,172 | $ | 332 | $ | — | ||||||||||
Cost of revenue | 32,932 | 24,441 | 13,211 | 78 | — | |||||||||||||||
Selling, general and administrative expenses | 42,314 | 26,642 | 16,444 | 10,981 | 7,303 | |||||||||||||||
Research and development expenses | 50,149 | 33,108 | 15,067 | 18,644 | 14,636 | |||||||||||||||
Other (expense) income, net (a) | (12,424 | ) | (370 | ) | (359 | ) | 5,607 | — | ||||||||||||
Provision for income taxes | — | — | — | — | — | |||||||||||||||
Net loss | (55,055 | ) | (29,397 | ) | (20,909 | ) | (23,764 | ) | (21,939 | ) | ||||||||||
Basic and diluted net loss per share | (3.94 | ) | (2.17 | ) | (2.15 | ) | (3.00 | ) | (3.60 | ) | ||||||||||
| (a) | In the year ended December 31, 2011, other expense includes approximately $10.7 million of interest expense associated with our 3.5% convertible senior notes due December 15, 2017. |
| September 30, | September 30, | September 30, | September 30, | September 30, | ||||||||||||||||
| As of December 31, | ||||||||||||||||||||
(In thousands) | 2011 | 2010 | 2009 | 2008 | 2007 | |||||||||||||||
Consolidated Balance Sheet Data: | ||||||||||||||||||||
Cash, cash equivalents and short-term investments | $ | 163,182 | $ | 213,478 | $ | 50,835 | $ | 20,804 | $ | 28,276 | ||||||||||
Total assets | 240,732 | 267,577 | 77,953 | 30,338 | 32,355 | |||||||||||||||
Convertible senior notes, net of discounts (b) | 94,277 | 88,922 | — | — | — | |||||||||||||||
Total stockholders’ equity | 126,784 | 167,764 | 70,983 | 26,756 | 29,272 | |||||||||||||||
| (b) | At December 31, 2011 and 2010, the aggregate principal amount of our 3.5% convertible senior notes due December 15, 2017 was $143.75 million. |
40
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read together with our consolidated financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K. This discussion and analysis contains forward-looking statements that involve risks, uncertainties, judgment and assumptions. You should review the “Risk Factors” section of this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. Certain abbreviated key terms have the meanings defined elsewhere in this Annual Report on Form 10-K.
Overview
HeartWare is a medical device company that develops and manufactures miniaturized implantable heart pumps, or ventricular assist devices, to treat patients suffering from advanced heart failure.
The HeartWare Ventricular Assist System (the “HeartWare System”), which includes a ventricular assist device (“VAD”), or blood pump, patient accessories and surgical tools, is designed to provide circulatory support for patients in the advanced stage of heart failure. The core of the HeartWare System is a proprietary continuous flow blood pump, the HVAD Pump, which is a full-output device capable of pumping up to 10 liters of blood per minute. The HeartWare System is designed to be implanted adjacent to the heart, avoiding the abdominal surgery generally required to implant similar devices.
In 2009, we received CE Marking for the HeartWare System in the European Union and in March 2011 we received approval from the Therapeutic Goods Administration in Australia allowing for commercial sale and distribution of our device. In the U.S., the device is the subject of clinical trials for two indications: bridge-to-transplant and destination therapy. Our device is also available in other countries around the world under special access programs and limited commercial availability.
Recent key milestones in the development and commercialization of the HeartWare System include the following:
| • | In February 2012, we were notified that a U.S. FDA Circulatory System Devices Panel of the Medical Devices Advisory Committee will review our Pre-Market Approval application for our HeartWare System for a bridge-to-transplant indication on April 25, 2012; |
| • | Implanted the HeartWare System in over 900 patients in 2011; |
| • | In January 2011, we were named to the REVIVE-IT study, a study to be completed by the Universities of Michigan and Pittsburgh on the benefits of LVAD’s in patients with earlier access to the device; |
| • | Approval of the HeartWare System by the Therapeutic Goods Administration (TGA) in Australia for listing on the Australian Register of Therapeutic Goods; |
| • | Reimbursement approval in France and Belgium of the HeartWare System; and |
| • | Presentation at the 25th European Association for Cardio-Thoracic Surgery of clinical data from our ADVANCE bridge-to-transplant clinical trial and the Continued Access Protocol (CAP). The updated data for 241 patients enrolled in either the pivotal trial ADVANCE or CAP demonstrated a 180-day survival of 93%. |
Beyond the HeartWare System, we are also evaluating our next generation device, the MVAD Pump. The MVAD Pump is a development-stage miniature ventricular assist device, approximately one-third the size of the HVAD Pump. The MVAD Pump is based on the same proprietary impeller suspension technology used in the HVAD Pump, with its single moving part held in place through a combination of passive-magnetic and hydrodynamic forces. Like the HVAD Pump, the MVAD Pump is designed to support the heart’s full cardiac output, yet also has the capability for partial support. On September 9, 2011, pre-clinical data was presented at the 19th Congress of the International Society for Rotary Blood Pumps (ISRBP), which demonstrated that the MVAD Pump attained the objectives for system performance, hemocompatability and biocompatibility in Good Laboratory Practice (“GLP”) animal studies, a precursor to human clinical trials. We are currently preparing to commence human clinical studies in 2012. The MVAD Pump is designed to be implantable by surgical techniques that are even less invasive than those required to implant the HVAD Pump.
41
We began generating revenue from our products in August 2008 and have incurred net losses in each year since our inception. We expect our losses to continue as we advance and expand our clinical trial activities in the U.S., continue to develop commercial markets outside of the U.S., and expand our research and development into next generation products including the MVAD Pump.
We have financed our operations primarily through the issuance of convertible notes and the issuance of shares of our common stock. Most recently, on December 15, 2010, we issued convertible notes with an aggregate principal amount of $143.75 million pursuant to the terms of an Indenture dated as of December 15, 2010. The convertible notes are senior unsecured obligations of the Company. The convertible notes bear interest at a rate of 3.5% per annum, payable semi-annually in arrears on June 15 and December 15 of each year. The convertible notes will mature on December 15, 2017, unless earlier repurchased or converted.
We are headquartered in Framingham, Massachusetts. We have operations and manufacturing facilities in Miami Lakes, Florida, a development and operations facility in Sydney, Australia and a distribution and customer service facility in Hannover, Germany.
Critical Accounting Policies and Estimates
We prepare our financial statements in accordance with accounting principles generally accepted in the United States. We are required to adopt various accounting policies and to make estimates and assumptions in preparing our financial statements that affect the reported amounts of our assets, liabilities, revenue and expenses. On an ongoing basis, we evaluate our estimates and assumptions. We base our estimates on our historical experience to the extent practicable and on various other assumptions that we believe are reasonable under the circumstances and at the time they are made. If our assumptions prove inaccurate or if our future results are not consistent with our historical experience, we may be required to make adjustments in our policies that affect our reported results. Our significant accounting policies are disclosed in Note 3 to the financial statements included in this report.
Our most critical accounting policies and estimates include: revenue recognition, inventory capitalization and valuation, accounting for share-based compensation, measurement of fair value, and the valuation of tax assets and liabilities. We also have other key accounting policies that are less subjective and, therefore, their application is less subject to variations that would have a material impact on our reported results of operations. The following is a discussion of our most critical policies, as well as the estimates and judgments involved.
Revenue recognition
We recognize revenue from product sales in accordance with FASB ASC 605 –Revenue Recognition. Pursuant to agreements or orders from customers, we ship product to our customers. Revenue from product sales is only recognized when persuasive evidence of an arrangement exists, substantially all the risks and rewards of ownership have transferred to our customers, the selling price is fixed and collection is reasonably assured. A majority of product sales are initially made on a consignment basis and as such, pursuant to the terms of the consignment arrangements, revenue is recognized on the date the consigned product is implanted or otherwise consumed. Revenue from product sales not sold on a consignment basis is recognized upon customer receipt and acceptance of the product. Revenue recognized to date is from sales of our devices in connection with our U.S. clinical trials and commercial sales in Europe and to a lesser extent under special access in other countries.
Inventory Capitalization
We expense costs relating to the production of inventories as research and development (“R&D”) expense in the period incurred until such time as we believe future commercialization is considered probable and future economic benefit is expected to be recognized, which generally is reliant upon receipt of regulatory approval. We then begin to capitalize subsequent inventory costs relating to that product. Inventories are stated at the lower of cost or market. Cost is determined on a first-in, first out, or FIFO, method. Work-in-process and finished goods include direct and indirect labor and manufacturing overhead. Finished goods include product which is ready-for-use and which is held by us or by our customers on a consignment basis.
42
We review our inventory for excess or obsolete items and write down obsolete or otherwise unmarketable inventory to its estimated net realizable value. Obsolescence may occur due to product expiring or product improvements rendering previous versions obsolete. The extent to which product improvements will cause obsolescence of existing inventory is difficult to determine as the rate of customer acceptance is dependent on many factors. We make judgments and estimates on matters, including forecasted sales volume. Our estimates and judgments in this area are subject to uncertainty and may differ from our actual experience in the future, which could have a material effect on recorded inventory values.
We include in inventory materials and finished goods that are held for sale. Certain materials and finished goods held in inventory may be used in research and development activities and are expensed as part of research and development costs when consumed.
Share-Based Compensation
We recognize share-based compensation expense in connection with our share-based awards, net of an estimated forfeiture rate, and therefore only recognize compensation cost for those awards expected to vest over the service period of the award. We estimate the forfeiture rate based on our historical experience of forfeitures and our employee retention rate. If our actual forfeiture rate is materially different from our estimate, the share-based compensation expense could be significantly different from what we have recorded in the current period. Calculating share-based compensation expense requires the input of highly subjective judgment and assumptions, including estimates of expected life of the share-based award, stock price volatility, forfeiture rates and risk-free interest rates. As a result, if factors change and we use different assumptions, our share-based compensation expense could be materially different in the future.
We value restricted stock units, or RSU’s, at their intrinsic value on the date of grant. We estimate the fair value of our stock options using a Black-Scholes option pricing model. The assumptions used in estimating the fair value of our stock options represent our best estimates, but these estimates involve inherent uncertainties and the application of management judgment. When appropriate, we estimate the expected life of a stock option by averaging the contractual term of the stock option (up to 10 years) with the associated vesting term (typically 4 years). We estimate the volatility of our shares on the date of grant considering several factors, including the historical volatility of our publicly-traded shares. We estimate the risk-free interest rate based on rates in effect for United States government bonds with terms similar to the expected lives of the awards, at the time of grant.
We have issued share-based awards with performance-based vesting criteria. Achievement of the milestones must be probable before we begin recording share-based compensation expense. At each reporting period, we review the likelihood that these awards will vest and if the vesting is deemed probable, we begin to recognize compensation expense at that time. In the period that achievement of the performance based criteria is deemed probable, U.S. GAAP requires the immediate recognition of all previously unrecognized compensation since the original grant date. As a result, compensation expense recorded in the period that achievement is deemed probable could include a substantial amount of previously unrecorded compensation expense related to the prior periods. During 2011, 2010 and 2009, we determined that achievement of certain performance-based vesting criteria for share-based awards originally granted in 2008 and 2007 was probable. Therefore, we began recording compensation expense during 2011, 2010 and 2009 in connection with certain share-based awards that had been outstanding but for which we had not previously recorded any compensation expense. If ultimately performance goals are not met, for any share-based awards where vesting was previously deemed probable, previously recognized compensation cost will be reversed.
Fair Value Measurements
FASB ASC 820 –Fair Value Measurements and Disclosures defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. FASB ASC 820 requires disclosures about the fair value of all financial instruments, whether or not recognized, for financial statement purposes. Disclosures about the fair value of financial instruments are based on pertinent information available to us as of the respective reporting dates. Accordingly, the estimates presented in our financial statements are not necessarily indicative of the amounts that could be realized on disposition of the financial instruments.
43
FASB ASC 820 specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement).
The three levels of the fair value hierarchy are as follows:
Level 1 – Quoted prices for identical instruments in active markets.
Level 2 – Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations whose inputs are observable or whose significant value drivers are observable.
Level 3 – Instruments with primarily unobservable value drivers.
The assumptions used in calculating the fair value of financial instruments represent our best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, the use of different estimates or assumptions would result in a higher or lower fair value and different amounts being recorded in our financial statements. Calculating fair value utilizing Level 3 inputs requires the input of highly subjective judgment and assumptions.
Income Taxes
We account for income taxes in accordance with the liability method presented by FASB ASC 740– Income Taxes. Under this method, deferred income taxes are determined based on the estimated future tax effects of differences between the financial statement and tax basis of assets and liabilities given the provisions of enacted tax laws. Deferred income tax provisions and benefits are based on changes to the assets or liabilities from year to year. In providing for deferred taxes, we consider tax regulations of the jurisdictions in which we operate, estimates of future taxable income, and available tax planning strategies. If tax regulations, operating results or the ability to implement tax-planning strategies vary, adjustments to the carrying value of deferred tax assets and liabilities may be required. Valuation allowances are recorded related to deferred tax assets based on the “more likely than not” criteria of FASB ASC 740. Through December 31, 2011, we have historically concluded that a full valuation allowance is required to offset our net deferred tax assets. We operate within multiple taxing jurisdictions and are subject to audit in those jurisdictions. Because of the complex issues involved, any claims can require an extended period to resolve.
FASB ASC 740 requires that we recognize the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the “more likely than not” threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority.
Reserves
Management must make estimates and assumptions to determine the amount of reserves to record in the financial statements. If any of these decisions proves incorrect, our consolidated financial statements could be materially and adversely affected.
We maintain allowances for doubtful accounts for estimated losses that may result from an inability to collect payments owed to us for product sales. We regularly review the allowance by considering factors such as historical experience, the age of the accounts receivable balances and current economic conditions that may affect a customer’s ability to pay.
44
Certain patient accessories sold with the HeartWare System are covered by a limited warranty ranging from one to two years. Estimated contractual warranty obligations are recorded as an expense when the related revenue is recognized and are included in cost of revenue on our consolidated statements of operations. Factors that affect estimated warranty liability include the number of units sold, historical and anticipated rates of warranty claims, cost per claim, and vendor supported warranty programs. We periodically assess the adequacy of our recorded warranty liabilities and adjust the amounts as necessary.
Results of Operations
The following is a description of significant components of our operations, including significant trends and uncertainties that we believe are important to an understanding of our business and results of operations.
Fiscal Years 2011 and 2010
Revenue, net
In 2011 and 2010, we generated revenue from commercial sales outside of the U.S. and sales in connection with our clinical trials in the U.S. The increase in revenue is primarily due to increased market penetration outside of the U.S., increased activity in our U.S. destination therapy study and continuing activity after completion of our U.S. bridge-to-transplant study through a Continued Access Protocol (“CAP”).
Net revenue for the years ended December 31, 2011 and 2010 was as follows:
| September 30, | September 30, | September 30, | ||||||||||
| 2011 | 2010 | Change | ||||||||||
| (in thousands) | ||||||||||||
Revenue, net | $ | 82,764 | $ | 55,164 | 50 | % | ||||||
Approximately 34% of our product sales in 2011 were derived in the U.S. as compared to 27% in the prior year. The increase in the portion of our revenue derived in the U.S. is due primarily to the increased enrollment in our U.S. destination therapy study.
Our sales outside of the U.S. are made in multiple currencies, with the majority of our international revenue denominated in the Euro. During 2011, our net international revenue denominated in foreign currencies increased by $9.6 million, or 25%, compared to 2010. The change in exchange rates for all foreign currencies for 2011 accounted for approximately $2.5 million of the increase for the year.
We expect to continue to generate and grow commercial revenue from product sales as we further expand our sales and marketing efforts outside of the United States and continue enrollment of our destination therapy clinical trial, ENDURANCE, in the U.S. Notwithstanding our plans to generally expand our U.S. clinical programs, revenue from U.S. sources in connection with our trials may vary from quarter to quarter as the recruitment of qualified patients that meet protocol criteria fluctuate, as we approach the enrollment capacity in our approved trials and because additional CAP cohorts are subject to FDA approval.
Future product sales are dependent on many factors, including receiving and maintaining the necessary regulatory approvals in the U.S. and internationally, perception of product performance and market acceptance among physicians, patients, health care payers and the medical community as well as our capacity to meet customer demand by manufacturing sufficient quantities of our products.
Cost of Revenue
Cost of revenue includes costs associated with manufacturing and distributing our product and consists of direct materials, labor and overheard expenses allocated to the manufacturing process, provisions for excess or obsolete inventory, and shipping costs. Cost of revenue totaled approximately $32.9 million for the year ended December 31, 2011 and approximately $24.4 million for the year ended December 31, 2010.
45
Gross profit and gross margin percentage for the years ended December 31, 2011 and 2010 were as follows:
| September 30, | September 30, | |||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
Gross profit | $ | 49,832 | $ | 30,723 | ||||
|
|
|
| |||||
Gross margin % | 60 | % | 56 | % | ||||
|
|
|
| |||||
The gross margin percentage for 2011 increased compared to 2010 as a result of lower per unit costs in 2011 primarily due to increased production volume and improved efficiencies in our manufacturing processes. In May 2011, we began shipping a sintered version of the HeartWare System on a global basis. Sintering is a process whereby minute beads are adhered to a titanium surface commonly used in medical devices to facilitate tissue adhesion. During the fourth quarter of 2011, we recorded a write-down of $0.6 million against our existing non-sintered inventory to reduce inventory to forecasted marketable levels, leaving a balance of $1.0 million as of December 31, 2011. This product continues to be implanted at certain customer sites. A write-down of all or a portion of this remaining inventory as obsolete could have a negative impact on our gross profit and gross margin percentage in future periods.
Selling, General and Administrative
Selling, general and administrative expenses include costs associated with selling and marketing our products and the general corporate administration of the Company. These costs are primarily related to salaries and wages and related employee costs, travel, external consultants and contractors, legal and accounting fees and general infrastructure costs, and include all operating costs not associated with or otherwise classified as research and development costs or cost of revenue.
Selling, general and administrative expenses for the years ended December 31, 2011 and 2010 were as follows:
| September 30, | September 30, | September 30, | ||||||||||
| 2011 | 2010 | Change | ||||||||||
| (in thousands) | ||||||||||||
Total selling, general and administrative expenses | $ | 42,314 | $ | 26,642 | 59 | % | ||||||
|
|
|
| |||||||||
% of operating expenses | 46 | % | 45 | % | ||||||||
|
|
|
| |||||||||
During 2011, we continued to experience significant growth as we expanded European sales and distribution capabilities and increased the number of implants in the U.S. under clinical trials. We also experienced growth in administrative costs as we expanded our administrative capabilities to support overall corporate growth. As a result, we experienced expansion of our staff, including senior management, and overall growth in selling and marketing and administrative functions and experienced a related expansion in infrastructure costs.
The increase of $15.7 million was a result of an increase in employee costs, including salaries and wages and related costs, of approximately $6.2 million, primarily due to increased headcount. We also experienced increases in office expenses of $2.2 million, travel expenses of $2.0 million, marketing expenses of $1.5 million and legal costs of $2.2 million. The increase in legal costs includes $0.9 million for the potential settlement of ongoing litigation as discussed in Note 14 to the accompanying consolidated financial statements.
We expect our selling, general and administrative expenses to continue to increase in 2012 compared to 2011 as we prepare for the launch of the HeartWare System in the United States, and continue to expand our sales and distribution capabilities as well as our administrative capabilities to support our overall corporate growth. We have and will continue to experience an increase in our employee headcount as well as an increase in costs associated with the necessary administrative infrastructure to support this expansion.
46
Research and Development
Research and development expenses are the direct and indirect costs associated with developing our products prior to commercialization and are expensed as incurred. These expenses fluctuate based on project level activity and consist primarily of salaries and wages and related employee costs of our research and development, clinical and regulatory staff, external research and development costs, and materials and expenses associated with clinical trials. Additional costs include travel, facilities and overhead allocations.
| September 30, | September 30, | September 30, | ||||||||||
| 2011 | 2010 | Change | ||||||||||
| (in thousands) | ||||||||||||
Total research and development expenses | $ | 50,149 | $ | 33,108 | 51 | % | ||||||
|
|
|
| |||||||||
% of operating expenses | 54 | % | 55 | % | ||||||||
|
|
|
| |||||||||
The increase of $17.0 million was due to an increase in costs associated with development projects, including consumables, animal studies, outside engineering, consultants and contractors, of $9.2 million, primarily related to MVAD development. We also experienced an increase in employee costs, including salaries and wages and related costs, of approximately $3.8 million and an increase in share-based compensation of $1.6 million. Costs associated with our U.S. clinical trials increased by $1.0 million.
Even with commercial approval of the HeartWare System in Europe, we expect that research and development expenses will continue to represent a significant portion of our operating expenses for the foreseeable future related to clinical trials in the U.S. and new product development, including costs related to the development of the MVAD system.
Foreign Exchange
We generate a substantial portion of our revenue and collect receivables in foreign currencies. Fluctuations in the exchange rate of the U.S. dollar against the Euro, British Pound and Australian dollar can result in foreign currency exchange gains and losses that may significantly impact our financial results. Continued fluctuation of these exchange rates could result in financial results that are not comparable from quarter to quarter. In general, we do not currently utilize foreign currency contracts to mitigate foreign exchange gains and losses.
In 2011, our net foreign exchange losses totaled approximately $2.3 million compared to $0.5 million in 2010. The increase in our net foreign exchange losses is primarily due to fluctuations in the rate of exchange between the Euro and U.S. dollar during 2011. In 2011 and 2010, the majority of our realized and unrealized foreign exchange gains and losses were experienced upon the collection of certain accounts receivable that were denominated in foreign currencies, and the translation to U.S. dollars at period end of certain balance sheet accounts denominated in foreign currencies, primarily the Euro. We expect to continue to realize foreign exchange gains and losses for the foreseeable future as the majority of our sales denominated in foreign currencies are settled in Euros.
