SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| |
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended October 31, 2011
OR
| |
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 000-54283
PATHEON INC.
(Exact name of registrant as specified in its charter)
|
| | |
| Canada | | Not Applicable |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | |
c/o Patheon Pharmaceuticals Services Inc. 4721 Emperor Boulevard, Suite 200 Durham, NC | | 27703 |
| (Address of principal executive offices) | | (Zip Code) |
(919) 226-3200
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None.
Securities registered pursuant to Section 12(g) of the Act:
Restricted Voting Shares
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
|
| | | | |
| Large accelerated filer | o | | Accelerated filer | o |
| | | | | |
| Non-accelerated filer | x (Do not check if a smaller reporting company) | | Smaller reporting company | o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of restricted voting shares held by non-affiliates of the registrant as of April 29, 2011, the last business day of the registrant's most recently completed second fiscal quarter, was $98,933,146 (based on the last reported closing sale price on the Toronto Stock Exchange on that date of $2.14 per share, as converted from C$2.27 using the closing rate of exchange from Reuters).
As of December 15, 2011, the registrant had 129,167,926 restricted voting shares outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive Proxy Statement and Information Circular to be delivered to shareholders in connection with the Annual and Special Meeting of Shareholders to be held March 22, 2012 are incorporated by reference into Part III.
TABLE OF CONTENTS
|
| | |
| | PART I | |
| Item 1. | Business | |
| Item 1A. | Risk Factors | |
| Item 1B. | Unresolved Staff Comments | |
| Item 2. | Properties | |
| Item 3. | Legal Proceedings | |
| Item 4. | Reserved and Removed | |
| | PART II | |
| Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | |
| Item 6. | Selected Financial Data | |
| Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | |
| Item 8. | Financial Statements and Supplementary Data | |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | |
| Item 9A. | Controls and Procedures | |
| Item 9B. | Other Information | |
| | PART III | |
| Item 10. | Directors, Executive Officers and Corporate Governance | |
| Item 11. | Executive Compensation | |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | |
| Item 14. | Principal Accountant Fees and Services | |
| | PART IV | |
| Item 15. | Exhibits and Financial Statement Schedules | |
| | SIGNATURES | |
| | INDEX TO CONSOLIDATED FINANCIAL STATEMENTS | |
| | EXHIBIT INDEX | |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This annual report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which reflect our expectations regarding our future growth, results of operations, performance (both operational and financial) and business prospects and opportunities. All statements, other than statements of historical fact, are forward-looking statements. Wherever possible, words such as “plans,” “expects,” or “does not expect,” “forecasts,” “anticipates” or “does not anticipate,” “believes,” “intends” and similar expressions or statements that certain actions, events or results “may,” “could,” “would,” “might” or “will” be taken, occur or be achieved have been used to identify these forward-looking statements. Although the forward-looking statements contained in this annual report on Form 10-K reflect our current assumptions based upon information currently available to us and based upon what we believe to be reasonable assumptions, we cannot be certain that actual results will be consistent with these forward-looking statements. Our current material assumptions include assumptions related to customer volumes, regulatory compliance and foreign exchange rates. Forward-looking statements necessarily involve significant known and unknown risks, assumptions and uncertainties that may cause our actual results, performance, prospects and opportunities in future periods to differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among other things, risks related to international operations and foreign currency fluctuations; customer demand for our services; regulatory matters affecting manufacturing and pharmaceutical development services; impacts of acquisitions, divestitures and restructurings; implementation of our new corporate strategy; the global economic environment; our exposure to complex production issues; our substantial financial leverage; interest rate risks; potential environmental, health and safety liabilities; credit and customer concentration; competition; rapid technological change; product liability claims; intellectual property; significant shareholder; supply arrangements; pension plans; derivative financial instruments; and our dependence upon key management, scientific and technical personnel. These and other risks are described in greater detail in “Item 1A. Risk Factors” of this annual report on Form 10-K. Although we have attempted to identify important risks and factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors and risks that cause actions, events or results not to be as anticipated, estimated or intended. There can be no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. These forward-looking statements are made as of the date of this annual report on Form 10-K, and except as required by law, we assume no obligation to update or revise them to reflect new events or circumstances.
General
All references to “$” or “dollars” in this annual report are to U.S. dollars unless otherwise indicated. References in this Form 10-K to “Patheon,” “we,” “us,” “our” and “our company” refer to Patheon Inc. and its consolidated subsidiaries.
PART I
Item 1. Business.
Overview
We are a leading provider of commercial manufacturing outsourcing services (“CMO”) and outsourced pharmaceutical development services (“PDS”) to the global pharmaceutical industry. We believe we are the world’s second-largest CMO provider and the world’s largest PDS provider based on calendar year 2010 revenues provided by PharmSource, a provider of pharmaceutical outsourcing business information. We offer a wide range of services from developing drug candidates at the pre-formulation stage through the launch, commercialization and production of approved drugs. During the fiscal year ended October 31, 2011 (“fiscal 2011”), we provided services to approximately 300 customers throughout the world, including 18 of the world’s 20 largest pharmaceutical companies, nine of the world’s 10 largest biotechnology companies and seven of the world’s 10 largest specialty pharmaceutical companies. In fiscal 2011, we manufactured 12 of the top 100 selling drug compounds in the world based on revenues for the products reported by Evaluate Pharma, a provider of pharmaceutical industry data, and our products were distributed in approximately 60 countries. We are also currently developing 17 of the top 100 developmental stage drugs in the world on behalf of our customers based on projected potential revenues for the products reported by Evaluate Pharma.
Our CMO business focuses primarily on prescription products in sterile dosage forms and solid, semi-solid and liquid conventional dosage forms. We have also developed a wide range of specialized capabilities in high potency, controlled substances and sustained release products. Our PDS business provides a broad range of development services, including finished dosage formulation across approximately 40 dosage forms, clinical trial packaging and associated analytical services.
We have established our position as a market leader by leveraging our scale, global reach, specialized capabilities, broad service offerings, scientific expertise and track record of product quality and regulatory compliance to provide cost-effective solutions to our customers.
Company History
The heritage of our company dates back to 1974, when we established Custom Pharmaceuticals Ltd., a contract manufacturing business, in Fort Erie, Canada. Since that time, we have expanded operations through acquisition of contract manufacturing facilities in Canada, Europe, Puerto Rico and the United States, and entered into the PDS business. In addition, we continue to assess our footprint and as market conditions warrant consolidate or dispose of facilities.
In 2007, we announced a plan to restructure our Canadian network of six pharmaceutical manufacturing facilities to align with our strategy of focusing on developing and manufacturing prescription, rather than over-the-counter, products. To improve capacity utilization and profitability at our Whitby facility, we began decommissioning our York Mills facility and transferring all services undertaken at that site to, primarily, our Whitby facility. In the fiscal year ended October 31, 2008 (“fiscal 2008”), we sold our Niagara-Burlington operations to Pharmetics Inc.
In fiscal 2008, we announced a plan to restructure our Puerto Rican operations. In January 2009, we closed our Carolina facility in Puerto Rico and are marketing the remaining assets for sale. Later in 2009, we announced our intention to consolidate our two remaining Puerto Rico operations into our manufacturing site in Manatí and ultimately close or sell our plant in Caguas. The consolidation is expected to continue beyond the end of calendar year 2012.
In 2006 and 2007, we conducted a review of strategic and financial alternatives that resulted in a $150,000,000 investment in us by JLL Partners Inc., a New York private equity firm (“JLL Partners”), and a refinancing of our North American indebtedness. As a result of this investment, JLL Patheon Holdings, LLC ("JLL Patheon Holdings"), an affiliate of JLL Partners, received two series of preferred stock, one of which it converted into 38,018,538 voting shares in 2009 and the other of which entitles it to elect up to three members of our Board of Directors (our “Board”).
JLL Patheon Holdings' also made an unsolicited offer to acquire any or all of the outstanding restricted voting shares of Patheon (“JLL Offer”) that it did not already own in 2009, which resulted in JLL Patheon Holdings and its affiliates (“JLL”) acquiring an additional 33,854,708 restricted voting shares that were validly deposited. The restricted voting shares that JLL purchased pursuant to the JLL Offer represented approximately 38% of the outstanding restricted voting shares of Patheon not already owned by JLL. As of October 31, 2011, JLL owned an aggregate of 72,077,781 restricted voting shares, representing approximately 56% of Patheon’s total restricted voting shares outstanding.
Our Segments
Although we were historically organized and managed as a single business segment providing commercial manufacturing and pharmaceutical development services, due to the continued growth in our operations and a change in our executive management structure, in the fiscal year ended October 31, 2008 (“fiscal 2008”) we organized ourselves into two operating segments: CMO and PDS. In addition, we categorize certain selling, general and administrative costs and certain foreign exchange gains and losses under a separate segment reporting line item referred to as “corporate costs.” In fiscal 2011, our CMO and PDS segments accounted for 81.8% and 18.2% of our total revenues, respectively. Financial information about these segments and information regarding net sales and long-lived assets attributable to operations in Canada, the United States, Europe and other countries is contained in “Note 16—Segmented Information” of our consolidated financial statements included in this Form 10-K. Additional financial information about our segments is contained in “Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.” For a discussion of risks attendant to our foreign operations, please see “Item 1A. Risk Factors—Risks Related to our Business and Industry.”
The illustration below sets forth the various stages of the drug development and manufacturing process; shaded processes are services that we provide.
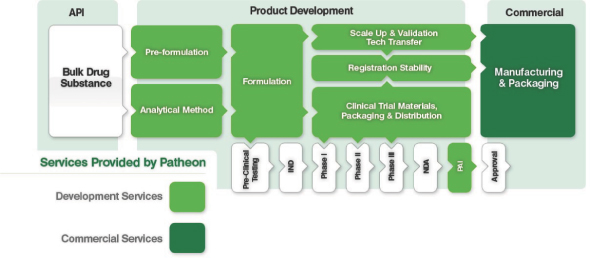 Note: API: Active Pharmaceutical Ingredient
Note: API: Active Pharmaceutical IngredientPAI: Pre-Approval Inspection(s)
Commercial Manufacturing
We believe we are the world’s second-largest CMO provider with an approximate 5% global market share in 2010 based on calendar year 2010 revenues provided by PharmSource. We operate nine facilities located throughout North America and Europe. We manufacture various sterile dosage forms, as well as solid, semi-solid and liquid conventional dosage forms. Our sterile dosage forms include aseptically (sterile) filled and terminally sterilized liquids and powders in ampoules, vials, bottles and pre-filled syringes and sterile lyophilized (freeze-dried) products in both vials and ampoules. Conventional dosage forms include both coated and uncoated compressed tablets, hard shell gelatin capsules, powders, ointments, creams, gels, syrups, suspensions, solutions and suppositories. Currently, our capacity utilization is higher for our facilities for sterile dosage forms than for conventional dosage forms. We further differentiate ourselves by offering specialized capabilities relating to high potency, controlled substance and sustained release products. In fiscal 2011, our CMO segment generated 81.8% of our total revenues.
Set forth below is a table illustrating our various dosage forms.
|
| | | | |
| Conventional dosage forms |
| Solids | | • Tablets • Capsules • Powders | Specialized capabilities | • High potency • Controlled substances |
| Semi-solids | | • Creams • Ointments • Suppositories • Gels | | • Sustained release products • Soft gels • Liquid filled hard shell capsules |
| Liquids | | • Syrups • Solutions • Suspensions | | • Nasal sprays |
| Sterile dosage forms |
| | | • Aseptically (sterile) filled and terminally sterilized liquids and powders (in ampoules, vials, bottles and pre-filled syringes) • Pre-filled syringes and sterile lyophilized (freeze-dried) products (in vials and ampoules) • Sterile (injectable) cephalosporin powder filling |
In fiscal 2011, we had a diverse CMO customer base with large pharmaceutical companies, mid-size companies and emerging companies comprising 55%, 24% and 7% of our fiscal 2011 CMO revenues, respectively, with the remainder being derived from our early stage pharmaceutical, generic and other customers.
Pharmaceutical Development Services
We believe we are the world’s largest PDS provider with an approximate 10% global market share in 2010 based on calendar year 2010 revenues provided by PharmSource, offering a broad range of development services across approximately 40 different dosage forms. We operate eight development centers and one clinical trial packaging facility located throughout North America and Europe. Our PDS offerings support customers across various stages of the drug development process, including (i) pre-formulation, formulation and development of dosage forms; (ii) manufacturing of development stage products during the regulatory drug approval process, including manufacturing of pilot batches; (iii) scale-up and technology transfer services designed to validate commercial-scale drug manufacturing processes; and (iv) development of analytical methods and delivery of analytical services. In fiscal 2011, our PDS offerings were provided to a diverse customer base with mid-size companies, large pharmaceutical companies and emerging companies comprising 33%, 28% and 37% of our fiscal 2011 PDS revenues, respectively, with the remaining 2% being derived from our early stage pharmaceutical, generic and other customers.
During fiscal 2011, we worked on approximately 419 projects for our customers, including seven drug candidates at the new drug application (“NDA”) stage. Among the projects we worked on during fiscal 2011, 117 projects were at Phase I, 104 projects were at Phase II, 100 projects were at Phase III, and 60 projects were at the pre-clinical or post-approval stage. During fiscal 2011 and 2010, we developed 10 products for customers that received new market approval. Since the beginning of fiscal 2001, our PDS business has developed, on behalf of our customers, 36 new molecular entities (“NME”) that have been approved for marketing by regulatory authorities, as well as numerous new formulations of existing NMEs. Any patent and drug approvals that we obtain, or help to obtain, belong to our customers, and we do not receive royalties or earn revenues from products or NMEs that we develop, or help to develop, other than for the development services we provide. Our development group, comprised of approximately 600 scientists and technicians, including approximately 80 holding doctoral degrees, has extensive development experience across a wide variety of pharmaceutical dosage forms. Our PDS business serves as a pipeline for future commercial manufacturing opportunities. Since most of these products are at the beginning of their patent life, these products typically present long-term manufacturing opportunities. From the beginning of fiscal 2008 through the end of fiscal 2011, we were awarded CMO contracts for 14 new products that had been developed by our PDS business. In fiscal 2011, our PDS segment generated 18.2% of our total revenues.
Performance Enhancement Initiatives
We are committed to providing quality products and services to our customers. We are undertaking a series of initiatives to reduce operating expenses and increase manufacturing efficiency, including launching the Patheon AdvantageTM II Lean 6 Sigma program and upgrading our information technology infrastructure.
Our new corporate strategy includes accelerating and revising the Patheon Advantage™ program, which combines “lean” manufacturing practices with “six sigma” manufacturing to streamline operations, remove production bottlenecks, increase capacity utilization and improve performance throughout the network; assessing strategic options for the Swindon commercial operation and the Burlington, Ontario facility; continuing the evolution of our existing commercial sites into centers of excellence focusing on specific technologies or production types; and focusing improvements in other areas of the business including working capital, pricing, and selling, general and administrative costs. We believe our pilot program in our Canadian sites has been very successful to date. A global roll out is being targeted for 2012.
In addition, we have developed an information technology master plan that sets the overall direction for systems and services for our business. It centers on the development of strategic information technology assets that will drive competitive advantages for our business and includes both the addition of new information technology assets and the enhancement of existing information technology assets.
Customers
In fiscal 2011, we provided services to approximately 300 customers throughout the world, including 18 of the world's 20 largest pharmaceutical companies, nine of the world's 10 largest biotechnology companies and seven of the world's 10 largest specialty pharmaceutical companies. We are also currently developing on behalf of our customers 17 of the 100 top developmental stage drugs in the world, based on the potential revenues for the products reported by EvaluatePharma®. During fiscal 2011, no single customer accounted for more than 10.0% of our consolidated revenues. In fiscal 2011, our top 20 customers in our CMO segment accounted for approximately 79% of our CMO revenues. As described above, in June 2009, we launched a new performance guarantee initiative designed to enhance our service to customers. The Patheon Performance Guarantee was added as a new feature in CMO contracts for customers with critical supply requirements.
We have entered into several master service agreements with customers that contemplate long-term multi-product and multi-site commercial manufacturing and/or PDS, including a seven-year manufacturing agreement that led to construction of a new manufacturing facility within one of our existing sites with significant financing from the customer, a five-year master supply agreement with a global pharmaceutical company to provide development and manufacturing services and “carve-out”
arrangements at certain of our facilities under which sizeable parts of our current production have been transferred to us from facilities owned by our customers that were slated for closure or downsizing. These arrangements are part of a trend towards developing broader and longer-term relationships with our customers.
Our CMO customers typically provide a yearly forecast of anticipated product demand. Customers also deliver firm purchase orders, typically three months prior to scheduled production, after which time they may adjust contract quantities or delivery dates within certain limits, provided that we are reimbursed for any expenses incurred in connection with such adjustment. Upon delivery to us of a customer purchase order confirming the quantity and delivery date, the order is scheduled for production. Our CMO customer contracts, typically with multi-year terms, formalize the standard business arrangements outlined above, including production based on the delivery of firm purchase orders. In addition, the contracts typically provide for 12 to 18 months’ advance notice for the transfer or discontinuance of any product. The customer assumes liability for all material commitments made in accordance with purchase orders. We maintain the right to pass on price increases to the customer over and above some predetermined minimum percentage. The actual revenues generated by our major customer agreements are based on volumes that are determined by market demands for the customer’s product from time to time.
Our PDS business provides services on a fee-for-service basis. We typically respond to a customer request and prepare a quotation which, if accepted, typically forms the basis of the contract with the customer. Our PDS contracts typically require us to perform development services within a designated scope. Frequently, the continuation of our work on a particular project will depend on various factors such as research results and the customer’s needs.
Sales and Marketing
Our global sales and marketing group is responsible for generating new business for our CMO and PDS businesses. Our sales team is broken into two distinct groups—territory-based sales executives and key account executives. Each of our territory-based sales teams is responsible for seeking potential customers and generating sales to all customers within its territory that are not named as a key account. Our North America territory-based sales team is comprised of 14 team members and covers the United States and Canada. We also have a territory-based sales team covering Europe and Japan, which is comprised of eight members. In addition, we have seven global key account executives who act as our primary interface with our most significant accounts; currently approximately 35 of our customers have key account status. Despite the functional and geographical delineation of our sales teams, each sales team or executive seeks to generate sales in both our CMO and PDS segments across our entire network. Determination of which site, or sites, will perform specific services is dictated by the nature of the customer’s product, our capabilities and customer preferences.
The projects of our existing customers are managed by site-based project managers and business managers, who also play an integral role in the sales process by ensuring that the existing projects are meeting customers’ expectations. Our sales executives work closely with the site-based teams to understand our customers’ projects and evolving needs, enabling the sales executives and site-based teams to obtain additional work on existing projects and to identify new projects.
Our sales team is supported by global marketing, sales operations and business intelligence groups located at our U.S. headquarters in Research Triangle Park, North Carolina, and regional support resources in Europe.
Our global marketing team supported the development and launch of two innovative programs in fiscal 2011:
| |
| • | SoluPath™- a fixed-price, multiplatform scalable solution to identify development technologies to increase bioavailability in formulations. By using parallel drug formulation screening and expert scientific analysis, the formulations are also able to move quickly to phase I trials. |
| |
| • | Sterile Backup Supply Program - a sterile backup supply program to eliminate the supply risks associated with increasing regulatory pressure on outsourced manufacturing. This program is specifically targeted to sterile manufacturing because of our exceptionally reliable sterile facilities in Italy with enhanced features of no required commitment volume and streamlined tech transfer processes. |
Supply Arrangements
For our contract manufacturing operations, we are required to source various active pharmaceutical ingredients ("APIs"), excipients, raw materials and packaging components from third-party suppliers and/or our actual customers. Our customers specify these components, raw materials and packaging materials in line with their product registration files, and, in some cases, they specify the actual supplier from whom we must purchase these inputs. In most cases, our customers manage the sourcing and physical delivery of the API to us at no cost. We generally source and procure all other input materials from
established local or regional suppliers specialized in serving the pharmaceutical sector. With the exception of certain patented APIs and excipients, most inputs are available from multiple sources.
Supply arrangements are an inherent part of our ability to produce products for our customers in a timely manner and thus create a degree of dependence that could negatively impact revenues if such supply is interrupted. Such interruptions can be either localized to a specific supplier issue or as a result of wider supply interruptions due to natural disasters or international disruptions caused by geopolitical issues or other events. See “Item 1A. Risk Factors—Risks Related to Our Business and Industry.” We work closely with suppliers at both a local and corporate level to establish clear supply agreements that set forth the supply relationship expectations and the legal terms and conditions of the agreements, including potential liabilities for supply interruption situations. These agreements are critical to our ability to manage and mitigate risk across our supply chain.
Competition
We operate in a market that is highly competitive. We compete to provide CMO and PDS to pharmaceutical companies around the world.
Our competition in the CMO market includes full-service pharmaceutical outsourcing companies; contract manufacturers focusing on a limited number of dosage forms; contract manufacturers providing multiple dosage forms; and large pharmaceutical companies offering third-party manufacturing services to fill their excess capacity. In addition, in Europe, there are a large number of privately owned, dedicated outsourcing companies that serve only their local or national markets. Also, large pharmaceutical companies have been seeking to divest portions of their manufacturing capacity, and any such divested businesses may compete with us in the future. We compete primarily on the basis of the security of supply (quality, regulatory compliance and financial stability), service (on-time delivery and manufacturing flexibility) and cost-effective manufacturing (prices and a commitment to continuous improvement).
Our competition in the PDS market includes a large number of laboratories that offer only a limited range of developmental services, generally at a small scale; providers focused on specific technologies and/or dosage forms; and a few fully integrated companies that can provide the full complement of services necessary to develop, scale-up and manufacture a wide range of dosage forms. We also compete in the PDS market with major pharmaceutical and chemical companies, specialized contract research organizations, research and development firms, universities and other research institutions. We may also compete with the internal operations of pharmaceutical companies that choose to source PDS internally. We compete primarily on the basis of scientific expertise, knowledge and experience in dosage form development, availability of a broad range of equipment, on-time delivery of clinical materials, compliance with current good manufacturing practices (“cGMPs”), regulatory compliance, cost effective services and financial stability.
Some of our competitors may have substantially greater financial, marketing, technical or other resources than we do. Additional competition may emerge and may, among other things, result in a decrease in the fees paid for our services.
One of the many factors affecting competition is the current excess capacity within the pharmaceutical industry of facilities capable of manufacturing drugs in solid and semi-solid dosage forms. Thus, customers currently have a wide range of supply alternatives for these dosage forms. Another factor causing increased competition is that a number of companies in Asia, particularly India, have been entering the CMO and PDS sectors over the past few years, have begun obtaining approval from the U.S. Food and Drug Administration (the “FDA”) for certain of their plants and have acquired additional plants in Europe and North America. One or more of these companies may become a significant competitor to us.
Employees
As of November 30, 2011, we had approximately 3,900 employees. National works councils are active at all of our facilities in the United Kingdom, France and Italy consistent with local labor laws. There is no union representation at any of our North American sites. Our management believes that we generally have a good relationship with our employees around the world and the works councils that represent a portion of our European employee base.
Intellectual Property
We rely on a combination of trademark, patent, trade secret and other intellectual property laws of the United States and other countries. We have applied in the United States and in certain foreign countries for registration of a limited number of trademarks and patents, some of which have been registered or issued. Also, many of the formulations used by us in manufacturing products to customer specifications are subject to patents or other intellectual property rights owned by or licensed to the relevant customer. Further, we rely on non-disclosure agreements and other contractual provisions to protect our intellectual property rights and typically enter into mutual confidentiality agreements with customers that own or are licensed users of patented formulations.
We have developed and continue to develop knowledge and expertise (“know-how”) and trade secrets in the provision of services in both our PDS and CMO businesses. Our know-how and trade secrets may not be patentable, but they are valuable in that they enhance our ability to provide high-quality services to our customers.
To the extent that we determine that certain aspects of the service provided by our CMO and PDS businesses are innovative and patentable, we have filed and pursued, and plan to continue to file and pursue, patent applications to protect such inventions, as well as applications for registration of other intellectual property rights, as appropriate. However, we do not consider any particular patent, trademark, license, franchise or concession to be material to our overall business.
Regulatory Matters
We are required to comply with the regulatory requirements of various local, state, provincial, national and international regulatory bodies having jurisdiction in the countries or localities where we manufacture products or where our customers' products are distributed. In particular, we are subject to laws and regulations concerning research and development, testing, manufacturing processes, equipment and facilities, including compliance with cGMPs, labeling and distribution, import and export, and product registration and listing. As a result, most of our facilities are subject to regulation by the FDA, as well as regulatory bodies of other jurisdictions, such as the European Medicines Agency of the European Union (“EMA”) and/or the National Health Surveillance Agency in Brazil (“Anvisa”), depending on the countries in which our customers market and sell the products we manufacture and/or package on their behalf. We are also required to comply with environmental, health and safety laws and regulations, as discussed in “Environmental Matters” below. These regulatory requirements impact many aspects of our operations, including manufacturing, developing, labeling, packaging, storage, distribution, import and export and record keeping related to customers' products. Noncompliance with any applicable regulatory requirements can result in government refusal to approve (i) facilities for testing or manufacturing products or (ii) products for commercialization. The FDA and other regulatory agencies can delay, limit or deny approval for many reasons, including:
| |
| • | Changes to the regulatory approval process, including new data requirements, for product candidates in those jurisdictions, including the United States, in which we or our customers may be seeking approval; |
| |
| • | A product candidate may not be deemed to be safe or effective; |
| |
| • | The ability of the regulatory agency to provide timely responses as a result of its resource constraints; and |
| |
| • | The manufacturing processes or facilities may not meet the applicable requirements. |
In addition, if new legislation or regulations are enacted or existing legislation or regulations are amended or are interpreted or enforced differently, we may be required to obtain additional approvals or operate according to different manufacturing or operating standards or pay additional product or establishment user fees. This may require a change in our research and development and manufacturing techniques or additional capital investments in our facilities.
Our pharmaceutical development and manufacturing projects generally involve products that must undergo pre-clinical and clinical evaluations relating to product safety and efficacy before they are approved as commercial therapeutic products. The regulatory authorities having jurisdiction in the countries in which our customers intend to market their products may delay or put on hold clinical trials, delay approval of a product or determine that the product is not approvable. The FDA or other regulatory agencies can delay approval of a drug if our manufacturing facility is not able to demonstrate compliance with cGMPs, pass other aspects of pre-approval inspections (i.e compliance with filed submissions) or properly scale up to produce commercial supplies. The FDA and comparable government authorities having jurisdiction in the countries in which our customers intend to market their products have the authority to withdraw product approval or suspend manufacture if there are significant problems with raw materials or supplies, quality control and assurance or the product is deemed adulterated or misbranded.
Some of our manufactured products are listed as controlled substances. Controlled substances are those products that present a risk of substance abuse. In the United States, these types of products are classified by the U.S. Drug Enforcement Agency (the “DEA”) as Schedule II, III, and IV substances under the Controlled Substances Act of 1970. The DEA classifies substances as Schedule I, II, III, IV or V substances, with Schedule I substances considered to present the highest risk of substance abuse and Schedule V substances the lowest risk. Scheduled substances are subject to DEA regulations relating to manufacturing, storage, distribution, import and export and physician prescription procedures. For example, scheduled drugs are subject to distribution limits and a higher level of recordkeeping requirements. Furthermore, the total amount of controlled substances for manufacture or commercial distribution is limited by the DEA and allocated through quotas. Our quotas or our customers' quotas, if any, may not be sufficient to meet commercial demand or to economically produce the product.
Entities must be registered annually with the DEA to manufacture, distribute, dispense, import, export and conduct research using controlled substances. State controlled substance laws also require registration for similar activities. In addition,
the DEA requires entities handling controlled substances to maintain records, file reports, follow specific labeling and packaging requirements and provide appropriate security measures to control against diversion of controlled substances. If we fail to follow these requirements, we may be subject to significant civil and/or criminal penalties and possibly a revocation of one of our DEA registrations.
Products containing controlled substances may generate significant public health and safety issues, and in such instances, federal or state authorities can withdraw or limit the marketing rights or regulatory approvals for these products. For some scheduled substances, the FDA may require us or our customers to develop product attributes or a risk evaluation and mitigation strategy to reduce the inappropriate use of the products, including the manner in which they are marketed and sold, so as to reduce the risk of diversion or abuse of the product. Developing such a program may be time-consuming and could delay approval of product candidates containing controlled substances. Such a program or delays of any approval from the FDA could adversely affect our business, results of operations and financial condition.
Audits are an important means by which prospective and existing customers gain confidence that our operations are conducted in accordance with applicable regulatory requirements. In fiscal 2011, our facilities and development centers were audited by 186 separate customer audit teams, representing both prospective and existing customers. These audits contribute to our ongoing improvement of our manufacturing and development practices. In addition to customer audits, we, like all commercial drug manufacturers, are subject to audits by various regulatory authorities. In fiscal 2011, 23 such audits by regulatory authorities were conducted at our sites in North America and Europe, involving multiple products. Responses to audit observations were submitted to address observations noted. We have yet to receive feedback from most of the inspections conducted in the third and fourth quarters of fiscal 2011. It is not unusual for regulatory agencies or customers to request further clarification and/or follow-up on the responses we provide.
Environmental Matters
Our operations are subject to a variety of environmental, health and safety laws and regulations in each of the jurisdictions in which we operate. These laws and regulations govern, among other things, air emissions, wastewater discharges, the handling and disposal of hazardous substances and wastes, soil and groundwater contamination and employee health and safety. We are also subject to laws and regulations governing the destruction and disposal of raw materials and non-compliant products, the handling of regulated material that is included in our offerings and the disposal of our offerings at the end of their useful life. These laws and regulations have increasingly become more stringent, and we may incur additional expenses to ensure compliance with existing or new requirements in the future. Any failure by us to comply with environmental, health and safety requirements could result in the limitation or suspension of our operations. We also could incur monetary fines, civil or criminal sanctions, third-party claims or cleanup or other costs as a result of violations of or liabilities under such requirements. In addition, compliance with environmental, health and safety requirements could restrict our ability to expand our facilities or require us to acquire costly pollution control equipment, incur other significant expenses or modify our manufacturing processes.
Our manufacturing facilities, in varying degrees, use, store and dispose of hazardous substances in connection with their processes. At some of our facilities, these substances are stored in underground storage tanks or used in refrigeration systems. Some of our facilities, including those in Puerto Rico, have been utilized over a period of years as manufacturing facilities, with operations that may have included on-site landfill or other waste disposal activities and have certain known or potential conditions that may require remediation in the future, and several of these have undergone remediation activities in the past by former owners or operators. Some of our facilities are located near third-party industrial sites and may be impacted by contamination migrating from such sites. A number of our facilities use groundwater from onsite wells for process and potable water, and if these onsite sources became contaminated or otherwise unavailable for future use, we could incur expenses for obtaining water from alternative sources. In addition, our operations have grown through acquisitions, and it is possible that facilities that we have acquired may expose us to environmental liabilities associated with historical site conditions that have not yet been discovered. Some environmental laws impose liability for contamination on current and former owners and operators of affected sites, regardless of fault. If remediation costs or potential claims for personal injury or property or natural resource damages resulting from contamination arise, they may be material and may not be recoverable under any contractual indemnity or otherwise from prior owners or operators or any insurance policy. Additionally, we may not be able to successfully enforce any such indemnity or insurance policy in the future. In the event that new or previously unknown contamination is discovered or new cleanup obligations are otherwise imposed at any of our currently or previously owned or operated facilities, we may be required to take additional, unplanned remedial measures and record charges for which no reserves have been recorded.
Seasonality
Revenues from some of our CMO and PDS operations have traditionally been lower in our first fiscal quarter, being the
three months ending January 31. We attribute this trend to several factors, including (i) the reassessment by many customers of their need for additional product in the last quarter of the calendar year in order to use existing inventories of products; (ii) the lower production of seasonal cough and cold remedies in the first fiscal quarter; (iii) limited project activity towards the end of the calendar year by many small pharmaceutical and biotechnology customers involved in PDS projects in order to reassess progress on their projects and manage cash resources; and (iv) the Patheon-wide facility shutdown during a portion of the traditional holiday period in December and January. In addition, we expect revenues in the first half of fiscal 2012 to be lower than in the first half of fiscal 2011, due to the impact in fiscal 2011 of $50.4 million in reservation fees and deferred revenue amortization from the amended manufacturing and supply agreement in the United Kingdom.
Research and Development
We have not spent any material amount in the last three fiscal years on company-sponsored research and development activities.
Risks Related to Our Business and Industry
We are dependent on our customers’ spending on and demand for our manufacturing and development services. A reduction in spending or demand could have a material adverse effect on our business.
The amount of customer spending on pharmaceutical development and manufacturing, particularly the amount our customers choose to spend on outsourcing these services, has a large impact on our sales and profitability. Consolidation in the pharmaceutical industry may impact such spending as customers integrate acquired operations, including research and development departments and manufacturing operations.
Many of our customers finance their research and development spending from private and public sources. We have experienced slowdowns in our customers’ spending on pharmaceutical development and related services, which we believe have been primarily due to the lack or decreased availability of capital for specialty and emerging pharmaceutical companies and the consolidation within the pharmaceutical industry, which resulted in the postponement of certain projects. Any reduction in customer and potential customer spending on pharmaceutical development and related services may have a material adverse effect on our business, results of operations and financial condition.
Furthermore, demand for our CMO segment is driven, in part, by products we bring to market for our PDS customers. Due to the long lead times associated with obtaining regulatory approvals for many of these products, particularly dosage forms, and the competitive advantage that can come from gaining early approval, it is important that we maintain a sufficiently large portfolio of pharmaceutical products and such products are brought to market on a timely basis. If we experience a reduction in research and development by our customers, the decrease in activity in our PDS segment could also negatively affect activity levels in our CMO business. Any decline in demand for our services may have a material adverse effect on our business, results of operations and financial condition.
The consumers of the products we manufacture for our customers may significantly influence our business, results of operations and financial condition.
We are dependent on demand for the products we manufacture for our customers and have no control or influence over the market demand for our customers’ products. Demand for our customers’ products can be adversely affected by, among other things, delays in health regulatory approval, the loss of patent and other intellectual property rights protection, the emergence of competing products, including generic drugs, the degree to which private and government drug plans subsidize payment for a particular product and changes in the marketing strategies for such products.
If the products we manufacture for our customers do not gain market acceptance, our revenues and profitability will be adversely affected. The degree of market acceptance of our customers’ products will depend on a number of factors, including:
| |
| • | the ability of our customers to publicly establish and demonstrate the efficacy and safety of such products, including compared to competing products; |
| |
| • | the costs to potential consumers of using such products; and |
| |
| • | marketing and distribution support for such products. |
If production volumes of key products that we manufacture for our customers and related revenues are not maintained, it may have a material adverse effect on our business, results of operations and financial condition. Additionally, any changes in product mix due to market acceptance of our customers’ products may adversely affect our margins.
Our services and offerings are highly complex, and if we are unable to provide quality and timely offerings to our customers, our business could suffer.
The services we offer are highly exacting and complex, due in part to strict regulatory requirements. A failure of our quality control systems in our business units and facilities could cause problems to arise in connection with facility operations or during preparation or provision of products, in both cases, for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, problems with raw materials or environmental factors. Such problems could affect production of a particular batch or series of batches, requiring the destruction of products, or could halt facility production altogether. In addition, our failure to meet required quality standards may result in our failure to timely deliver products to our customers, which in turn could damage our reputation for quality and service. Any such incident could, among other things, lead to increased costs, lost revenue, reimbursement to customers for lost APIs, damage to and possibly termination of existing customer relationships, time and expense spent investigating the cause and, depending on the cause, similar losses with respect to other batches or products. If problems are not discovered before the product is released to the market, we may be subject to regulatory actions, including product recalls, product seizures, injunctions to halt manufacture and distribution, restrictions on our operations, civil sanctions, including monetary sanctions, and criminal actions. In addition, such issues could subject us to litigation, the cost of which could be significant.
Our PDS projects are typically for a shorter term than our CMO projects, and any failure by us to maintain a high volume of PDS projects, including due to lower than expected success rates of the products for which we provide services, could adversely affect our business, results of operations and financial condition.
Unlike our CMO segment, where our contracts are typically multi-year in duration, our PDS segment contracts are generally shorter in term and typically require us to provide development services within a designated scope. Since our PDS business focuses on products that are still in the developmental stages, the viability of many of our PDS projects is not certain. As a result, many of these projects fail to progress to the subsequent development phase. Even if a customer wishes to proceed with a project, the product we are developing on its behalf may fail to receive necessary regulatory approval, or other factors, such as the development of a competing product, may hinder the development of the product.
If we are unable to continue to obtain new projects from existing and new customers, our PDS segment could be adversely affected. Furthermore, although our PDS business acts as a pipeline for our CMO segment, we cannot predict the turnover rate of our PDS projects or how successful we will be in winning new projects that lead to a viable product. As such, an increase in the turnover rate of our PDS projects may negatively affect our CMO segment at a later time. In addition, the discontinuation of a project as a result of our failure to satisfy a customer’s requirements may also affect our ability to obtain future projects from the customer involved or from new customers.
Continued volatility and disruption to the global capital and credit markets and the global economy have adversely affected, and may continue to adversely affect, our business and results of operations and have adversely affected, and may continue to adversely affect, our customers and suppliers.
In recent years, the global capital and credit markets and the global economy have experienced a period of significant uncertainty, characterized by the bankruptcy, failure, collapse or sale of various financial institutions and a considerable level of intervention from governments around the world. These conditions have adversely affected the demand for our products and services, which has negatively affected our business and results of operations. In addition, interest rate fluctuations, financial market volatility or credit market disruptions may limit our access to capital, and may also negatively affect our customers’ and our suppliers’ ability to obtain credit to finance their businesses on acceptable terms or at all. As a result, customers’ need for and ability to purchase our products or services may decrease. For example, certain of our customers have decreased their research and development spending due to their lack of access to capital. In addition, lack of access to capital may cause our suppliers to increase their prices, reduce their output or change their terms of sale. If our customers’ or suppliers’ operating and financial performance deteriorates, or if they are unable to make scheduled payments or obtain credit, our customers may not be able to pay, or may delay payment of, accounts receivable owed to us, and our suppliers may restrict credit or impose different payment terms. Any inability of our customers to pay us for our products and services or any demands by suppliers for different payment terms may adversely affect our earnings and cash flow.
As the contraction of the global capital and credit markets has spread throughout the broader economy, the United States and other major markets around the world have experienced very weak or negative economic growth. These recessionary conditions have impacted, and will continue to impact, consumer demand for the products we manufacture for our customers.
Our operations outside the United States and Canada are subject to a number of economic, political and regulatory risks.
We are an international company incorporated and listed in Canada with facilities and offices in seven countries. In fiscal 2011, we provided services to customers in approximately 60 countries, and nearly half of our revenues were attributable to customers outside the United States and Canada. Our operations outside the United States and Canada could be substantially affected by foreign economic, political and regulatory risks. These risks include:
| |
| • | fluctuations in currency exchange rates; |
| |
| • | the difficulty of enforcing agreements and collecting receivables through some foreign legal systems; |
| |
| • | customers in some foreign countries potentially having longer payment cycles; |
| |
| • | changes in local tax laws, tax rates in some countries that may exceed those of Canada or the United States and lower earnings due to withholding requirements or the imposition of tariffs, exchange controls or other restrictions; |
| |
| • | seasonal reductions in business activity; |
| |
| • | the credit risk of local customers and distributors; |
| |
| • | general economic and political conditions; |
| |
| • | unexpected changes in legal, regulatory or tax requirements; |
| |
| • | relationships with labor unions and works councils; |
| |
| • | the difficulties associated with managing a large global organization; |
| |
| • | the risk that certain governments may adopt regulations or take other actions that would have a direct or indirect adverse impact on our business and market opportunities, including nationalization of private enterprise; |
| |
| • | non-compliance with applicable currency exchange control regulations, transfer pricing regulations or other similar regulations; and |
| |
| • | violations of the Foreign Corrupt Practices Act by acts of agents and other intermediaries whom we have limited or no ability to control. |
If any of these economic or political risks materialize and we have failed to anticipate and effectively manage them, we may experience adverse effects on our business and results of operations. Additionally, if we do not remain in compliance with current regulatory requirements or fail to comply with future regulatory requirements, then such non-compliance may subject us to liability and have a material adverse effect on our business and results of operations.
Fluctuations in exchange rates could have a material adverse effect on our results of operations and financial performance.
Our most significant transaction exposures arise in our Canadian operations. Prior to the refinancing in the second quarter of the year ended October 31, 2010 ("fiscal 2010"), the balance sheet of our Canadian division included U.S. dollar denominated debt which was designated as a hedge against our investments in subsidiaries in the United States and Puerto Rico. The foreign exchange gains and losses related to the effective portion of this hedge were recorded in other comprehensive income. In the third quarter of fiscal 2010, we changed the functional currency of our corporate division in Canada to U.S. dollars, thereby eliminating the need to designate this U.S. dollar denominated debt as a hedge. In addition, approximately 90% of the revenues of the Canadian operations and approximately 10% of its operating expenses are transacted in U.S. dollars. As a result, we may experience transaction exposures because of volatility in the exchange rate between the Canadian and U.S. dollar. Based on our current U.S. denominated net inflows, as of October 31, 2011, fluctuations of +/-10% would, everything else being equal, have an annual effect on loss from continuing operations before taxes of approximately +/- $12.4 million, prior to hedging activities.
The objective of our foreign exchange risk management activities is to minimize transaction exposures and the resulting volatility of our earnings. To mitigate exchange-rate risk, we utilize foreign exchange forward contracts and collars in certain circumstances to lock in exchange rates with the objective that the gain or loss on the forward contracts and collars will approximately offset the loss or gain that results from the transaction or transactions being hedged. As of October 31, 2011, we had entered into foreign exchange forward contracts and collars to cover approximately 80% of our Canadian-U.S. dollar cash flow exposures for fiscal 2011.
Translation gains and losses related to certain foreign currency denominated intercompany loans are included as part of
the net investment in certain foreign subsidiaries and are included in accumulated other comprehensive income in shareholders’ equity. We do not currently hedge translation exposures.
While we attempt to mitigate our foreign exchange risk by engaging in foreign currency hedging activities using derivative financial instruments, we may not be successful. We may not be able to engage in hedging transactions in the future, and if we do, we may not be able to eliminate foreign currency risk, and foreign currency fluctuations may have a material adverse effect on our results of operations and financial performance.
We are, or may be, party to certain derivative financial instruments, and our results of operations may be negatively affected in the event of non-performance by the counterparties to such instruments.
From time to time, we enter into interest rate swaps and foreign exchange forward contracts and collars to limit our exposure to changes in variable interest rates and foreign exchange rates. When we enter into such swaps and contracts, we are exposed to credit-related losses, which could impact our results of operations and financial condition in the event of non-performance by the counterparties to such instruments. For more information about our foreign currency risks, please see “Item 7A. Quantitative and Qualitative Disclosures About Market Risk.”
Because a significant portion of our revenues comes from a limited number of customers, any decrease in sales to these customers could harm our business, results of operations and financial condition.
In fiscal 2011, our top 20 customers in our CMO segment accounted for approximately 79% of our CMO revenues. This customer concentration increases credit risk and other risks associated with particular customers and particular products, including risks related to market demand for customer products and regulatory and other operating risks. Disruptions in the production of major products could damage our customer relationships and adversely impact our results of operations in the future. Revenues from customers that have accounted for significant sales in the past, either individually or as a group, may not reach or exceed historical levels in any future period. The loss or a significant reduction of business from any of our major customers may have a material adverse effect on our business, results of operations and financial condition.
We operate in highly competitive markets and competition may adversely affect our business.
We operate in a market that is highly competitive. We compete to provide CMO and PDS to pharmaceutical companies around the world.
