Form 6-K
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF THE
SECURITIES EXCHANGE ACT OF 1934
For the month of June 2010
Commission File Number: 001-33623
WuXi PharmaTech (Cayman) Inc.
288 Fute Zhong Road, Waigaoqiao Free Trade Zone
Shanghai 200131
People’s Republic of China
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F x Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ¨
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes ¨ No x
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b):82-N/A
WuXi PharmaTech (Cayman) Inc.
Form 6-K
TABLE OF CONTENTS
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | |
| | WuXi PharmaTech (Cayman) Inc. |
| | |
| | By: | | /s/ GE LI |
| | Name: | | Ge Li |
| | Title: | | Chairman and Chief Executive Officer |
| | |
| Date: June 29, 2010 | | | | |
3

charles river
accelerating drug development. exactly.
Wuxi Pharma Tech
Creating a Leading Global CRO to Serve the Pharmaceutical Industry and Patients
Dr. Ge Li, WuXi Chairman/CEO Edward Hu, WuXi COO

Cautionary Note Regarding Forward-Looking Statements
Statements in this presentation contain “forward-looking” statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and as defined in the Private Securities Litigation Reform Act of 1995. These forward-looking statements, including those with respect to favorable industry trends, guidance for full-year 2010 performance, anticipated strong financial results for 2010 and beyond, and anticipated benefits of the proposed combination with Charles River Laboratories, are not historical facts but instead represent only our belief regarding future events, many of which, by their nature, are inherently uncertain and outside of our control. Our actual results and financial condition and other circumstances may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements. Risks with respect to the proposed combination include: (i) the possibility that the proposed combination may be delayed or not completed due to the failure to obtain stockholder or regulatory approvals or otherwise satisfy the conditions to the proposed combination as set forth in our acquisition agreement with Charles River; (ii) problems may arise in successfully integrating the businesses of the two companies; (iii) the acquisition may involve unexpected costs; (iv) the combined company may not achieve the anticipated transaction benefits; (v) restrictions in our acquisition agreement with Charles River that require us to conduct our business in the ordinary course consistent with past practices and in accordance with other specific limitations may delay or prevent us from taking advantage of business opportunities that may arise prior to the combination; and (vi) the businesses may suffer as a result of uncertainty surrounding the combination. In addition, the businesses may be subject to future regulatory or legislative actions and other risk factors. These other risk factors include: continued uncertainty in the global economy, the pressures being felt by our customers, and pharmaceutical industry consolidation may adversely impact our business and the trends for outsourced R&D and manufacturing for longer than expected or more severely than expected; we may be unable to successfully make our planned investments and capital expenditures on a timely basis, these investments may not yield the desired results, and we may need to modify the nature and level of our investments and capital expenditures; pharmaceutical companies may not change their business models as expected or in a manner favorable to us; we may fail to capitalize on the opportunities presented; we may not maintain our preferred provider status with our clients and may be unable to successfully expand our capabilities to meet client needs. In addition, other factors that could cause our actual results to differ from what we currently anticipate include failure to generate sufficient future cash flows or to secure any required future financing on acceptable terms or at all; failure to retain key personnel; effective integration of continuing products and services from AppTec; our reliance on a limited number of customers to continue to account for a high percentage of our revenues; risk of payment failure by any of our large customers, which could significantly harm our cash flows and profitability; dependence upon the continued service of our senior management and key scientific personnel; and our ability to retain our existing customers or expand our customer base. You should read the financial information contained in this presentation in conjunction with the consolidated financial statements and related notes thereto included in our 2009 Annual Report on Form 20-F filed with and available on the Securities and Exchange Commission’s (“SEC’s”) website at http://www.sec.gov. For additional information on these and other important factors that could adversely affect our or Charles River’s business, financial condition, results of operations and prospects, see “Risk Factors” (i) beginning on page 6 of our 2009 Annual Report on Form 20-F and (ii) beginning on page 18 of Charles River’s Annual Report on Form 10-K also filed at the SEC’s website. Our results of operations for first-quarter 2010 are not necessarily indicative of our operating results for any future periods. All projections in this presentation are based on limited information currently available to us, which is subject to change. Although these projections and the factors influencing them will likely change, we undertake no obligation to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this presentation, except as required by law. Such information speaks only as of the date of this presentation.