Interest Expense
Interest expense in 2011 consists of interest incurred on the principal amount of our convertible senior notes issued in December 2010, amortization of the related discount and amortization of the portion of the deferred financing costs allocated to the debt component. The convertible senior notes bear interest at a rate of 3.5% per annum. The discount on the convertible senior notes and the deferred financing costs are being amortized to interest expense through the December 15, 2017 maturity date of the convertible senior notes using the effective interest method.
Interest expense was approximately $10.7 million in 2011. Interest incurred on the principal amount of the convertible notes at the 3.5% coupon rate was approximately $5.0 million and non-cash amortization of the discount and deferred financing costs totaled approximately $5.7 million.
Investment Income, net
Investment income is primarily derived from investments and cash and short-term deposit accounts held in the U.S. The amortization of premium on our investments is also included in investment income, net. Investment income, net was approximately $0.5 million in 2011, compared to $0.6 million in 2010. While we had greater cash and investments balances during 2011 as a result of the issuance of our convertible senior notes in December 2010, we have experienced lower interest rates in 2011 compared to 2010.
47
Income Taxes
We are subject to taxation in the United States and jurisdictions outside of the United States. These jurisdictions have different marginal tax rates. While we have incurred losses since inception, changes in issued capital and share ownership, as well as other factors, may limit our ability to utilize any net operating loss carry-forwards, and as such a 100% valuation allowance has been recorded against our net deferred tax assets.
As of December 31, 2011, we did not have revenue or profit which would be sufficient to allow any portion of our deferred tax assets to be recorded. We intend to monitor closely whether to record a deferred tax asset as we further expand the commercialization of our products.
Fiscal Years 2010 and 2009
Revenue, net
In 2010 and 2009, we generated revenue from commercial sales outside of the U.S. and sales in connection with our U.S. clinical trials. The increase in revenue is primarily due to increased market penetration outside of the U.S. and increased activity in our U.S. bridge-to-transplant study including sales after completion of the study through a Continued Access Protocol (“CAP”).
Net revenue for the years ended December 31, 2010 and 2009 were as follows:
| September 30, | September 30, | September 30, | ||||||||||
| 2010 | 2009 | Change | ||||||||||
| (in thousands) | ||||||||||||
Revenue, net | $ | 55,164 | $ | 24,172 | 128 | % | ||||||
|
|
|
| |||||||||
Approximately 73% of our product sales in 2010 were derived outside of the U.S. as compared to 59% in the prior year. The increase in the portion of our revenue derived from outside of the United States is due to the continued commercial rollout of the HeartWare System in Europe and other countries with additional implants at existing sites and the addition of new sites.
Cost of Revenue
Cost of revenue totaled approximately $24.4 million for 2010 and approximately $13.2 million for 2009.
Gross profit and gross margin percentage for the years ended December 31, 2010 and 2009 were as follows:
| September 30, | September 30, | |||||||
| 2010 | 2009 | |||||||
| (in thousands) | ||||||||
Gross profit | $ | 30,723 | $ | 10,961 | ||||
|
|
|
| |||||
Gross margin % | 56 | % | 45 | % | ||||
|
|
|
| |||||
The increase in gross margin was primarily due to the increase in unit production reducing the overhead and labor costs applied on a per unit basis.
48
Selling, General and Administrative
Selling, general and administrative expenses for the years ended December 31, 2010 and 2009 were as follows:
| September 30, | September 30, | September 30, | ||||||||||
| 2010 | 2009 | Change | ||||||||||
| (in thousands) | ||||||||||||
Total selling, general and administrative expenses | $ | 26,642 | $ | 16,444 | 62 | % | ||||||
|
|
|
| |||||||||
% of operating expenses | 45 | % | 52 | % | ||||||||
|
|
|
| |||||||||
During 2010, we experienced significant growth as we expanded European sales and distribution capabilities and increased the number of implants in the U.S. under clinical trials. We also experienced growth in administrative costs as we continued to manage dual listings of our securities in the United States and Australia, raised additional capital and expanded our administrative capabilities to support overall corporate growth. As a result, we experienced expansion of our staff, including in senior management, and overall growth in selling and marketing and administrative functions and experienced a related expansion in infrastructure costs.
The increase of $10.2 million in selling, general and administrative costs are primarily a result of an increase in share-based payment expense of $5.6 million, an increase in employee costs, including salaries and wages and related costs, of $3.7 million, and an increase in travel of $1.2 million. In addition, we experienced increases in costs of $0.5 million related to accounting and tax matters, consultants and contractors of $0.5 million, office expenses of $0.4 million, marketing expenses of $0.4 million and bad debt expense related to the establishment of a reserve for doubtful accounts of $0.6 million. Increases in costs were partially offset by a decrease in legal fees in 2010 as compared to 2009 of approximately $4.4 million associated with a failed merger transaction in 2009. The decrease in sales and marketing expenses as a percentage of operating expenses is due to R&D expenses comprising a higher percentage of overall costs as discussed below.
Research and Development
Research and development expenses for the years ended December 31, 2010 and 2009 were as follows:
| September 30, | September 30, | September 30, | ||||||||||
| 2010 | 2009 | Change | ||||||||||
| (in thousands) | ||||||||||||
Total research and development expenses | $ | 33,108 | $ | 15,067 | 120 | % | ||||||
|
|
|
| |||||||||
% of operating expenses | 55 | % | 48 | % | ||||||||
|
|
|
| |||||||||
As discussed above, we experienced significant growth in 2010. We achieved significant research and development milestones including enrolling a substantial number of patients in our U.S. clinical trial, developing three design configurations for the MVAD and furthering animal studies for less invasive implantable techniques related to the MVAD. The increase in research and development expenses, in total and as a percentage of total operating expenses, was primarily a result of increased clinical trial activity, continued development of next generation products, and costs associated with preparing the PMA application submission to the FDA.
The increase in research and development expenses of approximately $18.0 million is primarily due to an increase in costs associated with our U.S. clinical trials of $5.5 million, an increase in costs associated with development projects including outside engineering, consultants and contractors of $6.9 million related to MVAD development and preparation for our PMA application for HVAD and an increase in headcount and related employee costs, including salaries and wages and related costs, of approximately $2.8 million.
Foreign Exchange
Foreign exchange losses totaled approximately $0.5 million in the year ended December 31, 2010, as compared to a loss of approximately $0.3 million in 2009. In 2010, the majority of our realized and unrealized foreign exchange gains and losses were experienced upon the collection of certain accounts receivable that were denominated in foreign currencies, and the translation to U.S. dollars of customer accounts receivable denominated in foreign currencies at period end, primarily the Euro. In 2009, the majority of our foreign exchange gains and losses were due to re-measurement of our cash holdings denominated in U.S. dollars held by our Australian subsidiary as a result of movements in the exchange rate between the Australian dollar and the U.S. dollar. During the first half of 2009, we maintained the majority of our cash and cash equivalents in Australia, denominated in both Australian and U.S. dollars. However, beginning in the second half of 2009, the majority of our cash and cash equivalents are in U.S. dollars on deposit with banks located in the United States.
49
Interest Expense
Interest expense was approximately $0.4 million in 2010. Interest incurred on the principal amount of our convertible senior notes at the 3.5% coupon rate was approximately $0.2 million and non-cash amortization of the related discount and deferred financing costs totaled approximately $0.2 million. Interest expense was approximately $0.8 million in 2009, which consists of interest on the principal amount of certain convertible loans of approximately $0.1 million and non-cash amortization of the related deferred financing costs of approximately $0.7 million.
Interest expense in 2009 consists of interest incurred on the principal amount of convertible loans that were drawn down in August and December 2009 and amortization of deferred (non cash) financing costs related to these convertible loans. Interest on the convertible loans was payable at a rate of 10% per annum. The deferred financing costs were being amortized over the term of the associated loan agreement, which was set to expire no later than November 1, 2011. However, with the repayment of all amounts available to be borrowed under the terms of the loan agreement, we effectively extinguished all debt under this loan agreement. There was no such interest expense in 2010 as the convertible loans were repaid and the loan agreement terminated in the fourth quarter of 2009.
Investment Income, net
Investment income, net was approximately $0.6 million for the year ended December 31, 2010 as compared to approximately $0.1 million for the prior year. The increase was primarily due to higher cash balances during the 2010 period resulting from the capital raises completed in the second half of 2009 and in February 2010. However, we experienced lower interest rates in 2010 compared to 2009.
Change in Fair Value of Derivative Instrument
As further discussed in Note 7 to the accompanying consolidated financial statements, we recorded the fair value of an embedded derivative instrument on July 31, 2009 related to the Australian dollar denominated conversion feature in a loan agreement. We were required to initially record this derivative at fair value on July 31, 2009 and re-measure fair value at each reporting period. During the year ended December 31, 2009, we recognized aggregate non-cash expenses of approximately $3.9 million due to the increase in the fair value of this derivative between July 31, 2009 and December 29, 2009, the date the derivative was extinguished. This increase in fair value was primarily due to an increase in the fair value of our common stock and is non-cash in nature.
Gain on Early Extinguishment of Debt
As further discussed in Note 7 to the accompanying consolidated financial statements, in 2009, we recorded a net gain (non-cash) on the early extinguishment of debt. Due to the repayment of all amounts borrowed under the related loan agreement, our inability to re-borrow amounts repaid and the lender’s inability to convert any repaid amounts into shares of our common stock, the fair value of the derivative instrument at the repayment dates, aggregating approximately $7.8 million, was recorded as a gain on the extinguishment of debt. Further, the unamortized balance of the deferred financing costs at the repayment dates, aggregating approximately $3.2 million, was recorded as a reduction of the gain on early extinguishment of debt, resulting in a net gain on the early extinguishment of debt of $4.6 million for the year ended December 31, 2009. Since there are no funds held in escrow, there are no amounts remaining for us to borrow and no amounts remaining for the lender to convert into shares of our common stock. Therefore, the lender’s conversion rights, and thus the derivative, were eliminated.
Income Taxes
As of December 31, 2010, we did not have revenue or profit which would be sufficient to allow any portion of our deferred tax assets to be recorded and we have incurred losses since inception. Therefore, a 100% valuation allowance was recorded against our net deferred tax assets.
50
Liquidity and Capital Resources
As of December 31, 2011, our cash and cash equivalents were approximately $71.3 million as compared to $192.1 million at December 31, 2010.
Following is a summary of our cash flow activities for the years ended December 31, 2011 and 2010:
| September 30, | September 30, | |||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
Net cash used in operating activities | $ | (39,392 | ) | $ | (27,635 | ) | ||
Net cash used in investing activities | (82,459 | ) | (32,133 | ) | ||||
Net cash provided by financing activities | 1,055 | 200,926 | ||||||
Effect of exchange rate changes on cash and cash equivalents | (95 | ) | 155 | |||||
|
|
|
| |||||
Net (decrease) increase in cash and cash equivalents | $ | (120,891 | ) | $ | 141,313 | |||
|
|
|
| |||||
Cash Used in Operating Activities
Cash used in operating activities for the year ended December 31, 2011 included a net loss of approximately $55.1 million and non-cash adjustments to net loss totaling approximately $23.0 million, which primarily consisted of $13.2 million of share-based compensation, $5.4 million for the amortization of the discount on our convertible notes and $2.6 million of depreciation and amortization on long-lived assets. Also included in cash used in operating activities in 2011 are approximately $17.6 million for the purchase and manufacture of inventories and $2.1 million for prepaid expenses. These amounts were partially offset by net collections of trade accounts receivable of $3.6 million, an increase in other accrued liabilities of $5.4 million, an increase in deferred rent of $2.2 million and an increase in trade accounts payable of $1.1 million.
Cash used in operating activities for the year ended December 31, 2010 included a net loss of approximately $29.4 million and non-cash adjustments to net loss totaling approximately $13.4 million, which primarily consisted of $10.6 million of share-based compensation, and $1.5 million of depreciation and amortization. Also included in cash used in operating activities in 2010 is approximately $8.2 million related to an increase in accounts receivable and $6.7 million for the purchase and manufacture of inventories offset by cash provided by the increase in other current liabilities of approximately $3.0 million.
Cash Used in Investing Activities
In 2011, cash used in investing activities included $67.7 million for the purchase (net of maturities) of available-for sale securities, $12.8 million to acquire property, plant and equipment, including the build-out of our new manufacturing facility located in Miami Lakes, Florida.
In 2010, cash used in investing activities included $25.8 million for the purchase of available-for-sale securities, $4.6 million to acquire property, plant and equipment and $1.25 million of cash paid for a security deposit on a new facility lease.
Cash Provided by Financing Activities
During 2010, cash provided by financing activities was primarily the result of the net cash proceeds from the issuance of convertible senior notes and an offering of our common stock.
In December 2010, we issued Convertible Senior Notes with an aggregate principal amount of $143.75 million pursuant to the terms of an Indenture dated as of December 15, 2010, and received net cash proceeds of approximately $139.0 million. The Convertible Senior Notes are senior unsecured obligations of the Company. The Convertible Senior Notes bear interest at a rate of 3.5% per annum, payable semi-annually in arrears on June 15 and December 15 of each year. The Convertible Senior Notes will mature on December 15, 2017, unless earlier repurchased by the Company or converted.
51
In February 2010, we closed a public offering, under a shelf registration on Form S-3 filed with the Securities and Exchange Commission on December 24, 2009, of approximately 1.77 million shares of our common stock at an offering price of $35.50 per share for aggregate gross proceeds of approximately $62.8 million. This amount includes the underwriter’s exercise of their over-allotment option to purchase an additional 230,595 shares of our common stock at the offering price
In 2011 and 2010, we also received approximately $1.1 million and $3.6 million, respectively, from the exercise of stock options.
Operating Capital and Capital Expenditure Requirements
We have incurred operating losses to date and anticipate that we will continue to incur substantial net losses as we expand our sales and marketing capabilities, develop new products and seek regulatory approvals for the HeartWare System in the U.S. In 2012, cash on hand is expected to primarily be used to fund our ongoing operations, including;
| • | expanding our sales and marketing capabilities on a global basis, |
| • | continuing our ENDURANCE clinical trial for destination therapy, |
| • | enrolling additional patients in our ADVANCE trial under a CAP, |
| • | preparing for the launch of the HeartWare System in the U.S., |
| • | continued product development, including first human implants of the MVAD Pump, |
| • | regulatory and other compliance functions, including costs related to our PMA application, and |
| • | general working capital. |
We expect to experience increased cash requirements for inventory and other working capital requirements to support continued growth.
Our convertible notes bear interest at a rate of 3.5% per annum, payable semi-annually in arrears on June 15 and December 15 of each year. The $2.5 million interest payments that were due on June 15 and December 15, 2011 were paid. Based on the outstanding principal amount of our convertible senior notes at December 31, 2011, the semi-annual interest payments due on June 15 and December 15, 2012 will be approximately $2.5 million each. These amounts are expected to be paid from cash on hand.
We believe cash on hand and investment balances as of December 31, 2011 are sufficient to support our planned operations through 2012.
Because of the numerous risks and uncertainties associated with the development of medical devices and the current economic situation, we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to obtain regulatory approvals in the U.S., fund commercial expansion outside of the U.S. and develop new products. Our future capital requirements will depend on many factors, including but not limited to the following:
| • | commercial acceptance of our products; |
| • | costs to manufacture our products; |
| • | expenses required to operate multiple clinical trials; |
| • | further product research and development for next generation products and peripherals and expanding indications for our products as well as efforts to sustain and maintain incremental improvements to existing products; |
| • | expanding our sales and marketing capabilities on a global basis, including building a team to support U.S. commercialization should the FDA approve our device for marketing in the U.S.; |
| • | broadening our infrastructure in order to meet the needs of our growing operations; and |
| • | complying with the requirements related to being a public company in both the United States and Australia. |
52
Contractual Obligations
At December 31, 2011, our contractual financial obligations and commitments by due dates were as follows:
| September 30, | September 30, | September 30, | September 30, | September 30, | ||||||||||||||||
| Total | Less than 1 year | 1-3 years | 3-5 years | Thereafter | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Convertible senior notes | $ | 173,938 | $ | 5,031 | $ | 10,063 | $ | 10,063 | $ | 148,781 | ||||||||||
Operating lease obligations | 18,378 | 2,211 | 3,973 | 2,783 | 9,411 | |||||||||||||||
Purchase obligations | 24,388 | 22,610 | 558 | 592 | 628 | |||||||||||||||
Other | 10,677 | 4,825 | 4,082 | 1,770 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total | $ | 227,381 | $ | 34,677 | $ | 18,676 | $ | 15,208 | $ | 158,820 | ||||||||||
As of January 15, 2011, we entered into a Public, Private Partnership Agreement with the Regents of the University of Michigan whereby we act as industry sponsor of a study being conducted by the University of Michigan Cardiovascular Center and the University of Pittsburgh exploring the potential benefits of VADs in patients who will be given earlier access to these devices under a grant awarded from the National Heart, Lung and Blood Institute. In the study, called REVIVE-IT, researchers will compare whether non-transplant eligible patients with heart failure less advanced than that of current VAD recipients do better with implanted devices than with current medical therapy. Pursuant to the terms of the agreement, we have committed to provide financial support up to $9.6 million over the five-year trial period. Through December 31, 2011, the total amount presented to us for payment was approximately $0.2 million. We have included the balance of this financial commitment in the above table based on the estimated timing of receipt of the requests for financial support, however, these estimates could change.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of changes in the value of market risk sensitive instruments caused by fluctuations in interest rates, foreign exchange rates and commodity prices. Changes in these factors could cause fluctuations in our results of operations and cash flows.
Interest Rate Risk
Our exposure to interest rate risk is currently confined to interest earnings on our cash and cash equivalents that are invested in highly liquid money market funds, short-term time deposits, short-term bank notes and short-term commercial paper. The primary objective of our investment activities is to preserve our capital to fund operations. We also seek to generate reasonable income from our investments without assuming significant risk. We do not presently use derivative financial instruments in our investment portfolio. Our cash and investments policy emphasizes liquidity and preservation of principal over other portfolio considerations.
If interest rates rise, the market value of our investment portfolio may decline, which could result in a loss if we choose or are forced to sell an investment before its scheduled maturity. We do not utilize derivative financial instruments to manage interest rate risks.
Our convertible notes do not bear interest rate risk as the notes were issued with a fixed interest rate of 3.5% per annum.
53
Foreign Currency Rate Fluctuations
We conduct business in foreign countries. For U.S. reporting purposes, we translate all assets and liabilities of our non-U.S. entities at the period-end exchange rate and revenue and expenses at the average exchange rates in effect during the periods. The net effect of these translation adjustments is shown in the accompanying consolidated financial statements as a component of stockholders’ equity.
We generate a significant portion of our revenue and collect receivables in foreign currencies. Fluctuations in the exchange rate of the U.S. dollar against major foreign currencies, including the Euro, British Pound and Australian dollar can result in foreign currency exchange gains and losses that may significantly impact our financial results. These foreign currency transaction and translation gains and losses are presented as a separate line item on our consolidated statements of operations. Continued fluctuation of these exchange rates could result in financial results that are not comparable from quarter to quarter. We do not currently utilize foreign currency contracts to mitigate the gains and losses generated by the re-measurement of non-functional currency assets and liabilities but do hold cash reserves in currencies in which those reserves are anticipated to be expended.
54
Item 8. Financial Statements and Supplementary Data
HEARTWARE INTERNATIONAL, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||
Financial Statements: | ||
| 56 | ||
| 57 | ||
| 58 | ||
| 59 | ||
| 60 | ||
| 61 | ||
| 62 | ||
55
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
HeartWare International, Inc.
We have audited the accompanying consolidated balance sheets of HeartWare International, Inc. (a Delaware corporation) and subsidiaries (the Company) as of December 31, 2011 and 2010, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of HeartWare International, Inc. and subsidiaries as of December 31, 2011 and 2010, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2011 in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2011, based on criteria established inInternal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated February 27, 2012 expressed an unqualified opinion.
/s/ Grant Thornton LLP
Fort Lauderdale, Florida
February 27, 2012
56
HEARTWARE INTERNATIONAL, INC.
(In thousands, except per share data)
| September 30, | September 30, | |||||||
| December 31, | ||||||||
| 2011 | 2010 | |||||||
ASSETS | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 71,257 | $ | 192,148 | ||||
Short-term investments, net | 91,925 | 21,330 | ||||||
Accounts receivable, net | 14,953 | 19,053 | ||||||
Inventories, net | 32,005 | 15,077 | ||||||
Prepaid expenses and other current assets | 4,507 | 2,406 | ||||||
|
|
|
| |||||
Total current assets | 214,647 | 250,014 | ||||||
Property, plant and equipment, net | 18,325 | 7,484 | ||||||
Long-term investments, net | — | 4,006 | ||||||
Other intangible assets, net | 2,014 | 1,596 | ||||||
Deferred financing costs, net | 2,653 | 2,939 | ||||||
Other assets | 3,093 | 1,538 | ||||||
|
|
|
| |||||
Total assets | $ | 240,732 | $ | 267,577 | ||||
|
|
|
| |||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 5,025 | $ | 3,890 | ||||
Other accrued liabilities | 12,436 | 7,001 | ||||||
|
|
|
| |||||
Total current liabilities | 17,461 | 10,891 | ||||||
Convertible senior notes, net | 94,277 | 88,922 | ||||||
Other long-term liabilities | 2,210 | — | ||||||
Commitments and contingencies – See Note 14 | ||||||||
Stockholders’ equity: | ||||||||
Preferred stock—$.001 par value; 5,000 shares authorized; no shares issued and outstanding at December 31, 2011 and 2010, respectively | — | — | ||||||
Common stock—$.001 par value; 25,000 shares authorized; 14,114 and 13,879 shares issued and outstanding at December 31, 2011 and 2010, respectively | 14 | 14 | ||||||
Additional paid-in capital | 316,748 | 302,533 | ||||||
Accumulated deficit | (182,324 | ) | (127,269 | ) | ||||
Accumulated other comprehensive loss: | ||||||||
Cumulative translation adjustments | (7,631 | ) | (7,548 | ) | ||||
Unrealized gain on investments | (23 | ) | 34 | |||||
|
|
|
| |||||
Total accumulated other comprehensive loss | (7,654 | ) | (7,514 | ) | ||||
|
|
|
| |||||
Total stockholders’ equity | 126,784 | 167,764 | ||||||
|
|
|
| |||||
Total liabilities and stockholders’ equity | $ | 240,732 | $ | 267,577 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these financial statements.