Our competition in the CMO market includes full-service pharmaceutical outsourcing companies; contract manufacturers focusing on a limited number of dosage forms; contract manufacturers providing multiple dosage forms; and large pharmaceutical companies offering third-party manufacturing services to fill their excess capacity. In addition, in Europe, there are a large number of privately owned, dedicated outsourcing companies that serve only their local or national markets. Also, large pharmaceutical companies have been seeking to divest portions of their manufacturing capacity, and any such divested businesses may compete with us in the future. We compete primarily on the basis of the security of supply (quality, regulatory compliance and financial stability), service (on-time delivery and manufacturing flexibility) and cost-effective manufacturing (prices and a commitment to continuous improvement).
Our competition in the PDS market includes a large number of laboratories that offer only a limited range of developmental services, generally at a small scale; providers focused on specific technologies and/or dosage forms; and a few fully integrated companies that can provide the full complement of services necessary to develop, scale-up and manufacture a wide range of dosage forms. We also compete in the PDS market with major pharmaceutical and chemical companies, specialized contract research organizations, research and development firms, universities and other research institutions. We may also compete with the internal operations of pharmaceutical companies that choose to source PDS services internally. We compete primarily on the basis of scientific expertise, knowledge and experience in dosage form development, availability of a broad range of equipment, on-time delivery of clinical materials, compliance with cGMPs, regulatory compliance, cost effective services and financial stability.
Some of our competitors may have substantially greater financial, marketing, technical or other resources than we do. Additional competition may emerge and may, among other things, result in a decrease in the fees paid for our services, which would affect our results of operations and financial condition.
One of the many factors affecting competition is the current excess capacity within the pharmaceutical industry of facilities capable of manufacturing drugs in solid and semi-solid dosage forms. Thus, customers currently have a wide range of supply alternatives for these dosage forms. Another factor causing increased competition is that a number of companies in Asia, particularly India, that have been entering the CMO and PDS sectors over the past few years, have begun obtaining approval from the FDA for certain of their plants and have acquired additional plants in Europe and North America. One or more of
these companies may become a significant competitor to us. Competition may mean lower prices and reduced demand for CMO and PDS, which could have an adverse effect on our business, results of operations and financial condition.
We may not be able to successfully offer new services.
In order to successfully compete, we will need to offer and develop new services. The related development costs may require a substantial investment, and we may not have the financial resources to fund such initiatives.
In addition, the success of enhanced or new services will depend on several factors, including our ability to:
| |
| • | properly anticipate and satisfy customer needs, including increasing demand for lower cost services; |
| |
| • | enhance, innovate, develop and manufacture new offerings in an economical and timely manner; |
| |
| • | differentiate our offerings from competitors’ offerings; |
| |
| • | meet quality requirements and other regulatory requirements of government agencies; |
| |
| • | obtain valid and enforceable intellectual property rights; and |
| |
| • | avoid infringing the proprietary rights of third parties. |
Even if we were to succeed in creating enhanced or new services, those services may not produce revenues in excess of the costs of development and capital investment and may be quickly rendered obsolete by changing customer preferences or by technologies or features offered by our competitors. In addition, innovations may not be accepted quickly in the marketplace because of, among other things, entrenched patterns of clinical practice, the need for regulatory clearance and uncertainty over third-party reimbursement.
We rely on our customers to supply many of the necessary ingredients for our products, and for other ingredients, we rely on other third parties. Our inability to obtain the necessary materials or ingredients for the products we manufacture on behalf of our customers may adversely impact our business, results of operations and financial condition.
Our operations require various APIs, components, compounds and raw materials supplied primarily by third parties, including our customers. Our customers specify the components, raw materials and packaging materials required for their products and, in some cases, specify the suppliers from which we must purchase these inputs. In most cases, the customers supply the APIs to us at no cost pursuant to our standard services agreements.
We generally source our components, compounds and raw materials locally, and most of the materials required by us for our CMO business are readily available from multiple sources.
In some cases, we manage the supply chain for our customers, including the sourcing of certain ingredients and packaging material from third-party suppliers. In certain instances, such ingredients or packaging material can only be supplied by a limited number of suppliers or in limited quantities. If our customers or third-party suppliers do not supply API or other raw materials on a timely basis, we may be unable to manufacture products for our customers. Although no one product or customer is material to our operations, a sustained disruption in the supply chain involving multiple customers or vendors at one time could have a material adverse effect on our results of operations.
Furthermore, customers or third-party suppliers may fail to provide us with raw materials and other components that meet the qualifications and standards required by us or our customers. If third-party suppliers are not able to provide us with products that meet our or our customers’ specifications on a timely basis, we may be unable to manufacture products, or products may be available only at a higher cost or after a long delay, which could prevent us from delivering products to our customers within required timeframes. Any such delay in delivering our products may create liability for us to our customers for breach of contract or cause us to experience order cancellations and loss of customers. In the event that we produce products with inferior quality components and raw materials, we may become subject to product liability or warranty claims caused by defective raw materials or components from a third-party supplier or from a customer, or our customer may be required to recall its products from the market.
It is also possible that any of our supplier relationships could be interrupted due to natural disasters, international supply disruptions caused by geopolitical issues or other events or could be terminated in the future. Any sustained interruption in our receipt of adequate supplies could have an adverse effect on our business and financial results. In addition, while we have supply chain processes intended to reduce volatility in component and material pricing, we may not be able to successfully manage price fluctuations. Price fluctuations or shortages may have an adverse effect on our results of operations and financial condition.
Technological change may cause our offerings to become obsolete over time. If customers decrease their purchases of
our offerings, our business, results of operations and financial condition may be adversely affected.
The healthcare industry is characterized by rapid technological change. Demand for our services may change in ways that we may not anticipate because of evolving industry standards or as a result of evolving customer needs that are increasingly sophisticated and varied or because of the introduction by competitors of new services and technologies. Any such decreased demand may adversely affect our business, results of operations and financial condition.
We are dependent on key management, scientific and technical personnel.
We are dependent upon the continued support and involvement of our key management, scientific and technical personnel, the majority of whom have employment agreements with us that impose noncompetition and nonsolicitation restrictions following cessation of employment. Because our ability to manage our business activities and, hence, our success depend in large part on the collective efforts of such personnel, our inability to continue to attract and retain such personnel could have a material adverse effect on our business.
Certain of our pension plans are underfunded, and additional cash contributions may be required, which may reduce the cash available for our business.
Certain of our employees in Canada, France and the United Kingdom are participants in defined benefit pension plans that we sponsor. As of October 31, 2011, the unfunded pension liability on our pension plans was approximately $30.6 million in the aggregate. The amount of future contributions to our defined benefit plans will depend upon asset returns and a number of other factors and, as a result, the amounts we will be required to contribute to such plans in the future may vary. Such cash contributions to the plans will reduce the cash available for our business.
In relation to our U.K. pension plan, the trustees are authorized to accelerate the required payment of future contribution obligations if they have received actuarial advice that the plan is incapable of paying all the benefits that have or will become due for payment as they become due. If the trustees of our U.K. pension plan were to be so advised and took such a step, our U.K. subsidiary would be required to meet the full balance of the cost of securing the benefits provided by the plan through the purchase of annuities from an insurance company, to the extent that it was able to do so. The cost would be likely to exceed the amount of any deficit under the plan while the plan was ongoing.
Any failure of our information systems, such as from data corruption, cyber-based attacks or network security breaches, could adversely affect our business and results of operations.
We rely on information systems in our business to obtain, rapidly process, analyze and manage data to:
| |
| • | facilitate the manufacture and distribution of thousands of inventory items to and from our facilities; |
| |
| • | receive, process and ship orders on a timely basis; |
| |
| • | manage the accurate billing of, and collections from, our customers; |
| |
| • | manage the accurate accounting for, and payment to, our vendors; and |
| |
| • | schedule and operate our global network of manufacturing and development facilities. |
Security breaches of this infrastructure can create system disruptions, shutdowns or unauthorized disclosure of confidential information. If we are unable to prevent such breaches, our operations could be disrupted, or we may suffer financial damage or loss because of lost or misappropriated information. We cannot be certain that advances in criminal capabilities, new discoveries in the field of cryptography or other developments will not compromise or breach the technology protecting the networks that access our products and services. If these systems are interrupted, damaged by unforeseen events or fail for any extended period of time, including due to the actions of third parties, then we may not be able to effectively manage our business, and our results of operations could be adversely affected.
From time to time, we may seek to restructure our operations, which may require us to incur restructuring charges, and we may not be able to achieve the cost savings that we expect from any such restructuring efforts.
To improve our profitability, we restructured our Canadian manufacturing operations during fiscal 2008. We also are in the process of restructuring our Puerto Rican operations as part of our efforts to eliminate operating losses and develop a long-term plan for our business. As part of our restructuring efforts, we incurred $7.0 million of restructuring charges during fiscal 2011, including approximately $1.9 million related to our Swindon facility and approximately $1.0 million related to the closure of our Zug offices. We expect to adopt additional restructuring plans in order to improve our operational efficiency.
We may not be able to achieve the level of benefits that we expect to realize from these or any future restructuring
activities, within expected timeframes, or at all. Furthermore, upon the closure of any facilities in connection with our restructuring efforts, we may not be able to divest such facilities at a fair price or in a timely manner. In addition, as part of any plant closures and the transfer of production to another facility, we are required to obtain the consents of our customers and the relevant regulatory agencies, which we may not be able to obtain. Changes in the amount, timing and character of charges related to our current and future restructurings and the failure to complete or a substantial delay in completing our current and any future restructuring plan could have a material adverse effect on our business.
We may in the future engage in acquisitions and joint ventures and may divest non-strategic businesses or assets. We may not be able to complete such transactions, and such transactions, if executed, pose significant risks.
Our future success may depend on our ability to acquire other businesses or technologies or enter into joint ventures that could complement, enhance or expand our current business or offerings and services or that might otherwise offer us growth opportunities. We may face competition from other companies in pursuing acquisitions and joint ventures. Our ability to enter into such transactions may also be limited by applicable antitrust laws and other regulations in the United States, Canada and foreign jurisdictions in which we do business. We may not be able to complete such transactions for reasons including, but not limited to, a failure to secure financing. Any transactions that we are able to identify and complete may involve a number of risks, including:
| |
| • | the diversion of management’s attention to negotiate the transaction and then integrate the acquired businesses or joint ventures; |
| |
| • | the possible adverse effects on our operating results during the negotiation and integration process; |
| |
| • | significant costs, charges or writedowns; |
| |
| • | the potential loss of customers or employees of the acquired business; and |
| |
| • | our potential inability to achieve our intended objectives for the transaction. |
In addition, we may be unable to maintain uniform standards, controls, procedures and policies with respect to the acquired business, and this may lead to operational inefficiencies. To the extent that we are successful in making acquisitions, we may have to expend substantial amounts of cash, incur debt and assume loss-making divisions.
We may also seek to sell some of our assets in connection with the divestiture of a non-strategic business or as part of internal restructuring efforts. In September 2011, we announced that we would be considering strategic alternatives for the Swindon U.K. commercial business and the Burlington, Ontario clinical packaging operation. To the extent that we are not successful in completing such divestitures or restructuring efforts, we may have to expend substantial amounts of cash, incur debt and continue to absorb loss-making or under-performing divisions. Any divestitures that we are unable to complete may involve a number of risks, including diversion of management’s attention, a negative impact on our customer relationships, costs associated with retaining the targeted divestiture, closing and disposing of the impacted business or transferring business to other facilities. Furthermore, our ability to initiate and complete such transactions may be hindered by our Investor Agreement (the “Investor Agreement”) with JLL Patheon Holdings. For example, under the terms of the Investor Agreement, we need majority independent director approval to engage in certain types of transactions.
JLL has significant influence over our business and affairs, and its interests may differ from ours and those of our other shareholders.
As of October 31, 2011, JLL owned an aggregate of 72,077,781 restricted voting shares, representing approximately 56% of our total restricted voting shares outstanding. JLL Patheon Holdings also owns an aggregate of 150,000 special voting Class I, Preferred Shares, Series D (“Series D Preferred Shares”), pursuant to which it is entitled to elect up to three of our directors based on the number of restricted voting shares that it holds.
In addition, in connection with the investment by JLL Patheon Holdings in our shares, on April 27, 2007, we entered into the Investor Agreement.
Under the Investor Agreement, we currently are required to seek the approval of JLL Patheon Holdings before we undertake certain actions, including share issuances, the payment of dividends, share repurchases, any merger, consolidation or sale of all or substantially all of our assets or a similar business combination transaction and the incurrence of certain indebtedness in excess of $20.0 million.
JLL exercises significant influence over us as a result of its majority shareholder position, voting rights, board appointment rights and its rights under the Investor Agreement. As a result, JLL has significant influence over our decisions to enter into corporate transactions and has the ability to prevent any transaction that requires shareholder approval. This
concentration of ownership and JLL's rights may prevent a change of control of us that might be considered to be in the interests of shareholders or other stakeholders. In addition, if we are unable to obtain requisite approvals from JLL, we may be prevented from executing critical elements of our business strategy.
Our stock price is volatile and could experience substantial declines.
The market price of our common stock has historically experienced, and may continue to experience, substantial volatility. Such volatility has resulted or may result from fluctuations in our quarterly operating results or anticipated future results, changes in general conditions in the economy or the financial markets, which both experienced uncertainty in fiscal 2010 and fiscal 2011 due to the effects of the global financial crisis, and other developments affecting us or our competitors. Some of these factors are beyond our control, such as changes in revenue and earnings estimates by analysts, market conditions within our industry, disclosures by product development partners and actions by regulatory authorities with respect to potential drug candidates and changes in pharmaceutical and biotechnology industries and the government sponsored clinical research sector. In addition, in recent years, the stock market has experienced significant price and volume fluctuations. The stock market, and in particular the market for pharmaceutical and biotechnology company stocks, has also experienced significant decreases in value in the past. This volatility and valuation decline have affected the market prices of securities issued by many companies, often for reasons unrelated to their operating performance, and might adversely affect the price of our common stock.
Our shareholders might have difficulty enforcing U.S. judgments against us, enforcing U.S. judgments in a Canadian court or bringing an original action in Canada to enforce liabilities based upon U.S. federal securities laws.
We are a corporation organized under the Canada Business Corporations Act, and some of our directors and officers reside principally outside of the United States. As a result, it may not be possible for our shareholders to enforce judgments obtained in U.S. courts against us or them within the United States because a substantial portion of our assets and the assets of these persons are located outside the United States. In addition, a Canadian court may not agree to recognize and enforce a judgment of a U.S. court. Accordingly, even if a shareholder obtains a favorable judgment in a U.S. court, he, she or it may be required to re-litigate the claim in other jurisdictions. In addition, it is possible that a Canadian court would not take jurisdiction over a matter involving a claim based on foreign laws, such as the federal securities laws of the United States.
Failure to implement our new corporate strategy or realize the expected benefits from this strategy could adversely affect our business and results of operations.
In September 2011, our Board reviewed and approved a new corporate strategy for our company that is focused on improving the performance of our core operations. This new corporate strategy includes, among other things, assessing our global footprint, accelerating our operational excellence programs for our CMO and PDS segments, and continuing the evolution of our existing commercial sites into centers of excellence that focus on specific technologies or production types.
We have incurred and will likely continue to incur expenses in connection with the design, review and implementation of our new corporate strategy, and these expenses may exceed our estimates, may be significant and could materially adversely impact our financial performance.
We have based the design of our new corporate strategy on certain assumptions regarding our business, markets, cost structures and customers. If our assumptions are incorrect, we may be unable to fully implement our new corporate strategy and, even if fully implemented, our new corporate strategy may not yield the benefits that we expect. For example, our new corporate strategy may involve the acquisition or disposition of assets, which we may not be able to consummate in a timely manner, on terms acceptable to us or at all, or which may not achieve the benefits or cost savings we anticipate. If we do not effectively manage our new corporate strategy, instead of resulting in growth for and enhanced value to our company, our new strategy may cause us to experience operational issues and expose us to operational and regulatory risk, each of which could have material adverse effects on our reputation, business, financial condition and results of operations.
Risks Relating to Regulatory and Legal Matters
Failure to comply with existing and future regulatory requirements could adversely affect our business, results of operations and financial condition.
Our industry is highly regulated. We are required to comply with the regulatory requirements of various local, state, provincial, national and international regulatory bodies having jurisdiction in the countries or localities in which we manufacture products or in which our customers’ products are distributed. In particular, we are subject to laws and regulations concerning research and development, testing, manufacturing processes, equipment and facilities, including compliance with cGMPs, labeling and distribution, import and export, and product registration and listing. As a result, most of our facilities are subject to regulation by the FDA, as well as regulatory bodies of other jurisdictions such as the EMEA and/or the NHSA,
depending on the countries in which our customers market and sell the products we manufacture and/or package on their behalf. These regulatory requirements impact many aspects of our operations, including manufacturing, developing, labeling, packaging, storage, distribution, import and export and record keeping related to customers’ products. Noncompliance with any applicable regulatory requirements can result in government refusal to approve (i) facilities for testing or manufacturing products or (ii) products for commercialization. The FDA and other regulatory agencies can delay, limit or deny approval for many reasons, including:
| |
| • | changes to the regulatory approval process, including new data requirements for product candidates in those jurisdictions, including the United States, in which we or our customers may be seeking approval; |
| |
| • | that a product candidate may not be deemed to be safe or effective; |
| |
| • | the ability of the regulatory agency to provide timely responses as a result of its resource constraints; and |
| |
| • | that the manufacturing processes or facilities may not meet the applicable requirements. |
Any delay in, or failure to receive, approval for any of our or our customers’ product candidates or the failure to maintain regulatory approval for our or our customers’ products could negatively impact our revenue growth and profitability.
In addition, if new legislation or regulations are enacted or existing legislation or regulations are amended or are interpreted or enforced differently, we may be required to obtain additional approvals, operate according to different manufacturing or operating standards or pay additional product or establishment user fees. This may require a change in our research and development and manufacturing techniques or additional capital investments in our facilities. Any related costs may be significant. If we fail to comply with applicable regulatory requirements in the future, then we may be subject to warning letters and/or civil or criminal penalties and fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, restrictions on the import and export of our products, debarment, exclusion, disgorgement of profits, operating restrictions and criminal prosecution and the loss of contracts, including government contracts, and resulting revenue losses. Inspections by regulatory authorities that identify any deficiencies could result in remedial actions, production stoppages or facility closure, which would disrupt the manufacturing process and supply of product to our customers. In addition, such failure to comply could expose us to contractual and product liability claims, including claims by customers for reimbursement for lost or damaged APIs or recall or other corrective actions, the cost of which could be significant.
Our pharmaceutical development and manufacturing projects generally involve products that must undergo pre-clinical and clinical evaluations relating to product safety and efficacy before they are approved as commercial therapeutic products. The regulatory authorities having jurisdiction in the countries in which our customers intend to market their products may delay or put on hold clinical trials or delay approval of a product or determine that the product is not approvable. The FDA or other regulatory agencies can delay approval of a drug if our manufacturing facility is not able to demonstrate compliance with cGMPs, pass other aspects of pre-approval inspections or properly scale up to produce commercial supplies. The FDA and comparable government authorities having jurisdiction in the countries in which our customers intend to market their products have the authority to withdraw product approval or suspend manufacture if there are significant problems with raw materials or supplies, quality control and assurance or the product we manufacture is adulterated or misbranded. If our pharmaceutical development projects and their related revenues are not maintained, it could materially adversely affect our results of operations and financial condition.
We are subject to regulatory requirements for controlled substances, which may adversely affect our business or subject us to liabilities if we fail to comply.
Some of our manufactured products are listed as controlled substances. Controlled substances are those products that present a risk of substance abuse. In the United States, these types of products are classified by the by the DEA as Schedule II, III, and IV substances under the Controlled Substances Act of 1970. The DEA classifies substances as Schedule I, II, III, IV or V substances, with Schedule I substances considered to present the highest risk of substance abuse and Schedule V substances the lowest risk. Scheduled substances are subject to DEA regulations relating to manufacturing, storage, distribution, import and export and physician prescription procedures. For example, scheduled drugs are subject to distribution limits and a higher level of recordkeeping requirements. Furthermore, the total amount of controlled substances for manufacture or commercial distribution is limited by the DEA and allocated through quotas, and we or our customers’ quotas, if any, may not be sufficient to meet commercial demand or to economically produce the product.
Entities must be registered annually with the DEA to manufacture, distribute, dispense, import, export and conduct research using controlled substances. State controlled substance laws also require registration for similar activities. In addition, the DEA requires entities handling controlled substances to maintain records, file reports, follow specific labeling and packaging requirements and provide appropriate security measures to control against diversion of controlled substances. In addition, certain of the non-U.S. jurisdictions in which our customers market their products have similar restrictions with
respect to controlled substances. If we fail to follow these requirements, we may be subject to significant civil and/or criminal penalties and possibly a revocation of a DEA registration.
Products containing controlled substances may generate significant public health and safety issues, and in such instances, federal or state authorities can withdraw or limit the marketing rights or regulatory approvals for these products. For some scheduled substances, the FDA may require us or our customers to develop product attributes or a risk evaluation and mitigation strategy to reduce the inappropriate use of the products, including the manner in which they are marketed and sold, so as to reduce the risk of diversion or abuse of the product. Developing such a program may be time-consuming and could delay approval of any product candidates. Such a program or delays of any approval from the FDA could adversely affect our business, results of operations and financial condition.
Decisions of the governmental agencies that regulate us and our customers may affect the demand for our products and significantly influence our business, results of operations and financial condition.
We are dependent on the ability of our customers to obtain regulatory approval and successfully market and obtain third-party coverage and reimbursement for their products and have no control or influence over the regulatory approval process. Delays in obtaining regulatory approval may have a material impact on our operations since our pharmaceutical development and manufacturing projects often involve products that must undergo safety and clinical evaluations before they are approved as commercial therapeutic products. In recent years, our revenues have been negatively impacted due to delays in the regulatory approval of certain of our customers’ products.
By way of example, on February 7, 2010, a unit of Johnson & Johnson (“J&J”) announced that it received a complete response letter from the FDA regarding an NDA for Ceftobiprole that requested additional information and recommended additional clinical studies before approval. The company originally submitted the application in May 2007, and Ceftobiprole has been approved in Canada and in Switzerland. On June 24, 2010, the Committee for Medicinal Products for Human Use (the “CHMP”), after re-examination, confirmed refusal of Janssen-Cilag International N.V.’s marketing authorization for Ceftobiprole. On September 9, 2010, Basilea Pharmaceutica Ltd. announced that Janssen-Cilag AG, a J&J company, will be discontinuing sale of Ceftobiprole (Zevtera™) for the treatment of complicated skin and soft tissue infections in Switzerland. Janssen-Cilag AG, the holder of the Marketing Authorization in Switzerland, has requested Swissmedic to withdraw the marketing authorization of Zevtera™ and discontinued sale of Zevtera™ as of September 17, 2010. This action was taken based on the unfavorable assessments of the marketing authorization applications for Ceftobiprole in the United States and the European Union. In the first quarter of fiscal 2011, we amended our manufacturing and supply agreement with J&J for Ceftobripole to terminate the agreement two and a half years earlier than was originally planned, which will negatively impact our future revenue streams from J&J for this product.
Since we develop and manufacture products that require regulatory approval, failure to gain all such regulatory approvals in a timely manner may adversely reduce our production levels, which would adversely affect our business, results of operations and financial condition. In the event that regulatory authorities fail to approve the products that we develop and/or manufacture, we may not receive payment from our customers under our contracts.
We are subject to environmental, health and safety laws and regulations, which could subject us to liabilities, increase our costs or restrict our operations in the future.
Our operations are subject to a variety of environmental, health and safety laws and regulations in each of the jurisdictions in which we operate. These laws and regulations govern, among other things, air emissions, wastewater discharges, the handling and disposal of hazardous substances and wastes, soil and groundwater contamination and employee health and safety. We are also subject to laws and regulations governing the destruction and disposal of raw materials and non-compliant products, the handling of regulated material that is included in our offerings and the disposal of our offerings at the end of their useful life. These laws and regulations have increasingly become more stringent, and we may incur additional expenses to ensure compliance with existing or new requirements in the future. Any failure by us to comply with environmental, health and safety requirements could result in the limitation or suspension of our operations. We also could incur monetary fines, civil or criminal sanctions, third-party claims or cleanup or other costs as a result of violations of or liabilities under such requirements. Although we maintain insurance coverage for environmental liabilities in the aggregate amount of $10 million, the costs of environmental remediation and other liabilities may exceed the amount of such coverage or may not be covered by such insurance. In addition, compliance with environmental, health and safety requirements could restrict our ability to expand our facilities or require us to acquire costly pollution control equipment, incur other significant expenses or modify our manufacturing processes.
Our manufacturing facilities, in varying degrees, use, store and dispose of hazardous substances in connection with their processes. At some of our facilities, these substances are stored in underground storage tanks or used in refrigeration systems.
Some of our facilities, including those in Puerto Rico, have been utilized over a period of years as manufacturing facilities, with operations that may have included on-site landfill or other waste disposal activities and have certain known or potential conditions that may require remediation in the future, and several of these have undergone remediation activities in the past by former owners or operators. Some of our facilities are located near third-party industrial sites and may be impacted by contamination migrating from such sites. A number of our facilities use groundwater from onsite wells for process and potable water, and if these onsite sources became contaminated or otherwise unavailable for future use, we could incur expenses for obtaining water from alternative sources. In addition, our operations have grown through acquisitions, and it is possible that facilities that we have acquired may expose us to environmental liabilities associated with historical site conditions that have not yet been discovered. Some environmental laws impose liability for contamination on current and former owners and operators of affected sites, regardless of fault. If remediation costs or potential claims for personal injury or property or natural resource damages resulting from contamination arise, they may be material and may not be recoverable under any contractual indemnity or otherwise from prior owners or operators or any insurance policy. Additionally, we may not be able to successfully enforce any such indemnity or insurance policy in the future. In the event that new or previously unknown contamination is discovered or new cleanup obligations are otherwise imposed at any of our currently or previously owned or operated facilities, we may be required to take additional, unplanned remedial measures and record charges for which no reserves have been recorded.
We are subject to product and other liability risks that could adversely affect our results of operations and financial condition.
We may be named as a defendant in product liability lawsuits, which may allege that products or services we have provided have resulted or could result in an unsafe condition or injury to consumers. We may also be exposed to other liability lawsuits, such as other tort, regulatory or intellectual property claims. Such lawsuits could be costly to defend and could result in reduced sales, significant liabilities and diversion of management’s time, attention and resources. Even claims without merit could subject us to adverse publicity and require us to incur significant legal fees.
Historically, we have sought to manage this risk through the combination of product liability insurance and contractual indemnities and liability limitations in our agreements with customers and vendors. We currently maintain insurance coverage for product and other liability claims in the aggregate amount of $75.0 million. If our existing liability insurance is inadequate or we are not able to maintain such insurance, there may be claims asserted against us that are not covered by such insurance. A partially or completely uninsured claim, if successful and of sufficient magnitude, could have a material adverse effect on our results of operations and financial condition.
We and our customers depend on trademarks, patents, trade secrets, copyrights and other forms of intellectual property protections, but these protections may not be adequate.
We rely on a combination of trademark, patent, trade secret and other intellectual property laws in Canada, the United States and other foreign countries. We have applied in Canada, the United States and in certain countries for registration of a limited number of patents and trademarks, some of which have been registered or issued. Our applications may not be approved by the applicable governmental authorities, and third parties may seek to oppose or otherwise challenge our registrations or applications. We also rely on unregistered proprietary rights, including know-how and trade secrets related to our PDS and CMO services. Although we require our employees to enter into confidentiality agreements prohibiting them from disclosing our proprietary information or technology, these agreements may not provide meaningful protection for our trade secrets and proprietary know-how. Further, third parties who are not party to confidentiality agreements may obtain access to our trade secrets or know-how, and others may independently develop similar or equivalent trade secrets or know-how. If our proprietary information is divulged to third parties, including our competitors, or our intellectual property rights are otherwise misappropriated or infringed, our competitive position could be harmed.
If we are unable to protect the confidentiality of our customers’ proprietary information, we may be subject to claims.
Many of the formulations used by us in manufacturing or developing products to customer specifications are subject to trade secret protection, patents or other protections owned or licensed by the relevant customer. We take significant efforts to protect our customer’s proprietary and confidential information, including requiring our employees to enter into agreements protecting such information. If, however, any of our employees breaches the non-disclosure provisions in such agreements, or if our customers make claims that their proprietary information has been disclosed, then our business may be materially adversely impacted.
Our services and our customers’ products may infringe on or misappropriate the intellectual property rights of third parties.
While we believe that our services do not infringe upon in any material respect or misappropriate the proprietary rights of
other parties and/or that meritorious defenses would exist with respect to any assertions to the contrary, our services may be found to infringe on the proprietary rights of others. Any claims that our services infringe third parties’ rights, including claims arising from our contracts with our customers, regardless of their merit or resolution, could be costly and may divert the efforts and attention of our management and technical personnel. We may not prevail in such proceedings given the complex technical issues and inherent uncertainties in intellectual property litigation. If such proceedings result in an adverse outcome, we could be required, among other things, to pay substantial damages, license such technology and/or cease the manufacture, use or sale of the infringing processes or offerings, any of which could adversely affect our business.
Tax legislation initiatives or challenges to our tax positions could adversely affect our results of operations and financial condition.
We are a multinational corporation with global operations. As such, we are subject to the tax laws and regulations of Canadian federal, provincial and local governments, the United States and many international jurisdictions. From time to time, various legislative initiatives may be proposed that could adversely affect our effective tax rate or tax payments. In addition, tax laws and regulations are extremely complex and subject to varying interpretations. If our tax positions are challenged by relevant tax authorities, we may not be successful in defending such a challenge and may experience an adverse impact on our results of operations and financial condition.
Changes in healthcare reimbursement in Canada, the United States or internationally could adversely affect customers’ demand for our services and our results of operations.
The healthcare industry has changed significantly over time, and we expect the industry to continue to evolve. Some of these changes, such as healthcare reform, adverse changes in government funding of healthcare products and services, legislation or regulations governing the privacy of patient information, or the delivery or pricing of pharmaceuticals and healthcare services or mandated benefits, may cause healthcare industry participants to reduce the amount of our services and products they purchase or the price they are willing to pay for our services and products. For example, the recent passage of healthcare reform legislation in the United States changes laws and regulations governing healthcare service providers and specifically includes certain cost containment measures that may adversely impact some or all of our customers and thus may have an adverse impact on our business. Changes in the healthcare industry’s pricing, selling, inventory, distribution or supply policies or practices could also significantly reduce our revenue and profitability. In particular, volatility in individual product demand may result from changes in public or private payer reimbursement or coverage.
Risks Relating to Our Debt
Our substantial level of indebtedness could adversely affect our financial health.
Our total interest-bearing debt as of October 31, 2011 was $275.7 million. As of October 31, 2011, we had approximately $70.5 million available for additional borrowings under our $75.0 million asset-based revolving credit facility ("ABL") and other lines of credit, taking into account our borrowing base limitations.
Our substantial financial leverage poses risks to us. Debt service requirements in future periods may be higher than in prior years as a result of a number of factors, including increased borrowing and increases in floating interest rates. In addition, we may incur substantial fees from time to time in connection with debt amendments or refinancing. If our cash flow is not sufficient to service our debt and adequately fund our business, we may be required to seek further additional financing or refinancing or dispose of assets. We may not be able to effect any of these alternatives on satisfactory terms or at all. In addition, our financial leverage could adversely affect our ability to raise additional capital to fund our operations, could impair our ability to respond to operational challenges, changing business and economic conditions and new business opportunities and may make us vulnerable in the event of a downturn in our business.
If we fail to satisfy our obligations under our indebtedness or fail to comply with the financial and other restrictive covenants contained in the agreements governing such indebtedness, such failure could result in an event of default in respect of any or all such indebtedness. An event of default under one or more of our material debt instruments could result in all of our indebtedness becoming immediately due and payable and could permit (i) the ABL lenders, (ii) the holders of our $280.0 million, 8.625% senior secured notes, due April 15, 2017 (the "Notes") and (iii) our other secured lenders to foreclose on our assets securing such indebtedness.
We may not be able to generate sufficient cash to service all of our indebtedness and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments or to refinance our debt obligations depends on our financial and operating performance, which is subject to prevailing economic and competitive conditions and to certain financial, business and other factors beyond our control. We may not be able to maintain a sufficient level of cash flow from operating activities to permit us
to pay the principal and interest on our Notes and our other indebtedness.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay capital expenditures, sell assets, seek additional capital or seek to restructure or refinance our indebtedness, including our ABL and Notes. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations. In the absence of such cash flows and resources, we could face substantial liquidity problems and might be required to sell material assets or operations to attempt to meet our debt service and other obligations. The instruments governing our indebtedness restrict our ability to conduct asset sales and/or use the proceeds from asset sales. We may not be able to consummate those asset sales to raise capital or sell assets at prices and on terms that we believe are fair and any proceeds that we receive may not be adequate to meet all debt service obligations then due. If we cannot meet our debt service obligations, the holders of our debt may accelerate our debt and, to the extent such debt is secured, foreclose on our assets. In such an event, we may not have sufficient assets to repay all of our debt.
Our debt agreements contain restrictions that limit our flexibility in operating our business and our ability to raise additional funds.
The agreements that govern the terms of our debt contain, and the agreements that govern our future debt may contain, covenants that restrict our ability and the ability of our subsidiaries to, among other things:
| |
| • | incur additional indebtedness; |
| |
| • | issue additional equity; |
| |
| • | pay dividends on or make distributions in respect of capital stock or make certain other restricted payments or investments; |
| |
| • | enter into agreements that restrict distributions from subsidiaries or restrict our ability to incur liens on certain of our assets; |
| |
| • | make capital expenditures; |
| |
| • | sell or otherwise dispose of assets, including capital stock of subsidiaries; |
| |
| • | enter into transactions with affiliates; |
| |
| • | create or incur liens; and |
A breach of the covenants or restrictions under our indebtedness could result in an event of default, which may allow our lenders and note holders to accelerate the related debt and may result in the acceleration of any other debt to which a cross-acceleration or cross-default provision applies. In the event our lenders and note holders accelerate the repayment of our indebtedness, we may not have sufficient assets to repay such indebtedness or be able to borrow sufficient funds to refinance it. Even if we are able to obtain new financing, it may not be on commercially reasonable terms or on terms acceptable to us. As a result of these restrictions, we may be:
| |
| • | limited in how we conduct our business and execute our business strategy; |
| |
| • | unable to raise additional debt or equity financing to operate during general economic or business downturns; |
| |
| • | unable to compete effectively or to take advantage of new business opportunities; or |
These restrictions may affect our ability to grow in accordance with our plans.
The amount of borrowings permitted under the ABL may fluctuate significantly, which may adversely affect our liquidity, results of operations and financial condition.
The amount of borrowings permitted at any time under the ABL is limited to a periodic borrowing base valuation of our and certain of our subsidiaries' eligible inventory and accounts receivable. As a result, our access to credit under the ABL is potentially subject to significant fluctuations depending on the value of the borrowing base eligible assets as of any valuation date and certain discretionary rights of the agent in respect of the calculation of such borrowing base valuation. The inability to borrow under, or the early termination of, the ABL may adversely affect our liquidity, results of operations and financial condition.
Despite our substantial level of indebtedness, we may still be able to incur significant additional amounts of debt, which
could further exacerbate the risks associated with our substantial debt.
We and our subsidiaries may be able to incur significant additional amounts of debt, including additional secured indebtedness, in the future. The terms of the ABL and Notes restrict, but do not completely prohibit, us from doing so. In addition, our ABL and Notes allow us to issue additional senior secured notes and other indebtedness under certain circumstances and generally do not prevent us from incurring other liabilities that do not constitute indebtedness. If new debt or other liabilities are added to our current debt levels, then the related risks that we and our subsidiaries now face could intensify.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 2. Properties.
We have a network of nine manufacturing facilities, and eight development centers located in North America and Europe and one clinical packaging facility in North America. The following table provides additional information about our principal manufacturing facilities and development centers:
|
| | | | | | | | |
| Facility sites | Country | | Segment | | Square Feet | | Owned/Leased |
| Burlington(1) | Canada | | PDS | | 45,496 |
| | Leased |
| Mississauga | Canada | | CMO/PDS | | 285,570 |
| | Owned |
| Whitby | Canada | | CMO/PDS | | 233,664 |
| | Owned |
| Cincinnati | U.S. | | CMO/PDS | | 495,700 |
| | Owned |
| Caguas | Puerto Rico | | CMO | | 209,336 |
| | Owned |
| Manatí | Puerto Rico | | CMO | | 546,872 |
| | Owned |
| Ferentino | Italy | | CMO/PDS | | 290,473 |
| | Owned |
| Monza | Italy | | CMO | | 463,229 |
| | Owned |
| Milton Park(2) | U.K. | | PDS | | 13,500 |
| | Leased |
| Swindon | U.K. | | CMO/PDS | | 355,511 |
| | Owned |
| Bourgoin-Jallieu | France | | CMO/PDS | | 355,228 |
| | Owned |
| San Francisco | U.S. | | PDS | | 5,063 |
| | Leased |
_________________________
| |
| (1) | Our Burlington facility is subject to a lease from Klaus Stephan Reeckmann until 2014, with a minimum annual rent of $256,410, based on an average foreign exchange rate of Canadian dollars (“CAD”) to USD for fiscal 2011 of 1.0137. |
| |
| (2) | Our Milton Park facility is subject to a lease from Lansdown Estates Group Limited until 2020, with a minimum annual rent of $133,000, based on an average foreign exchange rate of British pound sterling to USD for fiscal 2011 of 1.6085. |
We also lease facilities in Research Triangle Park, North Carolina (U.S. headquarters), Zug, Switzerland (European headquarters), Mississauga, Canada (regional administration) and Tokyo, Japan (sales office). Our primary facilities are pledged as collateral for the Notes and the ABL. See “Item 7. Management’s Discussion and Analysis—Liquidity and Capital Resources—Financing Arrangements.” We believe that our facilities are adequate for our operations and that suitable additional space will be available when needed.
Item 3. Legal Proceedings.
Neither we, nor any of our subsidiaries, are involved in any material pending legal proceeding. Additionally, no such proceedings are known to be contemplated by governmental authorities.
Item 4. Removed and Reserved
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity
Securities.
Market Information
Our restricted voting shares are traded on the TSX under the trading symbol “PTI.” There is no established public trading market for our shares in the United States. The following table sets forth the reported high and low trading prices (in Canadian dollars) and trading volumes of our restricted voting shares on the TSX for the following periods:
Toronto Stock Exchange
(Canadian $s)
|
| | | | | |
| | High | | Low |
| Fiscal year ending October 31, 2011: | | | |
| Quarter ended January 31, 2011 | 2.45 |
| | 2.03 |
|
| Quarter ended April 30, 2011 | 2.74 |
| | 2.27 |
|
| Quarter ended July 31, 2011 | 2.33 |
| | 1.80 |
|
| Quarter ended October 31, 2011 | 2.03 |
| | 1.33 |
|
| Fiscal year ended October 31, 2010: | | | |
| Quarter ended January 31, 2010 | 2.73 |
| | 2.11 |
|
| Quarter ended April 30, 2010 | 2.70 |
| | 2.46 |
|
| Quarter ended July 31, 2010 | 2.66 |
| | 2.38 |
|
| Quarter ended October 31, 2010 | 2.58 |
| | 2.19 |
|
Holders
As of December 15, 2011, there were approximately 539 holders of record of our restricted voting shares. This number does not include beneficial owners for whom shares are held by nominees in street name.
Dividends
We did not pay dividends on our restricted voting shares during fiscal 2011, fiscal 2010 or our fiscal year ended October 31, 2009 (“fiscal 2009”). We currently do not intend to pay cash dividends on our restricted voting shares for the foreseeable future, as we prefer to reinvest our cash to enhance our growth.
Our debt agreements include covenants that limit our ability to pay dividends. See “Note 8—Long-Term Debt” to our consolidated financial statements included in this Form 10-K. The Investor Agreement also prevents us from declaring or paying any dividends without the approval of JLL Patheon Holdings for so long as JLL Patheon Holdings holds 13,306,488 restricted voting shares.
Exchange Controls
There is no law or governmental decree or regulation in Canada that restricts the export or import of capital or affects the remittance of dividends, interest or other payments to non-resident holders of our common stock, other than withholding tax requirements. See “—Certain Canadian Federal Income Tax Considerations.”
There is no limitation imposed by Canadian law or by our articles of amalgamation on the right of a non-resident to hold or vote restricted voting shares, other than as provided by the “Investment Canada Act,” the “North American Free Trade
Agreement Implementation Act (Canada),” the “World Trade Organization Agreement Implementation Act” and the CBCA, which permits our shareholders, by special resolution, to amend our articles of amalgamation to constrain the issue or transfer of any class or series of our securities to persons who are not residents of Canada in certain limited circumstances.
The Investment Canada Act requires notification and, in certain cases, advance review and approval by the Government of Canada of the acquisition by a “non-Canadian” of “control” of a “Canadian business,” all as defined in the Investment Canada Act. Generally, the threshold for review will be higher in monetary terms for a member of the World Trade Organization or North American Free Trade Agreement.
Taxation
Certain Canadian Federal Income Tax Considerations
The following is a summary of the principal Canadian federal income tax considerations generally applicable to holders of our restricted voting shares who, at all relevant times, for purposes of the Income Tax Act (Canada) (the “Tax Act”) and the Canada-United States Tax Convention (1980) ( the "Canada-U.S. Tax Treaty") (i) are the beneficial owners of such restricted voting shares; (ii) are “qualifying persons” entitled to benefits under the Canada-U.S. Tax Treaty, (iii) are resident in the United States and are neither resident nor deemed to be resident in Canada; (iv) deal at arm's length with, and are not affiliated with, us; (v) hold their restricted voting shares as capital property; (vi) do not use or hold, and are not deemed to use or hold their restricted voting shares in connection with carrying on business in Canada; and (vii) do not hold or use restricted voting shares in connection with a permanent establishment or fixed base in Canada (each, a “U.S. Resident Holder”). Special rules, which are not discussed in this summary, may apply to a U.S. Resident Holder that is an insurer that carries on an insurance business in Canada and elsewhere.
Our restricted voting shares will generally be considered capital property to a U.S. Resident Holder unless either (i) the U.S. Resident Holder holds our restricted voting shares in the course of carrying on a business of buying and selling securities, or (ii) the U.S. Resident Holder has acquired our restricted voting shares in a transaction or transactions considered to be an adventure in the nature of trade.
Limited liability companies (“LLCs”) that are not taxed as corporations pursuant to the provisions of the Code generally do not qualify as resident in the United States and are not “qualified persons” for purposes of the Canada-U.S. Tax Treaty. Under the Canada-U.S. Tax Treaty, a resident of the United States who is a member of such an LLC and is otherwise a “qualified person” eligible for benefits under the Canada-U.S. Tax Treaty may be entitled to claim benefits under the Canada-U.S. Tax Treaty in respect of income, profits or gains derived through the LLC. A U.S. Resident Holder who is a member of an LLC should consult with his, her or its own tax advisors with respect to eligibility for benefits in respect of any income, profits or gains derived through such LLC.
The Canada-U.S. Tax Treaty includes limitation on benefits rules that restrict the ability of certain persons who are resident in the United States to claim any or all benefits under the Canada-U.S. Tax Treaty. A U.S. Resident Holder should consult his, her or its own tax advisors with respect to his, her or its eligibility for benefits under the Canada-U.S. Tax Treaty, having regard to these rules.
This summary is based on the current provisions of the Canada-U.S. Tax Treaty and the Tax Act, the regulations thereunder (the “Regulations”) and counsel's understanding of the current published administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”) made publicly available prior to the date of this registration statement. This summary also takes into account all specific proposals to amend the Tax Act and the Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Tax Proposals”), and assumes that all such Tax Proposals will be enacted in the form proposed. There is no assurance that the Tax Proposals will be enacted in their current form, or at all. This summary does not otherwise take into account or anticipate any changes in the law, whether by legislative, governmental or judicial action, or in the CRA's administrative policies or assessing practices.
This summary does not address the tax laws of any province or territory of, or any jurisdiction outside, Canada, which might materially differ from the Canadian federal considerations.