Use of Non-GAAP and Pro-Forma Financial Measures
We have provided 2009 and 2010 gross profit and operating income on a non-GAAP basis, which excludes share-based compensation expenses and amortization of acquired intangible assets and associated deferred tax impact. We believe both management and investors benefit from referring to these non-GAAP and pro-forma financial measures in assessing our financial performance and liquidity and when planning and forecasting future periods. These non-GAAP operating measures are useful for understanding and assessing underlying business performance and operating trends. We expect to continue providing gross profit and operating income on a non-GAAP basis using a consistent method on a quarterly basis. You should not view non-GAAP results on a stand-alone basis or as a substitute for results under GAAP, or as being comparable to results reported or forecasted by other companies, and should refer to the reconciliation of non-GAAP measures to GAAP measures for the indicated periods attached hereto.
WuXi PharmaTech
2
Key Discussion Points
Transaction Overview and Status Update
WuXi Overview
Positioning of Combined Company
Integration Update
WuXi PharmaTech
3
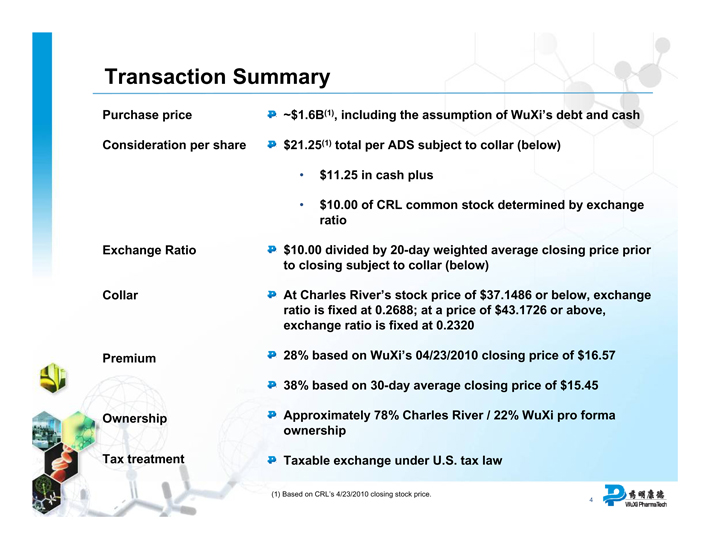
Transaction Summary
Purchase price ~$1.6B(1), including the assumption of WuXi’s debt and cash
Consideration per share $21.25(1) total per ADS subject to collar (below)
• $11.25 in cash plus
• $10.00 of CRL common stock determined by exchange ratio
Exchange Ratio $10.00 divided by 20-day weighted average closing price prior to closing subject to collar (below)
Collar At Charles River’s stock price of $37.1486 or below, exchange ratio is fixed at 0.2688; at a price of $43.1726 or above, exchange ratio is fixed at 0.2320
Premium 28% based on WuXi’s 04/23/2010 closing price of $16.57 38% based on 30-day average closing price of $15.45
Ownership Approximately 78% Charles River / 22% WuXi pro forma ownership
Tax treatment Taxable exchange under U.S. tax law
(1) Based on CRL’s 4/23/2010 closing stock price.
WuXi PharmaTech
4
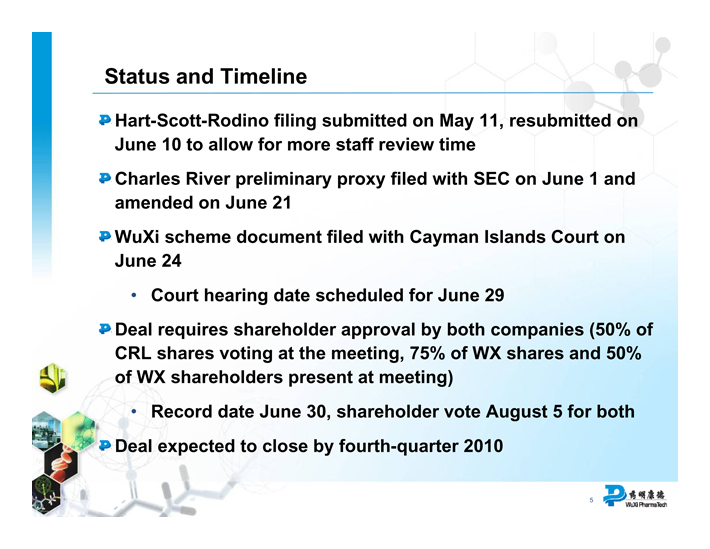
Status and Timeline
Hart-Scott-Rodino filing submitted on May 11, resubmitted on June 10 to allow for more staff review time Charles River preliminary proxy filed with SEC on June 1 and amended on June 21 WuXi scheme document filed with Cayman Islands Court on June 24
• Court hearing date scheduled for June 29
Deal requires shareholder approval by both companies (50% of CRL shares voting at the meeting, 75% of WX shares and 50% of WX shareholders present at meeting)
• Record date June 30, shareholder vote August 5 for both Deal expected to close by fourth-quarter 2010
WuXi PharmaTech
5
Key Discussion Points
Transaction Overview and Status Update
WuXi Overview
Positioning of Combined Company Integration Update
WuXi PharmaTech
6
Profile of WuXi
Leading China-based contract research organization serving the pharmaceutical, biotech, and medical device industries
• Virtually all of the largest pharmaceutical companies are customers, several for nearly a decade
World-class operations in China and the United States
• One of the largest employers of chemists in the pharmaceutical industry
Founded in 2000 to provide compound synthesis services
Has diversified throughout the past decade, adding downstream drug discovery and preclinical services
Now offers a broad and integrated portfolio of laboratory and manufacturing services
WuXi PharmaTech
7
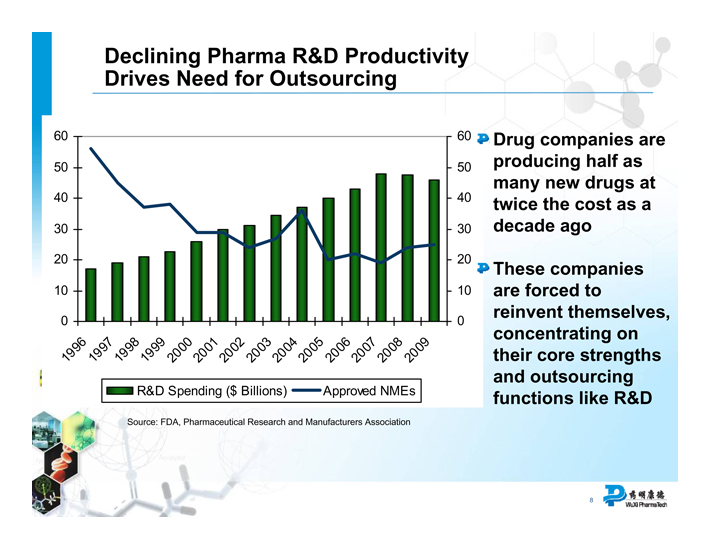
Declining Pharma R&D Productivity Drives Need for Outsourcing
60
50
40
30
20
10
0
1996
1997
1998
1999
2000
2001
2002
2003
2004
2005
2006
2007
2008
2009
60
50
40
30
20
10
0
R&D Spending ($ Billions) Approved NMEs
Source: FDA, Pharmaceutical Research and Manufacturers Association
Drug companies are producing half as many new drugs at twice the cost as a decade ago
These companies are forced to reinvent themselves, concentrating on their core strengths and outsourcing functions like R&D
WuXi PharmaTech
8
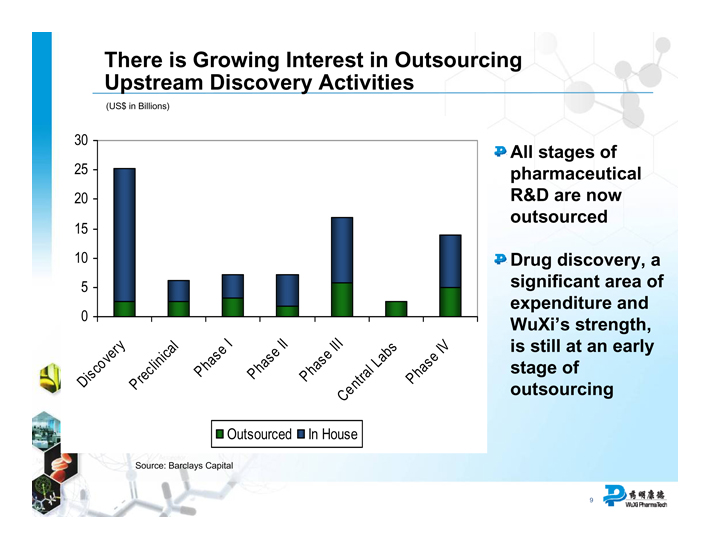
There is Growing Interest in Outsourcing Upstream Discovery Activities
(US$ in Billions)
30
25
20
15
10
5
0
Discovery Preclinical Phase I Phase II Phase III Central Labs Phase IV
Outsourced In House
Source: Barclays Capital
All stages of pharmaceutical R&D are now outsourced
Drug discovery, a significant area of expenditure and WuXi’s strength, is still at an early stage of outsourcing
WuXi PharmaTech
9