57
HEARTWARE INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| September 30, | September 30, | September 30, | ||||||||||
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
Revenue, net | $ | 82,764 | $ | 55,164 | $ | 24,172 | ||||||
Cost of revenue | 32,932 | 24,441 | 13,211 | |||||||||
|
|
|
|
|
| |||||||
Gross profit | 49,832 | 30,723 | 10,961 | |||||||||
Operating expenses: | ||||||||||||
Selling, general and administrative | 42,314 | 26,642 | 16,444 | |||||||||
Research and development | 50,149 | 33,108 | 15,067 | |||||||||
|
|
|
|
|
| |||||||
Total operating expenses | 92,463 | 59,750 | 31,511 | |||||||||
Loss from operations | (42,631 | ) | (29,027 | ) | (20,550 | ) | ||||||
Other income (expense): | ||||||||||||
Foreign exchange loss | (2,283 | ) | (503 | ) | (285 | ) | ||||||
Interest expense | (10,673 | ) | (431 | ) | (820 | ) | ||||||
Investment income, net | 532 | 564 | 100 | |||||||||
Change in fair value of derivative instrument | — | — | (3,935 | ) | ||||||||
Gain on early extinguishment of debt, net | — | — | 4,606 | |||||||||
Other, net | — | — | (25 | ) | ||||||||
|
|
|
|
|
| |||||||
Loss before income taxes | (55,055 | ) | (29,397 | ) | (20,909 | ) | ||||||
Provision for income taxes | — | — | — | |||||||||
|
|
|
|
|
| |||||||
Net loss | $ | (55,055 | ) | $ | (29,397 | ) | $ | (20,909 | ) | |||
|
|
|
|
|
| |||||||
Net loss per common share — basic and diluted | $ | (3.94 | ) | $ | (2.17 | ) | $ | (2.15 | ) | |||
|
|
|
|
|
| |||||||
Weighted average shares outstanding — basic and diluted | 13,959 | 13,570 | 9,714 | |||||||||
|
|
|
|
|
| |||||||
The accompanying notes are an integral part of these financial statements.
58
HEARTWARE INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
| September 30, | September 30, | September 30, | ||||||||||
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
Net loss | $ | (55,055 | ) | $ | (29,397 | ) | $ | (20,909 | ) | |||
Foreign currency translation adjustments | (83 | ) | 307 | 836 | ||||||||
Unrealized (loss) gain on investments | (57 | ) | 34 | — | ||||||||
|
|
|
|
|
| |||||||
Comprehensive loss | $ | (55,195 | ) | $ | (29,056 | ) | $ | (20,073 | ) | |||
|
|
|
|
|
| |||||||
The accompanying notes are an integral part of these financial statements.
59
HEARTWARE INTERNATIONAL, INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
(In thousands, except per share data)
| September 30, | September 30, | September 30, | September 30, | September 30, | September 30, | |||||||||||||||||||
| Common Shares, $.001 Par Value Per Share | Additional | Accumulated Other | ||||||||||||||||||||||
| Shares Issued | Amount | Paid-In Capital | Accumulated Deficit | Comprehensive Loss | Total | |||||||||||||||||||
Balance, December 31, 2008 | 8,867 | $ | 9 | $ | 112,401 | $ | (76,963 | ) | $ | (8,691 | ) | $ | 26,756 | |||||||||||
Issuance of common stock pursuant to private placement, net of offering costs | 2,737 | 3 | 58,619 | — | — | 58,622 | ||||||||||||||||||
Issuance of common stock pursuant to share-based awards | 182 | — | 1,546 | — | — | 1,546 | ||||||||||||||||||
Share-based compensation | — | — | 4,132 | — | — | 4,132 | ||||||||||||||||||
Net loss | — | — | — | (20,909 | ) | — | (20,909 | ) | ||||||||||||||||
Other comprehensive loss: | ||||||||||||||||||||||||
Foreign currency translation adjustment | — | — | — | — | 836 | 836 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balance, December 31, 2009 | 11,786 | 12 | 176,698 | (97,872 | ) | (7,855 | ) | 70,983 | ||||||||||||||||
Issuance of common stock pursuant to public offering, net of offering costs | 1,768 | 2 | 58,487 | — | — | 58,489 | ||||||||||||||||||
Issuance of common stock pursuant to share-based awards | 325 | — | 3,556 | — | — | 3,556 | ||||||||||||||||||
Allocation of fair value of equity component of convertible notes | — | — | 55,038 | — | — | 55,038 | ||||||||||||||||||
Allocation of a portion of convertible notes issuance costs to equity component of convertible notes | — | — | (1,831 | ) | — | — | (1,831 | ) | ||||||||||||||||
Share-based compensation | — | — | 10,585 | — | — | 10,585 | ||||||||||||||||||
Net loss | — | — | — | (29,397 | ) | — | (29,397 | ) | ||||||||||||||||
Other comprehensive loss: | ||||||||||||||||||||||||
Foreign currency translation adjustment | — | — | — | — | 307 | 307 | ||||||||||||||||||
Unrealized gain on investments | — | — | — | — | 34 | 34 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balance, December 31, 2010 | 13,879 | 14 | 302,533 | (127,269 | ) | (7,514 | ) | 167,764 | ||||||||||||||||
Issuance of common stock pursuant to share-based awards | 235 | — | 1,056 | — | — | 1,056 | ||||||||||||||||||
Share-based compensation | — | — | 13,159 | — | — | 13,159 | ||||||||||||||||||
Net loss | — | — | — | (55,055 | ) | — | (55,055 | ) | ||||||||||||||||
Other comprehensive loss: | ||||||||||||||||||||||||
Foreign currency translation adjustment | — | — | — | — | (83 | ) | (83 | ) | ||||||||||||||||
Unrealized loss on investments | — | — | — | — | (57 | ) | (57 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balance, December 31, 2011 | 14,114 | $ | 14 | $ | 316,748 | $ | (182,324 | ) | $ | (7,654 | ) | $ | 126,784 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
The accompanying notes are an integral part of these financial statements.
60
HEARTWARE INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| September 30, | September 30, | September 30, | ||||||||||
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||||||
Net loss | $ | (55,055 | ) | $ | (29,397 | ) | $ | (20,909 | ) | |||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
Depreciation of property, plant and equipment | 2,457 | 1,390 | 895 | |||||||||
Amortization of intangible assets | 136 | 102 | 76 | |||||||||
Share-based compensation expense | 13,159 | 10,585 | 4,132 | |||||||||
Bad debt expense | 473 | 600 | — | |||||||||
Amortization of premium on investments | 1,018 | 501 | — | |||||||||
Amortization of discount on convertible senior notes | 5,355 | 209 | — | |||||||||
Amortization of deferred financing costs | 287 | 11 | 671 | |||||||||
Change in fair value of derivative instrument | — | — | 3,935 | |||||||||
Gain on early extinguishment of debt, net | — | — | (4,606 | ) | ||||||||
Other | 73 | — | 25 | |||||||||
Change in operating assets and liabilities: | ||||||||||||
Accrued interest on convertible notes | — | 210 | — | |||||||||
Accounts receivable | 3,627 | (8,156 | ) | (11,140 | ) | |||||||
Inventories, net | (17,602 | ) | (6,741 | ) | (5,363 | ) | ||||||
Prepaid expenses and other current assets | (2,100 | ) | (733 | ) | (644 | ) | ||||||
Accounts payable | 1,135 | 766 | 2,523 | |||||||||
Other accrued liabilities | 5,435 | 3,018 | 863 | |||||||||
Other long-term liabilities | 2,210 | — | — | |||||||||
|
|
|
|
|
| |||||||
Net cash used in operating activities | (39,392 | ) | (27,635 | ) | (29,542 | ) | ||||||
CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||||||
Purchases of investments | (192,290 | ) | (25,802 | ) | — | |||||||
Maturities of investments | 123,125 | — | — | |||||||||
Additions to property, plant and equipment, net | (12,684 | ) | (4,576 | ) | (1,029 | ) | ||||||
Additions to patents | (554 | ) | (505 | ) | (444 | ) | ||||||
Cash paid for security deposits | (56 | ) | (1,250 | ) | — | |||||||
|
|
|
|
|
| |||||||
Net cash used in investing activities | (82,459 | ) | (32,133 | ) | (1,473 | ) | ||||||
CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||||||
Proceeds from issuance of common stock | — | 62,760 | 60,220 | |||||||||
Payment of common stock issuance costs | (1 | ) | (4,360 | ) | (1,510 | ) | ||||||
Proceeds from issuance of convertible debt | — | 143,750 | 20,000 | |||||||||
Repayment of convertible debt | — | — | (20,000 | ) | ||||||||
Payment of convertible debt issuance costs | — | (4,781 | ) | — | ||||||||
Proceeds from exercise of stock options | 1,056 | 3,557 | 1,547 | |||||||||
|
|
|
|
|
| |||||||
Net cash provided by financing activities | 1,055 | 200,926 | 60,257 | |||||||||
Effect of exchange rate changes on cash and cash equivalents | (95 | ) | 155 | 789 | ||||||||
|
|
|
|
|
| |||||||
CHANGE IN CASH AND CASH EQUIVALENTS | (120,891 | ) | 141,313 | 30,031 | ||||||||
CASH AND CASH EQUIVALENTS — BEGINNING OF PERIOD | 192,148 | 50,835 | 20,804 | |||||||||
|
|
|
|
|
| |||||||
CASH AND CASH EQUIVALENTS — END OF PERIOD | $ | 71,257 | $ | 192,148 | $ | 50,835 | ||||||
|
|
|
|
|
| |||||||
Supplemental disclosure of cash flow information: | ||||||||||||
Interest paid | $ | 5,031 | $ | — | $ | 149 | ||||||
|
|
|
|
|
| |||||||
Supplemental disclosure of non-cash investing and financing activities: | ||||||||||||
Transfers from inventory to property, plant and equipment | $ | 585 | $ | 535 | $ | — | ||||||
|
|
|
|
|
| |||||||
Recognition of fair value of derivative instrument | $ | — | $ | — | $ | 3,891 | ||||||
|
|
|
|
|
| |||||||
The accompanying notes are an integral part of these financial statements.
61
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
Note 1. Description of Business
HeartWare International, Inc., referred to in these notes collectively with its subsidiaries HeartWare Pty. Limited, HeartWare, Inc., HeartWare (UK) Limited, HeartWare GmbH and HeartWare France as “we,” “our,” “HeartWare” or the “Company”, is a medical device company that develops and manufactures miniaturized implantable heart pumps, or ventricular assist devices, to treat patients suffering from advanced heart failure.
The HeartWare Ventricular Assist System (the “HeartWare System”), which includes a ventricular assist device (“VAD”), or blood pump, patient accessories and surgical tools, is designed to provide circulatory support for patients in the advanced stage of heart failure. The core of the HeartWare System is a proprietary continuous flow blood pump, the HVAD Pump, which is a full-output device capable of pumping up to 10 liters of blood per minute. The HeartWare System is designed to be implanted adjacent to the heart, avoiding the abdominal surgery generally required to implant similar devices.
In 2009, we received CE Marking for the HeartWare System in the European Union allowing for commercial sale and distribution of our device. In the U.S., the device is the subject of clinical trials for two indications: bridge-to-transplant and destination therapy. Our device is also available in other countries around the world under special access programs. In February 2012, we were notified that a FDA Circulatory System Devices Panel of the Medical Devices Advisory Committee will review our application on April 25, 2012.
Beyond the HeartWare System, we are also evaluating our next generation device, the MVAD Pump. The MVAD Pump is a development-stage miniature ventricular assist device, approximately one-third the size of the HVAD Pump. The MVAD Pump is based on the same proprietary impeller suspension technology used in the HVAD Pump, with its single moving part held in place through a combination of passive-magnetic and hydrodynamic forces. Like the HVAD Pump, the MVAD Pump is designed to support the heart’s full cardiac output, yet also has the capability for partial support. We are currently preparing to commence human clinical studies in 2012. The MVAD Pump is designed to be implantable by surgical techniques that are even less invasive than those required to implant the HVAD Pump.
We are headquartered in Framingham, Massachusetts. We have operations and manufacturing facilities in Miami Lakes, Florida, a development and operations facility in Sydney, Australia and a distribution and customer service facility in Hannover, Germany.
HeartWare International, Inc. shares trade on the NASDAQ Stock Market under the symbol of “HTWR”. The Company’s shares also trade on the Australian Securities Exchange (“ASX”) in the form of CHESS Depositary Interests (“CDIs”) under the symbol of “HIN”. Each CDI represents 1/35th of a share of common stock.
Note 2. Liquidity
At December 31, 2011, we had approximately $163.2 million of cash, cash equivalents and investments. The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States, which contemplate continuation of the Company as a going concern. We have incurred substantial losses from operations since our inception, and such losses have continued through December 31, 2011. At December 31, 2011, we had an accumulated deficit of approximately $182.3 million.
We have financed our operations primarily through the issuance of shares of our common stock and the issuance of convertible notes. Most recently, in December 2010, we consummated the issuance and sale of $143.75 million aggregate principal amount of convertible notes. The convertible notes are the senior unsecured obligations of the Company. The convertible notes bear interest at a rate of 3.5% per annum, payable semi-annually in arrears on June 15 and December 15 of each year. The convertible notes will mature on December 15, 2017, unless earlier repurchased or converted. The convertible notes will be convertible at an initial conversion rate of 10 shares of common stock per $1,000 principal amount of convertible notes, which corresponds to an initial conversion price of $100.00 per share of common stock.
In 2012, our cash, cash equivalents and investments are expected to primarily be used to fund our ongoing operations including expanding our sales and marketing capabilities on a global basis, continuing our
62
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
ENDURANCE clinical trial for destination therapy, enrolling additional patients in our ADVANCE trial under a Continued Access Protocol (“CAP”), preparing for the launch of the HeartWare System in the United States, research and development of new products, regulatory and other compliance functions, including costs related to our Pre-Market Approval application, as well as for general working capital. We believe our cash, cash equivalents and investment balances are sufficient to support our planned operations through 2012.
Note 3. Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of HeartWare International, Inc., and its subsidiaries described in Note 1. All inter-company balances and transactions have been eliminated in consolidation.
Accounting Estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States (“U.S. GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting periods. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash and cash equivalents are recorded in the consolidated balance sheets at cost, which approximates fair value. All highly liquid investments purchased with an original maturity of three months or less at the date of purchase are considered to be cash equivalents.
Investments
Our investments classified as available-for-sale are stated at fair value with unrealized gains and losses reported in accumulated other comprehensive loss within stockholders’ equity. We classify our available-for-sale investments as short-term if their remaining time to maturity at purchase is beyond three months. Investments with maturities beyond one year may be classified as short-term based on their highly liquid nature and because such marketable securities represent the investment of cash that is available for current operations. Interest on investments classified as available-for-sale is included in investment income, net. Premiums paid on our short-term investments are amortized over the remaining term of the investment and such amortization is included in investment income, net.
Receivables
Accounts receivable consists of amounts due from the sale of our HeartWare System to our customers, which include hospitals, health research institutions and medical device distributors. We grant credit to customers in the normal course of business, but do not require collateral or any other security to support credit sales. Our receivables are geographically dispersed, with a significant portion from customers located in Europe and other foreign countries. At December 31, 2011, no customer had an accounts receivable balance greater than 10% of our total accounts receivable. At December 31, 2010, one customer had an accounts receivable balance greater than 10% of total accounts receivable representing approximately 13% of our total accounts receivable.
We maintain allowances for doubtful accounts for estimated losses that may result from an inability to collect payments owed to us for product sales. We regularly review the allowance by considering factors such as historical experience, the age of the accounts receivable balances and current economic conditions that may affect a customer’s ability to pay. Account balances are charged off against the allowance after appropriate collection efforts are exhausted and we feel it is probable that the receivable will not be recovered.
The following table summarizes the change in our allowance for doubtful accounts for the years ended December 31, 2011 and 2010:
63
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
| September 30, | September 30, | |||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
Beginning balance | $ | 600 | $ | — | ||||
Additions (bad debt expense) | 473 | 600 | ||||||
Deductions (charge-offs) | (573 | ) | — | |||||
|
|
|
| |||||
Ending balance | $ | 500 | $ | 600 | ||||
|
|
|
| |||||
As of December 31, 2011 and 2010, we did not have an allowance for returns.
Inventories, net
Inventories are stated at the lower of cost or market. Cost is determined using a first-in, first-out, or FIFO, method. Work-in-process and finished goods manufactured or assembled by us include direct and indirect labor and manufacturing overhead. Finished goods include product which is ready-for-use and which is held by us or by our customers on a consignment basis.
We review our inventory for excess or obsolete inventory and write-down obsolete or otherwise unmarketable inventory to its estimated net realizable value. Obsolescence may occur due to product expiring or product improvements rendering previous versions obsolete. In May 2011, we began shipping a sintered version of the HeartWare System on a global basis. Sintering is a process whereby minute beads are adhered to a titanium surface commonly used in medical devices to facilitate tissue adhesion. The extent to which this product enhancement will cause obsolescence of existing non-sintered inventory is difficult to determine at this time as the rate of customer acceptance is dependent on many factors and our ability to provide sintered product commercially in the U.S. is subject to FDA approval. During the fourth quarter of 2011, we recorded a write-down of $0.6 million against our existing non-sintered inventory, leaving a balance of $1.0 million as of December 31, 2011. This product continues to be implanted at select customer sites. However, a write-down of all or a portion of this remaining inventory as obsolete could have a material impact on our results of operations.
Components of inventories, net at December 31, 2011 and 2010 are as follows:
| September 30, | September 30, | |||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
Raw material | $ | 8,318 | $ | 4,279 | ||||
Work-in-process | 7,385 | 2,709 | ||||||
Finished goods | 16,302 | 8,089 | ||||||
|
|
|
| |||||
| $ | 32,005 | $ | 15,077 | |||||
|
|
|
| |||||
Finished goods inventories includes inventory held on consignment at customer sites of $7.2 million and $4.7 million, at December 31, 2011 and 2010, respectively.
Property, Plant and Equipment
We record property, plant and equipment and leasehold improvements at historical cost. Expenditures for maintenance and repairs are recorded to expense; additions and improvements are capitalized. We generally provide for depreciation using the straight-line method at rates that approximate the estimated useful lives of the assets. Leasehold improvements are amortized on a straight-line basis over the shorter of the useful life of the improvement or the remaining term of the lease.
Property, plant and equipment, net consists of the following at December 31, 2011 and 2010:
| September 30, | September 30, | September 30, | ||||||||||
| Estimated Useful Lives | 2011 | 2010 | ||||||||||
Property, plant and equipment | (in thousands) | |||||||||||
Machinery and equipment | 1.5 to 7 years | $ | 14,951 | $ | 8,967 | |||||||
Leasehold improvements | 3 to 10 years | 5,747 | 282 | |||||||||
Office equipment, furniture and fixtures | 5 to 7 years | 1,249 | 451 | |||||||||
Purchased software | 5 to 7 years | 2,733 | 1,741 | |||||||||
|
|
|
| |||||||||
| 24,680 | 11,441 | |||||||||||
Less: accumulated depreciation | (6,355 | ) | (3,957 | ) | ||||||||
|
|
|
| |||||||||
| $ | 18,325 | $ | 7,484 | |||||||||
|
|
|
| |||||||||
64
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
Depreciation expense was $2.5 million, $1.4 million and $0.9 million for the years ended December 31, 2011, 2010 and 2009, respectively.
We enter into agreements with medical centers participating in our U.S. clinical trials under which we loan certain equipment, including patient monitors, to the center to be used throughout the trials. The equipment loaned to the centers is classified as a long-lived asset and included as a component of property, plant and equipment (machinery and equipment) on our consolidated balance sheets. Depreciation expense on equipment subject to these agreements is classified in cost of revenue and is computed using the straight-line method based on the estimated useful life of three years.
We also enter into short-term cancellable rental agreements with certain commercial customers for components of the HeartWare System, including patient monitors and controllers. Under the terms of such agreements, we provide the equipment to the customers, but we retain title to the equipment. Equipment subject to rental agreements is classified as a long-lived asset and included as a component of property, plant and equipment (machinery and equipment). Depreciation expense on equipment subject to these agreements is classified in cost of revenue and is computed using the straight-line method based on the estimated useful life of one and one half years.
The net carrying value of equipment subject to the agreements discussed above was approximately $0.7 million and $0.5 million as of December 31, 2011 and 2010, respectively.
Deferred Financing Costs
Costs incurred in connection with the issuance of our convertible senior notes have been allocated between the liability component and the equity component as further discussed in Note 7. The liability component of the issuance costs incurred was capitalized and is included in deferred financing costs, net on our consolidated balance sheets. These costs are being amortized using the effective interest method through December 15, 2017, the maturity date of the notes, and such amortization expense is reflected in interest expense on our consolidated statements of operations. The amount of amortization for the years ended December 31, 2011 and 2010 was approximately $0.3 million and $0.01 million, respectively. The amount of accumulated amortization at December 31, 2011 and 2010 was approximately $0.3 million and $0.01 million, respectively.