This summary is of a general nature only and not intended to be, nor should it be construed to be, legal or tax advice to any particular U.S. Resident Holder, and no representations concerning the tax consequences to any particular U.S. Resident Holder are made. U.S. Resident Holders should consult their own tax advisers regarding the income tax consequences, arising from and relating to the acquisition, ownership and disposition of our restricted voting shares with respect to their own particular circumstances.
Currency Conversion
For purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of our restricted voting shares, including interest, dividends, adjusted cost base and proceeds of disposition, must be converted into Canadian dollars based on the relevant exchange rate applicable on the effective date (as determined in accordance with the Tax Act) of the related acquisition, disposition or recognition of income.
Disposition of Restricted Voting Shares
A U.S. Resident Holder will not be subject to tax under the Tax Act in respect of any capital gain (or entitled to deduct any capital loss) realized on a disposition of restricted voting shares unless our restricted voting shares constitute “taxable Canadian property” (as defined in the Tax Act) of the U.S. Resident Holder at the time of disposition and the U.S. Resident Holder is not entitled to relief under the Canada-U.S. Tax Treaty. So long as our restricted voting shares are then listed on a designated stock exchange (which currently includes the TSX), our restricted voting shares will generally not constitute taxable Canadian property of a U.S. Resident Holder.
A U.S. Resident Holder whose restricted voting shares are, or may be considered to be, taxable Canadian property should consult with his, her or its own tax advisors for advice having regard to such holder's particular circumstances.
Dividends
Dividends on restricted voting shares paid or credited, or deemed to be paid or credited, to a U.S. Resident Holder will be subject to a non-resident withholding tax under the Tax Act at a rate of 25%, subject to reduction under the provisions of an applicable tax treaty or convention. Pursuant to the Canada-U.S. Tax Treaty, the rate of withholding tax on dividends paid or credited to a U.S. Resident Holder that is the beneficial owner of such dividends generally is reduced to 15% or, if the U.S. Resident Holder is a corporation that is the beneficial owner of at least 10% of our voting stock, to 5%.
The Canada-U.S. Tax Treaty generally exempts from Canadian withholding tax dividends paid or credited to (i) a qualifying religious, scientific, literary, educational or charitable organization or (ii) a qualifying trust, company, organization or arrangement constituted and operated exclusively to administer or provide a pension, retirement or employee benefit fund or plan, if such organization or qualifying trust, company or arrangement is a resident of the United States and is exempt from income tax under the laws of the United States.
HOLDERS OF RESTRICTED VOTING SHARES ARE URGED TO CONSULT THEIR OWN TAX ADVISORS TO DETERMINE THE PARTICULAR TAX CONSEQUENCES TO THEM, INCLUDING THE APPLICATION AND EFFECT OF ANY STATE, LOCAL OR FOREIGN INCOME AND OTHER TAX LAWS, OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF RESTRICTED VOTING SHARES.
Recent Sales of Unregistered Securities.
The following securities were sold by us during fiscal 2011 and were not registered under the Securities Act.
Between November 1, 2010 and April 26, 2011, when our registration statement on Form 10 became effective, we issued options to purchase 5,042,000 restricted voting shares with an aggregate exercise price of $2.62 to our directors, officers, employees and consultants. The grants of these securities to persons residing outside the United States were made in reliance on Regulation S promulgated under the Securities Act. The grants of these securities to persons residing in the United States were made in reliance on Rule 701 promulgated under the Securities Act.
Stock Performance Graph
The information in this “Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity—Stock Performance Graph” is not deemed to be “soliciting material” or to be “filed” with the Securities and Exchange Commission (the “SEC”) or subject to Regulation 14A or 14C under the Exchange Act or to the liabilities of Section 18 of the Exchange Act, and will not be deemed to be incorporated by reference into any filing under the Securities Act or the Exchange Act, except to the extent we specifically incorporate it by reference into such filing.
The following graph compares our cumulative total shareholder return from October 31, 2006 with those of the TSX Index and the S&P/TSX Pharmaceutical Index. The measurement points utilized in the graph consist of the last trading day in each fiscal year. The historical stock performance presented below is not intended to and may not be indicative of future stock performance.
Comparison of 5-Year Cumulative Total Return*
Among Patheon Inc., the TSX Index and the S&P/TSX Pharmaceutical Index
* Assumes (1) $100 invested on October 31, 2006 in Patheon's restricted voting shares, the TSX Index and the S&P/TSX Pharmaceutical Index and (2) the immediate reinvestment of all dividends.
Item 6. Selected Financial Data.
The selected financial data set forth below as of and for the years ended October 31, 2011, 2010, 2009, 2008 and 2007 were derived from our consolidated financial statements and should be read in conjunction with “Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes thereto of this Form 10-K .
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in Canada (“Canadian GAAP”). See “Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations” and “Note 21—Additional Disclosures Required Under U.S. Generally Accepted Accounting Principles” to our consolidated financial statements included in this Form 10-K for a detailed description of the differences between Canadian GAAP and accounting principles generally accepted in the United States (“U.S. GAAP”) relating to our company.
Canadian GAAP
The following table shows selected financial information for the periods indicated in accordance with Canadian GAAP:
|
| | | | | | | | | | | | | | |
| | Years ended October 31, |
| | 2007(1) | | 2008(2) | | 2009(3) | | 2010(4) | | 2011(5) |
| | (Dollar information in millions of U.S. dollars (“USD”), except per share information) |
| | $ | | $ | | $ | | $ | | $ |
| Statement of (loss) income data: | | | | | | | | | |
| Revenues | 634.1 |
| | 717.3 |
| | 655.1 |
| | 671.2 |
| | 700.0 |
|
| (Loss) income before discontinued operations | (36.5 | ) | | 20.3 |
| | 1.0 |
| | (3.3 | ) | | (16.2 | ) |
| Adjusted EBITDA | 80.5 |
| | 82.6 |
| | 74.0 |
| | 91.7 |
| | 73.0 |
|
| Basic and diluted (loss) income per share from continuing operations | (0.39 | ) | | 0.21 |
| | (0.10 | ) | | (0.03 | ) | | (0.13 | ) |
| Weighted-average number of shares outstanding—basic and diluted (in thousands) | 92,834 |
| | 90,737 |
| | 100,964 |
| | 129,168 |
| | 129,168 |
|
| Balance sheet data (at period end): | | | | | | | | | |
| Total assets | 803.7 |
| | 701.9 |
| | 790.8 |
| | 808.9 |
| | 818.6 |
|
| Long-term debt | 203.6 |
| | 200.5 |
| | 221.1 |
| | 274.8 |
| | 274.6 |
|
| Deferred revenues | 26.0 |
| | 22.5 |
| | 41.7 |
| | 45.9 |
| | 36.5 |
|
| Convertible preferred shares—debt component | 139.9 |
| | — |
| | — |
| | — |
| | — |
|
| Other long-term liabilities | 22.1 |
| | 16.4 |
| | 21.5 |
| | 22.9 |
| | 21.7 |
|
| Total shareholders’ equity | 174.3 |
| | 237.2 |
| | 271.3 |
| | 273.0 |
| | 263.7 |
|
____________________________
| |
| (1) | Loss before discontinued operations included a non-cash impairment charge of $48.6 million related to the Puerto Rico operations, $15.8 million in repositioning expenses, a $12.4 million non-cash foreign exchange gain on the revaluation of certain U.S. dollar denominated debt in Canada and $7.1 million in non-cash accreted interest on the Series C Preferred Shares. Our liabilities as of October 31, 2007 included the debt component of JLL Patheon Holdings' purchase of the 150,000 units of Series C Preferred Shares for the price of $150.0 million. |
| |
| (2) | Income before discontinued operations included $19.9 million in repositioning expenses, a $6.4 million non-cash foreign exchange loss on the revaluation of certain U.S. dollar denominated debt in Canada, $13.5 million in non-cash accreted interest on the convertible Series C Preferred Shares and the $34.9 million non-cash gain on the deemed repayment of debt discussed below. The reduction in total liabilities from the fiscal year ended October 31, 2007 was primarily the result of the completion of our agreement with JLL Patheon Holdings pursuant to which JLL Patheon Holdings agreed to waive the mandatory redemption requirement in respect of the Series C Preferred Shares that it held (the “Redemption Waiver Agreement”) in fiscal 2008, which resulted in the full carrying value of the Series C Preferred Shares being classified within shareholders' equity on our balance sheet and the reclassification of $131.8 million of debt to equity. The entry into the Redemption Waiver Agreement resulted in a deemed repayment of the debt and equity components of the Series C Preferred Shares. As such, we recognized a non-cash gain of $34.9 million on the deemed repayment of the debt component. |
| |
| (3) | Income before discontinued operations included $2.1 million in repositioning expenses and $8.0 million in costs associated with the special committee of independent directors that we formed during fiscal 2009 (the “Special Committee”) and JLL Patheon Holdings' December 8, 2009 unsolicited offer to acquire any or all of our outstanding restricted voting shares that it did not already own at a price of $2.00 per share in cash (the “JLL Offer”). |
| |
| (4) | Loss before discontinued operations included $6.8 million in repositioning expenses, $12.2 million in refinancing costs, $3.6 million in non-cash impairment charges, $7.2 million in non-cash reduction in costs for the utilization of the prior fiscal years' investment tax credits, a non-cash tax benefit of $13.8 from the release of the valuation reserve in our Canadian operations and $3.0 million in costs associated with the Special Committee and the JLL Offer. The long-term debt increased from fiscal 2009 due to the issuance of the Notes for an aggregate principal amount of $280.0 million, the proceeds from which were used to repay all of the outstanding indebtedness under our then-existing senior secured term loan and our $75.0 million ABL, to repay certain other indebtedness and to pay related fees and expenses. We used the remaining proceeds for general corporate purposes. See “Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Summary of Cash Flows—Cash Provided by Financing Activities”). |
| |
| (5) | Loss before discontinued operations included $12.8 million in consulting and professional fees primarily related to our |
strategic initiatives and our SEC registration and $7.0 million in repositioning expenses, partially offset by proceeds from an insurance settlement of $4.9 million.
References to “Adjusted EBITDA” are to income (loss) before discontinued operations before repositioning expenses, interest expense, foreign exchange losses reclassified from other comprehensive loss, refinancing expenses, gains and losses on sale of fixed assets, gain on extinguishment of debt, income taxes, asset impairment charges, depreciation and amortization and other income and expenses.
Since Adjusted EBITDA is a non-GAAP measure that does not have a standardized meaning, it may not be comparable to similar measures presented by other issuers. Readers are cautioned that Adjusted EBITDA should not be construed as an alternative to net income (loss) determined in accordance with Canadian GAAP as an indicator of performance. Adjusted EBITDA is used by management as an internal measure of profitability. We have included Adjusted EBITDA because we believe that this measure is used by certain investors to assess our financial performance before non-cash charges and certain costs that we do not believe are reflective of our underlying business.
A reconciliation of Adjusted EBITDA to (loss) income before discontinued operations is set forth below:
|
| | | | | | | | | | | | | | |
| | Years ended October 31, |
| | 2007(1) | | 2008(2) | | 2009(3) | | 2010(4) | | 2011(5) |
| | (in millions of USD) |
| | $ | | $ | | $ | | $ | | $ |
| Net (loss) income for the period | (96.3 | ) | | 0.8 |
| | (6.8 | ) | | (5.0 | ) | | (16.8 | ) |
| Loss from discontinued operations | (59.8 | ) | | (19.5 | ) | | (7.8 | ) | | (1.7 | ) | | (0.6 | ) |
| (Loss) income before discontinued operations | (36.5 | ) | | 20.3 |
| | 1.0 |
| | (3.3 | ) | | (16.2 | ) |
| Add (deduct): | | | | | | | | | |
| Provision for (benefit from) income taxes | 19.7 |
| | 1.5 |
| | 12.5 |
| | (3.0 | ) | | 7.3 |
|
| Gain on extinguishment of debt | — |
| | (34.9 | ) | | — |
| | — |
| | — |
|
| (Gain) loss on sale of fixed assets | — |
| | (0.7 | ) | | — |
| | 0.2 |
| | 0.2 |
|
| Foreign exchange loss on foreign operations | 0.8 |
| | — |
| | — |
| | — |
| | — |
|
| Refinancing expenses | 13.5 |
| | — |
| | — |
| | 12.2 |
| | — |
|
| Interest expense, net | 29.1 |
| | 30.7 |
| | 15.4 |
| | 19.5 |
| | 25.4 |
|
| Repositioning expenses | 14.5 |
| | 19.9 |
| | 2.1 |
| | 6.8 |
| | 7.0 |
|
| Depreciation and amortization | 39.4 |
| | 45.3 |
| | 42.6 |
| | 55.8 |
| | 53.4 |
|
| Asset impairment charge | — |
| | 0.4 |
| | — |
| | 3.6 |
| | — |
|
| Other | — |
| | 0.1 |
| | 0.4 |
| | (0.1 | ) | | (4.1 | ) |
| Adjusted EBITDA | 80.5 |
| | 82.6 |
| | 74.0 |
| | 91.7 |
| | 73.0 |
|
U.S. GAAP
Our financial statements have been prepared in accordance with Canadian GAAP, which differs in certain respects from U.S. GAAP. The differences between Canadian GAAP and U.S. GAAP that affect our financial statements are described in detail in “Note 21—Additional Disclosures Required Under U.S. Generally Accepted Accounting Principles” to our consolidated financial statements included in this Form 10-K.
Had we followed U.S. GAAP, certain selected financial data reported above in accordance with Canadian GAAP would have been reported as follows:
|
| | | | | | | | | | | | | | |
| | Years ended October 31, |
| | 2007 | | 2008 | | 2009 | | 2010 | | 2011 |
| | (Dollar information in millions of USD, except per share information) |
| | $ | | $ | | $ | | $ | | $ |
| Statement of (loss) income data: | | | | | | | | | |
| Revenues | 634.1 |
| | 717.3 |
| | 655.1 |
| | 671.2 |
| | 700.0 |
|
| (Loss) income before discontinued operations | (41.5 | ) | | 4.9 |
| | 1.1 |
| | (2.9 | ) | | (15.8 | ) |
| Adjusted EBITDA | 66.4 |
| | 89.0 |
| | 74.0 |
| | 80.9 |
| | 66.4 |
|
| Basic (loss) income per share from continuing operations | (0.52 | ) | | 0.09 |
| | (0.10 | ) | | (0.02 | ) | | (0.12 | ) |
| Diluted income per share from continuing operations | — |
| | 0.01 |
| | — |
| | — |
| | — |
|
| Weighted-average number of shares outstanding during period—basic (in thousands) | 92,834 |
| | 90,737 |
| | 100,964 |
| | 129,168 |
| | 129,168 |
|
| Weighted-average number of shares outstanding during period—diluted (in thousands) | — |
| | 123,634 |
| | — |
| | — |
| | — |
|
| Balance sheet data (at period end): | | | | | | | | | |
| Total assets | 805.8 |
| | 703.5 |
| | 794.2 |
| | 813.2 |
| | 824.6 |
|
| Long-term debt | 207.5 |
| | 203.2 |
| | 223.5 |
| | 281.1 |
| | 280.1 |
|
| Deferred revenues | 26.0 |
| | 22.5 |
| | 41.7 |
| | 45.9 |
| | 36.5 |
|
| Other long-term liabilities | 28.4 |
| | 30.6 |
| | 49.5 |
| | 45.1 |
| | 53.7 |
|
| Convertible preferred shares—temporary equity | 155.2 |
| | — |
| | — |
| | — |
| | — |
|
| Total shareholders’ equity | 167.4 |
| | 222.2 |
| | 244.6 |
| | 248.1 |
| | 237.7 |
|
A reconciliation of Adjusted EBITDA to (loss) income before discontinued operations is set forth below:
|
| | | | | | | | | | | | | | |
| | Years ended October 31, |
| | 2007 | | 2008 | | 2009 | | 2010 | | 2011 |
| | (in millions of USD) |
| | $ | | $ | | $ | | $ | | $ |
| Net (loss) income for the period | (101.3 | ) | | (14.6 | ) | | (6.7 | ) | | (4.6 | ) | | (16.4 | ) |
| Loss from discontinued operations | (59.8 | ) | | (19.5 | ) | | (7.8 | ) | | (1.7 | ) | | (0.6 | ) |
| (Loss) income from continuing operations | (41.5 | ) | | 4.9 |
| | 1.1 |
| | (2.9 | ) | | (15.8 | ) |
| Add (deduct): | | | | | | | | | |
| Provision for (benefit from) income taxes | 18.0 |
| | 2.2 |
| | 12.6 |
| | (13.8 | ) | | 1.1 |
|
| (Gain) loss on sale of fixed assets | — |
| | (0.7 | ) | | — |
| | 0.2 |
| | 0.2 |
|
| Foreign exchange loss on foreign operations | 0.8 |
| | — |
| | — |
| | — |
| | — |
|
| Refinancing expenses | 13.5 |
| | — |
| | — |
| | 12.2 |
| | — |
|
| Interest expense, net | 22.0 |
| | 17.2 |
| | 15.4 |
| | 19.6 |
| | 25.6 |
|
| Repositioning expenses | 14.5 |
| | 19.9 |
| | 2.1 |
| | 6.8 |
| | 7.0 |
|
| Depreciation and amortization | 39.1 |
| | 45.0 |
| | 42.4 |
| | 55.6 |
| | 53.2 |
|
| Asset impairment charge | — |
| | 0.4 |
| | — |
| | 3.6 |
| | — |
|
| Other | — |
| | 0.1 |
| | 0.4 |
| | (0.4 | ) | | (4.9 | ) |
| Adjusted EBITDA | 66.4 |
| | 89.0 |
| | 74.0 |
| | 80.9 |
| | 66.4 |
|
The following is a summary of our unaudited quarterly results of operations under Canadian GAAP:
|
| | | | | | | |
| Year Ended October 31, 2011 |
| | 1st Quarter(1) | | 2nd Quarter(2) | | 3rd Quarter(3) | | 4th Quarter |
| | (Dollar information in millions of USD, except per share
information) |
| | $ | | $ | | $ | | $ |
| Revenues | 175.7 | | 170.0 | | 172.7 | | 181.6 |
| Gross profit | 43.0 | | 32.0 | | 27.4 | | 35.7 |
| Income (loss) before discontinued operations | 0.7 | | (11.1) | | (0.5) | | (5.3) |
| Loss from discontinued operations | (0.2) | | (0.1) | | (0.2) | | (0.1) |
| Net income (loss) attributable to restricted voting shareholders | 0.5 | | (11.2) | | (0.7) | | (5.4) |
| | | | | | | | |
| Basic and diluted income (loss) per share | | | | | | | |
| From continuing operations | 0.006 | | (0.086) | | (0.004) | | (0.041) |
| From discontinued operations | (0.002) | | (0.001) | | (0.002) | | (0.001) |
| | 0.004 | | (0.087) | | (0.006) | | (0.042) |
|
| | | | | | | | |
| Year Ended October 31, 2010 |
| | 1st Quarter(4) | | 2nd Quarter(5) | | 3rd Quarter | | 4th Quarter |
| | (Dollar information in millions of USD, except per share
information) |
| | $ | | $ | | $ | | $ |
| Revenues | 154.8 | | 175.4 | | 163.3 |
| | 177.7 |
| Gross profit | 24.6 | | 43.2 | | 34.4 |
| | 42.8 |
| (Loss) income before discontinued operations | (10.7) | | 11.3 | | (3.0) |
| | (0.9) |
| Loss from discontinued operations | (0.4) | | (0.4) | | — |
| | (0.9) |
| Net (loss) income attributable to restricted voting shareholders | (11.1) | | 10.9 | | (3.0) |
| | (1.8) |
| | | | | | | | |
| Basic and diluted (loss) income per share | | | | | | | |
| From continuing operations | (0.083) | | 0.087 | | (0.023) |
| | (0.041) |
| From discontinued operations | (0.003) | | (0.003) | | — |
| | (0.001) |
| | (0.086) | | 0.084 | | (0.023) |
| | (0.042) |
| |
| (1) | Revenues included the impact of approximately $32.9 million in reservation fees and deferred revenue amortization from the amended manufacturing and supply agreement in the United Kingdom. |
| |
| (2) | Revenues included the impact of approximately $17.5 million in reservation fees and deferred revenue amortization from the amended manufacturing and supply agreement in the United Kingdom. |
| |
| (3) | Loss before discontinued operations included an insurance settlement of $6.0 million in other income. |
| |
| (4) | Loss before discontinued operations included $3.0 million in special committee costs offset by $2.8 million of prior year's Canadian research and development investment tax credits |
| |
| (5) | Loss before discontinued operations included $13.8 million from releasing our valuation allowance pertaining to future tax assets in the Company's Canadian operations through income tax benefit in the income statement and $4.4 million of prior year's Canadian research and development investment tax credits through cost of goods sold. Loss also included $4.2 million in accelerated deferred revenue in Cincinnati. |
The following is a summary of our unaudited quarterly results of operations under U.S. GAAP:
|
| | | | | | | | | | | |
| Year Ended October 31, 2011 |
| | 1st Quarter | | 2nd Quarter | | 3rd Quarter | | 4th Quarter |
| | (Dollar information in millions of USD, except per share
information) |
| | $ | | $ | | $ | | $ |
| Revenues | 175.7 |
| | 170.0 |
| | 172.7 |
| | 181.6 |
|
| Gross profit | 42.2 |
| | 30.4 |
| | 25.8 |
| | 33.5 |
|
| Income (loss) before discontinued operations | 3.7 |
| | (10.3 | ) | | 0.6 |
| | (9.8 | ) |
| Loss from discontinued operations | (0.2 | ) | | (0.1 | ) | | (0.2 | ) | | (0.1 | ) |
| Net income (loss) attributable to restricted voting shareholders | 3.5 |
| | (10.4 | ) | | 0.4 |
| | (9.9 | ) |
| | | | | | | | |
| Basic and diluted income (loss) per share | | | | | | | |
| From continuing operations | 0.029 |
| | (0.080 | ) | | 0.005 |
| | (0.075 | ) |
| From discontinued operations | (0.002 | ) | | (0.001 | ) | | (0.002 | ) | | (0.001 | ) |
| | 0.027 |
| | (0.081 | ) | | 0.003 |
| | (0.076 | ) |
|
| | | | | | | | | | | |
| Year Ended October 31, 2010 |
| | 1st Quarter | | 2nd Quarter | | 3rd Quarter | | 4th Quarter |
| | (Dollar information in millions of USD, except per share
information) |
| | $ | | $ | | $ | | $ |
| Revenues | 154.8 |
| | 175.4 |
| | 163.3 |
| | 177.7 |
|
| Gross profit | 21.8 |
| | 38.8 |
| | 31.7 |
| | 42.1 |
|
| (Loss) income before discontinued operations | (1.7 | ) | | 4.6 |
| | (5.5 | ) | | (0.4 | ) |
| Loss from discontinued operations | (0.4 | ) | | (0.4 | ) | | — |
| | (0.9 | ) |
| Net (loss) income attributable to restricted voting shareholders | (2.1 | ) | | 4.2 |
| | (5.5 | ) | | (1.3 | ) |
| | | | | | | | |
| Basic and diluted (loss) income per share | | | | | | | |
| From continuing operations | (0.013 | ) | | 0.036 |
| | (0.043 | ) | | (0.003 | ) |
| From discontinued operations | (0.003 | ) | | (0.003 | ) | | — |
| | (0.007 | ) |
| | (0.016 | ) | | 0.033 |
| | (0.043 | ) | | (0.010 | ) |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion is designed to provide a better understanding of our consolidated financial statements, including a brief discussion of our business and products, key factors that impact our performance and a summary of our operating results. You should read the following discussion and analysis of financial condition and results of operations together with our consolidated financial statements and the related notes included in this Form 10-K. Our consolidated financial statements and MD&A have been prepared in accordance with Canadian GAAP. The impact of significant differences
between Canadian GAAP and U.S. GAAP on the financial statements is disclosed in “Note 21—Additional Disclosures Required Under U.S. Generally Accepted Accounting Principles” to our consolidated financial statements included in this Form 10-K. In addition to historical information, the following discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results could differ materially from those anticipated by the forward-looking statements due to important factors including, but not limited to, those set forth under “Item 1A. Risk Factors” of this Form 10-K.
Executive Overview
We are a leading provider of contract manufacturing and development services to the global pharmaceutical industry, offering a wide range of services from developing drug candidates at the pre-formulation stage through the launch, commercialization and production of approved drugs. We have established our position as a market leader by leveraging our scale, global reach, specialized capabilities, broad service offerings, scientific expertise and track record of product quality and regulatory compliance to provide cost-effective solutions to our customers.
We have two reportable segments, CMO and PDS. Our CMO business manufactures prescription products in sterile dosage forms as well as solid and liquid conventional dosage forms, and we differentiate ourselves by offering specialized manufacturing capabilities relating to high potency, controlled substance and sustained release products. Our PDS business provides a broad range of development services, including finished dosage formulation across approximately 40 dosage forms, clinical trial packaging and associated analytical services. Additionally, our PDS business serves as a pipeline for future commercial manufacturing opportunities.
Recent Business Highlights
The following is a summary of certain key financial results and non-financial events that occurred during and subsequent to fiscal 2011:
| |
| • | Revenues for fiscal 2011 increased $28.8 million, or 4.3%, to $700.0 million, from $671.2 million for fiscal 2010. Excluding currency fluctuations, revenues for fiscal 2011 would have been approximately 3.0% higher than prior year. |
| |
| • | Loss before discontinued operations for fiscal 2011 was $16.2 million, compared to a loss before discontinued operations of $3.3 million for fiscal 2010. |
| |
| • | Adjusted EBITDA for fiscal 2011 decreased $18.7 million, or 20.4%, to $73.0 million, from $91.7 million for fiscal 2010. |
| |
| • | On December 15, 2011, we announced the appointment of Michel Lagarde, Principal, JLL Partners, to our Board. Mr. Lagarde replaced Thomas S. Taylor, Managing Director, JLL Partners, who left our Board effective December 15, 2011. Mr. Lagarde replaced Mr. Taylor as the Chairman of our Compensation and Human Resources Committee ("CHR Committee") and is a member of our Audit and Corporate Governance Committees. |
| |
| • | On November 1, 2011, Eric W. Evans, our then Chief Financial Officer, resigned. In connection with Mr. Evans's employment agreement, we incurred a severance charge of approximately $0.5 million in the first quarter of fiscal year ended October 31, 2012 ("fiscal 2012"). |
| |
| • | In the fourth quarter of fiscal 2011, a customer of ours gave notice of its intent to seek indemnification against us pursuant to a Manufacturing Service Agreement (“MSA”) for all costs associated with a recall and associated defective products. We accrued $1.7 million, net of expected insurance proceeds, to cover recall costs and replace the returned products. In November, the customer gave further notice of its intent to seek indemnification pursuant to the MSA for all actual and potential third party claims filed against them in connection with the recall, as well as all costs and expenses of the defense and settlement of such claims. To date, three putative class actions related to the recall have been commenced in the U.S. against our customer. At this time, it is not possible to estimate the number of potential claimants or the amount of potential damages in the above actions. To date, we have not been named as a party in any action related to the product recall. |
| |
| • | On October 20, 2011, we announced that Boehringer Ingelheim, a leading globally operating pharmaceutical corporation, awarded us two projects with combined revenue of more than $18.0 million over a three year period. The projects are both fixed-dose combination drugs in development for the treatment of the growing population of type II diabetics. |
| |
| • | On October 5, 2011, Peter T. Bigelow, our then President, North American Operations, resigned effective as of November 1, 2011. In connection with his resignation, Mr. Bigelow agreed to provide transitional consulting services for up to one year. |
| |
| • | On September 8, 2011, our Board reviewed and approved our new corporate strategy which includes, among other |
things, assessing strategic options for the Swindon commercial operation and the Burlington, Ontario facility, accelerating our operational excellence programs for our CMO and PDS segments, and continuing the evolution of our existing commercial sites into centers of excellence focusing on specific technologies or production types. In addition, we are in the process of transferring our Zug, Switzerland European Headquarter operations to our U.K. operations. We expect these initiatives will reduce costs and make our company more efficient over the next several years.
| |
| • | On May 19, 2011, we settled an on-going insurance claim covering all current and future costs associated with water damage at our Swindon, U.K. facility for approximately $16.0 million. We recorded a settlement receivable of approximately $2.4 million against cost of goods sold in the fourth quarter of fiscal 2010, which was subsequently received in the first quarter of fiscal 2011. In the second quarter of fiscal 2011, we recorded an additional $2.6 million as a settlement receivable against cost of goods sold. In the third quarter of fiscal 2011, we received the final payout from the settlement of approximately $13.6 million. A portion of the settlement was used to offset capital expenditures and cover current and future remediation liabilities, with the remaining $4.9 million recorded as other income in the current fiscal year. |
| |
| • | On May 11, 2011, Michael E. Lytton joined our company as Executive Vice President, Corporate Development and Strategy, and General Counsel. |
| |
| • | On February 7, 2011, James C. Mullen was appointed as our Chief Executive Officer (“CEO”) and a member of our Board. |
| |
| • | In December 2010, we amended a manufacturing and supply agreement with a major customer, in which both parties agreed to a contract termination date in February 2011, approximately two and a half years earlier than was originally planned. The amendment reflected the customer's decision not to proceed with a product following receipt of a complete response letter from the FDA. As part of the amendment, the customer agreed to pay us a reservation fee of €21.6 million, and as a result of the shortened contract life, we accelerated the related deferred revenue recognition and were relieved of the obligation to repay certain customer-funded capital related to the original manufacturing and supply agreement. |
| |
| • | On November 30, 2010, Wesley P. Wheeler, our then President and Chief Executive Officer, left our company. We accrued approximately $1.4 million in the first quarter of fiscal 2011 for severance payments due to Mr. Wheeler under his employment agreement. |
Opportunities and Trends
Our target markets include the highly fragmented global market for the manufacture of finished pharmaceutical dosage forms and for PDS. According to PharmSource, a provider of pharmaceutical outsourcing business information, the CMO market totaled $11.7 billion in 2010, and could experience marginal growth of roughly 1% (in conservative scenarios) to as much as 3% - 5% annually during 2011 to 2015. PharmSource also estimates that the outsourced PDS market totaled approximately $1.3 billion in 2010, with growth projections in the 2011 to 2015 period approaching 3% annually. We are one of only a few industry participants that can provide a broad range of CMO and PDS services.
Pharmaceutical outsourcing service providers have faced challenges in recent years due to the uncertain economic environment. In the research and development area, emerging pharmaceutical companies have faced funding uncertainties due to limited access to capital, and many larger companies have decreased or delayed product development spending due to uncertainties surrounding industry consolidation, overall market weakness and the regulatory approval environment. As a result, decision-making related to the awarding of new outsourcing projects has slowed during recent years for similar reasons.
Puerto Rico Operations
We closed our Carolina facility in Puerto Rico effective January 31, 2009. In the second half of fiscal 2010, we performed an impairment analysis based on recent offers, which resulted in the complete write down as the fair value less the cost to sell was nil. We expect the sale of this property to be complete in the first half of fiscal 2012. The results of the Carolina operations have been reported in discontinued operations in fiscal 2011, 2010 and 2009.
In December 2009, we announced our plan to consolidate our Puerto Rico operations into our manufacturing site located in Manati and ultimately close or sell our plant in Caguas. Our current expectations with respect to the Caguas facility assume that we will be able to complete a sale during fiscal 2012 for a purchase price of approximately $7.0 million. In conjunction with this estimate we incurred an impairment charge of $3.6 million in fiscal 2010. The consolidation results in additional accelerated depreciation of Caguas assets of approximately $12.0 million by the end of the project. Because the business in our Caguas facility is being transferred within the existing site network, its results of operations are included in continuing operations in our consolidated financial statements.
As a result of the additional time required to fully transition manufacturing operations from Caguas to Manati due to longer than expected customer regulatory timelines and higher product demand, we now expect the transition to continue beyond the end of calendar year 2012. As a result of the additional time required to complete the transition, we now estimate that the restructuring program will cost $11.5 million, of which $10.8 million has been incurred as of October 31, 2011.
Selected Financial Information
|
| | | | | | | | |
| | Years ended October 31, |
| (in millions of USD, except per share information) | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Revenues | 700.0 |
| | 671.2 |
| | 655.1 |
|
| Adjusted EBITDA | 73.0 |
| | 91.7 |
| | 74.0 |
|
| Net loss attributable to restricted voting shareholders | (16.8 | ) | | (5.0 | ) | | (17.9 | ) |
| Basic and diluted loss per share | (0.13 | ) | | (0.04 | ) | | (0.18 | ) |
| Total assets | 818.6 |
| | 808.9 |
| | 790.8 |
|
| Total long-term liabilities | 357.7 |
| | 346.6 |
| | 311.2 |
|
Reconciliations of Adjusted EBITDA to income (loss) before discontinued operations are included in "Item 6—Selected Financial Data” and “Note 16—Segmented Information” to our consolidated financial statements included in this Form 10-K.
Results of Operations
The results of the Carolina operations have been segregated and reported as discontinued operations in fiscal 2011, 2010 and 2009.
Fiscal 2011 Compared to Fiscal 2010
Consolidated Statements of Loss
|
| | | | | | | | | | | | |
| | Years ended October 31, |
| (in millions of USD, except per share information) | 2011 | | 2010 | | Change | | Change |
| | $ | | $ | | $ | | % |
| Revenues | 700.0 |
| | 671.2 |
| | 28.8 |
| | 4.3 |
| Cost of goods sold | 561.9 |
| | 526.2 |
| | 35.7 |
| | 6.8 |
| Gross profit | 138.1 |
| | 145.0 |
| | (6.9 | ) | | 4.8 |
| Selling, general and administrative expenses | 120.2 |
| | 110.6 |
| | 9.6 |
| | 8.7 |
| Repositioning expenses | 7.0 |
| | 6.8 |
| | 0.2 |
| | 2.9 |
| Operating income | 10.9 |
| | 27.6 |
| | (16.7 | ) | | 60.5 |
| Interest expense, net | 25.4 |
| | 19.5 |
| | 5.9 |
| | 30.3 |
| Impairment charge | — |
| | 3.6 |
| | (3.6 | ) | | 100.0 |
| Foreign exchange gain | (1.6 | ) | | (1.5 | ) | | 0.1 |
| | 6.7 |
| Loss on sale of fixed assets | 0.2 |
| | 0.2 |
| | — |
| | — |
| Refinancing Expenses | — |
| | 12.2 |
| | | | |
| Other (income) expense, net | (4.2 | ) | | (0.1 | ) | | (4.1 | ) | | — |
| Loss from continuing operations before income taxes | (8.9 | ) | | (6.3 | ) | | (2.6 | ) | | 41.3 |
| Current | 1.6 |
| | 6.7 |
| | (5.1 | ) | | 76.1 |
| Future | 5.7 |
| | (9.7 | ) | | (15.4 | ) | | 158.8 |
| Provision for (benefit from) income taxes | 7.3 |
| | (3.0 | ) | | (10.3 | ) | | 343.3 |
| Loss before discontinued operations | (16.2 | ) | | (3.3 | ) | | (12.9 | ) | | 390.9 |
| Loss from discontinued operations | (0.6 | ) | | (1.7 | ) | | (1.1 | ) | | 64.7 |
| Net loss for the period | (16.8 | ) | | (5.0 | ) | | (11.8 | ) | | 236.0 |
| Dividends on convertible preferred shares | — |
| | — |
| | — |
| | — |
| Net loss attributable to restricted voting shareholders | (16.8 | ) | | (5.0 | ) | | (11.8 | ) | | 236.0 |
| Basic and diluted loss per share | | | | | | | |
| From continuing operations | $ | (0.125 | ) | | $ | (0.026 | ) | | | | |
| From discontinued operations | $ | (0.005 | ) | | $ | (0.013 | ) | | | | |
| | $ | (0.130 | ) | | $ | (0.039 | ) | | | | |
| Weighted-average number of shares outstanding during period—basic and diluted (in thousands) | 129,168 |
| | 129,168 |
| | | | |
Operating Income Summary
Revenues for fiscal 2011 increased $28.8 million, or 4.3%, to $700.0 million, from $671.2 million for fiscal 2010. Excluding currency fluctuations, revenues for fiscal 2011 would have been approximately 3.0% higher than the same period of the prior fiscal year. CMO revenues for fiscal 2011 increased $27.3 million, or 5.0%, to $572.6 million, from $545.3 million for fiscal 2010. PDS revenues for fiscal 2011 increased $1.5 million, or 1.2%, to $127.4 million, from $125.9 million for fiscal 2010.
Gross profit for fiscal 2011 decreased $6.9 million, or 4.8%, to $138.1 million, from $145.0 million for fiscal 2010. The decrease in gross profit was due to a decrease in gross profit margin to 19.7% for fiscal 2011 from 21.6% for fiscal 2010, partially offset by higher revenues. The decrease in gross profit margin was due to unfavorable foreign exchange impact on cost of goods sold related to the weakening of the U.S. dollar (-1.1%), higher labor costs (-0.7%), increase in supplies and maintenance (-0.7%), increase in inventory write-offs (-0.5%), and the impact of the prior fiscal years' research and development investment tax credits (-0.6%), partially offset by favorable mix resulting from the reservation fee and higher deferred revenue amortization related to the amended manufacturing and supply agreement in the United Kingdom.
Selling, general and administrative ("SG&A") expenses for fiscal 2011 increased $9.6 million, or 8.7%, to $120.2 million, from $110.6 million for fiscal 2010. The increase was primarily due to higher consulting and professional fees of $12.8 million, higher costs related to the former CEO's severance of $1.1 million and higher stock based compensation of $1.4 million, partially offset by elimination of costs associated with the special committee of independent directors (the “Special Committee”) of $3.0 million for fiscal 2010, and lower depreciation of $3.0 million. The impact of unfavorable foreign exchange rates on SG&A expense was approximately $3.4 million versus prior year.
Repositioning expenses for fiscal 2011 increased $0.2 million, or 2.9%, to $7.0 million, from $6.8 million for fiscal 2010.
The increase was due to increased expenses in connection with the Zug and Swindon facilities, partially offset by lower expenses in connection with the Caguas closure and consolidation in Puerto Rico during fiscal 2011 compared to fiscal 2010, as the prior period included the initial project accruals.
Operating income for fiscal 2011 decreased $16.7 million, or 60.5%, to $10.9 million (1.6% of revenues), from $27.6 million (4.1% of revenues) for fiscal 2010 as a result of the factors discussed above.
Interest Expense
Interest expense for fiscal 2011 increased $5.9 million, or 30.3%, to $25.4 million, from $19.5 million for fiscal 2010. The increase in interest expense primarily reflects the higher interest rates on the Notes versus the rates of our previous debt, as well as overall higher debt levels.
Impairment Charge
During fiscal 2010, we recorded an impairment charge of $3.6 million in connection with the consolidation of our Puerto Rico operations into our manufacturing site located in Manati. This charge wrote down the carrying value of the Caguas facility's long-lived assets to their anticipated fair value upon closure of the facility.
Refinancing Expenses
During fiscal 2010, we incurred expenses of $12.2 million in connection with our refinancing activities, which included fees paid to advisors and other related costs.
Other (Income) Expense, Net
Other income for fiscal 2011 was $4.2 million, compared to $0.1 million for fiscal 2010. The increase of other income was primarily due to the settlement of the insurance claim associated with water damage at our Swindon, U.K. facility, of which $4.9 million was recorded in other income.
Loss from Continuing Operations Before Income Taxes
We reported a loss from continuing operations before income taxes of $8.9 million for fiscal 2011, compared to a loss of $6.3 million for fiscal 2010. The $12.2 million of refinancing expenses during fiscal 2010, along with the other operating items discussed above, were the primary drivers of the year over year variance.
Income Taxes
Income taxes were an expense of $7.3 million for fiscal 2011, compared to a benefit of $3.0 million for fiscal 2010. The increase in tax expense for the period was primarily due to the benefit in fiscal 2010 of releasing $13.8 million of the valuation allowance pertaining to future tax assets in our Canadian operations and mix of earnings in various tax jurisdictions.
Loss before Discontinued Operations and Loss Per Share from Continuing Operations
We recorded a loss before discontinued operations for fiscal 2011 of $16.2 million, compared to a loss before discontinued operations of $3.3 million for fiscal 2010. The loss per share from continuing operations for fiscal 2011 was 12.5¢ compared to a loss per share of 2.6¢ for fiscal 2010.
Loss and Loss Per Share from Discontinued Operations
Discontinued operations for fiscal 2011 and 2010 include the results of the Carolina, Puerto Rico operations. Financial details of the operating activities of the Carolina operations are disclosed in “Note 3—Discontinued Operations and Plant Consolidations.” The loss from discontinued operations for fiscal 2011 was $0.6 million, or 0.5¢ per share, compared to a loss of $1.7 million, or 1.3¢ per share, for fiscal 2010. On-going costs of discontinued operations relate to maintaining the Carolina building for sale.
Net Loss, Loss Attributable to Restricted Voting Shareholders and Loss Per Share
Net loss attributable to restricted voting shares for fiscal 2011 increased $11.8 million, to $16.8 million, or 13.0¢ per share, from $5.0 million, or 3.9¢ per share, for fiscal 2010. Because we reported a loss for fiscal 2011 and 2010, there is no impact of dilution.
Revenues and Adjusted EBITDA by Business Segment
|
| | | | | | | | | | | |
| | Years ended October 31, |
| (in millions of USD) | 2011 | | 2010 | | Change | | Change |
| | $ | | $ | | $ | | % |
| Revenues | | | | | | | |
| Commercial Manufacturing | | | | | | | |
| North America | 274.5 |
| | 251.6 |
| | 22.9 |
| | 9.1 |
|
| Europe | 298.1 |
| | 293.7 |
| | 4.4 |
| | 1.5 |
|
| Total Commercial Manufacturing | 572.6 |
| | 545.3 |
| | 27.3 |
| | 5.0 |
|
| Pharmaceutical Development Services | 127.4 |
| | 125.9 |
| | 1.5 |
| | 1.2 |
|
| Total Revenues | 700.0 |
| | 671.2 |
| | 28.8 |
| | 4.3 |
|
| Adjusted EBITDA | | | | | | | |
| Commercial Manufacturing | | | | | | | |
| North America | 19.1 |
| | 20.6 |
| | (1.5 | ) | | (7.3 | ) |
| Europe | 60.9 |
| | 51.7 |
| | 9.2 |
| | 17.8 |
|
| Total Commercial Manufacturing | 80.0 |
| | 72.3 |
| | 7.7 |
| | 10.7 |
|
| Pharmaceutical Development Services | 29.9 |
| | 46.8 |
| | (16.9 | ) | | (36.1 | ) |
| Corporate Costs | (36.9 | ) | | (27.4 | ) | | (9.5 | ) | | 34.7 |
|
| Total Adjusted EBITDA | 73.0 |
| | 91.7 |
| | (18.7 | ) | | (20.4 | ) |
Commercial Manufacturing
Total CMO revenues for fiscal 2011 increased $27.3 million, or 5.0%, to $572.6 million, from $545.3 million for fiscal 2010. Had local currency exchange rates remained constant to the rates of fiscal 2010, CMO revenues for fiscal 2011 would have been approximately 3.6% higher than the same period of the prior fiscal year.
North American CMO revenues for fiscal 2011 increased $22.9 million, or 9.1%, to $274.5 million, from $251.6 million for fiscal 2010. Had Canadian dollar exchange rates remained constant to the rates of fiscal 2010, North American CMO revenues for fiscal 2011 would have been approximately 8.7% higher than the same period of the prior fiscal year. The increase was primarily due to increased worldwide demand for a customer's product manufactured in our Puerto Rico facility, higher volumes in Toronto and new product launch volumes in Cincinnati.
European CMO revenues for fiscal 2011 increased $4.4 million, or 1.5%, to $298.1 million, from $293.7 million for fiscal 2010. The increase was primarily due to higher revenues in the United Kingdom, from the reservation fee related to the amended manufacturing and supply agreement and accelerated deferred revenue versus take-or-pay revenue in the prior year, and higher volumes in Ferentino, partially offset by lower revenues across other sites. Had European currencies remained constant to the rates of fiscal 2010, European CMO revenues for fiscal 2011 would have been approximately 0.6% lower than the same period of the prior fiscal year.