WuXi’s Unique Competitive Strengths
Wuxi Apptec
Integrated R&D Service Platform
Experienced International Management Team
Highly Educated, Trained, & Motivated Workforce
Reputation for Operational Excellence
World-Class Facilities in China and the United States
Intense Focus on a Diversified, High-Quality Customer Base
Strong Protection of Customers’ Intellectual Property
China Cost Advantage
WuXi PharmaTech
10
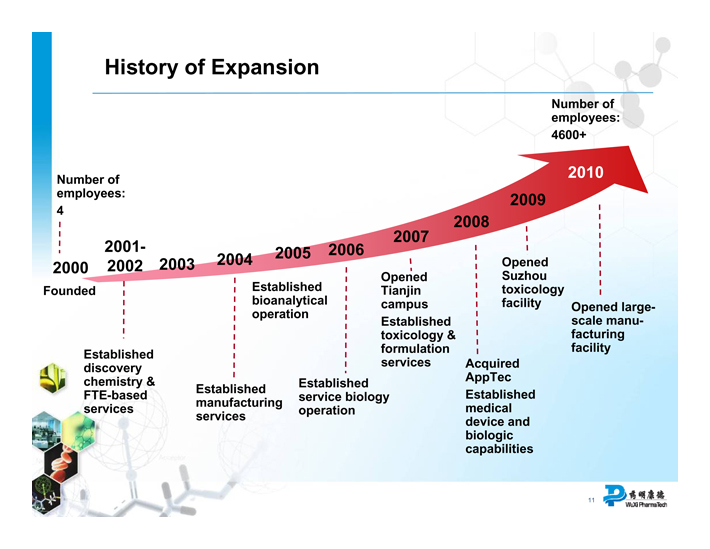
History of Expansion
Number of employees:
4
2000
Founded
2001-
2002
Established discovery chemistry & FTE-based services
2003
2004
Established manufacturing services
2005
Established bioanalytical operation
2006
Established service biology operation
2007
Opened Tianjin campus Established toxicology & formulation services
2008
Acquired AppTec Established medical device and biologic capabilities
2009
Opened Suzhou toxicology facility
Number of employees:
4600+
2010
Opened large-scale manufacturing facility
WuXi PharmaTech
11
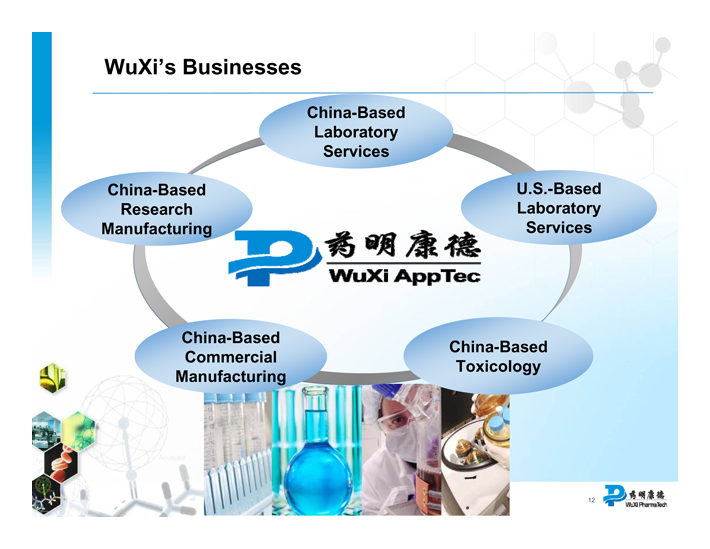
WuXi’s Businesses
Wuxi Apptec
China-Based Laboratory Services
U.S.-Based Laboratory Services
China-Based Toxicology
China-Based Commercial Manufacturing
China-Based Research Manufacturing
WuXi PharmaTech
12
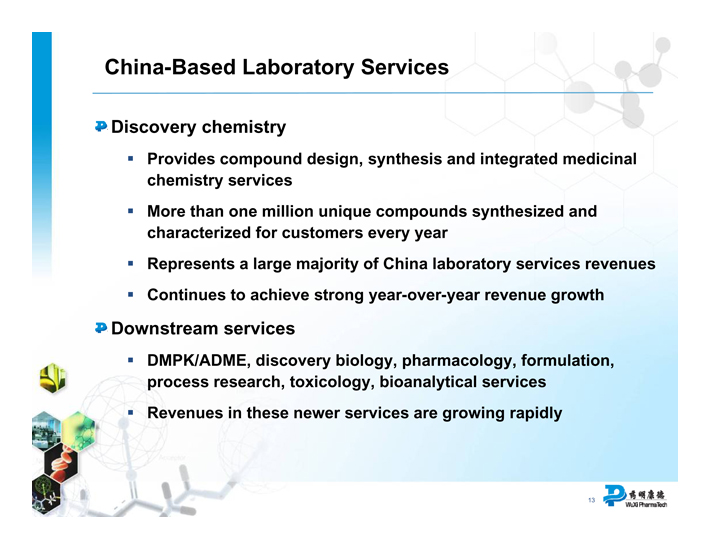
China-Based Laboratory Services
Discovery chemistry
Provides compound design, synthesis and integrated medicinal chemistry services
More than one million unique compounds synthesized and characterized for customers every year
Represents a large majority of China laboratory services revenues
Continues to achieve strong year-over-year revenue growth
Downstream services
• DMPK/ADME, discovery biology, pharmacology, formulation, process research, toxicology, bioanalytical services
• Revenues in these newer services are growing rapidly
WuXi PharmaTech
13
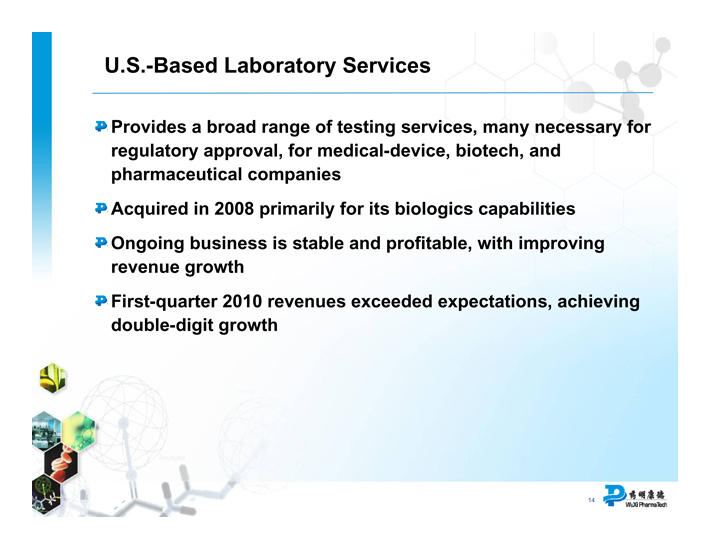
U.S.-Based Laboratory Services
Provides a broad range of testing services, many necessary for regulatory approval, for medical-device, biotech, and pharmaceutical companies
Acquired in 2008 primarily for its biologics capabilities
Ongoing business is stable and profitable, with improving revenue growth
First-quarter 2010 revenues exceeded expectations, achieving double-digit growth
WuXi PharmaTech
14