Other Intangible Assets, net
The gross carrying amount of intangible assets and the related accumulated amortization for intangible assets subject to amortization at December 31, 2011 and 2010 are as follows:
| September 30, | September 30, | September 30, | September 30, | September 30, | ||||||||||||||||
| 2011 | 2010 | |||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Amortizable Intangible Assets | Weighted Average Life (Years) | Gross Carrying Amount | Accumulated Amortization | Gross Carrying Amount | Accumulated Amortization | |||||||||||||||
Patents | 15 | $ | 2,416 | $ | (402 | ) | $ | 1,862 | $ | (266 | ) | |||||||||
Amortization expense for the years ended December 31, 2011, 2010 and 2009 was $0.1 million for each year.
Estimated amortization expense for each of the five succeeding fiscal years based upon our intangible asset portfolio at December 31, 2011 is approximately $0.2 million.
Revenue Recognition
We recognize revenue from product sales in accordance with FASB ASC 605 –Revenue Recognition. Pursuant to agreements or orders from customers, we ship product to our customers. Revenue from product sales is only recognized when persuasive evidence of an arrangement exists, substantially all the risks and rewards of ownership have transferred to our customers, the selling price is fixed and collection is reasonably assured. A majority of
65
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
product sales are initially made on a consignment basis and as such, pursuant to the terms of the consignment arrangements, revenue is recognized on the date the consigned product is implanted or otherwise consumed. Revenue from product sales not sold on a consignment basis is recognized upon customer receipt and acceptance of the product. Shipping fees billed to customers are included in revenue and the related shipping costs are included in cost of revenue. Value added taxes and other similar types of taxes collected from customers in connection with the sale of our products are recorded on a net basis and are not included in revenue. Revenue recognized to date is from sales of our devices in connection with our U.S. clinical trials, as commercial products to customers in Europe and under special access in other countries.
Product Warranty
Certain patient accessories sold with the HeartWare System are covered by a limited warranty ranging from one to two years. Estimated contractual warranty obligations are recorded as an expense when the related revenue is recognized and are included in cost of revenue on our consolidated statements of operations. Factors that affect estimated warranty liability include the number of units sold, historical and anticipated rates of warranty claims, cost per claim, and vendor supported warranty programs. We periodically assess the adequacy of our recorded warranty liabilities and adjust the amounts as necessary.
The amount of the liability recorded is equal to the estimated costs to repair or otherwise satisfy claims made by customers. Accrued warranty expense is included as a component of other accrued liabilities on the consolidated balance sheets.
The costs to repair or replace products associated with product recalls and voluntary service campaigns are recorded when they are determined to be probable and reasonably estimable as a cost of revenue and are not included in product warranty liability. No such costs were incurred in 2011 and approximately $0.4 million was incurred in 2010.
The following table summarizes the change in our warranty liability for the years ended December 31, 2011 and 2010:
| September 30, | September 30, | September 30, | ||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
Beginning balance | $ | 291 | $ | 99 | $ | — | ||||||
Accrual for (reversal of) warranty expense | (48 | ) | 312 | 99 | ||||||||
Warranty costs incurred during the period | (40 | ) | (120 | ) | — | |||||||
|
|
|
|
|
| |||||||
Ending balance | $ | 203 | $ | 291 | $ | 99 | ||||||
|
|
|
|
|
| |||||||
Share-Based Compensation
We recognize share-based compensation expense in connection with our share-based awards, net of an estimated forfeiture rate and therefore only recognize compensation cost for those awards expected to vest over the service period of the award. We value restricted stock units (“RSU’s”) at their intrinsic value on the date of grant. We use a Black-Scholes option pricing model to estimate the fair value of our stock options. Calculating share-based compensation expense requires the input of highly subjective judgment and assumptions, including estimates of expected life of the award, stock price volatility, forfeiture rates and risk-free interest rates. The assumptions used in calculating the fair value of share-based awards represent our best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and we use different assumptions, our share-based compensation expense could be materially different in the future.
When appropriate, we estimate the expected life of an option by averaging the contractual term of the stock option grants (up to 10 years) with the associated vesting term (typically 4 years).We estimate the volatility of our shares on the date of grant considering several factors, including the historical volatility of our publicly-traded shares. We estimate the forfeiture rate based on our historical experience of forfeitures and our employee retention rate. If our actual forfeiture rate is materially different from our estimate, share-based compensation expense could be significantly different from what we have recorded in the current period. We estimate the risk-free interest rate based on rates in effect for United States government bonds with similar lives, at the time of grant.
66
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
We have issued share-based awards with performance-based vesting criteria. Achievement of the milestones must be “probable” before we begin recording share-based compensation expense. At each reporting date, we review the likelihood that these awards will vest and if the vesting is deemed probable, we begin to recognize compensation expense at that time. If ultimately performance goals are not met, for any awards where vesting was previously deemed probable, previously recognized compensation cost will be reversed.
Impairment of Long-Lived Assets
We review our long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable from future undiscounted cash flows. Impairment losses are recorded for the excess, if any, of the carrying value over the fair value of the long-lived assets. As of December 31, 2011, none of our long-lived assets were impaired.
Income Taxes
We account for income taxes in accordance with FASB ASC 740– Income Taxes. Under this method, deferred tax assets and liabilities are provided for differences between the carrying amounts of assets and liabilities in the consolidated financial statements and the tax bases of assets and liabilities that will result in future taxable or deductible amounts. The deferred tax assets and liabilities are measured using the enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Income tax expense is computed as the tax payable or refundable for the period, plus or minus the change during the period in deferred tax assets and liabilities. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred taxes will not be realized.
FASB ASC 740 requires that we recognize the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the “more likely than not” threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority.
Translation of Foreign Currency
Assets and liabilities of our non-U.S. entities are translated at the period-end exchange rate and revenue and expenses are translated at the average exchange rates in effect during the respective periods. Equity transactions are translated at the spot rates on the dates of the original transactions. The net effect of these translation adjustments is shown in the accompanying consolidated financial statements as a component of stockholders’ equity, titled accumulated other comprehensive loss. Items in accumulated other comprehensive loss are not tax affected as we have incurred a net loss in each period since inception.
While most of the transactions of our domestic and international operations are denominated in the respective local currency, some transactions are denominated in other currencies. Transactions denominated in other currencies are accounted for in the respective local currency at the time of the transaction. Upon settlement of this type of transaction, any foreign currency gains or losses are included in our consolidated statements of operations.
Research and Development
Research and development costs, including new product development programs, regulatory compliance and clinical research, are expensed as incurred.
Marketing and Advertising Costs
Marketing, advertising and promotional costs are expensed when incurred. Advertising expenses were immaterial to our results of operations for the years ended December 31, 2011, 2010 and 2009.
67
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
Leases
We lease all of our administrative and manufacturing facilities. We recognize rent expense on a straight-line basis over the terms of our leases. Any scheduled rent increases, rent holidays and other related incentives are recognized on a straight-line basis over the terms of the leases. The difference between the cash rental payments and the straight-line recognition of rent expense over the terms of the leases results in a deferred rent asset or liability. As of December 31, 2011, the long-term portion of our deferred rent liability of approximately $2.2 million is included in other long-term liabilities on our consolidated balance sheets.
Fair Value Measurements
The carrying amounts reported in the consolidated balance sheets for cash and cash equivalents, accounts receivable, accounts payable and other current liabilities approximate their fair value based on the short-term maturity of these instruments. Investments are considered available-for-sale as of December 31, 2011 and 2010 and are carried at fair value. See Note 5, “Fair Value Measurements” and Note 7, “Debt” for more information.
Vendor Concentration
For the years ended December 31, 2011, 2010 and 2009, we purchased approximately 63%, 66% and 60%, respectively, of our inventory components and supplies from three vendors. In addition, one of the three vendors supplies consulting services and material used in research and development activities. As of December 31, 2011 and 2010, the amounts due to these vendors totaled approximately $1.2 million and $1.8 million, respectively.
Concentration of Credit Risk and other Risks and Uncertainties
Financial instruments that potentially expose us to concentrations of credit risk consist primarily of cash and cash equivalents, investments and trade accounts receivable. Cash and cash equivalents are primarily on deposit with financial institutions in the United States and these deposits generally exceed the amount of insurance provided by the Federal Deposit Insurance Corporation (the “FDIC”). The Company has not experienced any historical losses on its deposits of cash and cash equivalents. Our investments consist of investment grade rated corporate and government agency debt.
Concentration of credit risk with respect to our trade accounts receivable from our customers is primarily limited to hospitals, health research institutions and medical device distributors. Credit is extended to our customers based on an evaluation of a customer’s financial condition, and collateral is not required.
We are subject to certain risks and uncertainties including, but not limited to, our ability to achieve profitability, to generate cash flow sufficient to satisfy our indebtedness, to run clinical trials in order to receive and maintain FDA and foreign regulatory approvals for our products, the ability to achieve widespread acceptance of our products, our ability to manufacture our products in a sufficient volume and at a reasonable cost, the ability to protect our proprietary technologies and develop new products, the risks associated with operating in foreign countries, and general competitive and economic conditions. Changes in any of the preceding areas could have a material adverse effect on our business, results of operations or financial position.
New Accounting Standards
In May 2011, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2011-04,Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs. ASU No. 2011-04 clarifies some existing concepts, eliminates wording differences between U.S. GAAP and International Financial Reporting Standards (“IFRS”), and in some limited cases, changes some principles to achieve convergence between U.S. GAAP and IFRS. ASU No. 2011-04 results in a consistent definition of fair value and common requirements for measurement of and disclosure about fair value between U.S. GAAP and IFRS. ASU No. 2011-04 also expands the disclosures for fair value measurements that are estimated using significant unobservable (Level 3) inputs. The provisions of ASU No. 2011-04 will become effective for us on January 1, 2012 and are to be applied prospectively. We do not expect the adoption of the provisions of ASU No. 2011-04 to have a material effect on our consolidated financial position, results of operations or cash flows and we do not expect to materially modify or expand our financial statement footnote disclosures.
68
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
In June 2011, the FASB issued ASU No. 2011-05,Comprehensive Income (Topic 220): Presentation of Comprehensive Income. ASU No. 2011-05 requires an entity to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income, or in two separate but consecutive statements. ASU No. 2011-05 eliminates the option to present components of other comprehensive income as part of the statement of stockholders’ equity. The presentation requirements will become effective for us on January 1, 2012. As ASU No. 2011-05 applies to financial statement presentation matters, the adoption of ASU No. 2011-05 will not affect our consolidated financial position, results of operations or cash flows and we believe our current presentation of comprehensive loss complies with the new presentation requirements.
Note 4. Investments
We have cash investment policies that limit investments to investment grade rated securities. At December 31, 2011 and 2010, all of our investments were classified as available-for-sale and carried at fair value. Our investments in corporate debt are guaranteed by the FDIC or foreign governments. At December 31, 2011, our short-term investments had maturity dates of less than twenty-four months.
The amortized cost and fair value of our investments, with gross unrealized gains and losses, were as follows:
At December 31, 2011
| September 30, | September 30, | September 30, | September 30, | |||||||||||||
| Amortized Cost Basis | Gross Unrealized Gains | Gross Unrealized Losses | Aggregate Fair Value | |||||||||||||
| (in thousands) | ||||||||||||||||
Short-term investments: | ||||||||||||||||
U.S. government agency debt | $ | 31,290 | $ | 2 | $ | (28 | ) | $ | 31,264 | |||||||
Corporate debt | 5,023 | 3 | — | 5,026 | ||||||||||||
Certificates of deposit | 55,635 | — | — | 55,635 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total short-term investments | $ | 91,948 | $ | 5 | $ | (28 | ) | $ | 91,925 | |||||||
|
|
|
|
|
|
|
| |||||||||
At December 31, 2010
| September 30, | September 30, | September 30, | September 30, | |||||||||||||
| Amortized Cost Basis | Gross Unrealized Gains | Gross Unrealized Losses | Aggregate Fair Value | |||||||||||||
| (in thousands) | ||||||||||||||||
Short-term investments: | ||||||||||||||||
Corporate debt | $ | 21,295 | $ | 35 | $ | — | $ | 21,330 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Total short-term investments | $ | 21,295 | $ | 35 | $ | — | $ | 21,330 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Long-term investments: | ||||||||||||||||
U.S. government agency debt | $ | 4,007 | $ | — | $ | (1 | ) | $ | 4,006 | |||||||
|
|
|
|
|
|
|
| |||||||||
Total long-term investments | $ | 4,007 | $ | — | $ | (1 | ) | $ | 4,006 | |||||||
|
|
|
|
|
|
|
| |||||||||
In the years ended December 31, 2011 and 2010, we did not have any realized gains or losses upon the disposition of our investments.
Note 5. Fair Value Measurements
FASB ASC 820 –Fair Value Measurements and Disclosures, defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the
69
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
measurement date. FASB ASC 820 requires disclosures about the fair value of all financial instruments, whether or not recognized, for financial statement purposes. Disclosures about the fair value of financial instruments are based on pertinent information available to us as of the reporting dates. Accordingly, the estimates presented in these consolidated financial statements are not necessarily indicative of the amounts that could be realized on disposition of the financial instruments.
FASB ASC 820 specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement).
The three levels of the fair value hierarchy are as follows:
Level 1 – Quoted prices for identical instruments in active markets.
Level 2 – Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations whose inputs are observable or whose significant value drivers are observable.
Level 3 – Instruments with primarily unobservable value drivers.
The following table represents the fair value of our financial assets and financial liabilities measured at fair value on a recurring basis and which level was used in the fair value hierarchy.
| September 30, | September 30, | September 30, | September 30, | September 30, | ||||||||||||||||
At December 31, 2011 | ||||||||||||||||||||
| Carrying | Fair Value Measurements at the Reporting Date Using | |||||||||||||||||||
| Value | Fair Value | Level 1 | Level 2 | Level 3 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Assets | ||||||||||||||||||||
Short-term investments | $ | 91,925 | $ | 91,925 | $ | — | $ | 91,925 | $ | — | ||||||||||
Liabilities | ||||||||||||||||||||
Convertible senior notes | 94,277 | (1) | 145,259 | — | 145,259 | — | ||||||||||||||
| September 30, | September 30, | September 30, | September 30, | September 30, | ||||||||||||||||
At December 31, 2010 | ||||||||||||||||||||
| Carrying | Fair | Fair Value Measurements at the Reporting Date Using | ||||||||||||||||||
| Value | Value | Level 1 | Level 2 | Level 3 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Assets | ||||||||||||||||||||
Short-term investments | $ | 21,330 | $ | 21,330 | $ | — | $ | 21,330 | $ | — | ||||||||||
Long-term investments | 4,006 | 4,006 | — | 4,006 | — | |||||||||||||||
Liabilities | ||||||||||||||||||||
Convertible senior notes | 88,922 | (1) | 160,694 | — | 160,694 | — | ||||||||||||||
| (1) | The carrying amount of our convertible senior notes is net of unamortized discount. See Note 7, “Debt” for more information. |
The fair value of our investments and convertible senior notes was determined using quoted prices for the instruments in markets that are not active. The fair value of our convertible senior notes was determined using observable market data (including trade data) and is presented for disclosure purposes only.
Note 6. Other Balance Sheet Information
Other accrued liabilities consist of the following at December 31, 2011 and 2010:
70
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
| September 30, | September 30, | |||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
Accrued payroll and other employee costs | $ | 6,274 | $ | 4,153 | ||||
Accrued research and development costs | 1,627 | 672 | ||||||
Accrued material purchases | 1,332 | 256 | ||||||
Accrued litigation settlement | 1,125 | — | ||||||
Accrued professional fees | 1,100 | 577 | ||||||
Accrued VAT | 390 | 648 | ||||||
Other accrued expenses | 588 | 695 | ||||||
|
|
|
| |||||
| $ | 12,436 | $ | 7,001 | |||||
|
|
|
| |||||
Accrued payroll and other employee costs included year-end employee bonuses of approximately $4.4 million and $3.1 million at December 31, 2011 and 2010, respectively.
Note 7. Debt
Convertible Senior Notes
On December 15, 2010, we completed the sale of 3.5% convertible senior notes due 2017 (the “Convertible Notes”) for an aggregate principal amount of $143.75 million pursuant to the terms of an Indenture dated December 15, 2010. The Convertible Notes are the senior unsecured obligations of the Company. The Convertible Notes bear interest at a rate of 3.5% per annum, payable semi-annually in arrears on June 15 and December 15 of each year. The Convertible Notes will mature on December 15, 2017, unless earlier repurchased by us or converted.
The Convertible Notes will be convertible at an initial conversion rate of 10 shares of our common stock per $1,000 principal amount of Convertible Notes, which corresponds to an initial conversion price of $100.00 per share of our common stock. The conversion rate is subject to adjustment from time to time upon the occurrence of certain events.
Prior to June 15, 2017, holders may convert their Convertible Notes at their option only upon satisfaction of one or more conditions relating to the sale price of our common stock, the trading price per $1,000 principal amount of Convertible Notes or specified corporate events. On or after June 15, 2017 until the close of business of the business day immediately preceding the date the Convertible Notes mature, holders may convert their Convertible Notes at any time, regardless of whether any of the foregoing conditions have been met. As of the date of this report, none of the events that would allow holders to convert their Convertible Notes have occurred. Upon conversion, we will pay or deliver, as the case may be, cash, shares of our common stock or a combination thereof, at our election.
We may not redeem the Convertible Notes prior to maturity. Holders of the Convertible Notes may require us to purchase for cash all or a part of their Convertible Notes at a repurchase price equal to 100% of the principal amount of the Convertible Notes to be repurchased, plus accrued and unpaid interest, upon the occurrence of certain fundamental changes (as defined in the Indenture) involving the Company. The Indenture does not contain any financial or operating covenants or restrictions on the payments of dividends, the incurrence of indebtedness or the issuance or repurchase of securities by us or any of our subsidiaries.
The Indenture contains customary terms and nonfinancial covenants and defines events of default. If an event of default (other than certain events of bankruptcy, insolvency or reorganization) involving the Company occurs and is continuing, the Trustee (by notice to the Company) or the holders of at least 25% in principal amount of the outstanding Convertible Notes (by notice to the Company and the Trustee) may declare 100% of the principal of and accrued and unpaid interest, if any, on all the Convertible Notes to be due and payable. In case of certain events of bankruptcy, insolvency or reorganization, involving the Company, 100% of the principal of and accrued and unpaid interest on the Convertible Notes will automatically become due and payable. Upon a declaration of acceleration, principal and accrued and unpaid interest, if any, will be due and payable immediately. Notwithstanding the foregoing, the Indenture provides that, to the extent we elect, the sole remedy for an event of default relating to certain failures by us to comply with certain reporting covenants in the Indenture consists exclusively of the right to receive additional interest on the Convertible Notes.
71
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
In accordance with ASC 470-20,Debt, which applies to certain convertible debt instruments that may be settled in cash or other assets, or partially in cash, upon conversion, we recorded the long-term debt and equity components on our Convertible Notes separately on the issuance date. The amount recorded for long-term debt was determined by measuring the fair value of a similar liability that does not have an associated equity component. The measurement of fair value required the Company to make estimates and assumptions to determine the present value of the cash flows of the Convertible Notes, absent the conversion feature. This treatment increased interest expense associated with our Convertible Notes by adding a non-cash component to interest expense in the form of amortization of a debt discount calculated based on the difference between the 3.5% cash coupon rate and the effective interest rate on debt borrowing of approximately 12.5%. The discount is being amortized to interest expense through the December 15, 2017 maturity date of the Convertible Notes using the effective interest method and is included in interest expense on our consolidated statements of operations. Additionally, we allocated the costs related to issuance of the Convertible Notes on the same percentage as the long-term debt and equity components, such that a portion of the costs is allocated to the long-term debt component and the equity component included in additional paid-in capital. The portion of the costs allocated to the long-term debt component is presented as deferred financing costs, net on our consolidated balance sheets. These deferred financing costs are also being amortized to interest expense through the December 15, 2017 maturity date of the Convertible Notes using the effective interest method and such amortization is included in interest expense on our consolidated statements of operations
The Convertible Notes and the equity component, which is recorded in additional paid-in-capital, consisted of the following at December 31, 2011 and 2010:
| September 30, | September 30, | |||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
Principal amount | $ | 143,750 | $ | 143,750 | ||||
Unamortized discount | (49,473 | ) | (54,828 | ) | ||||
|
|
|
| |||||
Net carrying amount | $ | 94,277 | $ | 88,922 | ||||
|
|
|
| |||||
Equity component | $ | 55,038 | $ | 55,038 | ||||
|
|
|
| |||||
Based on the initial conversion rate of 10 shares of our common stock per $1,000 principal amount of Convertible Notes, which corresponds to an initial conversion price of $100.00 per share of our common stock, the number of shares issuable upon conversion of the Convertible Notes is 1,437,500. The value of these shares, based on the closing price of our common stock on December 31, 2011 of $69.00, was approximately $99.2 million. The fair value of our Convertible Notes as presented in Note 5 was $145.3 million at December 31, 2011.
Interest expense related to the Convertible Notes consisted of interest due on the principal amount, amortization of the discount and amortization of the portion of the deferred financing costs allocated to the long-term debt component. For the years ended December 31, 2011 and 2010, interest expense related to the Convertible Notes was as follows:
| September 30, | September 30, | |||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
Stated amount at 3.5% coupon rate | $ | 5,031 | $ | 210 | ||||
Amortization of discount | 5,355 | 209 | ||||||
Amortization of deferred financing costs | 287 | 11 | ||||||
|
|
|
| |||||
| $ | 10,673 | $ | 430 | |||||
|
|
|
| |||||
Loan Agreement
In February 2009, we entered into a loan agreement pursuant to which we were able to borrow up to $20.0 million, which amount was placed in escrow by the lender. As of December 31, 2009, the entire $20.0 million commitment under the loan agreement had been borrowed and repaid and was no longer available to us. The amount of interest expense incurred and paid on amounts borrowed under the loan agreement during the year ended December 31, 2009 was approximately $149,000.