Total CMO Adjusted EBITDA for fiscal 2011 increased $7.7 million, or 10.7%, to $80.0 million, from $72.3 million for fiscal 2010. This represents an Adjusted EBITDA margin of 14.0% for fiscal 2011 compared to 13.3% for fiscal 2010. Had local currencies remained constant to prior year rates, and after eliminating the impact of all foreign exchange gains and losses, CMO Adjusted EBITDA for fiscal 2011 would have been approximately $2.3 million higher.
North American Adjusted EBITDA for fiscal 2011 decreased $1.5 million, or 7.3%, to $19.1 million, from $20.6 million for fiscal 2010. The decrease was primarily driven by $5.0 million in consulting fees related to recent strategic initiatives, prior year recognition of accelerated deferred revenue of $4.2 million in Cincinnati and foreign exchange losses of $2.4 million as a result of the weakening of the U.S. dollar against the Canadian dollar. These were partially offset by a $6.7 million Adjusted EBITDA improvement in Puerto Rico, and better operating results in our Canadian operations. North American CMO had $4.0 million in repositioning costs relating to the Puerto Rican operations in fiscal 2011 that was not included in Adjusted EBITDA.
European Adjusted EBITDA for fiscal 2011 increased $9.2 million, or 17.8%, to $60.9 million, from $51.7 million million for fiscal 2010. This increase was primarily due to the recognition of the reservation fee related to the amended manufacturing and supply agreement in the United Kingdom and associated deferred revenue amortization, partially offset by lower operating results across other European sites. European CMO has $4.9 million in insurance proceeds relating to the U.K. operations that was recorded in other income and $2.9 million in repositioning costs for the Zug and U.K. operations, both of which were not included in Adjusted EBITDA.
Pharmaceutical Development Services
Total PDS revenues for fiscal 2011 increased by $1.5 million, or 1.2%, to $127.4 million, from $125.9 million for fiscal 2010. Had the local currency rates remained constant to fiscal 2010, PDS revenues for fiscal 2011 would have increased approximately 0.2% from fiscal 2010.
Total PDS Adjusted EBITDA for fiscal 2011 decreased by $16.9 million, or 36.1%, to $29.9 million, from $46.8 million for fiscal 2010. Had local currencies remained constant to the rates of the prior year and after eliminating the impact of all foreign exchange gains and losses, PDS Adjusted EBITDA for fiscal 2011 would have been approximately $2.2 million higher than reported. PDS Adjusted EBITDA for fiscal 2011 includes $5.8 million of research and development investment tax credits compared to $10.8 million in fiscal 2010. In addition, lower than expected sales at certain sites resulting from project cancellations related to customer regulatory approvals, clinical trial outcome issues, and industry consolidation contributed to the reduction in Adjusted EBITDA.
Corporate Costs
Corporate costs for fiscal 2011 increased $9.5 million, or 34.7%, to $36.9 million, from $27.4 million for fiscal 2010. The increase was primarily due to unfavorable foreign exchange of $3.4 million, $4.4 million of higher advisor fees due to registration with the SEC and corporate strategy initiatives, expenses related to the change in our CEO of $3.3 million and higher compensation expenses. These were partially offset by the non-recurrence of $3.0 million in Special Committee costs incurred in fiscal 2010.
Fiscal 2010 Compared to Fiscal 2009
Consolidated Statements of Loss
|
| | | | | | | | | | | | | |
| | Years ended October 31 |
| (in millions of USD, except per share information) | 2010 | | 2009 | | $ | | % |
| | $ | | $ | | Change | | Change |
| Revenues | 671.2 |
| | 655.1 |
| | 16.1 |
| | 2.5 |
|
| Cost of goods sold | 526.2 |
| | 511.2 |
| | 15.0 |
| | 2.9 |
|
| Gross profit | 145.0 |
| | 143.9 |
| | 1.1 |
| | 0.8 |
|
| Selling, general and administrative expenses | 110.6 |
| | 105.5 |
| | 5.1 |
| | 4.8 |
|
| Repositioning expenses | 6.8 |
| | 2.1 |
| | 4.7 |
| | 223.8 |
|
| Operating income | 27.6 |
| | 36.3 |
| | (8.7 | ) | | (24.0 | ) |
| Interest expense, net | 19.5 |
| | 15.4 |
| | 4.1 |
| | 26.6 |
|
| Impairment charge | 3.6 |
| | — |
| | 3.6 |
| | — |
|
| Foreign exchange (gain) loss | (1.5 | ) | | 7.0 |
| | (8.5 | ) | | (121.4 | ) |
| Loss on sale of fixed assets | 0.2 |
| | — |
| | 0.2 |
| | — |
|
| Refinancing Expenses | 12.2 |
| | — |
| | 12.2 |
| | — |
|
| Other (income) expense, net | (0.1 | ) | | 0.4 |
| | (0.5 | ) | | (125.0 | ) |
| (Loss) income from continuing operations before income taxes | (6.3 | ) | | 13.5 |
| | (19.8 | ) | | (146.7 | ) |
| Current | 6.7 |
| | 7.7 |
| | (1.0 | ) | | (13.0 | ) |
| Future | (9.7 | ) | | 4.8 |
| | (14.5 | ) | | (302.1 | ) |
| (Benefit from) provision for income taxes | (3.0 | ) | | 12.5 |
| | (15.5 | ) | | (124.0 | ) |
| (Loss) income before discontinued operations | (3.3 | ) | | 1.0 |
| | (4.3 | ) | | (430.0 | ) |
| Loss from discontinued operations | (1.7 | ) | | (7.8 | ) | | (6.1 | ) | | (78.2 | ) |
| Net loss for the period | (5.0 | ) | | (6.8 | ) | | (1.8 | ) | | (26.5 | ) |
| Dividends on convertible preferred shares | — |
| | 11.1 |
| | (11.1 | ) | | (100.0 | ) |
| Net loss attributable to restricted voting shareholders | (5.0 | ) | | (17.9 | ) | | (12.9 | ) | | (72.1 | ) |
| Basic and diluted loss per share | | | | | | | |
| From continuing operations | $ | (0.026 | ) | | $ | (0.100 | ) | | | | |
| From discontinued operations | $ | (0.013 | ) | | $ | (0.077 | ) | | | | |
| | $ | (0.039 | ) | | $ | (0.177 | ) | | | | |
| Weighted-average number of shares outstanding during period—basic and diluted (in thousands) | 129,168 |
| | 100,964 |
| | | | |
Operating Income Summary
Revenues for fiscal 2010 increased $16.1 million, or 2.5%, to $671.2 million, from $655.1 million for fiscal 2009. Excluding currency fluctuations, revenues for fiscal 2010 would have increased by approximately 2.3%. CMO revenues for fiscal 2010 increased $15.3 million, or 2.9%, to $545.3 million, from $530.0 million for fiscal 2009. PDS revenues for fiscal 2010 also increased $0.8 million, or 0.6%, to $125.9 million, from $125.1 million for fiscal 2009.
Gross profit for fiscal 2010 increased $1.1 million, or 0.8%, to $145.0 million, from $143.9 million for fiscal 2009. The increase in gross profit was due to higher volume, partially offset by a decrease in gross profit margin to 21.6% for fiscal 2010 from 22.0% for fiscal 2009. The decrease in gross profit margin was due to unfavorable foreign exchange impact on cost of goods sold (-1.7%), higher depreciation (-1.3%) and higher lease expense (-0.3%), partially offset by the favorable impact of the prior fiscal years' Canadian research and development investment tax credits (+1.1%) and higher volume.
Selling, general and administrative expenses for fiscal 2010 increased $5.1 million, or 4.8%, to $110.6 million, from $105.5 million for fiscal 2009. The increase was primarily due to higher compensation expenses and stock option amortization ($6.9 million), unfavorable foreign exchange ($1.9 million) and higher depreciation ($2.6 million), partially offset by costs associated with the Special Committee of $3.0 million for fiscal 2010 compared to $8.0 million for fiscal 2009. Fiscal 2009 also included $2.0 million of transitional expenses for the opening of our new U.S. headquarters.
Repositioning expenses for fiscal 2010 increased $4.7 million, or 223.8%, to $6.8 million, from $2.1 million for fiscal 2009. The increase was due to higher expenses in connection with the Caguas closure and consolidation in Puerto Rico in fiscal 2010 compared to expenses in connection with the ongoing shut down and transition of business out of our York Mills facility and manufacturing restructuring in Puerto Rico in fiscal 2009.
Operating income for fiscal 2010 decreased $8.7 million, or 24.0%, to $27.6 million (4.1% of revenues), from $36.3 million (5.5% of revenues) for fiscal 2009 as a result of the factors discussed above.
Interest Expense
Interest expense for fiscal 2010 increased $4.1 million, or 26.6%, to $19.5 million, from $15.4 million for fiscal 2009. The increase in interest expense primarily reflects the higher interest rates on the Notes versus the rates of our previous debt, as well as overall higher debt levels.
Impairment Charge
During fiscal 2010, we recorded an impairment charge of $3.6 million in connection with the consolidation of our Puerto Rico operations into our manufacturing site located in Manatí. This charge wrote down the carrying value of the Caguas facility’s long-lived assets to their anticipated fair value upon closure of the facility.
Foreign Exchange (Gains) Losses
Foreign exchange gain for fiscal 2010 was $1.5 million, compared to a loss of $7.0 million for fiscal 2009. The foreign exchange gain was primarily due to the overall strengthening of the Canadian dollar against the U.S. dollar during fiscal 2009 and favorable hedging contracts in the Canadian operations during fiscal 2010, which resulted in gains of $4.0 million for fiscal 2010 compared to losses of $9.2 million for fiscal 2009.
Refinancing Expenses
During fiscal 2010, we incurred expenses of $12.2 million in connection with our refinancing activities. These expenses include fees paid to advisors and other related costs.
(Loss) Income from Continuing Operations Before Income Taxes
We reported a loss from continuing operations before income taxes of $6.3 million for fiscal 2010, compared to income of $13.5 million for fiscal 2009. The $12.2 million of refinancing expenses during the second and third quarters of fiscal 2010, along with the other operating items discussed above, were the primary drivers of the year over year variance.
Income Taxes
Income taxes were a benefit of $3.0 million for fiscal 2010, compared to an income tax expense of $12.5 million for fiscal 2009. The benefit was primarily due to releasing the valuation allowance pertaining to future tax assets and recognition of the current net operating loss benefits in our Canadian operations. We have determined that this valuation allowance is no longer required based on our assessment of the future prospects of our Canadian operations.
(Loss) Income before Discontinued Operations and (Loss) Income Per Share from Continuing Operations
We recorded a loss before discontinued operations for fiscal 2010 of $3.3 million, compared to income before discontinued operations of $1.0 million for fiscal 2009. The loss per share before discontinued operations for fiscal 2010 was 2.6¢ compared to a loss per share of 10.0¢ for fiscal 2009, after taking into account the dividends of $11.1 million on the Class I Preferred Shares, Series C (the "Series C Preferred Shares") for fiscal 2009.
Loss and Loss Per Share from Discontinued Operations
Discontinued operations for fiscal 2010 and 2009 include the results of the Carolina, Puerto Rico operations. Financial details of the operating activities are disclosed in “Note 3—Discontinued Operations and Plant Consolidations” of our consolidated financial statements included in this Form 10-K. The loss from discontinued operations for fiscal 2010 was $1.7 million, or 1.3¢ per share, compared to a loss of $7.8 million, or 7.7¢ per share, for fiscal 2009. On-going costs of discontinued operations relate to maintaining the Carolina building for sale.
Net Loss, Loss Attributable to Restricted Voting Shareholders and Loss Per Share
Net loss attributable to restricted voting shares for fiscal 2010 decreased $12.9 million, or 72.1%, to $5.0 million, or 3.9¢ per share, from $17.9 million, or 17.7¢ per share, for fiscal 2009. Fiscal 2009 results include dividends on the Series C Preferred Shares of $11.1 million. Dividends were recorded until July 28, 2009, the date when the preferred shares were converted to restricted voting shares by JLL Patheon Holdings. Because we reported a loss for fiscal 2010 and 2009, there is no impact of dilution.
Revenues and Adjusted EBITDA by Business Segment
|
| | | | | | | | | | | |
| | Years ended October 31, |
| (in millions of USD) | 2010 | | 2009 | | Change | | Change |
| | $ | | $ | | $ | | % |
| Revenues | | | | | | | |
| Commercial Manufacturing | | | | | | | |
| North America | 251.6 |
| | 249.0 |
| | 2.6 |
| | 1.0 |
|
| Europe | 293.7 |
| | 281.0 |
| | 12.7 |
| | 4.5 |
|
| Total Commercial Manufacturing | 545.3 |
| | 530.0 |
| | 15.3 |
| | 2.9 |
|
| Pharmaceutical Development Services | 125.9 |
| | 125.1 |
| | 0.8 |
| | 0.6 |
|
| Total Revenues | 671.2 |
| | 655.1 |
| | 16.1 |
| | 2.5 |
|
| Adjusted EBITDA | | | | | | | |
| Commercial Manufacturing | | | | | | | |
| North America | 20.6 |
| | 19.2 |
| | 1.4 |
| | 7.3 |
|
| Europe | 51.7 |
| | 52.0 |
| | (0.3 | ) | | -0.6 |
|
| Total Commercial Manufacturing | 72.3 |
| | 71.2 |
| | 1.1 |
| | 1.5 |
|
| Pharmaceutical Development Services | 46.8 |
| | 32.7 |
| | 14.1 |
| | 43.1 |
|
| Corporate Costs | (27.4 | ) | | (29.9 | ) | | 2.5 |
| | -8.4 |
|
| Total Adjusted EBITDA | 91.7 |
| | 74.0 |
| | 17.7 |
| | 23.9 |
|
Commercial Manufacturing
Total CMO revenues for fiscal 2010 increased $15.3 million, or 2.9%, to $545.3 million, from $530.0 million for fiscal 2009. Changes in foreign exchange rates between fiscal 2009 and 2010 did not have a material impact on fiscal 2010 CMO revenues as compared to fiscal 2009 CMO revenues.
North American CMO revenues for fiscal 2010 increased $2.6 million, or 1.0%, to $251.6 million from $249.0 million for fiscal 2009. Had the Canadian dollar remained constant to the rates of fiscal 2009, North American CMO revenues for fiscal 2010 would have been approximately 0.2% higher than for fiscal 2009. The increase was primarily due to a favorable foreign exchange impact from Canada, higher revenues in Cincinnati as a result of accelerated deferred revenue recognition (+$7.2 million) and stronger performance in Puerto Rico (+$2.0 million), partially offset by lower revenues from the Canadian operations (-$9.0 million).
European CMO revenues for fiscal 2010 increased $12.7 million, or 4.5%, to $293.7 million, from $281.0 million for fiscal 2009. Had European currencies remained constant to the rates of fiscal 2009, European CMO revenues for fiscal 2010 would have been approximately 5.3% higher than for fiscal 2009. The increase was primarily due to higher revenues in the United Kingdom from increased take-or-pay and accelerated deferred revenue recognition, partially offset by the weakening of the Euro against the U.S. dollar.
Total CMO Adjusted EBITDA for fiscal 2010 increased $1.1 million, or 1.5%, to $72.3 million, from $71.2 million for fiscal 2009. This represents an Adjusted EBITDA margin (Adjusted EBITDA as a percentage of revenues) of 13.3% for fiscal 2010 compared to 13.4% for fiscal 2009. Had local currencies remained constant to fiscal 2009 rates, and after eliminating the impact of all foreign exchange gains and losses, CMO Adjusted EBITDA would have been approximately $3.5 million higher for fiscal 2010.
North American Adjusted EBITDA for fiscal 2010 increased $1.4 million, or 7.3%, to $20.6 million, from $19.2 million for fiscal 2009. The increase was primarily driven by higher revenue and favorable foreign exchange rates, inclusive of hedging (+$1.5 million), partially offset by unfavorable production mix and higher compensation costs. Included in the North American Adjusted EBITDA is a loss in the Puerto Rico operations of $11.9 million, up slightly from fiscal 2009. The continued weak Puerto Rico performance was due to the cost of operating two facilities, inventory provisions, high energy prices and various performance issues during the first half of fiscal 2010. We expect losses in Puerto Rico to be significantly reduced during fiscal 2011 due to improved operating performance and product mix, and the initial financial benefits of actions to integrate the Caguas site into Manatí during fiscal 2011. North American CMO had $6.8 million in repositioning expenses and $3.4 million in impairment charges relating to the Puerto Rican operations in fiscal 2010 that were not included in Adjusted EBITDA.
European Adjusted EBITDA for fiscal 2010 decreased $0.3 million, or 0.6%, to $51.7 million, from $52.0 million for
fiscal 2009. Higher revenues for fiscal 2010 were more than offset by unfavorable transactional foreign exchange ($4.9 million), higher employee benefit related costs ($2.0 million), higher lease expense ($0.8 million) and higher supplies and maintenance ($0.8 million).
Pharmaceutical Development Services
Total PDS revenues for fiscal 2010 increased by $0.8 million, or 0.6%, to $125.9 million, from $125.1 million for fiscal 2009. Had the local currency rates remained constant to fiscal 2009, PDS revenues for fiscal 2010 would have decreased approximately 0.2% from fiscal 2009.
Total PDS Adjusted EBITDA for fiscal 2010 increased by $14.1 million, or 43.1%, to $46.8 million, from $32.7 million for fiscal 2009. Had local currencies remained constant to the rates of fiscal 2009 and after eliminating the impact of all foreign exchange gains and losses, PDS Adjusted EBITDA for fiscal 2010 would have been approximately $2.6 million lower. PDS Adjusted EBITDA for fiscal 2010 includes $7.2 million in prior years Canadian research and development investment tax credits that were recognized this year and benefits from a tightly controlled cost structure.
Corporate Costs
Corporate costs for fiscal 2010 decreased $2.5 million, or 8.4%, to $27.4 million, from $29.9 million for fiscal 2009. This decrease was primarily due to lower Special Committee costs and the non recurrence of $2.0 million of transitional expenses for the opening our U.S. headquarters in Research Triangle Park, North Carolina, partially offset by higher compensation expenses and stock option amortization. Fiscal 2010 included $3.0 million associated with the Special Committee costs compared to $8.0 million of Special Committee costs for fiscal 2009.
Liquidity and Capital Resources
Overview
Our cash and cash equivalents totaled $33.4 at October 31, 2011 and $53.5 million at October 31, 2010. Our total debt was $275.7 million at October 31, 2011 and $278.3 million at October 31, 2010.
Our primary source of liquidity is cash flow from operations. Historically, we have also used availability under the ABL and other credit lines for any additional cash needs. Our principal uses of cash have been for capital expenditures, debt servicing requirements, working capital, employee benefit obligations, and in fiscal 2012, expenditures for consultants to assist in implementing our strategic initiatives.
From time to time, we evaluate strategic opportunities, including potential acquisitions, divestitures or investments in complementary businesses, and we anticipate continuing to make such evaluations. We may also access capital markets through the issuance of debt or equity in connection with the acquisition of complementary businesses or other significant assets or for other strategic opportunities.
Summary of Cash Flows
The following table summarizes our cash flows for the periods indicated:
|
| | | | | | | | |
| | Years ended October 31, |
| (in millions of USD) | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Cash provided by operating activities of continuing operations | 23.9 |
| | 50.7 |
| | 48.0 |
|
| Cash used in operating activities of discontinued operations | (1.0 | ) | | (0.7 | ) | | (8.9 | ) |
| Cash provided by operating activities | 22.9 |
| | 50.0 |
| | 39.1 |
|
| Cash used in investing activities of continuing operations | (47.4 | ) | | (50.0 | ) | | (49.5 | ) |
| Cash provided by investing activities of discontinued operations | — |
| | — |
| | 0.2 |
|
| Cash provided by financing activities | 2.7 |
| | 31.6 |
| | 13.6 |
|
| Other | 1.7 |
| | (0.4 | ) | | (1.3 | ) |
| Net (decrease) increase in cash and cash equivalents during the period | (20.1 | ) | | 31.2 |
| | 2.1 |
|
Cash Provided by Operating Activities
Cash provided by operating activities from continuing operations for fiscal 2011 decreased $26.8 million, or 52.9%, to $23.9 million, from $50.7 million for fiscal 2010. Prior year cash contributions included take or pay receipts in our Swindon operations of $53.1 million versus $29.3 million received from the reservation fee related to the amended manufacturing and supply agreement in the United Kingdom in fiscal 2011. Fiscal 2011 also included previously disclosed voluntary pension contributions in the U.K. of $4.9 million, and lower cash from operations, partially offset by $14.0 million from the insurance claim settlement.
Cash provided by operating activities from continuing operations for fiscal 2010 increased $2.7 million, or 5.6%, to $50.7 million, from $48.0 million for fiscal 2009. Year over year change in cash provided by operating activities from continuing operations was primarily due to higher deferred revenue (mainly an early payment for a take or pay amount) and better working capital usage, which was partially offset by refinancing costs, higher interest payments and lower cash flows from the commercial operations excluding the deferred revenue amounts.
Cash provided by operating activities from continuing operations for fiscal 2009 was primarily due to higher customer funded capital that increased deferred revenue and the use of deferred tax assets during fiscal 2009 which resulted in lower tax payments, partially offset by working capital usage.
Cash used in operating activities from discontinued operations for fiscal 2011 increased $0.3 million, or 42.9%, to $1.0 million, from $0.7 million for fiscal 2010.
Cash used in operating activities from discontinued operations for fiscal 2010 decreased $8.2 million, or 92.1%, to $0.7 million, from $8.9 million for fiscal 2009. The decrease in cash outflow for fiscal 2010 was due to our Carolina facility closing down operations for fiscal 2009 and fiscal 2010 expenses representing primarily utility costs, insurance and maintenance for the building while it is in the process of being sold.
Cash used in operating activities from discontinued operations for fiscal 2009 was due to severance payments and closing costs during the period.
Cash Used in Investing Activities
The following table summarizes the cash used in investing activities for the periods indicated:
|
| | | | | | | | |
| | Years ended October 31, |
| (in millions of USD) | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Total additions to capital assets | (47.8 | ) | | (48.7 | ) | | (49.1 | ) |
| Proceeds on sale of capital assets | 0.4 |
| | — |
| | 0.1 |
|
| Net increase in investments | — |
| | (1.1 | ) | | (0.3 | ) |
| Investment in intangibles | — |
| | (0.2 | ) | | (0.2 | ) |
| Cash used in investing activities of continuing operations | (47.4 | ) | | (50.0 | ) | | (49.5 | ) |
| Cash provided by investing activities of discontinued operations | — |
| | — |
| | 0.2 |
|
| Cash used in investing activities | (47.4 | ) | | (50.0 | ) | | (49.3 | ) |
Cash used in investing activities from continuing operations for fiscal 2011 decreased $2.6 million, or 5.2%, to $47.4 million, from $50.0 million for fiscal 2010. The decrease was primarily due to lower capital expenditures in fiscal 2011 and the non-recurrence of cash contributions in two Italian companies (BSP Pharmaceuticals) in fiscal 2010.
Cash used in investing activities from continuing operations for fiscal 2010 increased $0.5 million, or 1.0%, to $50.0 million, from $49.5 million for fiscal 2009.
Our principal ongoing investment activities are capital programs at our sites. The majority of our capital allocation is normally invested in projects that will support growth in capacity and revenues.
During fiscal 2011, our major capital projects (in millions of U.S. dollars) were:
|
| | | |
| • Facility infrastructure at Cincinnati to support introduction of new customer product | $ | 10.5 |
|
| • Consolidation of Caguas facility in Puerto Rico | $ | 2.3 |
|
| • Addition of PDS capabilities at the Bourgoin site | $ | 2.0 |
|
| • High potency packaging in Toronto | $ | 1.4 |
|
| • Equipment for customer product in Bourgoin | $ | 2.0 |
|
During fiscal 2010, our major capital projects (in millions of U.S. dollars) were:
|
| | | |
| • Facility infrastructure at Cincinnati to support introduction of new customer product | $ | 9.8 |
|
| • Consolidation of Caguas facility in Puerto Rico | $ | 4.9 |
|
| • Addition of PDS capabilities at the Bourgoin site | $ | 4.5 |
|
During fiscal 2009, our major project-related programs (in millions of U.S. dollars) were:
|
| | | |
| • New ERP system in Canadian operations | $ | 3.1 |
|
| • Additional pilot scale lyophilization capacity in Ferentino, Italy | $ | 5.7 |
|
| • Completion of capacity expansions in Mississauga, Canada | $ | 2.2 |
|
| • Completion of capacity expansions in Whitby, Canada | $ | 1.4 |
|
| • Completion of customer funded projects in Cincinnati, U.S.A. | $ | 5.3 |
|
Capital commitments to complete authorized capital projects were $5.9 million at the end of fiscal 2011. Based on current internal projections, we expect to make (or have made) these expenditures during fiscal 2012, and we expect to finance (or have financed) them with cash flows from operations, existing cash reserves, the ABL and customer funding.
Based on current management assessments, total capital expenditures (including expenditures to complete projects authorized at the end of fiscal 2011) for fiscal 2012 are expected to be near the amount of total capital expenditures for fiscal 2011, which was approximately $47.8 million. We expect to finance (or have financed) our capital expenditures with cash flows from operations, existing cash reserves, the ABL and customer funding. The major capital projects for fiscal 2012 consist of:
| |
| • | Expansion in Manati, Puerto Rico to be funded by the customer for increased capacity |
| |
| • | Expansion of the new PDS unit at the Bourgoin, France site |
| |
| • | Upgrade manufacturing equipment at Toronto site |
| |
| • | PDS Software enhancements |
Our principal ongoing investment activities are sustaining and project-related capital programs at our network of sites. The majority of our capital allocation is normally invested in project-related programs, which are defined as outlays that will generate growth in capacity and revenues, while sustaining expenditures relate to the preservation of existing assets and capacity.
Cash Provided by Financing Activities
The following table summarizes the cash provided by financing activities for the periods indicated:
|
| | | | | | | | |
| | Years ended October 31, |
| (in millions of USD) | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Increase (decrease) in short-term borrowings | 4.1 |
| | (10.7 | ) | | 3.0 |
|
| Increase in long-term debt | 9.0 |
| | 296.2 |
| | 50.5 |
|
| Increase in deferred financing costs | — |
| | (7.3 | ) | | — |
|
| Repayment of long-term debt | (10.4 | ) | | (246.6 | ) | | (39.9 | ) |
| Cash provided by financing activities of continuing operations | 2.7 |
| | 31.6 |
| | 13.6 |
|
| Cash provided by financing activities | 2.7 |
| | 31.6 |
| | 13.6 |
|
Cash provided by financing activities for fiscal 2011 decreased $28.9 million, or 91.5%, to $2.7 million, from $31.6 million for fiscal 2010, primarily due to the refinancing in the second quarter of fiscal 2010.
Cash provided by financing activities for fiscal 2010 increased $18.0 million, or 132.4%, to $31.6 million, from $13.6 million for fiscal 2009.
In April 2010, we issued the Notes for an aggregate principal amount of $280.0 million in a private placement. We used the net proceeds of the offering to repay all of the outstanding indebtedness under our then-existing senior secured term loan and our $75.0 million ABL, to repay certain other indebtedness and to pay related fees and expenses. We used the remaining proceeds for general corporate purposes.
We also amended and restated our existing $75.0 million ABL in connection with the offering to, among other things, extend the maturity date of this facility to 2014.
During fiscal 2010, the cash inflows were primarily due to the refinancing in the second quarter. For fiscal 2009, the cash inflows reflected net drawings on the then-existing credit facilities primarily to fund operations and capital expenditures.
Financing Arrangements
Historical Credit Arrangements
On April 27, 2007, we entered into credit facilities in the aggregate amount of $225.0 million, which were comprised of a seven year, $150.0 million senior secured term loan and the five-year, $75.0 million ABL. We were required to make quarterly installment payments of $0.4 million on the term loan, along with additional mandatory repayments based on certain excess cash flow measures. The interest rate applicable to each alternative base rate borrowing under the term loan was equal to 1.5% plus the greater of the prime rate and the federal funds effective rate plus 0.5%. The interest rate applicable to each Eurocurrency borrowing was equal to an adjusted LIBOR plus 2.5%. The interest rate applicable to the ABL was a floating rate determined by the currency of the loan, plus an applicable margin determined by the leverage ratio. The credit facilities were secured by substantially all of the assets of our operations in Canada, the United States, Puerto Rico and the United Kingdom and our investments in the shares of all other operating subsidiaries. The term loan and any borrowings under our then-existing ABL were paid off as part of the refinancing discussed below.
$280.0 Million Senior Secured Notes and $75.0 Million Amended ABL
In April 2010, we issued the Notes for an aggregate principal amount of $280.0 million. We used the net proceeds of the offering to repay all of the outstanding indebtedness under our then-existing senior secured term loan and the $75.0 million ABL, to repay certain other indebtedness and to pay related fees and expenses. We used the remaining proceeds for general corporate purposes.
We also amended and restated our then-existing $75.0 million ABL in connection with the Notes offering to, among other things, extend the maturity date of this facility to April 23, 2014.
The Notes and the ABL are secured by substantially all of our assets and are guaranteed by, and secured by substantially all of the assets of, our subsidiaries in the United States (including Puerto Rico), Canada, the United Kingdom (except Patheon UK Pension Trustees Limited) and the Netherlands. The Notes and the ABL are guaranteed on a limited basis by, and secured by certain assets of, our subsidiaries in France, Italy and Switzerland.
The agreements covering the Notes and ABL contain usual and customary covenants and events of default provisions.
The agreements that govern the terms of our debt, including the indenture that governs the Notes and the credit
agreement that governs the ABL, contain covenants that restrict our ability and the ability of our subsidiaries to, among other things:
| |
| • | incur additional indebtedness; |
| |
| • | issue additional equity; |
| |
| • | pay dividends on or make distributions in respect of capital stock or make certain other restricted payments or investments; |
| |
| • | enter into agreements that restrict distributions from subsidiaries or restrict our ability to incur liens on certain of our assets; |
| |
| • | make capital expenditures; |
| |
| • | sell or otherwise dispose of assets, including capital stock of subsidiaries; |
| |
| • | enter into transactions with affiliates; |
| |
| • | create or incur liens; and |
Provided that we are not in default under the ABL or the indenture governing the Notes and are able to demonstrate a certain Fixed Charge Coverage Ratio (as defined in the indenture governing the Notes), and will have a required minimum amount of remaining borrowing availability under the ABL after giving effect thereto, we are permitted to pay limited amounts of dividends or other distributions with respect to our restricted voting shares (as more particularly described in the ABL and the indenture governing the Notes, up to the lesser of (i) $15.0 million plus 50.0% of Excess Cash Flow (as defined in the ABL), plus net proceeds of additional permitted equity offerings under the ABL, and (ii) 50.0% of Consolidated Net Income (as defined in the indenture governing the Notes) plus net proceeds from additional permitted equity offerings or sales of restricted investments under the Notes.
In addition, under the ABL, if an event of default occurs or our borrowing availability falls below the greater of $10.0 million or 13.3% of total commitments under the ABL for any two consecutive days (which is defined under the ABL as an "Availability Trigger Event"), we will be required to satisfy and maintain a ratio of Consolidated EBITDA to Consolidated Fixed Charges (each as defined in the ABL) of not less than 1.10 to 1.00 until the first day thereafter on which, as applicable, either the event of default has been cured or our borrowing availability has exceeded the greater of $10.0 million or 13.3% of our total commitments for 30 consecutive days. Our ability to meet the required ratio can be affected by events beyond our control, and we may not be able to meet this ratio. A breach of any of these covenants could result in a default under the ABL.
Convertible Preferred Shares
The $150 million 8.5% preferred shares purchased by JLL Patheon Holdings on April 27, 2007 included 150,000 units, each consisting of one Series C Preferred Share (a convertible preferred share) and one Series D Preferred Share (a special voting preferred share). On July 29, 2009, JLL Patheon Holdings converted its 150,000 Series C Preferred Shares into a total of 38,018,538 of our restricted voting shares, in accordance with the convertible preferred share terms. As a result of the JLL Patheon Holdings conversion, we no longer have any Series C Preferred Shares outstanding. We recorded $11.1 million of dividends in fiscal 2009 related to the Series C Preferred Shares. Please refer to “Note 11—Shareholders’ Equity” of our consolidated financial statements included in this Form 10-K for more information.
Financing Ratios
Total interest-bearing debt at October 31, 2011 was $275.7 million and our consolidated ratio of interest-bearing debt to shareholders’ equity was 105%.
Total interest-bearing debt at the end of fiscal 2010 was $278.3 million and our consolidated ratio of interest-bearing debt to shareholders’ equity was 102%.
The following table summarizes the fixed and variable percentages of debt outstanding at the end of fiscal 2011 and 2010, after taking into account the impact of interest rate swap contracts that we had entered into, and the applicable interest rates at each quarter in fiscal 2011.
|
| | | | | | | | | | | | | |
| | % of Debt Outstanding | Interest Rates at End of Each Quarter in 2011 |
| | 10/31/2011 | | 10/31/2010 | | Q4 11 | | Q3 11 | | Q2 11 | | Q1 11 |
| | % | | % | | % | | % | | % | | % |
| Fixed rate | 99 |
| | 100 |
| | | | | | | | |
| Variable rate based on: | | | | | | | | | | | |
| Euribor (3 months) | 1.0 |
| | 0.0 |
| | 1.59 | | 1.61 | | 1.24 | | 1.07 |
Effects of Inflation
We do not believe that inflation has had a significant impact on our revenues or results of operations since inception. We expect our operating expenses will change in the future in line with periodic inflationary changes in price levels. Because we intend to retain and continue to use our property and equipment, we believe that the incremental inflation related to the replacement costs of such items will not materially affect our operations. However, the rate of inflation affects our expenses, such as those for employee compensation, which could increase our level of expenses and the rate at which we use our resources. While our management generally believes that we will be able to offset the effect of price-level changes by adjusting our service prices and implementing operating efficiencies, any material unfavorable changes in price levels could have a material adverse effect on our financial condition, results of operations and cash flows.
Off-Balance Sheet Arrangements
We do not use off-balance sheet entities to structure any of our financial arrangements. We do not have any interests in unconsolidated special-purpose or structured finance entities.
Tabular Disclosure of Contractual Obligations
Contractual repayments of long-term debt, commitments under operating leases, commitments under capital leases and purchase obligations are as follows:
|
| | | | | | | | | | | | | | |
| | Payments Due by Period |
| (in millions of USD) | Total | | Year 1 | | 2-3 Years | | 4-5 Years | | After 5 Years |
| | $ | | $ | | $ | | $ | | $ |
| Long-term debt | 281.1 |
| | 1.1 |
| | — |
| | — |
| | 280.0 |
|
| Interest on long-term debt | 133.1 |
| | 24.2 |
| | 48.4 |
| | 48.4 |
| | 12.1 |
|
| Operating leases | 22.7 |
| | 7.2 |
| | 9.5 |
| | 3.8 |
| | 2.2 |
|
| Capital leases | 0.1 |
| | 0.1 |
| | — |
| | — |
| | — |
|
| Purchase obligations(1) | 5.9 |
| | 5.9 |
| | — |
| | — |
| | — |
|
| Total contractual obligations(2) | 442.9 |
| | 38.5 |
| | 57.9 |
| | 52.2 |
| | 294.3 |
|
_________________________
| |
| (1) | Purchase obligations relate to capital commitments to complete authorized capital projects |
| |
| (2) | Not included in the table are other long-term liabilities of unfunded termination indemnities in the amount of $6.2 million, employee future benefits in the amount of $2.9 million and other long-term liabilities in the amount of $12.6 million. These other long-term liabilities either have no fixed payment dates or are not settled in cash. See “Note 9—Other Long-Term Liabilities” and “Note 10—Employee Future Benefits” to our consolidated financial statements included in this Form 10-K. |
Recent Accounting Pronouncements
We will convert to and report under U.S. GAAP beginning with fiscal 2012, and therefore will not be adopting any new Canadian GAAP pronouncements. See “Note 21— Additional Disclosures Required under U.S. Generally Accepted Accounting Principles" to our consolidated financial statements included in this Form 10-K for a description of recent U.S. GAAP accounting pronouncements, including the expected dates of adoption and estimated effects, if any, on our consolidated financial statements.
Critical Accounting Estimates
The preparation of our consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. These estimates and assumptions are based upon
management’s historical experience and are believed by management to be reasonable under the circumstances. Such estimates and assumptions are evaluated on an ongoing basis and form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ significantly from these estimates.
Our critical accounting estimates are those we believe are both most important to the portrayal of our financial condition and results and require our most difficult, subjective or complex judgments, often because we must make estimates about the effect of matters that are inherently uncertain. Judgments and uncertainties affecting the application of those policies may result in materially different amounts being reported under different conditions or using different assumptions. We believe the following estimates are the most critical in understanding the judgments that are involved in preparing our consolidated financial statements.
Impairment of Long-lived Depreciable Assets
We test for impairment annually and whenever events or circumstances make it more likely than not that the fair value of our capital assets and identifiable intangible assets (“long-lived depreciable assets”) has fallen below its carrying amount. If such indicators are present, we assess the recoverability of the assets or group of assets by determining whether the carrying value of such assets can be recovered through undiscounted future cash flows. In addition, the useful life over which cash flows will occur, their amount and the asset’s residual value, if any, are considered in the impairment calculation. In turn, measurement of an impairment loss requires a determination of fair value, which is based on the best information available. We derive the required undiscounted cash flow estimates from our historical experience and internal business plans. To determine fair value, we use quoted market prices when available, or our internal cash flow estimates discounted at an appropriate interest rate and independent appraisals, as appropriate. If the sum of undiscounted future cash flows is less than the carrying amount, the excess of the carrying amount over the estimated fair value, based on discounted future cash flows, is recorded as a charge to earnings.
During fiscal 2010, we recorded an impairment charge of $3.6 million in connection with the consolidation of our Puerto Rico operations into our manufacturing site located in Manatí. We recorded this charge to write down the carrying value of our Caguas facility’s long-lived assets to their anticipated fair value upon closure of the facility. Fair value was based on an offer from a third party to purchase the property less management’s best estimate of the costs to sell. In addition, an allocation of the purchase offer between land and building was performed based on current market conditions in Puerto Rico.
We also recorded an impairment charge of $0.8 million in discontinued operations to write down the remaining carrying value of the Carolina operations long-lived assets. This write-down was based on an offer from a third party to purchase the property less management’s best estimate on the costs to sell.
Reserve for Doubtful Accounts
We establish an appropriate provision for non-collectible or doubtful accounts. We consider several factors in estimating the allowance for uncollectible accounts receivable, including the age of the receivable, economic conditions that may have an impact on a specific group of customers or a specific customer and disputed services. Our risk management process includes standards and policies relating to customer credit limits, credit terms and customer deposits. Customer deposits relate primarily to our PDS business.
At October 31, 2011 and 2010, we had a reserve for doubtful accounts of $0.4 million and $0.9 million, respectively. These are specific reserves, not general reserves, and are based on factors discussed above.
Inventories
Inventories consisting of raw materials, packaging components, spare parts and work-in-process are valued at the lower of cost and net realizable value. These adjustments are customer specific estimates of net realizable value that we may ultimately realize upon the disposition of the inventories. We perform an assessment of excess, obsolete and problem products on an on-going basis.
We procure inventory based on specific customer orders and forecasts. Customers have limited rights of modification (for example, cancellations) with respect to these orders. Customer modifications to orders affecting inventory previously procured by us and purchases of inventory beyond customer needs may result in excess and obsolete inventory for the related customers. Although we may be able to use some excess components and raw materials for other products manufactured, a portion of the cost of this excess inventory may not be returned to the vendors or recovered from customers. Write-offs or write-downs of inventory could relate to:
| |
| • | declines in the market value of inventory; |
| |
| • | changes in customer demand for inventory, such as cancellation of orders; and |
| |
| • | our purchases of inventory beyond customer needs that result in excess quantities on hand that we may not be able to return to the vendor, use to fulfill orders from other customers or charge back to the customer. |
Adjustments above are recorded as an increase to cost of goods sold.
Payments received from customers for excess and obsolete inventories that have not been shipped to customers or otherwise disposed of are netted against inventory reserves.
Our practice is to dispose of excess and obsolete inventory as soon as practicable after such inventory has been identified as having no value to us.
Employee Future Benefits
We provide defined benefit pension plans to certain employees in our Canadian, U.K. and French operations and post-employment health and dental coverage to certain of our Canadian employees.
The determination of the obligation and expense for defined benefit pensions and other post-employment benefits is dependent on certain assumptions used by actuaries in calculating such amounts. The assumptions used in determining the accrued benefit obligation and the benefit expense as of and for the year ended October 31, 2011 were as follows:
|
| | | | | |
| | Defined Benefit Pension Plans % | | Other Benefit Plans % |
| Accrued benefit obligation | | | |
| Discount rate | 4.9 |
| | 5.2 |
|
| Rate of compensation increase | 3.7 |
| | — |
|
| Benefit costs recognized | | | |
| Discount rate | 5.2 |
| | 5.2 |
|
| Expected long-term rate of return on plan assets | 6.9 |
| | — |
|
| Rate of compensation increase | 3.9 |
| | — |
|
A 4% to 10% annual rate of increase in the per capita cost of covered health care and dental benefits was assumed for fiscal 2011, with the assumption that the rate will decrease gradually over the next five years to 6% and to remain at that level thereafter. The following table outlines the effects of a one-percentage-point increase and decrease in the assumed health care and dental benefit trend rates.
|
| | | | | |
| (in millions of USD) | Benefit Obligation | | Benefit Expense |
| | $ | | $ |
| Impact of: | | | |
| 1% increase | 1.0 |
| | 0.1 |
|
| 1% decrease | (0.8 | ) | | (0.1 | ) |
Stock-Based Compensation
We use the fair value method of accounting for stock-based compensation. We use the Black-Scholes option-pricing model to estimate the fair value of the options granted. The determination of the fair value of stock-based awards on the date of grant using an option-pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. These variables include our expected dividends, the risk-free interest rate, the expected life of the award and the expected stock price volatility over the term of the award. The principal assumptions we used in applying the Black-Scholes model are outlined below.
|
| | |
| | Fiscal 2011 |
| Expected dividend yield | None |
|
| Risk-free interest rate | 2.5 | % |
| Expected life | 5 years |
|
| Volatility | 59 | % |
We do not intend to pay dividends on our common stock in the foreseeable future and, accordingly, we use a dividend rate of zero in the option-pricing model. The Government of Canada five-year bond rate is used for the risk-free interest rate. The estimated life of the options is five years based on weighted-average life of these options, vesting period and management’s estimate based on stock volatility. The expected volatility is 59% based on the guidance for estimating expected volatility as set forth in Canadian Institute of Chartered Accountants Section 3870. In particular, volatility is a measure of the amount by which our common stock price has fluctuated or is expected to fluctuate during a period. We considered the historic volatility of our share price in estimating expected volatility.
If factors change and we employ different assumptions for estimating stock-based compensation expense in future periods or if we decide to use a different valuation model, the stock-based compensation expense we recognize in future periods may differ significantly from what we have previously recorded and could materially affect our operating income, net income and earnings per share. These differences may result in a lack of consistency in future periods and materially affect the fair value estimate of our stock-based awards. They may also result in a lack of comparability with other companies that use different models, methods and assumptions.
Income Taxes
We follow the liability method of income tax allocation. Under this method, future tax assets and liabilities are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the substantively enacted tax rates and laws that will be in effect when the differences are expected to reverse.
Preparation of our consolidated financial statements requires an estimate of income taxes in each of the jurisdictions in which we operate. The process involves an estimate of our current tax expense and an assessment of temporary differences resulting from differing treatment of items such as depreciation and amortization for tax and accounting purposes. These differences result in future tax assets and liabilities and are reflected in our consolidated balance sheet.