China-Based Research Manufacturing
Driven by superior capabilities of more than 200 WuXi process chemists
Revenue and profitability dependent on project mix
Depressed revenue in 2009 caused by constrained pharmaceutical R&D budgets; however, demand is substantially improving in 2010
Serves as a valuable feeder for future commercial manufacturing
WuXi PharmaTech
15

China-Based Commercial Manufacturing
Recently opened facility in Jinshan quadruples our manufacturing capacity
Expected to start generating revenues in second-half of 2010
Multiple commercial manufacturing programs being considered
Significant commercial manufacturing revenues expected in 2011 and beyond
WuXi PharmaTech
16

China-Based Toxicology
China, with less than 1% of the world’s toxicology market, is expected to be a major player in 5-10 years
Suzhou toxicology facility is the largest in China
Signed collaboration agreement with Johnson & Johnson
Non-GLP toxicology, client-sponsored GLP validation studies under way
GLP inspections by SFDA and OECD completed
Expect to offer GLP toxicology services in very near future
Significant revenue ramp-up beginning in 2011
WuXi PharmaTech
17

Management Team with Broad International Experience
Dr. Ge Li Chairman and CEO – US
Mr. Edward Hu COO Tanox – US
Dr. Shuhui Chen CSO – US
Dr. Suhan Tang CMO Schering Plough – US
Mr. Xiaozhong Liu EVP Entrepreneur
Mr. Felix Hsu SVP - US – US
Dr. Richard Soll SVP, Integrated Services – US
Mr. Hao Zhou CFO – US
Ms. Trabue Bryans VP/GM – Atlanta – US
Dr. Hui Cai VP, BD – US
Dr. Chichung Chan VP, Pharmacology – US
Mr. Wei-Min Chang VP, Operations GM STA – US
Dr. Minzhang Chen VP, Process R&D – US
Mr. Bob Coldreck VP, GLP QA – US
Dr. Tao Guo VP, Medicinal Chemistry – US
Dr. Deepak Hegde VP, PDS USV – India
Dr. Joseph Hughes VP, Testing- Philadelphia – US
Mr. Scott Kramer VP, Finance & Admin, US Operations – US
Dr. Qiang Lü VP, Biology – US
Dr. Rujian Ma VP, Synthetic Chemistry
Dr. Masai Naruhito VP, BD Japan – Japan
Ms. Lisa Olson VP/GM, St. Paul – US
Mr. Yifeng Shi VP, Operations Head of Waigaoqiao Site – US
Dr. Garry Takle VP Philadelphia Operations – US
Ms. Teri Tanquist VP, Process Improvement and Operations - US – US
Dr. Angela Wong VP, DMPK/ADME – US
Dr. Chengde Wu VP, Medicinal Chemistry – US
Dr. Jinsong Xing VP, Bioanalytical Services BMS – US
Mr. Zhaohui Zhang VP, Domestic Marketing Entrepreneur
Dr. Ning Zhao Lead Advisor, Analytical BMS – US
An average of 15 years of experience in major pharmaceutical, biotech and medical device companies; over 100 senior managers are oversea returnees.
WuXi PharmaTech
18
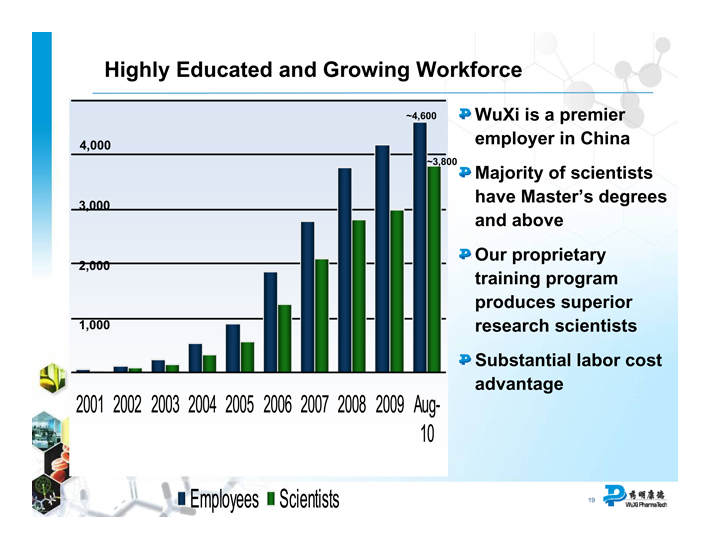
Highly Educated and Growing Workforce
4,000
3,000
2,000
1,000
2001
2002 2003 2004 2005 2006 2007 2008 2009 Aug-
10
Employees Scientists
WuXi is a premier employer in China
Majority of scientists have Master’s degrees and above
Our proprietary training program produces superior research scientists
Substantial labor cost advantage
WuXi PharmaTech
19
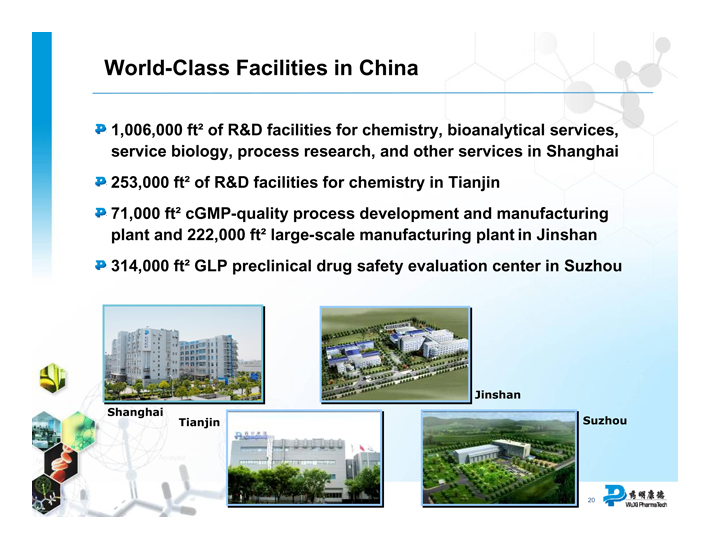
World-Class Facilities in China
1,006,000 ft2 of R&D facilities for chemistry, bioanalytical services, service biology, process research, and other services in Shanghai
253,000 ft2 of R&D facilities for chemistry in Tianjin
71,000 ft2 cGMP-quality process development and manufacturing plant and 222,000 ft2 large-scale manufacturing plant in Jinshan
314,000 ft2 GLP preclinical drug safety evaluation center in Suzhou
Shanghai
Tianjin
Jinshan
Suzhou
WuXi PharmaTech
20