72
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
Beginning July 31, 2009, until all monies were borrowed and repaid in December 2009, the lender was entitled to convert any outstanding loans or available escrow funds in whole or in part into shares of our common stock at any time prior to termination of the loan agreement at the U.S. dollar equivalent of AU$35.00 per share. The terms and conditions of this conversion provision were evaluated and determined to result in an embedded derivative within the host contract loan agreement. We computed the fair value of the embedded derivative to be approximately $3.9 million at July 31, 2009, the initial measurement date. Fair value was determined using a valuation model with observable market inputs to determine relevant assumptions including interest rates and stock and foreign currency volatilities. The initial fair value was capitalized as deferred financing costs and was being amortized over the contractual term of the loan agreement. The amount of deferred financing cost amortization for the year ended December 31, 2009 was approximately $671,000 and is included in interest expense on our consolidated statements of operations.
During the period of time monies were available under the loan agreement, the change in the fair value of the embedded derivative resulted in an expense aggregating approximately $3.9 million, which is presented as a separate line item on our consolidated statements of operations. Due to the repayment of all amounts borrowed under the loan agreement, our inability to re-borrow amounts repaid and the lender’s inability to convert any repaid amounts into shares of our common stock, the proportionate amount of the fair value of the embedded derivative net of the proportionate amount of the unamortized balance of the deferred financing costs at the repayment dates were recorded as a gain on the extinguishment of debt. For the year ended December 31, 2009, the net gain aggregating $4.6 million is presented as a gain on early extinguishment of debt, net on our consolidated statements of operations.
Note 8. Leases
On September 30, 2010, we amended and renewed our lease for our original facility in Miami Lakes, Florida. This facility contains our domestic operations and manufacturing. Under the amended lease, we maintained our existing space of approximately 59,000 square feet, extended the lease term to expire on June 30, 2013 and pay base rent of $9.00 per square foot starting in June 2011, subject to a 3% annual escalation thereafter. Under the amended lease, we have an option to renew for two additional three-year periods.
On December 9, 2010, we entered into a lease for a second facility in Miami Lakes, Florida as part of our planned expansion to support our efforts to prepare for U.S. commercialization. During 2011, we performed significant improvements on the facility and improvements will continue in the first quarter of 2012. Once completed, the facility will be used primarily for manufacturing, research and development and administrative functions. Under the lease, we rent approximately 131,000 square feet for a period ending February 28, 2022, with an option to renew for two five-year terms. The landlord will provide up to $1.75 million towards capital improvements of which $1.1 million had been provided as of December 31, 2011. Base rent will be $9.00 per square foot, payable starting March 1, 2012 and subject to a 3% annual escalation thereafter. A security deposit of $1.25 million was provided in the form of an unconditional stand-by letter of credit. The letter of credit is supported by a certificate of deposit for the same amount, which is included in other assets on our consolidated balance sheets. Under the provisions of the lease, subsequent to December 31, 2011, the security deposit was increased by $750,000.
On June 30, 2011, we amended and renewed our lease for our headquarters in Framingham, Massachusetts. Under the amended lease we began occupying additional space in the third quarter of 2011, increasing our total square footage from approximately 15,000 to 17,800. Base rent obligations increased to approximately $326,000 per year. The lease term expires on December 31, 2014 and we have an option to renew for an additional four-year period at fair market value, as defined in the lease agreement. We also have a right of first offer on an additional 3,002 square foot space in the building should it become available.
On November 1, 2011, we entered into a lease for a new facility in Sydney, Australia. This facility will replace our current facility in Australia that we are leasing month to month and will be used primarily for manufacturing, development and administrative functions. Under the lease, we rent approximately 15,100 square feet for a period ending October 31, 2014, with an option to renew for two three-year terms. Base rent obligations are approximately $190,000 per year payable starting February 1, 2012 and subject to a 3% annual escalation commencing November 1, 2012.
73
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
In addition to the leases discussed above, we have entered into various operating lease agreements for miscellaneous office space and equipment. The duration of these agreements is typically twenty-four to thirty-six months from origination. The aggregate base annual rental payment on these leases is less than $0.1 million.
Rent expense was approximately $2.1 million, $0.9 million and $0.9 million in 2011, 2010 and 2009, respectively. Future minimum rental commitments under non-cancelable operating lease agreements with remaining terms of at least one year as of December 31, 2011 are as follows:
| September 30, | ||||
| Operating Leases | ||||
Year Ending December 31, | (in thousands) | |||
2012 | $ | 2,211 | ||
2013 | 2,132 | |||
2014 | 1,841 | |||
2015 | 1,371 | |||
2016 | 1,412 | |||
Thereafter | 9,411 | |||
|
| |||
Total minimum lease payments | $ | 18,378 | ||
|
| |||
Note 9. Stockholders’ Equity
Preferred Stock
We are authorized to issue up to 5,000,000 shares of preferred stock, $.001 par value per share. Our board of directors is authorized, subject to any limitations prescribed by law, to provide for the issuance of the shares of preferred stock in series, and by filing a certificate pursuant to the applicable law of the state of Delaware, to establish from time to time the number of shares to be included in each such series, and to fix the designation, powers, preferences and rights of the shares of each such series and any qualifications, limitations or restrictions thereof. No shares of preferred stock have been issued or are outstanding.
Common Stock
We are authorized to issue up to 25,000,000 shares of common stock, $.001 par value per share. As of December 31, 2011, we had 14,114,055 shares outstanding. Holders are entitled to one vote for each share of common stock (or its equivalent).
Shares of our common stock reserved at December 31, 2011, for possible future issuance are as follows:
| September 30, | ||||
| (in thousands) | ||||
Convertible senior notes | 1,768 | |||
Equity award plans | 1,313 | |||
|
| |||
| 3,081 | ||||
|
| |||
See the Consolidated Statement of Stockholders’ Equity for details related to our equity transactions.
2010 Public Offering
In February 2010, we completed a public offering of approximately 1.77 million shares of our common stock, including the underwriter’s exercise of their overallotment to purchase 230,595 shares, at an offering price of $35.50 per share for aggregate gross proceeds of approximately $62.8 million. The underwriters for the transaction received a fee of 6% of the gross proceeds. After fees and related expenses, net proceeds from the offering were approximately $58.5 million.
The offering was completed pursuant to a prospectus supplement, dated January 27, 2010, to a shelf registration statement on Form S-3 that was previously filed with the SEC and which was declared effective on January 20, 2010. This shelf registration statement allows us to offer and sell from time to time, in one or more series or issuances and on terms that we will determine at the time of the offering, any combination of the securities described in the prospectus, up to an aggregate amount of $100 million.
74
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
2009 Private Placement
In August and September 2009, we entered into Purchase Agreements separately with a number of institutional and accredited investors for the private placement of approximately 2.74 million shares of our common stock at an issue price of $22.00 per share for aggregate gross proceeds of approximately $60.2 million. The placement agent for the transaction received a placement fee in aggregate of approximately 2% of the gross proceeds.
The issuance of approximately 1.4 million shares of the total number of shares sold to the investors in the Private Placement was subject to approval by our stockholders in accordance with NASDAQ Stock Market Rules and Australian Securities Exchange Listing Rules and, as a result, approximately $30.7 million of the proceeds from the Private Placement were held in escrow by an independent third party. Such proceeds were released to us and the 1.4 million shares were issued following stockholder approval which was obtained at a special meeting of stockholders held on October 26, 2009.
Note 10. Share-Based Compensation
We recognize share-based compensation expense for the portion of awards that are ultimately expected to vest using an accelerated accrual method over the vesting period from the date of grant. We estimate forfeitures at the time of grant. We have applied a forfeiture rate of approximately 12.5% to all unvested share-based awards as of December 31, 2011, which represents the portion that we expect will be forfeited over the vesting period. We reevaluate this estimated rate periodically and adjust the forfeiture rate as necessary. Vesting of share-based awards issued with performance-based vesting criteria must be probable before we begin recording share-based compensation expense. At each reporting period, we review the likelihood that these awards will vest and if vesting is deemed probable, we begin to recognize compensation expense at that time. If ultimately performance goals are not met, for any awards where vesting was previously deemed probable, previously recognized compensation expense will be reversed.
We allocate share-based compensation expense to cost of revenue, selling, general and administrative expense and research and development expense based on the award holders’ employment function. For the years ended December 31, 2011, 2010 and 2009, we recorded share-based compensation expenses as follows:
| September 30, | September 30, | September 30, | ||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
Cost of revenue | $ | 2,182 | $ | 937 | $ | 805 | ||||||
General and administrative | 7,378 | 7,630 | 2,007 | |||||||||
Research and development | 3,599 | 2,018 | 1,320 | |||||||||
|
|
|
|
|
| |||||||
| $ | 13,159 | $ | 10,585 | $ | 4,132 | |||||||
|
|
|
|
|
| |||||||
For the years ended December 31, 2011 and 2010, we experienced a significant increase in share-based compensation expense due primarily to increase in our number of employees and annual grants of equity awards to a large portion of our employees in December 2010 and September 2009.
No deferred tax benefits were attributed to our share-based compensation expense recorded in the accompanying consolidated financial statements because we are in a net operating loss position and a full valuation allowance is maintained for all net deferred tax assets. We receive a tax deduction for certain stock option exercises during the period the options are exercised, and for the vesting of restricted stock units during the period the restricted stock units vest. For stock options, the amount of the tax deduction is generally for the excess of the fair market value of our shares of common stock over the exercise price of the stock options at the date of exercise. For restricted stock units, the amount of the tax deduction is generally for the fair market value of our shares of common stock at the vesting date. Excess tax benefits are not included in the accompanying consolidated financial statements because we are in a net operating loss position and a full valuation allowance is maintained for all net deferred tax assets.
75
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
Equity Plans
We have issued share-based awards to employees, non-executive directors and outside consultants through various approved plans and outside of any formal plan. New shares are issued upon the exercise of share-based awards.
On August 5, 2008, we adopted the HeartWare International, Inc. 2008 Stock Incentive Plan (“2008 SIP”). The 2008 SIP allows for the issuance of share-based awards to employees, directors and consultants. We have issued options and restricted stock units (“RSU’s”) to employees and directors under the 2008 SIP. The plan allows for the issuance of share-based awards representing up to 13% of the prior fiscal year’s weighted average shares outstanding, less shares issued pursuant to awards under the 2008 SIP and share-based awards outstanding under our other equity plans. At December 31, 2011, there were approximately 369,000 shares available for future awards under the 2008 SIP. Under the terms of the 2008 SIP, the shares available for future issuance are adjusted on January 1st of each year based on the prior year’s weighted average shares outstanding. As of January 1, 2012, there were approximately 460,000 shares available for future awards under the 2008 SIP.
Stock Options
Each option allows the holder to subscribe for and be issued one share of our common stock at a specified price, which is generally the quoted market price of our common stock on the date the option is issued. Options generally vest on a pro-rata basis on each anniversary of the issuance date within four years of the date the option is issued or vest in accordance with performance-based criteria. Options may be exercised after they have vested and prior to the specified expiry date provided applicable exercise conditions are met, if any. The expiry date can be for periods of up to ten years from the date the option is issued.
Performance-based options vest in four equal tranches contingent upon the achievement of pre-determined corporate milestones related primarily to the development of our products and the achievement of certain prescribed clinical and regulatory objectives. Any performance-based options that have not vested after five years from the date of grant automatically expire.
The fair value of each option is estimated on the date of grant using the Black-Scholes option pricing model using the assumptions established at that time. The following table includes the weighted average assumptions used for options issued in the years ended December 31, 2011, 2010 and 2009.
| September 30, | September 30, | September 30, | ||||||||||
| 2011 | 2010 | 2009 | ||||||||||
Dividend yield | 0.00 | % | 0.00 | % | 0.00 | % | ||||||
Expected volatility | 58.23 | % | 60.94 | % | 60.50 | % | ||||||
Risk-free interest rate | 1.97 | % | 2.71 | % | 2.80 | % | ||||||
Estimated holding period (years) | 6.25 | 6.25 | 6.25 | |||||||||
Information related to options granted under all of our plans at December 31, 2011 and activity during the year then ended is as follows (certain amounts in U.S.$ were converted from AU$ at the then period-end spot rate):
| September 30, | September 30, | September 30, | September 30, | |||||||||||||
| Number of Options (in thousands) | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (Years) | Aggregate Intrinsic Value (in thousands) | |||||||||||||
Outstanding at December 31, 2010 | 404 | $ | 32.87 | |||||||||||||
Granted | 18 | 74.53 | ||||||||||||||
Exercised | (32 | ) | 32.54 | |||||||||||||
Forfeited | (9 | ) | 37.38 | |||||||||||||
Expired | — | — | ||||||||||||||
|
| |||||||||||||||
Outstanding at December 31, 2011 | 381 | $ | 34.79 | 5.84 | $ | 13,040 | ||||||||||
|
| |||||||||||||||
Exercisable at December 31, 2011 | 265 | $ | 33.61 | 5.16 | $ | 9,362 | ||||||||||
|
| |||||||||||||||
76
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
The aggregate intrinsic values at December 31, 2011 noted in the table above represent the closing price of our common stock traded on NASDAQ, less the weighted average exercise price at period end multiplied by the number of options outstanding and exercisable.
At December 31, 2011, 34,283 of the options outstanding that are not yet exercisable are subject to performance-based vesting criteria as described above.
The weighted average grant date fair value per share of options granted in the years ended December 31, 2011, 2010 and 2009 was $41.92, $28.62 and $16.50, respectively.
The total intrinsic value of options exercised during the years ended December 31, 2011, 2010 and 2009 was approximately $1.5 million, $4.0 million and $2.9 million, respectively. Cash received from option exercises in the years ended December 31, 2011, 2010 and 2009 was approximately $1.1 million, $3.6 million and $1.5 million.
At December 31, 2011, there was approximately $1.1 million of unrecognized compensation cost related to non-vested option awards, including performance-based options not yet deemed probable of vesting. The expense is expected to be recognized over a weighted average period of 1.0 years.
Restricted Stock Units
RSU’s issued under the plans vest on a pro-rata basis on each anniversary of the issuance date over three or four years or vest in accordance with performance-based criteria. The RSU’s with performance-based vesting criteria vest in tranches contingent upon the achievement of pre-determined corporate milestones related primarily to the development of our products and the achievement of certain prescribed clinical and regulatory objectives. RSU’s with performance-based vesting criteria not vested after five years from the date of grant automatically expire. There is no consideration payable on the vesting or exercise of RSU’s issued under the plans. Upon vesting, the RSU’s are exercised automatically and settled in shares of our common stock.
Information related to RSU’s at December 31, 2011 and activity during the year then ended is as follows:
| September 30, | September 30, | September 30, | ||||||||||
| Number of Units (in thousands) | Weighted Average Remaining Contractual Life (Years) | Aggregate Intrinsic Value (in thousands) | ||||||||||
Outstanding at December 31, 2010 | 545 | |||||||||||
Granted | 312 | |||||||||||
Vested/Exercised | (203 | ) | ||||||||||
Forfeited | (19 | ) | ||||||||||
Expired | — | |||||||||||
|
| |||||||||||
Outstanding at December 31, 2011 | 635 | 1.83 | $ | 43,774 | ||||||||
|
| |||||||||||
Exercisable at December 31, 2011 | — | — | $ | — | ||||||||
|
| |||||||||||
The aggregate intrinsic value at December 31, 2011 noted in the table above represents the closing price of our common stock traded on NASDAQ, multiplied by the number of RSU’s outstanding.
At December 31, 2011, 56,257 of the RSU’s outstanding that are not yet exercisable are subject to performance-based vesting criteria as described above.
The total intrinsic value of RSU’s vested during the years ended December 31, 2011, 2010 and 2009 was approximately $13.0 million, $11.6 million and $512,000, respectively.
The fair value of each RSU award equals the closing price of our common stock on the date of grant. The weighted average grant date fair value per share of RSU’s granted during the years ended December 31, 2011, 2010 and 2009 was $70.29, $73.87 and $27.21, respectively.
77
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
At December 31, 2011, we had approximately $25.0 million of unrecognized compensation cost related to non-vested RSU awards, including awards not yet deemed probable of vesting. The expense is expected to be recognized over a weighted average period of 1.8 years.
On December 21, 2011, our board of directors approved the grant of 36,000 RSU’s to our Chief Executive Officer, subject to stockholder approval. As this grant is subject to stockholder approval, it is not reflected in the above disclosures. We intend to seek stockholder approval at our 2012 annual meeting of stockholders.
Note 11. Income Taxes
The components of loss before income taxes for the years ended December 31, 2011, 2010 and 2009 were as follows:
| September 30, | September 30, | September 30, | ||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
United States | $ | (49,243 | ) | $ | (29,512 | ) | $ | (20,205 | ) | |||
Non-U.S. | (5,812 | ) | 115 | (704 | ) | |||||||
|
|
|
|
|
| |||||||
| $ | (55,055 | ) | $ | (29,397 | ) | $ | (20,909 | ) | ||||
|
|
|
|
|
| |||||||
The effective tax rate of 0% differs from the statutory United States federal income tax rate of 35% for all periods presented due primarily to a full valuation allowance on deferred tax assets.
The primary components of net deferred tax assets and liabilities at December 31, 2011 and 2010 were as follows:
| September 30, | September 30, | |||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
Deferred tax assets: | ||||||||
U.S. losses carried forward | $ | 52,681 | $ | 38,168 | ||||
Non-U.S. losses carried forward | 3,423 | 2,907 | ||||||
|
|
|
| |||||
Total net operating losses carried forward | 56,104 | 41,075 | ||||||
Equity awards | 3,453 | 3,881 | ||||||
Other deferred tax assets | 1,558 | 516 | ||||||
|
|
|
| |||||
Gross deferred tax assets | 61,115 | 45,472 | ||||||
Deferred tax liabilities: | ||||||||
Convertible debt | (18,617 | ) | (20,737 | ) | ||||
|
|
|
| |||||
Net deferred tax assets | 42,498 | 24,735 | ||||||
Less: valuation allowance | (42,498 | ) | (24,735 | ) | ||||
|
|
|
| |||||
Net deferred tax asset/(liability) | $ | — | $ | — | ||||
|
|
|
| |||||
FASB ASC 740—Income Taxes requires that a valuation allowance be established to reduce a deferred tax asset to its realizable value when it is more likely than not that all or a portion of a deferred tax asset will not be realized. A review of all available positive and negative evidence needs to be considered, including the utilization of past tax credits and length of carry-back and carry-forward periods, reversal of temporary differences, tax planning strategies, our current and past performance, the market environment in which we operate, and the evaluation of tax planning strategies to generate future taxable income.
At December 31, 2011 and 2010, we had gross deferred tax assets in excess of deferred tax liabilities of $42.5 million and $24.7 million, respectively. We determined that it is not “more likely than not” that such assets will be realized, and as such have applied a valuation allowance of $42.5 million and $24.7 million as of December 31, 2011 and 2010, respectively, to reduce our net deferred tax assets to their estimated realizable value. The valuation allowance primarily relates to the deferred tax assets arising from operating loss carry-forwards. The valuation
78
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
allowance on our net deferred tax assets increased by approximately $17.8 million for the year ended December 31, 2011, decreased by approximately $11.0 million for the year ended December 31, 2010 and increased by approximately $9.3 million for the year ended December 31, 2009.
We evaluate our ability to realize our deferred tax assets each period and adjust the amount of our valuation allowance, if necessary. If there is an ownership change, as defined under Internal Revenue Code Section 382, the use of operating loss and credit carry-forwards may be subject to limitation on use. We operate within multiple taxing jurisdictions and are subject to audit in those jurisdictions. Because of the complex issues involved, any claims can require an extended period to resolve.
Net operating losses representing excess tax benefits attributable to share based compensation are not included in the table of deferred tax asset and liabilities shown above because they have not been realized for financial statement purposes. Pursuant to ASC 718, excess tax benefits attributable to share based compensation will only be recorded to additional paid-in capital when they are realized through a reduction of taxes payable. As of December 31, 2011, the portion of the federal and state net operating loss related to share based compensation is approximately $11.5 million.
At December 31, 2011, we had net operating loss carry-forwards of approximately $155.6 million for U.S. federal and state income tax purposes that are available to offset future taxable income and begin to expire in the year 2025. We have foreign tax loss carry-forwards of approximately $14.3 million that do not expire. The adoption of ASC 740-10Accounting for Uncertainty in Income Taxes has had no impact on the reported carry-forwards at December 31, 2011. Net operating loss carry-forwards will expire as follows:
| September 30, | September 30, | September 30, | ||||||||||
Year of Expiration | Year Generated | U.S. Losses | Foreign Losses | |||||||||
| (in thousands) | ||||||||||||
Unlimited | 2006 | $ | — | $ | (3,968 | ) | ||||||
Unlimited | 2007 | — | (3,864 | ) | ||||||||
Unlimited | 2009 | — | (1,829 | ) | ||||||||
Unlimited | 2010 | — | (1,132 | ) | ||||||||
Unlimited | 2011 | — | (3,524 | ) | ||||||||
2025 | 2005 | (10,975 | ) | — | ||||||||
2026 | 2006 | (13,460 | ) | — | ||||||||
2027 | 2007 | (17,344 | ) | — | ||||||||
2028 | 2008 | (23,929 | ) | — | ||||||||
2029 | 2009 | (20,544 | ) | — | ||||||||
2030 | 2010 | (29,580 | ) | — | ||||||||
2031 | 2011 | (39,784 | ) | — | ||||||||
|
|
|
| |||||||||
| $ | (155,616 | ) | $ | (14,317 | ) | |||||||
|
|
|
| |||||||||
Uncertain tax positions
The amount of unrecognized tax benefits as of December 31, 2011 and December 31, 2010 was $0. There have been no material changes in unrecognized tax benefits through December 31, 2011. The fiscal years 2006 through 2011 are considered open tax years in U.S. federal and state and Australian tax jurisdictions. In addition, 2011 and 2010 are considered open tax years for German and United Kingdom jurisdictions. At December 31, 2011, we were not under examination by any of these taxing authorities for the open tax years.