While evaluating our future tax assets and liabilities during the first half of fiscal 2010, we concluded we would be able to utilize certain investment tax credits (“ITCs”) relating to scientific research and development costs. Therefore, we recorded a decrease of $7.2 million in the cost of goods sold relating to the utilization of all previous years’ ITCs.
During the first half of fiscal 2010 we also evaluated our valuation reserves. We determined that the valuation allowance on our net Canadian future tax assets is no longer required based on our assessment of the future prospects of our Canadian operations. As a result of this determination, we released $13.8 million of valuation reserves through income tax benefit in the consolidated statements of loss.
Prior to the second quarter of fiscal 2010, we recorded the ITCs as future tax assets. Of the $18.4 million on the balance sheet at October 31, 2010, $8.3 million was reclassed from future tax assets to other long-term assets for fiscal 2010 as we are in a position to utilize current and prior period ITCs.
Future tax assets of $20.1 and $ 20.2 million have been recorded at October 31, 2011 and 2010, respectively. These assets consist primarily of accounting provisions related to employee benefits not currently deductible for tax purposes, the tax benefit of net operating loss carryforwards, unclaimed research and development expenditures, deferred revenues and deferred financing and share issue costs. We evaluate our ability to realize future tax assets on a quarterly basis. The factors used to assess the likelihood of realization of these assets include our calculation of cumulative pre-tax book income or loss, turn-around of temporary timing differences, available tax planning strategies that could be implemented to realize the future tax assets, and forecasted pre-tax book income and taxable income by specific tax jurisdiction. Actual results may vary from these forecasts and result in a change in our ability to realize benefits of these tax assets in the future. If we are unable to meet our projected forecasts or implement certain tax planning strategies in jurisdictions for which there is currently no valuation allowance, we may be required to record additional valuation allowances. The future tax assets recorded at October 31, 2011 and 2010 are net of a valuation allowance of $9.7 million and $6.5 million, respectively.
Future tax liabilities of $33.7 million and $29.7 million have been recorded at October 31, 2011 and 2010, respectively.
These liabilities have arisen primarily on tax depreciation in excess of book depreciation.
Our tax filings are subject to audit by taxation authorities. Although our management believes that it has adequately provided for income taxes based on the information available, the outcome of audits cannot be known with certainty and the potential impact on our consolidated financial statements is not determinable.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Foreign Currency Risk
Our business is conducted in several currencies-Canadian dollars and U.S. dollars for our Canadian operations, U.S. dollars for our U.S. operations and Euros, U. S. dollars and British Sterling for our European operations. We are subject to foreign currency transaction risk because a significant portion of our revenues and operating expenses from our operations in certain countries are denominated in different currencies. Our material foreign currency transaction risk arises from our Canadian operations. Our Canadian operations negotiate sales contracts for payment in both U.S. and Canadian dollars, and materials and equipment are purchased in both U.S. and Canadian dollars. The majority of the non-material costs (including payroll, facilities' costs and costs of locally sourced supplies and inventory) of our Canadian operations are denominated in Canadian dollars. In fiscal 2011, approximately 90% of the revenues and 10% of the operating expenses of our Canadian operations were transacted in U.S. dollars. As a result, if we do not effectively hedge such foreign currency exposure, our results of operations will be adversely affected by an increase in the value of the Canadian dollar relative to such foreign currency. In addition, we may experience hedging and transactional gains or losses because of volatility in the exchange rate between the Canadian dollar and the U.S. dollar. Based on our current U.S. denominated net inflows, for each 10% change in the Canadian-U.S. dollar exchange rate, the impact on annual pre-tax income, excluding any hedging activities, would be approximately $12.4 million.
To mitigate exchange-rate risk, we utilize foreign exchange forward contracts and collars in certain circumstances to lock in exchange rates with the objective that the gain or loss on the forward contracts and collars will approximately offset the loss or gain that results from the transaction or transactions being hedged. As of October 31, 2011, we had entered into 131 foreign exchange forward contracts and collars covering approximately 80% of our Canadian-U.S. dollar cash flow exposures for fiscal 2011. For additional information please see “Note 8—Financial Instruments and Risk Management” to our consolidated financial statements included in this Form 10-K. We do not hedge any of our other foreign exchange exposures. Our foreign exchange forward contracts and collars mature at various dates through July 2013 and have an aggregate fair value of $139.6 million. As of October 31, 2011, an adverse exchange rate movement of 10% against our foreign exchange forward contracts and collars would result in a pre-tax loss of approximately $14.0 million.
Interest Rate Risk
As of October 31, 2011, our long-term debt consisted of the Notes, which have an aggregate principal amount of $280.0 million and bear interest at a fixed rate, and the $75.0 million ABL, which would bear interest at a variable rate. As of October 31, 2011, no borrowings were outstanding under the ABL. Assuming a fully drawn ABL and a 100 basis point increase in applicable interest rates, our interest expense, net, would increase by $0.75 million on an annual basis.
Item 8. Financial Statements and Supplementary Data.
See “Index to Consolidated Financial Statements.”
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
There have been no changes in or corresponding disagreements with our independent accountant during the last two fiscal years.
Item 9A. Controls and Procedures
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
As of October 31, 2011, our management, with the participation of our Chief Executive Officer and our Vice President and Corporate Controller (who is currently performing functions similar to those of a principal financial officer), evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on such evaluation, our Chief Executive Officer and our Vice President and Corporate Controller concluded that, as of October 31, 2011, our disclosure controls and procedures were effective in that they provided reasonable assurances that the information we are required to disclose in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods required by the SEC's rules and forms and that such information is accumulated and communicated to management, including our Chief Executive Officer and Vice President and Corporate Controller, as appropriate, to allow timely decisions regarding required disclosure.
Internal Control Over Financial Reporting
This annual report does not include a report of management's assessment regarding internal control over financial reporting due to a transition period established by rules of the Securities and Exchange Commission for newly public companies.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the quarter ended October 31, 2011 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
Patheon Global Bonus Plan
On December 15, 2011, the CHR Committee of our Board adopted the Patheon Global Bonus Plan (the “Bonus Plan”) to reward eligible participants (including our executive officers) for their collective and individual contributions to our success. The Bonus Plan will provide cash awards for the achievement of predetermined (i) corporate objectives for Adjusted Corporate EBITDA (as defined in the Bonus Plan), Net Free Cash Flow (as defined in the Bonus Plan) and revenue and (ii) site objectives for site budget attainment and Working Capital (as defined in the Bonus Plan).
Under the Bonus Plan, participants become eligible for bonus payouts based on (i) the achievement of corporate and, if applicable, site objectives and (ii) their individual performance ratings. Our executive officers' bonus eligibility is determined based solely on the achievement of corporate objectives and individual performance ratings. The CHR Committee approved the corporate and site performance objectives, each of which has three possible levels of achievement (minimum, target and maximum) that correspond to three levels of payouts (50%, 100% and 150% of the target payout for that objective).
Each participant's target award is equal to a percentage of his or her earned base pay (less certain benefit amounts) or a specified dollar value as (i) contained in the terms of his or her written employment agreement, (ii) approved by our human resources department for the participant's specific position or employment grade level, or (iii) approved by the CHR Committee for the participant. Provided that all necessary conditions for payout have been met, each participant's payout will equal the product of the participant's target award multiplied by the total achievement of the participant's designated weighted objectives times multiplied by a factor ranging from 0 to 1.75 based on his or her individual performance rating; provided, however, that the maximum possible payout to any eligible participant is 200% of target.
The foregoing summary is qualified in its entirety by reference to the Bonus Plan, a copy of which is filed as Exhibit 10.22 to this annual report on Form 10-K and is incorporated herein by reference.
Resignation of Thomas S. Taylor from Our Board
On December 15, 2011, Thomas S. Taylor, a member of our Board, notified our Board that he would be resigning from our Board, effective immediately.
Appointment of Michel Lagarde to Our Board
Background
On December 15, 2011, the Board appointed Michel Lagarde to serve as a director, effective immediately. Mr. Lagarde has been appointed as the Chairman of the CHR Committee and as a member of the Audit Committee and Corporate Governance Committee of our Board.
Mr. Lagarde will be compensated in accordance with our policy for the compensation of non-employee directors, which (i) is described under the heading Director Compensation in our amended registration statement on Form 10 filed with the SEC on April 13, 2011 and (ii) was included as Exhibit 10.19 to our registration statement on Form 10 filed with the SEC on February 25, 2011 (and which is incorporated by reference into this annual report on Form 10-K as Exhibit 10.19).
Mr. Lagarde, a Principal of JLL Partners, was appointed as a director pursuant to the Investor Agreement, which entitles JLL Patheon Holdings to designate a certain number of nominees for appointment or election to our Board from time to time depending on the number of our restricted voting shares it holds. In the event of the death, disability, resignation or removal of any director designated by JLL Patheon Holdings, the Investor Agreement provides that our Board must appoint a replacement director designated by the JLL Patheon Holdings to fill the vacancy.
JLL Patheon Holdings, together with its affiliates, currently owns 72,077,781 of our restricted voting shares, representing approximately 56% of the Company's total restricted voting shares outstanding. As a result of various arrangements with us, JLL Partners and its affiliates currently have the right to determine three of our nine board seats and the right to approve our entry into certain types of transactions. The following further describes certain of our transactions and relationships with JLL Partners and its affiliates.
Arrangements with JLL
Transactions with JLL
In 2007, we entered into a definitive agreement with JLL Partners Fund V L.P., under which its affiliate, JLL Patheon Holdings, purchased our convertible Series C Preferred Shares and special voting Series D Preferred Shares through a private placement with aggregate gross proceeds to us of $150 million. JLL Patheon Holdings also acquired a number of rights in connection with the private placement, including the right to elect up to three directors to our Board pursuant to the terms of the Series D Preferred Shares. In connection with certain rights under the terms of the Series C Preferred Shares held by JLL Patheon Holdings, we entered into an agreement with JLL Patheon Holdings on September 4, 2008, pursuant to which JLL Patheon Holdings waived its redemption rights under the Series C Preferred Shares in exchange for the issuance of additional restricted voting shares and the right to acquire, through the facilities of the TSX, over a one-year period, up to 1.26 million restricted voting shares.
In 2009, after JLL made the JLL Offer and pursuant to an agreement between JLL Holdings in respect of all of legal actions then outstanding in connection with the JLL Offer and related matters, JLL Patheon Holdings converted its 150,000 Series C Preferred Shares into a total of 38,018,538 restricted voting shares, and we entered into the Settlement Agreement with JLL Patheon Holdings in respect of all of legal actions then outstanding in connection with the JLL Offer and related matters. See “-Settlement Agreement.”
Series D Preferred Shares
The Series D Preferred Shares provide JLL Patheon Holdings the right to elect the following number of directors to our Board:
| |
| • | so long as JLL Patheon Holdings holds at least 22,811,123 restricted voting shares, it has the right to elect three members to our Board; |
| |
| • | so long as JLL Patheon Holdings holds at least 11,405,561 restricted voting shares, it has the right to elect two members to our Board; and |
| |
| • | so long as JLL Patheon Holdings holds at least 5,702,781 restricted voting shares, it has the right to elect one member to our Board. |
Investor Agreement
On April 27, 2007, we entered into the Investor Agreement with JLL Patheon Holdings in connection with its purchase of our Series C Preferred Shares and Series D Preferred Shares with aggregate gross proceeds to us of $150 million. The following is a summary of the key terms of the Investor Agreement:
Special Approval Rights
Provided that JLL Patheon Holdings holds at least 13,306,488 restricted voting shares, the approval of JLL Patheon Holdings is required before we may:
| |
| • | create or issue any shares of capital stock ranking pari passu with or senior to the Series C Preferred Shares, or issue any additional restricted voting shares or other equity securities, or securities convertible for or exchangeable into such securities, other than pursuant to our Incentive Stock Option Plan or any other security-based compensation arrangement consented to by JLL Patheon Holdings; |
| |
| • | declare or pay dividends or other distributions (including capital) on our restricted voting shares or other equity securities; |
| |
| • | redeem, repurchase or acquire any restricted voting shares or other equity securities; |
| |
| • | change our articles of amalgamation; |
| |
| • | change the rights of our existing classes of shares; |
| |
| • | merge, consolidate or sell all or substantially all of our assets or undertake any similar business combination transaction; |
| |
| • | incur any indebtedness for borrowed money in excess of $20 million, excluding borrowings under our credit facilities; |
| |
| • | initiate any insolvency, restructuring or reorganization process, voluntary liquidation, dissolution or winding-up of our company; |
| |
| • | change our Chief Executive Officer; or |
| |
| • | change the size of our Board. |
Standstill
Unless JLL Patheon Holdings or any of its affiliates makes an offer to acquire all of our outstanding restricted voting shares (or an offer, made in conjunction with the offer to acquire our restricted voting shares, to acquire all of any class or series of convertible securities then outstanding) by way of a take-over bid circular and in compliance with the terms of our shareholder rights plan (if we have such a plan then in effect), JLL Patheon Holdings agreed not to acquire or offer to acquire, directly or indirectly, any restricted voting shares or Series C Preferred Shares or direct or indirect rights or options to acquire any restricted voting shares, without our prior written approval, other than restricted voting shares received through (i) a stock dividend or a recapitalization of our company, (ii) any dividend reinvestment plan, (iii) a rights offering to all holders of restricted voting shares, (iv) our shareholders rights plan, or (v) conversion of the Series C Preferred Shares. JLL Patheon Holdings also agreed not to act jointly or in concert with any third party to propose or effect any take-over bid, amalgamation, merger, arrangement or other business combination with respect to us or to propose or effect any acquisition or purchase of any of our assets. JLL Patheon Holdings agreed not to solicit votes or proxies to attempt to alter the structure of our Board as it existed on April 27, 2007. Subsequent to the Investor Agreement, we have agreed to provide a limited waiver of these standstill provisions to permit an affiliate of JLL Patheon Holdings to acquire, through the facilities of the TSX, over a one-year period, up to 1,256,929 restricted voting shares (determined on a partially diluted basis, taking into account our restricted voting shares issuable on conversion of the Series C Preferred Shares).
The standstill provisions set forth above will expire on the earliest of (i) April 27, 2012, (ii) the date upon which JLL Patheon Holdings and its wholly owned subsidiary or subsidiaries that hold restricted voting shares or convertible shares (A) cease to own beneficially, directly or indirectly, restricted voting shares and Series C Preferred Shares that represent at least 20% of the number of restricted voting shares then issued and outstanding and (B) no longer have the right to nominate a representative to our Board, and (iii) the date on which our Board approves any of the following actions: (A) the sale of restricted voting shares or Series C Preferred Shares representing more than 35% of the fully diluted shares held by JLL Patheon Holdings to any third party other than a member of JLL Patheon Holdings and its wholly owned subsidiary or subsidiaries that hold restricted voting shares or convertible shares or any person acting jointly or in concert with any member of JLL Patheon Holdings and its wholly owned subsidiary or subsidiaries that hold restricted voting shares or convertible shares and its affiliates; (B) a consolidation, merger, arrangement or amalgamation (statutory or otherwise) of us with any such third party; or (C) the acquisition by any such third party or group of such third parties of restricted voting shares or Series C
Preferred Shares representing more than 35% of the fully diluted shares held by JLL Patheon Holdings.
Transfer of Series D Preferred Shares
The Series D Preferred Shares are not transferable, except to an affiliate of JLL Patheon Holdings.
Registration Rights
JLL Patheon Holdings may request us to effect a qualification under Canadian securities laws (or, if we are eligible to use Form F-10 and JLL Patheon Holdings so requests, under the Securities Act) of the distribution to the public in any or all of the provinces of Canada (or in the United States, if applicable) of all or part of the Series C Preferred Shares (or restricted voting shares received on conversion) held by JLL Patheon Holdings (a “Demand Registration”), subject to a maximum of two Demand Registrations. In addition, each time we elect to proceed with the preparation and filing of a prospectus under any Canadian securities laws in connection with a proposed distribution of any of our securities for cash, JLL Patheon Holdings will be entitled to request that we cause any or all of the shares held by JLL Patheon Holdings to be included in such prospectus (an “Incidental Registration”). We will bear all registration expenses, excluding underwriting or placement discounts and commissions. The Demand Registration rights terminate when JLL Patheon Holdings and its affiliates no longer beneficially own Series C Preferred Shares (or restricted voting shares received on conversion) representing at least 12,500,000 fully diluted restricted voting shares, and the Incidental Registration rights terminate when JLL Patheon Holdings and its affiliates no longer beneficially own Series C Preferred Shares (or restricted voting shares received on conversion) representing at least 6,250,000 fully diluted restricted voting shares.
The Investor Agreement also contains other customary provisions such as, among other things, general indemnification provisions by which we indemnify JLL Patheon Holdings and JLL Patheon Holdings indemnifies us.
Board Representation
In furtherance of the right to elect directors to our Board pursuant to the terms of the Series D Preferred Shares, the Investor Agreement provides that our Board will consist of up to nine members and that JLL Patheon Holdings has the right to designate nominees for election or appointment to our Board (the “JLL Representatives”) as follows:
| |
| • | so long as JLL Patheon Holdings holds at least 22,811,123 restricted voting shares, it has the right to designate three JLL Representatives; |
| |
| • | so long as JLL Patheon Holdings holds at least 11,405,561 restricted voting shares, it has the right to designate two JLL Representatives; and |
| |
| • | so long as JLL Patheon Holdings holds at least 5,702,781 restricted voting shares, it will be entitled to designate one JLL Representative. |
We have agreed to cause the JLL Representatives to be included as nominees proposed by our Board to the shareholders at future meetings and to use reasonable commercial efforts to cause the election of the JLL Representatives and solicit proxies in favor of their election.
In the event that JLL Patheon Holdings no longer holds any Series D Preferred Shares and is therefore not entitled to elect directors to our Board pursuant to the terms thereof, the board representation provisions of the Investor Agreement will be controlling.
Settlement Agreement
On November 30, 2009, we entered into the Settlement Agreement with JLL Patheon Holdings to settle all claims filed by us against JLL Patheon Holdings and by JLL Patheon Holdings against us in connection with the JLL Offer and related matters. The Settlement Agreement provides, among other things, that:
| |
| • | Upon the effective date of the Settlement Agreement, our Board would be modified to consist of (i) three nominees of JLL Patheon Holdings, (ii) one additional director selected by JLL Patheon Holdings, (iii) Roy T. Graydon and Derek J. Watchorn (the “Board Nominees”), (iv) Brian G. Shaw (the “Third Independent Nominee” and, collectively with the Board Nominees, the “Independent Directors”), (v) our Chief Executive Officer (who, at the time, was Wesley P. Wheeler and who is now James C. Mullen) and (vi) Joaquín B. Viso. At each meeting of shareholders held prior to March 1, 2011 at which directors were to be elected, JLL Patheon Holdings would vote all of its shares in favor of the re-election of, and would otherwise use its reasonable commercial efforts to re-elect, each of the Independent |
Directors and, at any requisitioned meeting called for the purpose of removing directors, JLL Patheon Holdings would vote all of its shares against the removal of any of the directors specified above. If either of the Board Nominees did not stand for re-election or vacated his office, a replacement nominee would be selected by our Board with the unanimous approval of the Independent Directors then in office, and JLL Patheon Holdings would vote all of its shares in favor of the election of, and would otherwise use its reasonable commercial efforts to elect or cause to be appointed, such replacement nominee as a director of our company. If the Third Independent Nominee did not stand for re-election or vacated his office, JLL Patheon Holdings would identify a replacement nominee who would be a qualifying independent director. At each meeting of shareholders held after March 1, 2011 and prior to March 1, 2012, JLL Patheon Holdings will vote all of its shares in favor of the election of, and against the removal of, and will otherwise use its reasonable commercial efforts to elect, at least three persons (who may or may not be one or more of the Independent Directors then in office) to our Board who would be “qualifying independent directors.” For these purposes, a “qualifying independent director” means a person who would be an independent director under the definition in the TSX Company Manual; provided, however, that the Third Independent Nominee will not be disqualified as an independent director under the definition in the TSX Company Manual solely by virtue of the fact that he or she has been identified by JLL Patheon Holdings. If any such Independent Director vacates his or her office, a replacement nominee will be selected by our Board with the unanimous approval of the Independent Directors then in office, and JLL Patheon Holdings will vote all of its shares in favor of the election of, and will otherwise use its reasonable commercial efforts to elect or cause to be appointed, such replacement nominee as a director of our company.
| |
| • | JLL Patheon Holdings agreed not to acquire any additional restricted voting shares of Patheon for a one-year period, which expired on November 29, 2010. Thereafter, and until April 27, 2012, JLL Patheon Holdings will not acquire any additional restricted voting shares unless, among other things, the acquisition complies with the standstill provisions of the Investor Agreement, and (i) if the acquisition is to be effected by means of a merger, consolidation, amalgamation or similar transaction requiring a vote of shareholders, the acquisition is approved by a majority vote of the holders of the outstanding restricted voting shares not already held by JLL Patheon Holdings or its associates, affiliates and/or joint actors and (ii) if the acquisition is to be effected by means of a takeover bid, the bid is subject to an irrevocable condition requiring the valid tender to the bid of at least a majority of the outstanding restricted voting shares not already held by JLL Patheon Holdings or its associates, affiliates and/or joint actors. |
| |
| • | Until April 27, 2012, certain transactions by us, including certain rights offerings, issuer bids and related party transactions, would require approval by a majority of the independent directors. |
| |
| • | Unless approved in advance by a majority of the independent directors, until April 27, 2012, JLL Patheon Holdings will not enter into, or agree to, any oral or written agreement, arrangement or understanding, formal or informal, direct or indirect, with any of our other shareholders, in respect of the acquisition of any of our securities or in respect of any merger, consolidation, amalgamation or similar transaction involving us. |
| |
| • | We agreed to pay JLL Patheon Holdings $1.5 million in connection with the settlement. |
Cost Sharing Arrangement
In fiscal 2010, we entered into a cost sharing arrangement with JLL Partners with respect to certain third-party consulting fees. The agreement terminated in the first quarter of fiscal 2011. During fiscal 2011, we reimbursed JLL Partners approximately $30,000 under this agreement, and JLL Partners did not provide us any reimbursements. Related to these transactions, there are currently no outstanding amounts payable to JLL Partners. These transactions were conducted in the normal course of business and were recorded at the exchanged amounts.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Directors and Executive Officers
Information regarding our directors and executive officers may be found under the captions “Business of the Meeting—Election of Directors” and “Executive Officers” in the Proxy Statement and Information Circular for our 2012 Annual and Special Meeting of Shareholders (our “2012 Proxy Statement”). Such information is incorporated herein by reference.
Compliance With Section 16(a) of the Exchange Act
Information regarding compliance with Section 16(a) of the Exchange Act by our directors, officers and beneficial owners of more than 10% of our restricted voting shares may be found under the caption “Section 16(a) Beneficial Ownership Reporting Compliance” in our 2012 Proxy Statement. Such information is incorporated herein by reference.
Code of Ethics
We have adopted a code of business conduct and ethics that applies to our directors, officers (including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions) and other employees. A copy of our code of business conduct and ethics is available on our website at www.patheon.com under “Investor Relations—Corporate Governance.” We intend to post on our website and (if required) file on Form 8-K all disclosures that are required by applicable law or the rules of the SEC concerning any amendment to, or waiver from, our code of business conduct and ethics.
Director Nominees
Information regarding procedures for recommending nominees to the board of directors may be found under the caption “Corporate Governance—The Board of Directors—Board Committees—Corporate Governance Committee” in our 2012 Proxy Statement. Such information is incorporated herein by reference.
Audit Committee
Information regarding our audit committee may be found under the caption “Corporate Governance—The Board of Directors—Board Committees—Audit Committee” in our 2012 Proxy Statement. Such information is incorporated herein by reference.
Item 11. Executive Compensation.
Information with respect to this item may be found under the caption “Executive Compensation” in the 2012 Proxy Statement. Such information is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information with respect to this item may be found under the captions “Principal Shareholders and Share Ownership by Management” and “Executive Compensation—Equity Compensation Plan Information” in the 2012 Proxy Statement. Such information is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Information with respect to this item may be found under the captions “Executive Compensation—Compensation Committee Interlocks and Insider Participation” and “Corporate Governance” in the 2012 Proxy Statement. Such information is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services.
Information with respect to this item may be found under the caption “Business of the Meeting—Appointment of Auditors—Independent Auditor Fee Information” in the 2012 Proxy Statement. Such information is incorporated herein by reference.
Item 15. Exhibits and Financial Statement Schedules.
(a)(1) Financial Statements
Our consolidated financial statements appear at the end of this annual report on Form 10-K. See “Index to Consolidated Financial Statements.”
(a)(2) Financial Statement Schedules
Schedules have been omitted because they are not applicable or the required information is shown in our consolidated financial statements or the related notes thereto. See "Index to Consolidated Financial Statements."
(a)(3) Exhibits
The list of exhibits filed as part of this annual report on Form 10-K is set forth on the Exhibit Index immediately preceding the exhibits hereto and is incorporated herein by reference.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | | |
| | | | |
| | PATHEON INC. |
| | | |
| | By: | | /s/ James C. Mullen |
| | James C. Mullen
|
| | Chief Executive Officer |
| | December 19, 2011 |
We, the undersigned officers and directors of Patheon Inc., hereby severally constitute and appoint James C. Mullen and Dean F. Wilson, and each of them singly, our true and lawful attorneys, with full power to them and each of them singly, to sign for us in our names in the capacities indicated below, all amendments to this report, and generally to do all things in our names and on our behalf in such capacities to enable Patheon Inc. to comply with the provisions of the Securities Exchange Act of 1934, as amended, and all requirements of the Securities and Exchange Commission.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
| | |
| Name | Title | Date |
| /s/ James C. Mullen | Chief Executive Officer (Principle Executive Officer) | December 19, 2011 |
| | | |
| /s/ Dean F. Wilson | Vice President, Corporate Controller (Principle Financial Officer and Principal Accounting Officer) | December 19, 2011 |
| | | |
| /s/ Ramsey A. Frank | Director | December 19, 2011 |
| | | |
| /s/ Paul S. Levy | Director | December 19, 2011 |
| | | |
| /s/ Michel Lagarde | Director | December 19, 2011 |
| | | |
| /s/ Daniel Agroskin | Director | December 19, 2011 |
| | | |
| /s/ Joaquín B. Viso | Director | December 19, 2011 |
| | | |
| /s/ Derek J. Watchorn | Director | December 19, 2011 |
| | | |
| /s/ Brian G. Shaw | Director | December 19, 2011 |
| | | |
| /s/ David E. Sutin | Director | December 19, 2011 |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
| |
| | Page |
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | F-1 |
| | |
| AUDITED FINANCIAL STATEMENTS: | |
| | |
| Consolidated Balance Sheets | F-2 |
| | |
| Consolidated Statements of Loss | F-3 |
| | |
| Consolidated Statements of Changes in Shareholders' Equity | F-4 |
| | |
| Consolidated Statements of Comprehensive (Loss) Income | F-5 |
| | |
| Consolidated Statements of Cash Flows | F-6 |
| | |
| Notes to Consolidated Financial Statements | F-7 |
| | |
| | |
INDEPENDENT AUDITORS' REPORT OF REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders of Patheon Inc.
We have audited the accompanying consolidated financial statements of Patheon Inc., which comprise the consolidated balance sheets as at October 31, 2011 and 2010, and the consolidated statements of loss, changes in shareholders' equity, comprehensive income (loss) and cash flows for each of the years in the three-year period ended October 31, 2011, and a summary of significant accounting policies and other explanatory information.
Management's responsibility for the consolidated financial statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with Canadian generally accepted accounting principles, and for such internal control as management determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditors' responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we comply with ethical requirements and plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on the auditors' judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained in our audits is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Patheon Inc. as at October 31, 2011 and 2010, and the results of its operations and its cash flows for each of the years in the three-year period ended October 31, 2011 in accordance with Canadian generally accepted accounting principles.
Raleigh, North Carolina /s/ Ernst & Young LLP
December 19, 2011
Patheon Inc.
CONSOLIDATED BALANCE SHEETS
|
| | | | | |
| | As of October 31, |
| (in millions of U.S. dollars ) | 2011 | | 2010 |
| | $ | | $ |
| Assets | | | |
| Current | | | |
| Cash and cash equivalents | 33.4 |
| | 53.5 |
|
| Accounts receivable | 158.0 |
| | 139.9 |
|
| Inventories | 81.8 |
| | 73.3 |
|
| Income taxes receivable | 3.1 |
| | 5.7 |
|
| Prepaid expenses and other | 10.7 |
| | 9.5 |
|
| Future tax assets—short term | 8.1 |
| | 9.0 |
|
| Total current assets | 295.1 |
| | 290.9 |
|
| Capital assets | 474.9 |
| | 478.3 |
|
| Intangible assets | — |
| | 1.4 |
|
| Future tax assets | 12.0 |
| | 11.2 |
|
| Goodwill | 3.5 |
| | 3.4 |
|
| Investments | 5.3 |
| | 5.3 |
|
| Long-term assets held for sale | 0.2 |
| | — |
|
| Other long-term assets | 27.6 |
| | 18.4 |
|
| Total assets | 818.6 |
| | 808.9 |
|
| Liabilities and shareholders’ equity | | | |
| Current | | | |
| Short-term borrowings | 6.1 |
| | 2.0 |
|
| Accounts payable and accrued liabilities | 181.2 |
| | 156.7 |
|
| Income taxes payable | — |
| | 0.4 |
|
| Deferred revenues—short term | 8.8 |
| | 26.7 |
|
| Current portion of long-term debt | 1.1 |
| | 3.5 |
|
| Total current liabilities | 197.2 |
| | 189.3 |
|
| Long-term debt | 274.6 |
| | 274.8 |
|
| Deferred revenues | 27.7 |
| | 19.2 |
|
| Future tax liabilities | 33.7 |
| | 29.7 |
|
| Other long-term liabilities | 21.7 |
| | 22.9 |
|
| Total liabilities | 554.9 |
| | 535.9 |
|
| Shareholders’ equity | | | |
| Restricted voting shares | 553.8 |
| | 553.8 |
|
| Contributed surplus | 13.5 |
| | 10.0 |
|
| Deficit | (347.5 | ) | | (330.7 | ) |
| Accumulated other comprehensive income | 43.9 |
| | 39.9 |
|
| Total shareholders’ equity | 263.7 |
| | 273.0 |
|
| Total liabilities and shareholders’ equity | 818.6 |
| | 808.9 |
|
On behalf of the Board:
|
| | |
| /s/ Ramsey A. Frank | | /s/ James C. Mullen |
RAMSEY A. FRANK | | JAMES C. MULLEN |
DIRECTOR | | CHIEF EXECUTIVE OFFICER |
see accompanying notes
Patheon Inc.
CONSOLIDATED STATEMENTS OF LOSS
|
| | | | | | | | | | | |
| | Years ended October 31, |
| (in millions of U.S. dollars, except loss per share) | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Revenues | 700.0 |
| | 671.2 |
| | 655.1 |
|
| Cost of goods sold | 561.9 |
| | 526.2 |
| | 511.2 |
|
| Gross profit | 138.1 |
| | 145.0 |
| | 143.9 |
|
| Selling, general and administrative expenses | 120.2 |
| | 110.6 |
| | 105.5 |
|
| Repositioning expenses | 7.0 |
| | 6.8 |
| | 2.1 |
|
| Operating income | 10.9 |
| | 27.6 |
| | 36.3 |
|
| Interest expense, net | 25.4 |
| | 19.5 |
| | 15.4 |
|
| Impairment charge | — |
| | 3.6 |
| | — |
|
| Foreign exchange (gain) loss | (1.6 | ) | | (1.5 | ) | | 7.0 |
|
| Loss on sale of fixed assets | 0.2 |
| | 0.2 |
| | — |
|
| Refinancing Expenses | — |
| | 12.2 |
| | — |
|
| Other (income) expense, net | (4.2 | ) | | (0.1 | ) | | 0.4 |
|
| (Loss) income from continuing operations before income taxes | (8.9 | ) | | (6.3 | ) | | 13.5 |
|
| Current | 1.6 |
| | 6.7 |
| | 7.7 |
|
| Future | 5.7 |
| | (9.7 | ) | | 4.8 |
|
| Provision for (benefit from) income taxes | 7.3 |
| | (3.0 | ) | | 12.5 |
|
| (Loss) income before discontinued operations | (16.2 | ) | | (3.3 | ) | | 1.0 |
|
| Loss from discontinued operations | (0.6 | ) | | (1.7 | ) | | (7.8 | ) |
| Net loss for the period | (16.8 | ) | | (5.0 | ) | | (6.8 | ) |
| Dividends on convertible preferred shares | — |
| | — |
| | 11.1 |
|
| Net loss attributable to restricted voting shareholders | (16.8 | ) | | (5.0 | ) | | (17.9 | ) |
| Basic and diluted loss per share | | | | | |
| From continuing operations | $ | (0.125 | ) | | $ | (0.026 | ) | | $ | (0.100 | ) |
| From discontinued operations | $ | (0.005 | ) | | $ | (0.013 | ) | | $ | (0.077 | ) |
| | $ | (0.130 | ) | | $ | (0.039 | ) | | $ | (0.177 | ) |
| Weighted-average number of shares outstanding during period—basic and diluted (in thousands) | 129,168 |
| | 129,168 |
| | 100,964 |
|
see accompanying notes
Patheon Inc.
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
|
| | | | | | | | |
| | Years ended October 31, |
| (in millions of U.S. dollars) | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Convertible preferred shares—equity component | | | | | |
| Balance at beginning of period | — |
| | — |
| | 149.2 |
|
| Paid in-kind dividend on shares | — |
| | — |
| | 11.1 |
|
| Conversion of convertible preferred shares | — |
| | — |
| | (160.3 | ) |
| Balance at end of period | — |
| | — |
| | — |
|
| Restricted voting shares | | | | | |
| Balance at beginning of period | 553.8 |
| | 553.8 |
| | 393.5 |
|
| Conversion of convertible preferred shares | — |
| | — |
| | 160.3 |
|
| Balance at end of period | 553.8 |
| | 553.8 |
| | 553.8 |
|
| Contributed surplus | | | | | |
| Balance at beginning of period | 10.0 |
| | 7.7 |
| | 6.7 |
|
| Stock-based compensation | 3.5 |
| | 2.3 |
| | 1.0 |
|
| Balance at end of period | 13.5 |
| | 10.0 |
| | 7.7 |
|
| Deficit | | | | | |
| Balance at beginning of period | (330.7 | ) | | (325.7 | ) | | (309.3 | ) |
| Adjustment related to change in accounting policy | — |
| | — |
| | 1.6 |
|
| Net loss attributable to restricted voting shareholders | (16.8 | ) | | (5.0 | ) | | (17.9 | ) |
| Balance at end of period | (347.5 | ) | | (330.7 | ) | | (325.7 | ) |
| Accumulated other comprehensive income (loss) | | | | | |
| Balance at beginning of period | 39.9 |
| | 35.5 |
| | (2.9 | ) |
| Other comprehensive income for the period | 4.0 |
| | 4.4 |
| | 38.4 |
|
| Balance at end of period | 43.9 |
| | 39.9 |
| | 35.5 |
|
| Total shareholders’ equity at end of period | 263.7 |
| | 273.0 |
| | 271.3 |
|
see accompanying notes
Patheon Inc.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE (LOSS) INCOME
|
| | | | | | | | |
| | Years ended October 31, |
| (in millions of U.S. dollars) | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Net loss attributable to restricted voting shareholders | (16.8 | ) | | (5.0 | ) | | (17.9 | ) |
| Other comprehensive income, net of income taxes | | | | | |
Change in foreign currency gains on investments in subsidiaries, net of hedging activities1 | 5.3 |
| | 1.6 |
| | 25.5 |
|
Change in value of derivatives designated as foreign currency and interest rate cash flow hedges2 | 1.7 |
| | 9.0 |
| | 8.0 |
|
(Losses) gains on foreign currency and interest rate cash flow hedges reclassified to consolidated statement of loss3 | (3.0 | ) | | (6.2 | ) | | 4.9 |
|
| Other comprehensive income for the period | 4.0 |
| | 4.4 |
| | 38.4 |
|
| | | | | | |
| Comprehensive (loss) income attributable to restricted voting shareholders | (12.8 | ) | | (0.6 | ) | | 20.5 |
|
_______________________
The amounts disclosed in other comprehensive income have been recorded net of income taxes as follows:
| |
1 | Net of an income tax benefit of $0.4 million, an expense of $0.1 million (2010) and nil (2009). |
| |
2 | Net of an income tax expense of $0.5 million, $1.9 million (2010) and $1.1 million (2009). |
| |
3 | Net of an income tax benefit of $1.1 million, $0.8 million (2010) and $0.9 million (2009). |
see accompanying notes
Patheon Inc.
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
| | | | | | | | |
| | Years ended October 31, |
| (in millions of U.S. dollars) | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Operating activities | | | | | |
| (Loss) income before discontinued operations | (16.2 | ) | | (3.3 | ) | | 1.0 |
|
| Add (deduct) charges to operations not requiring a current cash payment | | | | | |
| Depreciation and amortization | 53.4 |
| | 55.8 |
| | 42.6 |
|
| Impairment charge | — |
| | 3.6 |
| | — |
|
| Other non-cash interest | 1.1 |
| | 2.5 |
| | 0.6 |
|
| Change in other long-term assets and liabilities | (10.2 | ) | | (8.6 | ) | | (1.2 | ) |
| Future income taxes | 5.3 |
| | (8.9 | ) | | 4.8 |
|
| Amortization of deferred revenues | (45.0 | ) | | (37.4 | ) | | (1.0 | ) |
| Loss on sale of fixed assets | 0.2 |
| | 0.2 |
| | — |
|
| Stock-based compensation expense | 3.5 |
| | 2.3 |
| | 1.0 |
|
| Other | 0.4 |
| | (0.3 | ) | | 0.5 |
|
| | (7.5 | ) | | 5.9 |
| | 48.3 |
|
| Net change in non-cash working capital balances related to continuing operations | 1.0 |
| | (2.6 | ) | | (10.8 | ) |
| Increase in deferred revenues | 30.4 |
| | 47.4 |
| | 10.5 |
|
| Cash provided by operating activities of continuing operations | 23.9 |
| | 50.7 |
| | 48.0 |
|
| Cash used in operating activities of discontinued operations | (1.0 | ) | | (0.7 | ) | | (8.9 | ) |
| Cash provided by operating activities | 22.9 |
| | 50.0 |
| | 39.1 |
|
| Investing activities | | | | | |
| Additions to capital assets | (47.8 | ) | | (48.7 | ) | | (49.1 | ) |
| Proceeds on sale of capital assets | 0.4 |
| | — |
| | 0.1 |
|
| Net increase in investments | — |
| | (1.1 | ) | | (0.3 | ) |
| Investment in intangibles | — |
| | (0.2 | ) | | (0.2 | ) |
| Cash used in investing activities of continuing operations | (47.4 | ) | | (50.0 | ) | | (49.5 | ) |
| Cash provided by investing activities of discontinued operations | — |
| | — |
| | 0.2 |
|
| Cash used in investing activities | (47.4 | ) | | (50.0 | ) | | (49.3 | ) |
| Financing activities | | | | | |
| Increase (decrease) in short-term borrowings | 4.1 |
| | (10.7 | ) | | 3.0 |
|
| Increase in long-term debt | 9.0 |
| | 296.2 |
| | 50.5 |
|
| Increase in deferred financing costs | — |
| | (7.3 | ) | | — |
|
| Repayment of long-term debt | (10.4 | ) | | (246.6 | ) | | (39.9 | ) |
| Cash provided by financing activities of continuing operations | 2.7 |
| | 31.6 |
| | 13.6 |
|
| Cash provided by financing activities | 2.7 |
| | 31.6 |
| | 13.6 |
|
| Effect of exchange rate changes on cash and cash equivalents | 1.7 |
| | (0.4 | ) | | (1.3 | ) |
| Net (decrease) increase in cash and cash equivalents during the period | (20.1 | ) | | 31.2 |
| | 2.1 |
|
| Cash and cash equivalents, beginning of period | 53.5 |
| | 22.3 |
| | 20.2 |
|
| Cash and cash equivalents, end of period | 33.4 |
| | 53.5 |
| | 22.3 |
|
| Supplemental cash flow information | | | | | |
| Interest paid | 25.0 |
| | 21.1 |
| | 15.1 |
|
| Income taxes (received) paid, net | (1.3 | ) | | 9.1 |
| | 10.2 |
|
see accompanying notes
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
1. NATURE OF BUSINESS
Patheon Inc. (“Patheon” or the “Company”) is a Canadian public company, which trades under the symbol PTI on The Toronto Stock Exchange ("TSX"). The Company is an independent provider of drug development and manufacturing services to global pharmaceutical, biotechnology and specialty pharmaceutical companies.
Patheon’s commercial manufacturing activities relate primarily to products in solid, semi-solid, liquid and sterile dosage forms. The Company manufactures to customer specifications a wide variety of products in many packaging formats. The Company can be responsible for each aspect of the manufacturing and packaging process, from sourcing raw materials and packaging components to delivering the finished product in consumer-ready form to the customer's distribution facilities.
Patheon’s pharmaceutical development services include dosage form development, analytical methods development, pilot batch manufacture of new products for the regulatory drug approval process and the provision of scale-up services designed to demonstrate that a drug can be manufactured in commercial volumes.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The consolidated financial statements of the Company have been prepared by management in accordance with Canadian generally accepted accounting principles (“Canadian GAAP”). The significant accounting policies followed by the Company are summarized as follows:
Principles of consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany transactions have been eliminated.
Going concern
The consolidated financial statements have been prepared in accordance with Canadian GAAP using the going-concern assumption, which assumes the Company will be able to realize assets and discharge liabilities in the normal course of operations.
Use of estimates in the preparation of the consolidated financial statements
The preparation of the consolidated financial statements in conformity with Canadian GAAP requires management to make estimates and assumptions that affect: the reported amounts of assets and liabilities; the disclosure of contingent assets and liabilities at the date of the consolidated financial statements; and the reported amounts of revenue and expenses in the reporting period. Management believes that the estimates and assumptions used in preparing its consolidated financial statements are reasonable and prudent; however, actual results could differ from those estimates.
Changes in accounting policy
The Company had no changes in accounting policy from the consolidated financial statements for the year ended October 31, 2010 (“fiscal 2010”).
Foreign exchange translation
The Company’s assets and liabilities that are not denominated in U.S. dollars are translated into U.S. dollars as follows: non-monetary assets are translated using the exchange rate in effect at year end, non-monetary assets and liabilities are translated at the exchange rates in effect at the time of acquisition or issue, and revenues and expenses are translated at the average rate during each month. Translation gains and losses related to the carrying value of the Company’s foreign operations are included in accumulated other comprehensive income in shareholders’ equity. Foreign exchange gains and losses on transactions occurring in a currency different than the entity’s functional currency are reflected in income (loss).
Revenue recognition
The Company recognizes revenue for its commercial manufacturing and pharmaceutical development services when services are completed in accordance with specific agreements with its customers and when all costs connected with providing
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
these services have been incurred, the price is fixed or determinable and collectability is reasonably assured. Customer deposits on pharmaceutical development services in progress are included in accounts payable and accrued liabilities.
The Company does not receive any fees on signing of contracts. In the case of pharmaceutical development services, revenue is recognized on the achievement of specific milestones in accordance with the respective development service contracts. In the case of commercial manufacturing services, revenue is recognized when services are complete and the product has met rigorous quality assurance testing.
Deferred revenues
The costs of certain capital assets are reimbursed to the Company by the pharmaceutical companies that are to benefit from the improvements in connection with the manufacturing and packaging agreements in force. These reimbursements are recorded as deferred revenues and are recognized as income over the remaining minimum term of the agreements. In certain instances the Company receives prepayment for future services and these amounts are amortized over the future required service periods.