World-Class FDA-Registered Facilities in the United States
82,000 sq.ft. of facilities offers services in in vitro and in vivo biocompatibility, toxicology, and processing for tissue-based products in St. Paul
51,000 sq.ft. of facilities offers services in microbiology, medical- device chemistry, sterilization validations, and package testing in Atlanta
75,000 sq.ft. facility provides biologics testing services in Philadelphia
WuXi PharmaTech
21
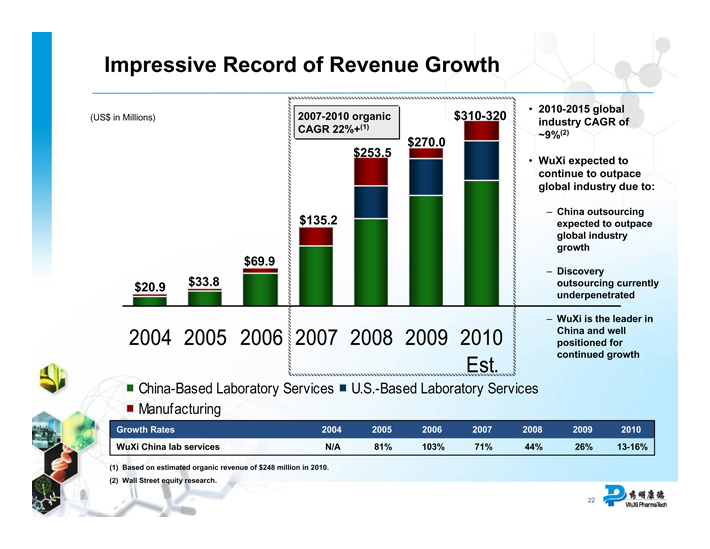
Impressive Record of Revenue Growth
(US$ in Millions)
$20.9
$33.8
$69.9
$135.2
$253.5
$270.0
$310-320
2007-2010 organic CAGR 22%+(1)
China-Based Laboratory Services
U.S.-Based Laboratory Services
Manufacturing
2010-2015 global industry CAGR of 9%(2)
WuXi expected to continue to outpace global industry due to:
– China outsourcing expected to outpace global industry growth
– Discovery outsourcing currently underpenetrated
– WuXi is the leader in China and well positioned for continued growth
Growth Rates 2004 2005 2006 2007 2008 2009 2010
WuXi China lab services N/A 81% 103% 71% 44% 26% 13-16%
(1) Based on estimated organic revenue of $248 million in 2010. (2) Wall Street equity research.
WuXi PharmaTech
22
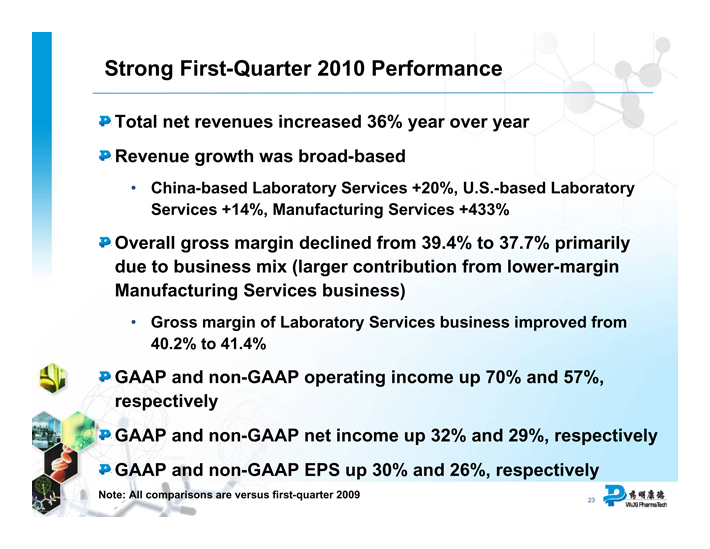
Strong First-Quarter 2010 Performance
Total net revenues increased 36% year over year
Revenue growth was broad-based
China-based Laboratory Services +20%, U.S.-based Laboratory Services +14%, Manufacturing Services +433%
Overall gross margin declined from 39.4% to 37.7% primarily due to business mix (larger contribution from lower-margin Manufacturing Services business)
Gross margin of Laboratory Services business improved from 40.2% to 41.4%
GAAP and non-GAAP operating income up 70% and 57%, respectively
GAAP and non-GAAP net income up 32% and 29%, respectively
GAAP and non-GAAP EPS up 30% and 26%, respectively
Note: All comparisons are versus first-quarter 2009
WuXi PharmaTech
23
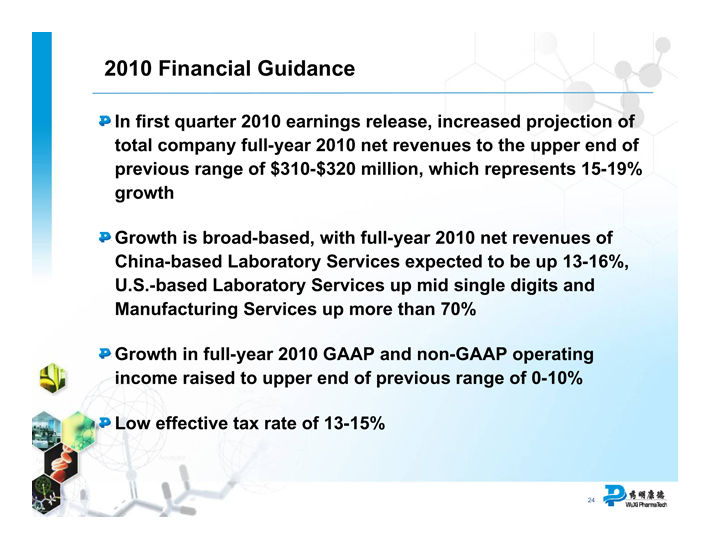
2010 Financial Guidance
In first quarter 2010 earnings release, increased projection of total company full-year 2010 net revenues to the upper end of previous range of $310-$320 million, which represents 15-19% growth
Growth is broad-based, with full-year 2010 net revenues of China-based Laboratory Services expected to be up 13-16%, U.S.-based Laboratory Services up mid single digits and Manufacturing Services up more than 70%
Growth in full-year 2010 GAAP and non-GAAP operating income raised to upper end of previous range of 0-10%
Low effective tax rate of 13-15%
WuXi PharmaTech
24