Note 12. Net Loss Per Common Share
Basic net loss per common share is computed by dividing net loss for the period by the weighted-average number of common shares outstanding during the period. Diluted net loss per common share adjusts basic net loss per share for the dilutive effects of convertible securities, share-based awards and other potentially dilutive instruments only in the periods in which such effect is dilutive. Due to our net loss for all periods presented, all potentially dilutive instruments were excluded because their inclusion would have been anti-dilutive. The following instruments have been excluded from the calculation of diluted net loss per share, as their effect would be anti-dilutive.
79
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
| September 30, | September 30, | September 30, | ||||||||||
Common shares issuable upon: | 2011 | 2010 | 2009 | |||||||||
| (in thousands) | ||||||||||||
Conversion of convertible senior notes | 1,438 | 1,438 | — | |||||||||
Exercise or vesting of share-based awards | 1,016 | 949 | 934 | |||||||||
Note 13. Business Segment, Geographic Areas and Major Customers
For financial reporting purposes, we have one reportable segment which designs, manufactures and markets medical devices for the treatment of advanced heart failure. Products are sold to customers located in the United States through our clinical trials, as commercial products to customers in Europe and under special access in other countries. Product sales attributed to a country or region are based on the location of the customer to whom the products are sold. Long-lived assets are primarily held in the United States.
Product sales by geographic location for the years ended December 31, 2011, 2010 and 2009 are as follows:
| September 30, | September 30, | September 30, | ||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
United States | $ | 28,199 | $ | 14,998 | $ | 9,967 | ||||||
Germany | 29,036 | 25,103 | 8,550 | |||||||||
International, excluding Germany | 25,529 | 15,063 | 5,655 | |||||||||
|
|
|
|
|
| |||||||
| $ | 82,764 | $ | 55,164 | $ | 24,172 | |||||||
|
|
|
|
|
| |||||||
For the year ended December 31, 2011, no customers individually accounted for more than 10% of product sales in the respective periods. For the year ended December 31, 2010, one customer exceeded 10% of product sales individually and accounted for approximately 16% of product sales in the aggregate. For the year ended December 31, 2009, two customers exceeded 10% of product sales individually and accounted for approximately 22% of product sales in the aggregate. As the majority of our revenue is generated outside of the U.S., we are dependent on favorable economic and regulatory environments for our products in Europe and other countries outside of the U.S.
The percentage of our revenue generated in the U.S. increased in 2011 as compared to 2010 primarily due to enrollment in our ENDURANCE destination therapy trial, which commenced in August 2010 and continued through 2011. The percentage of our revenue generated in the U.S. was lower in 2010 compared to 2009 due to the acceleration of our commercial efforts in Europe and the completion of enrollment in our ADVANCE trial in the U.S. in February 2010. While the FDA approved an Investigational Device Exemption Supplement that allowed us to enroll additional patients in our ADVANCE trial under a Continued Access Protocol, we experienced periods of inactivity while we awaited approval.
Note 14. Commitments and Contingencies
The following contingent liabilities resulting from the 2003 acquisition by HeartWare, Inc. of a business that previously held our technology existed as of December 31, 2011:
| • | a milestone payment of $1.25 million due within 6 months of the date when the first circulatory assist device is approved for sale in the United States, provided that we have at least $25 million in cash on hand and, if we do not have $25 million at that time, then the payment is deferred until such time that we have $25 million in cash on hand; and |
| • | a special payment of up to $500,000 upon a sale of our HeartWare, Inc. subsidiary if such sale generates proceeds in excess of the aggregate liquidation preferences of all of HeartWare, Inc.’s then outstanding preferred stock. |
We will record the effect of these payment obligations when and if these events occur or are deemed probable of occurring.
80
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
During 2009, we paid $750,000 as a result of our receipt of approval to sell our first circulatory assist device in Europe. This represented the first milestone payment resulting from the acquisition noted above and is included in research and development expense.
As of January 15, 2011, we entered into a Public, Private Partnership Agreement with the Regents of the University of Michigan whereby we will act as industry sponsor of a study conducted by the University of Michigan Cardiovascular Center and the University of Pittsburgh exploring the potential benefits of VADs in patients who will be given earlier access to these devices under a grant awarded from the National Heart, Lung and Blood Institute. In the study, called REVIVE-IT, researchers will compare whether non-transplant eligible patients with heart failure less advanced than that of current VAD recipients do better with implanted devices than with current medical therapy. Pursuant to the terms of the agreement, we have committed to provide financial support up to $9.6 million over the five-year trial period. Through December 31, 2011, the total amount presented to us for payment was approximately $0.2 million.
In December 2011, for $1.5 million, we purchased from parties unrelated to us, non-controlling interests in three development stage entities under common control, and obtained the rights to purchase the remaining ownership interests in two of the three entities. As of the date of this filing, we are waiting for additional information necessary to finalize the accounting treatment of these investments. As a result, we have been unable to conclude if the entities, individually or as a group, could be variable interest entities, and if so, whether or not they would require consolidation. We expect to obtain the necessary information to determine the appropriate accounting treatment of the investments no later than the first half of 2012. We have included the amount paid for the investments in other assets in the accompanying consolidated balance sheet as of December 31, 2011. The results of operations of these entities from the date of our investment have been immaterial. We do not believe finalizing the accounting treatment of these investments will have a material impact on the accompanying consolidated financial statements.
At December 31, 2011, we had purchase order commitments of approximately $22.6 million related to product costs, supplies, services and property, plant and equipment purchases. Many of our materials and supplies require long lead times and as such purchase order commitments reflect materials that may be received up to one year from the date of order.
In addition to the above, we have entered into employment agreements with all of our executive officers. These contracts do not have a fixed term and are constructed on an at will basis. Some of these contracts provide executives with the right to receive certain additional payments and benefits if their employment is terminated after a change of control, as defined in such agreements.
The taxation and customs requirements, together with other applicable laws and regulations of certain foreign jurisdictions, can be inherently complex and subject to differing interpretation by local authorities. We are subject to the risk that either we have misinterpreted applicable laws and regulations, or that foreign authorities may take inconsistent, unclear or changing positions on local law, customs practices or rules. In the event that we have misinterpreted any of the above, or that foreign authorities take positions contrary to ours, we may incur liabilities that may differ materially from the amounts accrued in the accompanying consolidated financial statements.
From time to time we may be involved in litigation or other contingencies arising in the ordinary course of business. Except as set forth below, and based on the information presently available, management believes that there are no contingencies, claims or actions pending or threatened, the ultimate resolution of which will have a material adverse effect on our financial position, liquidity or results of operations.
On February 24, 2010, we received a letter from two holders of Series A Preferred Stock in HeartWare, Inc., an indirect subsidiary of HeartWare International, Inc. These holders requested various financial and other information regarding HeartWare, Inc. for the purpose of determining the Company’s compliance with their rights as holders of Series A Preferred Stock, including whether a liquidation event has occurred since inception in 2003. HeartWare, Inc. issued Series A-1 and Series A-2 Preferred Stock to certain equity holders of Kriton Medical, Inc. when HeartWare, Inc. purchased out of bankruptcy substantially all of the assets of Kriton in July 2003. The Series A-1 and Series A-2 Preferred Stock do not have voting or dividend rights but entitle the holders thereof to receive, upon certain liquidation events of HeartWare, Inc. (but not the liquidation of or change of control of HeartWare International, Inc.), an amount equal to $10 per share of Series A-1 and $21 per share of Series A-2. The aggregate liquidation preference payment obligation totals approximately $15 million.
On June 27, 2011, HeartWare International, Inc. and HeartWare, Inc., along with HeartWare’s directors, certain officers and a significant stockholder, were named as defendants in a putative class action lawsuit filed in Massachusetts state court by two other Series A Preferred Stockholders on behalf of all holders of Series A Preferred Stock. The complaint alleges that the defendants breached their fiduciary and contractual obligations to Series A Preferred Stockholders by preventing them from receiving a payment of the liquidation preference in connection with certain corporate transactions, including a transaction in 2005 in which HeartWare, Inc. was acquired by HeartWare Limited, a subsidiary of HeartWare International, Inc. The plaintiffs seek monetary damages, interest, costs and limited equitable relief. We do not believe HeartWare International, Inc., HeartWare,
81
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
Inc. or any of our directors, officers or stockholders have abrogated the rights, or in any way failed to satisfy obligations owed to, any of our stockholders, including holders of Series A Preferred Stock. On September 12, 2011, the defendants served on plaintiffs a motion to dismiss the complaint with prejudice. On February 3, 2012, counsel for plaintiffs and defendants entered into a Memorandum of Understanding to settle the matter. Defendants have agreed to pay up to $1.1 million to participating putative class members in exchange for a full and unconditional release of all claims asserted in the litigation, including any and all claims arising from any right to receive a payment upon any liquidation or deemed liquidation event that has arisen or may arise in the future. We expect insurance to fund a significant portion of the settlement amount, although coverage is not assured. The settlement must be finally approved by the court following notice to putative class members.
In accordance with ASC 450, Contingencies, we accrue loss contingencies including costs of settlement, damages and defense related to litigation to the extent they are probable and reasonably estimable. Otherwise, we expense these costs as incurred. If the estimate of a probable loss is a range and no amount within the range is more likely, we accrue the minimum amount of the range. As of December 31, 2011, we have determined that settlement of the litigation discussed above is probable and that the reasonably estimable settlement amount is $1.1 million. At December 31, 2011, we recorded a liability for the $1.1 million, a $0.2 million receivable from one of the co-defendants, who is a related party, and expense of $0.9 million.
Note 15. Guarantees
On December 16, 2008, we entered into a Deed of Cross Guarantee (the “Deed”) by and among the Group’s then-existing entities; HeartWare International, Inc., HeartWare Pty. Limited (formerly HeartWare Limited) and HeartWare Inc., whereby the companies have agreed to cross-guarantee each other’s liabilities. The Deed was established as a condition to obtaining financial reporting relief under ASIC Class Order 98/1418 which provides relief for us from the requirement to prepare and lodge audited accounts for HeartWare Pty. Limited in Australia. HeartWare International, Inc. is the holding entity, HeartWare, Inc. is the alternative Trustee and HeartWare Pty. Limited is a member of the Closed Group for purposes of the Class Order.
Note 16. Retirement Savings Plan
We have established a 401(k) plan in which substantially all of our U.S. employees are eligible to participate. Contributions made by employees are limited to the maximum allowable for U.S. federal income tax purposes. Beginning in April 2010, we commenced a matching program whereby we match employee contributions at a rate of 100% of applicable contributions up to 3% of included compensation plus 50% of applicable contributions up to the next 2% of included compensation. Our contributions to the 401(k) plan were approximately $0.5 million and $0.3 million for the years ended December 31, 2011 and 2010. We did not make any contributions to the plan in 2009.
Note 17. Quarterly Financial Information (Unaudited)
The following table presents selected quarterly financial information for the periods indicated. This information has been derived from our unaudited quarterly consolidated financial statements, which in the opinion of management include all adjustments (consisting only of normal recurring adjustments) necessary for a fair presentation of such information. The quarterly per share data presented below was calculated separately and may not sum to the annual figures presented in the consolidated financial statements. These operating results are also not necessarily indicative of results for any future period.
| September 30, | September 30, | September 30, | September 30, | |||||||||||||
| Three Months Ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
2011 | ||||||||||||||||
Revenue, net | $ | 17,975 | $ | 20,389 | $ | 21,340 | $ | 23,060 | ||||||||
Gross profit | 10,379 | 12,476 | 13,456 | 13,521 | ||||||||||||
Net loss | (9,431 | ) | (10,096 | ) | (13,964 | ) | (21,564 | ) | ||||||||
Net loss per common share – basic and diluted (1) | $ | (0.68 | ) | $ | (0.73 | ) | $ | (1.00 | ) | $ | (1.53 | ) | ||||
82
HEARTWARE INTERNATIONAL, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
| September 30, | September 30, | September 30, | September 30, | |||||||||||||
| Three Months Ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
Weighted average shares outstanding – basic and diluted | 13,901 | 13,923 | 13,948 | 14,063 | ||||||||||||
2010 | ||||||||||||||||
Revenue, net | $ | 10,703 | $ | 9,757 | $ | 13,817 | $ | 20,887 | ||||||||
Gross profit | 5,023 | 5,464 | 7,814 | 12,422 | ||||||||||||
Net loss | (4,544 | ) | (9,982 | ) | (7,844 | ) | (7,027 | ) | ||||||||
Net loss per common share – basic and diluted (1) | $ | (0.35 | ) | $ | (0.73 | ) | $ | (0.57 | ) | $ | (0.51 | ) | ||||
Weighted average shares outstanding – basic and diluted | 12,958 | 13,683 | 13,753 | 13,874 | ||||||||||||
| (1) | Net loss per common share for each quarter is computed using the weighted-average number of shares outstanding during that quarter while net loss per common share for the full year is computed using the weighted-average number of shares outstanding during the year. Thus, the sum of the four quarters’ net loss per common share may not equal the full-year loss per share. |
Significant amounts in per quarter information listed above include:
| • | Net loss for the quarter ended March 31, 2011 included $2.9 million of share-based compensation expense, $2.6 million of interest expense and $0.6 million of foreign exchange gains. |
| • | Net loss for the quarter ended June 30, 2011 included $3.5 million of share-based compensation expense and $2.6 million of interest expense. |
| • | Net loss for the quarter ended September 30, 2011 included $3.6 million of share-based compensation expense, $2.7 million of interest expense and $1.4 million of foreign exchange losses. |
| • | Net loss for the quarter ended December 31, 2011 included $3.2 million of share-based compensation expense, $2.7 million of interest expense, $1.6 million of foreign exchange losses and $0.9 million related to an accrual for a potential litigation settlement. |
| • | Net loss for the quarters ended March 31, June 30, September 30 and December 31, 2010 include approximately $1.7 million, $4.3 million, $2.6 million and $2.0 million, respectively, of share-based compensation expense. |
Note 18. Subsequent Events
We have evaluated events and transactions that occurred subsequent to December 31, 2011 through the date the financial statements were issued, for potential recognition or disclosure in the accompanying consolidated financial statements.
Except as disclosed in Note 14, we did not identify any events or transactions that should be recognized or disclosed in the accompanying consolidated financial statements.
83
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of the Chief Executive Officer and the Vice President of Finance, carried out an evaluation required by the Securities Exchange Act of 1934, as amended (the “Exchange Act”), of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rule 13a-15(e) of the Exchange Act, as of December 31, 2011. Based on this evaluation, our Chief Executive Officer and Vice President of Finance concluded that, as of December 31, 2011, our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and to provide reasonable assurance that such information is accumulated and communicated to our management, including our Chief Executive Officer and Vice President of Finance, as appropriate to allow timely decisions regarding required disclosures.
Management’s Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on our financial statements.
Under the supervision and with the participation of our Chief Executive Officer and our Vice President of Finance, management conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2011 based on the framework inInternal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and in accordance with the interpretive guidance issued by the SEC in Release No. 34-55929. Based on that evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2011.
Our independent registered public accounting firm, Grant Thornton LLP, has issued a report on our internal control over financial reporting, which is presented below.
Attestation Report of Independent Registered Public Accounting Firm
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
HeartWare International, Inc.
We have audited HeartWare International, Inc. (a Delaware corporation) and subsidiaries’ (the Company) internal control over financial reporting as of December 31, 2011, based on criteria established inInternal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
84
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on criteria established inInternal Control-Integrated Framework issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of the Company as of December 31, 2011 and 2010, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2011, and our report dated February 27, 2012 expressed an unqualified opinion.
/s/ Grant Thornton LLP
Fort Lauderdale, Florida
February 27, 2012
85
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the fiscal quarter ended December 31, 2011, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Controls and Procedures
Our management, including the Chief Executive Officer and Vice President of Finance, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Thus, misstatements due to error or fraud may occur and not be detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of controls.
None.
86
Item 10. Directors, Executive Officers and Corporate Governance
Our executive officers and their respective ages are as follows:
Name | Age | Position | ||||
Douglas Godshall | 47 | Director, President and Chief Executive Officer | ||||
Jeffrey LaRose | 50 | Executive Vice President and Chief Scientific Officer | ||||
David Hathaway | 64 | Chief Medical Officer | ||||
Lauren Farrell | 44 | Vice President, Finance | ||||
Larry Knopf | 50 | Senior Vice President, General Counsel and Secretary | ||||
James Schuermann | 43 | Senior Vice President, Sales and Marketing | ||||
Robert Yocher | 61 | Senior Vice President, Regulatory & Quality Assurance | ||||
Biographical Summaries
Douglas Godshall. Mr. Godshall has been President and Chief Executive Officer since September 2006 and became a director in October 2006. Prior to joining HeartWare, Mr. Godshall served in various executive and managerial positions at Boston Scientific Corporation, where he had been employed since 1990, including as a member of Boston Scientific’s Operating Committee and since January 2005, as President, Vascular Surgery. Prior thereto, Mr. Godshall spent 5 years as Vice President, Business Development, at Boston Scientific, where he was focused on acquisition strategies for the cardiology, electrophysiology, neuroradiology and vascular surgery divisions. Mr. Godshall has a Bachelor of Arts in Business from Lafayette College and Masters of Business Administration from Northeastern University in Boston, Massachusetts.
Jeffrey LaRose. Mr. LaRose is our Chief Scientific Officer and has been with the Company since its inception. Prior to joining HeartWare, since April 1999, he was involved in the development of HeartWare’s technology through his employment with Kriton Medical, which the Company acquired in 2003. He is responsible for all aspects of the design and physiological controls for HeartWare’s left ventricular assist device, the HeartWare LVAD System. Mr. LaRose also leads the development of our miniaturization technology and has twenty years of experience in hydraulic technology development including roles with AEA Technology Engineering Software and Babcock and Wilcox. He holds a Master of Science in Mechanical Engineering from the University of Akron, Ohio.
Dr. David Hathaway.Dr. Hathaway joined HeartWare in June 2008 as our Chief Medical Officer responsible for all medical and clinical affairs, including the design and execution of HeartWare’s clinical trial program. Prior to joining HeartWare, Dr. Hathaway served as a private consultant in the biotechnology and medical device industry from October 2006 to June 2008. From June 2003 to September 2006, Dr. Hathaway was the Chief Medical Officer of Arginox Pharmaceuticals. Prior to joining Arginox, Dr. Hathaway was Vice President, Clinical Development at Restoragen from May 2001 to February 2003. Dr. Hathaway was previously Vice President of Medical Affairs with Knoll Pharmaceutical Company until it was acquired by Abbott Laboratories. He oversaw the Medical Affairs Department and was responsible for clinical research, regulatory affairs, medical information and drug advocacy. Prior to joining Knoll, Dr. Hathaway was Vice President, Cardiovascular Drug Discovery at Bristol-Myers Squibb, where he managed a team of 90 scientists. Before transitioning to a corporate career, he was Division Chief and Director of the Krannert Institute of Cardiology at the Indiana University School of Medicine, where he practiced for more than 14 years. He also served as a Clinical Associate and Cardiology Fellow at the National Institutes of Health in Bethesda, Md. Dr. Hathaway has been section editor (Cardiovascular Diseases) of Kelley’s Textbook of Medicine and a member of the editorial boards of the Journal of Clinical Investigation, the Journal of the American College of Cardiology and Circulation. He has authored over 80 scientific and medical publications and is an inventor on 13 U.S. patents and 8 pending U.S. patent applications. He is a member of the Association of American Physicians, the American College of Physicians and the American Society for Clinical Investigation and is a fellow in the American College of Cardiology. He earned his medical degree from the Indiana University School of Medicine.
87
Lauren Farrell.Ms. Farrell joined HeartWare in November 2006 as Group Director, Finance and was promoted to Vice President, Finance in August 2008. Ms. Farrell has overall responsibility for the Company’s accounting and finance activities. Ms. Farrell has over 20 years accounting and finance experience including roles in public accounting, financial management and reporting, and strategic financial consulting. Prior to joining HeartWare, Ms. Farrell was Chief Financial Officer of Ambient Corporation from March 2005 to January 2006. From January 2001 to July 2004, Ms. Farrell served as Vice President at Bingham Strategic Advisors. Ms. Farrell is a Certified Public Accountant and holds a Bachelors of Science in Accounting and a Masters of Business Administration from Bentley College.
Lawrence Knopf. Mr. Knopf joined HeartWare in March 2011 as our Senior Vice President, General Counsel and Secretary. Mr. Knopf has overall responsibility for the Company’s legal and compliance functions. Between 1993 and 2010, Mr. Knopf served in a variety of legal positions at Boston Scientific Corporation, a global medical device company. From 2007, Mr. Knopf was Senior Vice President and Deputy General Counsel, from 1994, Vice President and Assistant General Counsel and from 1993, Assistant General Counsel. Previously, Mr. Knopf was a corporate associate at the Boston law firms of Bingham McCutchen, LLP and Gaston & Snow. Mr. Knopf received a Juris Doctor from the University of Michigan School of Law and holds a Bachelor of Science, Accounting and Political Science, from The Wharton School of the University of Pennsylvania. He is admitted to the Bar in Massachusetts, New York and Connecticut and is admitted as a Certified Public Accountant in Connecticut.