Financial assets and liabilities
The Company’s financial instruments are classified into one of the following five categories: held-for-trading, held to maturity investments, loans and receivables, available-for-sale financial assets and other financial liabilities. All financial instruments, including derivatives, are included in the consolidated balance sheets and are measured at fair value except for held to maturity investments, loans and receivables and other financial liabilities, which are measured at amortized cost. Held-for-trading financial instruments are recorded at cost as they are initiated and are subsequently measured at fair value and all revaluation gains and losses are included in net income (loss) in the period in which they arise. Available-for-sale financial instruments are also recorded at cost and are subsequently measured at fair value with all revaluation gains and losses included in other comprehensive income. All financial instrument transactions are recorded on the settlement date. Please refer to Note 13—Financial Instruments and Risk Management.
The Company expenses as incurred all transaction costs, including fees paid to advisors and other related costs. Financing costs, including underwriting and arrangement fees paid to lenders are deferred and netted against the carrying value of the related debt and amortized into interest expense using the effective interest rate method.
Derivatives and hedge accounting
The Company enters into foreign exchange forward contracts and collars to reduce its exposure to foreign currency denominated cash flows and changes in the fair value of foreign denominated assets and liabilities. Please refer to Note 13—Financial Instruments and Risk Management. The Company previously entered into interest rate swap contracts to reduce its exposure to variable interest rates.
All derivative instruments are recorded on the consolidated balance sheets at fair value unless exempted from derivative treatment as a normal purchase and sale. All changes in their fair value are recorded in income (loss) unless cash flow hedge accounting is used, in which case the changes in the fair value associated with the effective portions of the hedge are recorded in other comprehensive income.
Prior to the refinancing in the second quarter of fiscal 2010, the Company held foreign currency denominated debt as a hedge against the carrying value of its equity investment in certain foreign currency denominated operations. The foreign exchange translation impact of foreign denominated debt that was designated as an effective hedge of the net investments in foreign operations was recognized in other comprehensive income. The foreign exchange translation impact of foreign denominated debt that was not considered to be an effective hedge was recorded in income (loss) from operations. In the third quarter of fiscal 2010, the Company changed the functional currency of its corporate division in Canada to U.S. dollars, thereby eliminating the need for the Company to designate this U.S. dollar denominated debt as a hedge.
Cash and cash equivalents
Cash and cash equivalents include cash in interest-bearing accounts and term deposits with remaining maturities of less than three months at the date the term deposit was acquired.
Inventories
Inventories consisting of raw materials, packaging components, spare parts and work-in-process are valued at the lower
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
of cost and net realizable value. Cost is principally determined by the standard cost method, which approximates average cost, and includes cost of purchased materials, costs of conversion, namely labor and overhead, and other costs, such as freight in, necessary to bringing the inventories to their present location and condition.
Capital assets
Capital assets are carried at cost less accumulated depreciation. The cost of assets disposed of and the related accumulated depreciation are removed from the accounts and any resulting gain or loss is reflected in earnings.
Depreciation is provided on the straight-line basis based on estimated useful lives as follows:
|
| |
| Buildings | 30 – 50 years |
| Building equipment | 15 years |
| Machinery and equipment | 5 – 15 years |
| Office equipment | 3 – 10 years |
| Computer software | 2 – 10 years |
| Furniture and fixtures | 7 – 10 years |
Repairs and maintenance costs are charged to operations as incurred.
Intangible assets
Intangible assets represent the values assigned to acquired customer contracts and relationships and certain royalty agreements entered into with customers. They are amortized on a straight-line basis over their estimated economic lives.
Impairment of long-lived depreciable assets
The Company reviews whether there are any indicators of impairment of its capital assets and identifiable intangible assets (“long-lived depreciable assets”). If such indicators are present, the Company assesses the recoverability of the assets or group of assets by determining whether the carrying value of such assets can be recovered through undiscounted future cash flows. If the sum of undiscounted future cash flows is less than the carrying amount, the excess of the carrying amount over the estimated fair value, based on discounted future cash flows, is recorded as a charge to earnings.
Goodwill
Goodwill represents the excess of the purchase price of the Company's interest in subsidiary companies over the fair value of the underlying net identifiable assets arising on acquisitions. Goodwill is not subject to amortization but is subject to an annual review for impairment, or more frequently if events or changes in circumstances indicate that goodwill is impaired. Goodwill impairment is assessed based on a comparison of the fair value of an individual reporting unit to the underlying carrying value of the reporting unit’s net assets including goodwill. When the carrying amount of the reporting unit exceeds its fair value, the fair value of the reporting unit’s goodwill, determined in the same manner as in a business combination, is compared with its carrying amount to measure the amount of the impairment loss, if any.
Employee benefit plans
The Company provides a number of benefit plans to its employees including:
(a) defined benefit pension plans; (b) post-employment benefit plans; (c) defined contribution pension plans; and (d) unfunded termination indemnities.
The cost of defined benefit pension plans and other post-employment benefits, which include health care and dental benefits, related to employees' current service is charged to earnings annually. The cost is computed on an actuarial basis using the projected benefit method pro-rated based on service and management's best estimates of various actuarial factors, including salary escalation, other cost escalation and retirement ages of employees.
The valuation of defined benefit pension plan assets is at current market value, based on an actuarial valuation, for purposes of calculating the expected return on plan assets. Past service costs resulting from plan amendments are deferred and amortized on a straight-line basis over the remaining service life of employees active at the time of amendment.
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
Actuarial gains and losses arise from the difference between the actual long-term rate of return on plan assets for a period and the expected long-term rate of return on plan assets for that period, or from changes in actuarial assumptions used to determine the accrued benefit obligation. The excess of the net accumulated actuarial gain or loss over 10% of the greater of the benefit obligations and the fair value of plan assets is amortized over the average remaining service period of active employees. The average remaining service period of the active employees covered by the pension plans and the other retirement benefit plans at the measurement date of October 31, 2011 is 18 years (2010—18 years). When the restructuring of a benefit plan gives rise to both a curtailment and a settlement of obligations, the curtailment is accounted for prior to the settlement.
The cost of defined contribution pension plans is charged to earnings as funds are contributed by the Company.
Unfunded termination indemnities for the employees of the Company’s subsidiary in Italy are accrued based on Italian severance pay statutes. The liability recorded on the consolidated balance sheets is the amount to which the employees would be entitled if the employees’ employment with the Company ceased.
Income taxes
The Company follows the liability method of income tax allocation. Under this method, future tax assets and liabilities are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the substantively enacted tax rates and laws that will be in effect when the differences are expected to reverse.
The Company evaluates its ability to realize future tax assets on a quarterly basis. The factors used to assess the likelihood of realization of these assets include the Company's calculation of cumulative pre-tax book income or loss, turn-around of temporary timing differences, available tax planning strategies that could be implemented to realize the future tax assets, and forecasted pre-tax book income and taxable income by specific tax jurisdiction. Actual results may vary from these forecasts and result in a change in the ability of the Company to realize benefits of these tax assets in the future. If the Company is unable to meet its projected forecasts or implement certain tax planning strategies in jurisdictions for which there is currently no valuation allowance, the recording of a valuation allowance may be required.
Convertible preferred shares
On July 29, 2009, JLL Patheon Holdings, LLC (“JLL Patheon Holdings”) converted its 150,000 Series C convertible preferred shares of the Company into a total of 38,018,538 restricted voting shares of the Company, in accordance with the convertible preferred share terms. As a result of this conversion, the Company no longer has any Series C preferred shares outstanding.
Stock options
The fair value of stock options granted, modified or settled on or after November 1, 2003 is recognized on a straight-line basis over the applicable stock option vesting period as stock-based compensation expense in the consolidated statements of loss and contributed surplus in the consolidated balance sheets. On the exercise of stock options, consideration received and the accumulated contributed surplus amount is credited to share capital. The Company expenses forfeitures as incurred.
For the purposes of calculating the stock-based compensation expense, the fair value of stock options is estimated at the date of the grant using the Black-Scholes option pricing model and the cost is amortized over the vesting period. This model requires the input of a number of assumptions including dividend yields, expected stock price volatility, expected time until exercise and risk-free interest rates. Although the assumptions used reflect management’s best estimates, they involve assumptions based on market conditions generally outside of the control of the Company.
Loss per share
The calculation of loss per share—from continuing and discontinued operations equals reported net loss attributable to restricted voting shareholders—from continuing and discontinued operations divided by the weighted-average number of restricted voting shares outstanding during the year. Diluted income per share would reflect the assumed conversion of all dilutive securities using the treasury stock method.
Under the treasury stock method:
| |
| • | the exercise of options is assumed to be at the beginning of the period (or at the time of issuance, if later); |
| |
| • | options for which the closing fair market value exceeds the option price are the only ones that are assumed to be |
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
dilutive;
| |
| • | the proceeds from the exercise of options, plus future period compensation expense on options granted on or after November 1, 2003, are assumed to be used to purchase restricted voting shares at the average price during the period; |
| |
| • | the number of restricted voting shares assumed to be dilutive, plus the weighted-average number of restricted voting shares outstanding during the year, is used in the denominator of the diluted earnings per share computation; and |
| |
| • | the convertible preferred shares are assumed to have been converted at the beginning of the year (or at time of issuance, if later), and the resulting restricted voting shares are included in the denominator. |
Since the Company was in a loss position for the fiscal year ended October 31, 2011 ("fiscal 2011") , fiscal 2010 and the fiscal year ended October 31, 2009 ("fiscal 2009"), there is no dilutive effect.
Government financing
The Company makes periodic applications for financial assistance under available government assistance programs in the various jurisdictions in which it operates. Grants relating to capital expenditures are reflected as a reduction of the cost of the related assets. Grants and tax credits relating to current operating expenditures are generally recorded as a reduction of expenses at the time the eligible expenses are incurred. In the case of certain foreign subsidiaries, the Company receives investment incentive allowances, which are accounted for as a reduction of income tax expense.
Recently issued accounting pronouncements
Future accounting changes (U.S. GAAP and International Financial Reporting Standards)
In February 2008, the Canadian Accounting Standards Board confirmed that publicly accountable enterprises will be required to adopt International Financial Reporting Standards (“IFRSs”) in place of Canadian GAAP for interim and annual reporting purposes for fiscal years beginning on or after January 1, 2011, unless, as permitted by Canadian securities regulations, registrants adopt U.S. generally accepted accounting principles (“U.S. GAAP”) on or before this date. The Company filed a registration statement with the United States Securities and Exchange Commission (the “SEC”) on February 25, 2011 that became effective on April 26, 2011. As a consequence, the Company will convert to and report under U.S. GAAP beginning with the fiscal year ending October 31, 2012 ("fiscal 2012"). As a result, the Company will not adopt IFRSs on November 1, 2011.
Additional Pronouncements
Canadian Institute of Chartered Accountants (“CICA”) Section 1582, “Business Combinations,” Section 1601, “Consolidations,” Section 1602, “Non-controlling Interests,” and Emerging Issues Committee ("EIC") abstract EIC-175, “Multiple Deliverable Revenue Arrangements” all apply to annual and interim financial statements for fiscal years beginning on or after January 1, 2011, with early adoption permitted. The Company will convert to and report under U.S. GAAP beginning with fiscal 2012, and therefore will not be adopting these pronouncements.
3. DISCONTINUED OPERATIONS AND PLANT CONSOLIDATIONS
Puerto Rico
The Company announced on December 10, 2009 its plan to consolidate its Puerto Rico operations into its manufacturing site located in Manati and ultimately close or sell its plant in Caguas. During fiscal 2010, the Company received a letter of intent for the purchase of its Caguas facility for a purchase price of $7.0 million, which resulted in the Company increasing the impairment charge related to the value of the land to $3.6 million from the initial impairment amount of $1.3 million recorded earlier in fiscal 2010. As a result of additional time required to transition manufacturing operations from Caguas to Manati due to longer than expected customer regulatory time lines and increased product demand, the Company now expects the transition to continue beyond the end of calendar year 2012. Therefore, the letter of intent was rescinded, and the extended time-frame will result in additional repositioning costs. The Company has increased its estimated total project repositioning expenses from $9.0 million to $11.5 million, of which an additional $4.1 million in repositioning expenses were incurred in fiscal 2011. The
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
consolidation also results in additional accelerated depreciation of Caguas assets of approximately $12.0 million over the life of the project. Because the business in the Caguas facility is being transferred within the existing site network, its results of operations are included in continuing operations.
The Company closed its Carolina facility in Puerto Rico effective January 31, 2009. In the second half of fiscal 2010 the Company received a letter of intent on the property which led management to complete another impairment analysis and completely write down the assets as the fair value less the cost to sell was zero. The results of the Carolina operations have been reported in discontinued operations in fiscal 2011, 2010 and 2009.
The results of discontinued operations for Carolina for fiscal 2011, 2010 and 2009 are as follows:
|
| | | | | | | | |
| | Years ended October 31, |
| | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Revenues | — |
| | — |
| | 2.6 |
|
| Cost of goods sold | — |
| | — |
| | 3.5 |
|
| Gross loss | — |
| | — |
| | (0.9 | ) |
| Selling, general and administrative expenses | 0.6 |
| | 0.9 |
| | 2.8 |
|
| Repositioning expenses (Note 18) | — |
| | — |
| | 3.4 |
|
| Operating loss | (0.6 | ) | | (0.9 | ) | | (7.1 | ) |
| Asset impairment charge | — |
| | 0.8 |
| | 0.7 |
|
| Loss before income taxes | (0.6 | ) | | (1.7 | ) | | (7.8 | ) |
| Net loss for the period | (0.6 | ) | | (1.7 | ) | | (7.8 | ) |
For fiscal 2010 and 2009, the Company recorded impairment charges of $0.8 million and $0.7 million, respectively, to write down the carrying value of the Carolina operations long-lived assets to their fair value less cost to sell, based on discussions with third parties interested in purchasing the facility.
Canada
In connection with the restructuring of its network of pharmaceutical manufacturing facilities within Canada, the Company closed its York Mills facility and transferred all commercial production and development services undertaken at its York Mills facility to its site in Whitby. The Company exited the York Mills facility in the third quarter of fiscal 2009. Because the business in the York Mills facility was transferred within the existing site network, its results of operations were included in continuing operations.
4. INVENTORIES
|
| | | | | |
| | As of October 31, |
| | 2011 | | 2010 |
| | $ | | $ |
| Raw materials, packaging components and spare parts | 53.0 |
| | 47.3 |
|
| Work-in-process | 28.8 |
| | 26.0 |
|
| Balance, end of the year | 81.8 |
| | 73.3 |
|
Below is a roll forward of the Company's inventory provisions for fiscal 2011 and 2010:
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | |
| | As of October 31, |
| | 2011 | | 2010 |
| | $ | | $ |
| Balance, beginning of the year | (7.8 | ) | | (5.0 | ) |
| Additions | (7.6 | ) | | (5.7 | ) |
| Write-offs | 2.8 |
| | 2.9 |
|
| Balance, end of the year | (12.6 | ) | | (7.8 | ) |
5. CAPITAL ASSETS
|
| | | | | | | | | | | | | | | | | |
| | As of October 31, |
| | 2011 | | 2010 |
| | Cost | | Accumulated Depreciation | | Net Book Value | | Cost | | Accumulated Depreciation | | Net Book Value |
| | $ | | $ | | $ | | $ | | $ | | $ |
| Land | 30.9 |
| | — |
| | 30.9 |
| | 30.6 |
| | — |
| | 30.6 |
|
| Buildings | 309.9 |
| | 86.7 |
| | 223.2 |
| | 283.1 |
| | 71.2 |
| | 211.9 |
|
| Machinery and equipment | 451.2 |
| | 269.8 |
| | 181.4 |
| | 430.1 |
| | 238.8 |
| | 191.3 |
|
| Office equipment (including software) | 43.6 |
| | 39.3 |
| | 4.3 |
| | 41.4 |
| | 36.0 |
| | 5.4 |
|
| Furniture and fixtures | 17.5 |
| | 12.9 |
| | 4.6 |
| | 17.7 |
| | 12.0 |
| | 5.7 |
|
| Construction in progress | 30.5 |
| | — |
| | 30.5 |
| | 33.4 |
| | — |
| | 33.4 |
|
| Balance, end of the year | 883.6 |
| | 408.7 |
| | 474.9 |
| | 836.3 |
| | 358.0 |
| | 478.3 |
|
The amount of open purchase commitments related to authorized capital projects at October 31, 2011 and 2010 was approximately $5.9 million and $13.3 million, respectively. The expenditures are expected to be incurred during fiscal 2012.
Included in capital assets are assets under capital leases with a cost of $7.5 million and $16.0 million at October 31, 2011 and 2010, respectively. The amount of accumulated depreciation for assets under capital leases is $7.5 million and $4.1 million at October 31, 2011 and 2010, respectively.
6. INTANGIBLE ASSETS
|
| | | | | |
| | As of October 31, |
| | 2011 | | 2010 |
| | $ | | $ |
| Balance, beginning of the year | 1.4 |
| | 3.2 |
|
| Additions | — |
| | 0.2 |
|
| Amortization | (1.4 | ) | | (2.0 | ) |
| Balance, end of the year | — |
| | 1.4 |
|
7. SHORT-TERM BORROWINGS
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | |
| | As of October 31, |
| | 2011 | | 2010 |
| | $ | | $ |
| Italian short-term operating credit facilities totaling €17.0 million (October 2010—€17.0 million), bearing interest at 3-month Euribor plus spreads between 0.5% and 1.25%. | 4.2 |
| | — |
|
| Short-term insurance premium financing | 1.9 |
| | 2.0 |
|
| Balance, end of the period | 6.1 |
| | 2.0 |
|
8. LONG-TERM DEBT
Long-term debt in the accompanying consolidated balance sheets at October 31, 2011 and 2010 consists of the following:
|
| | | | | |
| | As of October 31, |
| | 2011 | | 2010 |
| | $ | | $ |
| 8.625% senior secured notes due April 15, 2017 | 280.0 |
| | 280.0 |
|
| U.S. obligations under capital leases bearing interest at fixed rate of 5.6%, maturing November 2011. | 0.1 |
| | 0.6 |
|
| U.K. obligations under capital lease bearing interest at 6%, maturing in 2012, payable in Euros. | — |
| | 2.1 |
|
| Italian unsecured government loan, bearing interest at 0.9% per annum, maturing in 2012. | 1.0 |
| | 1.9 |
|
| Total long-term debt outstanding | 281.1 |
| | 284.6 |
|
| Add call option premium on high yield bonds | 0.8 |
| | 0.9 |
|
| Less unamortized transaction costs | (6.2 | ) | | (7.2 | ) |
| Less current portion | (1.1 | ) | | (3.5 | ) |
| Balance, end of the period | 274.6 |
| | 274.8 |
|
In April 2010, the Company issued $280.0 million in aggregate principal amount of 8.625% senior secured notes due April 15, 2017 (“the Notes”) in a private placement. The net proceeds from the Notes were used to repay all of the outstanding indebtedness under the Company’s then-existing senior secured term loan and $75.0 million asset-based revolving credit facility (“ABL”), repay certain other indebtedness and to pay related fees and expenses. The remaining proceeds were used for general corporate purposes.
The Company also amended and restated its existing $75.0 million ABL. Under the amended terms of the ABL, the maturity date was extended from 2012 to 2014.
Interest on the Notes accrues at the rate of 8.625% per annum and is payable semi-annually in arrears on April 15 and October 15, commencing on October 15, 2010 to the holders of Notes of record on the immediately preceding April 1 and October 1, respectively. Interest on the Notes accrues from the most recent date to which interest has been paid or, if no interest has been paid, from and including the issue date. Interest on the Notes is computed on the basis of a 360-day year comprised of twelve 30-day months.
At any time prior to April 15, 2013, the Company may redeem all or a part of the Notes, at a redemption price equal to the greater of (i) 101% of the principal amount of the Notes redeemed and (ii) the sum of the 2013 redemption price set forth in the table below and all interest payments on the Notes redeemed that would be required from the date of redemption (the “Redemption Date”) through April 15, 2013, discounted back to the Redemption date, subject to the rights of holders on the relevant record date to receive interest due on the relevant interest payment date.
In addition, until April 15, 2013, the Company may redeem up to 35.0% of the aggregate principal amount of the Notes at a redemption price equal to 108.625% of the aggregate principal amount thereof, plus accrued and unpaid interest thereon, if any, to the applicable redemption date, subject to the right of holders on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds of one or more equity offerings. At least 65.0% of the sum of the aggregate principal amount of the Notes originally issued under the indenture and any additional notes issued under the indenture after the original issue date must remain outstanding immediately after each redemption, and each redemption must occur within 90 days after the related equity offering.
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
Additionally, at any time prior to April 15, 2013, the Company may redeem a portion of the Notes, at a redemption price equal to 103.000% of the principal amount thereof, plus accrued and unpaid interest thereon, if any, to the applicable date of redemption, subject to the rights of holders on the relevant record date to receive interest due on the relevant interest payment date; provided that in no event may the Company redeem more than 10.0% of the original aggregate principal amount of the Notes and any additional notes during any twelve-month period.
On and after April 15, 2013, the Company may redeem the Notes, in whole or in part, at the redemption prices (expressed as percentages of the principal amount of the Notes to be redeemed) set forth below, plus accrued and unpaid interest thereon, if any, to the applicable redemption date, subject to the rights of holders on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on April 15 of each of the years indicated below:
|
| | | |
| Year | | % |
| 2013 | | 106.469 |
|
| 2014 | | 104.313 |
|
| 2015 | | 102.156 |
|
| 2016 and thereafter | | 100.000 |
|
The agreements that govern the terms of the Company’s debt, including the indenture that governs the Notes and the credit agreement that governs the ABL, contain covenants that restrict the Company’s ability and the ability of its subsidiaries to, among other things:
| |
| • | incur additional indebtedness; |
| |
| • | issue additional equity; |
| |
| • | pay dividends on or make distributions in respect of capital stock or make certain other restricted payments or investments; |
| |
| • | enter into agreements that restrict distributions from subsidiaries or restrict its ability to incur liens on certain of its assets; |
| |
| • | make capital expenditures; |
| |
| • | sell or otherwise dispose of assets, including capital stock of subsidiaries; |
| |
| • | enter into transactions with affiliates; |
| |
| • | create or incur liens; and |
Provided that the Company is not in default under the ABL or the indenture governing the Notes and is able to demonstrate a certain Fixed Charge Coverage Ratio (as defined in the indenture governing the Notes), and will have a required minimum amount of remaining borrowing availability under the ABL after giving effect thereto, the Company is permitted to pay limited amounts of dividends or other distributions with respect to its common stock (as more particularly described in the ABL and the indenture governing the Notes, up to the lesser of (i) $15.0 million plus 50.0% of Excess Cash Flow (as defined in the ABL), plus net proceeds of additional permitted equity offerings under the ABL, and (ii) 50.0% of Consolidated Net Income (as defined in the indenture governing the Notes) plus net proceeds from additional permitted equity offerings or sales of restricted investments under the Notes).
In addition, under the ABL, if an event of default occurs or the Company’s borrowing availability falls below the greater of $10.0 million and 13.3% of total commitments under the ABL for any two consecutive days (which is defined under the ABL as an "Availability Trigger Event"), the Company will be required to satisfy and maintain a ratio of Consolidated EBITDA to Consolidated Fixed Charges (each as defined in the ABL) of not less than 1.10 to 1.00 until the first day thereafter on which, as applicable, either the event of default has been cured or its borrowing availability has exceeded the greater of $10.0 million and 13.3% of its total commitments for 30 consecutive days. The Company’s ability to meet the required ratio can be affected by events beyond the Company’s control, and the Company may not be able to meet this ratio. A breach of any of these
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
covenants could result in a default under the ABL.
The Notes and the ABL are secured by substantially all of our assets and guaranteed by, and secured by substantially all of the assets of, the Company's subsidiaries in the United States (including Puerto Rico), Canada, the United Kingdom (except Patheon UK Pension Trustees Limited) and the Netherlands. The Notes and the ABL are guaranteed on a limited basis by, and secured by certain assets of, the Company’s subsidiaries in France, Italy and Switzerland.
The Notes indenture contains language consistent with the ABL, which contains usual and customary covenants and events of default provisions.
Estimated minimum annual repayments of long-term debt based on current exchange rates for the next five years are:
|
| | |
| | $ |
| 2012 | 1.1 |
|
| 2013 | — |
|
| 2014 | — |
|
| 2015 | — |
|
| 2016 | — |
|
| Thereafter | 280.0 |
|
| Total payments | 281.1 |
|
Included within the above future repayments of long-term debt are obligations under capital leases. Future minimum capital lease payments under capital leases in effect at October 31, 2011 are as follows:
|
| | |
| | $ |
| 2012 | 0.1 |
|
| 2013 | — |
|
| 2014 | — |
|
| 2015 | — |
|
| Total payments | 0.1 |
|
| Less capital lease minimum payments representing interest | — |
|
| Total payments | 0.1 |
|
In the second quarter of fiscal 2011, the Company and a major customer agreed to terminate a supply contract. As part of the agreement, the customer agreed to relieve the Company of the obligation to pay the final amount for equipment under a capital lease maturing in fiscal 2012. This debt obligation was reclassified and amortized through deferred revenues.
9. OTHER LONG-TERM LIABILITIES
|
| | | | | |
| | As of October 31, |
| | 2011 | | 2010 |
| | $ | | $ |
| Unfunded termination indemnities (2011- €4.5 million; 2010—€4.6 million) | 6.2 |
| | 6.5 |
|
| Employee future benefits | 6.1 |
| | 8.1 |
|
| Other long-term liabilities | 9.4 |
| | 8.3 |
|
| | 21.7 |
| | 22.9 |
|
The unfunded termination indemnities relate to the employees of the Company's Italian subsidiary. In accordance with Italian severance pay statutes, an employee benefit is accrued for service to date and is payable when the employee’s employment with the Company ceases. The termination indemnity liability is calculated in accordance with local civil and labor laws based on each employee's length of service, employment category and remuneration. The termination liability is adjusted annually by a cost-of-living index provided by the Italian government. Although there is no vesting period, the Italian
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
government has established private accounts for these benefits and has required the Company to contribute $3.4 million in fiscal 2011 and $3.3 million in fiscal 2010 to these accounts, with additional contributions in the future. The liability recorded in the consolidated balance sheets is the amount to which the employees would be entitled if their employment with the Company ceased. The related expenses for fiscal 2011, 2010 and 2009 amounted to $3.1 million, $2.5 million and $2.5 million, respectively.
Other long-term liabilities at October 31, 2011, include $4.0 million of customer funded capital liabilities, $1.9 million for a deferred compensation plan, $1.1 million of deferred rent liability, and $0.9 million of post employment benefits. Other long-term liabilities at October 31, 2010, include $3.1 million of customer funded capital liabilities, $0.5 million of post employment benefits, $1.9 million for a deferred compensation plan and $0.9 million of deferred rent liability.
10. EMPLOYEE FUTURE BENEFITS
The Company has a number of defined benefit pension plans. In addition, it has other benefit plans that provide post-retirement healthcare and dental benefits. The Company measured the accrued benefit obligation and the fair value of plan assets for accounting purposes as of October 31, 2011 for the defined benefit pension and other benefit plans.
Information about the Company's defined benefit pension and other benefit plans, in aggregate, is as follows:
|
| | | | | | | |
| | Defined Benefit Pension Plans 2011 | | Other Benefit Plans 2011 | | Defined Benefit Pension Plans 2010 | | Other Benefit Plans 2010 |
| | $ | | $ | | $ | | $ |
| Change in benefit obligation | | | | | | | |
| Benefit obligation, beginning of the year | 93.6 | | 6.4 | | 77.7 | | 5.3 |
| Current service cost | 4.0 | | 0.1 | | 3.1 | | 0.1 |
| Interest cost | 5.3 | | 0.4 | | 4.5 | | 0.3 |
| Member contributions during the year | 1.4 | | — | | 0.9 | | — |
| Benefits paid | (2.7) | | (0.2) | | (2.3) | | (0.1) |
| Actuarial loss | 8.7 | | 0.1 | | 9.5 | | 0.5 |
| Currency translation | 0.6 | | 0.1 | | 0.2 | | 0.3 |
| Benefit obligation, end of the year | 110.9 | | 6.9 | | 93.6 | | 6.4 |
| Change in plan assets | | | | | | | |
| Market value of plan assets, beginning of year | 66.0 | | — | | 55.5 | | — |
| Actual return on plan assets | 3.4 | | — | | 6.5 | | — |
| Member contributions during the year | 1.4 | | — | | 0.9 | | — |
| Employer contributions | 11.6 | | 0.2 | | 4.6 | | 0.1 |
| Benefits paid | (2.7) | | (0.2) | | (2.2) | | (0.1) |
| Currency translation | 0.6 | | — | | 0.7 | | — |
| Market value of plan assets, end of the year | 80.3 | | — | | 66.0 | | — |
| Reconciliation of funded status | | | | | | | |
| Funded status, deficit | (30.6) | | (7.0) | | (27.6) | | (6.4) |
| Unamortized net actuarial loss | 33.8 | | 0.9 | | 25.1 | | 0.8 |
| Prepaid asset (accrued benefit liability) | 3.2 | | (6.1) | | (2.5) | | (5.6) |
The accrued benefit liability of $6.1 million for the other benefit plan is in other long-term liabilities and the defined benefit plan plan is a prepaid asset of $3.2 million in other long-term assets. In fiscal 2010 the $8.1 million accrued benefit liability for both sets of plans was included in other long-term liabilities. For all of the Company’s plans, the accrued benefit obligations are in excess of plan assets as of October 31, 2011 and 2010. Please refer to Note 9—Other Long-Term Liabilities.
Defined benefit pension plan assets consist of:
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | |
| | 2011 | | 2010 |
| | % | | % |
| Equity securities | 76 |
| | 76 |
|
| Debt securities | 20 |
| | 21 |
|
| Other | 4 |
| | 3 |
|
| Total | 100 |
| | 100 |
|
The significant actuarial assumptions adopted in measuring the Company's accrued benefit obligation and benefit plan expense in connection with its defined benefit pension and other benefit plans is as follows (weighted-average assumptions as of October 31, 2011 and 2010):
|
| | | | | | | | | | | |
| | Defined Benefit Pension Plans 2011 | | Other Benefit Plans 2011 | | Defined Benefit Pension Plans 2010 | | Other Benefit Plans 2010 |
| | % | | % | | % | | % |
| Accrued benefit obligation | | | | | | | |
| Discount rate | 4.9 |
| | 5.2 |
| | 5.3 |
| | 5.3 |
|
| Rate of compensation increase | 3.7 |
| | — |
| | 3.9 |
| | — |
|
| Benefit costs recognized | | | | | | | |
| Discount rate | 5.2 |
| | 5.2 |
| | 5.8 |
| | 5.3 |
|
| Expected long-term rate of return on plan assets | 6.9 |
| | — |
| | 6.9 |
| | — |
|
| Rate of compensation increase | 3.9 |
| | — |
| | 4.1 |
| | — |
|
In connection with the other benefit plans, a 4% to 10% annual rate of increase in the per capita cost of covered health care and dental benefits was assumed for 2011 (4% to 11% 2010). The rate was assumed to decrease gradually over the next five years to 6% and remain at that level thereafter (2010—6% and thereafter). The following table outlines the effects of a one-percentage-point increase and decrease in the assumed health care and dental benefit trend rates:
|
| | | | | |
| | Benefit Obligation | | Benefit Expense |
| | $ | | $ |
| Impact of: | | | |
| 1% increase | 1.0 |
| | 0.1 |
|
| 1% decrease | (0.8 | ) | | (0.1 | ) |
The cost components of the Company's defined benefit pension plan and other benefit plans in aggregate are as follows:
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | | | | | | | | |
| | Defined Benefit Pension Plans 2011 | | Other Benefit Plans 2011 | | Defined Benefit Pension Plans 2010 | | Other Benefit Plans 2010 | | Defined Benefit Pension Plans 2009 | | Other Benefit Plans 2009 |
| | $ | | $ | | $ | | $ | | $ | | $ |
| Current service cost | 4.0 |
| | 0.1 |
| | 3.1 |
| | 0.1 |
| | 3.0 |
| | — |
|
| Interest cost | 5.3 |
| | 0.4 |
| | 4.5 |
| | 0.3 |
| | 4.1 |
| | 0.3 |
|
| Actual return on plan assets | (3.4 | ) | | — |
| | (6.5 | ) | | — |
| | — |
| | — |
|
| Actuarial loss | 8.7 |
| | 0.1 |
| | 9.5 |
| | 0.5 |
| | 2.4 |
| | 0.5 |
|
| Elements of employee future benefit costs before adjustments to recognize the long-term nature of employee future benefits | 14.6 |
| | 0.6 |
| | 10.6 |
| | 0.9 |
| | 9.5 |
| | 0.8 |
|
| Adjustments to recognize the long-term nature of employee future benefit costs: | | | | | | | | | | | |
| Difference between expected return and actual return on plan assets for the year | (1.6 | ) | | — |
| | 2.6 |
| | — |
| | (4.6 | ) | | — |
|
| Difference between amortization of the net actuarial loss for the year and the actual actuarial loss on accrued benefit obligation for the year | (8.2 | ) | | (0.1 | ) | | (8.7 | ) | | (0.5 | ) | | (2.2 | ) | | (0.5 | ) |
| Difference between amortization of past service costs for the year and actual plan amendments for the year | — |
| | — |
| | — |
| | — |
| | 0.3 |
| | — |
|
| Net benefit cost recognized | 4.8 |
| | 0.5 |
| | 4.5 |
| | 0.4 |
| | 3.0 |
| | 0.3 |
|
The Company also provides retirement benefits for the majority of its employees at its Canadian, U.S. and Puerto Rican sites under defined contribution pension plans. The total expense for the plans amounted to $2.8 million, $2.8 million and $3.4 million for fiscal 2011, 2010 and 2009, respectively.
The net benefit cost recognized for fiscal 2009, includes a curtailment gain of $1.2 million arising from a decision to conform certain post-employment benefits in the Company’s Canadian operations.
Total cash payments for employee future benefits totaled $14.6 million, $7.5 million and $6.9 million for fiscal 2011, 2010 and 2009, consisting of cash contributed by the Company to its defined benefit pension plans of $11.6 million, $4.6 million and $3.3 million, cash payments directly to beneficiaries for its other benefit plans of $0.2 million, $0.1 million and $0.2 million and cash contributed to its defined contribution plans of $2.8 million, $2.8 million and $3.4 million, respectively.
11. SHAREHOLDERS’ EQUITY
Share capital
Share capital consists of the following:
|
| | | | | |
| | 2011 | | 2010 |
| | $ | | $ |
| Authorized | — |
| | — |
|
| Unlimited Class I preferred shares—Issuable from time to time in one or more series, each series comprising the number of shares and having the designation, rights, privileges, restrictions and conditions determined by the Company’s board of directors. | — |
| | — |
|
| Unlimited restricted voting shares. | | | |
| Issued and outstanding | | | |
| 150,000 Class I preferred shares consisting of 150,000 Series C (“convertible preferred shares”—converted July 29, 2009) and 150,000 Series D (“special voting preferred shares”) | — |
| | — |
|
| Restricted voting shares of 129,167,926; (2010—129,167,926) | 553.8 |
| | 553.8 |
|
Convertible preferred shares
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
On July 29, 2009, JLL Patheon Holdings converted its 150,000 Series C preferred shares into a total of 38,018,538 restricted voting shares of Patheon, in accordance with the convertible preferred share terms. As a result of this conversion, the Company no longer has any Series C preferred shares outstanding.
Upon expiry of JLL Patheon Holdings' unsolicited offer to acquire any or all of the outstanding restricted voting shares of Patheon (“JLL Offer”) on August 26, 2009, JLL Patheon Holdings and its affiliates (“JLL”) had acquired an aggregate of 33,854,708 restricted voting shares that were validly deposited. The restricted voting shares that JLL purchased pursuant to the JLL Offer represented approximately 38% of the outstanding restricted voting shares of the Company not already owned by JLL. As of October 31, 2011, JLL owned an aggregate of 72,077,781 Patheon restricted voting shares, representing approximately 56% of Patheon’s total restricted voting shares outstanding.
Restricted voting shares
The Company’s articles were amended on April 26, 2007 to re-designate the common shares as restricted voting shares. This occurred in connection with the issuance of the Series D special voting preferred shares. The holders of the Series D special voting preferred shares have the right to elect three of nine members of the Board of Directors. The holders of Patheon’s restricted voting shares have the right to elect the remaining members of the Board of Directors. Under the rules of the TSX, voting equity securities are not to be designated, or called, common shares unless they have a right to vote in all circumstances that is not less, on a per share basis, than the voting rights of each other class of voting securities. Accordingly, the Company amended its articles to re-designate the common shares as restricted voting shares. This re-designation involved only a change in the name of the securities; the number of shares outstanding and the terms and conditions of the outstanding shares were not affected by the change.
Incentive stock option plan
The Company has an incentive stock option plan in which directors, officers and key employees of the Company and its consolidated subsidiaries, as well as other persons engaged to provide ongoing management or consulting services to Patheon, are eligible to participate. On March 10, 2011, the Company's shareholders approved an amendment to the stock option plan, which, among other things, provides that the maximum number of shares that may be issued under the plan is 15,500,151, which currently represents 12% of the issued and outstanding restricted voting shares. The plan previously provided that the maximum number of shares that may be issued under the plan was 7.5% of the sum, at any point in time, of the issued and outstanding restricted voting shares of the Company and the aggregate number of restricted voting shares issuable upon exercise of the conversion rights attached to the issued and outstanding Class I Preferred Shares, Series C of the Company. As of October 31, 2011, 2010 and 2009, the total number of restricted voting shares issuable under the plan was 15,500,151 shares, 9,687,594 shares and 9,687,594 shares, respectively, of which there were stock options outstanding to purchase 12,628,458 shares, 8,327,357 shares and 4,699,348 shares, respectively, under the plan. Before the March 2011 amendments, the plan provided that the exercise prices of options were determined at the time of grant and could not be less than the weighted-average market price of the Company's restricted voting shares on the TSX during the two trading days immediately preceding the grant date. Following the March 2011 amendments, the exercise prices of the options may not be less than the closing price of the restricted voting shares on the TSX (or on such other stock exchange in Canada or the United States on which restricted voting shares may be then listed and posted) on the grant date. Options generally expire in no more than 10 years after the grant date and are subject to early expiry in the event of death, resignation, dismissal or retirement of an optionee. Options have vesting periods of either three years or five years, with either one-third or one-fifth vesting on each anniversary of the grant date, respectively.
A summary of the plan and changes during each of fiscal 2011, 2010 and 2009 are as follows:
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | | | | | | | | |
| | 2011 | | 2010 | | 2009 |
| (Dollar amounts in Canadian dollars) | Shares Number | | Weighted- Average Exercise Price | | Shares Number | | Weighted- Average Exercise Price | | Shares Number | | Weighted- Average Exercise Price |
| | | | $ | | | | $ | | | | $ |
| Outstanding, beginning of the year | 8,327,357 |
| | 3.26 |
| | 4,699,348 |
| | 5.12 |
| | 5,987,965 |
| | 6.22 |
|
| Granted | 5,862,000 |
| | 2.50 |
| | 4,741,000 |
| | 2.59 |
| | 438,571 |
| | 2.58 |
|
| Exercised | — |
| | — |
| | — |
| | — |
| | — |
| | — |
|
| Forfeited | (1,560,899 | ) | | 3.34 |
| | (1,112,991 | ) | | 8.23 |
| | (1,727,188 | ) | | 8.32 |
|
| Outstanding, end of the year | 12,628,458 |
| | 2.88 |
| | 8,327,357 |
| | 3.26 |
| | 4,699,348 |
| | 5.12 |
|
| Exercisable, end of the year | 3,893,124 |
| | 3.65 |
| | 2,542,246 |
| | 4.52 |
| | 2,451,187 |
| | 6.88 |
|
The following table summarizes information about options outstanding at October 31, 2011:
|
| | | | | | | | | | | | | |
| (Dollar Amounts in Canadian dollars) | Options Outstanding | | Options Exercisable |
| Range of Exercise Prices | Number Outstanding | | Weighted- Average Remaining Contractual Life | | Weighted- Average Exercise Price | | Number Exercisable | | Weighted- Average Exercise Price |
| | | | | | $ | | | | $ |
| $1.54 - 2.59 | 2,930,667 |
| | 8.1 years | | 2.36 |
| | 743,467 |
| | 2.58 |
|
| $2.60- 2.88 | 7,043,967 |
| | 9.0 years | | 2.61 |
| | 495,833 |
| | 2.60 |
|
| $2.89 – 3.20 | 1,362,833 |
| | 3.2 years | | 3.14 |
| | 1,362,833 |
| | 3.14 |
|
| $3.21– 13.21 | 1,290,990 |
| | 3.2 years | | 5.20 |
| | 1,290,991 |
| | 5.20 |
|
| Total | 12,628,457 |
| | | | | | 3,893,124 |
| | |
The Company did not issue any restricted voting shares under the stock option plan during fiscal 2011, 2010 and 2009.
For the purposes of calculating the stock-based compensation expense in connection with the Company’s incentive stock option plan, the fair value of stock options is estimated at the date of the grant using the Black-Scholes option pricing model and the cost is amortized over the vesting period.
The weighted-average fair value of stock options granted during fiscal 2011, 2010 and 2009 was $1.30, $1.39 and $1.40, respectively. The fair value of stock options for purposes of determining stock-based compensation was estimated on the date of grant using the following assumptions:
|
| | | | | | | | |
| | 2011 | | 2010 | | 2009 |
| Risk free interest rate | 2.5 | % | | 2.7 | % | | 2.8 | % |
| Expected volatility | 59 | % | | 60 | % | | 62 | % |
| Expected weighted-average life of options | 5 years |
| | 5 years |
| | 5 years |
|
| Expected dividend yield | 0 | % | | 0 | % | | 0 | % |
The Company recorded stock-based compensation expense in fiscal 2011, 2010 and 2009 of $3.5 million, $2.3 million and $1.0 million, respectively, for options granted on or after November 1, 2003.
12. OTHER INFORMATION
Foreign exchange
During fiscal 2011, 2010 and 2009, the Company recorded a foreign exchange gain on cash flow hedges and transactions related to operating exposures of $1.6 million and $1.5 million, and a loss of $7.0 million, respectively.
Net change in non-cash working capital balances related to continuing operations
The net changes in non-cash working capital balances related to continuing operations are as follows:
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | |
| | 2011 | | 2010 |
| | $ | | $ |
| Accounts receivable | (17.4 | ) | | 9.4 |
|
| Inventories | (8.5 | ) | | 3.7 |
|
| Income taxes receivable | 2.6 |
| | (2.6 | ) |
| Prepaid expenses and other | (2.1 | ) | | 1.4 |
|
| Accounts payable and accrued liabilities | 26.7 |
| | (13.1 | ) |
| Income taxes payable | (0.3 | ) | | (1.4 | ) |
| | 1.0 |
| | (2.6 | ) |
Related party transactions
Revenues for contract manufacturing from a company controlled by Joaquin B. Viso (the “Viso Affiliate”), a director and significant shareholder of the Company, were approximately less then $0.1 million, $0.1 million and $0.8 million for fiscal 2011, 2010 and 2009, respectively. These transactions were conducted in the normal course of business and are recorded at the exchanged amounts. Accounts receivable at October 31, 2011 and 2010 were nil and less than $0.1 million, respectively, resulting from these transactions. In addition, Patheon manufactures a product for a third party for which the product’s intellectual property is owned by the Viso Affiliate. The manufacturing agreement was originally contracted between the third party and the Viso Affiliate, but has been administered directly between Patheon and the third party on normal commercial terms since 2003.
As of October 31, 2011 and 2010, the Company had an investment of $3.3 million and $3.3 million, respectively, representing an 18% interest in two Italian companies (collectively referred to as “BSP Pharmaceuticals”) whose largest investor was an officer of the Company until December 31, 2009. These companies specialize in the manufacture of cytotoxic pharmaceutical products. As a result of the shareholders’ agreement with the other investors in BSP Pharmaceuticals that provides the Company with significant influence over BSP Pharmaceuticals’ operations, the Company accounts for its investment in BSP Pharmaceuticals using the equity method. Accordingly, for fiscal 2011, 2010 and 2009, the Company recorded investment income of less than $0.1 million, $0.4 million and losses of $0.4 million, respectively.