2011-2012 Growth Prospects
Strong revenue growth, driven by
•Continued solid growth in discovery chemistry
•Continued rapid growth in newer, downstream China-based laboratory services such as DMPK/ADME, discovery biology, pharmacology, formulation, process research, bioanalytical services
• Beginning of substantial revenue ramp-up from toxicology
• Significant revenues contribution from large-scale manufacturing
• Steady growth in small-scale research manufacturing
• Steady growth in U.S. laboratory services
WuXi PharmaTech
25
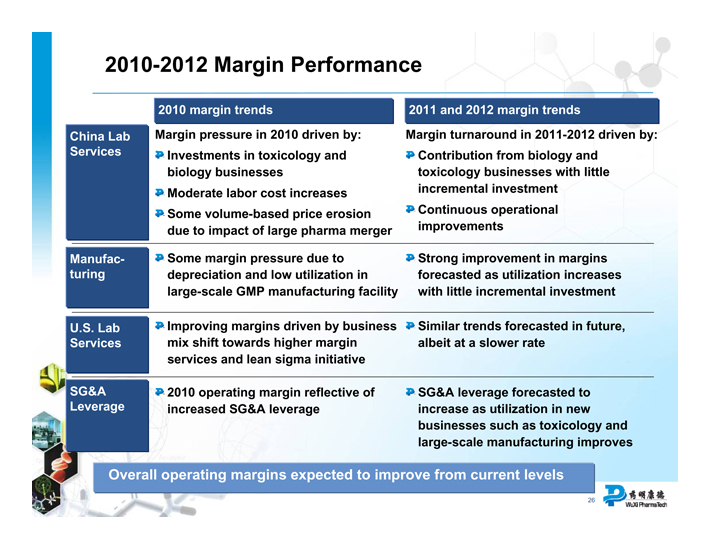
2010-2012 Margin Performance
China Lab Services
2010 margin trends
2011 and 2012 margin trends
Margin pressure in 2010 driven by:
Investments in toxicology and biology businesses
Moderate labor cost increases
Some volume-based price erosion due to impact of large pharma merger
Margin turnaround in 2011-2012 driven by:
Contribution from biology and toxicology businesses with little incremental investment
Continuous operational improvements
Manufacturing
Some margin pressure due to depreciation and low utilization in large-scale GMP manufacturing facility
Strong improvement in margins forecasted as utilization increases with little incremental investment
U.S. Lab Services
Improving margins driven by business
mix shift towards higher margin
services and lean sigma initiative
Similar trends forecasted in future,
albeit at a slower rate
SG&A Leverage
2010 operating margin reflective of increased SG&A leverage
SG&A leverage forecasted to
increase as utilization in new
businesses such as toxicology and
large-scale manufacturing improves
Overall operating margins expected to improve from current levels
WuXi PharmaTech
26

Key Discussion Points
Transaction Overview and Status Update
WuXi Overview
Positioning of Combined Company
Integration Update
WuXi PharmaTech
27
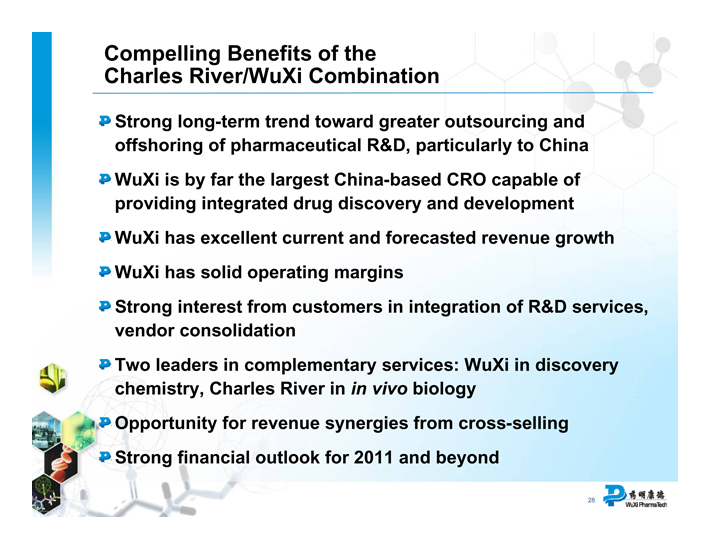
Compelling Benefits of the Charles River/WuXi Combination
Strong long-term trend toward greater outsourcing and offshoring of pharmaceutical R&D, particularly to China
WuXi is by far the largest China-based CRO capable of providing integrated drug discovery and development
WuXi has excellent current and forecasted revenue growth
WuXi has solid operating margins
Strong interest from customers in integration of R&D services, vendor consolidation
Two leaders in complementary services: WuXi in discovery chemistry, Charles River in in vivo biology
Opportunity for revenue synergies from cross-selling
Strong financial outlook for 2011 and beyond
WuXi PharmaTech
28
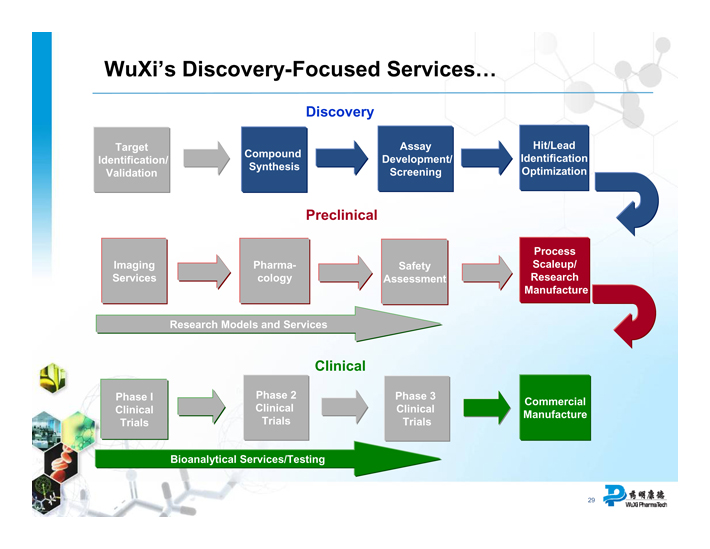
WuXi’s Discovery-Focused Services…
Discovery
Target Identification/ Validation
Compound Synthesis
Assay Development/ Screening
Hit/Lead Identification Optimization
Preclinical
Imaging Services
Pharmacology
Safety Assessment
Process Scaleup/ Research Manufacture
Research Models and Services
Clinical
Phase I Clinical Trials
Phase 2 Clinical Trials
Phase 3 Clinical Trials
Commercial Manufacture
Bioanalytical Services/Testing
WuXi PharmaTech
29
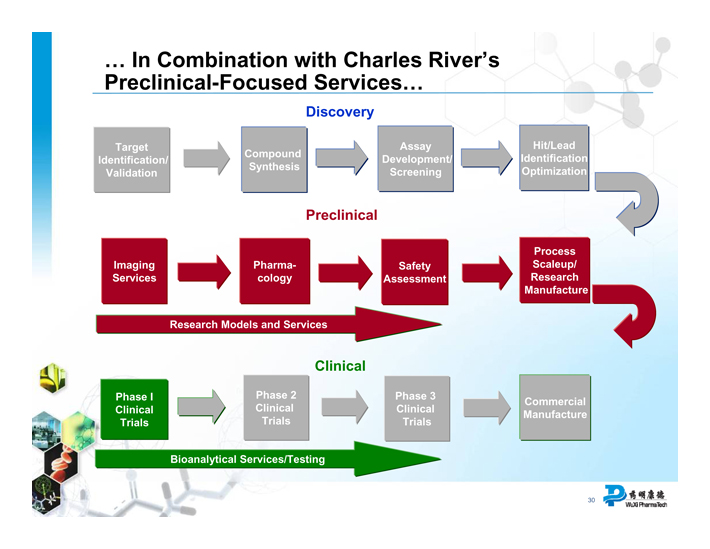
... In Combination with Charles River’s Preclinical-Focused Services...
Discovery
Target Identification/ Validation
Compound Synthesis
Assay Development/ Screening
Hit/Lead Identification Optimization
Preclinical
Imaging Services
Pharmacology
Safety Assessment
Process Scaleup/ Research Manufacture
Research Models and Services
Clinical
Phase I Clinical Trials
Phase 2 Clinical Trials
Phase 3 Clinical Trials
Commercial Manufacture
Bioanalytical Services/Testing
WuXi PharmaTech
30
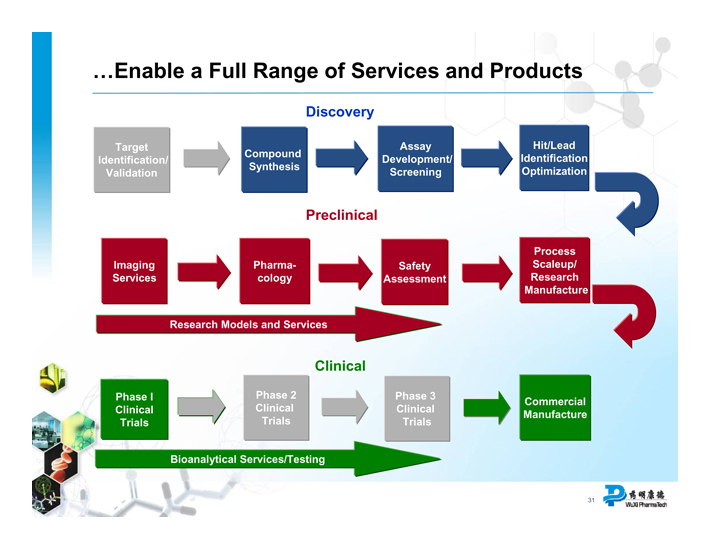
... Enable a Full Range of Services and Products
Discovery
Target Identification/ Validation
Compound Synthesis
Assay Development/ Screening
Hit/Lead Identification Optimization
Preclinical
Imaging Services
Pharmacology
Safety Assessment
Process Scaleup/ Research Manufacture
Research Models and Services
Clinical
Phase I Clinical Trials
Phase 2 Clinical Trials
Phase 3 Clinical Trials
Commercial Manufacture
Bioanalytical Services/Testing
WuXi PharmaTech
31
Revenue Synergies from Cross-Selling
WuXi’s China-based laboratory services business has about 150 customers, mainly large pharmaceutical companies
Charles River has about 5,000 customers, including many small and medium-sized companies, that WuXi could tap
WuXi’s sales force is largely its senior management; Charles River’s 200-person sales force could produce significant additional sales opportunities
R&D managers within pharmaceutical companies coordinate the advancement of molecules through development, and seek maximum speed and minimum disruption: Time is money
Pharmaceutical companies increasingly want broad, integrated service platforms from high-quality vendors
Revenue synergy opportunities from cross-selling and integrated services are large and real
WuXi PharmaTech
32