James Schuermann.Mr. Schuermann joined HeartWare in September 2007. He has overall responsibility for HeartWare’s sales and marketing activities across all markets. Mr. Schuermann has over 15 years of sales and marketing experience in the medical device arena. Prior to joining HeartWare, he spent nine years in sales and marketing at Boston Scientific Corporation. Over this time he progressed from sales through product management until being appointed Director of Marketing in 2005. Before joining Boston Scientific, he spent 5 years in medical sales and sales management at Sherwood Davis & Geck. Mr. Schuermann received his undergraduate degree in marketing from Kelley School of Business, Indiana University, Bloomington, and his MBA from Ageno School of Business, Golden Gate University, San Francisco.
Robert Yocher.Mr. Yocher joined HeartWare in June 2011 as our Senior Vice President Regulatory and Quality. Mr.Yocher has overall responsibility for the Company’s quality and regulatory activities. Between 1999 and 2011, Mr. Yocher was Vice President, Regulatory Affairs and Corporate Quality Compliance at Genzyme Corporation, a global biopharmaceuticals and medical device company. Previously, Mr. Yocher worked in a variety of senior regulatory, quality and clinical positions at EDAP Technomed, Inc., a urological medical device company, BioField Corporation, a development stage medical device company, and Dornier Medical Systems, a therapeutic device company, among others. Mr. Yocher holds a Master of Health Science, Public Health Microbiology and Epidemiology, from Quinnipiac University and a Bachelor of Arts, Microbiology/Chemistry, from the University of Connecticut.
Other Information
We have a code of business conduct and ethics that applies to each director, officer and employee of the Company, including the executive, financial and accounting officers. Our code of conduct is available on our website at www.heartware.com.
The other information required by this Item 10 is incorporated herein by reference to the applicable information in our definitive proxy statement for our 2012 annual meeting of stockholders to be filed with the Securities and Exchange Commission or is to be included in Item 10 of an amendment to this Annual Report on Form 10-K to be filed with the Securities and Exchange Commission.
Item 11. Executive Compensation
The information required by this Item 11 is incorporated herein by reference to the applicable information in our definitive proxy statement for our 2012 annual meeting of stockholders to be filed with the Securities and Exchange Commission, including the information set forth under the caption “Executive Compensation” or is to be included in Item 11 of an amendment to this Annual Report on Form 10-K to be filed with the Securities and Exchange Commission.
88
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item 12 is incorporated herein by reference to the applicable information in our definitive proxy statement for our 2012 annual meeting of stockholders to be filed with the Securities and Exchange Commission, including the information set forth under the caption “Security Ownership of Certain Beneficial Owners and Management” or is to be included in Item 12 of an amendment to this Annual Report on Form 10-K to be filed with the Securities and Exchange Commission.
Australian Disclosure Requirements
In addition to our primary listing on NASDAQ, we are also listed for quotation in the form of CHESS Depositary Interests (“CDIs”) on the Australian Securities Exchange (“ASX”) and trade under the symbol “HIN”. As part of our ASX listing, we are required to comply with various disclosure requirements as set out in the ASX Listing Rules. The following information is intended to comply with the ASX Listing Rules and is not intended to fulfill information required by Part III of this Annual Report on Form 10-K.
Substantial Shareholders
The number of CDIs held by our substantial shareholders (being shareholders who, together with their associates, have a relevant interest in at least 5% of our voting shares) should their stockholdings be converted from common stock into CDIs as at 31 December 2011 are set out below:
| September 30, | September 30, | |||||||
| Number of CDIs | Percentage % | |||||||
FMR LLC and affiliates | 71,128,085 | 14.4 | ||||||
Wellington Management Company, LLC | 65,131,185 | 13.2 | ||||||
Apple Tree Partners | 58,588,670 | 11.9 | ||||||
T. Rowe Price Associates, Inc. | 40,069,610 | 8.1 | ||||||
Mr. Muneer A. Satter | 36,872,500 | 7.5 | ||||||
Janus Capital Management | 27,855,170 | 5.6 | ||||||
The above amounts are based solely upon information furnished to us or contained in reports filed with the Securities and Exchange Commission.
Distribution of equity security holders as at 15 February 2012
As at 15 February 2012, there were 14,114,055 shares of our common stock outstanding, a portion of which were held as CDIs. The table below presents the number of shares of common stock and the number of CDIs held.
| September 30, | September 30, | September 30, | September 30, | September 30, | September 30, | September 30, | September 30, | |||||||||||||||||||||||||
| Common Stock | CDIs* | Options (unlisted) | RSU’s (unlisted) | |||||||||||||||||||||||||||||
| Number of holders | Number of Shares | Number of holders | Number of CDIs | Number of holders | Number of options | Number of holders | Number of RSU’s | |||||||||||||||||||||||||
1 – 1,000 | 3 | 1,133 | 108 | 67,019 | 41 | 22,629 | 59 | 33,633 | ||||||||||||||||||||||||
1,001 – 5,000 | 1 | 1,914 | 166 | 517,245 | 31 | 83,422 | 83 | 195,649 | ||||||||||||||||||||||||
5,001 – 10,000 | 0 | — | 146 | 1,242,060 | 9 | 48,074 | 15 | 106,425 | ||||||||||||||||||||||||
10,001 – 100,000 | 0 | — | 227 | 7,720,366 | 3 | 76,148 | 10 | 296,651 | ||||||||||||||||||||||||
100,001 – and over | 2 | 12,868,510 | 40 | 33,940,740 | 1 | 149,464 | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| 6 | 12,871,557 | 687 | 43,487,430 | 85 | 379,737 | 167 | 632,358 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
89
* Holders of CDIs may receive 1 share of common stock for every 35 CDIs held by them. The common stock equivalent of the number of CDIs outstanding at 15 February 2012 was 1,242,498 shares.
Unmarketable parcels
As at 15 February 2012, the number of shareholders holding less than a marketable parcel (for the purposes of the ASX Listing Rules) was 23.
Top 20 CDI Holders
| September 30, | September 30, | September 30, | ||||||||
No. | Holder | Number of CDIs held | % of CDIs Outstanding | |||||||
| 1. | J P Morgan Nominees Australia Limited | 9,314,710 | 21.42 | |||||||
| 2. | HSBC Custody Nominees (Australia) Limited | 6,510,974 | 14.97 | |||||||
| 3. | Warman Investments Pty. Ltd. | 6,227,057 | 14.32 | |||||||
| 4. | National Nominees Limited | 1,660,540 | 3.82 | |||||||
| 5. | Mr. Robert Thomas and Mrs. Kyrenia Thomas <Rob Thomas Super Fund A/C> | 747,950 | 1.72 | |||||||
| 6. | Viking Management Services Pty. Ltd. <VHK Superannuation Fund A/C> | 727,265 | 1.67 | |||||||
| 7. | JP Morgan Nominees Australia Limited <Cash Income A/C> | 681,318 | 1.57 | |||||||
| 8. | Asia Union Investments Pty. Ltd. | 500,000 | 1.15 | |||||||
| 9. | Mrs. Kyrenia Thomas | 500,000 | 1.15 | |||||||
| 10. | Spectrok Pty. Ltd. <Hedley Family A/C> | 499,975 | 1.15 | |||||||
| 11. | PBC Investments Pty. Limited <PBC Super Fund A/C> | 412,000 | 0.95 | |||||||
| 12. | Citicorp Nominees Pty. Limited | 404,911 | 0.93 | |||||||
| 13. | Mr. Alex Proimos | 389,066 | 0.89 | |||||||
| 14. | Merrill Lynch (Australia) Nominees Pty. Limited | 355,032 | 0.82 | |||||||
| 15. | Mr. Olev Rahn | 350,000 | 0.80 | |||||||
| 16. | Maerborg Pty. Ltd. <Stevenson Family S/F A/C> | 334,915 | 0.77 | |||||||
| 17. | Debuscey Pty. Ltd. | 299,990 | 0.69 | |||||||
| 18. | Mr. Robert Gordon Bruce Roberts <Rosbruce A/C> | 250,000 | 0.57 | |||||||
| 19. | Ms. Jiren Chen | 243,647 | 0.56 | |||||||
| 20. | Mr. Philip James Currie + Mrs. Anne Jennifer Currie <Currie Family S/Fund A/C> | 238,630 | 0.55 | |||||||
Total CDIs held by top 20 shareholders | 30,647,980 | 70.48 | ||||||||
Total CDIs held by all other shareholders | 12,839,450 | 29.52 | ||||||||
Two individuals, Todd Frazier and Stephen Boyce, own 20% or more of the Company’s options issued outside employee incentive plans, with each owning options to subscribe for 2,285 and 2,857 shares or common stock, respectively.
Voting Rights
HeartWare International’s by-laws provide that each stockholder has one vote for every share of common stock entitled to vote held of record by such stockholder and a proportionate vote for each fractional share of stock entitled to vote so held, unless otherwise provided by Delaware General Corporation Law or in the certificate of incorporation.
Holders of CDIs have one vote for every 35 CDIs held of record by such stockholder.
If holders of CDIs wish to attend HeartWare International general meetings, they will be able to do so. Under the ASX Listing Rules, HeartWare International, as an issuer of CDIs, must allow CDI holders to attend any meeting of the holders of the underlying securities unless relevant U.S. law at the time of the meeting prevents CDI holders from attending those meetings.
90
In order to vote at such meetings, CDI holders have the following options:
| (a) | instructing CDN, as the legal owner, to vote the HeartWare International shares of common stock underlying their CDIs in a particular manner. The instruction form must be completed and returned to HeartWare International’s share registry prior to the meeting; |
| (b) | informing HeartWare International that they wish to nominate themselves or another person to be appointed as CDN’s proxy for the purposes of attending and voting at the general meeting; |
| (c) | converting their CDIs into a holding of HeartWare International shares of common stock and voting these at the meeting (however, if thereafter the former CDI holder wishes to sell their investment on ASX, it would be necessary to convert HeartWare International shares of common stock back to CDIs). This must be done prior to the record date for the meeting. See section 7 below for further information regarding the conversion process. |
As holders of CDIs will not appear on HeartWare International’s share register as the legal holders of HeartWare International shares of common stock, they will not be entitled to vote at HeartWare International shareholder meetings unless one of the above steps is undertaken.
Proxy forms and details of these alternatives will be included in each notice of meeting sent to CDI holders by HeartWare International.
Holders of options and restricted stock units are not entitled to vote.
Required Statements
The Company makes the following disclosures:
| (a) | There is no current on-market buy-back of the Company’s securities. |
| (b) | HeartWare International, Inc. was incorporated in the state of Delaware in the United States of America. |
| (c) | The Company is not subject to Chapters 6, 6A, 6B or 6C of the Corporations Act 2001 (Cth) dealing with the acquisitions of shares (ie, substantial shareholdings and takeovers). |
| (d) | Under the Delaware General Corporation Law, shares are generally freely transferable subject to restrictions imposed by U.S. federal or state securities laws, by the certificate of incorporation or by-laws of the Company or by an agreement signed with the holders of the shares at issue. The Company’s certificate of incorporation and by-laws do not impose any specific restrictions on transfer. |
General Information
The name of the Company Secretary is Mr. Lawrence J. Knopf.
The address of the principal registered office in Australia is Unit 2, 3 Marshall Road, Kirrawee, NSW 2232 (02) 8078 6164.
Registers of securities are held at Computershare Investor Services Pty. Limited, Level 3, 60 Carrington Street, SYDNEY NSW 2000, Investor Enquiries: 1300 855 080. A list of registered holders of the common stock of HeartWare International entitled to vote at the general meeting of stockholders is available at the corporate headquarters of the Company, 205 Newbury Street, Suite 101, Framingham, MA 01701 USA.
91
Quotation has been granted for the Company’s CDIs on ASX Limited. In addition, the Company’s common stock became listed for quotation on NASDAQ on 24 February 2009.
Australian Corporate Governance Statement
The Board of Directors and employees of HeartWare International, Inc. (“HeartWare” or “the Company”) are committed to developing, promoting and maintaining a strong culture of good corporate governance and ethical conduct.
The Board of Directors is pleased to confirm that the Company’s corporate governance framework is generally consistent with the ASX’s Corporate Governance Council’s “Corporate Governance Principles and Recommendations with 2010 amendments (2nd Edition)” (“ASX Governance Recommendations”), other than as set out below. To this end, the Company provides below a review of its corporate governance framework using the same numbering as adopted for the Principles as set out in the ASX Governance Recommendations.
Copies of the Company’s codes and policies may be downloaded from the corporate governance section of the HeartWare website (www.heartware.com).
It should be noted that the Company redomiciled to the United States in November 2008 and listed on NASDAQ in late February 2009. As a result and to meet NASDAQ listing requirements, the policies and practices adopted by the Company have been adopted to be consistent with U.S. listing standards.
Principle 1—Lay solid foundations for management and oversight
Recommendation 1.1 – Establish the functions reserved to the Board of Directors and those delegated to senior executives and disclose those functions
The primary responsibility of:
| (a) | the Board of Directors is to provide effective governance over the business and affairs of HeartWare and its controlled entities (“the HeartWare Group”) so that the interests of all stakeholders are protected; and |
| (b) | the Chief Executive Officer is to oversee the day-to-day performance of the HeartWare Group (pursuant to Board delegated powers). |
The Board’s responsibilities are recognized and documented on an aggregated basis via the Corporate Governance Guidelines established by the Board of Directors and via Letters of Appointment for each individual director. A copy of the Guidelines is available on the corporate governance section of the Company’s website.
While day-to-day management has been delegated to the Chief Executive Officer, it is noted that the following matters are specifically reserved for the attention of the Board:
| (a) | decisions about corporate strategy and policies as well as commitments over prescribed limits; |
| (b) | setting major capital expenditure, acquisitions, divestments and funding arrangements; |
| (c) | setting the various internal controls and reporting framework for the management of the risks inherent in the operations of the HeartWare Group; |
| (d) | setting of discretionary financial and related operating limits for management; and |
| (e) | establishing and determining the powers and functions of the committees of the Board. |
Recommendation 1.2 – Disclose the process for evaluating the performance of senior executives
The Company’s 2011 definitive proxy statement filed with the Securities and Exchange Commission included extensive discussions in relation to the mechanics concerning the evaluation of performance of the Company’s
92
senior executives, including relevant benchmarking activities. Information regarding executive compensation for the year ended 31 December 2011, as required by Item 11, is incorporated by reference to the applicable information in our definitive proxy statement for our 2012 annual meeting of stockholders to be filed with the Securities and Exchange Commission, including the information set forth under the captions “Executive Compensation,” “Compensation of Directors” and “Compensation Committee Interlocks and Insider Participation,” or is to be included in Item 11 of an amendment to this Annual Report on Form 10-K to be filed with the Securities and Exchange Commission.
Recommendation 1.3 – Disclosure of information indicated in the guide to reporting on Principle 1 of the ASX Governance Recommendations
Reporting Requirement
The Company fully complied with Recommendation 1.1 to 1.3 during the year ended 31 December 2011.
Principle 2—Structure the Board to add value
Recommendation 2.1 – A majority of the Board of Directors should be independent
Recommendation 2.2 – The Chair should be an independent director
Recommendation 2.3 – The roles of Chairman and Chief Executive Officer should not be exercised by the same individual
At February 2012, the Board of Directors presently comprises eight (8) directors. The eight (8) directors include six (6) independent non-executive directors (including the Chairman of the Board), one (1) executive director (being the Chief Executive Officer) and one (1) non-independent, non-executive director (being the Deputy Chairman).
The current composition of the Board and length of tenure of each member of the Board is as follows:
Name | Position | Date Appointed | Tenure* | Independent | ||||||||||
Rob Thomas | Non-executive director | 26 Nov 2004 | 7.1 years | Yes | ||||||||||
Seth Harrison | Non-executive Deputy Chairman | 26 Nov 2004 | 7.1 years | No | ||||||||||
Denis Wade | Non-executive director | 15 Dec 2004 | 7.0 years | Yes | ||||||||||
Christine Bennett | Non-executive director | 15 Dec 2004 | 7.0 years | Yes | ||||||||||
Bob Stockman | Non-executive director | 11 Dec 2006 | 5.1 years | Yes | ||||||||||
Ray Larkin Jr | Non-executive Chairman | 3 Oct 2009 | 3.2 years | Yes | ||||||||||
Tim Barberich | Non-executive director | 29 Apr 2009 | 3.7 years | Yes | ||||||||||
Doug Godshall | Chief Executive Officer / President / Executive Director | 28 Oct 2006 | 5.2 years | No | ||||||||||
| * | Calculated as at 31 December 2011. |
93
Independent advice
At the Company’s expense, the Board collectively or directors (acting as individuals) are entitled to seek advice from independent external advisers in relation to any matter which is considered necessary to fulfill their relevant duties and responsibilities.
Individual directors seeking such advice must obtain the approval of the Chairman (which may not be unreasonably withheld). Any advice so obtained will be made available to all Board members.
Recommendation 2.4 – The Board should establish a Nomination Committee
The members of the Nominating and Governance Committee are Mr. Barberich, Mr. Larkin (Chair) and Mr. Thomas. A copy of the Nominating and Governance Committee Charter is available on the corporate governance section of the Company’s website. The Nominating and Governance Committee met four times during 2011 with each of Mr. Barberich, Mr. Larkin and Mr. Thomas attending on all occasions. HeartWare International’s Corporate Governance Guidelines and the Charter of the Nominating and Governance Committee establish a process for selecting candidates for nomination as a member of the Board, reviewing director qualifications and determining the appropriate mix of skills, experience and characteristics necessary to meet the current needs of the Board. The Committee may engage as it deems appropriate a third party search firm to assist in identifying qualified director candidates.
Reporting Requirement
The Company fully complied with Recommendation 2.1 to 2.4 during the year ended 31 December 2011.
Recommendation 2.5 – Disclose the process for evaluating the performance of the Board, its committees and individual directors
Reporting Requirement
The Company has not undertaken a formal review of the performance of the Board, its committees and individual directors. The Company has not therefore complied with Recommendation 2.5 during the year ended 31 December 2011. In 2012, the Nominating and Governance Committee distributed a written questionnaire to each member of the Board to solicit input as the performance and effectiveness of the Board and the Committees of the Board during 2011.
Recommendation 2.6 – Disclosure of information indicated in the guide to reporting on Principle 2 of the ASX Governance Recommendations
Reporting Requirement
Information regarding Directors, including biographical information, key attributes, experience and skills as required by Item 12, is incorporated herein by reference to the applicable information in our definitive proxy statement for our 2011 annual meeting of stockholders to be filed with the Securities and Exchange Commission, including the information set forth under the caption “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters,” or is to be included in Item 12 of an amendment to this Annual Report on Form 10-K to be filed with the Securities and Exchange Commission. The Nominating and Governance Committee is responsible for identifying and reviewing with the Board from time to time the appropriate skills and characteristics required of Board members in the context of the current makeup and needs of the Board. In accordance with HeartWare International’s Corporate Governance Guidelines, this assessment includes issues of diversity, age, skills such as understanding of manufacturing, technology, intellectual property, finance and marketing, and international background, all in the context of an assessment of the perceived needs of the Board at that point in time. Pursuant to the Guidelines, directors should possess the highest personal and professional ethics, integrity and values, and be committed to representing the long-term interests of stockholders. They must also have an inquisitive and objective perspective and mature judgment.
The Company has fully complied with Recommendation 2.6 during the year ended 31 December 2011.
94
Principle 3 – Promote ethical and responsible decision-making
Recommendation 3.1 – Establish a Code of Conduct and disclose it
The Company has adopted a Code of Business Conduct and Ethics, a copy of which is available on the corporate governance section of the Company’s website.
The Company fully complied with Recommendation 3.1 during the year ended 31 December 2011.
Recommendation 3.2—Establish a policy concerning diversity and disclose it. The policy should include requirements for the Board to establish measurable objectives for achieving gender diversity and for the Board to assess annually both the objectives and progress in achieving them.
During 2011, the Board of Directors adopted a Diversity Policy which includes the responsibility to establish appropriate and measurable diversity objectives and for the Board to assess regularly the overall effectiveness of the objectives and annually review the progress in achieving the diversity objectives. A copy of the Diversity Policy is available on the corporate governance section of the Company’s website.
Recommendation 3.3—Disclosure of measurable objectives for achieving gender diversity set by the Board in accordance with the Diversity Policy and progress towards achieving them.
During 2011, the Board of Directors adopted a Diversity Policy. Diversity at HeartWare signifies not only a blend of races, genders, ages, ethnicities, religions, cultural backgrounds, languages, social backgrounds and military service but also a range of experiences, perspectives, skill sets, capabilities, and thought. The Board, with the assistance of the Nominating and Governance Committee and management, intends to use 2011 data as a baseline to establish and measure diversity objectives for 2012 and beyond.
Recommendation 3.4—Disclosure of the proportion of women employees in the whole organisation, women in senior executive positions and women on the Board
HeartWare is committed to driving diversity across all levels of the Company. At 31 December 2011, women represented approximately 41% ( 134 of 330) of the total employee base, 18% (2 of 11) of executive management and 12.5% (1 of 8) of the Board of Directors.
Recommendation 3.5—Disclosure of information indicated in the guide to reporting on Principle 3 of the ASX Governance Recommendations
The Company fully complied with Recommendation 3.1 to 3.5 during the year ended 31 December 2011.
Principle 4 – Safeguard integrity in financial reporting
Recommendation 4.1 – The Board should establish an Audit Committee
Recommendation 4.2 – The Audit Committee should: (a) consist of non-executive directors only; (b) consist of a majority of independent directors; (c) be chaired by an independent chair who is not chair of the Board; and (d) have at least three members
Recommendation 4.3 – The Audit Committee should have a formal charter
The members of the Audit Committee are Dr. Bennett, Mr. Stockman (Chair), Mr. Thomas and Dr. Wade all of whom are independent non-executive directors. The Audit Committee met seven times during 2011 with each of Dr. Bennett, Mr. Stockman (Chair) and Dr. Wade attending on all occasions. Mr. Thomas attended on six occasions.