In connection with its investment in BSP Pharmaceuticals, the Company has a management services agreement with BSP Pharmaceuticals that provides on-going sales and marketing services, and provided engineering and operational services during the construction of the BSP facility which was completed in 2008. There were no management fees recorded under this agreement for fiscal 2011, 2010 and 2009. Accounts receivable at October 31, 2011 and 2010 include a balance of $1.2 million and $2.2 million, respectively, in connection with the management services agreement. These services were conducted in the normal course of business and are recorded at the exchanged amounts.
In connection with certain of BSP Pharmaceuticals’ bank financing, the Company had made commitments that it will not dispose of its interest in BSP Pharmaceuticals prior to January 1, 2011, and if needed, irrevocably inject equity (pro-rata) in order to ensure BSP complies with certain specific bank covenants.
On August 27, 2009, in response to the Company’s legal action, JLL Patheon Holdings commenced a legal action against each of the then current and former members of the Special Committee in respect of such members’ conduct in connection with the JLL Offer and other related matters. On November 30, 2009, the Special Committee of the Company’s Board of Directors and JLL Patheon Holdings announced that they entered into a settlement agreement in respect of the pending legal actions between the parties, which was confirmed by the courts on December 4, 2009. As part of this settlement the Company paid JLL Patheon Holdings $1.5 million and Joaquin B. Viso $0.4 million.
In fiscal 2010, Patheon and JLL Partners Inc. ("JLL Partners") entered into a cost sharing arrangement with respect to certain third party consulting fees. The cost sharing arrangement between JLL Partners and Patheon was terminated during the first quarter of fiscal 2011, and there are no outstanding payables to JLL Partners related to this arrangement.
13. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT
Categories of financial assets and liabilities
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
Under Canadian GAAP financial instruments are classified into one of the following five categories: held-for-trading, held to maturity investments, loans and receivables, available-for-sale financial assets and other financial liabilities. The Company has also designated certain of its derivatives as effective hedges. The carrying values of the Company’s financial instruments, including those held for sale on the consolidated balance sheets are classified into the following categories:
|
| | | | | |
| As of October 31, | 2011 | | 2010 |
| | $ | | $ |
Held-for-trading1 | 33.4 |
| | 53.5 |
|
Loans and receivables2 | 158.0 |
| | 139.9 |
|
Other financial liabilities3 | 463.0 |
| | 437.0 |
|
Derivatives designated as effective hedges4—gain (loss) | (0.5 | ) | | 1.3 |
|
Other derivatives5 | 0.1 |
| | 0.7 |
|
_____________________________
| |
| (1) | Includes cash and cash equivalents in bank accounts bearing interest rates up to 1%. |
| |
| (2) | Includes accounts receivable. |
| |
| (3) | Includes bank indebtedness, accounts payable, accrued liabilities and long-term debt. |
| |
| (4) | Includes the Company’s forward contracts and collars in 2011 and forward contracts in 2010. |
| |
| (5) | Includes the embedded call option on the Notes. |
The Company has determined the estimated fair values of its financial instruments based on appropriate valuation methodologies; however, considerable judgment is required to develop these estimates. The fair values of the Company’s financial instruments are not materially different from their carrying values.
As of October 31, 2011 and 2010, the carrying amount of the financial assets that the Company has pledged as collateral for its long-term debt facilities was $106.7 million and $101.5 million, respectively. Please refer to Note 8—Long Term Debt.
Fair value measurements
The Company classifies and discloses its fair value measurements using a fair value hierarchy that reflects the significance of the inputs used in making the measurements. The fair value hierarchy has the following levels: (a) quoted prices (unadjusted) in active markets for identical assets or liabilities (Level 1); (b) inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e., as prices) or indirectly (i.e., derived from prices) (Level 2); and (c) inputs for the asset or liability that are not based on observable market data (unobservable inputs) (Level 3).
The fair value is principally applied to financial assets and liabilities such as derivative instruments consisting of interest rate swaps and foreign exchange forward contracts. The following table provides a summary of the financial assets and liabilities that are measured at fair value as of October 31, 2011 and 2010:
|
| | | | | | | | | | | | | | | | | | | | | | | |
| | Fair value measurement at October 31, 2011 using: | | Fair value measurement at October 31, 2010 using: |
| Assets measured at fair value | Level 1 | | Level 2 | | Level 3 | | Total | | Level 1 | | Level 2 | | Level 3 | | Total |
| | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ |
| Derivatives designated as hedging instruments: | | | | | | | | | | | | | | | |
| Foreign exchange forward contracts | — |
| | 1.2 |
| | — |
| | 1.2 |
| | — |
| | 1.3 |
| | — |
| | 1.3 |
|
| Total assets | — |
| | 1.2 |
| | — |
| | 1.2 |
| | — |
| | 1.3 |
| | — |
| | 1.3 |
|
| Derivatives not designated as hedging instruments: | | | | | | | | | | | | | | | |
| Embedded call option on Notes | — |
| | — |
| | 0.1 |
| | 0.1 |
| | — |
| | — |
| | 0.7 |
| | 0.7 |
|
| Total assets | — |
| | — |
| | 0.1 |
| | 0.1 |
| | — |
| | — |
| | 0.7 |
| | 0.7 |
|
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | | | | | | | | | | | | | | |
| | Fair value measurement at October 31, 2011 using: | | Fair value measurement at October 31, 2010 using: |
| Liabilities measured at fair value | Level 1 | | Level 2 | | Level 3 | | Total | | Level 1 | | Level 2 | | Level 3 | | Total |
| | $ | | $ | | $ | | $ | | $ | | $ | | $ | | $ |
| Derivatives designated as hedging instruments: | | | | | | | | | | | | | | | |
| Foreign exchange collars | — |
| | 1.7 |
| | — |
| | 1.7 |
| | — |
| | — |
| | — |
| | — |
|
| | — |
| | 1.7 |
| | — |
| | 1.7 |
| | — |
| | — |
| | — |
| | — |
|
Level 1—Based on quoted market prices in active markets.
Level 2—Inputs, other than quoted prices in active markets, that are observable, either directly or indirectly.
Level 3—Unobservable inputs that are not corroborated by market data.
The following table presents the fair value of the Company’s derivative financial instruments as well as their classification on the consolidated balance sheets as of October 31, 2011 and 2010:
|
| | | | | | | | | |
| | Asset Derivatives as of October 31, 2011 | | Asset Derivatives as of October 31, 2010 |
| Fair values of derivative instruments | Balance Sheet Location | | Fair Value | | Balance Sheet Location | | Fair Value |
| | | | $ | | | | $ |
| Derivatives designated as hedging instruments: | | | | | | | |
| Foreign exchange forward contracts | Prepaid expenses and other | | 0.2 |
| | Prepaid expenses and other | | 1.3 |
|
| Foreign exchange forward contracts | Other long-term assets | | 1.0 |
| | | | — |
|
| Total designated derivatives | | | 1.2 |
| | | | 1.3 |
|
| Derivatives not designated as hedging instruments: | | | | | | | |
| Embedded call option on Notes | Other long-term assets | | 0.1 |
| | Other long-term assets | | 0.7 |
|
| Total Non-designated Derivatives | | | 0.1 |
| | | | 0.7 |
|
|
| | | | | | | | | |
| | Liability Derivatives as of October 31, 2011 | | Liability Derivatives as of October 31, 2010 |
| | Balance Sheet Location | | Fair Value | | Balance Sheet Location | | Fair Value |
| | | | $ | | | | $ |
| Derivatives designated as hedging instruments: | | | | | | | |
| Foreign exchange collars | Accounts payable and acrued liabilities | | 1.4 |
| | | | — |
|
| Foreign exchange collars | Other long-term liabilities | | 0.3 |
| | | | — |
|
| Total designated derivatives | | | 1.7 |
| | | | — |
|
The Company has optional pre-payment clauses on the Notes, and is therefore required to account for the value of these optional pre-payment clauses separately as an embedded derivative under Canadian GAAP. The embedded derivative has been bifurcated from the Notes and recorded separately at fair value. In each subsequent period any change in fair value will be recorded as income or expenses in the Company’s consolidated statements of loss.
The Company uses valuations from a third party evaluator to assist in estimating the fair value of the embedded call option on the Notes. These third party valuations are completed on a quarterly basis, and take into consideration current market rates and trends. For the debt instruments with embedded options, evaluators determine the price both with and without the option; the price without the option is the “base price.” In the case of debt instruments with calls, the final evaluation is the
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
lesser of “base price” and “price with call.” The evaluator uses models that use the income approach, which discounts future cash flows to the net present value of the security, as the valuation technique.
The following table presents a reconciliation of the closing balance with respect to the Company’s only Level 3 financial instrument as of October 31, 2011:
Assets measured at fair value based on Level 3
|
| | | | | |
| (in millions of USD) | Embedded call option on Notes | | Total |
| | $ | | $ |
| Opening balance | 0.7 |
| | 0.7 |
|
| Purchases | — |
| | — |
|
| Issues | — |
| | — |
|
| Total gains (losses) | | | |
| In net loss | (0.6 | ) | | (0.6 | ) |
| In other comprehensive income | — |
| | — |
|
| Settlements | — |
| | — |
|
| Transfers out of Level 3 | — |
| | — |
|
| Closing balance (October 31, 2011) | 0.1 |
| | 0.1 |
|
Foreign exchange forward contracts, interest rate swaps and other hedging arrangements
The Company utilizes financial instruments to manage the risk associated with fluctuations in foreign exchange and interest rates. The Company formally documents all relationships between hedging instruments and hedged items, as well as its risk management objective and strategy for undertaking various hedge transactions.
As of October 31, 2011, the Company’s Canadian operations had entered into foreign exchange forward contracts to sell an aggregate amount of $32.7 million. These contracts hedge the Canadian operations’ expected exposure to U.S. dollar denominated cash flows and mature at the latest on July 10, 2013, at an average exchange rate of $1.040 Canadian. The mark-to-market value of these financial instruments as of October 31, 2011 was an unrealized gain of $1.2 million, which has been recorded in accumulated other comprehensive income in shareholders’ equity, net of associated income tax.
As of October 31, 2011, the Company's Canadian operations had entered into foreign exchange collars to sell an aggregate amount of US$106.9 million. These contracts hedge the Canadian operations' expected exposure to U.S. dollar denominated cash flows and mature at the latest on January 8, 2013, at an average exchange rate of $0.9824 Canadian. The mark-to-market value of these financial instruments as of October 31, 2011 was an unrealized loss of $1.7 million, which has been recorded in accumulated other comprehensive income in shareholders' equity, net of associated income tax.
Prior to the refinancing, the Company had entered into interest rate swap contracts to convert all of the interest costs on its senior secured term loan from a floating to a fixed rate of interest until June 30, 2010. In April 2010, the Company completed the private placement of the Notes, with net proceeds of $268.5 million. The net proceeds of the offering were used to repay all of the outstanding indebtedness under the Company’s then-existing senior secured term loan and $75.0 million ABL, to repay other indebtedness and to pay related fees and expenses. The Company used the remaining proceeds for general corporate purposes. Currently approximately 1% of the Company's debt is variable rate debt. The Company cancelled its interest rate swap on June 30, 2010, and at that time paid $1.8 million in hedging losses related to these financial instruments. These losses were recorded as part of the refinancing costs.
Risks arising from financial instruments and risk management
The Company’s activities expose it to a variety of financial risks: market risk (including foreign exchange and interest rate), credit risk and liquidity risk. The Company’s overall risk management program focuses on the unpredictability of financial markets and seeks to minimize potential adverse effects on the Company’s financial performance. The Company uses derivative financial instruments to hedge certain risk exposures. The Company does not purchase any derivative financial instruments for speculative purposes.
Risk management is the responsibility of the Company’s corporate finance team. The corporate finance team works with
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
the Company’s operational personnel to identify, evaluate and, where appropriate, hedge financial risks. The Company’s corporate finance team also monitors material risks and discusses them with the audit committee of the board of directors.
Foreign exchange risk
The Company operates in Canada, the United States, Puerto Rico, Italy, France, Switzerland, the United Kingdom and Japan. Foreign exchange risk arises because the value of the local currency receivable or payable for transactions denominated in foreign currencies may vary due to changes in exchange rates (“transaction exposures”) and because the non-U.S. dollar denominated financial statements of the Company may vary on consolidation into the reporting currency of U.S. dollars (“translation exposures”).
The Company’s most significant transaction exposures arise in its Canadian operations. Prior to the refinancing in the second quarter of fiscal 2010, the balance sheet of the Company’s Canadian division included U.S. dollar denominated debt which was designated as a hedge against the Company’s investments in subsidiaries in the United States and Puerto Rico. The foreign exchange gains and losses related to the effective portion of this hedge were recorded in other comprehensive income. In the third quarter of fiscal 2010, the Company changed the functional currency of its corporate division in Canada to U.S. dollars, thereby eliminating the need for the Company to designate this U.S. dollar denominated debt as a hedge. In addition, approximately 90% of the revenues of the Canadian operations and approximately 10% of its operating expenses are transacted in U.S. dollars. As a result, the Company may experience transaction exposures because of volatility in the exchange rate between the Canadian and U.S. dollar. Based on the Company’s current U.S. denominated net inflows, as of October 31, 2011, fluctuations of +/-10% would, everything else being equal, have an effect on loss from continuing operations before taxes of approximately +/- $12.4 million, prior to hedging activities.
The objective of the Company’s foreign exchange risk management activities is to minimize transaction exposures and the resulting volatility of the Company’s earnings. The Company manages this risk by entering into foreign exchange forward contracts. As of October 31, 2011, the Company has entered into forward foreign exchange contracts to cover approximately 80% of its Canadian-U.S. dollar cash flow exposures for fiscal 2012. The Company does not currently hedge any translation exposures.
Translation gains and losses related to certain foreign currency denominated intercompany loans are included as part of the net investment in certain foreign subsidiaries, and are included in accumulated other comprehensive income (loss) in shareholders’ equity.
Interest rate risk
Prior to the issuance of the Notes, the Company’s interest rate risk primarily arose from its floating rate debt, in particular its revolving debt, demand lines, senior secured term loan in North America and its Italian mortgages. The objective of the Company’s interest rate management activities is to minimize the volatility of the Company’s earnings. In order to manage this risk, the Company had entered into interest rate swaps to convert the interest expense on its senior secured term loan, until June 2010, from a floating interest rate to a fixed interest rate. In April 2010, the Company completed the private placement of the Notes. The net proceeds from the Notes were used to repay all of the outstanding indebtedness under the Company’s then-existing senior secured term loan and $75.0 million ABL, to repay certain other indebtedness and to pay related fees and expenses. The Company used the remaining proceeds for general corporate purposes. Currently approximately 1% of the Company's debt is variable rate debt. The Company cancelled the interest rate swaps on June 30, 2010, and paid $1.8 million in related hedging losses. These losses were accrued as part of the refinancing costs.
Credit risk
Credit risk arises from cash and cash equivalents held with banks and financial institutions, derivative financial instruments (foreign exchange forward contracts and interest rate swaps with positive fair values), and credit exposure to customers, including outstanding accounts receivable. The maximum exposure to credit risk is equal to the carrying value of the financial assets.
The objective of managing counterparty credit risk is to prevent losses in financial assets. The Company assesses the credit quality of the counterparties, taking into account their financial position, past experience and other factors. Management also monitors the utilization of credit limits regularly. In cases where the credit quality of a customer does not meet the
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
Company’s requirements, a cash deposit is received before any services are provided. As of October 31, 2011 and 2010, the Company held deposits of $17.9 million and $14.6 million, respectively.
The carrying amount of accounts receivable are reduced through the use of an allowance account and the amount of the loss is recognized in the consolidated statements of loss within operating expenses. When a receivable balance is considered uncollectible, it is written off against the allowance for accounts receivable. Subsequent recoveries of amounts previously written off are credited against operating expenses in the consolidated statements of loss.
The following table sets forth details of the age of receivables that are not overdue as well as an analysis of overdue amounts and related allowance for the doubtful accounts:
|
| | | | | |
| | As of October 31, |
| | 2011 | | 2010 |
| | $ | | $ |
| Total accounts receivable | 158.4 |
| | 140.8 |
|
| Less: Allowance for doubtful accounts | (0.4 | ) | | (0.9 | ) |
| | 158.0 |
| | 139.9 |
|
| Of which: | | | |
| Not overdue | 130.6 |
| | 116.6 |
|
| Past due for more than one day but for not more than three months | 21.9 |
| | 19.4 |
|
| Past due more for than three months but for not more than six months | 2.7 |
| | 3.2 |
|
| Past due for more than six months but not for more than one year | 1.4 |
| | 1.4 |
|
| Past due for more than one year | 1.8 |
| | 0.2 |
|
| Less: Allowance for doubtful accounts | (0.4 | ) | | (0.9 | ) |
| Total accounts receivable, net | 158.0 |
| | 139.9 |
|
Liquidity risk
Liquidity risk arises through excess of financial obligations over available financial assets due at any point in time. The Company’s objective in managing liquidity risk is to maintain sufficient readily available reserves in order to meet its liquidity requirements at all times. The Company mitigates liquidity risk by maintaining cash and cash equivalents on hand and through the availability of funding from credit facilities. As of October 31, 2011, the Company was holding cash and cash equivalents of $33.4 million and had undrawn lines of credit available to it of $94.9 million.
The contractual maturities of the Company’s financial liabilities are presented in Note 8—Long-Term Debt and reflect the impact of the refinancing in second quarter of fiscal 2010.
14. MANAGEMENT OF CAPITAL
The Company defines the capital that it manages as the aggregate of its shareholders’ equity and interest bearing debt. The Company’s objectives when managing capital are to ensure that the Company has adequate capital to achieve its business plans, so that it can provide products and services to its customers and returns to its shareholders.
In order to maintain or adjust its capital structure, the Company may adjust the type of capital utilized, including purchase versus lease decisions and issuing debt or equity securities, all subject to market conditions and the terms of the underlying third party agreements.
As of October 31, 2011 and 2010, total managed capital was $539.4 million and $551.3 million, respectively, comprised of shareholders’ equity of $263.7 million and $273.0 million, respectively and cash interest-bearing debt of $275.7 million and $278.3 million, respectively.
15. INCOME TAXES
The following is a reconciliation of the expected income tax (recovery) expense obtained by applying a single statutory tax rate of 26.75% (which is a mixture of federal and provincial rates) of its Canadian parent, to the loss from continuing
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
operations before income taxes.
|
| | | | | | | | |
| | As of October 31, |
| | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Expected income tax expense (recovery) using statutory tax rates | (2.4 | ) | | (1.8 | ) | | 4.5 |
|
| Change in valuation allowance | 4.0 |
| | (12.0 | ) | | (0.2 | ) |
| Permanent differences and other: | | | | | |
| Foreign | 2.3 |
| | 2.5 |
| | 2.5 |
|
| Domestic | (0.6 | ) | | (0.1 | ) | | 0.4 |
|
| Foreign rate differentials | 2.5 |
| | 8.4 |
| | 5.3 |
|
| Other | 1.5 |
| | — |
| | — |
|
| Provision for income taxes | 7.3 |
| | (3.0 | ) | | 12.5 |
|
| Effective tax rate | (82.0 | )% | | 47.6 | % | | 92.6 | % |
Permanent foreign differences reconciling expected income tax expense using statutory tax rates to the provision for income taxes were primarily meals & entertainment costs, employee costs, and salary & benefits that are not deductible in Italy, France, Canada and the United States. Other permanent domestic differences were primarily due to the book versus tax treatment of foreign exchange gains in Canada (resulting from the change in functional currency of the Company's corporate division in Canada to U.S. dollars as disclosed in the third quarter of fiscal 2010).
The tax effects of significant items comprising the Company’s net future income tax liabilities are as follows:
|
| | | | | |
| | 2011 | | 2010 |
| | $ | | $ |
| Net operating loss carryforward | 15.4 |
| | 26.3 |
|
| Accounting provisions not currently deductible for tax purposes | 2.5 |
| | 4.3 |
|
| Unrealized foreign exchange losses on debt | 0.6 |
| | 1.1 |
|
| Share issue costs | 0.3 |
| | 1.0 |
|
| Deferred financing costs | 0.3 |
| | 0.9 |
|
| Deferred revenue | 8.2 |
| | 2.1 |
|
| Unclaimed R&D expenditures | 10.0 |
| | 7.4 |
|
| Investment tax credits | (5.5 | ) | | (4.4 | ) |
| Other | 10.0 |
| | 6.4 |
|
| Book depreciation in excess of tax depreciation | (45.7 | ) | | (48.1 | ) |
| Valuation allowance | (9.7 | ) | | (6.5 | ) |
| | (13.6 | ) | | (9.5 | ) |
The short-term and long-term future income tax assets and liabilities after netting are as follows:
|
| | | | | |
| | 2011 | | 2010 |
| | $ | | $ |
| Short-term future income tax assets | 8.1 |
| | 9.0 |
|
| Long-term future income tax assets | 12.0 |
| | 11.2 |
|
| Long-term future income tax liabilities | (33.7 | ) | | (29.7 | ) |
| | (13.6 | ) | | (9.5 | ) |
The Company has tax-effected net operating losses, consisting of federal, state and foreign, of $15.4 million; $6.4 million
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
of the losses have an indefinite life and $9.0 million have expiry dates ranging from October 31, 2016 to October 31, 2031.
While evaluating the Company’s future tax assets and liabilities during the first half of fiscal 2010, the Company concluded it would be able to utilize certain Investment Tax Credits (“ITCs”) relating to scientific research and development costs. Therefore, the Company recorded a decrease of $7.2 million in the cost of goods sold relating to the utilization of all previous years’ ITCs.
The Company has not recorded deferred tax liabilities on the unremitted earnings of foreign subsidiaries as the Company currently intends to reinvest these earnings in its foreign operations indefinitely. Under Canadian tax laws, the Company's unremitted earnings from its foreign subsidiaries are exempt from income tax but may be subject to withholding tax at the source upon distribution. Determination of this withholding tax liability is not practicable.
During the first half of fiscal 2010 the Company evaluated its valuation reserves. The Company determined that the valuation allowance on its net Canadian future tax assets is no longer required based on its assessment of the future prospects of its Canadian operations. As a result of this determination, the Company released $13.8 million of valuation reserves through the benefit from income taxes in its consolidated statement of loss for fiscal 2010.
Prior to the second quarter of fiscal 2010, the Company recorded the ITCs as future tax assets. Of the $23.4 million on the consolidated balance sheet at October 31, 2011, $8.3 million was reclassified from future tax assets to other long-term assets in fiscal 2010 as the Company was in a position to utilize current and prior period ITCs.
16. SEGMENTED INFORMATION
The Company is organized and managed in two business segments: commercial manufacturing and PDS. These segments are organized around the service activities provided to the Company's customers.
|
| | | | | | | | | | | |
| | As of and for the year ended October 31, 2011 |
| | Commercial | | PDS | | Corp. & Other | | Total |
| | $ | | $ | | $ | | $ |
| Revenues | 572.6 |
| | 127.4 |
| | — |
| | 700.0 |
|
| Adjusted EBITDA | 80.0 |
| | 29.9 |
| | (36.9 | ) | | 73.0 |
|
| Total assets | 668.1 |
| | 83.9 |
| | 66.6 |
| | 818.6 |
|
| Depreciation | 46.6 |
| | 5.8 |
| | 1.0 |
| | 53.4 |
|
| Goodwill | 3.5 |
| | — |
| | — |
| | 3.5 |
|
| Capital expenditures | 38.4 |
| | 8.8 |
| | 0.6 |
| | 47.8 |
|
|
| | | | | | | | | | | |
| | As of and for the year ended October 31, 2010 |
| | Commercial | | PDS | | Corp. & Other | | Total |
| | $ | | $ | | $ | | $ |
| Revenues | 545.3 |
| | 125.9 |
| | — |
| | 671.2 |
|
| Adjusted EBITDA | 72.3 |
| | 46.8 |
| | (27.4 | ) | | 91.7 |
|
| Total assets | 636.1 |
| | 81.7 |
| | 91.1 |
| | 808.9 |
|
| Depreciation | 49.3 |
| | 5.9 |
| | 0.6 |
| | 55.8 |
|
| Impairment | 3.6 |
| | — |
| | — |
| | 3.6 |
|
| Goodwill | 3.4 |
| | — |
| | — |
| | 3.4 |
|
| Capital expenditures | 38.8 |
| | 8.7 |
| | 1.2 |
| | 48.7 |
|
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | | |
| | As of and for the year ended October 31, 2009 |
| | Commercial | | PDS | | Corp. & Other | | Total |
| | $ | | $ | | $ | | $ |
| Revenues | 530.0 |
| | 125.1 |
| | — |
| | 655.1 |
|
| Adjusted EBITDA | 71.2 |
| | 32.7 |
| | (29.9 | ) | | 74.0 |
|
| Total assets | 671.3 |
| | 61.3 |
| | 58.2 |
| | 790.8 |
|
| Depreciation | 37.0 |
| | 5.1 |
| | 0.5 |
| | 42.6 |
|
| Goodwill | 3.2 |
| | — |
| | — |
| | 3.2 |
|
| Capital expenditures | 39 |
| | 8.2 | | 1.9 | | 49.1 |
|
Cash and cash equivalents as well as future tax assets are considered to be part of “Corp. & Other” in the breakout of total assets shown above. Total assets in the commercial segment include $0.7 million as of October 31, 2009 that are classified as held for sale. In fiscal 2010 the Company, following another impairment analysis, completely wrote down the assets held for sale to their fair value less the cost to sell, which was nil.
The Company evaluates the performance of its segments based on segment Adjusted EBITDA, which is defined as income (loss) before discontinued operations before repositioning expenses, interest expense, foreign exchange losses reclassified from other comprehensive loss, refinancing expenses, gains and losses on sale of fixed assets, gain on extinguishment of debt, income taxes, asset impairment charges, depreciation and amortization, and other non-cash expenses. The Company’s presentation of Adjusted EBITDA may not be comparable to similarly-titled measures used by other companies.
Below is a reconciliation of Adjusted EBITDA to its closest Canadian GAAP measure.
|
| | | | | | | | |
| | Years ended October 31, |
| | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Adjusted EBITDA: | | | | | |
| Total Adjusted EBITDA per above | 73.0 |
| | 91.7 |
| | 74.0 |
|
| Depreciation and amortization | (53.4 | ) | | (55.8 | ) | | (42.6 | ) |
| Repositioning expenses | (7.0 | ) | | (6.8 | ) | | (2.1 | ) |
| Interest expense, net | (25.4 | ) | | (19.5 | ) | | (15.4 | ) |
| Impairment charge | — |
| | (3.6 | ) | | — |
|
| (Loss) gain on sale of fixed assets | (0.2 | ) | | (0.2 | ) | | — |
|
| Refinancing expenses | — |
| | (12.2 | ) | | — |
|
| Benefit from (provision for) income taxes | (7.3 | ) | | 3.0 |
| | (12.5 | ) |
| Other | 4.1 |
| | 0.1 |
| | (0.4 | ) |
| (Loss) income before discontinued operations | (16.2 | ) | | (3.3 | ) | | 1.0 |
|
As illustrated in the table below, revenues are attributed to countries based on the location of the customer's billing address, capital assets are attributed to the country in which they are located and goodwill is attributed to the country in which the entity to which the goodwill pertains is located:
|
| | | | | | | | | | | | | | |
| | As of and year ended October 31, 2011 |
| | Canada | | U.S.* | | Europe | | Other | | Total |
| | $ | | $ | | $ | | $ | | $ |
| Revenues | 12.0 |
| | 348.9 |
| | 302.4 |
| | 36.7 |
| | 700.0 |
|
| Capital assets | 115.6 |
| | 133.6 |
| | 224.0 |
| | 1.7 |
| | 474.9 |
|
| Goodwill | 3.5 |
| | — |
| | — |
| | — |
| | 3.5 |
|
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | | | | | |
| | As of and year ended October 31, 2010 |
| | Canada | | U.S.* | | Europe | | Other | | Total |
| | $ | | $ | | $ | | $ | | $ |
| Revenues | 16.2 |
| | 319.7 |
| | 310.7 |
| | 24.6 |
| | 671.2 |
|
| Capital assets | 116.4 |
| | 129.4 |
| | 230.8 |
| | 1.7 |
| | 478.3 |
|
| Impairment | — |
| | 3.6 |
| | — |
| | — |
| | 3.6 |
|
| Goodwill | 3.4 |
| | — |
| | — |
| | — |
| | 3.4 |
|
|
| | | | | | | | | | | | | | |
| | As of and year ended October 31, 2009 |
| | Canada | | US* | | Europe | | Other | | Total |
| | $ | | $ | | $ | | $ | | $ |
| Revenues | 18.1 |
| | 345.5 |
| | 262.5 |
| | 29.0 |
| | 655.1 |
|
| Capital assets | 117.4 |
| | 131.4 |
| | 240.9 |
| | 1.1 |
| | 490.8 |
|
| Goodwill | 3.2 |
| | — |
| | — |
| | — |
| | 3.2 |
|
_________________________________
* Includes Puerto Rico
During fiscal 2011, 2010 and 2009, the Company did not have any one customer that accounted for more than 10% of total revenues.
17. COMMITMENTS AND CONTINGENCIES
Long-term operating leases
The Company has entered into long-term rental agreements expiring at various dates until 2020. The future rental payments for the next five years and thereafter are estimated as follows:
|
| | |
| | $ |
| 2011 | 7.2 |
|
| 2012 | 5.7 |
|
| 2013 | 3.8 |
|
| 2014 | 2.5 |
|
| 2015 | 1.2 |
|
| Thereafter | 2.3 |
|
| Total payments | 22.7 |
|
Other
The Company may be subject to lawsuits, investigations and other claims, including environmental, labor, product, customer disputes and other matters in the normal course of operations and otherwise. The Company believes that adequate provisions have been recorded in the accounts where required. Although it is not possible to estimate the extent of potential costs, if any, the Company believes that the ultimate resolution of such contingencies will not have a material adverse impact on the results of operations, financial position or liquidity.
The Company’s tax filings are subject to audit by taxation authorities. Although the Company believes that it has adequately provided for income taxes based on the information available, the outcome of audits cannot be known with certainty and the potential impact on the financial statements is not determinable.
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
18. REPOSITIONING EXPENSES
During fiscal 2011, the Company incurred $7.0 million in repositioning expenses, of which $4.0 million related to the shutdown of the Caguas facility. The remaining $3.0 million related to the Company's 2011 strategic initiatives in Zug and Swindon.
During fiscal 2010, the Company incurred $6.8 million in expenses associated with the shutdown of its Caguas facility. Included in the employee-related expenses within the commercial segment for fiscal 2009 are repositioning expenses related to the York Mills facility and $3.4 million of repositioning expenses related to the closure of the Company’s Carolina facility that are presented in discontinued operations.
The following is a summary of these expenses and other charges associated with operational improvements (collectively “repositioning expenses”) as of and for fiscal 2011, 2010 and 2009:
|
| | | | | | | | | | | |
| As of and for the year ended October 31, 2011 | Commercial | | PDS | | Corporate | | Total |
| | $ | | $ | | $ | | $ |
| Total repositioning liabilities at October 31, 2010 | | | | | | | 3.2 |
|
| Employee-related expenses | 3.3 |
| | — |
| | 1.0 |
| | 4.3 |
|
| Consulting, professional and project costs | 2.7 |
| | — |
| | — |
| | 2.7 |
|
| Total expenses | 6.0 |
| | — |
| | 1.0 |
| | 7.0 |
|
| Repositioning expenses paid | | | | | | | (3.5 | ) |
| Total repositioning liabilities at October 31, 2011 | | | | | | | 6.7 |
|
|
| | | | | | | | | | | |
| As of and for the year ended October 31, 2010 | Commercial | | PDS | | Corporate | | Total |
| | $ | | $ | | $ | | $ |
| Total repositioning liabilities at October 31, 2009 | | | | | | | 2.9 |
|
| Employee-related expenses | 3.7 |
| | — |
| | — |
| | 3.7 |
|
| Consulting, professional and project costs | 3.1 |
| | — |
| | — |
| | 3.1 |
|
| Total expenses | 6.8 |
| | — |
| | — |
| | 6.8 |
|
| Repositioning expenses paid | | | | | | | (6.4 | ) |
| Foreign exchange | | | | | | | (0.1 | ) |
| Total repositioning liabilities at October 31, 2010 | | | | | | | 3.2 |
|
|
| | | | | | | | | | | |
| As of and for the year ended October 31, 2009 | Commercial | | PDS | | Corporate | | Total |
| | $ | | $ | | $ | | $ |
| Total repositioning liabilities at October 31, 2008 | | | | | | | 8.0 |
|
| Employee-related expenses | 5.1 |
| | — |
| | — |
| | 5.1 |
|
| Consulting, professional and project management costs | 0.4 |
| | — |
| | — |
| | 0.4 |
|
| Total expenses | 5.5 |
| | — |
| | — |
| | 5.5 |
|
| Repositioning expenses paid | | | | | | | (10.8 | ) |
| Foreign exchange | | | | | | | 0.2 |
|
| Total repositioning liabilities at October 31, 2009 | | | | | | | 2.9 |
|
19. REFINANCING EXPENSES
During fiscal 2010, the Company incurred expenses of $12.2 million in connection with its refinancing activities. These transaction costs relate to expenses associated with obtaining debt financing, including fees paid to advisors and other related costs. Included in the refinancing expenses are $1.8 million in costs related to the previous senior secured term loan that were
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
written off and $1.8 million in losses for the interest rate swaps on the previous debt that were cancelled on June 30, 2010. Financing costs of $7.6 million, including fees paid to lenders, were capitalized and netted against the carrying value of the related debt and amortized to interest expense over the life of the Notes and credit agreement. Please refer to Note 8—Long-Term Debt.
20. SUBSEQUENT EVENTS
On November 1, 2011, the Company announced that Eric Evans, the Company's then Chief Financial Officer, resigned from the Company. Due to Mr. Evans's employment agreement, the Company incurred a severance charge of approximately $0.5 million in the first quarter of fiscal 2012 for severance and benefits.
In the fourth quarter of fiscal 2011, a customer gave notice of its intent to seek indemnification against the Company pursuant to a manufacturing service agreement (“MSA”) for all costs associated with a recall and associated defective products. The Company accrued $1.7 million, net of expected insurance proceeds, to cover recall costs and replace the returned products. In November, the customer gave further notice of its intent to seek indemnification pursuant to the MSA for all actual and potential third-party claims filed against them in connection with the recall, as well as all costs and expenses of the defense and settlement of such claims. To date, three putative class actions related to the recall have been commenced in the United States against the customer. At this time, it is not possible to estimate the number of potential claimants or the amount of potential damages in the above actions. To date, the Company has not been named as a party in any action related to the product recall.
On December 15, 2011, the Company announced the appointment of Michel Lagarde, Principal, JLL Partners, to its Board. Mr. Lagarde replaced Thomas S. Taylor, Managing Director, JLL Partners, who left the Board effective December 15, 2011. Mr. Lagarde replaced Mr. Taylor as the Chairman of the Company's Compensation and Human Resources Committee and is a member of the Company's Audit and Corporate Governance Committees.
21. ADDITIONAL DISCLOSURES REQUIRED UNDER U.S. GENERALLY ACCEPTED ACCOUNTING PRINCIPLES
The Company’s consolidated financial statements have been prepared in accordance with Canadian GAAP. In the case of the Company, Canadian GAAP conforms in all material respects with U.S. GAAP except for certain matters, the details of which are as follows:
Consolidated Balance Sheets
The application of U.S. GAAP has the following effects on consolidated balance sheet items as reported under Canadian GAAP:
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | | | | | | | | | | | | |
| | As of October 31, 2011 | | As of October 31, 2010 |
| | Canadian GAAP | | Increase (Decrease) | | Notes | | U.S. GAAP | | Canadian GAAP | | Increase (Decrease) | | Notes | | U.S. GAAP |
| | $ | | $ | | | | $ | | $ | | $ | | | | $ |
| Assets | | | | | | | | | | | | | | | |
| Current | | | | | | | | | | | | | | | |
| Cash and cash equivalents | 33.4 |
| | — |
| | | | 33.4 |
| | 53.5 |
| | — |
| | | | 53.5 |
|
| Accounts receivable | 158.0 |
| | — |
| | | | 158.0 |
| | 139.9 |
| | — |
| | | | 139.9 |
|
| Inventories | 81.8 |
| | — |
| | | | 81.8 |
| | 73.3 |
| | — |
| | | | 73.3 |
|
| Income taxes receivable | 3.1 |
| | — |
| | | | 3.1 |
| | 5.7 |
| | — |
| | | | 5.7 |
|
| Prepaid expenses and other | 10.7 |
| | — |
| | | | 10.7 |
| | 9.5 |
| | — |
| | | | 9.5 |
|
| Future tax assets—short term | 8.1 |
| | — |
| |
| | 8.1 |
| | 9.0 |
| | (1.3 | ) | | g | | 7.7 |
|
| Total current assets | 295.1 |
| | — |
| | | | 295.1 |
| | 290.9 |
| | (1.3 | ) | | | | 289.6 |
|
| Capital assets | 474.9 |
| | (0.7 | ) | | e | | 474.2 |
| | 478.3 |
| | (0.9 | ) | | e | | 477.4 |
|
| Intangible assets | — |
| | — |
| | | | — |
| | 1.4 |
| | — |
| | | | 1.4 |
|
| Deferred financing costs | — |
| | 6.2 |
| | f | | 6.2 |
| | — |
| | 7.2 |
| | f | | 7.2 |
|
| Future tax assets | 12.0 |
| | 27.1 |
| | b,c | | 39.1 |
| | 11.2 |
| | 17.7 |
| | c | | 28.9 |
|
| Goodwill | 3.5 |
| | — |
| | | | 3.5 |
| | 3.4 |
| | — |
| | | | 3.4 |
|
| Investments | 5.3 |
| | — |
| | | | 5.3 |
| | 5.3 |
| | — |
| | | | 5.3 |
|
| Long-term assets held for sale | 0.2 |
| | — |
| | | | 0.2 |
| | — |
| | — |
| | | | — |
|
| Other long-term assets | 27.6 |
| | (26.6 | ) | | b,c,d | | 1.0 |
| | 18.4 |
| | (18.4 | ) | | c,d | | — |
|
| Total assets | 818.6 |
| | 6.0 |
| | | | 824.6 |
| | 808.9 |
| | 4.3 |
| | | | 813.2 |
|
| Liabilities and shareholders’ equity | | | | | | | | | | | | | | | |
| Current | | | | | | | | | | | | | | | |
| Short term borrowings | 6.1 |
| | — |
| | | | 6.1 |
| | 2.0 |
| | — |
| | | | 2.0 |
|
| Accounts payable and accrued liabilities | 181.2 |
| | 0.3 |
| | b | | 181.5 |
| | 156.7 |
| | — |
| | | | 156.7 |
|
| Income taxes payable | — |
| | — |
| | | | — |
| | 0.4 |
| | 1.0 |
| | g | | 1.4 |
|
| Deferred revenues—short term | 8.8 |
| | — |
| | | | 8.8 |
| | 26.7 |
| | — |
| | | | 26.7 |
|
| Current portion of long-term debt | 1.1 |
| | — |
| | | | 1.1 |
| | 3.5 |
| | — |
| | | | 3.5 |
|
| Total current liabilities | 197.2 |
| | 0.3 |
| | | | 197.5 |
| | 189.3 |
| | 1.0 |
| | | | 190.3 |
|
| Long-term debt | 274.6 |
| | 5.5 |
| | d,f | | 280.1 |
| | 274.8 |
| | 6.3 |
| | d,f | | 281.1 |
|
| Deferred revenues | 27.7 |
| | — |
| | | | 27.7 |
| | 19.2 |
| | — |
| | | | 19.2 |
|
| Future tax liabilities | 33.7 |
| | (5.8 | ) | | b,e | | 27.9 |
| | 29.7 |
| | (0.3 | ) | | e | | 29.4 |
|
| Other long-term liabilities | 21.7 |
| | 32.0 |
| | b,g | | 53.7 |
| | 22.9 |
| | 22.2 |
| | b,g | | 45.1 |
|
| Total liabilities | 554.9 |
| | 32.0 |
| | | | 586.9 |
| | 535.9 |
| | 29.2 |
| | | | 565.1 |
|
| Shareholders’ equity | | | | | | | | | | | | | | | |
| Restricted voting shares | 553.8 |
| | 18.1 |
| | a | | 571.9 |
| | 553.8 |
| | 18.1 |
| | a | | 571.9 |
|
| Contributed surplus | 13.5 |
| | — |
| | | | 13.5 |
| | 10.0 |
| | — |
| | | | 10.0 |
|
| Deficit | (347.5 | ) | | (24.4 | ) | | a,d,e,g,i | | (371.9 | ) | | (330.7 | ) | | (25.8 | ) | | a,d,e,g | | (356.5 | ) |
| Accumulated other comprehensive income (loss) | 43.9 |
| | (19.7 | ) | | a,b,e | | 24.2 |
| | 39.9 |
| | (17.2 | ) | | a,b,e | | 22.7 |
|
| Total shareholders’ equity | 263.7 |
| | (26.0 | ) | | | | 237.7 |
| | 273.0 |
| | (24.9 | ) | | | | 248.1 |
|
| Total liabilities and shareholders’ equity | 818.6 |
| | 6.0 |
| | | | 824.6 |
| | 808.9 |
| | 4.3 |
| | | | 813.2 |
|
see accompanying notes
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
Consolidated Statements of Loss
The application of U.S. GAAP had the following effects on net loss and net loss per share as reported under Canadian GAAP:
|
| | | | | | | | | | | | |
| | Year Ended October 31, 2011 |
| | Canadian GAAP | | Increase (Decrease) | | Notes | | U.S. GAAP |
| | $ | | $ | | | | $ |
| Revenues | 700.0 |
| | — |
| | | | 700.0 |
|
| Cost of goods sold | 561.9 |
| | 6.3 |
| | c | | 568.2 |
|
| Gross profit | 138.1 |
| | (6.3 | ) | | | | 131.8 |
|
| Selling, general and administrative expenses | 120.2 |
| | — |
| | | | 120.2 |
|
| Repositioning expenses | 7.0 |
| | — |
| | | | 7.0 |
|
| Loss on sale of fixed assets | — |
| | 0.2 |
| | h | | 0.2 |
|
| Operating income | 10.9 |
| | (6.5 | ) | | | | 4.4 |
|
| Interest expense, net | 25.4 |
| | 0.2 |
| | d | | 25.6 |
|
| Foreign exchange gain | (1.6 | ) | | — |
| | | | (1.6 | ) |
| Loss on sale of fixed assets | 0.2 |
| | (0.2 | ) | | h | | — |
|
| Other (income) expense, net | (4.2 | ) | | (0.7 | ) | | d | | (4.9 | ) |
| Loss from continuing operations before income taxes | (8.9 | ) | | (5.8 | ) | | | | (14.7 | ) |
| Current | 1.6 |
| | — |
| | g | | 1.6 |
|
| Future | 5.7 |
| | (6.2 | ) | | c,e,g | | (0.5 | ) |
| Provision for income taxes | 7.3 |
| | (6.2 | ) | | | | 1.1 |
|
| Loss before discontinued operations | (16.2 | ) | | 0.4 |
| | | | (15.8 | ) |
| Loss from discontinued operations | (0.6 | ) | | — |
| | | | (0.6 | ) |
| Net loss for the period | (16.8 | ) | | 0.4 |
| | | | (16.4 | ) |
| Net loss attributable to restricted voting shareholders | (16.8 | ) | | 0.4 |
| | | | (16.4 | ) |
| Basic and diluted loss per share | | | | | | | |
| From continuing operations | $ | (0.125 | ) | | | | | | $ | (0.122 | ) |
| From discontinued operations | $ | (0.005 | ) | | | | | | $ | (0.005 | ) |
| | $ | (0.130 | ) | | | | | | $ | (0.127 | ) |
| Weighted-average number of shares outstanding during period—basic and diluted (in thousands) | 129,168 |
| | | | | | 129,168 |
|
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | | | |
| | Year Ended October 31, 2010 |
| | Canadian GAAP | | Increase (Decrease) | | Notes | | U.S. GAAP |
| | $ | | $ | | | | $ |
| Revenues | 671.2 |
| | — |
| | | | 671.2 |
|
| Cost of goods sold | 526.2 |
| | 10.6 |
| | c,e | | 536.8 |
|
| Gross profit | 145.0 |
| | (10.6 | ) | | | | 134.4 |
|
| Selling, general and administrative expenses | 110.6 |
| | — |
| | | | 110.6 |
|
| Repositioning expenses | 6.8 |
| | — |
| | | | 6.8 |
|
| Impairment charge | — |
| | 3.6 |
| | h | | 3.6 |
|
| Loss on sale of fixed assets | — |
| | 0.2 |
| | h | | 0.2 |
|
| Operating income | 27.6 |
| | (14.4 | ) | | | | 13.2 |
|
| Interest expense, net | 19.5 |
| | 0.1 |
| | d | | 19.6 |
|
| Impairment charge | 3.6 |
| | (3.6 | ) | | h | | — |
|
| Foreign exchange gain | (1.5 | ) | | — |
| | | | (1.5 | ) |
| Refinancing expenses | 12.2 |
| | — |
| | | | 12.2 |
|
| Loss on sale of fixed assets | 0.2 |
| | (0.2 | ) | | h | | — |
|
| Other (income) expense, net | (0.1 | ) | | (0.3 | ) | | d | | (0.4 | ) |
| Loss from continuing operations before income taxes | (6.3 | ) | | (10.4 | ) | | | | (16.7 | ) |
| Current | 6.7 |
| | — |
| | | | 6.7 |
|
| Future | (9.7 | ) | | (10.8 | ) | | c,e | | (20.5 | ) |
| Benefit from income taxes | (3.0 | ) | | (10.8 | ) | | | | (13.8 | ) |
| Loss before discontinued operations | (3.3 | ) | | 0.4 |
| | | | (2.9 | ) |
| Loss from discontinued operations | (1.7 | ) | | — |
| | | | (1.7 | ) |
| Net loss for the period | (5.0 | ) | | 0.4 |
| | | | (4.6 | ) |
| Net loss attributable to restricted voting shareholders | (5.0 | ) | | 0.4 |
| | | | (4.6 | ) |
| Basic and diluted loss per share | | | | | | | |
| From continuing operations | $ | (0.026 | ) | | | | | | $ | (0.023 | ) |
| From discontinued operations | $ | (0.013 | ) | | | | | | $ | (0.013 | ) |
| | $ | (0.039 | ) | | | | | | $ | (0.036 | ) |
| Weighted-average number of shares outstanding during period—basic and diluted (in thousands) | 129,168 |
| | | | | | 129,168 |
|
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | | | |
| | Year Ended October 31, 2009 |
| | Canadian GAAP | | Increase (Decrease) | | Notes | | U.S. GAAP |
| | $ | | $ | | | | $ |
| Revenues | 655.1 |
| | — |
| | | | 655.1 |
|
| Cost of goods sold | 511.2 |
| | (0.2 | ) | | e | | 511.0 |
|
| Gross profit | 143.9 |
| | 0.2 |
| | | | 144.1 |
|
| Selling, general and administrative expenses | 105.5 |
| | — |
| | | | 105.5 |
|
| Repositioning expenses | 2.1 |
| | — |
| | | | 2.1 |
|
| Operating income | 36.3 |
| | 0.2 |
| | | | 36.5 |
|
| Interest expense, net | 15.4 |
| | — |
| |
| | 15.4 |
|
| Foreign exchange loss | 7.0 |
| | — |
| |
| | 7.0 |
|
| Other (income) expense, net | 0.4 |
| | — |
| | | | 0.4 |
|
| Income from continuing operations before income taxes | 13.5 |
| | 0.2 |
| | | | 13.7 |
|
| Current | 7.7 |
| | — |
| |
| | 7.7 |
|
| Future | 4.8 |
| | 0.1 |
| | e | | 4.9 |
|
| Provision for income taxes | 12.5 |
| | 0.1 |
| | | | 12.6 |
|
| Income before discontinued operations | 1.0 |
| | 0.1 |
| | | | 1.1 |
|
| Loss from discontinued operations | (7.8 | ) | | — |
| | | | (7.8 | ) |
| Net loss for the period | (6.8 | ) | | 0.1 |
| | | | (6.7 | ) |
| Dividends on convertible preferred shares | 11.1 |
| | — |
| | | | 11.1 |
|
| Net loss attributable to restricted voting shareholders | (17.9 | ) | | 0.1 |
| | | | (17.8 | ) |
| Basic and diluted loss per share | | | | | | | |
| From continuing operations | $ | (0.100 | ) | | | | | | $ | (0.099 | ) |
| From discontinued operations | $ | (0.077 | ) | | | | | | $ | (0.077 | ) |
| | $ | (0.177 | ) | | | | | | $ | (0.176 | ) |
| Weighted-average number of shares outstanding during period—basic and diluted (in thousands) | 100,964 |
| | | | | | 100,964 |
|
see accompanying notes
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
Consolidated Statements of Cash Flows
There would be no material differences between Canadian GAAP and U.S. GAAP in total operating, investing or financing activities for fiscal 2011, 2010 and 2009. However, there were certain reclassifications within these activities.