Enthusiastic Client Response
Senior management of CRL and WX each have reviewed the transaction with their respective top 20 pharmaceutical and biotechnology clients
Response has been overwhelmingly positive
Follow-up meetings have been scheduled/initiated
Clients are viewing this transformational combination as a strategic fit for the combined company, which offers them the opportunity to acquire more products and services from a single entity
Clients are requesting a more integrated approach to buying end-to-end services across the drug development continuum
Wall Street research reporting channel checks that substantiate client views on integrated approach
Value of CRL brand seen as an advantage in terms of standardizing products and services in China
WuXi PharmaTech
33
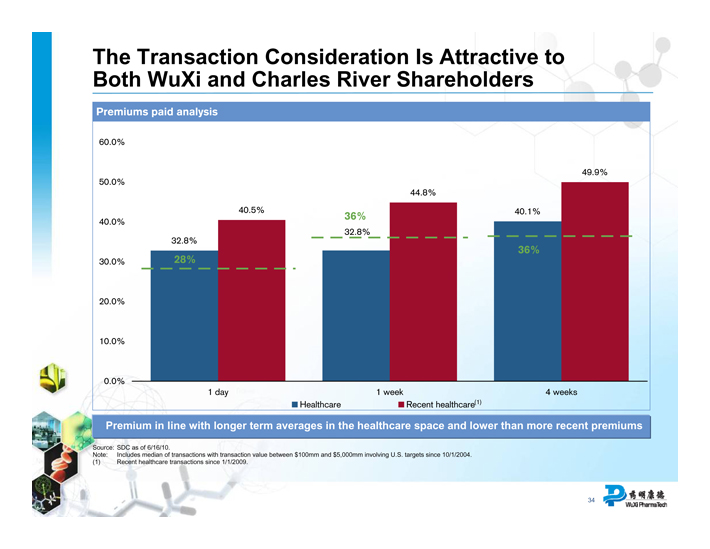
The Transaction Consideration Is Attractive to Both WuXi and Charles River Shareholders
Premiums paid analysis
60.0% 50.0% 40.0% 30.0% 20.0% 10.0% 0.0%
32.8%
28%
40.5%
36%
32.8%
44.8%
40.1%
36%
49.9%
1 day
Healthcare
1 week
Recent healthcare(1)
4 weeks
Premium in line with longer term averages in the healthcare space and lower than more recent premiums
Source: SDC as of 6/16/10.
Note: Includes median of transactions with transaction value between $100mm and $5,000mm involving U.S. targets since 10/1/2004. (1) Recent healthcare transactions since 1/1/2009.
WuXi PharmaTech
34
Key Discussion Points
Transaction Overview and Status Update
WuXi Overview
Positioning of Combined Company
Integration Update
WuXi PharmaTech
35

Limited Integration Risk
Four highest-ranking members of WuXi senior management will
remain, signed three-year contracts
All key members of WuXi senior management expected to stay
Three board members will join the CRL board
Both companies’ managements are very knowledgeable about
each others’ business
WuXi management is fully bi-cultural and bilingual
Most grew up in China and received extensive education at elite
U.S. universities and work experience in the U.S.
biopharmaceutical industry
Little overlap in operations, pointing to smooth transition
Integration planning is well advanced
WuXi PharmaTech
36