A copy of the Audit Committee Charter is available on the corporate governance section of the Company’s website.
Reporting Requirement
The Company fully complied with Recommendation 4.1 to 4.3 during the year ended 31 December 2011.
Recommendation 4.4 – Disclosure of information indicated in the guide to reporting on Principle 4 of the ASX Governance Recommendations
95
Reporting Requirement
Information regarding the skills, experience and expertise of directors, including audit committee members, in accordance with U.S. disclosure requirements is included by reference to the applicable information in our definitive proxy statement for our 2011 annual meeting of stockholders to be filed with the Securities and Exchange Commission. To the extent these disclosures are not specifically restated within in this Corporate Governance Statement then the Company has not complied with the requirement to include this information within the Corporate Governance Statement.
In Item 9A of this Annual Report on Form 10-K, we have disclosed information regarding the Company’s Controls and Procedures, including management’s evaluation of the effectiveness of our disclosure controls and procedures and management’s evaluation of the effectiveness of our internal control over financial reporting. To the extent these disclosures are not specifically restated within in this Corporate Governance Statement then the Company has not complied with the requirement to include this information within the Corporate Governance Statement.
Our independent registered public accounting firm, Grant Thornton LLP, has issued a report on our internal control over financial reporting, which is included in Item 9A of this Annual Report on Form 10-K.
The Company has not disclosed its policy for selection and appointment of the Company’s external auditor or for the rotation of external audit engagement partners.
In all other respects, the Company fully complied with Recommendation 4.4 during the year ended 31 December 2011.
Principle 5 – Make timely and balanced disclosure
Recommendation 5.1 – Establish written policies designed to ensure compliance with ASX Listing Rule disclosure requirements and to ensure accountability at a senior executive level for that compliance and disclose those policies
Recommendation 5.2 – Disclosure of information indicated in the guide to reporting on Principle 5 of the ASX Governance Recommendations
HeartWare is committed to providing timely and balanced disclosure to the market and, in consequence, to meeting its continuous disclosure requirements. In accordance with its commitment to fully comply with its continuous disclosure requirements, the Company has adopted a Continuous Disclosure Policy, together with other internal mechanisms and reporting requirements.
A copy of the Continuous Disclosure Policy is available on the corporate governance section of the Company’s website. In addition, a copy of all of the Company’s ASX announcements, financial reports and related public information are also available on the Company’s website.
Reporting Requirement
The Company fully complied with Recommendation 5.1 and 5.2 during the year ended 31 December 2011.
Principle 6 – Respect the rights of shareholders
Recommendation 6.1 – Design a communications policy for promoting effective communication with shareholders and encourage their participation at general meetings and disclose those policies
Recommendation 6.2 – Disclosure of information indicated in the guide to reporting on Principle 6 of the ASX Governance Recommendations
The Company has implemented a number of measures so as to facilitate what it believes to be the effective and efficient exercise of the rights of shareholders. The Company communicates information to shareholders through a range of media including annual reports, public (ASX) announcements, webcasts of earnings calls and other significant presentations to analysts and investors, and via the Company’s website including advance notification of
96
webcast investor presentations and earnings announcements. Key financial information and stock performance are also available on the Company’s website. Shareholders can raise questions with the Company by contacting the Company via telephone, facsimile, post or email, with relevant contact details being available on the Company’s website. Investor Relations maintains a record for internal use of significant presentations to analysts and investors, including conferences and individual briefings, as well as a general description of topics discussed.
All shareholders are invited to attend the Company’s Annual General Meeting, either in person or by proxy. The Board regards the Annual General Meeting as an excellent forum in which to discuss issues relevant to the Company and thereby encourages full participation by shareholders. Shareholders have an opportunity to submit questions to the Board and the Company’s auditors.
Reporting requirement
The Company substantially complied with Recommendation 6.1 and 6.2 for the year ended 31 December 2011.
Principle 7 – Recognise and manage risk
Recommendation 7.1 – Establish policies for the oversight and management of material business risks and disclose it
The risks that the Company faces are continually changing in line with the development of the Company. The primary risks faced by the Company during 2011 included liquidity or funding risk, operational risks associated with the manufacture of an implantable medical device, ongoing risks of the Company’s human clinical trials and achieving relevant regulatory hurdles which will unlock key markets for the Company’s products together with growing revenue and inventory management.
The above is set in an environment where the Company must actively manage fundamental risks such as the integrity of the Company’s intellectual property portfolio, disaster management, exchange rate risk and the risk of losing key management personnel.
In simple terms, risk is inherent in all activities undertaken by HeartWare. Unfortunately, many of these risks are beyond the control of the Company and, as such, it is important that risk be mitigated on a continuous basis, particularly if the Company is to preserve shareholder value.
The Board of Directors has approved a Risk Management Policy, a copy of which is available on the corporate governance page of the Company’s website. In summary, the Risk Management Policy is designed to ensure that risks including, amongst others, technology risks, economic risks, financial risks and other operational risks are identified, evaluated and mitigated to enable the achievement of the Company’s goals.
It would be remiss of the Board not to acknowledge that no risk management system can provide total assurance that HeartWare’s risks will be fully mitigated. This is particularly the case in organizations such as HeartWare where its pre-revenue status means that limited resources can be applied to the risk management process. HeartWare’s approach is therefore not to eliminate risk, rather to utilize available resources as effectively as possible in order to manage the risks inevitably involved in many corporate activities.
Reporting requirement
The Company fully complied with Recommendation 7.1 for the year ended 31 December 2011.
Recommendation 7.2 – Require management to design and implement the risk management and internal control system to manage the Company’s material business risks and report to it whether those risks are being managed effectively (and makes disclosures therein); Disclose that management has reported to the Board as to the effectiveness of the Company’s management of its material business risks
Management provides the Board with frequent (i.e. generally monthly) updates on the state of the Company’s business, including the risks that the Company faces from time-to-time. This update includes up-to-date financial information, operational activity, clinical status and competitor updates. These updates are founded on internal
97
communications that are fostered internally through weekly management meetings and other internal communications. These processes operate in addition to the Company’s system of internal controls over financial reporting, its Quality System, complaint handling processes, employee policies and standard operating procedures.
In addition, the Board of Directors holds regular meetings at the Company’s facility in Miami Lakes for the purposes of discussing and reviewing operational developments.
The Company fully complied with Recommendation 7.2 for the year ended 31 December 2011.
Recommendation 7.3 – Disclose whether the Board has received assurance from the Chief Executive Officer and the Chief Financial Officer that the declaration under Section 295A of the Corporations Act is founded on a sound system of risk management and internal control and is operating effectively in all material respects in relation to financial reporting risks
Reporting requirement
As the Company prepares and files its financial statements under U.S. accounting practices and laws, management is required to provide representations to the Board on a wide range of issues, including in relation to the effectiveness of the Company’s disclosure controls and procedures as well as the design or operation of internal control over financial reporting.
Notwithstanding the above and in consequence of its dual listed status and various waivers granted by the ASX and the Australian Securities and Investments Commission, no declaration is required under Section 295A of the Corporations Act. To this end, shareholders’ attention is drawn to Item 9A of this Annual Report on Form 10-K and the certifications provided by the Chief Executive Officer and the Vice President of Finance at the end of the Form 10-K. As stated above, Item 9A of this Annual Report on Form 10-K discloses information regarding the Company’s controls and procedures, including management’s evaluation of the effectiveness of our disclosure controls and procedures and management’s evaluation of the effectiveness of our internal control over financial reporting.
For the reasons stated above, the Company has not complied with Recommendation 7.3 for the year ended 31 December 2011.
Recommendation 7.4 – Disclosure of information indicated in the guide to reporting on Principle 7of the ASX Governance Recommendations
Reporting requirement
Except as disclosed above, the Company believes that the aforementioned reporting meets, or otherwise exceeds, the requirements of Recommendation 7.2 to 7.4 for the year ended 31 December 2011.
Principle 8 – Remunerate fairly and responsibly
Recommendation 8.1 – Establish a Remuneration Committee
Recommendation 8.2 – The Remuneration Committee should be structured so that it: (a) consist of a majority of independent directors; (b) is chaired by an independent chair; and (c) has at least three members
The members of the Compensation Committee are Mr. Barberich (Chair), Mr. Thomas, Dr. Bennett and Dr. Wade all of whom are independent non-executive directors. A copy of the Compensation Committee Charter is available on the corporate governance section of the Company’s website. The Compensation Committee met five times during 2011 with each of Mr. Barberich, Mr. Thomas, and Dr. Wade attending on all occasions and Dr. Bennett attended on four occasions.
During 2011, the Board of Directors adopted a Diversity Policy. The Board, with the assistance of the Nominating and Governance Committee and management, intends to use data from 2011 to establish a baseline to establish and measure diversity objectives for 2012 and beyond. Accordingly, the Compensation Committee did not assess remuneration by gender during 2011.
98
Recommendation 8.3 – Clearly distinguish the structure of non-executive directors’ remuneration from that of executive directors and senior executives
As noted above in the discussion regarding Recommendation 1.2, the definitive proxy statement for the Company’s 2011 annual meeting of stockholders includes extensive discussions in relation to the mechanics concerning the evaluation of performance of the Company’s senior executives. Information is also included in relation to the Company’s remuneration practices and policies, including its annual performance review process, its external benchmarking review and its meritorious approach to employee performance.
Reporting requirement
As previously disclosed no review or other form of assessment has been undertaken in relation to the non-executive directors.
Recommendation 8.4 – Disclosure of information indicated in the guide to reporting on Principle 8 of the ASX Governance Recommendations
Reporting requirement
With the exception noted above, the Company complied with Recommendation 8.1 to 8.3 during the year ended 31 December 2011.
This report is made in accordance with a resolution of the Board of Directors.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item 13 is incorporated herein by reference to the applicable information in our definitive proxy statement for our 2012 annual meeting of stockholders to be filed with the Securities and Exchange Commission, including the information set forth under the captions “Certain Relationships and Related Transactions, and Director Independence”, “Policies and Procedures for Review and Approval of Related Party Transactions”, “Corporate Governance” and “Compensation Committee Interlocks and Insider Participation,” or is to be included in Item 13 of an amendment to this Annual Report on Form 10-K to be filed with the Securities and Exchange Commission.
Item 14. Principal Accounting Fees and Services
The information required by this Item 14 is incorporated herein by reference to the applicable information in our definitive proxy statement for our 2012 annual meeting of stockholders to be filed with the Securities and Exchange Commission, including the information set forth under the captions “Principal Accounting Fees and Services” and “Audit Committee’s Pre-Approval Policy,” or is to be included in Item 14 of an amendment to this Annual Report on Form 10-K to be filed with the Securities and Exchange Commission.
99
Item 15. Exhibits, Financial Statement Schedules
The following documents are filed as part of this Annual Report on Form 10-K:
| 1. | Financial Statements: | |
| Report of Independent Registered Public Accounting Firm | ||
| Consolidated Balance Sheets | ||
| Consolidated Statements of Operations | ||
| Consolidated Statements of Comprehensive Loss | ||
| Consolidated Statement of Stockholders’ Equity | ||
| Consolidated Statements of Cash Flows | ||
| Notes to Consolidated Financial Statements | ||
| 2. | Financial Statement Schedules: | |
| Required schedule information is included in the Notes to Consolidated Financial Statements or is omitted because it is either not required or not applicable. | ||
| 3. | Exhibits: | |
| See Exhibit Index | ||
100
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| HeartWare International, Inc. | ||||||
| Date: February 27, 2012 | By | /s/ Douglas Godshall | ||||
| Name: | Douglas Godshall | |||||
| Title: | President and Chief Executive Officer | |||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Title | Date | ||
/s/ Douglas Godshall | President, Chief Executive Officer and | |||
Douglas Godshall | Director (Principal Executive Officer) | February 27, 2012 | ||
/s/ Lauren Farrell | Vice President of Finance | |||
Lauren Farrell | (Principal Financial and Accounting Officer) | February 27, 2012 | ||
101
Name | Title | Date | ||
/s/ C. Raymond Larkin, Jr. | ||||
C. Raymond Larkin, Jr. | Chairman and Director | February 22, 2012 | ||
/s/ Timothy J. Barberich | ||||
Timothy J. Barberich | Director | February 22, 2012 | ||
/s/ Christine Bennett | ||||
Christine Bennett | Director | February 22, 2012 | ||
/s/ Seth Harrison | ||||
Seth Harrison | Director | February 22, 2012 | ||
/s/ Robert Stockman | ||||
Robert Stockman | Director | February 21, 2012 | ||
/s/ Robert Thomas | ||||
Robert Thomas | Director | February 23, 2012 | ||
/s/ Denis Wade | ||||
Denis Wade | Director | February 20, 2012 | ||
102
Exhibit Index
Exhibit No. | Description | |
| 2.1 | Implementation Agreement, dated as of August 5, 2008, between HeartWare International, Inc. and HeartWare Limited. (13) | |
| 2.2 | Agreement and Plan of Merger, dated as of February 12, 2009, among HeartWare International, Inc., Thoratec Corporation, Thomas Merger Sub I, Inc. and Thomas Merger Sub II, Inc. (14) | |
| 3.1 | Certificate of Incorporation of HeartWare International, Inc.(4) | |
| 3.2 | Bylaws of HeartWare International, Inc. (4) | |
| 10.01 | Securities Exchange Agreement between Apple Tree Partners I, L.P., Anthony Low-Beer, Edward Nerssissian, Garrett and Carol Thunen, HeartWare, Inc. and HeartWare Limited dated December 13, 2004 (1) | |
| 10.02 | Amended and Restated Employment Agreement, dated as of December 16, 2009, by and between HeartWare International, Inc. and Douglas Godshall (11) + | |
| 10.03 | Amended and Restated Employment Agreement, dated as of December 16, 2009, by and between HeartWare, Inc. and David McIntyre (19)+ | |
| 10.04 | Amended and Restated Employment Agreement, dated as of December 16, 2009, by and between HeartWare, Inc. and Jeffrey LaRose (20)+ | |
| 10.05 | Employment Agreement, dated as of December 5, 2008, between HeartWare, Inc. and James Schuermann (5) + | |
| 10.07 | Employment Agreement, dated as of December 5, 2008 between HeartWare, Inc. and David R. Hathaway, M.D. (12) + | |
| 10.08 | Form of Amendment to Employment Agreement (for Section 16 officers), dated December 2010 (27)+ | |
| 10.09 | Form of Deed of Indemnity, Access and Insurance Agreement for directors and executive officers (1) + | |
| 10.10 | Letter of Appointment as a Director of the Company dated December 1, 2006 between HeartWare Limited and Robert Stockman (1) + | |
| 10.11 | Letter of Appointment as a Director of the Company dated December 15, 2004 between HeartWare Limited and Robert Thomas (1) + | |
| 10.12 | Letter of Appointment as a Director of the Company dated December 15, 2004 between HeartWare Limited and Christine Bennett (1) + | |
| 10.13 | Letter of Appointment as a Director of the Company dated December 15, 2004 between HeartWare Limited and Denis Wade (1) + | |
| 10.14 | Letter of Appointment as a Director of the Company dated September 3, 2008 between HeartWare International, Inc. and Ray Larkin (15) + | |
| 10.15 | Letter of Appointment as a Director of the Company dated April 16, 2008 between HeartWare International, Inc. and Timothy J. Barberich (16) + | |
| 10.18 | HeartWare International, Inc. 2008 Stock Incentive Plan (6) + | |
| 10.19 | HeartWare International, Inc. Employee Stock Option Plan (7) + | |
| 10.20 | HeartWare International, Inc. Restricted Stock Unit Plan (8) + | |
| 10.21 | Form of HeartWare International, Inc. Incentive Option Terms (9) + | |
| 10.22 | Nonstatutory Stock Option Notice and Agreement to 2008 Stock Incentive Plan (24) + | |
| 10.23 | Restricted Stock Units Notice and Agreement to 2008 Stock Incentive Plan (25) + | |
| 10.24 | Lease Agreement, dated as of April 17, 2008, between JDRP Associates No. 1, Ltd. and HeartWare, Inc. (“Lease”)(10) | |
| 10.25 | First Amendment to Lease dated September 30, 2010 (2) | |
| 10.26 | Business Lease, dated December 27, 2006, between HeartWare, Inc. and Atlantic-Philadelphia Realty LLC (17) | |
| 10.27 | First Amendment to Business Lease, dated August 19, 2008, between HeartWare, Inc. and Atlantic-Philadelphia Realty LLC (“Business Lease”) (18) | |
| 10.28 | Second Amendment to Business Lease, dated August 9, 2010 (26) | |
103
Exhibit No. | Description | |
| 10.29 | Lease Agreement dated December 8, 2010 by and between MCP EWE LLC, as Landlord, HeartWare, Inc., as Tenant, and guaranteed by HeartWare International, Inc., as Guarantor (28) | |
| 10.30 | Loan Agreement, dated as of February 12, 2009, among HeartWare International, Inc., the Guarantors thereto and Thoratec Corporation (14) | |
| 10.31 | Investor’s Rights Agreement, dated as of February 12, 2009, between HeartWare International, Inc. and Thoratec Corporation (14) | |
| 10.32 | Indenture dated as of December 15, 2010 between the Company and Wilmington Trust FSB, as trustee (21) | |
| 10.33 | First Supplemental Indenture dated as of December 15, 2010 between the Company and Wilmington Trust FSB, as trustee (22) | |
| 10.34 | Form of 3.50% Convertible Senior Notes due 2017 (23) | |
| 10.35 | HeartWare International, Inc. Bonus Plan for Executive Officers, dated February 22, 2011 (29) + | |
| 10.36 | Third amendment to Business Lease, between HeartWare, Inc. and Atlantic-Philadelphia Realty LLC, dated June 30, 2011 (30) | |
| 10.37 | Letter Agreement between HeartWare and David McIntyre dated December 26, 2011 (31) + | |
| 10.38 | Offer letter, dated as of March 21, 2011, between HeartWare, Inc. and Lawrence J Knopf +* | |
| 10.39 | Offer letter, dated as of June 6, 2011, between HeartWare, Inc. and Robert E. Yocher +* | |
| 21.1 | List of Subsidiaries * | |
| 23.1 | Consent of Independent Registered Public Accounting Firm * | |
| 31.1 | Certificate pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934 * | |
| 31.2 | Certificate pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934 * | |
| 32.1 | Certificate pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 ** | |
| 32.2 | Certificate pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 ** | |
| (1) | Incorporated by reference to the respective exhibits filed with the Company’s Registration Statement on Form 10 (File No. 000-52595) filed with the Securities and Exchange Commission on April 30, 2007. |
| (2) | Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on September 30, 2010. |
| (3) | Incorporated by reference to Exhibit 99.1 to the Company’s Registration Statement on Form S-8 (File No. 333-147506) filed with the Securities and Exchange Commission on November 19, 2007. |
| (4) | Incorporated by reference to the respective exhibits filed with the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 13, 2008. |
| (5) | Incorporated by reference to Exhibit 99 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 5, 2008. |
| (6) | Incorporated by reference to Appendix 12 to the Information Memorandum contained in the Company’s Proxy Statement on Form DEF 14A filed with the Securities and Exchange Commission on September 22, 2008. |
| (7) | Incorporated by reference to Appendix 9 to the Information Memorandum contained in the Company’s Proxy Statement on Form DEF 14A filed with the Securities and Exchange Commission on September 22, 2008. |
| (8) | Incorporated by reference to Appendix 10 to the Information Memorandum contained in the Company’s Proxy Statement on Form DEF 14A filed with the Securities and Exchange Commission on September 22, 2008. |
| (9) | Incorporated by reference to Exhibit 99.4 to the Company’s Registration Statement on Form S-8 (File No. 333-155359) filed with the Securities and Exchange Commission on November 13, 2008. |
| (10) | Incorporated by reference to Exhibit 10.01 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on April 18, 2008. |
| (11) | Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 18, 2009. |
| (12) | Incorporated by reference to Exhibit 10.13 to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 26, 2009. |
104
| (13) | Incorporated by reference to Appendix 1 to the Information Memorandum contained in the Company’s Proxy Statement on Form DEF 14A filed with the Securities and Exchange Commission on September 22, 2008. |
| (14) | Incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 13, 2009. |
| (15) | Incorporated by reference to Exhibit 10.27 to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 26, 2009. |
| (16) | Incorporated by reference to Exhibit 10.28 to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 26, 2009. |
| (17) | Incorporated by reference to Exhibit 10.40 to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 28, 2008. |
| (18) | Incorporated by reference to Exhibit 10.27 to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 23, 2010. |
| (19) | Incorporated by reference to Exhibit 10.5 to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 23, 2010. |
| (20) | Incorporated by reference to Exhibit 10.6 to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 23, 2010. |
| (21) | Incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 15, 2010. |
| (22) | Incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 15, 2010. |
| (23) | Incorporated by reference to Exhibit 4.3 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 15, 2010. |
| (24) | Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 6, 2010. |
| (25) | Incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 6, 2010. |
| (26) | Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 19, 2010. |
| (27) | Incorporated by reference to Exhibit 10.08 to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 24, 2011. |
| (28) | Incorporated by reference to Exhibit 10.29 to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 24, 2011. |
| (29) | Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 24, 2011. |
| (30) | Incorporated by reference to Exhibit 10.35 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 5, 2011. |
| (31) | Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 27, 2011. |
| * | Filed herewith |
| ** | Furnished herewith |
| + | Management contract or compensatory plan or arrangement. |
105