Consolidated Statements of Changes to Shareholders’ Equity
The application of U.S. GAAP had the following effects on shareholders’ equity as reported under Canadian GAAP:
|
| | | | | | | | | | | | | | | | | | | | | |
| | Year Ended October 31, 2011 | | Year Ended October 31, 2010 |
| Canadian GAAP | | Increase (Decrease) | | Notes | | U.S. GAAP | | Canadian GAAP | | Increase (Decrease) | | Notes | | U.S. GAAP |
| | $ | | $ | | | | $ | | $ | | $ | | | | $ |
| Restricted voting shares | | | | | | | | | | | | | | | |
| Balance at beginning of period | 553.8 |
| | 18.1 |
| | | | 571.9 |
| | 553.8 |
| | 18.1 |
| | | | 571.9 |
|
| Balance at end of period | 553.8 |
| | 18.1 |
| | | | 571.9 |
| | 553.8 |
| | 18.1 |
| | | | 571.9 |
|
| Contributed surplus | | | | | | | | | | | | | | | |
| Balance at beginning of period | 10.0 |
| | — |
| | | | 10.0 |
| | 7.7 |
| | — |
| | | | 7.7 |
|
| Stock-based compensation | 3.5 |
| | — |
| | | | 3.5 |
| | 2.3 |
| | — |
| | | | 2.3 |
|
| Balance at end of period | 13.5 |
| | — |
| | | | 13.5 |
| | 10.0 |
| | — |
| | | | 10.0 |
|
| Deficit | | | | | | | | | | | | | | | |
| Balance at beginning of period | (330.7 | ) | | (24.8 | ) | | | | (355.5 | ) | | (325.7 | ) | | (25.2 | ) | | | | (350.9 | ) |
| Adjustments for U.S. GAAP differences | — |
| | 0.4 |
| | d,e | | 0.4 |
| | — |
| | 0.4 |
| | d,e | | 0.4 |
|
| Net loss attributable to restricted voting shareholders | (16.8 | ) | | — |
| | | | (16.8 | ) | | (5.0 | ) | | — |
| | | | (5.0 | ) |
| Balance at end of period | (347.5 | ) | | (24.4 | ) | | | | (371.9 | ) | | (330.7 | ) | | (24.8 | ) | | | | (355.5 | ) |
| Accumulated other comprehensive income (loss) | | | | | | | | | | | | | | | |
| Balance at beginning of period | 39.9 |
| | (17.2 | ) | | | | 22.7 |
| | 35.5 |
| | (19.6 | ) | | | | 15.9 |
|
| Pension adjustment | — |
| | (2.5 | ) | | b | | (2.5 | ) | | — |
| | 2.4 |
| | b | | 2.4 |
|
| Other comprehensive income for the period | 4.0 |
| | — |
| | | | 4.0 |
| | 4.4 |
| | — |
| | | | 4.4 |
|
| Balance at end of period | 43.9 |
| | (19.7 | ) | | | | 24.2 |
| | 39.9 |
| | (17.2 | ) | | | | 22.7 |
|
| Total shareholders’ equity at end of period | 263.7 |
| | (26.0 | ) | | | | 237.7 |
| | 273.0 |
| | (23.9 | ) | | | | 249.1 |
|
see accompanying notes
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | |
| | Year Ended October 31, 2009 |
| Canadian GAAP | | Increase (Decrease) | | Notes | | U.S. GAAP |
| | $ | | $ | | | | $ |
| Convertible preferred shares—equity component | | | | | | | |
| Balance at beginning of period | 149.2 |
| | 18.6 |
| |
| | 167.8 |
|
| Paid in-kind dividend on shares | 11.1 |
| | — |
| | | | 11.1 |
|
| Conversion of convertible preferred shares | (160.3 | ) | | (18.6 | ) | | | | (178.9 | ) |
| Balance at end of period | — |
| | — |
| | | | — |
|
| Restricted voting shares | | | | | | | |
| Balance at beginning of period | 393.5 |
| | (0.5 | ) | | | | 393.0 |
|
| Conversion of convertible preferred shares | 160.3 |
| | 18.6 |
| | | | 178.9 |
|
| Balance at end of period | 553.8 |
| | 18.1 |
| | | | 571.9 |
|
| Contributed surplus | | | | | | | |
| Balance at beginning of period | 6.7 |
| | — |
| | | | 6.7 |
|
| Stock-based compensation | 1.0 |
| | — |
| | | | 1.0 |
|
| Balance at end of period | 7.7 |
| | — |
| | | | 7.7 |
|
| Deficit | | | | | | | |
| Balance at beginning of period | (309.3 | ) | | (25.3 | ) | |
| | (334.6 | ) |
| Elimination of gain on deemed redemption of preferred shares | 1.6 |
| | — |
| |
| | 1.6 |
|
| Foreign exchange on preferred shares | — |
| | 0.1 |
| | e | | 0.1 |
|
| Adjustments for U.S. GAAP differences | (17.9 | ) | | — |
| |
| | (17.9 | ) |
| Balance at end of period | (325.7 | ) | | (25.2 | ) | | | | (350.9 | ) |
| Accumulated other comprehensive income (loss) | | | | | | | |
| Balance at beginning of period | (2.9 | ) | | (7.8 | ) | |
| | (10.7 | ) |
| Pension adjustment | — |
| | (11.7 | ) | | b | | (11.7 | ) |
| Adjustments for U.S. GAAP differences | — |
| | (0.1 | ) | | e | | (0.1 | ) |
| Other comprehensive income for the period | 38.4 |
| | — |
| |
| | 38.4 |
|
| Balance at end of period | 35.5 |
| | (19.6 | ) | | | | 15.9 |
|
| Total shareholders’ equity at end of period | 271.3 |
| | (26.7 | ) | | | | 244.6 |
|
see accompanying notes
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
(a) Preferred shares
Under Canadian GAAP, the convertible preferred shares held by JLL were classified at inception as having both an equity component and a debt component. Therefore, the Company recognized foreign exchange gains and losses during fiscal 2007 and 2008 on the U.S. dollar denominated debt portion of the preferred shares as they were held by an entity with a Canadian dollar functional currency and marked to market the debt portion at current foreign exchange rates. Under U.S. GAAP, however, the preferred shares would have been deemed to be mezzanine equity at inception. Therefore, the Company would not have recognized any foreign exchange activity under U.S. GAAP.
The Company considered its U.S. dollar denominated debt held by the Canadian entity as a hedge against its U.S. dollar denominated investments . Therefore, under Canadian GAAP, changes in the fair value associated with the effective portions of the hedge were recorded as other comprehensive income, and changes in the ineffective portions of the hedge were recorded as foreign exchange gains and losses on the consolidated statements of loss.
Because JLL’s preferred shares were primarily recorded as debt, under Canadian GAAP, the Company recorded accrued interest on the debt portion of the preferred shares. Under U.S. GAAP Accounting Standards Codification (“ASC”) 480, “Distinguishing Liabilities from Equity” (“ASC 480”), the Company would have recorded accreted dividends on the entire amount.
As discussed above, under U.S. GAAP, the value of the preferred stock would be adjusted from its initial value on the April 27, 2007 issuance date to its redemption value over the period from issuance date to the redemption or conversion date using the method discussed in ASC 480.
In September 2008, the Company entered into an agreement (the “Redemption Waiver Agreement”) with JLL whereby JLL agreed to waive the mandatory redemption requirement contained in the terms of its preferred shares. The Redemption Waiver Agreement resulted in a deemed repayment of the debt and equity components of the preferred shares, as well as in a change in the accounting treatment for those shares. Completion of the Redemption Waiver Agreement resulted in the full carrying value of the preferred shares being classified within shareholders’ equity on the Company’s balance sheets, and no further accretive interest expense was recorded in the consolidated statements of loss. Paid-in-kind dividend equivalents on the preferred shares were reported below net loss to arrive at a loss attributable to the restricted voting shareholders.
Upon settlement of the debt portion of JLL’s preferred shares, the Company recognized a gain on the extinguishment of this debt. The Company reported this gain on its consolidated statement of loss below operating income and before income from continuing operations before income tax. Additionally, upon settlement of the equity portion of JLL’s preferred shares, the Company recognized a loss on the deemed redemption, which increased accumulated deficit. Under U.S. GAAP, there would be no gain or loss recognized since the preferred shares would have been recorded solely as equity from inception.
According to U.S. GAAP ASC 260, “Earnings Per Share,” under the inducement clause, the excess (deficit) of the fair value of all securities and other consideration transferred in the transaction by the Company to the holders of the convertible preferred stock over (under) the carrying value of securities issuable pursuant to the original conversion terms would be netted against net income (loss) in computing net income (loss) per share. The impact of this was a net gain for the restricted shareholders of $18.2 million, partially offset by the additional 400,000 restricted shares issued to JLL with a fair value of approximately $1.1 million.
The net impact on loss attributable to restricted voting shareholders was a gain (deemed dividend) of $17.1 million which was included in the net loss per share calculation for fiscal 2008.
On July 29, 2009, JLL converted its 150,000 convertible preferred shares of Patheon into a total of 38,018,538 restricted voting shares of Patheon, in accordance with the convertible preferred share terms. As a result of this conversion, the Company no longer has any Series C preferred shares outstanding.
(b) Pensions and post retirement plans
Under U.S. GAAP ASC 715, “Compensation—Retirement Benefits,” the Company is required to recognize the over or underfunded status of defined benefit pension and other post-retirement plans on its balance sheet. The over or under funded status is measured as the difference between the fair value of the plan assets and the benefit obligation, being the projected obligation for pension plans and the accumulated benefit obligation for other post-retirement plans. In addition, the Company is required to recognize any previously unrecognized actuarial gains and losses and prior service costs and credits
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
that arise during the applicable period in other comprehensive income, net of tax. No similar requirement currently exists under Canadian GAAP. In addition, overfunded plans are reported as non-current assets and underfunded plans are reported as non-current liabilities, with expected benefit payments over the next 12 months reclassified as short-term liabilities from non-current liabilities.
(c) Research & Development Tax Credits
Under U.S. GAAP ASC 740, “Income Taxes” (“ASC 740”) the Company's tax credits are credited against income tax expense, whereas under Canadian GAAP CICA Section 3805, “Investment Tax Credits,” ITCs are offset against the related operating expense. Because the Company’s tax credits are related to research and development costs, primarily labor, assets are not typically created as a part of the operations subject to the tax credit calculation pool.
Under U.S. GAAP, the Company has reclassified the credit to cost of goods related to its tax credits to income tax expense and has reclassified the related tax credits receivables to deferred tax assets, short-term or long-term, based upon when they are expected to be used. The tax credits will impact current tax expense when used and deferred tax expense when accumulated during the course of a fiscal year.
(d) Embedded derivative on call option premium
Under CICA Section 3855, “Financial Instruments—Recognition and Measurement,” if the economic characteristics of an embedded derivative (in this case the call option on the Notes) are not closely related to the economic characteristics of the host contract (the Notes), then bifurcation of the embedded derivative is required. CICA Section 3855 provides that the economic characteristics of a call option are not closely related to the economic characteristics of the host contract if the call option’s exercise price is not approximately equal, on each exercise date, to the amortized cost of the host contract. In determining whether the exercise price is approximately equal, the amortized cost of the host contract is assumed to be its par value at any given time. Under U.S. GAAP ASC 815; “Derivatives and Hedging,” the bond call provisions were considered clearly and closely related to the host instrument, and as such, the embedded derivative is not valued separately from the debt. Therefore, the Canadian GAAP valuations for the call options are reversed for the U.S. GAAP presentation.
(e) Deferred transaction costs
U.S. GAAP ASC 805, “Business Combinations,” and its predecessor, Statement of Financial Accounting Standards No. 141, “Business Combinations,” each require deferred transaction costs to be expensed as incurred. Under Canadian GAAP, such costs are capitalized and amortized over 15 years. As such, the effect of the deferred transaction costs has been reversed as of the first period presented and included in opening accumulated deficit.
(f) Deferred financing costs
In accordance with Canadian GAAP, the Company accounts for deferred financing costs, or transaction costs, as a reduction from the related liability and amortizes such costs using the effective interest method. However, for U.S. GAAP purposes, the Company accounts for these costs as an asset and amortizes them over the expected term of the financial liability using the effective interest method.
(g) Reserves for uncertain tax positions
The Company adopted the uncertain tax positions standard of ASC 740 on November 1, 2007. As a result of the implementation of this standard, the Company recognized no material adjustment in the liability for unrecognized income tax benefits or effect on accumulated deficit. As of October 31, 2011, 2010 and 2009, unrecognized tax benefits were $0.8 million, $1.4 million and $2.7 million, respectively.
(h) Long-lived assets classified as held and used
Under U.S. GAAP ASC 360, “Long-Lived Assets Classified as Held and Used” (“ASC 360”), impairments and gains/losses on sale of assets should be reported in operating income (loss).
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
Additional U.S. GAAP Disclosures
Accounts payable and accrued liabilities
The following is the breakdown of accounts payable and accrued liabilities:
|
| | | | | |
| | As of October 31, |
| | 2011 | | 2010 |
| | $ | | $ |
| Trade payables | 102.2 |
| | 88.9 |
|
| Interest payable | 1.0 |
| | 1.0 |
|
| Accrued salaries and related expenses | 44.0 |
| | 44.7 |
|
| Customer deposits | 17.9 |
| | 14.6 |
|
| Other accruals | 16.1 |
| | 7.5 |
|
| | 181.2 |
| | 156.7 |
|
Income taxes
During fiscal 2011, the Company adopted uncertain tax provisions standard ASC 740, applied retroactively to November 1, 2007. As a result of the implementation of this standard, the Company recognized no material adjustment in the liability for unrecognized income tax benefits or effect on accumulated deficit. As of October 31, 2011 and 2010, unrecognized tax benefits were $0.8 million and $1.4 million, respectively. At October 31, 2011 and 2010, unrecognized tax benefits of $0.8 million and $0.6 million, respectively, related to permanent income tax differences, will have a favorable effect on the Company's effective tax rate if recognized; the remaining balance would result in a reclassification on the balance sheet.
The Company classifies interest recognized under ASC 740 as a component of interest expense in the consolidated statements of loss. Penalties, if incurred, are recorded as other operating expenses in the consolidated statements of loss. The Company recorded no material amount of interest expense under ASC 740 for fiscal 2011 and 2010.
The Company for Canadian purposes is generally no longer subject to examinations for the fiscal years ended October 31, 2006 and prior. The Company is currently under examination by the Canada Revenue Agency for the fiscal years ended October 31, 2007 through October 31, 2008. During fiscal 2011, the Canada Revenue Agency concluded examination of the Company's transfer pricing position for the years ended October 31, 2005 through 2008 resulting in no adjustment to Canadian taxable income.
During fiscal 2011, the U.S. Internal Revenue Service concluded examination of the Company's October 31, 2007 and 2008 consolidated U.S. federal income tax returns. The Company acquiesced to the conclusions reached in the examination. The results of the examination have been reviewed and accepted by the Joint Committee on Taxation. The Company believes the issues addressed by this examination are settled and has, accordingly, reduced the reserve for these uncertain tax benefits.
During fiscal 2011, the French tax authority concluded examination of the Company's income tax returns for the years ended October 31, 2007 and 2008. The Company appealed the assessment related to transfer pricing, but the French authorities maintained the assessment and the Company has indicated it intends to further appeal and believes the result will be a nil or immaterial assessment. The Company anticipates no material change to unrecognized tax benefits resulting from this examination.
During fiscal 2011, the Hungarian tax authority initiated an examination for the years ended December 31, 2005 through August 12, 2010 as part of the liquidation process. The examination has concluded and resulted in a nil assessment.
Statutes related to foreign and state jurisdictions are open from October 31, 2004-October 31, 2010. Certain carry forward tax attributes generated in prior years remain subject to examination and adjustment.
During the next 12 months, the Company expects no material change in the total amount of unrecognized tax benefits. A reconciliation of the beginning and ending amounts of the tax reserves is as follows:
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | |
| Balance at November 1, 2008 | $ | 0.6 |
|
| Increase based on tax positions taken in the current year | 2.1 |
|
| Balance at October 31, 2009 | $ | 2.7 |
|
| Decrease related to settlements with taxing authorities and changes in law | (1.3 | ) |
| Balance at October 31, 2010 | $ | 1.4 |
|
| Decrease related to positions taken in the current year | (0.8 | ) |
| Increase based on tax positions taken in current year | 0.2 |
|
| Balance at October 31, 2011 | $ | 0.8 |
|
Below is a breakout of the Company’s (loss) income from continuing operations before income taxes:
|
| | | | | | | | |
| Years ended October 31, |
| 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Domestic | (12.5 | ) | | (15.7 | ) | | 1.2 |
|
| Foreign | (2.2 | ) | | (1.0 | ) | | 12.5 |
|
| | (14.7 | ) | | (16.7 | ) | | 13.7 |
|
Earnings per share
The Company did not include 12,628,457, 8,327,357, and 4,699,348 outstanding options in fiscal 2011, 2010 and 2009, respectively, because they were anti-dilutive in nature.
Pension plan assumptions
The following weighted-average assumptions were used to determine the projected benefit obligation of the Company's defined benefit and other post retirement plans at the end of the respective fiscal year:
|
| | | | | | | | | | | |
| | Defined Benefit Plans | | Other Benefit Plans |
| | 2011 | | 2010 | | 2011 | | 2010 |
| Discount rate | 4.9 | % | | 5.3 | % | | 5.2 | % | | 5.3 | % |
| Rate of future compensation increases | 3.7 | % | | 3.9 | % | | — | % | | — | % |
The following weighted-average assumptions were used to determine the net periodic benefit cost of the Company’s defined benefit and other post retirement plans during the respective fiscal year:
|
| | | | | | | | | | | | | | | | | |
| | Defined Benefit Plans | | Other Benefit Plans |
| | 2011 | | 2010 | | 2009 | | 2011 | | 2010 | | 2009 |
| Discount rate | 5.2 | % | | 5.8 | % | | 6.0 | % | | 5.2 | % | | 5.3 | % | | 7.0 | % |
| Expected long-term return on plan assets | 6.9 | % | | 6.9 | % | | 7.3 | % | | — | % | | — | % | | — | % |
| Rate of future compensation increases | 3.9 | % | | 4.1 | % | | 4.2 | % | | — | % | | — | % | | — | % |
The 4.9% weighted-average discount rate used to determine the projected benefit obligation of the Company’s plans at the end of fiscal 2011 was derived by reference to appropriate benchmark yields on high quality corporate bonds, with terms which approximate the duration of the benefit payments and the relevant benchmark bond indices considering the individual plan’s characteristics, to select a rate at which the Company believes the pension benefits could have been effectively settled.
The Company selects an expected long-term rate of return on its pension plan assets and, in doing so, considers a number of factors including, without limitation, recent and historical performance of plan assets, asset allocation and other third-party
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
studies and surveys. The Company considered the pension plan portfolios’ asset allocations over a variety of time periods and compared them with third-party studies and reviewed the performance of the capital markets in recent years and other factors and advice from various third parties, such as the pension plans’ advisors, investment managers and actuaries. While the Company considered both the recent performance and the historical performance of pension plan assets, the Company’s assumptions are based primarily on its estimates of long-term, prospective rates of return. Using the aforementioned methodologies, the Company selected the 6.9% long-term rate of return on plan assets assumption used for the pension plans during fiscal 2011. Differences between actual and expected asset returns are recognized in the net periodic benefit cost over the remaining service period of the active participating employees.
The rate of future compensation increases is an assumption used by the actuarial consultants for pension accounting and is determined based on the Company’s current expectation for such increases.
Pension plan assets
The following table presents information on the fair value of the defined benefit plans’ assets at October 31, 2011, 2010 and 2009, respectively:
|
| | | | | | | | |
| | Defined Benefit Plans |
| | 2011 | | 2010 | | 2009 |
| | $ | | $ | | $ |
| Fair value of plan assets | 80.3 |
| | 66.0 |
| | 55.5 |
|
The Pension Committee for the Company’s defined benefit plans (the “Pension Committee”) has adopted (and revises from time to time) an investment policy for the Canadian and U.K. defined benefit plans with the objective of meeting or exceeding, over time, the expected long-term rate of return on plan assets assumption, weighed against a reasonable risk level. In connection with this objective, the Pension Committee retains professional investment managers that invest plan assets in the following asset classes: equity and fixed income securities and cash and other investments, which may include hedge funds and private equity and global balanced strategies.
The Company’s defined benefit plans currently have the following targets for these asset classes, which are intended to be flexible guidelines for allocating the plans’ assets among various classes of assets, and are reviewed periodically and considered for readjustment when an asset class weighting is outside of its target (recognizing that these are flexible targets that may vary from time to time) with the objective of achieving the expected long-term rate of return on plan assets assumption, weighed against a reasonable risk level, as follows:
|
| | | |
| | Defined Benefit Plans Canada | Defined Benefit Plans U.K. |
| Asset Category: | | |
| Domestic equity securities | 25-55% | 38 | % |
| Foreign equity securities | 5-30% | 37 | % |
| Debt securities | 30-70% | 25 | % |
| Other | 0-20% | — | % |
The fair value hierarchy must have the following levels: (a) quoted prices (unadjusted) in active markets for identical assets or liabilities (Level 1); (b) inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e., as prices) or indirectly (i.e., derived from prices) (Level 2); and (c) inputs for the asset or liability that are not based on observable market data (unobservable inputs) (Level 3).
The fair values of the defined benefit plans’ assets at October 31, 2011, by asset categories were as follows:
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | | | | | | | | | |
| Defined Benefit Plans | Total | | Level 1 | | Level 2 | | Level 3 |
| | $ | | $ | | $ | | $ |
| Asset Category: | | | | | | | |
| Equity securities | 44.4 |
| | — |
| | 44.4 |
| | — |
|
| Debt securities | 13.9 |
| | — |
| | 13.9 |
| | — |
|
| Cash and other investments: | 22.0 |
| | — |
| | 22.0 |
| | — |
|
| Total pension plan assets at fair value | 80.3 |
| | — |
| | 80.3 |
| | — |
|
Within the equity securities asset class, the investment policy adopted by the Pension Committee provides for investments in a broad range of publicly-traded securities ranging from domestic and international stocks and small to large capitalization stocks. Within the debt securities asset class, the investment policy provides for investments in a broad range of publicly-traded debt securities, including domestic and international treasury issues, and corporate debt securities. In the cash and other investments asset class, investments may be in cash and cash equivalents and other investments, which may include hedge funds and private equity not covered in the classes listed above, provided that such investments are approved by the Pension Committee prior to their selection.
The Pension Committee’s investment policy does not allow the use of derivatives for speculative purposes, but such policy does allow its investment managers to use derivatives for the purpose of reducing risk exposures or to replicate exposures of a particular asset class.
Estimated future benefit payments
The following benefit payments, which reflect expected future service, as appropriate, are expected to be paid out of the Company's defined benefit plans and other post-retirement benefit plans:
|
| | | | | |
| | Total Defined Benefit Plan Payments | | Total Other Benefit Plan Payments |
| | $ | | $ |
| 2011 | 2.3 |
| | 0.2 |
|
| 2012 | 2.5 |
| | 0.2 |
|
| 2013 | 2.5 |
| | 0.2 |
|
| 2014 | 2.7 |
| | 0.3 |
|
| 2015 | 3.1 |
| | 0.3 |
|
| 2016 through 2020 | 14.4 |
| | 2.0 |
|
| | 27.5 |
| | 3.2 |
|
The Company expects to incur approximately $1.4 million of amortization from actuarial losses as part of its pension costs in fiscal 2012.
Deferred Revenues
The following table summarizes the deferred revenue activity for each of fiscal 2011, 2010 and 2009:
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
|
| | | |
| Balance at November 1, 2008 | $ | 22.5 |
|
| Cash received from customers | 10.5 |
|
| Cash repaid to customers | — |
|
| Amortization of deferred revenues | (1.0 | ) |
| Foreign exchange | 0.5 |
|
Other (1) | 9.2 |
|
| Balance at October 31, 2009 | $ | 41.7 |
|
| Cash received from customers | 47.4 |
|
| Cash repaid to customers | (1.5 | ) |
| Amortization of deferred revenues | (37.4 | ) |
| Foreign exchange | (0.2 | ) |
Other (2) | (4.1 | ) |
| Balance at October 31, 2010 | $ | 45.9 |
|
| Cash received from customers | 30.4 |
|
| Cash repaid to customers | (3.1 | ) |
| Amortization of deferred revenues | (45.0 | ) |
| Foreign exchange | 0.2 |
|
Other (2) | 8.1 |
|
| Balance at October 31, 2011 | $ | 36.5 |
|
(1) Other changes to deferred revenues primarily consist of equipment received from customers in relation to certain manufacturing agreements in which he Company will retain ownership.
(2) Other changes to deferred revenues primarily consist of movement between deferred revenue and other long-term liabilities associated with the amendments and eventual cancellation of a manufacturing agreement.
Lease Expenses
The Company’s total rental expenses related to its operating leases for fiscal 2011, 2010 and 2009 were $7.2 million, $6.0 million and $4.1 million, respectively.
Stock-Based Compensation
The total fair value of shares that vested during fiscal 2011, 2010 and 2009 was $2.0 million, $1.3 million and $1.6 million, respectively. As of October 31, 2011, the total unrecognized compensation cost related to the nonvested stock options was $7.5 million, which is expected to be recognized through fiscal 2016, with a weighted average remaining vesting period of 2.58 years.
Impact of new and pending U.S. GAAP accounting standards
In September 2011, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2011-08, “Intangibles—Goodwill and Other (Topic 350): Testing Goodwill for Impairment,” which amends U.S. GAAP ASC 350, “Intangible Assets—Goodwill and Other.” Under this new ASU, an entity may elect the option to assess qualitative factors to determine whether it is necessary to perform the first step in the two-step impairment testing process. This guidance is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011, with early adoption permitted. The Company does not expect this ASU would have a material impact on its consolidated financial statements if prepared under U.S. GAAP.
In June 2011, the FASB issued ASU 2011-05, “Comprehensive Income (Topic 220): Presentation of Comprehensive Income.” This standard eliminates the current option to report other comprehensive income and its components in the statement of changes in equity. Under this new ASU, an entity can elect to present items of net income and other comprehensive income in one continuous statement or in two separate, but consecutive, statements. This guidance is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. Early adoption is permitted, but full retrospective
Patheon Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
October 31, 2011, 2010 and 2009
(Dollar information in tabular form is expressed in millions of U.S. dollars)
application is required. As the Company reports net (loss) income and comprehensive (loss) income in two statements, the adoption of this ASU will not impact the presentation of the Company's consolidated financial statements if presented in accordance with U.S. GAAP.
In May 2011, the FASB issued ASU 2011-04, “Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs.” This ASU modifies the existing standards to include disclosure of all transfers between Level 1 and Level 2 asset and liability fair value categories. In addition, the ASU provides guidance on measuring the fair value of financial instruments managed within a portfolio and the application of premiums and discounts on fair value measurements. The ASU requires additional disclosure for Level 3 measurements regarding the sensitivity of fair value to changes in unobservable inputs and any interrelationships between those inputs. This guidance is effective for interim and annual periods beginning after December 15, 2011, with early adoption prohibited. The Company does not expect this ASU would have a material impact on its consolidated financial statements if prepared under U.S. GAAP.
EXHIBIT INDEX
|
| | | | | | | | | | | |
| | | Incorporated By Reference | |
Exhibit Number | Description | Form | Filing Date | Number | Filed Herewith |
| 3.1 | Articles of Amalgamation of Patheon Inc. (“Patheon”). | 10/A | 4/13/2011 | 3.1 | |
| 3.2 | Amendment, dated April 26, 2007, to Articles of Amalgamation of Patheon. | 10/A | 4/13/2011 | 3.2 | |
| 3.3 | By-Laws of Patheon dated March 27, 2008. | 10 | 2/25/2011 | 3.3 | |
| 4.1 | Form of Patheon's Share Certificate. | 10 | 2/25/2011 | 4.1 | |
| 4.2 | Indenture dated April 23, 2010 among Patheon, certain subsidiaries of Patheon as Guarantors, U.S. Bank National Association and Deutsche Bank Trust Company Americas, with respect to the 8.625% Senior Secured Notes due 2017. | 10 | 2/25/2011 | 4.2 | |
| 4.3 | Form of 8.625% Senior Secured Notes due 2017 (included in Exhibit 4.2). | 10 | 2/25/2011 | 4.3 | |
| 10.1 | Amended and Restated Revolving Credit Agreement dated April 23, 2010 among Patheon, certain subsidiaries of Patheon as Guarantors, the lenders party thereto, JPMorgan Chase Bank, N.A., as U.S. Administrative Agent, JPMorgan Chase Bank, N.A., as Canadian Administrative Agent, and Toronto Branch and J.P. Morgan Europe Limited, as European Administrative Agent. | 10 | 2/25/2011 | 10.1 | |
| 10.2 | Purchase Agreement dated March 1, 2007 between Patheon and JLL Partners Fund V, L.P. | 10 | 2/25/2011 | 10.2 | |
| 10.3 | Investor Agreement dated April 27, 2007 between Patheon and JLL Patheon Holdings, LLC. | 10 | 2/25/2011 | 10.3 | |
| 10.4 | Redemption Waiver Agreement dated September 4, 2008 between Patheon and JLL Patheon Holdings, LLC. | 10 | 2/25/2011 | 10.4 | |
| 10.5 | Settlement Agreement dated November 29, 2009 between Patheon and JLL Patheon Holdings, LLC. | 10 | 2/25/2011 | 10.5 | |
| 10.6 | Lease Agreement dated January 15, 1996 between Lansdown Estates Group Limited and Oxford Asymmetry Limited, assigned to Patheon U.K. Limited on May 3, 2006 in respect of the Milton Park Facility. | 10 | 2/25/2011 | 10.6 | |
| 10.7 | Licence to Assign Lease Agreement in respect of the Milton Park Facility dated April 28, 2006 among MEPC Milton Park No. 1 Limited and MEPC Milton Park No. 2 Limited, EVOTEC (UK) Limited and Patheon UK Limited. | 10 | 2/25/2011 | 10.7 | |
| 10.8 | Contract for the Sale of Leasehold Land in respect of the Milton Park Facility dated May 3, 2006 between EVOTEC (UK) Limited and Patheon UK Limited. | 10 | 2/25/2011 | 10.8 | |
| 10.9 | Assignment of Leasehold Property in respect of the Milton Park Facility dated May 3, 2006 between EVOTEC (UK) Limited and Patheon UK Limited. | 10 | 2/25/2011 | 10.9 | |
| 10.10 | Lease Agreement dated December 1, 1993 between Custom Pharmaceuticals and Promix Laboratories, Divisions of Patheon, and The Cadillac Fairview Corporation Limited in respect of the Burlington Facility. | 10 | 2/25/2011 | 10.10 | |
| 10.11 | Lease Renewal Agreement dated April 10, 2004 between Patheon and Klaus Stephan Reeckmann in respect of the Burlington Facility. | 10 | 2/25/2011 | 10.11 | |
| 10.12 | 2011 Amended and Restated Incentive Stock Option Plan.* | 10-Q | 9/9/2011 | 10.2 | |
| 10.13 | Form of Stock Option Agreement under the Incentive Stock Option Plan for certain awards granted on or before March 17, 2010. * | 10 | 2/25/2011 | 10.13 | |
| 10.14 | Form of Stock Option Agreement under the Incentive Stock Option Plan for certain awards granted on or after March 17, 2010. * | 10 | 2/25/2011 | 10.14 | |
| 10.15 | Amended and Restated Restricted Share Unit Plan of Patheon dated September 4, 2008 (the “Restricted Share Unit Plan”).* | 10 | 2/25/2011 | 10.15 | |
|
| | | | | | | | | | | |
| 10.16 |
| Performance Share Unit Plan of Patheon dated December 13, 2007 (the “Performance Share Unit Plan”).* | 10 | 2/25/2011 | 10.16 | | |
| 10.17 |
| Form of Performance Share Unit Grant Agreement under the Performance Share Unit Plan.* | 10 | 2/25/2011 | 10.17 | | |
| 10.18 |
| Directors Deferred Share Unit Plan of Patheon dated February 22, 2008, as amended March 27, 2008.* | 10 | 2/25/2011 | 10.18 | | |
| 10.19 |
| Description of Compensation for Non-Employee Directors of Patheon.* | 10 | 2/25/2011 | 10.19 | | |
| 10.20 |
| Deferred Compensation Plan of Patheon dated January 1, 2003, as amended December 18, 2008.* | 10 | 2/25/2011 | 10.20 | | |
| 10.21 |
| The Patheon Group's Leadership Incentive Plan.* | 10-Q | 9/9/2011 | 10.1 | | |
| 10.22 |
| The Patheon Global Bonus Plan.*
| | | | X | |
| 10.23 |
| Employment Agreement between Patheon Pharmaceuticals Services Inc. and James C. Mullen effective February 7, 2011.* | 10 | 2/25/2011 | 10.21 | | |
| 10.24 |
| Amended and Restated Employment Agreement dated April 25, 2011 between Patheon Pharmaceuticals Services Inc. and James C. Mullen effective February 7, 2011.* | 10-Q | 6/10/2011 | 10.2 | | |
| 10.25 |
| Employment Agreement between Patheon Pharmaceuticals Services Inc. and Wesley P. Wheeler dated December 3, 2007.* | 10 | 2/25/2011 | 10.22 | | |
| 10.26 |
| First Amendment, dated May 5, 2009, to Employment Agreement between Patheon Pharmaceuticals Services Inc. and Wesley P. Wheeler dated December 3, 2007.* | 10 | 2/25/2011 | 10.23 | | |
| 10.27 |
| Separation Agreement between Patheon Pharmaceuticals Services Inc. and Wesley P. Wheeler dated November 30, 2010.* | 10 | 2/25/2011 | 10.24 | | |
| 10.28 |
| Employment Agreement between Patheon Pharmaceuticals Services Inc. and Peter T. Bigelow dated December 31, 2009.* | 10 | 2/25/2011 | 10.25 | | |
| 10.29 |
| Consulting and Separation Agreement between Patheon Pharmaceuticals Services Inc. and Peter T. Bigelow dated October 5, 2011.* | 8-K | 10/6/2011 | 10.1 | | |
| 10.30 |
| Employment Agreement between Patheon Pharmaceuticals Services Inc. and Eric W. Evans effective May 27, 2008.* | 10 | 2/25/2011 | 10.26 | | |
| 10.31 |
| Severance and General Release Agreement between Patheon Pharmaceuticals Services Inc. and Eric Evans dated November 1, 2011.* | | | | X | |
| 10.32 |
| Employment Agreement between Patheon Pharmaceuticals Services Inc. and Warren A. Horton dated January 22, 2010.* | 10 | 2/25/2011 | 10.27 | | |
| 10.33 |
| Summary of Key Terms of the Employment Arrangement between Patheon Pharmaceuticals Services Inc. and Mark J. Kontny dated March 17, 2010.* | 10 | 2/25/2011 | 10.28 | | |
| 10.34 |
| Offer Letter from Patheon to Doaa A. Fathallah dated February 20, 2008.* | 10 | 2/25/2011 | 10.29 | | |
| 10.35 |
| Employment Agreement between Patheon International GmbH and Doaa A. Fathallah effective May 6, 2008.* | 10 | 2/25/2011 | 10.30 | | |
| 10.36 |
| First Amendment, dated June 29, 2010, to Employment Agreement between Patheon International AG, as successor entity to Patheon International GmbH, and Doaa A. Fathallah effective May 6, 2008.* | 10 | 2/25/2011 | 10.31 | | |
| 10.37 |
| Addendum, effective December 17, 2009, to Employment Agreement between Patheon International AG (GmbH) and Doaa A. Fathallah effective May 6, 2008.* | 10 | 2/25/2011 | 10.32 | | |
| 10.38 |
| Employment Agreement between Patheon Pharmaceuticals Services Inc. and Paul M. Garofolo dated May 12, 2008.* | 10 | 2/25/2011 | 10.33 | | |
| 10.39 |
| First Amendment, dated November 23, 2008, to Employment Agreement between Patheon Pharmaceuticals Services Inc. and Paul M. Garofolo dated May 12, 2008.* | 10 | 2/25/2011 | 10.34 | | |
| 10.40 |
| Second Amendment, dated August 1, 2011, to Employment Agreement between Patheon Pharmaceuticals Services Inc. and Paul M. Garofolo dated May 12, 2008.* | | | | X | |
| 10.41 |
| Employment Agreement between Patheon Pharmaceuticals Services Inc. and Geoffrey M. Glass dated March 17, 2009.* | 10 | 2/25/2011 | 10.35 | | |
|
| | | | | | | | | | | |
| 10.42 |
| Addendum, effective October 1, 2009, to Employment Agreement between Patheon Pharmaceuticals Services Inc. and Geoffrey M. Glass dated March 17, 2009.* | 10 | 2/25/2011 | 10.36 | | |
| 10.43 |
| Employment Agreement between Patheon Limited UK and Andrew Kelley effective November 1, 2005.* | 10 | 2/25/2011 | 10.37 | | |
| 10.44 |
| Amendment, dated August 16, 2006, to Employment Agreement between Patheon Limited UK and Andrew Kelley effective November 1, 2005.* | 10 | 2/25/2011 | 10.38 | | |
| 10.45 |
| Addendum, dated January 31, 2009, to Employment Agreement between Patheon Limited UK and Andrew Kelley effective November 1, 2005.* | 10 | 2/25/2011 | 10.39 | | |
| 10.46 |
| Amendment, dated January 28, 2011, to Employment Agreement between Patheon Limited UK and Andrew Kelley effective November 1, 2005.*
| 10 | 2/25/2011 | 10.4 | | |
| 10.47 |
| Employment Agreement between Patheon Pharmaceuticals Services Inc. and Michael E. Lytton effective May 9, 2011.*
| 10-Q | 9/9/2011 | 10.3 | | |
| 10.48 |
| Employment Agreement between Patheon Italia S.p.A. and Antonella Mancuso dated September 3, 2001.*
| 10 | 2/25/2011 | 10.41 | | |
| 10.49 |
| Employment Agreement between Rebecca Holland New and Patheon Pharmaceuticals Services Inc. dated August 15, 2011.*
| | | | X | |
| 10.50 |
| Form of Indemnification Agreement entered into between Patheon and each of Ramsey A. Frank, Paul S. Levy, Thomas S. Taylor and Derek J. Watchorn.*
| 10 | 2/25/2011 | 10.42 | | |
| 21.1 |
| Subsidiaries of Patheon.
| | | | X | |
| 23.1 |
| Consent of Ernst & Young LLP.
| | | | X | |
| 31.1 |
| Certification by Principal Executive Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
| | | | X | |
| 31.2 |
| Certification by Principal Financial Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
| | | | X | |
| 32.1 |
| Certification by Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
| | | | X | |
| 32.2 |
| Certification by Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
| | | | X | |
__________________
* Represents a management contract or compensatory plan or arrangement.
 Note: API: Active Pharmaceutical Ingredient
Note: API: Active Pharmaceutical Ingredient