Additional Information
This document may be deemed to be solicitation material used in connection with the solicitation of proxies from Charles River shareholders to approve the proposed combination of Charles River and WuXi. In connection with the proposed transaction, Charles River has filed a preliminary proxy statement and will file a definitive proxy statement with the SEC. The information contained in the preliminary filing is not complete and may be changed. Before making any voting or investment decisions, Charles River’s investors and security holders are urged to read the definitive proxy statement when it becomes available and any other relevant documents filed with the SEC because they will contain important information. The definitive proxy statement will be mailed to the shareholders of Charles River seeking their approval of the proposed transaction. Charles River’s shareholders will also be able to obtain a copy of the definitive proxy statement free of charge by directing a request to: Charles River Laboratories, 251 Ballardvale Street, Wilmington, MA 01887, Attention: General Counsel. In addition, the preliminary proxy statement is and the definitive proxy statement will be available free of charge at the SEC’s website, www.sec.gov. Charles River’s shareholders may also access copies of the documents filed with the SEC by Charles River on Charles River’s website at www.criver.com.
This document is not a solicitation of proxies from WuXi’s security holders to approve the proposed combination. In connection with the proposed transaction, WuXi has filed a preliminary scheme document and will file a final scheme document that has been approved by the Grand Court of the Cayman Islands with the SEC on Form 6-K. The information contained in the preliminary scheme document is not complete and may be changed. Before making any voting or investment decisions, WuXi’s security holders are urged to read the final scheme document when it becomes available and any other relevant documents filed with the SEC because they will contain important information. The final scheme document will be mailed to WuXi’s security holders seeking their approval of the proposed combination. WuXi’s security holders will also be able to obtain a copy of the final scheme document free of charge by directing a request to: 288 Fute Zhong Road, Waigaoqiao Free Trade Zone, Shanghai 200131, People’s Republic of China, Attention: Genyong Qiu. In addition, the final scheme document will be available free of charge at the SEC’s website, www.sec.gov. WuXi’s security holders may also access copies of the documents filed with the SEC by WuXi on WuXi’s website at www.wuxiapptec.com.
Charles River, WuXi and their respective directors and executive officers and other members of management may be deemed to be participants in the solicitation of proxies in respect of the proposed transaction. Information regarding Charles River’s directors and executive officers is available in Charles River’s proxy statement for its 2010 annual meeting of shareholders, which was filed with the SEC on March 30, 2010. Information regarding the interests of Charles River’s directors and certain members of Charles River’s management in the proposed transaction is set forth in the preliminary proxy statement filed with the SEC. Information regarding WuXi’s directors and executive officers is available in WuXi’s annual report on Form 20-F for the fiscal year ended December 31, 2009, which was filed with the SEC on April 23, 2010. Information regarding the interests of WuXi’s directors and certain members of WuXi’s management in the proposed transaction is available in WuXi’s preliminary scheme document, which was filed on Form 6-K with the SEC on June 24, 2010.
This document does not constitute an offer of any securities for sale or a solicitation of an offer to buy any securities. The Charles River shares to be issued in the proposed transaction have not been and will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements. Charles River intends to issue such Charles River shares pursuant to the exemption from registration set forth in Section 3(a)(10) of the Securities Act.
WuXi PharmaTech
37

charles river
accelerating drug development. exactly.
Wuxi Pharmatech
Creating a CRO Powerhouse to Serve the Pharmaceutical Industry and Patients
Dr. Ge Li, WuXi Chairman/CEO
Edward Hu, WuXi COO

charles river
accelerating drug development. exactly.
Wuxi PharmaTech
Appendix
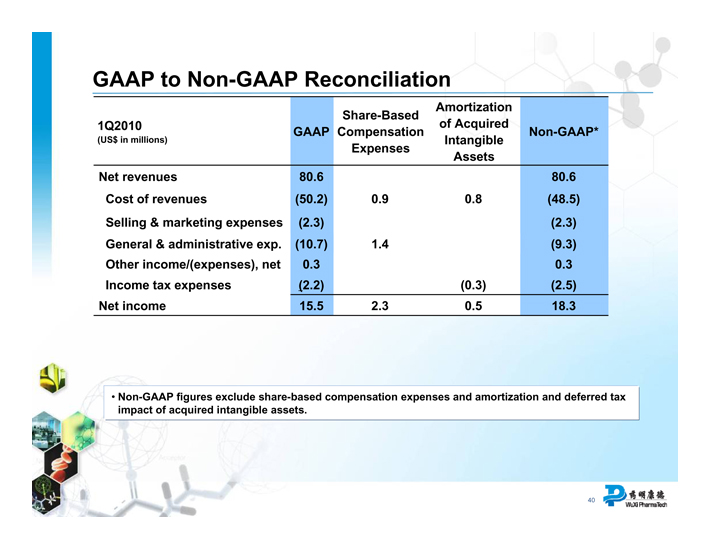
GAAP to Non-GAAP Reconciliation
Share-Based Amortization
1Q2010 of Acquired
GAAP Compensation Non-GAAP*
(US$ in millions) Intangible
Expenses Assets
Net revenues 80.6 80.6
Cost of revenues (50.2) 0.9 0.8 (48.5)
Selling & marketing expenses (2.3) (2.3)
General & administrative exp. (10.7) 1.4 (9.3)
Other income/(expenses), net 0.3 0.3
Income tax expenses (2.2) (0.3) (2.5)
Net income 15.5 2.3 0.5 18.3
Non-GAAP figures exclude share-based compensation expenses and amortization and deferred tax impact of acquired intangible assets.
WuXi PharmaTech
40
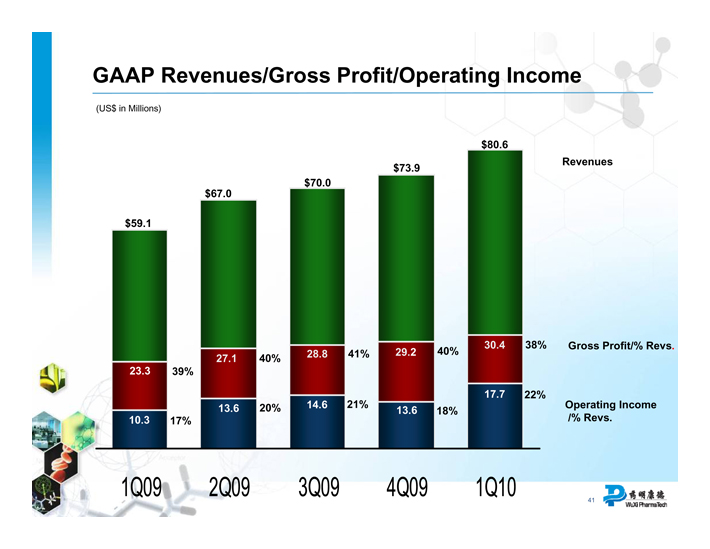
GAAP Revenues/Gross Profit/Operating Income
(US$ in Millions)
$59.1
$67.0
$70.0
$73.9
$80.6
23.3 39%
10.3 17%
27.1 40%
13.6 20%
28.8 41%
14.6 21%
29.2 40%
13.6 18%
30.4 38%
17.7 22%
Revenues
Gross Profit/% Revs.
Operating Income
/% Revs.
1Q09 2Q09 3Q09 4Q09 1Q10
WuXi PharmaTech
41

Non-GAAP Revenues/Gross Profit/Operating Income
(US$ in Millions)
$59.1
$67.0
$70.0
$73.9
$80.6
Revenues
24.7 42%
13.0 22%
28.4 42%
17.1 25%
30.2 43%
18.0 26%
30.8 42%
17.3 23%
32.1 40%
20.5 25%
Gross Profit/% Revs.
Operating Income/
% Revs.
1Q09 2Q09 3Q09 4Q09 1Q10
WuXi PharmaTech
42










































